KR20200037851A - 장내 항생제 내성 박테리아를 탈콜론화하기 위한 조성물 및 방법 - Google Patents
장내 항생제 내성 박테리아를 탈콜론화하기 위한 조성물 및 방법 Download PDFInfo
- Publication number
- KR20200037851A KR20200037851A KR1020207006802A KR20207006802A KR20200037851A KR 20200037851 A KR20200037851 A KR 20200037851A KR 1020207006802 A KR1020207006802 A KR 1020207006802A KR 20207006802 A KR20207006802 A KR 20207006802A KR 20200037851 A KR20200037851 A KR 20200037851A
- Authority
- KR
- South Korea
- Prior art keywords
- bacterial
- pharmaceutical composition
- rrna sequence
- bacterial strains
- subject
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
- 239000000203 mixture Substances 0.000 title claims abstract description 387
- 238000000034 method Methods 0.000 title claims abstract description 126
- 241000894006 Bacteria Species 0.000 title claims abstract description 57
- 229940079866 intestinal antibiotic Drugs 0.000 title description 2
- 230000001580 bacterial effect Effects 0.000 claims abstract description 506
- 239000008194 pharmaceutical composition Substances 0.000 claims abstract description 175
- 108020004465 16S ribosomal RNA Proteins 0.000 claims abstract description 116
- 230000003115 biocidal effect Effects 0.000 claims abstract description 54
- 208000015181 infectious disease Diseases 0.000 claims abstract description 47
- 244000052769 pathogen Species 0.000 claims abstract description 41
- 244000052616 bacterial pathogen Species 0.000 claims abstract description 33
- 239000003814 drug Substances 0.000 claims description 101
- 229940124597 therapeutic agent Drugs 0.000 claims description 83
- 238000011319 anticancer therapy Methods 0.000 claims description 58
- 239000002775 capsule Substances 0.000 claims description 54
- 239000000463 material Substances 0.000 claims description 54
- 230000000968 intestinal effect Effects 0.000 claims description 45
- 210000001035 gastrointestinal tract Anatomy 0.000 claims description 44
- 230000002550 fecal effect Effects 0.000 claims description 35
- 238000011282 treatment Methods 0.000 claims description 34
- 241000588921 Enterobacteriaceae Species 0.000 claims description 32
- 241000282414 Homo sapiens Species 0.000 claims description 31
- 241000588724 Escherichia coli Species 0.000 claims description 30
- 241000194033 Enterococcus Species 0.000 claims description 29
- 239000003795 chemical substances by application Substances 0.000 claims description 27
- 238000000576 coating method Methods 0.000 claims description 27
- 239000011248 coating agent Substances 0.000 claims description 23
- 229940079593 drug Drugs 0.000 claims description 23
- 230000000694 effects Effects 0.000 claims description 23
- 241000607142 Salmonella Species 0.000 claims description 22
- 108010059993 Vancomycin Proteins 0.000 claims description 22
- 210000000936 intestine Anatomy 0.000 claims description 22
- 230000001717 pathogenic effect Effects 0.000 claims description 22
- 229960003165 vancomycin Drugs 0.000 claims description 22
- MYPYJXKWCTUITO-LYRMYLQWSA-N vancomycin Chemical compound O([C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]1OC1=C2C=C3C=C1OC1=CC=C(C=C1Cl)[C@@H](O)[C@H](C(N[C@@H](CC(N)=O)C(=O)N[C@H]3C(=O)N[C@H]1C(=O)N[C@H](C(N[C@@H](C3=CC(O)=CC(O)=C3C=3C(O)=CC=C1C=3)C(O)=O)=O)[C@H](O)C1=CC=C(C(=C1)Cl)O2)=O)NC(=O)[C@@H](CC(C)C)NC)[C@H]1C[C@](C)(N)[C@H](O)[C@H](C)O1 MYPYJXKWCTUITO-LYRMYLQWSA-N 0.000 claims description 22
- MYPYJXKWCTUITO-UHFFFAOYSA-N vancomycin Natural products O1C(C(=C2)Cl)=CC=C2C(O)C(C(NC(C2=CC(O)=CC(O)=C2C=2C(O)=CC=C3C=2)C(O)=O)=O)NC(=O)C3NC(=O)C2NC(=O)C(CC(N)=O)NC(=O)C(NC(=O)C(CC(C)C)NC)C(O)C(C=C3Cl)=CC=C3OC3=CC2=CC1=C3OC1OC(CO)C(O)C(O)C1OC1CC(C)(N)C(O)C(C)O1 MYPYJXKWCTUITO-UHFFFAOYSA-N 0.000 claims description 22
- 230000003111 delayed effect Effects 0.000 claims description 20
- 238000004519 manufacturing process Methods 0.000 claims description 20
- 241000607598 Vibrio Species 0.000 claims description 19
- 239000003242 anti bacterial agent Substances 0.000 claims description 19
- 210000002429 large intestine Anatomy 0.000 claims description 19
- 238000001228 spectrum Methods 0.000 claims description 19
- 239000002246 antineoplastic agent Substances 0.000 claims description 18
- YZBQHRLRFGPBSL-RXMQYKEDSA-N carbapenem Chemical compound C1C=CN2C(=O)C[C@H]21 YZBQHRLRFGPBSL-RXMQYKEDSA-N 0.000 claims description 17
- 210000000813 small intestine Anatomy 0.000 claims description 17
- 241000736262 Microbiota Species 0.000 claims description 16
- 241000607768 Shigella Species 0.000 claims description 16
- 241000191940 Staphylococcus Species 0.000 claims description 16
- 229940124307 fluoroquinolone Drugs 0.000 claims description 16
- 229940088710 antibiotic agent Drugs 0.000 claims description 14
- 238000002512 chemotherapy Methods 0.000 claims description 14
- 108090000204 Dipeptidase 1 Proteins 0.000 claims description 13
- 102000006635 beta-lactamase Human genes 0.000 claims description 13
- 241000589876 Campylobacter Species 0.000 claims description 12
- 241000193163 Clostridioides difficile Species 0.000 claims description 12
- 241000590002 Helicobacter pylori Species 0.000 claims description 12
- 241000282412 Homo Species 0.000 claims description 12
- 241000186779 Listeria monocytogenes Species 0.000 claims description 12
- 241000194017 Streptococcus Species 0.000 claims description 12
- 229940037467 helicobacter pylori Drugs 0.000 claims description 12
- 208000019206 urinary tract infection Diseases 0.000 claims description 12
- 241000193403 Clostridium Species 0.000 claims description 11
- 210000000056 organ Anatomy 0.000 claims description 11
- 241000186000 Bifidobacterium Species 0.000 claims description 10
- 206010035664 Pneumonia Diseases 0.000 claims description 10
- 241000192142 Proteobacteria Species 0.000 claims description 10
- 238000011394 anticancer treatment Methods 0.000 claims description 10
- 241000606161 Chlamydia Species 0.000 claims description 9
- 241000588769 Proteus <enterobacteria> Species 0.000 claims description 9
- 241000589516 Pseudomonas Species 0.000 claims description 9
- 206010040047 Sepsis Diseases 0.000 claims description 9
- 210000004369 blood Anatomy 0.000 claims description 9
- 239000008280 blood Substances 0.000 claims description 9
- 208000037815 bloodstream infection Diseases 0.000 claims description 9
- 235000015097 nutrients Nutrition 0.000 claims description 9
- 241000588748 Klebsiella Species 0.000 claims description 8
- 229940126546 immune checkpoint molecule Drugs 0.000 claims description 8
- 230000001965 increasing effect Effects 0.000 claims description 8
- 239000000546 pharmaceutical excipient Substances 0.000 claims description 8
- 238000001356 surgical procedure Methods 0.000 claims description 8
- 238000002626 targeted therapy Methods 0.000 claims description 8
- 241000193830 Bacillus <bacterium> Species 0.000 claims description 7
- 241000193755 Bacillus cereus Species 0.000 claims description 7
- 241000606790 Haemophilus Species 0.000 claims description 7
- 241000589248 Legionella Species 0.000 claims description 7
- 208000007764 Legionnaires' Disease Diseases 0.000 claims description 7
- 241000589902 Leptospira Species 0.000 claims description 7
- 241000588771 Morganella <proteobacterium> Species 0.000 claims description 7
- 241000186359 Mycobacterium Species 0.000 claims description 7
- 241000204031 Mycoplasma Species 0.000 claims description 7
- 241000588653 Neisseria Species 0.000 claims description 7
- 241000191967 Staphylococcus aureus Species 0.000 claims description 7
- 241000607626 Vibrio cholerae Species 0.000 claims description 7
- 229940096118 ella Drugs 0.000 claims description 7
- 238000002560 therapeutic procedure Methods 0.000 claims description 7
- OOLLAFOLCSJHRE-ZHAKMVSLSA-N ulipristal acetate Chemical compound C1=CC(N(C)C)=CC=C1[C@@H]1C2=C3CCC(=O)C=C3CC[C@H]2[C@H](CC[C@]2(OC(C)=O)C(C)=O)[C@]2(C)C1 OOLLAFOLCSJHRE-ZHAKMVSLSA-N 0.000 claims description 7
- 241000588807 Bordetella Species 0.000 claims description 6
- 206010064687 Device related infection Diseases 0.000 claims description 6
- 241000589886 Treponema Species 0.000 claims description 6
- 238000009169 immunotherapy Methods 0.000 claims description 6
- 238000001959 radiotherapy Methods 0.000 claims description 6
- 230000028327 secretion Effects 0.000 claims description 6
- 150000003952 β-lactams Chemical class 0.000 claims description 6
- 241000607528 Aeromonas hydrophila Species 0.000 claims description 5
- 241000589968 Borrelia Species 0.000 claims description 5
- 241000589562 Brucella Species 0.000 claims description 5
- 241000589875 Campylobacter jejuni Species 0.000 claims description 5
- 241000193155 Clostridium botulinum Species 0.000 claims description 5
- 241000193468 Clostridium perfringens Species 0.000 claims description 5
- 241000186216 Corynebacterium Species 0.000 claims description 5
- 241000588722 Escherichia Species 0.000 claims description 5
- 241001646719 Escherichia coli O157:H7 Species 0.000 claims description 5
- 241000589989 Helicobacter Species 0.000 claims description 5
- 241000186781 Listeria Species 0.000 claims description 5
- 208000016604 Lyme disease Diseases 0.000 claims description 5
- 201000009906 Meningitis Diseases 0.000 claims description 5
- 241000984031 Orientia Species 0.000 claims description 5
- 241000293871 Salmonella enterica subsp. enterica serovar Typhi Species 0.000 claims description 5
- 241000607272 Vibrio parahaemolyticus Species 0.000 claims description 5
- 238000001794 hormone therapy Methods 0.000 claims description 5
- 241001453380 Burkholderia Species 0.000 claims description 4
- 241000589601 Francisella Species 0.000 claims description 4
- 208000008839 Kidney Neoplasms Diseases 0.000 claims description 4
- 241000607000 Plesiomonas Species 0.000 claims description 4
- 206010038389 Renal cancer Diseases 0.000 claims description 4
- 241000606701 Rickettsia Species 0.000 claims description 4
- 241000531795 Salmonella enterica subsp. enterica serovar Paratyphi A Species 0.000 claims description 4
- 206010062255 Soft tissue infection Diseases 0.000 claims description 4
- 208000031650 Surgical Wound Infection Diseases 0.000 claims description 4
- 206010062910 Vascular infections Diseases 0.000 claims description 4
- 206010048038 Wound infection Diseases 0.000 claims description 4
- 230000017531 blood circulation Effects 0.000 claims description 4
- 208000037976 chronic inflammation Diseases 0.000 claims description 4
- 208000037893 chronic inflammatory disorder Diseases 0.000 claims description 4
- 229950009791 durvalumab Drugs 0.000 claims description 4
- 230000002401 inhibitory effect Effects 0.000 claims description 4
- 201000010982 kidney cancer Diseases 0.000 claims description 4
- 230000000670 limiting effect Effects 0.000 claims description 4
- 229960003301 nivolumab Drugs 0.000 claims description 4
- 229960002621 pembrolizumab Drugs 0.000 claims description 4
- 238000002360 preparation method Methods 0.000 claims description 4
- 206010040872 skin infection Diseases 0.000 claims description 4
- 229940120293 vaginal suppository Drugs 0.000 claims description 4
- 239000006216 vaginal suppository Substances 0.000 claims description 4
- 241000588747 Klebsiella pneumoniae Species 0.000 claims description 3
- 241000607734 Yersinia <bacteria> Species 0.000 claims description 3
- 241000607447 Yersinia enterocolitica Species 0.000 claims description 3
- 208000020832 chronic kidney disease Diseases 0.000 claims description 3
- 239000000147 enterotoxin Substances 0.000 claims description 3
- 210000004996 female reproductive system Anatomy 0.000 claims description 3
- 229960005386 ipilimumab Drugs 0.000 claims description 3
- 230000008685 targeting Effects 0.000 claims description 3
- 229940098232 yersinia enterocolitica Drugs 0.000 claims description 3
- 206010006500 Brucellosis Diseases 0.000 claims description 2
- 241000589874 Campylobacter fetus Species 0.000 claims description 2
- 241001445332 Coxiella <snail> Species 0.000 claims description 2
- 241000605314 Ehrlichia Species 0.000 claims description 2
- 241000606999 Plesiomonas shigelloides Species 0.000 claims description 2
- 244000061458 Solanum melongena Species 0.000 claims description 2
- 235000002597 Solanum melongena Nutrition 0.000 claims description 2
- FZWLAAWBMGSTSO-UHFFFAOYSA-N Thiazole Chemical compound C1=CSC=N1 FZWLAAWBMGSTSO-UHFFFAOYSA-N 0.000 claims description 2
- 241000607265 Vibrio vulnificus Species 0.000 claims description 2
- 229960003852 atezolizumab Drugs 0.000 claims description 2
- 230000002008 hemorrhagic effect Effects 0.000 claims description 2
- 238000011866 long-term treatment Methods 0.000 claims description 2
- 229940118696 vibrio cholerae Drugs 0.000 claims description 2
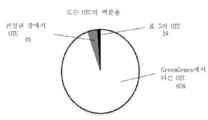
- 230000008029 eradication Effects 0.000 abstract description 6
- 238000009472 formulation Methods 0.000 description 99
- -1 sprinkles Substances 0.000 description 55
- 206010028980 Neoplasm Diseases 0.000 description 51
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 49
- 210000004027 cell Anatomy 0.000 description 44
- 210000003608 fece Anatomy 0.000 description 41
- 210000001072 colon Anatomy 0.000 description 36
- 201000011510 cancer Diseases 0.000 description 34
- 208000024891 symptom Diseases 0.000 description 34
- 230000001225 therapeutic effect Effects 0.000 description 34
- 230000037396 body weight Effects 0.000 description 29
- 230000002496 gastric effect Effects 0.000 description 29
- 229920000642 polymer Polymers 0.000 description 29
- 244000005700 microbiome Species 0.000 description 26
- 201000010099 disease Diseases 0.000 description 22
- 208000035475 disorder Diseases 0.000 description 22
- 235000013406 prebiotics Nutrition 0.000 description 21
- 206010012735 Diarrhoea Diseases 0.000 description 19
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 19
- 238000012360 testing method Methods 0.000 description 19
- 206010009887 colitis Diseases 0.000 description 18
- 239000012530 fluid Substances 0.000 description 18
- 208000002551 irritable bowel syndrome Diseases 0.000 description 17
- 239000000427 antigen Substances 0.000 description 16
- 102000036639 antigens Human genes 0.000 description 16
- 108091007433 antigens Proteins 0.000 description 16
- HDTRYLNUVZCQOY-UHFFFAOYSA-N α-D-glucopyranosyl-α-D-glucopyranoside Natural products OC1C(O)C(O)C(CO)OC1OC1C(O)C(O)C(O)C(CO)O1 HDTRYLNUVZCQOY-UHFFFAOYSA-N 0.000 description 15
- HDTRYLNUVZCQOY-WSWWMNSNSA-N Trehalose Natural products O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@@H](O)[C@@H](CO)O1 HDTRYLNUVZCQOY-WSWWMNSNSA-N 0.000 description 15
- HDTRYLNUVZCQOY-LIZSDCNHSA-N alpha,alpha-trehalose Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 HDTRYLNUVZCQOY-LIZSDCNHSA-N 0.000 description 15
- 239000002552 dosage form Substances 0.000 description 15
- 230000001363 autoimmune Effects 0.000 description 14
- 230000000813 microbial effect Effects 0.000 description 14
- 238000012216 screening Methods 0.000 description 14
- 241000701806 Human papillomavirus Species 0.000 description 13
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 13
- 229930006000 Sucrose Natural products 0.000 description 13
- 210000004215 spore Anatomy 0.000 description 13
- 239000005720 sucrose Substances 0.000 description 13
- 241000196324 Embryophyta Species 0.000 description 12
- 210000001198 duodenum Anatomy 0.000 description 12
- 230000036541 health Effects 0.000 description 12
- 239000000047 product Substances 0.000 description 12
- FERIUCNNQQJTOY-UHFFFAOYSA-N Butyric acid Natural products CCCC(O)=O FERIUCNNQQJTOY-UHFFFAOYSA-N 0.000 description 11
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 11
- NKANXQFJJICGDU-QPLCGJKRSA-N Tamoxifen Chemical compound C=1C=CC=CC=1C(/CC)=C(C=1C=CC(OCCN(C)C)=CC=1)/C1=CC=CC=C1 NKANXQFJJICGDU-QPLCGJKRSA-N 0.000 description 11
- 239000002502 liposome Substances 0.000 description 11
- 244000005706 microflora Species 0.000 description 11
- 229920001223 polyethylene glycol Polymers 0.000 description 11
- 241000894007 species Species 0.000 description 11
- 239000003826 tablet Substances 0.000 description 11
- BPYKTIZUTYGOLE-IFADSCNNSA-N Bilirubin Chemical compound N1C(=O)C(C)=C(C=C)\C1=C\C1=C(C)C(CCC(O)=O)=C(CC2=C(C(C)=C(\C=C/3C(=C(C=C)C(=O)N\3)C)N2)CCC(O)=O)N1 BPYKTIZUTYGOLE-IFADSCNNSA-N 0.000 description 10
- VVQNEPGJFQJSBK-UHFFFAOYSA-N Methyl methacrylate Chemical compound COC(=O)C(C)=C VVQNEPGJFQJSBK-UHFFFAOYSA-N 0.000 description 10
- 208000015914 Non-Hodgkin lymphomas Diseases 0.000 description 10
- 229920001577 copolymer Polymers 0.000 description 10
- 229940127089 cytotoxic agent Drugs 0.000 description 10
- 239000002702 enteric coating Substances 0.000 description 10
- 238000009505 enteric coating Methods 0.000 description 10
- 239000000194 fatty acid Substances 0.000 description 10
- 230000012010 growth Effects 0.000 description 10
- 239000002609 medium Substances 0.000 description 10
- 210000003097 mucus Anatomy 0.000 description 10
- 239000013589 supplement Substances 0.000 description 10
- FERIUCNNQQJTOY-UHFFFAOYSA-M Butyrate Chemical compound CCCC([O-])=O FERIUCNNQQJTOY-UHFFFAOYSA-M 0.000 description 9
- 208000011231 Crohn disease Diseases 0.000 description 9
- CMSMOCZEIVJLDB-UHFFFAOYSA-N Cyclophosphamide Chemical compound ClCCN(CCCl)P1(=O)NCCCO1 CMSMOCZEIVJLDB-UHFFFAOYSA-N 0.000 description 9
- 229920003134 Eudragit® polymer Polymers 0.000 description 9
- 208000022559 Inflammatory bowel disease Diseases 0.000 description 9
- XUJNEKJLAYXESH-REOHCLBHSA-N L-Cysteine Chemical compound SC[C@H](N)C(O)=O XUJNEKJLAYXESH-REOHCLBHSA-N 0.000 description 9
- 229930012538 Paclitaxel Natural products 0.000 description 9
- 239000002202 Polyethylene glycol Substances 0.000 description 9
- 239000008346 aqueous phase Substances 0.000 description 9
- 210000003405 ileum Anatomy 0.000 description 9
- 230000007246 mechanism Effects 0.000 description 9
- 229960001592 paclitaxel Drugs 0.000 description 9
- 239000000126 substance Substances 0.000 description 9
- RCINICONZNJXQF-MZXODVADSA-N taxol Chemical compound O([C@@H]1[C@@]2(C[C@@H](C(C)=C(C2(C)C)[C@H](C([C@]2(C)[C@@H](O)C[C@H]3OC[C@]3([C@H]21)OC(C)=O)=O)OC(=O)C)OC(=O)[C@H](O)[C@@H](NC(=O)C=1C=CC=CC=1)C=1C=CC=CC=1)O)C(=O)C1=CC=CC=C1 RCINICONZNJXQF-MZXODVADSA-N 0.000 description 9
- 208000007465 Giant cell arteritis Diseases 0.000 description 8
- 206010061218 Inflammation Diseases 0.000 description 8
- 208000031981 Thrombocytopenic Idiopathic Purpura Diseases 0.000 description 8
- 230000002159 abnormal effect Effects 0.000 description 8
- 239000004480 active ingredient Substances 0.000 description 8
- 201000003710 autoimmune thrombocytopenic purpura Diseases 0.000 description 8
- 229920002678 cellulose Polymers 0.000 description 8
- JCKYGMPEJWAADB-UHFFFAOYSA-N chlorambucil Chemical compound OC(=O)CCCC1=CC=C(N(CCCl)CCCl)C=C1 JCKYGMPEJWAADB-UHFFFAOYSA-N 0.000 description 8
- 235000014113 dietary fatty acids Nutrition 0.000 description 8
- 238000011156 evaluation Methods 0.000 description 8
- 229930195729 fatty acid Natural products 0.000 description 8
- 230000004054 inflammatory process Effects 0.000 description 8
- 238000002955 isolation Methods 0.000 description 8
- DNIAPMSPPWPWGF-UHFFFAOYSA-N monopropylene glycol Natural products CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 8
- 235000010987 pectin Nutrition 0.000 description 8
- 239000001814 pectin Substances 0.000 description 8
- 229920001277 pectin Polymers 0.000 description 8
- 239000000243 solution Substances 0.000 description 8
- 206010043207 temporal arteritis Diseases 0.000 description 8
- 206010071577 Autoimmune hyperlipidaemia Diseases 0.000 description 7
- DLGOEMSEDOSKAD-UHFFFAOYSA-N Carmustine Chemical compound ClCCNC(=O)N(N=O)CCCl DLGOEMSEDOSKAD-UHFFFAOYSA-N 0.000 description 7
- 206010009900 Colitis ulcerative Diseases 0.000 description 7
- UHDGCWIWMRVCDJ-CCXZUQQUSA-N Cytarabine Chemical compound O=C1N=C(N)C=CN1[C@H]1[C@@H](O)[C@H](O)[C@@H](CO)O1 UHDGCWIWMRVCDJ-CCXZUQQUSA-N 0.000 description 7
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 7
- AOJJSUZBOXZQNB-TZSSRYMLSA-N Doxorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 AOJJSUZBOXZQNB-TZSSRYMLSA-N 0.000 description 7
- 241000305071 Enterobacterales Species 0.000 description 7
- 206010072579 Granulomatosis with polyangiitis Diseases 0.000 description 7
- 229930195725 Mannitol Natural products 0.000 description 7
- VMHLLURERBWHNL-UHFFFAOYSA-M Sodium acetate Chemical compound [Na+].CC([O-])=O VMHLLURERBWHNL-UHFFFAOYSA-M 0.000 description 7
- 229920002472 Starch Polymers 0.000 description 7
- 201000006704 Ulcerative Colitis Diseases 0.000 description 7
- 235000010980 cellulose Nutrition 0.000 description 7
- 239000001913 cellulose Substances 0.000 description 7
- 229960004397 cyclophosphamide Drugs 0.000 description 7
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 description 7
- 235000018417 cysteine Nutrition 0.000 description 7
- 229960000684 cytarabine Drugs 0.000 description 7
- UREBDLICKHMUKA-CXSFZGCWSA-N dexamethasone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@@H](C)[C@@](C(=O)CO)(O)[C@@]1(C)C[C@@H]2O UREBDLICKHMUKA-CXSFZGCWSA-N 0.000 description 7
- 238000004108 freeze drying Methods 0.000 description 7
- 235000011187 glycerol Nutrition 0.000 description 7
- 239000000594 mannitol Substances 0.000 description 7
- 235000010355 mannitol Nutrition 0.000 description 7
- 210000000664 rectum Anatomy 0.000 description 7
- 230000000405 serological effect Effects 0.000 description 7
- 235000019698 starch Nutrition 0.000 description 7
- 239000008107 starch Substances 0.000 description 7
- 229940032147 starch Drugs 0.000 description 7
- 210000002784 stomach Anatomy 0.000 description 7
- 208000011580 syndromic disease Diseases 0.000 description 7
- 210000001519 tissue Anatomy 0.000 description 7
- UCFGDBYHRUNTLO-QHCPKHFHSA-N topotecan Chemical compound C1=C(O)C(CN(C)C)=C2C=C(CN3C4=CC5=C(C3=O)COC(=O)[C@]5(O)CC)C4=NC2=C1 UCFGDBYHRUNTLO-QHCPKHFHSA-N 0.000 description 7
- 210000004881 tumor cell Anatomy 0.000 description 7
- VBICKXHEKHSIBG-UHFFFAOYSA-N 1-monostearoylglycerol Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCC(O)CO VBICKXHEKHSIBG-UHFFFAOYSA-N 0.000 description 6
- 208000004998 Abdominal Pain Diseases 0.000 description 6
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 6
- 208000008190 Agammaglobulinemia Diseases 0.000 description 6
- 108010082126 Alanine transaminase Proteins 0.000 description 6
- 108010003415 Aspartate Aminotransferases Proteins 0.000 description 6
- 102000004625 Aspartate Aminotransferases Human genes 0.000 description 6
- 208000009299 Benign Mucous Membrane Pemphigoid Diseases 0.000 description 6
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 6
- 102000004190 Enzymes Human genes 0.000 description 6
- 108090000790 Enzymes Proteins 0.000 description 6
- 206010016100 Faeces discoloured Diseases 0.000 description 6
- GHASVSINZRGABV-UHFFFAOYSA-N Fluorouracil Chemical compound FC1=CNC(=O)NC1=O GHASVSINZRGABV-UHFFFAOYSA-N 0.000 description 6
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 6
- 206010020983 Hypogammaglobulinaemia Diseases 0.000 description 6
- 239000005536 L01XE08 - Nilotinib Substances 0.000 description 6
- RJKFOVLPORLFTN-LEKSSAKUSA-N Progesterone Chemical compound C1CC2=CC(=O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H](C(=O)C)[C@@]1(C)CC2 RJKFOVLPORLFTN-LEKSSAKUSA-N 0.000 description 6
- BPEGJWRSRHCHSN-UHFFFAOYSA-N Temozolomide Chemical compound O=C1N(C)N=NC2=C(C(N)=O)N=CN21 BPEGJWRSRHCHSN-UHFFFAOYSA-N 0.000 description 6
- 239000002253 acid Substances 0.000 description 6
- 208000002552 acute disseminated encephalomyelitis Diseases 0.000 description 6
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 6
- 229960004630 chlorambucil Drugs 0.000 description 6
- 239000002577 cryoprotective agent Substances 0.000 description 6
- 229960003957 dexamethasone Drugs 0.000 description 6
- 238000012377 drug delivery Methods 0.000 description 6
- 229940088598 enzyme Drugs 0.000 description 6
- 230000029142 excretion Effects 0.000 description 6
- 210000004051 gastric juice Anatomy 0.000 description 6
- 238000012423 maintenance Methods 0.000 description 6
- 208000008795 neuromyelitis optica Diseases 0.000 description 6
- HHZIURLSWUIHRB-UHFFFAOYSA-N nilotinib Chemical compound C1=NC(C)=CN1C1=CC(NC(=O)C=2C=C(NC=3N=C(C=CN=3)C=3C=NC=CC=3)C(C)=CC=2)=CC(C(F)(F)F)=C1 HHZIURLSWUIHRB-UHFFFAOYSA-N 0.000 description 6
- 229920001282 polysaccharide Polymers 0.000 description 6
- 239000005017 polysaccharide Substances 0.000 description 6
- 150000004804 polysaccharides Chemical class 0.000 description 6
- DEQANNDTNATYII-OULOTJBUSA-N (4r,7s,10s,13r,16s,19r)-10-(4-aminobutyl)-19-[[(2r)-2-amino-3-phenylpropanoyl]amino]-16-benzyl-n-[(2r,3r)-1,3-dihydroxybutan-2-yl]-7-[(1r)-1-hydroxyethyl]-13-(1h-indol-3-ylmethyl)-6,9,12,15,18-pentaoxo-1,2-dithia-5,8,11,14,17-pentazacycloicosane-4-carboxa Chemical compound C([C@@H](N)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](CC=2C3=CC=CC=C3NC=2)NC(=O)[C@H](CC=2C=CC=CC=2)NC1=O)C(=O)N[C@H](CO)[C@H](O)C)C1=CC=CC=C1 DEQANNDTNATYII-OULOTJBUSA-N 0.000 description 5
- FDKXTQMXEQVLRF-ZHACJKMWSA-N (E)-dacarbazine Chemical compound CN(C)\N=N\c1[nH]cnc1C(N)=O FDKXTQMXEQVLRF-ZHACJKMWSA-N 0.000 description 5
- UEJJHQNACJXSKW-UHFFFAOYSA-N 2-(2,6-dioxopiperidin-3-yl)-1H-isoindole-1,3(2H)-dione Chemical compound O=C1C2=CC=CC=C2C(=O)N1C1CCC(=O)NC1=O UEJJHQNACJXSKW-UHFFFAOYSA-N 0.000 description 5
- WYWHKKSPHMUBEB-UHFFFAOYSA-N 6-Mercaptoguanine Natural products N1C(N)=NC(=S)C2=C1N=CN2 WYWHKKSPHMUBEB-UHFFFAOYSA-N 0.000 description 5
- 102100036475 Alanine aminotransferase 1 Human genes 0.000 description 5
- 102000002260 Alkaline Phosphatase Human genes 0.000 description 5
- 108020004774 Alkaline Phosphatase Proteins 0.000 description 5
- BFYIZQONLCFLEV-DAELLWKTSA-N Aromasine Chemical compound O=C1C=C[C@]2(C)[C@H]3CC[C@](C)(C(CC4)=O)[C@@H]4[C@@H]3CC(=C)C2=C1 BFYIZQONLCFLEV-DAELLWKTSA-N 0.000 description 5
- 208000009137 Behcet syndrome Diseases 0.000 description 5
- 208000035473 Communicable disease Diseases 0.000 description 5
- 206010010774 Constipation Diseases 0.000 description 5
- HKVAMNSJSFKALM-GKUWKFKPSA-N Everolimus Chemical compound C1C[C@@H](OCCO)[C@H](OC)C[C@@H]1C[C@@H](C)[C@H]1OC(=O)[C@@H]2CCCCN2C(=O)C(=O)[C@](O)(O2)[C@H](C)CC[C@H]2C[C@H](OC)/C(C)=C/C=C/C=C/[C@@H](C)C[C@@H](C)C(=O)[C@H](OC)[C@H](O)/C(C)=C/[C@@H](C)C(=O)C1 HKVAMNSJSFKALM-GKUWKFKPSA-N 0.000 description 5
- 241000701044 Human gammaherpesvirus 4 Species 0.000 description 5
- VSNHCAURESNICA-UHFFFAOYSA-N Hydroxyurea Chemical compound NC(=O)NO VSNHCAURESNICA-UHFFFAOYSA-N 0.000 description 5
- XDXDZDZNSLXDNA-TZNDIEGXSA-N Idarubicin Chemical compound C1[C@H](N)[C@H](O)[C@H](C)O[C@H]1O[C@@H]1C2=C(O)C(C(=O)C3=CC=CC=C3C3=O)=C3C(O)=C2C[C@@](O)(C(C)=O)C1 XDXDZDZNSLXDNA-TZNDIEGXSA-N 0.000 description 5
- 229920001202 Inulin Polymers 0.000 description 5
- FBOZXECLQNJBKD-ZDUSSCGKSA-N L-methotrexate Chemical compound C=1N=C2N=C(N)N=C(N)C2=NC=1CN(C)C1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 FBOZXECLQNJBKD-ZDUSSCGKSA-N 0.000 description 5
- 239000005411 L01XE02 - Gefitinib Substances 0.000 description 5
- 239000002147 L01XE04 - Sunitinib Substances 0.000 description 5
- 239000002118 L01XE12 - Vandetanib Substances 0.000 description 5
- 239000002177 L01XE27 - Ibrutinib Substances 0.000 description 5
- NWIBSHFKIJFRCO-WUDYKRTCSA-N Mytomycin Chemical compound C1N2C(C(C(C)=C(N)C3=O)=O)=C3[C@@H](COC(N)=O)[C@@]2(OC)[C@@H]2[C@H]1N2 NWIBSHFKIJFRCO-WUDYKRTCSA-N 0.000 description 5
- 108010016076 Octreotide Proteins 0.000 description 5
- 229920001800 Shellac Polymers 0.000 description 5
- FOCVUCIESVLUNU-UHFFFAOYSA-N Thiotepa Chemical compound C1CN1P(N1CC1)(=S)N1CC1 FOCVUCIESVLUNU-UHFFFAOYSA-N 0.000 description 5
- 208000025851 Undifferentiated connective tissue disease Diseases 0.000 description 5
- 208000017379 Undifferentiated connective tissue syndrome Diseases 0.000 description 5
- RJURFGZVJUQBHK-UHFFFAOYSA-N actinomycin-C1 Natural products CC1OC(=O)C(C(C)C)N(C)C(=O)CN(C)C(=O)C2CCCN2C(=O)C(C(C)C)NC(=O)C1NC(=O)C1=C(N)C(=O)C(C)=C2OC(C(C)=CC=C3C(=O)NC4C(=O)NC(C(N5CCCC5C(=O)N(C)CC(=O)N(C)C(C(C)C)C(=O)OC4C)=O)C(C)C)=C3N=C21 RJURFGZVJUQBHK-UHFFFAOYSA-N 0.000 description 5
- 235000010443 alginic acid Nutrition 0.000 description 5
- 229920000615 alginic acid Polymers 0.000 description 5
- 230000001174 ascending effect Effects 0.000 description 5
- 238000004820 blood count Methods 0.000 description 5
- 229960005243 carmustine Drugs 0.000 description 5
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Natural products OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 5
- 150000001875 compounds Chemical class 0.000 description 5
- 210000001731 descending colon Anatomy 0.000 description 5
- 229920001971 elastomer Polymers 0.000 description 5
- 239000000806 elastomer Substances 0.000 description 5
- VJJPUSNTGOMMGY-MRVIYFEKSA-N etoposide Chemical compound COC1=C(O)C(OC)=CC([C@@H]2C3=CC=4OCOC=4C=C3[C@@H](O[C@H]3[C@@H]([C@@H](O)[C@@H]4O[C@H](C)OC[C@H]4O3)O)[C@@H]3[C@@H]2C(OC3)=O)=C1 VJJPUSNTGOMMGY-MRVIYFEKSA-N 0.000 description 5
- 229960005420 etoposide Drugs 0.000 description 5
- 229960005167 everolimus Drugs 0.000 description 5
- XGALLCVXEZPNRQ-UHFFFAOYSA-N gefitinib Chemical compound C=12C=C(OCCCN3CCOCC3)C(OC)=CC2=NC=NC=1NC1=CC=C(F)C(Cl)=C1 XGALLCVXEZPNRQ-UHFFFAOYSA-N 0.000 description 5
- 235000001727 glucose Nutrition 0.000 description 5
- 239000008103 glucose Substances 0.000 description 5
- BXWNKGSJHAJOGX-UHFFFAOYSA-N hexadecan-1-ol Chemical compound CCCCCCCCCCCCCCCCO BXWNKGSJHAJOGX-UHFFFAOYSA-N 0.000 description 5
- 239000001257 hydrogen Substances 0.000 description 5
- 229910052739 hydrogen Inorganic materials 0.000 description 5
- 229960001507 ibrutinib Drugs 0.000 description 5
- XYFPWWZEPKGCCK-GOSISDBHSA-N ibrutinib Chemical compound C1=2C(N)=NC=NC=2N([C@H]2CN(CCC2)C(=O)C=C)N=C1C(C=C1)=CC=C1OC1=CC=CC=C1 XYFPWWZEPKGCCK-GOSISDBHSA-N 0.000 description 5
- JYJIGFIDKWBXDU-MNNPPOADSA-N inulin Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)OC[C@]1(OC[C@]2(OC[C@]3(OC[C@]4(OC[C@]5(OC[C@]6(OC[C@]7(OC[C@]8(OC[C@]9(OC[C@]%10(OC[C@]%11(OC[C@]%12(OC[C@]%13(OC[C@]%14(OC[C@]%15(OC[C@]%16(OC[C@]%17(OC[C@]%18(OC[C@]%19(OC[C@]%20(OC[C@]%21(OC[C@]%22(OC[C@]%23(OC[C@]%24(OC[C@]%25(OC[C@]%26(OC[C@]%27(OC[C@]%28(OC[C@]%29(OC[C@]%30(OC[C@]%31(OC[C@]%32(OC[C@]%33(OC[C@]%34(OC[C@]%35(OC[C@]%36(O[C@@H]%37[C@@H]([C@@H](O)[C@H](O)[C@@H](CO)O%37)O)[C@H]([C@H](O)[C@@H](CO)O%36)O)[C@H]([C@H](O)[C@@H](CO)O%35)O)[C@H]([C@H](O)[C@@H](CO)O%34)O)[C@H]([C@H](O)[C@@H](CO)O%33)O)[C@H]([C@H](O)[C@@H](CO)O%32)O)[C@H]([C@H](O)[C@@H](CO)O%31)O)[C@H]([C@H](O)[C@@H](CO)O%30)O)[C@H]([C@H](O)[C@@H](CO)O%29)O)[C@H]([C@H](O)[C@@H](CO)O%28)O)[C@H]([C@H](O)[C@@H](CO)O%27)O)[C@H]([C@H](O)[C@@H](CO)O%26)O)[C@H]([C@H](O)[C@@H](CO)O%25)O)[C@H]([C@H](O)[C@@H](CO)O%24)O)[C@H]([C@H](O)[C@@H](CO)O%23)O)[C@H]([C@H](O)[C@@H](CO)O%22)O)[C@H]([C@H](O)[C@@H](CO)O%21)O)[C@H]([C@H](O)[C@@H](CO)O%20)O)[C@H]([C@H](O)[C@@H](CO)O%19)O)[C@H]([C@H](O)[C@@H](CO)O%18)O)[C@H]([C@H](O)[C@@H](CO)O%17)O)[C@H]([C@H](O)[C@@H](CO)O%16)O)[C@H]([C@H](O)[C@@H](CO)O%15)O)[C@H]([C@H](O)[C@@H](CO)O%14)O)[C@H]([C@H](O)[C@@H](CO)O%13)O)[C@H]([C@H](O)[C@@H](CO)O%12)O)[C@H]([C@H](O)[C@@H](CO)O%11)O)[C@H]([C@H](O)[C@@H](CO)O%10)O)[C@H]([C@H](O)[C@@H](CO)O9)O)[C@H]([C@H](O)[C@@H](CO)O8)O)[C@H]([C@H](O)[C@@H](CO)O7)O)[C@H]([C@H](O)[C@@H](CO)O6)O)[C@H]([C@H](O)[C@@H](CO)O5)O)[C@H]([C@H](O)[C@@H](CO)O4)O)[C@H]([C@H](O)[C@@H](CO)O3)O)[C@H]([C@H](O)[C@@H](CO)O2)O)[C@@H](O)[C@H](O)[C@@H](CO)O1 JYJIGFIDKWBXDU-MNNPPOADSA-N 0.000 description 5
- 229940029339 inulin Drugs 0.000 description 5
- 229960004338 leuprorelin Drugs 0.000 description 5
- 210000004185 liver Anatomy 0.000 description 5
- SGDBTWWWUNNDEQ-LBPRGKRZSA-N melphalan Chemical compound OC(=O)[C@@H](N)CC1=CC=C(N(CCCl)CCCl)C=C1 SGDBTWWWUNNDEQ-LBPRGKRZSA-N 0.000 description 5
- GLVAUDGFNGKCSF-UHFFFAOYSA-N mercaptopurine Chemical compound S=C1NC=NC2=C1NC=N2 GLVAUDGFNGKCSF-UHFFFAOYSA-N 0.000 description 5
- LBWFXVZLPYTWQI-IPOVEDGCSA-N n-[2-(diethylamino)ethyl]-5-[(z)-(5-fluoro-2-oxo-1h-indol-3-ylidene)methyl]-2,4-dimethyl-1h-pyrrole-3-carboxamide;(2s)-2-hydroxybutanedioic acid Chemical compound OC(=O)[C@@H](O)CC(O)=O.CCN(CC)CCNC(=O)C1=C(C)NC(\C=C/2C3=CC(F)=CC=C3NC\2=O)=C1C LBWFXVZLPYTWQI-IPOVEDGCSA-N 0.000 description 5
- XWXYUMMDTVBTOU-UHFFFAOYSA-N nilutamide Chemical compound O=C1C(C)(C)NC(=O)N1C1=CC=C([N+]([O-])=O)C(C(F)(F)F)=C1 XWXYUMMDTVBTOU-UHFFFAOYSA-N 0.000 description 5
- 239000006187 pill Substances 0.000 description 5
- 235000010482 polyoxyethylene sorbitan monooleate Nutrition 0.000 description 5
- 239000000244 polyoxyethylene sorbitan monooleate Substances 0.000 description 5
- 229940068968 polysorbate 80 Drugs 0.000 description 5
- 229920000053 polysorbate 80 Polymers 0.000 description 5
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 5
- 239000001267 polyvinylpyrrolidone Substances 0.000 description 5
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 5
- 229960004618 prednisone Drugs 0.000 description 5
- 239000006041 probiotic Substances 0.000 description 5
- 235000018291 probiotics Nutrition 0.000 description 5
- 230000035755 proliferation Effects 0.000 description 5
- 235000018102 proteins Nutrition 0.000 description 5
- 102000004169 proteins and genes Human genes 0.000 description 5
- 108090000623 proteins and genes Proteins 0.000 description 5
- 230000000306 recurrent effect Effects 0.000 description 5
- 230000004044 response Effects 0.000 description 5
- 239000000523 sample Substances 0.000 description 5
- 239000004208 shellac Substances 0.000 description 5
- 229940113147 shellac Drugs 0.000 description 5
- 235000013874 shellac Nutrition 0.000 description 5
- ZLGIYFNHBLSMPS-ATJNOEHPSA-N shellac Chemical compound OCCCCCC(O)C(O)CCCCCCCC(O)=O.C1C23[C@H](C(O)=O)CCC2[C@](C)(CO)[C@@H]1C(C(O)=O)=C[C@@H]3O ZLGIYFNHBLSMPS-ATJNOEHPSA-N 0.000 description 5
- 210000003491 skin Anatomy 0.000 description 5
- 239000007787 solid Substances 0.000 description 5
- 235000000346 sugar Nutrition 0.000 description 5
- 239000004094 surface-active agent Substances 0.000 description 5
- 239000000725 suspension Substances 0.000 description 5
- 239000000454 talc Substances 0.000 description 5
- 235000012222 talc Nutrition 0.000 description 5
- 229910052623 talc Inorganic materials 0.000 description 5
- LIRYPHYGHXZJBZ-UHFFFAOYSA-N trametinib Chemical compound CC(=O)NC1=CC=CC(N2C(N(C3CC3)C(=O)C3=C(NC=4C(=CC(I)=CC=4)F)N(C)C(=O)C(C)=C32)=O)=C1 LIRYPHYGHXZJBZ-UHFFFAOYSA-N 0.000 description 5
- 210000003384 transverse colon Anatomy 0.000 description 5
- UHTHHESEBZOYNR-UHFFFAOYSA-N vandetanib Chemical compound COC1=CC(C(/N=CN2)=N/C=3C(=CC(Br)=CC=3)F)=C2C=C1OCC1CCN(C)CC1 UHTHHESEBZOYNR-UHFFFAOYSA-N 0.000 description 5
- XRASPMIURGNCCH-UHFFFAOYSA-N zoledronic acid Chemical compound OP(=O)(O)C(P(O)(O)=O)(O)CN1C=CN=C1 XRASPMIURGNCCH-UHFFFAOYSA-N 0.000 description 5
- NMUSYJAQQFHJEW-KVTDHHQDSA-N 5-azacytidine Chemical compound O=C1N=C(N)N=CN1[C@H]1[C@H](O)[C@H](O)[C@@H](CO)O1 NMUSYJAQQFHJEW-KVTDHHQDSA-N 0.000 description 4
- FHVDTGUDJYJELY-UHFFFAOYSA-N 6-{[2-carboxy-4,5-dihydroxy-6-(phosphanyloxy)oxan-3-yl]oxy}-4,5-dihydroxy-3-phosphanyloxane-2-carboxylic acid Chemical compound O1C(C(O)=O)C(P)C(O)C(O)C1OC1C(C(O)=O)OC(OP)C(O)C1O FHVDTGUDJYJELY-UHFFFAOYSA-N 0.000 description 4
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 4
- 208000003343 Antiphospholipid Syndrome Diseases 0.000 description 4
- 102000015790 Asparaginase Human genes 0.000 description 4
- 108010024976 Asparaginase Proteins 0.000 description 4
- 208000023275 Autoimmune disease Diseases 0.000 description 4
- 208000031212 Autoimmune polyendocrinopathy Diseases 0.000 description 4
- 201000009030 Carcinoma Diseases 0.000 description 4
- 229920000623 Cellulose acetate phthalate Polymers 0.000 description 4
- 229920001661 Chitosan Polymers 0.000 description 4
- 208000030939 Chronic inflammatory demyelinating polyneuropathy Diseases 0.000 description 4
- 206010009657 Clostridium difficile colitis Diseases 0.000 description 4
- MWWSFMDVAYGXBV-RUELKSSGSA-N Doxorubicin hydrochloride Chemical compound Cl.O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 MWWSFMDVAYGXBV-RUELKSSGSA-N 0.000 description 4
- 108010069236 Goserelin Proteins 0.000 description 4
- 208000035186 Hemolytic Autoimmune Anemia Diseases 0.000 description 4
- 101001012157 Homo sapiens Receptor tyrosine-protein kinase erbB-2 Proteins 0.000 description 4
- 201000009794 Idiopathic Pulmonary Fibrosis Diseases 0.000 description 4
- 206010021245 Idiopathic thrombocytopenic purpura Diseases 0.000 description 4
- 206010061598 Immunodeficiency Diseases 0.000 description 4
- 208000003456 Juvenile Arthritis Diseases 0.000 description 4
- 206010059176 Juvenile idiopathic arthritis Diseases 0.000 description 4
- 244000199885 Lactobacillus bulgaricus Species 0.000 description 4
- 235000013960 Lactobacillus bulgaricus Nutrition 0.000 description 4
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 4
- 108010000817 Leuprolide Proteins 0.000 description 4
- 208000012309 Linear IgA disease Diseases 0.000 description 4
- GQYIWUVLTXOXAJ-UHFFFAOYSA-N Lomustine Chemical compound ClCCN(N=O)C(=O)NC1CCCCC1 GQYIWUVLTXOXAJ-UHFFFAOYSA-N 0.000 description 4
- AFVFQIVMOAPDHO-UHFFFAOYSA-N Methanesulfonic acid Chemical compound CS(O)(=O)=O AFVFQIVMOAPDHO-UHFFFAOYSA-N 0.000 description 4
- 208000003250 Mixed connective tissue disease Diseases 0.000 description 4
- ZDZOTLJHXYCWBA-VCVYQWHSSA-N N-debenzoyl-N-(tert-butoxycarbonyl)-10-deacetyltaxol Chemical compound O([C@H]1[C@H]2[C@@](C([C@H](O)C3=C(C)[C@@H](OC(=O)[C@H](O)[C@@H](NC(=O)OC(C)(C)C)C=4C=CC=CC=4)C[C@]1(O)C3(C)C)=O)(C)[C@@H](O)C[C@H]1OC[C@]12OC(=O)C)C(=O)C1=CC=CC=C1 ZDZOTLJHXYCWBA-VCVYQWHSSA-N 0.000 description 4
- 229910002651 NO3 Inorganic materials 0.000 description 4
- NHNBFGGVMKEFGY-UHFFFAOYSA-N Nitrate Chemical compound [O-][N+]([O-])=O NHNBFGGVMKEFGY-UHFFFAOYSA-N 0.000 description 4
- 208000000733 Paroxysmal Hemoglobinuria Diseases 0.000 description 4
- 206010034277 Pemphigoid Diseases 0.000 description 4
- 102100036050 Phosphatidylinositol N-acetylglucosaminyltransferase subunit A Human genes 0.000 description 4
- 102100024216 Programmed cell death 1 ligand 1 Human genes 0.000 description 4
- 102100030086 Receptor tyrosine-protein kinase erbB-2 Human genes 0.000 description 4
- 241000702670 Rotavirus Species 0.000 description 4
- 208000025865 Ulcer Diseases 0.000 description 4
- 206010046851 Uveitis Diseases 0.000 description 4
- 229940081735 acetylcellulose Drugs 0.000 description 4
- 230000001154 acute effect Effects 0.000 description 4
- 239000000654 additive Substances 0.000 description 4
- 229940072056 alginate Drugs 0.000 description 4
- 125000002947 alkylene group Chemical group 0.000 description 4
- 229940124650 anti-cancer therapies Drugs 0.000 description 4
- 210000001815 ascending colon Anatomy 0.000 description 4
- 208000027625 autoimmune inner ear disease Diseases 0.000 description 4
- 230000009286 beneficial effect Effects 0.000 description 4
- ONIQOQHATWINJY-UHFFFAOYSA-N cabozantinib Chemical compound C=12C=C(OC)C(OC)=CC2=NC=CC=1OC(C=C1)=CC=C1NC(=O)C1(C(=O)NC=2C=CC(F)=CC=2)CC1 ONIQOQHATWINJY-UHFFFAOYSA-N 0.000 description 4
- YKPUWZUDDOIDPM-SOFGYWHQSA-N capsaicin Chemical compound COC1=CC(CNC(=O)CCCC\C=C\C(C)C)=CC=C1O YKPUWZUDDOIDPM-SOFGYWHQSA-N 0.000 description 4
- 229920002301 cellulose acetate Polymers 0.000 description 4
- 229940081734 cellulose acetate phthalate Drugs 0.000 description 4
- 239000003638 chemical reducing agent Substances 0.000 description 4
- 229960004316 cisplatin Drugs 0.000 description 4
- OROGSEYTTFOCAN-DNJOTXNNSA-N codeine Chemical compound C([C@H]1[C@H](N(CC[C@@]112)C)C3)=C[C@H](O)[C@@H]1OC1=C2C3=CC=C1OC OROGSEYTTFOCAN-DNJOTXNNSA-N 0.000 description 4
- 238000002052 colonoscopy Methods 0.000 description 4
- 230000000295 complement effect Effects 0.000 description 4
- 229960000975 daunorubicin Drugs 0.000 description 4
- 201000001981 dermatomyositis Diseases 0.000 description 4
- 229960002918 doxorubicin hydrochloride Drugs 0.000 description 4
- 239000000839 emulsion Substances 0.000 description 4
- 239000002158 endotoxin Substances 0.000 description 4
- WXCXUHSOUPDCQV-UHFFFAOYSA-N enzalutamide Chemical compound C1=C(F)C(C(=O)NC)=CC=C1N1C(C)(C)C(=O)N(C=2C=C(C(C#N)=CC=2)C(F)(F)F)C1=S WXCXUHSOUPDCQV-UHFFFAOYSA-N 0.000 description 4
- 229920006227 ethylene-grafted-maleic anhydride Polymers 0.000 description 4
- 229960002949 fluorouracil Drugs 0.000 description 4
- 238000007710 freezing Methods 0.000 description 4
- 230000008014 freezing Effects 0.000 description 4
- 230000006870 function Effects 0.000 description 4
- ZZUFCTLCJUWOSV-UHFFFAOYSA-N furosemide Chemical compound C1=C(Cl)C(S(=O)(=O)N)=CC(C(O)=O)=C1NCC1=CC=CO1 ZZUFCTLCJUWOSV-UHFFFAOYSA-N 0.000 description 4
- 125000004474 heteroalkylene group Chemical group 0.000 description 4
- 125000004435 hydrogen atom Chemical group [H]* 0.000 description 4
- 230000002209 hydrophobic effect Effects 0.000 description 4
- 229920003088 hydroxypropyl methyl cellulose Polymers 0.000 description 4
- 229960001101 ifosfamide Drugs 0.000 description 4
- HOMGKSMUEGBAAB-UHFFFAOYSA-N ifosfamide Chemical compound ClCCNP1(=O)OCCCN1CCCl HOMGKSMUEGBAAB-UHFFFAOYSA-N 0.000 description 4
- 210000000987 immune system Anatomy 0.000 description 4
- 210000004347 intestinal mucosa Anatomy 0.000 description 4
- 229940004208 lactobacillus bulgaricus Drugs 0.000 description 4
- 239000008101 lactose Substances 0.000 description 4
- 108010021336 lanreotide Proteins 0.000 description 4
- 229920006008 lipopolysaccharide Polymers 0.000 description 4
- 230000003908 liver function Effects 0.000 description 4
- 206010025135 lupus erythematosus Diseases 0.000 description 4
- 239000012931 lyophilized formulation Substances 0.000 description 4
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 description 4
- 230000001404 mediated effect Effects 0.000 description 4
- RQZAXGRLVPAYTJ-GQFGMJRRSA-N megestrol acetate Chemical compound C1=C(C)C2=CC(=O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@@](C(C)=O)(OC(=O)C)[C@@]1(C)CC2 RQZAXGRLVPAYTJ-GQFGMJRRSA-N 0.000 description 4
- 229960001428 mercaptopurine Drugs 0.000 description 4
- 230000004682 mucosal barrier function Effects 0.000 description 4
- 206010028417 myasthenia gravis Diseases 0.000 description 4
- 239000002105 nanoparticle Substances 0.000 description 4
- 230000007935 neutral effect Effects 0.000 description 4
- 229960002700 octreotide Drugs 0.000 description 4
- 229960003359 palonosetron hydrochloride Drugs 0.000 description 4
- 244000045947 parasite Species 0.000 description 4
- 201000003045 paroxysmal nocturnal hemoglobinuria Diseases 0.000 description 4
- 108010044644 pegfilgrastim Proteins 0.000 description 4
- 208000033808 peripheral neuropathy Diseases 0.000 description 4
- 229920001515 polyalkylene glycol Polymers 0.000 description 4
- 239000000843 powder Substances 0.000 description 4
- 229960005205 prednisolone Drugs 0.000 description 4
- OIGNJSKKLXVSLS-VWUMJDOOSA-N prednisolone Chemical compound O=C1C=C[C@]2(C)[C@H]3[C@@H](O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 OIGNJSKKLXVSLS-VWUMJDOOSA-N 0.000 description 4
- XOFYZVNMUHMLCC-ZPOLXVRWSA-N prednisone Chemical compound O=C1C=C[C@]2(C)[C@H]3C(=O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 XOFYZVNMUHMLCC-ZPOLXVRWSA-N 0.000 description 4
- 230000000529 probiotic effect Effects 0.000 description 4
- 230000008569 process Effects 0.000 description 4
- 208000002574 reactive arthritis Diseases 0.000 description 4
- 150000004666 short chain fatty acids Chemical class 0.000 description 4
- 235000021391 short chain fatty acids Nutrition 0.000 description 4
- VZZJRYRQSPEMTK-CALCHBBNSA-N sonidegib Chemical compound C1[C@@H](C)O[C@@H](C)CN1C(N=C1)=CC=C1NC(=O)C1=CC=CC(C=2C=CC(OC(F)(F)F)=CC=2)=C1C VZZJRYRQSPEMTK-CALCHBBNSA-N 0.000 description 4
- 125000000547 substituted alkyl group Chemical group 0.000 description 4
- 230000009885 systemic effect Effects 0.000 description 4
- 229960001603 tamoxifen Drugs 0.000 description 4
- 229960004964 temozolomide Drugs 0.000 description 4
- 229960003433 thalidomide Drugs 0.000 description 4
- 229960001196 thiotepa Drugs 0.000 description 4
- 231100000397 ulcer Toxicity 0.000 description 4
- 229960005486 vaccine Drugs 0.000 description 4
- 229960004528 vincristine Drugs 0.000 description 4
- OGWKCGZFUXNPDA-UHFFFAOYSA-N vincristine Natural products C1C(CC)(O)CC(CC2(C(=O)OC)C=3C(=CC4=C(C56C(C(C(OC(C)=O)C7(CC)C=CCN(C67)CC5)(O)C(=O)OC)N4C=O)C=3)OC)CN1CCC1=C2NC2=CC=CC=C12 OGWKCGZFUXNPDA-UHFFFAOYSA-N 0.000 description 4
- AQTQHPDCURKLKT-JKDPCDLQSA-N vincristine sulfate Chemical compound OS(O)(=O)=O.C([C@@H](C[C@]1(C(=O)OC)C=2C(=CC3=C([C@]45[C@H]([C@@]([C@H](OC(C)=O)[C@]6(CC)C=CCN([C@H]56)CC4)(O)C(=O)OC)N3C=O)C=2)OC)C[C@@](C2)(O)CC)N2CCC2=C1NC1=CC=CC=C21 AQTQHPDCURKLKT-JKDPCDLQSA-N 0.000 description 4
- NAALWFYYHHJEFQ-ZASNTINBSA-N (2s,5r,6r)-6-[[(2r)-2-[[6-[4-[bis(2-hydroxyethyl)sulfamoyl]phenyl]-2-oxo-1h-pyridine-3-carbonyl]amino]-2-(4-hydroxyphenyl)acetyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid Chemical compound N([C@@H](C(=O)N[C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C=1C=CC(O)=CC=1)C(=O)C(C(N1)=O)=CC=C1C1=CC=C(S(=O)(=O)N(CCO)CCO)C=C1 NAALWFYYHHJEFQ-ZASNTINBSA-N 0.000 description 3
- LKJPYSCBVHEWIU-KRWDZBQOSA-N (R)-bicalutamide Chemical compound C([C@@](O)(C)C(=O)NC=1C=C(C(C#N)=CC=1)C(F)(F)F)S(=O)(=O)C1=CC=C(F)C=C1 LKJPYSCBVHEWIU-KRWDZBQOSA-N 0.000 description 3
- AILRADAXUVEEIR-UHFFFAOYSA-N 5-chloro-4-n-(2-dimethylphosphorylphenyl)-2-n-[2-methoxy-4-[4-(4-methylpiperazin-1-yl)piperidin-1-yl]phenyl]pyrimidine-2,4-diamine Chemical compound COC1=CC(N2CCC(CC2)N2CCN(C)CC2)=CC=C1NC(N=1)=NC=C(Cl)C=1NC1=CC=CC=C1P(C)(C)=O AILRADAXUVEEIR-UHFFFAOYSA-N 0.000 description 3
- STQGQHZAVUOBTE-UHFFFAOYSA-N 7-Cyan-hept-2t-en-4,6-diinsaeure Natural products C1=2C(O)=C3C(=O)C=4C(OC)=CC=CC=4C(=O)C3=C(O)C=2CC(O)(C(C)=O)CC1OC1CC(N)C(O)C(C)O1 STQGQHZAVUOBTE-UHFFFAOYSA-N 0.000 description 3
- MKBLHFILKIKSQM-UHFFFAOYSA-N 9-methyl-3-[(2-methyl-1h-imidazol-3-ium-3-yl)methyl]-2,3-dihydro-1h-carbazol-4-one;chloride Chemical compound Cl.CC1=NC=CN1CC1C(=O)C(C=2C(=CC=CC=2)N2C)=C2CC1 MKBLHFILKIKSQM-UHFFFAOYSA-N 0.000 description 3
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 3
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 3
- 229920001817 Agar Polymers 0.000 description 3
- 108010088751 Albumins Proteins 0.000 description 3
- 102000009027 Albumins Human genes 0.000 description 3
- 108010074708 B7-H1 Antigen Proteins 0.000 description 3
- VTYYLEPIZMXCLO-UHFFFAOYSA-L Calcium carbonate Chemical compound [Ca+2].[O-]C([O-])=O VTYYLEPIZMXCLO-UHFFFAOYSA-L 0.000 description 3
- PTOAARAWEBMLNO-KVQBGUIXSA-N Cladribine Chemical compound C1=NC=2C(N)=NC(Cl)=NC=2N1[C@H]1C[C@H](O)[C@@H](CO)O1 PTOAARAWEBMLNO-KVQBGUIXSA-N 0.000 description 3
- 208000015943 Coeliac disease Diseases 0.000 description 3
- 206010056979 Colitis microscopic Diseases 0.000 description 3
- 102000008186 Collagen Human genes 0.000 description 3
- 108010035532 Collagen Proteins 0.000 description 3
- 206010009944 Colon cancer Diseases 0.000 description 3
- 206010011409 Cross infection Diseases 0.000 description 3
- 241000223935 Cryptosporidium Species 0.000 description 3
- 241000179197 Cyclospora Species 0.000 description 3
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 3
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 description 3
- 108010092160 Dactinomycin Proteins 0.000 description 3
- ZBNZXTGUTAYRHI-UHFFFAOYSA-N Dasatinib Chemical compound C=1C(N2CCN(CCO)CC2)=NC(C)=NC=1NC(S1)=NC=C1C(=O)NC1=C(C)C=CC=C1Cl ZBNZXTGUTAYRHI-UHFFFAOYSA-N 0.000 description 3
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 3
- 102100025012 Dipeptidyl peptidase 4 Human genes 0.000 description 3
- XXPXYPLPSDPERN-UHFFFAOYSA-N Ecteinascidin 743 Natural products COc1cc2C(NCCc2cc1O)C(=O)OCC3N4C(O)C5Cc6cc(C)c(OC)c(O)c6C(C4C(S)c7c(OC(=O)C)c(C)c8OCOc8c37)N5C XXPXYPLPSDPERN-UHFFFAOYSA-N 0.000 description 3
- 102100023795 Elafin Human genes 0.000 description 3
- 241000792859 Enema Species 0.000 description 3
- 229930091371 Fructose Natural products 0.000 description 3
- 239000005715 Fructose Substances 0.000 description 3
- RFSUNEUAIZKAJO-ARQDHWQXSA-N Fructose Chemical compound OC[C@H]1O[C@](O)(CO)[C@@H](O)[C@@H]1O RFSUNEUAIZKAJO-ARQDHWQXSA-N 0.000 description 3
- 108010010803 Gelatin Proteins 0.000 description 3
- BLCLNMBMMGCOAS-URPVMXJPSA-N Goserelin Chemical compound C([C@@H](C(=O)N[C@H](COC(C)(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N1[C@@H](CCC1)C(=O)NNC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@H](CC=1NC=NC=1)NC(=O)[C@H]1NC(=O)CC1)C1=CC=C(O)C=C1 BLCLNMBMMGCOAS-URPVMXJPSA-N 0.000 description 3
- 208000005176 Hepatitis C Diseases 0.000 description 3
- 229940076838 Immune checkpoint inhibitor Drugs 0.000 description 3
- 102000003814 Interleukin-10 Human genes 0.000 description 3
- 108090000174 Interleukin-10 Proteins 0.000 description 3
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 3
- 241000567229 Isospora Species 0.000 description 3
- QIVBCDIJIAJPQS-VIFPVBQESA-N L-tryptophane Chemical compound C1=CC=C2C(C[C@H](N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-VIFPVBQESA-N 0.000 description 3
- 239000002067 L01XE06 - Dasatinib Substances 0.000 description 3
- 239000002145 L01XE14 - Bosutinib Substances 0.000 description 3
- 239000002137 L01XE24 - Ponatinib Substances 0.000 description 3
- 241000186660 Lactobacillus Species 0.000 description 3
- 102000004895 Lipoproteins Human genes 0.000 description 3
- 108090001030 Lipoproteins Proteins 0.000 description 3
- 206010025323 Lymphomas Diseases 0.000 description 3
- 240000003183 Manihot esculenta Species 0.000 description 3
- 235000016735 Manihot esculenta subsp esculenta Nutrition 0.000 description 3
- 102100022430 Melanocyte protein PMEL Human genes 0.000 description 3
- XOGTZOOQQBDUSI-UHFFFAOYSA-M Mesna Chemical compound [Na+].[O-]S(=O)(=O)CCS XOGTZOOQQBDUSI-UHFFFAOYSA-M 0.000 description 3
- RJQXTJLFIWVMTO-TYNCELHUSA-N Methicillin Chemical compound COC1=CC=CC(OC)=C1C(=O)N[C@@H]1C(=O)N2[C@@H](C(O)=O)C(C)(C)S[C@@H]21 RJQXTJLFIWVMTO-TYNCELHUSA-N 0.000 description 3
- 101100407308 Mus musculus Pdcd1lg2 gene Proteins 0.000 description 3
- 241001263478 Norovirus Species 0.000 description 3
- 208000003435 Optic Neuritis Diseases 0.000 description 3
- 229910019142 PO4 Inorganic materials 0.000 description 3
- 241000642506 Pemara Species 0.000 description 3
- 229920002845 Poly(methacrylic acid) Polymers 0.000 description 3
- 239000004372 Polyvinyl alcohol Substances 0.000 description 3
- 108700030875 Programmed Cell Death 1 Ligand 2 Proteins 0.000 description 3
- 102100024213 Programmed cell death 1 ligand 2 Human genes 0.000 description 3
- 206010037660 Pyrexia Diseases 0.000 description 3
- 206010072148 Stiff-Person syndrome Diseases 0.000 description 3
- 101800001271 Surface protein Proteins 0.000 description 3
- JXLYSJRDGCGARV-WWYNWVTFSA-N Vinblastine Natural products O=C(O[C@H]1[C@](O)(C(=O)OC)[C@@H]2N(C)c3c(cc(c(OC)c3)[C@]3(C(=O)OC)c4[nH]c5c(c4CCN4C[C@](O)(CC)C[C@H](C3)C4)cccc5)[C@@]32[C@H]2[C@@]1(CC)C=CCN2CC3)C JXLYSJRDGCGARV-WWYNWVTFSA-N 0.000 description 3
- 238000009825 accumulation Methods 0.000 description 3
- 230000002378 acidificating effect Effects 0.000 description 3
- 150000007513 acids Chemical class 0.000 description 3
- RJURFGZVJUQBHK-IIXSONLDSA-N actinomycin D Chemical compound C[C@H]1OC(=O)[C@H](C(C)C)N(C)C(=O)CN(C)C(=O)[C@@H]2CCCN2C(=O)[C@@H](C(C)C)NC(=O)[C@H]1NC(=O)C1=C(N)C(=O)C(C)=C2OC(C(C)=CC=C3C(=O)N[C@@H]4C(=O)N[C@@H](C(N5CCC[C@H]5C(=O)N(C)CC(=O)N(C)[C@@H](C(C)C)C(=O)O[C@@H]4C)=O)C(C)C)=C3N=C21 RJURFGZVJUQBHK-IIXSONLDSA-N 0.000 description 3
- 239000008186 active pharmaceutical agent Substances 0.000 description 3
- 235000010419 agar Nutrition 0.000 description 3
- 108700025316 aldesleukin Proteins 0.000 description 3
- YBBLVLTVTVSKRW-UHFFFAOYSA-N anastrozole Chemical compound N#CC(C)(C)C1=CC(C(C)(C#N)C)=CC(CN2N=CN=C2)=C1 YBBLVLTVTVSKRW-UHFFFAOYSA-N 0.000 description 3
- 230000000844 anti-bacterial effect Effects 0.000 description 3
- 230000003110 anti-inflammatory effect Effects 0.000 description 3
- 230000004596 appetite loss Effects 0.000 description 3
- 239000007864 aqueous solution Substances 0.000 description 3
- 229960003272 asparaginase Drugs 0.000 description 3
- DCXYFEDJOCDNAF-UHFFFAOYSA-M asparaginate Chemical compound [O-]C(=O)C(N)CC(N)=O DCXYFEDJOCDNAF-UHFFFAOYSA-M 0.000 description 3
- 238000003556 assay Methods 0.000 description 3
- 208000006673 asthma Diseases 0.000 description 3
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 3
- 229960002756 azacitidine Drugs 0.000 description 3
- WZPBZJONDBGPKJ-VEHQQRBSSA-N aztreonam Chemical compound O=C1N(S([O-])(=O)=O)[C@@H](C)[C@@H]1NC(=O)C(=N/OC(C)(C)C(O)=O)\C1=CSC([NH3+])=N1 WZPBZJONDBGPKJ-VEHQQRBSSA-N 0.000 description 3
- 229960003644 aztreonam Drugs 0.000 description 3
- 230000004888 barrier function Effects 0.000 description 3
- 230000008901 benefit Effects 0.000 description 3
- 229960000997 bicalutamide Drugs 0.000 description 3
- 238000009534 blood test Methods 0.000 description 3
- 210000004204 blood vessel Anatomy 0.000 description 3
- GXJABQQUPOEUTA-RDJZCZTQSA-N bortezomib Chemical compound C([C@@H](C(=O)N[C@@H](CC(C)C)B(O)O)NC(=O)C=1N=CC=NC=1)C1=CC=CC=C1 GXJABQQUPOEUTA-RDJZCZTQSA-N 0.000 description 3
- 229960003736 bosutinib Drugs 0.000 description 3
- UBPYILGKFZZVDX-UHFFFAOYSA-N bosutinib Chemical compound C1=C(Cl)C(OC)=CC(NC=2C3=CC(OC)=C(OCCCN4CCN(C)CC4)C=C3N=CC=2C#N)=C1Cl UBPYILGKFZZVDX-UHFFFAOYSA-N 0.000 description 3
- 210000000481 breast Anatomy 0.000 description 3
- 229950004272 brigatinib Drugs 0.000 description 3
- 239000000872 buffer Substances 0.000 description 3
- KVUAALJSMIVURS-ZEDZUCNESA-L calcium folinate Chemical compound [Ca+2].C1NC=2NC(N)=NC(=O)C=2N(C=O)C1CNC1=CC=C(C(=O)N[C@@H](CCC([O-])=O)C([O-])=O)C=C1 KVUAALJSMIVURS-ZEDZUCNESA-L 0.000 description 3
- 229960000541 cetyl alcohol Drugs 0.000 description 3
- 229940045110 chitosan Drugs 0.000 description 3
- 230000001684 chronic effect Effects 0.000 description 3
- 235000019506 cigar Nutrition 0.000 description 3
- DQLATGHUWYMOKM-UHFFFAOYSA-L cisplatin Chemical compound N[Pt](N)(Cl)Cl DQLATGHUWYMOKM-UHFFFAOYSA-L 0.000 description 3
- 229960002436 cladribine Drugs 0.000 description 3
- 238000011260 co-administration Methods 0.000 description 3
- 229920001436 collagen Polymers 0.000 description 3
- 229960002465 dabrafenib Drugs 0.000 description 3
- BFSMGDJOXZAERB-UHFFFAOYSA-N dabrafenib Chemical compound S1C(C(C)(C)C)=NC(C=2C(=C(NS(=O)(=O)C=3C(=CC=CC=3F)F)C=CC=2)F)=C1C1=CC=NC(N)=N1 BFSMGDJOXZAERB-UHFFFAOYSA-N 0.000 description 3
- 229960002448 dasatinib Drugs 0.000 description 3
- STQGQHZAVUOBTE-VGBVRHCVSA-N daunorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(C)=O)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 STQGQHZAVUOBTE-VGBVRHCVSA-N 0.000 description 3
- 229960003109 daunorubicin hydrochloride Drugs 0.000 description 3
- 230000007423 decrease Effects 0.000 description 3
- 238000004090 dissolution Methods 0.000 description 3
- 229960003668 docetaxel Drugs 0.000 description 3
- 229940088679 drug related substance Drugs 0.000 description 3
- 235000013601 eggs Nutrition 0.000 description 3
- 239000007920 enema Substances 0.000 description 3
- 229940095399 enema Drugs 0.000 description 3
- 229960004671 enzalutamide Drugs 0.000 description 3
- 150000002148 esters Chemical class 0.000 description 3
- LIQODXNTTZAGID-OCBXBXKTSA-N etoposide phosphate Chemical compound COC1=C(OP(O)(O)=O)C(OC)=CC([C@@H]2C3=CC=4OCOC=4C=C3[C@@H](O[C@H]3[C@@H]([C@@H](O)[C@@H]4O[C@H](C)OC[C@H]4O3)O)[C@@H]3[C@@H]2C(OC3)=O)=C1 LIQODXNTTZAGID-OCBXBXKTSA-N 0.000 description 3
- 229960000752 etoposide phosphate Drugs 0.000 description 3
- 230000007717 exclusion Effects 0.000 description 3
- 229960000255 exemestane Drugs 0.000 description 3
- 150000004665 fatty acids Chemical class 0.000 description 3
- ODKNJVUHOIMIIZ-RRKCRQDMSA-N floxuridine Chemical compound C1[C@H](O)[C@@H](CO)O[C@H]1N1C(=O)NC(=O)C(F)=C1 ODKNJVUHOIMIIZ-RRKCRQDMSA-N 0.000 description 3
- GIUYCYHIANZCFB-FJFJXFQQSA-N fludarabine phosphate Chemical compound C1=NC=2C(N)=NC(F)=NC=2N1[C@@H]1O[C@H](COP(O)(O)=O)[C@@H](O)[C@@H]1O GIUYCYHIANZCFB-FJFJXFQQSA-N 0.000 description 3
- MKXKFYHWDHIYRV-UHFFFAOYSA-N flutamide Chemical compound CC(C)C(=O)NC1=CC=C([N+]([O-])=O)C(C(F)(F)F)=C1 MKXKFYHWDHIYRV-UHFFFAOYSA-N 0.000 description 3
- 229960002074 flutamide Drugs 0.000 description 3
- FTSSQIKWUOOEGC-RULYVFMPSA-N fructooligosaccharide Chemical compound OC[C@H]1O[C@@](CO)(OC[C@@]2(OC[C@@]3(OC[C@@]4(OC[C@@]5(OC[C@@]6(OC[C@@]7(OC[C@@]8(OC[C@@]9(OC[C@@]%10(OC[C@@]%11(O[C@H]%12O[C@H](CO)[C@@H](O)[C@H](O)[C@H]%12O)O[C@H](CO)[C@@H](O)[C@@H]%11O)O[C@H](CO)[C@@H](O)[C@@H]%10O)O[C@H](CO)[C@@H](O)[C@@H]9O)O[C@H](CO)[C@@H](O)[C@@H]8O)O[C@H](CO)[C@@H](O)[C@@H]7O)O[C@H](CO)[C@@H](O)[C@@H]6O)O[C@H](CO)[C@@H](O)[C@@H]5O)O[C@H](CO)[C@@H](O)[C@@H]4O)O[C@H](CO)[C@@H](O)[C@@H]3O)O[C@H](CO)[C@@H](O)[C@@H]2O)[C@@H](O)[C@@H]1O FTSSQIKWUOOEGC-RULYVFMPSA-N 0.000 description 3
- 229940107187 fructooligosaccharide Drugs 0.000 description 3
- 229960002584 gefitinib Drugs 0.000 description 3
- 239000008273 gelatin Substances 0.000 description 3
- 229920000159 gelatin Polymers 0.000 description 3
- 235000019322 gelatine Nutrition 0.000 description 3
- 235000011852 gelatine desserts Nutrition 0.000 description 3
- 208000005017 glioblastoma Diseases 0.000 description 3
- 208000005252 hepatitis A Diseases 0.000 description 3
- 125000004404 heteroalkyl group Chemical group 0.000 description 3
- 229940088013 hycamtin Drugs 0.000 description 3
- 235000010979 hydroxypropyl methyl cellulose Nutrition 0.000 description 3
- 239000001866 hydroxypropyl methyl cellulose Substances 0.000 description 3
- YLMAHDNUQAMNNX-UHFFFAOYSA-N imatinib methanesulfonate Chemical compound CS(O)(=O)=O.C1CN(C)CCN1CC1=CC=C(C(=O)NC=2C=C(NC=3N=C(C=CN=3)C=3C=NC=CC=3)C(C)=CC=2)C=C1 YLMAHDNUQAMNNX-UHFFFAOYSA-N 0.000 description 3
- DOUYETYNHWVLEO-UHFFFAOYSA-N imiquimod Chemical compound C1=CC=CC2=C3N(CC(C)C)C=NC3=C(N)N=C21 DOUYETYNHWVLEO-UHFFFAOYSA-N 0.000 description 3
- 230000028993 immune response Effects 0.000 description 3
- 239000012274 immune-checkpoint protein inhibitor Substances 0.000 description 3
- 238000003018 immunoassay Methods 0.000 description 3
- 230000004957 immunoregulator effect Effects 0.000 description 3
- 238000000338 in vitro Methods 0.000 description 3
- 239000012678 infectious agent Substances 0.000 description 3
- 230000002458 infectious effect Effects 0.000 description 3
- 238000001802 infusion Methods 0.000 description 3
- 239000004615 ingredient Substances 0.000 description 3
- 230000005764 inhibitory process Effects 0.000 description 3
- 238000011221 initial treatment Methods 0.000 description 3
- 229940076144 interleukin-10 Drugs 0.000 description 3
- 238000001990 intravenous administration Methods 0.000 description 3
- GURKHSYORGJETM-WAQYZQTGSA-N irinotecan hydrochloride (anhydrous) Chemical compound Cl.C1=C2C(CC)=C3CN(C(C4=C([C@@](C(=O)OC4)(O)CC)C=4)=O)C=4C3=NC2=CC=C1OC(=O)N(CC1)CCC1N1CCCCC1 GURKHSYORGJETM-WAQYZQTGSA-N 0.000 description 3
- FABUFPQFXZVHFB-PVYNADRNSA-N ixabepilone Chemical compound C/C([C@@H]1C[C@@H]2O[C@]2(C)CCC[C@@H]([C@@H]([C@@H](C)C(=O)C(C)(C)[C@@H](O)CC(=O)N1)O)C)=C\C1=CSC(C)=N1 FABUFPQFXZVHFB-PVYNADRNSA-N 0.000 description 3
- 229940039696 lactobacillus Drugs 0.000 description 3
- 229960004942 lenalidomide Drugs 0.000 description 3
- GOTYRUGSSMKFNF-UHFFFAOYSA-N lenalidomide Chemical compound C1C=2C(N)=CC=CC=2C(=O)N1C1CCC(=O)NC1=O GOTYRUGSSMKFNF-UHFFFAOYSA-N 0.000 description 3
- 229960003881 letrozole Drugs 0.000 description 3
- HPJKCIUCZWXJDR-UHFFFAOYSA-N letrozole Chemical compound C1=CC(C#N)=CC=C1C(N1N=CN=C1)C1=CC=C(C#N)C=C1 HPJKCIUCZWXJDR-UHFFFAOYSA-N 0.000 description 3
- 210000000265 leukocyte Anatomy 0.000 description 3
- GFIJNRVAKGFPGQ-LIJARHBVSA-N leuprolide Chemical compound CCNC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@H](CC=1N=CNC=1)NC(=O)[C@H]1NC(=O)CC1)CC1=CC=C(O)C=C1 GFIJNRVAKGFPGQ-LIJARHBVSA-N 0.000 description 3
- RGLRXNKKBLIBQS-XNHQSDQCSA-N leuprolide acetate Chemical compound CC(O)=O.CCNC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@H](CC=1N=CNC=1)NC(=O)[C@H]1NC(=O)CC1)CC1=CC=C(O)C=C1 RGLRXNKKBLIBQS-XNHQSDQCSA-N 0.000 description 3
- 239000007788 liquid Substances 0.000 description 3
- 230000033001 locomotion Effects 0.000 description 3
- 229960002247 lomustine Drugs 0.000 description 3
- 208000019017 loss of appetite Diseases 0.000 description 3
- 235000021266 loss of appetite Nutrition 0.000 description 3
- 208000004341 lymphocytic colitis Diseases 0.000 description 3
- 229960001924 melphalan Drugs 0.000 description 3
- HEBKCHPVOIAQTA-UHFFFAOYSA-N meso ribitol Natural products OCC(O)C(O)C(O)CO HEBKCHPVOIAQTA-UHFFFAOYSA-N 0.000 description 3
- 230000002503 metabolic effect Effects 0.000 description 3
- 208000037819 metastatic cancer Diseases 0.000 description 3
- 208000011575 metastatic malignant neoplasm Diseases 0.000 description 3
- 229920003145 methacrylic acid copolymer Polymers 0.000 description 3
- OSWPMRLSEDHDFF-UHFFFAOYSA-N methyl salicylate Chemical compound COC(=O)C1=CC=CC=C1O OSWPMRLSEDHDFF-UHFFFAOYSA-N 0.000 description 3
- 229960003085 meticillin Drugs 0.000 description 3
- 229960004857 mitomycin Drugs 0.000 description 3
- 229950008835 neratinib Drugs 0.000 description 3
- 201000001119 neuropathy Diseases 0.000 description 3
- 230000007823 neuropathy Effects 0.000 description 3
- 229960001346 nilotinib Drugs 0.000 description 3
- 229960002653 nilutamide Drugs 0.000 description 3
- 229940021182 non-steroidal anti-inflammatory drug Drugs 0.000 description 3
- 231100000252 nontoxic Toxicity 0.000 description 3
- 230000003000 nontoxic effect Effects 0.000 description 3
- 239000003921 oil Substances 0.000 description 3
- 230000003204 osmotic effect Effects 0.000 description 3
- 229960001756 oxaliplatin Drugs 0.000 description 3
- 239000001301 oxygen Substances 0.000 description 3
- 229910052760 oxygen Inorganic materials 0.000 description 3
- OLDRWYVIKMSFFB-SSPJITILSA-N palonosetron hydrochloride Chemical compound Cl.C1N(CC2)CCC2[C@@H]1N1C(=O)C(C=CC=C2CCC3)=C2[C@H]3C1 OLDRWYVIKMSFFB-SSPJITILSA-N 0.000 description 3
- WRUUGTRCQOWXEG-UHFFFAOYSA-N pamidronate Chemical compound NCCC(O)(P(O)(O)=O)P(O)(O)=O WRUUGTRCQOWXEG-UHFFFAOYSA-N 0.000 description 3
- 229960005184 panobinostat Drugs 0.000 description 3
- FPOHNWQLNRZRFC-ZHACJKMWSA-N panobinostat Chemical compound CC=1NC2=CC=CC=C2C=1CCNCC1=CC=C(\C=C\C(=O)NO)C=C1 FPOHNWQLNRZRFC-ZHACJKMWSA-N 0.000 description 3
- 230000037361 pathway Effects 0.000 description 3
- 230000002085 persistent effect Effects 0.000 description 3
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 3
- 239000010452 phosphate Substances 0.000 description 3
- 239000004014 plasticizer Substances 0.000 description 3
- 229920003229 poly(methyl methacrylate) Polymers 0.000 description 3
- 229920000058 polyacrylate Polymers 0.000 description 3
- 229920001610 polycaprolactone Polymers 0.000 description 3
- 239000004632 polycaprolactone Substances 0.000 description 3
- 229920000193 polymethacrylate Polymers 0.000 description 3
- 229920002451 polyvinyl alcohol Polymers 0.000 description 3
- PHXJVRSECIGDHY-UHFFFAOYSA-N ponatinib Chemical compound C1CN(C)CCN1CC(C(=C1)C(F)(F)F)=CC=C1NC(=O)C1=CC=C(C)C(C#CC=2N3N=CC=CC3=NC=2)=C1 PHXJVRSECIGDHY-UHFFFAOYSA-N 0.000 description 3
- 239000000186 progesterone Substances 0.000 description 3
- 229960003387 progesterone Drugs 0.000 description 3
- 238000000746 purification Methods 0.000 description 3
- 230000006798 recombination Effects 0.000 description 3
- 238000005215 recombination Methods 0.000 description 3
- 230000008439 repair process Effects 0.000 description 3
- 150000003839 salts Chemical class 0.000 description 3
- 210000004911 serous fluid Anatomy 0.000 description 3
- 210000002966 serum Anatomy 0.000 description 3
- 229960005325 sonidegib Drugs 0.000 description 3
- 235000010356 sorbitol Nutrition 0.000 description 3
- 239000000600 sorbitol Substances 0.000 description 3
- 238000013268 sustained release Methods 0.000 description 3
- 239000012730 sustained-release form Substances 0.000 description 3
- 229940034785 sutent Drugs 0.000 description 3
- 229940069905 tasigna Drugs 0.000 description 3
- 229960003087 tioguanine Drugs 0.000 description 3
- MNRILEROXIRVNJ-UHFFFAOYSA-N tioguanine Chemical compound N1C(N)=NC(=S)C2=NC=N[C]21 MNRILEROXIRVNJ-UHFFFAOYSA-N 0.000 description 3
- PKVRCIRHQMSYJX-AIFWHQITSA-N trabectedin Chemical compound C([C@@]1(C(OC2)=O)NCCC3=C1C=C(C(=C3)O)OC)S[C@@H]1C3=C(OC(C)=O)C(C)=C4OCOC4=C3[C@H]2N2[C@@H](O)[C@H](CC=3C4=C(O)C(OC)=C(C)C=3)N(C)[C@H]4[C@@H]21 PKVRCIRHQMSYJX-AIFWHQITSA-N 0.000 description 3
- 229960004066 trametinib Drugs 0.000 description 3
- 229960003962 trifluridine Drugs 0.000 description 3
- VSQQQLOSPVPRAZ-RRKCRQDMSA-N trifluridine Chemical compound C1[C@H](O)[C@@H](CO)O[C@H]1N1C(=O)NC(=O)C(C(F)(F)F)=C1 VSQQQLOSPVPRAZ-RRKCRQDMSA-N 0.000 description 3
- 241000701161 unidentified adenovirus Species 0.000 description 3
- NQPDZGIKBAWPEJ-UHFFFAOYSA-N valeric acid Chemical compound CCCCC(O)=O NQPDZGIKBAWPEJ-UHFFFAOYSA-N 0.000 description 3
- 229960000241 vandetanib Drugs 0.000 description 3
- OGWKCGZFUXNPDA-XQKSVPLYSA-N vincristine Chemical compound C([N@]1C[C@@H](C[C@]2(C(=O)OC)C=3C(=CC4=C([C@]56[C@H]([C@@]([C@H](OC(C)=O)[C@]7(CC)C=CCN([C@H]67)CC5)(O)C(=O)OC)N4C=O)C=3)OC)C[C@@](C1)(O)CC)CC1=C2NC2=CC=CC=C12 OGWKCGZFUXNPDA-XQKSVPLYSA-N 0.000 description 3
- 229920002554 vinyl polymer Polymers 0.000 description 3
- 239000001993 wax Substances 0.000 description 3
- 208000016261 weight loss Diseases 0.000 description 3
- 230000004580 weight loss Effects 0.000 description 3
- 229960004276 zoledronic acid Drugs 0.000 description 3
- YBJHBAHKTGYVGT-ZKWXMUAHSA-N (+)-Biotin Chemical compound N1C(=O)N[C@@H]2[C@H](CCCCC(=O)O)SC[C@@H]21 YBJHBAHKTGYVGT-ZKWXMUAHSA-N 0.000 description 2
- BMKDZUISNHGIBY-ZETCQYMHSA-N (+)-dexrazoxane Chemical compound C([C@H](C)N1CC(=O)NC(=O)C1)N1CC(=O)NC(=O)C1 BMKDZUISNHGIBY-ZETCQYMHSA-N 0.000 description 2
- RWRDJVNMSZYMDV-SIUYXFDKSA-L (223)RaCl2 Chemical compound Cl[223Ra]Cl RWRDJVNMSZYMDV-SIUYXFDKSA-L 0.000 description 2
- QBADKJRRVGKRHP-JLXQGRKUSA-N (3as)-2-[(3s)-1-azabicyclo[2.2.2]octan-3-yl]-3a,4,5,6-tetrahydro-3h-benzo[de]isoquinolin-1-one;2-[3,5-bis(trifluoromethyl)phenyl]-n,2-dimethyl-n-[6-(4-methylpiperazin-1-yl)-4-[(3z)-penta-1,3-dien-3-yl]pyridin-3-yl]propanamide Chemical compound C1N(CC2)CCC2[C@@H]1N1C(=O)C(C=CC=C2CCC3)=C2[C@H]3C1.C\C=C(\C=C)C1=CC(N2CCN(C)CC2)=NC=C1N(C)C(=O)C(C)(C)C1=CC(C(F)(F)F)=CC(C(F)(F)F)=C1 QBADKJRRVGKRHP-JLXQGRKUSA-N 0.000 description 2
- IFGIYSGOEZJNBE-LHJYHSJWSA-N (3s,4r,4as,7ar,12bs)-3-(cyclopropylmethyl)-4a,9-dihydroxy-3-methyl-2,4,5,6,7a,13-hexahydro-1h-4,12-methanobenzofuro[3,2-e]isoquinoline-3-ium-7-one;bromide Chemical compound [Br-].C([N@@+]1(C)[C@@H]2CC=3C4=C(C(=CC=3)O)O[C@@H]3[C@]4([C@@]2(O)CCC3=O)CC1)C1CC1 IFGIYSGOEZJNBE-LHJYHSJWSA-N 0.000 description 2
- PUDHBTGHUJUUFI-SCTWWAJVSA-N (4r,7s,10s,13r,16s,19r)-10-(4-aminobutyl)-n-[(2s,3r)-1-amino-3-hydroxy-1-oxobutan-2-yl]-19-[[(2r)-2-amino-3-naphthalen-2-ylpropanoyl]amino]-16-[(4-hydroxyphenyl)methyl]-13-(1h-indol-3-ylmethyl)-6,9,12,15,18-pentaoxo-7-propan-2-yl-1,2-dithia-5,8,11,14,17-p Chemical compound C([C@H]1C(=O)N[C@H](CC=2C3=CC=CC=C3NC=2)C(=O)N[C@@H](CCCCN)C(=O)N[C@H](C(N[C@@H](CSSC[C@@H](C(=O)N1)NC(=O)[C@H](N)CC=1C=C2C=CC=CC2=CC=1)C(=O)N[C@@H]([C@@H](C)O)C(N)=O)=O)C(C)C)C1=CC=C(O)C=C1 PUDHBTGHUJUUFI-SCTWWAJVSA-N 0.000 description 2
- UWYZHKAOTLEWKK-UHFFFAOYSA-N 1,2,3,4-tetrahydroisoquinoline Chemical compound C1=CC=C2CNCCC2=C1 UWYZHKAOTLEWKK-UHFFFAOYSA-N 0.000 description 2
- HJTAZXHBEBIQQX-UHFFFAOYSA-N 1,5-bis(chloromethyl)naphthalene Chemical compound C1=CC=C2C(CCl)=CC=CC2=C1CCl HJTAZXHBEBIQQX-UHFFFAOYSA-N 0.000 description 2
- HZAXFHJVJLSVMW-UHFFFAOYSA-N 2-Aminoethan-1-ol Chemical compound NCCO HZAXFHJVJLSVMW-UHFFFAOYSA-N 0.000 description 2
- SMNDYUVBFMFKNZ-UHFFFAOYSA-N 2-furoic acid Chemical compound OC(=O)C1=CC=CO1 SMNDYUVBFMFKNZ-UHFFFAOYSA-N 0.000 description 2
- LVYLCBNXHHHPSB-UHFFFAOYSA-N 2-hydroxyethyl salicylate Chemical compound OCCOC(=O)C1=CC=CC=C1O LVYLCBNXHHHPSB-UHFFFAOYSA-N 0.000 description 2
- MEAPRSDUXBHXGD-UHFFFAOYSA-N 3-chloro-n-(4-propan-2-ylphenyl)propanamide Chemical compound CC(C)C1=CC=C(NC(=O)CCCl)C=C1 MEAPRSDUXBHXGD-UHFFFAOYSA-N 0.000 description 2
- XAUDJQYHKZQPEU-KVQBGUIXSA-N 5-aza-2'-deoxycytidine Chemical compound O=C1N=C(N)N=CN1[C@@H]1O[C@H](CO)[C@@H](O)C1 XAUDJQYHKZQPEU-KVQBGUIXSA-N 0.000 description 2
- ZGXJTSGNIOSYLO-UHFFFAOYSA-N 88755TAZ87 Chemical compound NCC(=O)CCC(O)=O ZGXJTSGNIOSYLO-UHFFFAOYSA-N 0.000 description 2
- 208000032194 Acute haemorrhagic leukoencephalitis Diseases 0.000 description 2
- 208000026872 Addison Disease Diseases 0.000 description 2
- 239000005995 Aluminium silicate Substances 0.000 description 2
- 206010001935 American trypanosomiasis Diseases 0.000 description 2
- 229920000856 Amylose Polymers 0.000 description 2
- 208000028185 Angioedema Diseases 0.000 description 2
- 206010002556 Ankylosing Spondylitis Diseases 0.000 description 2
- 206010003011 Appendicitis Diseases 0.000 description 2
- 206010003267 Arthritis reactive Diseases 0.000 description 2
- CIWBSHSKHKDKBQ-JLAZNSOCSA-N Ascorbic acid Chemical compound OC[C@H](O)[C@H]1OC(=O)C(O)=C1O CIWBSHSKHKDKBQ-JLAZNSOCSA-N 0.000 description 2
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 2
- 208000032116 Autoimmune Experimental Encephalomyelitis Diseases 0.000 description 2
- 206010071576 Autoimmune aplastic anaemia Diseases 0.000 description 2
- 206010003827 Autoimmune hepatitis Diseases 0.000 description 2
- 206010064539 Autoimmune myocarditis Diseases 0.000 description 2
- 206010069002 Autoimmune pancreatitis Diseases 0.000 description 2
- 208000022106 Autoimmune polyendocrinopathy type 2 Diseases 0.000 description 2
- 206010003840 Autonomic nervous system imbalance Diseases 0.000 description 2
- 102000006942 B-Cell Maturation Antigen Human genes 0.000 description 2
- 108010008014 B-Cell Maturation Antigen Proteins 0.000 description 2
- 102100024222 B-lymphocyte antigen CD19 Human genes 0.000 description 2
- 241000606125 Bacteroides Species 0.000 description 2
- 208000023328 Basedow disease Diseases 0.000 description 2
- KUVIULQEHSCUHY-XYWKZLDCSA-N Beclometasone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(Cl)[C@@H]1[C@@H]1C[C@H](C)[C@@](C(=O)COC(=O)CC)(OC(=O)CC)[C@@]1(C)C[C@@H]2O KUVIULQEHSCUHY-XYWKZLDCSA-N 0.000 description 2
- 208000008439 Biliary Liver Cirrhosis Diseases 0.000 description 2
- 208000033222 Biliary cirrhosis primary Diseases 0.000 description 2
- 108010006654 Bleomycin Proteins 0.000 description 2
- 206010006187 Breast cancer Diseases 0.000 description 2
- 208000026310 Breast neoplasm Diseases 0.000 description 2
- COVZYZSDYWQREU-UHFFFAOYSA-N Busulfan Chemical compound CS(=O)(=O)OCCCCOS(C)(=O)=O COVZYZSDYWQREU-UHFFFAOYSA-N 0.000 description 2
- QWOJMRHUQHTCJG-UHFFFAOYSA-N CC([CH2-])=O Chemical compound CC([CH2-])=O QWOJMRHUQHTCJG-UHFFFAOYSA-N 0.000 description 2
- 108010021064 CTLA-4 Antigen Proteins 0.000 description 2
- 229940045513 CTLA4 antagonist Drugs 0.000 description 2
- 241000282472 Canis lupus familiaris Species 0.000 description 2
- 208000031229 Cardiomyopathies Diseases 0.000 description 2
- 208000005024 Castleman disease Diseases 0.000 description 2
- 229930186147 Cephalosporin Natural products 0.000 description 2
- 208000024699 Chagas disease Diseases 0.000 description 2
- 229920002101 Chitin Polymers 0.000 description 2
- VEXZGXHMUGYJMC-UHFFFAOYSA-M Chloride anion Chemical compound [Cl-] VEXZGXHMUGYJMC-UHFFFAOYSA-M 0.000 description 2
- 206010008609 Cholangitis sclerosing Diseases 0.000 description 2
- 206010008631 Cholera Diseases 0.000 description 2
- 208000017667 Chronic Disease Diseases 0.000 description 2
- 206010057645 Chronic Inflammatory Demyelinating Polyradiculoneuropathy Diseases 0.000 description 2
- 108700022831 Clostridium difficile toxB Proteins 0.000 description 2
- 208000010007 Cogan syndrome Diseases 0.000 description 2
- 208000011038 Cold agglutinin disease Diseases 0.000 description 2
- 206010009868 Cold type haemolytic anaemia Diseases 0.000 description 2
- 208000013586 Complex regional pain syndrome type 1 Diseases 0.000 description 2
- 206010011258 Coxsackie myocarditis Diseases 0.000 description 2
- 102100039498 Cytotoxic T-lymphocyte protein 4 Human genes 0.000 description 2
- HEBKCHPVOIAQTA-QWWZWVQMSA-N D-arabinitol Chemical compound OC[C@@H](O)C(O)[C@H](O)CO HEBKCHPVOIAQTA-QWWZWVQMSA-N 0.000 description 2
- 208000034423 Delivery Diseases 0.000 description 2
- 201000004624 Dermatitis Diseases 0.000 description 2
- 206010012468 Dermatitis herpetiformis Diseases 0.000 description 2
- 206010048768 Dermatosis Diseases 0.000 description 2
- 206010012741 Diarrhoea haemorrhagic Diseases 0.000 description 2
- 208000021866 Dressler syndrome Diseases 0.000 description 2
- 206010014733 Endometrial cancer Diseases 0.000 description 2
- 206010014759 Endometrial neoplasm Diseases 0.000 description 2
- 201000009273 Endometriosis Diseases 0.000 description 2
- 241000588914 Enterobacter Species 0.000 description 2
- 206010014954 Eosinophilic fasciitis Diseases 0.000 description 2
- 206010064212 Eosinophilic oesophagitis Diseases 0.000 description 2
- 241000588698 Erwinia Species 0.000 description 2
- 206010015226 Erythema nodosum Diseases 0.000 description 2
- 239000004386 Erythritol Substances 0.000 description 2
- UNXHWFMMPAWVPI-UHFFFAOYSA-N Erythritol Natural products OCC(O)C(O)CO UNXHWFMMPAWVPI-UHFFFAOYSA-N 0.000 description 2
- 239000001856 Ethyl cellulose Substances 0.000 description 2
- ZZSNKZQZMQGXPY-UHFFFAOYSA-N Ethyl cellulose Chemical compound CCOCC1OC(OC)C(OCC)C(OCC)C1OC1C(O)C(O)C(OC)C(CO)O1 ZZSNKZQZMQGXPY-UHFFFAOYSA-N 0.000 description 2
- 208000004332 Evans syndrome Diseases 0.000 description 2
- 108010040721 Flagellin Proteins 0.000 description 2
- 102000010451 Folate receptor alpha Human genes 0.000 description 2
- 108050001931 Folate receptor alpha Proteins 0.000 description 2
- 206010016952 Food poisoning Diseases 0.000 description 2
- 208000019331 Foodborne disease Diseases 0.000 description 2
- VWUXBMIQPBEWFH-WCCTWKNTSA-N Fulvestrant Chemical compound OC1=CC=C2[C@H]3CC[C@](C)([C@H](CC4)O)[C@@H]4[C@@H]3[C@H](CCCCCCCCCS(=O)CCCC(F)(F)C(F)(F)F)CC2=C1 VWUXBMIQPBEWFH-WCCTWKNTSA-N 0.000 description 2
- 102100040578 G antigen 7 Human genes 0.000 description 2
- 208000018522 Gastrointestinal disease Diseases 0.000 description 2
- 241000206672 Gelidium Species 0.000 description 2
- 241000224467 Giardia intestinalis Species 0.000 description 2
- 101000930822 Giardia intestinalis Dipeptidyl-peptidase 4 Proteins 0.000 description 2
- 206010018364 Glomerulonephritis Diseases 0.000 description 2
- 102100041003 Glutamate carboxypeptidase 2 Human genes 0.000 description 2
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 2
- 208000024869 Goodpasture syndrome Diseases 0.000 description 2
- 208000015023 Graves' disease Diseases 0.000 description 2
- 229920002907 Guar gum Polymers 0.000 description 2
- 208000035895 Guillain-Barré syndrome Diseases 0.000 description 2
- 241000588731 Hafnia Species 0.000 description 2
- 208000030836 Hashimoto thyroiditis Diseases 0.000 description 2
- 206010019263 Heart block congenital Diseases 0.000 description 2
- 201000004331 Henoch-Schoenlein purpura Diseases 0.000 description 2
- 206010019617 Henoch-Schonlein purpura Diseases 0.000 description 2
- 206010019939 Herpes gestationis Diseases 0.000 description 2
- 101000980825 Homo sapiens B-lymphocyte antigen CD19 Proteins 0.000 description 2
- 101001048718 Homo sapiens Elafin Proteins 0.000 description 2
- 101000893968 Homo sapiens G antigen 7 Proteins 0.000 description 2
- 101000892862 Homo sapiens Glutamate carboxypeptidase 2 Proteins 0.000 description 2
- 101000874179 Homo sapiens Syndecan-1 Proteins 0.000 description 2
- 101000669460 Homo sapiens Toll-like receptor 5 Proteins 0.000 description 2
- 101000863873 Homo sapiens Tyrosine-protein phosphatase non-receptor type substrate 1 Proteins 0.000 description 2
- 240000005979 Hordeum vulgare Species 0.000 description 2
- 235000007340 Hordeum vulgare Nutrition 0.000 description 2
- 101000954493 Human papillomavirus type 16 Protein E6 Proteins 0.000 description 2
- 229920002153 Hydroxypropyl cellulose Polymers 0.000 description 2
- XDXDZDZNSLXDNA-UHFFFAOYSA-N Idarubicin Natural products C1C(N)C(O)C(C)OC1OC1C2=C(O)C(C(=O)C3=CC=CC=C3C3=O)=C3C(O)=C2CC(O)(C(C)=O)C1 XDXDZDZNSLXDNA-UHFFFAOYSA-N 0.000 description 2
- 208000031814 IgA Vasculitis Diseases 0.000 description 2
- 208000010159 IgA glomerulonephritis Diseases 0.000 description 2
- 206010021263 IgA nephropathy Diseases 0.000 description 2
- 208000014919 IgG4-related retroperitoneal fibrosis Diseases 0.000 description 2
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 2
- 208000029462 Immunodeficiency disease Diseases 0.000 description 2
- 108010065805 Interleukin-12 Proteins 0.000 description 2
- 102000013462 Interleukin-12 Human genes 0.000 description 2
- 102000004388 Interleukin-4 Human genes 0.000 description 2
- 108090000978 Interleukin-4 Proteins 0.000 description 2
- 206010022557 Intermediate uveitis Diseases 0.000 description 2
- 208000005615 Interstitial Cystitis Diseases 0.000 description 2
- 239000005517 L01XE01 - Imatinib Substances 0.000 description 2
- 239000005551 L01XE03 - Erlotinib Substances 0.000 description 2
- 239000002136 L01XE07 - Lapatinib Substances 0.000 description 2
- 239000003798 L01XE11 - Pazopanib Substances 0.000 description 2
- 244000199866 Lactobacillus casei Species 0.000 description 2
- 235000013958 Lactobacillus casei Nutrition 0.000 description 2
- 201000010743 Lambert-Eaton myasthenic syndrome Diseases 0.000 description 2
- 208000032514 Leukocytoclastic vasculitis Diseases 0.000 description 2
- CSNNHWWHGAXBCP-UHFFFAOYSA-L Magnesium sulfate Chemical compound [Mg+2].[O-][S+2]([O-])([O-])[O-] CSNNHWWHGAXBCP-UHFFFAOYSA-L 0.000 description 2
- 102100028389 Melanoma antigen recognized by T-cells 1 Human genes 0.000 description 2
- 208000027530 Meniere disease Diseases 0.000 description 2
- 229920000168 Microcrystalline cellulose Polymers 0.000 description 2
- 206010049567 Miller Fisher syndrome Diseases 0.000 description 2
- 208000012192 Mucous membrane pemphigoid Diseases 0.000 description 2
- 101100481579 Mus musculus Tlr11 gene Proteins 0.000 description 2
- 101100481580 Mus musculus Tlr12 gene Proteins 0.000 description 2
- 208000000112 Myalgia Diseases 0.000 description 2
- 208000009525 Myocarditis Diseases 0.000 description 2
- 201000002481 Myositis Diseases 0.000 description 2
- LKJPYSCBVHEWIU-UHFFFAOYSA-N N-[4-cyano-3-(trifluoromethyl)phenyl]-3-[(4-fluorophenyl)sulfonyl]-2-hydroxy-2-methylpropanamide Chemical compound C=1C=C(C#N)C(C(F)(F)F)=CC=1NC(=O)C(O)(C)CS(=O)(=O)C1=CC=C(F)C=C1 LKJPYSCBVHEWIU-UHFFFAOYSA-N 0.000 description 2
- 206010028813 Nausea Diseases 0.000 description 2
- 206010071579 Neuronal neuropathy Diseases 0.000 description 2
- 102000008299 Nitric Oxide Synthase Human genes 0.000 description 2
- 108010021487 Nitric Oxide Synthase Proteins 0.000 description 2
- 206010029803 Nosocomial infection Diseases 0.000 description 2
- 206010030113 Oedema Diseases 0.000 description 2
- 206010053869 POEMS syndrome Diseases 0.000 description 2
- KDLHZDBZIXYQEI-UHFFFAOYSA-N Palladium Chemical compound [Pd] KDLHZDBZIXYQEI-UHFFFAOYSA-N 0.000 description 2
- 208000004788 Pars Planitis Diseases 0.000 description 2
- 208000008223 Pemphigoid Gestationis Diseases 0.000 description 2
- 241000721454 Pemphigus Species 0.000 description 2
- 229930182555 Penicillin Natural products 0.000 description 2
- JGSARLDLIJGVTE-MBNYWOFBSA-N Penicillin G Chemical compound N([C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C(=O)CC1=CC=CC=C1 JGSARLDLIJGVTE-MBNYWOFBSA-N 0.000 description 2
- 208000031845 Pernicious anaemia Diseases 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 description 2
- NQRYJNQNLNOLGT-UHFFFAOYSA-N Piperidine Chemical compound C1CCNCC1 NQRYJNQNLNOLGT-UHFFFAOYSA-N 0.000 description 2
- 208000000766 Pityriasis Lichenoides Diseases 0.000 description 2
- 206010048895 Pityriasis lichenoides et varioliformis acuta Diseases 0.000 description 2
- 229920002732 Polyanhydride Polymers 0.000 description 2
- 206010065159 Polychondritis Diseases 0.000 description 2
- DLRVVLDZNNYCBX-UHFFFAOYSA-N Polydextrose Polymers OC1C(O)C(O)C(CO)OC1OCC1C(O)C(O)C(O)C(O)O1 DLRVVLDZNNYCBX-UHFFFAOYSA-N 0.000 description 2
- 229920000331 Polyhydroxybutyrate Polymers 0.000 description 2
- 208000031732 Post-Lyme Disease Syndrome Diseases 0.000 description 2
- 208000004347 Postpericardiotomy Syndrome Diseases 0.000 description 2
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 2
- 208000012654 Primary biliary cholangitis Diseases 0.000 description 2
- 101710089372 Programmed cell death protein 1 Proteins 0.000 description 2
- 102100040678 Programmed cell death protein 1 Human genes 0.000 description 2
- 208000037534 Progressive hemifacial atrophy Diseases 0.000 description 2
- XBDQKXXYIPTUBI-UHFFFAOYSA-M Propionate Chemical compound CCC([O-])=O XBDQKXXYIPTUBI-UHFFFAOYSA-M 0.000 description 2
- 101710120463 Prostate stem cell antigen Proteins 0.000 description 2
- 102100036735 Prostate stem cell antigen Human genes 0.000 description 2
- 102000007066 Prostate-Specific Antigen Human genes 0.000 description 2
- 108010072866 Prostate-Specific Antigen Proteins 0.000 description 2
- 208000003100 Pseudomembranous Enterocolitis Diseases 0.000 description 2
- 206010037128 Pseudomembranous colitis Diseases 0.000 description 2
- 201000004681 Psoriasis Diseases 0.000 description 2
- 201000001263 Psoriatic Arthritis Diseases 0.000 description 2
- 208000036824 Psoriatic arthropathy Diseases 0.000 description 2
- 208000003670 Pure Red-Cell Aplasia Diseases 0.000 description 2
- 241000700159 Rattus Species 0.000 description 2
- 208000012322 Raynaud phenomenon Diseases 0.000 description 2
- 208000015634 Rectal Neoplasms Diseases 0.000 description 2
- 201000001947 Reflex Sympathetic Dystrophy Diseases 0.000 description 2
- 102100037886 Regenerating islet-derived protein 3-gamma Human genes 0.000 description 2
- 101710086144 Regenerating islet-derived protein 3-gamma Proteins 0.000 description 2
- 208000033464 Reiter syndrome Diseases 0.000 description 2
- 208000005793 Restless legs syndrome Diseases 0.000 description 2
- 206010038979 Retroperitoneal fibrosis Diseases 0.000 description 2
- 208000025747 Rheumatic disease Diseases 0.000 description 2
- 206010039491 Sarcoma Diseases 0.000 description 2
- 206010039705 Scleritis Diseases 0.000 description 2
- 208000034189 Sclerosis Diseases 0.000 description 2
- 206010049416 Short-bowel syndrome Diseases 0.000 description 2
- 208000021386 Sjogren Syndrome Diseases 0.000 description 2
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 2
- DBMJMQXJHONAFJ-UHFFFAOYSA-M Sodium laurylsulphate Chemical compound [Na+].CCCCCCCCCCCCOS([O-])(=O)=O DBMJMQXJHONAFJ-UHFFFAOYSA-M 0.000 description 2
- 229930182558 Sterol Natural products 0.000 description 2
- 208000005718 Stomach Neoplasms Diseases 0.000 description 2
- 241000194020 Streptococcus thermophilus Species 0.000 description 2
- 206010042276 Subacute endocarditis Diseases 0.000 description 2
- 102000019197 Superoxide Dismutase Human genes 0.000 description 2
- 108010012715 Superoxide dismutase Proteins 0.000 description 2
- 208000002286 Susac Syndrome Diseases 0.000 description 2
- 206010042742 Sympathetic ophthalmia Diseases 0.000 description 2
- 102100035721 Syndecan-1 Human genes 0.000 description 2
- 108010008038 Synthetic Vaccines Proteins 0.000 description 2
- 208000001106 Takayasu Arteritis Diseases 0.000 description 2
- CBPNZQVSJQDFBE-FUXHJELOSA-N Temsirolimus Chemical compound C1C[C@@H](OC(=O)C(C)(CO)CO)[C@H](OC)C[C@@H]1C[C@@H](C)[C@H]1OC(=O)[C@@H]2CCCCN2C(=O)C(=O)[C@](O)(O2)[C@H](C)CC[C@H]2C[C@H](OC)/C(C)=C/C=C/C=C/[C@@H](C)C[C@@H](C)C(=O)[C@H](OC)[C@H](O)/C(C)=C/[C@@H](C)C(=O)C1 CBPNZQVSJQDFBE-FUXHJELOSA-N 0.000 description 2
- 206010071574 Testicular autoimmunity Diseases 0.000 description 2
- 206010043561 Thrombocytopenic purpura Diseases 0.000 description 2
- 102100039357 Toll-like receptor 5 Human genes 0.000 description 2
- 206010051526 Tolosa-Hunt syndrome Diseases 0.000 description 2
- DOOTYTYQINUNNV-UHFFFAOYSA-N Triethyl citrate Chemical compound CCOC(=O)CC(O)(C(=O)OCC)CC(=O)OCC DOOTYTYQINUNNV-UHFFFAOYSA-N 0.000 description 2
- 241000223109 Trypanosoma cruzi Species 0.000 description 2
- QIVBCDIJIAJPQS-UHFFFAOYSA-N Tryptophan Natural products C1=CC=C2C(CC(N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-UHFFFAOYSA-N 0.000 description 2
- 208000026928 Turner syndrome Diseases 0.000 description 2
- 206010067584 Type 1 diabetes mellitus Diseases 0.000 description 2
- 108700036309 Type I Plasminogen Deficiency Proteins 0.000 description 2
- 102100029948 Tyrosine-protein phosphatase non-receptor type substrate 1 Human genes 0.000 description 2
- 206010046274 Upper gastrointestinal haemorrhage Diseases 0.000 description 2
- 208000024780 Urticaria Diseases 0.000 description 2
- 108010053099 Vascular Endothelial Growth Factor Receptor-2 Proteins 0.000 description 2
- 102100033177 Vascular endothelial growth factor receptor 2 Human genes 0.000 description 2
- 206010047115 Vasculitis Diseases 0.000 description 2
- 241000700605 Viruses Species 0.000 description 2
- 229930003316 Vitamin D Natural products 0.000 description 2
- QYSXJUFSXHHAJI-XFEUOLMDSA-N Vitamin D3 Natural products C1(/[C@@H]2CC[C@@H]([C@]2(CCC1)C)[C@H](C)CCCC(C)C)=C/C=C1\C[C@@H](O)CCC1=C QYSXJUFSXHHAJI-XFEUOLMDSA-N 0.000 description 2
- 206010047642 Vitiligo Diseases 0.000 description 2
- 206010047700 Vomiting Diseases 0.000 description 2
- OUUYBRCCFUEMLH-YDALLXLXSA-N [(1s)-2-[4-[bis(2-chloroethyl)amino]phenyl]-1-carboxyethyl]azanium;chloride Chemical compound Cl.OC(=O)[C@@H](N)CC1=CC=C(N(CCCl)CCCl)C=C1 OUUYBRCCFUEMLH-YDALLXLXSA-N 0.000 description 2
- QVXFGVVYTKZLJN-KHPPLWFESA-N [(z)-hexadec-7-enyl] acetate Chemical compound CCCCCCCC\C=C/CCCCCCOC(C)=O QVXFGVVYTKZLJN-KHPPLWFESA-N 0.000 description 2
- JNWFIPVDEINBAI-UHFFFAOYSA-N [5-hydroxy-4-[4-(1-methylindol-5-yl)-5-oxo-1H-1,2,4-triazol-3-yl]-2-propan-2-ylphenyl] dihydrogen phosphate Chemical compound C1=C(OP(O)(O)=O)C(C(C)C)=CC(C=2N(C(=O)NN=2)C=2C=C3C=CN(C)C3=CC=2)=C1O JNWFIPVDEINBAI-UHFFFAOYSA-N 0.000 description 2
- 238000010521 absorption reaction Methods 0.000 description 2
- 229950009821 acalabrutinib Drugs 0.000 description 2
- WDENQIQQYWYTPO-IBGZPJMESA-N acalabrutinib Chemical compound CC#CC(=O)N1CCC[C@H]1C1=NC(C=2C=CC(=CC=2)C(=O)NC=2N=CC=CC=2)=C2N1C=CN=C2N WDENQIQQYWYTPO-IBGZPJMESA-N 0.000 description 2
- RUGAHXUZHWYHNG-NLGNTGLNSA-N acetic acid;(4r,7s,10s,13r,16s,19r)-10-(4-aminobutyl)-n-[(2s,3r)-1-amino-3-hydroxy-1-oxobutan-2-yl]-19-[[(2r)-2-amino-3-naphthalen-2-ylpropanoyl]amino]-16-[(4-hydroxyphenyl)methyl]-13-(1h-indol-3-ylmethyl)-6,9,12,15,18-pentaoxo-7-propan-2-yl-1,2-dithia-5, Chemical compound CC(O)=O.CC(O)=O.CC(O)=O.CC(O)=O.CC(O)=O.C([C@H]1C(=O)N[C@H](CC=2C3=CC=CC=C3NC=2)C(=O)N[C@@H](CCCCN)C(=O)N[C@H](C(N[C@@H](CSSC[C@@H](C(=O)N1)NC(=O)[C@H](N)CC=1C=C2C=CC=CC2=CC=1)C(=O)N[C@@H]([C@@H](C)O)C(N)=O)=O)C(C)C)C1=CC=C(O)C=C1.C([C@H]1C(=O)N[C@H](CC=2C3=CC=CC=C3NC=2)C(=O)N[C@@H](CCCCN)C(=O)N[C@H](C(N[C@@H](CSSC[C@@H](C(=O)N1)NC(=O)[C@H](N)CC=1C=C2C=CC=CC2=CC=1)C(=O)N[C@@H]([C@@H](C)O)C(N)=O)=O)C(C)C)C1=CC=C(O)C=C1 RUGAHXUZHWYHNG-NLGNTGLNSA-N 0.000 description 2
- 108010052004 acetyl-2-naphthylalanyl-3-chlorophenylalanyl-1-oxohexadecyl-seryl-4-aminophenylalanyl(hydroorotyl)-4-aminophenylalanyl(carbamoyl)-leucyl-ILys-prolyl-alaninamide Proteins 0.000 description 2
- 230000009471 action Effects 0.000 description 2
- 230000004913 activation Effects 0.000 description 2
- 239000000443 aerosol Substances 0.000 description 2
- 229960001686 afatinib Drugs 0.000 description 2
- ULXXDDBFHOBEHA-CWDCEQMOSA-N afatinib Chemical compound N1=CN=C2C=C(O[C@@H]3COCC3)C(NC(=O)/C=C/CN(C)C)=CC2=C1NC1=CC=C(F)C(Cl)=C1 ULXXDDBFHOBEHA-CWDCEQMOSA-N 0.000 description 2
- 108010081667 aflibercept Proteins 0.000 description 2
- 229960005310 aldesleukin Drugs 0.000 description 2
- SHGAZHPCJJPHSC-YCNIQYBTSA-N all-trans-retinoic acid Chemical compound OC(=O)\C=C(/C)\C=C\C=C(/C)\C=C\C1=C(C)CCCC1(C)C SHGAZHPCJJPHSC-YCNIQYBTSA-N 0.000 description 2
- 208000004631 alopecia areata Diseases 0.000 description 2
- 229960000473 altretamine Drugs 0.000 description 2
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 2
- 229910052782 aluminium Inorganic materials 0.000 description 2
- 235000012211 aluminium silicate Nutrition 0.000 description 2
- 229940024606 amino acid Drugs 0.000 description 2
- 235000001014 amino acid Nutrition 0.000 description 2
- 150000001413 amino acids Chemical class 0.000 description 2
- 229960002749 aminolevulinic acid Drugs 0.000 description 2
- 206010002022 amyloidosis Diseases 0.000 description 2
- 208000007502 anemia Diseases 0.000 description 2
- 125000000129 anionic group Chemical group 0.000 description 2
- 239000000730 antalgic agent Substances 0.000 description 2
- 229940121363 anti-inflammatory agent Drugs 0.000 description 2
- 239000002260 anti-inflammatory agent Substances 0.000 description 2
- 230000000845 anti-microbial effect Effects 0.000 description 2
- 239000007798 antifreeze agent Substances 0.000 description 2
- 238000013459 approach Methods 0.000 description 2
- ATALOFNDEOCMKK-OITMNORJSA-N aprepitant Chemical compound O([C@@H]([C@@H]1C=2C=CC(F)=CC=2)O[C@H](C)C=2C=C(C=C(C=2)C(F)(F)F)C(F)(F)F)CCN1CC1=NNC(=O)N1 ATALOFNDEOCMKK-OITMNORJSA-N 0.000 description 2
- 229960001372 aprepitant Drugs 0.000 description 2
- 229940078010 arimidex Drugs 0.000 description 2
- 229940087620 aromasin Drugs 0.000 description 2
- GOLCXWYRSKYTSP-UHFFFAOYSA-N arsenic trioxide Inorganic materials O1[As]2O[As]1O2 GOLCXWYRSKYTSP-UHFFFAOYSA-N 0.000 description 2
- 201000000448 autoimmune hemolytic anemia Diseases 0.000 description 2
- 208000006424 autoimmune oophoritis Diseases 0.000 description 2
- 201000009780 autoimmune polyendocrine syndrome type 2 Diseases 0.000 description 2
- 206010071578 autoimmune retinopathy Diseases 0.000 description 2
- 208000010928 autoimmune thyroid disease Diseases 0.000 description 2
- 229950002916 avelumab Drugs 0.000 description 2
- 230000003376 axonal effect Effects 0.000 description 2
- VSRXQHXAPYXROS-UHFFFAOYSA-N azanide;cyclobutane-1,1-dicarboxylic acid;platinum(2+) Chemical compound [NH2-].[NH2-].[Pt+2].OC(=O)C1(C(O)=O)CCC1 VSRXQHXAPYXROS-UHFFFAOYSA-N 0.000 description 2
- 229950000210 beclometasone dipropionate Drugs 0.000 description 2
- 239000000440 bentonite Substances 0.000 description 2
- 229910000278 bentonite Inorganic materials 0.000 description 2
- SVPXDRXYRYOSEX-UHFFFAOYSA-N bentoquatam Chemical compound O.O=[Si]=O.O=[Al]O[Al]=O SVPXDRXYRYOSEX-UHFFFAOYSA-N 0.000 description 2
- WGQKYBSKWIADBV-UHFFFAOYSA-N benzylamine Chemical compound NCC1=CC=CC=C1 WGQKYBSKWIADBV-UHFFFAOYSA-N 0.000 description 2
- 229960002537 betamethasone Drugs 0.000 description 2
- 230000015572 biosynthetic process Effects 0.000 description 2
- 229940031416 bivalent vaccine Drugs 0.000 description 2
- 229960001467 bortezomib Drugs 0.000 description 2
- 239000006085 branching agent Substances 0.000 description 2
- 208000000594 bullous pemphigoid Diseases 0.000 description 2
- 229960002092 busulfan Drugs 0.000 description 2
- BMQGVNUXMIRLCK-OAGWZNDDSA-N cabazitaxel Chemical compound O([C@H]1[C@@H]2[C@]3(OC(C)=O)CO[C@@H]3C[C@@H]([C@]2(C(=O)[C@H](OC)C2=C(C)[C@@H](OC(=O)[C@H](O)[C@@H](NC(=O)OC(C)(C)C)C=3C=CC=CC=3)C[C@]1(O)C2(C)C)C)OC)C(=O)C1=CC=CC=C1 BMQGVNUXMIRLCK-OAGWZNDDSA-N 0.000 description 2
- 229960001573 cabazitaxel Drugs 0.000 description 2
- 235000008207 calcium folinate Nutrition 0.000 description 2
- 239000011687 calcium folinate Substances 0.000 description 2
- 229940056434 caprelsa Drugs 0.000 description 2
- 229960002504 capsaicin Drugs 0.000 description 2
- 235000017663 capsaicin Nutrition 0.000 description 2
- BVKZGUZCCUSVTD-UHFFFAOYSA-N carbonic acid Chemical compound OC(O)=O BVKZGUZCCUSVTD-UHFFFAOYSA-N 0.000 description 2
- 229960004562 carboplatin Drugs 0.000 description 2
- BLMPQMFVWMYDKT-NZTKNTHTSA-N carfilzomib Chemical compound C([C@@H](C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CC(C)C)C(=O)[C@]1(C)OC1)NC(=O)CN1CCOCC1)CC1=CC=CC=C1 BLMPQMFVWMYDKT-NZTKNTHTSA-N 0.000 description 2
- 108010021331 carfilzomib Proteins 0.000 description 2
- 229960002438 carfilzomib Drugs 0.000 description 2
- 229940097647 casodex Drugs 0.000 description 2
- 210000004534 cecum Anatomy 0.000 description 2
- 229940124587 cephalosporin Drugs 0.000 description 2
- 150000001780 cephalosporins Chemical group 0.000 description 2
- 201000005795 chronic inflammatory demyelinating polyneuritis Diseases 0.000 description 2
- 208000025302 chronic primary adrenal insufficiency Diseases 0.000 description 2
- 201000010002 cicatricial pemphigoid Diseases 0.000 description 2
- 229960002271 cobimetinib Drugs 0.000 description 2
- RESIMIUSNACMNW-BXRWSSRYSA-N cobimetinib fumarate Chemical compound OC(=O)\C=C\C(O)=O.C1C(O)([C@H]2NCCCC2)CN1C(=O)C1=CC=C(F)C(F)=C1NC1=CC=C(I)C=C1F.C1C(O)([C@H]2NCCCC2)CN1C(=O)C1=CC=C(F)C(F)=C1NC1=CC=C(I)C=C1F RESIMIUSNACMNW-BXRWSSRYSA-N 0.000 description 2
- 229960004126 codeine Drugs 0.000 description 2
- 230000000112 colonic effect Effects 0.000 description 2
- 208000029742 colonic neoplasm Diseases 0.000 description 2
- 230000001332 colony forming effect Effects 0.000 description 2
- 238000012790 confirmation Methods 0.000 description 2
- 201000004395 congenital heart block Diseases 0.000 description 2
- 239000000599 controlled substance Substances 0.000 description 2
- 239000003246 corticosteroid Substances 0.000 description 2
- 229960001334 corticosteroids Drugs 0.000 description 2
- 201000003278 cryoglobulinemia Diseases 0.000 description 2
- 238000012258 culturing Methods 0.000 description 2
- 230000001085 cytostatic effect Effects 0.000 description 2
- 231100000433 cytotoxic Toxicity 0.000 description 2
- 230000001472 cytotoxic effect Effects 0.000 description 2
- 229960000640 dactinomycin Drugs 0.000 description 2
- 229940076705 defibrotide sodium Drugs 0.000 description 2
- 229960002272 degarelix Drugs 0.000 description 2
- MEUCPCLKGZSHTA-XYAYPHGZSA-N degarelix Chemical compound C([C@H](C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCNC(C)C)C(=O)N1[C@@H](CCC1)C(=O)N[C@H](C)C(N)=O)NC(=O)[C@H](CC=1C=CC(NC(=O)[C@H]2NC(=O)NC(=O)C2)=CC=1)NC(=O)[C@H](CO)NC(=O)[C@@H](CC=1C=NC=CC=1)NC(=O)[C@@H](CC=1C=CC(Cl)=CC=1)NC(=O)[C@@H](CC=1C=C2C=CC=CC2=CC=1)NC(C)=O)C1=CC=C(NC(N)=O)C=C1 MEUCPCLKGZSHTA-XYAYPHGZSA-N 0.000 description 2
- 230000003210 demyelinating effect Effects 0.000 description 2
- 230000001419 dependent effect Effects 0.000 description 2
- 238000011161 development Methods 0.000 description 2
- 230000018109 developmental process Effects 0.000 description 2
- 229960000605 dexrazoxane Drugs 0.000 description 2
- 235000005911 diet Nutrition 0.000 description 2
- 230000037213 diet Effects 0.000 description 2
- XBDQKXXYIPTUBI-UHFFFAOYSA-N dimethylselenoniopropionate Natural products CCC(O)=O XBDQKXXYIPTUBI-UHFFFAOYSA-N 0.000 description 2
- 239000006185 dispersion Substances 0.000 description 2
- 229960004679 doxorubicin Drugs 0.000 description 2
- 229940075117 droxia Drugs 0.000 description 2
- 239000003937 drug carrier Substances 0.000 description 2
- 208000019479 dysautonomia Diseases 0.000 description 2
- 208000001848 dysentery Diseases 0.000 description 2
- 206010014599 encephalitis Diseases 0.000 description 2
- 238000001839 endoscopy Methods 0.000 description 2
- 201000000708 eosinophilic esophagitis Diseases 0.000 description 2
- 229960001433 erlotinib Drugs 0.000 description 2
- AAKJLRGGTJKAMG-UHFFFAOYSA-N erlotinib Chemical compound C=12C=C(OCCOC)C(OCCOC)=CC2=NC=NC=1NC1=CC=CC(C#C)=C1 AAKJLRGGTJKAMG-UHFFFAOYSA-N 0.000 description 2
- 235000019414 erythritol Nutrition 0.000 description 2
- UNXHWFMMPAWVPI-ZXZARUISSA-N erythritol Chemical compound OC[C@H](O)[C@H](O)CO UNXHWFMMPAWVPI-ZXZARUISSA-N 0.000 description 2
- 229940009714 erythritol Drugs 0.000 description 2
- 229960001842 estramustine Drugs 0.000 description 2
- FRPJXPJMRWBBIH-RBRWEJTLSA-N estramustine Chemical compound ClCCN(CCCl)C(=O)OC1=CC=C2[C@H]3CC[C@](C)([C@H](CC4)O)[C@@H]4[C@@H]3CCC2=C1 FRPJXPJMRWBBIH-RBRWEJTLSA-N 0.000 description 2
- 235000019325 ethyl cellulose Nutrition 0.000 description 2
- 229920001249 ethyl cellulose Polymers 0.000 description 2
- 208000002980 facial hemiatrophy Diseases 0.000 description 2
- 229960005304 fludarabine phosphate Drugs 0.000 description 2
- 229960002258 fulvestrant Drugs 0.000 description 2
- 206010017758 gastric cancer Diseases 0.000 description 2
- 239000000499 gel Substances 0.000 description 2
- SDUQYLNIPVEERB-QPPQHZFASA-N gemcitabine Chemical compound O=C1N=C(N)C=CN1[C@H]1C(F)(F)[C@H](O)[C@@H](CO)O1 SDUQYLNIPVEERB-QPPQHZFASA-N 0.000 description 2
- 208000018090 giant cell myocarditis Diseases 0.000 description 2
- 229940085435 giardia lamblia Drugs 0.000 description 2
- RWSXRVCMGQZWBV-WDSKDSINSA-N glutathione Chemical compound OC(=O)[C@@H](N)CCC(=O)N[C@@H](CS)C(=O)NCC(O)=O RWSXRVCMGQZWBV-WDSKDSINSA-N 0.000 description 2
- YQEMORVAKMFKLG-UHFFFAOYSA-N glycerine monostearate Natural products CCCCCCCCCCCCCCCCCC(=O)OC(CO)CO YQEMORVAKMFKLG-UHFFFAOYSA-N 0.000 description 2
- SVUQHVRAGMNPLW-UHFFFAOYSA-N glycerol monostearate Natural products CCCCCCCCCCCCCCCCC(=O)OCC(O)CO SVUQHVRAGMNPLW-UHFFFAOYSA-N 0.000 description 2
- 230000036449 good health Effects 0.000 description 2
- 229960002913 goserelin Drugs 0.000 description 2
- 239000008187 granular material Substances 0.000 description 2
- 235000010417 guar gum Nutrition 0.000 description 2
- 239000000665 guar gum Substances 0.000 description 2
- 229960002154 guar gum Drugs 0.000 description 2
- CJNBYAVZURUTKZ-UHFFFAOYSA-N hafnium(IV) oxide Inorganic materials O=[Hf]=O CJNBYAVZURUTKZ-UHFFFAOYSA-N 0.000 description 2
- 230000035876 healing Effects 0.000 description 2
- 208000007475 hemolytic anemia Diseases 0.000 description 2
- 230000002440 hepatic effect Effects 0.000 description 2
- 208000002672 hepatitis B Diseases 0.000 description 2
- UUVWYPNAQBNQJQ-UHFFFAOYSA-N hexamethylmelamine Chemical compound CN(C)C1=NC(N(C)C)=NC(N(C)C)=N1 UUVWYPNAQBNQJQ-UHFFFAOYSA-N 0.000 description 2
- HNDVDQJCIGZPNO-UHFFFAOYSA-N histidine Natural products OC(=O)C(N)CC1=CN=CN1 HNDVDQJCIGZPNO-UHFFFAOYSA-N 0.000 description 2
- 230000003284 homeostatic effect Effects 0.000 description 2
- OROGSEYTTFOCAN-UHFFFAOYSA-N hydrocodone Natural products C1C(N(CCC234)C)C2C=CC(O)C3OC2=C4C1=CC=C2OC OROGSEYTTFOCAN-UHFFFAOYSA-N 0.000 description 2
- JYGXADMDTFJGBT-VWUMJDOOSA-N hydrocortisone Chemical compound O=C1CC[C@]2(C)[C@H]3[C@@H](O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 JYGXADMDTFJGBT-VWUMJDOOSA-N 0.000 description 2
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 2
- 229960001330 hydroxycarbamide Drugs 0.000 description 2
- 235000010977 hydroxypropyl cellulose Nutrition 0.000 description 2
- 239000001863 hydroxypropyl cellulose Substances 0.000 description 2
- UFVKGYZPFZQRLF-UHFFFAOYSA-N hydroxypropyl methyl cellulose Chemical compound OC1C(O)C(OC)OC(CO)C1OC1C(O)C(O)C(OC2C(C(O)C(OC3C(C(O)C(O)C(CO)O3)O)C(CO)O2)O)C(CO)O1 UFVKGYZPFZQRLF-UHFFFAOYSA-N 0.000 description 2
- 229920000639 hydroxypropylmethylcellulose acetate succinate Polymers 0.000 description 2
- 201000006362 hypersensitivity vasculitis Diseases 0.000 description 2
- 229960000908 idarubicin Drugs 0.000 description 2
- IFSDAJWBUCMOAH-HNNXBMFYSA-N idelalisib Chemical compound C1([C@@H](NC=2C=3N=CNC=3N=CN=2)CC)=NC2=CC=CC(F)=C2C(=O)N1C1=CC=CC=C1 IFSDAJWBUCMOAH-HNNXBMFYSA-N 0.000 description 2
- 229960002751 imiquimod Drugs 0.000 description 2
- 230000007813 immunodeficiency Effects 0.000 description 2
- 230000002163 immunogen Effects 0.000 description 2
- 208000015446 immunoglobulin a vasculitis Diseases 0.000 description 2
- 230000006872 improvement Effects 0.000 description 2
- 201000008319 inclusion body myositis Diseases 0.000 description 2
- CGIGDMFJXJATDK-UHFFFAOYSA-N indomethacin Chemical compound CC1=C(CC(O)=O)C2=CC(OC)=CC=C2N1C(=O)C1=CC=C(Cl)C=C1 CGIGDMFJXJATDK-UHFFFAOYSA-N 0.000 description 2
- 229940117681 interleukin-12 Drugs 0.000 description 2
- 229940028885 interleukin-4 Drugs 0.000 description 2
- 208000036971 interstitial lung disease 2 Diseases 0.000 description 2
- 244000000074 intestinal pathogen Species 0.000 description 2
- 238000007918 intramuscular administration Methods 0.000 description 2
- 238000007912 intraperitoneal administration Methods 0.000 description 2
- 229940084651 iressa Drugs 0.000 description 2
- 229960000779 irinotecan hydrochloride Drugs 0.000 description 2
- 230000000622 irritating effect Effects 0.000 description 2
- KQNPFQTWMSNSAP-UHFFFAOYSA-N isobutyric acid Chemical compound CC(C)C(O)=O KQNPFQTWMSNSAP-UHFFFAOYSA-N 0.000 description 2
- 229960002014 ixabepilone Drugs 0.000 description 2
- 229960003648 ixazomib Drugs 0.000 description 2
- MXAYKZJJDUDWDS-LBPRGKRZSA-N ixazomib Chemical compound CC(C)C[C@@H](B(O)O)NC(=O)CNC(=O)C1=CC(Cl)=CC=C1Cl MXAYKZJJDUDWDS-LBPRGKRZSA-N 0.000 description 2
- 201000002215 juvenile rheumatoid arthritis Diseases 0.000 description 2
- NLYAJNPCOHFWQQ-UHFFFAOYSA-N kaolin Chemical compound O.O.O=[Al]O[Si](=O)O[Si](=O)O[Al]=O NLYAJNPCOHFWQQ-UHFFFAOYSA-N 0.000 description 2
- BJHIKXHVCXFQLS-PQLUHFTBSA-N keto-D-tagatose Chemical compound OC[C@@H](O)[C@H](O)[C@H](O)C(=O)CO BJHIKXHVCXFQLS-PQLUHFTBSA-N 0.000 description 2
- HCZHHEIFKROPDY-UHFFFAOYSA-N kynurenic acid Chemical compound C1=CC=C2NC(C(=O)O)=CC(=O)C2=C1 HCZHHEIFKROPDY-UHFFFAOYSA-N 0.000 description 2
- JJTUDXZGHPGLLC-UHFFFAOYSA-N lactide Chemical compound CC1OC(=O)C(C)OC1=O JJTUDXZGHPGLLC-UHFFFAOYSA-N 0.000 description 2
- 229940017800 lactobacillus casei Drugs 0.000 description 2
- 229960001739 lanreotide acetate Drugs 0.000 description 2
- 229960004891 lapatinib Drugs 0.000 description 2
- BCFGMOOMADDAQU-UHFFFAOYSA-N lapatinib Chemical compound O1C(CNCCS(=O)(=O)C)=CC=C1C1=CC=C(N=CN=C2NC=3C=C(Cl)C(OCC=4C=C(F)C=CC=4)=CC=3)C2=C1 BCFGMOOMADDAQU-UHFFFAOYSA-N 0.000 description 2
- 229960002293 leucovorin calcium Drugs 0.000 description 2
- 208000032839 leukemia Diseases 0.000 description 2
- 229940063725 leukeran Drugs 0.000 description 2
- 201000011486 lichen planus Diseases 0.000 description 2
- 206010071570 ligneous conjunctivitis Diseases 0.000 description 2
- 201000007270 liver cancer Diseases 0.000 description 2
- 208000014018 liver neoplasm Diseases 0.000 description 2
- 230000007774 longterm Effects 0.000 description 2
- 210000004072 lung Anatomy 0.000 description 2
- 210000002751 lymph Anatomy 0.000 description 2
- 235000019359 magnesium stearate Nutrition 0.000 description 2
- 229960001786 megestrol Drugs 0.000 description 2
- 229960004296 megestrol acetate Drugs 0.000 description 2
- 229940083118 mekinist Drugs 0.000 description 2
- 201000001441 melanoma Diseases 0.000 description 2
- 229960002514 melphalan hydrochloride Drugs 0.000 description 2
- 210000004379 membrane Anatomy 0.000 description 2
- 239000012528 membrane Substances 0.000 description 2
- 229960004635 mesna Drugs 0.000 description 2
- 206010061289 metastatic neoplasm Diseases 0.000 description 2
- BDAGIHXWWSANSR-UHFFFAOYSA-N methanoic acid Natural products OC=O BDAGIHXWWSANSR-UHFFFAOYSA-N 0.000 description 2
- 229960000485 methotrexate Drugs 0.000 description 2
- 229960002834 methylnaltrexone bromide Drugs 0.000 description 2
- 239000013586 microbial product Substances 0.000 description 2
- 235000019813 microcrystalline cellulose Nutrition 0.000 description 2
- 239000008108 microcrystalline cellulose Substances 0.000 description 2
- 229940016286 microcrystalline cellulose Drugs 0.000 description 2
- 206010063344 microscopic polyangiitis Diseases 0.000 description 2
- BMGQWWVMWDBQGC-IIFHNQTCSA-N midostaurin Chemical compound CN([C@H]1[C@H]([C@]2(C)O[C@@H](N3C4=CC=CC=C4C4=C5C(=O)NCC5=C5C6=CC=CC=C6N2C5=C43)C1)OC)C(=O)C1=CC=CC=C1 BMGQWWVMWDBQGC-IIFHNQTCSA-N 0.000 description 2
- 229950010895 midostaurin Drugs 0.000 description 2
- KKZJGLLVHKMTCM-UHFFFAOYSA-N mitoxantrone Chemical compound O=C1C2=C(O)C=CC(O)=C2C(=O)C2=C1C(NCCNCCO)=CC=C2NCCNCCO KKZJGLLVHKMTCM-UHFFFAOYSA-N 0.000 description 2
- BQJCRHHNABKAKU-KBQPJGBKSA-N morphine Chemical compound O([C@H]1[C@H](C=C[C@H]23)O)C4=C5[C@@]12CCN(C)[C@@H]3CC5=CC=C4O BQJCRHHNABKAKU-KBQPJGBKSA-N 0.000 description 2
- 210000000214 mouth Anatomy 0.000 description 2
- 210000004400 mucous membrane Anatomy 0.000 description 2
- 201000006417 multiple sclerosis Diseases 0.000 description 2
- DQCKKXVULJGBQN-XFWGSAIBSA-N naltrexone Chemical compound N1([C@@H]2CC3=CC=C(C=4O[C@@H]5[C@](C3=4)([C@]2(CCC5=O)O)CC1)O)CC1CC1 DQCKKXVULJGBQN-XFWGSAIBSA-N 0.000 description 2
- 201000003631 narcolepsy Diseases 0.000 description 2
- 230000008693 nausea Effects 0.000 description 2
- 229960000801 nelarabine Drugs 0.000 description 2
- IXOXBSCIXZEQEQ-UHTZMRCNSA-N nelarabine Chemical compound C1=NC=2C(OC)=NC(N)=NC=2N1[C@@H]1O[C@H](CO)[C@@H](O)[C@@H]1O IXOXBSCIXZEQEQ-UHTZMRCNSA-N 0.000 description 2
- 201000008383 nephritis Diseases 0.000 description 2
- ZNHPZUKZSNBOSQ-BQYQJAHWSA-N neratinib Chemical compound C=12C=C(NC\C=C\CN(C)C)C(OCC)=CC2=NC=C(C#N)C=1NC(C=C1Cl)=CC=C1OCC1=CC=CC=N1 ZNHPZUKZSNBOSQ-BQYQJAHWSA-N 0.000 description 2
- 229940071846 neulasta Drugs 0.000 description 2
- 208000004235 neutropenia Diseases 0.000 description 2
- 229940099637 nilandron Drugs 0.000 description 2
- 208000015200 ocular cicatricial pemphigoid Diseases 0.000 description 2
- 235000019198 oils Nutrition 0.000 description 2
- 229950004864 olamine Drugs 0.000 description 2
- 229960000770 ondansetron hydrochloride Drugs 0.000 description 2
- 229940005483 opioid analgesics Drugs 0.000 description 2
- 239000006186 oral dosage form Substances 0.000 description 2
- DWAFYCQODLXJNR-BNTLRKBRSA-L oxaliplatin Chemical compound O1C(=O)C(=O)O[Pt]11N[C@@H]2CCCC[C@H]2N1 DWAFYCQODLXJNR-BNTLRKBRSA-L 0.000 description 2
- 239000012188 paraffin wax Substances 0.000 description 2
- 229960000639 pazopanib Drugs 0.000 description 2
- CUIHSIWYWATEQL-UHFFFAOYSA-N pazopanib Chemical compound C1=CC2=C(C)N(C)N=C2C=C1N(C)C(N=1)=CC=NC=1NC1=CC=C(C)C(S(N)(=O)=O)=C1 CUIHSIWYWATEQL-UHFFFAOYSA-N 0.000 description 2
- HQQSBEDKMRHYME-UHFFFAOYSA-N pefloxacin mesylate Chemical compound [H+].CS([O-])(=O)=O.C1=C2N(CC)C=C(C(O)=O)C(=O)C2=CC(F)=C1N1CCN(C)CC1 HQQSBEDKMRHYME-UHFFFAOYSA-N 0.000 description 2
- 229960001373 pegfilgrastim Drugs 0.000 description 2
- 229940049954 penicillin Drugs 0.000 description 2
- FPVKHBSQESCIEP-JQCXWYLXSA-N pentostatin Chemical compound C1[C@H](O)[C@@H](CO)O[C@H]1N1C(N=CNC[C@H]2O)=C2N=C1 FPVKHBSQESCIEP-JQCXWYLXSA-N 0.000 description 2
- 230000035699 permeability Effects 0.000 description 2
- 229940063179 platinol Drugs 0.000 description 2
- 229920002939 poly(N,N-dimethylacrylamides) Polymers 0.000 description 2
- 239000005015 poly(hydroxybutyrate) Substances 0.000 description 2
- 229920000747 poly(lactic acid) Polymers 0.000 description 2
- 229920002463 poly(p-dioxanone) polymer Polymers 0.000 description 2
- 229920002401 polyacrylamide Polymers 0.000 description 2
- 201000006292 polyarteritis nodosa Diseases 0.000 description 2
- 239000000622 polydioxanone Substances 0.000 description 2
- 208000005987 polymyositis Diseases 0.000 description 2
- 229920005862 polyol Polymers 0.000 description 2
- 150000003077 polyols Chemical class 0.000 description 2
- 229920001451 polypropylene glycol Polymers 0.000 description 2
- 229920001296 polysiloxane Polymers 0.000 description 2
- 229920000166 polytrimethylene carbonate Polymers 0.000 description 2
- 229920002635 polyurethane Polymers 0.000 description 2
- 239000004814 polyurethane Substances 0.000 description 2
- 229940100467 polyvinyl acetate phthalate Drugs 0.000 description 2
- 229960000688 pomalidomide Drugs 0.000 description 2
- UVSMNLNDYGZFPF-UHFFFAOYSA-N pomalidomide Chemical compound O=C1C=2C(N)=CC=CC=2C(=O)N1C1CCC(=O)NC1=O UVSMNLNDYGZFPF-UHFFFAOYSA-N 0.000 description 2
- 229960001131 ponatinib Drugs 0.000 description 2
- 208000017805 post-transplant lymphoproliferative disease Diseases 0.000 description 2
- 239000011591 potassium Substances 0.000 description 2
- 229910052700 potassium Inorganic materials 0.000 description 2
- 235000007686 potassium Nutrition 0.000 description 2
- BWHMMNNQKKPAPP-UHFFFAOYSA-L potassium carbonate Chemical compound [K+].[K+].[O-]C([O-])=O BWHMMNNQKKPAPP-UHFFFAOYSA-L 0.000 description 2
- OGSBUKJUDHAQEA-WMCAAGNKSA-N pralatrexate Chemical compound C1=NC2=NC(N)=NC(N)=C2N=C1CC(CC#C)C1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 OGSBUKJUDHAQEA-WMCAAGNKSA-N 0.000 description 2
- 229960000214 pralatrexate Drugs 0.000 description 2
- VJZLQIPZNBPASX-OJJGEMKLSA-L prednisolone sodium phosphate Chemical compound [Na+].[Na+].O=C1C=C[C@]2(C)[C@H]3[C@@H](O)C[C@](C)([C@@](CC4)(O)C(=O)COP([O-])([O-])=O)[C@@H]4[C@@H]3CCC2=C1 VJZLQIPZNBPASX-OJJGEMKLSA-L 0.000 description 2
- 208000018290 primary dysautonomia Diseases 0.000 description 2
- 201000000742 primary sclerosing cholangitis Diseases 0.000 description 2
- 229960000624 procarbazine Drugs 0.000 description 2
- CPTBDICYNRMXFX-UHFFFAOYSA-N procarbazine Chemical compound CNNCC1=CC=C(C(=O)NC(C)C)C=C1 CPTBDICYNRMXFX-UHFFFAOYSA-N 0.000 description 2
- 229960004604 propranolol hydrochloride Drugs 0.000 description 2
- AQHHHDLHHXJYJD-UHFFFAOYSA-N propranolol hydrochloride Natural products C1=CC=C2C(OCC(O)CNC(C)C)=CC=CC2=C1 AQHHHDLHHXJYJD-UHFFFAOYSA-N 0.000 description 2
- 210000002307 prostate Anatomy 0.000 description 2
- 208000005069 pulmonary fibrosis Diseases 0.000 description 2
- 229940092814 radium (223ra) dichloride Drugs 0.000 description 2
- GZUITABIAKMVPG-UHFFFAOYSA-N raloxifene Chemical compound C1=CC(O)=CC=C1C1=C(C(=O)C=2C=CC(OCCN3CCCCC3)=CC=2)C2=CC=C(O)C=C2S1 GZUITABIAKMVPG-UHFFFAOYSA-N 0.000 description 2
- 238000003753 real-time PCR Methods 0.000 description 2
- 206010038038 rectal cancer Diseases 0.000 description 2
- 201000001275 rectum cancer Diseases 0.000 description 2
- 230000002829 reductive effect Effects 0.000 description 2
- 208000009169 relapsing polychondritis Diseases 0.000 description 2
- 230000000552 rheumatic effect Effects 0.000 description 2
- 201000003068 rheumatic fever Diseases 0.000 description 2
- 206010039073 rheumatoid arthritis Diseases 0.000 description 2
- CVHZOJJKTDOEJC-UHFFFAOYSA-N saccharin Chemical compound C1=CC=C2C(=O)NS(=O)(=O)C2=C1 CVHZOJJKTDOEJC-UHFFFAOYSA-N 0.000 description 2
- YGSDEFSMJLZEOE-UHFFFAOYSA-N salicylic acid Chemical compound OC(=O)C1=CC=CC=C1O YGSDEFSMJLZEOE-UHFFFAOYSA-N 0.000 description 2
- 201000000306 sarcoidosis Diseases 0.000 description 2
- 208000010157 sclerosing cholangitis Diseases 0.000 description 2
- 238000007423 screening assay Methods 0.000 description 2
- 208000011581 secondary neoplasm Diseases 0.000 description 2
- QZAYGJVTTNCVMB-UHFFFAOYSA-N serotonin Chemical compound C1=C(O)C=C2C(CCN)=CNC2=C1 QZAYGJVTTNCVMB-UHFFFAOYSA-N 0.000 description 2
- 230000001568 sexual effect Effects 0.000 description 2
- 210000001599 sigmoid colon Anatomy 0.000 description 2
- 230000011664 signaling Effects 0.000 description 2
- 235000020183 skimmed milk Nutrition 0.000 description 2
- 208000017520 skin disease Diseases 0.000 description 2
- 239000011734 sodium Substances 0.000 description 2
- 229910052708 sodium Inorganic materials 0.000 description 2
- 235000019333 sodium laurylsulphate Nutrition 0.000 description 2
- VWDWKYIASSYTQR-UHFFFAOYSA-N sodium nitrate Chemical compound [Na+].[O-][N+]([O-])=O VWDWKYIASSYTQR-UHFFFAOYSA-N 0.000 description 2
- GEHJYWRUCIMESM-UHFFFAOYSA-L sodium sulfite Chemical compound [Na+].[Na+].[O-]S([O-])=O GEHJYWRUCIMESM-UHFFFAOYSA-L 0.000 description 2
- 229940034810 soltamox Drugs 0.000 description 2
- 239000002904 solvent Substances 0.000 description 2
- 206010041823 squamous cell carcinoma Diseases 0.000 description 2
- 150000003432 sterols Chemical class 0.000 description 2
- 235000003702 sterols Nutrition 0.000 description 2
- 201000011549 stomach cancer Diseases 0.000 description 2
- 238000002563 stool test Methods 0.000 description 2
- 208000008467 subacute bacterial endocarditis Diseases 0.000 description 2
- 229960001796 sunitinib Drugs 0.000 description 2
- WINHZLLDWRZWRT-ATVHPVEESA-N sunitinib Chemical compound CCN(CC)CCNC(=O)C1=C(C)NC(\C=C/2C3=CC(F)=CC=C3NC\2=O)=C1C WINHZLLDWRZWRT-ATVHPVEESA-N 0.000 description 2
- 229960002812 sunitinib malate Drugs 0.000 description 2
- 239000000375 suspending agent Substances 0.000 description 2
- 201000000596 systemic lupus erythematosus Diseases 0.000 description 2
- 229940095374 tabloid Drugs 0.000 description 2
- FQZYTYWMLGAPFJ-OQKDUQJOSA-N tamoxifen citrate Chemical compound [H+].[H+].[H+].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O.C=1C=CC=CC=1C(/CC)=C(C=1C=CC(OCCN(C)C)=CC=1)/C1=CC=CC=C1 FQZYTYWMLGAPFJ-OQKDUQJOSA-N 0.000 description 2
- 229960003454 tamoxifen citrate Drugs 0.000 description 2
- 229940061353 temodar Drugs 0.000 description 2
- 229960000235 temsirolimus Drugs 0.000 description 2
- QFJCIRLUMZQUOT-UHFFFAOYSA-N temsirolimus Natural products C1CC(O)C(OC)CC1CC(C)C1OC(=O)C2CCCCN2C(=O)C(=O)C(O)(O2)C(C)CCC2CC(OC)C(C)=CC=CC=CC(C)CC(C)C(=O)C(OC)C(O)C(C)=CC(C)C(=O)C1 QFJCIRLUMZQUOT-UHFFFAOYSA-N 0.000 description 2
- 238000011287 therapeutic dose Methods 0.000 description 2
- CWERGRDVMFNCDR-UHFFFAOYSA-N thioglycolic acid Chemical compound OC(=O)CS CWERGRDVMFNCDR-UHFFFAOYSA-N 0.000 description 2
- 229960002952 tipiracil Drugs 0.000 description 2
- QQHMKNYGKVVGCZ-UHFFFAOYSA-N tipiracil Chemical compound N1C(=O)NC(=O)C(Cl)=C1CN1C(=N)CCC1 QQHMKNYGKVVGCZ-UHFFFAOYSA-N 0.000 description 2
- 229960000303 topotecan Drugs 0.000 description 2
- 229960002190 topotecan hydrochloride Drugs 0.000 description 2
- 229960000977 trabectedin Drugs 0.000 description 2
- 238000002627 tracheal intubation Methods 0.000 description 2
- 238000002054 transplantation Methods 0.000 description 2
- 208000009174 transverse myelitis Diseases 0.000 description 2
- 229960001727 tretinoin Drugs 0.000 description 2
- URAYPUMNDPQOKB-UHFFFAOYSA-N triacetin Chemical compound CC(=O)OCC(OC(C)=O)COC(C)=O URAYPUMNDPQOKB-UHFFFAOYSA-N 0.000 description 2
- 239000001069 triethyl citrate Substances 0.000 description 2
- 235000013769 triethyl citrate Nutrition 0.000 description 2
- VMYFZRTXGLUXMZ-UHFFFAOYSA-N triethyl citrate Natural products CCOC(=O)C(O)(C(=O)OCC)C(=O)OCC VMYFZRTXGLUXMZ-UHFFFAOYSA-N 0.000 description 2
- 125000005591 trimellitate group Chemical group 0.000 description 2
- 210000003932 urinary bladder Anatomy 0.000 description 2
- 230000002568 urticarial effect Effects 0.000 description 2
- 230000009677 vaginal delivery Effects 0.000 description 2
- ZOCKGBMQLCSHFP-KQRAQHLDSA-N valrubicin Chemical compound O([C@H]1C[C@](CC2=C(O)C=3C(=O)C4=CC=CC(OC)=C4C(=O)C=3C(O)=C21)(O)C(=O)COC(=O)CCCC)[C@H]1C[C@H](NC(=O)C(F)(F)F)[C@H](O)[C@H](C)O1 ZOCKGBMQLCSHFP-KQRAQHLDSA-N 0.000 description 2
- 229940054937 valstar Drugs 0.000 description 2
- 230000035899 viability Effects 0.000 description 2
- KDQAABAKXDWYSZ-PNYVAJAMSA-N vinblastine sulfate Chemical compound OS(O)(=O)=O.C([C@H](C[C@]1(C(=O)OC)C=2C(=CC3=C([C@]45[C@H]([C@@]([C@H](OC(C)=O)[C@]6(CC)C=CCN([C@H]56)CC4)(O)C(=O)OC)N3C)C=2)OC)C[C@@](C2)(O)CC)N2CCC2=C1NC1=CC=CC=C21 KDQAABAKXDWYSZ-PNYVAJAMSA-N 0.000 description 2
- 229960004982 vinblastine sulfate Drugs 0.000 description 2
- 229960002110 vincristine sulfate Drugs 0.000 description 2
- GBABOYUKABKIAF-GHYRFKGUSA-N vinorelbine Chemical compound C1N(CC=2C3=CC=CC=C3NC=22)CC(CC)=C[C@H]1C[C@]2(C(=O)OC)C1=CC([C@]23[C@H]([C@]([C@H](OC(C)=O)[C@]4(CC)C=CCN([C@H]34)CC2)(O)C(=O)OC)N2C)=C2C=C1OC GBABOYUKABKIAF-GHYRFKGUSA-N 0.000 description 2
- 235000019166 vitamin D Nutrition 0.000 description 2
- 239000011710 vitamin D Substances 0.000 description 2
- 150000003710 vitamin D derivatives Chemical class 0.000 description 2
- 229940046008 vitamin d Drugs 0.000 description 2
- 230000008673 vomiting Effects 0.000 description 2
- WAEXFXRVDQXREF-UHFFFAOYSA-N vorinostat Chemical compound ONC(=O)CCCCCCC(=O)NC1=CC=CC=C1 WAEXFXRVDQXREF-UHFFFAOYSA-N 0.000 description 2
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 2
- 229920003176 water-insoluble polymer Polymers 0.000 description 2
- 229960002760 ziv-aflibercept Drugs 0.000 description 2
- 229940095188 zydelig Drugs 0.000 description 2
- GVJHHUAWPYXKBD-IEOSBIPESA-N α-tocopherol Chemical compound OC1=C(C)C(C)=C2O[C@@](CCC[C@H](C)CCC[C@H](C)CCCC(C)C)(C)CCC2=C1C GVJHHUAWPYXKBD-IEOSBIPESA-N 0.000 description 2
- KIUKXJAPPMFGSW-DNGZLQJQSA-N (2S,3S,4S,5R,6R)-6-[(2S,3R,4R,5S,6R)-3-Acetamido-2-[(2S,3S,4R,5R,6R)-6-[(2R,3R,4R,5S,6R)-3-acetamido-2,5-dihydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-2-carboxy-4,5-dihydroxyoxan-3-yl]oxy-5-hydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-3,4,5-trihydroxyoxane-2-carboxylic acid Chemical compound CC(=O)N[C@H]1[C@H](O)O[C@H](CO)[C@@H](O)[C@@H]1O[C@H]1[C@H](O)[C@@H](O)[C@H](O[C@H]2[C@@H]([C@@H](O[C@H]3[C@@H]([C@@H](O)[C@H](O)[C@H](O3)C(O)=O)O)[C@H](O)[C@@H](CO)O2)NC(C)=O)[C@@H](C(O)=O)O1 KIUKXJAPPMFGSW-DNGZLQJQSA-N 0.000 description 1
- JNYAEWCLZODPBN-JGWLITMVSA-N (2r,3r,4s)-2-[(1r)-1,2-dihydroxyethyl]oxolane-3,4-diol Chemical class OC[C@@H](O)[C@H]1OC[C@H](O)[C@H]1O JNYAEWCLZODPBN-JGWLITMVSA-N 0.000 description 1
- YXTKHLHCVFUPPT-YYFJYKOTSA-N (2s)-2-[[4-[(2-amino-5-formyl-4-oxo-1,6,7,8-tetrahydropteridin-6-yl)methylamino]benzoyl]amino]pentanedioic acid;(1r,2r)-1,2-dimethanidylcyclohexane;5-fluoro-1h-pyrimidine-2,4-dione;oxalic acid;platinum(2+) Chemical compound [Pt+2].OC(=O)C(O)=O.[CH2-][C@@H]1CCCC[C@H]1[CH2-].FC1=CNC(=O)NC1=O.C1NC=2NC(N)=NC(=O)C=2N(C=O)C1CNC1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 YXTKHLHCVFUPPT-YYFJYKOTSA-N 0.000 description 1
- SSOORFWOBGFTHL-OTEJMHTDSA-N (4S)-5-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-6-amino-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[2-[(2S)-2-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-6-amino-1-[[(2S)-1-[[(2S)-1-[[(2S,3S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-6-amino-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-5-amino-1-[[(2S)-1-[[(2S)-1-[[(2S)-6-amino-1-[[(2S)-6-amino-1-[[(2S)-1-[[(2S)-1-[[(2S)-5-amino-1-[[(2S)-5-carbamimidamido-1-[[(2S)-5-carbamimidamido-1-[[(1S)-4-carbamimidamido-1-carboxybutyl]amino]-1-oxopentan-2-yl]amino]-1-oxopentan-2-yl]amino]-1,5-dioxopentan-2-yl]amino]-5-carbamimidamido-1-oxopentan-2-yl]amino]-5-carbamimidamido-1-oxopentan-2-yl]amino]-1-oxohexan-2-yl]amino]-1-oxohexan-2-yl]amino]-5-carbamimidamido-1-oxopentan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-1,5-dioxopentan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-3-hydroxy-1-oxopropan-2-yl]amino]-3-hydroxy-1-oxopropan-2-yl]amino]-3-hydroxy-1-oxopropan-2-yl]amino]-1-oxopropan-2-yl]amino]-1-oxohexan-2-yl]amino]-3-hydroxy-1-oxopropan-2-yl]amino]-1-oxo-3-phenylpropan-2-yl]amino]-3-methyl-1-oxopentan-2-yl]amino]-3-methyl-1-oxobutan-2-yl]amino]-5-carbamimidamido-1-oxopentan-2-yl]amino]-1-oxohexan-2-yl]amino]-3-methyl-1-oxobutan-2-yl]amino]-5-carbamimidamido-1-oxopentan-2-yl]amino]-3-methyl-1-oxobutan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-1-oxopropan-2-yl]amino]-5-carbamimidamido-1-oxopentan-2-yl]carbamoyl]pyrrolidin-1-yl]-2-oxoethyl]amino]-3-(1H-indol-3-yl)-1-oxopropan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-1-oxo-3-phenylpropan-2-yl]amino]-5-carbamimidamido-1-oxopentan-2-yl]amino]-1-oxohexan-2-yl]amino]-3-methyl-1-oxobutan-2-yl]amino]-5-carbamimidamido-1-oxopentan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-1-oxo-3-phenylpropan-2-yl]amino]-3-(1H-imidazol-4-yl)-1-oxopropan-2-yl]amino]-3-methyl-1-oxobutan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-4-[[(2S)-2-[[(2S)-2-[[(2S)-2,6-diaminohexanoyl]amino]-3-methylbutanoyl]amino]propanoyl]amino]-5-oxopentanoic acid Chemical compound CC[C@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1C(=O)CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@@H](N)CCCCN)C(C)C)C(C)C)C(C)C)C(C)C)C(C)C)C(C)C)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O SSOORFWOBGFTHL-OTEJMHTDSA-N 0.000 description 1
- SGKRLCUYIXIAHR-AKNGSSGZSA-N (4s,4ar,5s,5ar,6r,12ar)-4-(dimethylamino)-1,5,10,11,12a-pentahydroxy-6-methyl-3,12-dioxo-4a,5,5a,6-tetrahydro-4h-tetracene-2-carboxamide Chemical compound C1=CC=C2[C@H](C)[C@@H]([C@H](O)[C@@H]3[C@](C(O)=C(C(N)=O)C(=O)[C@H]3N(C)C)(O)C3=O)C3=C(O)C2=C1O SGKRLCUYIXIAHR-AKNGSSGZSA-N 0.000 description 1
- FFTVPQUHLQBXQZ-KVUCHLLUSA-N (4s,4as,5ar,12ar)-4,7-bis(dimethylamino)-1,10,11,12a-tetrahydroxy-3,12-dioxo-4a,5,5a,6-tetrahydro-4h-tetracene-2-carboxamide Chemical compound C1C2=C(N(C)C)C=CC(O)=C2C(O)=C2[C@@H]1C[C@H]1[C@H](N(C)C)C(=O)C(C(N)=O)=C(O)[C@@]1(O)C2=O FFTVPQUHLQBXQZ-KVUCHLLUSA-N 0.000 description 1
- WKJGTOYAEQDNIA-IOOZKYRYSA-N (6r,7r)-7-[[(2r)-2-amino-2-phenylacetyl]amino]-3-chloro-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid;hydrate Chemical compound O.C1([C@H](C(=O)N[C@@H]2C(N3C(=C(Cl)CS[C@@H]32)C(O)=O)=O)N)=CC=CC=C1 WKJGTOYAEQDNIA-IOOZKYRYSA-N 0.000 description 1
- MWWSFMDVAYGXBV-FGBSZODSSA-N (7s,9s)-7-[(2r,4s,5r,6s)-4-amino-5-hydroxy-6-methyloxan-2-yl]oxy-6,9,11-trihydroxy-9-(2-hydroxyacetyl)-4-methoxy-8,10-dihydro-7h-tetracene-5,12-dione;hydron;chloride Chemical compound Cl.O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@@H](O)[C@H](C)O1 MWWSFMDVAYGXBV-FGBSZODSSA-N 0.000 description 1
- FPVKHBSQESCIEP-UHFFFAOYSA-N (8S)-3-(2-deoxy-beta-D-erythro-pentofuranosyl)-3,6,7,8-tetrahydroimidazo[4,5-d][1,3]diazepin-8-ol Natural products C1C(O)C(CO)OC1N1C(NC=NCC2O)=C2N=C1 FPVKHBSQESCIEP-UHFFFAOYSA-N 0.000 description 1
- NCCJWSXETVVUHK-ZYSAIPPVSA-N (z)-7-[(2r)-2-amino-2-carboxyethyl]sulfanyl-2-[[(1s)-2,2-dimethylcyclopropanecarbonyl]amino]hept-2-enoic acid;(5r,6s)-3-[2-(aminomethylideneamino)ethylsulfanyl]-6-[(1r)-1-hydroxyethyl]-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid Chemical compound C1C(SCC\N=C/N)=C(C(O)=O)N2C(=O)[C@H]([C@H](O)C)[C@H]21.CC1(C)C[C@@H]1C(=O)N\C(=C/CCCCSC[C@H](N)C(O)=O)C(O)=O NCCJWSXETVVUHK-ZYSAIPPVSA-N 0.000 description 1
- VXZCUHNJXSIJIM-MEBGWEOYSA-N (z)-but-2-enedioic acid;(e)-n-[4-[3-chloro-4-(pyridin-2-ylmethoxy)anilino]-3-cyano-7-ethoxyquinolin-6-yl]-4-(dimethylamino)but-2-enamide Chemical compound OC(=O)\C=C/C(O)=O.C=12C=C(NC(=O)\C=C\CN(C)C)C(OCC)=CC2=NC=C(C#N)C=1NC(C=C1Cl)=CC=C1OCC1=CC=CC=N1 VXZCUHNJXSIJIM-MEBGWEOYSA-N 0.000 description 1
- WEYNBWVKOYCCQT-UHFFFAOYSA-N 1-(3-chloro-4-methylphenyl)-3-{2-[({5-[(dimethylamino)methyl]-2-furyl}methyl)thio]ethyl}urea Chemical compound O1C(CN(C)C)=CC=C1CSCCNC(=O)NC1=CC=C(C)C(Cl)=C1 WEYNBWVKOYCCQT-UHFFFAOYSA-N 0.000 description 1
- OKMWKBLSFKFYGZ-UHFFFAOYSA-N 1-behenoylglycerol Chemical compound CCCCCCCCCCCCCCCCCCCCCC(=O)OCC(O)CO OKMWKBLSFKFYGZ-UHFFFAOYSA-N 0.000 description 1
- VSNHCAURESNICA-NJFSPNSNSA-N 1-oxidanylurea Chemical compound N[14C](=O)NO VSNHCAURESNICA-NJFSPNSNSA-N 0.000 description 1
- OWEGMIWEEQEYGQ-UHFFFAOYSA-N 100676-05-9 Natural products OC1C(O)C(O)C(CO)OC1OCC1C(O)C(O)C(O)C(OC2C(OC(O)C(O)C2O)CO)O1 OWEGMIWEEQEYGQ-UHFFFAOYSA-N 0.000 description 1
- FUFLCEKSBBHCMO-UHFFFAOYSA-N 11-dehydrocorticosterone Natural products O=C1CCC2(C)C3C(=O)CC(C)(C(CC4)C(=O)CO)C4C3CCC2=C1 FUFLCEKSBBHCMO-UHFFFAOYSA-N 0.000 description 1
- WHBHBVVOGNECLV-OBQKJFGGSA-N 11-deoxycortisol Chemical compound O=C1CC[C@]2(C)[C@H]3CC[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 WHBHBVVOGNECLV-OBQKJFGGSA-N 0.000 description 1
- WNWHHMBRJJOGFJ-UHFFFAOYSA-N 16-methylheptadecan-1-ol Chemical class CC(C)CCCCCCCCCCCCCCCO WNWHHMBRJJOGFJ-UHFFFAOYSA-N 0.000 description 1
- VOXZDWNPVJITMN-ZBRFXRBCSA-N 17β-estradiol Chemical compound OC1=CC=C2[C@H]3CC[C@](C)([C@H](CC4)O)[C@@H]4[C@@H]3CCC2=C1 VOXZDWNPVJITMN-ZBRFXRBCSA-N 0.000 description 1
- XYHKNCXZYYTLRG-UHFFFAOYSA-N 1h-imidazole-2-carbaldehyde Chemical compound O=CC1=NC=CN1 XYHKNCXZYYTLRG-UHFFFAOYSA-N 0.000 description 1
- KXLMYOMYMQMPJE-UHFFFAOYSA-N 2,5-diacetyloxy-3-benzylbenzoic acid Chemical compound OC(=O)C1=CC(OC(=O)C)=CC(CC=2C=CC=CC=2)=C1OC(C)=O KXLMYOMYMQMPJE-UHFFFAOYSA-N 0.000 description 1
- RFHAOTPXVQNOHP-UHFFFAOYSA-O 2-(2,4-difluorophenyl)-1-(1h-1,2,4-triazol-2-ium-2-yl)-3-(1,2,4-triazol-1-yl)propan-2-ol Chemical compound C([C@](O)(C[N+]=1NC=NC=1)C=1C(=CC(F)=CC=1)F)N1C=NC=N1 RFHAOTPXVQNOHP-UHFFFAOYSA-O 0.000 description 1
- PAWQVTBBRAZDMG-UHFFFAOYSA-N 2-(3-bromo-2-fluorophenyl)acetic acid Chemical compound OC(=O)CC1=CC=CC(Br)=C1F PAWQVTBBRAZDMG-UHFFFAOYSA-N 0.000 description 1
- FEBUJFMRSBAMES-UHFFFAOYSA-N 2-[(2-{[3,5-dihydroxy-2-(hydroxymethyl)-6-phosphanyloxan-4-yl]oxy}-3,5-dihydroxy-6-({[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}methyl)oxan-4-yl)oxy]-3,5-dihydroxy-6-(hydroxymethyl)oxan-4-yl phosphinite Chemical compound OC1C(O)C(O)C(CO)OC1OCC1C(O)C(OC2C(C(OP)C(O)C(CO)O2)O)C(O)C(OC2C(C(CO)OC(P)C2O)O)O1 FEBUJFMRSBAMES-UHFFFAOYSA-N 0.000 description 1
- DSKYSDCYIODJPC-UHFFFAOYSA-N 2-butyl-2-ethylpropane-1,3-diol Chemical compound CCCCC(CC)(CO)CO DSKYSDCYIODJPC-UHFFFAOYSA-N 0.000 description 1
- WROUWQQRXUBECT-UHFFFAOYSA-N 2-ethylacrylic acid Chemical compound CCC(=C)C(O)=O WROUWQQRXUBECT-UHFFFAOYSA-N 0.000 description 1
- GWYFCOCPABKNJV-UHFFFAOYSA-M 3-Methylbutanoic acid Natural products CC(C)CC([O-])=O GWYFCOCPABKNJV-UHFFFAOYSA-M 0.000 description 1
- WUIABRMSWOKTOF-OYALTWQYSA-N 3-[[2-[2-[2-[[(2s,3r)-2-[[(2s,3s,4r)-4-[[(2s,3r)-2-[[6-amino-2-[(1s)-3-amino-1-[[(2s)-2,3-diamino-3-oxopropyl]amino]-3-oxopropyl]-5-methylpyrimidine-4-carbonyl]amino]-3-[(2r,3s,4s,5s,6s)-3-[(2r,3s,4s,5r,6r)-4-carbamoyloxy-3,5-dihydroxy-6-(hydroxymethyl)ox Chemical compound OS([O-])(=O)=O.N([C@H](C(=O)N[C@H](C)[C@@H](O)[C@H](C)C(=O)N[C@@H]([C@H](O)C)C(=O)NCCC=1SC=C(N=1)C=1SC=C(N=1)C(=O)NCCC[S+](C)C)[C@@H](O[C@H]1[C@H]([C@@H](O)[C@H](O)[C@H](CO)O1)O[C@@H]1[C@H]([C@@H](OC(N)=O)[C@H](O)[C@@H](CO)O1)O)C=1NC=NC=1)C(=O)C1=NC([C@H](CC(N)=O)NC[C@H](N)C(N)=O)=NC(N)=C1C WUIABRMSWOKTOF-OYALTWQYSA-N 0.000 description 1
- AOJJSUZBOXZQNB-VTZDEGQISA-N 4'-epidoxorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@@H](O)[C@H](C)O1 AOJJSUZBOXZQNB-VTZDEGQISA-N 0.000 description 1
- OSWFIVFLDKOXQC-UHFFFAOYSA-N 4-(3-methoxyphenyl)aniline Chemical compound COC1=CC=CC(C=2C=CC(N)=CC=2)=C1 OSWFIVFLDKOXQC-UHFFFAOYSA-N 0.000 description 1
- ZHSKUOZOLHMKEA-UHFFFAOYSA-N 4-[5-[bis(2-chloroethyl)amino]-1-methylbenzimidazol-2-yl]butanoic acid;hydron;chloride Chemical compound Cl.ClCCN(CCCl)C1=CC=C2N(C)C(CCCC(O)=O)=NC2=C1 ZHSKUOZOLHMKEA-UHFFFAOYSA-N 0.000 description 1
- UHDGCWIWMRVCDJ-STUHELBRSA-N 4-amino-1-[(3s,4s,5r)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]pyrimidin-2-one Chemical compound O=C1N=C(N)C=CN1C1[C@@H](O)[C@H](O)[C@@H](CO)O1 UHDGCWIWMRVCDJ-STUHELBRSA-N 0.000 description 1
- 101710169336 5'-deoxyadenosine deaminase Proteins 0.000 description 1
- SQDAZGGFXASXDW-UHFFFAOYSA-N 5-bromo-2-(trifluoromethoxy)pyridine Chemical compound FC(F)(F)OC1=CC=C(Br)C=N1 SQDAZGGFXASXDW-UHFFFAOYSA-N 0.000 description 1
- PLIXOHWIPDGJEI-OJSHLMAWSA-N 5-chloro-6-[(2-iminopyrrolidin-1-yl)methyl]-1h-pyrimidine-2,4-dione;1-[(2r,4s,5r)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-5-(trifluoromethyl)pyrimidine-2,4-dione;hydrochloride Chemical compound Cl.N1C(=O)NC(=O)C(Cl)=C1CN1C(=N)CCC1.C1[C@H](O)[C@@H](CO)O[C@H]1N1C(=O)NC(=O)C(C(F)(F)F)=C1 PLIXOHWIPDGJEI-OJSHLMAWSA-N 0.000 description 1
- BSYNRYMUTXBXSQ-FOQJRBATSA-N 59096-14-9 Chemical compound CC(=O)OC1=CC=CC=C1[14C](O)=O BSYNRYMUTXBXSQ-FOQJRBATSA-N 0.000 description 1
- USSIQXCVUWKGNF-UHFFFAOYSA-N 6-(dimethylamino)-4,4-diphenylheptan-3-one Chemical compound C=1C=CC=CC=1C(CC(C)N(C)C)(C(=O)CC)C1=CC=CC=C1 USSIQXCVUWKGNF-UHFFFAOYSA-N 0.000 description 1
- PVXPPJIGRGXGCY-TZLCEDOOSA-N 6-O-alpha-D-glucopyranosyl-D-fructofuranose Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1OC[C@@H]1[C@@H](O)[C@H](O)C(O)(CO)O1 PVXPPJIGRGXGCY-TZLCEDOOSA-N 0.000 description 1
- VVIAGPKUTFNRDU-UHFFFAOYSA-N 6S-folinic acid Natural products C1NC=2NC(N)=NC(=O)C=2N(C=O)C1CNC1=CC=C(C(=O)NC(CCC(O)=O)C(O)=O)C=C1 VVIAGPKUTFNRDU-UHFFFAOYSA-N 0.000 description 1
- MYYIMZRZXIQBGI-HVIRSNARSA-N 6alpha-Fluoroprednisolone Chemical compound O=C1C=C[C@]2(C)[C@H]3[C@@H](O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3C[C@H](F)C2=C1 MYYIMZRZXIQBGI-HVIRSNARSA-N 0.000 description 1
- RHXHGRAEPCAFML-UHFFFAOYSA-N 7-cyclopentyl-n,n-dimethyl-2-[(5-piperazin-1-ylpyridin-2-yl)amino]pyrrolo[2,3-d]pyrimidine-6-carboxamide Chemical compound N1=C2N(C3CCCC3)C(C(=O)N(C)C)=CC2=CN=C1NC(N=C1)=CC=C1N1CCNCC1 RHXHGRAEPCAFML-UHFFFAOYSA-N 0.000 description 1
- SHGAZHPCJJPHSC-ZVCIMWCZSA-N 9-cis-retinoic acid Chemical compound OC(=O)/C=C(\C)/C=C/C=C(/C)\C=C\C1=C(C)CCCC1(C)C SHGAZHPCJJPHSC-ZVCIMWCZSA-N 0.000 description 1
- 102100031585 ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1 Human genes 0.000 description 1
- 208000002008 AIDS-Related Lymphoma Diseases 0.000 description 1
- 125000003345 AMP group Chemical group 0.000 description 1
- 206010000060 Abdominal distension Diseases 0.000 description 1
- 208000024893 Acute lymphoblastic leukemia Diseases 0.000 description 1
- 101710137115 Adenylyl cyclase-associated protein 1 Proteins 0.000 description 1
- 102100021879 Adenylyl cyclase-associated protein 2 Human genes 0.000 description 1
- 101710137132 Adenylyl cyclase-associated protein 2 Proteins 0.000 description 1
- 108010012934 Albumin-Bound Paclitaxel Proteins 0.000 description 1
- 102000003730 Alpha-catenin Human genes 0.000 description 1
- 108090000020 Alpha-catenin Proteins 0.000 description 1
- 102100023635 Alpha-fetoprotein Human genes 0.000 description 1
- 208000004881 Amebiasis Diseases 0.000 description 1
- 206010001980 Amoebiasis Diseases 0.000 description 1
- 229920000945 Amylopectin Polymers 0.000 description 1
- WZPBZJONDBGPKJ-UHFFFAOYSA-N Antibiotic SQ 26917 Natural products O=C1N(S(O)(=O)=O)C(C)C1NC(=O)C(=NOC(C)(C)C(O)=O)C1=CSC(N)=N1 WZPBZJONDBGPKJ-UHFFFAOYSA-N 0.000 description 1
- 102000044503 Antimicrobial Peptides Human genes 0.000 description 1
- 108700042778 Antimicrobial Peptides Proteins 0.000 description 1
- 208000016583 Anus disease Diseases 0.000 description 1
- 208000019901 Anxiety disease Diseases 0.000 description 1
- 101001005269 Arabidopsis thaliana Ceramide synthase 1 LOH3 Proteins 0.000 description 1
- 101001005312 Arabidopsis thaliana Ceramide synthase LOH1 Proteins 0.000 description 1
- 101100504181 Arabidopsis thaliana GCS1 gene Proteins 0.000 description 1
- 102000003984 Aryl Hydrocarbon Receptors Human genes 0.000 description 1
- 108090000448 Aryl Hydrocarbon Receptors Proteins 0.000 description 1
- 108010011485 Aspartame Proteins 0.000 description 1
- 206010003645 Atopy Diseases 0.000 description 1
- IVRMZWNICZWHMI-UHFFFAOYSA-N Azide Chemical compound [N-]=[N+]=[N-] IVRMZWNICZWHMI-UHFFFAOYSA-N 0.000 description 1
- 102100035526 B melanoma antigen 1 Human genes 0.000 description 1
- 102100029822 B- and T-lymphocyte attenuator Human genes 0.000 description 1
- 208000010839 B-cell chronic lymphocytic leukemia Diseases 0.000 description 1
- 208000003950 B-cell lymphoma Diseases 0.000 description 1
- 102100038080 B-cell receptor CD22 Human genes 0.000 description 1
- 102100022005 B-lymphocyte antigen CD20 Human genes 0.000 description 1
- MLDQJTXFUGDVEO-UHFFFAOYSA-N BAY-43-9006 Chemical compound C1=NC(C(=O)NC)=CC(OC=2C=CC(NC(=O)NC=3C=C(C(Cl)=CC=3)C(F)(F)F)=CC=2)=C1 MLDQJTXFUGDVEO-UHFFFAOYSA-N 0.000 description 1
- 241000606126 Bacteroidaceae Species 0.000 description 1
- 241000692822 Bacteroidales Species 0.000 description 1
- 241000605059 Bacteroidetes Species 0.000 description 1
- 241001141113 Bacteroidia Species 0.000 description 1
- 206010004146 Basal cell carcinoma Diseases 0.000 description 1
- 208000027496 Behcet disease Diseases 0.000 description 1
- 102000015735 Beta-catenin Human genes 0.000 description 1
- 108060000903 Beta-catenin Proteins 0.000 description 1
- KWIUHFFTVRNATP-UHFFFAOYSA-N Betaine Natural products C[N+](C)(C)CC([O-])=O KWIUHFFTVRNATP-UHFFFAOYSA-N 0.000 description 1
- 241000186016 Bifidobacterium bifidum Species 0.000 description 1
- 241000186012 Bifidobacterium breve Species 0.000 description 1
- 241001608472 Bifidobacterium longum Species 0.000 description 1
- 241000186015 Bifidobacterium longum subsp. infantis Species 0.000 description 1
- 206010005003 Bladder cancer Diseases 0.000 description 1
- 241001202853 Blautia Species 0.000 description 1
- 206010005949 Bone cancer Diseases 0.000 description 1
- 208000018084 Bone neoplasm Diseases 0.000 description 1
- 241000283690 Bos taurus Species 0.000 description 1
- 241000167854 Bourreria succulenta Species 0.000 description 1
- 208000003174 Brain Neoplasms Diseases 0.000 description 1
- 206010055113 Breast cancer metastatic Diseases 0.000 description 1
- 102000003930 C-Type Lectins Human genes 0.000 description 1
- 108090000342 C-Type Lectins Proteins 0.000 description 1
- 102100024263 CD160 antigen Human genes 0.000 description 1
- 102100027207 CD27 antigen Human genes 0.000 description 1
- 102100038078 CD276 antigen Human genes 0.000 description 1
- 101150013553 CD40 gene Proteins 0.000 description 1
- 102100025221 CD70 antigen Human genes 0.000 description 1
- 101150050673 CHK1 gene Proteins 0.000 description 1
- 102000000905 Cadherin Human genes 0.000 description 1
- 108050007957 Cadherin Proteins 0.000 description 1
- 101100455063 Caenorhabditis elegans lmp-1 gene Proteins 0.000 description 1
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 1
- 102100028801 Calsyntenin-1 Human genes 0.000 description 1
- 102100025570 Cancer/testis antigen 1 Human genes 0.000 description 1
- 102100039510 Cancer/testis antigen 2 Human genes 0.000 description 1
- GAGWJHPBXLXJQN-UORFTKCHSA-N Capecitabine Chemical compound C1=C(F)C(NC(=O)OCCCCC)=NC(=O)N1[C@H]1[C@H](O)[C@H](O)[C@@H](C)O1 GAGWJHPBXLXJQN-UORFTKCHSA-N 0.000 description 1
- GAGWJHPBXLXJQN-UHFFFAOYSA-N Capecitabine Natural products C1=C(F)C(NC(=O)OCCCCC)=NC(=O)N1C1C(O)C(O)C(C)O1 GAGWJHPBXLXJQN-UHFFFAOYSA-N 0.000 description 1
- BVKZGUZCCUSVTD-UHFFFAOYSA-L Carbonate Chemical compound [O-]C([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-L 0.000 description 1
- 229920002134 Carboxymethyl cellulose Polymers 0.000 description 1
- 208000009458 Carcinoma in Situ Diseases 0.000 description 1
- 206010062746 Carditis Diseases 0.000 description 1
- 241000723418 Carya Species 0.000 description 1
- 102100028906 Catenin delta-1 Human genes 0.000 description 1
- 241000700198 Cavia Species 0.000 description 1
- 241000700199 Cavia porcellus Species 0.000 description 1
- 102100025064 Cellular tumor antigen p53 Human genes 0.000 description 1
- 206010008342 Cervix carcinoma Diseases 0.000 description 1
- JWBOIMRXGHLCPP-UHFFFAOYSA-N Chloditan Chemical compound C=1C=CC=C(Cl)C=1C(C(Cl)Cl)C1=CC=C(Cl)C=C1 JWBOIMRXGHLCPP-UHFFFAOYSA-N 0.000 description 1
- 229920001287 Chondroitin sulfate Polymers 0.000 description 1
- 208000006332 Choriocarcinoma Diseases 0.000 description 1
- 208000005443 Circulating Neoplastic Cells Diseases 0.000 description 1
- KRKNYBCHXYNGOX-UHFFFAOYSA-K Citrate Chemical compound [O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O KRKNYBCHXYNGOX-UHFFFAOYSA-K 0.000 description 1
- 241000588923 Citrobacter Species 0.000 description 1
- 241001112696 Clostridia Species 0.000 description 1
- 241001112695 Clostridiales Species 0.000 description 1
- 241000193171 Clostridium butyricum Species 0.000 description 1
- 208000003322 Coinfection Diseases 0.000 description 1
- 208000002881 Colic Diseases 0.000 description 1
- 206010009895 Colitis ischaemic Diseases 0.000 description 1
- 206010055114 Colon cancer metastatic Diseases 0.000 description 1
- 208000001333 Colorectal Neoplasms Diseases 0.000 description 1
- 241001464948 Coprococcus Species 0.000 description 1
- MFYSYFVPBJMHGN-ZPOLXVRWSA-N Cortisone Chemical compound O=C1CC[C@]2(C)[C@H]3C(=O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 MFYSYFVPBJMHGN-ZPOLXVRWSA-N 0.000 description 1
- MFYSYFVPBJMHGN-UHFFFAOYSA-N Cortisone Natural products O=C1CCC2(C)C3C(=O)CC(C)(C(CC4)(O)C(=O)CO)C4C3CCC2=C1 MFYSYFVPBJMHGN-UHFFFAOYSA-N 0.000 description 1
- 229920002785 Croscarmellose sodium Polymers 0.000 description 1
- 208000019707 Cryoglobulinemic vasculitis Diseases 0.000 description 1
- 241000724252 Cucumber mosaic virus Species 0.000 description 1
- 108010025464 Cyclin-Dependent Kinase 4 Proteins 0.000 description 1
- 102100036252 Cyclin-dependent kinase 4 Human genes 0.000 description 1
- 229920000858 Cyclodextrin Polymers 0.000 description 1
- 241001541138 Cypho Species 0.000 description 1
- 102000004127 Cytokines Human genes 0.000 description 1
- 108090000695 Cytokines Proteins 0.000 description 1
- 241000701022 Cytomegalovirus Species 0.000 description 1
- 206010011831 Cytomegalovirus infection Diseases 0.000 description 1
- XUJNEKJLAYXESH-UWTATZPHSA-N D-Cysteine Chemical compound SC[C@@H](N)C(O)=O XUJNEKJLAYXESH-UWTATZPHSA-N 0.000 description 1
- HMFHBZSHGGEWLO-SOOFDHNKSA-N D-ribofuranose Chemical compound OC[C@H]1OC(O)[C@H](O)[C@@H]1O HMFHBZSHGGEWLO-SOOFDHNKSA-N 0.000 description 1
- 108010002069 Defensins Proteins 0.000 description 1
- 102000000541 Defensins Human genes 0.000 description 1
- 208000005156 Dehydration Diseases 0.000 description 1
- 206010012205 Delayed puberty Diseases 0.000 description 1
- 206010012559 Developmental delay Diseases 0.000 description 1
- 229920002307 Dextran Polymers 0.000 description 1
- PYGXAGIECVVIOZ-UHFFFAOYSA-N Dibutyl decanedioate Chemical compound CCCCOC(=O)CCCCCCCCC(=O)OCCCC PYGXAGIECVVIOZ-UHFFFAOYSA-N 0.000 description 1
- 235000019739 Dicalciumphosphate Nutrition 0.000 description 1
- 101100216227 Dictyostelium discoideum anapc3 gene Proteins 0.000 description 1
- HHJIUUAMYGBVSD-YTFFSALGSA-N Diflucortolone valerate Chemical compound C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@]1(F)[C@@H]2[C@@H]2C[C@@H](C)[C@H](C(=O)COC(=O)CCCC)[C@@]2(C)C[C@@H]1O HHJIUUAMYGBVSD-YTFFSALGSA-N 0.000 description 1
- WYQPLTPSGFELIB-JTQPXKBDSA-N Difluprednate Chemical compound C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@]1(F)[C@@H]2[C@@H]2CC[C@@](C(=O)COC(C)=O)(OC(=O)CCC)[C@@]2(C)C[C@@H]1O WYQPLTPSGFELIB-JTQPXKBDSA-N 0.000 description 1
- 208000002699 Digestive System Neoplasms Diseases 0.000 description 1
- 108010067722 Dipeptidyl Peptidase 4 Proteins 0.000 description 1
- 206010013554 Diverticulum Diseases 0.000 description 1
- 241001143779 Dorea Species 0.000 description 1
- 208000027244 Dysbiosis Diseases 0.000 description 1
- 206010013911 Dysgeusia Diseases 0.000 description 1
- 229930195710 D‐cysteine Natural products 0.000 description 1
- 108010015972 Elafin Proteins 0.000 description 1
- 206010058838 Enterocolitis infectious Diseases 0.000 description 1
- 101710146739 Enterotoxin Proteins 0.000 description 1
- HTIJFSOGRVMCQR-UHFFFAOYSA-N Epirubicin Natural products COc1cccc2C(=O)c3c(O)c4CC(O)(CC(OC5CC(N)C(=O)C(C)O5)c4c(O)c3C(=O)c12)C(=O)CO HTIJFSOGRVMCQR-UHFFFAOYSA-N 0.000 description 1
- 102000018651 Epithelial Cell Adhesion Molecule Human genes 0.000 description 1
- 108010066687 Epithelial Cell Adhesion Molecule Proteins 0.000 description 1
- 206010015108 Epstein-Barr virus infection Diseases 0.000 description 1
- 208000000461 Esophageal Neoplasms Diseases 0.000 description 1
- 241000186394 Eubacterium Species 0.000 description 1
- 229920003136 Eudragit® L polymer Polymers 0.000 description 1
- 229920003137 Eudragit® S polymer Polymers 0.000 description 1
- 241001608234 Faecalibacterium Species 0.000 description 1
- 239000004606 Fillers/Extenders Substances 0.000 description 1
- 241000192125 Firmicutes Species 0.000 description 1
- POPFMWWJOGLOIF-XWCQMRHXSA-N Flurandrenolide Chemical compound C1([C@@H](F)C2)=CC(=O)CC[C@]1(C)[C@@H]1[C@@H]2[C@@H]2C[C@H]3OC(C)(C)O[C@@]3(C(=O)CO)[C@@]2(C)C[C@@H]1O POPFMWWJOGLOIF-XWCQMRHXSA-N 0.000 description 1
- BDAGIHXWWSANSR-UHFFFAOYSA-M Formate Chemical compound [O-]C=O BDAGIHXWWSANSR-UHFFFAOYSA-M 0.000 description 1
- 102100039717 G antigen 1 Human genes 0.000 description 1
- 102100039699 G antigen 4 Human genes 0.000 description 1
- 102100039698 G antigen 5 Human genes 0.000 description 1
- 101710092267 G antigen 5 Proteins 0.000 description 1
- 102100039713 G antigen 6 Human genes 0.000 description 1
- 101710092269 G antigen 6 Proteins 0.000 description 1
- 102000040452 GAGE family Human genes 0.000 description 1
- 108091072337 GAGE family Proteins 0.000 description 1
- 101710198884 GATA-type zinc finger protein 1 Proteins 0.000 description 1
- 102100025101 GATA-type zinc finger protein 1 Human genes 0.000 description 1
- 102100029974 GTPase HRas Human genes 0.000 description 1
- 101710091881 GTPase HRas Proteins 0.000 description 1
- 102100031351 Galectin-9 Human genes 0.000 description 1
- 101100229077 Gallus gallus GAL9 gene Proteins 0.000 description 1
- 102100030525 Gap junction alpha-4 protein Human genes 0.000 description 1
- 229940124897 Gardasil Drugs 0.000 description 1
- 208000007882 Gastritis Diseases 0.000 description 1
- 206010017993 Gastrointestinal neoplasms Diseases 0.000 description 1
- 208000009139 Gilbert Disease Diseases 0.000 description 1
- 208000022412 Gilbert syndrome Diseases 0.000 description 1
- DTHNMHAUYICORS-KTKZVXAJSA-N Glucagon-like peptide 1 Chemical compound C([C@@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](C)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(N)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CCC(N)=O)NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CC=1N=CNC=1)[C@@H](C)O)[C@@H](C)O)C(C)C)C1=CC=CC=C1 DTHNMHAUYICORS-KTKZVXAJSA-N 0.000 description 1
- 102400000326 Glucagon-like peptide 2 Human genes 0.000 description 1
- 101800000221 Glucagon-like peptide 2 Proteins 0.000 description 1
- 108010024636 Glutathione Proteins 0.000 description 1
- 239000004471 Glycine Substances 0.000 description 1
- 102000007390 Glycogen Phosphorylase Human genes 0.000 description 1
- 108010046163 Glycogen Phosphorylase Proteins 0.000 description 1
- NMJREATYWWNIKX-UHFFFAOYSA-N GnRH Chemical class C1CCC(C(=O)NCC(N)=O)N1C(=O)C(CC(C)C)NC(=O)C(CC=1C2=CC=CC=C2NC=1)NC(=O)CNC(=O)C(NC(=O)C(CO)NC(=O)C(CC=1C2=CC=CC=C2NC=1)NC(=O)C(CC=1NC=NC=1)NC(=O)C1NC(=O)CC1)CC1=CC=C(O)C=C1 NMJREATYWWNIKX-UHFFFAOYSA-N 0.000 description 1
- 239000000579 Gonadotropin-Releasing Hormone Substances 0.000 description 1
- 208000012766 Growth delay Diseases 0.000 description 1
- 102100035943 HERV-H LTR-associating protein 2 Human genes 0.000 description 1
- 208000007521 HIV Seropositivity Diseases 0.000 description 1
- 102100030595 HLA class II histocompatibility antigen gamma chain Human genes 0.000 description 1
- 206010018852 Haematoma Diseases 0.000 description 1
- MUQNGPZZQDCDFT-JNQJZLCISA-N Halcinonide Chemical compound C1CC2=CC(=O)CC[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@H]3OC(C)(C)O[C@@]3(C(=O)CCl)[C@@]1(C)C[C@@H]2O MUQNGPZZQDCDFT-JNQJZLCISA-N 0.000 description 1
- 208000002250 Hematologic Neoplasms Diseases 0.000 description 1
- 102100029100 Hematopoietic prostaglandin D synthase Human genes 0.000 description 1
- 102000001554 Hemoglobins Human genes 0.000 description 1
- 108010054147 Hemoglobins Proteins 0.000 description 1
- GVGLGOZIDCSQPN-PVHGPHFFSA-N Heroin Chemical compound O([C@H]1[C@H](C=C[C@H]23)OC(C)=O)C4=C5[C@@]12CCN(C)[C@@H]3CC5=CC=C4OC(C)=O GVGLGOZIDCSQPN-PVHGPHFFSA-N 0.000 description 1
- 208000017604 Hodgkin disease Diseases 0.000 description 1
- 208000021519 Hodgkin lymphoma Diseases 0.000 description 1
- 208000010747 Hodgkins lymphoma Diseases 0.000 description 1
- 101000777636 Homo sapiens ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1 Proteins 0.000 description 1
- 101000874316 Homo sapiens B melanoma antigen 1 Proteins 0.000 description 1
- 101000864344 Homo sapiens B- and T-lymphocyte attenuator Proteins 0.000 description 1
- 101000884305 Homo sapiens B-cell receptor CD22 Proteins 0.000 description 1
- 101000897405 Homo sapiens B-lymphocyte antigen CD20 Proteins 0.000 description 1
- 101000914511 Homo sapiens CD27 antigen Proteins 0.000 description 1
- 101000934356 Homo sapiens CD70 antigen Proteins 0.000 description 1
- 101000856237 Homo sapiens Cancer/testis antigen 1 Proteins 0.000 description 1
- 101000889345 Homo sapiens Cancer/testis antigen 2 Proteins 0.000 description 1
- 101000721661 Homo sapiens Cellular tumor antigen p53 Proteins 0.000 description 1
- 101000886137 Homo sapiens G antigen 1 Proteins 0.000 description 1
- 101000886678 Homo sapiens G antigen 2D Proteins 0.000 description 1
- 101000886136 Homo sapiens G antigen 4 Proteins 0.000 description 1
- 101001082627 Homo sapiens HLA class II histocompatibility antigen gamma chain Proteins 0.000 description 1
- 101000988802 Homo sapiens Hematopoietic prostaglandin D synthase Proteins 0.000 description 1
- 101001055145 Homo sapiens Interleukin-2 receptor subunit beta Proteins 0.000 description 1
- 101000777628 Homo sapiens Leukocyte antigen CD37 Proteins 0.000 description 1
- 101000868279 Homo sapiens Leukocyte surface antigen CD47 Proteins 0.000 description 1
- 101001137987 Homo sapiens Lymphocyte activation gene 3 protein Proteins 0.000 description 1
- 101000578784 Homo sapiens Melanoma antigen recognized by T-cells 1 Proteins 0.000 description 1
- 101001036406 Homo sapiens Melanoma-associated antigen C1 Proteins 0.000 description 1
- 101001057156 Homo sapiens Melanoma-associated antigen C2 Proteins 0.000 description 1
- 101001057159 Homo sapiens Melanoma-associated antigen C3 Proteins 0.000 description 1
- 101001133056 Homo sapiens Mucin-1 Proteins 0.000 description 1
- 101000623901 Homo sapiens Mucin-16 Proteins 0.000 description 1
- 101000934338 Homo sapiens Myeloid cell surface antigen CD33 Proteins 0.000 description 1
- 101000884270 Homo sapiens Natural killer cell receptor 2B4 Proteins 0.000 description 1
- 101000581981 Homo sapiens Neural cell adhesion molecule 1 Proteins 0.000 description 1
- 101001098352 Homo sapiens OX-2 membrane glycoprotein Proteins 0.000 description 1
- 101001114057 Homo sapiens P antigen family member 1 Proteins 0.000 description 1
- 101000880770 Homo sapiens Protein SSX2 Proteins 0.000 description 1
- 101001062222 Homo sapiens Receptor-binding cancer antigen expressed on SiSo cells Proteins 0.000 description 1
- 101000633784 Homo sapiens SLAM family member 7 Proteins 0.000 description 1
- 101000777277 Homo sapiens Serine/threonine-protein kinase Chk2 Proteins 0.000 description 1
- 101000914514 Homo sapiens T-cell-specific surface glycoprotein CD28 Proteins 0.000 description 1
- 101000763579 Homo sapiens Toll-like receptor 1 Proteins 0.000 description 1
- 101000763537 Homo sapiens Toll-like receptor 10 Proteins 0.000 description 1
- 101000831567 Homo sapiens Toll-like receptor 2 Proteins 0.000 description 1
- 101000831496 Homo sapiens Toll-like receptor 3 Proteins 0.000 description 1
- 101000669447 Homo sapiens Toll-like receptor 4 Proteins 0.000 description 1
- 101000669406 Homo sapiens Toll-like receptor 6 Proteins 0.000 description 1
- 101000669402 Homo sapiens Toll-like receptor 7 Proteins 0.000 description 1
- 101000800483 Homo sapiens Toll-like receptor 8 Proteins 0.000 description 1
- 101000904724 Homo sapiens Transmembrane glycoprotein NMB Proteins 0.000 description 1
- 101000801433 Homo sapiens Trophoblast glycoprotein Proteins 0.000 description 1
- 101000801234 Homo sapiens Tumor necrosis factor receptor superfamily member 18 Proteins 0.000 description 1
- 101000851376 Homo sapiens Tumor necrosis factor receptor superfamily member 8 Proteins 0.000 description 1
- 101000851370 Homo sapiens Tumor necrosis factor receptor superfamily member 9 Proteins 0.000 description 1
- 101000666896 Homo sapiens V-type immunoglobulin domain-containing suppressor of T-cell activation Proteins 0.000 description 1
- 229920000869 Homopolysaccharide Polymers 0.000 description 1
- 229940123502 Hormone receptor antagonist Drugs 0.000 description 1
- 241000725303 Human immunodeficiency virus Species 0.000 description 1
- 241000713772 Human immunodeficiency virus 1 Species 0.000 description 1
- 102000004867 Hydro-Lyases Human genes 0.000 description 1
- 108090001042 Hydro-Lyases Proteins 0.000 description 1
- 206010020751 Hypersensitivity Diseases 0.000 description 1
- 102100034980 ICOS ligand Human genes 0.000 description 1
- HEFNNWSXXWATRW-UHFFFAOYSA-N Ibuprofen Chemical compound CC(C)CC1=CC=C(C(C)C(O)=O)C=C1 HEFNNWSXXWATRW-UHFFFAOYSA-N 0.000 description 1
- 102000037982 Immune checkpoint proteins Human genes 0.000 description 1
- 108091008036 Immune checkpoint proteins Proteins 0.000 description 1
- 229940123776 Immuno-oncology therapy Drugs 0.000 description 1
- 102000008070 Interferon-gamma Human genes 0.000 description 1
- 108010074328 Interferon-gamma Proteins 0.000 description 1
- 102100020793 Interleukin-13 receptor subunit alpha-2 Human genes 0.000 description 1
- 101710112634 Interleukin-13 receptor subunit alpha-2 Proteins 0.000 description 1
- 108010002350 Interleukin-2 Proteins 0.000 description 1
- 102000000588 Interleukin-2 Human genes 0.000 description 1
- 102100026879 Interleukin-2 receptor subunit beta Human genes 0.000 description 1
- 102100030703 Interleukin-22 Human genes 0.000 description 1
- 108010066979 Interleukin-27 Proteins 0.000 description 1
- JUZNIMUFDBIJCM-ANEDZVCMSA-N Invanz Chemical compound O=C([C@H]1NC[C@H](C1)SC=1[C@H](C)[C@@H]2[C@H](C(N2C=1C(O)=O)=O)[C@H](O)C)NC1=CC=CC(C(O)=O)=C1 JUZNIMUFDBIJCM-ANEDZVCMSA-N 0.000 description 1
- 206010022971 Iron Deficiencies Diseases 0.000 description 1
- 208000011200 Kawasaki disease Diseases 0.000 description 1
- ONIBWKKTOPOVIA-BYPYZUCNSA-N L-Proline Chemical compound OC(=O)[C@@H]1CCCN1 ONIBWKKTOPOVIA-BYPYZUCNSA-N 0.000 description 1
- QNAYBMKLOCPYGJ-REOHCLBHSA-N L-alanine Chemical compound C[C@H](N)C(O)=O QNAYBMKLOCPYGJ-REOHCLBHSA-N 0.000 description 1
- 239000004201 L-cysteine Substances 0.000 description 1
- 235000013878 L-cysteine Nutrition 0.000 description 1
- ZDXPYRJPNDTMRX-VKHMYHEASA-N L-glutamine Chemical compound OC(=O)[C@@H](N)CCC(N)=O ZDXPYRJPNDTMRX-VKHMYHEASA-N 0.000 description 1
- HNDVDQJCIGZPNO-YFKPBYRVSA-N L-histidine Chemical compound OC(=O)[C@@H](N)CC1=CN=CN1 HNDVDQJCIGZPNO-YFKPBYRVSA-N 0.000 description 1
- FFEARJCKVFRZRR-BYPYZUCNSA-N L-methionine Chemical compound CSCC[C@H](N)C(O)=O FFEARJCKVFRZRR-BYPYZUCNSA-N 0.000 description 1
- 239000005511 L01XE05 - Sorafenib Substances 0.000 description 1
- 239000002146 L01XE16 - Crizotinib Substances 0.000 description 1
- 239000002138 L01XE21 - Regorafenib Substances 0.000 description 1
- 102000017578 LAG3 Human genes 0.000 description 1
- 241001112693 Lachnospiraceae Species 0.000 description 1
- 241001112724 Lactobacillales Species 0.000 description 1
- 240000001046 Lactobacillus acidophilus Species 0.000 description 1
- 235000013956 Lactobacillus acidophilus Nutrition 0.000 description 1
- 240000006024 Lactobacillus plantarum Species 0.000 description 1
- 235000013965 Lactobacillus plantarum Nutrition 0.000 description 1
- 241000917009 Lactobacillus rhamnosus GG Species 0.000 description 1
- 206010023825 Laryngeal cancer Diseases 0.000 description 1
- 240000007472 Leucaena leucocephala Species 0.000 description 1
- 235000010643 Leucaena leucocephala Nutrition 0.000 description 1
- 102100031586 Leukocyte antigen CD37 Human genes 0.000 description 1
- 102100032913 Leukocyte surface antigen CD47 Human genes 0.000 description 1
- GSDSWSVVBLHKDQ-JTQLQIEISA-N Levofloxacin Chemical compound C([C@@H](N1C2=C(C(C(C(O)=O)=C1)=O)C=C1F)C)OC2=C1N1CCN(C)CC1 GSDSWSVVBLHKDQ-JTQLQIEISA-N 0.000 description 1
- 208000007820 Lichen Sclerosus et Atrophicus Diseases 0.000 description 1
- 206010058467 Lung neoplasm malignant Diseases 0.000 description 1
- 206010025312 Lymphoma AIDS related Diseases 0.000 description 1
- 108010010995 MART-1 Antigen Proteins 0.000 description 1
- 102100028198 Macrophage colony-stimulating factor 1 receptor Human genes 0.000 description 1
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 1
- 229920002774 Maltodextrin Polymers 0.000 description 1
- 239000005913 Maltodextrin Substances 0.000 description 1
- GUBGYTABKSRVRQ-PICCSMPSSA-N Maltose Natural products O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@@H](CO)OC(O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-PICCSMPSSA-N 0.000 description 1
- 208000025205 Mantle-Cell Lymphoma Diseases 0.000 description 1
- 208000006395 Meigs Syndrome Diseases 0.000 description 1
- 206010027139 Meigs' syndrome Diseases 0.000 description 1
- 206010027145 Melanocytic naevus Diseases 0.000 description 1
- 102100039447 Melanoma-associated antigen C1 Human genes 0.000 description 1
- 102100027252 Melanoma-associated antigen C2 Human genes 0.000 description 1
- 102100027248 Melanoma-associated antigen C3 Human genes 0.000 description 1
- YJPIGAIKUZMOQA-UHFFFAOYSA-N Melatonin Natural products COC1=CC=C2N(C(C)=O)C=C(CCN)C2=C1 YJPIGAIKUZMOQA-UHFFFAOYSA-N 0.000 description 1
- 244000246386 Mentha pulegium Species 0.000 description 1
- 235000016257 Mentha pulegium Nutrition 0.000 description 1
- 235000004357 Mentha x piperita Nutrition 0.000 description 1
- 102000003735 Mesothelin Human genes 0.000 description 1
- 108090000015 Mesothelin Proteins 0.000 description 1
- 206010027476 Metastases Diseases 0.000 description 1
- FQISKWAFAHGMGT-SGJOWKDISA-M Methylprednisolone sodium succinate Chemical compound [Na+].C([C@@]12C)=CC(=O)C=C1[C@@H](C)C[C@@H]1[C@@H]2[C@@H](O)C[C@]2(C)[C@@](O)(C(=O)COC(=O)CCC([O-])=O)CC[C@H]21 FQISKWAFAHGMGT-SGJOWKDISA-M 0.000 description 1
- 229930192392 Mitomycin Natural products 0.000 description 1
- 239000004909 Moisturizer Substances 0.000 description 1
- 102100034256 Mucin-1 Human genes 0.000 description 1
- 102100023123 Mucin-16 Human genes 0.000 description 1
- 108010063954 Mucins Proteins 0.000 description 1
- MSFSPUZXLOGKHJ-UHFFFAOYSA-N Muraminsaeure Natural products OC(=O)C(C)OC1C(N)C(O)OC(CO)C1O MSFSPUZXLOGKHJ-UHFFFAOYSA-N 0.000 description 1
- 101100219181 Mus musculus Cd160 gene Proteins 0.000 description 1
- 101100481581 Mus musculus Tlr13 gene Proteins 0.000 description 1
- 208000008238 Muscle Spasticity Diseases 0.000 description 1
- 102100025243 Myeloid cell surface antigen CD33 Human genes 0.000 description 1
- 102100031789 Myeloid-derived growth factor Human genes 0.000 description 1
- KWIUHFFTVRNATP-UHFFFAOYSA-O N,N,N-trimethylglycinium Chemical compound C[N+](C)(C)CC(O)=O KWIUHFFTVRNATP-UHFFFAOYSA-O 0.000 description 1
- WHNWPMSKXPGLAX-UHFFFAOYSA-N N-Vinyl-2-pyrrolidone Chemical compound C=CN1CCCC1=O WHNWPMSKXPGLAX-UHFFFAOYSA-N 0.000 description 1
- PLILLUUXAVKBPY-SBIAVEDLSA-N NCCO.NCCO.CC1=NN(C=2C=C(C)C(C)=CC=2)C(=O)\C1=N/NC(C=1O)=CC=CC=1C1=CC=CC(C(O)=O)=C1 Chemical compound NCCO.NCCO.CC1=NN(C=2C=C(C)C(C)=CC=2)C(=O)\C1=N/NC(C=1O)=CC=CC=1C1=CC=CC(C(O)=O)=C1 PLILLUUXAVKBPY-SBIAVEDLSA-N 0.000 description 1
- CMWTZPSULFXXJA-UHFFFAOYSA-N Naproxen Natural products C1=C(C(C)C(O)=O)C=CC2=CC(OC)=CC=C21 CMWTZPSULFXXJA-UHFFFAOYSA-N 0.000 description 1
- 102100029527 Natural cytotoxicity triggering receptor 3 ligand 1 Human genes 0.000 description 1
- 102100038082 Natural killer cell receptor 2B4 Human genes 0.000 description 1
- 208000003788 Neoplasm Micrometastasis Diseases 0.000 description 1
- 102100027347 Neural cell adhesion molecule 1 Human genes 0.000 description 1
- 102100024964 Neural cell adhesion molecule L1 Human genes 0.000 description 1
- 206010029260 Neuroblastoma Diseases 0.000 description 1
- 208000027626 Neurocognitive disease Diseases 0.000 description 1
- 208000029726 Neurodevelopmental disease Diseases 0.000 description 1
- 208000007256 Nevus Diseases 0.000 description 1
- 239000000020 Nitrocellulose Substances 0.000 description 1
- 102000019040 Nuclear Antigens Human genes 0.000 description 1
- 108010051791 Nuclear Antigens Proteins 0.000 description 1
- 102100037589 OX-2 membrane glycoprotein Human genes 0.000 description 1
- 208000008589 Obesity Diseases 0.000 description 1
- 206010030155 Oesophageal carcinoma Diseases 0.000 description 1
- 241000283973 Oryctolagus cuniculus Species 0.000 description 1
- 206010033128 Ovarian cancer Diseases 0.000 description 1
- 206010061535 Ovarian neoplasm Diseases 0.000 description 1
- 208000012868 Overgrowth Diseases 0.000 description 1
- 239000004100 Oxytetracycline Substances 0.000 description 1
- 102100023219 P antigen family member 1 Human genes 0.000 description 1
- 239000008118 PEG 6000 Substances 0.000 description 1
- 108060006580 PRAME Proteins 0.000 description 1
- 102000036673 PRAME Human genes 0.000 description 1
- 102100034640 PWWP domain-containing DNA repair factor 3A Human genes 0.000 description 1
- 108050007154 PWWP domain-containing DNA repair factor 3A Proteins 0.000 description 1
- 208000035467 Pancreatic insufficiency Diseases 0.000 description 1
- 206010061902 Pancreatic neoplasm Diseases 0.000 description 1
- 206010033645 Pancreatitis Diseases 0.000 description 1
- SHGAZHPCJJPHSC-UHFFFAOYSA-N Panrexin Chemical compound OC(=O)C=C(C)C=CC=C(C)C=CC1=C(C)CCCC1(C)C SHGAZHPCJJPHSC-UHFFFAOYSA-N 0.000 description 1
- MKPDWECBUAZOHP-AFYJWTTESA-N Paramethasone Chemical compound C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@@H]1[C@@H]2[C@@H]2C[C@@H](C)[C@@](C(=O)CO)(O)[C@@]2(C)C[C@@H]1O MKPDWECBUAZOHP-AFYJWTTESA-N 0.000 description 1
- 206010048705 Paraneoplastic cerebellar degeneration Diseases 0.000 description 1
- 241001494479 Pecora Species 0.000 description 1
- 229930195708 Penicillin V Natural products 0.000 description 1
- 208000008469 Peptic Ulcer Diseases 0.000 description 1
- 108010013639 Peptidoglycan Proteins 0.000 description 1
- 102100040283 Peptidyl-prolyl cis-trans isomerase B Human genes 0.000 description 1
- OAICVXFJPJFONN-UHFFFAOYSA-N Phosphorus Chemical compound [P] OAICVXFJPJFONN-UHFFFAOYSA-N 0.000 description 1
- 206010035226 Plasma cell myeloma Diseases 0.000 description 1
- 229920003171 Poly (ethylene oxide) Polymers 0.000 description 1
- 229920001100 Polydextrose Polymers 0.000 description 1
- 229920002584 Polyethylene Glycol 6000 Polymers 0.000 description 1
- 208000037062 Polyps Diseases 0.000 description 1
- 229920001213 Polysorbate 20 Polymers 0.000 description 1
- 229920001219 Polysorbate 40 Polymers 0.000 description 1
- 229920001214 Polysorbate 60 Polymers 0.000 description 1
- 241000036848 Porzana carolina Species 0.000 description 1
- 208000002389 Pouchitis Diseases 0.000 description 1
- 206010036774 Proctitis Diseases 0.000 description 1
- ONIBWKKTOPOVIA-UHFFFAOYSA-N Proline Natural products OC(=O)C1CCCN1 ONIBWKKTOPOVIA-UHFFFAOYSA-N 0.000 description 1
- 206010060862 Prostate cancer Diseases 0.000 description 1
- 208000000236 Prostatic Neoplasms Diseases 0.000 description 1
- 102100037686 Protein SSX2 Human genes 0.000 description 1
- 239000004373 Pullulan Substances 0.000 description 1
- 229920001218 Pullulan Polymers 0.000 description 1
- 206010037549 Purpura Diseases 0.000 description 1
- 241001672981 Purpura Species 0.000 description 1
- 208000006311 Pyoderma Diseases 0.000 description 1
- 101710100968 Receptor tyrosine-protein kinase erbB-2 Proteins 0.000 description 1
- 102100029165 Receptor-binding cancer antigen expressed on SiSo cells Human genes 0.000 description 1
- 206010038063 Rectal haemorrhage Diseases 0.000 description 1
- 201000000582 Retinoblastoma Diseases 0.000 description 1
- PYMYPHUHKUWMLA-LMVFSUKVSA-N Ribose Natural products OC[C@@H](O)[C@@H](O)[C@@H](O)C=O PYMYPHUHKUWMLA-LMVFSUKVSA-N 0.000 description 1
- 241000605947 Roseburia Species 0.000 description 1
- SKZKKFZAGNVIMN-UHFFFAOYSA-N Salicilamide Chemical compound NC(=O)C1=CC=CC=C1O SKZKKFZAGNVIMN-UHFFFAOYSA-N 0.000 description 1
- 206010061934 Salivary gland cancer Diseases 0.000 description 1
- 229920002305 Schizophyllan Polymers 0.000 description 1
- 206010039710 Scleroderma Diseases 0.000 description 1
- 102100031075 Serine/threonine-protein kinase Chk2 Human genes 0.000 description 1
- 208000000453 Skin Neoplasms Diseases 0.000 description 1
- 206010041067 Small cell lung cancer Diseases 0.000 description 1
- DWAQJAXMDSEUJJ-UHFFFAOYSA-M Sodium bisulfite Chemical compound [Na+].OS([O-])=O DWAQJAXMDSEUJJ-UHFFFAOYSA-M 0.000 description 1
- 229920002125 Sokalan® Polymers 0.000 description 1
- 235000002595 Solanum tuberosum Nutrition 0.000 description 1
- 244000061456 Solanum tuberosum Species 0.000 description 1
- 206010041349 Somnolence Diseases 0.000 description 1
- 101000668858 Spinacia oleracea 30S ribosomal protein S1, chloroplastic Proteins 0.000 description 1
- 101000857870 Squalus acanthias Gonadoliberin Proteins 0.000 description 1
- SSZBUIDZHHWXNJ-UHFFFAOYSA-N Stearinsaeure-hexadecylester Natural products CCCCCCCCCCCCCCCCCC(=O)OCCCCCCCCCCCCCCCC SSZBUIDZHHWXNJ-UHFFFAOYSA-N 0.000 description 1
- 101000898746 Streptomyces clavuligerus Clavaminate synthase 1 Proteins 0.000 description 1
- 241000282887 Suidae Species 0.000 description 1
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical compound [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 description 1
- 102100036234 Synaptonemal complex protein 1 Human genes 0.000 description 1
- 101710143177 Synaptonemal complex protein 1 Proteins 0.000 description 1
- 108091008874 T cell receptors Proteins 0.000 description 1
- 102000016266 T-Cell Antigen Receptors Human genes 0.000 description 1
- 102100027213 T-cell-specific surface glycoprotein CD28 Human genes 0.000 description 1
- 102100033082 TNF receptor-associated factor 3 Human genes 0.000 description 1
- 101001051488 Takifugu rubripes Neural cell adhesion molecule L1 Proteins 0.000 description 1
- NAVMQTYZDKMPEU-UHFFFAOYSA-N Targretin Chemical compound CC1=CC(C(CCC2(C)C)(C)C)=C2C=C1C(=C)C1=CC=C(C(O)=O)C=C1 NAVMQTYZDKMPEU-UHFFFAOYSA-N 0.000 description 1
- 208000024313 Testicular Neoplasms Diseases 0.000 description 1
- 206010057644 Testis cancer Diseases 0.000 description 1
- 239000004098 Tetracycline Substances 0.000 description 1
- 210000000068 Th17 cell Anatomy 0.000 description 1
- JZRWCGZRTZMZEH-UHFFFAOYSA-N Thiamine Natural products CC1=C(CCO)SC=[N+]1CC1=CN=C(C)N=C1N JZRWCGZRTZMZEH-UHFFFAOYSA-N 0.000 description 1
- 208000024770 Thyroid neoplasm Diseases 0.000 description 1
- 108010060818 Toll-Like Receptor 9 Proteins 0.000 description 1
- 102000002689 Toll-like receptor Human genes 0.000 description 1
- 108020000411 Toll-like receptor Proteins 0.000 description 1
- 102100027010 Toll-like receptor 1 Human genes 0.000 description 1
- 102100027009 Toll-like receptor 10 Human genes 0.000 description 1
- 102100024333 Toll-like receptor 2 Human genes 0.000 description 1
- 102100024324 Toll-like receptor 3 Human genes 0.000 description 1
- 102100039360 Toll-like receptor 4 Human genes 0.000 description 1
- 102100039387 Toll-like receptor 6 Human genes 0.000 description 1
- 102100039390 Toll-like receptor 7 Human genes 0.000 description 1
- 102100033110 Toll-like receptor 8 Human genes 0.000 description 1
- 102100033117 Toll-like receptor 9 Human genes 0.000 description 1
- 101710182223 Toxin B Proteins 0.000 description 1
- 102000046299 Transforming Growth Factor beta1 Human genes 0.000 description 1
- 102000011117 Transforming Growth Factor beta2 Human genes 0.000 description 1
- 101800002279 Transforming growth factor beta-1 Proteins 0.000 description 1
- 101800000304 Transforming growth factor beta-2 Proteins 0.000 description 1
- 102100023935 Transmembrane glycoprotein NMB Human genes 0.000 description 1
- 102000007641 Trefoil Factors Human genes 0.000 description 1
- 108010007389 Trefoil Factors Proteins 0.000 description 1
- 208000003721 Triple Negative Breast Neoplasms Diseases 0.000 description 1
- 102100033579 Trophoblast glycoprotein Human genes 0.000 description 1
- 102100028785 Tumor necrosis factor receptor superfamily member 14 Human genes 0.000 description 1
- 102100033728 Tumor necrosis factor receptor superfamily member 18 Human genes 0.000 description 1
- 102100022153 Tumor necrosis factor receptor superfamily member 4 Human genes 0.000 description 1
- 101710165473 Tumor necrosis factor receptor superfamily member 4 Proteins 0.000 description 1
- 102100040245 Tumor necrosis factor receptor superfamily member 5 Human genes 0.000 description 1
- 102100036857 Tumor necrosis factor receptor superfamily member 8 Human genes 0.000 description 1
- 102100036856 Tumor necrosis factor receptor superfamily member 9 Human genes 0.000 description 1
- 102000003425 Tyrosinase Human genes 0.000 description 1
- 108060008724 Tyrosinase Proteins 0.000 description 1
- 208000007097 Urinary Bladder Neoplasms Diseases 0.000 description 1
- 208000006105 Uterine Cervical Neoplasms Diseases 0.000 description 1
- 208000002495 Uterine Neoplasms Diseases 0.000 description 1
- 102100038282 V-type immunoglobulin domain-containing suppressor of T-cell activation Human genes 0.000 description 1
- 206010046996 Varicose vein Diseases 0.000 description 1
- 206010047741 Vulval cancer Diseases 0.000 description 1
- 208000033559 Waldenström macroglobulinemia Diseases 0.000 description 1
- 206010052428 Wound Diseases 0.000 description 1
- 208000027418 Wounds and injury Diseases 0.000 description 1
- TVXBFESIOXBWNM-UHFFFAOYSA-N Xylitol Natural products OCCC(O)C(O)C(O)CCO TVXBFESIOXBWNM-UHFFFAOYSA-N 0.000 description 1
- 229920002494 Zein Polymers 0.000 description 1
- SMEGJBVQLJJKKX-HOTMZDKISA-N [(2R,3S,4S,5R,6R)-5-acetyloxy-3,4,6-trihydroxyoxan-2-yl]methyl acetate Chemical compound CC(=O)OC[C@@H]1[C@H]([C@@H]([C@H]([C@@H](O1)O)OC(=O)C)O)O SMEGJBVQLJJKKX-HOTMZDKISA-N 0.000 description 1
- FJWGYAHXMCUOOM-QHOUIDNNSA-N [(2s,3r,4s,5r,6r)-2-[(2r,3r,4s,5r,6s)-4,5-dinitrooxy-2-(nitrooxymethyl)-6-[(2r,3r,4s,5r,6s)-4,5,6-trinitrooxy-2-(nitrooxymethyl)oxan-3-yl]oxyoxan-3-yl]oxy-3,5-dinitrooxy-6-(nitrooxymethyl)oxan-4-yl] nitrate Chemical compound O([C@@H]1O[C@@H]([C@H]([C@H](O[N+]([O-])=O)[C@H]1O[N+]([O-])=O)O[C@H]1[C@@H]([C@@H](O[N+]([O-])=O)[C@H](O[N+]([O-])=O)[C@@H](CO[N+]([O-])=O)O1)O[N+]([O-])=O)CO[N+](=O)[O-])[C@@H]1[C@@H](CO[N+]([O-])=O)O[C@@H](O[N+]([O-])=O)[C@H](O[N+]([O-])=O)[C@H]1O[N+]([O-])=O FJWGYAHXMCUOOM-QHOUIDNNSA-N 0.000 description 1
- 206010000059 abdominal discomfort Diseases 0.000 description 1
- 208000019790 abdominal distention Diseases 0.000 description 1
- 229960004103 abiraterone acetate Drugs 0.000 description 1
- UVIQSJCZCSLXRZ-UBUQANBQSA-N abiraterone acetate Chemical compound C([C@@H]1[C@]2(C)CC[C@@H]3[C@@]4(C)CC[C@@H](CC4=CC[C@H]31)OC(=O)C)C=C2C1=CC=CN=C1 UVIQSJCZCSLXRZ-UBUQANBQSA-N 0.000 description 1
- 229940028652 abraxane Drugs 0.000 description 1
- 239000002250 absorbent Substances 0.000 description 1
- 230000002745 absorbent Effects 0.000 description 1
- 239000003655 absorption accelerator Substances 0.000 description 1
- 239000000370 acceptor Substances 0.000 description 1
- DHKHKXVYLBGOIT-UHFFFAOYSA-N acetaldehyde Diethyl Acetal Natural products CCOC(C)OCC DHKHKXVYLBGOIT-UHFFFAOYSA-N 0.000 description 1
- 150000001241 acetals Chemical class 0.000 description 1
- 235000011054 acetic acid Nutrition 0.000 description 1
- XQEJFZYLWPSJOV-UHFFFAOYSA-N acetic acid;10-(4-aminobutyl)-19-[(2-amino-3-phenylpropanoyl)amino]-16-benzyl-n-(1,3-dihydroxybutan-2-yl)-7-(1-hydroxyethyl)-13-(1h-indol-3-ylmethyl)-6,9,12,15,18-pentaoxo-1,2-dithia-5,8,11,14,17-pentazacycloicosane-4-carboxamide Chemical compound CC(O)=O.O=C1NC(CC=2C=CC=CC=2)C(=O)NC(CC=2C3=CC=CC=C3NC=2)C(=O)NC(CCCCN)C(=O)NC(C(C)O)C(=O)NC(C(=O)NC(CO)C(O)C)CSSCC1NC(=O)C(N)CC1=CC=CC=C1 XQEJFZYLWPSJOV-UHFFFAOYSA-N 0.000 description 1
- 150000001252 acrylic acid derivatives Chemical class 0.000 description 1
- NIXOWILDQLNWCW-UHFFFAOYSA-N acrylic acid group Chemical group C(C=C)(=O)O NIXOWILDQLNWCW-UHFFFAOYSA-N 0.000 description 1
- 125000002252 acyl group Chemical group 0.000 description 1
- 238000009098 adjuvant therapy Methods 0.000 description 1
- 230000001919 adrenal effect Effects 0.000 description 1
- 229940009456 adriamycin Drugs 0.000 description 1
- USNRYVNRPYXCSP-JUGPPOIOSA-N afatinib dimaleate Chemical compound OC(=O)\C=C/C(O)=O.OC(=O)\C=C/C(O)=O.N1=CN=C2C=C(O[C@@H]3COCC3)C(NC(=O)/C=C/CN(C)C)=CC2=C1NC1=CC=C(F)C(Cl)=C1 USNRYVNRPYXCSP-JUGPPOIOSA-N 0.000 description 1
- 229960002736 afatinib dimaleate Drugs 0.000 description 1
- 239000008272 agar Substances 0.000 description 1
- 239000000556 agonist Substances 0.000 description 1
- 235000004279 alanine Nutrition 0.000 description 1
- 229940060265 aldara Drugs 0.000 description 1
- 229960001611 alectinib Drugs 0.000 description 1
- KDGFLJKFZUIJMX-UHFFFAOYSA-N alectinib Chemical compound CCC1=CC=2C(=O)C(C3=CC=C(C=C3N3)C#N)=C3C(C)(C)C=2C=C1N(CC1)CCC1N1CCOCC1 KDGFLJKFZUIJMX-UHFFFAOYSA-N 0.000 description 1
- 239000000783 alginic acid Substances 0.000 description 1
- 229960001126 alginic acid Drugs 0.000 description 1
- 150000004781 alginic acids Chemical class 0.000 description 1
- 125000001931 aliphatic group Chemical group 0.000 description 1
- 229960001445 alitretinoin Drugs 0.000 description 1
- 229940098174 alkeran Drugs 0.000 description 1
- 150000005215 alkyl ethers Chemical class 0.000 description 1
- 125000000217 alkyl group Chemical group 0.000 description 1
- 230000007815 allergy Effects 0.000 description 1
- 229940087168 alpha tocopherol Drugs 0.000 description 1
- HMFHBZSHGGEWLO-UHFFFAOYSA-N alpha-D-Furanose-Ribose Natural products OCC1OC(O)C(O)C1O HMFHBZSHGGEWLO-UHFFFAOYSA-N 0.000 description 1
- 108010026331 alpha-Fetoproteins Proteins 0.000 description 1
- SRHNADOZAAWYLV-XLMUYGLTSA-N alpha-L-Fucp-(1->2)-beta-D-Galp-(1->4)-[alpha-L-Fucp-(1->3)]-beta-D-GlcpNAc Chemical compound O[C@H]1[C@H](O)[C@H](O)[C@H](C)O[C@H]1O[C@H]1[C@H](O[C@H]2[C@@H]([C@@H](NC(C)=O)[C@H](O)O[C@@H]2CO)O[C@H]2[C@H]([C@H](O)[C@H](O)[C@H](C)O2)O)O[C@H](CO)[C@H](O)[C@@H]1O SRHNADOZAAWYLV-XLMUYGLTSA-N 0.000 description 1
- AZDRQVAHHNSJOQ-UHFFFAOYSA-N alumane Chemical class [AlH3] AZDRQVAHHNSJOQ-UHFFFAOYSA-N 0.000 description 1
- WNROFYMDJYEPJX-UHFFFAOYSA-K aluminium hydroxide Chemical compound [OH-].[OH-].[OH-].[Al+3] WNROFYMDJYEPJX-UHFFFAOYSA-K 0.000 description 1
- 229910000147 aluminium phosphate Inorganic materials 0.000 description 1
- 229960003437 aminoglutethimide Drugs 0.000 description 1
- ROBVIMPUHSLWNV-UHFFFAOYSA-N aminoglutethimide Chemical compound C=1C=C(N)C=CC=1C1(CC)CCC(=O)NC1=O ROBVIMPUHSLWNV-UHFFFAOYSA-N 0.000 description 1
- 229960003022 amoxicillin Drugs 0.000 description 1
- LSQZJLSUYDQPKJ-NJBDSQKTSA-N amoxicillin Chemical compound C1([C@@H](N)C(=O)N[C@H]2[C@H]3SC([C@@H](N3C2=O)C(O)=O)(C)C)=CC=C(O)C=C1 LSQZJLSUYDQPKJ-NJBDSQKTSA-N 0.000 description 1
- 229960000723 ampicillin Drugs 0.000 description 1
- AVKUERGKIZMTKX-NJBDSQKTSA-N ampicillin Chemical compound C1([C@@H](N)C(=O)N[C@H]2[C@H]3SC([C@@H](N3C2=O)C(O)=O)(C)C)=CC=CC=C1 AVKUERGKIZMTKX-NJBDSQKTSA-N 0.000 description 1
- 230000009604 anaerobic growth Effects 0.000 description 1
- 230000000202 analgesic effect Effects 0.000 description 1
- 229940035676 analgesics Drugs 0.000 description 1
- 229960002932 anastrozole Drugs 0.000 description 1
- 239000002333 angiotensin II receptor antagonist Substances 0.000 description 1
- 229940125364 angiotensin receptor blocker Drugs 0.000 description 1
- 238000010171 animal model Methods 0.000 description 1
- 208000022531 anorexia Diseases 0.000 description 1
- 229940069428 antacid Drugs 0.000 description 1
- 239000003159 antacid agent Substances 0.000 description 1
- 239000005557 antagonist Substances 0.000 description 1
- 230000001458 anti-acid effect Effects 0.000 description 1
- 230000002280 anti-androgenic effect Effects 0.000 description 1
- 230000001142 anti-diarrhea Effects 0.000 description 1
- 230000002924 anti-infective effect Effects 0.000 description 1
- 239000000051 antiandrogen Substances 0.000 description 1
- 229940030495 antiandrogen sex hormone and modulator of the genital system Drugs 0.000 description 1
- 229940125714 antidiarrheal agent Drugs 0.000 description 1
- 239000003793 antidiarrheal agent Substances 0.000 description 1
- 239000004599 antimicrobial Substances 0.000 description 1
- 230000036506 anxiety Effects 0.000 description 1
- 229960003982 apatinib Drugs 0.000 description 1
- 239000012736 aqueous medium Substances 0.000 description 1
- 239000003886 aromatase inhibitor Substances 0.000 description 1
- 229940046844 aromatase inhibitors Drugs 0.000 description 1
- 125000000732 arylene group Chemical group 0.000 description 1
- 235000010323 ascorbic acid Nutrition 0.000 description 1
- 229960005070 ascorbic acid Drugs 0.000 description 1
- 239000011668 ascorbic acid Substances 0.000 description 1
- 239000000605 aspartame Substances 0.000 description 1
- 235000010357 aspartame Nutrition 0.000 description 1
- IAOZJIPTCAWIRG-QWRGUYRKSA-N aspartame Chemical compound OC(=O)C[C@H](N)C(=O)N[C@H](C(=O)OC)CC1=CC=CC=C1 IAOZJIPTCAWIRG-QWRGUYRKSA-N 0.000 description 1
- 229960003438 aspartame Drugs 0.000 description 1
- 208000029560 autism spectrum disease Diseases 0.000 description 1
- 235000021302 avocado oil Nutrition 0.000 description 1
- 239000008163 avocado oil Substances 0.000 description 1
- 229960003005 axitinib Drugs 0.000 description 1
- RITAVMQDGBJQJZ-FMIVXFBMSA-N axitinib Chemical compound CNC(=O)C1=CC=CC=C1SC1=CC=C(C(\C=C\C=2N=CC=CC=2)=NN2)C2=C1 RITAVMQDGBJQJZ-FMIVXFBMSA-N 0.000 description 1
- 210000004666 bacterial spore Anatomy 0.000 description 1
- 210000003651 basophil Anatomy 0.000 description 1
- 239000011324 bead Substances 0.000 description 1
- 235000013871 bee wax Nutrition 0.000 description 1
- 239000012166 beeswax Substances 0.000 description 1
- 230000006399 behavior Effects 0.000 description 1
- 229960001215 bendamustine hydrochloride Drugs 0.000 description 1
- MMIMIFULGMZVPO-UHFFFAOYSA-N benzyl 3-bromo-2,6-dinitro-5-phenylmethoxybenzoate Chemical compound [O-][N+](=O)C1=C(C(=O)OCC=2C=CC=CC=2)C([N+](=O)[O-])=C(Br)C=C1OCC1=CC=CC=C1 MMIMIFULGMZVPO-UHFFFAOYSA-N 0.000 description 1
- YBHILYKTIRIUTE-UHFFFAOYSA-N berberine Chemical compound C1=C2CC[N+]3=CC4=C(OC)C(OC)=CC=C4C=C3C2=CC2=C1OCO2 YBHILYKTIRIUTE-UHFFFAOYSA-N 0.000 description 1
- 229940093265 berberine Drugs 0.000 description 1
- QISXPYZVZJBNDM-UHFFFAOYSA-N berberine Natural products COc1ccc2C=C3N(Cc2c1OC)C=Cc4cc5OCOc5cc34 QISXPYZVZJBNDM-UHFFFAOYSA-N 0.000 description 1
- 238000003339 best practice Methods 0.000 description 1
- GWYFCOCPABKNJV-UHFFFAOYSA-N beta-methyl-butyric acid Natural products CC(C)CC(O)=O GWYFCOCPABKNJV-UHFFFAOYSA-N 0.000 description 1
- 229960003237 betaine Drugs 0.000 description 1
- UREBDLICKHMUKA-DVTGEIKXSA-N betamethasone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@H](C)[C@@](C(=O)CO)(O)[C@@]1(C)C[C@@H]2O UREBDLICKHMUKA-DVTGEIKXSA-N 0.000 description 1
- 229960000870 betamethasone benzoate Drugs 0.000 description 1
- SOQJPQZCPBDOMF-YCUXZELOSA-N betamethasone benzoate Chemical compound O([C@]1([C@@]2(C)C[C@H](O)[C@]3(F)[C@@]4(C)C=CC(=O)C=C4CC[C@H]3[C@@H]2C[C@@H]1C)C(=O)CO)C(=O)C1=CC=CC=C1 SOQJPQZCPBDOMF-YCUXZELOSA-N 0.000 description 1
- 229960001102 betamethasone dipropionate Drugs 0.000 description 1
- CIWBQSYVNNPZIQ-XYWKZLDCSA-N betamethasone dipropionate Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@H](C)[C@@](C(=O)COC(=O)CC)(OC(=O)CC)[C@@]1(C)C[C@@H]2O CIWBQSYVNNPZIQ-XYWKZLDCSA-N 0.000 description 1
- 229960004311 betamethasone valerate Drugs 0.000 description 1
- SNHRLVCMMWUAJD-SUYDQAKGSA-N betamethasone valerate Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@H](C)[C@@](C(=O)CO)(OC(=O)CCCC)[C@@]1(C)C[C@@H]2O SNHRLVCMMWUAJD-SUYDQAKGSA-N 0.000 description 1
- 229960002938 bexarotene Drugs 0.000 description 1
- 229940108502 bicnu Drugs 0.000 description 1
- 229940002008 bifidobacterium bifidum Drugs 0.000 description 1
- 229940004120 bifidobacterium infantis Drugs 0.000 description 1
- 229940009291 bifidobacterium longum Drugs 0.000 description 1
- 210000000013 bile duct Anatomy 0.000 description 1
- 239000001986 bile esculin agar Substances 0.000 description 1
- 201000009036 biliary tract cancer Diseases 0.000 description 1
- 208000020790 biliary tract neoplasm Diseases 0.000 description 1
- 239000011230 binding agent Substances 0.000 description 1
- 239000000227 bioadhesive Substances 0.000 description 1
- 239000000090 biomarker Substances 0.000 description 1
- 238000001574 biopsy Methods 0.000 description 1
- 229960002685 biotin Drugs 0.000 description 1
- 235000020958 biotin Nutrition 0.000 description 1
- 239000011616 biotin Substances 0.000 description 1
- 229960000782 bismuth subsalicylate Drugs 0.000 description 1
- ZREIPSZUJIFJNP-UHFFFAOYSA-K bismuth subsalicylate Chemical compound C1=CC=C2O[Bi](O)OC(=O)C2=C1 ZREIPSZUJIFJNP-UHFFFAOYSA-K 0.000 description 1
- 229960001561 bleomycin Drugs 0.000 description 1
- OYVAGSVQBOHSSS-UAPAGMARSA-O bleomycin A2 Chemical compound N([C@H](C(=O)N[C@H](C)[C@@H](O)[C@H](C)C(=O)N[C@@H]([C@H](O)C)C(=O)NCCC=1SC=C(N=1)C=1SC=C(N=1)C(=O)NCCC[S+](C)C)[C@@H](O[C@H]1[C@H]([C@@H](O)[C@H](O)[C@H](CO)O1)O[C@@H]1[C@H]([C@@H](OC(N)=O)[C@H](O)[C@@H](CO)O1)O)C=1N=CNC=1)C(=O)C1=NC([C@H](CC(N)=O)NC[C@H](N)C(N)=O)=NC(N)=C1C OYVAGSVQBOHSSS-UAPAGMARSA-O 0.000 description 1
- 208000024330 bloating Diseases 0.000 description 1
- 229920001400 block copolymer Polymers 0.000 description 1
- 239000006161 blood agar Substances 0.000 description 1
- 230000036772 blood pressure Effects 0.000 description 1
- HXNZTJULPKRNPR-UHFFFAOYSA-N borinine Chemical compound B1=CC=CC=C1 HXNZTJULPKRNPR-UHFFFAOYSA-N 0.000 description 1
- 210000004556 brain Anatomy 0.000 description 1
- 201000000220 brain stem cancer Diseases 0.000 description 1
- RMRJXGBAOAMLHD-IHFGGWKQSA-N buprenorphine Chemical compound C([C@]12[C@H]3OC=4C(O)=CC=C(C2=4)C[C@@H]2[C@]11CC[C@]3([C@H](C1)[C@](C)(O)C(C)(C)C)OC)CN2CC1CC1 RMRJXGBAOAMLHD-IHFGGWKQSA-N 0.000 description 1
- 229960001736 buprenorphine Drugs 0.000 description 1
- 239000011575 calcium Substances 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- 229910000019 calcium carbonate Inorganic materials 0.000 description 1
- 235000010216 calcium carbonate Nutrition 0.000 description 1
- AXCZMVOFGPJBDE-UHFFFAOYSA-L calcium dihydroxide Chemical compound [OH-].[OH-].[Ca+2] AXCZMVOFGPJBDE-UHFFFAOYSA-L 0.000 description 1
- 239000000920 calcium hydroxide Substances 0.000 description 1
- 229910001861 calcium hydroxide Inorganic materials 0.000 description 1
- 239000001506 calcium phosphate Substances 0.000 description 1
- CJZGTCYPCWQAJB-UHFFFAOYSA-L calcium stearate Chemical compound [Ca+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O CJZGTCYPCWQAJB-UHFFFAOYSA-L 0.000 description 1
- 235000013539 calcium stearate Nutrition 0.000 description 1
- 239000008116 calcium stearate Substances 0.000 description 1
- MIOPJNTWMNEORI-UHFFFAOYSA-N camphorsulfonic acid Chemical compound C1CC2(CS(O)(=O)=O)C(=O)CC1C2(C)C MIOPJNTWMNEORI-UHFFFAOYSA-N 0.000 description 1
- 229960004117 capecitabine Drugs 0.000 description 1
- PGMBSCDPACPRSG-SCSDYSBLSA-N capiri Chemical compound C1=C(F)C(NC(=O)OCCCCC)=NC(=O)N1[C@H]1[C@H](O)[C@H](O)[C@@H](C)O1.C1=C2C(CC)=C3CN(C(C4=C([C@@](C(=O)OC4)(O)CC)C=4)=O)C=4C3=NC2=CC=C1OC(=O)N(CC1)CCC1N1CCCCC1 PGMBSCDPACPRSG-SCSDYSBLSA-N 0.000 description 1
- 229940001981 carac Drugs 0.000 description 1
- FPPNZSSZRUTDAP-UWFZAAFLSA-N carbenicillin Chemical compound N([C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C(=O)C(C(O)=O)C1=CC=CC=C1 FPPNZSSZRUTDAP-UWFZAAFLSA-N 0.000 description 1
- 229960003669 carbenicillin Drugs 0.000 description 1
- 150000001720 carbohydrates Chemical class 0.000 description 1
- 235000014633 carbohydrates Nutrition 0.000 description 1
- 125000004432 carbon atom Chemical group C* 0.000 description 1
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 1
- 239000001768 carboxy methyl cellulose Substances 0.000 description 1
- 235000010948 carboxy methyl cellulose Nutrition 0.000 description 1
- 239000008112 carboxymethyl-cellulose Substances 0.000 description 1
- 239000004203 carnauba wax Substances 0.000 description 1
- 235000013869 carnauba wax Nutrition 0.000 description 1
- 235000010418 carrageenan Nutrition 0.000 description 1
- 229920001525 carrageenan Polymers 0.000 description 1
- 239000000679 carrageenan Substances 0.000 description 1
- 229940113118 carrageenan Drugs 0.000 description 1
- 239000004359 castor oil Substances 0.000 description 1
- 235000019438 castor oil Nutrition 0.000 description 1
- 102000014509 cathelicidin Human genes 0.000 description 1
- 108060001132 cathelicidin Proteins 0.000 description 1
- POIUWJQBRNEFGX-XAMSXPGMSA-N cathelicidin Chemical compound C([C@@H](C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)CC)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(O)=O)NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CC(C)C)C1=CC=CC=C1 POIUWJQBRNEFGX-XAMSXPGMSA-N 0.000 description 1
- 150000001768 cations Chemical class 0.000 description 1
- 229960004841 cefadroxil Drugs 0.000 description 1
- NBFNMSULHIODTC-CYJZLJNKSA-N cefadroxil monohydrate Chemical compound O.C1([C@@H](N)C(=O)N[C@H]2[C@@H]3N(C2=O)C(=C(CS3)C)C(O)=O)=CC=C(O)C=C1 NBFNMSULHIODTC-CYJZLJNKSA-N 0.000 description 1
- XIURVHNZVLADCM-IUODEOHRSA-N cefalotin Chemical compound N([C@H]1[C@@H]2N(C1=O)C(=C(CS2)COC(=O)C)C(O)=O)C(=O)CC1=CC=CS1 XIURVHNZVLADCM-IUODEOHRSA-N 0.000 description 1
- 229960000603 cefalotin Drugs 0.000 description 1
- 229960001139 cefazolin Drugs 0.000 description 1
- MLYYVTUWGNIJIB-BXKDBHETSA-N cefazolin Chemical compound S1C(C)=NN=C1SCC1=C(C(O)=O)N2C(=O)[C@@H](NC(=O)CN3N=NN=C3)[C@H]2SC1 MLYYVTUWGNIJIB-BXKDBHETSA-N 0.000 description 1
- 229960001668 cefuroxime Drugs 0.000 description 1
- JFPVXVDWJQMJEE-IZRZKJBUSA-N cefuroxime Chemical compound N([C@@H]1C(N2C(=C(COC(N)=O)CS[C@@H]21)C(O)=O)=O)C(=O)\C(=N/OC)C1=CC=CO1 JFPVXVDWJQMJEE-IZRZKJBUSA-N 0.000 description 1
- 229960000590 celecoxib Drugs 0.000 description 1
- RZEKVGVHFLEQIL-UHFFFAOYSA-N celecoxib Chemical compound C1=CC(C)=CC=C1C1=CC(C(F)(F)F)=NN1C1=CC=C(S(N)(=O)=O)C=C1 RZEKVGVHFLEQIL-UHFFFAOYSA-N 0.000 description 1
- 210000000170 cell membrane Anatomy 0.000 description 1
- 230000003833 cell viability Effects 0.000 description 1
- 229920003086 cellulose ether Polymers 0.000 description 1
- 201000007455 central nervous system cancer Diseases 0.000 description 1
- 229940106164 cephalexin Drugs 0.000 description 1
- ZAIPMKNFIOOWCQ-UEKVPHQBSA-N cephalexin Chemical compound C1([C@@H](N)C(=O)N[C@H]2[C@@H]3N(C2=O)C(=C(CS3)C)C(O)=O)=CC=CC=C1 ZAIPMKNFIOOWCQ-UEKVPHQBSA-N 0.000 description 1
- 208000025434 cerebellar degeneration Diseases 0.000 description 1
- 208000026106 cerebrovascular disease Diseases 0.000 description 1
- 201000010881 cervical cancer Diseases 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 239000012829 chemotherapy agent Substances 0.000 description 1
- 235000019693 cherries Nutrition 0.000 description 1
- 125000001309 chloro group Chemical group Cl* 0.000 description 1
- NPSLCOWKFFNQKK-ZPSUVKRCSA-N chloroprednisone Chemical compound O=C1C=C[C@]2(C)[C@H]3C(=O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3C[C@H](Cl)C2=C1 NPSLCOWKFFNQKK-ZPSUVKRCSA-N 0.000 description 1
- 229950006229 chloroprednisone Drugs 0.000 description 1
- 229960001231 choline Drugs 0.000 description 1
- OEYIOHPDSNJKLS-UHFFFAOYSA-N choline Chemical compound C[N+](C)(C)CCO OEYIOHPDSNJKLS-UHFFFAOYSA-N 0.000 description 1
- 229940059329 chondroitin sulfate Drugs 0.000 description 1
- 208000022371 chronic pain syndrome Diseases 0.000 description 1
- 229940088516 cipro Drugs 0.000 description 1
- MYSWGUAQZAJSOK-UHFFFAOYSA-N ciprofloxacin Chemical compound C12=CC(N3CCNCC3)=C(F)C=C2C(=O)C(C(=O)O)=CN1C1CC1 MYSWGUAQZAJSOK-UHFFFAOYSA-N 0.000 description 1
- 239000004927 clay Substances 0.000 description 1
- 238000009535 clinical urine test Methods 0.000 description 1
- 229960002842 clobetasol Drugs 0.000 description 1
- CBGUOGMQLZIXBE-XGQKBEPLSA-N clobetasol propionate Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@H](C)[C@@](C(=O)CCl)(OC(=O)CC)[C@@]1(C)C[C@@H]2O CBGUOGMQLZIXBE-XGQKBEPLSA-N 0.000 description 1
- 238000004581 coalescence Methods 0.000 description 1
- 239000008199 coating composition Substances 0.000 description 1
- 229920001688 coating polymer Polymers 0.000 description 1
- 235000019868 cocoa butter Nutrition 0.000 description 1
- 229940110456 cocoa butter Drugs 0.000 description 1
- 239000003240 coconut oil Substances 0.000 description 1
- 235000019864 coconut oil Nutrition 0.000 description 1
- 239000003086 colorant Substances 0.000 description 1
- 238000002648 combination therapy Methods 0.000 description 1
- 238000004891 communication Methods 0.000 description 1
- 238000007906 compression Methods 0.000 description 1
- 230000006835 compression Effects 0.000 description 1
- 201000010918 connective tissue cancer Diseases 0.000 description 1
- 208000018631 connective tissue disease Diseases 0.000 description 1
- 108010015408 connexin 37 Proteins 0.000 description 1
- 238000013270 controlled release Methods 0.000 description 1
- STGQPVQAAFJJFX-UHFFFAOYSA-N copanlisib dihydrochloride Chemical compound Cl.Cl.C1=CC=2C3=NCCN3C(NC(=O)C=3C=NC(N)=NC=3)=NC=2C(OC)=C1OCCCN1CCOCC1 STGQPVQAAFJJFX-UHFFFAOYSA-N 0.000 description 1
- BMCQMVFGOVHVNG-TUFAYURCSA-N cortisol 17-butyrate Chemical compound C1CC2=CC(=O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@@](C(=O)CO)(OC(=O)CCC)[C@@]1(C)C[C@@H]2O BMCQMVFGOVHVNG-TUFAYURCSA-N 0.000 description 1
- ALEXXDVDDISNDU-JZYPGELDSA-N cortisol 21-acetate Chemical compound C1CC2=CC(=O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@@](C(=O)COC(=O)C)(O)[C@@]1(C)C[C@@H]2O ALEXXDVDDISNDU-JZYPGELDSA-N 0.000 description 1
- 229960004544 cortisone Drugs 0.000 description 1
- 229950002276 cortodoxone Drugs 0.000 description 1
- 229940088547 cosmegen Drugs 0.000 description 1
- 229940111134 coxibs Drugs 0.000 description 1
- 229960005061 crizotinib Drugs 0.000 description 1
- KTEIFNKAUNYNJU-GFCCVEGCSA-N crizotinib Chemical compound O([C@H](C)C=1C(=C(F)C=CC=1Cl)Cl)C(C(=NC=1)N)=CC=1C(=C1)C=NN1C1CCNCC1 KTEIFNKAUNYNJU-GFCCVEGCSA-N 0.000 description 1
- 229960000913 crospovidone Drugs 0.000 description 1
- 229920006037 cross link polymer Polymers 0.000 description 1
- 235000010947 crosslinked sodium carboxy methyl cellulose Nutrition 0.000 description 1
- 239000001767 crosslinked sodium carboxy methyl cellulose Substances 0.000 description 1
- 239000013078 crystal Substances 0.000 description 1
- IMBXRZKCLVBLBH-OGYJWPHRSA-N cvp protocol Chemical compound ClCCN(CCCl)P1(=O)NCCCO1.O=C1C=C[C@]2(C)[C@H]3C(=O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1.C([C@H](C[C@]1(C(=O)OC)C=2C(=C3C([C@]45[C@H]([C@@]([C@H](OC(C)=O)[C@]6(CC)C=CCN([C@H]56)CC4)(O)C(=O)OC)N3C=O)=CC=2)OC)C[C@@](C2)(O)CC)N2CCC2=C1NC1=CC=CC=C21 IMBXRZKCLVBLBH-OGYJWPHRSA-N 0.000 description 1
- 239000003255 cyclooxygenase 2 inhibitor Substances 0.000 description 1
- 108010048032 cyclophilin B Proteins 0.000 description 1
- 229960003901 dacarbazine Drugs 0.000 description 1
- 229940059359 dacogen Drugs 0.000 description 1
- 230000006378 damage Effects 0.000 description 1
- 229940026692 decadron Drugs 0.000 description 1
- 229960003603 decitabine Drugs 0.000 description 1
- 206010061428 decreased appetite Diseases 0.000 description 1
- 230000007812 deficiency Effects 0.000 description 1
- 230000002950 deficient Effects 0.000 description 1
- 230000006735 deficit Effects 0.000 description 1
- 230000000593 degrading effect Effects 0.000 description 1
- 230000018044 dehydration Effects 0.000 description 1
- 238000006297 dehydration reaction Methods 0.000 description 1
- 230000001934 delay Effects 0.000 description 1
- 108010031971 delta catenin Proteins 0.000 description 1
- 229960003662 desonide Drugs 0.000 description 1
- WBGKWQHBNHJJPZ-LECWWXJVSA-N desonide Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@@H]2[C@@H]1[C@@H]1C[C@H]3OC(C)(C)O[C@@]3(C(=O)CO)[C@@]1(C)C[C@@H]2O WBGKWQHBNHJJPZ-LECWWXJVSA-N 0.000 description 1
- 230000001627 detrimental effect Effects 0.000 description 1
- WDEFBBTXULIOBB-WBVHZDCISA-N dextilidine Chemical compound C=1C=CC=CC=1[C@@]1(C(=O)OCC)CCC=C[C@H]1N(C)C WDEFBBTXULIOBB-WBVHZDCISA-N 0.000 description 1
- 239000008121 dextrose Substances 0.000 description 1
- 238000002405 diagnostic procedure Methods 0.000 description 1
- 229960002069 diamorphine Drugs 0.000 description 1
- NEFBYIFKOOEVPA-UHFFFAOYSA-K dicalcium phosphate Chemical compound [Ca+2].[Ca+2].[O-]P([O-])([O-])=O NEFBYIFKOOEVPA-UHFFFAOYSA-K 0.000 description 1
- 229940038472 dicalcium phosphate Drugs 0.000 description 1
- 229910000390 dicalcium phosphate Inorganic materials 0.000 description 1
- 229960001585 dicloxacillin Drugs 0.000 description 1
- YFAGHNZHGGCZAX-JKIFEVAISA-N dicloxacillin Chemical compound N([C@@H]1C(N2[C@H](C(C)(C)S[C@@H]21)C(O)=O)=O)C(=O)C1=C(C)ON=C1C1=C(Cl)C=CC=C1Cl YFAGHNZHGGCZAX-JKIFEVAISA-N 0.000 description 1
- 150000005690 diesters Chemical class 0.000 description 1
- MTHSVFCYNBDYFN-UHFFFAOYSA-N diethylene glycol Chemical compound OCCOCCO MTHSVFCYNBDYFN-UHFFFAOYSA-N 0.000 description 1
- RGLYKWWBQGJZGM-ISLYRVAYSA-N diethylstilbestrol Chemical compound C=1C=C(O)C=CC=1C(/CC)=C(\CC)C1=CC=C(O)C=C1 RGLYKWWBQGJZGM-ISLYRVAYSA-N 0.000 description 1
- 229960000452 diethylstilbestrol Drugs 0.000 description 1
- 229960002124 diflorasone diacetate Drugs 0.000 description 1
- BOBLHFUVNSFZPJ-JOYXJVLSSA-N diflorasone diacetate Chemical compound C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@]1(F)[C@@H]2[C@@H]2C[C@H](C)[C@@](C(=O)COC(C)=O)(OC(C)=O)[C@@]2(C)C[C@@H]1O BOBLHFUVNSFZPJ-JOYXJVLSSA-N 0.000 description 1
- 229960003970 diflucortolone valerate Drugs 0.000 description 1
- 229960004875 difluprednate Drugs 0.000 description 1
- 210000002249 digestive system Anatomy 0.000 description 1
- FPAFDBFIGPHWGO-UHFFFAOYSA-N dioxosilane;oxomagnesium;hydrate Chemical compound O.[Mg]=O.[Mg]=O.[Mg]=O.O=[Si]=O.O=[Si]=O.O=[Si]=O.O=[Si]=O FPAFDBFIGPHWGO-UHFFFAOYSA-N 0.000 description 1
- HYPPXZBJBPSRLK-UHFFFAOYSA-N diphenoxylate Chemical compound C1CC(C(=O)OCC)(C=2C=CC=CC=2)CCN1CCC(C#N)(C=1C=CC=CC=1)C1=CC=CC=C1 HYPPXZBJBPSRLK-UHFFFAOYSA-N 0.000 description 1
- 229960004192 diphenoxylate Drugs 0.000 description 1
- 239000007884 disintegrant Substances 0.000 description 1
- NYDXNILOWQXUOF-UHFFFAOYSA-L disodium;2-[[4-[2-(2-amino-4-oxo-1,7-dihydropyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl]amino]pentanedioate Chemical compound [Na+].[Na+].C=1NC=2NC(N)=NC(=O)C=2C=1CCC1=CC=C(C(=O)NC(CCC([O-])=O)C([O-])=O)C=C1 NYDXNILOWQXUOF-UHFFFAOYSA-L 0.000 description 1
- 239000002270 dispersing agent Substances 0.000 description 1
- 238000007922 dissolution test Methods 0.000 description 1
- 201000008243 diversion colitis Diseases 0.000 description 1
- AVAACINZEOAHHE-VFZPANTDSA-N doripenem Chemical compound C=1([C@H](C)[C@@H]2[C@H](C(N2C=1C(O)=O)=O)[C@H](O)C)S[C@@H]1CN[C@H](CNS(N)(=O)=O)C1 AVAACINZEOAHHE-VFZPANTDSA-N 0.000 description 1
- 229960000895 doripenem Drugs 0.000 description 1
- 229960003722 doxycycline Drugs 0.000 description 1
- 239000006196 drop Substances 0.000 description 1
- 239000000890 drug combination Substances 0.000 description 1
- 229940126534 drug product Drugs 0.000 description 1
- 238000001035 drying Methods 0.000 description 1
- 230000002183 duodenal effect Effects 0.000 description 1
- 230000007140 dysbiosis Effects 0.000 description 1
- 235000019564 dysgeusia Nutrition 0.000 description 1
- 229940099302 efudex Drugs 0.000 description 1
- MDCUNMLZLNGCQA-HWOAGHQOSA-N elafin Chemical compound N([C@H](C(=O)N[C@@H](CCCCN)C(=O)NCC(=O)N1CCC[C@H]1C(=O)N[C@H](C(=O)N[C@@H](CO)C(=O)N[C@H](C(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)NCC(=O)N[C@@H](CO)C(=O)N[C@@H]1C(=O)N2CCC[C@H]2C(=O)N[C@H](C(=O)N[C@H](C(=O)N[C@@H](CC(C)C)C(=O)N[C@H](C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@H]2CSSC[C@H]3C(=O)NCC(=O)N[C@@H](CCSC)C(=O)N[C@@H](C)C(=O)N[C@@H](CSSC[C@H]4C(=O)N5CCC[C@H]5C(=O)NCC(=O)N[C@H](C(N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CSSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H]5N(CCC5)C(=O)[C@H]5N(CCC5)C(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCSC)NC(=O)[C@H](C)NC2=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(O)=O)C(=O)N4)C(=O)N[C@@H](CSSC1)C(=O)N[C@@H](CCC(O)=O)C(=O)NCC(=O)N[C@@H](CO)C(=O)N3)=O)[C@@H](C)CC)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](C(C)C)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCC(N)=O)C(O)=O)[C@@H](C)CC)[C@@H](C)CC)[C@@H](C)CC)[C@@H](C)O)C(C)C)C(C)C)C(=O)[C@@H]1CCCN1C(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](C)N MDCUNMLZLNGCQA-HWOAGHQOSA-N 0.000 description 1
- 239000003792 electrolyte Substances 0.000 description 1
- 229940120655 eloxatin Drugs 0.000 description 1
- 229940000733 emcyt Drugs 0.000 description 1
- 229940108890 emend Drugs 0.000 description 1
- DYLUUSLLRIQKOE-UHFFFAOYSA-N enasidenib Chemical compound N=1C(C=2N=C(C=CC=2)C(F)(F)F)=NC(NCC(C)(O)C)=NC=1NC1=CC=NC(C(F)(F)F)=C1 DYLUUSLLRIQKOE-UHFFFAOYSA-N 0.000 description 1
- 229950010133 enasidenib Drugs 0.000 description 1
- 230000002708 enhancing effect Effects 0.000 description 1
- 244000000021 enteric pathogen Species 0.000 description 1
- 231100000655 enterotoxin Toxicity 0.000 description 1
- 210000003979 eosinophil Anatomy 0.000 description 1
- 108010087914 epidermal growth factor receptor VIII Proteins 0.000 description 1
- 229960001904 epirubicin Drugs 0.000 description 1
- 229960003265 epirubicin hydrochloride Drugs 0.000 description 1
- 210000000981 epithelium Anatomy 0.000 description 1
- QAMYWGZHLCQOOJ-PWIVHLLHSA-N eribulin mesylate Chemical compound CS(O)(=O)=O.C([C@H]1CC[C@@H]2O[C@@H]3[C@H]4O[C@H]5C[C@](O[C@H]4[C@H]2O1)(O[C@@H]53)CC[C@@H]1O[C@H](C(C1)=C)CC1)C(=O)C[C@@H]2[C@@H](OC)[C@@H](C[C@H](O)CN)O[C@H]2C[C@@H]2C(=C)[C@H](C)C[C@H]1O2 QAMYWGZHLCQOOJ-PWIVHLLHSA-N 0.000 description 1
- 229960000439 eribulin mesylate Drugs 0.000 description 1
- GTTBEUCJPZQMDZ-UHFFFAOYSA-N erlotinib hydrochloride Chemical compound [H+].[Cl-].C=12C=C(OCCOC)C(OCCOC)=CC2=NC=NC=1NC1=CC=CC(C#C)=C1 GTTBEUCJPZQMDZ-UHFFFAOYSA-N 0.000 description 1
- 229960005073 erlotinib hydrochloride Drugs 0.000 description 1
- 230000003628 erosive effect Effects 0.000 description 1
- 229960002770 ertapenem Drugs 0.000 description 1
- 201000004101 esophageal cancer Diseases 0.000 description 1
- 210000003238 esophagus Anatomy 0.000 description 1
- 229940064258 estrace Drugs 0.000 description 1
- IIUMCNJTGSMNRO-VVSKJQCTSA-L estramustine sodium phosphate Chemical compound [Na+].[Na+].ClCCN(CCCl)C(=O)OC1=CC=C2[C@H]3CC[C@](C)([C@H](CC4)OP([O-])([O-])=O)[C@@H]4[C@@H]3CCC2=C1 IIUMCNJTGSMNRO-VVSKJQCTSA-L 0.000 description 1
- 235000019441 ethanol Nutrition 0.000 description 1
- LUJQXGBDWAGQHS-UHFFFAOYSA-N ethenyl acetate;phthalic acid Chemical compound CC(=O)OC=C.OC(=O)C1=CC=CC=C1C(O)=O LUJQXGBDWAGQHS-UHFFFAOYSA-N 0.000 description 1
- 229940085363 evista Drugs 0.000 description 1
- 229940060343 evomela Drugs 0.000 description 1
- 208000024519 eye neoplasm Diseases 0.000 description 1
- 206010016256 fatigue Diseases 0.000 description 1
- 235000019197 fats Nutrition 0.000 description 1
- 230000009567 fermentative growth Effects 0.000 description 1
- 239000000835 fiber Substances 0.000 description 1
- 210000002950 fibroblast Anatomy 0.000 description 1
- 239000000945 filler Substances 0.000 description 1
- 239000000706 filtrate Substances 0.000 description 1
- 238000009093 first-line therapy Methods 0.000 description 1
- 239000000796 flavoring agent Substances 0.000 description 1
- 229940115473 florajen3 Drugs 0.000 description 1
- 229960000961 floxuridine Drugs 0.000 description 1
- 229960000390 fludarabine Drugs 0.000 description 1
- AAXVEMMRQDVLJB-BULBTXNYSA-N fludrocortisone Chemical compound O=C1CC[C@]2(C)[C@@]3(F)[C@@H](O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 AAXVEMMRQDVLJB-BULBTXNYSA-N 0.000 description 1
- 229960002011 fludrocortisone Drugs 0.000 description 1
- 229960004511 fludroxycortide Drugs 0.000 description 1
- 229940042902 flumethasone pivalate Drugs 0.000 description 1
- JWRMHDSINXPDHB-OJAGFMMFSA-N flumethasone pivalate Chemical compound C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@]1(F)[C@@H]2[C@@H]2C[C@@H](C)[C@@](C(=O)COC(=O)C(C)(C)C)(O)[C@@]2(C)C[C@@H]1O JWRMHDSINXPDHB-OJAGFMMFSA-N 0.000 description 1
- 229960000676 flunisolide Drugs 0.000 description 1
- GAKMQHDJQHZUTJ-ULHLPKEOSA-N fluocortolone Chemical compound C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@@H]1[C@@H]2[C@@H]2C[C@@H](C)[C@H](C(=O)CO)[C@@]2(C)C[C@@H]1O GAKMQHDJQHZUTJ-ULHLPKEOSA-N 0.000 description 1
- 229960001751 fluoxymesterone Drugs 0.000 description 1
- YLRFCQOZQXIBAB-RBZZARIASA-N fluoxymesterone Chemical compound C1CC2=CC(=O)CC[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1CC[C@](C)(O)[C@@]1(C)C[C@@H]2O YLRFCQOZQXIBAB-RBZZARIASA-N 0.000 description 1
- 229960003590 fluperolone Drugs 0.000 description 1
- HHPZZKDXAFJLOH-QZIXMDIESA-N fluperolone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1CC[C@@](C(=O)[C@@H](OC(C)=O)C)(O)[C@@]1(C)C[C@@H]2O HHPZZKDXAFJLOH-QZIXMDIESA-N 0.000 description 1
- 229960003238 fluprednidene Drugs 0.000 description 1
- YVHXHNGGPURVOS-SBTDHBFYSA-N fluprednidene Chemical compound O=C1C=C[C@]2(C)[C@@]3(F)[C@@H](O)C[C@](C)([C@@](C(=C)C4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 YVHXHNGGPURVOS-SBTDHBFYSA-N 0.000 description 1
- 229960000618 fluprednisolone Drugs 0.000 description 1
- JYEFSHLLTQIXIO-SMNQTINBSA-N folfiri regimen Chemical compound FC1=CNC(=O)NC1=O.C1NC=2NC(N)=NC(=O)C=2N(C=O)C1CNC1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1.C1=C2C(CC)=C3CN(C(C4=C([C@@](C(=O)OC4)(O)CC)C=4)=O)C=4C3=NC2=CC=C1OC(=O)N(CC1)CCC1N1CCCCC1 JYEFSHLLTQIXIO-SMNQTINBSA-N 0.000 description 1
- PJZDLZXMGBOJRF-CXOZILEQSA-L folfirinox Chemical compound [Pt+4].[O-]C(=O)C([O-])=O.[NH-][C@H]1CCCC[C@@H]1[NH-].FC1=CNC(=O)NC1=O.C1NC=2NC(N)=NC(=O)C=2N(C=O)C1CNC1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1.C1=C2C(CC)=C3CN(C(C4=C([C@@](C(=O)OC4)(O)CC)C=4)=O)C=4C3=NC2=CC=C1OC(=O)N(CC1)CCC1N1CCCCC1 PJZDLZXMGBOJRF-CXOZILEQSA-L 0.000 description 1
- 235000008191 folinic acid Nutrition 0.000 description 1
- 239000011672 folinic acid Substances 0.000 description 1
- 201000003444 follicular lymphoma Diseases 0.000 description 1
- 235000013305 food Nutrition 0.000 description 1
- 235000013355 food flavoring agent Nutrition 0.000 description 1
- 230000037406 food intake Effects 0.000 description 1
- 235000003599 food sweetener Nutrition 0.000 description 1
- 235000019253 formic acid Nutrition 0.000 description 1
- 239000003205 fragrance Substances 0.000 description 1
- 229960002737 fructose Drugs 0.000 description 1
- 235000011389 fruit/vegetable juice Nutrition 0.000 description 1
- 125000000524 functional group Chemical group 0.000 description 1
- 108010084448 gamma Catenin Proteins 0.000 description 1
- 102000054078 gamma Catenin Human genes 0.000 description 1
- 229940044627 gamma-interferon Drugs 0.000 description 1
- 150000002270 gangliosides Chemical class 0.000 description 1
- 229940102767 gardasil 9 Drugs 0.000 description 1
- 239000007789 gas Substances 0.000 description 1
- XUBOMFCQGDBHNK-UHFFFAOYSA-N gatifloxacin Chemical compound FC1=CC(C(C(C(O)=O)=CN2C3CC3)=O)=C2C(OC)=C1N1CCNC(C)C1 XUBOMFCQGDBHNK-UHFFFAOYSA-N 0.000 description 1
- 239000007903 gelatin capsule Substances 0.000 description 1
- 229960005277 gemcitabine Drugs 0.000 description 1
- 229960005144 gemcitabine hydrochloride Drugs 0.000 description 1
- 230000002068 genetic effect Effects 0.000 description 1
- 229940080856 gleevec Drugs 0.000 description 1
- 229940084910 gliadel Drugs 0.000 description 1
- TWSALRJGPBVBQU-PKQQPRCHSA-N glucagon-like peptide 2 Chemical compound C([C@@H](C(=O)N[C@H](C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(O)=O)C(O)=O)[C@@H](C)CC)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCSC)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@H](CO)NC(=O)CNC(=O)[C@H](CC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CC=1NC=NC=1)[C@@H](C)O)[C@@H](C)CC)C1=CC=CC=C1 TWSALRJGPBVBQU-PKQQPRCHSA-N 0.000 description 1
- ZDXPYRJPNDTMRX-UHFFFAOYSA-N glutamine Natural products OC(=O)C(N)CCC(N)=O ZDXPYRJPNDTMRX-UHFFFAOYSA-N 0.000 description 1
- 229960002743 glutamine Drugs 0.000 description 1
- 229960003180 glutathione Drugs 0.000 description 1
- 235000003969 glutathione Nutrition 0.000 description 1
- ZEMPKEQAKRGZGQ-XOQCFJPHSA-N glycerol triricinoleate Natural products CCCCCC[C@@H](O)CC=CCCCCCCCC(=O)OC[C@@H](COC(=O)CCCCCCCC=CC[C@@H](O)CCCCCC)OC(=O)CCCCCCCC=CC[C@H](O)CCCCCC ZEMPKEQAKRGZGQ-XOQCFJPHSA-N 0.000 description 1
- 229940049654 glyceryl behenate Drugs 0.000 description 1
- 239000001087 glyceryl triacetate Substances 0.000 description 1
- 235000013773 glyceryl triacetate Nutrition 0.000 description 1
- 229960002389 glycol salicylate Drugs 0.000 description 1
- XLXSAKCOAKORKW-AQJXLSMYSA-N gonadorelin Chemical compound C([C@@H](C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1[C@@H](CCC1)C(=O)NCC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@H](CC=1N=CNC=1)NC(=O)[C@H]1NC(=O)CC1)C1=CC=C(O)C=C1 XLXSAKCOAKORKW-AQJXLSMYSA-N 0.000 description 1
- 229940035638 gonadotropin-releasing hormone Drugs 0.000 description 1
- 229960003690 goserelin acetate Drugs 0.000 description 1
- 244000000059 gram-positive pathogen Species 0.000 description 1
- 238000005469 granulation Methods 0.000 description 1
- 230000003179 granulation Effects 0.000 description 1
- 238000001631 haemodialysis Methods 0.000 description 1
- 201000009277 hairy cell leukemia Diseases 0.000 description 1
- 229960002383 halcinonide Drugs 0.000 description 1
- 201000010536 head and neck cancer Diseases 0.000 description 1
- 208000014829 head and neck neoplasm Diseases 0.000 description 1
- 230000007407 health benefit Effects 0.000 description 1
- 229940033776 hemangeol Drugs 0.000 description 1
- 238000005534 hematocrit Methods 0.000 description 1
- 201000005787 hematologic cancer Diseases 0.000 description 1
- 208000024200 hematopoietic and lymphoid system neoplasm Diseases 0.000 description 1
- 230000000322 hemodialysis Effects 0.000 description 1
- 206010073071 hepatocellular carcinoma Diseases 0.000 description 1
- 125000005549 heteroarylene group Chemical group 0.000 description 1
- 208000021760 high fever Diseases 0.000 description 1
- HHXHVIJIIXKSOE-QILQGKCVSA-N histrelin Chemical compound CCNC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CO)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@H](CC=1N=CNC=1)NC(=O)[C@H]1NC(=O)CC1)CC(N=C1)=CN1CC1=CC=CC=C1 HHXHVIJIIXKSOE-QILQGKCVSA-N 0.000 description 1
- 230000003054 hormonal effect Effects 0.000 description 1
- 229940088597 hormone Drugs 0.000 description 1
- 239000005556 hormone Substances 0.000 description 1
- 235000001050 hortel pimenta Nutrition 0.000 description 1
- 102000051957 human ERBB2 Human genes 0.000 description 1
- 244000005702 human microbiome Species 0.000 description 1
- 229920002674 hyaluronan Polymers 0.000 description 1
- 229960003160 hyaluronic acid Drugs 0.000 description 1
- 229960000890 hydrocortisone Drugs 0.000 description 1
- 229960001067 hydrocortisone acetate Drugs 0.000 description 1
- 229960001524 hydrocortisone butyrate Drugs 0.000 description 1
- 229910052588 hydroxylapatite Inorganic materials 0.000 description 1
- 229920003063 hydroxymethyl cellulose Polymers 0.000 description 1
- 229940031574 hydroxymethyl cellulose Drugs 0.000 description 1
- 229940031704 hydroxypropyl methylcellulose phthalate Drugs 0.000 description 1
- 229920003132 hydroxypropyl methylcellulose phthalate Polymers 0.000 description 1
- 206010020718 hyperplasia Diseases 0.000 description 1
- 229960003943 hypromellose Drugs 0.000 description 1
- 229960001680 ibuprofen Drugs 0.000 description 1
- 229940049235 iclusig Drugs 0.000 description 1
- 229940099279 idamycin Drugs 0.000 description 1
- 229960001176 idarubicin hydrochloride Drugs 0.000 description 1
- 239000002117 illicit drug Substances 0.000 description 1
- 229960002411 imatinib Drugs 0.000 description 1
- KTUFNOKKBVMGRW-UHFFFAOYSA-N imatinib Chemical compound C1CN(C)CCN1CC1=CC=C(C(=O)NC=2C=C(NC=3N=C(C=CN=3)C=3C=NC=CC=3)C(C)=CC=2)C=C1 KTUFNOKKBVMGRW-UHFFFAOYSA-N 0.000 description 1
- 229960003685 imatinib mesylate Drugs 0.000 description 1
- 239000012729 immediate-release (IR) formulation Substances 0.000 description 1
- 230000002519 immonomodulatory effect Effects 0.000 description 1
- 230000005934 immune activation Effects 0.000 description 1
- 230000036039 immunity Effects 0.000 description 1
- 238000001727 in vivo Methods 0.000 description 1
- 150000002475 indoles Chemical class 0.000 description 1
- 229960000905 indomethacin Drugs 0.000 description 1
- 230000006698 induction Effects 0.000 description 1
- 208000027139 infectious colitis Diseases 0.000 description 1
- 208000021646 inflammation of heart layer Diseases 0.000 description 1
- 230000004968 inflammatory condition Effects 0.000 description 1
- 208000027866 inflammatory disease Diseases 0.000 description 1
- 230000002757 inflammatory effect Effects 0.000 description 1
- 230000028709 inflammatory response Effects 0.000 description 1
- 239000003112 inhibitor Substances 0.000 description 1
- 210000005007 innate immune system Anatomy 0.000 description 1
- 229910052500 inorganic mineral Inorganic materials 0.000 description 1
- 108010074109 interleukin-22 Proteins 0.000 description 1
- 210000005027 intestinal barrier Anatomy 0.000 description 1
- 230000007358 intestinal barrier function Effects 0.000 description 1
- 210000002490 intestinal epithelial cell Anatomy 0.000 description 1
- 201000008267 intestinal tuberculosis Diseases 0.000 description 1
- 230000003834 intracellular effect Effects 0.000 description 1
- 208000020082 intraepithelial neoplasia Diseases 0.000 description 1
- 239000007927 intramuscular injection Substances 0.000 description 1
- 239000007928 intraperitoneal injection Substances 0.000 description 1
- 239000002563 ionic surfactant Substances 0.000 description 1
- 229960004768 irinotecan Drugs 0.000 description 1
- 229940048117 irinotecan hydrochloride liposome Drugs 0.000 description 1
- 201000008222 ischemic colitis Diseases 0.000 description 1
- FZWBNHMXJMCXLU-BLAUPYHCSA-N isomaltotriose Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1OC[C@@H]1[C@@H](O)[C@H](O)[C@@H](O)[C@@H](OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C=O)O1 FZWBNHMXJMCXLU-BLAUPYHCSA-N 0.000 description 1
- 229960002951 ixazomib citrate Drugs 0.000 description 1
- MBOMYENWWXQSNW-AWEZNQCLSA-N ixazomib citrate Chemical compound N([C@@H](CC(C)C)B1OC(CC(O)=O)(CC(O)=O)C(=O)O1)C(=O)CNC(=O)C1=CC(Cl)=CC=C1Cl MBOMYENWWXQSNW-AWEZNQCLSA-N 0.000 description 1
- 229940111707 ixempra Drugs 0.000 description 1
- 210000001503 joint Anatomy 0.000 description 1
- 229940119170 jojoba wax Drugs 0.000 description 1
- DKYWVDODHFEZIM-UHFFFAOYSA-N ketoprofen Chemical compound OC(=O)C(C)C1=CC=CC(C(=O)C=2C=CC=CC=2)=C1 DKYWVDODHFEZIM-UHFFFAOYSA-N 0.000 description 1
- 229960000991 ketoprofen Drugs 0.000 description 1
- 210000003734 kidney Anatomy 0.000 description 1
- 239000004922 lacquer Substances 0.000 description 1
- 229940039695 lactobacillus acidophilus Drugs 0.000 description 1
- 229940072205 lactobacillus plantarum Drugs 0.000 description 1
- 229940059406 lactobacillus rhamnosus gg Drugs 0.000 description 1
- 229960001375 lactose Drugs 0.000 description 1
- JCQLYHFGKNRPGE-FCVZTGTOSA-N lactulose Chemical compound OC[C@H]1O[C@](O)(CO)[C@@H](O)[C@@H]1O[C@H]1[C@H](O)[C@@H](O)[C@@H](O)[C@@H](CO)O1 JCQLYHFGKNRPGE-FCVZTGTOSA-N 0.000 description 1
- 229960000511 lactulose Drugs 0.000 description 1
- PFCRQPBOOFTZGQ-UHFFFAOYSA-N lactulose keto form Natural products OCC(=O)C(O)C(C(O)CO)OC1OC(CO)C(O)C(O)C1O PFCRQPBOOFTZGQ-UHFFFAOYSA-N 0.000 description 1
- 229960002437 lanreotide Drugs 0.000 description 1
- 229960001320 lapatinib ditosylate Drugs 0.000 description 1
- 206010023841 laryngeal neoplasm Diseases 0.000 description 1
- 201000004962 larynx cancer Diseases 0.000 description 1
- 229960001691 leucovorin Drugs 0.000 description 1
- 206010024378 leukocytosis Diseases 0.000 description 1
- 229940089519 levaquin Drugs 0.000 description 1
- 239000003446 ligand Substances 0.000 description 1
- 210000000088 lip Anatomy 0.000 description 1
- 210000005229 liver cell Anatomy 0.000 description 1
- 229940024740 lonsurf Drugs 0.000 description 1
- 210000003141 lower extremity Anatomy 0.000 description 1
- 210000003750 lower gastrointestinal tract Anatomy 0.000 description 1
- 239000000314 lubricant Substances 0.000 description 1
- 201000005249 lung adenocarcinoma Diseases 0.000 description 1
- 201000005202 lung cancer Diseases 0.000 description 1
- 208000020816 lung neoplasm Diseases 0.000 description 1
- 108010078259 luprolide acetate gel depot Proteins 0.000 description 1
- 210000004698 lymphocyte Anatomy 0.000 description 1
- 230000002535 lyotropic effect Effects 0.000 description 1
- 239000011777 magnesium Substances 0.000 description 1
- 229910052749 magnesium Inorganic materials 0.000 description 1
- ZLNQQNXFFQJAID-UHFFFAOYSA-L magnesium carbonate Chemical compound [Mg+2].[O-]C([O-])=O ZLNQQNXFFQJAID-UHFFFAOYSA-L 0.000 description 1
- 239000001095 magnesium carbonate Substances 0.000 description 1
- 229910000021 magnesium carbonate Inorganic materials 0.000 description 1
- 150000002681 magnesium compounds Chemical class 0.000 description 1
- VTHJTEIRLNZDEV-UHFFFAOYSA-L magnesium dihydroxide Chemical compound [OH-].[OH-].[Mg+2] VTHJTEIRLNZDEV-UHFFFAOYSA-L 0.000 description 1
- 239000000347 magnesium hydroxide Substances 0.000 description 1
- 229910001862 magnesium hydroxide Inorganic materials 0.000 description 1
- 239000000395 magnesium oxide Substances 0.000 description 1
- CPLXHLVBOLITMK-UHFFFAOYSA-N magnesium oxide Inorganic materials [Mg]=O CPLXHLVBOLITMK-UHFFFAOYSA-N 0.000 description 1
- 229910052943 magnesium sulfate Inorganic materials 0.000 description 1
- 235000019341 magnesium sulphate Nutrition 0.000 description 1
- AXZKOIWUVFPNLO-UHFFFAOYSA-N magnesium;oxygen(2-) Chemical compound [O-2].[Mg+2] AXZKOIWUVFPNLO-UHFFFAOYSA-N 0.000 description 1
- 229940049920 malate Drugs 0.000 description 1
- 230000003211 malignant effect Effects 0.000 description 1
- 208000015486 malignant pancreatic neoplasm Diseases 0.000 description 1
- 229940035034 maltodextrin Drugs 0.000 description 1
- 229960002160 maltose Drugs 0.000 description 1
- 238000007726 management method Methods 0.000 description 1
- FQXXSQDCDRQNQE-UHFFFAOYSA-N markiertes Thebain Natural products COC1=CC=C2C(N(CC3)C)CC4=CC=C(OC)C5=C4C23C1O5 FQXXSQDCDRQNQE-UHFFFAOYSA-N 0.000 description 1
- 229960004961 mechlorethamine Drugs 0.000 description 1
- HAWPXGHAZFHHAD-UHFFFAOYSA-N mechlorethamine Chemical compound ClCCN(C)CCCl HAWPXGHAZFHHAD-UHFFFAOYSA-N 0.000 description 1
- QZIQJVCYUQZDIR-UHFFFAOYSA-N mechlorethamine hydrochloride Chemical compound Cl.ClCCN(C)CCCl QZIQJVCYUQZDIR-UHFFFAOYSA-N 0.000 description 1
- 229960002868 mechlorethamine hydrochloride Drugs 0.000 description 1
- PSGAAPLEWMOORI-PEINSRQWSA-N medroxyprogesterone acetate Chemical compound C([C@@]12C)CC(=O)C=C1[C@@H](C)C[C@@H]1[C@@H]2CC[C@]2(C)[C@@](OC(C)=O)(C(C)=O)CC[C@H]21 PSGAAPLEWMOORI-PEINSRQWSA-N 0.000 description 1
- 229960002985 medroxyprogesterone acetate Drugs 0.000 description 1
- 208000004840 megacolon Diseases 0.000 description 1
- DRLFMBDRBRZALE-UHFFFAOYSA-N melatonin Chemical compound COC1=CC=C2NC=C(CCNC(C)=O)C2=C1 DRLFMBDRBRZALE-UHFFFAOYSA-N 0.000 description 1
- 229960003987 melatonin Drugs 0.000 description 1
- 229960001810 meprednisone Drugs 0.000 description 1
- PIDANAQULIKBQS-RNUIGHNZSA-N meprednisone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@@H]2[C@@H]1[C@@H]1C[C@H](C)[C@@](C(=O)CO)(O)[C@@]1(C)CC2=O PIDANAQULIKBQS-RNUIGHNZSA-N 0.000 description 1
- 229960002260 meropenem Drugs 0.000 description 1
- DMJNNHOOLUXYBV-PQTSNVLCSA-N meropenem Chemical compound C=1([C@H](C)[C@@H]2[C@H](C(N2C=1C(O)=O)=O)[C@H](O)C)S[C@@H]1CN[C@H](C(=O)N(C)C)C1 DMJNNHOOLUXYBV-PQTSNVLCSA-N 0.000 description 1
- 229940101533 mesnex Drugs 0.000 description 1
- 230000037323 metabolic rate Effects 0.000 description 1
- 150000002734 metacrylic acid derivatives Chemical class 0.000 description 1
- 230000001394 metastastic effect Effects 0.000 description 1
- 125000005397 methacrylic acid ester group Chemical group 0.000 description 1
- 229960001797 methadone Drugs 0.000 description 1
- 229930182817 methionine Natural products 0.000 description 1
- 229960004452 methionine Drugs 0.000 description 1
- 235000006109 methionine Nutrition 0.000 description 1
- 229920000609 methyl cellulose Polymers 0.000 description 1
- 229960001047 methyl salicylate Drugs 0.000 description 1
- 150000003956 methylamines Chemical class 0.000 description 1
- 235000010981 methylcellulose Nutrition 0.000 description 1
- 239000001923 methylcellulose Substances 0.000 description 1
- 229960004584 methylprednisolone Drugs 0.000 description 1
- 208000008275 microscopic colitis Diseases 0.000 description 1
- 235000013336 milk Nutrition 0.000 description 1
- 239000008267 milk Substances 0.000 description 1
- 210000004080 milk Anatomy 0.000 description 1
- 239000011707 mineral Substances 0.000 description 1
- 235000010755 mineral Nutrition 0.000 description 1
- 229960004023 minocycline Drugs 0.000 description 1
- 229960000350 mitotane Drugs 0.000 description 1
- 229960001156 mitoxantrone Drugs 0.000 description 1
- ZAHQPTJLOCWVPG-UHFFFAOYSA-N mitoxantrone dihydrochloride Chemical compound Cl.Cl.O=C1C2=C(O)C=CC(O)=C2C(=O)C2=C1C(NCCNCCO)=CC=C2NCCNCCO ZAHQPTJLOCWVPG-UHFFFAOYSA-N 0.000 description 1
- 229960004169 mitoxantrone hydrochloride Drugs 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000001333 moisturizer Effects 0.000 description 1
- 238000012544 monitoring process Methods 0.000 description 1
- LPUQAYUQRXPFSQ-DFWYDOINSA-M monosodium L-glutamate Chemical compound [Na+].[O-]C(=O)[C@@H](N)CCC(O)=O LPUQAYUQRXPFSQ-DFWYDOINSA-M 0.000 description 1
- 239000004223 monosodium glutamate Substances 0.000 description 1
- 235000013923 monosodium glutamate Nutrition 0.000 description 1
- PJUIMOJAAPLTRJ-UHFFFAOYSA-N monothioglycerol Chemical compound OCC(O)CS PJUIMOJAAPLTRJ-UHFFFAOYSA-N 0.000 description 1
- INAXVFBXDYWQFN-XHSDSOJGSA-N morphinan Chemical compound C1C2=CC=CC=C2[C@]23CCCC[C@H]3[C@@H]1NCC2 INAXVFBXDYWQFN-XHSDSOJGSA-N 0.000 description 1
- 229960005181 morphine Drugs 0.000 description 1
- 210000004877 mucosa Anatomy 0.000 description 1
- 239000002362 mulch Substances 0.000 description 1
- 239000000472 muscarinic agonist Substances 0.000 description 1
- 208000025113 myeloid leukemia Diseases 0.000 description 1
- 201000000050 myeloid neoplasm Diseases 0.000 description 1
- 208000010125 myocardial infarction Diseases 0.000 description 1
- AZBFJBJXUQUQLF-UHFFFAOYSA-N n-(1,5-dimethylpyrrolidin-3-yl)pyrrolidine-1-carboxamide Chemical compound C1N(C)C(C)CC1NC(=O)N1CCCC1 AZBFJBJXUQUQLF-UHFFFAOYSA-N 0.000 description 1
- PUPNJSIFIXXJCH-UHFFFAOYSA-N n-(4-hydroxyphenyl)-2-(1,1,3-trioxo-1,2-benzothiazol-2-yl)acetamide Chemical compound C1=CC(O)=CC=C1NC(=O)CN1S(=O)(=O)C2=CC=CC=C2C1=O PUPNJSIFIXXJCH-UHFFFAOYSA-N 0.000 description 1
- DAZSWUUAFHBCGE-KRWDZBQOSA-N n-[(2s)-3-methyl-1-oxo-1-pyrrolidin-1-ylbutan-2-yl]-3-phenylpropanamide Chemical compound N([C@@H](C(C)C)C(=O)N1CCCC1)C(=O)CCC1=CC=CC=C1 DAZSWUUAFHBCGE-KRWDZBQOSA-N 0.000 description 1
- BLCLNMBMMGCOAS-UHFFFAOYSA-N n-[1-[[1-[[1-[[1-[[1-[[1-[[1-[2-[(carbamoylamino)carbamoyl]pyrrolidin-1-yl]-5-(diaminomethylideneamino)-1-oxopentan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-3-[(2-methylpropan-2-yl)oxy]-1-oxopropan-2-yl]amino]-3-(4-hydroxyphenyl)-1-oxopropan-2-yl]amin Chemical compound C1CCC(C(=O)NNC(N)=O)N1C(=O)C(CCCN=C(N)N)NC(=O)C(CC(C)C)NC(=O)C(COC(C)(C)C)NC(=O)C(NC(=O)C(CO)NC(=O)C(CC=1C2=CC=CC=C2NC=1)NC(=O)C(CC=1NC=NC=1)NC(=O)C1NC(=O)CC1)CC1=CC=C(O)C=C1 BLCLNMBMMGCOAS-UHFFFAOYSA-N 0.000 description 1
- WPEWQEMJFLWMLV-UHFFFAOYSA-N n-[4-(1-cyanocyclopentyl)phenyl]-2-(pyridin-4-ylmethylamino)pyridine-3-carboxamide Chemical compound C=1C=CN=C(NCC=2C=CN=CC=2)C=1C(=O)NC(C=C1)=CC=C1C1(C#N)CCCC1 WPEWQEMJFLWMLV-UHFFFAOYSA-N 0.000 description 1
- 229960002009 naproxen Drugs 0.000 description 1
- CMWTZPSULFXXJA-VIFPVBQESA-N naproxen Chemical compound C1=C([C@H](C)C(O)=O)C=CC2=CC(OC)=CC=C21 CMWTZPSULFXXJA-VIFPVBQESA-N 0.000 description 1
- 230000002956 necrotizing effect Effects 0.000 description 1
- 230000006654 negative regulation of apoptotic process Effects 0.000 description 1
- 230000007302 negative regulation of cytokine production Effects 0.000 description 1
- WAXQNWCZJDTGBU-UHFFFAOYSA-N netupitant Chemical compound C=1N=C(N2CCN(C)CC2)C=C(C=2C(=CC=CC=2)C)C=1N(C)C(=O)C(C)(C)C1=CC(C(F)(F)F)=CC(C(F)(F)F)=C1 WAXQNWCZJDTGBU-UHFFFAOYSA-N 0.000 description 1
- 229960005163 netupitant Drugs 0.000 description 1
- 208000015122 neurodegenerative disease Diseases 0.000 description 1
- 230000000626 neurodegenerative effect Effects 0.000 description 1
- 230000001123 neurodevelopmental effect Effects 0.000 description 1
- 230000000926 neurological effect Effects 0.000 description 1
- 210000000440 neutrophil Anatomy 0.000 description 1
- 229940109551 nipent Drugs 0.000 description 1
- PCHKPVIQAHNQLW-CQSZACIVSA-N niraparib Chemical compound N1=C2C(C(=O)N)=CC=CC2=CN1C(C=C1)=CC=C1[C@@H]1CCCNC1 PCHKPVIQAHNQLW-CQSZACIVSA-N 0.000 description 1
- 229950011068 niraparib Drugs 0.000 description 1
- 229920001220 nitrocellulos Polymers 0.000 description 1
- 229910052757 nitrogen Inorganic materials 0.000 description 1
- 229940085033 nolvadex Drugs 0.000 description 1
- 208000002154 non-small cell lung carcinoma Diseases 0.000 description 1
- 239000000041 non-steroidal anti-inflammatory agent Substances 0.000 description 1
- QKQQEIVDLRUZRP-UHFFFAOYSA-N northebaine Natural products COC1=CC=C2C(NCC3)CC4=CC=C(OC)C5=C4C23C1O5 QKQQEIVDLRUZRP-UHFFFAOYSA-N 0.000 description 1
- XNGIFLGASWRNHJ-UHFFFAOYSA-N o-dicarboxybenzene Natural products OC(=O)C1=CC=CC=C1C(O)=O XNGIFLGASWRNHJ-UHFFFAOYSA-N 0.000 description 1
- 201000008106 ocular cancer Diseases 0.000 description 1
- 229940024847 odomzo Drugs 0.000 description 1
- 229960000572 olaparib Drugs 0.000 description 1
- FAQDUNYVKQKNLD-UHFFFAOYSA-N olaparib Chemical compound FC1=CC=C(CC2=C3[CH]C=CC=C3C(=O)N=N2)C=C1C(=O)N(CC1)CCN1C(=O)C1CC1 FAQDUNYVKQKNLD-UHFFFAOYSA-N 0.000 description 1
- 229920001542 oligosaccharide Polymers 0.000 description 1
- 150000002482 oligosaccharides Chemical class 0.000 description 1
- 239000004006 olive oil Substances 0.000 description 1
- 235000008390 olive oil Nutrition 0.000 description 1
- 229940127240 opiate Drugs 0.000 description 1
- 244000039328 opportunistic pathogen Species 0.000 description 1
- 201000005443 oral cavity cancer Diseases 0.000 description 1
- 229940003515 orapred Drugs 0.000 description 1
- KJIFKLIQANRMOU-UHFFFAOYSA-N oxidanium;4-methylbenzenesulfonate Chemical compound O.CC1=CC=C(S(O)(=O)=O)C=C1 KJIFKLIQANRMOU-UHFFFAOYSA-N 0.000 description 1
- 229960000625 oxytetracycline Drugs 0.000 description 1
- IWVCMVBTMGNXQD-PXOLEDIWSA-N oxytetracycline Chemical compound C1=CC=C2[C@](O)(C)[C@H]3[C@H](O)[C@H]4[C@H](N(C)C)C(O)=C(C(N)=O)C(=O)[C@@]4(O)C(O)=C3C(=O)C2=C1O IWVCMVBTMGNXQD-PXOLEDIWSA-N 0.000 description 1
- 235000019366 oxytetracycline Nutrition 0.000 description 1
- LSQZJLSUYDQPKJ-UHFFFAOYSA-N p-Hydroxyampicillin Natural products O=C1N2C(C(O)=O)C(C)(C)SC2C1NC(=O)C(N)C1=CC=C(O)C=C1 LSQZJLSUYDQPKJ-UHFFFAOYSA-N 0.000 description 1
- 229960004390 palbociclib Drugs 0.000 description 1
- AHJRHEGDXFFMBM-UHFFFAOYSA-N palbociclib Chemical compound N1=C2N(C3CCCC3)C(=O)C(C(=O)C)=C(C)C2=CN=C1NC(N=C1)=CC=C1N1CCNCC1 AHJRHEGDXFFMBM-UHFFFAOYSA-N 0.000 description 1
- 201000005580 palindromic rheumatism Diseases 0.000 description 1
- 229910052763 palladium Inorganic materials 0.000 description 1
- 229940046231 pamidronate Drugs 0.000 description 1
- 229960003978 pamidronic acid Drugs 0.000 description 1
- 208000014965 pancolitis Diseases 0.000 description 1
- 201000002528 pancreatic cancer Diseases 0.000 description 1
- 208000008443 pancreatic carcinoma Diseases 0.000 description 1
- 229940096763 panretin Drugs 0.000 description 1
- FJKROLUGYXJWQN-UHFFFAOYSA-N papa-hydroxy-benzoic acid Natural products OC(=O)C1=CC=C(O)C=C1 FJKROLUGYXJWQN-UHFFFAOYSA-N 0.000 description 1
- 235000019809 paraffin wax Nutrition 0.000 description 1
- 229960002858 paramethasone Drugs 0.000 description 1
- 230000003071 parasitic effect Effects 0.000 description 1
- 229940069533 paregoric Drugs 0.000 description 1
- 239000008414 paregoric Substances 0.000 description 1
- 238000007911 parenteral administration Methods 0.000 description 1
- 230000001314 paroxysmal effect Effects 0.000 description 1
- 239000002245 particle Substances 0.000 description 1
- 239000013618 particulate matter Substances 0.000 description 1
- 229940097097 pediapred Drugs 0.000 description 1
- 239000008188 pellet Substances 0.000 description 1
- 229960003349 pemetrexed disodium Drugs 0.000 description 1
- 230000035515 penetration Effects 0.000 description 1
- 229940056367 penicillin v Drugs 0.000 description 1
- XYJRXVWERLGGKC-UHFFFAOYSA-D pentacalcium;hydroxide;triphosphate Chemical compound [OH-].[Ca+2].[Ca+2].[Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O XYJRXVWERLGGKC-UHFFFAOYSA-D 0.000 description 1
- 229960002340 pentostatin Drugs 0.000 description 1
- 208000011906 peptic ulcer disease Diseases 0.000 description 1
- 108010044156 peptidyl-prolyl cis-trans isomerase b Proteins 0.000 description 1
- 210000004303 peritoneum Anatomy 0.000 description 1
- 201000002628 peritoneum cancer Diseases 0.000 description 1
- 206010034674 peritonitis Diseases 0.000 description 1
- 235000019271 petrolatum Nutrition 0.000 description 1
- 239000000825 pharmaceutical preparation Substances 0.000 description 1
- 230000003285 pharmacodynamic effect Effects 0.000 description 1
- 230000000144 pharmacologic effect Effects 0.000 description 1
- 210000003800 pharynx Anatomy 0.000 description 1
- BPLBGHOLXOTWMN-MBNYWOFBSA-N phenoxymethylpenicillin Chemical compound N([C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C(=O)COC1=CC=CC=C1 BPLBGHOLXOTWMN-MBNYWOFBSA-N 0.000 description 1
- 229960002895 phenylbutazone Drugs 0.000 description 1
- VYMDGNCVAMGZFE-UHFFFAOYSA-N phenylbutazonum Chemical compound O=C1C(CCCC)C(=O)N(C=2C=CC=CC=2)N1C1=CC=CC=C1 VYMDGNCVAMGZFE-UHFFFAOYSA-N 0.000 description 1
- GVKCHTBDSMQENH-UHFFFAOYSA-L phloxine B Chemical compound [Na+].[Na+].[O-]C(=O)C1=C(Cl)C(Cl)=C(Cl)C(Cl)=C1C1=C2C=C(Br)C(=O)C(Br)=C2OC2=C(Br)C([O-])=C(Br)C=C21 GVKCHTBDSMQENH-UHFFFAOYSA-L 0.000 description 1
- 150000003013 phosphoric acid derivatives Chemical class 0.000 description 1
- 229910052698 phosphorus Inorganic materials 0.000 description 1
- 239000011574 phosphorus Substances 0.000 description 1
- 229920001983 poloxamer Polymers 0.000 description 1
- 239000004584 polyacrylic acid Substances 0.000 description 1
- 235000013856 polydextrose Nutrition 0.000 description 1
- 239000001259 polydextrose Substances 0.000 description 1
- 229940035035 polydextrose Drugs 0.000 description 1
- 239000002861 polymer material Substances 0.000 description 1
- 239000004926 polymethyl methacrylate Substances 0.000 description 1
- 235000010486 polyoxyethylene sorbitan monolaurate Nutrition 0.000 description 1
- 239000000256 polyoxyethylene sorbitan monolaurate Substances 0.000 description 1
- 235000010483 polyoxyethylene sorbitan monopalmitate Nutrition 0.000 description 1
- 239000000249 polyoxyethylene sorbitan monopalmitate Substances 0.000 description 1
- 235000010989 polyoxyethylene sorbitan monostearate Nutrition 0.000 description 1
- 239000001818 polyoxyethylene sorbitan monostearate Substances 0.000 description 1
- 208000015768 polyposis Diseases 0.000 description 1
- 229920000136 polysorbate Polymers 0.000 description 1
- 229950008882 polysorbate Drugs 0.000 description 1
- 229940068977 polysorbate 20 Drugs 0.000 description 1
- 229940101027 polysorbate 40 Drugs 0.000 description 1
- 229940113124 polysorbate 60 Drugs 0.000 description 1
- 229920002744 polyvinyl acetate phthalate Polymers 0.000 description 1
- 235000019422 polyvinyl alcohol Nutrition 0.000 description 1
- 229920001290 polyvinyl ester Polymers 0.000 description 1
- 235000013809 polyvinylpolypyrrolidone Nutrition 0.000 description 1
- 229920000523 polyvinylpolypyrrolidone Polymers 0.000 description 1
- BWTNNZPNKQIADY-UHFFFAOYSA-N ponatinib hydrochloride Chemical compound Cl.C1CN(C)CCN1CC(C(=C1)C(F)(F)F)=CC=C1NC(=O)C1=CC=C(C)C(C#CC=2N3N=CC=CC3=NC=2)=C1 BWTNNZPNKQIADY-UHFFFAOYSA-N 0.000 description 1
- 229960002183 ponatinib hydrochloride Drugs 0.000 description 1
- 230000000291 postprandial effect Effects 0.000 description 1
- 229910000027 potassium carbonate Inorganic materials 0.000 description 1
- 235000011181 potassium carbonates Nutrition 0.000 description 1
- RWPGFSMJFRPDDP-UHFFFAOYSA-L potassium metabisulfite Chemical compound [K+].[K+].[O-]S(=O)S([O-])(=O)=O RWPGFSMJFRPDDP-UHFFFAOYSA-L 0.000 description 1
- 229940043349 potassium metabisulfite Drugs 0.000 description 1
- 235000010263 potassium metabisulphite Nutrition 0.000 description 1
- 230000035935 pregnancy Effects 0.000 description 1
- 239000003755 preservative agent Substances 0.000 description 1
- 230000002265 prevention Effects 0.000 description 1
- 229960001586 procarbazine hydrochloride Drugs 0.000 description 1
- 102000004196 processed proteins & peptides Human genes 0.000 description 1
- 108090000765 processed proteins & peptides Proteins 0.000 description 1
- RJKFOVLPORLFTN-UHFFFAOYSA-N progesterone acetate Natural products C1CC2=CC(=O)CCC2(C)C2C1C1CCC(C(=O)C)C1(C)CC2 RJKFOVLPORLFTN-UHFFFAOYSA-N 0.000 description 1
- 229940087463 proleukin Drugs 0.000 description 1
- 229940021945 promacta Drugs 0.000 description 1
- 235000019260 propionic acid Nutrition 0.000 description 1
- ZMRUPTIKESYGQW-UHFFFAOYSA-N propranolol hydrochloride Chemical compound [H+].[Cl-].C1=CC=C2C(OCC(O)CNC(C)C)=CC=CC2=C1 ZMRUPTIKESYGQW-UHFFFAOYSA-N 0.000 description 1
- BHMBVRSPMRCCGG-OUTUXVNYSA-N prostaglandin D2 Chemical compound CCCCC[C@H](O)\C=C\[C@@H]1[C@@H](C\C=C/CCCC(O)=O)[C@@H](O)CC1=O BHMBVRSPMRCCGG-OUTUXVNYSA-N 0.000 description 1
- BHMBVRSPMRCCGG-UHFFFAOYSA-N prostaglandine D2 Natural products CCCCCC(O)C=CC1C(CC=CCCCC(O)=O)C(O)CC1=O BHMBVRSPMRCCGG-UHFFFAOYSA-N 0.000 description 1
- 230000009430 psychological distress Effects 0.000 description 1
- 235000019423 pullulan Nutrition 0.000 description 1
- 208000009954 pyoderma gangrenosum Diseases 0.000 description 1
- MIXMJCQRHVAJIO-TZHJZOAOSA-N qk4dys664x Chemical compound O.C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@@H]1[C@@H]2[C@@H]2C[C@H]3OC(C)(C)O[C@@]3(C(=O)CO)[C@@]2(C)C[C@@H]1O.C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@@H]1[C@@H]2[C@@H]2C[C@H]3OC(C)(C)O[C@@]3(C(=O)CO)[C@@]2(C)C[C@@H]1O MIXMJCQRHVAJIO-TZHJZOAOSA-N 0.000 description 1
- 150000003856 quaternary ammonium compounds Chemical class 0.000 description 1
- 125000001453 quaternary ammonium group Chemical group 0.000 description 1
- IUVKMZGDUIUOCP-BTNSXGMBSA-N quinbolone Chemical compound O([C@H]1CC[C@H]2[C@H]3[C@@H]([C@]4(C=CC(=O)C=C4CC3)C)CC[C@@]21C)C1=CCCC1 IUVKMZGDUIUOCP-BTNSXGMBSA-N 0.000 description 1
- 229960004622 raloxifene Drugs 0.000 description 1
- BKXVVCILCIUCLG-UHFFFAOYSA-N raloxifene hydrochloride Chemical compound [H+].[Cl-].C1=CC(O)=CC=C1C1=C(C(=O)C=2C=CC(OCCN3CCCCC3)=CC=2)C2=CC=C(O)C=C2S1 BKXVVCILCIUCLG-UHFFFAOYSA-N 0.000 description 1
- 229960002119 raloxifene hydrochloride Drugs 0.000 description 1
- 229960000424 rasburicase Drugs 0.000 description 1
- 108010084837 rasburicase Proteins 0.000 description 1
- 102000005962 receptors Human genes 0.000 description 1
- 108020003175 receptors Proteins 0.000 description 1
- 229940107023 reclast Drugs 0.000 description 1
- 238000011084 recovery Methods 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 229960004836 regorafenib Drugs 0.000 description 1
- FNHKPVJBJVTLMP-UHFFFAOYSA-N regorafenib Chemical compound C1=NC(C(=O)NC)=CC(OC=2C=C(F)C(NC(=O)NC=3C=C(C(Cl)=CC=3)C(F)(F)F)=CC=2)=C1 FNHKPVJBJVTLMP-UHFFFAOYSA-N 0.000 description 1
- 210000003289 regulatory T cell Anatomy 0.000 description 1
- 230000001105 regulatory effect Effects 0.000 description 1
- 230000029058 respiratory gaseous exchange Effects 0.000 description 1
- 230000036387 respiratory rate Effects 0.000 description 1
- 210000002345 respiratory system Anatomy 0.000 description 1
- 230000004043 responsiveness Effects 0.000 description 1
- 230000000284 resting effect Effects 0.000 description 1
- 230000002441 reversible effect Effects 0.000 description 1
- 201000009410 rhabdomyosarcoma Diseases 0.000 description 1
- 229950003687 ribociclib Drugs 0.000 description 1
- 229960003452 romidepsin Drugs 0.000 description 1
- OHRURASPPZQGQM-GCCNXGTGSA-N romidepsin Chemical compound O1C(=O)[C@H](C(C)C)NC(=O)C(=C/C)/NC(=O)[C@H]2CSSCC\C=C\[C@@H]1CC(=O)N[C@H](C(C)C)C(=O)N2 OHRURASPPZQGQM-GCCNXGTGSA-N 0.000 description 1
- OHRURASPPZQGQM-UHFFFAOYSA-N romidepsin Natural products O1C(=O)C(C(C)C)NC(=O)C(=CC)NC(=O)C2CSSCCC=CC1CC(=O)NC(C(C)C)C(=O)N2 OHRURASPPZQGQM-UHFFFAOYSA-N 0.000 description 1
- 108010091666 romidepsin Proteins 0.000 description 1
- 235000019204 saccharin Nutrition 0.000 description 1
- 229940081974 saccharin Drugs 0.000 description 1
- 239000000901 saccharin and its Na,K and Ca salt Substances 0.000 description 1
- 229960002181 saccharomyces boulardii Drugs 0.000 description 1
- 229960000581 salicylamide Drugs 0.000 description 1
- 229960004889 salicylic acid Drugs 0.000 description 1
- 201000003804 salivary gland carcinoma Diseases 0.000 description 1
- 229940072272 sandostatin Drugs 0.000 description 1
- HFHDHCJBZVLPGP-UHFFFAOYSA-N schardinger α-dextrin Chemical compound O1C(C(C2O)O)C(CO)OC2OC(C(C2O)O)C(CO)OC2OC(C(C2O)O)C(CO)OC2OC(C(O)C2O)C(CO)OC2OC(C(C2O)O)C(CO)OC2OC2C(O)C(O)C1OC2CO HFHDHCJBZVLPGP-UHFFFAOYSA-N 0.000 description 1
- 201000000980 schizophrenia Diseases 0.000 description 1
- DCKVNWZUADLDEH-UHFFFAOYSA-N sec-butyl acetate Chemical compound CCC(C)OC(C)=O DCKVNWZUADLDEH-UHFFFAOYSA-N 0.000 description 1
- 229940095743 selective estrogen receptor modulator Drugs 0.000 description 1
- 239000000333 selective estrogen receptor modulator Substances 0.000 description 1
- 230000035945 sensitivity Effects 0.000 description 1
- 238000012163 sequencing technique Methods 0.000 description 1
- 238000009589 serological test Methods 0.000 description 1
- 229940076279 serotonin Drugs 0.000 description 1
- 230000035939 shock Effects 0.000 description 1
- 150000004760 silicates Chemical class 0.000 description 1
- RMAQACBXLXPBSY-UHFFFAOYSA-N silicic acid Chemical compound O[Si](O)(O)O RMAQACBXLXPBSY-UHFFFAOYSA-N 0.000 description 1
- 235000012239 silicon dioxide Nutrition 0.000 description 1
- 201000000849 skin cancer Diseases 0.000 description 1
- 208000000587 small cell lung carcinoma Diseases 0.000 description 1
- 235000010378 sodium ascorbate Nutrition 0.000 description 1
- PPASLZSBLFJQEF-RKJRWTFHSA-M sodium ascorbate Substances [Na+].OC[C@@H](O)[C@H]1OC(=O)C(O)=C1[O-] PPASLZSBLFJQEF-RKJRWTFHSA-M 0.000 description 1
- 229960005055 sodium ascorbate Drugs 0.000 description 1
- 229940001607 sodium bisulfite Drugs 0.000 description 1
- 229910000029 sodium carbonate Inorganic materials 0.000 description 1
- 239000001509 sodium citrate Substances 0.000 description 1
- NLJMYIDDQXHKNR-UHFFFAOYSA-K sodium citrate Chemical compound O.O.[Na+].[Na+].[Na+].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O NLJMYIDDQXHKNR-UHFFFAOYSA-K 0.000 description 1
- HRZFUMHJMZEROT-UHFFFAOYSA-L sodium disulfite Chemical compound [Na+].[Na+].[O-]S(=O)S([O-])(=O)=O HRZFUMHJMZEROT-UHFFFAOYSA-L 0.000 description 1
- 235000010267 sodium hydrogen sulphite Nutrition 0.000 description 1
- 229940001584 sodium metabisulfite Drugs 0.000 description 1
- 235000010262 sodium metabisulphite Nutrition 0.000 description 1
- 235000010344 sodium nitrate Nutrition 0.000 description 1
- 239000004317 sodium nitrate Substances 0.000 description 1
- 239000008109 sodium starch glycolate Substances 0.000 description 1
- 229940079832 sodium starch glycolate Drugs 0.000 description 1
- 229920003109 sodium starch glycolate Polymers 0.000 description 1
- 229940001482 sodium sulfite Drugs 0.000 description 1
- 235000010265 sodium sulphite Nutrition 0.000 description 1
- PPASLZSBLFJQEF-RXSVEWSESA-M sodium-L-ascorbate Chemical compound [Na+].OC[C@H](O)[C@H]1OC(=O)C(O)=C1[O-] PPASLZSBLFJQEF-RXSVEWSESA-M 0.000 description 1
- 239000007909 solid dosage form Substances 0.000 description 1
- 239000008247 solid mixture Substances 0.000 description 1
- 229960003787 sorafenib Drugs 0.000 description 1
- IVDHYUQIDRJSTI-UHFFFAOYSA-N sorafenib tosylate Chemical compound [H+].CC1=CC=C(S([O-])(=O)=O)C=C1.C1=NC(C(=O)NC)=CC(OC=2C=CC(NC(=O)NC=3C=C(C(Cl)=CC=3)C(F)(F)F)=CC=2)=C1 IVDHYUQIDRJSTI-UHFFFAOYSA-N 0.000 description 1
- 229960000487 sorafenib tosylate Drugs 0.000 description 1
- 208000018198 spasticity Diseases 0.000 description 1
- 239000007921 spray Substances 0.000 description 1
- 238000001694 spray drying Methods 0.000 description 1
- 208000017572 squamous cell neoplasm Diseases 0.000 description 1
- 239000002294 steroidal antiinflammatory agent Substances 0.000 description 1
- 150000003431 steroids Chemical class 0.000 description 1
- 230000000638 stimulation Effects 0.000 description 1
- 238000005728 strengthening Methods 0.000 description 1
- ZSJLQEPLLKMAKR-GKHCUFPYSA-N streptozocin Chemical compound O=NN(C)C(=O)N[C@H]1[C@@H](O)O[C@H](CO)[C@@H](O)[C@@H]1O ZSJLQEPLLKMAKR-GKHCUFPYSA-N 0.000 description 1
- 229940084642 strontium-89 chloride Drugs 0.000 description 1
- AHBGXTDRMVNFER-FCHARDOESA-L strontium-89(2+);dichloride Chemical compound [Cl-].[Cl-].[89Sr+2] AHBGXTDRMVNFER-FCHARDOESA-L 0.000 description 1
- 239000000758 substrate Substances 0.000 description 1
- 150000005846 sugar alcohols Chemical class 0.000 description 1
- 150000008163 sugars Chemical class 0.000 description 1
- 239000011593 sulfur Substances 0.000 description 1
- 229910052717 sulfur Inorganic materials 0.000 description 1
- 239000000829 suppository Substances 0.000 description 1
- 230000004083 survival effect Effects 0.000 description 1
- 230000002459 sustained effect Effects 0.000 description 1
- 239000003765 sweetening agent Substances 0.000 description 1
- 229940063683 taxotere Drugs 0.000 description 1
- 229940066453 tecentriq Drugs 0.000 description 1
- NRUKOCRGYNPUPR-QBPJDGROSA-N teniposide Chemical compound COC1=C(O)C(OC)=CC([C@@H]2C3=CC=4OCOC=4C=C3[C@@H](O[C@H]3[C@@H]([C@@H](O)[C@@H]4O[C@@H](OC[C@H]4O3)C=3SC=CC=3)O)[C@@H]3[C@@H]2C(OC3)=O)=C1 NRUKOCRGYNPUPR-QBPJDGROSA-N 0.000 description 1
- 229960001278 teniposide Drugs 0.000 description 1
- 229940061354 tequin Drugs 0.000 description 1
- IWVCMVBTMGNXQD-UHFFFAOYSA-N terramycin dehydrate Natural products C1=CC=C2C(O)(C)C3C(O)C4C(N(C)C)C(O)=C(C(N)=O)C(=O)C4(O)C(O)=C3C(=O)C2=C1O IWVCMVBTMGNXQD-UHFFFAOYSA-N 0.000 description 1
- 239000012085 test solution Substances 0.000 description 1
- 201000003120 testicular cancer Diseases 0.000 description 1
- 229960002180 tetracycline Drugs 0.000 description 1
- 229930101283 tetracycline Natural products 0.000 description 1
- 235000019364 tetracycline Nutrition 0.000 description 1
- 229940072172 tetracycline antibiotic Drugs 0.000 description 1
- 150000003522 tetracyclines Chemical class 0.000 description 1
- 229940031351 tetravalent vaccine Drugs 0.000 description 1
- 229940034915 thalomid Drugs 0.000 description 1
- FQXXSQDCDRQNQE-VMDGZTHMSA-N thebaine Chemical compound C([C@@H](N(CC1)C)C2=CC=C3OC)C4=CC=C(OC)C5=C4[C@@]21[C@H]3O5 FQXXSQDCDRQNQE-VMDGZTHMSA-N 0.000 description 1
- 230000004797 therapeutic response Effects 0.000 description 1
- 235000019157 thiamine Nutrition 0.000 description 1
- KYMBYSLLVAOCFI-UHFFFAOYSA-N thiamine Chemical compound CC1=C(CCO)SCN1CC1=CN=C(C)N=C1N KYMBYSLLVAOCFI-UHFFFAOYSA-N 0.000 description 1
- 229960003495 thiamine Drugs 0.000 description 1
- 239000011721 thiamine Substances 0.000 description 1
- 229940035024 thioglycerol Drugs 0.000 description 1
- 150000003573 thiols Chemical class 0.000 description 1
- 201000002510 thyroid cancer Diseases 0.000 description 1
- 210000001685 thyroid gland Anatomy 0.000 description 1
- 229940111100 tice bcg Drugs 0.000 description 1
- 229960001402 tilidine Drugs 0.000 description 1
- 229960001740 tipiracil hydrochloride Drugs 0.000 description 1
- KGHYQYACJRXCAT-UHFFFAOYSA-N tipiracil hydrochloride Chemical compound Cl.N1C(=O)NC(=O)C(Cl)=C1CN1C(=N)CCC1 KGHYQYACJRXCAT-UHFFFAOYSA-N 0.000 description 1
- 238000004448 titration Methods 0.000 description 1
- 229960000984 tocofersolan Drugs 0.000 description 1
- 210000002105 tongue Anatomy 0.000 description 1
- 230000000699 topical effect Effects 0.000 description 1
- XFCLJVABOIYOMF-QPLCGJKRSA-N toremifene Chemical compound C1=CC(OCCN(C)C)=CC=C1C(\C=1C=CC=CC=1)=C(\CCCl)C1=CC=CC=C1 XFCLJVABOIYOMF-QPLCGJKRSA-N 0.000 description 1
- 229960005026 toremifene Drugs 0.000 description 1
- 231100000033 toxigenic Toxicity 0.000 description 1
- 230000001551 toxigenic effect Effects 0.000 description 1
- 238000005809 transesterification reaction Methods 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
- 206010044412 transitional cell carcinoma Diseases 0.000 description 1
- 229960002622 triacetin Drugs 0.000 description 1
- 229960002117 triamcinolone acetonide Drugs 0.000 description 1
- YNDXUCZADRHECN-JNQJZLCISA-N triamcinolone acetonide Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@H]3OC(C)(C)O[C@@]3(C(=O)CO)[C@@]1(C)C[C@@H]2O YNDXUCZADRHECN-JNQJZLCISA-N 0.000 description 1
- 208000022679 triple-negative breast carcinoma Diseases 0.000 description 1
- VXKHXGOKWPXYNA-PGBVPBMZSA-N triptorelin Chemical compound C([C@@H](C(=O)N[C@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1[C@@H](CCC1)C(=O)NCC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@H](CC=1N=CNC=1)NC(=O)[C@H]1NC(=O)CC1)C1=CC=C(O)C=C1 VXKHXGOKWPXYNA-PGBVPBMZSA-N 0.000 description 1
- 229960004824 triptorelin Drugs 0.000 description 1
- 208000029729 tumor suppressor gene on chromosome 11 Diseases 0.000 description 1
- 230000004222 uncontrolled growth Effects 0.000 description 1
- 241001148471 unidentified anaerobic bacterium Species 0.000 description 1
- 210000002438 upper gastrointestinal tract Anatomy 0.000 description 1
- 230000003827 upregulation Effects 0.000 description 1
- AUFUWRKPQLGTGF-FMKGYKFTSA-N uridine triacetate Chemical compound CC(=O)O[C@@H]1[C@H](OC(C)=O)[C@@H](COC(=O)C)O[C@H]1N1C(=O)NC(=O)C=C1 AUFUWRKPQLGTGF-FMKGYKFTSA-N 0.000 description 1
- 229960003498 uridine triacetate Drugs 0.000 description 1
- LFOHPKKMDYSRLY-UHFFFAOYSA-N uridine triacetate Natural products CC(=O)OCC1OC(CN2C=CC(=O)NC2=O)C(OC(=O)C)C1OC(=O)C LFOHPKKMDYSRLY-UHFFFAOYSA-N 0.000 description 1
- 201000005112 urinary bladder cancer Diseases 0.000 description 1
- 230000002485 urinary effect Effects 0.000 description 1
- 210000002229 urogenital system Anatomy 0.000 description 1
- 208000023747 urothelial carcinoma Diseases 0.000 description 1
- 206010046766 uterine cancer Diseases 0.000 description 1
- 229940070710 valerate Drugs 0.000 description 1
- 229940005605 valeric acid Drugs 0.000 description 1
- ATCJTYORYKLVIA-SRXJVYAUSA-N vamp regimen Chemical compound O=C1C=C[C@]2(C)[C@H]3[C@@H](O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1.C=1N=C2N=C(N)N=C(N)C2=NC=1CN(C)C1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1.O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1.C([C@H](C[C@]1(C(=O)OC)C=2C(=CC3=C(C45[C@H]([C@@]([C@H](OC(C)=O)[C@]6(CC)C=CCN([C@H]56)CC4)(O)C(=O)OC)N3C=O)C=2)OC)C[C@@](C2)(O)CC)N2CCC2=C1NC1=CC=CC=C21 ATCJTYORYKLVIA-SRXJVYAUSA-N 0.000 description 1
- 229940097704 vantas Drugs 0.000 description 1
- 208000027185 varicose disease Diseases 0.000 description 1
- 230000004218 vascular function Effects 0.000 description 1
- 235000015112 vegetable and seed oil Nutrition 0.000 description 1
- 239000008158 vegetable oil Substances 0.000 description 1
- 229940099039 velcade Drugs 0.000 description 1
- LQBVNQSMGBZMKD-UHFFFAOYSA-N venetoclax Chemical compound C=1C=C(Cl)C=CC=1C=1CC(C)(C)CCC=1CN(CC1)CCN1C(C=C1OC=2C=C3C=CNC3=NC=2)=CC=C1C(=O)NS(=O)(=O)C(C=C1[N+]([O-])=O)=CC=C1NCC1CCOCC1 LQBVNQSMGBZMKD-UHFFFAOYSA-N 0.000 description 1
- 229960001183 venetoclax Drugs 0.000 description 1
- 229940061389 viadur Drugs 0.000 description 1
- 229940065658 vidaza Drugs 0.000 description 1
- 229960003048 vinblastine Drugs 0.000 description 1
- JXLYSJRDGCGARV-XQKSVPLYSA-N vincaleukoblastine Chemical compound C([C@@H](C[C@]1(C(=O)OC)C=2C(=CC3=C([C@]45[C@H]([C@@]([C@H](OC(C)=O)[C@]6(CC)C=CCN([C@H]56)CC4)(O)C(=O)OC)N3C)C=2)OC)C[C@@](C2)(O)CC)N2CCC2=C1NC1=CC=CC=C21 JXLYSJRDGCGARV-XQKSVPLYSA-N 0.000 description 1
- AQTQHPDCURKLKT-PNYVAJAMSA-N vincristine sulfate Chemical compound OS(O)(=O)=O.C([C@H](C[C@]1(C(=O)OC)C=2C(=CC3=C([C@]45[C@H]([C@@]([C@H](OC(C)=O)[C@]6(CC)C=CCN([C@H]56)CC4)(O)C(=O)OC)N3C=O)C=2)OC)C[C@@](C2)(O)CC)N2CCC2=C1NC1=CC=CC=C21 AQTQHPDCURKLKT-PNYVAJAMSA-N 0.000 description 1
- 229940034332 vincristine sulfate liposome Drugs 0.000 description 1
- 229960002066 vinorelbine Drugs 0.000 description 1
- 229960002166 vinorelbine tartrate Drugs 0.000 description 1
- GBABOYUKABKIAF-IWWDSPBFSA-N vinorelbinetartrate Chemical compound C1N(CC=2C3=CC=CC=C3NC=22)CC(CC)=C[C@H]1C[C@]2(C(=O)OC)C1=CC(C23[C@H]([C@@]([C@H](OC(C)=O)[C@]4(CC)C=CCN([C@H]34)CC2)(O)C(=O)OC)N2C)=C2C=C1OC GBABOYUKABKIAF-IWWDSPBFSA-N 0.000 description 1
- 230000003612 virological effect Effects 0.000 description 1
- 229940088594 vitamin Drugs 0.000 description 1
- 229930003231 vitamin Natural products 0.000 description 1
- 235000013343 vitamin Nutrition 0.000 description 1
- 239000011782 vitamin Substances 0.000 description 1
- 102000009310 vitamin D receptors Human genes 0.000 description 1
- 108050000156 vitamin D receptors Proteins 0.000 description 1
- 229960000237 vorinostat Drugs 0.000 description 1
- 201000005102 vulva cancer Diseases 0.000 description 1
- 230000003442 weekly effect Effects 0.000 description 1
- 239000000080 wetting agent Substances 0.000 description 1
- 239000009637 wintergreen oil Substances 0.000 description 1
- 229940085728 xtandi Drugs 0.000 description 1
- 239000000811 xylitol Substances 0.000 description 1
- 235000010447 xylitol Nutrition 0.000 description 1
- HEBKCHPVOIAQTA-SCDXWVJYSA-N xylitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)CO HEBKCHPVOIAQTA-SCDXWVJYSA-N 0.000 description 1
- 229960002675 xylitol Drugs 0.000 description 1
- 229940055760 yervoy Drugs 0.000 description 1
- 229940045208 yescarta Drugs 0.000 description 1
- 229940004212 yondelis Drugs 0.000 description 1
- 239000005019 zein Substances 0.000 description 1
- 229940093612 zein Drugs 0.000 description 1
- 229910052725 zinc Inorganic materials 0.000 description 1
- 239000011701 zinc Substances 0.000 description 1
- UHVMMEOXYDMDKI-JKYCWFKZSA-L zinc;1-(5-cyanopyridin-2-yl)-3-[(1s,2s)-2-(6-fluoro-2-hydroxy-3-propanoylphenyl)cyclopropyl]urea;diacetate Chemical compound [Zn+2].CC([O-])=O.CC([O-])=O.CCC(=O)C1=CC=C(F)C([C@H]2[C@H](C2)NC(=O)NC=2N=CC(=CC=2)C#N)=C1O UHVMMEOXYDMDKI-JKYCWFKZSA-L 0.000 description 1
- 229940072018 zofran Drugs 0.000 description 1
- 229940033942 zoladex Drugs 0.000 description 1
- 229940061261 zolinza Drugs 0.000 description 1
- 229940002005 zometa Drugs 0.000 description 1
- 239000002076 α-tocopherol Substances 0.000 description 1
- 235000004835 α-tocopherol Nutrition 0.000 description 1
- JLYXXMFPNIAWKQ-UHFFFAOYSA-N γ Benzene hexachloride Chemical compound ClC1C(Cl)C(Cl)C(Cl)C(Cl)C1Cl JLYXXMFPNIAWKQ-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K35/00—Medicinal preparations containing materials or reaction products thereof with undetermined constitution
- A61K35/66—Microorganisms or materials therefrom
- A61K35/74—Bacteria
- A61K35/741—Probiotics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K35/00—Medicinal preparations containing materials or reaction products thereof with undetermined constitution
- A61K35/66—Microorganisms or materials therefrom
- A61K35/74—Bacteria
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/04—Antibacterial agents
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/195—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from bacteria
- C07K14/24—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from bacteria from Enterobacteriaceae (F), e.g. Citrobacter, Serratia, Proteus, Providencia, Morganella, Yersinia
- C07K14/25—Shigella (G)
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N1/00—Microorganisms, e.g. protozoa; Compositions thereof; Processes of propagating, maintaining or preserving microorganisms or compositions thereof; Processes of preparing or isolating a composition containing a microorganism; Culture media therefor
- C12N1/20—Bacteria; Culture media therefor
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N1/00—Microorganisms, e.g. protozoa; Compositions thereof; Processes of propagating, maintaining or preserving microorganisms or compositions thereof; Processes of preparing or isolating a composition containing a microorganism; Culture media therefor
- C12N1/20—Bacteria; Culture media therefor
- C12N1/205—Bacterial isolates
-
- C12R1/01—
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12R—INDEXING SCHEME ASSOCIATED WITH SUBCLASSES C12C - C12Q, RELATING TO MICROORGANISMS
- C12R2001/00—Microorganisms ; Processes using microorganisms
- C12R2001/01—Bacteria or Actinomycetales ; using bacteria or Actinomycetales
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02A—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE
- Y02A50/00—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE in human health protection, e.g. against extreme weather
- Y02A50/30—Against vector-borne diseases, e.g. mosquito-borne, fly-borne, tick-borne or waterborne diseases whose impact is exacerbated by climate change
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Medicinal Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Pharmacology & Pharmacy (AREA)
- Engineering & Computer Science (AREA)
- Microbiology (AREA)
- Genetics & Genomics (AREA)
- Molecular Biology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Mycology (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Biochemistry (AREA)
- Zoology (AREA)
- Biotechnology (AREA)
- Wood Science & Technology (AREA)
- Epidemiology (AREA)
- Oncology (AREA)
- Communicable Diseases (AREA)
- Tropical Medicine & Parasitology (AREA)
- Biomedical Technology (AREA)
- Virology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- General Engineering & Computer Science (AREA)
- Biophysics (AREA)
- Gastroenterology & Hepatology (AREA)
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
- Medicinal Preparation (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201762542033P | 2017-08-07 | 2017-08-07 | |
| US62/542,033 | 2017-08-07 | ||
| PCT/US2018/045592 WO2019032572A1 (en) | 2017-08-07 | 2018-08-07 | COMPOSITIONS AND METHODS FOR DECOLONIZING ANTIBIOTIC-RESISTANT BACTERIA IN INTESTINES |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20200037851A true KR20200037851A (ko) | 2020-04-09 |
Family
ID=65272652
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020207006802A Ceased KR20200037851A (ko) | 2017-08-07 | 2018-08-07 | 장내 항생제 내성 박테리아를 탈콜론화하기 위한 조성물 및 방법 |
Country Status (8)
| Country | Link |
|---|---|
| US (2) | US20200093870A1 (enExample) |
| EP (1) | EP3665181A4 (enExample) |
| JP (1) | JP2020530493A (enExample) |
| KR (1) | KR20200037851A (enExample) |
| CN (1) | CN111328331A (enExample) |
| AU (1) | AU2018313765A1 (enExample) |
| CA (1) | CA3072029A1 (enExample) |
| WO (1) | WO2019032572A1 (enExample) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR20240072307A (ko) * | 2022-11-09 | 2024-05-24 | 주식회사 굿바이옴텍 | 베타-락탐계 항생제 내성 유전자 blaCTX-M-229 및 퀴놀론계 항생제 내성 유전자 qnrS를 갖는 신규한 다제내성 대장균 균주 및 이의 용도 |
Families Citing this family (24)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2011151941A1 (ja) | 2010-06-04 | 2011-12-08 | 国立大学法人東京大学 | 制御性t細胞の増殖または集積を誘導する作用を有する組成物 |
| JP6306507B2 (ja) | 2011-12-01 | 2018-04-18 | 国立大学法人 東京大学 | 制御性t細胞の増殖または集積を誘導するヒト由来細菌 |
| WO2019032573A1 (en) | 2017-08-07 | 2019-02-14 | Finch Therapeutics, Inc. | COMPOSITIONS AND METHODS FOR MAINTAINING AND RESTORING A HEALTHY INTESTINAL BARRIER |
| WO2019079629A1 (en) * | 2017-10-18 | 2019-04-25 | Ascus Biosciences, Inc. | IMPROVING POULTRY PRODUCTION BY ADMINISTERING A SYNTHETIC BIO-ASSEMBLY OF MICROBES OR PURIFIED STEM THEREFOR |
| CN113038957A (zh) | 2018-04-10 | 2021-06-25 | 谢尔塔治疗公司 | 微生物群 |
| WO2020132098A1 (en) * | 2018-12-19 | 2020-06-25 | Healthy Cow Corporation | Ready-to-use probiotic compositions and uses thereof |
| BR112022000875A2 (pt) | 2019-07-19 | 2022-03-29 | Finch Therapeutics Holdings Llc | Métodos e produtos para tratamento de distúrbios gastrointestinais |
| EP4021467A4 (en) | 2019-08-28 | 2023-09-13 | Xbiome Inc. | COMPOSITIONS CONTAINING BACTERIAL SPECIES AND ASSOCIATED METHODS |
| AU2020354581A1 (en) | 2019-09-24 | 2022-03-03 | Prolacta Bioscience, Inc. | Compositions and methods for treatment of inflammatory and immune diseases |
| KR20220108765A (ko) | 2019-10-07 | 2022-08-03 | 시올타 테라퓨틱스, 인크. | 치료용 약학 조성물 |
| GB201915144D0 (en) * | 2019-10-18 | 2019-12-04 | Multigerm Uk Entpr Ltd | Method of promoting SCFA production by gut microbiota |
| KR20220101637A (ko) * | 2019-10-18 | 2022-07-19 | 핀치 테라퓨틱스 홀딩스 엘엘씨 | 박테리아 대사산물을 대상체에 전달하기 위한 조성물 및 방법 |
| EP3838281A1 (en) * | 2019-12-20 | 2021-06-23 | 4D Pharma Research Limited | Compositions comprising bacterial strains |
| WO2021231804A2 (en) * | 2020-05-13 | 2021-11-18 | President And Fellows Of Harvard College | Methods and compositions for suppressing inflammation induced by gut microbes |
| GB202007452D0 (en) * | 2020-05-19 | 2020-07-01 | Microbiotica Ltd | Threrapeutic bacterial composition |
| MX2023001743A (es) | 2020-08-14 | 2023-04-21 | Prolacta Bioscience Inc | Composiciones de oligosacáridos de la leche humana para usarse con bacterioterapias. |
| EP4209577A4 (en) * | 2020-09-01 | 2024-06-26 | Tohoku University | AGENT FOR INCREASING THE CONTENT OF IGA ANTIBODIES IN MILK |
| EP4223868A4 (en) * | 2020-09-30 | 2025-02-12 | National Cancer Center | ENHANCEMENT OF THE ANTITUMOR EFFECT OF AN IMMUNE CHECKPOINT INHIBITOR BY ADMINISTRATION OF AN INTESTINAL RUMINOCOCCACEAE BACTERIUM |
| JP2023550652A (ja) * | 2020-11-25 | 2023-12-04 | セレス セラピューティクス インコーポレイテッド | 移植片対宿主病を処置するための設計された細菌組成物 |
| US20230149483A1 (en) * | 2021-11-12 | 2023-05-18 | Iowa State University Research Foundation, Inc. | Short-chain fatty acid use to mitigate antimicrobial resistance and virulence gene transfer in the gut |
| EP4442264A4 (en) * | 2021-12-02 | 2025-07-16 | Univ Tohoku | THERAPEUTIC AGENT FOR DIARRHEA AND METHOD FOR TREATING BOVINE DIARRHEA |
| CN116747227B (zh) * | 2023-08-12 | 2025-07-25 | 杭州市第一人民医院 | 泊马度胺在制备抗细菌感染药物中的应用 |
| WO2025093780A1 (en) * | 2023-11-05 | 2025-05-08 | Human Biome Institute S.A. | The concept of drugs made with the gut microbiota and its components and the method of their administration to obtain effective therapeutic effects |
| WO2025093778A1 (en) | 2023-11-05 | 2025-05-08 | Human Biome Institute S.A. | Functional compositions and methods of use thereof in eradication and/or decolonization of antibiotic-resistant bacteria and restoration of healthy microbiota |
Family Cites Families (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6727061B2 (en) * | 1997-02-20 | 2004-04-27 | Cabtec, Inc. | Methods for identifying species or Shigella and E. coli using operon sequence analysis |
| JP2003516151A (ja) * | 1999-11-29 | 2003-05-13 | エイブイアイ バイオファーマ, インコーポレイテッド | 細菌16Sおよび23SrRNAに標的化された、荷電していないアンチセンスオリゴヌクレオチド、ならびにその使用 |
| AU2012225305B2 (en) * | 2011-03-09 | 2017-06-08 | Regents Of The University Of Minnesota | Compositions and methods for transplantation of colon microbiota |
| JP6306507B2 (ja) * | 2011-12-01 | 2018-04-18 | 国立大学法人 東京大学 | 制御性t細胞の増殖または集積を誘導するヒト由来細菌 |
| US8921335B2 (en) * | 2012-01-31 | 2014-12-30 | The Regents Of The University Of California | Oral delivery of nucleic acid-based gene interfering agents by Salmonella |
| HK1220326A1 (zh) * | 2013-03-15 | 2017-05-05 | Seres Therapeutics, Inc. | 基於网络微生物组成和方法 |
| PT3003330T (pt) * | 2013-06-05 | 2018-10-10 | Rebiotix Inc | Terapêutica da restauração da microbiota (trm), composições e processos de fabrico |
| MA41020A (fr) * | 2014-11-25 | 2017-10-03 | Evelo Biosciences Inc | Compositions probiotiques et prébiotiques, et leurs procédés d'utilisation pour la modulation du microbiome |
| GB201519088D0 (en) * | 2015-10-28 | 2015-12-09 | Metabogen Ab | The use of bacteria formulations |
| WO2019032573A1 (en) * | 2017-08-07 | 2019-02-14 | Finch Therapeutics, Inc. | COMPOSITIONS AND METHODS FOR MAINTAINING AND RESTORING A HEALTHY INTESTINAL BARRIER |
-
2018
- 2018-08-07 CA CA3072029A patent/CA3072029A1/en active Pending
- 2018-08-07 CN CN201880064759.2A patent/CN111328331A/zh active Pending
- 2018-08-07 JP JP2020530423A patent/JP2020530493A/ja active Pending
- 2018-08-07 KR KR1020207006802A patent/KR20200037851A/ko not_active Ceased
- 2018-08-07 EP EP18843326.2A patent/EP3665181A4/en active Pending
- 2018-08-07 WO PCT/US2018/045592 patent/WO2019032572A1/en not_active Ceased
- 2018-08-07 AU AU2018313765A patent/AU2018313765A1/en not_active Abandoned
-
2019
- 2019-12-09 US US16/707,277 patent/US20200093870A1/en not_active Abandoned
-
2024
- 2024-02-28 US US18/590,022 patent/US20240307460A1/en not_active Abandoned
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR20240072307A (ko) * | 2022-11-09 | 2024-05-24 | 주식회사 굿바이옴텍 | 베타-락탐계 항생제 내성 유전자 blaCTX-M-229 및 퀴놀론계 항생제 내성 유전자 qnrS를 갖는 신규한 다제내성 대장균 균주 및 이의 용도 |
Also Published As
| Publication number | Publication date |
|---|---|
| CA3072029A1 (en) | 2019-02-14 |
| JP2020530493A (ja) | 2020-10-22 |
| CN111328331A (zh) | 2020-06-23 |
| US20200093870A1 (en) | 2020-03-26 |
| WO2019032572A1 (en) | 2019-02-14 |
| AU2018313765A1 (en) | 2020-02-27 |
| US20240307460A1 (en) | 2024-09-19 |
| EP3665181A4 (en) | 2021-06-09 |
| EP3665181A1 (en) | 2020-06-17 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR20200037851A (ko) | 장내 항생제 내성 박테리아를 탈콜론화하기 위한 조성물 및 방법 | |
| AU2021204574B2 (en) | Probiotic and prebiotic compositions, and methods of use thereof for modulation of the microbiome | |
| AU2020202312B2 (en) | Compositions and methods for inhibition of pathogenic bacterial growth | |
| KR20200038506A (ko) | 건강한 장 장벽을 유지 및 회복시키기 위한 조성물 및 방법 | |
| AU2021200287B2 (en) | Synergistic bacterial compositions and methods of production and use thereof | |
| KR102426653B1 (ko) | 상승작용적 박테리아 조성물, 그리고 이의 제조 방법 및 용도 | |
| AU2018338647B2 (en) | Novel platforms for co-stimulation, novel car designs and other enhancements for adoptive cellular therapy | |
| KR102869944B1 (ko) | 조작된 면역자극성 박테리아 균주 및 이의 용도 | |
| AU2014232370B2 (en) | Network-based microbial compositions and methods | |
| AU2017235661B2 (en) | Oligonucleotide probes and uses thereof | |
| AU2021218133A1 (en) | Ammonia-oxidizing nitrosomonas eutropha strain D23 | |
| TW202237826A (zh) | 基因編輯的自然殺手細胞 | |
| AU2014338859B2 (en) | Phage therapy of Pseudomonas infections | |
| AU2024201593A1 (en) | A genetically modified Lactobacillus and uses thereof | |
| AU2019316556B2 (en) | Methods for assessing the risk of developing progressive multifocal leukoencephalopathy caused by john cunningham virus by genetic testing | |
| KR20220004959A (ko) | 종양, 종양-상주 면역 세포, 및 종양 미세환경을 콜로니화하기 위해 조작된 면역자극성 박테리아 | |
| CN111867608A (zh) | 用于痤疮和生物膜的噬菌体治疗 | |
| RU2825990C1 (ru) | Композиции и способы | |
| RU2804664C2 (ru) | Hpv-специфические связывающие молекулы |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20200306 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| A201 | Request for examination | ||
| PA0201 | Request for examination |
Patent event code: PA02012R01D Patent event date: 20210720 Comment text: Request for Examination of Application |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20230703 Patent event code: PE09021S01D |
|
| E601 | Decision to refuse application | ||
| PE0601 | Decision on rejection of patent |
Patent event date: 20230906 Comment text: Decision to Refuse Application Patent event code: PE06012S01D Patent event date: 20230703 Comment text: Notification of reason for refusal Patent event code: PE06011S01I |