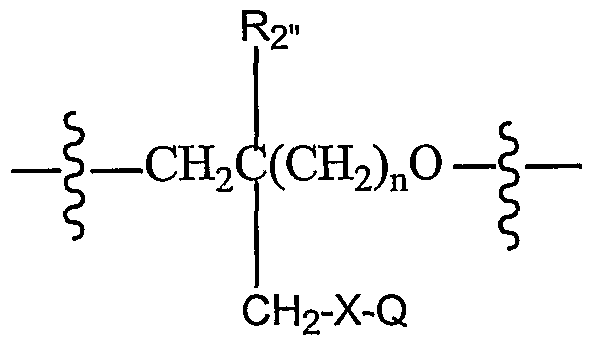SUGAR SURROGATE-CONTAINING OLIGOMERIC COMPOUNDS AND COMPOSITIONS FOR USE IN GENE MODULATION
Cross-Reference To Related Applications
[0001] The present application is a continuation in part of U.S. Serial Number 10/078,949 filed February 20, 2002 which is a continuation of 09/479,783 filed January 7, 2000, which is a divisional of U.S. Serial Number 08/870,608 filed June 6, 1997 which was issued as U.S. Patent 6,107,094 on August 22, 2002, which is a continuation-in-part of U.S. Serial Number 08/659,440 filed June 6, 1996 which was issued as U.S. Patent 5,898,031 on April 27, 1999, each of which is incorporated herein by reference in its entirety. The present application also claims benefit to U.S. Provisional Application Serial Number 60/423,760 filed November 5, 2002, which is incorporated herein by reference in its entirety.
Field of the Invention
[0002] The present invention provides modified oligomers that modulate gene expression via a RNA interference pathway. The oligomers of the invention include one or more modifications thereon resulting in differences in various physical properties and attributes compared to wild type nucleic acids. The modified oligomers are used alone or in compositions to modulate the targeted nucleic acids. In preferred embodiments of the invention, the modifications include replacement of the sugar moiety of an oligomer with a sugar surrogate.
Background of the Invention
[0003] In many species, introduction of double-stranded RNA (dsRNA) induces
potent and specific gene silencing. This phenomenon occurs in both plants and animals and has roles in viral defense and transposon silencing mechanisms. This phenomenon was originally described more than a decade ago by researchers working with the petunia flower. While trying to deepen the purple color of these flowers, Jorgensen et al. introduced a pigment-producing gene under the control of a powerful promoter. Instead of the expected deep purple color, many of the flowers appeared variegated or even white. Jorgensen named the observed phenomenon "cosuppression", since the expression of both the introduced gene and the homologous endogenous gene was suppressed Napoli et al., Plant Cell, 1990, 2, 279-289; Jorgensen et al., Plant Mol. Biol, 1996, 31, 957-973). [0004] Cosuppression has since been found to occur in many species of plants, fungi, and has been particularly well characterized in Neurospora crassa, where it is known as "quelling" (Cogoni and Macino, Genes Dev. 2000, 10, 638-643; Guru, Nature, 2000, 404, 804-808).
[0005] The first evidence that dsRNA could lead to gene silencing in animals came from work in the nematode, Caenorhabditis elegans. In 1995, researchers Guo and Kemphues were attempting to use antisense RNA to shut down expression of the par-1 gene in order to assess its function. As expected, injection of the antisense RNA disrupted expression of par-1, but quizzically, injection of the sense-strand control also disrupted expression (Guo and Kempheus, Cell, 1995, 81, 611-620). This result was a puzzle until Fire et al. injected dsRNA (a mixture of both sense and antisense strands) into C. elegans. This injection resulted in much more efficient silencing than injection of either the sense or the antisense strands alone. Injection of just a few molecules of dsRNA per cell was sufficient to completely silence the homologous gene's expression. Furthermore, injection of dsRNA into the gut of the worm caused gene silencing not only throughout the worm, but also in first generation offspring (Fire et al., Nature, 1998, 391, 806-811).
[0006] The potency of this phenomenon led Timmons and Fire to explore the limits of the dsRNA effects by feeding nematodes bacteria that had been engineered to express dsRNA homologous to the C. elegans unc-22 gene.
Surprisingly, these worms developed an unc-22 null-like phenotype (Timmons and Fire, Nature 1998, 395, 854; Timmons et al., Gene, 2001, 263, 103-112). Further work showed that soaking worms in dsRNA was also able to induce silencing (Tabara et al, Science, 1998, 282, 430-431). PCT publication WO 01/48183 discloses methods of inhibiting expression of a target gene in a nematode worm involving feeding to the worm a food organism which is capable of producing a double-stranded RNA structure having a nucleotide sequence substantially identical to a portion of the target gene following ingestion of the food organism by the nematode, or by introducing a DNA capable of producing the double-stranded RNA structure (Bogaert et al., 2001).
[0007] The posttranscriptional gene silencing defined in Caenorhabditis elegans resulting from exposure to double-stranded RNA (dsRNA) has since been designated as RNA interference (RNAi). This term has come to generalize all forms of gene silencing involving dsRNA leading to the sequence-specific reduction of endogenous targeted mRNA levels; unlike co-suppression, in which transgenic DNA leads to silencing of both the transgene and the endogenous gene. [0008] Introduction of exogenous double-stranded RNA (dsRNA) into Caenorhabditis elegans has been shown to specifically and potently disrupt the activity of genes containing homologous sequences. Montgomery et al. suggests that the primary interference affects of dsRNA are post-transcriptional. This conclusion being derived from examination of the primary DNA sequence after dsRNA-mediated interference and a finding of no evidence of alterations, followed by studies involving alteration of an upstream operon having no effect on the activity of its downstream gene. These results argue against an effect on initiation or elongation of transcription. Finally using in situ hybridization they observed that dsRNA-mediated interference produced a substantial, although not complete, reduction in accumulation of nascent transcripts in the nucleus, while cytoplasmic accumulation of transcripts was virtually eliminated. These results indicate that the endogenous mRNA is the primary target for interference and suggest a mechanism that degrades the targeted mRNA before translation can occur. It was also found that this mechanism is not dependent on the SMG system,
an mRNA surveillance system in C. elegans responsible for targeting and destroying aberrant messages. The authors further suggest a model of how dsRNA might function as a catalytic mechanism to target homologous mRNAs for degradation. (Montgomery et al., Proc. Natl. Acad. Sci. USA, 1998, 95, 15502- 15507).
[0009] Recently, the development of a cell-free system from syncytial blastoderm Drosophila embryos, which recapitulates many of the features of RNAi, has been reported. The interference observed in this reaction is sequence specific, is promoted by dsRNA but not single-stranded RNA, functions by specific mRNA degradation, and requires a minimum length of dsRNA. Furthermore, preincubation of dsRNA potentiates its activity demonstrating that RNAi can be mediated by sequence-specific processes in soluble reactions (Tuschl et al, Genes Dev., 1999, 13, 3191-3197).
[0010] In subsequent experiments, Tuschl et al, using the Drosophila in vitro system, demonstrated that 21- and 22-nt RNA fragments are the sequence-specific mediators of RNAi. These fragments, which they termed short interfering RNAs (siRNAs), were shown to be generated by an RNase Ill-like processing reaction from long dsRNA. They also showed that chemically synthesized siRNA duplexes with overhanging 3' ends mediate efficient target RNA cleavage in the Drosophila lysate, and that the cleavage site is located near the center of the region spanned by the guiding siRNA. In addition, they suggest that the direction of dsRNA processing determines whether sense or antisense target RNA can be cleaved by the siRNA-protein complex (Elbasbir et al, Genes Dev., 2001, 15, 188-200). Further characterization of the suppression of expression of endogenous and heterologous genes caused by the 21-23 nucleotide siRNAs have been investigated in several mammalian cell lines, including human embryonic kidney (293) and HeLa cells (Elbasbir et al., Nature, 2001, 411, 494-498). [0011] The Drosophila embryo extract system has been exploited, using green fluorescent protein and luciferase tagged siRNAs, to demonstrate that siRNAs can serve as primers to transform the target mRNA into dsRNA. The nascent dsRNA is degraded to eliminate the incorporated target mRNA while generating new
siRNAs in a cycle of dsRNA synthesis and degradation. Evidence is also presented that mRNA-dependent siRNA incorporation to form dsRNA is carried out by an RNA-dependent RNA polymerase activity (RdRP) (Lipardi et al, Cell, 2001, 107, 297-307).
[0012] The involvement of an RNA-directed RNA polymerase and siRNA primers as reported by Lipardi et al. (Lipardi et al, Cell, 2001, 107, 297-307) is one of the many intriguing features of gene silencing by RNA interference. This suggests an apparent catalytic nature to the phenomenon. New biochemical and genetic evidence reported by Nisbikura et al. also shows that an RNA-directed RNA polymerase chain reaction, primed by siRNA, amplifies the interference caused by a small amount of "trigger" dsRNA (Nishikura, Cell, 2001, 107, 415- 418).
[0013] Investigating the role of "trigger" RNA amplification during RNA interference (RNAi) in Caenorhabditis elegans, Sijen et al revealed a substantial fraction of siRNAs that cannot derive directly from input dsRNA. Instead, a population of siRNAs (termed secondary siRNAs) appeared to derive from the action of the previously reported cellular RNA-directed RNA polymerase (RdRP) on mRNAs that are being targeted by the RNAi mechanism. The distribution of secondary siRNAs exhibited a distinct polarity (5'-3'; on the antisense strand), suggesting a cyclic amplification process in which RdRP is primed by existing siRNAs. This amplification mechanism substantially augmented the potency of RNAi-based surveillance, while ensuring that the RNAi machinery will focus on expressed mRNAs (Sijen et al., Cell, 2001, 107, 465-476). [0014] Most recently, Tijsterman et al. have shown that, in fact, single-stranded RNA oligomers of antisense polarity can be potent inducers of gene silencing. As is the case for co-suppression, they showed that antisense RNAs act independently of the RNAi genes rde-1 and rde-4 but require the mutator/RNAi gene mut-7 and a putative DEAD box RNA helicase, mut-14. According to the authors, their data favor the hypothesis that gene silencing is accomplished by RNA primer extension using the mRNA as template, leading to dsRNA that is subsequently degraded suggesting that single-stranded RNA oligomers are ultimately
responsible for the RNAi phenomenon (Tijsterman et al, Science, 2002, 295, 694- 697).
[0015] Several recent publications have described the structural requirements for the dsRNA trigger required for RNAi activity. Recent reports have indicated that ideal dsRNA sequences are 21nt in length containing 2 nt 3 '-end overhangs (Elbashir et al, EMBO (2001), 20, 6877-6887, Sabine Brantl, Biochimica etBiophysica Acta, 2002, 1575, 15-25.) In this system, substitution of the 4 nucleosides from the 3 '-end with 2'-deoxynucleosides has been demonstrated to not affect activity. On the other hand, substitution with 2'-deoxynucleosides or 2'-OMe-nucleosides throughout the sequence (sense or antisense) was shown to be deleterious to RNAi activity.
[0016] Investigation of the structural requirements for RNA silencing in C. elegans has demonstrated modification of the internucleotide linkage (phosphorothioate) to not interfere with activity (Parrish et ah, Molecular Cell, 2000, 6, 1077-1087.) It was also shown by Parrish et al, that chemical modification like 2'-amino or 5-iodouridine are well tolerated in the sense strand but not the antisense strand of the dsRNA suggesting differing roles for the 2 strands in RNAi. Base modification such as guanine to inosine (where one hydrogen bond is lost) has been demonstrated to decrease RNAi activity independently of the position of the modification (sense or antisense). Some "position independent" loss of activity has been observed following the introduction of mismatches in the dsRNA trigger. Some types of modifications, for example introduction of sterically demanding bases such as 5-iodoU, have been shown to be deleterious to RNAi activity when positioned in the antisense strand, whereas modifications positioned in the sense strand were shown to be less detrimental to RNAi activity. As was the case for the 21 nt dsRNA sequences, RNA-DNA heteroduplexes did not serve as triggers for RNAi. However, dsRNA containing 2'-F-2'-deoxynucleosides appeared to be efficient in triggering RNAi response independent of the position (sense or antisense) of the 2'-F-2'- deoxynucleosides. [0017] In one study the reduction of gene expression was studied using
electroporated dsRNA and a 25mer morpholino oligomer in post implantation mouse embryos (Mellitzer et al, Mehanisms of Development, 2002, 118, 57-63).
The morpholino oligomer did show activity but was not as effective as the dsRNA.
[0018] A number of PCT applications have recently been published that relate to the RNAi phenomenon. These include: PCT publication WO 00/44895; PCT publication WO 00/49035; PCT publication WO 00/63364; PCT publication WO
01/36641; PCT publication WO 01/36646; PCT publication WO 99/32619; PCT publication WO 00/44914; PCT publication WO 01/29058; and PCT publication
WO 01/75164.
[0019] U.S. patents 5,898,031 and 6,107,094, each of which is commonly owned with this application and each of which is herein incorporated by reference, describe certain oligonucleotide having RNA like properties. When hybridized with RNA, these oligonucleotides serve as substrates for a dsRNase enzyme with resultant cleavage of the
RNA by the enzyme.
[0020] In another recently published paper (Martinez et al, Cell, 2002, 110, 563-574) it was shown that single stranded as well as double stranded siRNA resides in the RNA- induced silencing complex (RISC) together with elF2Cl and elf2C2 (human GERp950)
Argonaute proteins. The activity of 5'-phosphorylated single stranded siRNA was comparable to the double stranded siRNA in the system studied. In a related study, the inclusion of a 5'-phosphate moiety was shown to enhance activity of siRNA's in vivo in
Drosophilia embryos (Boutla, et al., Curr. Biol., 2001, 11, 1776-1780). In another study, it was reported that the 5'-phosphate was required for siRNA function in human HeLa cells (Schwarz et al, Molecular Cell, 2002, 10, 537-548).
[0021] In yet another recently published paper (Chiu et al, Molecular Cell, 2002, 10,
549-561) it was shown that the 5'-hydroxyl group of the siRNA is essential as it is phosphorylated for activity while the 3 '-hydroxyl group is not essential and tolerates substitute groups such as biotin. It was further shown that bulge structures in one or both of the sense or antisense strands either abolished or severely lowered the activity relative to the unmodified siRNA duplex. Also shown was severe lowering of activity when psoralen was used to cross link an siRNA duplex.
[0022] Like the RNAse H pathway, the RNA interference pathway for modulation of gene expression is an effective means for modulating the levels of specific gene products and, thus, would be useful in a number of therapeutic, diagnostic, and research applications involving gene silencing. The present invention therefore provides oligomeric compounds useful for modulating gene expression pathways, including those relying on mechanisms of action such as RNA interference and dsRNA enzymes, as well as antisense and non-antisense mechanisms. One having skill in the art, once armed with this disclosure will be able, without undue experimentation, to identify preferred oligonucleotide compounds for these uses.
Summary of the Invention
[0023] In certain aspects, the invention relates to oligomer compositions comprising a first oligomer and a second oligomer in which at least a portion of the first oligomer is capable of hybridizing with at least a portion of the second oligomer, and at least a portion of the first oligomer is complementary to and capable of hybridizing to a selected target nucleic acid. At least one of said first or said second oligomers includes at least one nucleoside having a sugar surrogate/nucleobase pair.
[0024] In some aspects, the first and second oliogmers comprise a complementary pair of siRNA oligomers.
[0025] In certain embodiments, the first and second oligomers comprise an antisense/sense pair of oligomers.
[0026] Each of the first and second oligomers have 10 to 40 nucleobases in some preferred embodiments. In other embodiments, each of the first and second oligomers have 18 to 30 nucleobases. In yet other embodiments, the first and second oliogmers have 21 to 24 nucleobases.
[0027] Certain aspects of the invention concern compositions in which the first oligomer is an antisense oliogmer. In these aspects, the second oligomer is a sense oligomer. In certain preferred embodiments, the second oliogmer has a plurality of ribose nucleoside units.
[0028] The sugar surrogate can be in the first oligomer. In other compounds, the sugar surrogate can be in the second oligomer. In yet other aspects, the sugar surrogate can appear in both the first and second oligomers. [0029] In some embodiments, the at least one oligomer includes a cyclobutyl nucleoside, cyclopentyl nucleoside, proline nucleoside, cyclohexene nucleoside, hexose nucleoside or a cyclohexane nucleoside. In certain embodiments, the sugar surrogate is an arabinonucleoside, xylonucleoside, lyxonucleoside, erythronucleoside, threonucleoside, 4'-thioribonucleoside, or 2'-deoxy-4'- thioribonucleside. As used herein, the aforementioned terms includes their deoxy derivatives. In some aspects, the invention concerns compositions where the sugar surrogate is arabinonucleoside.
[0030] In yet other aspects of the invention, the sugar surrogate is cyclobutyl nucleoside. In some embodiments, the cyclobutyl nucleoside is of the formula:
where the cyclobutyl ring is optionally substituted by at least one substituent at the C-2 and/or C-4 position, said substituent being halogen, Ci -Cio alkoxy, allyloxy, Ci -Cio alkyl or Ci -Cio alkylamine groups. As used in the above structure and elsewhere in this application, the curved line notation in the above structure indicates binding to another monomeric unit by way of a linking group or a terminal group.
[0031] The sugar surrogate may also be a cyclopentyl nucleoside. Some cyclopentyl nucleosides are of the formula:
Bx is a heterocyclic base moiety;
Q' is CH2, CHF, or CF2; and
R2 is OH; F; O-, S-, or N-alkyl; O-, S-, or N-alkenyl; O-, S- or N-alkynyl; or O- alkyl-O-alkyl, wherein the alkyl, alkenyl and alkynyl may be substituted or unsubstituted Ci to Cio alkyl or C2 to Cio alkenyl or alkynyl.
[0032] Other compounds of the invention are of the formula:
Bx is a heterocyclic base moiety;
Q is S, O, NH, N(Cι-C6 alkyl), CH2, CHF, or CF2;
R82 is a sugar substituent;
R
83 and R
85 are each independently OH, a protected hydroxyl group, an internucleoside linkage to an adjacent monomer, or a terminal group; and si K-83
'. Rδ
4 and R
85> are each independently H, alkyl, aralkyl, or aryl. [0033] In other embodiments, the sugar surrogate is a proline nucleoside. Some proline nucleosides are of the formula:
where X is H, a phosphate group, an activated phosphate group, an activated phosphite group, or a solid support;
Y is H or a hydroxyl protecting group;
Z is L8, L8 -Gi, L9, L9 -G, NR23R24, a nitrogen-containing heterocycle, a purine, a pyrimidine, a phosphate group, a polyether group, or a polyethylene glycol group;
L8 is Cι-C20 alkyl, C2-C20 alkenyl, or C2-C20 alkynyl;
L9 is C6-C aryl or C7-C15 aralkyl;
Gi is halogen, OR2ι, SR22, NR23R24, C(=NH)NR23R24, NHC(=NH)NR23R24,
CH=O, C(=O)OR25, CH(NR23 R24)(C(=O)OR25), C(=O)NR23R24, a metal coordination group, or a phosphate group;
G2 is halogen, OH, SH, SCH3, or NR23R24 ;
R2ι is H, Cι-C6 alkyl, or a hydroxyl protecting group;
R22 is H, Cι-C6 alkyl, or a thiol protecting group;
R 3 and, R24 are, independently, H, Cι-C6 alkyl, or an amine protecting group;
R25 is H, Ci-Ce alkyl, or an acid protecting group;
Q is Lι, G3, Lι -G3 or G3 -Lι -G3 ;
G3 is C(=O), C(=S), C(O)-O, C(O)-NH, C(S)-O, C(S)~NH or S(O)2; and n is O or 1.
[0034] In some compounds of the invention, the sugar surrogate is of the formula:
where each Bx is a heterocyclic base moiety; Ti is hydroxyl or a protected hydroxyl; Tr is hydroxyl or a protected hydroxyl; and L3 is an intemucleoside linkage. The above chemical structure shows two or more cyclohexene monomers. It is also within the scope of the invention that one monomer may also be present as indicated by the following structure.
[0035] In yet other compounds of the invention, the sugar surrogate is of formula:
wherein Bx is a heterocyclic nucleobase, R
95 is H, a hydroxyl protecting group, an intemucleoside linkage to an adjacent monomer, or a terminal group, and X
7 is a H or a sugar substitutent.
[0036] In certain embodiments, the sugar surrogate is a 4'-thioribonucleoside. In other embodiments, the sugar surrogate is a 4-thiodeoxyribonucleoside. [0037] Sugar surrogates may also be acyclic. In some embodiments, the sugar surrogate is a phosphoramidite derivative. Certain phosphoramidite derivative building blocks are of the formula:
where X is a conjugate and Y is a protecting group. Suitable protecting groups include 4,4'-dimethoxytrityl, trifluoroacetyl and fluorenylmethoxycarbonyl (Fmoc). In some embodiments, the conjugate is biotin. [0038] Other acyclic sugar surrogates include compounds with at least one monomer of the formula:
where Ri- is hydrogen, or a blocking group that is compatible with oligonucleotide synthesis; R
2» is hydrogen, nitro, lower alkyl amino, diloweralkyl amino or methyl; R
3" is hydrogen or --P(R »)OR
5» ; R-r is chlorine, 4- nitroimidazole, imidazole, tetrazole, triazole or di(lower-alkyl)amino-; R
5» is methyl, 2-cyanoethyl or 2,2,2-trichloroethyl; n is an integer from 0 to 2; X is oxygen, sulfur, or ~NR
6" ; R
6" is hydrogen or lower alkyl; Q is a heterocyclic nucleobase, and in some embodiments, is chosen from the group consisting of
where R
7» is lower-alkyl or loweralkyloxy methylene; and R
8» is hydrogen, benzoyl, anisoyl, or lower-alkyl carbonyl and its pharmaceutically acceptable addition salts are nucleotide analogs.
[0039] The invention also concerns compositions comprising an oligomer complementary to and capable of hybridizing to a selected target nucleic acid and at least one protein, said protein comprising at least a portion of a RNA-induced silencing complex (RISC), wherein said oligomer includes at least one nucleoside having a sugar surrogate discussed above.
[0040] In other aspects, the invention relates to oligomers having at least a first region and a second region, said first region of said oligomer complementary to and capable of hybridizing with said second region of said oligomer, at least a portion of said oligomer complementary to and capable of hybridizing to a selected target nucleic acid, said oligomer further including at least one nucleoside having a sugar surrogate disclosed above. [0041] In some embodiments, each of the first and second regions have at least
10 nucleosides. For certain compositions, the first region is in a 5' to 3' direction is complementary to the second region in a 3' to 5' direction.
[0042] Some compounds of the invention include a hairpin structure.
[0043] Certain aspects of the invention concern the first region of the oligomer being spaced from the second region of the oligomer by a third region that comprises at least two nucleosides.
[0044] In other aspects, the first region of the oligomer is spaced from the second region of the oligomer by a third region that comprises a non-nucleoside region.
[0045] Also provided by the present invention are pharmaceutical compositions comprising any of the disclosed compositions or oligomeric compounds and a pharmaceutically acceptable carrier.
[0046] Methods for modulating the expression of a target nucleic acid in a cell are also provided, wherein the methods comprise contacting the cell with any of the disclosed compositions or oligomeric compounds.
[0047] Methods of treating or preventing a disease or condition associated with a target nucleic acid are also provided, wherein the methods comprise administering to a patient having or predisposed to the disease or condition a therapeutically effective amount of any of the disclosed compositions or oligomeric compounds.
Detailed Description of the Invention
[0048] The present invention provides oligomeric compounds useful in the modulation of gene expression. Although not intending to be bound by theory, oligomeric compounds of the invention modulate gene expression by hybridizing to a nucleic acid target resulting in loss of normal function of the target nucleic acid. As used herein, the term "target nucleic acid" or "nucleic acid target" is used for convenience to encompass any nucleic acid capable of being targeted including without limitation DNA, RNA (including pre-mRNA and mRNA or portions thereof) transcribed from such DNA, and also cDNA derived from such RNA. In a preferred embodiment of this invention modulation of gene expression
is effected via modulation of a RNA associated with the particular gene RNA. [0049] The invention provides for modulation of a target nucleic acid that is a messenger RNA. The messenger RNA is degraded by the RNA interference mechanism as well as other mechanisms in which double stranded RNA RNA structures are recognized and degraded, cleaved or otherwise rendered inoperable. [0050] The functions of RNA to be interfered with can include replication and transcription. Replication and transcription, for example, can be from an endogenous cellular template, a vector, a plasmid construct or otherwise. The functions of RNA to be interfered with can include functions such as translocation of the RNA to a site of protein translation, translocation of the RNA to sites within the cell which are distant from the site of RNA synthesis, translation of protein from the RNA, splicing of the RNA to yield one or more RNA species, and catalytic activity or complex formation involving the RNA which may be engaged in or facilitated by the RNA. In the context of the present invention, "modulation" and "modulation of expression" mean either an increase (stimulation) or a decrease (inhibition) in the amount or levels of a nucleic acid molecule encoding the gene, e.g., DNA or RNA. Inhibition is often the preferred form of modulation of expression and mRNA is often a preferred target nucleic acid.
Compounds of the Invention
[0051] This invention is directed to certain molecular species which are related to oligonucleotides or oligonucleotide mimetics where at least one of the naturally occurring sugar moieties, ribose or deoxyribose, is replaced with non-naturally occurring sugars or non-sugar moieties. The non-naturally occurring sugars that may be used in the instant invention include arabinose, xylose, lyxose, erytl rose, and threose, as well as their deoxy derivatives. Certain xylose compositions are disclosed in U.S. Patent No. 6,329,346, the disclosure of which is incorporated in its entirety. Certain xylose structure are of the formula:
where Bx is a heterocyclic base moiety.
[0052] Threose nucleoside compositions are disclosed in Chaput et al., J. Am. Chem. Soc. 2003, 125, 856-57, Schoning et al., Science 2000, 290(5495), 1347-51 and Wu et al., Org. Lett. 2002, 4, 1279-82. In some embodiments, the thresose composition may be of the formula
where Bx is a heterocyclic base moiety. The monomeric units may be linked by intemucleoside linkages discussed herein. These linkages include phosphate and phosphoramidite linkages. The phosphoroamidite linkages include those of 2'- NH and 3'-NH isomers.
[0053] These sugar substitutes are illustrated by use of arabinonucleotides as building blocks for the compositions of the instant invention. In these compositions, an arabinose ring replaces the furanose ring that is normally present in RNA and DNA. Such building blocks are described in Damha et. al., J.A.C.S., 1998, 120, 12976-12977 and Damha et. al., Bioconjugate Chem., 1999, 10, 299- 305. In some embodiments, the nucleotides are joined via phosphodiester linkages. Certain compositions contain a sugar surrogate. In some preferred embodiments, the oligonucleotide is 2'-CN arabinonucleotide, a 2'-F arabinonucleotide, a 2'-Cl arabinonucleotide, a 2'-Br arabinonucleotide, a 2'-N3 arabinonucleotide, a 2'-OH arabinonucleotide, a 2'-O-CH3 arabinonucleotide or a 2'-dehydro-2'-CH arabinonucleotide. In some preferred embodiments, the oligonucleotide is 2'-F arabinonucleotide.
[0054] Analogous compositions may encompass the use of xylose, lyxose, erythrose, and threose sugars.
[0055] The invention also concerns cyclobutane rings as sugar surrogates.
These cyclobutyl moieties have heterocyclic bases attached thereto and may be connected by linking moieties into oligonucleotide-like stractures. A cyclobutane ring system is fixed when compared to a pentofuranose ring system because, unlike the cyclobutane ring system, the pentofuranose ring system permits rotation about intra-ring chemical bonds. Thus, the pentofuranosyl ring system can adopt a
"pucker" conformation while the cyclobutane ring can only adopt two conformations.
[0056] Like a pentofuranosyl ring, a cyclobutane ring system can have substituent functional groups at the different positions within the ring. These substituents mclude those substitutions on the sugar ring discussed elsewhere in this application.
[0057] Positional identification of the cyclobutane ring is made by reference to structure:
4
where the base is a heterocyclic base moiety.
[0058] The oligonucleotide surrogates of the invention are formed by linking together a plurality of cyclobutyl subunits via linking moieties. Each subunit includes a cyclobutane ring, a heterocyclic base, and a linking moiety for joining adjacent subunits.
[0059] In accordance with the invention, linking moieties are selected to covalently link individual heterocyclic-base-containing cyclobutyl moieties together in an orientation wherein the heterocyclic bases are positioned in space in a conformation which allows hybridization with a complementary strand of DNA or RNA. [0060] In certain preferred embodiments of the invention the linking moieties
are selected as 4 or 5 atom chains. Such 4 and 5 atom chains include the phosphodiester linkages of native DNA and RNA as well as the related synthetic phosphorothioate, phosplioramidate, alkyl phosphonate, phosphorodithioate and phosphotriester linkages of "oligonucleotide analogs." Other linking moieties include phosphate, carbamate, sulfonate, Ci -C6 -dialkylsilyl or formacetal linkages. Further linkages include an ~O~CH2 -CH2 — O~linkage. [0061] Monomers of the instant invention include those of the formula:
[0062] In preferred oligonucleotide surrogates the heterocyclic base is attached to each respective cyclobutyl moiety at the carbon- 1 (C-l) position of said cyclobutyl moiety and the linking moieties connect to each respective cyclobutyl moiety at the carbon-3 (C-3) position thereof. In these preferred embodiments, a substituent group can be located on one of the carbon-2 (C-2) or the carbon-4 (C- 4) positions of at least one of the cyclobutyl moieties. Prefened substituents include halogen, Ci -Cio alkoxy, allyloxy, Ci -Cio alkyl or Ci -Cio alkylamine groups. In certain embodiments, the substituent group is preferably positioned trans to the heterocyclic base.
[0063] In some preferred oligonucleotide surrogates of the invention, the linking moieties are 4 or 5 atom chains that connect adjacent cyclobutyl moieties. When the linking moieties are 5 atom chains, each of the linking moieties preferably is of the structure Li ~L2 ~L3, where Li and L3 are CH2 ; and L2 is phosphodiester, phosphorothioate, phosphoramidate, phosphotriester, d -C6 alkyl phosphonate, phosphorodithioate, phosphonate, carbamate, sulfonate, d -C6 -dialkylsilyl or formacetal. Preferably, each of the linking moieties is of the structure Li — L2 ~L , where L, and L are CH2 and L2 is phosphodiester or phosphorothioate. [0064] When the linking moieties are 4 atom chains, each of the linking moieties preferably is of the structure: L4 — L5 ~ L6— L7 where:
(a) L4 and L7 are CH2 ; and L5 and L6, independently, are CRιR2, C=CRιR2, C=NR3, C=O, C=S, O, S, SO, SO2, NR3 or SiR R5 ; or
(b) L4 and L7 are CH ; and L5 and L6, together, are CRι=CR2, C=C, part of a C6 aromatic ring, part of a C3 -C6 carbocyclic ring or part of a 3, 4, 5 or 6 membered heterocyclic ring; or
(c) L4 ~L5 ~L6 ~L7, together, are CH=N~NH~CH2 or CH2 -O— N=CH; wherein:
Ri and R2, independently, are H, OH, SH, NH2, Ci -Cio alkyl, d -Cι0 substituted alkyl, Ci -Cio alkenyl, C7 -Cio aralkyl, Ci -C6 alkoxy, Ci -C6 thioalkoxy, Ci -C6 alkylamino, C7 -Cio aralkylamino, Ci -Cio substituted alkylamino, heterocycloalkyl, heterocycloalkylamino, aminoalkylammo, polyalkylamino, halo, formyl, keto, benzoxy, carboxamido, thiocarboxamido, ester, thioester, carboxamidino, carbamyl, ureido, guanidino, an RNA cleaving group, a group for improving the pharmacokinetic properties of an oligonucleotide, or a group for improving the phamiacodynamic properties of an oligonucleotide;
R3 is H, OH, NH2, Ci -C6 alkyl, substituted lower alkyl, alkoxy, lower alkenyl, aralkyl, alkylamino, aralkylamino, substituted alkylamino, heterocycloalkyl, heterocycloalkylamino, aminoalkylamino, polyalkylamino, a RNA cleaving group, a group for improving the pharmacokinetic properties of an oligonucleotide and a group for improving the phamiacodynamic properties of an oligonucleotide; and
R and R5, independently, are Ci -C6 alkyl or alkoxy. Particularly preferred 4 atom linking moieties are CH=N~NH~CH2, CH2 -NH-NH-CH2, CH2 -O~ NH~CH2 or CH2 --O— N=CH.
[0065] U.S. Patent Nos. 5,359,044 and 6,001,841 describes the synthesis of the cyclobutane compositions.
[0066] The invention also can utilize compositions based on cyclopentane rings as sugar surrogates. These compositions may be of the formula:
Bx is a heterocyclic base moiety; Q' is CH2, CHF, or CF2; and
R2 is a sugar ring substituent described herein. In some embodiments, R2 is OH; F; O-, S-, or N-alkyl; O-, S-, or N-alkenyl; O-, S- or N-alkynyl; or O-alkyl-O- alkyl, wherein the alkyl, alkenyl and alkynyl may be substituted or unsubstituted Ci to Cio alkyl or C2 to Cio alkenyl or alkynyl '
[0067] The monomers may be linked by an intemucleotide linkage such as the phosphodiester linkage found in native nucleic acids. This linkage has not been the linkage of choice for synthetic oligonucleotides that are for the most part targeted to a portion of a nucleic acid such as mRNA because of stability problems e.g. degradation by nucleases. Preferred intemucleotide linkages or intemucleoside linkages as is the case for non phosphate ester type linkages include, for example, phosphorothioates, chiral phosphorothioates, phosphoro- dithioates, phosphotriesters, aminoalkylphosphotriesters, methyl and other alkyl phosphonates including 3 ' -alkylene phosphonates, 5'-alkylene phosphonates and chiral phosphonates, phosphinates, phosphoramidates including 3 '-amino phosphoramidate and aminoalkylphosphoramidates, thionophosphoramidates, thionoalkylphosphonates, thionoalkylphosphotriesters, selenophosphates and boranophosphates having normal 3'-5' linkages, 2'-5' linked analogs of these, and those having inverted polarity wherein one or more intemucleotide linkages is a 3' to 3', 5' to 5' or 2' to 2' linkage. Prefened oligonucleotides having inverted polarity comprise a single 3' to 3' linkage at the 3'-most intemucleotide linkage i.e. a single inverted nucleoside residue which may be abasic (the nucleobase is
missing or has a hydroxyl group in place thereof). Various salts, mixed salts and free acid forms are also included.
[0068] Representative United States patents that teach the preparation of the above phosphoras-containing linkages include, but are not limited to, U.S.: 3,687,808; 4,469,863; 4,476,301; 5,023,243; 5,177,196; 5,188,897; 5,264,423; 5,276,019; 5,278,302; 5,286,717; 5,321,131; 5,399,676; 5,405,939; 5,453,496; 5,455,233; 5,466,677; 5,476,925; 5,519,126; 5,536,821; 5,541,306; 5,550,111; 5,563,253; 5,571,799; 5,587,361; 5,194,599; 5,565,555; 5,527,899; 5,721,218; 5,672,697 and 5,625,050, certain of which are commonly owned with this application, and each of which is herein incorporated by reference. [0069] Prefened modified intemucleoside linkages that do not include a phosphorus atom therein include short chain alkyl or cycloalkyl intemucleoside linkages, mixed heteroatom and alkyl or cycloalkyl intemucleoside linkages, or one or more short chain heteroatomic or heterocyclic intemucleoside linkages. These include siloxane, sulfide, sulfoxide, sulfone, formacetal, thioformacetal, methylene formacetal, thioformacetal, alkenyl, sulfamate, methyleneimino, methylenehydrazino, sulfonate, sulfonamide, amide and others having mixed N, O, S and CH component parts.
[0070] Representative United States patents that teach the preparation of the above ohgonucleosides include, but are not limited to, U.S.: 5,034,506; 5,166,315; 5,185,444; 5,214,134; 5,216,141; 5,235,033; 5,264,562; 5,264,564; 5,405,938; 5,434,257; 5,466,677; 5,470,967; 5,489,677; 5,541,307; 5,561,225; 5,596,086; 5,602,240; 5,610,289; 5,602,240; 5,608,046; 5,610,289; 5,618,704; 5,623,070; 5,663,312; 5,633,360; 5,677,437; 5,792,608; 5,646,269 and 5,677,439, certain of which are commonly owned with this application, and each of which is herein incorporated by reference.
[0071] Some preferred embodiments of the invention are oligomeric compounds with phosphorothioate intemucleoside linkages and oligomeric compounds with heteroatom intemucleoside linkages, and in particular -CH2-NH-O-CH2-, -CH2- N(CH3)-O-CH2- [known as a methylene (methylimino) or MMI backbone], -CH2- O-N(CH3)-CH2-, -CH2-N(CH3)-N(CH3)-CH2- and -O-N(CH3)-CH2-CH2- [wherein
the native phosphodiester backbone is represented as -O-P(=O)(OH)-O-CH2-] of the above referenced U.S. patent 5,489,677, and the amide intemucleoside linkages of the above referenced U.S. patent 5,602,240.
[0072] The cyclopentane compositions can be synthesized as described in U.S.
Patent No. 5,602,240.
[0073] Other compounds of the invention are of the formula:
Bx is a heterocyclic base moiety;
Q is S, O, NH, N(Cι-C6 alkyl), CH2, CHF, or CF2;
R8 is a sugar substituent;
R83 and R85 are each independently OH, a protected hydroxyl group, an intemucleoside linkage to an adjacent monomer, or a terminal group; and
Rsr, R83'. R-84 and R85' are each independently H, alkyl, aralkyl, or aryl. [0074] In cetain embodiments, R82 is H, OH, alkoxy, aralkoxy or aryloxy; In other embodiments, at least one of R8r, R83>, R8 and R85> is other than H. Methods of synthesis of these monomers can be found in U.S. Patent Nos. 5,681,940 and 5,712,378, the disclosures of which are incorporated in their entirety. [0075] Compounds of the invention also include proline oligomers. These oligomeric compositions comprise one or more monomeric subunits of structure I:
Z is L8, L8 -Gi, L9, L9 -G, NR23R2 , a nitrogen-containing heterocycle, a purine, a pyrimidine, a phosphate group, a polyether group, or a polyethylene glycol group;
L8 is Cι-C20 alkyl, C2-C20 alkenyl, or C2-C20 alkynyl;
L9 is C6-Cι4 aryl or C7-Cι5 aralkyl;
Gi is halogen, OR2ι, SR22, NR23R24, C(=NH)NR23R24, NHC(=NH)NR23R24,
CH=O, C(=O)OR25, CH(NR23 R24)(C(=O)OR25), C(=O)NR23R24, a metal coordination group, or a phosphate group;
G2 is halogen, OH, SH, SCH3, or NR23R24;
R2ι is H, Cι-C6 alkyl, or a hydroxyl protecting group;
R22 is H, Cι-C6 alkyl, or a thiol protecting group;
R 3 and, R24 are, independently, H, Cι-C6 alkyl, or an amine protecting group;
R25 is H, Cι-C6 alkyl, or an acid protecting group;
Q is Li, G3, Li -G3 or G3 -Li -G3 ;
G3 is C(=O), C(=S), C(O)-O, C(O)-NH, C(S)-O, C(S)~NH or S(O)2; and n is O or 1.
[0076] In certain prefened embodiments, n is 1 and Q is carbonyl, thiocarbonyl, carboxy, acetyl or succinyl.
[0077] In one prefened group of compounds, Z includes a nitrogen-containing heterocycle such as an imidazole, pynole or carbazole ring. In a further prefened group, Z includes a purine or a pyrimidine nucleobase such as adenine, guanine, cytosine, uridine or thymine. In another prefened group of compounds, Z includes an unsubstituted or amine-substituted alkyl group, or an aryl group having 6 to
about 20 carbon atoms. In yet another prefened groups of compounds, Z includes fluorenylmethyl, phenyl, benzyl, alkyl-substituted benzyl, polyethylene glycol, glutamyl, or NR23R24 groups.
[0078] Further compounds of the invention include oligomeric compounds of structure II:
wherein: X is H, a phosphate group, an activated phosphate group, an activated phosphite group, a solid support, a conjugate group, or an oligonucleotide;
Y is H, a hydroxyl protecting group, a conjugate group or an oligonucleotide;
E is O or S;
Z is L8, L8 -Gi, L9, L9 -G , NR2 R 4, a nitrogen-containing heterocycle, a purine, a pyrimidine, a phosphate group, a polyether group, or a polyethylene glycol group;
L8 is Cι-C20 alkyl, C2-C20 alkenyl, or C2-C20 alkynyl;
L9 is C6-Cι4 aryl or C7-Cι5 aralkyl;
Gi is halogen, OR2j, SR22, NR23R24, C(=NH)NR23R24, NHC(-NH)NR23R24,
CH=O, C(=O)OR25, CH(NR23R24)(C(=O)OR25), C(=O)NR23R24, a metal coordination group, or a phosphate group;
G2 is halogen, OH, SH, SCH3, or NR23R24 ;
R2ι is H, Ci-Cβ alkyl, or a hydroxyl protecting group;
R22 is H, Cι-C6 alkyl, or a thiol protecting group;
R23 and R24 are, independently, H, Cι-C6 alkyl, or an amine protecting group;
R25 is H, Cι-C6 alkyl, or an acid protecting group;
Q is Li, G3, -G3 or G3 -Li -G3 ;
Li is Cι-C2o alkyl, C2-C20 alkenyl, or C2-C20 alkynyl;
G3 is C(=O), C(=S), C(O)-O, C(O)-NH, C(S)-O, C(S)-NH or S(O)2 ; n is O or 1; and m is 1 to about 50, preferably 1 to about 25.
[0079] U.S. Patent Nos. 5,519,134 and 5,714,606 describe synthesis of proline based compositions.
[0080] In some embodiments, A compound represented by one of the formula:
where:
G and K are each, independently, CR3A or N;
J is N or CR3B ;
Ri"isOHorNH2;
, 3A. 3B. and R3 » are H, NH2, lower alkyl, substituted lower alkyl, lower alkenyl, aralkyl, alkylamino, aralkylamino, substituted alkylamino, heterocycloalkyl, heterocycloalkylamino, aminoalkylamino, polyalkylamino, or a RNA cleaving moiety;
R4" and R5» are H, OH, NH2, lower alkyl, substituted lower alkyl, substituted amino, or a RNA cleaving moiety;
R6» and R7- are H, OH, NH2, SH, halogen, C(O)NH2, C(NH)NH2, C(O)O- alkyl, C(S)NH2, CN, C(NH)NHOH, lower alkyl, substituted lower alkyl, substituted amino, or a RNA cleaving moiety;
X is represented by one of the formulas:
Q is O or CHRn-;
R8» and R9" are H, lower alkyl, substituted lower alkyl, or a RNA cleaving moiety;
Rio- is H, OH, lower alkyl, substituted lower alkyl, F, CI, Br, CN, CF3, OCF3, OCN, O-alkyl, S-alkyl, SOMe, SO2Me, ONO2, NO2, N3, NH2, NH-alkyl, OCH2CH=CH2, OCH=CH2, OCH2C^CH, OC=CH, aralkyl, heteroaralkyl, heterocycloalkyl, aminoalkylamino, heterocycloalkyl, polyalkylamino, substituted silyl, or a RNA cleaving moiety; and
Rιι» is H, OH lower alkyl, substituted lower alkyl, or a RNA cleaving moiety.
[0081] In certain embodiments, when said compound is represented by Formula 4 and when G is N and Rio" is H or OH, then R2» is not H; when said compound is represented by Formula 6 and R6» is H and R2» is NH2 and R7» is C(O)NH2, C(S)NH2, C(O)O-alkyl, C(NH)NH2 or C(NH)NHOH then Rio" is not H or OH;
when said compound is represented by Formula 6 and R6" is H, OH or SH and Rr> is C(O)O-alkyl or C(NH)NH2 and R2- is ~CH2CN then Rι0» is not H or OH; and/or when said compound is represented by Formula 7 and R3» is H and G is C, Rio" is not H or OH. Additional preferences can be found in U.S. Patent No. 6,262,241, the disclosure of which is incorporated herein in its entirety. [0082] The invention also relates to 4'-thioribonucleosides. These compounds may have the natural anomeric configuration beta (b) or the non-natural anomeric configuration alpha (a).
where Bx is a heterocycle base moiety.
[0083] Hexose nucleosides are described in U.S. Patent No. 5,607,922, the disclosure of which is incorporated herein in its entirety. Compositions with hexose nucleosides can use any linking groups described herein. In certain embodiments, the invention is directed to oligonucleotides that contain at least one intemucleoside linkage comprised of a hexose sugar and an amide as described, for example, in U.S. Patent No. 5,780,607, hereby incorporated by reference in its entirety. In certain prefened embodiments, such oligonucleotides have the following formula:
wherein Bx is a heterocyclic nucleobase, R
95 is H, a hydroxyl protecting group, an intemucleoside linkage to an adjacent monomer, or a terminal group, and X
7 is a H or a sugar substitutent. In some embodiments, the sugar substituent is azido, F, CI, I, amino, -NHR
96, -N(R
96)
2, -OR
96, -SR
96, or CN where R
96 is Cι-C
20 alkyl, C
2- C
2o alkenyl, aroyl, Cι-C
2o alkanoyl, and phosphoryl.
[0084] Cyclohexenyl nucleic acids (CeNA) are compositions where the furanose ring normally present in an DNA/RNA molecule is replaced with a cyclohexenyl ring. CeNA DMT protected phosphoramidite monomers have been prepared and used for oligomeric compound synthesis following classical phosphoramidite chemistry. Fully modified CeNA oligomeric compounds and oligomers having specific positions modified with CeNA have been prepared and studied (see Wang et al, J. Am. Chem. Soc, 2000, 122, 8595-8602 and PCT Patent Application WO 01/49687). In general the incorporation of CeNA monomers into a DNA chain increases its stability of a DNA/RNA hybrid. CeNA oligoadenylates formed complexes with RNA and DNA complements with similar stability to the native complexes. The study of incorporating CeNA stractures into natural nucleic acid structures was shown by NMR and circular dichroism to proceed with easy conformational adaptation. Furthermore the incorporation of CeNA into a sequence targeting RNA was stable to serum and able to activate E. Coli RNase resulting in cleavage of the target RNA strand. [0085] The general formula of CeNA is shown by the following structures.

wherein each Bx is a heterocyclic base moiety;
Ti is hydroxyl or a protected hydroxyl; and
T2 is hydroxyl or a protected hydroxyl. [0086] U.S. Patent No. 5,591,722, which is incorporated herein by reference, discloses 2'-deoxy-4'-thioribonucleosides used as antiviral agents. [0087] hi some aspects, the present invention concerns compounds that include one or more 4-ribonucleoside or 2'-deoxy-4'-ribonucleoside. 4-Ribonucleoside and 2'-deoxy-4'-ribonucleoside compositions may be made by the method taught in U.S. Patent No. 5,639,873, which is incorporated by reference herein in its entirety.
[0088] Sugar surrogates may also be acyclic. In some embodiments, the sugar suπogate is a phosphoramidite derivative. Certain phosphoramidite derivatives are of the formula:
where X is a conjugate and Y is a protecting group. Suitable protecting groups include 4,4'-dimethoxytrityl, trifluoroacetyl and fluorenylmethoxycarbonyl (Fmoc). In some embodiments, the conjugate is biotin. Such compositions may comprise monomer units of the formula:
These compositions can be made by methods taught in U.S. Patent No. 5,567,811, which is incorporated by reference herein in its entirety. [0089] Other acyclic sugar sunogates include compounds of the formula:
where R
2» is hydrogen, nitro, lower alkyl amino, diloweralkyl amino or methyl; X is oxygen, sulfur, or ~NR
6» ; R
6" is hydrogen or lower alkyl; Q is a heterocyclcic nucleobase and, in some embodiments, is chosen from the group consisting of
where R
7» is lower-alkyl or loweralkyloxy methylene; and R
8" is hydrogen, benzoyl, anisoyl, or lower-alkyl carbonyl and its pharmaceutically acceptable addition salts are nucleotide analogs. These composition may be synthesized by methods taught by U.S. Patent No. 5,576,427, which is incorporated by reference herein in its entirety.
[0090] Further compounds of the invention include chimeric oligomeric compounds having a central region comprising a phosphodiester or a phosphorothioate oligodeoxynucleotide interspaced between flanking regions comprising the above-described monomeric or oligomeric structures. [0091] The invention further includes processes for preparing randomized oligomeric compounds including the steps of selecting a group of monomers as described above and covalently bonding at least two of the monomers of said group. In prefened processes, the Z moiety of at least one monomer of said group is different from the Z moiety of another monomer of said group.
Hybridization
[0092] In the context of this invention, "hybridization" means the pairing of
complementary strands of oligomeric compounds, hi the present invention, the prefened mechanism of pairing involves hydrogen bonding, which may be Watson-Crick, Hoogsteen or reversed Hoogsteen hydrogen bonding, between complementary nucleoside or nucleotide bases (nucleobases) of the strands of oligomeric compounds. For example, adenine and thymine are complementary nucleobases that pair through the formation of hydrogen bonds. Hybridization can occur under varying circumstances.
[0093] An oligomeric compound of the invention is believed to specifically hybridize to the target nucleic acid and interfere with its normal function to cause a loss of activity. There is preferably a sufficient degree of complementarity to avoid non-specific binding of the oligomeric compound to non-target nucleic acid sequences under conditions in which specific binding is desired, i.e., under physiological conditions in the case of in vivo assays or therapeutic treatment, and under conditions in which assays are performed in the case of in vitro assays. [0094] In the context of the present invention the phrase "stringent hybridization conditions" or "stringent conditions" refers to conditions under which an oligomeric compound of the invention will hybridize to its target sequence, but to a minimal number of other sequences. Stringent conditions are sequence- dependent and will vary with different circumstances and in the context of this invention; "stringent conditions" under which oligomeric compounds hybridize to a target sequence are detennined by the nature and composition of the oligomeric compounds and the assays in which they are being investigated. [0095] "Complementary," as used herein, refers to the capacity for precise pairing of two nucleobases regardless of where the two are located. For example, if a nucleobase at a certain position of an oligomeric compound is capable of hydrogen bonding with a nucleobase at a certain position of a target nucleic acid, then the position of hydrogen bonding between the oligonucleotide and the target nucleic acid is considered to be a complementary position. The oligomeric compound and the target nucleic acid are complementary to each other when a sufficient number of complementary positions in each molecule are occupied by nucleobases that can hydrogen bond with each other. Thus, "specifically
hybridizable" and "complementary" are terms which are used to indicate a sufficient degree of precise pairing or complementarity over a sufficient number of nucleobases such that stable and specific binding occurs between the oligonucleotide and a target nucleic acid.
[0096] It is understood in the art that the sequence of the oligomeric compound need not be 100% complementary to that of its target nucleic acid to be specifically hybridizable. Moreover, an oligomeric compound may hybridize over one or more segments such that intervening or adjacent segments are not involved in the hybridization event (e.g., a loop structure or hairpin structure). It is prefened that the oligomeric compounds of the present invention comprise at least 70% sequence complementarity to a target region within the target nucleic acid, more preferably that they comprise 90% sequence complementarity and even more preferably comprise 95% sequence complementarity to the target region within the target nucleic acid sequence to which they are targeted. For example, an oligomeric compound in which 18 of 20 nucleobases of the oligomeric compound are complementary to a target region, and would therefore specifically hybridize, would represent 90 percent complementarity. In this example, the remaining noncomplementary nucleobases may be clustered or interspersed with complementary nucleobases and need not be contiguous to each other or to complementary nucleobases. As such, an oligomeric compound which is 18 nucleobases in length having 4 (four) noncomplementary nucleobases which are flanked by two regions of complete complementarity with the target nucleic acid would have 77.8%) overall complementarity with the target nucleic acid and would thus fall within the scope of the present invention. Percent complementarity of an oligomeric compound with a region of a target nucleic acid can be determined routinely using BLAST programs (basic local alignment search tools) and PowerBLAST programs known in the art (Altschul et al., J. Mol. Biol., 1990, 215, 403-410; Zhang and Madden, Genome Res., 1997, 7, 649-656).
Targets of the invention
[0097] "Targeting" an oligomeric compound to a particular nucleic acid
molecule, in the context of this invention, can be a multistep process. The process usually begins with the identification of a target nucleic acid whose function is to be modulated. This target nucleic acid may be, for example, a mRNA transcribed from a cellular gene whose expression is associated with a particular disorder or disease state, or a nucleic acid molecule from an infectious agent. [0098] The targeting process usually also includes determination of at least one target region, segment, or site within the target nucleic acid for the interaction to occur such that the desired effect, e.g., modulation of expression, will result. Within the context of the present invention, the term "region" is defined as a portion of the target nucleic acid having at least one identifiable structure, function, or characteristic. Within regions of target nucleic acids are segments. "Segments" are defined as smaller or sub-portions of regions within a target nucleic acid. "Sites," as used in the present invention, are defined as positions within a target nucleic acid. The terms region, segment, and site can also be used to describe an oligomeric compound of the invention such as for example a gapped oligomeric compound having 3 separate segments. [0099] Since, as is known in the art, the translation initiation codon is typically 5'-AUG (in transcribed mRNA molecules; 5'-ATG in the conesponding DNA molecule), the translation initiation codon is also refened to as the "AUG codon," the "start codon" or the "AUG start codon". A minority of genes have a translation initiation codon having the RNA sequence 5'-GUG, 5'-UUG or 5'-CUG, and 5'-AUA, 5'- ACG and 5'-CUG have been shown to function in vivo. Thus, the terms "translation initiation codon" and "start codon" can encompass many codon sequences, even though the initiator amino acid in each instance is typically methionine (in eukaryotes) or formylmethionine (in prokaryotes). It is also known in the art that eukaryotic and prokaryotic genes may have two or more alternative start codons, any one of which may be preferentially utilized for translation initiation in a particular cell type or tissue, or under a particular set of conditions. In the context of the invention, "start codon" and "translation initiation codon" refer to the codon or codons that are used in vivo to initiate translation of an mRNA transcribed from a gene encoding a nucleic acid target,
regardless of the sequence(s) of such codons. It is also known in the art that a translation termination codon (or "stop codon") of a gene may have one of tliree sequences, i.e., 5'-UAA, 5'-UAG and 5'-UGA (the conesponding DNA sequences are 5'-TAA, 5'-TAG and 5'-TGA, respectively).
[0100] The terms "start codon region" and "translation initiation codon region" refer to a portion of such an mRNA or gene that encompasses from about 25 to about 50 contiguous nucleotides in either direction (i.e., 5' or 3') from a translation initiation codon. Similarly, the terms "stop codon region" and "translation teπnination codon region" refer to a portion of such an mRNA or gene that encompasses from about 25 to about 50 contiguous nucleotides in either direction (i.e., 5' or 3') from a translation termination codon. Consequently, the "start codon region" (or "translation initiation codon region") and the "stop codon region" (or "translation termination codon region") are all regions which may be targeted effectively with the antisense oligomeric compounds of the present invention. [0101] The open reading frame (ORF) or "coding region," which is known in the art to refer to the region between the translation initiation codon and the translation temiination codon, is also a region which may be targeted effectively. Within the context of the present invention, a prefened region is the intragenic region encompassing the translation initiation or teπnination codon of the open reading frame (ORF) of a gene.
[0102] Other target regions include the 5' untranslated region (5'UTR), known in the art to refer to the portion of an mRNA in the 5' direction from the translation initiation codon, and thus including nucleotides between the 5' cap site and the translation initiation codon of an mRNA (or conesponding nucleotides on the gene), and the 3' untranslated region (3'UTR), known in the art to refer to the portion of an mRNA in the 3' direction from the translation termination codon, and thus including nucleotides between the translation termination codon and 3' end of an mRNA (or conesponding nucleotides on the gene). The 5' cap site of an mRNA comprises an N7-methylated guanosine residue joined to the 5 '-most residue of the mRNA via a 5'-5' triphosphate linkage. The 5' cap region of an mRNA is considered to include the 5' cap structure itself as well as the first 50
nucleotides adjacent to the cap site. It is also prefened to target the 5' cap region. [0103] Although some eukaryotic mRNA transcripts are directly translated, many contain one or more regions, known as "introns," which are excised from a transcript before it is translated. The remaining (and therefore translated) regions are known as "exons" and are spliced together to form a continuous mRNA sequence. Targeting splice sites, i.e., intron-exon junctions or exon-intron junctions, may also be particularly useful in situations where abenant splicing is implicated in disease, or where an overproduction of a particular splice product is implicated in disease. Abenant fusion junctions due to reanangements or deletions are also prefened target sites. mRNA transcripts produced via the process of splicing of two (or more) mRNAs from different gene sources are known as "fusion transcripts". It is also known that introns can be effectively targeted using oligomeric compounds targeted to, for example, pre-mRNA. [0104] It is also known in the art that alternative RNA transcripts can be produced from the same genomic region of DNA. These alternative transcripts are generally known as "variants". More specifically, "pre-mRNA variants" are transcripts produced from the same genomic DNA that differ from other transcripts produced from the same genomic DNA in either their start or stop position and contain both intronic and exonic sequences.
[0105] Upon excision of one or more exon or intron regions, or portions thereof during splicing, pre-mRNA variants produce smaller "mRNA variants". Consequently, mRNA variants are processed pre-mRNA variants and each unique pre-mRNA variant must always produce a unique mRNA variant as a result of splicing. These mRNA variants are also known as "alternative splice variants". If no splicing of the pre-mRNA variant occurs then the pre-mRNA variant is identical to the mRNA variant.
[0106] It is also known in the art that variants can be produced through the use of alternative signals to start or stop transcription and that pre-mRNAs and mRNAs can possess more that one start codon or stop codon. Variants that originate from a pre-mRNA or mRNA that use alternative start codons are known as "alternative start variants" of that pre-mRNA or mRNA. Those transcripts that
use an alternative stop codon are known as "alternative stop variants" of that pre- mRNA or mRNA. One specific type of alternative stop variant is the "polyA variant" in which the multiple transcripts produced result from the alternative selection of one of the "polyA stop signals" by the transcription machinery, thereby producing transcripts that terminate at unique polyA sites. Within the context of the invention, the types of variants described herein are also prefened target nucleic acids.
[0107] The locations on the target nucleic acid to which prefened compounds and compositions of the invention hybridize are herein below refened to as "prefened target segments." As used herein the term "prefened target segment" is defined as at least an 8-nucleobase portion of a target region to which an active antisense oligomeric compound is targeted. While not wishing to be bound by theory, it is presently believed that these target segments represent portions of the target nucleic acid that are accessible for hybridization.
[0108] Once one or more target regions, segments or sites have been identified, oligomeric compounds are chosen which are sufficiently complementary to the target, i.e., hybridize sufficiently well and with sufficient specificity, to give the desired effect.
[0109] In accordance with an embodiment of the this invention, a series of nucleic acid duplexes comprising the antisense strand oligomeric compounds of the present invention and their representative complement sense strand compounds can be designed for a specific target or targets. The ends of the strands may be modified by the addition of one or more natural or modified nucleobases to form an overhang. The sense strand of the duplex is designed and synthesized as the complement of the antisense strand and may also contain modifications or additions to either terminus. For example, in one embodiment, both strands of the duplex would be complementary over the central nucleobases, each having overhangs at one or both termini.
[0110] For the purposes of describing an embodiment of this invention, the combination of an antisense strand and a sense strand, each of can be of a specified length, for example from 18 to 29. nucleotides long, is identified as a
complementary pair of siRNA oligonucleotides. This complementary pair of siRNA oligonucleotides can include additional nucleotides on either of their 5' or
3' ends. Further they can include other molecules or molecular structures on their
3' or 5' ends such as a phosphate group on the 5' end. A prefened group of compounds of the invention include a phosphate group on the 5' end of the antisense strand compound. Other prefened compounds also include a phosphate group on the 5' end of the sense strand compound. An even further prefened compounds would include additional nucleotides such as a two base overhang on the 3' end.
[0111] For example, a prefened siRNA complementary pair of oligonucleotides comprise antisense strand oligomeric compound having the sequence CGAGAGGCGGACGGGACC
(SEQ ID NO:l) and having a two-nucleobase overhang of deoxythymidine(dT) and its complement sense strand. These oligonucleotides would have the following structure:
5' c g a g a g g c g g a c g g g a c c g T T 3' Antisense Strand (SEQ ID NO:;
3' T T g c t c t c c g c c t g c c c t g g c 5' Complement Strand (SEQ ID N
[0112] In an additional embodiment of the invention, a single oligonucleotide having both the antisense portion as a first region in the oligonucleotide and the sense portion as a second region in the oligonucleotide is selected. The first and second regions are linked together by either a nucleotide linker (a string of one or more nucleotides that are linked together in a sequence) or by a non-nucleotide linker region or by a combination of both a nucleotide and non-nucleotide structure. In each of these stractures, the oligonucleotide, when folded back on itself, would be complementary at least between the first region, the antisense portion, and the second region, the sense portion. Thus the oligonucleotide would have a palindrome within it structure wherein the first region, the antisense portion in the 5' to 3' direction, is complementary to the second region, the sense portion in the 3' to 5' direction.
[0113] In a further embodiment, the invention includes oligonucleotide/protein compositions. Such compositions have both an oligonucleotide component and a
protein component. The oligonucleotide component comprises at least one oligonucleotide, either the antisense or the sense oligonucleotide but preferably the antisense oligonucleotide (the oligonucleotide that is antisense to the target nucleic acid). The oligonucleotide component can also comprise both the antisense and the sense strand oligonucleotides. The protein component of the composition comprises at least one protein that forms a portion of the RNA- induced silencing complex, i.e., the RISC complex.
[0114] RISC is a ribonucleoprotein complex that contains an oligonucleotide component and proteins of the Argonaute family of proteins, among others. While we do not wish to be bound by theory, the Argonaute proteins make up a highly conserved family whose members have been implicated in RNA interference and the regulation of related phenomena. Members of this family have been shown to possess the canonical PAZ and Piwi domains, thought to be a region of protein-protein interaction. Other proteins containing these domains have been shown to effect target cleavage, including the RNAse, Dicer. The Argonaute family of proteins includes, but depending on species, are not necessary limited to, elF2Cl and elF2C2. elF2C2 is also known as human GERp95. While we do not wish to be bound by theory, at least the antisense oligonucleotide strand is bound to the protein component of the RISC complex. Additional, the complex might also include the sense strand oligonucleotide. Carmell et al, Genes and Development 2002, 16, 2733-2742. [0115] Also while we do not wish to be bound by theory, it is further believe that the RISC complex may interact with one or more of the translation machinery components. Translation machinery components include but are not limited to proteins that effect or aid in the translation of an RNA into protein including the ribosomes or polyribosome complex. Therefore, in a further embodiment of the invention, the oligonucleotide component of the invention is associated with a RISC protein component and further associates with the translation machinery of a cell. Such interaction with the translation machinery of the cell would include interaction with structural and enzymatic proteins of the translation machinery including but not limited to the polyribosome and ribosomal subunits.
[0116] In a further embodiment of the invention, the oligonucleotide of the invention is associated with cellular factors such as transporters or chaperones. These cellular factors can be protein, lipid or carbohydrate based and can have stractural or enzymatic functions that may or may not require the complexation of one or more metal ions.
Furthermore, the oligonucleotide of the invention itself may have one or more moieties which are bound to the oligonucleotide which facilitate the active or passive transport, localization or compartmentalization of the oligonucleotide. Cellular localization includes, but is not limited to, localization to within the nucleus, the nucleolus or the cytoplasm. Compartmentalization includes, but is not limited to, any directed movement of the oligonucleotides of the invention to a cellular compartment including the nucleus, nucleolus, mitochondrion, or imbedding into a cellular membrane sunounding a compartment or the cell itself. [0117] In a further embodiment of the invention, the oligonucleotide of the invention is associated with cellular factors that affect gene expression, more specifically those involved in RNA modifications. These modifications include, but are not limited to posttrascriptional modifications such as methylation. Furthermore, the oligonucleotide of the invention itself may have one or more moieties which are bound to the oligonucleotide which facilitate the posttranscriptional modification.
[0118] The oligomeric compounds of the invention may be used in the form of single-stranded, double-stranded, circular or hairpin oligomeric compounds and may contain stractural elements such as internal or terminal bulges or loops. Once introduced to a system, the oligomeric compounds of the invention may interact with or elicit the action of one or more enzymes or may interact with one or more structural proteins to effect modification of the target nucleic acid. [0119] One non-limiting example of such an interaction is the RISC complex. Use of the RISC complex to effect cleavage of RNA targets thereby greatly enhances the efficiency of oligonucleotide-mediated inhibition of gene expression. Similar roles have been postulated for other ribonucleases such as those in the RNase III and ribonuclease L family of enzymes.
[0120] Prefened fonns of oligomeric compound of the invention include a single-stranded antisense oligonucleotide that binds in a RISC complex, a double stranded antisense/sense pair of oligonucleotide or a single strand oligonucleotide that includes both an antisense portion and a sense portion. Each of these compounds or compositions is used to induce potent and specific modulation of gene function. Such specific modulation of gene function has been shown in many species by the introduction of double-stranded stractures, such as double- stranded RNA (dsRNA) molecules and has been shown to induce potent and specific antisense-mediated reduction of the function of a gene or its associated gene products. This phenomenon occurs in both plants and animals and is believed to have an evolutionary connection to viral defense and transposon silencing.
[0121] The compounds and compositions of the invention are used to modulate the expression of a target nucleic acid. "Modulators" are those oligomeric compounds that decrease or increase the expression of a nucleic acid molecule encoding a target and which comprise at least an 8-nucleobase portion that is complementary to a prefened target segment. The screening method comprises the steps of contacting a prefened target segment of a nucleic acid molecule encoding a target with one or more candidate modulators, and selecting for one or more candidate modulators which decrease or increase the expression of a nucleic acid molecule encoding a target. Once it is shown that the candidate modulator or modulators are capable of modulating (e.g. either decreasing or increasing) the expression of a nucleic acid molecule encoding a target, the modulator may then be employed in further investigative studies of the function of a target, or for use as a research, diagnostic, or therapeutic agent in accordance with the present invention.
Oligomeric Compounds
[0122] In the context of the present invention, the term "oligomeric compound" refers to a polymeric structure capable of hybridizing a region of a nucleic acid molecule. This term includes oligonucleotides, ohgonucleosides, oligonucleotide analogs, oligonucleotide mimetics and combinations of these. Oligomeric
compounds routinely prepared linearly but can be joined or otherwise prepared to be circular and may also include branching. Oligomeric compounds can hybridized to form double stranded compounds that can be blunt ended or may include overhangs. In general an oligomeric compound comprises a backbone of linked momeric subunits where each linked momeric subunit is directly or indirectly attached to a heterocyclic base moiety. The linkages joining the monomeric subunits, the sugar moieties or suπogates and the heterocyclic base moieties can be independently modified giving rise to a plurality of motifs for the resulting oligomeric compounds including hemimers, gapmers and chimeras. [0123] As is known in the art, a nucleoside is a base-sugar combination. The base portion of the nucleoside is normally a heterocyclic base moiety. The two most common classes of such heterocyclic bases are purines and pyrimidines. Nucleotides are nucleosides that further include a phosphate group covalently linked to the sugar portion of the nucleoside. For those nucleosides that include a pentofuranosyl sugar, the phosphate group can be linked to either the 2', 3' or 5' hydroxyl moiety of the sugar. In forming oligonucleotides, the phosphate groups covalently link adjacent nucleosides to one another to form a linear polymeric compound. The respective ends of this linear polymeric structure can be joined to form a circular stracture by hybridization or by formation of a covalent bond, however, open linear structures are generally prefened. Within the oligonucleotide stracture, the phosphate groups are commonly refened to as foπning the intemucleoside linkages of the oligonucleotide. The normal intemucleoside linkage of RNA and DNA is a 3' to 5' phosphodiester linkage. [0124] In the context of this invention, the term "oligonucleotide" refers to an oligomer or polymer of ribonucleic acid (RNA) or deoxyribonucleic acid (DNA). This term includes oligonucleotides composed of naturally-occuning nucleobases, sugars and covalent intemucleoside linkages. The term "oligonucleotide analog" refers to oligonucleotides that have one or more non-naturally occurring portions which function in a similar manner to oligonulceotides. Such non-naturally occurring oligonucleotides are often prefened the naturally occurring forms because of desirable properties such as, for example, enhanced cellular uptake,
enhanced affinity for nucleic acid target and increased stability in the presence of nucleases.
[0125] In the context of this invention, the term " oligonucleoside" refers to nucleosides that are joined by intemucleoside linkages that do not have phosphorus atoms. Intemucleoside linkages of this type include short chain alkyl, cycloalkyl, mixed heteroatom alkyl, mixed heteroatom cycloalkyl, one or more short chain heteroatomic and one or more short chain heterocyclic. These intemucleoside linkages include but are not limited to siloxane, sulfide, sulfoxide, sulfone, acetal, formacetal, thioformacetal, methylene formacetal, thioformacetal, alkeneyl, sulfamate; methyleneimino, methylenehydrazino, sulfonate, sulfonamide, amide and others having mixed N, O, S and CH2 component parts. [0126] In addition to the modifications described above, the nucleosides of the oligomeric compounds of the invention can have a variety of other modification so long as these other modifications either alone or in combination with other nucleosides enhance one or more of the desired properties described above. Thus, for nucleotides that are incorporated into oligonucleotides of the invention, these nucleotides can have sugar portions that conespond to naturally-occurring sugars or modified sugars. Representative modified sugars include carbocyclic or acyclic sugars, sugars having substituent groups at one or more of their 2', 3' or 4' positions and sugars having substituents in place of one or more hydrogen atoms of the sugar. Additional nucleosides amenable to the present invention having altered base moieties and or altered sugar moieties are disclosed in United States Patent 3,687,808 and PCT application PCT/US 89/02323. [0127] Altered base moieties or altered sugar moieties also include other modifications consistent with the spirit of this invention. Such oligonucleotides are best described as being structurally distinguishable from, yet functionally interchangeable with, naturally occurring or synthetic wild type oligonucleotides. All such oligonucleotides are comprehended by this invention so long as they function effectively to mimic the structure of a desired RNA or DNA strand. A class of representative base modifications include tricyclic cytosine analog, termed "G clamp" (Lin, et al, J. Am. Chem. Soc. 1998, 120, 8531). This analog
makes four hydrogen bonds to a complementary guanine (G) within a helix by simultaneously recognizing the Watson-Crick and Hoogsteen faces of the targeted G. This G clamp modification when incorporated into phosphorothioate oligonucleotides, dramatically enhances antisense potencies in cell culture. The oligonucleotides of the invention also can include phenoxazine-substituted bases of the type disclosed by Flanagan, et al, Nat. Biotechnol 1999, 17(1), 48-52. [0128] The oligomeric compounds in accordance with this invention preferably comprise from about 8 to about 80 nucleobases (i.e. from about 8 to about 80 linked nucleosides). One of ordinary skill in the art will appreciate that the invention embodies oligomeric compounds of 8, 9, 10, 11, 12, 13, 14, 15, 16, 17,
18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, or 80 nucleobases in length.
[0129] In one prefened embodiment, the oligomeric compounds of the invention are 12 to 50 nucleobases in length. One having ordinary skill in the art will appreciate that this embodies oligomeric compounds of 12, 13, 14, 15, 16, 17, 18,
19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, or 50 nucleobases in length.
[0130] In another prefened embodiment, the oligomeric compounds of the invention are 15 to 30 nucleobases in length. One having ordinary skill in the art will appreciate that this embodies oligomeric compounds of 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, or 30 nucleobases in length.
[0131] Particularly prefened oligomeric compounds are oligonucleotides from about 12 to about 50 nucleobases, even more preferably those comprising from about 15 to about 30 nucleobases.
General Oligomer Synthesis
[0132] Oligomerization of modified and unmodified nucleosides is performed according to literature procedures for DNA like compounds (Protocols for
Oligonucleotides and Analogs, Ed. Agrawal (1993), Humana Press) and/or RNA like compounds (Scaringe, Methods (2001), 23, 206-217. Gait et al., Applications of Chemically synthesized RNA in RNA:Protein Interactions, Ed. Smith (1998), 1-36. Gallo et al., Tetrahedron (2001), 57, 5707-5713) synthesis as appropriate. In addition specific protocols for the synthesis of oligomeric compounds of the invention are illustrated in the examples below.
[0133] RNA oligomers can be synthesized by methods disclosed herein or purchased from various RNA synthesis companies such as for example Dharmacon Research Inc., (Lafayette, CO).
[0134] Inespective of the particular protocol used, the oligomeric compounds used in accordance with this invention may be conveniently and routinely made through the well-known technique of solid phase synthesis. Equipment for such synthesis is sold by several vendors including, for example, Applied Biosystems (Foster City, CA). Any other means for such synthesis known in the art may additionally or alternatively be employed.
[0135] For double stranded stractures of the invention, once synthesized, the complementary strands preferably are annealed. The single strands are aliquoted and diluted to a concentration of 50 uM. Once diluted, 30 uL of each strand is combined with 15uL of a 5X solution of annealing buffer. The final concentration of the buffer is 100 mM potassium acetate, 30 mM HEPES-KOH pH 7.4, and 2mM magnesium acetate. The final volume is 75 uL. This solution is incubated for 1 minute at 90°C and then centrifuged for 15 seconds. The tube is allowed to sit for 1 hour at 37°C at which time the dsRNA duplexes are used in experimentation. The final concentration of the dsRNA compound is 20 uM. This solution can be stored frozen (-20°C) and freeze-thawed up to 5 times. [0136] Once prepared, the desired synthetic duplexes are evaluated for their ability to modulate target expression. When cells reach 80% confluency, they are treated with synthetic duplexes comprising at least one oligomeric compound of the invention. For cells grown in 96-well plates, wells are washed once with 200 μL OPTI-MEM-1 reduced-serum medium (Gibco BRL) and then treated with 130 μL of OPTI-MEM-1 containing 12 μg/mL LIPOFECTIN (Gibco BRL) and the
desired dsRNA compound at a final concentration of 200 nM. After 5 hours of treatment, the medium is replaced with fresh medium. Cells are harvested 16 hours after treatment, at which time RNA is isolated and target reduction measured by RT-PCR.
Oligomer and Monomer Modifications
[0137] As is known in the art, a nucleoside is a base-sugar combination. The base portion of the nucleoside is normally a heterocyclic base. The two most common classes of such heterocyclic bases are the purines and the pyrimidines. Nucleotides are nucleosides that further include a phosphate group covalently linked to the sugar portion of the nucleoside. For those nucleosides that include a pentofuranosyl sugar, the phosphate group can be linked to either the 2', 3' or 5' hydroxyl moiety of the sugar. In forming oligonucleotides, the phosphate groups covalently link adjacent nucleosides to one another to form a linear polymeric compound. In turn, the respective ends of this linear polymeric compound can be further joined to form a circular compound, however, linear compounds are generally prefened. In addition, linear compounds may have internal nucleobase complementarity and may therefore fold in a manner as to produce a fully or partially double-stranded compound. Within oligonucleotides, the phosphate groups are commonly refened to as forming the intemucleoside linkage or in conjunction with the sugar ring the backbone of the oligonucleotide. The normal intemucleoside linkage that makes up the backbone of RNA and DNA is a 3' to 5' phosphodiester linkage.
Modified Intemucleoside Linkages
[0138] Specific examples of prefened antisense oligomeric compounds useful in this invention include oligonucleotides containing modified e.g. non-naturally occurring intemucleoside linkages. As defined in this specification, oligonucleotides having modified intemucleoside linkages include intemucleoside linkages that retain a phosphorus atom and intemucleoside linkages that do not have a phosphoras atom. For the purposes of this specification, and as sometimes
referenced in the art, modified oligonucleotides that do not have a phosphoras atom in their intemucleoside backbone can also be considered to be ohgonucleosides.
[0139] In the C. elegans system, modification of the intemucleotide linkage (phosphorothioate) did not significantly interfere with RNAi activity. Based on this observation, it is suggested that certain prefened oligomeric compounds of the invention can also have one or more modified intemucleoside linkages. A prefened phosphorus containing modified intemucleoside linkage is the phosphorothioate intemucleoside linkage.
[0140] Prefened modified oligonucleotide backbones containing a phosphorus atom therein include, for example, phosphorothioates, chiral phosphorothioates, phosphorodithioates, phosphotriesters, aminoalkylphosphotriesters, methyl and other alkyl phosphonates including 3 '-alkylene phosphonates, 5 '-alkylene phosphonates and chiral phosphonates, phosphinates, phosphoramidates including 3 '-amino phosphoramidate and aminoalkylphosphoramidates, thionophosphoramidates, thionoalkylphosphonates, thionoalkylphosphotriesters, selenophosphates and boranophosphates having normal 3'-5' linkages, 2'-5' linked analogs of these, and those having inverted polarity wherein one or more intemucleotide linkages is a 3' to 3', 5' to 5' or 2' to 2' linkage. Prefened oligonucleotides having inverted polarity comprise a single 3' to 3' linkage at the 3'-most intemucleotide linkage i.e. a single inverted nucleoside residue which may be abasic (the nucleobase is missing or has a hydroxyl group in place thereof). Various salts, mixed salts and free acid forms are also included. [0141] Representative United States patents that teach the preparation of the above phosphorus-containing linkages include, but are not limited to, U.S.: 3,687,808; 4,469,863; 4,476,301; 5,023,243; 5,177,196; 5,188,897; 5,264,423; 5,276,019; 5,278,302; 5,286,717; 5,321,131; 5,399,676; 5,405,939; 5,453,496; 5,455,233; 5,466,677; 5,476,925; 5,519,126; 5,536,821; 5,541,306; 5,550,111; 5,563,253; 5,571,799; 5,587,361; 5,194,599; 5,565,555; 5,527,899; 5,721,218; 5,672,697 and 5,625,050, certain of which are commonly owned with this application, and each of which is herein incorporated by reference.
[0142] In more prefened embodiments of the invention, oligomeric compounds have one or more phosphorothioate and/or heteroatom intemucleoside linkages, in particular -CH2-NH-O-CH2-, -CH2-N(CH3)-O-CH2- [known as a methylene (methylimino) or MMI backbone], -CH2-O-N(CH3)-CH2-, -CH2-N(CH3)-N(CH3)- CH2- and -O-N(CH3)-CH2-CH2- [wherein the native phosphodiester intemucleotide linkage is represented as -O-P(=O)(OH)-O-CH2-]. The MMI type intemucleoside linkages are disclosed in the above referenced U.S. patent 5,489,677. Prefened amide intemucleoside linkages are disclosed in the above referenced U.S. patent 5,602,240.
[0143] Prefened modified oligonucleotide backbones that do not include a phosphorus atom therein have backbones that are formed by short chain alkyl or cycloalkyl intemucleoside linkages, mixed heteroatom and alkyl or cycloalkyl intemucleoside linkages, or one or more short chain heteroatomic or heterocyclic intemucleoside linkages. These include those having morpholino linkages (formed in part from the sugar portion of a nucleoside); siloxane backbones; sulfide, sulfoxide and sulfone backbones; formacetal and thioformacetal backbones; methylene formacetal and thioformacetal backbones; riboacetal backbones; alkene containing backbones; sulfamate backbones; methyleneimino and methylenehydrazino backbones; sulfonate and sulfonamide backbones; amide backbones; and others having mixed N, O, S and CH2 component parts. [0144] Representative United States patents that teach the preparation of the above ohgonucleosides include, but are not limited to, U.S.: 5,034,506; 5,166,315; 5,185,444; 5,214,134; 5,216,141; 5,235,033; 5,264,562; 5,264,564; 5,405,938; 5,434,257; 5,466,677; 5,470,967; 5,489,677; 5,541,307; 5,561,225; 5,596,086; 5,602,240; 5,610,289; 5,602,240; 5,608,046; 5,610,289; 5,618,704; 5,623,070; 5,663,312; 5,633,360; 5,677,437; 5,792,608; 5,646,269 and 5,677,439, certain of which are commonly owned with this application, and each of which is herein incorporated by reference.
Oligomer Mimetics
[0145] Another prefened group of oligomeric compounds amenable to the
present invention includes oligonucleotide mimetics. The term mimetic as it is applied to oligonucleotides is intended to include oligomeric compounds wherein only the furanose ring or both the furanose ring and the intemucleotide linkage are replaced with novel groups, replacement of only the furanose ring is also refened to in the art as being a sugar sunogate. The heterocyclic base moiety or a modified heterocyclic base moiety is maintained for hybridization with an appropriate target nucleic acid. One such oligomeric compound, an oligonucleotide mimetic that has been shown to have excellent hybridization properties, is refened to as a peptide nucleic acid (PNA). In PNA oligomeric compounds, the sugar-backbone of an oligonucleotide is replaced with an amide containing backbone, in particular an aminoethylglycine backbone. The nucleobases are retained and are bound directly or indirectly to aza nitrogen atoms of the amide portion of the backbone. Representative United States patents that teach the preparation of PNA oligomeric compounds include, but are not limited to, U.S.: 5,539,082; 5,714,331; and 5,719,262, each of which is herein incorporated by reference. Further teaching of PNA oligomeric compounds can be found in Nielsen et al, Science, 1991, 254, 1497-1500. [0146] One class of oligonucleotide mimetic that has been studied is based on linked morpholino units (morpholino nucleic acid) having heterocyclic bases attached to the morpholino ring. A number of linking groups have been reported that link the morpholino monomeric units in a morpholino nucleic acid. A prefened class of linking groups have been selected to give a non-ionic oligomeric compound. The non-ionic morpholino-based oligomeric compounds are less likely to have undesired interactions with cellular proteins. Morpholino-based oligomeric compounds are non-ionic mimics of oligonucleotides which are less likely to form undesired interactions with cellular proteins (Dwaine A. Braasch and David R. Corey, Biochemistry, 2002, 41(14), 4503-4510). Morpholino-based oligomeric compounds are disclosed in United States Patent 5,034,506, issued July 23, 1991. The morpholino class of oligomeric compounds have been prepared having a variety of different linking groups joining the monomeric subunits.
[0147] Morpholino nucleic acids have been prepared having a variety of different linking groups (L2) joining the monomeric subunits. The basic formula is shown below:
wherein
Bx is a heterocycle base moiety;
Ti is hydroxyl or a protected hydroxyl;
T5 is hydrogen or a phosphate or phosphate derivative;
L2 is a linking group; and n is from 2 to about 50.
[0148] A further prefened modification includes Locked Nucleic Acids (LNAs) in which the 2'-hydroxyl group is linked to the 4' carbon atom of the sugar ring thereby forming a 2'-C,4'-C-oxymethylene linkage thereby forming a bicyclic sugar moiety. The linkage is preferably a methylene (-CH
2-)
n group bridging the 2' oxygen atom and the 4' carbon atom wherein n is 1 or 2 (Singh et al., Chem. Commun., 1998, 4, 455-456). LNA and LNA analogs display very high duplex thermal stabilities with complementary DNA and RNA (Tm = +3 to +10 C), stability towards 3'-exonucleolytic degradation and good solubility properties. The basic stracture of LNA showing the bicyclic ring system is shown below:
[0149] The conformations of LNAs determined by 2D NMR spectroscopy have shown that the locked orientation of the LNA nucleotides, both in single-stranded LNA and in duplexes, constrains the phosphate backbone in such a way as to introduce a higher population of the N-type conformation (Petersen et al., J. Mol. Recognit., 2000, 13, 44-53). These conformations are associated with improved stacking of the nucleobases (Wengel et al., Nucleosides Nucleotides, 1999, 18, 1365-1370).
[0150] LNA has been shown to form exceedingly stable LNA:LNA duplexes (Koshkin et al., J. Am. Chem. Soc, 1998, 120, 13252-13253). LNA:LNA hybridization was shown to be the most thermally stable nucleic acid type duplex system, and the RNA-mimicking character of LNA was established at the duplex level. Introduction of 3 LNA monomers (T or A) significantly increased melting points (Tm = +15/+11) toward DNA complements. The universality of LNA- mediated hybridization has been stressed by the formation of exceedingly stable LNA:LNA duplexes. The RNA-mimicking of LNA was reflected with regard to the N-type conformational restriction of the monomers and to the secondary stracture of the LNA:RNA duplex.
[0151] LNAs also form duplexes with complementary DNA, RNA or LNA with high thermal affinities. Circular dichroism (CD) spectra show that duplexes involving fully modified LNA (esp. LNA:RNA) structurally resemble an A-form RNA:RNA duplex. Nuclear magnetic resonance (NMR) examination of an LNA:DNA duplex confirmed the 3'-endo conformation of an LNA monomer.
Recognition of double-stranded DNA has also been demonstrated suggesting strand invasion by LNA. Studies of mismatched sequences show that LNAs obey the Watson-Crick base pairing rules with generally improved selectivity compared to the conesponding unmodified reference strands.
[0152] Novel types of LNA-oligomeric compounds, as well as the LNAs, are useful in a wide range of diagnostic and therapeutic applications. Among these are antisense applications, PCR applications, strand-displacement oligomers, substrates for nucleic acid polymerases and generally as nucleotide based drugs. [0153] Potent and nontoxic antisense oligonucleotides containing LNAs have been described (Wahlestedt et al., Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 5633- 5638.) The authors have demonstrated that LNAs confer several desired properties to antisense agents. LNA/DNA copolymers were not degraded readily in blood serum and cell extracts. LNA/DNA copolymers exhibited potent antisense activity in assay systems as disparate as G-protein-coupled receptor signaling in living rat brain and detection of reporter genes in Escherichia coli. Lipofectin-mediated efficient delivery of LNA into living human breast cancer cells has also been accomplished.
[0154] The synthesis and preparation of the LNA monomers adenine, cytosine, guanine, 5-methyl-cytosine, thymine and uracil, along with their oligomerization, and nucleic acid recognition properties have been described (Koshkin et al., Tetrahedron, 1998, 54, 3607-3630). LNAs and preparation thereof are also described in WO 98/39352 and WO 99/14226.
[0155] The first analogs of LNA, phosphorothioate-LNA and 2'-thio-LNAs, have also been prepared (Kumar et al., Bioorg. Med. Chem. Lett., 1998, 8, 2219- 2222). Preparation of locked nucleoside analogs containing oligodeoxyribonucleotide duplexes as substrates for nucleic acid polymerases has also been described (Wengel et al, PCT International Application WO 98-DK393 19980914). Furthermore, synthesis of 2'-amino-LNA, a novel conformationally restricted high-affinity oligonucleotide analog with a handle has been described in the art (Singh et al., J. Org. Chem., 1998, 63, 10035-10039). In addition, 2*- A-mino- and 2'-methylamino-LNA's have been prepared and the thermal stability
of their duplexes with complementary RNA and DNA strands has been previously reported.
[0156] Further oligonucleotide mimetics have been prepared to incude bicyclic and tricyclic nucleoside analogs having the formulas (amidite monomers shown):
(see Steffens et al, Helv. Chim. Acta, 1997, 80, 2426-2439; Steffens et al, J. Am. Chem. Soc, 1999, 121, 3249-3255; and Renneberg et al, J. Am. Chem. Soc, 2002, 124, 5993-6002). These modified nucleoside analogs have been oligomerized using the phosphoramidite approach and the resulting oligomeric compounds contaimng tricyclic nucleoside analogs have shown increased thermal stabilities (Tm's) when hybridized to DNA, RNA and itself. Oligomeric compounds containing bicyclic nucleoside analogs have shown thermal stabilities approaching that of DNA duplexes.
[0157] Another class of oligonucleotide mimetic is refened to as phosphonomonoester nucleic acids incorporate a phosphoras group in a backbone the backbone. This class of oligonucleotide mimetic is reported to have useful physical and biological and pharmacological properties in the areas of inhibiting gene expression (antisense oligonucleotides, ribozymes, sense oligonucleotides and triplex-forming oligonucleotides), as probes for the detection of nucleic acids and as auxiliaries for use in molecular biology.
[0158] The general fonnula (for definitions of variables see: United States Patents 5,874,553 and 6,127,346 herein incorporated by reference in their entirety) is shown below.
[0159] Another oligonucleotide mimetic has been reported wherein the furanosyl ring has been replaced by a cyclobutyl moiety.
Modified sugars
[0160] Oligomeric compounds of the invention may also contain one or more substituted sugar moieties. Prefened oligomeric compounds comprise a sugar substituent group selected from: OH; F; O-, S-, or N-alkyl; O-, S-, or N-alkenyl; O-, S- or N-alkynyl; or O-alkyl-O-alkyl, wherein the alkyl, alkenyl and alkynyl may be substituted or unsubstituted C\ to Cio alkyl or C2 to Cio alkenyl and alkynyl. Particularly prefened are O[(CH2)„O]mCH3, O(CH2)nOCH3, O(CH2)nNH2, O(CH2)nCH3, O(CH2)„ONH2, and O(CH2)nON[(CH2)nCH3J2, where n and m are from 1 to about 10. Other prefened oligonucleotides comprise a sugar substituent group selected from: Ci to Cio lower alkyl, substituted lower alkyl, alkenyl, alkynyl, alkaryl, aralkyl, O-alkaryl or O-aralkyl, SH, SCH3, OCN, CI, Br, CN, CF3, OCF3, SOCH3, SO2CH3, ONO2, NO2, N3, NH2, heterocycloalkyl, heterocycloalkaryl, aminoalkylamino, polyalkylamino, substituted silyl, an RNA cleaving group, a reporter group, an intercalator, a group for improving the pharmacokinetic properties of an oligonucleotide, or a group for improving the phamiacodynamic properties of an oligonucleotide, and other substituents having similar properties. A prefened modification includes 2'-methoxy ethoxy (2'-O- CH2CH2OCH3, also known as 2'-O-(2-methoxyethyl) or 2'-MOE) (Martin et al, Helv. Chim. Ada, 1995, 78, 486-504) i.e., an alkoxyalkoxy group. A further prefened modification includes 2'-dimethylaminooxyethoxy, i.e., a O(CH2)2ON(CH3)2 group, also known as 2'-DMAOE, as described in examples hereinbelow, and 2'-dimethylaminoethoxyethoxy (also known in the art as 2'-O- dimethyl-amino-ethoxy-ethyl or 2'-DMAEOE), i.e., 2'-O-CH2-O-CH2-N(CH3)2. [0161] Other prefened sugar substituent groups include methoxy (-O-CH3),
aminopropoxy (-OCH2CH2CH2NH2), allyl (-CH2-CH=CH2), -O-allyl (-O-CH2- CH=CH2) and fluoro (F). 2'-Sugar substituent groups may be in the arabino (up) position or ribo (down) position. A prefened 2'-arabino modification is 2'-F. Similar modifications may also be made at other positions on the oligomeric compound, particularly the 3' position of the sugar on the 3' terminal nucleoside or in 2'-5' linked oligonucleotides and the 5' position of 5' terminal nucleotide. Oligomeric compounds may also have sugar mimetics such as cyclobutyl moieties in place of the pentofuranosyl sugar. Representative United States patents that teach the preparation of such modified sugar structures include, but are not limited to, U.S.: 4,981,957; 5,118,800; 5,319,080; 5,359,044; 5,393,878; 5,446,137; 5,466,786; 5,514,785; 5,519,134; 5,567,811; 5,576,427; 5,591,722; 5,597,909; 5,610,300; 5,627,053; 5,639,873; 5,646,265; 5,658,873; 5,670,633; 5,792,747; and 5,700,920, certain of which are commonly owned with the instant application, and each of which is herein incorporated by reference in its entirety. [0162] Further representative sugar substituent groups include groups of formula Ia or IIa:
Rb is O, S or NH;
R is a single bond, O, S or C(=O);
R, is Ci-Cio alkyl, N(Rk)(Rffl), N(Rk)(R„), N=C(Rp)(Rq), N=C(Rp)(Rr) or has formula IIIa;
Ha
Rp and Rq are each independently hydrogen or Ci-Cio alkyl;
Rr is -Rx-Ry; each Rs, Rt, Ru and Rv is, independently, hydrogen, C(O)Rw, substituted or unsubstituted Ci-Cio alkyl, substituted or unsubstituted C2-Cιo alkenyl, substituted or unsubstituted C2-Cιo alkynyl, alkylsulfonyl, arylsulfonyl, a chemical functional group or a conjugate group, wherein the substituent groups are selected from hydroxyl, amino, alkoxy, carboxy, benzyl, phenyl, nitro, thiol, thioalkoxy, halogen, alkyl, aryl, alkenyl and alkynyl; or optionally, Ru and Rv, together form a phthalimido moiety with the nitrogen atom to which they are attached; each Rw is, independently, substituted or unsubstituted Ci-Cio alkyl, trifluoromethyl, cyanoethyloxy, methoxy, ethoxy, t-butoxy, allyloxy, 9- fluorenylmethoxy, 2-(trimethylsilyl)-ethoxy, 2,2,2-trichloroethoxy, benzyloxy, butyryl, iso-butyryl, phenyl or aryl;
Rk is hydrogen, a nitrogen protecting group or -Rx-Ry;
Rp is hydrogen, a nitrogen protecting group or -Rx-Ry;
Rx is a bond or a linking moiety;
Ry is a chemical functional group, a conjugate group or a solid support medium; each Rm and Rn is, independently, H, a nitrogen protecting group, substituted or unsubstituted Ci-Cio alkyl, substituted or unsubstituted C2-Cιo alkenyl, substituted or unsubstituted C2-Cιo alkynyl, wherein the substituent groups are selected from hydroxyl, amino, alkoxy, carboxy, benzyl, phenyl, nitro, thiol, thioalkoxy, halogen, alkyl, aryl, alkenyl, alkynyl; NH3 +, N(RU)(RV), guanidino and acyl where said acyl is an acid amide or an ester; or Rm and Rn, together, are a nitrogen protecting group, are joined in a ring stracture that optionally includes an additional heteroatom selected from N and O or are a chemical functional group;
Ri is OR2, SRz, orN(Rz)2; each Rz is, independently, H, Cι-C8 alkyl, Cι-C8 haloalkyl, C(=NH)N(H)RU, C(=O)N(H)Ru or OC(=O)N(H)Ru;
Rf, Rg and R comprise a ring system having from about 4 to about 7 carbon atoms or having from about 3 to about 6 carbon atoms and 1 or 2 heteroatoms wherein said heteroatoms are selected from oxygen, nitrogen and sulfur and wherein said ring system is aliphatic, unsaturated aliphatic, aromatic, or saturated or unsaturated heterocyclic;
Rj is alkyl or haloalkyl having 1 to about 10 carbon atoms, alkenyl having 2 to about 10 carbon atoms, alkynyl having 2 to about 10 carbon atoms, aryl having 6 to about 14 carbon atoms, N(Rk)(Rm) ORk, halo, SR or CN; ma is 1 to about 10; each mb is, independently, 0 or 1; mc is 0 or an integer from 1 to 10; md is an integer from 1 to 10; me is from 0, 1 or 2; and provided that when mc is 0, md is greater than 1. [0163] Representative substituents groups of Formula I are disclosed in United States Patent Application Serial No. 09/130,973, filed August 7, 1998, entitled "Capped 2'-Oxyethoxy Oligonucleotides," hereby incorporated by reference in its entirety.
[0164] Representative cyclic substituent groups of Formula II are disclosed in United States Patent Application Serial No. 09/123,108, filed July 27, 1998, entitled "RNA Targeted 2'-Oligomeric compounds that are Conformationally Preorganized," hereby incorporated by reference in its entirety. [0165] Particularly prefened sugar substituent groups include O[(CH2)nO]mCH , O(CH2)nOCH3, O(CH2)nNH2; O(CH2)nCH3, 0(CH2)nONH2; and O(CH2)nON[(CH2)nCH3)]25 where n and m are from 1 to about 10. [0166] Representative guanidino substituent groups that are shown in formula III and IV are disclosed in co-owned United States Patent Application 09/349,040, entitled "Functionalized Oligomers", filed July 7, 1999, hereby incorporated by reference in its entirety.
[0167] Representative acetamido substituent groups are disclosed in United States Patent 6,147,200 which is hereby incorporated by reference in its entirety.
[0168] Representative dimethylaminoethyloxyethyl substituent groups are disclosed in International Patent Application PCT/US99/17895, entitled "2'-O- Dimethylaminoethyloxyethyl-Oligomeric compounds", filed August 6, 1999, hereby incorporated by reference in its entirety.
Modified Nucleobases/Naturally occurring nucleobases
[0169] Oligomeric compounds may also include nucleobase (often refened to in the art simply as "base" or "heterocyclic base moiety") modifications or substitutions. As used herein, "unmodified" or "natural" nucleobases include the purine bases adenine (A) and guanine (G), and the pyrimidine bases thymine (T), cytosine (C) and uracil (U). Modified nucleobases also refened herein as heterocyclic base moieties include other synthetic and natural nucleobases such as 5-methylcytosine (5-me-C), 5-hydroxymethyl cytosine, xanthine, hypoxanthine, 2-aminoadenine, 6-methyl and other alkyl derivatives of adenine and\ guanine, 2- propyl and other alkyl derivatives of adenine and guanine, 2-thiouracil, 2- thiothymine and 2-thiocytosine, 5-halouracil and cytosine, 5-propynyl (-C≡C- CH3) uracil and cytosine and other alkynyl derivatives of pyrimidine bases, 6-azo uracil, cytosine and thymine, 5-uracil (pseudouracil), 4-thiouracil, 8-halo, 8- amino, 8-thiol, 8-thioalkyl, 8-hydroxyl and other 8-substituted adenines and guanines, 5-halo particularly 5-bromo, 5-trifluoromethyl and other 5-substituted uracils and cytosines, 7-methylguanine and 7-methyladenine, 2-F-adenine, 2- amino-adenine, 8-azaguanine and 8-azaadenine, 7-deazaguanine and 7- deazaadenine and 3-deazaguanine and 3-deazaadenine.
[0170] Heterocyclic base moieties may also include those in which the purine or pyrimidine base is replaced with other heterocycles, for example 7-deaza-adenine, 7-deazaguanosine, 2-aminopyridine and 2-pyridone. Further nucleobases include those disclosed in United States Patent No. 3,687,808, those disclosed in The Concise Encyclopedia Of Polymer Science And Engineering, pages 858-859, Kroschwitz, J.I., ed. John Wiley & Sons, 1990, those disclosed by Englisch et al, Angewandte Chemie, International Edition, 1991, 30, 613, and those disclosed by Sanghvi, Y.S., Chapter 15, Antisense Research and Applications, pages 289-302,
Crooke, S.T. and Lebleu, B. , ed., CRC Press, 1993. Certain of these nucleobases are particularly useful for increasing the binding affinity of the oligomeric compounds of the invention. These include 5 -substituted pyrimidines, 6- azapyrimidines and N-2, N-6 and O-6 substituted purines, including 2- aminopropyladenine, 5-propynyluracil and 5-propynylcytosine. 5-methylcytosine substitutions have been shown to increase nucleic acid duplex stability by 0.6- 1.2°C (Sanghvi, Y.S., Crooke, S.T. and Lebleu, B., eds., Antisense Research and Applications, CRC Press, Boca Raton, 1993, pp. 276-278) and are presently prefened base substitutions, even more particularly when combined with 2'-O- methoxyethyl sugar modifications.
[0171] In one aspect of the present invention oligomeric compounds are prepared having polycyclic heterocyclic compounds in place of one or more heterocyclic base moieties. A number of tricyclic heterocyclic compounds have been previously reported. These compounds are routinely used in antisense applications to increase the binding properties of the modified strand to a target strand. The most studied modifications are targeted to guanosines hence they have been termed G-clamps or cytidine analogs. Many of these polycyclic heterocyclic compounds have the general formula:
[0172] Representative cytosine analogs that make 3 hydrogen bonds with a guanosine in a second sfrand include l,3-diazaphenoxazine-2-one (Rιo= O, Rn - Rι4= H) [Kurchavov, et al, Nucleosides and Nucleotides, 1997, 16, 1837-1846], l,3-diazaphenothiazine-2-one (Rιo= S, n - Rι4= H), [Lin, K.-Y.; Jones, R. J.;
Matteucci, M. J. Am. Chem. Soc. 1995, 117, 3873-3874] and 6,7,8,9-tetrafluoro- l,3-diazaphenoxazine-2-one (Rι0 = O, Rn - R = F) [Wang, J.; Lin, K.-Y., Matteucci, M. Tetrahedron Lett. 1998, 39, 8385-8388]. Incorporated into oligonucleotides these base modifications were shown to hybridize with complementary guanine and the latter was also shown to hybridize with adenine and to enhance helical thermal stability by extended stacking mteractions(also see U.S. Patent Application entitled "Modified Peptide Nucleic Acids" filed May 24, 2002, Serial number 10/155,920; and U.S. Patent Application entitled "Nuclease Resistant Chimeric Oligonucleotides" filed May 24, 2002, Serial number 10/013,295, both of which are commonly owned with this application and are herein incorporated by reference in their entirety).
[0173] Further helix-stabilizing properties have been observed when a cytosine analog/substitute has an aminoethoxy moiety attached to the rigid 1,3-diaza- phenoxazine-2-one scaffold (Rιo= O, Rn = -O-(CH2)2-NH2, Rι2_ι4=H ) [Lin, K.- Y.; Matteucci, M. J. Am. Chem. Soc. 1998, 120, 8531-8532]. Binding studies demonstrated that a single incorporation could enhance the binding affinity of a model oligonucleotide to its complementary target DNA or RNA with a ΔTm of up to 18° relative to 5-methyl cytosine (dC5me), which is the highest known affinity enhancement for a single modification, yet. On the other hand, the gain in helical stability does not compromise the specificity of the oligonucleotides. The Tm data indicate an even greater discrimination between the perfect match and mismatched sequences compared to dC5me. It was suggested that the tethered amino group serves as an additional hydrogen bond donor to interact with the Hoogsteen face, namely the O6, of a complementary guanine thereby forming 4 hydrogen bonds. This means that the increased affinity of G-clamp is mediated by the combination of extended base stacking and additional specific hydrogen bonding. [0174] Further tricyclic heterocyclic compounds and methods of using them that are amenable to the present invention are disclosed in United States Patent Serial Number 6,028,183, which issued on May 22, 2000, and United States Patent Serial Number 6,007,992, which issued on December 28, 1999, the contents of both are commonly assigned with this application and are incorporated herein in
their entirety.
[0175] The enhanced binding affinity of the phenoxazine derivatives together with their uncompromised sequence specificity make them valuable nucleobase analogs for the development of more potent antisense-based drags. In fact, promising data have been derived from in vitro experiments demonstrating that heptanucleotides containing phenoxazine substitutions are capable to activate RNaseH, enhance cellular uptake and exhibit an increased antisense activity [Lin, K-Y; Matteucci, M. J. Am. Chem. Soc. 1998, 120, 8531-8532]. The activity enhancement was even more pronounced in case of G-clamp, as a single substitution was shown to significantly improve the in vitro potency of a 20mer 2'-deoxyphosphorothioate oligonucleotides [Flanagan, W. M.; Wolf, J.J.; Olson, P.; Grant, D.; Lin, K.-Y.; Wagner, R. W.; Matteucci, M. Proc. Natl. Acad. Sci. USA, 1999, 96, 3513-3518]. Nevertheless, to optimize oligonucleotide design and to better understand the impact of these heterocyclic modifications on the biological activity, it is important to evaluate their effect on the nuclease stability of the oligomers.
[0176] Further modified polycyclic heterocyclic compounds useful as heterocyclcic bases are disclosed in but not limited to, the above noted U.S. 3,687,808, as well as U.S.: 4,845,205; 5,130,302; 5,134,066; 5,175,273; 5,367,066; 5,432,272; 5,434,257; 5,457,187; 5,459,255; 5,484,908; 5,502,177; 5,525,711; 5,552,540; 5,587,469; 5,594,121, 5,596,091; 5,614,617; 5,645,985; 5,646,269; 5,750,692; 5,830,653; 5,763,588; 6,005,096; and 5,681,941, and Unites States Patent Application Serial number 09/996,292 filed November 28, 2001, certain of which are commonly owned with the instant application, and each of which is herein incorporated by reference.
Conjugates
[0177] A further prefened substitution that can be appended to the oligomeric compounds of the invention involves the linkage of one or more moieties or conjugates which enhance the activity, cellular distribution or cellular uptake of the resulting oligomeric compounds. In one embodiment such modified
oligomeric compounds are prepared by covalently attaching conjugate groups to functional groups such as hydroxyl or amino groups. Conjugate groups of the invention include intercalators, reporter molecules, polyamines, polyamides, polyethylene glycols, polyethers, groups that enhance the pharmacodynamic properties of oligomers, and groups that enhance the pharmacokinetic properties of oligomers. Typical conjugates groups include cholesterols, lipids, phospholipids, biotin, phenazine, folate, phenanthridine, anthraquinone, acridine, fiuoresceins, rhodamines, coumarins, and dyes. Groups that enhance the pharmacodynamic properties, in the context of this invention, include groups that improve oligomer uptake, enhance oligomer resistance to degradation, and/or strengthen sequence- specific hybridization with RNA. Groups that enhance the pharmacokinetic properties, in the context of this invention, include groups that improve oligomer uptake, distribution, metabolism or excretion. Representative conjugate groups are disclosed in International Patent Application PCT/US92/09196, filed October 23, 1992 the entire disclosure of which is incorporated herein by reference. [0178] Conjugate moieties include but are not limited to lipid moieties such as a cholesterol moiety (Letsinger et al., Proc. Natl Acad. Sci. USA, 1989, 86, 6553- 6556), cholic acid (Manoharan et al., Bioorg. Med. Chem. Let., 1994, 4, 1053- 1060), a thioether, e.g., hexyl-S-tritylthiol (Manoharan et al, Ann. NY. Acad. Sci., 1992, 660, 306-309; Manoharan et al., Bioorg. Med. Chem. Let, 1993, 3, 2765- 2770), a thiocholesterol (Oberhauser et al., Nucl Acids Res., 1992, 20, 533-538), an aliphatic chain, e.g., dodecandiol or undecyl residues (Saison-Behmoaras et al., EMBOJ., 1991, 10, 1111-1118; Kabanov et al., FEBSLett, 1990, 259, 327-330; Svinarchuk et al., Biochimie, 1993, 75, 49-54), a phospholipid, e.g., di-hexadecyl- rac-glycerol or triethylammonium l,2-di-O-hexadecyl-rac-glycero-3-H- phosphonate (Manoharan et al., Tetrahedron Lett, 1995, 36, 3651-3654; Shea et al., Nucl. Acids Res., 1990, 18, 3777-3783), apolyamine or a polyethylene glycol chain (Manoharan et al., Nucleosides & Nucleotides, 1995, 14, 969-973), or adamantane acetic acid (Manoharan et al., Tetrahedron Lett, 1995, 36, 3651- 3654), a palmityl moiety (Mishra et al., Biochim. Biophys. Ada, 1995, 1264, 229- 237), or an octadecylamine or hexylamino-carbonyl-oxycholesterol moiety
(Crooke et al., J. Pharmacol. Exp. Ther., 1996, 277, 923-937. [0179] The oligomeric compounds of the invention may also be conjugated to active drag substances, for example, aspirin, warfarin, phenylbutazone, ibuprofen, suprofen, fenbufen, ketoprofen, (S)-(+)-pranoprofen, carprofen, dansylsarcosine, 2,3,5-triiodobenzoic acid, flufenamic acid, folinic acid, a benzothiadiazide, chlorothiazide, a diazepine, indomethicin, a barbiturate, a cephalosporin, a sulfa drag, an antidiabetic, an antibacterial or an antibiotic. Oligonucleotide-drag conjugates and their preparation are described in United States Patent Application 09/334,130 (filed June 15, 1999) which is incorporated herein by reference in its entirety.
[0180] Representative United States patents that teach the preparation of such oligonucleotide conjugates include, but are not limited to, U.S.: 4,828,979;
4,948,882; 5,218,105; 5,525,465; 5,541,313; 5,545,730; 5,552,538; 5,578,717,
5,580,731; 5,580,731; 5,591,584; 5,109,124; 5,118,802; 5,138,045; 5,414,077
5,486,603; 5,512,439; 5,578,718; 5,608,046; 4,587,044; 4,605,735; 4,667,025
4,762,779; 4,789,737; 4,824,941; 4,835,263; 4,876,335; 4,904,582; 4,958,013
5,082,830; 5,112,963; 5,214,136; 5,082,830; 5,112,963; 5,214,136; 5,245,022
5,254,469; 5,258,506; 5,262,536; 5,272,250; 5,292,873; 5,317,098; 5,371,241
5,391,723; 5,416,203, 5,451,463; 5,510,475; 5,512,667; 5,514,785; 5,565,552
5,567,810; 5,574,142; 5,585,481; 5,587,371 ;; 5,595,726; 5,597,696; 5,599,923
5,599,928 and 5,688,941, certain of which are commonly owned with the instant application, and each of which is herein incorporated by reference.
Chimeric oligomeric compounds
[0181] It is not necessary for all positions in an oligomeric compound to be uniformly modified, and in fact more than one of the aforementioned modifications may be incorporated in a single oligomeric compound or even at a single monomeric subunit such as a nucleoside within a oligomeric compound. The present invention also includes oligomeric compounds which are chimeric oligomeric compounds. "Chimeric" oligomeric compounds or "chimeras," in the context of this invention, are oligomeric compounds that contain two or more
chemically distinct regions, each made up of at least one monomer unit, i.e., a nucleotide in the case of a nucleic acid based oligomer. [0182] Chimeric oligomeric compounds typically contain at least one region modified so as to confer increased resistance to nuclease degradation, increased cellular uptake, and/or increased binding affinity for the target nucleic acid. An additional region of the oligomeric compound may serve as a substrate for enzymes capable of cleaving RNA:DNA or RNA:RNA hybrids. By way of example, RNase H is a cellular endonuclease which cleaves the RNA strand of an RNA:DNA duplex. Activation of RNase H, therefore, results in cleavage of the RNA target, thereby greatly enhancing the efficiency of inhibition of gene expression. Consequently, comparable results can often be obtained with shorter oligomeric compounds when chimeras are used, compared to for example phosphorothioate deoxyoligonucleotides hybridizing to the same target region. Cleavage of the RNA target can be routinely detected by gel electrophoresis and, if necessary, associated nucleic acid hybridization techniques known in the art. [0183] Chimeric oligomeric compounds of the invention may be formed as composite structures of two or more oligonucleotides, oligonucleotide analogs, ohgonucleosides and/or oligonucleotide mimetics as described above. Such oligomeric compounds have also been refened to in the art as hybrids hemimers, gapmers or inverted gapmers. Representative United States patents that teach the preparation of such hybrid stractures include, but are not limited to, U.S.: 5,013,830; 5,149,797; 5,220,007; 5,256,775; 5,366,878; 5,403,711; 5,491,133; 5,565,350; 5,623,065; 5,652,355; 5,652,356; and 5,700,922, certain of which are commonly owned with the instant application, and each of which is herein incorporated by reference in its entirety.
3'-endo modifications
[0184] In one aspect of the present invention oligomeric compounds include nucleosides synthetically modified to induce a 3'-endo sugar conformation. A nucleoside can incorporate synthetic modifications of the heterocyclic base, the sugar moiety or both to induce a desired 3'-endo sugar conformation. These
modified nucleosides are used to mimic RNA like nucleosides so that particular properties of an oligomeric compound can be enhanced while maintaining the desirable 3'-endo conformational geometry. There is an apparent preference for an RNA type duplex (A form helix, predominantly 3'-endo) as a requirement (e.g. trigger) of RNA interference which is supported in part by the fact that duplexes composed of 2'-deoxy-2'-F-nucleosides appears efficient in triggering RNAi response in the C. elegans system. Properties that are enhanced by using more stable 3'-endo nucleosides include but aren't limited to modulation of pharmacokinetic properties through modification of protein binding, protein off- rate, absorption and clearance; modulation of nuclease stability as well as chemical stability; modulation of the binding affinity and specificity of the oligomer (affinity and specificity for enzymes as well as for complementary sequences); and increasing efficacy of RNA cleavage. The present invention provides oligomeric triggers of RNAi having one or more nucleosides modified in such a way as to favor a C3'-endo type conformation.
Scheme 1
C2'-endo/Southern C3'-endo/Northern
[0185] Nucleoside conformation is influenced by various factors including substitution at the 2', 3' or 4'-positions of the pentofuranosyl sugar. Electronegative substituents generally prefer the axial positions, while sterically demanding substituents generally prefer the equatorial positions (Principles of Nucleic Acid Stracture, Wolfgang Sanger, 1984, Springer-Verlag.) Modification of the 2' position to favor the 3'-endo conformation can be achieved while maintaining the 2'-OH as a recognition element, as illustrated in Figure 2, below (Gallo et al., Tetrahedron (2001), 57, 5707-5713. Harry-O'kuru et al., J. Org. Chem.,
(1997), 62(6), 1754-1759 and Tang et al., J. Org. Chem. (1999), 64, 747-754.) Alternatively, preference for the 3'-endo conformation can be achieved by deletion of the 2'-OH as exemplified by 2'deoxy-2'F-nucleosides (Kawasaki et al., J. Med. Chem. (1993), 36, 831-841), which adopts the 3'-endo conformation positioning the electronegative fluorine atom in the axial position. Other modifications of the ribose ring, for example substitution at the 4'-position to give 4'-F modified nucleosides (Guillerm et al., Bioorganic and Medicinal Chemistry Letters (1995), 5, 1455-1460 and Owen et al., J. Org. Chem. (1976), 41, 3010-3017), or for example modification to yield methanocarba nucleoside analogs (Jacobson et al., J. Med. Chem. Lett. (2000), 43, 2196-2203 and Lee et al., Bioorganic and Medicinal Chemistry Letters (2001), 11, 1333-1337) also induce preference for the 3'-endo conformation. Along similar lines, oligomeric triggers of RNAi response might be composed of one or more nucleosides modified in such a way that conformation is locked into a C3'-endo type conformation, i.e. Locked Nucleic Acid (LNA, Singh et al, Chem. Commun. (1998), 4, 455-456), and ethylene bridged Nucleic Acids (ENA, Morita et al, Bioorganic & Medicinal Chemistry Letters (2002), 12, 73-76.) Examples of modified nucleosides amenable to the present invention are shown below in Table I. These examples are meant to be representative and not exhaustive.
Table I
[0186] The prefened conformation of modified nucleosides and their oligomers can be estimated by various methods such as molecular dynamics calculations, nuclear magnetic resonance spectroscopy and CD measurements. Hence,
modifications predicted to induce RNA like conformations, A-form duplex geometry in an oligomeric context, are selected for use in the modified oligonucleotides of the present invention. The synthesis of numerous of the modified nucleosides amenable to the present invention are known in the art (see for example, Chemistry of Nucleosides and Nucleotides Nol 1-3, ed. Leroy B. Townsend, 1988, Plenum press., and the examples section below.) Nucleosides known to be inhibitors/substrates for RNA dependent RNA polymerases (for example HCV NS5B
[0187] In one aspect, the present invention is directed to oligonucleotides that are prepared having enhanced properties compared to native RNA against nucleic acid targets. A target is identified and an oligonucleotide is selected having an effective length and sequence that is complementary to a portion of the target sequence. Each nucleoside of the selected sequence is scrutinized for possible enhancing modifications. A prefened modification would be the replacement of one or more RNA nucleosides with nucleosides that have the same 3'-endo conformational geometry. Such modifications can enhance chemical and nuclease stability relative to native RNA while at the same time being much cheaper and easier to synthesize and/or incorporate into an oligonucleotide. The selected sequence can be further divided into regions and the nucleosides of each region evaluated for enhancing modifications that can be the result of a chimeric configuration. Consideration is also given to the 5' and 3 '-termini as there are often advantageous modifications that can be made to one or more of the terminal nucleosides. The oligomeric compounds of the present invention include at least one 5'-modified phosphate group on a single strand or on at least one 5'-position of a double stranded sequence or sequences. Further modifications are also considered such as intemucleoside linkages, conjugate groups, substitute sugars or bases, substitution of one or more nucleosides with nucleoside mimetics and any other modification that can enhance the selected sequence for its intended target. [0188] The terms used to describe the conformational geometry of homoduplex nucleic acids are "A Form" for RNA and "B Form" for DNA. The respective conformational geometry for RNA and DNA duplexes was determined from
X-ray diffraction analysis of nucleic acid fibers (Arnott and Hukins, Biochem. Biophys. Res. Comm., 1970, 47, 1504.) In general, RNA:RNA duplexes are more stable and have higher melting temperatures (Tm's) than DNA:DNA duplexes (Sanger et al., Principles of Nucleic Acid Stracture, 1984, Springer-Nerlag; New York, NY.; Lesnik et al., Biochemistry, 1995, 34, 10807-10815; Conte et al, Nucleic Acids Res., 1997, 25, 2627-2634). The increased stability of RNA has been attributed to several structural features, most notably the improved base stacking interactions that result from an A-form geometry (Searle et al., Nucleic Acids Res., 1993, 21, 2051-2056). The presence of the 2' hydroxyl in RNA biases the sugar toward a C3' endo pucker, i.e., also designated as Northern pucker, which causes the duplex to favor the A-form geometry. In addition, the 2' hydroxyl groups of RNA can form a network of water mediated hydrogen bonds that help stabilize the RNA duplex (Egli et al., Biochemistry, 1996, 35, 8489- 8494). On the other hand, deoxy nucleic acids prefer a C2' endo sugar pucker, i.e., also known as Southern pucker, which is thought to impart a less stable B- form geometry (Sanger, W. (1984) Principles of Nucleic Acid Stracture, Springer- Nerlag, New York, NY). As used herein, B-form geometry is inclusive of both C2'-endo pucker and O4'-endo pucker. This is consistent with Berger, et. al, Nucleic Acids Research, 1998, 26, 2473-2480, who pointed out that in considering the furanose conformations which give rise to B-form duplexes consideration should also be given to a O4'-endo pucker contribution. [0189] DNA:RNA hybrid duplexes, however, are usually less stable than pure RNA:RNA duplexes, and depending on their sequence may be either more or less stable thanDNA:DNA duplexes (Searle et al, Nucleic Acids Res., 1993, 21, 2051- 2056). The structure of a hybrid duplex is inteπnediate between A- and B-form geometries, which may result in poor stacking interactions (Lane et al, Eur. J. Biochem., 1993, 215, 297-306; Fedoroff et al, J. Mol. Biol, 1993, 233, 509-523; Gonzalez et al, Biochemistry, 1995, 34, 4969-4982; Horton et al, J. Mol. Biol, 1996, 264, 521-533). The stability of the duplex formed between a target RNA and a synthetic sequence is central to therapies such as but not limited to antisense and RNA interference as these mechanisms require the binding of a synthetic
oligonucleotide strand to an RNA target strand. In the case of antisense, effective inhibition of the mRNA requires that the antisense DNA have a very high binding affinity with the mRNA. Otherwise the desired interaction between the synthetic oligonucleotide strand and target mRNA strand will occur infrequently, resulting in decreased efficacy.
[0190] One routinely used method of modifying the sugar puckering is the substitution of the sugar at the 2'-position with a substituent group that influences the sugar geometry. The influence on ring conformation is dependant on the nature of the substituent at the 2'-position. A number of different substituents have been studied to determine their sugar puckering effect. For example, 2'- halogens have been studied showing that the 2'-fluoro derivative exhibits the largest population (65%) of the C3'-endo form, and the 2'-iodo exhibits the lowest population (7%). The populations of adenosine (2'-OH) versus deoxyadenosine (2'-H) are 36% and 19%, respectively. Furthermore, the effect of the 2'-fluoro group of adenosine dimers (2'-deoxy-2'-fluoroadenosine - 2'-deoxy-2,-fluoro- adenosine) is further conelated to the stabilization of the stacked conformation. [0191] As expected, the relative duplex stability can be enhanced by replacement of 2'-OH groups with 2'-F groups thereby increasing the C3'-endo population. It is assumed that the highly polar nature of the 2'-F bond and the extreme preference for C3'-endo puckering may stabilize the stacked conformation in an A-form duplex. Data from UN hypochromicity, circular dichroism, and 1H ΝMR also indicate that the degree of stacking decreases as the electronegativity of the halo substituent decreases. Furthermore, steric bulk at the 2'-position of the sugar moiety is better accommodated in an A-form duplex than a B-form duplex. Thus, a 2'-substituent on the 3'-terminus of a dinucleoside monophosphate is thought to exert a number of effects on the stacking conformation: steric repulsion, furanose puckering preference, electrostatic repulsion, hydrophobic attraction, and hydrogen bonding capabilities. These substituent effects are thought to be determined by the molecular size, electronegativity, and hydrophobicity of the substituent. Melting temperatures of complementary strands is also increased with the 2'-substituted adenosine
diphosphates. It is not clear whether the 3'-endo preference of the conformation or the presence of the substituent is responsible for the increased binding. However, greater overlap of adjacent bases (stacking) can be achieved with the 3'-endo conformation.
[0192] One synthetic 2'-modification that imparts increased nuclease resistance and a very high binding affinity to nucleotides is the 2-methoxyethoxy (2'-MOE, 2*-OCH2CH2OCH3) side chain (Baker et al, J. Biol. Chem., 1997, 272, 11944- 12000). One of the immediate advantages of the 2'-MOE substitution is the improvement in binding affinity, which is greater than many similar 2' modifications such as O-methyl, O-propyl, and O-aminopropyl. Oligonucleotides having the 2'-O-methoxyethyl substituent also have been shown to be antisense inhibitors of gene expression with promising features for in vivo use (Martin, P., Helv. Chim. Ada, 1995, 78, 486-504; Altmann et al, Chimia, 1996, 50, 168-176; Altmann et al, Biochem. Soc. Trans., 1996, 24, 630-637; and Altmann et al, Nucleosides Nucleotides, 1997, 16, 917-926). Relative to DNA, the oligonucleotides having the 2'-MOE modification displayed improved RNA affinity and higher nuclease resistance. Cliimeric oligonucleotides having 2'- MOE substituents in the wing nucleosides and an internal region of deoxy- phosphorothioate nucleotides (also termed a gapped oligonucleotide or gapmer) have shown effective reduction in the growth of tumors in animal models at low doses. 2'-MOE substituted oligonucleotides have also shown outstanding promise as antisense agents in several disease states. One such MOE substituted oligonucleotide is presently being investigated in clinical trials for the treatment of CMV retinitis.
Chemistries Defined
[0193] Unless otherwise defined herein, alkyl means Cι-Cι2, preferably Cι-C8, and more preferably Cι-C6, straight or (where possible) branched chain aliphatic hydrocarbyl. The term lower alky refers to Ci-Cio alkyl groups. Some prefened lower alkyl groups are Cι-C6.
[0194] Unless otherwise defined herein, heteroalkyl means Cι-Cι2, preferably
Ci-Cs, and more preferably Cι-C6, straight or (where possible) branched chain aliphatic hydrocarbyl containing at least one, and preferably about 1 to about 3, hetero atoms in the chain, including the terminal portion of the chain. Prefened heteroatoms include N, O and S.
[0195] Unless otherwise defined herein, cycloalkyl means C3-Cι2, preferably C3-
C8, and more preferably C -C6, aliphatic hydrocarbyl ring.
[0196] Unless otherwise defined herein, alkenyl means C2-Cι2, preferably C2-
C8, and more preferably C -C6 alkenyl, which may be sfraight or (where possible) branched hydrocarbyl moiety, which contains at least one carbon-carbon double bond.
[0197] Unless otherwise defined herein, alkynyl means C2-Cι2, preferably C2-
C8, and more preferably C2-C6 alkynyl, which may be straight or (where possible) branched hydrocarbyl moiety, which contains at least one carbon-carbon triple bond.
[0198] Unless otherwise defined herein, heterocycloalkyl means a ring moiety containing at least three ring members, at least one of which is carbon, and of which 1, 2 or three ring members are other than carbon. Preferably the number of carbon atoms varies from 1 to about 12, preferably 1 to about 6, and the total number of ring members varies from three to about 15, preferably from about 3 to about 8. Prefened ring heteroatoms are N, O and S. Prefened heterocycloalkyl groups include morpholino, thiomorpholino, piperidinyl, piperazinyl, homopiperidinyl, homopiperazinyl, homomorpholino, homothiomorpholino, pynolodinyl, tetrahydrooxazolyl, tetrahydroimidazolyl, tetrahydrothiazolyl, tetrahydroisoxazolyl, tetrahydropynazolyl, furanyl, pyranyl, and tetrahydroisothiazolyl.
[0199] Unless otherwise defined herein, alkoxy refers to an -O-alkyl group, where alkyl as defined herein.
[0200] Unless otherwise defined herein, aryl means any hydrocarbon ring structure containing at least one aryl ring. Prefened aryl rings have about 6 to about 20 ring carbons. Especially prefened aryl rings include phenyl, napthyl, anthracenyl, and phenanthrenyl.
[0201] Unless otherwise defined herein, hetaryl means a ring moiety containing at least one fully unsaturated ring, the ring consisting of carbon and non-carbon atoms. Preferably the ring system contains about 1 to about 4 rings. Preferably the number of carbon atoms varies from 1 to about 12, preferably 1 to about 6, and the total number of ring members varies from three to about 15, preferably from about 3 to about 8. Prefened ring heteroatoms are N, O and S. Prefened hetaryl moieties include pyrazolyl, thiophenyl, pyridyl, imidazolyl, tetrazolyl, pyridyl, pyrimidinyl, purinyl, qumazolinyl, quinoxalinyl, benzimidazolyl, benzothiophenyl, etc.
[0202] Unless otherwise defined herein, where a moiety is defined as a compound moiety, such as hetarylalkyl (hetaryl and alkyl), aralkyl (aryl and alkyl), etc., each of the sub-moieties is as defined herein. [0203] Unless otherwise defined herein, an electron withdrawing group is a group, such as the cyano or isocyanato group that draws electronic charge away from the carbon to which it is attached. Other electron withdrawing groups of note include those whose electronegativities exceed that of carbon, for example halogen, nitro, or phenyl substituted in the ortho- or para-position with one or more cyano, isothiocyanato, nitro or halo groups.
[0204] Unless otherwise defined herein, the terms halogen and halo have their ordinary meanings. Prefened halo (halogen) substituents are CI, Br, and I. The aforementioned optional substituents are, unless otherwise herein defined, suitable substituents depending upon desired properties. Included are halogens (CI, Br, I), alkyl, alkenyl, and alkynyl moieties, NO2, NH3 (substituted and unsubstituted), acid moieties (e.g. -CO2H, -OSO3H2, etc.), heterocycloalkyl moieties, hetaryl moieties, aryl moieties, etc.
In all the preceding formulae, the squiggle (~) indicates a bond to an oxygen or sulfur of the 5 '-phosphate.
[0205] Phosphate protecting groups include those described in US Patents No. US 5,760,209, US 5,614,621, US 6,051,699, US 6,020,475, US 6,326,478, US 6,169,177, US 6,121,437, US 6,465,628 each of which is expressly incorporated herein by reference in its entirety.
Screening, Target Validation and Drug Discovery
[0206] For use in screening and target validation, the compounds and compositions of the invention are used to modulate the expression of a selected protein. "Modulators" are those oligomeric compounds and compositions that decrease or increase the expression of a nucleic acid molecule encoding a protein and which comprise at least an 8-nucleobase portion which is complementary to a prefened target segment. The screening method comprises the steps of contacting a prefened target segment of a nucleic acid molecule encoding a protein with one or more candidate modulators, and selecting for one or more candidate modulators which decrease or increase the expression of a nucleic acid molecule encoding a protein. Once it is shown that the candidate modulator or modulators are capable of modulating (e.g. either decreasing or increasing) the expression of a nucleic acid molecule encoding a peptide, the modulator may then be employed in further investigative studies of the function of the peptide, or for use as a research, diagnostic, or therapeutic agent in accordance with the present invention. [0207] The conduction such screening and target validation studies, oligomeric compounds of invention can be used combined with their respective complementary strand oligomeric compound to form stabilized double-stranded (duplexed) oligonucleotides. Double stranded oligonucleotide moieties have been shown to modulate target expression and regulate translation as well as RNA processing via an antisense mechanism. Moreover, the double-stranded moieties may be subject to chemical modifications (Fire et al., Nature, 1998, 391, 806-811; Timmons and Fire, Nature 1998, 395, 854; Timmons et al., Gene, 2001, 263, 103- 112; Tabara et al., Science, 1998, 282, 430-431; Montgomery et al., Proc. Natl Acad. Sci. USA, 1998, 95, 15502-15507; Tuschl et al., Genes Dev., 1999, 13, 3191-3197; Elbashir et al., Nature, 2001, 411 , 494-498; Elbashir et al., Genes Dev. 2001, 15, 188-200 ; Nishikura et al., Cell (2001), 107, 415-416; and Bass et al., Cell (2000), 101, 235-238.) For example, such double-stranded moieties have been shown to inhibit the target by the classical hybridization of antisense strand of the duplex to the target, thereby triggering enzymatic degradation of the target
(Tijsterman et al., Science, 2002, 295, 694-697).
[0208] For use in drug discovery and target validation, oligomeric compounds of the present invention are used to elucidate relationships that exist between proteins and a disease state, phenotype, or condition. These methods include detecting or modulating a target peptide comprising contacting a sample, tissue, cell, or organism with the oligomeric compounds and compositions of the present invention, measuring the nucleic acid or protein level of the target and/or a related phenotypic or chemical endpoint at some time after treatment, and optionally comparing the measured value to a non-treated sample or sample treated with a further oligomeric compound of the invention. These methods can also be performed in parallel or in combination with other experiments to determine the function of unknown genes for the process of target validation or to determine the validity of a particular gene product as a target for treatment or prevention of a disease or disorder.
Kits, Research Reagents, Diagnostics, and Therapeutics [0209] The oligomeric compounds and compositions of the present invention can additionally be utilized for diagnostics, therapeutics, prophylaxis and as research reagents and kits. Such uses allows for those of ordinary skill to elucidate the function of particular genes or to distinguish between functions of various members of a biological pathway.
[0210] For use in kits and diagnostics, the oligomeric compounds and compositions of the present invention, either alone or in combination with other compounds or therapeutics, can be used as tools in differential and/or combinatorial analyses to elucidate expression patterns of a portion or the entire complement of genes expressed within cells and tissues.
[0211] As one non-limiting example, expression patterns within cells or tissues treated with one or more compounds or compositions of the invention are compared to control cells or tissues not treated with the compounds or compositions and the patterns produced are analyzed for differential levels of gene expression as they pertain, for example, to disease association, signaling pathway, cellular localization, expression level, size, structure or function of the genes
examined. These analyses can be performed on stimulated or unstimulated cells and in the presence or absence of other compounds that affect expression patterns. [0212] Examples of methods of gene expression analysis known in the art include DNA anays or microaπays (Brazma and Vilo, FEBSLett, 2000, 480, 17- 24; Celis, et al, FEBSLett, 2000, 480, 2-16), SAGE (serial analysis of gene expression)(Madden, et al, DrugDiscov. Today, 2000, 5, 415-425), READS (restriction enzyme amplification of digested cDNAs) (Prashar and Weissman, Methods Enzymol, 1999, 303, 258-72), TOGA (total gene expression analysis) (Sutcliffe, et al, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 1976-81), protein anays and proteomics (Celis, et al, FEBSLett, 2000, 480, 2-16; Jungblut, et al, Electrophoresis, 1999, 20, 2100-10), expressed sequence tag (EST) sequencing (Celis, et al, FEBSLett, 2000, 480, 2-16; Larsson, et al, J. Biotechnol, 2000, 80, 143-57), subfractive RNA fingerprinting (SuRF) (Fuchs, et al, Anal. Biochem., 2000, 286, 91-98; Larson, et al, Cytometry, 2000, 41, 203-208), subfractive cloning, differential display (DD) (Jurecic and Belmont, Curr. Opin. Microbiol, 2000, 3, 316-21), comparative genomic hybridization (Caralli, et al, J. Cell Biochem. Suppl, 1998, 31, 286-96), FISH (fluorescent in situ hybridization) techniques (Going and Gusterson, Eur. J. Cancer, 1999, 35, 1895-904) and mass spectrometry methods (To, Comb. Chem. High Throughput Screen, 2000, 3, 235- 41).
[0213] The compounds and compositions of the invention are useful for research and diagnostics, because these compounds and compositions hybridize to nucleic acids encoding proteins. Hybridization of the compounds and compositions of the invention with a nucleic acid can be detected by means known in the art. Such means may include conjugation of an enzyme to the compound or composition, radiolabelling or any other suitable detection means. Kits using such detection means for detecting the level of selected proteins in a sample may also be prepared.
[0214] The specificity and sensitivity of compounds and compositions can also be harnessed by those of skill in the art for therapeutic uses. Antisense oligomeric compounds have been employed as therapeutic moieties in the treatment of
disease states in animals, including humans. Antisense oligonucleotide drugs, including ribozymes, have been safely and effectively administered to humans and numerous clinical trials are presently underway. It is thus established that oligomeric compounds can be useful therapeutic modalities that can be configured to be useful in treatment regimes for the treatment of cells, tissues and animals, especially humans.
[0215] For therapeutics, an animal, preferably a human, suspected of having a disease or disorder that can be treated by modulating the expression of a selected protein is treated by administering the compounds and compositions. For example, in one non-limiting embodiment, the methods comprise the step of administering to the animal in need of treatment, a therapeutically effective amount of a protein inhibitor. The protein inhibitors of the present invention effectively inhibit the activity of the protein or inhibit the expression of the protein. In one embodiment, the activity or expression of a protein in an animal is inhibited by about 10%. Preferably, the activity or expression of a protein in an animal is inhibited by about 30%. More preferably, the activity or expression of a protein in an animal is inhibited by 50% or more. [0216] For example, the reduction of the expression of a protein may be measured in serum, adipose tissue, liver or any other body fluid, tissue or organ of the animal. Preferably, the cells contained within the fluids, tissues or organs being analyzed contain a nucleic acid molecule encoding a protein and/or the protein itself.
[0217] The compounds and compositions of the invention can be utilized in pharmaceutical compositions by adding an effective amount of the compound or composition to a suitable pharmaceutically acceptable diluent or carrier. Use of the oligomeric compounds and methods of the invention may also be useful prophylactically.
Formulations
[0218] The compounds and compositions of the invention may also be admixed, encapsulated, conjugated or otherwise associated with other molecules, molecule
structures or mixtures of compounds, as for example, liposomes, receptor-targeted molecules, oral, rectal, topical or other formulations, for assisting in uptake, distribution and/or absorption. Representative United States patents that teach the preparation of such uptake, distribution and/or absorption-assisting formulations include, but are not limited to, U.S.: 5,108,921; 5,354,844; 5,416,016; 5,459,127; 5,521,291; 5,543,158; 5,547,932; 5,583,020; 5,591,721; 4,426,330; 4,534,899; 5,013,556; 5,108,921; 5,213,804; 5,227,170; 5,264,221; 5,356,633; 5,395,619; 5,416,016; 5,417,978; 5,462,854; 5,469,854; 5,512,295; 5,527,528; 5,534,259; 5,543,152; 5,556,948; 5,580,575; and 5,595,756, each of which is herein incorporated by reference.
[0219] The compounds and compositions of the invention encompass any pharmaceutically acceptable salts, esters, or salts of such esters, or any other compound which, upon administration to an animal, including a human, is capable of providing (directly or indirectly) the biologically active metabolite or residue thereof. Accordingly, for example, the disclosure is also drawn to prodrugs and pharmaceutically acceptable salts of the oligomeric compounds of the invention, pharmaceutically acceptable salts of such prodrugs, and other bioequivalents. [0220] The term "prodrug" indicates a therapeutic agent that is prepared in an inactive form that is converted to an active form (i.e., drug) within the body or cells thereof by the action of endogenous enzymes or other chemicals and/or conditions. In particular, prodrag versions of the oligonucleotides of the invention are prepared as SATE [(S-acetyl-2-thioethyl) phosphate] derivatives according to the methods disclosed in WO 93/24510 to Gosselin et al, published December 9, 1993 or in WO 94/26764 and U.S. 5,770,713 to Imbach et al. [0221] The term "pharaiaceutically acceptable salts" refers to physiologically and pharmaceutically acceptable salts of the compounds and compositions of the invention: i.e., salts that retain the desired biological activity of the parent compound and do not impart undesired toxicological effects thereto. For oligonucleotides, prefened examples of pharmaceutically acceptable salts and their uses are further described in U.S. Patent 6,287,860, which is incorporated herein in its entirety.
[0222] The present invention also includes pharmaceutical compositions and formulations that include the compounds and compositions of the invention. The pharmaceutical compositions of the present invention may be administered in a number of ways depending upon whether local or systemic treatment is desired and upon the area to be treated. Administration may be topical (including ophthalmic and to mucous membranes including vaginal and rectal delivery), pulmonary, e.g., by inhalation or insufflation of powders or aerosols, including by nebulizer; infratracheal, intranasal, epidermal and transdermal), oral or parenteral. Parenteral administration includes intravenous, intraarterial, subcutaneous, intraperitoneal or intramuscular injection or infusion; or intracranial, e.g., intrathecal or intraventricular, administration. Pharmaceutical compositions and formulations for topical administration may include transdermal patches, ointments, lotions, creams, gels, drops, suppositories, sprays, liquids and powders. Conventional pharmaceutical carriers, aqueous, powder or oily bases, thickeners and the like may be necessary or desirable. Coated condoms, gloves and the like may also be useful.
[0223] The pharmaceutical formulations of the present invention, which may conveniently be presented in unit dosage form, may be prepared according to conventional techniques well known in the pharmaceutical industry. Such techniques include the step of bringing into association the active ingredients with the pham aceutical carrier(s) or excipient(s). In general, the formulations are prepared by uniformly and intimately bringing into association the active ingredients with liquid carriers or finely divided solid caniers or both, and then, if necessary, shaping the product.
[0224] The compounds and compositions of the present invention may be formulated into any of many possible dosage forms such as, but not limited to, tablets, capsules, gel capsules, liquid syrups, soft gels, suppositories, and enemas. The compositions of the present invention may also be formulated as suspensions in aqueous, non-aqueous or mixed media. Aqueous suspensions may further contain substances which increase the viscosity of the suspension including, for
example, sodium carboxymethylcellulose, sorbitol and/or dexfran. The suspension may also contain stabilizers.
[0225] Pharmaceutical compositions of the present invention include, but are not limited to, solutions, emulsions, foams and liposome-containing formulations. The pharmaceutical compositions and formulations of the present invention may comprise one or more penetration enhancers, caniers, excipients or other active or inactive ingredients.
[0226] Emulsions are typically heterogenous systems of one liquid dispersed in another in the form of droplets usually exceeding 0.1 μm in diameter. Emulsions may contain additional components in addition to the dispersed phases, and the active drag that may be present as a solution in either the aqueous phase, oily phase or itself as a separate phase. Micro emulsions are included as an embodiment of the present invention. Emulsions and their uses are well known in the art and are further described in U.S. Patent 6,287,860, which is incorporated herein in its entirety.
[0227] Formulations of the present invention include liposomal formulations. As used in the present invention, the term "liposome" means a vesicle composed of amphiphilic lipids ananged in a spherical bilayer or bilayers. Liposomes are unilamellar or multilamellar vesicles which have a membrane formed from a lipophilic material and an aqueous interior that contains the composition to be delivered. Cationic liposomes are positively charged liposomes which are believed to interact with negatively charged DNA molecules to form a stable complex. Liposomes that are pH-sensitive or negatively-charged are believed to entrap DNA rather than complex with it. Both cationic and noncationic liposomes have been used to deliver DNA to cells.
[0228] Liposomes also include "sterically stabilized" liposomes, a term which, as used herein, refers to liposomes comprising one or more specialized lipids that, when incorporated into liposomes, result in enhanced circulation lifetimes relative to liposomes lacking such specialized lipids. Examples of sterically stabilized liposomes are those in which part of the vesicle-forming lipid portion of the
liposome comprises one or more glycolipids or is derivatized with one or more hydrophilic polymers, such as a polyethylene glycol (PEG) moiety. Liposomes and their uses are further described in U.S. Patent 6,287,860, which is incorporated herein in its entirety.
[0229] The pharmaceutical formulations and compositions of the present invention may also include surfactants. The use of surfactants in drag products, formulations and in emulsions is well known in the art. Surfactants and their uses are further described in U.S. Patent 6,287,860, which is incorporated herein in its entirety.
[0230] In one embodiment, the present invention employs various penetration enhancers to effect the efficient delivery of nucleic acids, particularly oligonucleotides. In addition to aiding the diffusion of non-lipophilic drags across cell membranes, penetration enhancers also enhance the permeability of lipophilic drags. Penetration enhancers may be classified as belonging to one of five broad categories, i.e., surfactants, fatty acids, bile salts, chelating agents, andnon- chelating non-surfactants. Penetration enhancers and their uses are further described in U.S. Patent 6,287,860, which is incorporated herein in its entirety. [0231] One of skill in the art will recognize that formulations are routinely designed according to their intended use, i.e. route of administration. [0232] Prefened formulations for topical administration include those in which the oligonucleotides of the invention are in admixture with a topical delivery agent such as lipids, liposomes, fatty acids, fatty acid esters, steroids, chelating agents and surfactants. Prefened lipids and liposomes include neutral (e.g. dioleoylphosphatidyl DOPE ethanolamine, dimyristoylphosphatidyl choline DMPC, distearolyphosphatidyl choline) negative (e.g. dimyristoylphosphatidyl glycerol DMPG) and cationic (e.g. dioleoyltetramethylaminopropyl DOTAP and dioleoylphosphatidyl ethanolamine DOTMA).
[0233] For topical or other administration, compounds and compositions of the invention may be encapsulated within liposomes or may form complexes thereto, in particular to cationic liposomes. Alternatively, they may be complexed to lipids, in particular to cationic lipids. Prefened fatty acids and esters,
pharmaceutically acceptable salts thereof, and their uses are further described in U.S. Patent 6,287,860, which is incorporated herein in its entirety. Topical formulations are described in detail in United States patent application 09/315,298 filed on May 20, 1999, which is incorporated herein by reference in its entirety. [0234] Compositions and formulations for oral administration include powders or granules, microparticulates, nanoparticulates, suspensions or solutions in water or non-aqueous media, capsules, gel capsules, sachets, tablets or minitablets. Thickeners, flavoring agents, diluents, emulsifiers, dispersing aids or binders may be desirable. Prefened oral formulations are those in which oligonucleotides of the invention are administered in conjunction with one or more penetration enhancers surfactants and chelators. Prefened surfactants include fatty acids and/or esters or salts thereof, bile acids and/or salts thereof. Prefened bile acids/salts and fatty acids and their uses are further described in U.S. Patent 6,287,860, which is incorporated herein in its entirety. Also prefened are combinations of penetration enhancers, for example, fatty acids/salts in combination with bile acids/salts. A particularly prefened combination is the sodium salt of lauric acid, capric acid and UDCA. Further penetration enhancers include polyoxyethylene-9-lauryl ether, polyoxyethylene-20-cetyl ether. Compounds and compositions of the invention may be delivered orally, in granular form including sprayed dried particles, or complexed to form micro or nanoparticles. Complexing agents and their uses are further described in U.S. Patent 6,287,860, which is incorporated herein in its entirety. Certain oral formulations for oligonucleotides and their preparation are described in detail in United States applications 09/108,673 (filed July 1, 1998), 09/315,298 (filed May 20, 1999) and 10/071,822, filed February 8, 2002, each of which is incorporated herein by reference in their entirety.
[0235] Compositions and formulations for parenteral, intrathecal or intraventricular administration may include sterile aqueous solutions that may also contain buffers, diluents and other suitable additives such as, but not limited to, penetration enhancers, carrier compounds and other pharmaceutically acceptable carriers or excipients.
[0236] Certain embodiments of the invention provide pharmaceutical compositions containing one or more of the compounds and compositions of the invention and one or more other chemotherapeutic agents that function by a non- antisense mechanism. Examples of such chemotherapeutic agents include but are not limited to cancer chemotherapeutic drags such as daunorubicin, daunomycin, dactinomycin, doxorubicin, epirubicin, idarabicin, esorabicin, bleomycin, mafosfamide, ifosfamide, cytosine arabinoside, bis-chloroethylnitrosurea, busulfan, mitomycin C, actinomycin D, mithramycin, prednisone, hydroxyprogesterone, testosterone, tamoxifen, dacarbazine, procarbazine, hexamethylmelamine, pentamethylmelamine, mitoxantrone, amsacrine, chlorambucil, methylcyclohexylnitrosurea, nitrogen mustards, melphalan, cyclophosphamide, 6-mercaptopurine, 6-thioguanine, cytarabine, 5-azacytidine, hydroxyurea, deoxycoformycin, 4-hydroxyperoxycyclophosphoramide, 5- fluorouracil (5-FU), 5-fluorodeoxyuridine (5-FUdR), methotrexate (MTX), colchicine, taxol, vincristine, vinblastine, etoposide (VP-16), trimetrexate, irinotecan, topotecan, gemcitabine, teniposide, cisplatin and diethylstilbestrol (DES). When used with the oligomeric compounds of the invention, such chemotherapeutic agents may be used individually (e.g., 5-FU and oligonucleotide), sequentially (e.g., 5-FU and oligonucleotide for a period of time followed by MTX and oligonucleotide), or in combination with one or more other such chemotherapeutic agents (e.g., 5-FU, MTX and oligonucleotide, or 5-FU, radiotherapy and oligonucleotide). Anti-inflammatory drugs, including but not limited to nonsteroidal anti-inflammatory drugs and corticosteroids, and antiviral drags, including but not limited to ribivirin, vidarabine, acyclovir and ganciclovir, may also be combined in compositions of the invention. Combinations of compounds and compositions of the invention and other drags are also within the scope of this invention. Two or more combined compounds such as two oligomeric compounds or one oligomeric compound combined with further compounds may be used together or sequentially.
[0237] In another related embodiment, compositions of the invention may contain one or more of the compounds and compositions of the invention targeted
to a first nucleic acid and one or more additional compounds such as antisense oligomeric compounds targeted to a second nucleic acid target. Numerous examples of antisense oligomeric compounds are known in the art. Alternatively, compositions of the invention may contain two or more oligomeric compounds and compositions targeted to different regions of the same nucleic acid target. Two or more combined compounds may be used together or sequentially
Dosing
[0238] The formulation of therapeutic compounds and compositions of the invention and their subsequent administration (dosing) is believed to be within the skill of those in the art. Dosing is dependent on severity and responsiveness of the disease state to be treated, with the course of treatment lasting from several days to several months, or until a cure is effected or a diminution of the disease state is achieved. Optimal dosing schedules can be calculated from measurements of drug accumulation in the body of the patient. Persons of ordinary skill can easily determine optimum dosages, dosing methodologies and repetition rates. Optimum dosages may vary depending on the relative potency of individual oligonucleotides, and can generally be estimated based on EC5oS found to be effective in in vitro and in vivo animal models. In general, dosage is from 0.01 ug to 100 g per kg of body weight, and may be given once or more daily, weekly, monthly or yearly, or even once every 2 to 20 years. Persons of ordinary skill in the art can easily estimate repetition rates for dosing based on measured residence times and concentrations of the drag in bodily fluids or tissues. Following successful treatment, it may be desirable to have the patient undergo maintenance therapy to prevent the recuπence of the disease state, wherein the oligonucleotide is admimstered in maintenance doses, ranging from 0.01 ug to 100 g per kg of body weight, once or more daily, to once every 20 years. [0239] While the present invention has been described with specificity in accordance with certain of its prefened embodiments, the following examples serve only to illustrate the invention and are not intended to limit the same.
Example 1
Synthesis of Nucleoside Phosphoramidites
[0240] The following compounds, including amidites and their intermediates were prepared as described in US Patent 6,426,220 and published PCT WO 02/36743; 5'-O-Dimethoxytrityl-thymidine intermediate for 5 -methyl dC amidite, 5'-O-Dimethoxytrityl-2'-deoxy-5-methylcytidine intermediate for 5-methyl-dC amidite, 5'-O-Dimethoxytrityl-2'-deoxy-N4-benzoyl-5-methylcytidine penultimate intemiediate for 5-methyl dC amidite, [5'-O-(4,4'-Dimethoxytriphenylmethyl)-2'- deoxy-N4-benzoyl-5-methylcytidin-3'-O-yl]-2-cyanoethyl-NN- diisopropylphosphoramidite (5-methyl dC amidite), 2'-Fluorodeoxyadenosine, 2'- Fluorodeoxyguanosine, 2'-Fluorouridine, 2'-Fluorodeoxycytidine, 2'-O-(2- Methoxyethyl) modified amidites, 2'-O-(2-methoxyethyl)-5-methyluridine intermediate, 5'-O-DMT-2'-O-(2-methoxyethyl)-5-methyluridine penultimate intermediate, [5'-O-(4,4'-Dimethoxytriphenylmethyl)-2'-O-(2-methoxyethyl)-5- methyluridin-3'-O-yl]-2-cyanoethyl-NN-diisopropylphosphoramidite (MOE T amidite), 5'-O-Dimethoxytrityl-2'-O-(2-methoxyethyl)-5-methylcytidine intermediate, 5'-O-dimethoxytrityl-2'-O-(2-methoxyethyl)-Ν4-benzoyl-5-methyl- cytidine penultimate intermediate, [5'-O-(4,4'-Dimethoxytriphenylmethyl)-2'-O- (2-methoxyethyl)-N4-benzoyl-5-methylcytidin-3'-O-yl]-2-cyanoethyl-NN- diisopropylphosphoramidite (MOE 5-Me-C amidite), [5'-O-(4,4'- Dimethoxytriphenylmethyl)-2'-O-(2-methoxyethyl)-Ν6-benzoyladenosin-3'-O-yl]- 2-cyanoethyl-NN-diisopropylphosphoramidite (MOE A amdite), [5'-O-(4,4'- Dimethoxytriphenylmethyl)-2'-O-(2-methoxyethyl)-Ν4-isobutyrylguanosin-3'-O- yl]-2-cyanoethyl-NN-diisopropylphosphoramidite (MOE G amidite), 2'-O- (Aminooxyethyl) nucleoside amidites and 2'-O-(dimethylaminooxyethyl) nucleoside amidites, 2'-(Dimethylaminooxyethoxy) nucleoside amidites, 5'-O-tert- Butyldiphenylsilyl-O -2'-anhydro-5-methyluridine , 5'-O-tert-Butyldiphenylsilyl- 2,-O-(2-hydroxyethyl)-5-methyluridine, 2'-O-([2-phthalimidoxy)ethyl]-5'-t- butyldiphenylsilyl-5-methyluridine , 5'-O-tert-butyldiphenylsilyl-2'-O-[(2- formadoximinooxy)ethyl]-5-methyluridine, 5'-O-tert-Butyldiphenylsilyl-2'-O- [Ν,Ν dimethylaminooxyethyl]-5-methyluridine, 2'-O-(dimethylaminooxyethyl)-5-
methyluridine, 5'-O-DMT-2'-O-(dimethylaminooxyethyl)-5-methyluridine, 5'-O- DMT-2'-O-(2-N,N-dimethylaminooxyethyl)-5-methyluridine-3'-[(2-cyanoethyl)- N,N-diisopropylphosphoramidite], 2'-(Aminooxyethoxy) nucleoside amidites, N2- isobutyryl-6-O-diphenylcarbamoyl-2'-O-(2-ethylacetyl)-5'-O-(4,4'- dimethoxytrityl)guanosine-3'-[(2-cyanoethyl)-N,N-diisopropylphosphoramidite], 2'-dimethylaminoethoxyethoxy (2'-DMAEOE) nucleoside amidites, 2'-O-[2(2- N,N-dimethylaminoethoxy)ethyl]-5-methyl uridine, 5'-O-dimethoxytrityl-2'-O- [2(2-N,N-dimethylaminoethoxy)-ethyl)]-5-methyl uridine and 5'-O- Dimethoxytrityl-2'-O-[2(2-N,N-dimethylaminoethoxy)-ethyl)]-5-methyl uridine- 3'-O-(cyanoethyl-N,N-diisopropyl)phosphoramidite.
Example 2
Oligonucleotide and oligonucleoside synthesis
[0241] Oligonucleotides: Unsubstituted and substituted phosphodiester (P=O) oligonucleotides are synthesized on an automated DNA synthesizer (Applied
Biosystems model 394) using standard phosphoramidite chemistry with oxidation by iodine.
[0242] Phosphorothioates (P=S) are synthesized similar to phosphodiester oligonucleotides with the following exceptions: filiation was effected by utilizing a 10% w/v solution of 3,H-l,2-benzodithiole-3-one 1,1-dioxide in acetonitrile for the oxidation of the phosphite linkages. The filiation reaction step time was increased to 180 sec and preceded by the normal capping step. After cleavage from the CPG column and deblocking in concentrated ammonium hydroxide at
55°C (12-16 hr), the oligonucleotides were recovered by precipitating with >3 volumes of ethanol from a 1 M NH OAc solution. Phosphinate oligonucleotides are prepared as described in U.S. Patent 5,508,270, herein incorporated by reference.
[0243] Alkyl phosphonate oligonucleotides are prepared as described in U.S.
Patent 4,469,863, herein incorporated by reference.
[0244] 3'-Deoxy-3'-methylene phosphonate oligonucleotides are prepared as described in U.S. Patents 5,610,289 or 5,625,050, herein incorporated by
reference.
[0245] Phosphoramidite oligonucleotides are prepared as described in U.S.
Patent, 5,256,775 or U.S. Patent 5,366,878, herein incorporated by reference.
[0246] Alkylphosphonothioate oligonucleotides are prepared as described in published PCT applications PCT/US94/00902 and PCT/US93/06976 (published as WO 94/17093 and WO 94/02499, respectively), herein incorporated by reference.
[0247] 3'-Deoxy-3'-amino phosphoramidate oligonucleotides are prepared as described in U.S. Patent 5,476,925, herein incorporated by reference.
[0248] Phosphotriester oligonucleotides are prepared as described in U.S. Patent
5,023,243, herein incorporated by reference.
[0249] Borano phosphate oligonucleotides are prepared as described in U.S.
Patents 5,130,302 and 5,177,198, both herein incorporated by reference.
[0250] Ohgonucleosides: Methylenemethyhmmo linked ohgonucleosides, also identified as MMI linked ohgonucleosides, methylenedimethylhydrazo linked ohgonucleosides, also identified as MDH linked ohgonucleosides, and methylenecarbonylamino linked ohgonucleosides, also identified as amide-3 linked ohgonucleosides, and methyleneaminocarbonyl linked ohgonucleosides, also identified as amide-4 linked ohgonucleosides, as well as mixed backbone oligomeric compounds having, for instance, alternating MMI and P=O or P=S linkages are prepared as described in U.S. Patents 5,378,825, 5,386,023,
5,489,677, 5,602,240 and 5,610,289, all of which are herein incorporated by reference.
[0251] Formacetal and thioformacetal linked ohgonucleosides are prepared as described in U.S. Patents 5,264,562 and 5,264,564, herein incorporated by reference.
[0252] Ethylene oxide linked ohgonucleosides are prepared as described in U.S.
Patent 5,223,618, herein incorporated by reference.
Example 3
RNA Synthesis
[0253] In general, RNA synthesis chemistry is based on the selective incorporation of various protecting groups at strategic intermediary reactions. Although one of ordinary skill in the art will understand the use of protecting groups in organic synthesis, a useful class of protecting groups includes silyl ethers. In particular bulky silyl ethers are used to protect the 5 '-hydroxyl in combination with an acid-labile orthoester protecting group on the 2 '-hydroxyl. This set of protecting groups is then used with standard solid-phase synthesis technology. It is important to lastly remove the acid labile orthoester protecting group after all other synthetic steps. Moreover, the early use of the silyl protecting groups during synthesis ensures facile removal when desired, without undesired deprotection of 2' hydroxyl.
[0254] Following this procedure for the sequential protection of the 5 '-hydroxyl in combination with protection of the 2 '-hydroxyl by protecting groups that are differentially removed and are differentially chemically labile, RNA oligonucleotides were synthesized.
[0255] RNA oligonucleotides are synthesized in a stepwise fashion. Each nucleotide is added sequentially (3 '- to 5 '-direction) to a solid support-bound oligonucleotide. The first nucleoside at the 3 '-end of the chain is covalently attached to a solid support. The nucleotide precursor, a ribonucleoside phosphoramidite, and activator are added, coupling the second base onto the 5 '- end of the first nucleoside. The support is washed and any unreacted 5 '-hydroxyl groups are capped with acetic anhydride to yield 5 '-acetyl moieties. The linkage is then oxidized to the more stable and ultimately desired P(V) linkage. At the end of the nucleotide addition cycle, the 5 '-silyl group is cleaved with fluoride. The cycle is repeated for each subsequent nucleotide.
[0256] Following synthesis, the methyl protecting groups on the phosphates are cleaved in 30 minutes utilizing 1 M disodium-2-carbamoyl-2-cyanoethylene-l,l- dithiolate trihydrate (S2Na2) in DMF. The deprotection solution is washed from the solid support-bound oligonucleotide using water. The support is then treated with 40%) methylamine in water for 10 minutes at 55 °C. This releases the RNA oligonucleotides into solution, deprotects the exocyclic amines, and modifies the
2'- groups. The oligonucleotides can be analyzed by anion exchange HPLC at this stage.
[0257] The 2 '-orthoester groups are the last protecting groups to be removed. The ethylene glycol monoacetate orthoester protecting group developed by Dharmacon Research, Inc. (Lafayette, CO), is one example of a useful orthoester protecting group which, has the following important properties. It is stable to the conditions of nucleoside phosphoramidite synthesis and oligonucleotide synthesis. However, after oligonucleotide synthesis the oligonucleotide is treated with methylamine which not only cleaves the oligonucleotide from the solid support but also removes the acetyl groups from the orthoesters. The resulting 2-ethyl- hydroxyl substituents on the orthoester are less electron withdrawing than the acetylated precursor. As a result, the modified orthoester becomes more labile to acid-catalyzed hydrolysis. Specifically, the rate of cleavage is approximately 10 times faster after the acetyl groups are removed. Therefore, this orthoester possesses sufficient stability in order to be compatible with oligonucleotide synthesis and yet, when subsequently modified, permits deprotection to be carried out under relatively mild aqueous conditions compatible with the final RNA oligonucleotide product.
[0258] Additionally, methods of RNA synthesis are well known in the art (Scaringe, S. A. Ph.D. Thesis, University of Colorado, 1996; Scaringe, S. A., et al., J. Am. Chem. Soc, 1998, 120, 11820-11821; Matteucci, M. D. and Caruthers, M. H. J. Am. Chem. Soc, 1981, 103, 3185-3191; Beaucage, S. L. and Caruthers, M. H. Tetrahedron Lett, 1981, 22, 1859-1862; Dahl, B. J., et al., Ada Chem. Scand,. 1990, 44, 639-641; Reddy, M. P., et al., Tetrahedrom Lett, 1994, 25, 4311-4314; Wincott, F. et al., Nucleic Acids Res., 1995, 23, 2677-2684; Griffin, B. E., et al, Tetrahedron, 1967, 23, 2301-2313; Griffin, B. E., et al., Tetrahedron, 1967, 23, 2315-2331).
Example 4
Synthesis of Chimeric Oligonucleotides
[0259] Chimeric oligonucleotides, ohgonucleosides or mixed oligonucleotides/oligonucleosides of the invention can be of several different types. These include a first type wherein the "gap" segment of linked nucleosides is positioned between 5' and 3' "wing" segments of linked nucleosides and a second "open end" type wherein the "gap" segment is located at either the 3' or the 5' terminus of the oligomeric compound. Oligonucleotides of the first type are also known in the art as "gapmers" or gapped oligonucleotides. Oligonucleotides of the second type are also known in the art as "hemimers" or "wingmers".
[2'-O-Me]~[2'-deoxy]~[2,-O-Me] Chimeric Phosphorothioate
Oligonucleotides [0260] Chimeric oligonucleotides having 2'-O-alkyl phosphorothioate and 2'- deoxy phosphorothioate oligonucleotide segments are synthesized using an Applied Biosystems automated DNA synthesizer Model 394, as above. Oligonucleotides are synthesized using the automated synthesizer and 2'-deoxy- 5'-dimethoxytrityl-3'-O-phosphoramidite for the DNA portion and 5'-dimethoxy- trityl-2'-O-methyl-3'-O-phosphoramidite for 5' and 3' wings. The standard synthesis cycle is modified by incorporating coupling steps with increased reaction times for the 5'-dimethoxytrityl-2l-O-methyl-3'-O-phosphoramidite. The fully protected oligonucleotide is cleaved from the support and deprotected in concentrated ammonia (NH4OH) for 12-16 hr at 55°C. The deprotected oligo is then recovered by an appropriate method (precipitation, column chromatography, volume reduced in vacuo and analyzed spetrophotometrically for yield and for purity by capillary electrophoresis and by mass spectrometry.
[2'-O-(2-Methoxyethyl)]~[2'-deoxy]~[2'-O-(Methoxyethyl)] Chimeric
Phosphorothioate Oligonucleotides [0261] [2'-O-(2-methoxyethyl)]-[2'-deoxy]~[-2'-O-(methoxyethyl)] chimeric phosphorothioate oligonucleotides were prepared as per the procedure above for the 2 '-O-methyl chimeric oligonucleotide, with the substitution of 2'-O- (methoxyethyl) amidites for the 2'-O-methyl amidites.
[2'-O-(2-Methoxyethyl)Phosphodiester]~[2'-deoxy Phosphorothioate]-
-[2'-O-(2-MethoxyethyI) Phosphodiester] Chimeric Oligonucleotides
[0262] [2'-O-(2-methoxyethyl phosphodiester]~[2'-deoxy phosphorothioate]-- [2'-O-(methoxyethyl) phosphodiester] chimeric oligonucleotides are prepared as per the above procedure for the 2'-O-methyl chimeric oligonucleotide with the substitution of 2'-O-(methoxyethyl) amidites for the 2'-O-methyl amidites, oxidation with iodine to generate the phosphodiester intemucleotide linkages within the wing portions of the cliimeric stractures and sulfurization utilizing 3,H- 1,2 benzodithiole-3-one 1,1 dioxide (Beaucage Reagent) to generate the phosphorothioate intemucleotide linkages for the center gap. [0263] Other chimeric oligonucleotides, chimeric ohgonucleosides and mixed chimeric oligonucleotides/oligonucleosides.are synthesized according to United States patent 5,623,065, herein incorporated by reference.
Example 5
Design and screening of duplexed oligomeric compounds targeting a target [0264] In accordance with the present invention, a series of nucleic acid duplexes comprising the antisense oligomeric compounds of the present invention and their complements can be designed to target a target. The ends of the strands may be modified by the addition of one or more natural or modified nucleobases to form an overhang. The sense strand of the dsRNA is then designed and synthesized as the complement of the antisense strand and may also contain modifications or additions to either terminus. For example, in one embodiment, both strands of the dsRNA duplex would be complementary over the central nucleobases, each having overhangs at one or both termini. [0265] For example, a duplex comprising an antisense strand having the sequence CGAGAGGCGGACGGGACCG (SEQ ID NO:l) and having a two- nucleobase overhang of deoxythymidine(dT) would have the following stracture:
5' c g a g a g g c g g a c g g g a c c g T T 3' Antisense Strand (SEQ ID NO:
3' T T g c t c t c c g c c t g c c c t g g c 5' Compliment Strand (SEQ ID N
[0266] RNA strands of the duplex can be synthesized by methods disclosed
herein or purchased from Dharmacon Research Inc., (Lafayette, CO). Once synthesized, the complementary strands are annealed. The single strands are aliquoted and diluted to a concentration of 50 uM. Once diluted, 30 uL of each strand is combined with 15uL of a 5X solution of annealing buffer. The final concentration of said buffer is 100 mM potassium acetate, 30 mM HEPES-KOH pH 7.4, and 2mM magnesium acetate. The final volume is 75 uL. This solution is incubated for 1 minute at 90°C and then centrifuged for 15 seconds. The tube is allowed to sit for 1 hour at 37°C at which time the dsRNA duplexes are used in experimentation. The final concentration of the dsRNA duplex is 20 uM. This solution can be stored frozen (-20°C) and freeze-thawed up to 5 times. [0267] Once prepared, the duplexed antisense oligomeric compounds are evaluated for their ability to modulate a target expression. [0268] When cells reached 80% confluency, they are treated with duplexed antisense oligomeric compounds of the invention. For cells grown in 96-well plates, wells are washed once with 200 μL OPTI-MEM-1 reduced-serum medium (Gibco BRL) and then treated with 130 μL of OPTI-MEM-1 containing 12 μg/mL LIPOFECTIN (Gibco BRL) and the desired duplex antisense oligomeric compound at a final concentration of 200 nM. After 5 hours of treatment, the medium is replaced with fresh medium. Cells are harvested 16 hours after treatment, at which time RNA is isolated and target reduction measured by RT- PCR.
Example 6
Oligonucleotide Isolation
[0269] After cleavage from the controlled pore glass solid support and deblocking in concentrated ammonium hydroxide at 55°C for 12-16 hours, the oligonucleotides or ohgonucleosides are recovered by precipitation out of 1 M NH4OAc with >3 volumes of ethanol. Synthesized oligonucleotides were analyzed by elecfrospray mass spectroscopy (molecular weight determination) and by capillary gel electrophoresis and judged to be at least 70%o full length material. The relative amounts of phosphorothioate and phosphodiester linkages obtained in
the synthesis was determined by the ratio of conect molecular weight relative to the -16 amu product (+/-32 +/-48). For some studies oligonucleotides were purified by HPLC, as described by Chiang et al, J. Biol. Chem. 1991, 266, 18162- 18171. Results obtained with HPLC-purified material were similar to those obtained with non-HPLC purified material..
Example 7
Oligonucleotide Synthesis - 96 Well Plate Format
[0270] Oligonucleotides were synthesized via solid phase P(ffl) phosphoramidite chemistry on an automated synthesizer capable of assembling 96 sequences simultaneously in a 96-well format. Phosphodiester intemucleotide linkages were afforded by oxidation with aqueous iodine. Phosphorothioate intemucleotide linkages were generated by sulfurization utilizing 3,H-1,2 benzodithiole-3-one 1,1 dioxide (Beaucage Reagent) in anhydrous acetonitrile. Standard base-protected beta-cyanoethyl-diiso-propyl phosphoramidites were purchased from commercial vendors (e.g. PE- Applied Biosystems, Foster City, CA, or Pharmacia, Piscataway, NJ). Non-standard nucleosides are synthesized as per standard or patented methods. They are utilized as base protected beta- cyanoethyldiisopropyl phosphoramidites.
[0271] Oligonucleotides were cleaved from support and deprotected with concentrated NH4OH at elevated temperature (55-60°C) for 12-16 hours and the released product then dried in vacuo. The dried product was then re-suspended in sterile water to afford a master plate from which all analytical and test plate samples are then diluted utilizing robotic pipettors.
Example 8
Oligonucleotide Analysis - 96- Well Plate Format
[0272] The concentration of oligonucleotide in each well was assessed by dilution of samples and UN absorption spectroscopy. The full-length integrity of the individual products was evaluated by capillary electrophoresis (CE) in either the 96-well format (Beckman P/ACE™ MDQ) or, for individually prepared
samples, on a commercial CE apparatus (e.g., Beckman P/ACE™ 5000, ABI 270). Base and backbone composition was confirmed by mass analysis of the oligomeric compounds utilizing electrospray-mass spectroscopy. All assay test plates were diluted from the master plate using single and multi-channel robotic pipettors. Plates were judged to be acceptable if at least 85% of the oligomeric compounds on the plate were at least 85% full length.
Example 9
Cell culture and oligonucleotide treatment
[0273] The effect of oligomeric compounds on target nucleic acid expression can be tested in any of a variety of cell types provided that the target nucleic acid is present at measurable levels. This can be routinely determined using, for example, PCR or Northern blot analysis. The following cell types are provided for illustrative purposes, but other cell types can be routinely used, provided that the target is expressed in the cell type chosen. This can be readily determined by methods routine in the art, for example Northern blot analysis, ribonuclease protection assays, or RT-PCR. T-24 cells:
[0274] The human transitional cell bladder carcinoma cell line T-24 was obtained from the American Type Culture Collection (ATCC) (Manassas, VA). T-24 cells were routinely cultured in complete McCoy's 5 A basal media (Invitrogen Corporation, Carlsbad, CA) supplemented with 10% fetal calf serum (Invitrogen Corporation, Carlsbad, CA), penicillin 100 units per mL, and streptomycin 100 micrograms per mL (Invitrogen Corporation, Carlsbad, CA). Cells were routinely passaged by trypsinization and dilution when they reached 90% confluence. Cells were seeded into 96-well plates (Falcon-Primaria #353872) at a density of 7000 cells/well for use in RT-PCR analysis. [0275] For Northern blotting or other analysis, cells may be seeded onto 100 mm or other standard tissue culture plates and treated similarly, using appropriate volumes of medium and oligonucleotide. A549 cells:
[0276] The human lung carcinoma cell line A549 was obtained from the American Type Culture Collection (ATCC) (Manassas, VA). A549 cells were routinely cultured in DMEM basal media (Invitrogen Corporation, Carlsbad, CA) supplemented with 10%) fetal calf serum (Invitrogen Corporation, Carlsbad, CA), penicillin 100 units per mL, and streptomycin 100 micrograms per mL (Invitrogen Corporation, Carlsbad, CA). Cells were routinely passaged by trypsinization and dilution when they reached 90% confluence. NHDF cells:
[0277] Human neonatal dermal fibroblast (NHDF) were obtained from the Clonetics Corporation (WalkersviUe, MD). NHDFs were routinely maintained in Fibroblast Growth Medium (Clonetics Corporation, WalkersviUe, MD) supplemented as recommended by the supplier. Cells were maintained for up to 10 passages as recommended by the supplier. HEK cells:
[0278] Human embryonic keratinocytes (HEK) were obtained from the Clonetics Corporation (WalkersviUe, MD). HEKs were routinely maintained in Keratinocyte Growth Medium (Clonetics Corporation, WalkersviUe, MD) formulated as recommended by the supplier. Cells were routinely maintained for up to 10 passages as recommended by the supplier. Treatment with antisense oligomeric compoiinds: [0279] When cells reached 65-75% confluency, they were treated with oligonucleotide. For cells grown in 96-well plates, wells were washed once with 100 μL OPTI-MEM™-l reduced-serum medium (Invitrogen Corporation, Carlsbad, CA) and then treated with 130 μL of OPTI-MEM™-l containing 3.75 μg/mL LIPOFECTIN™ (Invitrogen Corporation, Carlsbad, CA) and the desired concentration of oligonucleotide. Cells are treated and data are obtained in triplicate. After 4-7 hours of treatment at 37°C, the medium was replaced with' fresh medium. Cells were harvested 16-24 hours after oligonucleotide treatment. [0280] The concentration of oligonucleotide used varies from cell line to cell line. To determine the optimal oligonucleotide concentration for a particular cell line, the cells are treated with a positive control oligonucleotide at a range of
concentrations. For human cells the positive control oligonucleotide is selected from either ISIS 13920 (TCCGTCATCGCTCCTCAGGG, SEQ ID NO: 4) which is targeted to human H-ras, or ISIS 18078,
(GTGCGCGCGAGCCCGAAATC, SEQ ID NO: 5) which is targeted to human Jun-N-terminal kinase-2 (JNK2). Both controls are 2'-O-methoxyethyl gapmers (2'-O-methoxyethyls shown in bold) with a phosphorothioate backbone. For mouse or rat cells the positive control oligonucleotide is ISIS 15770, ATGCATTCTGCCCCCAAGGA (SEQ ID NO: 6) a 2*-O-methoxyethyl gapmer (2'-O-methoxyethyls shown in bold) with a phosphorothioate backbone which is targeted to both mouse and rat c-raf. The concentration of positive control oligonucleotide that results in 80% inhibition of c-H-ras (for ISIS 13920), JNK2 (for ISIS 18078) or c-raf (for ISIS 15770) mRNA is then utilized as the screening concentration for new oligonucleotides in subsequent experiments for that cell line. If 80% inhibition is not achieved, the lowest concentration of positive control oligonucleotide that results in 60% inhibition of c-H-ras, JNK2 or c-raf mRNA is then utilized as the oligonucleotide screening concentration in subsequent experiments for that cell line. If 60% inhibition is not achieved, that particular cell line is deemed as unsuitable for oligonucleotide transfection experiments. The concentrations of antisense oligonucleotides used herein are

Example 10
Analysis of oligonucleotide inhibition of a target expression
[0281] Modulation of a target expression can be assayed in a variety of ways known in the art. For example, a target mRNA levels can be quantitated by, e.g., Northern blot analysis, competitive polymerase chain reaction (PCR), or real-time PCR (RT-PCR). Real-time quantitative PCR is presently prefened. RNA analysis can be performed on total cellular RNA or poly(A)+ mRNA. The prefened method of RNA analysis of the present invention is the use of total cellular RNA as described in other examples herein. Methods of RNA isolation are well known in the art. Northern blot analysis is also routine in the art. Real-
time quantitative (PCR) can be conveniently accomplished using the commercially available ABI PRISM™ 7600, 7700, or 7900 Sequence Detection System, available from PE- Applied Biosystems, Foster City, CA and used according to manufacturer's instructions.
[0282] Protein levels of a target can be quantitated in a variety of ways well known in the art, such as immunoprecipitation, Western blot analysis (immunoblotting), enzyme-linked immunosorbent assay (ELISA) or fluorescence- activated cell sorting (FACS). Antibodies directed to a target can be identified and obtained from a variety of sources, such as the MSRS catalog of antibodies (Aerie Corporation, Birmingham, MI), or can be prepared via conventional monoclonal or polyclonal antibody generation methods well known in the art.
Example 11
Design of phenotypic assays and in vivo studies for the use of a target inhibitors
Phenotypic assays
[0283] Once a target inhibitors have been identified by the methods disclosed herein, the oligomeric compounds are further investigated in one or more phenotypic assays, each having measurable endpoints predictive of efficacy in the treatment of a particular disease state or condition.
[0284] Phenotypic assays, kits and reagents for their use are well known to those skilled in the art and are herein used to investigate the role and/or association of a target in health and disease. Representative phenotypic assays, which can be purchased from any one of several commercial vendors, include those for determining cell viability, cytotoxicity, proliferation or cell survival (Molecular Probes, Eugene, OR; PerkinElmer, Boston, MA), protein-based assays including enzymatic assays (Panvera, LLC, Madison, WI; BD Biosciences, Franklin Lakes, NJ; Oncogene Research Products, San Diego, CA), cell regulation, signal transduction, inflammation, oxidative processes and apoptosis (Assay Designs Inc., Ann Arbor, MI), triglyceride accumulation (Sigma-Aldrich, St. Louis, MO), angiogenesis assays, tube formation assays, cytokine and hormone assays and
metabolic assays (Chemicon International Inc., Temecula, CA; Amersham Biosciences, Piscataway, NJ).
[0285] In one non-limiting example, cells determined to be appropriate for a particular phenotypic assay (i.e., MCF-7 cells selected for breast cancer studies; adipocytes for obesity studies) are treated with a target inhibitors identified from the in vitro studies as well as control compounds at optimal concentrations which are determined by the methods described above. At the end of the treatment period, treated and untreated cells are analyzed by one or more methods specific for the assay to determine phenotypic outcomes and endpoints. Phenotypic endpoints include changes in cell morphology over time or treatment dose as well as changes in levels of cellular components such as proteins, lipids, nucleic acids, hormones, saccharides or metals. Measurements of cellular status which include pH, stage of the cell cycle, intake or excretion of biological indicators by the cell, are also endpoints of interest.
[0286] Analysis of the geneotype of the cell (measurement of the expression of one or more of the genes of the cell) after treatment is also used as an indicator of the efficacy or potency of the target inliibitors. Hallmark genes, or those genes suspected to be associated with a specific disease state, condition, or phenotype, are measured in both treated and untreated cells. In vivo studies
[0287] The individual subjects of the in vivo studies described herein are warmblooded vertebrate animals, which includes humans.
The clinical trial is subjected to rigorous controls to ensure that individuals are not unnecessarily put at risk and that they are fully informed about their role in the study.
[0288] To account for the psychological effects of receiving treatments, volunteers are randomly given placebo or a target inhibitor. Furthermore, to prevent the doctors from being biased in treatments, they are not informed as to whether the medication they are administering is a a target inhibitor or a placebo. Using this randomization approach, each volunteer has the same chance of being given either the new treatment or the placebo.
[0289] Volunteers receive either the a target inhibitor or placebo for eight week period with biological parameters associated with the indicated disease state or condition being measured at the beginning (baseline measurements before any treatment), end (after the final treatment), and at regular intervals during the study period. Such measurements include the levels of nucleic acid molecules encoding a target or a target protein levels in body fluids, tissues or organs compared to pretreatment levels. Other measurements include, but are not limited to, indices of the disease state or condition being treated, body weight, blood pressure, serum titers of pharmacologic indicators of disease or toxicity as well as ADME (absorption, distribution, metabolism and excretion) measurements. Information recorded for each patient includes age (years), gender, height (cm), family history of disease state or condition (yes/no), motivation rating (some/moderate/great) and number and type of previous treatment regimens for the indicated disease or condition.
[0290] Volunteers taking part in this study are healthy adults (age 18 to 65 years) and roughly an equal number of males and females participate in the study. Volunteers with certain characteristics are equally distributed for placebo and a target inhibitor treatment. In general, the volunteers treated with placebo have little or no response to treatment, whereas the volunteers treated with the target inhibitor show positive trends in their disease state or condition index at the conclusion of the study.
Example 12 RNA Isolation
Poly (A) + mRNA isolation
[0291] Poly(A)+ mRNA was isolated according to Miura et al, (Clin. Chem., 1996, 42, 1758-1764). Other methods for poly(A)+ mRNA isolation are routine in the art. Briefly, for cells grown on 96-well plates, growth medium was removed from the cells and each well was washed with 200 μL cold PBS. 60 μL lysis buffer (10 mM Tris-HCl, pH 7.6, 1 mM EDTA, 0.5 M NaCl, 0.5% NP-40, 20 mM vanadyl-ribonucleoside complex) was added to each well, the plate was
gently agitated and then incubated at room temperature for five minutes. 55 μL of lysate was fransfened to Oligo d(T) coated 96-well plates (AGCT Inc., Irvine CA). Plates were incubated for 60 minutes at room temperature, washed 3 times with 200 μL of wash buffer (10 mM Tris-HCl pH 7.6, 1 mM EDTA, 0.3 M NaCl). After the final wash, the plate was blotted on paper towels to remove excess wash buffer and then air-dried for 5 minutes. 60 μL of elution buffer (5 mM Tris-HCl pH 7.6), preheated to 70°C, was added to each well, the plate was incubated on a 90°C hot plate for 5 minutes, and the eluate was then fransfened to a fresh 96-well plate.
[0292] Cells grown on 100 mm or other standard plates may be treated similarly, using appropriate volumes of all solutions. Total RNA Isolation
[0293] Total RNA was isolated using an RNEASY 96™ kit and buffers purchased from Qiagen Inc. (Valencia, CA) following the manufacturer's recommended procedures. Briefly, for cells grown on 96-well plates, growth medium was removed from the cells and each well was washed with 200 μL cold PB S . 150 μL Buffer RLT was added to each well and the plate vigorously agitated for 20 seconds. 150 μL of 70% ethanol was then added to each well and the contents mixed by pipetting three times up and down. The samples were then fransfened to the RNEASY 96™ well plate attached to a QIAVAC™ manifold fitted with a waste collection tray and attached to a vacuum source. Vacuum was applied for 1 minute. 500 μL of Buffer RW1 was added to each well of the RNEASY 96™ plate and incubated for 15 minutes and the vacuum was again applied for 1 minute. An additional 500 μL of Buffer RW1 was added to each well of the RNEASY 96™ plate and the vacuum was applied for 2 minutes. 1 mL of Buffer RPE was then added to each well of the RNEASY 96™ plate and the vacuum applied for a period of 90 seconds. The Buffer RPE wash was then repeated and the vacuum was applied for an additional 3 minutes. The plate was then removed from the QIAVAC™ manifold and blotted dry on paper towels. The plate was then re-attached to the QIAVAC™ manifold fitted with a collection tube rack containing 1.2 mL collection tubes. RNA was then eluted by pipetting 140 μL of RNAse free water into each well, incubating 1 minute, and then applying the vacuum for 3 minutes.
[0294] The repetitive pipetting and elution steps may be automated using a QIAGEN Bio-Robot 9604 (Qiagen, Inc., Valencia CA). Essentially, after lysing of the cells on the culture plate, the plate is fransfened to the robot deck where the pipetting, DNase treatment and elution steps are carried out.
Example 13
Real-time Quantitative PCR Analysis of a target mRNA Levels
[0295] Quantitation of a target mRNA levels was accomplished by real-time quantitative PCR using the ABI PRISM™ 7600, 7700, or 7900 Sequence Detection System (PE- Applied Biosystems, Foster City, CA) according to manufacturer's instructions. This is a closed-tube, non-gel-based, fluorescence detection system which allows high-throughput quantitation of polymerase chain reaction (PCR) products in real-time. As opposed to standard PCR in which amplification products are quantitated after the PCR is completed, products in real-time quantitative PCR are quantitated as they accumulate. This is accomplished by including in the PCR reaction an oligonucleotide probe that anneals specifically between the forward and reverse PCR primers, and contains two fluorescent dyes. A reporter dye (e.g., FAM or JOE, obtained from either PE- Applied Biosystems, Foster City, CA, Operon Technologies Inc., Alameda, CA or Integrated DNA Technologies Inc., Coralville, IA) is attached to the 5' end of the probe and a quencher dye (e.g., TAMRA, obtained from either PE-Applied Biosystems, Foster City, CA, Operon Technologies Inc., Alameda, CA or Integrated DNA Technologies Inc., Coralville, IA) is attached to the 3' end of the probe. When the probe and dyes are intact, reporter dye emission is quenched by the proximity of the 3' quencher dye. During amplification, annealing of the probe to the target sequence creates a substrate that can be cleaved by the 5'- exonuclease activity of Taq polymerase. During the extension phase of the PCR amplification cycle, cleavage of the probe by Taq polymerase releases the reporter dye from the remainder of the probe (and hence from the quencher moiety) and a sequence-specific fluorescent signal is generated. With each cycle, additional reporter dye molecules are cleaved from their respective probes, and the
fluorescence intensity is monitored at regular intervals by laser optics built into the ABI PRISM™ Sequence Detection System, h each assay, a series of parallel reactions containing serial dilutions of mRNA from untreated control samples generates a standard curve that is used to quantitate the percent inhibition after antisense oligonucleotide treatment of test samples.
[0296] Prior to quantitative PCR analysis, primer-probe sets specific to the target gene being measured are evaluated for their ability to be "multiplexed" with a GAPDH amplification reaction. In multiplexing, both the target gene and the internal standard gene GAPDH are amplified concunently in a single sample. In this analysis, mRNA isolated from untreated cells is serially diluted. Each dilution is amplified in the presence of primer-probe sets specific for GAPDH only, target gene only ("single-plexing"), or both (multiplexing). Following PCR amplification, standard curves of GAPDH and target mRNA signal as a function of dilution are generated from both the single-plexed and multiplexed samples. If both the slope and conelation coefficient of the GAPDH and target signals generated from the multiplexed samples fall within 10% of their conesponding values generated from the single-plexed samples, the primer-probe set specific for that target is deemed multiplexable. Other methods of PCR are also known in the art.
[0297] PCR reagents were obtained from Invitrogen Corporation, (Carlsbad, CA). RT-PCR reactions were carried out by adding 20 μL PCR cocktail (2.5x PCR buffer minus MgCl2, 6.6 mM MgCl2, 375 μM each of dATP, dCTP, dCTP and dGTP, 375 nM each of forward primer and reverse primer, 125 nM of probe, 4 Units RNAse inhibitor, 1.25 Units PLATINUM® Taq, 5 Units MuLV reverse transcriptase, and 2.5x ROX dye) to 96-well plates containing 30 μL total RNA solution (20-200 ng). The RT reaction was carried out by incubation for 30 minutes at 48°C. Following a 10 minute incubation at 95°C to activate the PLATINUM® Taq, 40 cycles of a two-step PCR protocol were carried out: 95°C for 15 seconds (denaturation) followed by 60°C for 1.5 minutes (annealing/extension) .
[0298] Gene target quantities obtained by real time RT-PCR are normalized using either the expression level of GAPDH, a gene whose expression is constant, or by quantifying total RNA using RiboGreen™ (Molecular Probes, Inc. Eugene, OR). GAPDH expression is quantified by real time RT-PCR, by being run simultaneously with the target, multiplexing, or separately. Total RNA is quantified using RiboGreen™ RNA quantification reagent (Molecular Probes, Inc. Eugene, OR). Methods of RNA quantification by RiboGreen™ are taught in Jones, L.J., et al, (Analytical Biochemistry, 1998, 265, 368-374). [0299] In this assay, 170 μL of RiboGreen™ working reagent (RiboGreen™ reagent diluted 1:350 in lOmM Tris-HCl, 1 mM EDTA, pH 7.5) is pipetted into a 96-well plate containing 30 μL purified, cellular RNA. The plate is read in a CytoFluor 4000 (PE Applied Biosystems) with excitation at 485nm and emission at 530nm.
[0300] Probes and primers are designed to hybridize to a human a target sequence, using published sequence information.
Example 14
Northern blot analysis of a target mRNA levels
[0301] Eighteen hours after treatment, cell monolayers were washed twice with cold PBS and lysed in 1 mL RNAZOL™ (TEL-TEST "B" Inc., Friendswood, TX). Total RNA was prepared following manufacturer's recommended protocols. Twenty micrograms of total RNA was fractionated by electrophoresis through 1.2% agarose gels containing 1.1% formaldehyde using a MOPS buffer system (AMRESCO, Inc. Solon, OH). RNA was fransfened from the gel to HYBOND™-N+ nylon membranes (Amersham Pharmacia Biotech, Piscataway, NJ) by overnight capillary fransfer using a Northern/Southem Transfer buffer system (TEL-TEST "B" Inc., Friendswood, TX). RNA transfer was confirmed by UN visualization. Membranes were fixed by UN cross-linking using a STRATALIΝKER™ UV Crosslinker 2400 (Stratagene, Inc, La Jolla, CA) and then probed using QUICIGIYB™ hybridization solution (Stratagene, La Jolla,
CA) using manufacturer's recommendations for stringent conditions.
[0302] To detect human a target, a human a target specific primer probe set is prepared by PCR To normalize for variations in loading and transfer efficiency membranes are stripped and probed for human glyceraldehyde-3 -phosphate dehydrogenase (GAPDH) RNA (Clontech, Palo Alto, CA).
[0303] Hybridized membranes were visualized and quantitated using a
PHOSPHORIMAGER™ and IMAGEQUANT™ Software V3.3 (Molecular
Dynamics, Sunnyvale, CA). Data was normalized to GAPDH levels in untreated controls.
Example 15
Inhibition of human a target expression by oligonucleotides
[0304] In accordance with the present invention, a series of oligomeric compounds are designed to target different regions of the human target RNA. The oligomeric compounds are analyzed for their effect on human target mRNA levels by quantitative real-time PCR as described in other examples herein. Data are averages from tliree experiments. The target regions to which these prefened sequences are complementary are herein refened to as "prefened target segments" and are therefore prefened for targeting by oligomeric compounds of the present invention. The sequences represent the reverse complement of the prefened antisense oligomeric compounds.
[0305] As these "prefened target segments" have been found by experimentation to be open to, and accessible for, hybridization with the antisense oligomeric compounds of the present invention, one of skill in the art will recognize or be able to ascertain, using no more than routine experimentation, further embodiments of the invention that encompass other oligomeric compounds that specifically hybridize to these prefened target segments and consequently inhibit the expression of a target.
[0306] According to the present invention, antisense oligomeric compounds include antisense oligomeric compounds, antisense oligonucleotides, ribozymes,
extemal guide sequence (EGS) oligonucleotides, alternate splicers, primers, probes, and other short oligomeric compounds that hybridize to at least a portion of the target nucleic acid.
Example 16
Western blot analysis of a target protein levels
[0307] Western blot analysis (immunoblot analysis) is carried out using standard methods. Cells are harvested 16-20 h after oligonucleotide treatment, washed once with PBS, suspended in Laemmli buffer (100 ul/well), boiled for 5 minutes and loaded on a 16% SDS-PAGE gel. Gels are run for 1.5 hours at 150 V, and fransfened to membrane for western blotting. Appropriate primary antibody directed to a target is used, with a radiolabeled or fluofescently labeled secondary antibody directed against the primary antibody species. Bands are visualized using a PHOSPHORJ-MAGER™ (Molecular Dynamics, Sunnyvale CA).
Example 17
Synthesis of Phosphoramidite Derivatives
[0308] Phosphoramidite derivatives are synthesized by the methods taught by
U.S. Patent No. 5,567,811.
Example 18
Synthesis of Arabinonucleotides
[0309] Arabinonucleotides are synthesized by methods taught by Damha et. al.,
J. Am. Chem. Soc. 1998, 120, 12976-12977 and Damha et. al., Bioconjugate
Chem. 1999, 10, 299-305.
Example 19
Synthesis of Cyclobutyl Nucleic Acids
[0310] Cyclobutyl nucleic acids are synthesized by the methods taught by U.S.
Patent No. 6,001,841.
Example 20
Synthesis of Cyclopentyl Nucleic Acids
[0311] Cyclopentyl nucleic acids are synthesized by the methods taught by U.S.
Patent No. 5,602,240.
Example 21
Synthesis of Proline Nucleic Acids
[0312] Proline nucleic acids are synthesized by the methods taught in U.S. Patent Nos. 5,519,134 and 5,714,606.
Example 22
Synthesis of 4'-Thioribonucleotides
[0313] 4 ' -Thioribonucleotides are synthesized by the methods taught by U. S . Patent No. 5,639,873.
Example 23
Synthesis of Oligonucleotides Containing a Hexose Sugar
[0314] Oligonucleotides containing a hexose sugar are synthesized as described in U.S. Patent No. 5,780,607.
Example 24
Synethsis of Cyclohexene Nucleic Acids
[0315] Cyclohexene nucleic acids are synthesized by the method of Wang et al.,
J. Am. Chem. Soc. 2000, 122, 8595-8602.
Example 25
Synthesis of Compostions Comprising a Xylonucleoside
[0316] Oligonucleotides containing a xylose sugar are synthesized as described in U.S. Patent No. 6,329,346.
Example 26
Synthesis of Compositions Comprising a Threonucleoside
[0317] Oligonucleotides containing a threose sugar are synthesized as described in. Chaput et al., J. Am. Chem. Soc. 2003, 125, 856-57, Schoning et al., Science 2000, 290(5495), 1347-51 and Wu et al., Org. Lett. 2002, 4, 1279-82.
Example 27
Preparation of l'-Ethoxy-3',5',5-tris(methoxybenzyl)thymidine
[0318] The title compound can be synthesized as described in U.S. Patent No.
5,712,378.
Example 28
4'-Thio modified constructs
[0319] Strands listed below can be made by methods of Example 22 and and can be duplexed with the complentary strand. Monomers in bold are 4'- thioribonucleosides. Non-bolded monomers are ribonucleosides. Underlined monomers have phosphothioate linkages. Other linkages are phosphodiester.
SEQIDNO. Sequence (5' 3')
7 UUUGUCUCUGGUCCUUACUU
7 UUU GUCUCUGGUCCUUAC UU
7 UUU GUC UCU GGU CCUUAC UU
7 UUU GUC UCU GGU CCUUACUU
[0320] The above constracts can be aassayed for PTEN mRNA level against an untreated control.
Example 29
4'-Thio modified nucleosides in the antisense strand of siRNAs
[0321] The antisense (AS) strands listed below were individually duplexed with the complementary RNA sense strand. Monomers in bold are 4'- thioribonucleosides (4'S). Oligomers with phosphothioate linkages are listed as
- HO ¬
PS. PO linkages are phosphodiester,
SEQ ID NO./ISIS NO. Sequence (3 ' 5 ') Linkage Sugar
8/303912 UUC AUU CCU GGU CUC UGU UU PS 2'OH
8/336675 UUC AUU CCU GGU CUC UGU UU PO 4'S
8/336671 UUC AUU CCU GGU CUC UGU UU PO 4'S
8/336674 UUC AUU CCU GGU CUC UGU UU PO 4'S
8/336672 UUC AUU CCU GGU CUC UGU UU PO 4'S
8/336673 UUC AUU CCU GGU CUC UGU UU PO 4'S
8/336676 UUC AUU CCU GGU CUC UGU UU PO 4'S
8/336678 UUC AUU CCU GGU CUC UGU UU PO 4'S
[0322] The compounds were assayed for PTEN mRNA level against an untreated control. The results are presented in the following graph.