WO2016167152A1 - Gas-barrier plastic molded product and method for manufacturing same - Google Patents
Gas-barrier plastic molded product and method for manufacturing same Download PDFInfo
- Publication number
- WO2016167152A1 WO2016167152A1 PCT/JP2016/061070 JP2016061070W WO2016167152A1 WO 2016167152 A1 WO2016167152 A1 WO 2016167152A1 JP 2016061070 W JP2016061070 W JP 2016061070W WO 2016167152 A1 WO2016167152 A1 WO 2016167152A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- content
- gas barrier
- plastic molded
- vacuum chamber
- equation
- Prior art date
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C23—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; CHEMICAL SURFACE TREATMENT; DIFFUSION TREATMENT OF METALLIC MATERIAL; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL; INHIBITING CORROSION OF METALLIC MATERIAL OR INCRUSTATION IN GENERAL
- C23C—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; SURFACE TREATMENT OF METALLIC MATERIAL BY DIFFUSION INTO THE SURFACE, BY CHEMICAL CONVERSION OR SUBSTITUTION; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL
- C23C16/00—Chemical coating by decomposition of gaseous compounds, without leaving reaction products of surface material in the coating, i.e. chemical vapour deposition [CVD] processes
- C23C16/22—Chemical coating by decomposition of gaseous compounds, without leaving reaction products of surface material in the coating, i.e. chemical vapour deposition [CVD] processes characterised by the deposition of inorganic material, other than metallic material
- C23C16/30—Deposition of compounds, mixtures or solid solutions, e.g. borides, carbides, nitrides
- C23C16/40—Oxides
- C23C16/401—Oxides containing silicon
-
- C—CHEMISTRY; METALLURGY
- C23—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; CHEMICAL SURFACE TREATMENT; DIFFUSION TREATMENT OF METALLIC MATERIAL; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL; INHIBITING CORROSION OF METALLIC MATERIAL OR INCRUSTATION IN GENERAL
- C23C—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; SURFACE TREATMENT OF METALLIC MATERIAL BY DIFFUSION INTO THE SURFACE, BY CHEMICAL CONVERSION OR SUBSTITUTION; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL
- C23C16/00—Chemical coating by decomposition of gaseous compounds, without leaving reaction products of surface material in the coating, i.e. chemical vapour deposition [CVD] processes
- C23C16/22—Chemical coating by decomposition of gaseous compounds, without leaving reaction products of surface material in the coating, i.e. chemical vapour deposition [CVD] processes characterised by the deposition of inorganic material, other than metallic material
- C23C16/30—Deposition of compounds, mixtures or solid solutions, e.g. borides, carbides, nitrides
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B65—CONVEYING; PACKING; STORING; HANDLING THIN OR FILAMENTARY MATERIAL
- B65D—CONTAINERS FOR STORAGE OR TRANSPORT OF ARTICLES OR MATERIALS, e.g. BAGS, BARRELS, BOTTLES, BOXES, CANS, CARTONS, CRATES, DRUMS, JARS, TANKS, HOPPERS, FORWARDING CONTAINERS; ACCESSORIES, CLOSURES, OR FITTINGS THEREFOR; PACKAGING ELEMENTS; PACKAGES
- B65D23/00—Details of bottles or jars not otherwise provided for
- B65D23/02—Linings or internal coatings
-
- C—CHEMISTRY; METALLURGY
- C23—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; CHEMICAL SURFACE TREATMENT; DIFFUSION TREATMENT OF METALLIC MATERIAL; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL; INHIBITING CORROSION OF METALLIC MATERIAL OR INCRUSTATION IN GENERAL
- C23C—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; SURFACE TREATMENT OF METALLIC MATERIAL BY DIFFUSION INTO THE SURFACE, BY CHEMICAL CONVERSION OR SUBSTITUTION; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL
- C23C16/00—Chemical coating by decomposition of gaseous compounds, without leaving reaction products of surface material in the coating, i.e. chemical vapour deposition [CVD] processes
- C23C16/04—Coating on selected surface areas, e.g. using masks
- C23C16/045—Coating cavities or hollow spaces, e.g. interior of tubes; Infiltration of porous substrates
-
- C—CHEMISTRY; METALLURGY
- C23—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; CHEMICAL SURFACE TREATMENT; DIFFUSION TREATMENT OF METALLIC MATERIAL; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL; INHIBITING CORROSION OF METALLIC MATERIAL OR INCRUSTATION IN GENERAL
- C23C—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; SURFACE TREATMENT OF METALLIC MATERIAL BY DIFFUSION INTO THE SURFACE, BY CHEMICAL CONVERSION OR SUBSTITUTION; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL
- C23C16/00—Chemical coating by decomposition of gaseous compounds, without leaving reaction products of surface material in the coating, i.e. chemical vapour deposition [CVD] processes
- C23C16/22—Chemical coating by decomposition of gaseous compounds, without leaving reaction products of surface material in the coating, i.e. chemical vapour deposition [CVD] processes characterised by the deposition of inorganic material, other than metallic material
- C23C16/30—Deposition of compounds, mixtures or solid solutions, e.g. borides, carbides, nitrides
- C23C16/32—Carbides
- C23C16/325—Silicon carbide
-
- C—CHEMISTRY; METALLURGY
- C23—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; CHEMICAL SURFACE TREATMENT; DIFFUSION TREATMENT OF METALLIC MATERIAL; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL; INHIBITING CORROSION OF METALLIC MATERIAL OR INCRUSTATION IN GENERAL
- C23C—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; SURFACE TREATMENT OF METALLIC MATERIAL BY DIFFUSION INTO THE SURFACE, BY CHEMICAL CONVERSION OR SUBSTITUTION; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL
- C23C16/00—Chemical coating by decomposition of gaseous compounds, without leaving reaction products of surface material in the coating, i.e. chemical vapour deposition [CVD] processes
- C23C16/22—Chemical coating by decomposition of gaseous compounds, without leaving reaction products of surface material in the coating, i.e. chemical vapour deposition [CVD] processes characterised by the deposition of inorganic material, other than metallic material
- C23C16/30—Deposition of compounds, mixtures or solid solutions, e.g. borides, carbides, nitrides
- C23C16/40—Oxides
-
- C—CHEMISTRY; METALLURGY
- C23—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; CHEMICAL SURFACE TREATMENT; DIFFUSION TREATMENT OF METALLIC MATERIAL; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL; INHIBITING CORROSION OF METALLIC MATERIAL OR INCRUSTATION IN GENERAL
- C23C—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; SURFACE TREATMENT OF METALLIC MATERIAL BY DIFFUSION INTO THE SURFACE, BY CHEMICAL CONVERSION OR SUBSTITUTION; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL
- C23C16/00—Chemical coating by decomposition of gaseous compounds, without leaving reaction products of surface material in the coating, i.e. chemical vapour deposition [CVD] processes
- C23C16/22—Chemical coating by decomposition of gaseous compounds, without leaving reaction products of surface material in the coating, i.e. chemical vapour deposition [CVD] processes characterised by the deposition of inorganic material, other than metallic material
- C23C16/30—Deposition of compounds, mixtures or solid solutions, e.g. borides, carbides, nitrides
- C23C16/42—Silicides
-
- C—CHEMISTRY; METALLURGY
- C23—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; CHEMICAL SURFACE TREATMENT; DIFFUSION TREATMENT OF METALLIC MATERIAL; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL; INHIBITING CORROSION OF METALLIC MATERIAL OR INCRUSTATION IN GENERAL
- C23C—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; SURFACE TREATMENT OF METALLIC MATERIAL BY DIFFUSION INTO THE SURFACE, BY CHEMICAL CONVERSION OR SUBSTITUTION; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL
- C23C16/00—Chemical coating by decomposition of gaseous compounds, without leaving reaction products of surface material in the coating, i.e. chemical vapour deposition [CVD] processes
- C23C16/44—Chemical coating by decomposition of gaseous compounds, without leaving reaction products of surface material in the coating, i.e. chemical vapour deposition [CVD] processes characterised by the method of coating
-
- C—CHEMISTRY; METALLURGY
- C23—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; CHEMICAL SURFACE TREATMENT; DIFFUSION TREATMENT OF METALLIC MATERIAL; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL; INHIBITING CORROSION OF METALLIC MATERIAL OR INCRUSTATION IN GENERAL
- C23C—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; SURFACE TREATMENT OF METALLIC MATERIAL BY DIFFUSION INTO THE SURFACE, BY CHEMICAL CONVERSION OR SUBSTITUTION; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL
- C23C16/00—Chemical coating by decomposition of gaseous compounds, without leaving reaction products of surface material in the coating, i.e. chemical vapour deposition [CVD] processes
- C23C16/44—Chemical coating by decomposition of gaseous compounds, without leaving reaction products of surface material in the coating, i.e. chemical vapour deposition [CVD] processes characterised by the method of coating
- C23C16/448—Chemical coating by decomposition of gaseous compounds, without leaving reaction products of surface material in the coating, i.e. chemical vapour deposition [CVD] processes characterised by the method of coating characterised by the method used for generating reactive gas streams, e.g. by evaporation or sublimation of precursor materials
-
- C—CHEMISTRY; METALLURGY
- C23—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; CHEMICAL SURFACE TREATMENT; DIFFUSION TREATMENT OF METALLIC MATERIAL; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL; INHIBITING CORROSION OF METALLIC MATERIAL OR INCRUSTATION IN GENERAL
- C23C—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; SURFACE TREATMENT OF METALLIC MATERIAL BY DIFFUSION INTO THE SURFACE, BY CHEMICAL CONVERSION OR SUBSTITUTION; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL
- C23C16/00—Chemical coating by decomposition of gaseous compounds, without leaving reaction products of surface material in the coating, i.e. chemical vapour deposition [CVD] processes
- C23C16/44—Chemical coating by decomposition of gaseous compounds, without leaving reaction products of surface material in the coating, i.e. chemical vapour deposition [CVD] processes characterised by the method of coating
- C23C16/448—Chemical coating by decomposition of gaseous compounds, without leaving reaction products of surface material in the coating, i.e. chemical vapour deposition [CVD] processes characterised by the method of coating characterised by the method used for generating reactive gas streams, e.g. by evaporation or sublimation of precursor materials
- C23C16/452—Chemical coating by decomposition of gaseous compounds, without leaving reaction products of surface material in the coating, i.e. chemical vapour deposition [CVD] processes characterised by the method of coating characterised by the method used for generating reactive gas streams, e.g. by evaporation or sublimation of precursor materials by activating reactive gas streams before their introduction into the reaction chamber, e.g. by ionisation or addition of reactive species
-
- C—CHEMISTRY; METALLURGY
- C23—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; CHEMICAL SURFACE TREATMENT; DIFFUSION TREATMENT OF METALLIC MATERIAL; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL; INHIBITING CORROSION OF METALLIC MATERIAL OR INCRUSTATION IN GENERAL
- C23C—COATING METALLIC MATERIAL; COATING MATERIAL WITH METALLIC MATERIAL; SURFACE TREATMENT OF METALLIC MATERIAL BY DIFFUSION INTO THE SURFACE, BY CHEMICAL CONVERSION OR SUBSTITUTION; COATING BY VACUUM EVAPORATION, BY SPUTTERING, BY ION IMPLANTATION OR BY CHEMICAL VAPOUR DEPOSITION, IN GENERAL
- C23C16/00—Chemical coating by decomposition of gaseous compounds, without leaving reaction products of surface material in the coating, i.e. chemical vapour deposition [CVD] processes
- C23C16/44—Chemical coating by decomposition of gaseous compounds, without leaving reaction products of surface material in the coating, i.e. chemical vapour deposition [CVD] processes characterised by the method of coating
- C23C16/52—Controlling or regulating the coating process
Definitions
- the present invention relates to a gas barrier plastic molding and a method for producing the same.
- a heating element CVD method is known as a technique for forming a thin film having gas barrier properties (hereinafter sometimes referred to as a gas barrier thin film).
- the heating element CVD method is also referred to as a Cat-CVD method or a hot wire CVD method, which decomposes by bringing a raw material gas into contact with a heated heating element and undergoes a reaction process directly or in a gas phase.
- This is a method of depositing as a thin film on the surface of a plastic molded body (see, for example, Patent Document 1).
- Patent Document 1 is excellent in gas barrier properties but lacks transparency.
- An object of the present invention is to provide a gas barrier plastic molded article excellent in gas barrier properties and transparency and a method for producing the same.
- the content of Si, O, or C is the content in the breakdown of the three elements of Si, O, and C.
- the content of Si, O, or C is the content in the breakdown of the three elements of Si, O, and C.
- the method for producing a gas barrier plastic molding according to the present invention includes an evacuation step in which the inside of a vacuum chamber is evacuated to adjust the inside of the vacuum chamber to an initial pressure P 0 or lower, and the pressure in the vacuum chamber is set to P 0 or lower.
- a silicon-containing hydrocarbon gas is introduced into the vacuum chamber to reduce the pressure in the vacuum chamber.
- a film forming step for forming the film for forming the film.
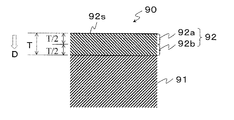
- Examples of the resin constituting the plastic molded body 91 include polyethylene terephthalate resin (PET), polybutylene terephthalate resin, polyethylene naphthalate resin, polyethylene resin, polypropylene resin (PP), and cycloolefin copolymer resin (COC, cyclic olefin copolymer).
- PET polyethylene terephthalate resin
- PP polypropylene resin
- COC cycloolefin copolymer resin
- Ionomer resin poly-4-methylpentene-1 resin, polymethyl methacrylate resin, polystyrene resin, ethylene-vinyl alcohol copolymer resin, acrylonitrile resin, polyvinyl chloride resin, polyvinylidene chloride resin, polyamide resin, polyamideimide resin
- Polyacetal resin polycarbonate resin, polysulfone resin, tetrafluoroethylene resin, acrylonitrile-styrene resin, or acrylonitrile-butadiene-styrene resin That.
- One of these may be used as a single layer, or two or more may be used as a laminate, but a single layer is preferable in terms of productivity.
- the type of resin is more preferably PET.
- the gas barrier plastic molded body 90 includes a form in which the plastic molded body 91 is a container, a film, or a sheet.
- the shape can be appropriately set according to the purpose and application, and is not particularly limited.
- the container includes a container that is used with a lid, a stopper, or a seal, or a container that is used without being used.
- the size of the opening can be appropriately set according to the contents.
- the plastic container includes a plastic container having a predetermined thickness having moderate rigidity and a plastic container formed by a sheet material having no rigidity. The present invention is not limited to the manufacturing method of the container.
- the contents are, for example, beverages such as water, tea beverages, soft drinks, carbonated beverages or fruit juice beverages, or liquid, viscous, powder or solid foods.
- the container may be either a returnable container or a one-way container.
- the film or sheet includes a long sheet or cut sheet. It does not matter whether the film or sheet is stretched or unstretched.
- the present invention is not limited to the method for manufacturing the plastic molded body 91.
- the thickness of the plastic molded body 91 can be appropriately set according to the purpose and application, and is not particularly limited.
- the thickness of the bottle is preferably 50 to 500 ⁇ m, more preferably 100 to 350 ⁇ m.
- the thickness of the film is preferably 3 to 300 ⁇ m, more preferably 10 to 100 ⁇ m.
- the thickness of the film is preferably 25 to 200 ⁇ m, more preferably 50 to 100 ⁇ m.
- the thickness of the sheet is preferably 50 to 500 ⁇ m, more preferably 100 to 350 ⁇ m.
- the gas barrier thin film 92 is provided on one or both of the inner wall surface and the outer wall surface. Further, when the plastic molded body 91 is a film, the gas barrier thin film 92 is provided on one side or both sides.
- the gas barrier thin film 92 contains silicon (Si), carbon (C), and oxygen (O) as constituent elements, and when the X-ray electron spectroscopic analysis is performed under the condition (1), the peak appearance position of the bond energy of Si—C And has a region where the main peak is observed.
- Condition (1) The measurement range is 95 to 105 eV.
- the gas barrier thin film 92 is a thin film having excellent transparency.
- the main peak means a peak having the highest intensity among peaks observed by separating the peaks in the condition (1).
- a peak observed at the peak appearance position of the Si—C bond energy is a Si—Si bond. It is preferably larger than the peak observed at the energy peak appearance position. Thereby, the transparency can be further enhanced.
- the content of Si, O, or C is the content in the breakdown of the three elements of Si, O, and C.
- Si content [%] ⁇ (Si content [atomic%]) / (total content of Si, O and C [atomic%]) ⁇ ⁇ 100
- the content of Si, O, or C is the content in the breakdown of the three elements of Si, O, and C.
- the O content represented by (Equation 3) in the upper layer 92a is lower than the Si content represented by (Equation 2) in the upper layer 92a (Condition 2). It is preferable.
- the upper layer 92a has the highest C content, then the Si content and the O content, and the transparency can be further enhanced.
- (Equation 3) O content [%] ⁇ (O content [atomic%]) / (total content of Si, O and C [atomic%]) ⁇ ⁇ 100
- the content of Si, O, or C is the content in the breakdown of the three elements of Si, O, and C.
- the C content represented by (Equation 1) in the lower layer 92b is higher than the O content represented by (Equation 3) in the lower layer 92b (Condition 3). Is preferred.
- the adhesion between the thin film and the plastic molded body can be increased.
- the gas barrier thin film 92 is preferably substantially colorless and transparent.
- substantially colorless and transparent means that the b * value is 2.0 or less, using the degree of coloration b *, which is the color difference in JIS K 7105-1981 “Testing methods for optical properties of plastics” as an index. Say things.
- the b * value is more preferably 1.7 or less.
- the b * value can be obtained by Equation 4.
- Equation 4 Y or Z is a tristimulus value.
- the correlation between the b * value and visual observation in the present invention is as shown in Table 1.
- the gas barrier plastic molded body 90 preferably has a barrier property improvement rate (Barrier Improvement Factor, hereinafter referred to as BIF) obtained by Equation 5 of 5 or more. More preferably, it is 10 or more.
- BIF Barrier Improvement Factor
- the oxygen permeability is set to 0.1. 0070 cc / container / day or less.
- the oxygen permeability can be 0.0098 cc / container / day or less.
- a method for producing a gas barrier plastic molded body according to this embodiment will be described by taking as an example a case where a gas barrier thin film is formed on the inner surface of a plastic bottle as the plastic molded body 4.
- the present invention is not limited to an apparatus, and for example, as shown in FIG. 2 of Patent Document 1, an apparatus having only one chamber may be used.
- silicon-containing hydrocarbon gas is introduced while the vacuum chamber 31 is evacuated to adjust the pressure in the vacuum chamber 31 to P 0 .
- the pressure in the vacuum chamber 31 increases rapidly, and the pressure in the vacuum chamber 31 may greatly exceed P 0 (for example, exceed P B ) in the initial stage of the preparation process.
- P 0 for example, exceed P B
- the pressure in the vacuum chamber 31 may be adjusted to be less than P 0 in the evacuation process so that the pressure in the vacuum chamber 31 does not become a large pressure exceeding, for example, P B in the preparation process.
- the heating element 42 is heated by energization, for example.
- the heating element 42 has a tantalum carbide phase.
- the tantalum carbide phase is, for example, a carbide obtained by carbonizing tantalum, a tantalum-based alloy, or tantalum or a tantalum-based alloy containing an additive.
- the tantalum carbide phase may contain, for example, Ta 2 C and TaC.
- the tantalum carbide phase may be present throughout the heating element 42 or may be present in a portion of the heating element 42.
- the heating element 42 is heated for the first time in the film forming process, and the heating element 42 is not heated in the exhaust process and the preparation process.
- the only gas remaining in the vacuum chamber 31 is air, and when the heating element 42 is heated in such an atmosphere, the heating element 42 is likely to be oxidized and deteriorated.
- the vacuum breaker valve (not shown) installed in the entrance / exit chamber 32 is operated to open the inside / outside chamber 32 to the atmosphere. At this time, it is preferable that the vacuum chamber 31 is always in a vacuum state, and the heating element 42 disposed in the vacuum chamber 31 is always kept in a vacuum state.
- the open / close gate 56 is opened, the gas barrier plastic molded body is taken out, and a new untreated plastic molded body is introduced. Then, the open / close gate 56 is closed, and the exhaust process, the preparation process, and the film forming process are repeated.
- the method for producing a gas barrier plastic molding having a substantially colorless and transparent gas barrier thin film has been described.
- the gas barrier thin film is not required to have a high degree of transparency (for example, b * exceeds 2 and is 6 or less). The case will be described.
- the method for producing a gas barrier plastic molded body according to the second embodiment includes an evacuation process in which the inside of the vacuum chamber 31 is evacuated to adjust the inside of the vacuum chamber 31 to an initial pressure P 0 or less, and the pressure in the vacuum chamber 31 is P.
- the heating element 42 is adjusted to 0 or less and is not heated, the source gas is introduced into the vacuum chamber 31 to adjust the pressure in the vacuum chamber 31 to P 0 .
- the manufacturing method according to the second embodiment is different from the manufacturing method according to the first embodiment in the following two points.
- the first point is the type of the heating element 42.
- the heating element 42 has a tantalum carbide phase, whereas in the second embodiment, the material of the heating element 42 is not limited.
- the second point is the type of source gas used. While the silicon-containing hydrocarbon gas is used in the first embodiment, the second embodiment is not limited to the silicon-containing hydrocarbon gas.
- the manufacturing method of the second embodiment has the same basic configuration as the manufacturing method according to the first embodiment except for the above two points. For this reason, here, description is omitted about a common structure, and only a different point is demonstrated.
- the material of the heating element is not particularly limited, but C, W, Ta, Nb, Ti, Hf, V, Cr, Mo, Mn, Tc, Re, Fe, Ru, Os, Co, Rh , Ir, Ni, Pd and Pt are preferably included.
- a heat generating body contains the 1 or 2 or more metal element chosen from the group of Ta, W, Mo, and Nb, for example.
- the material containing a metal element is a pure metal, an alloy, a metal or alloy containing an additive, or an intermetallic compound.
- the metal which forms an alloy or an intermetallic compound may be a combination of two or more of the aforementioned metals, or a combination of the aforementioned metals and other metals.
- the other metal is, for example, chromium.
- the alloy or intermetallic compound preferably contains a total of 80 atomic% or more of one or more metal elements selected from the group of Ta, W, Mo and Nb.
- the additive is, for example, an oxide such as zirconia, yttria, calcia, or silica. The addition amount of the additive is preferably 1% by mass or less.
- the source gas other than the above-mentioned silicon-containing hydrocarbon gas is, for example, alkane-based gases such as methane, ethane, propane, butane, pentane, or hexane, and alkene-based gases such as ethylene, propylene, or butyne.
- alkane-based gases such as methane, ethane, propane, butane, pentane, or hexane
- alkene-based gases such as ethylene, propylene, or butyne.
- Example 1 A gas barrier plastic molded body was manufactured using the film forming apparatus shown in FIG.
- a plastic bottle made of PET (with an internal volume of 500 ml) was used as the plastic molding, vinylsilane as the silicon-containing hydrocarbon gas, and tantalum carbide wire ( ⁇ 0.5 mm) as the heating element.
- the plastic bottle was lowered from the entrance / exit chamber, and the heating element and the source gas supply pipe were inserted into the plastic bottle.
- the film forming process was performed as follows. In the film forming process, heating of the heating element is started while continuing the introduction of the silicon-containing hydrocarbon gas, and the heating is performed up to 2100 to 2200 ° C. When the thin film deposited on the inner surface of the plastic bottle reaches 20 nm, the heating is generated. Body heating stopped. Thereafter, the plastic bottle was returned to the entrance / exit chamber, the gate valve was closed, and the gas supply was stopped. In the film forming process, the pressure in the vacuum chamber was allowed to reach P B Pa higher than P A Pa.
- Example 2 A gas barrier plastic molded article was produced in the same manner as in Example 1 except that the pressures P A and P B in each step were adjusted so that (P B ⁇ P A ) / P 0 was 0.15.
- Example 1 A gas barrier plastic molded article was prepared in the same manner as in Example 1 except that a tantalum wire ( ⁇ 0.5 mm) that was not carbonized was used as a heating element, the preparatory process was not performed, and the thickness of the thin film was changed to 36 nm. Manufactured.
- XPS analysis-composition analysis The surface of the thin film of the plastic bottle obtained in Example 1 and Comparative Example 1 in the first series of film forming operations was analyzed using an XPS apparatus (model: QUANTERASXM, manufactured by PHI). Table 2 shows the ratio of constituent elements on the surface of the thin film.
- the conditions of XPS analysis are as follows. Measurement conditions Excitation X-ray: Al mono Detection area: 100 ⁇ m ⁇ Extraction angle: 90deg Detection depth: about 8nm
- FIG. 4 is a narrow scan spectrum of Si2p obtained by XPS analysis of the thin film surface under the condition (1).
- (A) is the thin film of Example 1
- (b) is the thin film of Comparative Example 1.
- a main peak was observed at the peak appearance position of the Si—C bond energy
- Comparative Example 1 the main peak was observed at the peak appearance position of the Si—Si bond energy. A peak was observed.
- Example 1 XPS analysis-depth profile analysis
- Comparative Example 1 the depth profile of the thin film of the plastic bottle obtained in the first series of film forming operations was analyzed for Example 1 and Comparative Example 1 while performing argon ion etching.
- the test piece and analysis conditions were the same as in the composition analysis.
- the gas barrier thin film is divided into two equal parts in the depth direction, in Example 1, 10 nm on the opposite side to the plastic molded body is the upper layer, 10 nm on the plastic molded body side is the lower layer, and in Comparative Example 1, the plastic is 18 nm on the side opposite to the molded body was the upper layer, and 18 nm on the plastic molded body side was the lower layer.
- FIG. 5 is a depth profile of Example 1.
- the O and C profiles have one extreme value in each of the upper layer and the lower layer, the O profile having the highest priority is selected, and Si, C, and O at the extreme values of the O profile are selected.
- the content of was compared.
- the extreme value of the profile of O has a minimum value in Sputter Time 1.5 min included in the upper layer, and has a maximum value in Sputter Time 6.0 min included in the lower layer.
- the C content in Sputter Time 1.5 min was higher than the Si content in Sputter Time 1.5 min.
- the O content in Sputter Time 1.5 min was lower than the Si content in Sputter Time 1.5 min. From the above, in Example 1, it was confirmed that the composition at the extreme value of the O profile of the upper layer had the highest C content, followed by the Si content and the O content.
- FIG. 6 is a depth profile of Comparative Example 1.
- the O and C profiles have one extreme value in each of the upper layer and the lower layer, the O profile having the highest priority is selected and Si, C, and O at the extreme values of the O profile are selected. The content of was compared.
- the extreme value of the profile of O has a minimum value in Sputter Time 6.0 min included in the upper layer, and has a maximum value in Sputter Time 13.5 min included in the lower layer.
- the C content in Sputter Time 6.0 min was lower than the Si content in Sputter Time 6.0 min.
- the O content in Sputter Time 6.0 min was lower than the Si content in Sputter Time 6.0 min. From the above, in Comparative Example 1, it was confirmed that the composition at the extreme value of the O profile of the upper layer had the highest Si content.
- Example 1 as shown in FIG. 5, the C content in Sputter Time 6.0 min is higher than the Si content in Sputter Time 6.0 min, and the O content in Sputter Time 6.0 min is Sputter Time 6.0 min. It was higher than the Si content in.
- Comparative Example 1 as shown in FIG. 6, the C content in Sputter Time 13.5 min is lower than the Si content in Sutter Time 13.5 min, and the O content in Sputter Time 13.5 min is Sputter. It was higher than the Si content in Time 13.5 min.
- Example 1 when the integrated value of each atomic concentration of Si, C, and O in the lower layer was determined, in Example 1, the lower layer had the highest C content, and then the O content and the Si content. It could be confirmed. Moreover, in FIG. 6, when the integrated value of each atomic concentration of Si, C, and O in the lower layer was obtained, in Comparative Example 1, the lower layer had the highest O content, followed by the Si content and the C content. I was able to confirm.
- Transparency evaluation The transparency was evaluated using the plastic bottles obtained in the first series of film forming operations for Examples and Comparative Examples. Transparency was evaluated by b * value.
- the b * value was measured using a self-recording spectrophotometer (U-3900, manufactured by Hitachi) equipped with a 60 ⁇ integrating sphere attachment device (for infrared, visible and near infrared) manufactured by the same company.
- a self-recording spectrophotometer U-3900, manufactured by Hitachi
- a 60 ⁇ integrating sphere attachment device for infrared, visible and near infrared manufactured by the same company.
- an ultrasensitive photomultiplier tube R928: for ultraviolet and visible
- cooled PbS for near infrared region
- the transmittance measurement of only the gas barrier thin film can be calculated.
- the b * value in this example is the same as that calculated in the form including the absorption rate of the PET bottle. Show.
- the test piece used for the measurement of glossiness was used for the measurement.
- the average value of 3 sheets is shown in Table 3 as b * value.
- gas barrier property evaluation Regarding Examples and Comparative Examples, gas barrier properties were evaluated using each plastic bottle obtained when the number of repetitions of a series of film forming operations was 1st, 100th, and 200th. The gas barrier property was evaluated by BIF. First, oxygen permeability was measured for each plastic bottle of the example or comparative example. The oxygen permeability was measured under the conditions of 23 ° C. and 90% RH using an oxygen permeability measuring device (model: Oxtran 2/20, manufactured by Modern Control), conditioned for 24 hours from the start of measurement, and then started measurement. The value after 72 hours had passed.
- an oxygen permeability measuring device model: Oxtran 2/20, manufactured by Modern Control
- the oxygen permeability value of the unformed film bottle is defined as the oxygen permeability value of the plastic molded body where the thin film is not formed, and the oxygen permeability value of each plastic bottle of the example or comparative example is used as the gas barrier plastic molding. Calculated as oxygen permeability of the body.
- the evaluation criteria are as follows. The evaluation results are shown in Table 3. A: BIF of each plastic bottle is 10 or more (practical level). ⁇ : BIF of each plastic bottle is 5 or more and less than 10 (practical lower limit level). X: BIF of each plastic bottle is less than 5 (practical unsuitable level).
- each Example was substantially colorless and transparent and had high gas barrier properties. Moreover, compared with the comparative example 1, it has confirmed that it was a manufacturing method excellent in durability with respect to the catalyst activity and intensity
- Example 1 and Example 2 were able to form a thin film having a high gas barrier property without losing the catalytic activity of the heating element even when the heating element was repeatedly used 10,000 times or more.
- (P B -P A ) / P 0 was 0.11 or more, and a gas barrier thin film having high transparency and high gas barrier properties could be formed.
- (P B -P A ) / P 0 was 0.15 or more, and the gas barrier property was kept high even when the heating element was repeatedly used.
Abstract
Description
条件(1)測定範囲を95~105eVとする。 The gas barrier plastic molded article according to the present invention is a gas barrier plastic molded article comprising a plastic molded article and a gas barrier thin film provided on the surface of the plastic molded article, wherein the gas barrier thin film comprises silicon (Si) as a constituent element, It contains carbon (C) and oxygen (O), and when X-ray electron spectroscopy analysis is performed under the condition (1), it has a region where a main peak is observed at the peak appearance position of the bond energy of Si—C. Features.
Condition (1) The measurement range is 95 to 105 eV.
(数1)C含有率[%]={(C含有量[atomic%])/(Si,O及びCの合計含有量[atomic%])}×100
数1において、Si,O又はCの含有量は、Si,O及びCの3元素の内訳における含有量である。
(数2)Si含有率[%]={(Si含有量[atomic%])/(Si,O及びCの合計含有量[atomic%])}×100
数2において、Si,O又はCの含有量は、Si,O及びCの3元素の内訳における含有量である。 In the gas barrier plastic molded article according to the present invention, the gas barrier thin film has a gradient composition in the depth direction, the gas barrier thin film is divided into two equal parts in the depth direction, and the side opposite to the plastic molded article is the upper layer. When the plastic molding side is the lower layer, the C content represented by (Equation 1) in the upper layer is preferably higher than the Si content represented by (Equation 2) in the upper layer.
(Equation 1) C content [%] = {(C content [atomic%]) / (total content of Si, O and C [atomic%])} × 100
In Equation 1, the content of Si, O, or C is the content in the breakdown of the three elements of Si, O, and C.
(Expression 2) Si content [%] = {(Si content [atomic%]) / (total content of Si, O and C [atomic%])} × 100
In Formula 2, the content of Si, O, or C is the content in the breakdown of the three elements of Si, O, and C.
(数3)O含有率[%]={(O含有量[atomic%])/(Si,O及びCの合計含有量[atomic%])}×100
数3において、Si,O又はCの含有量は、Si,O及びCの3元素の内訳における含有量である。 In the gas barrier plastic molded article according to the present invention, it is preferable that the O content represented by (Equation 3) in the upper layer is lower than the Si content in the upper layer.
(Equation 3) O content [%] = {(O content [atomic%]) / (total content of Si, O and C [atomic%])} × 100
In Equation 3, the content of Si, O, or C is the content in the breakdown of the three elements of Si, O, and C.
(数1)C含有率[%]={(C含有量[atomic%])/(Si,O及びCの合計含有量[atomic%])}×100
数1において、Si,O又はCの含有量は、Si,O及びCの3元素の内訳における含有量である。
(数3)O含有率[%]={(O含有量[atomic%])/(Si,O及びCの合計含有量[atomic%])}×100
数3において、Si,O又はCの含有量は、Si,O及びCの3元素の内訳における含有量である。 In the gas barrier plastic molded article according to the present invention, the gas barrier thin film has a gradient composition in the depth direction, the gas barrier thin film is divided into two equal parts in the depth direction, and the side opposite to the plastic molded article is the upper layer. When the plastic molding side is the lower layer, the C content represented by (Equation 1) in the lower layer is preferably higher than the O content represented by (Equation 3) in the lower layer. By making C content rate higher than O content rate in a lower layer, the adhesiveness of a thin film and a plastic molding can be improved.
(Equation 1) C content [%] = {(C content [atomic%]) / (total content of Si, O and C [atomic%])} × 100
In Equation 1, the content of Si, O, or C is the content in the breakdown of the three elements of Si, O, and C.
(Equation 3) O content [%] = {(O content [atomic%]) / (total content of Si, O and C [atomic%])} × 100
In Equation 3, the content of Si, O, or C is the content in the breakdown of the three elements of Si, O, and C.
条件(1)測定範囲を95~105eVとする。 FIG. 1 is a cross-sectional view showing an example of a gas barrier plastic molded article according to this embodiment. The gas barrier plastic molded
Condition (1) The measurement range is 95 to 105 eV.
条件(1)測定範囲を95~105eVとする。 The gas barrier
Condition (1) The measurement range is 95 to 105 eV.
(数1)C含有率[%]={(C含有量[atomic%])/(Si,O及びCの合計含有量[atomic%])}×100
数1において、Si,O又はCの含有量は、Si,O及びCの3元素の内訳における含有量である。
(数2)Si含有率[%]={(Si含有量[atomic%])/(Si,O及びCの合計含有量[atomic%])}×100
数2において、Si,O又はCの含有量は、Si,O及びCの3元素の内訳における含有量である。 In the gas barrier plastic molded
(Equation 1) C content [%] = {(C content [atomic%]) / (total content of Si, O and C [atomic%])} × 100
In Equation 1, the content of Si, O, or C is the content in the breakdown of the three elements of Si, O, and C.
(Expression 2) Si content [%] = {(Si content [atomic%]) / (total content of Si, O and C [atomic%])} × 100
In
(数3)O含有率[%]={(O含有量[atomic%])/(Si,O及びCの合計含有量[atomic%])}×100
数3において、Si,O又はCの含有量は、Si,O及びCの3元素の内訳における含有量である。 In the gas barrier plastic molded
(Equation 3) O content [%] = {(O content [atomic%]) / (total content of Si, O and C [atomic%])} × 100
In Equation 3, the content of Si, O, or C is the content in the breakdown of the three elements of Si, O, and C.
(数5)BIF=[薄膜未形成のプラスチック成形体の酸素透過度]/[ガスバリア性プラスチック成形体の酸素透過度] The gas barrier plastic molded
(Equation 5) BIF = [Oxygen permeability of plastic molding without thin film] / [Oxygen permeability of gas barrier plastic molding]
図2に示す成膜装置を用いてガスバリア性プラスチック成形体を製造した。プラスチック成形体としてPET製のプラスチックボトル(内容量500ml)、珪素含有炭化水素ガスとしてビニルシラン、発熱体として炭化タンタル線(φ0.5mm)を用いた。まず、排気工程を次のとおり行った。排気工程では、真空チャンバの内部を排気して真空チャンバ内を初期圧力P0=1.5Pa以下に調整した。次いで、準備工程を次のとおり行った。準備工程では、ゲートバルブを開けた後、真空チャンバ内に珪素含有炭化水素ガスを導入して真空チャンバ内の圧力をP0Paに調整した後、P0Paより高いPAPaに到達させた。また、プラスチックボトルを出入用チャンバから降下させて発熱体及び原料ガス供給管をプラスチックボトルの内部に挿入した。次に、成膜工程を次のとおり行った。成膜工程では、珪素含有炭化水素ガスの導入を継続しながら発熱体の加熱を開始し、2100~2200℃まで加熱して、プラスチックボトルの内表面に堆積した薄膜が20nmに到達したところで、発熱体の加熱を停止した。その後、プラスチックボトルを出入チャンバに戻してゲートバルブを閉じるとともに、ガスの供給を停止した。また、成膜工程では、真空チャンバ内の圧力をPAPaより高いPBPaに到達させた。各工程での圧力PA,PBは、(PB-PA)/P0が0.16となるように調整した。成膜工程が完了した後、出入チャンバ内を大気解放して、得られたガスバリア性プラスチック成形体を取り出し、新たな未処理のプラスチックボトルを投入して開閉ゲートを閉じた。これら一連の成膜作業を繰り返し行った。 (Example 1)
A gas barrier plastic molded body was manufactured using the film forming apparatus shown in FIG. A plastic bottle made of PET (with an internal volume of 500 ml) was used as the plastic molding, vinylsilane as the silicon-containing hydrocarbon gas, and tantalum carbide wire (φ0.5 mm) as the heating element. First, the exhaust process was performed as follows. In the evacuation step, the inside of the vacuum chamber was evacuated and the inside of the vacuum chamber was adjusted to an initial pressure P 0 = 1.5 Pa or less. Subsequently, the preparatory process was performed as follows. In the preparation process, after opening the gate valve, silicon-containing hydrocarbon gas was introduced into the vacuum chamber to adjust the pressure in the vacuum chamber to P 0 Pa, and then reached P A Pa higher than P 0 Pa. . Further, the plastic bottle was lowered from the entrance / exit chamber, and the heating element and the source gas supply pipe were inserted into the plastic bottle. Next, the film forming process was performed as follows. In the film forming process, heating of the heating element is started while continuing the introduction of the silicon-containing hydrocarbon gas, and the heating is performed up to 2100 to 2200 ° C. When the thin film deposited on the inner surface of the plastic bottle reaches 20 nm, the heating is generated. Body heating stopped. Thereafter, the plastic bottle was returned to the entrance / exit chamber, the gate valve was closed, and the gas supply was stopped. In the film forming process, the pressure in the vacuum chamber was allowed to reach P B Pa higher than P A Pa. The pressures P A and P B in each step were adjusted so that (P B −P A ) / P 0 was 0.16. After the film formation process was completed, the inside of the inlet / outlet chamber was opened to the atmosphere, the resulting gas barrier plastic molded article was taken out, and a new untreated plastic bottle was put in to close the open / close gate. These series of film forming operations were repeated.
各工程での圧力PA,PBを(PB-PA)/P0が0.15となるように調整した以外は、実施例1と同様にしてガスバリア性プラスチック成形体を製造した。 (Example 2)
A gas barrier plastic molded article was produced in the same manner as in Example 1 except that the pressures P A and P B in each step were adjusted so that (P B −P A ) / P 0 was 0.15.
各工程での圧力PA,PBを(PB-PA)/P0が0.11となるように調整した以外は、実施例1と同様にしてガスバリア性プラスチック成形体を製造した。 (Example 3)
A gas barrier plastic molded article was produced in the same manner as in Example 1 except that the pressures P A and P B in each step were adjusted so that (P B −P A ) / P 0 was 0.11.
発熱体として炭化処理していないタンタル線(φ0.5mm)を用い、準備工程を行わなわず、薄膜の膜厚を36nmに変更した以外は、実施例1と同様にしてガスバリア性プラスチック成形体を製造した。 (Comparative Example 1)
A gas barrier plastic molded article was prepared in the same manner as in Example 1 except that a tantalum wire (φ0.5 mm) that was not carbonized was used as a heating element, the preparatory process was not performed, and the thickness of the thin film was changed to 36 nm. Manufactured.
実施例1及び比較例1について一連の成膜作業1回目で得られたプラスチックボトルの薄膜の表面をXPS装置(型式:QUANTERASXM、PHI社製)を用いて分析した。薄膜表面の構成元素の比率を表2に示す。XPS分析の条件は、次の通りである。
測定条件
励起X線:Al mono
検出領域:100μmφ
取出角:90deg
検出深さ:約8nm (XPS analysis-composition analysis)
The surface of the thin film of the plastic bottle obtained in Example 1 and Comparative Example 1 in the first series of film forming operations was analyzed using an XPS apparatus (model: QUANTERASXM, manufactured by PHI). Table 2 shows the ratio of constituent elements on the surface of the thin film. The conditions of XPS analysis are as follows.
Measurement conditions Excitation X-ray: Al mono
Detection area: 100 μmφ
Extraction angle: 90deg
Detection depth: about 8nm
実施例1及び比較例1について一連の成膜作業1回目で得られたプラスチックボトルの薄膜の表面を前記したXPS装置を用いて条件(1)で分析した。試験片及び分析条件は、組成分析と同様とした。 (XPS analysis-binding energy)
Regarding Example 1 and Comparative Example 1, the surface of the thin film of the plastic bottle obtained in the first series of film forming operations was analyzed under the condition (1) using the XPS apparatus described above. The test piece and analysis conditions were the same as in the composition analysis.
前記したXPS装置を用いて、アルゴンイオンエッチングを行いながら、実施例1及び比較例1について一連の成膜作業1回目で得られたプラスチックボトルの薄膜の深さプロファイルを分析した。試験片及び分析条件は、組成分析と同様とした。ここで、ガスバリア薄膜を深さ方向に二等分して考えたとき、実施例1ではプラスチック成形体とは反対側の10nmを上層とし、プラスチック成形体側の10nmを下層とし、比較例1ではプラスチック成形体とは反対側の18nmを上層とし、プラスチック成形体側の18nmを下層とした。 (XPS analysis-depth profile analysis)
Using the above-described XPS apparatus, the depth profile of the thin film of the plastic bottle obtained in the first series of film forming operations was analyzed for Example 1 and Comparative Example 1 while performing argon ion etching. The test piece and analysis conditions were the same as in the composition analysis. Here, when the gas barrier thin film is divided into two equal parts in the depth direction, in Example 1, 10 nm on the opposite side to the plastic molded body is the upper layer, 10 nm on the plastic molded body side is the lower layer, and in Comparative Example 1, the plastic is 18 nm on the side opposite to the molded body was the upper layer, and 18 nm on the plastic molded body side was the lower layer.
実施例及び比較例について一連の成膜作業1回目で得られたプラスチックボトルと用いて透明性を評価した。透明性はb*値で評価した。b*値は、自記分光光度計(U‐3900形、日立社製)に同社製60Φ積分球付属装置(赤外可視近赤外用)を取り付けたものを用いて測定した。検知器としては、超高感度光電子増倍管(R928:紫外可視用)と冷却型PbS(近赤外域用)を用いた。測定波長は、380nmから780nmの範囲で透過率を測定した。ペットボトルの透過率を測定することによって、ガスバリア薄膜のみの透過率測定を算出することができるが、本実施例のb*値は、ペットボトルの吸収率も含めた形で算出したものをそのまま示している。測定には、光沢度の測定で用いた試験片を使用した。3枚の平均値をb*値として表3に示した。 (Transparency evaluation)
The transparency was evaluated using the plastic bottles obtained in the first series of film forming operations for Examples and Comparative Examples. Transparency was evaluated by b * value. The b * value was measured using a self-recording spectrophotometer (U-3900, manufactured by Hitachi) equipped with a 60Φ integrating sphere attachment device (for infrared, visible and near infrared) manufactured by the same company. As the detector, an ultrasensitive photomultiplier tube (R928: for ultraviolet and visible) and cooled PbS (for near infrared region) were used. The transmittance was measured in the measurement wavelength range of 380 nm to 780 nm. By measuring the transmittance of the PET bottle, the transmittance measurement of only the gas barrier thin film can be calculated. However, the b * value in this example is the same as that calculated in the form including the absorption rate of the PET bottle. Show. The test piece used for the measurement of glossiness was used for the measurement. The average value of 3 sheets is shown in Table 3 as b * value.
実施例及び比較例について一連の成膜作業の繰返し回数が1回目、100回目、200回目で得られた各プラスチックボトルを用いてガスバリア性を評価した。ガスバリア性はBIFで評価した。まず、実施例又は比較例の各プラスチックボトルについて酸素透過度を測定した。酸素透過度は、酸素透過度測定装置(型式:Oxtran 2/20、Modern Control社製)を用いて、23℃、90%RHの条件にて測定し、測定開始から24時間コンディションし、測定開始から72時間経過後の値とした。BIFは、数5において、未成膜ボトルの酸素透過度の値を薄膜未形成のプラスチック成形体の酸素透過度とし、実施例又は比較例の各プラスチックボトルの酸素透過度の値をガスバリア性プラスチック成形体の酸素透過度として算出した。評価基準は、次のとおりである。評価結果を表3に示す。
◎:各プラスチックボトルのBIFが10以上である(実用レベル)。
○:各プラスチックボトルのBIFが5以上10未満である(実用下限レベル)。
×:各プラスチックボトルのBIFが5未満である(実用不適レベル)。 (Gas barrier property evaluation)
Regarding Examples and Comparative Examples, gas barrier properties were evaluated using each plastic bottle obtained when the number of repetitions of a series of film forming operations was 1st, 100th, and 200th. The gas barrier property was evaluated by BIF. First, oxygen permeability was measured for each plastic bottle of the example or comparative example. The oxygen permeability was measured under the conditions of 23 ° C. and 90% RH using an oxygen permeability measuring device (model:
A: BIF of each plastic bottle is 10 or more (practical level).
○: BIF of each plastic bottle is 5 or more and less than 10 (practical lower limit level).
X: BIF of each plastic bottle is less than 5 (practical unsuitable level).
一連の成膜作業を1万回繰り返した後、発熱体を成膜装置から外して、返し部から40~80mm部分を指で把持し、強度を確認した。評価基準は次のとおりである。評価結果を表3に示す。
○:発熱体直線部が±1.5mmの範囲を維持し、指で把持しても破損しない(実用レベル)。
△:発熱体直線部が±3.0mmの範囲を維持し、指で把持しても破損しない(実用下限レベル)。
×:発熱体直線部が±3.0mmの範囲を超え、指把持により破損する(実用不適レベル)。 (Mechanical durability evaluation)
After a series of film forming operations was repeated 10,000 times, the heating element was removed from the film forming apparatus, and a 40 to 80 mm portion from the return portion was grasped with a finger, and the strength was confirmed. The evaluation criteria are as follows. The evaluation results are shown in Table 3.
◯: The heating element straight line portion maintains a range of ± 1.5 mm and does not break even when gripped with a finger (practical level).
(Triangle | delta): A heating-element linear part maintains the range of +/- 3.0mm, and even if it hold | grips with a finger, it does not break (practical use lower limit level).
X: The heating element linear portion exceeds the range of ± 3.0 mm and is damaged by finger grip (practical unsuitable level).
31 成膜専用チャンバ(真空チャンバ)
32 出入用チャンバ
33 ゲートバルブ
42 発熱体
56 開閉ゲート
80 圧力検出部
VP1 真空ポンプ
VP2 真空ポンプ
90 ガスバリア性プラスチック成形体
91 プラスチック成形体
92 ガスバリア薄膜
92a 上層
92b 下層
92s ガスバリア薄膜の表面 4 Plastic molded body (plastic bottle)
31 Deposition chamber (vacuum chamber)
32 Entrance /
Claims (7)
- プラスチック成形体と、該プラスチック成形体の表面に設けたガスバリア薄膜とを備えるガスバリア性プラスチック成形体において、
前記ガスバリア薄膜は、構成元素として珪素(Si)、炭素(C)及び酸素(O)を含有し、かつ、条件(1)でX線電子分光分析すると、Si-Cの結合エネルギーのピーク出現位置に、メインピークが観察される領域を有することを特徴とするガスバリア性プラスチック成形体。
条件(1)測定範囲を95~105eVとする。 In a gas barrier plastic molded body comprising a plastic molded body and a gas barrier thin film provided on the surface of the plastic molded body,
The gas barrier thin film contains silicon (Si), carbon (C), and oxygen (O) as constituent elements, and when the X-ray electron spectroscopic analysis is performed under the condition (1), the peak appearance position of the bond energy of Si—C And a gas barrier plastic molded article having a region where a main peak is observed.
Condition (1) The measurement range is 95 to 105 eV. - 前記ガスバリア薄膜は、深さ方向に傾斜組成を有し、
前記ガスバリア薄膜を深さ方向に二等分し、前記プラスチック成形体とは反対側を上層とし、前記プラスチック成形体側を下層としたとき、
前記上層における(数1)で表されるC含有率が、前記上層における(数2)で表されるSi含有率よりも高いことを特徴とする請求項1に記載のガスバリア性プラスチック成形体。
(数1)C含有率[%]={(C含有量[atomic%])/(Si,O及びCの合計含有量[atomic%])}×100
数1において、Si,O又はCの含有量は、Si,O及びCの3元素の内訳における含有量である。
(数2)Si含有率[%]={(Si含有量[atomic%])/(Si,O及びCの合計含有量[atomic%])}×100
数2において、Si,O又はCの含有量は、Si,O及びCの3元素の内訳における含有量である。 The gas barrier thin film has a gradient composition in the depth direction,
When the gas barrier thin film is bisected in the depth direction, the side opposite to the plastic molded body is the upper layer, and the plastic molded body side is the lower layer,
2. The gas barrier plastic molded article according to claim 1, wherein the C content represented by (Equation 1) in the upper layer is higher than the Si content represented by (Equation 2) in the upper layer.
(Equation 1) C content [%] = {(C content [atomic%]) / (total content of Si, O and C [atomic%])} × 100
In Equation 1, the content of Si, O, or C is the content in the breakdown of the three elements of Si, O, and C.
(Expression 2) Si content [%] = {(Si content [atomic%]) / (total content of Si, O and C [atomic%])} × 100
In Formula 2, the content of Si, O, or C is the content in the breakdown of the three elements of Si, O, and C. - 前記上層における(数3)で表されるO含有率が、前記上層におけるSi含有率よりも低いことを特徴とする請求項2に記載のガスバリア性プラスチック成形体。
(数3)O含有率[%]={(O含有量[atomic%])/(Si,O及びCの合計含有量[atomic%])}×100
数3において、Si,O又はCの含有量は、Si,O及びCの3元素の内訳における含有量である。 3. The gas barrier plastic molded article according to claim 2, wherein an O content represented by (Equation 3) in the upper layer is lower than an Si content in the upper layer.
(Equation 3) O content [%] = {(O content [atomic%]) / (total content of Si, O and C [atomic%])} × 100
In Equation 3, the content of Si, O, or C is the content in the breakdown of the three elements of Si, O, and C. - 前記ガスバリア薄膜は、深さ方向に傾斜組成を有し、
前記ガスバリア薄膜を深さ方向に二等分し、前記プラスチック成形体とは反対側を上層とし、前記プラスチック成形体側を下層としたとき、
前記下層における(数1)で表されるC含有率が、前記下層における(数3)で表されるO含有率よりも高いことを特徴とする請求項1~3のいずれか1つに記載のガスバリア性プラスチック成形体。
(数1)C含有率[%]={(C含有量[atomic%])/(Si,O及びCの合計含有量[atomic%])}×100
数1において、Si,O又はCの含有量は、Si,O及びCの3元素の内訳における含有量である。
(数3)O含有率[%]={(O含有量[atomic%])/(Si,O及びCの合計含有量[atomic%])}×100
数3において、Si,O又はCの含有量は、Si,O及びCの3元素の内訳における含有量である。 The gas barrier thin film has a gradient composition in the depth direction,
When the gas barrier thin film is bisected in the depth direction, the side opposite to the plastic molded body is the upper layer, and the plastic molded body side is the lower layer,
4. The C content represented by (Equation 1) in the lower layer is higher than the O content represented by (Equation 3) in the lower layer. Gas barrier plastic molding.
(Equation 1) C content [%] = {(C content [atomic%]) / (total content of Si, O and C [atomic%])} × 100
In Equation 1, the content of Si, O, or C is the content in the breakdown of the three elements of Si, O, and C.
(Equation 3) O content [%] = {(O content [atomic%]) / (total content of Si, O and C [atomic%])} × 100
In Equation 3, the content of Si, O, or C is the content in the breakdown of the three elements of Si, O, and C. - 真空チャンバの内部を排気して前記真空チャンバ内を初期圧力P0以下に調整する排気工程と、
前記真空チャンバ内の圧力がP0以下に調整され、かつ、真空チャンバ内に配置された炭化タンタル相を有する発熱体が加熱されていないときに、珪素含有炭化水素ガスを前記真空チャンバ内に導入して該真空チャンバ内の圧力を前記P0に調整する準備工程と、
前記珪素含有炭化水素ガスを継続して前記真空チャンバに導入しながら前記発熱体を加熱して、前記真空チャンバ内に収容されているプラスチック成形体の表面にガスバリア薄膜を形成する成膜工程と、
を有することを特徴とするガスバリア性プラスチック成形体の製造方法。 Evacuating the inside of the vacuum chamber to adjust the inside of the vacuum chamber to an initial pressure P 0 or less;
Silicon-containing hydrocarbon gas is introduced into the vacuum chamber when the pressure in the vacuum chamber is adjusted to P 0 or less and the heating element having a tantalum carbide phase disposed in the vacuum chamber is not heated. A preparatory step of adjusting the pressure in the vacuum chamber to the P 0 ,
A film forming step of heating the heating element while continuously introducing the silicon-containing hydrocarbon gas into the vacuum chamber to form a gas barrier thin film on the surface of the plastic molded body housed in the vacuum chamber;
A method for producing a gas barrier plastic molded article, comprising: - 前記準備工程において、前記真空チャンバ内の圧力を前記P0に調整後、前記真空チャンバ内の圧力を前記P0より高い圧力PAに到達させ、
前記成膜工程において、前記真空チャンバ内の圧力を前記PAより高い圧力PBに到達させることを特徴とする請求項5に記載のガスバリア性プラスチック成形体の製造方法。 In the preparation step, wherein after adjusting the pressure in the vacuum chamber to said P 0, to reach the pressure of the vacuum chamber at a higher pressure P A from the P 0,
Wherein in the film forming step, the manufacturing method of the gas barrier plastic molded body according to claim 5, characterized in that to reach the pressure of said vacuum chamber to said P A higher pressures P B. - (PB-PA)/P0が0.11以上であることを特徴とする請求項6に記載のガスバリア性プラスチック成形体の製造方法。 7. The method for producing a gas barrier plastic molded article according to claim 6, wherein (P B -P A ) / P 0 is 0.11 or more.
Priority Applications (8)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR1020177032902A KR20170138476A (en) | 2015-04-17 | 2016-04-05 | Gas-barrier plastic molded article and manufacturing method thereof |
| MYPI2017703465A MY186446A (en) | 2015-04-17 | 2016-04-05 | Gas-barrier plastic molded product and method for manufacturing same |
| US15/566,515 US10487397B2 (en) | 2015-04-17 | 2016-04-05 | Gas-barrier plastic molded product and method for manufacturing same |
| SG11201708290QA SG11201708290QA (en) | 2015-04-17 | 2016-04-05 | Gas-barrier plastic molded product and method for manufacturing same |
| CN201680021428.1A CN107429392B (en) | 2015-04-17 | 2016-04-05 | Gas barrier property plastic shaped body and its manufacturing method |
| AU2016248605A AU2016248605A1 (en) | 2015-04-17 | 2016-04-05 | Gas-barrier plastic molded product and method for manufacturing same |
| EP16779943.6A EP3284846B1 (en) | 2015-04-17 | 2016-04-05 | Gas-barrier plastic molded product and method for manufacturing same |
| PH12017501752A PH12017501752A1 (en) | 2015-04-17 | 2017-09-25 | Gas-barrier plastic molded product and method for manufacturing same |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2015-085018 | 2015-04-17 | ||
| JP2015085018A JP6474673B2 (en) | 2015-04-17 | 2015-04-17 | Gas barrier plastic molded body and method for producing the same |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2016167152A1 true WO2016167152A1 (en) | 2016-10-20 |
Family
ID=57126273
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2016/061070 WO2016167152A1 (en) | 2015-04-17 | 2016-04-05 | Gas-barrier plastic molded product and method for manufacturing same |
Country Status (11)
| Country | Link |
|---|---|
| US (1) | US10487397B2 (en) |
| EP (1) | EP3284846B1 (en) |
| JP (1) | JP6474673B2 (en) |
| KR (1) | KR20170138476A (en) |
| CN (1) | CN107429392B (en) |
| AU (1) | AU2016248605A1 (en) |
| MY (1) | MY186446A (en) |
| PH (1) | PH12017501752A1 (en) |
| SG (1) | SG11201708290QA (en) |
| TW (1) | TWI686497B (en) |
| WO (1) | WO2016167152A1 (en) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2018095937A (en) * | 2016-12-15 | 2018-06-21 | 三菱重工機械システム株式会社 | Electrode state evaluation device, film deposition apparatus, and electrode state evaluation method |
| JP2019155704A (en) * | 2018-03-13 | 2019-09-19 | 東レエンジニアリング株式会社 | Barrier film and photoconversion member |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR20170118137A (en) * | 2015-02-18 | 2017-10-24 | 기린 가부시키가이샤 | Heating element and manufacturing method thereof |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2004107689A (en) * | 2002-09-13 | 2004-04-08 | Ulvac Japan Ltd | Diamond like carbon film deposition method and deposition system |
| WO2006126677A1 (en) * | 2005-05-27 | 2006-11-30 | Kirin Beer Kabushiki Kaisha | Apparatus for manufacturing gas barrier plastic container, method for manufacturing the container, and the container |
| JP2009120885A (en) * | 2007-11-13 | 2009-06-04 | Toyo Advanced Technologies Co Ltd | Carbonaceous thin film and production method therefor |
| WO2012091097A1 (en) * | 2010-12-28 | 2012-07-05 | 麒麟麦酒株式会社 | Gas-barrier plastic molded product and manufacturing process therefor |
| WO2012091095A1 (en) * | 2010-12-28 | 2012-07-05 | 麒麟麦酒株式会社 | Method for producing gas barrier plastic molded body |
Family Cites Families (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| AU2001250886A1 (en) * | 2000-03-20 | 2001-10-03 | N V. Bekaert S.A. | Materials having low dielectric constants and methods of making |
| US7288311B2 (en) * | 2003-02-10 | 2007-10-30 | Dai Nippon Printing Co., Ltd. | Barrier film |
| US20110274933A1 (en) * | 2008-12-12 | 2011-11-10 | Lintec Corporation | Laminate, method for producing same, electronic device member, and electronic device |
| KR101489551B1 (en) * | 2009-05-22 | 2015-02-03 | 린텍 가부시키가이샤 | Molded object, process for producing same, member for electronic device, and electronic device |
| JP5706777B2 (en) * | 2011-07-25 | 2015-04-22 | 麒麟麦酒株式会社 | Gas barrier plastic molding |
| MY167942A (en) | 2011-12-27 | 2018-10-04 | Kirin Brewery | Apparatus for forming thin film |
| TWI576242B (en) * | 2011-12-28 | 2017-04-01 | Kirin Brewery | Gas barrier plastic molded body and manufacturing method thereof |
| JP6009243B2 (en) * | 2012-06-27 | 2016-10-19 | 麒麟麦酒株式会社 | Carbonated beverage bottle and method for producing the same |
-
2015
- 2015-04-17 JP JP2015085018A patent/JP6474673B2/en active Active
-
2016
- 2016-04-05 WO PCT/JP2016/061070 patent/WO2016167152A1/en active Application Filing
- 2016-04-05 MY MYPI2017703465A patent/MY186446A/en unknown
- 2016-04-05 KR KR1020177032902A patent/KR20170138476A/en unknown
- 2016-04-05 EP EP16779943.6A patent/EP3284846B1/en active Active
- 2016-04-05 US US15/566,515 patent/US10487397B2/en active Active
- 2016-04-05 CN CN201680021428.1A patent/CN107429392B/en active Active
- 2016-04-05 SG SG11201708290QA patent/SG11201708290QA/en unknown
- 2016-04-05 AU AU2016248605A patent/AU2016248605A1/en not_active Abandoned
- 2016-04-11 TW TW105111263A patent/TWI686497B/en not_active IP Right Cessation
-
2017
- 2017-09-25 PH PH12017501752A patent/PH12017501752A1/en unknown
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2004107689A (en) * | 2002-09-13 | 2004-04-08 | Ulvac Japan Ltd | Diamond like carbon film deposition method and deposition system |
| WO2006126677A1 (en) * | 2005-05-27 | 2006-11-30 | Kirin Beer Kabushiki Kaisha | Apparatus for manufacturing gas barrier plastic container, method for manufacturing the container, and the container |
| JP2009120885A (en) * | 2007-11-13 | 2009-06-04 | Toyo Advanced Technologies Co Ltd | Carbonaceous thin film and production method therefor |
| WO2012091097A1 (en) * | 2010-12-28 | 2012-07-05 | 麒麟麦酒株式会社 | Gas-barrier plastic molded product and manufacturing process therefor |
| WO2012091095A1 (en) * | 2010-12-28 | 2012-07-05 | 麒麟麦酒株式会社 | Method for producing gas barrier plastic molded body |
Non-Patent Citations (1)
| Title |
|---|
| See also references of EP3284846A4 * |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2018095937A (en) * | 2016-12-15 | 2018-06-21 | 三菱重工機械システム株式会社 | Electrode state evaluation device, film deposition apparatus, and electrode state evaluation method |
| JP2019155704A (en) * | 2018-03-13 | 2019-09-19 | 東レエンジニアリング株式会社 | Barrier film and photoconversion member |
| WO2019176936A1 (en) * | 2018-03-13 | 2019-09-19 | 東レエンジニアリング株式会社 | Barrier film and light conversion member |
| JP7163041B2 (en) | 2018-03-13 | 2022-10-31 | 東レエンジニアリング株式会社 | Barrier film and light conversion material |
Also Published As
| Publication number | Publication date |
|---|---|
| KR20170138476A (en) | 2017-12-15 |
| JP6474673B2 (en) | 2019-02-27 |
| EP3284846A1 (en) | 2018-02-21 |
| US10487397B2 (en) | 2019-11-26 |
| US20180127872A1 (en) | 2018-05-10 |
| CN107429392A (en) | 2017-12-01 |
| JP2016204685A (en) | 2016-12-08 |
| CN107429392B (en) | 2019-08-20 |
| TW201641732A (en) | 2016-12-01 |
| EP3284846B1 (en) | 2021-02-17 |
| PH12017501752A1 (en) | 2018-04-11 |
| TWI686497B (en) | 2020-03-01 |
| EP3284846A4 (en) | 2018-12-19 |
| AU2016248605A1 (en) | 2017-10-19 |
| SG11201708290QA (en) | 2017-11-29 |
| MY186446A (en) | 2021-07-22 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| AU2011350429B2 (en) | Gas-barrier plastic molded product and manufacturing process therefor | |
| JP5260050B2 (en) | Gas barrier plastic container manufacturing apparatus and method for manufacturing the container | |
| JP5695673B2 (en) | Method for producing gas barrier plastic molding | |
| WO2016167152A1 (en) | Gas-barrier plastic molded product and method for manufacturing same | |
| JP2007261077A (en) | Dlc-film-coated biodegradable plastic container or film and its manufacturing method | |
| JP5706777B2 (en) | Gas barrier plastic molding | |
| JP6009243B2 (en) | Carbonated beverage bottle and method for producing the same | |
| JP5566334B2 (en) | Gas barrier plastic molded body and method for producing the same | |
| EP3093309B1 (en) | Process for depositing a gas barrier coating on a polymer film or polymer container, and polymer film or polymer container with coated with such a gas barrier | |
| JP5779044B2 (en) | Control method of wettability | |
| JP2007168882A (en) | Gas barrier plastic container | |
| JP7005256B2 (en) | Gas barrier container | |
| TWI537415B (en) | Production method of gas barrier plastic molded body |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 16779943 Country of ref document: EP Kind code of ref document: A1 |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 12017501752 Country of ref document: PH |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 11201708290Q Country of ref document: SG |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 15566515 Country of ref document: US |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| ENP | Entry into the national phase |
Ref document number: 2016248605 Country of ref document: AU Date of ref document: 20160405 Kind code of ref document: A |
|
| REEP | Request for entry into the european phase |
Ref document number: 2016779943 Country of ref document: EP |
|
| ENP | Entry into the national phase |
Ref document number: 20177032902 Country of ref document: KR Kind code of ref document: A |