KR20160138410A - 기체 상 포스겐화 플랜트의 가동 방법 - Google Patents
기체 상 포스겐화 플랜트의 가동 방법 Download PDFInfo
- Publication number
- KR20160138410A KR20160138410A KR1020167026160A KR20167026160A KR20160138410A KR 20160138410 A KR20160138410 A KR 20160138410A KR 1020167026160 A KR1020167026160 A KR 1020167026160A KR 20167026160 A KR20167026160 A KR 20167026160A KR 20160138410 A KR20160138410 A KR 20160138410A
- Authority
- KR
- South Korea
- Prior art keywords
- phosgene
- stream
- zone
- reaction
- amine
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
- 238000000034 method Methods 0.000 title claims abstract description 52
- YGYAWVDWMABLBF-UHFFFAOYSA-N Phosgene Chemical compound ClC(Cl)=O YGYAWVDWMABLBF-UHFFFAOYSA-N 0.000 claims abstract description 160
- 150000001412 amines Chemical class 0.000 claims abstract description 142
- 238000002156 mixing Methods 0.000 claims abstract description 81
- 150000002513 isocyanates Chemical class 0.000 claims abstract description 35
- 239000012948 isocyanate Substances 0.000 claims abstract description 29
- 239000011261 inert gas Substances 0.000 claims abstract description 28
- 238000006243 chemical reaction Methods 0.000 claims description 97
- 239000007789 gas Substances 0.000 claims description 79
- 239000000463 material Substances 0.000 claims description 36
- 239000000203 mixture Substances 0.000 claims description 18
- 230000008569 process Effects 0.000 claims description 18
- 239000007788 liquid Substances 0.000 claims description 13
- 238000009835 boiling Methods 0.000 claims description 7
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 claims description 5
- NAQMVNRVTILPCV-UHFFFAOYSA-N hexane-1,6-diamine Chemical compound NCCCCCCN NAQMVNRVTILPCV-UHFFFAOYSA-N 0.000 claims description 4
- 238000004064 recycling Methods 0.000 claims description 4
- 238000011084 recovery Methods 0.000 claims description 3
- RNLHGQLZWXBQNY-UHFFFAOYSA-N 3-(aminomethyl)-3,5,5-trimethylcyclohexan-1-amine Chemical compound CC1(C)CC(N)CC(C)(CN)C1 RNLHGQLZWXBQNY-UHFFFAOYSA-N 0.000 claims description 2
- DZIHTWJGPDVSGE-UHFFFAOYSA-N 4-[(4-aminocyclohexyl)methyl]cyclohexan-1-amine Chemical compound C1CC(N)CCC1CC1CCC(N)CC1 DZIHTWJGPDVSGE-UHFFFAOYSA-N 0.000 claims description 2
- CKDWPUIZGOQOOM-UHFFFAOYSA-N Carbamyl chloride Chemical compound NC(Cl)=O CKDWPUIZGOQOOM-UHFFFAOYSA-N 0.000 claims description 2
- ZZTCPWRAHWXWCH-UHFFFAOYSA-N diphenylmethanediamine Chemical compound C=1C=CC=CC=1C(N)(N)C1=CC=CC=C1 ZZTCPWRAHWXWCH-UHFFFAOYSA-N 0.000 claims description 2
- VOZKAJLKRJDJLL-UHFFFAOYSA-N tolylenediamine group Chemical group CC1=C(C=C(C=C1)N)N VOZKAJLKRJDJLL-UHFFFAOYSA-N 0.000 claims description 2
- 239000000376 reactant Substances 0.000 abstract description 28
- 239000012071 phase Substances 0.000 description 36
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 28
- 239000000047 product Substances 0.000 description 18
- 238000004519 manufacturing process Methods 0.000 description 17
- 229910052757 nitrogen Inorganic materials 0.000 description 13
- 239000011541 reaction mixture Substances 0.000 description 12
- RFFLAFLAYFXFSW-UHFFFAOYSA-N 1,2-dichlorobenzene Chemical compound ClC1=CC=CC=C1Cl RFFLAFLAYFXFSW-UHFFFAOYSA-N 0.000 description 11
- 239000012442 inert solvent Substances 0.000 description 11
- 238000001704 evaporation Methods 0.000 description 10
- 230000008020 evaporation Effects 0.000 description 10
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 7
- 229910000041 hydrogen chloride Inorganic materials 0.000 description 7
- IXCSERBJSXMMFS-UHFFFAOYSA-N hydrogen chloride Substances Cl.Cl IXCSERBJSXMMFS-UHFFFAOYSA-N 0.000 description 7
- XKRFYHLGVUSROY-UHFFFAOYSA-N Argon Chemical compound [Ar] XKRFYHLGVUSROY-UHFFFAOYSA-N 0.000 description 6
- 229920002396 Polyurea Polymers 0.000 description 6
- 230000015572 biosynthetic process Effects 0.000 description 6
- 239000006227 byproduct Substances 0.000 description 6
- MVPPADPHJFYWMZ-UHFFFAOYSA-N chlorobenzene Chemical compound ClC1=CC=CC=C1 MVPPADPHJFYWMZ-UHFFFAOYSA-N 0.000 description 6
- 150000004985 diamines Chemical class 0.000 description 6
- 238000010791 quenching Methods 0.000 description 6
- 238000005201 scrubbing Methods 0.000 description 6
- 239000002904 solvent Substances 0.000 description 6
- 238000001816 cooling Methods 0.000 description 5
- 238000004821 distillation Methods 0.000 description 5
- 238000002360 preparation method Methods 0.000 description 5
- 230000000171 quenching effect Effects 0.000 description 5
- 239000000126 substance Substances 0.000 description 5
- 239000012808 vapor phase Substances 0.000 description 5
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 description 4
- 230000004888 barrier function Effects 0.000 description 4
- 239000000460 chlorine Substances 0.000 description 4
- 238000010438 heat treatment Methods 0.000 description 4
- OCJBOOLMMGQPQU-UHFFFAOYSA-N 1,4-dichlorobenzene Chemical compound ClC1=CC=C(Cl)C=C1 OCJBOOLMMGQPQU-UHFFFAOYSA-N 0.000 description 3
- 238000010521 absorption reaction Methods 0.000 description 3
- 229910052786 argon Inorganic materials 0.000 description 3
- 150000004945 aromatic hydrocarbons Chemical class 0.000 description 3
- 229910052801 chlorine Inorganic materials 0.000 description 3
- 230000000052 comparative effect Effects 0.000 description 3
- 238000003795 desorption Methods 0.000 description 3
- 229940117389 dichlorobenzene Drugs 0.000 description 3
- 229910052736 halogen Inorganic materials 0.000 description 3
- 150000002367 halogens Chemical group 0.000 description 3
- 239000001307 helium Substances 0.000 description 3
- 229910052734 helium Inorganic materials 0.000 description 3
- SWQJXJOGLNCZEY-UHFFFAOYSA-N helium atom Chemical compound [He] SWQJXJOGLNCZEY-UHFFFAOYSA-N 0.000 description 3
- 239000012535 impurity Substances 0.000 description 3
- 150000003141 primary amines Chemical class 0.000 description 3
- 238000000926 separation method Methods 0.000 description 3
- 239000007858 starting material Substances 0.000 description 3
- 238000006467 substitution reaction Methods 0.000 description 3
- 238000013459 approach Methods 0.000 description 2
- 238000010924 continuous production Methods 0.000 description 2
- 239000012043 crude product Substances 0.000 description 2
- 238000009792 diffusion process Methods 0.000 description 2
- 125000005442 diisocyanate group Chemical group 0.000 description 2
- 229910001873 dinitrogen Inorganic materials 0.000 description 2
- 239000011552 falling film Substances 0.000 description 2
- 239000012467 final product Substances 0.000 description 2
- 239000007792 gaseous phase Substances 0.000 description 2
- 239000000543 intermediate Substances 0.000 description 2
- 230000003647 oxidation Effects 0.000 description 2
- 238000007254 oxidation reaction Methods 0.000 description 2
- 230000037361 pathway Effects 0.000 description 2
- 239000002244 precipitate Substances 0.000 description 2
- 230000009467 reduction Effects 0.000 description 2
- 239000007787 solid Substances 0.000 description 2
- 230000008646 thermal stress Effects 0.000 description 2
- 238000004448 titration Methods 0.000 description 2
- QCQCHGYLTSGIGX-GHXANHINSA-N 4-[[(3ar,5ar,5br,7ar,9s,11ar,11br,13as)-5a,5b,8,8,11a-pentamethyl-3a-[(5-methylpyridine-3-carbonyl)amino]-2-oxo-1-propan-2-yl-4,5,6,7,7a,9,10,11,11b,12,13,13a-dodecahydro-3h-cyclopenta[a]chrysen-9-yl]oxy]-2,2-dimethyl-4-oxobutanoic acid Chemical compound N([C@@]12CC[C@@]3(C)[C@]4(C)CC[C@H]5C(C)(C)[C@@H](OC(=O)CC(C)(C)C(O)=O)CC[C@]5(C)[C@H]4CC[C@@H]3C1=C(C(C2)=O)C(C)C)C(=O)C1=CN=CC(C)=C1 QCQCHGYLTSGIGX-GHXANHINSA-N 0.000 description 1
- UGFAIRIUMAVXCW-UHFFFAOYSA-N Carbon monoxide Chemical compound [O+]#[C-] UGFAIRIUMAVXCW-UHFFFAOYSA-N 0.000 description 1
- 125000003277 amino group Chemical group 0.000 description 1
- 229910002091 carbon monoxide Inorganic materials 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 239000007806 chemical reaction intermediate Substances 0.000 description 1
- 239000007795 chemical reaction product Substances 0.000 description 1
- NHYCGSASNAIGLD-UHFFFAOYSA-N chlorine monoxide Inorganic materials Cl[O] NHYCGSASNAIGLD-UHFFFAOYSA-N 0.000 description 1
- 238000004140 cleaning Methods 0.000 description 1
- 239000003086 colorant Substances 0.000 description 1
- 238000009833 condensation Methods 0.000 description 1
- 230000005494 condensation Effects 0.000 description 1
- 230000008602 contraction Effects 0.000 description 1
- 230000009849 deactivation Effects 0.000 description 1
- 238000000354 decomposition reaction Methods 0.000 description 1
- 230000007423 decrease Effects 0.000 description 1
- 238000011161 development Methods 0.000 description 1
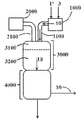
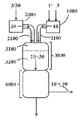
- 238000010586 diagram Methods 0.000 description 1
- 238000007865 diluting Methods 0.000 description 1
- 239000010408 film Substances 0.000 description 1
- 238000004817 gas chromatography Methods 0.000 description 1
- 238000010574 gas phase reaction Methods 0.000 description 1
- 239000008246 gaseous mixture Substances 0.000 description 1
- 238000000265 homogenisation Methods 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 230000000977 initiatory effect Effects 0.000 description 1
- 238000002347 injection Methods 0.000 description 1
- 239000007924 injection Substances 0.000 description 1
- 239000007791 liquid phase Substances 0.000 description 1
- 238000011068 loading method Methods 0.000 description 1
- 238000012423 maintenance Methods 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 238000005457 optimization Methods 0.000 description 1
- 239000003960 organic solvent Substances 0.000 description 1
- 239000005056 polyisocyanate Substances 0.000 description 1
- 229920001228 polyisocyanate Polymers 0.000 description 1
- 229920002635 polyurethane Polymers 0.000 description 1
- 239000004814 polyurethane Substances 0.000 description 1
- 230000008092 positive effect Effects 0.000 description 1
- 238000001556 precipitation Methods 0.000 description 1
- 150000003142 primary aromatic amines Chemical class 0.000 description 1
- 230000035484 reaction time Effects 0.000 description 1
- 238000010992 reflux Methods 0.000 description 1
- 239000013557 residual solvent Substances 0.000 description 1
- 238000005507 spraying Methods 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
- 239000006200 vaporizer Substances 0.000 description 1
- 239000002699 waste material Substances 0.000 description 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 1
- 238000010626 work up procedure Methods 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C263/00—Preparation of derivatives of isocyanic acid
- C07C263/10—Preparation of derivatives of isocyanic acid by reaction of amines with carbonyl halides, e.g. with phosgene
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J12/00—Chemical processes in general for reacting gaseous media with gaseous media; Apparatus specially adapted therefor
- B01J12/005—Chemical processes in general for reacting gaseous media with gaseous media; Apparatus specially adapted therefor carried out at high temperatures, e.g. by pyrolysis
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01B—NON-METALLIC ELEMENTS; COMPOUNDS THEREOF; METALLOIDS OR COMPOUNDS THEREOF NOT COVERED BY SUBCLASS C01C
- C01B32/00—Carbon; Compounds thereof
- C01B32/80—Phosgene
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C263/00—Preparation of derivatives of isocyanic acid
- C07C263/18—Separation; Purification; Stabilisation; Use of additives
- C07C263/20—Separation; Purification
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C265/00—Derivatives of isocyanic acid
- C07C265/14—Derivatives of isocyanic acid containing at least two isocyanate groups bound to the same carbon skeleton
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02P—CLIMATE CHANGE MITIGATION TECHNOLOGIES IN THE PRODUCTION OR PROCESSING OF GOODS
- Y02P20/00—Technologies relating to chemical industry
- Y02P20/50—Improvements relating to the production of bulk chemicals
- Y02P20/582—Recycling of unreacted starting or intermediate materials
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Inorganic Chemistry (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP14162004 | 2014-03-27 | ||
| EP14162004.7 | 2014-03-27 | ||
| PCT/EP2015/056214 WO2015144681A1 (de) | 2014-03-27 | 2015-03-24 | Verfahren zum betreiben einer gasphasenphosgenierungsanlage |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20160138410A true KR20160138410A (ko) | 2016-12-05 |
Family
ID=50382340
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020167026160A Withdrawn KR20160138410A (ko) | 2014-03-27 | 2015-03-24 | 기체 상 포스겐화 플랜트의 가동 방법 |
Country Status (7)
| Country | Link |
|---|---|
| US (1) | US9840461B2 (enExample) |
| EP (1) | EP3122719B1 (enExample) |
| JP (1) | JP6621760B2 (enExample) |
| KR (1) | KR20160138410A (enExample) |
| CN (1) | CN106458863B (enExample) |
| HU (1) | HUE039109T2 (enExample) |
| WO (1) | WO2015144681A1 (enExample) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2019245192A1 (ko) * | 2018-06-18 | 2019-12-26 | 한화케미칼 주식회사 | 지방족 이소시아네이트의 제조방법 |
Families Citing this family (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP3307708B1 (de) * | 2015-06-12 | 2021-12-01 | Covestro Intellectual Property GmbH & Co. KG | Verfahren zur herstellung von diisocyanaten in der gasphase |
| US10364214B1 (en) | 2016-09-01 | 2019-07-30 | Covestro Deutschland Ag | Method for producing isocyanates |
| EP3421426A1 (de) * | 2017-06-29 | 2019-01-02 | Covestro Deutschland AG | Energieeffizientes verfahren zur bereitstellung von phosgen-dampf |
| US11365172B2 (en) | 2017-07-03 | 2022-06-21 | Covestro Deutschland Ag | Production plant for producing a chemical product by reacting H-functional reactants with phosgene, and method for operating same with an interruption to production |
| EP4188592B1 (en) * | 2020-07-27 | 2025-09-10 | Basf Se | Phosgene production unit |
| US20230278000A1 (en) * | 2020-07-27 | 2023-09-07 | Basf Se | Phosgene production unit |
| KR102839457B1 (ko) * | 2020-12-30 | 2025-07-25 | 한화솔루션 주식회사 | 이소시아네이트 화합물의 제조 방법 |
| US20240228301A1 (en) * | 2021-04-21 | 2024-07-11 | Basf Se | Process for preparing phosgene |
| EP4296261A1 (de) | 2022-06-22 | 2023-12-27 | Covestro Deutschland AG | Verfahren und vorrichtung zur herstellung von isocyanaten |
| CN119365443A (zh) * | 2022-06-22 | 2025-01-24 | 科思创德国股份有限公司 | 通过(芳)脂族二胺和脂环族二胺的混合物的光气化生产异氰酸酯的方法 |
| EP4296260A1 (de) * | 2022-06-22 | 2023-12-27 | Covestro Deutschland AG | Herstellung spezieller isocyanate durch co-phosgenierung |
Family Cites Families (29)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS3510774B1 (enExample) * | 1954-12-01 | 1960-08-08 | ||
| DE3121036A1 (de) * | 1981-05-27 | 1982-12-16 | Bayer Ag, 5090 Leverkusen | Verfahren zur kontinuierlicehn herstellung von organischen mono- oder polyisocyanaten |
| DE3714439A1 (de) | 1987-04-30 | 1988-11-10 | Bayer Ag | Verfahren zur herstellung von (cyclo)aliphatischen diisocyanaten |
| FR2723585B1 (fr) | 1994-08-12 | 1996-09-27 | Rhone Poulenc Chimie | Procede de preparation de composes du type polyisocyanates aromatiques en phase gazeuse. |
| DE19942299A1 (de) * | 1999-09-04 | 2001-03-08 | Basf Ag | Verbessertes Verfahren zur Herstellung von Mono- und Oligo-Isocyanaten |
| DE10158160A1 (de) | 2001-11-28 | 2003-06-12 | Basf Ag | Herstellung von Isocyanaten in der Gasphase |
| EP1371634A1 (en) | 2002-06-14 | 2003-12-17 | Bayer Ag | Process for the purification of mixtures of toluenediisocyanate |
| EP1371633A1 (en) | 2002-06-14 | 2003-12-17 | Bayer Ag | Process for the purification of mixtures of toluenediisocyanate incorporating a dividing-wall distillation column |
| ES2271171T3 (es) | 2002-10-22 | 2007-04-16 | Bayer Materialscience Ag | Procedimiento para la purificacion de toluendiisocianato que incorpora una columna de destilacion de pared divisoria para la purificacion final. |
| DE10349504A1 (de) | 2003-10-23 | 2005-05-25 | Bayer Technology Services Gmbh | Verfahren zur Herstellung von Isocyanaten in der Gasphase |
| DE102005036870A1 (de) | 2005-08-02 | 2007-02-08 | Bayer Materialscience Ag | Verfahren zur Gasphasenphosgenierung |
| DE102005037328A1 (de) | 2005-08-04 | 2007-02-08 | Basf Ag | Verfahren zur Herstellung von Isocyanaten |
| DE102005042392A1 (de) | 2005-09-06 | 2007-03-08 | Basf Ag | Verfahren zur Herstellung von Isocyanaten |
| CN101583594B (zh) | 2006-11-07 | 2013-06-19 | 巴斯夫欧洲公司 | 生产异氰酸酯的方法 |
| ES2558857T3 (es) * | 2007-01-17 | 2016-02-09 | Basf Se | Procedimiento para la preparación de isocianatos |
| DE102007056511A1 (de) | 2007-11-22 | 2009-05-28 | Bayer Materialscience Ag | Verfahren zur Herstellung aromatischer Diisocyanate in der Gasphase |
| PL2323973T3 (pl) * | 2008-08-07 | 2012-08-31 | Basf Se | Sposób wytwarzania aromatycznych izocyjanianów |
| JP2012505850A (ja) | 2008-10-15 | 2012-03-08 | ビーエーエスエフ ソシエタス・ヨーロピア | イソシアネートの製造方法 |
| JP2010153365A (ja) * | 2008-11-19 | 2010-07-08 | Semiconductor Energy Lab Co Ltd | 発光素子、発光装置、電子機器及び照明装置 |
| CN101429139B (zh) * | 2008-12-18 | 2012-11-14 | 宁波万华聚氨酯有限公司 | 二环己基甲烷二异氰酸酯及其中间体的制备方法 |
| US20110301380A1 (en) * | 2009-03-06 | 2011-12-08 | Basf Se | Process and apparatus for preparing isocyanates |
| EP2408738B1 (de) | 2009-03-20 | 2017-07-26 | Basf Se | Verfahren zur herstellung von isocyanaten |
| DE102009032413A1 (de) | 2009-07-09 | 2011-01-13 | Bayer Materialscience Ag | Verfahren zur Herstellung von Isocyanaten |
| CN102933546B (zh) * | 2010-02-26 | 2014-12-10 | 巴斯夫欧洲公司 | 在气相中制备异氰酸酯的方法 |
| US8969615B2 (en) | 2011-03-31 | 2015-03-03 | Basf Se | Process for preparing isocyanates |
| WO2013029918A1 (de) * | 2011-09-02 | 2013-03-07 | Basf Se | Verfahren zur herstellung von isocyanaten |
| US8816126B2 (en) * | 2011-09-02 | 2014-08-26 | Basf Se | Process for preparing isocyanates |
| US9593075B2 (en) | 2012-03-19 | 2017-03-14 | Covestro Deutschland Ag | Method for producing isocyanates |
| JP6297602B2 (ja) | 2013-02-08 | 2018-03-20 | コベストロ、ドイチュラント、アクチエンゲゼルシャフトCovestro Deutschland Ag | ホスゲン化のガス状粗生成物からの、気相中の第一級アミンをホスゲン化することにより調製されたイソシアネートの分離方法 |
-
2015
- 2015-03-24 WO PCT/EP2015/056214 patent/WO2015144681A1/de not_active Ceased
- 2015-03-24 JP JP2016559280A patent/JP6621760B2/ja active Active
- 2015-03-24 US US15/128,179 patent/US9840461B2/en active Active
- 2015-03-24 HU HUE15711536A patent/HUE039109T2/hu unknown
- 2015-03-24 CN CN201580015894.4A patent/CN106458863B/zh active Active
- 2015-03-24 KR KR1020167026160A patent/KR20160138410A/ko not_active Withdrawn
- 2015-03-24 EP EP15711536.1A patent/EP3122719B1/de active Active
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2019245192A1 (ko) * | 2018-06-18 | 2019-12-26 | 한화케미칼 주식회사 | 지방족 이소시아네이트의 제조방법 |
| KR20190142642A (ko) * | 2018-06-18 | 2019-12-27 | 한화케미칼 주식회사 | 지방족 이소시아네이트의 제조방법 |
Also Published As
| Publication number | Publication date |
|---|---|
| WO2015144681A1 (de) | 2015-10-01 |
| US9840461B2 (en) | 2017-12-12 |
| JP6621760B2 (ja) | 2019-12-18 |
| US20170101367A1 (en) | 2017-04-13 |
| JP2017512798A (ja) | 2017-05-25 |
| HUE039109T2 (hu) | 2018-12-28 |
| EP3122719B1 (de) | 2018-05-16 |
| EP3122719A1 (de) | 2017-02-01 |
| CN106458863A (zh) | 2017-02-22 |
| CN106458863B (zh) | 2020-08-11 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR20160138410A (ko) | 기체 상 포스겐화 플랜트의 가동 방법 | |
| KR101433379B1 (ko) | 이소시아네이트의 기상 제조 방법 | |
| US10577311B2 (en) | Method for producing isocyanates | |
| KR101602495B1 (ko) | 엷게 착색된 이소시아네이트의 제조 | |
| US9796669B2 (en) | Process for preparing isocyanates | |
| CN110891932B (zh) | 在气相中制备异氰酸酯的方法 | |
| CN106715384A (zh) | 在气相中制备1,5‑戊二异氰酸酯的方法 | |
| KR101685699B1 (ko) | 이소시아네이트의 기상 제조 방법 | |
| CN101790510B (zh) | 制备异氰酸酯的方法 | |
| US10280135B2 (en) | Method for producing isocyanates | |
| WO2019145380A1 (en) | Process for the preparation of isocyanates | |
| KR20160137544A (ko) | 기체 상 포스겐화 플랜트의 가동 방법 | |
| KR20150114523A (ko) | 기체상에서 1차 아민의 포스겐화에 의해 제조된 이소시아네이트를 포스겐화 기체의 조생성물로부터 분리하는 방법 | |
| JP6893927B2 (ja) | イソシアネートの製造方法 | |
| US10858311B2 (en) | Method for producing isocyanates | |
| KR102733348B1 (ko) | 디이소시아네이트의 기체 상 제조 방법 | |
| HK1118532A (en) | Process for the preparation of isocyanates in the gas phase |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20160922 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| PC1203 | Withdrawal of no request for examination |