KR20130140358A - 살메테롤 지나포산염, 플루티카손 프로피오네이트 및 티오트로피움 브로마이드를 포함하는 흡입 제형용 건조 분말 및 이의 제조방법 - Google Patents
살메테롤 지나포산염, 플루티카손 프로피오네이트 및 티오트로피움 브로마이드를 포함하는 흡입 제형용 건조 분말 및 이의 제조방법 Download PDFInfo
- Publication number
- KR20130140358A KR20130140358A KR1020120063665A KR20120063665A KR20130140358A KR 20130140358 A KR20130140358 A KR 20130140358A KR 1020120063665 A KR1020120063665 A KR 1020120063665A KR 20120063665 A KR20120063665 A KR 20120063665A KR 20130140358 A KR20130140358 A KR 20130140358A
- Authority
- KR
- South Korea
- Prior art keywords
- carrier
- dry powder
- inhalation
- particle size
- inhalation formulation
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
- 239000000203 mixture Substances 0.000 title claims abstract description 109
- 238000009472 formulation Methods 0.000 title claims abstract description 79
- 239000000843 powder Substances 0.000 title claims abstract description 63
- GIIZNNXWQWCKIB-UHFFFAOYSA-N Serevent Chemical compound C1=C(O)C(CO)=CC(C(O)CNCCCCCCOCCCCC=2C=CC=CC=2)=C1 GIIZNNXWQWCKIB-UHFFFAOYSA-N 0.000 title claims abstract description 33
- 229960000289 fluticasone propionate Drugs 0.000 title claims abstract description 29
- WMWTYOKRWGGJOA-CENSZEJFSA-N fluticasone propionate Chemical compound C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@]1(F)[C@@H]2[C@@H]2C[C@@H](C)[C@@](C(=O)SCF)(OC(=O)CC)[C@@]2(C)C[C@@H]1O WMWTYOKRWGGJOA-CENSZEJFSA-N 0.000 title claims abstract description 29
- LERNTVKEWCAPOY-VOGVJGKGSA-N C[N+]1(C)[C@H]2C[C@H](C[C@@H]1[C@H]1O[C@@H]21)OC(=O)C(O)(c1cccs1)c1cccs1 Chemical compound C[N+]1(C)[C@H]2C[C@H](C[C@@H]1[C@H]1O[C@@H]21)OC(=O)C(O)(c1cccs1)c1cccs1 LERNTVKEWCAPOY-VOGVJGKGSA-N 0.000 title claims abstract description 17
- 229960000257 tiotropium bromide Drugs 0.000 title claims abstract description 17
- 238000000034 method Methods 0.000 title claims description 25
- 229960005018 salmeterol xinafoate Drugs 0.000 title description 2
- 239000002245 particle Substances 0.000 claims abstract description 102
- 239000002831 pharmacologic agent Substances 0.000 claims abstract description 47
- 229960004017 salmeterol Drugs 0.000 claims abstract description 31
- 230000008859 change Effects 0.000 claims abstract description 23
- 208000023504 respiratory system disease Diseases 0.000 claims abstract description 8
- 230000002265 prevention Effects 0.000 claims abstract description 4
- 238000002156 mixing Methods 0.000 claims description 18
- 238000000227 grinding Methods 0.000 claims description 6
- 238000004519 manufacturing process Methods 0.000 claims description 6
- WSVLPVUVIUVCRA-KPKNDVKVSA-N Alpha-lactose monohydrate Chemical group O.O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O WSVLPVUVIUVCRA-KPKNDVKVSA-N 0.000 claims description 3
- 150000002016 disaccharides Chemical class 0.000 claims description 3
- 150000004677 hydrates Chemical class 0.000 claims description 3
- 150000002772 monosaccharides Chemical group 0.000 claims description 3
- 150000004676 glycans Chemical class 0.000 claims description 2
- 229960001021 lactose monohydrate Drugs 0.000 claims description 2
- 229920001282 polysaccharide Polymers 0.000 claims description 2
- 239000005017 polysaccharide Substances 0.000 claims description 2
- 150000005846 sugar alcohols Polymers 0.000 claims description 2
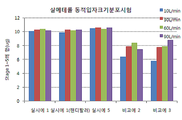
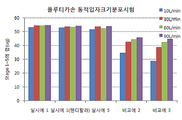
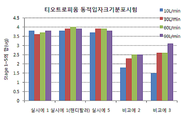
- 238000009826 distribution Methods 0.000 abstract description 20
- 208000006545 Chronic Obstructive Pulmonary Disease Diseases 0.000 abstract description 8
- 208000006673 asthma Diseases 0.000 abstract description 8
- 239000002775 capsule Substances 0.000 description 35
- 229960001375 lactose Drugs 0.000 description 22
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 21
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 21
- 239000003814 drug Substances 0.000 description 21
- 239000008101 lactose Substances 0.000 description 21
- 229940079593 drug Drugs 0.000 description 20
- 238000002360 preparation method Methods 0.000 description 19
- 238000012360 testing method Methods 0.000 description 18
- 229940110309 tiotropium Drugs 0.000 description 15
- LERNTVKEWCAPOY-DZZGSBJMSA-N tiotropium Chemical compound O([C@H]1C[C@@H]2[N+]([C@H](C1)[C@@H]1[C@H]2O1)(C)C)C(=O)C(O)(C=1SC=CC=1)C1=CC=CS1 LERNTVKEWCAPOY-DZZGSBJMSA-N 0.000 description 15
- 239000004480 active ingredient Substances 0.000 description 14
- 230000000052 comparative effect Effects 0.000 description 14
- 238000011049 filling Methods 0.000 description 12
- CPELXLSAUQHCOX-UHFFFAOYSA-M Bromide Chemical compound [Br-] CPELXLSAUQHCOX-UHFFFAOYSA-M 0.000 description 10
- 229940124748 beta 2 agonist Drugs 0.000 description 10
- 229940112141 dry powder inhaler Drugs 0.000 description 10
- 239000004615 ingredient Substances 0.000 description 9
- 239000000546 pharmaceutical excipient Substances 0.000 description 9
- 230000008569 process Effects 0.000 description 9
- 230000008901 benefit Effects 0.000 description 8
- 229940071648 metered dose inhaler Drugs 0.000 description 7
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 6
- 229940121948 Muscarinic receptor antagonist Drugs 0.000 description 5
- 239000000812 cholinergic antagonist Substances 0.000 description 5
- 229960002714 fluticasone Drugs 0.000 description 5
- MGNNYOODZCAHBA-GQKYHHCASA-N fluticasone Chemical compound C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@]1(F)[C@@H]2[C@@H]2C[C@@H](C)[C@@](C(=O)SCF)(O)[C@@]2(C)C[C@@H]1O MGNNYOODZCAHBA-GQKYHHCASA-N 0.000 description 5
- 229940125369 inhaled corticosteroids Drugs 0.000 description 5
- 229940127212 long-acting beta 2 agonist Drugs 0.000 description 5
- 210000004072 lung Anatomy 0.000 description 5
- YYAZJTUGSQOFHG-IAVNQIGZSA-N [(6s,8s,10s,11s,13s,14s,16r,17r)-6,9-difluoro-17-(fluoromethylsulfanylcarbonyl)-11-hydroxy-10,13,16-trimethyl-3-oxo-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-17-yl] propanoate;2-(hydroxymethyl)-4-[1-hydroxy-2-[6-(4-phenylbutoxy)hexylamino]eth Chemical compound C1=C(O)C(CO)=CC(C(O)CNCCCCCCOCCCCC=2C=CC=CC=2)=C1.C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)C1(F)[C@@H]2[C@@H]2C[C@@H](C)[C@@](C(=O)SCF)(OC(=O)CC)[C@@]2(C)C[C@@H]1O YYAZJTUGSQOFHG-IAVNQIGZSA-N 0.000 description 4
- 230000001078 anti-cholinergic effect Effects 0.000 description 4
- 239000003246 corticosteroid Substances 0.000 description 4
- 230000000694 effects Effects 0.000 description 4
- 206010061218 Inflammation Diseases 0.000 description 3
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 3
- 230000003182 bronchodilatating effect Effects 0.000 description 3
- 230000008602 contraction Effects 0.000 description 3
- 230000004054 inflammatory process Effects 0.000 description 3
- 238000010298 pulverizing process Methods 0.000 description 3
- 150000003839 salts Chemical class 0.000 description 3
- 239000000243 solution Substances 0.000 description 3
- 239000012453 solvate Substances 0.000 description 3
- 150000003431 steroids Chemical class 0.000 description 3
- 208000024891 symptom Diseases 0.000 description 3
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 description 2
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 2
- 241000950638 Symphysodon discus Species 0.000 description 2
- 230000009471 action Effects 0.000 description 2
- 238000005054 agglomeration Methods 0.000 description 2
- 230000002776 aggregation Effects 0.000 description 2
- 238000004458 analytical method Methods 0.000 description 2
- 229940124630 bronchodilator Drugs 0.000 description 2
- 239000003795 chemical substances by application Substances 0.000 description 2
- 238000002648 combination therapy Methods 0.000 description 2
- 229960001334 corticosteroids Drugs 0.000 description 2
- 201000010099 disease Diseases 0.000 description 2
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 2
- 239000006185 dispersion Substances 0.000 description 2
- 238000002347 injection Methods 0.000 description 2
- 239000007924 injection Substances 0.000 description 2
- 229960001361 ipratropium bromide Drugs 0.000 description 2
- KEWHKYJURDBRMN-ZEODDXGYSA-M ipratropium bromide hydrate Chemical compound O.[Br-].O([C@H]1C[C@H]2CC[C@@H](C1)[N@@+]2(C)C(C)C)C(=O)C(CO)C1=CC=CC=C1 KEWHKYJURDBRMN-ZEODDXGYSA-M 0.000 description 2
- HOQADATXFBOEGG-UHFFFAOYSA-N isofenphos Chemical compound CCOP(=S)(NC(C)C)OC1=CC=CC=C1C(=O)OC(C)C HOQADATXFBOEGG-UHFFFAOYSA-N 0.000 description 2
- 210000000056 organ Anatomy 0.000 description 2
- 230000000144 pharmacologic effect Effects 0.000 description 2
- 239000003380 propellant Substances 0.000 description 2
- 238000011160 research Methods 0.000 description 2
- 229940021597 salmeterol and fluticasone Drugs 0.000 description 2
- 238000012216 screening Methods 0.000 description 2
- 239000000741 silica gel Substances 0.000 description 2
- 229910002027 silica gel Inorganic materials 0.000 description 2
- 238000003860 storage Methods 0.000 description 2
- 230000002459 sustained effect Effects 0.000 description 2
- 230000001225 therapeutic effect Effects 0.000 description 2
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 2
- XWTYSIMOBUGWOL-UHFFFAOYSA-N (+-)-Terbutaline Chemical compound CC(C)(C)NCC(O)C1=CC(O)=CC(O)=C1 XWTYSIMOBUGWOL-UHFFFAOYSA-N 0.000 description 1
- NDAUXUAQIAJITI-LBPRGKRZSA-N (R)-salbutamol Chemical compound CC(C)(C)NC[C@H](O)C1=CC=C(O)C(CO)=C1 NDAUXUAQIAJITI-LBPRGKRZSA-N 0.000 description 1
- OWEGMIWEEQEYGQ-UHFFFAOYSA-N 100676-05-9 Natural products OC1C(O)C(O)C(CO)OC1OCC1C(O)C(O)C(O)C(OC2C(OC(O)C(O)C2O)CO)O1 OWEGMIWEEQEYGQ-UHFFFAOYSA-N 0.000 description 1
- YREYLAVBNPACJM-UHFFFAOYSA-N 2-(tert-butylamino)-1-(2-chlorophenyl)ethanol Chemical compound CC(C)(C)NCC(O)C1=CC=CC=C1Cl YREYLAVBNPACJM-UHFFFAOYSA-N 0.000 description 1
- LSLYOANBFKQKPT-DIFFPNOSSA-N 5-[(1r)-1-hydroxy-2-[[(2r)-1-(4-hydroxyphenyl)propan-2-yl]amino]ethyl]benzene-1,3-diol Chemical compound C([C@@H](C)NC[C@H](O)C=1C=C(O)C=C(O)C=1)C1=CC=C(O)C=C1 LSLYOANBFKQKPT-DIFFPNOSSA-N 0.000 description 1
- HBAQYPYDRFILMT-UHFFFAOYSA-N 8-[3-(1-cyclopropylpyrazol-4-yl)-1H-pyrazolo[4,3-d]pyrimidin-5-yl]-3-methyl-3,8-diazabicyclo[3.2.1]octan-2-one Chemical class C1(CC1)N1N=CC(=C1)C1=NNC2=C1N=C(N=C2)N1C2C(N(CC1CC2)C)=O HBAQYPYDRFILMT-UHFFFAOYSA-N 0.000 description 1
- USFZMSVCRYTOJT-UHFFFAOYSA-N Ammonium acetate Chemical compound N.CC(O)=O USFZMSVCRYTOJT-UHFFFAOYSA-N 0.000 description 1
- 239000005695 Ammonium acetate Substances 0.000 description 1
- VOVIALXJUBGFJZ-KWVAZRHASA-N Budesonide Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@@H]2[C@@H]1[C@@H]1C[C@H]3OC(CCC)O[C@@]3(C(=O)CO)[C@@]1(C)C[C@@H]2O VOVIALXJUBGFJZ-KWVAZRHASA-N 0.000 description 1
- 206010011732 Cyst Diseases 0.000 description 1
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 1
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 1
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 description 1
- 229920002307 Dextran Polymers 0.000 description 1
- 229920001353 Dextrin Polymers 0.000 description 1
- 239000004375 Dextrin Substances 0.000 description 1
- 208000000059 Dyspnea Diseases 0.000 description 1
- 206010013975 Dyspnoeas Diseases 0.000 description 1
- BDAGIHXWWSANSR-UHFFFAOYSA-M Formate Chemical compound [O-]C=O BDAGIHXWWSANSR-UHFFFAOYSA-M 0.000 description 1
- 108010010803 Gelatin Proteins 0.000 description 1
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 1
- GUBGYTABKSRVRQ-PICCSMPSSA-N Maltose Natural products O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@@H](CO)OC(O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-PICCSMPSSA-N 0.000 description 1
- 229930195725 Mannitol Natural products 0.000 description 1
- CBENFWSGALASAD-UHFFFAOYSA-N Ozone Chemical compound [O-][O+]=O CBENFWSGALASAD-UHFFFAOYSA-N 0.000 description 1
- 206010038687 Respiratory distress Diseases 0.000 description 1
- 229920002472 Starch Polymers 0.000 description 1
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 1
- 229930006000 Sucrose Natural products 0.000 description 1
- TVXBFESIOXBWNM-UHFFFAOYSA-N Xylitol Natural products OCCC(O)C(O)C(O)CCO TVXBFESIOXBWNM-UHFFFAOYSA-N 0.000 description 1
- 238000002835 absorbance Methods 0.000 description 1
- 230000009798 acute exacerbation Effects 0.000 description 1
- 239000000853 adhesive Substances 0.000 description 1
- 230000001070 adhesive effect Effects 0.000 description 1
- 239000000048 adrenergic agonist Substances 0.000 description 1
- 229940126157 adrenergic receptor agonist Drugs 0.000 description 1
- 239000000443 aerosol Substances 0.000 description 1
- 229940008126 aerosol Drugs 0.000 description 1
- NDAUXUAQIAJITI-UHFFFAOYSA-N albuterol Chemical compound CC(C)(C)NCC(O)C1=CC=C(O)C(CO)=C1 NDAUXUAQIAJITI-UHFFFAOYSA-N 0.000 description 1
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 1
- 229910052782 aluminium Inorganic materials 0.000 description 1
- 229910000147 aluminium phosphate Inorganic materials 0.000 description 1
- 229940043376 ammonium acetate Drugs 0.000 description 1
- 235000019257 ammonium acetate Nutrition 0.000 description 1
- 230000003110 anti-inflammatory effect Effects 0.000 description 1
- PYMYPHUHKUWMLA-WDCZJNDASA-N arabinose Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)C=O PYMYPHUHKUWMLA-WDCZJNDASA-N 0.000 description 1
- PYMYPHUHKUWMLA-UHFFFAOYSA-N arabinose Natural products OCC(O)C(O)C(O)C=O PYMYPHUHKUWMLA-UHFFFAOYSA-N 0.000 description 1
- 238000003556 assay Methods 0.000 description 1
- 229940092705 beclomethasone Drugs 0.000 description 1
- NBMKJKDGKREAPL-DVTGEIKXSA-N beclomethasone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(Cl)[C@@H]1[C@@H]1C[C@H](C)[C@@](C(=O)CO)(O)[C@@]1(C)C[C@@H]2O NBMKJKDGKREAPL-DVTGEIKXSA-N 0.000 description 1
- 102000016966 beta-2 Adrenergic Receptors Human genes 0.000 description 1
- 108010014499 beta-2 Adrenergic Receptors Proteins 0.000 description 1
- SRBFZHDQGSBBOR-UHFFFAOYSA-N beta-D-Pyranose-Lyxose Natural products OC1COC(O)C(O)C1O SRBFZHDQGSBBOR-UHFFFAOYSA-N 0.000 description 1
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 1
- GUBGYTABKSRVRQ-QUYVBRFLSA-N beta-maltose Chemical compound OC[C@H]1O[C@H](O[C@H]2[C@H](O)[C@@H](O)[C@H](O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@@H]1O GUBGYTABKSRVRQ-QUYVBRFLSA-N 0.000 description 1
- 210000000621 bronchi Anatomy 0.000 description 1
- 230000007883 bronchodilation Effects 0.000 description 1
- 239000000168 bronchodilator agent Substances 0.000 description 1
- 229960004436 budesonide Drugs 0.000 description 1
- 238000004364 calculation method Methods 0.000 description 1
- 239000007963 capsule composition Substances 0.000 description 1
- 230000000295 complement effect Effects 0.000 description 1
- 208000031513 cyst Diseases 0.000 description 1
- 230000007547 defect Effects 0.000 description 1
- 230000006866 deterioration Effects 0.000 description 1
- 235000019425 dextrin Nutrition 0.000 description 1
- 238000002845 discoloration Methods 0.000 description 1
- -1 discoloration Substances 0.000 description 1
- 230000005611 electricity Effects 0.000 description 1
- 230000007613 environmental effect Effects 0.000 description 1
- 238000011156 evaluation Methods 0.000 description 1
- 230000005713 exacerbation Effects 0.000 description 1
- 229960001022 fenoterol Drugs 0.000 description 1
- 238000001914 filtration Methods 0.000 description 1
- 229960000676 flunisolide Drugs 0.000 description 1
- 229940044170 formate Drugs 0.000 description 1
- 229960002848 formoterol Drugs 0.000 description 1
- BPZSYCZIITTYBL-UHFFFAOYSA-N formoterol Chemical compound C1=CC(OC)=CC=C1CC(C)NCC(O)C1=CC=C(O)C(NC=O)=C1 BPZSYCZIITTYBL-UHFFFAOYSA-N 0.000 description 1
- 239000008273 gelatin Substances 0.000 description 1
- 229920000159 gelatin Polymers 0.000 description 1
- 235000019322 gelatine Nutrition 0.000 description 1
- 235000011852 gelatine desserts Nutrition 0.000 description 1
- 238000012812 general test Methods 0.000 description 1
- 239000008103 glucose Substances 0.000 description 1
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 1
- 239000001866 hydroxypropyl methyl cellulose Substances 0.000 description 1
- 235000010979 hydroxypropyl methyl cellulose Nutrition 0.000 description 1
- 229920003088 hydroxypropyl methyl cellulose Polymers 0.000 description 1
- 229960003943 hypromellose Drugs 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 229960004078 indacaterol Drugs 0.000 description 1
- QZZUEBNBZAPZLX-QFIPXVFZSA-N indacaterol Chemical compound N1C(=O)C=CC2=C1C(O)=CC=C2[C@@H](O)CNC1CC(C=C(C(=C2)CC)CC)=C2C1 QZZUEBNBZAPZLX-QFIPXVFZSA-N 0.000 description 1
- 230000003434 inspiratory effect Effects 0.000 description 1
- 230000001788 irregular Effects 0.000 description 1
- 229950008204 levosalbutamol Drugs 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 238000004811 liquid chromatography Methods 0.000 description 1
- 230000007774 longterm Effects 0.000 description 1
- 239000000594 mannitol Substances 0.000 description 1
- 235000010355 mannitol Nutrition 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- HEBKCHPVOIAQTA-UHFFFAOYSA-N meso ribitol Natural products OCC(O)C(O)C(O)CO HEBKCHPVOIAQTA-UHFFFAOYSA-N 0.000 description 1
- 239000011259 mixed solution Substances 0.000 description 1
- 229960002744 mometasone furoate Drugs 0.000 description 1
- WOFMFGQZHJDGCX-ZULDAHANSA-N mometasone furoate Chemical compound O([C@]1([C@@]2(C)C[C@H](O)[C@]3(Cl)[C@@]4(C)C=CC(=O)C=C4CC[C@H]3[C@@H]2C[C@H]1C)C(=O)CCl)C(=O)C1=CC=CO1 WOFMFGQZHJDGCX-ZULDAHANSA-N 0.000 description 1
- 210000003097 mucus Anatomy 0.000 description 1
- 229940097496 nasal spray Drugs 0.000 description 1
- 239000007922 nasal spray Substances 0.000 description 1
- NVOYVOBDTVTBDX-PMEUIYRNSA-N oxitropium Chemical compound CC[N+]1(C)[C@H]2C[C@@H](C[C@@H]1[C@H]1O[C@@H]21)OC(=O)[C@H](CO)C1=CC=CC=C1 NVOYVOBDTVTBDX-PMEUIYRNSA-N 0.000 description 1
- 229960000797 oxitropium Drugs 0.000 description 1
- 238000004806 packaging method and process Methods 0.000 description 1
- 230000002085 persistent effect Effects 0.000 description 1
- 230000000704 physical effect Effects 0.000 description 1
- 229940002612 prodrug Drugs 0.000 description 1
- 239000000651 prodrug Substances 0.000 description 1
- 230000002685 pulmonary effect Effects 0.000 description 1
- 238000004080 punching Methods 0.000 description 1
- MIXMJCQRHVAJIO-TZHJZOAOSA-N qk4dys664x Chemical compound O.C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@@H]1[C@@H]2[C@@H]2C[C@H]3OC(C)(C)O[C@@]3(C(=O)CO)[C@@]2(C)C[C@@H]1O.C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@@H]1[C@@H]2[C@@H]2C[C@H]3OC(C)(C)O[C@@]3(C(=O)CO)[C@@]2(C)C[C@@H]1O MIXMJCQRHVAJIO-TZHJZOAOSA-N 0.000 description 1
- 230000000241 respiratory effect Effects 0.000 description 1
- 210000002345 respiratory system Anatomy 0.000 description 1
- 230000004044 response Effects 0.000 description 1
- 230000000284 resting effect Effects 0.000 description 1
- 229960002052 salbutamol Drugs 0.000 description 1
- 230000028327 secretion Effects 0.000 description 1
- 208000013220 shortness of breath Diseases 0.000 description 1
- XWZCREJRXRKIRQ-UHFFFAOYSA-M sodium;heptane-1-sulfonate;hydrate Chemical compound O.[Na+].CCCCCCCS([O-])(=O)=O XWZCREJRXRKIRQ-UHFFFAOYSA-M 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 239000002904 solvent Substances 0.000 description 1
- 239000000600 sorbitol Substances 0.000 description 1
- 235000010356 sorbitol Nutrition 0.000 description 1
- 238000005507 spraying Methods 0.000 description 1
- 229910001220 stainless steel Inorganic materials 0.000 description 1
- 239000010935 stainless steel Substances 0.000 description 1
- 239000008107 starch Substances 0.000 description 1
- 235000019698 starch Nutrition 0.000 description 1
- 230000003068 static effect Effects 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 239000005720 sucrose Substances 0.000 description 1
- 230000008685 targeting Effects 0.000 description 1
- 229960000195 terbutaline Drugs 0.000 description 1
- 229940124597 therapeutic agent Drugs 0.000 description 1
- 239000010409 thin film Substances 0.000 description 1
- 230000032258 transport Effects 0.000 description 1
- 229960005294 triamcinolone Drugs 0.000 description 1
- GFNANZIMVAIWHM-OBYCQNJPSA-N triamcinolone Chemical compound O=C1C=C[C@]2(C)[C@@]3(F)[C@@H](O)C[C@](C)([C@@]([C@H](O)C4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 GFNANZIMVAIWHM-OBYCQNJPSA-N 0.000 description 1
- 229960000859 tulobuterol Drugs 0.000 description 1
- 238000010792 warming Methods 0.000 description 1
- 239000000811 xylitol Substances 0.000 description 1
- 235000010447 xylitol Nutrition 0.000 description 1
- HEBKCHPVOIAQTA-SCDXWVJYSA-N xylitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)CO HEBKCHPVOIAQTA-SCDXWVJYSA-N 0.000 description 1
- 229960002675 xylitol Drugs 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/56—Compounds containing cyclopenta[a]hydrophenanthrene ring systems; Derivatives thereof, e.g. steroids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/13—Amines
- A61K31/135—Amines having aromatic rings, e.g. ketamine, nortriptyline
- A61K31/136—Amines having aromatic rings, e.g. ketamine, nortriptyline having the amino group directly attached to the aromatic ring, e.g. benzeneamine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/13—Amines
- A61K31/135—Amines having aromatic rings, e.g. ketamine, nortriptyline
- A61K31/137—Arylalkylamines, e.g. amphetamine, epinephrine, salbutamol, ephedrine or methadone
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/16—Amides, e.g. hydroxamic acids
- A61K31/165—Amides, e.g. hydroxamic acids having aromatic rings, e.g. colchicine, atenolol, progabide
- A61K31/166—Amides, e.g. hydroxamic acids having aromatic rings, e.g. colchicine, atenolol, progabide having the carbon of a carboxamide group directly attached to the aromatic ring, e.g. procainamide, procarbazine, metoclopramide, labetalol
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/439—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom the ring forming part of a bridged ring system, e.g. quinuclidine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/46—8-Azabicyclo [3.2.1] octane; Derivatives thereof, e.g. atropine, cocaine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/56—Compounds containing cyclopenta[a]hydrophenanthrene ring systems; Derivatives thereof, e.g. steroids
- A61K31/565—Compounds containing cyclopenta[a]hydrophenanthrene ring systems; Derivatives thereof, e.g. steroids not substituted in position 17 beta by a carbon atom, e.g. estrane, estradiol
- A61K31/568—Compounds containing cyclopenta[a]hydrophenanthrene ring systems; Derivatives thereof, e.g. steroids not substituted in position 17 beta by a carbon atom, e.g. estrane, estradiol substituted in positions 10 and 13 by a chain having at least one carbon atom, e.g. androstanes, e.g. testosterone
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/06—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite
- A61K47/26—Carbohydrates, e.g. sugar alcohols, amino sugars, nucleic acids, mono-, di- or oligo-saccharides; Derivatives thereof, e.g. polysorbates, sorbitan fatty acid esters or glycyrrhizin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/007—Pulmonary tract; Aromatherapy
- A61K9/0073—Sprays or powders for inhalation; Aerolised or nebulised preparations generated by other means than thermal energy
- A61K9/0075—Sprays or powders for inhalation; Aerolised or nebulised preparations generated by other means than thermal energy for inhalation via a dry powder inhaler [DPI], e.g. comprising micronized drug mixed with lactose carrier particles
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
- A61K9/141—Intimate drug-carrier mixtures characterised by the carrier, e.g. ordered mixtures, adsorbates, solid solutions, eutectica, co-dried, co-solubilised, co-kneaded, co-milled, co-ground products, co-precipitates, co-evaporates, co-extrudates, co-melts; Drug nanoparticles with adsorbed surface modifiers
- A61K9/145—Intimate drug-carrier mixtures characterised by the carrier, e.g. ordered mixtures, adsorbates, solid solutions, eutectica, co-dried, co-solubilised, co-kneaded, co-milled, co-ground products, co-precipitates, co-evaporates, co-extrudates, co-melts; Drug nanoparticles with adsorbed surface modifiers with organic compounds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
- A61K9/16—Agglomerates; Granulates; Microbeadlets ; Microspheres; Pellets; Solid products obtained by spray drying, spray freeze drying, spray congealing,(multiple) emulsion solvent evaporation or extraction
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
- A61P11/06—Antiasthmatics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
- A61P11/08—Bronchodilators
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Veterinary Medicine (AREA)
- Pharmacology & Pharmacy (AREA)
- Medicinal Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Epidemiology (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Pulmonology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Emergency Medicine (AREA)
- Otolaryngology (AREA)
- Biochemistry (AREA)
- Molecular Biology (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Pain & Pain Management (AREA)
- Rheumatology (AREA)
- Medicinal Preparation (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Priority Applications (20)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR1020120063665A KR20130140358A (ko) | 2012-06-14 | 2012-06-14 | 살메테롤 지나포산염, 플루티카손 프로피오네이트 및 티오트로피움 브로마이드를 포함하는 흡입 제형용 건조 분말 및 이의 제조방법 |
| HK15107131.8A HK1206602A1 (en) | 2012-06-14 | 2013-06-03 | Dry powder for inhalation formulation comprising salmeterol xinafoate, fluticasone propionate and tiotropium bromide, and method for preparing same |
| CA2876283A CA2876283A1 (en) | 2012-06-14 | 2013-06-03 | Dry powder for inhalation formulation comprising salmeterol xinafoate, fluticasone propionate and tiotropium bromide, and method for preparing same |
| RU2015100905A RU2015100905A (ru) | 2012-06-14 | 2013-06-03 | Сухой порошок для ингаляционного препарата, включающий сальметерола ксинафоат, флутиказона пропионат и тиотропия бромид, а также способ его изготовления |
| BR112014030910A BR112014030910A2 (pt) | 2012-06-14 | 2013-06-03 | pó seco para formulação de inalação compreendendo xinafoato de salmeterol, propionato de fluticasona e brometo de tiotrópio e método para preparar o mesmo |
| IN11257DEN2014 IN2014DN11257A (enExample) | 2012-06-14 | 2013-06-03 | |
| EP13804439.1A EP2861219A4 (en) | 2012-06-14 | 2013-06-03 | INHALED PREPARATION DRY POWDER COMPRISING SALMETEROL XINAFOATE, FLUTICASONE PROPIONATE AND TIOTROPIUM BROMIDE, AND PREPARATION METHOD THEREOF |
| MX2014014755A MX2014014755A (es) | 2012-06-14 | 2013-06-03 | Polvo seco para formulacion para inhalacion que comprende xinafoato de salmeterol, propionato de fluticasona y bromuro de tiotropio, y metodo para su preparacion. |
| SG11201408292YA SG11201408292YA (en) | 2012-06-14 | 2013-06-03 | Dry powder for inhalation formulation comprising salmeterol xinafoate, fluticasone propionate and tiotropium bromide, and method for preparing same |
| AU2013275113A AU2013275113A1 (en) | 2012-06-14 | 2013-06-03 | Dry powder for inhalation formulation comprising salmeterol xinafoate, fluticasone propionate and tiotropium bromide, and method for preparing same |
| PCT/KR2013/004880 WO2013187626A1 (en) | 2012-06-14 | 2013-06-03 | Dry powder for inhalation formulation comprising salmeterol xinafoate, fluticasone propionate and tiotropium bromide, and method for preparing same |
| US14/402,548 US9283232B2 (en) | 2012-06-14 | 2013-06-03 | Dry powder for inhalation formulation comprising salmeterol xinafoate, fluticasone propionate and tiotropium bromide, and method for preparing same |
| NZ703788A NZ703788A (en) | 2012-06-14 | 2013-06-03 | Dry powder for inhalation formulation comprising salmeterol xinafoate, fluticasone propionate and tiotropium bromide, and method for preparing same |
| JP2015517171A JP2015519394A (ja) | 2012-06-14 | 2013-06-03 | キシナホ酸サルメテロール、プロピオン酸フルチカゾンおよび臭化チオトロピウムを含む吸入製剤用ドライパウダー、ならびにその製造方法 |
| CN201380030898.0A CN104363895A (zh) | 2012-06-14 | 2013-06-03 | 用于吸入制剂的包含昔萘酸沙美特罗、丙酸氟替卡松和噻托溴铵的干粉及其制备方法 |
| TW102120909A TWI565481B (zh) | 2012-06-14 | 2013-06-13 | 包含沙美特羅羥萘甲酸鹽、弗提卡松丙酸鹽及托溴銨溴化物之吸入型調配物的乾粉末以及其製備方法 |
| PH12014502746A PH12014502746B1 (en) | 2012-06-14 | 2014-12-09 | Dry powder for inhalation formulation comprising salmeterol xinafoate, fluticasone propionate and tiotropium bromide, and method for preparing same |
| IL236198A IL236198A0 (en) | 2012-06-14 | 2014-12-11 | Dry powder for inhalation preparation containing salmatrol zinafoate, fluticasone propionate and tiotropium bromide, and its production method |
| ZA2015/00208A ZA201500208B (en) | 2012-06-14 | 2015-01-13 | Dry powder for inhalation formulation comprising salmeterol xinafoate, fluticasone propionate and tiotropium bromide, and method for preparing same |
| US14/746,225 US9549936B2 (en) | 2012-06-14 | 2015-06-22 | Method for preparing dry powder for inhalation formulation comprising salmeterol xinafoate, fluticasone propionate and tiotropium bromide |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR1020120063665A KR20130140358A (ko) | 2012-06-14 | 2012-06-14 | 살메테롤 지나포산염, 플루티카손 프로피오네이트 및 티오트로피움 브로마이드를 포함하는 흡입 제형용 건조 분말 및 이의 제조방법 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20130140358A true KR20130140358A (ko) | 2013-12-24 |
Family
ID=49758405
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020120063665A Ceased KR20130140358A (ko) | 2012-06-14 | 2012-06-14 | 살메테롤 지나포산염, 플루티카손 프로피오네이트 및 티오트로피움 브로마이드를 포함하는 흡입 제형용 건조 분말 및 이의 제조방법 |
Country Status (19)
| Country | Link |
|---|---|
| US (2) | US9283232B2 (enExample) |
| EP (1) | EP2861219A4 (enExample) |
| JP (1) | JP2015519394A (enExample) |
| KR (1) | KR20130140358A (enExample) |
| CN (1) | CN104363895A (enExample) |
| AU (1) | AU2013275113A1 (enExample) |
| BR (1) | BR112014030910A2 (enExample) |
| CA (1) | CA2876283A1 (enExample) |
| HK (1) | HK1206602A1 (enExample) |
| IL (1) | IL236198A0 (enExample) |
| IN (1) | IN2014DN11257A (enExample) |
| MX (1) | MX2014014755A (enExample) |
| NZ (1) | NZ703788A (enExample) |
| PH (1) | PH12014502746B1 (enExample) |
| RU (1) | RU2015100905A (enExample) |
| SG (1) | SG11201408292YA (enExample) |
| TW (1) | TWI565481B (enExample) |
| WO (1) | WO2013187626A1 (enExample) |
| ZA (1) | ZA201500208B (enExample) |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2016159542A1 (en) * | 2015-03-31 | 2016-10-06 | Hanmi Pharm. Co., Ltd. | Capsule for inhalation with improved stability of combined active ingredients |
| KR20200078162A (ko) * | 2018-12-21 | 2020-07-01 | 한미약품 주식회사 | 흡입 제형용 건조 분말 및 이의 제조방법 |
| KR20210044336A (ko) * | 2019-10-14 | 2021-04-23 | 한미약품 주식회사 | 흡입 제형용 건조 분말 및 이의 제조방법 |
Families Citing this family (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9360400B2 (en) | 2014-06-30 | 2016-06-07 | Proveris Scientific Corporation | Sampling apparatus for determining the amount and uniformity of a delivered dose of drug and related methods |
| WO2016052897A1 (en) * | 2014-09-30 | 2016-04-07 | Hanmi Pharm. Co., Ltd. | Dry powder for inhalation formulation having improved stability of combined active ingredients |
| MA41378A (fr) * | 2015-01-20 | 2017-11-28 | Teva Branded Pharmaceutical Prod R & D Inc | Inhalateur de poudre sèche comprenant du propionate de fluticasone et du xinafoate de salmétérol |
| WO2016178704A1 (en) * | 2015-05-01 | 2016-11-10 | Board Of Regents, The University Of Texas System | Multidrug brittle matrix compositions |
| WO2017156287A1 (en) * | 2016-03-09 | 2017-09-14 | Proveris Scientific Corporation | Methods for measuring dose content uniformity performance of inhaler and nasal devices |
| CN108066329B (zh) * | 2016-11-11 | 2021-11-16 | 江苏恒瑞医药股份有限公司 | 一种吸入用氟替卡松或其衍生物的微粒的制备方法 |
| US11774363B2 (en) * | 2018-08-07 | 2023-10-03 | Norton (Waterford) Limited | Application of raman spectroscopy for the manufacture of inhalation powders |
| CN116033893A (zh) * | 2020-06-26 | 2023-04-28 | 迈兰制药英国有限公司 | 包含5-[3-(3-羟基苯氧基)氮杂环丁烷-1-基]-5-甲基-2,2-二苯基己酰胺的制剂 |
| CN115266987B (zh) * | 2022-07-31 | 2023-06-30 | 浙江知一药业有限责任公司 | 治疗呼吸系统疾病的药物组合物 |
| CN115792071B (zh) * | 2022-12-01 | 2025-02-18 | 南京联智医药科技有限公司 | 一种噻格溴铵中间体的有关物质分析方法 |
Family Cites Families (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB9322014D0 (en) | 1993-10-26 | 1993-12-15 | Co Ordinated Drug Dev | Improvements in and relating to carrier particles for use in dry powder inhalers |
| GB0003935D0 (en) * | 2000-02-08 | 2000-04-12 | King S College London | Formulation for dry powder inhaler |
| DE10130371A1 (de) | 2001-06-23 | 2003-01-02 | Boehringer Ingelheim Pharma | Neue Arzneimittelkompositionen auf der Basis von Anticholinergika, Corticosteroiden und Betamimetika |
| GB0312148D0 (en) | 2003-05-28 | 2003-07-02 | Aventis Pharma Ltd | Stabilized pharmaceutical products |
| SE527190C2 (sv) | 2003-06-19 | 2006-01-17 | Microdrug Ag | Inhalatoranordning samt kombinerade doser av en beta2-agonist, ett antikolinergiskt medel och ett antiinflammatorisk steroid |
| GB0326632D0 (en) | 2003-11-14 | 2003-12-17 | Jagotec Ag | Dry powder formulations |
| JP2010519195A (ja) * | 2007-02-19 | 2010-06-03 | シプラ・リミテッド | 薬学的組成物 |
| KR20100063116A (ko) * | 2007-09-12 | 2010-06-10 | 글락소 그룹 리미티드 | 치료제들의 복합제 |
| WO2010007446A1 (en) * | 2008-07-18 | 2010-01-21 | Prosonix Limited | Process for improving crystallinity of fluticasone particles |
| AU2009276498A1 (en) * | 2008-07-30 | 2010-02-04 | Stc.Unm | Formulations containing large-size carrier particles for dry powder inhalation aerosols |
| TR201000681A2 (tr) | 2010-01-29 | 2011-08-22 | B�Lg�� Mahmut | İnhalasyon yoluyla alınan kuru toz formülasyonları. |
-
2012
- 2012-06-14 KR KR1020120063665A patent/KR20130140358A/ko not_active Ceased
-
2013
- 2013-06-03 CN CN201380030898.0A patent/CN104363895A/zh active Pending
- 2013-06-03 AU AU2013275113A patent/AU2013275113A1/en not_active Abandoned
- 2013-06-03 MX MX2014014755A patent/MX2014014755A/es unknown
- 2013-06-03 CA CA2876283A patent/CA2876283A1/en not_active Abandoned
- 2013-06-03 EP EP13804439.1A patent/EP2861219A4/en not_active Withdrawn
- 2013-06-03 BR BR112014030910A patent/BR112014030910A2/pt not_active IP Right Cessation
- 2013-06-03 NZ NZ703788A patent/NZ703788A/en not_active IP Right Cessation
- 2013-06-03 HK HK15107131.8A patent/HK1206602A1/xx unknown
- 2013-06-03 JP JP2015517171A patent/JP2015519394A/ja active Pending
- 2013-06-03 WO PCT/KR2013/004880 patent/WO2013187626A1/en not_active Ceased
- 2013-06-03 SG SG11201408292YA patent/SG11201408292YA/en unknown
- 2013-06-03 US US14/402,548 patent/US9283232B2/en not_active Expired - Fee Related
- 2013-06-03 IN IN11257DEN2014 patent/IN2014DN11257A/en unknown
- 2013-06-03 RU RU2015100905A patent/RU2015100905A/ru not_active Application Discontinuation
- 2013-06-13 TW TW102120909A patent/TWI565481B/zh not_active IP Right Cessation
-
2014
- 2014-12-09 PH PH12014502746A patent/PH12014502746B1/en unknown
- 2014-12-11 IL IL236198A patent/IL236198A0/en unknown
-
2015
- 2015-01-13 ZA ZA2015/00208A patent/ZA201500208B/en unknown
- 2015-06-22 US US14/746,225 patent/US9549936B2/en not_active Expired - Fee Related
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2016159542A1 (en) * | 2015-03-31 | 2016-10-06 | Hanmi Pharm. Co., Ltd. | Capsule for inhalation with improved stability of combined active ingredients |
| KR20160117069A (ko) | 2015-03-31 | 2016-10-10 | 한미약품 주식회사 | 복합 활성성분의 안정성이 개선된 흡입용 캡슐제 |
| KR20200078162A (ko) * | 2018-12-21 | 2020-07-01 | 한미약품 주식회사 | 흡입 제형용 건조 분말 및 이의 제조방법 |
| KR20210044336A (ko) * | 2019-10-14 | 2021-04-23 | 한미약품 주식회사 | 흡입 제형용 건조 분말 및 이의 제조방법 |
Also Published As
| Publication number | Publication date |
|---|---|
| AU2013275113A1 (en) | 2015-02-05 |
| TW201402154A (zh) | 2014-01-16 |
| IN2014DN11257A (enExample) | 2015-10-09 |
| US20150157566A1 (en) | 2015-06-11 |
| HK1206602A1 (en) | 2016-01-15 |
| US9283232B2 (en) | 2016-03-15 |
| SG11201408292YA (en) | 2015-01-29 |
| ZA201500208B (en) | 2016-10-26 |
| BR112014030910A2 (pt) | 2017-06-27 |
| NZ703788A (en) | 2016-10-28 |
| RU2015100905A (ru) | 2016-08-10 |
| US9549936B2 (en) | 2017-01-24 |
| CN104363895A (zh) | 2015-02-18 |
| TWI565481B (zh) | 2017-01-11 |
| PH12014502746A1 (en) | 2015-02-02 |
| IL236198A0 (en) | 2015-01-29 |
| US20150283151A1 (en) | 2015-10-08 |
| WO2013187626A1 (en) | 2013-12-19 |
| CA2876283A1 (en) | 2013-12-19 |
| MX2014014755A (es) | 2015-02-24 |
| PH12014502746B1 (en) | 2015-02-02 |
| EP2861219A4 (en) | 2015-10-28 |
| EP2861219A1 (en) | 2015-04-22 |
| JP2015519394A (ja) | 2015-07-09 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR20130140358A (ko) | 살메테롤 지나포산염, 플루티카손 프로피오네이트 및 티오트로피움 브로마이드를 포함하는 흡입 제형용 건조 분말 및 이의 제조방법 | |
| CN105101955B (zh) | 至少包含两种通过喷雾干燥以增加制剂稳定性而得到的干粉的组合物 | |
| CN104080444B (zh) | 用于吸入给药包含皮质类固醇和beta‑肾上腺素能药物的干粉配制剂 | |
| CN107205936B (zh) | 包含至少一种通过喷雾干燥得到的增加制剂稳定性的干粉的组合物 | |
| CN105338967A (zh) | 包含布地奈德和福莫特罗的药物组合物 | |
| JP5154732B2 (ja) | 薬剤 | |
| RU2823554C1 (ru) | Новые частицы носителя для сухих порошковых составов для ингаляции | |
| EP3203984B1 (en) | Pharmaceutical composition containing budesonide and formoterol. | |
| US12491156B2 (en) | Carrier particles for dry powder formulations for inhalation | |
| KR20180036459A (ko) | 복합 활성성분의 개선된 함량 균일성 및 높은 단위전달량을 갖는 흡입용 캡슐제 및 이의 제조방법 | |
| HK40064779A (en) | Novel carrier particles for dry powder formulations for inhalation | |
| KR20160038767A (ko) | 복합 활성성분의 안정성이 개선된 흡입 제형용 건조분말 | |
| HK1200021B (en) | Dry powder formulation comprising a corticosteroid and a beta-adrenergic for administration by inhalation |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0109 | Patent application |
Patent event code: PA01091R01D Comment text: Patent Application Patent event date: 20120614 |
|
| PG1501 | Laying open of application | ||
| A201 | Request for examination | ||
| PA0201 | Request for examination |
Patent event code: PA02012R01D Patent event date: 20170524 Comment text: Request for Examination of Application Patent event code: PA02011R01I Patent event date: 20120614 Comment text: Patent Application |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20180727 Patent event code: PE09021S01D |
|
| E601 | Decision to refuse application | ||
| PE0601 | Decision on rejection of patent |
Patent event date: 20181018 Comment text: Decision to Refuse Application Patent event code: PE06012S01D Patent event date: 20180727 Comment text: Notification of reason for refusal Patent event code: PE06011S01I |