WO2018193811A1 - タイヤ - Google Patents
タイヤ Download PDFInfo
- Publication number
- WO2018193811A1 WO2018193811A1 PCT/JP2018/013439 JP2018013439W WO2018193811A1 WO 2018193811 A1 WO2018193811 A1 WO 2018193811A1 JP 2018013439 W JP2018013439 W JP 2018013439W WO 2018193811 A1 WO2018193811 A1 WO 2018193811A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- tire
- thermoplastic elastomer
- resin material
- copolymer
- polyester
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B60—VEHICLES IN GENERAL
- B60C—VEHICLE TYRES; TYRE INFLATION; TYRE CHANGING; CONNECTING VALVES TO INFLATABLE ELASTIC BODIES IN GENERAL; DEVICES OR ARRANGEMENTS RELATED TO TYRES
- B60C1/00—Tyres characterised by the chemical composition or the physical arrangement or mixture of the composition
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B60—VEHICLES IN GENERAL
- B60C—VEHICLE TYRES; TYRE INFLATION; TYRE CHANGING; CONNECTING VALVES TO INFLATABLE ELASTIC BODIES IN GENERAL; DEVICES OR ARRANGEMENTS RELATED TO TYRES
- B60C1/00—Tyres characterised by the chemical composition or the physical arrangement or mixture of the composition
- B60C1/0016—Compositions of the tread
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B60—VEHICLES IN GENERAL
- B60C—VEHICLE TYRES; TYRE INFLATION; TYRE CHANGING; CONNECTING VALVES TO INFLATABLE ELASTIC BODIES IN GENERAL; DEVICES OR ARRANGEMENTS RELATED TO TYRES
- B60C1/00—Tyres characterised by the chemical composition or the physical arrangement or mixture of the composition
- B60C1/0025—Compositions of the sidewalls
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B60—VEHICLES IN GENERAL
- B60C—VEHICLE TYRES; TYRE INFLATION; TYRE CHANGING; CONNECTING VALVES TO INFLATABLE ELASTIC BODIES IN GENERAL; DEVICES OR ARRANGEMENTS RELATED TO TYRES
- B60C5/00—Inflatable pneumatic tyres or inner tubes
- B60C5/01—Inflatable pneumatic tyres or inner tubes without substantial cord reinforcement, e.g. cordless tyres, cast tyres
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L67/00—Compositions of polyesters obtained by reactions forming a carboxylic ester link in the main chain; Compositions of derivatives of such polymers
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B60—VEHICLES IN GENERAL
- B60C—VEHICLE TYRES; TYRE INFLATION; TYRE CHANGING; CONNECTING VALVES TO INFLATABLE ELASTIC BODIES IN GENERAL; DEVICES OR ARRANGEMENTS RELATED TO TYRES
- B60C9/00—Reinforcements or ply arrangement of pneumatic tyres
- B60C9/02—Carcasses
- B60C2009/0269—Physical properties or dimensions of the carcass coating rubber
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B60—VEHICLES IN GENERAL
- B60C—VEHICLE TYRES; TYRE INFLATION; TYRE CHANGING; CONNECTING VALVES TO INFLATABLE ELASTIC BODIES IN GENERAL; DEVICES OR ARRANGEMENTS RELATED TO TYRES
- B60C11/00—Tyre tread bands; Tread patterns; Anti-skid inserts
- B60C11/0008—Tyre tread bands; Tread patterns; Anti-skid inserts characterised by the tread rubber
- B60C2011/0016—Physical properties or dimensions
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B60—VEHICLES IN GENERAL
- B60C—VEHICLE TYRES; TYRE INFLATION; TYRE CHANGING; CONNECTING VALVES TO INFLATABLE ELASTIC BODIES IN GENERAL; DEVICES OR ARRANGEMENTS RELATED TO TYRES
- B60C13/00—Tyre sidewalls; Protecting, decorating, marking, or the like, thereof
- B60C2013/005—Physical properties of the sidewall rubber
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L2207/00—Properties characterising the ingredient of the composition
- C08L2207/04—Thermoplastic elastomer
Definitions
- This disclosure relates to tires.
- thermoplastic resins particularly thermoplastic resins and thermoplastic elastomers, as materials
- thermoplastic polymer materials that is, thermoplastic resins
- a tire that is formed of a thermoplastic resin material and has an annular tire skeleton, and has a reinforcing cord member that is wound in the circumferential direction around the outer periphery of the tire skeleton to form a reinforcing cord layer
- a plastic resin material contains at least a polyester-based thermoplastic elastomer
- the state of the resin material in the tire after manufacture affects the state of the tire skeleton. Therefore, if the state of the resin material can be appropriately controlled, it is considered that the characteristics (for example, durability, etc.) of the tire frame body and the tire can be improved to a desired state. However, there is still room for study as to how the state of the resin material is controlled to achieve the desired characteristics.
- an object of the present disclosure is to provide a tire having a tire skeleton body including a thermoplastic elastomer as a resin contained in a resin material and having excellent durability.
- thermoplastic resin is a concept including a thermoplastic resin, a thermoplastic elastomer, and a thermosetting resin, and does not include vulcanized rubber.
- “same species” means those having a skeleton that is common to the skeleton constituting the main chain of the resin, such as esters and styrenes.
- thermoplastic elastomer refers to a polymer that forms a crystalline hard segment with a high melting point, or a hard segment with a high cohesive force, and a polymer that forms an amorphous soft segment with a low glass transition temperature. The polymer compound which consists of a copolymer which has these.
- thermoplastic elastomer is softened and fluidized as the temperature rises, and becomes relatively hard and strong when cooled.
- the thermoplastic elastomer is a polymer compound having rubber-like elasticity.
- hard segment refers to a component that is relatively harder than the soft segment
- soft segment refers to a component that is relatively softer than the hard segment.
- the hard segment is preferably a molecular constraint component that serves as a crosslinking point of the crosslinked rubber that prevents plastic deformation.
- the soft segment is preferably a flexible component exhibiting rubber elasticity.
- a numerical range expressed using “to” means a range including numerical values described before and after “to” as a lower limit value and an upper limit value.
- the term “process” includes not only an independent process but also a process that can be clearly distinguished from other processes as long as the purpose is achieved. include.
- the tire according to the present embodiment has a tire skeleton formed of a resin material containing a thermoplastic elastomer (that is, part or all of which is formed only of the resin material).
- the thickness La of the amorphous portion measured by the small-angle X-ray scattering method of the thermoplastic elastomer is in the range of 12.3 nm to 13.9 nm.
- a tire having a tire skeleton formed using a thermoplastic elastomer having a non-crystalline portion thickness La in the above range is a thermoplastic having a non-crystalline portion thickness La in the above range. It has been clarified that the tire has a durability (particularly, crack resistance) as compared with a tire having a tire skeleton formed using only an elastomer.
- the thickness La of the amorphous part of the thermoplastic elastomer is the thickness of one amorphous part in the repeating structure of the crystal part and the amorphous part of the hard segment of the thermoplastic elastomer.
- the thickness La of the amorphous part is measured by a small-angle X-ray scattering method as described below using a sample collected from the tire frame or a sample prepared from a resin material used for forming the tire frame. Is done.
- the long period L is defined as 2 ⁇ / q max .
- q max is the wave number q [nm ⁇ 1 ] where the primary peak of small-angle X-ray scattering is shown.
- a tire having a tire skeleton formed using a thermoplastic elastomer having an amorphous part thickness La in the above range is excellent in durability.
- the reason is not clear, but is presumed as follows.
- a thickness La of the amorphous part of 12.3 nm or more is considered to indicate that the molecular chain of the amorphous part is extended in the repeating structure of the crystal part and the amorphous part of the hard segment.
- the orientation of the molecular chain in the amorphous part that is, the degree of alignment
- it is considered that the cushioning property of the thermoplastic elastomer is enhanced, and the durability of the tire, particularly the crack resistance against impact, is improved.
- the thickness La of the amorphous part is 13.9 nm or less, it indicates that the molecular chain of the amorphous part is not excessively extended in the repeating structure of the crystalline part and the amorphous part of the hard segment. Conceivable. Therefore, a decrease in the strength of the molecular chain in the amorphous part due to excessive elongation is suppressed. Thereby, it is considered that the strength of the thermoplastic elastomer is ensured and the durability of the tire, particularly the crack resistance against impact, is improved.
- the thickness La of the amorphous part is further preferably in the range of 12.5 nm to 13.7 nm, and more preferably in the range of 12.8 nm to 13.4 nm.
- the method for controlling the value of the thickness La of the amorphous part of the thermoplastic elastomer is not particularly limited.
- the value of the thickness La of the amorphous portion can be reduced by increasing the heating temperature at the time of forming the tire frame (for example, the cylinder temperature or mold temperature at the time of injection molding).
- the value of the thickness La of the amorphous part can be increased by lowering the heating temperature (for example, the cylinder temperature or the mold temperature) at the time of forming the tire skeleton.
- the long period L measured by the small-angle X-ray scattering method of the thermoplastic elastomer contained in the tire skeleton is in the range of 15.6 nm to 17.1 nm.
- the long period L of the thermoplastic elastomer is the repetitive structure of the crystal part and the amorphous part of the hard segment in the thermoplastic elastomer, and the crystal part in the repeating unit composed of one crystal part and one amorphous part. It is the total value of the thickness and the thickness of the amorphous part.
- the long period L is calculated as follows by a small angle X-ray scattering method using a sample collected from a tire frame or a sample prepared from a resin material used for forming the tire frame.
- the one-dimensional autocorrelation function ⁇ (r) obtained from the value of the one-dimensional autocorrelation function is plotted against r, and the value of r taking the primary peak is taken.
- Q in the formula represents the wave number [nm ⁇ 1 ] where the primary peak of the small-angle X-ray scattering is shown.
- R has a dimension of distance at any point in the polymer space.
- I (q) represents the X-ray scattering intensity.
- One-dimensional autocorrelation function ⁇ (r) ( ⁇ I (q) q 2 cos (rq) dq) / ( ⁇ I (q) q 2 dq)
- the durability of the tire is improved. This is thought to be because the long period L is 15.6 nm or more, which reduces the friction between molecules and improves the durability of the tire. Further, when the thickness is 17.1 nm or less, an increase in elastic modulus due to excessive elongation of the molecular chain is suppressed, and it is considered that the durability of the tire is also improved from this viewpoint.
- the long period L is further preferably in the range of 15.8 nm to 17.0 nm, and more preferably in the range of 16.1 nm to 16.7 nm.
- the value of the long period L increases as the thickness La of the amorphous part in the thermoplastic elastomer increases. Moreover, the growth of the crystal part in the thermoplastic elastomer is promoted, and the crystal part becomes thicker as the thickness of the crystal part increases. In addition, it exists in the tendency for melting
- the method for controlling the value of the long period L of the thermoplastic elastomer is not particularly limited.
- a method of controlling the value of the thickness La of the amorphous part can be mentioned.
- the long period L can be increased by increasing the heating temperature (for example, cylinder temperature or mold temperature) at the time of forming the tire skeleton and increasing the time required for cooling to promote crystal growth.
- the long period L can be reduced by lowering the heating temperature (for example, the cylinder temperature and the mold temperature) at the time of forming the tire skeleton to shorten the time required for cooling and suppressing the crystal growth.
- the degree of orientation f of the thermoplastic elastomer contained in the tire skeleton measured by the small angle X-ray scattering method is in the range of ⁇ 0.08 to 0.08 (that is, the degree of orientation f is The absolute value is preferably 0 or more and 0.08 or less).
- the orientation degree f of the thermoplastic elastomer means the orientation degree of molecules in the crystal part of the hard segment in the thermoplastic elastomer.
- orientation degree f is in the range of -0.08 to 0.08, the durability of the tire, particularly the crack resistance against impact, is improved. This is presumed to be because, when the degree of orientation f is within the above range, the mechanical input to the tire frame during traveling can be effectively dispersed, and the mechanical strength is increased.
- the orientation degree f is further preferably in the range of -0.08 to 0.04, and more preferably in the range of -0.02 to 0.02.
- the method for controlling the value of the orientation degree f of the thermoplastic elastomer is not particularly limited. For example, it can be carried out by changing the temperature of the thermoplastic elastomer, the cylinder temperature, the mold temperature, the cooling rate, etc. at the time of injection molding when forming the tire skeleton. For example, increasing the temperature at the time of injection molding of a thermoplastic elastomer (that is, increasing the temperature of the thermoplastic elastomer) decreases the viscosity, increases the cylinder temperature or mold temperature, and increases the time required for cooling, thereby increasing the molecular weight.
- the degree of orientation f can be reduced by relaxing the movement.
- the degree of crystallinity Xc of the thermoplastic elastomer contained in the tire skeleton measured by the wide angle X-ray scattering method is in the range of 12% to 45%.
- the crystallinity Xc of the thermoplastic elastomer represents the ratio of the crystal part in the hard segment of the thermoplastic elastomer. Note that the larger the value of the crystallinity Xc, the larger the proportion of crystal parts and the smaller the proportion of amorphous parts.
- the crystallinity Xc is in the range of 12% to 45%, the durability of the tire, particularly the crack resistance against impact, is improved. This is presumably because when the crystallinity Xc is 12% or more, the heat resistance is increased. Further, it is presumed that when the crystallinity degree Xc is 45% or less, the destruction phenomenon due to the crystal starting point is suppressed.
- the crystallinity Xc is more preferably 12% to 37%.
- the method for controlling the value of the crystallinity Xc of the thermoplastic elastomer is not particularly limited.
- the crystallinity Xc can be increased by increasing the heating temperature (for example, cylinder temperature or mold temperature) at the time of forming the tire skeleton and increasing the time required for cooling to promote crystal growth.
- the degree of crystallinity Xc can be reduced by lowering the mold temperature during cooling to shorten the time required for cooling and suppressing crystal growth.
- the resin material includes a thermoplastic elastomer, and may optionally include a component other than the thermoplastic elastomer such as an additive.
- the kind of thermoplastic elastomer used for formation of a tire frame is not particularly limited.
- polyester-based thermoplastic elastomer (TPC), polyamide-based thermoplastic elastomer (TPA), polystyrene-based thermoplastic elastomer (TPS), polyurethane-based thermoplastic elastomer (TPU), olefin-based thermoplastic elastomer (TPO), thermoplastic rubber A crosslinked body (TPV), other thermoplastic elastomers (TPZ), etc. are mentioned.
- JIS K6418 can be referred to.
- thermoplastic elastomer a polyester-based thermoplastic elastomer is preferable as the thermoplastic elastomer because of its advantage that the bending fatigue resistance is higher than that of other thermoplastic elastomers.
- a tire containing a polyester-based thermoplastic elastomer exhibits high durability by suppressing the occurrence and growth of fatigue cracks against repeated bending due to its high bending fatigue resistance.
- the polyester-based thermoplastic elastomer is a polymer compound having elasticity, a polymer containing a polyester that forms a crystalline hard segment with a high melting point, and a polymer that forms an amorphous soft segment with a low glass transition temperature, It is a thermoplastic resin material made of a copolymer having.
- the polyester-based thermoplastic elastomer means one having a partial structure made of polyester in the structure. Examples of the polyester-based thermoplastic elastomer include ester-based thermoplastic elastomer (TPC) defined in JIS K6418: 2007.
- polyester-based thermoplastic elastomer for example, at least a polyester is crystalline and a hard segment having a high melting point is formed, and another polymer (eg, polyester or polyether) is amorphous and has a low glass transition temperature.
- another polymer eg, polyester or polyether
- polyester forming the hard segment for example, aromatic polyester can be used.
- the aromatic polyester can be formed from, for example, an aromatic dicarboxylic acid or an ester-forming derivative thereof and an aliphatic diol.
- aromatic polyester examples include polybutylene terephthalate derived from terephthalic acid and / or dimethyl terephthalate and 1,4-butanediol. Further, isophthalic acid, phthalic acid, naphthalene-2,6-dicarboxylic acid, naphthalene-2,7-dicarboxylic acid, diphenyl-4,4′-dicarboxylic acid, diphenoxyethanedicarboxylic acid, 5-sulfoisophthalic acid, or these A dicarboxylic acid component such as an ester-forming derivative, and a diol having a molecular weight of 300 or less (for example, an aliphatic diol such as ethylene glycol, trimethylene glycol, pentamethylene glycol, hexamethylene glycol, neopentyl glycol, and decamethylene glycol, 1 , 4-cyclohexanedimethanol, and cycloaliphatic diols such as tricycl
- polyester forming the hard segment examples include polyethylene terephthalate, polybutylene terephthalate, polymethylene terephthalate, polyethylene naphthalate, and polybutylene naphthalate. Of these, polybutylene terephthalate is preferable.
- Aliphatic polyethers include poly (ethylene oxide) glycol, poly (propylene oxide) glycol, poly (tetramethylene oxide) glycol, poly (hexamethylene oxide) glycol, copolymers of ethylene oxide and propylene oxide, poly (propylene oxide) And ethylene oxide addition polymer of glycol, and a copolymer of ethylene oxide and tetrahydrofuran.
- the aliphatic polyester include poly ( ⁇ -caprolactone), polyenantlactone, polycaprylolactone, polybutylene adipate, and polyethylene adipate.
- poly (tetramethylene oxide) glycol poly (propylene oxide) glycol are polymers that form soft segments from the viewpoint of the elastic properties of the resulting polyester block copolymer.
- Preferred are ethylene oxide adducts, poly ( ⁇ -caprolactone), polybutylene adipate, and polyethylene adipate.
- the number average molecular weight of the polymer forming the hard segment (that is, polyester) is preferably 300 to 6000 from the viewpoint of toughness and low temperature flexibility.
- the number average molecular weight of the polymer forming the soft segment is preferably 300 to 6000 from the viewpoint of toughness and low temperature flexibility.
- the mass ratio (x: y) between the hard segment (x) and the soft segment (y) is preferably 99: 1 to 20:80, and 98: 2 to 30: from the viewpoint of moldability of the tire frame body. 70 is more preferable.
- the combination of the hard segment and the soft segment described above examples include, for example, combinations of the hard segment and the soft segment mentioned above.
- the combination of the hard segment and the soft segment described above is preferably a combination in which the hard segment is polybutylene terephthalate, the soft segment is an aliphatic polyether, and the hard segment is polybutylene terephthalate. More preferred is a combination wherein is poly (ethylene oxide) glycol.
- polyester-based thermoplastic elastomers examples include “Hytrel” series (for example, 3046, 5557, 6347, 4047N, and 4767N) manufactured by Toray DuPont Co., Ltd., and “Perprene” manufactured by Toyobo Co., Ltd. Series (for example, P30B, P40B, P40H, P55B, P70B, P150B, P280B, E450B, P150M, S1001, S2001, S5001, S6001, and S9001) can be used.
- Hytrel for example, 3046, 5557, 6347, 4047N, and 4767N
- Perprene manufactured by Toyobo Co., Ltd. Series
- the polyester-based thermoplastic elastomer can be synthesized by copolymerizing a polymer that forms a hard segment and a polymer that forms a soft segment by a known method.
- the content of the polyester thermoplastic elastomer in the total thermoplastic elastomer contained is not particularly limited, but the total amount of all resins Is preferably 50% by mass or more, more preferably 80% by mass or more, and still more preferably 90% by mass or more.
- the polyester-based thermoplastic elastomer can sufficiently exhibit the characteristics, and the durability of the tire is further improved. It becomes easy.
- the polyamide-based thermoplastic elastomer is a thermoplastic resin comprising a copolymer having a crystalline polymer having a high melting point and a non-crystalline polymer having a low glass transition temperature. It means a material having an amide bond (—CONH—) in the main chain of the polymer forming the hard segment.
- a soft segment in which at least polyamide is crystalline and a high melting point is formed and another polymer (for example, polyester or polyether) is amorphous and has a low glass transition temperature.
- the material which forms is mentioned.
- the polyamide-based thermoplastic elastomer may be formed using a chain extender such as dicarboxylic acid in addition to the hard segment and the soft segment.
- a chain extender such as dicarboxylic acid
- Specific examples of polyamide-based thermoplastic elastomers include amide-based thermoplastic elastomers (TPA) defined in JIS K6418: 2007, polyamide-based elastomers described in JP-A No. 2004-346273, and the like. it can.
- examples of the polyamide forming the hard segment include polyamides produced by monomers represented by the following general formula (1) or general formula (2).
- R 1 represents a molecular chain of a hydrocarbon having 2 to 20 carbon atoms (for example, an alkylene group having 2 to 20 carbon atoms).
- R 2 represents a hydrocarbon molecular chain having 3 to 20 carbon atoms (for example, an alkylene group having 3 to 20 carbon atoms).
- R 1 is preferably a hydrocarbon molecular chain having 3 to 18 carbon atoms (for example, an alkylene group having 3 to 18 carbon atoms), and a hydrocarbon molecular chain having 4 to 15 carbon atoms (for example, (Alkylene group having 4 to 15 carbon atoms) is more preferable, and a molecular chain of a hydrocarbon having 10 to 15 carbon atoms (for example, an alkylene group having 10 to 15 carbon atoms) is particularly preferable.
- R 2 is preferably a hydrocarbon molecular chain having 3 to 18 carbon atoms (eg, an alkylene group having 3 to 18 carbon atoms), and a hydrocarbon molecular chain having 4 to 15 carbon atoms.
- an alkylene group having 4 to 15 carbon atoms is more preferable, and a molecular chain of a hydrocarbon having 10 to 15 carbon atoms (for example, an alkylene group having 10 to 15 carbon atoms) is particularly preferable.
- the monomer represented by the general formula (1) or the general formula (2) include ⁇ -aminocarboxylic acid or lactam.
- the polyamide forming the hard segment include polycondensates of these ⁇ -aminocarboxylic acids or lactams, and copolycondensation polymers of diamines and dicarboxylic acids.
- Examples of ⁇ -aminocarboxylic acids include 6 to 20 carbon atoms such as 6-aminocaproic acid, 7-aminoheptanoic acid, 8-aminooctanoic acid, 10-aminocapric acid, 11-aminoundecanoic acid, and 12-aminododecanoic acid.
- Examples of the lactam include aliphatic lactams having 5 to 20 carbon atoms such as lauryl lactam, ⁇ -caprolactam, udecan lactam, ⁇ -enantolactam, and 2-pyrrolidone.
- diamine examples include ethylene diamine, trimethylene diamine, tetramethylene diamine, hexamethylene diamine, heptamethylene diamine, octamethylene diamine, nonamethylene diamine, decamethylene diamine, undecamethylene diamine, dodecamethylene diamine, 2, 2, 4
- diamine compounds such as aliphatic diamines having 2 to 20 carbon atoms such as trimethylhexamethylenediamine, 2,4,4-trimethylhexamethylenediamine, 3-methylpentamethylenediamine, and metaxylenediamine.
- the dicarboxylic acid can be represented by HOOC- (R 3 ) m —COOH (R 3 : a hydrocarbon molecular chain having 3 to 20 carbon atoms, m: 0 or 1).
- R 3 a hydrocarbon molecular chain having 3 to 20 carbon atoms, m: 0 or 1.
- oxalic acid, succinic acid And aliphatic dicarboxylic acids having 2 to 20 carbon atoms such as glutaric acid, adipic acid, pimelic acid, suberic acid, azelaic acid, sebacic acid, and dodecanedioic acid.
- a polyamide obtained by ring-opening polycondensation of lauryl lactam, ⁇ -caprolactam, or decane lactam can be preferably used.
- polyester, polyether, etc. are mentioned, for example.
- Specific examples include polyethylene glycol, polypropylene glycol, polytetramethylene ether glycol, and ABA type triblock polyether. These can be used alone or in combination of two or more.
- polyether diamine etc. which are obtained by making ammonia etc. react with the terminal of polyether can also be used.
- the “ABA type triblock polyether” means a polyether represented by the following general formula (3).
- x and z represent an integer of 1 to 20.
- y represents an integer of 4 to 50.
- each of x and z is preferably an integer of 1 to 18, more preferably an integer of 1 to 16, still more preferably an integer of 1 to 14, and particularly preferably an integer of 1 to 12.
- y is preferably an integer of 5 to 45, more preferably an integer of 6 to 40, still more preferably an integer of 7 to 35, and particularly preferably an integer of 8 to 30.
- combinations of the hard segment and the soft segment include a combination of a ring-opening polycondensate of lauryl lactam and polyethylene glycol, a combination of a ring-opening polycondensate of lauryl lactam and polypropylene glycol, and a ring opening of lauryl lactam.
- a combination of a polycondensate and a polytetramethylene ether glycol, or a ring-opening polycondensate of lauryl lactam and an ABA type triblock polyether is preferred, and a ring opening polycondensate of lauryl lactam and an ABA type triblock polyether The combination with is more preferable.
- the number average molecular weight of the polymer forming the hard segment is preferably 300 to 15000 from the viewpoint of melt moldability.
- the number average molecular weight of the polymer forming the soft segment is preferably 200 to 6000 from the viewpoint of toughness and low temperature flexibility.
- the mass ratio (x: y) of the hard segment (x) and the soft segment (y) is preferably 50:50 to 90:10, and preferably 50:50 to 80:20 from the viewpoint of moldability of the tire frame body. Is more preferable.
- the polyamide-based thermoplastic elastomer can be synthesized by copolymerizing a polymer forming a hard segment and a polymer forming a soft segment by a known method.
- Examples of commercially available products of polyamide-based thermoplastic elastomer include, for example, “UBESTA XPA” series (for example, XPA9063X1, XPA9055X1, XPA9048X2, XPA9048X1, XPA9040X1, XPA9040X2XPA9044, etc.) manufactured by Daicel Eponic Corporation. “Vestamide” series (for example, E40-S3, E47-S1, E47-S3, E55-S1, E55-S3, EX9200, E50-R2, etc.) can be used.
- polystyrene-based thermoplastic elastomer for example, at least polystyrene forms a hard segment, and other polymers (for example, polybutadiene, polyisoprene, polyethylene, hydrogenated polybutadiene, hydrogenated polyisoprene, etc.) Examples thereof include materials that form amorphous soft segment having a low glass transition temperature.
- polystyrene forming the hard segment for example, those obtained by a known radical polymerization method or ionic polymerization method are preferably used, and specifically, polystyrene having anion living polymerization can be mentioned.
- the polymer forming the soft segment include polybutadiene, polyisoprene, and poly (2,3-dimethyl-butadiene).
- the combination of the hard segment and the soft segment mentioned above can be mentioned.
- the combination of the hard segment and the soft segment is preferably a combination of polystyrene and polybutadiene or a combination of polystyrene and polyisoprene.
- the soft segment is preferably hydrogenated.
- the number average molecular weight of the polymer forming the hard segment is preferably from 5,000 to 500,000, more preferably from 10,000 to 200,000. Further, the number average molecular weight of the polymer forming the soft segment is preferably from 5,000 to 1,000,000, more preferably from 10,000 to 800,000, and even more preferably from 30,000 to 500,000. Further, the volume ratio (x: y) of the hard segment (x) and the soft segment (y) is preferably 5:95 to 80:20 from the viewpoint of moldability of the tire frame body, and 10:90 to 70:30. Is more preferable.
- the polystyrene-based thermoplastic elastomer can be synthesized by copolymerizing a polymer forming a hard segment and a polymer forming a soft segment by a known method.
- polystyrene-based thermoplastic elastomers include styrene-butadiene copolymers [for example, SBS (polystyrene-poly (butylene) block-polystyrene) and SEBS (polystyrene-poly (ethylene / butylene) block-polystyrene)], styrene.
- -Isoprene copolymer eg polystyrene-polyisoprene block-polystyrene
- styrene-propylene copolymer eg SEP (polystyrene- (ethylene / propylene) block), SEPS (polystyrene-poly (ethylene / propylene) block-polystyrene) ), SEEPS (polystyrene-poly (ethylene-ethylene / propylene) block-polystyrene), SEB (polystyrene (ethylene / butylene) block)] and the like.
- SEP polystyrene- (ethylene / propylene) block
- SEPS polystyrene-poly (ethylene / propylene) block-polystyrene
- SEEPS polystyrene-poly (ethylene-ethylene / propylene) block-polystyrene
- thermoplastic elastomer As a commercially available product of polystyrene-based thermoplastic elastomer, for example, “Tough Tech” series manufactured by Asahi Kasei Corporation (for example, H1031, H1041, H1043, H1051, H1052, H1053, H1062, H1082, H1141, H1221, and H1272 etc.) "SEBS” series (8007, 8076, etc.) and “SEPS” series (2002, 2063, etc.) manufactured by Kuraray Co., Ltd. can be used.
- “Tough Tech” series manufactured by Asahi Kasei Corporation for example, H1031, H1041, H1043, H1051, H1052, H1053, H1062, H1082, H1141, H1221, and H1272 etc.
- SEBS 8007, 8076, etc.
- SEPS 2002, 2063, etc.
- thermoplastic elastomer As polyurethane-based thermoplastic elastomers, for example, at least polyurethane forms a hard segment in which pseudo-crosslinking is formed by physical aggregation, and other polymers form a soft segment with a low glass transition temperature that is amorphous. Material.
- Specific examples of the polyurethane-based thermoplastic elastomer include a polyurethane-based thermoplastic elastomer (TPU) defined in JIS K6418: 2007.
- TPU polyurethane-based thermoplastic elastomer
- the polyurethane-based thermoplastic elastomer can be represented as a copolymer including a soft segment including a unit structure represented by the following formula A and a hard segment including a unit structure represented by the following formula B.
- P represents a long-chain aliphatic polyether or a long-chain aliphatic polyester.
- R represents an aliphatic hydrocarbon, an alicyclic hydrocarbon, or an aromatic hydrocarbon.
- P ′ represents a short chain aliphatic hydrocarbon, an alicyclic hydrocarbon, or an aromatic hydrocarbon.
- the long-chain aliphatic polyether or long-chain aliphatic polyester represented by P for example, those having a molecular weight of 500 to 5000 can be used.
- P is derived from a diol compound containing a long-chain aliphatic polyether represented by P or a long-chain aliphatic polyester.
- diol compounds include polyethylene glycol, polypropylene glycol, polytetramethylene ether glycol, poly (butylene abido) diol, poly- ⁇ -caprolactone diol, poly (hexamethylene carbonate) having a molecular weight within the above range.
- Diol, ABA type triblock polyether and the like These can be used alone or in combination of two or more.
- R is derived from a diisocyanate compound containing an aliphatic hydrocarbon, an alicyclic hydrocarbon, or an aromatic hydrocarbon represented by R.
- the aliphatic diisocyanate compound containing an aliphatic hydrocarbon represented by R include 1,2-ethylene diisocyanate, 1,3-propylene diisocyanate, 1,4-butane diisocyanate, and 1,6-hexamethylene diisocyanate. Is mentioned.
- Examples of the diisocyanate compound containing an alicyclic hydrocarbon represented by R include 1,4-cyclohexane diisocyanate and 4,4-cyclohexane diisocyanate.
- examples of the aromatic diisocyanate compound containing an aromatic hydrocarbon represented by R include 4,4′-diphenylmethane diisocyanate and tolylene diisocyanate. These can be used alone or in combination of two or more.
- P ′ is derived from a diol compound containing a short-chain aliphatic hydrocarbon, alicyclic hydrocarbon, or aromatic hydrocarbon represented by P ′.
- Examples of the aliphatic diol compound containing a short-chain aliphatic hydrocarbon represented by P ′ include glycol and polyalkylene glycol.
- Examples of the alicyclic diol compound containing an alicyclic hydrocarbon represented by P ′ include cyclopentane-1,2-diol, cyclohexane-1,2-diol, cyclohexane-1,3-diol, Examples include cyclohexane-1,4-diol and cyclohexane-1,4-dimethanol.
- examples of the aromatic diol compound containing an aromatic hydrocarbon represented by P ′ include hydroquinone, resorcin, chlorohydroquinone, bromohydroquinone, methylhydroquinone, phenylhydroquinone, methoxyhydroquinone, phenoxyhydroquinone, 4,4′- Dihydroxybiphenyl, 4,4′-dihydroxydiphenyl ether, 4,4′-dihydroxydiphenyl sulfide, 4,4′-dihydroxydiphenylsulfone, 4,4′-dihydroxybenzophenone, 4,4′-dihydroxydiphenylmethane, bisphenol A, 1, Examples include 1-di (4-hydroxyphenyl) cyclohexane, 1,2-bis (4-hydroxyphenoxy) ethane, 1,4-dihydroxynaphthalene, and 2,6-dihydroxynaphthalene. These can be used alone or in combination of two or more.
- the number average molecular weight of the polymer forming the hard segment is preferably 300 to 1500 from the viewpoint of melt moldability.
- the number average molecular weight of the polymer forming the soft segment is preferably 500 to 20000, more preferably 500 to 5000, and particularly preferably 500 to 3000, from the viewpoints of flexibility and thermal stability of the polyurethane-based thermoplastic elastomer.
- the mass ratio (x: y) of the hard segment (x) and the soft segment (y) is preferably 15:85 to 90:10, and preferably 30:70 to 90:10 from the viewpoint of moldability of the tire frame body. Is more preferable.
- the polyurethane-based thermoplastic elastomer can be synthesized by copolymerizing a polymer forming a hard segment and a polymer forming a soft segment by a known method.
- a polyurethane-based thermoplastic elastomer for example, thermoplastic polyurethane described in JP-A-5-331256 can be used.
- a combination of a hard segment composed only of an aromatic diol and an aromatic diisocyanate and a soft segment composed only of a polycarbonate ester is preferable.
- TDI isocyanate
- polyester polyol copolymer TDI / polyether polyol copolymer, TDI / caprolactone polyol copolymer, TDI / polycarbonate polyol copolymer, 4,4′-diphenylmethane diisocyanate (MDI) / Polyester polyol copolymer, MDI / polyether polyol copolymer, MDI / caprolactone polyol copolymer, MDI / polycarbonate polyol copolymer, and MDI + hydroquinone / polyhexa At least one selected from methylene carbonate copolymers is preferred.
- TDI / polyester polyol copolymer TDI / polyether polyol copolymer
- MDI / polyester polyol copolymer MDI / polyether polyol copolymer
- MDI + hydroquinone / polyhexamethylene carbonate copolymer At least one selected from is more preferable.
- thermoplastic elastomers examples include, for example, “Elastolan” series (for example, ET680, ET880, ET690, and ET890) manufactured by BASF, and “Clamiron U” series manufactured by Kuraray Co., Ltd. (For example, 2000 series, 3000 series, 8000 series, 9000 series, etc.), “Milactolan” series (for example, XN-2001, XN-2004, P390RSUP, P480RSUI, P26MRNAT, E490, E590, etc.) manufactured by Japan Miraclan Co., Ltd. And P890 etc.) can be used.
- “Elastolan” series for example, ET680, ET880, ET690, and ET890
- BASF BASF
- Clamiron U manufactured by Kuraray Co., Ltd.
- Milactolan for example, XN-2001, XN-2004, P390RSUP, P480RSUI, P26MRNAT, E490, E
- thermoplastic elastomer for example, at least a polyolefin forms a hard segment with a crystalline and high melting point, and other polymers (for example, other polyolefins and polyvinyl compounds) are amorphous and have a low glass transition temperature. Examples thereof include materials forming a soft segment. Examples of the polyolefin forming the hard segment include polyethylene, polypropylene, isotactic polypropylene, and polybutene. Examples of olefinic thermoplastic elastomers include olefin- ⁇ -olefin random copolymers, olefin block copolymers, and the like.
- propylene block copolymer ethylene-propylene copolymer, propylene-1-hexene copolymer, propylene-4-methyl-1-pentene copolymer, propylene-1-butene copolymer, ethylene- 1-hexene copolymer, ethylene-4-methyl-pentene copolymer, ethylene-1-butene copolymer, 1-butene-1-hexene copolymer, 1-butene-4-methyl-pentene, ethylene- Methacrylic acid copolymer, ethylene-methyl methacrylate copolymer, ethylene-ethyl methacrylate copolymer, ethylene-butyl methacrylate copolymer, ethylene-methyl acrylate copolymer, ethylene-ethyl acrylate copolymer, ethylene -Butyl acrylate copolymer, propylene-methacrylic acid copolymer, propylene-methacrylic Meth
- thermoplastic elastomers include propylene block copolymers, ethylene-propylene copolymers, propylene-1-hexene copolymers, propylene-4-methyl-1-pentene copolymers, propylene-1- Butene copolymer, ethylene-1-hexene copolymer, ethylene-4-methyl-pentene copolymer, ethylene-1-butene copolymer, ethylene-methacrylic acid copolymer, ethylene-methyl methacrylate copolymer , Ethylene-ethyl methacrylate copolymer, ethylene-butyl methacrylate copolymer, ethylene-methyl acrylate copolymer, ethylene-ethyl acrylate copolymer, ethylene-butyl acrylate copolymer, propylene-methacrylic acid copolymer , Propylene-methyl methacrylate copolymer, pro Lene-e
- olefin resins such as ethylene and propylene may be used.
- 50 mass% or more and 100 mass% or less of the olefin resin content rate in an olefin type thermoplastic elastomer are preferable.
- the number average molecular weight of the olefinic thermoplastic elastomer is preferably 5,000 to 10,000,000.
- the mechanical properties of the thermoplastic resin material are sufficient and the processability is excellent.
- the number average molecular weight of the olefinic thermoplastic elastomer is more preferably 7,000 to 1,000,000, and particularly preferably 10,000 to 1,000,000. Thereby, the mechanical properties and processability of the thermoplastic resin material can be further improved.
- the number average molecular weight of the polymer forming the soft segment is preferably 200 to 6000 from the viewpoint of toughness and low temperature flexibility.
- the mass ratio (x: y) of the hard segment (x) and the soft segment (y) is preferably 50:50 to 95:15, and preferably 50:50 to 90:10 from the viewpoint of moldability of the tire frame body. Is more preferable.
- the olefinic thermoplastic elastomer can be synthesized by copolymerization by a known method.
- an olefinic thermoplastic elastomer obtained by acid modification may be used as the olefinic thermoplastic elastomer.
- a product obtained by acid-modifying an olefinic thermoplastic elastomer means that an unsaturated compound having an acidic group such as a carboxylic acid group, a sulfuric acid group, and a phosphoric acid group is bonded to the olefinic thermoplastic elastomer.
- Examples of bonding an unsaturated compound having an acidic group such as a carboxylic acid group, a sulfuric acid group, and a phosphoric acid group to an olefin thermoplastic elastomer include, for example, an unsaturated compound having an acidic group as an olefin thermoplastic elastomer, Examples include bonding (for example, graft polymerization) an unsaturated bonding site of an unsaturated carboxylic acid (for example, generally maleic anhydride).
- the unsaturated compound having an acidic group is preferably an unsaturated compound having a carboxylic acid group which is a weak acid group from the viewpoint of suppressing deterioration of the olefin-based thermoplastic elastomer.
- acrylic acid, methacrylic acid, itaconic acid, croton include acids, isocrotonic acid, and maleic acid.
- thermoplastic elastomers examples include “Tuffmer” series (for example, A0550S, A1050S, A4050S, A1070S, A4070S, A35070S, A1085S, A4085S, A7090, A70090, MH7007, MH7010, manufactured by Mitsui Chemicals, Inc.
- the resin material may contain components other than the thermoplastic elastomer as desired.
- components other than the thermoplastic elastomer include rubber, thermoplastic resins, fillers (eg, silica, calcium carbonate, and clay), anti-aging agents, oils, plasticizers, color formers, and weathering agents.
- the content of the thermoplastic elastomer in the resin material is preferably 50% by mass or more from the viewpoint of sufficiently achieving the effects of the present disclosure, and is 80% by mass. More preferably, it is more preferably 90% by mass or more.
- the crystallization temperature of the thermoplastic elastomer contained in the resin material is preferably in the range of 148 ° C. to 160 ° C., more preferably in the range of 150 ° C. to 155 ° C., and in the range of 152 ° C. to 154 ° C. More preferably, it is within.
- the crystallization temperature is in the above range, the amorphous portion thickness La, the long period L, the orientation degree f, the crystallinity degree Xc, and the like can be easily controlled within the above-described ranges. In particular, the crack resistance against impact is easily improved.
- the crystallization temperature of the thermoplastic elastomer is measured by differential scanning calorimetry (DSC).
- the crystallization temperature of the thermoplastic elastomer having the highest content by mass is within the above range. Further, it is more preferable to include two or more thermoplastic elastomers having a crystallization temperature within the above range, including the thermoplastic elastomer having the highest content on a mass basis, and the crystallization temperatures of all the thermoplastic elastomers included. Is more preferably within the above range.
- the melting point of the resin material is usually about 100 ° C. to 350 ° C., but is preferably about 100 ° C. to 250 ° C., more preferably 120 ° C. to 250 ° C. from the viewpoint of tire durability and productivity.
- the tensile elastic modulus defined by JIS K7113: 1995 of the resin material (that is, the tire frame body) itself is preferably 50 MPa to 1000 MPa, more preferably 50 MPa to 800 MPa, and particularly preferably 50 MPa to 700 MPa.
- the tensile modulus of the resin material is 50 MPa to 1000 MPa, the rim can be assembled efficiently while maintaining the shape of the tire frame.
- the tensile strength specified in JIS K7113 (1995) of the resin material (that is, the tire skeleton) itself is usually about 15 MPa to 70 MPa, preferably 17 MPa to 60 MPa, and more preferably 20 MPa to 55 MPa.
- the tensile yield strength defined in JIS K7113 (1995) of the resin material (that is, the tire skeleton) itself is preferably 5 MPa or more, more preferably 5 MPa to 20 MPa, and particularly preferably 5 MPa to 17 MPa.
- the resin material can withstand deformation against a load applied to the tire during traveling.
- the tensile yield elongation defined by JIS K7113 (1995) of the resin material (that is, the tire frame body) itself is preferably 10% or more, more preferably 10% to 70%, and particularly preferably 15% to 60%.
- the tensile yield elongation of the resin material is 10% or more, the elastic region is large and the rim assembly property can be improved.
- the tensile elongation at break stipulated in JIS K7113 (1995) of the resin material (that is, the tire frame body) itself is preferably 50% or more, more preferably 100% or more, particularly preferably 150% or more, and most preferably 200% or more.
- the tensile elongation at break of the resin material is 50% or more, the rim assemblability is good, and it is difficult to break against a collision.
- the deflection temperature under load defined by ISO 75-2 or ASTM D648 of the resin material (that is, tire frame body) itself (condition: at 0.45 MPa load) is preferably 50 ° C. or higher, more preferably 50 ° C. to 150 ° C., 50 ° C. to 130 ° C. is particularly preferred.
- the deflection temperature under load of the resin material is 50 ° C. or higher, deformation of the tire skeleton can be suppressed even when vulcanization is performed in the manufacture of the tire.
- the Vicat softening temperature (Method A) defined in JIS K7206 (2016) of the resin material (that is, the tire skeleton) itself is preferably 130 ° C. or higher, preferably 130 to 250 ° C., and more preferably 130 to 220 ° C.
- the softening temperature (Method A) of the thermoplastic resin material is 130 ° C. or higher, softening and deformation of the tire in the use environment can be suppressed.
- deformation of the tire frame body can be suppressed.
- the tire according to the present embodiment may include a member other than the tire frame as necessary.
- a reinforcing member for reinforcing the tire frame body disposed on the outer periphery of the tire frame body or the like may be included.
- the reinforcing member include a cord member formed by including a metal member such as a steel cord, and a cord member coated with a coating resin material is also used.
- Examples of the resin used for the covering resin material in the reinforcing member include a thermosetting resin, a thermoplastic resin, and a thermoplastic elastomer.
- examples of the thermosetting resin include phenol resin, urea resin, melamine resin, epoxy resin, polyamide resin, and polyester resin.
- examples of the thermoplastic resin include urethane resin, olefin resin, vinyl chloride resin, polyamide resin, and polyester resin.
- thermoplastic elastomer examples include polyester-based thermoplastic elastomer (TPC), polyamide-based thermoplastic elastomer (TPA), polyolefin-based thermoplastic elastomer (TPO), and polystyrene-based thermoplastic elastomer (TPS) defined in JIS K6418: 2007. ), Polyurethane-based thermoplastic elastomer (TPU), crosslinked thermoplastic rubber (TPV), or other thermoplastic elastomer (TPZ). In view of elasticity required during running, moldability during production, and the like, it is preferable to use a thermoplastic elastomer among these. Moreover, it is preferable that the same kind of thermoplastic elastomer as the thermoplastic elastomer contained in the resin material which forms a tire frame is included.
- TPC polyester-based thermoplastic elastomer
- TPA polyamide-based thermoplastic elastomer
- TPO polyolefin-based thermoplastic elastomer
- a reinforcing member having a structure in which the cord member is coated with a coating resin material via an adhesive may be used, and the reinforcing member may be disposed on the tire frame body.
- the Martens hardness (d1) of the tire frame body, the Martens hardness (d2) of the coating resin material, and the Martens hardness (d3) of the adhesive layer satisfy the relationship of d1 ⁇ d2 ⁇ d3.
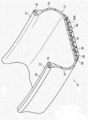
- FIG. 1A is a perspective view showing a cross section of a part of the tire 10 according to the first embodiment.
- FIG. 1B is a cross-sectional view of a bead portion when the tire 10 according to the first embodiment is mounted on a rim.
- the tire 10 has substantially the same cross-sectional shape as a rubber pneumatic tire generally used conventionally.
- FIG. 1A shows that the tire 10 has substantially the same cross-sectional shape as a rubber pneumatic tire generally used conventionally.
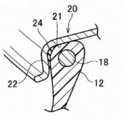
- the tire 10 includes a pair of bead portions 12 that contact the bead seat 21 and the rim flange 22 of the rim 20 shown in FIG. 1B, and side portions 14 that extend outward from the bead portion 12 in the tire radial direction.
- the tire case 17 includes only a crown portion 16 (that is, an outer peripheral portion) that connects an outer end in the tire radial direction of one side portion 14 and an outer end in the tire radial direction of the other side portion 14.
- the tire case 17 corresponds to the tire skeleton described above, and is formed of the resin material described above. In the first embodiment, the tire case 17 is entirely formed of the resin material described above, but the present disclosure is not limited to this configuration. Different resin materials may be used for each part of the tire case 17 (for example, the side portion 14, the crown portion 16, the bead portion 12, etc.) in the same manner as a rubber pneumatic tire that has been generally used conventionally. Further, in order to reinforce each part of the tire case 17, a reinforcing material (for example, a polymer material, a metal fiber, a cord, a nonwoven fabric, a woven fabric, or the like) may be embedded and disposed in each part of the tire case 17. Good.
- a reinforcing material for example, a polymer material, a metal fiber, a cord, a nonwoven fabric, a woven fabric, or the like
- two tire case halves that is, tire frame pieces
- the tire case 17 is not limited to the one formed by joining two members (that is, two tire case halves), and may be formed by joining three or more members.
- the tire case half can be produced by methods such as vacuum forming, pressure forming, injection molding, and melt casting. For this reason, it is not necessary to perform vulcanization compared to the case where a tire case is molded with rubber as in the prior art, the manufacturing process can be greatly simplified, and the molding time can be shortened.
- an annular bead core 18 is embedded in the bead portion 12 shown in FIG. 1B in the same manner as a conventionally used pneumatic tire.
- a steel cord is used as the bead core 18, but an organic fiber cord, a resin-coated organic fiber cord, a hard resin cord, or the like may be used.
- the bead core 18 can also be abbreviate
- the portion of the bead portion 12 that contacts the rim 20 and at least the portion that contacts the rim flange 22 of the rim 20 are made of a material that has better sealing properties than the resin material that forms the tire case 17.
- An annular seal layer 24 is formed.
- the seal layer 24 may also be formed at a portion where the bead portion 12 and the bead sheet 21 of the tire case 17 are in contact with each other. Note that the sealing layer 24 may be omitted if the sealing property between the rim 20 can be secured only with the resin material forming the tire case 17.
- Examples of the material having better sealing performance than the resin material forming the tire case 17 include materials softer than the resin material forming the tire case 17, such as rubber, thermoplastic resin and thermoplastic elastomer softer than the resin material. Can be mentioned.
- a reinforcement cord 26 having higher rigidity than the resin material forming the tire case 17 is wound around the crown portion 16 in the circumferential direction of the tire case 17.
- the reinforcing cord 26 is wound spirally in a state in which at least a part thereof is embedded in the crown portion 16 in a cross-sectional view along the axial direction of the tire case 17, thereby forming a reinforcing cord layer 28.
- a tread 30 made of a material superior in wear resistance to the resin material forming the tire case 17, for example, rubber, is disposed.
- the reinforcing cord 26 is in a state in which a metal member 26A such as a steel cord is covered with a covering resin material 27 (that is, a covering cord member).
- a metal member 26A such as a steel cord
- a covering resin material 27 that is, a covering cord member
- the same resin material as the resin material forming the tire case 17 is used as the coating resin material 27, but other thermoplastic resins or thermoplastic elastomers may be used.
- the reinforcement cord 26 is joined at a contact portion with the crown portion 16 by a method such as welding or adhesion with an adhesive.
- the reinforcing cord 26 may be a steel cord or the like that is not covered with the covering resin material 27.
- the elastic modulus of the coating resin material 27 is preferably set within a range of 0.1 to 10 times the elastic modulus of the resin material forming the tire case 17.
- the elastic modulus of the coating resin material 27 is 10 times or less than the elastic modulus of the resin material forming the tire case 17, the crown portion does not become too hard, and rim assembly is facilitated.
- the elastic modulus of the covering resin material 27 is 0.1 times or more of the elastic modulus of the resin material forming the tire case 17, the resin forming the reinforcing cord layer 28 is not too soft and has excellent in-plane shear rigidity. , Cornering power is improved.
- the reinforcing cord 26 has a substantially trapezoidal cross-sectional shape.
- the upper surface (that is, the outer surface in the tire radial direction) of the reinforcing cord 26 is denoted by reference numeral 26U
- the lower surface (that is, the inner surface in the tire radial direction) is denoted by reference numeral 26D.
- the cross-sectional shape of the reinforcing cord 26 is a substantially trapezoidal shape, but the present disclosure is not limited to this configuration.
- the reinforcing cord 26 may have any shape as long as the cross-sectional shape is a shape excluding a shape that becomes wider from the lower surface 26D side (that is, the inner side in the tire radial direction) toward the upper surface 26U side (that is, the outer side in the tire radial direction).
- the reinforcing cords 26 are arranged at intervals in the circumferential direction, a gap 28A is formed between adjacent reinforcing cords 26.
- the outer peripheral surface of the reinforcing cord layer 28 has a shape with irregularities, and the outer peripheral surface 17S of the tire case 17 in which the reinforcing cord layer 28 constitutes the outer peripheral portion also has an irregular shape.
- a fine roughened unevenness 96 is formed on the outer peripheral surface 17S (including the unevenness) of the tire case 17, and a cushion rubber 29 is bonded thereon via a bonding agent.
- the cushion rubber 29 flows into the contact surface with the reinforcing cord 26 so as to fill the rough unevenness 96.
- the above-mentioned tread 30 is joined on the cushion rubber 29 (that is, on the tire outer peripheral surface side).
- the tread 30 is formed with a tread pattern (not shown) including a plurality of grooves on the ground contact surface with the road surface in the same manner as a conventional rubber pneumatic tire.
- the tire manufacturing method according to the present embodiment will be described using the tire manufacturing method according to the first embodiment as an example.
- the tire case half is molded by injection molding or the like (molding process).
- the temperature of the resin material in this molding process for example, cylinder temperature, mold temperature, etc. in the case of injection molding, the thickness La and the length of the amorphous part of the thermoplastic elastomer contained in the tire case
- the period L, the orientation degree f, the crystallinity degree Xc, and the like can be controlled to be in the above-described ranges.
- the cylinder temperature is preferably in the range of 240 ° C.
- the mold temperature is preferably in the range of 50 ° C. to 110 ° C., more preferably in the range of 50 ° C. to 80 ° C., and further preferably in the range of 50 ° C. to 55 ° C.
- molding process influences control of the thickness La of the amorphous part of a thermoplastic elastomer, long period L, orientation degree f, crystallinity degree Xc, etc. .
- the cooling rate is preferably in the range of 140 ° C./second to 240 ° C./second, and more preferably in the range of 140 ° C./second to 230 ° C./second.
- the cooling time (that is, the time for cooling to the mold temperature) is preferably in the range of 1 second to 5 seconds, and more preferably in the range of 1 second to 1.5 seconds.
- the annular tire case halves obtained in the molding process are faced to each other and joined at the tire equatorial plane part to form a tire case (joining process).
- the tire case is not limited to the one formed by joining two members, and may be formed by joining three or more members.
- the joining will be described.
- tire case halves supported by a thin metal support ring face each other.
- a joining mold is installed so as to contact the outer peripheral surface of the abutting portion of the tire case half.
- the joining mold is configured to press the periphery of the joining portion (that is, the abutting portion) of the tire case half with a predetermined pressure.
- the periphery of the joint part of the tire case half is pressed at a temperature equal to or higher than the melting point of the resin material forming the tire case, so that the joint part is melted and the tire case halves are fused together to form a tire.
- Case 17 is formed.
- the temperature La of the amorphous part of the tire case 17, the long period L, the orientation degree f, the degree of crystallinity Xc, etc. can also be adjusted by adjusting the temperature of the resin material, for example, the temperature of the joining mold. Can be controlled to fall within the aforementioned range.
- the joining portion of the tire case half is heated using the joining mold, but the present disclosure is not limited thereto.
- the joining portion may be heated by a separately provided high-frequency heater or the like, or may be softened or melted beforehand by irradiation with hot air or infrared rays, and the tire case halves may be joined by pressing with a joining mold.
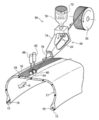
- FIG. 3 is an explanatory diagram for explaining the operation of embedding the reinforcing cord 26 in the crown portion of the tire case 17 using a cord heating device and rollers.
- the cord supply device 56 is disposed on the reel 58 around which the reinforcing cord 26 is wound, the cord heating device 59 disposed on the downstream side of the reel 58 in the cord transport direction, and the downstream side of the reinforcing cord 26 in the transport direction.
- the first roller 60, the first cylinder device 62 that moves the first roller 60 in the direction of contacting and separating from the outer peripheral surface of the tire, and the downstream side in the conveying direction of the reinforcing cord 26 of the first roller 60 A second roller 64, and a second cylinder device 66 that moves the second roller 64 in a direction in which the second roller 64 comes into contact with and separates from the tire outer peripheral surface.
- the second roller 64 can be used as a metal cooling roller.
- the surface of the first roller 60 or the second roller 64 is subjected to treatment (for example, fluororesin coating) for suppressing adhesion of the molten or softened coating resin material 27. .
- the present invention is not limited to this mode, and the roller itself may be formed of a material to which the coating resin material 27 is difficult to adhere.
- the cord supply device 56 includes the two rollers of the first roller 60 and the second roller 64.
- the present invention is not limited to this mode, and only one of the rollers is provided. You may do it.
- the cord heating device 59 includes a heater 70 and a fan 72 that generate hot air.
- the cord heating device 59 also includes a heating box 74 through which the reinforcing cord 26 passes and an outlet 76 through which the heated reinforcing cord 26 is discharged through an internal space in which hot air is supplied.
- the temperature of the heater 70 of the cord heating device 59 is raised, and the ambient air heated by the heater 70 is sent to the heating box 74 by the wind generated by the rotation of the fan 72.
- the reinforcing cord 26 unwound from the reel 58 is sent into a heating box 74 in which the internal space is heated with hot air and heated.
- the heating temperature is set to a temperature at which the covering resin material 27 of the reinforcing cord 26 is melted or softened.
- the heated reinforcing cord 26 passes through the discharge port 76 and is wound spirally around the outer peripheral surface of the crown portion 16 of the tire case 17 rotating in the direction of arrow R in FIG. At this time, the lower surface 26 ⁇ / b> D of the reinforcing cord 26 contacts the outer peripheral surface of the crown portion 16.
- the coating resin material 27 melted or softened by heating spreads on the outer peripheral surface of the crown portion 16, and the reinforcing cord 26 is welded to the outer peripheral surface of the crown portion 16. Thereby, the joint strength between the crown portion 16 and the reinforcing cord 26 is improved.
- the reinforcing cord 26 is joined to the outer peripheral surface of the crown portion 16 as described above.
- the invention is not limited to this aspect, and the joining may be performed by other methods.
- joining may be performed so that a part or the whole of the reinforcing cord 26 is embedded in the crown portion 16.
- a blasting device (not shown) emits a projection material at a high speed toward the outer peripheral surface 17S while rotating the tire case 17 side toward the outer peripheral surface 17S of the tire case 17.
- the ejected projection material collides with the outer peripheral surface 17S, and fine roughening unevenness 96 having an arithmetic average roughness Ra of 0.05 mm or more is formed on the outer peripheral surface 17S.
- the outer peripheral surface 17S becomes hydrophilic, and the wettability of the bonding agent described later is improved.
- a bonding agent for bonding the cushion rubber 29 is applied to the outer peripheral surface 17S of the tire case 17 subjected to the roughening treatment.
- the bonding agent is not particularly limited, and a triazine thiol adhesive, a chlorinated rubber adhesive, a phenolic resin adhesive, an isocyanate adhesive, a halogenated rubber adhesive, a rubber adhesive, or the like can be used. However, it is preferable to react at a temperature at which the cushion rubber 29 can be vulcanized (eg, 90 ° C. to 140 ° C.).
- the unvulcanized cushion rubber 29 is wound around the outer peripheral surface 17S to which the bonding agent has been applied for one round, and a bonding agent such as a rubber cement composition is applied onto the cushion rubber 29.
- a bonding agent such as a rubber cement composition
- the vulcanized or semi-cured tread rubber 30A is wound on the cushion rubber 29 to which the bonding agent has been applied for one round to form a raw tire case.
- the raw tire case is accommodated in a vulcanizing can or mold and vulcanized.
- the unvulcanized cushion rubber 29 flows into the roughened irregularities 96 formed on the outer peripheral surface 17S of the tire case 17 by the roughening treatment.
- the anchor rubber is exerted by the cushion rubber 29 flowing into the roughened unevenness 96, and the bonding strength between the tire case 17 and the cushion rubber 29 is improved. That is, the bonding strength between the tire case 17 and the tread 30 is improved via the cushion rubber 29.
- the tire skeleton body is formed of a resin material including a thermoplastic elastomer
- a tire is provided in which the amorphous portion has a thickness La in the range of 12.3 nm to 13.9 nm as measured by the small-angle X-ray scattering method of the thermoplastic elastomer.
- the first aspect is such that the long period L measured by the small-angle X-ray scattering method of the thermoplastic elastomer is in the range of 15.6 nm to 17.1 nm. Tires are provided.
- the degree of orientation f of the thermoplastic elastomer measured by a small angle X-ray scattering method is in the range of ⁇ 0.08 to 0.08.
- a tire according to a second aspect is provided.
- a fourth aspect of the present disclosure there is provided a tire according to any one of the first to third aspects, wherein the thermoplastic elastomer is a polyester-based thermoplastic elastomer.
- tires having the configuration shown in the first embodiment described above were produced by a known method.
- a resin material for forming the tire skeleton a polyester-based thermoplastic elastomer whose hard segment is polyester and whose soft segment is polyether is used.
- multifilaments having an average diameter of 1.15 mm (specifically, five monofilaments having an average diameter of 0.35 mm (made of steel, strength: 280 N, elongation: 3%)) were twisted.
- a heat-melted acid-modified polypropylene was adhered to the stranded wire to form an adhesive layer.
- the upper surface of the adhesive layer was covered with a polyamide-based thermoplastic elastomer extruded by an extruder (trade name “XPA9055” manufactured by UBE Co., Ltd.) and cooled to prepare a reinforcing cord.
- amorphous part thickness La, long period L, orientation degree f, and crystallinity degree Xc Using a sample prepared from the tire case of the prepared tire, the amorphous part thickness La and long period of the thermoplastic elastomer L and the degree of orientation f were measured by a small angle X-ray scattering method, and the crystallinity Xc was measured by a wide angle X-ray scattering method.
- product name: Nano-Viewer manufactured by Rigaku Corporation was used, and the measurement conditions were adjusted as follows. -Measurement condition- -Room temperature measurement-X-ray irradiation time 30 minutes Table 1 shows the measurement results.
- thermoplastic elastomer of Table 1 The detail of the thermoplastic elastomer of Table 1 is shown below.
- Hytrel 5557 polyyester-based thermoplastic elastomer, manufactured by Toray DuPont Co., Ltd., crystallization temperature: 163 ° C.
- Hytrel 4767N polyyester thermoplastic elastomer, manufactured by Toray DuPont Co., Ltd., crystallization temperature: 155 ° C.
- Example 6 Hytrel 5557 and Hytrel 4767N were mixed and used at a mass ratio of “25/75”.
- the tire of the example having the tire case formed using the thermoplastic elastomer having the amorphous portion thickness La in the range of 12.3 nm to 13.9 nm has the thickness of the amorphous portion. It turns out that it is excellent in durability compared with the tire of the comparative example which has the tire case formed using the thermoplastic elastomer which La does not exist in the said range.
Landscapes
- Engineering & Computer Science (AREA)
- Mechanical Engineering (AREA)
- Chemical & Material Sciences (AREA)
- Health & Medical Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Medicinal Chemistry (AREA)
- Polymers & Plastics (AREA)
- Organic Chemistry (AREA)
- Tires In General (AREA)
Priority Applications (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP18787330.2A EP3613611A4 (en) | 2017-04-18 | 2018-03-29 | PNEUMATIC |
| CN201880025491.1A CN110546018B (zh) | 2017-04-18 | 2018-03-29 | 轮胎 |
| US16/653,160 US20200039292A1 (en) | 2017-04-18 | 2019-10-15 | Tire |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2017082200A JP6817879B2 (ja) | 2017-04-18 | 2017-04-18 | タイヤ |
| JP2017-082200 | 2017-04-18 |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US16/653,160 Continuation US20200039292A1 (en) | 2017-04-18 | 2019-10-15 | Tire |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2018193811A1 true WO2018193811A1 (ja) | 2018-10-25 |
Family
ID=63856614
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2018/013439 Ceased WO2018193811A1 (ja) | 2017-04-18 | 2018-03-29 | タイヤ |
Country Status (5)
| Country | Link |
|---|---|
| US (1) | US20200039292A1 (enExample) |
| EP (1) | EP3613611A4 (enExample) |
| JP (1) | JP6817879B2 (enExample) |
| CN (1) | CN110546018B (enExample) |
| WO (1) | WO2018193811A1 (enExample) |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP7688515B2 (ja) * | 2021-05-06 | 2025-06-04 | 株式会社ブリヂストン | タイヤ及びタイヤ骨格体の製造方法 |
Citations (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH05331256A (ja) | 1992-06-02 | 1993-12-14 | Sanko Chem Co Ltd | 熱可塑性ポリウレタンの製造方法 |
| JP2000191928A (ja) * | 1998-12-28 | 2000-07-11 | Sumitomo Rubber Ind Ltd | ボ―ル用熱可塑性樹脂組成物及びこれを用いたワンピ―スゴルフボ―ル |
| JP2004346273A (ja) | 2003-05-26 | 2004-12-09 | Ube Ind Ltd | ポリアミド系エラストマー及びその製造方法 |
| WO2008026509A1 (en) * | 2006-08-29 | 2008-03-06 | National Institute Of Advanced Industrial Science And Technology | Thermoplastic elastomer composition having hierarchical structure and process for the production thereof |
| JP2008308636A (ja) * | 2007-06-18 | 2008-12-25 | Toyobo Co Ltd | 熱可塑性ポリエステルエラストマー |
| WO2011074473A1 (ja) * | 2009-12-14 | 2011-06-23 | アロン化成株式会社 | 熱可塑性エラストマー組成物及びその製造方法 |
| JP2012046025A (ja) | 2010-08-25 | 2012-03-08 | Bridgestone Corp | タイヤ |
| JP2014198779A (ja) * | 2013-03-29 | 2014-10-23 | 株式会社ブリヂストン | タイヤ |
| JP2017082200A (ja) | 2015-09-30 | 2017-05-18 | 京セラ株式会社 | 樹脂組成物、プリプレグ、金属張積層板、および配線基板 |
| WO2017146038A1 (ja) * | 2016-02-26 | 2017-08-31 | 株式会社ブリヂストン | タイヤ |
Family Cites Families (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP5893439B2 (ja) * | 2012-02-29 | 2016-03-23 | 株式会社ブリヂストン | タイヤの製造方法 |
| US20170298182A1 (en) * | 2014-09-24 | 2017-10-19 | Bridgestone Corporation | Tire |
-
2017
- 2017-04-18 JP JP2017082200A patent/JP6817879B2/ja active Active
-
2018
- 2018-03-29 CN CN201880025491.1A patent/CN110546018B/zh active Active
- 2018-03-29 WO PCT/JP2018/013439 patent/WO2018193811A1/ja not_active Ceased
- 2018-03-29 EP EP18787330.2A patent/EP3613611A4/en not_active Withdrawn
-
2019
- 2019-10-15 US US16/653,160 patent/US20200039292A1/en not_active Abandoned
Patent Citations (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH05331256A (ja) | 1992-06-02 | 1993-12-14 | Sanko Chem Co Ltd | 熱可塑性ポリウレタンの製造方法 |
| JP2000191928A (ja) * | 1998-12-28 | 2000-07-11 | Sumitomo Rubber Ind Ltd | ボ―ル用熱可塑性樹脂組成物及びこれを用いたワンピ―スゴルフボ―ル |
| JP2004346273A (ja) | 2003-05-26 | 2004-12-09 | Ube Ind Ltd | ポリアミド系エラストマー及びその製造方法 |
| WO2008026509A1 (en) * | 2006-08-29 | 2008-03-06 | National Institute Of Advanced Industrial Science And Technology | Thermoplastic elastomer composition having hierarchical structure and process for the production thereof |
| JP2008308636A (ja) * | 2007-06-18 | 2008-12-25 | Toyobo Co Ltd | 熱可塑性ポリエステルエラストマー |
| WO2011074473A1 (ja) * | 2009-12-14 | 2011-06-23 | アロン化成株式会社 | 熱可塑性エラストマー組成物及びその製造方法 |
| JP2012046025A (ja) | 2010-08-25 | 2012-03-08 | Bridgestone Corp | タイヤ |
| JP2014198779A (ja) * | 2013-03-29 | 2014-10-23 | 株式会社ブリヂストン | タイヤ |
| JP2017082200A (ja) | 2015-09-30 | 2017-05-18 | 京セラ株式会社 | 樹脂組成物、プリプレグ、金属張積層板、および配線基板 |
| WO2017146038A1 (ja) * | 2016-02-26 | 2017-08-31 | 株式会社ブリヂストン | タイヤ |
Non-Patent Citations (1)
| Title |
|---|
| See also references of EP3613611A4 |
Also Published As
| Publication number | Publication date |
|---|---|
| JP6817879B2 (ja) | 2021-01-20 |
| US20200039292A1 (en) | 2020-02-06 |
| EP3613611A1 (en) | 2020-02-26 |
| JP2018177091A (ja) | 2018-11-15 |
| EP3613611A4 (en) | 2020-11-25 |
| CN110546018A (zh) | 2019-12-06 |
| CN110546018B (zh) | 2021-12-07 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6057981B2 (ja) | タイヤ | |
| JP6086643B2 (ja) | タイヤ | |
| JP5818577B2 (ja) | タイヤ | |
| JP6138412B2 (ja) | タイヤ | |
| WO2013129632A1 (ja) | タイヤ | |
| JP5847555B2 (ja) | タイヤ | |
| JP2013220692A (ja) | タイヤ | |
| WO2019230822A1 (ja) | タイヤ用樹脂金属複合部材、及びその製造方法、並びにタイヤ | |
| WO2017104484A1 (ja) | タイヤ | |
| JP5818578B2 (ja) | タイヤ | |
| JP5911731B2 (ja) | タイヤ | |
| JP5778402B2 (ja) | タイヤ | |
| JP5627954B2 (ja) | タイヤ | |
| JP6014713B2 (ja) | タイヤ | |
| CN108495890B (zh) | 轮胎 | |
| JP5993544B2 (ja) | タイヤ | |
| JP6817879B2 (ja) | タイヤ | |
| JP7688515B2 (ja) | タイヤ及びタイヤ骨格体の製造方法 | |
| JP5905566B2 (ja) | タイヤ | |
| JP6014714B2 (ja) | タイヤ及びその製造方法 | |
| WO2017104483A1 (ja) | タイヤ | |
| WO2017104187A1 (ja) | タイヤ | |
| US20200238762A1 (en) | Tire | |
| JP2019001358A (ja) | タイヤ |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 18787330 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| ENP | Entry into the national phase |
Ref document number: 2018787330 Country of ref document: EP Effective date: 20191118 |