WO2022014639A1 - Egfr阻害剤 - Google Patents
Egfr阻害剤 Download PDFInfo
- Publication number
- WO2022014639A1 WO2022014639A1 PCT/JP2021/026461 JP2021026461W WO2022014639A1 WO 2022014639 A1 WO2022014639 A1 WO 2022014639A1 JP 2021026461 W JP2021026461 W JP 2021026461W WO 2022014639 A1 WO2022014639 A1 WO 2022014639A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- group
- alkyl group
- substituents
- egfr
- compound
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
- 0 *C(CC(C1)[n](c(C#C*)c2C(NC(*)(*)*)=O)c3c2c(N)ncn3)N1C(C=C)=O Chemical compound *C(CC(C1)[n](c(C#C*)c2C(NC(*)(*)*)=O)c3c2c(N)ncn3)N1C(C=C)=O 0.000 description 3
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/505—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim
- A61K31/519—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim ortho- or peri-condensed with heterocyclic rings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D487/00—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, not provided for by groups C07D451/00 - C07D477/00
- C07D487/02—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, not provided for by groups C07D451/00 - C07D477/00 in which the condensed system contains two hetero rings
- C07D487/04—Ortho-condensed systems
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02P—CLIMATE CHANGE MITIGATION TECHNOLOGIES IN THE PRODUCTION OR PROCESSING OF GOODS
- Y02P20/00—Technologies relating to chemical industry
- Y02P20/50—Improvements relating to the production of bulk chemicals
- Y02P20/55—Design of synthesis routes, e.g. reducing the use of auxiliary or protecting groups
Definitions
- the present invention relates to an EGFR inhibitor using a pyrimidine compound or a salt thereof.
- EGFR also called “ErbB1”
- ErbB1 is a receptor tyrosine kinase belonging to the ErbB family, and in normal tissues, it binds to Epidermal Growth Factor (also called “EGF”) mainly in epidermal tissues and contributes to cell proliferation and inhibition of apoptosis.
- EGF Epidermal Growth Factor
- EGFR is considered to be the causative gene of cancer, and gene amplification, overexpression, mutation, etc. of EGFR have been reported in various cancers. From non-clinical and clinical research data, it can be seen that activation of EGFR and downstream signals plays an important role in the survival and proliferation of cancer cells in cancer cells with these EGFR gene abnormalities and overexpressions. It is considered. For example, a mutation in which amino acids 746 to 750 of the EGFR exxon 19 region are deleted (also referred to as "exxon 19 deletion mutation”), and amino acid 858 of the exon 21 region is mutated from leucine to arginine (also referred to as "L858R mutation").
- Non-Patent Document 2 (Called) is thought to contribute to the survival and proliferation of cancer cells by inducing kinase activity by self-mutating EGFR in an EGF-independent manner. It has been reported that these mutations are present in about 30 to 50% of non-small cell lung cancer in East Asia and in about 10% of non-small cell lung cancer in Europe and the United States (Non-Patent Document 3).
- an inhibitor capable of controlling the kinase activity of EGFR may exert an antitumor effect by inhibiting EGFR and downstream signaling in cancer cells with gene amplification, overexpression, and / or mutation of EGFR. As it is expected, it is considered to be useful for treating cancer patients, prolonging their lives, and improving QOL.
- EGFR inhibitors as anticancer agents have been carried out conventionally, and they are used for the treatment of EGFR mutation-positive tumors.
- agents such as afatinib, gefitinib, and erlotinib have been approved as therapeutic agents for EGFR mutation-positive lung cancer with the Exxon 19 deletion mutation and the L858R mutation.
- Osimertinib is used as a therapeutic agent for EGFR mutation-positive lung cancer having an exon 19 deletion mutation and an L858R mutation, as well as an exon 20 region 790 amino acid mutated from threonine to methionine (also referred to as "T790M mutation"). Approved.
- Non-Patent Document 4 Mutations in which one or more amino acids are inserted into the exon 20 region (also called “exon 20 insertion mutation”) are also considered to be activation mutations in lung cancer and the like (Non-Patent Document 4), but these mutations are used.
- exon 20 insertion mutation For cancers with, it has been reported that they tend to be less sensitive to multiple existing EGFR inhibitors.
- afatinib as a clinical effect on EGFR mutation-positive lung cancer, it has been reported that the effect on exon 20 insertion mutation tends to be significantly lower than the effect on exon 19 deletion mutation and L858R mutation (Non-Patent Document 5).
- an EGFR inhibitor having an inhibitory activity against an exon 20 insertion mutation is required.
- Non-Patent Documents 6 and 7 It has also been reported that about 25 to 40% of lung cancers, about 15 to 30% of breast cancers, and a certain rate of other cancers cause brain metastasis.
- an EGFR inhibitor that has inhibitory activity against EGFR mutations and also has brain metastasis is desired.
- the present invention provides a therapeutic agent for a disease associated with EGFR, which contains a compound having EGFR inhibitory activity and brain transferability as an active ingredient.
- the present inventors have found that the compound represented by the following formula (I) or a salt thereof having pyrimidine as a basic skeleton has EGFR inhibitory activity and brain transferability, and EGFR is increased by inhibiting EGFR.
- EGFR inhibitory activity and brain transferability EGFR inhibitory activity and brain transferability
- EGFR is increased by inhibiting EGFR.
- it is useful as a therapeutic agent for related diseases (particularly malignant tumors), and have completed the present invention.
- R 1 may have a C1-C4 alkoxy groups as substituents C1-C4 alkyl group, or a C3-C4 cycloalkyl group
- R 2 indicates a C1-C6 alkyl group or a C1-C6 alkoxy group which may have 1 to 5 hydrogen atoms, halogen atoms, C1-C4 alkoxy groups or fluorine atoms as substituents, respectively
- R 3 represents a hydrogen atom or a C1-C4 alkyl group which may have 1 to 5 fluorine atoms as substituents
- R 4 represents a hydrogen atom, or a C1-C4 alkyl group
- R 5 indicates a phenyl group which may have 1 to 3 substituents selected from a fluorine atom and a chlorine atom.
- a therapeutic agent for a disease associated with EGFR which comprises a pyrimidine compound represented by (1) or a pharmaceutically acceptable salt thereof as an active ingredient.
- the pyrimidine compound has the following general formula (II).
- R 1 may have a C1-C4 alkoxy groups as substituents C1-C4 alkyl group, or a C3-C4 cycloalkyl group
- R 2 indicates a C1-C6 alkyl group or a C1-C6 alkoxy group which may have 1 to 5 hydrogen atoms, halogen atoms, C1-C4 alkoxy groups or fluorine atoms as substituents, respectively
- R 3 represents a hydrogen atom or a C1-C4 alkyl group which may have 1 to 5 fluorine atoms as substituents
- R 4 represents a hydrogen atom, or a C1-C4 alkyl group
- R 5 indicates a phenyl group which may have 1 to 3 substituents selected from
- the pyrimidine compound is contained in the general formula (I) or the general formula (II).
- R 2 is a compound which is C1-C4 alkoxy group five may have C1-C6 alkyl group from 1 as a substituent, the therapeutic agent according to [1] or [2].
- the pyrimidine compound is contained in the general formula (I) or the general formula (II).
- the pyrimidine compound is contained in the general formula (I) or the general formula (II).
- Agent. [6] The pyrimidine compound is contained in the general formula (I) or the general formula (II).
- the pyrimidine compound is contained in the general formula (I) or the general formula (II).
- R 2 is a methyl group, an ethyl group, a compound which is a methoxymethyl group, or an ethoxymethyl group, the therapeutic agent according to any one of [1] to [6].
- the pyrimidine compound is contained in the general formula (I) or the general formula (II).
- R 4 is a hydrogen atom, the therapeutic agent according to any one of [1] to [8].
- the pyrimidine compound is contained in the general formula (I) or the general formula (II).
- R 5 is a compound which is a phenyl group, the therapeutic agent according to any one of [1] to [9].
- the therapeutic agent according to any one of [1] to [10], wherein the pyrimidine compound is a compound selected from the following (1) to (3).
- Therapeutic agent [13] The therapeutic agent according to any one of [1] to [12], wherein the disease associated with EGFR is a malignant tumor having EGFR overexpression, EGFR gene amplification, or EGFR mutation.
- R 1 may have a C1-C4 alkoxy groups as substituents C1-C4 alkyl group, or a C3-C4 cycloalkyl group
- R 2 indicates a C1-C6 alkyl group or a C1-C6 alkoxy group which may have 1 to 5 hydrogen atoms, halogen atoms, C1-C4 alkoxy groups or fluorine atoms as substituents, respectively
- R 3 represents a hydrogen atom or a C1-C4 alkyl group which may have 1 to 5 fluorine atoms as substituents
- R 4 represents a hydrogen atom, or a C1-C4 alkyl group
- R 5 indicates a phenyl group which may have 1 to 3 substituents selected from a fluorine atom and a chlorine atom.
- R 1 may have a C1-C4 alkoxy groups as substituents C1-C4 alkyl group, or a C3-C4 cycloalkyl group
- R 2 indicates a C1-C6 alkyl group or a C1-C6 alkoxy group which may have 1 to 5 hydrogen atoms, halogen atoms, C1-C4 alkoxy groups or fluorine atoms as substituents, respectively
- R 3 represents a hydrogen atom or a C1-C4 alkyl group which may have 1 to 5 fluorine atoms as substituents
- R 4 represents a hydrogen atom, or a C1-C4 alkyl group
- R 5 indicates a phenyl group which may have 1 to 3 substituents selected from a fluorine atom and a chlorine atom.
- R 1 may have a C1-C4 alkoxy groups as substituents C1-C4 alkyl group, or a C3-C4 cycloalkyl group
- R 2 indicates a C1-C6 alkyl group or a C1-C6 alkoxy group which may have 1 to 5 hydrogen atoms, halogen atoms, C1-C4 alkoxy groups or fluorine atoms as substituents, respectively
- R 3 indicates a C1-C4 alkyl group which may have 1 to 5 hydrogen atoms or fluorine atoms as substituents
- R 4 represents a hydrogen atom, or a C1-C4 alkyl group
- R 5 indicates a phenyl group which may have 1 to 3 substituents selected from a fluorine atom and a chlorine
- a pharmaceutical composition for use in the treatment of diseases associated with EGFR which comprises a pyrimidine compound represented by the above or a pharmaceutically acceptable salt thereof and a pharmaceutically acceptable carrier.
- a pharmaceutical composition according to [16] wherein the disease involving EGFR is a malignant tumor having EGFR overexpression, EGFR gene amplification, or EGFR mutation.
- the present invention also relates to the following aspects.
- -Use of the compound represented by the above general formula (I) or a pharmaceutically acceptable salt thereof for producing a therapeutic agent for EGFR-positive tumor comprises administering to a subject in need thereof an effective amount of the compound represented by the above general formula (I) or a pharmaceutically acceptable salt thereof.
- Methodhod A method of treating an EGFR-positive tumor comprising administering to a subject in need thereof an effective amount of a compound represented by the above general formula (I) or a pharmaceutically acceptable salt thereof.
- -Use of the compound represented by the above general formula (I) or a pharmaceutically acceptable salt thereof for treating a malignant tumor having an exon 18 mutant EGFR -Use of the compound represented by the above general formula (I) or a pharmaceutically acceptable salt thereof for producing a therapeutic agent for a malignant tumor having an exon 18 mutant EGFR.
- a compound represented by the above general formula (I) or a pharmaceutically acceptable salt thereof for treating a malignant tumor having an exon 19 mutant EGFR a compound represented by the above general formula (I) or a pharmaceutically acceptable salt thereof for treating a malignant tumor having an exon 19 mutant EGFR.
- -Use of the compound represented by the above general formula (I) or a pharmaceutically acceptable salt thereof for treating a malignant tumor having an exon 19 mutant EGFR -Use of the compound represented by the above general formula (I) or a pharmaceutically acceptable salt thereof for producing a therapeutic agent for a malignant tumor having an exon 19 mutant EGFR.
- a compound represented by the above general formula (I) or a pharmaceutically acceptable salt thereof for treating a malignant tumor having an exon 20 mutant EGFR a pharmaceutically acceptable salt thereof for treating a malignant tumor having an exon 20 mutant EGFR.
- a compound represented by the above general formula (I) or a pharmaceutically acceptable salt thereof for treating a malignant tumor having an exon 20 mutant EGFR.
- -A method for treating a malignant tumor having an exon 20 mutant EGFR, and an effective amount of the compound represented by the above general formula (I) or a pharmaceutically acceptable salt thereof is administered to a subject who needs it. Methods, including doing.
- -Use of the compound represented by the above general formula (I) or a pharmaceutically acceptable salt thereof for treating a malignant tumor having an exon 21 mutant EGFR -Use of the compound represented by the above general formula (I) or a pharmaceutically acceptable salt thereof for producing a therapeutic agent for a malignant tumor having an exon 21 mutant EGFR.
- a compound represented by the above general formula (I) or a pharmaceutically acceptable salt thereof for treating an existing EGFR inhibitor-resistant malignant tumor a compound represented by the above general formula (I) or a pharmaceutically acceptable salt thereof for treating an existing EGFR inhibitor-resistant malignant tumor.
- -Use of the compound represented by the above general formula (I) or a pharmaceutically acceptable salt thereof for treating an existing EGFR inhibitor-resistant malignant tumor -Use of the compound represented by the above general formula (I) or a pharmaceutically acceptable salt thereof for producing a therapeutic agent for an existing EGFR inhibitor-resistant malignant tumor.
- a new therapeutic method for treating an EGFR-related disease or an EGFR-positive tumor is provided.
- the compound of the present invention or a salt thereof has excellent inhibitory activity against wild-type EGFR and mutant EGFR, and has excellent brain migration, antitumor effect against malignant tumors, and prolonging survival effect.
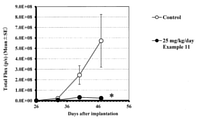
- FIG. 1 shows the antitumor effect of the compounds of Examples 2, 11 and 12 on a subcutaneous transplant model of the H1975-EGFRins SVD cell line.
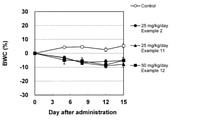
- FIG. 2 shows the rate of change in body weight of mice when the compounds of Examples 2, 11 and 12 were administered to a subcutaneous transplant model of the H1975-EGFRins SVD cell line.
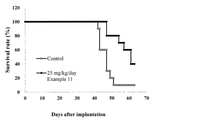
- FIG. 3 shows the antitumor effect of the Luciferase gene-introduced exon 20 insertion mutant EGFR-expressing cell line (H1975-EGFRinsSVD-Luc) of Example 11 on a direct brain transplant model.
- FIG. 1 shows the antitumor effect of the compounds of Examples 2, 11 and 12 on a subcutaneous transplant model of the H1975-EGFRins SVD cell line.
- FIG. 2 shows the rate of change in body weight of mice when the compounds of Examples 2, 11 and 12 were administered to a subcutaneous transplant model of the H1975-EGFRins SVD cell line.
- FIG. 3 shows the
- Example 4 shows the survival rate of mice when the compound of Example 11 was administered to a direct brain transplantation model of a Luciferase gene-introduced exon 20-inserted mutant EGFR-expressing cell line (H1975-EGFRinsSVD-Luc).
- One embodiment of the present invention has the following general formula (I). With respect to a compound represented by or a salt thereof. (In the formula, R 1 to R 5 are as defined above.)
- a preferred embodiment of the present invention is a pyrimidine compound represented by the following general formula (II) or a salt thereof. (In the formula, R 1 to R 5 are as defined above.)
- the compound represented by the above general formula (I) or general formula (II) of the present invention is a compound having a basic structure of pyrrolo [2,3-d] pyrimidine, and is also described in any of the above-mentioned prior art documents and the like. It is a novel compound not described.
- halogen atom examples include a chlorine atom, a bromine atom, a fluorine atom, and an iodine atom, preferably a chlorine atom and a fluorine atom, and more preferably a fluorine atom.
- alkyl group refers to a linear or branched saturated hydrocarbon group, specifically, a methyl group, an ethyl group, an n-propyl group, an isopropyl group, an n-butyl group, and the like. Examples thereof include isobutyl group, sec-butyl group, tert-butyl group, pentyl group, hexyl group and the like, preferably a linear or branched alkyl group having 1 to 4 carbon atoms, and more preferably a methyl group. It is a tert-butyl group.
- haloalkyl group refers to a group in which one to all hydrogen atoms are substituted with the above-mentioned halogen atom among linear or branched saturated hydrocarbon groups, and specifically. Is a monofluoromethyl group, a difluoromethyl group, a trifluoromethyl group, a 1-fluoroethyl group, a 2-fluoroethyl group, a 1,1-difluoroethyl group, a 1,2-difluoroethyl group, a 2,2-difluoroethyl group.
- 2,2,2-trifluoroethyl group monochloromethyl group, dichloromethyl group, trichloromethyl group, 1-chloroethyl group, 2-chloroethyl group, 1,1-dichloroethyl group and the like, preferably the number of carbon atoms.
- 1 to 6 linear or branched saturated hydrocarbon groups 1 to 3 hydrogen atoms are substituted with the above halogen atoms, and more preferably a monofluoromethyl group.
- cycloalkyl group refers to a monocyclic or polycyclic saturated hydrocarbon group having 3 to 7 carbon atoms, and specifically, a cyclopropyl group, a cyclobutyl group, a cyclopentyl group, or a cyclohexyl group. , Cycloheptyl group and the like, preferably a cyclopropyl group and a cyclobutyl group.
- the "aromatic hydrocarbon group” is a cyclic substituent composed of carbon and hydrogen having an unsaturated bond, and has 4e + 2 (e is an integer of 1 or more) in the cyclic ⁇ -electron system. Indicates a substituent containing an electron.
- C6-C14 aromatic hydrocarbon group refers to a monocyclic or polycyclic aromatic hydrocarbon group having 6 to 14 carbon atoms, specifically, a phenyl group, a naphthyl group, and the like. Examples thereof include a tetrahydronaphthyl group and an anthrasenyl group, and a phenyl group is preferable.
- aralkyl group refers to the alkyl group substituted with the aromatic hydrocarbon group, and specifically, a benzyl group, a phenylethyl group, a phenylpropyl group, a naphthylmethyl group and a naphthylethyl group.
- a benzyl group such as C7-C16 aralkyl group is mentioned, preferably a benzyl group.
- the term "unsaturated hydrocarbon group” refers to a linear or branched hydrocarbon group having 2 to 6 carbon atoms including at least one carbon-carbon double bond or triple bond.
- examples thereof include a vinyl group, an allyl group, a methyl vinyl group, a propenyl group, a butenyl group, a pentenyl group, a hexenyl group, an ethynyl group, a 2-propynyl group and the like, and a vinyl group, an allyl group and a 1-propenyl group are preferable.
- alkenyl group refers to a linear or branched hydrocarbon group having 2 to 6 carbon atoms containing at least one carbon-carbon double bond, specifically a vinyl group. Allyl group 2-methyl-2-propenyl group, isopropenyl group, 1-, 2- or 3-butenyl group, 2-, 3- or 4-pentenyl group, 2-methyl-2-butenyl group, 3-methyl- Examples thereof include a C2-C6 alkenyl group such as a 2-butenyl group and a 5-hexenyl group, preferably a vinyl group, an allyl group, a 1-propenyl group and a 2-methyl-2-propenyl group.
- alkynyl group refers to a linear or branched unsaturated hydrocarbon group having at least one triple bond (for example, 1 to 2, preferably 1). Examples thereof include C2-C6 alkynyl groups such as ethynyl group, 1- or 2-propynyl group, 1-, 2- or 3-butynyl group, 1-methyl-2-propynyl group, and preferably ethynyl group and 2 -Propynyl group.
- C3-C10 cyclic unsaturated hydrocarbon group refers to a monocyclic or polycyclic hydrocarbon group having at least one carbon-carbon double bond and having 3 to 10 carbon atoms. Specific examples thereof include a cyclopropenyl group, a cyclobutenyl group, a cyclopentenyl group, a cyclohexenyl group, a cycloheptenyl group, a cyclooctenyl group, a cyclononyl group and the like, preferably having 3 to 7 carbon atoms containing at least one carbon-carbon double bond. It is a monocyclic or polycyclic hydrocarbon group, more preferably a cyclopropenyl group.
- alkoxy group refers to the oxy group having an alkyl group, specifically, a methoxy group, an ethoxy group, an n-propoxy group, an isopropoxy group, an n-butoxy group, an isobutoxy group, sec. -C1-C6 alkoxy groups such as a butoxy group, a tert-butoxy group, a pentyloxy group, an isopentyloxy group and a hexyloxy group are mentioned, preferably a methoxy group and an ethoxy group, and more preferably a methoxy group.
- haloalkoxy group refers to the alkoxy group having at least one (preferably 1 to 13, more preferably 1 to 3) halogen atoms, and specifically, a fluoromethoxy group or a difluoro.
- cycloalkoxy group refers to the oxy group having the cycloalkyl group, and specifically, C3 such as a cyclopropoxy group, a cyclobutoxy group, a cyclopentyloxy group, a cyclohexyloxy group and a cycloheptyloxy group.
- C3 such as a cyclopropoxy group, a cyclobutoxy group, a cyclopentyloxy group, a cyclohexyloxy group and a cycloheptyloxy group.
- -C7 cycloalkoxy group is mentioned, preferably cyclobutoxy group, cyclopentyloxy group, cyclohexyloxy group.
- the "aralkyloxy group” refers to the oxygroup having the aralkyl group, and specifically, C7-C20 such as a benzyloxy group, a phenethyloxy group, a naphthylmethyloxy group, and a fluorenylmethyloxy group. Examples thereof include an aralkyloxy group, preferably a benzyloxy group.
- alkylthio group refers to a thioxy group having the above alkyl group, and specifically, a methylthio group, an ethylthio group, an n-propylthio group, an isopropylthio group, an n-butylthio group, an isobutylthio group, a tert.
- Examples thereof include C1-C6 alkylthio groups such as a butylthio group, an n-pentylthio group, an isopentylthio group and a hexylthio group, preferably a methylthio group, an ethylthio group and an n-propylthio group.
- alkoxyalkyl group refers to the alkyl group having at least one alkoxy group, and specifically, C1-C6 such as a methoxymethyl group, an ethoxyethyl group, a methoxyethyl group, and a methoxypropyl group.
- C1-C6 such as a methoxymethyl group, an ethoxyethyl group, a methoxyethyl group, and a methoxypropyl group.
- Alkoxy-C1-C6 alkyl groups can be mentioned.
- alkylamino group refers to an amino group substituted with a linear or branched hydrocarbon group having 1 to 6 carbon atoms instead of one or two hydrogen atoms. Specific examples thereof include a methylamino group, an ethylamino group, a dimethylamino group, a diethylamino group, an ethylmethylamino group and the like, and preferably a linear chain having 1 to 3 carbon atoms instead of one or two hydrogen atoms. Alternatively, it is an amino group substituted with an amino group substituted with a branched hydrocarbon group.
- the term "monoalkylamino group” refers to an amino group substituted with a linear or branched hydrocarbon group instead of a single hydrogen atom, and specifically, a methylamino group. Ethylamino group, n-propylamino group, isopropylamino group, n-butylamino group, isobutylamino group, sec-butylamino group, tert-butylamino group, pentylamino group, hexylamino group and the like are preferable. It is an amino group substituted with a linear or branched hydrocarbon group having 1 to 3 carbon atoms instead of one hydrogen atom.
- dialkylamino group refers to an amino group substituted with a linear or branched hydrocarbon group having 1 to 6 carbon atoms instead of two hydrogen atoms, and specifically, examples thereof include a dimethylamino group, a diethylamino group, an ethylmethylamino group, and the like, preferably an amino group substituted with a linear or branched hydrocarbon group having 1 to 3 carbon atoms instead of two hydrogen atoms. Yes, more preferably a dimethylamino group.
- acyl group refers to a formyl group substituted with a linear or branched hydrocarbon group instead of a hydrogen atom, and specifically, an acetyl group, an n-propanoyl group, or an isopro. Examples thereof include a panoyl group, an n-butyroyl group, a tert-butyroyl group and the like, preferably a group substituted with a linear or branched hydrocarbon group having 1 to 3 carbon atoms instead of the hydrogen atom of the formyl group. And more preferably an acetyl group.
- acyloxy group indicates the oxy group having the acyl group, and specific examples thereof include an alkylcarbonyloxy group and an arylcarbonyloxy group, preferably having 1 carbon atom instead of the hydrogen atom of the formyl group. It is an oxy group substituted with 3 linear or branched hydrocarbon groups, preferably an alkylcarbonyloxy group.
- alkoxycarbonyl group refers to a carbonyl group having the alkoxy group, specifically, a methoxycarbonyl group, an ethoxycarbonyl group, a propoxycarbonyl group, an isopropoxycarbonyl group, a butoxycarbonyl group, or an isobutoxycarbonyl group.
- Examples thereof include (C1-C6 alkoxy) carbonyl groups such as a group, a tert-butoxycarbonyl group, a pentyloxycarbonyl group, an isopentyloxycarbonyl group and a hexyloxycarbonyl group, preferably a tert-butoxycarbonyl group.
- the "aralkyloxycarbonyl group” refers to the carbonyl group having aralkyloxy, specifically, a benzyloxycarbonyl group, a phenethyloxycarbonyl group, a naphthylmethyloxycarbonyl group, a fluorenylmethyloxycarbonyl group.
- (C6-C20 aralkyl) such as (C6-C20 aralkyl) oxycarbonyl group is mentioned, and benzyloxycarbonyl group is preferable.
- the "saturated heterocyclic group” is a monocycle having at least one heteroatom (preferably 1 to 5, more preferably 1 to 3) selected from a nitrogen atom, an oxygen atom and a sulfur atom.
- Examples thereof include a phenyl group, a thiazolidinyl group, an oxazolidinyl group and the like, preferably an azetidinyl group, a pyrrolidinyl group and a piperidinyl group, and more preferably an azetidinyl group and a pyrrolidinyl group.
- the "unsaturated heterocyclic group” is simply a group having at least one heteroatom (preferably 1 to 5, more preferably 1 to 3) selected from a nitrogen atom, an oxygen atom and a sulfur atom. Shows a cyclic or polycyclic completely unsaturated or partially unsaturated heterocyclic group, specifically, an imidazolyl group, a thienyl group, a pyrrolyl group, an oxazolyl group, an isoxazolyl group, a thiazolyl group, an isothiazolyl group, a thiadiazolyl group, an oxadiazolyl group.
- pyrazolyl group triazolyl group, tetrazolyl group, pyridyl group, pyrazil group, pyrimidinyl group, pyridazinyl group, indolyl group, isoindrill group, indazolyl group, triazolopyridyl group, benzoimidazolyl group, benzoxazolyl group, benzothiazolyl group, benzo Examples thereof include a thienyl group, a furanyl group, a benzofuranyl group, a prynyl group, a quinolyl group, an isoquinolyl group, a quinazolinyl group, a quinoxalyl group, a methylenedioxyphenyl group, an ethylenedioxyphenyl group and a dihydrobenzofuranyl group, and an imidazolyl group is preferable.
- the "saturated heterocyclic oxy group” refers to the oxy group having the saturated heterocyclic group, and specifically, morpholinyloxy group, 1-pyrrolidinyloxy group, piperidinooxy group, piperazinyl.
- examples thereof include an oxy group, a 4-methyl-1-piperazinyloxy group, a tetrahydrofuranyloxy group, a tetrahydropyranyloxy group, a tetrahydrothiophenyloxy group, a thiazolidinyloxy group, an oxazolidinyloxy group, and the like. It is preferably a 1-pyrrolidinyloxy group, a piperidinooxy group, or a piperazinyloxy group.
- R 1 may have a C1-C4 alkoxy group as a substituent, or a C1-C4 alkyl group or a C3-C4 cyclo. It is an alkyl group.
- C1-C4 alkoxy group in the "may C1-C4 alkyl group which may have a C1-C4 alkoxy group as a substituent” represented by R 1, preferably methoxy group or ethoxy group, and most preferably Is a methoxy group.
- R 1 preferably methoxy group or ethoxy group, and most preferably Is a methoxy group.
- the number of substituents is preferably 1 to 3, and most preferably 1.
- the substituents may be the same or different.
- C1-C4 alkyl group of the “may C1-C4 alkyl group which may have a C1-C4 alkoxy group as a substituent" represented by R 1, preferably a methyl group, an ethyl group, n- propyl group, It is an isopropyl group or a tert-butyl group, more preferably a methyl group, an ethyl group, an isopropyl group or a tert-butyl group, and most preferably a methyl group or a tert-butyl group.
- C1-C4 alkyl group which may have a C1-C4 alkoxy group as a substituent for R 1, preferably three have been or C1-C4 alkyl from 1 methoxy group as a substituent It is a group, more preferably a methyl group, an ethyl group, an isopropyl group, a tert-butyl group, or a 1-methyl-1-methoxyethyl group, and most preferably a methyl group or a tert-butyl group.
- C3-C4 cycloalkyl group represented by R 1 is preferably a cyclopropyl group or cyclobutyl group, and most preferably a cyclopropyl group.
- R 1 is preferably, may have three of C1-C4 alkoxy group from 1 as a substituent a C1-C4 alkyl group, or C3-C4 cycloalkyl group.
- R 1 is more preferably a C1-C4 alkyl group or a C3-C4 cycloalkyl group which may have 1 to 3 methoxy groups as substituents.
- R 1 is more preferably a methyl group, an ethyl group, an isopropyl group, a tert-butyl group, a 1-methyl-1-methoxyethyl group, or a cyclopropyl group.
- R 1 is most preferably a methyl group, a tert-butyl group, or a cyclopropyl group.
- R 2 has 1 to 5 hydrogen atoms, halogen atoms, and 1 to 5 C1-C4 alkoxy groups or fluorine atoms as substituents, respectively. It may be a C1-C6 alkyl group or a C1-C6 alkoxy group.
- halogen atom is preferably a fluorine atom or a chlorine atom.
- C1-C4 alkoxy group of the "C1-C4 alkoxy group or a fluorine atom may C1-C6 alkyl group which may have five from each 1 as a substituent" represented by R 2 is preferably a methoxy group, It is an ethoxy group, most preferably a methoxy group.
- C1-C6 alkyl group of the "C1-C4 alkoxy group or a fluorine atom may be a have from one to five, respectively C1-C6 alkyl group as a substituent" represented by R 2 is preferably a methyl group, It is an ethyl group, an n-propyl group, an isopropyl group, or a tert-butyl group, and most preferably a methyl group.
- C1-C4 alkoxy group or a fluorine atom may C1-C6 alkyl group which may have five from each 1 as a substituent" represented by R 2 is preferably a methoxy group as a substituent group, an ethoxy group or a fluorine atom C1-C6 alkyl group (specifically, methyl group, methoxymethyl group, ethoxymethyl group, methoxyethyl group, ethoxyethyl group, fluoromethyl group, difluoromethyl group, tri) which may have 1 to 5 of each.
- Fluoromethyl group, etc. more preferably a C1-C6 alkyl group, more preferably a methyl group, an ethyl group, an n-propyl group, an isopropyl group, or a tert-butyl group, and most preferably a methyl group.
- C1-C6 alkoxy group represented by R 2 is preferably a methoxy group, or ethoxy group, most preferably a methoxy group.
- R 2 is preferably a substituent as C1-C4 alkoxy group or a fluorine atom five from 1 has been or C1-C6 alkyl group.
- R 2 is a C1-C6 alkyl group which may have 1 to 5 methoxy groups, ethoxy groups or fluorine atoms as substituents, respectively.
- R 2 may have 1 to 5 methoxy, ethoxy or fluorine atoms as substituents, respectively, from 1 to 5 methyl, ethyl, n-propyl, isopropyl or tert-butyl groups. (Preferably a methyl group or an ethyl group, more preferably a methyl group).
- R 2 is more preferably a C1-C4 alkoxy group from 1 to 5 have been or C1-C6 alkyl group as a substituent.
- R 2 is a C1-C6 alkyl group which may have 1 to 5 methoxy groups and 1 to 5 ethoxy groups as substituents, respectively.
- R 2 may have 1 to 5 methoxy or ethoxy groups as substituents, respectively, from 1 to 5 methyl groups, ethyl groups, n-propyl groups, isopropyl groups, or tert-butyl groups (preferably). , Methyl group or ethyl group, more preferably methyl group).
- R 2 is a methyl group, an ethyl group, a methoxymethyl group, or an ethoxymethyl group.
- R 2 is even more preferably at C1-C6 alkyl group.
- R 2 is more preferably methyl group, ethyl group, n- propyl group, an isopropyl group, or a tert- butyl group.
- R 2 is particularly preferably a methyl group or an ethyl group.
- R 2 is most preferably a methyl group.
- R 3 is a hydrogen atom, is from one to five have been or C1-C4 alkyl group and a fluorine atom as a substituent ..
- C1-C4 alkyl group of the "fluorine atom from one to five have been or C1-C4 alkyl group optionally as substituents" represented by R 3, preferably a methyl group, an ethyl group, n- propyl group , An isopropyl group, or a tert-butyl group, more preferably a methyl group or an ethyl group, and most preferably a methyl group.
- C1-C4 alkyl group from 1 fluorine atom as a substituent represented by R 3, preferably a methyl group, fluoromethyl group, difluoromethyl group, trifluoromethyl group, or an ethyl It is a group, more preferably a methyl group, a trifluoromethyl group, or an ethyl group, and most preferably a methyl group.
- R 3 is preferably, it may have from one to five fluorine atoms as a substituent a C1-C4 alkyl group.
- R 3 is more preferably a methyl group, a fluoromethyl group, a difluoromethyl group, a trifluoromethyl group, an ethyl group, a fluoroethyl group, a difluoroethyl group, a trifluoroethyl group, an n-propyl group, an isopropyl group, or a tert.
- -It is a butyl group.
- R 3 is more preferably a methyl group, fluoromethyl group, difluoromethyl group, trifluoromethyl group, or an ethyl group.
- R 3 is more preferably a methyl group, a trifluoromethyl group, or an ethyl group.
- R 3 is particularly preferably a methyl group or an ethyl group.
- R 3 is most preferably a methyl group.
- R 4 is a hydrogen atom, or a C1-C4 alkyl group.
- C1-C4 alkyl group represented by R 4 is preferably a methyl group, an ethyl group, an n- propyl group, an isopropyl group, or a tert- butyl group, more preferably a methyl group, or an ethyl group , Most preferably a methyl group.
- R 4 is preferably a hydrogen atom, a methyl group, an ethyl group, n- propyl group, an isopropyl group, or a tert- butyl group.
- R 4 is more preferably hydrogen atom, methyl group, or ethyl group.
- R 4 is more preferably a hydrogen atom, or a methyl group. R 4 is most preferably hydrogen atom.
- R 5 is 3 has may be a phenyl substituent from 1 selected from the group consisting of fluorine and chlorine atoms It is the basis.
- R 5 is preferably a phenyl group which may have one or two substituents selected from the group consisting of fluorine atoms and chlorine atoms.
- R 5 is more preferably a phenyl group, 2-fluorophenyl group, a 3-chlorophenyl group, 2,3-difluorophenyl group, 2,4-difluorophenyl group, or 3,5-difluorophenyl group.
- R 5 is most preferably a phenyl group.
- R 1 may have a C1-C4 alkoxy group as a substituent, or a C1-C4 alkyl group or a C3-C4 cycloalkyl group.
- R 2 is C1-C4 alkoxy group five may have C1-C6 alkyl group from 1 as a substituent
- R 3 is a C1-C4 alkyl group which may have 1 to 5 fluorine atoms as a substituent.
- R 4 is hydrogen atom, or a C1-C4 alkyl group
- R 5 is, have one or two substituents selected from the group consisting of fluorine atom and chlorine atom is also a phenyl group, the compound or a salt thereof.
- R 1 is a methyl group, an ethyl group, an n-propyl group, an isopropyl group, a tert-butyl group, a 1-methyl-1-methoxyethyl group, It is a cyclopropyl group or a cyclobutyl group
- R 2 is a methyl group, an ethyl group, an n- propyl group, tert- butyl group, a methoxymethyl group, or an ethoxymethyl group
- R 3 is a methyl group, a fluoromethyl group, a difluoromethyl group, a trifluoromethyl group, an ethyl group, a fluoroethyl group, a difluoroethyl group, a trifluoroethyl group, an n-propyl group, an isopropyl group, or a tert-
- R 4 is a hydrogen atom, a methyl group, an ethyl group, n- propyl group, an isopropyl group, a tert- butyl group
- R 5 is phenyl, 2-fluorophenyl group, 3-fluorophenyl group, 2,4-difluorophenyl group, 2,3-difluorophenyl group, 3,5-difluorophenyl group, 2-chlorophenyl group, 3- A compound or salt thereof, which is a chlorophenyl group, a 2,4-dichlorophenyl group, or a 3,5-dichlorophenyl group.
- R 1 is a methyl group, an ethyl group, an isopropyl group, a tert-butyl group, a 1-methyl-1-methoxyethyl group, or a cyclopropyl group.
- R 2 is a methyl group, an ethyl group, a methoxymethyl group, or an ethoxymethyl group.
- R 3 is a methyl group, a fluoromethyl group, a difluoromethyl group, a trifluoromethyl group, or an ethyl group.
- R 4 is a hydrogen atom, a methyl group, or ethyl group
- R 5 is phenyl, 2-fluorophenyl group, a 3-chlorophenyl group, 2,3-difluorophenyl group, 2,4-difluorophenyl group, or a 3,5-difluorophenyl group, a compound or a salt thereof be.
- R 1 is a methyl group, a tert-butyl group, or a cyclopropyl group.
- R 2 is a methyl group, an ethyl group, a methoxymethyl group, or an ethoxymethyl group.
- R 3 is a methyl group, a trifluoromethyl group, or an ethyl group.
- R 4 is hydrogen atom, or a methyl group,
- R 5 is a phenyl group, a compound or a salt thereof.
- R 1 is a methyl group, a tert-butyl group, or a cyclopropyl group.
- R 2 is a methyl group, an ethyl group, or a methoxymethyl group.
- R 3 is a methyl group
- R 4 is hydrogen atom
- R 5 is a phenyl group, a compound or a salt thereof.
- R 1 is a methyl group, a tert-butyl group, or a cyclopropyl group.
- R 2 is a methyl group
- R 3 is a methyl group
- R 4 is hydrogen atom
- R 5 is a phenyl group, a compound or a salt thereof.
- One embodiment of the present invention is a compound selected from the following (1) to (19), or a salt thereof.
- One embodiment of the present invention is a compound selected from the following (1) to (15), or a salt thereof.
- (1) 7-((3R, 5S) -1-acryloyl-5-methylpyrrolidin-3-yl) -4-amino-N-((R) -1-phenylethyl) -6- (prop-1-) In-1-yl) -7H-pyrrolo [2,3-d] pyrimidin-5-carboxamide
- (3) 7-((3R, 5S)- 1-acryloyl-5-methylpyr
- a compound selected from the following (1) to (3) or a salt thereof can be exemplified.
- the most suitable pyrimidine compound in the present invention is 7-((3R, 5S) -1-acryloyl-5-methylpyrrolidine-3-yl) -4-amino-6- (cyclopropylethynyl) -N-((R). ) -1-Phenylethyl) -7H-pyrrolo [2,3-d] pyrimidine-5-carboxamide.
- the compound according to the present invention can be produced, for example, by the following production method or the method shown in Examples. However, the method for producing a compound according to the present invention is not limited to these examples.
- the compounds (I) and (II) of the present invention can be produced, for example, by using the following production methods.
- ⁇ Manufacturing method> [In the formula, L 1 , L 2 , L 3 indicate the same or different leaving group, P 1 , P 2 indicate the same or different protecting group, and other symbols are synonymous with the above. ]
- This step is a method for obtaining a compound represented by the formula 3 by a Mitsunobu reaction between the compound represented by the formula 1 and a compound represented by the formula 2 which can be commercially available or can be produced by a known method.
- the Mitsunobu reaction is usually carried out in the presence of Mitsunobu and phosphine reagents.
- the amount of the compound represented by the formula 2 (P 1 wherein 2 represents a protecting group of amino group), the compound of formula 1 for (1 mole), can be used at 1 to 10 equivalents , Preferably 1 to 3 equivalents.
- the "protecting group for the amino group” is not particularly limited as long as it has the function, but for example, a benzyl group, a p-methoxybenzyl group, a 3,4-dimethoxybenzyl group, an o-nitrobenzyl group, a p-nitro.
- Aralkyl groups such as benzyl group, benzhydryl group, trityl group, cumyl group; for example, lower alkanoyl group such as formyl group, acetyl group, propionyl group, butyryl group, pivaloyl group, trifluoroacetyl group, trichloroacetyl group; for example, benzoyl group;
- an arylalkanoyl group such as a phenylacetyl group and a phenoxyacetyl group
- a lower alkoxycarbonyl group such as a methoxycarbonyl group, an ethoxycarbonyl group, a propyloxycarbonyl group and a tert-butoxycarbonyl group
- a p-nitrobenzyloxycarbonyl group and a phenethyl group for example, a p-nitrobenzyloxycarbonyl group and a phenethyl group.
- Aralkyloxycarbonyl groups such as oxycarbonyl groups; lower alkylsilyl groups such as trimethylsilyl group, tert-butyldimethylsilyl group; eg tetrahydropyranyl group; eg trimethylsilylethoxymethyl group; eg methylsulfonyl group, ethylsulfonyl group, tert- Lower alkylsulfonyl groups such as butylsulfonyl groups; for example lower alkylsulfinyl groups such as tert-butylsulfinyl groups; eg arylsulfonyl groups such as benzenesulfonyl groups and toluenesulfonyl groups, for example imide groups such as phthalimide groups.
- a trifluoroacetyl group an acetyl group, a tert-butoxycarbonyl group, a benzyloxycarbonyl group, a trimethylsilylethoxymethyl group, or a cumyl group is preferable.
- the amount of the Mitsunobu reagent used is usually about 1 to 100 mol, preferably about 1 to 10 mol, with respect to the compound (1 mol) represented by the formula 1.
- phosphine reagent triphenylphosphine, tributylphosphine, trifurylphosphine and the like are used.
- the amount of the phosphine reagent used is usually about 1 to 100 mol, preferably about 1 to 10 mol, with respect to the compound (1 mol) represented by the formula 1.
- the solvent may be any solvent as long as it does not adversely affect the reaction.
- hydrocarbons eg, benzene, toluene, xylene, etc.
- halogenated hydrocarbons eg, chloroform, 1,2-dichloroethane, etc.
- Nitriles eg, acetonitrile, etc.
- ethers eg, dimethoxyethane, hydrocarbon, etc.
- alcohols eg, methanol, ethanol, etc.
- aprotic polar solvents eg, N, N-dimethylformamide, dimethylsulfoxide, etc.
- Hexamethylphosphoramide etc. water or a mixture thereof and the like.
- the reaction time is 0.1 to 100 hours, preferably 0.5 to 24 hours.
- the reaction temperature is 0 ° C. to the boiling temperature of the solvent, preferably 0 ° C. to 100 ° C.
- the compound represented by the formula 3 thus obtained can be isolated and purified by a known separation and purification means, or can be subjected to the next step without isolation and purification.
- This step is a method for obtaining a compound represented by the formula 4 by reacting the compound represented by the formula 3 with ammonia or a salt thereof.
- the amount used can be 1 to 1000 equivalents, preferably 1 to 100 equivalents, relative to the compound (1 mol) represented by the formula 3.
- the solvent may be any solvent as long as it does not adversely affect the reaction.
- hydrocarbons eg, benzene, toluene, xylene, etc.
- halogenated hydrocarbons eg, chloroform, 1,2-dichloroethane, etc.
- Nitriles eg, acetonitrile, etc.
- ethers eg, dimethoxyethane, hydrocarbon, etc.
- alcohols eg, methanol, ethanol, etc.
- aprotic polar solvents eg, N, N-dimethylformamide, dimethylsulfoxide, etc.
- Hexamethylphosphoramide etc. water or a mixture thereof and the like.
- the reaction time is 0.1 to 100 hours, preferably 0.5 to 24 hours.
- the reaction temperature is 0 ° C. to the boiling temperature of the solvent, preferably 0 ° C. to 150 ° C.
- the compound represented by the formula 4 thus obtained can be isolated and purified by a known separation and purification means, or can be subjected to the next step without isolation and purification.
- This step is a method for obtaining a compound represented by the formula 5 in a carbon monoxide atmosphere, for example, in the presence of a transition metal catalyst, a base and an alcohol.
- the pressure of carbon monoxide is usually 1 atm to 20 atm, preferably 1 to 10 atm.
- Examples of the alcohol include methanol, ethanol, propanol, isopropanol, diethylaminoethanol, isobutanol, 4- (2-hydroxyethyl) morpholine, 3-morpholinopropanol, diethylaminopropanol and the like.
- the amount of alcohol used is usually 1 to 100 mol, preferably about 1 to 50 mol, with respect to the compound (1 mol) represented by the formula 4.
- a palladium catalyst eg, palladium acetate, palladium chloride, tetrakistriphenylphosphine palladium, palladium carbon, etc.
- a ligand eg, triphenylphosphine, tri-tert
- -Butylphosphine, etc. may be added and used.
- the amount of the transition metal catalyst used varies depending on the type of catalyst, but is usually about 0.0001 to 1 mol, preferably about 0.01 to 0.5 mol, with respect to compound 4 (1 mol), and the use of a ligand is used. The amount is usually about 0.0001 to 4 mol, preferably about 0.01 to 2 mol, relative to the compound (1 mol) represented by the formula 4.
- Examples of the base include organic amines (eg, trimethylamine, triethylamine, diisopropylethylamine, N-methylmorpholin, 1,8-diazapicyclo [5,4,0] undec-7-ene, pyridine, N, N-dimethylaniline).
- organic amines eg, trimethylamine, triethylamine, diisopropylethylamine, N-methylmorpholin, 1,8-diazapicyclo [5,4,0] undec-7-ene, pyridine, N, N-dimethylaniline).
- Alkali metal salts eg, sodium hydrogen carbonate, potassium hydrogen carbonate, sodium carbonate, potassium carbonate, cesium carbonate, sodium phosphate, potassium phosphate, sodium hydroxide, potassium hydroxide, etc.
- metal hydrides eg, potassium hydroxide, etc.
- alkali metal alkoxides eg, sodium methoxydo, sodium ethoxydo, sodium-tert-butoxide, potassium-tert-butoxide, etc.
- alkali metal disilazide eg, lithium disiradide, sodium, etc.
- alkali metal salts such as potassium carbonate, cesium carbonate, sodium phosphate and potassium phosphate
- alkali metal alkoxides such as sodium-tert-butoxide and potassium-tert-butoxide
- organic amines such as triethylamine and diisopropylethylamine are used. Suitable.
- the amount of the base used is usually about 0.1 to 50 mol, preferably about 1 to 20 mol, with respect to the compound (1 mol) represented by the formula 4.
- the solvent may be any solvent as long as it does not adversely affect the reaction.
- hydrocarbons eg, benzene, toluene, xylene, etc.
- halogenated hydrocarbons eg, chloroform, 1,2-dichloroethane, etc.
- Nitriles eg, acetonitrile, etc.
- ethers eg, dimethoxyethane, tetrahydrofuran, etc.
- alcohols eg, methanol, ethanol, etc.
- aprotic polar solvents eg, N, N-dimethylformamide, dimethyl sulfoxide, etc.
- Hexamethylphosphoramide, N-methylpyrrolidone, etc. water or a mixture thereof and the like can be mentioned.
- the reaction time is 0.1 to 100 hours, preferably 0.5 to 24 hours.
- the reaction temperature is 0 ° C. to the boiling temperature of the solvent, preferably 0 ° C. to 150 ° C.
- ester compound corresponding to the alcohol used or the mixture of the ester compound and the compound represented by the formula 5 is hydrolyzed to convert it into the compound represented by the formula 5. I can do things.
- sodium hydrogen carbonate, sodium carbonate, potassium carbonate, cesium carbonate, sodium hydroxide, potassium hydroxide, lithium hydroxide and the like are preferably used, and the amount used is relative to the compound (1 mol) represented by the formula 4. It is usually about 0.5 to 100 mol, preferably about 1 to 10 mol.
- the solvent may be any solvent as long as it does not adversely affect the reaction, and for example, water, methanol, ethanol, isopropanol, tetrahydrofuran, 1,4-dioxane, N, N-dimethylformamide and the like may be used alone or in combination. can.
- the reaction time is 0.1 to 100 hours, preferably 0.5 to 24 hours.
- the reaction temperature is 0 ° C. to the boiling temperature of the solvent, preferably 0 ° C. to 100 ° C.
- the compound represented by the formula 5 thus obtained can be isolated and purified by a known separation and purification means, or can be subjected to the next step without isolation and purification.
- This step is to introduce a protecting group into the carboxyl group of the compound represented by Formula 5, it is a method of obtaining the compound represented by the formula 6 (wherein 6 P 2 represents a protecting group of a carboxyl group).
- 6 P 2 represents a protecting group of a carboxyl group.
- a method of protection a commonly known method, for example, Protective Groups in Organic Synthesis third edition, T.I. W. Greene and P. G. M. It can be carried out by the method described in Wuts, John Wiley & Sons (1999), or a method similar thereto.
- the "protecting group for the carboxyl group” is not particularly limited as long as it has the function, but is, for example, a lower alkyl group such as a methyl group, an ethyl group, a propyl group, an isopropyl group, a tert-butyl group; for example, 2,2. , 2-Halo lower alkyl group such as trichloroethyl group; for example lower alkenyl group such as allyl group; for example trimethylsilylethoxymethyl group; for example benzyl group, p-methoxybenzyl group, p-nitrobenzyl group, benzhydryl group, trityl group and the like.
- a lower alkyl group such as a methyl group, an ethyl group, a propyl group, an isopropyl group, a tert-butyl group
- 2-Halo lower alkyl group such as trichloroethyl group
- Examples thereof include the aralkyl group of the above, and a methyl group, an ethyl group, a tert-butyl group, an allyl group, a benzyl group, a p-methoxybenzyl group, or a trimethylsilylethoxymethyl group is particularly preferable.
- a protecting group such as a tert-butyl ester group, a methyl ester group or an ethyl ester group.
- Examples of the protective base agent for this reaction include 2-tert-butyl-1,3-diisopropylisourea.
- the amount of these protecting base agents used is usually about 1 to 50 mol, preferably about 1 to 10 mol, with respect to the compound (1 mol) represented by the formula 5.
- the solvent may be any solvent as long as it does not adversely affect the reaction.
- hydrocarbons eg, benzene, toluene, xylene, etc.
- halogenated hydrocarbons eg, chloroform, 1,2-dichloroethane, etc.
- Nitriles eg, acetonitrile, etc.
- ethers eg, dimethoxyethane, tetrahydrofuran, tert-butylmethyl ether, etc.
- alcohols eg, methanol, ethanol, etc.
- aprotic polar solvents eg, N, N- Dimethylformamide, dimethylsulfoxide, hexamethylphosphoramide, etc.
- water or a mixture thereof, etc. may be mentioned.
- the reaction time is 0.1 to 100 hours, preferably 0.5 to 24 hours.
- the reaction temperature is 0 ° C. to the boiling temperature of the solvent, preferably 0 ° C. to 100 ° C.
- the compound represented by the formula 6 thus obtained can be isolated and purified by a known separation and purification means, or can be subjected to the next step without isolation and purification.
- This step is a method of halogenating a compound represented by the formula 6 to obtain a compound represented by the formula 7 (L 3 in the formula 7 represents a halogen atom).
- Halogenation can be carried out by, for example, a method using fluorine, chlorine, bromine, iodine or the like, a method using N-chlorosuccinimide, N-bromosuccinimide, N-iodosuccinimide or the like. In this reaction, a method using N-chlorosuccinimide, N-bromosuccinimide, N-iodosuccinimide or the like is preferable.
- N-chlorosuccinimide, N-bromosuccinimide, N-iodosuccinimide and the like can be used in an amount of 1 to 10 equivalents with respect to the compound (1 mol) represented by the formula 6, preferably 1 to 3 equivalents.
- the solvent may be any solvent as long as it does not adversely affect the reaction.
- hydrocarbons eg, benzene, toluene, xylene, etc.
- halogenated hydrocarbons eg, chloroform, 1,2-dichloroethane, etc.
- Nitriles eg, acetonitrile, etc.
- ethers eg, dimethoxyethane, tetrahydrofuran, etc.
- alcohols eg, methanol, ethanol, etc.
- aprotic polar solvents eg, N, N-dimethylformamide, dimethylsulfoxide, etc.
- Hexamethylphosphoramide etc. water or a mixture thereof and the like.
- the reaction time is 0.1 to 100 hours, preferably 0.5 to 24 hours.
- the reaction temperature is 0 ° C. to the boiling temperature of the solvent, preferably 0 ° C. to 100 ° C.
- the compound represented by the formula 7 thus obtained can be isolated and purified by a known separation and purification means, or can be subjected to the next step without isolation and purification.
- This step is a method of obtaining a compound protecting group of the amino group of the compound represented by the formula 7 (P 1 in the formula 7) is deprotected formula 8.
- a method of deprotection a commonly known method, for example, Protective Groups in Organic Synthesis third edition, T.I. W. Greene and P. G. M. It can be carried out by the method described in Wuts, John Wiley & Sons (1999), or a method similar thereto.
- Examples of the protecting group include tert-butyloxycarbonyl and the like.
- examples of the acid include hydrochloric acid, acetic acid, trifluoroacetic acid, sulfuric acid, tosylic acid and the like.
- the amount of acid used is preferably about 1 to 100 equivalents with respect to the compound (1 mol) represented by the formula 7.
- the solvent used in the reaction may be any solvent that does not adversely affect the reaction, and is, for example, alcohols (eg, methanol, etc.), hydrocarbons (eg, benzene, toluene, xylene, etc.), halogenated hydrocarbons (eg, benzene, toluene, xylene, etc.).
- alcohols eg, methanol, etc.
- hydrocarbons eg, benzene, toluene, xylene, etc.
- halogenated hydrocarbons eg, benzene, toluene, xylene, etc.
- reaction time is 0.1 to 100 hours, preferably 0.5 to 24 hours.
- reaction temperature is 0 to 100 ° C, preferably 0 to 50 ° C.
- the compound represented by the formula 8 thus obtained can be isolated and purified by a known separation and purification means, or can be subjected to the next step without isolation and purification.
- This step is a method for obtaining a compound represented by the formula 9 by an amidation reaction between the amino group of the compound represented by the formula 8 and an acrylic acid halide or an acrylic acid anhydride.
- the acrylic acid halide or the acrylic acid anhydride is usually 0.5 to 10 mol, preferably about 1 to 5 mol, with respect to the compound (1 mol) represented by the formula 8. It is about mol.
- the acrylic acid halide or acrylic acid anhydride can be produced by a commercially available product or by a known method.
- a base can be added as needed.
- the base include organic amines (eg, trimethylamine, triethylamine, iropropylethylamine, diisopropylethylamine, N-methylmorpholine, 1,8-diazapicyclo [5,4,0] undec-7-ene, pyridine, N, N-dimethylaniline, etc.), alkali metal salts (eg, sodium hydrogen carbonate, potassium hydrogen carbonate, sodium carbonate, potassium carbonate, cesium carbonate, sodium phosphate, potassium phosphate, sodium hydroxide, potassium hydroxide, etc.), metallic hydrogen Examples thereof include compounds (eg, potassium hydride, sodium hydride, etc.), alkali metal alkoxides (eg, sodium methoxydo, sodium ethoxydo, sodium-tert-butoxide, potassium-tert-butoxide, etc.) and the like.
- the amount of the base used is usually about 1 to 100 mol, preferably about 1 to
- the solvent used in the reaction may be any solvent as long as it does not adversely affect the reaction, for example, alcohols (eg, methanol, etc.) hydrocarbons (eg, benzene, toluene, xylene, etc.), halogenated hydrocarbons (eg, eg, methanol, etc.).
- alcohols eg, methanol, etc.
- hydrocarbons eg, benzene, toluene, xylene, etc.
- halogenated hydrocarbons eg, eg, methanol, etc.
- reaction time is 0.1 to 100 hours, preferably 0.5 to 24 hours.
- the reaction temperature is 0 ° C. to the boiling temperature of the solvent, preferably 0 ° C. to 100 ° C.
- the compound represented by the formula 9 thus obtained can be isolated and purified by a known separation and purification means, or can be subjected to the next step without isolation and purification.
- This step is a method for obtaining a compound represented by the formula 10 by reacting the compound represented by the formula 9 with a commercially available product or an acetylene derivative which can be produced by a known method.
- the amount used can be 1 to 50 equivalents, preferably 1 to 10 equivalents, relative to the compound (1 mol) represented by the formula 9.
- transition metal catalyst examples include palladium catalysts (eg, palladium acetate, palladium chloride, tetraxtriphenylphosphine palladium, dichlorobis (triphenylphosphine) palladium, dichlorobis (triphenylphosphine) dipalladium, etc.), nickel catalysts (eg, chloride). Nickel, etc.) is used, and if necessary, a ligand (eg, triphenylphosphine, tri-tert-butylphosphine, etc.) is added, and a copper catalyst (eg, copper iodide, copper bromide, copper chloride), etc. is added. May be used as a co-catalyst.
- palladium catalysts eg, palladium acetate, palladium chloride, tetraxtriphenylphosphine palladium, dichlorobis (triphenylphosphine) palladium, dichlorobis (triphen
- the amount of the transition metal catalyst used varies depending on the type of catalyst, but is usually about 0.0001 to 1 mol, preferably about 0.01 to 0.5 mol, with respect to the compound (1 mol) represented by the formula 9.
- the amount of the ligand used is usually about 0.0001 to 4 mol, preferably about 0.01 to 2 mol, and the amount of the copper catalyst used is about 0.0001 to 4 mol, based on the compound (1 mol) represented by the formula 9. It is usually about 0.0001 to 4 mol, preferably about 0.010 to 2 mol, relative to the compound (1 mol) represented by the formula 9.
- Examples of the base include organic amines (eg, trimethylamine, triethylamine, diisopropylethylamine, N-methylmorpholin, 1,8-diazapicyclo [5,4,0] undec-7-ene, pyridine, N, N-dimethylaniline).
- organic amines eg, trimethylamine, triethylamine, diisopropylethylamine, N-methylmorpholin, 1,8-diazapicyclo [5,4,0] undec-7-ene, pyridine, N, N-dimethylaniline).
- Alkali metal salts eg, sodium hydrogen carbonate, potassium hydrogen carbonate, sodium carbonate, potassium carbonate, cesium carbonate, sodium phosphate, potassium phosphate, sodium hydroxide, potassium hydroxide, etc.
- metal hydrides eg, potassium hydroxide, etc.
- alkali metal alkoxide eg, sodium methoxydo, sodium ethoxydo, sodium-tert-butoxide, potassium-tert-butoxide, etc.
- alkali metal disilazide eg, lithium disiradide, etc.
- alkali metal salts such as potassium carbonate, cesium carbonate, sodium phosphate and potassium phosphate
- alkali metal alkoxides such as sodium-tert-butoxide and potassium-tert-butoxide
- organic amines such as triethylamine and diisopropylethylamine are used. Suitable.
- the amount of the base used is usually about 0.1 to 10 mol, preferably about 1 to 5 mol, with respect to the compound (1 mol) represented by the formula 9.
- the solvent may be any solvent as long as it does not adversely affect the reaction.
- hydrocarbons eg, benzene, toluene, xylene, etc.
- halogenated hydrocarbons eg, chloroform, 1,2-dichloroethane, etc.
- Nitriles eg, acetonitrile, etc.
- ethers eg, dimethoxyethane, tetrahydrofuran, etc.
- alcohols eg, methanol, ethanol, etc.
- aprotic polar solvents eg, N, N-dimethylformamide, dimethylsulfoxide, etc.
- Hexamethylphosphoramide etc. water or a mixture thereof and the like.
- the reaction time is 0.1 to 100 hours, preferably 0.5 to 24 hours.
- the reaction temperature is 0 ° C. to the boiling temperature of the solvent, preferably 0 ° C. to 150 ° C.
- the compound represented by the formula 10 thus obtained can be isolated and purified by a known separation and purification means, or can be subjected to the next step without isolation and purification.
- This step is a method of obtaining a compound protecting group of the carboxyl group of the compound represented by the formula 10 (P 2 in the formula 10) was deprotected formula 11.
- a method of deprotection a commonly known method, for example, Protective Groups in Organic Synthesis, T.I. W. Greene and P. G. M. It can be carried out by the method described in Wuts, John Wiley & Sons (1981), or a method similar thereto.
- Examples of the protecting group include tert-butyl ester and the like.
- the acid include hydrochloric acid, acetic acid, trifluoroacetic acid, sulfuric acid, tosylic acid and the like.
- the amount of acid used is preferably about 1 to 100 equivalents with respect to the compound (1 mol) represented by the formula 10.
- the solvent used in the reaction may be any solvent that does not adversely affect the reaction, and is, for example, alcohols (eg, methanol, etc.), hydrocarbons (eg, benzene, toluene, xylene, etc.), halogenated hydrocarbons (eg, benzene, toluene, xylene, etc.).
- alcohols eg, methanol, etc.
- hydrocarbons eg, benzene, toluene, xylene, etc.
- halogenated hydrocarbons eg, benzene, toluene, xylene, etc.
- reaction time is 0.1 to 100 hours, preferably 0.5 to 24 hours.
- reaction temperature is 0 to 100 ° C, preferably 0 to 50 ° C.
- the compound represented by the formula 11 thus obtained can be isolated and purified by a known separation and purification means, or can be subjected to the next step without isolation and purification.
- This step is a method for obtaining a compound represented by the formula (I) by an amidation reaction between the carboxyl group of the compound represented by the formula 11 and an amine which can be produced by a commercially available product or a known method.
- the amidation method can be carried out by a conventionally known method, in which the reaction is carried out in the presence of a condensing agent, or the carboxylic acid moiety is activated by a conventionally known method to obtain a reactive derivative, and then the derivative is used.
- Examples are methods of amidating amines (see “Basics and Experiments of Peptide Synthesis” (Nobuo Izumiya et al., Maruzen Co., Ltd., 1983) for both methods).
- condensing agent examples include N, N'-dicyclohexylcarbodiimide (DCC), N, N'-diisopropylcarbodiimide (DIC), 1-ethyl-3- (3-dimethylaminopropyl) carbodiimide hydrochloride (WSC), and diphenylphosphoryl azide.
- DPPA benzotriazole-1-yl-oxytrisdimethylaminophosphonium hexafluorophosphate
- BOP benzotriazole-1-yl-oxytripyrrolidinophosphonium hexafluorophosphate
- PyAOP 7-azabenzotriazole-1- Iloxytrispyrrolidinophosphonium phosphate
- BroP bromotrispyrrolidinophosphonium hexafluorophosphate
- chlorotris pyrrolidin-1-yl) phosphonium hexafluorophosphate
- PyCroP 3- (diethoxyphosphoryloxy) -1 , 2,3-Benzotriazine-4 (3H) -one (DEPBT), O- (azabenzotriazole-1-yl) -N, N, N', N'-tetramethyluronium hexafluorophosphart (HATU) ), 4- (5,6-d
- HOBt 1-hydroxy-7-azabenzotriazole
- HOSu N-hydroxysuccinimide
- the amount of these used is usually about 1 to 100 mol, preferably about 1 to 10 mol, with respect to the compound (1 mol) represented by the formula 11.
- a base can be added as needed.
- the base include organic amines (eg, trimethylamine, triethylamine, diisopropylethylamine, N-methylmorpholin, 1,8-diazapicyclo [5,4,0] undec-7-ene, pyridine, N, N-dimethylaniline).
- Alkaline metal salts eg, sodium hydrogen carbonate, potassium hydrogen carbonate, sodium carbonate, potassium carbonate, cesium carbonate, sodium phosphate, potassium phosphate, sodium hydroxide, potassium hydroxide, etc.
- metal hydrides eg, potassium hydroxide, etc.
- examples thereof include potassium hydride (sodium hydride, etc.), alkali metal alkoxides (eg, sodium methoxydo, sodium ethoxydo, sodium-tert-butoxide, potassium-tert-butoxide, etc.) and the like.
- the amount of the base used is usually about 1 to 100 mol, preferably about 1 to 10 mol, with respect to the compound (1 mol) represented by the formula 11.
- the solvent used in the reaction may be any solvent as long as it does not adversely affect the reaction, for example, alcohols (eg, methanol, etc.) hydrocarbons (eg, benzene, toluene, xylene, etc.), halogenated hydrocarbons (eg, eg, methanol, etc.).
- alcohols eg, methanol, etc.
- hydrocarbons eg, benzene, toluene, xylene, etc.
- halogenated hydrocarbons eg, eg, methanol, etc.
- reaction time is 0.1 to 100 hours, preferably 0.5 to 24 hours.
- the reaction temperature is 0 ° C. to the boiling temperature of the solvent, preferably 0 ° C. to 100 ° C.
- the compounds (I) and (II) thus obtained can be isolated and purified by known separation and purification means, for example, concentration, concentration under reduced pressure, crystallization, solvent extraction, reprecipitation, chromatography and the like.
- step 10 "amidation reaction between the carboxyl group of the compound represented by the formula 11 and an amine which can be produced by a commercially available product or a known method” (step 10), "the compound represented by the formula 6 "Halogenization” (5th step), “Deprotection of the protective group of the amino group of the compound represented by the formula 7" (6th step), “Amino group and acrylic acid halide of the compound represented by the formula 8", or “Amidation reaction with acrylic acid anhydride” (7th step), "When L3 of the compound represented by the formula 9 has a removing group such as halogen, a commercially available product or an acetylene derivative that can be produced by a known method can be used. It is possible to lead to the compound represented by the formulas (I) and (II) through each step in the order of "Sono-head reaction" (8th step). The conditions of each step are the same as the above-mentioned conditions.
- any isomer or mixture is included in the compound of the present invention unless otherwise specified. Will be done.
- the racemate and the optical isomer divided from the racemate are also included in the compound of the present invention unless otherwise specified.
- the salt of the compound of the present invention means a pharmaceutically acceptable salt, and examples thereof include a base-added salt and an acid-added salt.
- the pyrimidine compound of the present invention or a salt thereof also contains a prodrug thereof.
- the prodrug is a compound that is converted into the compound of the present invention or a salt thereof by a reaction with an enzyme or gastric acid under physiological conditions in the living body, that is, the compound of the present invention or a compound thereof that is enzymatically oxidized, reduced, hydrolyzed or the like.
- the compound may be changed to the compound of the present invention or a salt thereof under physiological conditions as described in "Development of Pharmaceuticals", Vol. 7, Molecular Design, pp. 163 to 198, published by Hirokawa Shoten.
- the pyrimidine compound of the present invention or a salt thereof may be amorphous or crystalline, and whether the crystal form is single or a polymorphic mixture is included in the compound of the present invention or a salt thereof. .. Crystals can be produced by crystallization by applying a known crystallization method.
- the compound of the present invention or a salt thereof may be a solvate (for example, a hydrate or the like) or a non-solvate, and both are included in the compound of the present invention or a salt thereof.
- Isotopes e.g., 3 H, 14 C, 35 S, etc. 125 I
- compounds labeled with like are also encompassed in the compound or a salt thereof of the present invention.
- the compound of the present invention or a salt thereof has excellent EGFR inhibitory activity. Therefore, the compound of the present invention or a salt thereof is useful as an antitumor agent for malignant tumors having EGFR overexpression, EGFR gene amplification, EGFR mutation, etc., and no significant weight loss in mice was observed. Therefore, it has the advantage of having few side effects.
- EGFR includes human or non-human mammalian EGFR, preferably human EGFR.
- the NCBI Gene ID of human EGFR is 1956.
- the word "EGFR" includes isoforms.
- the compound of the present invention or a salt thereof is useful as a medicine for the prevention or treatment of diseases associated with EGFR due to its excellent EGFR inhibitory activity.
- Disease involving EGFR includes diseases in which the incidence rate is reduced, symptoms are ameliorated, alleviated, and / or completely cured by deleting, suppressing and / or inhibiting the function of EGFR.
- diseases include, but are not limited to, malignant tumors. Preferably, it is a malignant tumor having EGFR overexpression, EGFR gene amplification, or EGFR mutation.
- One embodiment of the present invention provides a therapeutic agent for a disease associated with EGFR, which comprises the compound of the present invention or a salt thereof. Also, one embodiment of the present invention provides an EGFR inhibitor comprising the compound of the present invention or a salt thereof. In addition, one embodiment of the present invention provides a therapeutic agent for an EGFR-positive tumor containing the compound of the present invention or a salt thereof. Also, one embodiment of the present invention provides a compound of the present invention or a salt thereof for treating a disease associated with EGFR. Also, one embodiment of the invention provides the use of a compound of the invention or a salt thereof for treating a disease associated with EGFR.
- one embodiment of the present invention provides the use of a compound of the present invention or a salt thereof for producing a therapeutic agent for a disease involving EGFR.
- one embodiment of the present invention provides a method for treating a disease involving EGFR, which comprises administering an effective amount of the compound of the present invention or a salt thereof to a subject in need thereof. ..
- one embodiment of the present invention provides a compound of the present invention or a salt thereof for treating an EGFR positive tumor.
- one embodiment of the invention provides the use of a compound of the invention or a salt thereof for treating an EGFR positive tumor.
- one embodiment of the present invention provides the use of a compound of the present invention or a salt thereof for producing a therapeutic agent for an EGFR positive tumor.
- one embodiment of the present invention provides a method for treating an EGFR-positive tumor, which comprises administering an effective amount of the compound of the present invention or a salt thereof to a subject in need thereof.
- One form of the compound of the present invention or a salt thereof inhibits a wild-type EGFR and a mutant EGFR having an insertion mutation, a point mutation, a deletion mutation, or the like.
- One embodiment of the present invention provides a wild-type EGFR, a compound having an inhibitory activity against mutant EGFR or a salt thereof, or a pharmaceutical or pharmaceutical composition containing the same.
- One embodiment of the invention provides an inhibitor of wild-type EGFR, and variant EGFR, comprising the compound of the invention or a salt thereof.
- one embodiment of the present invention is a method for inhibiting wild-type EGFR and mutant EGFR, which comprises administering an effective amount of the compound of the present invention or a salt thereof to a subject in need thereof.
- one embodiment of the invention provides the use of wild-type EGFR, and the compounds of the invention or salts thereof for producing inhibitors against mutant EGFR. Also, one embodiment of the invention provides a wild-type EGFR and a compound of the invention or a salt thereof for use as an inhibitor against mutant EGFR. Also, one embodiment of the invention provides wild-type EGFR and the use of compounds of the invention or salts thereof to inhibit mutant EGFR. In another embodiment of the invention, the invention provides the use of a compound of the invention or a salt thereof for the prevention or treatment of wild-type EGFR and diseases associated with variant EGFR.
- the human wild-type EGFR gene is, for example, the one shown in SEQ ID NO: 1, and the human wild-type EGFR protein is composed of, for example, the amino acid sequence shown in SEQ ID NO: 2.
- the nucleotide sequence information of the human wild-type EGFR gene can be obtained by NCBI Reference Sequence: NM_005228, and the amino acid sequence information of the human wild-type EGFR protein can be obtained by NCBI Reference Sequence: NP_005219 and the like.
- the pyrimidine compound of the present invention or a salt thereof exhibits inhibitory activity against mutant EGFR.
- mutant EGFR means one or more of insertion mutations, point mutations, deletion mutations, etc. in the exson 18 region, exxon 19 region, exon 20 region, exxon 21 region, etc. of human wild-type EGFR. EGFR with activation mutations or resistance acquisition mutations.
- exon 18 refers to the region of 688-728 in the amino acid sequence of a human wild-type EGFR protein (eg, a protein consisting of the amino acid sequence set forth in SEQ ID NO: 2).
- the "exxon 18 mutation” refers to a point mutation or deletion mutation of an amino acid in the exson 18 region of a human wild-type EGFR protein (for example, a protein consisting of the amino acid sequence shown in SEQ ID NO: 2).
- the point mutation of exon 18 include E709X or G719X, which is a point mutation in which glutamic acid at position 709 or glycine at position 719 in the exon 18 region is replaced with an arbitrary amino acid.
- E709X include E709K, which is a point mutation in which glutamic acid at position 709 of the exon 18 region is replaced with lysine, and E709A, which is a point mutation in which alanine is replaced.
- G719X examples include G719A, which is a point mutation in which glycine at position 719 in the exxon 18 region is replaced with alanine, G719S, which is a point mutation in which serine is replaced, and G719C, which is a point mutation in which cysteine is replaced.
- the deletion mutation in the exon 18 region includes not only mutations due to deletion of a part of amino acids in the exon 18 region but also mutations in which one or more arbitrary amino acids are inserted in addition to the amino acid deletion. do.
- Examples of the deletion mutation of exon 18 include a mutation (Del E709-T710insD) in which aspartic acid is inserted after deletion of glutamic acid at position 709 and threonine at position 710 in the exon 18 region.
- exon 19 refers to the region of 729-761 in the amino acid sequence of a human wild-type EGFR protein (eg, a protein consisting of the amino acid sequence set forth in SEQ ID NO: 2).
- exon 19 mutation refers to a mutation in which one or more amino acids are deleted in the exon 19 region of a human wild-type EGFR protein (for example, a protein consisting of the amino acid sequence shown in SEQ ID NO: 2).
- Deletion mutations in the exon 19 region include not only mutations due to deletion of some amino acids in the exon 19 region, but also mutations in which one or more arbitrary amino acids are inserted in addition to the amino acid deletion.
- Exxon 19 deletion mutations include, for example, a mutation in which 5 amino acids from 746th glutamic acid to 750th alanine in the exxon 19 region are deleted (Del E746-A750 (or d746-750)), and an exon 19 region.
- the exon 19 deletion mutation is a mutation (Del E746-A750) in which the 5 amino acids of alanine at position 750 are deleted from glutamic acid at position 746 in the exon 19 region.
- exon 20 refers to the region of 762-823 in the amino acid sequence of a human wild-type EGFR protein (eg, a protein consisting of the amino acid sequence set forth in SEQ ID NO: 2).
- the "exxon 20 mutation” refers to a point mutation, insertion mutation, deletion mutation, or the like of an amino acid in the exon 20 region of a human wild-type EGFR protein (for example, a protein consisting of the amino acid sequence shown in SEQ ID NO: 2). ..
- the exon 20 mutation include A763insFQEA, A767insASV, S768dupSVD, V769insASV, D770insNPG, D770insSVD, D773insNPH and the like (Nature medicine, 24, p638-6420).
- the exon 20 mutation is one or more insertion mutations or point mutations selected from V769_D770insASV, D770_N7771insNPG, D770_N7771insSVD, H773_V774insNPH, and T790M.
- exon 21 refers to a region of 824-875 in the amino acid sequence of a human wild-type EGFR protein (eg, a protein consisting of the amino acid sequence set forth in SEQ ID NO: 2).
- the "exon 21 mutation” refers to a point mutation in an amino acid in the exon 21 region of a human wild-type EGFR protein (for example, a protein consisting of the amino acid sequence shown in SEQ ID NO: 2).
- the point mutation of exon 21 include a point mutation in which one amino acid in the exon 21 region is replaced, preferably a point mutation in which leucine at position 858 or leucine at position 861 in the exon 21 region is replaced with an arbitrary amino acid.
- L858X or L861X which is.
- Examples of L858X include L858R, which is a point mutation in which leucine at position 858 of the exon 21 region is replaced with arginine.
- L861X include L861Q, which is a point mutation in which leucine at position 861 in the exon 21 region is replaced with glutamine.
- the point mutation in exon 21 is L858R.
- a mutation in an EGFR isoform of the amino acid set forth in SEQ ID NO: 2 may differ from the position of the amino acid set forth in SEQ ID NO: 2 due to deletion or insertion of the amino acid. It is understood to be similar to the mutation of the position corresponding to the position. Therefore, for example, the threonine at position 790 in the EGFR represented by SEQ ID NO: 2 corresponds to the threonine at position 745 in the EGFR consisting of the amino acid sequence represented by SEQ ID NO: 4.
- T790M means that the 790th threonine of EGFR represented by SEQ ID NO: 2 is mutated to methionine, but in EGFR consisting of the amino acid sequence represented by SEQ ID NO: 4, 745. Since it is the position corresponding to the second amino acid, "T745M” in the EGFR consisting of the amino acid sequence shown in SEQ ID NO: 4 corresponds to "T790M” in the EGFR represented by SEQ ID NO: 2. Further, for example, the threonine at position 790 in the EGFR represented by SEQ ID NO: 2 corresponds to the threonine at position 523 in the EGFR consisting of the amino acid sequence represented by SEQ ID NO: 6.
- T790M means that the 790th threonine of EGFR represented by SEQ ID NO: 2 is mutated to methionine, but in EGFR consisting of the amino acid sequence represented by SEQ ID NO: 6, 523. Since it is the position corresponding to the second amino acid, "T523M” in the EGFR consisting of the amino acid sequence shown in SEQ ID NO: 6 corresponds to "T790M” in the EGFR represented by SEQ ID NO: 2. Whether or not an amino acid of a certain EGFR isoform corresponds to which position of the amino acid shown in SEQ ID NO: 2 can be confirmed by, for example, Multiple Alignment of BLAST.
- SEQ ID NOs: 1 to 6 are as follows. EGFR variant 1
- the "EGFR-positive tumor” is a tumor in which an EGFR protein or an EGFR gene is detected.
- EGFR proteins and EGFR genes also include mutant EGFR proteins and mutant EGFR genes such as point mutations, insertion mutations, or deletion mutations.
- Examples of the EGFR protein detection method include commonly used detection methods such as an ELISA method using an antibody that specifically binds to the EGFR protein, a Western blotting method, or an immunostaining method.
- Antibodies that specifically bind to the EGFR protein can be prepared using commercially available products or by conventional methods.
- a method for detecting the EGFR gene for example, a Northern blotting method, a Southern blotting method, an RT-PCR method, a real-time PCR method, a digital PCR method, a DNA microarray method, an in situ hybridization method, a sequence analysis method, or the like, which are commonly used. Detection methods can be mentioned. Further, a detection method using a commercially available EGFR gene mutation detection kit such as Cobas EGFR mutation detection kit (Roche Diagnostics) can also be mentioned.
- a commercially available EGFR gene mutation detection kit such as Cobas EGFR mutation detection kit (Roche Diagnostics) can also be mentioned.
- the term "effective amount" of a pyrimidine compound of the invention causes a biological or medical response of a subject, such as a decrease or inhibition of enzyme or protein activity, or ameliorate symptoms and conditions. It refers to the amount (therapeutically effective amount) of the compound of the present invention, such as alleviating, slowing or delaying the progression of a disease, or preventing a disease.
- the term "subject” includes mammalian and non-mammalian animals.
- mammals include, but are not limited to, humans, chimpanzees, apes, monkeys, cows, horses, sheep, goats, pigs, rabbits, dogs, cats, rats, mice, guinea pigs, halinese, kangaroos, mogras, wild boars, bears. , Tigers, lions, etc.
- non-mammals include, but are not limited to, birds, fish, reptiles and the like.
- the subject is a human and may be a human diagnosed as requiring treatment for the symptoms, conditions, or diseases disclosed herein.
- a pharmaceutically acceptable carrier may be blended as necessary, and various administration forms can be adopted depending on the prophylactic or therapeutic purpose.
- any of oral preparations, injections, suppositories, ointments, patches and the like may be used, and oral preparations are preferably adopted.
- Each of these dosage forms can be produced by a conventional formulation method known to those skilled in the art.
- One embodiment of the present invention provides an antitumor agent for oral administration containing the compound of the present invention or a salt thereof as an active ingredient.
- one embodiment of the present invention provides a method for preventing and / or treating a tumor, which comprises orally administering an effective amount of the compound of the present invention or a salt thereof to a subject in need thereof. do.
- one embodiment of the present invention provides the use of a compound of the present invention or a salt thereof for producing an antitumor agent for oral administration.
- one embodiment of the present invention provides a compound of the present invention or a salt thereof for oral administration and use for the prevention and / or treatment of a tumor.
- One embodiment of the present invention provides a pharmaceutical composition containing the compound of the present invention or a salt thereof.
- the pharmaceutical composition of one embodiment of the present invention comprises the compound of the present invention or a salt thereof, and a pharmaceutically acceptable carrier.
- one embodiment of the present invention provides the use of a compound of the present invention or a salt thereof for producing a pharmaceutical composition.
- Another embodiment of the invention provides a compound of the invention or a salt thereof for use as a pharmaceutical.
- the pharmaceutically acceptable carrier various conventional organic or inorganic carrier substances are used as the preparation material, and excipients, binders, disintegrants, lubricants, coating agents, colorants, and liquid preparations in solid preparations are used. It is compounded as a solvent, a solubilizing agent, a suspending agent, an tonicity agent, a buffering agent, a pain-relieving agent, etc. Further, if necessary, pharmaceutical additives such as preservatives, antioxidants, sweeteners and stabilizers can be used.
- an excipient if necessary, a binder, a disintegrant, a lubricant, a colorant, a flavoring / odorant, etc. are added to the pyrimidine compound of the present invention, and then a conventional method is used.
- a conventional method is used.
- a pH regulator, buffer, stabilizer, tonicity agent, local anesthetic, etc. are added to the pyrimidine compound of the present invention, and subcutaneous, intramuscular and intravenous injections are performed by a conventional method.
- the agent can be manufactured.
- the amount of the pyrimidine compound of the present invention to be blended in each of the above-mentioned administration unit forms is not constant depending on the symptom of the subject to which this is applied, its dosage form, etc., but is generally 0 for oral preparations per administration unit form. It is preferably 0.05 to 1000 mg, 0.01 to 500 mg for injections, and 1 to 1000 mg for suppositories.
- the daily dose of the drug having the above-mentioned administration form varies depending on the subject's symptoms, body weight, age, gender, etc. and cannot be unconditionally determined. It may be 0.05 to 5000 mg, preferably 0.1 to 1000 mg.
- the malignant tumor that is the subject of the present invention is not particularly limited, and is, for example, brain tumor, head and neck cancer, gastrointestinal cancer (esophageal cancer, gastric cancer, duodenal cancer, liver cancer, biliary tract cancer (bile sac / bile duct cancer, etc.), pancreatic cancer, etc.).
- gastrointestinal cancer esophageal cancer, gastric cancer, duodenal cancer, liver cancer, biliary tract cancer (bile sac / bile duct cancer, etc.), pancreatic cancer, etc.
- Colon-rectal cancer colon cancer, rectal cancer, etc.
- Lung cancer non-small cell lung cancer, small cell lung cancer, mesotheloma, etc.
- breast cancer genital cancer (ovarian cancer, uterine cancer (cervical cancer, uterine body cancer, etc.)) Etc.), etc.
- Urological cancer renal cancer, bladder cancer, prostate cancer, testicular tumor, etc.
- hematopoietic tumor leukemia, malignant lymphoma, multiple myeloma, etc.
- bone / soft tumor skin cancer, etc.
- lung cancer breast cancer, gastric cancer, head and neck cancer, brain tumor, colorectal cancer, bladder cancer, biliary tract cancer, or uterine cancer, and more preferably lung cancer, breast cancer, colorectal cancer, or brain tumor.
- the tumor of interest of the invention is a malignant tumor with EGFR overexpression, EGFR gene amplification, or EGFR mutation, and specific malignant tumors are brain cancer, head and neck cancer, gastrointestinal cancer (esophageal tract).
- Cancer gastric cancer, duodenal cancer, liver cancer, biliary tract cancer (bile sac / bile duct cancer, etc.), pancreatic cancer, colon-rectal cancer (colon cancer, rectal cancer, etc.), lung cancer (non-small cell lung cancer, small cell lung cancer, mesentery) Tumors, etc.), breast cancer, genital cancer (ovarian cancer, uterine cancer (cervical cancer, uterine body cancer, etc.), etc.), urinary tract cancer (renal cancer, bladder cancer, prostate cancer, testicular tumor, etc.), hematopoietic tumor (leukemia, leukemia, etc.) Malignant lymphoma, multiple myeloma, etc.), bone / soft tumor, skin cancer, etc., preferably lung cancer, breast cancer, gastric cancer, head and neck cancer, brain cancer, colorectal cancer, bladder cancer, biliary tract cancer, or uterine cancer. Yes, more preferably lung cancer, breast cancer, colorectal cancer, or brain tumor
- the tumor of interest of the invention is an EGFR positive tumor
- the specific tumors are brain tumor, head and neck cancer, gastrointestinal cancer (esophageal cancer, gastric cancer, duodenal cancer, liver cancer, biliary tract cancer (esophageal cancer, gastric cancer, duodenal cancer, liver cancer, biliary tract cancer).
- the tumor is a brain tumor.
- the pyrimidine compounds of the present invention may be useful in the treatment of symptoms of the brain that require crossing the blood-brain barrier.
- the pyrimidine compound of one embodiment has a favorable passage through the blood-brain barrier for delivery to the brain, i.e., excellent brain transferability.
- As an index of the transferability of the compound to the brain there are the compound concentration in the brain and the Kp value (brain to plasma drug concentration ratio).
- Brain tumors treated with the pyrimidine compound of the present invention include metastatic brain tumors and primary brain tumors.
- Brain tumors are not particularly limited, but are, for example, metastatic brain tumors (eg, brain metastases of lung cancer, breast cancer, gastric cancer, colorectal cancer, bladder cancer, biliary tract cancer, uterine cancer, etc. (preferably lung cancer, breast cancer or gastric cancer)), hair.
- metastatic brain tumors eg, brain metastases of lung cancer, breast cancer, gastric cancer, colorectal cancer, bladder cancer, biliary tract cancer, uterine cancer, etc. (preferably lung cancer, breast cancer or gastric cancer)
- hair e.g, metastatic brain tumors (eg, brain metastases of lung cancer, breast cancer, gastric cancer, colorectal cancer, bladder cancer, biliary tract cancer, uterine cancer, etc. (preferably lung cancer, breast cancer or gastric cancer)), hair.
- room temperature usually indicates about 10 ° C to about 35 ° C. Further, in the examples of the following compounds,% indicates weight percent unless otherwise specified.
- KieselgelTM60F254, Art. 5744 or NH2 silica gel 60F254 plate Wako manufactured by Fuji Film Wako Pure Chemical Industries, Ltd. was used.
- 1 1 H-NMR was measured using AL400 (400 MHz) manufactured by JEOL, Mercury (400 MHz) manufactured by Varian, or Inova (400 MHz) manufactured by Varian, and tetramethylsilane was used as a standard substance.
- the mass spectrum was measured by electrospray ionization (ESI) or atmospheric chemical ionization (APCI) using Micromass ZQ or SQD manufactured by Waters.
- the microwave reaction was carried out using an Initiator manufactured by Biotage.
- Reference Example 1 (2) 4-Amino-7-((3R, 5S) -1- (tert-butoxycarbonyl) -5-methylpyrrolidine-3-yl) -7H-pyrrolo [2,3-d] pyrimidine-
- the compound (28.0 g) of Reference Example 1 (1), 10% palladium carbon catalyst (720 mg), NMP (84 mL), methanol (26 mL), and triethylamine (17.6 mL) were added to the 5-carboxylic acid pressure resistant tube. Then, it was substituted with carbon monoxide and stirred at 100 ° C. for 2 hours.
- reaction mixture was cooled to room temperature, 2M aqueous sodium hydroxide solution (79 mL) was added, and the mixture was stirred at 80 ° C. for 2 hours.
- the reaction mixture was cooled to room temperature, filtered through Celite, washed with methanol, and the filtrate was concentrated under reduced pressure. After adding more water, the aqueous layer was washed with tert-butyl methyl ether.
- a 1 M aqueous potassium hydrogensulfate solution was added to the aqueous layer to adjust the pH to about 3, and the precipitated solid was collected by filtration, washed with water and dried to obtain the desired product (23.4 g).
- Example 1 (1) tert-butyl-4-amino-6-bromo-7-((3R, 5S) -1- (tert-butoxycarbonyl) -5-methylpyrrolidine-3-yl) -7H-pyrroform [ 2,3-d] Pyrimidine-5-carboxylate Under a nitrogen atmosphere, the compound (15.0 g) of Reference Example 1 (2) was dissolved in chloroform (150 mL), and 2-tert-butyl-1,3-diisopropylisodium was dissolved. Rare (25 mL) was added, the temperature was raised to 60 ° C., and the mixture was stirred for 2 hours.
- the obtained tert-butyl ester compound was dissolved in chloroform (140 mL), N-bromosuccinimide (11.8 g) was added, and the mixture was stirred at room temperature for 24 hours. Chloroform and 10% aqueous sodium bisulfite solution were sequentially added to the reaction mixture, and the mixture was extracted with chloroform. The combined organic layers were washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure. The obtained residue was purified by silica gel chromatography (hexane: ethyl acetate) to obtain the desired product (13.8 g).
- Example 1 (2) tert-butyl-7-((3R, 5S) -1-acryloyl-5-methylpyrrolidine-3-yl) -4-amino-6-bromo-7H-pyrrolo [2,3-d] ] Pyrimidine-5-carboxylate
- the compound (11.4 g) of Example 1 (1) is dissolved in THF (57 mL), cooled to 0 ° C., and then a 1,4-dioxane solution (114 mL) of 4M hydrogen chloride is added. In addition, the mixture was stirred at 0 ° C. for 10 hours.
- Example 1 (3) tert-butyl-7-((3R, 5S) -1-acryloyl-5-methylpyrrolidine-3-yl) -4-amino-6- (prop-1-in-1-yl) -7H-Pyrrolidine [2,3-d] Pyrimidine-5-carboxylate
- Example 1 (2) compound (7.72 g), acetonitrile (154 mL), triethylamine (7.2 mL), PdCl 2 (PPh 3 ) 2 (1.2 g), copper (I) iodide (330 mg) was added with a DMF solution (85.7 mL) of 1.0 M propyne, replaced with nitrogen, and then stirred at 70 ° C. for 4 hours.
- Example 1 (4) 7-((3R, 5S) -1-acryloyl-5-methylpyrrolidin-3-yl) -4-amino-6- (prop-1-in-1-yl) -7H-pyrrolo [2,3-d] Pyrimidine-5-carboxylic acid
- chloroform 5 mL
- trifluoroacetic acid 5 mL
- Chloroform was added to the residue, and the mixture was concentrated again under reduced pressure.
- the residue was dried under reduced pressure to obtain the desired product (1.25 g).
- Example 1 7- (R)-((3R, 5S) -1-acryloyl-5-methylpyrrolidine-3-yl) -4-amino-N-((R) -1- (3,5) -Difluorophenyl) ethyl) -6- (prop-1-in-1-yl) -7H-pyrrolo [2,3-d] pyrimidine-5-carboxamide DMF (100 mg) of the compound of Example 1 (4).
- Example 2 7-((3R, 5S) -1-acryloyl-5-methylpyrrolidine-3-yl) -4-amino-N-((R) -1-phenylethyl) -6- (prop-1-) In-1-yl) -7H-pyrrolidine [2,3-d] pyrimidine-5-carboxamide
- (R) -1- (3,5-difluorophenyl) ethane-1-amine instead, the title compound was obtained in the same manner as in Example 1 except that (R) -1-phenylethane-1-amine was used.
- Example 3 7-((3R, 5S) -1-acryloyl-5-methylpyrrolidine-3-yl) -4-amino-N- (2-phenylpropan-2-yl) -6- (prop-1-yl) In-1-yl) -7H-pyrrolidine [2,3-d] pyrimidine-5-carboxamide
- Example 1 to (R) -1- (3.5-difluorophenyl) ethane-1-amine. Instead, the title compound was obtained in the same manner as in Example 1 except that 2-phenylpropane-2-amine was used.
- Example 4 7-((3R, 5S) -1-acryloyl-5-methylpyrrolidine-3-yl) -4-amino-N-((R) -1-phenylpropyl) -6- (prop-1-) In-1-yl) -7H-pyrrolidine [2,3-d] pyrimidine-5-carboxamide
- (R) -1- (3,5-difluorophenyl) ethane-1-amine instead, the title compound was obtained in the same manner as in Example 1 except that (R) -1-phenylpropane-1-amine was used.
- Example 5 7-((3R, 5S) -1-acryloyl-5-methylpyrrolidine-3-yl) -4-amino-N- (2- (2-fluorophenyl) propan-2-yl) -6- (Prop-1-in-1-yl) -7H-Pyrrolidine [2,3-d] Pyrimidine-5-Carboxamide
- (R) -1- (3,5-difluorophenyl) ethane The title compound was obtained in the same manner as in Example 1 except that 2- (2-fluorophenyl) propane-2-amine was used instead of -1-amine.
- Example 6 7-((3R, 5S) -1-acryloyl-5-methylpyrrolidine-3-yl) -4-amino-N-((R) -1- (3-chlorophenyl) ethyl) -6- ( Prop-1-in-1-yl) -7H-pyrrolidine [2,3-d] pyrimidine-5-carboxamide
- (R) -1- (3,5-difluorophenyl) ethane- The title compound was obtained in the same manner as in Example 1 except that (R)-(+)-1- (3-chlorophenyl) ethylamine hydrochloride was used instead of 1-amine.
- Example 7 7-((3R, 5S) -1-acryloyl-5-methylpyrrolidin-3-yl) -4-amino-N-((R) -1- (2,4-difluorophenyl) ethyl)- 6- (Prop-1-in-1-yl) -7H-pyrrolo [2,3-d] pyrimidin-5-carboxamide
- the title compound was obtained in the same manner as in Example 1 except that (R)-(+) -1- (2,4-difluorophenyl) ethylamine hydrochloride was used instead of ethane-1-amine. ..
- Example 8 7-((3R, 5S) -1-acryloyl-5-methylpyrrolidine-3-yl) -4-amino-6- (prop-1-in-1-yl) -N-((S)) -2,2,2-trifluoro-1-phenylethyl) -7H-pyrrolidine [2,3-d] pyrimidine-5-carboxamide
- (R) -1- (3,5-) The title compound was used in the same manner as in Example 1 except that (S) -2,2,2-trifluoro-1-phenylethan-1-amine was used instead of difluorophenyl) ethane-1-amine. Obtained.
- Example 9 7-((3R, 5S) -1-acryloyl-5-methylpyrrolidin-3-yl) -4-amino-6- (cyclopropylethynyl) -N- (2-phenylpropane-2-yl) -7H-Pyrrolo [2,3-d] pyrimidin-5-carboxamide
- cyclopropylacetylene was used instead of the DMF solution of 1.0 M propane, and in Example 1 (5), ( The title compound was obtained in the same manner as in Example 1 except that 2-phenylpropane-2-amine was used instead of R) -1- (3,5-difluorophenyl) ethane-1-amine.
- Example 10 7-((3R, 5S) -1-acryloyl-5-methylpyrrolidin-3-yl) -4-amino-6- (cyclopropylethynyl) -N-((R) -1- (2,) 3-Difluorophenyl) Ethyl) -7H-Pyrrolo [2,3-d] pyrimidin-5-carboxamide
- cyclopropylacetylene was used instead of the 1.0M propine DMF solution.
- Example 11 (1) tert-butyl (2S, 4R) -4- (4-amino-6-bromo-5-(((R) -1-phenylethyl) carbamoyl) -7H-pyrrolo [2,3- d] Pyrimidine-7-yl) -2-methylpyrrolidine-1-carboxylate Reference Example 1 (2) compound (1.00 g), (R)-(+) -1-phenylethylamine (0.503 g), Diisopropylethylamine (1.79 g), N, N-dimethylformamide (10 mL) were added, followed by HATU (1.58 g), and the mixture was stirred overnight at room temperature.
- Example 11 (2) 7-((3R, 5S) -1-acryloyl-5-methylpyrrolidine-3-yl) -4-amino-6-bromo-N-((R) -1-phenylethyl)- 7H-Pyrrolidine [2,3-d] Pyrimidine-5-carboxamide
- chloroform 3 mL
- acetonitrile 5 mL
- the obtained compound was used in the next reaction without further purification.
- Acetonitrile (3 mL) was added to the obtained amine body, the mixture was cooled to 0 ° C., acrylic chloride (99.9 mg) and diisopropylethylamine (713 mg) were added, and the mixture was stirred at 0 ° C. for 1 hour.
- the reaction mixture was concentrated under reduced pressure, and the obtained residue was purified by silica gel chromatography (ethyl acetate: methanol) to obtain the desired product (281 mg).
- Example 11 7-((3R, 5S) -1-acryloyl-5-methylpyrimidine-3-yl) -4-amino-6- (cyclopropylethynyl) -N-((R) -1-) Phenylethyl) -7H-pyrrolo [2,3-d] pyrimidine-5-carboxamide
- Example 12 7-((3R, 5S) -1-acryloyl-5-methylpyrrolidine-3-yl) -4-amino-6- (3,3-dimethylbuty-1-in-1-yl) -N- ((R) -1-Phenylethyl) -7H-pyrrolidine [2,3-d] pyrimidine-5-carboxamide
- Example 11 (3) 3,3-dimethyl-1-butyne was used instead of cyclopropylacetylene. The title compound was obtained in the same manner as in Example 11 except that the above was used.
- Example 13 7-((3R, 5S) -1-acryloyl-5-methylpyrrolidin-3-yl) -4-amino-6- (3-methoxy-3-methylbuty-1-in-1-yl)- N-((R) -1-phenylethyl) -7H-pyrrolo [2,3-d] pyrimidin-5-carboxamide
- Example 11 (3) instead of cyclopropylacetylene, 3-methoxy-3-methyl The title compound was obtained in the same manner as in Example 11 except that -1-butin was used.
- Example 14 7-((3R, 5S) -1-acryloyl-5-methylpyrrolidine-3-yl) -4-amino-6- (buty-1-in-1-yl) -N-((R)) -1-Phenylethyl) -7H-Pyrrolidine [2,3-d] Pyrimidine-5-Carboxamide
- Example 11 (3) 1-trimethylsilyl-1-butyne and tetra-fluorinated instead of cyclopropylacetylene. The title compound was obtained in the same manner as in Example 11 except that n-butylammonium was used.
- Example 15 7-((3R, 5S) -1-acryloyl-5-methylpyrrolidin-3-yl) -4-amino-N- (2- (2-fluorophenyl) propan-2-yl) -6- (3-Methylbuty-1-in-1-yl) -7H-pyrrolo [2,3-d] pyrimidin-5-carboxamide
- (R)-(+)-1-phenylethylamine Example 11 except that 2- (2-fluorophenyl) propane-2-amine was used instead and 3-methyl-1-butyne was used instead of cyclopropylacetylene in Example 11 (3). The title compound was obtained in the same manner as above.
- Example 16 (1) tert-butyl (2R, 4S) -4- (benzyloxy) -2-((tosiloxy) methyl) pyrrolidine-1-carboxylate tert-butyl (2R, 4S) -4- (benzyloxy) )-2- (Hydroxymethyl) pyrrolidine-1-carboxylate (2.0 g) is dissolved in methylene chloride (20 mL), cooled to 0 ° C., and then 1,4-diazabicyclo [2.2.2] octane (2.2.2) octane.
- Example 16 (2) tert-butyl (2S, 4S) -4- (benzyloxy) -2-ethylpyrrolidin-1-carboxylate Under a nitrogen atmosphere, copper iodide (2.04 g) was added to diethyl ether (12 mL). After suspending and cooling to 0 ° C., a diethyl ether solution (0.36 mL) of 1.04 M methyllithium was added, and the mixture was stirred at 0 ° C. for 30 minutes. Then, a solution of methylene chloride (4.0 mL) of the compound (1.98 g) of Example 16 (1) was added, the temperature was raised to room temperature, and the mixture was stirred for 1 hour.
- methylene chloride 4.0 mL
- the reaction mixture was cooled to 0 ° C., saturated aqueous ammonium chloride solution was added, and the mixture was extracted with ethyl acetate. The combined organic layers were washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure. The obtained residue was purified by silica gel chromatography (hexane: ethyl acetate) to obtain the desired product (707 mg).
- Example 16 (3) tert-butyl (2S, 4S) -2-ethyl-4-hydroxypyrrolidine-1-carboxylate Compound (1.06 g) of Example 16 (2), 10% palladium hydroxide carbon catalyst ( 160 mg) was suspended in ethanol (11 mL) and THF (11 mL), substituted with hydrogen, and stirred at room temperature for 20 hours. The reaction mixture was filtered through Celite, washed with ethanol, and the filtrate was concentrated under reduced pressure. The obtained residue was purified by silica gel chromatography (hexane: ethyl acetate) to obtain the desired product (709 mg).
- Example 16 (4) tert-butyl (2S, 4R) -4- (4-chloro-5-iodo-7H-pyrrolo [2,3-d] pyrimidin-7-yl) -2-ethylpyrrolidine-1-
- the compound (709 mg) of Carboxylate Example 16 (3) and 4-chloro-5-iodo-7H-pyrrolo [2,3-d] pyrimidine (1.11 g) were dissolved in THF (7.1 mL). After cooling to 0 ° C., triphenylphosphine (1.3 g) and diisopropylazodicarboxylate (1.00 mL) were added, the temperature was raised to room temperature, and the mixture was stirred for 1 hour.
- the reaction mixture was concentrated under reduced pressure, and the obtained residue was purified by silica gel chromatography (hexane: ethyl acetate) to obtain a corresponding coupling.
- the obtained compound was used in the next reaction without further purification.
- the obtained coupling body, THF (5.4 mL) and aqueous ammonia (5.4 mL) were added to the pressure resistant tube, and the mixture was stirred at 100 ° C. for 14 hours.
- the reaction mixture was cooled to room temperature, poured into water (12.8 mL) and extracted with ethyl acetate. The combined organic layers were washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure.
- Example 16 (5) tert-butyl (2S, 4R) -4- (4-amino-6-bromo-5-(((R) -1-phenylethyl) carbamoyl) -7H-pyrrolo [2,3- d] Pyrimidine-7-yl) -2-ethylpyrrolidine-1-carboxylate
- Example 16 (4) compound (797 mg), dichlorobis (triphenylphosphine) palladium (25 mg), (R)-(+) -1 -Phenylethylamine (0.55 mL) was suspended in DMF (8.0 mL), carbon monoxide was substituted, and the mixture was stirred at 80 ° C. for 2 hours.
- the reaction mixture was cooled to room temperature, water was added, and the mixture was extracted with ethyl acetate. The combined organic layers were washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure.
- the obtained residue was purified by silica gel chromatography (hexane: acetone) to obtain the corresponding amide.
- the obtained compound was used in the next reaction without further purification.
- the obtained amide is dissolved in acetonitrile (8.2 mL), cooled to ⁇ 10 ° C., and then a solution of N-bromosuccinimide (457 mg) in acetonitrile (8.2 mL) is slowly added dropwise to the reaction mixture at 30. Stir for minutes.
- Example 16 7-((3R, 5S) -1-acryloyl-5-ethylpyrrolidine-3-yl) -4-amino-6-bromo-N-((R) -1-phenylethyl)- 7H-Pyrrolidine [2,3-d] Pyrimidine-5-carboxamide
- acetonitrile 9.7 mL
- sodium iodide (1.05 g).
- trimethylsilyl chloride (0.89 mL) were added, and the mixture was stirred at 0 ° C. for 1 hour.
- Example 16 7-((3R, 5S) -1-acryloyl-5-ethylpyrrolidine-3-yl) -4-amino-N-((R) -1-phenylethyl) -6- (prop) -1-in-1-yl) -7H-pyrrolidine [2,3-d] pyrimidine-5-carboxamide
- Example 17 7-((3R, 5S) -1-acryloyl-5-ethylpyrrolidine-3-yl) -4-amino-6- (cyclopropylethynyl) -N-((R) -1-phenylethyl) -7H-Pyrrolidine [2,3-d] Pyrimidine-5-Carboxamide
- Example 16 (7) the same as in Example 16 except that cyclopropylacetylene was used instead of the DMF solution of 1.0 M propyne. The title compound was obtained.
- Example 16 187-((3R, 5R) -1-acryloyl-5- (methoxymethyl) pyrrolidine-3-yl) -4-amino-6- (cyclopropylethynyl) -N-((R) -1- Phenylethyl) -7H-pyrrolidine [2,3-d] pyrimidin-5-carboxamide
- tert-butyl (2R, 4S) -4- instead of the compound of Example 16 (3).
- hydroxy-2- (methoxymethyl) pyrrolidine-1-carboxylate was used and cyclopropylacetylene was used in Example 16 (7) instead of the DMF solution of 1.0 M compound.
- the title compound was obtained.
- Example 19 7-((3R, 5R) -1-acryloyl-5- (ethoxymethyl) pyrrolidine-3-yl) -4-amino-6- (cyclopropylethynyl) -N-((R) -1- Phenylethyl) -7H-pyrrolidine [2,3-d] pyrimidin-5-carboxamide
- Example 16 instead of the compound of Example 16 (3), tert-butyl (2R, 4S) -2- Same as in Example 16 except that (ethoxymethyl) -4-hydroxypyrrolidine-1-carboxamide was used and cyclopropylacetylene was used instead of the DMF solution of 1.0 M compound in Example 16 (7). The title compound was obtained.
- Comparative Example 6 Comparative Example 6 (1) tert-butyl (2S, 4R) -4- (4-amino-5-(((R) -1-phenylethyl) carbamoyl) -6- (prop-1-in-1-yl) ) -7H-pyrrolo [2,3-d] pyrimidin-7-yl) -2-methylpyrrolidine-1-carboxylate
- Example 11 (1) (230 mg), acetonitrile (4.6 mL), triethylamine (0.29 mL) ), PdCl 2 (PPh 3 ) 2 (5.9 mg), copper (I) iodide (1.6 mg), add 1.0 Mpropine DMF solution (2.1 mL), replace with nitrogen, and then bring to 70 ° C.
- the compound of the present invention was serially diluted with dimethyl sulfoxide (DMSO).
- DMSO dimethyl sulfoxide
- Final concentration is 5 mM
- manganese chloride final concentration is 1 mM
- ATP final concentration is near Km of each EGFR
- DMSO solution of the compound of the present invention final concentration of DMSO is 1%) is added at room temperature. After incubation for 1 hour, a kinase reaction was carried out.
- Termination Buffer was added thereto to stop the kinase reaction.
- S non-phosphorylated substrate peptide
- P phosphorylated peptide
- the amount of phosphorylation reaction was determined from the heights of the peaks of S and P, and the concentration of the compound capable of suppressing the phosphorylation reaction by 50% was defined as the IC50 value (nM) and is shown in the table below.
- the compound of the present invention has excellent inhibitory activity against wild-type and mutant EGFR.
- Test Example 2 Measurement of growth inhibitory activity against EGFR overexpressing cell line and Exxon 20-inserted mutant EGFR-expressing cell line
- the growth-inhibiting activity against EGFR overexpressing cell line and Exxon 20-inserted mutant EGFR-expressing cell line is EGFR overexpressing human breast cancer.
- the EGFR gene (WT, MCF10A_EGFR cells, MCF10A_EGFR / V769_D770insASV cells, MCF10A_EGFR / D777
- MDA-MB-468 cells were suspended in Leibovitz's L-15 medium containing deactivated 10% fetal bovine serum.
- NCI-H1975 cells were suspended in RPMI-1640 medium containing deactivated 10% fetal bovine serum.
- the cell suspension was inoculated into each well of the 96-well flat bottom plate so that the number of cells per well was 500, MDA-MB-468 cells were incubated in a carbon dioxide-free incubator, and the other cells were 5 cells.
- the cells were cultured at 37 ° C. for 1 day in an incubator containing% carbon dioxide gas.
- the compound of the present invention was prepared in DMSO to 1 mM and then diluted 1/200 in a medium to prepare a 5 ⁇ M solution. Then, the DMSO solution of the compound of the present invention was diluted with the medium used for cell suspension and added so that the final concentration of the maximum concentration of the test compound was 1000 nM, and the MDA-MB-468 cells did not contain carbon dioxide gas.
- the other cells were cultured at 37 ° C. for another 3 days in the incubator containing 5% carbon dioxide gas.
- the number of cells at the start of culturing (day 0) and after culturing (day 3) was measured using CellTiter-Glo® 2.0 Reagent (Promega) based on the protocol recommended by the manufacturer.
- the growth inhibition rate was calculated from the following formula, and the concentration of the test compound (GI50 (nM)) that inhibits 50% was determined. The results are shown in Table 4.
- the compound group of the present invention is MDA-MB-468 cell which is a wild type EGFR overexpressing strain, MCF10A_EGFR cell which gene-introduced and expressed wild type EGFR, L858R and T790M mutant EGFR positive cell.
- NCI-H1975 cells, Exxon 20-inserted mutant EGFR-expressing cell lines (MCF10A_EGFR / V769_D770insASV cells, MCF10A_EGFR / D770_N771insSVD cells, MCF10A_EGFR / H773_V774insNPH cells) also had excellent cell proliferation inhibitory activity.
- Test Example 3 Measurement of Growth Inhibitory Activity for Exxon 20 Insertion Mutant EGFR Expressing Cell Line
- the growth inhibitory activity for Exxon 20 Insertion Mutant EGFR expresses D770_N771insSVD mutant EGFR by gene modification using NCI-H1975 cells, and Evaluation was performed using H1975-EGFRinsSVD cells in which endogenous EGFR (T790M / L858R) was knocked out, and LXF 2478 cells (Charles river), which are tumors derived from V769_D770insASV mutant EGFR-positive human lung cancer patients.
- H1975-EGFRinsSVD cells are NCI-H1975 cells with PB-CMV-MCS-EF1-RFP + Puro vector encoding D770_N771insSVD (insSVD) with Super PiggyBacTransposase expression vector by Amaxa® Cell Registered Trademark (Registered Trademark) Cell After introduction by electroporation and selection with puromycin (SIGMA), XTN® TALENs Site-Specific Nucleases (Transposagen) is Amaxa® Cell. Cells introduced by electroporation with Line Nucleofector® Kit R and knocked out of endogenous EGFR (T790M / L858R) were sequenced.
- each cell was suspended in RPMI-1640 medium.
- the cell suspension was seeded in each well of a 96-well flat bottom plate so that the number of cells per well was 3,000, and the cells were cultured at 37 ° C. for one day in an incubator containing 5% carbon dioxide gas.
- the compound of the present invention and the compound of Comparative Example were dissolved in DMSO to 1 mM, and then added using a Tecan D300e digital dispenser (Tecan) so that the final concentration of the test compound was 1000 nM and the common ratio was 3. It was cultured at 37 ° C. for 3 days in an incubator containing 5% carbon dioxide gas.
- the number of cells at the start of culturing (day 0) and after culturing (day 3) was measured using CellTiter-Glo® 2.0 Reagent (Promega) based on the protocol recommended by the manufacturer.
- the growth inhibition rate was calculated from the following formula, and the concentration of the test compound (GI50 (nM)) that inhibits 50% was determined. The results are shown in Table 5.
- the compound group of the present invention also had excellent cell proliferation inhibitory activity in the exon 20-inserted mutant EGFR-expressing cell line (H1975-EGFRinsSVD and LXF 2478).
- Test Example 4 Evaluation of Oral Absorption The compound of the present invention was suspended or dissolved in 0.5% HPMC aqueous solution and 0.1N hydrochloric acid, and the dose was 50 mg / kg / day in BALB / cA mice (Nippon Claire Co., Ltd.). Was orally administered. After oral administration, blood was collected from the facial vein 0.5, 1, 2, 4 and 6 hours later to obtain plasma. The compound concentration in the obtained plasma was measured by LC-MS / MS, and the oral absorbability was evaluated. The results are shown in Table 6 below.
- the compound of the present invention was observed to have a sufficient plasma concentration and showed good oral absorbability.
- the oral absorbability was attenuated by 4 times or more as compared with the compound of the present invention.
- Test Example 5 Evaluation of brain transferability
- the compound of the present invention was suspended or dissolved in 0.5% HPMC aqueous solution and 0.1N hydrochloric acid, and was added to BALB / cA mouse Nippon Claire Co., Ltd. at a dose of 50 mg / kg / day. It was orally administered. After 0.5 hours after oral administration, blood was collected from the facial vein, and the entire brain was removed to obtain plasma and brain samples. After adding 3 times the amount of water to the obtained brain sample, homogenization was performed using an ultrasonic homogenizer to obtain a brain homogenate. The compound concentrations in the obtained plasma and brain homogenate were measured by LC-MS / MS, and the brain transferability was evaluated from the brain / plasma compound concentration. The results are shown in Table 7 below.
- the compound of the present invention had a higher brain / plasma compound concentration (Kp value) than that of Comparative Example 2, and showed good brain transferability. Further, in Comparative Example 2, the concentration of the compound in the brain was reduced by 80 times or more as compared with the compound of the present invention.
- Test Example 6 Antitumor effect confirmation test (in vivo) for a subcutaneous transplant model of H1975-EGFRins SVD cell line
- the H1975-EGFRinsSVD cell line contains RPMI-1640 (4.5 g / L glucose, 10 mM HEPES and 1 mM sodium pyruvate) containing deactivated 10% fetal bovine serum (FBS) (Fuji Film Wako Pure Chemical Industries, Ltd.) medium.
- FBS fetal bovine serum
- H1975-EGFRinsSVD cells were resuspended at a concentration of 8 ⁇ 10 7 cells / mL in PBS. Using a 1 mL tuberculin syringe and a 25 G injection needle, 8 x 10 6 cells / 0.1 mL each under the skin of the right chest of a 6-week-old nude mouse (BALB / cAJcl-nu / nu, Nippon Claire Co., Ltd.) The cell suspension was transplanted.
- Examples 2, 11 and 12 (Exple 2, 11, 12) were used as the test compound, and a 0.5% HPMC aqueous solution was used as the control.
- Examples 2, 11 and 12 (Excel 2, 11 and 12) were orally administered at doses of 25 mg / kg / day, 25 mg / kg / day and 50 mg / kg / day, respectively.
- test compound or control was orally administered once a day for 14 days (Day 1-14) from the day after grouping.
- the tumor volume (Tumor volume, hereinafter also referred to as "TV") was measured twice a week over time.
- an electronic balance for animals was used to measure the body weight.
- the body weight change rate (BWCn) on the nth day was calculated from the body weight (BWn) on the nth day by the following formula.
- the transition of the average value of TV and BWC of each individual is shown in FIGS. 1 and 2.
- BWCn (%) [(Weight on day n)-(Weight on grouping day)] / (Weight on grouping day) x 100
- the compound is effective when the average TV value of the compound-administered group on the final evaluation day (Day 15) is smaller than the average TV value of the Control group and shows a statistically significant difference (Dunnett type multiple comparison test) (Dunnett type multiple comparison test). It is determined that P ⁇ 0.001) and is indicated by * in the figure. The results are shown in FIG.
- Test Example 7 An antitumor effect confirmation test (in vivo) for a direct brain transplantation model of a Luciferase gene-introduced exon 20-inserted mutant EGFR-expressing cell line (H1975-EGFRinsSVD-Luc).
- the antitumor effect and life-prolonging effect of the invention compound by the direct brain transplantation model were evaluated using the H1975-EGFRinsSVD-Luc strain in which Luciferase was introduced into the human mutant EGFR-introduced cell line H1975-EGFRinsSVD.
- H1975-EGFRinsSVD-Luc cells are NCI-H1975-EGFRinsSVD cells with pJTI (registered trademark) FAST DES vector encoding Luciferase and pJTI (registered trademark) PhiC31 Integrase expression vector and Amaxa (registered trademark) CellLine. After introduction by the electroporation method using Kit R, the one selected by Hyglomycin B (Nakalitesk Co., Ltd.) was used.
- the H1975-EGFRinsSVD-Luc strain contains RPMI-1640 (4.5 g / L glucose, 10 mM HEPES and 1 mM sodium pyruvate) containing deactivated 10% fetal bovine serum (FBS) (Fuji Film Wako Pure Chemical Industries, Ltd.). Cell lines were cultured in medium at 37 ° C. in a 5% CO2 incubator.
- RPMI-1640 4.5 g / L glucose, 10 mM HEPES and 1 mM sodium pyruvate
- FBS fetal bovine serum
- the H1975-EGFRinsSVD-Luc cells were resuspended at a concentration of 12.5 ⁇ 10 7 cells / mL in PBS.
- a nude mouse (BALB / cAJcl-nu / nu, Nippon Claire Co., Ltd.) about 6 to 7 weeks old was fixed to a brain localization device using an ear bar for mice, and a sterile cotton swab was used to disinfect the skin on the crown. After disinfection by applying the liquid, an incision was made with a scalpel.
- Total Flux (Photon / sec) was measured using IVIS (PerkinElmer, Inc., model: Lumina II) for all surviving cases 26 days after transplantation. From the results, 10 animals per group were assigned by random stratification so that the average of Total Lux in each group was uniform.
- Example 11 The compound of Example 11 was used as the test compound, and a 0.5% HPMC aqueous solution was used as the control.
- Example 11 was administered at a dose of 12.5 mg / kg / day or 25 mg / kg / day.
- the compound or control of the present invention was orally administered once a day for 38 days (Day 27-64) from the day after the grouping day.
- the value obtained by logarithmically converting (Log10) the TotalFlux on the day of determining the antitumor effect (Day47), which is 3 weeks after the administration of the drug from the day after the grouping day (Day27), was used.
- a graph was created in which the average TotalFlux value of each group was set on the vertical axis and the number of days after transplantation (Day) was set on the horizontal axis, and the transition of TotalFlux during the drug administration period was observed.
- the survival days from the cell transplantation of the test compound group compared with the control group to the final evaluation date (Day 0-65) of the life-prolonging effect were analyzed using the Log-Rank test.
- the compound of the present invention or a salt thereof has EGFR inhibitory activity and brain transferability, and is useful as an EGFR inhibitor or a therapeutic agent for EGFR-positive tumors.
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Epidemiology (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Nitrogen Condensed Heterocyclic Rings (AREA)
Priority Applications (10)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| AU2021309779A AU2021309779B2 (en) | 2020-07-15 | 2021-07-14 | EGFR inhibitor |
| CA3189460A CA3189460A1 (en) | 2020-07-15 | 2021-07-14 | Egfr inhibitor |
| KR1020237001973A KR102873219B1 (ko) | 2020-07-15 | 2021-07-14 | Egfr 저해제 |
| PH1/2023/550119A PH12023550119A1 (en) | 2020-07-15 | 2021-07-14 | Egfr inhibitor |
| EP21842292.1A EP4197538A4 (en) | 2020-07-15 | 2021-07-14 | Egfr inhibitor |
| US18/016,103 US20230285397A1 (en) | 2020-07-15 | 2021-07-14 | Egfr inhibitor |
| BR112023000770A BR112023000770A2 (pt) | 2020-07-15 | 2021-07-14 | Inibidor de egfr |
| JP2022536419A JP7495983B2 (ja) | 2020-07-15 | 2021-07-14 | Egfr阻害剤 |
| MX2023000693A MX2023000693A (es) | 2020-07-15 | 2021-07-14 | Inhibidor de egfr. |
| CN202180061165.8A CN116368136B (zh) | 2020-07-15 | 2021-07-14 | Egfr抑制剂 |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2020-121525 | 2020-07-15 | ||
| JP2020121525 | 2020-07-15 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2022014639A1 true WO2022014639A1 (ja) | 2022-01-20 |
Family
ID=79554696
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2021/026461 Ceased WO2022014639A1 (ja) | 2020-07-15 | 2021-07-14 | Egfr阻害剤 |
Country Status (12)
| Country | Link |
|---|---|
| US (1) | US20230285397A1 (enExample) |
| EP (1) | EP4197538A4 (enExample) |
| JP (1) | JP7495983B2 (enExample) |
| KR (1) | KR102873219B1 (enExample) |
| CN (1) | CN116368136B (enExample) |
| AU (1) | AU2021309779B2 (enExample) |
| BR (1) | BR112023000770A2 (enExample) |
| CA (1) | CA3189460A1 (enExample) |
| MX (1) | MX2023000693A (enExample) |
| PH (1) | PH12023550119A1 (enExample) |
| TW (1) | TW202216152A (enExample) |
| WO (1) | WO2022014639A1 (enExample) |
Cited By (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPWO2022014638A1 (enExample) * | 2020-07-15 | 2022-01-20 | ||
| JPWO2022014640A1 (enExample) * | 2020-07-15 | 2022-01-20 | ||
| WO2024206858A1 (en) | 2023-03-30 | 2024-10-03 | Revolution Medicines, Inc. | Compositions for inducing ras gtp hydrolysis and uses thereof |
| WO2024229406A1 (en) | 2023-05-04 | 2024-11-07 | Revolution Medicines, Inc. | Combination therapy for a ras related disease or disorder |
| WO2025034702A1 (en) | 2023-08-07 | 2025-02-13 | Revolution Medicines, Inc. | Rmc-6291 for use in the treatment of ras protein-related disease or disorder |
| WO2025080946A2 (en) | 2023-10-12 | 2025-04-17 | Revolution Medicines, Inc. | Ras inhibitors |
| WO2025171296A1 (en) | 2024-02-09 | 2025-08-14 | Revolution Medicines, Inc. | Ras inhibitors |
| WO2025240847A1 (en) | 2024-05-17 | 2025-11-20 | Revolution Medicines, Inc. | Ras inhibitors |
| WO2025255438A1 (en) | 2024-06-07 | 2025-12-11 | Revolution Medicines, Inc. | Methods of treating a ras protein-related disease or disorder |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP4351737A4 (en) * | 2021-05-24 | 2025-05-14 | Taiho Pharmaceutical Co., Ltd. | Treatment methods for subjects with cancer having an aberration in egfr and/or her2 |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2010065898A2 (en) * | 2008-12-05 | 2010-06-10 | Principia Biopharma Inc. | Egfr kinase knockdown via electrophilically enhanced inhibitors |
| WO2013118817A1 (ja) * | 2012-02-07 | 2013-08-15 | 大鵬薬品工業株式会社 | キノリルピロロピリミジン化合物又はその塩 |
| WO2017038838A1 (ja) | 2015-09-01 | 2017-03-09 | 大鵬薬品工業株式会社 | 新規なピラゾロ[3,4-d]ピリミジン化合物又はその塩 |
| WO2017146116A1 (ja) | 2016-02-23 | 2017-08-31 | 大鵬薬品工業株式会社 | 新規縮合ピリミジン化合物又はその塩 |
| WO2020145374A1 (ja) * | 2019-01-11 | 2020-07-16 | 大鵬薬品工業株式会社 | ピリミジン化合物又はその塩 |
Family Cites Families (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9034885B2 (en) * | 2012-01-13 | 2015-05-19 | Acea Biosciences Inc. | EGFR modulators and uses thereof |
-
2021
- 2021-07-14 CA CA3189460A patent/CA3189460A1/en active Pending
- 2021-07-14 TW TW110125853A patent/TW202216152A/zh unknown
- 2021-07-14 PH PH1/2023/550119A patent/PH12023550119A1/en unknown
- 2021-07-14 WO PCT/JP2021/026461 patent/WO2022014639A1/ja not_active Ceased
- 2021-07-14 EP EP21842292.1A patent/EP4197538A4/en active Pending
- 2021-07-14 CN CN202180061165.8A patent/CN116368136B/zh active Active
- 2021-07-14 MX MX2023000693A patent/MX2023000693A/es unknown
- 2021-07-14 BR BR112023000770A patent/BR112023000770A2/pt unknown
- 2021-07-14 US US18/016,103 patent/US20230285397A1/en active Pending
- 2021-07-14 KR KR1020237001973A patent/KR102873219B1/ko active Active
- 2021-07-14 AU AU2021309779A patent/AU2021309779B2/en active Active
- 2021-07-14 JP JP2022536419A patent/JP7495983B2/ja active Active
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2010065898A2 (en) * | 2008-12-05 | 2010-06-10 | Principia Biopharma Inc. | Egfr kinase knockdown via electrophilically enhanced inhibitors |
| WO2013118817A1 (ja) * | 2012-02-07 | 2013-08-15 | 大鵬薬品工業株式会社 | キノリルピロロピリミジン化合物又はその塩 |
| WO2017038838A1 (ja) | 2015-09-01 | 2017-03-09 | 大鵬薬品工業株式会社 | 新規なピラゾロ[3,4-d]ピリミジン化合物又はその塩 |
| WO2017146116A1 (ja) | 2016-02-23 | 2017-08-31 | 大鵬薬品工業株式会社 | 新規縮合ピリミジン化合物又はその塩 |
| WO2020145374A1 (ja) * | 2019-01-11 | 2020-07-16 | 大鵬薬品工業株式会社 | ピリミジン化合物又はその塩 |
Non-Patent Citations (5)
| Title |
|---|
| "NCBI", Database accession no. NP_005219 |
| HIROKAWA SHOTEN: "lyakuhin no Kaihatsu (Development of Pharmaceutical Products", BUNSHI SEKKEI (MOLECULAR DESIGNING, vol. 7, 1990, pages 163 - 198 |
| NOBUO IZUMIYA ET AL.: "Peptide Gosei no Kiso to Jikken (Principle of Peptide Synthesis and Experiments", 1983, MARUZEN CO., LTD. |
| See also references of EP4197538A4 |
| T. W. GREENEP. G. M. WUTS: "Protective Groups in Organic Synthesis", 1981, JOHN WILEY & SONS |
Cited By (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPWO2022014638A1 (enExample) * | 2020-07-15 | 2022-01-20 | ||
| JPWO2022014640A1 (enExample) * | 2020-07-15 | 2022-01-20 | ||
| JP7373664B2 (ja) | 2020-07-15 | 2023-11-02 | 大鵬薬品工業株式会社 | 腫瘍の治療に使用されるピリミジン化合物を含む組み合わせ |
| JP7391226B2 (ja) | 2020-07-15 | 2023-12-04 | 大鵬薬品工業株式会社 | ピリミジン化合物の結晶 |
| WO2024206858A1 (en) | 2023-03-30 | 2024-10-03 | Revolution Medicines, Inc. | Compositions for inducing ras gtp hydrolysis and uses thereof |
| WO2024229406A1 (en) | 2023-05-04 | 2024-11-07 | Revolution Medicines, Inc. | Combination therapy for a ras related disease or disorder |
| WO2025034702A1 (en) | 2023-08-07 | 2025-02-13 | Revolution Medicines, Inc. | Rmc-6291 for use in the treatment of ras protein-related disease or disorder |
| WO2025080946A2 (en) | 2023-10-12 | 2025-04-17 | Revolution Medicines, Inc. | Ras inhibitors |
| WO2025171296A1 (en) | 2024-02-09 | 2025-08-14 | Revolution Medicines, Inc. | Ras inhibitors |
| WO2025240847A1 (en) | 2024-05-17 | 2025-11-20 | Revolution Medicines, Inc. | Ras inhibitors |
| WO2025255438A1 (en) | 2024-06-07 | 2025-12-11 | Revolution Medicines, Inc. | Methods of treating a ras protein-related disease or disorder |
Also Published As
| Publication number | Publication date |
|---|---|
| BR112023000770A2 (pt) | 2023-02-07 |
| KR102873219B1 (ko) | 2025-10-17 |
| TW202216152A (zh) | 2022-05-01 |
| KR20230026451A (ko) | 2023-02-24 |
| PH12023550119A1 (en) | 2024-06-24 |
| CN116368136B (zh) | 2025-09-05 |
| AU2021309779B2 (en) | 2024-06-27 |
| JPWO2022014639A1 (enExample) | 2022-01-20 |
| JP7495983B2 (ja) | 2024-06-05 |
| EP4197538A4 (en) | 2024-08-07 |
| CN116368136A (zh) | 2023-06-30 |
| AU2021309779A1 (en) | 2023-02-23 |
| MX2023000693A (es) | 2023-02-13 |
| US20230285397A1 (en) | 2023-09-14 |
| CA3189460A1 (en) | 2022-01-20 |
| EP4197538A1 (en) | 2023-06-21 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2022014639A1 (ja) | Egfr阻害剤 | |
| JP6783974B1 (ja) | ピリミジン化合物又はその塩 | |
| CN106414431A (zh) | 具有bace1抑制作用的二氢噻嗪和二氢噁嗪衍生物 | |
| KR101905295B1 (ko) | 나프티리딘디온 유도체 | |
| WO2019057103A1 (zh) | Jak激酶抑制剂及其制备方法和在医药领域的应用 | |
| JP7373664B2 (ja) | 腫瘍の治療に使用されるピリミジン化合物を含む組み合わせ | |
| RU2817044C1 (ru) | Ингибитор egfr | |
| RU2787992C1 (ru) | Пиримидиновое соединение или его соль | |
| WO2022014638A1 (ja) | ピリミジン化合物の結晶 | |
| BR122024008319A2 (pt) | Composto pirimidina, composição farmacêutica e agente antitumoral que compreendem o mesmo e usos do dito composto para o tratamento de tumor | |
| BR112021013460B1 (pt) | Composto pirimidina, composição farmacêutica e agente antitumoral que compreendem o mesmo e usos do dito composto para o tratamento de tumor | |
| HK40090490A (zh) | Egfr抑制剂 | |
| HK40056369B (zh) | 嘧啶化合物或其盐 | |
| HK40056369A (en) | Pyrimidine compound or salt thereof | |
| WO2024184316A1 (en) | Bifunctional protein targeting chimeras (protacs) comprising arylidene-indolinone scaffold as dcaf11 ligand and use thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 21842292 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 3189460 Country of ref document: CA Ref document number: 2022536419 Country of ref document: JP Kind code of ref document: A |
|
| ENP | Entry into the national phase |
Ref document number: 20237001973 Country of ref document: KR Kind code of ref document: A |
|
| REG | Reference to national code |
Ref country code: BR Ref legal event code: B01A Ref document number: 112023000770 Country of ref document: BR |
|
| ENP | Entry into the national phase |
Ref document number: 112023000770 Country of ref document: BR Kind code of ref document: A2 Effective date: 20230113 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| ENP | Entry into the national phase |
Ref document number: 2021309779 Country of ref document: AU Date of ref document: 20210714 Kind code of ref document: A |
|
| ENP | Entry into the national phase |
Ref document number: 2021842292 Country of ref document: EP Effective date: 20230215 |
|
| WWG | Wipo information: grant in national office |
Ref document number: 202180061165.8 Country of ref document: CN |