WO2019163943A1 - 医薬組成物 - Google Patents
医薬組成物 Download PDFInfo
- Publication number
- WO2019163943A1 WO2019163943A1 PCT/JP2019/006780 JP2019006780W WO2019163943A1 WO 2019163943 A1 WO2019163943 A1 WO 2019163943A1 JP 2019006780 W JP2019006780 W JP 2019006780W WO 2019163943 A1 WO2019163943 A1 WO 2019163943A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- group
- polymer
- compound
- cancer
- drug
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
- 0 CC(*1)(C1(*)C(*)(**)C(**)(O1)I)C1(C(*)=C)I Chemical compound CC(*1)(C1(*)C(*)(**)C(**)(O1)I)C1(C(*)=C)I 0.000 description 3
- SUDHEDJJFGYYPL-UHFFFAOYSA-N CCOC(N(C)C)=O Chemical compound CCOC(N(C)C)=O SUDHEDJJFGYYPL-UHFFFAOYSA-N 0.000 description 1
- QSCZSBGBDORDGM-UHFFFAOYSA-N COC(c(cc1)cc([N+]([O-])=O)c1S(Cl)(=O)=O)=O Chemical compound COC(c(cc1)cc([N+]([O-])=O)c1S(Cl)(=O)=O)=O QSCZSBGBDORDGM-UHFFFAOYSA-N 0.000 description 1
- OGHLGQSUFGNKDW-DYNHUECUSA-N C[C@@]1(C(C(C2)N(C)S(c(ccc(C(O)=O)c3)c3[N+]([O-])=O)(=O)=O)OC)O[C@H]2[n](c2ccccc22)c3c2c(C(NC2)=O)c2c2c3[n]1c1ccccc21 Chemical compound C[C@@]1(C(C(C2)N(C)S(c(ccc(C(O)=O)c3)c3[N+]([O-])=O)(=O)=O)OC)O[C@H]2[n](c2ccccc22)c3c2c(C(NC2)=O)c2c2c3[n]1c1ccccc21 OGHLGQSUFGNKDW-DYNHUECUSA-N 0.000 description 1
- CCUCCXOZLJSUQE-KWBLKEKNSA-N C[C@@]1(C(C(C2)N(C)S(c(ccc(C(OC)=O)c3)c3[N+]([O-])=O)(=O)=O)OC)O[C@H]2[n](c2ccccc22)c3c2c(C(NC2)=O)c2c2c3[n]1c1ccccc21 Chemical compound C[C@@]1(C(C(C2)N(C)S(c(ccc(C(OC)=O)c3)c3[N+]([O-])=O)(=O)=O)OC)O[C@H]2[n](c2ccccc22)c3c2c(C(NC2)=O)c2c2c3[n]1c1ccccc21 CCUCCXOZLJSUQE-KWBLKEKNSA-N 0.000 description 1
- HKSZLNNOFSGOKW-SKHIGLGASA-N C[C@@]1(C(C(C2)NC)OC)O[C@H]2[n](c2ccccc22)c3c2c(C(NC2)=O)c2c2c3[n]1c1ccccc21 Chemical compound C[C@@]1(C(C(C2)NC)OC)O[C@H]2[n](c2ccccc22)c3c2c(C(NC2)=O)c2c2c3[n]1c1ccccc21 HKSZLNNOFSGOKW-SKHIGLGASA-N 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K33/00—Medicinal preparations containing inorganic active ingredients
- A61K33/24—Heavy metals; Compounds thereof
- A61K33/243—Platinum; Compounds thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/505—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim
- A61K31/506—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim not condensed and containing further heterocyclic rings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/505—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim
- A61K31/517—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim ortho- or peri-condensed with carbocyclic ring systems, e.g. quinazoline, perimidine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/505—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim
- A61K31/519—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim ortho- or peri-condensed with heterocyclic rings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/535—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with at least one nitrogen and one oxygen as the ring hetero atoms, e.g. 1,2-oxazines
- A61K31/5375—1,4-Oxazines, e.g. morpholine
- A61K31/5377—1,4-Oxazines, e.g. morpholine not condensed and containing further heterocyclic rings, e.g. timolol
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/55—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having seven-membered rings, e.g. azelastine, pentylenetetrazole
- A61K31/553—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having seven-membered rings, e.g. azelastine, pentylenetetrazole having at least one nitrogen and one oxygen as ring hetero atoms, e.g. loxapine, staurosporine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7042—Compounds having saccharide radicals and heterocyclic rings
- A61K31/7052—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides
- A61K31/7056—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing five-membered rings with nitrogen as a ring hetero atom
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7042—Compounds having saccharide radicals and heterocyclic rings
- A61K31/7052—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides
- A61K31/706—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom
- A61K31/7064—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom containing condensed or non-condensed pyrimidines
- A61K31/7068—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom containing condensed or non-condensed pyrimidines having oxo groups directly attached to the pyrimidine ring, e.g. cytidine, cytidylic acid
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
Definitions
- the present invention relates to a pharmaceutical composition.
- the present invention relates to a pharmaceutical composition for treating or preventing cancer resistant to an anticancer drug.
- gefitinib is an anticancer agent that inhibits the tyrosine kinase activity of epidermal growth factor receptor (EGFR) and is used as a therapeutic agent for non-small cell lung cancer.
- Gefitinib is effective in non-small cell lung cancer that expresses EGFR with the L858R mutation, but when the T790M mutation occurs, it becomes a gefitinib resistant cancer.
- Osimertinib is effective for gefitinib-resistant cancers that express EGFR with the T790M mutation. However, when the C797S mutation occurs, the cancer becomes resistant to osmeltinib.
- an effective drug for cancer expressing EGFR having three mutations of L858R, T790M, and C797S has not been developed.
- staurosporine is a natural product isolated from Streptomyces genus actinomycetes (Non-Patent Document 1) and is known to have an antitumor action (for example, Patent Document 1). ).
- Patent Document 1 Non-Patent Document 1
- K252a To date, staurosporine analogues such as K252a have been reported (for example, Patent Document 2).
- X and Y are each independently H, —OH, Cl, propoxy, or ethylthiomethyl; R 6 is H, C 1-3 alkyl, —NH 2 , benzyl,
- R 7 and R 8 are each independently H, —OH, or methoxy, or may be taken together to form O ⁇ ;
- R 9 and R 10 are each independently H, methyl, ⁇ -D-glucopyranosyl, 4-O-methyl- ⁇ -D-glucopyranosyl, cyanoethyl, or
- R 11 is methyl;
- R 12 is H;
- R 13 and R 14 are each independently H, methoxy, —OH, hydroxymethyl, methylcarboxylate, methylamino, methylaminomethyl, propylaminomethyl, dimethylaminomethyl, methoxycarbonyl,
- R 15 and R 16 are each independently H, —OH, or
- R 17 and R 18 are H, —OH, methylamino, dimethylamino, oxime, or a group represented by any one of the following formulae:
- the anticancer agent is at least one anticancer agent selected from the group consisting of gefitinib, afatinib, osmeltinib, dasatinib, erlotinib, gemcitabine, cisplatin, pemetrexed, and midostaurin.
- the pharmaceutical composition as described.
- the kinase is at least one selected from the group consisting of EGFR, ABL1, ALK1, HER2, c-Kit, FGFR1, FGFR2, FGFR3, c-Src, PDGFRa, RET, DDR2, TRKA, and Flt-3.
- the pharmaceutical composition according to [5], wherein the kinase is EGFR having mutations of d746-750, T790M, and C797S.
- the compound (S) is staurosporine, 7-hydroxystautosporin, KT5926, staurosporine aglycone, SF2370, KT5823, 4′-N-benzoylstaurosporine, Go6976, N, N-dimethyl.
- Staurosporine NA 0359, N-ethoxycarbonyl-7-oxostaurosporine, KT-6124, CGP42700, 4'-demethylamino-4 ', 5'-dihydroxystaurosporine, 7-oxostaurosporine, CEP751, NA0346, NA0359, 3′-demethoxy-3′-hydroxystaurosporine, KT 6006, 7-O-methyl-UCN 01, TAN 999, NA 0346, NA 0345, NA 0344, CGP 44171A, SCH 47112, N, N -Jimee Rustaurosporin, TAN 1030A, Restaurtinib, 4′-demethylamino-4′-hydroxystaurosporine, AFN941, edtecalin, becatecarine, K252a, and K252a hydrazide, which is at least one compound selected from the group [1 ] To [6] The pharmaceutical composition according to any one of [6].
- a pharmaceutical composition that can be used for treatment or prevention of cancer resistant to an anticancer drug is provided.
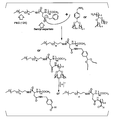
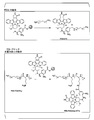
- FIG. 1 is a synthesis scheme of an aromatic aldehyde group-containing polymer and an aliphatic aldehyde group-containing polymer.
- FIG. 2 is a 1 H-NMR spectrum of an acetal group-containing polymer that is an intermediate of an aromatic aldehyde group-containing polymer.
- FIG. 3 is a 1 H-NMR spectrum of an aromatic aldehyde group-containing polymer.
- FIG. 4 is a 1 H-NMR spectrum of an acetal group-containing polymer that is an intermediate of an aliphatic aldehyde group-containing polymer.
- FIG. 5 is a 1 H-NMR spectrum of an aliphatic aldehyde group-containing polymer.
- FIG. 1 is a synthesis scheme of an aromatic aldehyde group-containing polymer and an aliphatic aldehyde group-containing polymer.
- FIG. 2 is a 1 H-NMR spectrum of an acetal group-containing polymer that is an intermediate
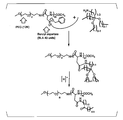
- FIG. 6 is a synthesis scheme of an aromatic ketone group-containing polymer and an aliphatic ketone group-containing polymer.
- FIG. 7 is a 1 H-NMR spectrum of an aliphatic ketone group-containing polymer.
- FIG. 8 is a 1 H-NMR spectrum of K252a and K252a hydrazide (K252a-H).
- FIG. 9 shows the results of HPLC analysis of K252a and K252a-H.
- FIG. 10 is a 1 H-NMR spectrum of a drug complex (K252a-H-bonded aliphatic polymer micelle) in which K252a-H is bound to an aliphatic ketone group-containing polymer.
- FIG. 10 is a 1 H-NMR spectrum of a drug complex (K252a-H-bonded aliphatic polymer micelle) in which K252a-H is bound to an aliphatic ketone group-containing polymer.
- FIG. 11 is a 1 H-NMR spectrum of a drug complex (K252a-H-bonded aromatic polymer micelle) in which K252a-H is bound to an aromatic aldehyde group-containing polymer.
- FIG. 12A shows the analysis results of the micelle size and the dispersion degree (PDI) of the K252a-H-bonded aromatic polymer micelle.
- FIG. 12B is an analysis result of the micelle size and the degree of dispersion (PDI) of K252a-H bonded aliphatic polymer micelles.
- FIG. 13 is an ultraviolet absorption spectrum of a sample prepared by dissolving K252a-H in distilled water or methanol.
- FIG. 14 is a HPLC analysis result of a sample after treatment of K252a-H linked aliphatic polymer micelles with an aqueous buffer at pH 1.
- FIG. 15 shows the results of cytotoxicity tests on lung cancer cells of K252a, K252a-H, K252a-H-linked aliphatic polymer micelles, and K252a-H-linked aromatic polymer micelles (hereinafter also referred to as “K252a class”). It is a graph.
- FIG. 16 is a graph showing the results of a cytotoxicity test for K252a class lung cancer cells.
- FIG. 17 is a graph showing the results of a cytotoxicity test for K252a class pancreatic cancer cells.
- FIG. 18 is a graph showing the results of a cytotoxicity test of K252a class on head and neck cancer cells.
- FIG. 19 is a graph showing the results of a cytotoxicity test for K252a class malignant mesothelioma cells.
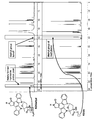
- FIG. 20 is a diagram showing first to third generation tyrosine kinase inhibitors (TKI) and resistance mutations thereof.
- FIG. 21 is a diagram showing the first to third generation TKIs and their resistance mutations.
- FIG. 22 shows the results of K252a-H kinase assay for EGFR mutation.
- FIG. 23A is a diagram showing the results of kinase profiling.
- FIG. 23B is a diagram showing the results of kinase profiling.
- FIG. 23A is a diagram showing the results of kinase profiling.
- FIG. 23C shows the results of kinase profiling.
- FIG. 24 is a diagram showing a list of mutant kinases whose kinase activity is suppressed to 30% or less by K252a-H.
- FIG. 25 is a diagram showing the results of kinase profiling.
- FIG. 26 shows the results of kinase profiling.
- FIG. 27 is a diagram showing the results of kinase profiling.
- FIG. 28 shows the results of kinase profiling.
- FIG. 29 is a diagram showing the results of kinase profiling.
- FIG. 30 shows the results of kinase profiling.
- FIG. 31 shows the results of kinase profiling.
- FIG. 32 shows the results of kinase profiling.
- FIG. 33A shows the results of kinase profiling.
- FIG. 33B shows the results of kinase profiling.
- FIG. 33C shows the results of kinase profiling.
- FIG. 33D shows the results of kinase profiling.
- FIG. 34A shows the results of kinase profiling.
- FIG. 34B shows the results of kinase profiling.
- FIG. 34B shows the results of kinase profiling.
- FIG. 34D shows the results of kinase profiling.
- FIG. 35 shows a list of kinases having an IC50 of staurosporine of 0.2 nM or less.
- the underlined kinase is a kinase that is important as a therapeutic target for cancer (particularly drug-resistant cancer).
- FIG. 36 is a graph showing the results of cytotoxicity tests of staurosporine and K252a class on non-small cell lung cancer.
- FIG. 37 is a graph showing the results of cytotoxicity tests of staurosporine and K252a family against pancreatic cancer.
- FIG. 38 is a graph showing the results of cytotoxicity tests of staurosporine and K252a family on head and neck cancer.
- FIG. 39 is a graph showing the results of cytotoxicity test for staurosporine and K252a malignant mesothelioma.
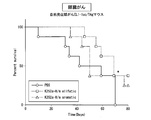
- FIG. 40 is a graph showing the results of an in vivo anti-tumor test for K252a species.
- FIG. 40 is a graph showing the results of an in vivo anti-tumor test for K252a species.
- FIG. 41 is a graph showing the results of an in vivo anti-tumor test for K252a species.
- FIG. 42 is a graph showing the results of an in vivo anti-tumor test for K252a species.
- FIG. 43 is a graph showing the results of cytotoxicity testing of staurosporine and K252a against spontaneous pancreatic cancer.
- FIG. 44 is a graph showing the results of an in vivo anti-tumor test for K252a species.
- FIG. 45 is a graph showing the results of an in vivo antitumor test for K252a species.
- FIG. 46 is a graph showing the results of a cytotoxicity test for K252a class lymphoma.
- FIG. 47 is a graph showing the results of a staurosporine and K252a class inhibitory activity test against MDR-1.
- FIG. 48 is a graph showing the results of a test evaluating the effect of human ⁇ 1-acid glycoprotein (hAGP) on the cytotoxicity of K252a species.
- FIG. 49 is a diagram showing an example of a reaction for binding a linker reagent (L1) to staurosporine.
- FIG. 50 is a diagram showing a 1 H-NMR spectrum of staurosporine bound to a linker reagent (L1).
- FIG. 51 is a diagram illustrating a scheme for preparing a drug complex of staurosporine and a polymer via a linker derived from a linker reagent (L1).
- the present invention provides the compound (S) represented by the following general formula (S), or a pharmaceutically acceptable salt thereof, or a pharmaceutically acceptable polymer, wherein the compound (S) or the Provided is a pharmaceutical composition for treating or preventing cancer resistant to an anticancer drug, which comprises a drug complex bound with a pharmaceutically acceptable salt.
- composition of the present embodiment comprises the compound (S) represented by the following general formula (S), or a pharmaceutically acceptable salt thereof, or a pharmaceutically acceptable polymer, the compound (S) or the It includes drug conjugates with pharmaceutically acceptable salts attached.
- X and Y are each independently H, —OH, Cl, propoxy, or ethylthiomethyl; R 6 is H, C 1-3 alkyl, —NH 2 , benzyl,
- R 7 and R 8 are each independently H, —OH, or methoxy, or may be taken together to form O ⁇ ;
- R 9 and R 10 are each independently H, methyl, ⁇ -D-glucopyranosyl, 4-O-methyl- ⁇ -D-glucopyranosyl, cyanoethyl, or
- R 11 is methyl;
- R 12 is H;
- R 13 and R 14 are each independently H, methoxy, —OH, hydroxymethyl, methylcarboxylate, methylamino, methylaminomethyl, propylaminomethyl, dimethylaminomethyl, methoxycarbonyl,
- R 15 and R 16 are each independently H, —OH, or
- R 17 and R 18 are each independently H, —OH, methylamino, dimethylamino, oxime, or a group represented by any one of the following formulae:
- R 19 is a group containing methoxy, —OH, —NH—NH 2 , or an active ester.
- Preferred examples of the compound (S) include compounds (S-1) and (S-2) represented by the following general formulas (S-1) and (S-2), respectively.
- X, Y, and R 6 are preferably H.
- R 13 and R 14 are each independently represented by H, methoxy, —OH, hydroxymethyl, methoxycarbonyl, or the following formula (h1). It is preferably a group.
- R 13 and R 14 in (S-1) are each independently H, methoxycarbonyl, or a group represented by the above formula (h1). More preferably, R 13 and R 14 in (S-2) are each independently H or methoxy.
- R 15 and R 16 are preferably each independently H or —OH.
- R 17 and R 18 are preferably H, —OH, methylamino, dimethylamino, oxime, or a group represented by the following formula (11). More preferred is amino or a group represented by the formula (l1).
- R 19 is methoxy, —OH, —NH—NH 2 , or a group containing an active ester.
- the group containing an active ester in the above formula (l1) includes an active esterifying agent (N-hydroxysuccinimide (NHS), 1-hydroxybenzotriazole (HOBt), 1-hydroxy-7-azabenzotriazole (HOAt). , Pentafluorophenol, p-nitrophenol, etc.).
- an active esterifying agent N-hydroxysuccinimide (NHS), 1-hydroxybenzotriazole (HOBt), 1-hydroxy-7-azabenzotriazole (HOAt).
- Pentafluorophenol p-nitrophenol, etc.
- Preferred examples of the compound (S-1) include a compound (S-1-1) represented by the following general formula (S-1-1).
- a compound (S-2-1) represented by the following general formula (S-2-1) is preferably exemplified.
- R 7 is H or —OH
- R 13 and R 14 are each independently H, methoxy, —OH, hydroxymethyl, methoxycarbonyl, or a group represented by the following formula. ]
- Examples of the compound (S) include the following compounds: staurosporine, 7-hydroxystautosporin, KT5926, staurosporine aglycone, SF2370, KT5823, 4′-N-benzoylstaurosporine, PKC412 , Go6976, N, N-dimethylstaurosporine, NA0359, N-ethoxycarbonyl-7-oxostaurosporine, KT-6124, CGP42700, 4'-demethylamino-4 ', 5'-dihydroxystaurosporine, 7- Oxostaurosporine, CEP751, NA0346, NA0359, 3′-demethoxy-3′-hydroxystaurosporine, KT6006, 7-O-methyl-UCN01, TAN999, NA03 6, NA0345, NA0344, CGP44171A, SCH47112, N, N-dimethylstaurosporine, TAN1030A, restaurtinib,
- Specific examples of the compound (S-1-1) include K252a and K252a hydrazide (hereinafter also referred to as “K252a-H”). Specific examples of the compound (S-2-1) include staurosporine.
- Compound (S) may be in the form of a pharmaceutically acceptable salt.
- the “pharmaceutically acceptable salt” means a salt that does not inhibit the pharmacological action (anticancer activity, kinase inhibitory activity, etc.) of the compound (S).
- the pharmaceutically acceptable salt is preferably one that does not cause side effects when administered to a living body.
- the pharmaceutically acceptable salt is not particularly limited, and for example, a salt with an alkali metal (sodium, potassium, etc.); a salt with an alkaline earth metal (magnesium, calcium, etc.); an organic base (pyridine, triethylamine, etc.) Salt with amine, salt with amine, salt with organic acid (acetic acid, formic acid, propionic acid, fumaric acid, maleic acid, succinic acid, tartaric acid, citric acid, malic acid, oxalic acid, benzoic acid, methanesulfonic acid, etc.) And salts with inorganic acids (hydrochloric acid, phosphoric acid, hydrobromic acid, sulfuric acid, nitric acid, etc.).
- the compound (S) may be a compound (K) or a pharmaceutically acceptable salt of the compound (K) described in [Second aspect] described later.
- Compound (S) may be used alone or in combination of two or more.
- the pharmaceutical composition of this embodiment may include a drug complex in which the compound (S) is bound to a pharmaceutically acceptable polymer.
- the “drug complex” refers to a molecule in which a pharmacological action (drug) is bound to another molecule (for example, a polymer).
- “Pharmaceutically acceptable polymer” means a polymer that does not inhibit the pharmacological action (anticancer activity, kinase inhibitory activity, etc.) of compound (S) when it forms a drug complex with compound (S). .
- a polymer that does not inhibit the pharmacological action of the compound (S) includes a compound in which the drug complex with the compound (S) exhibits a pharmacological action equivalent to or higher than that of the compound (S) in vitro, and in vivo And those having a pharmacological action equivalent to or higher than that of the compound (S).
- the pharmaceutically acceptable polymer is preferably a polymer that does not cause side effects when administered to a living body.
- the polymer that forms the drug complex with the compound (S) is not particularly limited as long as it does not inhibit the pharmacological action of the compound (S).
- the polymer may be a hydrophilic polymer, a hydrophobic polymer, or a copolymer including a hydrophilic polymer segment and a hydrophobic polymer segment.
- hydrophilic polymer segment examples include, for example, polyalkylene glycol, poly (2-oxazoline), polysaccharide, polyvinyl alcohol, polyvinyl pyrrolidone, polyacrylamide, polymethacrylamide, polyacrylic ester, polymethacrylic ester, poly (2-methacryloyloxyethyl phosphorylcholine), poly (N- (2-hydroxypropyl) methacrylamide) (PHPMA) and derivatives thereof.
- polyalkylene glycol and poly (2-oxazoline) are preferable, and polyalkylene glycol is more preferable.
- polyalkylene glycol examples include polyethylene glycol, polypropylene glycol, polyethylene glycol / polypropylene glycol copolymer, and the like, and polyethylene glycol is particularly preferable.
- the hydrophobic polymer segment include a polymer having a repeating unit derived from an amino acid and / or a derivative thereof. More specifically, a polyamino acid or a derivative thereof can be mentioned. Examples of polyamino acids and derivatives thereof include polyaspartic acid, polyglutamic acid, polylysine, poly (benzylaspartic acid), poly (benzylglutamic acid) and the like. Moreover, the hydrophobic polymer segment may contain a repeating unit derived from an amino acid having an alkyl group side chain or an aralkyl group side chain, for example. Examples of amino acids having an alkyl side chain include alanine, valine, leucine, and isoleucine.
- phenylalanine is mentioned as an amino acid which has an aralkyl group side chain.
- aralkyl group side chain When it has a repeating unit derived from two or more alkyl group side chain amino acids and / or aralkyl group side chain amino acids, these side chains may be the same or different.
- the ratio of the repeating unit derived from the alkyl group side chain amino acid or the aralkyl group side chain amino acid with respect to all the repeating units of the hydrophobic polymer segment is not particularly limited, and is, for example, 20% or more, 35% or more, 40% or more, 50 % Or more, 80% or more, 95% or more, 99% or more, or 100%.
- Preferred examples of the polymer that forms the drug complex with the compound (S) include the following polymer (P).
- the polymer (P) is a polymer having a repeating unit (I) represented by the following general formula (I) and a repeating unit (II) represented by the following general formula (II).
- L represents a divalent aromatic hydrocarbon group or a divalent aliphatic hydrocarbon group.
- R 1 represents a hydrogen atom, an aliphatic hydrocarbon group or an aromatic hydrocarbon group.
- X represents OR x , SR x or NR x1 R x2 .
- R x represents a hydrogen atom, an aliphatic hydrocarbon group or an aromatic hydrocarbon group.
- R x1 and R x2 each independently represent a hydrogen atom, an aliphatic hydrocarbon group or an aromatic hydrocarbon group.
- L represents a divalent aromatic hydrocarbon group or a divalent aliphatic hydrocarbon group.
- the divalent aromatic hydrocarbon group for L include a phenylene group and a benzylene group.
- the divalent aromatic hydrocarbon group for L may have a substituent. Examples of the substituent include a methyl group, an ethyl group, a propyl group, an isopropyl group, a tert-butyl group, a nitro group, and a halide.
- Examples of the divalent aliphatic hydrocarbon group for L include an ethylene group, a propylene group, a butylene group, and a pentylene group.
- the divalent aliphatic hydrocarbon group for L may have a substituent.
- the substituent include a methyl group, an ethyl group, a propyl group, an isopropyl group, a butyl group, a tert-butyl group, and a halide.
- L is preferably a methylene group, an ethylene group, a propylene group, or a benzylene group, and more preferably a methylene group, an ethylene group, or a benzylene group.
- L is preferably a divalent aliphatic hydrocarbon group, more preferably a methylene group, an ethylene group, or a propylene group, and even more preferably a methylene group or an ethylene group.
- R 1 represents a hydrogen atom, an aliphatic hydrocarbon group or an aromatic hydrocarbon group.
- the aliphatic hydrocarbon group for R 1 include an ethyl group, a propyl group, a butyl group, and a pentyl group.
- the aliphatic hydrocarbon group for R 1 may have a substituent.
- substituents examples include a methyl group, an ethyl group, a propyl group, an isopropyl group, a butyl group, an isobutyl group, a tert-butyl group, a pentyl group, an isopentyl group, a tert-pentyl group, a cyclohexyl group, and a trihalomethyl group.
- the aromatic hydrocarbon group for R 1 includes phenyl, benzyl, pyridyl, naphthyl, hydroxyphenyl, methoxyphenyl, ethoxyphenyl, xylyl, methylphenyl, nitrophenyl, chlorophenyl, fluoro Examples include an orophenyl group, an iodophenyl group, and a bromophenyl group.
- R 1 preferably a hydrogen atom or an aliphatic hydrocarbon group, more preferably a hydrogen atom or a methyl group, further preferably a methyl group.
- X represents OR x , SR x or NR x1 R x2 .
- R x represents a hydrogen atom, an aliphatic hydrocarbon group or an aromatic hydrocarbon group.
- the aliphatic hydrocarbon group for R x include methyl group, ethyl group, propyl group, isopropyl group, butyl group, isobutyl group, tert-butyl group, pentyl group, isopentyl group, tert-pentyl group, cyclohexyl group, and trifluoro group.
- a methyl group etc. are mentioned.
- the aromatic hydrocarbon group for R x includes phenyl, benzyl, pyridyl, naphthyl, hydroxyphenyl, methoxyphenyl, ethoxyphenyl, xylyl, methylphenyl, nitrophenyl, chlorophenyl, fluoro, Examples include an orophenyl group, an iodophenyl group, and a bromophenyl group.
- R x1 and R x2 each independently represent a hydrogen atom, an aliphatic hydrocarbon group or an aromatic hydrocarbon group.
- Examples of the aliphatic hydrocarbon group represented by R x1 and R x2 include methyl group, ethyl group, isopropyl group, butyl group, isobutyl group, tert-butyl group, pentyl group, isopentyl group, tert-pentyl group, cyclohexyl group, and trihalomethyl. Group.
- Examples of the aromatic hydrocarbon group represented by R x1 and R x2 include phenyl group, benzyl group, pyridyl group, naphthyl group, hydroxyphenyl group, methoxyphenyl group, ethoxyphenyl group, xylyl group, methylphenyl group, humanlophenyl group, and chlorophenyl. Group, fluorophenyl group, iodophenyl group, bromophenyl group and the like. Among them, X is preferably OR x and more preferably OH (hydroxy group).
- the polymer (P) may have a repeating unit other than the repeating units (I) and (II) (hereinafter sometimes referred to as “repeating unit (III)”).
- the repeating unit (III) is preferably a hydrophilic repeating unit.
- a repeating unit derived from polyethylene glycol a repeating unit derived from poly (ethylethylene phosphate), a repeating unit derived from polyvinyl alcohol, polyvinyl Examples thereof include a repeating unit derived from pyrrolidone, a repeating unit derived from poly (oxazoline), and a repeating unit derived from poly (N- (2-hydroxypropyl) methacrylamide) (PHPMA).
- the repeating unit (III) is preferably a repeating unit derived from polyethylene glycol.
- the content of the repeating units (I) to (III) is not particularly limited.
- the content of the repeating unit (I) is preferably 5 to 100 mol%, more preferably 10 to 80 mol%, more preferably 20 to 50 mol based on the total (100 mol%) of all repeating units constituting the polymer (P). More preferred is mol%.
- the content of the repeating unit (II) is preferably 0 to 80 mol%, more preferably 10 to 60 mol%, more preferably 20 to 40 mol based on the total (100 mol%) of all the repeating units constituting the polymer (P). More preferred is mol%.
- the content of the repeating unit (III) is preferably from 0 to 95 mol%, more preferably from 20 to 90 mol%, more preferably from 50 to 80, based on the total (100 mol%) of all repeating units constituting the polymer (P). More preferred is mol%.
- the molecular weight of the polymer (P) is preferably from 2,000 to 1,000,000 D, more preferably from 5,000 to 100,000 D, and even more preferably from 10,000 to 40,000 D.
- production method (1) includes the polymer (P1) having the repeating unit (II ′) represented by the following general formula (II ′) and the following general formula (II ′).
- R 2 represents a hydrogen atom, an aliphatic hydrocarbon group or an aromatic hydrocarbon group.
- L represents a divalent aromatic hydrocarbon group or a divalent aliphatic hydrocarbon group.
- R 1 represents a hydrogen atom, an aliphatic hydrocarbon group or an aromatic hydrocarbon group.
- Ra 11 and Ra 12 each independently represent a methyl group or an ethyl group, or Ra 11 and Ra 12 are bonded to each other to represent an ethylene group or a propylene group.
- R x represents a hydrogen atom, an aliphatic hydrocarbon group or an aromatic hydrocarbon group.
- m, L, R 1 and R x are m, L in the general formulas (I) and (II). , R 1 and R x .
- Ra 11 and Ra 12 each independently represent a methyl group or an ethyl group, or Ra 11 and Ra 12 are bonded to each other to represent an ethylene group or a propylene group.
- the compound (1a) becomes a cyclic acetal or a cyclic ketal.
- n1 and n2 are each independently 0 or 1, and 1 is preferable.
- Step (1) of production method (1) is an aminolysis reaction between polymer (P1) and compound (1a).
- the acetal structure or ketal structure of the compound (1a) is introduced into the side chain of the polymer (P1).
- the reaction temperature in the step (1) is not particularly limited as long as the acetal structure or ketal structure of the compound (1a) is introduced into the side chain of the polymer (P1), but is usually 4 ° C. to 100 ° C. ⁇ 40 ° C is preferred.
- the reaction time in the step (1) is not particularly limited as long as the acetal structure or ketal structure of the compound (1a) is introduced into the side chain of the polymer (P1), and the reaction time, the type of the compound (1a) although it can be selected depending on the amount, it is usually 4 hours to 5 days.
- step (2) of production method (1) polymer (P2) is hydrolyzed under neutral or weakly acidic conditions, and the acetal structure of repeating unit (I ′) of polymer (P2) is converted into an aldehyde or ketal. Convert structure to ketone.
- the hydrolysis is not particularly limited as long as the acetal structure of the repeating unit (I ′) of the polymer (P2) can be converted into an aldehyde or a ketal structure into a ketone.
- a method of treating with 0.1N hydrochloric acid for about 30 minutes (i) a method of treating in the presence of acetone and indium (III) trifluoromethanesulfonate (catalyst), and (iii) a catalytic amount in water at 30 ° C.
- a method using 1 to 5 mol% of Er (OTf) 3 in wet nitromethane at room temperature (v) a substantially neutral solution using tetrakis (3,5-trifluoromethylphenyl) borate
- Known methods such as a method using a catalytic amount of cerium (III) triflate in wet nitromethane at room temperature under pH conditions.
- production method (2) includes the polymer (P1) having the repeating unit (II ′) represented by the following general formula (II ′) and the following general formula (II ′).
- the polymer (P3) is hydrolyzed under neutral or weakly acidic conditions, and the repeating unit (I) represented by the following general formula (I) and the repeating unit represented by the following general formula (II) ( (2b) obtaining a polymer (P) having II); including.
- R 2 represents a hydrogen atom, an aliphatic hydrocarbon group or an aromatic hydrocarbon group.
- L represents a divalent aromatic hydrocarbon group or a divalent aliphatic hydrocarbon group.
- R 1 represents a hydrogen atom, an aliphatic hydrocarbon group or an aromatic hydrocarbon group.
- Ra 11 and Ra 12 each independently represent a methyl group or an ethyl group, or Ra 11 and Ra 12 are bonded to each other to represent an ethylene group or a propylene group.
- X represents OR x , SR x or NR x1 R x2 .
- R x represents a hydrogen atom, an aliphatic hydrocarbon group or an aromatic hydrocarbon group.
- R x1 and R x2 each independently represent a hydrogen atom, an aliphatic hydrocarbon group or an aromatic hydrocarbon group.
- m, L, X, R x , R x1 and R x2 are the same as those in the general formulas (I) and (II). The same as m, L, X, R x , R x1 and R x2 .
- R 1 , Ra 11 , and Ra 12 are the same as described above.
- Step (1) of production method (2) is the same as step (1) of production method (1).
- the side chain of the repeating unit (II ′) is obtained by subjecting the polymer (P2) to a predetermined treatment so that the repeating unit (I ′) is protected with an acetal structure.
- a desired functional group can be introduced into Hydrolysis under alkaline conditions is, for example, a method of treating in a mixture of 0.5N NaOH solution and DMSO (volume ratio: 50/50) at room temperature for 30 minutes, or treating with triethylamine in DMSO at room temperature for 1 hour. And a method of treating with diisopropylethylamine in DMSO for 1 hour at room temperature.
- Aminolysis can introduce an amino functional group by cleaving the ester with, for example, ethylenediamine or diaminopropane. By introducing an amino group, it can be combined with a fluorescent dye. Moreover, it can also attach
- the resulting carboxylic acid is subjected to transesterification or amide coupling using a known coupling agent.
- a known coupling agent for hydrolysis and amide coupling under alkaline conditions, for example, after the ester residue is treated by hydrolysis under alkaline conditions, the resulting carboxylic acid is subjected to transesterification or amide coupling using a known coupling agent. be able to.
- the hydrophilic / hydrophobic balance of the polymer can be made desirable, contributing to self-assembly in polar or non-polar solvents.
- step (2b) of the production method (2) the polymer (P3) is hydrolyzed under weakly acidic conditions to convert the acetal structure of the repeating unit (I ′) of the polymer (P3) into an aldehyde. Hydrolysis conditions are the same as in step (2) of production method (1).
- the bond between the polymer (P) and the compound (S) is a nitrogen atom-containing group capable of forming a Schiff base with an aldehyde group or a ketone group in the compound (S) (hereinafter sometimes referred to as “Schiff base-forming group”).
- the reaction can be performed by reacting the Schiff base-forming group with an aldehyde group or a ketone group contained in the repeating unit (I) of the polymer (P).
- Examples of such a Schiff base include an amino group, an imino group, and a hydrazide group.
- the Schiff base forming group may be introduced into the compound (S). The introduction of the Schiff base forming group can be performed by a known method.
- K252a since there is no Schiff base-forming group in K252a, it can be bonded to the polymer (P) by introducing a hydrazide group to form K252a-H as shown in the examples described later.
- the drug complex of the polymer (P) and K252a include a polymer having the repeating unit (ka-1) and the repeating unit (II) described in [Second embodiment] described later.
- the drug complex of the polymer (P) and the compound (S) has a repeating unit (Ia) represented by the following general formula (Ia) and a repeating unit (II) represented by the following general formula (II).
- a polymer is preferred.
- L represents a divalent aromatic hydrocarbon group or a divalent aliphatic hydrocarbon group.
- R 1 represents a hydrogen atom, an aliphatic hydrocarbon group or an aromatic hydrocarbon group.
- BM represents a group derived from the compound (S).
- X represents OR x , SR x or NR x1 R x2 .
- R x represents a hydrogen atom, an aliphatic hydrocarbon group or an aromatic hydrocarbon group.
- R x1 and R x2 each independently represent a hydrogen atom, an aliphatic hydrocarbon group or an aromatic hydrocarbon group.
- m, L, R 1 , X, R x , R x1 and R x2 are m, L, R 1 in the general formulas (I) and (II), The same as X, R x , R x1 and R x2 .
- BM represents a group derived from the compound (S).
- Preferred examples of the group derived from compound (S) include a group derived from K252a-H.
- the amount of the aldehyde group or ketone group to be introduced (the amount of the repeating unit (I)) can be controlled, and the amount of the drug bonded to the aldehyde group or the ketone group can also be controlled. Therefore, the dose of compound (S) can be appropriately controlled.
- an aldehyde group or a ketone group (a group represented by —LC ( ⁇ O) —R 1 in the above general formula (I)) to be introduced into the repeating unit (I)
- An aromatic aldehyde group (L in the general formula (I) is a divalent aromatic hydrocarbon group, R 1 is a hydrogen atom), an aliphatic aldehyde group (L in the general formula (I) is a divalent aliphatic carbonization
- an aromatic ketone group (L in the general formula (I) is a divalent aromatic hydrocarbon group, R 1 is an aliphatic hydrocarbon group or an aromatic hydrocarbon group; 1 is preferably an aliphatic hydrocarbon group, more preferably a methyl group), an aliphatic ketone group (L in the general formula (I) is a divalent aliphatic hydrocarbon group, R 1 is an aliphatic hydrocarbon group or aromatic R 1 is preferably selected from an
- the repeating unit (I) of the polymer (P) When the aromatic aldehyde group is introduced, the compound (S) is more stably held in the repeating unit (I) of the polymer (P). Therefore, the sustained release property of the drug can be controlled by selecting the type of aldehyde group or ketone group to be introduced into the repeating unit (I) of the polymer (P) according to the disease state and the type of drug.
- the compound (S) is stably maintained and the toxicity is reduced while the compound (S) is held by the polymer (P). Can reduce side effects and enhance the therapeutic effect.
- an aliphatic ketone group is introduced into the repeating unit (I) of the polymer (P) from the viewpoint of the antitumor effect when administered in vivo.
- L is preferably a divalent aliphatic hydrocarbon group (preferably a methylene group or ethylene group)
- R 1 is an aliphatic hydrocarbon group (preferably a methyl group).
- the compound (S) and the polymer (P) may be bonded using a known linker reagent.
- the linker reagent contains a Schiff base forming group
- the compound (S) can be bonded to the polymer (P) using the Schiff base forming group.
- a Schiff base-forming group may be introduced into the compound in which the linker is bound to the compound (S).
- the linker reagent that can be used is not particularly limited.
- a glutathione (GSH) / glutathione-S-transferase (GST) -sensitive linker reagent represented by the following formula (L1) (linker reagent (L1); 2012/039499, Huang CH et al., ChemMedChem. 2017 Jan 5; 12 (1): 19-22 .; Zhang J et al., J Am Chem Soc. 2011 Sep 7; 133 (35): 14109-19 .).
- the compound (SL1) represented by the following general formula (SL1) is a compound obtained by reacting the linker reagent (L1) with the compound (S-2-1).
- the following compound (SL1-H) containing a Schiff base-forming group can be obtained by refluxing the following compound (SL1) without solvent in the presence of anhydrous hydrazine or hydrazine hydrate.
- the compound (SL1-H) is obtained by, for example, converting the compound (SL1) into methanol, ethanol, propanol, isopropanol, butanol, tert-butanol, N, N-dimethylformamide (DMF), dimethyl sulfoxide (DMSO), tetrahydrofuran (THF).
- reaction temperature and reaction time are not particularly limited, and examples include room temperature to 50 ° C. for 30 minutes to 15 hours.
- R 7, R 13, and R 14 are the same as R 7, R 13, and R 14 in the general formula (S-2-1). ]
- the drug complex in which the compound (SL1-H) is bound to the polymer (P) is represented by the repeating unit (Ia-S) represented by the following general formula (Ia-S) and the following general formula (II). Having repeating unit (II).
- the drug complex having the repeating unit (Ia-S) and the repeating unit (II) includes a pH-sensitive bond (I) and a GSH / GST-sensitive bond (II). Therefore, when the above drug complex is administered in vivo, first, the bond (I) is cleaved around the tumor tissue in a low pH environment, and the compound (SL1-H) is released. Further, the compound (SL1-H) is taken up by tumor cells, and the bond (II) is cleaved by the action of GSH / GST to release the compound (S-2-1). Therefore, the release of the compound (S) from the polymer (P) in the living body can be controlled in two stages.
- the compound (S) may be a drug complex with a polymer other than the polymer (P).
- the bond between the compound (S) and the polymer can be performed using a known method depending on the functional group contained in the compound (S) and the polymer.
- the compound (S) may be directly bonded to the polymer, or a functional group capable of bonding to the polymer may be introduced into the compound (S) and bonded to the polymer via the functional group.
- a linker reagent is not specifically limited, For example, the said linker reagent (L1) is mentioned.
- an active ester may be introduced by an active esterifying agent and bonded to a polymer having a hydroxy group or an amino group. Conversely, an active ester may be introduced into the polymer.
- the linker reagent (L1) is bound to the compound (S) to obtain the compound (SL1), and then the compound (SL1) is demethylated to produce an active esterifying agent (N-hydroxysuccinimide (NHS)).
- an active esterifying agent N-hydroxysuccinimide (NHS)
- 1-hydroxybenzotriazole (HOBt), 1-hydroxy-7-azabenzotriazole (HOAt), pentafluorophenol, p-nitrophenol, etc.) to give an active ester represented by the following formula (SL2)
- R 7 , R 13 and R 14 are as defined above.
- E represents a group obtained by removing a hydroxy group from an active esterifying agent.
- R 7 , R 13 , R 14 and E are as defined above.
- Px represents an arbitrary polymer, and Ex represents a hydroxy group or an amino group.
- Ex ′ represents an ester bond or an amide bond.
- the drug complex of the compound (S) and polymer contained in the pharmaceutical composition of this embodiment may form a micelle.
- the polymer included in the drug complex preferably has a hydrophilic polymer segment and a hydrophobic polymer segment.
- the micelle of the drug complex of the compound (S) and the polymer can be prepared by a known method. For example, the drug conjugate is dissolved or suspended in a lipophilic or hydrophilic solvent, and the solution or suspension is dropped into the hydrophilic or lipophilic solvent and stirred to prepare a micelle of the drug conjugate. can do.
- the pharmaceutical composition of this embodiment may contain other components in addition to the compound (S).
- other components include pharmaceutically acceptable carriers.
- “Pharmaceutically acceptable carrier” means a carrier that does not inhibit the physiological activity of the active ingredient and does not exhibit substantial toxicity to the administration subject. By “not exhibiting substantial toxicity” is meant that the ingredient is not toxic to the administered subject at the doses normally used.
- the pharmaceutically acceptable carrier those commonly used in the pharmaceutical field can be used without particular limitation. For example, water, physiological saline, phosphate buffer, DMSO, dimethylacetamide, ethanol, glycerol, mineral An oil etc. can be mentioned.
- compositions of the present embodiment may contain an anticancer agent other than the compound (S) or an active ingredient other than the anticancer agent.
- active ingredients other than anticancer agents include, but are not limited to, anti-inflammatory agents, analgesics, antipyretic agents, anti-inflammatory agents, blood circulation promoters, stimulus-relaxing agents, antibiotics, and crude drugs.
- the compound (S) may be encapsulated or supported in known drug delivery particles such as liposomes and micelles. Encapsulation and loading in the drug delivery particles can be performed by a known method.
- the pharmaceutical composition of the present embodiment may also contain such drug delivery particles as an optional component.
- the pharmaceutical composition of this embodiment can be used for treating or preventing cancer resistant to an anticancer drug.
- Anticancer drug resistant cancer means a cancer that is resistant to one or more anticancer drugs.
- Anticancer agent means a compound used for the treatment or prevention of cancer and having an action of suppressing the growth of at least one kind of cancer cell.
- the anticancer agent having resistance to cancer is preferably an anticancer agent developed for the treatment of the cancer. More preferably, the anticancer agent is an anticancer agent approved for the treatment of the cancer. For example, it is an anticancer agent used for standard treatment of the cancer. Cancers that have acquired resistance to anticancer drugs used in such standard treatments are intractable due to ineffective drug therapy.
- the compound (S) exhibits high antitumor activity against cancer resistant to an anticancer drug. Therefore, the pharmaceutical composition of the present embodiment can be effectively used for treating or preventing cancer resistant to an anticancer drug. For example, if a cancer patient is treated with an existing anticancer drug and the effect of the anticancer drug cannot be obtained, or if the effect of the anticancer drug cannot be obtained
- the pharmaceutical composition in the form can be suitably used.
- the pharmaceutical composition of this embodiment can be suitably used as an anticancer agent used for cancer treatment after the second line.
- the pharmaceutical composition of this embodiment may be used for first-line cancer treatment.
- Whether or not a cancer is resistant to anticancer drugs is, for example, compared to the growth of cancer cells in the absence of anticancer drugs, the growth of cancer cells in the presence of anticancer drugs is suppressed. It can be determined by whether or not it is done. Compared with the growth rate of cancer cells in the absence of an anticancer agent, the growth inhibition rate of cancer cells in the presence of an anticancer agent is, for example, 50% or less, preferably 30% or less, more preferably 20%. Hereinafter, when the ratio is more preferably 10% or less, the cancer cell can be determined to be resistant to the anticancer agent.
- the growth rate is 2 times or more, preferably 5 times or more, more preferably 10 times or more, compared to the growth rate of cancer cells that are sensitive to anticancer agents. It can be determined that the drug is resistant to the anticancer drug. Alternatively, whether or not a cancer is resistant to an anticancer agent may be evaluated by, for example, the IC50 of the anticancer agent.
- the IC50 of the anticancer agent against cancer cells is, for example, 10 nM or more, preferably 20 nM or more, more preferably 30 nM or more, and even more preferably 50 nM or more, the cancer cells are against the anticancer agent. Can be determined to be resistant.
- the cancer cell is the anticancer agent.
- cancer resistant to an anticancer drug is a cancer refractory to an anticancer drug. That is, when an anticancer drug is administered to a cancer patient and the cancer patient's cancer does not shrink, the cancer grows, or the cancer growth rate is low, the cancer is It can be determined that the anticancer drug is resistant (refractory).
- the cancer to which the pharmaceutical composition of the present embodiment is applied is not particularly limited as long as it is an anticancer drug resistant cancer.
- cancer include lung cancer, pancreatic cancer, head and neck cancer, mesothelioma, neuroblastoma, liver cancer, malignant melanoma, uterine cancer, bladder cancer, biliary tract cancer, esophageal cancer, Examples include osteosarcoma, testicular tumor, thyroid cancer, acute myeloid leukemia, brain tumor, prostate cancer, squamous cell carcinoma of the head and neck, colon cancer, kidney cancer, ovarian cancer, and breast cancer.
- Preferred examples of cancer to which the pharmaceutical composition of this embodiment is applied include lung cancer, pancreatic cancer, head and neck cancer, and mesothelioma.
- anticancer drug-resistant cancer to which the pharmaceutical composition of the present embodiment can be applied are shown below.
- Cancer showing resistance to at least one anticancer drug selected from the group consisting of gefitinib, afatinib, osmeltinib, dasatinib, erlotinib, gemcitabine, cisplatin, pemetrexed, and midostaurin.
- Lung cancer particularly non-small cell lung cancer
- lung cancer that is resistant to osimertinib.
- lung cancer that is resistant to gefitinib, afitinib, and osimertinib.
- Pancreatic cancer having resistance to at least one anticancer agent selected from the group consisting of gemcitabine and erlotinib. More preferably, pancreatic cancer showing resistance to gemcitabine and erlotinib.
- Head and neck cancer that exhibits resistance to at least one anticancer drug selected from the group consisting of cisplatin, erlotinib, gefitinib, and midostaurin.
- a head and neck cancer resistant to cisplatin and erlotinib or a head and neck cancer resistant to erlotinib, gefitinib, and midostaurin.
- Examples of the molecular target drug include gefitinib, afatinib, osmeltinib, dasatinib, erlotinib, and midostaurin.
- Gatekeeper mutation refers to a mutation at a gatekeeper site.
- Gatekeeper site refers to the site of the amino acid residue located at the back of the ATP binding pocket of the kinase.
- deletion of amino acid residues for example, “d746-750” means deletion of amino acid residues at positions 746 to 750.
- deletion of amino acid residues is expressed as described above.
- the notation in which the one-letter code of amino acid is written on the left and right of the position number of the amino acid indicated by the number indicates the mutation of the amino acid residue of the position number indicated by the number, and the left side of the position number is the original amino acid residue.
- the right side of the position number indicates the amino acid residue after mutation.
- “T790M” indicates that the threonine (T) residue at position 790 is mutated to a methionine (M) residue.
- amino acid residue mutations are represented in the same manner as described above.
- a cancer that expresses at least one c-Kit selected from the group consisting of T670I and V559D (14) A cancer expressing FGFR1 having a V561M mutation. (15) A cancer expressing FGFR2 having a mutation of V564F. (16) A cancer expressing FGFR3 having a mutation of V565M. (17) A cancer expressing c-Src having a T341M mutation. (18) A cancer expressing PDGFRa having a mutation of T674I. (19) A cancer expressing RET having a mutation of V804L. (20) A cancer that expresses DDR2 having a mutation of T654M.
- a cancer that expresses a kinase having at least one mutation described in FIG. A cancer that expresses at least one of the kinases described in Table 3 below.
- AuroraA, AuroraB, CAMK2a, CAMK2d, CyclinC, CyclinA, CyclinA1, CHK1, DDR1, DYRK3, AK3, MEK3, MEK5, PDK1, PIM3, PKCmu, PKC1, and TRK are selected from TRB, TRK, and TRK.
- Cancers that express certain kinases. A cancer that expresses at least one kinase described in FIG.
- the cancer resistant to an anticancer drug preferably has at least one characteristic selected from the group consisting of (1) to (25) above, and from the group consisting of (1) to (25) above More preferably, it has a plurality of selected features.
- the dosage form of the pharmaceutical composition of the present embodiment is not particularly limited, and a known formulation method can be applied.
- Examples of the dosage form of the pharmaceutical composition of the present embodiment include emulsions, emulsions, liquids, gels, capsules, ointments, patches, patches, granules, tablets and the like. It is not limited.
- the administration route of the pharmaceutical composition of this embodiment is not particularly limited, and can be administered by oral or parenteral routes.
- the parenteral route includes all routes of administration other than oral, for example, intravenous, intramuscular, subcutaneous, intranasal, intradermal, ophthalmic, intracerebral, rectal, intravaginal and intraperitoneal. Further, the administration may be local administration or systemic administration.
- the pharmaceutical composition of the present embodiment can be administered in a single dose or multiple doses, and the administration period and interval are the type and condition of the disease, the administration route, the age of the administration subject, the body weight, the sex, etc. Can be selected as appropriate.
- the administration interval examples include, but are not limited to, 1 to 3 times a day, once every 2 to 3 days, 1 to 3 times a week, and once a day.
- the dosage of the pharmaceutical composition of this embodiment, its administration period and interval can be appropriately selected depending on the type of drug, the type and condition of the disease, the administration route, the age, weight and sex of the administration subject.
- the dosage of the pharmaceutical composition of the present embodiment can be a therapeutically effective amount of compound (S).
- “Therapeutically effective amount” means the amount of compound (S) effective for the treatment or prevention of cancer resistant to an anticancer drug.
- the dose of compound (S) per administration can be about 0.01 to 1000 mg, about 0.1 to 100 mg, about 0.5 to 50 mg, and about 1 to 10 mg per kg body weight.
- Rk 1 and Rk 2 are the same or different residues; (a) hydrogen, halogen, substituted or unsubstituted lower alkyl, substituted or unsubstituted lower alkenyl, substituted or unsubstituted lower alkynyl, hydroxy , Lower alkoxy, carboxy, lower alkoxycarbonyl, acyl, nitro, carbamoyl, lower alkylaminocarbonyl, —NR 5 R 6 [where R 5 and R 6 are hydrogen, substituted or unsubstituted lower alkyl, substituted or non-substituted, Substituted lower alkenyl, substituted or unsubstituted lower alkynyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, substituted or unsubstituted aralkyl, substituted or unsubstituted lower alkylaminocarbonyl, substituted or unsubstituted Lower aryla
- Rk 3 is hydrogen, halogen, acyl, carbamoyl, substituted or unsubstituted lower alkyl, substituted or unsubstituted alkenyl, substituted or unsubstituted lower alkynyl or amino;
- Wk 1 and Wk 2 are independently hydrogen, hydroxy, or Wk 1 and Wk 2 together represent oxygen.
- lower alkyl when used alone or in combination with other groups is 1 to 6 carbon atoms, preferably 1 to 5, more preferably 1 to 4 and particularly preferably 1 Means a linear or branched lower alkyl group containing ⁇ 3 or 1-2 carbon atoms.
- These groups include in particular methyl, ethyl, propyl, isopropyl, butyl, isobutyl, sec-butyl, tert-butyl, pentyl, amyl, isoamyl, neopentyl, 1-ethylpropyl, hexyl and the like.
- lower alkyl part of “lower alkoxy”, “lower alkoxycarbonyl”, “lower alkylaminocarbonyl”, “lower hydroxyalkyl” and “tri-lower alkylsilyl” has the same meaning as “lower alkyl” as defined above. .
- a “lower alkenyl” group is defined as a C 2 -C 6 alkenyl group, which may be straight or branched and may be Z or E type. Such groups include vinyl, propenyl, 1-butenyl, isobutenyl, 2-butenyl, 1-pentenyl, (Z) -2-pentenyl, (E) -2-pentenyl, (Z) -4-methyl-2 -Pentenyl, (E) -4-methyl-2-pentenyl, pentadienyl, such as 1,3 or 2,4-pentadienyl. More preferred C2-C6-alkenyl groups are C 2 -C 5- , C 2 -C 4 -alkenyl groups, even more preferred C 2 -C 3 -alkenyl groups.
- lower alkynyl refers to a C 2 -C 6 -alkynyl group which may be straight or branched and includes ethynyl, propynyl, 1-butynyl, 2-butynyl, 1-pentynyl, 2-pentynyl, 3 -Methyl-1-pentynyl, 3-pentynyl, 1-hexynyl, 2-hexynyl, 3-hexynyl and the like. More preferred C 2 -C 6 -alkynyl groups are C 2 -C 5- , C 2 -C 4 -alkynyl groups, even more preferred C 2 -C 3 -alkynyl groups.
- aryl group means a C 6 -C 14 -aryl group containing from 6 to 14 cyclic carbon atoms. These groups may be monocyclic, bicyclic, or tricyclic and are fused rings. Preferred aryl groups include phenyl, biphenyl, naphthyl, anthracenyl, phenanthrenyl and the like. The aryl part of the “arylcarbonyl” group and the “arylaminocarbonyl” group has the same meaning as defined above.
- heteroaryl group may contain 1 to 3 heteroatoms independently selected from nitrogen, sulfur or oxygen and refers to a C 3 -C 13 -heteroaryl group. These groups may be monocyclic, bicyclic or tricyclic.
- the C 3 -C 13 heteroaryl groups of the present invention include heteroaromatic and saturated and partially saturated heterocyclic groups. These heterocycles may be monocyclic, bicyclic, or tricyclic.
- Preferred 5- or 6-membered heterocyclic groups are thienyl, furyl, pyrrolyl, pyridyl, pyranyl, monophorinyl, pyrazinyl, methylpyrrolyl, and pyridazinyl.
- C 3 -C 13 -heteroaryl may be a bicyclic heterocyclic group.
- Preferred bicyclic heterocyclic groups are benzofuryl, benzothienyl, indolyl, imidazolyl, and pyrimidinyl.
- the most preferred C 3 -C 13 -heteroaryl is furyl and pyridyl.
- lower alkoxy includes alkoxy groups containing 1 to 6 carbon atoms, preferably 1 to 5, more preferably 1 to 4, particularly preferably 1 to 3 or 1 to 2 carbon atoms. And may be linear or branched. These groups include methoxy, ethoxy, propoxy, butoxy, isopropoxy, tert-butoxy, pentoxy, hexoxy and the like.
- acyl includes lower alkanoyl containing 1 to 6 carbon atoms, preferably 1 to 5, 1 to 4, 1 to 3 or 1 to 2 carbon atoms, linear or It may be a branch. These groups preferably include formyl, acetyl, propionyl, butyryl, isobutyryl, tert-butyryl, pentanoyl and hexanoyl.
- the acyl part of the “acyloxy” group has the same meaning as defined above.
- halogen includes fluoro, chloro, bromo, iodo and the like.
- aralkyl group refers to a C 7 -C 15 -aralkyl in which the alkyl group is substituted by aryl.
- the alkyl group and aryl can be selected from C 1 -C 6 -alkyl groups and C 6 -C 14 -aryl groups as defined above, wherein the total number of carbon atoms is from 7 to 15.
- Preferred C 7 -C 15 -aralkyl groups are benzyl, phenylethyl, phenylpropyl, phenylisopropyl, phenylbutyl, diphenylmethyl, 1,1-diphenylethyl, 1,2-diphenylethyl.
- the aralkyl part of the “aralkyloxy” group has the same meaning as defined above.
- Substituted lower alkyl group, substituted lower alkenyl group, and substituted lower alkynyl group are lower alkyl, hydroxy, lower alkoxy, carboxyl, lower alkoxycarbonyl, nitro, halogen, amino, mono- or di-lower alkylamino, dioxolane, dioxane, dithiolane, And having 1 to 3 independently selected substituents such as dithione.
- the lower alkyl portion of the lower alkylamino substituent has the same meaning as "lower alkyl" defined above.
- the substituted aryl group, substituted heteroaryl group and substituted aralkyl group are each independently selected from lower alkyl, hydroxy, lower alkoxy, carboxy, lower alkoxycarbonyl, nitro, amino, mono- or di-lower alkylamino, and halogen, etc. It has 1 to 3 substituents.
- Heterocyclic groups formed by R 5 and R 6 bonded to a nitrogen atom include pyrrolidinyl, piperidinyl, piperidino, morpholinyl, morpholino, thiomorpholino, N-methylpiperazinyl, indolyl, and isoindolyl.
- the ⁇ -amino acid group includes glycine, alanine, proline, glutamic acid and lysine, and may be L-type, D-type or racemic.
- Rk 1 and Rk 2 are hydrogen, halogen, nitro, —CH 2 OH, — (CH 2 ) k R 14 , —CH ⁇ CH (CH 2 ) m R 16 , —C ⁇ C (CH 2 ) n R 15 , —CO (CH 2 ) j R 4 (where R 4 is —SR 7 ), CH 2 O— (substituted or unsubstituted) lower alkyl (where substituted lower alkyl is preferably Is independently selected from the group consisting of —NR 5 R 6 . More preferably, Rk 1 and Rk 2 are hydrogen.
- residue R 14 is preferably phenyl, pyridyl, imidazolyl, thiazolyl, tetrazolyl, —COOR 15 , —OR 15 (where R 15 is preferably hydrogen, Selected from methyl, ethyl, phenyl or acyl), —SR 7 (wherein R 7 is preferably selected from substituted or unsubstituted lower alkyl, 2-thiazoline and pyridyl) and —NR 5 R 6 wherein R 5 and R 6 are preferably selected from hydrogen, methyl, ethyl, phenyl, carbamoyl and lower alkylaminocarbonyl.
- residue R 16 is preferably hydrogen, methyl, ethyl, phenyl, imidazole, thiazole, tetrazole, —COOR 15 , —OR 15 and —NR 5 R 6 (wherein residues R 15 , R 5 and R 6 is selected from the above preferred meanings).
- residue R 7 is preferably selected from the group consisting of substituted or unsubstituted lower alkyl, substituted or unsubstituted phenyl, pyridyl, pyrimidinyl, thiazole and tetrazole.
- k is preferably 2, 3 or 4
- j is preferably 1 or 2
- m1 and n are independently preferably 0 or 1.
- Rk 3 is hydrogen or acetyl, most preferably hydrogen.
- each Wk 1 and Wk 2 is hydrogen.
- the compound (K) is preferably represented by the following formula (k1-1).
- Compound (K) can be produced, for example, by refluxing a compound represented by the following general formula (k0) without a solvent in the presence of anhydrous hydrazine or hydrazine hydrate. Further, for example, a compound represented by the following general formula (k0) is converted into methanol, ethanol, propanol, isopropanol, butanol, tert-butanol, N, N-dimethylformamide (DMF), dimethyl sulfoxide (DMSO), tetrahydrofuran (THF).
- DMF N-dimethylformamide
- DMSO dimethyl sulfoxide
- THF tetrahydrofuran
- reaction temperature and reaction time are not particularly limited.
- the reaction can be performed at room temperature to 50 ° C. for 30 minutes to 15 hours.
- the second aspect of the present invention is a drug complex containing a polymer having a repeating unit (ka) represented by the following general formula (ka) and a repeating unit (II) represented by the following general formula (II) (Hereinafter sometimes referred to as “drug complex (K)”).
- m is preferably 1.
- L is preferably a methylene group, an ethylene group, a propylene group or a benzylene group, and more preferably a methylene group, an ethylene group or a benzylene group.
- R 1 is preferably a hydrogen atom or an aliphatic hydrocarbon group, and more preferably a hydrogen atom or a methyl group.
- Rk 1 and Rk 2 are each independently hydrogen, halogen, nitro, —CH 2 OH, — (CH 2 ) k R 14 , —CH ⁇ CH (CH 2 ) m R 16 , —C ⁇ C (CH 2 ) n R 15 , —CO (CH 2 ) j R 4 (where R 4 is —SR 7 ), CH 2 O— (substituted or unsubstituted) lower alkyl (here The substituted lower alkyl is preferably methoxymethyl, methoxyethyl or ethoxymethyl) or —NR 5 R 6 , more preferably hydrogen.
- Rk 3 is preferably hydrogen or acetyl, and more preferably hydrogen.
- Wk 1 and Wk 2 are preferably hydrogen.
- m is preferably 1.
- X is preferably OR x and more preferably OH (hydroxy group).
- the drug complex (K) contains a polymer having a repeating unit (ka-1) represented by the following general formula (ka-1) and a repeating unit (II) represented by the following general formula (II) It is preferable to do.
- m is preferably 1.
- L is preferably a methylene group, an ethylene group, a propylene group or a benzylene group, more preferably a methylene group, an ethylene group or a benzylene group.
- R 1 is preferably a hydrogen atom or an aliphatic hydrocarbon group, more preferably a hydrogen atom or a methyl group.
- X is preferably OR x and more preferably OH (hydroxy group).
- Compound (K) or drug conjugate (K) shows efficacy against malignant cancer and metastatic cancer for which existing cancer therapeutic agents do not show efficacy. Therefore, the compound (K) and the drug complex (K) are useful as cancer therapeutic agents that are resistant to existing cancer therapeutic agents.
- the sustained release property of the compound (K) can be controlled similarly to the drug complex of the first aspect. While the compound (K) is held in the polymer, the compound (K) is stably maintained and the toxicity is alleviated. Therefore, side effects can be reduced and the therapeutic effect can be enhanced. Further, by preparing micelles containing the drug complex (K), the compound (K) from the drug complex (K) depends on the in vivo pH environment, as in the micelle of the first aspect. The release of can be controlled.
- the micelle containing the drug complex (K) can be prepared by a known method in the same manner as the drug complex micelle of the first aspect.
- the drug conjugate (K) is dissolved or suspended in a lipophilic or hydrophilic solvent, and the solution or suspension is dropped into the hydrophilic or lipophilic solvent and stirred, whereby the drug conjugate ( Micelle containing K) can be prepared.
- the drug conjugate (K) is an embodiment in which the drug is the compound (K) in the drug conjugate of the first aspect, and is included in the drug conjugate of the first aspect. It can also be said that the drug conjugate (K) is an embodiment in which BM is a group derived from the compound (K) in the general formula (Ia) in the first embodiment.
- the compound (K) and the drug complex (K) can be used as the compound (S) and the drug complex in the pharmaceutical composition of the first aspect, respectively.
- the present invention provides a compound (S) or a pharmaceutically acceptable salt thereof, or a pharmaceutically acceptable salt in the manufacture of a pharmaceutical composition for treating or preventing cancer resistant to an anticancer drug.
- the present invention provides a compound (S) or a pharmaceutically acceptable salt thereof, or a drug in which the compound (S) or a pharmaceutically acceptable salt thereof is bound to a pharmaceutically acceptable polymer.
- a method for treating or preventing cancer resistant to an anticancer drug comprising administering the complex to a subject (for example, a patient suffering from cancer resistant to an anticancer drug).
- the present invention relates to a compound (S) or a pharmaceutically acceptable salt thereof, or a pharmaceutically acceptable polymer for use in the treatment or prevention of cancer resistant to an anticancer drug.
- a drug conjugate to which compound (S) or a pharmaceutically acceptable salt thereof is bound is bound.
- the present invention provides compound (S) or a pharmaceutically acceptable salt thereof, or a pharmaceutically acceptable polymer for treating or preventing anticancer drug-resistant cancer, S) or the use of a drug conjugate conjugated with a pharmaceutically acceptable salt thereof.
- K252a-hydrazide K252a-H
- K252a was purchased from BOC Science (US).
- K252a (20 mg) was dissolved in anhydrous methanol (200 ⁇ L) and added to anhydrous hydrazine (300 ⁇ L).
- the reaction mixture was stirred at 40 ° C. for 15 hours.
- the reaction mixture was evaporated to give a dry product.
- toluene was used as the co-evaporation solvent.
- the resulting product was used for the preparation of drug conjugates and micelles without further purification.
- FIG. 8 shows the 1 H-NMR analysis result of the obtained product.
- K252a-H was analyzed by HPLC. The analysis results are shown in FIG. Since K252a-H and K252a have different retention times, it was confirmed that they have different structures.
- the conditions for HPLC analysis are as follows. TSK-GEL ODS-100V column 4.6 ⁇ 150mm, particle size 5 ⁇ m (Tosoh Corporation) Column pressure: 10.7 MPa Column temperature: constant temperature around 40 ° C. Mobile phase: methanol / formate buffer (pH 3.0) mixture (3: 2) Flow rate: 1.2 mL / min, 20-30 minutes UV detection: wavelength 290 nm
- Example 2 Preparation of drug complex of K252a-H and polymer
- Aromatic aldehyde group-containing polymer or aliphatic ketone group-containing polymer and K252a-H were mixed at a mass ratio of 2: 1 and dissolved in DMSO. (Dissolve 15 mg drug / polymer mixture per 1 mL DMSO).
- the coupling reaction between the aromatic aldehyde group-containing polymer and K252a-H was carried out by stirring the reaction mixture at 40 ° C. overnight.
- the coupling reaction between the aliphatic ketone group-containing polymer and K252a-H was performed by stirring the reaction mixture for 96 hours.
- FIG. 10 shows the result of 1 H-NMR analysis confirming the bond between the aliphatic ketone group-containing polymer and K252a-H.
- FIG. 11 shows the results of 1 H-NMR analysis confirming the bond between the aliphatic aldehyde group-containing polymer and K252a-H.
- Example 3 Preparation of K252a-H bonded polymer micelle (Preparation of micelle) The reaction mixture in DMSO was dialyzed against dimethylacetamide (DMAc), and a solvent exchange from DMSO to DMAc was performed (dialysis for 4 hours, dialysis solvent was changed once). In addition, free K252a-H was removed by dialysis. A DMAc solution of the drug complex of polymer and K252a-H obtained as described above was used for the preparation of micelles. The DMAc solution was dropped into water at a ratio of 1 to 10 by volume and vortexed to prepare micelles.
- DMAc dimethylacetamide
- This solution was dialyzed against water for 24 hours in a dialysis bag with a molecular weight cut off (MWCO) of 3500 Da.
- the dialysis medium was changed 5 times during dialysis.
- the solution in the dialysis bag was filtered (0.22 ⁇ m) and concentrated by ultrafiltration using a 100 kDa MWCO filter membrane (Amicon).
- the micelle of the drug complex of K252a-H and the above polymer is sometimes referred to as “K252a-H-bonded polymer micelle”.
- FIGS. 12A and 12B The results are shown in FIGS. 12A and 12B.
- FIG. 12A is a drug complex micelle of an aromatic aldehyde group-containing polymer and K252a-H (hereinafter referred to as “K252a-H-bonded aromatic polymer micelle”)
- FIG. 12B is an aliphatic ketone group-containing polymer.
- K252a-H-linked aliphatic polymer micelle a drug complex micelle of K252a-H (hereinafter referred to as “K252a-H-linked aliphatic polymer micelle”). It was confirmed that the K252a-H-bonded aliphatic polymer micelle has a smaller micelle size than the K252a-H-bonded aromatic polymer micelle. Therefore, K252a-H-bonded aliphatic polymer micelles are expected to have higher cancer accumulation properties than K252a-H-bonded aromatic polymer micelles.
- Example 5 Release of K252a-H from K252a-H-bonded polymer micelles
- K252a-H K252a-H-bonded aromatic polymer micelles and K252a-H-bonded aliphatic polymer micelles
- K252a-H K252a-H-bonded aromatic polymer micelles
- K252a-H-bonded aliphatic polymer micelles Collectively referred to as “K252a”.
- the cell line used was American type. Purchased from Culture Collection (American Type Culture Collection, ATCC) or JCRB Cell Bank (National Research Institute for Pharmaceuticals, Health and Nutrition). PC14 PE6 was distributed by Prof. Hosho (Tottori University). Unless otherwise stated, DMEM, RPMI or E-MEM was used as the medium. Gefetinib, Afatinib, Erlotinib, Cisplatin, Pemetrexed, and Gemicitabine were purchased from Funakoshi Corporation. Osimertinib was purchased from Selleck Chemicals.
- 50 ⁇ L of the drug solution prepared above was added in order of increasing micelle concentration from row 3 to row 12 of a 96-well plate seeded with cancer cells.
- 50 ⁇ L of medium alone was added. Thereafter, it was incubated for 48 hours. After 48 hours, 10 ⁇ L of cell-counting kit-8 solution (DOJINDO) was added to each well. After incubating again, the absorbance at 450 nm was measured after 30 minutes, 1 hour, and 2 hours.
- results are shown in FIG. Gefenitib, Afatinib, and Osimetinib are molecularly targeted therapeutics for lung cancer that are currently approved by the FDA.
- the results shown in FIG. 15 indicate that K252a species (particularly, K252a, K252a-H, and K252a-H-linked aliphatic polymer micelles) exhibit high cytotoxic activity against lung cancer resistant to existing lung cancer therapeutic agents. It was done.
- FIG. 16 shows the extracted PC14 strain and PC14 / PE6 strain in FIG.
- the PC14 / PE6 strain is a cancer cell line obtained by administering the PC14 strain to the tail vein of mice and establishing cancer cells that have metastasized in the thoracic cavity (Yano et al., Oncology Research 9: 573-579 (1997)). Therefore, the PC14 / PE6 strain is a cancer cell line that has extremely high metastatic potential and is malignant. As shown in FIGS. 15 and 16, Gefenitib, Afatinib, and Osimetinib showed high cytotoxic activity against the PC14 strain. However, for existing malignant PC14 / PE6 strains, these existing molecular targeted drugs have significantly reduced cytotoxic activity.
- K252a compounds (particularly K252a-H and K252a-H-linked aliphatic polymer micelles) showed higher cytotoxic activity against the PC14 / PE6 strain. These results indicate that K252a is more effective against malignant metastatic cancer. Of the K252a-H-bonded aromatic polymer micelles and K252a-H-bonded aliphatic polymer micelles, K252a-H-bonded aliphatic polymer micelles tended to have higher cytotoxic activity against cancer cells.
- Example 7 Cytotoxicity test of K252a against pancreatic cancer cells Using various pancreatic cancer cell lines, the cytotoxicity test of K252a was performed in the same manner as described above. The results are shown in FIG.
- the BXPC3-F3 strain is a metastatic strain that has been malignant by repeating the establishment of a cancer cell line that has metastasized to the liver three times after orthotopic transplantation of the BXPC3 strain to mice.
- KP-3L is a metastatic strain established after transplanting human liver metastatic pancreatic cancer into the spleen of a nude mouse and then establishing a cancer cell line that has metastasized to the liver (JCRB, National Institute of Biomedical Innovation, Health and Nutrition) Obtained from cell bank).
- K252a-H-bonded aromatic polymer micelles K252a-H-bonded aliphatic polymer micelles tended to have higher cytotoxic activity against cancer cells.
- Example 8 Cytotoxicity test of K252a against head and neck cancer cells Using various head and neck cancer cell lines, the cytotoxicity test of K252a was performed in the same manner as described above. The results are shown in FIG. CDDP indicates cisplatin, which is a standard treatment for head and neck cancer.
- the SAS strain, FaDu strain, and HSC2 strain are all cisplatin resistant cell lines.
- K252a showed higher cytotoxic activity than any of cisplatin and Erlotinib against any head and neck cancer cell line.
- the FaDu strain the cytotoxic activity of K252a-H-linked aliphatic polymer micelles was high. This result shows that K252a is effective against a cisplatin resistant strain.
- Example 9 Cytotoxicity test of K252a against malignant mesothelioma cells Using the malignant mesothelioma cell line MSTO-211H, a cytotoxicity test of K252a was performed in the same manner as described above. The results are shown in FIG. CDDP indicates cisplatin and is a standard treatment for malignant mesothelioma. Pemetrexed is also a standard treatment for malignant mesothelioma. As shown in FIG. 19, K252a family showed higher cytotoxic activity than cisplatin, Pemetrexed and Midostaurin.
- Hot spot kinase profiling of K252a-H was carried out using the Monthly 202 mutual kinase panel consigned to Rection Biology.
- the conditions for the kinase assay are as follows. K252a was dissolved in DMSO and assayed at 100 nM. Buffer conditions: 20 mM HEPES, pH 7.5, 10 mM MgCl 2 , 1 mM EGTA, (2 mM MnCl 2 if applicable) ATP concentration: 10 ⁇ M Reaction time: 2 hours
- FIGS. 20 and 21 For reference, the first generation, second generation, and third generation tyrosine kinase inhibitors (TKI) and their resistance mutations are shown in FIGS. 20 and 21.
- FIG. 20 and 21 since the first to third generation TKIs are not effective for the C797S mutation, a TKI effective for the C797S mutation is required.
- K252a-H The results of the K252a-H kinase assay for the EGFR mutation are shown in FIG. As shown in FIG. 22, K252a-H is against kinases with gatekeeper mutations (d746-750 / T790M, d746-750 / T790M / C797S, L858R, T790M, etc.) that many TKIs do not show efficacy. In particular, it showed high inhibitory activity.
- Figures 23A-C show the results of kinase profiling of K252a-H.
- FIG. 22 The results of the K252a-H kinase assay for the EGFR mutation are shown in FIG. As shown in FIG. 22, K252a-H is against kinases with gatekeeper mutations (d746-750 / T790M, d746-750 / T790M / C797S, L858R, T790M, etc.) that many TKIs do
- K252a-H shows a list of mutant kinases whose kinase activity was suppressed to 30% or less by K252a-H as a result of kinase profiling for K252a-H.
- K252a-H was confirmed to be a potent inhibitor of the oncogene c-kit / Flt3 / RET.
- FIGS. 25 to 34D The results of kinase profiling using a Monthly Mutant Kinase panel are shown in FIGS. 25 to 34D.
- the kinase surrounded by a solid line has a mutation involved in drug resistance and shows a high inhibitory activity by the tested drug.
- These mutations are summarized in Table 2.
- Staurosporine and K252a were shown to have high inhibitory activity of kinases having the mutations listed in Table 2.
- staurosporine and K252a were shown to have a high inhibitory effect on the triple mutation of EGFR in oximeltinib resistance (d746-750 / T790M / C797S, L858R / T790M / C797S).
- HER2 gatekeeper mutation T798I
- HER2 is an EGFR homologue, and thus was inhibited by staurosporine and K252a in the same manner as the EGFR gatekeeper mutation.
- Figures 33A-D show the results of kinase profiling with 500 nM K252a-H.
- Figures 34A-D show the results of kinase profiling with 20 nM staurosporine.
- the IC50 of K252a-H for each wild type kinase was determined from the results of kinase profiling using the monthly virus type kinase panel. As a result, kinases having an IC50 of K252a-H of 5 nM or less are shown in Table 3. In Table 3, the underlined kinase is a kinase involved in drug resistance.
- IC50 of staurosporine with respect to each wild type kinase was determined from the result of kinase profiling using a monthly wild type kinase panel.
- the kinase in which IC50 of staurosporine was 0.2 nM or less is shown in FIG.
- the underlined kinase is a kinase that is important as a therapeutic target for cancer (particularly drug-resistant cancer).
- Staurosporine showed an especially high inhibitory effect with an IC50 of 0.1 nM or less for CaMK2a, CaMK2b, JAK3, ROS1, RSK4, STK22, and TRKB.
- BxPC3-F3 is a liver metastasis strain in which BxPC3 is malignant.
- BxPC3-F3 was prepared by isotopically transplanting BxPC3 into the pancreas of mice, isolating liver metastasized cells from the liver by trypsin treatment, establishing a cell line, and repeating the work (cell line establishment) three times. did.
- Anticancer agents (Gefetinib, Afetinib, Osimmertinib, Gemicitabine, Cisplatin, Erlotinib, Pemetrexed, Midostaurin) were purchased from Funakoshi.
- the cytotoxicity of the test drug was measured by the following method using cell counting kit 8 (DOJINDO, JAPAN). Cancer cell lines were seeded at 1 to 3 ⁇ 10 3 cells / well. The next day, the medium was changed and 50 ⁇ L of medium was added. Furthermore, a dilution series of the test drug was prepared, and 50 ⁇ L was added to the well and stirred. 72 hours later, 10 ⁇ L of cell counting kit 8 was added, and after 1 hour, absorbance at 450 nm was measured with a microplate reader (TECAN). Based on the measured absorbance, the cell viability at each dilution rate was calculated for each test drug by the following formula (1). Based on the calculated cell viability, the IC50 of the test drug was calculated.
- Non-small cell lung cancer The results of the cytotoxicity test for non-small cell lung cancer cell lines are shown in FIG. The characteristics of each cell line are as shown in Table 1 above.
- Known lung cancer therapies (Gefetinib, Afatinib, Osimertinib, Dasatinib) showed high cytotoxicity against PC14, while Gefetinib, Afatinib, and Osimertinib are against PC14-PE6, a malignant metastatic strain of PC14. The cytotoxicity was low.
- staurosporine, K252a, and K252a-H showed a higher effect on PC14-PE6 than on PC14.
- PC14 was sensitive to known lung cancer therapeutics (Gefetinib, Afatinib, Osimmertinib, Dasatinib), while PC14-PE6, A549, H358, NCI1650, and H520 were resistant to known lung cancer therapeutics. .
- staurosporine, K252a, and K252a-H tended to show high cytotoxicity against these lung cancer therapeutic drug resistant strains.
- pancreatic cancer The result of the cytotoxicity test for the pancreatic cancer cell line is shown in FIG. In FIG. 37, BxPC3-F3 is a malignant liver metastasis strain of BxPC3. KP3L is a metastatic strain that has metastasized to the liver of a mouse after transplanting human liver metastatic pancreatic cancer into the spleen of a nude mouse. Standard treatments for pancreatic cancer (Gemicitabine, Erlotinib) showed high cytotoxicity against BxPC3, but low cytotoxicity against BxPC3-F3 and KP3L. On the other hand, staurosporine, K252a, and K252a-H also showed high cytotoxicity against these pancreatic cancer therapeutic drug resistant strains.
- FIG. 38 The results of the cytotoxicity test against pancreatic cancer cell lines are shown in FIG. In FIG. 38, “CDDP” represents cisplatin.
- staurosporine, K252a, and K252a-H showed higher cytotoxicity than known head and neck cancer therapeutic agents (Midostaurin, cisplatin, Erlotinib, Gefetinib).
- staurosporine, K252a, and K252a-H also showed high cytotoxicity against SAS, which is a Midostaurin resistant strain.
- FIG. 39 The results of the cytotoxicity test on the malignant mesothelioma cell line are shown in FIG. In FIG. 39, “CDDP” represents cisplatin. Staurosporine, K252a, and K252a-H showed higher cytotoxicity than known malignant mesothelioma therapeutics (Midostaurin, cisplatin, Pemetrexed).
- mice In vivo antitumor activity of K252a-H (non-small cell lung cancer) Balb / c nude mice were anesthetized with isoflurane, and 5 ⁇ 10 5 cells / 20% Matrigel 50 ⁇ L of non-small cell lung cancer cell line (PC14-PE6-luc: cells into which luciferase gene was introduced into PC14-PE6) were directly added to the mice. Injected into the lungs. Mark 5mm from the tip of the injection needle, insert the injection needle from the 3rd to 4th position of the rib toward the lower lobe of the ribs, and suspend 20% Matrigel in non-small cell lung cancer cell line 50 ⁇ L of the solution was injected directly into the lung.
- PC14-PE6-luc cells into which luciferase gene was introduced into PC14-PE6
- a human non-small cell lung cancer orthotopic transplant model mouse was prepared. From 5 days after the cancer cell injection, the drug was injected into the human non-small cell lung cancer orthotopic transplantation mouse at a dose of 1 mg / kg body weight twice a week. Twice a week under isoflurane anesthesia, IVIS spectrum (Xwogen Corporation) was used to monitor tumor growth and evaluate the anti-tumor effect of the drug. Statistical processing of tumor size and survival days was performed using Prism7. K252a-H and K252a-H-linked aliphatic polymer micelles (prepared in Example 3) were used as test agents. PBS was used as a negative control.
- FIG. 40 shows tumor growth
- FIG. 41 shows the survival curve of mice by the Kaplan-Meier method.
- K252a-H-linked aliphatic polymer micelles were administered, tumor growth was significantly suppressed and survival time was significantly prolonged compared with PBS administration.
- K252a-H was administered, the survival time tended to be longer than when PBS was administered. From these results, it was confirmed that K252a (especially, K252a-H-bonded aliphatic polymer micelle) exhibits an antitumor effect even in vivo against osimertinib-resistant lung cancer.
- K252a-H, K252a-H linked aliphatic polymer micelles, K252a-H linked aromatic polymer micelles (prepared in Example 3), and gemcitabine were used.
- PBS was used as a negative control.
- the dose per dose of each drug is 3 mg / kg body weight for K252a-H, K252a-H linked aliphatic polymer micelles, and K252a-H linked aromatic polymer micelles, and 5 mg / kg body weight for gemcitabine. It was.
- pancreatic cancer Trial using pancreatic cancer spontaneous mice
- Transgenic mice that spontaneously develop pancreatic cancer Capiller corp
- pancreatic cancer spontaneously developing model mice EL-1-luc / TAg
- the drug administration was started from 13 to 15 weeks of age when pancreatic cancer occurred.
- the drug administration method was the same as the method described in “(Non-small cell lung cancer)”.
- drugs K252a-H-linked aliphatic polymer micelles and K252a-H-linked aromatic polymer micelles were used.
- PBS was used as a negative control.
- the dose per dose of each drug was 1 mg / kg body weight.
- FIG. 43 shows IC50 of staurosporine and K252a family for spontaneous pancreatic cancer EL1-luc / TAg cells taken from mouse pancreas and cell line. IC50 was calculated by the same method as described in Example 12. As shown in FIG. 43, EL-1 / TAg is resistant to gemcitabine and erlotinib. On the other hand, EL-1 / TAg was sensitive to staurosporine and K252a.
- FIG. 44 and 45 are diagrams showing the results of an antitumor effect evaluation test using a pancreatic cancer spontaneous model mouse.
- FIG. 44 shows tumor growth
- FIG. 45 shows the survival curve of mice by Kaplan-Meier method.
- K252a-H-bonded aliphatic polymer micelle or K252a-H-bonded aliphatic polymer micelle was administered, tumor growth was significantly suppressed as compared to PBS.
- the K252a-H-linked aliphatic polymer micelle was administered, the survival time was significantly prolonged as compared with the case where PBS was administered. Even when K252a-H-bonded aromatic polymer micelle was administered, the survival time tended to be longer than when PBS was administered. From these results, it was confirmed that K252a species (particularly, K252a-H-linked aliphatic polymer micelles) exhibited an antitumor effect in vivo against gemcitabine and erlotinib-resistant pancreatic cancer.
- Example 14 Cytotoxicity of K252a class to lymphoma
- the IC50 of K252a class to lymphoma cell line was calculated. The results are shown in FIG. Also for lymphoma cell lines, K252a species (particularly K252a, K252a-H, K252a-H-linked aliphatic polymer micelles) showed high cytotoxicity.
- Example 15 Drug transport inhibitory effect of ABC transporter (MDR-1) Human renal cancer cell line 786-0 is a cell that highly expresses MDR-1 (ABCB-1, p-gp) Strain (data not shown). Therefore, 786-0 was used to evaluate the effects of staurosporine and K252a on the drug excretion activity of MDR-1. The drug excretion activity was evaluated using an eFluxx-ID TM Green Multidrug Resistance Assay kit (Enzo Life Science). After culturing 786-0 cells in a well plate, trypsin / EDTA solution was added and incubated, and the cells were detached from the wells.
- the detached cells were collected and washed with the medium, and then the cells were suspended in the medium at a concentration of 1 ⁇ 10 6 cells / mL to obtain a cell suspension.
- the cell suspension was pre-warmed at 37 ° C. for 10 minutes or more, and a dilution series of drug was added to 250 ⁇ L of the cell suspension (2.5 ⁇ 10 5 cells).
- Staurosporine, K252a, K252a-H, and sunitinib were used as drugs.
- Verapamil final concentration 30 ⁇ M
- an MDR-1 inhibitor was used as a positive control.
- LD50 was calculated as a drug concentration at which the amount of eFluxx-GFP excretion was reduced to half.
- Example 16 Influence of human ⁇ 1-AGP
- human ⁇ 1-AGP acid glycoprotein
- human ⁇ 1-AGP human serum protein
- the cellular attributes of K252a-H and K252a-H linked aliphatic polymer micelles were evaluated. Cytotoxicity was evaluated using cell counting kit 8 (DOJINDO, JAPAN).
- DOJINDO cell counting kit 8
- the lung cancer cell line H460 was seeded in the wells at 1 to 3 ⁇ 10 3 cells / well and cultured. The next day, the medium was changed and hAGP was added to a final concentration of 0.5 mg / mL.
- a medium supplemented with 50 ⁇ L of hAGP-free medium was also prepared.
- a drug K252a-H or K252a-H-linked aliphatic polymer micelle
- a concentration of 1 ⁇ M was added to a concentration of 1 ⁇ M.
- 10 ⁇ L of cell counting kit 8 was added.
- the absorbance at 450 nm was measured with a microplate reader (TECAN). Based on the measured absorbance, the cell viability was calculated by the above formula (1).
- a linker reagent (L1) represented by the following formula (L1) was bound to staurosporine.
- a scheme of the linker binding reaction is shown in FIG. In FIG. 49, “DIC” represents diisopropylcarbodiimide.
- the activated esters in FIG. 49 include N-hydroxysuccinimide (NHS), 1-hydroxybenzotriazole (HOBt), 1-hydroxy-7-azabenzotriazole (HOAt), pentafluorophenol, p-nitrophenol. And active esters derived from these.
- FIG. 50 shows the 1 H NMR analysis result of the organic phase component.
- Staurosporine to which an active ester linker is bound can be bound to a polymer such as polyethylene glycol (PEG) to form a drug complex with the polymer.
- PEG polyethylene glycol
- the upper diagram of FIG. 51 shows a reaction in which polyethylene glycol is bonded to staurosporine via a linker.
- the lower diagram of FIG. 51 shows a reaction in which a PEG-polyamino acid block copolymer is bound to staurosporine via a linker. (Bottom view of FIG. 51).
- the polymer-linker-staurosporine complex reacts with glutathione (GSH) by glutathione transferase (GST) in the cell, and staurosporine is released.
- GSH glutathione
- GST glutathione transferase
- the compound (S′L1-H) can be bound to the polymers synthesized in Polymer Synthesis Examples 1 to 5.
- Examples of drug conjugates in which the compound (S′L1-H) is bound to an aromatic aldehyde group-containing polymer are shown below.
- bond (I) represents a pH-sensitive bond.
- Binding (II) represents a GSH sensitive binding that reacts with GSH in the presence of GST.
- the bond (I) is first cleaved under a low pH environment around the tumor tissue, and the compound (S′L1-H) becomes Released. Next, the compound (S′L1-H) is taken up into the tumor cell, and reacts with GSH by GST, whereby the bond (II) is cleaved to produce staurosporine. The staurosporine kills tumor cells.
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Veterinary Medicine (AREA)
- Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Epidemiology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Molecular Biology (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Organic Chemistry (AREA)
- Inorganic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicinal Preparation (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2018-030208 | 2018-02-22 | ||
| JP2018030208A JP7223502B6 (ja) | 2018-02-22 | 2018-02-22 | 医薬組成物 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2019163943A1 true WO2019163943A1 (ja) | 2019-08-29 |
Family
ID=67688423
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2019/006780 Ceased WO2019163943A1 (ja) | 2018-02-22 | 2019-02-22 | 医薬組成物 |
Country Status (2)
| Country | Link |
|---|---|
| JP (1) | JP7223502B6 (enExample) |
| WO (1) | WO2019163943A1 (enExample) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN111303165A (zh) * | 2019-09-19 | 2020-06-19 | 杭州科兴生物化工有限公司 | 一类星孢菌素类衍生物及其制备方法和应用 |
| WO2024156250A1 (zh) * | 2023-01-29 | 2024-08-02 | 杭州科兴生物化工有限公司 | 7-羰基星孢菌素衍生物及其制备方法和应用 |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH10501526A (ja) * | 1994-06-01 | 1998-02-10 | チバ−ガイギー アクチェンゲゼルシャフト | 多薬物耐性に対する作用物質としてのカルバゾール誘導体 |
| JP2009506000A (ja) * | 2005-08-25 | 2009-02-12 | クレアビリス・セラピューティクス・エスピーエー | K‐252aおよびその誘導体のポリマー結合体 |
| WO2016024595A1 (ja) * | 2014-08-11 | 2016-02-18 | 国立大学法人東京大学 | エピルビシン複合化ブロック共重合体と、抗癌剤とを含むミセル、及び当該ミセルを含む癌又は耐性癌、転移癌の治療に適用可能な医薬組成物 |
| WO2018038165A1 (ja) * | 2016-08-23 | 2018-03-01 | 公益財団法人川崎市産業振興財団 | ポリマー、ポリマーの製造方法、及び薬物複合体 |
-
2018
- 2018-02-22 JP JP2018030208A patent/JP7223502B6/ja active Active
-
2019
- 2019-02-22 WO PCT/JP2019/006780 patent/WO2019163943A1/ja not_active Ceased
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH10501526A (ja) * | 1994-06-01 | 1998-02-10 | チバ−ガイギー アクチェンゲゼルシャフト | 多薬物耐性に対する作用物質としてのカルバゾール誘導体 |
| JP2009506000A (ja) * | 2005-08-25 | 2009-02-12 | クレアビリス・セラピューティクス・エスピーエー | K‐252aおよびその誘導体のポリマー結合体 |
| WO2016024595A1 (ja) * | 2014-08-11 | 2016-02-18 | 国立大学法人東京大学 | エピルビシン複合化ブロック共重合体と、抗癌剤とを含むミセル、及び当該ミセルを含む癌又は耐性癌、転移癌の治療に適用可能な医薬組成物 |
| WO2018038165A1 (ja) * | 2016-08-23 | 2018-03-01 | 公益財団法人川崎市産業振興財団 | ポリマー、ポリマーの製造方法、及び薬物複合体 |
Non-Patent Citations (10)
| Title |
|---|
| CONSEIL, G. ET AL.: "Protein Kinase C Effectors Bind to Mul tidrug ABC Transporters and Inhibit Their Activity", BIOCHEMISTRY, vol. 40, no. 8, 2001, pages 2564 - 2571, XP055633336, DOI: 10.1021/bi002453m * |
| FESTUCCIA, C. ET AL.: "Uncoupling of the epidermal growth factor receptor from downstream signal transduction molecules guides the acquired resistance to gefitinib in prostate cancer cells", ONCOLOGY REPORTS, vol. 18, no. 2, 2007, pages 503 - 511, XP055633345 * |
| KONG, L. ET AL.: "Structural pharmacological studies on EGFR T790M/C797S", BIOCHEM BIOPHYS RES COMMUN., vol. 488, no. 2, 2017, pages 266 - 272, XP085033851, DOI: 10.1016/j.bbrc.2017.04.138 * |
| LAI, P. ET AL.: "Lestaurtinib is Cytotoxic to Oxaliplatin-resistant Transitional Cell Carcinoma Cell Line T24 In Vitro", TZU CHI MEDICAL JOURNAL, vol. 22, no. 3, 2010, pages 125 - 130, XP027457833, DOI: 10.1016/S1016-3190(10)60056-0 * |
| LEE, H. ET AL.: "Noncovalent Wild-type-Sparing Inhibitors of EGFR T790M", CANCER DISCOVERY, vol. 3, no. 2, 2012, pages 168 - 181, XP002725587 * |
| MIYAMOTO, K. ET AL.: "Inhibition of multi drug resistance by a new staurosporine derivative, NA- 382, in vitro and in vivo", CANCER RESEARCH, vol. 53, no. 7, 1993, pages 1555 - 1559, XP055633350 * |
| SHI, Z. ET AL.: "S-Phase arrest by nucleoside analogues and abrogation of survival without cell cycle progression by 7-hydroxystaurosporine", CANCER RESEARCH, vol. 61, no. 3, 2001, pages 1065 - 1072, XP055633340 * |
| SONG, X. ET AL.: "Staurosporine scaffold-based rational discovery of the wild-type sparing reversible inhibitors of EGFR T790M gatekeeper mutant in lung cancer with analog-sensitive kinase technology", JOURNAL OF MOLECULAR RECOGNITION., vol. 30, no. 4, 2017, pages e2590, XP055633338 * |
| UTZ, I. ET AL.: "The protein kinase C inhibitor CGP 41251, a staurosporine derivative with antitumor activity, reverses multidrug resistance", INT J CANCER., vol. 57, no. 1, 1994, pages 104 - 110, XP055406335, DOI: 10.1002/ijc.2910570119 * |
| YAO, J. ET AL.: "Systematic profiling of chemotherapeutic drug response to EGFR gatekeeper mutation in non-small cell lung cancer", COMPUTATIONAL BIOLOGY AND CHEMISTRY, vol. 64, 2016, pages 126 - 133, XP055633341 * |
Cited By (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN111303165A (zh) * | 2019-09-19 | 2020-06-19 | 杭州科兴生物化工有限公司 | 一类星孢菌素类衍生物及其制备方法和应用 |
| CN111333659A (zh) * | 2019-09-19 | 2020-06-26 | 杭州科兴生物化工有限公司 | 一类星孢菌素类衍生物及其制备方法和应用 |
| CN111471050A (zh) * | 2019-09-19 | 2020-07-31 | 杭州科兴生物化工有限公司 | 一类星孢菌素类衍生物及其制备方法和应用 |
| CN111471050B (zh) * | 2019-09-19 | 2021-07-06 | 杭州科兴生物化工有限公司 | 一类星孢菌素类衍生物及其制备方法和应用 |
| WO2024156250A1 (zh) * | 2023-01-29 | 2024-08-02 | 杭州科兴生物化工有限公司 | 7-羰基星孢菌素衍生物及其制备方法和应用 |
Also Published As
| Publication number | Publication date |
|---|---|
| JP7223502B2 (ja) | 2023-02-16 |
| JP7223502B6 (ja) | 2024-02-08 |
| JP2019142823A (ja) | 2019-08-29 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| Sanches et al. | Is prodrug design an approach to increase water solubility? | |
| Belhadj et al. | Design of Y-shaped targeting material for liposome-based multifunctional glioblastoma-targeted drug delivery | |
| CN103687624B (zh) | 靶向的聚合缀合物和其用途 | |
| JP6013424B2 (ja) | Hpma−ドセタキセルコンジュゲートおよびその使用 | |
| KR101721865B1 (ko) | 항암제의 전달을 위한 폴리머 시스템 | |
| JP2010526091A (ja) | 癌の処置のための生物学的な標的基の改変 | |
| JP2010526091A5 (enExample) | ||
| WO2018038165A1 (ja) | ポリマー、ポリマーの製造方法、及び薬物複合体 | |
| JP2022512826A (ja) | MetAP2阻害剤のバイオマーカーとその応用 | |
| Zhou et al. | A linear polyethylenimine (LPEI) drug conjugate with reversible charge to overcome multidrug resistance in cancer cells | |
| CN114980932A (zh) | Sortilin结合缀合化合物、其组合物及其用于治疗癌症的用途 | |
| CN105131039B (zh) | 一种喜树碱类磷脂化合物、其药物组合物及应用 | |
| WO2019163943A1 (ja) | 医薬組成物 | |
| Liu et al. | Bioresponsive nanocomplex integrating cancer-associated fibroblast deactivation and immunogenic chemotherapy for rebuilding immune-excluded tumors | |
| EP3419668B1 (en) | Polymeric conjugates and uses thereof | |
| US20240156971A1 (en) | Methods and sortilin binding conjugate compounds for targeting cancer stem cells | |
| KR20180118226A (ko) | 증식성 질환을 위한 조합 치료 | |
| Cao et al. | Peptide-drug conjugates with linker-dependent payload release coordinate dual antitumor efficacy: Intrinsic RGDS-mediated metastasis suppression synergizes with camptothecin cytotoxicity | |
| JP7084740B2 (ja) | 薬物複合体、ミセル、及び医薬組成物 | |
| WO2025001852A1 (zh) | 多肽偶联物、包含所述多肽偶联物的药物组合物及其用途 | |
| CN116925084A (zh) | 对肿瘤过表达的细胞周期调节蛋白wee1有双重抑制作用的化合物 | |
| CN117304424A (zh) | 一种还原响应性的纳米递送系统及其在制备治疗耐药性肿瘤的药物中的用途 | |
| CN111868093A (zh) | 抗癌剂 | |
| PL238778B1 (pl) | Peptydomimetyki i ich zastosowanie w terapii nowotworu trzustki |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 19757059 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 19757059 Country of ref document: EP Kind code of ref document: A1 |