WO2011115211A1 - Method for producing lithium manganese iron phosphate particulate powder, lithium manganese iron phosphate particulate powder and non-aqueous electrolyte secondary battery using that particulate powder - Google Patents
Method for producing lithium manganese iron phosphate particulate powder, lithium manganese iron phosphate particulate powder and non-aqueous electrolyte secondary battery using that particulate powder Download PDFInfo
- Publication number
- WO2011115211A1 WO2011115211A1 PCT/JP2011/056397 JP2011056397W WO2011115211A1 WO 2011115211 A1 WO2011115211 A1 WO 2011115211A1 JP 2011056397 W JP2011056397 W JP 2011056397W WO 2011115211 A1 WO2011115211 A1 WO 2011115211A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- compound
- olivine
- lithium
- powder
- limn
- Prior art date
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01B—NON-METALLIC ELEMENTS; COMPOUNDS THEREOF; METALLOIDS OR COMPOUNDS THEREOF NOT COVERED BY SUBCLASS C01C
- C01B25/00—Phosphorus; Compounds thereof
- C01B25/16—Oxyacids of phosphorus; Salts thereof
- C01B25/26—Phosphates
- C01B25/45—Phosphates containing plural metal, or metal and ammonium
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M10/00—Secondary cells; Manufacture thereof
- H01M10/05—Accumulators with non-aqueous electrolyte
- H01M10/052—Li-accumulators
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/13—Electrodes for accumulators with non-aqueous electrolyte, e.g. for lithium-accumulators; Processes of manufacture thereof
- H01M4/134—Electrodes based on metals, Si or alloys
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/36—Selection of substances as active materials, active masses, active liquids
- H01M4/48—Selection of substances as active materials, active masses, active liquids of inorganic oxides or hydroxides
- H01M4/50—Selection of substances as active materials, active masses, active liquids of inorganic oxides or hydroxides of manganese
- H01M4/505—Selection of substances as active materials, active masses, active liquids of inorganic oxides or hydroxides of manganese of mixed oxides or hydroxides containing manganese for inserting or intercalating light metals, e.g. LiMn2O4 or LiMn2OxFy
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01M—PROCESSES OR MEANS, e.g. BATTERIES, FOR THE DIRECT CONVERSION OF CHEMICAL ENERGY INTO ELECTRICAL ENERGY
- H01M4/00—Electrodes
- H01M4/02—Electrodes composed of, or comprising, active material
- H01M4/36—Selection of substances as active materials, active masses, active liquids
- H01M4/58—Selection of substances as active materials, active masses, active liquids of inorganic compounds other than oxides or hydroxides, e.g. sulfides, selenides, tellurides, halogenides or LiCoFy; of polyanionic structures, e.g. phosphates, silicates or borates
- H01M4/5825—Oxygenated metallic salts or polyanionic structures, e.g. borates, phosphates, silicates, olivines
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02E—REDUCTION OF GREENHOUSE GAS [GHG] EMISSIONS, RELATED TO ENERGY GENERATION, TRANSMISSION OR DISTRIBUTION
- Y02E60/00—Enabling technologies; Technologies with a potential or indirect contribution to GHG emissions mitigation
- Y02E60/10—Energy storage using batteries
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02P—CLIMATE CHANGE MITIGATION TECHNOLOGIES IN THE PRODUCTION OR PROCESSING OF GOODS
- Y02P70/00—Climate change mitigation technologies in the production process for final industrial or consumer products
- Y02P70/50—Manufacturing or production processes characterised by the final manufactured product
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02T—CLIMATE CHANGE MITIGATION TECHNOLOGIES RELATED TO TRANSPORTATION
- Y02T10/00—Road transport of goods or passengers
- Y02T10/60—Other road transportation technologies with climate change mitigation effect
- Y02T10/70—Energy storage systems for electromobility, e.g. batteries
Definitions
- the present invention is a method for producing olivine-type lithium iron manganese phosphate particles that can be easily produced at low cost, and has a high energy density when used as a high-power secondary battery.
- Provided are lithium iron manganese oxide particles and a secondary battery using the same.
- LiFePO 4 3.5V class olivine type structure LiFePO 4 to 4.1V class LiMnPO 4 as a positive electrode active material useful for a high energy density type lithium ion secondary battery.
- LiMnPO 4 is less liable to enter and exit Li than LiFePO 4 , improvement of charge / discharge characteristics is required.
- LiMnPO 4 having an olivine structure is composed of a strong phosphoric acid tetrahedral skeleton, an oxygen octahedron centered on manganese ions that contribute to redox, and lithium ions that are current carriers.
- grain surface there exists a tendency which coat
- it functions as an electrode of a secondary battery, and at the time of charge / discharge using Li as a negative electrode, it is said that it follows a two-phase reaction of the following formula due to the presence of a plateau region in charge / discharge characteristics indicated by capacity and voltage.
- Non-Patent Documents 1 to 6 are methods in which Fe is dissolved in Mn sites.
- LiMn 1-x Fe x PO 4 (0.05 ⁇ x ⁇ 0.5) also has better charge / discharge characteristics at low current as the particle surface is coated with carbon.
- the smaller the crystallite size satisfies the above condition the better the charge / discharge characteristics at a high current load.
- each aggregate in order to obtain a high molding density, each aggregate is formed so as to form a network of secondary particles that are appropriately aggregated and a conductive auxiliary agent such as carbon having a high graphitization rate. The state needs to be controlled.
- the positive electrode combined with a large amount of carbon is bulky, and there is a disadvantage that the substantial lithium ion density that can be filled per unit volume is lowered. Therefore, in order to secure charge / discharge capacity per unit volume, olivine-type lithium iron manganese phosphate coated with fine and moderate carbon was obtained and high density was obtained through a small amount of conductive auxiliary agent. There is a need to form aggregates.
- lithium manganese iron phosphate particles having an olivine structure with a small change in particle size, low electrical resistance, and high filling property due to charge / discharge are used as the positive electrode active material powder for non-aqueous electrolyte secondary batteries. It is required to produce by an industrial method with a small load.
- Patent Document 1 technology to reduce deterioration after charge / discharge cycle test
- Patent Document 2 technology to add different metal elements to reduce electrical resistance
- Patent Document 3 technology synthesized by hydrothermal method
- Patent Document 1 is a technique for improving charge / discharge repetition characteristics when LiMn 1-x Fe x PO 4 particle powder having an olivine type structure is used as a positive electrode. There is no mention of collective control.
- Patent Document 2 is not a technique related to the composite of LiMnPO 4 particle powder having an olivine structure and carbon.
- Patent Document 3 is a production method using a solid-phase reaction method, and since it has two heat treatments, it is difficult to say that the cost is low.
- the present invention establishes an industrial method of olivine-type lithium iron phosphate particles with a small olivine structure, which has a small change in particle size due to charge / discharge, low electrical resistance, and high packing properties, As a non-aqueous electrolyte secondary battery containing a high positive electrode active material, it is a technical subject to obtain a high capacity also in current load characteristics.
- the present invention relates to a method for producing olivine-type lithium manganese iron phosphate LiMn 1-x Fe x PO 4 (0.05 ⁇ x ⁇ 0.5) particle powder, wherein the raw material is Li compound, Mn compound , Fe compound, P compound and sugar or organic acid, and the raw material charge ratio is 0.95 ⁇ Li / (Mn + Fe) ⁇ 2.0, 0.95 ⁇ P / (Mn + Fe) ⁇ 1.3 in terms of mol ratio Obtained in the first step and the first step of obtaining an aqueous slurry having a pH of 5.5 to 12.5 by a neutralization reaction of a mixed solution containing 1 to 20 mol% of sugar or organic acid with respect to (Mn + Fe) The slurry is hydrothermally treated at a reaction temperature of 120 to 220 ° C., and the resulting compound has a production rate of LiMn 1-x Fe x PO 4 (0.05 ⁇ x ⁇ 0.5) of 75 wt% or more, Size 10-250
- the median diameter of the aggregated particles in the slurry is 0.1 to 10 ⁇ m
- the crystal phase is (NH 4 ) Mn 1- ⁇ Fe ⁇ PO 4 (0 ⁇ ⁇ ⁇ 1) or a manufacturing method according to the first aspect of the present invention for obtaining an aqueous slurry containing aggregated particles satisfying HMn 1- ⁇ Fe ⁇ PO 4 (0 ⁇ ⁇ ⁇ 1) (Invention 2).
- this invention is the manufacturing method of this invention 1 or 2 whose saccharide
- the present invention is the production method according to any one of the present inventions 1 to 3, wherein in the second step, washing is performed so that the sulfur content in the compound is 0.1 wt% or less (Invention 4). .
- the present invention is the production method according to any one of the present inventions 1 to 4, wherein in the third step, the organic substance to be added is at least one of carbon black, an oil and fat compound, a sugar compound, and a synthetic resin. (Invention 5).
- the present invention also relates to olivine type lithium manganese iron phosphate LiMn 1-x Fe x PO 4 (0.05 ⁇ x ⁇ 0.5) particle powder, wherein the content of lithium and phosphorus is in a molar ratio.
- the crystallite size is 25 to 300 nm, the median diameter of the aggregated particles is 0.3 to 20 ⁇ m, and the electrical resistivity of the powder is 1 to 1.0 ⁇ 10 6 ⁇ ⁇ cm.
- This is an olivine-type lithium iron manganese phosphate powder (this Invention 6).
- the present invention also relates to lithium iron manganese phosphate particles having an olivine structure according to the present invention 6, wherein the lithium and phosphorus contents satisfy Li ⁇ P (the present invention 7).
- the present invention also relates to lithium iron manganese phosphate particles having an olivine structure according to the present invention 6 or 7, wherein the unit cell volume V UC has the relationship represented by the following formula (1) (the present invention 8).
- the present invention is a non-aqueous electrolyte secondary battery produced using the olivine-type lithium manganese iron phosphate particles according to any one of the present inventions 6 to 8 (Invention 9).
- the method for producing olivine-type lithium iron manganese phosphate particles according to the present invention is low-cost and can be produced efficiently from almost equal amounts of raw materials. Is preferred.
- lithium olivine manganese phosphate particles having an olivine structure according to the present invention have little change in particle size due to charge / discharge, low electrical resistance, and high filling properties, and are used for non-aqueous electrolyte secondary batteries. Suitable as a positive electrode active material.
- the secondary battery using the olivine-type lithium manganese iron phosphate particles having the olivine structure as the positive electrode active material according to the present invention has a high capacity in the current load characteristics and can sufficiently withstand repeated charge and discharge.
- Example 2 is a secondary electron image of the olivine-type lithium manganese iron phosphate particles obtained in Example 1 by a scanning electron microscope. It is the discharge characteristic which made the positive electrode the olivine type structure lithium iron iron phosphate particle powder obtained in Example 1, and evaluated it by the coin cell. It is a secondary electron image by the scanning electron microscope of the lithium iron manganese phosphate particle powder of the olivine type structure obtained in Comparative Example 4. It is a secondary electron image by the scanning electron microscope of the particle powder obtained by chemically oxidizing the lithium iron manganese phosphate particle powder of the olivine type structure obtained in Comparative Example 4. It is a Rietveld analysis result of the X-ray-diffraction pattern of the particle powder obtained by chemically oxidizing the lithium iron manganese phosphate particle powder of the olivine type structure obtained in Comparative Example 4.
- the method for producing olivine-type lithium manganese iron phosphate LiMn 1-x Fe x PO 4 (0.05 ⁇ x ⁇ 0.5) particle powder according to the present invention includes a Li compound, a Mn compound, an Fe compound, Using a P compound and a sugar or an organic acid, the raw material charge ratio is 0.95 ⁇ Li / (Mn + Fe) ⁇ 2.0, 0.95 ⁇ P / (Mn + Fe) ⁇ 1.3 in terms of mol ratio, and (Mn + Fe)
- the raw materials used in the first step are preferably LiOH and Li 3 PO 4 as the Li compound, MnSO 4 and MnCO 3 as the Mn compound, FeSO 4 and FeCO 3 as the Fe compound, and P compound as the P compound.
- H 3 PO 4 , (NH 4 ) H 2 PO 4 , (NH 4 ) 2 HPO 4 , NaH 2 PO 4 , Na 2 HPO 4 , and Na 3 PO 4 are preferred.
- the Li compound, Mn compound, Fe compound, and P compound in the first step have a raw material charge ratio of 0.95 ⁇ Li / (Mn + Fe) ⁇ 2.0 and 0.95 ⁇ P / (Mn + Fe) ⁇ 1. 3 to be mixed.
- the raw material charge ratio is outside the above range, the target lithium iron manganese phosphate cannot be obtained.
- the molar ratio is 0.98 ⁇ Li / (Mn + Fe) ⁇ 1.8 and 0.98 ⁇ P / (Mn + Fe) ⁇ 1.2.
- the sugar or organic acid used in the first step is preferably sucrose, ascorbic acid, or citric acid.
- the addition amount of sugar or organic acid is 1 to 20 mol%, more preferably 1.5 to 18 mol%, based on (Mn + Fe).
- the added sugar or organic acid works as a transition metal reducing agent, and after hydrothermal reaction.
- the production rate of LiMn 1-x Fe x PO 4 (0.05 ⁇ x ⁇ 0.5) is increased, and the primary particle diameter of the product is refined.
- LiOH, NaOH, Na 2 CO 3 , NH 3 , urea, ethanolamine or the like is used as the alkali source used in the first step.
- the aqueous slurry in the first step has a pH of 5.5 in order to increase the production rate of LiMn 1-x Fe x PO 4 (0.05 ⁇ x ⁇ 0.5) of the compound obtained by the hydrothermal treatment in the second step.
- the median diameter of the aggregated particles in the slurry in the first step is preferably 0.1 to 10 ⁇ m.
- the crystal phase of the aggregated particles in the slurry is (NH 4 ) Mn 1- ⁇ Fe ⁇ PO 4 (0 ⁇ ⁇ ⁇ 1) or HMn 1- ⁇ Fe ⁇ PO 4 (0 ⁇ ⁇ ⁇ 1). It is preferable that the agglomerated particles are included. When one of the two phases is not included, the target lithium manganese iron phosphate cannot be obtained.
- the agglomerated particles having the median diameter and the crystal phase can be obtained by mixing raw materials under conditions where crystal nuclei are generated and grown (generation of a large number of crystal nuclei) or by pulverization of the product.
- Examples of the apparatus used for pulverization include a ball mill and a medium stirring mill.
- the hydrothermal treatment in the second step is preferably performed at 120 to 220 ° C.
- the hydrothermal treatment time is preferably 1 to 10 hours.
- the production rate of LiMn 1-x Fe x PO 4 (0.05 ⁇ x ⁇ 0.5) is 75 wt% or more.
- the production rate of LiMn 1-x Fe x PO 4 (0.05 ⁇ x ⁇ 0.5) remains low even after the subsequent steps.
- a more preferable production rate is 80 wt% or more.
- the crystallite size of the obtained compound based on LiMn 1-x Fe x PO 4 (0.05 ⁇ x ⁇ 0.5) is 10 to 250 nm.
- a compound having a crystallite size of less than 10 nm is industrially difficult to produce, and a compound having a crystallite size of more than 250 nm cannot obtain good battery characteristics.
- a more preferable crystallite size is 30 to 150 nm.
- the hydrothermal treatment in the second step needs to be performed by appropriately selecting the treatment temperature and the treatment time so as to satisfy the above generation rate and crystallite size. If the hydrothermal treatment temperature is low and the treatment time is short, the above-mentioned production rate may not be reached, or the crystallite size may not be reached, and if the hydrothermal treatment temperature is high and the treatment time is long, the above crystals May exceed child size.
- the compound obtained by the hydrothermal treatment in the second step is subjected to filtration washing or decantation washing in order to remove impurity sulfate ions and control the composition ratio.
- the apparatus used for cleaning include a press filter and a filter thickener.
- the washing of the compound obtained by the hydrothermal treatment in the second step is preferably performed so that the sulfur content in the compound is 0.1 wt% or less. Washing is sufficient if sulfur in the compound can be sufficiently removed, and usually washing with water may be performed.
- lithium compounds such as LiOH and Li 2 CO 3 are used for adjusting Li
- manganese compounds such as MnCO 3 and MnC 2 O 4 are used for adjusting Mn
- FeCO 3 and FeC are used for adjusting Fe.
- an iron compound such as 2 O 4 and a PO 4 -containing compound such as H 3 PO 4 , (NH 4 ) H 2 PO 4 , (NH 4 ) 2 HPO 4 for adjusting P.
- a fine lithium iron manganese phosphate solid solution coated with carbon 3 to 40 wt% of an organic substance is added in the third step to obtain a precursor powder. At that time, it is necessary to reduce the primary particle size of the precursor powder and to uniformly mix with the organic matter.
- the apparatus used for mixing include a Henschel mixer, a raking machine, a high speed mixer, a medium stirring mill, and the like.
- the organic substance to be added is preferably at least one of carbon black, an oil and fat compound, a sugar compound, and a synthetic resin.
- the organic substance added is preferably at least one of an oil and fat compound, a sugar compound, and a synthetic resin.
- the resulting compacted compact of olivine-type lithium manganese iron phosphate particles even at low temperatures such as 400 to 500 ° C. has an electrical resistivity of 1 to 1.0 ⁇ 10 6 ⁇ ⁇ cm. Satisfying and high performance secondary battery characteristics.
- oil and fat compound examples include stearic acid and oleic acid
- sugar compound examples include sucrose and dextrin
- synthetic resin examples include polyethylene, polypropylene, and polyvinyl alcohol (PVA).
- carbon black examples include acetylene black (manufactured by Denki Kagaku Kogyo Co., Ltd.) and ketjen black (manufactured by Lion Corporation).
- Preferred binders include polyvinyl alcohol (PVA), polyvinyl butyral, starch, carboxymethyl cellulose and the like.
- the precursor powder obtained in the third step is fired at a temperature of 250 to 850 ° C. in an inert gas or reducing gas atmosphere having an oxygen concentration of 0.1% or less.
- an inert gas N 2 , Ar, H 2 O, CO 2 or a mixed gas thereof is used.
- the reducing gas H 2 , CO, or a mixed gas of these gases and the inert gas is used.
- the apparatus used for firing include a gas flow type box muffle furnace, a gas flow type rotary furnace, and a fluidized heat treatment furnace.
- the calcination temperature may be 250 ° C. or higher, but the reaction of unreacted substances is completed, and the added organic matter
- it is preferably calcined at 350 to 850 ° C., more preferably at 400 to 750 ° C. for 1 to 10 hours.
- the lithium iron manganese phosphate particles according to the present invention can produce LiMn 1-x Fe x PO 4 (0.05 ⁇ x ⁇ 0.5) after hydrothermal treatment by adjusting an appropriate precursor slurry. The rate can be increased. Thereafter, the mixture is uniformly mixed with an organic substance and further baked, whereby the production rate of LiMn 1-x Fe x PO 4 (0.05 ⁇ x ⁇ 0.5) is high, and fine manganese phosphate sufficiently covered with carbon Iron lithium particle powder can be obtained.
- the lithium iron manganese phosphate particles according to the present invention are preferably produced by the production method according to the present invention 1 described above.
- the content of lithium and phosphorus is 0.9 ⁇ Li / (Mn + Fe) ⁇ 1.2 and 0.9 ⁇ P / (Mn + Fe) ⁇ 1.2 in terms of mol ratio. It is.
- the content of lithium and phosphorus is outside the above range, a heterogeneous phase is likely to be formed, and in some cases, grain growth is promoted and lithium manganese iron phosphate particles having high battery characteristics cannot be obtained.
- Preferable lithium and phosphorus contents are 0.98 ⁇ Li / (Mn + Fe) ⁇ 1.05, 0.98 ⁇ P / (Mn + Fe) ⁇ 1.05, and more preferably 1 ⁇ Li / (Mn + Fe) in molar ratio. ) ⁇ 1.05, 1 ⁇ P / (Mn + Fe) ⁇ 1.05.
- the lithium manganese iron particle powder according to the present invention preferably has a lithium and phosphorus content of Li ⁇ P.
- the BET specific surface area of the lithium iron manganese phosphate particles according to the present invention is 6 to 70 m 2 / g.
- the BET specific surface area is less than 6 m 2 / g, the movement of Li ions in the lithium iron manganese phosphate particle powder is slow, so that it is difficult to take out the current.
- a preferred BET specific surface area is 10 to 65 m 2 / g, more preferably 15 to 60 m 2 / g.
- the carbon content of the lithium iron manganese phosphate particles according to the present invention is 0.5 to 8.0 wt%.
- the carbon content is less than 0.5 wt%, the particle growth during the heat treatment cannot be suppressed, and the electric resistance of the obtained powder is increased, which deteriorates the charge / discharge characteristics of the secondary battery.
- the carbon content exceeds 8.0 wt%, the packing density of the positive electrode is lowered, and the energy density per volume of the secondary battery is reduced.
- a more preferable carbon content is 1.0 to 6.0 wt%.
- the lithium iron manganese phosphate particles according to the present invention have an impurity sulfur content of 0.08 wt% or less, and good storage characteristics can be obtained in a nonaqueous electrolyte secondary battery.
- impurities such as lithium sulfate are formed, and these impurities undergo a decomposition reaction during charge and discharge, and the reaction with the electrolyte during high-temperature storage is promoted, and after storage Resistance rises intensely.
- a more preferable sulfur content is 500 ppm or less.
- the crystal phase of LiMn 1-x Fe x PO 4 (0.05 ⁇ x ⁇ 0.5) having an olivine structure is 95 wt% or more.
- LiMn 1-x Fe x PO 4 (0.05 ⁇ x ⁇ 0.5) particles obtained after firing may be fine and the discharge capacity may be high.
- Li 3 PO 4 itself does not contribute to charging and discharging, it is desirable that it be less than 5 wt%.
- the crystallite size of the lithium iron manganese phosphate particles according to the present invention is 25 to 300 nm. It is extremely difficult to mass-produce a powder having a crystallite size of less than 25 nm while satisfying other powder characteristics by the production method of the present invention, and Li moves inside the particle at a crystallite size exceeding 300 nm. Time is required and the current load characteristics of the secondary battery are deteriorated.
- the preferred crystallite size is 30 nm to 200 nm, more preferably 40 nm to 150 nm.
- the median diameter of the aggregated particles of the lithium iron manganese phosphate particles according to the present invention is 0.3 to 30 ⁇ m.
- the median diameter is less than 0.3 ⁇ m, the positive electrode packing density is decreased and the reactivity with the electrolytic solution is increased.
- the median diameter exceeds 30 ⁇ m, it is too large for the electrode film thickness and it is extremely difficult to form a sheet.
- a preferable median diameter of the aggregated particles is 0.5 to 15 ⁇ m.
- the density of the compression molded body of the lithium iron manganese phosphate particles according to the present invention is preferably 1.8 g / cc or more.
- the true density of LiMn 1-x Fe x PO 4 (0.05 ⁇ x ⁇ 0.5) is 3.6 g / cc, and the closer to the true density, the better the filling property. Therefore, a preferable compression molding density is 2.0 g / cc or more exceeding 50% of the true density.
- the lithium manganese iron phosphate particle powder according to the present invention is considered to have a high compression-molded body density because the amount of remaining carbon is small and primary particles are appropriately aggregated.
- the powder electrical resistivity of the lithium iron manganese phosphate particles according to the present invention is 1 to 1.0 ⁇ 10 6 ⁇ ⁇ cm.
- the powder electrical resistivity is preferably 1 to 5.0 ⁇ 10 5 ⁇ ⁇ cm, more preferably 5 to 1.0 ⁇ 10 5 ⁇ ⁇ cm.
- the unit cell volume V UC of the lithium iron manganese phosphate particles according to the present invention is preferably in the relationship of formula (1).
- the lattice constant and the unit cell volume decrease linearly with the increase of the value of x in LiMn 1-x Fe x PO 4 having an olivine structure, and follow Vegard's law.
- the unit cell volume of LiMn 1-x Fe x PO 4 (0.05 ⁇ x ⁇ 0.5) having an olivine structure inferred from the linearity of the Vegard rule is 10.9 ⁇ (1-x) +291.36 Met. The closer to the unit cell volume, the transition metal substitution to the Li site and the loss of P and O are reduced. When the volume is smaller than the unit cell volume, Li is substituted to the transition metal site, and the theoretical capacity as an electrode is reduced. I think it will increase.
- the present inventors According to the heat treatment of a precursor having a composition ratio deviated from the stoichiometric ratio of LiMn 1-x Fe x PO 4 having an olivine structure, the present inventors have made LiMn 1-x Fe x PO 4 ( It was confirmed that an impurity crystal phase that exists more stably than 0.05 ⁇ x ⁇ 0.5) tends to be generated.
- the composition ratio of Li, Mn, Fe, and P is slight and a olivine-type LiMn 1-x Fe x PO 4 single crystal phase is formed, different lattice constants are obtained.
- the unit cell volume is more than the above formula (1). In that case, it is assumed that the transition metal substitution to the Li site and the loss of P and O occur, and the battery characteristics deteriorate.
- a conductive agent and a binder are added and mixed according to a conventional method.
- the conductive agent acetylene black, carbon black, graphite and the like are preferable, and as the binder, polytetrafluoroethylene, polyvinylidene fluoride and the like are preferable.
- the solvent for example, N-methyl-pyrrolidone is used, and the positive electrode active material sieved to 45 to 105 ⁇ m or less and the slurry containing the additive are kneaded until they become honey.
- the obtained slurry is applied onto the current collector with a doctor blade having a groove of 25 ⁇ m to 500 ⁇ m.
- the coating speed is about 60 cm / sec, and an Al foil of about 20 ⁇ m is usually used as a current collector.
- drying is performed at 80 to 180 ° C. in a non-oxidizing atmosphere of Fe 2+ .
- the sheet is subjected to a calender roll treatment so as to have a pressure of 1 to 3 t / cm 2 . In the step of forming the sheet, an oxidation reaction of Fe 2+ to Fe 3+ occurs even at room temperature.
- the density of the compression molded body of the positive electrode active material is as high as 1.8 g / cc or more, and the electrical resistivity of the compression molded body of the positive electrode active material is 1 to 1.0 ⁇ 10 6 ⁇ . ⁇ Because it is as low as cm, the amount of carbon added during sheet preparation can be suppressed, and since the BET specific surface area of the positive electrode active material is as low as 6 to 70 m 2 / g, the amount of binder added can be suppressed, resulting in density A high positive electrode sheet is obtained.
- lithium metal lithium metal, lithium / aluminum alloy, lithium / tin alloy, graphite or the like can be used, and the negative electrode sheet is produced by the same doctor blade method or metal rolling as the positive electrode.
- an organic solvent containing at least one of carbonates such as propylene carbonate and dimethyl carbonate and ethers such as dimethoxyethane can be used as the solvent for the electrolytic solution.
- At least one lithium salt such as lithium perchlorate and lithium tetrafluoroborate can be dissolved in the above solvent and used.
- the olivine-type lithium iron manganese phosphate LiMn 1-x Fe x PO 4 (0.05 ⁇ x ⁇ 0.5) particle powder according to the present invention is produced by hydrothermal reaction and firing, and is low in cost and in a small environment Can be manufactured with load.
- the present invention is the manufacturing method of LiMn 1-x Fe x PO 4 of olivine (0.05 ⁇ x ⁇ 0.5), LiMn 1-x Fe x PO 4 by hydrothermal reaction (0.05 ⁇ x ⁇ 0.5) is produced at a high production rate, then uniformly mixed with an organic substance to control the aggregated particle size, and fired under conditions of an inert or reducing atmosphere, and is finely coated with carbon. In addition, it is possible to obtain a particle powder having a high filling property.
- the nonaqueous electrolyte secondary battery using this as a positive electrode active material also has a current load characteristic.
- the present inventor estimates that a high capacity can be obtained and that it can sufficiently withstand repeated charge and discharge.
- the non-aqueous electrolyte secondary battery using the olivine-type lithium iron manganese phosphate particles according to the present invention as a positive electrode active material has a discharge capacity at 115 Ch / g at room temperature (25 ° C.) of 115 mAh / g or more.
- the discharge capacity at 1 C at 95 ° C. is at least 95 mAh / g, and the discharge capacity at 5 C at room temperature is at least 80 mAh / g.
- a typical embodiment of the present invention is as follows.
- the Li and P concentrations of the lithium and phosphorus-containing main raw materials were measured by neutralization titration using a pH meter and hydrochloric acid or NaOH reagent.
- the Fe concentration of the iron raw material was quantified by titration (JIS K5109), and the Mn concentration of the manganese raw material was also quantified by titration (Analytical Chemistry Handbook, edited by the Japan Analytical Chemical Society). Based on these analysis results, the reaction concentration and the raw material charge ratio were determined.
- Particle size of the aggregated particles in the slurry in the first step were measured median diameter D 50 by a wet method.
- the crystal phase of the aggregated particles in the slurry in the first step was obtained by filtering the aggregated particles in the slurry, and using Cu-K ⁇ , a wet cake using an X-ray diffractometer RINT-2500 [manufactured by Rigaku Corporation]. Measurement was performed under conditions of 40 kV and 300 mA, and the crystal phase was identified.
- the TCH pseudo-void function is used as the profile function, the method such as Finger is used for asymmetry of the function, and the reliability factor S value is 2.0. Analyzed to cut.
- the unit cell volume of the lithium iron manganese phosphate particles having the olivine structure (space group Pnma) according to the present invention was calculated from the product of the lattice constants a, b and c calculated by the Rietveld analysis.
- the composition ratio of the compound powder after adjusting the composition ratio of the compound obtained by the hydrothermal treatment in the second step and the lithium manganese iron phosphate particle powder according to the present invention is determined by the emission plasma analyzer ICAP-6500 [Thermo [Fischer Scientific]. The sample was dissolved in an acid solution at 200 ° C. using an autoclave.
- the specific surface area of the lithium iron manganese phosphate particles according to the present invention was measured using MONOSORB [manufactured by Yuasa Ionics Co., Ltd.] after drying and deaeration of the sample under nitrogen gas at 120 ° C. for 45 minutes.
- Carbon and sulfur amounts were quantified by burning them in an oxygen stream in a combustion furnace using EMIA-820 [manufactured by Horiba Ltd.].
- the density of the compression molded body of the lithium iron manganese phosphate particles according to the present invention was calculated from the weight and volume of the compression molded body when compacted to 1.5 t / cm 2 with a 13 mm ⁇ jig. Moreover, the powder electrical resistivity was measured by the two-terminal method using the compression molded body.
- NO 2 BF 4 having a molar number twice that of Li in the powder iron manganese phosphate is added to the powder of lithium manganese phosphate, left to stand for chemical oxidation, washed with acetonitrile and washed with Li. A particle powder with all removed was obtained.
- the primary particle diameter was measured by SEM photographs obtained with a scanning electron microscope (SEM) of Hitachi S-4800 type, and the change rate of the primary particle diameter before and after chemical oxidation was calculated.
- the secondary battery characteristics of the CR2032-type coin cell were evaluated using the lithium iron manganese phosphate particles having an olivine structure according to the present invention.
- the positive electrode material slurry was adjusted to 8 wt%, applied onto an Al current collector with a doctor blade, dried in air at 120 ° C. for 10 minutes, and the dried sheet was pressurized to 3 t / cm 2 to form a positive electrode A sheet was produced.
- the charge / discharge characteristics of the non-aqueous electrolyte secondary battery using the olivine type lithium iron manganese phosphate particles according to the present invention as the positive electrode active material were measured by measuring the discharge capacity at C / 10, 1C, and 5C at room temperature. And evaluated.
- C / 10 is 10 hours
- 1C is 1 hour
- 5C is 1/5 hour
- the theoretical capacity of LiMn 1-x Fe x PO 4 (0.05 ⁇ x ⁇ 0.5).
- the current value is fixed so that a current of 170 mAh / g flows.
- a higher C coefficient means higher current load characteristics.
- the voltage range during charging and discharging is not particularly limited, but in the present invention, the voltage range was 2.0 to 4.5V.
- Example 1 A solution of MnSO 4, H 3 PO 4 , and NH 4 OH was mixed, and NH 4 MnPO 4 was precipitated by a neutralization reaction at room temperature. After the precipitate was filtered off and washed with pure water, a solution of LiOH.H 2 O, FeSO 4 , H 3 PO 4 , and ascorbic acid was added, and the aqueous slurry adjusted to the raw material charge ratio shown in Table 1 Got.
- the slurry was mixed with a ball mill, and the aggregated particles were pulverized to adjust the particle size.
- the slurry was hydrothermally treated at 180 ° C. for 3 hours, and the resulting compound was filtered off, washed with pure water, and dried at 70 ° C. overnight. From the Rietveld analysis of the XRD pattern, the obtained dry powder has a production rate of LiMn 1-x Fe x PO 4 of 95% and 5 wt% of Li 3 PO 4 as an impurity crystal phase other than the olivine structure. confirmed.
- the crystallite size was 70 nm according to Scherrer's equation. Further, the content of impurity sulfur in the dry powder was 0.1 wt% or less (second step).
- the precursor powder obtained in the third step was put in an alumina crucible and baked in a nitrogen atmosphere at 650 ° C. for 5 hours.
- the temperature increase rate was 200 ° C./hr, and the N 2 gas flow rate was 1 L / min (fourth step).
- the obtained powder was lithium iron manganese phosphate particles having an olivine structure, and the composition ratio thereof was the same as the composition ratio of Li, Mn, Fe, and P adjusted in the third step.
- FIG. 1 shows an SEM photograph (secondary electron image) of the olivine-type lithium iron manganese phosphate particles obtained.
- Table 1 shows the manufacturing conditions of the first step and the properties of the obtained slurry
- Table 2 shows the characteristics of the powder in the second step and the manufacturing conditions of the third step
- Table 3 shows the obtained lithium iron manganese phosphate.
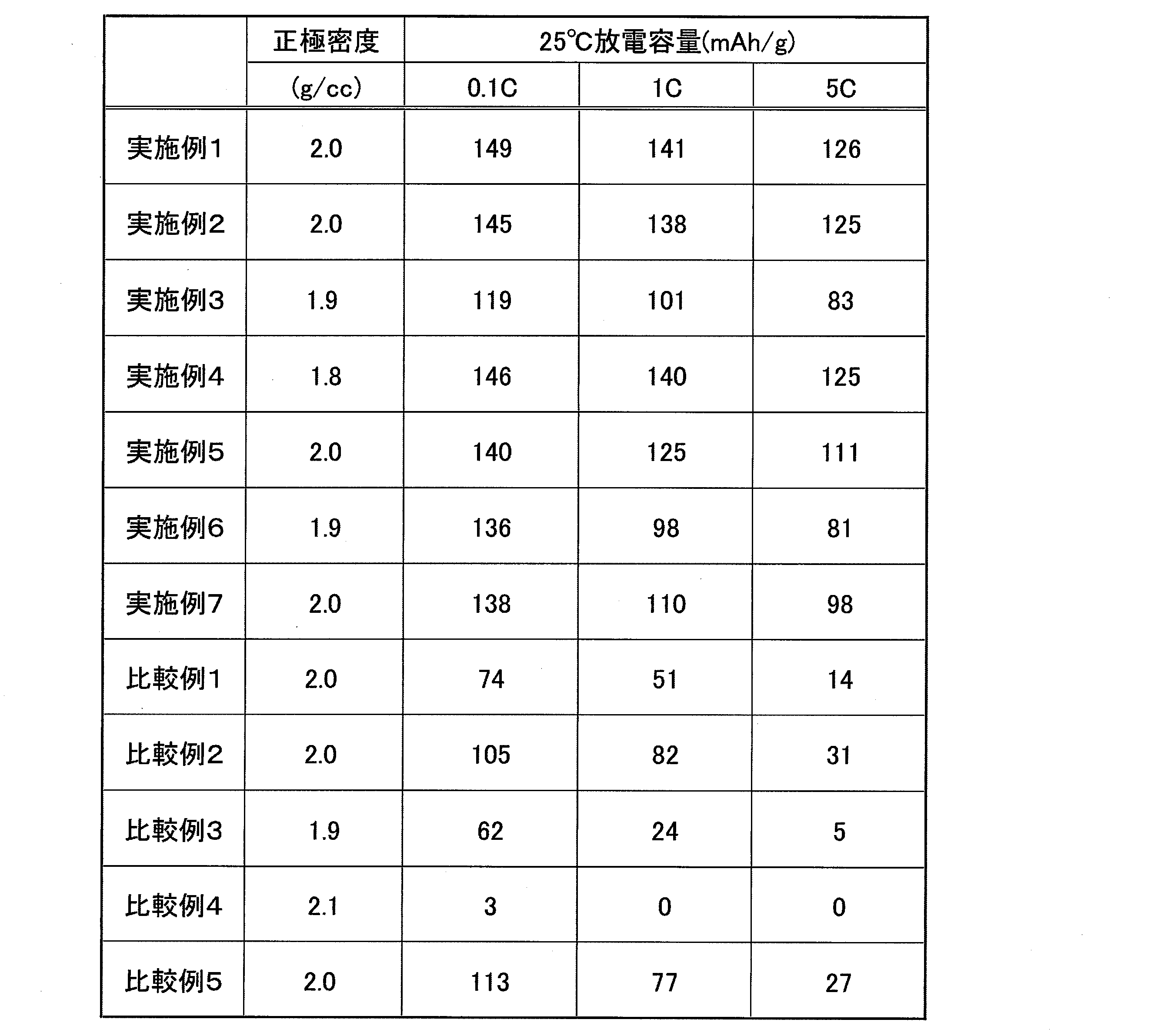
- the powder characteristics of the particle powder are shown in Table 4 and FIG. 2 as the battery characteristics evaluated by a coin cell using the obtained lithium iron manganese phosphate particle powder as a positive electrode. Further, the change rate of the primary particle size before and after the removal of Li by chemical oxidation was about 10%.
- Example 2 The same processing as in Example 1 was performed except that the raw material charging ratio was changed as shown in Table 1.
- Example 3 A solution of Fe 3 (PO 4 ) 2 .8H 2 O, Li 3 PO 4 and LiOH.H 2 O was added to NH 4 MnPO 4 obtained in the same manner as in Example 1, and the raw material charging ratios are as shown in Table 1.
- An aqueous slurry was obtained in the same manner as in Example 1 except that Thereafter, PVA was added as an organic substance in the third step to the slurry after the hydrothermal reaction, and the same treatment as in Example 1 was performed except that the slurry was evaporated to dryness.
- Example 4 A solution of MnSO 4 , FeSO 4 , H 3 PO 4 , NH 4 OH was mixed, and NH 4 Mn 0.7 Fe 0.3 PO 4 was precipitated by a neutralization reaction at room temperature. The obtained precipitate was separated by filtration and washed with pure water, and then LiOH.H 2 O, H 3 PO 4 and sucrose were added to obtain an aqueous slurry adjusted to the raw material charging ratio shown in Table 1. Thereafter, the same treatment as in Example 1 was carried out except that 15 wt% of polyethylene and stearic acid were used as organic substances in the third step.
- Example 5 A solution of MnSO 4 and Na 2 CO 3 was mixed, and MnCO 3 was precipitated by a neutralization reaction at room temperature. The obtained precipitate was separated by filtration and washed with pure water, and then H 3 PO 4 was added to precipitate HMnPO 4 . Thereafter, the same processing as in Example 1 was performed except that the raw material charging ratio was changed as shown in Table 1.
- Example 6 A solution of MnSO 4 , FeSO 4 , and Na 2 CO 3 was mixed, and HMn 0.9 Fe 0.1 CO 3 was precipitated by a neutralization reaction at room temperature. The resulting precipitate was filtered and washed with pure water, and then a solution of H 3 PO 4 was added to precipitate HMn 0.9 Fe 0.1 PO 4 . Thereafter, the same processing as in Example 1 was performed except that the raw material charging ratio was changed as shown in Table 1.
- Example 7 The raw material charging ratio was changed as shown in Table 1, and treatment was performed in the same manner as in Example 1 except that 20 wt% of sucrose and carbon black were used as organic substances in the third step.
- the agglomerated particles of the slurry were not pulverized, so the median diameter D 50 of the agglomerated particles was as large as 16 ⁇ m, and the yield of LiMnPO 4 having a olivine structure of the compound obtained after hydrothermal treatment was as low as 71 wt%. It was. Even in the lithium manganese iron phosphate particles powder obtained by firing, 7 wt% of Li 3 PO 4 which is an impurity crystal phase was present, and the battery characteristics deteriorated.
- Example 2 An aqueous system adjusted to the raw material charge ratio shown in Table 1, including a precipitate obtained by mixing a solution of MnSO 4, FeSO 4 , H 3 PO 4 , and LiOH ⁇ H 2 O and neutralizing reaction at room temperature A slurry was obtained. The slurry was mixed with a ball mill, the aggregated particles were pulverized to adjust the particle size, then hydrothermally treated at 180 ° C. for 3 hours, the resulting precipitate was filtered, washed with pure water, and dried at 70 ° C. overnight. . Thereafter, the same processing as in Example 1 was performed. The crystallite size of the obtained dry powder was as large as 260 nm, and impurity sulfur in the dry powder was 0.12 wt%. The lithium manganese iron phosphate particle powder obtained by firing had a large sulfur content and crystallite size, and the battery characteristics were poor.
- Example 3 The same process as in Example 2 was performed except that the amount of sucrose, which is an organic substance, was changed to 1 wt% in the third step of Example 2.
- the lithium manganese iron phosphate particles obtained by firing were coarse particles, had high electric resistance, and had poor battery characteristics.
- Li 2 CO 3 , MnCO 3 , (NH 4 ) H 2 PO 4 are mixed so that the ratio of Li, Mn, and P is 1: 1: 1, and a mixed solvent composed of ethanol and water to which PVA is further added.
- the obtained compound was separated by filtration and fired in air at 700 ° C. for 2 hours to obtain lithium iron manganese phosphate particles.
- the obtained lithium iron manganese phosphate particles were coarse particles, had high electric resistance, and had poor battery characteristics.
- FIG. 3 shows an SEM photograph of the obtained lithium iron manganese phosphate particle powder
- FIG. 4 shows an SEM photograph when Li is eliminated by chemical oxidation
- FIG. 5 shows a Rietveld analysis result of the XRD pattern. It was confirmed that LiMnPO 4 was changed to fine MnPO 4 by chemical oxidation.
- Example 5 An aqueous slurry was obtained in the same manner as in Example 3. Without performing the hydrothermal reaction in the second step, the precipitate in the slurry was separated by filtration, washed with pure water, and dried at 70 ° C. overnight. Thereafter, the same processing as in Example 1 was performed.
- the lithium manganese iron phosphate particle powder obtained by firing contained a large amount of impurity crystal phase Li 3 PO 4 , and the battery characteristics were poor.
- the non-aqueous electrolyte secondary battery using the olivine type lithium iron manganese phosphate particles according to the present invention as a positive electrode active material has a discharge capacity of 115 mAh / g or more at C / 10 at room temperature and 1 C at room temperature.
- the discharge capacity was 95 mAh / g or more, and the discharge capacity at 5 C at room temperature was 80 mAh / g or more.
- Example 8 to 15 The precursor powder obtained in the second step was changed to Li 2 CO 3 , MnCO 3 , FeC 2 O 4 , (NH 4 ) in the same manner as in Example 1 except that the raw material charging ratio was changed as shown in Table 5. Fine powders of H 2 PO 4 and (NH 4 ) 2 HPO 4 were added as necessary and mixed by a ball mill, and the composition ratios of Li, Mn, Fe, and P were adjusted as shown in Table 5. Thereafter, the same processing as in Example 1 was performed.
- the main component of the obtained powder was lithium iron manganese phosphate particles having an olivine structure, and the composition ratio was the same as the composition ratio of Li, Mn, Fe, and P adjusted in the third step.
- Table 5 shows the manufacturing conditions of the first step and the properties of the obtained slurry
- Table 6 shows the characteristics of the powder in the second step and the manufacturing conditions of the third step
- Tables 7 and 8 show the phosphoric acid obtained.
- the battery characteristics of the manganese iron lithium particle powder are shown in Table 9 and evaluated by a coin cell using the obtained lithium manganese iron phosphate particle powder as a positive electrode.
- lithium iron manganese phosphate particles having an olivine structure when the lithium and phosphorus contents satisfy Li ⁇ P, the formation of an Mn 2 P 2 O 7 impurity crystal phase that deteriorates battery characteristics Tended to be suppressed.
- the impurity crystal phase is not detected, but when Li and P are present out of the stoichiometric ratio, the excess Li It is believed that P and P do not adversely affect battery characteristics by forming an ion conductive amorphous phase.
- a coin cell using an electrode in which the amount of the conductive material is doubled with respect to the evaluation method is 5C.
- the discharge capacity was improved by 10 to 30%, and a further increase in capacity was confirmed.
- the method for producing olivine-type lithium iron manganese phosphate particles according to the present invention can be achieved by using an approximately equal amount of raw material without using an excessive amount of raw material, and a low-cost and efficient production method. is there.
- the olivine-type lithium manganese iron particle powder having the olivine structure according to the present invention makes it possible to produce a positive electrode sheet having a high filling property, and a secondary battery using the same has a high capacity in terms of current load characteristics. It was confirmed that it was obtained.
- the present invention uses olivine-type lithium iron manganese phosphate particles produced by a low-cost and efficient production method as a positive electrode active material, so that the energy density per volume is high and the capacity is high even in high current load characteristics.
- a non-aqueous electrolyte secondary battery can be obtained.
Abstract
Description
放電:MnPO4+Li++e-→LiMnPO4 Charging: LiMnPO 4 → MnPO 4 + Li + + e −
Discharge: MnPO 4 + Li + + e − → LiMnPO 4
VUC(Å3)<11.4×(1-x)+291.21 ・・・(1) The present invention also relates to lithium iron manganese phosphate particles having an olivine structure according to the present invention 6 or 7, wherein the unit cell volume V UC has the relationship represented by the following formula (1) (the present invention 8).
V UC (Å 3 ) <11.4 × (1-x) +291.21 (1)
VUC(Å3)<11.4×(1-x)+291.21 ・・・(1) The unit cell volume V UC of the lithium iron manganese phosphate particles according to the present invention is preferably in the relationship of formula (1).
V UC (Å 3 ) <11.4 × (1-x) +291.21 (1)
本発明に係るオリビン型構造のリン酸マンガン鉄リチウムLiMn1-xFexPO4(0.05≦x≦0.5)粒子粉末は水熱反応と焼成により製造され、低コストで、小さい環境負荷で製造できる。 <Action>
The olivine-type lithium iron manganese phosphate LiMn 1-x Fe x PO 4 (0.05 ≦ x ≦ 0.5) particle powder according to the present invention is produced by hydrothermal reaction and firing, and is low in cost and in a small environment Can be manufactured with load.
F.Izumi and T.Ikeda, Mater.Sci.Forum, 2000,Vol.198,p.321-324. <References>
F. Izumi and T. Ikeda, Mater. Sci. Forum, 2000, Vol. 198, p. 321-324.
MnSO4、H3PO4、NH4OHの溶液を混合し、室温での中和反応によりNH4MnPO4を沈殿させた。沈殿物を濾別し、純水で洗浄した後、LiOH・H2O、FeSO4、H3PO4、及びアスコルビン酸の溶液を加え、表1に記載の原料仕込み比に調整された水系スラリーを得た。 [Example 1]
A solution of MnSO 4, H 3 PO 4 , and NH 4 OH was mixed, and NH 4 MnPO 4 was precipitated by a neutralization reaction at room temperature. After the precipitate was filtered off and washed with pure water, a solution of LiOH.H 2 O, FeSO 4 , H 3 PO 4 , and ascorbic acid was added, and the aqueous slurry adjusted to the raw material charge ratio shown in Table 1 Got.
また、化学酸化による脱Li前後の一次粒子サイズの変化率は10%程度であった。 FIG. 1 shows an SEM photograph (secondary electron image) of the olivine-type lithium iron manganese phosphate particles obtained. Table 1 shows the manufacturing conditions of the first step and the properties of the obtained slurry, Table 2 shows the characteristics of the powder in the second step and the manufacturing conditions of the third step, and Table 3 shows the obtained lithium iron manganese phosphate. The powder characteristics of the particle powder are shown in Table 4 and FIG. 2 as the battery characteristics evaluated by a coin cell using the obtained lithium iron manganese phosphate particle powder as a positive electrode.
Further, the change rate of the primary particle size before and after the removal of Li by chemical oxidation was about 10%.
原料仕込み比を表1のように変えたほかは実施例1と同様に処理した。 [Example 2]
The same processing as in Example 1 was performed except that the raw material charging ratio was changed as shown in Table 1.
実施例1と同様にして得られたNH4MnPO4にFe3(PO4)2・8H2O、Li3PO4及びLiOH・H2Oの溶液を加え、原料仕込み比を表1のように変えたほかは実施例1と同様にして水系スラリーを得た。以後、水熱反応後のスラリーに第三工程における有機物としてPVAを添加し、蒸発乾固したほかは実施例1と同様に処理した。 [Example 3]
A solution of Fe 3 (PO 4 ) 2 .8H 2 O, Li 3 PO 4 and LiOH.H 2 O was added to NH 4 MnPO 4 obtained in the same manner as in Example 1, and the raw material charging ratios are as shown in Table 1. An aqueous slurry was obtained in the same manner as in Example 1 except that Thereafter, PVA was added as an organic substance in the third step to the slurry after the hydrothermal reaction, and the same treatment as in Example 1 was performed except that the slurry was evaporated to dryness.
MnSO4、FeSO4、H3PO4、NH4OHの溶液を混合し、室温での中和反応によりNH4Mn0.7Fe0.3PO4を沈殿させた。得られた沈殿物を濾別して純水で洗浄後、LiOH・H2O、H3PO4、及びショ糖を加え、表1に記載の原料仕込み比に調整された水系スラリーを得た。以後、第三工程における有機物としてポリエチレンとステアリン酸を合計15wt%用いたほかは実施例1と同様に処理した。 [Example 4]
A solution of MnSO 4 , FeSO 4 , H 3 PO 4 , NH 4 OH was mixed, and NH 4 Mn 0.7 Fe 0.3 PO 4 was precipitated by a neutralization reaction at room temperature. The obtained precipitate was separated by filtration and washed with pure water, and then LiOH.H 2 O, H 3 PO 4 and sucrose were added to obtain an aqueous slurry adjusted to the raw material charging ratio shown in Table 1. Thereafter, the same treatment as in Example 1 was carried out except that 15 wt% of polyethylene and stearic acid were used as organic substances in the third step.
MnSO4とNa2CO3の溶液を混合し、室温での中和反応によりMnCO3を沈殿させた。得られた沈殿物を濾別して純水で洗浄後、H3PO4を加え、HMnPO4を沈殿させた。以後、原料仕込み比を表1のように変えたほかは実施例1と同様に処理した。 [Example 5]
A solution of MnSO 4 and Na 2 CO 3 was mixed, and MnCO 3 was precipitated by a neutralization reaction at room temperature. The obtained precipitate was separated by filtration and washed with pure water, and then H 3 PO 4 was added to precipitate HMnPO 4 . Thereafter, the same processing as in Example 1 was performed except that the raw material charging ratio was changed as shown in Table 1.
MnSO4、FeSO4、Na2CO3の溶液を混合し、室温での中和反応によりHMn0.9Fe0.1CO3を沈殿させた。得られた沈殿物を濾別して純水で洗浄後、H3PO4の溶液を加え、HMn0.9Fe0.1PO4を沈殿させた。以後、原料仕込み比を表1のように変えたほかは実施例1と同様に処理した。 [Example 6]
A solution of MnSO 4 , FeSO 4 , and Na 2 CO 3 was mixed, and HMn 0.9 Fe 0.1 CO 3 was precipitated by a neutralization reaction at room temperature. The resulting precipitate was filtered and washed with pure water, and then a solution of H 3 PO 4 was added to precipitate HMn 0.9 Fe 0.1 PO 4 . Thereafter, the same processing as in Example 1 was performed except that the raw material charging ratio was changed as shown in Table 1.
原料仕込み比を表1のように変え、第三工程での有機物としてショ糖とカーボンブラックを合計20wt%用いたほかは実施例1と同様に処理した。 [Example 7]
The raw material charging ratio was changed as shown in Table 1, and treatment was performed in the same manner as in Example 1 except that 20 wt% of sucrose and carbon black were used as organic substances in the third step.
MnSO4、H3PO4、NH4OHの溶液を混合し、室温での中和反応によりNH4MnPO4を沈殿させた。得られた沈殿物を濾別して純水で洗浄後、LiOH・H2O及びアスコルビン酸の溶液を加え、表1に記載の原料仕込み比に調整された水系スラリーを得た。スラリーの凝集粒子の粒度を調整しなかったほかは実施例1と同様に処理した。 [Comparative Example 1]
A solution of MnSO 4, H 3 PO 4 , and NH 4 OH was mixed, and NH 4 MnPO 4 was precipitated by a neutralization reaction at room temperature. The obtained precipitate was separated by filtration and washed with pure water, and then a solution of LiOH.H 2 O and ascorbic acid was added to obtain an aqueous slurry adjusted to the raw material charge ratio shown in Table 1. The same treatment as in Example 1 was performed except that the particle size of the aggregated particles of the slurry was not adjusted.
MnSO4、FeSO4、H3PO4、LiOH・H2Oの溶液を混合し、室温での中和反応により得られた沈殿物を含む、表1に記載の原料仕込み比に調整された水系スラリーを得た。前記スラリーをボールミルで混合し、凝集粒子を粉砕して粒度を調整した後、180℃、3時間水熱処理し、得られた沈殿物を濾別して純水で洗浄し、70℃で一晩乾燥した。以後、実施例1と同様に処理した。得られた乾燥粉末の結晶子サイズは260nmと大きく、また、乾燥粉末中の不純物硫黄は0.12wt%であった。焼成して得られたリン酸マンガン鉄リチウム粒子粉末は硫黄含有量と結晶子サイズが大きく、電池特性が不良であった。 [Comparative Example 2]
An aqueous system adjusted to the raw material charge ratio shown in Table 1, including a precipitate obtained by mixing a solution of MnSO 4, FeSO 4 , H 3 PO 4 , and LiOH · H 2 O and neutralizing reaction at room temperature A slurry was obtained. The slurry was mixed with a ball mill, the aggregated particles were pulverized to adjust the particle size, then hydrothermally treated at 180 ° C. for 3 hours, the resulting precipitate was filtered, washed with pure water, and dried at 70 ° C. overnight. . Thereafter, the same processing as in Example 1 was performed. The crystallite size of the obtained dry powder was as large as 260 nm, and impurity sulfur in the dry powder was 0.12 wt%. The lithium manganese iron phosphate particle powder obtained by firing had a large sulfur content and crystallite size, and the battery characteristics were poor.
実施例2の第三工程において有機物であるショ糖量を1wt%としたほかは実施例2と同様に処理をした。焼成して得られたリン酸マンガン鉄リチウム粒子粉末は粗大粒子であり、電気抵抗が高く、電池特性は不良であった。 [Comparative Example 3]
The same process as in Example 2 was performed except that the amount of sucrose, which is an organic substance, was changed to 1 wt% in the third step of Example 2. The lithium manganese iron phosphate particles obtained by firing were coarse particles, had high electric resistance, and had poor battery characteristics.
Li、Mn、Pの比が1:1:1となるように、Li2CO3、MnCO3、(NH4)H2PO4を混合し、さらにPVAを添加したエタノールと水からなる混合溶媒中、ボールミルで粉砕、混合後、得られた化合物を濾別して空気中700℃、2時間で焼成し、リン酸マンガン鉄リチウム粒子粉末を得た。得られたリン酸マンガン鉄リチウム粒子粉末は粗大粒子であり、電気抵抗が高く、電池特性は不良であった。 [Comparative Example 4]
Li 2 CO 3 , MnCO 3 , (NH 4 ) H 2 PO 4 are mixed so that the ratio of Li, Mn, and P is 1: 1: 1, and a mixed solvent composed of ethanol and water to which PVA is further added. After pulverizing and mixing with a ball mill, the obtained compound was separated by filtration and fired in air at 700 ° C. for 2 hours to obtain lithium iron manganese phosphate particles. The obtained lithium iron manganese phosphate particles were coarse particles, had high electric resistance, and had poor battery characteristics.
実施例3と同様にして水系スラリーを得た。第二工程の水熱反応を行わず、前記スラリー中の沈殿物を濾別して純水で洗浄し、70℃で一晩乾燥した。以後、実施例1と同様に処理をした。焼成して得られたリン酸マンガン鉄リチウム粒子粉末は不純物結晶相であるLi3PO4を多く含み、電池特性は不良であった。 [Comparative Example 5]
An aqueous slurry was obtained in the same manner as in Example 3. Without performing the hydrothermal reaction in the second step, the precipitate in the slurry was separated by filtration, washed with pure water, and dried at 70 ° C. overnight. Thereafter, the same processing as in Example 1 was performed. The lithium manganese iron phosphate particle powder obtained by firing contained a large amount of impurity crystal phase Li 3 PO 4 , and the battery characteristics were poor.
原料仕込み比を表5のように変えたほかは実施例1と同様にして第二工程で得られた前駆体の粉末に、Li2CO3、MnCO3、FeC2O4、(NH4)H2PO4、(NH4)2HPO4の微細な粉末を必要に応じ添加してボールミルで混合し、Li、Mn、Fe、P組成比を表5のとおりに調整した。以後、実施例1と同様に処理した。 [Examples 8 to 15]
The precursor powder obtained in the second step was changed to Li 2 CO 3 , MnCO 3 , FeC 2 O 4 , (NH 4 ) in the same manner as in Example 1 except that the raw material charging ratio was changed as shown in Table 5. Fine powders of H 2 PO 4 and (NH 4 ) 2 HPO 4 were added as necessary and mixed by a ball mill, and the composition ratios of Li, Mn, Fe, and P were adjusted as shown in Table 5. Thereafter, the same processing as in Example 1 was performed.
The present invention uses olivine-type lithium iron manganese phosphate particles produced by a low-cost and efficient production method as a positive electrode active material, so that the energy density per volume is high and the capacity is high even in high current load characteristics. A non-aqueous electrolyte secondary battery can be obtained.
Claims (9)
- オリビン型構造のリン酸マンガン鉄リチウムLiMn1-xFexPO4(0.05≦x≦0.5)粒子粉末の製造方法であって、原料としてLi化合物、Mn化合物、Fe化合物、P化合物及び糖又は有機酸を用い、原料仕込み比がmol比で0.95≦Li/(Mn+Fe)≦2.0、0.95≦P/(Mn+Fe)≦1.3であり、(Mn+Fe)に対し1~20mol%の糖又は有機酸を含む混合溶液の中和反応によってpHが5.5~12.5である水系スラリーを得る第一工程、第一工程で得られたスラリーを反応温度120~220℃で水熱処理を行い、得られる化合物のLiMn1-xFexPO4(0.05≦x≦0.5)の生成率が75wt%以上であって、結晶子サイズを10~250nmとした後、該化合物を洗浄する第二工程、第二工程で得られた化合物に有機物を3~40wt%添加して前駆体粉末を得る第三工程、第三工程で得られた前駆体粉末を酸素濃度0.1%以下の不活性ガス又は還元性ガス雰囲気下で、温度250~850℃で焼成する第四工程からなるオリビン型構造のリン酸マンガン鉄リチウム粒子粉末の製造方法。 Lithium iron manganese phosphate LiMn 1-x Fe x PO 4 (0.05 ≦ x ≦ 0.5) particle powder manufacturing method of olivine type structure, with Li compound, Mn compound, Fe compound, P compound as raw material And sugar or organic acid, the raw material charge ratio is 0.95 ≦ Li / (Mn + Fe) ≦ 2.0, 0.95 ≦ P / (Mn + Fe) ≦ 1.3 in terms of mol ratio, and (Mn + Fe) A first step of obtaining an aqueous slurry having a pH of 5.5 to 12.5 by a neutralization reaction of a mixed solution containing 1 to 20 mol% of sugar or organic acid, the slurry obtained in the first step is reacted at a reaction temperature of 120 to Hydrothermal treatment is performed at 220 ° C., and the resulting compound has a production rate of LiMn 1-x Fe x PO 4 (0.05 ≦ x ≦ 0.5) of 75 wt% or more, and the crystallite size is 10 to 250 nm. After the compound In the second step, the organic compound is added to the compound obtained in the second step in an amount of 3 to 40 wt% to obtain a precursor powder, and the precursor powder obtained in the third step has an oxygen concentration of 0.1. A method for producing olivine-type lithium iron manganese phosphate particles comprising a fourth step of baking at a temperature of 250 to 850 ° C. in an inert gas or reducing gas atmosphere of not more than 10%.
- 第一工程において、スラリー中の凝集粒子のメジアン径が0.1~10μmであって、結晶相が(NH4)Mn1-αFeαPO4(0≦α<1)又はHMn1-βFeβPO4(0≦β<1)である凝集粒子を含む水系スラリーを得る請求項1に記載の製造方法。 In the first step, the median diameter of the aggregated particles in the slurry is 0.1 to 10 μm, and the crystal phase is (NH 4 ) Mn 1-α Fe α PO 4 (0 ≦ α <1) or HMn 1-β Fe beta PO 4 the method according to claim 1 to obtain an aqueous slurry containing the aggregated particles is (0 ≦ β <1).
- 第一工程において用いる糖又は有機酸が、ショ糖、アスコルビン酸、及びクエン酸のうち少なくとも1種である請求項1又は2に記載の製造方法。 The method according to claim 1 or 2, wherein the sugar or organic acid used in the first step is at least one of sucrose, ascorbic acid, and citric acid.
- 第二工程において、化合物中の硫黄含有量が0.1wt%以下になるように洗浄する請求項1~3のいずれかに記載の製造方法。 The production method according to any one of claims 1 to 3, wherein in the second step, washing is performed so that the sulfur content in the compound is 0.1 wt% or less.
- 第三工程において、添加する有機物がカーボンブラック、油脂化合物、糖化合物、及び合成樹脂のうち少なくとも1種である請求項1~4のいずれかに記載の製造方法。 The method according to any one of claims 1 to 4, wherein in the third step, the organic substance to be added is at least one of carbon black, an oil and fat compound, a sugar compound, and a synthetic resin.
- オリビン型構造のリン酸マンガン鉄リチウムLiMn1-xFexPO4(0.05≦x≦0.5)粒子粉末であって、リチウムとリンの含有量がmol比で0.9≦Li/(Mn+Fe)≦1.2、0.9≦P/(Mn+Fe)≦1.2であり、BET比表面積が6~70m2/gであり、炭素含有量が0.5~8wt%であり、硫黄含有量が0.08wt%以下であり、オリビン型構造の結晶相LiMn1-xFexPO4(0.05≦x≦0.5)の量が95wt%以上であり、結晶子サイズが25~300nmであり、凝集粒子のメジアン径が0.3~20μmであり、粉体電気抵抗率が1~1.0×106Ω・cmであることを特徴とするオリビン型構造のリン酸マンガン鉄リチウム粒子粉末。 Lithium manganese iron phosphate LiMn 1-x Fe x PO 4 (0.05 ≦ x ≦ 0.5) particle powder having an olivine type structure, wherein the lithium and phosphorus content is 0.9 ≦ Li / mol (Mn + Fe) ≦ 1.2, 0.9 ≦ P / (Mn + Fe) ≦ 1.2, the BET specific surface area is 6 to 70 m 2 / g, and the carbon content is 0.5 to 8 wt%. The sulfur content is 0.08 wt% or less, the amount of the crystalline phase LiMn 1-x Fe x PO 4 (0.05 ≦ x ≦ 0.5) of the olivine structure is 95 wt% or more, and the crystallite size is Phosphoric acid having an olivine structure characterized in that it has a median diameter of 25 to 300 nm, a median diameter of aggregated particles of 0.3 to 20 μm, and a powder electrical resistivity of 1 to 1.0 × 10 6 Ω · cm Manganese iron lithium particle powder.
- リチウムとリンの含有量がLi≧Pを満たす請求項6に記載のオリビン型構造のリン酸マンガン鉄リチウム粒子粉末。 The olivine-type lithium manganese iron phosphate particle powder according to claim 6, wherein the lithium and phosphorus contents satisfy Li ≧ P.
- 単位胞体積VUCが式(1)の関係にある請求項6又は7に記載のオリビン型構造のリン酸マンガン鉄リチウム粒子粉末。
VUC(Å3)<11.4×(1-x)+291.21・・・(1) The olivine type lithium manganese iron phosphate particle powder according to claim 6 or 7, wherein the unit cell volume V UC is in the relationship of the formula (1).
V UC (Å 3 ) <11.4 × (1-x) +291.21 (1) - 請求項6~8のいずれかに記載のオリビン型構造のリン酸マンガン鉄リチウム粒子粉末を用いて作製した非水電解質二次電池。 A non-aqueous electrolyte secondary battery produced using the olivine-type lithium iron manganese phosphate particles according to any one of claims 6 to 8.
Priority Applications (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| CN201180011603.6A CN102781827B (en) | 2010-03-19 | 2011-03-17 | Manufacture method, the iron manganese phosphate for lithium particle powder of iron manganese phosphate for lithium particle powder and use the rechargeable nonaqueous electrolytic battery of this particle powder |
| KR1020127022574A KR101810259B1 (en) | 2010-03-19 | 2011-03-17 | Method for producing lithium manganese iron phosphate particulate powder, lithium manganese iron phosphate particulate powder and non-aqueous electrolyte secondary battery using that particulate powder |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2010-063626 | 2010-03-19 | ||
| JP2010063626 | 2010-03-19 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2011115211A1 true WO2011115211A1 (en) | 2011-09-22 |
Family
ID=44649294
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2011/056397 WO2011115211A1 (en) | 2010-03-19 | 2011-03-17 | Method for producing lithium manganese iron phosphate particulate powder, lithium manganese iron phosphate particulate powder and non-aqueous electrolyte secondary battery using that particulate powder |
Country Status (4)
| Country | Link |
|---|---|
| JP (1) | JP5817963B2 (en) |
| KR (1) | KR101810259B1 (en) |
| CN (1) | CN102781827B (en) |
| WO (1) | WO2011115211A1 (en) |
Cited By (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2012229147A (en) * | 2011-04-27 | 2012-11-22 | Nichia Corp | Olivine-type lithium transition metal oxide, and method of producing the same |
| JP2013120621A (en) * | 2011-12-06 | 2013-06-17 | Toyota Motor Corp | Nonaqueous secondary battery |
| CN103708434A (en) * | 2012-10-09 | 2014-04-09 | 上海交通大学 | Lithium iron phosphate material and preparation method thereof |
| WO2014098933A1 (en) * | 2012-12-21 | 2014-06-26 | Dow Global Technologies Llc | Method for making lithium transition metal olivines using water/cosolvent mixtures |
| JP2015076155A (en) * | 2013-10-07 | 2015-04-20 | 太平洋セメント株式会社 | Method of producing lithium manganese phosphate positive electrode active material |
| CN104718657A (en) * | 2012-11-21 | 2015-06-17 | 株式会社Lg化学 | Lithium secondary battery |
| US9660266B2 (en) | 2012-11-21 | 2017-05-23 | Lg Chem, Ltd. | Lithium secondary battery |
| JP2018008877A (en) * | 2010-10-08 | 2018-01-18 | 株式会社半導体エネルギー研究所 | Method for manufacturing lithium iron phosphate |
| CN116081589A (en) * | 2022-10-12 | 2023-05-09 | 北京钠谛科技有限公司 | Lithium-rich lithium iron manganese phosphate material and preparation method thereof |
| EP4282827A1 (en) * | 2022-05-25 | 2023-11-29 | Hubei RT Advanced Materials Co., Ltd. | Preparation method of high-safety high-capacity lithium manganese iron phosphate |
Families Citing this family (35)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9318741B2 (en) | 2010-04-28 | 2016-04-19 | Semiconductor Energy Laboratory Co., Ltd. | Positive electrode active material of power storage device, power storage device, electrically propelled vehicle, and method for manufacturing power storage device |
| JP2012059570A (en) * | 2010-09-09 | 2012-03-22 | Gs Yuasa Corp | Cathode active material for lithium secondary battery and method for manufacturing the same |
| KR20140053875A (en) * | 2011-03-28 | 2014-05-08 | 미쯔이 죠센 가부시키가이샤 | Electrode material for secondary battery, method for producing electrode material for secondary battery, and secondary battery |
| CN103503206B (en) * | 2011-04-22 | 2016-03-02 | 昭和电工株式会社 | The manufacture method of positive active material for lithium secondary battery |
| US10096821B2 (en) | 2011-05-02 | 2018-10-09 | Toyota Jidosha Kabushiki Kaisha | Lithium secondary battery |
| JP2013101883A (en) * | 2011-11-09 | 2013-05-23 | Hitachi Ltd | Positive electrode active material for lithium ion secondary battery |
| JP5839227B2 (en) | 2011-11-10 | 2016-01-06 | トヨタ自動車株式会社 | Lithium secondary battery and manufacturing method thereof |
| JP5853662B2 (en) * | 2011-12-15 | 2016-02-09 | 株式会社Gsユアサ | Method of using nonaqueous electrolyte battery and plug-in hybrid vehicle equipped with nonaqueous electrolyte battery |
| JP2013175374A (en) * | 2012-02-27 | 2013-09-05 | Gs Yuasa Corp | Positive electrode active material for secondary battery, active material particle, and positive electrode and secondary battery using those |
| JP5712959B2 (en) * | 2012-03-30 | 2015-05-07 | 住友金属鉱山株式会社 | Precursor of positive electrode active material for lithium secondary battery, method for producing the same, and method for producing positive electrode active material for lithium secondary battery using the precursor |
| TW201342697A (en) * | 2012-03-30 | 2013-10-16 | Sumitomo Osaka Cement Co Ltd | Electrode material |
| JP2013246936A (en) * | 2012-05-24 | 2013-12-09 | Hitachi Ltd | Positive-electrode active material for nonaqueous secondary batteries |
| EP2698346A1 (en) * | 2012-08-14 | 2014-02-19 | Clariant International Ltd. | Mixed sulphate containing lithium-manganese-metal phosphate |
| KR101787212B1 (en) * | 2012-08-28 | 2017-10-18 | 어드밴스드 리튬 일렉트로케미스트리 컴퍼니 리미티드 | Method of producing battery composite material and its precursor |
| JP6143216B2 (en) * | 2012-08-29 | 2017-06-07 | 株式会社デンソー | Method for producing positive electrode active material for non-aqueous electrolyte secondary battery |
| WO2014034775A1 (en) * | 2012-08-31 | 2014-03-06 | 戸田工業株式会社 | Method for producing carbon composite lithium manganese iron phosphate particle powder, carbon composite lithium manganese iron phosphate particle powder, and nonaqueous electrolyte secondary battery using carbon composite lithium manganese iron phosphate particle powder |
| KR101498971B1 (en) * | 2012-10-17 | 2015-03-04 | 한화케미칼 주식회사 | Novel method for preparing olivine type electrode material using formic acid derivative |
| JP2014216240A (en) * | 2013-04-26 | 2014-11-17 | 住友大阪セメント株式会社 | Electrode active material and electrode material, electrode, lithium ion battery, and method for producing electrode material |
| JP5783295B2 (en) * | 2013-04-30 | 2015-09-24 | 住友大阪セメント株式会社 | Electrode material, paste, electrode plate and lithium ion battery |
| JP6232493B2 (en) * | 2013-05-08 | 2017-11-15 | 台湾立凱電能科技股▲ふん▼有限公司 | Battery composite material and method for producing precursor thereof |
| TWI617074B (en) | 2013-05-08 | 2018-03-01 | 台灣立凱電能科技股份有限公司 | Preparation method of battery composite material and precursor thereof |
| KR101631736B1 (en) * | 2013-08-01 | 2016-06-17 | 주식회사 엘지화학 | Lithium manganese phosphate based anode active material, preparation method thereof, and lithium secondary battery or hybrid capacitor comprising the same |
| KR101925041B1 (en) * | 2013-11-14 | 2018-12-04 | 주식회사 엘지화학 | Cathode Active Material and Lithium Secondary Battery |
| JP5836461B1 (en) * | 2014-09-29 | 2015-12-24 | 太平洋セメント株式会社 | Positive electrode material for lithium secondary battery |
| JP5892270B1 (en) * | 2015-01-30 | 2016-03-23 | 住友大阪セメント株式会社 | Method for producing positive electrode material for lithium ion secondary battery, positive electrode material for lithium ion secondary battery, positive electrode for lithium ion secondary battery, lithium ion secondary battery |
| JP6341516B2 (en) * | 2015-02-09 | 2018-06-13 | 株式会社三井E&Sホールディングス | Method for producing positive electrode material for lithium secondary battery |
| JP2015143189A (en) * | 2015-04-23 | 2015-08-06 | 日亜化学工業株式会社 | Olivine-type lithium transition metal oxide and manufacturing method therefor |
| CN105226245B (en) * | 2015-08-27 | 2018-03-09 | 青海泰丰先行锂能科技有限公司 | A kind of anode material for lithium-ion batteries and preparation method thereof |
| JP2018041683A (en) * | 2016-09-09 | 2018-03-15 | 太平洋セメント株式会社 | Method for manufacturing olivine type lithium phosphate-based positive electrode material |
| TWI625888B (en) * | 2017-07-14 | 2018-06-01 | Hcm Co Ltd | Lithium iron manganese phosphate particles, lithium iron manganese phosphate powder and preparation method thereof |
| JP6527201B2 (en) * | 2017-09-08 | 2019-06-05 | 太平洋セメント株式会社 | Method of manufacturing positive electrode active material complex for lithium ion secondary battery |
| JP6501014B1 (en) * | 2018-03-13 | 2019-04-17 | 住友大阪セメント株式会社 | Positive electrode material for lithium ion secondary battery, method for producing the same, electrode for lithium ion secondary battery, and lithium ion secondary battery |
| CN108408709B (en) * | 2018-03-30 | 2021-12-14 | 南阳逢源新能源科技有限公司 | Preparation process of pollution-free low-cost lithium manganese iron phosphate crystal material |
| JP6627932B1 (en) * | 2018-08-21 | 2020-01-08 | 住友大阪セメント株式会社 | Positive electrode material for lithium ion secondary battery, electrode for lithium ion secondary battery, and lithium ion secondary battery |
| WO2024054046A1 (en) * | 2022-09-06 | 2024-03-14 | 주식회사 엘지에너지솔루션 | Lithium secondary battery |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2008077448A1 (en) * | 2006-12-22 | 2008-07-03 | Umicore | SYNTHESIS OF CRYSTALLINE NANOMETRIC LiFeMPO4 |
| WO2009122686A1 (en) * | 2008-03-31 | 2009-10-08 | 戸田工業株式会社 | Lithium iron phosphate powder manufacturing method, olivine structured lithium iron phosphate powder, cathode sheet using said lithium iron phosphate powder, and non-aqueous solvent secondary battery |
Family Cites Families (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR101401836B1 (en) * | 2006-12-22 | 2014-05-29 | 썽뜨르 나쇼날르 드 라 르쉐르쉐 씨엉띠삐끄 | SYNTHESIS OF CRYSTALLINE NANOMETRIC LiFeMPO4 |
-
2011
- 2011-03-17 CN CN201180011603.6A patent/CN102781827B/en active Active
- 2011-03-17 JP JP2011059211A patent/JP5817963B2/en active Active
- 2011-03-17 KR KR1020127022574A patent/KR101810259B1/en active IP Right Grant
- 2011-03-17 WO PCT/JP2011/056397 patent/WO2011115211A1/en active Application Filing
Patent Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2008077448A1 (en) * | 2006-12-22 | 2008-07-03 | Umicore | SYNTHESIS OF CRYSTALLINE NANOMETRIC LiFeMPO4 |
| EP2094605A1 (en) * | 2006-12-22 | 2009-09-02 | Umicore | SYNTHESIS OF CRYSTALLINE NANOMETRIC LiFeMPO4 |
| US20100084615A1 (en) * | 2006-12-22 | 2010-04-08 | Stephane Levasseur | Synthesis of Crystalline Nanometric LiFeMPO4 |
| JP2010513193A (en) * | 2006-12-22 | 2010-04-30 | ユミコア ソシエテ アノニム | Synthesis of crystalline nano LiFeMPO4 |
| US20100327222A1 (en) * | 2006-12-22 | 2010-12-30 | Umicore | Synthesis of Crystalline Nanometric LiFeMPO4 |
| WO2009122686A1 (en) * | 2008-03-31 | 2009-10-08 | 戸田工業株式会社 | Lithium iron phosphate powder manufacturing method, olivine structured lithium iron phosphate powder, cathode sheet using said lithium iron phosphate powder, and non-aqueous solvent secondary battery |
| JP2009263222A (en) * | 2008-03-31 | 2009-11-12 | Toda Kogyo Corp | Method for producing lithium iron phosphate powder, olivine-structured lithium iron phosphate powder, cathode sheet using the lithium iron phosphate powder and non-aqueous solvent secondary battery |
Cited By (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US10135069B2 (en) | 2010-10-08 | 2018-11-20 | Semiconductor Energy Laboratory Co., Ltd. | Electrode material and method for manufacturing power storage device |
| JP2018008877A (en) * | 2010-10-08 | 2018-01-18 | 株式会社半導体エネルギー研究所 | Method for manufacturing lithium iron phosphate |
| JP2012229147A (en) * | 2011-04-27 | 2012-11-22 | Nichia Corp | Olivine-type lithium transition metal oxide, and method of producing the same |
| JP2013120621A (en) * | 2011-12-06 | 2013-06-17 | Toyota Motor Corp | Nonaqueous secondary battery |
| CN103708434A (en) * | 2012-10-09 | 2014-04-09 | 上海交通大学 | Lithium iron phosphate material and preparation method thereof |
| US9660266B2 (en) | 2012-11-21 | 2017-05-23 | Lg Chem, Ltd. | Lithium secondary battery |
| CN104718657A (en) * | 2012-11-21 | 2015-06-17 | 株式会社Lg化学 | Lithium secondary battery |
| US9853288B2 (en) | 2012-11-21 | 2017-12-26 | Lg Chem, Ltd. | Lithium secondary battery |
| CN104718657B (en) * | 2012-11-21 | 2018-01-12 | 株式会社Lg 化学 | Lithium secondary battery |
| WO2014098933A1 (en) * | 2012-12-21 | 2014-06-26 | Dow Global Technologies Llc | Method for making lithium transition metal olivines using water/cosolvent mixtures |
| JP2015076155A (en) * | 2013-10-07 | 2015-04-20 | 太平洋セメント株式会社 | Method of producing lithium manganese phosphate positive electrode active material |
| EP4282827A1 (en) * | 2022-05-25 | 2023-11-29 | Hubei RT Advanced Materials Co., Ltd. | Preparation method of high-safety high-capacity lithium manganese iron phosphate |
| CN116081589A (en) * | 2022-10-12 | 2023-05-09 | 北京钠谛科技有限公司 | Lithium-rich lithium iron manganese phosphate material and preparation method thereof |
| CN116081589B (en) * | 2022-10-12 | 2024-03-29 | 北京钠谛科技有限公司 | Lithium-rich lithium iron manganese phosphate material and preparation method thereof |
Also Published As
| Publication number | Publication date |
|---|---|
| CN102781827A (en) | 2012-11-14 |
| JP5817963B2 (en) | 2015-11-18 |
| KR20130057414A (en) | 2013-05-31 |
| JP2011213587A (en) | 2011-10-27 |
| KR101810259B1 (en) | 2017-12-18 |
| CN102781827B (en) | 2016-05-04 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5817963B2 (en) | Method for producing lithium manganese iron phosphate particle powder, lithium manganese iron phosphate particle powder, and nonaqueous electrolyte secondary battery using the particle powder | |
| JP5464322B2 (en) | Method for producing lithium iron phosphate particle powder, lithium iron phosphate particle powder having olivine structure, positive electrode material sheet using the lithium iron phosphate particle powder, and nonaqueous solvent secondary battery | |
| KR101369658B1 (en) | Li-Ni COMPOSITE OXIDE PARTICLE POWDER FOR RECHARGEABLE BATTERY WITH NONAQUEOUS ELECTROLYTE, PROCESS FOR PRODUCING THE Li-Ni COMPOSITE OXIDE PARTICLE POWDER, AND RECHARGEABLE BATTERY WITH NONAQUEOUS ELECTROLYTE | |
| JP6107832B2 (en) | Li-Ni composite oxide particle powder, method for producing the same, and nonaqueous electrolyte secondary battery | |
| JP6260535B2 (en) | Method for producing carbon composite lithium manganese iron phosphate particle powder, and method for producing a non-aqueous electrolyte secondary battery using the particle powder | |
| JP6112118B2 (en) | Li-Ni composite oxide particle powder and non-aqueous electrolyte secondary battery | |
| JP5835334B2 (en) | Ammonium manganese iron phosphate, method for producing the same, and method for producing positive electrode active material for lithium secondary battery using the ammonium manganese iron phosphate | |
| WO2013035572A1 (en) | Method for producing lithium- or sodium-containing oxoate compound | |
| WO2010104137A1 (en) | Process for producing lithium borate compound | |
| JP5120523B1 (en) | Ammonium manganese iron magnesium phosphate and its production method, positive electrode active material for lithium secondary battery using said ammonium manganese iron magnesium magnesium, its production method, and lithium secondary battery using said positive electrode active material | |
| JP2006286208A (en) | Lithium-ion secondary battery and cathode active material | |
| JP5505868B2 (en) | Precursor of positive electrode active material for lithium secondary battery and method for producing the same | |
| JP2010232091A (en) | Method for manufacturing positive active material for lithium ion battery, positive active material for lithium ion battery, electrode for lithium ion battery, and lithium ion battery | |
| JP5901492B2 (en) | Method for producing lithium silicate compound, method for producing lithium silicate compound aggregate, and method for producing lithium ion battery | |
| JP5364865B2 (en) | Method for producing positive electrode active material for lithium secondary battery | |
| JP5909131B2 (en) | Active material for lithium secondary battery, electrode for lithium secondary battery and lithium secondary battery using the same | |
| JP5988095B2 (en) | Precursor of positive electrode active material for lithium secondary battery and method for producing the same, positive electrode active material for lithium secondary battery using the precursor and method for producing the same, and lithium secondary battery using the positive electrode active material | |
| JP5862172B2 (en) | Secondary battery active material, secondary battery active material electrode, and secondary battery using the same | |
| Yamashita et al. | Hydrothermal synthesis and electrochemical properties of Li2FexMnxCo1− 2xSiO4/C cathode materials for lithium-ion batteries | |
| JP5769140B2 (en) | Method for producing positive electrode active material for lithium secondary battery | |
| JP6064309B2 (en) | Precursor of positive electrode active material for lithium secondary battery and method for producing the same, positive electrode active material for lithium secondary battery using the precursor and method for producing the same, and lithium secondary battery using the positive electrode active material | |
| JP2018147696A (en) | Positive electrode active material for nonaqueous electrolyte secondary battery |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| WWE | Wipo information: entry into national phase |
Ref document number: 201180011603.6 Country of ref document: CN |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 11756395 Country of ref document: EP Kind code of ref document: A1 |
|
| ENP | Entry into the national phase |
Ref document number: 20127022574 Country of ref document: KR Kind code of ref document: A |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 11756395 Country of ref document: EP Kind code of ref document: A1 |