CN100540503C - 制造陶瓷制品的方法和制造的陶瓷制品 - Google Patents
制造陶瓷制品的方法和制造的陶瓷制品 Download PDFInfo
- Publication number
- CN100540503C CN100540503C CNB2005800264282A CN200580026428A CN100540503C CN 100540503 C CN100540503 C CN 100540503C CN B2005800264282 A CNB2005800264282 A CN B2005800264282A CN 200580026428 A CN200580026428 A CN 200580026428A CN 100540503 C CN100540503 C CN 100540503C
- Authority
- CN
- China
- Prior art keywords
- weight
- ceramic
- batch
- green compact
- silicate mineral
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C04—CEMENTS; CONCRETE; ARTIFICIAL STONE; CERAMICS; REFRACTORIES
- C04B—LIME, MAGNESIA; SLAG; CEMENTS; COMPOSITIONS THEREOF, e.g. MORTARS, CONCRETE OR LIKE BUILDING MATERIALS; ARTIFICIAL STONE; CERAMICS; REFRACTORIES; TREATMENT OF NATURAL STONE
- C04B35/00—Shaped ceramic products characterised by their composition; Ceramics compositions; Processing powders of inorganic compounds preparatory to the manufacturing of ceramic products
- C04B35/01—Shaped ceramic products characterised by their composition; Ceramics compositions; Processing powders of inorganic compounds preparatory to the manufacturing of ceramic products based on oxide ceramics
- C04B35/16—Shaped ceramic products characterised by their composition; Ceramics compositions; Processing powders of inorganic compounds preparatory to the manufacturing of ceramic products based on oxide ceramics based on silicates other than clay
- C04B35/18—Shaped ceramic products characterised by their composition; Ceramics compositions; Processing powders of inorganic compounds preparatory to the manufacturing of ceramic products based on oxide ceramics based on silicates other than clay rich in aluminium oxide
- C04B35/195—Alkaline earth aluminosilicates, e.g. cordierite or anorthite
-
- C—CHEMISTRY; METALLURGY
- C04—CEMENTS; CONCRETE; ARTIFICIAL STONE; CERAMICS; REFRACTORIES
- C04B—LIME, MAGNESIA; SLAG; CEMENTS; COMPOSITIONS THEREOF, e.g. MORTARS, CONCRETE OR LIKE BUILDING MATERIALS; ARTIFICIAL STONE; CERAMICS; REFRACTORIES; TREATMENT OF NATURAL STONE
- C04B35/00—Shaped ceramic products characterised by their composition; Ceramics compositions; Processing powders of inorganic compounds preparatory to the manufacturing of ceramic products
- C04B35/01—Shaped ceramic products characterised by their composition; Ceramics compositions; Processing powders of inorganic compounds preparatory to the manufacturing of ceramic products based on oxide ceramics
- C04B35/16—Shaped ceramic products characterised by their composition; Ceramics compositions; Processing powders of inorganic compounds preparatory to the manufacturing of ceramic products based on oxide ceramics based on silicates other than clay
- C04B35/18—Shaped ceramic products characterised by their composition; Ceramics compositions; Processing powders of inorganic compounds preparatory to the manufacturing of ceramic products based on oxide ceramics based on silicates other than clay rich in aluminium oxide
- C04B35/185—Mullite 3Al2O3-2SiO2
-
- C—CHEMISTRY; METALLURGY
- C04—CEMENTS; CONCRETE; ARTIFICIAL STONE; CERAMICS; REFRACTORIES
- C04B—LIME, MAGNESIA; SLAG; CEMENTS; COMPOSITIONS THEREOF, e.g. MORTARS, CONCRETE OR LIKE BUILDING MATERIALS; ARTIFICIAL STONE; CERAMICS; REFRACTORIES; TREATMENT OF NATURAL STONE
- C04B35/00—Shaped ceramic products characterised by their composition; Ceramics compositions; Processing powders of inorganic compounds preparatory to the manufacturing of ceramic products
- C04B35/622—Forming processes; Processing powders of inorganic compounds preparatory to the manufacturing of ceramic products
- C04B35/626—Preparing or treating the powders individually or as batches ; preparing or treating macroscopic reinforcing agents for ceramic products, e.g. fibres; mechanical aspects section B
- C04B35/62605—Treating the starting powders individually or as mixtures
- C04B35/62625—Wet mixtures
- C04B35/6263—Wet mixtures characterised by their solids loadings, i.e. the percentage of solids
-
- C—CHEMISTRY; METALLURGY
- C04—CEMENTS; CONCRETE; ARTIFICIAL STONE; CERAMICS; REFRACTORIES
- C04B—LIME, MAGNESIA; SLAG; CEMENTS; COMPOSITIONS THEREOF, e.g. MORTARS, CONCRETE OR LIKE BUILDING MATERIALS; ARTIFICIAL STONE; CERAMICS; REFRACTORIES; TREATMENT OF NATURAL STONE
- C04B35/00—Shaped ceramic products characterised by their composition; Ceramics compositions; Processing powders of inorganic compounds preparatory to the manufacturing of ceramic products
- C04B35/622—Forming processes; Processing powders of inorganic compounds preparatory to the manufacturing of ceramic products
- C04B35/626—Preparing or treating the powders individually or as batches ; preparing or treating macroscopic reinforcing agents for ceramic products, e.g. fibres; mechanical aspects section B
- C04B35/63—Preparing or treating the powders individually or as batches ; preparing or treating macroscopic reinforcing agents for ceramic products, e.g. fibres; mechanical aspects section B using additives specially adapted for forming the products, e.g.. binder binders
- C04B35/632—Organic additives
-
- C—CHEMISTRY; METALLURGY
- C04—CEMENTS; CONCRETE; ARTIFICIAL STONE; CERAMICS; REFRACTORIES
- C04B—LIME, MAGNESIA; SLAG; CEMENTS; COMPOSITIONS THEREOF, e.g. MORTARS, CONCRETE OR LIKE BUILDING MATERIALS; ARTIFICIAL STONE; CERAMICS; REFRACTORIES; TREATMENT OF NATURAL STONE
- C04B35/00—Shaped ceramic products characterised by their composition; Ceramics compositions; Processing powders of inorganic compounds preparatory to the manufacturing of ceramic products
- C04B35/622—Forming processes; Processing powders of inorganic compounds preparatory to the manufacturing of ceramic products
- C04B35/626—Preparing or treating the powders individually or as batches ; preparing or treating macroscopic reinforcing agents for ceramic products, e.g. fibres; mechanical aspects section B
- C04B35/63—Preparing or treating the powders individually or as batches ; preparing or treating macroscopic reinforcing agents for ceramic products, e.g. fibres; mechanical aspects section B using additives specially adapted for forming the products, e.g.. binder binders
- C04B35/632—Organic additives
- C04B35/636—Polysaccharides or derivatives thereof
- C04B35/6365—Cellulose or derivatives thereof
-
- C—CHEMISTRY; METALLURGY
- C04—CEMENTS; CONCRETE; ARTIFICIAL STONE; CERAMICS; REFRACTORIES
- C04B—LIME, MAGNESIA; SLAG; CEMENTS; COMPOSITIONS THEREOF, e.g. MORTARS, CONCRETE OR LIKE BUILDING MATERIALS; ARTIFICIAL STONE; CERAMICS; REFRACTORIES; TREATMENT OF NATURAL STONE
- C04B2235/00—Aspects relating to ceramic starting mixtures or sintered ceramic products
- C04B2235/02—Composition of constituents of the starting material or of secondary phases of the final product
- C04B2235/30—Constituents and secondary phases not being of a fibrous nature
- C04B2235/32—Metal oxides, mixed metal oxides, or oxide-forming salts thereof, e.g. carbonates, nitrates, (oxy)hydroxides, chlorides
- C04B2235/3205—Alkaline earth oxides or oxide forming salts thereof, e.g. beryllium oxide
- C04B2235/3206—Magnesium oxides or oxide-forming salts thereof
-
- C—CHEMISTRY; METALLURGY
- C04—CEMENTS; CONCRETE; ARTIFICIAL STONE; CERAMICS; REFRACTORIES
- C04B—LIME, MAGNESIA; SLAG; CEMENTS; COMPOSITIONS THEREOF, e.g. MORTARS, CONCRETE OR LIKE BUILDING MATERIALS; ARTIFICIAL STONE; CERAMICS; REFRACTORIES; TREATMENT OF NATURAL STONE
- C04B2235/00—Aspects relating to ceramic starting mixtures or sintered ceramic products
- C04B2235/02—Composition of constituents of the starting material or of secondary phases of the final product
- C04B2235/30—Constituents and secondary phases not being of a fibrous nature
- C04B2235/32—Metal oxides, mixed metal oxides, or oxide-forming salts thereof, e.g. carbonates, nitrates, (oxy)hydroxides, chlorides
- C04B2235/3217—Aluminum oxide or oxide forming salts thereof, e.g. bauxite, alpha-alumina
-
- C—CHEMISTRY; METALLURGY
- C04—CEMENTS; CONCRETE; ARTIFICIAL STONE; CERAMICS; REFRACTORIES
- C04B—LIME, MAGNESIA; SLAG; CEMENTS; COMPOSITIONS THEREOF, e.g. MORTARS, CONCRETE OR LIKE BUILDING MATERIALS; ARTIFICIAL STONE; CERAMICS; REFRACTORIES; TREATMENT OF NATURAL STONE
- C04B2235/00—Aspects relating to ceramic starting mixtures or sintered ceramic products
- C04B2235/02—Composition of constituents of the starting material or of secondary phases of the final product
- C04B2235/30—Constituents and secondary phases not being of a fibrous nature
- C04B2235/32—Metal oxides, mixed metal oxides, or oxide-forming salts thereof, e.g. carbonates, nitrates, (oxy)hydroxides, chlorides
- C04B2235/3217—Aluminum oxide or oxide forming salts thereof, e.g. bauxite, alpha-alumina
- C04B2235/3218—Aluminium (oxy)hydroxides, e.g. boehmite, gibbsite, alumina sol
-
- C—CHEMISTRY; METALLURGY
- C04—CEMENTS; CONCRETE; ARTIFICIAL STONE; CERAMICS; REFRACTORIES
- C04B—LIME, MAGNESIA; SLAG; CEMENTS; COMPOSITIONS THEREOF, e.g. MORTARS, CONCRETE OR LIKE BUILDING MATERIALS; ARTIFICIAL STONE; CERAMICS; REFRACTORIES; TREATMENT OF NATURAL STONE
- C04B2235/00—Aspects relating to ceramic starting mixtures or sintered ceramic products
- C04B2235/02—Composition of constituents of the starting material or of secondary phases of the final product
- C04B2235/30—Constituents and secondary phases not being of a fibrous nature
- C04B2235/34—Non-metal oxides, non-metal mixed oxides, or salts thereof that form the non-metal oxides upon heating, e.g. carbonates, nitrates, (oxy)hydroxides, chlorides
- C04B2235/3418—Silicon oxide, silicic acids or oxide forming salts thereof, e.g. silica sol, fused silica, silica fume, cristobalite, quartz or flint
-
- C—CHEMISTRY; METALLURGY
- C04—CEMENTS; CONCRETE; ARTIFICIAL STONE; CERAMICS; REFRACTORIES
- C04B—LIME, MAGNESIA; SLAG; CEMENTS; COMPOSITIONS THEREOF, e.g. MORTARS, CONCRETE OR LIKE BUILDING MATERIALS; ARTIFICIAL STONE; CERAMICS; REFRACTORIES; TREATMENT OF NATURAL STONE
- C04B2235/00—Aspects relating to ceramic starting mixtures or sintered ceramic products
- C04B2235/02—Composition of constituents of the starting material or of secondary phases of the final product
- C04B2235/30—Constituents and secondary phases not being of a fibrous nature
- C04B2235/34—Non-metal oxides, non-metal mixed oxides, or salts thereof that form the non-metal oxides upon heating, e.g. carbonates, nitrates, (oxy)hydroxides, chlorides
- C04B2235/3427—Silicates other than clay, e.g. water glass
- C04B2235/3436—Alkaline earth metal silicates, e.g. barium silicate
- C04B2235/3445—Magnesium silicates, e.g. forsterite
-
- C—CHEMISTRY; METALLURGY
- C04—CEMENTS; CONCRETE; ARTIFICIAL STONE; CERAMICS; REFRACTORIES
- C04B—LIME, MAGNESIA; SLAG; CEMENTS; COMPOSITIONS THEREOF, e.g. MORTARS, CONCRETE OR LIKE BUILDING MATERIALS; ARTIFICIAL STONE; CERAMICS; REFRACTORIES; TREATMENT OF NATURAL STONE
- C04B2235/00—Aspects relating to ceramic starting mixtures or sintered ceramic products
- C04B2235/02—Composition of constituents of the starting material or of secondary phases of the final product
- C04B2235/30—Constituents and secondary phases not being of a fibrous nature
- C04B2235/34—Non-metal oxides, non-metal mixed oxides, or salts thereof that form the non-metal oxides upon heating, e.g. carbonates, nitrates, (oxy)hydroxides, chlorides
- C04B2235/3427—Silicates other than clay, e.g. water glass
- C04B2235/3463—Alumino-silicates other than clay, e.g. mullite
-
- C—CHEMISTRY; METALLURGY
- C04—CEMENTS; CONCRETE; ARTIFICIAL STONE; CERAMICS; REFRACTORIES
- C04B—LIME, MAGNESIA; SLAG; CEMENTS; COMPOSITIONS THEREOF, e.g. MORTARS, CONCRETE OR LIKE BUILDING MATERIALS; ARTIFICIAL STONE; CERAMICS; REFRACTORIES; TREATMENT OF NATURAL STONE
- C04B2235/00—Aspects relating to ceramic starting mixtures or sintered ceramic products
- C04B2235/02—Composition of constituents of the starting material or of secondary phases of the final product
- C04B2235/30—Constituents and secondary phases not being of a fibrous nature
- C04B2235/34—Non-metal oxides, non-metal mixed oxides, or salts thereof that form the non-metal oxides upon heating, e.g. carbonates, nitrates, (oxy)hydroxides, chlorides
- C04B2235/3427—Silicates other than clay, e.g. water glass
- C04B2235/3463—Alumino-silicates other than clay, e.g. mullite
- C04B2235/3481—Alkaline earth metal alumino-silicates other than clay, e.g. cordierite, beryl, micas such as margarite, plagioclase feldspars such as anorthite, zeolites such as chabazite
-
- C—CHEMISTRY; METALLURGY
- C04—CEMENTS; CONCRETE; ARTIFICIAL STONE; CERAMICS; REFRACTORIES
- C04B—LIME, MAGNESIA; SLAG; CEMENTS; COMPOSITIONS THEREOF, e.g. MORTARS, CONCRETE OR LIKE BUILDING MATERIALS; ARTIFICIAL STONE; CERAMICS; REFRACTORIES; TREATMENT OF NATURAL STONE
- C04B2235/00—Aspects relating to ceramic starting mixtures or sintered ceramic products
- C04B2235/02—Composition of constituents of the starting material or of secondary phases of the final product
- C04B2235/30—Constituents and secondary phases not being of a fibrous nature
- C04B2235/34—Non-metal oxides, non-metal mixed oxides, or salts thereof that form the non-metal oxides upon heating, e.g. carbonates, nitrates, (oxy)hydroxides, chlorides
- C04B2235/349—Clays, e.g. bentonites, smectites such as montmorillonite, vermiculites or kaolines, e.g. illite, talc or sepiolite
-
- C—CHEMISTRY; METALLURGY
- C04—CEMENTS; CONCRETE; ARTIFICIAL STONE; CERAMICS; REFRACTORIES
- C04B—LIME, MAGNESIA; SLAG; CEMENTS; COMPOSITIONS THEREOF, e.g. MORTARS, CONCRETE OR LIKE BUILDING MATERIALS; ARTIFICIAL STONE; CERAMICS; REFRACTORIES; TREATMENT OF NATURAL STONE
- C04B2235/00—Aspects relating to ceramic starting mixtures or sintered ceramic products
- C04B2235/02—Composition of constituents of the starting material or of secondary phases of the final product
- C04B2235/30—Constituents and secondary phases not being of a fibrous nature
- C04B2235/44—Metal salt constituents or additives chosen for the nature of the anions, e.g. hydrides or acetylacetonate
- C04B2235/449—Organic acids, e.g. EDTA, citrate, acetate, oxalate
-
- C—CHEMISTRY; METALLURGY
- C04—CEMENTS; CONCRETE; ARTIFICIAL STONE; CERAMICS; REFRACTORIES
- C04B—LIME, MAGNESIA; SLAG; CEMENTS; COMPOSITIONS THEREOF, e.g. MORTARS, CONCRETE OR LIKE BUILDING MATERIALS; ARTIFICIAL STONE; CERAMICS; REFRACTORIES; TREATMENT OF NATURAL STONE
- C04B2235/00—Aspects relating to ceramic starting mixtures or sintered ceramic products
- C04B2235/02—Composition of constituents of the starting material or of secondary phases of the final product
- C04B2235/50—Constituents or additives of the starting mixture chosen for their shape or used because of their shape or their physical appearance
- C04B2235/52—Constituents or additives characterised by their shapes
- C04B2235/5208—Fibers
- C04B2235/5216—Inorganic
- C04B2235/522—Oxidic
- C04B2235/5228—Silica and alumina, including aluminosilicates, e.g. mullite
-
- C—CHEMISTRY; METALLURGY
- C04—CEMENTS; CONCRETE; ARTIFICIAL STONE; CERAMICS; REFRACTORIES
- C04B—LIME, MAGNESIA; SLAG; CEMENTS; COMPOSITIONS THEREOF, e.g. MORTARS, CONCRETE OR LIKE BUILDING MATERIALS; ARTIFICIAL STONE; CERAMICS; REFRACTORIES; TREATMENT OF NATURAL STONE
- C04B2235/00—Aspects relating to ceramic starting mixtures or sintered ceramic products
- C04B2235/02—Composition of constituents of the starting material or of secondary phases of the final product
- C04B2235/50—Constituents or additives of the starting mixture chosen for their shape or used because of their shape or their physical appearance
- C04B2235/52—Constituents or additives characterised by their shapes
- C04B2235/5296—Constituents or additives characterised by their shapes with a defined aspect ratio, e.g. indicating sphericity
-
- C—CHEMISTRY; METALLURGY
- C04—CEMENTS; CONCRETE; ARTIFICIAL STONE; CERAMICS; REFRACTORIES
- C04B—LIME, MAGNESIA; SLAG; CEMENTS; COMPOSITIONS THEREOF, e.g. MORTARS, CONCRETE OR LIKE BUILDING MATERIALS; ARTIFICIAL STONE; CERAMICS; REFRACTORIES; TREATMENT OF NATURAL STONE
- C04B2235/00—Aspects relating to ceramic starting mixtures or sintered ceramic products
- C04B2235/60—Aspects relating to the preparation, properties or mechanical treatment of green bodies or pre-forms
- C04B2235/602—Making the green bodies or pre-forms by moulding
- C04B2235/6021—Extrusion moulding
-
- C—CHEMISTRY; METALLURGY
- C04—CEMENTS; CONCRETE; ARTIFICIAL STONE; CERAMICS; REFRACTORIES
- C04B—LIME, MAGNESIA; SLAG; CEMENTS; COMPOSITIONS THEREOF, e.g. MORTARS, CONCRETE OR LIKE BUILDING MATERIALS; ARTIFICIAL STONE; CERAMICS; REFRACTORIES; TREATMENT OF NATURAL STONE
- C04B2235/00—Aspects relating to ceramic starting mixtures or sintered ceramic products
- C04B2235/70—Aspects relating to sintered or melt-casted ceramic products
- C04B2235/80—Phases present in the sintered or melt-cast ceramic products other than the main phase
-
- C—CHEMISTRY; METALLURGY
- C04—CEMENTS; CONCRETE; ARTIFICIAL STONE; CERAMICS; REFRACTORIES
- C04B—LIME, MAGNESIA; SLAG; CEMENTS; COMPOSITIONS THEREOF, e.g. MORTARS, CONCRETE OR LIKE BUILDING MATERIALS; ARTIFICIAL STONE; CERAMICS; REFRACTORIES; TREATMENT OF NATURAL STONE
- C04B2235/00—Aspects relating to ceramic starting mixtures or sintered ceramic products
- C04B2235/70—Aspects relating to sintered or melt-casted ceramic products
- C04B2235/96—Properties of ceramic products, e.g. mechanical properties such as strength, toughness, wear resistance
-
- C—CHEMISTRY; METALLURGY
- C04—CEMENTS; CONCRETE; ARTIFICIAL STONE; CERAMICS; REFRACTORIES
- C04B—LIME, MAGNESIA; SLAG; CEMENTS; COMPOSITIONS THEREOF, e.g. MORTARS, CONCRETE OR LIKE BUILDING MATERIALS; ARTIFICIAL STONE; CERAMICS; REFRACTORIES; TREATMENT OF NATURAL STONE
- C04B2235/00—Aspects relating to ceramic starting mixtures or sintered ceramic products
- C04B2235/70—Aspects relating to sintered or melt-casted ceramic products
- C04B2235/96—Properties of ceramic products, e.g. mechanical properties such as strength, toughness, wear resistance
- C04B2235/9607—Thermal properties, e.g. thermal expansion coefficient
Landscapes
- Engineering & Computer Science (AREA)
- Chemical & Material Sciences (AREA)
- Ceramic Engineering (AREA)
- Manufacturing & Machinery (AREA)
- Organic Chemistry (AREA)
- Structural Engineering (AREA)
- Materials Engineering (AREA)
- Inorganic Chemistry (AREA)
- Compositions Of Oxide Ceramics (AREA)
- Press-Shaping Or Shaping Using Conveyers (AREA)
- Filtering Of Dispersed Particles In Gases (AREA)
- Porous Artificial Stone Or Porous Ceramic Products (AREA)
- Filtering Materials (AREA)
Abstract
制造陶瓷制品的方法,该方法包括提供包含以下组分的批料:(1)包含滑石、氧化铝和二氧化硅的无机原料的混合物,(2)包含水溶性有机粘结剂和具有高长径比以及大表面积的纤维状硅酸盐矿物的粘结剂,(3)极性溶剂;混合批料组分,形成均相增塑体;将增塑体成形为生坯体,该生坯体具有提高的强度;通过加热到一定温度并保持一定时间烧结该生坯体,使生坯体发生转化并充分转化为煅烧陶瓷制品。
Description
发明领域
本发明总体上涉及由可模塑粉状混合物制造陶瓷制品的方法,所述混合物通过对无机颗粒原料与包含水溶性有机粘结剂及无机粘结剂的粘结剂体系以及作为溶剂的水进行混合形成。更具体地,所述方法涉及制造堇青石制品,该制品具有提高的强度,能够在烧结过程中抗碎裂和抗损伤。
发明背景
通常由挤塑方法形成的普通独块多孔陶瓷体,如能应用于催化转化炉、柴油机颗粒过滤器、电加热的催化剂和化学处理催化剂的堇青石蜂窝状基材,需要粘结剂和其他类似助剂进行适当处理。通常,粘结剂是有机材料,必须满足许多要求。
例如,粘结剂必须与陶瓷材料相适应,以便形成在粘结剂中具有较高陶瓷材料含量的可流动的分散体。另外,通过使粘结剂中的陶瓷分散体成形而产生的“生坯”预成形体应当具有合理的强度,以便能够被搬运。
为能按需要烧除粘结剂,粘结剂应当能够从成形的陶瓷件中除去,而不会导致陶瓷件变形或碎裂。另外,不含粘结剂的预成形体应当具有足够的强度,进行无缺陷的固结。满足这些要求的粘结剂配方十分复杂,而且本领域已知有大量不同粘结剂配方。
形成堇青石的批料中一般采用水溶性纤维素醚的粘结剂。用这些粘结剂,结果可以得到具有良好“湿”强度和良好的尺寸及形状完整性的成形生坯体。“湿”强度用来描述生坯体在挤塑之后但在干燥之前的强度。“生坯”强度指生坯体在干燥之后但在煅烧之前的强度。
在100-600℃温度范围,更具体地在约300℃能烧除的纤维素醚粘结剂,在除去时很容易导致陶瓷件变形或破裂。在煅烧过程中除去有机组分涉及一系列同时发生的反应,这些反应相当复杂,例如,包括氧化、挥发和热降解。
因此,处理含有机粘结剂的增塑混合物的主要障碍是,后面形成的陶瓷制品的生坯在煅烧时会产生裂纹,尤其是在具有薄壁的蜂窝结构中。碎裂是在除去大量有机物的过程中产生内应力的结果,该过程使坯体内部产生过高的温度或压力梯度。
因此,在煅烧过程中必须考虑采取特殊措施,以避免陶瓷体产生裂纹。例如,采用长煅烧周期、专门设计的窑炉以及类似措施,以控制有机粘结剂的烧除,降低热应力、收缩差异和高发碎裂频率。不过,这些方法需要昂贵的复杂设备,增加了煅烧和制造成本。
考虑到本领域碰到的前述问题,仍然需要一种以成本经济和高效的方式制造陶瓷制品,特别是堇青石陶瓷体的方法,所得陶瓷体具有提高的强度,在烧结过程中能够抵抗在陶瓷体内产生的热应力和收缩,从而使这些制品在煅烧后几乎没有裂纹和缺陷。
发明概述
本发明是通过提供包含粉末无机原料、粘结剂和溶剂的批料来制造陶瓷制品的方法。所述粘结剂包含诸如纤维素醚粘结剂的水溶性有机粘结剂和纤维状硅酸盐矿物。纤维状硅酸盐材料优选具有高长径比(aspect)(优选高于500)并有大表面积(优选大于100平方米/克)。纤维状硅酸盐材料优选进一步具有1-2微米中值粒径的特征。所述批料还包含极性溶剂。例如,一种合适的极性溶剂是水。
在一个实施方式中,无机粉末材料是形成堇青石原料的混合物,包括二氧化硅、滑石和氧化铝,任选包含粘土和形成堇青石的其他原料,每种原料以有效量存在,能够与其他批料组分组合,形成主相是堇青石的煅烧陶瓷制品,该制品的。
将批料组分混合在一起,形成均相增塑体,然后成形为生坯体。成形过程可采用本领域已知的任何方法进行。在一个实施方式中,生坯体是独块蜂窝体。为形成这种结构,优选将增塑体从蜂窝体模具中挤出。最后,将生坯体烧结到一定温度保持一定时间,使生坯体发生转化,并充分转化为煅烧的陶瓷制品。
已经发现,通过使用有机和无机组分作为粘结剂,所述类型的陶瓷制品可以煅烧得更快,且裂纹更少或没有裂纹。特别地,生坯体在300-900℃的温度区间提高了强度,因此在后面的烧结过程中,更能抗碎裂和破损,不容易产生裂纹和破损。无机组分,优选为纤维状硅酸盐矿物的优选用量为2-10%,以在300-900℃的温度区间提供提高的强度。一种优选的纤维状硅酸盐矿物是绿坡缕石粘土。此外,当用少量(例如1-3%)的有机和无机组分作粘结剂时,该结构的煅烧强度可得到提高。
本发明另一方面提供一种陶瓷制品,该陶瓷制品包含堇青石的主相,其组成以氧化物基准表示,为33-41%氧化铝、46-53%二氧化硅和11-17%氧化镁,其中所述制品由包含以下组分的批料制造:包含滑石、氧化铝和二氧化硅的无机原料混合物;包含水溶性有机粘结剂和长径比大于500、表面积大于100平方米/克的纤维状硅酸盐矿物的粘结剂;极性溶剂。
附图简述
在阅读后面的详细描述时结合考虑以下附图,将能完整地理解本发明:
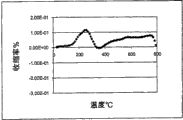
图1所示为挤塑形成的不含绿坡缕石粘土的堇青石生坯样品的收缩率与温度(最高至800℃)的关系图。
图2所示为挤塑形成的含5重量%绿坡缕石粘土的堇青石生坯样品的收缩率与温度(最高至800℃)的关系图。
发明详述
本发明适用于对陶瓷粉末加工,用来由可模塑批料制造成形制品,所述批料包含无机原料、粘结剂和溶剂。然而,本发明特别适用于形成包含堇青石和/或富铝红柱石的陶瓷制品。这种陶瓷制品的例子包括2-60%富铝红柱石和30-97%堇青石的混合物,还允许包含其他相,其含量通常最多达10重量%。
特别适合在本发明中用来形成堇青石的一些陶瓷批料组合物是美国专利6541407中揭示的那些,该专利被参考结合于此。煅烧后最终形成堇青石的陶瓷原料的一个实例如下(重量百分比,总计100重量%):33-41%氧化铝、46-53%二氧化硅和11-17%氧化镁。
在批料组合物中采用的无机原料可以是合成制得的原料,如氧化物、氢氧化物等,也可以是天然原料,如粘土、滑石,还可以是它们的组合。本发明不限于粉末或原料的类型,它们可根据陶瓷体所需的性质进行选择。
适合形成含堇青石的陶瓷制品的形成堇青石的无机陶瓷粉末原料可选自任何来源,优选包含高纯滑石、二氧化硅、氧化铝、粘土和能产生氧化镁的原料。优选原料是滑石、二氧化铝和氧化硅。
滑石具有大于15微米(μm)但小于35微米的中值粒径(MPS)。它具有小片型形态,可促进低CTE的煅烧陶瓷制品。合适的滑石形态指数(即衡量滑石扁平程度(platiness)的指标)大于0.75,如美国专利第5141686号所进一步揭示的。许多合适种类的氧化铝都可用作氧化铝源,如α-氧化铝、γ-氧化铝、ρ-氧化铝、氢氧化铝、勃姆石和它们的混合物。氧化铝具有5-25微米的中值粒径。二氧化硅包括但不限于石英、方石英、非晶二氧化硅(如熔凝二氧化硅或溶胶-凝胶二氧化硅)、沸石、硅藻土和它们的组合。二氧化硅具有10-35微米的中值粒径。无机原料还任选包含粘土,如高岭土。
本发明的粘结剂包含诸如纤维素醚粘结剂的水溶性有机粘结剂和无机组分。无机组分优选纤维状硅酸盐矿物。它优选具有高长径比(优选高于500)并有大表面积(优选大于100平方米/克),其表面带有大量电荷且与极性溶剂(如水)具有强相互作用。纤维状硅酸盐矿物优选的另一个特征是具有1-2微米的中值粒径。
对于形成堇青石的批料,合适的纤维状硅酸盐矿物是绿坡缕石粘土,它是水合硅铝酸镁粘土。绿坡缕石粘土中含有非常细的纤维状或针状颗粒,因而具有高长径比和大表面积。其长径比通常至少大于500。此材料的来源可以商品名Acti-gelTM 208得自Active Materials Company,Hunt Valley,Maryland。为达到如前所述的坯体提高的强度,向批料中加入纤维状硅酸盐矿物的量至少2.0重量%但不超过10.0重量%,优选至少5.0重量%但不超过10.0重量%。然而,如下面将要介绍的,加入少量(1-3%)即可增加陶瓷制品的最终强度。
合适的纤维素醚粘结剂是甲基纤维素、乙羟基乙基纤维素、羟丁基甲基纤维素、羟甲基纤维素、羟丙基甲基纤维素、羟乙基甲基纤维素、羟丁基纤维素、羟乙基纤维素、羟丙基纤维素、羧甲基纤维素钠和它们的混合物。甲基纤维素和/或甲基纤维素衍生物特别合适,优选甲基纤维素、羟丙基甲基纤维素或它们的组合(可以商品名MetholTM得自Dow Chemical Co.)。
有机粘结剂优选以2.5-10.0重量%的量加入,更优选2.5-5.0重量%。如果有机粘结剂的含量太低,将损害批料的可塑性,这将导致批料在挤塑过程中产生裂纹,因为批料在拉伸时不可避免要产生断裂。有机粘结剂是作为超添加物(super-addition)加入到无机原料粉末混合物中的。超添加是指在100克无机原料混合物中加入(例如)2.5-10克金属氧化物。
粘结剂体系和粉末材料通过溶剂如水混合在一起,所述溶剂润湿粉末材料,并作为溶解粘结剂的介质,从而赋予批料以可塑性。批料还可包含其他有机或无机组分作为任选的加工助剂。这些助剂包括表面活性剂、润滑剂、分散剂、油等。油组分提供了使混合物成形所需的流动性,同时保持粘结剂在溶剂中的强度。合适的油包括如矿物油的石蜡油、氢化聚丁烯、α-烯烃、内烯烃、聚苯醚、聚丁烯和聚异丁烯。
如果存在表面活性剂,它将促进溶剂与油组分之间的乳化作用。它能分散或湿润无机粉末。通常,在无其他物质存在的情况下,表面活性剂本身在室温下是不溶于溶剂的。合适的表面活性剂是油酸、月桂酸、硬脂酸和它们的组合。增塑批料中的润滑助剂是本领域已知的。这种组分的一个合适例子是硬脂酸钠。
批料还可包含成孔剂,它是可以在煅烧阶段自生坯体中烧掉的任何颗粒物质(非粘结剂)。合适类型的成孔剂包括石墨、淀粉、聚合物、纤维素、面粉等。石墨是一种优选的成孔剂,因为它对加工过程的负作用最小。也可采用成孔剂的组合。
在制备本发明的陶瓷体时,可模塑批料优选通过混合粉末原料与粘结剂体系以及其他任选组分形成增塑混合物来制备。批料组分按选定的任何所需量混合。
在一个实施方式中,批料包含100重量%形成堇青石的无机原料,2.0-10.0重量%绿坡缕石粘土,并且以100重量%形成堇青石的原料为基准,包含2.5-10重量%甲基纤维素,最多和包含3重量%硬脂酸钠,最多和包含30重量%石墨,以及25.0-40.0重量%作为溶剂的水。
在另一个实施方式中,可模塑粉末包含100重量%形成堇青石的无机原料,5.0-10.0重量%绿坡缕石,并且以100重量%形成堇青石的原料为基准,包含2.5-5.0重量%甲基纤维素,最多和包含3重量%硬脂酸钠,最多和包含30重量%石墨,以及25.0-40.0重量%作为溶剂的水。
粘结剂体系的各组分与陶瓷粉末原料、其他任选批料组分和足量的溶剂(即水)混合,形成均匀的可成形混合物。特别地,对于用来形成陶瓷产品的批料,该批料在成形步骤之前分两个阶段形成。在形成批料的第一阶段即润湿阶段,陶瓷材料与粘结剂组分和其他干组分一起进行干混合,然后加入水。举例来说,在本领域已知的Littleford混合器中进行混合。
第二阶段涉及批料的增塑。通常,第一阶段形成的湿混合物在任何合适的用来增塑批料的混合器中进行剪切,所述混合器如双螺杆挤出机/混合器、螺旋混合器、研磨混合器或双臂混合器等。
然后用已知对增塑混合物进行成形的方法,最好是通过模具挤塑的方法将所得增塑混合物成形为生坯体。挤塑操作中,可以采用液压式挤压机,或双段排气的单螺旋挤出机,或在出料端连有模具组件的双螺杆混合器进行垂直或者水平的挤塑。如果采用后者,合适的螺杆元件是根据原料和其他加工条件选择的,以产生足够的压力,迫使批料通过模具。
接下来,挤出的生坯体优选按照本领域熟知的条件进行干燥,并在合适的气氛和选定的温度下进行煅烧,煅烧时间取决于其组成、尺寸和几何形状,从而得到所需陶瓷的陶瓷制品。例如,对于主要形成堇青石蜂窝结构的组合物,随后形成的陶瓷件通常以15-100℃/小时的速率升温到最高1405-1430℃的温度下进行煅烧,在该温度范围保持约6-25小时的保留时间。煅烧时间和温度取决于原料的类型和用量以及所用设备的类型等因素。
在一个实施方式中,本发明的陶瓷制品是由堇青石-陶瓷体组成的蜂窝状基材。蜂窝结构是本领域熟知的结构。它们被设计成具有进口端或面和与进口端相对的出口端或面,废气通过进口端或面进入陶瓷体,在出口端排出。进口端与出口端之间有许多孔室,这些孔室具有多孔壁。为用于柴油机颗粒过滤器,进口端的孔室总量中有一部分孔室在其长度的一部分上被堵塞,而剩下的在进口端开放的那部分孔室在出口端被堵塞,因而通过蜂窝体孔室的废气流入开放的孔室,经过孔室壁,再通过出口端的开放孔室流出该蜂窝结构。
具有上述综合特征的本发明的益处和优点包括:(1)生坯体从模具出来后具有良好的湿强度和外形保持性;(2)生坯体在300-900℃的温度区间具有提高的强度,在烧结过程中,尤其是在除去或烧除批料中所含有机物的煅烧周期开始时,能承受热应力和不均匀收缩;(3)能够采用较短的煅烧周期;(4)制造过程更加有效、成本更经济;(5)浪费的器具较少。
因此,本发明适用于制造复杂的成形陶瓷体,尤其是堇青石,它们常通过挤塑形成;本发明还适用于制造相应的煅烧体,如具有高孔室密度和薄孔室壁尺寸的多孔室蜂窝结构。
为更完整地理解本发明,现提供以下非限制性实施例。
实施例
根据下面表I所示的组成制备样品。称取干组分,与水和其他批料组分混合,然后在不锈钢研磨机中捏合,形成增塑的批料,再将其挤塑成多孔室蜂窝体,该蜂窝体由许多具有方形截面的平行孔道组成。多孔室蜂窝体包含约200个孔室/平方英寸(csi),壁厚为0.019英寸。
表I
干燥之后,根据预定的煅烧周期对陶瓷件进行煅烧,最高温度达1425℃,在此温度保持15小时。多孔室蜂窝体的直径约5.66英寸,将其切成长度为10英寸。煅烧之后,目力检测样品是否有裂纹,并用膨胀仪在室温(RT)至975℃之间测定的热膨胀系数(CTE)以及通过圆棒或多孔棒上四点弯曲测试法测定的断裂模量(MOR)的强度来表征。CTE的单位是10-6℃-1。MOR在400-800℃测定,以确定样品在高温下的强度。MOR的单位是磅/平方英寸(psi)。所测得的性质列于下表II。
表II
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| 比较例 | 本发明 | 本发明 | 本发明 | 本发明 | 本发明 | 本发明 | |
| 性质 | |||||||
| MOR@400℃(psi) | 5<sup>*</sup> | 179 | 301 | 579 | 165 | 387 | 522 |
| MOR@800℃(psi) | 5<sup>*</sup> | 180 | 301 | 715 | 182 | 500 | 462 |
| CET(RT-975℃)(10<sup>-6</sup>℃<sup>-1</sup>) | 0.93 | 1.07 | 1.01 | 1.13 | 0.98 | 1.15 | 1.22 |
| 裂纹 | 许多 | 无 | 无 | 无 | 无 | 无 | 无 |
比较例的棒状样品在400℃和800℃的断裂模量都很低,它们在进行任何测定之前就已经破碎。多孔棒上的MOR测定显示读数为5psi,该数值列于表11。
本发明样品表明,在堇青石体的制备中组合使用绿坡缕石粘土和甲基纤维素,在400℃和800℃下的高温生坯强度都得到提高,因而在随后的煅烧过程中,堇青石体能够抵抗可能在煅烧过程中引发裂纹的热应力。因此,本发明样品均经受住煅烧而没有产生裂纹。相反,比较样品产生了裂纹。
图1所示为在制备中不加绿坡缕石粘土的比较样品1时,最高到800℃测定的收缩率。收缩率在约275-590℃之间出现了较大的变化,它与甲基纤维素粘结剂的燃烧有关。据信,该收缩率变化导致的热梯度和应力甚至使样品产生裂纹,因为该样品不具有能够适应这种大尺寸变化的高温生坯强度。
现在参见图2,该图显示了在制备中加入5重量%绿坡缕石粘土粘结剂的本发明样品3时,最高到800℃测定的收缩率。与图1相比,在约275-590℃之间发生的尺寸变化大为减小。因此,据信本发明样品3没有产生裂纹,原因是在前述温度范围内尺寸变化减小而且强度增加。
还看到,本发明样品的CTE随着绿坡缕石粘土加入量的增加而稍有增加。然而,只要此材料的加入量不超过10重量%,所得CTE仍然在可用限度内,蜂窝体仍然可用于催化转化炉、柴油机颗粒过滤器等。
本发明陶瓷制品的另一实施方式优选为堇青石-陶瓷体组成的蜂窝状基材,特别是上述柴油机颗粒过滤器。用来形成这种制品的批料仅包含极少量的纤维状硅酸盐矿物,如绿坡缕石粘土。特别地,已经发现组合加入1-3%纤维状硅酸盐矿物和甲基纤维素粘结剂可提高制品最终的煅烧强度。因此,本发明这一方面的优点是,煅烧体与不用纤维状硅酸盐矿物制造的类似(孔隙率、CTE相同)陶瓷制品相比具有提高的最终强度。特别地,与不用少量纤维状硅酸盐矿物制造的类似陶瓷制品相比,强度可提高25%(基于上述MOR值)或以上。使用少量纤维状硅酸盐矿物如绿坡缕石粘土的另一个好处是陶瓷制品的CTE受影响较小。特别地,使用该批料组合物可得到孔隙率大于45%而CTE小于0.5×10-6℃-1(RT-800℃)的组合物。
根据其最终陶瓷强度得到提高的本发明优选实施例,批料优选包含100重量%形成堇青石的无机原料,1.0-3.0重量%纤维状硅酸盐矿物(以100重量%无机原料为基准计)和2.5-10.0重量%水溶性有机粘结剂。高强度陶瓷制品更优选通过包含100重量%形成堇青石的无机原料,1.0-3.0重量%绿坡缕石粘土(以100重量%无机原料为基准计)和2.5-10.0重量%甲基纤维素的本发明批料制造。在最优选的实施方式中,本发明包括制造陶瓷制品的方法,该方法包括提供包含各组分混合物的批料。所述组分包括无机原料,包括滑石、氧化铝和二氧化硅;粘结剂,它包含1)和2)的组合,1)水溶性有机粘结剂和2)具有高长径比、大表面积的纤维状硅酸盐矿物;和极性溶剂。该方法还包括混合批料组分,形成均相增塑体;使增塑体成形为生坯体;烧结生坯体,使其转化为煅烧陶瓷制品。最优选地,批料还包含最多为30%,优选5-20%的成孔剂,如石墨。该制品包含的主相优选为堇青石。
虽然结合有限的实施方式描述了本发明,本领域的技术人员阅读本说明后将会理解,在不背离本说明书所揭示的本发明范围的前提下,可设计其他实施方式。因此,本发明的范围仅受所附权利要求的限制。
Claims (14)
1.一种制造陶瓷制品的方法,所述陶瓷制品包含30-97%的堇青石,以氧化物为基准计,所述堇青石的组成是33-41重量%的氧化铝、46-53重量%的二氧化硅和11-17重量%的氧化镁,所述方法包括:
提供包含以下组分的批料:(i)包含滑石、氧化铝和二氧化硅的无机原料的混合物,(ii)粘结剂,其包含水溶性有机粘结剂和具有大于500的长径比和大于100平方米/克的表面积的纤维状硅酸盐矿物,(iii)极性溶剂;
混合批料组分,形成均相增塑体;
将增塑体成形为生坯体;
通过加热到一定温度并保持一定时间烧结该生坯体,使生坯体发生转化并充分转化为煅烧的陶瓷制品。
2.如权利要求1所述的方法,其特征在于,所述生坯体与不含纤维状硅酸盐矿物的类似生坯体相比,在300-900℃的温度区间具有提高的强度,在烧结过程中抗碎裂。
3.如权利要求1所述的方法,其特征在于,所述陶瓷制品与不含纤维状硅酸盐矿物的类似陶瓷体相比,烧结后提高了最终强度。
4.如权利要求1所述的方法,其特征在于,所述有机粘结剂是纤维素醚粘结剂。
5.如权利要求1所述的方法,其特征在于,所述纤维状硅酸盐矿物是绿坡缕石粘土。
6.如权利要求1所述的方法,其特征在于,以100重量%的无机原料为基准计,所述纤维状硅酸盐矿物以2-10重量%的量加入。
7.如权利要求6所述的方法,其特征在于,以100重量%的无机原料为基准计,所述纤维状硅酸盐矿物以5-10重量%的量加入。
8.如权利要求1所述的方法,其特征在于,以100重量%的无机原料为基准计,所述纤维状硅酸盐矿物以1-3重量%的量加入。
9.如权利要求1所述的方法,其特征在于,所述批料包含成孔剂。
10.如权利要求1所述的方法,其特征在于,所述生坯体是蜂窝结构。
11.如权利要求1所述的方法,其特征在于,以100重量%形成堇青石的无机原料为基准计,所述批料包含2.0-10.0重量%绿坡缕石粘土,2.5-10重量%甲基纤维素,不大于3重量%硬脂酸钠,不大于30重量%石墨,以及25.0-40.0重量%作为溶剂的水。
12.如权利要求1所述的方法,其特征在于,以100重量%形成堇青石的无机原料为基准计,所述批料包含1.0-3.0重量%纤维状硅酸盐矿物,和2.5-10.0重量%水溶性有机粘结剂。
13.如权利要求1所述的方法,其特征在于,所述纤维状硅酸盐矿物还具有中值粒径为1-2微米的特性。
14.一种陶瓷制品,它由权利要求1所述的方法制得。
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US10/911,083 US7445745B2 (en) | 2004-08-03 | 2004-08-03 | Method for fabricating ceramic articles |
| US10/911,083 | 2004-08-03 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| CN1993300A CN1993300A (zh) | 2007-07-04 |
| CN100540503C true CN100540503C (zh) | 2009-09-16 |
Family
ID=35756633
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| CNB2005800264282A Expired - Fee Related CN100540503C (zh) | 2004-08-03 | 2005-08-03 | 制造陶瓷制品的方法和制造的陶瓷制品 |
Country Status (5)
| Country | Link |
|---|---|
| US (2) | US7445745B2 (zh) |
| EP (1) | EP1773550A2 (zh) |
| JP (1) | JP2008509067A (zh) |
| CN (1) | CN100540503C (zh) |
| WO (1) | WO2006017676A2 (zh) |
Families Citing this family (28)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP4358662B2 (ja) * | 2004-03-23 | 2009-11-04 | 日本碍子株式会社 | コーディエライト質ハニカム構造体の製造方法 |
| US7474286B2 (en) | 2005-04-01 | 2009-01-06 | Spudnik, Inc. | Laser displays using UV-excitable phosphors emitting visible colored light |
| US8000005B2 (en) | 2006-03-31 | 2011-08-16 | Prysm, Inc. | Multilayered fluorescent screens for scanning beam display systems |
| US8089425B2 (en) | 2006-03-03 | 2012-01-03 | Prysm, Inc. | Optical designs for scanning beam display systems using fluorescent screens |
| US8451195B2 (en) | 2006-02-15 | 2013-05-28 | Prysm, Inc. | Servo-assisted scanning beam display systems using fluorescent screens |
| JP4811189B2 (ja) * | 2006-08-21 | 2011-11-09 | 株式会社デンソー | 排ガス浄化用フィルタ基材の製造方法 |
| US7964262B2 (en) | 2006-08-29 | 2011-06-21 | Corning Incorporated | Layered silicate modified cordierite and method |
| CN101688979B (zh) | 2007-05-17 | 2011-02-09 | Prysm公司 | 用于扫描光束显示系统的具有发光带的多层屏幕 |
| US8556430B2 (en) | 2007-06-27 | 2013-10-15 | Prysm, Inc. | Servo feedback control based on designated scanning servo beam in scanning beam display systems with light-emitting screens |
| US8894917B2 (en) * | 2008-05-30 | 2014-11-25 | Corning Incorporated | High porosity cordierite honeycomb articles |
| AU2010215085A1 (en) * | 2009-02-23 | 2011-08-18 | Refire Glass Research Pty Limited | A process and method for producing a silica based product |
| US9227878B2 (en) | 2009-04-30 | 2016-01-05 | Corning Incorporated | Selected binders for the extrusion of ultra-thin wall cellular ceramics |
| CN106631088A (zh) | 2009-06-05 | 2017-05-10 | 康宁股份有限公司 | 形成堇青石的批料及其使用方法 |
| US8257623B2 (en) * | 2009-08-27 | 2012-09-04 | Corning Incorporated | Extrusion die flow modification and use |
| WO2011066072A1 (en) | 2009-11-30 | 2011-06-03 | Corning Incorporated | Method for pore size distribution control in a fired ceramic article |
| US8148297B2 (en) * | 2009-11-30 | 2012-04-03 | Corning Incorporated | Reticular cordierite composition, article and manufacture thereof |
| US8440586B2 (en) * | 2010-02-26 | 2013-05-14 | Corning Incorporated | Low pressure drop extruded catalyst filter |
| US9334191B2 (en) | 2010-05-28 | 2016-05-10 | Corning Incorporated | Methods for forming ceramic honeycomb articles |
| US9856177B2 (en) | 2010-05-28 | 2018-01-02 | Corning Incorporated | Cordierite porous ceramic honeycomb articles |
| US8680344B2 (en) | 2011-01-25 | 2014-03-25 | Zeochem Llc | Molecular sieve adsorbent blends and uses thereof |
| US9878958B2 (en) * | 2012-02-29 | 2018-01-30 | Corning Incorporated | Dimensional control of ceramic structures via composition |
| CN103521001B (zh) * | 2013-09-06 | 2016-02-24 | 安徽青阳县雄伟泵阀制造有限公司 | 一种珍珠岩滤芯及其制备方法 |
| CN104846252A (zh) * | 2015-04-27 | 2015-08-19 | 苏州统明机械有限公司 | 一种含氧化铜的耐高温的特种陶瓷及其制备方法 |
| CN113263605B (zh) * | 2015-06-29 | 2023-02-03 | 康宁股份有限公司 | 生产线、方法、以及烧结制品 |
| US10486332B2 (en) | 2015-06-29 | 2019-11-26 | Corning Incorporated | Manufacturing system, process, article, and furnace |
| JP6982555B2 (ja) * | 2018-08-02 | 2021-12-17 | 日本碍子株式会社 | ハニカム構造体の製造方法 |
| CN113929433A (zh) * | 2021-09-27 | 2022-01-14 | 蒙娜丽莎集团股份有限公司 | 一种低黏土体系高白陶瓷板及其制备方法 |
| CN116621589B (zh) * | 2023-05-12 | 2025-05-27 | 上海宝九和耐火材料有限公司 | 一种自流型高炉出铁沟用修补材料及其制备方法和用途 |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN1355776A (zh) * | 1999-06-11 | 2002-06-26 | 康宁股份有限公司 | 低热膨胀高孔隙率高强度堇青石体及其制造方法 |
Family Cites Families (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3885977A (en) * | 1973-11-05 | 1975-05-27 | Corning Glass Works | Anisotropic cordierite monolith |
| US5252272A (en) * | 1989-07-28 | 1993-10-12 | Engelhard Corporation | Thermal shock and creep resistant porous mullite articles prepared from topaz and process for manufacture |
| US6171373B1 (en) | 1996-04-23 | 2001-01-09 | Applied Ceramics, Inc. | Adsorptive monolith including activated carbon, method for making said monolith, and method for adsorbing chemical agents from fluid streams |
| ES2196243T3 (es) | 1997-11-27 | 2003-12-16 | Ferro Italia Srl | Teja ceramica y esmalte para utilizacion en una teja de este tipo. |
| US6132832A (en) * | 1998-05-07 | 2000-10-17 | Ferro Corporation | Tile glaze |
| US6444601B1 (en) | 1998-11-12 | 2002-09-03 | Itc, Inc. | Purified attapulgite clay |
| US6132671A (en) * | 1999-05-27 | 2000-10-17 | Corning Incorporated | Method for producing honeycomb ceramic bodies |
| US6207101B1 (en) * | 1999-09-30 | 2001-03-27 | Corning Incorporated | Method of making fired bodies |
| US6544913B2 (en) | 2001-01-19 | 2003-04-08 | Agency For Defense Development | Alumina-silica ceramic |
| US6743383B2 (en) | 2002-03-26 | 2004-06-01 | Council Of Scientific And Industrial Research | Process for the production of ceramic tiles |
| JP4358662B2 (ja) * | 2004-03-23 | 2009-11-04 | 日本碍子株式会社 | コーディエライト質ハニカム構造体の製造方法 |
-
2004
- 2004-08-03 US US10/911,083 patent/US7445745B2/en not_active Expired - Fee Related
-
2005
- 2005-08-03 US US11/197,050 patent/US20060030475A1/en not_active Abandoned
- 2005-08-03 JP JP2007525001A patent/JP2008509067A/ja not_active Withdrawn
- 2005-08-03 CN CNB2005800264282A patent/CN100540503C/zh not_active Expired - Fee Related
- 2005-08-03 WO PCT/US2005/027784 patent/WO2006017676A2/en not_active Ceased
- 2005-08-03 EP EP05778442A patent/EP1773550A2/en not_active Withdrawn
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN1355776A (zh) * | 1999-06-11 | 2002-06-26 | 康宁股份有限公司 | 低热膨胀高孔隙率高强度堇青石体及其制造方法 |
Also Published As
| Publication number | Publication date |
|---|---|
| WO2006017676A2 (en) | 2006-02-16 |
| JP2008509067A (ja) | 2008-03-27 |
| EP1773550A2 (en) | 2007-04-18 |
| US7445745B2 (en) | 2008-11-04 |
| US20060027951A1 (en) | 2006-02-09 |
| WO2006017676A3 (en) | 2006-08-24 |
| US20060030475A1 (en) | 2006-02-09 |
| CN1993300A (zh) | 2007-07-04 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CN100540503C (zh) | 制造陶瓷制品的方法和制造的陶瓷制品 | |
| JP4549449B2 (ja) | 焼成時間の速いコージエライト体の製造方法 | |
| EP2254678B1 (en) | Honeycomb manufacturing method using ground nut shells | |
| CN102858717B (zh) | 用于挤出模塑体的组合物 | |
| EP2364283B1 (en) | Cordierite honeycomb body and manufacturing method | |
| US11591265B2 (en) | Batch compositions comprising pre-reacted inorganic particles and methods of manufacture of green bodies therefrom | |
| JPH05330943A (ja) | ディーゼル粒子フィルターとして好適な多孔質セラミックの製造方法 | |
| JPH11100259A (ja) | 焼成時間が実質的に減少したコージエライト体の製造方法 | |
| JP2011529846A5 (zh) | ||
| JP2011529846A (ja) | 製造可能性が改善されたセラミック前駆体 | |
| JP2000239059A (ja) | 狭細孔寸法分布をもつ低熱膨張係数コージェライト体およびその作成方法 | |
| US9932272B2 (en) | Compositions and methods for making low thermal expansion ceramic bodies | |
| JP2012501287A (ja) | セル型モノリシック構造体の気体造孔剤 | |
| JP4745964B2 (ja) | 多孔質ハニカム構造体の製造方法及び多孔質ハニカム構造体 | |
| WO2024097046A1 (en) | Higher temperature extrusion of ceramic precursor paste |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| C06 | Publication | ||
| PB01 | Publication | ||
| C10 | Entry into substantive examination | ||
| SE01 | Entry into force of request for substantive examination | ||
| C14 | Grant of patent or utility model | ||
| GR01 | Patent grant | ||
| CF01 | Termination of patent right due to non-payment of annual fee |
Granted publication date: 20090916 Termination date: 20140803 |
|
| EXPY | Termination of patent right or utility model |