WO2020071554A1 - がん幹細胞特異的抗体 - Google Patents
がん幹細胞特異的抗体Info
- Publication number
- WO2020071554A1 WO2020071554A1 PCT/JP2019/039400 JP2019039400W WO2020071554A1 WO 2020071554 A1 WO2020071554 A1 WO 2020071554A1 JP 2019039400 W JP2019039400 W JP 2019039400W WO 2020071554 A1 WO2020071554 A1 WO 2020071554A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- antibody
- antigen
- cancer
- present disclosure
- seq
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/30—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants from tumour cells
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/0005—Vertebrate antigens
- A61K39/0011—Cancer antigens
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/705—Receptors; Cell surface antigens; Cell surface determinants
- C07K14/70503—Immunoglobulin superfamily
- C07K14/70532—B7 molecules, e.g. CD80, CD86
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/705—Receptors; Cell surface antigens; Cell surface determinants
- C07K14/70503—Immunoglobulin superfamily
- C07K14/70539—MHC-molecules, e.g. HLA-molecules
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2803—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily
- C07K16/2809—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily against the T-cell receptor (TcR)-CD3 complex
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2803—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily
- C07K16/2833—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily against MHC-molecules, e.g. HLA-molecules
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/51—Medicinal preparations containing antigens or antibodies comprising whole cells, viruses or DNA/RNA
- A61K2039/515—Animal cells
- A61K2039/5156—Animal cells expressing foreign proteins
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/30—Immunoglobulins specific features characterized by aspects of specificity or valency
- C07K2317/31—Immunoglobulins specific features characterized by aspects of specificity or valency multispecific
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/52—Constant or Fc region; Isotype
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/60—Immunoglobulins specific features characterized by non-natural combinations of immunoglobulin fragments
- C07K2317/62—Immunoglobulins specific features characterized by non-natural combinations of immunoglobulin fragments comprising only variable region components
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/60—Immunoglobulins specific features characterized by non-natural combinations of immunoglobulin fragments
- C07K2317/62—Immunoglobulins specific features characterized by non-natural combinations of immunoglobulin fragments comprising only variable region components
- C07K2317/622—Single chain antibody (scFv)
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/60—Immunoglobulins specific features characterized by non-natural combinations of immunoglobulin fragments
- C07K2317/64—Immunoglobulins specific features characterized by non-natural combinations of immunoglobulin fragments comprising a combination of variable region and constant region components
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/73—Inducing cell death, e.g. apoptosis, necrosis or inhibition of cell proliferation
- C07K2317/734—Complement-dependent cytotoxicity [CDC]
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/01—Fusion polypeptide containing a localisation/targetting motif
- C07K2319/03—Fusion polypeptide containing a localisation/targetting motif containing a transmembrane segment
Definitions
- the present invention relates to an antibody that recognizes an antigen that is specifically presented as an antigen to cancer stem cells, a pharmaceutical composition containing the antibody, particularly a pharmaceutical composition for treating cancer, and uses thereof.
- Cancer stem cells are considered causal cells involved in the development, recurrence, and metastasis of cancer, so targeting cancer stem cells could potentially suppress cancer growth, recurrence, and metastasis Is expected to be high. That is, the detection technology of cancer stem cells and the development of novel therapeutic agents targeting cancer stem cells are important issues for cancer medicine.
- cancer stem cell antigens genes specifically expressed in cancer stem cells (cancer stem cell antigens) (for example, Patent Documents 1 to 3). Partial peptides of the expressed proteins of these genes are presented as antigens on the cell surface. Therefore, development of cancer vaccine immunotherapy targeting cancer stem cells using such peptides has been attempted. In order for cancer vaccine therapy using a peptide derived from a cancer stem cell antigen to be effective, it is necessary to induce many vaccine peptide-specific T cells in a patient. However, it is known that immunity is low in terminal cases and that many T cells are difficult to induce.
- the present invention relates to an antibody that recognizes an antigen that is specifically presented as an antigen on cancer stem cells, a pharmaceutical composition containing the antibody, particularly a pharmaceutical composition for treating cancer and use thereof, and a cancer stem cell. It relates to methods for treating targeted cancer.
- the present inventors have been developing cancer vaccine immunotherapy targeting cancer stem cells, which are responsible for tumorigenicity and drug resistance of cancer, and vaccine peptide-specific T cells are mediated by T cell receptors. Attention was paid to the fact that cancer cells are killed by recognizing peptides presented on HLA-class I molecules on the surface of cancer cells. If there is an antibody that mimics the specificity of this T cell receptor, immunity will increase. We believe that immunotherapy targeting cancer stem cell antigens will be possible even in patients with reduced levels. Thus, while continuing to develop such antibodies, the inventors succeeded in separating an antibody fragment (scFv) that recognizes a complex of a cancer stem cell antigen peptide and HLA class I from human peripheral blood using a phage display antibody library. When these antibodies were converted to human IgG1 type, they found a new finding that they show complement and antibody-dependent cytotoxic activity against cancer cell lines, and as a result of further research, they have completed the present invention. Was.
- scFv antibody fragment
- the present invention relates to the following: [1] A multispecific antibody having an antigen-binding site that recognizes a complex of an antigen peptide derived from a protein selected from the group consisting of DNAJB8 protein, BORIS sf6 protein and FAM83B protein, and an MHC molecule. [2] The multispecific antibody of [1], further comprising an antigen-binding site that recognizes CD3. [3] The multispecific antibody of [1] or [2], wherein the antigen peptide is a peptide having the amino acid sequence of SEQ ID NO: 5, 23 or 6.
- An antigen binding site that recognizes a complex of an antigen peptide derived from a protein selected from the group consisting of DNAJB8 protein, BORIS sf6 protein and FAM83B protein with an MHC molecule has SEQ ID NOs: 7-14 and 24 to 38 Or the amino acid sequence of any one of SEQ ID NOs: 7-14 and SEQ ID NOs: 24-38, wherein one or two amino acids are substituted.
- the multispecific antibody according to [1] to [3].
- a pharmaceutical composition comprising an antibody that recognizes a complex of an antigen peptide derived from a protein selected from the group consisting of DNAJB8 protein, BORIS sf6 protein and FAM83B protein, and an MHC molecule.
- the pharmaceutical composition of [6] which is a pharmaceutical composition for preventing and / or treating cancer.
- the pharmaceutical composition of [6] to [8], wherein the antigenic peptide has the amino acid sequence of SEQ ID NO: 5, 23 or 6.
- ADVANTAGE OF THE INVENTION it becomes possible to perform an immunotherapy targeting cancer stem cells to patients with intractable cancers and sarcomas that are resistant to existing therapies.
- unlike conventional cancer vaccine immunotherapy there is no need to induce cytotoxic T cells, and thus high immunity, which is essential for cancer vaccine therapy, is not required.
- large-scale adjustment (10 10 or more) of cells required for adoptive immunotherapy is unnecessary.
- the antibody of the present invention since the antibody of the present invention has higher affinity than the T cell receptor, a high drug effect can be expected as an anticancer agent.
- FIG. 1 is a schematic diagram showing an outline of screening of scFv antibodies by the phage display method.
- the scFv phage display library is first screened for whether it reacts with the complex of HLA and HIV, and the reactant is removed as a non-specific antibody phage. Thereafter, those that react with the HLA-A24 / natural antigen peptide complex are selected as specific antibody phages, and those that do not react are selected as nonspecific antibody phages, and this is repeated three times. Soluble scFv is obtained from specific antibody phage.
- FIG. 2 shows the results of a peptide titration assay.
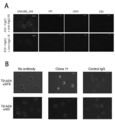
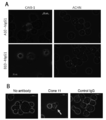
- FIG. 3 is a photographic view showing a state in which the antibody of the present disclosure is reacted with peptide-pulsed T2-A24 cells.
- A is a photograph showing the case where clone A10 and clone B10 are used as antibodies, DNAJB8-143 is used as a natural antigen peptide, and B is a photograph when clone 11 is used as an antibody and SF9 is used as a natural antigen peptide.
- FIG. 4 is a photographic diagram showing a state in which an antibody of the present disclosure is reacted with a cancer cell endogenously presenting a natural antigen peptide.
- A is a photograph when clone A10 and clone B10 are used as antibodies, and B is a photograph when clone 11 is used as an antibody. It is observed that the antibody binds to a part of the cell membrane expressing HLA-A24 and DNAJB8 or FAM83B.
- FIG. 4 is a photographic diagram showing a state in which an antibody of the present disclosure is reacted with a cancer cell endogenously presenting a natural antigen peptide.
- A is a photograph when clone A10 and clone B10 are used as antibodies
- B is a photograph when clone 11 is used as an antibody. It is observed that the antibody binds to a part of the cell membrane expressing HLA-A24 and DNAJB8 or FAM83B.
- FIG. 5 is a photograph showing the observed cytotoxic activity when the antibody of the present disclosure was reacted with peptide-pulsed T2-A24 cells.
- A is a photograph showing the case where clone A10 and clone B10 are used as antibodies, DNAJB8-143 is used as a natural antigen peptide, and B is a photograph when clone 11 is used as an antibody and SF9 is used as a natural antigen peptide.
- Complement binds to the periphery of the cells pulsed with the cancer stem cell antigen peptide and is stained red, and it is found that the nucleus is stained blue by DAPI that has broken down the cell membrane and flowed into the cells.
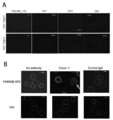
- FIG. 6 is a photograph showing observation of cytotoxic activity when the antibody of the present disclosure is reacted with a cancer cell endogenously presenting a natural antigen peptide.
- A is a photograph when clone A10 and clone B10 are used as antibodies, and B is a photograph when clone 11 is used as an antibody.
- Complement binds around cells expressing HLA-A24 and DNAJB8 or FAM83B and stains red. The nucleus is stained blue by DAPI that has broken down the cell membrane and flowed into the cells.
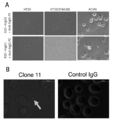
- FIG. 7 is a graph showing the results of FACS analysis of the reactivity of the antibody of the present disclosure in which scFv was converted to hIgG1 type with respect to the HLA-A02 / LV9 peptide complex.
- the antibodies of clone 11, clone 13 and clone 19 were all highly reactive at a concentration of 200 ⁇ g / mL, but clone 19 was the most reactive.
- epitope peptide refers to a major histocompatibility complex (MHC (human leukocyte antigen (HLA) in humans)) molecule, which is presented as an antigen on the cell surface, And a peptide having antigenicity (which can be recognized by T cells).
- MHC human leukocyte antigen
- the epitope peptide is a CTL epitope peptide, which is an epitope peptide that is presented by binding to MHC class I and is recognized by CD8-positive T cells, and is presented by antigen by binding to MHC class II and is recognized by CD4-positive T cells.
- Helper epitope peptide which is the epitope peptide to be used.
- tumor antigen peptides peptides derived from proteins specifically or excessively expressed in tumor cells are particularly referred to as tumor antigen peptides.
- a peptide derived from a protein specifically or excessively expressed in a cancer stem cell is referred to as a cancer stem cell antigen peptide.
- Antigen presentation refers to a phenomenon in which a peptide present in a cell binds to MHC, and this MHC / antigen peptide complex is localized on the cell surface. It is known that antigens presented on the cell surface activate cell-mediated immunity and humoral immunity after being recognized by T cells and the like, and antigens presented on MHC class I activate cell-mediated immunity.
- tumor antigen peptides generally bind to MHC class I. And a peptide to be presented as an antigen.
- binding motif Many peptides that bind to MHC are known to have certain characteristics. In the present disclosure, this feature is referred to as a “binding motif”. It is known in the art which MHC binds to a peptide having what binding motif. For example, in the binding motif of HLA-A24, which is a kind of human MHC, the second amino acid from the N-terminus is tyrosine, phenylalanine, methionine or tryptophan, and the C-terminal amino acid is leucine, isoleucine or phenylalanine.
- the second amino acid from the N-terminus is leucine, isoleucine or methionine, and / or the amino acid at the C-terminus is valine, leucine or isoleucine.
- natural peptide refers to a peptide that is actually presented as an antigen on the cell surface.
- natural antigen peptide refers to a natural peptide whose antigenicity has been confirmed.
- DNAJB8 is a member of the DNAJ / HSP40 family and encodes a 26 KD protein, but its localization and function have not been reported in detail except for its high expression in testis. Many of the DNAJ / HSP40 families have a J domain at the N-terminus, and the J domain binds to HSP70 to promote hydrolysis of ATP, resulting in a structural change in the substrate binding region of HSP70. It is thought to regulate the activity of HSP40 itself also has a peptide binding region, and some have a function of delivering a peptide to HSP70.
- FAM83B family with sequence similarity 83, member B
- EGFR epidermal growth factor receptor
- the BORIS (Brother of the Regulator of Imprinted Sites) gene is a paralog of the CTCF gene, also called 11-zinc finger protein, which contains 11 N-terminal peptide regions and a C-terminal peptide region. It has a zinc finger region.
- BORIS is known to function as a general transcription factor such as a repressor and activator of various gene expressions, and is also known to be expressed in various tumor cells, particularly cancer stem cells. ing. BORIS is classified into six subfamilies (sf1 to 6) according to the sequence of the C-terminal peptide region.
- sequence of the C-terminal peptide region of each subfamily is a sequence unique to each subfamily (for example, the unique sequence of BORIS sf6 is represented by the amino acid sequence of SEQ ID NO: 22), and It is highly conserved among its isoforms. It has been reported that BORIS is not expressed in normal tissues other than testis.
- a gene name such as “DNAJB8”
- it means a gene having a known nucleic acid sequence represented by the gene name, unless otherwise specified, typically, cDNA or Represents the mRNA sequence, but is not limited thereto, as long as one skilled in the art can recognize the sequence as the gene.
- examples of preferred genes and their nucleic acid sequences in the present disclosure include the following genes represented by the following sequences, such as polymorphisms of these genes (for example, SNPs and the like).
- the gene of the present disclosure also includes a sequence that can be recognized as DNAJB8: Gene accession No. NM_153330 (SEQ ID NO: 1)
- FAM83B Gene accession No. NM_001010872 (SEQ ID NO: 3) Therefore, mRNA as a gene expression product of the present disclosure may be represented simply by describing the gene name.
- a gene name such as “DNAJB8 protein” is appended with “protein”, it means the protein encoded by the gene, its isoform, and its homolog.
- the isoform include splicing variants and variants such as SNPs based on individual differences.
- One or more, preferably one to several, more preferably one to ten, one to five, one to three, one or two amino acids are substituted or deleted in the amino acid sequence of the encoded protein.
- an antigen peptide derived from a specific protein such as “an antigen peptide derived from a DNAJB8 protein”, is a partial peptide consisting of a continuous partial sequence in the amino acid sequence constituting the specific protein. And those having the properties of the above antigen peptide.
- Antibodies of the Present Disclosure is an antigen peptide derived from a protein that is an expression product of a gene specifically expressed in cancer stem cells, for example, DNAJB8, FAM83B, BORIS, PVT1, ASB4, LIN28B, and the like. And an antibody that specifically recognizes a complex with an MHC molecule.
- Such an antibody has, in principle, an antigen-binding site that recognizes a complex between the antigen peptide and an MHC molecule.
- antibody refers to a protein having an antigen-binding site and having a property of binding to a molecule recognized by the antigen-binding site, and is typically an immunoglobulin, but is not limited thereto. Not done. Therefore, not only immunoglobulin molecules but also functional fragments (antigen-binding fragments) of antibodies that can be generated from, for example, the antigen-binding site of immunoglobulins are included in the antibodies of the present disclosure.
- Such antigen-binding fragments typically include F (ab ′) 2 fragments, Fab ′ fragments, Fab fragments, Fv fragments, rIgG fragments, etc., as well as scFv, dsFv, diabodies and sc (Fv) 2. Also included.
- these fragments may be linked by a disulfide bond in the constant region or hinge region, or may be single-stranded (scFv) in which each region is linked by a linker.
- the antibodies of the present disclosure are single chain antibodies (scFv).
- the antibodies of the present disclosure are of the IgG1 type.
- the antibodies of the present disclosure also include multimers of immunoglobulins and functional fragments thereof (eg, dimers, trimers, tetramers, and polymers) in the antibodies of the present disclosure.
- the antibody of the present disclosure is not particularly limited as long as it can recognize a complex of a cancer stem cell antigen peptide and an MHC molecule present on the cell surface, and may be a polyclonal antibody or a monoclonal antibody. Good. There is no particular limitation as long as it has an antigen-binding site that recognizes a complex between a cancer stem cell antigen peptide and an MHC molecule. For example, a binding region for binding to another antigen-binding site or another protein may be used. You may have. Thus, in one aspect, the antibodies of the present disclosure may be multispecific antibodies. In one preferred aspect, the antibodies of the present disclosure are bispecific antibodies.
- the antibody of the present disclosure can recognize and bind to a complex between a cancer stem cell antigen peptide and an MHC molecule existing on the surface of a cancer stem cell, and thus can be used for various applications.
- the antibodies of the present disclosure can be used for detecting cancer stem cells.
- the antibodies of the present disclosure can be used for treating cancer stem cells (ie, treating cancer).
- the antibody of the present disclosure is used for treating cancer stem cells, it may be a method utilizing cell-mediated immunity or a method utilizing humoral immunity.
- adoptive immune cell therapy using T cells into which a chimeric antigen receptor (CAR) incorporating the antigen-binding site of the antibody of the present disclosure has been introduced, and the like can be mentioned.
- humoral immunity include, for example, a method utilizing the antibody-dependent cytotoxicity (ADCC) activity and the complement-dependent cytotoxicity (CDCC) activity of an antibody.
- ADCC antibody-dependent cytotoxicity
- CDCC complement-dependent cytotoxicity
- the antibodies of the present disclosure have ADCC activity and / or CDCC activity.
- the antibody of the present disclosure includes a chimeric antigen receptor (CAR) incorporating an antigen-binding site that recognizes a complex of a cancer stem cell antigen peptide and an MHC molecule.
- the antibody of the present disclosure has an antigen-binding site that specifically recognizes a complex of an antigen peptide derived from a protein that is an expression product of a gene specifically expressed in cancer stem cells and an MHC molecule. doing.
- a method for selecting an antigen-binding site that recognizes a specific antigen or an antibody having such an antigen-binding site any method known in the art may be used. Such a method includes, for example, a phage display method.
- any gene known in the art to be specifically expressed in cancer stem cells may be used.
- the gene specifically expressed in cancer stem cells is DNAJB8, FAM83B or BORISBsf6.
- the antigen peptide is not particularly limited as long as it binds to MHC.
- Peptides that bind to MHC have different characteristics depending on the type of MHC.
- HLA which is a human MHC
- a peptide that binds to HLA class I is about 8 to 14 amino acids in length, preferably about 8 to 10 amino acids in length
- HLA class II is 10 or more amino acids in length.
- it binds to a peptide of about 10 to 30 amino acids in length.
- a peptide that binds to HLA-A02 is such that the second amino acid from the N-terminus is leucine, isoleucine or methionine, and / or the C-terminal amino acid is valine, leucine or isoleucine.
- Peptides having a binding motif and binding to HLA-A24 are those wherein the second amino acid from the N-terminus is tyrosine, phenylalanine, methionine or tryptophan and / or the C-terminal amino acid is leucine, isoleucine or phenylalanine. Has a motif.
- the antigen peptide may be determined by predicting an MHC-restricted peptide based on the binding motif from the full-length sequence of the protein, or may be determined by identifying a natural peptide actually presented as an antigen.
- the method for identifying a natural peptide may be a method known in the art or a combination thereof, and includes, for example, the method described in Patent Document 2. Since the antibody of the present disclosure recognizes an antigen peptide presented as an antigen on the cell surface, preferably, the antigen peptide is a natural peptide.
- DNAJB8-143 (AFMEAFSSF (SEQ ID NO: 5)) as a natural peptide of DNAJB8, SF9 (SYQPNENKF (SEQ ID NO: 6)) as a natural peptide of FAM83B, and BORIS sf6 as a natural peptide in human cancer stem cells.
- LV9 LFIGTIKV (SEQ ID NO: 23)
- the antigenic peptide is DNAJB8-143, SF9, or LV9.
- the MHC is HLA. In one more preferred aspect, the MHC is HLA class I. In a further preferred embodiment, the MHC is HLA-A02. In another more preferred aspect, the MHC is HLA-A24.
- the antibody of the present disclosure is not particularly limited as long as it can specifically recognize the complex between the antigen peptide and the MHC molecule, and may be an antibody that recognizes and binds to another molecule. Therefore, in a preferred embodiment, the antibody of the present disclosure may have, in addition to an antigen-binding site that recognizes a complex of a cancer stem cell antigen peptide and an MHC molecule, a binding region for another molecule. Such a binding region is typically, but not limited to, an antigen binding site, and may be, for example, a ligand for a cell surface receptor. In a preferred embodiment, the antibodies of the present disclosure are antibodies that specifically bind to two or more molecules, ie, multispecific antibodies.
- multispecific antibody means an antibody that has at least one antigen-binding site and specifically binds to two or more molecules.
- An antigen-binding site that recognizes a complex between a stem cell antigen peptide and an MHC molecule refers to an antibody that can specifically bind to another molecule.
- Specific binding to another molecule may be achieved by another antigen binding site or by other methods, such as a ligand region for another molecule.
- another antigen recognized by the antibody of the present disclosure is not particularly limited, but is preferably one that contributes to treatment of cancer stem cells.
- antigens include proteins expressed on the cell surface of immune cells, such as CD3, CD28, CD40, PD1, CTLA4, TIGIT, OX40, and CD137.
- another antigen includes a T cell surface protein such as CD3 or CD28.
- the multispecific antibody of the present disclosure has a binding region for CD3 and / or CD28 in addition to an antigen binding site that recognizes a complex between a cancer stem cell antigen peptide and an MHC molecule.
- a binding region may be an antigen binding site, for example, CD80 or the like as a ligand for CD28.
- the present inventors screened the above-mentioned natural antigen peptide DNAs JB8-143, SF9, and LV9 for antibodies having an antigen-binding site that recognizes a complex of these antigen peptides with HLA-A24 or HLA-A02. . Therefore, in one preferred embodiment of the present disclosure, the antigen-binding site that recognizes the complex of the cancer stem cell antigen peptide and the MHC molecule is an amino acid sequence represented by any of SEQ ID NOs: 7-14 and SEQ ID NOs: 24-38, or , GNT, DGT or HDS.
- the antigen-binding site that recognizes a complex of the cancer stem cell antigen peptide and the MHC molecule is an amino acid sequence represented by any one of SEQ ID NOs: 7-14 and SEQ ID NOs: 24-38. It may include an amino acid sequence in which one or two amino acids have been substituted.
- the amino acid substitution is preferably a conservative substitution such as a substitution between acidic amino acids, a substitution between basic amino acids, or a substitution between neutral amino acids.
- Typical examples of the antibody of the present disclosure include a specific antibody having an antigen-binding site that recognizes a complex of a cancer stem cell antigen peptide (DNAJB8-143) having the amino acid sequence of SEQ ID NO: 5 and HLA-A24; A specific antibody having an antigen-binding site that recognizes a complex of a cancer stem cell antigen peptide (SF9) comprising the amino acid sequence of SEQ ID NO: 6 and HLA-A24; a cancer stem cell antigen peptide comprising the amino acid sequence of SEQ ID NO: 5 ( A bispecific antibody having an antigen-binding site that recognizes a complex of DNAJB8-143) and HLA-A24 and an antigen-binding site that recognizes CD3; a cancer stem cell antigen peptide (SF9) comprising the amino acid sequence of SEQ ID NO: 6; ) Having an antigen-binding site that recognizes a complex of HLA-A24 and an antigen-binding site that recognizes CD3 Antibody, an anti
- a trispecific antibody having an antigen-binding site to recognize recognizes an antigen-binding site that recognizes a complex of a cancer stem cell antigen peptide (DNAJB8-143) consisting of the amino acid sequence of SEQ ID NO: 5 and HLA-A24, and recognizes CD3.
- Ranaru cancer stem cell antigen peptide (SF9) and HLA-A24 antigen-binding site recognizes a complex of, such as trispecific antibodies having an antigen binding site and CD80 region recognize CD3 and the like.
- Another typical example of the antibody of the present disclosure includes a specific antibody having an antigen binding site that recognizes a complex of a cancer stem cell antigen peptide (LV9) having the amino acid sequence set forth in SEQ ID NO: 23 and HLA-A02, A bispecific antibody having an antigen-binding site that recognizes a complex of the cancer stem cell antigen peptide (LV9) and HLA-A02 having the amino acid sequence of No.
- the antibody of the present disclosure includes at least one of a complementarity-determining region (CDR) 1, a complementarity-determining region (CDR) 2, and a complementarity-determining region (CDR) 3 at an antigen-binding site; Preferably comprises at least one of a complementarity determining region (CDR) 1, a complementarity determining region (CDR) 2, and a complementarity determining region (CDR) 3.
- the antibodies of the present disclosure in one preferred aspect, comprise the amino acid sequence of SEQ ID NO: 7 and / or SEQ ID NO: 8 at the antigen binding site, particularly as the complementarity determining region (CDR) 3.
- the antigen binding site comprises, in particular as the complementarity determining region (CDR) 3, the amino acid sequence according to SEQ ID NO: 9 and / or SEQ ID NO: 10.
- the antigen binding site comprises the amino acid sequence set forth in SEQ ID NO: 11 and / or 12 as complementarity determining region (CDR) 3.
- the antigen binding site comprises the amino acid sequence set forth in SEQ ID NO: 13 and / or SEQ ID NO: 14, particularly as complementarity determining region (CDR) 3.
- the antibody of the present disclosure has an antigen-binding site comprising an amino acid sequence in which one or two amino acids have been substituted in the amino acid sequence set forth in any one of amino acid sequence numbers 7-14. It may be something.
- the antibody of the present disclosure includes, in a preferred embodiment, an amino acid sequence described in SEQ ID NO: 24 and / or SEQ ID NO: 27 as a complementarity determining region (CDR) 1 at an antigen binding site, and a complementarity determining region (CDR).
- the complementarity determining region (CDR) 3 includes the amino acid sequence of SEQ ID NO: 26 and / or SEQ ID NO: 28.
- the amino acid sequence as set forth in SEQ ID NO: 29 and / or SEQ ID NO: 32, in particular the complementarity determining region (CDR) 2, as the complementarity determining region (CDR) 2, 30 and / or the amino acid sequence represented by DGT, and the complementarity determining region (CDR) 3 include the amino acid sequence of SEQ ID NO: 31 and / or 33.
- the antigen binding site in particular as the complementarity determining region (CDR) 1, the amino acid sequence set forth in SEQ ID NO: 34 and / or SEQ ID NO: 37, the complementarity determining region (CDR) 2 as the sequence
- the amino acid sequence of SEQ ID NO: 35 and / or the amino acid sequence represented by HDS, and the complementarity determining region (CDR) 3 include the amino acid sequence of SEQ ID NO: 36 and / or SEQ ID NO: 38.
- the antibody of the present disclosure has an antigen-binding site comprising an amino acid sequence in which one or two amino acids have been substituted in the amino acid sequence of any of SEQ ID NOs: 24 to 38. It may be.
- CDR3 is particularly important for antigen binding.
- composition of the present disclosure One aspect of the present disclosure relates to a pharmaceutical composition containing the antibody of the present disclosure.
- the antibody of the present disclosure can specifically recognize a cancer stem cell antigen peptide presented as an antigen on the surface of a cancer stem cell, it can be used as an active ingredient of a pharmaceutical composition for various uses. . Therefore, as the antibody of the present disclosure contained as an active ingredient in the pharmaceutical composition of the present disclosure, any of the antibodies described in detail in the above ⁇ 1> can be used.
- the pharmaceutical composition of the present disclosure contains the antibody of the present disclosure as an active ingredient.
- the pharmaceutical composition of the present disclosure can be used not only as a therapeutic agent for treating cancer stem cells (ie, a cancer therapeutic agent), but also, for example, an agent for detecting cancer stem cells, a cancer vaccine immunotherapy for a patient to be treated. Can also be used for applications such as a companion diagnostic agent for diagnosing the efficacy against.
- the antibody of the present disclosure has ADCC activity and / or CDCC activity. That is, the antibody of the present disclosure recognizes a cancer stem cell antigen peptide presented on the surface of a cancer stem cell and binds to the surface of the cancer stem cell, whereby the antibody of the present disclosure exerts its cytotoxic activity on the cancer stem cell. Can be demonstrated. Therefore, in one preferred aspect, the pharmaceutical composition of the present disclosure is a pharmaceutical composition for preventing and / or treating cancer.
- prevention of cancer includes not only prevention of cancer in patients, but also prevention of recurrence in patients whose primary tumor has been removed by surgery, cancer treatment such as surgery, radiation therapy or drug therapy. To prevent metastasis of tumors that could not be completely removed.
- treatment of cancer includes not only cancer healing and improvement of symptoms that reduce the size of the cancer, but also progression that suppresses cancer cell growth, tumor expansion, or metastasis of cancer cells from the primary tumor. Prevention, etc. are included.
- compositions of the present disclosure can be administered to any living individual that can have a tumor, but are preferably human and non-human mammals (eg, rodents such as mice, rats, guinea pigs, hamsters, Primates such as chimpanzees, artiodactyls such as cattle, goats and sheep, equinoids such as horses, rabbits, dogs and cats), and more preferably humans.
- rodents such as mice, rats, guinea pigs, hamsters, Primates such as chimpanzees, artiodactyls such as cattle, goats and sheep, equinoids such as horses, rabbits, dogs and cats
- rodents such as mice, rats, guinea pigs, hamsters, Primates such as chimpanzees, artiodactyls such as cattle, goats and sheep, equinoids such as horses, rabbits, dogs and cats
- primates such as chimpanzees
- the cell population to be detected can be used for a cell population derived from any biological sample obtained from the biological individual, but is preferably used.
- a preferred embodiment of the antibody of the present disclosure has an antigen-binding site that recognizes a complex of a cancer stem cell antigen peptide derived from DNAJB8 protein or FAM83B protein and HLA-A24. Further, another preferred embodiment of the antibody of the present disclosure has an antigen-binding site that recognizes a complex of a cancer stem cell antigen peptide derived from the BORIS ⁇ sf6 protein and HLA-A02. Therefore, the pharmaceutical composition of the present disclosure can be suitably used particularly for subjects suffering from a cancer expressing DNAJB8, a cancer expressing FAM83B, or a cancer expressing BORIS sf6.
- it can be suitably used for a subject having HLA-A24 or HLA-A02 as HLA.
- it can be used for the prevention or treatment of cancer (tumor) such as colon cancer, lung cancer, breast cancer, myeloma, oral cancer, pancreatic cancer, skin cancer, and prostate cancer.
- cancer tumor
- cancer such as colon cancer, lung cancer, breast cancer, myeloma, oral cancer, pancreatic cancer, skin cancer, and prostate cancer.
- the pharmaceutical composition of the present disclosure exerts an antitumor effect by utilizing an immune cell capable of damaging a cancer stem cell to which an antibody serving as an active ingredient is bound. It is considered that a higher therapeutic effect can be exerted by suppressing the function of the above.
- the pharmaceutical compositions of the present disclosure are used with an immune checkpoint inhibitor.
- agent A and another agent B when one agent A and another agent B are "used together” or “used together", it means that the agent B is in a state where the agent B exerts its effect while the agent A is exerting its effect. . Therefore, the agent B may be administered simultaneously with the administration of the agent A, or the agent B may be administered at a certain interval after the administration of the agent A.
- the agent A and the agent B may be in the same dosage form or different dosage forms. Furthermore, as long as the agent A or the agent B does not lose its effect, the agent A and the agent B may be mixed to form one composition.
- immune checkpoint inhibitor in the present embodiment, any agent known as an immune checkpoint inhibitor can be used as long as the antigen recognition ability of the pharmaceutical composition of the present disclosure is not inhibited.
- Known as immune checkpoint inhibitors include, but are not limited to, for example, anti-PD-1, anti-PD-L1, anti-CTLA-4, anti-TIM-3, anti-LAG- 3 antibodies, anti-B7-H3 antibodies, anti-B7-H4 antibodies, anti-B7-H5 antibodies, anti-TIGIT antibodies and the like.
- the dosage form of the pharmaceutical composition of the present disclosure is not particularly limited, but may be an oil emulsion (emulsion formulation), polymer nanoparticles, a liposome formulation, or a particulate formulation bound to beads having a diameter of several ⁇ m. , Lipid-bound preparations, microsphere preparations, microcapsule preparations and the like.
- the administration method includes any known administration method such as intradermal administration, subcutaneous administration, intramuscular administration, and intravenous administration.
- the dose of the pharmaceutical composition of the present disclosure in the formulation can be appropriately adjusted depending on the disease to be treated, the age and weight of the patient, etc., but is usually 0.0001 mg to 1000 mg, preferably 0.001 mg to 1000 mg, It is preferably 0.1 mg to 10 mg, which is preferably administered once every several days to several months.
- chimeric antigen receptor refers to a single-chain antibody (scFv) in which a light chain and a heavy chain of an antibody variable region of an antibody recognizing a molecule present on the cell surface of a cancer cell are linked in series. It is a chimeric protein molecule designed to have a CD3 ⁇ chain on the C-terminal side among molecules constituting the T cell receptor (TCR) / CD3 complex on the terminal side.
- scFv single-chain antibody
- TCR T cell receptor
- CAR can be produced using the antibody of the present disclosure as a scFv.
- CAR which recognizes a complex between a cancer stem cell antigen peptide and MHC, is a cancer antigen that presents a cancer stem cell antigen peptide that can be targeted by CTLs. Since a dendritic cell presenting a peptide can be recognized, a pharmaceutical composition containing the genetically modified T cell (CAR-T) into which the CAR has been introduced is also included in the pharmaceutical composition of the present disclosure. .
- Tumor detection method (test method, diagnostic method)
- test method diagnostic method
- the detection method (diagnosis method) of the present disclosure using the antibody of the present disclosure typically, the blood of a subject is collected, or a part of a test tissue suspected of having a tumor is collected by biopsy or the like, and contained therein.
- colon cancer By detecting and measuring the amount of cells having a complex of a cancer stem cell antigen peptide and an MHC molecule with the antibody of the present disclosure, colon cancer, lung cancer, breast cancer, myeloma, oral cancer, pancreatic cancer, skin cancer, It detects, tests or diagnoses the presence or absence or degree of cancer (tumor) such as prostate cancer.
- Particular embodiments of the detection (test) method of the present disclosure using the antibodies of the present disclosure comprise the following steps (a) and (b), and optionally (c): (A) contacting a biological sample obtained from a subject with a tumor detection agent of the present disclosure; (B) measuring the amount of cells presenting a complex of a cancer stem cell antigen peptide and an HLA antigen in the biological sample using the amount of cells to which the tumor detection agent has been bound as an index; (C) a step of determining the presence of cancer based on the result of (b).
- a specific embodiment of the diagnostic method of the present disclosure using the antibody of the present disclosure includes the steps (a), (b), and (c) described above.
- the biological sample used herein include a sample prepared from a biological tissue of a subject (a tissue suspected of containing cancer cells and its surrounding tissue, blood, or the like). Specific examples include a sample containing tissue cells collected from the tissue.
- Prediction, determination, judgment or diagnosis of the presence or absence of a tumor can be performed, for example, by measuring the amount of cells to which the antibody of the present disclosure is bound in the blood of a subject or a test tissue suspected of having a tumor. At that time, in some cases, the level of cells to which the antibody of the present disclosure is bound in a normal corresponding tissue is used as a reference value, and the reference value is compared with the level in a sample obtained from a subject to determine a difference between the two.
- the comparison between the test tissue of the test subject and the normal corresponding tissue can be performed by performing measurements on the biological sample of the test subject and the biological sample of a normal subject in parallel.
- the antibody of the present disclosure obtained by measuring a plurality of (at least two, preferably three or more, more preferably five or more) normal tissues under uniform measurement conditions binds.
- the average or statistical median value of the amount of cells can be used for comparison as a normal or reference value.
- the determination as to whether or not the subject has cancer is made, for example, by comparing the level of the cells to which the antibody of the present disclosure is bound in the tissue of the subject with, for example, 2 times or more, preferably 3 times, the level of those in a normal person.
- the above can be performed as an index.
- One aspect of the present disclosure is also a method for preventing and / or treating cancer in a subject, comprising the steps of: determining an effective amount of the antibody or CAR-T cell of the present disclosure; It also relates to a method comprising the step of administering to a subject in need thereof.
- the “subject” in the present disclosure may be any biological individual that can suffer from cancer, and is preferably a human or non-human mammal (eg, a mouse, rat, guinea pig, hamster, or other rodent).
- the subject may be healthy or suffer from any disease, but typically suffers from cancer if prevention and / or treatment of cancer is contemplated.
- the subject is HLA-A24 or HLA-A02 positive.
- the subject has or is at risk of having DNAJB8 or FAM83B or BORIS sf6 positive cancer.
- the subject is HLA-A24 positive and has, or is at risk of having, DNAJB8 or FAM83B positive cancer.
- the subject is HLA-A02 positive and has or is at risk of having BORIS sf6 positive cancer.
- Antibodies and CAR-T cells of the present disclosure for use in the prophylaxis / treatment methods of the present disclosure include any of those described herein.
- An effective amount in the present disclosure is, for example, an amount that reduces the symptoms of cancer, or delays or stops the progress thereof, and is preferably an amount that suppresses or cures cancer. Also preferred is an amount that does not cause adverse effects beyond the benefit of administration. Such an amount can be appropriately determined by an in vitro test using cultured cells or the like, or a test using a model animal such as a mouse or rat, and such a test method is well known to those skilled in the art.
- the specific dose of the active ingredient depends on various conditions related to the subject in need thereof, such as the severity of symptoms, general health of the subject, age, weight, sex of the subject, diet, timing and frequency of administration, The determination can be made in consideration of the concomitant drug, reactivity to treatment, dosage form, and compliance with treatment.
- the antibody of the present disclosure As a specific dose, for example, in the case of the antibody of the present disclosure, it is generally 0.0001 mg to 2000 mg, preferably 0.001 mg to 2000 mg, and it is preferable to administer the dose once every one to four weeks.
- the number is usually 1 ⁇ 10 4 to 1 ⁇ 10 8 cells, preferably 1 ⁇ 10 5 to 1 ⁇ 10 7 cells, which is administered once a day to 4 weeks. Is preferred.
- any known appropriate administration method such as intradermal administration, subcutaneous administration, intramuscular administration, or intravenous administration can be used.
- One embodiment of the prevention / treatment method of the present disclosure further includes a step of selecting an HLA-A24 or HLA-A02-positive subject as a prevention / treatment subject before the administering step.
- This aspect of the present disclosure may further include, prior to the selecting step, determining the HLA type of the subject. The determination of the HLA type of a subject can be performed by any known method.
- One embodiment of the prevention / treatment method of the present disclosure further includes, before the administering step, a step of selecting a subject having DNAJB8, FAM83B, or BORIS ⁇ ⁇ sf6-positive cancer as a target of prevention / treatment.
- This aspect of the present disclosure may further include, prior to the selecting step, detecting a DNAJB8 or FAM83B or BORIS sf6-positive cancer in the subject.
- the tumor detection method described in the above ⁇ 3> can be used.
- One embodiment of the prevention / treatment method of the present disclosure is that, prior to the administering step, the subject is HLA-A24-positive and has DNAJB8 or FAM83B-positive cancer, or is HLA-A02-positive, and The method further includes a step of selecting a subject having BORIS sf6-positive cancer as a subject for prevention / treatment.
- This aspect of the disclosure may further comprise, prior to the selecting step, determining the HLA type of the subject and detecting DNAJB8 or FAM83B or BORIS sf6-positive cancer in the subject.
- Example 1 Screening of antibodies specific to HLA-A24 / natural antigen peptide complex (1) Preparation of antibody phage As cancer stem cell antigen peptides, natural antigen peptides DNAJB8-143 (SEQ ID NO: 5) derived from DNAJB8 protein and FAM83B protein are derived. Using the natural antigen peptide SF9 (SEQ ID NO: 6), scFv that recognizes the HLA-A24 / DNAJB8-143 complex and the HLA-A24 / SF9 complex was synthesized by Tsukahara et al., J Biol Chem., 2014, Aug 8 ; 289 (32): 22035-47.
- cancer stem cell antigen peptide means DNAJB8-143 and SF9.
- a phagemid vector incorporating a DNA encoding a peptide in which the heavy chain variable region (VH region) and the light chain variable region (VL region) of an antibody are linked by a linker is prepared from the scFv library, and the resulting phagemid vector is used in E. coli. Infected. This was further infected with a helper phage to prepare an M13 phage (antibody phage) displaying an scFv in which the VH region and the VL region were linked by a linker.
- a phage library was prepared using a biotin-modified complex of HLA-A24 and an HIV peptide (RYLRDQQLGI (SEQ ID NO: 15)) and streptavidin-conjugated magnetic beads. Specific binding phages were removed. Using the biotin-modified HLA-A24 / DNAJB8-143 complex or the biotin-modified HLA-A24 / SF9 complex and streptavidin-bound magnetic beads, antibody phages that bind to the respective complexes are selected from the remaining antibody phages. The (positive panning) step was repeated three times to obtain a candidate antibody phage that specifically binds to the HLA-A24 / natural antigen peptide complex.
- FACS analysis was further performed using an IgG1-type converted antibody (scFv-hIgG1), and clones with particularly high reactivity (DNAJB8-143: clone A10 and clone B10, SF9: clone 4 and clone 11) were selected. .
- Example 2 Peptide titration assay Various concentrations (50 ⁇ g / mL, 5 ⁇ g / mL, 500 ng / mL, 50 ng / mL, 5 ng / mL, 500 ng / mL, 500 pg / mL, 50 pg / mL, respectively) were added to T2 cells (T2-A24 cells) expressing HLA-A24. (mL, 5 pg / mL) and the antibody clone obtained in Example 1 were allowed to react with each other, and the reaction limit concentration of the antibody was calculated.
- HIV SEQ ID NO: 15
- CMV QYDPVAALF
- Example 3 Reactivity with antigen presenting cells
- Reactivity with peptide-pulsed T2-A24 cells Test whether or not the scFv-hIgG1 antibody obtained in Example 2 actually reacts with cells that present antigen on the cell surface did.
- the T2-A24 cells were pulsed with DNAJB8-143, HIV, CMV and EBV adjusted to a final concentration of 50 ⁇ g / mL, and reacted with the A10 antibody and B10 antibody obtained in Example 2 at a final concentration of 10 ⁇ g / mL. Observation was performed by immunostaining with hIgG-PE.
- the clone 11 antibody was similarly observed except that T2-A24 cells were pulsed with SF9 and HIV adjusted to a final concentration of 100 ⁇ g / mL. The results are shown in FIG. It was observed that scFv-IgG1 antibody was bound to the surface of T2-A24 cells pulsed with each cancer stem cell antigen peptide.
- the antibodies of the present disclosure can also recognize endogenously presented natural antigenic peptides.
- Example 4 Confirmation of complement-dependent cytotoxicity (CDCC) activity (1) Cytotoxic activity against peptide-pulsed T2-A24 cells T2-A24 cells pulsed with peptide as in Example 3 (1) were prepared to a final concentration of 10 ⁇ g / mL. Each antibody was reacted and observed using a PE-conjugated anti-C3b antibody and DAPI as fluorescent labels. When CDCC activity is confirmed, C3b binds around the cell and stains red, the cell membrane is destroyed by the CDCC activity, DAPI flows into the cell, and the nucleus stains blue. FIG. 5 shows the results. For all antibodies, CDCC activity against T2-A24 pulsed with a cancer stem cell antigen peptide was observed.
- Example 5 Analysis of Bispecific Antibody BiTE (1) Preparation of BiTE and Binding to Each Cell Using a method similar to that described in Stadler et al., Nature Medicine volume 23, pages 815-817 (2017), The anti-CD3 scFv (SEQ ID NO: 21) was ligated to the scFv of each antibody of clone A10, clone B10, clone 4 and clone 11 to prepare each bispecific antibody BiTE. Using this BiTE, the binding property to T2-A24 cells, T cells and NK cells pulsed with a cancer stem cell antigen peptide was tested. Each BiTE showed binding to T2-A24 cells pulsed with the cancer stem cell antigen peptide. It also showed high binding to T cells, but no binding to NK cells.
- BiTE prepared in (1) was mixed with T cells, and T2-A24 cells were pulsed with a cancer stem cell antigen peptide, in the presence of FITC-labeled annexin V and propidium iodide (PI). Co-cultured. The fluorescence was analyzed by FACS after 2.5 hours and 5 hours, respectively. In the case of the control (untreated and T cells only), cells in which both FITC and PI fluorescence were observed after 2.5 hours and 5 hours were obtained. In contrast, when co-cultured in the presence of BiTE, cells in which both FITC and PI fluorescence were observed showed a marked increase at 5 hours compared to 2.5 hours. It was observed.
- Example 6 Screening of Antibody Specific to HLA-A02 / Natural Antigen Peptide Complex (1) Preparation of Antibody Phage
- a cancer stem cell antigen peptide a natural polypeptide derived from a polypeptide represented by SEQ ID NO: 22 which is a sequence unique to BORIS sf6 ScFv that recognizes the HLA-A02 / LV9 complex using the antigen peptide LV9 (SEQ ID NO: 23) is described in Tsukahara et al., J Biol Chem., 2014, Aug 8; 289 (32): 22035-47. Using the same method as the phage display method.
- “cancer stem cell antigen peptide” means LV9.
- a phagemid vector incorporating a DNA encoding a peptide in which the heavy chain variable region (VH region) and the light chain variable region (VL region) of an antibody are linked by a linker is prepared from the scFv library, and the resulting phagemid vector is used in E. coli. Infected. This was further infected with a helper phage to prepare an M13 phage (antibody phage) displaying an scFv in which the VH region and the VL region were linked by a linker.
- a phage library was prepared using a biotin-modified complex of HLA-A02 and an HIV peptide (SLYNTVATL (SEQ ID NO: 39)) and streptavidin-conjugated magnetic beads. Specific binding phages were removed. From the remaining antibody phages, using a biotin-modified HLA-A02 / LV9 complex and streptavidin-bound magnetic beads, a step of selecting antibody phages that bind to each complex (positive panning) was repeated three times, and HLA- A candidate antibody phage that specifically binds to the A02 / natural antigen peptide complex was obtained.
- sequences of the CDR1 to 3 regions of these clones were as follows. Sequence of clone 11 VH region CDR1 region: GTFFDKYG (SEQ ID NO: 24) Sequence of CDR2 region: ITWNSGKV (SEQ ID NO: 25) Sequence of CDR3 region: CARDLGYYYDSSGYLKPTGSGFDYW (SEQ ID NO: 26) Sequence of clone 11 VL region CDR1 region: SSNIGAGYD (SEQ ID NO: 27) Sequence of CDR2 region: GNT Sequence of CDR3 region: CGTWDSSSLVVLF (SEQ ID NO: 28) Sequence of clone 13 VH region CDR1 region: GYTFTGYY (SEQ ID NO: 29) Sequence of CDR2 region: ISTVFNTP (SEQ ID NO: 30) Sequence of CDR3 region: CARSYSGSLQDAFDIW (SEQ ID NO: 31) Sequence of clone 13 VL region CDR
- the antibodies of the present disclosure can react with endogenously presented natural antigenic peptides and further exhibit cytotoxic activity by humoral immune response. Therefore, it becomes possible to perform immunotherapy targeting cancer stem cells on patients with refractory cancers and sarcomas that are resistant to existing therapies.
- the cytotoxic mechanism is humoral immunity
- cancer vaccine immunotherapy that does not depend on the immunity of the administration target can be performed, and high effects are expected as both anticancer drugs and cancer immunotherapy it can.
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Immunology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Organic Chemistry (AREA)
- Medicinal Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Molecular Biology (AREA)
- Genetics & Genomics (AREA)
- Biophysics (AREA)
- Biochemistry (AREA)
- Cell Biology (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Pharmacology & Pharmacy (AREA)
- Toxicology (AREA)
- Zoology (AREA)
- Gastroenterology & Hepatology (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Oncology (AREA)
- Microbiology (AREA)
- Mycology (AREA)
- Epidemiology (AREA)
- Peptides Or Proteins (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
Priority Applications (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP19870017.1A EP3862366A4 (en) | 2018-10-05 | 2019-10-04 | Cancer-stem-cell-specific antibody |
| US17/279,965 US20220033513A1 (en) | 2018-10-05 | 2019-10-04 | Cancer-stem-cell-specific antibody |
| JP2020551131A JPWO2020071554A1 (ja) | 2018-10-05 | 2019-10-04 | がん幹細胞特異的抗体 |
| JP2024067659A JP2024144409A (ja) | 2018-10-05 | 2024-04-18 | がん幹細胞特異的抗体 |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2018189834 | 2018-10-05 | ||
| JP2018-189834 | 2018-10-05 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2020071554A1 true WO2020071554A1 (ja) | 2020-04-09 |
Family
ID=70055296
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2019/039400 Ceased WO2020071554A1 (ja) | 2018-10-05 | 2019-10-04 | がん幹細胞特異的抗体 |
Country Status (4)
| Country | Link |
|---|---|
| US (1) | US20220033513A1 (enExample) |
| EP (1) | EP3862366A4 (enExample) |
| JP (2) | JPWO2020071554A1 (enExample) |
| WO (1) | WO2020071554A1 (enExample) |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2023010068A3 (en) * | 2021-07-28 | 2023-03-02 | Cartesian Therapeutics, Inc. | Multiprotein-engineered cells secreting a multispecific antibody |
| WO2023204290A1 (ja) * | 2022-04-21 | 2023-10-26 | 愛知県 | 多重特異性ナノ粒子 |
| WO2025047920A1 (ja) * | 2023-09-01 | 2025-03-06 | 小野薬品工業株式会社 | Hla/がん抗原pvt1由来ペプチド複合体特異的抗体 |
Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2002521053A (ja) * | 1998-07-28 | 2002-07-16 | マイクロメット アーゲー | ヘテロミニ体 |
| WO2010050190A1 (ja) | 2008-10-27 | 2010-05-06 | 北海道公立大学法人札幌医科大学 | がん幹細胞分子マーカー |
| WO2014153002A1 (en) * | 2013-03-14 | 2014-09-25 | The California Institute For Biomedical Research | Bispecific antibodies and uses thereof |
| JP2014529600A (ja) * | 2011-08-23 | 2014-11-13 | ロシュ グリクアート アーゲー | T細胞活性化抗原に対して特異的な二重特異性抗体及び腫瘍抗原および使用方法 |
| WO2015050259A1 (ja) | 2013-10-03 | 2015-04-09 | 大日本住友製薬株式会社 | 腫瘍抗原ペプチド |
| WO2016047715A1 (ja) | 2014-09-24 | 2016-03-31 | 北海道公立大学法人札幌医科大学 | 腫瘍抗原ペプチド |
| JP2016516049A (ja) * | 2013-03-15 | 2016-06-02 | メモリアル スローン−ケタリング キャンサー センター | 多量体化技術 |
-
2019
- 2019-10-04 EP EP19870017.1A patent/EP3862366A4/en active Pending
- 2019-10-04 JP JP2020551131A patent/JPWO2020071554A1/ja active Pending
- 2019-10-04 US US17/279,965 patent/US20220033513A1/en active Pending
- 2019-10-04 WO PCT/JP2019/039400 patent/WO2020071554A1/ja not_active Ceased
-
2024
- 2024-04-18 JP JP2024067659A patent/JP2024144409A/ja active Pending
Patent Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2002521053A (ja) * | 1998-07-28 | 2002-07-16 | マイクロメット アーゲー | ヘテロミニ体 |
| WO2010050190A1 (ja) | 2008-10-27 | 2010-05-06 | 北海道公立大学法人札幌医科大学 | がん幹細胞分子マーカー |
| JP2014529600A (ja) * | 2011-08-23 | 2014-11-13 | ロシュ グリクアート アーゲー | T細胞活性化抗原に対して特異的な二重特異性抗体及び腫瘍抗原および使用方法 |
| WO2014153002A1 (en) * | 2013-03-14 | 2014-09-25 | The California Institute For Biomedical Research | Bispecific antibodies and uses thereof |
| JP2016516049A (ja) * | 2013-03-15 | 2016-06-02 | メモリアル スローン−ケタリング キャンサー センター | 多量体化技術 |
| WO2015050259A1 (ja) | 2013-10-03 | 2015-04-09 | 大日本住友製薬株式会社 | 腫瘍抗原ペプチド |
| WO2016047715A1 (ja) | 2014-09-24 | 2016-03-31 | 北海道公立大学法人札幌医科大学 | 腫瘍抗原ペプチド |
Non-Patent Citations (8)
| Title |
|---|
| HORIBE R. ET AL.: "Brother of the regulator of the imprinted site (BORIS) variant subfamily 6 is a novel target of lung cancer stem-like cell immunotherapy", PLOS ONE, vol. 12, no. 3, 2017, pages 1 - 14, XP002777592 * |
| SAITO SHIMPEI : "ST-A-6: Development of HLA/peptide complex-specific artificial antibody targeting cancer stem cell antigen FAM83B", PROGRAMS AND ABSTRACTS OF 46TH ANNUAL MEETING OF THE JAPAN SOCIETY FOR CLINICAL IMMUNOLOGY; NOVEMBER 8TH TO 10TH, 2018, vol. 46, 8 November 2018 (2018-11-08), JP, pages 139, XP009528245 * |
| See also references of EP3862366A4 |
| SHIMPEI : "Development of HLA/peptide complex-specific artificial antibody targeting cancer stem cell antigen FAM83B", PROCEEDINGS OF THE JAPANESE SOCIETY OF PATHOLOGY, vol. 107, no. 1, 26 April 2018 (2018-04-26), JP, pages 522, XP009526902, ISSN: 0300-9181 * |
| STADLER ET AL., NATURE MEDICINE, vol. 23, 2017, pages 815 - 817 |
| TADANO HIROKI: "The antibody targeting cancer stem-like cell antigen DNAJB8-drived peptide/HLA-A24 complex", PROCEEDINGS OF THE JAPANESE SOCIETY OF PATHOLOGY, vol. 107, no. 1, 26 April 2018 (2018-04-26), pages 397, XP009526903, ISSN: 0300-9181 * |
| TSUKAHARA ET AL., J BIOL CHEM., vol. 289, no. 32, 8 August 2014 (2014-08-08), pages 22035 - 47 |
| TSUKAHARA TOMOHIDE : "2-8-1 Development of HLA/peptide complex-specific artificial antibody targeting cancer stem cell antigen DNAJB8 against bone and soft tissue sarcoma", THE JOURNAL OF JAPANESE ORTHOPAEDIC SURGICAL SOCIETY, vol. 92, no. 8, 30 August 2018 (2018-08-30), pages S1942, XP009527162, ISSN: 0021-5325 * |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2023010068A3 (en) * | 2021-07-28 | 2023-03-02 | Cartesian Therapeutics, Inc. | Multiprotein-engineered cells secreting a multispecific antibody |
| WO2023204290A1 (ja) * | 2022-04-21 | 2023-10-26 | 愛知県 | 多重特異性ナノ粒子 |
| WO2025047920A1 (ja) * | 2023-09-01 | 2025-03-06 | 小野薬品工業株式会社 | Hla/がん抗原pvt1由来ペプチド複合体特異的抗体 |
Also Published As
| Publication number | Publication date |
|---|---|
| EP3862366A4 (en) | 2022-06-29 |
| US20220033513A1 (en) | 2022-02-03 |
| EP3862366A1 (en) | 2021-08-11 |
| JPWO2020071554A1 (ja) | 2021-09-09 |
| JP2024144409A (ja) | 2024-10-11 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| AU2019204921C1 (en) | Chimeric Antigen Receptor (CAR) with Antigen Binding Domains to the T Cell Receptor Beta Constant Region. | |
| JP2025032089A (ja) | 腫瘍微小環境を標的とするキメラ抗原受容体 | |
| US20230190796A1 (en) | Engineered cells expressing prostate-specific membrane antigen (psma) or a modified form thereof and related methods | |
| US11385233B2 (en) | Methods of depleting malignant T-cells | |
| JP2024144409A (ja) | がん幹細胞特異的抗体 | |
| JP7308190B2 (ja) | 抗cd47及び抗pd-l1による卵巣癌の処置 | |
| JP2018525421A (ja) | Cd47遮断及び免疫同時刺激アゴニストを用いた標的細胞の枯渇亢進 | |
| JP2013535963A (ja) | Mhcクラスiiと糖尿病関連自己抗原性ペプチドとの天然型複合体に対するt細胞受容体様特異性を有する単離された高親和性実体 | |
| US20250314655A1 (en) | Methods | |
| CN114340659B (zh) | 联合治疗 | |
| AU2020244862B2 (en) | Semaphorin-4D antagonists for use in cancer therapy | |
| JP5566374B2 (ja) | 形質細胞の腫瘍性増殖をきたす疾患の治療薬 | |
| TWI805792B (zh) | 抗腫瘤劑及其評估方法 | |
| JP2024530059A (ja) | 個別化されたがん免疫療法を創出するためのプロセス | |
| WO2025047920A1 (ja) | Hla/がん抗原pvt1由来ペプチド複合体特異的抗体 | |
| RU2803148C2 (ru) | Противоопухолевое средство и способ его оценки | |
| HK40037513A (en) | Antitumor agent and evaluation method thereof | |
| HK40000286A (en) | Gene products differentially expressed in tumors and their uses | |
| HK40000286B (en) | Gene products differentially expressed in tumors and their uses |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 19870017 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 2020551131 Country of ref document: JP Kind code of ref document: A |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2019870017 Country of ref document: EP |
|
| ENP | Entry into the national phase |
Ref document number: 2019870017 Country of ref document: EP Effective date: 20210506 |