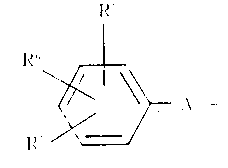KR940000008B1 - 고지질혈증 치료용의 약학 조성물 - Google Patents
고지질혈증 치료용의 약학 조성물 Download PDFInfo
- Publication number
- KR940000008B1 KR940000008B1 KR1019870015255A KR870015255A KR940000008B1 KR 940000008 B1 KR940000008 B1 KR 940000008B1 KR 1019870015255 A KR1019870015255 A KR 1019870015255A KR 870015255 A KR870015255 A KR 870015255A KR 940000008 B1 KR940000008 B1 KR 940000008B1
- Authority
- KR
- South Korea
- Prior art keywords
- group
- benzamide
- diethoxyphosphinylmethyl
- alkyl group
- pharmaceutical composition
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/66—Phosphorus compounds
- A61K31/675—Phosphorus compounds having nitrogen as a ring hetero atom, e.g. pyridoxal phosphate
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/66—Phosphorus compounds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/06—Antihyperlipidemics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
Landscapes
- Health & Medical Sciences (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Medicinal Chemistry (AREA)
- Epidemiology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Organic Chemistry (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Obesity (AREA)
- Hematology (AREA)
- Diabetes (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Abstract
Description
Claims (10)
- 활성 성분으로 하기 일반식(1)로 표시되는 카르복사미드 화합물을 함유하는 고지질혈증 치료용의 약학 조성물.식중에서, R1및 R2각각은 수소원자, C1~15알킬기, C3~8시클로알킬기, 디페닐 C1~6알킬기 또는 하기식의 기 :(식중에서, R5,R6및 R7각각은 수소원자, 할로겐원자, 니트로기, C1~6알콕시기, C1~6알콕시카르보닐기, C1~6알킬기, 할로겐-치환된 C1~6알킬기, 시아노기, 카르복실기 또는 히드록시기이고 ; A는 C1~4알킬렌기이며, ℓ은 0 또는 1이다)이고 ; 더우기, R1및 R2는 결합되어 있는 질소원자와 함께, 추가의 질소원자 또는 산소원자와 함께 또는 이것 없이, 헤테로시클릭기를 형성하며, 상기 헤테로시클릭기는 비치환되어 있거나, 또는 C1~6알킬기, 페닐 C1~6알킬기, 페닐기, 또는 C1~6알킬기, C1~6알콕시기, 할로겐원자 및 할로겐-치환된 C1~6알킬기로 이루어진 군으로부터 선택되는 치환기를 가지는 치환된 페닐기로 치환되어 있으며 ; R3는 수소원자, C1~15알킬기 또는 페닐-C1~6알킬기이고 ; R4는 C1~6알킬기 또는 페닐기이며, X는 산소 또는 황원자이다.
- 제2항에 있어서, 식중에서, R1및 R2각각이 수소원자, C1~6알킬기, 또는 하기식의 기 : (식중에서, R5, R6및 R7각각이 수소원자, 할로겐원자, C1~6알킬기, 할로겐-치환된C1~6알킬기, 또는 시아노기이며; A는 C1-4알킬렌기이고 ;ℓ은 0 또는 1이다)이며 ; R3는 수소원자이고 ; R4는 C1~6알킬기인 일반식(1)로 표시되는 카르복사미드 화합물이 활성 성분임을 특징으로 하는 고지질혈증 치료용 약학 조성물.
- 제3항에 있어서, 식중에서 R1이 수소원자인 일반식(1)로 표시되는 카르복사미드 화합물이 활성성분임을 특징으로 하는 고지질혈증 치료용 약학 조성물.
- 제3항에 있어서, 식중에서 R1이 비치환된 페닐 -C1~6알킬기 또는 페닐고리상의 치환기(들)로서 할로겐원자(들)를 가지는 치환된 페닐 -C1~6알킬기인 일반식(1)로 표시되는 카르복사미드 화합물이 활성 성분임을 특징으로 하는 고지질혈증 치료용 약학 조성물.
- 제3항에 있어서, 식중에서, R1이 C1~6알킬기인 일반식(1)로 표시되는 카르복사미드 화합물이 활성 성분임을 특징으로 하는 고지질혈증 치료용 약학 조성물.
- 제4항에 있어서, 다음으로 이루어진 군으로부터 선택되는 일반식(1)로 표시되는 카르복사미드 화합물이 활성성분임을 특징으로 하는 고지질혈증 치료용 약학 조성물 : 4-디에톡시포스피닐메틸-N-페닐벤즈아미드, 4-디에톡시포스피닐메틸-N-(4-클로로페닐)-벤즈아미드, 4-디에톡시포스피닐메틸-N-(4-브로모페닐)-벤즈아미드, 4-디에톡시포스피닐메틸-N-(4-요오도페닐) 벤즈아미드, 4-디에톡시포스피닐메틸-N-(4-플루오로페닐)-벤즈아미드, 4-디에톡시포스피닐메틸-N-(4-트리플루오로메틸페닐) 벤즈아미드, 4-디에톡시포스피닐메틸-N-(4-시아노페닐)-벤즈아미드, 4-디에톡시포스피닐메틸-N-(3,4-디클로로페닐)-벤즈아미드, 및 4-디에톡시포스피닐메틸-N-(4-클로로-3-트리플루오로메틸페닐) 벤즈아미드.
- 제5항에 있어서, 다음으로 이루어진 군으로부터 선택되는 일반식(1)로 표시되는 카르복사미드 화합물이 활성성분임을 특징으로 하는 고지질혈증 치료용 약학 조성물 : 4-디에톡시포스피닐메틸-N-벤질-N-(4-클로로페닐)벤즈아미드, 4-디에톡시포스피닐메틸-N-(4-클로로페닐)-N-(4-클로로페닐)벤즈아미드, 4-디에톡시포스피닐메틸-N-벤질-N-(4-트리플루오로페닐)벤즈아미드, 4-디에톡시포스피닐메틸-N-벤질-N-(3,4-디클로로페닐)벤즈아미드, 4-디에톡시포스피닐메틸-N-(4-클로로벤질)-N-(3,4-디클로로페닐)벤즈아미드, 및 4-디에톡시포스피닐메틸-N-벤질-N-(4-클로로-3-메틸페닐) 벤즈아미드.
- 제6항에 있어서, 다음으로 이루어진 군으로부터 선택되는 일반식(1)로 표시되는 카르복사미드 화합물이 활성성분임을 특징으로 하는 고지혈증 치료용 약학 조성물 : 4-디에톡시포스피닐메틸-N-메틸-N-페닐-벤즈아미드, 및 4-디에톡시포스피닐메틸-N-(4-클로로페닐)-N-메틸-벤즈아미드.
- 제2항에 있어서, 다음으로 이루어진 군으로부터 선택되는 일반식(1)로 표시되는 카르복사미드 화합물이 활성성분임을 특징으로 하는 고지질혈증 치료용 약학 조성물 : 4-디에톡시포스피닐메틸-N-(4-메틸페닐)-벤즈아미드, 4-디에톡시포스피닐메틸-N-(4-메톡시페닐)-벤즈아미드, 4-디에톡시포스피닐메틸-N-(4-니트로페닐)-벤즈아미드, 4-디아소프로폭시포스피닐메틸-N-(2-페닐에틸)-벤즈아미드, 4-디에톡시포스피닐메틸-N-(4-클로로페닐)-N-시클로펜틸벤즈아미드, 및 4-디에톡시포스피닐메틸-N-벤질-N-(4-니트로페닐)벤즈아미드.
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP31326786 | 1986-12-29 | ||
| JP61-313267 | 1986-12-29 | ||
| JP313267/86 | 1986-12-29 | ||
| JP62317246A JPH0651625B2 (ja) | 1986-12-29 | 1987-12-14 | 高脂質血症治療剤 |
| JP317246/87 | 1987-12-14 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| KR880007085A KR880007085A (ko) | 1988-08-26 |
| KR940000008B1 true KR940000008B1 (ko) | 1994-01-05 |
Family
ID=26567502
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1019870015255A Expired - Fee Related KR940000008B1 (ko) | 1986-12-29 | 1987-12-29 | 고지질혈증 치료용의 약학 조성물 |
Country Status (5)
| Country | Link |
|---|---|
| US (1) | US5081112A (ko) |
| EP (1) | EP0273444B1 (ko) |
| JP (1) | JPH0651625B2 (ko) |
| KR (1) | KR940000008B1 (ko) |
| DE (1) | DE3785740T2 (ko) |
Families Citing this family (19)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH0651625B2 (ja) * | 1986-12-29 | 1994-07-06 | 株式会社大塚製薬工場 | 高脂質血症治療剤 |
| AU606808B2 (en) * | 1988-06-29 | 1991-02-14 | Otsuka Pharmaceutical Factory, Inc. | Arylcarboxamide substituted by alkylphosphonates, process for preparing the same and a pharmaceutical composition containing the same |
| JP2584336B2 (ja) * | 1989-05-30 | 1997-02-26 | 株式会社大塚製薬工場 | カルボン酸アミド誘導体 |
| JP2835545B2 (ja) * | 1990-11-27 | 1998-12-14 | 株式会社大塚製薬工場 | 白内障予防及び治療剤 |
| JP2835547B2 (ja) * | 1991-12-25 | 1998-12-14 | 株式会社大塚製薬工場 | 糖尿病治療剤 |
| TW260664B (ko) * | 1993-02-15 | 1995-10-21 | Otsuka Pharma Factory Inc | |
| CN1048018C (zh) * | 1993-06-17 | 2000-01-05 | 株式会社大塚制药工场 | 膦酸二酯衍生物 |
| JP3074332B2 (ja) * | 1993-08-20 | 2000-08-07 | 株式会社大塚製薬工場 | ホスホン酸ジエステル誘導体 |
| JP3500468B2 (ja) * | 1995-12-27 | 2004-02-23 | 株式会社大塚製薬工場 | ホスホン酸ジエステル誘導体 |
| TW570799B (en) * | 1998-02-17 | 2004-01-11 | Otsuka Pharma Co Ltd | The agent for preventing and curing fatty liver |
| UA85194C2 (ru) * | 2003-08-11 | 2009-01-12 | Ф.Хоффманн-Ля Рош Аг | Пиперазины с or-замещенной фенильной группой и их применение как ингибиторов glyt 1 |
| US9409856B2 (en) | 2005-11-28 | 2016-08-09 | Gtx, Inc. | Estrogen receptor ligands and methods of use thereof |
| US8546451B2 (en) | 2005-11-28 | 2013-10-01 | Gtx, Inc. | Estrogen receptor ligands and methods of use thereof |
| US8637706B2 (en) * | 2005-11-28 | 2014-01-28 | Gtx, Inc. | Nuclear receptor binding agents |
| JP5572549B2 (ja) | 2007-08-13 | 2014-08-13 | リガンド・ファーマシューティカルズ・インコーポレイテッド | グルコキナーゼの新規な活性化剤 |
| US9427418B2 (en) | 2009-02-23 | 2016-08-30 | Gtx, Inc. | Estrogen receptor ligands and methods of use thereof |
| US9624161B2 (en) | 2009-02-23 | 2017-04-18 | Gtx, Inc. | Estrogen receptor ligands and methods of use thereof |
| WO2013078413A1 (en) * | 2011-11-22 | 2013-05-30 | The United States Of America, As Represented By The Secretary, Department Of Health And Human Services | Modulators of lipid storage |
| KR101432012B1 (ko) * | 2013-02-19 | 2014-08-21 | 한국식품연구원 | 항비만용 조성물 |
Family Cites Families (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3073740A (en) * | 1958-10-06 | 1963-01-15 | Smith Kline French Lab | Method of reducing cholesterol levels with tri- (dialkylaminoalkyl) phosphates |
| AU523040B2 (en) * | 1978-05-15 | 1982-07-08 | Kanebo Limited | Calcium -antagonistic composition |
| US4416877A (en) * | 1979-02-13 | 1983-11-22 | Symphar S.A. | Anti-atherosclerotic pharmaceutical compositions containing diphosphonate compounds |
| GB2043073B (en) * | 1979-02-13 | 1983-05-11 | Symphar Sa | Mono-and diphosphonate compounds |
| GB2043072B (en) * | 1979-02-13 | 1983-11-23 | Symphar Sa | Diphosphonate compounds |
| DE3564274D1 (en) * | 1984-12-12 | 1988-09-15 | Kanebo Ltd | Novel ethyl benzylphosphinate derivatives, process for production thereof, and their use as calcium antagonist |
| JPS61151199A (ja) * | 1984-12-26 | 1986-07-09 | Otsuka Pharmaceut Factory Inc | カルボン酸アミド誘導体 |
| US4704382A (en) * | 1985-07-29 | 1987-11-03 | G. D. Searle & Co. | Phenylpiperazine phosphonates |
| JPH0651625B2 (ja) * | 1986-12-29 | 1994-07-06 | 株式会社大塚製薬工場 | 高脂質血症治療剤 |
| US4822780A (en) * | 1987-07-08 | 1989-04-18 | Otsuka Pharmaceutical Factory, Inc. | Carboxamide compounds |
-
1987
- 1987-12-14 JP JP62317246A patent/JPH0651625B2/ja not_active Expired - Lifetime
- 1987-12-29 KR KR1019870015255A patent/KR940000008B1/ko not_active Expired - Fee Related
- 1987-12-29 EP EP87119319A patent/EP0273444B1/en not_active Expired - Lifetime
- 1987-12-29 DE DE87119319T patent/DE3785740T2/de not_active Expired - Lifetime
-
1989
- 1989-07-05 US US07/375,632 patent/US5081112A/en not_active Expired - Lifetime
Also Published As
| Publication number | Publication date |
|---|---|
| EP0273444A3 (en) | 1990-07-11 |
| KR880007085A (ko) | 1988-08-26 |
| JPS63264421A (ja) | 1988-11-01 |
| US5081112A (en) | 1992-01-14 |
| JPH0651625B2 (ja) | 1994-07-06 |
| DE3785740D1 (de) | 1993-06-09 |
| EP0273444B1 (en) | 1993-05-05 |
| DE3785740T2 (de) | 1993-10-21 |
| EP0273444A2 (en) | 1988-07-06 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR940000008B1 (ko) | 고지질혈증 치료용의 약학 조성물 | |
| EP0714660B1 (en) | Ranolazine and related piperazines for protecting skeletal muscles | |
| AU686706B2 (en) | Inhibition of smooth muscle migration and proliferation with hydroxy carbazole compounds | |
| AU2011202135A1 (en) | Use of ranolazine for the preparation of a medicament for the treatment of arrhythmias | |
| EP0109036B1 (en) | Anti-inflammatory/analgesic combination of alpha-fluoromethylhistidine and a selected non-steroidal anti-inflammatory drug (nsaid) | |
| EP0295637A2 (en) | Lipid regulating compositions | |
| JPWO1996031211A1 (ja) | 臓器または組織保護剤 | |
| KR900012892A (ko) | 항-염증성 아릴 화합물 | |
| US5866574A (en) | Pancreatitis remedy | |
| EP0273375A2 (en) | Propiophenone derivatives for treatment of pollakiuria (frequency urination) | |
| ZA200106199B (en) | Utilization of polycyclic 2-amino-thiazole systems in the production of medicaments for prophylaxis or treatment of obesity. | |
| KR900006993B1 (ko) | 아테롬성 동맥경화증 치료제로서의 dl-5-[(2-벤질-3,4-디하이드로-2H-벤조피란-6-일)메틸]티아졸리딘-2,4-디온 | |
| US4895851A (en) | Use of oxoquinazoline derivatives in the treatment of hyperuricaemia | |
| EP0448029A2 (en) | Novel pharmaceutical uses of forskolin derivatives | |
| KR20010034114A (ko) | 칼륨 채널 활성화제 | |
| EP0261439B1 (en) | Medicament for curing arteriosclerosis, comprising pyrimido (2,1-b) benzothiazole derivative | |
| US4198409A (en) | Triazinones for veterinary use | |
| US3466377A (en) | Aralkyl aliphatic sulfoxide oral,parenteral and rectal dosage units for pain,fever and inflammation | |
| JPS6245525A (ja) | 脂質低下剤 | |
| EP0916340A1 (en) | Suppositories | |
| JPH0231693B2 (ko) | ||
| US5141933A (en) | Treatment for hyperglycaemia | |
| KR960014874B1 (ko) | 요산 배출용 조성물 | |
| US4988720A (en) | Novel treatment of hyperglycaemia | |
| JPS60208914A (ja) | 肝障害の予防、治療剤 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0109 | Patent application |
St.27 status event code: A-0-1-A10-A12-nap-PA0109 |
|
| R17-X000 | Change to representative recorded |
St.27 status event code: A-3-3-R10-R17-oth-X000 |
|
| P11-X000 | Amendment of application requested |
St.27 status event code: A-2-2-P10-P11-nap-X000 |
|
| P13-X000 | Application amended |
St.27 status event code: A-2-2-P10-P13-nap-X000 |
|
| PG1501 | Laying open of application |
St.27 status event code: A-1-1-Q10-Q12-nap-PG1501 |
|
| A201 | Request for examination | ||
| PA0201 | Request for examination |
St.27 status event code: A-1-2-D10-D11-exm-PA0201 |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
St.27 status event code: A-1-2-D10-D21-exm-PE0902 |
|
| P11-X000 | Amendment of application requested |
St.27 status event code: A-2-2-P10-P11-nap-X000 |
|
| P13-X000 | Application amended |
St.27 status event code: A-2-2-P10-P13-nap-X000 |
|
| G160 | Decision to publish patent application | ||
| PG1605 | Publication of application before grant of patent |
St.27 status event code: A-2-2-Q10-Q13-nap-PG1605 |
|
| E701 | Decision to grant or registration of patent right | ||
| PE0701 | Decision of registration |
St.27 status event code: A-1-2-D10-D22-exm-PE0701 |
|
| GRNT | Written decision to grant | ||
| PR0701 | Registration of establishment |
St.27 status event code: A-2-4-F10-F11-exm-PR0701 |
|
| PR1002 | Payment of registration fee |
St.27 status event code: A-2-2-U10-U11-oth-PR1002 Fee payment year number: 1 |
|
| PR1001 | Payment of annual fee |
St.27 status event code: A-4-4-U10-U11-oth-PR1001 Fee payment year number: 4 |
|
| PR1001 | Payment of annual fee |
St.27 status event code: A-4-4-U10-U11-oth-PR1001 Fee payment year number: 5 |
|
| PR1001 | Payment of annual fee |
St.27 status event code: A-4-4-U10-U11-oth-PR1001 Fee payment year number: 6 |
|
| PR1001 | Payment of annual fee |
St.27 status event code: A-4-4-U10-U11-oth-PR1001 Fee payment year number: 7 |
|
| PR1001 | Payment of annual fee |
St.27 status event code: A-4-4-U10-U11-oth-PR1001 Fee payment year number: 8 |
|
| PR1001 | Payment of annual fee |
St.27 status event code: A-4-4-U10-U11-oth-PR1001 Fee payment year number: 9 |
|
| PR1001 | Payment of annual fee |
St.27 status event code: A-4-4-U10-U11-oth-PR1001 Fee payment year number: 10 |
|
| PR1001 | Payment of annual fee |
St.27 status event code: A-4-4-U10-U11-oth-PR1001 Fee payment year number: 11 |
|
| PR1001 | Payment of annual fee |
St.27 status event code: A-4-4-U10-U11-oth-PR1001 Fee payment year number: 12 |
|
| PR1001 | Payment of annual fee |
St.27 status event code: A-4-4-U10-U11-oth-PR1001 Fee payment year number: 13 |
|
| FPAY | Annual fee payment |
Payment date: 20061226 Year of fee payment: 14 |
|
| PR1001 | Payment of annual fee |
St.27 status event code: A-4-4-U10-U11-oth-PR1001 Fee payment year number: 14 |
|
| LAPS | Lapse due to unpaid annual fee | ||
| PC1903 | Unpaid annual fee |
St.27 status event code: A-4-4-U10-U13-oth-PC1903 Not in force date: 20080106 Payment event data comment text: Termination Category : DEFAULT_OF_REGISTRATION_FEE |
|
| PC1903 | Unpaid annual fee |
St.27 status event code: N-4-6-H10-H13-oth-PC1903 Ip right cessation event data comment text: Termination Category : DEFAULT_OF_REGISTRATION_FEE Not in force date: 20080106 |
|
| P22-X000 | Classification modified |
St.27 status event code: A-4-4-P10-P22-nap-X000 |