WO2021006291A1 - 薬剤溶出型ステント - Google Patents
薬剤溶出型ステント Download PDFInfo
- Publication number
- WO2021006291A1 WO2021006291A1 PCT/JP2020/026688 JP2020026688W WO2021006291A1 WO 2021006291 A1 WO2021006291 A1 WO 2021006291A1 JP 2020026688 W JP2020026688 W JP 2020026688W WO 2021006291 A1 WO2021006291 A1 WO 2021006291A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- stent
- cilostazol
- layer
- bioabsorbable polymer
- mass
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/82—Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/10—Drugs for disorders of the cardiovascular system for treating ischaemic or atherosclerotic diseases, e.g. antianginal drugs, coronary vasodilators, drugs for myocardial infarction, retinopathy, cerebrovascula insufficiency, renal arteriosclerosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/82—Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/86—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure
- A61F2/88—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure the wire-like elements formed as helical or spiral coils
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/47—Quinolines; Isoquinolines
- A61K31/4709—Non-condensed quinolines and containing further heterocyclic rings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L31/00—Materials for other surgical articles, e.g. stents, stent-grafts, shunts, surgical drapes, guide wires, materials for adhesion prevention, occluding devices, surgical gloves, tissue fixation devices
- A61L31/02—Inorganic materials
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L31/00—Materials for other surgical articles, e.g. stents, stent-grafts, shunts, surgical drapes, guide wires, materials for adhesion prevention, occluding devices, surgical gloves, tissue fixation devices
- A61L31/02—Inorganic materials
- A61L31/022—Metals or alloys
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L31/00—Materials for other surgical articles, e.g. stents, stent-grafts, shunts, surgical drapes, guide wires, materials for adhesion prevention, occluding devices, surgical gloves, tissue fixation devices
- A61L31/04—Macromolecular materials
- A61L31/06—Macromolecular materials obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L31/00—Materials for other surgical articles, e.g. stents, stent-grafts, shunts, surgical drapes, guide wires, materials for adhesion prevention, occluding devices, surgical gloves, tissue fixation devices
- A61L31/08—Materials for coatings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L31/00—Materials for other surgical articles, e.g. stents, stent-grafts, shunts, surgical drapes, guide wires, materials for adhesion prevention, occluding devices, surgical gloves, tissue fixation devices
- A61L31/08—Materials for coatings
- A61L31/10—Macromolecular materials
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L31/00—Materials for other surgical articles, e.g. stents, stent-grafts, shunts, surgical drapes, guide wires, materials for adhesion prevention, occluding devices, surgical gloves, tissue fixation devices
- A61L31/12—Composite materials, i.e. containing one material dispersed in a matrix of the same or different material
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L31/00—Materials for other surgical articles, e.g. stents, stent-grafts, shunts, surgical drapes, guide wires, materials for adhesion prevention, occluding devices, surgical gloves, tissue fixation devices
- A61L31/14—Materials characterised by their function or physical properties, e.g. injectable or lubricating compositions, shape-memory materials, surface modified materials
- A61L31/148—Materials at least partially resorbable by the body
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L31/00—Materials for other surgical articles, e.g. stents, stent-grafts, shunts, surgical drapes, guide wires, materials for adhesion prevention, occluding devices, surgical gloves, tissue fixation devices
- A61L31/14—Materials characterised by their function or physical properties, e.g. injectable or lubricating compositions, shape-memory materials, surface modified materials
- A61L31/16—Biologically active materials, e.g. therapeutic substances
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/82—Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/86—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2300/00—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices
- A61L2300/20—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices containing or releasing organic materials
- A61L2300/204—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices containing or releasing organic materials with nitrogen-containing functional groups, e.g. aminoxides, nitriles, guanidines
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2300/00—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices
- A61L2300/40—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices characterised by a specific therapeutic activity or mode of action
- A61L2300/42—Anti-thrombotic agents, anticoagulants, anti-platelet agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2300/00—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices
- A61L2300/40—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices characterised by a specific therapeutic activity or mode of action
- A61L2300/422—Anti-atherosclerotic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2300/00—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices
- A61L2300/60—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices characterised by a special physical form
- A61L2300/602—Type of release, e.g. controlled, sustained, slow
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2300/00—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices
- A61L2300/60—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices characterised by a special physical form
- A61L2300/606—Coatings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2300/00—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices
- A61L2300/60—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices characterised by a special physical form
- A61L2300/63—Crystals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2420/00—Materials or methods for coatings medical devices
- A61L2420/08—Coatings comprising two or more layers
Definitions
- the present invention relates to a cilostazol-coated stent and a method for producing the same, and more particularly to a stent having a plurality of layers containing cilostazol and a method for producing the same.
- PTA percutaneous transluminal coronary angioplasty
- PTCA is a thin tube (balloon catheter) or stent with a balloon at the tip inserted through the artery in the arm or thigh, passed through the stenosis of the coronary artery, and then the balloon at the tip is inflated.
- It is a technique to restore blood flow by expanding the narrowed blood vessels. This dilates the vascular lumen at the lesion, thereby increasing blood flow through the vascular lumen.
- This PTCA is used not only for atherosclerotic diseases but also for stenosis treatment of shunt blood vessels formed in the arms of hemodialysis patients.
- the vascular site where PTCA is performed is damaged such as detachment of endothelial cells or damage to the elastic plate, and proliferation of the intima of the blood vessel, which is a healing reaction of the blood vessel wall, occurs. Restenosis occurs in about 30-40% of successful cases.
- the causes of restenosis in humans are mainly the inflammatory process seen in monocyte adhesion and / or infiltration that occurs 1 to 3 days after PTCA, and intimal thickening due to smooth muscle cells that peak proliferatively after about 45 days. The formation process is being considered. When restenosis occurs, it is necessary to perform PTCA again, so there is an urgent need to establish preventive and therapeutic methods.
- a drug-eluting type medical device for intraluminal indwelling in which an anticancer agent, an immunosuppressant, an antiinflammatory agent, or a smooth muscle cell growth inhibitor is carried on the surface of a stent or the like, the lumen is used. Attempts have been actively made to reduce the restenosis rate by locally releasing the drug at the indwelling site for several days.
- a limus-based drug that can act as an anticancer agent and an immunosuppressant is common. Due to their strong cytotoxicity, these drugs have the effect of strongly suppressing the proliferation of vascular smooth muscle cells, which is the main cause of restenosis, so-called intima thickening. However, since it also strongly suppresses the regeneration of vascular endothelial cells, there is a major clinical problem that it can induce delayed intrastent thrombosis.
- Patent Document 1 is a drug-eluting type in which a mixture containing a bioabsorbable polymer having a molecular weight of 40,000 to 600,000 and cilostazol is coated on the surface of a stent body made of a metal or a polymer material. Propose a stent (see claims and [0015] etc.).
- Patent Document 1 states that the stent elutes a drug at a time when restenosis occurs in the inflammatory process or intima thickening process after stent placement and acts on intravascular cells to effectively suppress intima thickening. We disclose that it has an effect and can greatly improve restenosis after stent placement, which has occurred with high probability (see [0028]).
- the stent disclosed in Patent Document 1 is used for relatively large arteries such as coronary coronary arteries, and is required to act on the inflammatory process observed in the adhesion and infiltration of monocytes occurring 1 to 3 days after PTCA. .. Therefore, the stent described in Patent Document 1 is required to exert its effect by releasing cilostazol for several days after installation.
- peripheral arterial disease due to infarction of a thinner peripheral artery has been attracting attention.
- PID peripheral arterial disease
- arteriosclerosis occurs in the blood vessels of the foot, and the blood vessels become thin or clogged, so that sufficient blood does not flow to the foot.
- symptoms such as numbness, pain, and coldness appear when walking.
- the foot may become ulcerated or necrotic, and in severe cases, the foot may have to be operated on.
- arteriosclerosis may extend not only to limbs but also to blood vessels throughout the body. If the PAD is left untreated, it may cause myocardial infarction, angina pectoris, cerebral infarction and the like.
- treatment methods for PAD such as drug therapy, physical therapy, and surgery, depending on the progress of the medical condition and the treatment goal. If a drug indwelling stent for peripheral arteries can be realized, it is possible to provide a new therapeutic method for treating PAD with minimal invasiveness.
- the present inventors may have an active ingredient in the pathological vascular artery for a longer period of time (for example, 6 to 12 months) than that of a drug indwelling stent for a coronary artery (for example, Patent Document 1). I thought I needed a stent.
- An object of the present invention is to provide a drug indwelling stent in which the active ingredient can be present in pathological blood vessels for a longer period of time (for example, 6 to 12 months).
- Such indwelling drug stents can be suitably used for the treatment of peripheral blood vessels (eg, peripheral arterial blood vessels) and can provide a less invasive treatment method.
- the present inventors have a deposited layer in which a plurality of layers are deposited on the stent skeleton, and each layer of the deposited layer contains crystalline cilostazol, and at least one of the plurality of layers.
- the crystalline cilostazol contained a bioabsorbable polymer, and the elution rate was tested in vitro, and it was found that after 24 hours, a drug-indwelling stent having an elution rate of 5% by mass or less could be obtained. Furthermore, they have found that such indwelling drug stents are suitable for peripheral vascular applications and have completed the present invention.
- the present specification includes the following aspects.
- 1. With the stent skeleton It has a sedimentary layer with multiple layers deposited on the stent skeleton. Each layer of the sedimentary layer contains crystalline cilostazol (CLZ) and contains A stent in which at least one of the plurality of layers contains a bioabsorbable polymer.
- CLZ crystalline cilostazol
- the crystalline cilostazol elutes up to 5% by weight 24 hours after contacting the stent in vitro with the elution medium of a phosphate buffered sodium chloride solution containing 0.25% by weight sodium lauryl sulfate at 37 ° C. Stent.
- 2. 2.
- each layer of the sedimentary layer contains crystalline cilostazol (CLZ) and contains A stent in which at least one of the plurality of layers contains a bioabsorbable polymer.
- CLZ crystalline cilostazol
- the crystalline cilostazol elutes up to 20% by weight 15 days after contacting the stent in vitro with the elution medium of a phosphate buffered sodium chloride solution containing 0.25% by weight sodium lauryl sulfate at 37 ° C. Stent. 3. 3.
- the deposition layer has at least two layers, and the cilostazol content of the first layer close to the stent is larger than the cilostazol content of the second layer far from the stent, and both layers contain a bioabsorbable polymer.
- 9. With the stent skeleton Includes a first layer deposited on the stent skeleton and a second layer deposited on it.
- the first and second layers contain cilostazol and a bioabsorbable polymer, respectively.
- the bioabsorbable polymer contains L-lactide and DL-lactide in a mass ratio of 6: 4 to 8: 2 and has a viscosity of 1.8 to 4.5 dL / g.
- the first layer contains 470 ⁇ 47 ⁇ g of cilostazol and 313 ⁇ 31 ⁇ g of the bioabsorbable polymer
- the second layer contains 30 ⁇ 3 ⁇ g of cilostazol and 270 ⁇ 27 ⁇ g of the bioabsorbable polymer.
- crystalline cilostazol can elute 5% by mass or less 24 hours after the elution rate is tested in vitro. Therefore, the indwelling drug stent of the embodiment of the present invention can release crystalline cilostazol over a longer period of time and can be used more preferably for peripheral blood vessels.
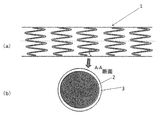
- FIG. 1 schematically shows the entire shape (a) of the stent according to the embodiment of the present invention and the cross section (b) of the stent between A and A.
- FIG. 2 schematically shows how a coating agent is coated on a stent using an ultrasonic atomizer.
- the stent of one embodiment of the present invention With the stent skeleton It has a sedimentary layer with multiple layers deposited on the stent skeleton. Each layer of the sedimentary layer contains crystalline cilostazol and contains At least one of the plurality of layers contains a bioabsorbable polymer.
- the crystalline cilostazol elutes up to 5% by weight 24 hours after contacting the stent in vitro with the elution medium of a phosphate buffered sodium chloride solution containing 0.25% by weight sodium lauryl sulfate at 37 ° C.
- the stent of another embodiment of the present invention With the stent skeleton It has a sedimentary layer with multiple layers deposited on the stent skeleton.
- Each layer of the sedimentary layer contains crystalline cilostazol and contains A stent in which at least one of the plurality of layers contains a bioabsorbable polymer.
- the crystalline cilostazol elutes up to 20% by weight 15 days after contacting the stent in vitro with the elution medium of a phosphate buffered sodium chloride solution containing 0.25% by weight sodium lauryl sulfate at 37 ° C.
- the stent of a further embodiment of the present invention With the stent skeleton Includes a first layer deposited on the stent skeleton and a second layer deposited on it.
- the first and second layers contain cilostazol and a bioabsorbable polymer, respectively.
- the bioabsorbable polymer contains L-lactide and DL-lactide in a mass ratio of 6: 4 to 8: 2 and has a viscosity of 1.8 to 4.5 dL / g.
- the first layer contains 470 ⁇ 47 ⁇ g of cilostazol and 313 ⁇ 31 ⁇ g of the bioabsorbable polymer
- the second layer contains 30 ⁇ 3 ⁇ g of cilostazol and 270 ⁇ 27 ⁇ g of the bioabsorbable polymer.
- the stent of the embodiment of the present invention has a stent skeleton and a deposited layer in which a plurality of layers are deposited on the stent skeleton.
- the "stent skeleton” means a skeleton forming a stent, which is usually formed into a coarse cylindrical shape using, for example, a metal or a polymer material, and is the object of the present invention. There are no particular restrictions as long as a stent can be obtained.
- metal stent skeleton for example, a stent skeleton made of an appropriate alloy such as nickel, cobalt, chromium, titanium, and stainless steel can be exemplified, and a metal stent skeleton containing a cobalt-chromium alloy as a main component is preferable. Is.
- the stent of the embodiment of the present invention has a deposited layer in which a plurality of layers are deposited on the stent skeleton.
- Each layer of the deposited layer contains crystalline cilostazol, and at least one of the plurality of layers contains a bioabsorbable polymer.
- Cilostazol is 6- [4- (1-cyclohexyl-1H-tetrazol-5-yl) butoxy] -3,4-dihydrocarbostyryl.
- Cilostazol has an inhibitory effect on platelet aggregation, an inhibitory effect on phosphodiesterase (PDE), an anti-ulcer effect, an antihypertensive effect and an anti-inflammatory effect, and has an antithrombotic agent, a cerebral circulation improving agent, an anti-inflammatory agent, an anti-ulcer agent, an antihypertensive agent and an anti-inflammatory agent.
- Cilostazol also contains a pharmaceutically acceptable salt thereof.
- cilostazol is preferably crystalline. It is preferable that cilostazol has a crystal structure rather than having no crystal structure (non-crystal) because the elution rate can be suppressed to a low level.
- the bioabsorbable polymer is not particularly limited as long as the stent intended by the present invention can be obtained.
- the bioabsorbable polymer include polylactic acid containing lactide, and the molecular weight (Mw: weight average molecular weight) thereof may be 40,000 to 700,000. The viscosity may be 0.4 to 5.0 dL / g, and may be 0.4 to 4.2 dL / g.
- the bioabsorbable polymer contains DL lactide, L lactide and the like, and may contain glycolide, caprolactone and the like.
- a polymer (including 100% by mass of L-lactide poly-L lactic acid) can be exemplified.
- Commercially available products can be used as the bioabsorbable polymer, and examples thereof include LR704S (trade name), L206S (trade name), and LR706S (trade name). The bioabsorbable polymer can be used alone or in combination.
- the bioabsorbable polymer preferably contains 90% by mass or more of polylactic acid, more preferably 93% by mass or more, and even more preferably 96% by mass.
- the bioabsorbable polymer has an advantageous effect that it can be slowly released when it contains 90% by mass or more of polylactic acid.
- the sedimentary layer has at least two layers, and the cilostazol content of the first layer close to the stent skeleton is preferably larger than the cilostazol content of the second layer far from the stent skeleton. Layers closer to the stent skeleton have the beneficial effect of long-term sustained release when the cilostazol content is higher.
- the content of cilostazol in the first layer is preferably 300 to 750 ⁇ g, more preferably 350 to 550 ⁇ g, and even more preferably 440 to 480 ⁇ g. Further, the first layer preferably contains 470 ⁇ 47 ⁇ g of cilostazol.
- the content of cilostazol in the second layer is preferably 0 to 100 ⁇ g, more preferably 10 to 80 ⁇ g, and even more preferably 20 to 60 ⁇ g. Further, the second layer preferably contains 30 ⁇ 3 ⁇ g of cilostazol.
- the content of cilostazol in the first layer is preferably 40 to 100% by mass, more preferably 50 to 70% by mass, and even more preferably 55 to 65% by mass.
- the content of cilostazol in the second layer is preferably 0 to 50% by mass, more preferably 2 to 20% by mass, and even more preferably 5 to 10% by mass.
- the deposited layer has at least two layers, and the cilostazol content of the first layer close to the stent is larger than the cilostazol content of the second layer far from the stent, and it is preferable that both layers contain a bioabsorbable polymer.
- the content of the bioabsorbable polymer in the first layer is preferably 0 to 500 ⁇ g, more preferably 250 to 350 ⁇ g, and even more preferably 300 to 320 ⁇ g. Further, the first layer preferably contains 313 ⁇ 31 ⁇ g of the bioabsorbable polymer.
- the content of the bioabsorbable polymer in the second layer is preferably 180 to 540 ⁇ g, more preferably 200 to 300 ⁇ g, and even more preferably 260 to 285 ⁇ g. Further, the second layer preferably contains 270 ⁇ 27 ⁇ g of the bioabsorbable polymer.
- the content of the bioabsorbable polymer in the first layer is preferably 0 to 60% by mass, more preferably 25 to 50% by mass, and even more preferably 35 to 45% by mass.
- the content of the bioabsorbable polymer in the second layer is preferably 70% by mass or more, more preferably 80% by mass or more, and further preferably 90% by mass or more.
- the crystalline silostazole is brought into contact with an elution medium of a phosphate buffered sodium chloride solution containing 0.25% by mass sodium lauryl sulfate at 37 ° C. in vitro, and then 15 After a day, the crystalline silostazole is preferably eluted in an amount of 20% by mass or less, and 8 days after the stent is brought into contact, the crystalline silostazole is preferably eluted in an amount of 7% by mass or less, and the stent is contacted. One day after the aging, the crystalline silostazole is preferably eluted in an amount of 5% by mass or less, and more preferably 3% by mass or less.
- the crystalline silostazole is brought into contact with an elution medium of a phosphate buffered sodium chloride solution containing 0.25% by mass sodium lauryl sulfate at 37 ° C. in vitro, and then 15 After a day, the crystalline syrostazole may be eluted, for example, 1.0% by mass or more, 0.1% by mass or more, and 8 days after the stent is brought into contact, the crystalline syrostazole may be eluted.
- the crystalline silostazole is eluted, for example, 0.1% by mass or more. It may be eluted in an amount of 0.01% by mass or more.
- the crystalline cilostazol elutes in the above proportions or less after the above time after contacting the stent with the elution medium of a phosphate buffered sodium chloride solution containing 0.25% by mass sodium lauryl sulfate at 37 ° C.
- cilostazol can be released for more than 3 months, so that cilostazol can be present in the living body for a very long period of time. Therefore, the stent of the embodiment of the present invention can be suitably used, for example, for peripheral blood vessels, preferably for peripheral arterial blood vessels.
- the stent of the embodiment of the present invention can also be used for, for example, a relatively thick artery in which a stent has been conventionally used, such as a coronary artery of the heart and a lower limb artery.
- the method for producing the stent according to the embodiment of the present invention is not particularly limited.
- the stent of the embodiment of the present invention is, for example, (i) preparing a stent skeleton; (ii) preparing a mixture containing cilostazol; (iii) coating the stent skeleton with the mixture, (ii) and. It can be produced by using a production method including repeating (iii) (however, the content of cilostazol and the like are adjusted).
- the mixture containing cilostazol can contain the bioabsorbable polymer in addition to cilostazol.
- the mixture can further contain a solvent such as an additive. Since cilostazol is poorly soluble in bioabsorbable polymers, it is necessary to prevent the coating from peeling off and maintain high strength.
- the mixed mass ratio of cilostazol and a bioabsorbable polymer, for example polylactic acid, is preferably 1: 0.5 to 1: 1.5. When it is within this ratio range, a better intimal thickening effect can be obtained. Further, when the mixed mass ratio is 1: 1.1 to 1: 1.5, the coating strength and the effect of sustained release can be further enhanced.
- the method of coating the stent skeleton with a mixture of cilostazol and a bioabsorbable polymer is not particularly limited as long as the stent of the present invention can be obtained, and has been conventionally used.
- a simple spray method, a dipping method, an electrodeposition method, an ultrasonic spray method and the like can be used, but it is preferable to use the ultrasonic spray method in terms of coating. If possible, the above-described embodiments can be combined as appropriate.
- FIG. 1A schematically shows a drug-eluting stent 1 of one embodiment of the present invention.
- the drug-eluting stent 1 has a cylindrical shape with a longitudinal axis and has a lumen.
- the drug-eluting stent 1 has a coarse mesh-like side surface in a cylindrical form and is formed so as to be expandable laterally.
- the mesh can be formed by a member 2 (a line of a metal, a polymer material, or the like) forming a stent skeleton.
- the drug-eluting stent 1 is usually inserted into the body in a non-dilated form, expanded at a therapeutic site in a blood vessel, and placed in the blood vessel. Dilation may be achieved intravascularly with a balloon catheter.
- FIG. 1A schematically describes the mesh. As long as the stent of the present invention can be obtained, the mesh pattern is not particularly limited.
- FIG. 1 (b) schematically shows a cross section (AA cross section) of the line forming the stent skeleton in FIG. 1 (a).
- a deposition layer 3 is formed on the stent skeleton member 2.
- the stent skeleton member 2 can be manufactured by any method. It can be made from hollow or formed stainless steel pipes by, for example, laser, discharge milling, chemical etching or other means.
- the stent skeleton member 2 can be formed of an appropriate alloy of nickel, cobalt, chromium, titanium, stainless steel or the like.
- the sedimentary layer 3 is formed of at least two layers. In FIG. 1 (b), a plurality of layers are not shown.
- FIG. 2 schematically shows an ultrasonic spray coating device 4 formed by coating a sedimentary layer 3 on a stent skeleton member 2.
- the surface of the stent skeleton member 2 is first plasma-treated by a plasma treatment device (not shown) before the coating step.
- the stent skeleton member 2 is attached to the mandrel and attached to the ultrasonic spray coating device 4.
- the coating liquid is sent through the pipe 6 by a syringe pump, atomized by the ultrasonic spray nozzle 5, and ejected.
- the stent skeleton member 2 is linearly moved while rotating under the ultrasonic nozzle 5 to deposit the sedimentary layer 3 on the stent skeleton member 2. After that, the stent skeleton member 2 is rotated and linearly moved, dried in a nitrogen stream, and further dried in a desiccator under reduced pressure to prepare a drug-eluting stent 1.
- the coating liquid is changed according to the number of layers contained in the deposited layer 3, and the stent skeleton member is coated a plurality of times to form the deposited layer 3.
- a coating solution using a mixture of cilostazol and a bioabsorbable polymer dissolved in a solvent at a ratio corresponding to the deposited layer 3 to be formed. Since the deposited layer 3 contains a plurality of layers, it is necessary to prepare a plurality of coating liquids.
- a volatile solvent having a low boiling point can be used so that it can be easily removed after coating.
- a solvent can be exemplified.
- the viscosity of the above-mentioned polymer means the ultimate viscosity [ ⁇ ] (dL / g) and was measured by a capillary viscometer method.
- the ultimate viscosity was calculated by the following formula from the measured values of the sample solution having a concentration of C (g / dL) and the flow time (t) of the sample solution and the flow time of the solvent (t0).
- An Ubbelohde viscometer was used as an apparatus, and chloroform (25 ° C.) was used as a solvent.
- Mw weight average molecular weight
- Example 1 A cobalt-chromium alloy was used as the stent skeleton member 2, and a solution in which cilostazol (CLZ) was dissolved in methylene chloride was prepared. This solution was applied by ultrasonic spray coating onto a cobalt-chromium alloy base material to form a first layer made of 440 ⁇ g cilostazol. Next, a solution was prepared in which cilostazol and the polymer (a) were mixed at a ratio of 1: 9 (mass ratio) and dissolved in methylene chloride. This solution was applied onto the first layer by ultrasonic spray coating to form a second layer of 540 ⁇ g of polymer (a) and 60 ⁇ g of cilostazol to obtain the stent of Example 1. It was.
- CLZ cilostazol
- Examples 2-3 Stents of Examples 2 and 3 were produced using the same method as described in Example 1 except that the polymer (a) was changed to the polymer (b) or (c).
- Comparative Examples 1 to 3 The stents of Comparative Examples 1 to 3 were produced by using the same method as that described in Example 1 except that the polymer (a) was changed to the polymers (d) to (f).
- Example 4 A cobalt-chromium alloy was used as the stent skeleton member 2, and cilostazol and the polymer (b) were mixed at a ratio of 3: 2 to prepare a solution dissolved in methylene chloride. This solution was applied by ultrasonic spray coating onto the cobalt-chromium alloy of the stent backbone member to form a first layer of 313 ⁇ g of polymer (b) and 470 ⁇ g of cilostazol. Next, cilostazol and the polymer (b) were mixed at a ratio of 1: 9 (mass ratio) to prepare a solution dissolved in methylene chloride. This solution was applied onto the first layer by ultrasonic spray coating to form a second layer of 270 ⁇ g of polymer (b) and 30 ⁇ g of cilostazol to obtain the stent of Example 4. It was.
- Example 5 A cobalt-chromium alloy was used as the stent skeleton member 2, and cilostazol and the polymer (b) were mixed at a ratio of 3: 2 to prepare a solution dissolved in methylene chloride. This solution was applied by ultrasonic spray coating onto a cobalt-chromium alloy base material to form a first layer of 323 ⁇ g of polymer (b) and 485 ⁇ g of cilostazol. Next, cilostazol and the polymer (b) were mixed at a ratio of 1:19 (mass ratio) to prepare a solution dissolved in methylene chloride. This solution was applied onto the first layer by ultrasonic spray coating to form a second layer made of 285 ⁇ g polymer (b) and 15 ⁇ g cilostazol to obtain the stent of Example 5. It was.
- Example 6 A cobalt-chromium alloy was used as the stent skeleton member 2, and cilostazol and the polymer (b) were mixed at a ratio of 3: 2 to prepare a solution dissolved in methylene chloride. This solution was applied by ultrasonic spray coating onto a cobalt-chromium alloy base material to form a first layer of 313 ⁇ g polymer (b) and 470 ⁇ g cilostazol. Next, cilostazol and the polymer (c) were mixed at a ratio of 1: 9 (mass ratio) to prepare a solution dissolved in methylene chloride. This solution was applied onto the first layer by ultrasonic spray coating to form a second layer of 270 ⁇ g of polymer (c) and 30 ⁇ g of cilostazol to obtain the stent of Example 6. It was.
- Example 7 A cobalt-chromium alloy was used as the stent skeleton member 2, and cilostazol and the polymer (b) were mixed at a ratio of 3: 2 to prepare a solution dissolved in methylene chloride. This solution was applied by ultrasonic spray coating onto a cobalt-chromium alloy base material to form a first layer of 490 ⁇ g polymer (b) and 735 ⁇ g cilostazol. Next, cilostazol and the polymer (b) were mixed at a ratio of 1:19 (mass ratio) to prepare a solution dissolved in methylene chloride. This solution was applied onto the first layer by ultrasonic spray coating to form a second layer made of 285 ⁇ g polymer (b) and 15 ⁇ g cilostazol to obtain the stent of Example 7. It was.
- Example 8 A stent of Example 8 was obtained using a method similar to that described in Example 4, except that a first layer made of 180 ⁇ g of polymer (b) and 270 ⁇ g of cilostazol was formed.
- Example 9 A stent of Example 9 was obtained using a method similar to that described in Example 4, except that a first layer made of 247 ⁇ g of polymer (b) and 370 ⁇ g of cilostazol was formed.
- Cilostazol dissolution test (in vitro) 1.
- Elution test method of syrostazole Using an dissolution tester 400-DS (Apparatus 7), use 10 mL of phosphate buffered sodium chloride solution containing 0.25 mass% sodium lauryl sulfate as the test solution, and set the temperature of the dissolution test solution to 37 ° C. Tested at Dip Speed 10. Sampling was performed 0.5, 1, 3, 6, 9, 12, 18, 24 hours later, and the entire amount of the test solution was replaced at each sampling time.
- Cilostazol elution rate measurement HPLC measurement was performed on 10 ⁇ L of each sample solution and standard solution under the following conditions, and the elution rate was calculated from the peak area values At and As of cilostazol. The dissolution rate was calculated using the following formula.
- Measurement conditions Detector Ultraviolet absorptiometer (measurement wavelength: 254 nm)
- Column Stainless steel tube with an inner diameter of 4.6 mm and a length of 150 mm filled with octadecylsilylated silica gel for liquid chromatography of 5 ⁇ m
- Column temperature Constant temperature around 25 ° C
- Mobile phase Water / acetonitrile / methanol mixed solution (10: 7 :) 3, v / v / v)
- Flow rate Adjusted so that the retention time of cilostazol is about 9 minutes
- Cilostazol concentration in arterial tissue and residual cilostazol on stent by rabbit iliac artery implantation (in vivo) Each stent of the example and the comparative example was placed in the rabbit iliac artery. Implantation was performed as follows. First, an incision is made in the neck of the rabbit to expose the right carotid artery and an intoducer is placed. A guide wire for a balloon catheter is inserted from the intoducer and moved to the distal part of the treatment site of the iliac artery under fluoroscopy. Then, a contrast catheter is inserted along the guide wire to perform angiography of the treated site of the iliac artery.
- the balloon catheter of the sample is inserted to the treatment site along the guide wire for the balloon catheter under fluoroscopy.
- the balloon is 14 atm (over-expansion) using an indeflator.
- the balloon is contracted to remove the indeflator, and the balloon catheter is guided by the balloon catheter. Pull out along the wire.
- Treat the left and right iliac arteries in a similar manner Next, the contrast medium is moved along the balloon catheter guide wire to the front of the treatment site, and angiography is performed using a diluted contrast medium. After treating the left and right iliac arteries in the same way, the contrast catheter is pulled out. Finally, the blood vessels at the sheath insertion site are ligated and the skin and muscular layer are sutured. As a result, the stent is placed in the iliac blood vessel of the rabbit.
- the cilostazol concentration and the residual amount of the stent in the arterial tissue at the implantation site of each stent were analyzed.
- the arterial tissue at the implantation site of each stent was separated into a stent and an arterial tissue.
- the organic layer obtained by the liquid-liquid extraction method was dried from each of the separated samples to prepare a sample.
- Cilostazol was quantified for the obtained sample by LC / MS / MS method using an electrospray ionization method, and the cilostazol concentration in the arterial tissue ( ⁇ g number of cilostazol in 1 g of tissue: ⁇ g / g tissue) and residual cilostazol.
- the amount (residual rate of cilostazol on the stent (%)) was calculated.
- Cilostazol concentration in arterial tissue and residual cilostazol on stent by porcine iliac artery implantation (in vivo) Evaluation of cilostazol concentration and residual amount of cilostazol in arterial tissue by porcine iliac artery implantation was performed using the same method as that used for rabbits, and cilostazol concentration in arterial tissue by porcine iliac artery implantation ( The number of ⁇ g of cilostazol in 1 g of tissue: ⁇ g / g tissue) and the residual amount of cilostazol (residual rate of cilostazol on the stent (%)) were analyzed.
- Each of the stents of Examples 1 to 9 has a stent skeleton and a deposited layer in which a plurality of layers are deposited on the stent skeleton, and each layer of the deposited layer contains crystalline silostazole and is composed of the plurality of layers. After at least one layer contains a bioabsorbable polymer and the crystalline silostazole contacts the stent in vitro with an elution medium of a phosphate buffered sodium chloride solution containing 0.25 mass% sodium lauryl sulfate at 37 ° C. After 24 hours, 5% by mass or less is eluted.
- each of the stents of Examples 1 to 9 has a stent skeleton and a deposited layer in which a plurality of layers are deposited on the stent skeleton, and each layer of the deposited layer contains crystalline silostazole, and the plurality of the above-mentioned stents.
- At least one of the layers contains a bioabsorbable polymer, the crystalline silostazole contacting the stent in vitro with an elution medium of a phosphate buffered sodium chloride solution containing 0.25 mass% sodium lauryl sulfate at 37 ° C. After 15 days, 20% by mass or less is eluted. Therefore, the stents of Examples 1-9 are capable of sustained release of cilostazol for more than 3 months. Needless to say, the plurality of layers is not limited to two layers, and three or more layers can be applied.
- crystalline cilostazol can elute 5% by mass or less 24 hours after the elution rate is tested in vitro.
- crystalline cilostazol can elute 20% by mass or less after 15 days after testing the dissolution rate in vitro. Therefore, the indwelling drug stent of the embodiment of the present invention can release crystalline cilostazol over a longer period of time and can be used more preferably for peripheral blood vessels.
- Stent 2 Stent skeleton member 3: Deposit layer 4: Ultrasonic spray coating device 5: Ultrasonic spray nozzle 6: Piping
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Veterinary Medicine (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Vascular Medicine (AREA)
- Heart & Thoracic Surgery (AREA)
- Epidemiology (AREA)
- Surgery (AREA)
- Engineering & Computer Science (AREA)
- Chemical & Material Sciences (AREA)
- Biomedical Technology (AREA)
- Medicinal Chemistry (AREA)
- Cardiology (AREA)
- Inorganic Chemistry (AREA)
- Molecular Biology (AREA)
- Pharmacology & Pharmacy (AREA)
- Oral & Maxillofacial Surgery (AREA)
- Transplantation (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Composite Materials (AREA)
- Materials Engineering (AREA)
- Urology & Nephrology (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Organic Chemistry (AREA)
- Materials For Medical Uses (AREA)
- Media Introduction/Drainage Providing Device (AREA)
- Building Environments (AREA)
- Load-Bearing And Curtain Walls (AREA)
- Shielding Devices Or Components To Electric Or Magnetic Fields (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Priority Applications (8)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| ES20837247T ES2987979T3 (es) | 2019-07-09 | 2020-07-08 | Endoprótesis vascular de elución de fármacos |
| MX2022000389A MX2022000389A (es) | 2019-07-09 | 2020-07-08 | Stent de elución de fármaco. |
| JP2021530714A JP7033694B2 (ja) | 2019-07-09 | 2020-07-08 | 薬剤溶出型ステント |
| CN202080048831.XA CN114051417A (zh) | 2019-07-09 | 2020-07-08 | 药剂溶出型支架 |
| US17/597,449 US11806257B2 (en) | 2019-07-09 | 2020-07-08 | Drug-eluting stent including crystalline cilostazol |
| EP20837247.4A EP3998049B1 (en) | 2019-07-09 | 2020-07-08 | Drug-eluting stent |
| JP2022029319A JP7200412B2 (ja) | 2019-07-09 | 2022-02-28 | 薬剤溶出型ステント |
| US18/465,332 US12485026B2 (en) | 2019-07-09 | 2023-09-12 | Drug-eluting stent including crystalline cilostazol |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2019-127529 | 2019-07-09 | ||
| JP2019127529 | 2019-07-09 |
Related Child Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US17/597,449 A-371-Of-International US11806257B2 (en) | 2019-07-09 | 2020-07-08 | Drug-eluting stent including crystalline cilostazol |
| US18/465,332 Continuation US12485026B2 (en) | 2019-07-09 | 2023-09-12 | Drug-eluting stent including crystalline cilostazol |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2021006291A1 true WO2021006291A1 (ja) | 2021-01-14 |
Family
ID=74113786
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2020/026688 Ceased WO2021006291A1 (ja) | 2019-07-09 | 2020-07-08 | 薬剤溶出型ステント |
Country Status (8)
| Country | Link |
|---|---|
| US (2) | US11806257B2 (enExample) |
| EP (1) | EP3998049B1 (enExample) |
| JP (2) | JP7033694B2 (enExample) |
| CN (1) | CN114051417A (enExample) |
| ES (1) | ES2987979T3 (enExample) |
| MX (2) | MX2022000389A (enExample) |
| TW (2) | TW202322815A (enExample) |
| WO (1) | WO2021006291A1 (enExample) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2023038112A1 (ja) * | 2021-09-10 | 2023-03-16 | テルモ株式会社 | 薬剤溶出性の医療機器およびその製造方法 |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2024222834A1 (zh) * | 2023-04-27 | 2024-10-31 | 北京信立泰医疗器械有限公司 | 药物涂层及含有其的医疗装置、系统和制备方法 |
Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2005531391A (ja) * | 2002-06-27 | 2005-10-20 | 微創医療器械(上海)有限公司 | 薬剤放出ステント |
| JP2006198390A (ja) * | 2005-01-17 | 2006-08-03 | Kyung Bum Lee | 薬物放出調節型多層コーティングステント及びその製造方法 |
| JP2014502193A (ja) * | 2010-11-18 | 2014-01-30 | コーディス・コーポレイション | 心筋障害を低減するためのアデノシンa2a受容体作動薬/ホスホジエステラーゼ阻害剤の組み合わせの局所血管送達 |
| WO2015020139A1 (ja) * | 2013-08-07 | 2015-02-12 | 学校法人近畿大学 | ナノ粒子及びナノ粒子組成物並びにその製造方法 |
| WO2016067994A1 (ja) | 2014-10-28 | 2016-05-06 | 株式会社Jimro | 薬剤溶出型ステント |
| JP2019127529A (ja) | 2018-01-24 | 2019-08-01 | 出光興産株式会社 | 潤滑油組成物及び冷凍機用組成物 |
Family Cites Families (19)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5824048A (en) | 1993-04-26 | 1998-10-20 | Medtronic, Inc. | Method for delivering a therapeutic substance to a body lumen |
| CN100471469C (zh) | 2002-06-27 | 2009-03-25 | 微创医疗器械(上海)有限公司 | 一种具有多层涂层的药物洗脱支架 |
| US7318945B2 (en) | 2003-07-09 | 2008-01-15 | Medtronic Vascular, Inc. | Laminated drug-polymer coated stent having dipped layers |
| CN101052362A (zh) | 2004-09-08 | 2007-10-10 | 株式会社钟化 | 生物体留置用支架 |
| KR101265625B1 (ko) * | 2006-09-11 | 2013-05-22 | 엘지전자 주식회사 | 멀티 채널을 정의하는 방송 신호를 처리하는 방송 수신기 및 그 제어방법 |
| KR20090122202A (ko) | 2007-01-30 | 2009-11-26 | 헤모텍 아게 | 생분해성 혈관 지지체 |
| CN101641059B (zh) * | 2007-02-14 | 2011-10-19 | 山东瑞安泰医疗技术有限公司 | 一种不对称药物控释涂层冠脉内支架 |
| KR20100085179A (ko) | 2007-09-04 | 2010-07-28 | 가부시키가이샤 니혼 스텐토 테크놀로지 | 약제 서방성 스텐트 |
| US8067111B2 (en) * | 2008-06-30 | 2011-11-29 | Lg Chem, Ltd. | Battery module having battery cell assembly with heat exchanger |
| WO2010111196A2 (en) | 2009-03-23 | 2010-09-30 | Micell Technologies, Inc. | Peripheral stents having layers |
| US20100280600A1 (en) * | 2009-04-30 | 2010-11-04 | Vipul Bhupendra Dave | Dual drug stent |
| JP5784940B2 (ja) | 2011-03-18 | 2015-09-24 | テルモ株式会社 | 薬剤溶出ステント |
| KR101903443B1 (ko) * | 2012-02-02 | 2018-10-02 | 삼성전자주식회사 | 멀티미디어 통신 시스템에서 장면 구성 정보 송수신 장치 및 방법 |
| JP6139665B2 (ja) | 2012-10-25 | 2017-05-31 | ボストン サイエンティフィック サイムド,インコーポレイテッドBoston Scientific Scimed,Inc. | ステント |
| CN103948458A (zh) * | 2014-05-05 | 2014-07-30 | 加奇生物科技(上海)有限公司 | 颅内药物洗脱支架 |
| CN203829101U (zh) * | 2014-05-05 | 2014-09-17 | 加奇生物科技(上海)有限公司 | 颅内药物洗脱支架 |
| US9475326B2 (en) * | 2014-09-09 | 2016-10-25 | Trodat Gmbh | Removable die plate for self-inking stamps |
| CN204542477U (zh) * | 2015-02-10 | 2015-08-12 | 东莞颠覆产品设计有限公司 | 一种多层可扩张血管支架 |
| PL3421253T3 (pl) * | 2017-06-28 | 2020-01-31 | Hid Global Rastede Gmbh | Okno termochromowe |
-
2020
- 2020-07-08 EP EP20837247.4A patent/EP3998049B1/en active Active
- 2020-07-08 US US17/597,449 patent/US11806257B2/en active Active
- 2020-07-08 ES ES20837247T patent/ES2987979T3/es active Active
- 2020-07-08 MX MX2022000389A patent/MX2022000389A/es unknown
- 2020-07-08 JP JP2021530714A patent/JP7033694B2/ja active Active
- 2020-07-08 WO PCT/JP2020/026688 patent/WO2021006291A1/ja not_active Ceased
- 2020-07-08 CN CN202080048831.XA patent/CN114051417A/zh active Pending
- 2020-07-08 TW TW112105824A patent/TW202322815A/zh unknown
- 2020-07-08 TW TW109123057A patent/TWI794616B/zh active
-
2022
- 2022-01-07 MX MX2023001542A patent/MX2023001542A/es unknown
- 2022-02-28 JP JP2022029319A patent/JP7200412B2/ja active Active
-
2023
- 2023-09-12 US US18/465,332 patent/US12485026B2/en active Active
Patent Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2005531391A (ja) * | 2002-06-27 | 2005-10-20 | 微創医療器械(上海)有限公司 | 薬剤放出ステント |
| JP2006198390A (ja) * | 2005-01-17 | 2006-08-03 | Kyung Bum Lee | 薬物放出調節型多層コーティングステント及びその製造方法 |
| JP2014502193A (ja) * | 2010-11-18 | 2014-01-30 | コーディス・コーポレイション | 心筋障害を低減するためのアデノシンa2a受容体作動薬/ホスホジエステラーゼ阻害剤の組み合わせの局所血管送達 |
| WO2015020139A1 (ja) * | 2013-08-07 | 2015-02-12 | 学校法人近畿大学 | ナノ粒子及びナノ粒子組成物並びにその製造方法 |
| WO2016067994A1 (ja) | 2014-10-28 | 2016-05-06 | 株式会社Jimro | 薬剤溶出型ステント |
| JP2019127529A (ja) | 2018-01-24 | 2019-08-01 | 出光興産株式会社 | 潤滑油組成物及び冷凍機用組成物 |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2023038112A1 (ja) * | 2021-09-10 | 2023-03-16 | テルモ株式会社 | 薬剤溶出性の医療機器およびその製造方法 |
Also Published As
| Publication number | Publication date |
|---|---|
| US20230414383A1 (en) | 2023-12-28 |
| CN114051417A (zh) | 2022-02-15 |
| EP3998049A4 (en) | 2023-06-28 |
| ES2987979T3 (es) | 2024-11-19 |
| MX2023001542A (es) | 2023-04-10 |
| TW202116317A (zh) | 2021-05-01 |
| MX2022000389A (es) | 2023-02-07 |
| US12485026B2 (en) | 2025-12-02 |
| JP7033694B2 (ja) | 2022-03-10 |
| JP2022078154A (ja) | 2022-05-24 |
| US20220160525A1 (en) | 2022-05-26 |
| EP3998049B1 (en) | 2024-08-21 |
| JP7200412B2 (ja) | 2023-01-06 |
| EP3998049A1 (en) | 2022-05-18 |
| US11806257B2 (en) | 2023-11-07 |
| TW202322815A (zh) | 2023-06-16 |
| TWI794616B (zh) | 2023-03-01 |
| JPWO2021006291A1 (enExample) | 2021-01-14 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| RU2360646C2 (ru) | Эндолюминальный протез, содержащий лечебное средство | |
| JP5579353B2 (ja) | 抗炎症剤および薬物配給装置 | |
| CN102641239B (zh) | 用于治疗cad的西罗莫司及其衍生物的溶液制剂 | |
| JP5615474B2 (ja) | Cad治療のためのタキサンの注入可能な配合物 | |
| JP4832787B2 (ja) | 薬物溶出式の医療装置における酸化を防ぎ薬物の劣化を減少するための酸化防止剤の使用 | |
| JP4994558B2 (ja) | 脈管の病気の治療のための配給装置 | |
| JP2006006938A (ja) | 調整された薬物放出のためのヘパリン・バリア被膜 | |
| US12485026B2 (en) | Drug-eluting stent including crystalline cilostazol | |
| JP2005334646A (ja) | 抗増殖薬および配給装置 | |
| KR20190057059A (ko) | 고분자-없는 약물 용출 혈관 스텐트 | |
| KR20050092757A (ko) | 생체 유치용 스텐트 | |
| JP2005312967A (ja) | 脈管の病気の予防および治療のための薬物/薬物配給システム | |
| JP6820745B2 (ja) | 薬剤溶出型ステント | |
| Thierry et al. | Effects of Surface Modification Induced by Sterilization Processes on the Thrombogenicity of Nickel-Titanium Stents | |
| JP2003320036A (ja) | ステント | |
| HK1243309B (en) | Drug-eluting stent | |
| HK1150229A1 (en) | Drug-delivery endovascular stent and method for treating restenosis | |
| HK1150229B (en) | Drug-delivery endovascular stent and method for treating restenosis |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 20837247 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 2021530714 Country of ref document: JP Kind code of ref document: A |
|
| ENP | Entry into the national phase |
Ref document number: 2020837247 Country of ref document: EP Effective date: 20220209 |