WO2013180589A1 - Synergistic fungicidal mixture containing dimethomorph and propamocarb-hydrochloride - Google Patents
Synergistic fungicidal mixture containing dimethomorph and propamocarb-hydrochloride Download PDFInfo
- Publication number
- WO2013180589A1 WO2013180589A1 PCT/PT2013/000034 PT2013000034W WO2013180589A1 WO 2013180589 A1 WO2013180589 A1 WO 2013180589A1 PT 2013000034 W PT2013000034 W PT 2013000034W WO 2013180589 A1 WO2013180589 A1 WO 2013180589A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- dimethomorph
- propamocarb
- hydrochloride
- compound
- composition according
- Prior art date
Links
- 0 COC(C(OC)=C1)=CCC1C(c(cc1)ccc1[N-])=CC(*1CCOCC1)=O Chemical compound COC(C(OC)=C1)=CCC1C(c(cc1)ccc1[N-])=CC(*1CCOCC1)=O 0.000 description 3
Classifications
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01N—PRESERVATION OF BODIES OF HUMANS OR ANIMALS OR PLANTS OR PARTS THEREOF; BIOCIDES, e.g. AS DISINFECTANTS, AS PESTICIDES OR AS HERBICIDES; PEST REPELLANTS OR ATTRACTANTS; PLANT GROWTH REGULATORS
- A01N37/00—Biocides, pest repellants or attractants, or plant growth regulators containing organic compounds containing a carbon atom having three bonds to hetero atoms with at the most two bonds to halogen, e.g. carboxylic acids
- A01N37/36—Biocides, pest repellants or attractants, or plant growth regulators containing organic compounds containing a carbon atom having three bonds to hetero atoms with at the most two bonds to halogen, e.g. carboxylic acids containing at least one carboxylic group or a thio analogue, or a derivative thereof, and a singly bound oxygen or sulfur atom attached to the same carbon skeleton, this oxygen or sulfur atom not being a member of a carboxylic group or of a thio analogue, or of a derivative thereof, e.g. hydroxy-carboxylic acids
- A01N37/38—Biocides, pest repellants or attractants, or plant growth regulators containing organic compounds containing a carbon atom having three bonds to hetero atoms with at the most two bonds to halogen, e.g. carboxylic acids containing at least one carboxylic group or a thio analogue, or a derivative thereof, and a singly bound oxygen or sulfur atom attached to the same carbon skeleton, this oxygen or sulfur atom not being a member of a carboxylic group or of a thio analogue, or of a derivative thereof, e.g. hydroxy-carboxylic acids having at least one oxygen or sulfur atom attached to an aromatic ring system
Definitions
- the present invention relates to a novel fungicidal synergistic composition consisting of a fungicidal active ingredient combination suitable for the selective control of fungi in crops of useful plants, for example in potato, tomato and lettuce.
- the invention also relates to a method for controlling fungi in crops of useful plants and to the use of the novel composition for that purpose.
- a novel synergistic composition for fungi control hich in addition to crop formulation additives, comprises as active ingredients a mixture of dimethomorph and propamocarb- HCl.
- dimethomorph is a systemic fungicide which moves up the treated plant stem and into growing leaves, acting on the cell wall biosynthesis. Overall, it acts on all the stages in which there is formation of cell membrane, spore germination, germ tube formation, the haustoria, hyphal growth and spore formation. Furthermore, it is effective against strains resistant to downy mildew phenylamines. Finally, dimethomorph is a fungicide with curative and preventive activity, being considered a reference in the market due to its eradicative ability.
- Dimethomorph was disclosed for the first time in European Patent EP 120321.
- European Patent EP 120321 There are several patent documents describing mixtures of dimethomorph with other fungicides.
- EP0901322 describes a fungicidal mixture of dimethomorph with several carbamates. The cited document is silent regarding propamocarb-HCl.
- EP0753258, EP0985346, US6316446 describe mixtures with N-acetonylbenzamides, oxazolidinones and phenyl benzyl ethers derivatives, respectively.
- Compound II is a carbamate systemic fungicide having a preventive effect. It is absorbed by roots and leaves and transported upwards in the plant. This active ingredient has activity against several fungi from the Oomycete family which cause "damping-off ' and foliar diseases. Propamocarb hydrochloride disrupts the formation of fungal cell walls by interfering with the synthesis of phospholipids and fatty acids. It affects mycelial growth and also spore production and germination. The biochemical mode of action is exercised at the level of cell membrane permeability, affecting its integrity.
- British patent GB- 1212708 describes propamocarb and its activity is reported in the control of Pythium ultimum. This document does not mention any result or biological activity of the mixture claimed in the present invention.
- EP835055, W01998/26654, WO 1998/044801, WO2006/040123 and WO2007/ 128541 describe compositions comprising propamocarb and ofurace, benalaxyl, a derivative of phosphorous acid (e.g. fosetyl-Al), fluoxastrobin and flutriafol, respectively.
- WO2009/082939 describes an aqueous suspension concentrate (SC) composition comprising tebuconazole and propamocarb.
- EP2338341 describes a composition comprising: A) propamocarb-HCl and B) an insecticide compound selected from the group of flubendiamide or rynaxapyr.
- WO8805630 claims the mixture of dimethomorph with several pesticides. Although propamocarb is mentioned as part of a long list of fungicides to be used in association with dimethomorph, no reference is made to the specific association of dimethomorph and propamocarb in its hydrochloride form, nor examples or evidence of the formulation according to the invention are mentioned.
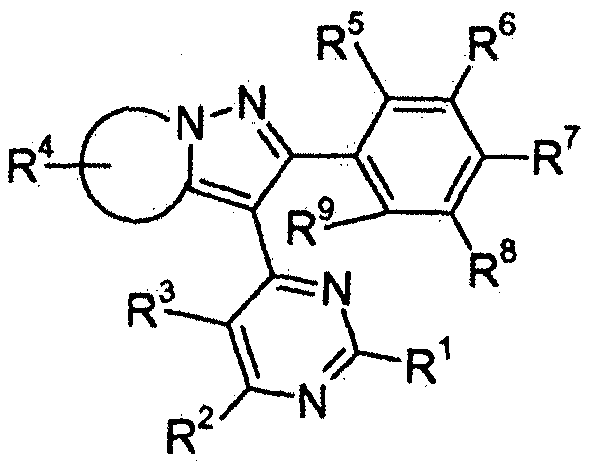
- EP2409570A2 claims a fungicidal mixture comprising a compound of general formula:
- R1- 9 have the meanings cited therein, and several compounds commonly used in crop protection.
- the present invention does not make use in anyway of the general compound depicted above.
- WO2010/128003 A2 relates to a method for increasing the vigor and/or yield of agricultural crops under the conditions of an essentially non-existent pressure from pathogenic agents, comprising a Bacillus subtilis strain, or a derivative thereof, and optionally at least one compound selected from strobilurins, carboxamides, azoles, heterocyclic compounds, carbamates, other active substances, growth regulators, herbicides and insecticides.
- WO2009/037242A2 relates to fungicidal mixtures, comprising Bacillus subtilis and Bacillus pumilus strains, or derivatives thereof, and optionally at least one compound selected from azoles, strobilurins, carboxamides, heterocyclic compounds, carbamates and other fungicides.
- the present invention falls off the scope of the mentioned patent applications, as it describes a mixture of dimethomorph and propamocarb-HCI, not including the Bacillus sp. strains mentioned.
- the concentrations used therein for the tank mixture "propamocarb +dimethomorph" were 1,01 kg/ha for propamocarb and 0,22 kg/ha for dimethomorph.
- Table 3 line 5 vs. line 9) of the cited document, the values mentioned for RAUDPC (Relative Area Under the Disease Progress Curve) are 0.761 and 0.8 1 for dimethomorph + propamocarb and dimethomorph alone, respectively. Therefore, the mixture of dimethomorph and propamocarb did not show a statistically significant biological interaction between the two active substances when compared to the result of dimethomorph alone, not showing as such any benefit for crop protection purposes.
- the present invention does not fall in the scope of the mentioned closest state of the art as it relates to an unique product in the form of a readily usable suspension concentrate of dimethomorph and propamocarb hydrochloride.
- the present composition makes use of lower field application doses (propamocarb-HCI: 0,8-1,0 kg/ha; dimethomorph: 0,144-0,180 kg/ha), it shows a surprisingly biological interaction between the two active ingredients resulting in a robust synergistic behavior.
- the references mentioned in the prior art do not disclose nor the synergistic composition according to the present invention, nor the concentration ranges of the active ingredients in the mixture, nor the application rates described herein for an effective fungicidal action.
- dimethomorph exists commercially associated to other fungicides siich as mancozeb (under the name ACROBAT M DG * , marketed by BASF * ) or a strobilurin, such as pyraclostrobin (under the name COACH PLUS * , marketed by BASF * ).
- propamocarb-HCl exists commercially associated to other fungicides such as fenamidone (under the name CONSENTO * , marketed by Bayer Cropscience * ), fluopicolide (under the name INFINITO * , marketed by Bayer Cropscience * ), fosetyl-Al (under the name PREVICUR ENERGY * , marketed by Bayer Cropscience * ), chlorothalonil (under the name TATTOO C * , marketed by Bayer Cropscience * ) and cymoxanil (under the name PROXANIL * . marketed by Agriphar SA * ).
- fenamidone under the name CONSENTO * , marketed by Bayer Cropscience *
- fluopicolide under the name INFINITO * , marketed by Bayer Cropscience *
- fosetyl-Al under the name PREVICUR ENERGY * , marketed by Bayer Cropscience *
- chlorothalonil under
- the present invention relates to a binary fungicidal combination, consisting of dimethomorph and propamocarb-hydrochloride, to the preparation thereof and use of said composition for the control of fungi pathogens.
- the fungicidal composition consists of dimethomorph (compound I) and propamocarb hydrochloride (compound II) in the form of a suspension concentrate (SC) containing 90 g/L of dimethomorph and 500 g/L of propamocarb hydrochloride.
- SC suspension concentrate
- the fungicidal composition according to the present invention is used to treat the following plant diseases: potato late blight (Phythophtora infestans), tomato downy mildew (Phythophtora infestans) and lettuce downy mildew (Bremia lact cae).
- the invention also relates to a process to treat crops affected by pathogen fungi by applying to said crops, 144 g/ha to 180 g/ha of compound I and 800 g/ha to 1000 g/ha of Compound II.
- concentrations of the active ingredients in the composition according to the present invention are in the range of 72 g/L to 90 g/L for compound I, and in the range of 400 g/L to 500 g/L for compound II.
- the concentration of compound I is 90 g/L and the concentration of compound II is 500 g/L.
- the fungicidal composition according to the present invention further comprises surfactants, anti-freeze agents and rheology modifiers.
- the surfactants are selected from the group comprising dispersing agents, biological activators and anti-foam agents.
- the preferred dispersing agents are non-ionic polymethyl methacrylate- polyethylene oxide graft copolymers, such as Atlox 4913 commercially available from Croda ® , alone or combined with a polyoxyethylene alkyl ether such as Atlox 4894 commercially available from Croda ® , and the preferred anti-foam agents are emulsions of polydimethylsiloxane in water such as AF9030 commercially available from Momentive ® .
- Biological activators used in the present invention are branched C9-G ethoxylated alcohols, preferably C9-C11 branched ethoxylated alcohols, such as Rhodasurf 860/P commercially available from Rhodia ® , or Cn to CM branched ethoxylated alcohols, such as Rhodasurf 870 commercially available from Rhodia ® .
- the preparation of the suspension concentrate according to the invention is carried out using the following composition:
- Non-ionic surfactant (Atlox 4894) 7.0 Ethoxylated alcohol (Rhodasurf 860/P) 50.0 Antifreeze agent (Propylene Glycol) 30.0 Anti-Foam agent (AF9030) 10.0
- the Thickener solution is prepared by mixing Preventol Bit 20 N (1.0% (w/w)), xantham gum (2.0% (w/W)) and water (97.0% (w/w)).
- the process for preparing the suspension concentrate according to the invention comprises the following steps: mixing Compound II with propylene glycol water, a polymeric surfactant (e.g. Atlox 4913), a nonionic proprietary surfactant blend (e.g. Atlox 4894), an ethoxylated isodecyl alcohol (e.g. Rhodasurf 860/P) and a anti- foam (e.g. AF 9030); dispersing Compound I, with stirring, in the previously prepared solution; pumping the suspension thus obtained through a refrigerated bead mill (for example* a Dyno-mill) in order to reduce the particle size; adjusting viscosity by adding the thickner solution with stirring.
- a polymeric surfactant e.g. Atlox 4913
- a nonionic proprietary surfactant blend e.g. Atlox 4894
- an ethoxylated isodecyl alcohol e.g. Rhodasurf 860/P
- An accelerated storage test in which a formulation sample is stored in an oven for 2 weeks at 54 ⁇ 2°C, and their physical-chemical and technical properties are compared to those of a sample of the same batch which was not submitted to the test. This test is intended to represent a simulation of the behavior of a formulation after a 2 years storage period.
- a low temperature stability test was carried out at 0 ⁇ 2°C during one week.
- Table 1 shows the physical and chemical properties of the dimethomorph plus propamocarb hydrochloride SC formulation prepared according to the example above before and after the accelerated storage test.
- the spontaneity of dispersion in % was determined according to CIPAC MT 160, using CIPAC standard water D at 30 ⁇ 1°C and shows that both active substances are well dispersed (above 90%), before and after the accelerated test.
- the pourability in % was determined according to CIPAC MT 148,1.
- the test of pourability is a direct measure of how the viscosity can influence the product, namely the handling, in particular, the easiness or difficultness to remove all of the content from the agrochemical package and rinse the package for environmental reasons.
- the residue of the composition according to the invention after pouring out the packaging contents was of 2.2% and 3.2% before and after the accelerated test, very low and interesting value in terms of end-use and environment issues.
- Table 2 shows the physical and chemical properties of the above dimethomorph plus propamocarb-HCI SC formulation before and after the low temperature stability test.
- E expected efficacy
- M measured efficacy
- the sensitivity analysis f an individual, mono-sporangia isolate of Phytophthora infestans forms the basis of the investigations and the resulting conclusions.
- This isolate results of an original, unselected "wild-type” sensitivity towards dimethomorph and is a representative of the current predominating and probably also unselected ("wild type") sensitivity level towards propamocarb hydrochloride.
- the base of the in vivo sensitivity analysis is the detached leaf test system with the treatment of whole, intaet plants one day before preparing the test sets and before inoculation (protective test system).
- the fungicide treatments are prepared with a 0.05% Uniperol-solution.
- the different fungicide concentrations are graded logarithmically by a factor of 2 in order to obtain an accurate EC evaluation for the sensitivity towards the active compound combination (first test run: DMM + PMC).
- Young potato plants variety "Bintje" are sprayed with the respective fungicide solutions to run-off conditions.
- test sets are prepared by cutting leaf parts from the treated plant material and disposed onto water agar (0.6% agar) in disposable Petri dishes of 6 cm diameter. For each concentration, a separate Petri dish is used to avoid gas phase interactions between differently treated plant material. Each Petri dish contains 3-5 leaf parts as replicates from different leaves treated with the same fungicide concentration. During the time of inoculation, the leafs are exposed to the zoospore suspension of the isolate for about 1-2 minutes. The test isolate is directly obtained from the "propagation leaves" which are transferred into 7.5 mL tubes. Approximately 5 mL of distilled H2O is added into the tubes and the suspension is prepared by shaking the tubes several times.
- each test set is inoculated from above by dispersing the spore suspension of the test isolate with a micro-sprayer in an airbrush system. After inoculation, the Petri dishes with the differently treated leaf parts are again separated from each other to avoid gas phase interactions.
- test material is scored macroscopically regarding disease coverage/development (sporangia formation) at each fungicide concentration relative to the respective untreated control (in %).
- EC50, EC70 and EC90 values are calculated for the single compounds as well as for the compound combination (Calculated) by Probit analysis [WEBER, E., GrundriB der biologischen Statising; 8) Auflage, Gustav-Fischer-Verlag, Stuttgart-New York, 1980]. These concentrations are then used for analyzing the respective efficacy of the single compounds as well as the compound combination (Measured). From the measured efficacy values of the single compounds, the Expected efficacy (in %) of the combination is calculated using the Colby equation (COLBY, S.R., Calculating synergistic and antagonistic responses of herbicide combination, Weeds, 15, pp 20-22, 1967).
- E is the expected efficacy, in percentage, when using the mixture of the active compounds I and II at the concentrations a and b.
- x is the efficacy, in percentage, when using active compound I at the concentration a;
- y is the efficacy, in percentage, when using active compound II at the concentration b.
- the observed efficacy is higher at every EC when compared to the results obtained by the active ingredients alone.
- the fungicidal composition of the present invention is not just an admixture of both ingredients resulting in the aggregation of the properties shown individually, but rather the formulation according to the invention shows synergism providing an unexpected property for a wide range of relevant EC's.
- the test results show that mixtures according to the invention have synergistically increased activity against potato late blight (Phythophtora infestdns). This is due to an enhanced biological action and consequently an improved toxic effect regarding the target fungi, when compared to the effect of both active substances alone.
- the object of the present invention has therefore reached a surprisingly technical and practical effect;
Abstract
The present invention provides a fungicidal composition comprising a mixture of dimethomorph (compound I) and propamocarb hydrochloride (compound II) in the form of a suspension concentrate containing 72 g/L to 90 g/L of dimethomorph and 400 g/L to 500 g/L of propamocarb-HCl. The present invention also relates to a method to treat crops by applying 144 g/ha to 180 g/ha of dimethomorph and 800 g/ha to 1000 g/ha of propamocarb hydrochloride. The composition is preferably used for treating potato late blight (Phythophtora infestans), tomato downy mildew (Phythophtora infestans) and lettuce downy mildew (Bremia lactucae).
Description
I
"SYNERGISTIC FUNGICIDAL MIXTURE CONTAINING DIMETHOMORPH
AND PROPAMOCARB-HYDROCHLORIDE"
FIELD OF THE INVENTION
The present invention relates to a novel fungicidal synergistic composition consisting of a fungicidal active ingredient combination suitable for the selective control of fungi in crops of useful plants, for example in potato, tomato and lettuce. The invention also relates to a method for controlling fungi in crops of useful plants and to the use of the novel composition for that purpose.
BACKGROUND OF THE INVENTION
In the field of crop protection, the use of a particular active substance for the control of fungi may become increasingly difficult in time due to the adaptation of the mentioned fungi to the fungicide being used. This leads to the loss of effectiveness in the control of the pathogen, overuse of the fungicide and, consequently, downfall in crop production and profitability.
A very well-known and widely used form of approaching this problem is by using a combination of different active substances presenting different mechanisms of action (EPPO Standards, Good plant protection practices, EPPO Bulletin 32, pages 367-369, 2002). In fact, in many cases only the combination of the characteristics and properties of more than one active substance makes it possible to obtain a product of high performance in all the aspects of biological action (preventive action, curative action and eradicative action) while keeping the probability of the development of resistance to these molecules to a minimum. Nevertheless, the effect of such a combination may result in an antagonistic, additive or yet a synergistic activity.
Surprisingly, it has now been found that the combination of a carbamate such as the systemic propamocarb-HCl with dimethomorph shows very positive characteristics, including a curative, preventive and eradicative effect, associated to a surprisingly synergistic effect. The present invention constitutes for this reason a gain in the range of plant protection solutions aiming at the downy mildew control.
Therefore, it is proposed in accordance with the present invention a novel synergistic composition for fungi control hich, in addition to crop formulation additives, comprises as active ingredients a mixture of dimethomorph and propamocarb- HCl.
Dimethomorph, compound (I), [IUPAC: (E,Z)-4-[3-(4-chlorophenyl)-3-(3,4- dimethoxyphenyl)acryloyl]morpholine], is a mixture of E and Z isomers whose structural formulas are:
Compound I and a CAA (Carboxylic Acid Amide) fungicide with biological action on fungi of the water mold family, (see "The Pesticide Manual" 14th. Edition (2006), The British Crop Protection Council, pages 347 f.). Regarding the biochemical mode of action, dimethomorph is a systemic fungicide which moves up the treated plant stem and into growing leaves, acting on the cell wall biosynthesis. Overall, it acts on all the stages in which there is formation of cell membrane, spore germination, germ tube formation, the haustoria, hyphal growth and spore formation. Furthermore, it is effective against strains resistant to downy mildew phenylamines. Finally, dimethomorph is a fungicide with
curative and preventive activity, being considered a reference in the market due to its eradicative ability.
Dimethomorph was disclosed for the first time in European Patent EP 120321. There are several patent documents describing mixtures of dimethomorph with other fungicides. EP0901322 describes a fungicidal mixture of dimethomorph with several carbamates. The cited document is silent regarding propamocarb-HCl. On the other hand, EP0753258, EP0985346, US6316446 describe mixtures with N-acetonylbenzamides, oxazolidinones and phenyl benzyl ethers derivatives, respectively.
Propamocarb-HCl, Compound (II), [IUPAC: propyl
3-(dimethylamino)propylcarbamate hydrochloride], whose structural formula is:
Compound II is a carbamate systemic fungicide having a preventive effect. It is absorbed by roots and leaves and transported upwards in the plant. This active ingredient has activity against several fungi from the Oomycete family which cause "damping-off ' and foliar diseases. Propamocarb hydrochloride disrupts the formation of fungal cell walls by interfering with the synthesis of phospholipids and fatty acids. It affects mycelial growth and also spore production and germination. The biochemical mode of action is exercised at the level of cell membrane permeability, affecting its integrity.
British patent GB- 1212708 describes propamocarb and its activity is reported in the control of Pythium ultimum. This document does not mention any result or biological activity of the mixture claimed in the present invention.
EP835055, W01998/26654, WO 1998/044801, WO2006/040123 and WO2007/ 128541 describe compositions comprising propamocarb and ofurace, benalaxyl, a derivative of phosphorous acid (e.g. fosetyl-Al), fluoxastrobin and flutriafol, respectively. On the Other hand WO2009/082939 describes an aqueous suspension concentrate (SC) composition comprising tebuconazole and propamocarb. EP2338341 describes a composition comprising: A) propamocarb-HCl and B) an insecticide compound selected from the group of flubendiamide or rynaxapyr.
WO8805630 claims the mixture of dimethomorph with several pesticides. Although propamocarb is mentioned as part of a long list of fungicides to be used in association with dimethomorph, no reference is made to the specific association of dimethomorph and propamocarb in its hydrochloride form, nor examples or evidence of the formulation according to the invention are mentioned. EP2409570A2 claims a fungicidal mixture comprising a compound of general formula:
in which R1- 9 have the meanings cited therein, and several compounds commonly used in crop protection. The present invention does not make use in anyway of the general compound depicted above.
WO2010/128003 A2 relates to a method for increasing the vigor and/or yield of agricultural crops under the conditions of an essentially non-existent pressure from pathogenic agents, comprising a Bacillus subtilis strain, or a derivative thereof, and optionally at least one compound selected from strobilurins, carboxamides, azoles,
heterocyclic compounds, carbamates, other active substances, growth regulators, herbicides and insecticides. On the other hand, WO2009/037242A2 relates to fungicidal mixtures, comprising Bacillus subtilis and Bacillus pumilus strains, or derivatives thereof, and optionally at least one compound selected from azoles, strobilurins, carboxamides, heterocyclic compounds, carbamates and other fungicides. The present invention falls off the scope of the mentioned patent applications, as it describes a mixture of dimethomorph and propamocarb-HCI, not including the Bacillus sp. strains mentioned. The document that was considered the closest state of the art is "Containment of existing potato late blight (Phytophthora infestans) foliar epidemics with fungicides, CROP PROTECTION 21 (7), pp. 575-582, August 2002". This document reports the efficacy against potato mildew of the mixture propamocarb, in the form of a suspension concentrate, and dimethomorph in the form of a wettable powder. The stated mixture was prepared by the "tank mix" method, a very widely used form in crop protection of aggregating two fungicides, with different modes of action, for later application to the fields being protected. The concentrations used therein for the tank mixture "propamocarb +dimethomorph" were 1,01 kg/ha for propamocarb and 0,22 kg/ha for dimethomorph. In Table 3 (line 5 vs. line 9) of the cited document, the values mentioned for RAUDPC (Relative Area Under the Disease Progress Curve) are 0.761 and 0.8 1 for dimethomorph + propamocarb and dimethomorph alone, respectively. Therefore, the mixture of dimethomorph and propamocarb did not show a statistically significant biological interaction between the two active substances when compared to the result of dimethomorph alone, not showing as such any benefit for crop protection purposes.
The present invention does not fall in the scope of the mentioned closest state of the art as it relates to an unique product in the form of a readily usable suspension concentrate of dimethomorph and propamocarb hydrochloride. Also, and although the present composition makes use of lower field application doses (propamocarb-HCI: 0,8-1,0 kg/ha; dimethomorph: 0,144-0,180 kg/ha), it shows a surprisingly biological interaction between the two active ingredients resulting in a robust synergistic behavior.
The references mentioned in the prior art do not disclose nor the synergistic composition according to the present invention, nor the concentration ranges of the active ingredients in the mixture, nor the application rates described herein for an effective fungicidal action.
In the market of anti-mildew potato products, the virulence of the disease and the problems of resistance to some systemic fungicides, that often causes the decrease in potato culture yields, has led to the development of various products in the form of mixtures of active substances. The objective of such is to achieve superior efficacy combined with the minimization of resistance development associated with the biochemical mode of action of these molecules enhanced by high mutagenic capacity that potato mildew shows.
For the control of potato mildew, dimethomorph exists commercially associated to other fungicides siich as mancozeb (under the name ACROBAT M DG*, marketed by BASF*) or a strobilurin, such as pyraclostrobin (under the name COACH PLUS*, marketed by BASF*).
For the control of potato mildew, propamocarb-HCl exists commercially associated to other fungicides such as fenamidone (under the name CONSENTO*, marketed by Bayer Cropscience*), fluopicolide (under the name INFINITO*, marketed by Bayer Cropscience*), fosetyl-Al (under the name PREVICUR ENERGY*, marketed by Bayer Cropscience*), chlorothalonil (under the name TATTOO C*, marketed by Bayer Cropscience*) and cymoxanil (under the name PROXANIL*. marketed by Agriphar SA*).
The number of existing commercial solutions presently in the market is a clear sign that the subject matter described in the present invention is a general concern regarding the need for the development of new synergistic mixtures to overcome fungicidal resistance issues. Also, the various patent documents cited herein with respect to the mixtures of dimethomorph or propamocarb with other active substances show a clear interest for the finding of new synergistic solutions for crop protection purposes, namely fungicidal solutions.
In conclusion, there is still an opportunity for novel and innovative mix product formulations with a surprisingly synergistic effect for the sustainable control of plant pathogenic fungi, in particular resistant strains, with an effective application rate as low as possible, benefiting of a better toxicological and ecotoxicological profile.
DETAILED DESCRIPTION OF THE INVENTION The present invention relates to a binary fungicidal combination, consisting of dimethomorph and propamocarb-hydrochloride, to the preparation thereof and use of said composition for the control of fungi pathogens.
In a preferred embodiment, the fungicidal composition consists of dimethomorph (compound I) and propamocarb hydrochloride (compound II) in the form of a suspension concentrate (SC) containing 90 g/L of dimethomorph and 500 g/L of propamocarb hydrochloride.
The fungicidal composition according to the present invention is used to treat the following plant diseases: potato late blight (Phythophtora infestans), tomato downy mildew (Phythophtora infestans) and lettuce downy mildew (Bremia lact cae).
The invention also relates to a process to treat crops affected by pathogen fungi by applying to said crops, 144 g/ha to 180 g/ha of compound I and 800 g/ha to 1000 g/ha of Compound II.
The concentrations of the active ingredients in the composition according to the present invention are in the range of 72 g/L to 90 g/L for compound I, and in the range of 400 g/L to 500 g/L for compound II.
In a preferred embodiment, the concentration of compound I is 90 g/L and the concentration of compound II is 500 g/L.
The fungicidal composition according to the present invention further comprises surfactants, anti-freeze agents and rheology modifiers. The surfactants are selected from the group comprising dispersing agents, biological activators and anti-foam agents.
The preferred dispersing agents are non-ionic polymethyl methacrylate- polyethylene oxide graft copolymers, such as Atlox 4913 commercially available from Croda®, alone or combined with a polyoxyethylene alkyl ether such as Atlox 4894 commercially available from Croda®, and the preferred anti-foam agents are emulsions of polydimethylsiloxane in water such as AF9030 commercially available from Momentive®. Biological activators used in the present invention are branched C9-G ethoxylated alcohols, preferably C9-C11 branched ethoxylated alcohols, such as Rhodasurf 860/P commercially available from Rhodia®, or Cn to CM branched ethoxylated alcohols, such as Rhodasurf 870 commercially available from Rhodia®. As an example, the preparation of the suspension concentrate according to the invention is carried out using the following composition:
g/L
Compound I 90.0 Compound II 500.0
Polymeric surfactant (Atlox 4913) 11.0 Non-ionic surfactant (Atlox 4894) 7.0 Ethoxylated alcohol (Rhodasurf 860/P) 50.0 Antifreeze agent (Propylene Glycol) 30.0 Anti-Foam agent (AF9030) 10.0
Water 255.0
Thickener Solution* 147.0
* The Thickener solution is prepared by mixing Preventol Bit 20 N (1.0% (w/w)), xantham gum (2.0% (w/W)) and water (97.0% (w/w)).
The process for preparing the suspension concentrate according to the invention comprises the following steps: mixing Compound II with propylene glycol water, a polymeric surfactant (e.g. Atlox 4913), a nonionic proprietary surfactant blend (e.g. Atlox 4894), an ethoxylated isodecyl alcohol (e.g. Rhodasurf 860/P) and a anti- foam (e.g. AF 9030); dispersing Compound I, with stirring, in the previously prepared solution; pumping the suspension thus obtained through a refrigerated bead mill (for example* a Dyno-mill) in order to reduce the particle size; adjusting viscosity by adding the thickner solution with stirring.
The example of formulation described above is meant to be illustrative and riot limit the present invention in any way.
STABILITY TESTS;
Stability tests were carried out under the following conditions:
An accelerated storage test, in which a formulation sample is stored in an oven for 2 weeks at 54 ± 2°C, and their physical-chemical and technical properties are compared to those of a sample of the same batch which was not submitted to the test. This test is intended to represent a simulation of the behavior of a formulation after a 2 years storage period. A low temperature stability test was carried out at 0 ± 2°C during one week.
The present tests were conducted according to the following legislation and guidelines, which are included herein as references: · Regulation (EC) No. 1107/2009 of 21 October 2009 and Commission
Regulation (EU) No. 545/2011 of 10 June 2011.
• Manual on the Development and Use of FAO and WHO Specifications for Pesticides, November 2010 - second revision of the first edition.
• CIPAC Handbooks Vol. 1A to N, Collaborative international Pesticides Analytical Council.
A. Dimethomorph plus Propamocarb hydrochloride SC
Table 1 shows the physical and chemical properties of the dimethomorph plus propamocarb hydrochloride SC formulation prepared according to the example above before and after the accelerated storage test.
Table 1 - Accelerated storage test results.
The above results demonstrate that dimethomorph and propamocarb-HCl in the SC formulation do not show, after the accelerated test, a percentage of relative degradation higher than 5 ; which complies with the requirements of the "Manual on the Development and Use of FAO and WHO Specifications for Pesticides, November 2010" .
The suspensibility of the composition was evaluated by CIPAC MT 184. FAO specifies a minimum of 60% found in suspension after 30 minutes at 30°C in standard CIPAC water D. Table 1 shows suspensibility results, before and after the accelerated test for both active substances of close 100%, which is well above the minimum required.
The spontaneity of dispersion in % was determined according to CIPAC MT 160, using CIPAC standard water D at 30 ± 1°C and shows that both active substances are well dispersed (above 90%), before and after the accelerated test.
The pourability in % was determined according to CIPAC MT 148,1. The test of pourability is a direct measure of how the viscosity can influence the product, namely the handling, in particular, the easiness or difficultness to remove all of the content from the agrochemical package and rinse the package for environmental reasons. The residue of the composition according to the invention after pouring out the packaging contents was of 2.2% and 3.2% before and after the accelerated test, very low and interesting value in terms of end-use and environment issues.
Table 2 shows the physical and chemical properties of the above dimethomorph plus propamocarb-HCI SC formulation before and after the low temperature stability test.
Table 2: Low temperature stability test results.
After the low temperature stability test, the contents of both active substances remained practically unchanged.
It can also be seen that no significant changes at the particle size level were found after the tests. This is of major importance in the end-use of the composition according to the invention, particularly as regards the spraying efficacy in the field.
FUNGICIDAL ACTION
The following study was performed in order to highlight the synergistic effects of the fungicidal compound combination dimethomorph plus propamocarb hydrochloride.
The results of the bioassays of both active substances towards Phytophthora infestans for the two single compounds dimethomorph (DMM) and propamocarb-HCl (PMC) as well as their combination are presented in table 3. The potential synergistic effect of said combination is determined at the EC50, EC70 and EC90 levels calculated from the full dose-response of the compound combination.
Table 3 presents the calculations of the expected efficacy (E) for the compound combination (dimethomorph + propamocarb-HCl) with the data of the fungicidal activity (= efficacy) following the formula of Colby. The comparison of this value (E) to the measured (bio-assayed) efficacy of the compound combination (M) demonstrates an existing synergistic effect if E is (remarkably) lower than M. The data shows a clear synergistic effect of the combination of dimethomorph and propamocarb hydrochloride (DMM: 90 g/L + PMC: 500 g/L) at all three selected test concentration levels EC50, EC70 and EC90, since E is in all cases lower than M.
Material and methods
The sensitivity analysis f an individual, mono-sporangia isolate of Phytophthora infestans forms the basis of the investigations and the resulting conclusions. This isolate results of an original, unselected "wild-type" sensitivity towards dimethomorph and is a
representative of the current predominating and probably also unselected ("wild type") sensitivity level towards propamocarb hydrochloride.
The base of the in vivo sensitivity analysis is the detached leaf test system with the treatment of whole, intaet plants one day before preparing the test sets and before inoculation (protective test system). The fungicide treatments are prepared with a 0.05% Uniperol-solution. The different fungicide concentrations are graded logarithmically by a factor of 2 in order to obtain an accurate EC evaluation for the sensitivity towards the active compound combination (first test run: DMM + PMC). Young potato plants variety "Bintje" are sprayed with the respective fungicide solutions to run-off conditions.
One day after spraying and just before inoculation, the test sets are prepared by cutting leaf parts from the treated plant material and disposed onto water agar (0.6% agar) in disposable Petri dishes of 6 cm diameter. For each concentration, a separate Petri dish is used to avoid gas phase interactions between differently treated plant material. Each Petri dish contains 3-5 leaf parts as replicates from different leaves treated with the same fungicide concentration. During the time of inoculation, the leafs are exposed to the zoospore suspension of the isolate for about 1-2 minutes. The test isolate is directly obtained from the "propagation leaves" which are transferred into 7.5 mL tubes. Approximately 5 mL of distilled H2O is added into the tubes and the suspension is prepared by shaking the tubes several times. The concentration for the sporangia suspension is adjusted at ca. 100.000 sporangia/mL. After a dark, wet and cold period of 2 h at 2-4°C to promote the zoospore release, each test set is inoculated from above by dispersing the spore suspension of the test isolate with a micro-sprayer in an airbrush system. After inoculation, the Petri dishes with the differently treated leaf parts are again separated from each other to avoid gas phase interactions.
The test is then incubated in the climate chamber for 6 days (18°C, ca. 50 mol/m2s, 16/8 h light/darkness period, 80% relative humidity). The Petri dishes with the differently treated plant material, corresponding to the various modalities tested, are separated from each other to avoid gas phase interactions.
After the incubation period, the test material is scored macroscopically regarding disease coverage/development (sporangia formation) at each fungicide concentration relative to the respective untreated control (in %). From the resulting dose-response, EC50, EC70 and EC90 values are calculated for the single compounds as well as for the compound combination (Calculated) by Probit analysis [WEBER, E., GrundriB der biologischen Statistik; 8) Auflage, Gustav-Fischer-Verlag, Stuttgart-New York, 1980]. These concentrations are then used for analyzing the respective efficacy of the single compounds as well as the compound combination (Measured). From the measured efficacy values of the single compounds, the Expected efficacy (in %) of the combination is calculated using the Colby equation (COLBY, S.R., Calculating synergistic and antagonistic responses of herbicide combination, Weeds, 15, pp 20-22, 1967).
E = x + y - x X y/100 wherein:
E is the expected efficacy, in percentage, when using the mixture of the active compounds I and II at the concentrations a and b. x is the efficacy, in percentage, when using active compound I at the concentration a; y is the efficacy, in percentage, when using active compound II at the concentration b.
If the expected value (E) of the combination is lower than the measured value (M) a synergistic fungicidal effect is expressed with the combination of the two compounds I and II. The results from the assays were computed using the above formulas and are presented in Table 3.
Table 3: Preventative efficacy on leaves inoculated with Phytophthora infestans
When the leaves are treated with a suspension containing the mixture of the two active ingredients according to the invention, the observed efficacy is higher at every EC when compared to the results obtained by the active ingredients alone.
Surprisingly, there is a higher fungicide control when the leaves are treated with the composition according to the present invention, in comparison to the active ingredients applied by per se. The fungicidal composition of the present invention is not just an admixture of both ingredients resulting in the aggregation of the properties shown individually, but rather the formulation according to the invention shows synergism providing an unexpected property for a wide range of relevant EC's.
The test results show that mixtures according to the invention have synergistically increased activity against potato late blight (Phythophtora infestdns). This is due to an enhanced biological action and consequently an improved toxic effect regarding the target fungi, when compared to the effect of both active substances alone. The object of the present invention has therefore reached a surprisingly technical and practical effect;
Claims
1. Synergistic fungicidal composition characterized in that it consists of dimethomorph and propamocarb hydrochloride in the form of a suspension concentrate in which: the dose of dimethomorph is in the range from 72 g/L to 90 g/L, and - the dose of propamocarb hydrochloride is in the range from 400 g/L to
500 g/L.
2. The fungicidal composition according to claim I, characterized in that the dose of dimethomorph is 90 g/L and the dose of propamocarb hydrochloride is 500 g/L.
3. The fungicidal composition according to any of the preceding claims, characterized in that it further comprises surfactants, anti-freeze agents and rheology modifiers.
4. The fungicidal composition according to claim 3, characterized in that said surfactants are selected from the group comprising dispersing agents, biological activators and anti-foam agents.
5. The fungicidal composition according to claim 4, characterized in that said biological activators are Cn to Cu branched ethdxylated alcohols.
6. Use of the fungicidal composition characterized in that it is used to treat potato late blight (Phythophtora infes(ans), tomato downy mildew (Phythop tora infestans) and lettuce downy mildew (Bremia lactucae).
7. A process to treat crops from pathogen fungi, characterized in that a composition according to any of the claims 1 to 5 is applied to the crops in the range of 144 g/ha to 180 g/ha of dimethomorph and 800 g/ha to 1000 g/ha of propamocafb hydrochloride.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| PT106351 | 2012-06-01 | ||
| PT106351A PT106351B (en) | 2012-06-01 | 2012-06-01 | SYNERGIC FUNGICIDE MIXTURE CONTAINING DIMETHOMORF AND PROPAMOCARBE-HYDROCHLORIDE |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2013180589A1 true WO2013180589A1 (en) | 2013-12-05 |
Family
ID=48700672
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/PT2013/000034 WO2013180589A1 (en) | 2012-06-01 | 2013-05-30 | Synergistic fungicidal mixture containing dimethomorph and propamocarb-hydrochloride |
Country Status (2)
| Country | Link |
|---|---|
| PT (1) | PT106351B (en) |
| WO (1) | WO2013180589A1 (en) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP3045042A1 (en) | 2015-01-15 | 2016-07-20 | Omya International AG | Use of surface-reacted calcium carbonate as carrier for agrochemical compounds |
| WO2022128912A1 (en) * | 2020-12-18 | 2022-06-23 | Syngenta Crop Protection Ag | Isocycloseram formulation |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN103798067A (en) * | 2014-01-24 | 2014-05-21 | 曾彩莲 | Potato late blight preventing method |
Citations (18)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3513241A (en) * | 1966-12-17 | 1970-05-19 | Schering Ag | Fungicidal and fungistatic n-(dialkylaminoalkyl) - carbamic acid and thiocarbamic acid esters |
| EP0120321A1 (en) | 1983-02-28 | 1984-10-03 | Shell Agrar GmbH & Co. KG | Acrylic-acid amides, their preparation and use |
| WO1988005630A1 (en) | 1987-01-30 | 1988-08-11 | Shell Agrar Gmbh & Co. Kg | Fungicidal compositions |
| EP0753258A2 (en) | 1995-07-12 | 1997-01-15 | Rohm And Haas Company | Synergistic fungicidal compositions of N-acetonyl-benzamides |
| EP0835055A1 (en) | 1995-06-24 | 1998-04-15 | Agrevo Uk Limited | Fungicide mixtures |
| WO1998026654A2 (en) | 1996-12-19 | 1998-06-25 | Isagro S.P.A. | Fungicidal compositions based on (n-phenylacetyl-n-2,6-xylyl)methyl alaninate |
| WO1998044801A1 (en) | 1997-04-04 | 1998-10-15 | Rhone Poulenc Agro | Synergetic fungicide composition |
| EP0901322A1 (en) | 1996-05-09 | 1999-03-17 | Basf Aktiengesellschaft | Fungicidal mixtures |
| EP0985346A1 (en) | 1998-08-31 | 2000-03-15 | American Cyanamid Company | Fungicidal mixtures |
| US6316446B1 (en) | 1998-05-18 | 2001-11-13 | Basf Aktiengesellschaft | Fungicidal mixture |
| WO2006040123A2 (en) | 2004-10-12 | 2006-04-20 | Bayer Cropscience Ag | Fungicidal active ingredient combinations containing fluoxastrobin |
| WO2007128541A2 (en) | 2006-05-08 | 2007-11-15 | Syngenta Participations Ag | Pesticidal combinations comprising flutriafol |
| WO2008077926A2 (en) * | 2006-12-22 | 2008-07-03 | Bayer Cropscience Ag | Pesticide composition comprising propamocarb-hydrochloride and an insecticide active substance |
| WO2009037242A2 (en) | 2007-09-20 | 2009-03-26 | Basf Se | Combinations comprising a fungicidal strain and an active compound |
| WO2009082939A1 (en) | 2007-12-19 | 2009-07-09 | Rotam Agrochem International Co., Ltd | Agrochemical composition and method for preparing the same |
| WO2010128003A2 (en) | 2009-05-06 | 2010-11-11 | Basf Se | A method for increasing the vigor and/or crop yield of agricultural plants under essentially non-existent pathogen pressure |
| EP2409570A2 (en) | 2010-06-29 | 2012-01-25 | Basf Se | Fungicidal mixtures based on pyrazolopyridine compounds |
| CN102613218A (en) * | 2012-03-09 | 2012-08-01 | 陕西先农生物科技有限公司 | Sterilizing composition containing dimethomorph and propamocarb |
-
2012
- 2012-06-01 PT PT106351A patent/PT106351B/en active IP Right Grant
-
2013
- 2013-05-30 WO PCT/PT2013/000034 patent/WO2013180589A1/en active Application Filing
Patent Citations (20)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3513241A (en) * | 1966-12-17 | 1970-05-19 | Schering Ag | Fungicidal and fungistatic n-(dialkylaminoalkyl) - carbamic acid and thiocarbamic acid esters |
| GB1212708A (en) | 1966-12-17 | 1970-11-18 | Schering Ag | Fungicidal and fungistatic preparations |
| EP0120321A1 (en) | 1983-02-28 | 1984-10-03 | Shell Agrar GmbH & Co. KG | Acrylic-acid amides, their preparation and use |
| WO1988005630A1 (en) | 1987-01-30 | 1988-08-11 | Shell Agrar Gmbh & Co. Kg | Fungicidal compositions |
| EP0835055A1 (en) | 1995-06-24 | 1998-04-15 | Agrevo Uk Limited | Fungicide mixtures |
| EP0753258A2 (en) | 1995-07-12 | 1997-01-15 | Rohm And Haas Company | Synergistic fungicidal compositions of N-acetonyl-benzamides |
| EP0901322A1 (en) | 1996-05-09 | 1999-03-17 | Basf Aktiengesellschaft | Fungicidal mixtures |
| WO1998026654A2 (en) | 1996-12-19 | 1998-06-25 | Isagro S.P.A. | Fungicidal compositions based on (n-phenylacetyl-n-2,6-xylyl)methyl alaninate |
| WO1998044801A1 (en) | 1997-04-04 | 1998-10-15 | Rhone Poulenc Agro | Synergetic fungicide composition |
| US6316446B1 (en) | 1998-05-18 | 2001-11-13 | Basf Aktiengesellschaft | Fungicidal mixture |
| EP0985346A1 (en) | 1998-08-31 | 2000-03-15 | American Cyanamid Company | Fungicidal mixtures |
| WO2006040123A2 (en) | 2004-10-12 | 2006-04-20 | Bayer Cropscience Ag | Fungicidal active ingredient combinations containing fluoxastrobin |
| WO2007128541A2 (en) | 2006-05-08 | 2007-11-15 | Syngenta Participations Ag | Pesticidal combinations comprising flutriafol |
| WO2008077926A2 (en) * | 2006-12-22 | 2008-07-03 | Bayer Cropscience Ag | Pesticide composition comprising propamocarb-hydrochloride and an insecticide active substance |
| EP2338341A2 (en) | 2006-12-22 | 2011-06-29 | Bayer CropScience AG | Pesticide composition comprising propamocarb-hydrochloride and an insecticide active substance |
| WO2009037242A2 (en) | 2007-09-20 | 2009-03-26 | Basf Se | Combinations comprising a fungicidal strain and an active compound |
| WO2009082939A1 (en) | 2007-12-19 | 2009-07-09 | Rotam Agrochem International Co., Ltd | Agrochemical composition and method for preparing the same |
| WO2010128003A2 (en) | 2009-05-06 | 2010-11-11 | Basf Se | A method for increasing the vigor and/or crop yield of agricultural plants under essentially non-existent pathogen pressure |
| EP2409570A2 (en) | 2010-06-29 | 2012-01-25 | Basf Se | Fungicidal mixtures based on pyrazolopyridine compounds |
| CN102613218A (en) * | 2012-03-09 | 2012-08-01 | 陕西先农生物科技有限公司 | Sterilizing composition containing dimethomorph and propamocarb |
Non-Patent Citations (8)
| Title |
|---|
| "CIPAC Handbooks", vol. 1A, article "N, Collaborative International Pesticides Analytical Council" |
| "Manual on the Development and Use of FAO and WHO Specifications for Pesticides", November 2010 |
| "The Pesticide Manual" 14"'. Edition", 2006, THE BRITISH CROP PROTECTION COUNCIL, pages: 347 |
| COLBY, S.R.: "Calculating synergistic and antagonistic responses of herbicide combination", WEEDS, vol. 15, 1967, pages 20 - 22 |
| CROP PROTECTION, vol. 21, no. 7, August 2002 (2002-08-01), pages 575 - 582 |
| DATABASE WPI Week 201270, Derwent World Patents Index; AN 2012-M86019, XP002710401 * |
| EPPO STANDARDS: "Good plant protection practices", EPPO BULLETIN, vol. 32, 2002, pages 367 - 369 |
| JEFFREY M STEIN ET AL: "Containment of existing potato late blight (Phytophthora infestans) foliar epidemics with fungicides", CROP PROTECTION, vol. 21, no. 7, 1 August 2002 (2002-08-01), pages 575 - 582, XP055028413, ISSN: 0261-2194, DOI: 10.1016/S0261-2194(01)00147-8 * |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP3045042A1 (en) | 2015-01-15 | 2016-07-20 | Omya International AG | Use of surface-reacted calcium carbonate as carrier for agrochemical compounds |
| WO2016113289A1 (en) | 2015-01-15 | 2016-07-21 | Omya International Ag | Use of surface-reacted calcium carbonate as carrier for agrochemical compounds |
| US10292383B2 (en) | 2015-01-15 | 2019-05-21 | Omya International Ag | Use of surface-reacted calcium carbonate as carrier for agrochemical compounds |
| WO2022128912A1 (en) * | 2020-12-18 | 2022-06-23 | Syngenta Crop Protection Ag | Isocycloseram formulation |
Also Published As
| Publication number | Publication date |
|---|---|
| PT106351A (en) | 2013-12-02 |
| PT106351B (en) | 2014-05-21 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP2013500981A (en) | Plant pathogen inhibitor combinations and methods of use | |
| JP2005530829A (en) | Active substance fungicidal mixture | |
| EP1280408B1 (en) | Fungicidal composition containing n-(alpha-cyano-2-thenyl)-4-ethyl-2-(ethylamino)-5-thiazolecarboxamide | |
| CN108935486A (en) | A kind of bactericidal composition | |
| RU2662289C2 (en) | Method of controlling strobilurine resistant septoria tritici | |
| CN102106355A (en) | Antifungal composition containing pyraoxystrobin | |
| KR20010012566A (en) | Fungicidal combinations comprising a 4-phenoxyquinoline | |
| CN104686540B (en) | A kind of bactericidal composition containing metconazole and amine benzene pyrrole bacterium ketone and application thereof | |
| CN106922703A (en) | A kind of bactericidal composition | |
| WO2013180589A1 (en) | Synergistic fungicidal mixture containing dimethomorph and propamocarb-hydrochloride | |
| MXPA02010331A (en) | Fungicidal active ingredient combinations. | |
| TWI220381B (en) | A method of combating phytopathogenic diseases on crop plants and a fungicidal composition having synergistic fungicidal activity | |
| CN105104397A (en) | Pyraclostrobin and metiram compound water dispersible granules and preparation method thereof | |
| CN104336036B (en) | Fungicidal composition and application thereof | |
| RU2357416C2 (en) | Synergetic herbicide composition as emulsifying concentrate and weed control method | |
| WO2019243994A1 (en) | A fungicidal composition and a process for preparation thereof | |
| CN106212480B (en) | A kind of microbicide compositions | |
| CN108260593A (en) | A kind of bactericidal composition | |
| CN105284843B (en) | Bactericidal composition | |
| CN102318626A (en) | Germicidal composition containing bupirimate and EBDC compounds | |
| EP2642853B1 (en) | Fungicidal mixture | |
| CN109380240B (en) | Pesticide composition containing oxathiapiprolin and application thereof | |
| RU2815385C2 (en) | Synergistic fungicidal composition | |
| CN103503895A (en) | Antifungal composition containing pyrazole compound and triazole compound | |
| CN107087609A (en) | Microbicide compositions containing anthraquinone analog compound and S-Ethyl ethylthio sulfonate |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 13732266 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 13732266 Country of ref document: EP Kind code of ref document: A1 |