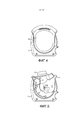
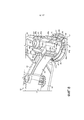
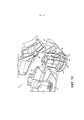
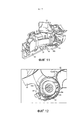
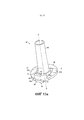
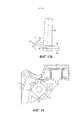
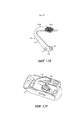
RU2582755C1 - Ингалятор - Google Patents
Ингалятор Download PDFInfo
- Publication number
- RU2582755C1 RU2582755C1 RU2014152832/14A RU2014152832A RU2582755C1 RU 2582755 C1 RU2582755 C1 RU 2582755C1 RU 2014152832/14 A RU2014152832/14 A RU 2014152832/14A RU 2014152832 A RU2014152832 A RU 2014152832A RU 2582755 C1 RU2582755 C1 RU 2582755C1
- Authority
- RU
- Russia
- Prior art keywords
- blister
- drive
- actuator
- axis
- rotation
- Prior art date
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0028—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up
- A61M15/0045—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using multiple prepacked dosages on a same carrier, e.g. blisters
- A61M15/0053—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using multiple prepacked dosages on a same carrier, e.g. blisters characterized by the type or way of disposal
- A61M15/0058—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using multiple prepacked dosages on a same carrier, e.g. blisters characterized by the type or way of disposal the used dosages being cut from the carrier
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0028—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up
- A61M15/0045—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using multiple prepacked dosages on a same carrier, e.g. blisters
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M11/00—Sprayers or atomisers specially adapted for therapeutic purposes
- A61M11/001—Particle size control
- A61M11/003—Particle size control by passing the aerosol trough sieves or filters
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0001—Details of inhalators; Constructional features thereof
- A61M15/0021—Mouthpieces therefor
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0001—Details of inhalators; Constructional features thereof
- A61M15/0021—Mouthpieces therefor
- A61M15/0025—Mouthpieces therefor with caps
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0001—Details of inhalators; Constructional features thereof
- A61M15/0021—Mouthpieces therefor
- A61M15/0025—Mouthpieces therefor with caps
- A61M15/0026—Hinged caps
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0028—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up
- A61M15/003—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using capsules, e.g. to be perforated or broken-up
- A61M15/0033—Details of the piercing or cutting means
- A61M15/0035—Piercing means
- A61M15/0036—Piercing means hollow piercing means
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0028—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up
- A61M15/003—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using capsules, e.g. to be perforated or broken-up
- A61M15/0033—Details of the piercing or cutting means
- A61M15/0041—Details of the piercing or cutting means with movable piercing or cutting means
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0028—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up
- A61M15/0045—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using multiple prepacked dosages on a same carrier, e.g. blisters
- A61M15/0053—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using multiple prepacked dosages on a same carrier, e.g. blisters characterized by the type or way of disposal
- A61M15/0056—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using multiple prepacked dosages on a same carrier, e.g. blisters characterized by the type or way of disposal the used dosages being crushed
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0028—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up
- A61M15/0045—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using multiple prepacked dosages on a same carrier, e.g. blisters
- A61M15/0053—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using multiple prepacked dosages on a same carrier, e.g. blisters characterized by the type or way of disposal
- A61M15/006—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using multiple prepacked dosages on a same carrier, e.g. blisters characterized by the type or way of disposal the used dosages being discarded out of the inhaler's housing
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0065—Inhalators with dosage or measuring devices
- A61M15/0068—Indicating or counting the number of dispensed doses or of remaining doses
- A61M15/0081—Locking means
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/08—Inhaling devices inserted into the nose
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0028—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up
- A61M15/0045—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using multiple prepacked dosages on a same carrier, e.g. blisters
- A61M15/0046—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using multiple prepacked dosages on a same carrier, e.g. blisters characterized by the type of carrier
- A61M15/0051—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using multiple prepacked dosages on a same carrier, e.g. blisters characterized by the type of carrier the dosages being arranged on a tape, e.g. strips
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2202/00—Special media to be introduced, removed or treated
- A61M2202/06—Solids
- A61M2202/064—Powder
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2205/00—General characteristics of the apparatus
- A61M2205/19—Constructional features of carpules, syringes or blisters
Landscapes
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Life Sciences & Earth Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Biomedical Technology (AREA)
- Heart & Thoracic Surgery (AREA)
- Hematology (AREA)
- Anesthesiology (AREA)
- Animal Behavior & Ethology (AREA)
- Veterinary Medicine (AREA)
- Pulmonology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Chemical & Material Sciences (AREA)
- Dispersion Chemistry (AREA)
- Biophysics (AREA)
- Otolaryngology (AREA)
- Medical Preparation Storing Or Oral Administration Devices (AREA)
- Medicinal Preparation (AREA)
- Respiratory Apparatuses And Protective Means (AREA)
- Infusion, Injection, And Reservoir Apparatuses (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| GB1209267.2A GB2502350A (en) | 2012-05-25 | 2012-05-25 | Inhaler having means to control the force applied to an actuating lever |
| GB1209267.2 | 2012-05-25 | ||
| PCT/GB2013/051261 WO2013175176A1 (en) | 2012-05-25 | 2013-05-16 | Inhaler |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| RU2582755C1 true RU2582755C1 (ru) | 2016-04-27 |
Family
ID=46546684
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| RU2014152832/14A RU2582755C1 (ru) | 2012-05-25 | 2013-05-16 | Ингалятор |
Country Status (26)
| Country | Link |
|---|---|
| US (1) | US10183133B2 (enExample) |
| EP (2) | EP2846860B1 (enExample) |
| JP (1) | JP6255393B2 (enExample) |
| KR (1) | KR101849181B1 (enExample) |
| CN (1) | CN104507521B (enExample) |
| AR (1) | AR091361A1 (enExample) |
| AU (1) | AU2013264972B2 (enExample) |
| BR (1) | BR112014029111B1 (enExample) |
| CA (1) | CA2871173C (enExample) |
| DK (1) | DK2846860T3 (enExample) |
| ES (2) | ES2836882T3 (enExample) |
| GB (1) | GB2502350A (enExample) |
| HU (1) | HUE039761T2 (enExample) |
| IL (1) | IL235825A (enExample) |
| IN (1) | IN2014DN10634A (enExample) |
| MX (1) | MX359344B (enExample) |
| NZ (1) | NZ700622A (enExample) |
| PL (1) | PL2846860T3 (enExample) |
| PT (1) | PT2846860T (enExample) |
| RU (1) | RU2582755C1 (enExample) |
| SG (1) | SG11201406764UA (enExample) |
| SI (1) | SI2846860T1 (enExample) |
| TR (1) | TR201808143T4 (enExample) |
| TW (1) | TWI632925B (enExample) |
| WO (1) | WO2013175176A1 (enExample) |
| ZA (1) | ZA201408102B (enExample) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| RU211036U1 (ru) * | 2021-11-01 | 2022-05-18 | Федеральное государственное бюджетное учреждение "48 Центральный научно-исследовательский институт" Министерства обороны Российской Федерации | Диспергирующее устройство порошкообразных препаратов |
Families Citing this family (21)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| TWI568463B (zh) * | 2013-07-12 | 2017-02-01 | H 斯圖爾特 坎貝爾 | 用於吸入器之吹嘴 |
| EP2878326A1 (en) * | 2013-11-27 | 2015-06-03 | Vectura Delivery Devices Limited | Inhaler |
| CN106139335B (zh) * | 2015-04-09 | 2022-12-16 | 正大天晴药业集团股份有限公司 | 一种药粉释放装置 |
| NZ739315A (en) * | 2015-07-20 | 2018-09-28 | Vectura Delivery Devices Ltd | Dry powder inhaler |
| JP7057285B2 (ja) | 2016-01-19 | 2022-04-19 | ノバルティス アーゲー | 複数投与吸入器 |
| AR108513A1 (es) * | 2016-05-25 | 2018-08-29 | Vectura Delivery Devices Ltd | Inhalador de polvo seco con dispositivo de ruptura de blíster |
| KR102534558B1 (ko) * | 2016-12-06 | 2023-05-19 | 노턴 (워터포드) 리미티드 | 통합된 전자 모듈을 가진 흡입 디바이스 |
| GB201812671D0 (en) * | 2018-08-03 | 2018-09-19 | Coalesce Product Dev Ltd | An inhaler device |
| WO2020058206A1 (en) | 2018-09-17 | 2020-03-26 | Vectura Delivery Devices Limited | Dry powder inhaler |
| EP3622990A1 (en) | 2018-09-17 | 2020-03-18 | Vectura Delivery Devices Limited | Dry powder inhaler |
| EP3628358A1 (en) | 2018-09-25 | 2020-04-01 | Vectura Delivery Devices Limited | Inhaler |
| WO2020236658A1 (en) | 2019-05-17 | 2020-11-26 | Impel Neuropharma, Inc. | Single-use nasal delivery device |
| CN114728137B (zh) | 2019-11-18 | 2024-07-12 | 维克多瑞传送设备有限公司 | 具有依从性监视器的干粉吸入器 |
| KR102755134B1 (ko) | 2019-11-18 | 2025-01-14 | 벡투라 딜리버리 디바이시스 리미티드 | 엄수/준수 모니터를 갖는 건조 분말 흡입기 |
| US12427270B2 (en) | 2019-11-18 | 2025-09-30 | Vectura Delivery Devices Limited | Inhaler for use with a compliance monitor |
| CA195576S (en) * | 2019-11-18 | 2022-03-08 | Vectura Delivery Devices Ltd | Inhaler |
| EP4114743B1 (en) | 2020-03-06 | 2024-11-13 | Vectura Delivery Devices Limited | Process for manufacturing a blister strip for a dry powder inhaler |
| CN117412786A (zh) | 2021-05-17 | 2024-01-16 | 维克多瑞传送设备有限公司 | 具有坚持/依从监测器的吸入器 |
| JP7629544B2 (ja) * | 2021-05-17 | 2025-02-13 | ヴェクトュラ・デリヴァリー・ディヴァイスィズ・リミテッド | 乾燥粉末吸入器のためのアドヒアランスモニタ |
| FI4188491T3 (fi) * | 2021-08-13 | 2024-06-07 | Norton Waterford Ltd | Kuivajauhelääkeinhalaattori |
| EP4137184A1 (en) | 2021-08-16 | 2023-02-22 | Vectura Delivery Devices Limited | Adherence / compliance monitor for an inhaler |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB2407042A (en) * | 2003-10-17 | 2005-04-20 | Vectura Ltd | Pocket blister inhaler with piercing actuator |
| RU2010135348A (ru) * | 2008-01-24 | 2012-02-27 | Вектура Деливери Дивайсиз Лимитед (Gb) | Ингалятор |
Family Cites Families (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB9004781D0 (en) | 1990-03-02 | 1990-04-25 | Glaxo Group Ltd | Device |
| EP2011535A1 (en) * | 2007-07-06 | 2009-01-07 | Boehringer Ingelheim Pharma GmbH & Co. KG | Inhaler |
| EP2082771A1 (en) * | 2008-01-24 | 2009-07-29 | Vectura Delivery Devices Limited | Inhaler |
| WO2010039200A2 (en) * | 2008-09-30 | 2010-04-08 | Oriel Therapeutics, Inc. | Dry powder inhalers with endless strips and cooperating piercers and related methods |
| GB201020130D0 (en) | 2010-11-26 | 2011-01-12 | Vectura Delivery Devices Ltd | Inhaler |
| ATE549052T1 (de) * | 2009-03-30 | 2012-03-15 | Sanovel Ilac Sanayi Ve Ticaret As | Trockenpulverinhalatorvorrichtung |
-
2012
- 2012-05-25 GB GB1209267.2A patent/GB2502350A/en not_active Withdrawn
-
2013
- 2013-05-16 PL PL13723923T patent/PL2846860T3/pl unknown
- 2013-05-16 AU AU2013264972A patent/AU2013264972B2/en active Active
- 2013-05-16 CN CN201380027248.0A patent/CN104507521B/zh active Active
- 2013-05-16 DK DK13723923.2T patent/DK2846860T3/en active
- 2013-05-16 US US14/402,179 patent/US10183133B2/en active Active
- 2013-05-16 EP EP13723923.2A patent/EP2846860B1/en active Active
- 2013-05-16 KR KR1020147030330A patent/KR101849181B1/ko active Active
- 2013-05-16 IN IN10634DEN2014 patent/IN2014DN10634A/en unknown
- 2013-05-16 TR TR2018/08143T patent/TR201808143T4/tr unknown
- 2013-05-16 WO PCT/GB2013/051261 patent/WO2013175176A1/en not_active Ceased
- 2013-05-16 RU RU2014152832/14A patent/RU2582755C1/ru active
- 2013-05-16 MX MX2014014367A patent/MX359344B/es active IP Right Grant
- 2013-05-16 PT PT137239232T patent/PT2846860T/pt unknown
- 2013-05-16 ES ES18168085T patent/ES2836882T3/es active Active
- 2013-05-16 BR BR112014029111-0A patent/BR112014029111B1/pt active IP Right Grant
- 2013-05-16 NZ NZ700622A patent/NZ700622A/en unknown
- 2013-05-16 HU HUE13723923A patent/HUE039761T2/hu unknown
- 2013-05-16 SI SI201331112T patent/SI2846860T1/sl unknown
- 2013-05-16 CA CA2871173A patent/CA2871173C/en active Active
- 2013-05-16 JP JP2015513257A patent/JP6255393B2/ja active Active
- 2013-05-16 EP EP18168085.1A patent/EP3388099B1/en active Active
- 2013-05-16 ES ES13723923.2T patent/ES2673208T3/es active Active
- 2013-05-16 SG SG11201406764UA patent/SG11201406764UA/en unknown
- 2013-05-24 AR ARP130101816 patent/AR091361A1/es active IP Right Grant
- 2013-05-24 TW TW102118341A patent/TWI632925B/zh active
-
2014
- 2014-11-05 ZA ZA2014/08102A patent/ZA201408102B/en unknown
- 2014-11-20 IL IL235825A patent/IL235825A/en active IP Right Grant
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB2407042A (en) * | 2003-10-17 | 2005-04-20 | Vectura Ltd | Pocket blister inhaler with piercing actuator |
| WO2005037353A1 (en) * | 2003-10-17 | 2005-04-28 | Vectura Limited | Inhaler |
| RU2010135348A (ru) * | 2008-01-24 | 2012-02-27 | Вектура Деливери Дивайсиз Лимитед (Gb) | Ингалятор |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| RU211036U1 (ru) * | 2021-11-01 | 2022-05-18 | Федеральное государственное бюджетное учреждение "48 Центральный научно-исследовательский институт" Министерства обороны Российской Федерации | Диспергирующее устройство порошкообразных препаратов |
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| RU2582755C1 (ru) | Ингалятор | |
| RU2569706C2 (ru) | Ингалятор | |
| AU2014253494B2 (en) | Inhaler | |
| HK1261478A1 (en) | Inhaler | |
| HK1261478B (en) | Inhaler | |
| HK1203424B (en) | Inhaler | |
| HK1197586B (en) | Inhaler |