KR20180098672A - 자가-가교결합 항체 - Google Patents
자가-가교결합 항체 Download PDFInfo
- Publication number
- KR20180098672A KR20180098672A KR1020187022826A KR20187022826A KR20180098672A KR 20180098672 A KR20180098672 A KR 20180098672A KR 1020187022826 A KR1020187022826 A KR 1020187022826A KR 20187022826 A KR20187022826 A KR 20187022826A KR 20180098672 A KR20180098672 A KR 20180098672A
- Authority
- KR
- South Korea
- Prior art keywords
- mediator
- antibody
- antigen
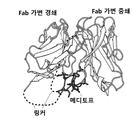
- mediotope
- binding fragment
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
- 238000004132 cross linking Methods 0.000 title claims abstract description 158
- 230000027455 binding Effects 0.000 claims abstract description 430
- 238000000034 method Methods 0.000 claims abstract description 153
- 239000000203 mixture Substances 0.000 claims abstract description 37
- 238000011282 treatment Methods 0.000 claims abstract description 8
- 239000000427 antigen Substances 0.000 claims description 300
- 108091007433 antigens Proteins 0.000 claims description 300
- 102000036639 antigens Human genes 0.000 claims description 300
- 239000012634 fragment Substances 0.000 claims description 282
- 210000004027 cell Anatomy 0.000 claims description 165
- 108090000765 processed proteins & peptides Proteins 0.000 claims description 114
- 150000007523 nucleic acids Chemical class 0.000 claims description 70
- COLNVLDHVKWLRT-QMMMGPOBSA-N L-phenylalanine Chemical compound OC(=O)[C@@H](N)CC1=CC=CC=C1 COLNVLDHVKWLRT-QMMMGPOBSA-N 0.000 claims description 69
- 108020004707 nucleic acids Proteins 0.000 claims description 61
- 102000039446 nucleic acids Human genes 0.000 claims description 61
- -1 Tac Proteins 0.000 claims description 60
- 239000013598 vector Substances 0.000 claims description 55
- 108010069514 Cyclic Peptides Proteins 0.000 claims description 54
- 102000001189 Cyclic Peptides Human genes 0.000 claims description 54
- 150000001413 amino acids Chemical group 0.000 claims description 53
- 230000014509 gene expression Effects 0.000 claims description 48
- 101710160107 Outer membrane protein A Proteins 0.000 claims description 47
- 125000002915 carbonyl group Chemical group [*:2]C([*:1])=O 0.000 claims description 47
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 claims description 41
- KGBXLFKZBHKPEV-UHFFFAOYSA-N boric acid Chemical compound OB(O)O KGBXLFKZBHKPEV-UHFFFAOYSA-N 0.000 claims description 40
- 239000004327 boric acid Substances 0.000 claims description 40
- OUYCCCASQSFEME-QMMMGPOBSA-N L-tyrosine Chemical compound OC(=O)[C@@H](N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-QMMMGPOBSA-N 0.000 claims description 39
- 239000002773 nucleotide Substances 0.000 claims description 37
- 125000003729 nucleotide group Chemical group 0.000 claims description 37
- 239000003814 drug Substances 0.000 claims description 36
- 102000005962 receptors Human genes 0.000 claims description 34
- 108020003175 receptors Proteins 0.000 claims description 33
- CKLJMWTZIZZHCS-REOHCLBHSA-N L-aspartic acid Chemical compound OC(=O)[C@@H](N)CC(O)=O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 claims description 29
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims description 27
- OUYCCCASQSFEME-UHFFFAOYSA-N tyrosine Natural products OC(=O)C(N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-UHFFFAOYSA-N 0.000 claims description 27
- QIVBCDIJIAJPQS-UHFFFAOYSA-N Tryptophan Natural products C1=CC=C2C(CC(N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-UHFFFAOYSA-N 0.000 claims description 26
- 201000010099 disease Diseases 0.000 claims description 26
- QIVBCDIJIAJPQS-VIFPVBQESA-N L-tryptophane Chemical compound C1=CC=C2C(C[C@H](N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-VIFPVBQESA-N 0.000 claims description 25
- 239000000126 substance Substances 0.000 claims description 25
- 230000001225 therapeutic effect Effects 0.000 claims description 25
- COLNVLDHVKWLRT-UHFFFAOYSA-N phenylalanine Natural products OC(=O)C(N)CC1=CC=CC=C1 COLNVLDHVKWLRT-UHFFFAOYSA-N 0.000 claims description 24
- 229960005190 phenylalanine Drugs 0.000 claims description 24
- 101000934338 Homo sapiens Myeloid cell surface antigen CD33 Proteins 0.000 claims description 23
- 102100025243 Myeloid cell surface antigen CD33 Human genes 0.000 claims description 23
- 230000001965 increasing effect Effects 0.000 claims description 23
- 238000004519 manufacturing process Methods 0.000 claims description 22
- 102000052116 epidermal growth factor receptor activity proteins Human genes 0.000 claims description 21
- 108700015053 epidermal growth factor receptor activity proteins Proteins 0.000 claims description 21
- 229960000578 gemtuzumab Drugs 0.000 claims description 21
- YOHYSYJDKVYCJI-UHFFFAOYSA-N n-[3-[[6-[3-(trifluoromethyl)anilino]pyrimidin-4-yl]amino]phenyl]cyclopropanecarboxamide Chemical compound FC(F)(F)C1=CC=CC(NC=2N=CN=C(NC=3C=C(NC(=O)C4CC4)C=CC=3)C=2)=C1 YOHYSYJDKVYCJI-UHFFFAOYSA-N 0.000 claims description 21
- 229960000575 trastuzumab Drugs 0.000 claims description 21
- 210000004899 c-terminal region Anatomy 0.000 claims description 20
- 229940124597 therapeutic agent Drugs 0.000 claims description 19
- 235000004279 alanine Nutrition 0.000 claims description 18
- 206010028980 Neoplasm Diseases 0.000 claims description 17
- 230000001939 inductive effect Effects 0.000 claims description 16
- 102000004190 Enzymes Human genes 0.000 claims description 15
- 108090000790 Enzymes Proteins 0.000 claims description 15
- QNAYBMKLOCPYGJ-REOHCLBHSA-N L-alanine Chemical compound C[C@H](N)C(O)=O QNAYBMKLOCPYGJ-REOHCLBHSA-N 0.000 claims description 15
- 201000011510 cancer Diseases 0.000 claims description 15
- 239000002253 acid Substances 0.000 claims description 14
- 235000003704 aspartic acid Nutrition 0.000 claims description 14
- OQFSQFPPLPISGP-UHFFFAOYSA-N beta-carboxyaspartic acid Natural products OC(=O)C(N)C(C(O)=O)C(O)=O OQFSQFPPLPISGP-UHFFFAOYSA-N 0.000 claims description 14
- JFVLNTLXEZDFHW-QMMMGPOBSA-N (2s)-2-amino-3-(2-bromophenyl)propanoic acid Chemical compound OC(=O)[C@@H](N)CC1=CC=CC=C1Br JFVLNTLXEZDFHW-QMMMGPOBSA-N 0.000 claims description 13
- GDMOHOYNMWWBAU-QMMMGPOBSA-N (2s)-2-amino-3-(3-bromophenyl)propanoic acid Chemical compound OC(=O)[C@@H](N)CC1=CC=CC(Br)=C1 GDMOHOYNMWWBAU-QMMMGPOBSA-N 0.000 claims description 13
- 102100024222 B-lymphocyte antigen CD19 Human genes 0.000 claims description 13
- 101000980825 Homo sapiens B-lymphocyte antigen CD19 Proteins 0.000 claims description 13
- 229910052751 metal Inorganic materials 0.000 claims description 13
- 239000002184 metal Substances 0.000 claims description 13
- 230000001603 reducing effect Effects 0.000 claims description 13
- XDOLZJYETYVRKV-UHFFFAOYSA-N 7-Aminoheptanoic acid Chemical compound NCCCCCCC(O)=O XDOLZJYETYVRKV-UHFFFAOYSA-N 0.000 claims description 12
- 239000012216 imaging agent Substances 0.000 claims description 12
- 210000001519 tissue Anatomy 0.000 claims description 12
- BXRLWGXPSRYJDZ-UHFFFAOYSA-N 3-cyanoalanine Chemical compound OC(=O)C(N)CC#N BXRLWGXPSRYJDZ-UHFFFAOYSA-N 0.000 claims description 11
- DGYHPLMPMRKMPD-UHFFFAOYSA-N L-propargyl glycine Natural products OC(=O)C(N)CC#C DGYHPLMPMRKMPD-UHFFFAOYSA-N 0.000 claims description 11
- 241000829100 Macaca mulatta polyomavirus 1 Species 0.000 claims description 11
- 229940127089 cytotoxic agent Drugs 0.000 claims description 11
- 239000000032 diagnostic agent Substances 0.000 claims description 11
- 229940039227 diagnostic agent Drugs 0.000 claims description 11
- 101001012157 Homo sapiens Receptor tyrosine-protein kinase erbB-2 Proteins 0.000 claims description 10
- 102100030086 Receptor tyrosine-protein kinase erbB-2 Human genes 0.000 claims description 10
- 241000725643 Respiratory syncytial virus Species 0.000 claims description 10
- 229940079593 drug Drugs 0.000 claims description 10
- 230000000694 effects Effects 0.000 claims description 10
- 108020004684 Internal Ribosome Entry Sites Proteins 0.000 claims description 9
- 239000002246 antineoplastic agent Substances 0.000 claims description 9
- 239000002738 chelating agent Substances 0.000 claims description 9
- 229960004641 rituximab Drugs 0.000 claims description 9
- 238000009175 antibody therapy Methods 0.000 claims description 8
- 239000004098 Tetracycline Substances 0.000 claims description 7
- 238000012258 culturing Methods 0.000 claims description 7
- 238000010494 dissociation reaction Methods 0.000 claims description 7
- 230000005593 dissociations Effects 0.000 claims description 7
- 210000003527 eukaryotic cell Anatomy 0.000 claims description 7
- 230000002401 inhibitory effect Effects 0.000 claims description 7
- 239000008194 pharmaceutical composition Substances 0.000 claims description 7
- 230000002285 radioactive effect Effects 0.000 claims description 7
- 229960002180 tetracycline Drugs 0.000 claims description 7
- 239000003053 toxin Substances 0.000 claims description 7
- 231100000765 toxin Toxicity 0.000 claims description 7
- 108700012359 toxins Proteins 0.000 claims description 7
- 102000003886 Glycoproteins Human genes 0.000 claims description 6
- 108090000288 Glycoproteins Proteins 0.000 claims description 6
- 239000000611 antibody drug conjugate Substances 0.000 claims description 6
- 229940049595 antibody-drug conjugate Drugs 0.000 claims description 6
- 229960000397 bevacizumab Drugs 0.000 claims description 6
- 230000001268 conjugating effect Effects 0.000 claims description 6
- 238000009826 distribution Methods 0.000 claims description 6
- 210000004962 mammalian cell Anatomy 0.000 claims description 6
- 229930101283 tetracycline Natural products 0.000 claims description 6
- 235000019364 tetracycline Nutrition 0.000 claims description 6
- 150000003522 tetracyclines Chemical class 0.000 claims description 6
- 102000006992 Interferon-alpha Human genes 0.000 claims description 5
- 108010047761 Interferon-alpha Proteins 0.000 claims description 5
- 230000000890 antigenic effect Effects 0.000 claims description 5
- 230000001413 cellular effect Effects 0.000 claims description 5
- 230000007423 decrease Effects 0.000 claims description 5
- 108700041286 delta Proteins 0.000 claims description 5
- 108020001507 fusion proteins Proteins 0.000 claims description 5
- 102000037865 fusion proteins Human genes 0.000 claims description 5
- SGKRLCUYIXIAHR-AKNGSSGZSA-N (4s,4ar,5s,5ar,6r,12ar)-4-(dimethylamino)-1,5,10,11,12a-pentahydroxy-6-methyl-3,12-dioxo-4a,5,5a,6-tetrahydro-4h-tetracene-2-carboxamide Chemical compound C1=CC=C2[C@H](C)[C@@H]([C@H](O)[C@@H]3[C@](C(O)=C(C(N)=O)C(=O)[C@H]3N(C)C)(O)C3=O)C3=C(O)C2=C1O SGKRLCUYIXIAHR-AKNGSSGZSA-N 0.000 claims description 4
- 102100022005 B-lymphocyte antigen CD20 Human genes 0.000 claims description 4
- 108010001857 Cell Surface Receptors Proteins 0.000 claims description 4
- 108010066687 Epithelial Cell Adhesion Molecule Proteins 0.000 claims description 4
- 102000018651 Epithelial Cell Adhesion Molecule Human genes 0.000 claims description 4
- 101000897405 Homo sapiens B-lymphocyte antigen CD20 Proteins 0.000 claims description 4
- 108010002350 Interleukin-2 Proteins 0.000 claims description 4
- 102000000588 Interleukin-2 Human genes 0.000 claims description 4
- 102000010789 Interleukin-2 Receptors Human genes 0.000 claims description 4
- 108010038453 Interleukin-2 Receptors Proteins 0.000 claims description 4
- QPCDCPDFJACHGM-UHFFFAOYSA-N N,N-bis{2-[bis(carboxymethyl)amino]ethyl}glycine Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(=O)O)CCN(CC(O)=O)CC(O)=O QPCDCPDFJACHGM-UHFFFAOYSA-N 0.000 claims description 4
- 102000009524 Vascular Endothelial Growth Factor A Human genes 0.000 claims description 4
- 229960000446 abciximab Drugs 0.000 claims description 4
- 229960000548 alemtuzumab Drugs 0.000 claims description 4
- 150000001639 boron compounds Chemical class 0.000 claims description 4
- 229950001178 capromab Drugs 0.000 claims description 4
- 229950002595 clivatuzumab tetraxetan Drugs 0.000 claims description 4
- 229960003722 doxycycline Drugs 0.000 claims description 4
- 229960000598 infliximab Drugs 0.000 claims description 4
- 230000010837 receptor-mediated endocytosis Effects 0.000 claims description 4
- 101150047061 tag-72 gene Proteins 0.000 claims description 4
- 229960003989 tocilizumab Drugs 0.000 claims description 4
- 229960005267 tositumomab Drugs 0.000 claims description 4
- MZOFCQQQCNRIBI-VMXHOPILSA-N (3s)-4-[[(2s)-1-[[(2s)-1-[[(1s)-1-carboxy-2-hydroxyethyl]amino]-4-methyl-1-oxopentan-2-yl]amino]-5-(diaminomethylideneamino)-1-oxopentan-2-yl]amino]-3-[[2-[[(2s)-2,6-diaminohexanoyl]amino]acetyl]amino]-4-oxobutanoic acid Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@@H](N)CCCCN MZOFCQQQCNRIBI-VMXHOPILSA-N 0.000 claims description 3
- MJZJYWCQPMNPRM-UHFFFAOYSA-N 6,6-dimethyl-1-[3-(2,4,5-trichlorophenoxy)propoxy]-1,6-dihydro-1,3,5-triazine-2,4-diamine Chemical compound CC1(C)N=C(N)N=C(N)N1OCCCOC1=CC(Cl)=C(Cl)C=C1Cl MJZJYWCQPMNPRM-UHFFFAOYSA-N 0.000 claims description 3
- 102100035248 Alpha-(1,3)-fucosyltransferase 4 Human genes 0.000 claims description 3
- 102100024217 CAMPATH-1 antigen Human genes 0.000 claims description 3
- 108010065524 CD52 Antigen Proteins 0.000 claims description 3
- 108010021064 CTLA-4 Antigen Proteins 0.000 claims description 3
- 229940045513 CTLA4 antagonist Drugs 0.000 claims description 3
- 108010022366 Carcinoembryonic Antigen Proteins 0.000 claims description 3
- 102100025475 Carcinoembryonic antigen-related cell adhesion molecule 5 Human genes 0.000 claims description 3
- 102000000844 Cell Surface Receptors Human genes 0.000 claims description 3
- 102000000989 Complement System Proteins Human genes 0.000 claims description 3
- 108010069112 Complement System Proteins Proteins 0.000 claims description 3
- 102100039498 Cytotoxic T-lymphocyte protein 4 Human genes 0.000 claims description 3
- 101001022185 Homo sapiens Alpha-(1,3)-fucosyltransferase 4 Proteins 0.000 claims description 3
- 101001002657 Homo sapiens Interleukin-2 Proteins 0.000 claims description 3
- 101000868279 Homo sapiens Leukocyte surface antigen CD47 Proteins 0.000 claims description 3
- 101000623901 Homo sapiens Mucin-16 Proteins 0.000 claims description 3
- 101000851376 Homo sapiens Tumor necrosis factor receptor superfamily member 8 Proteins 0.000 claims description 3
- 101000851370 Homo sapiens Tumor necrosis factor receptor superfamily member 9 Proteins 0.000 claims description 3
- 108010041012 Integrin alpha4 Proteins 0.000 claims description 3
- 102000008607 Integrin beta3 Human genes 0.000 claims description 3
- 108010020950 Integrin beta3 Proteins 0.000 claims description 3
- 108010074328 Interferon-gamma Proteins 0.000 claims description 3
- 102000008070 Interferon-gamma Human genes 0.000 claims description 3
- 102000013462 Interleukin-12 Human genes 0.000 claims description 3
- 108010065805 Interleukin-12 Proteins 0.000 claims description 3
- 108090000176 Interleukin-13 Proteins 0.000 claims description 3
- 102000003816 Interleukin-13 Human genes 0.000 claims description 3
- 102000013691 Interleukin-17 Human genes 0.000 claims description 3
- 108050003558 Interleukin-17 Proteins 0.000 claims description 3
- 108090001005 Interleukin-6 Proteins 0.000 claims description 3
- 102100032913 Leukocyte surface antigen CD47 Human genes 0.000 claims description 3
- 102100023123 Mucin-16 Human genes 0.000 claims description 3
- 108010025020 Nerve Growth Factor Proteins 0.000 claims description 3
- 102000015336 Nerve Growth Factor Human genes 0.000 claims description 3
- 108010025832 RANK Ligand Proteins 0.000 claims description 3
- 102000014128 RANK Ligand Human genes 0.000 claims description 3
- 108060008682 Tumor Necrosis Factor Proteins 0.000 claims description 3
- 102000000852 Tumor Necrosis Factor-alpha Human genes 0.000 claims description 3
- 102100036857 Tumor necrosis factor receptor superfamily member 8 Human genes 0.000 claims description 3
- 102100036856 Tumor necrosis factor receptor superfamily member 9 Human genes 0.000 claims description 3
- 108010073929 Vascular Endothelial Growth Factor A Proteins 0.000 claims description 3
- 108010019530 Vascular Endothelial Growth Factors Proteins 0.000 claims description 3
- 229950009106 altumomab Drugs 0.000 claims description 3
- 210000004978 chinese hamster ovary cell Anatomy 0.000 claims description 3
- 230000008045 co-localization Effects 0.000 claims description 3
- 229960002806 daclizumab Drugs 0.000 claims description 3
- 229960001251 denosumab Drugs 0.000 claims description 3
- 229930182830 galactose Natural products 0.000 claims description 3
- 210000003714 granulocyte Anatomy 0.000 claims description 3
- 102000055277 human IL2 Human genes 0.000 claims description 3
- 229960003130 interferon gamma Drugs 0.000 claims description 3
- OHDXDNUPVVYWOV-UHFFFAOYSA-N n-methyl-1-(2-naphthalen-1-ylsulfanylphenyl)methanamine Chemical compound CNCC1=CC=CC=C1SC1=CC=CC2=CC=CC=C12 OHDXDNUPVVYWOV-UHFFFAOYSA-N 0.000 claims description 3
- 229940053128 nerve growth factor Drugs 0.000 claims description 3
- 229960000470 omalizumab Drugs 0.000 claims description 3
- 239000000906 photoactive agent Substances 0.000 claims description 3
- 210000002307 prostate Anatomy 0.000 claims description 3
- 241001430294 unidentified retrovirus Species 0.000 claims description 3
- 229960003824 ustekinumab Drugs 0.000 claims description 3
- 239000013603 viral vector Substances 0.000 claims description 3
- RTQWWZBSTRGEAV-PKHIMPSTSA-N 2-[[(2s)-2-[bis(carboxymethyl)amino]-3-[4-(methylcarbamoylamino)phenyl]propyl]-[2-[bis(carboxymethyl)amino]propyl]amino]acetic acid Chemical compound CNC(=O)NC1=CC=C(C[C@@H](CN(CC(C)N(CC(O)=O)CC(O)=O)CC(O)=O)N(CC(O)=O)CC(O)=O)C=C1 RTQWWZBSTRGEAV-PKHIMPSTSA-N 0.000 claims description 2
- 102000013455 Amyloid beta-Peptides Human genes 0.000 claims description 2
- 108010090849 Amyloid beta-Peptides Proteins 0.000 claims description 2
- 108010074708 B7-H1 Antigen Proteins 0.000 claims description 2
- 108700012439 CA9 Proteins 0.000 claims description 2
- 102100024423 Carbonic anhydrase 9 Human genes 0.000 claims description 2
- 241001663880 Gammaretrovirus Species 0.000 claims description 2
- 108010054278 Lac Repressors Proteins 0.000 claims description 2
- 241000713666 Lentivirus Species 0.000 claims description 2
- 102100024216 Programmed cell death 1 ligand 1 Human genes 0.000 claims description 2
- XYVNHPYNSPGYLI-UUOKFMHZSA-N [(2r,3s,4r,5r)-5-(2-amino-6-oxo-3h-purin-9-yl)-4-hydroxy-2-(phosphonooxymethyl)oxolan-3-yl] dihydrogen phosphate Chemical compound C1=2NC(N)=NC(=O)C=2N=CN1[C@@H]1O[C@H](COP(O)(O)=O)[C@@H](OP(O)(O)=O)[C@H]1O XYVNHPYNSPGYLI-UUOKFMHZSA-N 0.000 claims description 2
- 229950005186 abagovomab Drugs 0.000 claims description 2
- 229960002964 adalimumab Drugs 0.000 claims description 2
- 229950005794 anrukinzumab Drugs 0.000 claims description 2
- 229950001863 bapineuzumab Drugs 0.000 claims description 2
- 229960004669 basiliximab Drugs 0.000 claims description 2
- 229950003269 bectumomab Drugs 0.000 claims description 2
- 229960003270 belimumab Drugs 0.000 claims description 2
- 229950000321 benralizumab Drugs 0.000 claims description 2
- 229960000455 brentuximab vedotin Drugs 0.000 claims description 2
- 229960003735 brodalumab Drugs 0.000 claims description 2
- 229960001838 canakinumab Drugs 0.000 claims description 2
- 229940034605 capromab pendetide Drugs 0.000 claims description 2
- 229960000419 catumaxomab Drugs 0.000 claims description 2
- 229960003115 certolizumab pegol Drugs 0.000 claims description 2
- 239000000562 conjugate Substances 0.000 claims description 2
- 229960002482 dalotuzumab Drugs 0.000 claims description 2
- 229950007998 demcizumab Drugs 0.000 claims description 2
- 229960001776 edrecolomab Drugs 0.000 claims description 2
- 229960000284 efalizumab Drugs 0.000 claims description 2
- 229950008579 ertumaxomab Drugs 0.000 claims description 2
- 229950009569 etaracizumab Drugs 0.000 claims description 2
- 229940093443 fanolesomab Drugs 0.000 claims description 2
- 229950004923 fontolizumab Drugs 0.000 claims description 2
- 229950004896 ganitumab Drugs 0.000 claims description 2
- 229950002026 girentuximab Drugs 0.000 claims description 2
- 229960001001 ibritumomab tiuxetan Drugs 0.000 claims description 2
- 229950002200 igovomab Drugs 0.000 claims description 2
- 229960005386 ipilimumab Drugs 0.000 claims description 2
- 229950000518 labetuzumab Drugs 0.000 claims description 2
- 229950007254 mavrilimumab Drugs 0.000 claims description 2
- 229960005108 mepolizumab Drugs 0.000 claims description 2
- 229950000720 moxetumomab pasudotox Drugs 0.000 claims description 2
- 229960005027 natalizumab Drugs 0.000 claims description 2
- 229960000513 necitumumab Drugs 0.000 claims description 2
- 210000002569 neuron Anatomy 0.000 claims description 2
- 229950010203 nimotuzumab Drugs 0.000 claims description 2
- 229960002450 ofatumumab Drugs 0.000 claims description 2
- 229950007283 oregovomab Drugs 0.000 claims description 2
- 229960000402 palivizumab Drugs 0.000 claims description 2
- 229960001972 panitumumab Drugs 0.000 claims description 2
- 229940067082 pentetate Drugs 0.000 claims description 2
- 229960003876 ranibizumab Drugs 0.000 claims description 2
- 229950003238 rilotumumab Drugs 0.000 claims description 2
- 229950007308 satumomab Drugs 0.000 claims description 2
- 229950010077 sifalimumab Drugs 0.000 claims description 2
- 229950010708 sulesomab Drugs 0.000 claims description 2
- 230000000946 synaptic effect Effects 0.000 claims description 2
- 229950008160 tanezumab Drugs 0.000 claims description 2
- CBPNZQVSJQDFBE-HGVVHKDOSA-N temsirolimus Chemical compound C1C[C@@H](OC(=O)C(C)(CO)CO)[C@H](OC)C[C@@H]1C[C@@H](C)[C@H]1OC(=O)[C@@H]2CCCCN2C(=O)C(=O)[C@](O)(O2)[C@H](C)CCC2C[C@H](OC)/C(C)=C/C=C/C=C/[C@@H](C)C[C@@H](C)C(=O)[C@H](OC)[C@H](O)/C(C)=C/[C@@H](C)C(=O)C1 CBPNZQVSJQDFBE-HGVVHKDOSA-N 0.000 claims description 2
- 229950000835 tralokinumab Drugs 0.000 claims description 2
- 229950007217 tremelimumab Drugs 0.000 claims description 2
- 229950005972 urelumab Drugs 0.000 claims description 2
- 229950004393 visilizumab Drugs 0.000 claims description 2
- 229950003511 votumumab Drugs 0.000 claims description 2
- 229950008250 zalutumumab Drugs 0.000 claims description 2
- SKWCZPYWFRTSDD-UHFFFAOYSA-N 2,3-bis(azaniumyl)propanoate;chloride Chemical compound Cl.NCC(N)C(O)=O SKWCZPYWFRTSDD-UHFFFAOYSA-N 0.000 claims 10
- 101001133056 Homo sapiens Mucin-1 Proteins 0.000 claims 1
- 108010028921 Lipopeptides Proteins 0.000 claims 1
- 108010058398 Macrophage Colony-Stimulating Factor Receptor Proteins 0.000 claims 1
- 102100034256 Mucin-1 Human genes 0.000 claims 1
- 241000751958 Omalium Species 0.000 claims 1
- 102000008022 Proto-Oncogene Proteins c-met Human genes 0.000 claims 1
- 101710183280 Topoisomerase Proteins 0.000 claims 1
- 229950006061 anatumomab mafenatox Drugs 0.000 claims 1
- 229950005725 arcitumomab Drugs 0.000 claims 1
- 229960001743 golimumab Drugs 0.000 claims 1
- 238000012216 screening Methods 0.000 abstract description 5
- 238000012360 testing method Methods 0.000 abstract description 4
- 238000003745 diagnosis Methods 0.000 abstract 1
- 125000005647 linker group Chemical group 0.000 description 224
- 125000003275 alpha amino acid group Chemical group 0.000 description 128
- 108010047041 Complementarity Determining Regions Proteins 0.000 description 95
- 125000000217 alkyl group Chemical group 0.000 description 75
- 206010057190 Respiratory tract infections Diseases 0.000 description 62
- 235000001014 amino acid Nutrition 0.000 description 60
- 229940024606 amino acid Drugs 0.000 description 51
- 108090000623 proteins and genes Proteins 0.000 description 50
- 241000282414 Homo sapiens Species 0.000 description 45
- 230000004048 modification Effects 0.000 description 44
- 238000012986 modification Methods 0.000 description 44
- 102000004196 processed proteins & peptides Human genes 0.000 description 43
- 229960005395 cetuximab Drugs 0.000 description 41
- 102000004169 proteins and genes Human genes 0.000 description 32
- 235000018102 proteins Nutrition 0.000 description 31
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical group NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 25
- 239000003795 chemical substances by application Substances 0.000 description 23
- 240000004808 Saccharomyces cerevisiae Species 0.000 description 22
- 235000014680 Saccharomyces cerevisiae Nutrition 0.000 description 22
- 229920001184 polypeptide Polymers 0.000 description 22
- ODKSFYDXXFIFQN-BYPYZUCNSA-N L-arginine Chemical compound OC(=O)[C@@H](N)CCCN=C(N)N ODKSFYDXXFIFQN-BYPYZUCNSA-N 0.000 description 20
- 229950000128 lumiliximab Drugs 0.000 description 20
- 150000001875 compounds Chemical class 0.000 description 19
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 19
- 238000007363 ring formation reaction Methods 0.000 description 18
- MTCFGRXMJLQNBG-REOHCLBHSA-N (2S)-2-Amino-3-hydroxypropansäure Chemical compound OC[C@H](N)C(O)=O MTCFGRXMJLQNBG-REOHCLBHSA-N 0.000 description 17
- 102100038080 B-cell receptor CD22 Human genes 0.000 description 17
- 101000884305 Homo sapiens B-cell receptor CD22 Proteins 0.000 description 17
- 230000009471 action Effects 0.000 description 17
- 210000003719 b-lymphocyte Anatomy 0.000 description 17
- 125000001424 substituent group Chemical group 0.000 description 17
- 229960004441 tyrosine Drugs 0.000 description 17
- 235000002374 tyrosine Nutrition 0.000 description 17
- XUJNEKJLAYXESH-REOHCLBHSA-N L-Cysteine Chemical group SC[C@H](N)C(O)=O XUJNEKJLAYXESH-REOHCLBHSA-N 0.000 description 16
- 230000003993 interaction Effects 0.000 description 16
- 230000035772 mutation Effects 0.000 description 16
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 description 15
- 102100029000 Prolactin receptor Human genes 0.000 description 15
- 238000004422 calculation algorithm Methods 0.000 description 15
- 239000003623 enhancer Substances 0.000 description 15
- 229950000121 otlertuzumab Drugs 0.000 description 15
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical compound OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 description 14
- KDXKERNSBIXSRK-YFKPBYRVSA-N L-lysine Chemical compound NCCCC[C@H](N)C(O)=O KDXKERNSBIXSRK-YFKPBYRVSA-N 0.000 description 14
- 229910052799 carbon Inorganic materials 0.000 description 14
- 229940088598 enzyme Drugs 0.000 description 14
- 229950002950 lintuzumab Drugs 0.000 description 14
- 230000035897 transcription Effects 0.000 description 14
- 238000013518 transcription Methods 0.000 description 14
- 101000777628 Homo sapiens Leukocyte antigen CD37 Proteins 0.000 description 13
- DCXYFEDJOCDNAF-REOHCLBHSA-N L-asparagine Chemical group OC(=O)[C@@H](N)CC(N)=O DCXYFEDJOCDNAF-REOHCLBHSA-N 0.000 description 13
- 102100031586 Leukocyte antigen CD37 Human genes 0.000 description 13
- 102000007298 Mucin-1 Human genes 0.000 description 13
- 108010008707 Mucin-1 Proteins 0.000 description 13
- 229950011428 indatuximab ravtansine Drugs 0.000 description 13
- 229950005674 modotuximab Drugs 0.000 description 13
- PECYZEOJVXMISF-UHFFFAOYSA-N 3-aminoalanine Chemical compound [NH3+]CC(N)C([O-])=O PECYZEOJVXMISF-UHFFFAOYSA-N 0.000 description 12
- 241000699666 Mus <mouse, genus> Species 0.000 description 12
- 101100109397 Neurospora crassa (strain ATCC 24698 / 74-OR23-1A / CBS 708.71 / DSM 1257 / FGSC 987) arg-8 gene Proteins 0.000 description 12
- 108010002519 Prolactin Receptors Proteins 0.000 description 12
- 239000013604 expression vector Substances 0.000 description 12
- RAXXELZNTBOGNW-UHFFFAOYSA-N imidazole Natural products C1=CNC=N1 RAXXELZNTBOGNW-UHFFFAOYSA-N 0.000 description 12
- 230000001105 regulatory effect Effects 0.000 description 12
- WHUUTDBJXJRKMK-UHFFFAOYSA-N Glutamic acid Natural products OC(=O)C(N)CCC(O)=O WHUUTDBJXJRKMK-UHFFFAOYSA-N 0.000 description 11
- AYFVYJQAPQTCCC-GBXIJSLDSA-N L-threonine Chemical compound C[C@@H](O)[C@H](N)C(O)=O AYFVYJQAPQTCCC-GBXIJSLDSA-N 0.000 description 11
- 102100038007 Low affinity immunoglobulin epsilon Fc receptor Human genes 0.000 description 11
- MTCFGRXMJLQNBG-UHFFFAOYSA-N Serine Natural products OCC(N)C(O)=O MTCFGRXMJLQNBG-UHFFFAOYSA-N 0.000 description 11
- 150000001241 acetals Chemical class 0.000 description 11
- 125000003118 aryl group Chemical group 0.000 description 11
- 230000002209 hydrophobic effect Effects 0.000 description 11
- 150000002905 orthoesters Chemical class 0.000 description 11
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 10
- 229910052739 hydrogen Inorganic materials 0.000 description 10
- 230000005847 immunogenicity Effects 0.000 description 10
- 229960000310 isoleucine Drugs 0.000 description 10
- AGPKZVBTJJNPAG-UHFFFAOYSA-N isoleucine Natural products CCC(C)C(N)C(O)=O AGPKZVBTJJNPAG-UHFFFAOYSA-N 0.000 description 10
- 241000588724 Escherichia coli Species 0.000 description 9
- ZDXPYRJPNDTMRX-VKHMYHEASA-N L-glutamine Chemical compound OC(=O)[C@@H](N)CCC(N)=O ZDXPYRJPNDTMRX-VKHMYHEASA-N 0.000 description 9
- 108091028043 Nucleic acid sequence Proteins 0.000 description 9
- 108010076504 Protein Sorting Signals Proteins 0.000 description 9
- 125000003295 alanine group Chemical group N[C@@H](C)C(=O)* 0.000 description 9
- 238000003776 cleavage reaction Methods 0.000 description 9
- 125000003630 glycyl group Chemical group [H]N([H])C([H])([H])C(*)=O 0.000 description 9
- 229910052736 halogen Inorganic materials 0.000 description 9
- 239000002609 medium Substances 0.000 description 9
- 230000007017 scission Effects 0.000 description 9
- DCXYFEDJOCDNAF-UHFFFAOYSA-N Asparagine Natural products OC(=O)C(N)CC(N)=O DCXYFEDJOCDNAF-UHFFFAOYSA-N 0.000 description 8
- 229920002567 Chondroitin Polymers 0.000 description 8
- 239000004471 Glycine Substances 0.000 description 8
- ROHFNLRQFUQHCH-YFKPBYRVSA-N L-leucine Chemical compound CC(C)C[C@H](N)C(O)=O ROHFNLRQFUQHCH-YFKPBYRVSA-N 0.000 description 8
- 239000004472 Lysine Substances 0.000 description 8
- AYFVYJQAPQTCCC-UHFFFAOYSA-N Threonine Natural products CC(O)C(N)C(O)=O AYFVYJQAPQTCCC-UHFFFAOYSA-N 0.000 description 8
- 239000004473 Threonine Substances 0.000 description 8
- DHKHKXVYLBGOIT-UHFFFAOYSA-N acetaldehyde Diethyl Acetal Chemical group CCOC(C)OCC DHKHKXVYLBGOIT-UHFFFAOYSA-N 0.000 description 8
- 229960001230 asparagine Drugs 0.000 description 8
- 235000009582 asparagine Nutrition 0.000 description 8
- 125000001246 bromo group Chemical group Br* 0.000 description 8
- 238000006243 chemical reaction Methods 0.000 description 8
- 125000001309 chloro group Chemical group Cl* 0.000 description 8
- DLGJWSVWTWEWBJ-HGGSSLSASA-N chondroitin Chemical compound CC(O)=N[C@@H]1[C@H](O)O[C@H](CO)[C@H](O)[C@@H]1OC1[C@H](O)[C@H](O)C=C(C(O)=O)O1 DLGJWSVWTWEWBJ-HGGSSLSASA-N 0.000 description 8
- 230000021615 conjugation Effects 0.000 description 8
- 235000018977 lysine Nutrition 0.000 description 8
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 8
- 125000006552 (C3-C8) cycloalkyl group Chemical group 0.000 description 7
- 102100038796 E3 ubiquitin-protein ligase TRIM13 Human genes 0.000 description 7
- 101000664589 Homo sapiens E3 ubiquitin-protein ligase TRIM13 Proteins 0.000 description 7
- 210000001744 T-lymphocyte Anatomy 0.000 description 7
- KZSNJWFQEVHDMF-UHFFFAOYSA-N Valine Chemical compound CC(C)C(N)C(O)=O KZSNJWFQEVHDMF-UHFFFAOYSA-N 0.000 description 7
- 241000700605 Viruses Species 0.000 description 7
- 125000002947 alkylene group Chemical group 0.000 description 7
- 229940009098 aspartate Drugs 0.000 description 7
- 238000005516 engineering process Methods 0.000 description 7
- 125000000404 glutamine group Chemical group N[C@@H](CCC(N)=O)C(=O)* 0.000 description 7
- 230000001976 improved effect Effects 0.000 description 7
- 230000010076 replication Effects 0.000 description 7
- 238000006467 substitution reaction Methods 0.000 description 7
- 125000004178 (C1-C4) alkyl group Chemical group 0.000 description 6
- 241000894006 Bacteria Species 0.000 description 6
- 108020004414 DNA Proteins 0.000 description 6
- 101000878605 Homo sapiens Low affinity immunoglobulin epsilon Fc receptor Proteins 0.000 description 6
- AGPKZVBTJJNPAG-WHFBIAKZSA-N L-isoleucine Chemical compound CC[C@H](C)[C@H](N)C(O)=O AGPKZVBTJJNPAG-WHFBIAKZSA-N 0.000 description 6
- 241001529936 Murinae Species 0.000 description 6
- 102000035195 Peptidases Human genes 0.000 description 6
- 108091005804 Peptidases Proteins 0.000 description 6
- 108010020346 Polyglutamic Acid Proteins 0.000 description 6
- 239000004365 Protease Substances 0.000 description 6
- 238000007792 addition Methods 0.000 description 6
- 238000013459 approach Methods 0.000 description 6
- 230000008901 benefit Effects 0.000 description 6
- 238000010367 cloning Methods 0.000 description 6
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 6
- 125000001153 fluoro group Chemical group F* 0.000 description 6
- 230000012010 growth Effects 0.000 description 6
- 150000002367 halogens Chemical class 0.000 description 6
- 150000002500 ions Chemical class 0.000 description 6
- 230000001404 mediated effect Effects 0.000 description 6
- 150000002739 metals Chemical class 0.000 description 6
- 239000002105 nanoparticle Substances 0.000 description 6
- YBYRMVIVWMBXKQ-UHFFFAOYSA-N phenylmethanesulfonyl fluoride Chemical compound FS(=O)(=O)CC1=CC=CC=C1 YBYRMVIVWMBXKQ-UHFFFAOYSA-N 0.000 description 6
- 239000013612 plasmid Substances 0.000 description 6
- 229920002643 polyglutamic acid Polymers 0.000 description 6
- 108091033319 polynucleotide Proteins 0.000 description 6
- 102000040430 polynucleotide Human genes 0.000 description 6
- 239000002157 polynucleotide Substances 0.000 description 6
- 230000002829 reductive effect Effects 0.000 description 6
- 150000003384 small molecules Chemical class 0.000 description 6
- 238000003860 storage Methods 0.000 description 6
- 238000002560 therapeutic procedure Methods 0.000 description 6
- NFGXHKASABOEEW-UHFFFAOYSA-N 1-methylethyl 11-methoxy-3,7,11-trimethyl-2,4-dodecadienoate Chemical compound COC(C)(C)CCCC(C)CC=CC(C)=CC(=O)OC(C)C NFGXHKASABOEEW-UHFFFAOYSA-N 0.000 description 5
- 102000002260 Alkaline Phosphatase Human genes 0.000 description 5
- 108020004774 Alkaline Phosphatase Proteins 0.000 description 5
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 5
- XUKUURHRXDUEBC-KAYWLYCHSA-N Atorvastatin Chemical compound C=1C=CC=CC=1C1=C(C=2C=CC(F)=CC=2)N(CC[C@@H](O)C[C@@H](O)CC(O)=O)C(C(C)C)=C1C(=O)NC1=CC=CC=C1 XUKUURHRXDUEBC-KAYWLYCHSA-N 0.000 description 5
- XUKUURHRXDUEBC-UHFFFAOYSA-N Atorvastatin Natural products C=1C=CC=CC=1C1=C(C=2C=CC(F)=CC=2)N(CCC(O)CC(O)CC(O)=O)C(C(C)C)=C1C(=O)NC1=CC=CC=C1 XUKUURHRXDUEBC-UHFFFAOYSA-N 0.000 description 5
- 241000701022 Cytomegalovirus Species 0.000 description 5
- 102100024746 Dihydrofolate reductase Human genes 0.000 description 5
- 241000233866 Fungi Species 0.000 description 5
- 108010021625 Immunoglobulin Fragments Proteins 0.000 description 5
- 102000008394 Immunoglobulin Fragments Human genes 0.000 description 5
- 125000003342 alkenyl group Chemical group 0.000 description 5
- 125000000539 amino acid group Chemical group 0.000 description 5
- 239000003242 anti bacterial agent Substances 0.000 description 5
- 229940088710 antibiotic agent Drugs 0.000 description 5
- 206010003246 arthritis Diseases 0.000 description 5
- 229960005370 atorvastatin Drugs 0.000 description 5
- 230000015572 biosynthetic process Effects 0.000 description 5
- 125000000484 butyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 5
- 208000032852 chronic lymphocytic leukemia Diseases 0.000 description 5
- 239000010949 copper Substances 0.000 description 5
- 125000004122 cyclic group Chemical group 0.000 description 5
- 108020001096 dihydrofolate reductase Proteins 0.000 description 5
- 239000000975 dye Substances 0.000 description 5
- 230000006870 function Effects 0.000 description 5
- 229930195712 glutamate Natural products 0.000 description 5
- 230000013595 glycosylation Effects 0.000 description 5
- 238000006206 glycosylation reaction Methods 0.000 description 5
- 238000010348 incorporation Methods 0.000 description 5
- 125000002346 iodo group Chemical group I* 0.000 description 5
- 125000000741 isoleucyl group Chemical group [H]N([H])C(C(C([H])([H])[H])C([H])([H])C([H])([H])[H])C(=O)O* 0.000 description 5
- 210000003292 kidney cell Anatomy 0.000 description 5
- 150000003951 lactams Chemical class 0.000 description 5
- 239000003446 ligand Substances 0.000 description 5
- 125000001624 naphthyl group Chemical group 0.000 description 5
- 230000004962 physiological condition Effects 0.000 description 5
- 235000019419 proteases Nutrition 0.000 description 5
- 230000003252 repetitive effect Effects 0.000 description 5
- 150000003839 salts Chemical class 0.000 description 5
- 239000004474 valine Substances 0.000 description 5
- 235000014393 valine Nutrition 0.000 description 5
- FWMNVWWHGCHHJJ-SKKKGAJSSA-N 4-amino-1-[(2r)-6-amino-2-[[(2r)-2-[[(2r)-2-[[(2r)-2-amino-3-phenylpropanoyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoyl]amino]hexanoyl]piperidine-4-carboxylic acid Chemical compound C([C@H](C(=O)N[C@H](CC(C)C)C(=O)N[C@H](CCCCN)C(=O)N1CCC(N)(CC1)C(O)=O)NC(=O)[C@H](N)CC=1C=CC=CC=1)C1=CC=CC=C1 FWMNVWWHGCHHJJ-SKKKGAJSSA-N 0.000 description 4
- 102000017420 CD3 protein, epsilon/gamma/delta subunit Human genes 0.000 description 4
- 108050005493 CD3 protein, epsilon/gamma/delta subunit Proteins 0.000 description 4
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 4
- 241000196324 Embryophyta Species 0.000 description 4
- 101150065691 FRL2 gene Proteins 0.000 description 4
- 102100032789 Formin-like protein 3 Human genes 0.000 description 4
- 108010031794 IGF Type 1 Receptor Proteins 0.000 description 4
- 102000038455 IGF Type 1 Receptor Human genes 0.000 description 4
- 108010073816 IgE Receptors Proteins 0.000 description 4
- 241000235649 Kluyveromyces Species 0.000 description 4
- NNJVILVZKWQKPM-UHFFFAOYSA-N Lidocaine Chemical compound CCN(CC)CC(=O)NC1=C(C)C=CC=C1C NNJVILVZKWQKPM-UHFFFAOYSA-N 0.000 description 4
- 102000003792 Metallothionein Human genes 0.000 description 4
- 108090000157 Metallothionein Proteins 0.000 description 4
- ONIBWKKTOPOVIA-UHFFFAOYSA-N Proline Natural products OC(=O)C1CCCN1 ONIBWKKTOPOVIA-UHFFFAOYSA-N 0.000 description 4
- 102100035721 Syndecan-1 Human genes 0.000 description 4
- 230000004913 activation Effects 0.000 description 4
- 125000000304 alkynyl group Chemical group 0.000 description 4
- 230000003321 amplification Effects 0.000 description 4
- 125000000613 asparagine group Chemical group N[C@@H](CC(N)=O)C(=O)* 0.000 description 4
- CKLJMWTZIZZHCS-REOHCLBHSA-L aspartate group Chemical group N[C@@H](CC(=O)[O-])C(=O)[O-] CKLJMWTZIZZHCS-REOHCLBHSA-L 0.000 description 4
- ZADPBFCGQRWHPN-UHFFFAOYSA-N boronic acid Chemical compound OBO ZADPBFCGQRWHPN-UHFFFAOYSA-N 0.000 description 4
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 4
- 238000012217 deletion Methods 0.000 description 4
- 230000037430 deletion Effects 0.000 description 4
- 230000004069 differentiation Effects 0.000 description 4
- 150000002148 esters Chemical class 0.000 description 4
- 101150029401 fmnl3 gene Proteins 0.000 description 4
- 238000009472 formulation Methods 0.000 description 4
- 235000013922 glutamic acid Nutrition 0.000 description 4
- 239000004220 glutamic acid Substances 0.000 description 4
- 102000005396 glutamine synthetase Human genes 0.000 description 4
- 108020002326 glutamine synthetase Proteins 0.000 description 4
- 238000003384 imaging method Methods 0.000 description 4
- 230000016784 immunoglobulin production Effects 0.000 description 4
- 238000001727 in vivo Methods 0.000 description 4
- 125000001041 indolyl group Chemical group 0.000 description 4
- 239000003112 inhibitor Substances 0.000 description 4
- NOESYZHRGYRDHS-UHFFFAOYSA-N insulin Chemical compound N1C(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(NC(=O)CN)C(C)CC)CSSCC(C(NC(CO)C(=O)NC(CC(C)C)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CCC(N)=O)C(=O)NC(CC(C)C)C(=O)NC(CCC(O)=O)C(=O)NC(CC(N)=O)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CSSCC(NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2C=CC(O)=CC=2)NC(=O)C(CC(C)C)NC(=O)C(C)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2NC=NC=2)NC(=O)C(CO)NC(=O)CNC2=O)C(=O)NCC(=O)NC(CCC(O)=O)C(=O)NC(CCCNC(N)=N)C(=O)NCC(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC(O)=CC=3)C(=O)NC(C(C)O)C(=O)N3C(CCC3)C(=O)NC(CCCCN)C(=O)NC(C)C(O)=O)C(=O)NC(CC(N)=O)C(O)=O)=O)NC(=O)C(C(C)CC)NC(=O)C(CO)NC(=O)C(C(C)O)NC(=O)C1CSSCC2NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CC(N)=O)NC(=O)C(NC(=O)C(N)CC=1C=CC=CC=1)C(C)C)CC1=CN=CN1 NOESYZHRGYRDHS-UHFFFAOYSA-N 0.000 description 4
- 125000000959 isobutyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])* 0.000 description 4
- 238000002955 isolation Methods 0.000 description 4
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 4
- 238000000111 isothermal titration calorimetry Methods 0.000 description 4
- 229960004194 lidocaine Drugs 0.000 description 4
- 239000003550 marker Substances 0.000 description 4
- 229910052757 nitrogen Inorganic materials 0.000 description 4
- 238000003199 nucleic acid amplification method Methods 0.000 description 4
- 239000012071 phase Substances 0.000 description 4
- 230000008569 process Effects 0.000 description 4
- 125000001500 prolyl group Chemical group [H]N1C([H])(C(=O)[*])C([H])([H])C([H])([H])C1([H])[H] 0.000 description 4
- 125000001436 propyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])[H] 0.000 description 4
- 238000000746 purification Methods 0.000 description 4
- 125000002914 sec-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 4
- 238000002198 surface plasmon resonance spectroscopy Methods 0.000 description 4
- 230000002195 synergetic effect Effects 0.000 description 4
- 125000000999 tert-butyl group Chemical group [H]C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 description 4
- 125000000341 threoninyl group Chemical group [H]OC([H])(C([H])([H])[H])C([H])(N([H])[H])C(*)=O 0.000 description 4
- 230000005030 transcription termination Effects 0.000 description 4
- PEMUHKUIQHFMTH-QMMMGPOBSA-N (2s)-2-amino-3-(4-bromophenyl)propanoic acid Chemical compound OC(=O)[C@@H](N)CC1=CC=C(Br)C=C1 PEMUHKUIQHFMTH-QMMMGPOBSA-N 0.000 description 3
- GZCWLCBFPRFLKL-UHFFFAOYSA-N 1-prop-2-ynoxypropan-2-ol Chemical compound CC(O)COCC#C GZCWLCBFPRFLKL-UHFFFAOYSA-N 0.000 description 3
- 125000006163 5-membered heteroaryl group Chemical group 0.000 description 3
- 102000043853 ADAMTS13 Human genes 0.000 description 3
- 108091005670 ADAMTS13 Proteins 0.000 description 3
- 102000013563 Acid Phosphatase Human genes 0.000 description 3
- 108010051457 Acid Phosphatase Proteins 0.000 description 3
- 239000004475 Arginine Substances 0.000 description 3
- UHOVQNZJYSORNB-UHFFFAOYSA-N Benzene Chemical compound C1=CC=CC=C1 UHOVQNZJYSORNB-UHFFFAOYSA-N 0.000 description 3
- 241000699802 Cricetulus griseus Species 0.000 description 3
- 101150074155 DHFR gene Proteins 0.000 description 3
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 3
- 238000012413 Fluorescence activated cell sorting analysis Methods 0.000 description 3
- 241000287828 Gallus gallus Species 0.000 description 3
- 108700028146 Genetic Enhancer Elements Proteins 0.000 description 3
- 102100031132 Glucose-6-phosphate isomerase Human genes 0.000 description 3
- 108010070600 Glucose-6-phosphate isomerase Proteins 0.000 description 3
- 102100041003 Glutamate carboxypeptidase 2 Human genes 0.000 description 3
- 241000238631 Hexapoda Species 0.000 description 3
- 101000892862 Homo sapiens Glutamate carboxypeptidase 2 Proteins 0.000 description 3
- 108060003951 Immunoglobulin Proteins 0.000 description 3
- SHGAZHPCJJPHSC-NUEINMDLSA-N Isotretinoin Chemical compound OC(=O)C=C(C)/C=C/C=C(C)C=CC1=C(C)CCCC1(C)C SHGAZHPCJJPHSC-NUEINMDLSA-N 0.000 description 3
- 241001138401 Kluyveromyces lactis Species 0.000 description 3
- FBOZXECLQNJBKD-ZDUSSCGKSA-N L-methotrexate Chemical compound C=1N=C2N=C(N)N=C(N)C2=NC=1CN(C)C1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 FBOZXECLQNJBKD-ZDUSSCGKSA-N 0.000 description 3
- KZSNJWFQEVHDMF-BYPYZUCNSA-N L-valine Chemical compound CC(C)[C@H](N)C(O)=O KZSNJWFQEVHDMF-BYPYZUCNSA-N 0.000 description 3
- NWIBSHFKIJFRCO-WUDYKRTCSA-N Mytomycin Chemical compound C1N2C(C(C(C)=C(N)C3=O)=O)=C3[C@@H](COC(N)=O)[C@@]2(OC)[C@@H]2[C@H]1N2 NWIBSHFKIJFRCO-WUDYKRTCSA-N 0.000 description 3
- 229930193140 Neomycin Natural products 0.000 description 3
- 241000589516 Pseudomonas Species 0.000 description 3
- 241000700159 Rattus Species 0.000 description 3
- 108020004459 Small interfering RNA Proteins 0.000 description 3
- 101150006914 TRP1 gene Proteins 0.000 description 3
- WDLRUFUQRNWCPK-UHFFFAOYSA-N Tetraxetan Chemical compound OC(=O)CN1CCN(CC(O)=O)CCN(CC(O)=O)CCN(CC(O)=O)CC1 WDLRUFUQRNWCPK-UHFFFAOYSA-N 0.000 description 3
- 108091023040 Transcription factor Proteins 0.000 description 3
- 108090000435 Urokinase-type plasminogen activator Proteins 0.000 description 3
- 102000003990 Urokinase-type plasminogen activator Human genes 0.000 description 3
- 241000251539 Vertebrata <Metazoa> Species 0.000 description 3
- 238000005865 alkene metathesis reaction Methods 0.000 description 3
- 125000003277 amino group Chemical group 0.000 description 3
- 239000007864 aqueous solution Substances 0.000 description 3
- ODKSFYDXXFIFQN-UHFFFAOYSA-N arginine Natural products OC(=O)C(N)CCCNC(N)=N ODKSFYDXXFIFQN-UHFFFAOYSA-N 0.000 description 3
- 238000003556 assay Methods 0.000 description 3
- 230000001580 bacterial effect Effects 0.000 description 3
- 238000004113 cell culture Methods 0.000 description 3
- 230000004663 cell proliferation Effects 0.000 description 3
- 238000004587 chromatography analysis Methods 0.000 description 3
- 239000013599 cloning vector Substances 0.000 description 3
- 230000002860 competitive effect Effects 0.000 description 3
- 239000002299 complementary DNA Substances 0.000 description 3
- 125000000151 cysteine group Chemical group N[C@@H](CS)C(=O)* 0.000 description 3
- 230000003247 decreasing effect Effects 0.000 description 3
- 238000013461 design Methods 0.000 description 3
- 239000000539 dimer Substances 0.000 description 3
- 230000012202 endocytosis Effects 0.000 description 3
- 230000004927 fusion Effects 0.000 description 3
- UIWYJDYFSGRHKR-UHFFFAOYSA-N gadolinium atom Chemical compound [Gd] UIWYJDYFSGRHKR-UHFFFAOYSA-N 0.000 description 3
- 239000010931 gold Substances 0.000 description 3
- 239000001963 growth medium Substances 0.000 description 3
- 229940088597 hormone Drugs 0.000 description 3
- 239000005556 hormone Substances 0.000 description 3
- 125000001165 hydrophobic group Chemical group 0.000 description 3
- 102000018358 immunoglobulin Human genes 0.000 description 3
- 239000002955 immunomodulating agent Substances 0.000 description 3
- 229960005280 isotretinoin Drugs 0.000 description 3
- 238000005304 joining Methods 0.000 description 3
- 239000000463 material Substances 0.000 description 3
- 229930182817 methionine Chemical group 0.000 description 3
- 229960004452 methionine Drugs 0.000 description 3
- 125000001360 methionine group Chemical group N[C@@H](CCSC)C(=O)* 0.000 description 3
- 229960000485 methotrexate Drugs 0.000 description 3
- 239000000178 monomer Substances 0.000 description 3
- 229960004927 neomycin Drugs 0.000 description 3
- 210000001672 ovary Anatomy 0.000 description 3
- 230000005298 paramagnetic effect Effects 0.000 description 3
- 230000036961 partial effect Effects 0.000 description 3
- 230000037361 pathway Effects 0.000 description 3
- 230000003285 pharmacodynamic effect Effects 0.000 description 3
- 230000009467 reduction Effects 0.000 description 3
- 230000028327 secretion Effects 0.000 description 3
- 125000003607 serino group Chemical group [H]N([H])[C@]([H])(C(=O)[*])C(O[H])([H])[H] 0.000 description 3
- 108091008023 transcriptional regulators Proteins 0.000 description 3
- 239000013638 trimer Substances 0.000 description 3
- 241000701161 unidentified adenovirus Species 0.000 description 3
- 229910052720 vanadium Inorganic materials 0.000 description 3
- 230000003612 virological effect Effects 0.000 description 3
- DIGQNXIGRZPYDK-WKSCXVIASA-N (2R)-6-amino-2-[[2-[[(2S)-2-[[2-[[(2R)-2-[[(2S)-2-[[(2R,3S)-2-[[2-[[(2S)-2-[[2-[[(2S)-2-[[(2S)-2-[[(2R)-2-[[(2S,3S)-2-[[(2R)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[2-[[(2S)-2-[[(2R)-2-[[2-[[2-[[2-[(2-amino-1-hydroxyethylidene)amino]-3-carboxy-1-hydroxypropylidene]amino]-1-hydroxy-3-sulfanylpropylidene]amino]-1-hydroxyethylidene]amino]-1-hydroxy-3-sulfanylpropylidene]amino]-1,3-dihydroxypropylidene]amino]-1-hydroxyethylidene]amino]-1-hydroxypropylidene]amino]-1,3-dihydroxypropylidene]amino]-1,3-dihydroxypropylidene]amino]-1-hydroxy-3-sulfanylpropylidene]amino]-1,3-dihydroxybutylidene]amino]-1-hydroxy-3-sulfanylpropylidene]amino]-1-hydroxypropylidene]amino]-1,3-dihydroxypropylidene]amino]-1-hydroxyethylidene]amino]-1,5-dihydroxy-5-iminopentylidene]amino]-1-hydroxy-3-sulfanylpropylidene]amino]-1,3-dihydroxybutylidene]amino]-1-hydroxy-3-sulfanylpropylidene]amino]-1,3-dihydroxypropylidene]amino]-1-hydroxyethylidene]amino]-1-hydroxy-3-sulfanylpropylidene]amino]-1-hydroxyethylidene]amino]hexanoic acid Chemical compound C[C@@H]([C@@H](C(=N[C@@H](CS)C(=N[C@@H](C)C(=N[C@@H](CO)C(=NCC(=N[C@@H](CCC(=N)O)C(=NC(CS)C(=N[C@H]([C@H](C)O)C(=N[C@H](CS)C(=N[C@H](CO)C(=NCC(=N[C@H](CS)C(=NCC(=N[C@H](CCCCN)C(=O)O)O)O)O)O)O)O)O)O)O)O)O)O)O)N=C([C@H](CS)N=C([C@H](CO)N=C([C@H](CO)N=C([C@H](C)N=C(CN=C([C@H](CO)N=C([C@H](CS)N=C(CN=C(C(CS)N=C(C(CC(=O)O)N=C(CN)O)O)O)O)O)O)O)O)O)O)O)O DIGQNXIGRZPYDK-WKSCXVIASA-N 0.000 description 2
- OGYGFUAIIOPWQD-UHFFFAOYSA-N 1,3-thiazolidine Chemical compound C1CSCN1 OGYGFUAIIOPWQD-UHFFFAOYSA-N 0.000 description 2
- GVNVAWHJIKLAGL-UHFFFAOYSA-N 2-(cyclohexen-1-yl)cyclohexan-1-one Chemical compound O=C1CCCCC1C1=CCCCC1 GVNVAWHJIKLAGL-UHFFFAOYSA-N 0.000 description 2
- JHALWMSZGCVVEM-UHFFFAOYSA-N 2-[4,7-bis(carboxymethyl)-1,4,7-triazonan-1-yl]acetic acid Chemical compound OC(=O)CN1CCN(CC(O)=O)CCN(CC(O)=O)CC1 JHALWMSZGCVVEM-UHFFFAOYSA-N 0.000 description 2
- 125000006276 2-bromophenyl group Chemical group [H]C1=C([H])C(Br)=C(*)C([H])=C1[H] 0.000 description 2
- 125000006275 3-bromophenyl group Chemical group [H]C1=C([H])C(Br)=C([H])C(*)=C1[H] 0.000 description 2
- OSJPPGNTCRNQQC-UWTATZPHSA-N 3-phospho-D-glyceric acid Chemical compound OC(=O)[C@H](O)COP(O)(O)=O OSJPPGNTCRNQQC-UWTATZPHSA-N 0.000 description 2
- 125000004800 4-bromophenyl group Chemical group [H]C1=C([H])C(*)=C([H])C([H])=C1Br 0.000 description 2
- NMUSYJAQQFHJEW-KVTDHHQDSA-N 5-azacytidine Chemical compound O=C1N=C(N)N=CN1[C@H]1[C@H](O)[C@H](O)[C@@H](CO)O1 NMUSYJAQQFHJEW-KVTDHHQDSA-N 0.000 description 2
- STQGQHZAVUOBTE-UHFFFAOYSA-N 7-Cyan-hept-2t-en-4,6-diinsaeure Natural products C1=2C(O)=C3C(=O)C=4C(OC)=CC=CC=4C(=O)C3=C(O)C=2CC(O)(C(C)=O)CC1OC1CC(N)C(O)C(C)O1 STQGQHZAVUOBTE-UHFFFAOYSA-N 0.000 description 2
- 229920000936 Agarose Polymers 0.000 description 2
- 101710154825 Aminoglycoside 3'-phosphotransferase Proteins 0.000 description 2
- 208000023275 Autoimmune disease Diseases 0.000 description 2
- 230000003844 B-cell-activation Effects 0.000 description 2
- 241000283690 Bos taurus Species 0.000 description 2
- 241000701822 Bovine papillomavirus Species 0.000 description 2
- WKBOTKDWSSQWDR-UHFFFAOYSA-N Bromine atom Chemical compound [Br] WKBOTKDWSSQWDR-UHFFFAOYSA-N 0.000 description 2
- 125000004649 C2-C8 alkynyl group Chemical group 0.000 description 2
- 102000003847 Carboxypeptidase B2 Human genes 0.000 description 2
- 108090000201 Carboxypeptidase B2 Proteins 0.000 description 2
- 241000282693 Cercopithecidae Species 0.000 description 2
- 101150065749 Churc1 gene Proteins 0.000 description 2
- PTOAARAWEBMLNO-KVQBGUIXSA-N Cladribine Chemical compound C1=NC=2C(N)=NC(Cl)=NC=2N1[C@H]1C[C@H](O)[C@@H](CO)O1 PTOAARAWEBMLNO-KVQBGUIXSA-N 0.000 description 2
- 108091026890 Coding region Proteins 0.000 description 2
- 102000036364 Cullin Ring E3 Ligases Human genes 0.000 description 2
- UHDGCWIWMRVCDJ-CCXZUQQUSA-N Cytarabine Chemical compound O=C1N=C(N)C=CN1[C@H]1[C@@H](O)[C@H](O)[C@@H](CO)O1 UHDGCWIWMRVCDJ-CCXZUQQUSA-N 0.000 description 2
- QNAYBMKLOCPYGJ-UHFFFAOYSA-N D-alpha-Ala Natural products CC([NH3+])C([O-])=O QNAYBMKLOCPYGJ-UHFFFAOYSA-N 0.000 description 2
- 230000004568 DNA-binding Effects 0.000 description 2
- 241000255925 Diptera Species 0.000 description 2
- AOJJSUZBOXZQNB-TZSSRYMLSA-N Doxorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 AOJJSUZBOXZQNB-TZSSRYMLSA-N 0.000 description 2
- 239000006144 Dulbecco’s modified Eagle's medium Substances 0.000 description 2
- ULGZDMOVFRHVEP-RWJQBGPGSA-N Erythromycin Chemical compound O([C@@H]1[C@@H](C)C(=O)O[C@@H]([C@@]([C@H](O)[C@@H](C)C(=O)[C@H](C)C[C@@](C)(O)[C@H](O[C@H]2[C@@H]([C@H](C[C@@H](C)O2)N(C)C)O)[C@H]1C)(C)O)CC)[C@H]1C[C@@](C)(OC)[C@@H](O)[C@H](C)O1 ULGZDMOVFRHVEP-RWJQBGPGSA-N 0.000 description 2
- 241000206602 Eukaryota Species 0.000 description 2
- 239000004230 Fast Yellow AB Substances 0.000 description 2
- GHASVSINZRGABV-UHFFFAOYSA-N Fluorouracil Chemical compound FC1=CNC(=O)NC1=O GHASVSINZRGABV-UHFFFAOYSA-N 0.000 description 2
- 101150074355 GS gene Proteins 0.000 description 2
- 229910052688 Gadolinium Inorganic materials 0.000 description 2
- 102100039289 Glial fibrillary acidic protein Human genes 0.000 description 2
- 101710193519 Glial fibrillary acidic protein Proteins 0.000 description 2
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 2
- 108010092372 Granulocyte-Macrophage Colony-Stimulating Factor Receptors Proteins 0.000 description 2
- 102000016355 Granulocyte-Macrophage Colony-Stimulating Factor Receptors Human genes 0.000 description 2
- 108090000100 Hepatocyte Growth Factor Proteins 0.000 description 2
- 102100021866 Hepatocyte growth factor Human genes 0.000 description 2
- 101001123448 Homo sapiens Prolactin receptor Proteins 0.000 description 2
- 206010020751 Hypersensitivity Diseases 0.000 description 2
- 108010054477 Immunoglobulin Fab Fragments Proteins 0.000 description 2
- 102000001706 Immunoglobulin Fab Fragments Human genes 0.000 description 2
- 108090001061 Insulin Proteins 0.000 description 2
- 102000004877 Insulin Human genes 0.000 description 2
- 102000003777 Interleukin-1 beta Human genes 0.000 description 2
- 108090000193 Interleukin-1 beta Proteins 0.000 description 2
- 102000003815 Interleukin-11 Human genes 0.000 description 2
- 108090000177 Interleukin-11 Proteins 0.000 description 2
- 102100039897 Interleukin-5 Human genes 0.000 description 2
- 108010002616 Interleukin-5 Proteins 0.000 description 2
- 244000285963 Kluyveromyces fragilis Species 0.000 description 2
- 108090001090 Lectins Proteins 0.000 description 2
- 102000004856 Lectins Human genes 0.000 description 2
- ROHFNLRQFUQHCH-UHFFFAOYSA-N Leucine Chemical group CC(C)CC(N)C(O)=O ROHFNLRQFUQHCH-UHFFFAOYSA-N 0.000 description 2
- 241001465754 Metazoa Species 0.000 description 2
- UGJBHEZMOKVTIM-UHFFFAOYSA-N N-formylglycine Chemical compound OC(=O)CNC=O UGJBHEZMOKVTIM-UHFFFAOYSA-N 0.000 description 2
- 229910003827 NRaRb Inorganic materials 0.000 description 2
- 101710163270 Nuclease Proteins 0.000 description 2
- 238000012879 PET imaging Methods 0.000 description 2
- 102000016387 Pancreatic elastase Human genes 0.000 description 2
- 108010067372 Pancreatic elastase Proteins 0.000 description 2
- 229920000805 Polyaspartic acid Polymers 0.000 description 2
- 241000288906 Primates Species 0.000 description 2
- 102100024819 Prolactin Human genes 0.000 description 2
- 108010057464 Prolactin Proteins 0.000 description 2
- 102100038239 Protein Churchill Human genes 0.000 description 2
- 241000588769 Proteus <enterobacteria> Species 0.000 description 2
- 108091030071 RNAI Proteins 0.000 description 2
- 108020005091 Replication Origin Proteins 0.000 description 2
- 102000006382 Ribonucleases Human genes 0.000 description 2
- 108010083644 Ribonucleases Proteins 0.000 description 2
- 241000607142 Salmonella Species 0.000 description 2
- 241000293869 Salmonella enterica subsp. enterica serovar Typhimurium Species 0.000 description 2
- 241000607720 Serratia Species 0.000 description 2
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 2
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 2
- 241000256251 Spodoptera frugiperda Species 0.000 description 2
- PJANXHGTPQOBST-VAWYXSNFSA-N Stilbene Natural products C=1C=CC=CC=1/C=C/C1=CC=CC=C1 PJANXHGTPQOBST-VAWYXSNFSA-N 0.000 description 2
- 102000001435 Synapsin Human genes 0.000 description 2
- 108050009621 Synapsin Proteins 0.000 description 2
- 108090000058 Syndecan-1 Proteins 0.000 description 2
- IQFYYKKMVGJFEH-XLPZGREQSA-N Thymidine Chemical compound O=C1NC(=O)C(C)=CN1[C@@H]1O[C@H](CO)[C@@H](O)C1 IQFYYKKMVGJFEH-XLPZGREQSA-N 0.000 description 2
- 102000006601 Thymidine Kinase Human genes 0.000 description 2
- 108020004440 Thymidine kinase Proteins 0.000 description 2
- 102000005789 Vascular Endothelial Growth Factors Human genes 0.000 description 2
- 238000007259 addition reaction Methods 0.000 description 2
- 230000000996 additive effect Effects 0.000 description 2
- OIRDTQYFTABQOQ-KQYNXXCUSA-N adenosine Chemical compound C1=NC=2C(N)=NC=NC=2N1[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O OIRDTQYFTABQOQ-KQYNXXCUSA-N 0.000 description 2
- 238000001042 affinity chromatography Methods 0.000 description 2
- 150000001299 aldehydes Chemical class 0.000 description 2
- 125000004450 alkenylene group Chemical group 0.000 description 2
- 230000007815 allergy Effects 0.000 description 2
- 238000004458 analytical method Methods 0.000 description 2
- 239000005557 antagonist Substances 0.000 description 2
- 230000000259 anti-tumor effect Effects 0.000 description 2
- 210000000612 antigen-presenting cell Anatomy 0.000 description 2
- 230000006907 apoptotic process Effects 0.000 description 2
- 125000000732 arylene group Chemical group 0.000 description 2
- 229960002756 azacitidine Drugs 0.000 description 2
- 244000052616 bacterial pathogen Species 0.000 description 2
- 239000002585 base Substances 0.000 description 2
- 238000005452 bending Methods 0.000 description 2
- 230000004071 biological effect Effects 0.000 description 2
- 230000033228 biological regulation Effects 0.000 description 2
- AIXAANGOTKPUOY-UHFFFAOYSA-N carbachol Chemical compound [Cl-].C[N+](C)(C)CCOC(N)=O AIXAANGOTKPUOY-UHFFFAOYSA-N 0.000 description 2
- 238000003570 cell viability assay Methods 0.000 description 2
- 238000005119 centrifugation Methods 0.000 description 2
- 239000003153 chemical reaction reagent Substances 0.000 description 2
- 230000001276 controlling effect Effects 0.000 description 2
- 229910052802 copper Inorganic materials 0.000 description 2
- 230000001086 cytosolic effect Effects 0.000 description 2
- 231100000433 cytotoxic Toxicity 0.000 description 2
- 239000002254 cytotoxic agent Substances 0.000 description 2
- 231100000599 cytotoxic agent Toxicity 0.000 description 2
- 230000001472 cytotoxic effect Effects 0.000 description 2
- 230000006378 damage Effects 0.000 description 2
- STQGQHZAVUOBTE-VGBVRHCVSA-N daunorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(C)=O)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 STQGQHZAVUOBTE-VGBVRHCVSA-N 0.000 description 2
- 230000000593 degrading effect Effects 0.000 description 2
- 230000001419 dependent effect Effects 0.000 description 2
- 238000002405 diagnostic procedure Methods 0.000 description 2
- 238000001962 electrophoresis Methods 0.000 description 2
- 230000002708 enhancing effect Effects 0.000 description 2
- GNBHRKFJIUUOQI-UHFFFAOYSA-N fluorescein Chemical compound O1C(=O)C2=CC=CC=C2C21C1=CC=C(O)C=C1OC1=CC(O)=CC=C21 GNBHRKFJIUUOQI-UHFFFAOYSA-N 0.000 description 2
- 229960002949 fluorouracil Drugs 0.000 description 2
- 239000000499 gel Substances 0.000 description 2
- 230000009368 gene silencing by RNA Effects 0.000 description 2
- 210000005046 glial fibrillary acidic protein Anatomy 0.000 description 2
- WHUUTDBJXJRKMK-VKHMYHEASA-L glutamate group Chemical group N[C@@H](CCC(=O)[O-])C(=O)[O-] WHUUTDBJXJRKMK-VKHMYHEASA-L 0.000 description 2
- ZDXPYRJPNDTMRX-UHFFFAOYSA-N glutamine Natural products OC(=O)C(N)CCC(N)=O ZDXPYRJPNDTMRX-UHFFFAOYSA-N 0.000 description 2
- 108020004445 glyceraldehyde-3-phosphate dehydrogenase Proteins 0.000 description 2
- 102000006602 glyceraldehyde-3-phosphate dehydrogenase Human genes 0.000 description 2
- 125000005843 halogen group Chemical group 0.000 description 2
- 210000003494 hepatocyte Anatomy 0.000 description 2
- 125000004474 heteroalkylene group Chemical group 0.000 description 2
- 125000005549 heteroarylene group Chemical group 0.000 description 2
- 125000006588 heterocycloalkylene group Chemical group 0.000 description 2
- 210000004408 hybridoma Anatomy 0.000 description 2
- 239000001257 hydrogen Substances 0.000 description 2
- 230000028993 immune response Effects 0.000 description 2
- 210000000987 immune system Anatomy 0.000 description 2
- 230000002163 immunogen Effects 0.000 description 2
- 230000002998 immunogenetic effect Effects 0.000 description 2
- 229940121354 immunomodulator Drugs 0.000 description 2
- 229940125396 insulin Drugs 0.000 description 2
- 102000006495 integrins Human genes 0.000 description 2
- 108010044426 integrins Proteins 0.000 description 2
- 229940079322 interferon Drugs 0.000 description 2
- 229940074383 interleukin-11 Drugs 0.000 description 2
- 229910052742 iron Inorganic materials 0.000 description 2
- 239000002523 lectin Substances 0.000 description 2
- 125000001909 leucine group Chemical group [H]N(*)C(C(*)=O)C([H])([H])C(C([H])([H])[H])C([H])([H])[H] 0.000 description 2
- 230000000670 limiting effect Effects 0.000 description 2
- 229960003646 lysine Drugs 0.000 description 2
- 125000003588 lysine group Chemical group [H]N([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])(N([H])[H])C(*)=O 0.000 description 2
- 210000003519 mature b lymphocyte Anatomy 0.000 description 2
- 231100000682 maximum tolerated dose Toxicity 0.000 description 2
- 229910001092 metal group alloy Inorganic materials 0.000 description 2
- MYWUZJCMWCOHBA-VIFPVBQESA-N methamphetamine Chemical compound CN[C@@H](C)CC1=CC=CC=C1 MYWUZJCMWCOHBA-VIFPVBQESA-N 0.000 description 2
- 229940126619 mouse monoclonal antibody Drugs 0.000 description 2
- FABPRXSRWADJSP-MEDUHNTESA-N moxifloxacin Chemical compound COC1=C(N2C[C@H]3NCCC[C@H]3C2)C(F)=CC(C(C(C(O)=O)=C2)=O)=C1N2C1CC1 FABPRXSRWADJSP-MEDUHNTESA-N 0.000 description 2
- 229960003702 moxifloxacin Drugs 0.000 description 2
- 235000015097 nutrients Nutrition 0.000 description 2
- 125000004043 oxo group Chemical group O=* 0.000 description 2
- 230000000849 parathyroid Effects 0.000 description 2
- QOFFJEBXNKRSPX-ZDUSSCGKSA-N pemetrexed Chemical compound C1=N[C]2NC(N)=NC(=O)C2=C1CCC1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 QOFFJEBXNKRSPX-ZDUSSCGKSA-N 0.000 description 2
- 229960005079 pemetrexed Drugs 0.000 description 2
- 229960003330 pentetic acid Drugs 0.000 description 2
- 229920001481 poly(stearyl methacrylate) Polymers 0.000 description 2
- 108010064470 polyaspartate Proteins 0.000 description 2
- 230000023603 positive regulation of transcription initiation, DNA-dependent Effects 0.000 description 2
- 229940002612 prodrug Drugs 0.000 description 2
- 239000000651 prodrug Substances 0.000 description 2
- 229940097325 prolactin Drugs 0.000 description 2
- 230000035755 proliferation Effects 0.000 description 2
- 230000001737 promoting effect Effects 0.000 description 2
- 239000012857 radioactive material Substances 0.000 description 2
- 238000010188 recombinant method Methods 0.000 description 2
- 238000011160 research Methods 0.000 description 2
- 230000004044 response Effects 0.000 description 2
- 230000001177 retroviral effect Effects 0.000 description 2
- 230000002441 reversible effect Effects 0.000 description 2
- 238000012552 review Methods 0.000 description 2
- 102220005928 rs121434556 Human genes 0.000 description 2
- 210000002966 serum Anatomy 0.000 description 2
- 230000019491 signal transduction Effects 0.000 description 2
- 230000011664 signaling Effects 0.000 description 2
- 239000002904 solvent Substances 0.000 description 2
- 230000000392 somatic effect Effects 0.000 description 2
- 241000894007 species Species 0.000 description 2
- PJANXHGTPQOBST-UHFFFAOYSA-N stilbene Chemical compound C=1C=CC=CC=1C=CC1=CC=CC=C1 PJANXHGTPQOBST-UHFFFAOYSA-N 0.000 description 2
- 235000021286 stilbenes Nutrition 0.000 description 2
- 125000000547 substituted alkyl group Chemical group 0.000 description 2
- 150000003573 thiols Chemical group 0.000 description 2
- 230000002103 transcriptional effect Effects 0.000 description 2
- 238000001890 transfection Methods 0.000 description 2
- 230000009466 transformation Effects 0.000 description 2
- 230000009261 transgenic effect Effects 0.000 description 2
- 210000004881 tumor cell Anatomy 0.000 description 2
- 238000000108 ultra-filtration Methods 0.000 description 2
- 238000011144 upstream manufacturing Methods 0.000 description 2
- 125000002987 valine group Chemical group [H]N([H])C([H])(C(*)=O)C([H])(C([H])([H])[H])C([H])([H])[H] 0.000 description 2
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 2
- QGVLYPPODPLXMB-UBTYZVCOSA-N (1aR,1bS,4aR,7aS,7bS,8R,9R,9aS)-4a,7b,9,9a-tetrahydroxy-3-(hydroxymethyl)-1,1,6,8-tetramethyl-1,1a,1b,4,4a,7a,7b,8,9,9a-decahydro-5H-cyclopropa[3,4]benzo[1,2-e]azulen-5-one Chemical compound C1=C(CO)C[C@]2(O)C(=O)C(C)=C[C@H]2[C@@]2(O)[C@H](C)[C@@H](O)[C@@]3(O)C(C)(C)[C@H]3[C@@H]21 QGVLYPPODPLXMB-UBTYZVCOSA-N 0.000 description 1
- TZEGAVSWQUEHAQ-QHCPKHFHSA-N (2,3,4,5,6-pentafluorophenyl) (2s)-2-(9h-fluoren-9-ylmethoxycarbonylamino)-3-methylbutanoate Chemical compound O=C([C@@H](NC(=O)OCC1C2=CC=CC=C2C2=CC=CC=C21)C(C)C)OC1=C(F)C(F)=C(F)C(F)=C1F TZEGAVSWQUEHAQ-QHCPKHFHSA-N 0.000 description 1
- SXJJSALQDJZYKA-UHFFFAOYSA-N (2,5-dioxocyclopentyl) 3-(pyridin-2-yldisulfanyl)propanoate Chemical compound O=C1CCC(=O)C1OC(=O)CCSSC1=CC=CC=N1 SXJJSALQDJZYKA-UHFFFAOYSA-N 0.000 description 1
- LQAQWVUOOSWWST-UHFFFAOYSA-N (2,5-dioxopyrrolidin-1-yl) 3-[2-[2-[2-[3-(2,5-dioxopyrrol-1-yl)propanoylamino]ethoxy]ethoxy]ethoxy]propanoate Chemical compound O=C(CCN1C(=O)C=CC1=O)NCCOCCOCCOCCC(=O)ON1C(=O)CCC1=O LQAQWVUOOSWWST-UHFFFAOYSA-N 0.000 description 1
- TZPDZOJURBVWHS-UHFFFAOYSA-N (2,5-dioxopyrrolidin-1-yl) 3-[2-[2-[3-(2,5-dioxopyrrol-1-yl)propanoylamino]ethoxy]ethoxy]propanoate Chemical compound O=C1CCC(=O)N1OC(=O)CCOCCOCCNC(=O)CCN1C(=O)C=CC1=O TZPDZOJURBVWHS-UHFFFAOYSA-N 0.000 description 1
- MFRNYXJJRJQHNW-DEMKXPNLSA-N (2s)-2-[[(2r,3r)-3-methoxy-3-[(2s)-1-[(3r,4s,5s)-3-methoxy-5-methyl-4-[methyl-[(2s)-3-methyl-2-[[(2s)-3-methyl-2-(methylamino)butanoyl]amino]butanoyl]amino]heptanoyl]pyrrolidin-2-yl]-2-methylpropanoyl]amino]-3-phenylpropanoic acid Chemical compound CN[C@@H](C(C)C)C(=O)N[C@@H](C(C)C)C(=O)N(C)[C@@H]([C@@H](C)CC)[C@H](OC)CC(=O)N1CCC[C@H]1[C@H](OC)[C@@H](C)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 MFRNYXJJRJQHNW-DEMKXPNLSA-N 0.000 description 1
- VEGGTWZUZGZKHY-GJZGRUSLSA-N (2s)-2-[[(2s)-2-amino-3-methylbutanoyl]amino]-5-(carbamoylamino)-n-[4-(hydroxymethyl)phenyl]pentanamide Chemical compound NC(=O)NCCC[C@H](NC(=O)[C@@H](N)C(C)C)C(=O)NC1=CC=C(CO)C=C1 VEGGTWZUZGZKHY-GJZGRUSLSA-N 0.000 description 1
- JSXMFBNJRFXRCX-NSHDSACASA-N (2s)-2-amino-3-(4-prop-2-ynoxyphenyl)propanoic acid Chemical group OC(=O)[C@@H](N)CC1=CC=C(OCC#C)C=C1 JSXMFBNJRFXRCX-NSHDSACASA-N 0.000 description 1
- XYZKFPPYAROAPU-JTQLQIEISA-N (2s)-2-amino-3-(4-propanethioylphenyl)propanoic acid Chemical compound CCC(=S)C1=CC=C(C[C@H](N)C(O)=O)C=C1 XYZKFPPYAROAPU-JTQLQIEISA-N 0.000 description 1
- XBTVUBOTGRKKPO-NSHDSACASA-N (2s)-2-amino-3-[4-(2-methylpropanethioyl)phenyl]propanoic acid Chemical compound CC(C)C(=S)C1=CC=C(C[C@H](N)C(O)=O)C=C1 XBTVUBOTGRKKPO-NSHDSACASA-N 0.000 description 1
- NEMHIKRLROONTL-QMMMGPOBSA-N (2s)-2-azaniumyl-3-(4-azidophenyl)propanoate Chemical group OC(=O)[C@@H](N)CC1=CC=C(N=[N+]=[N-])C=C1 NEMHIKRLROONTL-QMMMGPOBSA-N 0.000 description 1
- ZXSBHXZKWRIEIA-JTQLQIEISA-N (2s)-3-(4-acetylphenyl)-2-azaniumylpropanoate Chemical compound CC(=O)C1=CC=C(C[C@H](N)C(O)=O)C=C1 ZXSBHXZKWRIEIA-JTQLQIEISA-N 0.000 description 1
- LOGFVTREOLYCPF-KXNHARMFSA-N (2s,3r)-2-[[(2r)-1-[(2s)-2,6-diaminohexanoyl]pyrrolidine-2-carbonyl]amino]-3-hydroxybutanoic acid Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@H]1CCCN1C(=O)[C@@H](N)CCCCN LOGFVTREOLYCPF-KXNHARMFSA-N 0.000 description 1
- DEQANNDTNATYII-OULOTJBUSA-N (4r,7s,10s,13r,16s,19r)-10-(4-aminobutyl)-19-[[(2r)-2-amino-3-phenylpropanoyl]amino]-16-benzyl-n-[(2r,3r)-1,3-dihydroxybutan-2-yl]-7-[(1r)-1-hydroxyethyl]-13-(1h-indol-3-ylmethyl)-6,9,12,15,18-pentaoxo-1,2-dithia-5,8,11,14,17-pentazacycloicosane-4-carboxa Chemical compound C([C@@H](N)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](CC=2C3=CC=CC=C3NC=2)NC(=O)[C@H](CC=2C=CC=CC=2)NC1=O)C(=O)N[C@H](CO)[C@H](O)C)C1=CC=CC=C1 DEQANNDTNATYII-OULOTJBUSA-N 0.000 description 1
- XIYOPDCBBDCGOE-IWVLMIASSA-N (4s,4ar,5s,5ar,12ar)-4-(dimethylamino)-1,5,10,11,12a-pentahydroxy-6-methylidene-3,12-dioxo-4,4a,5,5a-tetrahydrotetracene-2-carboxamide Chemical compound C=C1C2=CC=CC(O)=C2C(O)=C2[C@@H]1[C@H](O)[C@H]1[C@H](N(C)C)C(=O)C(C(N)=O)=C(O)[C@@]1(O)C2=O XIYOPDCBBDCGOE-IWVLMIASSA-N 0.000 description 1
- FFTVPQUHLQBXQZ-KVUCHLLUSA-N (4s,4as,5ar,12ar)-4,7-bis(dimethylamino)-1,10,11,12a-tetrahydroxy-3,12-dioxo-4a,5,5a,6-tetrahydro-4h-tetracene-2-carboxamide Chemical compound C1C2=C(N(C)C)C=CC(O)=C2C(O)=C2[C@@H]1C[C@H]1[C@H](N(C)C)C(=O)C(C(N)=O)=C(O)[C@@]1(O)C2=O FFTVPQUHLQBXQZ-KVUCHLLUSA-N 0.000 description 1
- FPVKHBSQESCIEP-UHFFFAOYSA-N (8S)-3-(2-deoxy-beta-D-erythro-pentofuranosyl)-3,6,7,8-tetrahydroimidazo[4,5-d][1,3]diazepin-8-ol Natural products C1C(O)C(CO)OC1N1C(NC=NCC2O)=C2N=C1 FPVKHBSQESCIEP-UHFFFAOYSA-N 0.000 description 1
- FDKXTQMXEQVLRF-ZHACJKMWSA-N (E)-dacarbazine Chemical compound CN(C)\N=N\c1[nH]cnc1C(N)=O FDKXTQMXEQVLRF-ZHACJKMWSA-N 0.000 description 1
- LKJPYSCBVHEWIU-KRWDZBQOSA-N (R)-bicalutamide Chemical compound C([C@@](O)(C)C(=O)NC=1C=C(C(C#N)=CC=1)C(F)(F)F)S(=O)(=O)C1=CC=C(F)C=C1 LKJPYSCBVHEWIU-KRWDZBQOSA-N 0.000 description 1
- 108091032973 (ribonucleotides)n+m Proteins 0.000 description 1
- JYEUMXHLPRZUAT-UHFFFAOYSA-N 1,2,3-triazine Chemical compound C1=CN=NN=C1 JYEUMXHLPRZUAT-UHFFFAOYSA-N 0.000 description 1
- NWUYHJFMYQTDRP-UHFFFAOYSA-N 1,2-bis(ethenyl)benzene;1-ethenyl-2-ethylbenzene;styrene Chemical compound C=CC1=CC=CC=C1.CCC1=CC=CC=C1C=C.C=CC1=CC=CC=C1C=C NWUYHJFMYQTDRP-UHFFFAOYSA-N 0.000 description 1
- GEYOCULIXLDCMW-UHFFFAOYSA-N 1,2-phenylenediamine Chemical compound NC1=CC=CC=C1N GEYOCULIXLDCMW-UHFFFAOYSA-N 0.000 description 1
- OWEGMIWEEQEYGQ-UHFFFAOYSA-N 100676-05-9 Natural products OC1C(O)C(O)C(CO)OC1OCC1C(O)C(O)C(O)C(OC2C(OC(O)C(O)C2O)CO)O1 OWEGMIWEEQEYGQ-UHFFFAOYSA-N 0.000 description 1
- FUFLCEKSBBHCMO-UHFFFAOYSA-N 11-dehydrocorticosterone Natural products O=C1CCC2(C)C3C(=O)CC(C)(C(CC4)C(=O)CO)C4C3CCC2=C1 FUFLCEKSBBHCMO-UHFFFAOYSA-N 0.000 description 1
- VILCJCGEZXAXTO-UHFFFAOYSA-N 2,2,2-tetramine Chemical compound NCCNCCNCCN VILCJCGEZXAXTO-UHFFFAOYSA-N 0.000 description 1
- JKMHFZQWWAIEOD-UHFFFAOYSA-N 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid Chemical compound OCC[NH+]1CCN(CCS([O-])(=O)=O)CC1 JKMHFZQWWAIEOD-UHFFFAOYSA-N 0.000 description 1
- GOJUJUVQIVIZAV-UHFFFAOYSA-N 2-amino-4,6-dichloropyrimidine-5-carbaldehyde Chemical group NC1=NC(Cl)=C(C=O)C(Cl)=N1 GOJUJUVQIVIZAV-UHFFFAOYSA-N 0.000 description 1
- AZKSAVLVSZKNRD-UHFFFAOYSA-M 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide Chemical compound [Br-].S1C(C)=C(C)N=C1[N+]1=NC(C=2C=CC=CC=2)=NN1C1=CC=CC=C1 AZKSAVLVSZKNRD-UHFFFAOYSA-M 0.000 description 1
- AOJJSUZBOXZQNB-VTZDEGQISA-N 4'-epidoxorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@@H](O)[C@H](C)O1 AOJJSUZBOXZQNB-VTZDEGQISA-N 0.000 description 1
- 101710169336 5'-deoxyadenosine deaminase Proteins 0.000 description 1
- NMUSYJAQQFHJEW-UHFFFAOYSA-N 5-Azacytidine Natural products O=C1N=C(N)N=CN1C1C(O)C(O)C(CO)O1 NMUSYJAQQFHJEW-UHFFFAOYSA-N 0.000 description 1
- XAUDJQYHKZQPEU-KVQBGUIXSA-N 5-aza-2'-deoxycytidine Chemical compound O=C1N=C(N)N=CN1[C@@H]1O[C@H](CO)[C@@H](O)C1 XAUDJQYHKZQPEU-KVQBGUIXSA-N 0.000 description 1
- DALMAZHDNFCDRP-VMPREFPWSA-N 9h-fluoren-9-ylmethyl n-[(2s)-1-[[(2s)-5-(carbamoylamino)-1-[4-(hydroxymethyl)anilino]-1-oxopentan-2-yl]amino]-3-methyl-1-oxobutan-2-yl]carbamate Chemical compound O=C([C@H](CCCNC(N)=O)NC(=O)[C@@H](NC(=O)OCC1C2=CC=CC=C2C2=CC=CC=C21)C(C)C)NC1=CC=C(CO)C=C1 DALMAZHDNFCDRP-VMPREFPWSA-N 0.000 description 1
- 108091022885 ADAM Proteins 0.000 description 1
- 102000029791 ADAM Human genes 0.000 description 1
- 102000007469 Actins Human genes 0.000 description 1
- 108010085238 Actins Proteins 0.000 description 1
- ORILYTVJVMAKLC-UHFFFAOYSA-N Adamantane Natural products C1C(C2)CC3CC1CC2C3 ORILYTVJVMAKLC-UHFFFAOYSA-N 0.000 description 1
- 102100036664 Adenosine deaminase Human genes 0.000 description 1
- 241000256118 Aedes aegypti Species 0.000 description 1
- 241000256173 Aedes albopictus Species 0.000 description 1
- 102100027211 Albumin Human genes 0.000 description 1
- 108010088751 Albumins Proteins 0.000 description 1
- 102000009027 Albumins Human genes 0.000 description 1
- 101710187573 Alcohol dehydrogenase 2 Proteins 0.000 description 1
- 101710133776 Alcohol dehydrogenase class-3 Proteins 0.000 description 1
- 108700028369 Alleles Proteins 0.000 description 1
- 102100023635 Alpha-fetoprotein Human genes 0.000 description 1
- 108010079054 Amyloid beta-Protein Precursor Proteins 0.000 description 1
- 102000014303 Amyloid beta-Protein Precursor Human genes 0.000 description 1
- 108010083359 Antigen Receptors Proteins 0.000 description 1
- 102000006306 Antigen Receptors Human genes 0.000 description 1
- 108020000948 Antisense Oligonucleotides Proteins 0.000 description 1
- 108091023037 Aptamer Proteins 0.000 description 1
- CIWBSHSKHKDKBQ-JLAZNSOCSA-N Ascorbic acid Chemical compound OC[C@H](O)[C@H]1OC(=O)C(O)=C1O CIWBSHSKHKDKBQ-JLAZNSOCSA-N 0.000 description 1
- 241000228212 Aspergillus Species 0.000 description 1
- 241000351920 Aspergillus nidulans Species 0.000 description 1
- 241000228245 Aspergillus niger Species 0.000 description 1
- 241000972773 Aulopiformes Species 0.000 description 1
- 241001203868 Autographa californica Species 0.000 description 1
- 241000713842 Avian sarcoma virus Species 0.000 description 1
- 108090001008 Avidin Proteins 0.000 description 1
- 241000193830 Bacillus <bacterium> Species 0.000 description 1
- 241000194108 Bacillus licheniformis Species 0.000 description 1
- 235000014469 Bacillus subtilis Nutrition 0.000 description 1
- DWRXFEITVBNRMK-UHFFFAOYSA-N Beta-D-1-Arabinofuranosylthymine Natural products O=C1NC(=O)C(C)=CN1C1C(O)C(O)C(CO)O1 DWRXFEITVBNRMK-UHFFFAOYSA-N 0.000 description 1
- 108010006654 Bleomycin Proteins 0.000 description 1
- 241000255789 Bombyx mori Species 0.000 description 1
- 241000409811 Bombyx mori nucleopolyhedrovirus Species 0.000 description 1
- 206010006187 Breast cancer Diseases 0.000 description 1
- 239000002126 C01EB10 - Adenosine Substances 0.000 description 1
- 108091016585 CD44 antigen Proteins 0.000 description 1
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 1
- 241000222122 Candida albicans Species 0.000 description 1
- 241000282465 Canis Species 0.000 description 1
- 208000024172 Cardiovascular disease Diseases 0.000 description 1
- 102000004225 Cathepsin B Human genes 0.000 description 1
- 108090000712 Cathepsin B Proteins 0.000 description 1
- 102000004171 Cathepsin K Human genes 0.000 description 1
- 108090000625 Cathepsin K Proteins 0.000 description 1
- 102000005600 Cathepsins Human genes 0.000 description 1
- 108010084457 Cathepsins Proteins 0.000 description 1
- 229910052684 Cerium Inorganic materials 0.000 description 1
- 206010008342 Cervix carcinoma Diseases 0.000 description 1
- JWBOIMRXGHLCPP-UHFFFAOYSA-N Chloditan Chemical compound C=1C=CC=C(Cl)C=1C(C(Cl)Cl)C1=CC=C(Cl)C=C1 JWBOIMRXGHLCPP-UHFFFAOYSA-N 0.000 description 1
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 description 1
- 241000282552 Chlorocebus aethiops Species 0.000 description 1
- 108090000746 Chymosin Proteins 0.000 description 1
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 1
- MFYSYFVPBJMHGN-ZPOLXVRWSA-N Cortisone Chemical compound O=C1CC[C@]2(C)[C@H]3C(=O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 MFYSYFVPBJMHGN-ZPOLXVRWSA-N 0.000 description 1
- MFYSYFVPBJMHGN-UHFFFAOYSA-N Cortisone Natural products O=C1CCC2(C)C3C(=O)CC(C)(C(CC4)(O)C(=O)CO)C4C3CCC2=C1 MFYSYFVPBJMHGN-UHFFFAOYSA-N 0.000 description 1
- 229920000742 Cotton Polymers 0.000 description 1
- 241000699800 Cricetinae Species 0.000 description 1
- CMSMOCZEIVJLDB-UHFFFAOYSA-N Cyclophosphamide Chemical compound ClCCN(CCCl)P1(=O)NCCCO1 CMSMOCZEIVJLDB-UHFFFAOYSA-N 0.000 description 1
- 102100030497 Cytochrome c Human genes 0.000 description 1
- 108010075031 Cytochromes c Proteins 0.000 description 1
- QNAYBMKLOCPYGJ-UWTATZPHSA-N D-alanine Chemical compound C[C@@H](N)C(O)=O QNAYBMKLOCPYGJ-UWTATZPHSA-N 0.000 description 1
- 108090000626 DNA-directed RNA polymerases Proteins 0.000 description 1
- 102000004163 DNA-directed RNA polymerases Human genes 0.000 description 1
- 108010019673 Darbepoetin alfa Proteins 0.000 description 1
- ZBNZXTGUTAYRHI-UHFFFAOYSA-N Dasatinib Chemical compound C=1C(N2CCN(CCO)CC2)=NC(C)=NC=1NC(S1)=NC=C1C(=O)NC1=C(C)C=CC=C1Cl ZBNZXTGUTAYRHI-UHFFFAOYSA-N 0.000 description 1
- WEAHRLBPCANXCN-UHFFFAOYSA-N Daunomycin Natural products CCC1(O)CC(OC2CC(N)C(O)C(C)O2)c3cc4C(=O)c5c(OC)cccc5C(=O)c4c(O)c3C1 WEAHRLBPCANXCN-UHFFFAOYSA-N 0.000 description 1
- 102000007260 Deoxyribonuclease I Human genes 0.000 description 1
- 108010008532 Deoxyribonuclease I Proteins 0.000 description 1
- BWGNESOTFCXPMA-UHFFFAOYSA-N Dihydrogen disulfide Chemical compound SS BWGNESOTFCXPMA-UHFFFAOYSA-N 0.000 description 1
- 102000016607 Diphtheria Toxin Human genes 0.000 description 1
- 108010053187 Diphtheria Toxin Proteins 0.000 description 1
- 241000255601 Drosophila melanogaster Species 0.000 description 1
- 206010059866 Drug resistance Diseases 0.000 description 1
- 229910052692 Dysprosium Inorganic materials 0.000 description 1
- 102100037024 E3 ubiquitin-protein ligase XIAP Human genes 0.000 description 1
- 241000588914 Enterobacter Species 0.000 description 1
- 241000588921 Enterobacteriaceae Species 0.000 description 1
- 102400001368 Epidermal growth factor Human genes 0.000 description 1
- 101800003838 Epidermal growth factor Proteins 0.000 description 1
- YQYJSBFKSSDGFO-UHFFFAOYSA-N Epihygromycin Natural products OC1C(O)C(C(=O)C)OC1OC(C(=C1)O)=CC=C1C=C(C)C(=O)NC1C(O)C(O)C2OCOC2C1O YQYJSBFKSSDGFO-UHFFFAOYSA-N 0.000 description 1
- HTIJFSOGRVMCQR-UHFFFAOYSA-N Epirubicin Natural products COc1cccc2C(=O)c3c(O)c4CC(O)(CC(OC5CC(N)C(=O)C(C)O5)c4c(O)c3C(=O)c12)C(=O)CO HTIJFSOGRVMCQR-UHFFFAOYSA-N 0.000 description 1
- 108010074604 Epoetin Alfa Proteins 0.000 description 1
- 229910052691 Erbium Inorganic materials 0.000 description 1
- 241000588698 Erwinia Species 0.000 description 1
- 241000588722 Escherichia Species 0.000 description 1
- 241001522878 Escherichia coli B Species 0.000 description 1
- 241001302584 Escherichia coli str. K-12 substr. W3110 Species 0.000 description 1
- 108010008165 Etanercept Proteins 0.000 description 1
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 1
- 229910052693 Europium Inorganic materials 0.000 description 1
- HKVAMNSJSFKALM-GKUWKFKPSA-N Everolimus Chemical compound C1C[C@@H](OCCO)[C@H](OC)C[C@@H]1C[C@@H](C)[C@H]1OC(=O)[C@@H]2CCCCN2C(=O)C(=O)[C@](O)(O2)[C@H](C)CC[C@H]2C[C@H](OC)/C(C)=C/C=C/C=C/[C@@H](C)C[C@@H](C)C(=O)[C@H](OC)[C@H](O)/C(C)=C/[C@@H](C)C(=O)C1 HKVAMNSJSFKALM-GKUWKFKPSA-N 0.000 description 1
- 108010037362 Extracellular Matrix Proteins Proteins 0.000 description 1
- 102000010834 Extracellular Matrix Proteins Human genes 0.000 description 1
- 108010000196 Factor XIIIa Proteins 0.000 description 1
- 108010088842 Fibrinolysin Proteins 0.000 description 1
- 241000700662 Fowlpox virus Species 0.000 description 1
- VWUXBMIQPBEWFH-WCCTWKNTSA-N Fulvestrant Chemical compound OC1=CC=C2[C@H]3CC[C@](C)([C@H](CC4)O)[C@@H]4[C@@H]3[C@H](CCCCCCCCCS(=O)CCCC(F)(F)C(F)(F)F)CC2=C1 VWUXBMIQPBEWFH-WCCTWKNTSA-N 0.000 description 1
- GYHNNYVSQQEPJS-UHFFFAOYSA-N Gallium Chemical compound [Ga] GYHNNYVSQQEPJS-UHFFFAOYSA-N 0.000 description 1
- 108700039691 Genetic Promoter Regions Proteins 0.000 description 1
- 229930182566 Gentamicin Natural products 0.000 description 1
- CEAZRRDELHUEMR-URQXQFDESA-N Gentamicin Chemical compound O1[C@H](C(C)NC)CC[C@@H](N)[C@H]1O[C@H]1[C@H](O)[C@@H](O[C@@H]2[C@@H]([C@@H](NC)[C@@](C)(O)CO2)O)[C@H](N)C[C@@H]1N CEAZRRDELHUEMR-URQXQFDESA-N 0.000 description 1
- 108010073178 Glucan 1,4-alpha-Glucosidase Proteins 0.000 description 1
- 102100022624 Glucoamylase Human genes 0.000 description 1
- 102000030595 Glucokinase Human genes 0.000 description 1
- 108010021582 Glucokinase Proteins 0.000 description 1
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 1
- 239000004366 Glucose oxidase Substances 0.000 description 1
- 108010015776 Glucose oxidase Proteins 0.000 description 1
- 244000299507 Gossypium hirsutum Species 0.000 description 1
- ZRALSGWEFCBTJO-UHFFFAOYSA-N Guanidine Chemical group NC(N)=N ZRALSGWEFCBTJO-UHFFFAOYSA-N 0.000 description 1
- 239000007995 HEPES buffer Substances 0.000 description 1
- 102000025850 HLA-A2 Antigen Human genes 0.000 description 1
- 108010074032 HLA-A2 Antigen Proteins 0.000 description 1
- 102000006354 HLA-DR Antigens Human genes 0.000 description 1
- 108010058597 HLA-DR Antigens Proteins 0.000 description 1
- 101100082540 Haemophilus influenzae (strain ATCC 51907 / DSM 11121 / KW20 / Rd) pcp gene Proteins 0.000 description 1
- 102100031573 Hematopoietic progenitor cell antigen CD34 Human genes 0.000 description 1
- 102000008055 Heparan Sulfate Proteoglycans Human genes 0.000 description 1
- 229920002971 Heparan sulfate Polymers 0.000 description 1
- HTTJABKRGRZYRN-UHFFFAOYSA-N Heparin Chemical compound OC1C(NC(=O)C)C(O)OC(COS(O)(=O)=O)C1OC1C(OS(O)(=O)=O)C(O)C(OC2C(C(OS(O)(=O)=O)C(OC3C(C(O)C(O)C(O3)C(O)=O)OS(O)(=O)=O)C(CO)O2)NS(O)(=O)=O)C(C(O)=O)O1 HTTJABKRGRZYRN-UHFFFAOYSA-N 0.000 description 1
- 241000700721 Hepatitis B virus Species 0.000 description 1
- 208000009889 Herpes Simplex Diseases 0.000 description 1
- 102000005548 Hexokinase Human genes 0.000 description 1
- 108700040460 Hexokinases Proteins 0.000 description 1
- 229910052689 Holmium Inorganic materials 0.000 description 1
- 241000282412 Homo Species 0.000 description 1
- 101000971171 Homo sapiens Apoptosis regulator Bcl-2 Proteins 0.000 description 1
- 101100390517 Homo sapiens FCER2 gene Proteins 0.000 description 1
- 101000777663 Homo sapiens Hematopoietic progenitor cell antigen CD34 Proteins 0.000 description 1
- 101100346929 Homo sapiens MUC1 gene Proteins 0.000 description 1
- 108010001336 Horseradish Peroxidase Proteins 0.000 description 1
- 108091006905 Human Serum Albumin Proteins 0.000 description 1
- 102000008100 Human Serum Albumin Human genes 0.000 description 1
- 241000701109 Human adenovirus 2 Species 0.000 description 1
- 241000701024 Human betaherpesvirus 5 Species 0.000 description 1
- 241000713772 Human immunodeficiency virus 1 Species 0.000 description 1
- 206010020843 Hyperthermia Diseases 0.000 description 1
- 102000009438 IgE Receptors Human genes 0.000 description 1
- 108700005091 Immunoglobulin Genes Proteins 0.000 description 1
- 102000014150 Interferons Human genes 0.000 description 1
- 108010050904 Interferons Proteins 0.000 description 1
- 102000000589 Interleukin-1 Human genes 0.000 description 1
- 108010002352 Interleukin-1 Proteins 0.000 description 1
- 108010038484 Interleukin-5 Receptors Proteins 0.000 description 1
- 102000010786 Interleukin-5 Receptors Human genes 0.000 description 1
- 101000913968 Ipomoea purpurea Chalcone synthase C Proteins 0.000 description 1
- 241000588748 Klebsiella Species 0.000 description 1
- 241000235058 Komagataella pastoris Species 0.000 description 1
- ONIBWKKTOPOVIA-BYPYZUCNSA-N L-Proline Chemical compound OC(=O)[C@@H]1CCCN1 ONIBWKKTOPOVIA-BYPYZUCNSA-N 0.000 description 1
- RHGKLRLOHDJJDR-BYPYZUCNSA-N L-citrulline Chemical compound NC(=O)NCCC[C@H]([NH3+])C([O-])=O RHGKLRLOHDJJDR-BYPYZUCNSA-N 0.000 description 1
- FFEARJCKVFRZRR-BYPYZUCNSA-N L-methionine Chemical group CSCC[C@H](N)C(O)=O FFEARJCKVFRZRR-BYPYZUCNSA-N 0.000 description 1
- SXTAYKAGBXMACB-DPVSGNNYSA-N L-methionine sulfoximine Chemical compound CS(=N)(=O)CC[C@H](N)C(O)=O SXTAYKAGBXMACB-DPVSGNNYSA-N 0.000 description 1
- 125000002435 L-phenylalanyl group Chemical group O=C([*])[C@](N([H])[H])([H])C([H])([H])C1=C([H])C([H])=C([H])C([H])=C1[H] 0.000 description 1
- 125000000174 L-prolyl group Chemical group [H]N1C([H])([H])C([H])([H])C([H])([H])[C@@]1([H])C(*)=O 0.000 description 1
- 125000000510 L-tryptophano group Chemical group [H]C1=C([H])C([H])=C2N([H])C([H])=C(C([H])([H])[C@@]([H])(C(O[H])=O)N([H])[*])C2=C1[H] 0.000 description 1
- 239000005551 L01XE03 - Erlotinib Substances 0.000 description 1
- 239000002067 L01XE06 - Dasatinib Substances 0.000 description 1
- 239000002136 L01XE07 - Lapatinib Substances 0.000 description 1
- 241000481961 Lachancea thermotolerans Species 0.000 description 1
- 241000235651 Lachancea waltii Species 0.000 description 1
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 1
- XNRVGTHNYCNCFF-UHFFFAOYSA-N Lapatinib ditosylate monohydrate Chemical compound O.CC1=CC=C(S(O)(=O)=O)C=C1.CC1=CC=C(S(O)(=O)=O)C=C1.O1C(CNCCS(=O)(=O)C)=CC=C1C1=CC=C(N=CN=C2NC=3C=C(Cl)C(OCC=4C=C(F)C=CC=4)=CC=3)C2=C1 XNRVGTHNYCNCFF-UHFFFAOYSA-N 0.000 description 1
- GQYIWUVLTXOXAJ-UHFFFAOYSA-N Lomustine Chemical compound ClCCN(N=O)C(=O)NC1CCCCC1 GQYIWUVLTXOXAJ-UHFFFAOYSA-N 0.000 description 1
- 101710109756 Low affinity immunoglobulin epsilon Fc receptor Proteins 0.000 description 1
- 235000007688 Lycopersicon esculentum Nutrition 0.000 description 1
- 102000043129 MHC class I family Human genes 0.000 description 1
- 108091054437 MHC class I family Proteins 0.000 description 1
- 239000002616 MRI contrast agent Substances 0.000 description 1
- 241000282567 Macaca fascicularis Species 0.000 description 1
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 1
- GUBGYTABKSRVRQ-PICCSMPSSA-N Maltose Natural products O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@@H](CO)OC(O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-PICCSMPSSA-N 0.000 description 1
- 102000000424 Matrix Metalloproteinase 2 Human genes 0.000 description 1
- 108010016165 Matrix Metalloproteinase 2 Proteins 0.000 description 1
- 102000002274 Matrix Metalloproteinases Human genes 0.000 description 1
- 108010000684 Matrix Metalloproteinases Proteins 0.000 description 1
- 102000001776 Matrix metalloproteinase-9 Human genes 0.000 description 1
- 108010015302 Matrix metalloproteinase-9 Proteins 0.000 description 1
- 241000219828 Medicago truncatula Species 0.000 description 1
- 102000018697 Membrane Proteins Human genes 0.000 description 1
- 108010052285 Membrane Proteins Proteins 0.000 description 1
- XOGTZOOQQBDUSI-UHFFFAOYSA-M Mesna Chemical compound [Na+].[O-]S(=O)(=O)CCS XOGTZOOQQBDUSI-UHFFFAOYSA-M 0.000 description 1
- FQISKWAFAHGMGT-SGJOWKDISA-M Methylprednisolone sodium succinate Chemical compound [Na+].C([C@@]12C)=CC(=O)C=C1[C@@H](C)C[C@@H]1[C@@H]2[C@@H](O)C[C@]2(C)[C@@](O)(C(=O)COC(=O)CCC([O-])=O)CC[C@H]21 FQISKWAFAHGMGT-SGJOWKDISA-M 0.000 description 1
- 101100508818 Mus musculus Inpp5k gene Proteins 0.000 description 1
- 101000596538 Mus musculus THAP domain-containing protein 11 Proteins 0.000 description 1
- 241000699670 Mus sp. Species 0.000 description 1
- 102100030856 Myoglobin Human genes 0.000 description 1
- 108010062374 Myoglobin Proteins 0.000 description 1
- ZDZOTLJHXYCWBA-VCVYQWHSSA-N N-debenzoyl-N-(tert-butoxycarbonyl)-10-deacetyltaxol Chemical compound O([C@H]1[C@H]2[C@@](C([C@H](O)C3=C(C)[C@@H](OC(=O)[C@H](O)[C@@H](NC(=O)OC(C)(C)C)C=4C=CC=CC=4)C[C@]1(O)C3(C)C)=O)(C)[C@@H](O)C[C@H]1OC[C@]12OC(=O)C)C(=O)C1=CC=CC=C1 ZDZOTLJHXYCWBA-VCVYQWHSSA-N 0.000 description 1
- RHGKLRLOHDJJDR-UHFFFAOYSA-N Ndelta-carbamoyl-DL-ornithine Natural products OC(=O)C(N)CCCNC(N)=O RHGKLRLOHDJJDR-UHFFFAOYSA-N 0.000 description 1
- 229910052779 Neodymium Inorganic materials 0.000 description 1
- 241001181114 Neta Species 0.000 description 1
- 241000221960 Neurospora Species 0.000 description 1
- 241000221961 Neurospora crassa Species 0.000 description 1
- 244000061176 Nicotiana tabacum Species 0.000 description 1
- 235000002637 Nicotiana tabacum Nutrition 0.000 description 1
- 208000015914 Non-Hodgkin lymphomas Diseases 0.000 description 1
- 108010016076 Octreotide Proteins 0.000 description 1
- 108091034117 Oligonucleotide Proteins 0.000 description 1
- 108020005187 Oligonucleotide Probes Proteins 0.000 description 1
- 102100021079 Ornithine decarboxylase Human genes 0.000 description 1
- 108700005126 Ornithine decarboxylases Proteins 0.000 description 1
- 241000283973 Oryctolagus cuniculus Species 0.000 description 1
- 208000001132 Osteoporosis Diseases 0.000 description 1
- 239000004100 Oxytetracycline Substances 0.000 description 1
- 229910019142 PO4 Inorganic materials 0.000 description 1
- 108010087702 Penicillinase Proteins 0.000 description 1
- 241000228143 Penicillium Species 0.000 description 1
- 108091093037 Peptide nucleic acid Proteins 0.000 description 1
- 101000907988 Petunia hybrida Chalcone-flavanone isomerase C Proteins 0.000 description 1
- 240000007377 Petunia x hybrida Species 0.000 description 1
- 244000046052 Phaseolus vulgaris Species 0.000 description 1
- 235000010627 Phaseolus vulgaris Nutrition 0.000 description 1
- 102000001105 Phosphofructokinases Human genes 0.000 description 1
- 108010069341 Phosphofructokinases Proteins 0.000 description 1
- 102000012288 Phosphopyruvate Hydratase Human genes 0.000 description 1
- 108010022181 Phosphopyruvate Hydratase Proteins 0.000 description 1
- 108091000080 Phosphotransferase Proteins 0.000 description 1
- 108010004729 Phycoerythrin Proteins 0.000 description 1
- 241000709664 Picornaviridae Species 0.000 description 1
- 206010035226 Plasma cell myeloma Diseases 0.000 description 1
- 108010001014 Plasminogen Activators Proteins 0.000 description 1
- 102000001938 Plasminogen Activators Human genes 0.000 description 1
- 108091036407 Polyadenylation Proteins 0.000 description 1
- 108010039918 Polylysine Proteins 0.000 description 1
- 241001505332 Polyomavirus sp. Species 0.000 description 1
- 229910052777 Praseodymium Inorganic materials 0.000 description 1
- TUZYXOIXSAXUGO-UHFFFAOYSA-N Pravastatin Natural products C1=CC(C)C(CCC(O)CC(O)CC(O)=O)C2C(OC(=O)C(C)CC)CC(O)C=C21 TUZYXOIXSAXUGO-UHFFFAOYSA-N 0.000 description 1
- 101710117018 Prolactin receptor Proteins 0.000 description 1
- 229940124158 Protease/peptidase inhibitor Drugs 0.000 description 1
- 101800004937 Protein C Proteins 0.000 description 1
- 102000017975 Protein C Human genes 0.000 description 1
- 101710149951 Protein Tat Proteins 0.000 description 1
- 102000004022 Protein-Tyrosine Kinases Human genes 0.000 description 1
- 108090000412 Protein-Tyrosine Kinases Proteins 0.000 description 1
- 102000016611 Proteoglycans Human genes 0.000 description 1
- 108010067787 Proteoglycans Proteins 0.000 description 1
- 101000762949 Pseudomonas aeruginosa (strain ATCC 15692 / DSM 22644 / CIP 104116 / JCM 14847 / LMG 12228 / 1C / PRS 101 / PAO1) Exotoxin A Proteins 0.000 description 1
- 101100084022 Pseudomonas aeruginosa (strain ATCC 15692 / DSM 22644 / CIP 104116 / JCM 14847 / LMG 12228 / 1C / PRS 101 / PAO1) lapA gene Proteins 0.000 description 1
- 102000013009 Pyruvate Kinase Human genes 0.000 description 1
- 108020005115 Pyruvate Kinase Proteins 0.000 description 1
- 239000012980 RPMI-1640 medium Substances 0.000 description 1
- 102000004879 Racemases and epimerases Human genes 0.000 description 1
- 108090001066 Racemases and epimerases Proteins 0.000 description 1
- 241000700157 Rattus norvegicus Species 0.000 description 1
- 101100366438 Rattus norvegicus Sphkap gene Proteins 0.000 description 1
- 108091081062 Repeated sequence (DNA) Proteins 0.000 description 1
- 241000714474 Rous sarcoma virus Species 0.000 description 1
- 241000235070 Saccharomyces Species 0.000 description 1
- 229910052772 Samarium Inorganic materials 0.000 description 1
- 101800001700 Saposin-D Proteins 0.000 description 1
- 241000235347 Schizosaccharomyces pombe Species 0.000 description 1
- 241001123650 Schwanniomyces occidentalis Species 0.000 description 1
- 229920002684 Sepharose Polymers 0.000 description 1
- 241000607715 Serratia marcescens Species 0.000 description 1
- 241000607768 Shigella Species 0.000 description 1
- 101710084578 Short neurotoxin 1 Proteins 0.000 description 1
- 108010047827 Sialic Acid Binding Immunoglobulin-like Lectins Proteins 0.000 description 1
- 102000007073 Sialic Acid Binding Immunoglobulin-like Lectins Human genes 0.000 description 1
- BQCADISMDOOEFD-UHFFFAOYSA-N Silver Chemical compound [Ag] BQCADISMDOOEFD-UHFFFAOYSA-N 0.000 description 1
- 241000700584 Simplexvirus Species 0.000 description 1
- 108091027967 Small hairpin RNA Proteins 0.000 description 1
- KEAYESYHFKHZAL-UHFFFAOYSA-N Sodium Chemical compound [Na] KEAYESYHFKHZAL-UHFFFAOYSA-N 0.000 description 1
- VMHLLURERBWHNL-UHFFFAOYSA-M Sodium acetate Chemical compound [Na+].CC([O-])=O VMHLLURERBWHNL-UHFFFAOYSA-M 0.000 description 1
- 240000003768 Solanum lycopersicum Species 0.000 description 1
- 244000061456 Solanum tuberosum Species 0.000 description 1
- 235000002595 Solanum tuberosum Nutrition 0.000 description 1
- 108010023197 Streptokinase Proteins 0.000 description 1
- 241000187747 Streptomyces Species 0.000 description 1
- 108090000054 Syndecan-2 Proteins 0.000 description 1
- 230000006044 T cell activation Effects 0.000 description 1
- 230000033540 T cell apoptotic process Effects 0.000 description 1
- 108091008874 T cell receptors Proteins 0.000 description 1
- 102000016266 T-Cell Antigen Receptors Human genes 0.000 description 1
- 241000255588 Tephritidae Species 0.000 description 1
- 229910052771 Terbium Inorganic materials 0.000 description 1
- 108700031126 Tetraspanins Proteins 0.000 description 1
- 102000043977 Tetraspanins Human genes 0.000 description 1
- FZWLAAWBMGSTSO-UHFFFAOYSA-N Thiazole Chemical compound C1=CSC=N1 FZWLAAWBMGSTSO-UHFFFAOYSA-N 0.000 description 1
- 108090000190 Thrombin Proteins 0.000 description 1
- 108010000499 Thromboplastin Proteins 0.000 description 1
- 102000002262 Thromboplastin Human genes 0.000 description 1
- 229910052775 Thulium Inorganic materials 0.000 description 1
- 102000003978 Tissue Plasminogen Activator Human genes 0.000 description 1
- 108090000373 Tissue Plasminogen Activator Proteins 0.000 description 1
- 241001149964 Tolypocladium Species 0.000 description 1
- 101710120037 Toxin CcdB Proteins 0.000 description 1
- 101710182532 Toxin a Proteins 0.000 description 1
- 102000040945 Transcription factor Human genes 0.000 description 1
- 102000004338 Transferrin Human genes 0.000 description 1
- 108090000901 Transferrin Proteins 0.000 description 1
- 108700019146 Transgenes Proteins 0.000 description 1
- 102100023935 Transmembrane glycoprotein NMB Human genes 0.000 description 1
- 102000005924 Triose-Phosphate Isomerase Human genes 0.000 description 1
- 108700015934 Triose-phosphate isomerases Proteins 0.000 description 1
- 108090000631 Trypsin Proteins 0.000 description 1
- 102000004142 Trypsin Human genes 0.000 description 1
- 108091023045 Untranslated Region Proteins 0.000 description 1
- 208000006105 Uterine Cervical Neoplasms Diseases 0.000 description 1
- 244000000188 Vaccinium ovalifolium Species 0.000 description 1
- JXLYSJRDGCGARV-WWYNWVTFSA-N Vinblastine Natural products O=C(O[C@H]1[C@](O)(C(=O)OC)[C@@H]2N(C)c3c(cc(c(OC)c3)[C@]3(C(=O)OC)c4[nH]c5c(c4CCN4C[C@](O)(CC)C[C@H](C3)C4)cccc5)[C@@]32[C@H]2[C@@]1(CC)C=CCN2CC3)C JXLYSJRDGCGARV-WWYNWVTFSA-N 0.000 description 1
- 108020005202 Viral DNA Proteins 0.000 description 1
- IXKSXJFAGXLQOQ-XISFHERQSA-N WHWLQLKPGQPMY Chemical compound C([C@@H](C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N1CCC[C@H]1C(=O)NCC(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(O)=O)NC(=O)[C@@H](N)CC=1C2=CC=CC=C2NC=1)C1=CNC=N1 IXKSXJFAGXLQOQ-XISFHERQSA-N 0.000 description 1
- 108700031544 X-Linked Inhibitor of Apoptosis Proteins 0.000 description 1
- 238000002441 X-ray diffraction Methods 0.000 description 1
- 241000235013 Yarrowia Species 0.000 description 1
- 240000008042 Zea mays Species 0.000 description 1
- 235000005824 Zea mays ssp. parviglumis Nutrition 0.000 description 1
- 235000002017 Zea mays subsp mays Nutrition 0.000 description 1
- IEDXPSOJFSVCKU-HOKPPMCLSA-N [4-[[(2S)-5-(carbamoylamino)-2-[[(2S)-2-[6-(2,5-dioxopyrrolidin-1-yl)hexanoylamino]-3-methylbutanoyl]amino]pentanoyl]amino]phenyl]methyl N-[(2S)-1-[[(2S)-1-[[(3R,4S,5S)-1-[(2S)-2-[(1R,2R)-3-[[(1S,2R)-1-hydroxy-1-phenylpropan-2-yl]amino]-1-methoxy-2-methyl-3-oxopropyl]pyrrolidin-1-yl]-3-methoxy-5-methyl-1-oxoheptan-4-yl]-methylamino]-3-methyl-1-oxobutan-2-yl]amino]-3-methyl-1-oxobutan-2-yl]-N-methylcarbamate Chemical compound CC[C@H](C)[C@@H]([C@@H](CC(=O)N1CCC[C@H]1[C@H](OC)[C@@H](C)C(=O)N[C@H](C)[C@@H](O)c1ccccc1)OC)N(C)C(=O)[C@@H](NC(=O)[C@H](C(C)C)N(C)C(=O)OCc1ccc(NC(=O)[C@H](CCCNC(N)=O)NC(=O)[C@@H](NC(=O)CCCCCN2C(=O)CCC2=O)C(C)C)cc1)C(C)C IEDXPSOJFSVCKU-HOKPPMCLSA-N 0.000 description 1
- QTBSBXVTEAMEQO-UHFFFAOYSA-N acetic acid Substances CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 1
- 125000002339 acetoacetyl group Chemical group O=C([*])C([H])([H])C(=O)C([H])([H])[H] 0.000 description 1
- 239000012190 activator Substances 0.000 description 1
- 125000002015 acyclic group Chemical group 0.000 description 1
- 229950009084 adecatumumab Drugs 0.000 description 1
- 229960005305 adenosine Drugs 0.000 description 1
- 230000002411 adverse Effects 0.000 description 1
- 230000009824 affinity maturation Effects 0.000 description 1
- 230000002776 aggregation Effects 0.000 description 1
- 238000004220 aggregation Methods 0.000 description 1
- 125000003172 aldehyde group Chemical group 0.000 description 1
- 150000001336 alkenes Chemical group 0.000 description 1
- 125000003545 alkoxy group Chemical group 0.000 description 1
- 229940100198 alkylating agent Drugs 0.000 description 1
- 239000002168 alkylating agent Substances 0.000 description 1
- SHGAZHPCJJPHSC-YCNIQYBTSA-N all-trans-retinoic acid Chemical compound OC(=O)\C=C(/C)\C=C\C=C(/C)\C=C\C1=C(C)CCCC1(C)C SHGAZHPCJJPHSC-YCNIQYBTSA-N 0.000 description 1
- 208000026935 allergic disease Diseases 0.000 description 1
- WNNNWFKQCKFSDK-UHFFFAOYSA-N allylglycine Chemical group OC(=O)C(N)CC=C WNNNWFKQCKFSDK-UHFFFAOYSA-N 0.000 description 1
- WQZGKKKJIJFFOK-PHYPRBDBSA-N alpha-D-galactose Chemical compound OC[C@H]1O[C@H](O)[C@H](O)[C@@H](O)[C@H]1O WQZGKKKJIJFFOK-PHYPRBDBSA-N 0.000 description 1
- 108010026331 alpha-Fetoproteins Proteins 0.000 description 1
- 230000004075 alteration Effects 0.000 description 1
- 229960000473 altretamine Drugs 0.000 description 1
- 229950010817 alvocidib Drugs 0.000 description 1
- BIIVYFLTOXDAOV-YVEFUNNKSA-N alvocidib Chemical compound O[C@@H]1CN(C)CC[C@@H]1C1=C(O)C=C(O)C2=C1OC(C=1C(=CC=CC=1)Cl)=CC2=O BIIVYFLTOXDAOV-YVEFUNNKSA-N 0.000 description 1
- 150000001412 amines Chemical class 0.000 description 1
- 238000012870 ammonium sulfate precipitation Methods 0.000 description 1
- 229960000723 ampicillin Drugs 0.000 description 1
- AVKUERGKIZMTKX-NJBDSQKTSA-N ampicillin Chemical compound C1([C@@H](N)C(=O)N[C@H]2[C@H]3SC([C@@H](N3C2=O)C(O)=O)(C)C)=CC=CC=C1 AVKUERGKIZMTKX-NJBDSQKTSA-N 0.000 description 1
- 239000003957 anion exchange resin Substances 0.000 description 1
- 150000001450 anions Chemical class 0.000 description 1
- 230000001263 anti-prolactin effect Effects 0.000 description 1
- 230000000840 anti-viral effect Effects 0.000 description 1
- 230000009833 antibody interaction Effects 0.000 description 1
- 230000030741 antigen processing and presentation Effects 0.000 description 1
- 239000000074 antisense oligonucleotide Substances 0.000 description 1
- 238000012230 antisense oligonucleotides Methods 0.000 description 1
- 230000001640 apoptogenic effect Effects 0.000 description 1
- 125000000637 arginyl group Chemical group N[C@@H](CCCNC(N)=N)C(=O)* 0.000 description 1
- 229960003272 asparaginase Drugs 0.000 description 1
- 230000001363 autoimmune Effects 0.000 description 1
- IVRMZWNICZWHMI-UHFFFAOYSA-N azide group Chemical group [N-]=[N+]=[N-] IVRMZWNICZWHMI-UHFFFAOYSA-N 0.000 description 1
- 108700000711 bcl-X Proteins 0.000 description 1
- 102000055104 bcl-X Human genes 0.000 description 1
- 229960002707 bendamustine Drugs 0.000 description 1
- YTKUWDBFDASYHO-UHFFFAOYSA-N bendamustine Chemical compound ClCCN(CCCl)C1=CC=C2N(C)C(CCCC(O)=O)=NC2=C1 YTKUWDBFDASYHO-UHFFFAOYSA-N 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- RWCCWEUUXYIKHB-UHFFFAOYSA-N benzophenone Chemical compound C=1C=CC=CC=1C(=O)C1=CC=CC=C1 RWCCWEUUXYIKHB-UHFFFAOYSA-N 0.000 description 1
- 239000012965 benzophenone Substances 0.000 description 1
- 108010051210 beta-Fructofuranosidase Proteins 0.000 description 1
- IQFYYKKMVGJFEH-UHFFFAOYSA-N beta-L-thymidine Natural products O=C1NC(=O)C(C)=CN1C1OC(CO)C(O)C1 IQFYYKKMVGJFEH-UHFFFAOYSA-N 0.000 description 1
- UCMIRNVEIXFBKS-UHFFFAOYSA-N beta-alanine Chemical compound NCCC(O)=O UCMIRNVEIXFBKS-UHFFFAOYSA-N 0.000 description 1
- GUBGYTABKSRVRQ-QUYVBRFLSA-N beta-maltose Chemical compound OC[C@H]1O[C@H](O[C@H]2[C@H](O)[C@@H](O)[C@H](O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@@H]1O GUBGYTABKSRVRQ-QUYVBRFLSA-N 0.000 description 1
- 229960000997 bicalutamide Drugs 0.000 description 1
- 230000000975 bioactive effect Effects 0.000 description 1
- 239000003124 biologic agent Substances 0.000 description 1
- 238000005460 biophysical method Methods 0.000 description 1
- 229910052797 bismuth Inorganic materials 0.000 description 1
- JCXGWMGPZLAOME-UHFFFAOYSA-N bismuth atom Chemical compound [Bi] JCXGWMGPZLAOME-UHFFFAOYSA-N 0.000 description 1
- 229960001561 bleomycin Drugs 0.000 description 1
- OYVAGSVQBOHSSS-UAPAGMARSA-O bleomycin A2 Chemical compound N([C@H](C(=O)N[C@H](C)[C@@H](O)[C@H](C)C(=O)N[C@@H]([C@H](O)C)C(=O)NCCC=1SC=C(N=1)C=1SC=C(N=1)C(=O)NCCC[S+](C)C)[C@@H](O[C@H]1[C@H]([C@@H](O)[C@H](O)[C@H](CO)O1)O[C@@H]1[C@H]([C@@H](OC(N)=O)[C@H](O)[C@@H](CO)O1)O)C=1N=CNC=1)C(=O)C1=NC([C@H](CC(N)=O)NC[C@H](N)C(N)=O)=NC(N)=C1C OYVAGSVQBOHSSS-UAPAGMARSA-O 0.000 description 1
- 210000004369 blood Anatomy 0.000 description 1
- 239000008280 blood Substances 0.000 description 1
- 230000023555 blood coagulation Effects 0.000 description 1
- 210000001772 blood platelet Anatomy 0.000 description 1
- 210000002798 bone marrow cell Anatomy 0.000 description 1
- GXJABQQUPOEUTA-RDJZCZTQSA-N bortezomib Chemical compound C([C@@H](C(=O)N[C@@H](CC(C)C)B(O)O)NC(=O)C=1N=CC=NC=1)C1=CC=CC=C1 GXJABQQUPOEUTA-RDJZCZTQSA-N 0.000 description 1
- 229960001467 bortezomib Drugs 0.000 description 1
- 108010006025 bovine growth hormone Proteins 0.000 description 1
- GDTBXPJZTBHREO-UHFFFAOYSA-N bromine Substances BrBr GDTBXPJZTBHREO-UHFFFAOYSA-N 0.000 description 1
- 229910052794 bromium Inorganic materials 0.000 description 1
- 239000000872 buffer Substances 0.000 description 1
- UHYPYGJEEGLRJD-UHFFFAOYSA-N cadmium(2+);selenium(2-) Chemical compound [Se-2].[Cd+2] UHYPYGJEEGLRJD-UHFFFAOYSA-N 0.000 description 1
- 239000011575 calcium Substances 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- 238000004364 calculation method Methods 0.000 description 1
- 244000309466 calf Species 0.000 description 1
- 239000000969 carrier Substances 0.000 description 1
- 239000003729 cation exchange resin Substances 0.000 description 1
- 230000010261 cell growth Effects 0.000 description 1
- 230000022534 cell killing Effects 0.000 description 1
- 230000008619 cell matrix interaction Effects 0.000 description 1
- 230000012292 cell migration Effects 0.000 description 1
- 230000015861 cell surface binding Effects 0.000 description 1
- 230000003833 cell viability Effects 0.000 description 1
- 230000005754 cellular signaling Effects 0.000 description 1
- 201000010881 cervical cancer Diseases 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 230000000739 chaotic effect Effects 0.000 description 1
- 238000012512 characterization method Methods 0.000 description 1
- 239000013522 chelant Substances 0.000 description 1
- 229910052801 chlorine Inorganic materials 0.000 description 1
- 239000000460 chlorine Substances 0.000 description 1
- 238000011098 chromatofocusing Methods 0.000 description 1
- 229910052804 chromium Inorganic materials 0.000 description 1
- 239000013611 chromosomal DNA Substances 0.000 description 1
- 210000000349 chromosome Anatomy 0.000 description 1
- 229940080701 chymosin Drugs 0.000 description 1
- 238000002983 circular dichroism Methods 0.000 description 1
- 238000000978 circular dichroism spectroscopy Methods 0.000 description 1
- 238000001142 circular dichroism spectrum Methods 0.000 description 1
- DQLATGHUWYMOKM-UHFFFAOYSA-L cisplatin Chemical compound N[Pt](N)(Cl)Cl DQLATGHUWYMOKM-UHFFFAOYSA-L 0.000 description 1
- 229960004316 cisplatin Drugs 0.000 description 1
- 229960002173 citrulline Drugs 0.000 description 1
- 235000013477 citrulline Nutrition 0.000 description 1
- 229960002436 cladribine Drugs 0.000 description 1
- 238000012875 competitive assay Methods 0.000 description 1
- 230000009918 complex formation Effects 0.000 description 1
- 230000000536 complexating effect Effects 0.000 description 1
- 238000004590 computer program Methods 0.000 description 1
- 238000012790 confirmation Methods 0.000 description 1
- 239000000470 constituent Substances 0.000 description 1
- 239000000356 contaminant Substances 0.000 description 1
- 239000005289 controlled pore glass Substances 0.000 description 1
- 235000005822 corn Nutrition 0.000 description 1
- 229960004544 cortisone Drugs 0.000 description 1
- 239000003431 cross linking reagent Substances 0.000 description 1
- 150000003983 crown ethers Chemical class 0.000 description 1
- 239000013078 crystal Substances 0.000 description 1
- 125000002993 cycloalkylene group Chemical group 0.000 description 1
- 229960004397 cyclophosphamide Drugs 0.000 description 1
- 235000018417 cysteine Nutrition 0.000 description 1
- 229960000684 cytarabine Drugs 0.000 description 1
- 210000004292 cytoskeleton Anatomy 0.000 description 1
- GVJHHUAWPYXKBD-UHFFFAOYSA-N d-alpha-tocopherol Natural products OC1=C(C)C(C)=C2OC(CCCC(C)CCCC(C)CCCC(C)C)(C)CCC2=C1C GVJHHUAWPYXKBD-UHFFFAOYSA-N 0.000 description 1
- 229960005029 darbepoetin alfa Drugs 0.000 description 1
- 229960002448 dasatinib Drugs 0.000 description 1
- 229960000975 daunorubicin Drugs 0.000 description 1
- 230000034994 death Effects 0.000 description 1
- 229960003603 decitabine Drugs 0.000 description 1
- 230000002950 deficient Effects 0.000 description 1
- 238000001212 derivatisation Methods 0.000 description 1
- 238000001514 detection method Methods 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 229960003957 dexamethasone Drugs 0.000 description 1
- UREBDLICKHMUKA-CXSFZGCWSA-N dexamethasone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@@H](C)[C@@](C(=O)CO)(O)[C@@]1(C)C[C@@H]2O UREBDLICKHMUKA-CXSFZGCWSA-N 0.000 description 1
- 238000000502 dialysis Methods 0.000 description 1
- 238000000113 differential scanning calorimetry Methods 0.000 description 1
- 238000006471 dimerization reaction Methods 0.000 description 1
- 208000035475 disorder Diseases 0.000 description 1
- 230000006334 disulfide bridging Effects 0.000 description 1
- 239000003534 dna topoisomerase inhibitor Substances 0.000 description 1
- 229960003668 docetaxel Drugs 0.000 description 1
- 239000002552 dosage form Substances 0.000 description 1
- 230000003828 downregulation Effects 0.000 description 1
- ZWAOHEXOSAUJHY-ZIYNGMLESA-N doxifluridine Chemical compound O[C@@H]1[C@H](O)[C@@H](C)O[C@H]1N1C(=O)NC(=O)C(F)=C1 ZWAOHEXOSAUJHY-ZIYNGMLESA-N 0.000 description 1
- 229950005454 doxifluridine Drugs 0.000 description 1
- 229960004679 doxorubicin Drugs 0.000 description 1
- 239000003937 drug carrier Substances 0.000 description 1
- 238000012377 drug delivery Methods 0.000 description 1
- 238000009510 drug design Methods 0.000 description 1
- 229960002224 eculizumab Drugs 0.000 description 1
- 239000012636 effector Substances 0.000 description 1
- 230000008030 elimination Effects 0.000 description 1
- 238000003379 elimination reaction Methods 0.000 description 1
- 229940096118 ella Drugs 0.000 description 1
- 239000002158 endotoxin Substances 0.000 description 1
- JOZGNYDSEBIJDH-UHFFFAOYSA-N eniluracil Chemical compound O=C1NC=C(C#C)C(=O)N1 JOZGNYDSEBIJDH-UHFFFAOYSA-N 0.000 description 1
- 229950010213 eniluracil Drugs 0.000 description 1
- 210000003979 eosinophil Anatomy 0.000 description 1
- 229940116977 epidermal growth factor Drugs 0.000 description 1
- 229960001904 epirubicin Drugs 0.000 description 1
- 229960003388 epoetin alfa Drugs 0.000 description 1
- 229960001433 erlotinib Drugs 0.000 description 1
- AAKJLRGGTJKAMG-UHFFFAOYSA-N erlotinib Chemical compound C=12C=C(OCCOC)C(OCCOC)=CC2=NC=NC=1NC1=CC=CC(C#C)=C1 AAKJLRGGTJKAMG-UHFFFAOYSA-N 0.000 description 1
- 229960003276 erythromycin Drugs 0.000 description 1
- 229960000403 etanercept Drugs 0.000 description 1
- 238000012869 ethanol precipitation Methods 0.000 description 1
- ZMMJGEGLRURXTF-UHFFFAOYSA-N ethidium bromide Chemical compound [Br-].C12=CC(N)=CC=C2C2=CC=C(N)C=C2[N+](CC)=C1C1=CC=CC=C1 ZMMJGEGLRURXTF-UHFFFAOYSA-N 0.000 description 1
- 229960005542 ethidium bromide Drugs 0.000 description 1
- 229960005420 etoposide Drugs 0.000 description 1
- VJJPUSNTGOMMGY-MRVIYFEKSA-N etoposide Chemical compound COC1=C(O)C(OC)=CC([C@@H]2C3=CC=4OCOC=4C=C3[C@@H](O[C@H]3[C@@H]([C@@H](O)[C@@H]4O[C@H](C)OC[C@H]4O3)O)[C@@H]3[C@@H]2C(OC3)=O)=C1 VJJPUSNTGOMMGY-MRVIYFEKSA-N 0.000 description 1
- 229960005167 everolimus Drugs 0.000 description 1
- 230000007717 exclusion Effects 0.000 description 1
- 210000002950 fibroblast Anatomy 0.000 description 1
- 230000004992 fission Effects 0.000 description 1
- 238000000684 flow cytometry Methods 0.000 description 1
- 229960000390 fludarabine Drugs 0.000 description 1
- GIUYCYHIANZCFB-FJFJXFQQSA-N fludarabine phosphate Chemical compound C1=NC=2C(N)=NC(F)=NC=2N1[C@@H]1O[C@H](COP(O)(O)=O)[C@@H](O)[C@@H]1O GIUYCYHIANZCFB-FJFJXFQQSA-N 0.000 description 1
- 238000000799 fluorescence microscopy Methods 0.000 description 1
- 238000001943 fluorescence-activated cell sorting Methods 0.000 description 1
- 239000007850 fluorescent dye Substances 0.000 description 1
- 238000012632 fluorescent imaging Methods 0.000 description 1
- 229910052731 fluorine Inorganic materials 0.000 description 1
- MKXKFYHWDHIYRV-UHFFFAOYSA-N flutamide Chemical compound CC(C)C(=O)NC1=CC=C([N+]([O-])=O)C(C(F)(F)F)=C1 MKXKFYHWDHIYRV-UHFFFAOYSA-N 0.000 description 1
- 229960002074 flutamide Drugs 0.000 description 1
- 230000003325 follicular Effects 0.000 description 1
- 210000000285 follicular dendritic cell Anatomy 0.000 description 1
- 229960002258 fulvestrant Drugs 0.000 description 1
- 125000000524 functional group Chemical group 0.000 description 1
- 230000002538 fungal effect Effects 0.000 description 1
- 229910052733 gallium Inorganic materials 0.000 description 1
- 238000001502 gel electrophoresis Methods 0.000 description 1
- BRZYSWJRSDMWLG-CAXSIQPQSA-N geneticin Natural products O1C[C@@](O)(C)[C@H](NC)[C@@H](O)[C@H]1O[C@@H]1[C@@H](O)[C@H](O[C@@H]2[C@@H]([C@@H](O)[C@H](O)[C@@H](C(C)O)O2)N)[C@@H](N)C[C@H]1N BRZYSWJRSDMWLG-CAXSIQPQSA-N 0.000 description 1
- 210000001280 germinal center Anatomy 0.000 description 1
- 102000018146 globin Human genes 0.000 description 1
- 108060003196 globin Proteins 0.000 description 1
- 239000008103 glucose Substances 0.000 description 1
- 229940116332 glucose oxidase Drugs 0.000 description 1
- 235000019420 glucose oxidase Nutrition 0.000 description 1
- 150000004676 glycans Chemical class 0.000 description 1
- 235000011187 glycerol Nutrition 0.000 description 1
- 230000002414 glycolytic effect Effects 0.000 description 1
- 229940126613 gomiliximab Drugs 0.000 description 1
- 239000003102 growth factor Substances 0.000 description 1
- 108010067006 heat stable toxin (E coli) Proteins 0.000 description 1
- 210000003958 hematopoietic stem cell Anatomy 0.000 description 1
- 229920000669 heparin Polymers 0.000 description 1
- 229960002897 heparin Drugs 0.000 description 1
- 108010038082 heparin proteoglycan Proteins 0.000 description 1
- 206010073071 hepatocellular carcinoma Diseases 0.000 description 1
- 229940022353 herceptin Drugs 0.000 description 1
- UUVWYPNAQBNQJQ-UHFFFAOYSA-N hexamethylmelamine Chemical compound CN(C)C1=NC(N(C)C)=NC(N(C)C)=N1 UUVWYPNAQBNQJQ-UHFFFAOYSA-N 0.000 description 1
- 238000004128 high performance liquid chromatography Methods 0.000 description 1
- 238000004191 hydrophobic interaction chromatography Methods 0.000 description 1
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 1
- 238000012872 hydroxylapatite chromatography Methods 0.000 description 1
- 230000036031 hyperthermia Effects 0.000 description 1
- 229960003685 imatinib mesylate Drugs 0.000 description 1
- YLMAHDNUQAMNNX-UHFFFAOYSA-N imatinib methanesulfonate Chemical compound CS(O)(=O)=O.C1CN(C)CCN1CC1=CC=C(C(=O)NC=2C=C(NC=3N=C(C=CN=3)C=3C=NC=CC=3)C(C)=CC=2)C=C1 YLMAHDNUQAMNNX-UHFFFAOYSA-N 0.000 description 1
- 210000003297 immature b lymphocyte Anatomy 0.000 description 1
- 230000014726 immortalization of host cell Effects 0.000 description 1
- 230000001900 immune effect Effects 0.000 description 1
- 229940127121 immunoconjugate Drugs 0.000 description 1
- 230000002584 immunomodulator Effects 0.000 description 1
- 230000003308 immunostimulating effect Effects 0.000 description 1
- 230000001506 immunosuppresive effect Effects 0.000 description 1
- 238000009169 immunotherapy Methods 0.000 description 1
- 238000000338 in vitro Methods 0.000 description 1
- 238000011534 incubation Methods 0.000 description 1
- 230000006698 induction Effects 0.000 description 1
- 208000027866 inflammatory disease Diseases 0.000 description 1
- 230000005764 inhibitory process Effects 0.000 description 1
- 150000002484 inorganic compounds Chemical class 0.000 description 1
- 229910010272 inorganic material Inorganic materials 0.000 description 1
- 229950000038 interferon alfa Drugs 0.000 description 1
- 229910000765 intermetallic Inorganic materials 0.000 description 1
- 230000000968 intestinal effect Effects 0.000 description 1
- 230000004068 intracellular signaling Effects 0.000 description 1
- 238000001990 intravenous administration Methods 0.000 description 1
- 230000006623 intrinsic pathway Effects 0.000 description 1
- 239000001573 invertase Substances 0.000 description 1
- 235000011073 invertase Nutrition 0.000 description 1
- 238000005342 ion exchange Methods 0.000 description 1
- 230000003447 ipsilateral effect Effects 0.000 description 1
- UWKQSNNFCGGAFS-XIFFEERXSA-N irinotecan Chemical compound C1=C2C(CC)=C3CN(C(C4=C([C@@](C(=O)OC4)(O)CC)C=4)=O)C=4C3=NC2=CC=C1OC(=O)N(CC1)CCC1N1CCCCC1 UWKQSNNFCGGAFS-XIFFEERXSA-N 0.000 description 1
- 229960004768 irinotecan Drugs 0.000 description 1
- SZVJSHCCFOBDDC-UHFFFAOYSA-N iron(II,III) oxide Inorganic materials O=[Fe]O[Fe]O[Fe]=O SZVJSHCCFOBDDC-UHFFFAOYSA-N 0.000 description 1
- 229960000318 kanamycin Drugs 0.000 description 1
- 229930027917 kanamycin Natural products 0.000 description 1
- SBUJHOSQTJFQJX-NOAMYHISSA-N kanamycin Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CN)O[C@@H]1O[C@H]1[C@H](O)[C@@H](O[C@@H]2[C@@H]([C@@H](N)[C@H](O)[C@@H](CO)O2)O)[C@H](N)C[C@@H]1N SBUJHOSQTJFQJX-NOAMYHISSA-N 0.000 description 1
- 229930182823 kanamycin A Natural products 0.000 description 1
- 150000002576 ketones Chemical class 0.000 description 1
- 210000003734 kidney Anatomy 0.000 description 1
- 239000008101 lactose Substances 0.000 description 1
- 229910052747 lanthanoid Inorganic materials 0.000 description 1
- 229910052746 lanthanum Inorganic materials 0.000 description 1
- 229960004891 lapatinib Drugs 0.000 description 1
- 229960004942 lenalidomide Drugs 0.000 description 1
- GOTYRUGSSMKFNF-UHFFFAOYSA-N lenalidomide Chemical compound C1C=2C(N)=CC=CC=2C(=O)N1C1CCC(=O)NC1=O GOTYRUGSSMKFNF-UHFFFAOYSA-N 0.000 description 1
- 230000003902 lesion Effects 0.000 description 1
- 239000002502 liposome Substances 0.000 description 1
- 230000004807 localization Effects 0.000 description 1
- 230000033001 locomotion Effects 0.000 description 1
- 229960002247 lomustine Drugs 0.000 description 1
- 101150074251 lpp gene Proteins 0.000 description 1
- 210000005265 lung cell Anatomy 0.000 description 1
- 210000001165 lymph node Anatomy 0.000 description 1
- 230000002132 lysosomal effect Effects 0.000 description 1
- 108010026228 mRNA guanylyltransferase Proteins 0.000 description 1
- 230000012976 mRNA stabilization Effects 0.000 description 1
- 210000002540 macrophage Anatomy 0.000 description 1
- 239000011777 magnesium Substances 0.000 description 1
- 229910052749 magnesium Inorganic materials 0.000 description 1
- 230000003211 malignant effect Effects 0.000 description 1
- 229910052748 manganese Inorganic materials 0.000 description 1
- 239000011572 manganese Substances 0.000 description 1
- WPBNNNQJVZRUHP-UHFFFAOYSA-L manganese(2+);methyl n-[[2-(methoxycarbonylcarbamothioylamino)phenyl]carbamothioyl]carbamate;n-[2-(sulfidocarbothioylamino)ethyl]carbamodithioate Chemical compound [Mn+2].[S-]C(=S)NCCNC([S-])=S.COC(=O)NC(=S)NC1=CC=CC=C1NC(=S)NC(=O)OC WPBNNNQJVZRUHP-UHFFFAOYSA-L 0.000 description 1
- 239000011159 matrix material Substances 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 229960004961 mechlorethamine Drugs 0.000 description 1
- HAWPXGHAZFHHAD-UHFFFAOYSA-N mechlorethamine Chemical compound ClCCN(C)CCCl HAWPXGHAZFHHAD-UHFFFAOYSA-N 0.000 description 1
- 229960001786 megestrol Drugs 0.000 description 1
- RQZAXGRLVPAYTJ-GQFGMJRRSA-N megestrol acetate Chemical compound C1=C(C)C2=CC(=O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@@](C(C)=O)(OC(=O)C)[C@@]1(C)CC2 RQZAXGRLVPAYTJ-GQFGMJRRSA-N 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- GLVAUDGFNGKCSF-UHFFFAOYSA-N mercaptopurine Chemical compound S=C1NC=NC2=C1NC=N2 GLVAUDGFNGKCSF-UHFFFAOYSA-N 0.000 description 1
- 229960001428 mercaptopurine Drugs 0.000 description 1
- 229960004635 mesna Drugs 0.000 description 1
- 108020004999 messenger RNA Proteins 0.000 description 1
- 230000002503 metabolic effect Effects 0.000 description 1
- 230000004060 metabolic process Effects 0.000 description 1
- 229940042016 methacycline Drugs 0.000 description 1
- 229960004584 methylprednisolone Drugs 0.000 description 1
- HPNSFSBZBAHARI-UHFFFAOYSA-N micophenolic acid Natural products OC1=C(CC=C(C)CCC(O)=O)C(OC)=C(C)C2=C1C(=O)OC2 HPNSFSBZBAHARI-UHFFFAOYSA-N 0.000 description 1
- 230000000813 microbial effect Effects 0.000 description 1
- 244000005700 microbiome Species 0.000 description 1
- 230000003278 mimic effect Effects 0.000 description 1
- 229960004023 minocycline Drugs 0.000 description 1
- 230000002438 mitochondrial effect Effects 0.000 description 1
- 229960004857 mitomycin Drugs 0.000 description 1
- 229960000350 mitotane Drugs 0.000 description 1
- KKZJGLLVHKMTCM-UHFFFAOYSA-N mitoxantrone Chemical compound O=C1C2=C(O)C=CC(O)=C2C(=O)C2=C1C(NCCNCCO)=CC=C2NCCNCCO KKZJGLLVHKMTCM-UHFFFAOYSA-N 0.000 description 1
- 229960001156 mitoxantrone Drugs 0.000 description 1
- 108091005601 modified peptides Proteins 0.000 description 1
- 238000003032 molecular docking Methods 0.000 description 1
- 210000003097 mucus Anatomy 0.000 description 1
- 229960000951 mycophenolic acid Drugs 0.000 description 1
- HPNSFSBZBAHARI-RUDMXATFSA-N mycophenolic acid Chemical compound OC1=C(C\C=C(/C)CCC(O)=O)C(OC)=C(C)C2=C1C(=O)OC2 HPNSFSBZBAHARI-RUDMXATFSA-N 0.000 description 1
- 210000000066 myeloid cell Anatomy 0.000 description 1
- 201000000050 myeloid neoplasm Diseases 0.000 description 1
- GNOLWGAJQVLBSM-UHFFFAOYSA-N n,n,5,7-tetramethyl-1,2,3,4-tetrahydronaphthalen-1-amine Chemical compound C1=C(C)C=C2C(N(C)C)CCCC2=C1C GNOLWGAJQVLBSM-UHFFFAOYSA-N 0.000 description 1
- 239000013642 negative control Substances 0.000 description 1
- 230000011234 negative regulation of signal transduction Effects 0.000 description 1
- 108010072514 neuramide Proteins 0.000 description 1
- 230000007935 neutral effect Effects 0.000 description 1
- 230000003472 neutralizing effect Effects 0.000 description 1
- 229910052759 nickel Inorganic materials 0.000 description 1
- 125000004433 nitrogen atom Chemical group N* 0.000 description 1
- 231100000957 no side effect Toxicity 0.000 description 1
- 229960002700 octreotide Drugs 0.000 description 1
- JRZJOMJEPLMPRA-UHFFFAOYSA-N olefin Natural products CCCCCCCC=C JRZJOMJEPLMPRA-UHFFFAOYSA-N 0.000 description 1
- 239000002751 oligonucleotide probe Substances 0.000 description 1
- 238000005457 optimization Methods 0.000 description 1
- 229920000620 organic polymer Polymers 0.000 description 1
- 229940043515 other immunoglobulins in atc Drugs 0.000 description 1
- 230000002611 ovarian Effects 0.000 description 1
- 230000002018 overexpression Effects 0.000 description 1
- 125000004430 oxygen atom Chemical group O* 0.000 description 1
- 229960000625 oxytetracycline Drugs 0.000 description 1
- IWVCMVBTMGNXQD-PXOLEDIWSA-N oxytetracycline Chemical compound C1=CC=C2[C@](O)(C)[C@H]3[C@H](O)[C@H]4[C@H](N(C)C)C(O)=C(C(N)=O)C(=O)[C@@]4(O)C(O)=C3C(=O)C2=C1O IWVCMVBTMGNXQD-PXOLEDIWSA-N 0.000 description 1
- 235000019366 oxytetracycline Nutrition 0.000 description 1
- TVIDEEHSOPHZBR-AWEZNQCLSA-N para-(benzoyl)-phenylalanine Chemical compound C1=CC(C[C@H](N)C(O)=O)=CC=C1C(=O)C1=CC=CC=C1 TVIDEEHSOPHZBR-AWEZNQCLSA-N 0.000 description 1
- 244000045947 parasite Species 0.000 description 1
- 229950009506 penicillinase Drugs 0.000 description 1
- 229960002340 pentostatin Drugs 0.000 description 1
- FPVKHBSQESCIEP-JQCXWYLXSA-N pentostatin Chemical compound C1[C@H](O)[C@@H](CO)O[C@H]1N1C(N=CNC[C@H]2O)=C2N=C1 FPVKHBSQESCIEP-JQCXWYLXSA-N 0.000 description 1
- LQRJAEQXMSMEDP-XCHBZYMASA-N peptide a Chemical group N([C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](C)C(=O)NCCCC[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)C(\NC(=O)[C@@H](CCCCN)NC(=O)CNC(C)=O)=C/C=1C=CC=CC=1)C(N)=O)C(=O)C(\NC(=O)[C@@H](CCCCN)NC(=O)CNC(C)=O)=C\C1=CC=CC=C1 LQRJAEQXMSMEDP-XCHBZYMASA-N 0.000 description 1
- 239000000137 peptide hydrolase inhibitor Substances 0.000 description 1
- 238000010647 peptide synthesis reaction Methods 0.000 description 1
- 125000001151 peptidyl group Chemical group 0.000 description 1
- 210000005259 peripheral blood Anatomy 0.000 description 1
- 239000011886 peripheral blood Substances 0.000 description 1
- 210000005105 peripheral blood lymphocyte Anatomy 0.000 description 1
- 210000001322 periplasm Anatomy 0.000 description 1
- 230000035699 permeability Effects 0.000 description 1
- 238000002823 phage display Methods 0.000 description 1
- 230000000144 pharmacologic effect Effects 0.000 description 1
- 150000002993 phenylalanine derivatives Chemical class 0.000 description 1
- 101150009573 phoA gene Proteins 0.000 description 1
- QGVLYPPODPLXMB-QXYKVGAMSA-N phorbol Natural products C[C@@H]1[C@@H](O)[C@]2(O)[C@H]([C@H]3C=C(CO)C[C@@]4(O)[C@H](C=C(C)C4=O)[C@@]13O)C2(C)C QGVLYPPODPLXMB-QXYKVGAMSA-N 0.000 description 1
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 1
- 239000010452 phosphate Substances 0.000 description 1
- 229910052698 phosphorus Inorganic materials 0.000 description 1
- 230000026731 phosphorylation Effects 0.000 description 1
- 238000006366 phosphorylation reaction Methods 0.000 description 1
- 102000020233 phosphotransferase Human genes 0.000 description 1
- 229940012957 plasmin Drugs 0.000 description 1
- 229940127126 plasminogen activator Drugs 0.000 description 1
- 210000004180 plasmocyte Anatomy 0.000 description 1
- 108700028325 pokeweed antiviral Proteins 0.000 description 1
- 230000008488 polyadenylation Effects 0.000 description 1
- 229920000768 polyamine Polymers 0.000 description 1
- 229920000570 polyether Polymers 0.000 description 1
- 229920000656 polylysine Polymers 0.000 description 1
- 229920000642 polymer Polymers 0.000 description 1
- 229920001282 polysaccharide Polymers 0.000 description 1
- 239000005017 polysaccharide Substances 0.000 description 1
- 150000004032 porphyrins Chemical class 0.000 description 1
- 239000013641 positive control Substances 0.000 description 1
- 238000012636 positron electron tomography Methods 0.000 description 1
- 229910052700 potassium Inorganic materials 0.000 description 1
- 230000003389 potentiating effect Effects 0.000 description 1
- 229960002965 pravastatin Drugs 0.000 description 1
- TUZYXOIXSAXUGO-PZAWKZKUSA-N pravastatin Chemical compound C1=C[C@H](C)[C@H](CC[C@@H](O)C[C@@H](O)CC(O)=O)[C@H]2[C@@H](OC(=O)[C@@H](C)CC)C[C@H](O)C=C21 TUZYXOIXSAXUGO-PZAWKZKUSA-N 0.000 description 1
- 239000002244 precipitate Substances 0.000 description 1
- 239000002243 precursor Substances 0.000 description 1
- 229960004618 prednisone Drugs 0.000 description 1
- XOFYZVNMUHMLCC-ZPOLXVRWSA-N prednisone Chemical compound O=C1C=C[C@]2(C)[C@H]3C(=O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 XOFYZVNMUHMLCC-ZPOLXVRWSA-N 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- CPTBDICYNRMXFX-UHFFFAOYSA-N procarbazine Chemical compound CNNCC1=CC=C(C(=O)NC(C)C)C=C1 CPTBDICYNRMXFX-UHFFFAOYSA-N 0.000 description 1
- 229960000624 procarbazine Drugs 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- 210000001236 prokaryotic cell Anatomy 0.000 description 1
- 230000002062 proliferating effect Effects 0.000 description 1
- 238000011321 prophylaxis Methods 0.000 description 1
- 229960000856 protein c Drugs 0.000 description 1
- 230000004853 protein function Effects 0.000 description 1
- 238000001742 protein purification Methods 0.000 description 1
- 230000017854 proteolysis Effects 0.000 description 1
- 239000002534 radiation-sensitizing agent Substances 0.000 description 1
- 238000001959 radiotherapy Methods 0.000 description 1
- 229910052705 radium Inorganic materials 0.000 description 1
- HCWPIIXVSYCSAN-UHFFFAOYSA-N radium atom Chemical compound [Ra] HCWPIIXVSYCSAN-UHFFFAOYSA-N 0.000 description 1
- 229960004622 raloxifene Drugs 0.000 description 1
- GZUITABIAKMVPG-UHFFFAOYSA-N raloxifene Chemical compound C1=CC(O)=CC=C1C1=C(C(=O)C=2C=CC(OCCN3CCCCC3)=CC=2)C2=CC=C(O)C=C2S1 GZUITABIAKMVPG-UHFFFAOYSA-N 0.000 description 1
- 230000013878 renal filtration Effects 0.000 description 1
- BOLDJAUMGUJJKM-LSDHHAIUSA-N renifolin D Natural products CC(=C)[C@@H]1Cc2c(O)c(O)ccc2[C@H]1CC(=O)c3ccc(O)cc3O BOLDJAUMGUJJKM-LSDHHAIUSA-N 0.000 description 1
- 230000003362 replicative effect Effects 0.000 description 1
- 229920005989 resin Polymers 0.000 description 1
- 239000011347 resin Substances 0.000 description 1
- 210000003705 ribosome Anatomy 0.000 description 1
- 235000019515 salmon Nutrition 0.000 description 1
- 229950006896 sapacitabine Drugs 0.000 description 1
- LBGFKUUHOPIEMA-PEARBKPGSA-N sapacitabine Chemical compound O=C1N=C(NC(=O)CCCCCCCCCCCCCCC)C=CN1[C@H]1[C@@H](C#N)[C@H](O)[C@@H](CO)O1 LBGFKUUHOPIEMA-PEARBKPGSA-N 0.000 description 1
- 231100000241 scar Toxicity 0.000 description 1
- 230000003248 secreting effect Effects 0.000 description 1
- 210000000717 sertoli cell Anatomy 0.000 description 1
- 230000035939 shock Effects 0.000 description 1
- 239000002924 silencing RNA Substances 0.000 description 1
- 239000000377 silicon dioxide Substances 0.000 description 1
- 229910052709 silver Inorganic materials 0.000 description 1
- 239000004332 silver Substances 0.000 description 1
- 238000002741 site-directed mutagenesis Methods 0.000 description 1
- 238000001542 size-exclusion chromatography Methods 0.000 description 1
- 239000004055 small Interfering RNA Substances 0.000 description 1
- 239000001632 sodium acetate Substances 0.000 description 1
- 235000017281 sodium acetate Nutrition 0.000 description 1
- 239000011780 sodium chloride Substances 0.000 description 1
- 238000002415 sodium dodecyl sulfate polyacrylamide gel electrophoresis Methods 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 239000007790 solid phase Substances 0.000 description 1
- 239000000243 solution Substances 0.000 description 1
- 230000009870 specific binding Effects 0.000 description 1
- 230000003595 spectral effect Effects 0.000 description 1
- 125000004079 stearyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 230000004936 stimulating effect Effects 0.000 description 1
- 230000000638 stimulation Effects 0.000 description 1
- 229960005202 streptokinase Drugs 0.000 description 1
- 125000005717 substituted cycloalkylene group Chemical group 0.000 description 1
- GECHUMIMRBOMGK-UHFFFAOYSA-N sulfapyridine Chemical compound C1=CC(N)=CC=C1S(=O)(=O)NC1=CC=CC=N1 GECHUMIMRBOMGK-UHFFFAOYSA-N 0.000 description 1
- 229910052717 sulfur Inorganic materials 0.000 description 1
- 239000006228 supernatant Substances 0.000 description 1
- 239000013589 supplement Substances 0.000 description 1
- 230000004083 survival effect Effects 0.000 description 1
- 238000004114 suspension culture Methods 0.000 description 1
- 102000042996 syndecan proteoglycan family Human genes 0.000 description 1
- 108091077880 syndecan proteoglycan family Proteins 0.000 description 1
- 231100000337 synergistic cytotoxicity Toxicity 0.000 description 1
- 238000003786 synthesis reaction Methods 0.000 description 1
- 238000002626 targeted therapy Methods 0.000 description 1
- 230000008685 targeting Effects 0.000 description 1
- 235000012976 tarts Nutrition 0.000 description 1
- IWVCMVBTMGNXQD-UHFFFAOYSA-N terramycin dehydrate Natural products C1=CC=C2C(O)(C)C3C(O)C4C(N(C)C)C(O)=C(C(N)=O)C(=O)C4(O)C(O)=C3C(=O)C2=C1O IWVCMVBTMGNXQD-UHFFFAOYSA-N 0.000 description 1
- 238000010998 test method Methods 0.000 description 1
- 108700020534 tetracycline resistance-encoding transposon repressor Proteins 0.000 description 1
- 230000004797 therapeutic response Effects 0.000 description 1
- 229960004072 thrombin Drugs 0.000 description 1
- 229940104230 thymidine Drugs 0.000 description 1
- 229960000187 tissue plasminogen activator Drugs 0.000 description 1
- 238000004448 titration Methods 0.000 description 1
- 235000010384 tocopherol Nutrition 0.000 description 1
- 229960001295 tocopherol Drugs 0.000 description 1
- 229930003799 tocopherol Natural products 0.000 description 1
- 239000011732 tocopherol Substances 0.000 description 1
- 229940044693 topoisomerase inhibitor Drugs 0.000 description 1
- 230000001988 toxicity Effects 0.000 description 1
- 231100000419 toxicity Toxicity 0.000 description 1
- 239000011573 trace mineral Substances 0.000 description 1
- 235000013619 trace mineral Nutrition 0.000 description 1
- 230000005758 transcription activity Effects 0.000 description 1
- 230000005026 transcription initiation Effects 0.000 description 1
- 239000012581 transferrin Substances 0.000 description 1
- 229910052723 transition metal Inorganic materials 0.000 description 1
- 150000003624 transition metals Chemical class 0.000 description 1
- 230000014621 translational initiation Effects 0.000 description 1
- 108091007466 transmembrane glycoproteins Proteins 0.000 description 1
- 102000027257 transmembrane receptors Human genes 0.000 description 1
- 108091008578 transmembrane receptors Proteins 0.000 description 1
- 238000002054 transplantation Methods 0.000 description 1
- 229960001727 tretinoin Drugs 0.000 description 1
- 239000012588 trypsin Substances 0.000 description 1
- 229960004799 tryptophan Drugs 0.000 description 1
- 125000000430 tryptophan group Chemical group [H]N([H])C(C(=O)O*)C([H])([H])C1=C([H])N([H])C2=C([H])C([H])=C([H])C([H])=C12 0.000 description 1
- 230000004614 tumor growth Effects 0.000 description 1
- 108010087967 type I signal peptidase Proteins 0.000 description 1
- OOLLAFOLCSJHRE-ZHAKMVSLSA-N ulipristal acetate Chemical compound C1=CC(N(C)C)=CC=C1[C@@H]1C2=C3CCC(=O)C=C3CC[C@H]2[C@H](CC[C@]2(OC(C)=O)C(C)=O)[C@]2(C)C1 OOLLAFOLCSJHRE-ZHAKMVSLSA-N 0.000 description 1
- 241000701447 unidentified baculovirus Species 0.000 description 1
- 230000003827 upregulation Effects 0.000 description 1
- VBEQCZHXXJYVRD-GACYYNSASA-N uroanthelone Chemical compound C([C@@H](C(=O)N[C@H](C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CS)C(=O)N[C@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)NCC(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N[C@@H](CO)C(=O)NCC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CS)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O)C(C)C)[C@@H](C)O)NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CC=1NC=NC=1)NC(=O)[C@H](CCSC)NC(=O)[C@H](CS)NC(=O)[C@@H](NC(=O)CNC(=O)CNC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CS)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)CNC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@H]1N(CCC1)C(=O)[C@H](CS)NC(=O)CNC(=O)[C@H]1N(CCC1)C(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC(N)=O)C(C)C)[C@@H](C)CC)C1=CC=C(O)C=C1 VBEQCZHXXJYVRD-GACYYNSASA-N 0.000 description 1
- 229960005356 urokinase Drugs 0.000 description 1
- 239000003981 vehicle Substances 0.000 description 1
- 229960003048 vinblastine Drugs 0.000 description 1
- JXLYSJRDGCGARV-XQKSVPLYSA-N vincaleukoblastine Chemical compound C([C@@H](C[C@]1(C(=O)OC)C=2C(=CC3=C([C@]45[C@H]([C@@]([C@H](OC(C)=O)[C@]6(CC)C=CCN([C@H]56)CC4)(O)C(=O)OC)N3C)C=2)OC)C[C@@](C2)(O)CC)N2CCC2=C1NC1=CC=CC=C21 JXLYSJRDGCGARV-XQKSVPLYSA-N 0.000 description 1
- 229960004528 vincristine Drugs 0.000 description 1
- OGWKCGZFUXNPDA-XQKSVPLYSA-N vincristine Chemical compound C([N@]1C[C@@H](C[C@]2(C(=O)OC)C=3C(=CC4=C([C@]56[C@H]([C@@]([C@H](OC(C)=O)[C@]7(CC)C=CCN([C@H]67)CC5)(O)C(=O)OC)N4C=O)C=3)OC)C[C@@](C1)(O)CC)CC1=C2NC2=CC=CC=C12 OGWKCGZFUXNPDA-XQKSVPLYSA-N 0.000 description 1
- OGWKCGZFUXNPDA-UHFFFAOYSA-N vincristine Natural products C1C(CC)(O)CC(CC2(C(=O)OC)C=3C(=CC4=C(C56C(C(C(OC(C)=O)C7(CC)C=CCN(C67)CC5)(O)C(=O)OC)N4C=O)C=3)OC)CN1CCC1=C2NC2=CC=CC=C12 OGWKCGZFUXNPDA-UHFFFAOYSA-N 0.000 description 1
- GBABOYUKABKIAF-GHYRFKGUSA-N vinorelbine Chemical compound C1N(CC=2C3=CC=CC=C3NC=22)CC(CC)=C[C@H]1C[C@]2(C(=O)OC)C1=CC([C@]23[C@H]([C@]([C@H](OC(C)=O)[C@]4(CC)C=CCN([C@H]34)CC2)(O)C(=O)OC)N2C)=C2C=C1OC GBABOYUKABKIAF-GHYRFKGUSA-N 0.000 description 1
- 229960002066 vinorelbine Drugs 0.000 description 1
- 230000029812 viral genome replication Effects 0.000 description 1
- 238000005406 washing Methods 0.000 description 1
- 238000001262 western blot Methods 0.000 description 1
- 238000012447 xenograft mouse model Methods 0.000 description 1
- 229910052727 yttrium Inorganic materials 0.000 description 1
- VWQVUPCCIRVNHF-UHFFFAOYSA-N yttrium atom Chemical compound [Y] VWQVUPCCIRVNHF-UHFFFAOYSA-N 0.000 description 1
- XRASPMIURGNCCH-UHFFFAOYSA-N zoledronic acid Chemical compound OP(=O)(O)C(P(O)(O)=O)(O)CN1C=CN=C1 XRASPMIURGNCCH-UHFFFAOYSA-N 0.000 description 1
- 229960004276 zoledronic acid Drugs 0.000 description 1
- GVJHHUAWPYXKBD-IEOSBIPESA-N α-tocopherol Chemical compound OC1=C(C)C(C)=C2O[C@@](CCC[C@H](C)CCC[C@H](C)CCCC(C)C)(C)CCC2=C1C GVJHHUAWPYXKBD-IEOSBIPESA-N 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2803—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2863—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against receptors for growth factors, growth regulators
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/30—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants from tumour cells
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/32—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against translation products of oncogenes
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K7/00—Peptides having 5 to 20 amino acids in a fully defined sequence; Derivatives thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/505—Medicinal preparations containing antigens or antibodies comprising antibodies
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/20—Immunoglobulins specific features characterized by taxonomic origin
- C07K2317/24—Immunoglobulins specific features characterized by taxonomic origin containing regions, domains or residues from different species, e.g. chimeric, humanized or veneered
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/52—Constant or Fc region; Isotype
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/55—Fab or Fab'
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/56—Immunoglobulins specific features characterized by immunoglobulin fragments variable (Fv) region, i.e. VH and/or VL
- C07K2317/567—Framework region [FR]
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/76—Antagonist effect on antigen, e.g. neutralization or inhibition of binding
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/77—Internalization into the cell
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/90—Immunoglobulins specific features characterized by (pharmaco)kinetic aspects or by stability of the immunoglobulin
- C07K2317/92—Affinity (KD), association rate (Ka), dissociation rate (Kd) or EC50 value
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/30—Non-immunoglobulin-derived peptide or protein having an immunoglobulin constant or Fc region, or a fragment thereof, attached thereto
Landscapes
- Chemical & Material Sciences (AREA)
- Health & Medical Sciences (AREA)
- Organic Chemistry (AREA)
- Immunology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Medicinal Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Biophysics (AREA)
- Genetics & Genomics (AREA)
- Biochemistry (AREA)
- Molecular Biology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Oncology (AREA)
- Cell Biology (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Peptides Or Proteins (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Preparation Of Compounds By Using Micro-Organisms (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
- Medicinal Preparation (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201662276803P | 2016-01-08 | 2016-01-08 | |
| US62/276,803 | 2016-01-08 | ||
| US201662317342P | 2016-04-01 | 2016-04-01 | |
| US62/317,342 | 2016-04-01 | ||
| PCT/US2017/012754 WO2017120599A1 (en) | 2016-01-08 | 2017-01-09 | Self-crosslinking antibodies |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20180098672A true KR20180098672A (ko) | 2018-09-04 |
Family
ID=59274084
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020187022826A Ceased KR20180098672A (ko) | 2016-01-08 | 2017-01-09 | 자가-가교결합 항체 |
Country Status (8)
| Country | Link |
|---|---|
| US (1) | US11459397B2 (enExample) |
| EP (1) | EP3399992A4 (enExample) |
| JP (1) | JP6983808B2 (enExample) |
| KR (1) | KR20180098672A (enExample) |
| CN (1) | CN108712908A (enExample) |
| AU (1) | AU2017205343A1 (enExample) |
| CA (1) | CA3010632A1 (enExample) |
| WO (1) | WO2017120599A1 (enExample) |
Families Citing this family (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| AU2017215350A1 (en) * | 2016-02-02 | 2018-08-09 | Meditope Biosciences, Inc. | Anti-EGFR antibody drug conjugate |
| KR20200130711A (ko) * | 2018-03-12 | 2020-11-19 | 메모리얼 슬로안 케터링 캔서 센터 | 이중특이적 결합제 및 이의 용도 |
| CN109521192B (zh) * | 2018-11-27 | 2021-07-13 | 柏荣诊断产品(上海)有限公司 | 一种提高胶乳比浊试剂检测灵敏度的方法 |
| WO2021072264A1 (en) * | 2019-10-10 | 2021-04-15 | Arizona Board Of Regents On Behalf Of Arizona State University | Oncolytic viruses that express multi-specific immune cell engagers |
| WO2021068971A1 (en) * | 2019-10-12 | 2021-04-15 | Bio-Thera Solutions, Ltd. | Anti-cd20 antibody formulation and use of anti-cd20 antibody for treatment of cd20 positive diseases |
| JPWO2021112249A1 (enExample) * | 2019-12-06 | 2021-06-10 | ||
| KR20220157446A (ko) * | 2020-03-23 | 2022-11-29 | 브리스톨-마이어스 스큅 컴퍼니 | 암을 치료하기 위한 항-ccr8 항체 |
| CN115210262B (zh) * | 2020-09-29 | 2023-07-14 | 昆明赛诺制药股份有限公司 | 人源化抗-cd22重组免疫毒素及其应用 |
| CN112661828B (zh) * | 2021-01-15 | 2022-04-01 | 武汉大学 | 一种突触素的抗原肽及其抗体与应用 |
| WO2022158554A1 (ja) * | 2021-01-22 | 2022-07-28 | 味の素株式会社 | 二量体化した環状ペプチドをスクリーニングする方法 |
| CA3212386A1 (en) * | 2021-03-18 | 2022-09-22 | Alessandro ARCOVITO | Ferritin variants with increased stability and complexation ability |
| CN115677856B (zh) * | 2021-07-29 | 2023-11-28 | 东莞市朋志生物科技有限公司 | 抗人IgM抗体及其应用 |
| JP2024025400A (ja) * | 2022-08-12 | 2024-02-26 | 浜松ホトニクス株式会社 | 膜貫通タンパク質を標的オルガネラに局在化させるためのペプチドリンカー及び局在化方法並びに局在化する融合タンパク質 |
Family Cites Families (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE60124912T2 (de) * | 2001-09-14 | 2007-06-14 | Affimed Therapeutics Ag | Multimerische, einzelkettige, Tandem-Fv-Antikörper |
| NZ553224A (en) * | 2004-07-26 | 2009-05-31 | Asterion Ltd | Linkers |
| US20140127200A1 (en) * | 2008-01-03 | 2014-05-08 | The Scripps Research Institute | Multispecific Antibody Targeting and Multivalency Through Modular Recognition Domains |
| US8962804B2 (en) * | 2010-10-08 | 2015-02-24 | City Of Hope | Meditopes and meditope-binding antibodies and uses thereof |
| CA2814040C (en) * | 2010-10-08 | 2021-03-23 | Joshua Michael Donaldson | A monoclonal antibody framework binding interface for meditopes, meditope delivery systems and methods for their use |
| CN114634572A (zh) * | 2011-10-10 | 2022-06-17 | 希望之城公司 | 中间位和中间位结合抗体及其用途 |
| US9428553B2 (en) * | 2012-02-10 | 2016-08-30 | City Of Hope | Meditopes and meditope-binding antibodies and uses thereof |
| US20180148514A1 (en) * | 2014-10-02 | 2018-05-31 | City Of Hope | Multivalent meditopes, meditope-binding antibodies and uses thereof |
| WO2016187158A1 (en) * | 2015-05-15 | 2016-11-24 | City Of Hope | Chimeric antigen receptor compositions |
| AU2017215350A1 (en) * | 2016-02-02 | 2018-08-09 | Meditope Biosciences, Inc. | Anti-EGFR antibody drug conjugate |
-
2017
- 2017-01-09 EP EP17736526.9A patent/EP3399992A4/en not_active Withdrawn
- 2017-01-09 AU AU2017205343A patent/AU2017205343A1/en not_active Abandoned
- 2017-01-09 US US16/068,892 patent/US11459397B2/en active Active
- 2017-01-09 CA CA3010632A patent/CA3010632A1/en active Pending
- 2017-01-09 CN CN201780016120.2A patent/CN108712908A/zh active Pending
- 2017-01-09 WO PCT/US2017/012754 patent/WO2017120599A1/en not_active Ceased
- 2017-01-09 JP JP2018554653A patent/JP6983808B2/ja not_active Expired - Fee Related
- 2017-01-09 KR KR1020187022826A patent/KR20180098672A/ko not_active Ceased
Also Published As
| Publication number | Publication date |
|---|---|
| JP6983808B2 (ja) | 2021-12-17 |
| CN108712908A (zh) | 2018-10-26 |
| US20190016822A1 (en) | 2019-01-17 |
| EP3399992A4 (en) | 2019-07-31 |
| CA3010632A1 (en) | 2017-07-13 |
| EP3399992A1 (en) | 2018-11-14 |
| WO2017120599A1 (en) | 2017-07-13 |
| JP2019509054A (ja) | 2019-04-04 |
| AU2017205343A1 (en) | 2018-07-19 |
| US11459397B2 (en) | 2022-10-04 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| AU2020289727B2 (en) | Multivalent meditopes, meditope-binding antibodies and uses thereof | |
| KR20180098672A (ko) | 자가-가교결합 항체 | |
| JP7312168B2 (ja) | Cd137に結合するシングルドメイン抗体 | |
| JP7198761B2 (ja) | 抗icosアゴニスト抗体およびそれらの使用 | |
| JP7051692B2 (ja) | Pd-1に特異的に結合する抗体及びその使用 | |
| KR20200110358A (ko) | 항-pd-1 항체 및 치료 방법 | |
| TWI894360B (zh) | 抗tigit抗體及雙抗體和它們的應用 | |
| TW202342501A (zh) | 介白素-21突變蛋白及治療方法 | |
| HK1242589B (en) | Multivalent meditopes, meditope-binding antibodies and uses thereof | |
| HK1242589A1 (en) | Multivalent meditopes, meditope-binding antibodies and uses thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20180808 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| A201 | Request for examination | ||
| PA0201 | Request for examination |
Patent event code: PA02012R01D Patent event date: 20220110 Comment text: Request for Examination of Application |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20231110 Patent event code: PE09021S01D |
|
| PE0601 | Decision on rejection of patent |
Patent event date: 20240626 Comment text: Decision to Refuse Application Patent event code: PE06012S01D |