KR20170002550A - 티에노트리아졸로디아제핀 화합물을 사용한 내성 비호지킨 림프종, 수아세포종, 및/또는 alk+ 비소세포 폐암의 치료 방법 - Google Patents
티에노트리아졸로디아제핀 화합물을 사용한 내성 비호지킨 림프종, 수아세포종, 및/또는 alk+ 비소세포 폐암의 치료 방법 Download PDFInfo
- Publication number
- KR20170002550A KR20170002550A KR1020167033941A KR20167033941A KR20170002550A KR 20170002550 A KR20170002550 A KR 20170002550A KR 1020167033941 A KR1020167033941 A KR 1020167033941A KR 20167033941 A KR20167033941 A KR 20167033941A KR 20170002550 A KR20170002550 A KR 20170002550A
- Authority
- KR
- South Korea
- Prior art keywords
- compound
- solid dispersion
- lymphoma
- pharmaceutically acceptable
- formula
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
- 150000001875 compounds Chemical class 0.000 title claims abstract description 327
- 208000015914 Non-Hodgkin lymphomas Diseases 0.000 title claims abstract description 43
- 208000002154 non-small cell lung carcinoma Diseases 0.000 title claims abstract description 29
- 208000029729 tumor suppressor gene on chromosome 11 Diseases 0.000 title claims abstract description 29
- 238000000034 method Methods 0.000 title claims description 90
- 208000000172 Medulloblastoma Diseases 0.000 title 1
- 125000004432 carbon atom Chemical group C* 0.000 claims abstract description 51
- 125000000217 alkyl group Chemical group 0.000 claims abstract description 49
- 125000005843 halogen group Chemical group 0.000 claims abstract description 37
- 125000004435 hydrogen atom Chemical group [H]* 0.000 claims abstract description 19
- 125000003545 alkoxy group Chemical group 0.000 claims abstract description 15
- 125000004093 cyano group Chemical group *C#N 0.000 claims abstract description 12
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 claims abstract description 11
- 125000002887 hydroxy group Chemical group [H]O* 0.000 claims abstract description 8
- 125000004076 pyridyl group Chemical group 0.000 claims abstract description 6
- 239000007962 solid dispersion Substances 0.000 claims description 222
- 239000000203 mixture Substances 0.000 claims description 102
- 229920000642 polymer Polymers 0.000 claims description 62
- 150000003839 salts Chemical class 0.000 claims description 57
- -1 methyl (S) - {4- (3'-cyanobiphenyl-4-yl) -2,3,9-trimethyl-6H-thieno [3,2- f] [1 , 2,4] triazolo [4,3-a] [1,4] diazepin-6-yl} acetate Chemical compound 0.000 claims description 42
- 239000012453 solvate Substances 0.000 claims description 42
- 229920000639 hydroxypropylmethylcellulose acetate succinate Polymers 0.000 claims description 39
- 102100033793 ALK tyrosine kinase receptor Human genes 0.000 claims description 32
- ZUAAPNNKRHMPKG-UHFFFAOYSA-N acetic acid;butanedioic acid;methanol;propane-1,2-diol Chemical compound OC.CC(O)=O.CC(O)CO.OC(=O)CCC(O)=O ZUAAPNNKRHMPKG-UHFFFAOYSA-N 0.000 claims description 29
- 239000003814 drug Substances 0.000 claims description 27
- 238000000634 powder X-ray diffraction Methods 0.000 claims description 26
- 230000009477 glass transition Effects 0.000 claims description 20
- 241000124008 Mammalia Species 0.000 claims description 18
- 101150023956 ALK gene Proteins 0.000 claims description 11
- 239000002955 immunomodulating agent Substances 0.000 claims description 11
- 229940121354 immunomodulator Drugs 0.000 claims description 11
- 210000001161 mammalian embryo Anatomy 0.000 claims description 11
- 210000004881 tumor cell Anatomy 0.000 claims description 11
- 229940124291 BTK inhibitor Drugs 0.000 claims description 10
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 claims description 10
- 238000001694 spray drying Methods 0.000 claims description 10
- 229940124292 CD20 monoclonal antibody Drugs 0.000 claims description 9
- 230000008569 process Effects 0.000 claims description 9
- 210000004027 cell Anatomy 0.000 claims description 8
- 239000003276 histone deacetylase inhibitor Substances 0.000 claims description 8
- 125000003854 p-chlorophenyl group Chemical group [H]C1=C([H])C(*)=C([H])C([H])=C1Cl 0.000 claims description 8
- 206010018852 Haematoma Diseases 0.000 claims description 7
- 208000031671 Large B-Cell Diffuse Lymphoma Diseases 0.000 claims description 7
- 206010012818 diffuse large B-cell lymphoma Diseases 0.000 claims description 7
- 150000004677 hydrates Chemical class 0.000 claims description 7
- 230000002584 immunomodulator Effects 0.000 claims description 7
- 229940124302 mTOR inhibitor Drugs 0.000 claims description 7
- 239000003628 mammalian target of rapamycin inhibitor Substances 0.000 claims description 7
- 229940121372 histone deacetylase inhibitor Drugs 0.000 claims description 6
- 201000001441 melanoma Diseases 0.000 claims description 6
- 208000028564 B-cell non-Hodgkin lymphoma Diseases 0.000 claims description 5
- 208000025205 Mantle-Cell Lymphoma Diseases 0.000 claims description 5
- 239000003795 chemical substances by application Substances 0.000 claims description 5
- 239000000843 powder Substances 0.000 claims description 5
- 201000006845 reticulosarcoma Diseases 0.000 claims description 5
- 208000029922 reticulum cell sarcoma Diseases 0.000 claims description 5
- 201000004085 CLL/SLL Diseases 0.000 claims description 4
- 208000031422 Lymphocytic Chronic B-Cell Leukemia Diseases 0.000 claims description 4
- 206010052178 Lymphocytic lymphoma Diseases 0.000 claims description 4
- 206010025323 Lymphomas Diseases 0.000 claims description 4
- 206010029260 Neuroblastoma Diseases 0.000 claims description 4
- 206010039491 Sarcoma Diseases 0.000 claims description 4
- 208000027585 T-cell non-Hodgkin lymphoma Diseases 0.000 claims description 4
- 208000023738 chronic lymphocytic leukemia/small lymphocytic lymphoma Diseases 0.000 claims description 4
- 210000002808 connective tissue Anatomy 0.000 claims description 4
- 239000003112 inhibitor Substances 0.000 claims description 4
- 101150010028 TFG gene Proteins 0.000 claims description 3
- 230000000694 effects Effects 0.000 claims description 3
- 101150068690 eml4 gene Proteins 0.000 claims description 3
- 201000003444 follicular lymphoma Diseases 0.000 claims description 3
- 101150030839 Klc1 gene Proteins 0.000 claims description 2
- 208000003906 hydrocephalus Diseases 0.000 claims 2
- 108700026220 vif Genes Proteins 0.000 claims 1
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 54
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 54
- 239000001267 polyvinylpyrrolidone Substances 0.000 description 53
- 239000006185 dispersion Substances 0.000 description 51
- 238000009472 formulation Methods 0.000 description 41
- 239000008194 pharmaceutical composition Substances 0.000 description 39
- 229940079593 drug Drugs 0.000 description 26
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 24
- 239000002904 solvent Substances 0.000 description 21
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 20
- 239000000243 solution Substances 0.000 description 19
- 239000002775 capsule Substances 0.000 description 18
- HBAQYPYDRFILMT-UHFFFAOYSA-N 8-[3-(1-cyclopropylpyrazol-4-yl)-1H-pyrazolo[4,3-d]pyrimidin-5-yl]-3-methyl-3,8-diazabicyclo[3.2.1]octan-2-one Chemical class C1(CC1)N1N=CC(=C1)C1=NNC2=C1N=C(N=C2)N1C2C(N(CC1CC2)C)=O HBAQYPYDRFILMT-UHFFFAOYSA-N 0.000 description 17
- 238000012360 testing method Methods 0.000 description 17
- 125000001424 substituent group Chemical group 0.000 description 16
- 238000001990 intravenous administration Methods 0.000 description 15
- 238000002360 preparation method Methods 0.000 description 15
- 239000007921 spray Substances 0.000 description 15
- 238000004090 dissolution Methods 0.000 description 14
- 239000002552 dosage form Substances 0.000 description 12
- 239000002609 medium Substances 0.000 description 12
- 241001465754 Metazoa Species 0.000 description 11
- IYKJEILNJZQJPU-UHFFFAOYSA-N acetic acid;butanedioic acid Chemical compound CC(O)=O.OC(=O)CCC(O)=O IYKJEILNJZQJPU-UHFFFAOYSA-N 0.000 description 11
- 229920003088 hydroxypropyl methyl cellulose Polymers 0.000 description 11
- 239000000546 pharmaceutical excipient Substances 0.000 description 11
- 241000700159 Rattus Species 0.000 description 10
- 238000002474 experimental method Methods 0.000 description 10
- 238000000338 in vitro Methods 0.000 description 10
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 description 10
- 238000002844 melting Methods 0.000 description 10
- 230000008018 melting Effects 0.000 description 10
- 206010028980 Neoplasm Diseases 0.000 description 9
- 239000002253 acid Substances 0.000 description 9
- 239000003968 dna methyltransferase inhibitor Substances 0.000 description 9
- 239000012530 fluid Substances 0.000 description 9
- 239000008187 granular material Substances 0.000 description 9
- 230000036470 plasma concentration Effects 0.000 description 9
- 230000015572 biosynthetic process Effects 0.000 description 8
- 239000000314 lubricant Substances 0.000 description 8
- 239000002245 particle Substances 0.000 description 8
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 description 7
- 229920000168 Microcrystalline cellulose Polymers 0.000 description 7
- 239000002202 Polyethylene glycol Substances 0.000 description 7
- 239000001866 hydroxypropyl methyl cellulose Substances 0.000 description 7
- 235000010979 hydroxypropyl methyl cellulose Nutrition 0.000 description 7
- 239000008108 microcrystalline cellulose Substances 0.000 description 7
- 229940016286 microcrystalline cellulose Drugs 0.000 description 7
- 235000019813 microcrystalline cellulose Nutrition 0.000 description 7
- 229920001223 polyethylene glycol Polymers 0.000 description 7
- 239000000725 suspension Substances 0.000 description 7
- LRANPJDWHYRCER-UHFFFAOYSA-N 1,2-diazepine Chemical compound N1C=CC=CC=N1 LRANPJDWHYRCER-UHFFFAOYSA-N 0.000 description 6
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 6
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 6
- 229940126190 DNA methyltransferase inhibitor Drugs 0.000 description 6
- 229920003134 Eudragit® polymer Polymers 0.000 description 6
- VVQNEPGJFQJSBK-UHFFFAOYSA-N Methyl methacrylate Chemical compound COC(=O)C(C)=C VVQNEPGJFQJSBK-UHFFFAOYSA-N 0.000 description 6
- 102000013814 Wnt Human genes 0.000 description 6
- 108050003627 Wnt Proteins 0.000 description 6
- 229910052799 carbon Inorganic materials 0.000 description 6
- 239000000945 filler Substances 0.000 description 6
- UFVKGYZPFZQRLF-UHFFFAOYSA-N hydroxypropyl methyl cellulose Chemical compound OC1C(O)C(OC)OC(CO)C1OC1C(O)C(O)C(OC2C(C(O)C(OC3C(C(O)C(O)C(CO)O3)O)C(CO)O2)O)C(CO)O1 UFVKGYZPFZQRLF-UHFFFAOYSA-N 0.000 description 6
- 238000001727 in vivo Methods 0.000 description 6
- 239000000825 pharmaceutical preparation Substances 0.000 description 6
- KDYFGRWQOYBRFD-UHFFFAOYSA-L succinate(2-) Chemical compound [O-]C(=O)CCC([O-])=O KDYFGRWQOYBRFD-UHFFFAOYSA-L 0.000 description 6
- 241000282472 Canis lupus familiaris Species 0.000 description 5
- 241000282412 Homo Species 0.000 description 5
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 5
- 238000010521 absorption reaction Methods 0.000 description 5
- 239000000654 additive Substances 0.000 description 5
- 125000003342 alkenyl group Chemical group 0.000 description 5
- 239000011203 carbon fibre reinforced carbon Substances 0.000 description 5
- 229920001577 copolymer Polymers 0.000 description 5
- 208000035250 cutaneous malignant susceptibility to 1 melanoma Diseases 0.000 description 5
- 238000001035 drying Methods 0.000 description 5
- 239000004615 ingredient Substances 0.000 description 5
- 235000019359 magnesium stearate Nutrition 0.000 description 5
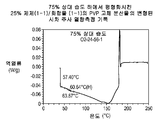
- 238000001565 modulated differential scanning calorimetry Methods 0.000 description 5
- 239000003826 tablet Substances 0.000 description 5
- WSVLPVUVIUVCRA-KPKNDVKVSA-N Alpha-lactose monohydrate Chemical compound O.O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O WSVLPVUVIUVCRA-KPKNDVKVSA-N 0.000 description 4
- 229920002785 Croscarmellose sodium Polymers 0.000 description 4
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 4
- VZCYOOQTPOCHFL-OWOJBTEDSA-N Fumaric acid Chemical compound OC(=O)\C=C\C(O)=O VZCYOOQTPOCHFL-OWOJBTEDSA-N 0.000 description 4
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 4
- DPXJVFZANSGRMM-UHFFFAOYSA-N acetic acid;2,3,4,5,6-pentahydroxyhexanal;sodium Chemical compound [Na].CC(O)=O.OCC(O)C(O)C(O)C(O)C=O DPXJVFZANSGRMM-UHFFFAOYSA-N 0.000 description 4
- 239000002168 alkylating agent Substances 0.000 description 4
- 229940100198 alkylating agent Drugs 0.000 description 4
- 238000004458 analytical method Methods 0.000 description 4
- 239000007900 aqueous suspension Substances 0.000 description 4
- 239000011230 binding agent Substances 0.000 description 4
- 229940075614 colloidal silicon dioxide Drugs 0.000 description 4
- 238000001816 cooling Methods 0.000 description 4
- 229960001681 croscarmellose sodium Drugs 0.000 description 4
- 235000010947 crosslinked sodium carboxy methyl cellulose Nutrition 0.000 description 4
- 238000011161 development Methods 0.000 description 4
- 238000000113 differential scanning calorimetry Methods 0.000 description 4
- 239000007884 disintegrant Substances 0.000 description 4
- 150000002148 esters Chemical class 0.000 description 4
- 230000003203 everyday effect Effects 0.000 description 4
- 238000012750 in vivo screening Methods 0.000 description 4
- 229960001021 lactose monohydrate Drugs 0.000 description 4
- 238000004519 manufacturing process Methods 0.000 description 4
- 238000002156 mixing Methods 0.000 description 4
- 230000004048 modification Effects 0.000 description 4
- 238000012986 modification Methods 0.000 description 4
- 239000000651 prodrug Substances 0.000 description 4
- 229940002612 prodrug Drugs 0.000 description 4
- 108090000623 proteins and genes Proteins 0.000 description 4
- 238000000935 solvent evaporation Methods 0.000 description 4
- 238000010922 spray-dried dispersion Methods 0.000 description 4
- 230000000707 stereoselective effect Effects 0.000 description 4
- 239000000126 substance Substances 0.000 description 4
- 238000003786 synthesis reaction Methods 0.000 description 4
- GNMUEVRJHCWKTO-FQEVSTJZSA-N 6h-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepine-6-acetamide, 4-(4-chlorophenyl)-n-(4-hydroxyphenyl)-2,3,9-trimethyl-, (6s)- Chemical compound C([C@@H]1N=C(C2=C(N3C(C)=NN=C31)SC(=C2C)C)C=1C=CC(Cl)=CC=1)C(=O)NC1=CC=C(O)C=C1 GNMUEVRJHCWKTO-FQEVSTJZSA-N 0.000 description 3
- 229920003135 Eudragit® L 100-55 Polymers 0.000 description 3
- 102000003964 Histone deacetylase Human genes 0.000 description 3
- 108090000353 Histone deacetylase Proteins 0.000 description 3
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 3
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 3
- 208000022559 Inflammatory bowel disease Diseases 0.000 description 3
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 3
- OFOBLEOULBTSOW-UHFFFAOYSA-N Propanedioic acid Natural products OC(=O)CC(O)=O OFOBLEOULBTSOW-UHFFFAOYSA-N 0.000 description 3
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 3
- 125000000218 acetic acid group Chemical group C(C)(=O)* 0.000 description 3
- 230000009471 action Effects 0.000 description 3
- 230000000996 additive effect Effects 0.000 description 3
- 125000004429 atom Chemical group 0.000 description 3
- 238000000889 atomisation Methods 0.000 description 3
- 210000004369 blood Anatomy 0.000 description 3
- 239000008280 blood Substances 0.000 description 3
- BTANRVKWQNVYAZ-UHFFFAOYSA-N butan-2-ol Chemical compound CCC(C)O BTANRVKWQNVYAZ-UHFFFAOYSA-N 0.000 description 3
- 201000011510 cancer Diseases 0.000 description 3
- 239000001569 carbon dioxide Substances 0.000 description 3
- 229910002092 carbon dioxide Inorganic materials 0.000 description 3
- 239000001913 cellulose Substances 0.000 description 3
- 239000000460 chlorine Substances 0.000 description 3
- 238000004587 chromatography analysis Methods 0.000 description 3
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 3
- 238000002425 crystallisation Methods 0.000 description 3
- 230000008025 crystallization Effects 0.000 description 3
- 230000002354 daily effect Effects 0.000 description 3
- 150000004683 dihydrates Chemical class 0.000 description 3
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 3
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 3
- GDCRSXZBSIRSFR-UHFFFAOYSA-N ethyl prop-2-enoate;2-methylprop-2-enoic acid Chemical compound CC(=C)C(O)=O.CCOC(=O)C=C GDCRSXZBSIRSFR-UHFFFAOYSA-N 0.000 description 3
- 238000011049 filling Methods 0.000 description 3
- 210000001035 gastrointestinal tract Anatomy 0.000 description 3
- 238000005469 granulation Methods 0.000 description 3
- 230000003179 granulation Effects 0.000 description 3
- 238000004128 high performance liquid chromatography Methods 0.000 description 3
- 238000004895 liquid chromatography mass spectrometry Methods 0.000 description 3
- 239000002207 metabolite Substances 0.000 description 3
- 150000007522 mineralic acids Chemical class 0.000 description 3
- 239000008185 minitablet Substances 0.000 description 3
- 150000007524 organic acids Chemical class 0.000 description 3
- 239000008188 pellet Substances 0.000 description 3
- 229920000191 poly(N-vinyl pyrrolidone) Polymers 0.000 description 3
- 239000002244 precipitate Substances 0.000 description 3
- 239000000047 product Substances 0.000 description 3
- 238000001953 recrystallisation Methods 0.000 description 3
- 239000007787 solid Substances 0.000 description 3
- 235000000346 sugar Nutrition 0.000 description 3
- 150000008163 sugars Chemical class 0.000 description 3
- 239000000454 talc Substances 0.000 description 3
- 229910052623 talc Inorganic materials 0.000 description 3
- 235000012222 talc Nutrition 0.000 description 3
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 description 3
- 208000001072 type 2 diabetes mellitus Diseases 0.000 description 3
- 239000000080 wetting agent Substances 0.000 description 3
- GHOKWGTUZJEAQD-ZETCQYMHSA-N (D)-(+)-Pantothenic acid Chemical compound OCC(C)(C)[C@@H](O)C(=O)NCCC(O)=O GHOKWGTUZJEAQD-ZETCQYMHSA-N 0.000 description 2
- CYSGHNMQYZDMIA-UHFFFAOYSA-N 1,3-Dimethyl-2-imidazolidinon Chemical compound CN1CCN(C)C1=O CYSGHNMQYZDMIA-UHFFFAOYSA-N 0.000 description 2
- WNXJIVFYUVYPPR-UHFFFAOYSA-N 1,3-dioxolane Chemical compound C1COCO1 WNXJIVFYUVYPPR-UHFFFAOYSA-N 0.000 description 2
- VBICKXHEKHSIBG-UHFFFAOYSA-N 1-monostearoylglycerol Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCC(O)CO VBICKXHEKHSIBG-UHFFFAOYSA-N 0.000 description 2
- WXTMDXOMEHJXQO-UHFFFAOYSA-N 2,5-dihydroxybenzoic acid Chemical compound OC(=O)C1=CC(O)=CC=C1O WXTMDXOMEHJXQO-UHFFFAOYSA-N 0.000 description 2
- 125000003903 2-propenyl group Chemical group [H]C([*])([H])C([H])=C([H])[H] 0.000 description 2
- XAUDJQYHKZQPEU-KVQBGUIXSA-N 5-aza-2'-deoxycytidine Chemical group O=C1N=C(N)N=CN1[C@@H]1O[C@H](CO)[C@@H](O)C1 XAUDJQYHKZQPEU-KVQBGUIXSA-N 0.000 description 2
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 2
- 241000272517 Anseriformes Species 0.000 description 2
- CIWBSHSKHKDKBQ-JLAZNSOCSA-N Ascorbic acid Chemical compound OC[C@H](O)[C@H]1OC(=O)C(O)=C1O CIWBSHSKHKDKBQ-JLAZNSOCSA-N 0.000 description 2
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 2
- 208000023275 Autoimmune disease Diseases 0.000 description 2
- 102000001805 Bromodomains Human genes 0.000 description 2
- 108050009021 Bromodomains Proteins 0.000 description 2
- 208000011691 Burkitt lymphomas Diseases 0.000 description 2
- 239000004322 Butylated hydroxytoluene Substances 0.000 description 2
- NLZUEZXRPGMBCV-UHFFFAOYSA-N Butylhydroxytoluene Chemical compound CC1=CC(C(C)(C)C)=C(O)C(C(C)(C)C)=C1 NLZUEZXRPGMBCV-UHFFFAOYSA-N 0.000 description 2
- 125000002853 C1-C4 hydroxyalkyl group Chemical group 0.000 description 2
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 2
- 239000004215 Carbon black (E152) Substances 0.000 description 2
- 208000024172 Cardiovascular disease Diseases 0.000 description 2
- 229920000623 Cellulose acetate phthalate Polymers 0.000 description 2
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 2
- RGHNJXZEOKUKBD-SQOUGZDYSA-N D-gluconic acid Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C(O)=O RGHNJXZEOKUKBD-SQOUGZDYSA-N 0.000 description 2
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 2
- 229920003152 Eudragit® RS polymer Polymers 0.000 description 2
- IAJILQKETJEXLJ-UHFFFAOYSA-N Galacturonsaeure Natural products O=CC(O)C(O)C(O)C(O)C(O)=O IAJILQKETJEXLJ-UHFFFAOYSA-N 0.000 description 2
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 2
- AEMRFAOFKBGASW-UHFFFAOYSA-N Glycolic acid Chemical compound OCC(O)=O AEMRFAOFKBGASW-UHFFFAOYSA-N 0.000 description 2
- 208000002250 Hematologic Neoplasms Diseases 0.000 description 2
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 2
- 101150023321 KIF5B gene Proteins 0.000 description 2
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 2
- 229930195725 Mannitol Natural products 0.000 description 2
- AFVFQIVMOAPDHO-UHFFFAOYSA-N Methanesulfonic acid Chemical compound CS(O)(=O)=O AFVFQIVMOAPDHO-UHFFFAOYSA-N 0.000 description 2
- LRHPLDYGYMQRHN-UHFFFAOYSA-N N-Butanol Chemical compound CCCCO LRHPLDYGYMQRHN-UHFFFAOYSA-N 0.000 description 2
- 238000005481 NMR spectroscopy Methods 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical class OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 description 2
- ZTHYODDOHIVTJV-UHFFFAOYSA-N Propyl gallate Chemical compound CCCOC(=O)C1=CC(O)=C(O)C(O)=C1 ZTHYODDOHIVTJV-UHFFFAOYSA-N 0.000 description 2
- 235000021355 Stearic acid Nutrition 0.000 description 2
- 229930006000 Sucrose Natural products 0.000 description 2
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 2
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical class OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 2
- 102000013530 TOR Serine-Threonine Kinases Human genes 0.000 description 2
- 108010065917 TOR Serine-Threonine Kinases Proteins 0.000 description 2
- WYURNTSHIVDZCO-UHFFFAOYSA-N Tetrahydrofuran Chemical compound C1CCOC1 WYURNTSHIVDZCO-UHFFFAOYSA-N 0.000 description 2
- DTQVDTLACAAQTR-UHFFFAOYSA-N Trifluoroacetic acid Chemical compound OC(=O)C(F)(F)F DTQVDTLACAAQTR-UHFFFAOYSA-N 0.000 description 2
- 239000013543 active substance Substances 0.000 description 2
- 230000002776 aggregation Effects 0.000 description 2
- RWZYAGGXGHYGMB-UHFFFAOYSA-N anthranilic acid Chemical compound NC1=CC=CC=C1C(O)=O RWZYAGGXGHYGMB-UHFFFAOYSA-N 0.000 description 2
- 229940121363 anti-inflammatory agent Drugs 0.000 description 2
- 239000002260 anti-inflammatory agent Substances 0.000 description 2
- 239000011324 bead Substances 0.000 description 2
- WPYMKLBDIGXBTP-UHFFFAOYSA-N benzoic acid Chemical compound OC(=O)C1=CC=CC=C1 WPYMKLBDIGXBTP-UHFFFAOYSA-N 0.000 description 2
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 2
- 235000010354 butylated hydroxytoluene Nutrition 0.000 description 2
- 229940095259 butylated hydroxytoluene Drugs 0.000 description 2
- 150000001721 carbon Chemical group 0.000 description 2
- 229940081734 cellulose acetate phthalate Drugs 0.000 description 2
- 239000003153 chemical reaction reagent Substances 0.000 description 2
- 229910052801 chlorine Inorganic materials 0.000 description 2
- 238000000576 coating method Methods 0.000 description 2
- 230000000052 comparative effect Effects 0.000 description 2
- 229960003603 decitabine Drugs 0.000 description 2
- 230000003247 decreasing effect Effects 0.000 description 2
- 239000008121 dextrose Substances 0.000 description 2
- 230000004069 differentiation Effects 0.000 description 2
- 239000003085 diluting agent Substances 0.000 description 2
- XBDQKXXYIPTUBI-UHFFFAOYSA-N dimethylselenoniopropionate Natural products CCC(O)=O XBDQKXXYIPTUBI-UHFFFAOYSA-N 0.000 description 2
- 201000010099 disease Diseases 0.000 description 2
- 239000002270 dispersing agent Substances 0.000 description 2
- 238000007922 dissolution test Methods 0.000 description 2
- GUVUOGQBMYCBQP-UHFFFAOYSA-N dmpu Chemical compound CN1CCCN(C)C1=O GUVUOGQBMYCBQP-UHFFFAOYSA-N 0.000 description 2
- FSXVSUSRJXIJHB-UHFFFAOYSA-M ethyl prop-2-enoate;methyl 2-methylprop-2-enoate;trimethyl-[2-(2-methylprop-2-enoyloxy)ethyl]azanium;chloride Chemical compound [Cl-].CCOC(=O)C=C.COC(=O)C(C)=C.CC(=C)C(=O)OCC[N+](C)(C)C FSXVSUSRJXIJHB-UHFFFAOYSA-M 0.000 description 2
- 238000011156 evaluation Methods 0.000 description 2
- 238000013213 extrapolation Methods 0.000 description 2
- 238000001125 extrusion Methods 0.000 description 2
- 229910052731 fluorine Inorganic materials 0.000 description 2
- 239000001530 fumaric acid Substances 0.000 description 2
- 235000011087 fumaric acid Nutrition 0.000 description 2
- 230000006870 function Effects 0.000 description 2
- 125000000524 functional group Chemical group 0.000 description 2
- 230000004927 fusion Effects 0.000 description 2
- 229920000159 gelatin Polymers 0.000 description 2
- 239000007903 gelatin capsule Substances 0.000 description 2
- 235000019322 gelatine Nutrition 0.000 description 2
- 239000011521 glass Substances 0.000 description 2
- 230000003394 haemopoietic effect Effects 0.000 description 2
- 229910052736 halogen Inorganic materials 0.000 description 2
- 150000002367 halogens Chemical class 0.000 description 2
- 238000010438 heat treatment Methods 0.000 description 2
- 239000011874 heated mixture Substances 0.000 description 2
- 210000003958 hematopoietic stem cell Anatomy 0.000 description 2
- 125000001072 heteroaryl group Chemical group 0.000 description 2
- 239000008241 heterogeneous mixture Substances 0.000 description 2
- 229930195733 hydrocarbon Natural products 0.000 description 2
- 150000002430 hydrocarbons Chemical class 0.000 description 2
- JYGXADMDTFJGBT-VWUMJDOOSA-N hydrocortisone Chemical compound O=C1CC[C@]2(C)[C@H]3[C@@H](O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 JYGXADMDTFJGBT-VWUMJDOOSA-N 0.000 description 2
- 229910052739 hydrogen Inorganic materials 0.000 description 2
- 239000001257 hydrogen Substances 0.000 description 2
- 239000012052 hydrophilic carrier Substances 0.000 description 2
- 230000002209 hydrophobic effect Effects 0.000 description 2
- 229920003132 hydroxypropyl methylcellulose phthalate Polymers 0.000 description 2
- 229940031704 hydroxypropyl methylcellulose phthalate Drugs 0.000 description 2
- 230000002757 inflammatory effect Effects 0.000 description 2
- 230000000968 intestinal effect Effects 0.000 description 2
- 125000000959 isobutyl group Chemical group [H]C([H])([H])C([H])(C([H])([H])[H])C([H])([H])* 0.000 description 2
- TWBYWOBDOCUKOW-UHFFFAOYSA-N isonicotinic acid Chemical compound OC(=O)C1=CC=NC=C1 TWBYWOBDOCUKOW-UHFFFAOYSA-N 0.000 description 2
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 2
- JVTAAEKCZFNVCJ-UHFFFAOYSA-N lactic acid Chemical compound CC(O)C(O)=O JVTAAEKCZFNVCJ-UHFFFAOYSA-N 0.000 description 2
- 229960001375 lactose Drugs 0.000 description 2
- 239000008101 lactose Substances 0.000 description 2
- 230000003902 lesion Effects 0.000 description 2
- 230000000670 limiting effect Effects 0.000 description 2
- 239000007791 liquid phase Substances 0.000 description 2
- 238000011068 loading method Methods 0.000 description 2
- 239000000594 mannitol Substances 0.000 description 2
- 235000010355 mannitol Nutrition 0.000 description 2
- 239000000463 material Substances 0.000 description 2
- 238000005259 measurement Methods 0.000 description 2
- BDAGIHXWWSANSR-UHFFFAOYSA-N methanoic acid Natural products OC=O BDAGIHXWWSANSR-UHFFFAOYSA-N 0.000 description 2
- 238000003801 milling Methods 0.000 description 2
- 230000009826 neoplastic cell growth Effects 0.000 description 2
- QIQXTHQIDYTFRH-UHFFFAOYSA-N octadecanoic acid Chemical compound CCCCCCCCCCCCCCCCCC(O)=O QIQXTHQIDYTFRH-UHFFFAOYSA-N 0.000 description 2
- OQCDKBAXFALNLD-UHFFFAOYSA-N octadecanoic acid Natural products CCCCCCCC(C)CCCCCCCCC(O)=O OQCDKBAXFALNLD-UHFFFAOYSA-N 0.000 description 2
- 231100000822 oral exposure Toxicity 0.000 description 2
- 230000008506 pathogenesis Effects 0.000 description 2
- 230000002093 peripheral effect Effects 0.000 description 2
- 239000012071 phase Substances 0.000 description 2
- XNGIFLGASWRNHJ-UHFFFAOYSA-L phthalate(2-) Chemical compound [O-]C(=O)C1=CC=CC=C1C([O-])=O XNGIFLGASWRNHJ-UHFFFAOYSA-L 0.000 description 2
- 239000004014 plasticizer Substances 0.000 description 2
- 229920003124 powdered cellulose Polymers 0.000 description 2
- 235000019814 powdered cellulose Nutrition 0.000 description 2
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 2
- 238000012545 processing Methods 0.000 description 2
- 125000001436 propyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])[H] 0.000 description 2
- 235000018102 proteins Nutrition 0.000 description 2
- 102000004169 proteins and genes Human genes 0.000 description 2
- 230000002285 radioactive effect Effects 0.000 description 2
- 239000000376 reactant Substances 0.000 description 2
- 238000000611 regression analysis Methods 0.000 description 2
- 206010039073 rheumatoid arthritis Diseases 0.000 description 2
- YGSDEFSMJLZEOE-UHFFFAOYSA-N salicylic acid Chemical compound OC(=O)C1=CC=CC=C1O YGSDEFSMJLZEOE-UHFFFAOYSA-N 0.000 description 2
- 238000000926 separation method Methods 0.000 description 2
- 239000011780 sodium chloride Substances 0.000 description 2
- 239000007909 solid dosage form Substances 0.000 description 2
- 239000012265 solid product Substances 0.000 description 2
- 238000013112 stability test Methods 0.000 description 2
- 239000008117 stearic acid Substances 0.000 description 2
- 210000000130 stem cell Anatomy 0.000 description 2
- 239000008223 sterile water Substances 0.000 description 2
- 238000006467 substitution reaction Methods 0.000 description 2
- 239000000758 substrate Substances 0.000 description 2
- 239000005720 sucrose Substances 0.000 description 2
- 239000004094 surface-active agent Substances 0.000 description 2
- 230000002195 synergetic effect Effects 0.000 description 2
- 201000000596 systemic lupus erythematosus Diseases 0.000 description 2
- 125000000999 tert-butyl group Chemical group [H]C([H])([H])C(*)(C([H])([H])[H])C([H])([H])[H] 0.000 description 2
- 238000002560 therapeutic procedure Methods 0.000 description 2
- 210000001519 tissue Anatomy 0.000 description 2
- KSOVGRCOLZZTPF-QMKUDKLTSA-N (1s,2s,3r,4r)-3-[[5-fluoro-2-[3-methyl-4-(4-methylpiperazin-1-yl)anilino]pyrimidin-4-yl]amino]bicyclo[2.2.1]hept-5-ene-2-carboxamide Chemical compound N([C@H]1[C@H]([C@@]2([H])C[C@@]1(C=C2)[H])C(N)=O)C(C(=CN=1)F)=NC=1NC(C=C1C)=CC=C1N1CCN(C)CC1 KSOVGRCOLZZTPF-QMKUDKLTSA-N 0.000 description 1
- QBYIENPQHBMVBV-HFEGYEGKSA-N (2R)-2-hydroxy-2-phenylacetic acid Chemical compound O[C@@H](C(O)=O)c1ccccc1.O[C@@H](C(O)=O)c1ccccc1 QBYIENPQHBMVBV-HFEGYEGKSA-N 0.000 description 1
- YOVVNQKCSKSHKT-HNNXBMFYSA-N (2s)-1-[4-[[2-(2-aminopyrimidin-5-yl)-7-methyl-4-morpholin-4-ylthieno[3,2-d]pyrimidin-6-yl]methyl]piperazin-1-yl]-2-hydroxypropan-1-one Chemical compound C1CN(C(=O)[C@@H](O)C)CCN1CC1=C(C)C2=NC(C=3C=NC(N)=NC=3)=NC(N3CCOCC3)=C2S1 YOVVNQKCSKSHKT-HNNXBMFYSA-N 0.000 description 1
- QDITZBLZQQZVEE-YBEGLDIGSA-N (5z)-5-[(4-pyridin-4-ylquinolin-6-yl)methylidene]-1,3-thiazolidine-2,4-dione Chemical compound S1C(=O)NC(=O)\C1=C\C1=CC=C(N=CC=C2C=3C=CN=CC=3)C2=C1 QDITZBLZQQZVEE-YBEGLDIGSA-N 0.000 description 1
- LAMIXXKAWNLXOC-INIZCTEOSA-N (S)-HDAC-42 Chemical compound O=C([C@@H](C(C)C)C=1C=CC=CC=1)NC1=CC=C(C(=O)NO)C=C1 LAMIXXKAWNLXOC-INIZCTEOSA-N 0.000 description 1
- MIOPJNTWMNEORI-GMSGAONNSA-N (S)-camphorsulfonic acid Chemical compound C1C[C@@]2(CS(O)(=O)=O)C(=O)C[C@@H]1C2(C)C MIOPJNTWMNEORI-GMSGAONNSA-N 0.000 description 1
- BJEPYKJPYRNKOW-REOHCLBHSA-N (S)-malic acid Chemical compound OC(=O)[C@@H](O)CC(O)=O BJEPYKJPYRNKOW-REOHCLBHSA-N 0.000 description 1
- BWDQBBCUWLSASG-MDZDMXLPSA-N (e)-n-hydroxy-3-[4-[[2-hydroxyethyl-[2-(1h-indol-3-yl)ethyl]amino]methyl]phenyl]prop-2-enamide Chemical compound C=1NC2=CC=CC=C2C=1CCN(CCO)CC1=CC=C(\C=C\C(=O)NO)C=C1 BWDQBBCUWLSASG-MDZDMXLPSA-N 0.000 description 1
- NAOLWIGVYRIGTP-UHFFFAOYSA-N 1,3,5-trihydroxyanthracene-9,10-dione Chemical compound C1=CC(O)=C2C(=O)C3=CC(O)=CC(O)=C3C(=O)C2=C1 NAOLWIGVYRIGTP-UHFFFAOYSA-N 0.000 description 1
- OKMWKBLSFKFYGZ-UHFFFAOYSA-N 1-behenoylglycerol Chemical compound CCCCCCCCCCCCCCCCCCCCCC(=O)OCC(O)CO OKMWKBLSFKFYGZ-UHFFFAOYSA-N 0.000 description 1
- IIZPXYDJLKNOIY-JXPKJXOSSA-N 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCC\C=C/C\C=C/C\C=C/C\C=C/CCCCC IIZPXYDJLKNOIY-JXPKJXOSSA-N 0.000 description 1
- FGRBYDKOBBBPOI-UHFFFAOYSA-N 10,10-dioxo-2-[4-(N-phenylanilino)phenyl]thioxanthen-9-one Chemical compound O=C1c2ccccc2S(=O)(=O)c2ccc(cc12)-c1ccc(cc1)N(c1ccccc1)c1ccccc1 FGRBYDKOBBBPOI-UHFFFAOYSA-N 0.000 description 1
- UEJJHQNACJXSKW-UHFFFAOYSA-N 2-(2,6-dioxopiperidin-3-yl)-1H-isoindole-1,3(2H)-dione Chemical compound O=C1C2=CC=CC=C2C(=O)N1C1CCC(=O)NC1=O UEJJHQNACJXSKW-UHFFFAOYSA-N 0.000 description 1
- RGHYDLZMTYDBDT-UHFFFAOYSA-N 2-amino-8-ethyl-4-methyl-6-(1H-pyrazol-5-yl)-7-pyrido[2,3-d]pyrimidinone Chemical compound O=C1N(CC)C2=NC(N)=NC(C)=C2C=C1C=1C=CNN=1 RGHYDLZMTYDBDT-UHFFFAOYSA-N 0.000 description 1
- RMZNXRYIFGTWPF-UHFFFAOYSA-N 2-nitrosoacetic acid Chemical compound OC(=O)CN=O RMZNXRYIFGTWPF-UHFFFAOYSA-N 0.000 description 1
- WLJVXDMOQOGPHL-PPJXEINESA-N 2-phenylacetic acid Chemical compound O[14C](=O)CC1=CC=CC=C1 WLJVXDMOQOGPHL-PPJXEINESA-N 0.000 description 1
- JUSFANSTBFGBAF-IRXDYDNUSA-N 3-[2,4-bis[(3s)-3-methylmorpholin-4-yl]pyrido[2,3-d]pyrimidin-7-yl]-n-methylbenzamide Chemical compound CNC(=O)C1=CC=CC(C=2N=C3N=C(N=C(C3=CC=2)N2[C@H](COCC2)C)N2[C@H](COCC2)C)=C1 JUSFANSTBFGBAF-IRXDYDNUSA-N 0.000 description 1
- OSWFIVFLDKOXQC-UHFFFAOYSA-N 4-(3-methoxyphenyl)aniline Chemical compound COC1=CC=CC(C=2C=CC(N)=CC=2)=C1 OSWFIVFLDKOXQC-UHFFFAOYSA-N 0.000 description 1
- VVLHQJDAUIPZFH-UHFFFAOYSA-N 4-[4-[[5-fluoro-4-[3-(prop-2-enoylamino)anilino]pyrimidin-2-yl]amino]phenoxy]-n-methylpyridine-2-carboxamide Chemical compound C1=NC(C(=O)NC)=CC(OC=2C=CC(NC=3N=C(NC=4C=C(NC(=O)C=C)C=CC=4)C(F)=CN=3)=CC=2)=C1 VVLHQJDAUIPZFH-UHFFFAOYSA-N 0.000 description 1
- FPEIJQLXFHKLJV-UHFFFAOYSA-N 4-[6-(1h-indol-5-yl)-1-[1-(pyridin-3-ylmethyl)piperidin-4-yl]pyrazolo[3,4-d]pyrimidin-4-yl]morpholine Chemical compound C=1C=CN=CC=1CN(CC1)CCC1N(C1=NC(=N2)C=3C=C4C=CNC4=CC=3)N=CC1=C2N1CCOCC1 FPEIJQLXFHKLJV-UHFFFAOYSA-N 0.000 description 1
- IMXHGCRIEAKIBU-UHFFFAOYSA-N 4-[6-[4-(methoxycarbonylamino)phenyl]-4-(4-morpholinyl)-1-pyrazolo[3,4-d]pyrimidinyl]-1-piperidinecarboxylic acid methyl ester Chemical compound C1=CC(NC(=O)OC)=CC=C1C1=NC(N2CCOCC2)=C(C=NN2C3CCN(CC3)C(=O)OC)C2=N1 IMXHGCRIEAKIBU-UHFFFAOYSA-N 0.000 description 1
- JIFCFQDXHMUPGP-UHFFFAOYSA-N 4-tert-butyl-n-[2-methyl-3-[4-methyl-6-[4-(morpholine-4-carbonyl)anilino]-5-oxopyrazin-2-yl]phenyl]benzamide Chemical compound C1=CC=C(C=2N=C(NC=3C=CC(=CC=3)C(=O)N3CCOCC3)C(=O)N(C)C=2)C(C)=C1NC(=O)C1=CC=C(C(C)(C)C)C=C1 JIFCFQDXHMUPGP-UHFFFAOYSA-N 0.000 description 1
- GYLDXIAOMVERTK-UHFFFAOYSA-N 5-(4-amino-1-propan-2-yl-3-pyrazolo[3,4-d]pyrimidinyl)-1,3-benzoxazol-2-amine Chemical compound C12=C(N)N=CN=C2N(C(C)C)N=C1C1=CC=C(OC(N)=N2)C2=C1 GYLDXIAOMVERTK-UHFFFAOYSA-N 0.000 description 1
- JEGHXKRHKHPBJD-UHFFFAOYSA-N 5-(7-methylsulfonyl-2-morpholin-4-yl-5,6-dihydropyrrolo[2,3-d]pyrimidin-4-yl)pyrimidin-2-amine Chemical compound CS(=O)(=O)N1CCC2=C1N=C(N1CCOCC1)N=C2C1=CN=C(N)N=C1 JEGHXKRHKHPBJD-UHFFFAOYSA-N 0.000 description 1
- JTDYUFSDZATMKU-UHFFFAOYSA-N 6-(1,3-dioxo-2-benzo[de]isoquinolinyl)-N-hydroxyhexanamide Chemical compound C1=CC(C(N(CCCCCC(=O)NO)C2=O)=O)=C3C2=CC=CC3=C1 JTDYUFSDZATMKU-UHFFFAOYSA-N 0.000 description 1
- PLIVFNIUGLLCEK-UHFFFAOYSA-N 7-[4-(3-ethynylanilino)-7-methoxyquinazolin-6-yl]oxy-n-hydroxyheptanamide Chemical compound C=12C=C(OCCCCCCC(=O)NO)C(OC)=CC2=NC=NC=1NC1=CC=CC(C#C)=C1 PLIVFNIUGLLCEK-UHFFFAOYSA-N 0.000 description 1
- ZCYVEMRRCGMTRW-UHFFFAOYSA-N 7553-56-2 Chemical compound [I] ZCYVEMRRCGMTRW-UHFFFAOYSA-N 0.000 description 1
- KVLFRAWTRWDEDF-IRXDYDNUSA-N AZD-8055 Chemical compound C1=C(CO)C(OC)=CC=C1C1=CC=C(C(=NC(=N2)N3[C@H](COCC3)C)N3[C@H](COCC3)C)C2=N1 KVLFRAWTRWDEDF-IRXDYDNUSA-N 0.000 description 1
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 1
- 241000271566 Aves Species 0.000 description 1
- YUXMAKUNSXIEKN-BTJKTKAUSA-N BGT226 Chemical compound OC(=O)\C=C/C(O)=O.C1=NC(OC)=CC=C1C1=CC=C(N=CC2=C3N(C=4C=C(C(N5CCNCC5)=CC=4)C(F)(F)F)C(=O)N2C)C3=C1 YUXMAKUNSXIEKN-BTJKTKAUSA-N 0.000 description 1
- 108091005625 BRD4 Proteins 0.000 description 1
- 239000005711 Benzoic acid Substances 0.000 description 1
- 241000283690 Bos taurus Species 0.000 description 1
- 102100033641 Bromodomain-containing protein 2 Human genes 0.000 description 1
- 102100033642 Bromodomain-containing protein 3 Human genes 0.000 description 1
- 102100029895 Bromodomain-containing protein 4 Human genes 0.000 description 1
- 241000283707 Capra Species 0.000 description 1
- OKTJSMMVPCPJKN-NJFSPNSNSA-N Carbon-14 Chemical compound [14C] OKTJSMMVPCPJKN-NJFSPNSNSA-N 0.000 description 1
- 241000700198 Cavia Species 0.000 description 1
- GHOKWGTUZJEAQD-UHFFFAOYSA-N Chick antidermatitis factor Natural products OCC(C)(C)C(O)C(=O)NCCC(O)=O GHOKWGTUZJEAQD-UHFFFAOYSA-N 0.000 description 1
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 description 1
- 206010009900 Colitis ulcerative Diseases 0.000 description 1
- 208000011231 Crohn disease Diseases 0.000 description 1
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 1
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 description 1
- RGHNJXZEOKUKBD-UHFFFAOYSA-N D-gluconic acid Natural products OCC(O)C(O)C(O)C(O)C(O)=O RGHNJXZEOKUKBD-UHFFFAOYSA-N 0.000 description 1
- 206010012438 Dermatitis atopic Diseases 0.000 description 1
- YZCKVEUIGOORGS-OUBTZVSYSA-N Deuterium Chemical compound [2H] YZCKVEUIGOORGS-OUBTZVSYSA-N 0.000 description 1
- 208000032765 Device extrusion Diseases 0.000 description 1
- 239000004375 Dextrin Substances 0.000 description 1
- 229920001353 Dextrin Polymers 0.000 description 1
- FEWJPZIEWOKRBE-JCYAYHJZSA-N Dextrotartaric acid Chemical compound OC(=O)[C@H](O)[C@@H](O)C(O)=O FEWJPZIEWOKRBE-JCYAYHJZSA-N 0.000 description 1
- 241000283086 Equidae Species 0.000 description 1
- VGGSQFUCUMXWEO-UHFFFAOYSA-N Ethene Chemical compound C=C VGGSQFUCUMXWEO-UHFFFAOYSA-N 0.000 description 1
- HKVAMNSJSFKALM-GKUWKFKPSA-N Everolimus Chemical compound C1C[C@@H](OCCO)[C@H](OC)C[C@@H]1C[C@@H](C)[C@H]1OC(=O)[C@@H]2CCCCN2C(=O)C(=O)[C@](O)(O2)[C@H](C)CC[C@H]2C[C@H](OC)/C(C)=C/C=C/C=C/[C@@H](C)C[C@@H](C)C(=O)[C@H](OC)[C@H](O)/C(C)=C/[C@@H](C)C(=O)C1 HKVAMNSJSFKALM-GKUWKFKPSA-N 0.000 description 1
- 241000282326 Felis catus Species 0.000 description 1
- PXGOKWXKJXAPGV-UHFFFAOYSA-N Fluorine Chemical compound FF PXGOKWXKJXAPGV-UHFFFAOYSA-N 0.000 description 1
- DSLZVSRJTYRBFB-UHFFFAOYSA-N Galactaric acid Natural products OC(=O)C(O)C(O)C(O)C(O)C(O)=O DSLZVSRJTYRBFB-UHFFFAOYSA-N 0.000 description 1
- 241000287828 Gallus gallus Species 0.000 description 1
- 108010010803 Gelatin Proteins 0.000 description 1
- 239000001828 Gelatine Substances 0.000 description 1
- WHUUTDBJXJRKMK-UHFFFAOYSA-N Glutamic acid Natural products OC(=O)C(N)CCC(O)=O WHUUTDBJXJRKMK-UHFFFAOYSA-N 0.000 description 1
- 102000003693 Hedgehog Proteins Human genes 0.000 description 1
- 108090000031 Hedgehog Proteins Proteins 0.000 description 1
- 102100021489 Histone H4-like protein type G Human genes 0.000 description 1
- 108010033040 Histones Proteins 0.000 description 1
- 101000871850 Homo sapiens Bromodomain-containing protein 2 Proteins 0.000 description 1
- 101000871851 Homo sapiens Bromodomain-containing protein 3 Proteins 0.000 description 1
- 241000521257 Hydrops Species 0.000 description 1
- 239000004354 Hydroxyethyl cellulose Substances 0.000 description 1
- 229920000663 Hydroxyethyl cellulose Polymers 0.000 description 1
- 229920002153 Hydroxypropyl cellulose Polymers 0.000 description 1
- 241000370541 Idia Species 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- 206010061218 Inflammation Diseases 0.000 description 1
- 102100023422 Kinesin-1 heavy chain Human genes 0.000 description 1
- QAQJMLQRFWZOBN-LAUBAEHRSA-N L-ascorbyl-6-palmitate Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](O)[C@H]1OC(=O)C(O)=C1O QAQJMLQRFWZOBN-LAUBAEHRSA-N 0.000 description 1
- 239000011786 L-ascorbyl-6-palmitate Substances 0.000 description 1
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical compound OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 description 1
- QQDIFLSJMFDTCQ-UHFFFAOYSA-N MC1568 Chemical compound CN1C(C=CC(=O)NO)=CC=C1C=CC(=O)C1=CC=CC(F)=C1 QQDIFLSJMFDTCQ-UHFFFAOYSA-N 0.000 description 1
- UEZVMMHDMIWARA-UHFFFAOYSA-N Metaphosphoric acid Chemical class OP(=O)=O UEZVMMHDMIWARA-UHFFFAOYSA-N 0.000 description 1
- 241000699670 Mus sp. Species 0.000 description 1
- 206010051141 Myeloblastoma Diseases 0.000 description 1
- WHNWPMSKXPGLAX-UHFFFAOYSA-N N-Vinyl-2-pyrrolidone Chemical compound C=CN1CCCC1=O WHNWPMSKXPGLAX-UHFFFAOYSA-N 0.000 description 1
- AJRGHIGYPXNABY-UHFFFAOYSA-N N-hydroxy-1-[(4-methoxyphenyl)methyl]-6-indolecarboxamide Chemical compound C1=CC(OC)=CC=C1CN1C2=CC(C(=O)NO)=CC=C2C=C1 AJRGHIGYPXNABY-UHFFFAOYSA-N 0.000 description 1
- PAWIYAYFNXQGAP-UHFFFAOYSA-N N-hydroxy-2-[4-[[(1-methyl-3-indolyl)methylamino]methyl]-1-piperidinyl]-5-pyrimidinecarboxamide Chemical compound C12=CC=CC=C2N(C)C=C1CNCC(CC1)CCN1C1=NC=C(C(=O)NO)C=N1 PAWIYAYFNXQGAP-UHFFFAOYSA-N 0.000 description 1
- 208000033383 Neuroendocrine tumor of pancreas Diseases 0.000 description 1
- GRYLNZFGIOXLOG-UHFFFAOYSA-N Nitric acid Chemical class O[N+]([O-])=O GRYLNZFGIOXLOG-UHFFFAOYSA-N 0.000 description 1
- JWOGUUIOCYMBPV-UHFFFAOYSA-N OT-Key 11219 Natural products N1C(=O)C(CCCCCC(=O)CC)NC(=O)C2CCCCN2C(=O)C(C(C)CC)NC(=O)C1CC1=CN(OC)C2=CC=CC=C12 JWOGUUIOCYMBPV-UHFFFAOYSA-N 0.000 description 1
- 208000008589 Obesity Diseases 0.000 description 1
- 206010030113 Oedema Diseases 0.000 description 1
- 241000283973 Oryctolagus cuniculus Species 0.000 description 1
- TUVCWJQQGGETHL-UHFFFAOYSA-N PI-103 Chemical compound OC1=CC=CC(C=2N=C3C4=CC=CN=C4OC3=C(N3CCOCC3)N=2)=C1 TUVCWJQQGGETHL-UHFFFAOYSA-N 0.000 description 1
- 241001494479 Pecora Species 0.000 description 1
- 241000286209 Phasianidae Species 0.000 description 1
- 241000233805 Phoenix Species 0.000 description 1
- 239000004698 Polyethylene Substances 0.000 description 1
- 229920002565 Polyethylene Glycol 400 Polymers 0.000 description 1
- 241000183024 Populus tremula Species 0.000 description 1
- 229920003078 Povidone K 12 Polymers 0.000 description 1
- 229920003079 Povidone K 17 Polymers 0.000 description 1
- 241000288906 Primates Species 0.000 description 1
- 102000001708 Protein Isoforms Human genes 0.000 description 1
- 108010029485 Protein Isoforms Proteins 0.000 description 1
- 102100033661 Protein TFG Human genes 0.000 description 1
- IWYDHOAUDWTVEP-UHFFFAOYSA-N R-2-phenyl-2-hydroxyacetic acid Natural products OC(=O)C(O)C1=CC=CC=C1 IWYDHOAUDWTVEP-UHFFFAOYSA-N 0.000 description 1
- 241000283984 Rodentia Species 0.000 description 1
- 229920002472 Starch Polymers 0.000 description 1
- KDYFGRWQOYBRFD-UHFFFAOYSA-N Succinic acid Natural products OC(=O)CCC(O)=O KDYFGRWQOYBRFD-UHFFFAOYSA-N 0.000 description 1
- 241000282887 Suidae Species 0.000 description 1
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical compound [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 description 1
- 210000001744 T-lymphocyte Anatomy 0.000 description 1
- FEWJPZIEWOKRBE-UHFFFAOYSA-N Tartaric acid Natural products [H+].[H+].[O-]C(=O)C(O)C(O)C([O-])=O FEWJPZIEWOKRBE-UHFFFAOYSA-N 0.000 description 1
- BPEGJWRSRHCHSN-UHFFFAOYSA-N Temozolomide Chemical compound O=C1N(C)N=NC2=C(C(N)=O)N=CN21 BPEGJWRSRHCHSN-UHFFFAOYSA-N 0.000 description 1
- 108091023040 Transcription factor Proteins 0.000 description 1
- 102000040945 Transcription factor Human genes 0.000 description 1
- RTKIYFITIVXBLE-UHFFFAOYSA-N Trichostatin A Natural products ONC(=O)C=CC(C)=CC(C)C(=O)C1=CC=C(N(C)C)C=C1 RTKIYFITIVXBLE-UHFFFAOYSA-N 0.000 description 1
- YZCKVEUIGOORGS-NJFSPNSNSA-N Tritium Chemical compound [3H] YZCKVEUIGOORGS-NJFSPNSNSA-N 0.000 description 1
- 201000006704 Ulcerative Colitis Diseases 0.000 description 1
- 230000004156 Wnt signaling pathway Effects 0.000 description 1
- 238000002441 X-ray diffraction Methods 0.000 description 1
- 240000008042 Zea mays Species 0.000 description 1
- 235000005824 Zea mays ssp. parviglumis Nutrition 0.000 description 1
- 235000002017 Zea mays subsp mays Nutrition 0.000 description 1
- 230000002378 acidificating effect Effects 0.000 description 1
- 150000007513 acids Chemical class 0.000 description 1
- 239000004480 active ingredient Substances 0.000 description 1
- 239000002671 adjuvant Substances 0.000 description 1
- 230000002411 adverse Effects 0.000 description 1
- 238000005054 agglomeration Methods 0.000 description 1
- 238000004220 aggregation Methods 0.000 description 1
- 238000013019 agitation Methods 0.000 description 1
- IAJILQKETJEXLJ-RSJOWCBRSA-N aldehydo-D-galacturonic acid Chemical compound O=C[C@H](O)[C@@H](O)[C@@H](O)[C@H](O)C(O)=O IAJILQKETJEXLJ-RSJOWCBRSA-N 0.000 description 1
- IAJILQKETJEXLJ-QTBDOELSSA-N aldehydo-D-glucuronic acid Chemical compound O=C[C@H](O)[C@@H](O)[C@H](O)[C@H](O)C(O)=O IAJILQKETJEXLJ-QTBDOELSSA-N 0.000 description 1
- 239000000783 alginic acid Substances 0.000 description 1
- 235000010443 alginic acid Nutrition 0.000 description 1
- 229920000615 alginic acid Polymers 0.000 description 1
- 229960001126 alginic acid Drugs 0.000 description 1
- 150000004781 alginic acids Chemical class 0.000 description 1
- 125000000304 alkynyl group Chemical group 0.000 description 1
- HZVVJJIYJKGMFL-UHFFFAOYSA-N almasilate Chemical compound O.[Mg+2].[Al+3].[Al+3].O[Si](O)=O.O[Si](O)=O HZVVJJIYJKGMFL-UHFFFAOYSA-N 0.000 description 1
- BJEPYKJPYRNKOW-UHFFFAOYSA-N alpha-hydroxysuccinic acid Natural products OC(=O)C(O)CC(O)=O BJEPYKJPYRNKOW-UHFFFAOYSA-N 0.000 description 1
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 1
- 229910052782 aluminium Inorganic materials 0.000 description 1
- 235000010210 aluminium Nutrition 0.000 description 1
- 229910000147 aluminium phosphate Inorganic materials 0.000 description 1
- 239000004599 antimicrobial Substances 0.000 description 1
- 239000002246 antineoplastic agent Substances 0.000 description 1
- 229940041181 antineoplastic drug Drugs 0.000 description 1
- 239000003963 antioxidant agent Substances 0.000 description 1
- 235000006708 antioxidants Nutrition 0.000 description 1
- 239000012736 aqueous medium Substances 0.000 description 1
- 125000003118 aryl group Chemical group 0.000 description 1
- 235000010323 ascorbic acid Nutrition 0.000 description 1
- 239000011668 ascorbic acid Substances 0.000 description 1
- 229960005070 ascorbic acid Drugs 0.000 description 1
- 235000010385 ascorbyl palmitate Nutrition 0.000 description 1
- 229910052789 astatine Inorganic materials 0.000 description 1
- 238000011914 asymmetric synthesis Methods 0.000 description 1
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 1
- 201000008937 atopic dermatitis Diseases 0.000 description 1
- SRSXLGNVWSONIS-UHFFFAOYSA-N benzenesulfonic acid Chemical compound OS(=O)(=O)C1=CC=CC=C1 SRSXLGNVWSONIS-UHFFFAOYSA-N 0.000 description 1
- 229940092714 benzenesulfonic acid Drugs 0.000 description 1
- 235000010233 benzoic acid Nutrition 0.000 description 1
- 230000004071 biological effect Effects 0.000 description 1
- 229950000080 birabresib Drugs 0.000 description 1
- 210000004556 brain Anatomy 0.000 description 1
- SXDBWCPKPHAZSM-UHFFFAOYSA-N bromic acid Chemical class OBr(=O)=O SXDBWCPKPHAZSM-UHFFFAOYSA-N 0.000 description 1
- 229910052794 bromium Inorganic materials 0.000 description 1
- KDYFGRWQOYBRFD-NUQCWPJISA-N butanedioic acid Chemical compound O[14C](=O)CC[14C](O)=O KDYFGRWQOYBRFD-NUQCWPJISA-N 0.000 description 1
- 125000004369 butenyl group Chemical group C(=CCC)* 0.000 description 1
- 239000006227 byproduct Substances 0.000 description 1
- CJZGTCYPCWQAJB-UHFFFAOYSA-L calcium stearate Chemical compound [Ca+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O CJZGTCYPCWQAJB-UHFFFAOYSA-L 0.000 description 1
- 239000008116 calcium stearate Substances 0.000 description 1
- 235000013539 calcium stearate Nutrition 0.000 description 1
- 239000007894 caplet Substances 0.000 description 1
- 239000007963 capsule composition Substances 0.000 description 1
- 125000002837 carbocyclic group Chemical group 0.000 description 1
- 235000011089 carbon dioxide Nutrition 0.000 description 1
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 1
- 239000000969 carrier Substances 0.000 description 1
- 239000004359 castor oil Substances 0.000 description 1
- 235000019438 castor oil Nutrition 0.000 description 1
- 229960001777 castor oil Drugs 0.000 description 1
- 235000010980 cellulose Nutrition 0.000 description 1
- 229920002678 cellulose Polymers 0.000 description 1
- 210000001638 cerebellum Anatomy 0.000 description 1
- XDLYKKIQACFMJG-WKILWMFISA-N chembl1234354 Chemical compound C1=NC(OC)=CC=C1C(C1=O)=CC2=C(C)N=C(N)N=C2N1[C@@H]1CC[C@@H](OCCO)CC1 XDLYKKIQACFMJG-WKILWMFISA-N 0.000 description 1
- 238000006243 chemical reaction Methods 0.000 description 1
- 235000013330 chicken meat Nutrition 0.000 description 1
- 238000000633 chiral stationary phase gas chromatography Methods 0.000 description 1
- 210000000349 chromosome Anatomy 0.000 description 1
- 235000015165 citric acid Nutrition 0.000 description 1
- 238000000975 co-precipitation Methods 0.000 description 1
- 239000008119 colloidal silica Substances 0.000 description 1
- 210000001072 colon Anatomy 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 235000005822 corn Nutrition 0.000 description 1
- 125000000753 cycloalkyl group Chemical group 0.000 description 1
- GVJHHUAWPYXKBD-UHFFFAOYSA-N d-alpha-tocopherol Natural products OC1=C(C)C(C)=C2OC(CCCC(C)CCCC(C)CCCC(C)C)(C)CCC2=C1C GVJHHUAWPYXKBD-UHFFFAOYSA-N 0.000 description 1
- 229950006418 dactolisib Drugs 0.000 description 1
- JOGKUKXHTYWRGZ-UHFFFAOYSA-N dactolisib Chemical group O=C1N(C)C2=CN=C3C=CC(C=4C=C5C=CC=CC5=NC=4)=CC3=C2N1C1=CC=C(C(C)(C)C#N)C=C1 JOGKUKXHTYWRGZ-UHFFFAOYSA-N 0.000 description 1
- 238000000354 decomposition reaction Methods 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 238000001212 derivatisation Methods 0.000 description 1
- 229910052805 deuterium Inorganic materials 0.000 description 1
- 229940096516 dextrates Drugs 0.000 description 1
- 235000019425 dextrin Nutrition 0.000 description 1
- 125000002576 diazepinyl group Chemical class N1N=C(C=CC=C1)* 0.000 description 1
- FKGKZBBDJSKCIS-UHFFFAOYSA-N diethyl-[[6-[[4-(hydroxycarbamoyl)phenyl]carbamoyloxymethyl]naphthalen-2-yl]methyl]azanium;chloride;hydrate Chemical compound O.[Cl-].C1=CC2=CC(C[NH+](CC)CC)=CC=C2C=C1COC(=O)NC1=CC=C(C(=O)NO)C=C1 FKGKZBBDJSKCIS-UHFFFAOYSA-N 0.000 description 1
- FSBVERYRVPGNGG-UHFFFAOYSA-N dimagnesium dioxido-bis[[oxido(oxo)silyl]oxy]silane hydrate Chemical compound O.[Mg+2].[Mg+2].[O-][Si](=O)O[Si]([O-])([O-])O[Si]([O-])=O FSBVERYRVPGNGG-UHFFFAOYSA-N 0.000 description 1
- 208000035475 disorder Diseases 0.000 description 1
- 238000009826 distribution Methods 0.000 description 1
- 231100000673 dose–response relationship Toxicity 0.000 description 1
- 239000003937 drug carrier Substances 0.000 description 1
- 238000007876 drug discovery Methods 0.000 description 1
- 230000008030 elimination Effects 0.000 description 1
- 238000003379 elimination reaction Methods 0.000 description 1
- RDYMFSUJUZBWLH-UHFFFAOYSA-N endosulfan Chemical compound C12COS(=O)OCC2C2(Cl)C(Cl)=C(Cl)C1(Cl)C2(Cl)Cl RDYMFSUJUZBWLH-UHFFFAOYSA-N 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 239000002662 enteric coated tablet Substances 0.000 description 1
- INVTYAOGFAGBOE-UHFFFAOYSA-N entinostat Chemical compound NC1=CC=CC=C1NC(=O)C(C=C1)=CC=C1CNC(=O)OCC1=CC=CN=C1 INVTYAOGFAGBOE-UHFFFAOYSA-N 0.000 description 1
- 229950005837 entinostat Drugs 0.000 description 1
- 230000002255 enzymatic effect Effects 0.000 description 1
- 238000006911 enzymatic reaction Methods 0.000 description 1
- 239000002532 enzyme inhibitor Substances 0.000 description 1
- 229940125532 enzyme inhibitor Drugs 0.000 description 1
- 238000011067 equilibration Methods 0.000 description 1
- CCIVGXIOQKPBKL-UHFFFAOYSA-M ethanesulfonate Chemical compound CCS([O-])(=O)=O CCIVGXIOQKPBKL-UHFFFAOYSA-M 0.000 description 1
- MVPICKVDHDWCJQ-UHFFFAOYSA-N ethyl 3-pyrrolidin-1-ylpropanoate Chemical compound CCOC(=O)CCN1CCCC1 MVPICKVDHDWCJQ-UHFFFAOYSA-N 0.000 description 1
- 239000000374 eutectic mixture Substances 0.000 description 1
- 238000001704 evaporation Methods 0.000 description 1
- 230000008020 evaporation Effects 0.000 description 1
- 229960005167 everolimus Drugs 0.000 description 1
- 239000011737 fluorine Substances 0.000 description 1
- 230000003325 follicular Effects 0.000 description 1
- 235000003599 food sweetener Nutrition 0.000 description 1
- 235000019253 formic acid Nutrition 0.000 description 1
- 238000004108 freeze drying Methods 0.000 description 1
- 229960002598 fumaric acid Drugs 0.000 description 1
- DSLZVSRJTYRBFB-DUHBMQHGSA-N galactaric acid Chemical compound OC(=O)[C@H](O)[C@@H](O)[C@@H](O)[C@H](O)C(O)=O DSLZVSRJTYRBFB-DUHBMQHGSA-N 0.000 description 1
- 239000007789 gas Substances 0.000 description 1
- 230000002496 gastric effect Effects 0.000 description 1
- 239000008273 gelatin Substances 0.000 description 1
- 235000011852 gelatine desserts Nutrition 0.000 description 1
- 229960005219 gentisic acid Drugs 0.000 description 1
- 230000002518 glial effect Effects 0.000 description 1
- 239000000174 gluconic acid Substances 0.000 description 1
- 235000012208 gluconic acid Nutrition 0.000 description 1
- 229960001031 glucose Drugs 0.000 description 1
- 229940097043 glucuronic acid Drugs 0.000 description 1
- 235000013922 glutamic acid Nutrition 0.000 description 1
- 239000004220 glutamic acid Substances 0.000 description 1
- ZEMPKEQAKRGZGQ-XOQCFJPHSA-N glycerol triricinoleate Natural products CCCCCC[C@@H](O)CC=CCCCCCCCC(=O)OC[C@@H](COC(=O)CCCCCCCC=CC[C@@H](O)CCCCCC)OC(=O)CCCCCCCC=CC[C@H](O)CCCCCC ZEMPKEQAKRGZGQ-XOQCFJPHSA-N 0.000 description 1
- 229940049654 glyceryl behenate Drugs 0.000 description 1
- 229940075507 glyceryl monostearate Drugs 0.000 description 1
- QFWPJPIVLCBXFJ-UHFFFAOYSA-N glymidine Chemical class N1=CC(OCCOC)=CN=C1NS(=O)(=O)C1=CC=CC=C1 QFWPJPIVLCBXFJ-UHFFFAOYSA-N 0.000 description 1
- 238000000227 grinding Methods 0.000 description 1
- LHGVFZTZFXWLCP-UHFFFAOYSA-N guaiacol Chemical class COC1=CC=CC=C1O LHGVFZTZFXWLCP-UHFFFAOYSA-N 0.000 description 1
- 229940093915 gynecological organic acid Drugs 0.000 description 1
- 125000000623 heterocyclic group Chemical group 0.000 description 1
- 229920001903 high density polyethylene Polymers 0.000 description 1
- 239000004700 high-density polyethylene Substances 0.000 description 1
- 229960000890 hydrocortisone Drugs 0.000 description 1
- 150000002431 hydrogen Chemical class 0.000 description 1
- 235000019447 hydroxyethyl cellulose Nutrition 0.000 description 1
- 239000001863 hydroxypropyl cellulose Substances 0.000 description 1
- 235000010977 hydroxypropyl cellulose Nutrition 0.000 description 1
- 229960003943 hypromellose Drugs 0.000 description 1
- 238000005470 impregnation Methods 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 238000007901 in situ hybridization Methods 0.000 description 1
- 230000004054 inflammatory process Effects 0.000 description 1
- 230000002401 inhibitory effect Effects 0.000 description 1
- 230000000977 initiatory effect Effects 0.000 description 1
- 150000007529 inorganic bases Chemical class 0.000 description 1
- 229910052740 iodine Inorganic materials 0.000 description 1
- 239000011630 iodine Substances 0.000 description 1
- 210000004731 jugular vein Anatomy 0.000 description 1
- 239000004310 lactic acid Substances 0.000 description 1
- 235000014655 lactic acid Nutrition 0.000 description 1
- 210000000867 larynx Anatomy 0.000 description 1
- 239000000787 lecithin Substances 0.000 description 1
- 235000010445 lecithin Nutrition 0.000 description 1
- 229940067606 lecithin Drugs 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 229920001684 low density polyethylene Polymers 0.000 description 1
- 239000004702 low-density polyethylene Substances 0.000 description 1
- 206010025135 lupus erythematosus Diseases 0.000 description 1
- ZLNQQNXFFQJAID-UHFFFAOYSA-L magnesium carbonate Chemical compound [Mg+2].[O-]C([O-])=O ZLNQQNXFFQJAID-UHFFFAOYSA-L 0.000 description 1
- 239000001095 magnesium carbonate Substances 0.000 description 1
- 229910000021 magnesium carbonate Inorganic materials 0.000 description 1
- 235000014380 magnesium carbonate Nutrition 0.000 description 1
- HSSGNVITVKZRHD-UHFFFAOYSA-L magnesium octadecanoate octadecanoic acid Chemical compound [Mg++].CCCCCCCCCCCCCCCCCC(O)=O.CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HSSGNVITVKZRHD-UHFFFAOYSA-L 0.000 description 1
- 239000000395 magnesium oxide Substances 0.000 description 1
- CPLXHLVBOLITMK-UHFFFAOYSA-N magnesium oxide Inorganic materials [Mg]=O CPLXHLVBOLITMK-UHFFFAOYSA-N 0.000 description 1
- 235000012245 magnesium oxide Nutrition 0.000 description 1
- 239000000391 magnesium silicate Substances 0.000 description 1
- 235000019793 magnesium trisilicate Nutrition 0.000 description 1
- 229940099273 magnesium trisilicate Drugs 0.000 description 1
- 229910000386 magnesium trisilicate Inorganic materials 0.000 description 1
- AXZKOIWUVFPNLO-UHFFFAOYSA-N magnesium;oxygen(2-) Chemical compound [O-2].[Mg+2] AXZKOIWUVFPNLO-UHFFFAOYSA-N 0.000 description 1
- VZCYOOQTPOCHFL-UPHRSURJSA-N maleic acid Chemical compound OC(=O)\C=C/C(O)=O VZCYOOQTPOCHFL-UPHRSURJSA-N 0.000 description 1
- 239000011976 maleic acid Substances 0.000 description 1
- 239000001630 malic acid Substances 0.000 description 1
- 235000011090 malic acid Nutrition 0.000 description 1
- 229960002510 mandelic acid Drugs 0.000 description 1
- 229960001855 mannitol Drugs 0.000 description 1
- 239000000155 melt Substances 0.000 description 1
- 238000007909 melt granulation Methods 0.000 description 1
- 230000002503 metabolic effect Effects 0.000 description 1
- 229940117841 methacrylic acid copolymer Drugs 0.000 description 1
- 229920003145 methacrylic acid copolymer Polymers 0.000 description 1
- 229940098779 methanesulfonic acid Drugs 0.000 description 1
- VDOCQQKGPJENHJ-UHFFFAOYSA-N methyl n-[4-[4-morpholin-4-yl-1-[1-(pyridin-3-ylmethyl)piperidin-4-yl]pyrazolo[3,4-d]pyrimidin-6-yl]phenyl]carbamate Chemical compound C1=CC(NC(=O)OC)=CC=C1C1=NC(N2CCOCC2)=C(C=NN2C3CCN(CC=4C=NC=CC=4)CC3)C2=N1 VDOCQQKGPJENHJ-UHFFFAOYSA-N 0.000 description 1
- 239000001788 mono and diglycerides of fatty acids Substances 0.000 description 1
- IJMHHZDBRUGXNO-UHFFFAOYSA-N n-[3-(8-anilinoimidazo[1,2-a]pyrazin-6-yl)phenyl]-4-tert-butylbenzamide Chemical compound C1=CC(C(C)(C)C)=CC=C1C(=O)NC1=CC=CC(C=2N=C(NC=3C=CC=CC=3)C3=NC=CN3C=2)=C1 IJMHHZDBRUGXNO-UHFFFAOYSA-N 0.000 description 1
- CDOOFZZILLRUQH-GDLZYMKVSA-N n-[3-[6-[4-[(2r)-1,4-dimethyl-3-oxopiperazin-2-yl]anilino]-4-methyl-5-oxopyrazin-2-yl]-2-methylphenyl]-4,5,6,7-tetrahydro-1-benzothiophene-2-carboxamide Chemical compound CN1CCN(C)C(=O)[C@H]1C(C=C1)=CC=C1NC1=NC(C=2C(=C(NC(=O)C=3SC=4CCCCC=4C=3)C=CC=2)C)=CN(C)C1=O CDOOFZZILLRUQH-GDLZYMKVSA-N 0.000 description 1
- 125000004108 n-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- JOWXJLIFIIOYMS-UHFFFAOYSA-N n-hydroxy-2-[[2-(6-methoxypyridin-3-yl)-4-morpholin-4-ylthieno[3,2-d]pyrimidin-6-yl]methyl-methylamino]pyrimidine-5-carboxamide Chemical compound C1=NC(OC)=CC=C1C1=NC(N2CCOCC2)=C(SC(CN(C)C=2N=CC(=CN=2)C(=O)NO)=C2)C2=N1 JOWXJLIFIIOYMS-UHFFFAOYSA-N 0.000 description 1
- 201000011519 neuroendocrine tumor Diseases 0.000 description 1
- 230000004031 neuronal differentiation Effects 0.000 description 1
- 229910017604 nitric acid Inorganic materials 0.000 description 1
- 229910052757 nitrogen Inorganic materials 0.000 description 1
- 231100000252 nontoxic Toxicity 0.000 description 1
- 230000003000 nontoxic effect Effects 0.000 description 1
- 210000004940 nucleus Anatomy 0.000 description 1
- 235000020824 obesity Nutrition 0.000 description 1
- CGBJSGAELGCMKE-UHFFFAOYSA-N omipalisib Chemical compound COC1=NC=C(C=2C=C3C(C=4C=NN=CC=4)=CC=NC3=CC=2)C=C1NS(=O)(=O)C1=CC=C(F)C=C1F CGBJSGAELGCMKE-UHFFFAOYSA-N 0.000 description 1
- 230000003287 optical effect Effects 0.000 description 1
- 238000003305 oral gavage Methods 0.000 description 1
- 235000005985 organic acids Nutrition 0.000 description 1
- 150000007530 organic bases Chemical class 0.000 description 1
- 229910052760 oxygen Inorganic materials 0.000 description 1
- 239000001301 oxygen Substances 0.000 description 1
- 239000003002 pH adjusting agent Substances 0.000 description 1
- 238000010979 pH adjustment Methods 0.000 description 1
- 238000004806 packaging method and process Methods 0.000 description 1
- 239000011713 pantothenic acid Substances 0.000 description 1
- 229940055726 pantothenic acid Drugs 0.000 description 1
- 235000019161 pantothenic acid Nutrition 0.000 description 1
- FJKROLUGYXJWQN-UHFFFAOYSA-N papa-hydroxy-benzoic acid Natural products OC(=O)C1=CC=C(O)C=C1 FJKROLUGYXJWQN-UHFFFAOYSA-N 0.000 description 1
- 230000036961 partial effect Effects 0.000 description 1
- 238000011056 performance test Methods 0.000 description 1
- 238000005191 phase separation Methods 0.000 description 1
- 239000004417 polycarbonate Substances 0.000 description 1
- 229920000515 polycarbonate Polymers 0.000 description 1
- 229920000573 polyethylene Polymers 0.000 description 1
- 229920001282 polysaccharide Polymers 0.000 description 1
- 239000005017 polysaccharide Substances 0.000 description 1
- 150000004804 polysaccharides Chemical class 0.000 description 1
- 229920002689 polyvinyl acetate Polymers 0.000 description 1
- 239000011118 polyvinyl acetate Substances 0.000 description 1
- LZMJNVRJMFMYQS-UHFFFAOYSA-N poseltinib Chemical compound C1CN(C)CCN1C(C=C1)=CC=C1NC1=NC(OC=2C=C(NC(=O)C=C)C=CC=2)=C(OC=C2)C2=N1 LZMJNVRJMFMYQS-UHFFFAOYSA-N 0.000 description 1
- 238000002600 positron emission tomography Methods 0.000 description 1
- 229940069328 povidone Drugs 0.000 description 1
- 238000001556 precipitation Methods 0.000 description 1
- 208000029340 primitive neuroectodermal tumor Diseases 0.000 description 1
- 230000035755 proliferation Effects 0.000 description 1
- 125000004368 propenyl group Chemical group C(=CC)* 0.000 description 1
- 235000019260 propionic acid Nutrition 0.000 description 1
- 239000000473 propyl gallate Substances 0.000 description 1
- 235000010388 propyl gallate Nutrition 0.000 description 1
- 229940075579 propyl gallate Drugs 0.000 description 1
- 239000003223 protective agent Substances 0.000 description 1
- IUVKMZGDUIUOCP-BTNSXGMBSA-N quinbolone Chemical compound O([C@H]1CC[C@H]2[C@H]3[C@@H]([C@]4(C=CC(=O)C=C4CC3)C)CC[C@@]21C)C1=CCCC1 IUVKMZGDUIUOCP-BTNSXGMBSA-N 0.000 description 1
- 229950010654 quisinostat Drugs 0.000 description 1
- ZAHRKKWIAAJSAO-UHFFFAOYSA-N rapamycin Natural products COCC(O)C(=C/C(C)C(=O)CC(OC(=O)C1CCCCN1C(=O)C(=O)C2(O)OC(CC(OC)C(=CC=CC=CC(C)CC(C)C(=O)C)C)CCC2C)C(C)CC3CCC(O)C(C3)OC)C ZAHRKKWIAAJSAO-UHFFFAOYSA-N 0.000 description 1
- 230000008707 rearrangement Effects 0.000 description 1
- 102000005962 receptors Human genes 0.000 description 1
- 108020003175 receptors Proteins 0.000 description 1
- 230000002829 reductive effect Effects 0.000 description 1
- 239000013557 residual solvent Substances 0.000 description 1
- FECGNJPYVFEKOD-VMPITWQZSA-N resminostat Chemical compound C1=CC(CN(C)C)=CC=C1S(=O)(=O)N1C=C(\C=C\C(=O)NO)C=C1 FECGNJPYVFEKOD-VMPITWQZSA-N 0.000 description 1
- 229950002821 resminostat Drugs 0.000 description 1
- 230000002441 reversible effect Effects 0.000 description 1
- 238000009490 roller compaction Methods 0.000 description 1
- 229960004889 salicylic acid Drugs 0.000 description 1
- 229950009216 sapanisertib Drugs 0.000 description 1
- 229920006395 saturated elastomer Polymers 0.000 description 1
- 238000013341 scale-up Methods 0.000 description 1
- 238000012216 screening Methods 0.000 description 1
- 125000002914 sec-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 1
- 210000002966 serum Anatomy 0.000 description 1
- 229960002930 sirolimus Drugs 0.000 description 1
- QFJCIRLUMZQUOT-HPLJOQBZSA-N sirolimus Chemical compound C1C[C@@H](O)[C@H](OC)C[C@@H]1C[C@@H](C)[C@H]1OC(=O)[C@@H]2CCCCN2C(=O)C(=O)[C@](O)(O2)[C@H](C)CC[C@H]2C[C@H](OC)/C(C)=C/C=C/C=C/[C@@H](C)C[C@@H](C)C(=O)[C@H](OC)[C@H](O)/C(C)=C/[C@@H](C)C(=O)C1 QFJCIRLUMZQUOT-HPLJOQBZSA-N 0.000 description 1
- 230000008410 smoothened signaling pathway Effects 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- VPZRWNZGLKXFOE-UHFFFAOYSA-M sodium phenylbutyrate Chemical compound [Na+].[O-]C(=O)CCCC1=CC=CC=C1 VPZRWNZGLKXFOE-UHFFFAOYSA-M 0.000 description 1
- 229960002232 sodium phenylbutyrate Drugs 0.000 description 1
- 229940045902 sodium stearyl fumarate Drugs 0.000 description 1
- JAJWGJBVLPIOOH-IZYKLYLVSA-M sodium taurocholate Chemical compound [Na+].C([C@H]1C[C@H]2O)[C@H](O)CC[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@H]([C@@H](CCC(=O)NCCS([O-])(=O)=O)C)[C@@]2(C)[C@@H](O)C1 JAJWGJBVLPIOOH-IZYKLYLVSA-M 0.000 description 1
- AEQFSUDEHCCHBT-UHFFFAOYSA-M sodium valproate Chemical class [Na+].CCCC(C([O-])=O)CCC AEQFSUDEHCCHBT-UHFFFAOYSA-M 0.000 description 1
- 239000006104 solid solution Substances 0.000 description 1
- 230000003381 solubilizing effect Effects 0.000 description 1
- 238000000638 solvent extraction Methods 0.000 description 1
- 239000000600 sorbitol Substances 0.000 description 1
- 238000005507 spraying Methods 0.000 description 1
- 239000003381 stabilizer Substances 0.000 description 1
- 239000008107 starch Substances 0.000 description 1
- 235000019698 starch Nutrition 0.000 description 1
- 239000007858 starting material Substances 0.000 description 1
- 210000002784 stomach Anatomy 0.000 description 1
- 125000000547 substituted alkyl group Chemical group 0.000 description 1
- BUUPQKDIAURBJP-UHFFFAOYSA-N sulfinic acid Chemical compound OS=O BUUPQKDIAURBJP-UHFFFAOYSA-N 0.000 description 1
- 229910052717 sulfur Inorganic materials 0.000 description 1
- 239000011593 sulfur Substances 0.000 description 1
- 239000003765 sweetening agent Substances 0.000 description 1
- 208000024891 symptom Diseases 0.000 description 1
- 239000006188 syrup Substances 0.000 description 1
- 235000020357 syrup Nutrition 0.000 description 1
- 230000009897 systematic effect Effects 0.000 description 1
- 230000009885 systemic effect Effects 0.000 description 1
- 238000004885 tandem mass spectrometry Methods 0.000 description 1
- 230000008685 targeting Effects 0.000 description 1
- 239000011975 tartaric acid Substances 0.000 description 1
- 235000002906 tartaric acid Nutrition 0.000 description 1
- 229960004964 temozolomide Drugs 0.000 description 1
- YLQBMQCUIZJEEH-UHFFFAOYSA-N tetrahydrofuran Natural products C=1C=COC=1 YLQBMQCUIZJEEH-UHFFFAOYSA-N 0.000 description 1
- 229960003433 thalidomide Drugs 0.000 description 1
- 235000010384 tocopherol Nutrition 0.000 description 1
- 239000011732 tocopherol Substances 0.000 description 1
- 229960001295 tocopherol Drugs 0.000 description 1
- 229930003799 tocopherol Natural products 0.000 description 1
- JOXIMZWYDAKGHI-UHFFFAOYSA-N toluene-4-sulfonic acid Chemical class CC1=CC=C(S(O)(=O)=O)C=C1 JOXIMZWYDAKGHI-UHFFFAOYSA-N 0.000 description 1
- AKCRNFFTGXBONI-UHFFFAOYSA-N torin 1 Chemical compound C1CN(C(=O)CC)CCN1C1=CC=C(N2C(C=CC3=C2C2=CC(=CC=C2N=C3)C=2C=C3C=CC=CC3=NC=2)=O)C=C1C(F)(F)F AKCRNFFTGXBONI-UHFFFAOYSA-N 0.000 description 1
- GUXXEUUYCAYESJ-UHFFFAOYSA-N torin 2 Chemical compound C1=NC(N)=CC=C1C1=CC=C(N=CC2=C3N(C=4C=C(C=CC=4)C(F)(F)F)C(=O)C=C2)C3=C1 GUXXEUUYCAYESJ-UHFFFAOYSA-N 0.000 description 1
- MFAQYJIYDMLAIM-UHFFFAOYSA-N torkinib Chemical compound C12=C(N)N=CN=C2N(C(C)C)N=C1C1=CC2=CC(O)=CC=C2N1 MFAQYJIYDMLAIM-UHFFFAOYSA-N 0.000 description 1
- QORWJWZARLRLPR-UHFFFAOYSA-H tricalcium bis(phosphate) Chemical compound [Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O QORWJWZARLRLPR-UHFFFAOYSA-H 0.000 description 1
- 235000019731 tricalcium phosphate Nutrition 0.000 description 1
- RTKIYFITIVXBLE-QEQCGCAPSA-N trichostatin A Chemical compound ONC(=O)/C=C/C(/C)=C/[C@@H](C)C(=O)C1=CC=C(N(C)C)C=C1 RTKIYFITIVXBLE-QEQCGCAPSA-N 0.000 description 1
- 229910052722 tritium Inorganic materials 0.000 description 1
- 238000001291 vacuum drying Methods 0.000 description 1
- 125000000391 vinyl group Chemical group [H]C([*])=C([H])[H] 0.000 description 1
- 230000003442 weekly effect Effects 0.000 description 1
- GVJHHUAWPYXKBD-IEOSBIPESA-N α-tocopherol Chemical compound OC1=C(C)C(C)=C2O[C@@](CCC[C@H](C)CCC[C@H](C)CCCC(C)C)(C)CCC2=C1C GVJHHUAWPYXKBD-IEOSBIPESA-N 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/55—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having seven-membered rings, e.g. azelastine, pentylenetetrazole
- A61K31/551—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having seven-membered rings, e.g. azelastine, pentylenetetrazole having two nitrogen atoms, e.g. dilazep
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/55—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having seven-membered rings, e.g. azelastine, pentylenetetrazole
- A61K31/551—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having seven-membered rings, e.g. azelastine, pentylenetetrazole having two nitrogen atoms, e.g. dilazep
- A61K31/5513—1,4-Benzodiazepines, e.g. diazepam or clozapine
- A61K31/5517—1,4-Benzodiazepines, e.g. diazepam or clozapine condensed with five-membered rings having nitrogen as a ring hetero atom, e.g. imidazobenzodiazepines, triazolam
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K45/00—Medicinal preparations containing active ingredients not provided for in groups A61K31/00 - A61K41/00
- A61K45/06—Mixtures of active ingredients without chemical characterisation, e.g. antiphlogistics and cardiaca
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0053—Mouth and digestive tract, i.e. intraoral and peroral administration
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
- A61K9/141—Intimate drug-carrier mixtures characterised by the carrier, e.g. ordered mixtures, adsorbates, solid solutions, eutectica, co-dried, co-solubilised, co-kneaded, co-milled, co-ground products, co-precipitates, co-evaporates, co-extrudates, co-melts; Drug nanoparticles with adsorbed surface modifiers
- A61K9/146—Intimate drug-carrier mixtures characterised by the carrier, e.g. ordered mixtures, adsorbates, solid solutions, eutectica, co-dried, co-solubilised, co-kneaded, co-milled, co-ground products, co-precipitates, co-evaporates, co-extrudates, co-melts; Drug nanoparticles with adsorbed surface modifiers with organic macromolecular compounds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
- A61K9/16—Agglomerates; Granulates; Microbeadlets ; Microspheres; Pellets; Solid products obtained by spray drying, spray freeze drying, spray congealing,(multiple) emulsion solvent evaporation or extraction
- A61K9/1605—Excipients; Inactive ingredients
- A61K9/1629—Organic macromolecular compounds
- A61K9/1652—Polysaccharides, e.g. alginate, cellulose derivatives; Cyclodextrin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
- A61K9/4841—Filling excipients; Inactive ingredients
- A61K9/4866—Organic macromolecular compounds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
- A61K9/4891—Coated capsules; Multilayered drug free capsule shells
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
- A61P35/02—Antineoplastic agents specific for leukemia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Epidemiology (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Organic Chemistry (AREA)
- Nutrition Science (AREA)
- Physiology (AREA)
- Hematology (AREA)
- Oncology (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Medicinal Preparation (AREA)
- Heterocyclic Carbon Compounds Containing A Hetero Ring Having Oxygen Or Sulfur (AREA)
Applications Claiming Priority (7)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201461987813P | 2014-05-02 | 2014-05-02 | |
| US61/987,813 | 2014-05-02 | ||
| US201461990459P | 2014-05-08 | 2014-05-08 | |
| US201461990469P | 2014-05-08 | 2014-05-08 | |
| US61/990,459 | 2014-05-08 | ||
| US61/990,469 | 2014-05-08 | ||
| PCT/US2015/028798 WO2015168555A1 (en) | 2014-05-02 | 2015-05-01 | Method of treating resistant non-hodgkin lymphoma, medulloblastoma, and/or alk+non-small cell lung cancer using thienotriazolodiazepine compounds |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20170002550A true KR20170002550A (ko) | 2017-01-06 |
Family
ID=54359380
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020167033941A Withdrawn KR20170002550A (ko) | 2014-05-02 | 2015-05-01 | 티에노트리아졸로디아제핀 화합물을 사용한 내성 비호지킨 림프종, 수아세포종, 및/또는 alk+ 비소세포 폐암의 치료 방법 |
Country Status (10)
| Country | Link |
|---|---|
| US (1) | US20170095484A1 (enExample) |
| EP (1) | EP3137086A4 (enExample) |
| JP (1) | JP2017514907A (enExample) |
| KR (1) | KR20170002550A (enExample) |
| CN (1) | CN106687117A (enExample) |
| AU (1) | AU2015252940A1 (enExample) |
| CA (1) | CA2947593A1 (enExample) |
| MX (1) | MX2016014299A (enExample) |
| RU (1) | RU2016146102A (enExample) |
| WO (1) | WO2015168555A1 (enExample) |
Families Citing this family (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| MX366703B (es) | 2013-03-15 | 2019-07-22 | Incyte Holdings Corp | Heterociclos tricíclicos como inhibidores de la proteína bet. |
| EP3019502B1 (en) | 2013-07-08 | 2017-05-17 | Incyte Holdings Corporation | Tricyclic heterocycles as bet protein inhibitors |
| WO2015081189A1 (en) | 2013-11-26 | 2015-06-04 | Incyte Corporation | Bicyclic heterocycles as bet protein inhibitors |
| US9399640B2 (en) | 2013-11-26 | 2016-07-26 | Incyte Corporation | Substituted pyrrolo[2,3-c]pyridines and pyrazolo[3,4-c]pyridines as BET protein inhibitors |
| WO2015095492A1 (en) | 2013-12-19 | 2015-06-25 | Incyte Corporation | Tricyclic heterocycles as bet protein inhibitors |
| KR102702503B1 (ko) | 2014-04-23 | 2024-09-05 | 인사이트 홀딩스 코포레이션 | BET 단백질의 저해제로서의 1H-피롤로[2,3-c]피리딘-7(6H)-온 및 피라졸로[3,4-c]피리딘-7(6H)-온 |
| JP2017528446A (ja) * | 2014-08-19 | 2017-09-28 | オンコエシックス ゲーエムベーハー | チエノトリアゾロジアゼピン化合物を用いるリンパ腫の治療方法 |
| WO2016044130A1 (en) | 2014-09-15 | 2016-03-24 | Incyte Corporation | Tricyclic heterocycles for use as bet protein inhibitors |
| US10683305B2 (en) | 2015-04-27 | 2020-06-16 | Concert Pharmaceuticals, Inc. | Deuterated OTX-015 |
| US20170121347A1 (en) | 2015-10-29 | 2017-05-04 | Incyte Corporation | Amorphous solid form of a bet protein inhibitor |
| PH12021551886A1 (en) | 2016-06-20 | 2023-07-17 | Incyte Corp | Crystalline solid forms of a bet inhibitor |
| IL271658B2 (en) * | 2017-06-30 | 2023-09-01 | Onxeo | New oral formulations of belinostat |
| US11833155B2 (en) | 2020-06-03 | 2023-12-05 | Incyte Corporation | Combination therapy for treatment of myeloproliferative neoplasms |
Family Cites Families (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CA2412776C (en) * | 2000-06-16 | 2011-03-15 | Mitsubishi Pharma Corporation | Compositions controlling release ph range and/or speed |
| JP5478262B2 (ja) * | 2007-12-28 | 2014-04-23 | 田辺三菱製薬株式会社 | 抗癌剤 |
| DK2571503T3 (en) * | 2010-05-14 | 2015-04-20 | Dana Farber Cancer Inst Inc | COMPOSITIONS AND THEIR USE IN THE TREATMENT OF NEOPLASIA, INFLAMMATORY DISEASE AND OTHER DISORDERS |
| BR112014032105A2 (pt) * | 2012-06-25 | 2017-08-01 | Oncoethix Sa | método para o tratamento de câncer |
| KR20150100613A (ko) * | 2012-09-28 | 2015-09-02 | 온코에틱스 게엠베하 | 티에노트리아졸로디아제핀 화합물을 함유하는 약제학적 제제 |
| WO2015018520A1 (en) * | 2013-08-06 | 2015-02-12 | Oncoethix Sa | A bet-brd inhibitor represents a novel agent for alk positive anaplastic large cell lymphoma |
| WO2015018522A1 (en) * | 2013-08-06 | 2015-02-12 | Oncoethix Sa | Bet-bromodomain inhibitor shows synergism with several anti-cancer agents in pre-clinical models of diffuse large b-cell lymphoma (dlbcl) |
| EP3074019A1 (en) * | 2013-11-27 | 2016-10-05 | Oncoethix GmbH | Method of treating non-small-cell lung cancer using pharmaceutical formulation containing thienotriazolodiazepine compounds |
| WO2015078929A1 (en) * | 2013-11-27 | 2015-06-04 | Oncoethix Sa | Method of treating leukemia using pharmaceutical formulation containing thienotriazolodiazepine compounds |
-
2015
- 2015-05-01 WO PCT/US2015/028798 patent/WO2015168555A1/en not_active Ceased
- 2015-05-01 JP JP2017510449A patent/JP2017514907A/ja active Pending
- 2015-05-01 AU AU2015252940A patent/AU2015252940A1/en not_active Abandoned
- 2015-05-01 CA CA2947593A patent/CA2947593A1/en not_active Abandoned
- 2015-05-01 RU RU2016146102A patent/RU2016146102A/ru not_active Application Discontinuation
- 2015-05-01 KR KR1020167033941A patent/KR20170002550A/ko not_active Withdrawn
- 2015-05-01 EP EP15786309.3A patent/EP3137086A4/en not_active Withdrawn
- 2015-05-01 US US15/308,548 patent/US20170095484A1/en not_active Abandoned
- 2015-05-01 CN CN201580036829.XA patent/CN106687117A/zh active Pending
- 2015-05-01 MX MX2016014299A patent/MX2016014299A/es unknown
Also Published As
| Publication number | Publication date |
|---|---|
| CA2947593A1 (en) | 2015-11-05 |
| MX2016014299A (es) | 2017-01-27 |
| AU2015252940A1 (en) | 2016-11-17 |
| CN106687117A (zh) | 2017-05-17 |
| RU2016146102A3 (enExample) | 2018-12-17 |
| JP2017514907A (ja) | 2017-06-08 |
| EP3137086A1 (en) | 2017-03-08 |
| RU2016146102A (ru) | 2018-06-05 |
| WO2015168555A1 (en) | 2015-11-05 |
| US20170095484A1 (en) | 2017-04-06 |
| EP3137086A4 (en) | 2017-12-27 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR20170002550A (ko) | 티에노트리아졸로디아제핀 화합물을 사용한 내성 비호지킨 림프종, 수아세포종, 및/또는 alk+ 비소세포 폐암의 치료 방법 | |
| CN104968334B (zh) | 包含噻吩并三唑并二氮杂卓化合物的药物制剂 | |
| KR20160145833A (ko) | 티에노트리아졸로디아제핀 화합물을 사용한 급성 골수성 백혈병 및/또는 급성 림프아구성 백혈병의 치료 방법 | |
| KR20170002610A (ko) | 티에노트리아졸로디아제핀 화합물을 사용한 삼중 음성 유방암의 치료 방법 | |
| KR20170044172A (ko) | 티에노트리아졸로디아제핀 화합물을 함유하는 약학적 조성물을 사용하여 급성 골수성 백혈병 또는 급성 림프구성 백혈병을 치료하는 방법 | |
| KR20160037201A (ko) | Bet-브로모도메인 억제제를 사용한 미만성 거대 b-세포 림프종 (dlbcl)의 치료 방법 | |
| WO2015018522A1 (en) | Bet-bromodomain inhibitor shows synergism with several anti-cancer agents in pre-clinical models of diffuse large b-cell lymphoma (dlbcl) | |
| KR20160079823A (ko) | 티에노트리아졸로디아제핀 화합물을 함유하는 제약 제제를 사용하여 백혈병을 치료하는 방법 | |
| WO2015169953A1 (en) | Method of treating glioma using thienotriazolodiazepine compounds | |
| US9820992B2 (en) | Method of treating non-small-cell lung cancer using pharmaceutical formulation containing thienotriazolodiazepine compounds | |
| KR20170037670A (ko) | 티에노트리아졸로디아제핀 화합물을 사용한 림프종의 치료 방법 | |
| WO2015168587A1 (en) | Method of treating resistant multiple myeloma and mantle cell lymphoma using thienotriazolodiazepine compounds |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20161202 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| PC1203 | Withdrawal of no request for examination |