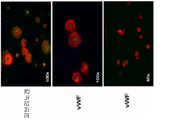
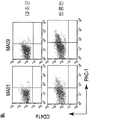
KR20190142424A - 기질 없는 조건 하에서 인간 배아줄기세포로부터 기능적 거핵구 및 혈소판의 대규모 생성 - Google Patents
기질 없는 조건 하에서 인간 배아줄기세포로부터 기능적 거핵구 및 혈소판의 대규모 생성 Download PDFInfo
- Publication number
- KR20190142424A KR20190142424A KR1020197037186A KR20197037186A KR20190142424A KR 20190142424 A KR20190142424 A KR 20190142424A KR 1020197037186 A KR1020197037186 A KR 1020197037186A KR 20197037186 A KR20197037186 A KR 20197037186A KR 20190142424 A KR20190142424 A KR 20190142424A
- Authority
- KR
- South Korea
- Prior art keywords
- cells
- platelets
- medium
- megakaryocytes
- tpo
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/06—Animal cells or tissues; Human cells or tissues
- C12N5/0602—Vertebrate cells
- C12N5/0634—Cells from the blood or the immune system
- C12N5/0644—Platelets; Megakaryocytes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P7/00—Drugs for disorders of the blood or the extracellular fluid
- A61P7/04—Antihaemorrhagics; Procoagulants; Haemostatic agents; Antifibrinolytic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P7/00—Drugs for disorders of the blood or the extracellular fluid
- A61P7/08—Plasma substitutes; Perfusion solutions; Dialytics or haemodialytics; Drugs for electrolytic or acid-base disorders, e.g. hypovolemic shock
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2500/00—Specific components of cell culture medium
- C12N2500/90—Serum-free medium, which may still contain naturally-sourced components
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2501/00—Active agents used in cell culture processes, e.g. differentation
- C12N2501/10—Growth factors
- C12N2501/115—Basic fibroblast growth factor (bFGF, FGF-2)
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2501/00—Active agents used in cell culture processes, e.g. differentation
- C12N2501/10—Growth factors
- C12N2501/125—Stem cell factor [SCF], c-kit ligand [KL]
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2501/00—Active agents used in cell culture processes, e.g. differentation
- C12N2501/10—Growth factors
- C12N2501/145—Thrombopoietin [TPO]
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2501/00—Active agents used in cell culture processes, e.g. differentation
- C12N2501/10—Growth factors
- C12N2501/155—Bone morphogenic proteins [BMP]; Osteogenins; Osteogenic factor; Bone inducing factor
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2501/00—Active agents used in cell culture processes, e.g. differentation
- C12N2501/10—Growth factors
- C12N2501/165—Vascular endothelial growth factor [VEGF]
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2501/00—Active agents used in cell culture processes, e.g. differentation
- C12N2501/20—Cytokines; Chemokines
- C12N2501/23—Interleukins [IL]
- C12N2501/2311—Interleukin-11 (IL-11)
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2501/00—Active agents used in cell culture processes, e.g. differentation
- C12N2501/20—Cytokines; Chemokines
- C12N2501/26—Flt-3 ligand (CD135L, flk-2 ligand)
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2501/00—Active agents used in cell culture processes, e.g. differentation
- C12N2501/90—Polysaccharides
- C12N2501/91—Heparin
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2502/00—Coculture with; Conditioned medium produced by
- C12N2502/13—Coculture with; Conditioned medium produced by connective tissue cells; generic mesenchyme cells, e.g. so-called "embryonic fibroblasts"
- C12N2502/1394—Bone marrow stromal cells; whole marrow
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2506/00—Differentiation of animal cells from one lineage to another; Differentiation of pluripotent cells
- C12N2506/02—Differentiation of animal cells from one lineage to another; Differentiation of pluripotent cells from embryonic cells
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2506/00—Differentiation of animal cells from one lineage to another; Differentiation of pluripotent cells
- C12N2506/45—Differentiation of animal cells from one lineage to another; Differentiation of pluripotent cells from artificially induced pluripotent stem cells
Landscapes
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Life Sciences & Earth Sciences (AREA)
- Biomedical Technology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Hematology (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Zoology (AREA)
- Wood Science & Technology (AREA)
- Genetics & Genomics (AREA)
- Biotechnology (AREA)
- General Health & Medical Sciences (AREA)
- Biochemistry (AREA)
- General Engineering & Computer Science (AREA)
- Microbiology (AREA)
- Cell Biology (AREA)
- Immunology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Medicinal Chemistry (AREA)
- Veterinary Medicine (AREA)
- Pharmacology & Pharmacy (AREA)
- Public Health (AREA)
- General Chemical & Material Sciences (AREA)
- Diabetes (AREA)
- Animal Behavior & Ethology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
- Measuring Or Testing Involving Enzymes Or Micro-Organisms (AREA)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR1020217016080A KR20210066020A (ko) | 2009-12-04 | 2010-12-03 | 기질 없는 조건 하에서 인간 배아줄기세포로부터 기능적 거핵구 및 혈소판의 대규모 생성 |
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US26693909P | 2009-12-04 | 2009-12-04 | |
| US61/266,939 | 2009-12-04 | ||
| US38416510P | 2010-09-17 | 2010-09-17 | |
| US61/384,165 | 2010-09-17 | ||
| PCT/US2010/058990 WO2011069127A1 (en) | 2009-12-04 | 2010-12-03 | Large scale generation of functional megakaryocytes and platelets from human embryonic stem cells under stromal-free conditions |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020187021160A Division KR20180086301A (ko) | 2009-12-04 | 2010-12-03 | 기질 없는 조건 하에서 인간 배아줄기세포로부터 기능적 거핵구 및 혈소판의 대규모 생성 |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020217016080A Division KR20210066020A (ko) | 2009-12-04 | 2010-12-03 | 기질 없는 조건 하에서 인간 배아줄기세포로부터 기능적 거핵구 및 혈소판의 대규모 생성 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20190142424A true KR20190142424A (ko) | 2019-12-26 |
Family
ID=44115332
Family Applications (4)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020197037186A Ceased KR20190142424A (ko) | 2009-12-04 | 2010-12-03 | 기질 없는 조건 하에서 인간 배아줄기세포로부터 기능적 거핵구 및 혈소판의 대규모 생성 |
| KR1020127017368A Ceased KR20120128605A (ko) | 2009-12-04 | 2010-12-03 | 기질 없는 조건 하에서 인간 배아줄기세포로부터 기능적 거핵구 및 혈소판의 대규모 생성 |
| KR1020217016080A Ceased KR20210066020A (ko) | 2009-12-04 | 2010-12-03 | 기질 없는 조건 하에서 인간 배아줄기세포로부터 기능적 거핵구 및 혈소판의 대규모 생성 |
| KR1020187021160A Ceased KR20180086301A (ko) | 2009-12-04 | 2010-12-03 | 기질 없는 조건 하에서 인간 배아줄기세포로부터 기능적 거핵구 및 혈소판의 대규모 생성 |
Family Applications After (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020127017368A Ceased KR20120128605A (ko) | 2009-12-04 | 2010-12-03 | 기질 없는 조건 하에서 인간 배아줄기세포로부터 기능적 거핵구 및 혈소판의 대규모 생성 |
| KR1020217016080A Ceased KR20210066020A (ko) | 2009-12-04 | 2010-12-03 | 기질 없는 조건 하에서 인간 배아줄기세포로부터 기능적 거핵구 및 혈소판의 대규모 생성 |
| KR1020187021160A Ceased KR20180086301A (ko) | 2009-12-04 | 2010-12-03 | 기질 없는 조건 하에서 인간 배아줄기세포로부터 기능적 거핵구 및 혈소판의 대규모 생성 |
Country Status (10)
| Country | Link |
|---|---|
| US (2) | US9988603B2 (enExample) |
| EP (1) | EP2507359A4 (enExample) |
| JP (4) | JP2013512676A (enExample) |
| KR (4) | KR20190142424A (enExample) |
| AU (4) | AU2010325811A1 (enExample) |
| BR (1) | BR112012013497A2 (enExample) |
| CA (2) | CA2782013C (enExample) |
| IN (1) | IN2012DN05054A (enExample) |
| RU (1) | RU2012127810A (enExample) |
| WO (1) | WO2011069127A1 (enExample) |
Families Citing this family (16)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP3712258B1 (en) | 2006-04-14 | 2025-01-01 | Astellas Institute for Regenerative Medicine | Hemangio-colony forming cells |
| KR20190142424A (ko) | 2009-12-04 | 2019-12-26 | 스템 셀 앤드 리제너러티브 메디신 인터내셔날, 인크. | 기질 없는 조건 하에서 인간 배아줄기세포로부터 기능적 거핵구 및 혈소판의 대규모 생성 |
| KR102108245B1 (ko) | 2011-11-30 | 2020-05-08 | 아스텔라스 인스티튜트 포 리제너러티브 메디슨 | 중간엽 간질 세포 및 이에 관련된 용도 |
| US8961956B2 (en) | 2011-11-30 | 2015-02-24 | Ocata Therapeutics, Inc. | Mesenchymal stromal cells and uses related thereto |
| GB201210857D0 (en) | 2012-06-19 | 2012-08-01 | Cambridge Entpr Ltd | Transcription factor mediated programming towards megakaryocytes |
| CN104471059B (zh) | 2012-07-12 | 2018-04-17 | 珠海横琴爱姆斯坦生物科技有限公司 | 人胚胎干细胞衍生的间充质样干细胞、方法及其应用 |
| US9074186B2 (en) | 2012-08-15 | 2015-07-07 | Boston Medical Center Corporation | Production of red blood cells and platelets from stem cells |
| EP3973967A1 (en) * | 2012-12-21 | 2022-03-30 | Astellas Institute for Regenerative Medicine | Methods for production of platelets from pluripotent stem cells and compositions thereof |
| JP6580329B2 (ja) | 2014-03-31 | 2019-09-25 | シスメックス株式会社 | 未分化細胞から分化細胞および/または分化細胞の産生物を取得する方法 |
| WO2015179301A1 (en) | 2014-05-19 | 2015-11-26 | Eleftherios Papoutsakis | Megakaryocytic particles and microparticles for cell therapy & fate modification of stem and progenitor cells |
| AU2016242823A1 (en) * | 2015-03-30 | 2017-09-21 | Jeffrey Thomas Loh | Methods for producing modified red blood cell compositions, compositions and uses thereof |
| AU2016308818B2 (en) | 2015-08-18 | 2022-04-21 | Astellas Institute For Regenerative Medicine | Clinical formulations |
| CN108135940B (zh) * | 2015-10-14 | 2022-04-15 | 株式会社美加细胞 | 纯化血小板的制造方法 |
| MX2020007071A (es) | 2018-01-05 | 2020-11-11 | Platelet Biogenesis Inc | Composiciones y metodos para producir megacariocitos. |
| WO2020006539A1 (en) | 2018-06-29 | 2020-01-02 | Platelet Biogenesis, Inc. | Compositions for drug delivery and methods of use thereof |
| CA3178268A1 (en) | 2020-05-11 | 2021-11-18 | Jonathan Thon | Compositions and methods related to megakaryocyte-derived extracellular vesicles |
Family Cites Families (18)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5128259A (en) | 1989-10-27 | 1992-07-07 | Hahnemann University | Factor-dependent hematopoietic cell line exhibiting epo-induced erythrocyte maturation |
| EP1364197B1 (en) * | 2001-01-29 | 2010-04-14 | Ivan N. Rich | ATP-based assay for the determination of the proliferation status of hematopoietic stem and progenitor cells |
| WO2002078449A2 (en) | 2001-04-02 | 2002-10-10 | Advanced Cell Technology, Inc. | Method for facilitating the production of differentiated cell types and tissues from embryonic and adult pluripotent and multipotent cells |
| WO2003046141A2 (en) | 2001-11-26 | 2003-06-05 | Advanced Cell Technology, Inc. | Methods for making and using reprogrammed human somatic cell nuclei and autologous and isogenic human stem cells |
| US20040052771A1 (en) | 2002-07-12 | 2004-03-18 | Lim Sai Kiang | Hemangioblast progenitor cells |
| US20040229350A1 (en) | 2003-05-12 | 2004-11-18 | Nikolai Strelchenko | Morula derived embryonic stem cells |
| CN1286970C (zh) | 2003-12-30 | 2006-11-29 | 上海伯瑞生物技术发展有限公司 | 由脐血cd34+细胞体外诱导生成巨核细胞的方法 |
| EP1735429A4 (en) | 2004-03-31 | 2008-06-11 | Newlink Genetics Corp | METHOD AND COMPOSITIONS FOR OBTAINING EMBRYONAL STEM CELLS OF ABSTINENT HEMATOPOETIC STEM CELLS AND USES THEREOF |
| WO2006050330A2 (en) * | 2004-11-01 | 2006-05-11 | Wisconsin Alumni Research Foundation | Platelets from stem cells |
| US7893315B2 (en) | 2004-11-04 | 2011-02-22 | Advanced Cell Technology, Inc. | Derivation of embryonic stem cells and embryo-derived cells |
| WO2007095064A2 (en) | 2006-02-09 | 2007-08-23 | Wisconsin Alumni Research Foundation | ERYTHROID CELLS PRODUCING ADULT-TYPE β-HEMOGLOBIN GENERATED FROM HUMAN EMBRYONIC STEM CELLS |
| CN101045914A (zh) | 2006-03-29 | 2007-10-03 | 中国人民解放军军事医学科学院野战输血研究所 | 体外诱导造血干/祖细胞分化为成熟红细胞的方法与应用 |
| EP3712258B1 (en) * | 2006-04-14 | 2025-01-01 | Astellas Institute for Regenerative Medicine | Hemangio-colony forming cells |
| JP2008307007A (ja) | 2007-06-15 | 2008-12-25 | Bayer Schering Pharma Ag | 出生後のヒト組織由来未分化幹細胞から誘導したヒト多能性幹細胞 |
| US9683232B2 (en) * | 2007-12-10 | 2017-06-20 | Kyoto University | Efficient method for nuclear reprogramming |
| EP3441462A1 (en) | 2008-05-06 | 2019-02-13 | Astellas Institute for Regenerative Medicine | Hemangio colony forming cells and non-engrafting hemangio cells |
| CN112280739A (zh) | 2008-05-06 | 2021-01-29 | 安斯泰来再生医药协会 | 用于制备衍生自多能干细胞的去核类红细胞的方法 |
| KR20190142424A (ko) | 2009-12-04 | 2019-12-26 | 스템 셀 앤드 리제너러티브 메디신 인터내셔날, 인크. | 기질 없는 조건 하에서 인간 배아줄기세포로부터 기능적 거핵구 및 혈소판의 대규모 생성 |
-
2010
- 2010-12-03 KR KR1020197037186A patent/KR20190142424A/ko not_active Ceased
- 2010-12-03 IN IN5054DEN2012 patent/IN2012DN05054A/en unknown
- 2010-12-03 JP JP2012542230A patent/JP2013512676A/ja active Pending
- 2010-12-03 CA CA2782013A patent/CA2782013C/en active Active
- 2010-12-03 KR KR1020127017368A patent/KR20120128605A/ko not_active Ceased
- 2010-12-03 AU AU2010325811A patent/AU2010325811A1/en not_active Abandoned
- 2010-12-03 WO PCT/US2010/058990 patent/WO2011069127A1/en not_active Ceased
- 2010-12-03 BR BR112012013497A patent/BR112012013497A2/pt not_active Application Discontinuation
- 2010-12-03 KR KR1020217016080A patent/KR20210066020A/ko not_active Ceased
- 2010-12-03 EP EP10835231.1A patent/EP2507359A4/en not_active Withdrawn
- 2010-12-03 RU RU2012127810/10A patent/RU2012127810A/ru not_active Application Discontinuation
- 2010-12-03 CA CA3115233A patent/CA3115233A1/en not_active Abandoned
- 2010-12-03 US US13/512,827 patent/US9988603B2/en active Active
- 2010-12-03 KR KR1020187021160A patent/KR20180086301A/ko not_active Ceased
-
2016
- 2016-04-25 JP JP2016086761A patent/JP2016135138A/ja active Pending
-
2017
- 2017-03-24 AU AU2017201995A patent/AU2017201995B2/en not_active Ceased
-
2018
- 2018-05-01 US US15/968,591 patent/US20190002829A1/en not_active Abandoned
-
2019
- 2019-03-05 JP JP2019039160A patent/JP2019122385A/ja active Pending
- 2019-05-24 AU AU2019203670A patent/AU2019203670B2/en not_active Ceased
-
2022
- 2022-01-13 AU AU2022200188A patent/AU2022200188A1/en not_active Abandoned
- 2022-01-28 JP JP2022011675A patent/JP2022064953A/ja active Pending
Also Published As
| Publication number | Publication date |
|---|---|
| US9988603B2 (en) | 2018-06-05 |
| EP2507359A1 (en) | 2012-10-10 |
| JP2016135138A (ja) | 2016-07-28 |
| AU2017201995B2 (en) | 2019-06-06 |
| CA2782013A1 (en) | 2011-06-09 |
| US20120315338A1 (en) | 2012-12-13 |
| KR20180086301A (ko) | 2018-07-30 |
| KR20210066020A (ko) | 2021-06-04 |
| US20190002829A1 (en) | 2019-01-03 |
| RU2012127810A (ru) | 2014-01-10 |
| AU2022200188A1 (en) | 2022-02-10 |
| IN2012DN05054A (enExample) | 2015-10-09 |
| WO2011069127A1 (en) | 2011-06-09 |
| KR20120128605A (ko) | 2012-11-27 |
| AU2019203670B2 (en) | 2021-10-14 |
| JP2022064953A (ja) | 2022-04-26 |
| EP2507359A4 (en) | 2015-05-13 |
| CA3115233A1 (en) | 2011-06-09 |
| AU2010325811A1 (en) | 2012-06-21 |
| JP2013512676A (ja) | 2013-04-18 |
| AU2019203670A1 (en) | 2019-06-13 |
| BR112012013497A2 (pt) | 2016-05-31 |
| AU2017201995A1 (en) | 2017-04-13 |
| CA2782013C (en) | 2021-06-08 |
| JP2019122385A (ja) | 2019-07-25 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20250099505A1 (en) | Methods for production of platelets from pluripotent stem cells and compositions thereof | |
| AU2019203670B2 (en) | Large scale generation of functional megakaryocytes and platelets from human embryonic stem cells under stromal-free conditions | |
| US20200263132A1 (en) | Methods for producing enucleated erythroid cells derived from pluripotent stem cells | |
| US20200157503A1 (en) | Hemangio colony forming cells and non-engrafting hemangio cells | |
| US20110151554A1 (en) | Method for culturing and subculturing primate embryonic stem cell, as well as method for inducing differentiation thereof | |
| US20120295347A1 (en) | Methods and Compositions for Producing Endothelial Progenitor Cells from Pluripotent Stem Cells | |
| WO2005097979A2 (en) | Methods and compositions for obtaining hematopoietic stem cells derived from embryonic stem cells and uses thereof | |
| Marshall | The origin and regulation of haematopoietic stem cells during mammalian embryogenesis | |
| WO2012103098A2 (en) | Compositions and methods for treating hematological cytopenias |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A107 | Divisional application of patent | ||
| A201 | Request for examination | ||
| PA0104 | Divisional application for international application |
Comment text: Divisional Application for International Patent Patent event code: PA01041R01D Patent event date: 20191216 Application number text: 1020187021160 Filing date: 20180723 |
|
| PA0201 | Request for examination | ||
| PG1501 | Laying open of application | ||
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20200131 Patent event code: PE09021S01D |
|
| AMND | Amendment | ||
| N231 | Notification of change of applicant | ||
| PN2301 | Change of applicant |
Patent event date: 20200810 Comment text: Notification of Change of Applicant Patent event code: PN23011R01D |
|
| E601 | Decision to refuse application | ||
| PE0601 | Decision on rejection of patent |
Patent event date: 20201030 Comment text: Decision to Refuse Application Patent event code: PE06012S01D Patent event date: 20200131 Comment text: Notification of reason for refusal Patent event code: PE06011S01I |
|
| AMND | Amendment | ||
| PX0901 | Re-examination |
Patent event code: PX09011S01I Patent event date: 20201030 Comment text: Decision to Refuse Application Patent event code: PX09012R01I Patent event date: 20200331 Comment text: Amendment to Specification, etc. |
|
| PX0601 | Decision of rejection after re-examination |
Comment text: Decision to Refuse Application Patent event code: PX06014S01D Patent event date: 20210224 Comment text: Amendment to Specification, etc. Patent event code: PX06012R01I Patent event date: 20210129 Comment text: Decision to Refuse Application Patent event code: PX06011S01I Patent event date: 20201030 Comment text: Amendment to Specification, etc. Patent event code: PX06012R01I Patent event date: 20200331 Comment text: Notification of reason for refusal Patent event code: PX06013S01I Patent event date: 20200131 |
|
| X601 | Decision of rejection after re-examination | ||
| PA0104 | Divisional application for international application |
Comment text: Divisional Application for International Patent Patent event code: PA01041R01D Patent event date: 20210526 Application number text: 1020187021160 Filing date: 20180723 |