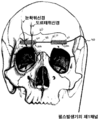
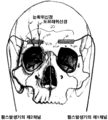
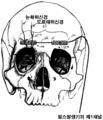
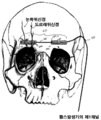
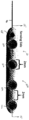KR20150014445A - 뇌신경 자극용 피하 전극 - Google Patents
뇌신경 자극용 피하 전극 Download PDFInfo
- Publication number
- KR20150014445A KR20150014445A KR20147031070A KR20147031070A KR20150014445A KR 20150014445 A KR20150014445 A KR 20150014445A KR 20147031070 A KR20147031070 A KR 20147031070A KR 20147031070 A KR20147031070 A KR 20147031070A KR 20150014445 A KR20150014445 A KR 20150014445A
- Authority
- KR
- South Korea
- Prior art keywords
- electrical contacts
- electrode
- pair
- nerve
- electrode body
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
- 230000007383 nerve stimulation Effects 0.000 title claims description 14
- 238000007920 subcutaneous administration Methods 0.000 title abstract description 8
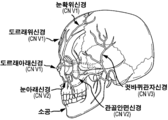
- 210000003792 cranial nerve Anatomy 0.000 title description 2
- 238000000034 method Methods 0.000 claims abstract description 30
- 210000005036 nerve Anatomy 0.000 claims description 95
- WABPQHHGFIMREM-UHFFFAOYSA-N lead(0) Chemical compound [Pb] WABPQHHGFIMREM-UHFFFAOYSA-N 0.000 claims description 25
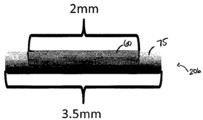
- 210000000988 bone and bone Anatomy 0.000 claims description 20
- 239000011148 porous material Substances 0.000 claims description 19
- 238000010079 rubber tapping Methods 0.000 claims description 16
- 210000002808 connective tissue Anatomy 0.000 claims description 14
- 210000003205 muscle Anatomy 0.000 claims description 14
- 210000001328 optic nerve Anatomy 0.000 claims description 7
- 238000002955 isolation Methods 0.000 claims description 6
- 230000000638 stimulation Effects 0.000 description 36
- 210000003491 skin Anatomy 0.000 description 30
- 210000004556 brain Anatomy 0.000 description 18
- 210000004126 nerve fiber Anatomy 0.000 description 18
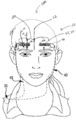
- 210000001061 forehead Anatomy 0.000 description 14
- 230000004936 stimulating effect Effects 0.000 description 10
- 238000011282 treatment Methods 0.000 description 10
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 9
- 238000004873 anchoring Methods 0.000 description 8
- 238000013459 approach Methods 0.000 description 8
- 208000006735 Periostitis Diseases 0.000 description 7
- 239000000835 fiber Substances 0.000 description 7
- 238000002513 implantation Methods 0.000 description 7
- 230000001939 inductive effect Effects 0.000 description 7
- 210000003460 periosteum Anatomy 0.000 description 7
- 210000003625 skull Anatomy 0.000 description 7
- 230000008901 benefit Effects 0.000 description 6
- 230000008878 coupling Effects 0.000 description 6
- 238000010168 coupling process Methods 0.000 description 6
- 238000005859 coupling reaction Methods 0.000 description 6
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 6
- 206010010904 Convulsion Diseases 0.000 description 5
- 201000010099 disease Diseases 0.000 description 5
- 230000000694 effects Effects 0.000 description 5
- 210000003478 temporal lobe Anatomy 0.000 description 5
- 210000001519 tissue Anatomy 0.000 description 5
- 208000002193 Pain Diseases 0.000 description 4
- 208000035475 disorder Diseases 0.000 description 4
- 210000004709 eyebrow Anatomy 0.000 description 4
- 210000003128 head Anatomy 0.000 description 4
- 210000004872 soft tissue Anatomy 0.000 description 4
- 238000002560 therapeutic procedure Methods 0.000 description 4
- 238000012546 transfer Methods 0.000 description 4
- 208000003098 Ganglion Cysts Diseases 0.000 description 3
- 206010040880 Skin irritation Diseases 0.000 description 3
- 208000005400 Synovial Cyst Diseases 0.000 description 3
- 230000000712 assembly Effects 0.000 description 3
- 238000000429 assembly Methods 0.000 description 3
- 230000007177 brain activity Effects 0.000 description 3
- 210000003710 cerebral cortex Anatomy 0.000 description 3
- 230000007012 clinical effect Effects 0.000 description 3
- 238000002635 electroconvulsive therapy Methods 0.000 description 3
- 206010015037 epilepsy Diseases 0.000 description 3
- 230000005284 excitation Effects 0.000 description 3
- 230000001815 facial effect Effects 0.000 description 3
- 229910052741 iridium Inorganic materials 0.000 description 3
- GKOZUEZYRPOHIO-UHFFFAOYSA-N iridium atom Chemical compound [Ir] GKOZUEZYRPOHIO-UHFFFAOYSA-N 0.000 description 3
- 230000008447 perception Effects 0.000 description 3
- 229910052697 platinum Inorganic materials 0.000 description 3
- 210000004761 scalp Anatomy 0.000 description 3
- 230000036556 skin irritation Effects 0.000 description 3
- 231100000475 skin irritation Toxicity 0.000 description 3
- 210000004304 subcutaneous tissue Anatomy 0.000 description 3
- 210000001103 thalamus Anatomy 0.000 description 3
- 238000002054 transplantation Methods 0.000 description 3
- 208000012902 Nervous system disease Diseases 0.000 description 2
- 208000025966 Neurological disease Diseases 0.000 description 2
- KDLHZDBZIXYQEI-UHFFFAOYSA-N Palladium Chemical compound [Pd] KDLHZDBZIXYQEI-UHFFFAOYSA-N 0.000 description 2
- 230000009471 action Effects 0.000 description 2
- 238000013528 artificial neural network Methods 0.000 description 2
- 230000002146 bilateral effect Effects 0.000 description 2
- 230000015572 biosynthetic process Effects 0.000 description 2
- 210000000133 brain stem Anatomy 0.000 description 2
- 239000003990 capacitor Substances 0.000 description 2
- 238000010276 construction Methods 0.000 description 2
- 230000009977 dual effect Effects 0.000 description 2
- 210000000744 eyelid Anatomy 0.000 description 2
- 239000007943 implant Substances 0.000 description 2
- 239000012528 membrane Substances 0.000 description 2
- 230000035515 penetration Effects 0.000 description 2
- 230000002360 prefrontal effect Effects 0.000 description 2
- 230000035807 sensation Effects 0.000 description 2
- 210000003582 temporal bone Anatomy 0.000 description 2
- 210000001186 vagus nerve Anatomy 0.000 description 2
- 208000001654 Drug Resistant Epilepsy Diseases 0.000 description 1
- HBBGRARXTFLTSG-UHFFFAOYSA-N Lithium ion Chemical compound [Li+] HBBGRARXTFLTSG-UHFFFAOYSA-N 0.000 description 1
- 201000009906 Meningitis Diseases 0.000 description 1
- 208000028389 Nerve injury Diseases 0.000 description 1
- 206010033799 Paralysis Diseases 0.000 description 1
- 208000018737 Parkinson disease Diseases 0.000 description 1
- 208000008765 Sciatica Diseases 0.000 description 1
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 description 1
- NRTOMJZYCJJWKI-UHFFFAOYSA-N Titanium nitride Chemical compound [Ti]#N NRTOMJZYCJJWKI-UHFFFAOYSA-N 0.000 description 1
- 230000001154 acute effect Effects 0.000 description 1
- 230000003044 adaptive effect Effects 0.000 description 1
- 239000000853 adhesive Substances 0.000 description 1
- 230000001070 adhesive effect Effects 0.000 description 1
- 229910045601 alloy Inorganic materials 0.000 description 1
- 239000000956 alloy Substances 0.000 description 1
- 210000003423 ankle Anatomy 0.000 description 1
- 230000004872 arterial blood pressure Effects 0.000 description 1
- 230000001746 atrial effect Effects 0.000 description 1
- 230000000740 bleeding effect Effects 0.000 description 1
- 230000000747 cardiac effect Effects 0.000 description 1
- 230000003727 cerebral blood flow Effects 0.000 description 1
- 238000006243 chemical reaction Methods 0.000 description 1
- 230000001684 chronic effect Effects 0.000 description 1
- 210000000860 cochlear nerve Anatomy 0.000 description 1
- 230000001149 cognitive effect Effects 0.000 description 1
- 150000001875 compounds Chemical class 0.000 description 1
- 230000003750 conditioning effect Effects 0.000 description 1
- 230000007797 corrosion Effects 0.000 description 1
- 238000005260 corrosion Methods 0.000 description 1
- 239000002537 cosmetic Substances 0.000 description 1
- 210000003464 cuspid Anatomy 0.000 description 1
- 230000006378 damage Effects 0.000 description 1
- 230000002939 deleterious effect Effects 0.000 description 1
- 210000004513 dentition Anatomy 0.000 description 1
- 238000013461 design Methods 0.000 description 1
- 230000035487 diastolic blood pressure Effects 0.000 description 1
- 239000003814 drug Substances 0.000 description 1
- 229940079593 drug Drugs 0.000 description 1
- 238000002651 drug therapy Methods 0.000 description 1
- 208000010515 dystocia Diseases 0.000 description 1
- 230000000632 dystonic effect Effects 0.000 description 1
- 210000000613 ear canal Anatomy 0.000 description 1
- 230000005684 electric field Effects 0.000 description 1
- 238000005868 electrolysis reaction Methods 0.000 description 1
- 210000002615 epidermis Anatomy 0.000 description 1
- 210000003195 fascia Anatomy 0.000 description 1
- 238000000799 fluorescence microscopy Methods 0.000 description 1
- 238000002594 fluoroscopy Methods 0.000 description 1
- 210000002454 frontal bone Anatomy 0.000 description 1
- 210000001652 frontal lobe Anatomy 0.000 description 1
- 230000006870 function Effects 0.000 description 1
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 1
- 229910052737 gold Inorganic materials 0.000 description 1
- 239000010931 gold Substances 0.000 description 1
- 208000015181 infectious disease Diseases 0.000 description 1
- 238000011221 initial treatment Methods 0.000 description 1
- 229910001416 lithium ion Inorganic materials 0.000 description 1
- 210000004072 lung Anatomy 0.000 description 1
- 208000024714 major depressive disease Diseases 0.000 description 1
- 210000002698 mandibular nerve Anatomy 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 210000004228 maxillary nerve Anatomy 0.000 description 1
- 210000001259 mesencephalon Anatomy 0.000 description 1
- 230000000116 mitigating effect Effects 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 238000012544 monitoring process Methods 0.000 description 1
- 230000008764 nerve damage Effects 0.000 description 1
- 210000000944 nerve tissue Anatomy 0.000 description 1
- 210000000653 nervous system Anatomy 0.000 description 1
- 210000002569 neuron Anatomy 0.000 description 1
- 239000002858 neurotransmitter agent Substances 0.000 description 1
- 230000007935 neutral effect Effects 0.000 description 1
- 229910001120 nichrome Inorganic materials 0.000 description 1
- 229910052758 niobium Inorganic materials 0.000 description 1
- 239000010955 niobium Substances 0.000 description 1
- GUCVJGMIXFAOAE-UHFFFAOYSA-N niobium atom Chemical compound [Nb] GUCVJGMIXFAOAE-UHFFFAOYSA-N 0.000 description 1
- NJPPVKZQTLUDBO-UHFFFAOYSA-N novaluron Chemical compound C1=C(Cl)C(OC(F)(F)C(OC(F)(F)F)F)=CC=C1NC(=O)NC(=O)C1=C(F)C=CC=C1F NJPPVKZQTLUDBO-UHFFFAOYSA-N 0.000 description 1
- 230000003287 optical effect Effects 0.000 description 1
- 230000008058 pain sensation Effects 0.000 description 1
- 229910052763 palladium Inorganic materials 0.000 description 1
- 206010033675 panniculitis Diseases 0.000 description 1
- 230000007170 pathology Effects 0.000 description 1
- 210000002976 pectoralis muscle Anatomy 0.000 description 1
- 230000002093 peripheral effect Effects 0.000 description 1
- 210000000578 peripheral nerve Anatomy 0.000 description 1
- 230000000144 pharmacologic effect Effects 0.000 description 1
- 230000002035 prolonged effect Effects 0.000 description 1
- 238000011321 prophylaxis Methods 0.000 description 1
- 238000002106 pulse oximetry Methods 0.000 description 1
- 238000010926 purge Methods 0.000 description 1
- 230000007115 recruitment Effects 0.000 description 1
- 239000003870 refractory metal Substances 0.000 description 1
- 230000021670 response to stimulus Effects 0.000 description 1
- 230000002441 reversible effect Effects 0.000 description 1
- 229910052702 rhenium Inorganic materials 0.000 description 1
- WUAPFZMCVAUBPE-UHFFFAOYSA-N rhenium atom Chemical compound [Re] WUAPFZMCVAUBPE-UHFFFAOYSA-N 0.000 description 1
- 230000011664 signaling Effects 0.000 description 1
- 210000001032 spinal nerve Anatomy 0.000 description 1
- 229910001220 stainless steel Inorganic materials 0.000 description 1
- 239000010935 stainless steel Substances 0.000 description 1
- 210000002784 stomach Anatomy 0.000 description 1
- 208000024891 symptom Diseases 0.000 description 1
- 210000000225 synapse Anatomy 0.000 description 1
- 230000035488 systolic blood pressure Effects 0.000 description 1
- 229910052715 tantalum Inorganic materials 0.000 description 1
- GUVRBAGPIYLISA-UHFFFAOYSA-N tantalum atom Chemical compound [Ta] GUVRBAGPIYLISA-UHFFFAOYSA-N 0.000 description 1
- 230000002123 temporal effect Effects 0.000 description 1
- 230000001225 therapeutic effect Effects 0.000 description 1
- 239000010936 titanium Substances 0.000 description 1
- 229910052719 titanium Inorganic materials 0.000 description 1
- 230000036346 tooth eruption Effects 0.000 description 1
- 238000012549 training Methods 0.000 description 1
- 210000003901 trigeminal nerve Anatomy 0.000 description 1
- 238000002604 ultrasonography Methods 0.000 description 1
- 230000002861 ventricular Effects 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N1/00—Electrotherapy; Circuits therefor
- A61N1/02—Details
- A61N1/04—Electrodes
- A61N1/05—Electrodes for implantation or insertion into the body, e.g. heart electrode
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N1/00—Electrotherapy; Circuits therefor
- A61N1/02—Details
- A61N1/04—Electrodes
- A61N1/05—Electrodes for implantation or insertion into the body, e.g. heart electrode
- A61N1/0551—Spinal or peripheral nerve electrodes
- A61N1/0558—Anchoring or fixation means therefor
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/34—Trocars; Puncturing needles
- A61B17/3417—Details of tips or shafts, e.g. grooves, expandable, bendable; Multiple coaxial sliding cannulas, e.g. for dilating
- A61B17/3421—Cannulas
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F9/00—Methods or devices for treatment of the eyes; Devices for putting in contact-lenses; Devices to correct squinting; Apparatus to guide the blind; Protective devices for the eyes, carried on the body or in the hand
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N1/00—Electrotherapy; Circuits therefor
- A61N1/02—Details
- A61N1/04—Electrodes
- A61N1/05—Electrodes for implantation or insertion into the body, e.g. heart electrode
- A61N1/0504—Subcutaneous electrodes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N1/00—Electrotherapy; Circuits therefor
- A61N1/02—Details
- A61N1/04—Electrodes
- A61N1/05—Electrodes for implantation or insertion into the body, e.g. heart electrode
- A61N1/0526—Head electrodes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N1/00—Electrotherapy; Circuits therefor
- A61N1/02—Details
- A61N1/04—Electrodes
- A61N1/05—Electrodes for implantation or insertion into the body, e.g. heart electrode
- A61N1/0551—Spinal or peripheral nerve electrodes
- A61N1/0553—Paddle shaped electrodes, e.g. for laminotomy
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N1/00—Electrotherapy; Circuits therefor
- A61N1/18—Applying electric currents by contact electrodes
- A61N1/32—Applying electric currents by contact electrodes alternating or intermittent currents
- A61N1/36—Applying electric currents by contact electrodes alternating or intermittent currents for stimulation
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N1/00—Electrotherapy; Circuits therefor
- A61N1/18—Applying electric currents by contact electrodes
- A61N1/32—Applying electric currents by contact electrodes alternating or intermittent currents
- A61N1/36—Applying electric currents by contact electrodes alternating or intermittent currents for stimulation
- A61N1/3605—Implantable neurostimulators for stimulating central or peripheral nerve system
- A61N1/36128—Control systems
- A61N1/36146—Control systems specified by the stimulation parameters
- A61N1/36182—Direction of the electrical field, e.g. with sleeve around stimulating electrode
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N1/00—Electrotherapy; Circuits therefor
- A61N1/18—Applying electric currents by contact electrodes
- A61N1/32—Applying electric currents by contact electrodes alternating or intermittent currents
- A61N1/36—Applying electric currents by contact electrodes alternating or intermittent currents for stimulation
- A61N1/3605—Implantable neurostimulators for stimulating central or peripheral nerve system
- A61N1/3606—Implantable neurostimulators for stimulating central or peripheral nerve system adapted for a particular treatment
- A61N1/36064—Epilepsy
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- General Health & Medical Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Heart & Thoracic Surgery (AREA)
- Radiology & Medical Imaging (AREA)
- Cardiology (AREA)
- Neurology (AREA)
- Neurosurgery (AREA)
- Surgery (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Pathology (AREA)
- Medical Informatics (AREA)
- Molecular Biology (AREA)
- Ophthalmology & Optometry (AREA)
- Vascular Medicine (AREA)
- Electrotherapy Devices (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201261620879P | 2012-04-05 | 2012-04-05 | |
| US61/620,879 | 2012-04-05 | ||
| PCT/US2013/035499 WO2013152316A1 (en) | 2012-04-05 | 2013-04-05 | Subcutaneous electrodes for cranial nerve stimulation |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20150014445A true KR20150014445A (ko) | 2015-02-06 |
Family
ID=49301098
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR20147031070A Withdrawn KR20150014445A (ko) | 2012-04-05 | 2013-04-05 | 뇌신경 자극용 피하 전극 |
Country Status (7)
| Country | Link |
|---|---|
| US (1) | US10035013B2 (enExample) |
| EP (1) | EP2833960B1 (enExample) |
| JP (1) | JP2015513980A (enExample) |
| KR (1) | KR20150014445A (enExample) |
| AU (1) | AU2013243309B2 (enExample) |
| CA (1) | CA2869612A1 (enExample) |
| WO (1) | WO2013152316A1 (enExample) |
Families Citing this family (34)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP2485799A4 (en) | 2009-10-05 | 2013-05-15 | Univ California | EXTRACRANIAL IMPLANTABLE DEVICES, SYSTEMS AND METHOD FOR THE TREATMENT OF NEUROLOGICAL ILLNESSES |
| WO2012075192A2 (en) | 2010-11-30 | 2012-06-07 | The Regents Of The University Of California | Pulse generator for cranial nerve stimulation |
| EP2651497B1 (en) | 2010-12-14 | 2019-02-20 | The Regents of The University of California | Extracranial implantable systems for the treatment of medical disorders |
| KR20140037803A (ko) | 2010-12-14 | 2014-03-27 | 더 리젠트스 오브 더 유니이버시티 오브 캘리포니아 | 의료 질환 치료용 장치, 시스템 및 방법 |
| US9731127B2 (en) * | 2013-04-24 | 2017-08-15 | Neurosigma, Inc. | Modulation of autonomic nervous system activity and integrated electrode assemblies for trigeminal neurostimulation |
| US9731122B2 (en) | 2013-04-29 | 2017-08-15 | Rainbow Medical Ltd. | Electroosmotic tissue treatment |
| WO2016093321A1 (ja) * | 2014-12-11 | 2016-06-16 | 株式会社アイカムス・ラボ | 細胞培養方法及び細胞培養装置 |
| KR102427652B1 (ko) | 2015-03-30 | 2022-08-01 | 세팔리 테크놀로지 에스피알엘 | 삼차 신경의 경피 전기 자극을 위한 장치 |
| US9616221B2 (en) | 2015-07-08 | 2017-04-11 | Rainbow Medical Ltd. | Electrical treatment of Alzheimer's disease |
| US10898716B2 (en) | 2015-10-29 | 2021-01-26 | Rainbow Medical Ltd. | Electrical substance clearance from the brain |
| US9724515B2 (en) | 2015-10-29 | 2017-08-08 | Rainbow Medical Ltd. | Electrical substance clearance from the brain for treatment of Alzheimer's disease |
| US10518085B2 (en) | 2015-12-29 | 2019-12-31 | Rainbow Medical Ltd. | Disc therapy |
| US9950156B2 (en) | 2016-09-13 | 2018-04-24 | Rainbow Medical Ltd. | Disc therapy |
| US9770591B2 (en) | 2015-12-29 | 2017-09-26 | Rainbow Medical Ltd. | Disc therapy |
| US11484706B2 (en) | 2015-12-29 | 2022-11-01 | Discure Technologies Ltd | Disc therapy |
| US10569086B2 (en) | 2017-01-11 | 2020-02-25 | Rainbow Medical Ltd. | Electrical microglial cell activation |
| US10758722B2 (en) | 2017-05-03 | 2020-09-01 | Rainbow Medical Ltd. | Electrical treatment of Parkinson's disease |
| DE202018001803U1 (de) | 2017-05-19 | 2018-06-27 | Cefaly Technology Sprl | Externe Trigeminusnervenstimulation für die Akutbehandlung von Migräneattacken |
| US11723579B2 (en) | 2017-09-19 | 2023-08-15 | Neuroenhancement Lab, LLC | Method and apparatus for neuroenhancement |
| US11717686B2 (en) | 2017-12-04 | 2023-08-08 | Neuroenhancement Lab, LLC | Method and apparatus for neuroenhancement to facilitate learning and performance |
| US11478603B2 (en) | 2017-12-31 | 2022-10-25 | Neuroenhancement Lab, LLC | Method and apparatus for neuroenhancement to enhance emotional response |
| US12280219B2 (en) | 2017-12-31 | 2025-04-22 | NeuroLight, Inc. | Method and apparatus for neuroenhancement to enhance emotional response |
| WO2019175879A1 (en) | 2018-03-14 | 2019-09-19 | Rainbow Medical Ltd. | Electrical substance clearance from the brain |
| US11364361B2 (en) | 2018-04-20 | 2022-06-21 | Neuroenhancement Lab, LLC | System and method for inducing sleep by transplanting mental states |
| DE102018110239A1 (de) * | 2018-04-27 | 2019-10-31 | Precisis Ag | Elektrodenpad zur elektrischen Stimulation |
| WO2020056418A1 (en) | 2018-09-14 | 2020-03-19 | Neuroenhancement Lab, LLC | System and method of improving sleep |
| US12357792B2 (en) | 2019-01-04 | 2025-07-15 | Shifamed Holdings, Llc | Internal recharging systems and methods of use |
| US11123197B2 (en) | 2019-09-03 | 2021-09-21 | Rainbow Medical Ltd. | Hydropneumatic artificial intervertebral disc |
| US10881858B1 (en) | 2019-09-18 | 2021-01-05 | Rainbow Medical Ltd. | Electrical substance clearance from the brain |
| EP4138981A4 (en) | 2020-04-23 | 2024-05-22 | Shifamed Holdings, LLC | Power management for interatrial shunts and associated systems and methods |
| US11298530B1 (en) | 2021-05-03 | 2022-04-12 | Discure Technologies Ltd. | Synergistic therapies for intervertebral disc degeneration |
| US11344721B1 (en) | 2021-08-16 | 2022-05-31 | Rainbow Medical Ltd. | Cartilage treatment |
| US11413455B1 (en) | 2022-02-08 | 2022-08-16 | Rainbow Medical Ltd. | Electrical treatment of Alzheimer's disease |
| US12208267B1 (en) | 2024-04-19 | 2025-01-28 | Yossi Gross | Blood flow enhancement therapy system |
Family Cites Families (22)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20010025192A1 (en) * | 1999-04-29 | 2001-09-27 | Medtronic, Inc. | Single and multi-polar implantable lead for sacral nerve electrical stimulation |
| US6952613B2 (en) | 2001-01-31 | 2005-10-04 | Medtronic, Inc. | Implantable gastrointestinal lead with active fixation |
| US6792314B2 (en) * | 2001-06-18 | 2004-09-14 | Alfred E. Mann Foundation For Scientific Research | Miniature implantable array and stimulation system suitable for eyelid stimulation |
| WO2005000090A2 (en) * | 2003-05-30 | 2005-01-06 | Medi-Screw, Inc. | Medical implant systems |
| WO2005062829A2 (en) * | 2003-12-19 | 2005-07-14 | Advanced Bionics Corporation | Skull-mounted electrical stimulation system and method for treating patients |
| US7920915B2 (en) * | 2005-11-16 | 2011-04-05 | Boston Scientific Neuromodulation Corporation | Implantable stimulator |
| US8204607B2 (en) * | 2005-06-09 | 2012-06-19 | Medtronic, Inc. | Implantable medical lead |
| EP1931418B1 (en) * | 2005-10-03 | 2012-11-21 | Washington University | Electrode for stimulating bone growth, tissue healing and/or pain control |
| GB0604613D0 (en) * | 2006-03-07 | 2006-04-19 | Simpson Brian A | Electrostimulation device |
| US9314618B2 (en) * | 2006-12-06 | 2016-04-19 | Spinal Modulation, Inc. | Implantable flexible circuit leads and methods of use |
| US20080140151A1 (en) * | 2006-12-11 | 2008-06-12 | Brodkey Jason A | Nerve stimulation apparatus and method for the treatment of head pain |
| KR101485815B1 (ko) * | 2007-07-04 | 2015-01-23 | 고쿠리츠다이가쿠호진 히로시마다이가쿠 | 경두개 전기 자극 장치 |
| US8214057B2 (en) * | 2007-10-16 | 2012-07-03 | Giancarlo Barolat | Surgically implantable electrodes |
| EP2092951A1 (fr) * | 2008-02-20 | 2009-08-26 | Stx-Med Sprl | Appareil pour le traîtement électrothérapeutique des céphalées de tension |
| CN102202725B (zh) * | 2008-10-21 | 2016-06-01 | Med-El电气医疗器械有限公司 | 用于面神经刺激的系统和方法 |
| US8494641B2 (en) | 2009-04-22 | 2013-07-23 | Autonomic Technologies, Inc. | Implantable neurostimulator with integral hermetic electronic enclosure, circuit substrate, monolithic feed-through, lead assembly and anchoring mechanism |
| EP2485799A4 (en) * | 2009-10-05 | 2013-05-15 | Univ California | EXTRACRANIAL IMPLANTABLE DEVICES, SYSTEMS AND METHOD FOR THE TREATMENT OF NEUROLOGICAL ILLNESSES |
| US9101766B2 (en) * | 2009-10-16 | 2015-08-11 | The Board Of Trustees Of The Leland Stanford Junior University | Eliciting analgesia by transcranial electrical stimulation |
| EP2651497B1 (en) | 2010-12-14 | 2019-02-20 | The Regents of The University of California | Extracranial implantable systems for the treatment of medical disorders |
| US20120203301A1 (en) * | 2011-02-07 | 2012-08-09 | Advanced Neuromodulation Systems, Inc. | Methods using trigeminal nerve stimulation to treat neurological diseases |
| WO2012145244A1 (en) * | 2011-04-20 | 2012-10-26 | Medtronic, Inc. | Method and apparatus for assessing neural activation |
| US10220211B2 (en) * | 2013-01-22 | 2019-03-05 | Livanova Usa, Inc. | Methods and systems to diagnose depression |
-
2013
- 2013-04-05 JP JP2015504752A patent/JP2015513980A/ja not_active Ceased
- 2013-04-05 EP EP13772822.6A patent/EP2833960B1/en active Active
- 2013-04-05 WO PCT/US2013/035499 patent/WO2013152316A1/en not_active Ceased
- 2013-04-05 CA CA2869612A patent/CA2869612A1/en not_active Abandoned
- 2013-04-05 AU AU2013243309A patent/AU2013243309B2/en not_active Ceased
- 2013-04-05 US US14/390,986 patent/US10035013B2/en active Active
- 2013-04-05 KR KR20147031070A patent/KR20150014445A/ko not_active Withdrawn
Also Published As
| Publication number | Publication date |
|---|---|
| CA2869612A1 (en) | 2013-10-10 |
| WO2013152316A1 (en) | 2013-10-10 |
| US20150119898A1 (en) | 2015-04-30 |
| EP2833960B1 (en) | 2018-03-07 |
| AU2013243309A1 (en) | 2014-10-30 |
| US10035013B2 (en) | 2018-07-31 |
| EP2833960A1 (en) | 2015-02-11 |
| AU2013243309B2 (en) | 2017-05-11 |
| EP2833960A4 (en) | 2015-12-09 |
| JP2015513980A (ja) | 2015-05-18 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR20150014445A (ko) | 뇌신경 자극용 피하 전극 | |
| JP7541547B2 (ja) | 神経精神障害を治療するための装置、システム及び方法 | |
| JP2019166378A (ja) | 医学的障害の治療のためのデバイス | |
| JP2014506501A (ja) | 運動障害の標的治療のための装置、システム、および方法 | |
| JP2018501078A (ja) | 電気刺激による頭痛治療 | |
| AU2015264879B2 (en) | Extracranial Implantable Devices, Systems and Methods for the Treatment of Neuropsychiatric Disorders |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20141104 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| N231 | Notification of change of applicant | ||
| PN2301 | Change of applicant |
Patent event date: 20141218 Comment text: Notification of Change of Applicant Patent event code: PN23011R01D |
|
| PG1501 | Laying open of application | ||
| PC1203 | Withdrawal of no request for examination | ||
| WITN | Application deemed withdrawn, e.g. because no request for examination was filed or no examination fee was paid |