JP7082050B2 - 脊髄性筋萎縮症の処置において有用なアデノ-関連ウイルスベクター - Google Patents
脊髄性筋萎縮症の処置において有用なアデノ-関連ウイルスベクター Download PDFInfo
- Publication number
- JP7082050B2 JP7082050B2 JP2018531163A JP2018531163A JP7082050B2 JP 7082050 B2 JP7082050 B2 JP 7082050B2 JP 2018531163 A JP2018531163 A JP 2018531163A JP 2018531163 A JP2018531163 A JP 2018531163A JP 7082050 B2 JP7082050 B2 JP 7082050B2
- Authority
- JP
- Japan
- Prior art keywords
- aav
- aav vector
- sequence
- composition
- vector
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 208000002320 spinal muscular atrophy Diseases 0.000 title claims description 37
- 238000011282 treatment Methods 0.000 title claims description 23
- 239000013603 viral vector Substances 0.000 title description 27
- 108090000623 proteins and genes Proteins 0.000 claims description 47
- 239000000203 mixture Substances 0.000 claims description 42
- 210000000234 capsid Anatomy 0.000 claims description 41
- 230000014509 gene expression Effects 0.000 claims description 40
- 239000013598 vector Substances 0.000 claims description 35
- 241000282414 Homo sapiens Species 0.000 claims description 28
- 239000013607 AAV vector Substances 0.000 claims description 27
- 150000007523 nucleic acids Chemical group 0.000 claims description 26
- 102100021244 Integral membrane protein GPR180 Human genes 0.000 claims description 23
- 241000700605 Viruses Species 0.000 claims description 17
- 210000004027 cell Anatomy 0.000 claims description 17
- 102000004169 proteins and genes Human genes 0.000 claims description 17
- 108091028043 Nucleic acid sequence Proteins 0.000 claims description 16
- 230000001105 regulatory effect Effects 0.000 claims description 16
- 210000002569 neuron Anatomy 0.000 claims description 12
- FWMNVWWHGCHHJJ-SKKKGAJSSA-N 4-amino-1-[(2r)-6-amino-2-[[(2r)-2-[[(2r)-2-[[(2r)-2-amino-3-phenylpropanoyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoyl]amino]hexanoyl]piperidine-4-carboxylic acid Chemical compound C([C@H](C(=O)N[C@H](CC(C)C)C(=O)N[C@H](CCCCN)C(=O)N1CCC(N)(CC1)C(O)=O)NC(=O)[C@H](N)CC=1C=CC=CC=1)C1=CC=CC=C1 FWMNVWWHGCHHJJ-SKKKGAJSSA-N 0.000 claims description 6
- 241000702423 Adeno-associated virus - 2 Species 0.000 claims description 6
- 210000002161 motor neuron Anatomy 0.000 claims description 6
- 239000008194 pharmaceutical composition Substances 0.000 claims description 6
- 108091093126 WHP Posttrascriptional Response Element Proteins 0.000 claims description 3
- 239000003937 drug carrier Substances 0.000 claims description 3
- 241000124008 Mammalia Species 0.000 claims description 2
- 229940079593 drug Drugs 0.000 claims description 2
- 239000003814 drug Substances 0.000 claims description 2
- 230000001124 posttranscriptional effect Effects 0.000 claims description 2
- 238000002560 therapeutic procedure Methods 0.000 claims description 2
- 230000037396 body weight Effects 0.000 claims 4
- 125000003275 alpha amino acid group Chemical group 0.000 claims 1
- 239000003795 chemical substances by application Substances 0.000 claims 1
- 102100021947 Survival motor neuron protein Human genes 0.000 description 47
- 238000000034 method Methods 0.000 description 47
- 238000012384 transportation and delivery Methods 0.000 description 26
- 108091026890 Coding region Proteins 0.000 description 21
- 108020004414 DNA Proteins 0.000 description 16
- 230000003612 virological effect Effects 0.000 description 16
- 239000003623 enhancer Substances 0.000 description 15
- 235000018102 proteins Nutrition 0.000 description 15
- 241000702421 Dependoparvovirus Species 0.000 description 14
- 150000001413 amino acids Chemical group 0.000 description 14
- 238000004519 manufacturing process Methods 0.000 description 14
- 230000010076 replication Effects 0.000 description 14
- 238000001415 gene therapy Methods 0.000 description 13
- 238000001802 infusion Methods 0.000 description 12
- 210000003169 central nervous system Anatomy 0.000 description 11
- 239000013612 plasmid Substances 0.000 description 11
- 208000002267 Anti-neutrophil cytoplasmic antibody-associated vasculitis Diseases 0.000 description 9
- 241000699670 Mus sp. Species 0.000 description 9
- 230000002950 deficient Effects 0.000 description 9
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 9
- 108091032973 (ribonucleotides)n+m Proteins 0.000 description 8
- 101000617738 Homo sapiens Survival motor neuron protein Proteins 0.000 description 8
- 108700019146 Transgenes Proteins 0.000 description 8
- 239000012634 fragment Substances 0.000 description 8
- 239000005090 green fluorescent protein Substances 0.000 description 8
- 108020004707 nucleic acids Proteins 0.000 description 8
- 102000039446 nucleic acids Human genes 0.000 description 8
- 239000002245 particle Substances 0.000 description 8
- 241000701022 Cytomegalovirus Species 0.000 description 7
- 108091034117 Oligonucleotide Proteins 0.000 description 7
- 235000001014 amino acid Nutrition 0.000 description 7
- 229940024606 amino acid Drugs 0.000 description 7
- 230000015572 biosynthetic process Effects 0.000 description 7
- 239000002773 nucleotide Substances 0.000 description 7
- 125000003729 nucleotide group Chemical group 0.000 description 7
- 230000004083 survival effect Effects 0.000 description 7
- 241001465754 Metazoa Species 0.000 description 6
- 210000001175 cerebrospinal fluid Anatomy 0.000 description 6
- 239000002299 complementary DNA Substances 0.000 description 6
- 201000010099 disease Diseases 0.000 description 6
- 230000006870 function Effects 0.000 description 6
- 210000000278 spinal cord Anatomy 0.000 description 6
- 238000003786 synthesis reaction Methods 0.000 description 6
- 238000010361 transduction Methods 0.000 description 6
- 230000026683 transduction Effects 0.000 description 6
- 230000014616 translation Effects 0.000 description 6
- 241001164825 Adeno-associated virus - 8 Species 0.000 description 5
- 102000004190 Enzymes Human genes 0.000 description 5
- 108090000790 Enzymes Proteins 0.000 description 5
- 101000834253 Gallus gallus Actin, cytoplasmic 1 Proteins 0.000 description 5
- 239000000969 carrier Substances 0.000 description 5
- 230000000295 complement effect Effects 0.000 description 5
- 229940088598 enzyme Drugs 0.000 description 5
- 230000001404 mediated effect Effects 0.000 description 5
- 108020004999 messenger RNA Proteins 0.000 description 5
- 230000035772 mutation Effects 0.000 description 5
- 108090000765 processed proteins & peptides Proteins 0.000 description 5
- 238000013519 translation Methods 0.000 description 5
- 241000701161 unidentified adenovirus Species 0.000 description 5
- 241001634120 Adeno-associated virus - 5 Species 0.000 description 4
- 108020004705 Codon Proteins 0.000 description 4
- 102000053602 DNA Human genes 0.000 description 4
- 102000016911 Deoxyribonucleases Human genes 0.000 description 4
- 108010053770 Deoxyribonucleases Proteins 0.000 description 4
- 102000012288 Phosphopyruvate Hydratase Human genes 0.000 description 4
- 108010022181 Phosphopyruvate Hydratase Proteins 0.000 description 4
- 241000282898 Sus scrofa Species 0.000 description 4
- 101150013568 US16 gene Proteins 0.000 description 4
- 230000000694 effects Effects 0.000 description 4
- 210000003754 fetus Anatomy 0.000 description 4
- 208000015181 infectious disease Diseases 0.000 description 4
- 230000004048 modification Effects 0.000 description 4
- 238000012986 modification Methods 0.000 description 4
- 238000004806 packaging method and process Methods 0.000 description 4
- 239000000546 pharmaceutical excipient Substances 0.000 description 4
- 102000004196 processed proteins & peptides Human genes 0.000 description 4
- 238000000746 purification Methods 0.000 description 4
- 238000003753 real-time PCR Methods 0.000 description 4
- 230000001225 therapeutic effect Effects 0.000 description 4
- 238000012546 transfer Methods 0.000 description 4
- 210000004291 uterus Anatomy 0.000 description 4
- 241001655883 Adeno-associated virus - 1 Species 0.000 description 3
- 241001164823 Adeno-associated virus - 7 Species 0.000 description 3
- 108010014303 DNA-directed DNA polymerase Proteins 0.000 description 3
- 102000016928 DNA-directed DNA polymerase Human genes 0.000 description 3
- 241000713666 Lentivirus Species 0.000 description 3
- 241000699666 Mus <mouse, genus> Species 0.000 description 3
- 108700026244 Open Reading Frames Proteins 0.000 description 3
- 108010092799 RNA-directed DNA polymerase Proteins 0.000 description 3
- 208000004756 Respiratory Insufficiency Diseases 0.000 description 3
- JLCPHMBAVCMARE-UHFFFAOYSA-N [3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-hydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methyl [5-(6-aminopurin-9-yl)-2-(hydroxymethyl)oxolan-3-yl] hydrogen phosphate Polymers Cc1cn(C2CC(OP(O)(=O)OCC3OC(CC3OP(O)(=O)OCC3OC(CC3O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c3nc(N)[nH]c4=O)C(COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3CO)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cc(C)c(=O)[nH]c3=O)n3cc(C)c(=O)[nH]c3=O)n3ccc(N)nc3=O)n3cc(C)c(=O)[nH]c3=O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)O2)c(=O)[nH]c1=O JLCPHMBAVCMARE-UHFFFAOYSA-N 0.000 description 3
- 238000004458 analytical method Methods 0.000 description 3
- 230000008901 benefit Effects 0.000 description 3
- 208000035475 disorder Diseases 0.000 description 3
- 230000001815 facial effect Effects 0.000 description 3
- 230000002068 genetic effect Effects 0.000 description 3
- 239000003018 immunosuppressive agent Substances 0.000 description 3
- 230000006872 improvement Effects 0.000 description 3
- 238000002347 injection Methods 0.000 description 3
- 239000007924 injection Substances 0.000 description 3
- 238000007913 intrathecal administration Methods 0.000 description 3
- 238000001990 intravenous administration Methods 0.000 description 3
- 238000010172 mouse model Methods 0.000 description 3
- 238000002887 multiple sequence alignment Methods 0.000 description 3
- 238000005457 optimization Methods 0.000 description 3
- 229920001184 polypeptide Polymers 0.000 description 3
- 239000013608 rAAV vector Substances 0.000 description 3
- 230000009467 reduction Effects 0.000 description 3
- 230000000241 respiratory effect Effects 0.000 description 3
- 201000004193 respiratory failure Diseases 0.000 description 3
- 210000003594 spinal ganglia Anatomy 0.000 description 3
- 210000002330 subarachnoid space Anatomy 0.000 description 3
- 239000000126 substance Substances 0.000 description 3
- 208000024891 symptom Diseases 0.000 description 3
- 210000001519 tissue Anatomy 0.000 description 3
- 230000001052 transient effect Effects 0.000 description 3
- 241001529453 unidentified herpesvirus Species 0.000 description 3
- 210000002845 virion Anatomy 0.000 description 3
- 241000251468 Actinopterygii Species 0.000 description 2
- 241000202702 Adeno-associated virus - 3 Species 0.000 description 2
- 102100036475 Alanine aminotransferase 1 Human genes 0.000 description 2
- 108010082126 Alanine transaminase Proteins 0.000 description 2
- 108010003415 Aspartate Aminotransferases Proteins 0.000 description 2
- 102000004625 Aspartate Aminotransferases Human genes 0.000 description 2
- 108090000565 Capsid Proteins Proteins 0.000 description 2
- 241000282693 Cercopithecidae Species 0.000 description 2
- 102100023321 Ceruloplasmin Human genes 0.000 description 2
- 108010010803 Gelatin Proteins 0.000 description 2
- 102000008214 Glutamate decarboxylase Human genes 0.000 description 2
- 108091022930 Glutamate decarboxylase Proteins 0.000 description 2
- 102100030652 Glutamate receptor 1 Human genes 0.000 description 2
- 101710087628 Glutamate receptor 1 Proteins 0.000 description 2
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 2
- 241000282412 Homo Species 0.000 description 2
- XQFRJNBWHJMXHO-RRKCRQDMSA-N IDUR Chemical compound C1[C@H](O)[C@@H](CO)O[C@H]1N1C(=O)NC(=O)C(I)=C1 XQFRJNBWHJMXHO-RRKCRQDMSA-N 0.000 description 2
- 102100034343 Integrase Human genes 0.000 description 2
- 108091092195 Intron Proteins 0.000 description 2
- 108091061960 Naked DNA Proteins 0.000 description 2
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N Phenol Chemical compound OC1=CC=CC=C1 ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 description 2
- ZTHYODDOHIVTJV-UHFFFAOYSA-N Propyl gallate Chemical compound CCCOC(=O)C1=CC(O)=C(O)C(O)=C1 ZTHYODDOHIVTJV-UHFFFAOYSA-N 0.000 description 2
- 208000033526 Proximal spinal muscular atrophy type 3 Diseases 0.000 description 2
- 101150081851 SMN1 gene Proteins 0.000 description 2
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 2
- RAHZWNYVWXNFOC-UHFFFAOYSA-N Sulphur dioxide Chemical compound O=S=O RAHZWNYVWXNFOC-UHFFFAOYSA-N 0.000 description 2
- 102000001435 Synapsin Human genes 0.000 description 2
- 108050009621 Synapsin Proteins 0.000 description 2
- 239000002253 acid Substances 0.000 description 2
- 230000003321 amplification Effects 0.000 description 2
- 230000001580 bacterial effect Effects 0.000 description 2
- 230000009286 beneficial effect Effects 0.000 description 2
- 230000004071 biological effect Effects 0.000 description 2
- 108010006025 bovine growth hormone Proteins 0.000 description 2
- 210000004556 brain Anatomy 0.000 description 2
- 239000000872 buffer Substances 0.000 description 2
- OSASVXMJTNOKOY-UHFFFAOYSA-N chlorobutanol Chemical compound CC(C)(O)C(Cl)(Cl)Cl OSASVXMJTNOKOY-UHFFFAOYSA-N 0.000 description 2
- HVYWMOMLDIMFJA-DPAQBDIFSA-N cholesterol Chemical compound C1C=C2C[C@@H](O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H]([C@H](C)CCCC(C)C)[C@@]1(C)CC2 HVYWMOMLDIMFJA-DPAQBDIFSA-N 0.000 description 2
- 239000013599 cloning vector Substances 0.000 description 2
- 238000002648 combination therapy Methods 0.000 description 2
- 238000004590 computer program Methods 0.000 description 2
- 230000007812 deficiency Effects 0.000 description 2
- 238000012217 deletion Methods 0.000 description 2
- 230000037430 deletion Effects 0.000 description 2
- 238000005516 engineering process Methods 0.000 description 2
- CBOQJANXLMLOSS-UHFFFAOYSA-N ethyl vanillin Chemical compound CCOC1=CC(C=O)=CC=C1O CBOQJANXLMLOSS-UHFFFAOYSA-N 0.000 description 2
- 239000008273 gelatin Substances 0.000 description 2
- 229920000159 gelatin Polymers 0.000 description 2
- 235000019322 gelatine Nutrition 0.000 description 2
- 235000011852 gelatine desserts Nutrition 0.000 description 2
- 238000010353 genetic engineering Methods 0.000 description 2
- 229960003444 immunosuppressant agent Drugs 0.000 description 2
- 238000000338 in vitro Methods 0.000 description 2
- 239000002502 liposome Substances 0.000 description 2
- 239000003550 marker Substances 0.000 description 2
- 239000000463 material Substances 0.000 description 2
- 239000011159 matrix material Substances 0.000 description 2
- 238000003199 nucleic acid amplification method Methods 0.000 description 2
- 230000008488 polyadenylation Effects 0.000 description 2
- 239000003755 preservative agent Substances 0.000 description 2
- 238000012545 processing Methods 0.000 description 2
- 206010039722 scoliosis Diseases 0.000 description 2
- 238000002864 sequence alignment Methods 0.000 description 2
- 239000011780 sodium chloride Substances 0.000 description 2
- 239000003381 stabilizer Substances 0.000 description 2
- 238000010561 standard procedure Methods 0.000 description 2
- 150000003431 steroids Chemical class 0.000 description 2
- 238000001308 synthesis method Methods 0.000 description 2
- 238000012385 systemic delivery Methods 0.000 description 2
- 230000001988 toxicity Effects 0.000 description 2
- 231100000419 toxicity Toxicity 0.000 description 2
- 238000001890 transfection Methods 0.000 description 2
- 208000032527 type III spinal muscular atrophy Diseases 0.000 description 2
- 238000011144 upstream manufacturing Methods 0.000 description 2
- 210000003462 vein Anatomy 0.000 description 2
- 230000009385 viral infection Effects 0.000 description 2
- CHHHXKFHOYLYRE-UHFFFAOYSA-M 2,4-Hexadienoic acid, potassium salt (1:1), (2E,4E)- Chemical compound [K+].CC=CC=CC([O-])=O CHHHXKFHOYLYRE-UHFFFAOYSA-M 0.000 description 1
- KISWVXRQTGLFGD-UHFFFAOYSA-N 2-[[2-[[6-amino-2-[[2-[[2-[[5-amino-2-[[2-[[1-[2-[[6-amino-2-[(2,5-diamino-5-oxopentanoyl)amino]hexanoyl]amino]-5-(diaminomethylideneamino)pentanoyl]pyrrolidine-2-carbonyl]amino]-3-hydroxypropanoyl]amino]-5-oxopentanoyl]amino]-5-(diaminomethylideneamino)p Chemical compound C1CCN(C(=O)C(CCCN=C(N)N)NC(=O)C(CCCCN)NC(=O)C(N)CCC(N)=O)C1C(=O)NC(CO)C(=O)NC(CCC(N)=O)C(=O)NC(CCCN=C(N)N)C(=O)NC(CO)C(=O)NC(CCCCN)C(=O)NC(C(=O)NC(CC(C)C)C(O)=O)CC1=CC=C(O)C=C1 KISWVXRQTGLFGD-UHFFFAOYSA-N 0.000 description 1
- WXNZTHHGJRFXKQ-UHFFFAOYSA-N 4-chlorophenol Chemical compound OC1=CC=C(Cl)C=C1 WXNZTHHGJRFXKQ-UHFFFAOYSA-N 0.000 description 1
- 108010011619 6-Phytase Proteins 0.000 description 1
- 241000580270 Adeno-associated virus - 4 Species 0.000 description 1
- 241000972680 Adeno-associated virus - 6 Species 0.000 description 1
- 241000649045 Adeno-associated virus 10 Species 0.000 description 1
- 229920001817 Agar Polymers 0.000 description 1
- 102100027211 Albumin Human genes 0.000 description 1
- 108010088751 Albumins Proteins 0.000 description 1
- 108700028369 Alleles Proteins 0.000 description 1
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 1
- 108020000948 Antisense Oligonucleotides Proteins 0.000 description 1
- 102000006734 Beta-Globulins Human genes 0.000 description 1
- 108010087504 Beta-Globulins Proteins 0.000 description 1
- 241000283690 Bos taurus Species 0.000 description 1
- 101710116137 Calcium/calmodulin-dependent protein kinase II Proteins 0.000 description 1
- 241000700199 Cavia porcellus Species 0.000 description 1
- 241000588919 Citrobacter freundii Species 0.000 description 1
- 101150026402 DBP gene Proteins 0.000 description 1
- 230000006820 DNA synthesis Effects 0.000 description 1
- 101150110160 DRD1 gene Proteins 0.000 description 1
- 229920002307 Dextran Polymers 0.000 description 1
- 206010013033 Diplegia Diseases 0.000 description 1
- 101150118728 Dlx5 gene Proteins 0.000 description 1
- 101710107143 Dopamine receptor 1 Proteins 0.000 description 1
- 241000283073 Equus caballus Species 0.000 description 1
- 241000282326 Felis catus Species 0.000 description 1
- 108700028146 Genetic Enhancer Elements Proteins 0.000 description 1
- 241000282575 Gorilla Species 0.000 description 1
- 108010043121 Green Fluorescent Proteins Proteins 0.000 description 1
- 102000004144 Green Fluorescent Proteins Human genes 0.000 description 1
- 101150001754 Gusb gene Proteins 0.000 description 1
- 101150064935 HELI gene Proteins 0.000 description 1
- 241000238631 Hexapoda Species 0.000 description 1
- 108010000521 Human Growth Hormone Proteins 0.000 description 1
- 102000002265 Human Growth Hormone Human genes 0.000 description 1
- 239000000854 Human Growth Hormone Substances 0.000 description 1
- 241000700588 Human alphaherpesvirus 1 Species 0.000 description 1
- 241000701024 Human betaherpesvirus 5 Species 0.000 description 1
- 206010023201 Joint contracture Diseases 0.000 description 1
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 1
- 239000012097 Lipofectamine 2000 Substances 0.000 description 1
- 241000282567 Macaca fascicularis Species 0.000 description 1
- 108010063312 Metalloproteins Proteins 0.000 description 1
- 102000010750 Metalloproteins Human genes 0.000 description 1
- 101710081079 Minor spike protein H Proteins 0.000 description 1
- 208000010428 Muscle Weakness Diseases 0.000 description 1
- 206010028372 Muscular weakness Diseases 0.000 description 1
- 108091005461 Nucleic proteins Proteins 0.000 description 1
- 241000283973 Oryctolagus cuniculus Species 0.000 description 1
- 241000282577 Pan troglodytes Species 0.000 description 1
- 241001504519 Papio ursinus Species 0.000 description 1
- 235000019483 Peanut oil Nutrition 0.000 description 1
- 102000007079 Peptide Fragments Human genes 0.000 description 1
- 108010033276 Peptide Fragments Proteins 0.000 description 1
- 241000009328 Perro Species 0.000 description 1
- 241000364051 Pima Species 0.000 description 1
- 102000010780 Platelet-Derived Growth Factor Human genes 0.000 description 1
- 108010038512 Platelet-Derived Growth Factor Proteins 0.000 description 1
- 241000288906 Primates Species 0.000 description 1
- 102100024304 Protachykinin-1 Human genes 0.000 description 1
- 101710136297 Protein VP2 Proteins 0.000 description 1
- 101710118046 RNA-directed RNA polymerase Proteins 0.000 description 1
- 241000700159 Rattus Species 0.000 description 1
- 206010039491 Sarcoma Diseases 0.000 description 1
- 101150113275 Smn gene Proteins 0.000 description 1
- 208000003954 Spinal Muscular Atrophies of Childhood Diseases 0.000 description 1
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 1
- 229930006000 Sucrose Natural products 0.000 description 1
- 101710171779 Survival motor neuron protein Proteins 0.000 description 1
- 210000001744 T-lymphocyte Anatomy 0.000 description 1
- 101150108167 TAC1 gene Proteins 0.000 description 1
- 108010006785 Taq Polymerase Proteins 0.000 description 1
- 206010044565 Tremor Diseases 0.000 description 1
- 102000004243 Tubulin Human genes 0.000 description 1
- 108090000704 Tubulin Proteins 0.000 description 1
- 101150008036 UL29 gene Proteins 0.000 description 1
- 101150099617 UL5 gene Proteins 0.000 description 1
- 101150011902 UL52 gene Proteins 0.000 description 1
- 101150033561 UL8 gene Proteins 0.000 description 1
- 108020005202 Viral DNA Proteins 0.000 description 1
- 108020000999 Viral RNA Proteins 0.000 description 1
- 208000036142 Viral infection Diseases 0.000 description 1
- 230000002159 abnormal effect Effects 0.000 description 1
- 150000007513 acids Chemical class 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- 230000000996 additive effect Effects 0.000 description 1
- 239000008272 agar Substances 0.000 description 1
- 235000010419 agar Nutrition 0.000 description 1
- 229940100198 alkylating agent Drugs 0.000 description 1
- 239000002168 alkylating agent Substances 0.000 description 1
- VREFGVBLTWBCJP-UHFFFAOYSA-N alprazolam Chemical compound C12=CC(Cl)=CC=C2N2C(C)=NN=C2CN=C1C1=CC=CC=C1 VREFGVBLTWBCJP-UHFFFAOYSA-N 0.000 description 1
- 239000003957 anion exchange resin Substances 0.000 description 1
- 230000000340 anti-metabolite Effects 0.000 description 1
- 229940100197 antimetabolite Drugs 0.000 description 1
- 239000002256 antimetabolite Substances 0.000 description 1
- 239000000074 antisense oligonucleotide Substances 0.000 description 1
- 238000012230 antisense oligonucleotides Methods 0.000 description 1
- 238000003556 assay Methods 0.000 description 1
- 238000007846 asymmetric PCR Methods 0.000 description 1
- 238000009227 behaviour therapy Methods 0.000 description 1
- 230000027455 binding Effects 0.000 description 1
- 230000008499 blood brain barrier function Effects 0.000 description 1
- 210000001218 blood-brain barrier Anatomy 0.000 description 1
- 238000011095 buffer preparation Methods 0.000 description 1
- 239000007853 buffer solution Substances 0.000 description 1
- 239000001506 calcium phosphate Substances 0.000 description 1
- 229910000389 calcium phosphate Inorganic materials 0.000 description 1
- 235000011010 calcium phosphates Nutrition 0.000 description 1
- 125000002091 cationic group Chemical group 0.000 description 1
- 238000004113 cell culture Methods 0.000 description 1
- 239000013592 cell lysate Substances 0.000 description 1
- 210000002230 centromere Anatomy 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 238000006243 chemical reaction Methods 0.000 description 1
- 229960004926 chlorobutanol Drugs 0.000 description 1
- 235000012000 cholesterol Nutrition 0.000 description 1
- 210000000349 chromosome Anatomy 0.000 description 1
- 230000001684 chronic effect Effects 0.000 description 1
- 238000011278 co-treatment Methods 0.000 description 1
- 238000005056 compaction Methods 0.000 description 1
- 239000012141 concentrate Substances 0.000 description 1
- 238000010276 construction Methods 0.000 description 1
- 230000001086 cytosolic effect Effects 0.000 description 1
- 238000001514 detection method Methods 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 230000018109 developmental process Effects 0.000 description 1
- 239000003085 diluting agent Substances 0.000 description 1
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 1
- 238000009826 distribution Methods 0.000 description 1
- 231100000673 dose–response relationship Toxicity 0.000 description 1
- 230000004064 dysfunction Effects 0.000 description 1
- 238000004520 electroporation Methods 0.000 description 1
- BEFDCLMNVWHSGT-UHFFFAOYSA-N ethenylcyclopentane Chemical compound C=CC1CCCC1 BEFDCLMNVWHSGT-UHFFFAOYSA-N 0.000 description 1
- 229940073505 ethyl vanillin Drugs 0.000 description 1
- 230000001605 fetal effect Effects 0.000 description 1
- 230000004927 fusion Effects 0.000 description 1
- 150000004676 glycans Chemical class 0.000 description 1
- 235000011187 glycerol Nutrition 0.000 description 1
- 229960005150 glycerol Drugs 0.000 description 1
- 239000003102 growth factor Substances 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 239000005556 hormone Substances 0.000 description 1
- 229940088597 hormone Drugs 0.000 description 1
- 102000053565 human SMN1 Human genes 0.000 description 1
- 230000028993 immune response Effects 0.000 description 1
- 238000003125 immunofluorescent labeling Methods 0.000 description 1
- 229940125721 immunosuppressive agent Drugs 0.000 description 1
- 238000001727 in vivo Methods 0.000 description 1
- 238000010348 incorporation Methods 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 239000003112 inhibitor Substances 0.000 description 1
- 238000003780 insertion Methods 0.000 description 1
- 230000037431 insertion Effects 0.000 description 1
- 239000000543 intermediate Substances 0.000 description 1
- 201000006913 intermediate spinal muscular atrophy Diseases 0.000 description 1
- 238000001361 intraarterial administration Methods 0.000 description 1
- 230000003834 intracellular effect Effects 0.000 description 1
- 238000000185 intracerebroventricular administration Methods 0.000 description 1
- 238000007918 intramuscular administration Methods 0.000 description 1
- 238000010253 intravenous injection Methods 0.000 description 1
- 238000007914 intraventricular administration Methods 0.000 description 1
- 238000002955 isolation Methods 0.000 description 1
- 238000011901 isothermal amplification Methods 0.000 description 1
- 201000004815 juvenile spinal muscular atrophy Diseases 0.000 description 1
- 239000008101 lactose Substances 0.000 description 1
- 150000002632 lipids Chemical class 0.000 description 1
- 101710121537 mRNA (guanine-N(7))-methyltransferase Proteins 0.000 description 1
- 238000012423 maintenance Methods 0.000 description 1
- 230000034217 membrane fusion Effects 0.000 description 1
- 239000000693 micelle Substances 0.000 description 1
- 238000010369 molecular cloning Methods 0.000 description 1
- 238000009126 molecular therapy Methods 0.000 description 1
- 239000000178 monomer Substances 0.000 description 1
- 230000008111 motor development Effects 0.000 description 1
- 210000003205 muscle Anatomy 0.000 description 1
- 230000003387 muscular Effects 0.000 description 1
- 238000002703 mutagenesis Methods 0.000 description 1
- 231100000350 mutagenesis Toxicity 0.000 description 1
- 238000007857 nested PCR Methods 0.000 description 1
- 230000004770 neurodegeneration Effects 0.000 description 1
- 210000004498 neuroglial cell Anatomy 0.000 description 1
- 208000018360 neuromuscular disease Diseases 0.000 description 1
- 230000002981 neuropathic effect Effects 0.000 description 1
- 230000003472 neutralizing effect Effects 0.000 description 1
- 230000000422 nocturnal effect Effects 0.000 description 1
- 235000016709 nutrition Nutrition 0.000 description 1
- 230000035764 nutrition Effects 0.000 description 1
- 238000002515 oligonucleotide synthesis Methods 0.000 description 1
- 230000003204 osmotic effect Effects 0.000 description 1
- 238000007500 overflow downdraw method Methods 0.000 description 1
- 238000002638 palliative care Methods 0.000 description 1
- -1 parabens Chemical compound 0.000 description 1
- 229940090668 parachlorophenol Drugs 0.000 description 1
- 239000000312 peanut oil Substances 0.000 description 1
- 239000001814 pectin Substances 0.000 description 1
- 235000010987 pectin Nutrition 0.000 description 1
- 229920001277 pectin Polymers 0.000 description 1
- 239000008188 pellet Substances 0.000 description 1
- 210000001428 peripheral nervous system Anatomy 0.000 description 1
- 210000002856 peripheral neuron Anatomy 0.000 description 1
- 230000002085 persistent effect Effects 0.000 description 1
- 229960003742 phenol Drugs 0.000 description 1
- 239000002953 phosphate buffered saline Substances 0.000 description 1
- 229920000642 polymer Polymers 0.000 description 1
- 108091033319 polynucleotide Proteins 0.000 description 1
- 102000040430 polynucleotide Human genes 0.000 description 1
- 239000002157 polynucleotide Substances 0.000 description 1
- 235000010241 potassium sorbate Nutrition 0.000 description 1
- 239000004302 potassium sorbate Substances 0.000 description 1
- 229940069338 potassium sorbate Drugs 0.000 description 1
- 230000035935 pregnancy Effects 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 108010041634 preprotachykinin Proteins 0.000 description 1
- 230000002265 prevention Effects 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 230000000750 progressive effect Effects 0.000 description 1
- 239000000473 propyl gallate Substances 0.000 description 1
- 229940075579 propyl gallate Drugs 0.000 description 1
- 235000010388 propyl gallate Nutrition 0.000 description 1
- 210000001938 protoplast Anatomy 0.000 description 1
- 208000022074 proximal spinal muscular atrophy Diseases 0.000 description 1
- 238000011002 quantification Methods 0.000 description 1
- 230000011514 reflex Effects 0.000 description 1
- 230000004202 respiratory function Effects 0.000 description 1
- 230000029058 respiratory gaseous exchange Effects 0.000 description 1
- 230000004044 response Effects 0.000 description 1
- 108091008146 restriction endonucleases Proteins 0.000 description 1
- 238000012552 review Methods 0.000 description 1
- 150000003839 salts Chemical class 0.000 description 1
- 239000000523 sample Substances 0.000 description 1
- 230000028327 secretion Effects 0.000 description 1
- 239000008159 sesame oil Substances 0.000 description 1
- 235000011803 sesame oil Nutrition 0.000 description 1
- 235000010199 sorbic acid Nutrition 0.000 description 1
- 229940075582 sorbic acid Drugs 0.000 description 1
- 239000004334 sorbic acid Substances 0.000 description 1
- 241000894007 species Species 0.000 description 1
- 210000001324 spliceosome Anatomy 0.000 description 1
- 230000000087 stabilizing effect Effects 0.000 description 1
- 238000006467 substitution reaction Methods 0.000 description 1
- 239000005720 sucrose Substances 0.000 description 1
- 229940044609 sulfur dioxide Drugs 0.000 description 1
- 235000010269 sulphur dioxide Nutrition 0.000 description 1
- 238000001356 surgical procedure Methods 0.000 description 1
- 239000000725 suspension Substances 0.000 description 1
- 230000002194 synthesizing effect Effects 0.000 description 1
- 238000010189 synthetic method Methods 0.000 description 1
- 238000007910 systemic administration Methods 0.000 description 1
- 230000008685 targeting Effects 0.000 description 1
- 238000012360 testing method Methods 0.000 description 1
- 230000003582 thrombocytopenic effect Effects 0.000 description 1
- 231100000027 toxicology Toxicity 0.000 description 1
- 238000013518 transcription Methods 0.000 description 1
- 230000035897 transcription Effects 0.000 description 1
- 230000005026 transcription initiation Effects 0.000 description 1
- 238000003146 transient transfection Methods 0.000 description 1
- QORWJWZARLRLPR-UHFFFAOYSA-H tricalcium bis(phosphate) Chemical compound [Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O QORWJWZARLRLPR-UHFFFAOYSA-H 0.000 description 1
- 241000701447 unidentified baculovirus Species 0.000 description 1
- 241001430294 unidentified retrovirus Species 0.000 description 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 1
- 238000005303 weighing Methods 0.000 description 1
- 230000004584 weight gain Effects 0.000 description 1
- 235000019786 weight gain Nutrition 0.000 description 1
- 238000001262 western blot Methods 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/63—Introduction of foreign genetic material using vectors; Vectors; Use of hosts therefor; Regulation of expression
- C12N15/79—Vectors or expression systems specially adapted for eukaryotic hosts
- C12N15/85—Vectors or expression systems specially adapted for eukaryotic hosts for animal cells
- C12N15/86—Viral vectors
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/1703—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates
- A61K38/1709—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates from mammals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K48/00—Medicinal preparations containing genetic material which is inserted into cells of the living body to treat genetic diseases; Gene therapy
- A61K48/005—Medicinal preparations containing genetic material which is inserted into cells of the living body to treat genetic diseases; Gene therapy characterised by an aspect of the 'active' part of the composition delivered, i.e. the nucleic acid delivered
- A61K48/0066—Manipulation of the nucleic acid to modify its expression pattern, e.g. enhance its duration of expression, achieved by the presence of particular introns in the delivered nucleic acid
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0085—Brain, e.g. brain implants; Spinal cord
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P21/00—Drugs for disorders of the muscular or neuromuscular system
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/46—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates
- C07K14/47—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates from mammals
- C07K14/4701—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates from mammals not used
- C07K14/4702—Regulators; Modulating activity
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2750/00—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA ssDNA viruses
- C12N2750/00011—Details
- C12N2750/14011—Parvoviridae
- C12N2750/14111—Dependovirus, e.g. adenoassociated viruses
- C12N2750/14141—Use of virus, viral particle or viral elements as a vector
- C12N2750/14143—Use of virus, viral particle or viral elements as a vector viral genome or elements thereof as genetic vector
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2800/00—Nucleic acids vectors
- C12N2800/22—Vectors comprising a coding region that has been codon optimised for expression in a respective host
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2810/00—Vectors comprising a targeting moiety
- C12N2810/50—Vectors comprising as targeting moiety peptide derived from defined protein
- C12N2810/60—Vectors comprising as targeting moiety peptide derived from defined protein from viruses
- C12N2810/6027—Vectors comprising as targeting moiety peptide derived from defined protein from viruses ssDNA viruses
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Genetics & Genomics (AREA)
- General Health & Medical Sciences (AREA)
- Organic Chemistry (AREA)
- Medicinal Chemistry (AREA)
- Zoology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Molecular Biology (AREA)
- Animal Behavior & Ethology (AREA)
- Pharmacology & Pharmacy (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Biochemistry (AREA)
- Biotechnology (AREA)
- Epidemiology (AREA)
- Biomedical Technology (AREA)
- Biophysics (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Gastroenterology & Hepatology (AREA)
- Wood Science & Technology (AREA)
- General Engineering & Computer Science (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Neurology (AREA)
- Toxicology (AREA)
- Microbiology (AREA)
- Virology (AREA)
- Plant Pathology (AREA)
- Physics & Mathematics (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Marine Sciences & Fisheries (AREA)
- Immunology (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Physical Education & Sports Medicine (AREA)
- Psychology (AREA)
- Neurosurgery (AREA)
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
Description
出願人は、電子形態で添付して申請される配列表材料を、本明細書の一部を構成するものとして援用する。このファイルは“16-7655PCT_SEQ_Listing_ST25.txt”と標識される。
脊髄性筋萎縮症(SMA)は、スプライセオソーム(splicesome)生合成に含まれる普遍的に発現されるタンパク質(survival of motor neuron-SMN)をコードする遺伝子であるテロメアSMN1中の突然変異により引き起こされる神経筋疾患である。不明確な理由で、SMN欠乏は下位運動ニューロンへの選択的な毒性を生じ、進行性のニューロン消失及び筋力低下を生ずる。疾患の重篤度は、少量の全長SMN転写物の産生しか生じないスプライス部位の突然変異を有する相同遺伝子(SMN2)のセントロメア複製のコピー数により変化する。1-2個のSMN2のコピーを有する患者は、SMAの重症を呈し、それは生後数か月内の発症及び呼吸不全への急速な進行を特徴とする。3個のSMN2のコピーを有する患者は、一般に軽症の疾患を示し、典型的には6月齢後に発症する。多くは歩行能力を得ることはないが、めったに呼吸不全に進行せず、多くの場合に成人まで生きる。4個のSMN2のコピーを有する患者は成人まで発症せず、筋力低下が徐々に発症する。緩和ケア以外にSMAのための現在の処置はない。
非特許文献4及び非特許文献5)。AAV9の全身的送達は血液脳関門を通過し、CNSへのGFPの広範囲の遺伝子導入を達成することも示された。(非特許文献6;非特許文献7)。
和されない。ある態様において、核酸配列は配列番号1又はそれと少なくとも95%の同一性を共有する配列をコードする。
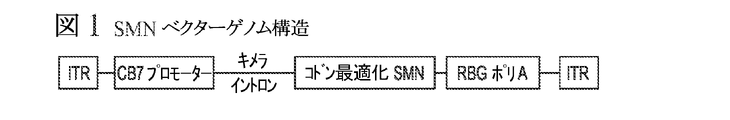
提供され、それは天然のhSMN1配列と比較して翻訳を最大にするように設計された(図5及び配列番号3に示される通り)。5’キャッピング及びmRNAの安定性を向上させるためにイントロンをコード配列の上流に導入した(図5及び配列番号4を参照されたい)。
とも約36個以上のヌクレオチドのより短いフラグメント間の同一性が望ましい場合もある。
Manual,Cold Spring Harbor Press,Cold Spring Harbor,NYを参照されたい。
及び血小板-由来成長因子ベータ鎖プロモーターが含まれるがこれらに限られない。引用によりその記載事項が本明細書に取り込まれるHiokiら、Gene Therapy,June 2007,14(11):872-82を参照されたい。他のニューロン特異的なプロモーターには67kDaグルタミン酸デカルボキシラーゼ(GAD67)、ホメオボックスDlx5/6、グルタメートレセプター1(GluR1)、プレプロタキキニン1(Tac1)プロモーター、ニューロン-特異的エノラーゼ(NSE)及びドーパミンレセプター1(Drd1a)プロモーターが含まれる。例えばDelzorら、Human Gene Therapy Method.August 2012,23(4):242-254を参照されたい。別の態様において、プロモーターはGUSbプロモーター http://www.jci.org/articles/view/41615#B30である。
いられる場合、「作動可能に連結」という用語は、問題の遺伝子と連続している発現調節配列と、トランスで(in trans)又は問題の遺伝子を調節する距離で働く発現調節配列との両方を指す。
で提示された及び/又は当該技術分野において既知のAAVキャプシドに約90%の同一性~約99.9%の同一性、約95%~約99%の同一性又は約97%~約98%の同一性を共有する。1つの態様において、AAVキャプシドはAAVキャプシドと少なくとも95%の同一性を共有する。AAVキャプシドのパーセント同一性を決定する場合、様々な(variable)タンパク質(例えばvp1、vp2又はvp3)のいずれかについて比較を行う場合もある。1つの態様において、AAVキャプシドはAAV8 vp3と少なくとも95%同一性を共有する。別の態様において、自己-相補性AAVが用いられる。
ロブタノール、ソルビン酸カリウム、ソルビン酸、二酸化硫黄、没食子酸プロピル、パラベン、エチルバニリン、グリセリン、フェノール及びパラクロロフェノールが含まれる。適切な化学的安定剤にはゼラチン及びアルブミンが含まれる。
10も参照されたい。オリゴヌクレオチド合成及び遺伝子合成についての以下の特許も参照されたい。Gene Seq.2012 Apr;6(1):10-21;米国特許第8008005号明細書;及び米国特許第7985565号明細書。それらの文書のそれぞれは引用によりその記載事項が本明細書に取り込まれる。さらに、PCRを介してDNAを作製するためのキット及びプロトコルは商業的に入手可能である。これらはTaqポリメラーゼ;OneTaq(登録商標)(New England Biolabs);Q5(登録商標)High-Fidelity DNAポリメラーゼ(New England Biolabs);及びGoTaq(登録商標)G2ポリメラーゼ(Promega)を含むがこれらに限られないポリメラーゼの使用を含む。本明細書に記載されるhSMN配列を含有するプラスミドを用いてトランスフェクションされた細胞からDNAを作製する場合もある。キット及びプロトコルは既知でありかつ商業的に入手可能であり、QIAGENプラスミドキット;Chargeswitch(登録商標)Pro Filter Plasmid Kits(Invitrogen);及びGenElute(商標)Plamid Kits(Sigma Aldrich)が含まれるがこれらに限られない。本明細書において有用な他の方法には、熱サイクルの必要性を取り除いた配列-特異的等温増幅法が含まれる。これらの方法は、二重鎖DNAを分離するために、熱の代わりに典型的にBst DNA Polymerase、Large Fragme
nt(New England Biolabs)のような鎖置換DNAポリメラーゼを用いる。RNA依存性DNAポリメラーゼである逆転写酵素(RT)の使用を介する増幅を介してRNA分子からDNAを作成する場合もある。RTsは、最初のRNAテンプレートに相補的でありcDNAと呼ばれるDNAの鎖を重合させる。このcDNAを次いでPCR又は上記で概述した等温法を介してさらに増幅することができる。GenScript;GENEWIZ(登録商標);GeneArt(登録商標)(life Technologies);及びIntegrated DNA Technologiesを含むがこれらに限られない会社から商業的にカスタムDNA(custom DNA)を作成することもできる。
Feb 2を参照されたい。
栄養が懸念である場合、胃瘻管の造設が適している。呼吸機能が悪化する時、気管切開又は非侵襲性呼吸支持器(respiratory support)が提供される。睡眠時呼吸障害は持続気道陽圧の夜間使用を用いて処置され得る。SMA II及びSMA IIIの患者における脊柱側弯症のための外科手術は、努力肺活量が30%-40%より高い場合、安全に行われ得る。電動車いす(power chair)及び他の装置は生活の質の向上を可能にする。引用によりその記載事項が本明細書に取り込まれる米国特許第8211631号明細書も参照されたい。
SMNΔ7マウスモデルを用い、我々はSMAの処置のためのAAV媒介遺伝子療法を評価した。遍在性CBプロモーターの調節下にコドン最適化ヒトSMN1 cDNAを保有する向神経性AAVrh.10ベクターを構築した(図1)。SMNΔ7新生仔に顔面静脈を介してベクターの5x1010個のゲノムコピー(kg当たり5x1013個のゲノムコピー)を注入した。処置はこの用量において後根神経節のような末梢ニューロンにおける健常な発現(図2)ならびに脊髄内における形質導入を生じた。生存期間のいくらかの向上(未処置マウスにおける14日に対して21日)も観察された。
SMNΔ7新生仔に1尾当たり5x1012個のゲノムコピーのベクターを静脈内注入を介して注入した。仔マウスのメジアン生存期間は10日であった。アスパラギン酸アミノトランスフェラーゼ(AST)及びアラニンアミノトランスフェラーゼ(ALT)レベル
は上昇した。図6。
1回の注入を介して脳脊髄液(CSF)に直接送達されるAAVrh.10.SMNの投薬及び薬効を評価する。
Claims (21)
- 配列番号5のアミノ酸配列又はそれに少なくとも99%同一な配列を含むAAVrh10キャプシドと、機能がある生存運動ニューロン(SMN)タンパク質をコードする配列番号2により定義される核酸配列及び宿主細胞におけるSMN配列の発現を方向付ける発現調節配列を含むベクターゲノムとを含む、組換えアデノ関連ウイルス(AAV)ベクター。
- 前記発現調節配列はプロモーターを含む、請求項1に記載のAAVベクター。
- 前記プロモーターはCBプロモーターである、請求項2に記載のAAVベクター。
- 前記プロモーターはCB7プロモーターである、請求項3に記載のAAVベクター。
- 前記発現調節配列は組織特異的プロモーターを含む、請求項2に記載のAAVベクター。
- 組織特異的プロモーターはニューロン-特異的プロモーターである、請求項5に記載のAAVベクター。
- イントロン、コザック配列、ポリA、WPRE及び転写後調節エレメントの1つ以上をさらに含む、請求項1~6のいずれかに記載のAAVベクター。
- AAV逆位末端反復(ITRs)配列をさらに含む、請求項1~7のいずれかに記載のAAVベクター。
- 前記ITRsは、キャプシドを供給するAAVと異なるAAVに由来する、請求項8に記載のAAVベクター。
- 前記ITRsはAAV2に由来する、請求項8に記載のAAVベクター。
- 製薬学的に許容され得る担体及び請求項1~10のいずれか1つに記載のAAVベクターを含む、医薬組成物。
- ヒト患者の脊髄性筋萎縮症の処置に使用するための、請求項1~10のいずれか1つに記載のAAVベクター又は請求項11に記載の組成物。
- 前記AAVベクター又は組成物は別の療法と併用して投与可能である、請求項12に記載の使用のための、AAVベクター又は組成物。
- 前記AAVベクター又は組成物は体重kg当たり約1x1010個のゲノムコピー(GC)ないし体重kg当たり約1x1014個のGCの用量で投与可能である、請求項13に記載の使用のための、AAVベクター又は組成物。
- 前記AAVベクター又は組成物は体重kg当たり約5x1013個のGCの用量で投与可能である、請求項14に記載の使用のための、AAVベクター又は組成物。
- 前記AAVベクター又は組成物は体重kg当たり約2.5x1012個のGCの用量で投与可能である、請求項14に記載の使用のための、AAVベクター又は組成物。
- 前記AAVベクター又は組成物は1回以上投与可能である、請求項12~16のいずれかに記載の使用のための、AAVベクター又は組成物。
- 患者における脊髄性筋萎縮症を処置するための薬剤を製造するための、請求項1~10のいずれか1に記載のAAVベクター又は請求項11に記載の組成物の使用。
- 前記薬剤がクモ膜下腔内投与のために調整される、請求項18に記載の使用。
- 前記患者が哺乳類である、請求項18又は19に記載の使用。
- 前記患者がヒトである、請求項20に記載の使用。
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201562267012P | 2015-12-14 | 2015-12-14 | |
| US62/267,012 | 2015-12-14 | ||
| PCT/US2016/066669 WO2017106354A1 (en) | 2015-12-14 | 2016-12-14 | Adeno-associated viral vectors useful in treatment of spinal muscular atropy |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2018537984A JP2018537984A (ja) | 2018-12-27 |
| JP2018537984A5 JP2018537984A5 (ja) | 2020-01-23 |
| JP7082050B2 true JP7082050B2 (ja) | 2022-06-07 |
Family
ID=57737990
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2018531163A Active JP7082050B2 (ja) | 2015-12-14 | 2016-12-14 | 脊髄性筋萎縮症の処置において有用なアデノ-関連ウイルスベクター |
Country Status (13)
| Country | Link |
|---|---|
| US (2) | US20180353624A1 (ja) |
| EP (1) | EP3394270A1 (ja) |
| JP (1) | JP7082050B2 (ja) |
| KR (1) | KR20180086266A (ja) |
| CN (1) | CN109072254A (ja) |
| AU (1) | AU2016370630B2 (ja) |
| BR (1) | BR112018011975A2 (ja) |
| CA (1) | CA3008280A1 (ja) |
| IL (1) | IL259877A (ja) |
| MA (1) | MA44119A (ja) |
| MX (1) | MX2018007234A (ja) |
| WO (1) | WO2017106354A1 (ja) |
| ZA (1) | ZA201803956B (ja) |
Families Citing this family (18)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| AU2017301819B2 (en) * | 2016-07-26 | 2024-09-26 | Cornell University | Gene therapy for the treatment of aldehyde dehydrogenase deficiency |
| JOP20190200A1 (ar) | 2017-02-28 | 2019-08-27 | Univ Pennsylvania | تركيبات نافعة في معالجة ضمور العضل النخاعي |
| LT3589730T (lt) | 2017-02-28 | 2024-03-12 | The Trustees Of The University Of Pennsylvania | Adenoasocijuoto viruso (aav) monofiletinės grupės f vektorius, ir jo panaudojimo būdai |
| JP2020533968A (ja) * | 2017-08-25 | 2020-11-26 | オービッド・セラピューティクス・インコーポレイテッドOvid Therapeutics Inc. | 組換えアデノ随伴ベクター |
| EP3837374A4 (en) * | 2018-08-15 | 2022-06-08 | Biogen MA Inc. | COMBINATION THERAPY FOR SPINAL MUSCLE ATROPHY |
| US20220042045A1 (en) * | 2018-12-21 | 2022-02-10 | Genethon | Expression cassettes for gene therapy vectors |
| US20220280548A1 (en) * | 2019-08-15 | 2022-09-08 | Biogen Ma Inc. | Combination therapy for spinal muscular atrophy |
| CN112011571A (zh) * | 2020-04-26 | 2020-12-01 | 辉大(上海)生物科技有限公司 | 一种用于治疗脊髓性肌萎缩的基因治疗药物 |
| EP4142800A1 (en) * | 2020-04-28 | 2023-03-08 | Genethon | Use of a synthetic aav capsid for gene therapy of muscle and central nervous system disorders |
| CA3177407A1 (en) | 2020-05-12 | 2021-11-18 | James M. Wilson | Compositions for drg-specific reduction of transgene expression |
| RU2742837C1 (ru) * | 2020-06-02 | 2021-02-11 | Общество С Ограниченной Ответственностью "Анабион" | Кодон-оптимизированная нуклеиновая кислота, которая кодирует белок SMN1, и ее применение |
| CN113755524B (zh) * | 2020-06-02 | 2023-11-03 | 舒泰神(北京)生物制药股份有限公司 | 用于治疗脊髓性肌萎缩的腺相关病毒载体及其用途 |
| AU2022214429A1 (en) | 2021-02-01 | 2023-09-14 | Regenxbio Inc. | Gene therapy for neuronal ceroid lipofuscinoses |
| CN112852882A (zh) * | 2021-02-04 | 2021-05-28 | 中吉智药(南京)生物技术有限公司 | 一种杆状病毒感染昆虫细胞生产aav基因药物的系统及方法 |
| WO2023086822A2 (en) * | 2021-11-09 | 2023-05-19 | Asimov Inc. | Stable production systems for aav vector production |
| WO2023087019A2 (en) | 2021-11-15 | 2023-05-19 | The Trustees Of The University Of Pennsylvania | Compositions for drg-specific reduction of transgene expression |
| WO2023219394A1 (ko) * | 2022-05-10 | 2023-11-16 | 서울대학교산학협력단 | 인간 smn1 단백질 변이체 및 이의 용도 |
| WO2024165839A1 (en) * | 2023-02-06 | 2024-08-15 | Royal Holloway And Bedford New College | Transgenes |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20150110857A1 (en) | 2013-10-22 | 2015-04-23 | Shire Human Genetic Therapies, Inc. | Cns delivery of mrna and uses thereof |
| JP2015521612A (ja) | 2012-06-21 | 2015-07-30 | アソシアシオン・アンスティテュ・ドゥ・ミオロジーAssociation Institut De Myologie | 遺伝子治療ベクターの広範な遺伝子送達 |
| WO2015121501A1 (en) | 2014-02-17 | 2015-08-20 | King's College London | Adeno-associated virus vector |
Family Cites Families (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| ES2648241T3 (es) * | 2003-09-30 | 2017-12-29 | The Trustees Of The University Of Pennsylvania | Clados de virus adenoasociados (AAV), secuencias, vectores que contienen el mismo, y usos de los mismos |
| WO2014178863A1 (en) * | 2013-05-01 | 2014-11-06 | Genzyme Corporation | Compositions and methods for treating spinal muscular atrophy |
-
2016
- 2016-12-14 KR KR1020187019828A patent/KR20180086266A/ko unknown
- 2016-12-14 CN CN201680081819.2A patent/CN109072254A/zh active Pending
- 2016-12-14 MX MX2018007234A patent/MX2018007234A/es unknown
- 2016-12-14 US US16/061,109 patent/US20180353624A1/en not_active Abandoned
- 2016-12-14 JP JP2018531163A patent/JP7082050B2/ja active Active
- 2016-12-14 AU AU2016370630A patent/AU2016370630B2/en not_active Expired - Fee Related
- 2016-12-14 CA CA3008280A patent/CA3008280A1/en active Pending
- 2016-12-14 EP EP16822581.1A patent/EP3394270A1/en not_active Withdrawn
- 2016-12-14 MA MA044119A patent/MA44119A/fr unknown
- 2016-12-14 WO PCT/US2016/066669 patent/WO2017106354A1/en active Application Filing
- 2016-12-14 BR BR112018011975A patent/BR112018011975A2/pt not_active Application Discontinuation
-
2018
- 2018-06-07 IL IL259877A patent/IL259877A/en unknown
- 2018-06-13 ZA ZA2018/03956A patent/ZA201803956B/en unknown
-
2021
- 2021-09-30 US US17/490,611 patent/US20220265861A1/en not_active Abandoned
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2015521612A (ja) | 2012-06-21 | 2015-07-30 | アソシアシオン・アンスティテュ・ドゥ・ミオロジーAssociation Institut De Myologie | 遺伝子治療ベクターの広範な遺伝子送達 |
| US20150110857A1 (en) | 2013-10-22 | 2015-04-23 | Shire Human Genetic Therapies, Inc. | Cns delivery of mrna and uses thereof |
| WO2015121501A1 (en) | 2014-02-17 | 2015-08-20 | King's College London | Adeno-associated virus vector |
Non-Patent Citations (1)
| Title |
|---|
| HU, C. et al.,RH10 provides superior transgene expression in mice when compared with natural AAV serotypes for neonatal gene therapy,Journal of Gene Medicine,2010年,Vol.12, No.9,P.766-778 |
Also Published As
| Publication number | Publication date |
|---|---|
| US20180353624A1 (en) | 2018-12-13 |
| WO2017106354A1 (en) | 2017-06-22 |
| US20220265861A1 (en) | 2022-08-25 |
| BR112018011975A2 (pt) | 2018-12-11 |
| JP2018537984A (ja) | 2018-12-27 |
| MX2018007234A (es) | 2018-11-09 |
| IL259877A (en) | 2018-07-31 |
| EP3394270A1 (en) | 2018-10-31 |
| KR20180086266A (ko) | 2018-07-30 |
| AU2016370630A1 (en) | 2018-06-28 |
| CN109072254A (zh) | 2018-12-21 |
| CA3008280A1 (en) | 2017-06-22 |
| ZA201803956B (en) | 2019-04-24 |
| MA44119A (fr) | 2018-10-31 |
| AU2016370630B2 (en) | 2023-04-13 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP7082050B2 (ja) | 脊髄性筋萎縮症の処置において有用なアデノ-関連ウイルスベクター | |
| JP7037574B2 (ja) | 脊髄性筋萎縮症の治療に有用な組成物 | |
| BR112019013576A2 (pt) | terapia genica para o tratamento da fenilcetonuria | |
| JP7061067B2 (ja) | クリグラー・ナジャー症候群の処置のための組成物 | |
| JP2024111074A (ja) | 脊髄性筋萎縮症のための併用療法 | |
| AU2017362491B2 (en) | Intrathecal delivery of recombinant Adeno-associated virus encoding Methyl-CpG binding protein 2 | |
| CA3185281A1 (en) | Compositions useful for treatment of charcot-marie-tooth disease | |
| JP2022519596A (ja) | Cln3ポリヌクレオチドのアデノ随伴ウイルス送達 | |
| US20230167455A1 (en) | Compositions useful in treatment of cdkl5 deficiency disorder (cdd) | |
| US20220226502A1 (en) | Adeno-associated virus vector delivery of cystathionine beta-synthase (cbs) enzyme for treating cbs deficiency | |
| JP2020518269A (ja) | 繊毛病のための遺伝子治療 | |
| WO2020257731A1 (en) | Compositions and methods for treatment of maple syrup urine disease | |
| CN113574176A (zh) | 腺相关病毒对cln6多核苷酸的递送 | |
| TW202045728A (zh) | 用於治療克拉培氏病之組成物 | |
| CN112011571A (zh) | 一种用于治疗脊髓性肌萎缩的基因治疗药物 | |
| JP2024515623A (ja) | 髄腔内送達によってピット・ホプキンス症候群を治療するためのメチル-cpg結合タンパク質2をコードする組換えアデノ随伴ウイルス | |
| WO2024197073A2 (en) | Aav-mediated gene therapy | |
| TW202246513A (zh) | Cln3多核苷酸的腺相關病毒遞送 | |
| TW202208622A (zh) | 用於治療克拉培氏病之組成物 | |
| EP4405469A1 (en) | Compositions useful for treatment of charcot-marie-tooth disease | |
| CN116670159A (zh) | 组合物及其用于治疗安格尔曼综合征的用途 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20191209 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20191209 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20201105 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20201223 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20210311 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20210521 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20210917 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20211214 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20220209 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20220316 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20220427 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20220526 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 7082050 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |