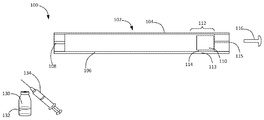
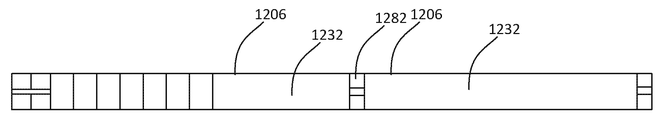
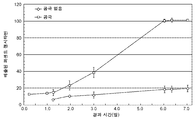
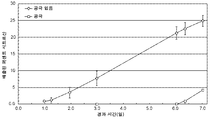
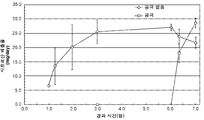
KR20160083061A - 삼투 약물 전달 디바이스들, 키트, 및 방법들 - Google Patents
삼투 약물 전달 디바이스들, 키트, 및 방법들 Download PDFInfo
- Publication number
- KR20160083061A KR20160083061A KR1020167014800A KR20167014800A KR20160083061A KR 20160083061 A KR20160083061 A KR 20160083061A KR 1020167014800 A KR1020167014800 A KR 1020167014800A KR 20167014800 A KR20167014800 A KR 20167014800A KR 20160083061 A KR20160083061 A KR 20160083061A
- Authority
- KR
- South Korea
- Prior art keywords
- fluid
- elongated tube
- drug
- water
- reservoir
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
- 238000000034 method Methods 0.000 title claims abstract description 78
- 230000003204 osmotic effect Effects 0.000 title claims description 50
- 238000012377 drug delivery Methods 0.000 title claims description 49
- 239000012530 fluid Substances 0.000 claims abstract description 149
- 239000003814 drug Substances 0.000 claims abstract description 141
- 229940079593 drug Drugs 0.000 claims abstract description 128
- 238000003780 insertion Methods 0.000 claims abstract description 21
- 230000037431 insertion Effects 0.000 claims abstract description 21
- 210000003708 urethra Anatomy 0.000 claims abstract description 19
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 81
- 239000002357 osmotic agent Substances 0.000 claims description 58
- 230000014759 maintenance of location Effects 0.000 claims description 40
- 239000002243 precursor Substances 0.000 claims description 40
- 239000007787 solid Substances 0.000 claims description 30
- 229960005277 gemcitabine Drugs 0.000 claims description 29
- SDUQYLNIPVEERB-QPPQHZFASA-N gemcitabine Chemical compound O=C1N=C(N)C=CN1[C@H]1C(F)(F)[C@H](O)[C@@H](CO)O1 SDUQYLNIPVEERB-QPPQHZFASA-N 0.000 claims description 29
- 239000000463 material Substances 0.000 claims description 20
- 239000000203 mixture Substances 0.000 claims description 17
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 claims description 16
- 238000009472 formulation Methods 0.000 claims description 16
- 238000013022 venting Methods 0.000 claims description 15
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 claims description 12
- 239000002904 solvent Substances 0.000 claims description 12
- 238000004891 communication Methods 0.000 claims description 10
- 238000007599 discharging Methods 0.000 claims description 10
- 239000003193 general anesthetic agent Substances 0.000 claims description 9
- 229920000642 polymer Polymers 0.000 claims description 9
- 239000000126 substance Substances 0.000 claims description 9
- 229920001296 polysiloxane Polymers 0.000 claims description 8
- 239000011780 sodium chloride Substances 0.000 claims description 8
- XIQVNETUBQGFHX-UHFFFAOYSA-N Ditropan Chemical compound C=1C=CC=CC=1C(O)(C(=O)OCC#CCN(CC)CC)C1CCCCC1 XIQVNETUBQGFHX-UHFFFAOYSA-N 0.000 claims description 7
- XSQUKJJJFZCRTK-UHFFFAOYSA-N Urea Chemical compound NC(N)=O XSQUKJJJFZCRTK-UHFFFAOYSA-N 0.000 claims description 7
- 229960005434 oxybutynin Drugs 0.000 claims description 7
- NNJVILVZKWQKPM-UHFFFAOYSA-N Lidocaine Chemical compound CCN(CC)CC(=O)NC1=C(C)C=CC=C1C NNJVILVZKWQKPM-UHFFFAOYSA-N 0.000 claims description 6
- 229960004194 lidocaine Drugs 0.000 claims description 6
- 239000012528 membrane Substances 0.000 claims description 6
- 229940127089 cytotoxic agent Drugs 0.000 claims description 5
- 229920001971 elastomer Polymers 0.000 claims description 5
- 239000000806 elastomer Substances 0.000 claims description 5
- 229920002635 polyurethane Polymers 0.000 claims description 5
- 239000004814 polyurethane Substances 0.000 claims description 5
- 229960001491 trospium Drugs 0.000 claims description 5
- OYYDSUSKLWTMMQ-JKHIJQBDSA-N trospium Chemical compound [N+]12([C@@H]3CC[C@H]2C[C@H](C3)OC(=O)C(O)(C=2C=CC=CC=2)C=2C=CC=CC=2)CCCC1 OYYDSUSKLWTMMQ-JKHIJQBDSA-N 0.000 claims description 5
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 claims description 4
- 229930006000 Sucrose Natural products 0.000 claims description 4
- 239000000556 agonist Substances 0.000 claims description 4
- 239000002246 antineoplastic agent Substances 0.000 claims description 4
- 239000004202 carbamide Substances 0.000 claims description 4
- 239000005720 sucrose Substances 0.000 claims description 4
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 claims description 3
- 229920000954 Polyglycolide Polymers 0.000 claims description 3
- 230000001022 anti-muscarinic effect Effects 0.000 claims description 3
- 229920001477 hydrophilic polymer Polymers 0.000 claims description 3
- 239000008101 lactose Substances 0.000 claims description 3
- 229960001756 oxaliplatin Drugs 0.000 claims description 3
- DWAFYCQODLXJNR-BNTLRKBRSA-L oxaliplatin Chemical compound O1C(=O)C(=O)O[Pt]11N[C@@H]2CCCC[C@H]2N1 DWAFYCQODLXJNR-BNTLRKBRSA-L 0.000 claims description 3
- 229920000747 poly(lactic acid) Polymers 0.000 claims description 3
- 229920000052 poly(p-xylylene) Polymers 0.000 claims description 3
- 239000010703 silicon Substances 0.000 claims description 3
- 229910052710 silicon Inorganic materials 0.000 claims description 3
- 239000001509 sodium citrate Substances 0.000 claims description 3
- HRXKRNGNAMMEHJ-UHFFFAOYSA-K trisodium citrate Chemical compound [Na+].[Na+].[Na+].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O HRXKRNGNAMMEHJ-UHFFFAOYSA-K 0.000 claims description 3
- 229940038773 trisodium citrate Drugs 0.000 claims description 3
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 claims description 2
- 239000004433 Thermoplastic polyurethane Substances 0.000 claims description 2
- 239000011248 coating agent Substances 0.000 claims description 2
- 238000000576 coating method Methods 0.000 claims description 2
- 239000002526 disodium citrate Substances 0.000 claims description 2
- 235000019262 disodium citrate Nutrition 0.000 claims description 2
- CEYULKASIQJZGP-UHFFFAOYSA-L disodium;2-(carboxymethyl)-2-hydroxybutanedioate Chemical compound [Na+].[Na+].[O-]C(=O)CC(O)(C(=O)O)CC([O-])=O CEYULKASIQJZGP-UHFFFAOYSA-L 0.000 claims description 2
- HWPKGOGLCKPRLZ-UHFFFAOYSA-M monosodium citrate Chemical compound [Na+].OC(=O)CC(O)(C([O-])=O)CC(O)=O HWPKGOGLCKPRLZ-UHFFFAOYSA-M 0.000 claims description 2
- 239000002524 monosodium citrate Substances 0.000 claims description 2
- 235000018342 monosodium citrate Nutrition 0.000 claims description 2
- 229920001606 poly(lactic acid-co-glycolic acid) Polymers 0.000 claims description 2
- 229920002803 thermoplastic polyurethane Polymers 0.000 claims description 2
- 235000019263 trisodium citrate Nutrition 0.000 claims description 2
- XUIMIQQOPSSXEZ-UHFFFAOYSA-N Silicon Chemical compound [Si] XUIMIQQOPSSXEZ-UHFFFAOYSA-N 0.000 claims 1
- 239000013583 drug formulation Substances 0.000 description 33
- 239000007788 liquid Substances 0.000 description 28
- 239000000243 solution Substances 0.000 description 21
- 239000003826 tablet Substances 0.000 description 17
- 238000011282 treatment Methods 0.000 description 15
- AOJJSUZBOXZQNB-TZSSRYMLSA-N Doxorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 AOJJSUZBOXZQNB-TZSSRYMLSA-N 0.000 description 12
- 239000011148 porous material Substances 0.000 description 12
- -1 polydimethylsiloxane Polymers 0.000 description 11
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 9
- 206010057190 Respiratory tract infections Diseases 0.000 description 7
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 7
- 108091006146 Channels Proteins 0.000 description 6
- ZMXDDKWLCZADIW-UHFFFAOYSA-N N,N-Dimethylformamide Chemical compound CN(C)C=O ZMXDDKWLCZADIW-UHFFFAOYSA-N 0.000 description 6
- 239000002202 Polyethylene glycol Substances 0.000 description 6
- YKPUWZUDDOIDPM-SOFGYWHQSA-N capsaicin Chemical compound COC1=CC(CNC(=O)CCCC\C=C\C(C)C)=CC=C1O YKPUWZUDDOIDPM-SOFGYWHQSA-N 0.000 description 6
- 229960004679 doxorubicin Drugs 0.000 description 6
- 229920003171 Poly (ethylene oxide) Polymers 0.000 description 5
- 229940035674 anesthetics Drugs 0.000 description 5
- 239000003795 chemical substances by application Substances 0.000 description 5
- 238000013461 design Methods 0.000 description 5
- 230000000694 effects Effects 0.000 description 5
- 230000004907 flux Effects 0.000 description 5
- 230000001965 increasing effect Effects 0.000 description 5
- 238000002347 injection Methods 0.000 description 5
- 239000007924 injection Substances 0.000 description 5
- 125000006850 spacer group Chemical group 0.000 description 5
- 206010021639 Incontinence Diseases 0.000 description 4
- 208000005615 Interstitial Cystitis Diseases 0.000 description 4
- NWIBSHFKIJFRCO-WUDYKRTCSA-N Mytomycin Chemical compound C1N2C(C(C(C)=C(N)C3=O)=O)=C3[C@@H](COC(N)=O)[C@@]2(OC)[C@@H]2[C@H]1N2 NWIBSHFKIJFRCO-WUDYKRTCSA-N 0.000 description 4
- QPCVHQBVMYCJOM-UHFFFAOYSA-N Propiverine Chemical compound C=1C=CC=CC=1C(C=1C=CC=CC=1)(OCCC)C(=O)OC1CCN(C)CC1 QPCVHQBVMYCJOM-UHFFFAOYSA-N 0.000 description 4
- PPBRXRYQALVLMV-UHFFFAOYSA-N Styrene Chemical compound C=CC1=CC=CC=C1 PPBRXRYQALVLMV-UHFFFAOYSA-N 0.000 description 4
- NKANXQFJJICGDU-QPLCGJKRSA-N Tamoxifen Chemical compound C=1C=CC=CC=1C(/CC)=C(C=1C=CC(OCCN(C)C)=CC=1)/C1=CC=CC=C1 NKANXQFJJICGDU-QPLCGJKRSA-N 0.000 description 4
- 239000007864 aqueous solution Substances 0.000 description 4
- 229960000190 bacillus calmette–guérin vaccine Drugs 0.000 description 4
- 150000001875 compounds Chemical class 0.000 description 4
- 238000007906 compression Methods 0.000 description 4
- 230000006835 compression Effects 0.000 description 4
- 201000010099 disease Diseases 0.000 description 4
- 230000003628 erosive effect Effects 0.000 description 4
- 230000005484 gravity Effects 0.000 description 4
- 208000015181 infectious disease Diseases 0.000 description 4
- 230000027939 micturition Effects 0.000 description 4
- 239000003149 muscarinic antagonist Substances 0.000 description 4
- 229960003510 propiverine Drugs 0.000 description 4
- 238000003860 storage Methods 0.000 description 4
- SGTNSNPWRIOYBX-UHFFFAOYSA-N 2-(3,4-dimethoxyphenyl)-5-{[2-(3,4-dimethoxyphenyl)ethyl](methyl)amino}-2-(propan-2-yl)pentanenitrile Chemical compound C1=C(OC)C(OC)=CC=C1CCN(C)CCCC(C#N)(C(C)C)C1=CC=C(OC)C(OC)=C1 SGTNSNPWRIOYBX-UHFFFAOYSA-N 0.000 description 3
- 206010005003 Bladder cancer Diseases 0.000 description 3
- 241001467552 Mycobacterium bovis BCG Species 0.000 description 3
- 208000007097 Urinary Bladder Neoplasms Diseases 0.000 description 3
- 239000002253 acid Substances 0.000 description 3
- 239000000048 adrenergic agonist Substances 0.000 description 3
- 230000003444 anaesthetic effect Effects 0.000 description 3
- 210000001124 body fluid Anatomy 0.000 description 3
- 239000010839 body fluid Substances 0.000 description 3
- 229960002504 capsaicin Drugs 0.000 description 3
- 235000017663 capsaicin Nutrition 0.000 description 3
- 239000000812 cholinergic antagonist Substances 0.000 description 3
- 229950010160 dimethocaine Drugs 0.000 description 3
- OWQIUQKMMPDHQQ-UHFFFAOYSA-N dimethocaine Chemical compound CCN(CC)CC(C)(C)COC(=O)C1=CC=C(N)C=C1 OWQIUQKMMPDHQQ-UHFFFAOYSA-N 0.000 description 3
- 208000035475 disorder Diseases 0.000 description 3
- 229960000855 flavoxate Drugs 0.000 description 3
- SPIUTQOUKAMGCX-UHFFFAOYSA-N flavoxate Chemical compound C1=CC=C2C(=O)C(C)=C(C=3C=CC=CC=3)OC2=C1C(=O)OCCN1CCCCC1 SPIUTQOUKAMGCX-UHFFFAOYSA-N 0.000 description 3
- 238000001727 in vivo Methods 0.000 description 3
- 230000000670 limiting effect Effects 0.000 description 3
- 229960004857 mitomycin Drugs 0.000 description 3
- HLXZNVUGXRDIFK-UHFFFAOYSA-N nickel titanium Chemical compound [Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni] HLXZNVUGXRDIFK-UHFFFAOYSA-N 0.000 description 3
- 229910001000 nickel titanium Inorganic materials 0.000 description 3
- 230000035699 permeability Effects 0.000 description 3
- 230000008569 process Effects 0.000 description 3
- 238000011084 recovery Methods 0.000 description 3
- 238000012360 testing method Methods 0.000 description 3
- 201000005112 urinary bladder cancer Diseases 0.000 description 3
- 210000004291 uterus Anatomy 0.000 description 3
- 229960000653 valrubicin Drugs 0.000 description 3
- ZOCKGBMQLCSHFP-KQRAQHLDSA-N valrubicin Chemical compound O([C@H]1C[C@](CC2=C(O)C=3C(=O)C4=CC=CC(OC)=C4C(=O)C=3C(O)=C21)(O)C(=O)COC(=O)CCCC)[C@H]1C[C@H](NC(=O)C(F)(F)F)[C@H](O)[C@H](C)O1 ZOCKGBMQLCSHFP-KQRAQHLDSA-N 0.000 description 3
- 229960001722 verapamil Drugs 0.000 description 3
- LEBVLXFERQHONN-UHFFFAOYSA-N 1-butyl-N-(2,6-dimethylphenyl)piperidine-2-carboxamide Chemical compound CCCCN1CCCCC1C(=O)NC1=C(C)C=CC=C1C LEBVLXFERQHONN-UHFFFAOYSA-N 0.000 description 2
- AOJJSUZBOXZQNB-VTZDEGQISA-N 4'-epidoxorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@@H](O)[C@H](C)O1 AOJJSUZBOXZQNB-VTZDEGQISA-N 0.000 description 2
- LRFVTYWOQMYALW-UHFFFAOYSA-N 9H-xanthine Chemical class O=C1NC(=O)NC2=C1NC=N2 LRFVTYWOQMYALW-UHFFFAOYSA-N 0.000 description 2
- RZVAJINKPMORJF-UHFFFAOYSA-N Acetaminophen Chemical compound CC(=O)NC1=CC=C(O)C=C1 RZVAJINKPMORJF-UHFFFAOYSA-N 0.000 description 2
- MXPOCMVWFLDDLZ-NSCUHMNNSA-N Apaziquone Chemical compound CN1C(\C=C\CO)=C(CO)C(C2=O)=C1C(=O)C=C2N1CC1 MXPOCMVWFLDDLZ-NSCUHMNNSA-N 0.000 description 2
- QTGIAADRBBLJGA-UHFFFAOYSA-N Articaine Chemical compound CCCNC(C)C(=O)NC=1C(C)=CSC=1C(=O)OC QTGIAADRBBLJGA-UHFFFAOYSA-N 0.000 description 2
- 229930003347 Atropine Natural products 0.000 description 2
- KPYSYYIEGFHWSV-UHFFFAOYSA-N Baclofen Chemical compound OC(=O)CC(CN)C1=CC=C(Cl)C=C1 KPYSYYIEGFHWSV-UHFFFAOYSA-N 0.000 description 2
- 108030001720 Bontoxilysin Proteins 0.000 description 2
- 229940127291 Calcium channel antagonist Drugs 0.000 description 2
- HTIJFSOGRVMCQR-UHFFFAOYSA-N Epirubicin Natural products COc1cccc2C(=O)c3c(O)c4CC(O)(CC(OC5CC(N)C(=O)C(C)O5)c4c(O)c3C(=O)c12)C(=O)CO HTIJFSOGRVMCQR-UHFFFAOYSA-N 0.000 description 2
- ULGZDMOVFRHVEP-RWJQBGPGSA-N Erythromycin Chemical compound O([C@@H]1[C@@H](C)C(=O)O[C@@H]([C@@]([C@H](O)[C@@H](C)C(=O)[C@H](C)C[C@@](C)(O)[C@H](O[C@H]2[C@@H]([C@H](C[C@@H](C)O2)N(C)C)O)[C@H]1C)(C)O)CC)[C@H]1C[C@@](C)(OC)[C@@H](O)[C@H](C)O1 ULGZDMOVFRHVEP-RWJQBGPGSA-N 0.000 description 2
- GHASVSINZRGABV-UHFFFAOYSA-N Fluorouracil Chemical compound FC1=CNC(=O)NC1=O GHASVSINZRGABV-UHFFFAOYSA-N 0.000 description 2
- UGJMXCAKCUNAIE-UHFFFAOYSA-N Gabapentin Chemical compound OC(=O)CC1(CN)CCCCC1 UGJMXCAKCUNAIE-UHFFFAOYSA-N 0.000 description 2
- RKUNBYITZUJHSG-UHFFFAOYSA-N Hyosciamin-hydrochlorid Natural products CN1C(C2)CCC1CC2OC(=O)C(CO)C1=CC=CC=C1 RKUNBYITZUJHSG-UHFFFAOYSA-N 0.000 description 2
- 208000000913 Kidney Calculi Diseases 0.000 description 2
- 102000043136 MAP kinase family Human genes 0.000 description 2
- 108091054455 MAP kinase family Proteins 0.000 description 2
- 229930192392 Mitomycin Natural products 0.000 description 2
- 102000014415 Muscarinic acetylcholine receptor Human genes 0.000 description 2
- 108050003473 Muscarinic acetylcholine receptor Proteins 0.000 description 2
- WHNWPMSKXPGLAX-UHFFFAOYSA-N N-Vinyl-2-pyrrolidone Chemical compound C=CN1CCCC1=O WHNWPMSKXPGLAX-UHFFFAOYSA-N 0.000 description 2
- DEXMFYZAHXMZNM-UHFFFAOYSA-N Narceine Chemical compound OC(=O)C1=C(OC)C(OC)=CC=C1C(=O)CC1=C(CCN(C)C)C=C(OCO2)C2=C1OC DEXMFYZAHXMZNM-UHFFFAOYSA-N 0.000 description 2
- 101710204212 Neocarzinostatin Proteins 0.000 description 2
- 206010029148 Nephrolithiasis Diseases 0.000 description 2
- 208000000693 Neurogenic Urinary Bladder Diseases 0.000 description 2
- 206010029279 Neurogenic bladder Diseases 0.000 description 2
- 208000002193 Pain Diseases 0.000 description 2
- VVWYOYDLCMFIEM-UHFFFAOYSA-N Propantheline Chemical compound C1=CC=C2C(C(=O)OCC[N+](C)(C(C)C)C(C)C)C3=CC=CC=C3OC2=C1 VVWYOYDLCMFIEM-UHFFFAOYSA-N 0.000 description 2
- 210000001744 T-lymphocyte Anatomy 0.000 description 2
- 239000004098 Tetracycline Substances 0.000 description 2
- FOCVUCIESVLUNU-UHFFFAOYSA-N Thiotepa Chemical compound C1CN1P(N1CC1)(=S)N1CC1 FOCVUCIESVLUNU-UHFFFAOYSA-N 0.000 description 2
- 206010046458 Urethral neoplasms Diseases 0.000 description 2
- 206010046543 Urinary incontinence Diseases 0.000 description 2
- XQAXGZLFSSPBMK-UHFFFAOYSA-M [7-(dimethylamino)phenothiazin-3-ylidene]-dimethylazanium;chloride;trihydrate Chemical compound O.O.O.[Cl-].C1=CC(=[N+](C)C)C=C2SC3=CC(N(C)C)=CC=C3N=C21 XQAXGZLFSSPBMK-UHFFFAOYSA-M 0.000 description 2
- 229910045601 alloy Inorganic materials 0.000 description 2
- 239000000956 alloy Substances 0.000 description 2
- 239000002160 alpha blocker Substances 0.000 description 2
- 229960000836 amitriptyline Drugs 0.000 description 2
- KRMDCWKBEZIMAB-UHFFFAOYSA-N amitriptyline Chemical compound C1CC2=CC=CC=C2C(=CCCN(C)C)C2=CC=CC=C21 KRMDCWKBEZIMAB-UHFFFAOYSA-N 0.000 description 2
- 239000003708 ampul Substances 0.000 description 2
- 229940035676 analgesics Drugs 0.000 description 2
- 239000000730 antalgic agent Substances 0.000 description 2
- 239000003242 anti bacterial agent Substances 0.000 description 2
- 230000002921 anti-spasmodic effect Effects 0.000 description 2
- 229940065524 anticholinergics inhalants for obstructive airway diseases Drugs 0.000 description 2
- 229940125681 anticonvulsant agent Drugs 0.000 description 2
- 239000001961 anticonvulsive agent Substances 0.000 description 2
- 229940124575 antispasmodic agent Drugs 0.000 description 2
- 229950002465 apaziquone Drugs 0.000 description 2
- 229960003831 articaine Drugs 0.000 description 2
- RKUNBYITZUJHSG-SPUOUPEWSA-N atropine Chemical compound O([C@H]1C[C@H]2CC[C@@H](C1)N2C)C(=O)C(CO)C1=CC=CC=C1 RKUNBYITZUJHSG-SPUOUPEWSA-N 0.000 description 2
- 229960000396 atropine Drugs 0.000 description 2
- 229960000794 baclofen Drugs 0.000 description 2
- BLFLLBZGZJTVJG-UHFFFAOYSA-N benzocaine Chemical compound CCOC(=O)C1=CC=C(N)C=C1 BLFLLBZGZJTVJG-UHFFFAOYSA-N 0.000 description 2
- 210000000476 body water Anatomy 0.000 description 2
- 229940053031 botulinum toxin Drugs 0.000 description 2
- 229960003150 bupivacaine Drugs 0.000 description 2
- 239000000480 calcium channel blocker Substances 0.000 description 2
- 239000002775 capsule Substances 0.000 description 2
- 230000015556 catabolic process Effects 0.000 description 2
- 210000004027 cell Anatomy 0.000 description 2
- 229960001231 choline Drugs 0.000 description 2
- OEYIOHPDSNJKLS-UHFFFAOYSA-N choline Chemical compound C[N+](C)(C)CCO OEYIOHPDSNJKLS-UHFFFAOYSA-N 0.000 description 2
- MYSWGUAQZAJSOK-UHFFFAOYSA-N ciprofloxacin Chemical compound C12=CC(N3CCNCC3)=C(F)C=C2C(=O)C(C(=O)O)=CN1C1CC1 MYSWGUAQZAJSOK-UHFFFAOYSA-N 0.000 description 2
- ZPUCINDJVBIVPJ-LJISPDSOSA-N cocaine Chemical compound O([C@H]1C[C@@H]2CC[C@@H](N2C)[C@H]1C(=O)OC)C(=O)C1=CC=CC=C1 ZPUCINDJVBIVPJ-LJISPDSOSA-N 0.000 description 2
- 230000008602 contraction Effects 0.000 description 2
- 238000006731 degradation reaction Methods 0.000 description 2
- XYYVYLMBEZUESM-UHFFFAOYSA-N dihydrocodeine Natural products C1C(N(CCC234)C)C2C=CC(=O)C3OC2=C4C1=CC=C2OC XYYVYLMBEZUESM-UHFFFAOYSA-N 0.000 description 2
- 239000004205 dimethyl polysiloxane Substances 0.000 description 2
- 229940126534 drug product Drugs 0.000 description 2
- 229960001904 epirubicin Drugs 0.000 description 2
- 229940011871 estrogen Drugs 0.000 description 2
- 239000000262 estrogen Substances 0.000 description 2
- 230000029142 excretion Effects 0.000 description 2
- 230000004761 fibrosis Effects 0.000 description 2
- 238000005429 filling process Methods 0.000 description 2
- 229960002949 fluorouracil Drugs 0.000 description 2
- 239000012634 fragment Substances 0.000 description 2
- 230000006870 function Effects 0.000 description 2
- XPXMKIXDFWLRAA-UHFFFAOYSA-N hydrazinide Chemical compound [NH-]N XPXMKIXDFWLRAA-UHFFFAOYSA-N 0.000 description 2
- OROGSEYTTFOCAN-UHFFFAOYSA-N hydrocodone Natural products C1C(N(CCC234)C)C2C=CC(O)C3OC2=C4C1=CC=C2OC OROGSEYTTFOCAN-UHFFFAOYSA-N 0.000 description 2
- 229960004801 imipramine Drugs 0.000 description 2
- BCGWQEUPMDMJNV-UHFFFAOYSA-N imipramine Chemical compound C1CC2=CC=CC=C2N(CCCN(C)C)C2=CC=CC=C21 BCGWQEUPMDMJNV-UHFFFAOYSA-N 0.000 description 2
- 239000007943 implant Substances 0.000 description 2
- 238000002513 implantation Methods 0.000 description 2
- CGIGDMFJXJATDK-UHFFFAOYSA-N indomethacin Chemical compound CC1=C(CC(O)=O)C2=CC(OC)=CC=C2N1C(=O)C1=CC=C(Cl)C=C1 CGIGDMFJXJATDK-UHFFFAOYSA-N 0.000 description 2
- 230000004941 influx Effects 0.000 description 2
- 238000001802 infusion Methods 0.000 description 2
- 229940043355 kinase inhibitor Drugs 0.000 description 2
- 229960001375 lactose Drugs 0.000 description 2
- 244000144972 livestock Species 0.000 description 2
- 229920002521 macromolecule Polymers 0.000 description 2
- 210000002540 macrophage Anatomy 0.000 description 2
- ZGEGCLOFRBLKSE-UHFFFAOYSA-N methylene hexane Natural products CCCCCC=C ZGEGCLOFRBLKSE-UHFFFAOYSA-N 0.000 description 2
- 229960000907 methylthioninium chloride Drugs 0.000 description 2
- 238000012986 modification Methods 0.000 description 2
- 230000004048 modification Effects 0.000 description 2
- BQJCRHHNABKAKU-KBQPJGBKSA-N morphine Chemical compound O([C@H]1[C@H](C=C[C@H]23)O)C4=C5[C@@]12CCN(C)[C@@H]3CC5=CC=C4O BQJCRHHNABKAKU-KBQPJGBKSA-N 0.000 description 2
- 229960000805 nalbuphine Drugs 0.000 description 2
- NETZHAKZCGBWSS-CEDHKZHLSA-N nalbuphine Chemical compound C([C@]12[C@H]3OC=4C(O)=CC=C(C2=4)C[C@@H]2[C@]1(O)CC[C@@H]3O)CN2CC1CCC1 NETZHAKZCGBWSS-CEDHKZHLSA-N 0.000 description 2
- QZGIWPZCWHMVQL-UIYAJPBUSA-N neocarzinostatin chromophore Chemical compound O1[C@H](C)[C@H](O)[C@H](O)[C@@H](NC)[C@H]1O[C@@H]1C/2=C/C#C[C@H]3O[C@@]3([C@@H]3OC(=O)OC3)C#CC\2=C[C@H]1OC(=O)C1=C(O)C=CC2=C(C)C=C(OC)C=C12 QZGIWPZCWHMVQL-UIYAJPBUSA-N 0.000 description 2
- 230000001272 neurogenic effect Effects 0.000 description 2
- 230000036961 partial effect Effects 0.000 description 2
- 239000008188 pellet Substances 0.000 description 2
- 239000000546 pharmaceutical excipient Substances 0.000 description 2
- 239000000825 pharmaceutical preparation Substances 0.000 description 2
- 239000003757 phosphotransferase inhibitor Substances 0.000 description 2
- 229920000435 poly(dimethylsiloxane) Polymers 0.000 description 2
- 229920001223 polyethylene glycol Polymers 0.000 description 2
- 239000000843 powder Substances 0.000 description 2
- 229960000697 propantheline Drugs 0.000 description 2
- 229960003981 proparacaine Drugs 0.000 description 2
- 201000007608 radiation cystitis Diseases 0.000 description 2
- 230000002829 reductive effect Effects 0.000 description 2
- 230000004044 response Effects 0.000 description 2
- NBFQYHKHPBMJJV-UHFFFAOYSA-N risocaine Chemical compound CCCOC(=O)C1=CC=C(N)C=C1 NBFQYHKHPBMJJV-UHFFFAOYSA-N 0.000 description 2
- 150000003839 salts Chemical group 0.000 description 2
- 229960004499 scopolamine hydrobromide Drugs 0.000 description 2
- WTGQALLALWYDJH-MOUKNHLCSA-N scopolamine hydrobromide (anhydrous) Chemical compound Br.C1([C@@H](CO)C(=O)O[C@H]2C[C@@H]3N([C@H](C2)[C@@H]2[C@H]3O2)C)=CC=CC=C1 WTGQALLALWYDJH-MOUKNHLCSA-N 0.000 description 2
- 239000000333 selective estrogen receptor modulator Substances 0.000 description 2
- 229940095743 selective estrogen receptor modulator Drugs 0.000 description 2
- 239000003772 serotonin uptake inhibitor Substances 0.000 description 2
- 239000013464 silicone adhesive Substances 0.000 description 2
- 150000003384 small molecules Chemical class 0.000 description 2
- 238000001356 surgical procedure Methods 0.000 description 2
- 229960001603 tamoxifen Drugs 0.000 description 2
- 229960005383 terodiline Drugs 0.000 description 2
- UISARWKNNNHPGI-UHFFFAOYSA-N terodiline Chemical compound C=1C=CC=CC=1C(CC(C)NC(C)(C)C)C1=CC=CC=C1 UISARWKNNNHPGI-UHFFFAOYSA-N 0.000 description 2
- 229960002180 tetracycline Drugs 0.000 description 2
- 229930101283 tetracycline Natural products 0.000 description 2
- 235000019364 tetracycline Nutrition 0.000 description 2
- 150000003522 tetracyclines Chemical class 0.000 description 2
- 229920002725 thermoplastic elastomer Polymers 0.000 description 2
- 229960001196 thiotepa Drugs 0.000 description 2
- 230000036962 time dependent Effects 0.000 description 2
- 210000001519 tissue Anatomy 0.000 description 2
- 229960004045 tolterodine Drugs 0.000 description 2
- OOGJQPCLVADCPB-HXUWFJFHSA-N tolterodine Chemical compound C1([C@@H](CCN(C(C)C)C(C)C)C=2C(=CC=C(C)C=2)O)=CC=CC=C1 OOGJQPCLVADCPB-HXUWFJFHSA-N 0.000 description 2
- 210000002700 urine Anatomy 0.000 description 2
- BDIAUFOIMFAIPU-UHFFFAOYSA-N valepotriate Natural products CC(C)CC(=O)OC1C=C(C(=COC2OC(=O)CC(C)C)COC(C)=O)C2C11CO1 BDIAUFOIMFAIPU-UHFFFAOYSA-N 0.000 description 2
- 229950009268 zinostatin Drugs 0.000 description 2
- RJMIEHBSYVWVIN-LLVKDONJSA-N (2r)-2-[4-(3-oxo-1h-isoindol-2-yl)phenyl]propanoic acid Chemical compound C1=CC([C@H](C(O)=O)C)=CC=C1N1C(=O)C2=CC=CC=C2C1 RJMIEHBSYVWVIN-LLVKDONJSA-N 0.000 description 1
- GRYSXUXXBDSYRT-WOUKDFQISA-N (2r,3r,4r,5r)-2-(hydroxymethyl)-4-methoxy-5-[6-(methylamino)purin-9-yl]oxolan-3-ol Chemical compound C1=NC=2C(NC)=NC=NC=2N1[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1OC GRYSXUXXBDSYRT-WOUKDFQISA-N 0.000 description 1
- BWNLUIXQIHPUGO-RDTXWAMCSA-N (2r,4r)-4-(dimethylamino)-2-phenyl-2-pyridin-2-ylpentanamide Chemical compound C1([C@](C(N)=O)(C[C@@H](C)N(C)C)C=2N=CC=CC=2)=CC=CC=C1 BWNLUIXQIHPUGO-RDTXWAMCSA-N 0.000 description 1
- WRRSFOZOETZUPG-FFHNEAJVSA-N (4r,4ar,7s,7ar,12bs)-9-methoxy-3-methyl-2,4,4a,7,7a,13-hexahydro-1h-4,12-methanobenzofuro[3,2-e]isoquinoline-7-ol;hydrate Chemical compound O.C([C@H]1[C@H](N(CC[C@@]112)C)C3)=C[C@H](O)[C@@H]1OC1=C2C3=CC=C1OC WRRSFOZOETZUPG-FFHNEAJVSA-N 0.000 description 1
- SGKRLCUYIXIAHR-AKNGSSGZSA-N (4s,4ar,5s,5ar,6r,12ar)-4-(dimethylamino)-1,5,10,11,12a-pentahydroxy-6-methyl-3,12-dioxo-4a,5,5a,6-tetrahydro-4h-tetracene-2-carboxamide Chemical compound C1=CC=C2[C@H](C)[C@@H]([C@H](O)[C@@H]3[C@](C(O)=C(C(N)=O)C(=O)[C@H]3N(C)C)(O)C3=O)C3=C(O)C2=C1O SGKRLCUYIXIAHR-AKNGSSGZSA-N 0.000 description 1
- TVYLLZQTGLZFBW-ZBFHGGJFSA-N (R,R)-tramadol Chemical compound COC1=CC=CC([C@]2(O)[C@H](CCCC2)CN(C)C)=C1 TVYLLZQTGLZFBW-ZBFHGGJFSA-N 0.000 description 1
- ZKMNUMMKYBVTFN-HNNXBMFYSA-N (S)-ropivacaine Chemical compound CCCN1CCCC[C@H]1C(=O)NC1=C(C)C=CC=C1C ZKMNUMMKYBVTFN-HNNXBMFYSA-N 0.000 description 1
- MWHXMIASLKXGBU-RNCYCKTQSA-N (e)-but-2-enedioic acid;[2-[(1r)-3-[di(propan-2-yl)amino]-1-phenylpropyl]-4-(hydroxymethyl)phenyl] 2-methylpropanoate Chemical compound OC(=O)\C=C\C(O)=O.C1([C@@H](CCN(C(C)C)C(C)C)C=2C(=CC=C(CO)C=2)OC(=O)C(C)C)=CC=CC=C1 MWHXMIASLKXGBU-RNCYCKTQSA-N 0.000 description 1
- KQODQNJLJQHFQV-MKWZWQCGSA-N (e,4s)-4-[[(2s)-3,3-dimethyl-2-[[(2s)-3-methyl-2-(methylamino)-3-(1-methylindol-3-yl)butanoyl]amino]butanoyl]-methylamino]-2,5-dimethylhex-2-enoic acid Chemical class C1=CC=C2C(C(C)(C)[C@@H](C(=O)N[C@H](C(=O)N(C)[C@H](\C=C(/C)C(O)=O)C(C)C)C(C)(C)C)NC)=CN(C)C2=C1 KQODQNJLJQHFQV-MKWZWQCGSA-N 0.000 description 1
- CNTMOLDWXSVYKD-PSRNMDMQSA-N (e,4s)-4-[[(2s)-3,3-dimethyl-2-[[(2s)-3-methyl-2-(methylamino)-3-phenylbutanoyl]amino]butanoyl]-methylamino]-2,5-dimethylhex-2-enoic acid Chemical compound OC(=O)C(/C)=C/[C@H](C(C)C)N(C)C(=O)[C@H](C(C)(C)C)NC(=O)[C@@H](NC)C(C)(C)C1=CC=CC=C1 CNTMOLDWXSVYKD-PSRNMDMQSA-N 0.000 description 1
- ZUBLNWRGQSNWGQ-UHFFFAOYSA-N 1-azabicyclo[2.2.2]octan-4-yl n-(2-phenylphenyl)carbamate;hydrochloride Chemical compound Cl.C1CN(CC2)CCC12OC(=O)NC1=CC=CC=C1C1=CC=CC=C1 ZUBLNWRGQSNWGQ-UHFFFAOYSA-N 0.000 description 1
- BFUUJUGQJUTPAF-UHFFFAOYSA-N 2-(3-amino-4-propoxybenzoyl)oxyethyl-diethylazanium;chloride Chemical compound [Cl-].CCCOC1=CC=C(C(=O)OCC[NH+](CC)CC)C=C1N BFUUJUGQJUTPAF-UHFFFAOYSA-N 0.000 description 1
- JMLXKDZVBYFSBK-UHFFFAOYSA-N 2-(hexylamino)-4-oxopentanoic acid Chemical compound CCCCCCNC(C(O)=O)CC(C)=O JMLXKDZVBYFSBK-UHFFFAOYSA-N 0.000 description 1
- GKNPSSNBBWDAGH-UHFFFAOYSA-N 2-hydroxy-2,2-diphenylacetic acid (1,1-dimethyl-3-piperidin-1-iumyl) ester Chemical compound C1[N+](C)(C)CCCC1OC(=O)C(O)(C=1C=CC=CC=1)C1=CC=CC=C1 GKNPSSNBBWDAGH-UHFFFAOYSA-N 0.000 description 1
- UJABSZITRMATFL-UHFFFAOYSA-N 2-methyl-5-phenylfuran-3-carbonyl chloride Chemical compound ClC(=O)C1=C(C)OC(C=2C=CC=CC=2)=C1 UJABSZITRMATFL-UHFFFAOYSA-N 0.000 description 1
- VRBFTYUMFJWSJY-UHFFFAOYSA-N 28804-46-8 Chemical group ClC1CC(C=C2)=CC=C2C(Cl)CC2=CC=C1C=C2 VRBFTYUMFJWSJY-UHFFFAOYSA-N 0.000 description 1
- IYNWSQDZXMGGGI-NUEKZKHPSA-N 3-hydroxymorphinan Chemical compound C1CCC[C@H]2[C@H]3CC4=CC=C(O)C=C4[C@]21CCN3 IYNWSQDZXMGGGI-NUEKZKHPSA-N 0.000 description 1
- 239000003185 4 aminobutyric acid B receptor stimulating agent Substances 0.000 description 1
- DSUFPYCILZXJFF-UHFFFAOYSA-N 4-[[4-[[4-(pentoxycarbonylamino)cyclohexyl]methyl]cyclohexyl]carbamoyloxy]butyl n-[4-[[4-(butoxycarbonylamino)cyclohexyl]methyl]cyclohexyl]carbamate Chemical compound C1CC(NC(=O)OCCCCC)CCC1CC1CCC(NC(=O)OCCCCOC(=O)NC2CCC(CC3CCC(CC3)NC(=O)OCCCC)CC2)CC1 DSUFPYCILZXJFF-UHFFFAOYSA-N 0.000 description 1
- AMJLLDSZOICXMS-WLMVGHMQSA-N 4-amino-1-[(2r,4r,5r)-3,3-difluoro-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]pyrimidin-2-one Chemical compound O=C1N=C(N)C=CN1[C@H]1C(F)(F)[C@H](O)[C@@H](CO)O1.O=C1N=C(N)C=CN1[C@H]1C(F)(F)[C@H](O)[C@@H](CO)O1 AMJLLDSZOICXMS-WLMVGHMQSA-N 0.000 description 1
- WZRJTRPJURQBRM-UHFFFAOYSA-N 4-amino-n-(5-methyl-1,2-oxazol-3-yl)benzenesulfonamide;5-[(3,4,5-trimethoxyphenyl)methyl]pyrimidine-2,4-diamine Chemical compound O1C(C)=CC(NS(=O)(=O)C=2C=CC(N)=CC=2)=N1.COC1=C(OC)C(OC)=CC(CC=2C(=NC(N)=NC=2)N)=C1 WZRJTRPJURQBRM-UHFFFAOYSA-N 0.000 description 1
- HQFWVSGBVLEQGA-UHFFFAOYSA-N 4-aminobenzoic acid 3-(dibutylamino)propyl ester Chemical compound CCCCN(CCCC)CCCOC(=O)C1=CC=C(N)C=C1 HQFWVSGBVLEQGA-UHFFFAOYSA-N 0.000 description 1
- SQDAZGGFXASXDW-UHFFFAOYSA-N 5-bromo-2-(trifluoromethoxy)pyridine Chemical compound FC(F)(F)OC1=CC=C(Br)C=N1 SQDAZGGFXASXDW-UHFFFAOYSA-N 0.000 description 1
- BSYNRYMUTXBXSQ-FOQJRBATSA-N 59096-14-9 Chemical compound CC(=O)OC1=CC=CC=C1[14C](O)=O BSYNRYMUTXBXSQ-FOQJRBATSA-N 0.000 description 1
- USSIQXCVUWKGNF-UHFFFAOYSA-N 6-(dimethylamino)-4,4-diphenylheptan-3-one Chemical compound C=1C=CC=CC=1C(CC(C)N(C)C)(C(=O)CC)C1=CC=CC=C1 USSIQXCVUWKGNF-UHFFFAOYSA-N 0.000 description 1
- SLXKOJJOQWFEFD-UHFFFAOYSA-N 6-aminohexanoic acid Chemical compound NCCCCCC(O)=O SLXKOJJOQWFEFD-UHFFFAOYSA-N 0.000 description 1
- ZGXJTSGNIOSYLO-UHFFFAOYSA-N 88755TAZ87 Chemical compound NCC(=O)CCC(O)=O ZGXJTSGNIOSYLO-UHFFFAOYSA-N 0.000 description 1
- GSDSWSVVBLHKDQ-UHFFFAOYSA-N 9-fluoro-3-methyl-10-(4-methylpiperazin-1-yl)-7-oxo-2,3-dihydro-7H-[1,4]oxazino[2,3,4-ij]quinoline-6-carboxylic acid Chemical compound FC1=CC(C(C(C(O)=O)=C2)=O)=C3N2C(C)COC3=C1N1CCN(C)CC1 GSDSWSVVBLHKDQ-UHFFFAOYSA-N 0.000 description 1
- 229930000680 A04AD01 - Scopolamine Natural products 0.000 description 1
- WSVLPVUVIUVCRA-KPKNDVKVSA-N Alpha-lactose monohydrate Chemical compound O.O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O WSVLPVUVIUVCRA-KPKNDVKVSA-N 0.000 description 1
- CPELXLSAUQHCOX-UHFFFAOYSA-M Bromide Chemical compound [Br-] CPELXLSAUQHCOX-UHFFFAOYSA-M 0.000 description 1
- 108090000312 Calcium Channels Proteins 0.000 description 1
- 102000003922 Calcium Channels Human genes 0.000 description 1
- 229930186147 Cephalosporin Natural products 0.000 description 1
- 229920001287 Chondroitin sulfate Polymers 0.000 description 1
- KRKNYBCHXYNGOX-UHFFFAOYSA-K Citrate Chemical compound [O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O KRKNYBCHXYNGOX-UHFFFAOYSA-K 0.000 description 1
- CMSMOCZEIVJLDB-UHFFFAOYSA-N Cyclophosphamide Chemical compound ClCCN(CCCl)P1(=O)NCCCO1 CMSMOCZEIVJLDB-UHFFFAOYSA-N 0.000 description 1
- 206010011796 Cystitis interstitial Diseases 0.000 description 1
- 108010000437 Deamino Arginine Vasopressin Proteins 0.000 description 1
- 229920002307 Dextran Polymers 0.000 description 1
- IJVCSMSMFSCRME-KBQPJGBKSA-N Dihydromorphine Chemical compound O([C@H]1[C@H](CC[C@H]23)O)C4=C5[C@@]12CCN(C)[C@@H]3CC5=CC=C4O IJVCSMSMFSCRME-KBQPJGBKSA-N 0.000 description 1
- 102000004190 Enzymes Human genes 0.000 description 1
- 108090000790 Enzymes Proteins 0.000 description 1
- 208000010228 Erectile Dysfunction Diseases 0.000 description 1
- OGDVEMNWJVYAJL-LEPYJNQMSA-N Ethyl morphine Chemical compound C([C@H]1[C@H](N(CC[C@@]112)C)C3)=C[C@H](O)[C@@H]1OC1=C2C3=CC=C1OCC OGDVEMNWJVYAJL-LEPYJNQMSA-N 0.000 description 1
- OGDVEMNWJVYAJL-UHFFFAOYSA-N Ethylmorphine Natural products C1C(N(CCC234)C)C2C=CC(O)C3OC2=C4C1=CC=C2OCC OGDVEMNWJVYAJL-UHFFFAOYSA-N 0.000 description 1
- VTUSIVBDOCDNHS-UHFFFAOYSA-N Etidocaine Chemical compound CCCN(CC)C(CC)C(=O)NC1=C(C)C=CC=C1C VTUSIVBDOCDNHS-UHFFFAOYSA-N 0.000 description 1
- IKYCZSUNGFRBJS-UHFFFAOYSA-N Euphorbia factor RL9 = U(1) = Resiniferatoxin Natural products COC1=CC(O)=CC(CC(=O)OCC=2CC3(O)C(=O)C(C)=CC3C34C(C)CC5(OC(O4)(CC=4C=CC=CC=4)OC5C3C=2)C(C)=C)=C1 IKYCZSUNGFRBJS-UHFFFAOYSA-N 0.000 description 1
- 206010016654 Fibrosis Diseases 0.000 description 1
- WSFSSNUMVMOOMR-UHFFFAOYSA-N Formaldehyde Chemical compound O=C WSFSSNUMVMOOMR-UHFFFAOYSA-N 0.000 description 1
- 241000237858 Gastropoda Species 0.000 description 1
- 229920002683 Glycosaminoglycan Polymers 0.000 description 1
- NMJREATYWWNIKX-UHFFFAOYSA-N GnRH Chemical class C1CCC(C(=O)NCC(N)=O)N1C(=O)C(CC(C)C)NC(=O)C(CC=1C2=CC=CC=C2NC=1)NC(=O)CNC(=O)C(NC(=O)C(CO)NC(=O)C(CC=1C2=CC=CC=C2NC=1)NC(=O)C(CC=1NC=NC=1)NC(=O)C1NC(=O)CC1)CC1=CC=C(O)C=C1 NMJREATYWWNIKX-UHFFFAOYSA-N 0.000 description 1
- 108010072471 HTI-286 Proteins 0.000 description 1
- HTTJABKRGRZYRN-UHFFFAOYSA-N Heparin Chemical compound OC1C(NC(=O)C)C(O)OC(COS(O)(=O)=O)C1OC1C(OS(O)(=O)=O)C(O)C(OC2C(C(OS(O)(=O)=O)C(OC3C(C(O)C(O)C(O3)C(O)=O)OS(O)(=O)=O)C(CO)O2)NS(O)(=O)=O)C(C(O)=O)O1 HTTJABKRGRZYRN-UHFFFAOYSA-N 0.000 description 1
- GVGLGOZIDCSQPN-PVHGPHFFSA-N Heroin Chemical compound O([C@H]1[C@H](C=C[C@H]23)OC(C)=O)C4=C5[C@@]12CCN(C)[C@@H]3CC5=CC=C4OC(C)=O GVGLGOZIDCSQPN-PVHGPHFFSA-N 0.000 description 1
- DKLKMKYDWHYZTD-UHFFFAOYSA-N Hexylcaine Chemical compound C=1C=CC=CC=1C(=O)OC(C)CNC1CCCCC1 DKLKMKYDWHYZTD-UHFFFAOYSA-N 0.000 description 1
- 241000372132 Hydrometridae Species 0.000 description 1
- STECJAGHUSJQJN-GAUPFVANSA-N Hyoscine Natural products C1([C@H](CO)C(=O)OC2C[C@@H]3N([C@H](C2)[C@@H]2[C@H]3O2)C)=CC=CC=C1 STECJAGHUSJQJN-GAUPFVANSA-N 0.000 description 1
- 206010020853 Hypertonic bladder Diseases 0.000 description 1
- 206010021118 Hypotonia Diseases 0.000 description 1
- HEFNNWSXXWATRW-UHFFFAOYSA-N Ibuprofen Chemical compound CC(C)CC1=CC=C(C(C)C(O)=O)C=C1 HEFNNWSXXWATRW-UHFFFAOYSA-N 0.000 description 1
- XDXDZDZNSLXDNA-TZNDIEGXSA-N Idarubicin Chemical compound C1[C@H](N)[C@H](O)[C@H](C)O[C@H]1O[C@@H]1C2=C(O)C(C(=O)C3=CC=CC=C3C3=O)=C3C(O)=C2C[C@@](O)(C(C)=O)C1 XDXDZDZNSLXDNA-TZNDIEGXSA-N 0.000 description 1
- XDXDZDZNSLXDNA-UHFFFAOYSA-N Idarubicin Natural products C1C(N)C(O)C(C)OC1OC1C2=C(O)C(C(=O)C3=CC=CC=C3C3=O)=C3C(O)=C2CC(O)(C(C)=O)C1 XDXDZDZNSLXDNA-UHFFFAOYSA-N 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- 206010061218 Inflammation Diseases 0.000 description 1
- VDJHFHXMUKFKET-UHFFFAOYSA-N Ingenol mebutate Natural products CC1CC2C(C)(C)C2C2C=C(CO)C(O)C3(O)C(OC(=O)C(C)=CC)C(C)=CC31C2=O VDJHFHXMUKFKET-UHFFFAOYSA-N 0.000 description 1
- 108010002350 Interleukin-2 Proteins 0.000 description 1
- ALFGKMXHOUSVAD-UHFFFAOYSA-N Ketobemidone Chemical compound C=1C=CC(O)=CC=1C1(C(=O)CC)CCN(C)CC1 ALFGKMXHOUSVAD-UHFFFAOYSA-N 0.000 description 1
- 206010023421 Kidney fibrosis Diseases 0.000 description 1
- FBOZXECLQNJBKD-ZDUSSCGKSA-N L-methotrexate Chemical compound C=1N=C2N=C(N)N=C(N)C2=NC=1CN(C)C1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 FBOZXECLQNJBKD-ZDUSSCGKSA-N 0.000 description 1
- 239000005411 L01XE02 - Gefitinib Substances 0.000 description 1
- STECJAGHUSJQJN-USLFZFAMSA-N LSM-4015 Chemical compound C1([C@@H](CO)C(=O)OC2C[C@@H]3N([C@H](C2)[C@@H]2[C@H]3O2)C)=CC=CC=C1 STECJAGHUSJQJN-USLFZFAMSA-N 0.000 description 1
- 108010000817 Leuprolide Proteins 0.000 description 1
- JAQUASYNZVUNQP-USXIJHARSA-N Levorphanol Chemical compound C1C2=CC=C(O)C=C2[C@]23CCN(C)[C@H]1[C@@H]2CCCC3 JAQUASYNZVUNQP-USXIJHARSA-N 0.000 description 1
- 241000124008 Mammalia Species 0.000 description 1
- XADCESSVHJOZHK-UHFFFAOYSA-N Meperidine Chemical compound C=1C=CC=CC=1C1(C(=O)OCC)CCN(C)CC1 XADCESSVHJOZHK-UHFFFAOYSA-N 0.000 description 1
- 241001465754 Metazoa Species 0.000 description 1
- BAVYZALUXZFZLV-UHFFFAOYSA-N Methylamine Chemical compound NC BAVYZALUXZFZLV-UHFFFAOYSA-N 0.000 description 1
- 206010027566 Micturition urgency Diseases 0.000 description 1
- IDBPHNDTYPBSNI-UHFFFAOYSA-N N-(1-(2-(4-Ethyl-5-oxo-2-tetrazolin-1-yl)ethyl)-4-(methoxymethyl)-4-piperidyl)propionanilide Chemical compound C1CN(CCN2C(N(CC)N=N2)=O)CCC1(COC)N(C(=O)CC)C1=CC=CC=C1 IDBPHNDTYPBSNI-UHFFFAOYSA-N 0.000 description 1
- STECJAGHUSJQJN-UHFFFAOYSA-N N-Methyl-scopolamin Natural products C1C(C2C3O2)N(C)C3CC1OC(=O)C(CO)C1=CC=CC=C1 STECJAGHUSJQJN-UHFFFAOYSA-N 0.000 description 1
- MBBZMMPHUWSWHV-BDVNFPICSA-N N-methylglucamine Chemical compound CNC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO MBBZMMPHUWSWHV-BDVNFPICSA-N 0.000 description 1
- UIQMVEYFGZJHCZ-SSTWWWIQSA-N Nalorphine Chemical compound C([C@@H](N(CC1)CC=C)[C@@H]2C=C[C@@H]3O)C4=CC=C(O)C5=C4[C@@]21[C@H]3O5 UIQMVEYFGZJHCZ-SSTWWWIQSA-N 0.000 description 1
- CMWTZPSULFXXJA-UHFFFAOYSA-N Naproxen Natural products C1=C(C(C)C(O)=O)C=CC2=CC(OC)=CC=C21 CMWTZPSULFXXJA-UHFFFAOYSA-N 0.000 description 1
- 108010025020 Nerve Growth Factor Proteins 0.000 description 1
- 102000015336 Nerve Growth Factor Human genes 0.000 description 1
- 229940122467 Nerve growth factor antagonist Drugs 0.000 description 1
- ONBWJWYUHXVEJS-ZTYRTETDSA-N Normorphine Chemical compound C([C@@H](NCC1)[C@@H]2C=C[C@@H]3O)C4=CC=C(O)C5=C4[C@@]21[C@H]3O5 ONBWJWYUHXVEJS-ZTYRTETDSA-N 0.000 description 1
- 229940127450 Opioid Agonists Drugs 0.000 description 1
- 102000003840 Opioid Receptors Human genes 0.000 description 1
- 108090000137 Opioid Receptors Proteins 0.000 description 1
- 239000008896 Opium Substances 0.000 description 1
- 206010068319 Oropharyngeal pain Diseases 0.000 description 1
- VNQABZCSYCTZMS-UHFFFAOYSA-N Orthoform Chemical compound COC(=O)C1=CC=C(O)C(N)=C1 VNQABZCSYCTZMS-UHFFFAOYSA-N 0.000 description 1
- 208000009722 Overactive Urinary Bladder Diseases 0.000 description 1
- BRUQQQPBMZOVGD-XFKAJCMBSA-N Oxycodone Chemical compound O=C([C@@H]1O2)CC[C@@]3(O)[C@H]4CC5=CC=C(OC)C2=C5[C@@]13CCN4C BRUQQQPBMZOVGD-XFKAJCMBSA-N 0.000 description 1
- UQCNKQCJZOAFTQ-ISWURRPUSA-N Oxymorphone Chemical compound O([C@H]1C(CC[C@]23O)=O)C4=C5[C@@]12CCN(C)[C@@H]3CC5=CC=C4O UQCNKQCJZOAFTQ-ISWURRPUSA-N 0.000 description 1
- DUDKAZCAISNGQN-UHFFFAOYSA-N Oxyphencyclimine Chemical compound CN1CCCN=C1COC(=O)C(O)(C=1C=CC=CC=1)C1CCCCC1 DUDKAZCAISNGQN-UHFFFAOYSA-N 0.000 description 1
- 229930012538 Paclitaxel Natural products 0.000 description 1
- 206010033372 Pain and discomfort Diseases 0.000 description 1
- 208000000450 Pelvic Pain Diseases 0.000 description 1
- 229930182555 Penicillin Natural products 0.000 description 1
- 108010081690 Pertussis Toxin Proteins 0.000 description 1
- 201000007100 Pharyngitis Diseases 0.000 description 1
- YQKAVWCGQQXBGW-UHFFFAOYSA-N Piperocaine Chemical compound CC1CCCCN1CCCOC(=O)C1=CC=CC=C1 YQKAVWCGQQXBGW-UHFFFAOYSA-N 0.000 description 1
- 229920003072 Plasdone™ povidone Polymers 0.000 description 1
- 206010036018 Pollakiuria Diseases 0.000 description 1
- 229920002614 Polyether block amide Polymers 0.000 description 1
- 229930182780 Polyphenon E Natural products 0.000 description 1
- 239000004743 Polypropylene Substances 0.000 description 1
- 208000004550 Postoperative Pain Diseases 0.000 description 1
- 229940127315 Potassium Channel Openers Drugs 0.000 description 1
- RJKFOVLPORLFTN-LEKSSAKUSA-N Progesterone Chemical class C1CC2=CC(=O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H](C(=O)C)[C@@]1(C)CC2 RJKFOVLPORLFTN-LEKSSAKUSA-N 0.000 description 1
- KCLANYCVBBTKTO-UHFFFAOYSA-N Proparacaine Chemical compound CCCOC1=CC=C(C(=O)OCCN(CC)CC)C=C1N KCLANYCVBBTKTO-UHFFFAOYSA-N 0.000 description 1
- CAJIGINSTLKQMM-UHFFFAOYSA-N Propoxycaine Chemical compound CCCOC1=CC(N)=CC=C1C(=O)OCCN(CC)CC CAJIGINSTLKQMM-UHFFFAOYSA-N 0.000 description 1
- 241001195377 Prorates Species 0.000 description 1
- 206010060862 Prostate cancer Diseases 0.000 description 1
- 208000000236 Prostatic Neoplasms Diseases 0.000 description 1
- 241000220259 Raphanus Species 0.000 description 1
- 235000006140 Raphanus sativus var sativus Nutrition 0.000 description 1
- 229920000439 Sulodexide Polymers 0.000 description 1
- 108700012920 TNF Proteins 0.000 description 1
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 description 1
- 102000002689 Toll-like receptor Human genes 0.000 description 1
- 108020000411 Toll-like receptor Proteins 0.000 description 1
- 229940123445 Tricyclic antidepressant Drugs 0.000 description 1
- 108010057266 Type A Botulinum Toxins Proteins 0.000 description 1
- 208000025865 Ulcer Diseases 0.000 description 1
- 206010046431 Urethral cancer Diseases 0.000 description 1
- 206010046798 Uterine leiomyoma Diseases 0.000 description 1
- JXLYSJRDGCGARV-WWYNWVTFSA-N Vinblastine Natural products O=C(O[C@H]1[C@](O)(C(=O)OC)[C@@H]2N(C)c3c(cc(c(OC)c3)[C@]3(C(=O)OC)c4[nH]c5c(c4CCN4C[C@](O)(CC)C[C@H](C3)C4)cccc5)[C@@]32[C@H]2[C@@]1(CC)C=CCN2CC3)C JXLYSJRDGCGARV-WWYNWVTFSA-N 0.000 description 1
- 235000010724 Wisteria floribunda Nutrition 0.000 description 1
- 208000027418 Wounds and injury Diseases 0.000 description 1
- LUVOJBWJNHWVNG-UHFFFAOYSA-N [Na].[Na].[Na].OC(=O)CC(O)(C(O)=O)CC(O)=O Chemical compound [Na].[Na].[Na].OC(=O)CC(O)(C(O)=O)CC(O)=O LUVOJBWJNHWVNG-UHFFFAOYSA-N 0.000 description 1
- 230000009102 absorption Effects 0.000 description 1
- 238000010521 absorption reaction Methods 0.000 description 1
- 239000004480 active ingredient Substances 0.000 description 1
- 239000011149 active material Substances 0.000 description 1
- 230000001154 acute effect Effects 0.000 description 1
- 239000000853 adhesive Substances 0.000 description 1
- 230000001070 adhesive effect Effects 0.000 description 1
- 239000000695 adrenergic alpha-agonist Substances 0.000 description 1
- 229940009456 adriamycin Drugs 0.000 description 1
- 229960001391 alfentanil Drugs 0.000 description 1
- KGYFOSCXVAXULR-UHFFFAOYSA-N allylprodine Chemical compound C=1C=CC=CC=1C1(OC(=O)CC)CCN(C)CC1CC=C KGYFOSCXVAXULR-UHFFFAOYSA-N 0.000 description 1
- 229950004361 allylprodine Drugs 0.000 description 1
- 229940124308 alpha-adrenoreceptor antagonist Drugs 0.000 description 1
- 229960001349 alphaprodine Drugs 0.000 description 1
- UVAZQQHAVMNMHE-XJKSGUPXSA-N alphaprodine Chemical compound C=1C=CC=CC=1[C@@]1(OC(=O)CC)CCN(C)C[C@@H]1C UVAZQQHAVMNMHE-XJKSGUPXSA-N 0.000 description 1
- WMGSQTMJHBYJMQ-UHFFFAOYSA-N aluminum;magnesium;silicate Chemical compound [Mg+2].[Al+3].[O-][Si]([O-])([O-])[O-] WMGSQTMJHBYJMQ-UHFFFAOYSA-N 0.000 description 1
- 150000001408 amides Chemical class 0.000 description 1
- 229960002684 aminocaproic acid Drugs 0.000 description 1
- 229940126575 aminoglycoside Drugs 0.000 description 1
- 229960002749 aminolevulinic acid Drugs 0.000 description 1
- 229960003022 amoxicillin Drugs 0.000 description 1
- LSQZJLSUYDQPKJ-NJBDSQKTSA-N amoxicillin Chemical compound C1([C@@H](N)C(=O)N[C@H]2[C@H]3SC([C@@H](N3C2=O)C(O)=O)(C)C)=CC=C(O)C=C1 LSQZJLSUYDQPKJ-NJBDSQKTSA-N 0.000 description 1
- AVKUERGKIZMTKX-NJBDSQKTSA-N ampicillin Chemical compound C1([C@@H](N)C(=O)N[C@H]2[C@H]3SC([C@@H](N3C2=O)C(O)=O)(C)C)=CC=CC=C1 AVKUERGKIZMTKX-NJBDSQKTSA-N 0.000 description 1
- 229960000723 ampicillin Drugs 0.000 description 1
- 230000000202 analgesic effect Effects 0.000 description 1
- 238000004458 analytical method Methods 0.000 description 1
- LKYQLAWMNBFNJT-UHFFFAOYSA-N anileridine Chemical compound C1CC(C(=O)OCC)(C=2C=CC=CC=2)CCN1CCC1=CC=C(N)C=C1 LKYQLAWMNBFNJT-UHFFFAOYSA-N 0.000 description 1
- 229960002512 anileridine Drugs 0.000 description 1
- 239000005557 antagonist Substances 0.000 description 1
- 230000000844 anti-bacterial effect Effects 0.000 description 1
- 230000000708 anti-progestin effect Effects 0.000 description 1
- 230000001028 anti-proliverative effect Effects 0.000 description 1
- 230000001754 anti-pyretic effect Effects 0.000 description 1
- 229940088710 antibiotic agent Drugs 0.000 description 1
- 239000000935 antidepressant agent Substances 0.000 description 1
- 229940005513 antidepressants Drugs 0.000 description 1
- 229940121375 antifungal agent Drugs 0.000 description 1
- 239000003429 antifungal agent Substances 0.000 description 1
- 229960005475 antiinfective agent Drugs 0.000 description 1
- 239000004599 antimicrobial Substances 0.000 description 1
- 239000003418 antiprogestin Substances 0.000 description 1
- 239000002221 antipyretic Substances 0.000 description 1
- 229940125716 antipyretic agent Drugs 0.000 description 1
- 239000003443 antiviral agent Substances 0.000 description 1
- RMTMMKNSPRRFHW-SVAVBUBPSA-N apatorsen Chemical compound N1([C@@H]2O[C@H](COP(O)(=S)OC3C([C@@H](O[C@@H]3COP(O)(=S)OC3C([C@@H](O[C@@H]3COP(O)(=S)OC3C([C@@H](O[C@@H]3COP(O)(=S)OC3[C@H](O[C@H](C3)N3C4=C(C(NC(N)=N4)=O)N=C3)COP(O)(=S)OC3[C@H](O[C@H](C3)N3C4=C(C(NC(N)=N4)=O)N=C3)COP(O)(=S)OC3[C@H](O[C@H](C3)N3C(N=C(N)C(C)=C3)=O)COP(O)(=S)OC3[C@H](O[C@H](C3)N3C(NC(=O)C(C)=C3)=O)COP(O)(=S)OC3[C@H](O[C@H](C3)N3C(N=C(N)C(C)=C3)=O)COP(O)(=S)OC3[C@H](O[C@H](C3)N3C4=C(C(NC(N)=N4)=O)N=C3)COP(O)(=S)OC3[C@H](O[C@H](C3)N3C(N=C(N)C(C)=C3)=O)COP(O)(=S)OC3[C@H](O[C@H](C3)N3C4=C(C(NC(N)=N4)=O)N=C3)COP(O)(=S)OC3[C@H](O[C@H](C3)N3C4=C(C(NC(N)=N4)=O)N=C3)COP(O)(=S)OC3[C@H](O[C@H](C3)N3C(N=C(N)C(C)=C3)=O)COP(S)(=O)OC3[C@H](O[C@H](C3)N3C4=C(C(NC(N)=N4)=O)N=C3)COP(O)(=S)OC3[C@H](O[C@H](C3)N3C(N=C(N)C(C)=C3)=O)COP(O)(=S)OC3C([C@@H](O[C@@H]3COP(O)(=S)OC3C([C@@H](O[C@@H]3COP(O)(=S)OC3C([C@@H](O[C@@H]3COP(O)(=S)OC3C([C@@H](O[C@@H]3CO)N3C4=C(C(NC(N)=N4)=O)N=C3)OCCOC)N3C4=C(C(NC(N)=N4)=O)N=C3)OCCOC)N3C4=C(C(NC(N)=N4)=O)N=C3)OCCOC)N3C4=NC=NC(N)=C4N=C3)OCCOC)N3C(NC(=O)C(C)=C3)=O)OCCOC)N3C(N=C(N)C(C)=C3)=O)OCCOC)N3C4=C(C(NC=N4)=N)N=C3)OCCOC)C(O)C2OCCOC)C=C(C)C(=O)NC1=O RMTMMKNSPRRFHW-SVAVBUBPSA-N 0.000 description 1
- 239000003899 bactericide agent Substances 0.000 description 1
- 239000011324 bead Substances 0.000 description 1
- NCNRHFGMJRPRSK-MDZDMXLPSA-N belinostat Chemical compound ONC(=O)\C=C\C1=CC=CC(S(=O)(=O)NC=2C=CC=CC=2)=C1 NCNRHFGMJRPRSK-MDZDMXLPSA-N 0.000 description 1
- 229960003094 belinostat Drugs 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- 230000008901 benefit Effects 0.000 description 1
- 229960005274 benzocaine Drugs 0.000 description 1
- 229940049706 benzodiazepine Drugs 0.000 description 1
- 150000001557 benzodiazepines Chemical class 0.000 description 1
- VXJABHHJLXLNMP-UHFFFAOYSA-N benzoic acid [2-methyl-2-(propylamino)propyl] ester Chemical compound CCCNC(C)(C)COC(=O)C1=CC=CC=C1 VXJABHHJLXLNMP-UHFFFAOYSA-N 0.000 description 1
- RDJGWRFTDZZXSM-RNWLQCGYSA-N benzylmorphine Chemical compound O([C@@H]1[C@]23CCN([C@H](C4)[C@@H]3C=C[C@@H]1O)C)C1=C2C4=CC=C1OCC1=CC=CC=C1 RDJGWRFTDZZXSM-RNWLQCGYSA-N 0.000 description 1
- 229940124748 beta 2 agonist Drugs 0.000 description 1
- FLKWNFFCSSJANB-UHFFFAOYSA-N bezitramide Chemical compound O=C1N(C(=O)CC)C2=CC=CC=C2N1C(CC1)CCN1CCC(C#N)(C=1C=CC=CC=1)C1=CC=CC=C1 FLKWNFFCSSJANB-UHFFFAOYSA-N 0.000 description 1
- 229960004611 bezitramide Drugs 0.000 description 1
- 239000000560 biocompatible material Substances 0.000 description 1
- 239000003124 biologic agent Substances 0.000 description 1
- 239000012620 biological material Substances 0.000 description 1
- 230000000740 bleeding effect Effects 0.000 description 1
- 230000000903 blocking effect Effects 0.000 description 1
- RMRJXGBAOAMLHD-IHFGGWKQSA-N buprenorphine Chemical compound C([C@]12[C@H]3OC=4C(O)=CC=C(C2=4)C[C@@H]2[C@]11CC[C@]3([C@H](C1)[C@](C)(O)C(C)(C)C)OC)CN2CC1CC1 RMRJXGBAOAMLHD-IHFGGWKQSA-N 0.000 description 1
- 229960001736 buprenorphine Drugs 0.000 description 1
- 229960003369 butacaine Drugs 0.000 description 1
- QLHULAHOXSSASE-UHFFFAOYSA-N butan-2-yl 2-(2-hydroxyethyl)piperidine-1-carboxylate Chemical compound CCC(C)OC(=O)N1CCCCC1CCO QLHULAHOXSSASE-UHFFFAOYSA-N 0.000 description 1
- IFKLAQQSCNILHL-QHAWAJNXSA-N butorphanol Chemical compound N1([C@@H]2CC3=CC=C(C=C3[C@@]3([C@]2(CCCC3)O)CC1)O)CC1CCC1 IFKLAQQSCNILHL-QHAWAJNXSA-N 0.000 description 1
- 229960001113 butorphanol Drugs 0.000 description 1
- 229960000590 celecoxib Drugs 0.000 description 1
- RZEKVGVHFLEQIL-UHFFFAOYSA-N celecoxib Chemical compound C1=CC(C)=CC=C1C1=CC(C(F)(F)F)=NN1C1=CC=C(S(N)(=O)=O)C=C1 RZEKVGVHFLEQIL-UHFFFAOYSA-N 0.000 description 1
- 229940124587 cephalosporin Drugs 0.000 description 1
- 150000001780 cephalosporins Chemical class 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 230000003034 chemosensitisation Effects 0.000 description 1
- 239000006114 chemosensitizer Substances 0.000 description 1
- 229960002023 chloroprocaine Drugs 0.000 description 1
- VDANGULDQQJODZ-UHFFFAOYSA-N chloroprocaine Chemical compound CCN(CC)CCOC(=O)C1=CC=C(N)C=C1Cl VDANGULDQQJODZ-UHFFFAOYSA-N 0.000 description 1
- 229940059329 chondroitin sulfate Drugs 0.000 description 1
- 229960001747 cinchocaine Drugs 0.000 description 1
- PUFQVTATUTYEAL-UHFFFAOYSA-N cinchocaine Chemical compound C1=CC=CC2=NC(OCCCC)=CC(C(=O)NCCN(CC)CC)=C21 PUFQVTATUTYEAL-UHFFFAOYSA-N 0.000 description 1
- 229960003405 ciprofloxacin Drugs 0.000 description 1
- DQLATGHUWYMOKM-UHFFFAOYSA-L cisplatin Chemical compound N[Pt](N)(Cl)Cl DQLATGHUWYMOKM-UHFFFAOYSA-L 0.000 description 1
- 229960004316 cisplatin Drugs 0.000 description 1
- STJMRWALKKWQGH-UHFFFAOYSA-N clenbuterol Chemical compound CC(C)(C)NCC(O)C1=CC(Cl)=C(N)C(Cl)=C1 STJMRWALKKWQGH-UHFFFAOYSA-N 0.000 description 1
- 229960001117 clenbuterol Drugs 0.000 description 1
- GKEGFOKQMZHVOW-KUTGSRRKSA-M clidinium bromide Chemical compound [Br-].C1([C@H]2CC[N@+](CC2)(C1)C)OC(=O)C(O)(C=1C=CC=CC=1)C1=CC=CC=C1 GKEGFOKQMZHVOW-KUTGSRRKSA-M 0.000 description 1
- 229960005098 clidinium bromide Drugs 0.000 description 1
- GPZLDQAEBHTMPG-UHFFFAOYSA-N clonitazene Chemical compound N=1C2=CC([N+]([O-])=O)=CC=C2N(CCN(CC)CC)C=1CC1=CC=C(Cl)C=C1 GPZLDQAEBHTMPG-UHFFFAOYSA-N 0.000 description 1
- 229950001604 clonitazene Drugs 0.000 description 1
- 229940047766 co-trimoxazole Drugs 0.000 description 1
- 229960003920 cocaine Drugs 0.000 description 1
- 229960004126 codeine Drugs 0.000 description 1
- OROGSEYTTFOCAN-DNJOTXNNSA-N codeine Natural products C([C@H]1[C@H](N(CC[C@@]112)C)C3)=C[C@H](O)[C@@H]1OC1=C2C3=CC=C1OC OROGSEYTTFOCAN-DNJOTXNNSA-N 0.000 description 1
- 230000000052 comparative effect Effects 0.000 description 1
- 238000010276 construction Methods 0.000 description 1
- 238000013270 controlled release Methods 0.000 description 1
- 229920001577 copolymer Polymers 0.000 description 1
- 230000008878 coupling Effects 0.000 description 1
- 238000010168 coupling process Methods 0.000 description 1
- 238000005859 coupling reaction Methods 0.000 description 1
- 229960004741 cyclomethycaine Drugs 0.000 description 1
- YLRNESBGEGGQBK-UHFFFAOYSA-N cyclomethycaine Chemical compound CC1CCCCN1CCCOC(=O)C(C=C1)=CC=C1OC1CCCCC1 YLRNESBGEGGQBK-UHFFFAOYSA-N 0.000 description 1
- 229960004397 cyclophosphamide Drugs 0.000 description 1
- 229960003843 cyproterone Drugs 0.000 description 1
- DUSHUSLJJMDGTE-ZJPMUUANSA-N cyproterone Chemical compound C1=C(Cl)C2=CC(=O)[C@@H]3C[C@@H]3[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@@](C(=O)C)(O)[C@@]1(C)CC2 DUSHUSLJJMDGTE-ZJPMUUANSA-N 0.000 description 1
- 201000003146 cystitis Diseases 0.000 description 1
- 239000002254 cytotoxic agent Substances 0.000 description 1
- 231100000599 cytotoxic agent Toxicity 0.000 description 1
- POZRVZJJTULAOH-LHZXLZLDSA-N danazol Chemical compound C1[C@]2(C)[C@H]3CC[C@](C)([C@](CC4)(O)C#C)[C@@H]4[C@@H]3CCC2=CC2=C1C=NO2 POZRVZJJTULAOH-LHZXLZLDSA-N 0.000 description 1
- 229960000766 danazol Drugs 0.000 description 1
- 229960002677 darifenacin Drugs 0.000 description 1
- HXGBXQDTNZMWGS-RUZDIDTESA-N darifenacin Chemical compound C=1C=CC=CC=1C([C@H]1CN(CCC=2C=C3CCOC3=CC=2)CC1)(C(=O)N)C1=CC=CC=C1 HXGBXQDTNZMWGS-RUZDIDTESA-N 0.000 description 1
- LKFHUFAEFBRVQX-UHFFFAOYSA-N decanedioic acid;propane-1,2,3-triol Chemical compound OCC(O)CO.OC(=O)CCCCCCCCC(O)=O LKFHUFAEFBRVQX-UHFFFAOYSA-N 0.000 description 1
- 230000006735 deficit Effects 0.000 description 1
- 239000008367 deionised water Substances 0.000 description 1
- 229910021641 deionized water Inorganic materials 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- NFLWUMRGJYTJIN-NXBWRCJVSA-N desmopressin Chemical compound C([C@H]1C(=O)N[C@H](C(N[C@@H](CC(N)=O)C(=O)N[C@@H](CSSCCC(=O)N[C@@H](CC=2C=CC(O)=CC=2)C(=O)N1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(N)=O)=O)CCC(=O)N)C1=CC=CC=C1 NFLWUMRGJYTJIN-NXBWRCJVSA-N 0.000 description 1
- 229960004281 desmopressin Drugs 0.000 description 1
- 229950003851 desomorphine Drugs 0.000 description 1
- LNNWVNGFPYWNQE-GMIGKAJZSA-N desomorphine Chemical compound C1C2=CC=C(O)C3=C2[C@]24CCN(C)[C@H]1[C@@H]2CCC[C@@H]4O3 LNNWVNGFPYWNQE-GMIGKAJZSA-N 0.000 description 1
- 238000001784 detoxification Methods 0.000 description 1
- WDEFBBTXULIOBB-WBVHZDCISA-N dextilidine Chemical compound C=1C=CC=CC=1[C@@]1(C(=O)OCC)CCC=C[C@H]1N(C)C WDEFBBTXULIOBB-WBVHZDCISA-N 0.000 description 1
- INUNXTSAACVKJS-OAQYLSRUSA-N dextromoramide Chemical compound C([C@@H](C)C(C(=O)N1CCCC1)(C=1C=CC=CC=1)C=1C=CC=CC=1)N1CCOCC1 INUNXTSAACVKJS-OAQYLSRUSA-N 0.000 description 1
- 229960003701 dextromoramide Drugs 0.000 description 1
- 229960004193 dextropropoxyphene Drugs 0.000 description 1
- XLMALTXPSGQGBX-GCJKJVERSA-N dextropropoxyphene Chemical compound C([C@](OC(=O)CC)([C@H](C)CN(C)C)C=1C=CC=CC=1)C1=CC=CC=C1 XLMALTXPSGQGBX-GCJKJVERSA-N 0.000 description 1
- 229960003461 dezocine Drugs 0.000 description 1
- VTMVHDZWSFQSQP-VBNZEHGJSA-N dezocine Chemical compound C1CCCC[C@H]2CC3=CC=C(O)C=C3[C@]1(C)[C@H]2N VTMVHDZWSFQSQP-VBNZEHGJSA-N 0.000 description 1
- 239000000032 diagnostic agent Substances 0.000 description 1
- 229960002069 diamorphine Drugs 0.000 description 1
- RXTHKWVSXOIHJS-UHFFFAOYSA-N diampromide Chemical compound C=1C=CC=CC=1N(C(=O)CC)CC(C)N(C)CCC1=CC=CC=C1 RXTHKWVSXOIHJS-UHFFFAOYSA-N 0.000 description 1
- 229950001059 diampromide Drugs 0.000 description 1
- GUBNMFJOJGDCEL-UHFFFAOYSA-N dicyclomine hydrochloride Chemical compound [Cl-].C1CCCCC1C1(C(=O)OCC[NH+](CC)CC)CCCCC1 GUBNMFJOJGDCEL-UHFFFAOYSA-N 0.000 description 1
- RGLYKWWBQGJZGM-ISLYRVAYSA-N diethylstilbestrol Chemical compound C=1C=C(O)C=CC=1C(/CC)=C(\CC)C1=CC=C(O)C=C1 RGLYKWWBQGJZGM-ISLYRVAYSA-N 0.000 description 1
- 229960000452 diethylstilbestrol Drugs 0.000 description 1
- 238000009792 diffusion process Methods 0.000 description 1
- 229960000920 dihydrocodeine Drugs 0.000 description 1
- RBOXVHNMENFORY-DNJOTXNNSA-N dihydrocodeine Chemical compound C([C@H]1[C@H](N(CC[C@@]112)C)C3)C[C@H](O)[C@@H]1OC1=C2C3=CC=C1OC RBOXVHNMENFORY-DNJOTXNNSA-N 0.000 description 1
- 239000003085 diluting agent Substances 0.000 description 1
- 238000010790 dilution Methods 0.000 description 1
- 239000012895 dilution Substances 0.000 description 1
- RHUWRJWFHUKVED-UHFFFAOYSA-N dimenoxadol Chemical compound C=1C=CC=CC=1C(C(=O)OCCN(C)C)(OCC)C1=CC=CC=C1 RHUWRJWFHUKVED-UHFFFAOYSA-N 0.000 description 1
- 229950011187 dimenoxadol Drugs 0.000 description 1
- 238000004141 dimensional analysis Methods 0.000 description 1
- QIRAYNIFEOXSPW-UHFFFAOYSA-N dimepheptanol Chemical compound C=1C=CC=CC=1C(CC(C)N(C)C)(C(O)CC)C1=CC=CC=C1 QIRAYNIFEOXSPW-UHFFFAOYSA-N 0.000 description 1
- 229950004655 dimepheptanol Drugs 0.000 description 1
- CANBGVXYBPOLRR-UHFFFAOYSA-N dimethylthiambutene Chemical compound C=1C=CSC=1C(=CC(C)N(C)C)C1=CC=CS1 CANBGVXYBPOLRR-UHFFFAOYSA-N 0.000 description 1
- 229950005563 dimethylthiambutene Drugs 0.000 description 1
- LQGIXNQCOXNCRP-UHFFFAOYSA-N dioxaphetyl butyrate Chemical compound C=1C=CC=CC=1C(C=1C=CC=CC=1)(C(=O)OCC)CCN1CCOCC1 LQGIXNQCOXNCRP-UHFFFAOYSA-N 0.000 description 1
- 229950008972 dioxaphetyl butyrate Drugs 0.000 description 1
- SVDHSZFEQYXRDC-UHFFFAOYSA-N dipipanone Chemical compound C=1C=CC=CC=1C(C=1C=CC=CC=1)(C(=O)CC)CC(C)N1CCCCC1 SVDHSZFEQYXRDC-UHFFFAOYSA-N 0.000 description 1
- 229960002500 dipipanone Drugs 0.000 description 1
- 238000006073 displacement reaction Methods 0.000 description 1
- 238000002224 dissection Methods 0.000 description 1
- 238000004090 dissolution Methods 0.000 description 1
- 238000009826 distribution Methods 0.000 description 1
- 229960003722 doxycycline Drugs 0.000 description 1
- 239000003684 drug solvent Substances 0.000 description 1
- 230000009977 dual effect Effects 0.000 description 1
- 230000004064 dysfunction Effects 0.000 description 1
- 239000013013 elastic material Substances 0.000 description 1
- 239000013536 elastomeric material Substances 0.000 description 1
- 229960002236 emepronium Drugs 0.000 description 1
- JEJBJBKVPOWOQK-UHFFFAOYSA-N emepronium Chemical compound C=1C=CC=CC=1C(CC(C)[N+](C)(C)CC)C1=CC=CC=C1 JEJBJBKVPOWOQK-UHFFFAOYSA-N 0.000 description 1
- ZOWQTJXNFTWSCS-IAQYHMDHSA-N eptazocine Chemical compound C1N(C)CC[C@@]2(C)C3=CC(O)=CC=C3C[C@@H]1C2 ZOWQTJXNFTWSCS-IAQYHMDHSA-N 0.000 description 1
- 229950010920 eptazocine Drugs 0.000 description 1
- 229960003276 erythromycin Drugs 0.000 description 1
- 150000002148 esters Chemical class 0.000 description 1
- FRPJXPJMRWBBIH-RBRWEJTLSA-N estramustine Chemical compound ClCCN(CCCl)C(=O)OC1=CC=C2[C@H]3CC[C@](C)([C@H](CC4)O)[C@@H]4[C@@H]3CCC2=C1 FRPJXPJMRWBBIH-RBRWEJTLSA-N 0.000 description 1
- 229960001842 estramustine Drugs 0.000 description 1
- WGJHHMKQBWSQIY-UHFFFAOYSA-N ethoheptazine Chemical compound C=1C=CC=CC=1C1(C(=O)OCC)CCCN(C)CC1 WGJHHMKQBWSQIY-UHFFFAOYSA-N 0.000 description 1
- 229960000569 ethoheptazine Drugs 0.000 description 1
- MORSAEFGQPDBKM-UHFFFAOYSA-N ethylmethylthiambutene Chemical compound C=1C=CSC=1C(=CC(C)N(C)CC)C1=CC=CS1 MORSAEFGQPDBKM-UHFFFAOYSA-N 0.000 description 1
- 229950006111 ethylmethylthiambutene Drugs 0.000 description 1
- 229960004578 ethylmorphine Drugs 0.000 description 1
- 229960003976 etidocaine Drugs 0.000 description 1
- PXDBZSCGSQSKST-UHFFFAOYSA-N etonitazene Chemical compound C1=CC(OCC)=CC=C1CC1=NC2=CC([N+]([O-])=O)=CC=C2N1CCN(CC)CC PXDBZSCGSQSKST-UHFFFAOYSA-N 0.000 description 1
- 229950004538 etonitazene Drugs 0.000 description 1
- 230000001747 exhibiting effect Effects 0.000 description 1
- 229960002428 fentanyl Drugs 0.000 description 1
- PJMPHNIQZUBGLI-UHFFFAOYSA-N fentanyl Chemical compound C=1C=CC=CC=1N(C(=O)CC)C(CC1)CCN1CCC1=CC=CC=C1 PJMPHNIQZUBGLI-UHFFFAOYSA-N 0.000 description 1
- 229960004524 fesoterodine fumarate Drugs 0.000 description 1
- MKXKFYHWDHIYRV-UHFFFAOYSA-N flutamide Chemical compound CC(C)C(=O)NC1=CC=C([N+]([O-])=O)C(C(F)(F)F)=C1 MKXKFYHWDHIYRV-UHFFFAOYSA-N 0.000 description 1
- 229960002074 flutamide Drugs 0.000 description 1
- 239000012458 free base Substances 0.000 description 1
- 229960002870 gabapentin Drugs 0.000 description 1
- XGALLCVXEZPNRQ-UHFFFAOYSA-N gefitinib Chemical compound C=12C=C(OCCCN3CCOCC3)C(OC)=CC2=NC=NC=1NC1=CC=C(F)C(Cl)=C1 XGALLCVXEZPNRQ-UHFFFAOYSA-N 0.000 description 1
- 229960002584 gefitinib Drugs 0.000 description 1
- 210000004907 gland Anatomy 0.000 description 1
- 229940015042 glycopyrrolate Drugs 0.000 description 1
- 239000003102 growth factor Substances 0.000 description 1
- 229920000669 heparin Polymers 0.000 description 1
- 229960002897 heparin Drugs 0.000 description 1
- 229960005388 hexylcaine Drugs 0.000 description 1
- 150000004677 hydrates Chemical class 0.000 description 1
- LLPOLZWFYMWNKH-CMKMFDCUSA-N hydrocodone Chemical compound C([C@H]1[C@H](N(CC[C@@]112)C)C3)CC(=O)[C@@H]1OC1=C2C3=CC=C1OC LLPOLZWFYMWNKH-CMKMFDCUSA-N 0.000 description 1
- 229960000240 hydrocodone Drugs 0.000 description 1
- 230000007062 hydrolysis Effects 0.000 description 1
- 238000006460 hydrolysis reaction Methods 0.000 description 1
- WVLOADHCBXTIJK-YNHQPCIGSA-N hydromorphone Chemical compound O([C@H]1C(CC[C@H]23)=O)C4=C5[C@@]12CCN(C)[C@@H]3CC5=CC=C4O WVLOADHCBXTIJK-YNHQPCIGSA-N 0.000 description 1
- 229960001410 hydromorphone Drugs 0.000 description 1
- WTJBNMUWRKPFRS-UHFFFAOYSA-N hydroxypethidine Chemical compound C=1C=CC(O)=CC=1C1(C(=O)OCC)CCN(C)CC1 WTJBNMUWRKPFRS-UHFFFAOYSA-N 0.000 description 1
- 229950008496 hydroxypethidine Drugs 0.000 description 1
- 229960001680 ibuprofen Drugs 0.000 description 1
- 229960000908 idarubicin Drugs 0.000 description 1
- 239000012216 imaging agent Substances 0.000 description 1
- 229960002751 imiquimod Drugs 0.000 description 1
- DOUYETYNHWVLEO-UHFFFAOYSA-N imiquimod Chemical compound C1=CC=CC2=C3N(CC(C)C)C=NC3=C(N)N=C21 DOUYETYNHWVLEO-UHFFFAOYSA-N 0.000 description 1
- 239000002955 immunomodulating agent Substances 0.000 description 1
- 229940121354 immunomodulator Drugs 0.000 description 1
- 230000002584 immunomodulator Effects 0.000 description 1
- 201000001881 impotence Diseases 0.000 description 1
- 229960000905 indomethacin Drugs 0.000 description 1
- 229960004187 indoprofen Drugs 0.000 description 1
- 230000001939 inductive effect Effects 0.000 description 1
- 230000004968 inflammatory condition Effects 0.000 description 1
- 230000004054 inflammatory process Effects 0.000 description 1
- VDJHFHXMUKFKET-WDUFCVPESA-N ingenol mebutate Chemical compound C[C@@H]1C[C@H]2C(C)(C)[C@H]2[C@@H]2C=C(CO)[C@@H](O)[C@]3(O)[C@@H](OC(=O)C(\C)=C/C)C(C)=C[C@]31C2=O VDJHFHXMUKFKET-WDUFCVPESA-N 0.000 description 1
- 229960002993 ingenol mebutate Drugs 0.000 description 1
- 208000014674 injury Diseases 0.000 description 1
- 229960001361 ipratropium bromide Drugs 0.000 description 1
- KEWHKYJURDBRMN-ZEODDXGYSA-M ipratropium bromide hydrate Chemical compound O.[Br-].O([C@H]1C[C@H]2CC[C@@H](C1)[N@@+]2(C)C(C)C)C(=O)C(CO)C1=CC=CC=C1 KEWHKYJURDBRMN-ZEODDXGYSA-M 0.000 description 1
- IFKPLJWIEQBPGG-UHFFFAOYSA-N isomethadone Chemical compound C=1C=CC=CC=1C(C(C)CN(C)C)(C(=O)CC)C1=CC=CC=C1 IFKPLJWIEQBPGG-UHFFFAOYSA-N 0.000 description 1
- 229950009272 isomethadone Drugs 0.000 description 1
- 229960000318 kanamycin Drugs 0.000 description 1
- 229930027917 kanamycin Natural products 0.000 description 1
- SBUJHOSQTJFQJX-NOAMYHISSA-N kanamycin Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CN)O[C@@H]1O[C@H]1[C@H](O)[C@@H](O[C@@H]2[C@@H]([C@@H](N)[C@H](O)[C@@H](CO)O2)O)[C@H](N)C[C@@H]1N SBUJHOSQTJFQJX-NOAMYHISSA-N 0.000 description 1
- 229930182823 kanamycin A Natural products 0.000 description 1
- 229960003029 ketobemidone Drugs 0.000 description 1
- 229960004752 ketorolac Drugs 0.000 description 1
- OZWKMVRBQXNZKK-UHFFFAOYSA-N ketorolac Chemical compound OC(=O)C1CCN2C1=CC=C2C(=O)C1=CC=CC=C1 OZWKMVRBQXNZKK-UHFFFAOYSA-N 0.000 description 1
- 108010045069 keyhole-limpet hemocyanin Proteins 0.000 description 1
- 210000003734 kidney Anatomy 0.000 description 1
- 229960001021 lactose monohydrate Drugs 0.000 description 1
- RYZCWZZJFAKYHX-LLVKDONJSA-N lanperisone Chemical compound C([C@@H](C)C(=O)C=1C=CC(=CC=1)C(F)(F)F)N1CCCC1 RYZCWZZJFAKYHX-LLVKDONJSA-N 0.000 description 1
- 229950004624 lanperisone Drugs 0.000 description 1
- 150000002605 large molecules Chemical class 0.000 description 1
- GFIJNRVAKGFPGQ-LIJARHBVSA-N leuprolide Chemical compound CCNC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@H](CC=1N=CNC=1)NC(=O)[C@H]1NC(=O)CC1)CC1=CC=C(O)C=C1 GFIJNRVAKGFPGQ-LIJARHBVSA-N 0.000 description 1
- 229960004338 leuprorelin Drugs 0.000 description 1
- 229960004288 levobupivacaine Drugs 0.000 description 1
- LEBVLXFERQHONN-INIZCTEOSA-N levobupivacaine Chemical compound CCCCN1CCCC[C@H]1C(=O)NC1=C(C)C=CC=C1C LEBVLXFERQHONN-INIZCTEOSA-N 0.000 description 1
- RCYBMSQOSGJZLO-BGWNEDDSSA-N levophenacylmorphan Chemical compound C([C@]12CCCC[C@H]1[C@H]1CC3=CC=C(C=C32)O)CN1CC(=O)C1=CC=CC=C1 RCYBMSQOSGJZLO-BGWNEDDSSA-N 0.000 description 1
- 229950007939 levophenacylmorphan Drugs 0.000 description 1
- 229960003406 levorphanol Drugs 0.000 description 1
- 229960004488 linolenic acid Drugs 0.000 description 1
- 239000003589 local anesthetic agent Substances 0.000 description 1
- 229950010274 lofentanil Drugs 0.000 description 1
- IMYHGORQCPYVBZ-NLFFAJNJSA-N lofentanil Chemical compound CCC(=O)N([C@@]1([C@@H](CN(CCC=2C=CC=CC=2)CC1)C)C(=O)OC)C1=CC=CC=C1 IMYHGORQCPYVBZ-NLFFAJNJSA-N 0.000 description 1
- 238000004519 manufacturing process Methods 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- 229920002529 medical grade silicone Polymers 0.000 description 1
- RQZAXGRLVPAYTJ-GQFGMJRRSA-N megestrol acetate Chemical compound C1=C(C)C2=CC(=O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@@](C(C)=O)(OC(=O)C)[C@@]1(C)CC2 RQZAXGRLVPAYTJ-GQFGMJRRSA-N 0.000 description 1
- 229960004296 megestrol acetate Drugs 0.000 description 1
- 229960003194 meglumine Drugs 0.000 description 1
- 229960003869 mepenzolate bromide Drugs 0.000 description 1
- 229960002409 mepivacaine Drugs 0.000 description 1
- INWLQCZOYSRPNW-UHFFFAOYSA-N mepivacaine Chemical compound CN1CCCCC1C(=O)NC1=C(C)C=CC=C1C INWLQCZOYSRPNW-UHFFFAOYSA-N 0.000 description 1
- 229950007594 meprylcaine Drugs 0.000 description 1
- 229960000365 meptazinol Drugs 0.000 description 1
- JLICHNCFTLFZJN-HNNXBMFYSA-N meptazinol Chemical compound C=1C=CC(O)=CC=1[C@@]1(CC)CCCCN(C)C1 JLICHNCFTLFZJN-HNNXBMFYSA-N 0.000 description 1
- 239000002207 metabolite Substances 0.000 description 1
- 229950004316 metabutoxycaine Drugs 0.000 description 1
- LJQWYEFHNLTPBZ-UHFFFAOYSA-N metabutoxycaine Chemical compound CCCCOC1=C(N)C=CC=C1C(=O)OCCN(CC)CC LJQWYEFHNLTPBZ-UHFFFAOYSA-N 0.000 description 1
- 229950009131 metazocine Drugs 0.000 description 1
- YGSVZRIZCHZUHB-COLVAYQJSA-N metazocine Chemical compound C1C2=CC=C(O)C=C2[C@]2(C)CCN(C)[C@@]1([H])[C@@H]2C YGSVZRIZCHZUHB-COLVAYQJSA-N 0.000 description 1
- 229960001797 methadone Drugs 0.000 description 1
- 229960000485 methotrexate Drugs 0.000 description 1
- LZCOQTDXKCNBEE-IKIFYQGPSA-N methscopolamine Chemical compound C1([C@@H](CO)C(=O)O[C@H]2C[C@@H]3[N+]([C@H](C2)[C@@H]2[C@H]3O2)(C)C)=CC=CC=C1 LZCOQTDXKCNBEE-IKIFYQGPSA-N 0.000 description 1
- 229960001383 methylscopolamine Drugs 0.000 description 1
- NPZXCTIHHUUEEJ-CMKMFDCUSA-N metopon Chemical compound O([C@@]1(C)C(=O)CC[C@@H]23)C4=C5[C@@]13CCN(C)[C@@H]2CC5=CC=C4O NPZXCTIHHUUEEJ-CMKMFDCUSA-N 0.000 description 1
- 229950006080 metopon Drugs 0.000 description 1
- 229960000282 metronidazole Drugs 0.000 description 1
- VAOCPAMSLUNLGC-UHFFFAOYSA-N metronidazole Chemical compound CC1=NC=C([N+]([O-])=O)N1CCO VAOCPAMSLUNLGC-UHFFFAOYSA-N 0.000 description 1
- 230000036453 micturition reflex Effects 0.000 description 1
- 229960001156 mitoxantrone Drugs 0.000 description 1
- KKZJGLLVHKMTCM-UHFFFAOYSA-N mitoxantrone Chemical compound O=C1C2=C(O)C=CC(O)=C2C(=O)C2=C1C(NCCNCCO)=CC=C2NCCNCCO KKZJGLLVHKMTCM-UHFFFAOYSA-N 0.000 description 1
- 229960005181 morphine Drugs 0.000 description 1
- 239000000472 muscarinic agonist Substances 0.000 description 1
- 210000003205 muscle Anatomy 0.000 description 1
- 229940035363 muscle relaxants Drugs 0.000 description 1
- 230000036640 muscle relaxation Effects 0.000 description 1
- 239000003158 myorelaxant agent Substances 0.000 description 1
- GODGZZGKTZQSAL-VXFFQEMOSA-N myrophine Chemical compound C([C@@H]1[C@@H]2C=C[C@@H]([C@@H]3OC4=C5[C@]23CCN1C)OC(=O)CCCCCCCCCCCCC)C5=CC=C4OCC1=CC=CC=C1 GODGZZGKTZQSAL-VXFFQEMOSA-N 0.000 description 1
- 229950007471 myrophine Drugs 0.000 description 1
- 229960000515 nafcillin Drugs 0.000 description 1
- GPXLMGHLHQJAGZ-JTDSTZFVSA-N nafcillin Chemical compound C1=CC=CC2=C(C(=O)N[C@@H]3C(N4[C@H](C(C)(C)S[C@@H]43)C(O)=O)=O)C(OCC)=CC=C21 GPXLMGHLHQJAGZ-JTDSTZFVSA-N 0.000 description 1
- 229960000938 nalorphine Drugs 0.000 description 1
- 229960002009 naproxen Drugs 0.000 description 1
- CMWTZPSULFXXJA-VIFPVBQESA-N naproxen Chemical compound C1=C([C@H](C)C(O)=O)C=CC2=CC(OC)=CC=C21 CMWTZPSULFXXJA-VIFPVBQESA-N 0.000 description 1
- 210000005036 nerve Anatomy 0.000 description 1
- 229940053128 nerve growth factor Drugs 0.000 description 1
- 210000000653 nervous system Anatomy 0.000 description 1
- 230000002981 neuropathic effect Effects 0.000 description 1
- 239000002547 new drug Substances 0.000 description 1
- 229960004300 nicomorphine Drugs 0.000 description 1
- HNDXBGYRMHRUFN-CIVUWBIHSA-N nicomorphine Chemical compound O([C@H]1C=C[C@H]2[C@H]3CC=4C5=C(C(=CC=4)OC(=O)C=4C=NC=CC=4)O[C@@H]1[C@]52CCN3C)C(=O)C1=CC=CN=C1 HNDXBGYRMHRUFN-CIVUWBIHSA-N 0.000 description 1
- NXFQHRVNIOXGAQ-YCRREMRBSA-N nitrofurantoin Chemical compound O1C([N+](=O)[O-])=CC=C1\C=N\N1C(=O)NC(=O)C1 NXFQHRVNIOXGAQ-YCRREMRBSA-N 0.000 description 1
- 229960000564 nitrofurantoin Drugs 0.000 description 1
- 230000003040 nociceptive effect Effects 0.000 description 1
- 239000000041 non-steroidal anti-inflammatory agent Substances 0.000 description 1
- 229940021182 non-steroidal anti-inflammatory drug Drugs 0.000 description 1
- 239000002767 noradrenalin uptake inhibitor Substances 0.000 description 1
- OGJPXUAPXNRGGI-UHFFFAOYSA-N norfloxacin Chemical compound C1=C2N(CC)C=C(C(O)=O)C(=O)C2=CC(F)=C1N1CCNCC1 OGJPXUAPXNRGGI-UHFFFAOYSA-N 0.000 description 1
- 229960001180 norfloxacin Drugs 0.000 description 1
- 229950011519 norlevorphanol Drugs 0.000 description 1
- WCJFBSYALHQBSK-UHFFFAOYSA-N normethadone Chemical compound C=1C=CC=CC=1C(CCN(C)C)(C(=O)CC)C1=CC=CC=C1 WCJFBSYALHQBSK-UHFFFAOYSA-N 0.000 description 1
- 229960004013 normethadone Drugs 0.000 description 1
- 229950006134 normorphine Drugs 0.000 description 1
- WCDSHELZWCOTMI-UHFFFAOYSA-N norpipanone Chemical compound C=1C=CC=CC=1C(C=1C=CC=CC=1)(C(=O)CC)CCN1CCCCC1 WCDSHELZWCOTMI-UHFFFAOYSA-N 0.000 description 1
- 229950007418 norpipanone Drugs 0.000 description 1
- QIQXTHQIDYTFRH-UHFFFAOYSA-N octadecanoic acid Chemical compound CCCCCCCCCCCCCCCCCC(O)=O QIQXTHQIDYTFRH-UHFFFAOYSA-N 0.000 description 1
- 229960001699 ofloxacin Drugs 0.000 description 1
- 229940124636 opioid drug Drugs 0.000 description 1
- 229960001027 opium Drugs 0.000 description 1
- 229950006098 orthocaine Drugs 0.000 description 1
- 230000001151 other effect Effects 0.000 description 1
- 210000001672 ovary Anatomy 0.000 description 1
- 208000020629 overactive bladder Diseases 0.000 description 1
- 229960002085 oxycodone Drugs 0.000 description 1
- 229960005118 oxymorphone Drugs 0.000 description 1
- 229960002369 oxyphencyclimine Drugs 0.000 description 1
- LSQZJLSUYDQPKJ-UHFFFAOYSA-N p-Hydroxyampicillin Natural products O=C1N2C(C(O)=O)C(C)(C)SC2C1NC(=O)C(N)C1=CC=C(O)C=C1 LSQZJLSUYDQPKJ-UHFFFAOYSA-N 0.000 description 1
- 229960001592 paclitaxel Drugs 0.000 description 1
- 229960005489 paracetamol Drugs 0.000 description 1
- 229940055076 parasympathomimetics choline ester Drugs 0.000 description 1
- 150000002960 penicillins Chemical class 0.000 description 1
- 210000003899 penis Anatomy 0.000 description 1
- NQPDZGIKBAWPEJ-UHFFFAOYSA-N pentanoic acid group Chemical group C(CCCC)(=O)O NQPDZGIKBAWPEJ-UHFFFAOYSA-N 0.000 description 1
- VOKSWYLNZZRQPF-GDIGMMSISA-N pentazocine Chemical compound C1C2=CC=C(O)C=C2[C@@]2(C)[C@@H](C)[C@@H]1N(CC=C(C)C)CC2 VOKSWYLNZZRQPF-GDIGMMSISA-N 0.000 description 1
- 229960005301 pentazocine Drugs 0.000 description 1
- 230000000737 periodic effect Effects 0.000 description 1
- 229960000482 pethidine Drugs 0.000 description 1
- LOXCOAXRHYDLOW-UHFFFAOYSA-N phenadoxone Chemical compound C=1C=CC=CC=1C(C=1C=CC=CC=1)(C(=O)CC)CC(C)N1CCOCC1 LOXCOAXRHYDLOW-UHFFFAOYSA-N 0.000 description 1
- 229950004540 phenadoxone Drugs 0.000 description 1
- ZQHYKVKNPWDQSL-KNXBSLHKSA-N phenazocine Chemical compound C([C@@]1(C)C2=CC(O)=CC=C2C[C@@H]2[C@@H]1C)CN2CCC1=CC=CC=C1 ZQHYKVKNPWDQSL-KNXBSLHKSA-N 0.000 description 1
- 229960000897 phenazocine Drugs 0.000 description 1
- 229960003799 phenazopyridine hydrochloride Drugs 0.000 description 1
- CFBQYWXPZVQQTN-QPTUXGOLSA-N phenomorphan Chemical compound C([C@]12CCCC[C@H]1[C@H]1CC3=CC=C(C=C32)O)CN1CCC1=CC=CC=C1 CFBQYWXPZVQQTN-QPTUXGOLSA-N 0.000 description 1
- 229950011496 phenomorphan Drugs 0.000 description 1
- IPOPQVVNCFQFRK-UHFFFAOYSA-N phenoperidine Chemical compound C1CC(C(=O)OCC)(C=2C=CC=CC=2)CCN1CCC(O)C1=CC=CC=C1 IPOPQVVNCFQFRK-UHFFFAOYSA-N 0.000 description 1
- 229960004315 phenoperidine Drugs 0.000 description 1
- 229940043441 phosphoinositide 3-kinase inhibitor Drugs 0.000 description 1
- PXXKIYPSXYFATG-UHFFFAOYSA-N piminodine Chemical compound C1CC(C(=O)OCC)(C=2C=CC=CC=2)CCN1CCCNC1=CC=CC=C1 PXXKIYPSXYFATG-UHFFFAOYSA-N 0.000 description 1
- 229950006445 piminodine Drugs 0.000 description 1
- 229960001045 piperocaine Drugs 0.000 description 1
- 239000002985 plastic film Substances 0.000 description 1
- 229920006255 plastic film Polymers 0.000 description 1
- 229920001155 polypropylene Polymers 0.000 description 1
- 235000012434 pretzels Nutrition 0.000 description 1
- 230000002265 prevention Effects 0.000 description 1
- 125000002924 primary amino group Chemical class [H]N([H])* 0.000 description 1
- 229960004919 procaine Drugs 0.000 description 1
- MFDFERRIHVXMIY-UHFFFAOYSA-N procaine Chemical compound CCN(CC)CCOC(=O)C1=CC=C(N)C=C1 MFDFERRIHVXMIY-UHFFFAOYSA-N 0.000 description 1
- 239000000583 progesterone congener Substances 0.000 description 1
- 229950003779 propiram Drugs 0.000 description 1
- ZBAFFZBKCMWUHM-UHFFFAOYSA-N propiram Chemical compound C=1C=CC=NC=1N(C(=O)CC)C(C)CN1CCCCC1 ZBAFFZBKCMWUHM-UHFFFAOYSA-N 0.000 description 1
- 229950003255 propoxycaine Drugs 0.000 description 1
- 210000002307 prostate Anatomy 0.000 description 1
- 201000007094 prostatitis Diseases 0.000 description 1
- 239000000018 receptor agonist Substances 0.000 description 1
- 229940044601 receptor agonist Drugs 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 230000001105 regulatory effect Effects 0.000 description 1
- 210000004994 reproductive system Anatomy 0.000 description 1
- DSDNAKHZNJAGHN-UHFFFAOYSA-N resinferatoxin Natural products C1=C(O)C(OC)=CC(CC(=O)OCC=2CC3(O)C(=O)C(C)=CC3C34C(C)CC5(OC(O4)(CC=4C=CC=CC=4)OC5C3C=2)C(C)=C)=C1 DSDNAKHZNJAGHN-UHFFFAOYSA-N 0.000 description 1
- DSDNAKHZNJAGHN-MXTYGGKSSA-N resiniferatoxin Chemical compound C1=C(O)C(OC)=CC(CC(=O)OCC=2C[C@]3(O)C(=O)C(C)=C[C@H]3[C@@]34[C@H](C)C[C@@]5(O[C@@](O4)(CC=4C=CC=CC=4)O[C@@H]5[C@@H]3C=2)C(C)=C)=C1 DSDNAKHZNJAGHN-MXTYGGKSSA-N 0.000 description 1
- 229940073454 resiniferatoxin Drugs 0.000 description 1
- 229950003447 risocaine Drugs 0.000 description 1
- XPYLKZZOBVLVHB-QDKIRNHSSA-N rociverine Chemical compound CCN(CC)CC(C)OC(=O)[C@H]1CCCC[C@]1(O)C1CCCCC1 XPYLKZZOBVLVHB-QDKIRNHSSA-N 0.000 description 1
- 229960001538 rociverine Drugs 0.000 description 1
- 229960001549 ropivacaine Drugs 0.000 description 1
- CQRYARSYNCAZFO-UHFFFAOYSA-N salicyl alcohol Chemical compound OCC1=CC=CC=C1O CQRYARSYNCAZFO-UHFFFAOYSA-N 0.000 description 1
- 229920006395 saturated elastomer Polymers 0.000 description 1
- 229960002646 scopolamine Drugs 0.000 description 1
- STECJAGHUSJQJN-FWXGHANASA-N scopolamine Chemical compound C1([C@@H](CO)C(=O)O[C@H]2C[C@@H]3N([C@H](C2)[C@@H]2[C@H]3O2)C)=CC=CC=C1 STECJAGHUSJQJN-FWXGHANASA-N 0.000 description 1
- 210000001625 seminal vesicle Anatomy 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 239000012781 shape memory material Substances 0.000 description 1
- 229920000431 shape-memory polymer Polymers 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 210000005070 sphincter Anatomy 0.000 description 1
- 210000000278 spinal cord Anatomy 0.000 description 1
- 229950002227 stilonium iodide Drugs 0.000 description 1
- 229960004739 sufentanil Drugs 0.000 description 1
- GGCSSNBKKAUURC-UHFFFAOYSA-N sufentanil Chemical compound C1CN(CCC=2SC=CC=2)CCC1(COC)N(C(=O)CC)C1=CC=CC=C1 GGCSSNBKKAUURC-UHFFFAOYSA-N 0.000 description 1
- 229940124530 sulfonamide Drugs 0.000 description 1
- 150000003456 sulfonamides Chemical class 0.000 description 1
- 229960003491 sulodexide Drugs 0.000 description 1
- 239000000725 suspension Substances 0.000 description 1
- 208000024891 symptom Diseases 0.000 description 1
- 208000011580 syndromic disease Diseases 0.000 description 1
- 229950008160 tanezumab Drugs 0.000 description 1
- RCINICONZNJXQF-MZXODVADSA-N taxol Chemical compound O([C@@H]1[C@@]2(C[C@@H](C(C)=C(C2(C)C)[C@H](C([C@]2(C)[C@@H](O)C[C@H]3OC[C@]3([C@H]21)OC(C)=O)=O)OC(=O)C)OC(=O)[C@H](O)[C@@H](NC(=O)C=1C=CC=CC=1)C=1C=CC=CC=1)O)C(=O)C1=CC=CC=C1 RCINICONZNJXQF-MZXODVADSA-N 0.000 description 1
- 210000001550 testis Anatomy 0.000 description 1
- 229960002372 tetracaine Drugs 0.000 description 1
- GKCBAIGFKIBETG-UHFFFAOYSA-N tetracaine Chemical compound CCCCNC1=CC=C(C(=O)OCCN(C)C)C=C1 GKCBAIGFKIBETG-UHFFFAOYSA-N 0.000 description 1
- 229940124597 therapeutic agent Drugs 0.000 description 1
- 229960001402 tilidine Drugs 0.000 description 1
- 229940110309 tiotropium Drugs 0.000 description 1
- LERNTVKEWCAPOY-DZZGSBJMSA-N tiotropium Chemical compound O([C@H]1C[C@@H]2[N+]([C@H](C1)[C@@H]1[C@H]2O1)(C)C)C(=O)C(O)(C=1SC=CC=1)C1=CC=CS1 LERNTVKEWCAPOY-DZZGSBJMSA-N 0.000 description 1
- 229910052719 titanium Inorganic materials 0.000 description 1
- 239000010936 titanium Substances 0.000 description 1
- 229940044616 toll-like receptor 7 agonist Drugs 0.000 description 1
- 238000011200 topical administration Methods 0.000 description 1
- 229960004380 tramadol Drugs 0.000 description 1
- TVYLLZQTGLZFBW-GOEBONIOSA-N tramadol Natural products COC1=CC=CC([C@@]2(O)[C@@H](CCCC2)CN(C)C)=C1 TVYLLZQTGLZFBW-GOEBONIOSA-N 0.000 description 1
- LLPOLZWFYMWNKH-UHFFFAOYSA-N trans-dihydrocodeinone Natural products C1C(N(CCC234)C)C2CCC(=O)C3OC2=C4C1=CC=C2OC LLPOLZWFYMWNKH-UHFFFAOYSA-N 0.000 description 1
- LOIYMIARKYCTBW-OWOJBTEDSA-N trans-urocanic acid Chemical compound OC(=O)\C=C\C1=CNC=N1 LOIYMIARKYCTBW-OWOJBTEDSA-N 0.000 description 1
- LOIYMIARKYCTBW-UHFFFAOYSA-N trans-urocanic acid Natural products OC(=O)C=CC1=CNC=N1 LOIYMIARKYCTBW-UHFFFAOYSA-N 0.000 description 1
- 230000008733 trauma Effects 0.000 description 1
- 239000003029 tricyclic antidepressant agent Substances 0.000 description 1
- RDTKUZXIHMTSJO-UEIGIMKUSA-M triethyl-[2-[4-[(e)-2-phenylethenyl]phenoxy]ethyl]azanium;iodide Chemical compound [I-].C1=CC(OCC[N+](CC)(CC)CC)=CC=C1\C=C\C1=CC=CC=C1 RDTKUZXIHMTSJO-UEIGIMKUSA-M 0.000 description 1
- 229950002569 trimecaine Drugs 0.000 description 1
- GOZBHBFUQHMKQB-UHFFFAOYSA-N trimecaine Chemical compound CCN(CC)CC(=O)NC1=C(C)C=C(C)C=C1C GOZBHBFUQHMKQB-UHFFFAOYSA-N 0.000 description 1
- IEDVJHCEMCRBQM-UHFFFAOYSA-N trimethoprim Chemical compound COC1=C(OC)C(OC)=CC(CC=2C(=NC(N)=NC=2)N)=C1 IEDVJHCEMCRBQM-UHFFFAOYSA-N 0.000 description 1
- 229960001082 trimethoprim Drugs 0.000 description 1
- 229930004668 tropane alkaloid Natural products 0.000 description 1
- 229940046728 tumor necrosis factor alpha inhibitor Drugs 0.000 description 1
- 239000002451 tumor necrosis factor inhibitor Substances 0.000 description 1
- 230000036269 ulceration Effects 0.000 description 1
- 241000701161 unidentified adenovirus Species 0.000 description 1
- 208000000143 urethritis Diseases 0.000 description 1
- 210000003932 urinary bladder Anatomy 0.000 description 1
- 208000022934 urinary frequency Diseases 0.000 description 1
- 230000036318 urination frequency Effects 0.000 description 1
- 210000001215 vagina Anatomy 0.000 description 1
- 229950008617 vamicamide Drugs 0.000 description 1
- 210000005166 vasculature Anatomy 0.000 description 1
- 201000010653 vesiculitis Diseases 0.000 description 1
- 229960003048 vinblastine Drugs 0.000 description 1
- JXLYSJRDGCGARV-XQKSVPLYSA-N vincaleukoblastine Chemical compound C([C@@H](C[C@]1(C(=O)OC)C=2C(=CC3=C([C@]45[C@H]([C@@]([C@H](OC(C)=O)[C@]6(CC)C=CCN([C@H]56)CC4)(O)C(=O)OC)N3C)C=2)OC)C[C@@](C2)(O)CC)N2CCC2=C1NC1=CC=CC=C21 JXLYSJRDGCGARV-XQKSVPLYSA-N 0.000 description 1
- 230000000007 visual effect Effects 0.000 description 1
- 239000011800 void material Substances 0.000 description 1
- 229940075420 xanthine Drugs 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M31/00—Devices for introducing or retaining media, e.g. remedies, in cavities of the body
- A61M31/002—Devices for releasing a drug at a continuous and controlled rate for a prolonged period of time
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7042—Compounds having saccharide radicals and heterocyclic rings
- A61K31/7052—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides
- A61K31/706—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom
- A61K31/7064—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom containing condensed or non-condensed pyrimidines
- A61K31/7068—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom containing condensed or non-condensed pyrimidines having oxo groups directly attached to the pyrimidine ring, e.g. cytidine, cytidylic acid
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0002—Galenical forms characterised by the drug release technique; Application systems commanded by energy
- A61K9/0004—Osmotic delivery systems; Sustained release driven by osmosis, thermal energy or gas
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0034—Urogenital system, e.g. vagina, uterus, cervix, penis, scrotum, urethra, bladder; Personal lubricants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/2004—Excipients; Inactive ingredients
- A61K9/2009—Inorganic compounds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/2004—Excipients; Inactive ingredients
- A61K9/2013—Organic compounds, e.g. phospholipids, fats
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/2004—Excipients; Inactive ingredients
- A61K9/2013—Organic compounds, e.g. phospholipids, fats
- A61K9/2018—Sugars, or sugar alcohols, e.g. lactose, mannitol; Derivatives thereof, e.g. polysorbates
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L31/00—Materials for other surgical articles, e.g. stents, stent-grafts, shunts, surgical drapes, guide wires, materials for adhesion prevention, occluding devices, surgical gloves, tissue fixation devices
- A61L31/04—Macromolecular materials
- A61L31/06—Macromolecular materials obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L31/00—Materials for other surgical articles, e.g. stents, stent-grafts, shunts, surgical drapes, guide wires, materials for adhesion prevention, occluding devices, surgical gloves, tissue fixation devices
- A61L31/08—Materials for coatings
- A61L31/10—Macromolecular materials
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L31/00—Materials for other surgical articles, e.g. stents, stent-grafts, shunts, surgical drapes, guide wires, materials for adhesion prevention, occluding devices, surgical gloves, tissue fixation devices
- A61L31/14—Materials characterised by their function or physical properties, e.g. injectable or lubricating compositions, shape-memory materials, surface modified materials
- A61L31/16—Biologically active materials, e.g. therapeutic substances
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P13/00—Drugs for disorders of the urinary system
- A61P13/10—Drugs for disorders of the urinary system of the bladder
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P23/00—Anaesthetics
- A61P23/02—Local anaesthetics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61J—CONTAINERS SPECIALLY ADAPTED FOR MEDICAL OR PHARMACEUTICAL PURPOSES; DEVICES OR METHODS SPECIALLY ADAPTED FOR BRINGING PHARMACEUTICAL PRODUCTS INTO PARTICULAR PHYSICAL OR ADMINISTERING FORMS; DEVICES FOR ADMINISTERING FOOD OR MEDICINES ORALLY; BABY COMFORTERS; DEVICES FOR RECEIVING SPITTLE
- A61J1/00—Containers specially adapted for medical or pharmaceutical purposes
- A61J1/14—Details; Accessories therefor
- A61J1/20—Arrangements for transferring or mixing fluids, e.g. from vial to syringe
- A61J1/2096—Combination of a vial and a syringe for transferring or mixing their contents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2300/00—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices
- A61L2300/20—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices containing or releasing organic materials
- A61L2300/23—Carbohydrates
- A61L2300/232—Monosaccharides, disaccharides, polysaccharides, lipopolysaccharides
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2300/00—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices
- A61L2300/40—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices characterised by a specific therapeutic activity or mode of action
- A61L2300/402—Anaestetics, analgesics, e.g. lidocaine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2300/00—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices
- A61L2300/40—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices characterised by a specific therapeutic activity or mode of action
- A61L2300/416—Anti-neoplastic or anti-proliferative or anti-restenosis or anti-angiogenic agents, e.g. paclitaxel, sirolimus
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L2300/00—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices
- A61L2300/40—Biologically active materials used in bandages, wound dressings, absorbent pads or medical devices characterised by a specific therapeutic activity or mode of action
- A61L2300/432—Inhibitors, antagonists
- A61L2300/436—Inhibitors, antagonists of receptors
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2205/00—General characteristics of the apparatus
- A61M2205/04—General characteristics of the apparatus implanted
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2209/00—Ancillary equipment
- A61M2209/04—Tools for specific apparatus
- A61M2209/045—Tools for specific apparatus for filling, e.g. for filling reservoirs
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Epidemiology (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Heart & Thoracic Surgery (AREA)
- Molecular Biology (AREA)
- Anesthesiology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Biomedical Technology (AREA)
- Urology & Nephrology (AREA)
- Organic Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Surgery (AREA)
- Vascular Medicine (AREA)
- Hematology (AREA)
- Reproductive Health (AREA)
- Gynecology & Obstetrics (AREA)
- Biophysics (AREA)
- Inorganic Chemistry (AREA)
- Medicinal Preparation (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Infusion, Injection, And Reservoir Apparatuses (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
- Materials For Medical Uses (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201361899982P | 2013-11-05 | 2013-11-05 | |
| US61/899,982 | 2013-11-05 | ||
| PCT/US2014/064063 WO2015069723A1 (en) | 2013-11-05 | 2014-11-05 | Osmotic drug delivery devices, kits, and methods |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20160083061A true KR20160083061A (ko) | 2016-07-11 |
Family
ID=51946057
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020167014800A Withdrawn KR20160083061A (ko) | 2013-11-05 | 2014-11-05 | 삼투 약물 전달 디바이스들, 키트, 및 방법들 |
Country Status (10)
| Country | Link |
|---|---|
| US (1) | US20160279399A1 (enExample) |
| EP (1) | EP3065807A1 (enExample) |
| JP (1) | JP2017500171A (enExample) |
| KR (1) | KR20160083061A (enExample) |
| CN (1) | CN105792880A (enExample) |
| BR (1) | BR112016010103A8 (enExample) |
| CA (1) | CA2929554A1 (enExample) |
| IL (1) | IL245445A0 (enExample) |
| RU (1) | RU2016120199A (enExample) |
| WO (1) | WO2015069723A1 (enExample) |
Families Citing this family (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2016508842A (ja) | 2013-03-05 | 2016-03-24 | タリス バイオメディカル エルエルシー | 装置の開口部を通じて制御された薬物放出のための薬物送達装置及び方法 |
| HRP20251264T1 (hr) * | 2014-03-06 | 2025-12-05 | Taris Biomedical Llc | Sustavi za primjenu lijeka i metode za liječenje karcinoma mokraćnog mjehura gemcitabinom |
| JP6743041B2 (ja) | 2015-03-30 | 2020-08-19 | タリス バイオメディカル エルエルシー | 上部尿路への薬剤の局所的な送達装置及び方法 |
| US9867974B2 (en) * | 2015-06-01 | 2018-01-16 | Wisconsin Alumni Research Foundation | Microfluidic device for multiplexed point source administration of compounds |
| LT3432970T (lt) | 2017-02-01 | 2021-05-10 | Taris Biomedical Llc | In vivo vaistų vartojimo įtaisai |
| WO2019023388A1 (en) | 2017-07-25 | 2019-01-31 | Taris Biomedical Llc | METHODS OF TREATING TUMOR METASTASIS |
| CN111278476B (zh) | 2017-09-22 | 2023-01-17 | 贝克顿·迪金森公司 | 用作导管封管液的4%柠檬酸三钠溶液 |
| PE20210041A1 (es) | 2017-11-08 | 2021-01-08 | Taris Biomedical Llc | Metodos de tratamiento y terapia de mantenimiento para el cancer de vejiga con gemcitabina |
| CA3107461A1 (en) | 2018-08-01 | 2020-02-06 | Taris Biomedical Llc | Methods of treating overactive bladder using trospium |
| CN109589491B (zh) * | 2019-01-29 | 2024-01-09 | 上海安翰医疗技术有限公司 | 自动给药装置 |
| EP3952973B1 (en) | 2019-06-13 | 2023-06-07 | Hollister Incorporated | Reusable urinary catheter products |
| AU2020304005B2 (en) | 2019-06-25 | 2025-12-04 | Hollister Incorporated | Reusable urinary catheter products |
| EP4221657A4 (en) * | 2020-09-30 | 2024-10-09 | Modibodi Australia Pty Ltd | TEXTILE FRAME |
| WO2025147450A1 (en) * | 2024-01-02 | 2025-07-10 | Manta Pharma Llc | Implantable device for adjustable dose using pressure sensor for piston displacement monitoring |
Family Cites Families (21)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB2155889B (en) * | 1984-03-21 | 1988-03-02 | Alza Corp | Dispenser-capsule |
| MY125870A (en) * | 1997-07-25 | 2006-08-30 | Alza Corp | Osmotic delivery system flow modulator apparatus and method |
| US6436091B1 (en) * | 1999-11-16 | 2002-08-20 | Microsolutions, Inc. | Methods and implantable devices and systems for long term delivery of a pharmaceutical agent |
| US8133501B2 (en) * | 2002-02-08 | 2012-03-13 | Boston Scientific Scimed, Inc. | Implantable or insertable medical devices for controlled drug delivery |
| EP1613389B8 (en) * | 2003-03-31 | 2008-05-07 | Intarcia Therapeutics, Inc. | Osmotic pump with means for dissipating internal pressure |
| US8257963B2 (en) * | 2007-06-01 | 2012-09-04 | Depuy Mitek, Inc. | Chondrocyte container and method of use |
| DK1933810T3 (da) | 2005-08-11 | 2013-01-28 | Childrens Medical Center | Intravesikal lægemiddelafgivelsesindretning og fremgangsmåde |
| US20100003297A1 (en) * | 2005-08-11 | 2010-01-07 | Massachusetts Institute Of Technology | Implantable Drug Delivery Device and Methods of Treating Male Genitourinary and Surrounding Tissues |
| TW200927141A (en) * | 2007-11-22 | 2009-07-01 | Bayer Schering Pharma Oy | Vaginal delivery system |
| DK2231254T3 (da) | 2007-12-11 | 2014-07-21 | Massachusetts Inst Technology | Implanterbar lægemiddelafgivelsesindretning |
| US8343516B2 (en) * | 2009-06-26 | 2013-01-01 | Taris Biomedical, Inc. | Solid drug tablets for implantable drug delivery devices |
| US9017312B2 (en) | 2009-09-10 | 2015-04-28 | Taris Biomedical Llc | Implantable device for controlled drug delivery |
| US8721621B2 (en) | 2009-09-10 | 2014-05-13 | Taris Biomedical, Inc. | Systems and methods for deploying devices to genitourinary sites |
| US8679094B2 (en) | 2009-12-17 | 2014-03-25 | Taris Biomedical, Inc. | Implantable device with intravesical tolerability and methods of treatment |
| CA2795837A1 (en) * | 2010-03-09 | 2011-09-15 | Woonsup Shin | Electro-osmotic pumps, systems, methods, and compositions |
| PT2600800T (pt) | 2010-08-05 | 2020-11-05 | Taris Biomedical Llc | Dispositivo de administração de drogas por stent ureteral, kit e método |
| US8690840B2 (en) | 2010-10-06 | 2014-04-08 | Taris Biomedical, Inc. | Time-selective bioresorbable or collapsible drug delivery systems and methods |
| US9457176B2 (en) | 2010-10-06 | 2016-10-04 | Taris Biomedical Llc | Implantable drug delivery device with bladder retention feature |
| US9114111B2 (en) | 2011-01-10 | 2015-08-25 | Allergan, Inc. | Methods for sustained treatment of bladder pain and irritative voiding |
| TWI615155B (zh) * | 2011-11-01 | 2018-02-21 | 拜耳股份有限公司 | 滲透活性的陰道遞送系統 |
| SG11201507294WA (en) | 2013-03-15 | 2015-10-29 | Taris Biomedical Llc | Drug delivery devices with drug-permeable component and methods |
-
2014
- 2014-11-05 KR KR1020167014800A patent/KR20160083061A/ko not_active Withdrawn
- 2014-11-05 WO PCT/US2014/064063 patent/WO2015069723A1/en not_active Ceased
- 2014-11-05 JP JP2016552464A patent/JP2017500171A/ja active Pending
- 2014-11-05 US US15/033,692 patent/US20160279399A1/en not_active Abandoned
- 2014-11-05 CN CN201480063520.5A patent/CN105792880A/zh active Pending
- 2014-11-05 EP EP14802296.5A patent/EP3065807A1/en not_active Withdrawn
- 2014-11-05 RU RU2016120199A patent/RU2016120199A/ru not_active Application Discontinuation
- 2014-11-05 CA CA2929554A patent/CA2929554A1/en not_active Abandoned
- 2014-11-05 BR BR112016010103A patent/BR112016010103A8/pt not_active IP Right Cessation
-
2016
- 2016-05-03 IL IL245445A patent/IL245445A0/en unknown
Also Published As
| Publication number | Publication date |
|---|---|
| CA2929554A1 (en) | 2015-05-14 |
| IL245445A0 (en) | 2016-06-30 |
| BR112016010103A8 (pt) | 2018-01-30 |
| US20160279399A1 (en) | 2016-09-29 |
| EP3065807A1 (en) | 2016-09-14 |
| WO2015069723A1 (en) | 2015-05-14 |
| RU2016120199A (ru) | 2017-12-11 |
| CN105792880A (zh) | 2016-07-20 |
| RU2016120199A3 (enExample) | 2018-06-29 |
| JP2017500171A (ja) | 2017-01-05 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP7122999B2 (ja) | 薬物透過性構成部材を備えた薬物送達デバイス、及び方法 | |
| US11135161B2 (en) | Intravesical device for controlled drug delivery | |
| RU2669407C2 (ru) | Устройства для доставки лекарственных средств и способы доставки лекарственных средств | |
| KR20160083061A (ko) | 삼투 약물 전달 디바이스들, 키트, 및 방법들 | |
| JP7425534B2 (ja) | 薬物透過性構成要素を有する薬物送達デバイス及び方法 | |
| US11992583B2 (en) | Drug delivery devices and methods | |
| US10137287B2 (en) | Drug delivery devices and methods for controlled drug release through device orifice | |
| EP3432970B1 (en) | In vivo drug delivery devices | |
| US20200345976A1 (en) | Drug Delivery Devices and Methods for Use with a Urinary Catheter | |
| HK40047951A (en) | Drug delivery devices |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20160602 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| PC1203 | Withdrawal of no request for examination | ||
| WITN | Application deemed withdrawn, e.g. because no request for examination was filed or no examination fee was paid |