KR20250021652A - 생체내 수용체 점유율을 결정하기 위한 검정 - Google Patents
생체내 수용체 점유율을 결정하기 위한 검정 Download PDFInfo
- Publication number
- KR20250021652A KR20250021652A KR1020257003448A KR20257003448A KR20250021652A KR 20250021652 A KR20250021652 A KR 20250021652A KR 1020257003448 A KR1020257003448 A KR 1020257003448A KR 20257003448 A KR20257003448 A KR 20257003448A KR 20250021652 A KR20250021652 A KR 20250021652A
- Authority
- KR
- South Korea
- Prior art keywords
- btk
- leu
- glu
- lys
- bound
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 238000003556 assay Methods 0.000 title abstract description 36
- 238000001727 in vivo Methods 0.000 title description 5
- 239000003814 drug Substances 0.000 abstract description 80
- 229940079593 drug Drugs 0.000 abstract description 77
- 210000004369 blood Anatomy 0.000 abstract description 65
- 239000008280 blood Substances 0.000 abstract description 65
- 108090000765 processed proteins & peptides Proteins 0.000 abstract description 56
- 150000001875 compounds Chemical class 0.000 abstract description 51
- 239000003795 chemical substances by application Substances 0.000 abstract description 26
- 102000004196 processed proteins & peptides Human genes 0.000 abstract description 21
- 230000027455 binding Effects 0.000 abstract description 15
- 230000002934 lysing effect Effects 0.000 abstract description 3
- 238000012544 monitoring process Methods 0.000 abstract description 2
- 102000001714 Agammaglobulinaemia Tyrosine Kinase Human genes 0.000 description 79
- 108010029445 Agammaglobulinaemia Tyrosine Kinase Proteins 0.000 description 79
- 108020003175 receptors Proteins 0.000 description 60
- 102000005962 receptors Human genes 0.000 description 60
- 239000006166 lysate Substances 0.000 description 38
- 239000000523 sample Substances 0.000 description 34
- 239000000243 solution Substances 0.000 description 30
- 241000282693 Cercopithecidae Species 0.000 description 28
- 239000011324 bead Substances 0.000 description 23
- 238000003312 immunocapture Methods 0.000 description 22
- 238000001294 liquid chromatography-tandem mass spectrometry Methods 0.000 description 19
- 238000004090 dissolution Methods 0.000 description 17
- 150000002500 ions Chemical class 0.000 description 16
- 230000003285 pharmacodynamic effect Effects 0.000 description 13
- 239000012139 lysis buffer Substances 0.000 description 12
- 238000002360 preparation method Methods 0.000 description 12
- 241000282414 Homo sapiens Species 0.000 description 11
- 102000035195 Peptidases Human genes 0.000 description 11
- 108091005804 Peptidases Proteins 0.000 description 11
- 239000004365 Protease Substances 0.000 description 11
- 238000000034 method Methods 0.000 description 11
- 239000000872 buffer Substances 0.000 description 10
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 9
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 9
- 108090000631 Trypsin Proteins 0.000 description 9
- 102000004142 Trypsin Human genes 0.000 description 9
- 230000002427 irreversible effect Effects 0.000 description 9
- 238000002552 multiple reaction monitoring Methods 0.000 description 9
- 239000012588 trypsin Substances 0.000 description 9
- 238000004458 analytical method Methods 0.000 description 8
- 238000001514 detection method Methods 0.000 description 8
- 238000011161 development Methods 0.000 description 8
- 238000010790 dilution Methods 0.000 description 8
- 239000012895 dilution Substances 0.000 description 8
- 239000000137 peptide hydrolase inhibitor Substances 0.000 description 8
- 229940124158 Protease/peptidase inhibitor Drugs 0.000 description 7
- 230000029087 digestion Effects 0.000 description 7
- 238000011002 quantification Methods 0.000 description 7
- 230000004044 response Effects 0.000 description 7
- 239000000758 substrate Substances 0.000 description 7
- 108010090804 Streptavidin Proteins 0.000 description 6
- 108010062796 arginyllysine Proteins 0.000 description 6
- 238000006243 chemical reaction Methods 0.000 description 6
- UQLDLKMNUJERMK-UHFFFAOYSA-L di(octadecanoyloxy)lead Chemical compound [Pb+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O UQLDLKMNUJERMK-UHFFFAOYSA-L 0.000 description 6
- 108010034529 leucyl-lysine Proteins 0.000 description 6
- 238000004895 liquid chromatography mass spectrometry Methods 0.000 description 6
- BDAGIHXWWSANSR-UHFFFAOYSA-N methanoic acid Natural products OC=O BDAGIHXWWSANSR-UHFFFAOYSA-N 0.000 description 6
- 239000000047 product Substances 0.000 description 6
- 238000004885 tandem mass spectrometry Methods 0.000 description 6
- 238000010200 validation analysis Methods 0.000 description 6
- LMKSBGIUPVRHEH-FXQIFTODSA-N Met-Ala-Asn Chemical compound CSCC[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@H](C(O)=O)CC(N)=O LMKSBGIUPVRHEH-FXQIFTODSA-N 0.000 description 5
- 229920001213 Polysorbate 20 Polymers 0.000 description 5
- KOSRFJWDECSPRO-UHFFFAOYSA-N alpha-L-glutamyl-L-glutamic acid Natural products OC(=O)CCC(N)C(=O)NC(CCC(O)=O)C(O)=O KOSRFJWDECSPRO-UHFFFAOYSA-N 0.000 description 5
- 229940000406 drug candidate Drugs 0.000 description 5
- 238000007876 drug discovery Methods 0.000 description 5
- 108010055341 glutamyl-glutamic acid Proteins 0.000 description 5
- 238000005259 measurement Methods 0.000 description 5
- 235000010486 polyoxyethylene sorbitan monolaurate Nutrition 0.000 description 5
- 239000000256 polyoxyethylene sorbitan monolaurate Substances 0.000 description 5
- 102000004169 proteins and genes Human genes 0.000 description 5
- 108090000623 proteins and genes Proteins 0.000 description 5
- MDNAVFBZPROEHO-UHFFFAOYSA-N Ala-Lys-Val Natural products CC(C)C(C(O)=O)NC(=O)C(NC(=O)C(C)N)CCCCN MDNAVFBZPROEHO-UHFFFAOYSA-N 0.000 description 4
- UULLJGQFCDXVTQ-CYDGBPFRSA-N Arg-Pro-Ile Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H]([C@@H](C)CC)C(O)=O UULLJGQFCDXVTQ-CYDGBPFRSA-N 0.000 description 4
- DATSKXOXPUAOLK-KKUMJFAQSA-N Asn-Tyr-Leu Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC(C)C)C(O)=O DATSKXOXPUAOLK-KKUMJFAQSA-N 0.000 description 4
- WVLZTXGTNGHPBO-SRVKXCTJSA-N Cys-Leu-Leu Chemical compound [H]N[C@@H](CS)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O WVLZTXGTNGHPBO-SRVKXCTJSA-N 0.000 description 4
- 238000002965 ELISA Methods 0.000 description 4
- IQACOVZVOMVILH-FXQIFTODSA-N Glu-Glu-Ser Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(O)=O IQACOVZVOMVILH-FXQIFTODSA-N 0.000 description 4
- WXHFZJFZWNCDNB-KKUMJFAQSA-N Leu-Asn-Tyr Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 WXHFZJFZWNCDNB-KKUMJFAQSA-N 0.000 description 4
- PESQCPHRXOFIPX-UHFFFAOYSA-N N-L-methionyl-L-tyrosine Natural products CSCCC(N)C(=O)NC(C(O)=O)CC1=CC=C(O)C=C1 PESQCPHRXOFIPX-UHFFFAOYSA-N 0.000 description 4
- HNFUGJUZJRYUHN-JSGCOSHPSA-N Phe-Gly-Val Chemical compound CC(C)[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H](N)CC1=CC=CC=C1 HNFUGJUZJRYUHN-JSGCOSHPSA-N 0.000 description 4
- GXDPQJUBLBZKDY-IAVJCBSLSA-N Phe-Ile-Ile Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O GXDPQJUBLBZKDY-IAVJCBSLSA-N 0.000 description 4
- LKEKWDJCJSPXNI-IRIUXVKKSA-N Thr-Glu-Tyr Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 LKEKWDJCJSPXNI-IRIUXVKKSA-N 0.000 description 4
- 108010005233 alanylglutamic acid Proteins 0.000 description 4
- 150000001413 amino acids Chemical group 0.000 description 4
- 108010038633 aspartylglutamate Proteins 0.000 description 4
- 238000011953 bioanalysis Methods 0.000 description 4
- 230000005754 cellular signaling Effects 0.000 description 4
- 238000005516 engineering process Methods 0.000 description 4
- 238000002156 mixing Methods 0.000 description 4
- 210000003819 peripheral blood mononuclear cell Anatomy 0.000 description 4
- 238000003908 quality control method Methods 0.000 description 4
- 230000035945 sensitivity Effects 0.000 description 4
- 238000001228 spectrum Methods 0.000 description 4
- 239000011550 stock solution Substances 0.000 description 4
- 238000001195 ultra high performance liquid chromatography Methods 0.000 description 4
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Chemical compound O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 4
- OSWFIVFLDKOXQC-UHFFFAOYSA-N 4-(3-methoxyphenyl)aniline Chemical compound COC1=CC=CC(C=2C=CC(N)=CC=2)=C1 OSWFIVFLDKOXQC-UHFFFAOYSA-N 0.000 description 3
- PLVAAIPKSGUXDV-WHFBIAKZSA-N Asn-Gly-Cys Chemical compound C([C@@H](C(=O)NCC(=O)N[C@@H](CS)C(=O)O)N)C(=O)N PLVAAIPKSGUXDV-WHFBIAKZSA-N 0.000 description 3
- XWKBWZXGNXTDKY-ZKWXMUAHSA-N Asp-Val-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@H](C(C)C)NC(=O)[C@@H](N)CC(O)=O XWKBWZXGNXTDKY-ZKWXMUAHSA-N 0.000 description 3
- XOKGKOQWADCLFQ-GARJFASQSA-N Gln-Arg-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CCC(=O)N)N)C(=O)O XOKGKOQWADCLFQ-GARJFASQSA-N 0.000 description 3
- XFAUJGNLHIGXET-AVGNSLFASA-N Gln-Leu-Leu Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O XFAUJGNLHIGXET-AVGNSLFASA-N 0.000 description 3
- JJKKWYQVHRUSDG-GUBZILKMSA-N Glu-Ala-Lys Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCCN)C(O)=O JJKKWYQVHRUSDG-GUBZILKMSA-N 0.000 description 3
- XXCDTYBVGMPIOA-FXQIFTODSA-N Glu-Asp-Glu Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O XXCDTYBVGMPIOA-FXQIFTODSA-N 0.000 description 3
- ILWHFUZZCFYSKT-AVGNSLFASA-N Glu-Lys-Leu Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(O)=O ILWHFUZZCFYSKT-AVGNSLFASA-N 0.000 description 3
- LGWUJBCIFGVBSJ-CIUDSAMLSA-N Glu-Met-Cys Chemical compound CSCC[C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CCC(=O)O)N LGWUJBCIFGVBSJ-CIUDSAMLSA-N 0.000 description 3
- JLJLBWDKDRYOPA-RYUDHWBXSA-N Gly-Gln-Tyr Chemical compound NCC(=O)N[C@@H](CCC(N)=O)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 JLJLBWDKDRYOPA-RYUDHWBXSA-N 0.000 description 3
- WVUDHMBJNBWZBU-XUXIUFHCSA-N Ile-Lys-Met Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCSC)C(=O)O)N WVUDHMBJNBWZBU-XUXIUFHCSA-N 0.000 description 3
- HASRFYOMVPJRPU-SRVKXCTJSA-N Leu-Arg-Glu Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC(O)=O)C(O)=O HASRFYOMVPJRPU-SRVKXCTJSA-N 0.000 description 3
- ADJWHHZETYAAAX-SRVKXCTJSA-N Leu-Ser-His Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)N ADJWHHZETYAAAX-SRVKXCTJSA-N 0.000 description 3
- VAGCEUUEMMXFEX-GUBZILKMSA-N Met-Met-Asn Chemical compound CSCC[C@H](N)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(N)=O)C(O)=O VAGCEUUEMMXFEX-GUBZILKMSA-N 0.000 description 3
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 3
- XMBSYZWANAQXEV-UHFFFAOYSA-N N-alpha-L-glutamyl-L-phenylalanine Natural products OC(=O)CCC(N)C(=O)NC(C(O)=O)CC1=CC=CC=C1 XMBSYZWANAQXEV-UHFFFAOYSA-N 0.000 description 3
- KRYSMKKRRRWOCZ-QEWYBTABSA-N Phe-Ile-Glu Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCC(O)=O)C(O)=O KRYSMKKRRRWOCZ-QEWYBTABSA-N 0.000 description 3
- CJXURNZYNHCYFD-WDCWCFNPSA-N Thr-Lys-Gln Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(=O)N)C(=O)O)N)O CJXURNZYNHCYFD-WDCWCFNPSA-N 0.000 description 3
- CTDPLKMBVALCGN-JSGCOSHPSA-N Tyr-Gly-Val Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)NCC(=O)N[C@@H](C(C)C)C(O)=O CTDPLKMBVALCGN-JSGCOSHPSA-N 0.000 description 3
- SBLZVFCEOCWRLS-BPNCWPANSA-N Tyr-Met-Ala Chemical compound C[C@@H](C(=O)O)NC(=O)[C@H](CCSC)NC(=O)[C@H](CC1=CC=C(C=C1)O)N SBLZVFCEOCWRLS-BPNCWPANSA-N 0.000 description 3
- VFOHXOLPLACADK-GVXVVHGQSA-N Val-Gln-Leu Chemical compound CC(C)C[C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)N)NC(=O)[C@H](C(C)C)N VFOHXOLPLACADK-GVXVVHGQSA-N 0.000 description 3
- 235000001014 amino acid Nutrition 0.000 description 3
- 230000015572 biosynthetic process Effects 0.000 description 3
- 150000001768 cations Chemical class 0.000 description 3
- 239000003153 chemical reaction reagent Substances 0.000 description 3
- 230000009089 cytolysis Effects 0.000 description 3
- 239000008367 deionised water Substances 0.000 description 3
- 229910021641 deionized water Inorganic materials 0.000 description 3
- 235000019253 formic acid Nutrition 0.000 description 3
- 238000000338 in vitro Methods 0.000 description 3
- 238000000670 ligand binding assay Methods 0.000 description 3
- 239000002243 precursor Substances 0.000 description 3
- 230000008569 process Effects 0.000 description 3
- 235000018102 proteins Nutrition 0.000 description 3
- 238000011160 research Methods 0.000 description 3
- 239000006228 supernatant Substances 0.000 description 3
- 238000002877 time resolved fluorescence resonance energy transfer Methods 0.000 description 3
- HHGYNJRJIINWAK-FXQIFTODSA-N Ala-Ala-Arg Chemical compound C[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@H](C(O)=O)CCCN=C(N)N HHGYNJRJIINWAK-FXQIFTODSA-N 0.000 description 2
- CVGNCMIULZNYES-WHFBIAKZSA-N Ala-Asn-Gly Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(=O)NCC(O)=O CVGNCMIULZNYES-WHFBIAKZSA-N 0.000 description 2
- PCIFXPRIFWKWLK-YUMQZZPRSA-N Ala-Gly-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)CNC(=O)[C@H](C)N PCIFXPRIFWKWLK-YUMQZZPRSA-N 0.000 description 2
- RGQCNKIDEQJEBT-CQDKDKBSSA-N Ala-Leu-Tyr Chemical compound C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 RGQCNKIDEQJEBT-CQDKDKBSSA-N 0.000 description 2
- NINQYGGNRIBFSC-CIUDSAMLSA-N Ala-Lys-Ser Chemical compound NCCCC[C@H](NC(=O)[C@@H](N)C)C(=O)N[C@@H](CO)C(O)=O NINQYGGNRIBFSC-CIUDSAMLSA-N 0.000 description 2
- YHBDGLZYNIARKJ-GUBZILKMSA-N Ala-Pro-Val Chemical compound CC(C)[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](C)N YHBDGLZYNIARKJ-GUBZILKMSA-N 0.000 description 2
- MSWSRLGNLKHDEI-ACZMJKKPSA-N Ala-Ser-Glu Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(O)=O)C(O)=O MSWSRLGNLKHDEI-ACZMJKKPSA-N 0.000 description 2
- LYILPUNCKACNGF-NAKRPEOUSA-N Ala-Val-Ile Chemical compound CC[C@H](C)[C@@H](C(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@H](C)N LYILPUNCKACNGF-NAKRPEOUSA-N 0.000 description 2
- SGYSTDWPNPKJPP-GUBZILKMSA-N Arg-Ala-Arg Chemical compound NC(=N)NCCC[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O SGYSTDWPNPKJPP-GUBZILKMSA-N 0.000 description 2
- IASNWHAGGYTEKX-IUCAKERBSA-N Arg-Arg-Gly Chemical compound NC(N)=NCCC[C@H](N)C(=O)N[C@@H](CCCN=C(N)N)C(=O)NCC(O)=O IASNWHAGGYTEKX-IUCAKERBSA-N 0.000 description 2
- JCAISGGAOQXEHJ-ZPFDUUQYSA-N Arg-Gln-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)N)NC(=O)[C@H](CCCN=C(N)N)N JCAISGGAOQXEHJ-ZPFDUUQYSA-N 0.000 description 2
- DJAIOAKQIOGULM-DCAQKATOSA-N Arg-Glu-Met Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCSC)C(O)=O DJAIOAKQIOGULM-DCAQKATOSA-N 0.000 description 2
- WVNFNPGXYADPPO-BQBZGAKWSA-N Arg-Gly-Ser Chemical compound NC(N)=NCCC[C@H](N)C(=O)NCC(=O)N[C@@H](CO)C(O)=O WVNFNPGXYADPPO-BQBZGAKWSA-N 0.000 description 2
- YBIAYFFIVAZXPK-AVGNSLFASA-N Arg-His-Arg Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O YBIAYFFIVAZXPK-AVGNSLFASA-N 0.000 description 2
- UZGFHWIJWPUPOH-IHRRRGAJSA-N Arg-Leu-Lys Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CCCN=C(N)N)N UZGFHWIJWPUPOH-IHRRRGAJSA-N 0.000 description 2
- VEAIMHJZTIDCIH-KKUMJFAQSA-N Arg-Phe-Gln Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CCC(N)=O)C(O)=O VEAIMHJZTIDCIH-KKUMJFAQSA-N 0.000 description 2
- LXMKTIZAGIBQRX-HRCADAONSA-N Arg-Phe-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CC2=CC=CC=C2)NC(=O)[C@H](CCCN=C(N)N)N)C(=O)O LXMKTIZAGIBQRX-HRCADAONSA-N 0.000 description 2
- AWMAZIIEFPFHCP-RCWTZXSCSA-N Arg-Pro-Thr Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H]([C@@H](C)O)C(O)=O AWMAZIIEFPFHCP-RCWTZXSCSA-N 0.000 description 2
- ORXCYAFUCSTQGY-FXQIFTODSA-N Asn-Ala-Met Chemical compound C[C@@H](C(=O)N[C@@H](CCSC)C(=O)O)NC(=O)[C@H](CC(=O)N)N ORXCYAFUCSTQGY-FXQIFTODSA-N 0.000 description 2
- XHFXZQHTLJVZBN-FXQIFTODSA-N Asn-Arg-Asn Chemical compound C(C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)O)NC(=O)[C@H](CC(=O)N)N)CN=C(N)N XHFXZQHTLJVZBN-FXQIFTODSA-N 0.000 description 2
- UGXVKHRDGLYFKR-CIUDSAMLSA-N Asn-Asp-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](N)CC(N)=O UGXVKHRDGLYFKR-CIUDSAMLSA-N 0.000 description 2
- ZPMNECSEJXXNBE-CIUDSAMLSA-N Asn-Cys-Leu Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(C)C)C(O)=O ZPMNECSEJXXNBE-CIUDSAMLSA-N 0.000 description 2
- GQRDIVQPSMPQME-ZPFDUUQYSA-N Asn-Ile-Leu Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(C)C)C(O)=O GQRDIVQPSMPQME-ZPFDUUQYSA-N 0.000 description 2
- KHCNTVRVAYCPQE-CIUDSAMLSA-N Asn-Lys-Asn Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(O)=O KHCNTVRVAYCPQE-CIUDSAMLSA-N 0.000 description 2
- XTMZYFMTYJNABC-ZLUOBGJFSA-N Asn-Ser-Ala Chemical compound C[C@@H](C(=O)O)NC(=O)[C@H](CO)NC(=O)[C@H](CC(=O)N)N XTMZYFMTYJNABC-ZLUOBGJFSA-N 0.000 description 2
- VWADICJNCPFKJS-ZLUOBGJFSA-N Asn-Ser-Asp Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(O)=O)C(O)=O VWADICJNCPFKJS-ZLUOBGJFSA-N 0.000 description 2
- RRKCPMGSRIDLNC-AVGNSLFASA-N Asp-Glu-Tyr Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O RRKCPMGSRIDLNC-AVGNSLFASA-N 0.000 description 2
- ORRJQLIATJDMQM-HJGDQZAQSA-N Asp-Leu-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CC(O)=O ORRJQLIATJDMQM-HJGDQZAQSA-N 0.000 description 2
- MGSVBZIBCCKGCY-ZLUOBGJFSA-N Asp-Ser-Ser Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(O)=O MGSVBZIBCCKGCY-ZLUOBGJFSA-N 0.000 description 2
- GXIUDSXIUSTSLO-QXEWZRGKSA-N Asp-Val-Met Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCSC)C(=O)O)NC(=O)[C@H](CC(=O)O)N GXIUDSXIUSTSLO-QXEWZRGKSA-N 0.000 description 2
- 108091005502 Aspartic proteases Proteins 0.000 description 2
- 102000035101 Aspartic proteases Human genes 0.000 description 2
- 108090000317 Chymotrypsin Proteins 0.000 description 2
- 229940126062 Compound A Drugs 0.000 description 2
- ATPDEYTYWVMINF-ZLUOBGJFSA-N Cys-Cys-Ser Chemical compound [H]N[C@@H](CS)C(=O)N[C@@H](CS)C(=O)N[C@@H](CO)C(O)=O ATPDEYTYWVMINF-ZLUOBGJFSA-N 0.000 description 2
- YUZPQIQWXLRFBW-ACZMJKKPSA-N Cys-Glu-Ala Chemical compound [H]N[C@@H](CS)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(O)=O YUZPQIQWXLRFBW-ACZMJKKPSA-N 0.000 description 2
- SRUKWJMBAALPQV-IHPCNDPISA-N Cys-Phe-Trp Chemical compound [H]N[C@@H](CS)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(O)=O SRUKWJMBAALPQV-IHPCNDPISA-N 0.000 description 2
- DQGIAOGALAQBGK-BWBBJGPYSA-N Cys-Ser-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CO)NC(=O)[C@H](CS)N)O DQGIAOGALAQBGK-BWBBJGPYSA-N 0.000 description 2
- 108010005843 Cysteine Proteases Proteins 0.000 description 2
- 102000005927 Cysteine Proteases Human genes 0.000 description 2
- 108010090461 DFG peptide Proteins 0.000 description 2
- KCJJFESQRXGTGC-BQBZGAKWSA-N Gln-Glu-Gly Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)NCC(O)=O KCJJFESQRXGTGC-BQBZGAKWSA-N 0.000 description 2
- FGYPOQPQTUNESW-IUCAKERBSA-N Gln-Gly-Leu Chemical compound CC(C)C[C@@H](C(=O)O)NC(=O)CNC(=O)[C@H](CCC(=O)N)N FGYPOQPQTUNESW-IUCAKERBSA-N 0.000 description 2
- ORYMMTRPKVTGSJ-XVKPBYJWSA-N Gln-Gly-Val Chemical compound CC(C)[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H](N)CCC(N)=O ORYMMTRPKVTGSJ-XVKPBYJWSA-N 0.000 description 2
- FTIJVMLAGRAYMJ-MNXVOIDGSA-N Gln-Ile-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H]([C@@H](C)CC)NC(=O)[C@@H](N)CCC(N)=O FTIJVMLAGRAYMJ-MNXVOIDGSA-N 0.000 description 2
- HWEINOMSWQSJDC-SRVKXCTJSA-N Gln-Leu-Arg Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O HWEINOMSWQSJDC-SRVKXCTJSA-N 0.000 description 2
- XZUUUKNKNWVPHQ-JYJNAYRXSA-N Gln-Phe-Leu Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC(C)C)C(O)=O XZUUUKNKNWVPHQ-JYJNAYRXSA-N 0.000 description 2
- PAOHIZNRJNIXQY-XQXXSGGOSA-N Gln-Thr-Ala Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(O)=O PAOHIZNRJNIXQY-XQXXSGGOSA-N 0.000 description 2
- JKDBRTNMYXYLHO-JYJNAYRXSA-N Gln-Tyr-Leu Chemical compound NC(=O)CC[C@H](N)C(=O)N[C@H](C(=O)N[C@@H](CC(C)C)C(O)=O)CC1=CC=C(O)C=C1 JKDBRTNMYXYLHO-JYJNAYRXSA-N 0.000 description 2
- HPBKQFJXDUVNQV-FHWLQOOXSA-N Gln-Tyr-Tyr Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)N[C@@H](CC2=CC=C(C=C2)O)C(=O)O)NC(=O)[C@H](CCC(=O)N)N)O HPBKQFJXDUVNQV-FHWLQOOXSA-N 0.000 description 2
- SOEXCCGNHQBFPV-DLOVCJGASA-N Gln-Val-Val Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C(C)C)C(O)=O SOEXCCGNHQBFPV-DLOVCJGASA-N 0.000 description 2
- NLKVNZUFDPWPNL-YUMQZZPRSA-N Glu-Arg-Gly Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(O)=O NLKVNZUFDPWPNL-YUMQZZPRSA-N 0.000 description 2
- LTUVYLVIZHJCOQ-KKUMJFAQSA-N Glu-Arg-Phe Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O LTUVYLVIZHJCOQ-KKUMJFAQSA-N 0.000 description 2
- JRCUFCXYZLPSDZ-ACZMJKKPSA-N Glu-Asp-Ser Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(O)=O JRCUFCXYZLPSDZ-ACZMJKKPSA-N 0.000 description 2
- UMIRPYLZFKOEOH-YVNDNENWSA-N Glu-Gln-Ile Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O UMIRPYLZFKOEOH-YVNDNENWSA-N 0.000 description 2
- PVBBEKPHARMPHX-DCAQKATOSA-N Glu-Gln-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](N)CCC(O)=O PVBBEKPHARMPHX-DCAQKATOSA-N 0.000 description 2
- NKLRYVLERDYDBI-FXQIFTODSA-N Glu-Glu-Asp Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(O)=O NKLRYVLERDYDBI-FXQIFTODSA-N 0.000 description 2
- OAGVHWYIBZMWLA-YFKPBYRVSA-N Glu-Gly-Gly Chemical compound OC(=O)CC[C@H](N)C(=O)NCC(=O)NCC(O)=O OAGVHWYIBZMWLA-YFKPBYRVSA-N 0.000 description 2
- KRGZZKWSBGPLKL-IUCAKERBSA-N Glu-Gly-Lys Chemical compound C(CCN)C[C@@H](C(=O)O)NC(=O)CNC(=O)[C@H](CCC(=O)O)N KRGZZKWSBGPLKL-IUCAKERBSA-N 0.000 description 2
- KRRFFAHEAOCBCQ-SIUGBPQLSA-N Glu-Ile-Tyr Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O KRRFFAHEAOCBCQ-SIUGBPQLSA-N 0.000 description 2
- VSRCAOIHMGCIJK-SRVKXCTJSA-N Glu-Leu-Arg Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O VSRCAOIHMGCIJK-SRVKXCTJSA-N 0.000 description 2
- ATVYZJGOZLVXDK-IUCAKERBSA-N Glu-Leu-Gly Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)NCC(O)=O ATVYZJGOZLVXDK-IUCAKERBSA-N 0.000 description 2
- IVGJYOOGJLFKQE-AVGNSLFASA-N Glu-Leu-Lys Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CCC(=O)O)N IVGJYOOGJLFKQE-AVGNSLFASA-N 0.000 description 2
- QJVZSVUYZFYLFQ-CIUDSAMLSA-N Glu-Pro-Ala Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(O)=O QJVZSVUYZFYLFQ-CIUDSAMLSA-N 0.000 description 2
- QOXDAWODGSIDDI-GUBZILKMSA-N Glu-Ser-Lys Chemical compound C(CCN)C[C@@H](C(=O)O)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(=O)O)N QOXDAWODGSIDDI-GUBZILKMSA-N 0.000 description 2
- HZISRJBYZAODRV-XQXXSGGOSA-N Glu-Thr-Ala Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(O)=O HZISRJBYZAODRV-XQXXSGGOSA-N 0.000 description 2
- DLISPGXMKZTWQG-IFFSRLJSSA-N Glu-Thr-Val Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(O)=O DLISPGXMKZTWQG-IFFSRLJSSA-N 0.000 description 2
- QEJKKJNDDDPSMU-KKUMJFAQSA-N Glu-Tyr-Met Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CCSC)C(O)=O QEJKKJNDDDPSMU-KKUMJFAQSA-N 0.000 description 2
- VIPDPMHGICREIS-GVXVVHGQSA-N Glu-Val-Leu Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O VIPDPMHGICREIS-GVXVVHGQSA-N 0.000 description 2
- 108091005503 Glutamic proteases Proteins 0.000 description 2
- 108010051815 Glutamyl endopeptidase Proteins 0.000 description 2
- MQVNVZUEPUIAFA-WDSKDSINSA-N Gly-Cys-Gln Chemical compound C(CC(=O)N)[C@@H](C(=O)O)NC(=O)[C@H](CS)NC(=O)CN MQVNVZUEPUIAFA-WDSKDSINSA-N 0.000 description 2
- IXKRSKPKSLXIHN-YUMQZZPRSA-N Gly-Cys-Leu Chemical compound [H]NCC(=O)N[C@@H](CS)C(=O)N[C@@H](CC(C)C)C(O)=O IXKRSKPKSLXIHN-YUMQZZPRSA-N 0.000 description 2
- HFPVRZWORNJRRC-UWVGGRQHSA-N Gly-Pro-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)CN HFPVRZWORNJRRC-UWVGGRQHSA-N 0.000 description 2
- WNGHUXFWEWTKAO-YUMQZZPRSA-N Gly-Ser-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)CN WNGHUXFWEWTKAO-YUMQZZPRSA-N 0.000 description 2
- HQSKKSLNLSTONK-JTQLQIEISA-N Gly-Tyr-Gly Chemical compound OC(=O)CNC(=O)[C@@H](NC(=O)CN)CC1=CC=C(O)C=C1 HQSKKSLNLSTONK-JTQLQIEISA-N 0.000 description 2
- JBCLFWXMTIKCCB-UHFFFAOYSA-N H-Gly-Phe-OH Natural products NCC(=O)NC(C(O)=O)CC1=CC=CC=C1 JBCLFWXMTIKCCB-UHFFFAOYSA-N 0.000 description 2
- NLDMNSXOCDLTTB-UHFFFAOYSA-N Heterophylliin A Natural products O1C2COC(=O)C3=CC(O)=C(O)C(O)=C3C3=C(O)C(O)=C(O)C=C3C(=O)OC2C(OC(=O)C=2C=C(O)C(O)=C(O)C=2)C(O)C1OC(=O)C1=CC(O)=C(O)C(O)=C1 NLDMNSXOCDLTTB-UHFFFAOYSA-N 0.000 description 2
- SVHKVHBPTOMLTO-DCAQKATOSA-N His-Arg-Asp Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(O)=O)C(O)=O SVHKVHBPTOMLTO-DCAQKATOSA-N 0.000 description 2
- LCNNHVQNFNJLGK-AVGNSLFASA-N His-Gln-His Chemical compound C1=C(NC=N1)C[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](CC2=CN=CN2)C(=O)O)N LCNNHVQNFNJLGK-AVGNSLFASA-N 0.000 description 2
- FSOXZQBMPBQKGJ-QSFUFRPTSA-N His-Ile-Ala Chemical compound [O-]C(=O)[C@H](C)NC(=O)[C@H]([C@@H](C)CC)NC(=O)[C@@H]([NH3+])CC1=CN=CN1 FSOXZQBMPBQKGJ-QSFUFRPTSA-N 0.000 description 2
- WSEITRHJRVDTRX-QTKMDUPCSA-N His-Met-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CCSC)NC(=O)[C@H](CC1=CN=CN1)N)O WSEITRHJRVDTRX-QTKMDUPCSA-N 0.000 description 2
- BCSGDNGNHKBRRJ-ULQDDVLXSA-N His-Tyr-Val Chemical compound CC(C)[C@@H](C(=O)O)NC(=O)[C@H](CC1=CC=C(C=C1)O)NC(=O)[C@H](CC2=CN=CN2)N BCSGDNGNHKBRRJ-ULQDDVLXSA-N 0.000 description 2
- CWJQMCPYXNVMBS-STECZYCISA-N Ile-Arg-Tyr Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)O)N CWJQMCPYXNVMBS-STECZYCISA-N 0.000 description 2
- LEDRIAHEWDJRMF-CFMVVWHZSA-N Ile-Asn-Tyr Chemical compound CC[C@H](C)[C@H](N)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 LEDRIAHEWDJRMF-CFMVVWHZSA-N 0.000 description 2
- NKRJALPCDNXULF-BYULHYEWSA-N Ile-Asp-Gly Chemical compound [H]N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(O)=O)C(=O)NCC(O)=O NKRJALPCDNXULF-BYULHYEWSA-N 0.000 description 2
- NPROWIBAWYMPAZ-GUDRVLHUSA-N Ile-Asp-Pro Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N1CCC[C@@H]1C(=O)O)N NPROWIBAWYMPAZ-GUDRVLHUSA-N 0.000 description 2
- TVSPLSZTKTUYLV-ZPFDUUQYSA-N Ile-Glu-Met Chemical compound N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCC(=O)O)C(=O)N[C@@H](CCSC)C(=O)O TVSPLSZTKTUYLV-ZPFDUUQYSA-N 0.000 description 2
- HYLIOBDWPQNLKI-HVTMNAMFSA-N Ile-His-Gln Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)N[C@@H](CCC(=O)N)C(=O)O)N HYLIOBDWPQNLKI-HVTMNAMFSA-N 0.000 description 2
- AXNGDPAKKCEKGY-QPHKQPEJSA-N Ile-Ile-Thr Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)O)N AXNGDPAKKCEKGY-QPHKQPEJSA-N 0.000 description 2
- HUORUFRRJHELPD-MNXVOIDGSA-N Ile-Leu-Glu Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(=O)O)C(=O)O)N HUORUFRRJHELPD-MNXVOIDGSA-N 0.000 description 2
- WYUHAXJAMDTOAU-IAVJCBSLSA-N Ile-Phe-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)O)N WYUHAXJAMDTOAU-IAVJCBSLSA-N 0.000 description 2
- XLXPYSDGMXTTNQ-DKIMLUQUSA-N Ile-Phe-Leu Chemical compound CC[C@H](C)[C@H](N)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC(C)C)C(O)=O XLXPYSDGMXTTNQ-DKIMLUQUSA-N 0.000 description 2
- XLXPYSDGMXTTNQ-UHFFFAOYSA-N Ile-Phe-Leu Natural products CCC(C)C(N)C(=O)NC(C(=O)NC(CC(C)C)C(O)=O)CC1=CC=CC=C1 XLXPYSDGMXTTNQ-UHFFFAOYSA-N 0.000 description 2
- RKQAYOWLSFLJEE-SVSWQMSJSA-N Ile-Thr-Cys Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CS)C(=O)O)N RKQAYOWLSFLJEE-SVSWQMSJSA-N 0.000 description 2
- COWHUQXTSYTKQC-RWRJDSDZSA-N Ile-Thr-Glu Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCC(=O)O)C(=O)O)N COWHUQXTSYTKQC-RWRJDSDZSA-N 0.000 description 2
- JTBFQNHKNRZJDS-SYWGBEHUSA-N Ile-Trp-Ala Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CNC2=CC=CC=C21)C(=O)N[C@@H](C)C(=O)O)N JTBFQNHKNRZJDS-SYWGBEHUSA-N 0.000 description 2
- IPFKIGNDTUOFAF-CYDGBPFRSA-N Ile-Val-Arg Chemical compound CC[C@H](C)[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](C(O)=O)CCCN=C(N)N IPFKIGNDTUOFAF-CYDGBPFRSA-N 0.000 description 2
- FADYJNXDPBKVCA-UHFFFAOYSA-N L-Phenylalanyl-L-lysin Natural products NCCCCC(C(O)=O)NC(=O)C(N)CC1=CC=CC=C1 FADYJNXDPBKVCA-UHFFFAOYSA-N 0.000 description 2
- TYYLDKGBCJGJGW-UHFFFAOYSA-N L-tryptophan-L-tyrosine Natural products C=1NC2=CC=CC=C2C=1CC(N)C(=O)NC(C(O)=O)CC1=CC=C(O)C=C1 TYYLDKGBCJGJGW-UHFFFAOYSA-N 0.000 description 2
- 241000880493 Leptailurus serval Species 0.000 description 2
- CQQGCWPXDHTTNF-GUBZILKMSA-N Leu-Ala-Glu Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@H](C(O)=O)CCC(O)=O CQQGCWPXDHTTNF-GUBZILKMSA-N 0.000 description 2
- MDVZJYGNAGLPGJ-KKUMJFAQSA-N Leu-Asn-Phe Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 MDVZJYGNAGLPGJ-KKUMJFAQSA-N 0.000 description 2
- WIDZHJTYKYBLSR-DCAQKATOSA-N Leu-Glu-Glu Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O WIDZHJTYKYBLSR-DCAQKATOSA-N 0.000 description 2
- WQWSMEOYXJTFRU-GUBZILKMSA-N Leu-Glu-Ser Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(O)=O WQWSMEOYXJTFRU-GUBZILKMSA-N 0.000 description 2
- APFJUBGRZGMQFF-QWRGUYRKSA-N Leu-Gly-Lys Chemical compound CC(C)C[C@H](N)C(=O)NCC(=O)N[C@H](C(O)=O)CCCCN APFJUBGRZGMQFF-QWRGUYRKSA-N 0.000 description 2
- HRTRLSRYZZKPCO-BJDJZHNGSA-N Leu-Ile-Ser Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CO)C(O)=O HRTRLSRYZZKPCO-BJDJZHNGSA-N 0.000 description 2
- IAJFFZORSWOZPQ-SRVKXCTJSA-N Leu-Leu-Asn Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(O)=O IAJFFZORSWOZPQ-SRVKXCTJSA-N 0.000 description 2
- RXGLHDWAZQECBI-SRVKXCTJSA-N Leu-Leu-Ser Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(O)=O RXGLHDWAZQECBI-SRVKXCTJSA-N 0.000 description 2
- ZGUMORRUBUCXEH-AVGNSLFASA-N Leu-Lys-Gln Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(N)=O)C(O)=O ZGUMORRUBUCXEH-AVGNSLFASA-N 0.000 description 2
- GSSMYQHXZNERFX-WDSOQIARSA-N Leu-Met-Trp Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC1=CNC2=CC=CC=C21)C(=O)O)N GSSMYQHXZNERFX-WDSOQIARSA-N 0.000 description 2
- DRWMRVFCKKXHCH-BZSNNMDCSA-N Leu-Phe-Leu Chemical compound CC(C)C[C@H]([NH3+])C(=O)N[C@H](C(=O)N[C@@H](CC(C)C)C([O-])=O)CC1=CC=CC=C1 DRWMRVFCKKXHCH-BZSNNMDCSA-N 0.000 description 2
- DPURXCQCHSQPAN-AVGNSLFASA-N Leu-Pro-Pro Chemical compound CC(C)C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N1[C@H](C(O)=O)CCC1 DPURXCQCHSQPAN-AVGNSLFASA-N 0.000 description 2
- UCRJTSIIAYHOHE-ULQDDVLXSA-N Leu-Tyr-Arg Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)O)N UCRJTSIIAYHOHE-ULQDDVLXSA-N 0.000 description 2
- MVJRBCJCRYGCKV-GVXVVHGQSA-N Leu-Val-Gln Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O MVJRBCJCRYGCKV-GVXVVHGQSA-N 0.000 description 2
- MPGHETGWWWUHPY-CIUDSAMLSA-N Lys-Ala-Asp Chemical compound OC(=O)C[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CCCCN MPGHETGWWWUHPY-CIUDSAMLSA-N 0.000 description 2
- XFIHDSBIPWEYJJ-YUMQZZPRSA-N Lys-Ala-Gly Chemical compound OC(=O)CNC(=O)[C@H](C)NC(=O)[C@@H](N)CCCCN XFIHDSBIPWEYJJ-YUMQZZPRSA-N 0.000 description 2
- NQCJGQHHYZNUDK-DCAQKATOSA-N Lys-Arg-Ser Chemical compound NCCCC[C@H](N)C(=O)N[C@H](C(=O)N[C@@H](CO)C(O)=O)CCCN=C(N)N NQCJGQHHYZNUDK-DCAQKATOSA-N 0.000 description 2
- NLOZZWJNIKKYSC-WDSOQIARSA-N Lys-Arg-Trp Chemical compound C1=CC=C2C(C[C@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](N)CCCCN)C(O)=O)=CNC2=C1 NLOZZWJNIKKYSC-WDSOQIARSA-N 0.000 description 2
- HQVDJTYKCMIWJP-YUMQZZPRSA-N Lys-Asn-Gly Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(=O)NCC(O)=O HQVDJTYKCMIWJP-YUMQZZPRSA-N 0.000 description 2
- NCTDKZKNBDZDOL-GARJFASQSA-N Lys-Asn-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CC(=O)N)NC(=O)[C@H](CCCCN)N)C(=O)O NCTDKZKNBDZDOL-GARJFASQSA-N 0.000 description 2
- LZWNAOIMTLNMDW-NHCYSSNCSA-N Lys-Asn-Val Chemical compound CC(C)[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)N)NC(=O)[C@H](CCCCN)N LZWNAOIMTLNMDW-NHCYSSNCSA-N 0.000 description 2
- GKFNXYMAMKJSKD-NHCYSSNCSA-N Lys-Asp-Val Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C(C)C)C(O)=O GKFNXYMAMKJSKD-NHCYSSNCSA-N 0.000 description 2
- GCMWRRQAKQXDED-IUCAKERBSA-N Lys-Glu-Gly Chemical compound [NH3+]CCCC[C@H]([NH3+])C(=O)N[C@@H](CCC([O-])=O)C(=O)NCC([O-])=O GCMWRRQAKQXDED-IUCAKERBSA-N 0.000 description 2
- GPJGFSFYBJGYRX-YUMQZZPRSA-N Lys-Gly-Asp Chemical compound NCCCC[C@H](N)C(=O)NCC(=O)N[C@H](C(O)=O)CC(O)=O GPJGFSFYBJGYRX-YUMQZZPRSA-N 0.000 description 2
- OWRUUFUVXFREBD-KKUMJFAQSA-N Lys-His-Leu Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CC(C)C)C(O)=O OWRUUFUVXFREBD-KKUMJFAQSA-N 0.000 description 2
- WRODMZBHNNPRLN-SRVKXCTJSA-N Lys-Leu-Ser Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(O)=O WRODMZBHNNPRLN-SRVKXCTJSA-N 0.000 description 2
- ZJWIXBZTAAJERF-IHRRRGAJSA-N Lys-Lys-Arg Chemical compound NCCCC[C@H](N)C(=O)N[C@@H](CCCCN)C(=O)N[C@H](C(O)=O)CCCN=C(N)N ZJWIXBZTAAJERF-IHRRRGAJSA-N 0.000 description 2
- YUAXTFMFMOIMAM-QWRGUYRKSA-N Lys-Lys-Gly Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)NCC(O)=O YUAXTFMFMOIMAM-QWRGUYRKSA-N 0.000 description 2
- WBSCNDJQPKSPII-KKUMJFAQSA-N Lys-Lys-Lys Chemical compound NCCCC[C@H](N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(O)=O WBSCNDJQPKSPII-KKUMJFAQSA-N 0.000 description 2
- YXPJCVNIDDKGOE-MELADBBJSA-N Lys-Lys-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CCCCN)NC(=O)[C@H](CCCCN)N)C(=O)O YXPJCVNIDDKGOE-MELADBBJSA-N 0.000 description 2
- AEIIJFBQVGYVEV-YESZJQIVSA-N Lys-Phe-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CC2=CC=CC=C2)NC(=O)[C@H](CCCCN)N)C(=O)O AEIIJFBQVGYVEV-YESZJQIVSA-N 0.000 description 2
- LUAJJLPHUXPQLH-KKUMJFAQSA-N Lys-Phe-Ser Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CO)C(=O)O)NC(=O)[C@H](CCCCN)N LUAJJLPHUXPQLH-KKUMJFAQSA-N 0.000 description 2
- HYSVGEAWTGPMOA-IHRRRGAJSA-N Lys-Pro-Leu Chemical compound [H]N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(O)=O HYSVGEAWTGPMOA-IHRRRGAJSA-N 0.000 description 2
- MGKFCQFVPKOWOL-CIUDSAMLSA-N Lys-Ser-Asp Chemical compound C(CCN)C[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(=O)O)C(=O)O)N MGKFCQFVPKOWOL-CIUDSAMLSA-N 0.000 description 2
- YKBSXQFZWFXFIB-VOAKCMCISA-N Lys-Thr-Lys Chemical compound NCCCC[C@H](N)C(=O)N[C@@H]([C@H](O)C)C(=O)N[C@@H](CCCCN)C(O)=O YKBSXQFZWFXFIB-VOAKCMCISA-N 0.000 description 2
- RYOLKFYZBHMYFW-WDSOQIARSA-N Lys-Trp-Arg Chemical compound C1=CC=C2C(C[C@H](NC(=O)[C@@H](N)CCCCN)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O)=CNC2=C1 RYOLKFYZBHMYFW-WDSOQIARSA-N 0.000 description 2
- RQILLQOQXLZTCK-KBPBESRZSA-N Lys-Tyr-Gly Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)NCC(O)=O RQILLQOQXLZTCK-KBPBESRZSA-N 0.000 description 2
- PSVAVKGDUAKZKU-BZSNNMDCSA-N Lys-Tyr-His Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)N[C@@H](CC2=CN=CN2)C(=O)O)NC(=O)[C@H](CCCCN)N)O PSVAVKGDUAKZKU-BZSNNMDCSA-N 0.000 description 2
- PPNCMJARTHYNEC-MEYUZBJRSA-N Lys-Tyr-Thr Chemical compound NCCCC[C@H](N)C(=O)N[C@H](C(=O)N[C@@H]([C@H](O)C)C(O)=O)CC1=CC=C(O)C=C1 PPNCMJARTHYNEC-MEYUZBJRSA-N 0.000 description 2
- HMZPYMSEAALNAE-ULQDDVLXSA-N Lys-Val-Tyr Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O HMZPYMSEAALNAE-ULQDDVLXSA-N 0.000 description 2
- IKXQOBUBZSOWDY-AVGNSLFASA-N Lys-Val-Val Chemical compound CC(C)[C@@H](C(=O)N[C@@H](C(C)C)C(=O)O)NC(=O)[C@H](CCCCN)N IKXQOBUBZSOWDY-AVGNSLFASA-N 0.000 description 2
- 101001018085 Lysobacter enzymogenes Lysyl endopeptidase Proteins 0.000 description 2
- VIZLHGTVGKBBKO-AVGNSLFASA-N Met-Arg-His Chemical compound CSCC[C@@H](C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)N VIZLHGTVGKBBKO-AVGNSLFASA-N 0.000 description 2
- DCHHUGLTVLJYKA-FXQIFTODSA-N Met-Asn-Ala Chemical compound CSCC[C@H](N)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](C)C(O)=O DCHHUGLTVLJYKA-FXQIFTODSA-N 0.000 description 2
- STTRPDDKDVKIDF-KKUMJFAQSA-N Met-Glu-Tyr Chemical compound CSCC[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 STTRPDDKDVKIDF-KKUMJFAQSA-N 0.000 description 2
- SBFPAAPFKZPDCZ-JYJNAYRXSA-N Met-Pro-Tyr Chemical compound [H]N[C@@H](CCSC)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O SBFPAAPFKZPDCZ-JYJNAYRXSA-N 0.000 description 2
- PNHRPOWKRRJATF-IHRRRGAJSA-N Met-Tyr-Ser Chemical compound CSCC[C@H](N)C(=O)N[C@H](C(=O)N[C@@H](CO)C(O)=O)CC1=CC=C(O)C=C1 PNHRPOWKRRJATF-IHRRRGAJSA-N 0.000 description 2
- 108010006035 Metalloproteases Proteins 0.000 description 2
- 102000005741 Metalloproteases Human genes 0.000 description 2
- WYBVBIHNJWOLCJ-UHFFFAOYSA-N N-L-arginyl-L-leucine Natural products CC(C)CC(C(O)=O)NC(=O)C(N)CCCN=C(N)N WYBVBIHNJWOLCJ-UHFFFAOYSA-N 0.000 description 2
- AUEJLPRZGVVDNU-UHFFFAOYSA-N N-L-tyrosyl-L-leucine Natural products CC(C)CC(C(O)=O)NC(=O)C(N)CC1=CC=C(O)C=C1 AUEJLPRZGVVDNU-UHFFFAOYSA-N 0.000 description 2
- 108010002311 N-glycylglutamic acid Proteins 0.000 description 2
- 101100068676 Neurospora crassa (strain ATCC 24698 / 74-OR23-1A / CBS 708.71 / DSM 1257 / FGSC 987) gln-1 gene Proteins 0.000 description 2
- 101100342977 Neurospora crassa (strain ATCC 24698 / 74-OR23-1A / CBS 708.71 / DSM 1257 / FGSC 987) leu-1 gene Proteins 0.000 description 2
- NKLDZIPTGKBDBB-HTUGSXCWSA-N Phe-Gln-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)N)NC(=O)[C@H](CC1=CC=CC=C1)N)O NKLDZIPTGKBDBB-HTUGSXCWSA-N 0.000 description 2
- LRBSWBVUCLLRLU-BZSNNMDCSA-N Phe-Leu-Lys Chemical compound CC(C)C[C@H](NC(=O)[C@@H](N)Cc1ccccc1)C(=O)N[C@@H](CCCCN)C(O)=O LRBSWBVUCLLRLU-BZSNNMDCSA-N 0.000 description 2
- ZUQACJLOHYRVPJ-DKIMLUQUSA-N Phe-Lys-Ile Chemical compound CC[C@H](C)[C@@H](C(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](N)CC1=CC=CC=C1 ZUQACJLOHYRVPJ-DKIMLUQUSA-N 0.000 description 2
- ZPPVJIJMIKTERM-YUMQZZPRSA-N Pro-Gln-Gly Chemical compound OC(=O)CNC(=O)[C@H](CCC(=O)N)NC(=O)[C@@H]1CCCN1 ZPPVJIJMIKTERM-YUMQZZPRSA-N 0.000 description 2
- DIFXZGPHVCIVSQ-CIUDSAMLSA-N Pro-Gln-Ser Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CO)C(O)=O DIFXZGPHVCIVSQ-CIUDSAMLSA-N 0.000 description 2
- NXEYSLRNNPWCRN-SRVKXCTJSA-N Pro-Glu-Leu Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O NXEYSLRNNPWCRN-SRVKXCTJSA-N 0.000 description 2
- DXTOOBDIIAJZBJ-BQBZGAKWSA-N Pro-Gly-Ser Chemical compound [H]N1CCC[C@H]1C(=O)NCC(=O)N[C@@H](CO)C(O)=O DXTOOBDIIAJZBJ-BQBZGAKWSA-N 0.000 description 2
- STASJMBVVHNWCG-IHRRRGAJSA-N Pro-His-Leu Chemical compound C([C@@H](C(=O)N[C@@H](CC(C)C)C([O-])=O)NC(=O)[C@H]1[NH2+]CCC1)C1=CN=CN1 STASJMBVVHNWCG-IHRRRGAJSA-N 0.000 description 2
- RFWXYTJSVDUBBZ-DCAQKATOSA-N Pro-Pro-Glu Chemical compound OC(=O)CC[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H]1NCCC1 RFWXYTJSVDUBBZ-DCAQKATOSA-N 0.000 description 2
- RCYUBVHMVUHEBM-RCWTZXSCSA-N Pro-Pro-Thr Chemical compound [H]N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H]([C@@H](C)O)C(O)=O RCYUBVHMVUHEBM-RCWTZXSCSA-N 0.000 description 2
- KWMZPPWYBVZIER-XGEHTFHBSA-N Pro-Ser-Thr Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(O)=O KWMZPPWYBVZIER-XGEHTFHBSA-N 0.000 description 2
- IURWWZYKYPEANQ-HJGDQZAQSA-N Pro-Thr-Glu Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCC(O)=O)C(O)=O IURWWZYKYPEANQ-HJGDQZAQSA-N 0.000 description 2
- MCPXQHVVCPTRIM-HJOGWXRNSA-N Pro-Trp-Trp Chemical compound N([C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)O)C(=O)[C@@H]1CCCN1 MCPXQHVVCPTRIM-HJOGWXRNSA-N 0.000 description 2
- FIDMVVBUOCMMJG-CIUDSAMLSA-N Ser-Asn-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](N)CO FIDMVVBUOCMMJG-CIUDSAMLSA-N 0.000 description 2
- ICHZYBVODUVUKN-SRVKXCTJSA-N Ser-Asn-Tyr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O ICHZYBVODUVUKN-SRVKXCTJSA-N 0.000 description 2
- CRZRTKAVUUGKEQ-ACZMJKKPSA-N Ser-Gln-Ala Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](C)C(O)=O CRZRTKAVUUGKEQ-ACZMJKKPSA-N 0.000 description 2
- IXUGADGDCQDLSA-FXQIFTODSA-N Ser-Gln-Gln Chemical compound C(CC(=O)N)[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)O)NC(=O)[C@H](CO)N IXUGADGDCQDLSA-FXQIFTODSA-N 0.000 description 2
- GRSLLFZTTLBOQX-CIUDSAMLSA-N Ser-Glu-Met Chemical compound CSCC[C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)O)NC(=O)[C@H](CO)N GRSLLFZTTLBOQX-CIUDSAMLSA-N 0.000 description 2
- QBUWQRKEHJXTOP-DCAQKATOSA-N Ser-His-Arg Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O QBUWQRKEHJXTOP-DCAQKATOSA-N 0.000 description 2
- BKZYBLLIBOBOOW-GHCJXIJMSA-N Ser-Ile-Asp Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(O)=O)C(O)=O BKZYBLLIBOBOOW-GHCJXIJMSA-N 0.000 description 2
- HBTCFCHYALPXME-HTFCKZLJSA-N Ser-Ile-Ile Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O HBTCFCHYALPXME-HTFCKZLJSA-N 0.000 description 2
- VIIJCAQMJBHSJH-FXQIFTODSA-N Ser-Met-Ser Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CO)C(O)=O VIIJCAQMJBHSJH-FXQIFTODSA-N 0.000 description 2
- DINQYZRMXGWWTG-GUBZILKMSA-N Ser-Pro-Pro Chemical compound OC[C@H](N)C(=O)N1CCC[C@H]1C(=O)N1[C@H](C(O)=O)CCC1 DINQYZRMXGWWTG-GUBZILKMSA-N 0.000 description 2
- FLMYSKVSDVHLEW-SVSWQMSJSA-N Ser-Thr-Ile Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O FLMYSKVSDVHLEW-SVSWQMSJSA-N 0.000 description 2
- SNXUIBACCONSOH-BWBBJGPYSA-N Ser-Thr-Ser Chemical compound OC[C@H](N)C(=O)N[C@@H]([C@H](O)C)C(=O)N[C@@H](CO)C(O)=O SNXUIBACCONSOH-BWBBJGPYSA-N 0.000 description 2
- SDFUZKIAHWRUCS-QEJZJMRPSA-N Ser-Trp-Glu Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)O)NC(=O)[C@H](CO)N SDFUZKIAHWRUCS-QEJZJMRPSA-N 0.000 description 2
- 102000012479 Serine Proteases Human genes 0.000 description 2
- 108010022999 Serine Proteases Proteins 0.000 description 2
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 2
- OJRNZRROAIAHDL-LKXGYXEUSA-N Thr-Asn-Ser Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(O)=O OJRNZRROAIAHDL-LKXGYXEUSA-N 0.000 description 2
- ZQUKYJOKQBRBCS-GLLZPBPUSA-N Thr-Gln-Gln Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](CCC(=O)N)C(=O)O)N)O ZQUKYJOKQBRBCS-GLLZPBPUSA-N 0.000 description 2
- NIEWSKWFURSECR-FOHZUACHSA-N Thr-Gly-Asp Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)NCC(=O)N[C@@H](CC(O)=O)C(O)=O NIEWSKWFURSECR-FOHZUACHSA-N 0.000 description 2
- VYEHBMMAJFVTOI-JHEQGTHGSA-N Thr-Gly-Gln Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)NCC(=O)N[C@@H](CCC(N)=O)C(O)=O VYEHBMMAJFVTOI-JHEQGTHGSA-N 0.000 description 2
- LCCSEJSPBWKBNT-OSUNSFLBSA-N Thr-Ile-Met Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCSC)C(=O)O)NC(=O)[C@H]([C@@H](C)O)N LCCSEJSPBWKBNT-OSUNSFLBSA-N 0.000 description 2
- VUXIQSUQQYNLJP-XAVMHZPKSA-N Thr-Ser-Pro Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CO)C(=O)N1CCC[C@@H]1C(=O)O)N)O VUXIQSUQQYNLJP-XAVMHZPKSA-N 0.000 description 2
- WPSKTVVMQCXPRO-BWBBJGPYSA-N Thr-Ser-Ser Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(O)=O WPSKTVVMQCXPRO-BWBBJGPYSA-N 0.000 description 2
- CURFABYITJVKEW-QTKMDUPCSA-N Thr-Val-His Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)N)O CURFABYITJVKEW-QTKMDUPCSA-N 0.000 description 2
- 108091005501 Threonine proteases Proteins 0.000 description 2
- 102000035100 Threonine proteases Human genes 0.000 description 2
- PGPCENKYTLDIFM-SZMVWBNQSA-N Trp-His-Glu Chemical compound [H]N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CCC(O)=O)C(O)=O PGPCENKYTLDIFM-SZMVWBNQSA-N 0.000 description 2
- YGKVNUAKYPGORG-AVGNSLFASA-N Tyr-Asp-Glu Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O YGKVNUAKYPGORG-AVGNSLFASA-N 0.000 description 2
- WPVGRKLNHJJCEN-BZSNNMDCSA-N Tyr-Asp-Phe Chemical compound C([C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC=1C=CC=CC=1)C(O)=O)C1=CC=C(O)C=C1 WPVGRKLNHJJCEN-BZSNNMDCSA-N 0.000 description 2
- OSMTVLSRTQDWHJ-JBACZVJFSA-N Tyr-Glu-Trp Chemical compound C([C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(O)=O)C1=CC=C(O)C=C1 OSMTVLSRTQDWHJ-JBACZVJFSA-N 0.000 description 2
- OHOVFPKXPZODHS-SJWGOKEGSA-N Tyr-Ile-Pro Chemical compound CC[C@H](C)[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CC2=CC=C(C=C2)O)N OHOVFPKXPZODHS-SJWGOKEGSA-N 0.000 description 2
- GYBVHTWOQJMYAM-HRCADAONSA-N Tyr-Met-Pro Chemical compound CSCC[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CC2=CC=C(C=C2)O)N GYBVHTWOQJMYAM-HRCADAONSA-N 0.000 description 2
- FASACHWGQBNSRO-ZEWNOJEFSA-N Tyr-Phe-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)[C@H](CC1=CC=CC=C1)NC(=O)[C@H](CC2=CC=C(C=C2)O)N FASACHWGQBNSRO-ZEWNOJEFSA-N 0.000 description 2
- RCMWNNJFKNDKQR-UFYCRDLUSA-N Tyr-Pro-Phe Chemical compound C([C@H](N)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CC=1C=CC=CC=1)C(O)=O)C1=CC=C(O)C=C1 RCMWNNJFKNDKQR-UFYCRDLUSA-N 0.000 description 2
- RGYCVIZZTUBSSG-JYJNAYRXSA-N Tyr-Pro-Val Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(O)=O RGYCVIZZTUBSSG-JYJNAYRXSA-N 0.000 description 2
- KWKJGBHDYJOVCR-SRVKXCTJSA-N Tyr-Ser-Cys Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CS)C(=O)O)N)O KWKJGBHDYJOVCR-SRVKXCTJSA-N 0.000 description 2
- NHOVZGFNTGMYMI-KKUMJFAQSA-N Tyr-Ser-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 NHOVZGFNTGMYMI-KKUMJFAQSA-N 0.000 description 2
- JQOMHZMWQHXALX-FHWLQOOXSA-N Tyr-Tyr-Glu Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CCC(O)=O)C(O)=O JQOMHZMWQHXALX-FHWLQOOXSA-N 0.000 description 2
- CWOSXNKDOACNJN-BZSNNMDCSA-N Val-Arg-Trp Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CC1=CNC2=CC=CC=C21)C(=O)O)N CWOSXNKDOACNJN-BZSNNMDCSA-N 0.000 description 2
- AUMNPAUHKUNHHN-BYULHYEWSA-N Val-Asn-Asp Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CC(=O)O)C(=O)O)N AUMNPAUHKUNHHN-BYULHYEWSA-N 0.000 description 2
- ZXAGTABZUOMUDO-GVXVVHGQSA-N Val-Glu-Lys Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)N[C@@H](CCCCN)C(=O)O)N ZXAGTABZUOMUDO-GVXVVHGQSA-N 0.000 description 2
- LAYSXAOGWHKNED-XPUUQOCRSA-N Val-Gly-Ser Chemical compound CC(C)[C@H](N)C(=O)NCC(=O)N[C@@H](CO)C(O)=O LAYSXAOGWHKNED-XPUUQOCRSA-N 0.000 description 2
- KDKLLPMFFGYQJD-CYDGBPFRSA-N Val-Ile-Arg Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCCN=C(N)N)C(=O)O)NC(=O)[C@H](C(C)C)N KDKLLPMFFGYQJD-CYDGBPFRSA-N 0.000 description 2
- AGXGCFSECFQMKB-NHCYSSNCSA-N Val-Leu-Asp Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)O)NC(=O)[C@H](C(C)C)N AGXGCFSECFQMKB-NHCYSSNCSA-N 0.000 description 2
- VPGCVZRRBYOGCD-AVGNSLFASA-N Val-Lys-Val Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(O)=O VPGCVZRRBYOGCD-AVGNSLFASA-N 0.000 description 2
- KISFXYYRKKNLOP-IHRRRGAJSA-N Val-Phe-Ser Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CO)C(=O)O)N KISFXYYRKKNLOP-IHRRRGAJSA-N 0.000 description 2
- RYQUMYBMOJYYDK-NHCYSSNCSA-N Val-Pro-Glu Chemical compound CC(C)[C@@H](C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(=O)O)C(=O)O)N RYQUMYBMOJYYDK-NHCYSSNCSA-N 0.000 description 2
- NZYNRRGJJVSSTJ-GUBZILKMSA-N Val-Ser-Val Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(O)=O NZYNRRGJJVSSTJ-GUBZILKMSA-N 0.000 description 2
- UVHFONIHVHLDDQ-IFFSRLJSSA-N Val-Thr-Glu Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)O)NC(=O)[C@H](C(C)C)N)O UVHFONIHVHLDDQ-IFFSRLJSSA-N 0.000 description 2
- 108010044940 alanylglutamine Proteins 0.000 description 2
- 108010047495 alanylglycine Proteins 0.000 description 2
- 238000010171 animal model Methods 0.000 description 2
- 238000013459 approach Methods 0.000 description 2
- 108010069926 arginyl-glycyl-serine Proteins 0.000 description 2
- 108010040443 aspartyl-aspartic acid Proteins 0.000 description 2
- 108010093581 aspartyl-proline Proteins 0.000 description 2
- 230000008901 benefit Effects 0.000 description 2
- 239000000090 biomarker Substances 0.000 description 2
- 229960000074 biopharmaceutical Drugs 0.000 description 2
- 238000011088 calibration curve Methods 0.000 description 2
- 230000015556 catabolic process Effects 0.000 description 2
- 239000008004 cell lysis buffer Substances 0.000 description 2
- 229960002376 chymotrypsin Drugs 0.000 description 2
- 238000004163 cytometry Methods 0.000 description 2
- 230000003247 decreasing effect Effects 0.000 description 2
- 238000006731 degradation reaction Methods 0.000 description 2
- 238000007865 diluting Methods 0.000 description 2
- 238000009509 drug development Methods 0.000 description 2
- 230000000694 effects Effects 0.000 description 2
- 238000010828 elution Methods 0.000 description 2
- 238000000684 flow cytometry Methods 0.000 description 2
- 108010006664 gamma-glutamyl-glycyl-glycine Proteins 0.000 description 2
- 108010049041 glutamylalanine Proteins 0.000 description 2
- XBGGUPMXALFZOT-UHFFFAOYSA-N glycyl-L-tyrosine hemihydrate Natural products NCC(=O)NC(C(O)=O)CC1=CC=C(O)C=C1 XBGGUPMXALFZOT-UHFFFAOYSA-N 0.000 description 2
- 108010010147 glycylglutamine Proteins 0.000 description 2
- 108010050848 glycylleucine Proteins 0.000 description 2
- 108010015792 glycyllysine Proteins 0.000 description 2
- 108010087823 glycyltyrosine Proteins 0.000 description 2
- 238000004896 high resolution mass spectrometry Methods 0.000 description 2
- 108010085325 histidylproline Proteins 0.000 description 2
- 230000002779 inactivation Effects 0.000 description 2
- 238000004989 laser desorption mass spectroscopy Methods 0.000 description 2
- 108010083708 leucyl-aspartyl-valine Proteins 0.000 description 2
- 108010047926 leucyl-lysyl-tyrosine Proteins 0.000 description 2
- 108010073472 leucyl-prolyl-proline Proteins 0.000 description 2
- 108010091871 leucylmethionine Proteins 0.000 description 2
- 108010057821 leucylproline Proteins 0.000 description 2
- 239000007788 liquid Substances 0.000 description 2
- 108010054155 lysyllysine Proteins 0.000 description 2
- 238000007885 magnetic separation Methods 0.000 description 2
- 238000004519 manufacturing process Methods 0.000 description 2
- 239000000463 material Substances 0.000 description 2
- 230000007246 mechanism Effects 0.000 description 2
- 108010005942 methionylglycine Proteins 0.000 description 2
- 108010031719 prolyl-serine Proteins 0.000 description 2
- 108010079317 prolyl-tyrosine Proteins 0.000 description 2
- 108010070643 prolylglutamic acid Proteins 0.000 description 2
- 238000012292 receptor occupancy assay Methods 0.000 description 2
- 238000012552 review Methods 0.000 description 2
- 239000007921 spray Substances 0.000 description 2
- 238000013097 stability assessment Methods 0.000 description 2
- 238000003860 storage Methods 0.000 description 2
- 108010029599 tyrosyl-glutamyl-tryptophan Proteins 0.000 description 2
- 238000004704 ultra performance liquid chromatography Methods 0.000 description 2
- 108010009962 valyltyrosine Proteins 0.000 description 2
- 238000005406 washing Methods 0.000 description 2
- 239000003643 water by type Substances 0.000 description 2
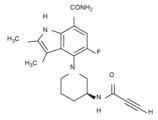
- VJPPLCNBDLZIFG-ZDUSSCGKSA-N 4-[(3S)-3-(but-2-ynoylamino)piperidin-1-yl]-5-fluoro-2,3-dimethyl-1H-indole-7-carboxamide Chemical compound C(C#CC)(=O)N[C@@H]1CN(CCC1)C1=C2C(=C(NC2=C(C=C1F)C(=O)N)C)C VJPPLCNBDLZIFG-ZDUSSCGKSA-N 0.000 description 1
- YRNWIFYIFSBPAU-UHFFFAOYSA-N 4-[4-(dimethylamino)phenyl]-n,n-dimethylaniline Chemical compound C1=CC(N(C)C)=CC=C1C1=CC=C(N(C)C)C=C1 YRNWIFYIFSBPAU-UHFFFAOYSA-N 0.000 description 1
- 229920000936 Agarose Polymers 0.000 description 1
- MDNAVFBZPROEHO-DCAQKATOSA-N Ala-Lys-Val Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(O)=O MDNAVFBZPROEHO-DCAQKATOSA-N 0.000 description 1
- HPKSHFSEXICTLI-CIUDSAMLSA-N Arg-Glu-Ala Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(O)=O HPKSHFSEXICTLI-CIUDSAMLSA-N 0.000 description 1
- DJIMLSXHXKWADV-CIUDSAMLSA-N Asn-Leu-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CC(N)=O DJIMLSXHXKWADV-CIUDSAMLSA-N 0.000 description 1
- OVPHVTCDVYYTHN-AVGNSLFASA-N Asp-Glu-Phe Chemical compound OC(=O)C[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 OVPHVTCDVYYTHN-AVGNSLFASA-N 0.000 description 1
- QJHOOKBAHRJPPX-QWRGUYRKSA-N Asp-Phe-Gly Chemical compound OC(=O)C[C@H](N)C(=O)N[C@H](C(=O)NCC(O)=O)CC1=CC=CC=C1 QJHOOKBAHRJPPX-QWRGUYRKSA-N 0.000 description 1
- 108091008875 B cell receptors Proteins 0.000 description 1
- 101100505161 Caenorhabditis elegans mel-32 gene Proteins 0.000 description 1
- 101100315624 Caenorhabditis elegans tyr-1 gene Proteins 0.000 description 1
- 241000283707 Capra Species 0.000 description 1
- IRKLTAKLAFUTLA-KATARQTJSA-N Cys-Thr-Lys Chemical compound C[C@@H](O)[C@H](NC(=O)[C@@H](N)CS)C(=O)N[C@@H](CCCCN)C(O)=O IRKLTAKLAFUTLA-KATARQTJSA-N 0.000 description 1
- 238000012286 ELISA Assay Methods 0.000 description 1
- -1 Fc receptor- Proteins 0.000 description 1
- 102000009109 Fc receptors Human genes 0.000 description 1
- HSHCEAUPUPJPTE-JYJNAYRXSA-N Gln-Leu-Tyr Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)O)NC(=O)[C@H](CCC(=O)N)N HSHCEAUPUPJPTE-JYJNAYRXSA-N 0.000 description 1
- YRHZWVKUFWCEPW-GLLZPBPUSA-N Gln-Thr-Gln Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)O)NC(=O)[C@H](CCC(=O)N)N)O YRHZWVKUFWCEPW-GLLZPBPUSA-N 0.000 description 1
- AOCARQDSFTWWFT-DCAQKATOSA-N Glu-Met-Arg Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O AOCARQDSFTWWFT-DCAQKATOSA-N 0.000 description 1
- NNCSJUBVFBDDLC-YUMQZZPRSA-N Gly-Leu-Ser Chemical compound NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(O)=O NNCSJUBVFBDDLC-YUMQZZPRSA-N 0.000 description 1
- YABRDIBSPZONIY-BQBZGAKWSA-N Gly-Ser-Met Chemical compound [H]NCC(=O)N[C@@H](CO)C(=O)N[C@@H](CCSC)C(O)=O YABRDIBSPZONIY-BQBZGAKWSA-N 0.000 description 1
- DKJWUIYLMLUBDX-XPUUQOCRSA-N Gly-Val-Cys Chemical compound NCC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CS)C(=O)O DKJWUIYLMLUBDX-XPUUQOCRSA-N 0.000 description 1
- 101710088172 HTH-type transcriptional regulator RipA Proteins 0.000 description 1
- CJGDTAHEMXLRMB-ULQDDVLXSA-N His-Arg-Phe Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O CJGDTAHEMXLRMB-ULQDDVLXSA-N 0.000 description 1
- 241000282412 Homo Species 0.000 description 1
- 108010001336 Horseradish Peroxidase Proteins 0.000 description 1
- PHIXPNQDGGILMP-YVNDNENWSA-N Ile-Glu-Glu Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)N[C@@H](CCC(=O)O)C(=O)O)N PHIXPNQDGGILMP-YVNDNENWSA-N 0.000 description 1
- ADDYYRVQQZFIMW-MNXVOIDGSA-N Ile-Lys-Glu Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(=O)O)C(=O)O)N ADDYYRVQQZFIMW-MNXVOIDGSA-N 0.000 description 1
- 102000004310 Ion Channels Human genes 0.000 description 1
- 239000002177 L01XE27 - Ibrutinib Substances 0.000 description 1
- PRZVBIAOPFGAQF-SRVKXCTJSA-N Leu-Glu-Met Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCSC)C(O)=O PRZVBIAOPFGAQF-SRVKXCTJSA-N 0.000 description 1
- QNBVTHNJGCOVFA-AVGNSLFASA-N Leu-Leu-Glu Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@H](C(O)=O)CCC(O)=O QNBVTHNJGCOVFA-AVGNSLFASA-N 0.000 description 1
- IDGZVZJLYFTXSL-DCAQKATOSA-N Leu-Ser-Arg Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CCCN=C(N)N IDGZVZJLYFTXSL-DCAQKATOSA-N 0.000 description 1
- WTZUSCUIVPVCRH-SRVKXCTJSA-N Lys-Gln-Arg Chemical compound NCCCC[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@H](C(O)=O)CCCN=C(N)N WTZUSCUIVPVCRH-SRVKXCTJSA-N 0.000 description 1
- LJADEBULDNKJNK-IHRRRGAJSA-N Lys-Leu-Val Chemical compound CC(C)C[C@H](NC(=O)[C@@H](N)CCCCN)C(=O)N[C@@H](C(C)C)C(O)=O LJADEBULDNKJNK-IHRRRGAJSA-N 0.000 description 1
- 241000282567 Macaca fascicularis Species 0.000 description 1
- 241000218605 Macacine betaherpesvirus 3 Species 0.000 description 1
- 241001465754 Metazoa Species 0.000 description 1
- 241000283973 Oryctolagus cuniculus Species 0.000 description 1
- BCNRNJWSRFDPTQ-HJWJTTGWSA-N Pro-Ile-Phe Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O BCNRNJWSRFDPTQ-HJWJTTGWSA-N 0.000 description 1
- 102000014128 RANK Ligand Human genes 0.000 description 1
- 108010025832 RANK Ligand Proteins 0.000 description 1
- OLIJLNWFEQEFDM-SRVKXCTJSA-N Ser-Asp-Phe Chemical compound OC[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 OLIJLNWFEQEFDM-SRVKXCTJSA-N 0.000 description 1
- LHUBVKCLOVALIA-HJGDQZAQSA-N Thr-Arg-Gln Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(N)=O)C(O)=O LHUBVKCLOVALIA-HJGDQZAQSA-N 0.000 description 1
- WHVSJHJTMUHYBT-SRVKXCTJSA-N Val-Met-Met Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CCSC)C(=O)O)N WHVSJHJTMUHYBT-SRVKXCTJSA-N 0.000 description 1
- 238000002835 absorbance Methods 0.000 description 1
- 229950009821 acalabrutinib Drugs 0.000 description 1
- WDENQIQQYWYTPO-IBGZPJMESA-N acalabrutinib Chemical compound CC#CC(=O)N1CCC[C@H]1C1=NC(C=2C=CC(=CC=2)C(=O)NC=2N=CC=CC=2)=C2N1C=CN=C2N WDENQIQQYWYTPO-IBGZPJMESA-N 0.000 description 1
- 230000004913 activation Effects 0.000 description 1
- 238000000540 analysis of variance Methods 0.000 description 1
- 239000012491 analyte Substances 0.000 description 1
- 239000005557 antagonist Substances 0.000 description 1
- 108010060035 arginylproline Proteins 0.000 description 1
- 238000011948 assay development Methods 0.000 description 1
- 230000000903 blocking effect Effects 0.000 description 1
- 238000004364 calculation method Methods 0.000 description 1
- 210000004027 cell Anatomy 0.000 description 1
- 239000013592 cell lysate Substances 0.000 description 1
- 230000006037 cell lysis Effects 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 238000012398 clinical drug development Methods 0.000 description 1
- 230000000593 degrading effect Effects 0.000 description 1
- 238000010586 diagram Methods 0.000 description 1
- 229940042399 direct acting antivirals protease inhibitors Drugs 0.000 description 1
- 238000010494 dissociation reaction Methods 0.000 description 1
- 230000005593 dissociations Effects 0.000 description 1
- 238000009510 drug design Methods 0.000 description 1
- 238000000132 electrospray ionisation Methods 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 108010079547 glutamylmethionine Proteins 0.000 description 1
- 229960001507 ibrutinib Drugs 0.000 description 1
- XYFPWWZEPKGCCK-GOSISDBHSA-N ibrutinib Chemical compound C1=2C(N)=NC=NC=2N([C@H]2CN(CCC2)C(=O)C=C)N=C1C(C=C1)=CC=C1OC1=CC=CC=C1 XYFPWWZEPKGCCK-GOSISDBHSA-N 0.000 description 1
- 230000001900 immune effect Effects 0.000 description 1
- 210000000987 immune system Anatomy 0.000 description 1
- 238000001114 immunoprecipitation Methods 0.000 description 1
- 230000010354 integration Effects 0.000 description 1
- 238000002955 isolation Methods 0.000 description 1
- 230000007774 longterm Effects 0.000 description 1
- 230000014759 maintenance of location Effects 0.000 description 1
- 239000011159 matrix material Substances 0.000 description 1
- 230000009149 molecular binding Effects 0.000 description 1
- 108010087904 neutravidin Proteins 0.000 description 1
- 239000002547 new drug Substances 0.000 description 1
- 238000012856 packing Methods 0.000 description 1
- 230000037361 pathway Effects 0.000 description 1
- 238000002203 pretreatment Methods 0.000 description 1
- 238000010814 radioimmunoprecipitation assay Methods 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 238000002864 sequence alignment Methods 0.000 description 1
- 108010048818 seryl-histidine Proteins 0.000 description 1
- 230000019491 signal transduction Effects 0.000 description 1
- 229940126586 small molecule drug Drugs 0.000 description 1
- 150000003384 small molecules Chemical class 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 230000004083 survival effect Effects 0.000 description 1
- 238000003786 synthesis reaction Methods 0.000 description 1
- 238000012360 testing method Methods 0.000 description 1
- 230000001225 therapeutic effect Effects 0.000 description 1
- 230000007704 transition Effects 0.000 description 1
- 239000011534 wash buffer Substances 0.000 description 1
- 238000001262 western blot Methods 0.000 description 1
Images
Classifications
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/543—Immunoassay; Biospecific binding assay; Materials therefor with an insoluble carrier for immobilising immunochemicals
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/68—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving proteins, peptides or amino acids
- G01N33/6803—General methods of protein analysis not limited to specific proteins or families of proteins
- G01N33/6848—Methods of protein analysis involving mass spectrometry
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N30/00—Investigating or analysing materials by separation into components using adsorption, absorption or similar phenomena or using ion-exchange, e.g. chromatography or field flow fractionation
- G01N30/02—Column chromatography
- G01N30/62—Detectors specially adapted therefor
- G01N30/72—Mass spectrometers
- G01N30/7233—Mass spectrometers interfaced to liquid or supercritical fluid chromatograph
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/543—Immunoassay; Biospecific binding assay; Materials therefor with an insoluble carrier for immobilising immunochemicals
- G01N33/54393—Improving reaction conditions or stability, e.g. by coating or irradiation of surface, by reduction of non-specific binding, by promotion of specific binding
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/68—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving proteins, peptides or amino acids
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/68—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving proteins, peptides or amino acids
- G01N33/6803—General methods of protein analysis not limited to specific proteins or families of proteins
- G01N33/6848—Methods of protein analysis involving mass spectrometry
- G01N33/6851—Methods of protein analysis involving laser desorption ionisation mass spectrometry
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N30/00—Investigating or analysing materials by separation into components using adsorption, absorption or similar phenomena or using ion-exchange, e.g. chromatography or field flow fractionation
- G01N30/02—Column chromatography
- G01N2030/022—Column chromatography characterised by the kind of separation mechanism
- G01N2030/027—Liquid chromatography
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2333/00—Assays involving biological materials from specific organisms or of a specific nature
- G01N2333/90—Enzymes; Proenzymes
- G01N2333/91—Transferases (2.)
- G01N2333/912—Transferases (2.) transferring phosphorus containing groups, e.g. kinases (2.7)
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Immunology (AREA)
- Molecular Biology (AREA)
- Chemical & Material Sciences (AREA)
- Physics & Mathematics (AREA)
- Biomedical Technology (AREA)
- Hematology (AREA)
- Urology & Nephrology (AREA)
- Analytical Chemistry (AREA)
- Pathology (AREA)
- General Physics & Mathematics (AREA)
- General Health & Medical Sciences (AREA)
- Biochemistry (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Bioinformatics & Computational Biology (AREA)
- Microbiology (AREA)
- Biotechnology (AREA)
- Cell Biology (AREA)
- Food Science & Technology (AREA)
- Medicinal Chemistry (AREA)
- Spectroscopy & Molecular Physics (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Biophysics (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Optics & Photonics (AREA)
- Investigating Or Analysing Biological Materials (AREA)
- Peptides Or Proteins (AREA)
- Other Investigation Or Analysis Of Materials By Electrical Means (AREA)
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201862669442P | 2018-05-10 | 2018-05-10 | |
| US62/669,442 | 2018-05-10 | ||
| PCT/US2019/031524 WO2019217684A1 (en) | 2018-05-10 | 2019-05-09 | Assay to determine in vivo receptor occupancy |
| KR1020207035120A KR102764357B1 (ko) | 2018-05-10 | 2019-05-09 | 생체내 수용체 점유율을 결정하기 위한 검정 |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020207035120A Division KR102764357B1 (ko) | 2018-05-10 | 2019-05-09 | 생체내 수용체 점유율을 결정하기 위한 검정 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20250021652A true KR20250021652A (ko) | 2025-02-13 |
Family
ID=66821369
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020257003448A Pending KR20250021652A (ko) | 2018-05-10 | 2019-05-09 | 생체내 수용체 점유율을 결정하기 위한 검정 |
| KR1020207035120A Active KR102764357B1 (ko) | 2018-05-10 | 2019-05-09 | 생체내 수용체 점유율을 결정하기 위한 검정 |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020207035120A Active KR102764357B1 (ko) | 2018-05-10 | 2019-05-09 | 생체내 수용체 점유율을 결정하기 위한 검정 |
Country Status (7)
| Country | Link |
|---|---|
| US (2) | US12085564B2 (enExample) |
| EP (1) | EP3791183B1 (enExample) |
| JP (2) | JP7570925B2 (enExample) |
| KR (2) | KR20250021652A (enExample) |
| CN (2) | CN113804873B (enExample) |
| ES (1) | ES2992942T3 (enExample) |
| WO (1) | WO2019217684A1 (enExample) |
Families Citing this family (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR20250021652A (ko) | 2018-05-10 | 2025-02-13 | 브리스톨-마이어스 스큅 컴퍼니 | 생체내 수용체 점유율을 결정하기 위한 검정 |
| CN115932261B (zh) * | 2022-08-15 | 2025-07-25 | 广州医科大学附属第五医院 | 一种btk受体占有率的检测方法 |
| CN116482345B (zh) * | 2023-06-16 | 2023-09-22 | 军科正源(北京)药物研究有限责任公司 | 用于检测双靶点药物受体占有率的方法 |
Family Cites Families (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20090062255A1 (en) * | 2007-08-17 | 2009-03-05 | Thallion Pharmaceuticals Inc. | Tumor-targeting evaluation methodology and compounds related thereto |
| WO2012010240A1 (en) * | 2010-07-19 | 2012-01-26 | Cellzome Ag | In vivo method for the evaluation of a compound-target interaction |
| EP3033625B1 (en) * | 2013-08-13 | 2020-01-22 | The Scripps Research Institute | Cysteine-reactive ligand discovery in proteomes |
| WO2016100593A1 (en) * | 2014-12-17 | 2016-06-23 | Pharmacyclics Llc | Methods and assays for quantification and normalization of kinase and ligand binding |
| KR20250021652A (ko) | 2018-05-10 | 2025-02-13 | 브리스톨-마이어스 스큅 컴퍼니 | 생체내 수용체 점유율을 결정하기 위한 검정 |
-
2019
- 2019-05-09 KR KR1020257003448A patent/KR20250021652A/ko active Pending
- 2019-05-09 JP JP2020563439A patent/JP7570925B2/ja active Active
- 2019-05-09 KR KR1020207035120A patent/KR102764357B1/ko active Active
- 2019-05-09 US US17/051,466 patent/US12085564B2/en active Active
- 2019-05-09 EP EP19729943.1A patent/EP3791183B1/en active Active
- 2019-05-09 CN CN202110955643.1A patent/CN113804873B/zh active Active
- 2019-05-09 WO PCT/US2019/031524 patent/WO2019217684A1/en not_active Ceased
- 2019-05-09 CN CN201980031353.9A patent/CN112105930B/zh active Active
- 2019-05-09 ES ES19729943T patent/ES2992942T3/es active Active
-
2024
- 2024-07-31 US US18/791,084 patent/US20250085279A1/en active Pending
- 2024-10-09 JP JP2024177293A patent/JP2025011208A/ja not_active Withdrawn
Also Published As
| Publication number | Publication date |
|---|---|
| US12085564B2 (en) | 2024-09-10 |
| CN113804873B (zh) | 2024-06-18 |
| ES2992942T3 (en) | 2024-12-20 |
| JP7570925B2 (ja) | 2024-10-22 |
| KR20210008028A (ko) | 2021-01-20 |
| KR102764357B1 (ko) | 2025-02-10 |
| WO2019217684A1 (en) | 2019-11-14 |
| CN112105930A (zh) | 2020-12-18 |
| EP3791183A1 (en) | 2021-03-17 |
| EP3791183B1 (en) | 2024-07-24 |
| JP2021523367A (ja) | 2021-09-02 |
| CN112105930B (zh) | 2025-02-28 |
| US20210156854A1 (en) | 2021-05-27 |
| JP2025011208A (ja) | 2025-01-23 |
| US20250085279A1 (en) | 2025-03-13 |
| CN113804873A (zh) | 2021-12-17 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20250085279A1 (en) | Assay to determine in vivo receptor occupancy | |
| Kulyyassov et al. | Targeted liquid chromatography‐tandem mass spectrometry analysis of proteins: basic principles, applications, and perspectives | |
| Percy et al. | Comparison of standard-and nano-flow liquid chromatography platforms for MRM-based quantitation of putative plasma biomarker proteins | |
| Cox et al. | Multiple reaction monitoring as a method for identifying protein posttranslational modifications | |
| Whiteaker et al. | Sequential multiplexed analyte quantification using peptide immunoaffinity enrichment coupled to mass spectrometry | |
| Faria et al. | Comparison of a stable isotope labeled (SIL) peptide and an extended SIL peptide as internal standards to track digestion variability of an unstable signature peptide during quantification of a cancer biomarker, human osteopontin, from plasma using capillary microflow LC–MS/MS | |
| Ocaña et al. | An immunoaffinity liquid chromatography–tandem mass spectrometry assay for the quantitation of matrix metalloproteinase 9 in mouse serum | |
| Wu et al. | A strategy for liquid chromatography/tandem mass spectrometry based quantitation of pegylated protein drugs in plasma using plasma protein precipitation with water‐miscible organic solvents and subsequent trypsin digestion to generate surrogate peptides for detection | |
| CA2732109A1 (en) | Mass spectrometry assay for plasma-renin | |
| Zhang et al. | Immunoaffinity LC–MS/MS for quantitative determination of a free and total protein target as a target engagement biomarker | |
| Li et al. | Peptide and protein quantification using automated immuno-MALDI (iMALDI) | |
| Huang et al. | Identification and quantification of immunoglobulin G (G1, G2, G3 and G4) in human blood plasma by high-resolution quadrupole-Orbitrap mass spectrometry | |
| Kim et al. | Quantitative analysis of low-abundance serological proteins with peptide affinity-based enrichment and pseudo-multiple reaction monitoring by hybrid quadrupole time-of-flight mass spectrometry | |
| Zhi et al. | Selected reaction monitoring (SRM) mass spectrometry without isotope labeling can be used for rapid protein quantification | |
| Grote et al. | Using pure protein to build a multiple reaction monitoring mass spectrometry assay for targeted detection and quantitation | |
| JP7629278B2 (ja) | Aβペプチドを測定するための抗体セット、Aβペプチドの測定方法及び試薬キット | |
| Chen et al. | Sequential immunoaffinity-LC/MS assay for quantitation of a therapeutic protein in monkey plasma | |
| Kuhn et al. | Multiplexed immunoaffinity enrichment of peptides with anti-peptide antibodies and quantification by stable isotope dilution multiple reaction monitoring mass spectrometry | |
| Dillen et al. | A screening UHPLC–MS/MS method for the analysis of amyloid peptides in cerebrospinal fluid of preclinical species | |
| Geumann et al. | A sandwich enzyme-linked immunosorbent assay for the quantification of insoluble membrane and scaffold proteins | |
| Park et al. | Qualification and application of a liquid chromatography–quadrupole time‐of‐flight mass spectrometric method for the determination of trastuzumab in rat plasma | |
| Tao et al. | High-throughput protein modification quantitation analysis using intact protein MRM and its application on hENGase inhibitor screening | |
| Dahabiyeh et al. | Measurement of the total angiotensinogen and its reduced and oxidised forms in human plasma using targeted LC-MS/MS | |
| Mesmin et al. | Mass spectrometric quantification of AcSDKP‐NH2 in human plasma and urine and comparison with an immunoassay | |
| Anselm et al. | Immunoaffinity-Based Liquid Chromatography Mass Spectrometric Assay to Accurately Quantify the Protein Concentration of HMGB1 in EDTA Plasma |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A107 | Divisional application of patent | ||
| PA0104 | Divisional application for international application |
Comment text: Divisional Application for International Patent Patent event code: PA01041R01D Patent event date: 20250203 Application number text: 1020207035120 Filing date: 20201207 |
|
| PG1501 | Laying open of application |