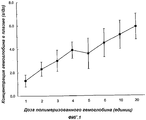
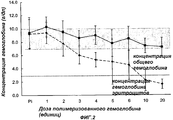
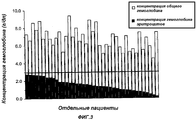
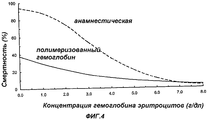
RU2325924C2 - Способ лечения пациентов с массивной потерей крови - Google Patents
Способ лечения пациентов с массивной потерей крови Download PDFInfo
- Publication number
- RU2325924C2 RU2325924C2 RU2005113240/14A RU2005113240A RU2325924C2 RU 2325924 C2 RU2325924 C2 RU 2325924C2 RU 2005113240/14 A RU2005113240/14 A RU 2005113240/14A RU 2005113240 A RU2005113240 A RU 2005113240A RU 2325924 C2 RU2325924 C2 RU 2325924C2
- Authority
- RU
- Russia
- Prior art keywords
- hemoglobin
- solution
- polymerized
- blood
- molecular weight
- Prior art date
Links
- 210000004369 blood Anatomy 0.000 title claims abstract description 71
- 239000008280 blood Substances 0.000 title claims abstract description 71
- 238000000034 method Methods 0.000 title claims description 64
- 230000036772 blood pressure Effects 0.000 claims abstract description 11
- 241000124008 Mammalia Species 0.000 claims abstract description 10
- 230000002427 irreversible effect Effects 0.000 claims abstract description 4
- 108010054147 Hemoglobins Proteins 0.000 claims description 266
- 102000001554 Hemoglobins Human genes 0.000 claims description 266
- 210000003743 erythrocyte Anatomy 0.000 claims description 77
- 229920000642 polymer Polymers 0.000 claims description 20
- 238000009826 distribution Methods 0.000 claims description 11
- 208000007502 anemia Diseases 0.000 claims description 8
- 239000012535 impurity Substances 0.000 claims description 8
- 210000000496 pancreas Anatomy 0.000 claims description 7
- 231100000419 toxicity Toxicity 0.000 claims description 7
- 230000001988 toxicity Effects 0.000 claims description 7
- 230000025033 vasoconstriction Effects 0.000 claims description 7
- 206010062237 Renal impairment Diseases 0.000 claims description 6
- 210000001035 gastrointestinal tract Anatomy 0.000 claims description 6
- 210000002216 heart Anatomy 0.000 claims description 6
- 231100000857 poor renal function Toxicity 0.000 claims description 6
- 206010021138 Hypovolaemic shock Diseases 0.000 claims description 4
- 206010040560 shock Diseases 0.000 claims description 4
- 208000028867 ischemia Diseases 0.000 claims description 3
- 230000000694 effects Effects 0.000 abstract description 4
- 230000006378 damage Effects 0.000 abstract description 3
- 210000000748 cardiovascular system Anatomy 0.000 abstract description 2
- 238000002347 injection Methods 0.000 abstract description 2
- 239000007924 injection Substances 0.000 abstract description 2
- QSHDDOUJBYECFT-UHFFFAOYSA-N mercury Chemical compound [Hg] QSHDDOUJBYECFT-UHFFFAOYSA-N 0.000 abstract description 2
- 229910052753 mercury Inorganic materials 0.000 abstract description 2
- 239000000126 substance Substances 0.000 abstract description 2
- 239000003814 drug Substances 0.000 abstract 2
- 239000000243 solution Substances 0.000 description 125
- 108010034539 PolyHeme Proteins 0.000 description 36
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 21
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 19
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 17
- 239000001301 oxygen Substances 0.000 description 17
- 229910052760 oxygen Inorganic materials 0.000 description 17
- 208000032843 Hemorrhage Diseases 0.000 description 16
- 208000034158 bleeding Diseases 0.000 description 16
- 230000000740 bleeding effect Effects 0.000 description 16
- 230000034994 death Effects 0.000 description 13
- 231100000517 death Toxicity 0.000 description 13
- 238000001802 infusion Methods 0.000 description 13
- 208000014674 injury Diseases 0.000 description 11
- 239000011780 sodium chloride Substances 0.000 description 10
- 230000008733 trauma Effects 0.000 description 9
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Chemical compound O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 9
- 239000008215 water for injection Substances 0.000 description 9
- 229910000033 sodium borohydride Inorganic materials 0.000 description 8
- 239000012279 sodium borohydride Substances 0.000 description 8
- 108010003320 Carboxyhemoglobin Proteins 0.000 description 7
- 238000001356 surgical procedure Methods 0.000 description 7
- CIWBSHSKHKDKBQ-JLAZNSOCSA-N Ascorbic acid Chemical compound OC[C@H](O)[C@H]1OC(=O)C(O)=C1O CIWBSHSKHKDKBQ-JLAZNSOCSA-N 0.000 description 6
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 6
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 6
- 238000001914 filtration Methods 0.000 description 6
- 238000002560 therapeutic procedure Methods 0.000 description 6
- SXRSQZLOMIGNAQ-UHFFFAOYSA-N Glutaraldehyde Chemical compound O=CCCCC=O SXRSQZLOMIGNAQ-UHFFFAOYSA-N 0.000 description 5
- 238000004458 analytical method Methods 0.000 description 5
- 239000007864 aqueous solution Substances 0.000 description 5
- 210000004027 cell Anatomy 0.000 description 5
- 230000007423 decrease Effects 0.000 description 5
- 231100000331 toxic Toxicity 0.000 description 5
- 230000002588 toxic effect Effects 0.000 description 5
- 238000006243 chemical reaction Methods 0.000 description 4
- 230000003247 decreasing effect Effects 0.000 description 4
- 239000000706 filtrate Substances 0.000 description 4
- 230000003993 interaction Effects 0.000 description 4
- 238000007477 logistic regression Methods 0.000 description 4
- 239000000463 material Substances 0.000 description 4
- 239000000203 mixture Substances 0.000 description 4
- 239000000047 product Substances 0.000 description 4
- 239000004471 Glycine Substances 0.000 description 3
- 229960005070 ascorbic acid Drugs 0.000 description 3
- 235000010323 ascorbic acid Nutrition 0.000 description 3
- 239000011668 ascorbic acid Substances 0.000 description 3
- 238000006392 deoxygenation reaction Methods 0.000 description 3
- 239000007789 gas Substances 0.000 description 3
- 238000005259 measurement Methods 0.000 description 3
- 229910052757 nitrogen Inorganic materials 0.000 description 3
- 238000006116 polymerization reaction Methods 0.000 description 3
- 239000011148 porous material Substances 0.000 description 3
- 238000002360 preparation method Methods 0.000 description 3
- 230000000750 progressive effect Effects 0.000 description 3
- 238000005406 washing Methods 0.000 description 3
- UGFAIRIUMAVXCW-UHFFFAOYSA-N Carbon monoxide Chemical compound [O+]#[C-] UGFAIRIUMAVXCW-UHFFFAOYSA-N 0.000 description 2
- 208000024172 Cardiovascular disease Diseases 0.000 description 2
- VEXZGXHMUGYJMC-UHFFFAOYSA-M Chloride anion Chemical compound [Cl-] VEXZGXHMUGYJMC-UHFFFAOYSA-M 0.000 description 2
- 229930186217 Glycolipid Natural products 0.000 description 2
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 2
- 108010061951 Methemoglobin Proteins 0.000 description 2
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 2
- WCUXLLCKKVVCTQ-UHFFFAOYSA-M Potassium chloride Chemical compound [Cl-].[K+] WCUXLLCKKVVCTQ-UHFFFAOYSA-M 0.000 description 2
- 206010047139 Vasoconstriction Diseases 0.000 description 2
- 241000700605 Viruses Species 0.000 description 2
- 208000027418 Wounds and injury Diseases 0.000 description 2
- 230000001154 acute effect Effects 0.000 description 2
- 239000012298 atmosphere Substances 0.000 description 2
- 239000003633 blood substitute Substances 0.000 description 2
- 229910002091 carbon monoxide Inorganic materials 0.000 description 2
- 238000007385 chemical modification Methods 0.000 description 2
- 239000003153 chemical reaction reagent Substances 0.000 description 2
- 239000003795 chemical substances by application Substances 0.000 description 2
- 230000001447 compensatory effect Effects 0.000 description 2
- 230000001186 cumulative effect Effects 0.000 description 2
- 238000010438 heat treatment Methods 0.000 description 2
- 238000004128 high performance liquid chromatography Methods 0.000 description 2
- 230000001771 impaired effect Effects 0.000 description 2
- 230000003907 kidney function Effects 0.000 description 2
- 150000003904 phospholipids Chemical class 0.000 description 2
- 230000000379 polymerizing effect Effects 0.000 description 2
- 229910052700 potassium Inorganic materials 0.000 description 2
- 239000011591 potassium Substances 0.000 description 2
- 238000011045 prefiltration Methods 0.000 description 2
- 239000012264 purified product Substances 0.000 description 2
- 230000002829 reductive effect Effects 0.000 description 2
- 238000006467 substitution reaction Methods 0.000 description 2
- 238000000108 ultra-filtration Methods 0.000 description 2
- INGWEZCOABYORO-UHFFFAOYSA-N 2-(furan-2-yl)-7-methyl-1h-1,8-naphthyridin-4-one Chemical compound N=1C2=NC(C)=CC=C2C(O)=CC=1C1=CC=CO1 INGWEZCOABYORO-UHFFFAOYSA-N 0.000 description 1
- PREOBXYMXLETCA-UHFFFAOYSA-N 2-[4-(2-carboxyphenoxy)-4-oxobutanoyl]oxybenzoic acid Chemical compound OC(=O)C1=CC=CC=C1OC(=O)CCC(=O)OC1=CC=CC=C1C(O)=O PREOBXYMXLETCA-UHFFFAOYSA-N 0.000 description 1
- HVCOBJNICQPDBP-UHFFFAOYSA-N 3-[3-[3,5-dihydroxy-6-methyl-4-(3,4,5-trihydroxy-6-methyloxan-2-yl)oxyoxan-2-yl]oxydecanoyloxy]decanoic acid;hydrate Chemical compound O.OC1C(OC(CC(=O)OC(CCCCCCC)CC(O)=O)CCCCCCC)OC(C)C(O)C1OC1C(O)C(O)C(O)C(C)O1 HVCOBJNICQPDBP-UHFFFAOYSA-N 0.000 description 1
- CYDQOEWLBCCFJZ-UHFFFAOYSA-N 4-(4-fluorophenyl)oxane-4-carboxylic acid Chemical compound C=1C=C(F)C=CC=1C1(C(=O)O)CCOCC1 CYDQOEWLBCCFJZ-UHFFFAOYSA-N 0.000 description 1
- 206010002091 Anaesthesia Diseases 0.000 description 1
- 102000015081 Blood Coagulation Factors Human genes 0.000 description 1
- 108010039209 Blood Coagulation Factors Proteins 0.000 description 1
- 206010010071 Coma Diseases 0.000 description 1
- 102100037815 Fas apoptotic inhibitory molecule 3 Human genes 0.000 description 1
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 1
- 206010019196 Head injury Diseases 0.000 description 1
- 101000878510 Homo sapiens Fas apoptotic inhibitory molecule 3 Proteins 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- 108010064719 Oxyhemoglobins Proteins 0.000 description 1
- 206010033799 Paralysis Diseases 0.000 description 1
- FKNQFGJONOIPTF-UHFFFAOYSA-N Sodium cation Chemical compound [Na+] FKNQFGJONOIPTF-UHFFFAOYSA-N 0.000 description 1
- 238000010521 absorption reaction Methods 0.000 description 1
- 230000037005 anaesthesia Effects 0.000 description 1
- 230000003276 anti-hypertensive effect Effects 0.000 description 1
- 239000003963 antioxidant agent Substances 0.000 description 1
- 230000003078 antioxidant effect Effects 0.000 description 1
- 235000006708 antioxidants Nutrition 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- 230000004872 arterial blood pressure Effects 0.000 description 1
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 1
- 229960000074 biopharmaceutical Drugs 0.000 description 1
- 239000003114 blood coagulation factor Substances 0.000 description 1
- 230000000747 cardiac effect Effects 0.000 description 1
- 210000002421 cell wall Anatomy 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 238000005352 clarification Methods 0.000 description 1
- 239000000306 component Substances 0.000 description 1
- 238000004132 cross linking Methods 0.000 description 1
- 230000009089 cytolysis Effects 0.000 description 1
- 238000007872 degassing Methods 0.000 description 1
- 108010002255 deoxyhemoglobin Proteins 0.000 description 1
- 238000011026 diafiltration Methods 0.000 description 1
- 201000010099 disease Diseases 0.000 description 1
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 1
- 230000002526 effect on cardiovascular system Effects 0.000 description 1
- 239000008151 electrolyte solution Substances 0.000 description 1
- 229940021013 electrolyte solution Drugs 0.000 description 1
- 238000002297 emergency surgery Methods 0.000 description 1
- 239000002158 endotoxin Substances 0.000 description 1
- 238000011156 evaluation Methods 0.000 description 1
- 230000007717 exclusion Effects 0.000 description 1
- 239000012530 fluid Substances 0.000 description 1
- 230000006870 function Effects 0.000 description 1
- 230000007661 gastrointestinal function Effects 0.000 description 1
- 239000008103 glucose Substances 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 230000004217 heart function Effects 0.000 description 1
- 238000005534 hematocrit Methods 0.000 description 1
- 230000023597 hemostasis Effects 0.000 description 1
- 229920006158 high molecular weight polymer Polymers 0.000 description 1
- 230000002631 hypothermal effect Effects 0.000 description 1
- 150000002463 imidates Chemical class 0.000 description 1
- 208000015181 infectious disease Diseases 0.000 description 1
- 150000002500 ions Chemical class 0.000 description 1
- 229910052742 iron Inorganic materials 0.000 description 1
- 210000000265 leukocyte Anatomy 0.000 description 1
- 230000000670 limiting effect Effects 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 208000019423 liver disease Diseases 0.000 description 1
- 238000011068 loading method Methods 0.000 description 1
- 238000001471 micro-filtration Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 210000003205 muscle Anatomy 0.000 description 1
- 210000000056 organ Anatomy 0.000 description 1
- 230000004768 organ dysfunction Effects 0.000 description 1
- 238000002103 osmometry Methods 0.000 description 1
- 238000002496 oximetry Methods 0.000 description 1
- 238000006213 oxygenation reaction Methods 0.000 description 1
- 230000004203 pancreatic function Effects 0.000 description 1
- 230000000149 penetrating effect Effects 0.000 description 1
- 210000004180 plasmocyte Anatomy 0.000 description 1
- 239000001103 potassium chloride Substances 0.000 description 1
- 235000011164 potassium chloride Nutrition 0.000 description 1
- 239000002244 precipitate Substances 0.000 description 1
- 230000035935 pregnancy Effects 0.000 description 1
- 239000003755 preservative agent Substances 0.000 description 1
- 230000002335 preservative effect Effects 0.000 description 1
- 230000007425 progressive decline Effects 0.000 description 1
- 208000005069 pulmonary fibrosis Diseases 0.000 description 1
- 238000000746 purification Methods 0.000 description 1
- WHOMFKWHIQZTHY-UHFFFAOYSA-N pyridoxine 5'-phosphate Chemical compound CC1=NC=C(COP(O)(O)=O)C(CO)=C1O WHOMFKWHIQZTHY-UHFFFAOYSA-N 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 230000000717 retained effect Effects 0.000 description 1
- 229940125723 sedative agent Drugs 0.000 description 1
- 239000000932 sedative agent Substances 0.000 description 1
- 238000001542 size-exclusion chromatography Methods 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- 229910001415 sodium ion Inorganic materials 0.000 description 1
- 239000001540 sodium lactate Substances 0.000 description 1
- 235000011088 sodium lactate Nutrition 0.000 description 1
- 229940005581 sodium lactate Drugs 0.000 description 1
- 241000894007 species Species 0.000 description 1
- 238000007619 statistical method Methods 0.000 description 1
- 238000003860 storage Methods 0.000 description 1
- 108010001708 stroma free hemoglobin Proteins 0.000 description 1
- 239000000758 substrate Substances 0.000 description 1
- 230000004083 survival effect Effects 0.000 description 1
- 208000024891 symptom Diseases 0.000 description 1
- 230000035488 systolic blood pressure Effects 0.000 description 1
- 238000012360 testing method Methods 0.000 description 1
- 238000004809 thin layer chromatography Methods 0.000 description 1
- 210000001519 tissue Anatomy 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
- 230000000472 traumatic effect Effects 0.000 description 1
- 238000005303 weighing Methods 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/41—Porphyrin- or corrin-ring-containing peptides
- A61K38/42—Haemoglobins; Myoglobins
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/18—Drugs for disorders of the alimentary tract or the digestive system for pancreatic disorders, e.g. pancreatic enzymes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P13/00—Drugs for disorders of the urinary system
- A61P13/12—Drugs for disorders of the urinary system of the kidneys
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P7/00—Drugs for disorders of the blood or the extracellular fluid
- A61P7/08—Plasma substitutes; Perfusion solutions; Dialytics or haemodialytics; Drugs for electrolytic or acid-base disorders, e.g. hypovolemic shock
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10S—TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10S530/00—Chemistry: natural resins or derivatives; peptides or proteins; lignins or reaction products thereof
- Y10S530/827—Proteins from mammals or birds
- Y10S530/829—Blood
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Chemical & Material Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Animal Behavior & Ethology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Gastroenterology & Hepatology (AREA)
- Epidemiology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Immunology (AREA)
- Heart & Thoracic Surgery (AREA)
- Hematology (AREA)
- Diabetes (AREA)
- Cardiology (AREA)
- Urology & Nephrology (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US41593502P | 2002-10-03 | 2002-10-03 | |
| US60/415,935 | 2002-10-03 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| RU2005113240A RU2005113240A (ru) | 2005-12-10 |
| RU2325924C2 true RU2325924C2 (ru) | 2008-06-10 |
Family
ID=32176456
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| RU2005113240/14A RU2325924C2 (ru) | 2002-10-03 | 2003-10-03 | Способ лечения пациентов с массивной потерей крови |
Country Status (11)
| Country | Link |
|---|---|
| US (1) | US7291592B2 (enExample) |
| EP (1) | EP1553968A4 (enExample) |
| JP (1) | JP2006502231A (enExample) |
| CN (1) | CN1703235A (enExample) |
| AU (1) | AU2003272827B2 (enExample) |
| CA (1) | CA2499459A1 (enExample) |
| MX (1) | MXPA05003340A (enExample) |
| NO (1) | NO20051390L (enExample) |
| RU (1) | RU2325924C2 (enExample) |
| WO (1) | WO2004037279A1 (enExample) |
| ZA (1) | ZA200502307B (enExample) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| RU2452519C1 (ru) * | 2011-03-25 | 2012-06-10 | Государственное учреждение здравоохранения Научно-исследовательский институт скорой помощи имени Н.В. Склифосовского Департамента здравоохранения г. Москвы | Способ компенсации глобулярного объема крови и иммуномодулирующего воздействия при трансплантации |
Families Citing this family (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20050049707A1 (en) * | 2003-08-29 | 2005-03-03 | Ferree Bret A. | Cemented artificial disc replacements |
| EP1471821B1 (en) * | 2002-01-15 | 2013-06-26 | Board Of Regents, The University Of Texas System | Compositions to reduce scattering of light during therapeutic and diagnostic imaging procedures |
| US20090004159A1 (en) * | 2006-01-24 | 2009-01-01 | The Board Of Trustees Of The University Of Illinoi | Polymerized Hemoglobin Media and Its Use in Isolation and Transplantation of Islet Cells |
| JP2009524436A (ja) | 2006-01-24 | 2009-07-02 | ノースフィールド ラボラトリーズ、インコーポレイテッド | 重合したヘモグロビンの培地、並びに島細胞の単離及び移植におけるその使用 |
| WO2008009634A2 (en) * | 2006-07-17 | 2008-01-24 | Novo Nordisk Health Care Ag | Factor viia analogues with increased activity for treating thrombocytopenia |
| RU2361608C1 (ru) * | 2008-03-18 | 2009-07-20 | Общество С Ограниченной Ответственностью "Научно-Производственная Компания "Медбиофарм" | Кровезаменитель с функцией переноса кислорода, фармацевтическая композиция (варианты) |
| EP3655019A4 (en) | 2017-07-18 | 2021-04-21 | Virtech Bio, Inc. | BLOOD SUBSTITUTES INCLUDING HEMOGLOBIN AND METHODS OF MANUFACTURING |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5194590A (en) * | 1986-06-20 | 1993-03-16 | Northfield Laboratories, Inc. | Acellular red blood cell substitute |
| US5464814A (en) * | 1986-06-20 | 1995-11-07 | Northfield Laboratories, Inc. | Acellular red blood cell substitute |
| EP1093720A1 (en) * | 1995-03-23 | 2001-04-25 | Biopure Corporation | Stable polymerized hemoglobin blood-substitute |
Family Cites Families (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4826811A (en) * | 1986-06-20 | 1989-05-02 | Northfield Laboratories, Inc. | Acellular red blood cell substitute |
| US5955581A (en) * | 1986-11-10 | 1999-09-21 | Biopure Corporation | Method for producing a stable polymerized hemoglobin blood-substitute |
| US5854209A (en) | 1995-03-23 | 1998-12-29 | Biopure Corporation | Method for oxygenating tissue having reduced red blood cell flow |
| US5084558A (en) * | 1987-10-13 | 1992-01-28 | Biopure Corporation | Extra pure semi-synthetic blood substitute |
| US5895810A (en) * | 1995-03-23 | 1999-04-20 | Biopure Corporation | Stable polymerized hemoglobin and use thereof |
| US20020065211A1 (en) * | 1995-03-23 | 2002-05-30 | Biopure Corporation | Increasing function of organs having reduced red blood cell flow |
| US6271351B1 (en) * | 1995-03-23 | 2001-08-07 | Biopure Corporation | Method for preserving a hemoglobin blood substitute |
| US5691452A (en) * | 1995-03-23 | 1997-11-25 | Biopure Corporation | Method for preserving a hemoglobin blood substitute |
| US6150507A (en) * | 1995-03-23 | 2000-11-21 | Biopure Corporation | Method for producing a purified hemoglobin product |
| US6288027B1 (en) * | 1995-03-23 | 2001-09-11 | Biopure Corporation | Preserving a hemoglobin blood substitute with a transparent overwrap |
| US5691453A (en) * | 1995-06-07 | 1997-11-25 | Biopure Corporation | Separation of polymerized hemoglobin from unpolymerized hemoglobin on hydroxyapatite using HPLC |
| RO121089B1 (ro) * | 1996-03-28 | 2006-12-29 | Northfield Laboratories, Inc. | Soluţie apoasă de hemoglobină polimerată cu glutaraldehida, procedeu de preparare şi utilizarea acesteia |
| US5890852A (en) * | 1998-03-17 | 1999-04-06 | Emerson Electric Company | Thread cutting die and method of manufacturing same |
-
2003
- 2003-10-03 CN CNA2003801009126A patent/CN1703235A/zh active Pending
- 2003-10-03 AU AU2003272827A patent/AU2003272827B2/en not_active Expired - Fee Related
- 2003-10-03 JP JP2004546786A patent/JP2006502231A/ja active Pending
- 2003-10-03 EP EP03755029A patent/EP1553968A4/en not_active Withdrawn
- 2003-10-03 MX MXPA05003340A patent/MXPA05003340A/es active IP Right Grant
- 2003-10-03 RU RU2005113240/14A patent/RU2325924C2/ru not_active IP Right Cessation
- 2003-10-03 WO PCT/US2003/031377 patent/WO2004037279A1/en not_active Ceased
- 2003-10-03 CA CA002499459A patent/CA2499459A1/en not_active Abandoned
- 2003-10-03 US US10/678,927 patent/US7291592B2/en not_active Expired - Fee Related
-
2005
- 2005-03-16 NO NO20051390A patent/NO20051390L/no not_active Application Discontinuation
- 2005-03-18 ZA ZA2005/02307A patent/ZA200502307B/en unknown
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5194590A (en) * | 1986-06-20 | 1993-03-16 | Northfield Laboratories, Inc. | Acellular red blood cell substitute |
| US5464814A (en) * | 1986-06-20 | 1995-11-07 | Northfield Laboratories, Inc. | Acellular red blood cell substitute |
| EP1093720A1 (en) * | 1995-03-23 | 2001-04-25 | Biopure Corporation | Stable polymerized hemoglobin blood-substitute |
Non-Patent Citations (4)
| Title |
|---|
| описание с.26, 33. * |
| реферат, формула, описание. * |
| формула, описание. * |
| формула, описание. Клиническая хирургия. / Под ред. Р.КОНДЕНА и др. - М.: Практика, 1998, с.204-208; 433-434. Интенсивная терапия. Реанимация. Первая помощь. / Под ред. В.Д.МАЛЫШЕВА. - М.: Медицина, 2000, с.142). * |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| RU2452519C1 (ru) * | 2011-03-25 | 2012-06-10 | Государственное учреждение здравоохранения Научно-исследовательский институт скорой помощи имени Н.В. Склифосовского Департамента здравоохранения г. Москвы | Способ компенсации глобулярного объема крови и иммуномодулирующего воздействия при трансплантации |
Also Published As
| Publication number | Publication date |
|---|---|
| MXPA05003340A (es) | 2005-07-05 |
| US7291592B2 (en) | 2007-11-06 |
| NO20051390L (no) | 2005-05-30 |
| EP1553968A1 (en) | 2005-07-20 |
| RU2005113240A (ru) | 2005-12-10 |
| CA2499459A1 (en) | 2004-05-06 |
| EP1553968A4 (en) | 2009-06-24 |
| WO2004037279A1 (en) | 2004-05-06 |
| JP2006502231A (ja) | 2006-01-19 |
| AU2003272827B2 (en) | 2009-03-19 |
| ZA200502307B (en) | 2005-11-30 |
| AU2003272827A1 (en) | 2004-05-13 |
| CN1703235A (zh) | 2005-11-30 |
| US20040067876A1 (en) | 2004-04-08 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US6552173B2 (en) | Acellular red blood cell substitute | |
| Vlahakes et al. | Hemodynamic effects and oxygen transport properties of a new blood substitute in a model of massive blood replacement | |
| US5084558A (en) | Extra pure semi-synthetic blood substitute | |
| US4826811A (en) | Acellular red blood cell substitute | |
| EP0277289B1 (en) | Extra pure semi-synthetic blood substitute | |
| Bjorkholm et al. | A phase I single blind clinical trial of a new oxygen transport agent (MP4), human hemoglobin modified with maleimide-activated polyethylene glycol | |
| US7521417B2 (en) | Method and apparatus for preparing an acellular red blood cell substitute | |
| RU2325924C2 (ru) | Способ лечения пациентов с массивной потерей крови | |
| Kasper et al. | Failure of autologous fresh frozen plasma to reduce blood loss and transfusion requirements in coronary artery bypass surgery | |
| Stollings et al. | Oxygen therapeutics: Oxygen delivery without blood | |
| Gould et al. | The development of polymerized pyridoxylated hemoglobin solution as a red cell substitute | |
| King et al. | Treating anemia | |
| US6046170A (en) | Therapeutic use of hemoglobin to treat head injury | |
| Standl | Artificial oxygen carriers as red blood cell substitutes–perfluorocarbons and cell-free hemoglobin | |
| HK1084875A (en) | Method for treating patients with massive blood loss | |
| Rao | Blood Conservation-How Practical? | |
| Muirhead et al. | The advantages and clinical uses of desiccated plasma prepared by the Adtevac process | |
| Ala | Transfusion of Red Cells | |
| RU2185154C2 (ru) | Способ лечения ожогового шока | |
| Murphy | The Use of Blood and Blood Products | |
| Anthony et al. | Indications for Transfusion | |
| Winslow | Clinical indications for blood substitutes and optimal properties | |
| Laiken et al. | Acute renal failure associated with hemodialysis and hypophosphatemia | |
| Biffl et al. | Transfusion alternatives in trauma |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| MM4A | The patent is invalid due to non-payment of fees |
Effective date: 20141004 |