WO2022014670A1 - 膵臓がんのバイオマーカーとしてのマイクロrnaの使用 - Google Patents
膵臓がんのバイオマーカーとしてのマイクロrnaの使用 Download PDFInfo
- Publication number
- WO2022014670A1 WO2022014670A1 PCT/JP2021/026597 JP2021026597W WO2022014670A1 WO 2022014670 A1 WO2022014670 A1 WO 2022014670A1 JP 2021026597 W JP2021026597 W JP 2021026597W WO 2022014670 A1 WO2022014670 A1 WO 2022014670A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- microrna
- pancreatic cancer
- subject
- seq
- amount
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q1/00—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions
- C12Q1/68—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving nucleic acids
- C12Q1/6876—Nucleic acid products used in the analysis of nucleic acids, e.g. primers or probes
- C12Q1/6883—Nucleic acid products used in the analysis of nucleic acids, e.g. primers or probes for diseases caused by alterations of genetic material
- C12Q1/6886—Nucleic acid products used in the analysis of nucleic acids, e.g. primers or probes for diseases caused by alterations of genetic material for cancer
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/11—DNA or RNA fragments; Modified forms thereof; Non-coding nucleic acids having a biological activity
- C12N15/113—Non-coding nucleic acids modulating the expression of genes, e.g. antisense oligonucleotides; Antisense DNA or RNA; Triplex- forming oligonucleotides; Catalytic nucleic acids, e.g. ribozymes; Nucleic acids used in co-suppression or gene silencing
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q1/00—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions
- C12Q1/68—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving nucleic acids
- C12Q1/6809—Methods for determination or identification of nucleic acids involving differential detection
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q1/00—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions
- C12Q1/68—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving nucleic acids
- C12Q1/6844—Nucleic acid amplification reactions
- C12Q1/6851—Quantitative amplification
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/574—Immunoassay; Biospecific binding assay; Materials therefor for cancer
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q2600/00—Oligonucleotides characterized by their use
- C12Q2600/106—Pharmacogenomics, i.e. genetic variability in individual responses to drugs and drug metabolism
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q2600/00—Oligonucleotides characterized by their use
- C12Q2600/158—Expression markers
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q2600/00—Oligonucleotides characterized by their use
- C12Q2600/178—Oligonucleotides characterized by their use miRNA, siRNA or ncRNA
Definitions
- the present disclosure relates to the use of microRNA as a biomarker for pancreatic cancer.
- the present disclosure also presents a method for selecting microRNAs for determining whether a subject has pancreatic cancer, a method for determining whether a subject has pancreatic cancer, and whether or not the subject has pancreatic cancer.
- the present invention relates to a method for determining the pathological condition of a subject who has been suffering from pancreatic cancer, and a method for identifying a test substance that can treat pancreatic cancer.
- the present disclosure also relates to kits for use in said uses or methods, biomarkers for detecting pancreatic cancer or for monitoring the effects of pancreatic cancer treatment, and compositions for detecting pancreatic cancer. ..
- Pancreatic cancer is one of the malignant tumors with an extremely poor prognosis.
- diagnosis of pancreatic cancer the importance of pancreatic juice cytology by endoscopic retrograde cholangiopancreatography for patients with localized pancreatic duct stenosis has been reported.
- diagnostic ability is 30 to 84.7%, which is different between facilities, and its usefulness is not constant.
- Non-Patent Document 1 presents a method of detecting Glypican-1 positive exosomes in the serum of a pancreatic cancer patient by a flow cytometry method, which has 100% sensitivity and 100% specificity.
- Non-Patent Document 2 reports that a method for detecting a KRAS mutation in DNA derived from exosomes in the serum of pancreatic cancer patients and that the mutation was found in 66.7% of resectable pancreatic cancer cases.
- Non-Patent Document 3 reports a study focusing on exosomes in pancreatic juice of patients with pancreatic cancer.
- Non-Patent Document 1 has problems such as a small number of cases of early-stage pancreatic cancer that truly contributes to prognosis prolongation, and it is considered that a follow-up examination by a large cohort is necessary.
- a positive rate of 7.4% was observed even in a healthy person, and the sensitivity was also 75.4%.
- the invention described in Non-Patent Document 2 has a problem in its specificity and sensitivity.
- the invention described in Non-Patent Document 3 is intended for analysis of known microRNAs (miR-21 and miR-155).
- One object of the present disclosure is to provide a novel use of microRNA as a biomarker for pancreatic cancer.
- the present disclosure also includes a novel method for selecting microRNAs for determining whether a subject has pancreatic cancer, a novel method for determining whether a subject has pancreatic cancer, and a new method for determining whether a subject has pancreatic cancer.
- one object is to provide a new method for determining the pathological condition of a affected subject and a new method for identifying a test substance that can treat pancreatic cancer.
- the invention is also a novel kit for use in said uses or methods, novel biomarkers for detecting pancreatic cancer or for monitoring the effects of pancreatic cancer treatment, and novels for detecting pancreatic cancer.
- One purpose is to provide the composition.
- One aspect of the present disclosure is the use of at least one microRNA comprising the nucleotide sequence set forth in any of SEQ ID NOs: 1 to 15 in a biological sample derived from a subject as a biomarker for pancreatic cancer.
- the pancreatic cancer biomarker is used to determine whether the subject has pancreatic cancer, to determine the pathological condition of the subject who has or has suffered from pancreatic cancer, or to determine the pathology of the subject.
- One aspect of the present disclosure is a method for selecting microRNAs for determining whether or not they have pancreatic cancer, and is a group A of microRNAs in a biological sample derived from a subject suffering from pancreatic cancer. And the microRNA from the group B of microRNA in the culture medium in which pancreatic cancer cells were cultured and the group C of microRNA in a biological sample derived from a subject with no signs associated with pancreatic cancer.
- a method comprising selecting a microRNA that is common to the group A of the microRNA and the group B of the microRNA and is not common to the group C of the microRNA.
- One aspect of the present disclosure is a method for determining whether a subject has pancreatic cancer, a nucleotide sequence set forth in any of SEQ ID NOs: 1 to 15 in a biological sample derived from the subject. Comparing the amount of at least one microRNA comprising, with the threshold of at least one corresponding to said at least one microRNA, and the amount of said at least one microRNA being said at least one threshold. If larger, the subject provides a method comprising determining that the subject has pancreatic cancer.
- One aspect of the present disclosure is a method for determining the pathological condition of a subject suffering from or suffering from pancreatic cancer, wherein SEQ ID NOs: 1 to 15 in the first biological sample derived from the subject.
- the first amount of at least one microRNA comprising the nucleotide sequence shown in any of the above is compared with the second amount of said at least one microRNA in the subject's second biological sample. This includes determining that the condition of the subject is improved when the first amount is smaller than the second amount, and the first biological sample is the second living body.
- a method of taking a sample after taking it is provided.
- One aspect of the present disclosure is a method for identifying a test substance that can treat pancreatic cancer, wherein the test substance is administered to a subject suffering from pancreatic cancer.
- a first amount of at least one microRNA comprising the nucleotide sequence set forth in any of SEQ ID NOs: 1 to 15 in the subject-derived first biological sample after administration and the test substance are administered. Comparing with a second amount of the at least one microRNA in a second biological sample of the previous subject, and if the first amount is less than the second amount, said Provided are methods comprising identifying a test substance as a test substance capable of treating pancreatic cancer.
- One aspect of the present disclosure is a biomarker for detecting pancreatic cancer, which is at least one microRNA comprising the nucleotide sequence set forth in any of SEQ ID NOs: 1-15 in a biological sample derived from a subject. I will provide a.
- One aspect of the present disclosure is pancreatic cancer treatment, which is at least one microRNA comprising the nucleotide sequence set forth in any of SEQ ID NOs: 1-5, 9 and 15 in a serum or plasma sample from a subject. Provides biomarkers for monitoring the effects of.
- kits are provided that include reagents for measuring microRNAs.
- compositions for detecting pancreatic cancer comprising a reagent for measuring at least one microRNA comprising the nucleotide sequence set forth in any of SEQ ID NOs: 1-15. do.
- FIG. 1 is a bar graph showing the results of real-time PCR for microRNA miR-6858-5p.
- FIG. 2 is a bar graph showing the results of real-time PCR for microRNA miR-4516 (SEQ ID NO: 1).
- FIG. 3 is a bar graph showing the results of real-time PCR for the microRNA miR-4674 (SEQ ID NO: 2).
- FIG. 4 is a bar graph showing the results of real-time PCR for microRNA miR-6800-5p (SEQ ID NO: 3).
- FIG. 5 is a bar graph showing the results of real-time PCR for microRNA miR-149-3p (SEQ ID NO: 4).
- FIG. 6 is a bar graph showing the results of real-time PCR for the microRNA miR-3621 (SEQ ID NO: 5).
- FIG. 7 is a bar graph showing the results of real-time PCR for the microRNA miR-4484 (SEQ ID NO: 6).
- FIG. 8 is a bar graph showing the results of real-time PCR for the microRNA miR-3940-5p (SEQ ID NO: 9).
- FIG. 9 is a bar graph showing the results of real-time PCR for microRNA miR-3656 (SEQ ID NO: 15).
- FIG. 10 is a bar graph showing the results of real-time PCR for microRNA miR-4516 (SEQ ID NO: 1) in a pancreatic juice sample.
- FIG. 11 is a bar graph showing the results of real-time PCR for microRNA miR-4674 (SEQ ID NO: 2) in a pancreatic juice sample.
- FIG. 12 is a bar graph showing the results of real-time PCR for microRNA miR-4516 (SEQ ID NO: 1) in a large number of pancreatic juice samples.
- FIG. 13 is a bar graph showing the results of real-time PCR for microRNA miR-4674 (SEQ ID NO: 2) in a large number of pancreatic juice samples.
- FIG. 14A is a graph of a receiver operating characteristic (ROC) curve for miR-4516 (SEQ ID NO: 1) in a pancreatic juice sample.
- FIG. ROC receiver operating characteristic
- FIG. 14B is a graph of a receiver operating characteristic (ROC) curve for miR-4674 (SEQ ID NO: 2).
- FIG. 15 is a bar graph showing the results of real-time PCR for microRNA miR-4516 (SEQ ID NO: 1) in a serum sample.
- FIG. 16 is a bar graph showing the results of real-time PCR for microRNA miR-4674 (SEQ ID NO: 2) in a serum sample.
- FIG. 17 is a bar graph showing the expression level of miR-4516 (SEQ ID NO: 1) in serum samples collected from PDAC-8 before and after surgery.
- FIG. 18 is a bar graph showing the expression level of miR-4674 (SEQ ID NO: 2) in serum samples collected from PDAC-4 before and after surgery.
- FIG. 19 is a bar graph showing the expression level of miR-4516 (SEQ ID NO: 1) in a large number of serum samples.
- FIG. 20 is a graph of receiver operating characteristic (ROC) curves for miR-4516 (SEQ ID NO: 1) in a large number of serum samples.
- ROC receiver operating characteristic
- One aspect of the present disclosure is as a biomarker for pancreatic cancer of at least one microRNA comprising or consisting of the nucleotide sequence set forth in any of SEQ ID NOs: 1-15 in a biological sample derived from a subject.
- the pancreatic cancer biomarker is for determining whether a subject has pancreatic cancer.
- the pancreatic cancer biomarker is for determining the pathology of a subject suffering from or suffering from pancreatic cancer.
- the pancreatic cancer biomarker is for identifying a test substance that can treat pancreatic cancer.
- pancreatic cancer means cancer that originated in the pancreas.
- surgery radiation therapy
- chemotherapy chemotherapy.
- Surgery for pancreatic cancer is performed by excising the pancreas, depending on where the pancreatic cancer is formed, without limitation.
- Radiation therapy for pancreatic cancer is not limited, but there are a method of irradiating pancreatic cancer from outside the body and a method of irradiating the site where pancreatic cancer is formed during surgery.
- Chemotherapy for pancreatic cancer is not limited, but there is a method of instilling an anticancer drug.
- Other treatments for pancreatic cancer include immunotherapy and gene therapy.
- biomarker for pancreatic cancer means something that reflects information about pancreatic cancer.
- the information about pancreatic cancer may be, for example, information related to the presence or pathophysiology of pancreatic cancer in humans or non-human mammals, or information for identifying substances that can treat pancreatic cancer.
- the information regarding pancreatic cancer may be, for example, the concentration or content of the microRNA according to the present disclosure in a biological sample derived from a human or non-human mammal.
- subject means a human or non-human mammal.
- subject suffering from pancreatic cancer refers to human or non-human mammals diagnosed with pancreatic cancer by a person with expertise, such as a doctor or veterinarian, based on, for example, known diagnostic criteria. means.
- the subject suffering from pancreatic cancer may be, for example, a subject before or after receiving treatment such as surgery, radiation therapy, and chemotherapy.
- subject suffering from pancreatic cancer means a human or non-human mammal after being treated for pancreatic cancer.
- Subjects suffering from pancreatic cancer are humans or non-humans who have been diagnosed with pancreatic cancer in remission or complete cure, for example, by a person with expertise such as a doctor or veterinarian, based on, for example, known diagnostic criteria. It may be a human mammal.
- the non-human mammal may be, for example, a mouse, rat, rabbit, dog, sheep, pig, or non-human primate.
- the subject is preferably a human.
- the subject may be, for example, a human diagnosed with pancreatic cancer based on known diagnostic criteria, or a human or non-human mammal diagnosed with chronic pancreatitis.
- the subject may be, for example, a human or non-human mammal with no signs associated with pancreatic cancer.
- the subject is preferably a human.
- the term "biological sample” means a composition obtained from a subject that may contain at least one microRNA to be measured.
- the biological sample may be, for example, a pancreatic juice sample, a serum or plasma sample, a stool sample, a duodenal juice sample, or a bile fluid sample.
- the pancreatic juice sample may be pancreatic juice collected from a subject, or may be a sample that has been treated to separate and / or concentrate exosomes that may contain at least one microRNA to be measured in pancreatic juice. ..
- the method for collecting pancreatic juice is not particularly limited, and pancreatic juice can be collected by using a known method (for example, a method of intubating and collecting pancreatic duct).
- the serum or plasma sample may be serum or plasma from which blood cell components have been removed by using a known method such as centrifugation from whole blood collected from a subject, or at least plasma or plasma to be measured. It may be a sample that has been treated to separate and / or concentrate exosomes that may contain one type of microRNA.
- the stool sample, duodenal fluid sample, or bile sample may be stool, duodenal fluid, or bile collected from a subject, or at least one micro of measurement in stool, duodenal fluid, or bile.
- the sample may be a sample that has been treated to separate and / or concentrate the exosomes that may contain RNA.
- the method for collecting pancreatic juice is not particularly limited, and pancreatic juice can be collected using a known method.
- the biological sample may contain additives as long as it does not interfere with the measurement of at least one type of microRNA to be measured.
- additives may be, for example, buffers, nuclease inhibitors (eg DNase inhibitors and RNase inhibitors), pH regulators, surfactants, and chelating agents, or combinations thereof.
- the use of the at least one microRNA as a biomarker for pancreatic cancer is from pancreatic juice, serum or plasma, stool, duodenal juice or bile collected from a subject, pancreatic juice sample, serum. Alternatively, it may include preparing a plasma sample, a stool sample, a duodenal juice sample or a bile sample.
- the biological sample is a pancreatic juice sample or a serum or plasma sample. In one embodiment, the biological sample is a pancreatic juice sample or a serum sample. In one embodiment, the biological sample is a pancreatic juice sample. In one embodiment, the biological sample is a pancreatic juice sample.
- the biological sample is a serum or plasma sample, preferably a serum sample, with respect to at least one microRNA comprising the nucleotide sequence set forth in SEQ ID NO: 1.
- exosome means vesicle granules of about 100 nm surrounded by a lipid bilayer membrane. Exosomes are indistinguishable by their mechanism of development or size. Exosomes in pancreatic juice, serum or plasma, stool, duodenal juice or bile can be separated and / or concentrated by known methods such as centrifugation or by commercially available extraction kits.
- microRNA means RNA consisting of 16 to 25 bases. MicroRNAs are contained, for example, in exosomes and are present in body fluids or blood.
- the microRNA to be measured contains or consists of the nucleotide sequence shown in any of SEQ ID NOs: 1 to 15. At least one microRNA containing or consisting of the nucleotide sequence set forth in any of SEQ ID NOs: 1-15 can be a marker for pancreatic cancer.
- MicroRNAs can be separated and / or concentrated by known methods or commercially available extraction columns.
- a predetermined nucleotide sequence "microRNA consisting of” means that the main component consists only of polynucleotides constituting the predetermined nucleotide sequence and does not contain other nucleotides.
- the microRNA consisting of a predetermined nucleotide sequence may contain only the polynucleotide constituting the predetermined nucleotide sequence as a nucleotide component, and may further contain an addition such as a sugar chain and a methyl group.
- a microRNA that "consists essentially of" a given nucleotide sequence may further contain 5 to 1 bases, such as 3 bases, 2 bases, or 1 base at the boundaries of the given nucleotide sequence.
- At least one type of microRNA may be one type of microRNA containing or consisting of the nucleotide sequence shown in any of SEQ ID NOs: 1 to 15, and may be a combination of two or more types.
- the at least one microRNA may be a microRNA comprising or consisting of the nucleotide sequence set forth in any of SEQ ID NOs: 1-15, eg, the nucleotides set forth in any of SEQ ID NOs: 1-5, 9 and 15. It may be a microRNA containing or consisting of a sequence, preferably the nucleotide sequence set forth in any of SEQ ID NOs: 1 to 3, 5 and 15, and more preferably containing or consisting of the nucleotide sequence set forth in SEQ ID NO: 1 or 2. It may be a microRNA consisting of.
- the at least one microRNA in a biological sample may be, for example, one of the microRNAs comprising or consisting of the nucleotide sequence set forth in any of SEQ ID NOs: 1-15 in a pancreatic fluid sample, serum or plasma sample. It may be a combination of two or more kinds.
- the at least one microRNA in a biological sample is any of SEQ ID NOs: 1-5, 9 and 15 in a pancreatic fluid sample, serum or plasma sample, eg, SEQ ID NOs: 1-3, 5 and 15.
- microRNA may be one kind of microRNA containing or consisting of the nucleotide sequence shown in any of the above, preferably the nucleotide sequence shown in SEQ ID NO: 1 or 2, and may be a combination of two or more kinds.
- the at least one microRNA in the biological sample is set forth in the nucleotide sequence set forth in any of SEQ ID NOs: 1-3, 5 and 15 in the pancreatic fluid sample, preferably SEQ ID NO: 1 or 2. It may be one of microRNAs containing or consisting of a nucleotide sequence, and may be a combination of two or more.
- the at least one microRNA in a biological sample comprises or consists of the nucleotide sequence set forth in any of SEQ ID NOs: 1, 3-5, 9 and 15 in a serum or plasma sample. It may be one kind of, and it may be a combination of two or more kinds. In one embodiment, the at least one microRNA in a biological sample may be one of microRNAs comprising or consisting of the nucleotide sequence set forth in SEQ ID NO: 1 in a serum or plasma sample.
- microRNAs as biomarkers to determine if a subject has pancreatic cancer
- the use of microRNAs as biomarkers for pancreatic cancer to determine if a subject has pancreatic cancer is described above.
- the term "amount of microRNA” is, for example, the content or concentration of microRNA to be measured in a pancreatic juice sample, serum or plasma sample, stool sample, duodenal juice sample, or bile sample per unit amount. good.
- the amount of microRNA can be measured by a method that can quantitatively measure a nucleotide having a specific sequence (for example, quantitative PCR and immunoenzyme measurement method).
- the amount of microRNA may be the signal intensity obtained by the measurement method, or the content or concentration calculated from the signal intensity obtained from the biological sample using the signal intensity obtained from the control sample at a known concentration. May be.
- use as a pancreatic cancer biomarker to determine if a person has pancreatic cancer comprises measuring the amount of at least one microRNA in a biological sample derived from a subject. It's fine.
- the amount of microRNA can be measured, for example, by quantitative PCR or enzyme-linked immunosorbent assay (ELISA).
- the term "threshold” is a value set for determining whether or not a person has pancreatic cancer based on the amount of microRNA to be measured.
- the threshold value can be appropriately set according to the type of microRNA to be measured, the type of sample, gender, age, and race.
- the threshold value may be, for example, a value (diagnosis threshold value) for discriminating between pancreatic cancer and a healthy person or a patient with chronic pancreatitis.
- the diagnostic threshold measures, for example, the amount of microRNA to be measured in a group of pancreatic cancer patients and the amount of said microRNA in a healthy subject or a group of patients with chronic pancreatitis, and has a false negative rate, a false positive rate, a cost, and a presence. It can be set in consideration of the disease rate.
- a ROC curve Receiveiver Operator Characteristic Curve
- the threshold value may be set from an empirical rule, for example.
- the term "judgment" can be performed automatically or mechanically without the judgment of a person with specialized knowledge such as a doctor or a laboratory technician. Therefore, the determination is made automatically or mechanically by comparing the amount of microRNA to be measured (measured value) with the threshold value corresponding to the microRNA.
- the determination of whether or not the subject has pancreatic cancer can be determined, for example, when the measured value is larger than the threshold value, it can be determined that the subject has pancreatic cancer. In another example, the determination of whether or not the subject has pancreatic cancer may determine that the subject is unlikely to have pancreatic cancer if the measured value is smaller than the threshold value.
- the comparison between the amount of the microRNA and the threshold may be whether or not the microRNA is present.
- the determination method may determine that the subject suffers from pancreatic cancer in the presence of microRNA.
- the comparison comprises or consists of, for example, the nucleotide sequence set forth in any of SEQ ID NOs: 1-15 in two or more different biological samples derived from a subject (eg, pancreatic fluid sample, or serum or plasma sample).
- a combination of comparing the amount of microRNA with the threshold may be combined.
- the comparison is the first of a first microRNA consisting of the nucleotide sequence set forth in any of SEQ ID NOs: 1-15 in a subject-derived pancreatic fluid sample (eg, SEQ ID NO: 1).
- the comparison comprises, for example, two or more nucleotide sequences shown in any of SEQ ID NOs: 1 to 15 in a specific biological sample derived from a subject (eg, pancreatic fluid sample, or serum or plasma sample). It may be combined to compare the amount of microRNA and the threshold.
- the comparison is for a first microRNA consisting of the nucleotide sequence set forth in any of SEQ ID NOs: 1-15 in a first pancreatic fluid sample from a subject (eg, SEQ ID NO: 1). Comparing the amount with the first threshold of the first microRNA (SEQ ID NO: 1) for pancreatic fluid; and SEQ ID NOs: 1-15 other than said SEQ ID NO: in the second pancreatic fluid sample from the subject. Includes comparing the amount of second microRNA comprising the nucleotide sequence set forth in any of (eg, SEQ ID NO: 2) with the second threshold of said second microRNA for pancreatic fluid (SEQ ID NO: 2). It's fine.
- the comparison is, for example, the nucleotide sequence shown in any of two or more SEQ ID NOs: 1 to 15 in two or more different biological samples (eg, pancreatic fluid sample, or serum or plasma sample) derived from a subject. It may be combined to compare the amount of microRNA containing or consisting with the threshold.
- the comparison comprises the amount of first microRNA consisting of the nucleotide sequence set forth in any of SEQ ID NOs: 1-15 in a subject-derived pancreatic fluid sample (eg, SEQ ID NO: 1). Comparing with the first threshold of said first microRNA (SEQ ID NO: 1) for pancreatic fluid; and set forth in any of SEQ ID NOs: 1-15 other than said SEQ ID NO in the subject-derived serum sample. It may include comparing the amount of the second microRNA containing a nucleotide sequence (eg, SEQ ID NO: 2) with the second threshold of said second microRNA for serum (SEQ ID NO: 2).
- the determination is the test when the first amount of the microRNA is greater than the first threshold and the second amount of the microRNA is greater than the second threshold.
- the body may be determined to have pancreatic cancer.
- the determination is that if the first amount of the microRNA is greater than the first threshold, but the second amount of the microRNA is less than the second threshold, the subject. May require re-examination or follow-up.
- the determination is the test when the first amount of the microRNA is smaller than the first threshold and the second amount of the microRNA is smaller than the second threshold. It may be determined that the body does not have pancreatic cancer.
- microRNAs as biomarkers to determine the pathology of subjects with or suffering from pancreatic cancer
- Biomarkers for determining the pathology of subjects with or suffering from pancreatic cancer The use of microRNA as is with the first amount of at least one microRNA comprising or consisting of the nucleotide sequence set forth in any of SEQ ID NOs: 1-15 in the subject-derived first biosample. Compare with a second amount of the at least one microRNA in a second biological sample of the subject, and if the first amount is less than the second amount, the test. The first biological sample is measured after the second biological sample is measured, including determining that the condition of the body is improved.
- the invention provides the use of microRNAs as biomarkers to determine the pathology of a subject suffering from pancreatic cancer.
- the first biological sample is preferably a biological sample of the same type as the second biological sample.
- the second biological sample is also a pancreatic juice sample.
- the first biological sample is a serum sample
- the second biological sample is also a serum sample.
- the first biological sample may be a different type of biological sample than the second biological sample.
- the second biological sample may be a serum sample.
- the first biological sample may be collected, for example, after a subject suffering from or suffering from pancreatic cancer has undergone treatment such as surgery, radiation therapy, and chemotherapy.
- the second biological sample may be taken prior to receiving the treatment.
- the interval between the time of collecting the first biological sample and the time of collecting the second biological sample may be, for example, 2 weeks, 1 month, 2 months, or 3 months.
- the first biological sample and the second biological sample may each be taken after receiving the treatment.
- the "pathology" of a subject means the state of pancreatic cancer in a human or non-human mammal suffering from or suffering from pancreatic cancer.
- the condition of the subject may be a resolved pancreatic cancer condition in a human or non-human mammal suffering from pancreatic cancer.
- the condition of the subject when the first amount is smaller than the second amount, it can be determined that the condition of the subject is improved.
- the determination of the pathological condition can determine that the condition of the subject is aggravated when the first amount is larger than the second amount.
- the condition can be determined that the subject's condition is stable if the first amount is equal to the second amount.
- the comparison comprises, for example, the nucleotide sequence set forth in any of SEQ ID NOs: 1-15 in two or more first biological samples derived from a subject (eg, pancreatic fluid sample, or serum or plasma sample). It may be combined to compare a first amount of at least one microRNA consisting of the subject with a second amount of said at least one microRNA in a second biological sample derived from the subject. In one embodiment, the comparison is the first of a microRNA consisting of the nucleotide sequence set forth in any of SEQ ID NOs: 1-15 in a subject-derived first pancreatic fluid sample (eg, SEQ ID NO: 1).
- the comparison comprises, for example, two or more nucleotide sequences set forth in any of SEQ ID NOs: 1-15 in a first biological sample derived from a subject (eg, pancreatic fluid sample, or serum or plasma sample). It may be combined to compare a first amount of microRNA consisting of the subject with a second amount of said microRNA in a second biological sample of the same species (pancreatic fluid sample) derived from the subject. In one embodiment, the comparison is the first of a microRNA consisting of the nucleotide sequence set forth in any of SEQ ID NOs: 1-15 in a first pancreatic fluid sample from said subject (eg, SEQ ID NO: 1).
- a third amount of microRNA consisting of the nucleotide sequence shown in any of 1 to 15 eg, SEQ ID NO: 2
- a fourth of the microRNA (SEQ ID NO: 2) in the second pancreatic fluid sample of the subject May include comparing with the amount of.
- the comparison is, for example, two or more nucleotides shown in any one of SEQ ID NOs: 1 to 15 in two or more first biological samples (eg, pancreatic fluid sample, or serum or plasma sample) derived from a subject.
- a first amount of microRNA containing or consisting of a sequence may be combined to compare a second amount of said microRNA in a second biological sample derived from said subject.
- the comparison is the first of a microRNA consisting of the nucleotide sequence set forth in any of SEQ ID NOs: 1-15 in a subject-derived first pancreatic fluid sample (eg, SEQ ID NO: 1).
- a third amount of microRNA consisting of the nucleotide sequence shown in any of 15 to 15 eg, SEQ ID NO: 2
- a fourth of the microRNA (SEQ ID NO: 2) in a second serum sample derived from the subject May include comparing with the amount of.
- the determination is when the first amount of the microRNA is greater than the second amount of the microRNA and the third amount of the microRNA is greater than the fourth threshold.
- the condition of the subject suffering from pancreatic cancer may be determined to be exacerbated.
- the determination is that the first amount of the microRNA is greater than the second amount of the microRNA, but the third amount of the microRNA is greater than the fourth amount of the microRNA. If it is small, it may be determined that the condition of the subject suffering from pancreatic cancer remains unchanged or requires follow-up.
- the determination is that the first amount of the microRNA is smaller than the second amount of the microRNA and the third amount of the microRNA is greater than the fourth amount of the microRNA. If it is small, it may be determined that the condition of the subject suffering from pancreatic cancer has improved.
- microRNA as a biomarker to identify test substances that can treat pancreatic cancer
- the use of microRNA as a biomarker to identify test substances that can treat pancreatic cancer can be used to treat pancreatic cancer.
- Administration of the test substance to the affected subject a first amount of at least one microRNA comprising the nucleotide sequence set forth in any of SEQ ID NOs: 1-15 after administration of the test substance, and Compare with a second amount of the at least one microRNA in a second biological sample of the subject prior to administration of the test substance, and the first amount is greater than the second amount.
- the test substance is a test substance capable of treating the pancreatic cancer when it is also small.
- test substance capable of treating pancreatic cancer means a substance that is expected to slow down or maintain the progression of pancreatic cancer or to retreat pancreatic cancer.
- the test substance that can treat pancreatic cancer may be, for example, a small molecule compound, a protein (eg, an antibody), DNA, or RNA.
- the test substance that can treat pancreatic cancer may be, for example, a disease other than pancreatic cancer or a drug for treating cancer.
- the test substance may be, for example, one kind or a mixture of two or more kinds.
- Administration of the test substance can be carried out by a known method. Administration of the test substance can be carried out, for example, by oral administration, infusion, or injection.
- subject suffering from pancreatic cancer is a non-human mammal.
- the non-human mammal may be, for example, a mouse, rat, rabbit, dog, sheep, pig, or non-human primate (eg, monkey and orangutan).
- the first biological sample is preferably a biological sample of the same type as the second biological sample.
- the second biological sample is also a pancreatic juice sample.
- the first biological sample is a serum sample
- the second biological sample is also a serum sample.
- the interval between the collection time of the first biological sample and the collection time of the second biological sample may be, for example, 1 hour, 3 hours, 12 hours, 1 day, 3 days, 1 week, or 1 month. ..
- the identification of a test substance capable of treating pancreatic cancer specifies, for example, that the test substance is a test substance capable of treating pancreatic cancer when the first amount is smaller than the second amount. be able to.
- the identification of a test substance that can treat pancreatic cancer is that if the first amount is greater than the second amount, the test substance is a test substance that can exacerbate pancreatic cancer. Can be specified.
- the comparison is made, for example, to any of SEQ ID NOs: 1 to 15 in two or more first biological samples (eg, pancreatic fluid sample, or serum or plasma sample) derived from the subject after administration of the test substance.
- the comparison is from the nucleotide sequence set forth in any of SEQ ID NOs: 1-15 in the first pancreatic fluid sample from the subject after administration of the test substance (eg, SEQ ID NO: 1).
- the comparison is, for example, two kinds shown in any one of SEQ ID NOs: 1 to 15 in the first biological sample (for example, pancreatic juice sample, or serum or plasma sample) derived from the subject after administration of the test substance.
- a first amount of microRNA containing or consisting of the above nucleotide sequence and a second of the microRNA in a second biological sample of the same species (pancreatic fluid sample) derived from the subject prior to administration of the test substance. May be combined to compare with the amount of.
- the comparison is from the nucleotide sequence set forth in any of SEQ ID NOs: 1-15 in the first pancreatic fluid sample from the subject after administration of the test substance (eg, SEQ ID NO: 1).
- a third amount of microRNA consisting of the nucleotide sequence (eg, SEQ ID NO: 2) set forth in any of SEQ ID NOs: 1-15 in the first pancreatic fluid sample derived from the subject after administration of the test substance. It may include comparing with a fourth amount of the microRNA (SEQ ID NO: 2) in the second pancreatic fluid sample of the subject prior to administration of the test substance.
- the comparison is, for example, to any of SEQ ID NOs: 1 to 15 in two or more first biological samples (eg, pancreatic fluid sample, or serum or plasma sample) derived from the subject after administration of the test substance.
- a first amount of microRNA containing or consisting of the indicated two or more nucleotide sequences and a second amount of said microRNA in a second biological sample derived from said subject prior to administration of said test substance. May be combined to compare with.
- the comparison is from the nucleotide sequence set forth in any of SEQ ID NOs: 1-15 in the first pancreatic fluid sample from the subject after administration of the test substance (eg, SEQ ID NO: 1).
- a third amount of microRNA consisting of the nucleotide sequence (eg, SEQ ID NO: 2) set forth in any of SEQ ID NOs: 1-15 in the first serum sample derived from the subject after administration of the test substance. It may include comparing with a fourth amount of the microRNA (SEQ ID NO: 2) in a second serum sample from the subject prior to administration of the test substance.
- the identification as a test substance may be specified, for example, as a test substance capable of treating pancreatic cancer when the first amount is smaller than the second amount.
- the identification of a test substance may be identified as a test substance that cannot treat pancreatic cancer if the first amount is greater than the second amount.
- pancreatic cancer "Use as a biomarker for pancreatic cancer", its exemplary embodiments "use to determine if a subject has pancreatic cancer", “have or suffer from pancreatic cancer” Descriptions of terms and embodiments in “uses for determining the pathology of a subject” or “uses for identifying a test substance capable of treating pancreatic cancer” (eg, a combination of a biological sample and microRNA) are provided. , Appropriately apply between them, unless otherwise noted.
- One aspect of the present disclosure is a method for selecting microRNAs for determining whether or not they have pancreatic cancer, and is a group A of microRNAs in a biological sample derived from a subject suffering from pancreatic cancer. And the microRNA from the group B of microRNA in the culture medium in which pancreatic cancer cells were cultured and the group C of microRNA in a biological sample derived from a subject with no signs associated with pancreatic cancer.
- a method comprising selecting a microRNA that is common to the group A of the microRNA and the group B of the microRNA and is not common to the group C of the microRNA.
- pancreatic cancer cell may be, for example, a known pancreatic cancer cell line.
- Pancreatic cancer cells are commercially available or can be prepared by culturing pancreatic cancer cells taken from a subject suffering from pancreatic cancer.
- Pancreatic cancer cells may be, for example, one type of cell line or a combination or mixture of two or more types of cell lines.
- Pancreatic cancer cells include, for example, KLM-1, MIA Paca2, Panc-1, PK-1, PK-45H, PK-45P, PK-59, PK-8, T3M-4, 58-Pan, Hc48, HPC.
- pancreatic cancer cells are at least one selected from the group consisting of the human pancreatic cancer cell lines Panc-1, BxPC-3, and MIAPaCa-2, or a combination or mixture of two or more. include.
- pancreatic cancer cells means maintaining or proliferating pancreatic cancer cells in vitro (ex vivo).
- Pancreatic cancer cells can be cultured, for example, under known conditions using a known culture medium.
- culture medium means a liquid containing components necessary for cell culture.
- the culture solution can be prepared, for example, by mixing a predetermined amount of each component (solid) constituting the culture solution and an amount of pure water having a predetermined concentration.
- the culture medium may be, for example, Dulbecco's modified Eagle's medium (DMEM), minimum essential medium (MEM), basal medium Eagle (BME), or DMEM / F12.
- the culture broth may further contain certain additives (such as antibiotics).
- group of microRNAs contains information about the nucleotide sequences of at least two microRNAs.
- the group of microRNAs may further contain information regarding the expression level of each microRNA.
- the group of microRNAs can be prepared, for example, by microarray analysis of microRNAs in a sample or culture medium.
- the group of microRNAs contains, for example, information on the nucleotide sequences of at least 100, 300, 500, 1000, 1500, or 2000 microRNAs.
- common microRNA means that at least two microRNAs are both identical in terms of nucleotide sequence. In one example, the common microRNAs are such that at least two microRNAs are both 20 nucleotides in length and the types of each of the 1st to 20th nucleotides are the same.
- non-common microRNA means that at least two microRNAs differ in terms of nucleotide sequence.
- the dissimilar microRNAs are one microRNA 18 nucleotides in length and the other 20 nucleotides in length. In one example, dissimilar microRNAs are such that at least two microRNAs are both 20 nucleotides in length and the first to 20th nucleotide type in any microRNA is at least one with the other microRNAs. different.
- pancreatic cancer means a subject who has no signs suggestive of pancreatic cancer or whose signs do not meet the prescribed criteria.
- Signs suggestive of pancreatic cancer are, for example, abdominal ultrasonography, CT, MRI, endoscopic ultrasonography (EUS), MR cholangiopancreatography (MRCP), or PET, or a combination thereof. Investigate by.
- selection means to select the one that suits the purpose from a large number, or to exclude the one that does not suit the purpose from the large number.
- selection microRNAs refers to selecting from a large number of microRNAs a microRNA that is useful as an indicator for determining whether a subject has pancreatic cancer, or from the large number of microRNAs. It means excluding microRNAs that are not useful as the index.
- selecting the microRNA obtains a first group of microRNAs common to the A group of the microRNAs and the B group of the microRNAs, and the first group of the microRNAs. This includes removing from one group the microRNA common to the A group of the microRNA, the B group of the microRNA, and the C group of the microRNA.
- selecting the microRNA means that the microRNA is obtained by removing the microRNA common to the group A of the microRNA and the group C of the microRNA from the group A of the microRNA. Obtaining two groups, obtaining a third group of microRNAs from the B group of the microRNA, excluding the microRNA common to the B group of the microRNA and the C group of the microRNA, and It involves selecting microRNAs that are common to the second group of microRNAs and the third group of microRNAs.
- the selected microRNA is expressed using either or both of a biological sample derived from a subject suffering from pancreatic cancer and a culture medium in which pancreatic cancer cells are cultured. You may verify that. In the method for selecting microRNA, it may be verified that the selected microRNA is not expressed by using a biological sample derived from a subject in which no sign related to pancreatic cancer is observed. Verification of expression or non-expression can be performed, for example, by quantitative PCR.
- the expression of the selected microRNA may be expressed, for example, when the expression level of the selected microRNA is equal to or higher than a predetermined threshold value. The fact that the selected microRNA is not expressed may mean that the selected microRNA is not expressed, for example, when the expression level of the selected microRNA is below a predetermined threshold value.
- One aspect of the present disclosure is a method for determining whether a subject has pancreatic cancer, a nucleotide sequence set forth in any of SEQ ID NOs: 1 to 15 in a biological sample derived from the subject. Comparing the amount of at least one microRNA containing, with the threshold of at least one corresponding to the microRNA, and when the amount of the at least one microRNA is greater than the threshold of at least one. , The subject provides a method comprising determining that the subject has pancreatic cancer.
- a subject determined to have pancreatic cancer may be treated for pancreatic cancer. Therefore, one embodiment according to this aspect provides a method of treating pancreatic cancer in a subject.
- the embodiment provides a method of treating pancreatic cancer in a subject, wherein the method comprises at least one of the nucleotide sequences set forth in any of SEQ ID NOs: 1-15 in a biological sample derived from the subject. Comparing the amount of microRNA with at least one threshold corresponding to the microRNA, if the amount of at least one microRNA is greater than the at least one threshold, the subject is pancreatic. It includes determining that the patient has pancreatic cancer and treating the pancreatic cancer in the subject determined to have pancreatic cancer.
- treatment of pancreatic cancer may be, for example, chemotherapy, immunotherapy, gene therapy, radiation therapy or surgery, or a combination thereof for pancreatic cancer in a subject.
- One aspect of the present disclosure is a method for determining the pathological condition of a subject suffering from or suffering from pancreatic cancer, wherein SEQ ID NOs: 1 to 15 in the first biological sample derived from the subject.
- the first amount of at least one microRNA comprising the nucleotide sequence shown in any of the above is compared with the second amount of said at least one microRNA in the subject's second biological sample. This includes determining that the condition of the subject is improved when the first amount is smaller than the second amount, and the first biological sample is the second living body.
- a method of taking a sample after taking it is provided.
- the invention provides a method for determining the pathology of a subject suffering from pancreatic cancer.
- One aspect of the present disclosure is a method for identifying a test substance that can treat pancreatic cancer, wherein the test substance is administered to a subject suffering from pancreatic cancer.
- a first amount of at least one microRNA comprising the nucleotide sequence set forth in any of SEQ ID NOs: 1 to 15 in the subject-derived first biological sample after administration and the test substance are administered. Comparing with a second amount of the at least one microRNA in a second biological sample of the previous subject, and if the first amount is less than the second amount, said Provided are methods comprising identifying a test substance as a test substance capable of treating pancreatic cancer.
- One aspect of the present disclosure is at least one microRNA comprising the nucleotide sequence set forth in any of SEQ ID NOs: 1-15 in a biological sample derived from a subject, for detecting pancreatic cancer or pancreas. Provides biomarkers for monitoring the effects of cancer treatment.
- One aspect of the present disclosure is pancreatic cancer treatment, which is at least one microRNA comprising the nucleotide sequence set forth in any of SEQ ID NOs: 1-5, 9 and 15 in a serum or plasma sample from a subject. Provides biomarkers for monitoring the effects of.
- the biomarker to monitor the effect of pancreatic cancer treatment is the nucleotide sequence set forth in any of SEQ ID NOs: 1, 3-5, 9 and 15 in serum or plasma samples from the subject. Is a microRNA containing. In one embodiment, the biomarker for monitoring the effect of pancreatic cancer treatment is a microRNA comprising the nucleotide sequence set forth in SEQ ID NO: 1 in a serum or plasma sample from a subject.
- detecting pancreatic cancer means getting information about pancreatic cancer.
- Biomarkers for detecting pancreatic cancer or monitoring the effects of pancreatic cancer treatment are used to obtain information about pancreatic cancer or the effects of pancreatic cancer treatment. Means something that reflects information about.
- Information about pancreatic cancer includes, for example, information related to the presence or pathophysiology of pancreatic cancer in humans or non-human mammals, information related to the effects of treatment for pancreatic cancer, or substances capable of treating pancreatic cancer. It may be information for identification.
- the information regarding pancreatic cancer may be, for example, the concentration or content of the microRNA according to the present disclosure in a biological sample derived from a human or non-human mammal.
- Biomarkers for detecting pancreatic cancer or monitoring the effects of pancreatic cancer treatment include, for example, methods for determining whether a subject according to the present disclosure has pancreatic cancer, pancreatic cancer. It can be used as a method for determining the pathological condition of a subject suffering from or suffering from pancreatic cancer, or as a method for identifying a test substance that can treat pancreatic cancer.
- Biomarkers for monitoring the treatment of pancreatic cancer are, for example, the biomarkers disclosed herein in compositions obtained from subjects treated for pancreatic cancer.
- the biomarker should be prepared, for example, from a composition obtained from a subject (eg, pancreatic juice sample, serum or plasma sample, stool sample, duodenal juice sample, or bile sample) which may contain the microRNA according to the present disclosure. Can be done.
- a composition obtained from a subject eg, pancreatic juice sample, serum or plasma sample, stool sample, duodenal juice sample, or bile sample
- pancreatic juice sample serum or plasma sample
- stool sample e.g, duodenal juice sample, or bile sample
- bile sample e.g., bile sample
- treatment of pancreatic cancer may be, but is not limited to, chemotherapy, immunotherapy, gene therapy, radiation therapy or surgery, or a combination thereof.
- kits One aspect of the present disclosure measures at least one microRNA comprising or consisting of the nucleotide sequence set forth in any of SEQ ID NOs: 1-15 for use as a biomarker for pancreatic cancer described above.
- a kit containing reagents for this is provided.
- the term "reagent for measurement” or “measurement reagent” includes a probe that can specifically bind to the microRNA to be measured.
- the probe may be labeled with, for example, a fluorescent material.
- Probes include, for example, at least one nucleotide (eg, DNA or RNA) containing a sequence complementary to the nucleotide sequence of microRNA. Such nucleotides are, for example, commercially available.
- the probe may be a primer set for use in, for example, a nucleic acid amplification method such as PCR. Such primer sets can be appropriately set and prepared by those skilled in the art based on the sequence of the microRNA to be amplified.
- the kit may further include, for example, a buffer in liquid or solid form, and a nuclease inhibitor (eg, DNase inhibitor and RNase inhibitor).
- a nuclease inhibitor eg, DNase inhibitor and RNase inhibitor.
- the characteristics of the measurement reagent can be appropriately set depending on the method for measuring the microRNA to be measured.
- the measurement reagent may contain a nucleotide having a sequence complementary to the microRNA to be measured as a probe, and the probe may be labeled with a fluorescent substance.
- composition for detecting pancreatic cancer comprising a reagent for measuring at least one microRNA comprising or consisting of the nucleotide sequence set forth in any of SEQ ID NOs: 1-15. Provide things.
- the composition comprises a reagent for measuring any or a combination of at least one microRNA comprising or consisting of the nucleotide sequences set forth in any of SEQ ID NOs: 1-5.
- the composition measures a reagent for measuring a microRNA comprising or consisting of the nucleotide sequence set forth in SEQ ID NO: 1 and a microRNA comprising or consisting of the nucleotide sequence set forth in SEQ ID NO: 2. Contains both reagents to be used.
- Microarray analysis was performed on the microRNA and the microRNA derived from exosomes in the pancreatic fluid samples of the above-mentioned three patients with chronic pancreatitis. The microarray analysis was carried out by Cell Innovator Co., Ltd. More specifically, 1000 ng of total RNA for each microRNA was labeled using FlashTag® Biotin HSR RNA Labeling Kit. The labeled RNA was hybridized to Affymetrix GeneChip® miRNA 4.0 Array, and the hybridized Array was scanned. The scanned data was analyzed using Affymetrix Transcriptome Analysis Console 4.0.
- the RNA was compared with the microRNA sequence information obtained by microarray analysis, and multiple types of microRNA sequences that were highly expressed in both samples were obtained. From the obtained multiple types of microRNA sequences, microRNA sequences overlapping with exosome-derived microRNA sequences in the pancreatic fluid samples of the three patients with chronic pancreatitis were removed.
- microRNA is highly expressed in pancreatic cancer patients and / or pancreatic cancer cell lines, and is low in non-cancer patients (chronic pancreatitis patients), and is used as a biomarker for pancreatic cancer. It is speculated that it can be done.
- the 15 types of microRNAs obtained in this test 1 are shown in the table below.
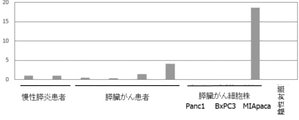
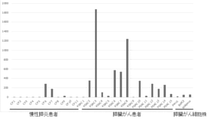
- FIG. 1 shows a large amount of miR-6858-5p in any of the pancreatic fluid (2 specimens) of chronic pancreatitis, the pancreatic fluid (4 specimens) of a pancreatic cancer patient, and the culture fluid (3 specimens) of a pancreatic cancer cell line. There is no difference, indicating that the condition that the expression level is high in pancreatic cancer patients and / or pancreatic cancer cell lines is not satisfied as compared with the expression level in non-cancer patients (chronic pancreatitis patients).
- FIG. 1 suggests that microRNA miR-6858-5p is not useful as a biomarker for pancreatic cancer.
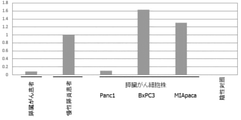
- FIG. 2 shows pancreatic juice (3 samples) and pancreatic cancer cell lines (2 bodies) of pancreatic cancer patients compared to the amount of miR-4516 in pancreatic juice (2 samples) of non-cancer patients (chronic pancreatitis patients). It shows that the amount of miR-4516 in is larger.
- FIG. 3 shows pancreatic juice (3 samples) and pancreatic cancer cells of pancreatic cancer patients compared to the amount of miR-4674 (SEQ ID NO: 2) in pancreatic juice (2 samples) of non-cancer patients (chronic pancreatitis patients). It is shown that the amount of miR-4674 in the culture solution (3 samples) of the strain is larger.
- pancreatic cancer patients and / or pancreatic cancer cell lines than in non-cancer patients (chronic pancreatitis patients), and miR-4674 is used in the pancreas. It suggests that it can be a biomarker for pancreatic cancer.
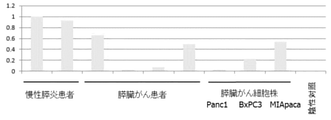
- FIG. 4 shows pancreatic juice (1 sample) and pancreas of pancreatic cancer patients compared to the amount of miR-6800-5p (SEQ ID NO: 3) in pancreatic juice (2 samples) of non-cancer patients (chronic pancreatitis patients). It is shown that the amount of miR-6800-5p in the pancreatic juice (1 sample) of the pancreatic cell line is larger. This result shows that for miR-6800-5p, the expression level in non-cancer patients (chronic pancreatitis patients) was compared with that in pancreatic cancer patients (1 sample) and / or pancreatic cancer cell lines (1 sample). It satisfies the condition of high expression level and suggests that miR-6800-5p can be a biomarker for pancreatic cancer.
- FIG. 5 shows the amount of miR-149-3p (SEQ ID NO: 4) in the pancreatic juice (1 sample) of a non-cancer patient (chronic pancreatitis patient) in the culture medium (2 samples) of a pancreatic cancer cell line. It is shown that the amount of miR-149-3p in the above is larger. This result satisfies the condition that the expression level of miR-149-3p is higher in pancreatic cancer patients and / or pancreatic cancer cell lines than in non-cancer patients (chronic pancreatitis patients). , MiR-149-3p suggests that it can be a biomarker for pancreatic cancer.
- FIG. 6 shows the pancreatic juice (2 samples) and pancreatic cancer cells of a pancreatic cancer patient as compared with the amount of miR-3621 (SEQ ID NO: 5) in the pancreatic juice (2 samples) of a non-cancer patient (chronic pancreatitis patient). It shows that the amount of miR-3621 in the culture solution (2 samples) of the strain is larger. This result satisfies the condition that the expression level of miR-3621 is higher in pancreatic cancer patients and / or pancreatic cancer cell lines than in non-cancer patients (chronic pancreatitis patients), and miR It suggests that -3621 can be a biomarker for pancreatic cancer.
- the amount of miR-4484 (SEQ ID NO: 6) in the pancreatic juice (2 samples) of a non-cancer patient (chronic pancreatitis patient) is the pancreatic juice (4 samples) of a pancreatic cancer patient and a pancreatic cancer cell line. It shows that it is larger than the amount of miR-4484 in the culture solution (3 samples). This result did not satisfy the condition that the expression level of miR-4484 was higher in pancreatic cancer patients and / or pancreatic cancer cell lines than in non-cancer patients (chronic pancreatitis patients). It shows that miR-4484 in pancreatic fluid is not available as a biomarker for pancreatic cancer.
- FIG. 8 shows the amount of miR-3940-5p (SEQ ID NO: 9) in the pancreatic juice (2 samples) of a non-cancer patient (chronic pancreatitis patient) in the culture medium (2 samples) of a pancreatic cancer cell line. It shows that the amount of miR-3940-5p is larger. This result satisfies the condition that the expression level of miR-3940-5p is higher in pancreatic cancer patients and / or pancreatic cancer cell lines than in non-cancer patients (chronic pancreatitis patients). , MiR-3940-5p suggests that it may be a biomarker for pancreatic cancer.
- FIG. 9 shows the pancreatic juice (1 sample) and pancreatic cancer cells of a pancreatic cancer patient as compared with the amount of miR-3656 (SEQ ID NO: 15) in the pancreatic juice (1 sample) of a non-cancer patient (chronic pancreatitis patient). It is shown that the amount of miR-3656 in the culture solution (2 samples) of the strain is larger. This result satisfies the condition that the expression level of miR-3656 is higher in pancreatic cancer patients and / or pancreatic cancer cell lines than in non-cancer patients (chronic pancreatitis patients), and miR It suggests that -3656 can be a biomarker for pancreatic cancer.
- 2 to 6 and 8 to 9 show the results of real-time PCR of Test 2 and the results of microarray analysis of Test 1 with respect to the microRNA consisting of the nucleotide sequences shown in SEQ ID NOs: 1 to 5, 9 and 15. Shows consistency. 2 to 6 and 8 to 9 also show that the microRNA consisting of the nucleotide sequences shown in SEQ ID NOs: 1 to 3, 5 and 15 is expressed in a non-cancer patient (chronic pancreatitis patient) as compared with the expression level. It is shown that the expression level is high in pancreatic cancer cell lines and patients with pancreatic cancer, suggesting that the microRNA is preferable as a biomarker for pancreatic cancer.
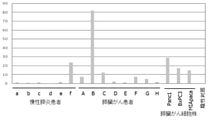
- FIG. 10 shows the relative value of miR-4516 in the pancreatic juice sample of each patient standardized with respect to the measured value of the microRNA miR-4516 (SEQ ID NO: 1) in the pancreatic juice sample of the chronic pancreatitis patient a.
- FIG. 10 shows the relative value of miR-4516 in the pancreatic juice sample of each patient standardized with respect to the measured value of the microRNA miR-4516 (SEQ ID NO: 1) in the pancreatic juice sample of the chronic pancreatitis patient a.
- FIG. 11 shows the relative value of miR-4674 in the pancreatic juice sample of each patient standardized with respect to the measured value of the microRNA miR-4674 (SEQ ID NO: 2) in the pancreatic juice sample of the chronic pancreatitis patient a.
- the relative value 2 when the relative value 2 is used as the threshold value, 5 out of 6 patients with chronic pancreatitis are judged to be negative (specificity: 83.3%), and 5 out of 8 patients with pancreatic cancer are positive. It shows that it is judged (sensitivity: 62.5%) and that 3 out of 3 types of pancreatic cancer cells are judged to be positive (positive rate: 100%).
- This result indicates that the microRNA miR-4674 (SEQ ID NO: 2) in the pancreatic juice sample is useful as a biomarker for pancreatic cancer.
- FIGS. 12 and 13 show the results of the same test as in Test 3.
- FIG. 12 shows the relative value of miR-4516 in the pancreatic juice sample of each patient, which was standardized with respect to the measured value of the microRNA miR-4516 (SEQ ID NO: 1) in the pancreatic juice sample of the chronic pancreatitis patient CP1.
- FIG. 13 shows the relative value of miR-4674 in the pancreatic juice sample of each patient, which was standardized with respect to the measured value of the microRNA miR-4674 (SEQ ID NO: 2) in the pancreatic juice sample of the chronic pancreatitis patient CP1.
- Indicators sensitivity, specificity, positive predictive value, negative predictive value and correct diagnosis
- miR-4516 SEQ ID NO: 1
- miR-4674 SEQ ID NO: 2
- miR-4516 (SEQ ID NO: 1) and miR-4674 (SEQ ID NO: 2) can be used for the diagnosis of pancreatic cancer, especially early diagnosis, with high sensitivity, high specificity and high accuracy rate, respectively. Suggests.
- Sensitivity, specificity and accuracy in tests using a combination of miR-4516 (SEQ ID NO: 1) and pancreatic juice cytology (PJC) (miR-4516 / PJC) were 93.3%, 81.8% and correct diagnosis rates, respectively. It was 88.5%.
- the sensitivity, specificity, and correctness rate in the examination using the combination of miR-4674 (SEQ ID NO: 2) and pancreatic juice cytology (PJC) (miR-4674 / PJC) are 100%, 81.8%, and the correct diagnosis rate, respectively. It was 92.3%.
- the sensitivity, specificity and accuracy rate in the examination using the combination of miR-4516, miR-4674 and pancreatic juice cytology (PJC) are 100% and 72.7%, respectively. And 88.5%.
- PJC pancreatic juice cytology
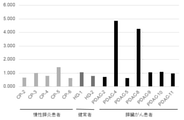
- Amount of microRNA miR-4516 (SEQ ID NO: 1) and miR-4674 (SEQ ID NO: 1) in serum of patients with chronic pancreatitis (5 samples), serum of healthy subjects (2 samples) and serum of patients with pancreatic cancer (7 samples) was examined by quantitative real-time PCR. These results are shown in FIGS. 15 and 16, respectively.
- FIG. 15 shows the relative value of microRNA miR-4516 (SEQ ID NO: 1) in the serum of each patient.
- a relative value of 1.5 is used as a threshold value
- 2 out of 7 pancreatic cancer patients are judged to be positive (sensitivity: about 28.6%), and 5 out of 5 patients with chronic pancreatitis. Indicates that is negative or that 2 out of 2 healthy subjects are negative (specificity: 100%).
- This result indicates that the microRNA miR-4516 (SEQ ID NO: 1) in serum is useful as a biomarker for pancreatic cancer.
- FIG. 16 shows the relative value of microRNA miR-4674 (SEQ ID NO: 2) in the serum of each patient.
- a relative value of 1.5 when a relative value of 1.5 is used as a threshold, 0 out of 7 pancreatic cancer patients are determined to be positive (sensitivity: 0%), and 5 out of 5 patients with chronic pancreatitis are negative. It indicates that it is judged or that 2 out of 2 healthy subjects are judged as negative (specificity: 100%). This result indicates that the microRNA miR-4674 (SEQ ID NO: 2) in serum cannot be said to be usable as a biomarker for pancreatic cancer.
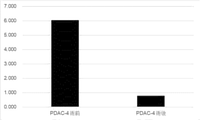
- FIG. 17 shows that the expression level of miR-4516 (SEQ ID NO: 1) decreased after treatment for pancreatic cancer.
- Pancreatic cancer patient PDAC-4 underwent tumor removal surgery.
- the expression level of miR-4516 (SEQ ID NO: 1) was low in the pancreatic juice collected before surgery (Fig. 12) and high in the collected serum (Fig. 15).
- Serum was collected from PDAC-4 after undergoing surgery, and the expression level of miR-4516 (SEQ ID NO: 1) in the serum was examined (FIG. 18).
- FIG. 18 shows that the expression level of miR-4516 (SEQ ID NO: 1) decreased after treatment for pancreatic cancer.
- pancreatic cancer-specific serum marker miR-4516 (SEQ ID NO: 1) is a pancreatic cancer as a biomarker in serum for discriminating pancreatic cancer patients from other subjects. I checked if it could be used for the inspection of. Serum was collected from 39 pancreatic cancer patients (PDAC) and 32 other subjects. Thirty-two subjects were 2 healthy subjects (HD), 5 patients with chronic pancreatitis (CP), 1 patient with intraductal papillary mucinous tumor (IPMN), and 3 patients with intraductal papillary mucinous adenocarcinoma.
- HD healthy subjects
- CP chronic pancreatitis
- IPMN intraductal papillary mucinous tumor
- 3 patients with intraductal papillary mucinous adenocarcinoma 3 patients with intraductal papillary mucinous adenocarcinoma.
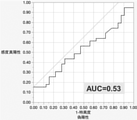
- FIG. 19 The expression level of miR-4516 (SEQ ID NO: 1) in the collected serum was examined (FIG. 19). Based on the above results, a ROC curve was created (FIG. 20).
- FIG. 20 shows that the area under the curve of miR-4516 (SEQ ID NO: 1) is 0.53.
- the sensitivity and correct diagnosis rate in the examination using miR-4516 (SEQ ID NO: 1) were 15.4% and 53.5%, respectively.
- the specificity was 100%, which was a good result.
Landscapes
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Organic Chemistry (AREA)
- Zoology (AREA)
- Wood Science & Technology (AREA)
- Genetics & Genomics (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Immunology (AREA)
- Molecular Biology (AREA)
- Biotechnology (AREA)
- Analytical Chemistry (AREA)
- Bioinformatics & Cheminformatics (AREA)
- General Engineering & Computer Science (AREA)
- Microbiology (AREA)
- Physics & Mathematics (AREA)
- Biochemistry (AREA)
- General Health & Medical Sciences (AREA)
- Biophysics (AREA)
- Biomedical Technology (AREA)
- Pathology (AREA)
- Oncology (AREA)
- Hospice & Palliative Care (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Plant Pathology (AREA)
- Hematology (AREA)
- Urology & Nephrology (AREA)
- Cell Biology (AREA)
- Food Science & Technology (AREA)
- Medicinal Chemistry (AREA)
- General Physics & Mathematics (AREA)
- Measuring Or Testing Involving Enzymes Or Micro-Organisms (AREA)
- Investigating Or Analysing Biological Materials (AREA)
Priority Applications (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US18/016,049 US20230272481A1 (en) | 2020-07-16 | 2021-07-15 | Use of microrna as pancreatic cancer biomarker |
| JP2022536442A JPWO2022014670A1 (enExample) | 2020-07-16 | 2021-07-15 |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2020122124 | 2020-07-16 | ||
| JP2020-122124 | 2020-07-16 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2022014670A1 true WO2022014670A1 (ja) | 2022-01-20 |
Family
ID=79555676
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2021/026597 Ceased WO2022014670A1 (ja) | 2020-07-16 | 2021-07-15 | 膵臓がんのバイオマーカーとしてのマイクロrnaの使用 |
Country Status (3)
| Country | Link |
|---|---|
| US (1) | US20230272481A1 (enExample) |
| JP (1) | JPWO2022014670A1 (enExample) |
| WO (1) | WO2022014670A1 (enExample) |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2009528070A (ja) * | 2006-03-02 | 2009-08-06 | ザ オハイオ ステイト ユニバーシティ | 膵臓癌に関連するマイクロrna発現プロファイル |
Family Cites Families (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP6611411B2 (ja) * | 2013-12-05 | 2019-11-27 | 東レ株式会社 | 膵臓がんの検出キット及び検出方法 |
| JP2016123340A (ja) * | 2014-12-26 | 2016-07-11 | 株式会社エバンス | 膵癌治療感受性の診断方法及び膵癌治療感受性の増強剤 |
| WO2019117269A1 (ja) * | 2017-12-13 | 2019-06-20 | 国立大学法人広島大学 | 膵がんの検出を補助する方法 |
-
2021
- 2021-07-15 WO PCT/JP2021/026597 patent/WO2022014670A1/ja not_active Ceased
- 2021-07-15 JP JP2022536442A patent/JPWO2022014670A1/ja active Pending
- 2021-07-15 US US18/016,049 patent/US20230272481A1/en active Pending
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2009528070A (ja) * | 2006-03-02 | 2009-08-06 | ザ オハイオ ステイト ユニバーシティ | 膵臓癌に関連するマイクロrna発現プロファイル |
Non-Patent Citations (3)
| Title |
|---|
| BUNSO KYO, YUICHI NAGAKAWA, SHINOBU UEDA, KOHSUKE KANEKURA, KAZUHIKO KASUYA, MASAHIKO KURODA, AKIHIKO TSUCHIDA: "Comprehensive analysis of circulating microRNA and detection of novel biomarkers in patients with pancreatic cancer", TOKYO IKA DAIGAKU ZASSHI - THE JOURNAL OF TOKYO MEDICAL UNIVERSITY, vol. 75, no. 2, 1 January 2017 (2017-01-01), Japan, pages 234 - 240, XP009533238, ISSN: 0040-8905 * |
| SHUICHI MITSUNAGA, MOTOHIRO KOJIMA, MASAFUMI IKEDA, ATSUSHI OCHIAI: "Serum microRNAs as tumor markers for diagnosis of pancreatic cancer", JOURNAL OF THE JAPAN PANCREAS SOCIETY, vol. 32, no. 1, 25 February 2017 (2017-02-25), pages 56 - 61, XP055886946, ISSN: 0913-0071, DOI: 10.2958/suizo.32.56 * |
| SHUO CHEN, MENG XU, JING ZHAO, JIAQI SHEN, JUNHUI LI, YANG LIU, GANG CAO, JIANCANG MA, WEIZHOU HE, XI CHEN,TAO SHAN: "MicroRNA-4516 suppresses pancreatic cancer development via negatively regulating orthodenticle homeobox 1", INTERNATIONAL JOURNAL OF BIOLOGICAL SCIENCES, vol. 16, no. 12, 18 May 2020 (2020-05-18), pages 2159 - 2169, XP055886949, ISSN: 1449-2288, DOI: 10.7150/ijbs.45933 * |
Also Published As
| Publication number | Publication date |
|---|---|
| US20230272481A1 (en) | 2023-08-31 |
| JPWO2022014670A1 (enExample) | 2022-01-20 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| Jin et al. | Liquid biopsy in uveal melanoma: are we there yet? | |
| JPWO2014003053A1 (ja) | 膵臓がんの検出方法及び検出用キット | |
| CN109825586A (zh) | 用于肺癌检测的DNA甲基化qPCR试剂盒及使用方法 | |
| KR20210026852A (ko) | 비용종을 갖는 비부비동염의 진단을 위한 정보 제공 방법 | |
| Qiu et al. | Analysis on expression level and diagnostic value of miR-19 and miR-21 in peripheral blood of patients with undifferentiated lung cancer | |
| CN106834447A (zh) | 一种用于检测神经母细胞瘤的引物组及检测方法 | |
| KR101657051B1 (ko) | 만성폐쇄성폐질환 진단용 마커 조성물 | |
| WO2022014670A1 (ja) | 膵臓がんのバイオマーカーとしてのマイクロrnaの使用 | |
| WO2015137406A1 (ja) | 肺扁平上皮癌と肺腺癌の鑑別評価方法 | |
| JP7610265B2 (ja) | 大腸癌診断用マーカー、大腸癌の診断を補助する方法、大腸癌の診断のためにデータを収集する方法、大腸癌の診断用キット、大腸癌治療薬、大腸癌の治療方法、大腸癌の診断方法 | |
| CN115261476A (zh) | 筛选血清外泌体LncRNA HULC作为肝癌早期标记物的方法及其制备试剂盒的用途 | |
| WO2018212288A1 (ja) | パーキンソン病の判定マーカーおよび判定方法 | |
| JP5316749B2 (ja) | シスプラチン耐性遺伝子診断方法及びシスプラチン治療効果遺伝子診断キット | |
| KR20230059846A (ko) | 원형탈모증의 진단 또는 예후 평가를 위한 정보 제공 방법 | |
| WO2015105191A1 (ja) | リンパ節腫脹病変の評価方法 | |
| CN106520924A (zh) | 一种用于检测卵巢癌的引物组及检测方法 | |
| WO2014151465A1 (en) | Brain-specific gene signature of tumor cells | |
| CN109554490A (zh) | 一种与复发性流产相关的微生物及其应用 | |
| CN116790760B (zh) | 结肠癌特异性环状rna标记物及其检测引物与应用 | |
| JP6041297B2 (ja) | 犬リンパ腫の診断方法及び診断キット | |
| CN109182514A (zh) | 肺癌诊断或转移诊断标志物LncRNA Loc729658和试剂盒及其应用 | |
| JP2005160354A (ja) | 神経芽腫の診断方法 | |
| CN117120631A (zh) | 滤泡性甲状腺癌特异性标志物 | |
| CN106755459A (zh) | 一种用于检测乳腺癌的引物组及检测方法 | |
| US20250298015A1 (en) | Diagnostic method of detecting inflammation biomarker(s) |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 21841671 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 2022536442 Country of ref document: JP Kind code of ref document: A |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 21841671 Country of ref document: EP Kind code of ref document: A1 |