WO2023219141A1 - ガスバリアフィルム、包装フィルム及び包装袋 - Google Patents
ガスバリアフィルム、包装フィルム及び包装袋 Download PDFInfo
- Publication number
- WO2023219141A1 WO2023219141A1 PCT/JP2023/017786 JP2023017786W WO2023219141A1 WO 2023219141 A1 WO2023219141 A1 WO 2023219141A1 JP 2023017786 W JP2023017786 W JP 2023017786W WO 2023219141 A1 WO2023219141 A1 WO 2023219141A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- layer
- gas barrier
- film
- adhesive
- hardness
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B27/00—Layered products comprising a layer of synthetic resin
- B32B27/06—Layered products comprising a layer of synthetic resin as the main or only constituent of a layer, which is next to another layer of the same or of a different material
- B32B27/08—Layered products comprising a layer of synthetic resin as the main or only constituent of a layer, which is next to another layer of the same or of a different material of synthetic resin
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B1/00—Layered products having a non-planar shape
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B27/00—Layered products comprising a layer of synthetic resin
- B32B27/30—Layered products comprising a layer of synthetic resin comprising vinyl (co)polymers; comprising acrylic (co)polymers
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B27/00—Layered products comprising a layer of synthetic resin
- B32B27/32—Layered products comprising a layer of synthetic resin comprising polyolefins
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B33/00—Layered products characterised by particular properties or particular surface features, e.g. particular surface coatings; Layered products designed for particular purposes not covered by another single class
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B38/00—Ancillary operations in connection with laminating processes
- B32B38/0036—Heat treatment
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B7/00—Layered products characterised by the relation between layers; Layered products characterised by the relative orientation of features between layers, or by the relative values of a measurable parameter between layers, i.e. products comprising layers having different physical, chemical or physicochemical properties; Layered products characterised by the interconnection of layers
- B32B7/04—Interconnection of layers
- B32B7/12—Interconnection of layers using interposed adhesives or interposed materials with bonding properties
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B65—CONVEYING; PACKING; STORING; HANDLING THIN OR FILAMENTARY MATERIAL
- B65D—CONTAINERS FOR STORAGE OR TRANSPORT OF ARTICLES OR MATERIALS, e.g. BAGS, BARRELS, BOTTLES, BOXES, CANS, CARTONS, CRATES, DRUMS, JARS, TANKS, HOPPERS, FORWARDING CONTAINERS; ACCESSORIES, CLOSURES, OR FITTINGS THEREFOR; PACKAGING ELEMENTS; PACKAGES
- B65D65/00—Wrappers or flexible covers; Packaging materials of special type or form
- B65D65/38—Packaging materials of special type or form
- B65D65/40—Applications of laminates for particular packaging purposes
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08J—WORKING-UP; GENERAL PROCESSES OF COMPOUNDING; AFTER-TREATMENT NOT COVERED BY SUBCLASSES C08B, C08C, C08F, C08G or C08H
- C08J7/00—Chemical treatment or coating of shaped articles made of macromolecular substances
- C08J7/04—Coating
- C08J7/042—Coating with two or more layers, where at least one layer of a composition contains a polymer binder
- C08J7/0423—Coating with two or more layers, where at least one layer of a composition contains a polymer binder with at least one layer of inorganic material and at least one layer of a composition containing a polymer binder
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08J—WORKING-UP; GENERAL PROCESSES OF COMPOUNDING; AFTER-TREATMENT NOT COVERED BY SUBCLASSES C08B, C08C, C08F, C08G or C08H
- C08J7/00—Chemical treatment or coating of shaped articles made of macromolecular substances
- C08J7/04—Coating
- C08J7/048—Forming gas barrier coatings
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2250/00—Layers arrangement
- B32B2250/02—2 layers
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2250/00—Layers arrangement
- B32B2250/24—All layers being polymeric
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2250/00—Layers arrangement
- B32B2250/24—All layers being polymeric
- B32B2250/242—All polymers belonging to those covered by group B32B27/32
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2255/00—Coating on the layer surface
- B32B2255/10—Coating on the layer surface on synthetic resin layer or on natural or synthetic rubber layer
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2255/00—Coating on the layer surface
- B32B2255/20—Inorganic coating
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2255/00—Coating on the layer surface
- B32B2255/20—Inorganic coating
- B32B2255/205—Metallic coating
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2255/00—Coating on the layer surface
- B32B2255/26—Polymeric coating
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2255/00—Coating on the layer surface
- B32B2255/28—Multiple coating on one surface
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2272/00—Resin or rubber layer comprising scrap, waste or recycling material
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2305/00—Condition, form or state of the layers or laminate
- B32B2305/72—Cured, e.g. vulcanised, cross-linked
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2307/00—Properties of the layers or laminate
- B32B2307/30—Properties of the layers or laminate having particular thermal properties
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2307/00—Properties of the layers or laminate
- B32B2307/30—Properties of the layers or laminate having particular thermal properties
- B32B2307/306—Resistant to heat
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2307/00—Properties of the layers or laminate
- B32B2307/30—Properties of the layers or laminate having particular thermal properties
- B32B2307/308—Heat stability
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2307/00—Properties of the layers or laminate
- B32B2307/30—Properties of the layers or laminate having particular thermal properties
- B32B2307/31—Heat sealable
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2307/00—Properties of the layers or laminate
- B32B2307/50—Properties of the layers or laminate having particular mechanical properties
- B32B2307/51—Elastic
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2307/00—Properties of the layers or laminate
- B32B2307/50—Properties of the layers or laminate having particular mechanical properties
- B32B2307/514—Oriented
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2307/00—Properties of the layers or laminate
- B32B2307/50—Properties of the layers or laminate having particular mechanical properties
- B32B2307/536—Hardness
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2307/00—Properties of the layers or laminate
- B32B2307/50—Properties of the layers or laminate having particular mechanical properties
- B32B2307/558—Impact strength, toughness
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2307/00—Properties of the layers or laminate
- B32B2307/50—Properties of the layers or laminate having particular mechanical properties
- B32B2307/58—Cuttability
- B32B2307/581—Resistant to cut
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2307/00—Properties of the layers or laminate
- B32B2307/70—Other properties
- B32B2307/72—Density
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2307/00—Properties of the layers or laminate
- B32B2307/70—Other properties
- B32B2307/724—Permeability to gases, adsorption
- B32B2307/7242—Non-permeable
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2307/00—Properties of the layers or laminate
- B32B2307/70—Other properties
- B32B2307/724—Permeability to gases, adsorption
- B32B2307/7242—Non-permeable
- B32B2307/7244—Oxygen barrier
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2307/00—Properties of the layers or laminate
- B32B2307/70—Other properties
- B32B2307/724—Permeability to gases, adsorption
- B32B2307/7242—Non-permeable
- B32B2307/7246—Water vapor barrier
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2307/00—Properties of the layers or laminate
- B32B2307/70—Other properties
- B32B2307/732—Dimensional properties
- B32B2307/734—Dimensional stability
- B32B2307/736—Shrinkable
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2307/00—Properties of the layers or laminate
- B32B2307/70—Other properties
- B32B2307/732—Dimensional properties
- B32B2307/737—Dimensions, e.g. volume or area
- B32B2307/7375—Linear, e.g. length, distance or width
- B32B2307/7376—Thickness
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2307/00—Properties of the layers or laminate
- B32B2307/70—Other properties
- B32B2307/748—Releasability
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2309/00—Parameters for the laminating or treatment process; Apparatus details
- B32B2309/02—Temperature
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2309/00—Parameters for the laminating or treatment process; Apparatus details
- B32B2309/04—Time
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2311/00—Metals, their alloys or their compounds
- B32B2311/24—Aluminium
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2323/00—Polyalkenes
- B32B2323/10—Polypropylene
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2439/00—Containers; Receptacles
- B32B2439/02—Open containers
- B32B2439/06—Bags, sacks, sachets
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2439/00—Containers; Receptacles
- B32B2439/40—Closed containers
- B32B2439/46—Bags
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2439/00—Containers; Receptacles
- B32B2439/70—Food packaging
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2439/00—Containers; Receptacles
- B32B2439/80—Medical packaging
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B32—LAYERED PRODUCTS
- B32B—LAYERED PRODUCTS, i.e. PRODUCTS BUILT-UP OF STRATA OF FLAT OR NON-FLAT, e.g. CELLULAR OR HONEYCOMB, FORM
- B32B2553/00—Packaging equipment or accessories not otherwise provided for
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08J—WORKING-UP; GENERAL PROCESSES OF COMPOUNDING; AFTER-TREATMENT NOT COVERED BY SUBCLASSES C08B, C08C, C08F, C08G or C08H
- C08J2323/00—Characterised by the use of homopolymers or copolymers of unsaturated aliphatic hydrocarbons having only one carbon-to-carbon double bond; Derivatives of such polymers
- C08J2323/02—Characterised by the use of homopolymers or copolymers of unsaturated aliphatic hydrocarbons having only one carbon-to-carbon double bond; Derivatives of such polymers not modified by chemical after treatment
- C08J2323/10—Homopolymers or copolymers of propene
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08J—WORKING-UP; GENERAL PROCESSES OF COMPOUNDING; AFTER-TREATMENT NOT COVERED BY SUBCLASSES C08B, C08C, C08F, C08G or C08H
- C08J2323/00—Characterised by the use of homopolymers or copolymers of unsaturated aliphatic hydrocarbons having only one carbon-to-carbon double bond; Derivatives of such polymers
- C08J2323/02—Characterised by the use of homopolymers or copolymers of unsaturated aliphatic hydrocarbons having only one carbon-to-carbon double bond; Derivatives of such polymers not modified by chemical after treatment
- C08J2323/10—Homopolymers or copolymers of propene
- C08J2323/12—Polypropene
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08J—WORKING-UP; GENERAL PROCESSES OF COMPOUNDING; AFTER-TREATMENT NOT COVERED BY SUBCLASSES C08B, C08C, C08F, C08G or C08H
- C08J2429/00—Characterised by the use of homopolymers or copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and at least one being terminated by an alcohol, ether, aldehydo, ketonic, acetal, or ketal radical; Hydrolysed polymers of esters of unsaturated alcohols with saturated carboxylic acids; Derivatives of such polymer
- C08J2429/02—Homopolymers or copolymers of unsaturated alcohols
- C08J2429/04—Polyvinyl alcohol; Partially hydrolysed homopolymers or copolymers of esters of unsaturated alcohols with saturated carboxylic acids
Definitions
- the present disclosure relates to a gas barrier film, a packaging film, and a packaging bag.
- Gas barrier films are widely used in food and pharmaceutical packaging materials that undergo heat sterilization treatments such as boiling and retort processing.
- heat sterilization treatments such as boiling and retort processing.
- a film using a polyethylene terephthalate film base material which is heat resistant and has a low oxygen permeability, has conventionally been used.
- Patent Document 1 proposes a method of blending an ethylene- ⁇ -olefin copolymer with a polypropylene film
- Patent Documents 2 and 3 propose a method of laminating different polypropylene films.
- One aspect of the present disclosure aims to provide a gas barrier film that is capable of achieving both good oxygen barrier properties and interlayer adhesion after heat sterilization treatment, while having a polypropylene base material.
- One aspect of the present disclosure also aims to provide a packaging film and a packaging bag using the gas barrier film.
- One aspect of the present disclosure includes a base layer containing a polypropylene resin, a vapor deposition layer containing an inorganic oxide, and a gas barrier coating layer in this order, and the base layer includes a first skin layer and a core layer.
- a vapor deposited layer is formed on the first skin layer side, the hardness of the first skin layer measured by nanoindentation method is 0.02 to 0.15 GPa, and the hardness of the core layer is 0.
- the hardness of the core layer is 0.
- Such a gas barrier film can have both good oxygen barrier properties and interlayer adhesion after heat sterilization treatment, while having a polypropylene base material.
- the first skin layer may include a propylene- ⁇ -olefin copolymer.
- the ratio of the thickness of the first skin layer to the thickness of the core layer may be 1/100 to 1/5.
- the first skin layer may have a composite modulus of elasticity of 1.2 to 2.5 GPa
- the core layer may have a composite modulus of 2.0 GPa or more, as measured by a nanoindentation method.
- the inorganic oxide may include at least one of aluminum oxide and silicon oxide.
- the gas barrier coating layer is a cured product of a composition comprising a water-soluble polymer having a hydroxyl group and at least one selected from the group consisting of metal alkoxides, silane coupling agents, and hydrolysates thereof. It may consist of
- the gas barrier film may further include an anchor coat layer between the base layer and the vapor deposition layer.
- the base layer may include a first skin layer, a core layer, and a second skin layer in this order.
- the second skin layer may include a propylene- ⁇ -olefin copolymer.
- the second skin layer may have a hardness of 0.02 to 0.15 GPa as measured by a nanoindentation method.
- the thickness of the deposited layer may be 5 nm or more and 80 nm or less.
- Another aspect of the present disclosure is a packaging film comprising the above gas barrier film, a sealant layer overlapping the gas barrier coating layer, and an adhesive layer bonding the gas barrier coating layer and the sealant layer, After performing retort treatment, the hardness of the adhesive layer measured by a nanoindentation method is 0.1 MPa or more and less than 0.9 MPa.
- the hardness of the adhesive layer measured by a nanoindentation method before the retort treatment may be 1.0 MPa or more.
- the thickness of the adhesive layer may be 0.5 ⁇ m or more and 10 ⁇ m or less.
- each of the base layer and the sealant layer is a polyolefin film, and the total mass ratio of polyolefin in the packaging film may be 90% by mass or more.
- the base layer may be a stretched polypropylene film
- the sealant layer may be an unstretched polypropylene film
- the packaging film further includes an outermost layer overlapping the base layer, and a second adhesive layer that adheres the outermost layer and the base layer, and the adhesive layer adheres the base layer and the sealant layer.
- the second adhesive layer may adhere the base layer and the outermost layer.
- the outermost layer after the outermost layer is exposed at 120° C. for 15 minutes, the outermost layer has a heat shrinkage rate in the MD direction of 1% or more as determined by the following formula (1), and the packaging film is subjected to retort treatment.
- the hardness of the second adhesive layer measured by a nanoindentation method may be 0.1 MPa or more and less than 0.9 MPa.
- Thermal contraction rate in MD direction (%) (MD direction length before heating - MD direction length after heating) / MD direction length before heating ⁇ 100 ... (1)
- One aspect of the present disclosure provides a packaging bag that is a product of the packaging film described above.
- a gas barrier film that is capable of achieving both good oxygen barrier properties and interlayer adhesion after heat sterilization treatment, while having a polypropylene base material. Further, according to one aspect of the present disclosure, a packaging film and a packaging bag using the gas barrier film can be provided.
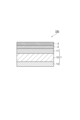
- FIG. 1 is a schematic cross-sectional view showing a gas barrier film according to a first embodiment.
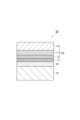
- FIG. 3 is a schematic cross-sectional view showing a gas barrier film according to a modification of the first embodiment.
- FIG. 1 is a schematic cross-sectional view showing a packaging film according to a first embodiment. It is a schematic sectional view showing a packaging film concerning a modification of a 1st embodiment.
- 5 is a diagram showing a laminate according to a second embodiment
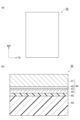
- FIG. 5(a) is a schematic plan view of the laminate according to the second embodiment
- FIG. FIG. 3 is a schematic cross-sectional view showing such a laminate.
- It is a schematic front view of an example of the packaging bag concerning 2nd Embodiment.
- FIG. 7 is a schematic cross-sectional view showing a laminate according to a modification of the second embodiment. It is a schematic diagram which shows the measuring method of the thermal shrinkage rate at the time of oven heating.
- FIG. 1 is a schematic cross-sectional view showing a gas barrier film according to a first embodiment.
- the gas barrier film 10a includes a base layer 1, a vapor deposition layer 2, and a gas barrier coating layer 3 in this order.
- the base material layer 1 includes a first skin layer 11 and a core layer 12, and a vapor deposition layer 2 is formed on the first skin layer 11 side. That is, the vapor deposition layer 2 is formed on the first skin layer 11.
- FIG. 2 is a schematic cross-sectional view showing a gas barrier film according to a modification of the first embodiment.
- the gas barrier film 10b includes a base layer 1, a vapor deposition layer 2, and a gas barrier coating layer 3 in this order.
- the base material layer 1 includes a first skin layer 11, a core layer 12, and a second skin layer 13, and the vapor deposition layer 2 is formed on the first skin layer 11 side. That is, the vapor deposition layer 2 is formed on the first skin layer 11.
- the base material layer is a film (base film) that serves as a support, and contains a polypropylene resin.
- polypropylene resins include homopolypropylene and propylene copolymers.
- propylene copolymers include polypropylene copolymers such as propylene-ethylene random copolymers, propylene-ethylene block copolymers, and propylene- ⁇ -olefin copolymers.
- recycled resin may be used, or resin obtained by polymerizing raw materials derived from biomass such as plants may be used. These resins may be used alone or in combination with resins polymerized from ordinary fossil fuels.
- the base material layer may be a stretched film or an unstretched film, but from the viewpoint of oxygen barrier properties, it may be a stretched film.
- the stretched film include a uniaxially stretched film and a biaxially stretched film, but the biaxially stretched film may improve heat resistance.
- the base material layer is, for example, a polyolefin film.
- the base layer may include or be made of a polypropylene film.
- the polypropylene film constituting the base layer may be a stretched film or a non-stretched film.
- the polypropylene film may be a stretched polypropylene film.
- the gas barrier film can be more suitably used in applications where heat treatment such as retort treatment and boiling treatment is performed.
- the stretching method is not particularly limited, and any method may be used as long as it can provide a dimensionally stable film, such as inflation stretching, uniaxial stretching, or biaxial stretching.
- the base material layer contains known additives such as antioxidants, stabilizers, lubricants such as calcium stearate, fatty acid amide, and erucic acid amide, organic additives such as antistatic agents, silica, zeolite, thyroid, and hydrotalcite. , inorganic additives such as particulate lubricants such as silicon particles.
- the thickness (total thickness) of the base material layer is not particularly limited, and may be, for example, 3 to 200 ⁇ m, or 6 to 50 ⁇ m.
- the polypropylene resin contained in the core layer may be crystalline polypropylene. From the viewpoint of further improving heat resistance, the polypropylene resin contained in the core layer may be homopolypropylene, which is a homopolymer of propylene.
- the polypropylene resin contained in the core layer may include a mixture of homopolypropylene and a propylene- ⁇ -olefin random copolymer.
- the polypropylene resin contained in the core layer can contain homopolypropylene in an amount of 80% by mass or more, and may contain 100% by mass.
- the first skin layer can alleviate the difference between the shrinkage stress of the core layer and the shrinkage stress of the gas barrier coating layer due to heat sterilization treatment, and can prevent the occurrence of cracks in the gas barrier coating layer.
- another layer having an adhesive function including acid-modified polyolefin, ethylene-vinyl alcohol copolymer, polyamide, etc., may be provided.
- the core layer and the first skin layer may be in contact with each other without intervening.
- the first skin layer (the surface of the base material layer on the vapor deposited layer side) may be subjected to surface treatment such as plasma treatment or corona treatment.
- the second skin layer can increase the lamination strength with the adjacent layer (outer film or sealant layer).
- Another layer may be provided between the core layer and the second skin layer as described above.
- the core layer and the second skin layer may be in contact with each other without interposing any other layer.
- the surface of the core layer may be subjected to surface treatment such as plasma treatment or corona treatment, or an easy-adhesion coating layer may be provided.
- the polypropylene resin contained in each skin layer may contain a propylene copolymer (a copolymer of propylene and other monomers) from the viewpoint of improving adhesion with the core layer.
- a propylene copolymer a copolymer of propylene and other monomers
- examples of other monomers include ⁇ -olefins such as ethylene, 1-butene, and 1-hexene.
- the propylene copolymer may specifically be a propylene-ethylene random copolymer, a propylene-ethylene block copolymer, a propylene- ⁇ -olefin copolymer, or the like.
- each skin layer may contain a propylene- ⁇ -olefin copolymer.
- the content of the propylene monomer in the propylene copolymer can be 90 mol% or more, and may be 92 mol% or more, based on all monomers constituting the copolymer.
- the content of propylene monomer in the propylene copolymer can be 99.8 mol% or less, and may be 99.5 mol% or less, based on all monomers constituting the copolymer. .
- the hardness and composite modulus of the core layer and each skin layer represent the hardness and composite modulus measured by the nanoindentation method.
- the nanoindentation method is a measurement method that performs a quasi-static indentation test on a measurement target to obtain the mechanical properties of the sample.
- Hysitron TI-Premier (trade name) manufactured by Bruker Japan Co., Ltd. can be used as the measuring device, and a Berkovich type diamond indenter manufactured by Bruker Japan Co., Ltd. can be used as the indenter.
- indentation is performed at a pushing speed of 30 nm/sec to a depth of 30 nm, held at the maximum depth for 1 second, and then unloaded at a speed of 30 nm/sec. Do by doing.
- the measurement is performed by acquiring a shape image of the cross section of the sample using the shape measurement function of the measuring device that scans the sample surface with an indenter, and from the shape image, specifying 20 points on the target layer at intervals of 1 ⁇ m or more.
- Measurements using the nanoindentation method are performed on the cross section of the base layer by embedding the base layer in resin, cutting it to obtain a cross section.
- corona treatment may be performed as a surface treatment on the front and back surfaces of the base layer to prevent separation between the embedding resin and the base layer. Note that the sample used for measurement does not need to be in the state of only the base material layer, and may be in the state of a gas barrier film or packaging film.
- the hardness of the core layer is 0.07 GPa or more.
- a core layer having a hardness within this range is resistant to heat and can suppress shrinkage of the base layer during heat sterilization.
- the hardness of the core layer may be 0.072 GPa or more, or 0.075 GPa or more.
- the upper limit of the hardness is not particularly limited, but since polypropylene resin is used as the material, it can be set to 0.2 GPa or less.
- the hardness of the first skin layer is in the range of 0.02 to 0.15 GPa.
- the first skin layer is prevented from becoming too soft, and deterioration in adhesion and gas barrier properties are less likely to occur.
- the first skin layer is prevented from becoming too hard. This alleviates the stress difference between the core layer and the gas barrier coating layer during heat sterilization treatment, making it difficult for gas barrier properties to deteriorate.
- the adhesion strength between the core layer and the first skin layer is not easily reduced.
- the hardness of the first skin layer may be 0.025 to 0.10 GPa, or 0.030 to 0.098 GPa.
- the hardness of the second skin layer can be set similarly to the first skin layer 11. This not only increases the lamination strength with adjacent layers, but also increases the adhesion strength with the core layer.
- the hardness of the core layer is set to be the highest in the base layer. That is, the hardness of the core layer is set higher than the hardness of the first skin layer, and the hardness of the core layer is set higher than the hardness of the second skin layer.
- the hardness of the first skin layer and the second skin layer may be the same or different.
- the composite modulus of the core layer may be 2.0 GPa or more.
- a core layer having a composite modulus of elasticity within this range becomes stronger by heat, and therefore it becomes easier to suppress shrinkage of the base material layer during heat sterilization treatment.
- the composite modulus of the core layer may be 2.1 GPa or more, or 2.13 GPa or more.
- the upper limit of the composite elastic modulus is not particularly limited, but since polypropylene resin is used as the material, it is 4.5 GPa or less.
- the composite modulus of the first skin layer may be in the range of 1.2 to 2.5 GPa.
- the composite modulus of the first skin layer is 1.2 GPa or more, the first skin layer is prevented from becoming too soft, and decreases in adhesion, gas barrier properties, etc. are less likely to occur.
- the first skin layer is prevented from becoming too hard. This makes it easier to alleviate the stress difference between the core layer and the gas barrier coating layer during heat sterilization treatment, making it more difficult for the gas barrier properties to deteriorate.
- the adhesion strength between the core layer and the first skin layer is also less likely to decrease.
- the composite modulus of the first skin layer may be 1.22 to 2.48 GPa, or 1.25 to 2.45 GPa.
- the composite modulus of the second skin layer can be set in the same way as the first skin layer. This not only makes it possible to further increase the lamination strength with adjacent layers, but also makes it possible to further increase the adhesion strength with the core layer.
- the composite modulus of the core layer may be set to be the largest among the base material layers. . That is, the composite modulus of the core layer may be set to be greater than the composite modulus of the first skin layer, and the composite modulus of the core layer may be greater than the composite modulus of the second skin layer. .
- the composite modulus of the first skin layer and the second skin layer may be the same or different.
- the method for adjusting the hardness and composite modulus of the core layer and each skin layer is not particularly limited.
- the hardness and composite modulus of each layer containing polypropylene resin can be adjusted by, for example, the type of resin constituting each layer, their mixing ratio when using multiple resins, and the monomer ratio when using a copolymer. , and can be performed by adjusting the manufacturing method of each layer.
- each skin layer may be 0.1 ⁇ m or more.
- the skin layer can be uniformly laminated on the core layer, and variations in the thickness of the skin layer can be suppressed. Moreover, it is thought that this makes it possible to sufficiently relieve stress on the vapor deposited layer during heat sterilization treatment, and to suppress deterioration of gas barrier properties. From this point of view, the thickness of each skin layer is, for example, 0.3 ⁇ m or more.
- each skin layer is not particularly limited, it is, for example, 2.0 ⁇ m or less, and may be 1.8 ⁇ m or less, from the viewpoint of ensuring more sufficient heat resistance of the entire base layer.
- the ratio of the thickness of the first skin layer to the thickness of the core layer may be 1/100 to 1/5, and may be 1/70 to 1. /10 may be sufficient.
- the thickness ratio is within the above range, the heat resistance of the entire base layer can be more fully ensured, and the adhesion between each layer in the gas barrier film and the packaging film can be further improved.
- the ratio of the thickness of the second skin layer to the thickness of the core layer may be 1/100 to 1/5, and may be 1/70 to 1. /10 may be sufficient.
- the thickness ratio is within the above range, the heat resistance of the entire base layer can be more fully ensured, and the adhesion between each layer in the gas barrier film and the packaging film can be further improved.
- the vapor deposition layer contains an inorganic oxide, and is a layer provided on the base material layer from the viewpoint of improving gas barrier properties against, for example, water vapor and oxygen.
- the deposited layer may be transparent.
- the inorganic oxide include aluminum oxide, silicon oxide, tin oxide, magnesium oxide, and mixtures thereof. From the viewpoint of heat resistance during heat sterilization, at least one of aluminum oxide and silicon oxide may be used as the inorganic oxide.
- the thickness of the deposited layer may be 5 to 300 nm. When the thickness of the vapor deposited layer is 5 nm or more, it is easier to make the layer thickness uniform, and it is easier to ensure the function as a gas barrier film. Further, if the thickness of the vapor deposited layer is 300 nm or less, flexibility can be easily imparted to the vapor deposited layer, and even if external factors such as bending or pulling are applied after the layer is formed, the vapor deposited layer is less likely to crack. From this point of view, the thickness of the deposited layer may be, for example, 6 nm or more, 150 nm or less, or 100 nm or less. The thickness of the deposited layer may be 5 to 80 nm, or 20 to 40 nm.
- the vapor deposition layer 2 can be formed by a normal vacuum vapor deposition method. It is also possible to use other thin film forming methods such as sputtering, ion plating, and plasma vapor deposition (CVD). However, in terms of productivity, vacuum evaporation is currently superior.
- any one of an electron beam heating method, a resistance heating method, and an induction heating method may be used.
- an electron beam heating method may be used as the heating means.
- vapor deposited layer In order to improve the adhesion between the vapor deposited layer and the base material layer and the density of the vapor deposited layer, it is also possible to form the vapor deposited layer using a plasma assist method or an ion beam assist method. Furthermore, in order to increase the transparency of the vapor deposited layer, reactive vapor deposition may be used in which various gases such as oxygen are blown during vapor deposition.
- An anchor coat layer can be further provided between the base material layer and the vapor deposition layer. Thereby, it is possible to further improve the adhesion, gas barrier properties, etc. of both layers after heat sterilization treatment.
- coating agents for providing the anchor coat layer include acrylic resins, epoxy resins, acrylic urethane resins, polyester polyurethane resins, and polyether polyurethane resins. From the viewpoint of heat resistance and interlayer adhesion strength, an acrylic urethane resin or a polyester polyurethane resin may be used as the coating agent.
- the thickness of the anchor coat layer may be 0.05 to 2 ⁇ m. If the thickness of the anchor coat layer is 0.05 ⁇ m or more, it is possible to further improve the adhesion between the base material layer and the vapor deposition layer. Further, if the thickness of the anchor coat layer is 2 ⁇ m or less, flexibility can be easily imparted to the anchor coat layer, and gas barrier properties can be easily maintained even if external factors such as bending or pulling are applied after the layer is formed. From this point of view, the thickness of the anchor coat layer may be, for example, 0.08 ⁇ m or more and 1 ⁇ m or less.
- the gas barrier coating layer is provided for the purpose of protecting the deposited layer and supplementing the gas barrier properties.
- the gas barrier coating layer is made of a cured product of a composition containing a water-soluble polymer having a hydroxyl group and at least one member selected from the group consisting of metal alkoxides, silane coupling agents, and hydrolysates thereof. good.
- water-soluble polymers having hydroxyl groups examples include polyvinyl alcohol, polyvinylpyrrolidone, starch, methylcellulose, carboxymethylcellulose, and sodium alginate.
- PVA polyvinyl alcohol
- gas barrier properties tend to be better.
- Examples of metal alkoxides include compounds represented by the following general formula.
- M (OR 1 ) m (R 2 ) nm In the above formula, R 1 is a monovalent organic group having 1 to 8 carbon atoms, and may be an alkyl group such as a methyl group or an ethyl group (OR 1 is a hydrolyzable group).
- R 2 is a monovalent organic group having 1 to 8 carbon atoms, and may be an alkyl group such as a methyl group or an ethyl group.
- M represents an n-valent metal atom such as Si, Ti, Al, and Zr.
- m is an integer from 1 to n.
- R 1 or R 2 may be the same or different.
- metal alkoxide examples include tetraethoxysilane [Si(OC 2 H 5 ) 4 ], triisopropoxyaluminum [Al(O-2′-C 3 H 7 ) 3 ], and the like.
- Tetraethoxysilane (TEOS) and triisopropoxyaluminum tend to be relatively stable in aqueous solvents after hydrolysis.
- Examples of the silane coupling agent include compounds represented by the following general formula. Si(OR 21 ) p (R 22 ) 3-p R 23 ...(2)
- R 21 represents an alkyl group such as a methyl group or an ethyl group
- R 22 represents an alkyl group, an aralkyl group, an aryl group, an alkenyl group, an alkyl group substituted with an acryloxy group, or an alkyl group substituted with a methacryloxy group.
- R 23 represents a monovalent organic functional group
- p represents an integer of 1 to 3.
- R 21 or R 22 may be the same or different.
- the monovalent organic functional group represented by R 23 is a monovalent organic functional group containing a glycidyloxy group, an epoxy group, a mercapto group, a hydroxyl group, an amino group, an alkyl group substituted with a halogen atom, or an isocyanate group. Examples include groups. Multimer compounds such as dimers and trimers of these silane coupling agents may be used.
- the silane coupling agent includes vinyltrimethoxysilane, ⁇ -chloropropylmethyldimethoxysilane, ⁇ -chloropropyltrimethoxysilane, 3-glycidoxypropylmethyldimethoxysilane, 3-glycidoxypropyltrimethoxysilane. , 3-glycidoxypropylmethyldiethoxysilane, 3-glycidoxypropylmethyltriethoxysilane, ⁇ -methacryloxypropyltrimethoxysilane, ⁇ -methacryloxypropylmethyldimethoxysilane, 1,3,5-tris(3 -Methoxysilylpropyl) isocyanurate and other silane coupling agents.
- the gas barrier coating layer can be formed by coating the composition for forming a gas barrier coating layer on the vapor deposited layer and then heating and drying it.
- the composition for forming a gas barrier coating layer is prepared by dissolving a water-soluble polymer in an aqueous solvent (water, water/alcohol mixed solvent, etc.) and adding at least one of a metal alkoxide and a silane coupling agent, or a mixture thereof. It can be prepared by mixing pre-hydrolyzed products. If necessary, known additives such as isocyanate compounds, dispersants, stabilizers, viscosity modifiers, colorants, etc. may be added to this composition (mixed solution) to the extent that gas barrier properties are not impaired. It is possible.
- the amount of PVA in the composition may be 20 to 70% by mass, or 25 to 60% by mass, based on the total solid content of the composition.
- the amount of PVA is 20% by mass or more, the flexibility of the layer is maintained and it becomes easier to form a gas barrier coating layer.
- the amount of PVA is 70% by mass or less, it becomes easier to impart sufficient gas barrier properties to the gas barrier film.
- the amount of TEOS in the composition may be 30 to 80% by mass, or 40 to 75% by mass, based on the total solid content of the composition.
- the amount of TEOS is 30% by mass or more, high gas barrier properties are likely to be exhibited.
- the amount of TEOS is 80% by mass or less, it becomes easier to maintain the flexibility of the layer.
- the amount of TEOS is calculated in terms of SiO2 .
- the mass ratio may be 1 to 20% by mass, or 5 to 15% by mass, based on the total solid content of the composition.
- the amount of isocyanurate silane is 1% by mass or more, hot water resistance is easily obtained and adhesion strength is unlikely to decrease.
- the amount of isocyanurate silane is 20% by mass or less, the amounts of other components in the composition will not become too low, and as a result, high gas barrier properties can be easily obtained.
- the thickness of the gas barrier coating layer may be 0.1 to 5 ⁇ m. If the thickness of the gas barrier coating layer is 0.1 ⁇ m or more, high gas barrier properties are likely to be exhibited. In addition, if the thickness of the gas barrier coating layer is 5 ⁇ m or less, it is possible to suppress the deterioration of gas barrier properties due to cracks in the layer during coating.From this viewpoint, the thickness of the gas barrier coating layer is, for example, 0 ⁇ m or less. .2 ⁇ m or more, and may be 1 ⁇ m or less.
- FIG. 3 is a schematic cross-sectional view showing the packaging film according to the first embodiment.
- the packaging film 20 shown in FIG. 3 has a structure in which a sealant layer 23 is laminated on the gas barrier coating layer 3 of the gas barrier film 10a via an adhesive layer 24.
- FIG. 4 is a schematic cross-sectional view showing a packaging film according to a modification of the first embodiment.
- a sealant layer 23 is laminated on the second skin layer 13 of the gas barrier film 10b via an adhesive layer 24, and a second adhesive layer 25 is laminated on the gas barrier coating layer 3 of the gas barrier film 10b.
- An outer layer film (outermost layer) 22 is laminated therebetween.
- the direction of the gas barrier film 10b may be reversed. That is, the outer layer film (second base material layer) 22 is laminated on the second skin layer 13 of the gas barrier film 10b via the second adhesive layer 25, and the adhesive layer 24 is laminated on the gas barrier coating layer 3 of the gas barrier film 10b.
- the sealant layer 23 may be laminated with the sealant layer 23 interposed therebetween.
- the outer layer film is a layer provided for the purpose of increasing the rigidity of the packaging bag. Therefore, the outer layer film can be referred to as a second base layer.
- the outer layer film contains a polyolefin resin.
- the polyolefin resin include polyethylene and polypropylene, but polypropylene may be used from the viewpoint of retort treatment resistance.
- the polypropylene may be homopolypropylene or a propylene copolymer, but from the viewpoint of heat resistance, homopolypropylene may be used.
- the outer layer film may be a stretched film or an unstretched film, but from the viewpoint of oxygen barrier properties, it may be a stretched film.
- the thickness of the outer layer film is not particularly limited, but can be, for example, 15 to 200 ⁇ m.
- the sealant layer contains polyolefin resin.
- polyolefin resins include low density polyethylene resin (LDPE), medium density polyethylene resin (MDPE), linear low density polyethylene resin (LLDPE), ethylene-vinyl acetate copolymer (EVA), and ethylene-vinyl acetate copolymer (EVA).
- Ethylene resins such as ⁇ -olefin copolymers, ethylene-(meth)acrylic acid copolymers, homopolypropylene resins (PP), propylene-ethylene random copolymers, propylene-ethylene block copolymers, propylene- ⁇ -olefin copolymers, etc.
- Polypropylene resins such as polymers, mixtures thereof, etc. can be used.
- the material of the sealant layer can be appropriately selected from the above-mentioned thermoplastic resins depending on the intended use and temperature conditions such as boiling treatment and retort treatment.
- the sealant layer may be a polyolefin film like the base layer.
- the sealant layer is, for example, a resin layer having a single layer structure and mainly made of polypropylene, but is not limited thereto.
- the sealant layer may include or consist of a polypropylene film.
- the thermoplastic resin constituting the sealant layer may or may not be stretched. From the viewpoint of lowering the melting point and facilitating heat sealing, it may not be stretched.
- the polypropylene film constituting the sealant layer may be an unstretched polypropylene film from the viewpoint of improving sealing performance by heat sealing.
- the thickness of the sealant layer is not particularly limited, but can be, for example, 15 to 200 ⁇ m.
- the adhesive layer bonds the films together.
- the adhesive constituting the adhesive layer include polyurethane resins in which a difunctional or higher-functional isocyanate compound is applied to a base material such as polyester polyol, polyether polyol, acrylic polyol, or carbonate polyol.
- Various polyols may be used alone or in combination of two or more. From the viewpoint of heat resistance (retort treatment resistance) during heat sterilization treatment, a two-component curing type urethane adhesive may be used.
- a carbodiimide compound, an oxazoline compound, an epoxy compound, a phosphorus compound, a silane coupling agent, etc. may be blended with the above-mentioned polyurethane resin.
- the adhesive from the viewpoint of environmental consideration, an adhesive whose polymer component is derived from biomass or a biodegradable adhesive may be used. Further, the adhesive may be an adhesive having barrier properties.
- the amount of adhesive applied may be, for example, 0.5 to 10 g/m 2 from the viewpoint of obtaining desired adhesive strength, followability, processability, etc.
- the thickness of the adhesive layer is, for example, 0.5 ⁇ m or more and 10 ⁇ m or less.
- the thickness of the adhesive layer may be 1 ⁇ m or more, 2 ⁇ m or more, 8 ⁇ m or less, 6 ⁇ m or less, or 5 ⁇ m or less.
- the second adhesive layer is a layered member that adheres the gas barrier film and the outer layer film.
- the material of the adhesive included in the second adhesive layer is the same as the material of the adhesive included in the adhesive layer. Therefore, a description of the materials included in the second adhesive layer will be omitted.
- the thickness of the second adhesive layer is similar to the thickness of the adhesive layer, and is, for example, 0.5 ⁇ m or more and 10 ⁇ m or less.
- the total mass ratio of polyolefin (polypropylene in this embodiment) in the packaging film is 90% by mass or more.
- the packaging film can be called a packaging material made of a single material (monomaterial), and has excellent recyclability.
- the polyolefin content in the packaging film may be 92.5% by mass or more, or 95% by mass or more, based on the total amount of the packaging film.
- the base layer After the base layer is exposed to 120° C. for 15 minutes (hereinafter simply referred to as "after heating"), the base layer has a heat shrinkage rate in the MD direction of 1% or more as determined by the following formula (1).
- the heat shrinkage rate of the base material layer in the MD direction determined by the following formula (1) is 1% or more.
- the heat shrinkage rate in the MD direction is, for example, 12% or less, 11% or less, 10% or less, 9% or less, 8%.
- a biaxially stretched polypropylene film has a heat shrinkage rate of 1% or more, while a PET film has a heat shrinkage rate of less than 1%.
- Thermal contraction rate in MD direction (%) (MD direction length before heating - MD direction length after heating) / MD direction length before heating ⁇ 100 ... (1)
- the thermal contraction rate of the base layer in the TD direction determined by the following formula (2) is not particularly limited, but is, for example, 1% or more. From the viewpoint of reducing deformation during bag manufacturing and suppressing peeling between the gas barrier film and the sealant layer, the above heat shrinkage rate in the TD direction is, for example, 12% or less, 11% or less, 10% or less, 9% or less, 8%. or 7% or less.
- Thermal contraction rate in TD direction (%) (TD length before heating - TD length after heating) / TD direction length before heating x 100...(2)
- the hardness of the adhesive layer is 0.1 MPa or more and less than 0.9 MPa.
- the adhesive layer can satisfactorily follow the expansion and contraction of the base material layer due to heat treatment such as retort treatment and boiling treatment. For this reason, even if the packaging film including the base material layer having the above-mentioned heat shrinkage rate is subjected to the above-mentioned heat treatment, peeling between the gas barrier film and the sealant layer is unlikely to occur.
- the hardness of the adhesive layer before the retort treatment is not particularly limited, but is, for example, 1.0 MPa or more.
- the hardness of the adhesive layer can be controlled by the adhesive material, amount of curing agent, aging time, etc. The narrower the distance between the molecular chains of the adhesive, the harder the adhesive layer tends to be. The bulkier the adhesive, the softer the adhesive layer tends to be.
- the hardness of an aliphatic adhesive layer tends to be higher than that of an alicyclic or aromatic adhesive layer.
- the hardness of an alicyclic adhesive layer tends to be higher than that of an aromatic adhesive layer.
- the larger the amount of curing agent contained in the adhesive layer the harder the adhesive layer tends to be.
- the hardness of the adhesive layer in the packaging film is obtained by measuring the hardness of the part exposed from the sealant layer (exposed part). Removal of the sealant layer to expose the adhesive layer is performed using, for example, an oblique cutting device. In this embodiment, the hardness of the adhesive layer is obtained by measuring with a nanoindentation method.
- the hardness of a sample such as an adhesive layer is calculated, for example, by the following method. First, fused silica, which will serve as a standard sample, is tested in advance to calibrate the relationship between the contact depth and the projected contact area between the indenter and the sample. Thereafter, the hardness of the sample is calculated by analyzing the unloading curve in the range of 20 to 95% of the maximum load during unloading using the Oliver-Pharr method.
- the outer layer film After the outer layer film is exposed to 120° C. for 15 minutes, the outer layer film has a heat shrinkage rate in the MD direction of 1% or more as determined by the above formula (1).
- the heat shrinkage rate of the outer layer film in the MD direction determined by the following formula (1) is 1% or more. It is.
- the following heat shrinkage rate in the MD direction is, for example, 12% or less, 11% or less, 10% or less, 9% or less, 8% or less, or 7% or less.
- the thermal shrinkage rate of the outer layer film in the TD direction determined by the above formula (2) is not particularly limited, but is, for example, 1% or more and 12% or less.
- the hardness of the second adhesive layer is 0.1 MPa or more and less than 0.9 MPa.
- the second adhesive layer can satisfactorily follow the expansion and contraction of the outer layer film due to heat treatment such as retort treatment and boil treatment. Therefore, even if the packaging film including the second adhesive layer having the above-mentioned heat shrinkage rate is subjected to the heat treatment, peeling between the gas barrier film and the outer layer film is less likely to occur.
- the hardness of the second adhesive layer before the retort treatment is not particularly limited, but is, for example, 1.0 MPa or more.
- the hardness of the second adhesive layer can be controlled by the adhesive material, the amount of curing agent, aging time, etc. The narrower the distance between the molecular chains of the adhesive, the harder the second adhesive layer tends to be. The bulkier the adhesive, the softer the second adhesive layer tends to be.
- the hardness of the aliphatic second adhesive layer tends to be higher than that of the alicyclic or aromatic second adhesive layer.
- the hardness of the alicyclic second adhesive layer tends to be higher than that of the aromatic second adhesive layer.
- the larger the amount of curing agent contained in the second adhesive layer the harder the second adhesive layer tends to be.
- the packaging bag is made by bag-making the above-mentioned packaging film.
- the packaging bag can contain contents such as food and medicine.
- the packaging bag may be made into a bag shape by folding one packaging film in half so that the sealant layers face each other and then heat-sealing the three sides.
- the bags may be formed into a bag shape by stacking them so that they are facing each other and then heat-sealing the four sides.
- the packaging bag may have a shape such as a standing pouch having a bent portion (folded portion).
- the packaging bag according to this embodiment can maintain high gas barrier properties even if it has a shape having a bent portion.
- FIG. 5(a) is a schematic plan view of a laminate according to one embodiment
- FIG. 5(b) is a schematic cross-sectional view showing the laminate according to one embodiment.
- the laminate 35 shown in FIGS. 5(a) and 5(b) is a sheet-like member (gas barrier laminate) having gas barrier properties, and is a sheet-like packaging material used, for example, in manufacturing packaging bags.
- the laminate 35 can be suitably used, for example, in applications where heat treatment such as retort treatment and boiling treatment is performed.
- the laminate 35 may correspond to the packaging film of the first embodiment.
- Retort processing is a method that sterilizes microorganisms such as mold, yeast, and bacteria under pressure in order to generally preserve foods, medicines, etc.
- packaging bags containing foods, etc. are subjected to pressure sterilization treatment at 105 to 140°C and 0.15 to 0.30 MPa for 10 to 120 minutes.
- pressure sterilization treatment There are two types of retort devices: a steam type that uses heated steam and a hot water type that uses pressurized heated water.
- Boiling is a method of sterilizing foods, medicines, etc. with moist heat to preserve them.
- a packaging bag containing foods, etc. is subjected to moist heat sterilization treatment at 60 to 100°C and under atmospheric pressure for 10 to 120 minutes, depending on the contents.
- the boiling process is usually performed at 100°C or lower using a hot water bath.
- a hot water bath There are two methods: a batch method, in which the material is immersed in a hot water tank at a constant temperature and treated for a certain period of time, and then taken out, and a continuous method, in which the material is passed through the hot water tank in a tunnel.
- the laminate 35 includes a barrier layer 40, a sealant layer 50, and an adhesive layer 60.
- the barrier layer 40 and the sealant layer 50 are laminated on each other and bonded together with an adhesive layer 60.
- a barrier layer 40, an adhesive layer 60, and a sealant layer 50 are laminated in this order.
- the direction MD is defined as the flow direction (longitudinal direction) of the laminate 35
- the direction TD is defined as the width direction (lateral direction) of the laminate 35.
- a direction perpendicular to both the directions MD and TD is defined as the lamination direction of the members included in the laminated body 35.
- the barrier layer 40 is a member that functions as a support in the laminate 35 and exhibits gas barrier properties against gases such as water vapor and oxygen.
- the barrier layer 40 may correspond to the gas barrier film of the first embodiment.
- the barrier layer 40 includes a base material 41, an adhesion layer 42, a vapor deposition layer 43, and a barrier coat 44.
- a base material 41, an adhesive layer 42, a vapor deposition layer 43, and a barrier coat 44 are laminated in this order. Therefore, the adhesion layer 42 and the vapor deposition layer 43 are located between the base material 41 and the barrier coat 44 in the lamination direction, and the adhesion layer 42 is located between the base material 41 and the vapor deposition layer 43.
- the barrier coat 44 of the barrier layers 40 is closest to the adhesive layer 60 in the lamination direction. Therefore, among the barrier layers 40, the base material 41 is farthest from the adhesive layer 60 in the lamination direction.
- the base material 41 is a plastic member (base material layer) that functions as the outermost layer in the laminate 35.
- the thickness of the base material 41 is not particularly limited. Depending on the application, the thickness can be set to 6 to 200 ⁇ m, but from the viewpoint of reducing material to reduce environmental impact, and from the viewpoint of obtaining excellent heat resistance, impact resistance, and excellent gas barrier properties, It may be 9-50 ⁇ m, it may be 12-38 ⁇ m, it may be 18-30 ⁇ m.
- the base material 41 is, for example, a polyolefin film.
- the base material 41 may include or be made of a polypropylene film.
- the polypropylene film may be an acid-modified polypropylene film obtained by graft-modifying polypropylene using an unsaturated carboxylic acid, an acid anhydride of an unsaturated carboxylic acid, an ester of an unsaturated carboxylic acid, or the like.
- polypropylene resins such as homopolypropylene resin (PP), propylene-ethylene random copolymer, propylene-ethylene block copolymer, propylene- ⁇ -olefin copolymer, etc. can be used.
- additives such as a flame retardant, a slip agent, an anti-blocking agent, an antioxidant, a light stabilizer, a tackifier, and an antistatic agent may be added to the polypropylene film constituting the base material 41.
- the polypropylene film constituting the base material 41 may be a stretched film or a non-stretched film.
- the polypropylene film may be a stretched polypropylene film.
- the laminate 35 can be more suitably used in applications where heat treatment such as retort treatment and boiling treatment is performed.
- the stretching method is not particularly limited, and any method may be used as long as it can provide a dimensionally stable film, such as inflation stretching, uniaxial stretching, or biaxial stretching.
- the laminated surface of the base material 41 may be subjected to various pretreatments such as corona treatment, plasma treatment, flame treatment, etc., or provided with a coating layer such as an easy-to-adhesion layer, as long as the barrier performance is not impaired.
- the adhesion layer 42 functions as a layer (anchor coat layer) that can improve the adhesion performance of the vapor deposited layer 43 on the base material 41 and is provided directly above the base material 41 . Therefore, the adhesive layer 42 is located between the base material 41 and the vapor deposition layer 43.
- the smoothness of the surface of the barrier layer 40 on which the vapor deposition layer 43 is provided can be improved. In addition, by improving the smoothness, it becomes easier to form the vapor deposition layer 43 uniformly without defects, and it becomes easier to exhibit high barrier properties.
- the adhesion layer 42 can be formed using, for example, an anchor coating agent.
- the anchor coating agent examples include polyester polyurethane resins, polyether polyurethane resins, and the like.
- a polyester polyurethane resin may be used from the viewpoint of heat resistance and interlayer adhesive strength.
- the thickness of the adhesive layer 42 is not particularly limited, but may be in the range of 0.01 to 5 ⁇ m, may be in the range of 0.03 to 3 ⁇ m, or may be in the range of 0.05 to 2 ⁇ m.
- the thickness of the adhesion layer 42 is at least the above lower limit value, more sufficient interlayer adhesive strength tends to be obtained, while when it is at most the above upper limit value, desired gas barrier properties tend to be easily expressed.
- any known coating method can be used without any particular restriction, and examples include a dipping method, a method using a spray, a coater, a printing machine, a brush, etc. Can be mentioned.
- the types of coaters and printing machines used in these methods and their coating methods include gravure coaters such as direct gravure method, reverse gravure method, kiss reverse gravure method, and offset gravure method, reverse roll coater, and microgravure. Examples include a coater, a chamber doctor coater, an air knife coater, a dip coater, a bar coater, a comma coater, and a die coater.
- the coating amount of the adhesive layer 42 may be 0.01 to 5 g/m 2 or 0.03 to 3 g/m 2 in mass per 1 m 2 after the anchor coating agent is applied and dried.
- mass per 1 m 2 after coating and drying the anchor coating agent is above the above lower limit, film formation tends to be sufficient, while when it is below the above upper limit, it is sufficiently easy to dry and the solvent is removed. It tends to be difficult to remain.
- Methods for drying the adhesive layer 42 are not particularly limited, but include natural drying, drying in an oven set at a predetermined temperature, and a dryer attached to the coater, such as an arch dryer, a floating dryer, and a drum dryer. , a method using an infrared dryer, etc. can be mentioned. Further, the drying conditions can be appropriately selected depending on the drying method. For example, in the case of drying in an oven, drying may be performed at a temperature of 60 to 100° C. for about 1 second to 2 minutes.
- a polyvinyl alcohol resin can be used instead of the polyurethane resin described above.
- the polyvinyl alcohol resin may be any resin having a vinyl alcohol unit formed by saponifying a vinyl ester unit, and examples thereof include polyvinyl alcohol (PVA) and ethylene-vinyl alcohol copolymer (EVOH).
- vinyl esters such as vinyl acetate, vinyl formate, vinyl propionate, vinyl valerate, vinyl caprate, vinyl laurate, vinyl stearate, vinyl pivalate, and vinyl versatate can be polymerized alone. , followed by saponified resins.
- the PVA may be a copolymerized or post-modified modified PVA. Modified PVA can be obtained, for example, by copolymerizing a vinyl ester and an unsaturated monomer copolymerizable with the vinyl ester, followed by saponification.
- Examples of unsaturated monomers copolymerizable with vinyl ester include olefins such as ethylene, propylene, isobutylene, ⁇ -octene, ⁇ -dodecene, and ⁇ -octadecene; 3-buten-1-ol, 4-pentyn-1-ol , 5-hexen-1-ol, etc.; unsaturated acids such as acrylic acid, methacrylic acid, crotonic acid, maleic acid, maleic anhydride, itaconic acid, undecylenic acid; acrylonitrile, methacrylonitrile, etc.
- olefins such as ethylene, propylene, isobutylene, ⁇ -octene, ⁇ -dodecene, and ⁇ -octadecene
- unsaturated acids such as acrylic acid
- Nitriles such as diacetone acrylamide, acrylamide, and methacrylamide; olefin sulfonic acids such as ethylene sulfonic acid, allyl sulfonic acid, and methallyl sulfonic acid; alkyl vinyl ethers, dimethylallyl vinyl ketone, N-vinyl pyrrolidone, vinyl chloride, vinyl ethylene Vinyl compounds such as carbonate, 2,2-dialkyl-4-vinyl-1,3-dioquinrane, glycerin monoallyl ether, 3,4-diacetoxy-1-butene; vinylidene chloride, 1,4-diacetoxy-2-butene, Examples include vinylene carbonate.
- the degree of polymerization of PVA is, for example, 300 to 3000. If the degree of polymerization is less than 300, the barrier properties tend to deteriorate, and if it exceeds 3000, the viscosity is too high and the coating suitability tends to deteriorate.
- the degree of saponification of PVA may be 90 mol% or more, 95 mol% or more, or 99 mol% or more. Moreover, the degree of saponification of PVA may be 100 mol% or less, or 99.9 mol% or less.
- the degree of polymerization and saponification of PVA can be measured according to the method described in JIS K 6726 (1994).
- EVOH is generally a copolymer of ethylene and acid vinyl esters such as vinyl acetate, vinyl formate, vinyl propionate, vinyl valerate, vinyl caprate, vinyl laurate, vinyl stearate, vinyl pivalate, and vinyl versatate. Obtained by saponifying the union.
- the degree of polymerization of EVOH is, for example, 300 to 3000. If the degree of polymerization is less than 300, the barrier properties tend to deteriorate, and if it exceeds 3000, the viscosity is too high and the coating suitability tends to deteriorate.
- the degree of saponification of the vinyl ester component of EVOH may be 90 mol% or more, 95 mol% or more, or 99 mol% or more. Further, the degree of saponification of EVOH may be 100 mol% or less, or 99.9 mol% or less.
- the degree of saponification of EVOH is determined by nuclear magnetic resonance (1H-NMR) measurement from the peak area of hydrogen atoms contained in the vinyl ester structure and the peak area of hydrogen atoms contained in the vinyl alcohol structure.
- the ethylene unit content of EVOH is, for example, 10 mol% or more, may be 15 mol% or more, may be 20 mol% or more, or may be 25 mol% or more. Further, the ethylene unit content of EVOH may be 65 mol% or less, 55 mol% or less, or 50 mol% or less. When the ethylene unit content is 10 mol % or more, gas barrier properties or dimensional stability under high humidity can be maintained favorably. On the other hand, when the ethylene unit content is 65 mol% or less, gas barrier properties can be improved.
- the ethylene unit content of EVOH can be determined by NMR method.
- methods for forming the adhesive layer 42 include coating using a polyvinyl alcohol resin solution, multilayer extrusion, and the like.
- the vapor deposition layer 43 is a layer (gas barrier layer) that exhibits gas barrier properties against water vapor and oxygen, and includes at least one of a metal and an inorganic oxide.
- the vapor deposition layer 43 is provided directly above the adhesive layer 42 .
- the vapor deposition layer 43 may have a single layer structure or a laminated structure. Therefore, the vapor deposition layer 43 includes at least one of a metal vapor deposition layer and an inorganic oxide layer.
- examples of the metal contained in the metal vapor deposition layer include aluminum, stainless steel, and the like.
- the inorganic oxide contained in the inorganic oxide layer include aluminum oxide, silicon oxide, magnesium oxide, and tin oxide.
- the inorganic oxide may be selected from the group consisting of aluminum oxide, silicon oxide, and magnesium oxide.
- the inorganic oxide layer may be a layer using silicon oxide.
- the O/Si ratio of the inorganic oxide layer is preferably 1.7 or more.
- the O/Si ratio is 1.7 or more, the metal Si content is suppressed and good transparency is easily obtained.
- the O/Si ratio may be 2.0 or less.
- the crystallinity of SiO becomes high and the inorganic oxide layer can be prevented from becoming too hard, and good tensile resistance can be obtained. Thereby, it is possible to suppress the occurrence of cracks in the inorganic oxide layer when layering the barrier coat 44.
- the base material 41 may shrink due to heat during boiling or retort processing, but since the O/Si ratio is 2.0 or less, the inorganic oxide layer can follow the shrinkage. It is possible to suppress the deterioration of barrier properties. From the viewpoint of obtaining these effects more fully, the O/Si ratio of the inorganic oxide layer may be 1.75 or more and 1.9 or less, or 1.8 or more and 1.85 or less.
- the O/Si ratio of the inorganic oxide layer can be determined by X-ray photoelectron spectroscopy (XPS).
- XPS X-ray photoelectron spectroscopy
- the measurement device is an X-ray photoelectron spectrometer (manufactured by JEOL Ltd., product name: JPS-90MXV)
- the X-ray source is non-monochromatic MgK ⁇ (1253.6eV)
- the X-ray source is 100W (10kV-10mA).
- Relative sensitivity factors of 2.28 for O1s and 0.9 for Si2p can be used for quantitative analysis to determine the O/Si ratio, respectively.
- the thickness of the vapor deposition layer 43 is, for example, 5 nm or more and 80 nm or less. When the thickness of the vapor deposition layer 43 is 5 nm or more, sufficient water vapor barrier properties can be obtained. Moreover, when the thickness of the vapor deposition layer 43 is 80 nm or less, it is possible to suppress the occurrence of cracks due to deformation due to internal stress of the thin film, and to suppress a decrease in water vapor barrier properties. It should be noted that if the thickness of the vapor deposited layer 43 exceeds 80 nm, the cost tends to increase due to an increase in the amount of material used, a longer time for film formation, etc., which is not preferable from an economic point of view. From the same viewpoint as above, the thickness of the vapor deposition layer 43 may be 20 nm or more and 40 nm or less.
- the vapor deposition layer 43 can be formed, for example, by vacuum film formation.
- a physical vapor deposition method or a chemical vapor deposition method can be used.
- the physical vapor deposition method include, but are not limited to, a vacuum evaporation method, a sputtering method, an ion plating method, and the like.
- the chemical vapor deposition method include, but are not limited to, a thermal CVD method, a plasma CVD method, a photo CVD method, and the like.
- the above vacuum film formation methods include resistance heating vacuum evaporation method, EB (Electron Beam) heating vacuum evaporation method, induction heating vacuum evaporation method, sputtering method, reactive sputtering method, dual magnetron sputtering method, and plasma chemical vapor deposition method. (PECVD method) etc. may be used. However, if productivity is taken into consideration, the vacuum evaporation method may be the most superior at present.
- a heating means for the vacuum evaporation method any one of an electron beam heating method, a resistance heating method, and an induction heating method may be used.
- the barrier coat 44 is a coating layer having gas barrier properties (gas barrier coating layer), and is provided on the base material 41 .
- the barrier coat 44 is made of, for example, a gas barrier coating layer-forming composition ( This layer is formed using a coating agent (hereinafter also referred to as a coating agent).
- the coating agent may contain at least a silane coupling agent or a hydrolyzate thereof, and may contain a hydroxyl group-containing polymer compound, a metal alkoxide and It may contain at least one selected from the group consisting of those hydrolysates, a silane coupling agent or its hydrolysate, and a hydroxyl group-containing polymer compound or its hydrolysate, and a metal alkoxide or its hydrolysate. It may contain a hydrolyzate and a silane coupling agent or a hydrolyzate thereof.
- the coating agent can be prepared by directly or pre-hydrolyzing a metal alkoxide and a silane coupling agent in a solution in which a water-soluble polymer containing a hydroxyl group is dissolved in an aqueous (water or water/alcohol mixed) solvent. It can be prepared by mixing those that have been subjected to a treatment such as oxidation.
- hydroxyl group-containing polymer compound used in the coating agent examples include polyvinyl alcohol, polyvinylpyrrolidone, starch, methylcellulose, carboxymethylcellulose, and sodium alginate.
- PVA polyvinyl alcohol
- gas barrier properties tend to be particularly excellent.
- the barrier coat 44 has a composition containing at least one member selected from the group consisting of metal alkoxides represented by the general formula (I) shown in the above embodiments and hydrolysates thereof. May be formed from objects.
- metal alkoxide examples include tetraethoxysilane [Si(OC 2 H 5 ) 4 ], triisopropoxyaluminum [Al(O-2′-C 3 H 7 ) 3 ], and the like. Tetraethoxysilane and triisopropoxyaluminum tend to be relatively stable in aqueous solvents after hydrolysis.
- silane coupling agent examples include the compound represented by the above general formula (II) shown in the above embodiment.
- silane coupling agent examples include vinyltrimethoxysilane, ⁇ -chloropropylmethyldimethoxysilane, ⁇ -chloropropyltrimethoxysilane, glycidoxypropyltrimethoxysilane, ⁇ -methacryloxypropyltrimethoxysilane, ⁇ - Examples include silane coupling agents such as methacryloxypropylmethyldimethoxysilane.
- the silane coupling agent may be a polymer obtained by polymerizing the compound represented by the above general formula (II).
- the multimer may be a trimer or 1,3,5-tris(3-trialkoxysilylalkyl)isocyanurate. This is a condensation polymer of 3-isocyanatealkylalkoxysilane. It is known that in this 1,3,5-tris(3-trialkoxysilylalkyl) isocyanurate, the isocyan moiety has no chemical reactivity, but the reactivity is ensured by the polarity of the nurate moiety.
- 3-isocyanate alkyl alkoxylane like 3-isocyanate alkyl alkoxylane, it is added to adhesives, etc., and is known as an adhesion improver. Therefore, by adding 1,3,5-tris(3-trialkoxysilylalkyl)isocyanurate to a hydroxyl group-containing polymer compound, the water resistance of the gas barrier coating layer can be improved due to hydrogen bonding.
- 3-Isocyanate alkyl alkoxyrane has high reactivity and low liquid stability, whereas 1,3,5-tris(3-trialkoxysilylalkyl) isocyanurate is not water-soluble due to the polarity of the nurate moiety. However, it is easily dispersed in aqueous solutions and can maintain stable liquid viscosity. Furthermore, the water resistance properties of 3-isocyanatealkylalkoxyrane and 1,3,5-tris(3-trialkoxysilylalkyl)isocyanurate are equivalent.
- 1,3,5-Tris(3-trialkoxysilylalkyl)isocyanurate is sometimes produced by thermal condensation of 3-isocyanatepropylalkoxysilane, and may also contain the raw material 3-isocyanatepropylalkoxysilane. However, there is no particular problem. It may be 1,3,5-tris(3-trialkoxysilylpropyl)isocyanurate or 1,3,5-tris(3-trimethoxysilylpropyl)isocyanurate. 1,3,5-tris(3-trimethoxysilylpropyl)isocyanurate is practically advantageous because the methoxy group has a high hydrolysis rate and those containing a propyl group are available at relatively low cost.
- additives such as isocyanate compounds, dispersants, stabilizers, viscosity modifiers, colorants, etc. can be added to the coating agent as necessary, within a range that does not impair gas barrier properties. .
- the thickness of the barrier coat 44 may be 50 to 1000 nm, or 100 to 500 nm. When the thickness of the barrier coat 44 is 50 nm or more, it tends to be able to obtain more sufficient gas barrier properties, and when it is 1000 nm or less, it tends to be able to maintain sufficient flexibility.
- the coating liquid for forming the barrier coat 44 can be used, for example, by a dipping method, a roll coating method, a gravure coating method, a reverse gravure coating method, an air knife coating method, a comma coating method, a die coating method, a screen printing method, a spray coating method, or a gravure coating method. It can be applied by an offset method or the like.
- a coating formed by applying this coating liquid can be dried by, for example, a hot air drying method, a hot roll drying method, a high frequency irradiation method, an infrared irradiation method, a UV irradiation method, or a combination thereof.
- the temperature at which the coating film is dried can be, for example, 50 to 150°C, or 70 to 100°C. By setting the drying temperature within the above range, it is possible to further suppress the occurrence of cracks in the vapor deposited layer 43 and the barrier coat 44, and it is possible to exhibit excellent barrier properties.
- the barrier coat 44 may be formed using a coating agent containing a polyvinyl alcohol resin and a silane compound.
- An acid catalyst, an alkali catalyst, a photoheavy initiator, etc. may be added to the coating agent as necessary.
- the polyvinyl alcohol resin is as described above.
- examples of the silane compound include silane coupling agents, polysilazane, siloxane, etc. Specifically, tetramethoxysilane, tetraethoxysilane, glycidoxypropyltrimethoxysilane, acryloxypropyltrimethoxysilane, hexamethyl Examples include disilazane.
- the laminate 35 may include a printed layer.
- the printed layer can be provided on at least one surface of the base material 41, for example.
- the printing layer is provided at a position visible from the outside of the laminate 35 for the purpose of displaying information about the contents, identifying the contents, improving concealability, or improving the design of the packaging bag.
- the printing method and printing ink are not particularly limited, and are appropriately selected from among known printing methods and printing inks, taking into consideration suitability for printing onto a film, design characteristics such as color tone, adhesion, safety as a food container, etc. Ru.
- As the printing method for example, a gravure printing method, an offset printing method, a gravure offset printing method, a flexo printing method, an inkjet printing method, etc. can be used. Among them, the gravure printing method is likely to be used from the viewpoint of productivity and high definition of images.
- the surface of the layer on which the printed layer is provided may be subjected to various pre-treatments such as corona treatment, plasma treatment, flame treatment, etc., or a coat layer such as an easy-to-adhesion layer may be provided.
- the sealant layer 50 is a layer that provides sealing properties by heat sealing in the laminate 35 . From the viewpoint of suitability for recycling of the laminate 35, the sealant layer 50 is a polyolefin film like the base material 41. In this embodiment, the sealant layer 50 is a resin layer having a single layer structure and mainly made of polypropylene, but is not limited thereto. Sealant layer 50 may include or consist of a polypropylene film.
- the polypropylene film may be an acid-modified polypropylene film obtained by graft-modifying polypropylene using an unsaturated carboxylic acid, an acid anhydride of an unsaturated carboxylic acid, an ester of an unsaturated carboxylic acid, or the like.
- polypropylene resins such as homopolypropylene resin (PP), propylene-ethylene random copolymer, propylene-ethylene block copolymer, propylene- ⁇ -olefin copolymer, etc. can be used.
- the polypropylene film constituting the sealant layer 50 may be an unstretched polypropylene film from the viewpoint of improving sealing performance by heat sealing.
- additives such as flame retardants, slip agents, anti-blocking agents, antioxidants, light stabilizers, tackifiers, and antistatic agents may be added to the polypropylene film constituting the sealant layer 50.
- the thickness of the sealant layer 50 is determined by the mass of the contents, the shape of the packaging bag, etc., but may be approximately 30 to 150 ⁇ m thick, or 50 to 80 ⁇ m thick.
- the sealant layer 50 can be formed by a dry lamination method in which the film-like sealant layer made of polypropylene described above is bonded together with an adhesive such as a one-component or two-component urethane adhesive; It can be formed by any known lamination method, such as a non-solvent dry lamination method in which the sheets are laminated using a solvent-free adhesive, or an extrusion lamination method in which the above-mentioned polypropylene is heated and melted, extruded into a curtain shape, and laminated together.
- the dry lamination method has the highest resistance to retort treatment, especially high-temperature hydrothermal treatment at 120° C. or higher.
- the lamination method is not particularly limited.
- the adhesive layer 60 is a layered member that adheres the barrier layer 40 and the sealant layer 50.
- As the material of the adhesive included in the adhesive layer 60 for example, polyester-isocyanate resin, urethane resin, polyether resin, etc. can be used.
- a two-component curing type urethane adhesive that is resistant to retort use can be used. Note that from the viewpoint of environmental considerations, the adhesive does not need to contain 3-glycidyloxypropyltrimethoxysilane (GPTMS).
- the adhesive layer 60 does not need to contain chlorine.
- the adhesive layer 60 may be formed of a biomass material and may not contain a solvent.
- the thickness of the adhesive layer 60 is 0.5 ⁇ m or more and 10 ⁇ m or less. When the thickness of the adhesive layer 60 is 0.5 ⁇ m or more, peeling between the barrier layer 40 and the sealant layer 50 can be suppressed well. When the thickness of the adhesive layer 60 is 10 ⁇ m or less, the laminate 35 can be easily made into a monomaterial (details will be described later).
- the thickness of the adhesive layer 60 may be 1 ⁇ m or more, 2 ⁇ m or more, 8 ⁇ m or less, 6 ⁇ m or less, or 5 ⁇ m or less.
- the ratio of the total mass of polyolefin (polypropylene in this embodiment) in the laminate 35 is 90% by mass or more.
- the laminate 35 can be said to be a packaging material made of a single material (monomaterial), and has excellent recyclability.
- the content of polyolefin in the laminate 35 may be 92.5% by mass or more, or 95% by mass or more, based on the total amount of the laminate 35.
- Heat shrinkage rate of base material 41 After exposing the base material 41 at 120° C. for 15 minutes (hereinafter simply referred to as "after heating"), the base material 41 undergoes thermal contraction in the MD direction as determined by the above formula (1) described in the above first embodiment. rate is 1% or more. For example, after heating the base material 41 or the barrier layer 40 including the base material 41 in an oven at 120° C. for 15 minutes, the heat shrinkage rate of the base material 41 in the MD direction determined by the following formula (1) is 1% or more. be.
- the heat shrinkage rate in the MD direction is, for example, 12% or less, 11% or less, 10% or less, 9% or less, It is 8% or less, or 7% or less.
- the heat shrinkage rate of a biaxially stretched polypropylene film is 1%, while the heat shrinkage rate of a PET film is less than 1%.
- the thermal contraction rate of the base material 41 in the TD direction determined by the above formula (2) described in the first embodiment is, for example, 1% or more, although it is not particularly limited.
- the heat shrinkage rate in the TD direction is, for example, 12% or less, 11% or less, 10% or less, 9% or less, It is 8% or less, or 7% or less.
- the hardness of the adhesive layer 60 is 0.1 MPa or more and less than 0.9 MPa.
- the adhesive layer 60 can satisfactorily follow the expansion and contraction of the base material 41 due to heat treatment such as retort treatment and boiling treatment. Therefore, even if the laminate 35 including the base material 41 having the above-mentioned heat shrinkage rate is subjected to the heat treatment, peeling between the barrier layer 40 and the sealant layer 50 is less likely to occur.
- the hardness of the adhesive layer 60 before the retort treatment is not particularly limited, but is, for example, 1.0 MPa or more.
- the hardness of the adhesive layer 60 can be controlled by the adhesive material, the amount of curing agent, the aging time, etc. The narrower the distance between the molecular chains of the adhesive, the harder the adhesive layer 60 tends to be. The bulkier the adhesive, the softer the adhesive layer 60 tends to be.
- the hardness of the aliphatic adhesive layer 60 tends to be higher than that of the alicyclic or aromatic adhesive layer 60.
- the hardness of the alicyclic adhesive layer 60 tends to be higher than that of the aromatic adhesive layer 60.
- the larger the amount of curing agent contained in the adhesive layer 60 the harder the adhesive layer 60 tends to be.
- the hardness of the adhesive layer 60 in the laminate 35 is obtained by measuring the hardness of the portion exposed from the sealant layer 50 (exposed portion). Removal of the sealant layer 50 to expose the adhesive layer 60 is performed using, for example, a diagonal cutting device.
- the hardness of the adhesive layer 60 is obtained by measuring with a nanoindentation method.
- the nanoindentation method is a measurement method that obtains the mechanical properties of a target object (sample) to be measured by performing a quasi-static indentation test using an indenter.
- the hardness of a sample such as the adhesive layer 60 is calculated, for example, by the following method.
- fused silica which will serve as a standard sample, is tested in advance to calibrate the relationship between the contact depth and the projected contact area between the indenter and the sample. Thereafter, the hardness of the sample is calculated by analyzing the unloading curve in the range of 20 to 95% of the maximum load during unloading using the Oliver-Pharr method.
- FIG. 6 is a schematic plan view of an example of a packaging bag.
- the packaging bag 100 shown in FIG. 6 is formed into a bag shape by, for example, sealing the ends of a laminate 35 that is folded in two so as to sandwich the contents therebetween.
- the packaging bag 100 is a three-sided bag having a main body part 101 in which the contents are stored, a seal part 102 located at the end of the main body part 101, and a folding part 103 in which the laminate 35 is bent.
- the shape of the main body portion 101 is not particularly limited, and has a rectangular shape when viewed from a predetermined direction, for example. At least a portion of the outer surface of the main body portion 101 may be printed.
- the main body portion 101 may contain a specific gas such as nitrogen in addition to the contents.
- the seal portion 102 is a portion where a portion of the sealant layer 50 included in the laminate 35 and another portion are bonded together.
- the seal portion 102 a portion of the sealant layer 50 included in the laminate 35 and another portion are in close contact with each other.
- the seal portion 102 is formed, for example, by heating and compressing (that is, heat-sealing) a part of the sealant layer 50 and another part of the laminate 35, but is not limited thereto.
- the seal portion 102 may be formed by cold sealing or the like.
- the bent portion 103 constitutes one side of the main body 101, and the seal portion 102 constitutes the remaining three sides of the main body 101. Both ends of the bent portion 103 and the seal portion 102 overlap.
- the hardness of the adhesive layer 60 measured by a nanoindentation method is 0.1 MPa or more and less than 0.9 MPa. Accordingly, by using the base material 41 having a heat shrinkage rate of 1% or more in the MD direction, even if the base material 41 expands and contracts due to retort processing, the adhesive layer 60 can follow it well. Therefore, damage to the barrier coat 44 due to the occurrence of cracks in the adhesive layer 60 can be suppressed. Therefore, it is possible to provide a laminate 35 that can exhibit excellent gas barrier properties (especially oxygen permeation prevention performance) even after retort treatment.
- the barrier layer 40 includes an adhesion layer 42 and a vapor deposition layer 43 located between the base material 41 and the barrier coat 44, and the adhesion layer 42 is located between the base material 41 and the vapor deposition layer 43. . Therefore, the gas barrier properties of the laminate 35 can be improved compared to the case where the barrier layer has only the base material 41 and the barrier coat 44.
- the thickness of the vapor deposition layer 43 may be 5 nm or more and 80 nm or less. In this case, gas barrier properties can be improved while preventing the vapor deposited layer 43 from cracking.
- the thickness of the adhesive layer 60 may be 0.5 ⁇ m or more and 10 ⁇ m or less.
- the laminate 35 can be easily made into a monomaterial while satisfactorily suppressing separation between the barrier layer 40 and the sealant layer 50.
- each of the base material 41 and the sealant layer 50 is a polyolefin film, and the proportion of the total mass of polyolefin in the laminate 35 may be 90% by mass or more. In this case, monomaterialization is realized.
- the base material 41 may be a stretched polypropylene film
- the sealant layer 50 may be an unstretched polypropylene film.
- the laminate 35 has excellent heat resistance against retort processing and the like.
- FIG. 7 is a schematic cross-sectional view showing a laminate according to a modified example.
- the laminate (packaging film) 35A includes, in addition to the barrier layer 40, the sealant layer 50, and the adhesive layer 60, an outermost layer 70 that overlaps the barrier layer 40, and an outermost layer 70 and the barrier layer 40. It has a second adhesive layer 60A to which it adheres.
- the sealant layer 50, the adhesive layer 60, the barrier layer 40, the second adhesive layer 60A, and the outermost layer 70 are laminated in this order.
- the adhesive layer 60, the base material 41, the adhesive layer 42, the vapor deposition layer 43, the barrier coat 44, the second adhesive layer 60A, and the outermost layer 70 are laminated in this order. Therefore, the adhesive layer 60 adheres the base material 41 and the sealant layer 50, and the second adhesive layer 60A adheres the barrier coat 44 and the outermost layer 70.
- the barrier layer 40 functions as an intermediate layer in the laminate 35A.
- the outermost layer 70 is a plastic member that functions as the outermost material in the laminate 35A.
- the outermost layer 70 has the same function and performance as the base material 41.
- the thickness of the outermost layer 70 is approximately the same as the thickness of the base material 41.
- the outermost layer 70 is, for example, a polyolefin film.
- the outermost layer 70 may contain or be made of a polypropylene film.
- the thermal contraction rate of the outermost layer 70 in the MD direction determined by the above formula (1) is 1% or more.
- the thermal contraction rate of the outermost layer 70 in the MD direction determined by the above formula (1) is It is 1% or more.
- the heat shrinkage rate in the MD direction is, for example, 12% or less, 11% or less, 10% or less, 9% or less, 8% or less, or 7% or less.
- the thermal contraction rate of the outermost layer 70 in the TD direction determined by the above formula (2) is not particularly limited, but is, for example, 1% or more and 12% or less.
- the second adhesive layer 60A is a layered member that adheres the barrier layer 40 and the outermost layer 70.
- the material of the adhesive included in the second adhesive layer 60A is the same as the material of the adhesive included in the adhesive layer 60.
- the thickness of the second adhesive layer 60A is similar to the thickness of the adhesive layer 60, and is 0.5 ⁇ m or more and 10 ⁇ m or less.
- the hardness of the second adhesive layer 60A is 0.1 MPa or more and less than 0.9 MPa. In this case, the second adhesive layer 60A can satisfactorily follow the expansion and contraction of the outermost layer 70 due to heat treatment such as retort treatment and boil treatment.
- the hardness of the second adhesive layer 60A before the retort treatment is not particularly limited, but is, for example, 1.0 MPa or more.
- the hardness of the second adhesive layer 60A can be controlled by the adhesive material, the amount of curing agent, the aging time, etc. The narrower the distance between the molecular chains of the adhesive, the harder the second adhesive layer 60A tends to be. The bulkier the adhesive, the softer the second adhesive layer 60A tends to be.
- the hardness of the aliphatic second adhesive layer 60A tends to be harder than that of the alicyclic or aromatic second adhesive layer 60A.
- the hardness of the second adhesive layer 60A, which is alicyclic tends to be harder than the second adhesive layer 60A, which is aromatic.
- the larger the amount of curing agent contained in the second adhesive layer 60A the harder the second adhesive layer 60A tends to be. The longer the aging time, the harder the second adhesive layer 60A tends to become.
- the outermost layer 70 can protect the barrier layer 40.
- the base material layer includes a first skin layer and a core layer, and the vapor deposition layer is formed on the first skin layer side,
- the hardness of the first skin layer measured by a nanoindentation method is 0.02 to 0.15 GPa
- the hardness of the core layer is 0.07 GPa or more
- the hardness of the first skin layer is 0.02 to 0.15 GPa.
- a gas barrier film in which the hardness of the core layer is greater than that of the core layer [2] The gas barrier film according to [1], wherein the first skin layer contains a propylene- ⁇ -olefin copolymer. [3] The gas barrier film according to [1] or [2], wherein the ratio of the thickness of the first skin layer to the thickness of the core layer is 1/100 to 1/5. [4] The first skin layer has a composite modulus of elasticity of 1.2 to 2.5 GPa, and the core layer has a composite modulus of 2.0 GPa or more, as measured by a nanoindentation method, [1 ] to [3]. The gas barrier film according to any one of [3].
- a packaging film comprising: an adhesive layer that adheres the gas barrier coating layer and the sealant layer and includes a two-component curing urethane adhesive,
- the adhesive layer has a hardness of 0.1 MPa or more and less than 0.9 MPa, as measured by a nanoindentation method after the packaging film is subjected to retort treatment.
- the hardness of the adhesive layer measured by a nanoindentation method before the retort treatment is 1.0 MPa or more.
- each of the base layer and the sealant layer is a polyolefin film, The packaging film according to any one of [12] to [14], wherein the total mass ratio of polyolefin in the packaging film is 90% by mass or more.
- the base layer is a stretched polypropylene film, The packaging film according to any one of [12] to [15], wherein the sealant layer is an unstretched polypropylene film.
- one aspect of the present disclosure is not limited to the above embodiment, the above modification, and [1] to [19] above.
- One aspect of the present disclosure can be further modified without departing from the gist thereof.
- the proportion of the total mass of polypropylene in the laminate may be 90% by mass or more. Therefore, for example, in the laminate according to the above modification, the mass ratio of polypropylene in one of the base material and the outermost layer may be less than 90 mass%. Alternatively, in the laminate, the mass proportion of polypropylene in the barrier layer may be less than 90% by mass.
- the anchor coat layer and the vapor deposition layer are provided on the base material, but the present invention is not limited thereto.
- a vapor deposition layer may be provided on the sealant layer.
- an anchor coat layer may be provided between the sealant layer and the vapor deposition layer.
- the sealant layer and the anchor coat layer may be coextruded layers.
- an anchor coat layer and a vapor deposition layer may be included on the base material, and a vapor deposition layer may be provided on the sealant layer.
- the anchor coat layer may not be provided.
- Example 1 Ethylene-1-butene-propylene random copolymer resin (ethylene content: 2.5 mol%, 1-butene content: 3.5 mol%) was used as the material for the skin layer, and homopolypropylene resin was used as the material for the core layer. prepared. After coextruding these resins, they were biaxially stretched to produce a base film (base layer) with a total thickness of 20 ⁇ m. The thickness of the skin layer was 0.7 ⁇ m, and the thickness of the core layer was 19.3 ⁇ m.
- An acrylic primer solution was applied on the skin layer of the base layer by gravure coating and dried to form an anchor coat layer with a thickness of 0.1 ⁇ m.
- a thin film of silicon oxide with a thickness of 30 nm was deposited on the anchor coat layer by reactive vapor deposition using radio-frequency excited ion plating in an oxygen atmosphere under reduced pressure to form a vapor deposited layer made of an inorganic oxide.
- Tetraethoxysilane hereinafter referred to as "TEOS”
- methanol methanol
- hydrochloric acid 0.1N hydrochloric acid
- PVA polyvinyl alcohol
- IPA 1,3,5-tris(3-methoxysilylpropyl)isocyanurate
- a coating liquid (composition for forming a gas barrier coating layer) was prepared by mixing a solution diluted with a solution of 1/1 to have a solid content of 5% by mass (calculated as R 2 Si(OH) 3 ).
- the coating liquid was prepared so that the mass ratio of the SiO 2 solid content (converted value) of TEOS, the R 2 Si(OH) 3 solid content (converted value) of isocyanurate silane, and the PVA solid content was 40/5/55.
- the solution was prepared as follows. This coating liquid was applied onto the vapor deposited layer by gravure coating, and then dried under conditions of 80° C. and 60 seconds to form a gas barrier coating layer with a thickness of 0.3 ⁇ m.
- Example 1 having a laminated structure of gas barrier coating layer/deposited layer/anchor coat layer/skin layer/core layer was obtained.
- Example 2 A gas barrier film was obtained in the same manner as in Example 1, except that ethylene-propylene random copolymer resin (ethylene content: 5.0 mol%) was used as the material for the skin layer.
- Example 3 A gas barrier film was obtained in the same manner as in Example 1, except that ethylene-propylene random copolymer resin (ethylene content: 3.2 mol%) was used as the material for the skin layer.
- Example 4 A gas barrier film was obtained in the same manner as in Example 1, except that an ethylene-propylene random copolymer resin (ethylene content: 7.5 mol%) was used as the material for the skin layer.
- Example 1 A gas barrier film was obtained in the same manner as in Example 1, except that a base film having only a core layer without forming a skin layer was used.
- Example 2 The same procedure as in Example 1 was performed except that ethylene-1-butene-propylene random copolymer resin (ethylene content: 5 mol%, 1-butene content: 6 mol%) was used as the material for the skin layer. A gas barrier film was obtained.
- Example 3 A gas barrier film was obtained in the same manner as in Example 1, except that ethylene-propylene random copolymer resin (ethylene content: 0.5 mol%) was used as the material for the skin layer.
- Ethylene-1-butene-propylene random copolymer resin (ethylene content: 2.5 mol%, 1-butene content: 3.5 mol%) was used as the material for the first skin layer, and homogeneous as the material for the core layer.
- an ethylene-propylene random copolymer resin (ethylene content: 7.5 mol %) was prepared as a material for the second skin layer. After coextruding these resins, they were biaxially stretched to produce a base film (base layer) with a total thickness of 20 ⁇ m. The thickness of both the first skin layer and the second skin layer was 0.7 ⁇ m, and the thickness of the core layer was 18.6 ⁇ m.
- each layer is laminated on the first skin layer of the base material layer by performing the same operation as in Example 1, thereby forming a gas barrier coating layer/vapor deposition layer/anchor coat layer/first skin layer/core layer/ A gas barrier film of Example 5 having a laminated structure of a second skin layer was obtained.
- Example 6 A gas barrier film was obtained in the same manner as in Example 5, except that an ethylene-propylene random copolymer resin (ethylene content: 5.6 mol%) was used as the material for the second skin layer.
- Example 7 A gas barrier film was obtained in the same manner as in Example 1, except that the surface of the core layer opposite to the first skin layer lamination surface was subjected to corona treatment.
- a stretched polypropylene film having a thickness of 20 ⁇ m was laminated on the gas barrier coating layer side of the gas barrier film produced in each example by a dry lamination method via a two-component curable urethane adhesive. Further, an unstretched polypropylene film having a thickness of 60 ⁇ m was laminated on the base layer side of the gas barrier film by a dry lamination method via a two-component curable urethane adhesive. Thereby, a packaging film was obtained.
- Example 8 On the skin layer of the base material layer, a water-based polyurethane resin (manufactured by Mitsui Chemicals, Inc., Takelac WPB-341) was applied by gravure coating, and dried to form an anchor coat layer with a thickness of 1.5 ⁇ m. A gas barrier film was obtained by the same operation as in Example 1.
- Example 9 A gas barrier film was obtained in the same manner as in Example 1, except that the skin layer thickness was 0.3 ⁇ m and the core layer thickness was 19.7 ⁇ m.
- Example 10 The same operation as in Example 1 except that the skin layer thickness was 1.5 ⁇ m, the core layer thickness was 16.5 ⁇ m, and the anchor coat layer thickness was 1.1 ⁇ m. A gas barrier film was obtained.
- Example 11 The material of the skin layer was a butene-propylene random copolymer resin (butene content: 2.5 mol%), the thickness of the skin layer was 0.7 ⁇ m, and the thickness of the anchor coat layer was 2.
- a gas barrier film was obtained in the same manner as in Example 1 except that the thickness was 1 ⁇ m.
- Example 12 The material of the skin layer was a butene-propylene random copolymer resin (butene content: 1.0 mol%), the thickness of the skin layer was 0.7 ⁇ m, and the thickness of the anchor coat layer was 3. A gas barrier film was obtained in the same manner as in Example 1 except that the thickness was 1 ⁇ m.
- Example 13 The thickness of the anchor coat layer was set to 4.1 ⁇ m, and the coating liquid (composition for forming a gas barrier coating layer) was composed of SiO 2 solid content (converted value) of TEOS and R 2 Si (OH) of isocyanurate silane.
- a gas barrier film was obtained by the same operation as in Example 1, except that the solution was prepared so that the mass ratio of PVA solid content (converted value) to PVA solid content was 45/10/45.
- Example 14 The thickness of the anchor coat layer was set to 5.1 ⁇ m, and the coating liquid (composition for forming a gas barrier coating layer) was composed of SiO 2 solid content (converted value) of TEOS and R 2 Si (OH) of isocyanurate silane.
- a gas barrier film was obtained in the same manner as in Example 1, except that the mass ratio of PVA solid content to PVA solid content was 45/7/48.
- the cut film was embedded in a photocurable resin and cured using a halogen lamp KTX-100R (manufactured by Kenko Tokina Co., Ltd.). D-800 manufactured by Toagosei Co., Ltd. was used as the photocurable resin.
- the film-embedded resin after photocuring was fixed with an insert for an AFM sample holder, and the cross-section of the film was cut with a glass knife at room temperature (25°C). Thereafter, final cross-sectional cutting was performed with a diamond knife at room temperature at a cutting speed of 1.0 mm/s and a cutting layer thickness of 100 nm, and cutting was completed when a mirror surface was obtained.
- EM UC7 ultramicrotome
- EM FC7 manufactured by Leica
- Hysitron TI-Premier (trade name) manufactured by Bruker Japan Co., Ltd. was used as a measuring device, and a Berkovich type diamond indenter manufactured by Bruker Japan Co., Ltd. was used as an indenter.
- indentation is performed at a pushing speed of 30 nm/sec to a depth of 30 nm, held at the maximum depth for 1 second, and then unloaded at a speed of 30 nm/sec. I went by doing that.
- the measurement was performed by acquiring a shape image of the cross section of the sample using the shape measurement function of the measuring device that scans the sample surface with an indenter, and from the shape image, specifying 20 points on the target layer at intervals of 1 ⁇ m or more.
- Oxygen permeability measurement was performed on the packaging film after retort treatment. The measurement was performed using an oxygen permeability measuring device (OXTRAN 2/20, manufactured by Modern Control) at a temperature of 30° C. and a relative humidity of 70%. The measurement method was based on JIS K-7126, B method (isobaric method), and ASTM D3985-81, and the measured value was expressed in the unit [cm 3 (STP)/m 2 ⁇ day ⁇ atm].
- the lamination strength between the gas barrier film and the unstretched polypropylene film was measured for the packaging film after the retort treatment. The measurement was conducted in accordance with JIS K6854 at a test width of 15 mm, a peeling speed of 300 mm/min, and a peeling angle of 180 degrees. The measured value was expressed in the unit [N/15mm]. In Examples 1 to 4 and Comparative Examples 1 to 3, the lamination strength between the first skin layer and the unstretched polypropylene film was measured. In Examples 5 to 7 and Reference Examples 1 to 3, the laminate strength between the second skin layer and the unstretched polypropylene film was measured.
- ethylene-1-butene-propylene random copolymer resin is "EBP”
- ethylene-propylene random copolymer resin is "EP”
- butene-propylene random copolymer resin is "BP”
- homopolypropylene Resin is written as "HPP”
- acrylic primer solution as "acrylic”
- water-based polyurethane resin as "urethane”
- silicon oxide as "SiOx”
- isocyanurate silane as "SC”.
- HPP corona treatment
- the packaging film using the gas barrier film of the example can keep the oxygen permeability low even after heat sterilization, and the lamination strength between each layer can be kept low. It was confirmed that it is excellent.
- Example 15 A 60 ⁇ m thick unstretched polypropylene film was laminated on the gas barrier coating layer side of the gas barrier film prepared in Example 1 using a two-component curing urethane adhesive (a) by a dry lamination method to form a packaging film. I got it.
- Examples 16-20 Packaging films of Examples 16 to 20 were obtained in the same manner as in Example 15, except that two-component curing type urethane adhesives (b) to (f) were used instead of adhesive (a). .
- Example 21 An unstretched polypropylene film with a thickness of 60 ⁇ m was laminated on the gas barrier coating layer side of the gas barrier film prepared in Example 2 by a dry lamination method via a two-part curable urethane adhesive (a) to form a packaging film. I got it.
- Examples 22 to 26 Packaging films of Examples 24 to 28 were obtained in the same manner as in Example 21, except that two-component curing type urethane adhesives (b) to (f) were used instead of the adhesive (a). .
- Example 27 An unstretched polypropylene film with a thickness of 60 ⁇ m was laminated on the gas barrier coating layer side of the gas barrier film prepared in Example 3 by a dry lamination method via a two-part curable urethane adhesive (a) to form a packaging film. I got it.
- Example 28-32 Packaging films of Examples 28 to 32 were obtained in the same manner as in Example 27, except that two-component curing type urethane adhesives (b) to (f) were used instead of the adhesive (a). .
- Example 33 An unstretched polypropylene film with a thickness of 60 ⁇ m was laminated on the gas barrier coating layer side of the gas barrier film prepared in Example 4 by a dry lamination method via a two-component curing urethane adhesive (a) to form a packaging film. I got it.
- Examples 34 to 38 Packaging films of Examples 34 to 48 were obtained in the same manner as in Example 33, except that two-component curing type urethane adhesives (b) to (f) were used instead of the adhesive (a). .
- the sample was placed in an oblique cutting device (manufactured by Daipla Wintes Co., Ltd., trade name: SAICAS DN-GS).
- SAICAS DN-GS oblique cutting device
- a diamond cutting blade having a V-shaped tip was attached to an oblique cutting device, and side cut lines were formed on the surface of the sealant layer at 1 mm width intervals.
- a diamond knife with a blade width of 1 mm, a rake angle of 20 degrees, and a relief angle of 10 degrees was brought into contact with the surface of the sealant layer under a load of 0.05 N, and then at a horizontal speed of 50 ⁇ m/s and a vertical speed of 1 ⁇ m/s.
- the sealant layer was cut diagonally under the following conditions.
- Hysitron TI-Premier (trade name) manufactured by Bruker Japan Co., Ltd. was used as a device for measuring the hardness of the adhesive layer by the nanoindentation method. Further, as an indenter, a Berkovich type diamond indenter manufactured by Bruker Japan Co., Ltd. was used. After installing the sample with the adhesive layer exposed on the above device, set the indentation speed: 100 nm/sec, test depth: 125 nm in displacement control mode, and place the indenter on the sample at room temperature (25°C). I pushed it in. Subsequently, after holding the maximum displacement for 2 seconds, the load was unloaded at a speed of 50 nm/second.
- the surface load was 1 ⁇ N, and surface correction was performed using TriboScan software.
- the measurement point was determined by observing the optical microscope image and measuring the sample surface, that is, the exposed surface of the adhesive layer (when the distance between the sealant layer and the base material layer on the exposed surface is divided into 4 equal parts, the distance from the sealant side is 1/4). Measurement was performed using the nanoindentation method by specifying 30 points at intervals of 30 ⁇ m or more.
- To calculate the hardness of the adhesive layer first, a standard sample of fused quartz is tested in advance, and the relationship between the contact depth and the projected contact area between the indenter and the sample is calibrated. Then, the unloading curve in the range of 20 to 95% of the maximum load at unloading was analyzed using the Oliver-Pharr method, and the hardness was calculated.
- Oxygen permeability measurement was performed on the packaging film after the retort treatment in the same manner as described above.
- Table 7 shows the conditions of each example and the evaluation results of each example. As is clear from the results shown in Table 7, the harder the adhesive layer is, the higher the oxygen permeability measurement results are. Furthermore, it was confirmed that the laminate strength was excellent as in Examples 1 to 14.
- a biaxially oriented polypropylene (OPP) film (manufactured by Mitsui Chemicals Tohcello Co., Ltd., trade name: ME-1, thickness: 20 ⁇ m) was prepared as a base material and an outermost layer. Further, as a sealant layer, an unstretched polypropylene (CPP) film (manufactured by Toray Film Kako Co., Ltd., trade name: Torephan ZK93KM, thickness: 60 ⁇ m) was prepared.
- OPP organic polypropylene
- TDI tolylene diisocyanate
- a coating liquid ⁇ was prepared by preparing and mixing 10 g each of the following liquids A, B, and C.
- Solution A 72.1 g of 0.1N hydrochloric acid was added to 17.9 g of tetraethoxysilane (Si(OC 2 H 5 ) 4 ) and 10 g of methanol, and the mixture was stirred for 30 minutes to be hydrolyzed, resulting in a solid content of 5% by mass (SiO 2 (conversion) hydrolysis solution.
- Liquid B 5% by mass water/methanol solution of polyvinyl alcohol (mass ratio of water:methanol is 95:5).
- Solution C 1,3,5-tris(3-trialkoxysilylpropyl)isocyanurate was diluted to a solid content of 5% by mass with a mixed solution of water/isopropyl alcohol (mass ratio of water:isopropyl alcohol was 1:1). Hydrolysis solution.
- Coating liquid ⁇ 1/1 solution diluted to a solid content of 5% by mass (calculated as R2Si (OH) 3 ), and a solution diluted with a 1/1 solution, and a coating liquid ⁇ was prepared by mixing three solutions. Coating liquid ⁇ was prepared so that the mass ratio of SiO 2 solid content (converted value) of TEOS, R 2 Si(OH) 3 solid content (converted value) of isocyanurate silane, and PVA solid content was 40/5/55. The solution was prepared as follows.
- aqueous polyurethane resin 40 to 75% by mass
- water-soluble polymer 10 to 40% by mass
- silane coupling agent 5 to 20% by mass
- Water-based polyurethane resin Aqueous dispersion of water-based polyurethane resin containing acid group-containing polyurethane resin and polyamine compound, water-based polyurethane dispersion "Takelac (registered trademark) WPB-341" manufactured by Mitsui Chemicals, Inc., solid content 30% .
- Water-soluble polymer polyvinyl alcohol with a degree of saponification of 98 to 99% and a degree of polymerization of 500 (trade name: Poval PVA-105, manufactured by Kuraray Co., Ltd.).
- Silane coupling agent 3-glycidoxypropyltriethoxysilane (trade name: KBE-403, manufactured by Shin-Etsu Chemical Co., Ltd.).
- Example 45 The composition for forming an adhesive layer was applied to the base material using a bar coat, and the composition for forming an adhesive layer was dried at 50°C. Thereby, an adhesion layer (anchor coat layer) with a thickness of 0.2 ⁇ m was formed. Next, a transparent vapor deposition layer (silica vapor deposition layer) made of silicon oxide and having a thickness of 40 nm was formed using a vacuum vapor deposition apparatus using an electron beam heating method.
- a barrier coat with a thickness of 300 nm was formed on the vapor deposited layer.
- a barrier layer including a base material, an adhesive layer, a vapor deposition layer, and a barrier coat was formed.
- a two-component curable urethane adhesive (a) was applied on the barrier coat of the barrier layer to form an adhesive layer with a thickness of 3 ⁇ m.
- a sealant layer was laminated via the adhesive layer by a dry lamination method.
- a laminate gas barrier laminate having a laminate structure of a base material, an adhesive layer, a vapor deposition layer, a barrier coat, an adhesive layer, and a sealant layer was manufactured.
- the content of polypropylene in the obtained laminate was 90% by mass or more.
- Example 46-50 Laminated bodies of Examples 46 to 50 were produced in the same manner as in Example 1, except that two-component curable urethane adhesives (b) to (f) were used instead of adhesive (a). did.
- Example 51 to 56 Laminated bodies of Examples 51 to 56 were produced in the same manner as Examples 45 to 50, except that coating liquid ⁇ was used instead of coating liquid ⁇ .
- Laminates of Examples 57 to 62 were produced in the same manner as Examples 45 to 50, except that coating liquid ⁇ was used instead of coating liquid ⁇ .
- Example 63 After forming a barrier layer in the same manner as in Example 45, a two-component curable urethane adhesive (a) was applied on the barrier coat of the barrier layer to form an adhesive layer with a thickness of 3 ⁇ m. Next, the outermost layer was laminated with an adhesive layer in between by a dry lamination method. Next, a two-component curable urethane adhesive (a) was applied onto the base material of the barrier layer to form an adhesive layer with a thickness of 3 ⁇ m. Next, a sealant layer was laminated via the adhesive layer by a dry lamination method.
- a laminate gas barrier laminate having a laminate structure of the outermost layer, the second adhesive layer, the barrier coat, the vapor deposited layer, the adhesive layer, the base material, the adhesive layer, and the sealant layer was manufactured.
- the content of polypropylene in the obtained laminate was 90% by mass or more.
- Examples 64-68 Laminated bodies of Examples 64 to 68 were produced in the same manner as in Example 63, except that two-component curing type urethane adhesives (b) to (f) were used instead of adhesive (a). did.
- Examples 69-74 Laminated bodies of Examples 69 to 74 were produced in the same manner as Examples 63 to 68, except that coating liquid ⁇ was used instead of coating liquid ⁇ .
- Laminates of Examples 75 to 80 were produced in the same manner as Examples 63 to 68, except that coating liquid ⁇ was used instead of coating liquid ⁇ .
- Comparative Examples 14 to 16 Laminates of Comparative Examples 14 to 16 were produced in the same manner as in Example 63, except that adhesives (g) to (i) were used instead of adhesive (a).
- Comparative Example 23 A laminate of Comparative Example 19 was produced in the same manner as in Example 45, except that a PET film (thickness: 20 ⁇ m) was used as the base material instead of the OPP film.
- Comparative example 24 A laminate of Comparative Example 20 was produced in the same manner as Comparative Example 5, except that a PET film (thickness: 20 ⁇ m) was used as the base material instead of the OPP film.
- the thermal shrinkage rate of the base material included in the barrier layer was measured according to the following procedure.
- the measurement results of the heat shrinkage rates of the base materials in Examples 45 to 50 and Comparative Examples 5 to 7 are shown in Table 8 below.
- the measurement results of the heat shrinkage rates of the base materials in Examples 51 to 56 and Comparative Examples 8 to 10 are shown in Table 9 below.
- the measurement results of the heat shrinkage rates of the base materials in Examples 57 to 62 and Comparative Examples 11 to 13 are shown in Table 10 below.
- the measurement results of the heat shrinkage rates of the base materials in Examples 63 to 68 and Comparative Examples 14 to 16 are shown in Table 11 below.
- the measurement results of the heat shrinkage rates of the base materials in Examples 69 to 74 and Comparative Examples 17 to 19 are shown in Table 12 below.
- the measurement results of the heat shrinkage rates of the base materials in Examples 75 to 80 and Comparative Examples 20 to 22 are shown in Table 13 below.
- the measurement results of the heat shrinkage rates of the base materials in Comparative Examples 23 and 24 are shown in Table 14 below.
- a measurement sample 500 was obtained by cutting out a base material to be measured into a size of 200 mm x 200 mm.
- two straight lines L1 and L2 with a length of 120 mm or more parallel to the TD direction of the measurement sample 500 were written with an interval of 100 mm.
- two straight lines L3 and L4 with a length of 120 mm or more parallel to the MD direction of the measurement sample 500 were written with an interval of 100 mm.
- scales N1 to N7 were written on the straight line L1 at seven locations at intervals of 20 mm. Scales were similarly drawn on the straight lines L2 to L4.
- MD direction heat shrinkage rate (%) (MD direction length before heating - MD direction length after heating) / MD direction length before heating ⁇ 100 ... (1)
- the sample was placed in an oblique cutting device (manufactured by Daipla Wintes Co., Ltd., trade name: SAICAS DN-GS).
- SAICAS DN-GS oblique cutting device
- a diamond cutting blade having a V-shaped tip was attached to an oblique cutting device, and side cut lines were formed on the surface of the sealant layer at 1 mm width intervals.
- a diamond knife with a blade width of 1 mm, a rake angle of 20 degrees, and a relief angle of 10 degrees was brought into contact with the surface of the sealant layer under a load of 0.05 N, and then at a horizontal speed of 50 ⁇ m/s and a vertical speed of 1 ⁇ m/s.
- the sealant layer was cut diagonally under the following conditions.
- Hysitron TI-Premier (trade name) manufactured by Bruker Japan Co., Ltd. was used as a device for measuring the hardness of the adhesive layer by the nanoindentation method. Further, as an indenter, a Berkovich type diamond indenter manufactured by Bruker Japan Co., Ltd. was used. After installing the sample with the adhesive layer exposed on the above device, set the indentation speed: 100 nm/sec, test depth: 125 nm in displacement control mode, and place the indenter on the sample at room temperature (25°C). I pushed it in. Subsequently, after holding the maximum displacement for 2 seconds, the load was unloaded at a speed of 50 nm/second.
- the surface load was 1 ⁇ N, and surface correction was performed using TriboScan software.
- the measurement point was determined by observing the optical microscope image and measuring the sample surface, that is, the exposed surface of the adhesive layer (when the distance between the sealant layer and the base material layer on the exposed surface is divided into 4 equal parts, the distance from the sealant side is 1/4). Measurement was performed using the nanoindentation method by specifying 30 points at intervals of 30 ⁇ m or more. To calculate the hardness of the adhesive layer, first, a standard sample of fused quartz is tested in advance, and the relationship between the contact depth and the projected contact area between the indenter and the sample is calibrated.
- Oxygen permeability (OTR) was measured for the laminate after the retort treatment. The measurement was performed using an oxygen permeability measuring device (OXTRAN 2/20, manufactured by Modern Control) at a temperature of 30° C. and a relative humidity of 70%. The OTR measurement method was based on JIS K-7126, B method (isobaric method), and ASTM D3985-81, and the OTR measurement value was expressed in the unit [cc/m 2 ⁇ day ⁇ atm]. The OTR measurement results for Examples 45 to 80 and Comparative Examples 5 to 24 are shown in Tables 8 to 14 below.
- the absolute value of oxygen permeability varies greatly depending on whether the heat shrinkage rate of the base material is 1% or more. Specifically, when a base material with a heat shrinkage rate of 1% or more is used, as shown in Tables 8 to 13, the oxygen permeability when the hardness of the adhesive layer is 0.9 MPa or more. is higher than the oxygen permeability when the hardness of the adhesive is 0.8 MPa. In addition, when the hardness of the adhesive layer is 0.9 MPa or more, the oxygen permeability is at least 3.5 or more.
- the oxygen permeability of the examples and comparative examples using coating liquid ⁇ or coating liquid ⁇ is lower than that of the examples and comparative examples using coating liquid ⁇ . It has become. Further, the oxygen permeability of the Examples and Comparative Examples having the outermost layer is lower than that of the Examples and Comparative Examples having no outermost layer.
- SYMBOLS 1 Base material layer, 2, 43... Vapor deposition layer, 3... Gas barrier coating layer, 10a, 10b... Gas barrier film, 11... First skin layer, 12... Core layer, 13... Second skin layer, 20, 30... Packaging film, 22...Outer layer film (outermost layer), 23,50...Sealant layer, 24,60...Adhesive layer, 25,60A...Second adhesive layer, 35,35A...Laminated body (packaging film), 40...Barrier layer (Gas barrier film), 41... Base material (base material layer), 44... Barrier coat (gas barrier coating layer), 70... Outermost layer, 100... Packaging bag.
- Base material layer 2, 43... Vapor deposition layer, 3... Gas barrier coating layer, 10a, 10b... Gas barrier film, 11... First skin layer, 12... Core layer, 13... Second skin layer, 20, 30... Packaging film, 22...Outer layer film (outermost layer), 23,50...Sealant layer, 24,60...Adhesive layer, 25,60A...Second adhesive
Landscapes
- Chemical & Material Sciences (AREA)
- Polymers & Plastics (AREA)
- Health & Medical Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Medicinal Chemistry (AREA)
- Organic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Mechanical Engineering (AREA)
- Thermal Sciences (AREA)
- Physics & Mathematics (AREA)
- Inorganic Chemistry (AREA)
- Laminated Bodies (AREA)
- Wrappers (AREA)
Priority Applications (6)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN202380039674.XA CN119384355A (zh) | 2022-05-12 | 2023-05-11 | 阻气膜、包装膜和包装袋 |
| JP2023553018A JP7473088B2 (ja) | 2022-05-12 | 2023-05-11 | ガスバリアフィルム、包装フィルム及び包装袋 |
| EP23803615.6A EP4520526A4 (en) | 2022-05-12 | 2023-05-11 | GAS BARRIER FILM, WRAPPING FILM AND WRAPPING BAG |
| US18/863,930 US20250296306A1 (en) | 2022-05-12 | 2023-05-11 | Gas barrier film, packaging film, and packaging bag |
| JP2024061722A JP7658492B2 (ja) | 2022-05-12 | 2024-04-05 | 包装フィルム及び包装袋 |
| JP2025044869A JP2025094120A (ja) | 2022-05-12 | 2025-03-19 | ガスバリアフィルム、包装フィルム及び包装袋 |
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2022-078669 | 2022-05-12 | ||
| JP2022078669 | 2022-05-12 | ||
| JP2022-105772 | 2022-06-30 | ||
| JP2022105772A JP7296507B1 (ja) | 2022-06-30 | 2022-06-30 | ガスバリア積層体及び包装袋 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2023219141A1 true WO2023219141A1 (ja) | 2023-11-16 |
Family
ID=88730360
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2023/017786 Ceased WO2023219141A1 (ja) | 2022-05-12 | 2023-05-11 | ガスバリアフィルム、包装フィルム及び包装袋 |
Country Status (5)
| Country | Link |
|---|---|
| US (1) | US20250296306A1 (enExample) |
| EP (1) | EP4520526A4 (enExample) |
| JP (3) | JP7473088B2 (enExample) |
| CN (1) | CN119384355A (enExample) |
| WO (1) | WO2023219141A1 (enExample) |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2024150748A1 (ja) * | 2023-01-11 | 2024-07-18 | Toppanホールディングス株式会社 | ガスバリア性積層体、包装容器及び包装製品 |
| JP7605357B1 (ja) | 2023-10-20 | 2024-12-24 | 大日本印刷株式会社 | 積層体および包装袋 |
| EP4424507A4 (en) * | 2021-10-28 | 2025-02-19 | Toppan Holdings Inc. | GAS BARRIER FILM AND PACKAGING MATERIAL |
| WO2025243797A1 (ja) * | 2024-05-21 | 2025-11-27 | Toppanホールディングス株式会社 | 包装材、包装袋、ガスバリア積層体及びガスバリア積層体の製造方法 |
Families Citing this family (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN119300977A (zh) * | 2022-06-15 | 2025-01-10 | 凸版控股株式会社 | 阻气性层叠体、包装膜、包装容器以及包装制品 |
| EP4541581A4 (en) * | 2022-06-15 | 2025-09-17 | Toppan Holdings Inc | MULTILAYER FILM AND PACKAGING FILM |
| WO2025070674A1 (ja) * | 2023-09-27 | 2025-04-03 | 大日本印刷株式会社 | 積層体、包装袋および加熱殺菌パウチ |
Citations (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS63291929A (ja) | 1987-05-25 | 1988-11-29 | Idemitsu Petrochem Co Ltd | 金属蒸着用ポリプロピレン系フィルム |
| JPH09290477A (ja) * | 1996-03-01 | 1997-11-11 | Toppan Printing Co Ltd | バリア性フィルムおよびそれを用いた包装材料 |
| JP2969657B2 (ja) | 1989-07-18 | 1999-11-02 | 東レ株式会社 | ポリプロピレン複合フィルム及び金属蒸着ポリプロピレン複合フィルム |
| JP2000233476A (ja) * | 1999-02-16 | 2000-08-29 | Dainippon Printing Co Ltd | 透明バリア性フィルム |
| JP2006159801A (ja) * | 2004-12-10 | 2006-06-22 | Oji Paper Co Ltd | ガスバリア積層体 |
| JP2017069203A (ja) * | 2015-09-30 | 2017-04-06 | 大日本印刷株式会社 | 電池用包装材料及び電池 |
| JP2017177685A (ja) * | 2016-03-31 | 2017-10-05 | 大日本印刷株式会社 | 建材用防湿フィルム |
| WO2020085463A1 (ja) * | 2018-10-24 | 2020-04-30 | 大日本印刷株式会社 | 蓄電デバイス用外装材、その製造方法、及び蓄電デバイス |
| WO2020129291A1 (ja) * | 2018-12-21 | 2020-06-25 | 凸版印刷株式会社 | ガスバリアフィルム及びその製造方法、包装フィルム、並びに、包装袋 |
| JP2021028394A (ja) * | 2018-11-01 | 2021-02-25 | 東レ株式会社 | ポリプロピレンフィルム、および離型フィルム |
| JP2021041620A (ja) * | 2019-09-11 | 2021-03-18 | 大日本印刷株式会社 | バリアフィルム及び包装材料 |
| WO2021230319A1 (ja) * | 2020-05-14 | 2021-11-18 | 凸版印刷株式会社 | ガスバリアフィルム |
| WO2022085586A1 (ja) * | 2020-10-23 | 2022-04-28 | 凸版印刷株式会社 | ガスバリア積層体及び包装材 |
| JP2023056928A (ja) * | 2021-10-08 | 2023-04-20 | 大日本印刷株式会社 | 積層体及び包装材料 |
Family Cites Families (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP7088138B2 (ja) | 2019-07-29 | 2022-06-21 | 凸版印刷株式会社 | 積層体及び包装袋 |
| US12083774B2 (en) | 2019-08-09 | 2024-09-10 | Toppan Inc. | Barrier film and barrier packaging material |
| WO2021065890A1 (ja) | 2019-09-30 | 2021-04-08 | 大日本印刷株式会社 | バリア性積層体、該バリア性積層体を備える包装容器 |
| JP6895135B2 (ja) | 2019-09-30 | 2021-06-30 | 大日本印刷株式会社 | バリア性積層体、該バリア性積層体を備える包装容器 |
| CN114728512B (zh) * | 2019-12-06 | 2024-08-20 | 凸版印刷株式会社 | 阻气膜 |
| JP2023007518A (ja) * | 2019-12-16 | 2023-01-19 | 凸版印刷株式会社 | 積層体及びこのリサイクル方法、並びに再生樹脂組成物及びこれを含む物品 |
| JP7784053B2 (ja) | 2020-03-31 | 2025-12-11 | 大日本印刷株式会社 | 積層体、レトルト用またはボイル用パウチ |
| JP7238912B2 (ja) | 2020-03-31 | 2023-03-14 | 大日本印刷株式会社 | 積層体、レトルト用またはボイル用パウチ |
| WO2021220935A1 (ja) * | 2020-04-28 | 2021-11-04 | 凸版印刷株式会社 | ガスバリアフィルム |
| JP7296507B1 (ja) | 2022-06-30 | 2023-06-22 | 凸版印刷株式会社 | ガスバリア積層体及び包装袋 |
-
2023
- 2023-05-11 EP EP23803615.6A patent/EP4520526A4/en active Pending
- 2023-05-11 US US18/863,930 patent/US20250296306A1/en active Pending
- 2023-05-11 CN CN202380039674.XA patent/CN119384355A/zh active Pending
- 2023-05-11 WO PCT/JP2023/017786 patent/WO2023219141A1/ja not_active Ceased
- 2023-05-11 JP JP2023553018A patent/JP7473088B2/ja active Active
-
2024
- 2024-04-05 JP JP2024061722A patent/JP7658492B2/ja active Active
-
2025
- 2025-03-19 JP JP2025044869A patent/JP2025094120A/ja active Pending
Patent Citations (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS63291929A (ja) | 1987-05-25 | 1988-11-29 | Idemitsu Petrochem Co Ltd | 金属蒸着用ポリプロピレン系フィルム |
| JP2969657B2 (ja) | 1989-07-18 | 1999-11-02 | 東レ株式会社 | ポリプロピレン複合フィルム及び金属蒸着ポリプロピレン複合フィルム |
| JPH09290477A (ja) * | 1996-03-01 | 1997-11-11 | Toppan Printing Co Ltd | バリア性フィルムおよびそれを用いた包装材料 |
| JP2000233476A (ja) * | 1999-02-16 | 2000-08-29 | Dainippon Printing Co Ltd | 透明バリア性フィルム |
| JP2006159801A (ja) * | 2004-12-10 | 2006-06-22 | Oji Paper Co Ltd | ガスバリア積層体 |
| JP2017069203A (ja) * | 2015-09-30 | 2017-04-06 | 大日本印刷株式会社 | 電池用包装材料及び電池 |
| JP2017177685A (ja) * | 2016-03-31 | 2017-10-05 | 大日本印刷株式会社 | 建材用防湿フィルム |
| WO2020085463A1 (ja) * | 2018-10-24 | 2020-04-30 | 大日本印刷株式会社 | 蓄電デバイス用外装材、その製造方法、及び蓄電デバイス |
| JP2021028394A (ja) * | 2018-11-01 | 2021-02-25 | 東レ株式会社 | ポリプロピレンフィルム、および離型フィルム |
| WO2020129291A1 (ja) * | 2018-12-21 | 2020-06-25 | 凸版印刷株式会社 | ガスバリアフィルム及びその製造方法、包装フィルム、並びに、包装袋 |
| JP2021041620A (ja) * | 2019-09-11 | 2021-03-18 | 大日本印刷株式会社 | バリアフィルム及び包装材料 |
| WO2021230319A1 (ja) * | 2020-05-14 | 2021-11-18 | 凸版印刷株式会社 | ガスバリアフィルム |
| WO2022085586A1 (ja) * | 2020-10-23 | 2022-04-28 | 凸版印刷株式会社 | ガスバリア積層体及び包装材 |
| JP2023056928A (ja) * | 2021-10-08 | 2023-04-20 | 大日本印刷株式会社 | 積層体及び包装材料 |
Non-Patent Citations (1)
| Title |
|---|
| See also references of EP4520526A4 |
Cited By (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP4424507A4 (en) * | 2021-10-28 | 2025-02-19 | Toppan Holdings Inc. | GAS BARRIER FILM AND PACKAGING MATERIAL |
| WO2024150748A1 (ja) * | 2023-01-11 | 2024-07-18 | Toppanホールディングス株式会社 | ガスバリア性積層体、包装容器及び包装製品 |
| JP7605357B1 (ja) | 2023-10-20 | 2024-12-24 | 大日本印刷株式会社 | 積層体および包装袋 |
| JP2025070928A (ja) * | 2023-10-20 | 2025-05-02 | 大日本印刷株式会社 | 積層体および包装袋 |
| WO2025243797A1 (ja) * | 2024-05-21 | 2025-11-27 | Toppanホールディングス株式会社 | 包装材、包装袋、ガスバリア積層体及びガスバリア積層体の製造方法 |
Also Published As
| Publication number | Publication date |
|---|---|
| JPWO2023219141A1 (enExample) | 2023-11-16 |
| JP2025094120A (ja) | 2025-06-24 |
| EP4520526A1 (en) | 2025-03-12 |
| EP4520526A4 (en) | 2025-08-06 |
| CN119384355A (zh) | 2025-01-28 |
| JP7473088B2 (ja) | 2024-04-23 |
| JP2024094345A (ja) | 2024-07-09 |
| JP7658492B2 (ja) | 2025-04-08 |
| US20250296306A1 (en) | 2025-09-25 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP7658492B2 (ja) | 包装フィルム及び包装袋 | |
| US20220153006A1 (en) | Laminate and packaging bag | |
| JP7332070B1 (ja) | ガスバリア積層体及び包装袋 | |
| US20240025612A1 (en) | Laminate, packaging bag, and standing pouch | |
| JP7677515B2 (ja) | ガスバリア積層体 | |
| JP7231095B2 (ja) | 積層体及び包装袋 | |
| JP7771601B2 (ja) | 積層体、包装材料、包装体及び包装物品 | |
| JP7632775B1 (ja) | ガスバリア積層体及び包装袋 | |
| JP7473036B2 (ja) | 積層体及び包装袋 | |
| EP4559677A1 (en) | Laminate for packaging, method for selecting same, method for evaluating same, packaging bag, and method for producing same | |
| WO2025115849A1 (ja) | 包装用積層体及び包装袋 | |
| WO2023181852A1 (ja) | 包装用積層体及び包装袋 | |
| JP2025001761A (ja) | ガスバリア積層体、包装袋及び包装体 | |
| JP7673883B1 (ja) | 積層体、包装袋及び包装体 | |
| JP2023178816A (ja) | 包装用積層体及び包装袋 | |
| WO2024247593A1 (ja) | シーラントフィルム、包装材及び包装体 | |
| JP2025110394A (ja) | 積層体及び包装袋 | |
| CN118265610A (zh) | 阻隔膜、层叠体以及包装袋 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| ENP | Entry into the national phase |
Ref document number: 2023553018 Country of ref document: JP Kind code of ref document: A |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 23803615 Country of ref document: EP Kind code of ref document: A1 |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 18863930 Country of ref document: US |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 202380039674.X Country of ref document: CN |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 202447087174 Country of ref document: IN |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2023803615 Country of ref document: EP |
|
| ENP | Entry into the national phase |
Ref document number: 2023803615 Country of ref document: EP Effective date: 20241202 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| WWP | Wipo information: published in national office |
Ref document number: 202380039674.X Country of ref document: CN |
|
| WWP | Wipo information: published in national office |
Ref document number: 18863930 Country of ref document: US |