KR20110097923A - 항-지질 항체 결정 구조를 이용한 항체 설계 - Google Patents
항-지질 항체 결정 구조를 이용한 항체 설계 Download PDFInfo
- Publication number
- KR20110097923A KR20110097923A KR1020117015390A KR20117015390A KR20110097923A KR 20110097923 A KR20110097923 A KR 20110097923A KR 1020117015390 A KR1020117015390 A KR 1020117015390A KR 20117015390 A KR20117015390 A KR 20117015390A KR 20110097923 A KR20110097923 A KR 20110097923A
- Authority
- KR
- South Korea
- Prior art keywords
- antibody
- lipid
- antibodies
- amino acid
- optionally
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
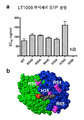
- 239000013078 crystal Substances 0.000 title claims abstract description 24
- 238000013461 design Methods 0.000 title abstract description 8
- 150000002632 lipids Chemical class 0.000 claims abstract description 209
- 230000027455 binding Effects 0.000 claims abstract description 187
- 238000000034 method Methods 0.000 claims abstract description 134
- 230000000975 bioactive effect Effects 0.000 claims abstract description 103
- 150000003408 sphingolipids Chemical class 0.000 claims abstract description 61
- 239000012634 fragment Substances 0.000 claims abstract description 53
- 239000003446 ligand Substances 0.000 claims abstract description 30
- 229910052751 metal Inorganic materials 0.000 claims abstract description 23
- 239000002184 metal Substances 0.000 claims abstract description 23
- 150000003839 salts Chemical class 0.000 claims abstract description 23
- 238000000126 in silico method Methods 0.000 claims abstract description 7
- 150000002739 metals Chemical class 0.000 claims abstract description 7
- 108090000623 proteins and genes Proteins 0.000 claims description 91
- 239000000203 mixture Substances 0.000 claims description 83
- 125000003275 alpha amino acid group Chemical group 0.000 claims description 68
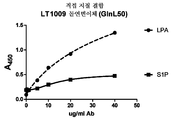
- 238000002965 ELISA Methods 0.000 claims description 61
- 102000008394 Immunoglobulin Fragments Human genes 0.000 claims description 47
- 108010021625 Immunoglobulin Fragments Proteins 0.000 claims description 47
- 230000000694 effects Effects 0.000 claims description 44
- 235000001014 amino acid Nutrition 0.000 claims description 37
- 108010047041 Complementarity Determining Regions Proteins 0.000 claims description 36
- 238000004519 manufacturing process Methods 0.000 claims description 36
- 229940024606 amino acid Drugs 0.000 claims description 33
- 150000001413 amino acids Chemical class 0.000 claims description 33
- 108010054477 Immunoglobulin Fab Fragments Proteins 0.000 claims description 24
- 102000001706 Immunoglobulin Fab Fragments Human genes 0.000 claims description 24
- 150000007523 nucleic acids Chemical group 0.000 claims description 22
- 125000000539 amino acid group Chemical group 0.000 claims description 16
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Natural products NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 claims description 14
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 claims description 13
- 108091028043 Nucleic acid sequence Proteins 0.000 claims description 12
- 239000004472 Lysine Substances 0.000 claims description 11
- CKLJMWTZIZZHCS-REOHCLBHSA-N aspartic acid group Chemical group N[C@@H](CC(=O)O)C(=O)O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 claims description 11
- 239000004473 Threonine Substances 0.000 claims description 9
- 125000003295 alanine group Chemical group N[C@@H](C)C(=O)* 0.000 claims description 9
- 235000003704 aspartic acid Nutrition 0.000 claims description 9
- HNDVDQJCIGZPNO-UHFFFAOYSA-N histidine Natural products OC(=O)C(N)CC1=CN=CN1 HNDVDQJCIGZPNO-UHFFFAOYSA-N 0.000 claims description 9
- 230000001976 improved effect Effects 0.000 claims description 9
- 238000002198 surface plasmon resonance spectroscopy Methods 0.000 claims description 9
- OUYCCCASQSFEME-QMMMGPOBSA-N L-tyrosine Chemical compound OC(=O)[C@@H](N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-QMMMGPOBSA-N 0.000 claims description 8
- MTCFGRXMJLQNBG-UHFFFAOYSA-N Serine Natural products OCC(N)C(O)=O MTCFGRXMJLQNBG-UHFFFAOYSA-N 0.000 claims description 8
- AYFVYJQAPQTCCC-UHFFFAOYSA-N Threonine Natural products CC(O)C(N)C(O)=O AYFVYJQAPQTCCC-UHFFFAOYSA-N 0.000 claims description 8
- 235000004279 alanine Nutrition 0.000 claims description 8
- OQFSQFPPLPISGP-UHFFFAOYSA-N beta-carboxyaspartic acid Natural products OC(=O)C(N)C(C(O)=O)C(O)=O OQFSQFPPLPISGP-UHFFFAOYSA-N 0.000 claims description 8
- 125000003588 lysine group Chemical group [H]N([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])(N([H])[H])C(*)=O 0.000 claims description 8
- COLNVLDHVKWLRT-QMMMGPOBSA-N phenylalanine group Chemical group N[C@@H](CC1=CC=CC=C1)C(=O)O COLNVLDHVKWLRT-QMMMGPOBSA-N 0.000 claims description 8
- 239000004471 Glycine Substances 0.000 claims description 7
- ROHFNLRQFUQHCH-YFKPBYRVSA-N L-leucine Chemical compound CC(C)C[C@H](N)C(O)=O ROHFNLRQFUQHCH-YFKPBYRVSA-N 0.000 claims description 7
- ROHFNLRQFUQHCH-UHFFFAOYSA-N Leucine Natural products CC(C)CC(N)C(O)=O ROHFNLRQFUQHCH-UHFFFAOYSA-N 0.000 claims description 7
- 238000002441 X-ray diffraction Methods 0.000 claims description 7
- COLNVLDHVKWLRT-UHFFFAOYSA-N phenylalanine Natural products OC(=O)C(N)CC1=CC=CC=C1 COLNVLDHVKWLRT-UHFFFAOYSA-N 0.000 claims description 7
- WHUUTDBJXJRKMK-UHFFFAOYSA-N Glutamic acid Natural products OC(=O)C(N)CCC(O)=O WHUUTDBJXJRKMK-UHFFFAOYSA-N 0.000 claims description 5
- 125000000341 threoninyl group Chemical group [H]OC([H])(C([H])([H])[H])C([H])(N([H])[H])C(*)=O 0.000 claims description 5
- OUYCCCASQSFEME-UHFFFAOYSA-N tyrosine Natural products OC(=O)C(N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-UHFFFAOYSA-N 0.000 claims description 5
- QIVBCDIJIAJPQS-UHFFFAOYSA-N Tryptophan Natural products C1=CC=C2C(CC(N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-UHFFFAOYSA-N 0.000 claims description 4
- 235000013922 glutamic acid Nutrition 0.000 claims description 3
- 239000004220 glutamic acid Substances 0.000 claims description 3
- 229960000310 isoleucine Drugs 0.000 claims description 3
- AGPKZVBTJJNPAG-UHFFFAOYSA-N isoleucine Natural products CCC(C)C(N)C(O)=O AGPKZVBTJJNPAG-UHFFFAOYSA-N 0.000 claims description 3
- 125000000487 histidyl group Chemical group [H]N([H])C(C(=O)O*)C([H])([H])C1=C([H])N([H])C([H])=N1 0.000 claims 4
- 238000000302 molecular modelling Methods 0.000 claims 3
- 125000003607 serino group Chemical group [H]N([H])[C@]([H])(C(=O)[*])C(O[H])([H])[H] 0.000 claims 3
- 125000000291 glutamic acid group Chemical group N[C@@H](CCC(O)=O)C(=O)* 0.000 claims 2
- 125000000741 isoleucyl group Chemical group [H]N([H])C(C(C([H])([H])[H])C([H])([H])C([H])([H])[H])C(=O)O* 0.000 claims 2
- 125000000430 tryptophan group Chemical group [H]N([H])C(C(=O)O*)C([H])([H])C1=C([H])N([H])C2=C([H])C([H])=C([H])C([H])=C12 0.000 claims 2
- 238000013500 data storage Methods 0.000 claims 1
- 125000003630 glycyl group Chemical group [H]N([H])C([H])([H])C(*)=O 0.000 claims 1
- 125000001909 leucine group Chemical group [H]N(*)C(C(*)=O)C([H])([H])C(C([H])([H])[H])C([H])([H])[H] 0.000 claims 1
- GZQKNULLWNGMCW-PWQABINMSA-N lipid A (E. coli) Chemical compound O1[C@H](CO)[C@@H](OP(O)(O)=O)[C@H](OC(=O)C[C@@H](CCCCCCCCCCC)OC(=O)CCCCCCCCCCCCC)[C@@H](NC(=O)C[C@@H](CCCCCCCCCCC)OC(=O)CCCCCCCCCCC)[C@@H]1OC[C@@H]1[C@@H](O)[C@H](OC(=O)C[C@H](O)CCCCCCCCCCC)[C@@H](NC(=O)C[C@H](O)CCCCCCCCCCC)[C@@H](OP(O)(O)=O)O1 GZQKNULLWNGMCW-PWQABINMSA-N 0.000 claims 1
- 238000005457 optimization Methods 0.000 abstract description 4
- DUYSYHSSBDVJSM-KRWOKUGFSA-N sphingosine 1-phosphate Chemical compound CCCCCCCCCCCCC\C=C\[C@@H](O)[C@@H](N)COP(O)(O)=O DUYSYHSSBDVJSM-KRWOKUGFSA-N 0.000 description 250
- 241000282414 Homo sapiens Species 0.000 description 111
- 210000004027 cell Anatomy 0.000 description 110
- 239000000427 antigen Substances 0.000 description 96
- 108091007433 antigens Proteins 0.000 description 95
- 102000036639 antigens Human genes 0.000 description 95
- 235000018102 proteins Nutrition 0.000 description 73
- 102000004169 proteins and genes Human genes 0.000 description 72
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 68
- 241001529936 Murinae Species 0.000 description 58
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 55
- 108090000765 processed proteins & peptides Proteins 0.000 description 49
- 238000011282 treatment Methods 0.000 description 44
- XUJNEKJLAYXESH-REOHCLBHSA-N L-Cysteine Chemical compound SC[C@H](N)C(O)=O XUJNEKJLAYXESH-REOHCLBHSA-N 0.000 description 43
- 108060003951 Immunoglobulin Proteins 0.000 description 42
- 102000018358 immunoglobulin Human genes 0.000 description 42
- 241001465754 Metazoa Species 0.000 description 41
- 201000010099 disease Diseases 0.000 description 39
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical group OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 37
- 230000035772 mutation Effects 0.000 description 37
- 102000004196 processed proteins & peptides Human genes 0.000 description 37
- 206010028980 Neoplasm Diseases 0.000 description 36
- 239000003795 chemical substances by application Substances 0.000 description 36
- 229920001184 polypeptide Polymers 0.000 description 36
- 230000014509 gene expression Effects 0.000 description 34
- 210000001508 eye Anatomy 0.000 description 32
- 238000009472 formulation Methods 0.000 description 32
- 229940027941 immunoglobulin g Drugs 0.000 description 32
- 150000001875 compounds Chemical class 0.000 description 31
- 239000000523 sample Substances 0.000 description 31
- 239000011780 sodium chloride Substances 0.000 description 30
- -1 HE- Chemical compound 0.000 description 29
- 208000035475 disorder Diseases 0.000 description 29
- 239000003814 drug Substances 0.000 description 29
- 239000002953 phosphate buffered saline Substances 0.000 description 29
- 210000004408 hybridoma Anatomy 0.000 description 28
- 238000006467 substitution reaction Methods 0.000 description 28
- 230000003993 interaction Effects 0.000 description 26
- 239000013612 plasmid Substances 0.000 description 26
- 238000003556 assay Methods 0.000 description 25
- 239000000872 buffer Substances 0.000 description 25
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 24
- 102000004190 Enzymes Human genes 0.000 description 24
- 108090000790 Enzymes Proteins 0.000 description 24
- 241000699670 Mus sp. Species 0.000 description 24
- 239000011575 calcium Substances 0.000 description 24
- 230000000875 corresponding effect Effects 0.000 description 24
- 229940088598 enzyme Drugs 0.000 description 24
- 241000894007 species Species 0.000 description 24
- 239000013598 vector Substances 0.000 description 24
- 108020004414 DNA Proteins 0.000 description 23
- 238000000746 purification Methods 0.000 description 23
- 230000001225 therapeutic effect Effects 0.000 description 23
- WWUZIQQURGPMPG-UHFFFAOYSA-N (-)-D-erythro-Sphingosine Natural products CCCCCCCCCCCCCC=CC(O)C(N)CO WWUZIQQURGPMPG-UHFFFAOYSA-N 0.000 description 22
- WWUZIQQURGPMPG-KRWOKUGFSA-N sphingosine Chemical compound CCCCCCCCCCCCC\C=C\[C@@H](O)[C@@H](N)CO WWUZIQQURGPMPG-KRWOKUGFSA-N 0.000 description 22
- 238000002360 preparation method Methods 0.000 description 21
- 210000002966 serum Anatomy 0.000 description 21
- 230000011664 signaling Effects 0.000 description 21
- 230000004071 biological effect Effects 0.000 description 20
- 238000006243 chemical reaction Methods 0.000 description 20
- 241000588724 Escherichia coli Species 0.000 description 19
- 239000000463 material Substances 0.000 description 19
- 230000033115 angiogenesis Effects 0.000 description 18
- 108020004707 nucleic acids Proteins 0.000 description 18
- 102000039446 nucleic acids Human genes 0.000 description 18
- 230000002441 reversible effect Effects 0.000 description 18
- 239000000243 solution Substances 0.000 description 18
- 239000000126 substance Substances 0.000 description 18
- 241000699666 Mus <mouse, genus> Species 0.000 description 17
- 201000011510 cancer Diseases 0.000 description 17
- 229940106189 ceramide Drugs 0.000 description 17
- 239000002609 medium Substances 0.000 description 17
- 238000002560 therapeutic procedure Methods 0.000 description 17
- YDNKGFDKKRUKPY-JHOUSYSJSA-N C16 ceramide Natural products CCCCCCCCCCCCCCCC(=O)N[C@@H](CO)[C@H](O)C=CCCCCCCCCCCCCC YDNKGFDKKRUKPY-JHOUSYSJSA-N 0.000 description 16
- CRJGESKKUOMBCT-VQTJNVASSA-N N-acetylsphinganine Chemical compound CCCCCCCCCCCCCCC[C@@H](O)[C@H](CO)NC(C)=O CRJGESKKUOMBCT-VQTJNVASSA-N 0.000 description 16
- 229910019142 PO4 Inorganic materials 0.000 description 16
- ZVEQCJWYRWKARO-UHFFFAOYSA-N ceramide Natural products CCCCCCCCCCCCCCC(O)C(=O)NC(CO)C(O)C=CCCC=C(C)CCCCCCCCC ZVEQCJWYRWKARO-UHFFFAOYSA-N 0.000 description 16
- 229940079593 drug Drugs 0.000 description 16
- VVGIYYKRAMHVLU-UHFFFAOYSA-N newbouldiamide Natural products CCCCCCCCCCCCCCCCCCCC(O)C(O)C(O)C(CO)NC(=O)CCCCCCCCCCCCCCCCC VVGIYYKRAMHVLU-UHFFFAOYSA-N 0.000 description 16
- 239000006228 supernatant Substances 0.000 description 16
- 230000015572 biosynthetic process Effects 0.000 description 15
- 238000010790 dilution Methods 0.000 description 15
- 239000012895 dilution Substances 0.000 description 15
- 238000001727 in vivo Methods 0.000 description 15
- 238000002347 injection Methods 0.000 description 15
- 239000007924 injection Substances 0.000 description 15
- 238000011160 research Methods 0.000 description 15
- YBJHBAHKTGYVGT-ZKWXMUAHSA-N (+)-Biotin Chemical compound N1C(=O)N[C@@H]2[C@H](CCCCC(=O)O)SC[C@@H]21 YBJHBAHKTGYVGT-ZKWXMUAHSA-N 0.000 description 14
- 241000283707 Capra Species 0.000 description 14
- 210000004978 chinese hamster ovary cell Anatomy 0.000 description 14
- 238000002474 experimental method Methods 0.000 description 14
- 229910052739 hydrogen Inorganic materials 0.000 description 14
- 229940072221 immunoglobulins Drugs 0.000 description 14
- 210000004698 lymphocyte Anatomy 0.000 description 14
- 239000002207 metabolite Substances 0.000 description 14
- 239000002773 nucleotide Substances 0.000 description 14
- 125000003729 nucleotide group Chemical group 0.000 description 14
- 239000010452 phosphate Substances 0.000 description 14
- 230000002829 reductive effect Effects 0.000 description 14
- 239000000758 substrate Substances 0.000 description 14
- 238000012360 testing method Methods 0.000 description 14
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical group [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 13
- 238000010367 cloning Methods 0.000 description 13
- 150000002500 ions Chemical class 0.000 description 13
- 239000002502 liposome Substances 0.000 description 13
- 102000005962 receptors Human genes 0.000 description 13
- 108020003175 receptors Proteins 0.000 description 13
- 210000001519 tissue Anatomy 0.000 description 13
- 208000005590 Choroidal Neovascularization Diseases 0.000 description 12
- 206010060823 Choroidal neovascularisation Diseases 0.000 description 12
- 241000282412 Homo Species 0.000 description 12
- 239000012148 binding buffer Substances 0.000 description 12
- 239000013604 expression vector Substances 0.000 description 12
- 238000011534 incubation Methods 0.000 description 12
- 230000003287 optical effect Effects 0.000 description 12
- 206010035226 Plasma cell myeloma Diseases 0.000 description 11
- 239000004480 active ingredient Substances 0.000 description 11
- 238000004458 analytical method Methods 0.000 description 11
- 238000013459 approach Methods 0.000 description 11
- 230000008901 benefit Effects 0.000 description 11
- 239000002738 chelating agent Substances 0.000 description 11
- 238000005755 formation reaction Methods 0.000 description 11
- 230000002163 immunogen Effects 0.000 description 11
- 238000000338 in vitro Methods 0.000 description 11
- 230000001965 increasing effect Effects 0.000 description 11
- 201000000050 myeloid neoplasm Diseases 0.000 description 11
- 239000002243 precursor Substances 0.000 description 11
- 239000000047 product Substances 0.000 description 11
- 230000009467 reduction Effects 0.000 description 11
- 230000004044 response Effects 0.000 description 11
- 239000011734 sodium Substances 0.000 description 11
- JLVSPVFPBBFMBE-HXSWCURESA-O sphingosylphosphocholine acid Chemical compound CCCCCCCCCCCCC\C=C\[C@@H](O)[C@@H]([NH3+])COP([O-])(=O)OCC[N+](C)(C)C JLVSPVFPBBFMBE-HXSWCURESA-O 0.000 description 11
- 206010064930 age-related macular degeneration Diseases 0.000 description 10
- 230000008859 change Effects 0.000 description 10
- 230000002860 competitive effect Effects 0.000 description 10
- 238000004132 cross linking Methods 0.000 description 10
- 229940127089 cytotoxic agent Drugs 0.000 description 10
- 230000006870 function Effects 0.000 description 10
- 230000013595 glycosylation Effects 0.000 description 10
- 238000006206 glycosylation reaction Methods 0.000 description 10
- 208000019622 heart disease Diseases 0.000 description 10
- 238000003780 insertion Methods 0.000 description 10
- 230000037431 insertion Effects 0.000 description 10
- 208000002780 macular degeneration Diseases 0.000 description 10
- 238000002703 mutagenesis Methods 0.000 description 10
- 231100000350 mutagenesis Toxicity 0.000 description 10
- 230000008569 process Effects 0.000 description 10
- 230000035755 proliferation Effects 0.000 description 10
- 239000011347 resin Substances 0.000 description 10
- 229920005989 resin Polymers 0.000 description 10
- 229940124597 therapeutic agent Drugs 0.000 description 10
- 238000005406 washing Methods 0.000 description 10
- NFGXHKASABOEEW-UHFFFAOYSA-N 1-methylethyl 11-methoxy-3,7,11-trimethyl-2,4-dodecadienoate Chemical compound COC(C)(C)CCCC(C)CC=CC(C)=CC(=O)OC(C)C NFGXHKASABOEEW-UHFFFAOYSA-N 0.000 description 9
- LYCAIKOWRPUZTN-UHFFFAOYSA-N Ethylene glycol Chemical compound OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 description 9
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 9
- 102000011011 Sphingosine 1-phosphate receptors Human genes 0.000 description 9
- 108050001083 Sphingosine 1-phosphate receptors Proteins 0.000 description 9
- 239000002253 acid Substances 0.000 description 9
- 239000002246 antineoplastic agent Substances 0.000 description 9
- 210000004369 blood Anatomy 0.000 description 9
- 239000008280 blood Substances 0.000 description 9
- 229910052791 calcium Inorganic materials 0.000 description 9
- 230000007423 decrease Effects 0.000 description 9
- 230000012010 growth Effects 0.000 description 9
- 230000000670 limiting effect Effects 0.000 description 9
- 230000002503 metabolic effect Effects 0.000 description 9
- 230000004048 modification Effects 0.000 description 9
- 238000012986 modification Methods 0.000 description 9
- 239000008194 pharmaceutical composition Substances 0.000 description 9
- 150000003904 phospholipids Chemical class 0.000 description 9
- 229920000642 polymer Polymers 0.000 description 9
- RYCNUMLMNKHWPZ-SNVBAGLBSA-N 1-acetyl-sn-glycero-3-phosphocholine Chemical compound CC(=O)OC[C@@H](O)COP([O-])(=O)OCC[N+](C)(C)C RYCNUMLMNKHWPZ-SNVBAGLBSA-N 0.000 description 8
- 108091026890 Coding region Proteins 0.000 description 8
- JZNWSCPGTDBMEW-UHFFFAOYSA-N Glycerophosphorylethanolamin Natural products NCCOP(O)(=O)OCC(O)CO JZNWSCPGTDBMEW-UHFFFAOYSA-N 0.000 description 8
- 229920001213 Polysorbate 20 Polymers 0.000 description 8
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 8
- YZXBAPSDXZZRGB-DOFZRALJSA-N arachidonic acid Chemical compound CCCCC\C=C/C\C=C/C\C=C/C\C=C/CCCC(O)=O YZXBAPSDXZZRGB-DOFZRALJSA-N 0.000 description 8
- 210000004899 c-terminal region Anatomy 0.000 description 8
- 238000012512 characterization method Methods 0.000 description 8
- 239000003153 chemical reaction reagent Substances 0.000 description 8
- 238000011161 development Methods 0.000 description 8
- 230000018109 developmental process Effects 0.000 description 8
- 208000037765 diseases and disorders Diseases 0.000 description 8
- 238000005516 engineering process Methods 0.000 description 8
- 239000003623 enhancer Substances 0.000 description 8
- 210000004602 germ cell Anatomy 0.000 description 8
- 238000003306 harvesting Methods 0.000 description 8
- 230000002209 hydrophobic effect Effects 0.000 description 8
- 238000005259 measurement Methods 0.000 description 8
- 230000007246 mechanism Effects 0.000 description 8
- 235000021317 phosphate Nutrition 0.000 description 8
- 150000008104 phosphatidylethanolamines Chemical class 0.000 description 8
- 239000000256 polyoxyethylene sorbitan monolaurate Substances 0.000 description 8
- 235000010486 polyoxyethylene sorbitan monolaurate Nutrition 0.000 description 8
- 230000002265 prevention Effects 0.000 description 8
- 230000001105 regulatory effect Effects 0.000 description 8
- 102200082919 rs35857380 Human genes 0.000 description 8
- 239000007787 solid Substances 0.000 description 8
- 150000003410 sphingosines Chemical class 0.000 description 8
- 229910052720 vanadium Inorganic materials 0.000 description 8
- QAPSNMNOIOSXSQ-YNEHKIRRSA-N 1-[(2r,4s,5r)-4-[tert-butyl(dimethyl)silyl]oxy-5-(hydroxymethyl)oxolan-2-yl]-5-methylpyrimidine-2,4-dione Chemical compound O=C1NC(=O)C(C)=CN1[C@@H]1O[C@H](CO)[C@@H](O[Si](C)(C)C(C)(C)C)C1 QAPSNMNOIOSXSQ-YNEHKIRRSA-N 0.000 description 7
- YRNWIFYIFSBPAU-UHFFFAOYSA-N 4-[4-(dimethylamino)phenyl]-n,n-dimethylaniline Chemical compound C1=CC(N(C)C)=CC=C1C1=CC=C(N(C)C)C=C1 YRNWIFYIFSBPAU-UHFFFAOYSA-N 0.000 description 7
- 241000894006 Bacteria Species 0.000 description 7
- 108091035707 Consensus sequence Proteins 0.000 description 7
- KDXKERNSBIXSRK-YFKPBYRVSA-N L-lysine Chemical compound NCCCC[C@H](N)C(O)=O KDXKERNSBIXSRK-YFKPBYRVSA-N 0.000 description 7
- ZQQLMECVOXKFJK-NXCSZAMKSA-N N-octadecanoylsphingosine 1-phosphate Chemical compound CCCCCCCCCCCCCCCCCC(=O)N[C@@H](COP(O)(O)=O)[C@H](O)\C=C\CCCCCCCCCCCCC ZQQLMECVOXKFJK-NXCSZAMKSA-N 0.000 description 7
- 108091007491 NSP3 Papain-like protease domains Proteins 0.000 description 7
- 239000002202 Polyethylene glycol Substances 0.000 description 7
- 241000251539 Vertebrata <Metazoa> Species 0.000 description 7
- 241000700605 Viruses Species 0.000 description 7
- 238000001042 affinity chromatography Methods 0.000 description 7
- AWUCVROLDVIAJX-UHFFFAOYSA-N alpha-glycerophosphate Natural products OCC(O)COP(O)(O)=O AWUCVROLDVIAJX-UHFFFAOYSA-N 0.000 description 7
- 125000004429 atom Chemical group 0.000 description 7
- 229960002685 biotin Drugs 0.000 description 7
- 235000020958 biotin Nutrition 0.000 description 7
- 239000011616 biotin Substances 0.000 description 7
- 239000000969 carrier Substances 0.000 description 7
- 238000004113 cell culture Methods 0.000 description 7
- 238000004587 chromatography analysis Methods 0.000 description 7
- 230000021615 conjugation Effects 0.000 description 7
- 238000004925 denaturation Methods 0.000 description 7
- 230000036425 denaturation Effects 0.000 description 7
- 238000001514 detection method Methods 0.000 description 7
- 150000004665 fatty acids Chemical class 0.000 description 7
- 230000004927 fusion Effects 0.000 description 7
- 102000005396 glutamine synthetase Human genes 0.000 description 7
- 108020002326 glutamine synthetase Proteins 0.000 description 7
- 230000016784 immunoglobulin production Effects 0.000 description 7
- 230000005764 inhibitory process Effects 0.000 description 7
- 208000014674 injury Diseases 0.000 description 7
- 210000003292 kidney cell Anatomy 0.000 description 7
- 239000007788 liquid Substances 0.000 description 7
- 239000000546 pharmaceutical excipient Substances 0.000 description 7
- 229920001223 polyethylene glycol Polymers 0.000 description 7
- 239000000244 polyoxyethylene sorbitan monooleate Substances 0.000 description 7
- 235000010482 polyoxyethylene sorbitan monooleate Nutrition 0.000 description 7
- 229940068968 polysorbate 80 Drugs 0.000 description 7
- 229920000053 polysorbate 80 Polymers 0.000 description 7
- 238000012552 review Methods 0.000 description 7
- 102220040182 rs587778219 Human genes 0.000 description 7
- 238000001356 surgical procedure Methods 0.000 description 7
- 238000007910 systemic administration Methods 0.000 description 7
- 125000003396 thiol group Chemical group [H]S* 0.000 description 7
- MTCFGRXMJLQNBG-REOHCLBHSA-N (2S)-2-Amino-3-hydroxypropansäure Chemical compound OC[C@H](N)C(O)=O MTCFGRXMJLQNBG-REOHCLBHSA-N 0.000 description 6
- PORPENFLTBBHSG-MGBGTMOVSA-N 1,2-dihexadecanoyl-sn-glycerol-3-phosphate Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP(O)(O)=O)OC(=O)CCCCCCCCCCCCCCC PORPENFLTBBHSG-MGBGTMOVSA-N 0.000 description 6
- TZCPCKNHXULUIY-RGULYWFUSA-N 1,2-distearoyl-sn-glycero-3-phosphoserine Chemical compound CCCCCCCCCCCCCCCCCC(=O)OC[C@H](COP(O)(=O)OC[C@H](N)C(O)=O)OC(=O)CCCCCCCCCCCCCCCCC TZCPCKNHXULUIY-RGULYWFUSA-N 0.000 description 6
- 108010088751 Albumins Proteins 0.000 description 6
- 102000009027 Albumins Human genes 0.000 description 6
- 206010003445 Ascites Diseases 0.000 description 6
- BVKZGUZCCUSVTD-UHFFFAOYSA-L Carbonate Chemical compound [O-]C([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-L 0.000 description 6
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical compound OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 description 6
- ZDXPYRJPNDTMRX-VKHMYHEASA-N L-glutamine Chemical compound OC(=O)[C@@H](N)CCC(N)=O ZDXPYRJPNDTMRX-VKHMYHEASA-N 0.000 description 6
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 6
- 108090000526 Papain Proteins 0.000 description 6
- 239000004365 Protease Substances 0.000 description 6
- 239000007983 Tris buffer Substances 0.000 description 6
- 108010073929 Vascular Endothelial Growth Factor A Proteins 0.000 description 6
- 102000005789 Vascular Endothelial Growth Factors Human genes 0.000 description 6
- 108010019530 Vascular Endothelial Growth Factors Proteins 0.000 description 6
- 238000013357 binding ELISA Methods 0.000 description 6
- 230000005754 cellular signaling Effects 0.000 description 6
- 238000005119 centrifugation Methods 0.000 description 6
- 238000002983 circular dichroism Methods 0.000 description 6
- 238000003776 cleavage reaction Methods 0.000 description 6
- 125000000151 cysteine group Chemical group N[C@@H](CS)C(=O)* 0.000 description 6
- 235000014113 dietary fatty acids Nutrition 0.000 description 6
- OTKJDMGTUTTYMP-UHFFFAOYSA-N dihydrosphingosine Natural products CCCCCCCCCCCCCCCC(O)C(N)CO OTKJDMGTUTTYMP-UHFFFAOYSA-N 0.000 description 6
- 230000002526 effect on cardiovascular system Effects 0.000 description 6
- 239000012636 effector Substances 0.000 description 6
- DEFVIWRASFVYLL-UHFFFAOYSA-N ethylene glycol bis(2-aminoethyl)tetraacetic acid Chemical compound OC(=O)CN(CC(O)=O)CCOCCOCCN(CC(O)=O)CC(O)=O DEFVIWRASFVYLL-UHFFFAOYSA-N 0.000 description 6
- 229930195729 fatty acid Natural products 0.000 description 6
- 239000000194 fatty acid Substances 0.000 description 6
- 150000002339 glycosphingolipids Chemical class 0.000 description 6
- 239000001963 growth medium Substances 0.000 description 6
- 150000002430 hydrocarbons Chemical group 0.000 description 6
- 239000001257 hydrogen Substances 0.000 description 6
- 230000003463 hyperproliferative effect Effects 0.000 description 6
- 239000003112 inhibitor Substances 0.000 description 6
- 239000011777 magnesium Substances 0.000 description 6
- SXTAYKAGBXMACB-UHFFFAOYSA-N methionine sulfoximine Chemical compound CS(=N)(=O)CCC(N)C(O)=O SXTAYKAGBXMACB-UHFFFAOYSA-N 0.000 description 6
- 102000013415 peroxidase activity proteins Human genes 0.000 description 6
- 108040007629 peroxidase activity proteins Proteins 0.000 description 6
- YBYRMVIVWMBXKQ-UHFFFAOYSA-N phenylmethanesulfonyl fluoride Chemical compound FS(=O)(=O)CC1=CC=CC=C1 YBYRMVIVWMBXKQ-UHFFFAOYSA-N 0.000 description 6
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 6
- 125000002467 phosphate group Chemical group [H]OP(=O)(O[H])O[*] 0.000 description 6
- 238000011084 recovery Methods 0.000 description 6
- 230000007017 scission Effects 0.000 description 6
- 238000000926 separation method Methods 0.000 description 6
- 238000003998 size exclusion chromatography high performance liquid chromatography Methods 0.000 description 6
- 238000002415 sodium dodecyl sulfate polyacrylamide gel electrophoresis Methods 0.000 description 6
- OTKJDMGTUTTYMP-ZWKOTPCHSA-N sphinganine Chemical compound CCCCCCCCCCCCCCC[C@@H](O)[C@@H](N)CO OTKJDMGTUTTYMP-ZWKOTPCHSA-N 0.000 description 6
- YHEDRJPUIRMZMP-ZWKOTPCHSA-N sphinganine 1-phosphate Chemical compound CCCCCCCCCCCCCCC[C@@H](O)[C@@H](N)COP(O)(O)=O YHEDRJPUIRMZMP-ZWKOTPCHSA-N 0.000 description 6
- 230000004083 survival effect Effects 0.000 description 6
- 230000000699 topical effect Effects 0.000 description 6
- 230000001988 toxicity Effects 0.000 description 6
- 231100000419 toxicity Toxicity 0.000 description 6
- LENZDBCJOHFCAS-UHFFFAOYSA-N tris Chemical compound OCC(N)(CO)CO LENZDBCJOHFCAS-UHFFFAOYSA-N 0.000 description 6
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 6
- 238000002424 x-ray crystallography Methods 0.000 description 6
- 108091032973 (ribonucleotides)n+m Proteins 0.000 description 5
- HVAUUPRFYPCOCA-AREMUKBSSA-N 2-O-acetyl-1-O-hexadecyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCCOC[C@@H](OC(C)=O)COP([O-])(=O)OCC[N+](C)(C)C HVAUUPRFYPCOCA-AREMUKBSSA-N 0.000 description 5
- OKTWQKXBJUBAKS-WQADZSDSSA-N 2-[[(e,2r,3s)-2-amino-3-hydroxyoctadec-4-enoxy]-hydroxyphosphoryl]oxyethyl-trimethylazanium;chloride Chemical compound [Cl-].CCCCCCCCCCCCC\C=C\[C@H](O)[C@H](N)COP(O)(=O)OCC[N+](C)(C)C OKTWQKXBJUBAKS-WQADZSDSSA-N 0.000 description 5
- 241000283690 Bos taurus Species 0.000 description 5
- 239000004971 Cross linker Substances 0.000 description 5
- BWGNESOTFCXPMA-UHFFFAOYSA-N Dihydrogen disulfide Chemical compound SS BWGNESOTFCXPMA-UHFFFAOYSA-N 0.000 description 5
- 238000012286 ELISA Assay Methods 0.000 description 5
- 206010016654 Fibrosis Diseases 0.000 description 5
- 102000003688 G-Protein-Coupled Receptors Human genes 0.000 description 5
- 108090000045 G-Protein-Coupled Receptors Proteins 0.000 description 5
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Chemical class OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 5
- ZWZWYGMENQVNFU-UHFFFAOYSA-N Glycerophosphorylserin Natural products OC(=O)C(N)COP(O)(=O)OCC(O)CO ZWZWYGMENQVNFU-UHFFFAOYSA-N 0.000 description 5
- 206010061218 Inflammation Diseases 0.000 description 5
- HNDVDQJCIGZPNO-YFKPBYRVSA-N L-histidine Chemical compound OC(=O)[C@@H](N)CC1=CN=CN1 HNDVDQJCIGZPNO-YFKPBYRVSA-N 0.000 description 5
- AYFVYJQAPQTCCC-GBXIJSLDSA-N L-threonine Chemical compound C[C@@H](O)[C@H](N)C(O)=O AYFVYJQAPQTCCC-GBXIJSLDSA-N 0.000 description 5
- 206010029113 Neovascularisation Diseases 0.000 description 5
- 108010003541 Platelet Activating Factor Proteins 0.000 description 5
- 108020004511 Recombinant DNA Proteins 0.000 description 5
- 230000002159 abnormal effect Effects 0.000 description 5
- 230000009471 action Effects 0.000 description 5
- 239000002671 adjuvant Substances 0.000 description 5
- 230000002411 adverse Effects 0.000 description 5
- 230000004075 alteration Effects 0.000 description 5
- 239000012491 analyte Substances 0.000 description 5
- 230000009286 beneficial effect Effects 0.000 description 5
- 150000001720 carbohydrates Chemical class 0.000 description 5
- 210000000170 cell membrane Anatomy 0.000 description 5
- 239000011248 coating agent Substances 0.000 description 5
- 238000000576 coating method Methods 0.000 description 5
- 238000002648 combination therapy Methods 0.000 description 5
- 230000000295 complement effect Effects 0.000 description 5
- 239000000356 contaminant Substances 0.000 description 5
- 238000002425 crystallisation Methods 0.000 description 5
- 230000008025 crystallization Effects 0.000 description 5
- 229960002433 cysteine Drugs 0.000 description 5
- 235000018417 cysteine Nutrition 0.000 description 5
- 238000012217 deletion Methods 0.000 description 5
- 230000037430 deletion Effects 0.000 description 5
- 230000001419 dependent effect Effects 0.000 description 5
- 238000012377 drug delivery Methods 0.000 description 5
- 230000008622 extracellular signaling Effects 0.000 description 5
- 230000004761 fibrosis Effects 0.000 description 5
- ZDXPYRJPNDTMRX-UHFFFAOYSA-N glutamine Natural products OC(=O)C(N)CCC(N)=O ZDXPYRJPNDTMRX-UHFFFAOYSA-N 0.000 description 5
- 239000003102 growth factor Substances 0.000 description 5
- NIOYUNMRJMEDGI-UHFFFAOYSA-N hexadecanal Chemical compound CCCCCCCCCCCCCCCC=O NIOYUNMRJMEDGI-UHFFFAOYSA-N 0.000 description 5
- 230000028993 immune response Effects 0.000 description 5
- 238000002649 immunization Methods 0.000 description 5
- 230000003053 immunization Effects 0.000 description 5
- 229940099472 immunoglobulin a Drugs 0.000 description 5
- 238000010348 incorporation Methods 0.000 description 5
- 230000004054 inflammatory process Effects 0.000 description 5
- 230000003834 intracellular effect Effects 0.000 description 5
- 210000004962 mammalian cell Anatomy 0.000 description 5
- 239000011159 matrix material Substances 0.000 description 5
- 239000012528 membrane Substances 0.000 description 5
- 210000004379 membrane Anatomy 0.000 description 5
- 229910021645 metal ion Inorganic materials 0.000 description 5
- 210000000056 organ Anatomy 0.000 description 5
- 230000037361 pathway Effects 0.000 description 5
- 239000012071 phase Substances 0.000 description 5
- 210000002381 plasma Anatomy 0.000 description 5
- 238000012545 processing Methods 0.000 description 5
- 102220118106 rs886041171 Human genes 0.000 description 5
- 239000001488 sodium phosphate Substances 0.000 description 5
- 229910000162 sodium phosphate Inorganic materials 0.000 description 5
- 239000007790 solid phase Substances 0.000 description 5
- 238000003860 storage Methods 0.000 description 5
- 208000024891 symptom Diseases 0.000 description 5
- 238000001890 transfection Methods 0.000 description 5
- 230000008733 trauma Effects 0.000 description 5
- RYFMWSXOAZQYPI-UHFFFAOYSA-K trisodium phosphate Chemical compound [Na+].[Na+].[Na+].[O-]P([O-])([O-])=O RYFMWSXOAZQYPI-UHFFFAOYSA-K 0.000 description 5
- ZDRVLAOYDGQLFI-UHFFFAOYSA-N 4-[[4-(4-chlorophenyl)-1,3-thiazol-2-yl]amino]phenol;hydrochloride Chemical group Cl.C1=CC(O)=CC=C1NC1=NC(C=2C=CC(Cl)=CC=2)=CS1 ZDRVLAOYDGQLFI-UHFFFAOYSA-N 0.000 description 4
- 229920000936 Agarose Polymers 0.000 description 4
- 102100033620 Calponin-1 Human genes 0.000 description 4
- 208000031229 Cardiomyopathies Diseases 0.000 description 4
- HAYVTMHUNMMXCV-IMJSIDKUSA-N Cys-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@@H](N)CS HAYVTMHUNMMXCV-IMJSIDKUSA-N 0.000 description 4
- 108090000695 Cytokines Proteins 0.000 description 4
- 102000004127 Cytokines Human genes 0.000 description 4
- 102220573621 Galectin-1_F4V_mutation Human genes 0.000 description 4
- 206010019280 Heart failures Diseases 0.000 description 4
- 101000945318 Homo sapiens Calponin-1 Proteins 0.000 description 4
- 101000935587 Homo sapiens Flavin reductase (NADPH) Proteins 0.000 description 4
- 101000652736 Homo sapiens Transgelin Proteins 0.000 description 4
- 108010001336 Horseradish Peroxidase Proteins 0.000 description 4
- 108010091358 Hypoxanthine Phosphoribosyltransferase Proteins 0.000 description 4
- 102100029098 Hypoxanthine-guanine phosphoribosyltransferase Human genes 0.000 description 4
- 108700005091 Immunoglobulin Genes Proteins 0.000 description 4
- 206010027476 Metastases Diseases 0.000 description 4
- 108091034117 Oligonucleotide Proteins 0.000 description 4
- 241000283973 Oryctolagus cuniculus Species 0.000 description 4
- 206010057190 Respiratory tract infections Diseases 0.000 description 4
- 240000004808 Saccharomyces cerevisiae Species 0.000 description 4
- 235000014680 Saccharomyces cerevisiae Nutrition 0.000 description 4
- ATBOMIWRCZXYSZ-XZBBILGWSA-N [1-[2,3-dihydroxypropoxy(hydroxy)phosphoryl]oxy-3-hexadecanoyloxypropan-2-yl] (9e,12e)-octadeca-9,12-dienoate Chemical compound CCCCCCCCCCCCCCCC(=O)OCC(COP(O)(=O)OCC(O)CO)OC(=O)CCCCCCC\C=C\C\C=C\CCCCC ATBOMIWRCZXYSZ-XZBBILGWSA-N 0.000 description 4
- 230000003321 amplification Effects 0.000 description 4
- 230000002491 angiogenic effect Effects 0.000 description 4
- 238000010171 animal model Methods 0.000 description 4
- 230000006907 apoptotic process Effects 0.000 description 4
- 229940114079 arachidonic acid Drugs 0.000 description 4
- 235000021342 arachidonic acid Nutrition 0.000 description 4
- 230000001413 cellular effect Effects 0.000 description 4
- 239000007795 chemical reaction product Substances 0.000 description 4
- 239000002299 complementary DNA Substances 0.000 description 4
- 230000009260 cross reactivity Effects 0.000 description 4
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 description 4
- 230000006378 damage Effects 0.000 description 4
- 230000003247 decreasing effect Effects 0.000 description 4
- 230000029087 digestion Effects 0.000 description 4
- 239000000539 dimer Substances 0.000 description 4
- 239000006196 drop Substances 0.000 description 4
- 150000002066 eicosanoids Chemical class 0.000 description 4
- 239000000839 emulsion Substances 0.000 description 4
- 210000003527 eukaryotic cell Anatomy 0.000 description 4
- 150000002270 gangliosides Chemical class 0.000 description 4
- 239000011521 glass Substances 0.000 description 4
- 230000001900 immune effect Effects 0.000 description 4
- 230000005847 immunogenicity Effects 0.000 description 4
- 230000008595 infiltration Effects 0.000 description 4
- 238000001764 infiltration Methods 0.000 description 4
- 230000002401 inhibitory effect Effects 0.000 description 4
- 239000003550 marker Substances 0.000 description 4
- 230000001404 mediated effect Effects 0.000 description 4
- 230000009401 metastasis Effects 0.000 description 4
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 4
- 239000003094 microcapsule Substances 0.000 description 4
- 239000004005 microsphere Substances 0.000 description 4
- 238000003199 nucleic acid amplification method Methods 0.000 description 4
- 229940055729 papain Drugs 0.000 description 4
- 235000019834 papain Nutrition 0.000 description 4
- 239000002245 particle Substances 0.000 description 4
- 238000012510 peptide mapping method Methods 0.000 description 4
- 238000002823 phage display Methods 0.000 description 4
- WTJKGGKOPKCXLL-RRHRGVEJSA-N phosphatidylcholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCCCCCCC=CCCCCCCCC WTJKGGKOPKCXLL-RRHRGVEJSA-N 0.000 description 4
- 150000003905 phosphatidylinositols Chemical class 0.000 description 4
- 230000000069 prophylactic effect Effects 0.000 description 4
- 230000010076 replication Effects 0.000 description 4
- 238000004366 reverse phase liquid chromatography Methods 0.000 description 4
- 230000035945 sensitivity Effects 0.000 description 4
- 230000019491 signal transduction Effects 0.000 description 4
- 239000000377 silicon dioxide Substances 0.000 description 4
- 239000002904 solvent Substances 0.000 description 4
- 150000003409 sphingosine 1-phosphates Chemical class 0.000 description 4
- 108010035597 sphingosine kinase Proteins 0.000 description 4
- 239000003381 stabilizer Substances 0.000 description 4
- 239000011550 stock solution Substances 0.000 description 4
- 238000003786 synthesis reaction Methods 0.000 description 4
- 230000008685 targeting Effects 0.000 description 4
- 239000003053 toxin Substances 0.000 description 4
- 231100000765 toxin Toxicity 0.000 description 4
- 108700012359 toxins Proteins 0.000 description 4
- 238000013518 transcription Methods 0.000 description 4
- 230000035897 transcription Effects 0.000 description 4
- 210000004881 tumor cell Anatomy 0.000 description 4
- 238000011144 upstream manufacturing Methods 0.000 description 4
- 230000003612 virological effect Effects 0.000 description 4
- 239000011534 wash buffer Substances 0.000 description 4
- 230000029663 wound healing Effects 0.000 description 4
- WRGQSWVCFNIUNZ-GDCKJWNLSA-N 1-oleoyl-sn-glycerol 3-phosphate Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)OC[C@@H](O)COP(O)(O)=O WRGQSWVCFNIUNZ-GDCKJWNLSA-N 0.000 description 3
- JKMHFZQWWAIEOD-UHFFFAOYSA-N 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid Chemical compound OCC[NH+]1CCN(CCS([O-])(=O)=O)CC1 JKMHFZQWWAIEOD-UHFFFAOYSA-N 0.000 description 3
- 108020004774 Alkaline Phosphatase Proteins 0.000 description 3
- 102000002260 Alkaline Phosphatase Human genes 0.000 description 3
- 108090001008 Avidin Proteins 0.000 description 3
- UHOVQNZJYSORNB-UHFFFAOYSA-N Benzene Chemical compound C1=CC=CC=C1 UHOVQNZJYSORNB-UHFFFAOYSA-N 0.000 description 3
- WVDDGKGOMKODPV-UHFFFAOYSA-N Benzyl alcohol Chemical compound OCC1=CC=CC=C1 WVDDGKGOMKODPV-UHFFFAOYSA-N 0.000 description 3
- 208000024172 Cardiovascular disease Diseases 0.000 description 3
- 241000282693 Cercopithecidae Species 0.000 description 3
- 241000699800 Cricetinae Species 0.000 description 3
- 241000699802 Cricetulus griseus Species 0.000 description 3
- 239000006144 Dulbecco’s modified Eagle's medium Substances 0.000 description 3
- 241000196324 Embryophyta Species 0.000 description 3
- 102000003974 Fibroblast growth factor 2 Human genes 0.000 description 3
- 108090000379 Fibroblast growth factor 2 Proteins 0.000 description 3
- 239000007995 HEPES buffer Substances 0.000 description 3
- 108010091135 Immunoglobulin Fc Fragments Proteins 0.000 description 3
- 102000018071 Immunoglobulin Fc Fragments Human genes 0.000 description 3
- QNAYBMKLOCPYGJ-REOHCLBHSA-N L-alanine Chemical compound C[C@H](N)C(O)=O QNAYBMKLOCPYGJ-REOHCLBHSA-N 0.000 description 3
- DCXYFEDJOCDNAF-REOHCLBHSA-N L-asparagine Chemical compound OC(=O)[C@@H](N)CC(N)=O DCXYFEDJOCDNAF-REOHCLBHSA-N 0.000 description 3
- 241000282567 Macaca fascicularis Species 0.000 description 3
- 241000124008 Mammalia Species 0.000 description 3
- SUHOOTKUPISOBE-UHFFFAOYSA-N O-phosphoethanolamine Chemical compound NCCOP(O)(O)=O SUHOOTKUPISOBE-UHFFFAOYSA-N 0.000 description 3
- 108010038512 Platelet-Derived Growth Factor Proteins 0.000 description 3
- 102000010780 Platelet-Derived Growth Factor Human genes 0.000 description 3
- 241000288906 Primates Species 0.000 description 3
- 238000002835 absorbance Methods 0.000 description 3
- 150000007513 acids Chemical class 0.000 description 3
- 239000000556 agonist Substances 0.000 description 3
- 229960000723 ampicillin Drugs 0.000 description 3
- AVKUERGKIZMTKX-NJBDSQKTSA-N ampicillin Chemical compound C1([C@@H](N)C(=O)N[C@H]2[C@H]3SC([C@@H](N3C2=O)C(O)=O)(C)C)=CC=CC=C1 AVKUERGKIZMTKX-NJBDSQKTSA-N 0.000 description 3
- 108010005774 beta-Galactosidase Proteins 0.000 description 3
- 229930003827 cannabinoid Natural products 0.000 description 3
- 239000003557 cannabinoid Substances 0.000 description 3
- 125000004432 carbon atom Chemical group C* 0.000 description 3
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 3
- 230000015556 catabolic process Effects 0.000 description 3
- 230000010261 cell growth Effects 0.000 description 3
- 230000004663 cell proliferation Effects 0.000 description 3
- 230000033077 cellular process Effects 0.000 description 3
- 208000026106 cerebrovascular disease Diseases 0.000 description 3
- 239000003593 chromogenic compound Substances 0.000 description 3
- 238000005345 coagulation Methods 0.000 description 3
- 230000015271 coagulation Effects 0.000 description 3
- 230000008602 contraction Effects 0.000 description 3
- 229920001577 copolymer Polymers 0.000 description 3
- 230000008878 coupling Effects 0.000 description 3
- 238000010168 coupling process Methods 0.000 description 3
- 238000005859 coupling reaction Methods 0.000 description 3
- 239000003431 cross linking reagent Substances 0.000 description 3
- 238000006731 degradation reaction Methods 0.000 description 3
- 238000010586 diagram Methods 0.000 description 3
- 238000000502 dialysis Methods 0.000 description 3
- 230000004069 differentiation Effects 0.000 description 3
- 239000003085 diluting agent Substances 0.000 description 3
- 238000011143 downstream manufacturing Methods 0.000 description 3
- 239000012149 elution buffer Substances 0.000 description 3
- 230000002255 enzymatic effect Effects 0.000 description 3
- 238000006911 enzymatic reaction Methods 0.000 description 3
- 238000011156 evaluation Methods 0.000 description 3
- 208000030533 eye disease Diseases 0.000 description 3
- 230000035876 healing Effects 0.000 description 3
- 230000036541 health Effects 0.000 description 3
- 239000012145 high-salt buffer Substances 0.000 description 3
- 239000000017 hydrogel Substances 0.000 description 3
- 238000003384 imaging method Methods 0.000 description 3
- 229940127121 immunoconjugate Drugs 0.000 description 3
- 238000001802 infusion Methods 0.000 description 3
- 230000000977 initiatory effect Effects 0.000 description 3
- 230000004068 intracellular signaling Effects 0.000 description 3
- 238000001990 intravenous administration Methods 0.000 description 3
- 238000002955 isolation Methods 0.000 description 3
- 230000003902 lesion Effects 0.000 description 3
- 150000002617 leukotrienes Chemical class 0.000 description 3
- 125000003473 lipid group Chemical group 0.000 description 3
- 230000014759 maintenance of location Effects 0.000 description 3
- 238000013507 mapping Methods 0.000 description 3
- 230000037353 metabolic pathway Effects 0.000 description 3
- 230000004060 metabolic process Effects 0.000 description 3
- 230000005012 migration Effects 0.000 description 3
- 238000013508 migration Methods 0.000 description 3
- 230000004001 molecular interaction Effects 0.000 description 3
- 238000010172 mouse model Methods 0.000 description 3
- 201000006417 multiple sclerosis Diseases 0.000 description 3
- 230000007935 neutral effect Effects 0.000 description 3
- 238000006386 neutralization reaction Methods 0.000 description 3
- 229910052757 nitrogen Inorganic materials 0.000 description 3
- 239000002736 nonionic surfactant Substances 0.000 description 3
- 210000001672 ovary Anatomy 0.000 description 3
- 230000007170 pathology Effects 0.000 description 3
- 230000004983 pleiotropic effect Effects 0.000 description 3
- 239000011148 porous material Substances 0.000 description 3
- 239000003755 preservative agent Substances 0.000 description 3
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 3
- 239000012562 protein A resin Substances 0.000 description 3
- 230000005180 public health Effects 0.000 description 3
- 230000022532 regulation of transcription, DNA-dependent Effects 0.000 description 3
- 230000003248 secreting effect Effects 0.000 description 3
- 230000028327 secretion Effects 0.000 description 3
- 239000012679 serum free medium Substances 0.000 description 3
- 238000002741 site-directed mutagenesis Methods 0.000 description 3
- 238000001228 spectrum Methods 0.000 description 3
- 230000006641 stabilisation Effects 0.000 description 3
- 238000011105 stabilization Methods 0.000 description 3
- 238000007920 subcutaneous administration Methods 0.000 description 3
- JJAHTWIKCUJRDK-UHFFFAOYSA-N succinimidyl 4-(N-maleimidomethyl)cyclohexane-1-carboxylate Chemical compound C1CC(CN2C(C=CC2=O)=O)CCC1C(=O)ON1C(=O)CCC1=O JJAHTWIKCUJRDK-UHFFFAOYSA-N 0.000 description 3
- 239000000725 suspension Substances 0.000 description 3
- 238000013268 sustained release Methods 0.000 description 3
- 230000009885 systemic effect Effects 0.000 description 3
- 238000004885 tandem mass spectrometry Methods 0.000 description 3
- 238000004448 titration Methods 0.000 description 3
- 231100000331 toxic Toxicity 0.000 description 3
- 230000002588 toxic effect Effects 0.000 description 3
- 231100000027 toxicology Toxicity 0.000 description 3
- 230000000381 tumorigenic effect Effects 0.000 description 3
- 238000000108 ultra-filtration Methods 0.000 description 3
- 230000002792 vascular Effects 0.000 description 3
- 238000011179 visual inspection Methods 0.000 description 3
- OZFAFGSSMRRTDW-UHFFFAOYSA-N (2,4-dichlorophenyl) benzenesulfonate Chemical compound ClC1=CC(Cl)=CC=C1OS(=O)(=O)C1=CC=CC=C1 OZFAFGSSMRRTDW-UHFFFAOYSA-N 0.000 description 2
- FDKXTQMXEQVLRF-ZHACJKMWSA-N (E)-dacarbazine Chemical compound CN(C)\N=N\c1[nH]cnc1C(N)=O FDKXTQMXEQVLRF-ZHACJKMWSA-N 0.000 description 2
- GEYOCULIXLDCMW-UHFFFAOYSA-N 1,2-phenylenediamine Chemical compound NC1=CC=CC=C1N GEYOCULIXLDCMW-UHFFFAOYSA-N 0.000 description 2
- DIYPCWKHSODVAP-UHFFFAOYSA-N 1-[3-(2,5-dioxopyrrol-1-yl)benzoyl]oxy-2,5-dioxopyrrolidine-3-sulfonic acid Chemical compound O=C1C(S(=O)(=O)O)CC(=O)N1OC(=O)C1=CC=CC(N2C(C=CC2=O)=O)=C1 DIYPCWKHSODVAP-UHFFFAOYSA-N 0.000 description 2
- YHHSONZFOIEMCP-UHFFFAOYSA-N 2-(trimethylazaniumyl)ethyl hydrogen phosphate Chemical compound C[N+](C)(C)CCOP(O)([O-])=O YHHSONZFOIEMCP-UHFFFAOYSA-N 0.000 description 2
- NNDIXBJHNLFJJP-UHFFFAOYSA-N 20-Hydroxyeicosatetraenoic acid Chemical compound OCCCCCC=CCC=CCC=CCC=CCCCC(O)=O NNDIXBJHNLFJJP-UHFFFAOYSA-N 0.000 description 2
- UAIUNKRWKOVEES-UHFFFAOYSA-N 3,3',5,5'-tetramethylbenzidine Chemical compound CC1=C(N)C(C)=CC(C=2C=C(C)C(N)=C(C)C=2)=C1 UAIUNKRWKOVEES-UHFFFAOYSA-N 0.000 description 2
- HRDVWDQQYABHGH-UHFFFAOYSA-N 4-[4-(dimethylamino)phenyl]-N,N-dimethylaniline hydrochloride Chemical compound Cl.CN(C)c1ccc(cc1)-c1ccc(cc1)N(C)C HRDVWDQQYABHGH-UHFFFAOYSA-N 0.000 description 2
- GANZODCWZFAEGN-UHFFFAOYSA-N 5-mercapto-2-nitro-benzoic acid Chemical compound OC(=O)C1=CC(S)=CC=C1[N+]([O-])=O GANZODCWZFAEGN-UHFFFAOYSA-N 0.000 description 2
- QGZKDVFQNNGYKY-UHFFFAOYSA-N Ammonia Chemical compound N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 description 2
- XKRFYHLGVUSROY-UHFFFAOYSA-N Argon Chemical compound [Ar] XKRFYHLGVUSROY-UHFFFAOYSA-N 0.000 description 2
- DCXYFEDJOCDNAF-UHFFFAOYSA-N Asparagine Natural products OC(=O)C(N)CC(N)=O DCXYFEDJOCDNAF-UHFFFAOYSA-N 0.000 description 2
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 2
- 208000023275 Autoimmune disease Diseases 0.000 description 2
- 102000004506 Blood Proteins Human genes 0.000 description 2
- 108010017384 Blood Proteins Proteins 0.000 description 2
- 208000002381 Brain Hypoxia Diseases 0.000 description 2
- 201000006474 Brain Ischemia Diseases 0.000 description 2
- 208000026310 Breast neoplasm Diseases 0.000 description 2
- 229940124638 COX inhibitor Drugs 0.000 description 2
- 241000282832 Camelidae Species 0.000 description 2
- 241000282472 Canis lupus familiaris Species 0.000 description 2
- 102000014914 Carrier Proteins Human genes 0.000 description 2
- 206010008120 Cerebral ischaemia Diseases 0.000 description 2
- 241000282552 Chlorocebus aethiops Species 0.000 description 2
- HEDRZPFGACZZDS-UHFFFAOYSA-N Chloroform Chemical compound ClC(Cl)Cl HEDRZPFGACZZDS-UHFFFAOYSA-N 0.000 description 2
- 102000036364 Cullin Ring E3 Ligases Human genes 0.000 description 2
- UHDGCWIWMRVCDJ-CCXZUQQUSA-N Cytarabine Chemical compound O=C1N=C(N)C=CN1[C@H]1[C@@H](O)[C@H](O)[C@@H](CO)O1 UHDGCWIWMRVCDJ-CCXZUQQUSA-N 0.000 description 2
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 2
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 description 2
- SRBFZHDQGSBBOR-IOVATXLUSA-N D-xylopyranose Chemical compound O[C@@H]1COC(O)[C@H](O)[C@H]1O SRBFZHDQGSBBOR-IOVATXLUSA-N 0.000 description 2
- 102000053602 DNA Human genes 0.000 description 2
- 102000016928 DNA-directed DNA polymerase Human genes 0.000 description 2
- 108010014303 DNA-directed DNA polymerase Proteins 0.000 description 2
- 241000255925 Diptera Species 0.000 description 2
- AOJJSUZBOXZQNB-TZSSRYMLSA-N Doxorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 AOJJSUZBOXZQNB-TZSSRYMLSA-N 0.000 description 2
- 239000012591 Dulbecco’s Phosphate Buffered Saline Substances 0.000 description 2
- 102000036530 EDG receptors Human genes 0.000 description 2
- 108091007263 EDG receptors Proteins 0.000 description 2
- 241000283086 Equidae Species 0.000 description 2
- 108010008177 Fd immunoglobulins Proteins 0.000 description 2
- 241000724791 Filamentous phage Species 0.000 description 2
- WSFSSNUMVMOOMR-UHFFFAOYSA-N Formaldehyde Chemical compound O=C WSFSSNUMVMOOMR-UHFFFAOYSA-N 0.000 description 2
- 241000233866 Fungi Species 0.000 description 2
- SXRSQZLOMIGNAQ-UHFFFAOYSA-N Glutaraldehyde Chemical compound O=CCCCC=O SXRSQZLOMIGNAQ-UHFFFAOYSA-N 0.000 description 2
- 244000068988 Glycine max Species 0.000 description 2
- 235000010469 Glycine max Nutrition 0.000 description 2
- 102000003886 Glycoproteins Human genes 0.000 description 2
- 108090000288 Glycoproteins Proteins 0.000 description 2
- 241000238631 Hexapoda Species 0.000 description 2
- 101000693265 Homo sapiens Sphingosine 1-phosphate receptor 1 Proteins 0.000 description 2
- 101000693269 Homo sapiens Sphingosine 1-phosphate receptor 3 Proteins 0.000 description 2
- 241000701024 Human betaherpesvirus 5 Species 0.000 description 2
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 2
- 102000013463 Immunoglobulin Light Chains Human genes 0.000 description 2
- 108010065825 Immunoglobulin Light Chains Proteins 0.000 description 2
- 108010067060 Immunoglobulin Variable Region Proteins 0.000 description 2
- 102000017727 Immunoglobulin Variable Region Human genes 0.000 description 2
- 102000004310 Ion Channels Human genes 0.000 description 2
- 108090000862 Ion Channels Proteins 0.000 description 2
- 244000285963 Kluyveromyces fragilis Species 0.000 description 2
- 241001138401 Kluyveromyces lactis Species 0.000 description 2
- AGPKZVBTJJNPAG-WHFBIAKZSA-N L-isoleucine Chemical compound CC[C@H](C)[C@H](N)C(O)=O AGPKZVBTJJNPAG-WHFBIAKZSA-N 0.000 description 2
- QIVBCDIJIAJPQS-VIFPVBQESA-N L-tryptophane Chemical compound C1=CC=C2C(C[C@H](N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-VIFPVBQESA-N 0.000 description 2
- 108090001030 Lipoproteins Proteins 0.000 description 2
- 102000004895 Lipoproteins Human genes 0.000 description 2
- 229930184725 Lipoxin Natural products 0.000 description 2
- 239000006142 Luria-Bertani Agar Substances 0.000 description 2
- 102000004137 Lysophosphatidic Acid Receptors Human genes 0.000 description 2
- 108090000642 Lysophosphatidic Acid Receptors Proteins 0.000 description 2
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 2
- PEEHTFAAVSWFBL-UHFFFAOYSA-N Maleimide Chemical compound O=C1NC(=O)C=C1 PEEHTFAAVSWFBL-UHFFFAOYSA-N 0.000 description 2
- ZDZOTLJHXYCWBA-VCVYQWHSSA-N N-debenzoyl-N-(tert-butoxycarbonyl)-10-deacetyltaxol Chemical compound O([C@H]1[C@H]2[C@@](C([C@H](O)C3=C(C)[C@@H](OC(=O)[C@H](O)[C@@H](NC(=O)OC(C)(C)C)C=4C=CC=CC=4)C[C@]1(O)C3(C)C)=O)(C)[C@@H](O)C[C@H]1OC[C@]12OC(=O)C)C(=O)C1=CC=CC=C1 ZDZOTLJHXYCWBA-VCVYQWHSSA-N 0.000 description 2
- 230000004988 N-glycosylation Effects 0.000 description 2
- 206010061309 Neoplasm progression Diseases 0.000 description 2
- 101710163270 Nuclease Proteins 0.000 description 2
- 230000004989 O-glycosylation Effects 0.000 description 2
- 208000022873 Ocular disease Diseases 0.000 description 2
- 108020005187 Oligonucleotide Probes Proteins 0.000 description 2
- 102000004316 Oxidoreductases Human genes 0.000 description 2
- 108090000854 Oxidoreductases Proteins 0.000 description 2
- 241001494479 Pecora Species 0.000 description 2
- 108091005804 Peptidases Proteins 0.000 description 2
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N Phenol Natural products OC1=CC=CC=C1 ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 description 2
- 239000012980 RPMI-1640 medium Substances 0.000 description 2
- 241000700159 Rattus Species 0.000 description 2
- 229920002684 Sepharose Polymers 0.000 description 2
- 241000607720 Serratia Species 0.000 description 2
- XUIMIQQOPSSXEZ-UHFFFAOYSA-N Silicon Chemical compound [Si] XUIMIQQOPSSXEZ-UHFFFAOYSA-N 0.000 description 2
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 2
- VMHLLURERBWHNL-UHFFFAOYSA-M Sodium acetate Chemical compound [Na+].CC([O-])=O VMHLLURERBWHNL-UHFFFAOYSA-M 0.000 description 2
- 102100025750 Sphingosine 1-phosphate receptor 1 Human genes 0.000 description 2
- 102100025747 Sphingosine 1-phosphate receptor 3 Human genes 0.000 description 2
- 108010090804 Streptavidin Proteins 0.000 description 2
- 108091008874 T cell receptors Proteins 0.000 description 2
- 102000016266 T-Cell Antigen Receptors Human genes 0.000 description 2
- 210000001744 T-lymphocyte Anatomy 0.000 description 2
- NKANXQFJJICGDU-QPLCGJKRSA-N Tamoxifen Chemical compound C=1C=CC=CC=1C(/CC)=C(C=1C=CC(OCCN(C)C)=CC=1)/C1=CC=CC=C1 NKANXQFJJICGDU-QPLCGJKRSA-N 0.000 description 2
- 206010043376 Tetanus Diseases 0.000 description 2
- IQFYYKKMVGJFEH-XLPZGREQSA-N Thymidine Chemical compound O=C1NC(=O)C(C)=CN1[C@@H]1O[C@H](CO)[C@@H](O)C1 IQFYYKKMVGJFEH-XLPZGREQSA-N 0.000 description 2
- 206010047513 Vision blurred Diseases 0.000 description 2
- 208000027418 Wounds and injury Diseases 0.000 description 2
- 230000002378 acidificating effect Effects 0.000 description 2
- 230000003213 activating effect Effects 0.000 description 2
- 125000002252 acyl group Chemical group 0.000 description 2
- 239000000654 additive Substances 0.000 description 2
- OIRDTQYFTABQOQ-KQYNXXCUSA-N adenosine Chemical compound C1=NC=2C(N)=NC=NC=2N1[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O OIRDTQYFTABQOQ-KQYNXXCUSA-N 0.000 description 2
- 238000012867 alanine scanning Methods 0.000 description 2
- 125000000217 alkyl group Chemical group 0.000 description 2
- 150000001414 amino alcohols Chemical class 0.000 description 2
- 125000003277 amino group Chemical group 0.000 description 2
- 239000004037 angiogenesis inhibitor Substances 0.000 description 2
- 238000005571 anion exchange chromatography Methods 0.000 description 2
- 239000003242 anti bacterial agent Substances 0.000 description 2
- 230000002424 anti-apoptotic effect Effects 0.000 description 2
- 230000001093 anti-cancer Effects 0.000 description 2
- 229940088710 antibiotic agent Drugs 0.000 description 2
- 230000009830 antibody antigen interaction Effects 0.000 description 2
- 238000011091 antibody purification Methods 0.000 description 2
- 230000005875 antibody response Effects 0.000 description 2
- 229940124691 antibody therapeutics Drugs 0.000 description 2
- 210000000628 antibody-producing cell Anatomy 0.000 description 2
- 239000007864 aqueous solution Substances 0.000 description 2
- 125000003118 aryl group Chemical group 0.000 description 2
- 229960001230 asparagine Drugs 0.000 description 2
- 235000009582 asparagine Nutrition 0.000 description 2
- 229940120638 avastin Drugs 0.000 description 2
- VSRXQHXAPYXROS-UHFFFAOYSA-N azanide;cyclobutane-1,1-dicarboxylic acid;platinum(2+) Chemical compound [NH2-].[NH2-].[Pt+2].OC(=O)C1(C(O)=O)CCC1 VSRXQHXAPYXROS-UHFFFAOYSA-N 0.000 description 2
- 210000003719 b-lymphocyte Anatomy 0.000 description 2
- 230000001580 bacterial effect Effects 0.000 description 2
- 239000011324 bead Substances 0.000 description 2
- 102000005936 beta-Galactosidase Human genes 0.000 description 2
- 238000002306 biochemical method Methods 0.000 description 2
- 230000008827 biological function Effects 0.000 description 2
- 239000000090 biomarker Substances 0.000 description 2
- 229960000074 biopharmaceutical Drugs 0.000 description 2
- 238000001574 biopsy Methods 0.000 description 2
- 238000007413 biotinylation Methods 0.000 description 2
- 230000000903 blocking effect Effects 0.000 description 2
- 210000001124 body fluid Anatomy 0.000 description 2
- 239000010839 body fluid Substances 0.000 description 2
- 230000037396 body weight Effects 0.000 description 2
- DQXBYHZEEUGOBF-UHFFFAOYSA-N but-3-enoic acid;ethene Chemical compound C=C.OC(=O)CC=C DQXBYHZEEUGOBF-UHFFFAOYSA-N 0.000 description 2
- 238000004364 calculation method Methods 0.000 description 2
- 229940065144 cannabinoids Drugs 0.000 description 2
- 235000014633 carbohydrates Nutrition 0.000 description 2
- 229960004562 carboplatin Drugs 0.000 description 2
- 125000002843 carboxylic acid group Chemical group 0.000 description 2
- YCIMNLLNPGFGHC-UHFFFAOYSA-N catechol Chemical compound OC1=CC=CC=C1O YCIMNLLNPGFGHC-UHFFFAOYSA-N 0.000 description 2
- 230000030833 cell death Effects 0.000 description 2
- 230000006037 cell lysis Effects 0.000 description 2
- 206010008118 cerebral infarction Diseases 0.000 description 2
- 229930183167 cerebroside Natural products 0.000 description 2
- 150000001784 cerebrosides Chemical class 0.000 description 2
- 239000013522 chelant Substances 0.000 description 2
- 238000007385 chemical modification Methods 0.000 description 2
- 229940044683 chemotherapy drug Drugs 0.000 description 2
- HVYWMOMLDIMFJA-DPAQBDIFSA-N cholesterol Chemical compound C1C=C2C[C@@H](O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H]([C@H](C)CCCC(C)C)[C@@]1(C)CC2 HVYWMOMLDIMFJA-DPAQBDIFSA-N 0.000 description 2
- OEYIOHPDSNJKLS-UHFFFAOYSA-N choline Chemical compound C[N+](C)(C)CCO OEYIOHPDSNJKLS-UHFFFAOYSA-N 0.000 description 2
- 230000002759 chromosomal effect Effects 0.000 description 2
- 238000001142 circular dichroism spectrum Methods 0.000 description 2
- 230000004087 circulation Effects 0.000 description 2
- 230000000052 comparative effect Effects 0.000 description 2
- 238000012875 competitive assay Methods 0.000 description 2
- 230000009918 complex formation Effects 0.000 description 2
- 238000013270 controlled release Methods 0.000 description 2
- 238000007796 conventional method Methods 0.000 description 2
- 239000012228 culture supernatant Substances 0.000 description 2
- 230000001351 cycling effect Effects 0.000 description 2
- 239000002254 cytotoxic agent Substances 0.000 description 2
- 231100000599 cytotoxic agent Toxicity 0.000 description 2
- 231100000135 cytotoxicity Toxicity 0.000 description 2
- 230000003013 cytotoxicity Effects 0.000 description 2
- 229960003901 dacarbazine Drugs 0.000 description 2
- 230000020176 deacylation Effects 0.000 description 2
- 238000005947 deacylation reaction Methods 0.000 description 2
- 230000022811 deglycosylation Effects 0.000 description 2
- 229960005156 digoxin Drugs 0.000 description 2
- XSDVOEIEBUGRQX-RBUKOAKNSA-N dihydroceramide Chemical compound CCCCCCCCCCCCCCC[C@@H](O)[C@H](CO)NC=O XSDVOEIEBUGRQX-RBUKOAKNSA-N 0.000 description 2
- 238000007865 diluting Methods 0.000 description 2
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 2
- ZGSPNIOCEDOHGS-UHFFFAOYSA-L disodium [3-[2,3-di(octadeca-9,12-dienoyloxy)propoxy-oxidophosphoryl]oxy-2-hydroxypropyl] 2,3-di(octadeca-9,12-dienoyloxy)propyl phosphate Chemical compound [Na+].[Na+].CCCCCC=CCC=CCCCCCCCC(=O)OCC(OC(=O)CCCCCCCC=CCC=CCCCCC)COP([O-])(=O)OCC(O)COP([O-])(=O)OCC(OC(=O)CCCCCCCC=CCC=CCCCCC)COC(=O)CCCCCCCC=CCC=CCCCCC ZGSPNIOCEDOHGS-UHFFFAOYSA-L 0.000 description 2
- 150000002019 disulfides Chemical class 0.000 description 2
- 150000004662 dithiols Chemical class 0.000 description 2
- 239000002552 dosage form Substances 0.000 description 2
- 229940126534 drug product Drugs 0.000 description 2
- 239000000975 dye Substances 0.000 description 2
- 210000002889 endothelial cell Anatomy 0.000 description 2
- 239000002158 endotoxin Substances 0.000 description 2
- 150000002148 esters Chemical class 0.000 description 2
- 239000005038 ethylene vinyl acetate Substances 0.000 description 2
- 210000002950 fibroblast Anatomy 0.000 description 2
- 230000003176 fibrotic effect Effects 0.000 description 2
- 238000001914 filtration Methods 0.000 description 2
- 239000013020 final formulation Substances 0.000 description 2
- 239000012530 fluid Substances 0.000 description 2
- GNBHRKFJIUUOQI-UHFFFAOYSA-N fluorescein Chemical compound O1C(=O)C2=CC=CC=C2C21C1=CC=C(O)C=C1OC1=CC(O)=CC=C21 GNBHRKFJIUUOQI-UHFFFAOYSA-N 0.000 description 2
- 239000007850 fluorescent dye Substances 0.000 description 2
- 235000012631 food intake Nutrition 0.000 description 2
- 238000004108 freeze drying Methods 0.000 description 2
- 239000000499 gel Substances 0.000 description 2
- 238000001502 gel electrophoresis Methods 0.000 description 2
- 230000002068 genetic effect Effects 0.000 description 2
- 239000008103 glucose Substances 0.000 description 2
- 229960002989 glutamic acid Drugs 0.000 description 2
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 2
- 229940093915 gynecological organic acid Drugs 0.000 description 2
- 238000010438 heat treatment Methods 0.000 description 2
- 210000003630 histaminocyte Anatomy 0.000 description 2
- 229940088597 hormone Drugs 0.000 description 2
- 239000005556 hormone Substances 0.000 description 2
- 125000004435 hydrogen atom Chemical class [H]* 0.000 description 2
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 2
- FDGQSTZJBFJUBT-UHFFFAOYSA-N hypoxanthine Chemical compound O=C1NC=NC2=C1NC=N2 FDGQSTZJBFJUBT-UHFFFAOYSA-N 0.000 description 2
- 230000007954 hypoxia Effects 0.000 description 2
- 210000002865 immune cell Anatomy 0.000 description 2
- 210000000987 immune system Anatomy 0.000 description 2
- 230000009851 immunogenic response Effects 0.000 description 2
- 238000001114 immunoprecipitation Methods 0.000 description 2
- 239000012535 impurity Substances 0.000 description 2
- 238000011065 in-situ storage Methods 0.000 description 2
- 230000002779 inactivation Effects 0.000 description 2
- 230000006698 induction Effects 0.000 description 2
- 238000009616 inductively coupled plasma Methods 0.000 description 2
- 208000027866 inflammatory disease Diseases 0.000 description 2
- NOESYZHRGYRDHS-UHFFFAOYSA-N insulin Chemical compound N1C(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(NC(=O)CN)C(C)CC)CSSCC(C(NC(CO)C(=O)NC(CC(C)C)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CCC(N)=O)C(=O)NC(CC(C)C)C(=O)NC(CCC(O)=O)C(=O)NC(CC(N)=O)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CSSCC(NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2C=CC(O)=CC=2)NC(=O)C(CC(C)C)NC(=O)C(C)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2NC=NC=2)NC(=O)C(CO)NC(=O)CNC2=O)C(=O)NCC(=O)NC(CCC(O)=O)C(=O)NC(CCCNC(N)=N)C(=O)NCC(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC(O)=CC=3)C(=O)NC(C(C)O)C(=O)N3C(CCC3)C(=O)NC(CCCCN)C(=O)NC(C)C(O)=O)C(=O)NC(CC(N)=O)C(O)=O)=O)NC(=O)C(C(C)CC)NC(=O)C(CO)NC(=O)C(C(C)O)NC(=O)C1CSSCC2NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CC(N)=O)NC(=O)C(NC(=O)C(N)CC=1C=CC=CC=1)C(C)C)CC1=CN=CN1 NOESYZHRGYRDHS-UHFFFAOYSA-N 0.000 description 2
- 238000007912 intraperitoneal administration Methods 0.000 description 2
- 238000010253 intravenous injection Methods 0.000 description 2
- 238000005040 ion trap Methods 0.000 description 2
- 108010045069 keyhole-limpet hemocyanin Proteins 0.000 description 2
- 210000003734 kidney Anatomy 0.000 description 2
- 150000002639 lipoxins Chemical class 0.000 description 2
- 210000005229 liver cell Anatomy 0.000 description 2
- 230000007774 longterm Effects 0.000 description 2
- RLSSMJSEOOYNOY-UHFFFAOYSA-N m-cresol Chemical compound CC1=CC=CC(O)=C1 RLSSMJSEOOYNOY-UHFFFAOYSA-N 0.000 description 2
- 238000012423 maintenance Methods 0.000 description 2
- 230000003211 malignant effect Effects 0.000 description 2
- 238000013411 master cell bank Methods 0.000 description 2
- GLVAUDGFNGKCSF-UHFFFAOYSA-N mercaptopurine Chemical compound S=C1NC=NC2=C1NC=N2 GLVAUDGFNGKCSF-UHFFFAOYSA-N 0.000 description 2
- 108020004999 messenger RNA Proteins 0.000 description 2
- MYWUZJCMWCOHBA-VIFPVBQESA-N methamphetamine Chemical compound CN[C@@H](C)CC1=CC=CC=C1 MYWUZJCMWCOHBA-VIFPVBQESA-N 0.000 description 2
- 125000001360 methionine group Chemical group N[C@@H](CCSC)C(=O)* 0.000 description 2
- 239000004530 micro-emulsion Substances 0.000 description 2
- 230000000116 mitigating effect Effects 0.000 description 2
- 239000000178 monomer Substances 0.000 description 2
- 230000004899 motility Effects 0.000 description 2
- 210000004400 mucous membrane Anatomy 0.000 description 2
- 231100000219 mutagenic Toxicity 0.000 description 2
- 230000003505 mutagenic effect Effects 0.000 description 2
- 238000001728 nano-filtration Methods 0.000 description 2
- 239000002105 nanoparticle Substances 0.000 description 2
- 230000001613 neoplastic effect Effects 0.000 description 2
- 210000002241 neurite Anatomy 0.000 description 2
- 150000002812 neutral glycosphingolipids Chemical class 0.000 description 2
- 230000003472 neutralizing effect Effects 0.000 description 2
- 239000002547 new drug Substances 0.000 description 2
- 239000002751 oligonucleotide probe Substances 0.000 description 2
- 150000007524 organic acids Chemical class 0.000 description 2
- 235000005985 organic acids Nutrition 0.000 description 2
- 238000007254 oxidation reaction Methods 0.000 description 2
- 125000004430 oxygen atom Chemical group O* 0.000 description 2
- 229940094443 oxytocics prostaglandins Drugs 0.000 description 2
- 230000008506 pathogenesis Effects 0.000 description 2
- AQIXEPGDORPWBJ-UHFFFAOYSA-N pentan-3-ol Chemical compound CCC(O)CC AQIXEPGDORPWBJ-UHFFFAOYSA-N 0.000 description 2
- 238000010647 peptide synthesis reaction Methods 0.000 description 2
- 210000005105 peripheral blood lymphocyte Anatomy 0.000 description 2
- 210000001322 periplasm Anatomy 0.000 description 2
- 239000000825 pharmaceutical preparation Substances 0.000 description 2
- YHHSONZFOIEMCP-UHFFFAOYSA-O phosphocholine Chemical compound C[N+](C)(C)CCOP(O)(O)=O YHHSONZFOIEMCP-UHFFFAOYSA-O 0.000 description 2
- 150000003010 phosphonosphingolipids Chemical class 0.000 description 2
- 229950004354 phosphorylcholine Drugs 0.000 description 2
- 150000003019 phosphosphingolipids Chemical class 0.000 description 2
- 229920001200 poly(ethylene-vinyl acetate) Polymers 0.000 description 2
- 229920000136 polysorbate Polymers 0.000 description 2
- 229920002451 polyvinyl alcohol Polymers 0.000 description 2
- 230000004481 post-translational protein modification Effects 0.000 description 2
- 229940002612 prodrug Drugs 0.000 description 2
- 239000000651 prodrug Substances 0.000 description 2
- 210000001236 prokaryotic cell Anatomy 0.000 description 2
- QELSKZZBTMNZEB-UHFFFAOYSA-N propylparaben Chemical compound CCCOC(=O)C1=CC=C(O)C=C1 QELSKZZBTMNZEB-UHFFFAOYSA-N 0.000 description 2
- 150000003180 prostaglandins Chemical class 0.000 description 2
- 238000000159 protein binding assay Methods 0.000 description 2
- 230000002797 proteolythic effect Effects 0.000 description 2
- 238000011002 quantification Methods 0.000 description 2
- 238000003127 radioimmunoassay Methods 0.000 description 2
- 238000001959 radiotherapy Methods 0.000 description 2
- 238000010188 recombinant method Methods 0.000 description 2
- GHMLBKRAJCXXBS-UHFFFAOYSA-N resorcinol Chemical compound OC1=CC=CC(O)=C1 GHMLBKRAJCXXBS-UHFFFAOYSA-N 0.000 description 2
- 238000004007 reversed phase HPLC Methods 0.000 description 2
- 102220089752 rs146378222 Human genes 0.000 description 2
- 239000012146 running buffer Substances 0.000 description 2
- 230000036573 scar formation Effects 0.000 description 2
- 230000037390 scarring Effects 0.000 description 2
- 238000012216 screening Methods 0.000 description 2
- 239000010703 silicon Substances 0.000 description 2
- 229910052710 silicon Inorganic materials 0.000 description 2
- 238000004467 single crystal X-ray diffraction Methods 0.000 description 2
- 238000009097 single-agent therapy Methods 0.000 description 2
- 239000001632 sodium acetate Substances 0.000 description 2
- 235000017281 sodium acetate Nutrition 0.000 description 2
- DAEPDZWVDSPTHF-UHFFFAOYSA-M sodium pyruvate Chemical compound [Na+].CC(=O)C([O-])=O DAEPDZWVDSPTHF-UHFFFAOYSA-M 0.000 description 2
- 239000000600 sorbitol Substances 0.000 description 2
- 238000004611 spectroscopical analysis Methods 0.000 description 2
- 125000002657 sphingoid group Chemical group 0.000 description 2
- 230000004137 sphingolipid metabolism Effects 0.000 description 2
- 210000000952 spleen Anatomy 0.000 description 2
- 239000007858 starting material Substances 0.000 description 2
- UCSJYZPVAKXKNQ-HZYVHMACSA-N streptomycin Chemical compound CN[C@H]1[C@H](O)[C@@H](O)[C@H](CO)O[C@H]1O[C@@H]1[C@](C=O)(O)[C@H](C)O[C@H]1O[C@@H]1[C@@H](NC(N)=N)[C@H](O)[C@@H](NC(N)=N)[C@H](O)[C@H]1O UCSJYZPVAKXKNQ-HZYVHMACSA-N 0.000 description 2
- 235000000346 sugar Nutrition 0.000 description 2
- 150000008163 sugars Chemical class 0.000 description 2
- 230000002459 sustained effect Effects 0.000 description 2
- 239000012730 sustained-release form Substances 0.000 description 2
- WYWHKKSPHMUBEB-UHFFFAOYSA-N tioguanine Chemical compound N1C(N)=NC(=S)C2=C1N=CN2 WYWHKKSPHMUBEB-UHFFFAOYSA-N 0.000 description 2
- 231100000041 toxicology testing Toxicity 0.000 description 2
- 230000005030 transcription termination Effects 0.000 description 2
- 230000009261 transgenic effect Effects 0.000 description 2
- 238000013519 translation Methods 0.000 description 2
- 238000002054 transplantation Methods 0.000 description 2
- 230000032258 transport Effects 0.000 description 2
- LWIHDJKSTIGBAC-UHFFFAOYSA-K tripotassium phosphate Chemical compound [K+].[K+].[K+].[O-]P([O-])([O-])=O LWIHDJKSTIGBAC-UHFFFAOYSA-K 0.000 description 2
- 230000005751 tumor progression Effects 0.000 description 2
- 231100000588 tumorigenic Toxicity 0.000 description 2
- 238000002562 urinalysis Methods 0.000 description 2
- 239000003981 vehicle Substances 0.000 description 2
- 230000035899 viability Effects 0.000 description 2
- DGVVWUTYPXICAM-UHFFFAOYSA-N β‐Mercaptoethanol Chemical compound OCCS DGVVWUTYPXICAM-UHFFFAOYSA-N 0.000 description 2
- HDTRYLNUVZCQOY-UHFFFAOYSA-N α-D-glucopyranosyl-α-D-glucopyranoside Natural products OC1C(O)C(O)C(CO)OC1OC1C(O)C(O)C(O)C(CO)O1 HDTRYLNUVZCQOY-UHFFFAOYSA-N 0.000 description 1
- XMQUEQJCYRFIQS-YFKPBYRVSA-N (2s)-2-amino-5-ethoxy-5-oxopentanoic acid Chemical compound CCOC(=O)CC[C@H](N)C(O)=O XMQUEQJCYRFIQS-YFKPBYRVSA-N 0.000 description 1
- KYBXNPIASYUWLN-WUCPZUCCSA-N (2s)-5-hydroxypyrrolidine-2-carboxylic acid Chemical compound OC1CC[C@@H](C(O)=O)N1 KYBXNPIASYUWLN-WUCPZUCCSA-N 0.000 description 1
- FPVKHBSQESCIEP-UHFFFAOYSA-N (8S)-3-(2-deoxy-beta-D-erythro-pentofuranosyl)-3,6,7,8-tetrahydroimidazo[4,5-d][1,3]diazepin-8-ol Natural products C1C(O)C(CO)OC1N1C(NC=NCC2O)=C2N=C1 FPVKHBSQESCIEP-UHFFFAOYSA-N 0.000 description 1
- NWUYHJFMYQTDRP-UHFFFAOYSA-N 1,2-bis(ethenyl)benzene;1-ethenyl-2-ethylbenzene;styrene Chemical compound C=CC1=CC=CC=C1.CCC1=CC=CC=C1C=C.C=CC1=CC=CC=C1C=C NWUYHJFMYQTDRP-UHFFFAOYSA-N 0.000 description 1
- VILFTWLXLYIEMV-UHFFFAOYSA-N 1,5-difluoro-2,4-dinitrobenzene Chemical compound [O-][N+](=O)C1=CC([N+]([O-])=O)=C(F)C=C1F VILFTWLXLYIEMV-UHFFFAOYSA-N 0.000 description 1
- MEAZTWJVOWHKJM-CIAPRIGGSA-N 1-(3-O-sulfo-beta-D-galactosyl)-N-tetracosanoylsphingosine Chemical compound CCCCCCCCCCCCCCCCCCCCCCCC(=O)N[C@H]([C@H](O)\C=C\CCCCCCCCCCCCC)CO[C@@H]1O[C@H](CO)[C@H](O)[C@H](OS(O)(=O)=O)[C@H]1O MEAZTWJVOWHKJM-CIAPRIGGSA-N 0.000 description 1
- VSNHCAURESNICA-NJFSPNSNSA-N 1-oxidanylurea Chemical compound N[14C](=O)NO VSNHCAURESNICA-NJFSPNSNSA-N 0.000 description 1
- ZNHVWPKMFKADKW-LQWMCKPYSA-N 12(S)-HETE Chemical compound CCCCC\C=C/C[C@H](O)\C=C\C=C/C\C=C/CCCC(O)=O ZNHVWPKMFKADKW-LQWMCKPYSA-N 0.000 description 1
- ZNHVWPKMFKADKW-ZYBDYUKJSA-N 12-HETE Natural products CCCCC\C=C/C[C@@H](O)\C=C\C=C/C\C=C/CCCC(O)=O ZNHVWPKMFKADKW-ZYBDYUKJSA-N 0.000 description 1
- JSFATNQSLKRBCI-VAEKSGALSA-N 15-HETE Natural products CCCCC[C@H](O)\C=C\C=C/C\C=C/C\C=C/CCCC(O)=O JSFATNQSLKRBCI-VAEKSGALSA-N 0.000 description 1
- JSFATNQSLKRBCI-UHFFFAOYSA-N 15-Hydroxyeicosatetraenoic acid Chemical compound CCCCCC(O)C=CC=CCC=CCC=CCCCC(O)=O JSFATNQSLKRBCI-UHFFFAOYSA-N 0.000 description 1
- YBBNVCVOACOHIG-UHFFFAOYSA-N 2,2-diamino-1,4-bis(4-azidophenyl)-3-butylbutane-1,4-dione Chemical compound C=1C=C(N=[N+]=[N-])C=CC=1C(=O)C(N)(N)C(CCCC)C(=O)C1=CC=C(N=[N+]=[N-])C=C1 YBBNVCVOACOHIG-UHFFFAOYSA-N 0.000 description 1
- KGLPWQKSKUVKMJ-UHFFFAOYSA-N 2,3-dihydrophthalazine-1,4-dione Chemical compound C1=CC=C2C(=O)NNC(=O)C2=C1 KGLPWQKSKUVKMJ-UHFFFAOYSA-N 0.000 description 1
- BLSAPDZWVFWUTL-UHFFFAOYSA-N 2,5-dioxopyrrolidine-3-sulfonic acid Chemical class OS(=O)(=O)C1CC(=O)NC1=O BLSAPDZWVFWUTL-UHFFFAOYSA-N 0.000 description 1
- UEJJHQNACJXSKW-UHFFFAOYSA-N 2-(2,6-dioxopiperidin-3-yl)-1H-isoindole-1,3(2H)-dione Chemical compound O=C1C2=CC=CC=C2C(=O)N1C1CCC(=O)NC1=O UEJJHQNACJXSKW-UHFFFAOYSA-N 0.000 description 1
- FALRKNHUBBKYCC-UHFFFAOYSA-N 2-(chloromethyl)pyridine-3-carbonitrile Chemical compound ClCC1=NC=CC=C1C#N FALRKNHUBBKYCC-UHFFFAOYSA-N 0.000 description 1
- NGNBDVOYPDDBFK-UHFFFAOYSA-N 2-[2,4-di(pentan-2-yl)phenoxy]acetyl chloride Chemical group CCCC(C)C1=CC=C(OCC(Cl)=O)C(C(C)CCC)=C1 NGNBDVOYPDDBFK-UHFFFAOYSA-N 0.000 description 1
- RTQWWZBSTRGEAV-PKHIMPSTSA-N 2-[[(2s)-2-[bis(carboxymethyl)amino]-3-[4-(methylcarbamoylamino)phenyl]propyl]-[2-[bis(carboxymethyl)amino]propyl]amino]acetic acid Chemical compound CNC(=O)NC1=CC=C(C[C@@H](CN(CC(C)N(CC(O)=O)CC(O)=O)CC(O)=O)N(CC(O)=O)CC(O)=O)C=C1 RTQWWZBSTRGEAV-PKHIMPSTSA-N 0.000 description 1
- QKNYBSVHEMOAJP-UHFFFAOYSA-N 2-amino-2-(hydroxymethyl)propane-1,3-diol;hydron;chloride Chemical compound Cl.OCC(N)(CO)CO QKNYBSVHEMOAJP-UHFFFAOYSA-N 0.000 description 1
- 125000000979 2-amino-2-oxoethyl group Chemical group [H]C([*])([H])C(=O)N([H])[H] 0.000 description 1
- NEAQRZUHTPSBBM-UHFFFAOYSA-N 2-hydroxy-3,3-dimethyl-7-nitro-4h-isoquinolin-1-one Chemical compound C1=C([N+]([O-])=O)C=C2C(=O)N(O)C(C)(C)CC2=C1 NEAQRZUHTPSBBM-UHFFFAOYSA-N 0.000 description 1
- XBBVURRQGJPTHH-UHFFFAOYSA-N 2-hydroxyacetic acid;2-hydroxypropanoic acid Chemical compound OCC(O)=O.CC(O)C(O)=O XBBVURRQGJPTHH-UHFFFAOYSA-N 0.000 description 1
- CQOQDQWUFQDJMK-SSTWWWIQSA-N 2-methoxy-17beta-estradiol Chemical compound C([C@@H]12)C[C@]3(C)[C@@H](O)CC[C@H]3[C@@H]1CCC1=C2C=C(OC)C(O)=C1 CQOQDQWUFQDJMK-SSTWWWIQSA-N 0.000 description 1
- NDMPLJNOPCLANR-UHFFFAOYSA-N 3,4-dihydroxy-15-(4-hydroxy-18-methoxycarbonyl-5,18-seco-ibogamin-18-yl)-16-methoxy-1-methyl-6,7-didehydro-aspidospermidine-3-carboxylic acid methyl ester Natural products C1C(CC)(O)CC(CC2(C(=O)OC)C=3C(=CC4=C(C56C(C(C(O)C7(CC)C=CCN(C67)CC5)(O)C(=O)OC)N4C)C=3)OC)CN1CCC1=C2NC2=CC=CC=C12 NDMPLJNOPCLANR-UHFFFAOYSA-N 0.000 description 1
- AOJJSUZBOXZQNB-VTZDEGQISA-N 4'-epidoxorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@@H](O)[C@H](C)O1 AOJJSUZBOXZQNB-VTZDEGQISA-N 0.000 description 1
- XZKIHKMTEMTJQX-UHFFFAOYSA-N 4-Nitrophenyl Phosphate Chemical compound OP(O)(=O)OC1=CC=C([N+]([O-])=O)C=C1 XZKIHKMTEMTJQX-UHFFFAOYSA-N 0.000 description 1
- QFVHZQCOUORWEI-UHFFFAOYSA-N 4-[(4-anilino-5-sulfonaphthalen-1-yl)diazenyl]-5-hydroxynaphthalene-2,7-disulfonic acid Chemical compound C=12C(O)=CC(S(O)(=O)=O)=CC2=CC(S(O)(=O)=O)=CC=1N=NC(C1=CC=CC(=C11)S(O)(=O)=O)=CC=C1NC1=CC=CC=C1 QFVHZQCOUORWEI-UHFFFAOYSA-N 0.000 description 1
- TVZGACDUOSZQKY-LBPRGKRZSA-N 4-aminofolic acid Chemical compound C1=NC2=NC(N)=NC(N)=C2N=C1CNC1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 TVZGACDUOSZQKY-LBPRGKRZSA-N 0.000 description 1
- KGIJOOYOSFUGPC-CABOLEKPSA-N 5-HETE Natural products CCCCC\C=C/C\C=C/C\C=C/C=C/[C@H](O)CCCC(O)=O KGIJOOYOSFUGPC-CABOLEKPSA-N 0.000 description 1
- KGIJOOYOSFUGPC-MSFIICATSA-N 5-Hydroxyeicosatetraenoic acid Chemical compound CCCCCC=CCC=CCC=C\C=C\[C@@H](O)CCCC(O)=O KGIJOOYOSFUGPC-MSFIICATSA-N 0.000 description 1
- 229940117976 5-hydroxylysine Drugs 0.000 description 1
- 102100031126 6-phosphogluconolactonase Human genes 0.000 description 1
- 108010029731 6-phosphogluconolactonase Proteins 0.000 description 1
- WLCZTRVUXYALDD-IBGZPJMESA-N 7-[[(2s)-2,6-bis(2-methoxyethoxycarbonylamino)hexanoyl]amino]heptoxy-methylphosphinic acid Chemical compound COCCOC(=O)NCCCC[C@H](NC(=O)OCCOC)C(=O)NCCCCCCCOP(C)(O)=O WLCZTRVUXYALDD-IBGZPJMESA-N 0.000 description 1
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 1
- 241000251468 Actinopterygii Species 0.000 description 1
- 102000007469 Actins Human genes 0.000 description 1
- 108010085238 Actins Proteins 0.000 description 1
- 241000256118 Aedes aegypti Species 0.000 description 1
- 241000256173 Aedes albopictus Species 0.000 description 1
- HJCMDXDYPOUFDY-WHFBIAKZSA-N Ala-Gln Chemical compound C[C@H](N)C(=O)N[C@H](C(O)=O)CCC(N)=O HJCMDXDYPOUFDY-WHFBIAKZSA-N 0.000 description 1
- 108010031132 Alcohol Oxidoreductases Proteins 0.000 description 1
- 102000005751 Alcohol Oxidoreductases Human genes 0.000 description 1
- 102000016912 Aldehyde Reductase Human genes 0.000 description 1
- 108010053754 Aldehyde reductase Proteins 0.000 description 1
- 241000272525 Anas platyrhynchos Species 0.000 description 1
- 208000019901 Anxiety disease Diseases 0.000 description 1
- 108091023037 Aptamer Proteins 0.000 description 1
- 101100107610 Arabidopsis thaliana ABCF4 gene Proteins 0.000 description 1
- 239000004475 Arginine Substances 0.000 description 1
- 208000002109 Argyria Diseases 0.000 description 1
- BFYIZQONLCFLEV-DAELLWKTSA-N Aromasine Chemical compound O=C1C=C[C@]2(C)[C@H]3CC[C@](C)(C(CC4)=O)[C@@H]4[C@@H]3CC(=C)C2=C1 BFYIZQONLCFLEV-DAELLWKTSA-N 0.000 description 1
- CIWBSHSKHKDKBQ-JLAZNSOCSA-N Ascorbic acid Natural products OC[C@H](O)[C@H]1OC(=O)C(O)=C1O CIWBSHSKHKDKBQ-JLAZNSOCSA-N 0.000 description 1
- 241000228212 Aspergillus Species 0.000 description 1
- 241000351920 Aspergillus nidulans Species 0.000 description 1
- 241000228245 Aspergillus niger Species 0.000 description 1
- 201000001320 Atherosclerosis Diseases 0.000 description 1
- 241001203868 Autographa californica Species 0.000 description 1
- 241000271566 Aves Species 0.000 description 1
- MLDQJTXFUGDVEO-UHFFFAOYSA-N BAY-43-9006 Chemical compound C1=NC(C(=O)NC)=CC(OC=2C=CC(NC(=O)NC=3C=C(C(Cl)=CC=3)C(F)(F)F)=CC=2)=C1 MLDQJTXFUGDVEO-UHFFFAOYSA-N 0.000 description 1
- 241000193830 Bacillus <bacterium> Species 0.000 description 1
- 235000014469 Bacillus subtilis Nutrition 0.000 description 1
- 108090000363 Bacterial Luciferases Proteins 0.000 description 1
- DWRXFEITVBNRMK-UHFFFAOYSA-N Beta-D-1-Arabinofuranosylthymine Natural products O=C1NC(=O)C(C)=CN1C1C(O)C(O)C(CO)O1 DWRXFEITVBNRMK-UHFFFAOYSA-N 0.000 description 1
- 102100026189 Beta-galactosidase Human genes 0.000 description 1
- 201000004569 Blindness Diseases 0.000 description 1
- 240000008564 Boehmeria nivea Species 0.000 description 1
- 241000255789 Bombyx mori Species 0.000 description 1
- 241000409811 Bombyx mori nucleopolyhedrovirus Species 0.000 description 1
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 1
- 206010006187 Breast cancer Diseases 0.000 description 1
- COVZYZSDYWQREU-UHFFFAOYSA-N Busulfan Chemical compound CS(=O)(=O)OCCCCOS(C)(=O)=O COVZYZSDYWQREU-UHFFFAOYSA-N 0.000 description 1
- 125000001433 C-terminal amino-acid group Chemical group 0.000 description 1
- 239000002126 C01EB10 - Adenosine Substances 0.000 description 1
- 241000222120 Candida <Saccharomycetales> Species 0.000 description 1
- GAGWJHPBXLXJQN-UORFTKCHSA-N Capecitabine Chemical compound C1=C(F)C(NC(=O)OCCCCC)=NC(=O)N1[C@H]1[C@H](O)[C@H](O)[C@@H](C)O1 GAGWJHPBXLXJQN-UORFTKCHSA-N 0.000 description 1
- GAGWJHPBXLXJQN-UHFFFAOYSA-N Capecitabine Natural products C1=C(F)C(NC(=O)OCCCCC)=NC(=O)N1C1C(O)C(O)C(C)O1 GAGWJHPBXLXJQN-UHFFFAOYSA-N 0.000 description 1
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 1
- 239000004215 Carbon black (E152) Substances 0.000 description 1
- DLGOEMSEDOSKAD-UHFFFAOYSA-N Carmustine Chemical compound ClCCNC(=O)N(N=O)CCCl DLGOEMSEDOSKAD-UHFFFAOYSA-N 0.000 description 1
- 108010078791 Carrier Proteins Proteins 0.000 description 1
- 241000700198 Cavia Species 0.000 description 1
- 102000000844 Cell Surface Receptors Human genes 0.000 description 1
- 108010001857 Cell Surface Receptors Proteins 0.000 description 1
- JWBOIMRXGHLCPP-UHFFFAOYSA-N Chloditan Chemical compound C=1C=CC=C(Cl)C=1C(C(Cl)Cl)C1=CC=C(Cl)C=C1 JWBOIMRXGHLCPP-UHFFFAOYSA-N 0.000 description 1
- VEXZGXHMUGYJMC-UHFFFAOYSA-M Chloride anion Chemical compound [Cl-] VEXZGXHMUGYJMC-UHFFFAOYSA-M 0.000 description 1
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 description 1
- 208000002691 Choroiditis Diseases 0.000 description 1
- KRKNYBCHXYNGOX-UHFFFAOYSA-K Citrate Chemical compound [O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O KRKNYBCHXYNGOX-UHFFFAOYSA-K 0.000 description 1
- PTOAARAWEBMLNO-KVQBGUIXSA-N Cladribine Chemical compound C1=NC=2C(N)=NC(Cl)=NC=2N1[C@H]1C[C@H](O)[C@@H](CO)O1 PTOAARAWEBMLNO-KVQBGUIXSA-N 0.000 description 1
- 108020004705 Codon Proteins 0.000 description 1
- 108010035532 Collagen Proteins 0.000 description 1
- 102000008186 Collagen Human genes 0.000 description 1
- 102000010970 Connexin Human genes 0.000 description 1
- 108050001175 Connexin Proteins 0.000 description 1
- 229920000742 Cotton Polymers 0.000 description 1
- CMSMOCZEIVJLDB-UHFFFAOYSA-N Cyclophosphamide Chemical compound ClCCN(CCCl)P1(=O)NCCCO1 CMSMOCZEIVJLDB-UHFFFAOYSA-N 0.000 description 1
- 102000018832 Cytochromes Human genes 0.000 description 1
- 108010052832 Cytochromes Proteins 0.000 description 1
- 241000701022 Cytomegalovirus Species 0.000 description 1
- IGXWBGJHJZYPQS-SSDOTTSWSA-N D-Luciferin Chemical compound OC(=O)[C@H]1CSC(C=2SC3=CC=C(O)C=C3N=2)=N1 IGXWBGJHJZYPQS-SSDOTTSWSA-N 0.000 description 1
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 1
- SHZGCJCMOBCMKK-UHFFFAOYSA-N D-mannomethylose Natural products CC1OC(O)C(O)C(O)C1O SHZGCJCMOBCMKK-UHFFFAOYSA-N 0.000 description 1
- WQZGKKKJIJFFOK-QTVWNMPRSA-N D-mannopyranose Chemical compound OC[C@H]1OC(O)[C@@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-QTVWNMPRSA-N 0.000 description 1
- AERBNCYCJBRYDG-UHFFFAOYSA-N D-ribo-phytosphingosine Natural products CCCCCCCCCCCCCCC(O)C(O)C(N)CO AERBNCYCJBRYDG-UHFFFAOYSA-N 0.000 description 1
- 108010041986 DNA Vaccines Proteins 0.000 description 1
- 239000012623 DNA damaging agent Substances 0.000 description 1
- 238000001712 DNA sequencing Methods 0.000 description 1
- 230000006820 DNA synthesis Effects 0.000 description 1
- 229940021995 DNA vaccine Drugs 0.000 description 1
- CYCGRDQQIOGCKX-UHFFFAOYSA-N Dehydro-luciferin Natural products OC(=O)C1=CSC(C=2SC3=CC(O)=CC=C3N=2)=N1 CYCGRDQQIOGCKX-UHFFFAOYSA-N 0.000 description 1
- 208000016192 Demyelinating disease Diseases 0.000 description 1
- 206010012305 Demyelination Diseases 0.000 description 1
- 229920002307 Dextran Polymers 0.000 description 1
- 239000004375 Dextrin Substances 0.000 description 1
- 229920001353 Dextrin Polymers 0.000 description 1
- 206010012689 Diabetic retinopathy Diseases 0.000 description 1
- 238000009007 Diagnostic Kit Methods 0.000 description 1
- 101000703517 Dictyostelium discoideum Sphingosine-1-phosphate lyase Proteins 0.000 description 1
- LTMHDMANZUZIPE-AMTYYWEZSA-N Digoxin Natural products O([C@H]1[C@H](C)O[C@H](O[C@@H]2C[C@@H]3[C@@](C)([C@@H]4[C@H]([C@]5(O)[C@](C)([C@H](O)C4)[C@H](C4=CC(=O)OC4)CC5)CC3)CC2)C[C@@H]1O)[C@H]1O[C@H](C)[C@@H](O[C@H]2O[C@@H](C)[C@H](O)[C@@H](O)C2)[C@@H](O)C1 LTMHDMANZUZIPE-AMTYYWEZSA-N 0.000 description 1
- 102000016607 Diphtheria Toxin Human genes 0.000 description 1
- 108010053187 Diphtheria Toxin Proteins 0.000 description 1
- 241000255601 Drosophila melanogaster Species 0.000 description 1
- 201000009273 Endometriosis Diseases 0.000 description 1
- 241000792859 Enema Species 0.000 description 1
- 241000588914 Enterobacter Species 0.000 description 1
- 241000588921 Enterobacteriaceae Species 0.000 description 1
- 101800003838 Epidermal growth factor Proteins 0.000 description 1
- 102400001368 Epidermal growth factor Human genes 0.000 description 1
- HTIJFSOGRVMCQR-UHFFFAOYSA-N Epirubicin Natural products COc1cccc2C(=O)c3c(O)c4CC(O)(CC(OC5CC(N)C(=O)C(C)O5)c4c(O)c3C(=O)c12)C(=O)CO HTIJFSOGRVMCQR-UHFFFAOYSA-N 0.000 description 1
- 241000588698 Erwinia Species 0.000 description 1
- 241000588722 Escherichia Species 0.000 description 1
- 241001302584 Escherichia coli str. K-12 substr. W3110 Species 0.000 description 1
- 108090000371 Esterases Proteins 0.000 description 1
- QUSNBJAOOMFDIB-UHFFFAOYSA-N Ethylamine Chemical compound CCN QUSNBJAOOMFDIB-UHFFFAOYSA-N 0.000 description 1
- 241000206602 Eukaryota Species 0.000 description 1
- 229910052693 Europium Inorganic materials 0.000 description 1
- 108091029865 Exogenous DNA Proteins 0.000 description 1
- 241000282326 Felis catus Species 0.000 description 1
- 102000016359 Fibronectins Human genes 0.000 description 1
- 108010067306 Fibronectins Proteins 0.000 description 1
- 108090000331 Firefly luciferases Proteins 0.000 description 1
- BJGNCJDXODQBOB-UHFFFAOYSA-N Fivefly Luciferin Natural products OC(=O)C1CSC(C=2SC3=CC(O)=CC=C3N=2)=N1 BJGNCJDXODQBOB-UHFFFAOYSA-N 0.000 description 1
- GHASVSINZRGABV-UHFFFAOYSA-N Fluorouracil Chemical compound FC1=CNC(=O)NC1=O GHASVSINZRGABV-UHFFFAOYSA-N 0.000 description 1
- PNNNRSAQSRJVSB-SLPGGIOYSA-N Fucose Natural products C[C@H](O)[C@@H](O)[C@H](O)[C@H](O)C=O PNNNRSAQSRJVSB-SLPGGIOYSA-N 0.000 description 1
- 101150074355 GS gene Proteins 0.000 description 1
- 108010015133 Galactose oxidase Proteins 0.000 description 1
- 241000287828 Gallus gallus Species 0.000 description 1
- 108010010803 Gelatin Proteins 0.000 description 1
- 108700028146 Genetic Enhancer Elements Proteins 0.000 description 1
- 229930182566 Gentamicin Natural products 0.000 description 1
- CEAZRRDELHUEMR-URQXQFDESA-N Gentamicin Chemical compound O1[C@H](C(C)NC)CC[C@@H](N)[C@H]1O[C@H]1[C@H](O)[C@@H](O[C@@H]2[C@@H]([C@@H](NC)[C@@](C)(O)CO2)O)[C@H](N)C[C@@H]1N CEAZRRDELHUEMR-URQXQFDESA-N 0.000 description 1
- 208000010412 Glaucoma Diseases 0.000 description 1
- 108010073178 Glucan 1,4-alpha-Glucosidase Proteins 0.000 description 1
- 102100022624 Glucoamylase Human genes 0.000 description 1
- 239000004366 Glucose oxidase Substances 0.000 description 1
- 108010015776 Glucose oxidase Proteins 0.000 description 1
- 108010018962 Glucosephosphate Dehydrogenase Proteins 0.000 description 1
- 244000299507 Gossypium hirsutum Species 0.000 description 1
- 102000009465 Growth Factor Receptors Human genes 0.000 description 1
- 108010009202 Growth Factor Receptors Proteins 0.000 description 1
- 206010018910 Haemolysis Diseases 0.000 description 1
- 102000002812 Heat-Shock Proteins Human genes 0.000 description 1
- 108010004889 Heat-Shock Proteins Proteins 0.000 description 1
- HTTJABKRGRZYRN-UHFFFAOYSA-N Heparin Chemical compound OC1C(NC(=O)C)C(O)OC(COS(O)(=O)=O)C1OC1C(OS(O)(=O)=O)C(O)C(OC2C(C(OS(O)(=O)=O)C(OC3C(C(O)C(O)C(O3)C(O)=O)OS(O)(=O)=O)C(CO)O2)NS(O)(=O)=O)C(C(O)=O)O1 HTTJABKRGRZYRN-UHFFFAOYSA-N 0.000 description 1
- 206010019695 Hepatic neoplasm Diseases 0.000 description 1
- 101000998953 Homo sapiens Immunoglobulin heavy variable 1-2 Proteins 0.000 description 1
- 101001038001 Homo sapiens Lysophosphatidic acid receptor 2 Proteins 0.000 description 1
- 101001038006 Homo sapiens Lysophosphatidic acid receptor 3 Proteins 0.000 description 1
- 101000864393 Homo sapiens Protein BUD31 homolog Proteins 0.000 description 1
- 101000653759 Homo sapiens Sphingosine 1-phosphate receptor 5 Proteins 0.000 description 1
- 101000939387 Homo sapiens Urocortin-3 Proteins 0.000 description 1
- 108090000144 Human Proteins Proteins 0.000 description 1
- 102000003839 Human Proteins Human genes 0.000 description 1
- UGQMRVRMYYASKQ-UHFFFAOYSA-N Hypoxanthine nucleoside Natural products OC1C(O)C(CO)OC1N1C(NC=NC2=O)=C2N=C1 UGQMRVRMYYASKQ-UHFFFAOYSA-N 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- 102000009786 Immunoglobulin Constant Regions Human genes 0.000 description 1
- 108010009817 Immunoglobulin Constant Regions Proteins 0.000 description 1
- 102000006496 Immunoglobulin Heavy Chains Human genes 0.000 description 1
- 108010019476 Immunoglobulin Heavy Chains Proteins 0.000 description 1
- 102100036887 Immunoglobulin heavy variable 1-2 Human genes 0.000 description 1
- 102000012960 Immunoglobulin kappa-Chains Human genes 0.000 description 1
- 108010090227 Immunoglobulin kappa-Chains Proteins 0.000 description 1
- 108090001061 Insulin Proteins 0.000 description 1
- 102000004877 Insulin Human genes 0.000 description 1
- 238000012695 Interfacial polymerization Methods 0.000 description 1
- 102100026720 Interferon beta Human genes 0.000 description 1
- 108090000467 Interferon-beta Proteins 0.000 description 1
- 108090001007 Interleukin-8 Proteins 0.000 description 1
- 208000002260 Keloid Diseases 0.000 description 1
- 241000588748 Klebsiella Species 0.000 description 1
- 241000235649 Kluyveromyces Species 0.000 description 1
- 241000235058 Komagataella pastoris Species 0.000 description 1
- ODKSFYDXXFIFQN-BYPYZUCNSA-P L-argininium(2+) Chemical compound NC(=[NH2+])NCCC[C@H]([NH3+])C(O)=O ODKSFYDXXFIFQN-BYPYZUCNSA-P 0.000 description 1
- SHZGCJCMOBCMKK-DHVFOXMCSA-N L-fucopyranose Chemical compound C[C@@H]1OC(O)[C@@H](O)[C@H](O)[C@@H]1O SHZGCJCMOBCMKK-DHVFOXMCSA-N 0.000 description 1
- FFEARJCKVFRZRR-BYPYZUCNSA-N L-methionine Chemical compound CSCC[C@H](N)C(O)=O FFEARJCKVFRZRR-BYPYZUCNSA-N 0.000 description 1
- SXTAYKAGBXMACB-DPVSGNNYSA-N L-methionine sulfoximine Chemical compound CS(=N)(=O)CC[C@H](N)C(O)=O SXTAYKAGBXMACB-DPVSGNNYSA-N 0.000 description 1
- FBOZXECLQNJBKD-ZDUSSCGKSA-N L-methotrexate Chemical compound C=1N=C2N=C(N)N=C(N)C2=NC=1CN(C)C1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 FBOZXECLQNJBKD-ZDUSSCGKSA-N 0.000 description 1
- LRQKBLKVPFOOQJ-YFKPBYRVSA-N L-norleucine Chemical compound CCCC[C@H]([NH3+])C([O-])=O LRQKBLKVPFOOQJ-YFKPBYRVSA-N 0.000 description 1
- 125000000174 L-prolyl group Chemical group [H]N1C([H])([H])C([H])([H])C([H])([H])[C@@]1([H])C(*)=O 0.000 description 1
- 125000000510 L-tryptophano group Chemical group [H]C1=C([H])C([H])=C2N([H])C([H])=C(C([H])([H])[C@@]([H])(C(O[H])=O)N([H])[*])C2=C1[H] 0.000 description 1
- 239000005551 L01XE03 - Erlotinib Substances 0.000 description 1
- 239000002147 L01XE04 - Sunitinib Substances 0.000 description 1
- 239000005511 L01XE05 - Sorafenib Substances 0.000 description 1
- 241000481961 Lachancea thermotolerans Species 0.000 description 1
- 241000235651 Lachancea waltii Species 0.000 description 1
- 108010023244 Lactoperoxidase Proteins 0.000 description 1
- 102000045576 Lactoperoxidases Human genes 0.000 description 1
- 108091026898 Leader sequence (mRNA) Proteins 0.000 description 1
- 108060001084 Luciferase Proteins 0.000 description 1
- 239000005089 Luciferase Substances 0.000 description 1
- DDWFXDSYGUXRAY-UHFFFAOYSA-N Luciferin Natural products CCc1c(C)c(CC2NC(=O)C(=C2C=C)C)[nH]c1Cc3[nH]c4C(=C5/NC(CC(=O)O)C(C)C5CC(=O)O)CC(=O)c4c3C DDWFXDSYGUXRAY-UHFFFAOYSA-N 0.000 description 1
- 235000007688 Lycopersicon esculentum Nutrition 0.000 description 1
- 102100040387 Lysophosphatidic acid receptor 2 Human genes 0.000 description 1
- 102100040388 Lysophosphatidic acid receptor 3 Human genes 0.000 description 1
- 241000282553 Macaca Species 0.000 description 1
- 239000004907 Macro-emulsion Substances 0.000 description 1
- 206010025421 Macule Diseases 0.000 description 1
- 108010026217 Malate Dehydrogenase Proteins 0.000 description 1
- 102000013460 Malate Dehydrogenase Human genes 0.000 description 1
- 229930195725 Mannitol Natural products 0.000 description 1
- 102000002274 Matrix Metalloproteinases Human genes 0.000 description 1
- 108010000684 Matrix Metalloproteinases Proteins 0.000 description 1
- 229930192392 Mitomycin Natural products 0.000 description 1
- 102100035971 Molybdopterin molybdenumtransferase Human genes 0.000 description 1
- 101710119577 Molybdopterin molybdenumtransferase Proteins 0.000 description 1
- 102000016943 Muramidase Human genes 0.000 description 1
- 108010014251 Muramidase Proteins 0.000 description 1
- 101000935589 Mus musculus Flavin reductase (NADPH) Proteins 0.000 description 1
- NWIBSHFKIJFRCO-WUDYKRTCSA-N Mytomycin Chemical compound C1N2C(C(C(C)=C(N)C3=O)=O)=C3[C@@H](COC(N)=O)[C@@]2(OC)[C@@H]2[C@H]1N2 NWIBSHFKIJFRCO-WUDYKRTCSA-N 0.000 description 1
- OVRNDRQMDRJTHS-CBQIKETKSA-N N-Acetyl-D-Galactosamine Chemical compound CC(=O)N[C@H]1[C@@H](O)O[C@H](CO)[C@H](O)[C@@H]1O OVRNDRQMDRJTHS-CBQIKETKSA-N 0.000 description 1
- 108010062010 N-Acetylmuramoyl-L-alanine Amidase Proteins 0.000 description 1
- NQTADLQHYWFPDB-UHFFFAOYSA-N N-Hydroxysuccinimide Chemical compound ON1C(=O)CCC1=O NQTADLQHYWFPDB-UHFFFAOYSA-N 0.000 description 1
- MBLBDJOUHNCFQT-UHFFFAOYSA-N N-acetyl-D-galactosamine Natural products CC(=O)NC(C=O)C(O)C(O)C(O)CO MBLBDJOUHNCFQT-UHFFFAOYSA-N 0.000 description 1
- YDNKGFDKKRUKPY-TURZORIXSA-N N-hexadecanoylsphingosine Chemical compound CCCCCCCCCCCCCCCC(=O)N[C@@H](CO)[C@H](O)\C=C\CCCCCCCCCCCCC YDNKGFDKKRUKPY-TURZORIXSA-N 0.000 description 1
- 101150071357 NPP2 gene Proteins 0.000 description 1
- 108091061960 Naked DNA Proteins 0.000 description 1
- 238000011887 Necropsy Methods 0.000 description 1
- 229930193140 Neomycin Natural products 0.000 description 1
- 241000221960 Neurospora Species 0.000 description 1
- 241000221961 Neurospora crassa Species 0.000 description 1
- 244000061176 Nicotiana tabacum Species 0.000 description 1
- 235000002637 Nicotiana tabacum Nutrition 0.000 description 1
- 108091005461 Nucleic proteins Proteins 0.000 description 1
- 239000004677 Nylon Substances 0.000 description 1
- MSHZHSPISPJWHW-UHFFFAOYSA-N O-(chloroacetylcarbamoyl)fumagillol Chemical compound O1C(CC=C(C)C)C1(C)C1C(OC)C(OC(=O)NC(=O)CCl)CCC21CO2 MSHZHSPISPJWHW-UHFFFAOYSA-N 0.000 description 1
- WSDRAZIPGVLSNP-UHFFFAOYSA-N O.P(=O)(O)(O)O.O.O.P(=O)(O)(O)O Chemical compound O.P(=O)(O)(O)O.O.O.P(=O)(O)(O)O WSDRAZIPGVLSNP-UHFFFAOYSA-N 0.000 description 1
- 208000008589 Obesity Diseases 0.000 description 1
- 108700020796 Oncogene Proteins 0.000 description 1
- 239000002033 PVDF binder Substances 0.000 description 1
- 229930012538 Paclitaxel Natural products 0.000 description 1
- 208000002193 Pain Diseases 0.000 description 1
- 229930182555 Penicillin Natural products 0.000 description 1
- JGSARLDLIJGVTE-MBNYWOFBSA-N Penicillin G Chemical compound N([C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C(=O)CC1=CC=CC=C1 JGSARLDLIJGVTE-MBNYWOFBSA-N 0.000 description 1
- 241000228143 Penicillium Species 0.000 description 1
- 102000057297 Pepsin A Human genes 0.000 description 1
- 108090000284 Pepsin A Proteins 0.000 description 1
- 102000035195 Peptidases Human genes 0.000 description 1
- 108700020962 Peroxidase Proteins 0.000 description 1
- 102000003992 Peroxidases Human genes 0.000 description 1
- 240000007377 Petunia x hybrida Species 0.000 description 1
- ABLZXFCXXLZCGV-UHFFFAOYSA-N Phosphorous acid Chemical compound OP(O)=O ABLZXFCXXLZCGV-UHFFFAOYSA-N 0.000 description 1
- 108010004729 Phycoerythrin Proteins 0.000 description 1
- 108010076986 Phytochelatins Proteins 0.000 description 1
- 101100080097 Phytophthora capsici NLP2 gene Proteins 0.000 description 1
- 241000158500 Platanus racemosa Species 0.000 description 1
- 108010069381 Platelet Endothelial Cell Adhesion Molecule-1 Proteins 0.000 description 1
- 102100024616 Platelet endothelial cell adhesion molecule Human genes 0.000 description 1
- 229920000805 Polyaspartic acid Polymers 0.000 description 1
- 239000004793 Polystyrene Substances 0.000 description 1
- 239000004372 Polyvinyl alcohol Substances 0.000 description 1
- 208000003971 Posterior uveitis Diseases 0.000 description 1
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 1
- ONIBWKKTOPOVIA-UHFFFAOYSA-N Proline Natural products OC(=O)C1CCCN1 ONIBWKKTOPOVIA-UHFFFAOYSA-N 0.000 description 1
- 102100030160 Protein BUD31 homolog Human genes 0.000 description 1
- 108010076504 Protein Sorting Signals Proteins 0.000 description 1
- 241000588769 Proteus <enterobacteria> Species 0.000 description 1
- 241000589516 Pseudomonas Species 0.000 description 1
- 238000011530 RNeasy Mini Kit Methods 0.000 description 1
- 241000700157 Rattus norvegicus Species 0.000 description 1
- 208000007135 Retinal Neovascularization Diseases 0.000 description 1
- 206010038848 Retinal detachment Diseases 0.000 description 1
- 206010038923 Retinopathy Diseases 0.000 description 1
- 206010038933 Retinopathy of prematurity Diseases 0.000 description 1
- 102100037486 Reverse transcriptase/ribonuclease H Human genes 0.000 description 1
- 102000042463 Rho family Human genes 0.000 description 1
- 108091078243 Rho family Proteins 0.000 description 1
- 238000011579 SCID mouse model Methods 0.000 description 1
- 101100068078 Saccharomyces cerevisiae (strain ATCC 204508 / S288c) GCN4 gene Proteins 0.000 description 1
- 241000607142 Salmonella Species 0.000 description 1
- 241000293869 Salmonella enterica subsp. enterica serovar Typhimurium Species 0.000 description 1
- 241000235347 Schizosaccharomyces pombe Species 0.000 description 1
- 241000311088 Schwanniomyces Species 0.000 description 1
- 241001123650 Schwanniomyces occidentalis Species 0.000 description 1
- 229920005654 Sephadex Polymers 0.000 description 1
- 239000012507 Sephadex™ Substances 0.000 description 1
- 108010071390 Serum Albumin Proteins 0.000 description 1
- 102000007562 Serum Albumin Human genes 0.000 description 1
- 241000607768 Shigella Species 0.000 description 1
- BQCADISMDOOEFD-UHFFFAOYSA-N Silver Chemical compound [Ag] BQCADISMDOOEFD-UHFFFAOYSA-N 0.000 description 1
- 108010003723 Single-Domain Antibodies Proteins 0.000 description 1
- 108020004459 Small interfering RNA Proteins 0.000 description 1
- 240000003768 Solanum lycopersicum Species 0.000 description 1
- 244000061456 Solanum tuberosum Species 0.000 description 1
- 235000002595 Solanum tuberosum Nutrition 0.000 description 1
- 102100029802 Sphingosine 1-phosphate receptor 5 Human genes 0.000 description 1
- 241000252794 Sphinx Species 0.000 description 1
- 241000256251 Spodoptera frugiperda Species 0.000 description 1
- 238000003070 Statistical process control Methods 0.000 description 1
- 241000187747 Streptomyces Species 0.000 description 1
- 229930006000 Sucrose Natural products 0.000 description 1
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 1
- 241000282887 Suidae Species 0.000 description 1
- 210000000662 T-lymphocyte subset Anatomy 0.000 description 1
- 208000023809 Tenon cyst Diseases 0.000 description 1
- 241000255588 Tephritidae Species 0.000 description 1
- 108010055044 Tetanus Toxin Proteins 0.000 description 1
- 239000004098 Tetracycline Substances 0.000 description 1
- FOCVUCIESVLUNU-UHFFFAOYSA-N Thiotepa Chemical compound C1CN1P(N1CC1)(=S)N1CC1 FOCVUCIESVLUNU-UHFFFAOYSA-N 0.000 description 1
- 108091036066 Three prime untranslated region Proteins 0.000 description 1
- 108010022394 Threonine synthase Proteins 0.000 description 1
- 240000002657 Thymus vulgaris Species 0.000 description 1
- 235000007303 Thymus vulgaris Nutrition 0.000 description 1
- 108010034949 Thyroglobulin Proteins 0.000 description 1
- 102000009843 Thyroglobulin Human genes 0.000 description 1
- 241001149964 Tolypocladium Species 0.000 description 1
- 101710120037 Toxin CcdB Proteins 0.000 description 1
- 102000004338 Transferrin Human genes 0.000 description 1
- 108090000901 Transferrin Proteins 0.000 description 1
- 108090001012 Transforming Growth Factor beta Proteins 0.000 description 1
- 102000004887 Transforming Growth Factor beta Human genes 0.000 description 1
- HDTRYLNUVZCQOY-WSWWMNSNSA-N Trehalose Natural products O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@@H](O)[C@@H](CO)O1 HDTRYLNUVZCQOY-WSWWMNSNSA-N 0.000 description 1
- 241000223259 Trichoderma Species 0.000 description 1
- 239000013504 Triton X-100 Substances 0.000 description 1
- 229920004890 Triton X-100 Polymers 0.000 description 1
- 108010092464 Urate Oxidase Proteins 0.000 description 1
- 108010046334 Urease Proteins 0.000 description 1
- 102100029794 Urocortin-3 Human genes 0.000 description 1
- 244000000188 Vaccinium ovalifolium Species 0.000 description 1
- KZSNJWFQEVHDMF-UHFFFAOYSA-N Valine Chemical compound CC(C)C(N)C(O)=O KZSNJWFQEVHDMF-UHFFFAOYSA-N 0.000 description 1
- 238000005411 Van der Waals force Methods 0.000 description 1
- JXLYSJRDGCGARV-WWYNWVTFSA-N Vinblastine Natural products O=C(O[C@H]1[C@](O)(C(=O)OC)[C@@H]2N(C)c3c(cc(c(OC)c3)[C@]3(C(=O)OC)c4[nH]c5c(c4CCN4C[C@](O)(CC)C[C@H](C3)C4)cccc5)[C@@]32[C@H]2[C@@]1(CC)C=CCN2CC3)C JXLYSJRDGCGARV-WWYNWVTFSA-N 0.000 description 1
- 208000034698 Vitreous haemorrhage Diseases 0.000 description 1
- 108010093894 Xanthine oxidase Proteins 0.000 description 1
- 102100033220 Xanthine oxidase Human genes 0.000 description 1
- 238000012452 Xenomouse strains Methods 0.000 description 1
- 241000235013 Yarrowia Species 0.000 description 1
- 240000008042 Zea mays Species 0.000 description 1
- 235000005824 Zea mays ssp. parviglumis Nutrition 0.000 description 1
- 235000002017 Zea mays subsp mays Nutrition 0.000 description 1
- IERHLVCPSMICTF-CCXZUQQUSA-N [(2r,3s,4s,5r)-5-(4-amino-2-oxopyrimidin-1-yl)-3,4-dihydroxyoxolan-2-yl]methyl dihydrogen phosphate Chemical compound O=C1N=C(N)C=CN1[C@H]1[C@@H](O)[C@H](O)[C@@H](COP(O)(O)=O)O1 IERHLVCPSMICTF-CCXZUQQUSA-N 0.000 description 1
- JLCPHMBAVCMARE-UHFFFAOYSA-N [3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-hydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methyl [5-(6-aminopurin-9-yl)-2-(hydroxymethyl)oxolan-3-yl] hydrogen phosphate Polymers Cc1cn(C2CC(OP(O)(=O)OCC3OC(CC3OP(O)(=O)OCC3OC(CC3O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c3nc(N)[nH]c4=O)C(COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3CO)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cc(C)c(=O)[nH]c3=O)n3cc(C)c(=O)[nH]c3=O)n3ccc(N)nc3=O)n3cc(C)c(=O)[nH]c3=O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)O2)c(=O)[nH]c1=O JLCPHMBAVCMARE-UHFFFAOYSA-N 0.000 description 1
- 238000002679 ablation Methods 0.000 description 1
- 230000005856 abnormality Effects 0.000 description 1
- DPXJVFZANSGRMM-UHFFFAOYSA-N acetic acid;2,3,4,5,6-pentahydroxyhexanal;sodium Chemical compound [Na].CC(O)=O.OCC(O)C(O)C(O)C(O)C=O DPXJVFZANSGRMM-UHFFFAOYSA-N 0.000 description 1
- 239000003929 acidic solution Substances 0.000 description 1
- 230000004913 activation Effects 0.000 description 1
- 239000008186 active pharmaceutical agent Substances 0.000 description 1
- 239000013543 active substance Substances 0.000 description 1
- 230000006978 adaptation Effects 0.000 description 1
- 229960005305 adenosine Drugs 0.000 description 1
- 230000009824 affinity maturation Effects 0.000 description 1
- 238000001261 affinity purification Methods 0.000 description 1
- 230000002776 aggregation Effects 0.000 description 1
- 238000004220 aggregation Methods 0.000 description 1
- 150000001299 aldehydes Chemical class 0.000 description 1
- 150000001336 alkenes Chemical class 0.000 description 1
- 150000001345 alkine derivatives Chemical class 0.000 description 1
- 229940100198 alkylating agent Drugs 0.000 description 1
- 239000002168 alkylating agent Substances 0.000 description 1
- SHGAZHPCJJPHSC-YCNIQYBTSA-N all-trans-retinoic acid Chemical compound OC(=O)\C=C(/C)\C=C\C=C(/C)\C=C\C1=C(C)CCCC1(C)C SHGAZHPCJJPHSC-YCNIQYBTSA-N 0.000 description 1
- HDTRYLNUVZCQOY-LIZSDCNHSA-N alpha,alpha-trehalose Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 HDTRYLNUVZCQOY-LIZSDCNHSA-N 0.000 description 1
- WQZGKKKJIJFFOK-PHYPRBDBSA-N alpha-D-galactose Chemical compound OC[C@H]1O[C@H](O)[C@H](O)[C@@H](O)[C@H]1O WQZGKKKJIJFFOK-PHYPRBDBSA-N 0.000 description 1
- 229960000473 altretamine Drugs 0.000 description 1
- 229940037003 alum Drugs 0.000 description 1
- 150000001408 amides Chemical class 0.000 description 1
- 229960003896 aminopterin Drugs 0.000 description 1
- 229910021529 ammonia Inorganic materials 0.000 description 1
- 238000012870 ammonium sulfate precipitation Methods 0.000 description 1
- 230000019552 anatomical structure morphogenesis Effects 0.000 description 1
- 239000002870 angiogenesis inducing agent Substances 0.000 description 1
- 239000003957 anion exchange resin Substances 0.000 description 1
- 150000001450 anions Chemical class 0.000 description 1
- 229940045799 anthracyclines and related substance Drugs 0.000 description 1
- 230000003527 anti-angiogenesis Effects 0.000 description 1
- 230000001772 anti-angiogenic effect Effects 0.000 description 1
- 229940046836 anti-estrogen Drugs 0.000 description 1
- 230000001833 anti-estrogenic effect Effects 0.000 description 1
- 230000003510 anti-fibrotic effect Effects 0.000 description 1
- 229940121363 anti-inflammatory agent Drugs 0.000 description 1
- 239000002260 anti-inflammatory agent Substances 0.000 description 1
- 229940124599 anti-inflammatory drug Drugs 0.000 description 1
- 230000000340 anti-metabolite Effects 0.000 description 1
- 230000002927 anti-mitotic effect Effects 0.000 description 1
- 229940125644 antibody drug Drugs 0.000 description 1
- 238000009175 antibody therapy Methods 0.000 description 1
- 230000000890 antigenic effect Effects 0.000 description 1
- 229940100197 antimetabolite Drugs 0.000 description 1
- 239000002256 antimetabolite Substances 0.000 description 1
- 239000004599 antimicrobial Substances 0.000 description 1
- 239000003963 antioxidant agent Substances 0.000 description 1
- 235000006708 antioxidants Nutrition 0.000 description 1
- 210000000436 anus Anatomy 0.000 description 1
- 230000036506 anxiety Effects 0.000 description 1
- 239000012062 aqueous buffer Substances 0.000 description 1
- 239000013011 aqueous formulation Substances 0.000 description 1
- 239000007900 aqueous suspension Substances 0.000 description 1
- PYMYPHUHKUWMLA-UHFFFAOYSA-N arabinose Natural products OCC(O)C(O)C(O)C=O PYMYPHUHKUWMLA-UHFFFAOYSA-N 0.000 description 1
- ODKSFYDXXFIFQN-UHFFFAOYSA-N arginine Natural products OC(=O)C(N)CCCNC(N)=N ODKSFYDXXFIFQN-UHFFFAOYSA-N 0.000 description 1
- 229910052786 argon Inorganic materials 0.000 description 1
- 239000003886 aromatase inhibitor Substances 0.000 description 1
- 229940046844 aromatase inhibitors Drugs 0.000 description 1
- 235000010323 ascorbic acid Nutrition 0.000 description 1
- 229960005070 ascorbic acid Drugs 0.000 description 1
- 239000011668 ascorbic acid Substances 0.000 description 1
- 125000000613 asparagine group Chemical group N[C@@H](CC(N)=O)C(=O)* 0.000 description 1
- 150000001510 aspartic acids Chemical class 0.000 description 1
- 208000006673 asthma Diseases 0.000 description 1
- 239000012298 atmosphere Substances 0.000 description 1
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 1
- 238000011888 autopsy Methods 0.000 description 1
- 230000003376 axonal effect Effects 0.000 description 1
- 210000003651 basophil Anatomy 0.000 description 1
- YTKUWDBFDASYHO-UHFFFAOYSA-N bendamustine Chemical compound ClCCN(CCCl)C1=CC=C2N(C)C(CCCC(O)=O)=NC2=C1 YTKUWDBFDASYHO-UHFFFAOYSA-N 0.000 description 1
- 229960002707 bendamustine Drugs 0.000 description 1
- 229960000686 benzalkonium chloride Drugs 0.000 description 1
- 229960003872 benzethonium Drugs 0.000 description 1
- UREZNYTWGJKWBI-UHFFFAOYSA-M benzethonium chloride Chemical compound [Cl-].C1=CC(C(C)(C)CC(C)(C)C)=CC=C1OCCOCC[N+](C)(C)CC1=CC=CC=C1 UREZNYTWGJKWBI-UHFFFAOYSA-M 0.000 description 1
- 235000019445 benzyl alcohol Nutrition 0.000 description 1
- CADWTSSKOVRVJC-UHFFFAOYSA-N benzyl(dimethyl)azanium;chloride Chemical compound [Cl-].C[NH+](C)CC1=CC=CC=C1 CADWTSSKOVRVJC-UHFFFAOYSA-N 0.000 description 1
- SRBFZHDQGSBBOR-UHFFFAOYSA-N beta-D-Pyranose-Lyxose Natural products OC1COC(O)C(O)C1O SRBFZHDQGSBBOR-UHFFFAOYSA-N 0.000 description 1
- WQZGKKKJIJFFOK-FPRJBGLDSA-N beta-D-galactose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@H]1O WQZGKKKJIJFFOK-FPRJBGLDSA-N 0.000 description 1
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 1
- IQFYYKKMVGJFEH-UHFFFAOYSA-N beta-L-thymidine Natural products O=C1NC(=O)C(C)=CN1C1OC(CO)C(O)C1 IQFYYKKMVGJFEH-UHFFFAOYSA-N 0.000 description 1
- 229960000397 bevacizumab Drugs 0.000 description 1
- 239000011230 binding agent Substances 0.000 description 1
- 108091008324 binding proteins Proteins 0.000 description 1
- 239000000227 bioadhesive Substances 0.000 description 1
- 239000003124 biologic agent Substances 0.000 description 1
- 230000008033 biological extinction Effects 0.000 description 1
- 230000033228 biological regulation Effects 0.000 description 1
- 230000008512 biological response Effects 0.000 description 1
- 239000012472 biological sample Substances 0.000 description 1
- 238000005460 biophysical method Methods 0.000 description 1
- 230000006287 biotinylation Effects 0.000 description 1
- HUTDDBSSHVOYJR-UHFFFAOYSA-H bis[(2-oxo-1,3,2$l^{5},4$l^{2}-dioxaphosphaplumbetan-2-yl)oxy]lead Chemical compound [Pb+2].[Pb+2].[Pb+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O HUTDDBSSHVOYJR-UHFFFAOYSA-H 0.000 description 1
- 210000004204 blood vessel Anatomy 0.000 description 1
- 238000006664 bond formation reaction Methods 0.000 description 1
- 238000010322 bone marrow transplantation Methods 0.000 description 1
- 239000005388 borosilicate glass Substances 0.000 description 1
- 229960001467 bortezomib Drugs 0.000 description 1
- GXJABQQUPOEUTA-RDJZCZTQSA-N bortezomib Chemical compound C([C@@H](C(=O)N[C@@H](CC(C)C)B(O)O)NC(=O)C=1N=CC=NC=1)C1=CC=CC=C1 GXJABQQUPOEUTA-RDJZCZTQSA-N 0.000 description 1
- 229940098773 bovine serum albumin Drugs 0.000 description 1
- 210000000621 bronchi Anatomy 0.000 description 1
- 210000001775 bruch membrane Anatomy 0.000 description 1
- 229960002092 busulfan Drugs 0.000 description 1
- LRHPLDYGYMQRHN-UHFFFAOYSA-N butyl alcohol Substances CCCCO LRHPLDYGYMQRHN-UHFFFAOYSA-N 0.000 description 1
- 125000000484 butyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 229940088954 camptosar Drugs 0.000 description 1
- 230000004611 cancer cell death Effects 0.000 description 1
- 208000035269 cancer or benign tumor Diseases 0.000 description 1
- 229960004117 capecitabine Drugs 0.000 description 1
- 239000002775 capsule Substances 0.000 description 1
- 229910052799 carbon Inorganic materials 0.000 description 1
- 229910002091 carbon monoxide Inorganic materials 0.000 description 1
- 239000001768 carboxy methyl cellulose Substances 0.000 description 1
- 229940030602 cardiac therapy drug Drugs 0.000 description 1
- 230000001451 cardiotoxic effect Effects 0.000 description 1
- 239000002327 cardiovascular agent Substances 0.000 description 1
- 229940125692 cardiovascular agent Drugs 0.000 description 1
- 230000009084 cardiovascular function Effects 0.000 description 1
- 210000000748 cardiovascular system Anatomy 0.000 description 1
- 229960005243 carmustine Drugs 0.000 description 1
- 238000012219 cassette mutagenesis Methods 0.000 description 1
- 239000003729 cation exchange resin Substances 0.000 description 1
- 150000001768 cations Chemical class 0.000 description 1
- 230000020411 cell activation Effects 0.000 description 1
- 230000021164 cell adhesion Effects 0.000 description 1
- 229940076006 cell cycle modulator Drugs 0.000 description 1
- 230000006721 cell death pathway Effects 0.000 description 1
- 230000003915 cell function Effects 0.000 description 1
- 230000036755 cellular response Effects 0.000 description 1
- 229920002301 cellulose acetate Polymers 0.000 description 1
- 125000001549 ceramide group Chemical group 0.000 description 1
- 150000001783 ceramides Chemical class 0.000 description 1
- 230000002490 cerebral effect Effects 0.000 description 1
- 208000019065 cervical carcinoma Diseases 0.000 description 1
- 230000009920 chelation Effects 0.000 description 1
- 238000012412 chemical coupling Methods 0.000 description 1
- 230000035605 chemotaxis Effects 0.000 description 1
- 238000002512 chemotherapy Methods 0.000 description 1
- 239000012829 chemotherapy agent Substances 0.000 description 1
- 235000013330 chicken meat Nutrition 0.000 description 1
- 235000012000 cholesterol Nutrition 0.000 description 1
- 229960001231 choline Drugs 0.000 description 1
- 210000003161 choroid Anatomy 0.000 description 1
- 238000011210 chromatographic step Methods 0.000 description 1
- DQLATGHUWYMOKM-UHFFFAOYSA-L cisplatin Chemical compound N[Pt](N)(Cl)Cl DQLATGHUWYMOKM-UHFFFAOYSA-L 0.000 description 1
- 229960004316 cisplatin Drugs 0.000 description 1
- 238000005354 coacervation Methods 0.000 description 1
- 229920001436 collagen Polymers 0.000 description 1
- 150000004814 combretastatins Chemical class 0.000 description 1
- 238000004891 communication Methods 0.000 description 1
- 230000006854 communication Effects 0.000 description 1
- 238000010835 comparative analysis Methods 0.000 description 1
- 230000000536 complexating effect Effects 0.000 description 1
- 239000008139 complexing agent Substances 0.000 description 1
- 239000002131 composite material Substances 0.000 description 1
- 238000005094 computer simulation Methods 0.000 description 1
- 239000003636 conditioned culture medium Substances 0.000 description 1
- 230000001268 conjugating effect Effects 0.000 description 1
- 210000000795 conjunctiva Anatomy 0.000 description 1
- 238000011109 contamination Methods 0.000 description 1
- 230000001276 controlling effect Effects 0.000 description 1
- 235000005822 corn Nutrition 0.000 description 1
- 210000004087 cornea Anatomy 0.000 description 1
- 230000002596 correlated effect Effects 0.000 description 1
- 239000007822 coupling agent Substances 0.000 description 1
- 239000006071 cream Substances 0.000 description 1
- 238000009295 crossflow filtration Methods 0.000 description 1
- 238000005520 cutting process Methods 0.000 description 1
- 125000004093 cyano group Chemical group *C#N 0.000 description 1
- HPXRVTGHNJAIIH-UHFFFAOYSA-N cyclohexanol Chemical compound OC1CCCCC1 HPXRVTGHNJAIIH-UHFFFAOYSA-N 0.000 description 1
- 229960004397 cyclophosphamide Drugs 0.000 description 1
- UFULAYFCSOUIOV-UHFFFAOYSA-N cysteamine Chemical compound NCCS UFULAYFCSOUIOV-UHFFFAOYSA-N 0.000 description 1
- 229960000684 cytarabine Drugs 0.000 description 1
- 210000004292 cytoskeleton Anatomy 0.000 description 1
- 231100000433 cytotoxic Toxicity 0.000 description 1
- 230000001472 cytotoxic effect Effects 0.000 description 1
- 125000001295 dansyl group Chemical group [H]C1=C([H])C(N(C([H])([H])[H])C([H])([H])[H])=C2C([H])=C([H])C([H])=C(C2=C1[H])S(*)(=O)=O 0.000 description 1
- 238000013480 data collection Methods 0.000 description 1
- 230000034994 death Effects 0.000 description 1
- 230000002950 deficient Effects 0.000 description 1
- 230000007850 degeneration Effects 0.000 description 1
- 238000005238 degreasing Methods 0.000 description 1
- 239000003405 delayed action preparation Substances 0.000 description 1
- YSMODUONRAFBET-UHFFFAOYSA-N delta-DL-hydroxylysine Natural products NCC(O)CCC(N)C(O)=O YSMODUONRAFBET-UHFFFAOYSA-N 0.000 description 1
- 239000000412 dendrimer Substances 0.000 description 1
- 229920000736 dendritic polymer Polymers 0.000 description 1
- 238000001212 derivatisation Methods 0.000 description 1
- 230000001066 destructive effect Effects 0.000 description 1
- UREBDLICKHMUKA-CXSFZGCWSA-N dexamethasone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@@H](C)[C@@](C(=O)CO)(O)[C@@]1(C)C[C@@H]2O UREBDLICKHMUKA-CXSFZGCWSA-N 0.000 description 1
- 229960003957 dexamethasone Drugs 0.000 description 1
- 235000019425 dextrin Nutrition 0.000 description 1
- 238000011026 diafiltration Methods 0.000 description 1
- 238000003745 diagnosis Methods 0.000 description 1
- 238000002405 diagnostic procedure Methods 0.000 description 1
- 238000000188 dichroism spectroscopy Methods 0.000 description 1
- 238000009792 diffusion process Methods 0.000 description 1
- LTMHDMANZUZIPE-PUGKRICDSA-N digoxin Chemical compound C1[C@H](O)[C@H](O)[C@@H](C)O[C@H]1O[C@@H]1[C@@H](C)O[C@@H](O[C@@H]2[C@H](O[C@@H](O[C@@H]3C[C@@H]4[C@]([C@@H]5[C@H]([C@]6(CC[C@@H]([C@@]6(C)[C@H](O)C5)C=5COC(=O)C=5)O)CC4)(C)CC3)C[C@@H]2O)C)C[C@@H]1O LTMHDMANZUZIPE-PUGKRICDSA-N 0.000 description 1
- LTMHDMANZUZIPE-UHFFFAOYSA-N digoxine Natural products C1C(O)C(O)C(C)OC1OC1C(C)OC(OC2C(OC(OC3CC4C(C5C(C6(CCC(C6(C)C(O)C5)C=5COC(=O)C=5)O)CC4)(C)CC3)CC2O)C)CC1O LTMHDMANZUZIPE-UHFFFAOYSA-N 0.000 description 1
- 102000004419 dihydrofolate reductase Human genes 0.000 description 1
- 125000005442 diisocyanate group Chemical group 0.000 description 1
- 238000004141 dimensional analysis Methods 0.000 description 1
- 125000000118 dimethyl group Chemical group [H]C([H])([H])* 0.000 description 1
- 229960003983 diphtheria toxoid Drugs 0.000 description 1
- 229940042399 direct acting antivirals protease inhibitors Drugs 0.000 description 1
- 150000002016 disaccharides Chemical class 0.000 description 1
- 230000006806 disease prevention Effects 0.000 description 1
- VXNQMUVMEIGUJW-XNOMRPDFSA-L disodium;[2-methoxy-5-[(z)-2-(3,4,5-trimethoxyphenyl)ethenyl]phenyl] phosphate Chemical compound [Na+].[Na+].C1=C(OP([O-])([O-])=O)C(OC)=CC=C1\C=C/C1=CC(OC)=C(OC)C(OC)=C1 VXNQMUVMEIGUJW-XNOMRPDFSA-L 0.000 description 1
- 238000010494 dissociation reaction Methods 0.000 description 1
- 230000005593 dissociations Effects 0.000 description 1
- 238000004090 dissolution Methods 0.000 description 1
- 239000012153 distilled water Substances 0.000 description 1
- 239000003534 dna topoisomerase inhibitor Substances 0.000 description 1
- 229960003668 docetaxel Drugs 0.000 description 1
- POULHZVOKOAJMA-UHFFFAOYSA-M dodecanoate Chemical compound CCCCCCCCCCCC([O-])=O POULHZVOKOAJMA-UHFFFAOYSA-M 0.000 description 1
- 231100000673 dose–response relationship Toxicity 0.000 description 1
- 238000012444 downstream purification process Methods 0.000 description 1
- ZWAOHEXOSAUJHY-ZIYNGMLESA-N doxifluridine Chemical compound O[C@@H]1[C@H](O)[C@@H](C)O[C@H]1N1C(=O)NC(=O)C(F)=C1 ZWAOHEXOSAUJHY-ZIYNGMLESA-N 0.000 description 1
- 229950005454 doxifluridine Drugs 0.000 description 1
- 229960004679 doxorubicin Drugs 0.000 description 1
- 229940000406 drug candidate Drugs 0.000 description 1
- 239000003937 drug carrier Substances 0.000 description 1
- 238000009509 drug development Methods 0.000 description 1
- 238000007876 drug discovery Methods 0.000 description 1
- 229940088679 drug related substance Drugs 0.000 description 1
- 239000003596 drug target Substances 0.000 description 1
- 102220481278 eIF5-mimic protein 2_Y27F_mutation Human genes 0.000 description 1
- 230000000678 effect on lipid Effects 0.000 description 1
- 238000002565 electrocardiography Methods 0.000 description 1
- 238000004520 electroporation Methods 0.000 description 1
- 230000009881 electrostatic interaction Effects 0.000 description 1
- 238000009261 endocrine therapy Methods 0.000 description 1
- 229940034984 endocrine therapy antineoplastic and immunomodulating agent Drugs 0.000 description 1
- 206010014801 endophthalmitis Diseases 0.000 description 1
- 210000002472 endoplasmic reticulum Anatomy 0.000 description 1
- RDYMFSUJUZBWLH-UHFFFAOYSA-N endosulfan Chemical compound C12COS(=O)OCC2C2(Cl)C(Cl)=C(Cl)C1(Cl)C2(Cl)Cl RDYMFSUJUZBWLH-UHFFFAOYSA-N 0.000 description 1
- 239000007920 enema Substances 0.000 description 1
- 229940079360 enema for constipation Drugs 0.000 description 1
- 210000003979 eosinophil Anatomy 0.000 description 1
- 229940116977 epidermal growth factor Drugs 0.000 description 1
- 229960001904 epirubicin Drugs 0.000 description 1
- 210000000981 epithelium Anatomy 0.000 description 1
- 229940082789 erbitux Drugs 0.000 description 1
- AAKJLRGGTJKAMG-UHFFFAOYSA-N erlotinib Chemical compound C=12C=C(OCCOC)C(OCCOC)=CC2=NC=NC=1NC1=CC=CC(C#C)=C1 AAKJLRGGTJKAMG-UHFFFAOYSA-N 0.000 description 1
- 229960001433 erlotinib Drugs 0.000 description 1
- 230000003628 erosive effect Effects 0.000 description 1
- YSMODUONRAFBET-UHNVWZDZSA-N erythro-5-hydroxy-L-lysine Chemical compound NC[C@H](O)CC[C@H](N)C(O)=O YSMODUONRAFBET-UHNVWZDZSA-N 0.000 description 1
- 210000003238 esophagus Anatomy 0.000 description 1
- 239000000328 estrogen antagonist Substances 0.000 description 1
- 238000012869 ethanol precipitation Methods 0.000 description 1
- OGPBJKLSAFTDLK-UHFFFAOYSA-N europium atom Chemical compound [Eu] OGPBJKLSAFTDLK-UHFFFAOYSA-N 0.000 description 1
- 238000001704 evaporation Methods 0.000 description 1
- 229960000255 exemestane Drugs 0.000 description 1
- 230000001747 exhibiting effect Effects 0.000 description 1
- 210000003722 extracellular fluid Anatomy 0.000 description 1
- 210000001723 extracellular space Anatomy 0.000 description 1
- 239000000284 extract Substances 0.000 description 1
- 238000000605 extraction Methods 0.000 description 1
- 239000003889 eye drop Substances 0.000 description 1
- 229940012356 eye drops Drugs 0.000 description 1
- 239000003885 eye ointment Substances 0.000 description 1
- 210000000744 eyelid Anatomy 0.000 description 1
- 230000001605 fetal effect Effects 0.000 description 1
- 239000012467 final product Substances 0.000 description 1
- 229930003944 flavone Natural products 0.000 description 1
- 150000002213 flavones Chemical class 0.000 description 1
- 235000011949 flavones Nutrition 0.000 description 1
- 229930003935 flavonoid Natural products 0.000 description 1
- 150000002215 flavonoids Chemical class 0.000 description 1
- 235000017173 flavonoids Nutrition 0.000 description 1
- 239000008394 flocculating agent Substances 0.000 description 1
- 229960000390 fludarabine Drugs 0.000 description 1
- GIUYCYHIANZCFB-FJFJXFQQSA-N fludarabine phosphate Chemical compound C1=NC=2C(N)=NC(F)=NC=2N1[C@@H]1O[C@H](COP(O)(O)=O)[C@@H](O)[C@@H]1O GIUYCYHIANZCFB-FJFJXFQQSA-N 0.000 description 1
- 150000002222 fluorine compounds Chemical class 0.000 description 1
- 229960002949 fluorouracil Drugs 0.000 description 1
- 239000006260 foam Substances 0.000 description 1
- 238000005194 fractionation Methods 0.000 description 1
- 235000021588 free fatty acids Nutrition 0.000 description 1
- 230000005714 functional activity Effects 0.000 description 1
- 230000002538 fungal effect Effects 0.000 description 1
- 108020001507 fusion proteins Proteins 0.000 description 1
- 102000037865 fusion proteins Human genes 0.000 description 1
- 229930182830 galactose Natural products 0.000 description 1
- 210000003976 gap junction Anatomy 0.000 description 1
- 210000001035 gastrointestinal tract Anatomy 0.000 description 1
- 239000008273 gelatin Substances 0.000 description 1
- 229920000159 gelatin Polymers 0.000 description 1
- 235000019322 gelatine Nutrition 0.000 description 1
- 235000011852 gelatine desserts Nutrition 0.000 description 1
- SDUQYLNIPVEERB-QPPQHZFASA-N gemcitabine Chemical compound O=C1N=C(N)C=CN1[C@H]1C(F)(F)[C@H](O)[C@@H](CO)O1 SDUQYLNIPVEERB-QPPQHZFASA-N 0.000 description 1
- 229960005277 gemcitabine Drugs 0.000 description 1
- 238000003500 gene array Methods 0.000 description 1
- 238000001415 gene therapy Methods 0.000 description 1
- 238000007429 general method Methods 0.000 description 1
- 210000004392 genitalia Anatomy 0.000 description 1
- 229940116332 glucose oxidase Drugs 0.000 description 1
- 235000019420 glucose oxidase Nutrition 0.000 description 1
- 229940049906 glutamate Drugs 0.000 description 1
- 229930195712 glutamate Natural products 0.000 description 1
- 150000004676 glycans Chemical class 0.000 description 1
- 150000002321 glycerophosphoglycerophosphoglycerols Chemical class 0.000 description 1
- 230000034659 glycolysis Effects 0.000 description 1
- 239000008187 granular material Substances 0.000 description 1
- 230000005484 gravity Effects 0.000 description 1
- 125000005843 halogen group Chemical group 0.000 description 1
- 235000015220 hamburgers Nutrition 0.000 description 1
- 201000010235 heart cancer Diseases 0.000 description 1
- 208000024348 heart neoplasm Diseases 0.000 description 1
- 210000002443 helper t lymphocyte Anatomy 0.000 description 1
- 201000011066 hemangioma Diseases 0.000 description 1
- 230000002489 hematologic effect Effects 0.000 description 1
- 230000008588 hemolysis Effects 0.000 description 1
- 229960002897 heparin Drugs 0.000 description 1
- 229920000669 heparin Polymers 0.000 description 1
- 229940022353 herceptin Drugs 0.000 description 1
- 125000000623 heterocyclic group Chemical group 0.000 description 1
- 239000000833 heterodimer Substances 0.000 description 1
- UUVWYPNAQBNQJQ-UHFFFAOYSA-N hexamethylmelamine Chemical compound CN(C)C1=NC(N(C)C)=NC(N(C)C)=N1 UUVWYPNAQBNQJQ-UHFFFAOYSA-N 0.000 description 1
- 238000004128 high performance liquid chromatography Methods 0.000 description 1
- 238000000589 high-performance liquid chromatography-mass spectrometry Methods 0.000 description 1
- 229940121372 histone deacetylase inhibitor Drugs 0.000 description 1
- 239000003276 histone deacetylase inhibitor Substances 0.000 description 1
- 239000000710 homodimer Substances 0.000 description 1
- 238000002744 homologous recombination Methods 0.000 description 1
- 230000006801 homologous recombination Effects 0.000 description 1
- 229940048921 humira Drugs 0.000 description 1
- 229930195733 hydrocarbon Natural products 0.000 description 1
- 230000007062 hydrolysis Effects 0.000 description 1
- 238000006460 hydrolysis reaction Methods 0.000 description 1
- 229920001477 hydrophilic polymer Polymers 0.000 description 1
- 238000004191 hydrophobic interaction chromatography Methods 0.000 description 1
- 229920001600 hydrophobic polymer Polymers 0.000 description 1
- 125000002349 hydroxyamino group Chemical group [H]ON([H])[*] 0.000 description 1
- 229910052588 hydroxylapatite Inorganic materials 0.000 description 1
- 238000012872 hydroxylapatite chromatography Methods 0.000 description 1
- 229920003063 hydroxymethyl cellulose Polymers 0.000 description 1
- 229940031574 hydroxymethyl cellulose Drugs 0.000 description 1
- 229960001001 ibritumomab tiuxetan Drugs 0.000 description 1
- HOMGKSMUEGBAAB-UHFFFAOYSA-N ifosfamide Chemical compound ClCCNP1(=O)OCCCN1CCCl HOMGKSMUEGBAAB-UHFFFAOYSA-N 0.000 description 1
- 229960001101 ifosfamide Drugs 0.000 description 1
- KTUFNOKKBVMGRW-UHFFFAOYSA-N imatinib Chemical compound C1CN(C)CCN1CC1=CC=C(C(=O)NC=2C=C(NC=3N=C(C=CN=3)C=3C=NC=CC=3)C(C)=CC=2)C=C1 KTUFNOKKBVMGRW-UHFFFAOYSA-N 0.000 description 1
- 150000002463 imidates Chemical class 0.000 description 1
- 238000003018 immunoassay Methods 0.000 description 1
- 238000003364 immunohistochemistry Methods 0.000 description 1
- 239000002955 immunomodulating agent Substances 0.000 description 1
- 229940121354 immunomodulator Drugs 0.000 description 1
- 239000003547 immunosorbent Substances 0.000 description 1
- 238000009169 immunotherapy Methods 0.000 description 1
- 238000002513 implantation Methods 0.000 description 1
- 238000000099 in vitro assay Methods 0.000 description 1
- 238000010874 in vitro model Methods 0.000 description 1
- 230000006882 induction of apoptosis Effects 0.000 description 1
- 230000001939 inductive effect Effects 0.000 description 1
- 210000004969 inflammatory cell Anatomy 0.000 description 1
- 230000002757 inflammatory effect Effects 0.000 description 1
- 230000028709 inflammatory response Effects 0.000 description 1
- 238000011081 inoculation Methods 0.000 description 1
- 150000007529 inorganic bases Chemical class 0.000 description 1
- 150000002484 inorganic compounds Chemical class 0.000 description 1
- 229910010272 inorganic material Inorganic materials 0.000 description 1
- 229940125396 insulin Drugs 0.000 description 1
- 230000010354 integration Effects 0.000 description 1
- 102000006495 integrins Human genes 0.000 description 1
- 108010044426 integrins Proteins 0.000 description 1
- 210000000936 intestine Anatomy 0.000 description 1
- 238000007918 intramuscular administration Methods 0.000 description 1
- 239000007928 intraperitoneal injection Substances 0.000 description 1
- 238000007913 intrathecal administration Methods 0.000 description 1
- 238000011835 investigation Methods 0.000 description 1
- PGLTVOMIXTUURA-UHFFFAOYSA-N iodoacetamide Chemical compound NC(=O)CI PGLTVOMIXTUURA-UHFFFAOYSA-N 0.000 description 1
- 238000005342 ion exchange Methods 0.000 description 1
- UWKQSNNFCGGAFS-XIFFEERXSA-N irinotecan Chemical compound C1=C2C(CC)=C3CN(C(C4=C([C@@](C(=O)OC4)(O)CC)C=4)=O)C=4C3=NC2=CC=C1OC(=O)N(CC1)CCC1N1CCCCC1 UWKQSNNFCGGAFS-XIFFEERXSA-N 0.000 description 1
- 208000028867 ischemia Diseases 0.000 description 1
- 235000015110 jellies Nutrition 0.000 description 1
- 239000008274 jelly Substances 0.000 description 1
- 210000001117 keloid Anatomy 0.000 description 1
- TYQCGQRIZGCHNB-JLAZNSOCSA-N l-ascorbic acid Chemical compound OC[C@H](O)[C@H]1OC(O)=C(O)C1=O TYQCGQRIZGCHNB-JLAZNSOCSA-N 0.000 description 1
- 229940057428 lactoperoxidase Drugs 0.000 description 1
- 238000011031 large-scale manufacturing process Methods 0.000 description 1
- 230000002045 lasting effect Effects 0.000 description 1
- 229940070765 laurate Drugs 0.000 description 1
- 239000010410 layer Substances 0.000 description 1
- GOTYRUGSSMKFNF-UHFFFAOYSA-N lenalidomide Chemical compound C1C=2C(N)=CC=CC=2C(=O)N1C1CCC(=O)NC1=O GOTYRUGSSMKFNF-UHFFFAOYSA-N 0.000 description 1
- 229960004942 lenalidomide Drugs 0.000 description 1
- 208000032839 leukemia Diseases 0.000 description 1
- RGLRXNKKBLIBQS-XNHQSDQCSA-N leuprolide acetate Chemical compound CC(O)=O.CCNC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@H](CC=1N=CNC=1)NC(=O)[C@H]1NC(=O)CC1)CC1=CC=C(O)C=C1 RGLRXNKKBLIBQS-XNHQSDQCSA-N 0.000 description 1
- 230000004576 lipid-binding Effects 0.000 description 1
- 210000004185 liver Anatomy 0.000 description 1
- 208000014018 liver neoplasm Diseases 0.000 description 1
- 210000005228 liver tissue Anatomy 0.000 description 1
- 244000144972 livestock Species 0.000 description 1
- 230000033001 locomotion Effects 0.000 description 1
- 229940076783 lucentis Drugs 0.000 description 1
- 210000004072 lung Anatomy 0.000 description 1
- 210000005265 lung cell Anatomy 0.000 description 1
- 210000002751 lymph Anatomy 0.000 description 1
- 210000000207 lymphocyte subset Anatomy 0.000 description 1
- 210000003563 lymphoid tissue Anatomy 0.000 description 1
- 239000012139 lysis buffer Substances 0.000 description 1
- 239000004325 lysozyme Substances 0.000 description 1
- 229960000274 lysozyme Drugs 0.000 description 1
- 235000010335 lysozyme Nutrition 0.000 description 1
- 229910052749 magnesium Inorganic materials 0.000 description 1
- 229940049920 malate Drugs 0.000 description 1
- BJEPYKJPYRNKOW-UHFFFAOYSA-L malate(2-) Chemical compound [O-]C(=O)C(O)CC([O-])=O BJEPYKJPYRNKOW-UHFFFAOYSA-L 0.000 description 1
- 239000000594 mannitol Substances 0.000 description 1
- 235000010355 mannitol Nutrition 0.000 description 1
- 238000004949 mass spectrometry Methods 0.000 description 1
- 108010082117 matrigel Proteins 0.000 description 1
- 229960001924 melphalan Drugs 0.000 description 1
- SGDBTWWWUNNDEQ-LBPRGKRZSA-N melphalan Chemical compound OC(=O)[C@@H](N)CC1=CC=C(N(CCCl)CCCl)C=C1 SGDBTWWWUNNDEQ-LBPRGKRZSA-N 0.000 description 1
- 238000002844 melting Methods 0.000 description 1
- 230000008018 melting Effects 0.000 description 1
- 229960003151 mercaptamine Drugs 0.000 description 1
- 229960001428 mercaptopurine Drugs 0.000 description 1
- 229930182817 methionine Natural products 0.000 description 1
- 229960004452 methionine Drugs 0.000 description 1
- 229960000485 methotrexate Drugs 0.000 description 1
- 239000004292 methyl p-hydroxybenzoate Substances 0.000 description 1
- 235000010270 methyl p-hydroxybenzoate Nutrition 0.000 description 1
- 229960002216 methylparaben Drugs 0.000 description 1
- 239000000693 micelle Substances 0.000 description 1
- 244000005700 microbiome Species 0.000 description 1
- 108010029942 microperoxidase Proteins 0.000 description 1
- 150000007522 mineralic acids Chemical class 0.000 description 1
- 229960004857 mitomycin Drugs 0.000 description 1
- 229960000350 mitotane Drugs 0.000 description 1
- 239000003607 modifier Substances 0.000 description 1
- 238000012544 monitoring process Methods 0.000 description 1
- 150000002772 monosaccharides Chemical class 0.000 description 1
- 238000000465 moulding Methods 0.000 description 1
- 210000000214 mouth Anatomy 0.000 description 1
- 210000004877 mucosa Anatomy 0.000 description 1
- 230000002107 myocardial effect Effects 0.000 description 1
- 208000010125 myocardial infarction Diseases 0.000 description 1
- 208000031225 myocardial ischemia Diseases 0.000 description 1
- 239000002088 nanocapsule Substances 0.000 description 1
- 210000000822 natural killer cell Anatomy 0.000 description 1
- 229930014626 natural product Natural products 0.000 description 1
- 229960004927 neomycin Drugs 0.000 description 1
- 230000007372 neural signaling Effects 0.000 description 1
- 208000004296 neuralgia Diseases 0.000 description 1
- 208000015122 neurodegenerative disease Diseases 0.000 description 1
- 208000021722 neuropathic pain Diseases 0.000 description 1
- 239000002353 niosome Substances 0.000 description 1
- 239000000041 non-steroidal anti-inflammatory agent Substances 0.000 description 1
- 229940021182 non-steroidal anti-inflammatory drug Drugs 0.000 description 1
- 231100000252 nontoxic Toxicity 0.000 description 1
- 230000003000 nontoxic effect Effects 0.000 description 1
- 238000010899 nucleation Methods 0.000 description 1
- 235000015097 nutrients Nutrition 0.000 description 1
- 229920001778 nylon Polymers 0.000 description 1
- 235000020824 obesity Nutrition 0.000 description 1
- 239000002674 ointment Substances 0.000 description 1
- 150000002482 oligosaccharides Polymers 0.000 description 1
- IXWNTLSTOZFSCM-YVACAVLKSA-N ombrabulin Chemical compound C1=C(NC(=O)[C@@H](N)CO)C(OC)=CC=C1\C=C/C1=CC(OC)=C(OC)C(OC)=C1 IXWNTLSTOZFSCM-YVACAVLKSA-N 0.000 description 1
- 229950003600 ombrabulin Drugs 0.000 description 1
- 238000011275 oncology therapy Methods 0.000 description 1
- 230000014207 opsonization Effects 0.000 description 1
- 150000007530 organic bases Chemical class 0.000 description 1
- BWKDAMBGCPRVPI-ZQRPHVBESA-N ortataxel Chemical compound O([C@@H]1[C@]23OC(=O)O[C@H]2[C@@H](C(=C([C@@H](OC(C)=O)C(=O)[C@]2(C)[C@@H](O)C[C@H]4OC[C@]4([C@H]21)OC(C)=O)C3(C)C)C)OC(=O)[C@H](O)[C@@H](NC(=O)OC(C)(C)C)CC(C)C)C(=O)C1=CC=CC=C1 BWKDAMBGCPRVPI-ZQRPHVBESA-N 0.000 description 1
- 230000003647 oxidation Effects 0.000 description 1
- 230000001590 oxidative effect Effects 0.000 description 1
- 239000001301 oxygen Substances 0.000 description 1
- 229910052760 oxygen Inorganic materials 0.000 description 1
- LXCFILQKKLGQFO-UHFFFAOYSA-N p-hydroxybenzoic acid methyl ester Natural products COC(=O)C1=CC=C(O)C=C1 LXCFILQKKLGQFO-UHFFFAOYSA-N 0.000 description 1
- 239000006174 pH buffer Substances 0.000 description 1
- 238000012856 packing Methods 0.000 description 1
- 229960001592 paclitaxel Drugs 0.000 description 1
- 239000012188 paraffin wax Substances 0.000 description 1
- 230000036961 partial effect Effects 0.000 description 1
- 230000004963 pathophysiological condition Effects 0.000 description 1
- 229960003407 pegaptanib Drugs 0.000 description 1
- 230000006320 pegylation Effects 0.000 description 1
- 230000000149 penetrating effect Effects 0.000 description 1
- 230000035515 penetration Effects 0.000 description 1
- 239000003961 penetration enhancing agent Substances 0.000 description 1
- 229940049954 penicillin Drugs 0.000 description 1
- XYJRXVWERLGGKC-UHFFFAOYSA-D pentacalcium;hydroxide;triphosphate Chemical compound [OH-].[Ca+2].[Ca+2].[Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O XYJRXVWERLGGKC-UHFFFAOYSA-D 0.000 description 1
- 229960002340 pentostatin Drugs 0.000 description 1
- FPVKHBSQESCIEP-JQCXWYLXSA-N pentostatin Chemical compound C1[C@H](O)[C@@H](CO)O[C@H]1N1C(N=CNC[C@H]2O)=C2N=C1 FPVKHBSQESCIEP-JQCXWYLXSA-N 0.000 description 1
- 229940111202 pepsin Drugs 0.000 description 1
- 239000000137 peptide hydrolase inhibitor Substances 0.000 description 1
- 230000003836 peripheral circulation Effects 0.000 description 1
- 230000002093 peripheral effect Effects 0.000 description 1
- 239000008255 pharmaceutical foam Substances 0.000 description 1
- 230000003285 pharmacodynamic effect Effects 0.000 description 1
- 239000008363 phosphate buffer Substances 0.000 description 1
- 125000004437 phosphorous atom Chemical group 0.000 description 1
- 230000001766 physiological effect Effects 0.000 description 1
- 230000006461 physiological response Effects 0.000 description 1
- 229930000756 phytoceramide Natural products 0.000 description 1
- AERBNCYCJBRYDG-KSZLIROESA-N phytosphingosine Chemical compound CCCCCCCCCCCCCC[C@@H](O)[C@@H](O)[C@@H](N)CO AERBNCYCJBRYDG-KSZLIROESA-N 0.000 description 1
- 229940033329 phytosphingosine Drugs 0.000 description 1
- 229920003023 plastic Polymers 0.000 description 1
- 239000004033 plastic Substances 0.000 description 1
- 150000003057 platinum Chemical class 0.000 description 1
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Substances [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 1
- 230000010287 polarization Effects 0.000 description 1
- 229920001983 poloxamer Polymers 0.000 description 1
- 229920000747 poly(lactic acid) Polymers 0.000 description 1
- 229920003229 poly(methyl methacrylate) Polymers 0.000 description 1
- 229920002401 polyacrylamide Polymers 0.000 description 1
- 230000008488 polyadenylation Effects 0.000 description 1
- 108010054442 polyalanine Proteins 0.000 description 1
- 108010064470 polyaspartate Proteins 0.000 description 1
- 229920000728 polyester Polymers 0.000 description 1
- 229940050929 polyethylene glycol 3350 Drugs 0.000 description 1
- 229920002338 polyhydroxyethylmethacrylate Polymers 0.000 description 1
- 239000004926 polymethyl methacrylate Substances 0.000 description 1
- 229920001451 polypropylene glycol Polymers 0.000 description 1
- 229920001282 polysaccharide Polymers 0.000 description 1
- 239000005017 polysaccharide Substances 0.000 description 1
- 229920001296 polysiloxane Polymers 0.000 description 1
- 229920002223 polystyrene Polymers 0.000 description 1
- 239000004810 polytetrafluoroethylene Substances 0.000 description 1
- 229920001343 polytetrafluoroethylene Polymers 0.000 description 1
- 229920002981 polyvinylidene fluoride Polymers 0.000 description 1
- 239000001267 polyvinylpyrrolidone Substances 0.000 description 1
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 1
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 1
- 230000013155 positive regulation of cell migration Effects 0.000 description 1
- 231100000683 possible toxicity Toxicity 0.000 description 1
- 239000011591 potassium Substances 0.000 description 1
- 229910052700 potassium Inorganic materials 0.000 description 1
- 229910000160 potassium phosphate Inorganic materials 0.000 description 1
- 235000011009 potassium phosphates Nutrition 0.000 description 1
- 235000012015 potatoes Nutrition 0.000 description 1
- 230000003389 potentiating effect Effects 0.000 description 1
- 244000144977 poultry Species 0.000 description 1
- 235000013594 poultry meat Nutrition 0.000 description 1
- 239000000843 powder Substances 0.000 description 1
- 238000001556 precipitation Methods 0.000 description 1
- 230000002335 preservative effect Effects 0.000 description 1
- 150000003141 primary amines Chemical class 0.000 description 1
- CPTBDICYNRMXFX-UHFFFAOYSA-N procarbazine Chemical compound CNNCC1=CC=C(C(=O)NC(C)C)C=C1 CPTBDICYNRMXFX-UHFFFAOYSA-N 0.000 description 1
- 229960000624 procarbazine Drugs 0.000 description 1
- 238000003672 processing method Methods 0.000 description 1
- 230000002062 proliferating effect Effects 0.000 description 1
- 125000001500 prolyl group Chemical group [H]N1C([H])(C(=O)[*])C([H])([H])C([H])([H])C1([H])[H] 0.000 description 1
- 235000010232 propyl p-hydroxybenzoate Nutrition 0.000 description 1
- 239000004405 propyl p-hydroxybenzoate Substances 0.000 description 1
- 229960003415 propylparaben Drugs 0.000 description 1
- 235000019419 proteases Nutrition 0.000 description 1
- 230000001012 protector Effects 0.000 description 1
- 239000012268 protein inhibitor Substances 0.000 description 1
- 229940121649 protein inhibitor Drugs 0.000 description 1
- 238000001742 protein purification Methods 0.000 description 1
- 230000012743 protein tagging Effects 0.000 description 1
- 230000017854 proteolysis Effects 0.000 description 1
- 230000005855 radiation Effects 0.000 description 1
- 238000002708 random mutagenesis Methods 0.000 description 1
- 229960003876 ranibizumab Drugs 0.000 description 1
- 229910052761 rare earth metal Inorganic materials 0.000 description 1
- 150000002910 rare earth metals Chemical class 0.000 description 1
- 239000000376 reactant Substances 0.000 description 1
- 230000009257 reactivity Effects 0.000 description 1
- 230000007115 recruitment Effects 0.000 description 1
- 238000007670 refining Methods 0.000 description 1
- 230000008929 regeneration Effects 0.000 description 1
- 238000011069 regeneration method Methods 0.000 description 1
- 108091006091 regulatory enzymes Proteins 0.000 description 1
- 229940116176 remicade Drugs 0.000 description 1
- 238000007634 remodeling Methods 0.000 description 1
- 230000033458 reproduction Effects 0.000 description 1
- 208000037803 restenosis Diseases 0.000 description 1
- 230000000717 retained effect Effects 0.000 description 1
- 210000001525 retina Anatomy 0.000 description 1
- 230000004264 retinal detachment Effects 0.000 description 1
- 230000002207 retinal effect Effects 0.000 description 1
- 229930002330 retinoic acid Natural products 0.000 description 1
- PYWVYCXTNDRMGF-UHFFFAOYSA-N rhodamine B Chemical compound [Cl-].C=12C=CC(=[N+](CC)CC)C=C2OC2=CC(N(CC)CC)=CC=C2C=1C1=CC=CC=C1C(O)=O PYWVYCXTNDRMGF-UHFFFAOYSA-N 0.000 description 1
- 210000003705 ribosome Anatomy 0.000 description 1
- 102200156936 rs28934878 Human genes 0.000 description 1
- 102220047535 rs587783040 Human genes 0.000 description 1
- 239000011833 salt mixture Substances 0.000 description 1
- 238000005070 sampling Methods 0.000 description 1
- 238000013391 scatchard analysis Methods 0.000 description 1
- 238000003345 scintillation counting Methods 0.000 description 1
- 238000012163 sequencing technique Methods 0.000 description 1
- 238000013207 serial dilution Methods 0.000 description 1
- 210000000717 sertoli cell Anatomy 0.000 description 1
- 229910052709 silver Inorganic materials 0.000 description 1
- 239000004332 silver Substances 0.000 description 1
- 239000002356 single layer Substances 0.000 description 1
- 102000030938 small GTPase Human genes 0.000 description 1
- 108060007624 small GTPase Proteins 0.000 description 1
- 150000003384 small molecules Chemical class 0.000 description 1
- 210000000329 smooth muscle myocyte Anatomy 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- PTLRDCMBXHILCL-UHFFFAOYSA-M sodium arsenite Chemical compound [Na+].[O-][As]=O PTLRDCMBXHILCL-UHFFFAOYSA-M 0.000 description 1
- 229910000029 sodium carbonate Inorganic materials 0.000 description 1
- 235000019812 sodium carboxymethyl cellulose Nutrition 0.000 description 1
- 229920001027 sodium carboxymethylcellulose Polymers 0.000 description 1
- 239000001509 sodium citrate Substances 0.000 description 1
- NLJMYIDDQXHKNR-UHFFFAOYSA-K sodium citrate Chemical compound O.O.[Na+].[Na+].[Na+].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O NLJMYIDDQXHKNR-UHFFFAOYSA-K 0.000 description 1
- 239000012064 sodium phosphate buffer Substances 0.000 description 1
- 229940054269 sodium pyruvate Drugs 0.000 description 1
- ALPWRKFXEOAUDR-GKEJWYBXSA-M sodium;[(2r)-2,3-di(octadecanoyloxy)propyl] hydrogen phosphate Chemical compound [Na+].CCCCCCCCCCCCCCCCCC(=O)OC[C@H](COP(O)([O-])=O)OC(=O)CCCCCCCCCCCCCCCCC ALPWRKFXEOAUDR-GKEJWYBXSA-M 0.000 description 1
- 230000003381 solubilizing effect Effects 0.000 description 1
- 229960003787 sorafenib Drugs 0.000 description 1
- 108010066791 sphingosine-1-phosphate phosphatase Proteins 0.000 description 1
- 238000012421 spiking Methods 0.000 description 1
- 238000009987 spinning Methods 0.000 description 1
- 239000007921 spray Substances 0.000 description 1
- 238000012289 standard assay Methods 0.000 description 1
- 238000010561 standard procedure Methods 0.000 description 1
- SFVFIFLLYFPGHH-UHFFFAOYSA-M stearalkonium chloride Chemical compound [Cl-].CCCCCCCCCCCCCCCCCC[N+](C)(C)CC1=CC=CC=C1 SFVFIFLLYFPGHH-UHFFFAOYSA-M 0.000 description 1
- 238000011146 sterile filtration Methods 0.000 description 1
- 150000003431 steroids Chemical class 0.000 description 1
- 230000004936 stimulating effect Effects 0.000 description 1
- 210000002784 stomach Anatomy 0.000 description 1
- 239000012089 stop solution Substances 0.000 description 1
- 229960005322 streptomycin Drugs 0.000 description 1
- ZSJLQEPLLKMAKR-GKHCUFPYSA-N streptozocin Chemical compound O=NN(C)C(=O)N[C@H]1[C@@H](O)O[C@H](CO)[C@@H](O)[C@@H]1O ZSJLQEPLLKMAKR-GKHCUFPYSA-N 0.000 description 1
- 238000010254 subcutaneous injection Methods 0.000 description 1
- 239000007929 subcutaneous injection Substances 0.000 description 1
- 229940014800 succinic anhydride Drugs 0.000 description 1
- 239000005720 sucrose Substances 0.000 description 1
- WINHZLLDWRZWRT-ATVHPVEESA-N sunitinib Chemical compound CCN(CC)CCNC(=O)C1=C(C)NC(\C=C/2C3=CC(F)=CC=C3NC\2=O)=C1C WINHZLLDWRZWRT-ATVHPVEESA-N 0.000 description 1
- 229960001796 sunitinib Drugs 0.000 description 1
- 239000013589 supplement Substances 0.000 description 1
- 230000008093 supporting effect Effects 0.000 description 1
- 239000000829 suppository Substances 0.000 description 1
- 239000004094 surface-active agent Substances 0.000 description 1
- 230000004654 survival pathway Effects 0.000 description 1
- 238000004114 suspension culture Methods 0.000 description 1
- 230000005469 synchrotron radiation Effects 0.000 description 1
- 230000002195 synergetic effect Effects 0.000 description 1
- 235000020357 syrup Nutrition 0.000 description 1
- 239000006188 syrup Substances 0.000 description 1
- 239000003826 tablet Substances 0.000 description 1
- 229960001603 tamoxifen Drugs 0.000 description 1
- AYUNIORJHRXIBJ-TXHRRWQRSA-N tanespimycin Chemical compound N1C(=O)\C(C)=C\C=C/[C@H](OC)[C@@H](OC(N)=O)\C(C)=C\[C@H](C)[C@@H](O)[C@@H](OC)C[C@H](C)CC2=C(NCC=C)C(=O)C=C1C2=O AYUNIORJHRXIBJ-TXHRRWQRSA-N 0.000 description 1
- 229950007866 tanespimycin Drugs 0.000 description 1
- RCINICONZNJXQF-MZXODVADSA-N taxol Chemical compound O([C@@H]1[C@@]2(C[C@@H](C(C)=C(C2(C)C)[C@H](C([C@]2(C)[C@@H](O)C[C@H]3OC[C@]3([C@H]21)OC(C)=O)=O)OC(=O)C)OC(=O)[C@H](O)[C@@H](NC(=O)C=1C=CC=CC=1)C=1C=CC=CC=1)O)C(=O)C1=CC=CC=C1 RCINICONZNJXQF-MZXODVADSA-N 0.000 description 1
- 229940063683 taxotere Drugs 0.000 description 1
- 239000003277 telomerase inhibitor Substances 0.000 description 1
- IMCGHZIGRANKHV-AJNGGQMLSA-N tert-butyl (3s,5s)-2-oxo-5-[(2s,4s)-5-oxo-4-propan-2-yloxolan-2-yl]-3-propan-2-ylpyrrolidine-1-carboxylate Chemical compound O1C(=O)[C@H](C(C)C)C[C@H]1[C@H]1N(C(=O)OC(C)(C)C)C(=O)[C@H](C(C)C)C1 IMCGHZIGRANKHV-AJNGGQMLSA-N 0.000 description 1
- 229960000814 tetanus toxoid Drugs 0.000 description 1
- 229960002180 tetracycline Drugs 0.000 description 1
- 229930101283 tetracycline Natural products 0.000 description 1
- 235000019364 tetracycline Nutrition 0.000 description 1
- 150000003522 tetracyclines Chemical class 0.000 description 1
- MPLHNVLQVRSVEE-UHFFFAOYSA-N texas red Chemical compound [O-]S(=O)(=O)C1=CC(S(Cl)(=O)=O)=CC=C1C(C1=CC=2CCCN3CCCC(C=23)=C1O1)=C2C1=C(CCC1)C3=[N+]1CCCC3=C2 MPLHNVLQVRSVEE-UHFFFAOYSA-N 0.000 description 1
- 229960003433 thalidomide Drugs 0.000 description 1
- 229940126585 therapeutic drug Drugs 0.000 description 1
- 238000011285 therapeutic regimen Methods 0.000 description 1
- CNHYKKNIIGEXAY-UHFFFAOYSA-N thiolan-2-imine Chemical compound N=C1CCCS1 CNHYKKNIIGEXAY-UHFFFAOYSA-N 0.000 description 1
- 229960001196 thiotepa Drugs 0.000 description 1
- 229940104230 thymidine Drugs 0.000 description 1
- 239000001585 thymus vulgaris Substances 0.000 description 1
- 229960002175 thyroglobulin Drugs 0.000 description 1
- 229960003087 tioguanine Drugs 0.000 description 1
- 230000007838 tissue remodeling Effects 0.000 description 1
- RUELTTOHQODFPA-UHFFFAOYSA-N toluene 2,6-diisocyanate Chemical compound CC1=C(N=C=O)C=CC=C1N=C=O RUELTTOHQODFPA-UHFFFAOYSA-N 0.000 description 1
- 238000011200 topical administration Methods 0.000 description 1
- 229940044693 topoisomerase inhibitor Drugs 0.000 description 1
- 230000002110 toxicologic effect Effects 0.000 description 1
- 239000011573 trace mineral Substances 0.000 description 1
- 235000013619 trace mineral Nutrition 0.000 description 1
- 210000003437 trachea Anatomy 0.000 description 1
- 230000002103 transcriptional effect Effects 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
- 239000012581 transferrin Substances 0.000 description 1
- 230000001052 transient effect Effects 0.000 description 1
- 238000003146 transient transfection Methods 0.000 description 1
- 238000011269 treatment regimen Methods 0.000 description 1
- IUCJMVBFZDHPDX-UHFFFAOYSA-N tretamine Chemical compound C1CN1C1=NC(N2CC2)=NC(N2CC2)=N1 IUCJMVBFZDHPDX-UHFFFAOYSA-N 0.000 description 1
- 229950001353 tretamine Drugs 0.000 description 1
- 239000013638 trimer Substances 0.000 description 1
- 229960001099 trimetrexate Drugs 0.000 description 1
- NOYPYLRCIDNJJB-UHFFFAOYSA-N trimetrexate Chemical compound COC1=C(OC)C(OC)=CC(NCC=2C(=C3C(N)=NC(N)=NC3=CC=2)C)=C1 NOYPYLRCIDNJJB-UHFFFAOYSA-N 0.000 description 1
- 239000002753 trypsin inhibitor Substances 0.000 description 1
- 230000004614 tumor growth Effects 0.000 description 1
- 229940121358 tyrosine kinase inhibitor Drugs 0.000 description 1
- 239000005483 tyrosine kinase inhibitor Substances 0.000 description 1
- 238000000870 ultraviolet spectroscopy Methods 0.000 description 1
- 241000701447 unidentified baculovirus Species 0.000 description 1
- 241001515965 unidentified phage Species 0.000 description 1
- 210000002700 urine Anatomy 0.000 description 1
- VBEQCZHXXJYVRD-GACYYNSASA-N uroanthelone Chemical compound C([C@@H](C(=O)N[C@H](C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CS)C(=O)N[C@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)NCC(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N[C@@H](CO)C(=O)NCC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CS)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O)C(C)C)[C@@H](C)O)NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CC=1NC=NC=1)NC(=O)[C@H](CCSC)NC(=O)[C@H](CS)NC(=O)[C@@H](NC(=O)CNC(=O)CNC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CS)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)CNC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@H]1N(CCC1)C(=O)[C@H](CS)NC(=O)CNC(=O)[C@H]1N(CCC1)C(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC(N)=O)C(C)C)[C@@H](C)CC)C1=CC=C(O)C=C1 VBEQCZHXXJYVRD-GACYYNSASA-N 0.000 description 1
- XGOYIMQSIKSOBS-UHFFFAOYSA-N vadimezan Chemical compound C1=CC=C2C(=O)C3=CC=C(C)C(C)=C3OC2=C1CC(O)=O XGOYIMQSIKSOBS-UHFFFAOYSA-N 0.000 description 1
- 229950008737 vadimezan Drugs 0.000 description 1
- 239000004066 vascular targeting agent Substances 0.000 description 1
- 230000006442 vascular tone Effects 0.000 description 1
- 210000005166 vasculature Anatomy 0.000 description 1
- 210000003462 vein Anatomy 0.000 description 1
- 229960003048 vinblastine Drugs 0.000 description 1
- JXLYSJRDGCGARV-XQKSVPLYSA-N vincaleukoblastine Chemical compound C([C@@H](C[C@]1(C(=O)OC)C=2C(=CC3=C([C@]45[C@H]([C@@]([C@H](OC(C)=O)[C@]6(CC)C=CCN([C@H]56)CC4)(O)C(=O)OC)N3C)C=2)OC)C[C@@](C2)(O)CC)N2CCC2=C1NC1=CC=CC=C21 JXLYSJRDGCGARV-XQKSVPLYSA-N 0.000 description 1
- 229960004528 vincristine Drugs 0.000 description 1
- OGWKCGZFUXNPDA-XQKSVPLYSA-N vincristine Chemical compound C([N@]1C[C@@H](C[C@]2(C(=O)OC)C=3C(=CC4=C([C@]56[C@H]([C@@]([C@H](OC(C)=O)[C@]7(CC)C=CCN([C@H]67)CC5)(O)C(=O)OC)N4C=O)C=3)OC)C[C@@](C1)(O)CC)CC1=C2NC2=CC=CC=C12 OGWKCGZFUXNPDA-XQKSVPLYSA-N 0.000 description 1
- OGWKCGZFUXNPDA-UHFFFAOYSA-N vincristine Natural products C1C(CC)(O)CC(CC2(C(=O)OC)C=3C(=CC4=C(C56C(C(C(OC(C)=O)C7(CC)C=CCN(C67)CC5)(O)C(=O)OC)N4C=O)C=3)OC)CN1CCC1=C2NC2=CC=CC=C12 OGWKCGZFUXNPDA-UHFFFAOYSA-N 0.000 description 1
- 229960004355 vindesine Drugs 0.000 description 1
- UGGWPQSBPIFKDZ-KOTLKJBCSA-N vindesine Chemical compound C([C@@H](C[C@]1(C(=O)OC)C=2C(=CC3=C([C@]45[C@H]([C@@]([C@H](O)[C@]6(CC)C=CCN([C@H]56)CC4)(O)C(N)=O)N3C)C=2)OC)C[C@@](C2)(O)CC)N2CCC2=C1N=C1[C]2C=CC=C1 UGGWPQSBPIFKDZ-KOTLKJBCSA-N 0.000 description 1
- 229960002066 vinorelbine Drugs 0.000 description 1
- GBABOYUKABKIAF-GHYRFKGUSA-N vinorelbine Chemical compound C1N(CC=2C3=CC=CC=C3NC=22)CC(CC)=C[C@H]1C[C@]2(C(=O)OC)C1=CC([C@]23[C@H]([C@]([C@H](OC(C)=O)[C@]4(CC)C=CCN([C@H]34)CC2)(O)C(=O)OC)N2C)=C2C=C1OC GBABOYUKABKIAF-GHYRFKGUSA-N 0.000 description 1
- 230000000007 visual effect Effects 0.000 description 1
- 230000004393 visual impairment Effects 0.000 description 1
- 239000003643 water by type Substances 0.000 description 1
- 230000003442 weekly effect Effects 0.000 description 1
- 230000037314 wound repair Effects 0.000 description 1
- UGBMEXLBFDAOGL-INIZCTEOSA-N zd6126 Chemical compound C1C[C@H](NC(C)=O)C2=CC(OP(O)(O)=O)=CC=C2C2=C1C=C(OC)C(OC)=C2OC UGBMEXLBFDAOGL-INIZCTEOSA-N 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/395—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum
- A61K39/39591—Stabilisation, fragmentation
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/505—Medicinal preparations containing antigens or antibodies comprising antibodies
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2299/00—Coordinates from 3D structures of peptides, e.g. proteins or enzymes
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/20—Immunoglobulins specific features characterized by taxonomic origin
- C07K2317/24—Immunoglobulins specific features characterized by taxonomic origin containing regions, domains or residues from different species, e.g. chimeric, humanized or veneered
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/55—Fab or Fab'
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/56—Immunoglobulins specific features characterized by immunoglobulin fragments variable (Fv) region, i.e. VH and/or VL
- C07K2317/565—Complementarity determining region [CDR]
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/90—Immunoglobulins specific features characterized by (pharmaco)kinetic aspects or by stability of the immunoglobulin
- C07K2317/92—Affinity (KD), association rate (Ka), dissociation rate (Kd) or EC50 value
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- Organic Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Immunology (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Veterinary Medicine (AREA)
- Pharmacology & Pharmacy (AREA)
- Genetics & Genomics (AREA)
- Biochemistry (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Molecular Biology (AREA)
- Biophysics (AREA)
- Epidemiology (AREA)
- Mycology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Microbiology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Rheumatology (AREA)
- Pain & Pain Management (AREA)
- Peptides Or Proteins (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
Applications Claiming Priority (6)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US12031808P | 2008-12-05 | 2008-12-05 | |
| US61/120,318 | 2008-12-05 | ||
| US15589509P | 2009-02-26 | 2009-02-26 | |
| US61/155,895 | 2009-02-26 | ||
| US23125809P | 2009-08-04 | 2009-08-04 | |
| US61/231,258 | 2009-08-04 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20110097923A true KR20110097923A (ko) | 2011-08-31 |
Family
ID=42233904
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020117015390A Withdrawn KR20110097923A (ko) | 2008-12-05 | 2009-12-04 | 항-지질 항체 결정 구조를 이용한 항체 설계 |
Country Status (9)
| Country | Link |
|---|---|
| US (1) | US20110044990A1 (enExample) |
| EP (1) | EP2374001A4 (enExample) |
| JP (1) | JP2012511026A (enExample) |
| KR (1) | KR20110097923A (enExample) |
| CN (1) | CN102573905A (enExample) |
| AU (1) | AU2009322185A1 (enExample) |
| CA (1) | CA2745436A1 (enExample) |
| IL (1) | IL213358A0 (enExample) |
| WO (1) | WO2010065921A2 (enExample) |
Families Citing this family (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2011153416A2 (en) * | 2010-06-04 | 2011-12-08 | Lpath, Inc. | Novel anti-s1p antibody variants of lt1009 |
| ITRM20100441A1 (it) * | 2010-08-05 | 2012-02-06 | Michele Pitaro | Procedimento per la produzione di anticorpi monoclonali anti-idiotipo ad uso diagnostico e/o terapeutico |
| BR112015002605B1 (pt) | 2012-08-07 | 2023-03-07 | Massachusetts Institute Of Technology | Agente de anticorpo específico para o vírus da dengue, seu uso, kit, composição farmacêutica e método para sua produção |
| EP3104888A4 (en) | 2014-02-11 | 2017-08-09 | Massachusetts Institute Of Technology | Novel full spectrum anti-dengue antibody |
| CA3003397A1 (en) | 2014-10-30 | 2016-05-06 | Textile-Based Delivery, Inc. | Delivery systems |
| CN106916227B (zh) * | 2015-12-24 | 2019-12-13 | 凯惠科技发展(上海)有限公司 | 一种tpbg抗体及其制备方法、其偶联物和应用 |
| JP6778932B2 (ja) * | 2016-09-16 | 2020-11-04 | 国立大学法人大阪大学 | 免疫実体クラスタリングソフトウェア |
| EP3625254B1 (en) | 2017-07-31 | 2023-12-13 | F. Hoffmann-La Roche AG | Three-dimensional structure-based humanization method |
| CN107480360B (zh) * | 2017-08-03 | 2020-04-24 | 中北大学 | 光束细分和相界面漫反射的激光烧熔的数值计算方法 |
| CN109837250B (zh) * | 2019-01-15 | 2021-10-12 | 中国农业科学院兰州兽医研究所 | 羔羊睾丸支持细胞永生化细胞系及其建立方法和应用 |
Family Cites Families (43)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB8308235D0 (en) * | 1983-03-25 | 1983-05-05 | Celltech Ltd | Polypeptides |
| US4816567A (en) * | 1983-04-08 | 1989-03-28 | Genentech, Inc. | Recombinant immunoglobin preparations |
| US5225539A (en) * | 1986-03-27 | 1993-07-06 | Medical Research Council | Recombinant altered antibodies and methods of making altered antibodies |
| US6548640B1 (en) * | 1986-03-27 | 2003-04-15 | Btg International Limited | Altered antibodies |
| US5567610A (en) * | 1986-09-04 | 1996-10-22 | Bioinvent International Ab | Method of producing human monoclonal antibodies and kit therefor |
| AU600575B2 (en) * | 1987-03-18 | 1990-08-16 | Sb2, Inc. | Altered antibodies |
| GB8823869D0 (en) * | 1988-10-12 | 1988-11-16 | Medical Res Council | Production of antibodies |
| US5175384A (en) * | 1988-12-05 | 1992-12-29 | Genpharm International | Transgenic mice depleted in mature t-cells and methods for making transgenic mice |
| US20040049014A1 (en) * | 1988-12-28 | 2004-03-11 | Protein Design Labs, Inc. | Humanized immunoglobulins |
| US5530101A (en) * | 1988-12-28 | 1996-06-25 | Protein Design Labs, Inc. | Humanized immunoglobulins |
| US5229275A (en) * | 1990-04-26 | 1993-07-20 | Akzo N.V. | In-vitro method for producing antigen-specific human monoclonal antibodies |
| EP0590058B1 (en) * | 1991-06-14 | 2003-11-26 | Genentech, Inc. | HUMANIZED Heregulin ANTIBODy |
| US5565332A (en) * | 1991-09-23 | 1996-10-15 | Medical Research Council | Production of chimeric antibodies - a combinatorial approach |
| US5932448A (en) * | 1991-11-29 | 1999-08-03 | Protein Design Labs., Inc. | Bispecific antibody heterodimers |
| US5777085A (en) * | 1991-12-20 | 1998-07-07 | Protein Design Labs, Inc. | Humanized antibodies reactive with GPIIB/IIIA |
| EP0626012B1 (en) * | 1992-02-11 | 2003-07-09 | Cell Genesys, Inc. | Homogenotization of gene-targeting events |
| US5714350A (en) * | 1992-03-09 | 1998-02-03 | Protein Design Labs, Inc. | Increasing antibody affinity by altering glycosylation in the immunoglobulin variable region |
| US6129914A (en) * | 1992-03-27 | 2000-10-10 | Protein Design Labs, Inc. | Bispecific antibody effective to treat B-cell lymphoma and cell line |
| US5573905A (en) * | 1992-03-30 | 1996-11-12 | The Scripps Research Institute | Encoded combinatorial chemical libraries |
| GB9223377D0 (en) * | 1992-11-04 | 1992-12-23 | Medarex Inc | Humanized antibodies to fc receptors for immunoglobulin on human mononuclear phagocytes |
| US6210671B1 (en) * | 1992-12-01 | 2001-04-03 | Protein Design Labs, Inc. | Humanized antibodies reactive with L-selectin |
| GB9325182D0 (en) * | 1993-12-08 | 1994-02-09 | T Cell Sciences Inc | Humanized antibodies or binding proteins thereof specific for t cell subpopulations exhibiting select beta chain variable regions |
| US5534615A (en) * | 1994-04-25 | 1996-07-09 | Genentech, Inc. | Cardiac hypertrophy factor and uses therefor |
| US5731168A (en) * | 1995-03-01 | 1998-03-24 | Genentech, Inc. | Method for making heteromultimeric polypeptides |
| US6096871A (en) * | 1995-04-14 | 2000-08-01 | Genentech, Inc. | Polypeptides altered to contain an epitope from the Fc region of an IgG molecule for increased half-life |
| US7060808B1 (en) * | 1995-06-07 | 2006-06-13 | Imclone Systems Incorporated | Humanized anti-EGF receptor monoclonal antibody |
| US5882644A (en) * | 1996-03-22 | 1999-03-16 | Protein Design Labs, Inc. | Monoclonal antibodies specific for the platelet derived growth factor β receptor and methods of use thereof |
| US5834597A (en) * | 1996-05-20 | 1998-11-10 | Protein Design Labs, Inc. | Mutated nonactivating IgG2 domains and anti CD3 antibodies incorporating the same |
| US6013256A (en) * | 1996-09-24 | 2000-01-11 | Protein Design Labs, Inc. | Method of preventing acute rejection following solid organ transplantation |
| JP4460155B2 (ja) * | 1997-12-05 | 2010-05-12 | ザ・スクリプス・リサーチ・インステイチユート | マウス抗体のヒト化 |
| WO1999045959A1 (en) * | 1998-03-13 | 1999-09-16 | Dana-Farber Cancer Institute, Inc. | Humanized antibody and uses thereof |
| ATE477273T1 (de) * | 1998-12-01 | 2010-08-15 | Facet Biotech Corp | Humanisierte antikoerper gegen gamma-interferon |
| US6858383B2 (en) * | 2000-12-22 | 2005-02-22 | Medlyte, Inc. | Compositions and methods for the treatment and prevention of cardiovascular diseases and disorders, and for identifying agents therapeutic therefor |
| WO2003074679A2 (en) * | 2002-03-01 | 2003-09-12 | Xencor | Antibody optimization |
| US7678386B2 (en) * | 2002-07-15 | 2010-03-16 | Board Of Regents The University Of Texas | Liposomes coated with selected antibodies that bind to aminophospholipids |
| US7794713B2 (en) * | 2004-04-07 | 2010-09-14 | Lpath, Inc. | Compositions and methods for the treatment and prevention of hyperproliferative diseases |
| CA2623517A1 (en) * | 2005-09-23 | 2007-04-05 | Walter Reed Army Institute Of Research (Wrair) | Antibodies with simultaneous subsite specificities to protein and lipid epitopes |
| US20080213274A1 (en) * | 2005-10-28 | 2008-09-04 | Sabbadini Roger A | Compositions and methods for the treatment and prevention of fibrotic, inflammatory, and neovascularization conditions of the eye |
| US8796429B2 (en) * | 2006-05-31 | 2014-08-05 | Lpath, Inc. | Bioactive lipid derivatives, and methods of making and using same |
| US9274129B2 (en) * | 2006-05-31 | 2016-03-01 | Lpath, Inc. | Methods and reagents for detecting bioactive lipids |
| US9217749B2 (en) * | 2006-05-31 | 2015-12-22 | Lpath, Inc. | Immune-derived moieties reactive against lysophosphatidic acid |
| US8444970B2 (en) * | 2006-10-27 | 2013-05-21 | Lpath, Inc. | Compositions and methods for treating ocular diseases and conditions |
| PT2087002E (pt) * | 2006-10-27 | 2014-11-26 | Lpath Inc | Composições e métodos para a ligação de esfingosina-1- fosfato |
-
2009
- 2009-12-04 KR KR1020117015390A patent/KR20110097923A/ko not_active Withdrawn
- 2009-12-04 JP JP2011539761A patent/JP2012511026A/ja active Pending
- 2009-12-04 WO PCT/US2009/066862 patent/WO2010065921A2/en not_active Ceased
- 2009-12-04 CA CA2745436A patent/CA2745436A1/en not_active Abandoned
- 2009-12-04 US US12/631,784 patent/US20110044990A1/en not_active Abandoned
- 2009-12-04 AU AU2009322185A patent/AU2009322185A1/en not_active Abandoned
- 2009-12-04 CN CN2009801562855A patent/CN102573905A/zh active Pending
- 2009-12-04 EP EP09831241A patent/EP2374001A4/en not_active Withdrawn
-
2011
- 2011-06-05 IL IL213358A patent/IL213358A0/en unknown
Also Published As
| Publication number | Publication date |
|---|---|
| WO2010065921A2 (en) | 2010-06-10 |
| JP2012511026A (ja) | 2012-05-17 |
| US20110044990A1 (en) | 2011-02-24 |
| WO2010065921A3 (en) | 2011-12-29 |
| EP2374001A4 (en) | 2013-03-13 |
| CA2745436A1 (en) | 2010-06-10 |
| EP2374001A2 (en) | 2011-10-12 |
| CN102573905A (zh) | 2012-07-11 |
| IL213358A0 (en) | 2011-07-31 |
| AU2009322185A1 (en) | 2011-07-21 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US8026342B2 (en) | Compositions and methods for binding sphingosine-1-phosphate | |
| US20100292443A1 (en) | Humanized platelet activating factor antibody design using anti-lipid antibody templates | |
| US8614103B2 (en) | Compositions and methods for treating sphingosine-1-phosphate (S1P) related ocular diseases and conditions | |
| KR20110097923A (ko) | 항-지질 항체 결정 구조를 이용한 항체 설계 | |
| TW201041594A (en) | Compositions and methods for increasing muscle growth | |
| KR101947758B1 (ko) | 포스포릴콜린에 대한 신규 항체 | |
| US20130261287A1 (en) | Antibody design using anti-lipid antibody crystal structures | |
| US20140186339A1 (en) | Compositions and methods for treating ocular diseases and conditions | |
| WO2011153416A2 (en) | Novel anti-s1p antibody variants of lt1009 | |
| AU2013273727A1 (en) | Compositions and methods for treating ocular diseases and conditions | |
| US20120034631A1 (en) | Anti-lysophospholipid antibody design using antibody structures | |
| AU2016204486A1 (en) | Composition and Methods for Treating Ocular Diseases and Conditions |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20110704 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| PC1203 | Withdrawal of no request for examination | ||
| WITN | Application deemed withdrawn, e.g. because no request for examination was filed or no examination fee was paid |