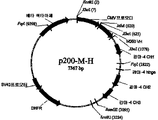
KR20110140143A - 혈관형성을 조절하는 α5β1 인테그린에 대한 키메라성 및 인간화 항체 - Google Patents
혈관형성을 조절하는 α5β1 인테그린에 대한 키메라성 및 인간화 항체 Download PDFInfo
- Publication number
- KR20110140143A KR20110140143A KR1020117029575A KR20117029575A KR20110140143A KR 20110140143 A KR20110140143 A KR 20110140143A KR 1020117029575 A KR1020117029575 A KR 1020117029575A KR 20117029575 A KR20117029575 A KR 20117029575A KR 20110140143 A KR20110140143 A KR 20110140143A
- Authority
- KR
- South Korea
- Prior art keywords
- ser
- antibody
- thr
- leu
- gly
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
- 102000006495 integrins Human genes 0.000 title claims abstract description 93
- 108010044426 integrins Proteins 0.000 title claims abstract description 93
- 230000033115 angiogenesis Effects 0.000 title abstract description 40
- 238000000034 method Methods 0.000 claims abstract description 105
- 108090000765 processed proteins & peptides Proteins 0.000 claims description 59
- 108090000623 proteins and genes Proteins 0.000 claims description 57
- 102000004169 proteins and genes Human genes 0.000 claims description 51
- 102000004196 processed proteins & peptides Human genes 0.000 claims description 46
- 229920001184 polypeptide Polymers 0.000 claims description 40
- 239000011159 matrix material Substances 0.000 claims description 37
- 239000000243 solution Substances 0.000 claims description 18
- 239000000758 substrate Substances 0.000 claims description 14
- 241000700605 Viruses Species 0.000 claims description 8
- 238000010828 elution Methods 0.000 claims description 8
- 102000009109 Fc receptors Human genes 0.000 claims description 4
- 108010087819 Fc receptors Proteins 0.000 claims description 4
- 101710120037 Toxin CcdB Proteins 0.000 claims description 4
- 150000002632 lipids Chemical class 0.000 claims description 4
- 239000012528 membrane Substances 0.000 claims description 4
- 229930186217 Glycolipid Natural products 0.000 claims description 3
- 102000003886 Glycoproteins Human genes 0.000 claims description 3
- 108090000288 Glycoproteins Proteins 0.000 claims description 3
- 102000004856 Lectins Human genes 0.000 claims description 3
- 108090001030 Lipoproteins Proteins 0.000 claims description 3
- 102000004895 Lipoproteins Human genes 0.000 claims description 3
- 108010076039 Polyproteins Proteins 0.000 claims description 3
- 239000003637 basic solution Substances 0.000 claims description 3
- 235000014113 dietary fatty acids Nutrition 0.000 claims description 3
- 229930195729 fatty acid Natural products 0.000 claims description 3
- 239000000194 fatty acid Substances 0.000 claims description 3
- 150000004665 fatty acids Chemical class 0.000 claims description 3
- 150000004676 glycans Chemical class 0.000 claims description 3
- 239000002523 lectin Substances 0.000 claims description 3
- 229920001282 polysaccharide Polymers 0.000 claims description 3
- 239000005017 polysaccharide Substances 0.000 claims description 3
- 108091008104 nucleic acid aptamers Proteins 0.000 claims description 2
- HVCOBJNICQPDBP-UHFFFAOYSA-N 3-[3-[3,5-dihydroxy-6-methyl-4-(3,4,5-trihydroxy-6-methyloxan-2-yl)oxyoxan-2-yl]oxydecanoyloxy]decanoic acid;hydrate Chemical compound O.OC1C(OC(CC(=O)OC(CCCCCCC)CC(O)=O)CCCCCCC)OC(C)C(O)C1OC1C(O)C(O)C(O)C(C)O1 HVCOBJNICQPDBP-UHFFFAOYSA-N 0.000 claims 1
- 108010052006 Mitogen Receptors Proteins 0.000 claims 1
- 230000003472 neutralizing effect Effects 0.000 claims 1
- 239000008194 pharmaceutical composition Substances 0.000 abstract description 13
- 210000001508 eye Anatomy 0.000 description 88
- 150000001413 amino acids Chemical group 0.000 description 83
- 150000007523 nucleic acids Chemical class 0.000 description 82
- 102000039446 nucleic acids Human genes 0.000 description 67
- 108020004707 nucleic acids Proteins 0.000 description 67
- 235000018102 proteins Nutrition 0.000 description 49
- 230000027455 binding Effects 0.000 description 48
- 238000009739 binding Methods 0.000 description 47
- 210000001519 tissue Anatomy 0.000 description 45
- 235000001014 amino acid Nutrition 0.000 description 39
- 229940024606 amino acid Drugs 0.000 description 39
- 210000004027 cell Anatomy 0.000 description 39
- 108020004414 DNA Proteins 0.000 description 38
- 239000000427 antigen Substances 0.000 description 33
- 108091007433 antigens Proteins 0.000 description 33
- 102000036639 antigens Human genes 0.000 description 33
- 108060003951 Immunoglobulin Proteins 0.000 description 29
- 102000018358 immunoglobulin Human genes 0.000 description 29
- 239000013598 vector Substances 0.000 description 26
- 238000011282 treatment Methods 0.000 description 24
- 208000005590 Choroidal Neovascularization Diseases 0.000 description 23
- 206010060823 Choroidal neovascularisation Diseases 0.000 description 23
- 241000880493 Leptailurus serval Species 0.000 description 22
- 241001465754 Metazoa Species 0.000 description 22
- 206010028980 Neoplasm Diseases 0.000 description 21
- 238000012360 testing method Methods 0.000 description 21
- 230000001225 therapeutic effect Effects 0.000 description 21
- 239000013612 plasmid Substances 0.000 description 19
- ANHVRCNNGJMJNG-BZSNNMDCSA-N Tyr-Tyr-Cys Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)N[C@@H](CC2=CC=C(C=C2)O)C(=O)N[C@@H](CS)C(=O)O)N)O ANHVRCNNGJMJNG-BZSNNMDCSA-N 0.000 description 18
- PZTZYZUTCPZWJH-FXQIFTODSA-N Val-Ser-Ser Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)O)N PZTZYZUTCPZWJH-FXQIFTODSA-N 0.000 description 18
- 239000000126 substance Substances 0.000 description 18
- 241000699666 Mus <mouse, genus> Species 0.000 description 17
- 108091028043 Nucleic acid sequence Proteins 0.000 description 16
- 230000000694 effects Effects 0.000 description 16
- 241001529936 Murinae Species 0.000 description 15
- XKUKSGPZAADMRA-UHFFFAOYSA-N glycyl-glycyl-glycine Natural products NCC(=O)NCC(=O)NCC(O)=O XKUKSGPZAADMRA-UHFFFAOYSA-N 0.000 description 15
- 231100000241 scar Toxicity 0.000 description 15
- FULZDMOZUZKGQU-ONGXEEELSA-N Gly-Val-His Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)NC(=O)CN FULZDMOZUZKGQU-ONGXEEELSA-N 0.000 description 14
- 239000012634 fragment Substances 0.000 description 14
- 108091034117 Oligonucleotide Proteins 0.000 description 13
- FQPDRTDDEZXCEC-SVSWQMSJSA-N Thr-Ile-Ser Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CO)C(O)=O FQPDRTDDEZXCEC-SVSWQMSJSA-N 0.000 description 13
- 230000005764 inhibitory process Effects 0.000 description 13
- 239000000203 mixture Substances 0.000 description 13
- 108020004705 Codon Proteins 0.000 description 12
- 238000002965 ELISA Methods 0.000 description 12
- QYSFWUIXDFJUDW-DCAQKATOSA-N Ser-Leu-Arg Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O QYSFWUIXDFJUDW-DCAQKATOSA-N 0.000 description 12
- PYTKULIABVRXSC-BWBBJGPYSA-N Ser-Ser-Thr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(O)=O PYTKULIABVRXSC-BWBBJGPYSA-N 0.000 description 12
- 201000011510 cancer Diseases 0.000 description 12
- 230000021615 conjugation Effects 0.000 description 12
- 229940072221 immunoglobulins Drugs 0.000 description 12
- MSILNNHVVMMTHZ-UWVGGRQHSA-N Arg-His-Gly Chemical compound NC(N)=NCCC[C@H](N)C(=O)N[C@H](C(=O)NCC(O)=O)CC1=CN=CN1 MSILNNHVVMMTHZ-UWVGGRQHSA-N 0.000 description 11
- WHUUTDBJXJRKMK-UHFFFAOYSA-N Glutamic acid Natural products OC(=O)C(N)CCC(O)=O WHUUTDBJXJRKMK-UHFFFAOYSA-N 0.000 description 11
- MIIVFRCYJABHTQ-ONGXEEELSA-N Gly-Leu-Val Chemical compound [H]NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(O)=O MIIVFRCYJABHTQ-ONGXEEELSA-N 0.000 description 11
- PDUHNKAFQXQNLH-ZETCQYMHSA-N Gly-Lys-Gly Chemical compound NCCCC[C@H](NC(=O)CN)C(=O)NCC(O)=O PDUHNKAFQXQNLH-ZETCQYMHSA-N 0.000 description 11
- UWQDKRIZSROAKS-FJXKBIBVSA-N Gly-Met-Thr Chemical compound [H]NCC(=O)N[C@@H](CCSC)C(=O)N[C@@H]([C@@H](C)O)C(O)=O UWQDKRIZSROAKS-FJXKBIBVSA-N 0.000 description 11
- GGAPHLIUUTVYMX-QWRGUYRKSA-N Gly-Phe-Ser Chemical compound OC[C@@H](C([O-])=O)NC(=O)[C@@H](NC(=O)C[NH3+])CC1=CC=CC=C1 GGAPHLIUUTVYMX-QWRGUYRKSA-N 0.000 description 11
- PMGDADKJMCOXHX-UHFFFAOYSA-N L-Arginyl-L-glutamin-acetat Natural products NC(=N)NCCCC(N)C(=O)NC(CCC(N)=O)C(O)=O PMGDADKJMCOXHX-UHFFFAOYSA-N 0.000 description 11
- UQCNIMDPYICBTR-KYNKHSRBSA-N Thr-Thr-Gly Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(=O)NCC(O)=O UQCNIMDPYICBTR-KYNKHSRBSA-N 0.000 description 11
- YOPQYBJJNSIQGZ-JNPHEJMOSA-N Thr-Tyr-Tyr Chemical compound C([C@H](NC(=O)[C@@H](N)[C@H](O)C)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(O)=O)C1=CC=C(O)C=C1 YOPQYBJJNSIQGZ-JNPHEJMOSA-N 0.000 description 11
- MBLJBGZWLHTJBH-SZMVWBNQSA-N Trp-Val-Arg Chemical compound C1=CC=C2C(C[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O)=CNC2=C1 MBLJBGZWLHTJBH-SZMVWBNQSA-N 0.000 description 11
- NUQZCPSZHGIYTA-HKUYNNGSSA-N Tyr-Trp-Gly Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)NCC(=O)O)NC(=O)[C@H](CC3=CC=C(C=C3)O)N NUQZCPSZHGIYTA-HKUYNNGSSA-N 0.000 description 11
- 108010073929 Vascular Endothelial Growth Factor A Proteins 0.000 description 11
- 102000005789 Vascular Endothelial Growth Factors Human genes 0.000 description 11
- 108010019530 Vascular Endothelial Growth Factors Proteins 0.000 description 11
- 108010086434 alanyl-seryl-glycine Proteins 0.000 description 11
- 238000002583 angiography Methods 0.000 description 11
- 108010008355 arginyl-glutamine Proteins 0.000 description 11
- 230000006378 damage Effects 0.000 description 11
- 239000003102 growth factor Substances 0.000 description 11
- 229910052739 hydrogen Inorganic materials 0.000 description 11
- 238000002347 injection Methods 0.000 description 11
- 239000007924 injection Substances 0.000 description 11
- 108010033670 threonyl-aspartyl-tyrosine Proteins 0.000 description 11
- KXEVYGKATAMXJJ-ACZMJKKPSA-N Ala-Glu-Asp Chemical compound C[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(O)=O KXEVYGKATAMXJJ-ACZMJKKPSA-N 0.000 description 10
- AWZKCUCQJNTBAD-SRVKXCTJSA-N Ala-Leu-Lys Chemical compound C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@H](C(O)=O)CCCCN AWZKCUCQJNTBAD-SRVKXCTJSA-N 0.000 description 10
- NRVQLLDIJJEIIZ-VZFHVOOUSA-N Cys-Thr-Ala Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](C)C(=O)O)NC(=O)[C@H](CS)N)O NRVQLLDIJJEIIZ-VZFHVOOUSA-N 0.000 description 10
- JXFLPKSDLDEOQK-JHEQGTHGSA-N Gln-Gly-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H](N)CCC(N)=O JXFLPKSDLDEOQK-JHEQGTHGSA-N 0.000 description 10
- XZLLTYBONVKGLO-SDDRHHMPSA-N Gln-Lys-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CCCCN)NC(=O)[C@H](CCC(=O)N)N)C(=O)O XZLLTYBONVKGLO-SDDRHHMPSA-N 0.000 description 10
- SOEGEPHNZOISMT-BYPYZUCNSA-N Gly-Ser-Gly Chemical compound NCC(=O)N[C@@H](CO)C(=O)NCC(O)=O SOEGEPHNZOISMT-BYPYZUCNSA-N 0.000 description 10
- ZGKVPOSSTGHJAF-HJPIBITLSA-N Ile-Tyr-Ser Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)N[C@@H](CO)C(=O)O)N ZGKVPOSSTGHJAF-HJPIBITLSA-N 0.000 description 10
- BQSLGJHIAGOZCD-CIUDSAMLSA-N Leu-Ala-Ser Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(O)=O BQSLGJHIAGOZCD-CIUDSAMLSA-N 0.000 description 10
- KPJJOZUXFOLGMQ-CIUDSAMLSA-N Lys-Asp-Asn Chemical compound C(CCN)C[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N[C@@H](CC(=O)N)C(=O)O)N KPJJOZUXFOLGMQ-CIUDSAMLSA-N 0.000 description 10
- JDJMFMVVJHLWDP-UNQGMJICSA-N Pro-Thr-Phe Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O JDJMFMVVJHLWDP-UNQGMJICSA-N 0.000 description 10
- RZUOXAKGNHXZTB-GUBZILKMSA-N Ser-Arg-Met Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCSC)C(O)=O RZUOXAKGNHXZTB-GUBZILKMSA-N 0.000 description 10
- BNFVPSRLHHPQKS-WHFBIAKZSA-N Ser-Asp-Gly Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(O)=O)C(=O)NCC(O)=O BNFVPSRLHHPQKS-WHFBIAKZSA-N 0.000 description 10
- SFTZWNJFZYOLBD-ZDLURKLDSA-N Ser-Gly-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H](N)CO SFTZWNJFZYOLBD-ZDLURKLDSA-N 0.000 description 10
- STUAPCLEDMKXKL-LKXGYXEUSA-N Thr-Ser-Asn Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(O)=O STUAPCLEDMKXKL-LKXGYXEUSA-N 0.000 description 10
- PKZIWSHDJYIPRH-JBACZVJFSA-N Trp-Tyr-Gln Chemical compound [H]N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CCC(N)=O)C(O)=O PKZIWSHDJYIPRH-JBACZVJFSA-N 0.000 description 10
- SCCKSNREWHMKOJ-SRVKXCTJSA-N Tyr-Asn-Ser Chemical compound N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CO)C(O)=O SCCKSNREWHMKOJ-SRVKXCTJSA-N 0.000 description 10
- YKCXQOBTISTQJD-BZSNNMDCSA-N Tyr-Leu-His Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)NC(=O)[C@H](CC2=CC=C(C=C2)O)N YKCXQOBTISTQJD-BZSNNMDCSA-N 0.000 description 10
- MYLNLEIZWHVENT-VKOGCVSHSA-N Val-Ile-Trp Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CNC2=CC=CC=C21)C(=O)O)NC(=O)[C@H](C(C)C)N MYLNLEIZWHVENT-VKOGCVSHSA-N 0.000 description 10
- 210000004204 blood vessel Anatomy 0.000 description 10
- 210000002889 endothelial cell Anatomy 0.000 description 10
- 239000013604 expression vector Substances 0.000 description 10
- 108010089804 glycyl-threonine Proteins 0.000 description 10
- PBVLJOIPOGUQQP-CIUDSAMLSA-N Asp-Ala-Leu Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(O)=O PBVLJOIPOGUQQP-CIUDSAMLSA-N 0.000 description 9
- 208000032544 Cicatrix Diseases 0.000 description 9
- ZFBBMCKQSNJZSN-AUTRQRHGSA-N Gln-Val-Gln Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O ZFBBMCKQSNJZSN-AUTRQRHGSA-N 0.000 description 9
- 108010054477 Immunoglobulin Fab Fragments Proteins 0.000 description 9
- 102000001706 Immunoglobulin Fab Fragments Human genes 0.000 description 9
- FEHQLKKBVJHSEC-SZMVWBNQSA-N Leu-Glu-Trp Chemical compound C1=CC=C2C(C[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](N)CC(C)C)C(O)=O)=CNC2=C1 FEHQLKKBVJHSEC-SZMVWBNQSA-N 0.000 description 9
- ICYRCNICGBJLGM-HJGDQZAQSA-N Leu-Thr-Asp Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@H](C(O)=O)CC(O)=O ICYRCNICGBJLGM-HJGDQZAQSA-N 0.000 description 9
- LCNASHSOFMRYFO-WDCWCFNPSA-N Leu-Thr-Gln Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@H](C(O)=O)CCC(N)=O LCNASHSOFMRYFO-WDCWCFNPSA-N 0.000 description 9
- 241000283984 Rodentia Species 0.000 description 9
- YUJLIIRMIAGMCQ-CIUDSAMLSA-N Ser-Leu-Ser Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(O)=O YUJLIIRMIAGMCQ-CIUDSAMLSA-N 0.000 description 9
- 238000000338 in vitro Methods 0.000 description 9
- 108010017391 lysylvaline Proteins 0.000 description 9
- 238000004519 manufacturing process Methods 0.000 description 9
- 230000035772 mutation Effects 0.000 description 9
- 239000000047 product Substances 0.000 description 9
- 230000037387 scars Effects 0.000 description 9
- 108010026333 seryl-proline Proteins 0.000 description 9
- XTZDZAXYPDISRR-MNXVOIDGSA-N Glu-Ile-Lys Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CCC(=O)O)N XTZDZAXYPDISRR-MNXVOIDGSA-N 0.000 description 8
- YGHSQRJSHKYUJY-SCZZXKLOSA-N Gly-Val-Pro Chemical compound CC(C)[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)CN YGHSQRJSHKYUJY-SCZZXKLOSA-N 0.000 description 8
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 8
- LJBVRCDPWOJOEK-PPCPHDFISA-N Leu-Thr-Ile Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O LJBVRCDPWOJOEK-PPCPHDFISA-N 0.000 description 8
- QESXLSQLQHHTIX-RHYQMDGZSA-N Leu-Val-Thr Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O QESXLSQLQHHTIX-RHYQMDGZSA-N 0.000 description 8
- 241000288906 Primates Species 0.000 description 8
- FPCGZYMRFFIYIH-CIUDSAMLSA-N Ser-Lys-Ser Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(O)=O FPCGZYMRFFIYIH-CIUDSAMLSA-N 0.000 description 8
- 108010008685 alanyl-glutamyl-aspartic acid Proteins 0.000 description 8
- 238000003556 assay Methods 0.000 description 8
- 230000015572 biosynthetic process Effects 0.000 description 8
- 239000000872 buffer Substances 0.000 description 8
- 238000006243 chemical reaction Methods 0.000 description 8
- 239000003153 chemical reaction reagent Substances 0.000 description 8
- 238000010276 construction Methods 0.000 description 8
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 8
- 230000001965 increasing effect Effects 0.000 description 8
- 239000003446 ligand Substances 0.000 description 8
- FMRKUXFLLPKVPG-JYJNAYRXSA-N His-Gln-Tyr Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)N)NC(=O)[C@H](CC2=CN=CN2)N)O FMRKUXFLLPKVPG-JYJNAYRXSA-N 0.000 description 7
- UCOCBWDBHCUPQP-DCAQKATOSA-N Leu-Arg-Ser Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(O)=O UCOCBWDBHCUPQP-DCAQKATOSA-N 0.000 description 7
- PESQCPHRXOFIPX-UHFFFAOYSA-N N-L-methionyl-L-tyrosine Natural products CSCCC(N)C(=O)NC(C(O)=O)CC1=CC=C(O)C=C1 PESQCPHRXOFIPX-UHFFFAOYSA-N 0.000 description 7
- 206010029113 Neovascularisation Diseases 0.000 description 7
- XQJCEKXQUJQNNK-ZLUOBGJFSA-N Ser-Ser-Ser Chemical compound OC[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(O)=O XQJCEKXQUJQNNK-ZLUOBGJFSA-N 0.000 description 7
- KPNSNVTUVKSBFL-ZJDVBMNYSA-N Thr-Met-Thr Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCSC)C(=O)N[C@@H]([C@@H](C)O)C(=O)O)N)O KPNSNVTUVKSBFL-ZJDVBMNYSA-N 0.000 description 7
- 125000000539 amino acid group Chemical group 0.000 description 7
- 239000003795 chemical substances by application Substances 0.000 description 7
- 238000013461 design Methods 0.000 description 7
- 201000010099 disease Diseases 0.000 description 7
- 230000004927 fusion Effects 0.000 description 7
- 230000012010 growth Effects 0.000 description 7
- 210000004408 hybridoma Anatomy 0.000 description 7
- 238000003018 immunoassay Methods 0.000 description 7
- 230000001575 pathological effect Effects 0.000 description 7
- 238000002360 preparation method Methods 0.000 description 7
- 108010031719 prolyl-serine Proteins 0.000 description 7
- 210000001525 retina Anatomy 0.000 description 7
- 108010069117 seryl-lysyl-aspartic acid Proteins 0.000 description 7
- 241000894007 species Species 0.000 description 7
- 238000006467 substitution reaction Methods 0.000 description 7
- 239000013603 viral vector Substances 0.000 description 7
- OYTPNWYZORARHL-XHNCKOQMSA-N Gln-Ala-Pro Chemical compound C[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CCC(=O)N)N OYTPNWYZORARHL-XHNCKOQMSA-N 0.000 description 6
- MSHXWFKYXJTLEZ-CIUDSAMLSA-N Gln-Met-Asn Chemical compound CSCC[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)O)NC(=O)[C@H](CCC(=O)N)N MSHXWFKYXJTLEZ-CIUDSAMLSA-N 0.000 description 6
- YQPFCZVKMUVZIN-AUTRQRHGSA-N Glu-Val-Gln Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O YQPFCZVKMUVZIN-AUTRQRHGSA-N 0.000 description 6
- AIMGJYMCTAABEN-GVXVVHGQSA-N Leu-Val-Glu Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O AIMGJYMCTAABEN-GVXVVHGQSA-N 0.000 description 6
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 description 6
- 206010025421 Macule Diseases 0.000 description 6
- BQVUABVGYYSDCJ-UHFFFAOYSA-N Nalpha-L-Leucyl-L-tryptophan Natural products C1=CC=C2C(CC(NC(=O)C(N)CC(C)C)C(O)=O)=CNC2=C1 BQVUABVGYYSDCJ-UHFFFAOYSA-N 0.000 description 6
- 108091005461 Nucleic proteins Proteins 0.000 description 6
- MGDFPGCFVJFITQ-CIUDSAMLSA-N Pro-Glu-Asp Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(O)=O MGDFPGCFVJFITQ-CIUDSAMLSA-N 0.000 description 6
- QGMLKFGTGXWAHF-IHRRRGAJSA-N Ser-Arg-Phe Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O QGMLKFGTGXWAHF-IHRRRGAJSA-N 0.000 description 6
- KDGARKCAKHBEDB-NKWVEPMBSA-N Ser-Gly-Pro Chemical compound C1C[C@@H](N(C1)C(=O)CNC(=O)[C@H](CO)N)C(=O)O KDGARKCAKHBEDB-NKWVEPMBSA-N 0.000 description 6
- SPVHQURZJCUDQC-VOAKCMCISA-N Thr-Lys-Leu Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(O)=O SPVHQURZJCUDQC-VOAKCMCISA-N 0.000 description 6
- HTONZBWRYUKUKC-RCWTZXSCSA-N Val-Thr-Val Chemical compound CC(C)[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(O)=O HTONZBWRYUKUKC-RCWTZXSCSA-N 0.000 description 6
- 108010069020 alanyl-prolyl-glycine Proteins 0.000 description 6
- 108010087924 alanylproline Proteins 0.000 description 6
- 108010069205 aspartyl-phenylalanine Proteins 0.000 description 6
- 210000002919 epithelial cell Anatomy 0.000 description 6
- 230000002401 inhibitory effect Effects 0.000 description 6
- 238000013532 laser treatment Methods 0.000 description 6
- 230000008569 process Effects 0.000 description 6
- 230000035755 proliferation Effects 0.000 description 6
- 108010020755 prolyl-glycyl-glycine Proteins 0.000 description 6
- 238000000746 purification Methods 0.000 description 6
- 210000002966 serum Anatomy 0.000 description 6
- 238000010186 staining Methods 0.000 description 6
- 238000013518 transcription Methods 0.000 description 6
- 230000035897 transcription Effects 0.000 description 6
- XWFWAXPOLRTDFZ-FXQIFTODSA-N Ala-Pro-Ser Chemical compound C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(O)=O XWFWAXPOLRTDFZ-FXQIFTODSA-N 0.000 description 5
- AOHKLEBWKMKITA-IHRRRGAJSA-N Arg-Phe-Ser Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CO)C(=O)O)NC(=O)[C@H](CCCN=C(N)N)N AOHKLEBWKMKITA-IHRRRGAJSA-N 0.000 description 5
- XYBJLTKSGFBLCS-QXEWZRGKSA-N Asp-Arg-Val Chemical compound NC(N)=NCCC[C@@H](C(=O)N[C@@H](C(C)C)C(O)=O)NC(=O)[C@@H](N)CC(O)=O XYBJLTKSGFBLCS-QXEWZRGKSA-N 0.000 description 5
- QNFRBNZGVVKBNJ-PEFMBERDSA-N Asp-Ile-Gln Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)O)NC(=O)[C@H](CC(=O)O)N QNFRBNZGVVKBNJ-PEFMBERDSA-N 0.000 description 5
- 101100505161 Caenorhabditis elegans mel-32 gene Proteins 0.000 description 5
- 108091026890 Coding region Proteins 0.000 description 5
- FQCILXROGNOZON-YUMQZZPRSA-N Gln-Pro-Gly Chemical compound NC(=O)CC[C@H](N)C(=O)N1CCC[C@H]1C(=O)NCC(O)=O FQCILXROGNOZON-YUMQZZPRSA-N 0.000 description 5
- WLRYGVYQFXRJDA-DCAQKATOSA-N Gln-Pro-Pro Chemical compound NC(=O)CC[C@H](N)C(=O)N1CCC[C@H]1C(=O)N1[C@H](C(O)=O)CCC1 WLRYGVYQFXRJDA-DCAQKATOSA-N 0.000 description 5
- BYYNJRSNDARRBX-YFKPBYRVSA-N Gly-Gln-Gly Chemical compound NCC(=O)N[C@@H](CCC(N)=O)C(=O)NCC(O)=O BYYNJRSNDARRBX-YFKPBYRVSA-N 0.000 description 5
- VBOBNHSVQKKTOT-YUMQZZPRSA-N Gly-Lys-Ala Chemical compound [H]NCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(O)=O VBOBNHSVQKKTOT-YUMQZZPRSA-N 0.000 description 5
- HAOUOFNNJJLVNS-BQBZGAKWSA-N Gly-Pro-Ser Chemical compound NCC(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(O)=O HAOUOFNNJJLVNS-BQBZGAKWSA-N 0.000 description 5
- RVNOXPZHMUWCLW-GMOBBJLQSA-N Ile-Met-Asn Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(=O)N)C(=O)O)N RVNOXPZHMUWCLW-GMOBBJLQSA-N 0.000 description 5
- RKQAYOWLSFLJEE-SVSWQMSJSA-N Ile-Thr-Cys Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CS)C(=O)O)N RKQAYOWLSFLJEE-SVSWQMSJSA-N 0.000 description 5
- YQEZLKZALYSWHR-UHFFFAOYSA-N Ketamine Chemical compound C=1C=CC=C(Cl)C=1C1(NC)CCCCC1=O YQEZLKZALYSWHR-UHFFFAOYSA-N 0.000 description 5
- HVHRPWQEQHIQJF-AVGNSLFASA-N Leu-Lys-Glu Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(O)=O HVHRPWQEQHIQJF-AVGNSLFASA-N 0.000 description 5
- BRTVHXHCUSXYRI-CIUDSAMLSA-N Leu-Ser-Ser Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(O)=O BRTVHXHCUSXYRI-CIUDSAMLSA-N 0.000 description 5
- BKWJQWJPZMUWEG-LFSVMHDDSA-N Phe-Ala-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CC1=CC=CC=C1 BKWJQWJPZMUWEG-LFSVMHDDSA-N 0.000 description 5
- MKGIILKDUGDRRO-FXQIFTODSA-N Pro-Ser-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H]1CCCN1 MKGIILKDUGDRRO-FXQIFTODSA-N 0.000 description 5
- 108010003201 RGH 0205 Proteins 0.000 description 5
- IYCBDVBJWDXQRR-FXQIFTODSA-N Ser-Ala-Met Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C)C(=O)N[C@@H](CCSC)C(O)=O IYCBDVBJWDXQRR-FXQIFTODSA-N 0.000 description 5
- YMTLKLXDFCSCNX-BYPYZUCNSA-N Ser-Gly-Gly Chemical compound OC[C@H](N)C(=O)NCC(=O)NCC(O)=O YMTLKLXDFCSCNX-BYPYZUCNSA-N 0.000 description 5
- GJFYFGOEWLDQGW-GUBZILKMSA-N Ser-Leu-Gln Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)O)NC(=O)[C@H](CO)N GJFYFGOEWLDQGW-GUBZILKMSA-N 0.000 description 5
- XNCUYZKGQOCOQH-YUMQZZPRSA-N Ser-Leu-Gly Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)NCC(O)=O XNCUYZKGQOCOQH-YUMQZZPRSA-N 0.000 description 5
- ADJDNJCSPNFFPI-FXQIFTODSA-N Ser-Pro-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)CO ADJDNJCSPNFFPI-FXQIFTODSA-N 0.000 description 5
- AZWNCEBQZXELEZ-FXQIFTODSA-N Ser-Pro-Ser Chemical compound OC[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(O)=O AZWNCEBQZXELEZ-FXQIFTODSA-N 0.000 description 5
- YOSLMIPKOUAHKI-OLHMAJIHSA-N Thr-Asp-Asp Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(O)=O)C(O)=O YOSLMIPKOUAHKI-OLHMAJIHSA-N 0.000 description 5
- WPAKPLPGQNUXGN-OSUNSFLBSA-N Thr-Ile-Arg Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O WPAKPLPGQNUXGN-OSUNSFLBSA-N 0.000 description 5
- BPGDJSUFQKWUBK-KJEVXHAQSA-N Thr-Val-Tyr Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 BPGDJSUFQKWUBK-KJEVXHAQSA-N 0.000 description 5
- 208000027418 Wounds and injury Diseases 0.000 description 5
- 230000006907 apoptotic process Effects 0.000 description 5
- 230000030833 cell death Effects 0.000 description 5
- 238000003776 cleavage reaction Methods 0.000 description 5
- 230000001186 cumulative effect Effects 0.000 description 5
- 108010060199 cysteinylproline Proteins 0.000 description 5
- 239000003814 drug Substances 0.000 description 5
- 108010030074 endodeoxyribonuclease MluI Proteins 0.000 description 5
- 239000007850 fluorescent dye Substances 0.000 description 5
- 230000006870 function Effects 0.000 description 5
- 108010067216 glycyl-glycyl-glycine Proteins 0.000 description 5
- 108010050848 glycylleucine Proteins 0.000 description 5
- 208000014674 injury Diseases 0.000 description 5
- 230000003993 interaction Effects 0.000 description 5
- 229960003299 ketamine Drugs 0.000 description 5
- 239000002609 medium Substances 0.000 description 5
- 108010070409 phenylalanyl-glycyl-glycine Proteins 0.000 description 5
- 108010051242 phenylalanylserine Proteins 0.000 description 5
- 230000001766 physiological effect Effects 0.000 description 5
- 229920000642 polymer Polymers 0.000 description 5
- 108010087846 prolyl-prolyl-glycine Proteins 0.000 description 5
- 102000005962 receptors Human genes 0.000 description 5
- 108020003175 receptors Proteins 0.000 description 5
- 108091008146 restriction endonucleases Proteins 0.000 description 5
- 230000007017 scission Effects 0.000 description 5
- 239000007790 solid phase Substances 0.000 description 5
- 230000009466 transformation Effects 0.000 description 5
- YBJHBAHKTGYVGT-ZKWXMUAHSA-N (+)-Biotin Chemical compound N1C(=O)N[C@@H]2[C@H](CCCCC(=O)O)SC[C@@H]21 YBJHBAHKTGYVGT-ZKWXMUAHSA-N 0.000 description 4
- JBVSSSZFNTXJDX-YTLHQDLWSA-N Ala-Ala-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@H](C)N JBVSSSZFNTXJDX-YTLHQDLWSA-N 0.000 description 4
- VNYMOTCMNHJGTG-JBDRJPRFSA-N Ala-Ile-Ser Chemical compound [H]N[C@@H](C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CO)C(O)=O VNYMOTCMNHJGTG-JBDRJPRFSA-N 0.000 description 4
- ARHJJAAWNWOACN-FXQIFTODSA-N Ala-Ser-Val Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(O)=O ARHJJAAWNWOACN-FXQIFTODSA-N 0.000 description 4
- 102000000412 Annexin Human genes 0.000 description 4
- 108050008874 Annexin Proteins 0.000 description 4
- BPAUXFVCSYQDQX-JRQIVUDYSA-N Asp-Tyr-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CC1=CC=C(C=C1)O)NC(=O)[C@H](CC(=O)O)N)O BPAUXFVCSYQDQX-JRQIVUDYSA-N 0.000 description 4
- 241000282693 Cercopithecidae Species 0.000 description 4
- 206010012689 Diabetic retinopathy Diseases 0.000 description 4
- 108090000790 Enzymes Proteins 0.000 description 4
- 102000004190 Enzymes Human genes 0.000 description 4
- 108010037362 Extracellular Matrix Proteins Proteins 0.000 description 4
- 102000010834 Extracellular Matrix Proteins Human genes 0.000 description 4
- FFVXLVGUJBCKRX-UKJIMTQDSA-N Gln-Ile-Val Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](C(C)C)C(=O)O)NC(=O)[C@H](CCC(=O)N)N FFVXLVGUJBCKRX-UKJIMTQDSA-N 0.000 description 4
- DYFJZDDQPNIPAB-NHCYSSNCSA-N Glu-Arg-Val Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C(C)C)C(O)=O DYFJZDDQPNIPAB-NHCYSSNCSA-N 0.000 description 4
- 239000004471 Glycine Substances 0.000 description 4
- FFEARJCKVFRZRR-BYPYZUCNSA-N L-methionine Chemical compound CSCC[C@H](N)C(O)=O FFEARJCKVFRZRR-BYPYZUCNSA-N 0.000 description 4
- 241000699670 Mus sp. Species 0.000 description 4
- SITLTJHOQZFJGG-UHFFFAOYSA-N N-L-alpha-glutamyl-L-valine Natural products CC(C)C(C(O)=O)NC(=O)C(N)CCC(O)=O SITLTJHOQZFJGG-UHFFFAOYSA-N 0.000 description 4
- 206010030113 Oedema Diseases 0.000 description 4
- IHCXPSYCHXFXKT-DCAQKATOSA-N Pro-Arg-Glu Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(O)=O IHCXPSYCHXFXKT-DCAQKATOSA-N 0.000 description 4
- RMODQFBNDDENCP-IHRRRGAJSA-N Pro-Lys-Leu Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(O)=O RMODQFBNDDENCP-IHRRRGAJSA-N 0.000 description 4
- FDMKYQQYJKYCLV-GUBZILKMSA-N Pro-Pro-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H]1NCCC1 FDMKYQQYJKYCLV-GUBZILKMSA-N 0.000 description 4
- BMKNXTJLHFIAAH-CIUDSAMLSA-N Ser-Ser-Leu Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(O)=O BMKNXTJLHFIAAH-CIUDSAMLSA-N 0.000 description 4
- AABIBDJHSKIMJK-FXQIFTODSA-N Ser-Ser-Met Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCSC)C(O)=O AABIBDJHSKIMJK-FXQIFTODSA-N 0.000 description 4
- VVKVHAOOUGNDPJ-SRVKXCTJSA-N Ser-Tyr-Ser Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CO)C(O)=O VVKVHAOOUGNDPJ-SRVKXCTJSA-N 0.000 description 4
- SIEBDTCABMZCLF-XGEHTFHBSA-N Ser-Val-Thr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O SIEBDTCABMZCLF-XGEHTFHBSA-N 0.000 description 4
- XSLXHSYIVPGEER-KZVJFYERSA-N Thr-Ala-Val Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](C(C)C)C(O)=O XSLXHSYIVPGEER-KZVJFYERSA-N 0.000 description 4
- DXPURPNJDFCKKO-RHYQMDGZSA-N Thr-Lys-Val Chemical compound CC(C)[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](N)[C@@H](C)O)C(O)=O DXPURPNJDFCKKO-RHYQMDGZSA-N 0.000 description 4
- HJSLDXZAZGFPDK-ULQDDVLXSA-N Val-Phe-Leu Chemical compound CC(C)C[C@@H](C(=O)O)NC(=O)[C@H](CC1=CC=CC=C1)NC(=O)[C@H](C(C)C)N HJSLDXZAZGFPDK-ULQDDVLXSA-N 0.000 description 4
- 238000004458 analytical method Methods 0.000 description 4
- 239000007853 buffer solution Substances 0.000 description 4
- 230000008859 change Effects 0.000 description 4
- 230000000295 complement effect Effects 0.000 description 4
- 150000001875 compounds Chemical class 0.000 description 4
- 238000011161 development Methods 0.000 description 4
- 230000018109 developmental process Effects 0.000 description 4
- 229940079593 drug Drugs 0.000 description 4
- 238000005516 engineering process Methods 0.000 description 4
- 229940088598 enzyme Drugs 0.000 description 4
- 239000013613 expression plasmid Substances 0.000 description 4
- 210000002744 extracellular matrix Anatomy 0.000 description 4
- 238000001990 intravenous administration Methods 0.000 description 4
- 238000002955 isolation Methods 0.000 description 4
- 108020004999 messenger RNA Proteins 0.000 description 4
- 229930182817 methionine Natural products 0.000 description 4
- 238000012544 monitoring process Methods 0.000 description 4
- 239000002773 nucleotide Substances 0.000 description 4
- 125000003729 nucleotide group Chemical group 0.000 description 4
- 230000004044 response Effects 0.000 description 4
- 229960004641 rituximab Drugs 0.000 description 4
- 241000701161 unidentified adenovirus Species 0.000 description 4
- 108010009962 valyltyrosine Proteins 0.000 description 4
- 239000011534 wash buffer Substances 0.000 description 4
- NFGXHKASABOEEW-UHFFFAOYSA-N 1-methylethyl 11-methoxy-3,7,11-trimethyl-2,4-dodecadienoate Chemical compound COC(C)(C)CCCC(C)CC=CC(C)=CC(=O)OC(C)C NFGXHKASABOEEW-UHFFFAOYSA-N 0.000 description 3
- MNZHHDPWDWQJCQ-YUMQZZPRSA-N Ala-Leu-Gly Chemical compound C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)NCC(O)=O MNZHHDPWDWQJCQ-YUMQZZPRSA-N 0.000 description 3
- HOVPGJUNRLMIOZ-CIUDSAMLSA-N Ala-Ser-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@H](C)N HOVPGJUNRLMIOZ-CIUDSAMLSA-N 0.000 description 3
- WQKAQKZRDIZYNV-VZFHVOOUSA-N Ala-Ser-Thr Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(O)=O WQKAQKZRDIZYNV-VZFHVOOUSA-N 0.000 description 3
- 102100024075 Alpha-internexin Human genes 0.000 description 3
- 108090000672 Annexin A5 Proteins 0.000 description 3
- 102000004121 Annexin A5 Human genes 0.000 description 3
- ICRHGPYYXMWHIE-LPEHRKFASA-N Arg-Ser-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CO)NC(=O)[C@H](CCCN=C(N)N)N)C(=O)O ICRHGPYYXMWHIE-LPEHRKFASA-N 0.000 description 3
- ULBHWNVWSCJLCO-NHCYSSNCSA-N Arg-Val-Glu Chemical compound OC(=O)CC[C@@H](C(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@@H](N)CCCN=C(N)N ULBHWNVWSCJLCO-NHCYSSNCSA-N 0.000 description 3
- PUUPMDXIHCOPJU-HJGDQZAQSA-N Asn-Thr-Lys Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CC(=O)N)N)O PUUPMDXIHCOPJU-HJGDQZAQSA-N 0.000 description 3
- 102000000844 Cell Surface Receptors Human genes 0.000 description 3
- 108010001857 Cell Surface Receptors Proteins 0.000 description 3
- OHLLDUNVMPPUMD-DCAQKATOSA-N Cys-Leu-Val Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](C(C)C)C(=O)O)NC(=O)[C@H](CS)N OHLLDUNVMPPUMD-DCAQKATOSA-N 0.000 description 3
- BCFXQBXXDSEHRS-FXQIFTODSA-N Cys-Ser-Arg Chemical compound [H]N[C@@H](CS)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O BCFXQBXXDSEHRS-FXQIFTODSA-N 0.000 description 3
- 238000001712 DNA sequencing Methods 0.000 description 3
- 102100024785 Fibroblast growth factor 2 Human genes 0.000 description 3
- 108090000379 Fibroblast growth factor 2 Proteins 0.000 description 3
- WSFSSNUMVMOOMR-UHFFFAOYSA-N Formaldehyde Chemical compound O=C WSFSSNUMVMOOMR-UHFFFAOYSA-N 0.000 description 3
- JKDBRTNMYXYLHO-JYJNAYRXSA-N Gln-Tyr-Leu Chemical compound NC(=O)CC[C@H](N)C(=O)N[C@H](C(=O)N[C@@H](CC(C)C)C(O)=O)CC1=CC=C(O)C=C1 JKDBRTNMYXYLHO-JYJNAYRXSA-N 0.000 description 3
- ZMXZGYLINVNTKH-DZKIICNBSA-N Gln-Val-Phe Chemical compound NC(=O)CC[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 ZMXZGYLINVNTKH-DZKIICNBSA-N 0.000 description 3
- 101000833549 Homo sapiens Alpha-internexin Proteins 0.000 description 3
- FBGXMKUWQFPHFB-JBDRJPRFSA-N Ile-Ser-Cys Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CS)C(=O)O)N FBGXMKUWQFPHFB-JBDRJPRFSA-N 0.000 description 3
- 206010061218 Inflammation Diseases 0.000 description 3
- 208000022559 Inflammatory bowel disease Diseases 0.000 description 3
- QNAYBMKLOCPYGJ-REOHCLBHSA-N L-alanine Chemical compound C[C@H](N)C(O)=O QNAYBMKLOCPYGJ-REOHCLBHSA-N 0.000 description 3
- KZSNJWFQEVHDMF-BYPYZUCNSA-N L-valine Chemical compound CC(C)[C@H](N)C(O)=O KZSNJWFQEVHDMF-BYPYZUCNSA-N 0.000 description 3
- WSGXUIQTEZDVHJ-GARJFASQSA-N Leu-Ala-Pro Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](C)C(=O)N1CCC[C@@H]1C(O)=O WSGXUIQTEZDVHJ-GARJFASQSA-N 0.000 description 3
- KIZIOFNVSOSKJI-CIUDSAMLSA-N Leu-Ser-Cys Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CS)C(=O)O)N KIZIOFNVSOSKJI-CIUDSAMLSA-N 0.000 description 3
- ILDSIMPXNFWKLH-KATARQTJSA-N Leu-Thr-Ser Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(O)=O ILDSIMPXNFWKLH-KATARQTJSA-N 0.000 description 3
- VUBIPAHVHMZHCM-KKUMJFAQSA-N Leu-Tyr-Ser Chemical compound CC(C)C[C@H](N)C(=O)N[C@H](C(=O)N[C@@H](CO)C(O)=O)CC1=CC=C(O)C=C1 VUBIPAHVHMZHCM-KKUMJFAQSA-N 0.000 description 3
- NTBFKPBULZGXQL-KKUMJFAQSA-N Lys-Asp-Tyr Chemical compound NCCCC[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 NTBFKPBULZGXQL-KKUMJFAQSA-N 0.000 description 3
- YTJFXEDRUOQGSP-DCAQKATOSA-N Lys-Pro-Ser Chemical compound [H]N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(O)=O YTJFXEDRUOQGSP-DCAQKATOSA-N 0.000 description 3
- RQILLQOQXLZTCK-KBPBESRZSA-N Lys-Tyr-Gly Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)NCC(O)=O RQILLQOQXLZTCK-KBPBESRZSA-N 0.000 description 3
- KZNQNBZMBZJQJO-UHFFFAOYSA-N N-glycyl-L-proline Natural products NCC(=O)N1CCCC1C(O)=O KZNQNBZMBZJQJO-UHFFFAOYSA-N 0.000 description 3
- 101100068676 Neurospora crassa (strain ATCC 24698 / 74-OR23-1A / CBS 708.71 / DSM 1257 / FGSC 987) gln-1 gene Proteins 0.000 description 3
- 241000283973 Oryctolagus cuniculus Species 0.000 description 3
- JLLJTMHNXQTMCK-UBHSHLNASA-N Phe-Pro-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)CC1=CC=CC=C1 JLLJTMHNXQTMCK-UBHSHLNASA-N 0.000 description 3
- QARPMYDMYVLFMW-KKUMJFAQSA-N Phe-Pro-Glu Chemical compound C([C@H](N)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCC(O)=O)C(O)=O)C1=CC=CC=C1 QARPMYDMYVLFMW-KKUMJFAQSA-N 0.000 description 3
- AMBLXEMWFARNNQ-DCAQKATOSA-N Pro-Asn-Leu Chemical compound CC(C)C[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)N)NC(=O)[C@@H]1CCCN1 AMBLXEMWFARNNQ-DCAQKATOSA-N 0.000 description 3
- HWLKHNDRXWTFTN-GUBZILKMSA-N Pro-Pro-Cys Chemical compound C1C[C@H](NC1)C(=O)N2CCC[C@H]2C(=O)N[C@@H](CS)C(=O)O HWLKHNDRXWTFTN-GUBZILKMSA-N 0.000 description 3
- 241000700159 Rattus Species 0.000 description 3
- 108020004511 Recombinant DNA Proteins 0.000 description 3
- UQFYNFTYDHUIMI-WHFBIAKZSA-N Ser-Gly-Ala Chemical compound OC(=O)[C@H](C)NC(=O)CNC(=O)[C@@H](N)CO UQFYNFTYDHUIMI-WHFBIAKZSA-N 0.000 description 3
- SRSPTFBENMJHMR-WHFBIAKZSA-N Ser-Ser-Gly Chemical compound OC[C@H](N)C(=O)N[C@@H](CO)C(=O)NCC(O)=O SRSPTFBENMJHMR-WHFBIAKZSA-N 0.000 description 3
- XJDMUQCLVSCRSJ-VZFHVOOUSA-N Ser-Thr-Ala Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(O)=O XJDMUQCLVSCRSJ-VZFHVOOUSA-N 0.000 description 3
- SNXUIBACCONSOH-BWBBJGPYSA-N Ser-Thr-Ser Chemical compound OC[C@H](N)C(=O)N[C@@H]([C@H](O)C)C(=O)N[C@@H](CO)C(O)=O SNXUIBACCONSOH-BWBBJGPYSA-N 0.000 description 3
- AXKJPUBALUNJEO-UBHSHLNASA-N Ser-Trp-Asn Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H](CC(N)=O)C(O)=O AXKJPUBALUNJEO-UBHSHLNASA-N 0.000 description 3
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 3
- 241000191967 Staphylococcus aureus Species 0.000 description 3
- JWQNAFHCXKVZKZ-UVOCVTCTSA-N Thr-Lys-Thr Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)O)C(O)=O JWQNAFHCXKVZKZ-UVOCVTCTSA-N 0.000 description 3
- QFHRUCJIRVILCK-YJRXYDGGSA-N Tyr-Thr-Cys Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CC1=CC=C(C=C1)O)N)O QFHRUCJIRVILCK-YJRXYDGGSA-N 0.000 description 3
- ZLFHAAGHGQBQQN-GUBZILKMSA-N Val-Ala-Pro Natural products CC(C)[C@H](N)C(=O)N[C@@H](C)C(=O)N1CCC[C@H]1C(O)=O ZLFHAAGHGQBQQN-GUBZILKMSA-N 0.000 description 3
- ZQGPWORGSNRQLN-NHCYSSNCSA-N Val-Asp-His Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)N ZQGPWORGSNRQLN-NHCYSSNCSA-N 0.000 description 3
- BMGOFDMKDVVGJG-NHCYSSNCSA-N Val-Asp-Lys Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N[C@@H](CCCCN)C(=O)O)N BMGOFDMKDVVGJG-NHCYSSNCSA-N 0.000 description 3
- HGJRMXOWUWVUOA-GVXVVHGQSA-N Val-Leu-Gln Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)O)NC(=O)[C@H](C(C)C)N HGJRMXOWUWVUOA-GVXVVHGQSA-N 0.000 description 3
- VCIYTVOBLZHFSC-XHSDSOJGSA-N Val-Phe-Pro Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N2CCC[C@@H]2C(=O)O)N VCIYTVOBLZHFSC-XHSDSOJGSA-N 0.000 description 3
- KZSNJWFQEVHDMF-UHFFFAOYSA-N Valine Natural products CC(C)C(N)C(O)=O KZSNJWFQEVHDMF-UHFFFAOYSA-N 0.000 description 3
- 238000007792 addition Methods 0.000 description 3
- 230000002411 adverse Effects 0.000 description 3
- 238000001261 affinity purification Methods 0.000 description 3
- 235000004279 alanine Nutrition 0.000 description 3
- 230000002491 angiogenic effect Effects 0.000 description 3
- 238000000137 annealing Methods 0.000 description 3
- 108010047857 aspartylglycine Proteins 0.000 description 3
- 239000002585 base Substances 0.000 description 3
- 230000000903 blocking effect Effects 0.000 description 3
- 230000017531 blood circulation Effects 0.000 description 3
- 230000010261 cell growth Effects 0.000 description 3
- 230000002860 competitive effect Effects 0.000 description 3
- 239000000470 constituent Substances 0.000 description 3
- 239000006196 drop Substances 0.000 description 3
- 239000003889 eye drop Substances 0.000 description 3
- 108020001507 fusion proteins Proteins 0.000 description 3
- 102000037865 fusion proteins Human genes 0.000 description 3
- 108010000434 glycyl-alanyl-leucine Proteins 0.000 description 3
- 108010050475 glycyl-leucyl-tyrosine Proteins 0.000 description 3
- 108010077515 glycylproline Proteins 0.000 description 3
- 210000003128 head Anatomy 0.000 description 3
- 230000001976 improved effect Effects 0.000 description 3
- 238000001727 in vivo Methods 0.000 description 3
- 208000015181 infectious disease Diseases 0.000 description 3
- 230000004054 inflammatory process Effects 0.000 description 3
- 238000010253 intravenous injection Methods 0.000 description 3
- 238000005342 ion exchange Methods 0.000 description 3
- 239000002502 liposome Substances 0.000 description 3
- 230000004807 localization Effects 0.000 description 3
- 208000002780 macular degeneration Diseases 0.000 description 3
- 206010061289 metastatic neoplasm Diseases 0.000 description 3
- 230000014399 negative regulation of angiogenesis Effects 0.000 description 3
- 239000012071 phase Substances 0.000 description 3
- 108010024654 phenylalanyl-prolyl-alanine Proteins 0.000 description 3
- 238000007747 plating Methods 0.000 description 3
- 229920000729 poly(L-lysine) polymer Polymers 0.000 description 3
- 108010090894 prolylleucine Proteins 0.000 description 3
- 238000003127 radioimmunoassay Methods 0.000 description 3
- 206010039073 rheumatoid arthritis Diseases 0.000 description 3
- 239000000523 sample Substances 0.000 description 3
- 238000012216 screening Methods 0.000 description 3
- 125000006850 spacer group Chemical group 0.000 description 3
- 230000009870 specific binding Effects 0.000 description 3
- 210000000952 spleen Anatomy 0.000 description 3
- 238000010561 standard procedure Methods 0.000 description 3
- 238000002198 surface plasmon resonance spectroscopy Methods 0.000 description 3
- 210000004881 tumor cell Anatomy 0.000 description 3
- 239000004474 valine Substances 0.000 description 3
- 108010073969 valyllysine Proteins 0.000 description 3
- 238000001262 western blot Methods 0.000 description 3
- 229920000936 Agarose Polymers 0.000 description 2
- YYSWCHMLFJLLBJ-ZLUOBGJFSA-N Ala-Ala-Ser Chemical compound C[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(O)=O YYSWCHMLFJLLBJ-ZLUOBGJFSA-N 0.000 description 2
- LBYMZCVBOKYZNS-CIUDSAMLSA-N Ala-Leu-Asp Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(O)=O LBYMZCVBOKYZNS-CIUDSAMLSA-N 0.000 description 2
- SUMYEVXWCAYLLJ-GUBZILKMSA-N Ala-Leu-Gln Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O SUMYEVXWCAYLLJ-GUBZILKMSA-N 0.000 description 2
- SUHLZMHFRALVSY-YUMQZZPRSA-N Ala-Lys-Gly Chemical compound NCCCC[C@H](NC(=O)[C@@H](N)C)C(=O)NCC(O)=O SUHLZMHFRALVSY-YUMQZZPRSA-N 0.000 description 2
- MDNAVFBZPROEHO-DCAQKATOSA-N Ala-Lys-Val Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(O)=O MDNAVFBZPROEHO-DCAQKATOSA-N 0.000 description 2
- MDNAVFBZPROEHO-UHFFFAOYSA-N Ala-Lys-Val Natural products CC(C)C(C(O)=O)NC(=O)C(NC(=O)C(C)N)CCCCN MDNAVFBZPROEHO-UHFFFAOYSA-N 0.000 description 2
- YJHKTAMKPGFJCT-NRPADANISA-N Ala-Val-Glu Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O YJHKTAMKPGFJCT-NRPADANISA-N 0.000 description 2
- 108700028369 Alleles Proteins 0.000 description 2
- AUFHLLPVPSMEOG-YUMQZZPRSA-N Arg-Gly-Glu Chemical compound NC(N)=NCCC[C@H](N)C(=O)NCC(=O)N[C@@H](CCC(O)=O)C(O)=O AUFHLLPVPSMEOG-YUMQZZPRSA-N 0.000 description 2
- XRNXPIGJPQHCPC-RCWTZXSCSA-N Arg-Thr-Val Chemical compound CC(C)[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)CCCNC(N)=N)[C@@H](C)O)C(O)=O XRNXPIGJPQHCPC-RCWTZXSCSA-N 0.000 description 2
- ZUVMUOOHJYNJPP-XIRDDKMYSA-N Arg-Trp-Gln Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H](CCC(N)=O)C(O)=O ZUVMUOOHJYNJPP-XIRDDKMYSA-N 0.000 description 2
- NVGWESORMHFISY-SRVKXCTJSA-N Asn-Asn-Phe Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O NVGWESORMHFISY-SRVKXCTJSA-N 0.000 description 2
- SRUUBQBAVNQZGJ-LAEOZQHASA-N Asn-Gln-Val Chemical compound CC(C)[C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)N)NC(=O)[C@H](CC(=O)N)N SRUUBQBAVNQZGJ-LAEOZQHASA-N 0.000 description 2
- WONGRTVAMHFGBE-WDSKDSINSA-N Asn-Gly-Gln Chemical compound C(CC(=O)N)[C@@H](C(=O)O)NC(=O)CNC(=O)[C@H](CC(=O)N)N WONGRTVAMHFGBE-WDSKDSINSA-N 0.000 description 2
- NUCUBYIUPVYGPP-XIRDDKMYSA-N Asn-Leu-Trp Chemical compound CC(C)C[C@H](NC(=O)[C@@H](N)CC(N)=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O NUCUBYIUPVYGPP-XIRDDKMYSA-N 0.000 description 2
- HNXWVVHIGTZTBO-LKXGYXEUSA-N Asn-Ser-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC(N)=O HNXWVVHIGTZTBO-LKXGYXEUSA-N 0.000 description 2
- QNNBHTFDFFFHGC-KKUMJFAQSA-N Asn-Tyr-Lys Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CC(=O)N)N)O QNNBHTFDFFFHGC-KKUMJFAQSA-N 0.000 description 2
- HSWYMWGDMPLTTH-FXQIFTODSA-N Asp-Glu-Gln Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O HSWYMWGDMPLTTH-FXQIFTODSA-N 0.000 description 2
- SNDBKTFJWVEVPO-WHFBIAKZSA-N Asp-Gly-Ser Chemical compound [H]N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H](CO)C(O)=O SNDBKTFJWVEVPO-WHFBIAKZSA-N 0.000 description 2
- DPNWSMBUYCLEDG-CIUDSAMLSA-N Asp-Lys-Ser Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(O)=O DPNWSMBUYCLEDG-CIUDSAMLSA-N 0.000 description 2
- JSNWZMFSLIWAHS-HJGDQZAQSA-N Asp-Thr-Leu Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC(C)C)C(=O)O)NC(=O)[C@H](CC(=O)O)N)O JSNWZMFSLIWAHS-HJGDQZAQSA-N 0.000 description 2
- KNDCWFXCFKSEBM-AVGNSLFASA-N Asp-Tyr-Glu Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CCC(O)=O)C(O)=O KNDCWFXCFKSEBM-AVGNSLFASA-N 0.000 description 2
- 201000009030 Carcinoma Diseases 0.000 description 2
- 206010068051 Chimerism Diseases 0.000 description 2
- 206010009944 Colon cancer Diseases 0.000 description 2
- 108010047041 Complementarity Determining Regions Proteins 0.000 description 2
- WVLZTXGTNGHPBO-SRVKXCTJSA-N Cys-Leu-Leu Chemical compound [H]N[C@@H](CS)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O WVLZTXGTNGHPBO-SRVKXCTJSA-N 0.000 description 2
- NDNZRWUDUMTITL-FXQIFTODSA-N Cys-Ser-Val Chemical compound [H]N[C@@H](CS)C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(O)=O NDNZRWUDUMTITL-FXQIFTODSA-N 0.000 description 2
- 229920002307 Dextran Polymers 0.000 description 2
- 102000009123 Fibrin Human genes 0.000 description 2
- 108010073385 Fibrin Proteins 0.000 description 2
- BWGVNKXGVNDBDI-UHFFFAOYSA-N Fibrin monomer Chemical compound CNC(=O)CNC(=O)CN BWGVNKXGVNDBDI-UHFFFAOYSA-N 0.000 description 2
- 102000016359 Fibronectins Human genes 0.000 description 2
- 108010067306 Fibronectins Proteins 0.000 description 2
- 208000032612 Glial tumor Diseases 0.000 description 2
- 206010018338 Glioma Diseases 0.000 description 2
- WLODHVXYKYHLJD-ACZMJKKPSA-N Gln-Asp-Ser Chemical compound C(CC(=O)N)[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N[C@@H](CO)C(=O)O)N WLODHVXYKYHLJD-ACZMJKKPSA-N 0.000 description 2
- DHNWZLGBTPUTQQ-QEJZJMRPSA-N Gln-Asp-Trp Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)O)NC(=O)[C@H](CCC(=O)N)N DHNWZLGBTPUTQQ-QEJZJMRPSA-N 0.000 description 2
- SNLOOPZHAQDMJG-CIUDSAMLSA-N Gln-Glu-Glu Chemical compound NC(=O)CC[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O SNLOOPZHAQDMJG-CIUDSAMLSA-N 0.000 description 2
- ZNZPKVQURDQFFS-FXQIFTODSA-N Gln-Glu-Ser Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(O)=O ZNZPKVQURDQFFS-FXQIFTODSA-N 0.000 description 2
- ZEEPYMXTJWIMSN-GUBZILKMSA-N Gln-Lys-Ser Chemical compound NCCCC[C@@H](C(=O)N[C@@H](CO)C(O)=O)NC(=O)[C@@H](N)CCC(N)=O ZEEPYMXTJWIMSN-GUBZILKMSA-N 0.000 description 2
- BETSEXMYBWCDAE-SZMVWBNQSA-N Gln-Trp-Lys Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CCC(=O)N)N BETSEXMYBWCDAE-SZMVWBNQSA-N 0.000 description 2
- CKOFNWCLWRYUHK-XHNCKOQMSA-N Glu-Asp-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CC(=O)O)NC(=O)[C@H](CCC(=O)O)N)C(=O)O CKOFNWCLWRYUHK-XHNCKOQMSA-N 0.000 description 2
- RFDHKPSHTXZKLL-IHRRRGAJSA-N Glu-Gln-Phe Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)N)NC(=O)[C@H](CCC(=O)O)N RFDHKPSHTXZKLL-IHRRRGAJSA-N 0.000 description 2
- UHVIQGKBMXEVGN-WDSKDSINSA-N Glu-Gly-Asn Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)NCC(=O)N[C@@H](CC(N)=O)C(O)=O UHVIQGKBMXEVGN-WDSKDSINSA-N 0.000 description 2
- QDMVXRNLOPTPIE-WDCWCFNPSA-N Glu-Lys-Thr Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)O)C(O)=O QDMVXRNLOPTPIE-WDCWCFNPSA-N 0.000 description 2
- HGJREIGJLUQBTJ-SZMVWBNQSA-N Glu-Trp-Leu Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H](CC(C)C)C(O)=O HGJREIGJLUQBTJ-SZMVWBNQSA-N 0.000 description 2
- WGYHAAXZWPEBDQ-IFFSRLJSSA-N Glu-Val-Thr Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O WGYHAAXZWPEBDQ-IFFSRLJSSA-N 0.000 description 2
- IRJWAYCXIYUHQE-WHFBIAKZSA-N Gly-Ser-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)CN IRJWAYCXIYUHQE-WHFBIAKZSA-N 0.000 description 2
- FFJQHWKSGAWSTJ-BFHQHQDPSA-N Gly-Thr-Ala Chemical compound [H]NCC(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(O)=O FFJQHWKSGAWSTJ-BFHQHQDPSA-N 0.000 description 2
- SYOJVRNQCXYEOV-XVKPBYJWSA-N Gly-Val-Glu Chemical compound [H]NCC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O SYOJVRNQCXYEOV-XVKPBYJWSA-N 0.000 description 2
- MAABHGXCIBEYQR-XVYDVKMFSA-N His-Asn-Ala Chemical compound C[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)N)NC(=O)[C@H](CC1=CN=CN1)N MAABHGXCIBEYQR-XVYDVKMFSA-N 0.000 description 2
- HVCRQRQPIIRNLY-IUCAKERBSA-N His-Gln-Gly Chemical compound C1=C(NC=N1)C[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)NCC(=O)O)N HVCRQRQPIIRNLY-IUCAKERBSA-N 0.000 description 2
- HIAHVKLTHNOENC-HGNGGELXSA-N His-Glu-Ala Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(O)=O HIAHVKLTHNOENC-HGNGGELXSA-N 0.000 description 2
- CSTDQOOBZBAJKE-BWAGICSOSA-N His-Tyr-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CC1=CC=C(C=C1)O)NC(=O)[C@H](CC2=CN=CN2)N)O CSTDQOOBZBAJKE-BWAGICSOSA-N 0.000 description 2
- 241000282412 Homo Species 0.000 description 2
- 101000690301 Homo sapiens Aldo-keto reductase family 1 member C4 Proteins 0.000 description 2
- 101001116548 Homo sapiens Protein CBFA2T1 Proteins 0.000 description 2
- UWBDLNOCIDGPQE-GUBZILKMSA-N Ile-Lys Chemical compound CC[C@H](C)[C@H](N)C(=O)N[C@H](C(O)=O)CCCCN UWBDLNOCIDGPQE-GUBZILKMSA-N 0.000 description 2
- NPAYJTAXWXJKLO-NAKRPEOUSA-N Ile-Met-Ser Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CO)C(=O)O)N NPAYJTAXWXJKLO-NAKRPEOUSA-N 0.000 description 2
- FGBRXCZYVRFNKQ-MXAVVETBSA-N Ile-Phe-Ser Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CO)C(=O)O)N FGBRXCZYVRFNKQ-MXAVVETBSA-N 0.000 description 2
- YKZAMJXNJUWFIK-JBDRJPRFSA-N Ile-Ser-Ala Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](C)C(=O)O)N YKZAMJXNJUWFIK-JBDRJPRFSA-N 0.000 description 2
- JHNJNTMTZHEDLJ-NAKRPEOUSA-N Ile-Ser-Arg Chemical compound CC[C@H](C)[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O JHNJNTMTZHEDLJ-NAKRPEOUSA-N 0.000 description 2
- VGSPNSSCMOHRRR-BJDJZHNGSA-N Ile-Ser-Lys Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)O)N VGSPNSSCMOHRRR-BJDJZHNGSA-N 0.000 description 2
- 206010065042 Immune reconstitution inflammatory syndrome Diseases 0.000 description 2
- 102000009786 Immunoglobulin Constant Regions Human genes 0.000 description 2
- 108010009817 Immunoglobulin Constant Regions Proteins 0.000 description 2
- 108010067060 Immunoglobulin Variable Region Proteins 0.000 description 2
- 102000017727 Immunoglobulin Variable Region Human genes 0.000 description 2
- RCFDOSNHHZGBOY-UHFFFAOYSA-N L-isoleucyl-L-alanine Natural products CCC(C)C(N)C(=O)NC(C)C(O)=O RCFDOSNHHZGBOY-UHFFFAOYSA-N 0.000 description 2
- LRQKBLKVPFOOQJ-YFKPBYRVSA-N L-norleucine Chemical compound CCCC[C@H]([NH3+])C([O-])=O LRQKBLKVPFOOQJ-YFKPBYRVSA-N 0.000 description 2
- TYYLDKGBCJGJGW-UHFFFAOYSA-N L-tryptophan-L-tyrosine Natural products C=1NC2=CC=CC=C2C=1CC(N)C(=O)NC(C(O)=O)CC1=CC=C(O)C=C1 TYYLDKGBCJGJGW-UHFFFAOYSA-N 0.000 description 2
- QIVBCDIJIAJPQS-VIFPVBQESA-N L-tryptophane Chemical compound C1=CC=C2C(C[C@H](N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-VIFPVBQESA-N 0.000 description 2
- 108090001090 Lectins Proteins 0.000 description 2
- OIARJGNVARWKFP-YUMQZZPRSA-N Leu-Asn-Gly Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)NCC(O)=O OIARJGNVARWKFP-YUMQZZPRSA-N 0.000 description 2
- PVMPDMIKUVNOBD-CIUDSAMLSA-N Leu-Asp-Ser Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(O)=O PVMPDMIKUVNOBD-CIUDSAMLSA-N 0.000 description 2
- HYIFFZAQXPUEAU-QWRGUYRKSA-N Leu-Gly-Leu Chemical compound CC(C)C[C@H](N)C(=O)NCC(=O)N[C@H](C(O)=O)CC(C)C HYIFFZAQXPUEAU-QWRGUYRKSA-N 0.000 description 2
- APFJUBGRZGMQFF-QWRGUYRKSA-N Leu-Gly-Lys Chemical compound CC(C)C[C@H](N)C(=O)NCC(=O)N[C@H](C(O)=O)CCCCN APFJUBGRZGMQFF-QWRGUYRKSA-N 0.000 description 2
- BTNXKBVLWJBTNR-SRVKXCTJSA-N Leu-His-Asn Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CC(N)=O)C(O)=O BTNXKBVLWJBTNR-SRVKXCTJSA-N 0.000 description 2
- IFMPDNRWZZEZSL-SRVKXCTJSA-N Leu-Leu-Cys Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CS)C(O)=O IFMPDNRWZZEZSL-SRVKXCTJSA-N 0.000 description 2
- VCHVSKNMTXWIIP-SRVKXCTJSA-N Leu-Lys-Ser Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(O)=O VCHVSKNMTXWIIP-SRVKXCTJSA-N 0.000 description 2
- XOWMDXHFSBCAKQ-SRVKXCTJSA-N Leu-Ser-Leu Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CC(C)C XOWMDXHFSBCAKQ-SRVKXCTJSA-N 0.000 description 2
- LINKCQUOMUDLKN-KATARQTJSA-N Leu-Thr-Cys Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CC(C)C)N)O LINKCQUOMUDLKN-KATARQTJSA-N 0.000 description 2
- QWWPYKKLXWOITQ-VOAKCMCISA-N Leu-Thr-Leu Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@H](C(O)=O)CC(C)C QWWPYKKLXWOITQ-VOAKCMCISA-N 0.000 description 2
- AIQWYVFNBNNOLU-RHYQMDGZSA-N Leu-Thr-Val Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(O)=O AIQWYVFNBNNOLU-RHYQMDGZSA-N 0.000 description 2
- YQFZRHYZLARWDY-IHRRRGAJSA-N Leu-Val-Lys Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](C(O)=O)CCCCN YQFZRHYZLARWDY-IHRRRGAJSA-N 0.000 description 2
- NRQRKMYZONPCTM-CIUDSAMLSA-N Lys-Asp-Ser Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(O)=O NRQRKMYZONPCTM-CIUDSAMLSA-N 0.000 description 2
- MWVUEPNEPWMFBD-SRVKXCTJSA-N Lys-Cys-Lys Chemical compound NCCCC[C@H](N)C(=O)N[C@@H](CS)C(=O)N[C@H](C(O)=O)CCCCN MWVUEPNEPWMFBD-SRVKXCTJSA-N 0.000 description 2
- ODUQLUADRKMHOZ-JYJNAYRXSA-N Lys-Glu-Tyr Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)O)NC(=O)[C@H](CCCCN)N)O ODUQLUADRKMHOZ-JYJNAYRXSA-N 0.000 description 2
- GQFDWEDHOQRNLC-QWRGUYRKSA-N Lys-Gly-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H](N)CCCCN GQFDWEDHOQRNLC-QWRGUYRKSA-N 0.000 description 2
- FGMHXLULNHTPID-KKUMJFAQSA-N Lys-His-Lys Chemical compound NCCCC[C@H](N)C(=O)N[C@H](C(=O)N[C@@H](CCCCN)C(O)=O)CC1=CN=CN1 FGMHXLULNHTPID-KKUMJFAQSA-N 0.000 description 2
- LUTDBHBIHHREDC-IHRRRGAJSA-N Lys-Pro-Lys Chemical compound NCCCC[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(O)=O LUTDBHBIHHREDC-IHRRRGAJSA-N 0.000 description 2
- YKBSXQFZWFXFIB-VOAKCMCISA-N Lys-Thr-Lys Chemical compound NCCCC[C@H](N)C(=O)N[C@@H]([C@H](O)C)C(=O)N[C@@H](CCCCN)C(O)=O YKBSXQFZWFXFIB-VOAKCMCISA-N 0.000 description 2
- 238000000134 MTT assay Methods 0.000 description 2
- DTICLBJHRYSJLH-GUBZILKMSA-N Met-Ala-Val Chemical compound CSCC[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](C(C)C)C(O)=O DTICLBJHRYSJLH-GUBZILKMSA-N 0.000 description 2
- XOMXAVJBLRROMC-IHRRRGAJSA-N Met-Asp-Phe Chemical compound CSCC[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 XOMXAVJBLRROMC-IHRRRGAJSA-N 0.000 description 2
- CIDICGYKRUTYLE-FXQIFTODSA-N Met-Ser-Ala Chemical compound CSCC[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](C)C(O)=O CIDICGYKRUTYLE-FXQIFTODSA-N 0.000 description 2
- IHRFZLQEQVHXFA-RHYQMDGZSA-N Met-Thr-Lys Chemical compound CSCC[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@H](C(O)=O)CCCCN IHRFZLQEQVHXFA-RHYQMDGZSA-N 0.000 description 2
- 238000005481 NMR spectroscopy Methods 0.000 description 2
- KPKZJLCSROULON-QKGLWVMZSA-N Phalloidin Chemical compound N1C(=O)[C@@H]([C@@H](O)C)NC(=O)[C@H](C)NC(=O)[C@H](C[C@@](C)(O)CO)NC(=O)[C@H](C2)NC(=O)[C@H](C)NC(=O)[C@@H]3C[C@H](O)CN3C(=O)[C@@H]1CSC1=C2C2=CC=CC=C2N1 KPKZJLCSROULON-QKGLWVMZSA-N 0.000 description 2
- JOXIIFVCSATTDH-IHPCNDPISA-N Phe-Asn-Trp Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CC2=CNC3=CC=CC=C32)C(=O)O)N JOXIIFVCSATTDH-IHPCNDPISA-N 0.000 description 2
- YTILBRIUASDGBL-BZSNNMDCSA-N Phe-Leu-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CC1=CC=CC=C1 YTILBRIUASDGBL-BZSNNMDCSA-N 0.000 description 2
- JDMKQHSHKJHAHR-UHFFFAOYSA-N Phe-Phe-Leu-Tyr Natural products C=1C=C(O)C=CC=1CC(C(O)=O)NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(N)CC=1C=CC=CC=1)CC1=CC=CC=C1 JDMKQHSHKJHAHR-UHFFFAOYSA-N 0.000 description 2
- ZJPGOXWRFNKIQL-JYJNAYRXSA-N Phe-Pro-Pro Chemical compound C([C@H](N)C(=O)N1[C@@H](CCC1)C(=O)N1[C@@H](CCC1)C(O)=O)C1=CC=CC=C1 ZJPGOXWRFNKIQL-JYJNAYRXSA-N 0.000 description 2
- NJJBATPLUQHRBM-IHRRRGAJSA-N Phe-Pro-Ser Chemical compound C1C[C@H](N(C1)C(=O)[C@H](CC2=CC=CC=C2)N)C(=O)N[C@@H](CO)C(=O)O NJJBATPLUQHRBM-IHRRRGAJSA-N 0.000 description 2
- SJRQWEDYTKYHHL-SLFFLAALSA-N Phe-Tyr-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CC2=CC=C(C=C2)O)NC(=O)[C@H](CC3=CC=CC=C3)N)C(=O)O SJRQWEDYTKYHHL-SLFFLAALSA-N 0.000 description 2
- 229920001213 Polysorbate 20 Polymers 0.000 description 2
- UAYHMOIGIQZLFR-NHCYSSNCSA-N Pro-Gln-Val Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](C(C)C)C(O)=O UAYHMOIGIQZLFR-NHCYSSNCSA-N 0.000 description 2
- KIPIKSXPPLABPN-CIUDSAMLSA-N Pro-Glu-Asn Chemical compound NC(=O)C[C@@H](C(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]1CCCN1 KIPIKSXPPLABPN-CIUDSAMLSA-N 0.000 description 2
- CZCCVJUUWBMISW-FXQIFTODSA-N Pro-Ser-Cys Chemical compound C1C[C@H](NC1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CS)C(=O)O CZCCVJUUWBMISW-FXQIFTODSA-N 0.000 description 2
- SEZGGSHLMROBFX-CIUDSAMLSA-N Pro-Ser-Gln Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(N)=O)C(O)=O SEZGGSHLMROBFX-CIUDSAMLSA-N 0.000 description 2
- YDTUEBLEAVANFH-RCWTZXSCSA-N Pro-Val-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@@H]1CCCN1 YDTUEBLEAVANFH-RCWTZXSCSA-N 0.000 description 2
- 206010060862 Prostate cancer Diseases 0.000 description 2
- 208000000236 Prostatic Neoplasms Diseases 0.000 description 2
- 108010076504 Protein Sorting Signals Proteins 0.000 description 2
- 108010008281 Recombinant Fusion Proteins Proteins 0.000 description 2
- 102000007056 Recombinant Fusion Proteins Human genes 0.000 description 2
- 206010039491 Sarcoma Diseases 0.000 description 2
- 238000012300 Sequence Analysis Methods 0.000 description 2
- BRKHVZNDAOMAHX-BIIVOSGPSA-N Ser-Ala-Pro Chemical compound C[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CO)N BRKHVZNDAOMAHX-BIIVOSGPSA-N 0.000 description 2
- HQTKVSCNCDLXSX-BQBZGAKWSA-N Ser-Arg-Gly Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(O)=O HQTKVSCNCDLXSX-BQBZGAKWSA-N 0.000 description 2
- QPFJSHSJFIYDJZ-GHCJXIJMSA-N Ser-Asp-Ile Chemical compound CC[C@H](C)[C@@H](C(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](N)CO QPFJSHSJFIYDJZ-GHCJXIJMSA-N 0.000 description 2
- BPMRXBZYPGYPJN-WHFBIAKZSA-N Ser-Gly-Asn Chemical compound [H]N[C@@H](CO)C(=O)NCC(=O)N[C@@H](CC(N)=O)C(O)=O BPMRXBZYPGYPJN-WHFBIAKZSA-N 0.000 description 2
- GZSZPKSBVAOGIE-CIUDSAMLSA-N Ser-Lys-Ala Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(O)=O GZSZPKSBVAOGIE-CIUDSAMLSA-N 0.000 description 2
- HDBOEVPDIDDEPC-CIUDSAMLSA-N Ser-Lys-Asn Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(O)=O HDBOEVPDIDDEPC-CIUDSAMLSA-N 0.000 description 2
- NIOYDASGXWLHEZ-CIUDSAMLSA-N Ser-Met-Glu Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CCC(O)=O)C(O)=O NIOYDASGXWLHEZ-CIUDSAMLSA-N 0.000 description 2
- GDUZTEQRAOXYJS-SRVKXCTJSA-N Ser-Phe-Asn Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)O)NC(=O)[C@H](CO)N GDUZTEQRAOXYJS-SRVKXCTJSA-N 0.000 description 2
- WLJPJRGQRNCIQS-ZLUOBGJFSA-N Ser-Ser-Asn Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(O)=O WLJPJRGQRNCIQS-ZLUOBGJFSA-N 0.000 description 2
- VGQVAVQWKJLIRM-FXQIFTODSA-N Ser-Ser-Val Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(O)=O VGQVAVQWKJLIRM-FXQIFTODSA-N 0.000 description 2
- HSWXBJCBYSWBPT-GUBZILKMSA-N Ser-Val-Val Chemical compound CC(C)[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)CO)C(C)C)C(O)=O HSWXBJCBYSWBPT-GUBZILKMSA-N 0.000 description 2
- GFDUZZACIWNMPE-KZVJFYERSA-N Thr-Ala-Met Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCSC)C(O)=O GFDUZZACIWNMPE-KZVJFYERSA-N 0.000 description 2
- DCLBXIWHLVEPMQ-JRQIVUDYSA-N Thr-Asp-Tyr Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 DCLBXIWHLVEPMQ-JRQIVUDYSA-N 0.000 description 2
- GXUWHVZYDAHFSV-FLBSBUHZSA-N Thr-Ile-Thr Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(O)=O GXUWHVZYDAHFSV-FLBSBUHZSA-N 0.000 description 2
- YOOAQCZYZHGUAZ-KATARQTJSA-N Thr-Leu-Ser Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(O)=O YOOAQCZYZHGUAZ-KATARQTJSA-N 0.000 description 2
- XKWABWFMQXMUMT-HJGDQZAQSA-N Thr-Pro-Glu Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(O)=O XKWABWFMQXMUMT-HJGDQZAQSA-N 0.000 description 2
- ZESGVALRVJIVLZ-VFCFLDTKSA-N Thr-Thr-Pro Chemical compound C[C@H]([C@@H](C(=O)N[C@@H]([C@@H](C)O)C(=O)N1CCC[C@@H]1C(=O)O)N)O ZESGVALRVJIVLZ-VFCFLDTKSA-N 0.000 description 2
- CYCGARJWIQWPQM-YJRXYDGGSA-N Thr-Tyr-Ser Chemical compound C[C@@H](O)[C@H]([NH3+])C(=O)N[C@H](C(=O)N[C@@H](CO)C([O-])=O)CC1=CC=C(O)C=C1 CYCGARJWIQWPQM-YJRXYDGGSA-N 0.000 description 2
- BGDKAVGWHJFAGW-UHFFFAOYSA-N Tropicamide Chemical compound C=1C=CC=CC=1C(CO)C(=O)N(CC)CC1=CC=NC=C1 BGDKAVGWHJFAGW-UHFFFAOYSA-N 0.000 description 2
- UDCHKDYNMRJYMI-QEJZJMRPSA-N Trp-Glu-Ser Chemical compound [H]N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(O)=O UDCHKDYNMRJYMI-QEJZJMRPSA-N 0.000 description 2
- QIVBCDIJIAJPQS-UHFFFAOYSA-N Tryptophan Natural products C1=CC=C2C(CC(N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-UHFFFAOYSA-N 0.000 description 2
- QJBWZNTWJSZUOY-UWJYBYFXSA-N Tyr-Ala-Cys Chemical compound C[C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CC1=CC=C(C=C1)O)N QJBWZNTWJSZUOY-UWJYBYFXSA-N 0.000 description 2
- SMLCYZYQFRTLCO-UWJYBYFXSA-N Tyr-Cys-Ala Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CS)C(=O)N[C@@H](C)C(O)=O SMLCYZYQFRTLCO-UWJYBYFXSA-N 0.000 description 2
- AUZADXNWQMBZOO-JYJNAYRXSA-N Tyr-Pro-Arg Chemical compound C([C@H](N)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCCN=C(N)N)C(O)=O)C1=CC=C(O)C=C1 AUZADXNWQMBZOO-JYJNAYRXSA-N 0.000 description 2
- RWOKVQUCENPXGE-IHRRRGAJSA-N Tyr-Ser-Arg Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O RWOKVQUCENPXGE-IHRRRGAJSA-N 0.000 description 2
- PWKMJDQXKCENMF-MEYUZBJRSA-N Tyr-Thr-Leu Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(O)=O PWKMJDQXKCENMF-MEYUZBJRSA-N 0.000 description 2
- SQUMHUZLJDUROQ-YDHLFZDLSA-N Tyr-Val-Asp Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(O)=O)C(O)=O SQUMHUZLJDUROQ-YDHLFZDLSA-N 0.000 description 2
- CGGVNFJRZJUVAE-BYULHYEWSA-N Val-Asp-Asn Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N[C@@H](CC(=O)N)C(=O)O)N CGGVNFJRZJUVAE-BYULHYEWSA-N 0.000 description 2
- PYXQBKJPHNCTNW-CYDGBPFRSA-N Val-Ile-Met Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCSC)C(=O)O)NC(=O)[C@H](C(C)C)N PYXQBKJPHNCTNW-CYDGBPFRSA-N 0.000 description 2
- XTDDIVQWDXMRJL-IHRRRGAJSA-N Val-Leu-His Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)NC(=O)[C@H](C(C)C)N XTDDIVQWDXMRJL-IHRRRGAJSA-N 0.000 description 2
- BTWMICVCQLKKNR-DCAQKATOSA-N Val-Leu-Ser Chemical compound CC(C)[C@H]([NH3+])C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C([O-])=O BTWMICVCQLKKNR-DCAQKATOSA-N 0.000 description 2
- SYSWVVCYSXBVJG-RHYQMDGZSA-N Val-Leu-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C(C)C)N)O SYSWVVCYSXBVJG-RHYQMDGZSA-N 0.000 description 2
- FMQGYTMERWBMSI-HJWJTTGWSA-N Val-Phe-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)[C@H](CC1=CC=CC=C1)NC(=O)[C@H](C(C)C)N FMQGYTMERWBMSI-HJWJTTGWSA-N 0.000 description 2
- KISFXYYRKKNLOP-IHRRRGAJSA-N Val-Phe-Ser Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CO)C(=O)O)N KISFXYYRKKNLOP-IHRRRGAJSA-N 0.000 description 2
- XBJKAZATRJBDCU-GUBZILKMSA-N Val-Pro-Ala Chemical compound CC(C)[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(O)=O XBJKAZATRJBDCU-GUBZILKMSA-N 0.000 description 2
- KSFXWENSJABBFI-ZKWXMUAHSA-N Val-Ser-Asn Chemical compound [H]N[C@@H](C(C)C)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(O)=O KSFXWENSJABBFI-ZKWXMUAHSA-N 0.000 description 2
- RYHUIHUOYRNNIE-NRPADANISA-N Val-Ser-Gln Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(=O)N)C(=O)O)N RYHUIHUOYRNNIE-NRPADANISA-N 0.000 description 2
- BZDGLJPROOOUOZ-XGEHTFHBSA-N Val-Thr-Cys Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](C(C)C)N)O BZDGLJPROOOUOZ-XGEHTFHBSA-N 0.000 description 2
- UVHFONIHVHLDDQ-IFFSRLJSSA-N Val-Thr-Glu Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)O)NC(=O)[C@H](C(C)C)N)O UVHFONIHVHLDDQ-IFFSRLJSSA-N 0.000 description 2
- ZLNYBMWGPOKSLW-LSJOCFKGSA-N Val-Val-Asp Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(O)=O)C(O)=O ZLNYBMWGPOKSLW-LSJOCFKGSA-N 0.000 description 2
- LLJLBRRXKZTTRD-GUBZILKMSA-N Val-Val-Ser Chemical compound CC(C)[C@@H](C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CO)C(=O)O)N LLJLBRRXKZTTRD-GUBZILKMSA-N 0.000 description 2
- 230000002159 abnormal effect Effects 0.000 description 2
- -1 about 60% identity Chemical class 0.000 description 2
- 238000002835 absorbance Methods 0.000 description 2
- 238000010521 absorption reaction Methods 0.000 description 2
- 238000009825 accumulation Methods 0.000 description 2
- 230000035508 accumulation Effects 0.000 description 2
- 238000001042 affinity chromatography Methods 0.000 description 2
- 108010041407 alanylaspartic acid Proteins 0.000 description 2
- 230000004075 alteration Effects 0.000 description 2
- 230000003444 anaesthetic effect Effects 0.000 description 2
- 230000010056 antibody-dependent cellular cytotoxicity Effects 0.000 description 2
- 108010052670 arginyl-glutamyl-glutamic acid Proteins 0.000 description 2
- 108010009111 arginyl-glycyl-glutamic acid Proteins 0.000 description 2
- 108010068265 aspartyltyrosine Proteins 0.000 description 2
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 2
- 230000008901 benefit Effects 0.000 description 2
- 229960002685 biotin Drugs 0.000 description 2
- 235000020958 biotin Nutrition 0.000 description 2
- 239000011616 biotin Substances 0.000 description 2
- 210000004369 blood Anatomy 0.000 description 2
- 239000008280 blood Substances 0.000 description 2
- 230000037396 body weight Effects 0.000 description 2
- 239000008366 buffered solution Substances 0.000 description 2
- 230000015556 catabolic process Effects 0.000 description 2
- 230000004663 cell proliferation Effects 0.000 description 2
- 238000001516 cell proliferation assay Methods 0.000 description 2
- 238000004587 chromatography analysis Methods 0.000 description 2
- 230000002759 chromosomal effect Effects 0.000 description 2
- 230000004540 complement-dependent cytotoxicity Effects 0.000 description 2
- 239000002299 complementary DNA Substances 0.000 description 2
- 230000008878 coupling Effects 0.000 description 2
- 238000010168 coupling process Methods 0.000 description 2
- 238000005859 coupling reaction Methods 0.000 description 2
- 238000006731 degradation reaction Methods 0.000 description 2
- 238000012217 deletion Methods 0.000 description 2
- 230000037430 deletion Effects 0.000 description 2
- 230000001419 dependent effect Effects 0.000 description 2
- FSXRLASFHBWESK-UHFFFAOYSA-N dipeptide phenylalanyl-tyrosine Natural products C=1C=C(O)C=CC=1CC(C(O)=O)NC(=O)C(N)CC1=CC=CC=C1 FSXRLASFHBWESK-UHFFFAOYSA-N 0.000 description 2
- 238000010494 dissociation reaction Methods 0.000 description 2
- 230000005593 dissociations Effects 0.000 description 2
- 230000013020 embryo development Effects 0.000 description 2
- HKSZLNNOFSGOKW-UHFFFAOYSA-N ent-staurosporine Natural products C12=C3N4C5=CC=CC=C5C3=C3CNC(=O)C3=C2C2=CC=CC=C2N1C1CC(NC)C(OC)C4(C)O1 HKSZLNNOFSGOKW-UHFFFAOYSA-N 0.000 description 2
- 230000007613 environmental effect Effects 0.000 description 2
- 238000011156 evaluation Methods 0.000 description 2
- 238000002474 experimental method Methods 0.000 description 2
- 229940012356 eye drops Drugs 0.000 description 2
- 210000000744 eyelid Anatomy 0.000 description 2
- 229950003499 fibrin Drugs 0.000 description 2
- 238000000684 flow cytometry Methods 0.000 description 2
- 230000002068 genetic effect Effects 0.000 description 2
- 108010080575 glutamyl-aspartyl-alanine Proteins 0.000 description 2
- 108010013768 glutamyl-aspartyl-proline Proteins 0.000 description 2
- 108010049041 glutamylalanine Proteins 0.000 description 2
- 108010082286 glycyl-seryl-alanine Proteins 0.000 description 2
- 108010010147 glycylglutamine Proteins 0.000 description 2
- 239000005556 hormone Substances 0.000 description 2
- 229940088597 hormone Drugs 0.000 description 2
- 102000054751 human RUNX1T1 Human genes 0.000 description 2
- 230000001900 immune effect Effects 0.000 description 2
- 210000000987 immune system Anatomy 0.000 description 2
- 230000003053 immunization Effects 0.000 description 2
- 230000006872 improvement Effects 0.000 description 2
- 208000027866 inflammatory disease Diseases 0.000 description 2
- 230000004941 influx Effects 0.000 description 2
- 238000007918 intramuscular administration Methods 0.000 description 2
- 238000007919 intrasynovial administration Methods 0.000 description 2
- 108010073472 leucyl-prolyl-proline Proteins 0.000 description 2
- 108010057821 leucylproline Proteins 0.000 description 2
- 210000004698 lymphocyte Anatomy 0.000 description 2
- 108010003700 lysyl aspartic acid Proteins 0.000 description 2
- 229920002521 macromolecule Polymers 0.000 description 2
- 238000013507 mapping Methods 0.000 description 2
- 238000005259 measurement Methods 0.000 description 2
- 230000007246 mechanism Effects 0.000 description 2
- 238000002844 melting Methods 0.000 description 2
- 230000008018 melting Effects 0.000 description 2
- 230000001394 metastastic effect Effects 0.000 description 2
- 238000000520 microinjection Methods 0.000 description 2
- 230000005012 migration Effects 0.000 description 2
- 238000013508 migration Methods 0.000 description 2
- 230000001613 neoplastic effect Effects 0.000 description 2
- 230000007935 neutral effect Effects 0.000 description 2
- 239000001301 oxygen Substances 0.000 description 2
- 229910052760 oxygen Inorganic materials 0.000 description 2
- 239000000256 polyoxyethylene sorbitan monolaurate Substances 0.000 description 2
- 235000010486 polyoxyethylene sorbitan monolaurate Nutrition 0.000 description 2
- 238000001556 precipitation Methods 0.000 description 2
- 230000002062 proliferating effect Effects 0.000 description 2
- 108010077112 prolyl-proline Proteins 0.000 description 2
- 210000001747 pupil Anatomy 0.000 description 2
- 238000010188 recombinant method Methods 0.000 description 2
- 238000011084 recovery Methods 0.000 description 2
- 230000002829 reductive effect Effects 0.000 description 2
- 230000004043 responsiveness Effects 0.000 description 2
- 230000037390 scarring Effects 0.000 description 2
- 230000028327 secretion Effects 0.000 description 2
- 230000037432 silent mutation Effects 0.000 description 2
- 239000011780 sodium chloride Substances 0.000 description 2
- 239000001488 sodium phosphate Substances 0.000 description 2
- 229910000162 sodium phosphate Inorganic materials 0.000 description 2
- 239000002904 solvent Substances 0.000 description 2
- HKSZLNNOFSGOKW-FYTWVXJKSA-N staurosporine Chemical compound C12=C3N4C5=CC=CC=C5C3=C3CNC(=O)C3=C2C2=CC=CC=C2N1[C@H]1C[C@@H](NC)[C@@H](OC)[C@]4(C)O1 HKSZLNNOFSGOKW-FYTWVXJKSA-N 0.000 description 2
- CGPUWJWCVCFERF-UHFFFAOYSA-N staurosporine Natural products C12=C3N4C5=CC=CC=C5C3=C3CNC(=O)C3=C2C2=CC=CC=C2N1C1CC(NC)C(OC)C4(OC)O1 CGPUWJWCVCFERF-UHFFFAOYSA-N 0.000 description 2
- 230000000638 stimulation Effects 0.000 description 2
- 230000004083 survival effect Effects 0.000 description 2
- 230000009885 systemic effect Effects 0.000 description 2
- 238000002560 therapeutic procedure Methods 0.000 description 2
- 108010071097 threonyl-lysyl-proline Proteins 0.000 description 2
- 238000002627 tracheal intubation Methods 0.000 description 2
- 238000012546 transfer Methods 0.000 description 2
- RYFMWSXOAZQYPI-UHFFFAOYSA-K trisodium phosphate Chemical compound [Na+].[Na+].[Na+].[O-]P([O-])([O-])=O RYFMWSXOAZQYPI-UHFFFAOYSA-K 0.000 description 2
- 229960004791 tropicamide Drugs 0.000 description 2
- 108010080629 tryptophan-leucine Proteins 0.000 description 2
- 108010044292 tryptophyltyrosine Proteins 0.000 description 2
- 108010079202 tyrosyl-alanyl-cysteine Proteins 0.000 description 2
- 108010051110 tyrosyl-lysine Proteins 0.000 description 2
- 108010071635 tyrosyl-prolyl-arginine Proteins 0.000 description 2
- 241001529453 unidentified herpesvirus Species 0.000 description 2
- 108010052774 valyl-lysyl-glycyl-phenylalanyl-tyrosine Proteins 0.000 description 2
- 238000011179 visual inspection Methods 0.000 description 2
- 210000004127 vitreous body Anatomy 0.000 description 2
- 238000005406 washing Methods 0.000 description 2
- 108091032973 (ribonucleotides)n+m Proteins 0.000 description 1
- UKAUYVFTDYCKQA-UHFFFAOYSA-N -2-Amino-4-hydroxybutanoic acid Natural products OC(=O)C(N)CCO UKAUYVFTDYCKQA-UHFFFAOYSA-N 0.000 description 1
- ZGEGCLOFRBLKSE-UHFFFAOYSA-N 1-Heptene Chemical class CCCCCC=C ZGEGCLOFRBLKSE-UHFFFAOYSA-N 0.000 description 1
- CPKVUHPKYQGHMW-UHFFFAOYSA-N 1-ethenylpyrrolidin-2-one;molecular iodine Chemical compound II.C=CN1CCCC1=O CPKVUHPKYQGHMW-UHFFFAOYSA-N 0.000 description 1
- BFSVOASYOCHEOV-UHFFFAOYSA-N 2-diethylaminoethanol Chemical compound CCN(CC)CCO BFSVOASYOCHEOV-UHFFFAOYSA-N 0.000 description 1
- UAIUNKRWKOVEES-UHFFFAOYSA-N 3,3',5,5'-tetramethylbenzidine Chemical compound CC1=C(N)C(C)=CC(C=2C=C(C)C(N)=C(C)C=2)=C1 UAIUNKRWKOVEES-UHFFFAOYSA-N 0.000 description 1
- AJBVYEYZVYPFCF-CIUDSAMLSA-N Ala-Lys-Asn Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(O)=O AJBVYEYZVYPFCF-CIUDSAMLSA-N 0.000 description 1
- ZDILXFDENZVOTL-BPNCWPANSA-N Ala-Val-Tyr Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O ZDILXFDENZVOTL-BPNCWPANSA-N 0.000 description 1
- 102000002260 Alkaline Phosphatase Human genes 0.000 description 1
- 108020004774 Alkaline Phosphatase Proteins 0.000 description 1
- 241000143060 Americamysis bahia Species 0.000 description 1
- NLXLAEXVIDQMFP-UHFFFAOYSA-N Ammonium chloride Substances [NH4+].[Cl-] NLXLAEXVIDQMFP-UHFFFAOYSA-N 0.000 description 1
- VHUUQVKOLVNVRT-UHFFFAOYSA-N Ammonium hydroxide Chemical compound [NH4+].[OH-] VHUUQVKOLVNVRT-UHFFFAOYSA-N 0.000 description 1
- 206010002091 Anaesthesia Diseases 0.000 description 1
- 108091023037 Aptamer Proteins 0.000 description 1
- PQWTZSNVWSOFFK-FXQIFTODSA-N Arg-Asp-Asn Chemical compound C(C[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N[C@@H](CC(=O)N)C(=O)O)N)CN=C(N)N PQWTZSNVWSOFFK-FXQIFTODSA-N 0.000 description 1
- 239000004475 Arginine Substances 0.000 description 1
- USNJAPJZSGTTPX-XVSYOHENSA-N Asp-Phe-Thr Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H]([C@@H](C)O)C(O)=O USNJAPJZSGTTPX-XVSYOHENSA-N 0.000 description 1
- DCXYFEDJOCDNAF-UHFFFAOYSA-N Asparagine Natural products OC(=O)C(N)CC(N)=O DCXYFEDJOCDNAF-UHFFFAOYSA-N 0.000 description 1
- 206010003571 Astrocytoma Diseases 0.000 description 1
- 208000037260 Atherosclerotic Plaque Diseases 0.000 description 1
- 241000700663 Avipoxvirus Species 0.000 description 1
- 241000894006 Bacteria Species 0.000 description 1
- 201000004569 Blindness Diseases 0.000 description 1
- 206010006187 Breast cancer Diseases 0.000 description 1
- 208000026310 Breast neoplasm Diseases 0.000 description 1
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 1
- 102000003952 Caspase 3 Human genes 0.000 description 1
- 108090000397 Caspase 3 Proteins 0.000 description 1
- 208000018380 Chemical injury Diseases 0.000 description 1
- 206010009900 Colitis ulcerative Diseases 0.000 description 1
- 206010010904 Convulsion Diseases 0.000 description 1
- 241000557626 Corvus corax Species 0.000 description 1
- 241000699800 Cricetinae Species 0.000 description 1
- 208000011231 Crohn disease Diseases 0.000 description 1
- SHIBSTMRCDJXLN-UHFFFAOYSA-N Digoxigenin Natural products C1CC(C2C(C3(C)CCC(O)CC3CC2)CC2O)(O)C2(C)C1C1=CC(=O)OC1 SHIBSTMRCDJXLN-UHFFFAOYSA-N 0.000 description 1
- 238000012286 ELISA Assay Methods 0.000 description 1
- 108010041308 Endothelial Growth Factors Proteins 0.000 description 1
- 241000588724 Escherichia coli Species 0.000 description 1
- 108091029865 Exogenous DNA Proteins 0.000 description 1
- 108091006020 Fc-tagged proteins Proteins 0.000 description 1
- 108010049003 Fibrinogen Proteins 0.000 description 1
- 102000008946 Fibrinogen Human genes 0.000 description 1
- YCKRFDGAMUMZLT-UHFFFAOYSA-N Fluorine atom Chemical compound [F] YCKRFDGAMUMZLT-UHFFFAOYSA-N 0.000 description 1
- AVZHGSCDKIQZPQ-CIUDSAMLSA-N Glu-Arg-Ala Chemical compound C[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CCC(O)=O)C(O)=O AVZHGSCDKIQZPQ-CIUDSAMLSA-N 0.000 description 1
- INGJLBQKTRJLFO-UKJIMTQDSA-N Glu-Ile-Val Chemical compound CC(C)[C@@H](C(O)=O)NC(=O)[C@H]([C@@H](C)CC)NC(=O)[C@@H](N)CCC(O)=O INGJLBQKTRJLFO-UKJIMTQDSA-N 0.000 description 1
- QNJNPKSWAHPYGI-JYJNAYRXSA-N Glu-Phe-Leu Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@H](C(=O)N[C@@H](CC(C)C)C(O)=O)CC1=CC=CC=C1 QNJNPKSWAHPYGI-JYJNAYRXSA-N 0.000 description 1
- CQZDZKRHFWJXDF-WDSKDSINSA-N Gly-Gln-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@H](CCC(N)=O)NC(=O)CN CQZDZKRHFWJXDF-WDSKDSINSA-N 0.000 description 1
- WCORRBXVISTKQL-WHFBIAKZSA-N Gly-Ser-Ser Chemical compound NCC(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(O)=O WCORRBXVISTKQL-WHFBIAKZSA-N 0.000 description 1
- 102000009465 Growth Factor Receptors Human genes 0.000 description 1
- 108010009202 Growth Factor Receptors Proteins 0.000 description 1
- 208000009889 Herpes Simplex Diseases 0.000 description 1
- PMMYEEVYMWASQN-DMTCNVIQSA-N Hydroxyproline Chemical compound O[C@H]1CN[C@H](C(O)=O)C1 PMMYEEVYMWASQN-DMTCNVIQSA-N 0.000 description 1
- 206010020675 Hypermetropia Diseases 0.000 description 1
- 206010021143 Hypoxia Diseases 0.000 description 1
- 108010058683 Immobilized Proteins Proteins 0.000 description 1
- 102000018071 Immunoglobulin Fc Fragments Human genes 0.000 description 1
- 108010091135 Immunoglobulin Fc Fragments Proteins 0.000 description 1
- 102000008394 Immunoglobulin Fragments Human genes 0.000 description 1
- 108010021625 Immunoglobulin Fragments Proteins 0.000 description 1
- 102000006496 Immunoglobulin Heavy Chains Human genes 0.000 description 1
- 108010019476 Immunoglobulin Heavy Chains Proteins 0.000 description 1
- 102000013463 Immunoglobulin Light Chains Human genes 0.000 description 1
- 108010065825 Immunoglobulin Light Chains Proteins 0.000 description 1
- 102000012745 Immunoglobulin Subunits Human genes 0.000 description 1
- 108010079585 Immunoglobulin Subunits Proteins 0.000 description 1
- 208000007766 Kaposi sarcoma Diseases 0.000 description 1
- PWKSKIMOESPYIA-BYPYZUCNSA-N L-N-acetyl-Cysteine Chemical compound CC(=O)N[C@@H](CS)C(O)=O PWKSKIMOESPYIA-BYPYZUCNSA-N 0.000 description 1
- DCXYFEDJOCDNAF-REOHCLBHSA-N L-asparagine Chemical compound OC(=O)[C@@H](N)CC(N)=O DCXYFEDJOCDNAF-REOHCLBHSA-N 0.000 description 1
- CKLJMWTZIZZHCS-REOHCLBHSA-N L-aspartic acid Chemical compound OC(=O)[C@@H](N)CC(O)=O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 description 1
- UKAUYVFTDYCKQA-VKHMYHEASA-N L-homoserine Chemical compound OC(=O)[C@@H](N)CCO UKAUYVFTDYCKQA-VKHMYHEASA-N 0.000 description 1
- AGPKZVBTJJNPAG-WHFBIAKZSA-N L-isoleucine Chemical compound CC[C@H](C)[C@H](N)C(O)=O AGPKZVBTJJNPAG-WHFBIAKZSA-N 0.000 description 1
- ROHFNLRQFUQHCH-YFKPBYRVSA-N L-leucine Chemical compound CC(C)C[C@H](N)C(O)=O ROHFNLRQFUQHCH-YFKPBYRVSA-N 0.000 description 1
- KDXKERNSBIXSRK-YFKPBYRVSA-N L-lysine Chemical compound NCCCC[C@H](N)C(O)=O KDXKERNSBIXSRK-YFKPBYRVSA-N 0.000 description 1
- QEFRNWWLZKMPFJ-ZXPFJRLXSA-N L-methionine (R)-S-oxide Chemical compound C[S@@](=O)CC[C@H]([NH3+])C([O-])=O QEFRNWWLZKMPFJ-ZXPFJRLXSA-N 0.000 description 1
- QEFRNWWLZKMPFJ-UHFFFAOYSA-N L-methionine sulphoxide Natural products CS(=O)CCC(N)C(O)=O QEFRNWWLZKMPFJ-UHFFFAOYSA-N 0.000 description 1
- COLNVLDHVKWLRT-QMMMGPOBSA-N L-phenylalanine Chemical compound OC(=O)[C@@H](N)CC1=CC=CC=C1 COLNVLDHVKWLRT-QMMMGPOBSA-N 0.000 description 1
- OUYCCCASQSFEME-QMMMGPOBSA-N L-tyrosine Chemical compound OC(=O)[C@@H](N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-QMMMGPOBSA-N 0.000 description 1
- SBANPBVRHYIMRR-GARJFASQSA-N Leu-Ser-Pro Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CO)C(=O)N1CCC[C@@H]1C(=O)O)N SBANPBVRHYIMRR-GARJFASQSA-N 0.000 description 1
- SBANPBVRHYIMRR-UHFFFAOYSA-N Leu-Ser-Pro Natural products CC(C)CC(N)C(=O)NC(CO)C(=O)N1CCCC1C(O)=O SBANPBVRHYIMRR-UHFFFAOYSA-N 0.000 description 1
- ROHFNLRQFUQHCH-UHFFFAOYSA-N Leucine Natural products CC(C)CC(N)C(O)=O ROHFNLRQFUQHCH-UHFFFAOYSA-N 0.000 description 1
- 206010058467 Lung neoplasm malignant Diseases 0.000 description 1
- 239000004472 Lysine Substances 0.000 description 1
- 108090000988 Lysostaphin Proteins 0.000 description 1
- 231100000002 MTT assay Toxicity 0.000 description 1
- SPSSJSICDYYTQN-HJGDQZAQSA-N Met-Thr-Gln Chemical compound CSCC[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@H](C(O)=O)CCC(N)=O SPSSJSICDYYTQN-HJGDQZAQSA-N 0.000 description 1
- 206010027476 Metastases Diseases 0.000 description 1
- 241000713869 Moloney murine leukemia virus Species 0.000 description 1
- 108091061960 Naked DNA Proteins 0.000 description 1
- 206010028851 Necrosis Diseases 0.000 description 1
- 206010029260 Neuroblastoma Diseases 0.000 description 1
- 208000022873 Ocular disease Diseases 0.000 description 1
- 108700026244 Open Reading Frames Proteins 0.000 description 1
- 206010033128 Ovarian cancer Diseases 0.000 description 1
- 206010061535 Ovarian neoplasm Diseases 0.000 description 1
- 238000012408 PCR amplification Methods 0.000 description 1
- 206010061902 Pancreatic neoplasm Diseases 0.000 description 1
- 108090000526 Papain Proteins 0.000 description 1
- 102000003992 Peroxidases Human genes 0.000 description 1
- 108010009711 Phalloidine Proteins 0.000 description 1
- 108010039918 Polylysine Proteins 0.000 description 1
- 102000029797 Prion Human genes 0.000 description 1
- 108091000054 Prion Proteins 0.000 description 1
- CGBYDGAJHSOGFQ-LPEHRKFASA-N Pro-Ala-Pro Chemical compound C[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@@H]2CCCN2 CGBYDGAJHSOGFQ-LPEHRKFASA-N 0.000 description 1
- VCYJKOLZYPYGJV-AVGNSLFASA-N Pro-Arg-Leu Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(O)=O VCYJKOLZYPYGJV-AVGNSLFASA-N 0.000 description 1
- WFHYFCWBLSKEMS-KKUMJFAQSA-N Pro-Glu-Phe Chemical compound N([C@@H](CCC(=O)O)C(=O)N[C@@H](CC=1C=CC=CC=1)C(O)=O)C(=O)[C@@H]1CCCN1 WFHYFCWBLSKEMS-KKUMJFAQSA-N 0.000 description 1
- HAAQQNHQZBOWFO-LURJTMIESA-N Pro-Gly-Gly Chemical compound OC(=O)CNC(=O)CNC(=O)[C@@H]1CCCN1 HAAQQNHQZBOWFO-LURJTMIESA-N 0.000 description 1
- KCLANYCVBBTKTO-UHFFFAOYSA-N Proparacaine Chemical compound CCCOC1=CC=C(C(=O)OCCN(CC)CC)C=C1N KCLANYCVBBTKTO-UHFFFAOYSA-N 0.000 description 1
- 102000007327 Protamines Human genes 0.000 description 1
- 108010007568 Protamines Proteins 0.000 description 1
- 201000004681 Psoriasis Diseases 0.000 description 1
- 206010038848 Retinal detachment Diseases 0.000 description 1
- 201000000582 Retinoblastoma Diseases 0.000 description 1
- 206010039897 Sedation Diseases 0.000 description 1
- 229920002684 Sepharose Polymers 0.000 description 1
- HRNQLKCLPVKZNE-CIUDSAMLSA-N Ser-Ala-Leu Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(O)=O HRNQLKCLPVKZNE-CIUDSAMLSA-N 0.000 description 1
- VIIJCAQMJBHSJH-FXQIFTODSA-N Ser-Met-Ser Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CO)C(O)=O VIIJCAQMJBHSJH-FXQIFTODSA-N 0.000 description 1
- JURQXQBJKUHGJS-UHFFFAOYSA-N Ser-Ser-Ser-Ser Chemical compound OCC(N)C(=O)NC(CO)C(=O)NC(CO)C(=O)NC(CO)C(O)=O JURQXQBJKUHGJS-UHFFFAOYSA-N 0.000 description 1
- OQSQCUWQOIHECT-YJRXYDGGSA-N Ser-Tyr-Thr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H]([C@@H](C)O)C(O)=O OQSQCUWQOIHECT-YJRXYDGGSA-N 0.000 description 1
- MFQMZDPAZRZAPV-NAKRPEOUSA-N Ser-Val-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)[C@H](C(C)C)NC(=O)[C@H](CO)N MFQMZDPAZRZAPV-NAKRPEOUSA-N 0.000 description 1
- MTCFGRXMJLQNBG-UHFFFAOYSA-N Serine Natural products OCC(N)C(O)=O MTCFGRXMJLQNBG-UHFFFAOYSA-N 0.000 description 1
- 108010090804 Streptavidin Proteins 0.000 description 1
- 108010008038 Synthetic Vaccines Proteins 0.000 description 1
- 206010043276 Teratoma Diseases 0.000 description 1
- IJVNLNRVDUTWDD-MEYUZBJRSA-N Thr-Leu-Tyr Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O IJVNLNRVDUTWDD-MEYUZBJRSA-N 0.000 description 1
- NJGMALCNYAMYCB-JRQIVUDYSA-N Thr-Tyr-Asn Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC(N)=O)C(O)=O NJGMALCNYAMYCB-JRQIVUDYSA-N 0.000 description 1
- AYFVYJQAPQTCCC-UHFFFAOYSA-N Threonine Natural products CC(O)C(N)C(O)=O AYFVYJQAPQTCCC-UHFFFAOYSA-N 0.000 description 1
- 239000004473 Threonine Substances 0.000 description 1
- 108090000190 Thrombin Proteins 0.000 description 1
- 208000007536 Thrombosis Diseases 0.000 description 1
- 108091023040 Transcription factor Proteins 0.000 description 1
- 102000040945 Transcription factor Human genes 0.000 description 1
- RNDWCRUOGGQDKN-UBHSHLNASA-N Trp-Ser-Asp Chemical compound [H]N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(O)=O)C(O)=O RNDWCRUOGGQDKN-UBHSHLNASA-N 0.000 description 1
- BVOCLAPFOBSJHR-KKUMJFAQSA-N Tyr-Cys-His Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)N[C@@H](CS)C(=O)N[C@@H](CC2=CN=CN2)C(=O)O)N)O BVOCLAPFOBSJHR-KKUMJFAQSA-N 0.000 description 1
- 201000006704 Ulcerative Colitis Diseases 0.000 description 1
- 206010052428 Wound Diseases 0.000 description 1
- 229960004308 acetylcysteine Drugs 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- 230000002378 acidificating effect Effects 0.000 description 1
- 150000007513 acids Chemical class 0.000 description 1
- 230000004913 activation Effects 0.000 description 1
- 206010064930 age-related macular degeneration Diseases 0.000 description 1
- 239000000556 agonist Substances 0.000 description 1
- 150000001412 amines Chemical class 0.000 description 1
- 125000003277 amino group Chemical group 0.000 description 1
- 235000011114 ammonium hydroxide Nutrition 0.000 description 1
- 230000037005 anaesthesia Effects 0.000 description 1
- 239000012491 analyte Substances 0.000 description 1
- 210000004102 animal cell Anatomy 0.000 description 1
- 238000010171 animal model Methods 0.000 description 1
- 239000005557 antagonist Substances 0.000 description 1
- 239000003242 anti bacterial agent Substances 0.000 description 1
- 230000001772 anti-angiogenic effect Effects 0.000 description 1
- 230000001028 anti-proliverative effect Effects 0.000 description 1
- 230000002137 anti-vascular effect Effects 0.000 description 1
- 229940088710 antibiotic agent Drugs 0.000 description 1
- 230000009830 antibody antigen interaction Effects 0.000 description 1
- 238000011091 antibody purification Methods 0.000 description 1
- 210000000628 antibody-producing cell Anatomy 0.000 description 1
- 239000002246 antineoplastic agent Substances 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- ODKSFYDXXFIFQN-UHFFFAOYSA-N arginine Natural products OC(=O)C(N)CCCNC(N)=N ODKSFYDXXFIFQN-UHFFFAOYSA-N 0.000 description 1
- 210000001367 artery Anatomy 0.000 description 1
- 206010003246 arthritis Diseases 0.000 description 1
- 235000009582 asparagine Nutrition 0.000 description 1
- 229960001230 asparagine Drugs 0.000 description 1
- 235000003704 aspartic acid Nutrition 0.000 description 1
- 230000001580 bacterial effect Effects 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- OQFSQFPPLPISGP-UHFFFAOYSA-N beta-carboxyaspartic acid Natural products OC(=O)C(N)C(C(O)=O)C(O)=O OQFSQFPPLPISGP-UHFFFAOYSA-N 0.000 description 1
- 229940064804 betadine Drugs 0.000 description 1
- 239000003124 biologic agent Substances 0.000 description 1
- 230000000740 bleeding effect Effects 0.000 description 1
- 210000000601 blood cell Anatomy 0.000 description 1
- 210000000988 bone and bone Anatomy 0.000 description 1
- 210000004556 brain Anatomy 0.000 description 1
- 239000006172 buffering agent Substances 0.000 description 1
- 230000003139 buffering effect Effects 0.000 description 1
- 210000005252 bulbus oculi Anatomy 0.000 description 1
- 230000036952 cancer formation Effects 0.000 description 1
- 230000000711 cancerogenic effect Effects 0.000 description 1
- 229910052799 carbon Inorganic materials 0.000 description 1
- 239000011545 carbonate/bicarbonate buffer Substances 0.000 description 1
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 1
- UHBYWPGGCSDKFX-UHFFFAOYSA-N carboxyglutamic acid Chemical compound OC(=O)C(N)CC(C(O)=O)C(O)=O UHBYWPGGCSDKFX-UHFFFAOYSA-N 0.000 description 1
- 231100000357 carcinogen Toxicity 0.000 description 1
- 239000003183 carcinogenic agent Substances 0.000 description 1
- 239000000969 carrier Substances 0.000 description 1
- 210000000845 cartilage Anatomy 0.000 description 1
- 239000006285 cell suspension Substances 0.000 description 1
- 230000003833 cell viability Effects 0.000 description 1
- 230000001413 cellular effect Effects 0.000 description 1
- 238000012512 characterization method Methods 0.000 description 1
- 238000009614 chemical analysis method Methods 0.000 description 1
- 238000002512 chemotherapy Methods 0.000 description 1
- 210000003161 choroid Anatomy 0.000 description 1
- 230000007012 clinical effect Effects 0.000 description 1
- 238000010367 cloning Methods 0.000 description 1
- 208000029742 colonic neoplasm Diseases 0.000 description 1
- 230000009137 competitive binding Effects 0.000 description 1
- 230000030944 contact inhibition Effects 0.000 description 1
- 239000000356 contaminant Substances 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 238000005520 cutting process Methods 0.000 description 1
- ATDGTVJJHBUTRL-UHFFFAOYSA-N cyanogen bromide Chemical compound BrC#N ATDGTVJJHBUTRL-UHFFFAOYSA-N 0.000 description 1
- 235000018417 cysteine Nutrition 0.000 description 1
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 description 1
- 210000000805 cytoplasm Anatomy 0.000 description 1
- 239000000824 cytostatic agent Substances 0.000 description 1
- 230000001085 cytostatic effect Effects 0.000 description 1
- 231100000433 cytotoxic Toxicity 0.000 description 1
- 229940127089 cytotoxic agent Drugs 0.000 description 1
- 230000001472 cytotoxic effect Effects 0.000 description 1
- 238000004925 denaturation Methods 0.000 description 1
- 230000036425 denaturation Effects 0.000 description 1
- 239000007933 dermal patch Substances 0.000 description 1
- 238000003745 diagnosis Methods 0.000 description 1
- 238000002405 diagnostic procedure Methods 0.000 description 1
- 230000029087 digestion Effects 0.000 description 1
- QONQRTHLHBTMGP-UHFFFAOYSA-N digitoxigenin Natural products CC12CCC(C3(CCC(O)CC3CC3)C)C3C11OC1CC2C1=CC(=O)OC1 QONQRTHLHBTMGP-UHFFFAOYSA-N 0.000 description 1
- SHIBSTMRCDJXLN-KCZCNTNESA-N digoxigenin Chemical compound C1([C@@H]2[C@@]3([C@@](CC2)(O)[C@H]2[C@@H]([C@@]4(C)CC[C@H](O)C[C@H]4CC2)C[C@H]3O)C)=CC(=O)OC1 SHIBSTMRCDJXLN-KCZCNTNESA-N 0.000 description 1
- 239000012895 dilution Substances 0.000 description 1
- 238000010790 dilution Methods 0.000 description 1
- NJDNXYGOVLYJHP-UHFFFAOYSA-L disodium;2-(3-oxido-6-oxoxanthen-9-yl)benzoate Chemical compound [Na+].[Na+].[O-]C(=O)C1=CC=CC=C1C1=C2C=CC(=O)C=C2OC2=CC([O-])=CC=C21 NJDNXYGOVLYJHP-UHFFFAOYSA-L 0.000 description 1
- 238000009826 distribution Methods 0.000 description 1
- PMMYEEVYMWASQN-UHFFFAOYSA-N dl-hydroxyproline Natural products OC1C[NH2+]C(C([O-])=O)C1 PMMYEEVYMWASQN-UHFFFAOYSA-N 0.000 description 1
- 239000002552 dosage form Substances 0.000 description 1
- 231100000673 dose–response relationship Toxicity 0.000 description 1
- 239000000975 dye Substances 0.000 description 1
- 230000003511 endothelial effect Effects 0.000 description 1
- 239000002158 endotoxin Substances 0.000 description 1
- 239000003623 enhancer Substances 0.000 description 1
- 230000006862 enzymatic digestion Effects 0.000 description 1
- 230000002255 enzymatic effect Effects 0.000 description 1
- 230000005713 exacerbation Effects 0.000 description 1
- 238000013401 experimental design Methods 0.000 description 1
- 208000030533 eye disease Diseases 0.000 description 1
- 229940012952 fibrinogen Drugs 0.000 description 1
- 210000002950 fibroblast Anatomy 0.000 description 1
- 239000012530 fluid Substances 0.000 description 1
- GNBHRKFJIUUOQI-UHFFFAOYSA-N fluorescein Chemical compound O1C(=O)C2=CC=CC=C2C21C1=CC=C(O)C=C1OC1=CC(O)=CC=C21 GNBHRKFJIUUOQI-UHFFFAOYSA-N 0.000 description 1
- 238000002073 fluorescence micrograph Methods 0.000 description 1
- 229910052731 fluorine Inorganic materials 0.000 description 1
- 239000011737 fluorine Substances 0.000 description 1
- 238000013467 fragmentation Methods 0.000 description 1
- 238000006062 fragmentation reaction Methods 0.000 description 1
- 239000007789 gas Substances 0.000 description 1
- 238000010353 genetic engineering Methods 0.000 description 1
- 208000005017 glioblastoma Diseases 0.000 description 1
- 235000013922 glutamic acid Nutrition 0.000 description 1
- 239000004220 glutamic acid Substances 0.000 description 1
- ZDXPYRJPNDTMRX-UHFFFAOYSA-N glutamine Natural products OC(=O)C(N)CCC(N)=O ZDXPYRJPNDTMRX-UHFFFAOYSA-N 0.000 description 1
- 108010078144 glutaminyl-glycine Proteins 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 230000003862 health status Effects 0.000 description 1
- 201000011066 hemangioma Diseases 0.000 description 1
- 238000004128 high performance liquid chromatography Methods 0.000 description 1
- 239000001257 hydrogen Substances 0.000 description 1
- 125000004435 hydrogen atom Chemical group [H]* 0.000 description 1
- 230000002209 hydrophobic effect Effects 0.000 description 1
- 229960002591 hydroxyproline Drugs 0.000 description 1
- 230000001146 hypoxic effect Effects 0.000 description 1
- 238000003384 imaging method Methods 0.000 description 1
- 230000000984 immunochemical effect Effects 0.000 description 1
- 238000000760 immunoelectrophoresis Methods 0.000 description 1
- 230000016784 immunoglobulin production Effects 0.000 description 1
- 238000003364 immunohistochemistry Methods 0.000 description 1
- 238000010324 immunological assay Methods 0.000 description 1
- 238000011534 incubation Methods 0.000 description 1
- 230000006698 induction Effects 0.000 description 1
- 230000001939 inductive effect Effects 0.000 description 1
- 239000012678 infectious agent Substances 0.000 description 1
- 230000002757 inflammatory effect Effects 0.000 description 1
- 238000001802 infusion Methods 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 239000003112 inhibitor Substances 0.000 description 1
- 238000011221 initial treatment Methods 0.000 description 1
- 230000000977 initiatory effect Effects 0.000 description 1
- 238000003780 insertion Methods 0.000 description 1
- 230000037431 insertion Effects 0.000 description 1
- 230000000968 intestinal effect Effects 0.000 description 1
- 210000000936 intestine Anatomy 0.000 description 1
- 230000003834 intracellular effect Effects 0.000 description 1
- 238000007912 intraperitoneal administration Methods 0.000 description 1
- 239000007928 intraperitoneal injection Substances 0.000 description 1
- 229960000310 isoleucine Drugs 0.000 description 1
- AGPKZVBTJJNPAG-UHFFFAOYSA-N isoleucine Natural products CCC(C)C(N)C(O)=O AGPKZVBTJJNPAG-UHFFFAOYSA-N 0.000 description 1
- 230000002147 killing effect Effects 0.000 description 1
- 238000012933 kinetic analysis Methods 0.000 description 1
- 150000002605 large molecules Chemical class 0.000 description 1
- 108010034529 leucyl-lysine Proteins 0.000 description 1
- 210000000265 leukocyte Anatomy 0.000 description 1
- 239000003589 local anesthetic agent Substances 0.000 description 1
- 230000033001 locomotion Effects 0.000 description 1
- 201000005202 lung cancer Diseases 0.000 description 1
- 208000020816 lung neoplasm Diseases 0.000 description 1
- 210000001165 lymph node Anatomy 0.000 description 1
- 210000003563 lymphoid tissue Anatomy 0.000 description 1
- 230000036210 malignancy Effects 0.000 description 1
- 230000003211 malignant effect Effects 0.000 description 1
- 208000015486 malignant pancreatic neoplasm Diseases 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 230000001404 mediated effect Effects 0.000 description 1
- 238000002483 medication Methods 0.000 description 1
- 230000002503 metabolic effect Effects 0.000 description 1
- 230000009401 metastasis Effects 0.000 description 1
- 208000011645 metastatic carcinoma Diseases 0.000 description 1
- MYWUZJCMWCOHBA-VIFPVBQESA-N methamphetamine Chemical compound CN[C@@H](C)CC1=CC=CC=C1 MYWUZJCMWCOHBA-VIFPVBQESA-N 0.000 description 1
- LSDPWZHWYPCBBB-UHFFFAOYSA-O methylsulfide anion Chemical compound [SH2+]C LSDPWZHWYPCBBB-UHFFFAOYSA-O 0.000 description 1
- 238000000386 microscopy Methods 0.000 description 1
- 239000004005 microsphere Substances 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 108091005601 modified peptides Proteins 0.000 description 1
- 238000000302 molecular modelling Methods 0.000 description 1
- 230000002911 mydriatic effect Effects 0.000 description 1
- 208000010125 myocardial infarction Diseases 0.000 description 1
- 229940097496 nasal spray Drugs 0.000 description 1
- 239000007922 nasal spray Substances 0.000 description 1
- 230000017074 necrotic cell death Effects 0.000 description 1
- 210000002569 neuron Anatomy 0.000 description 1
- 231100000252 nontoxic Toxicity 0.000 description 1
- 230000003000 nontoxic effect Effects 0.000 description 1
- 235000015097 nutrients Nutrition 0.000 description 1
- 239000002674 ointment Substances 0.000 description 1
- 230000008520 organization Effects 0.000 description 1
- 201000008482 osteoarthritis Diseases 0.000 description 1
- 201000008968 osteosarcoma Diseases 0.000 description 1
- 230000036407 pain Effects 0.000 description 1
- 201000002528 pancreatic cancer Diseases 0.000 description 1
- 208000008443 pancreatic carcinoma Diseases 0.000 description 1
- 239000002245 particle Substances 0.000 description 1
- 230000037361 pathway Effects 0.000 description 1
- 230000002093 peripheral effect Effects 0.000 description 1
- 108040007629 peroxidase activity proteins Proteins 0.000 description 1
- COLNVLDHVKWLRT-UHFFFAOYSA-N phenylalanine Natural products OC(=O)C(N)CC1=CC=CC=C1 COLNVLDHVKWLRT-UHFFFAOYSA-N 0.000 description 1
- 150000003904 phospholipids Chemical class 0.000 description 1
- BZQFBWGGLXLEPQ-REOHCLBHSA-N phosphoserine Chemical compound OC(=O)[C@@H](N)COP(O)(O)=O BZQFBWGGLXLEPQ-REOHCLBHSA-N 0.000 description 1
- INAAIJLSXJJHOZ-UHFFFAOYSA-N pibenzimol Chemical compound C1CN(C)CCN1C1=CC=C(N=C(N2)C=3C=C4NC(=NC4=CC=3)C=3C=CC(O)=CC=3)C2=C1 INAAIJLSXJJHOZ-UHFFFAOYSA-N 0.000 description 1
- 230000028742 placenta development Effects 0.000 description 1
- 238000002264 polyacrylamide gel electrophoresis Methods 0.000 description 1
- 229920000656 polylysine Polymers 0.000 description 1
- 239000013641 positive control Substances 0.000 description 1
- 230000003389 potentiating effect Effects 0.000 description 1
- 239000000843 powder Substances 0.000 description 1
- 239000002244 precipitate Substances 0.000 description 1
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 1
- 229960003981 proparacaine Drugs 0.000 description 1
- 239000003380 propellant Substances 0.000 description 1
- 210000002307 prostate Anatomy 0.000 description 1
- 229940048914 protamine Drugs 0.000 description 1
- 238000000159 protein binding assay Methods 0.000 description 1
- 230000002797 proteolythic effect Effects 0.000 description 1
- 210000001938 protoplast Anatomy 0.000 description 1
- 230000001179 pupillary effect Effects 0.000 description 1
- 238000011002 quantification Methods 0.000 description 1
- 238000003156 radioimmunoprecipitation Methods 0.000 description 1
- 230000009257 reactivity Effects 0.000 description 1
- 230000008707 rearrangement Effects 0.000 description 1
- 230000006798 recombination Effects 0.000 description 1
- 238000005215 recombination Methods 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 230000008929 regeneration Effects 0.000 description 1
- 238000011069 regeneration method Methods 0.000 description 1
- 230000006884 regulation of angiogenesis Effects 0.000 description 1
- 230000026267 regulation of growth Effects 0.000 description 1
- 229920005989 resin Polymers 0.000 description 1
- 239000011347 resin Substances 0.000 description 1
- 230000000717 retained effect Effects 0.000 description 1
- 230000004264 retinal detachment Effects 0.000 description 1
- 230000002207 retinal effect Effects 0.000 description 1
- 230000001177 retroviral effect Effects 0.000 description 1
- 230000000250 revascularization Effects 0.000 description 1
- 210000003786 sclera Anatomy 0.000 description 1
- 230000036280 sedation Effects 0.000 description 1
- 230000035945 sensitivity Effects 0.000 description 1
- 230000001953 sensory effect Effects 0.000 description 1
- 239000012679 serum free medium Substances 0.000 description 1
- 238000002741 site-directed mutagenesis Methods 0.000 description 1
- HAEPBEMBOAIUPN-UHFFFAOYSA-L sodium tetrathionate Chemical compound O.O.[Na+].[Na+].[O-]S(=O)(=O)SSS([O-])(=O)=O HAEPBEMBOAIUPN-UHFFFAOYSA-L 0.000 description 1
- POECFFCNUXZPJT-UHFFFAOYSA-M sodium;carbonic acid;hydrogen carbonate Chemical compound [Na+].OC(O)=O.OC([O-])=O POECFFCNUXZPJT-UHFFFAOYSA-M 0.000 description 1
- 238000001179 sorption measurement Methods 0.000 description 1
- 208000014618 spinal cord cancer Diseases 0.000 description 1
- 230000002269 spontaneous effect Effects 0.000 description 1
- 239000007858 starting material Substances 0.000 description 1
- 238000007619 statistical method Methods 0.000 description 1
- 210000000130 stem cell Anatomy 0.000 description 1
- 238000003860 storage Methods 0.000 description 1
- 238000007920 subcutaneous administration Methods 0.000 description 1
- 239000007929 subcutaneous injection Substances 0.000 description 1
- 238000010254 subcutaneous injection Methods 0.000 description 1
- 239000006228 supernatant Substances 0.000 description 1
- 239000013589 supplement Substances 0.000 description 1
- 239000000829 suppository Substances 0.000 description 1
- 125000003831 tetrazolyl group Chemical group 0.000 description 1
- 230000008467 tissue growth Effects 0.000 description 1
- NLVFBUXFDBBNBW-PBSUHMDJSA-N tobramycin Chemical compound N[C@@H]1C[C@H](O)[C@@H](CN)O[C@@H]1O[C@H]1[C@H](O)[C@@H](O[C@@H]2[C@@H]([C@@H](N)[C@H](O)[C@@H](CO)O2)O)[C@H](N)C[C@@H]1N NLVFBUXFDBBNBW-PBSUHMDJSA-N 0.000 description 1
- 229940035275 tobrex Drugs 0.000 description 1
- 229940100611 topical cream Drugs 0.000 description 1
- 230000000699 topical effect Effects 0.000 description 1
- 229940041677 topical spray Drugs 0.000 description 1
- 231100000331 toxic Toxicity 0.000 description 1
- 230000002588 toxic effect Effects 0.000 description 1
- 239000003053 toxin Substances 0.000 description 1
- 231100000765 toxin Toxicity 0.000 description 1
- 108700012359 toxins Proteins 0.000 description 1
- FGMPLJWBKKVCDB-UHFFFAOYSA-N trans-L-hydroxy-proline Natural products ON1CCCC1C(O)=O FGMPLJWBKKVCDB-UHFFFAOYSA-N 0.000 description 1
- 238000010361 transduction Methods 0.000 description 1
- 230000026683 transduction Effects 0.000 description 1
- 238000001890 transfection Methods 0.000 description 1
- 230000001131 transforming effect Effects 0.000 description 1
- 238000013519 translation Methods 0.000 description 1
- 230000032258 transport Effects 0.000 description 1
- 230000008733 trauma Effects 0.000 description 1
- 230000001960 triggered effect Effects 0.000 description 1
- OUYCCCASQSFEME-UHFFFAOYSA-N tyrosine Natural products OC(=O)C(N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-UHFFFAOYSA-N 0.000 description 1
- 230000002792 vascular Effects 0.000 description 1
- 210000005166 vasculature Anatomy 0.000 description 1
- 210000003462 vein Anatomy 0.000 description 1
- 230000007998 vessel formation Effects 0.000 description 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 1
- 230000003442 weekly effect Effects 0.000 description 1
- 230000029663 wound healing Effects 0.000 description 1
- 238000002424 x-ray crystallography Methods 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/42—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against immunoglobulins
- C07K16/4208—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against immunoglobulins against an idiotypic determinant on Ig
- C07K16/4241—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against immunoglobulins against an idiotypic determinant on Ig against anti-human or anti-animal Ig
- C07K16/4258—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against immunoglobulins against an idiotypic determinant on Ig against anti-human or anti-animal Ig against anti-receptor Ig
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/395—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
- A61P19/02—Drugs for skeletal disorders for joint disorders, e.g. arthritis, arthrosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P27/00—Drugs for disorders of the senses
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P27/00—Drugs for disorders of the senses
- A61P27/02—Ophthalmic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2839—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the integrin superfamily
- C07K16/2842—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the integrin superfamily against integrin beta1-subunit-containing molecules, e.g. CD29, CD49
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/505—Medicinal preparations containing antigens or antibodies comprising antibodies
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/20—Immunoglobulins specific features characterized by taxonomic origin
- C07K2317/24—Immunoglobulins specific features characterized by taxonomic origin containing regions, domains or residues from different species, e.g. chimeric, humanized or veneered
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/30—Immunoglobulins specific features characterized by aspects of specificity or valency
- C07K2317/32—Immunoglobulins specific features characterized by aspects of specificity or valency specific for a neo-epitope on a complex, e.g. antibody-antigen or ligand-receptor
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/55—Fab or Fab'
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/56—Immunoglobulins specific features characterized by immunoglobulin fragments variable (Fv) region, i.e. VH and/or VL
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/73—Inducing cell death, e.g. apoptosis, necrosis or inhibition of cell proliferation
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/76—Antagonist effect on antigen, e.g. neutralization or inhibition of binding
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/90—Immunoglobulins specific features characterized by (pharmaco)kinetic aspects or by stability of the immunoglobulin
- C07K2317/92—Affinity (KD), association rate (Ka), dissociation rate (Kd) or EC50 value
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Medicinal Chemistry (AREA)
- Organic Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Pharmacology & Pharmacy (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Immunology (AREA)
- Genetics & Genomics (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Molecular Biology (AREA)
- Biochemistry (AREA)
- Biophysics (AREA)
- Heart & Thoracic Surgery (AREA)
- Ophthalmology & Optometry (AREA)
- Cardiology (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Rheumatology (AREA)
- Physical Education & Sports Medicine (AREA)
- Microbiology (AREA)
- Mycology (AREA)
- Epidemiology (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Peptides Or Proteins (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US42974302P | 2002-11-26 | 2002-11-26 | |
| US60/429,743 | 2002-11-26 |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020057009577A Division KR20050089151A (ko) | 2002-11-26 | 2003-11-26 | 혈관형성을 조절하는 α5β1 인테그린에 대한 키메라성 및인간화 항체 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20110140143A true KR20110140143A (ko) | 2011-12-30 |
Family
ID=32681936
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020117029575A Abandoned KR20110140143A (ko) | 2002-11-26 | 2003-11-26 | 혈관형성을 조절하는 α5β1 인테그린에 대한 키메라성 및 인간화 항체 |
| KR1020057009577A Abandoned KR20050089151A (ko) | 2002-11-26 | 2003-11-26 | 혈관형성을 조절하는 α5β1 인테그린에 대한 키메라성 및인간화 항체 |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020057009577A Abandoned KR20050089151A (ko) | 2002-11-26 | 2003-11-26 | 혈관형성을 조절하는 α5β1 인테그린에 대한 키메라성 및인간화 항체 |
Country Status (14)
| Country | Link |
|---|---|
| EP (2) | EP1575516B1 (enExample) |
| JP (2) | JP4741242B2 (enExample) |
| KR (2) | KR20110140143A (enExample) |
| CN (1) | CN100369930C (enExample) |
| AU (1) | AU2003298783B2 (enExample) |
| BR (1) | BR0316670A (enExample) |
| CA (1) | CA2507099C (enExample) |
| ES (1) | ES2387985T3 (enExample) |
| MX (1) | MXPA05005558A (enExample) |
| NO (1) | NO20053101L (enExample) |
| NZ (1) | NZ540562A (enExample) |
| RU (1) | RU2346701C2 (enExample) |
| WO (1) | WO2004056308A2 (enExample) |
| ZA (1) | ZA200505114B (enExample) |
Families Citing this family (38)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| MY147019A (en) | 2002-03-13 | 2012-10-15 | Biogen Idec Inc | Anti-alpha v beta 6 antibodies |
| US7276589B2 (en) | 2002-11-26 | 2007-10-02 | Pdl Biopharma, Inc. | Chimeric and humanized antibodies to α5β1 integrin that modulate angiogenesis |
| EP1755659B1 (en) | 2004-03-24 | 2011-11-02 | Abbott Biotherapeutics Corp. | Use of anti-alpha5beta1 antibodies to inhibit cancer cell proliferation |
| US20060008415A1 (en) * | 2004-06-25 | 2006-01-12 | Protein Design Labs, Inc. | Stable liquid and lyophilized formulation of proteins |
| CN102875681A (zh) * | 2005-07-08 | 2013-01-16 | 拜奥根Idec马萨诸塞公司 | 抗-αvβ6抗体及其用途 |
| CA2646611A1 (en) * | 2006-03-21 | 2008-05-22 | Genentech, Inc. | Combinatorial therapy |
| CN101448858B (zh) * | 2006-03-21 | 2013-06-12 | 健泰科生物技术公司 | 牵涉α5β1拮抗剂的联合疗法 |
| CA2652886A1 (en) * | 2006-05-24 | 2007-11-29 | Bayer Schering Pharma Aktiengesellschaft | High affinity human and humanized anti-.alpha.5.beta.1 integrin function blocking antibodies with reduced immunogenicity |
| CN101563105B (zh) | 2006-07-10 | 2013-01-23 | 拜奥根Idec马萨诸塞公司 | 用于抑制smad4-缺陷癌症的组合物和方法 |
| EP2526967A1 (en) * | 2007-07-17 | 2012-11-28 | Merck Patent GmbH | Engineered anti-alpha v-integrin hybrid antibodies |
| WO2009018226A2 (en) * | 2007-07-27 | 2009-02-05 | Facet Biotech Corporation | Pharmaceutical combinations comprising a tyrosine kinase inhibitor and an antibody against integrin alpha 1 beta 5 (cd49e) |
| AR066170A1 (es) * | 2007-09-26 | 2009-07-29 | Genentech Inc | Anti integrina alfa 5 beta 1 |
| AU2009212442C1 (en) | 2008-02-05 | 2014-07-17 | Bristol-Myers Squibb Company | Alpha 5 - beta 1 antibodies and their uses |
| KR101054725B1 (ko) * | 2008-07-31 | 2011-08-05 | 재단법인서울대학교산학협력재단 | 세포외기질 성분을 함유하는 노화조절용 조성물 및 이를이용한 노화세포의 노화조절방법 |
| CA2748161A1 (en) * | 2008-12-23 | 2010-07-01 | Astrazeneca Ab | Targeted binding agents directed to .alpha.5.beta.1 and uses thereof |
| US20120114667A1 (en) | 2008-12-23 | 2012-05-10 | Medimmune Limited | TARGETED BINDING AGENTS DIRECTED TO a5BETA1 AND USES THEREOF |
| WO2010095031A2 (en) * | 2009-02-23 | 2010-08-26 | Glenmark Pharmaceuticals S.A. | Humanized antibodies that bind to cd19 and their uses |
| MA33210B1 (fr) * | 2009-03-25 | 2012-04-02 | Genentech Inc | Nouveaux anticorps anti-a5b1 et leurs utilisations |
| CN101638429B (zh) * | 2009-08-28 | 2012-01-25 | 南方医科大学 | 一种特异性识别细胞表面整合素α3β1的小分子肽 |
| CA2784278C (en) * | 2009-12-18 | 2019-02-26 | Novartis Ag | Wash solution and method for affinity chromatography |
| RU2015102845A (ru) * | 2010-02-23 | 2015-06-10 | Санофи | АНТИТЕЛА К ИНТЕГРИНУ α2 И ИХ ПРИМЕНЕНИЯ |
| SG192667A1 (en) | 2011-02-11 | 2013-09-30 | Merck Patent Gmbh | Anti-alpha-v integrin antibody for the treatment of prostate cancer |
| EP2834273B1 (en) * | 2012-04-05 | 2018-08-22 | F.Hoffmann-La Roche Ag | Bispecific antibodies against human tweak and human il17 and uses thereof |
| EP3530284B1 (en) | 2012-12-26 | 2023-10-25 | OncoSynergy, Inc. | Anti-integrin beta1 antibody compositions and methods of use thereof |
| US10035860B2 (en) | 2013-03-15 | 2018-07-31 | Biogen Ma Inc. | Anti-alpha V beta 6 antibodies and uses thereof |
| US10035859B2 (en) | 2013-03-15 | 2018-07-31 | Biogen Ma Inc. | Anti-alpha V beta 6 antibodies and uses thereof |
| JP7414529B2 (ja) | 2017-06-07 | 2024-01-16 | シファメド・ホールディングス・エルエルシー | 血管内流体移動デバイス、システム、および使用方法 |
| EP3710076B1 (en) | 2017-11-13 | 2023-12-27 | Shifamed Holdings, LLC | Intravascular fluid movement devices, systems, and methods of use |
| EP4085965A1 (en) | 2018-02-01 | 2022-11-09 | Shifamed Holdings, LLC | Intravascular blood pumps and methods of use and manufacture |
| WO2020028537A1 (en) | 2018-07-31 | 2020-02-06 | Shifamed Holdings, Llc | Intravascaular blood pumps and methods of use |
| US12220570B2 (en) | 2018-10-05 | 2025-02-11 | Shifamed Holdings, Llc | Intravascular blood pumps and methods of use |
| WO2021011473A1 (en) | 2019-07-12 | 2021-01-21 | Shifamed Holdings, Llc | Intravascular blood pumps and methods of manufacture and use |
| WO2021016372A1 (en) | 2019-07-22 | 2021-01-28 | Shifamed Holdings, Llc | Intravascular blood pumps with struts and methods of use and manufacture |
| US12465748B2 (en) | 2019-08-07 | 2025-11-11 | Supira Medical, Inc. | Catheter blood pumps and collapsible pump housings |
| WO2021062260A1 (en) | 2019-09-25 | 2021-04-01 | Shifamed Holdings, Llc | Catheter blood pumps and collapsible blood conduits |
| US11724089B2 (en) | 2019-09-25 | 2023-08-15 | Shifamed Holdings, Llc | Intravascular blood pump systems and methods of use and control thereof |
| US12102815B2 (en) | 2019-09-25 | 2024-10-01 | Shifamed Holdings, Llc | Catheter blood pumps and collapsible pump housings |
| US12409310B2 (en) | 2019-12-11 | 2025-09-09 | Shifamed Holdings, Llc | Descending aorta and vena cava blood pumps |
Family Cites Families (28)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB8308235D0 (en) | 1983-03-25 | 1983-05-05 | Celltech Ltd | Polypeptides |
| US4816567A (en) | 1983-04-08 | 1989-03-28 | Genentech, Inc. | Recombinant immunoglobin preparations |
| US4704366A (en) * | 1984-06-22 | 1987-11-03 | Bio-Rad Laboratories, Inc. | Process for binding IgG to protein A |
| US5807715A (en) | 1984-08-27 | 1998-09-15 | The Board Of Trustees Of The Leland Stanford Junior University | Methods and transformed mammalian lymphocyte cells for producing functional antigen-binding protein including chimeric immunoglobulin |
| JPS61134325A (ja) | 1984-12-04 | 1986-06-21 | Teijin Ltd | ハイブリツド抗体遺伝子の発現方法 |
| US5618920A (en) | 1985-11-01 | 1997-04-08 | Xoma Corporation | Modular assembly of antibody genes, antibodies prepared thereby and use |
| GB8607679D0 (en) | 1986-03-27 | 1986-04-30 | Winter G P | Recombinant dna product |
| US5225539A (en) | 1986-03-27 | 1993-07-06 | Medical Research Council | Recombinant altered antibodies and methods of making altered antibodies |
| US4946778A (en) | 1987-09-21 | 1990-08-07 | Genex Corporation | Single polypeptide chain binding molecules |
| US4801687A (en) * | 1986-10-27 | 1989-01-31 | Bioprobe International, Inc. | Monoclonal antibody purification process using protein A |
| US5530101A (en) | 1988-12-28 | 1996-06-25 | Protein Design Labs, Inc. | Humanized immunoglobulins |
| GB8928874D0 (en) | 1989-12-21 | 1990-02-28 | Celltech Ltd | Humanised antibodies |
| WO1992015683A1 (en) | 1991-03-06 | 1992-09-17 | MERCK Patent Gesellschaft mit beschränkter Haftung | Humanized and chimeric monoclonal antibodies |
| DE69233482T2 (de) | 1991-05-17 | 2006-01-12 | Merck & Co., Inc. | Verfahren zur Verminderung der Immunogenität der variablen Antikörperdomänen |
| KR100252547B1 (ko) | 1991-09-05 | 2000-09-01 | 프레드 마리얀스키 | 폴리-또는 올리고누클레오티드의 세포로의 표적화된 전달 |
| CA2078539C (en) | 1991-09-18 | 2005-08-02 | Kenya Shitara | Process for producing humanized chimera antibody |
| US5565332A (en) | 1991-09-23 | 1996-10-15 | Medical Research Council | Production of chimeric antibodies - a combinatorial approach |
| GB9125979D0 (en) | 1991-12-06 | 1992-02-05 | Wellcome Found | Antibody |
| AU684041B2 (en) | 1992-02-19 | 1997-12-04 | Schering Corporation | Cloning and expression of humanized monoclonal antibodies against human interleukin-4 |
| US5733743A (en) | 1992-03-24 | 1998-03-31 | Cambridge Antibody Technology Limited | Methods for producing members of specific binding pairs |
| US5639641A (en) | 1992-09-09 | 1997-06-17 | Immunogen Inc. | Resurfacing of rodent antibodies |
| WO1995022618A1 (en) | 1994-02-22 | 1995-08-24 | Dana-Farber Cancer Institute | Nucleic acid delivery system, method of synthesis and uses thereof |
| US5705154A (en) | 1995-03-08 | 1998-01-06 | Schering Corporation | Humanized monoclonal antibodies against human interleukin-4 |
| US5961955A (en) | 1997-06-03 | 1999-10-05 | Coulter Pharmaceutical, Inc. | Radioprotectant for peptides labeled with radioisotope |
| AU746662B2 (en) * | 1998-05-08 | 2002-05-02 | Regents Of The University Of California, The | Methods for detecting and inhibiting angiogenesis |
| US6852318B1 (en) * | 1998-05-08 | 2005-02-08 | The Regents Of The University Of California | Methods for detecting and inhibiting angiogenesis |
| US7285268B2 (en) * | 2002-11-26 | 2007-10-23 | Pdl Biopharma, Inc. | Chimeric and humanized antibodies to α5β1 integrin that modulate angiogenesis |
| US9111624B2 (en) | 2013-03-22 | 2015-08-18 | Katsuyuki Fujita | Semiconductor memory device |
-
2003
- 2003-11-26 KR KR1020117029575A patent/KR20110140143A/ko not_active Abandoned
- 2003-11-26 JP JP2004562249A patent/JP4741242B2/ja not_active Expired - Lifetime
- 2003-11-26 MX MXPA05005558A patent/MXPA05005558A/es active IP Right Grant
- 2003-11-26 RU RU2005120019/15A patent/RU2346701C2/ru active
- 2003-11-26 CN CNB2003801091807A patent/CN100369930C/zh not_active Expired - Lifetime
- 2003-11-26 NZ NZ540562A patent/NZ540562A/en not_active IP Right Cessation
- 2003-11-26 WO PCT/US2003/038172 patent/WO2004056308A2/en not_active Ceased
- 2003-11-26 CA CA2507099A patent/CA2507099C/en not_active Expired - Lifetime
- 2003-11-26 ES ES03796541T patent/ES2387985T3/es not_active Expired - Lifetime
- 2003-11-26 EP EP03796541A patent/EP1575516B1/en not_active Expired - Lifetime
- 2003-11-26 AU AU2003298783A patent/AU2003298783B2/en not_active Expired
- 2003-11-26 EP EP10012456A patent/EP2351583A1/en not_active Withdrawn
- 2003-11-26 KR KR1020057009577A patent/KR20050089151A/ko not_active Abandoned
- 2003-11-26 BR BR0316670-8A patent/BR0316670A/pt not_active IP Right Cessation
-
2005
- 2005-06-23 ZA ZA200505114A patent/ZA200505114B/en unknown
- 2005-06-24 NO NO20053101A patent/NO20053101L/no not_active Application Discontinuation
-
2011
- 2011-01-06 JP JP2011000939A patent/JP2011120593A/ja active Pending
Also Published As
| Publication number | Publication date |
|---|---|
| WO2004056308A2 (en) | 2004-07-08 |
| JP2011120593A (ja) | 2011-06-23 |
| EP2351583A1 (en) | 2011-08-03 |
| ES2387985T3 (es) | 2012-10-05 |
| CN100369930C (zh) | 2008-02-20 |
| MXPA05005558A (es) | 2005-07-26 |
| EP1575516A4 (en) | 2007-09-26 |
| ZA200505114B (en) | 2007-03-28 |
| NZ540562A (en) | 2008-04-30 |
| JP2006516098A (ja) | 2006-06-22 |
| RU2346701C2 (ru) | 2009-02-20 |
| RU2005120019A (ru) | 2006-01-20 |
| WO2004056308A3 (en) | 2005-09-01 |
| AU2003298783A1 (en) | 2004-07-14 |
| AU2003298783B2 (en) | 2010-11-04 |
| CA2507099C (en) | 2013-09-24 |
| NO20053101D0 (no) | 2005-06-24 |
| BR0316670A (pt) | 2005-10-11 |
| NO20053101L (no) | 2005-08-26 |
| EP1575516B1 (en) | 2012-05-30 |
| CN1753911A (zh) | 2006-03-29 |
| EP1575516A2 (en) | 2005-09-21 |
| JP4741242B2 (ja) | 2011-08-03 |
| KR20050089151A (ko) | 2005-09-07 |
| CA2507099A1 (en) | 2004-07-08 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| CA2507099C (en) | Chimeric and humanized antibodies to .alpha.5.beta.1 integrin that modulate angiogenesis | |
| US8017116B2 (en) | Chimeric and humanized antibodies to α5β1 integrin that modulate angiogenesis | |
| US8309084B2 (en) | Chimeric and humanized antibodies to α5β1 integrin that modulate angiogenesis | |
| CN1795009B (zh) | 抗-cd33抗体及其用途 | |
| TWI852077B (zh) | 用於移除造血幹細胞的抗體藥物結合物 | |
| KR102476846B1 (ko) | 항―c―MET 항체 및 이의 용도 | |
| CN101636168A (zh) | Sp35抗体及其用途 | |
| JP2015038109A (ja) | 眼科疾患を治療する方法 | |
| JP2025166101A (ja) | 新規抗Nogo-A抗体 | |
| JP2024527982A (ja) | ヒト化抗ヒトβig-h3タンパク質とその利用 | |
| JP7136468B2 (ja) | 抗セクレトグラニンiii(scg3)抗体およびその使用 | |
| HK1089189A1 (en) | CHIMERIC AND HUMANIZED ANTIBODIES TO α5β1 INTEGRIN THAT MODULATE ANGIOGENESIS | |
| HK1089189B (en) | CHIMERIC AND HUMANIZED ANTIBODIES TO α5β1 INTEGRIN THAT MODULATE ANGIOGENESIS | |
| KR20200088061A (ko) | 혈관형성을조절하는인테그린에대한키메라성및인간화항체 | |
| HK1036016B (en) | Inhibitors of complement activation | |
| HK1036016A1 (zh) | 补体活化的抑制剂 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A107 | Divisional application of patent | ||
| A201 | Request for examination | ||
| PA0104 | Divisional application for international application |
Comment text: Divisional Application for International Patent Patent event code: PA01041R01D Patent event date: 20111209 |
|
| PA0201 | Request for examination | ||
| PG1501 | Laying open of application | ||
| E701 | Decision to grant or registration of patent right | ||
| PE0701 | Decision of registration |
Patent event code: PE07011S01D Comment text: Decision to Grant Registration Patent event date: 20120228 |
|
| NORF | Unpaid initial registration fee | ||
| PC1904 | Unpaid initial registration fee |