WO2021187526A1 - Procede de production de composition de resine absorbante particulaire, de corps absorbant et d'article absorbant - Google Patents
Procede de production de composition de resine absorbante particulaire, de corps absorbant et d'article absorbant Download PDFInfo
- Publication number
- WO2021187526A1 WO2021187526A1 PCT/JP2021/010818 JP2021010818W WO2021187526A1 WO 2021187526 A1 WO2021187526 A1 WO 2021187526A1 JP 2021010818 W JP2021010818 W JP 2021010818W WO 2021187526 A1 WO2021187526 A1 WO 2021187526A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- water
- absorbent resin
- particles
- mass
- granulated
- Prior art date
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F13/00—Bandages or dressings; Absorbent pads
- A61F13/15—Absorbent pads, e.g. sanitary towels, swabs or tampons for external or internal application to the body; Supporting or fastening means therefor; Tampon applicators
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F13/00—Bandages or dressings; Absorbent pads
- A61F13/15—Absorbent pads, e.g. sanitary towels, swabs or tampons for external or internal application to the body; Supporting or fastening means therefor; Tampon applicators
- A61F13/53—Absorbent pads, e.g. sanitary towels, swabs or tampons for external or internal application to the body; Supporting or fastening means therefor; Tampon applicators characterised by the absorbing medium
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08J—WORKING-UP; GENERAL PROCESSES OF COMPOUNDING; AFTER-TREATMENT NOT COVERED BY SUBCLASSES C08B, C08C, C08F, C08G or C08H
- C08J3/00—Processes of treating or compounding macromolecular substances
- C08J3/12—Powdering or granulating
Definitions
- the present invention relates to a method for producing a particulate water-absorbent resin composition, an absorber and an absorbent article.
- Absorbents containing water-absorbent resin particles are used for absorbent articles for absorbing water-based liquids such as urine.
- a step of pulverizing the block-shaped polymer obtained by the polymerization into particles by a treatment such as pulverization is performed.
- Small particles such as particles having a particle size of less than 180 ⁇ m generated by pulverization are used by increasing the particle size by granulation (for example, Patent Document 1).
- Absorbent articles are required to have excellent liquid absorbency under pressure in consideration of the usage mode in which pressure is applied due to body weight or the like.
- An object of the present invention is to provide a particulate water-absorbent resin composition capable of providing an absorbent article having excellent absorbency under pressure.
- the method for producing a particulate water-absorbent resin composition of the present invention comprises polymerizing a monomer containing an ethylenically unsaturated monomer to obtain a hydrogel polymer, and granulating the hydrogel polymer. To obtain a particle group containing fine particles having a particle size of less than 180 ⁇ m, to obtain a granulated particle group containing granulated particles by granulating the particle group, and to re-particle the granulated particle group to obtain a polymer.
- Water-absorbent resin particles containing polymer particles obtained through a granulation step can be obtained by a method including obtaining particles, and water-absorbent resin particles containing polymer particles obtained by obtaining the granulation step.
- the water-absorbent resin particles containing the polymer particles obtained without going through the granulation step are mixed to obtain a particulate water-absorbent resin composition.
- the abundance rate of the granulated particles that do not pass through the JIS standard sieve having an opening of 850 ⁇ m is 70% by mass or more
- the mixing ratio of the water-absorbent resin particles obtained through the granulation step is the particulate water-absorbent resin composition. It is 40 to 80% by mass with respect to the total amount.
- the abundance rate of the granulated particles that do not pass through the JIS standard sieve having a mesh size of 31.5 mm is 5% by mass or more in the granulated particle group before repartitioning.
- the present invention also provides a method for producing an absorber, which comprises obtaining a particulate water-absorbent resin composition by the above method and obtaining an absorber containing the particulate water-absorbent resin composition.
- the present invention also provides a method for producing an absorbent article, which comprises obtaining an absorber by the above method and arranging the absorber between a liquid permeable sheet and a liquid permeable sheet.
- Water-soluble means that it exhibits a solubility in water of 5% by mass or more at 25 ° C.
- the materials exemplified in the present specification may be used alone or in combination of two or more.
- the content of each component in the composition means the total amount of the plurality of substances present in the composition when a plurality of substances corresponding to each component are present in the composition, unless otherwise specified.
- “Saline” refers to a 0.9% by mass sodium chloride aqueous solution.
- the particles that pass through the sieve of the opening X mean that the particles have a size that allows them to pass through the sieve of the opening X, and a step of actually passing the particles through the sieve of the opening X is required. It is not something to do.
- the method for producing a particulate water-absorbent resin composition according to the present embodiment includes water-absorbent resin particles containing polymer particles obtained by obtaining a granulation step (hereinafter, also referred to as “granulated water-absorbent resin particles”). Includes mixing with water-absorbent resin particles (hereinafter, also referred to as “non-granulated water-absorbent resin particles”) obtained without undergoing a granulation step.
- the particulate water-absorbent resin composition according to the present embodiment is a mixture containing at least the above two types of water-absorbent resin particles.
- the method for producing the granulated water-absorbent resin particles is to polymerize a monomer containing an ethylenically unsaturated monomer to obtain a hydrogel polymer, and to granulate the hydrogel polymer to have a particle diameter of 180 ⁇ m. To obtain a particle group containing less than fine powder, to obtain a granulated particle group containing granulated particles by granulating the particle group, and to obtain a polymer particle by granulating the granulated particle group again. And, including.
- the method for producing the granulated water-absorbent resin particles will be described in detail.
- a monomer containing an ethylenically unsaturated monomer is polymerized to obtain a hydrogel polymer.
- the hydrogel-like polymer may be a crosslinked polymer formed by polymerization of a monomer containing an ethylenically unsaturated monomer, which contains water and becomes a gel.
- the water-absorbent resin particles can contain a crosslinked polymer formed by polymerizing a monomer containing an ethylenically unsaturated monomer.
- the crosslinked polymer has a monomeric unit derived from an ethylenically unsaturated monomer. That is, the water-absorbent resin particles can have a structural unit derived from the ethylenically unsaturated monomer.
- Polymerization can be carried out by, for example, an aqueous solution polymerization method.
- an aqueous solution polymerization method the polymerization of the monomer by the aqueous solution polymerization method will be described.
- the ethylenically unsaturated monomer is preferably water-soluble.
- examples of the ethylenically unsaturated monomer include ⁇ , ⁇ -unsaturated carboxylic acids such as (meth) acrylic acid, maleic acid, maleic anhydride and fumaric acid, and carboxylic acid-based monomers such as salts thereof; (meth).
- Nonionic monomers such as acrylamide, N, N-dimethyl (meth) acrylamide, 2-hydroxyethyl (meth) acrylate, N-methylol (meth) acrylamide, polyethylene glycol mono (meth) acrylate; N, N- Amino group-containing unsaturated monomers such as diethylaminoethyl (meth) acrylate, N, N-diethylaminopropyl (meth) acrylate, diethylaminopropyl (meth) acrylamide and quaternized products thereof; vinyl sulfonic acid, styrene sulfonic acid, 2 Examples thereof include sulfonic acid-based monomers such as- (meth) acrylamide-2-methylpropanesulfonic acid, 2- (meth) acryloylethanesulfonic acid and salts thereof.
- the ethylenically unsaturated monomer one type may be used alone, or two or more types may be used
- the ethylenically unsaturated monomer preferably contains at least one selected from the group consisting of (meth) acrylic acid and salts thereof, (meth) acrylamide, and N, N-dimethylacrylamide, and is preferably (meth) acrylic acid. It is more preferable to contain at least one selected from and salts thereof. Further, (meth) acrylic acid and a salt thereof may be copolymerized with another ethylenically unsaturated monomer. In this case, 70 to 100 mol% of the above (meth) acrylic acid and a salt thereof are preferably used, more preferably 80 to 100 mol%, and 90 to 90 to 100 mol% of the total amount of the ethylenically unsaturated monomer. It is more preferable to use 100 mol%.
- the ethylenically unsaturated monomer preferably contains at least one of acrylic acid and a salt thereof.
- the acid group is an alkaline neutralizer in advance if necessary.
- an alkaline neutralizer include alkali metal salts such as sodium hydroxide, sodium carbonate, sodium hydrogencarbonate, potassium hydroxide and potassium carbonate; ammonia and the like.
- alkaline neutralizers may be used in the form of an aqueous solution in order to simplify the neutralization operation.
- One type of alkaline neutralizer may be used alone, or two or more types may be used in combination.
- the acid group may be neutralized before the polymerization of the ethylenically unsaturated monomer as a raw material, or during or after the polymerization.
- the degree of neutralization of the ethylenically unsaturated monomer by the alkaline neutralizer enhances the water absorption performance by increasing the osmotic pressure of the obtained water-absorbent resin particles, and is safe due to the presence of the excess alkaline neutralizer. From the viewpoint of preventing problems such as, usually, it is preferably 10 to 100 mol%, more preferably 30 to 90 mol%, further preferably 40 to 85 mol%, and 50. It is even more preferably ⁇ 80 mol%.
- the degree of neutralization is the degree of neutralization for all the acid groups of the ethylenically unsaturated monomer.
- the ethylenically unsaturated monomer is usually preferably used in the state of an aqueous solution.
- concentration of the ethylenically unsaturated monomer in the aqueous solution containing the ethylenically unsaturated monomer may be 20% by mass or more and the saturation concentration or less, and is 25 to 70% by mass. %, More preferably 30 to 50% by mass.
- the amount of the ethylenically unsaturated monomer used is the total amount of the monomers (the total amount of the monomers for obtaining the water-absorbent resin particles. For example, the total amount of the monomers giving the structural unit of the crosslinked polymer. The same shall apply hereinafter). It may be 70 to 100 mol%, 80 to 100 mol%, 90 to 100 mol%, 95 to 100 mol%, or 100 mol%. Among them, the ratio of (meth) acrylic acid and its salt may be 70 to 100 mol% with respect to the total amount of the monomer, 80 to 100 mol%, 90 to 100 mol%, 95 to 100 mol%, or It may be 100 mol%. "Ratio of (meth) acrylic acid and its salt” means the ratio of the total amount of (meth) acrylic acid and its salt.
- the water-absorbent resin particles are, for example, water-absorbent resin particles containing a crosslinked polymer having a structural unit derived from an ethylenically unsaturated monomer, and the ethylenically unsaturated monomer is composed of (meth) acrylic acid and It contains at least one compound selected from the group consisting of the salts, and the ratio of (meth) acrylic acid and its salts is 70 to 100 mol% with respect to the total amount of monomers for obtaining water-absorbent resin particles. It may be a thing.
- the monomer aqueous solution may contain a polymerization initiator.
- the polymerization of the monomer contained in the aqueous monomer solution is started by adding a polymerization initiator to the aqueous monomer solution and, if necessary, heating, irradiating with light or the like.
- the polymerization initiator include a photopolymerization initiator and a radical polymerization initiator, and among them, a water-soluble radical polymerization initiator is preferably used.
- the polymerization initiator may be, for example, an azo compound, a peroxide or the like.
- Examples of the azo compound include 2,2'-azobis [2- (N-phenylamidino) propane] dihydrochloride and 2,2'-azobis ⁇ 2- [N- (4-chlorophenyl) amidino] propane ⁇ .
- 2,2'-azobis (2-amidinopropane) dihydrochloride 2,2'-azobis ⁇ 2- [1- (2-hydroxy) Ethyl) -2-imidazolin-2-yl] propane ⁇ dihydrochloride and 2,2'-azobis [N- (2-carboxyethyl) -2-methylpropion amidine] tetrahydrate are preferred.
- One of these azo compounds may be used alone, or two or more thereof may be used in combination.
- peroxide examples include persulfates such as potassium persulfate, ammonium persulfate, and sodium persulfate; methyl ethyl ketone peroxide, methyl isobutyl ketone peroxide, di-t-butyl peroxide, t-butyl cumyl peroxide, t. -Organic peroxides such as butylperoxyacetate, t-butylperoxyisobutyrate, t-butylperoxypivalate; peroxides such as hydrogen peroxide can be mentioned.
- persulfates such as potassium persulfate, ammonium persulfate, and sodium persulfate
- methyl ethyl ketone peroxide methyl isobutyl ketone peroxide
- di-t-butyl peroxide di-t-butyl peroxide
- t-butyl cumyl peroxide t.
- potassium persulfate, ammonium persulfate, sodium persulfate or hydrogen peroxide is preferably used from the viewpoint of obtaining water-absorbent resin particles having good water absorption performance, and potassium persulfate and persulfate are preferable. It is more preferable to use ammonium sulfate or sodium persulfate.
- One of these peroxides may be used alone, or two or more thereof may be used in combination.
- a redox polymerization initiator can also be used as a redox polymerization initiator by using a polymerization initiator and a reducing agent in combination.
- the reducing agent include sodium sulfite, sodium hydrogen sulfite, ferrous sulfate, L-ascorbic acid and the like.
- the total amount of the polymerization initiator used is preferably 0.001 to 1 mol with respect to 100 mol of the ethylenically unsaturated monomer used for the polymerization from the viewpoint of avoiding a rapid polymerization reaction and shortening the polymerization reaction time. , 0.005 to 0.5 mol, more preferably 0.008 to 0.3 mol, even more preferably 0.01 to 0.2 mol.
- the monomer aqueous solution preferably contains an internal cross-linking agent.
- the obtained cross-linked polymer can have cross-linking by the internal cross-linking agent in addition to self-cross-linking by the polymerization reaction as its internal cross-linking structure.
- the internal cross-linking agent for example, a compound having two or more polymerizable unsaturated groups is used, and preferably, a compound having two polymerizable unsaturated groups is used.
- di or tri (meth) acrylic acid esters of polyols such as (poly) ethylene glycol, (poly) propylene glycol, trimethylpropane, glycerin polyoxyethylene glycol, polyoxypropylene glycol, and (poly) glycerin; Unsaturated polyesters obtained by reacting the above polyol with unsaturated acids such as maleic acid and fumaric acid; bisacrylamides such as N, N'-methylenebis (meth) acrylamide; polyepoxide and (meth) acrylic acid.
- Di or tri (meth) acrylic acid esters obtained by reaction carbamil di (meth) acrylic acid obtained by reacting polyisocyanate such as tolylene diisocyanate or hexamethylene diisocyanate with hydroxyethyl (meth) acrylic acid.
- Esters allylicated starch; allylated cellulose; diallyl phthalate; N, N', N "-triallyl isocyanurate; divinylbenzene and the like.
- a compound having two or more reactive functional groups can be used as an internal cross-linking agent.
- glycidyl group-containing compounds such as (poly) ethylene glycol diglycidyl ether, (poly) propylene glycol diglycidyl ether, and (poly) glycerin diglycidyl ether; (poly) ethylene glycol, (poly) propylene glycol, (poly). Examples thereof include glycerin, pentaerythritol, ethylenediamine, polyethyleneimine, and glycidyl (meth) acrylate.
- (poly) ethylene glycol diglycidyl ether, (poly) propylene glycol diglycidyl ether, and (poly) glycerin diglycidyl ether are preferable from the viewpoint of excellent reactivity at low temperature.
- These internal cross-linking agents may be used alone or in combination of two or more.
- the amount used is 0.0001 mol with respect to 100 mol of the ethylenically unsaturated monomer in order to sufficiently enhance the water absorption performance such as the water absorption capacity of the obtained water-absorbent resin particles.
- the above is preferable, 0.001 mol or more is more preferable, 0.003 mol or more is further preferable, and 0.01 mol or more is further preferable.
- the addition of the internal cross-linking agent insolubilizes the cross-linked polymer and brings about a suitable water absorption capacity, but an increase in the amount of the internal cross-linking agent added leads to a decrease in the water absorption capacity of the obtained water-absorbent resin particles. Therefore, the amount of the internal cross-linking agent is preferably, for example, 0.50 mol or less, more preferably 0.25 mol or less, and further preferably 0. It is 10 mol or less.
- the monomer aqueous solution may contain additives such as a chain transfer agent and a thickener, if necessary.
- a chain transfer agent include thiols, thiol acids, secondary alcohols, hypophosphorous acid, phosphorous acid and the like.
- the thickener include carboxymethyl cellulose, hydroxyethyl cellulose, hydroxypropyl cellulose, methyl cellulose, polyethylene glycol, polyacrylic acid, neutralized polyacrylic acid, polyacrylamide and the like. One of these may be used alone, or two or more thereof may be used in combination.
- a solvent other than water such as a water-soluble organic solvent, may be appropriately added to the monomer aqueous solution.
- the polymerization method is, for example, a static polymerization method in which the monomer aqueous solution is polymerized without stirring (for example, a static state), or a stirring polymerization method in which the monomer aqueous solution is polymerized while being stirred in the reaction apparatus. It's okay. It is preferable to obtain a hydrogel-like polymer by aqueous solution static polymerization, which is a static polymerization method. In the static polymerization method, when the polymerization is completed, a single block-shaped hydrogel-like polymer occupying substantially the same volume as the monomer aqueous solution existing in the reaction vessel can be obtained.
- the form of production may be batch, semi-continuous, continuous, etc.
- a polymerization reaction can be carried out while continuously supplying a monomer aqueous solution to a belt conveyor-shaped continuous polymerization apparatus to obtain a hydrogel having a continuous shape such as a band shape. ..
- the polymerization temperature varies depending on the polymerization initiator used, but is preferably 0 to 130 ° C., more preferably 10 to 110 ° C. from the viewpoint of increasing the productivity by rapidly advancing the polymerization and shortening the polymerization time.
- the polymerization time is appropriately set according to the type and amount of the polymerization initiator used, the reaction temperature, and the like, but is preferably 1 to 200 minutes, more preferably 5 to 100 minutes.
- the water content of the block-shaped water-containing gel-like polymer obtained by polymerizing the monomers is preferably 30 to 80% by mass, more preferably 40 to 75% by mass, from the viewpoint that the coarse crushing step can be easily carried out. 50 to 70% by mass is more preferable.
- the water content of the water-containing gel-like polymer is adjusted by operations such as the water content of the monomer aqueous solution, drying after polymerization, and humidification.
- the water content of the hydrogel polymer is the content of water in the total mass of the hydrogel polymer in% by mass.
- the hydrogel polymer is atomized to obtain a particle group containing fine particles having a particle size of less than 180 ⁇ m.
- a group of particles containing fine particles having a particle diameter of less than 180 ⁇ m is finally obtained.
- a step of obtaining a particle group and a step of further crushing the coarsely crushed product to obtain a particle group may be included.
- the particle swarming step may include the step of drying the hydrogel polymer, the crude product and / or the particle group.
- the coarsely crushed product is preferably subjected to crushing after undergoing a drying step.
- the particle swarming step may further include a step of classifying the particle group obtained by pulverization.
- the particle group obtained by the particle swarming step may consist only of fine particles having a particle size of less than 180 ⁇ m.
- the particleization step can include a step of coarsely crushing the hydrogel polymer to obtain a coarsely crushed product.
- the crude product obtained by coarsely crushing the hydrogel-like polymer may be in the form of a hydrogel.
- the coarsely crushed product may be in the form of particles, or may have an elongated shape in which particles are connected.
- the size of the minimum side of the coarsely crushed product may be, for example, about 0.1 to 15 mm, preferably about 1.0 to 10 mm.
- the size of the maximum side of the coarsely crushed product may be about 0.1 to 200 mm, preferably about 1.0 to 150 mm.
- a kneader for example, a pressurized kneader, a double-armed kneader, etc.
- a meat chopper for example, a meat chopper, and a cutter mill are more preferable.
- the crushing device may be of the same type as the crushing device described later.
- the hydrogel polymer may be cut into a size of, for example, about 5 cm square using a cutting machine. After cutting the hydrogel polymer with a cutting machine, it may be further coarsely crushed using a crushing device.
- the polymerization step and the coarse crushing step may be carried out substantially at the same time.
- the particleization step can include the step of drying the hydrogel polymer, the coarsely crushed product and / or the pulverized product. These dried products can be obtained by removing the solvent containing water in the hydrogel polymer, coarsely crushed product or crushed product by heating and / or blowing air. Drying is preferably carried out after the hydrogel polymer is roughly crushed and before crushing. That is, the particle-forming step preferably includes a step of drying the hydrogel-like coarse crushed product to obtain a dry crushed product.
- the drying method may be natural drying, heat drying, or vacuum drying.
- the drying may be performed under normal pressure or reduced pressure, for example, and may be performed under an air flow such as nitrogen in order to improve the drying efficiency.
- a plurality of methods may be used in combination.
- the drying temperature is preferably 70 to 250 ° C, more preferably 80 to 200 ° C.
- the drying step is carried out until the water content of the coarsely crushed product is 20% by mass or less, preferably 10% by mass or less, and more preferably 5% by mass or less.
- the drying temperature may be 120 ° C. or higher, 150 ° C. or higher, or 180 ° C. or higher.
- the particleization step can include a step of crushing a hydrogel polymer, a coarsely crushed product and / or a dried product thereof. By pulverizing the hydrogel polymer, coarsely crushed product and / or dried product thereof, a particle group containing fine powder can be obtained.
- the pulverization is preferably carried out after coarse crushing and drying. That is, the particle swarming step preferably includes a step of pulverizing the dried coarse crushed product to obtain a particle group containing fine powder.
- roller mill roller mill
- stamp mill stamp mill
- jet mill high-speed rotary crusher
- hammer mill pin mill, rotor beater mill, etc.
- container-driven mill rotary mill, vibration mill, planetary mill, etc.
- Crusher can be used.
- a high speed rotary grinder is used.
- the crusher may have an opening on the outlet side, such as a perforated plate, a screen, or a grid, for controlling the maximum particle size of the crushed particles.
- the shape of the opening may be polygonal, circular, or the like, and the maximum diameter of the opening may be 0.1 to 5 mm, 0.3 to 3.0 mm, or 0.5 to 1.5 mm.
- Grinding may be performed so that at least a part of the particle group becomes fine powder having a particle size of less than 180 ⁇ m.
- the pulverization for example, while pulverizing for the main purpose of obtaining polymer particles having a particle size of less than 850 ⁇ m and having an appropriate particle size that can be used without granulation, some fine particles having a particle size of less than 180 ⁇ m are generated. It can be done in such a way.
- the abundance of fine particles having a particle size of less than 180 ⁇ m in the total amount of the particle group obtained by the particle swarming step may be, for example, 1 to 100% by mass, preferably 30 to 60% by mass.
- the particle size of less than 180 ⁇ m means that the particle size can pass through a JIS standard sieve having a mesh size of 180 ⁇ m.
- the particle group obtained by the particle formation step may include particles having a particle diameter of 180 ⁇ m or more and less than 850 ⁇ m (particles that pass through a JIS standard sieve having a mesh size of 850 ⁇ m and do not pass through a JIS standard sieve having a mesh size of 180 ⁇ m).
- Particles having a particle diameter of 250 ⁇ m or more and less than 850 ⁇ m may be included.
- the particle swarming step may further include a step of classifying a group of particles containing fine particles having a particle size of less than 180 ⁇ m obtained by grinding.
- Classification refers to an operation of dividing a certain particle group into two or more particle groups having different particle size distributions according to the particle size.
- a particle group consisting of only fine particles having a particle size of less than 180 ⁇ m may be separated, or the abundance of fine particles having a particle size of less than 180 ⁇ m in the particle group may be increased. If necessary, a part of the classified particles may be pulverized again, or the pulverization step and the classification step may be repeated.
- a known classification method can be used for the classification of the particle group, and for example, screen classification, wind power classification, or the like may be used.
- Screen classification is a method of classifying particles on a screen into particles that pass through the mesh of the screen and particles that do not pass through the screen by vibrating the screen.
- Wind power classification is a method of classifying particles using the flow of air.
- the classification method it is preferable to use screen classification. Examples of the screen classification include a vibrating sieve, a rotary shifter, a cylindrical stirring sieve, a blower shifter, a low-tap type shaker, an electric vibration type shaker, and the like.
- a group of particles containing fine particles having a particle size of less than 180 ⁇ m is granulated to obtain a group of granulated particles containing the granulated particles.
- Granulation is performed so that the abundance of granulated particles that do not pass through the JIS standard sieve having a mesh size of 850 ⁇ m is 70% by mass or more in the obtained granulated particle group.
- granulation means agglomerating particles to obtain particles having a larger particle size than the original particles.
- the abundance of fine powder having a particle diameter of less than 180 ⁇ m is 10% by mass or more, 20% by mass or more, 30% by mass or more, 40% by mass or more, 50% by mass or more, 60% by mass or more. It may be 70% by mass or more, 80% by mass or more, 90% by mass or more, 95% by mass or more or 98% by mass or more, 100% by mass, 98% by mass or less, 95% by mass or less, 90% by mass or less, 80% by mass. % Or less, 70% by mass or less, 60% by mass or less, 50% by mass or less, 40% by mass or less, or 30% by mass or less.
- Granulation can be performed, for example, by mixing a group of particles and water.
- Water may be used as an aqueous solution containing components such as water-soluble polymerizable monomers such as water-soluble salts and ethylenically unsaturated monomers, cross-linking agents and hydrophilic organic solvents.
- the cross-linking agent for example, the above-mentioned internal cross-linking agent or the later-described surface cross-linking agent can be used.
- the proportion of water in the aqueous liquid may be, for example, 90 to 100% by mass.
- a method of mixing the particle group and water for example, water may be dropped little by little, the whole amount may be added at once, sprayed, or mixed in the state of steam.
- the mixed amount of water is preferably 55 parts by mass or more, and more preferably 60 parts by mass or more with respect to 100 parts by mass of the particle group. When the mixing amount of water is 55 parts by mass or more, the particle group and water can be mixed more uniformly.
- the mixing amount of water is preferably 115 parts by mass or less, and more preferably 105 parts by mass or less with respect to 100 parts by mass of the particle group. When the mixing amount of water is 115 parts by mass or less, the subsequent drying can be performed more efficiently.
- the temperature at the time of mixing may be, for example, 40 to 150 ° C, preferably 60 to 100 ° C.
- the temperature at the time of mixing may be 80 to 95 ° C.
- Granulation is preferably carried out by mixing water that has been preheated to a mixing temperature with a group of particles. From the viewpoint of more uniform granulation, it is preferable that the granulation is carried out by putting all the preheated water into the pre-stirred particle group in the mixing container at one time.
- the mixing time of the particle group and water is such that the entire amount of the particle group and water used for mixing is put into the same mixing container. After that, it is preferably 10 to 100 seconds, and more preferably 15 to 90 seconds.
- Mixing of the particle group and water can be performed using, for example, various stirrers having stirring blades.
- the stirring blade flat plate blades, lattice blades, paddle blades, propeller blades, anchor blades, turbine blades, Faudler blades, ribbon blades, full zone blades, Maxblend blades and the like can be used.
- the flat plate blade has a shaft (stirring shaft) and a flat plate portion (stirring portion) arranged around the shaft. Further, the flat plate portion may have a slit or the like.
- the stirring type mixer include a mortar mixer, a continuous kneader, a ladyge mixer and the like.
- the mixing conditions of the particle group and water are as follows, for example, the mixing amount of water is 55 to 115 parts by mass with respect to 100 parts by mass of the particle group, and the mixing temperature.
- the mixing time may be 40 to 150 ° C., and the mixing time may be 10 to 100 seconds after the entire amount of the particle group and water is put into the same container, and the mixing amount of water is 60 to 100 parts by mass with respect to 100 parts by mass of the particle group. It may be a combination of 105 parts by mass, a mixing temperature of 60 to 100 ° C., and a mixing time of 15 to 90 seconds after the entire amount of the particle group and water is put into the same container.
- the mixing conditions of the particle group and water may be set so that the ratio of the granulated particles having a predetermined particle size in the obtained granulated particle group is a certain value or more.
- the particles may be confirmed with the naked eye during or after mixing, and if necessary, further mixing may be performed by heating, adding water, adding particle groups, or the like.
- the particle size distribution of the granulated particles can be measured after the granulation step.
- the granulated particle group obtained by granulation it is preferable to use the granulated particle group obtained by granulation as it is without drying.
- the particle size distribution can be measured using all or part of the granulated particle group obtained by granulation.
- the amount of the granulated particle group used for measuring the particle size distribution may be, for example, 30 g or more or 100 g or more, and may be 30 to 100 g.
- the particle size distribution of the granulated particle group is measured by classifying the granulated particle group using a JIS standard sieve with each opening.
- classification for example, using an electromagnetic vibration type sieve shaker Octagon 200 (manufactured by endcotts) in which the vibration intensity is set to 7, a JIS standard sieve and a saucer having a predetermined opening into which measurement samples are placed are placed in the vertical direction. This can be done by vibrating for 10 minutes to shake the particles.
- the abundance rate of the granulated particles that do not pass through the JIS standard sieve having an opening of 850 ⁇ m is 70% by mass or more, preferably 75% by mass or more, and 77% by mass or more. It may be 80% by mass or more, 85% by mass or more, or 88% by mass or more.
- the abundance rate of the granulated particles that do not pass through the JIS standard sieve having an opening of 850 ⁇ m may be, for example, 100% by mass, 98% by mass or less, 95% by mass or less, 90% by mass or less, 88% by mass. % Or less, 85% by mass or less, or 80% by mass or less.
- the abundance rate of the granulated particles that do not pass through the JIS standard sieve having a mesh size of 9.5 mm may be, for example, 55% by mass or more, preferably 60% by mass or more. .. The abundance rate may be 70% by mass or more, 80% by mass or more, or 85% by mass or more.
- the abundance rate of the granulated particles that do not pass through the JIS standard sieve having a mesh size of 9.5 mm may be, for example, 100% by mass, 98% by mass or less, 95% by mass or less, 93% by mass or less. It may be 90% by mass or less, 88% by mass or less, 85% by mass or less, 80% by mass or less, 75% by mass or less, or 70% by mass or less.
- the abundance rate of the granulated particles that do not pass through the JIS standard sieve having a mesh size of 31.5 mm may be, for example, 5% by mass or more, and 10% by mass or more or 15% by mass or more. Is preferable.
- the abundance rate is 20% by mass or more, 25% by mass or more, 30% by mass or more, 35% by mass or more, 40% by mass or more, 45% by mass or more, 50% by mass or more, 55% by mass or more, 60% by mass or more. It may be 65% by mass or more, 70% by mass or more, 75% by mass or more, 80% by mass or more, 85% by mass or more, or 90% by mass or more.
- the abundance rate of the granulated particles that do not pass through the JIS standard sieve having a mesh size of 31.5 mm may be, for example, 100% by mass, 98% by mass or less, 95% by mass or less. 93% by mass or less, 90% by mass or less, 85% by mass or less, 80% by mass or less, 75% by mass or less, 70% by mass or less, 65% by mass or less, 60% by mass or less, 55% by mass or less, 50% by mass or less, It may be 45% by mass or less, 40% by mass or less, 35% by mass or less, 30% by mass or less, or 25% by mass or less.
- the granulated particles used for measuring the particle size distribution may be returned to the manufacturing process of the granulated water-absorbent resin particles and used continuously in the production of the granulated water-absorbent resin particles.
- the granulated particles used for measuring the particle size distribution do not need to be used for producing the granulated water-absorbent resin particles. good.
- the granulated particle group obtained by granulation is re-granulated to obtain polymer particles.
- Polymer particles having a desired particle size can be obtained by re-granulating the particles whose particle size has been increased by granulation.
- the step of further crushing the granulated coarse crushed product to obtain the granulated crushed product (polymer particles) may be included.
- the re-granulation step may include a step of drying the granulated particles, the granulated coarse crushed product, or the pulverized granulated product.
- the granulated coarse crushed product is preferably subjected to pulverization after undergoing a drying step.
- the repartitioning step may further include a step of classifying the granulated pulverized product obtained by pulverization.
- the re-granulation step can include a step of coarsely crushing the granulated particles to obtain a granulated coarse crushed product.
- the granulated coarse crushed product may be in the form of a hydrogel.
- the size of the minimum side of the granulated coarse crushed product may be, for example, about 0.1 to 15 mm, preferably about 1.0 to 10 mm.
- the coarse crushing may be performed only on granulated particles having a large size, such as those having a minimum side size of more than 5 mm or more than 10 mm.
- the coarsening apparatus used in the repartitioning may be the same as that used for the coarsening in the above-mentioned particleization step.
- the re-granulation step can include a step of drying the granulated particles, the granulated coarse crushed product, or the pulverized granulated product. By drying, a granulated dried product can be obtained. Drying in the repartitioning step is preferably performed on the granulated coarse crushed product. That is, the drying step in the repartitioning step is preferably performed after coarse crushing.
- the drying temperature may be 120 ° C. or higher, 150 ° C. or higher, or 180 ° C. or higher. When the drying is carried out at normal pressure, the drying temperature is preferably 70 to 250 ° C, more preferably 80 to 200 ° C. The drying is preferably carried out until the water content of the finally obtained polymer particles is 10% by mass or less, and more preferably 5% by mass or less.
- the same method as the drying in the above-mentioned particleization step can be applied.
- the re-granulation step can include a step of crushing granulated particles, granulated coarse crushed products and / or dried products thereof.
- pulverization is preferably performed after coarse pulverization and drying.
- the repartitioning step preferably includes a step of drying the granulated coarse crushed product and then pulverizing it to obtain polymer particles.
- the pulverizer used for pulverization in the repartitioning step may be the same as that used for pulverization in the above-mentioned particleization step.
- the particle size distribution of the polymer particles obtained by repartitioning tends to be more suitable. Specifically, in the polymer particles obtained by repartitioning, the yield of particles having a particle size of 180 ⁇ m or more and less than 850 ⁇ m can be improved, and the generation rate of fine particles having a particle size of less than 180 ⁇ m can be suppressed. .. A high yield of polymer particles having a particle size of 180 ⁇ m or more and less than 850 ⁇ m indicates that the granulation strength is higher.
- the repartitioning step may include a step of further classifying the polymer particles after crushing.
- the method for classifying the particles after re-particle formation may be the same as the classification method shown in the above-mentioned particle-forming step, and it is preferable to use screen classification.
- the particle size may be increased to a desired particle size by performing granulation again.
- the granulation performed after the re-granulation it is not necessary to obtain granulated particles having a large particle size as in the first granulation described above, and the final required particle size (for example, the particle size is 180 ⁇ m or more and less than 850 ⁇ m).
- Granulation may be performed until the range) is reached. The steps of reparticle formation and granulation may be repeated a plurality of times.
- the method for producing the water-absorbent resin particles may include a step of performing surface cross-linking of the polymer particles.
- the surface cross-linking can be performed, for example, by adding a cross-linking agent (surface cross-linking agent) for performing the surface cross-linking to the polymer particles and reacting them.
- the surface cross-linking agent may be added at any timing after pulverization in the repartitioning step, and may be performed before or after drying in the reparticlening step, or before or after classification. good.
- the addition of the surface cross-linking agent is preferably performed after the drying and classification in the reparticle step.
- the surface cross-linking may be performed on the granulated water-absorbent resin particles alone, or may be performed collectively after mixing the granulated water-absorbent resin particles and the non-granulated water-absorbent resin particles described later.
- a surface cross-linking agent By adding a surface cross-linking agent and performing the surface cross-linking treatment, the cross-linking density in the vicinity of the surface of the polymer particles is increased, so that the water absorption performance of the obtained water-absorbent resin particles can be improved.
- the surface cross-linking agent can be added, for example, by adding a surface cross-linking agent solution or by spraying the surface cross-linking agent solution.
- the surface cross-linking agent is preferably added as a surface cross-linking agent solution by dissolving the surface cross-linking agent in a solvent such as water and / or alcohol.
- the surface cross-linking step may be carried out once or divided into a plurality of times of two or more times.
- the surface cross-linking agent may contain, for example, two or more functional groups (reactive functional groups) having reactivity with a functional group derived from an ethylenically unsaturated monomer.
- functional groups reactive functional groups
- examples of the surface cross-linking agent include polyols such as ethylene glycol, propylene glycol, 1,4-butanediol, trimethylolpropane, glycerin, polyoxyethylene glycol, polyoxypropylene glycol, and polyglycerin; (poly) ethylene glycol di.
- Polyglycidyl compounds such as glycidyl ether, (poly) glycerin diglycidyl ether, (poly) glycerin triglycidyl ether, (poly) propylene glycol polyglycidyl ether, (poly) glycerol polyglycidyl ether; epichlorohydrin, epibromhydrin, ⁇ - Haloepoxy compounds such as methyl epichlorohydrin; compounds having two or more reactive functional groups such as isocyanate compounds such as 2,4-tolylene diisocyanate and hexamethylene diisocyanate; 3-methyl-3-oxetanemethanol, 3-ethyl-3- Oxetane compounds such as oxetane methanol, 3-butyl-3-oxetane methanol, 3-methyl-3-oxetane ethanol, 3-ethyl-3-oxetane ethanol, 3-butyl-3-ox
- the amount of the surface cross-linking agent added is usually based on 100 mol of the total amount of the ethylenically unsaturated monomer used for the polymerization from the viewpoint of appropriately increasing the cross-linking density near the surface of the water-absorbent resin particles (polymer particles). , Preferably 0.0001 to 4.0 mol, more preferably 0.001 to 2.0 mol.
- the surface cross-linking step is preferably carried out in the presence of water in the range of 1 to 200 parts by mass with respect to 100 parts by mass of the ethylenically unsaturated monomer.
- the amount of water can be adjusted by appropriately using a water-soluble organic solvent such as water and / or alcohol.
- the water-absorbent resin particles can be more preferably cross-linked in the vicinity of the particle surface.
- the treatment temperature of the surface cross-linking agent is appropriately set according to the surface cross-linking agent used, and may be 20 to 250 ° C.
- the treatment time with the surface cross-linking agent may be 1 to 200 minutes or 5 to 100 minutes.
- Surface cross-linking may be performed only once or at multiple timings.
- the surface cross-linking may be carried out after the pulverization step and before the pulverization in addition to the execution after the pulverization step or after the pulverization step.
- the method for producing the non-granulated water-absorbent resin particles is, for example, polymerizing an ethylenically unsaturated monomer to obtain a hydrogel polymer (polymerization step), and granulating the hydrogel polymer into a polymer. It may include obtaining particles (particulation step).
- the polymerization step in the production of the non-granulated water-absorbent resin particles the same embodiment as the polymerization in the above-mentioned granulated water-absorbent resin particles can be applied.
- the particle-forming step in the production of non-granulated water-absorbent resin particles is, for example, a step of coarsely crushing a hydrogel polymer obtained by polymerization to obtain a coarse crushed product, and a step of further crushing the coarse crushed product to obtain a crushed product. It may include a step of obtaining (polymer particles).
- the particleization step may include a step of drying the hydrogel polymer, coarsely crushed product and / or crushed product. The coarsely crushed product is preferably subjected to crushing after undergoing a drying step.
- the particleization step may further include a step of classifying the polymer particles obtained by pulverization.
- the particle formation step for the non-granulated water-absorbent resin particles has the same embodiment as the particle formation step for the granulated water-absorbent resin particles described above, except that the obtained particle group does not need to contain fine particles having a particle size of less than 180 ⁇ m. Applicable.
- the method for producing the non-granulated water-absorbent resin particles may further include a step of surface cross-linking of the polymer particles.
- the surface cross-linking agent may be added at any time after pulverization, before or after drying, or before or after classification.
- the same embodiments as those of the surface cross-linking step for the above-mentioned granulated water-absorbent resin particles can be applied.
- the particle size is 180 ⁇ m or more and 850 ⁇ m or less by adjusting the particle size such as polymer particle classification obtained without going through the granulation step.
- the granulated water-absorbent resin particles and the non-granulated water-absorbent resin particles contained in the particulate water-absorbent resin composition can be obtained without the above-mentioned polymer particles obtained through the granulation step and the granulation step. Each contains polymer particles.
- Granulated water-absorbent resin particles and non-granulated water-absorbent resin particles may be collectively referred to simply as water-absorbent resin particles.
- the granulated water-absorbent resin particles and the non-granulated water-absorbent resin particles may be composed of only the respective polymer particles, and in addition to the polymer particles, for example, a gel stabilizer and a metal chelating agent (ethylenediamine 4). Additional components such as acetic acid and salts thereof, diethylenetriamine-5 acetic acid and salts thereof, such as diethylenetriamine-5 sodium acetate), and fluidity improvers (lubricants) may be further included. Additional components may be placed inside, on the surface, or both of the polymer particles.
- the water-absorbent resin particles may contain a plurality of inorganic particles arranged on the surface of the polymer particles.
- the inorganic particles may be silica particles such as amorphous silica.
- Inorganic particles usually have a small size as compared with the size of polymer particles.
- the average particle size of the inorganic particles may be 0.1 to 50 ⁇ m, 0.5 to 30 ⁇ m, or 1 to 20 ⁇ m.
- the average particle size can be measured by the pore electric resistance method or the laser diffraction / scattering method depending on the characteristics of the particles.
- the content of the inorganic particles is 0.05 parts by mass or more and 0.1 parts by mass or more based on 100 parts by mass of the total mass of the polymer particles. , 0.15 parts by mass or more or 0.2 parts by mass or more, 5.0 parts by mass or less, 3.0 parts by mass or less, 1.0 parts by mass or less, 0.5 parts by mass or less or 0.3 It may be less than or equal to a mass part.
- the shape of the water-absorbent resin particles may be, for example, a crushed shape, an amorphous shape, an amorphous crushed shape, or a shape formed by aggregating these particles.
- the medium particle size of these water-absorbent resin particles may be 130 to 800 ⁇ m, 200 to 850 ⁇ m, 250 to 700 ⁇ m, 300 to 600 ⁇ m, or 300 to 450 ⁇ m.
- the CRC (centrifuge retention capacity) of the water-absorbent resin particles may be, for example, 25 g / g or more, 28 g / g or more, 30 g / g or more, or 32 g / g or more, and 40 g / g or less. It may be 38 g / g or less, 36 g / g or less, 34 g / g or less, or 33 g / g or less. CRC is measured by the method described in Examples described later with reference to the EDANA method (NWSP 241.0.R2 (15), page.769-778).
- the absorption ratio (AAP, Absorption against Pressure) of the water-absorbent resin particles under pressure of 2.07 kPa is, for example, 10 g / g or more, 18 g / g or more, 20 g / g or more, or 22 g / g or more. It may be 30 g / g or less, 28 g / g or less, 26 g / g or less, 24 g / g or less, 20 g / g or less, or 15 g / g or less.
- the absorption ratio of the water-absorbent resin particles under 2.07 kPa pressurization is measured by the method described in Examples described later.
- the water absorption rate of the water-absorbent resin particles by Vortex is, for example, 30 seconds or more, 32 seconds or more, 34 seconds or more, 36 seconds or more, 38 seconds, 40 seconds or more, 42 seconds or more, 44 seconds or more, 46 seconds or more, 48. It may be 90 seconds or less, 80 seconds or less, 70 seconds or less, 60 seconds or less, 55 seconds or less, 50 seconds or less, or 40 seconds or less.
- the water absorption rate by the Vortex method is measured in accordance with (Japanese Industrial Standard JIS K 7224 (1996)).
- the method for producing a particulate water-absorbent resin composition according to the present embodiment includes a step of mixing granulated water-absorbent resin particles and non-granulated water-absorbent resin particles.
- the mixing ratio of the granulated water-absorbent resin particles and the non-granulated water-absorbent resin particles was 40 to 80% by mass of the granulated water-absorbent resin particles with respect to the total amount of the particulate water-absorbent resin composition, and the granulated water-absorbent resin particles were not granulated.
- the water-absorbent resin particles are 20 to 60% by mass.
- Mixing of the water-absorbent resin particles can be performed, for example, by stirring the two types of water-absorbent resin particles using a commercially available shaker.
- the mixing ratio of the granulated water-absorbent resin particles is preferably 45% by mass or more, preferably 48% by mass or more, 50% by mass or more, 60% by mass or more, or 70% by mass with respect to the total amount of the particulate water-absorbent resin composition. It may be% or more.
- the mixing ratio of the granulated water-absorbent resin particles is preferably 77% by mass or less, preferably 75% by mass or less, 70% by mass or less, 65% by mass or less, or 60% by mass with respect to the total amount of the particulate water-absorbent resin composition. It may be less than or equal to%.
- the mixing ratio of the granulated water-absorbent resin particles is preferably 45% by mass or more, preferably 48% by mass or more and 50% by mass, based on the total amount of the granulated water-absorbent resin particles and the non-granulated water-absorbent resin particles. % Or more, 60% by mass or more, or 70% by mass or more.
- the mixing ratio of the granulated water-absorbent resin particles is preferably 77% by mass or less, preferably 75% by mass or less, 70% by mass or less, 65% by mass or less, or 60% by mass with respect to the total amount of the particulate water-absorbent resin composition. It may be less than or equal to%.
- the medium particle size of the particulate water-absorbent resin composition obtained by the production method according to the present embodiment may be 130 to 800 ⁇ m, 200 to 850 ⁇ m, 250 to 700 ⁇ m, 300 to 600 ⁇ m, or 400 to 500 ⁇ m.
- the CRC (centrifuge retention capacity) of the particulate water-absorbent resin composition may be, for example, 25 g / g or more, 28 g / g or more, 30 g / g or more, or 32 g / g or more, and 40 g / g / g. It may be g or less, 38 g / g or less, 36 g / g or less, 34 g / g or less, or 33 g / g or less.
- the absorption ratio of the particulate water-absorbent resin composition under pressure of 2.07 kPa may be, for example, 15 g / g or more, 18 g / g or more, 20 g / g or more, or 22 g / g or more, and is 30 g. It may be / g or less, 28 g / g or less, 26 g / g or less, 24 g / g or less, 22 g / g or less, or 18 g / g or less.
- the water absorption rate of the particulate water-absorbent resin composition by Vortex is, for example, 30 seconds or more, 32 seconds or more, 34 seconds or more, 36 seconds or more, 38 seconds, 40 seconds or more, 42 seconds or more, 44 seconds or more, 46 seconds or more. , 48 seconds or more, 90 seconds or less, 80 seconds or less, 70 seconds or less, 60 seconds or less, 55 seconds or less, 50 seconds or less, or 40 seconds or less.
- the three-minute value of the absorption amount (AUL, Absorption Under Load) of the particulate water-absorbent resin composition under a load of 2.07 kPa is, for example, 8 ml / g or more, 10 ml / g or more, 13 ml / g or more, 15 ml / g. It may be more than or equal to 17 ml / g or more, and may be 22 ml / g or less, 20 ml / g or less, 18 ml / g or less, or 15 ml / g or less.
- the 15-minute value of the absorption amount of the particulate water-absorbent resin composition under a load of 2.07 kPa is, for example, 14 ml / g or more, 16 ml / g or more, 20 ml / g or more, 22 ml / g or more, or 24 ml / g or more. It may be 30 ml / g or less, 28 ml / g or less, 26 ml / g or less, 24 ml / g or less, or 20 ml / g or less. Specific measurement methods for the 3-minute value and the 15-minute value of the absorbed amount under load will be shown in Examples described later.
- the ratio of the three-minute value to the 15-minute value of the absorption amount under a load of 2.07 kPa of the particulate water-absorbent resin composition is, for example, 50% or more, 55% or more, 60% or more, 65% or more, or 70% or more. It may be 80% or less, 75% or less, 70% or less, or 65% or less.
- the particulate water-absorbent resin composition obtained by the production method according to the present embodiment has excellent absorption performance when used under pressure.
- the reason why such an effect is obtained is not clear, but the present inventor speculates as follows.
- the present invention is not limited to the following mechanism.
- granulation is performed by sufficiently kneading granulated particles having a large particle size to a certain ratio or more. ..
- the fact that more granulated particles with a large particle size can be obtained means that the degree of kneading is higher.
- the particles that have undergone such a granulation step have a more complicated shape, such that the particles obtained by re-granulation after that have more fine voids inside and have a plurality of protrusions.
- the particulate water-absorbent resin composition obtained by the production method according to the present embodiment has excellent absorbability of body fluids such as urine and blood, and includes, for example, disposable diapers, sanitary napkins, sanitary products such as tampons, pet sheets, and the like. It can be applied to fields such as animal excrement treatment materials such as toilet formulations for dogs or cats.
- the particulate water-absorbent resin composition can be suitably used for an absorber.
- the absorber containing the particulate water-absorbent resin composition obtained by the production method according to the present embodiment has excellent water-absorbing performance.
- the method for producing an absorber according to the present embodiment includes, for example, obtaining a particulate water-absorbent resin composition by the above-mentioned method and obtaining an absorber containing the particulate water-absorbent resin composition.
- the content of the particulate water-absorbent resin composition in the absorber is 100 to 1000 g (that is, 100 to 100 g) per square meter of the absorber from the viewpoint of obtaining sufficient liquid absorption performance when the absorber is used for an absorbent article. It is preferably 1000 g / m 2 ), more preferably 150 to 800 g / m 2 , and even more preferably 200 to 700 g / m 2 . From the viewpoint of exhibiting sufficient liquid absorption performance as an absorbent article, the content is preferably 100 g / m 2 or more. From the viewpoint of suppressing the occurrence of the gel blocking phenomenon, the content is preferably 1000 g / m 2 or less.
- the mass ratio of the particulate water-absorbent resin composition in the absorber may be 2% to 100% and 10% to 80% with respect to the total of the particulate water-absorbent resin composition and the fibrous material. It is preferably 20% to 70%, more preferably 20% to 70%.
- the structure of the absorber may be, for example, a form in which the particulate water-absorbent resin composition and the fibrous material are uniformly mixed, and the particulate water-absorbent resin is formed between the fibrous material formed in a sheet or layer.
- the composition may be in a sandwiched form or in any other form.
- fibrous material examples include finely pulverized wood pulp, cotton, cotton linter, rayon, cellulosic fibers such as cellulose acetate, and synthetic fibers such as polyamide, polyester, and polyolefin.
- the fibers may be adhered to each other by adding an adhesive binder to the fibrous material.
- the adhesive binder include heat-sealing synthetic fibers, hot melt adhesives, adhesive emulsions, and the like.
- the heat-bondable synthetic fiber examples include a total fusion type binder such as polyethylene, polypropylene, and an ethylene-propylene copolymer, and a non-total fusion type binder having a side-by-side or core-sheath structure of polypropylene and polyethylene.
- a total fusion type binder such as polyethylene, polypropylene, and an ethylene-propylene copolymer
- non-total fusion type binder having a side-by-side or core-sheath structure of polypropylene and polyethylene.
- hot melt adhesives examples include ethylene-vinyl acetate copolymer, styrene-isoprene-styrene block copolymer, styrene-butadiene-styrene block copolymer, styrene-ethylene-butylene-styrene block copolymer, and styrene-ethylene-propylene-styrene block copolymer.
- a compounding of a base polymer such as amorphous polypropylene and a tackifier, a plasticizer, an antioxidant and the like.
- Adhesive emulsions include, for example, polymers of at least one monomer selected from the group consisting of methyl methacrylate, styrene, acrylonitrile, 2-ethylhexyl acrylate, butyl acrylate, butadiene, ethylene, and vinyl acetate. Be done. These adhesive binders may be used alone or in combination of two or more.
- the absorber may further contain additives such as inorganic particles (for example, amorphous silica), deodorants, pigments, dyes, antibacterial agents, fragrances, and adhesives. These additives can impart various functions to the absorber.
- inorganic particles for example, amorphous silica
- deodorants for example, amorphous silica
- pigments for example, amorphous silica
- dyes for example, amorphous silica
- antibacterial agents for example, a amorphous silica
- fragrances for example, amorphous silica
- adhesives such as inorganic particles (for example, amorphous silica), deodorants, pigments, dyes, antibacterial agents, fragrances, and adhesives. These additives can impart various functions to the absorber.
- the absorber may contain inorganic particles in addition to the inorganic particles in the water-absorbent resin particles. Examples of the inorganic particles include silicon dioxide, zeolite, kaolin,
- the shape of the absorber is not particularly limited, and may be, for example, a sheet shape.
- the thickness of the absorber (for example, the thickness of the sheet-shaped absorber) may be, for example, 0.1 to 20 mm and 0.3 to 15 mm.
- the above absorber can be suitably used for an absorbent article.
- the method for producing an absorbent article according to the present embodiment includes, for example, arranging an absorber between a liquid permeable sheet and a liquid permeable sheet.
- the absorbent article containing the particulate water-absorbent resin composition obtained by the production method according to the present embodiment exhibits excellent water-absorbing performance particularly under pressure.
- the absorbent article may include an absorber, a liquid permeable sheet and a liquid permeable sheet.
- the liquid permeable sheet (liquid permeable top sheet) is arranged on the outermost side on the side where the liquid to be absorbed enters.
- the liquid impermeable sheet (liquid impermeable back sheet) is arranged on the outermost side opposite to the side on which the liquid to be absorbed enters.
- the absorbent article may further include a core wrap. The core wrap retains the shape of the absorber.
- absorbent articles examples include diapers (for example, paper diapers), toilet training pants, incontinence pads, sanitary products (sanitary napkins, tampons, etc.), sweat pads, pet sheets, toilet materials, animal excrement treatment materials, and the like. ..
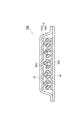
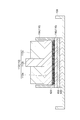
- FIG. 1 is a cross-sectional view showing an example of an absorbent article.
- the absorbent article 100 shown in FIG. 1 includes an absorbent body 10, core wraps 20a and 20b, a liquid permeable top sheet 30, and a liquid permeable back sheet 40.
- the liquid permeable back sheet 40, the core wrap 20b, the absorbent body 10, the core wrap 20a, and the liquid permeable top sheet 30 are laminated in this order.
- FIG. 1 there is a portion shown so that there is a gap between the members, but the members may be in close contact with each other without the gap.
- the absorber 10 has a particulate water-absorbent resin composition 10a and a fiber layer 10b containing a fibrous material.
- the particulate water-absorbent resin composition 10a is dispersed in the fiber layer 10b.
- the core wrap 20a is arranged on one side of the absorber 10 (upper side of the absorber 10 in FIG. 1) in contact with the absorber 10.
- the core wrap 20b is arranged on the other side of the absorber 10 (lower side of the absorber 10 in FIG. 1) in contact with the absorber 10.
- the absorber 10 is arranged between the core wrap 20a and the core wrap 20b.
- the core wrap 20a and the core wrap 20b have, for example, a main surface having the same size as the absorber 10.
- the core wrap By using the core wrap, it is possible to maintain the shape retention of the absorber and prevent the particulate water-absorbent resin composition or the like constituting the absorber from falling off or flowing.
- the core wrap include non-woven fabrics, woven fabrics, tissues, synthetic resin films having liquid permeation holes, net-like sheets having a mesh, and the like, and from the viewpoint of economy, a tissue made by wet-molding crushed pulp is preferable. Used.
- the liquid permeable top sheet 30 is arranged on the outermost side on the side where the liquid to be absorbed enters.
- the liquid permeable top sheet 30 is arranged on the core wrap 20a in contact with the core wrap 20a.
- the liquid permeable back sheet 40 is arranged on the outermost side of the absorbent article 100 on the opposite side of the liquid permeable top sheet 30.
- the liquid impermeable back sheet 40 is arranged under the core wrap 20b in contact with the core wrap 20b.
- the liquid permeable top sheet 30 and the liquid permeable back sheet 40 have, for example, a main surface wider than the main surface of the absorber 10, and the liquid permeable top sheet 30 and the liquid permeable back sheet 40 have.
- the outer edge extends around the absorber 10 and the core wraps 20a, 20b.
- liquid permeable top sheet 30 examples include non-woven fabrics and porous sheets.
- non-woven fabric examples include thermal-bonded non-woven fabric, air-through non-woven fabric, resin-bonded non-woven fabric, spunbond non-woven fabric, melt-blow non-woven fabric, spunbond / melt-blow / spunbond non-woven fabric, air-laid non-woven fabric, spunlace non-woven fabric, point-bond non-woven fabric and the like.
- thermal bond non-woven fabrics, air-through non-woven fabrics, spunbond non-woven fabrics, and spunbond / melt blow / spunbond non-woven fabrics are preferably used.
- a resin or fiber known in the art can be used, and polyethylene (from the viewpoint of liquid permeability, flexibility and strength when used in an absorbent article, polyethylene ( Polyester such as PE), polypropylene (PP), polyethylene terephthalate (PET), polytrimethylene terephthalate (PTT), polyester such as polyethylene naphthalate (PEN), polyamide such as nylon, rayon, and other synthetic resins or fibers. Examples include cotton, silk, linen and pulp (cellulose) fibers.
- synthetic fibers are preferably used from the viewpoint of increasing the strength of the liquid permeable top sheet 30, and among them, polyolefin and polyester are preferable. These materials may be used alone or in combination of two or more kinds of materials.
- the non-woven fabric used for the liquid permeable top sheet 30 has appropriate hydrophilicity from the viewpoint of improving the liquid absorption performance of the absorbent article. From this point of view, it is preferable that the hydrophilicity when measured according to the "hydrophilicity of the non-woven fabric" described in International Publication No. 2011/086843 (based on the pulp and paper test method No. 68 (2000)) is 5 to 200. Those of 10 to 150 are more preferable.
- the non-woven fabric having such hydrophilicity among the above-mentioned non-woven fabrics, those in which the material itself exhibits appropriate hydrophilicity such as rayon fiber may be used, and hydrophobic chemistry such as polyolefin fiber and polyester fiber may be used.
- a fiber may be used which has been hydrophilized by a known method to impart an appropriate degree of hydrophilicity.
- Examples of the method for hydrophilizing chemical fibers include a method of obtaining a non-woven fabric by a spunbond method obtained by mixing a hydrophobic chemical fiber with a hydrophilic agent in a spunbonded non-woven fabric, and a spunbonded non-woven fabric using hydrophobic chemical fibers. Examples thereof include a method of accommodating a hydrophilic agent when producing the above, a method of impregnating the spunbonded non-woven fabric with a hydrophobic chemical fiber, and then impregnating the hydrophilic agent.
- Hydrophilic agents include anionic surfactants such as aliphatic sulfonates and higher alcohol sulfates, cationic surfactants such as quaternary ammonium salts, polyethylene glycol fatty acid esters, polyglycerin fatty acid esters, and sorbitan fatty acids.
- Nonionic surfactants such as esters, silicone-based surfactants such as polyoxyalkylene-modified silicones, and stain-releasing agents made of polyester-based, polyamide-based, acrylic-based, and urethane-based resins are used.
- the non-woven fabric used for the liquid permeable top sheet 30 is appropriately bulky from the viewpoint of imparting good liquid permeability, flexibility, strength and cushioning property to the absorbent article and increasing the liquid penetration rate of the absorbent article. It is preferably high and has a large amount of grain.
- the basis weight of the non-woven fabric is preferably 5 to 200 g / m 2 , more preferably 8 to 150 g / m 2 , and even more preferably 10 to 100 g / m 2 .
- the thickness of the non-woven fabric is preferably 20 to 1400 ⁇ m, more preferably 50 to 1200 ⁇ m, and even more preferably 80 to 1000 ⁇ m.
- the liquid impermeable back sheet 40 prevents the liquid absorbed by the absorber 10 from leaking from the back sheet 40 side to the outside.
- the liquid impermeable back sheet 40 is made of a liquid impermeable film mainly composed of a polyolefin resin such as polyethylene (PE) and polypropylene (PP), a breathable resin film, and a non-woven fabric such as spunbond or spunlace.
- PE polyethylene
- PP polypropylene
- a non-woven fabric such as spunbond or spunlace.
- a composite film to which the above resin films are bonded, a spunbond / melt blow / spunbond (SMS) non-woven fabric in which a water-resistant melt-blown non-woven fabric is sandwiched between high-strength spun-bonded non-woven fabrics can be used.
- the back sheet 40 should use a resin film having a basis weight of 10 to 50 g / m 2 mainly made of low density polyethylene (LDPE) resin. Can be done. In addition, when a breathable material is used, the stuffiness at the time of wearing is reduced, and the discomfort given to the wearer can be reduced.
- LDPE low density polyethylene
- the magnitude relationship between the absorbent body 10, the core wraps 20a and 20b, the liquid permeable top sheet 30, and the liquid permeable back sheet 40 is not particularly limited, and is appropriately adjusted according to the use of the absorbent article and the like. Further, the method of retaining the shape of the absorber 10 by using the core wraps 20a and 20b is not particularly limited, and as shown in FIG. 1, the absorber may be sandwiched by a plurality of core wraps, and the absorber may be sandwiched by one core wrap. May be coated.
- the absorber 10 may be adhered to the liquid permeable top sheet 30.
- the liquid is guided to the absorbent body more smoothly, so that it is easy to obtain an excellent absorbent article by preventing liquid leakage.
- the absorber 10 is sandwiched or covered by the core wrap, it is preferable that at least the core wrap and the liquid permeable top sheet 30 are adhered to each other, and it is more preferable that the core wrap and the absorber 10 are adhered to each other.
- Examples of the bonding method include a method of applying a hot melt adhesive to the liquid permeable top sheet 30 at predetermined intervals in the width direction in a vertical stripe shape, a spiral shape, or the like, and bonding starch or carboxymethyl cellulose. , Polyvinyl alcohol, polyvinylpyrrolidone, and other methods of bonding using a water-soluble binder selected from water-soluble polymers.
- a method of adhering by heat-sealing may be adopted.
- Production Example 1 (Production of non-granulated water-absorbent resin particles) [polymerization] 509.71 g (7.07 mol) of 100% acrylic acid was placed in a round bottom cylindrical separable flask having an inner diameter of 11 cm and an internal volume of 2 L equipped with a stirrer. After adding 436.47 g of ion-exchanged water into the separable flask while stirring this acrylic acid, 444.68 g of 48% by mass sodium hydroxide was added dropwise under an ice bath to give a monomer concentration of 45.1. 1390.86 g of a mass% acrylic acid partial neutralizing solution (neutralization rate 75.4 mol%) was prepared. The preparation of this acrylic acid partial neutralizing solution was repeated to obtain a required amount.
- the above reaction solution was supplied to the stainless steel double-armed kneader, and the system was replaced with nitrogen gas while keeping the reaction solution at 30 ° C. Subsequently, while stirring the reaction solution, 92.63 g (7.780 mmol) of a 2.0 mass% sodium persulfate aqueous solution and 15.85 g of a 0.5 mass% L-ascorbic acid aqueous solution were added. After 1 minute, the temperature began to rise and polymerization started. After 6 minutes, the maximum temperature during the polymerization was 93 ° C., and then stirring was continued while maintaining the jacket temperature at 60 ° C., and 60 minutes after the start of the polymerization, the produced hydrogel-like polymer was taken out.
- the obtained hydrogel-like polymer was sequentially charged into a meat chopper 12VR-750SDX manufactured by Kiren Royal Co., Ltd. and subdivided (coarse) to obtain a hydrogel-like coarsely crushed product.
- the diameter of the hole in the plate located at the outlet of the meat chopper was 6.4 mm.
- the hydrogel-like coarsely crushed product was spread on a wire mesh having a mesh size of 0.8 cm ⁇ 0.8 cm and dried with hot air at 160 ° C. for 60 minutes to obtain a coarsely crushed and dried product.
- the particle group (A) was classified using a JIS standard sieve having a mesh size of 850 ⁇ m, a JIS standard sieve having a mesh size of 250 ⁇ m, and a JIS standard sieve having a mesh size of 180 ⁇ m.
- the fraction passed through a JIS standard sieve with a mesh size of 850 ⁇ m and did not pass through a JIS standard sieve with a mesh size of 250 ⁇ m, and passed through a water-absorbent resin particle (A1) and a JIS standard sieve with a mesh size of 180 ⁇ m.
- a fraction (a1) was obtained.
- a surface cross-linking agent solution consisting of 0.0783 g of ethylene carbonate, 0.125 g of propylene glycol, and 0.5 g of ion-exchanged water is mixed and mixed with 25 g of the water-absorbent resin particles (A1) after classification at 25 ° C. for 35 minutes. I got the liquid. Then, the mixture was heat-treated at 200 ° C. for 35 minutes to obtain surface-crosslinked water-absorbent resin particles (A2).
- the surface-crosslinked water-absorbent resin particles (A2) were classified with a JIS standard sieve having an opening of 850 ⁇ m and a JIS standard sieve having an opening of 250 ⁇ m.
- water-absorbent resin particles (A3) were obtained, which were fractions that passed through a JIS standard sieve having a mesh size of 850 ⁇ m and did not pass through a JIS standard sieve having a mesh size of 250 ⁇ m.
- non-granulated water-absorbent resin particles (A4) were obtained as Production Example 1. ..
- 25 g of water-absorbent resin particles were placed in a mayonnaise bottle having a capacity of 225 ml together with silicon dioxide, and the mixture was shaken for 3 minutes under the condition of 750 (cycle / min (CPM)) using a paint shaker (manufactured by Toyo Seiki Seisakusho). This was done by stirring well.
- the CRC of the water-absorbent resin particles (A4) was 34 g / g, and the medium particle size was 467 ⁇ m.
- Production Example 2 (Production of granulated water-absorbent resin particles) [Granulation] 40 g of the fine powder (a1) was placed in a round-bottomed cylindrical separable flask having an inner diameter of 11 cm and an internal volume of 2 L (the 2 L container was kept warm in a bath at 80 ° C.) equipped with a stirrer.
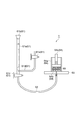
- the stirrer was equipped with a stirrer 200 as a stirrer, which is generally shown in FIG.
- the stirring blade 200 includes a shaft 200a and a flat plate portion 200b.
- the flat plate portion 200b is welded to the shaft 200a and has a curved tip.
- the flat plate portion 200b is formed with four slits S extending along the axial direction of the shaft 200a.
- the four slits S are arranged in the width direction of the flat plate portion 200b, the width of the two inner slits S is 1 cm, and the width of the two outer slits S is 0.5 cm.
- the length of the flat plate portion 200b is about 10 cm, and the width of the flat plate portion 200b is about 6 cm.
- the water-absorbent resin particles (B1) were classified with a JIS standard sieve having an opening of 850 ⁇ m and a JIS standard sieve having an opening of 180 ⁇ m.
- water-absorbent resin particles (B2) were obtained, which were fractions that passed through a JIS standard sieve having a mesh size of 850 ⁇ m and did not pass through a JIS standard sieve having a mesh size of 180 ⁇ m.
- the particle yield after granulation and crushing described later was measured.
- a surface cross-linking agent solution consisting of 0.0783 g of ethylene carbonate, 0.125 g of propylene glycol, and 0.5 g of ion-exchanged water is mixed with 25 g of water-absorbent resin particles (B2) at 25 ° C. for 35 minutes to obtain a mixed solution. rice field. Then, the mixture was heat-treated at 200 ° C. for 35 minutes to obtain surface-crosslinked water-absorbent resin particles (B3).
- the surface-crosslinked water-absorbent resin particles (B3) were classified with a JIS standard sieve having an opening of 850 ⁇ m and a JIS standard sieve having an opening of 250 ⁇ m.
- water-absorbent resin particles (B4) were obtained, which were fractions that passed through a JIS standard sieve having a mesh size of 850 ⁇ m and did not pass through a JIS standard sieve having a mesh size of 250 ⁇ m.
- Granulated water-absorbent resin particles (B5) were obtained as Production Example 2 by mixing 0.075 g of silicon dioxide (product name Aerosil 200, manufactured by Nippon Aerosil) with 25 g of water-absorbent resin particles (B4). Mixing was carried out in the same manner as in Production Example 1.
- the CRC of the water-absorbent resin particles (B5) was 31 g / g, and the medium particle size was 419 ⁇ m.
- Production Example 3 (Production of granulated water-absorbent resin particles)
- the same procedure as in Production Example 2 was carried out except that the stirring time in the round-bottomed cylindrical separable flask having an internal volume of 2 L was changed to 15 seconds. (B6) was obtained.
- the CRC of the water-absorbent resin particles (B6) was 30 g / g, and the medium particle size was 467 ⁇ m.
- Production Example 4 (Production of granulated water-absorbent resin particles)
- the same procedure as in Production Example 2 was carried out except that the amount of ion-exchanged water heated to 90 ° C. was changed to 25 g, and granulated water-absorbent resin particles (B7) were obtained as Production Example 4.
- the CRC of the water-absorbent resin particles (B7) was 32 g / g, and the medium particle size was 446 ⁇ m.
- Production Example 5 (Production of non-granulated water-absorbent resin particles) [polymerization] 509.09 g (7.06 mol) of 100% acrylic acid was placed in a round bottom cylindrical separable flask having an inner diameter of 11 cm and an internal volume of 2 L equipped with a stirrer. After adding 435.98 g of ion-exchanged water into the separable flask while stirring this acrylic acid, 447.03 g of 48% by mass sodium hydroxide was added dropwise under an ice bath to give a monomer concentration of 45.0. 1392.10 g of a mass% acrylic acid partial neutralizing solution (neutralization rate 75.9 mol%) was prepared. The preparation of this acrylic acid partial neutralizing solution was repeated to obtain a required amount.
- the above reaction solution was supplied to the kneader, and the system was replaced with nitrogen gas while keeping the reaction solution at 30 ° C. Subsequently, while stirring the reaction solution, 92.52 g (7.771 mmol) of a 2.0 mass% sodium persulfate aqueous solution and 15.83 g of a 0.5 mass% L-ascorbic acid aqueous solution were added. After minutes, the temperature began to rise and polymerization began. After 3 minutes, the maximum temperature during polymerization was 90 ° C., after which stirring was continued while maintaining the jacket temperature at 60 ° C. 60 minutes after the start of the polymerization, the produced hydrogel-like polymer was taken out.
- the hydrogel-like coarsely crushed product was spread on a wire mesh having a mesh size of 0.8 cm ⁇ 0.8 cm and dried with hot air at 160 ° C. for 60 minutes to obtain a coarsely crushed and dried product.
- An amorphous crushed pulverized product (D) was classified using a JIS standard sieve having a mesh size of 850 ⁇ m, a JIS standard sieve having a mesh size of 250 ⁇ m, and a JIS standard sieve having a mesh size of 180 ⁇ m.
- water-absorbent resin particles (D1) were obtained, which were fractions that passed through a JIS standard sieve having a mesh size of 850 ⁇ m and did not pass through a JIS standard sieve having a mesh size of 250 ⁇ m.
- a surface cross-linking agent solution consisting of 0.235 g of ethylene carbonate, 0.125 g of propylene glycol, and 2.0 g of ion-exchanged water was mixed with 25 g of water-absorbent resin particles (D1) at 25 ° C. for 35 minutes to obtain a mixture. .. Then, the mixture was heat-treated at 200 ° C. for 35 minutes to obtain surface-crosslinked water-absorbent resin particles (D2).
- Production Example 6 (Production of granulated water-absorbent resin particles) [polymerization] 509.71 g (7.07 mol) of 100% acrylic acid was placed in a round bottom cylindrical separable flask having an inner diameter of 11 cm and an internal volume of 2 L equipped with a stirrer. After adding 436.47 g of ion-exchanged water into the separable flask while stirring this acrylic acid, 444.68 g of 48% by mass sodium hydroxide was added dropwise under an ice bath to give a monomer concentration of 45.1. 1390.86 g of a mass% acrylic acid partial neutralizing solution (neutralization rate 75.4 mol%) was prepared. The preparation of this acrylic acid partial neutralizing solution was repeated to obtain a required amount.
- the above reaction solution was supplied to the kneader, and the system was replaced with nitrogen gas while keeping the reaction solution at 30 ° C. Subsequently, while stirring the reaction solution, 92.63 g (7.780 mmol) of a 2.0 mass% sodium persulfate aqueous solution and 15.85 g of a 0.5 mass% L-ascorbic acid aqueous solution were added. After 1 minute, the temperature began to rise and polymerization started. After 5 minutes, the maximum temperature during polymerization was 80.9 ° C, after which stirring was continued while maintaining the jacket temperature at 60 ° C. 60 minutes after the start of the polymerization, the produced hydrogel-like polymer was taken out.
- the hydrogel-like coarsely crushed product was spread on a wire mesh having a mesh size of 0.8 cm ⁇ 0.8 cm and dried with hot air at 160 ° C. for 60 minutes to obtain a coarsely crushed and dried product.
- An amorphous crushed pulverized product (E) was classified using a JIS standard sieve having a mesh size of 850 ⁇ m, a JIS standard sieve having a mesh size of 250 ⁇ m, and a JIS standard sieve having a mesh size of 180 ⁇ m.
- the water-absorbent resin particles (E1) which are fractions that passed through a JIS standard sieve with a mesh size of 850 ⁇ m and did not pass through a JIS standard sieve with a mesh size of 250 ⁇ m, and a JIS standard sieve with a mesh size of 180 ⁇ m were passed.
- a fraction (e1) was obtained.
- the water-absorbent resin particles (F1) were further classified with a JIS standard sieve having an opening of 850 ⁇ m and a JIS standard sieve having an opening of 180 ⁇ m.
- water-absorbent resin particles (F2) were obtained, which were fractions that passed through a JIS standard sieve having a mesh size of 850 ⁇ m and did not pass through a JIS standard sieve having a mesh size of 180 ⁇ m.
- a surface cross-linking agent solution consisting of 0.0783 g of ethylene carbonate, 0.125 g of propylene glycol, and 0.5 g of ion-exchanged water was mixed with 25 g of water-absorbent resin particles (F2) at 25 ° C. for 35 minutes to obtain a mixture. .. Then, the mixture was heat-treated at 200 ° C. for 35 minutes to obtain surface-crosslinked water-absorbent resin particles (F3).
- the surface-crosslinked water-absorbent resin particles (F3) were classified with a JIS standard sieve having an opening of 850 ⁇ m and a JIS standard sieve having an opening of 250 ⁇ m.
- water-absorbent resin particles (F4) were obtained, which were fractions that passed through a JIS standard sieve having a mesh size of 850 ⁇ m and did not pass through a JIS standard sieve having a mesh size of 250 ⁇ m.
- Granulated water-absorbent resin particles (F5) were obtained as Production Example 6 by mixing 0.075 g of silicon dioxide (product name Aerosil 200, manufactured by Nippon Aerosil) with 25 g of water-absorbent resin particles (F4). Mixing was carried out in the same manner as in Production Example 1.
- the CRC of the water-absorbent resin particles (F5) was 37 g / g, and the medium particle size was 413 ⁇ m.
- ⁇ Measurement of particle size distribution of granulated particles In the process of producing granulated water-absorbent resin particles, the total amount of the granulated particle group obtained by granulation or an arbitrary amount (about 30 g to 100 g) is sampled for measuring the particle size distribution of the granulated particle group. It was used as a sample. Sieves with JIS standard meshes of 106 mm, 53 mm, 31.5 mm, 9.5 mm, 2.8 mm, 850 ⁇ m, and 180 ⁇ m, and a saucer are placed in this order from the top, and the measurement sample is placed on a sieve with a mesh size of 106 mm. I put it in.
- Formula (2): "% of granulated particles abundance of 850 ⁇ m or more” (total of granulated particles remaining on each sieve having a mesh size of 106 mm, 53 mm, 31.5 mm, 9.5 mm, 2.8 mm, and 850 ⁇ m) Amount) / (Amount of sample used for measurement)
- Formula (3): "% of granulated particles abundance of 9.5 mm or more” (total amount of granulated particles remaining on each sieve having a mesh size of 106 mm, 53 mm, 31.5 mm, and 9.5 mm) / ( Sample amount used for measurement)
- the medium particle size of the water-absorbent resin particles and the particulate water-absorbent resin composition was measured by the following procedure. From the top of the JIS standard sieve, a sieve with a mesh size of 600 ⁇ m, a sieve with a mesh size of 500 ⁇ m, a sieve with a mesh size of 425 ⁇ m, a sieve with a mesh size of 300 ⁇ m, a sieve with a mesh size of 250 ⁇ m, a sieve with a mesh size of 180 ⁇ m, a sieve with a mesh size of 150 ⁇ m and a saucer. Combined in the order of.
- a non-woven fabric with a size of 60 mm x 170 mm (product name: Heat Pack MWA-18, manufactured by Nippon Paper Papylia Co., Ltd.) was folded in half in the longitudinal direction to adjust the size to 60 mm x 85 mm.
- a 60 mm ⁇ 85 mm non-woven fabric bag was produced by crimping the non-woven fabrics to each other on both sides extending in the longitudinal direction with a heat seal (a crimped portion having a width of 5 mm was formed on both sides along the longitudinal direction). 0.2 g of the particles to be measured were precisely weighed and contained inside the non-woven fabric bag. Then, the non-woven fabric bag was closed by crimping the remaining one side extending in the lateral direction with a heat seal.
- the entire non-woven fabric bag was completely moistened by floating the non-woven fabric bag on 1000 g of physiological saline contained in a stainless steel vat (240 mm ⁇ 320 mm ⁇ 45 mm) without folding the non-woven fabric bag.
- a stainless steel vat 240 mm ⁇ 320 mm ⁇ 45 mm
- the measuring device 110 provided with the wire mesh 116 of the above was prepared.
- the weight 112 includes a disc portion 112a, a rod-shaped portion 112b extending from the center of the disc portion 112a in a direction perpendicular to the disc portion 112a, and a cylindrical portion 112c having a through hole inserted into the rod-shaped portion 112b in the center. have.
- the disk portion 112a of the weight 112 has a diameter substantially equal to the inner diameter of the cylinder 114 so that it can be moved in the longitudinal direction of the cylinder 114 inside the cylinder 114.
- the diameter of the cylindrical portion 112c is smaller than the diameter of the disc portion 112a.
- One end of the cylinder 114 is open but shielded by a wire mesh 116, and the other end of the cylinder 114 is open so that the weight 112 can be inserted.
- 0.90 g of particles 120 to be measured were uniformly sprayed on the wire mesh 116.
- the total mass of the measuring device 110 (the total mass of the measuring device 110 and the measurement target particle 120 before liquid absorption). Wa [g] was measured.
- a glass filter 140 (ISO4793 P-250) with a diameter of 90 mm and a thickness of 7 mm in the center of the bottom surface of the recess of the stainless petri dish 130 with a diameter of 150 mm, 0 so that the water surface is at the same height as the upper surface of the glass filter 140.
- a 90% by mass aqueous sodium chloride solution (25 ° C ⁇ 2 ° C) was added.
- a sheet of filter paper 150 (ADVANTEC Toyo Co., Ltd., product name: (No. 3), thickness 0.23 mm, reserved particle diameter 5 ⁇ m) with a diameter of 90 mm is placed on the glass filter 140 so that the entire surface is wet and the surface is completely wet. , Excess liquid was removed.
- the above-mentioned measuring device 110 was placed on the filter paper 150, and the liquid was absorbed by the particles 120 to be measured under a load. After 1 hour, the measuring device 110 was lifted, and the total mass of the measuring device 110 (total mass of the measuring device 110 and the particles 120 to be measured after absorbing the liquid) Wb [g] was measured.
- ⁇ Vortex water absorption rate The water absorption rate of the water-absorbent resin particles and the physiological saline of the particulate water-absorbent resin composition was measured by the following procedure based on the Vortex method. The measurement was carried out in an environment where the temperature was 25 ° C. ⁇ 2 ° C. and the humidity was 50% ⁇ 10%. First, 50 ⁇ 0.1 g of a 0.9 mass% sodium chloride aqueous solution (physiological saline) adjusted to a temperature of 25 ⁇ 0.2 ° C. in a constant temperature water tank was weighed in a beaker having an internal volume of 100 mL.
- a vortex was generated by stirring at a rotation speed of 600 rpm using a magnetic stirrer bar (8 mm ⁇ ⁇ 30 mm, without ring).
- 2.0 ⁇ 0.002 g of measurement particles were added to the aqueous sodium chloride solution at one time.
- the time [seconds] from the addition of the measurement particles to the time when the vortex on the liquid surface converged was measured, and the time was obtained as the water absorption rate of the water-absorbent resin particles or the particulate water-absorbent resin composition.
- Tables 1 and 3 The results are shown in Tables 1 and 3.
- Example 1 After putting 10 g of the water-absorbent resin particles (A4) of Production Example 1 and 10 g of the water-absorbent resin particles (B5) of Production Example 2 into a 100 mL mayonnaise bottle, shake with a paint shaker (manufactured by Toyo Seiki Seisakusho Co., Ltd.) for 30 minutes. The particles were uniformly mixed to obtain the particulate water-absorbent resin composition of Example 1. The CRC of this particulate water-absorbent resin composition was 32 g / g, and the medium particle size was 443 ⁇ m.
- Example 2 After putting 5 g of the water-absorbent resin particles (A4) of Production Example 1 and 15 g of the water-absorbent resin particles (B5) of Production Example 2 into a 100 mL mayonnaise bottle, shake with a paint shaker (manufactured by Toyo Seiki Seisakusho Co., Ltd.) for 30 minutes. The mixture was uniformly mixed to obtain a particulate water-absorbent resin composition of Example 2. The CRC of this particulate water-absorbent resin composition was 32 g / g, and the medium particle size was 431 ⁇ m.
- Example 3 After putting 10 g of the water-absorbent resin particles (A4) of Production Example 1 and 10 g of the water-absorbent resin particles (B7) of Production Example 4 into a 100 mL mayonnaise bottle, shake with a paint shaker (manufactured by Toyo Seiki Seisakusho Co., Ltd.) for 30 minutes. The mixture was uniformly mixed to obtain a particulate water-absorbent resin composition of Example 3. The CRC of this particulate water-absorbent resin composition was 33 g / g, and the medium particle size was 467 ⁇ m.
- Example 4 After putting 10 g of the water-absorbent resin particles (D4) of Production Example 5 and 10 g of the water-absorbent resin particles (F5) of Production Example 6 into a 100 mL mayonnaise bottle, shake for 30 minutes with a paint shaker (manufactured by Toyo Seiki Seisakusho Co., Ltd.). The particles were uniformly mixed to obtain the particulate water-absorbent resin composition of Example 4. The CRC of this particulate water-absorbent resin composition was 36 g / g, and the medium particle size was 459 ⁇ m.
- the water absorption amount (room temperature, 25 ° C. ⁇ 2 ° C.) of the physiological saline under the load of the water-absorbent resin particles and the particulate water-absorbent resin composition was measured using the measuring device Y shown in FIG. The measurement was carried out in an environment where the temperature was 25 ° C. ⁇ 2 ° C. and the humidity was 50% ⁇ 10%.
- the measuring device Y is composed of a burette unit 61, a conduit 62, a measuring table 63, and a measuring unit 64 placed on the measuring table 63.
- the burette portion 61 has a burette 61a extending in the vertical direction, a rubber stopper 61b arranged at the upper end of the burette 61a, a cock 61c arranged at the lower end of the burette 61a, and one end extending into the burette 61a in the vicinity of the cock 61c. It has an air introduction pipe 61d and a cock 61e arranged on the other end side of the air introduction pipe 61d.
- the conduit 62 is attached between the burette portion 61 and the measuring table 63.
- the inner diameter of the conduit 62 is 6 mm.
- a hole having a diameter of 2 mm is formed in the central portion of the measuring table 63, and the conduit 62 is connected to the hole.
- the measuring unit 64 has a cylinder 64a (made of acrylic resin), a nylon mesh 64b adhered to the bottom of the cylinder 64a, and a weight 64c.
- the inner diameter of the cylinder 64a is 20 mm.
- the opening of the nylon mesh 64b is 75 ⁇ m (200 mesh).
- the weight 64c has a diameter of 19 mm and the weight 64c has a mass of 59.8 g.
- the weight 64c is placed on the particle 65 to be measured, and a load of 2.07 kPa can be applied to the particle 65 to be measured.
- the weight 64c was placed and the measurement was started. Since the same volume of air as the physiological saline absorbed by the particles 65 to be measured is quickly and smoothly supplied to the inside of the burette 61a from the air introduction pipe, the water level of the physiological saline inside the burette 61a is reduced. , The amount of physiological saline absorbed by the particle 65 to be measured.
- the scale of the burette 61a is engraved from top to bottom in 0 mL to 0.5 mL increments.
- Ratio of 3-minute value to 15-minute value (%) (3-minute value of saline water absorption under load / 15-minute value of saline water absorption under load) x 100
- an air-through type porous liquid permeable sheet made of polyethylene-polypropylene having a basis weight of 22 g / m 2 and having the same size as the absorber is placed on the upper surface of the laminate, and both ends are cut to a size of 30 cm ⁇ 10 cm.
- the absorption time under short-time pressurization was measured by the method outlined in FIG.
- the absorbent article was placed on a horizontal table in a room with a temperature of 25 ⁇ 2 ° C. and a humidity of 50% ⁇ 10%.
- a measuring jig provided with a cylinder 71 and an acrylic plate 75 was placed at the center of the main surface of the absorbent article.
- the cylinder 71 has an inner diameter of 4 cm, an outer diameter of 5 cm, and a height of 18 cm.
- the cylinder 71 has a mesh 73 with an opening of 2 mm on the bottom surface.
- the acrylic plate 75 has a size of 30 cm ⁇ 10 cm and has a hole with a diameter of 5 cm in the center.
- a cylinder 71 is installed in the hole of the acrylic plate 75. Subsequently, by placing two weights 77 of 10 cm ⁇ 10 cm and a weight of 2492.25 g on both ends of the measuring jig, a load of 2.07 kPa (0.3 psi) is applied to the entire 30 cm ⁇ 10 cm absorbent article. I called.
- the absorbent article using the particulate water-absorbent resin composition obtained in the examples had a short absorption time under pressure and had high absorption performance.
- Weight 100 ... Absorbent article, 110 ... Measuring device, 112a ... Disc part, 112b ... Rod-shaped part, 112c ... Cylindrical part, 114 ... Cylinder, 116 ... Wire mesh, 120 ... Particles to be measured, 130 ... Stainless steel, 140 ... Glass filter, 150 ... Filter paper, 200 ... Stirring blade, 200a ... Shaft, 200b ... Flat plate, S ... Slit.
Landscapes
- Health & Medical Sciences (AREA)
- Epidemiology (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Heart & Thoracic Surgery (AREA)
- Vascular Medicine (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Medicinal Chemistry (AREA)
- Polymers & Plastics (AREA)
- Organic Chemistry (AREA)
- Solid-Sorbent Or Filter-Aiding Compositions (AREA)
Abstract
L'invention concerne un procédé de production d'une composition de résine absorbante particulaire, lequel comporte l'obtention d'une composition de résine absorbante particulaire par obtention de particules de résine absorbantes contenant des particules polymères obtenues à l'aide d'un processus de granulation, et par mélange de ces particules de résine absorbantes contenant des particules polymères obtenues à l'aide d'un processus de granulation et de particules absorbantes contenant des particules polymères obtenues sans processus de granulation. Dans l'ensemble de particules obtenues par granulation, avant une nouvelle granulation, le taux de particules ne passant pas à travers un tamis selon les normes JIS dont l'ouverture de maille est de 850μm est supérieur ou égal à 70% en masse, et la proportion de particules de résine absorbantes obtenues par le procédé de granulation est compris entre 40 et 80% en masse par rapport à la quantité totale de composition de résine absorbante particulaire.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2022508411A JPWO2021187526A1 (fr) | 2020-03-18 | 2021-03-17 |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2020-047376 | 2020-03-18 | ||
| JP2020047376 | 2020-03-18 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2021187526A1 true WO2021187526A1 (fr) | 2021-09-23 |
Family
ID=77772100
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2021/010818 WO2021187526A1 (fr) | 2020-03-18 | 2021-03-17 | Procede de production de composition de resine absorbante particulaire, de corps absorbant et d'article absorbant |
Country Status (2)
| Country | Link |
|---|---|
| JP (1) | JPWO2021187526A1 (fr) |
| WO (1) | WO2021187526A1 (fr) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2023153402A1 (fr) * | 2022-02-14 | 2023-08-17 | ユニ・チャーム株式会社 | Couche absorbante et procédé de production de couche absorbante |
Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1996013542A1 (fr) * | 1994-10-26 | 1996-05-09 | Nippon Shokubai Co., Ltd. | Composition de resine absorbant l'eau et procede pour la fabriquer |
| JPH11106514A (ja) * | 1997-06-18 | 1999-04-20 | Nippon Shokubai Co Ltd | 吸水性樹脂造粒物の製造方法 |
| JPH11140194A (ja) * | 1997-06-23 | 1999-05-25 | Nippon Shokubai Co Ltd | 吸水性樹脂組成物およびその製造方法 |
| JP2005015787A (ja) * | 2003-06-03 | 2005-01-20 | Nippon Shokubai Co Ltd | 吸水材の製造方法 |
| JP2013034942A (ja) * | 2011-08-08 | 2013-02-21 | Nippon Shokubai Co Ltd | 粒子状吸水剤の製造方法 |
| JP2016529368A (ja) * | 2013-08-27 | 2016-09-23 | エルジー・ケム・リミテッド | 高吸水性樹脂の製造方法 |
-
2021
- 2021-03-17 WO PCT/JP2021/010818 patent/WO2021187526A1/fr active Application Filing
- 2021-03-17 JP JP2022508411A patent/JPWO2021187526A1/ja active Pending
Patent Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1996013542A1 (fr) * | 1994-10-26 | 1996-05-09 | Nippon Shokubai Co., Ltd. | Composition de resine absorbant l'eau et procede pour la fabriquer |
| JPH11106514A (ja) * | 1997-06-18 | 1999-04-20 | Nippon Shokubai Co Ltd | 吸水性樹脂造粒物の製造方法 |
| JPH11140194A (ja) * | 1997-06-23 | 1999-05-25 | Nippon Shokubai Co Ltd | 吸水性樹脂組成物およびその製造方法 |
| JP2005015787A (ja) * | 2003-06-03 | 2005-01-20 | Nippon Shokubai Co Ltd | 吸水材の製造方法 |
| JP2013034942A (ja) * | 2011-08-08 | 2013-02-21 | Nippon Shokubai Co Ltd | 粒子状吸水剤の製造方法 |
| JP2016529368A (ja) * | 2013-08-27 | 2016-09-23 | エルジー・ケム・リミテッド | 高吸水性樹脂の製造方法 |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2023153402A1 (fr) * | 2022-02-14 | 2023-08-17 | ユニ・チャーム株式会社 | Couche absorbante et procédé de production de couche absorbante |
Also Published As
| Publication number | Publication date |
|---|---|
| JPWO2021187526A1 (fr) | 2021-09-23 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP4261853B2 (ja) | 吸水性樹脂、吸水性樹脂粒子、およびその製造方法 | |
| WO2020184389A1 (fr) | Particules de résine absorbant l'eau | |
| WO2020184394A1 (fr) | Particule de résine absorbant l'eau, absorbeur, article absorbant, procédé de mesure de taux de rétention de perméation de particule de résine absorbant l'eau, et procédé de production de particule de résine absorbant l'eau | |
| US20220314194A1 (en) | Particulate water-absorbent resin composition | |
| JP6828222B1 (ja) | 吸水性樹脂粒子、吸収性物品、吸水性樹脂粒子を製造する方法、及び吸収体の加圧下での吸収量を高める方法 | |
| WO2021187526A1 (fr) | Procede de production de composition de resine absorbante particulaire, de corps absorbant et d'article absorbant | |
| JP7443330B2 (ja) | 吸水性樹脂粒子及びその製造方法、吸収体、吸収性物品、並びに、浸透速度の調整方法 | |
| EP3936540A1 (fr) | Particules de resine hydro-absorbantes, procede de production de celles-ci, corps absorbant ainsi qu'article absorbant | |
| WO2020184386A1 (fr) | Particules de résine absorbant l'eau, article absorbant, procédé de fabrication de particules de résine absorbant l'eau, procédé pour faciliter la perméation d'une solution saline physiologique dans un corps absorbant | |
| WO2020122219A1 (fr) | Particules de résine absorbantes, corps absorbant, article absorbant, et procédé de mesure de puissance d'absorption de liquide | |
| US20220219140A1 (en) | Water-absorbent resin particles | |
| EP3936530A1 (fr) | Corps absorbant, article absorbant et procédé d'ajustement de vitesse de perméation | |
| EP3936537A1 (fr) | Particules de resine hydro-absorbantes et procede de production de celles-ci | |
| WO2020218158A1 (fr) | Particules de resine absorbante | |
| WO2021006149A1 (fr) | Procédé de production de particules de polymère réticulé et gel de polymère réticulé | |
| JP7470496B2 (ja) | 粒子状吸水性樹脂組成物 | |
| WO2021187525A1 (fr) | Procede de production de particules de resine absorbantes, de corps absorbant et d'article absorbant | |
| EP3896117A1 (fr) | Particules de résine absorbante, procédé d'évaluation de propriétés de fuite de liquide de ces particules, procédé de fabrication de ces particules, et article absorbant | |
| WO2021049465A1 (fr) | Procédé d'amélioration de la quantité d'absorption d'eau sous charge, procédé de fabrication de particules de polymère réticulé, et procédé de fabrication de particules de résine absorbant l'eau | |
| JP7448553B2 (ja) | 架橋重合体粒子の製造方法、吸水性樹脂粒子の製造方法、及び、荷重下吸水量の向上方法 | |
| WO2021006148A1 (fr) | Gel de polymère réticulé, procédé pour le produire, composition de monomère, et procédé de production de particules de polymère réticulé | |
| JP6775050B2 (ja) | 吸収性物品 | |
| JP7470494B2 (ja) | 吸水性樹脂粒子 | |
| WO2021187501A1 (fr) | Feuille absorbant l'eau et article absorbant | |
| JP7091556B2 (ja) | 吸水性樹脂粒子、及び吸水シート |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 21771076 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 2022508411 Country of ref document: JP Kind code of ref document: A |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 21771076 Country of ref document: EP Kind code of ref document: A1 |