WO2022149487A1 - 前立腺がんの罹患の可能性を検査する方法 - Google Patents
前立腺がんの罹患の可能性を検査する方法 Download PDFInfo
- Publication number
- WO2022149487A1 WO2022149487A1 PCT/JP2021/048082 JP2021048082W WO2022149487A1 WO 2022149487 A1 WO2022149487 A1 WO 2022149487A1 JP 2021048082 W JP2021048082 W JP 2021048082W WO 2022149487 A1 WO2022149487 A1 WO 2022149487A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- ions
- prostate cancer
- group
- measuring
- propan
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/483—Physical analysis of biological material
- G01N33/487—Physical analysis of biological material of liquid biological material
- G01N33/493—Physical analysis of biological material of liquid biological material urine
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/574—Immunoassay; Biospecific binding assay; Materials therefor for cancer
- G01N33/57407—Specifically defined cancers
- G01N33/57434—Specifically defined cancers of prostate
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N30/00—Investigating or analysing materials by separation into components using adsorption, absorption or similar phenomena or using ion-exchange, e.g. chromatography or field flow fractionation
- G01N30/02—Column chromatography
- G01N30/62—Detectors specially adapted therefor
- G01N30/72—Mass spectrometers
- G01N30/7206—Mass spectrometers interfaced to gas chromatograph
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N30/00—Investigating or analysing materials by separation into components using adsorption, absorption or similar phenomena or using ion-exchange, e.g. chromatography or field flow fractionation
- G01N30/02—Column chromatography
- G01N30/86—Signal analysis
- G01N30/8624—Detection of slopes or peaks; baseline correction
- G01N30/8631—Peaks
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/574—Immunoassay; Biospecific binding assay; Materials therefor for cancer
- G01N33/57484—Immunoassay; Biospecific binding assay; Materials therefor for cancer involving compounds serving as markers for tumor, cancer, neoplasia, e.g. cellular determinants, receptors, heat shock/stress proteins, A-protein, oligosaccharides, metabolites
- G01N33/57488—Immunoassay; Biospecific binding assay; Materials therefor for cancer involving compounds serving as markers for tumor, cancer, neoplasia, e.g. cellular determinants, receptors, heat shock/stress proteins, A-protein, oligosaccharides, metabolites involving compounds identifable in body fluids
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2496/00—Reference solutions for assays of biological material
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2560/00—Chemical aspects of mass spectrometric analysis of biological material
Definitions
- This disclosure relates to a method for examining the possibility of developing prostate cancer.
- Prostate cancer has a high incidence in men, and the annual number of cases exceeds 100,000. In addition, it is predicted that the incidence of prostate cancer and the number of those who develop it will continue to increase with the westernization and aging of eating habits. As with any cancer, early detection and treatment are important.
- prostate cancer test a screening test using the blood PSA (Prostate Specific Antigen) value as an index is performed.
- PSA Prostate Specific Antigen
- a test is also performed in which prostate tissue is collected by needle biopsy to check for the presence of cancer cells. Although used as a definitive diagnostic method, this test is highly invasive and has a risk of causing infections.
- Non-Patent Document 1 various techniques for discriminating prostate cancer using the sense of smell of animals have been reported.
- the subject of this disclosure is to provide prostate cancer testing technology.
- the present inventor has Dymethylglutarate, 2,6-Xylidine, 2-Hydroxy-2-methylpropiophenone, 2,6-di (propan) in body fluid samples collected from the subject.
- At least one selected from the group consisting of -2-yl) phenol, Dimethylsuccinate, Acetophenone, 2-Phenyl-2-propanol, and 3,5,5-Trimetyl-2-cyclohexenone is a biomarker for prostate cancer.
- Item 1 Dymethyl glutarate, 2,6-Xylidine, 2-Hydroxy-2-methylpropiophenone, 2,6-di (propan-2-yl) phenol, Dimethyl succinate, Acetophenone, 2 in body fluid samples collected from the subject.
- -For prostate cancer prevalence including the step of measuring the amount or concentration of at least one biomarker selected from the group consisting of Phenyl-2-propanol and 3,5,5-Trimetyl-2-cyclohexenone. How to test the possibility.
- Item 2 Moreover, (2a) A step of determining that the subject is likely to have prostate cancer when the amount or concentration of the biomarker detected in the step (1) is equal to or higher than the reference value A. / Or (2b) A step of determining that the subject is unlikely to have prostate cancer when the amount or concentration of the biomarker detected in the step (1) is equal to or less than the reference value B. , Item 1. The method according to Item 1.
- Item 3 The biomarker in a body fluid sample in which the reference value A and the reference value B are a subject determined not to have prostate cancer or a subject determined to have prostate cancer.
- Item 2. The method according to Item 2, wherein the value is based on the maximum value, the average value, the percentile value, or the minimum value of the amount or concentration of.
- Item 4. The method according to any one of Items 1 to 3, wherein the measurement method in the step (1) is a gas chromatography mass spectrometry method.
- the monitor ion in the gas chromatography mass spectrometry method (A) When measuring Dymethyl glutarate, it is at least one selected from the group consisting of 129 ions with m / z, 59 ions with m / z, and 100 ions with m / z. (B) When measuring 2,6-Xylidine, it is at least one selected from the group consisting of 121 ions with m / z, 106 ions with m / z, and 91 ions with m / z.
- Item 6. The method according to any one of Items 1 to 5, wherein the biomarker is two or more kinds.
- Item 7. The method according to any one of Items 1 to 6, wherein the body fluid sample is a urine sample.
- Item 8 Dymethylglutarate, 2,6-Xylidine, 2-Hydroxy-2-methylpropiophenone, 2,6-di (propan-2-yl) phenol, Dimethylsuccinate, Acetophenone, 2-Phenyl-2-propanol, and A biomarker for prostate cancer consisting of at least one selected from the group consisting of 3,5,5-Trimetyl-2-cyclohexenone.
- Item 9 Dymethylglutarate, 2,6-Xylidine, 2-Hydroxy-2-methylpropiophenone, 2,6-di (propan-2-yl) phenol, Dimethylsuccinate, Acetophenone, 2-Phenyl-2-propanol, and 3,5,5 -Prostate cancer test agent comprising at least one standard sample and / or detection agent selected from the group consisting of Trimetyl-2-cyclohexenone.
- the present disclosure also includes the following aspects as another aspect: Item A.
- Item A Dymethyl glutarate, 2,6-Xylidine, 2-Hydroxy-2-methylpropiophenone, 2,6-di (propan-2-yl) phenol, Dimethyl succinate, Acetophenone, 2 in body fluid samples collected from the subject.
- -For prostate cancer prevalence including the step of measuring the amount or concentration of at least one biomarker selected from the group consisting of Phenyl-2-propanol and 3,5,5-Trimetyl-2-cyclohexenone.
- a method to assist in determining the possibility is determining the possibility.
- Item B (1) Dymethylglutarate, 2,6-Xylidine, 2-Hydroxy-2-methylpropiophenone, 2,6-di (propan-2-yl) phenol, Dimethylsuccinate, Acetophenone, 2 in body fluid samples collected from the subject.
- -A method for measuring a prostate cancer biomarker comprising the step of measuring the amount or concentration of at least one selected from the group consisting of Phenyl-2-propanol and 3,5,5-Trimetyl-2-cyclohexe none.
- Item C Dymethylglutarate, 2,6-Xylidine, 2-Hydroxy-2-methylpropiophenone, 2,6-di (propan-2-yl) phenol, Dimethylsuccinate, Acetophenone, 2 for use as a test for prostate cancer -At least one standard sample and / or detection agent selected from the group consisting of Phenyl-2-propanol and 3,5,5-Trimetyl-2-cyclohexenone.
- Item D Dymethylglutarate, 2,6-Xylidine, 2-Hydroxy-2-methylpropiophenone, 2,6-di (propan-2-yl) phenol, Dimethylsuccinate, Acetophenone, 2-Phenyl-2 for prostate cancer testing -Use of at least one standard sample and / or detector selected from the group consisting of propanol and 3,5,5-Trimetyl-2-cyclohexenone.
- Item E Dymethylglutarate, 2,6-Xylidine, 2-Hydroxy-2-methylpropiophenone, 2,6-di (propan-2-yl) phenol, Dimethylsuccinate, Acetophenone, 2- Use of at least one standard sample and / or detector selected from the group consisting of Phenyl-2-propanol and 3,5,5-Trimetyl-2-cyclohexenone.
- a box plot showing the measurement result of Dymethylglutarate in Example 2 is shown.
- the vertical axis shows the urinary concentration
- the horizontal axis shows the result of urine collected from a prostate cancer patient before undergoing radical prostatectomy before surgery, and after undergoing radical prostatectomy after surgery.
- the results of urine collected from a patient with prostate cancer are shown.
- the subjects are the same before and after surgery.
- the range shown by the box shows the concentration distribution range of the sample corresponding to 25-75% of all the samples
- the range shown by the horizontal line shows the concentration distribution range of the sample corresponding to 10-90% of all the samples.
- the horizontal bar in the box shows the median concentration in each population (preoperative and postoperative).
- the "+" in the box indicates the average value.
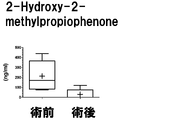
- Example 2 The measurement result of 2-Hydroxy-2-methylpropiophenone in Example 2 is shown.
- the vertical axis, the horizontal axis, the bar, and the dotted line are the same as those in FIG.
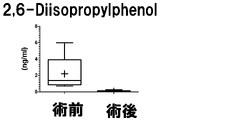
- the measurement result of 2,6-Diisopropylphenol (2,6-di (propan-2-yl) phenol) in Example 2 is shown.
- the vertical axis, the horizontal axis, the bar, and the dotted line are the same as those in FIG.
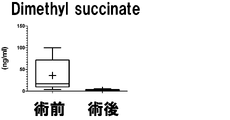
- Dimethyl succinate in Example 2 is shown.
- the vertical axis, the horizontal axis, the bar, and the dotted line are the same as those in FIG.
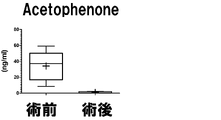
- the measurement result of Acetophenone in Example 2 is shown.
- the vertical axis, the horizontal axis, the bar, and the dotted line are the same as those in FIG.
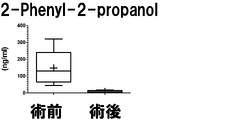
- the measurement result of 2-Phenyl-2-propanol in Example 2 is shown.
- the vertical axis, the horizontal axis, the bar, and the dotted line are the same as those in FIG.
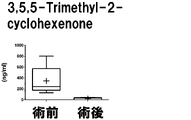
- the measurement results of 3,5,5-Trimetyl-2-cyclohexenone in Example 2 are shown.
- the vertical axis, the horizontal axis, the bar, and the dotted line are the same as those in FIG.
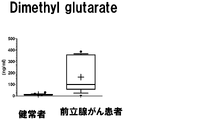
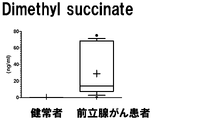
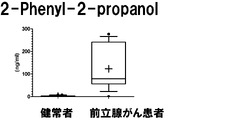
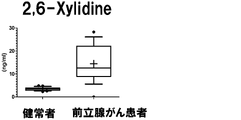
- a box plot showing the measurement result of Dymethylglutarate in Example 3 is shown.
- the vertical axis shows the urine concentration
- the horizontal axis shows the result of urine collected from a healthy person
- the prostate cancer patient shows the result of urine collected from a prostate cancer patient.
- the range shown by the box shows the concentration distribution range of the sample corresponding to 25-75% of all the samples
- the range shown by the horizontal line shows the concentration distribution range of the sample corresponding to 10-90% of all the samples.
- the horizontal bar in the box shows the median concentration in each population (preoperative and postoperative).
- the "+" in the box indicates the average value.
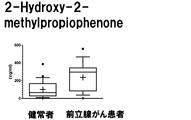
- the measurement result of 2-Hydroxy-2-methylpropiophenone in Example 3 is shown.
- the vertical axis, the horizontal axis, the bar, and the dotted line are the same as those in FIG.
- Example 3 The measurement result of 2,6-Diisopropylphenol (2,6-di (propan-2-yl) phenol) in Example 3 is shown.
- the vertical axis, the horizontal axis, the bar, and the dotted line are the same as those in FIG.
- the measurement result of Dimethyl succinate in Example 3 is shown.
- the vertical axis, the horizontal axis, the bar, and the dotted line are the same as those in FIG.
- the measurement result of Acetophenone in Example 3 is shown.
- the vertical axis, the horizontal axis, the bar, and the dotted line are the same as those in FIG.
- the measurement result of 2-Phenyl-2-propanol in Example 3 is shown.
- the vertical axis, the horizontal axis, the bar, and the dotted line are the same as those in FIG.
- the measurement results of 3,5,5-Trimetyl-2-cyclohexenone in Example 3 are shown.
- the vertical axis, the horizontal axis, the bar, and the dotted line are the same as those in FIG.
- the measurement result of 2,6-Xylidine in Example 3 is shown.
- the vertical axis, the horizontal axis, the bar, and the dotted line are the same as those in FIG.
- This disclosure describes (1) Dymethyl glutarate, 2,6-Xylidine, 2-Hydroxy-2-methylpropiophenone, 2,6-in body fluid samples taken from a subject. At least one biomarker selected from the group consisting of di (propan-2-yl) phenol, Dimethyl succinate, Acetophenone, 2-Phenyl-2-propanol, and 3,5,5-Trimetyl-2-cyclohexenone (book).
- a method of testing for the likelihood of developing prostate cancer comprising the step of measuring the amount or concentration of (sometimes referred to as a "target biomarker") herein (in the specification, "the disclosure of the present disclosure”. It may be referred to as "inspection method").
- the present disclosure describes Dymethylglutarate, 2,6-Xylidine, 2-Hydroxy-2-methylpropiophenone, 2,6-di (propan-2-yl) phenol, Dimethylsuccinate, Acetophenone, 2-Phenyl in body fluid samples.
- -2-propanol, and a biomarker for prostate cancer consisting of at least one selected from the group consisting of 3,5,5-Trimetyl-2-cyclohexenone.
- Prostate cancer to be tested is not particularly limited and includes all types, degrees (eg, mild, moderate, severe), stages and the like.
- the subject is the target organism of the test method of the present disclosure, and the species thereof is not particularly limited.
- Examples of the organism species of the subject include various mammals such as humans, monkeys, mice, rats, dogs, cats, and rabbits, and humans are preferable.
- the condition of the subject regarding prostate cancer is not particularly limited.
- Subjects include, for example, a sample for which it is unknown whether or not the patient has prostate cancer, a sample for which there is no history of prostate cancer, a sample for which the patient has a history of prostate cancer and has been treated for prostate cancer, and other test methods (for example, a sample that has already been determined to have (or does not have) prostate cancer by PSA test or the like can be mentioned.
- the subject is a human, any person can be the subject of the test, regardless of the medical history so far, including those who are considered to be healthy. In the case of a person who seems to be a healthy person, it is effective for early detection and early diagnosis of prostate cancer in general health examinations and human docks.
- the examination method of the present disclosure can assist the diagnosis of prostate cancer.
- the body fluid sample is not particularly limited as long as it can contain the target biomarker.
- the body fluid sample include body fluids such as blood that has passed through prostate cancer tissue, body fluids produced from the body fluids, and samples derived from these body fluids.
- a sample obtained by purifying the body fluid can be used as the sample derived from the body fluid.
- the purification treatment is not particularly limited as long as the amount or concentration of the target biomarker is not significantly reduced, and examples thereof include removal treatment of salts, proteins and the like (for example, enzyme treatment, chromatography column purification treatment, centrifugation treatment and the like).
- Specific examples of the body fluid sample include urine, blood, cerebrospinal fluid, body secretions, saliva, sputum; and samples derived from these body fluids.
- the method for collecting body fluid is not particularly limited, and for example, a method according to or similar to a method carried out in a general regular health examination can be adopted.
- the timing of collecting body fluid is not particularly limited, and may be, for example, first thing in the morning, evening, before going to bed, after meals, or before meals.
- the body fluid sample can be used immediately after collection or preparation, but it can also be used after cryopreservation. 2,6-di (propan-2-yl) phenol is reduced by denaturation due to oxidation at room temperature, and the half-life at room temperature is said to be 3-12 hours. Not suitable.
- the cryopreservation method is not particularly limited.
- the body fluid sample may be placed in a closed glass container, frozen as soon as possible (for example, at about -80 ° C), and stored at a general freezer temperature (for example, about -20 ° C). can.
- the storage period is not particularly limited and may be one year or longer, but is preferably shorter.
- the body fluid sample one type may be adopted alone, or two or more types may be adopted in combination.
- the target biomarkers are Dymethylglutarate, 2,6-Xylidine, 2-Hydroxy-2-methylpropiophenone, 2,6-di (propan-2-yl) phenol, Dimethylsuccinate, Acetophenone, 2-Phenyl-2-propanol, and It is at least one selected from the group consisting of 3,5,5-Trimetyl-2-cyclohexe none. These structural formulas are shown in Table 1.
- the target biomarker to be measured in the step (1) may be only one type, but may be two or more types, three or more types, four or more types, five or more types, six or more types, seven or more types, or eight types. be able to. By combining more target biomarkers, it becomes possible to more accurately test for prostate cancer and the like.
- the method for measuring the amount or concentration of the target biomarker is not particularly limited as long as the target biomarker can be detected.
- Examples of the method include a mass spectrometry method (MS method), and more preferably a gas chromatography mass spectrometry method (GC / MS method) from the viewpoint of detection sensitivity and the like.
- the GC / MS method is performed by separating the body fluid sample of the subject by gas chromatography and mass spectrometrically analyzing each separated fraction.
- Gas chromatography can be performed according to or according to a known method.
- an appropriate method may be selected from known methods according to the state of the body fluid sample.
- the components of the body fluid sample can be adsorbed on a volatile organic substance adsorbing material containing a general-purpose solid-phase microextraction fiber, and then desorbed by heating.
- the volatile organic substance adsorbing material is not particularly limited, and examples thereof include silicone resins, and among them, silicone elastomers are preferable.
- silicone resin and the silicone elastomer examples include polydimethylsiloxane (PDMS) and an adsorbent made of a modified silicone compound in which the methyl group of the polymer chain in the silicone compound is replaced with a vinyl group or a phenyl group.
- PDMS polydimethylsiloxane
- adsorbent made of a modified silicone compound in which the methyl group of the polymer chain in the silicone compound is replaced with a vinyl group or a phenyl group.
- the volatile organic substance adsorbing material may be used in combination with a suitable carrier.
- the carrier gas in gas chromatography is not particularly limited as long as it is an inert gas.
- Examples of the carrier gas include helium, nitrogen, argon and the like, and helium is preferable.
- the stationary phase of gas chromatography is not particularly limited.
- Examples of the stationary phase include polyethylene glycol, polymethylcyanoalkylsiloxane, polydimethylsiloxane / diphenylsiloxane, and polydimethylsiloxane.
- a stationary phase having a higher polarity is preferable, specifically, polyethylene glycol, polymethylcyanoalkylsiloxane, polydimethylsiloxane / diphenylsiloxane, etc.
- polyethylene glycol polymethylcyanoalkylsiloxane, etc. are more preferable. Polyethylene glycol is even more preferred.
- silica gel, activated carbon, zeolite, activated alumina and the like can be exemplified.
- the gas chromatography detector is not particularly limited as long as it can widely detect various components. Examples of the detector include a thermal conductivity type detector (TCD), a hydrogen flame ionization type detector (FID), an electron capture type detector (ECD) and the like.
- Mass spectrometry can be performed according to or according to a known method.
- the ionization method in mass spectrometry is not particularly limited.
- Examples of the ionization method include an electron ionization method (EI method), a positive chemical ionization method (PCI method), a negative chemical ionization method (NCI method), and the like, preferably the EI method.
- the ionization voltage in the EI method is not particularly limited, but is typically about 70V.
- the method for separating ionized sample molecules in mass spectrometry is not particularly limited.
- a flight time type for example, a flight time type, a magnetic field deflection type, a quadrupole type, an ion trap type, a Fourier transform ion cyclotron resonance type, a tandem type and the like can be adopted.
- the amount or concentration of the target biomarker can be quantified.
- the amount or concentration of the target biomarker can be calculated based on the amplitude value and area of the measurement peak of the ion in the mass spectrum obtained by mass spectrometry.
- the monitor ion used for quantification include the following monitor ions.
- At least 1 type (G) 2 selected from the group consisting of 105 ions with m / z, 77 ions with m / z, and 120 ions with m / z.
- Phenyl-2-propanol at least one selected from the group consisting of 43 ions with m / z, 121 ions with m / z, and 77 ions with m / z (H) 3
- the underlined ion can be used as a quantitative ion, and the other ion can be used as a reference ion.
- the method for measuring the amount or concentration of the target biomarker includes, for example, a surface plasmon resonance method, a metal oxide semiconductor, a conductive polymer, a polymer thin film, a cholesteric liquid crystal, a shotkey diode, a MOSFET, and a sir. It is also possible to adopt a detection method using Mr., Verister, thermocouple, crystal transducer, SAW device, fluorescent substance, light-absorbing substance, olfactory cell and the like.
- test method of the present disclosure including step (1), it is possible to provide a measured value for calculating a determination index of the possibility of developing prostate cancer, and preferably further provide the determination index. Can be done. This can assist in determining the possibility of developing prostate cancer.
- target biomarker can be used for prostate cancer testing in various aspects.
- target biomarkers are used for monitoring the therapeutic effect during or after prostate cancer treatment, detecting recurrence after prostate cancer treatment, determining the prognosis of patients, etc. can do.
- process (2) In one aspect of the test method of the present disclosure, further, when the amount or concentration of the biomarker detected in (2a) step (1) is equal to or higher than the reference value A, the subject suffers from prostate cancer. If the amount or concentration of the biomarker detected in step (2b) step (1) is less than or equal to the reference value B, the subject suffers from prostate cancer. It is preferable to include a step (these steps (2a) and (2b) may be collectively referred to as “step (2)”), which is determined to be less likely to be present.
- “possibility of suffering from prostate cancer” means “possibility of suffering from prostate cancer at the time of collecting body fluid sample”.
- the inspection method of the present disclosure including step (2) it is possible to determine the possibility of suffering from prostate cancer.
- the test method of the present disclosure can determine the possibility of having prostate cancer with high accuracy, the test method of the present disclosure including step (2) can be used to determine a subject suffering from prostate cancer. , More reliably determine "have prostate cancer (or have no prostate cancer)" (ie, "do not have prostate cancer (or have prostate cancer)” ) ”Can be further reduced).
- the reference value (reference value A and reference value B, respectively) can be appropriately set by those skilled in the art from the viewpoints of judgment sensitivity, judgment specificity, positive predictive value, negative predictive value, and the like.
- the reference value may be either a value set each time or a preset value according to race, age, and the like.
- the reference value is, for example, the amount or concentration of the target biomarker in a body fluid sample collected from a subject determined not to have prostate cancer or a subject determined to have prostate cancer. It can be a value based on a maximum value, an average value, a percentile value, or a minimum value.
- the "reference value” refers to a value in which both the determination sensitivity (presence accuracy rate) and the determination specificity (disease-free accuracy rate) are sufficiently high when the presence or absence of disease onset is determined based on the value. ..
- a value showing a high positive rate in an individual suffering from prostate cancer and showing a high negative rate in an individual not suffering from prostate cancer can be set as a reference value.
- the "judgment sensitivity” refers to the ratio (true positive ratio) showing a positive (outlier value) when a test is performed on a population developing a specific disease.
- the “judgment specificity” refers to a ratio (true negative ratio) showing a negative (normal value) when a test is performed on a population not suffering from a specific disease.
- the “positive predictive value” refers to the proportion of individuals who are actually suffering from the disease among the subjects who showed positive in the test, and the negative predictive value is the subject who showed negative in the test. Of these, it means the proportion of individuals who are not actually affected by the disease.
- ROC receiver operating characteristic
- Such a reference value does not take a specific value, but varies depending on the subject population used when setting the reference value.
- the measured value may differ depending on the analysis method used, so the reference value is set according to the analysis method used.
- a reference value based on the measured value in the body fluid sample collected from the same subject a certain period of time before.
- the "fixed period” is not particularly limited as long as the measured value can change within the same subject. For example, a period of about 1 month to 10 years, 2 months to 5 years, 3 months to 2 years, and 4 months to 1 year can be mentioned. Further, when the subject is undergoing treatment and / or surgery, a reference value can be set based on the measured value in the body fluid sample collected before the treatment and / or surgery.
- test method of the present disclosure includes the step of diagnosing prostate cancer with higher accuracy (2a) that the subject is likely to have prostate cancer.
- the test method of the present disclosure is used.
- step (3) the step of applying the prostate cancer diagnostic method to the subject determined to have a high possibility of having prostate cancer in step (2a), with higher accuracy.
- the morbidity of prostate cancer can be diagnosed.
- step (3) it is possible to truly suffer from prostate cancer.
- the diagnostic method for prostate cancer applied in step (3) is not particularly limited, and various known diagnostic methods can be adopted. Examples of the diagnostic method include rectal palpation, ultrasonography, MRI examination, CT examination, needle biopsy and the like. The diagnostic method may be one type alone or a combination of two or more types.
- test method of the present disclosure including the prostate cancer treatment step (2a) determines that the subject is likely to have prostate cancer, or step (3) causes the subject to have prostate cancer. If it is diagnosed, the subject is further determined to have prostate cancer in step (4) step (2a), or is diagnosed as having prostate cancer in step (3).
- step (step (4)) of treating the prostate cancer on the subject it becomes possible to treat the prostate cancer of the subject.
- the inspection method of the present disclosure can determine the possibility of suffering from prostate cancer with high sensitivity, a further step is applied to the inspection method of the present disclosure or the combination of the inspection method of the present disclosure and the step (3). By combining (4), a subject who is truly suffering from prostate cancer can be treated more reliably (that is, a subject who is truly suffering from prostate cancer may be excluded from the treatment target. Can be further reduced).
- the treatment method for prostate cancer is not particularly limited, and various known treatment methods can be adopted.
- Examples of the treatment method include chemotherapy, surgical treatment, radiotherapy, and immunotherapy. These can be carried out according to known methods.
- the therapeutic agent used for chemotherapy is not particularly limited, and various anticancer agents can be used.
- the anticancer agent include an alkylating agent, an antimetabolite, a microtube inhibitor, an antibiotic anticancer agent, a topoisomerase inhibitor, a platinum preparation, a molecular target drug, a hormonal agent, a biological preparation and the like.
- the alkylating agent include cyclophosphamide, iphosphamide, nitrosourea, dacarbazine, temozolomide, nimustine, busulfan, melphalan, procarbazine, and ranimustine.
- Antimetabolites include, for example, enocitabine, carmofur, capecitabine, tegafur, tegafur uracil, tegafur gimeracil oteracil potassium, gemcitabine, cytarabine, cytarabine ocphosphat, nerarabine, fluorouracil, fludarabine, pemetrexet.
- Examples include cladribine, doxifludine, hydroxycarbamide, mercaptopurine and the like.
- the microtubule inhibitor include alkaloid-based anticancer agents such as vincristine and taxane-based anticancer agents such as docetaxel and paclitaxel.
- antibiotic anticancer agent examples include mitomycin C, doxorubicin, epirubicin, daunorubicin, bleomycin, actinomycin D, acralubicin, idarubicin, pirarubicin, peplomycin, mitoxantrone, amrubicin, and dinostatinstimalamar.
- topoisomerase inhibitor examples include CPT-11 having a topoisomerase I inhibitory action, irinotecan, nogitecan, and etoposide and sobzoxane having a topoisomerase II inhibitory action.
- platinum preparation examples include cisplatin, nedaplatin, oxaliplatin, carboplatin and the like.
- hormonal agents include dexamethasone, finasteride, tamoxifen, astrosol, exemethan, ethinyl estradiol, chlormaginone, goseleline, bicalutamide, flutamide, bredonizolone, leuprorelin, retrozol, estramustine, toremifene, fosfestrol, and mittan.
- Examples include methyltestosterone, medroxyprogesterone, and mepitiostane.
- Bioforms include, for example, interferon ⁇ , ⁇ and ⁇ , interleukin 2, ubenimex, dried BCG and the like.
- Molecular-targeted drugs include, for example, nibolumab, penbrolizumab, rituximab, alemtuzumab, trusszumab, cetuximab, panitzummab, imatinib, dasatinib, nirotinib, gemtuzumab, elrotinib, gemtuzumab , Ibritumomab ozogamicin, Ibritumomab tiuxetan, Tamivarotene, tretinoin and the like.
- human epithelial growth factor receptor 2 inhibitor In addition to the molecular-targeted drugs specified here, human epithelial growth factor receptor 2 inhibitor, epithelial growth factor receptor inhibitor, Bcr-Abl tyrosine kinase inhibitor, epithelial growth factor tyrosine kinase inhibitor, mTOR inhibition Agents, inhibitors targeting angiogenesis such as vascular endothelial growth factor receptor 2 inhibitors ( ⁇ -VEGFR-2 antibody), various tyrosine kinase inhibitors such as MAP kinase inhibitors, inhibitors targeting cytokines, Molecular-targeted drugs such as proteasome inhibitors, antibody-anticancer drug formulations and the like can also be included. These inhibitors also include antibodies.
- the therapeutic agent may be one kind alone, two kinds, or a combination of three or more kinds.
- the test agent of this disclosure This will be described below.
- Standard samples are Dymethylglutarate, 2,6-Xylidine, 2-Hydroxy-2-methylpropiophenone, 2,6-di (propan-2-yl) phenol, Dimethylsuccinate, Acetophenone, 2-Phenyl-2-propanol, and 3 , 5,5-Trimetyl-2-cyclohexenone is at least one compound selected from the group, or a modification thereof, and is not particularly limited as long as this is the case.
- the standard sample can be used, for example, to prepare a calibration curve for the quantification of the biomarker of interest.
- the standard sample is a known compound or can be obtained from a known compound by an existing compound modification method.
- the detection agent is not particularly limited as long as the target biomarker can be used for detection.
- a substance (coloring reagent) that causes the chemical reaction is used as the detection agent. be able to.
- the color-developing reagent include phosphomolybdic acid, anisaldehyde, cerium / ammonium molybdate, sulfuric acid, ninhydrin, dragendorff, dinitrophenylhydrazine, palladium chloride, basic potassium permanganate, bromocresol green, and iron chloride (II). ) Etc. can be mentioned.
- an antibody against a compound constituting the target biomarker, a detection tag that assists the detection of the target biomarker by mass spectrometry, and the like can also be used as the detection agent.
- the test agent of the present disclosure may be in the form of a composition containing a standard sample and / or a detection agent.
- the composition may contain other components, if necessary.
- Other ingredients include, for example, bases, carriers, solvents, dispersants, emulsifiers, buffers, stabilizers, excipients, binders, disintegrants, lubricants, thickeners, moisturizers, colorants, fragrances. , Chelating agent and the like.
- the test agent of the present disclosure may be used in a kit containing a standard sample and / or a detection agent.
- the kit may include instruments, reagents and the like that can be used to carry out the inspection methods of the present disclosure.
- Reference example 1 Preparation of urine samples
- the urine samples of the subjects (6 healthy subjects, 14 prostate cancer patients (hereinafter, also referred to simply as "patients")) used in Examples 1 and 2 below are , 5 days worth of urine provided by a man who obtained an informed outlet.
- Table 2 shows the age of each subject, the stage of prostate cancer, and the treatment history.
- stage II cancer localized inside the prostate
- stage II cancer localized inside the prostate
- Example 1 Solid-phase microextraction-specification of prostate cancer markers using gas chromatograph mass spectrometry
- Five patients ages 62-77 to reduce personal odors due to diet and genetic predisposition, and odors due to occult blood.
- a mixed urine was prepared by mixing equal amounts of urine for 5 days with stage II and Gleason score 7.2 (average).
- 6 healthy subjects 52-75 years old were divided into 2 groups of 3 each, and mixed urine was prepared by mixing equal amounts of urine for 6 days of 3 individuals in each group.
- a large amount of volatile components were collected by putting mixed urine in a closed glass container and heating it, and analyzed the components with a mass spectrometer.
- the compound was adsorbed on solid-phase microextraction fibers coated with divinylbenzene / carboxen / polyvinyldimethylisomer (DVB / CAR / PDMS) (supelco, # 57348-U, Sigma-Aldrich) to concentrate the dilute components.
- DVD / CAR / PDMS polyvinyldimethylisomer
- GC-MS GCMS-TQ8030, Shimadzu
- the analysis conditions for GC-MS are as follows.
- % RSD relative standard deviation
- Specimens obtained from healthy subjects and prostate cancer patient specimens (preoperative) were plotted at different positions along the PC1 axis, indicating that the profiles of volatile components were clearly different. Comparing the healthy subject sample with the prostate cancer patient + endocrine treatment patient sample (preoperative) was also plotted at different positions, showing that the profile of the volatile component was clearly different, but it was different from the healthy subject sample. Prostate cancer patient specimen (preoperative) The difference was not as great as the specimen obtained from the patient, and the prostate cancer patient + endocrine treatment patient specimen (preoperative) was closer to the healthy subject specimen along the PC1 axis. As for the first principal component, healthy subjects were positively large, and on the contrary, prostate cancer patients were negatively large.
- the first principal component was considered to be an axis indicating the state of health. Based on this, the result that the prostate cancer patient + endocrine treatment patient sample (preoperative) was closer to a healthy person is peculiar to cancer released from cancer tissue due to the effect of endocrine treatment to suppress the progression of cancer. It can be considered that the number of volatile compounds in the disease may have decreased, and it may have become closer to the profile of volatile compounds released from the cancer tissue of healthy subjects.
- Prostate cancer patient specimens (postoperative) and prostate cancer patient specimens + endocrine treatment (postoperative) specimens, which are patients after cancer removal, are along the PC1 axis rather than preoperative cancer patients. It was plotted near a healthy person in the positive direction. The presence or absence of cancer was thought to change the profile of volatile components released into the urine.
- the principal component scores of the individual peaks obtained by GC-MS analysis were plotted on the graph of PC1 axis-PC2 axis. From the above results, it was considered that the peak with a negatively large first principal component score is the component group that characterizes prostate cancer.
- Eight compounds in this population (Dymethylglutarate, 2,6-Xylidine, 2-Hydroxy-2-methylpropiophenone, 2,6-di (propan-2-yl) phenol, Dimethylsuccinate, Acetophenone, 2-Phenyl-2- Phenol and 3,5,5-Trimetyl-2-cyclohexenone) were identified as prostate cancer markers.
- Example 2 Quantitative analysis of volatile organic compounds in human urine (change in urinary concentration before and after surgery) Using the preoperative urine of 5 cancer patients and the postoperative urine of these 5 patients, the 7 target compounds identified in Example 1 (Dymethyl glutarate, 2-Hydroxy-2-methylpropiophenone, 2,6- The concentrations of di (propan-2-yl) phenol, Dimethyl succinate, Acetophenone, 2-Phenyl-2-propanol, and 3,5,5-Trimetyl-2-cyclohexenone) were quantitatively analyzed. Each urine sample was obtained by mixing an equal amount of urine for 5 doses. The details of the quantitative analysis method are as follows.
- Standard stock solution Dilute the target compound with DMSO and dilute it with 10, 20, 50, 200, 500, 2000, 5000, 10000 and 20000 ng / mL (equivalent concentration in the sample is 1, 2, 5, 20, 50, 200). , 500, 1000 and 2000 ng / mL) and used as a standard stock solution.
- 20 mL of standard solution 250 ⁇ L of purified water was added to the headspace vial, and 25 ⁇ L of the standard stock solution for each calibration curve was added.
- 20 mL of sample solution and blank solution 250 ⁇ L of sample was added to a headspace vial, and 25 ⁇ L of DMSO was added.
- a solution prepared by performing the same operation using TOC purified water instead of the sample was used as a blank solution.
- 20 mL of added sample solution 250 ⁇ L of the sample was added to the headspace vial, and 25 ⁇ L of the standard stock solution 200, 500 and 2000 ng / mL was added.
- the results are shown in Figures 1-7.
- the seven target compounds are components that show higher values in preoperative specimens than in postoperative specimens. From this, it was found that these compounds are compounds derived from prostate cancer tissue.
- Example 3 Quantitative analysis of volatile organic compounds in human urine (comparison of urinary concentrations of healthy subjects and patients) Eight target compounds identified in Example 1 using 4 days'worth of urine (24 samples in total) from 6 healthy subjects and 2 to 5 days' worth of urine (13 samples in total) from 4 prostate cancer patients. (Dymethyl glutarate, 2-Hydroxy-2-methylpropiophenone, 2,6-di (propan-2-yl) phenol, Dimethyl succinate, Acetophenone, 2-Phenyl-2-propanol, 3,5,5-Trimetyl-2-cyclohexe none , And 2,6-Xylidine) were quantitatively analyzed. The quantitative analysis method is the same as in Example 2.
- the monitor ions of 2,6-Xylidine not selected in Example 2 are as follows.
- the eight target compounds are components that show higher values in the specimens of prostate cancer patients than in the specimens of healthy subjects. From this, it was found that these compounds are compounds derived from prostate cancer tissue.
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Immunology (AREA)
- Chemical & Material Sciences (AREA)
- Biomedical Technology (AREA)
- Physics & Mathematics (AREA)
- Urology & Nephrology (AREA)
- Biochemistry (AREA)
- Pathology (AREA)
- General Physics & Mathematics (AREA)
- General Health & Medical Sciences (AREA)
- Analytical Chemistry (AREA)
- Hematology (AREA)
- Molecular Biology (AREA)
- Food Science & Technology (AREA)
- Medicinal Chemistry (AREA)
- Cell Biology (AREA)
- Biotechnology (AREA)
- Hospice & Palliative Care (AREA)
- Oncology (AREA)
- Microbiology (AREA)
- Biophysics (AREA)
- Investigating Or Analysing Biological Materials (AREA)
- Measuring And Recording Apparatus For Diagnosis (AREA)
- Other Investigation Or Analysis Of Materials By Electrical Means (AREA)
Priority Applications (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| AU2021417970A AU2021417970A1 (en) | 2021-01-07 | 2021-12-24 | Method for testing possibility of having contracted prostate cancer |
| EP21917707.8A EP4276456A4 (en) | 2021-01-07 | 2021-12-24 | METHODS OF TESTING THE POSSIBILITY OF HAVING CONTRACTED PROSTATE CANCER |
| JP2022574004A JP7769179B2 (ja) | 2021-01-07 | 2021-12-24 | 前立腺がんの罹患の可能性を検査する方法 |
| US18/260,701 US20240085400A1 (en) | 2021-01-07 | 2021-12-24 | Method for testing possibility of having contracted prostate cancer |
| CN202180089607.XA CN116783484A (zh) | 2021-01-07 | 2021-12-24 | 检测前列腺癌的罹患可能性的方法 |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2021-001676 | 2021-01-07 | ||
| JP2021001676 | 2021-01-07 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2022149487A1 true WO2022149487A1 (ja) | 2022-07-14 |
Family
ID=82357872
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2021/048082 Ceased WO2022149487A1 (ja) | 2021-01-07 | 2021-12-24 | 前立腺がんの罹患の可能性を検査する方法 |
Country Status (7)
| Country | Link |
|---|---|
| US (1) | US20240085400A1 (enExample) |
| EP (1) | EP4276456A4 (enExample) |
| JP (1) | JP7769179B2 (enExample) |
| CN (1) | CN116783484A (enExample) |
| AU (1) | AU2021417970A1 (enExample) |
| TW (1) | TW202242412A (enExample) |
| WO (1) | WO2022149487A1 (enExample) |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2013515270A (ja) * | 2009-12-22 | 2013-05-02 | ザ リージェンツ オブ ザ ユニバーシティ オブ ミシガン | 前立腺癌の代謝学的プロファイリング |
| JP2013532830A (ja) * | 2010-07-28 | 2013-08-19 | メタボロン,インコーポレイテッド | 前立腺癌に関するバイオマーカー及びそれを使用する方法 |
| US20160025734A1 (en) * | 2014-07-23 | 2016-01-28 | Children's Medical Center Corporation | Use of urinary protein biomarkers to distinguish between neoplastic and non-neoplastic disease of the prostate |
| JP2017203649A (ja) * | 2016-05-09 | 2017-11-16 | 国立大学法人大阪大学 | 前立腺がん判定方法 |
| CN108344830A (zh) * | 2017-01-22 | 2018-07-31 | 中国科学院大连化学物理研究所 | 用于诊断前列腺癌的尿样组合标志物及检测试剂盒和方法 |
Family Cites Families (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US10908162B2 (en) * | 2017-10-27 | 2021-02-02 | The Board Of Regents Of The University Of Texas System | Methods related to volatile compounds in genitourinary cancers |
-
2021
- 2021-12-24 US US18/260,701 patent/US20240085400A1/en active Pending
- 2021-12-24 TW TW110148710A patent/TW202242412A/zh unknown
- 2021-12-24 EP EP21917707.8A patent/EP4276456A4/en active Pending
- 2021-12-24 AU AU2021417970A patent/AU2021417970A1/en active Pending
- 2021-12-24 JP JP2022574004A patent/JP7769179B2/ja active Active
- 2021-12-24 WO PCT/JP2021/048082 patent/WO2022149487A1/ja not_active Ceased
- 2021-12-24 CN CN202180089607.XA patent/CN116783484A/zh active Pending
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2013515270A (ja) * | 2009-12-22 | 2013-05-02 | ザ リージェンツ オブ ザ ユニバーシティ オブ ミシガン | 前立腺癌の代謝学的プロファイリング |
| JP2013532830A (ja) * | 2010-07-28 | 2013-08-19 | メタボロン,インコーポレイテッド | 前立腺癌に関するバイオマーカー及びそれを使用する方法 |
| US20160025734A1 (en) * | 2014-07-23 | 2016-01-28 | Children's Medical Center Corporation | Use of urinary protein biomarkers to distinguish between neoplastic and non-neoplastic disease of the prostate |
| JP2017203649A (ja) * | 2016-05-09 | 2017-11-16 | 国立大学法人大阪大学 | 前立腺がん判定方法 |
| CN108344830A (zh) * | 2017-01-22 | 2018-07-31 | 中国科学院大连化学物理研究所 | 用于诊断前列腺癌的尿样组合标志物及检测试剂盒和方法 |
Non-Patent Citations (2)
| Title |
|---|
| GORDON, R.T ET AL.: "The use of canines in the detection of human cancer", J. ALTERN. COMPLEMENT. MED, vol. 14, 2008, pages 61 - 67 |
| See also references of EP4276456A4 |
Also Published As
| Publication number | Publication date |
|---|---|
| EP4276456A4 (en) | 2025-02-26 |
| US20240085400A1 (en) | 2024-03-14 |
| EP4276456A1 (en) | 2023-11-15 |
| CN116783484A (zh) | 2023-09-19 |
| JP7769179B2 (ja) | 2025-11-13 |
| JPWO2022149487A1 (enExample) | 2022-07-14 |
| TW202242412A (zh) | 2022-11-01 |
| AU2021417970A1 (en) | 2023-07-27 |
| AU2021417970A9 (en) | 2024-05-16 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| Shigeyama et al. | Identification of volatile metabolites in human saliva from patients with oral squamous cell carcinoma via zeolite-based thin-film microextraction coupled with GC–MS | |
| EP2863227B1 (en) | Means and methods for metabolic differentiation of non-alcoholic steatohepatitis from liver disease | |
| US20050101023A1 (en) | Methods for diagnosing urinary tract and prostatic disorders | |
| JP7288283B2 (ja) | 小児がん検査用尿中代謝物マーカー | |
| Venäläinen et al. | Altered polyamine profiles in colorectal cancer | |
| JP6983221B2 (ja) | 大腸癌の併用検査 | |
| US20120316076A1 (en) | Biomarkers for the diagnosis of lacunar stroke | |
| WO2008063479A2 (en) | Pancreatic cancer biomarkers | |
| KR102700025B1 (ko) | 결핵의 대사체 바이오마커 | |
| Barroso et al. | Proper zinc evaluation in clinical practice: Effect of sample type and it's stability | |
| Tungkijanansin et al. | Gas chromatography-flame ionization detector for sweat based COVID-19 screening | |
| JP7769179B2 (ja) | 前立腺がんの罹患の可能性を検査する方法 | |
| JP2008533979A (ja) | 生体細胞中の時間依存的な発現を防止するための試薬及び方法 | |
| KR101799985B1 (ko) | S1p 및 스핑고신을 이용한 중증천식의 진단방법 | |
| MX2011001615A (es) | Metodo para el diagnostico de esteatohepatitis no alcoholica basado en un perfil metabolomico. | |
| CN114062532A (zh) | 一种类风湿关节炎血液诊断试剂盒及其应用 | |
| JP6769596B2 (ja) | 膀胱癌診断薬及び生体試料の判定方法 | |
| Komarevtseva et al. | Alfa-1-Antitrypsin Predicts Severe COVID-19, Gastric and Renal Cancer in Conditions of Hyperglycemia | |
| CN105102986A (zh) | 用于评价前列腺癌进度的分析方法、前列腺癌进度的评价方法、前列腺癌的检测方法以及检查试剂盒 | |
| PL241608B1 (pl) | Diagnostyczny panel biomarkerów metabolicznych do monitorowania skuteczności leczenia chemioterapeutycznego raka płuca | |
| EP4628896A1 (en) | Biomarker for diagnosing sarcopenia and sarcopenia diagnosis method using same | |
| EP4535004A1 (en) | Method for assisting diagnosis of blood tumor, method for obtaining data for diagnosing blood tumor, and kit for said methods | |
| Chuachaina | Identification of volatile markers in sweat for screening of COVID-19 infection by gas chromatography-mass spectrometry | |
| Zhang et al. | Simultaneous detection and quantification of free phenol and three structural isomers of cresol in human blood using gas chromatography-triple quadrupole mass spectrometry (GC-MS/MS) and their medical reference value ranges | |
| WO2025253002A1 (en) | Sample collection for liquid biopsy |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 21917707 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 2022574004 Country of ref document: JP Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 202180089607.X Country of ref document: CN |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 18260701 Country of ref document: US |
|
| ENP | Entry into the national phase |
Ref document number: 2021417970 Country of ref document: AU Date of ref document: 20211224 Kind code of ref document: A |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| ENP | Entry into the national phase |
Ref document number: 2021917707 Country of ref document: EP Effective date: 20230807 |