KR20180098247A - 가향된 니코틴 분말 - Google Patents
가향된 니코틴 분말 Download PDFInfo
- Publication number
- KR20180098247A KR20180098247A KR1020187016365A KR20187016365A KR20180098247A KR 20180098247 A KR20180098247 A KR 20180098247A KR 1020187016365 A KR1020187016365 A KR 1020187016365A KR 20187016365 A KR20187016365 A KR 20187016365A KR 20180098247 A KR20180098247 A KR 20180098247A
- Authority
- KR
- South Korea
- Prior art keywords
- nicotine
- particles
- powder
- flavor
- powder system
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/465—Nicotine; Derivatives thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/06—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite
- A61K47/08—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite containing oxygen, e.g. ethers, acetals, ketones, quinones, aldehydes, peroxides
- A61K47/12—Carboxylic acids; Salts or anhydrides thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/06—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite
- A61K47/16—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite containing nitrogen, e.g. nitro-, nitroso-, azo-compounds, nitriles, cyanates
- A61K47/18—Amines; Amides; Ureas; Quaternary ammonium compounds; Amino acids; Oligopeptides having up to five amino acids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/06—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite
- A61K47/16—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite containing nitrogen, e.g. nitro-, nitroso-, azo-compounds, nitriles, cyanates
- A61K47/18—Amines; Amides; Ureas; Quaternary ammonium compounds; Amino acids; Oligopeptides having up to five amino acids
- A61K47/183—Amino acids, e.g. glycine, EDTA or aspartame
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/007—Pulmonary tract; Aromatherapy
- A61K9/0073—Sprays or powders for inhalation; Aerolised or nebulised preparations generated by other means than thermal energy
- A61K9/0075—Sprays or powders for inhalation; Aerolised or nebulised preparations generated by other means than thermal energy for inhalation via a dry powder inhaler [DPI], e.g. comprising micronized drug mixed with lactose carrier particles
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
- A61K9/141—Intimate drug-carrier mixtures characterised by the carrier, e.g. ordered mixtures, adsorbates, solid solutions, eutectica, co-dried, co-solubilised, co-kneaded, co-milled, co-ground products, co-precipitates, co-evaporates, co-extrudates, co-melts; Drug nanoparticles with adsorbed surface modifiers
- A61K9/145—Intimate drug-carrier mixtures characterised by the carrier, e.g. ordered mixtures, adsorbates, solid solutions, eutectica, co-dried, co-solubilised, co-kneaded, co-milled, co-ground products, co-precipitates, co-evaporates, co-extrudates, co-melts; Drug nanoparticles with adsorbed surface modifiers with organic compounds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
- A61K9/50—Microcapsules having a gas, liquid or semi-solid filling; Solid microparticles or pellets surrounded by a distinct coating layer, e.g. coated microspheres, coated drug crystals
- A61K9/5005—Wall or coating material
- A61K9/5015—Organic compounds, e.g. fats, sugars
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0001—Details of inhalators; Constructional features thereof
- A61M15/0011—Details of inhalators; Constructional features thereof with microcapsules, e.g. several in one dose
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0001—Details of inhalators; Constructional features thereof
- A61M15/002—Details of inhalators; Constructional features thereof with air flow regulating means
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0001—Details of inhalators; Constructional features thereof
- A61M15/0021—Mouthpieces therefor
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0028—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up
- A61M15/003—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using capsules, e.g. to be perforated or broken-up
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0028—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up
- A61M15/003—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using capsules, e.g. to be perforated or broken-up
- A61M15/0033—Details of the piercing or cutting means
- A61M15/0035—Piercing means
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0028—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up
- A61M15/0063—Storages for pre-packed dosages
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/06—Inhaling appliances shaped like cigars, cigarettes or pipes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2202/00—Special media to be introduced, removed or treated
- A61M2202/06—Solids
- A61M2202/064—Powder
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2205/00—General characteristics of the apparatus
- A61M2205/33—Controlling, regulating or measuring
- A61M2205/3331—Pressure; Flow
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2210/00—Anatomical parts of the body
- A61M2210/10—Trunk
- A61M2210/1025—Respiratory system
- A61M2210/1039—Lungs
Landscapes
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Life Sciences & Earth Sciences (AREA)
- Veterinary Medicine (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Chemical & Material Sciences (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Epidemiology (AREA)
- Pulmonology (AREA)
- Anesthesiology (AREA)
- Biomedical Technology (AREA)
- Heart & Thoracic Surgery (AREA)
- Hematology (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Otolaryngology (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicinal Preparation (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
- Saccharide Compounds (AREA)
- Plural Heterocyclic Compounds (AREA)
- Seasonings (AREA)
- Manufacture Of Tobacco Products (AREA)
Abstract
Description
Claims (18)
- 분말 시스템으로서,
약 10㎛ 이하의 입자 크기를 가지고 니코틴 및 아미노산을 포함하는 제1 복수의 입자; 및
약 20㎛ 이상의 입자 크기를 가지고 향미를 포함하는 제2 복수의 입자를 포함하는 분말 시스템. - 분말 시스템으로서,
약 10㎛ 이하의 입자 크기를 가지고 니코틴 피루빈산염, 니코틴 모노-피루빈산염, 니코틴 아스파르트산염 및 니코틴 젖산염으로 이루어지는 군에서 선택된 니코틴을 포함하는 제1 복수의 입자; 및
약 20㎛ 이상의 입자 크기를 가지고 향미를 포함하는 제2 복수의 입자를 포함하는 분말 시스템. - 제1항 또는 제2항에 있어서, 상기 분말 시스템의 상기 니코틴의 적어도 약 60wt%는 약 10㎛ 이하의 입자 크기를 가지는 입자에 포함되는, 분말 시스템.
- 제1항 내지 제3항 중 어느 한 항에 있어서, 상기 분말 시스템의 상기 향미의 적어도 약 60wt%는 약 20㎛ 이상의 입자 크기를 가지는 입자에 포함되는, 분말 시스템.
- 제1항 내지 제4항 중 어느 한 항에 있어서, 상기 제1 복수의 입자는 약 5㎛ 이하, 약 3㎛ 이하, 또는 약 1㎛ 내지 약 3㎛ 범위의 질량 중앙 공기역학적 직경을 가지고, 상기 제2 복수의 입자는 약 50㎛ 이상, 또는 약 50㎛ 내지 약 150㎛ 범위의 질량 중앙 공기역학적 직경을 가지는 분말 시스템.
- 제1항 내지 제5항 중 어느 한 항에 있어서, 상기 니코틴은 니코틴 염 또는 니코틴 염 수화물을 포함하는 분말 시스템.
- 제1항 및 제3항 내지 제6항 중 어느 한 항에 있어서, 상기 아미노산은 니코틴 상에 배치되는 분말 시스템.
- 제1항 및 제3항 내지 제7항 중 어느 한 항에 있어서, 상기 아미노산은 류신을 포함하는 분말 시스템.
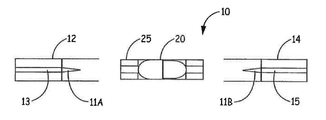
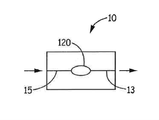
- 제1항 내지 제8항 중 어느 한 항에 있어서, 상기 제1 복수의 입자 및 상기 제2 복수의 입자는 단일 캡슐에 담기는 분말 시스템.
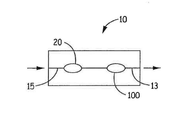
- 제1항 내지 제8항 중 어느 한 항에 있어서, 상기 제1 복수의 입자는 제1 캡슐에 담기고, 상기 제2 복수의 입자는 제2 캡슐에 담기는 분말 시스템.
- 제1항 내지 제10항 중 어느 한 항에 있어서, 상기 제1 복수의 입자는 상기 분말 시스템 총 중량의 약 50wt% 내지 약 99wt%이고, 상기 제2 복수의 입자는 상기 분말 시스템 총 중량의 약 50wt% 내지 약 1wt%인 분말 시스템.
- 제1항 내지 제11항 중 어느 한 항에 있어서, 상기 제2 복수의 입자는 스테아린산 마그네슘(magnesium stearate)을 포함하는 분말 시스템.
- 니코틴 분말 흡입기로서,
마우스피스부와 원위 단부 사이에서 연장되는 바디부;
상기 마우스피스부와 상기 원위 단부 사이에서 연장되는 기류 채널; 및
상기 기류 채널을 따라 배치된 니코틴 분말 수용부 및 상기 니코틴 분말 수용부 내에 배치된 제1항 내지 제12항 중 어느 한 항의 상기 분말 시스템을 포함하는 니코틴 분말 흡입기. - 제13항에 있어서, 상기 제1 복수의 입자 및 상기 제2 복수의 입자는 단일 캡슐 내에 담기고, 상기 캡슐은 상기 니코틴 분말 수용부 내에 배치되며, 상기 제1 복수의 입자 및 상기 제2 복수의 입자는 상기 단일 캡슐로부터 상기 기류 채널 내에 방출되는 니코틴 분말 흡입기.
- 제13항에 있어서, 상기 제1 복수의 입자는 제1 캡슐 내에 담기고, 상기 제2 복수의 입자는 제2 캡슐 내에 담기며, 상기 제2 캡슐은 상기 제1 캡슐의 상류 또는 하류에 있으며 상기 기류 채널 내에 있는 니코틴 분말 흡입기.
- 제13항에 있어서, 상기 니코틴 분말 수용부와 평행한 기류 관계에 있는 제2 기류 채널을 더 포함하되, 상기 제1 복수의 입자는 상기 니코틴 분말 수용부 내의 제1 캡슐에 담기고, 상기 제2 복수의 입자는 상기 제2 기류 채널 내의 제2 캡슐에 담기는 니코틴 분말 흡입기.
- 제13항 내지 제16항 중 어느 한 항에 있어서, 상기 제1 복수의 입자는 분당 약 5리터 미만의 흡입 속도로 사용자에 폐에 흡입되는 니코틴 분말 흡입기.
- 니코틴을 사용자의 폐에 흡입시키는 방법으로서,
상기 제1 복수의 입자를 사용자의 폐에 전달하기 위해 제12항 내지 제17항 중 어느 한 항의 상기 니코틴 분말 흡입기를 통해 분당 약 2리터 미만의 유속으로 공기를 흡입하는 단계를 포함하되, 상기 제2 복수의 입자는 상기 사용자의 폐에 전달되지 않는 방법.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR1020257012414A KR20250058060A (ko) | 2015-12-24 | 2016-12-08 | 가향된 니코틴 분말 |
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP15202728 | 2015-12-24 | ||
| EP15202728.0 | 2015-12-24 | ||
| PCT/IB2016/057452 WO2017109625A1 (en) | 2015-12-24 | 2016-12-08 | Flavoured nicotine powder |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020257012414A Division KR20250058060A (ko) | 2015-12-24 | 2016-12-08 | 가향된 니코틴 분말 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20180098247A true KR20180098247A (ko) | 2018-09-03 |
Family
ID=55066404
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020187016365A Ceased KR20180098247A (ko) | 2015-12-24 | 2016-12-08 | 가향된 니코틴 분말 |
| KR1020257012414A Pending KR20250058060A (ko) | 2015-12-24 | 2016-12-08 | 가향된 니코틴 분말 |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020257012414A Pending KR20250058060A (ko) | 2015-12-24 | 2016-12-08 | 가향된 니코틴 분말 |
Country Status (19)
| Country | Link |
|---|---|
| US (2) | US10751336B2 (ko) |
| EP (1) | EP3393451B1 (ko) |
| JP (3) | JP2019505479A (ko) |
| KR (2) | KR20180098247A (ko) |
| CN (2) | CN117695207A (ko) |
| AU (1) | AU2016376027B2 (ko) |
| CA (1) | CA3008208A1 (ko) |
| ES (1) | ES2952674T3 (ko) |
| HU (1) | HUE063028T2 (ko) |
| IL (1) | IL259936B2 (ko) |
| MX (1) | MX386252B (ko) |
| MY (1) | MY186270A (ko) |
| PH (1) | PH12018501142A1 (ko) |
| PL (1) | PL3393451T3 (ko) |
| RU (1) | RU2762084C2 (ko) |
| SG (1) | SG11201804598RA (ko) |
| UA (1) | UA122699C2 (ko) |
| WO (1) | WO2017109625A1 (ko) |
| ZA (1) | ZA201803584B (ko) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2024090890A1 (ko) * | 2022-10-27 | 2024-05-02 | 주식회사 케이티앤지 | 흡입용 저용량 니코틴 건조 분말 조성물 |
Families Citing this family (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| PL3393451T3 (pl) | 2015-12-24 | 2023-12-27 | Philip Morris Products S.A. | Aromatyzowany proszek nikotynowy |
| KR20200023285A (ko) * | 2017-06-28 | 2020-03-04 | 필립모리스 프로덕츠 에스.에이. | 흡입기와 함께 사용하기 위한 입자를 가진 컨테이너 |
| ES2965397T3 (es) * | 2019-11-14 | 2024-04-15 | Philip Morris Products Sa | Formulación mejorada de polvo seco con sabor a tabaco |
| USD1081739S1 (en) | 2021-04-06 | 2025-07-01 | Altria Client Services Llc | Die for gum forming |
| WO2023138670A1 (en) * | 2022-01-21 | 2023-07-27 | The University Of Hong Kong | Dual targeting powder formulation of antiviral agent for nasal and lung deposition through single intranasal administration |
| US12295412B2 (en) | 2022-01-28 | 2025-05-13 | Altria Client Services Llc | Oral pouch product |
| WO2025052299A1 (en) * | 2023-09-08 | 2025-03-13 | Philip Morris Products S.A. | Hybrid aerosol and powder generating consumable article and system |
Family Cites Families (24)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4655229A (en) * | 1984-01-30 | 1987-04-07 | R. J. Reynolds Tobacco Company | Flavor delivery system |
| US4735217A (en) | 1986-08-21 | 1988-04-05 | The Procter & Gamble Company | Dosing device to provide vaporized medicament to the lungs as a fine aerosol |
| NO168921C (no) | 1989-07-31 | 1992-04-22 | Svein Knudsen | Roekfri sigaretterstatning til bruk ved roekavvenning ellertil bruk i roekfrie miljoeer |
| US5441060A (en) * | 1993-02-08 | 1995-08-15 | Duke University | Dry powder delivery system |
| US6102036A (en) * | 1994-04-12 | 2000-08-15 | Smoke-Stop | Breath activated inhaler |
| GB9515182D0 (en) * | 1995-07-24 | 1995-09-20 | Co Ordinated Drug Dev | Improvements in and relating to powders for use in dry powder inhalers |
| MXPA02005666A (es) | 2000-10-12 | 2002-11-29 | Boehringer Ingelheim Pharma | Nuevos polvos para anhalacion que contienen tiotropio. |
| CA2538997A1 (en) | 2003-09-15 | 2005-03-24 | Vectura Limited | Pharmaceutical compositions for treating premature ejaculation by pulmonary inhalation |
| GB0426301D0 (en) | 2004-11-30 | 2004-12-29 | Vectura Ltd | Pharmaceutical formulations |
| GB2461008B (en) | 2006-12-08 | 2011-08-10 | Exchange Supplies Ltd | Nicotine inhalation device |
| EP2708256A3 (en) | 2007-03-30 | 2014-04-02 | Philip Morris Products S.A. | Device and method for delivery of a medicament |
| GB0712308D0 (en) | 2007-06-25 | 2007-08-01 | Kind Group Ltd | An inhalable composition |
| RU2011138941A (ru) * | 2009-02-23 | 2013-11-20 | Джапан Тобакко Инк. | Ненагреваемое устройство для всасывания табачного аромата |
| GB0905840D0 (en) * | 2009-04-06 | 2009-05-20 | Sagentia Ltd | Apparatus and methods |
| US20110268809A1 (en) * | 2010-04-28 | 2011-11-03 | Paul Andrew Brinkley | Nicotine-Containing Pharmaceutical Compositions |
| EP2648788B1 (en) | 2010-12-07 | 2017-08-09 | Respira Therapeutics, Inc. | Dry powder inhaler |
| US20150136160A1 (en) | 2012-06-08 | 2015-05-21 | Foundation Brands, Llc | Oral Stimulatory Product |
| US20140088045A1 (en) | 2012-09-21 | 2014-03-27 | Basil Rigas | Product comprising a nicotine-containing material and an anti-cancer agent |
| US20140261488A1 (en) * | 2013-03-15 | 2014-09-18 | Altria Client Services Inc. | Electronic smoking article |
| US20150283070A1 (en) | 2014-04-08 | 2015-10-08 | Sansa Corporation (Barbados) Inc. | Nicotine Formulations and Methods of Making the Same |
| EP3136895B1 (en) | 2014-04-28 | 2023-06-21 | Philip Morris Products S.A. | Flavoured nicotine powder inhaler |
| WO2015166344A1 (en) | 2014-04-28 | 2015-11-05 | Philip Morris Products S.A. | Nicotine powder inhaler |
| SG11201610498PA (en) | 2014-06-20 | 2017-01-27 | Philip Morris Products Sa | Nicotine powder delivery system with airflow management means |
| PL3393451T3 (pl) | 2015-12-24 | 2023-12-27 | Philip Morris Products S.A. | Aromatyzowany proszek nikotynowy |
-
2016
- 2016-12-08 PL PL16812864.3T patent/PL3393451T3/pl unknown
- 2016-12-08 MY MYPI2018702108A patent/MY186270A/en unknown
- 2016-12-08 JP JP2018529277A patent/JP2019505479A/ja active Pending
- 2016-12-08 KR KR1020187016365A patent/KR20180098247A/ko not_active Ceased
- 2016-12-08 HU HUE16812864A patent/HUE063028T2/hu unknown
- 2016-12-08 MX MX2018007507A patent/MX386252B/es unknown
- 2016-12-08 CN CN202311657737.6A patent/CN117695207A/zh active Pending
- 2016-12-08 CA CA3008208A patent/CA3008208A1/en active Pending
- 2016-12-08 CN CN201680070878.XA patent/CN108289841A/zh active Pending
- 2016-12-08 EP EP16812864.3A patent/EP3393451B1/en active Active
- 2016-12-08 UA UAA201803888A patent/UA122699C2/uk unknown
- 2016-12-08 KR KR1020257012414A patent/KR20250058060A/ko active Pending
- 2016-12-08 US US16/064,170 patent/US10751336B2/en active Active
- 2016-12-08 ES ES16812864T patent/ES2952674T3/es active Active
- 2016-12-08 SG SG11201804598RA patent/SG11201804598RA/en unknown
- 2016-12-08 WO PCT/IB2016/057452 patent/WO2017109625A1/en not_active Ceased
- 2016-12-08 RU RU2018126872A patent/RU2762084C2/ru active
- 2016-12-08 AU AU2016376027A patent/AU2016376027B2/en active Active
-
2018
- 2018-05-30 ZA ZA2018/03584A patent/ZA201803584B/en unknown
- 2018-05-31 PH PH12018501142A patent/PH12018501142A1/en unknown
- 2018-06-11 IL IL259936A patent/IL259936B2/en unknown
-
2020
- 2020-08-17 US US16/994,932 patent/US12414946B2/en active Active
-
2023
- 2023-05-30 JP JP2023088626A patent/JP2023113755A/ja active Pending
-
2025
- 2025-06-26 JP JP2025107898A patent/JP2025126285A/ja active Pending
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2024090890A1 (ko) * | 2022-10-27 | 2024-05-02 | 주식회사 케이티앤지 | 흡입용 저용량 니코틴 건조 분말 조성물 |
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US12233204B2 (en) | Nicotine powder delivery system | |
| US12478580B2 (en) | Flavoured nicotine powder inhaler | |
| US12414946B2 (en) | Flavored nicotine powder | |
| KR20190026656A (ko) | 니코틴 입자 전달 소모품 | |
| RU2824230C2 (ru) | Система доставки никотинового порошка | |
| HK1261338A1 (en) | Flavoured nicotine powder | |
| HK1261338B (en) | Flavoured nicotine powder | |
| HK40029937A (en) | Nicotine powder delivery system |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
St.27 status event code: A-0-1-A10-A15-nap-PA0105 |
|
| R17-X000 | Change to representative recorded |
St.27 status event code: A-3-3-R10-R17-oth-X000 |
|
| PG1501 | Laying open of application |
St.27 status event code: A-1-1-Q10-Q12-nap-PG1501 |
|
| R17-X000 | Change to representative recorded |
St.27 status event code: A-3-3-R10-R17-oth-X000 |
|
| R17-X000 | Change to representative recorded |
St.27 status event code: A-3-3-R10-R17-oth-X000 |
|
| A201 | Request for examination | ||
| PA0201 | Request for examination |
St.27 status event code: A-1-2-D10-D11-exm-PA0201 |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
St.27 status event code: A-1-2-D10-D21-exm-PE0902 |
|
| E13-X000 | Pre-grant limitation requested |
St.27 status event code: A-2-3-E10-E13-lim-X000 |
|
| P11-X000 | Amendment of application requested |
St.27 status event code: A-2-2-P10-P11-nap-X000 |
|
| P13-X000 | Application amended |
St.27 status event code: A-2-2-P10-P13-nap-X000 |
|
| R18-X000 | Changes to party contact information recorded |
St.27 status event code: A-3-3-R10-R18-oth-X000 |
|
| PE0601 | Decision on rejection of patent |
St.27 status event code: N-2-6-B10-B15-exm-PE0601 |
|
| E13-X000 | Pre-grant limitation requested |
St.27 status event code: A-2-3-E10-E13-lim-X000 |
|
| P11-X000 | Amendment of application requested |
St.27 status event code: A-2-2-P10-P11-nap-X000 |
|
| PX0601 | Decision of rejection after re-examination |
St.27 status event code: N-2-6-B10-B17-rex-PX0601 |
|
| X601 | Decision of rejection after re-examination | ||
| PA0104 | Divisional application for international application |
St.27 status event code: A-0-1-A10-A16-div-PA0104 |