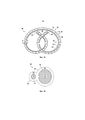
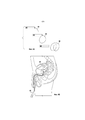
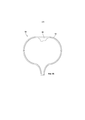
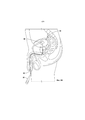
RU2694902C1 - Системы и способы доставки лекарственных средств для лечения рака мочевого пузыря гемцитабином - Google Patents
Системы и способы доставки лекарственных средств для лечения рака мочевого пузыря гемцитабином Download PDFInfo
- Publication number
- RU2694902C1 RU2694902C1 RU2016136103A RU2016136103A RU2694902C1 RU 2694902 C1 RU2694902 C1 RU 2694902C1 RU 2016136103 A RU2016136103 A RU 2016136103A RU 2016136103 A RU2016136103 A RU 2016136103A RU 2694902 C1 RU2694902 C1 RU 2694902C1
- Authority
- RU
- Russia
- Prior art keywords
- gemcitabine
- bladder
- drug
- patient
- day
- Prior art date
Links
- SDUQYLNIPVEERB-QPPQHZFASA-N gemcitabine Chemical compound O=C1N=C(N)C=CN1[C@H]1C(F)(F)[C@H](O)[C@@H](CO)O1 SDUQYLNIPVEERB-QPPQHZFASA-N 0.000 title claims abstract description 165
- 239000003814 drug Substances 0.000 title claims abstract description 164
- 229960005277 gemcitabine Drugs 0.000 title claims abstract description 163
- 238000000034 method Methods 0.000 title claims abstract description 36
- 208000007097 Urinary Bladder Neoplasms Diseases 0.000 title claims abstract description 20
- 206010005003 Bladder cancer Diseases 0.000 title claims abstract description 18
- 201000005112 urinary bladder cancer Diseases 0.000 title claims abstract description 15
- 229940079593 drug Drugs 0.000 title claims description 147
- 210000002700 urine Anatomy 0.000 claims abstract description 36
- 238000012377 drug delivery Methods 0.000 claims abstract description 23
- XSQUKJJJFZCRTK-UHFFFAOYSA-N Urea Chemical compound NC(N)=O XSQUKJJJFZCRTK-UHFFFAOYSA-N 0.000 claims description 14
- 239000004202 carbamide Substances 0.000 claims description 14
- 230000001225 therapeutic effect Effects 0.000 claims description 13
- 230000014759 maintenance of location Effects 0.000 claims description 11
- 229940124597 therapeutic agent Drugs 0.000 claims description 10
- 239000002562 thickening agent Substances 0.000 claims description 9
- 210000003708 urethra Anatomy 0.000 claims description 8
- 239000002357 osmotic agent Substances 0.000 claims description 7
- 239000002552 dosage form Substances 0.000 claims description 6
- 238000011200 topical administration Methods 0.000 claims description 6
- 239000003795 chemical substances by application Substances 0.000 claims description 5
- 239000012458 free base Substances 0.000 claims description 5
- 239000002775 capsule Substances 0.000 claims description 3
- 239000008297 liquid dosage form Substances 0.000 claims description 3
- 239000008187 granular material Substances 0.000 claims description 2
- 239000002904 solvent Substances 0.000 claims description 2
- 210000003932 urinary bladder Anatomy 0.000 abstract description 97
- 239000000126 substance Substances 0.000 abstract description 18
- 210000005068 bladder tissue Anatomy 0.000 abstract description 4
- 238000012423 maintenance Methods 0.000 abstract description 3
- 230000000694 effects Effects 0.000 abstract description 2
- 230000002035 prolonged effect Effects 0.000 abstract description 2
- 229920001296 polysiloxane Polymers 0.000 description 21
- 239000003826 tablet Substances 0.000 description 21
- 239000000463 material Substances 0.000 description 17
- 238000003780 insertion Methods 0.000 description 14
- 230000037431 insertion Effects 0.000 description 14
- 239000007787 solid Substances 0.000 description 14
- 210000001519 tissue Anatomy 0.000 description 12
- 206010028980 Neoplasm Diseases 0.000 description 11
- 238000009792 diffusion process Methods 0.000 description 11
- 230000010412 perfusion Effects 0.000 description 11
- 239000000203 mixture Substances 0.000 description 10
- 241001465754 Metazoa Species 0.000 description 9
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 9
- 238000013461 design Methods 0.000 description 8
- 241001467552 Mycobacterium bovis BCG Species 0.000 description 7
- 229960000190 bacillus calmette–guérin vaccine Drugs 0.000 description 7
- 239000011248 coating agent Substances 0.000 description 7
- 238000002513 implantation Methods 0.000 description 7
- 238000000338 in vitro Methods 0.000 description 7
- 244000309715 mini pig Species 0.000 description 7
- 238000012360 testing method Methods 0.000 description 7
- 239000004433 Thermoplastic polyurethane Substances 0.000 description 6
- 238000010521 absorption reaction Methods 0.000 description 6
- 238000000576 coating method Methods 0.000 description 6
- 239000007924 injection Substances 0.000 description 6
- 238000002347 injection Methods 0.000 description 6
- 238000001990 intravenous administration Methods 0.000 description 6
- 239000007788 liquid Substances 0.000 description 6
- 230000003204 osmotic effect Effects 0.000 description 6
- 229920002803 thermoplastic polyurethane Polymers 0.000 description 6
- 239000002246 antineoplastic agent Substances 0.000 description 5
- 229940127089 cytotoxic agent Drugs 0.000 description 5
- 239000000499 gel Substances 0.000 description 5
- 239000000243 solution Substances 0.000 description 5
- FIRDBEQIJQERSE-QPPQHZFASA-N 2',2'-Difluorodeoxyuridine Chemical compound FC1(F)[C@H](O)[C@@H](CO)O[C@H]1N1C(=O)NC(=O)C=C1 FIRDBEQIJQERSE-QPPQHZFASA-N 0.000 description 4
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 4
- 229920003171 Poly (ethylene oxide) Polymers 0.000 description 4
- 238000010276 construction Methods 0.000 description 4
- 238000001727 in vivo Methods 0.000 description 4
- 229920000642 polymer Polymers 0.000 description 4
- 229920002635 polyurethane Polymers 0.000 description 4
- 239000004814 polyurethane Substances 0.000 description 4
- 238000002360 preparation method Methods 0.000 description 4
- 230000001839 systemic circulation Effects 0.000 description 4
- 241000282472 Canis lupus familiaris Species 0.000 description 3
- 201000011510 cancer Diseases 0.000 description 3
- 238000013270 controlled release Methods 0.000 description 3
- 230000004069 differentiation Effects 0.000 description 3
- 229920001477 hydrophilic polymer Polymers 0.000 description 3
- 239000002955 immunomodulating agent Substances 0.000 description 3
- 230000007246 mechanism Effects 0.000 description 3
- 210000003205 muscle Anatomy 0.000 description 3
- 239000000546 pharmaceutical excipient Substances 0.000 description 3
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 3
- 239000001267 polyvinylpyrrolidone Substances 0.000 description 3
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 3
- 210000002307 prostate Anatomy 0.000 description 3
- 238000012216 screening Methods 0.000 description 3
- 238000001356 surgical procedure Methods 0.000 description 3
- 231100000331 toxic Toxicity 0.000 description 3
- 230000002588 toxic effect Effects 0.000 description 3
- 210000003741 urothelium Anatomy 0.000 description 3
- 229920002153 Hydroxypropyl cellulose Polymers 0.000 description 2
- 239000004952 Polyamide Substances 0.000 description 2
- 229920002614 Polyether block amide Polymers 0.000 description 2
- 241000282887 Suidae Species 0.000 description 2
- 239000002671 adjuvant Substances 0.000 description 2
- 239000012620 biological material Substances 0.000 description 2
- 239000008280 blood Substances 0.000 description 2
- 210000004369 blood Anatomy 0.000 description 2
- 210000004027 cell Anatomy 0.000 description 2
- 229920002678 cellulose Polymers 0.000 description 2
- 235000010980 cellulose Nutrition 0.000 description 2
- 230000008602 contraction Effects 0.000 description 2
- 239000006071 cream Substances 0.000 description 2
- 230000001186 cumulative effect Effects 0.000 description 2
- 238000005520 cutting process Methods 0.000 description 2
- 201000010099 disease Diseases 0.000 description 2
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 2
- 239000013536 elastomeric material Substances 0.000 description 2
- 239000000839 emulsion Substances 0.000 description 2
- 239000003623 enhancer Substances 0.000 description 2
- 230000003628 erosive effect Effects 0.000 description 2
- 239000012530 fluid Substances 0.000 description 2
- 239000001863 hydroxypropyl cellulose Substances 0.000 description 2
- 235000010977 hydroxypropyl cellulose Nutrition 0.000 description 2
- 230000003902 lesion Effects 0.000 description 2
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 description 2
- 229920000609 methyl cellulose Polymers 0.000 description 2
- 239000001923 methylcellulose Substances 0.000 description 2
- 238000012986 modification Methods 0.000 description 2
- 230000004048 modification Effects 0.000 description 2
- 230000003232 mucoadhesive effect Effects 0.000 description 2
- HLXZNVUGXRDIFK-UHFFFAOYSA-N nickel titanium Chemical compound [Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni] HLXZNVUGXRDIFK-UHFFFAOYSA-N 0.000 description 2
- 229910001000 nickel titanium Inorganic materials 0.000 description 2
- 239000002674 ointment Substances 0.000 description 2
- 239000006072 paste Substances 0.000 description 2
- 230000035515 penetration Effects 0.000 description 2
- 229920002647 polyamide Polymers 0.000 description 2
- 235000012434 pretzels Nutrition 0.000 description 2
- 238000002271 resection Methods 0.000 description 2
- 239000008137 solubility enhancer Substances 0.000 description 2
- 238000003860 storage Methods 0.000 description 2
- 208000024891 symptom Diseases 0.000 description 2
- 230000009885 systemic effect Effects 0.000 description 2
- 229920002725 thermoplastic elastomer Polymers 0.000 description 2
- 230000007704 transition Effects 0.000 description 2
- 206010044412 transitional cell carcinoma Diseases 0.000 description 2
- 241000304886 Bacilli Species 0.000 description 1
- 241000283690 Bos taurus Species 0.000 description 1
- 241000283707 Capra Species 0.000 description 1
- 229920002134 Carboxymethyl cellulose Polymers 0.000 description 1
- 229920001661 Chitosan Polymers 0.000 description 1
- 241000282326 Felis catus Species 0.000 description 1
- 102000004856 Lectins Human genes 0.000 description 1
- 108090001090 Lectins Proteins 0.000 description 1
- 241000124008 Mammalia Species 0.000 description 1
- 208000029549 Muscle injury Diseases 0.000 description 1
- 241001494479 Pecora Species 0.000 description 1
- 229920000148 Polycarbophil calcium Polymers 0.000 description 1
- 239000002202 Polyethylene glycol Substances 0.000 description 1
- 239000004721 Polyphenylene oxide Substances 0.000 description 1
- 208000007660 Residual Neoplasm Diseases 0.000 description 1
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 1
- 229920002125 Sokalan® Polymers 0.000 description 1
- 229920002807 Thiomer Polymers 0.000 description 1
- 238000009825 accumulation Methods 0.000 description 1
- DPXJVFZANSGRMM-UHFFFAOYSA-N acetic acid;2,3,4,5,6-pentahydroxyhexanal;sodium Chemical compound [Na].CC(O)=O.OCC(O)C(O)C(O)C(O)C=O DPXJVFZANSGRMM-UHFFFAOYSA-N 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 239000013543 active substance Substances 0.000 description 1
- 125000001931 aliphatic group Chemical group 0.000 description 1
- 229910045601 alloy Inorganic materials 0.000 description 1
- 239000000956 alloy Substances 0.000 description 1
- WMGSQTMJHBYJMQ-UHFFFAOYSA-N aluminum;magnesium;silicate Chemical compound [Mg+2].[Al+3].[O-][Si]([O-])([O-])[O-] WMGSQTMJHBYJMQ-UHFFFAOYSA-N 0.000 description 1
- 150000001408 amides Chemical class 0.000 description 1
- 239000000730 antalgic agent Substances 0.000 description 1
- 239000002260 anti-inflammatory agent Substances 0.000 description 1
- 229940121363 anti-inflammatory agent Drugs 0.000 description 1
- 239000008135 aqueous vehicle Substances 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 210000000746 body region Anatomy 0.000 description 1
- 210000001185 bone marrow Anatomy 0.000 description 1
- 239000001768 carboxy methyl cellulose Substances 0.000 description 1
- 238000005266 casting Methods 0.000 description 1
- 239000001913 cellulose Substances 0.000 description 1
- 230000000349 chemoimmunotherapeutic effect Effects 0.000 description 1
- 230000000973 chemotherapeutic effect Effects 0.000 description 1
- 238000002512 chemotherapy Methods 0.000 description 1
- 230000004087 circulation Effects 0.000 description 1
- 150000001875 compounds Chemical class 0.000 description 1
- 239000012141 concentrate Substances 0.000 description 1
- 230000001143 conditioned effect Effects 0.000 description 1
- 210000002808 connective tissue Anatomy 0.000 description 1
- 239000004035 construction material Substances 0.000 description 1
- 230000001276 controlling effect Effects 0.000 description 1
- 201000003146 cystitis Diseases 0.000 description 1
- 238000002574 cystoscopy Methods 0.000 description 1
- 239000002254 cytotoxic agent Substances 0.000 description 1
- 231100000599 cytotoxic agent Toxicity 0.000 description 1
- 239000008367 deionised water Substances 0.000 description 1
- 229910021641 deionized water Inorganic materials 0.000 description 1
- 239000002270 dispersing agent Substances 0.000 description 1
- 238000004090 dissolution Methods 0.000 description 1
- 238000009826 distribution Methods 0.000 description 1
- 238000010828 elution Methods 0.000 description 1
- 210000000981 epithelium Anatomy 0.000 description 1
- 150000002148 esters Chemical class 0.000 description 1
- -1 etc.) Polymers 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 239000010408 film Substances 0.000 description 1
- 238000009093 first-line therapy Methods 0.000 description 1
- 229920005570 flexible polymer Polymers 0.000 description 1
- 238000004108 freeze drying Methods 0.000 description 1
- 210000001035 gastrointestinal tract Anatomy 0.000 description 1
- 239000003292 glue Substances 0.000 description 1
- 239000012456 homogeneous solution Substances 0.000 description 1
- 239000000017 hydrogel Substances 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 238000001802 infusion Methods 0.000 description 1
- 230000007794 irritation Effects 0.000 description 1
- 238000002955 isolation Methods 0.000 description 1
- 239000002523 lectin Substances 0.000 description 1
- 150000002632 lipids Chemical class 0.000 description 1
- 239000006193 liquid solution Substances 0.000 description 1
- 238000011068 loading method Methods 0.000 description 1
- 239000000314 lubricant Substances 0.000 description 1
- 235000019359 magnesium stearate Nutrition 0.000 description 1
- 239000011159 matrix material Substances 0.000 description 1
- 230000010534 mechanism of action Effects 0.000 description 1
- 229940126601 medicinal product Drugs 0.000 description 1
- 230000001394 metastastic effect Effects 0.000 description 1
- 206010061289 metastatic neoplasm Diseases 0.000 description 1
- 230000027939 micturition Effects 0.000 description 1
- 230000003278 mimic effect Effects 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 210000004877 mucosa Anatomy 0.000 description 1
- 210000004400 mucous membrane Anatomy 0.000 description 1
- 210000000056 organ Anatomy 0.000 description 1
- 230000007170 pathology Effects 0.000 description 1
- 230000035699 permeability Effects 0.000 description 1
- 230000036470 plasma concentration Effects 0.000 description 1
- 229950005134 polycarbophil Drugs 0.000 description 1
- 229920000728 polyester Polymers 0.000 description 1
- 229920000570 polyether Polymers 0.000 description 1
- 229920001223 polyethylene glycol Polymers 0.000 description 1
- 239000000843 powder Substances 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 229940002612 prodrug Drugs 0.000 description 1
- 239000000651 prodrug Substances 0.000 description 1
- 150000003230 pyrimidines Chemical class 0.000 description 1
- 230000001105 regulatory effect Effects 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 230000004044 response Effects 0.000 description 1
- 230000000717 retained effect Effects 0.000 description 1
- 150000003839 salts Chemical class 0.000 description 1
- 239000000523 sample Substances 0.000 description 1
- 235000019812 sodium carboxymethyl cellulose Nutrition 0.000 description 1
- 229920001027 sodium carboxymethylcellulose Polymers 0.000 description 1
- 239000011780 sodium chloride Substances 0.000 description 1
- 239000007909 solid dosage form Substances 0.000 description 1
- 239000012453 solvate Substances 0.000 description 1
- 238000013222 sprague-dawley male rat Methods 0.000 description 1
- 230000000087 stabilizing effect Effects 0.000 description 1
- 208000014794 superficial urinary bladder carcinoma Diseases 0.000 description 1
- 239000004094 surface-active agent Substances 0.000 description 1
- 238000011477 surgical intervention Methods 0.000 description 1
- 239000000725 suspension Substances 0.000 description 1
- 231100000901 systemic toxic effect Toxicity 0.000 description 1
- 239000007916 tablet composition Substances 0.000 description 1
- 229940126585 therapeutic drug Drugs 0.000 description 1
- 210000004881 tumor cell Anatomy 0.000 description 1
- 230000002485 urinary effect Effects 0.000 description 1
- 208000019206 urinary tract infection Diseases 0.000 description 1
- 210000002229 urogenital system Anatomy 0.000 description 1
- 208000023747 urothelial carcinoma Diseases 0.000 description 1
- 238000012800 visualization Methods 0.000 description 1
- 230000003442 weekly effect Effects 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7042—Compounds having saccharide radicals and heterocyclic rings
- A61K31/7052—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides
- A61K31/706—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom
- A61K31/7064—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom containing condensed or non-condensed pyrimidines
- A61K31/7068—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom containing condensed or non-condensed pyrimidines having oxo groups directly attached to the pyrimidine ring, e.g. cytidine, cytidylic acid
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K45/00—Medicinal preparations containing active ingredients not provided for in groups A61K31/00 - A61K41/00
- A61K45/06—Mixtures of active ingredients without chemical characterisation, e.g. antiphlogistics and cardiaca
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/06—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite
- A61K47/16—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite containing nitrogen, e.g. nitro-, nitroso-, azo-compounds, nitriles, cyanates
- A61K47/18—Amines; Amides; Ureas; Quaternary ammonium compounds; Amino acids; Oligopeptides having up to five amino acids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0002—Galenical forms characterised by the drug release technique; Application systems commanded by energy
- A61K9/0004—Osmotic delivery systems; Sustained release driven by osmosis, thermal energy or gas
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0019—Injectable compositions; Intramuscular, intravenous, arterial, subcutaneous administration; Compositions to be administered through the skin in an invasive manner
- A61K9/0024—Solid, semi-solid or solidifying implants, which are implanted or injected in body tissue
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0034—Urogenital system, e.g. vagina, uterus, cervix, penis, scrotum, urethra, bladder; Personal lubricants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0087—Galenical forms not covered by A61K9/02 - A61K9/7023
- A61K9/0092—Hollow drug-filled fibres, tubes of the core-shell type, coated fibres, coated rods, microtubules or nanotubes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M31/00—Devices for introducing or retaining media, e.g. remedies, in cavities of the body
- A61M31/002—Devices for releasing a drug at a continuous and controlled rate for a prolonged period of time
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P13/00—Drugs for disorders of the urinary system
- A61P13/10—Drugs for disorders of the urinary system of the bladder
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07H—SUGARS; DERIVATIVES THEREOF; NUCLEOSIDES; NUCLEOTIDES; NUCLEIC ACIDS
- C07H19/00—Compounds containing a hetero ring sharing one ring hetero atom with a saccharide radical; Nucleosides; Mononucleotides; Anhydro-derivatives thereof
- C07H19/02—Compounds containing a hetero ring sharing one ring hetero atom with a saccharide radical; Nucleosides; Mononucleotides; Anhydro-derivatives thereof sharing nitrogen
- C07H19/04—Heterocyclic radicals containing only nitrogen atoms as ring hetero atom
- C07H19/06—Pyrimidine radicals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2300/00—Mixtures or combinations of active ingredients, wherein at least one active ingredient is fully defined in groups A61K31/00 - A61K41/00
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Pharmacology & Pharmacy (AREA)
- Epidemiology (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Organic Chemistry (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Urology & Nephrology (AREA)
- Molecular Biology (AREA)
- Biomedical Technology (AREA)
- Reproductive Health (AREA)
- Gynecology & Obstetrics (AREA)
- Neurosurgery (AREA)
- Dermatology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Anesthesiology (AREA)
- Biotechnology (AREA)
- Genetics & Genomics (AREA)
- Biochemistry (AREA)
- Heart & Thoracic Surgery (AREA)
- Hematology (AREA)
- Nanotechnology (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicinal Preparation (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Infusion, Injection, And Reservoir Apparatuses (AREA)
- Media Introduction/Drainage Providing Device (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201461949215P | 2014-03-06 | 2014-03-06 | |
| US61/949,215 | 2014-03-06 | ||
| PCT/US2015/019262 WO2015134911A1 (en) | 2014-03-06 | 2015-03-06 | Drug delivery systems and methods for treatment of bladder cancer with gemcitabine |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| RU2694902C1 true RU2694902C1 (ru) | 2019-07-18 |
Family
ID=52697553
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| RU2016136103A RU2694902C1 (ru) | 2014-03-06 | 2015-03-06 | Системы и способы доставки лекарственных средств для лечения рака мочевого пузыря гемцитабином |
Country Status (24)
Families Citing this family (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CA2882575C (en) | 2012-08-31 | 2020-05-26 | Dennis GIESING | Drug delivery systems and methods for treatment of prostate |
| HRP20201421T1 (hr) | 2013-08-19 | 2020-12-11 | Taris Biomedical Llc | Uređaji za davanje više jedinica lijeka |
| LT3113782T (lt) * | 2014-03-06 | 2022-11-25 | Taris Biomedical Llc | Vaisto tiekimo sistemos ir būdai, skirti šlapimo pūslės vėžio gydymui gemcitabinu |
| MX389296B (es) * | 2016-05-06 | 2025-03-20 | Taris Biomedical Llc | Metodo para tratar cancer urotelial del tracto inferior. |
| CA3071014A1 (en) * | 2017-07-25 | 2019-01-31 | Taris Biomedical Llc | Methods of treating tumor metastasis |
| JOP20200124A1 (ar) * | 2017-11-08 | 2020-05-20 | Taris Biomedical Llc | طُرق لعلاج سرطان المثانة باستخدام جيمسيتابين وعلاج مدوامة لذلك |
| CA3107461A1 (en) | 2018-08-01 | 2020-02-06 | Taris Biomedical Llc | Methods of treating overactive bladder using trospium |
| US20220370630A1 (en) * | 2019-10-03 | 2022-11-24 | Seikagaku Corporation | Transmucosal delivery system for pharmaceutical active ingredient to submucosal tissue of bladder |
| RU2713443C2 (ru) * | 2019-11-01 | 2020-02-05 | Федеральное государственное бюджетное учреждение "Национальный медицинский исследовательский центр радиологии" Министерства здравоохранения Российской Федерации (ФГБУ "НМИЦ радиологии" Минздрава России) | Способ комбинированного лечения мышечно-инвазивного рака мочевого пузыря т3-т4 n0-+m0 |
| WO2021154682A1 (en) * | 2020-01-27 | 2021-08-05 | Convergascent Llc | Tissue dosing for intraluminal local drug delivery |
| CN112169066B (zh) * | 2020-10-29 | 2023-05-12 | 安徽省立医院(中国科学技术大学附属第一医院) | 具有控制器的连续缓释给药留置管 |
| CN113616666A (zh) * | 2021-08-24 | 2021-11-09 | 傅广波 | 膀胱黏膜下注射吉西他滨在治疗膀胱癌中的应用 |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2011089604A2 (en) * | 2010-01-20 | 2011-07-28 | Theracoat Ltd | Material and method for treating internal cavities |
| US20120203203A1 (en) * | 2011-02-04 | 2012-08-09 | Taris Biomedical, Inc. | Implantable device for controlled dissolution and diffusion of low solubility drug |
Family Cites Families (21)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4111203A (en) * | 1976-11-22 | 1978-09-05 | Alza Corporation | Osmotic system with means for improving delivery kinetics of system |
| WO2004026281A2 (en) * | 2002-09-23 | 2004-04-01 | Microchips, Inc. | Micro-reservoir osmotic release systems and microtube array device |
| KR20060017749A (ko) * | 2003-03-31 | 2006-02-27 | 알자 코포레이션 | 내압 소멸 수단을 갖는 삼투성 펌프 |
| US8361490B2 (en) * | 2004-09-16 | 2013-01-29 | Theracoat Ltd. | Biocompatible drug delivery apparatus and methods |
| JP5265359B2 (ja) | 2005-08-11 | 2013-08-14 | マサチューセッツ インスティテュート オブ テクノロジー | 膀胱内薬物送達デバイスおよび方法 |
| WO2008013589A2 (en) * | 2006-04-24 | 2008-01-31 | Gloucester Pharmaceuticals | Treatment of ras-expressing tumors |
| EP2231254B9 (en) | 2007-12-11 | 2015-04-08 | Massachusetts Institute of Technology | Implantable drug delivery device |
| BRPI0917135A2 (pt) | 2008-08-09 | 2015-11-10 | Massachusetts Inst Technology | dispositivo médico para extensão e retenção em uma vesícula seminal, duto ejaculatório, próstata ou vaso deferente de um paciente, uso de um elastômero reabsorvível, e, dispositivo de bomba osmótica. |
| WO2010151896A1 (en) | 2009-06-26 | 2010-12-29 | Taris Biomedical, Inc. | Solid drug tablets for implantable drug delivery devices |
| US9017312B2 (en) | 2009-09-10 | 2015-04-28 | Taris Biomedical Llc | Implantable device for controlled drug delivery |
| US8721621B2 (en) | 2009-09-10 | 2014-05-13 | Taris Biomedical, Inc. | Systems and methods for deploying devices to genitourinary sites |
| EP2512581B1 (en) * | 2009-12-17 | 2021-02-17 | TARIS Biomedical LLC | Implantable device with intravesical tolerability |
| US20110218488A1 (en) | 2010-03-05 | 2011-09-08 | Taris Biomedical, Inc. | Systems and Methods for Implanting Devices in the Bladder and Other Genitourinary Sites |
| JP5945544B2 (ja) | 2010-10-06 | 2016-07-05 | タリス バイオメディカル エルエルシー | 時間選択的に生体吸収可能または崩壊可能な薬剤送達システムおよび方法 |
| WO2012048104A1 (en) | 2010-10-06 | 2012-04-12 | Taris Biomedical, Inc. | Implantable drug delivery device with bladden retention feature |
| CA2882575C (en) * | 2012-08-31 | 2020-05-26 | Dennis GIESING | Drug delivery systems and methods for treatment of prostate |
| CN105209012B (zh) | 2013-03-15 | 2019-02-01 | 塔里斯生物医药公司 | 用于药物递送的药物递送装置和方法 |
| BR112015022433B1 (pt) | 2013-03-15 | 2022-06-21 | Taris Biomedical Llc | Dispositivo de liberação de droga intravesical |
| HRP20201421T1 (hr) * | 2013-08-19 | 2020-12-11 | Taris Biomedical Llc | Uređaji za davanje više jedinica lijeka |
| RU2016120199A (ru) * | 2013-11-05 | 2017-12-11 | ТАРИС Биомедикал ЛЛК | Осмотические устройства, комплекты и способы для доставки лекарственных средств |
| LT3113782T (lt) * | 2014-03-06 | 2022-11-25 | Taris Biomedical Llc | Vaisto tiekimo sistemos ir būdai, skirti šlapimo pūslės vėžio gydymui gemcitabinu |
-
2015
- 2015-03-06 LT LTEPPCT/US2015/019262T patent/LT3113782T/lt unknown
- 2015-03-06 RS RS20220937A patent/RS63675B1/sr unknown
- 2015-03-06 MX MX2016011333A patent/MX2016011333A/es unknown
- 2015-03-06 ES ES15711373T patent/ES2930435T3/es active Active
- 2015-03-06 PL PL22196596.5T patent/PL4124339T3/pl unknown
- 2015-03-06 PT PT157113739T patent/PT3113782T/pt unknown
- 2015-03-06 HU HUE15711373A patent/HUE060704T2/hu unknown
- 2015-03-06 US US14/641,009 patent/US20150250717A1/en not_active Abandoned
- 2015-03-06 JP JP2016555554A patent/JP6901858B2/ja active Active
- 2015-03-06 LT LTEP22196596.5T patent/LT4124339T/lt unknown
- 2015-03-06 SM SM20220449T patent/SMT202200449T1/it unknown
- 2015-03-06 SI SI201532080T patent/SI4124339T1/sl unknown
- 2015-03-06 HR HRP20221252TT patent/HRP20221252T1/hr unknown
- 2015-03-06 CN CN201580011259.9A patent/CN106102751A/zh active Pending
- 2015-03-06 CA CA2939979A patent/CA2939979C/en active Active
- 2015-03-06 DK DK22196596.5T patent/DK4124339T3/da active
- 2015-03-06 PT PT221965965T patent/PT4124339T/pt unknown
- 2015-03-06 SG SG10202101978SA patent/SG10202101978SA/en unknown
- 2015-03-06 EP EP15711373.9A patent/EP3113782B1/en active Active
- 2015-03-06 SG SG11201607342QA patent/SG11201607342QA/en unknown
- 2015-03-06 ES ES22196596T patent/ES3051417T3/es active Active
- 2015-03-06 CN CN202510128139.2A patent/CN119950539A/zh active Pending
- 2015-03-06 RS RS20251017A patent/RS67337B1/sr unknown
- 2015-03-06 BR BR112016020381A patent/BR112016020381A8/pt not_active Application Discontinuation
- 2015-03-06 EP EP22196596.5A patent/EP4124339B1/en active Active
- 2015-03-06 WO PCT/US2015/019262 patent/WO2015134911A1/en not_active Ceased
- 2015-03-06 PL PL15711373.9T patent/PL3113782T3/pl unknown
- 2015-03-06 SM SM20250385T patent/SMT202500385T1/it unknown
- 2015-03-06 FI FIEP22196596.5T patent/FI4124339T3/fi active
- 2015-03-06 AU AU2015226901A patent/AU2015226901B2/en active Active
- 2015-03-06 IL IL247347A patent/IL247347B2/en unknown
- 2015-03-06 DK DK15711373.9T patent/DK3113782T3/da active
- 2015-03-06 RU RU2016136103A patent/RU2694902C1/ru active
- 2015-03-06 HR HRP20251264TT patent/HRP20251264T1/hr unknown
- 2015-03-06 KR KR1020167027303A patent/KR102338079B1/ko active Active
-
2019
- 2019-10-04 JP JP2019184023A patent/JP7061595B2/ja active Active
-
2020
- 2020-08-12 AU AU2020217383A patent/AU2020217383B2/en active Active
-
2021
- 2021-11-11 JP JP2021183995A patent/JP7383681B2/ja active Active
- 2021-12-23 US US17/561,540 patent/US20220117886A1/en not_active Abandoned
-
2022
- 2022-07-07 US US17/811,296 patent/US20220347091A1/en active Pending
- 2022-08-08 AU AU2022215156A patent/AU2022215156B2/en active Active
-
2023
- 2023-11-08 JP JP2023190543A patent/JP2024009034A/ja active Pending
-
2024
- 2024-03-11 IL IL311410A patent/IL311410B2/en unknown
- 2024-11-08 AU AU2024259822A patent/AU2024259822A1/en active Pending
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2011089604A2 (en) * | 2010-01-20 | 2011-07-28 | Theracoat Ltd | Material and method for treating internal cavities |
| US20120203203A1 (en) * | 2011-02-04 | 2012-08-09 | Taris Biomedical, Inc. | Implantable device for controlled dissolution and diffusion of low solubility drug |
Non-Patent Citations (1)
| Title |
|---|
| SHELLEY M. et al., Gemcitabine for unresectable, locally advanced or metastatic bladder cancer. Cochrane Database Syst Rev. 2011 Apr N 13 (4), CD008976. * |
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| RU2694902C1 (ru) | Системы и способы доставки лекарственных средств для лечения рака мочевого пузыря гемцитабином | |
| US10406335B2 (en) | Drug delivery systems and methods for treatment of prostate | |
| HK40088274A (en) | Drug delivery systems and methods for treatment of bladder cancer with gemcitabine | |
| HK40042011A (en) | Drug delivery systems and methods for treatment of prostate | |
| NZ723303B2 (en) | Drug delivery systems and methods for treatment of bladder cancer with gemcitabine |