KR20150077460A - 폐 가성 물질의 처리 및 매질의 재생을 위한 방법 및 시스템 - Google Patents
폐 가성 물질의 처리 및 매질의 재생을 위한 방법 및 시스템 Download PDFInfo
- Publication number
- KR20150077460A KR20150077460A KR1020157013245A KR20157013245A KR20150077460A KR 20150077460 A KR20150077460 A KR 20150077460A KR 1020157013245 A KR1020157013245 A KR 1020157013245A KR 20157013245 A KR20157013245 A KR 20157013245A KR 20150077460 A KR20150077460 A KR 20150077460A
- Authority
- KR
- South Korea
- Prior art keywords
- stream
- vessel
- inlet
- pulmonary
- medium
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
- 238000000034 method Methods 0.000 title claims abstract description 112
- 239000003518 caustics Substances 0.000 title claims abstract description 71
- 230000001172 regenerating effect Effects 0.000 title description 10
- 239000000463 material Substances 0.000 claims abstract description 179
- 230000008929 regeneration Effects 0.000 claims abstract description 173
- 238000011069 regeneration method Methods 0.000 claims abstract description 173
- 230000002685 pulmonary effect Effects 0.000 claims abstract description 138
- 238000011282 treatment Methods 0.000 claims abstract description 81
- 239000002253 acid Substances 0.000 claims abstract description 62
- 235000009496 Juglans regia Nutrition 0.000 claims abstract description 53
- 235000020234 walnut Nutrition 0.000 claims abstract description 53
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 claims abstract description 46
- 239000002699 waste material Substances 0.000 claims abstract description 31
- 230000008569 process Effects 0.000 claims abstract description 30
- 238000001179 sorption measurement Methods 0.000 claims abstract description 17
- 239000003463 adsorbent Substances 0.000 claims description 144
- 239000000126 substance Substances 0.000 claims description 60
- 241000758789 Juglans Species 0.000 claims description 52
- 238000002156 mixing Methods 0.000 claims description 38
- 238000000926 separation method Methods 0.000 claims description 30
- 239000013626 chemical specie Substances 0.000 claims description 23
- 239000002594 sorbent Substances 0.000 claims description 23
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N phenol group Chemical group C1(=CC=CC=C1)O ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 claims description 22
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 claims description 21
- 229910052760 oxygen Inorganic materials 0.000 claims description 21
- 239000001301 oxygen Substances 0.000 claims description 21
- 230000020477 pH reduction Effects 0.000 claims description 17
- 230000002829 reductive effect Effects 0.000 claims description 16
- 239000012530 fluid Substances 0.000 claims description 12
- 238000011144 upstream manufacturing Methods 0.000 claims description 12
- 150000002894 organic compounds Chemical class 0.000 claims description 8
- 238000004891 communication Methods 0.000 claims description 6
- 150000001875 compounds Chemical class 0.000 claims description 6
- 125000000853 cresyl group Chemical group C1(=CC=C(C=C1)C)* 0.000 claims description 4
- 238000011001 backwashing Methods 0.000 claims description 3
- 230000001737 promoting effect Effects 0.000 claims description 3
- 229920000642 polymer Polymers 0.000 abstract description 57
- 239000012071 phase Substances 0.000 abstract description 14
- 239000003208 petroleum Substances 0.000 abstract description 7
- 150000003568 thioethers Chemical class 0.000 abstract description 7
- RWSOTUBLDIXVET-UHFFFAOYSA-N Dihydrogen sulfide Chemical class S RWSOTUBLDIXVET-UHFFFAOYSA-N 0.000 abstract description 6
- CHRJZRDFSQHIFI-UHFFFAOYSA-N 1,2-bis(ethenyl)benzene;styrene Chemical compound C=CC1=CC=CC=C1.C=CC1=CC=CC=C1C=C CHRJZRDFSQHIFI-UHFFFAOYSA-N 0.000 abstract description 3
- 239000008346 aqueous phase Substances 0.000 abstract description 3
- 150000007513 acids Chemical class 0.000 abstract description 2
- 238000001914 filtration Methods 0.000 abstract description 2
- 210000004072 lung Anatomy 0.000 abstract description 2
- 240000007049 Juglans regia Species 0.000 abstract 1
- 230000000274 adsorptive effect Effects 0.000 abstract 1
- 125000005608 naphthenic acid group Chemical group 0.000 abstract 1
- 150000002989 phenols Chemical class 0.000 abstract 1
- 238000011045 prefiltration Methods 0.000 abstract 1
- 239000007790 solid phase Substances 0.000 abstract 1
- PPBRXRYQALVLMV-UHFFFAOYSA-N Styrene Chemical compound C=CC1=CC=CC=C1 PPBRXRYQALVLMV-UHFFFAOYSA-N 0.000 description 58
- 239000003921 oil Substances 0.000 description 37
- 238000012360 testing method Methods 0.000 description 35
- 239000000356 contaminant Substances 0.000 description 27
- 241000894007 species Species 0.000 description 27
- 238000012545 processing Methods 0.000 description 26
- HNNQYHFROJDYHQ-UHFFFAOYSA-N 3-(4-ethylcyclohexyl)propanoic acid 3-(3-ethylcyclopentyl)propanoic acid Chemical compound CCC1CCC(CCC(O)=O)C1.CCC1CCC(CCC(O)=O)CC1 HNNQYHFROJDYHQ-UHFFFAOYSA-N 0.000 description 25
- 230000009467 reduction Effects 0.000 description 25
- 238000011068 loading method Methods 0.000 description 23
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 21
- QTWJRLJHJPIABL-UHFFFAOYSA-N 2-methylphenol;3-methylphenol;4-methylphenol Chemical compound CC1=CC=C(O)C=C1.CC1=CC=CC(O)=C1.CC1=CC=CC=C1O QTWJRLJHJPIABL-UHFFFAOYSA-N 0.000 description 13
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 13
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 10
- 229930003836 cresol Natural products 0.000 description 10
- 238000010586 diagram Methods 0.000 description 10
- 239000011347 resin Substances 0.000 description 10
- 229920005989 resin Polymers 0.000 description 10
- 239000012267 brine Substances 0.000 description 9
- HPALAKNZSZLMCH-UHFFFAOYSA-M sodium;chloride;hydrate Chemical compound O.[Na+].[Cl-] HPALAKNZSZLMCH-UHFFFAOYSA-M 0.000 description 9
- 239000007787 solid Substances 0.000 description 9
- 239000002023 wood Substances 0.000 description 9
- 238000004064 recycling Methods 0.000 description 8
- 239000011148 porous material Substances 0.000 description 7
- 238000000746 purification Methods 0.000 description 7
- 239000011734 sodium Substances 0.000 description 7
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 6
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 description 6
- 238000004062 sedimentation Methods 0.000 description 6
- 239000002351 wastewater Substances 0.000 description 6
- 238000004065 wastewater treatment Methods 0.000 description 6
- 230000015556 catabolic process Effects 0.000 description 5
- 230000006378 damage Effects 0.000 description 5
- 239000000203 mixture Substances 0.000 description 5
- 238000006386 neutralization reaction Methods 0.000 description 5
- 239000011368 organic material Substances 0.000 description 5
- 230000002572 peristaltic effect Effects 0.000 description 5
- 238000011084 recovery Methods 0.000 description 5
- 150000003839 salts Chemical class 0.000 description 5
- 238000005201 scrubbing Methods 0.000 description 5
- 239000011324 bead Substances 0.000 description 4
- 230000003247 decreasing effect Effects 0.000 description 4
- 238000013461 design Methods 0.000 description 4
- 230000000694 effects Effects 0.000 description 4
- -1 mercaptides Chemical compound 0.000 description 4
- 239000002904 solvent Substances 0.000 description 4
- 235000013162 Cocos nucifera Nutrition 0.000 description 3
- 244000060011 Cocos nucifera Species 0.000 description 3
- 229910000147 aluminium phosphate Inorganic materials 0.000 description 3
- 239000010426 asphalt Substances 0.000 description 3
- 230000008901 benefit Effects 0.000 description 3
- 229910052799 carbon Inorganic materials 0.000 description 3
- 238000003795 desorption Methods 0.000 description 3
- 238000004821 distillation Methods 0.000 description 3
- 238000005187 foaming Methods 0.000 description 3
- 239000011521 glass Substances 0.000 description 3
- 230000005484 gravity Effects 0.000 description 3
- 238000009533 lab test Methods 0.000 description 3
- 239000003077 lignite Substances 0.000 description 3
- 238000004519 manufacturing process Methods 0.000 description 3
- 235000019645 odor Nutrition 0.000 description 3
- 230000003647 oxidation Effects 0.000 description 3
- 238000007254 oxidation reaction Methods 0.000 description 3
- 238000010979 pH adjustment Methods 0.000 description 3
- 239000002245 particle Substances 0.000 description 3
- 239000000243 solution Substances 0.000 description 3
- 239000011345 viscous material Substances 0.000 description 3
- QGZKDVFQNNGYKY-UHFFFAOYSA-N Ammonia Chemical compound N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 description 2
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 2
- 239000004215 Carbon black (E152) Substances 0.000 description 2
- 241000196324 Embryophyta Species 0.000 description 2
- VGGSQFUCUMXWEO-UHFFFAOYSA-N Ethene Chemical compound C=C VGGSQFUCUMXWEO-UHFFFAOYSA-N 0.000 description 2
- 239000005977 Ethylene Substances 0.000 description 2
- UCKMPCXJQFINFW-UHFFFAOYSA-N Sulphide Chemical compound [S-2] UCKMPCXJQFINFW-UHFFFAOYSA-N 0.000 description 2
- 230000002378 acidificating effect Effects 0.000 description 2
- 238000007792 addition Methods 0.000 description 2
- 238000013459 approach Methods 0.000 description 2
- 239000011260 aqueous acid Substances 0.000 description 2
- 230000001580 bacterial effect Effects 0.000 description 2
- 230000031018 biological processes and functions Effects 0.000 description 2
- 238000004523 catalytic cracking Methods 0.000 description 2
- 238000004140 cleaning Methods 0.000 description 2
- 230000007423 decrease Effects 0.000 description 2
- 238000006731 degradation reaction Methods 0.000 description 2
- 230000003111 delayed effect Effects 0.000 description 2
- 230000001419 dependent effect Effects 0.000 description 2
- 238000009826 distribution Methods 0.000 description 2
- 238000011010 flushing procedure Methods 0.000 description 2
- 229930195733 hydrocarbon Natural products 0.000 description 2
- 150000002430 hydrocarbons Chemical class 0.000 description 2
- 230000007935 neutral effect Effects 0.000 description 2
- 239000005416 organic matter Substances 0.000 description 2
- 239000003960 organic solvent Substances 0.000 description 2
- 239000011236 particulate material Substances 0.000 description 2
- 230000000737 periodic effect Effects 0.000 description 2
- 239000002994 raw material Substances 0.000 description 2
- 238000007670 refining Methods 0.000 description 2
- 229910052708 sodium Inorganic materials 0.000 description 2
- 239000000758 substrate Substances 0.000 description 2
- 231100000331 toxic Toxicity 0.000 description 2
- 230000002588 toxic effect Effects 0.000 description 2
- 238000004438 BET method Methods 0.000 description 1
- BWGNESOTFCXPMA-UHFFFAOYSA-N Dihydrogen disulfide Chemical compound SS BWGNESOTFCXPMA-UHFFFAOYSA-N 0.000 description 1
- 239000012028 Fenton's reagent Substances 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- UFWIBTONFRDIAS-UHFFFAOYSA-N Naphthalene Chemical compound C1=CC=CC2=CC=CC=C21 UFWIBTONFRDIAS-UHFFFAOYSA-N 0.000 description 1
- YGYAWVDWMABLBF-UHFFFAOYSA-N Phosgene Chemical compound ClC(Cl)=O YGYAWVDWMABLBF-UHFFFAOYSA-N 0.000 description 1
- OAICVXFJPJFONN-UHFFFAOYSA-N Phosphorus Chemical compound [P] OAICVXFJPJFONN-UHFFFAOYSA-N 0.000 description 1
- 239000003082 abrasive agent Substances 0.000 description 1
- 230000002411 adverse Effects 0.000 description 1
- 238000005273 aeration Methods 0.000 description 1
- 238000013019 agitation Methods 0.000 description 1
- 229910021529 ammonia Inorganic materials 0.000 description 1
- 238000010923 batch production Methods 0.000 description 1
- 238000006065 biodegradation reaction Methods 0.000 description 1
- 230000033228 biological regulation Effects 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 239000007844 bleaching agent Substances 0.000 description 1
- 150000004649 carbonic acid derivatives Chemical class 0.000 description 1
- 239000003054 catalyst Substances 0.000 description 1
- 230000003197 catalytic effect Effects 0.000 description 1
- 230000005465 channeling Effects 0.000 description 1
- 230000000295 complement effect Effects 0.000 description 1
- 238000009833 condensation Methods 0.000 description 1
- 230000005494 condensation Effects 0.000 description 1
- 239000004035 construction material Substances 0.000 description 1
- 229920006037 cross link polymer Polymers 0.000 description 1
- 239000010779 crude oil Substances 0.000 description 1
- 125000004122 cyclic group Chemical group 0.000 description 1
- 238000000354 decomposition reaction Methods 0.000 description 1
- 239000008367 deionised water Substances 0.000 description 1
- 229910021641 deionized water Inorganic materials 0.000 description 1
- 238000012217 deletion Methods 0.000 description 1
- 230000037430 deletion Effects 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 239000007789 gas Substances 0.000 description 1
- 231100001261 hazardous Toxicity 0.000 description 1
- MGZTXXNFBIUONY-UHFFFAOYSA-N hydrogen peroxide;iron(2+);sulfuric acid Chemical compound [Fe+2].OO.OS(O)(=O)=O MGZTXXNFBIUONY-UHFFFAOYSA-N 0.000 description 1
- 229910000037 hydrogen sulfide Inorganic materials 0.000 description 1
- 230000005764 inhibitory process Effects 0.000 description 1
- 238000005342 ion exchange Methods 0.000 description 1
- 238000006317 isomerization reaction Methods 0.000 description 1
- 239000003350 kerosene Substances 0.000 description 1
- 230000000670 limiting effect Effects 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 125000005609 naphthenate group Chemical group 0.000 description 1
- 229910052757 nitrogen Inorganic materials 0.000 description 1
- 230000036961 partial effect Effects 0.000 description 1
- 239000013618 particulate matter Substances 0.000 description 1
- 239000003209 petroleum derivative Substances 0.000 description 1
- 238000005504 petroleum refining Methods 0.000 description 1
- 150000004707 phenolate Chemical class 0.000 description 1
- 229910052698 phosphorus Inorganic materials 0.000 description 1
- 239000011574 phosphorus Substances 0.000 description 1
- 238000011020 pilot scale process Methods 0.000 description 1
- 229920003023 plastic Polymers 0.000 description 1
- 239000004033 plastic Substances 0.000 description 1
- 229920001467 poly(styrenesulfonates) Polymers 0.000 description 1
- 238000006116 polymerization reaction Methods 0.000 description 1
- 238000005086 pumping Methods 0.000 description 1
- 238000010926 purge Methods 0.000 description 1
- 238000005067 remediation Methods 0.000 description 1
- 230000029058 respiratory gaseous exchange Effects 0.000 description 1
- 238000013341 scale-up Methods 0.000 description 1
- 239000010802 sludge Substances 0.000 description 1
- 159000000000 sodium salts Chemical class 0.000 description 1
- 238000000638 solvent extraction Methods 0.000 description 1
- 230000006641 stabilisation Effects 0.000 description 1
- 238000011105 stabilization Methods 0.000 description 1
- 150000003464 sulfur compounds Chemical class 0.000 description 1
- 238000003786 synthesis reaction Methods 0.000 description 1
- 239000008399 tap water Substances 0.000 description 1
- 235000020679 tap water Nutrition 0.000 description 1
- 230000002123 temporal effect Effects 0.000 description 1
- 238000004227 thermal cracking Methods 0.000 description 1
- 230000007704 transition Effects 0.000 description 1
- 239000003039 volatile agent Substances 0.000 description 1
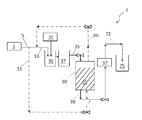
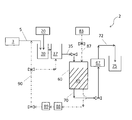
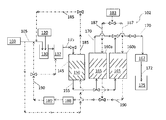
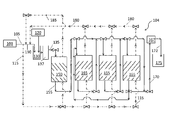
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F9/00—Multistage treatment of water, waste water or sewage
-
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F1/00—Treatment of water, waste water, or sewage
- C02F1/28—Treatment of water, waste water, or sewage by sorption
- C02F1/288—Treatment of water, waste water, or sewage by sorption using composite sorbents, e.g. coated, impregnated, multi-layered
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01D—SEPARATION
- B01D17/00—Separation of liquids, not provided for elsewhere, e.g. by thermal diffusion
- B01D17/02—Separation of non-miscible liquids
- B01D17/0208—Separation of non-miscible liquids by sedimentation
- B01D17/0214—Separation of non-miscible liquids by sedimentation with removal of one of the phases
-
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F1/00—Treatment of water, waste water, or sewage
- C02F1/28—Treatment of water, waste water, or sewage by sorption
-
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F1/00—Treatment of water, waste water, or sewage
- C02F1/28—Treatment of water, waste water, or sewage by sorption
- C02F1/283—Treatment of water, waste water, or sewage by sorption using coal, charred products, or inorganic mixtures containing them
-
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F1/00—Treatment of water, waste water, or sewage
- C02F1/28—Treatment of water, waste water, or sewage by sorption
- C02F1/285—Treatment of water, waste water, or sewage by sorption using synthetic organic sorbents
-
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F1/00—Treatment of water, waste water, or sewage
- C02F1/28—Treatment of water, waste water, or sewage by sorption
- C02F1/286—Treatment of water, waste water, or sewage by sorption using natural organic sorbents or derivatives thereof
-
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F1/00—Treatment of water, waste water, or sewage
- C02F1/40—Devices for separating or removing fatty or oily substances or similar floating material
-
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F1/00—Treatment of water, waste water, or sewage
- C02F1/66—Treatment of water, waste water, or sewage by neutralisation; pH adjustment
-
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F3/00—Biological treatment of water, waste water, or sewage
-
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F3/00—Biological treatment of water, waste water, or sewage
- C02F3/02—Aerobic processes
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10G—CRACKING HYDROCARBON OILS; PRODUCTION OF LIQUID HYDROCARBON MIXTURES, e.g. BY DESTRUCTIVE HYDROGENATION, OLIGOMERISATION, POLYMERISATION; RECOVERY OF HYDROCARBON OILS FROM OIL-SHALE, OIL-SAND, OR GASES; REFINING MIXTURES MAINLY CONSISTING OF HYDROCARBONS; REFORMING OF NAPHTHA; MINERAL WAXES
- C10G19/00—Refining hydrocarbon oils in the absence of hydrogen, by alkaline treatment
- C10G19/02—Refining hydrocarbon oils in the absence of hydrogen, by alkaline treatment with aqueous alkaline solutions
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10G—CRACKING HYDROCARBON OILS; PRODUCTION OF LIQUID HYDROCARBON MIXTURES, e.g. BY DESTRUCTIVE HYDROGENATION, OLIGOMERISATION, POLYMERISATION; RECOVERY OF HYDROCARBON OILS FROM OIL-SHALE, OIL-SAND, OR GASES; REFINING MIXTURES MAINLY CONSISTING OF HYDROCARBONS; REFORMING OF NAPHTHA; MINERAL WAXES
- C10G19/00—Refining hydrocarbon oils in the absence of hydrogen, by alkaline treatment
- C10G19/08—Recovery of used refining agents
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10G—CRACKING HYDROCARBON OILS; PRODUCTION OF LIQUID HYDROCARBON MIXTURES, e.g. BY DESTRUCTIVE HYDROGENATION, OLIGOMERISATION, POLYMERISATION; RECOVERY OF HYDROCARBON OILS FROM OIL-SHALE, OIL-SAND, OR GASES; REFINING MIXTURES MAINLY CONSISTING OF HYDROCARBONS; REFORMING OF NAPHTHA; MINERAL WAXES
- C10G25/00—Refining of hydrocarbon oils in the absence of hydrogen, with solid sorbents
- C10G25/12—Recovery of used adsorbent
-
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F1/00—Treatment of water, waste water, or sewage
- C02F1/72—Treatment of water, waste water, or sewage by oxidation
- C02F1/74—Treatment of water, waste water, or sewage by oxidation with air
-
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F2101/00—Nature of the contaminant
- C02F2101/10—Inorganic compounds
- C02F2101/101—Sulfur compounds
-
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F2101/00—Nature of the contaminant
- C02F2101/30—Organic compounds
- C02F2101/32—Hydrocarbons, e.g. oil
- C02F2101/327—Polyaromatic Hydrocarbons [PAH's]
-
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F2101/00—Nature of the contaminant
- C02F2101/30—Organic compounds
- C02F2101/34—Organic compounds containing oxygen
- C02F2101/345—Phenols
-
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F2103/00—Nature of the water, waste water, sewage or sludge to be treated
- C02F2103/34—Nature of the water, waste water, sewage or sludge to be treated from industrial activities not provided for in groups C02F2103/12 - C02F2103/32
- C02F2103/36—Nature of the water, waste water, sewage or sludge to be treated from industrial activities not provided for in groups C02F2103/12 - C02F2103/32 from the manufacture of organic compounds
- C02F2103/365—Nature of the water, waste water, sewage or sludge to be treated from industrial activities not provided for in groups C02F2103/12 - C02F2103/32 from the manufacture of organic compounds from petrochemical industry (e.g. refineries)
-
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F2209/00—Controlling or monitoring parameters in water treatment
- C02F2209/06—Controlling or monitoring parameters in water treatment pH
-
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F2209/00—Controlling or monitoring parameters in water treatment
- C02F2209/08—Chemical Oxygen Demand [COD]; Biological Oxygen Demand [BOD]
-
- C—CHEMISTRY; METALLURGY
- C02—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F—TREATMENT OF WATER, WASTE WATER, SEWAGE, OR SLUDGE
- C02F2303/00—Specific treatment goals
- C02F2303/16—Regeneration of sorbents, filters
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02W—CLIMATE CHANGE MITIGATION TECHNOLOGIES RELATED TO WASTEWATER TREATMENT OR WASTE MANAGEMENT
- Y02W10/00—Technologies for wastewater treatment
- Y02W10/10—Biological treatment of water, waste water, or sewage
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Engineering & Computer Science (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Water Supply & Treatment (AREA)
- Environmental & Geological Engineering (AREA)
- Hydrology & Water Resources (AREA)
- Biodiversity & Conservation Biology (AREA)
- Microbiology (AREA)
- Physics & Mathematics (AREA)
- Thermal Sciences (AREA)
- Water Treatment By Sorption (AREA)
- Solid-Sorbent Or Filter-Aiding Compositions (AREA)
- Analytical Chemistry (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201261718774P | 2012-10-26 | 2012-10-26 | |
| US61/718,774 | 2012-10-26 | ||
| PCT/US2013/066102 WO2014066338A1 (en) | 2012-10-26 | 2013-10-22 | Methods and systems for treating spent caustic and regenerating media |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20150077460A true KR20150077460A (ko) | 2015-07-07 |
Family
ID=49515558
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020157013245A Withdrawn KR20150077460A (ko) | 2012-10-26 | 2013-10-22 | 폐 가성 물질의 처리 및 매질의 재생을 위한 방법 및 시스템 |
Country Status (8)
| Country | Link |
|---|---|
| US (1) | US10053375B2 (enExample) |
| EP (1) | EP2911984A1 (enExample) |
| KR (1) | KR20150077460A (enExample) |
| CN (1) | CN104870380B (enExample) |
| BR (1) | BR112015009248A2 (enExample) |
| IN (1) | IN2015DN02901A (enExample) |
| RU (1) | RU2015119652A (enExample) |
| WO (1) | WO2014066338A1 (enExample) |
Families Citing this family (18)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9718712B2 (en) * | 2013-07-12 | 2017-08-01 | Uop Llc | Methods and systems for treating caustic materials |
| CN105278330A (zh) * | 2015-11-06 | 2016-01-27 | 哈尔滨商业大学 | 基于WebAccess的石油污水处理系统及处理方法 |
| CN106746100B (zh) * | 2015-11-19 | 2020-09-11 | 中国石油化工股份有限公司 | 一种乙烯精制废碱液的处理方法 |
| CN106746101B (zh) * | 2015-11-19 | 2020-09-11 | 中国石油化工股份有限公司 | 一种乙烯废碱液的处理方法 |
| EP3468924B1 (en) * | 2016-08-31 | 2023-05-03 | Siemens Energy, Inc. | Process for treatment of high total dissolved solids wastewater |
| US10435362B2 (en) | 2016-12-21 | 2019-10-08 | Uop Llc | Process for oxidizing one or more thiol compounds and subsequent separation in a single vessel |
| US10240096B1 (en) | 2017-10-25 | 2019-03-26 | Saudi Arabian Oil Company | Integrated process for activating hydroprocessing catalysts with in-situ produced sulfides and disulphides |
| US12139425B2 (en) | 2018-08-28 | 2024-11-12 | Lummus Technology Llc | Wet air oxidation of a spent material with spent caustic addition |
| CN109777741B (zh) * | 2019-01-15 | 2022-01-04 | 昆明理工大学 | 一种核桃壳高效利用的方法 |
| CN110331302B (zh) * | 2019-08-13 | 2021-06-08 | 包头稀土研究院 | 减少中重稀土萃取分离中酸用量的方法 |
| CN110306045B (zh) * | 2019-08-13 | 2021-04-16 | 包头稀土研究院 | 中重稀土氯化物溶液中的有机杂质的去除方法 |
| CN110317963B (zh) * | 2019-08-13 | 2021-07-13 | 包头稀土研究院 | 萃取分离由酸法制备的稀土氯化物溶液的方法 |
| CN110331285B (zh) * | 2019-08-13 | 2020-10-02 | 包头稀土研究院 | 由碱法制备的稀土氯化物溶液萃取分离轻稀土氯化物溶液的方法 |
| CN110306050B (zh) * | 2019-08-13 | 2021-04-16 | 包头稀土研究院 | 中重稀土萃取分离的方法 |
| CN110813983B (zh) * | 2019-10-31 | 2021-09-17 | 金能科技股份有限公司 | 烷基苯酚生产工艺所产固废酚渣的回收处理方法及其装置 |
| FR3124744A1 (fr) * | 2021-07-02 | 2023-01-06 | Suez Groupe | Procede de regeneration in situ d’un media adsorbant |
| WO2024206639A1 (en) * | 2023-03-31 | 2024-10-03 | Carollo Engineers, Inc. | Systems and methods for water treatment using ion exchange and water softening techniques |
| CN117964178B (zh) * | 2024-03-25 | 2024-06-21 | 矿冶科技集团有限公司 | 一种萃余液蒸汽除油的方法 |
Family Cites Families (18)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4049546A (en) * | 1974-11-11 | 1977-09-20 | Rohm And Haas Company | Decolorization of effluents from pulp mills |
| JPS5778927U (enExample) * | 1980-11-01 | 1982-05-15 | ||
| JPS5778927A (en) * | 1980-11-04 | 1982-05-17 | Hitachi Ltd | Treatment of offensive odor |
| US4695386A (en) * | 1985-05-20 | 1987-09-22 | Advanced Separation Technologies Incorporated | Process for the decolorization of pulp mill process streams |
| US5635078A (en) * | 1993-05-12 | 1997-06-03 | Mobil Oil Corporation | Rejuvenated catalytic oxidation of waste water, particularly for removal of cyanide and sulfur compounds |
| US6398965B1 (en) * | 1998-03-31 | 2002-06-04 | United States Filter Corporation | Water treatment system and process |
| US6929942B2 (en) * | 1999-08-10 | 2005-08-16 | Council Of Scientific And Industrial Research | Process for the treatment of industrial effluents using marine algae to produce potable wafer |
| US7005076B2 (en) * | 2003-06-06 | 2006-02-28 | Rmt, Inc. | Caustic solution treatment process |
| CN1187275C (zh) | 2003-06-19 | 2005-02-02 | 上海交通大学 | 辛醇合成工艺系统排放废碱液的二级回收处理方法 |
| US8097163B1 (en) * | 2006-04-06 | 2012-01-17 | Produced Water Development, Llc | Purification of oil field production water for beneficial use |
| CN101134616B (zh) * | 2006-08-30 | 2011-02-09 | 中国石油天然气股份有限公司 | 一种综合治理炼厂含酸碱废液的设备系统 |
| US20100051556A1 (en) * | 2008-08-29 | 2010-03-04 | Grott Gerald J | Methods of purifiying water using waste brines to regenerate ion-exchange resins |
| US7828962B2 (en) * | 2008-11-20 | 2010-11-09 | Merichem Company | Apparatus for treating a waste stream |
| CN101475274A (zh) * | 2008-12-31 | 2009-07-08 | 大连力达环境工程有限公司 | 综合电镀废水处理工艺 |
| CN101570370B (zh) * | 2009-04-26 | 2011-08-10 | 赵志军 | 高资源化处理环己酮皂化废碱液的方法 |
| CA2764112C (en) * | 2009-07-08 | 2018-01-16 | Saudi Arabian Oil Company | Low concentration wastewater treatment system and process |
| CN201648046U (zh) * | 2009-12-08 | 2010-11-24 | 江苏三星化工有限公司 | 一种核桃壳过滤污水处理装置 |
| US8734650B2 (en) * | 2010-12-01 | 2014-05-27 | Veolia Water Solutions & Technologies North America, Inc. | Method for recovering gas from shale reservoirs and purifying resulting produced water to allow the produced water to be used as drilling or frac water, or discharged to the environment |
-
2013
- 2013-10-22 CN CN201380064226.1A patent/CN104870380B/zh active Active
- 2013-10-22 IN IN2901DEN2015 patent/IN2015DN02901A/en unknown
- 2013-10-22 EP EP13785781.9A patent/EP2911984A1/en not_active Withdrawn
- 2013-10-22 KR KR1020157013245A patent/KR20150077460A/ko not_active Withdrawn
- 2013-10-22 WO PCT/US2013/066102 patent/WO2014066338A1/en not_active Ceased
- 2013-10-22 RU RU2015119652A patent/RU2015119652A/ru not_active Application Discontinuation
- 2013-10-22 BR BR112015009248A patent/BR112015009248A2/pt not_active IP Right Cessation
- 2013-10-22 US US14/436,918 patent/US10053375B2/en active Active
Also Published As
| Publication number | Publication date |
|---|---|
| US20150284264A1 (en) | 2015-10-08 |
| EP2911984A1 (en) | 2015-09-02 |
| US10053375B2 (en) | 2018-08-21 |
| RU2015119652A (ru) | 2016-12-20 |
| CN104870380A (zh) | 2015-08-26 |
| CN104870380B (zh) | 2017-08-04 |
| WO2014066338A1 (en) | 2014-05-01 |
| IN2015DN02901A (enExample) | 2015-09-11 |
| BR112015009248A2 (pt) | 2017-07-04 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR20150077460A (ko) | 폐 가성 물질의 처리 및 매질의 재생을 위한 방법 및 시스템 | |
| Xu et al. | Applications of porous resin sorbents in industrial wastewater treatment and resource recovery | |
| US5614100A (en) | Method for water remediation | |
| US9593032B2 (en) | Produced water treatment to remove organic compounds | |
| US7291272B2 (en) | Inorganic contaminant removal from water | |
| US5707528A (en) | System and process for treating organic-contaminated aqueous waste streams | |
| CA2908789A1 (en) | Method and installation for treating water coming from the petroleum and gas industries, especially production water from petroleum and/or gas fields | |
| US5681476A (en) | Process for the purification of groundwater | |
| US8092687B2 (en) | Method and installation for treating an aqueous phase containing an adsorbent used material | |
| EP2291330B1 (en) | Process for the treatment of the aqueous stream coming from the fischer-tropsch reaction by means of ion exchange resins | |
| WO2021159185A1 (en) | A process and a plant | |
| AU2023304153A1 (en) | Use of supercritical carbon dioxide for sorbent extraction | |
| US6270676B1 (en) | Process for removing ethers and/or polycyclic aromatic hydrocarbons from water containing them | |
| CA2908804A1 (en) | Method for treating industrial water by physical separation, adsorption on resin and reverse osmosis, and corresponding plant | |
| Feeney | Removal of organic materials from wastewaters with polymeric adsorbents | |
| JPH1057949A (ja) | 有機含有物を有する水の浄化法 | |
| US20250388492A1 (en) | Use of supercritical carbon dioxide for sorbent extraction | |
| FI67720B (fi) | Avlaegsnande av smao syramaengder | |
| Das et al. | Phenol removal from aqueous solution by adsorption with resin technology | |
| Woodard et al. | Ion Exchange for PFAS Removal | |
| AlBatrni | Novel Adsorbents for the Removal of Oil from Produced Water | |
| Liu et al. | Adsorption selectivity of salicylic acid and 5-sulfosalicylic acid onto hypercrosslinked polymeric adsorbents | |
| Wang et al. | Polymeric adsorption and regenerant distillation | |
| Boodoo et al. | Disinfection byproducts control with ion exchange | |
| Ying et al. | Section Four |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20150520 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| PC1203 | Withdrawal of no request for examination | ||
| WITN | Application deemed withdrawn, e.g. because no request for examination was filed or no examination fee was paid |