JP6831455B2 - 操向可能な医療機器及びその作製方法 - Google Patents
操向可能な医療機器及びその作製方法 Download PDFInfo
- Publication number
- JP6831455B2 JP6831455B2 JP2019520807A JP2019520807A JP6831455B2 JP 6831455 B2 JP6831455 B2 JP 6831455B2 JP 2019520807 A JP2019520807 A JP 2019520807A JP 2019520807 A JP2019520807 A JP 2019520807A JP 6831455 B2 JP6831455 B2 JP 6831455B2
- Authority
- JP
- Japan
- Prior art keywords
- medical device
- core
- polymer electrolyte
- electrolyte member
- electrodes
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 238000004519 manufacturing process Methods 0.000 title description 3
- 239000005518 polymer electrolyte Substances 0.000 claims description 102
- 229920001746 electroactive polymer Polymers 0.000 claims description 74
- 150000002500 ions Chemical class 0.000 claims description 54
- 229920000642 polymer Polymers 0.000 claims description 54
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 claims description 20
- 239000002131 composite material Substances 0.000 claims description 16
- 239000003575 carbonaceous material Substances 0.000 claims description 13
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 claims description 12
- 238000000576 coating method Methods 0.000 claims description 11
- 229910021393 carbon nanotube Inorganic materials 0.000 claims description 10
- 239000002041 carbon nanotube Substances 0.000 claims description 10
- 229910052737 gold Inorganic materials 0.000 claims description 10
- 239000010931 gold Substances 0.000 claims description 10
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 claims description 10
- 239000011248 coating agent Substances 0.000 claims description 9
- 229920002313 fluoropolymer Polymers 0.000 claims description 8
- 239000004811 fluoropolymer Substances 0.000 claims description 8
- 239000003792 electrolyte Substances 0.000 claims description 7
- 229920002981 polyvinylidene fluoride Polymers 0.000 claims description 7
- 239000002033 PVDF binder Substances 0.000 claims description 6
- 229910021401 carbide-derived carbon Inorganic materials 0.000 claims description 6
- 229920001940 conductive polymer Polymers 0.000 claims description 6
- 239000007769 metal material Substances 0.000 claims description 6
- 229910052697 platinum Inorganic materials 0.000 claims description 5
- 229920001609 Poly(3,4-ethylenedioxythiophene) Polymers 0.000 claims description 4
- 229920001577 copolymer Polymers 0.000 claims description 4
- 229910021389 graphene Inorganic materials 0.000 claims description 4
- 229920000767 polyaniline Polymers 0.000 claims description 4
- 229920000069 polyphenylene sulfide Polymers 0.000 claims description 3
- 229920000128 polypyrrole Polymers 0.000 claims description 2
- 230000004044 response Effects 0.000 claims description 2
- 238000009941 weaving Methods 0.000 claims description 2
- 230000001568 sexual effect Effects 0.000 claims 1
- 238000005452 bending Methods 0.000 description 18
- 238000000034 method Methods 0.000 description 18
- 239000000463 material Substances 0.000 description 14
- 229920001343 polytetrafluoroethylene Polymers 0.000 description 10
- 239000004810 polytetrafluoroethylene Substances 0.000 description 10
- 230000007704 transition Effects 0.000 description 10
- 229920005569 poly(vinylidene fluoride-co-hexafluoropropylene) Polymers 0.000 description 8
- 230000033001 locomotion Effects 0.000 description 7
- ZPTRYWVRCNOTAS-UHFFFAOYSA-M 1-ethyl-3-methylimidazol-3-ium;trifluoromethanesulfonate Chemical compound CC[N+]=1C=CN(C)C=1.[O-]S(=O)(=O)C(F)(F)F ZPTRYWVRCNOTAS-UHFFFAOYSA-M 0.000 description 6
- 229910052799 carbon Inorganic materials 0.000 description 6
- 239000002608 ionic liquid Substances 0.000 description 6
- 229910052751 metal Inorganic materials 0.000 description 6
- 239000002184 metal Substances 0.000 description 6
- 239000007787 solid Substances 0.000 description 6
- -1 1-ethyl-3-methylimidazolium tetrafluoroborate Chemical compound 0.000 description 5
- 150000001768 cations Chemical class 0.000 description 5
- 239000006185 dispersion Substances 0.000 description 5
- 239000012528 membrane Substances 0.000 description 5
- 229910001000 nickel titanium Inorganic materials 0.000 description 5
- 238000001356 surgical procedure Methods 0.000 description 5
- 238000003466 welding Methods 0.000 description 5
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 description 4
- 241001465754 Metazoa Species 0.000 description 4
- FXHOOIRPVKKKFG-UHFFFAOYSA-N N,N-Dimethylacetamide Chemical compound CN(C)C(C)=O FXHOOIRPVKKKFG-UHFFFAOYSA-N 0.000 description 4
- 230000008878 coupling Effects 0.000 description 4
- 238000010168 coupling process Methods 0.000 description 4
- 238000005859 coupling reaction Methods 0.000 description 4
- HLXZNVUGXRDIFK-UHFFFAOYSA-N nickel titanium Chemical compound [Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni] HLXZNVUGXRDIFK-UHFFFAOYSA-N 0.000 description 4
- 239000002904 solvent Substances 0.000 description 4
- 229910001020 Au alloy Inorganic materials 0.000 description 3
- 239000004952 Polyamide Substances 0.000 description 3
- 229920002614 Polyether block amide Polymers 0.000 description 3
- 239000004642 Polyimide Substances 0.000 description 3
- LRESCJAINPKJTO-UHFFFAOYSA-N bis(trifluoromethylsulfonyl)azanide;1-ethyl-3-methylimidazol-3-ium Chemical compound CCN1C=C[N+](C)=C1.FC(F)(F)S(=O)(=O)[N-]S(=O)(=O)C(F)(F)F LRESCJAINPKJTO-UHFFFAOYSA-N 0.000 description 3
- 230000005684 electric field Effects 0.000 description 3
- 239000003353 gold alloy Substances 0.000 description 3
- 238000000227 grinding Methods 0.000 description 3
- 229910001092 metal group alloy Inorganic materials 0.000 description 3
- 229920002647 polyamide Polymers 0.000 description 3
- 229920001721 polyimide Polymers 0.000 description 3
- 238000005476 soldering Methods 0.000 description 3
- 238000005507 spraying Methods 0.000 description 3
- 229910001220 stainless steel Inorganic materials 0.000 description 3
- 239000000126 substance Substances 0.000 description 3
- 239000000758 substrate Substances 0.000 description 3
- BQCIDUSAKPWEOX-UHFFFAOYSA-N 1,1-Difluoroethene Chemical compound FC(F)=C BQCIDUSAKPWEOX-UHFFFAOYSA-N 0.000 description 2
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 2
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 2
- 239000004593 Epoxy Substances 0.000 description 2
- 239000004812 Fluorinated ethylene propylene Substances 0.000 description 2
- PXHVJJICTQNCMI-UHFFFAOYSA-N Nickel Chemical compound [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 description 2
- 239000004677 Nylon Substances 0.000 description 2
- 239000004696 Poly ether ether ketone Substances 0.000 description 2
- 239000004697 Polyetherimide Substances 0.000 description 2
- BQCADISMDOOEFD-UHFFFAOYSA-N Silver Chemical compound [Ag] BQCADISMDOOEFD-UHFFFAOYSA-N 0.000 description 2
- 239000000853 adhesive Substances 0.000 description 2
- 230000001070 adhesive effect Effects 0.000 description 2
- 229910045601 alloy Inorganic materials 0.000 description 2
- 239000000956 alloy Substances 0.000 description 2
- 238000013459 approach Methods 0.000 description 2
- 210000001367 artery Anatomy 0.000 description 2
- 239000001569 carbon dioxide Substances 0.000 description 2
- 229910002092 carbon dioxide Inorganic materials 0.000 description 2
- 238000005260 corrosion Methods 0.000 description 2
- 230000007797 corrosion Effects 0.000 description 2
- 238000002788 crimping Methods 0.000 description 2
- 238000007606 doctor blade method Methods 0.000 description 2
- 238000010438 heat treatment Methods 0.000 description 2
- 229920001477 hydrophilic polymer Polymers 0.000 description 2
- 230000001050 lubricating effect Effects 0.000 description 2
- 150000002739 metals Chemical class 0.000 description 2
- 238000005459 micromachining Methods 0.000 description 2
- 238000012986 modification Methods 0.000 description 2
- 230000004048 modification Effects 0.000 description 2
- 229920001778 nylon Polymers 0.000 description 2
- 229920009441 perflouroethylene propylene Polymers 0.000 description 2
- 229920000052 poly(p-xylylene) Polymers 0.000 description 2
- 229920006260 polyaryletherketone Polymers 0.000 description 2
- 229920000728 polyester Polymers 0.000 description 2
- 229920002530 polyetherether ketone Polymers 0.000 description 2
- 229920001601 polyetherimide Polymers 0.000 description 2
- 238000003825 pressing Methods 0.000 description 2
- 230000008569 process Effects 0.000 description 2
- 238000000926 separation method Methods 0.000 description 2
- 229910052709 silver Inorganic materials 0.000 description 2
- 239000004332 silver Substances 0.000 description 2
- 229920001169 thermoplastic Polymers 0.000 description 2
- 229920001187 thermosetting polymer Polymers 0.000 description 2
- 238000012546 transfer Methods 0.000 description 2
- 210000003708 urethra Anatomy 0.000 description 2
- 210000003462 vein Anatomy 0.000 description 2
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 2
- 238000004804 winding Methods 0.000 description 2
- ODPYDILFQYARBK-UHFFFAOYSA-N 7-thiabicyclo[4.1.0]hepta-1,3,5-triene Chemical compound C1=CC=C2SC2=C1 ODPYDILFQYARBK-UHFFFAOYSA-N 0.000 description 1
- 229920003934 Aciplex® Polymers 0.000 description 1
- 229910001316 Ag alloy Inorganic materials 0.000 description 1
- 229910000531 Co alloy Inorganic materials 0.000 description 1
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 1
- 229910000640 Fe alloy Inorganic materials 0.000 description 1
- 229920003935 Flemion® Polymers 0.000 description 1
- 229920000557 Nafion® Polymers 0.000 description 1
- 229920001774 Perfluoroether Polymers 0.000 description 1
- 239000004698 Polyethylene Substances 0.000 description 1
- 239000004721 Polyphenylene oxide Substances 0.000 description 1
- 239000004372 Polyvinyl alcohol Substances 0.000 description 1
- 229910001260 Pt alloy Inorganic materials 0.000 description 1
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 1
- 229910001069 Ti alloy Inorganic materials 0.000 description 1
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 description 1
- 101150071882 US17 gene Proteins 0.000 description 1
- HZEWFHLRYVTOIW-UHFFFAOYSA-N [Ti].[Ni] Chemical compound [Ti].[Ni] HZEWFHLRYVTOIW-UHFFFAOYSA-N 0.000 description 1
- 230000004888 barrier function Effects 0.000 description 1
- 230000005540 biological transmission Effects 0.000 description 1
- 210000004204 blood vessel Anatomy 0.000 description 1
- 238000005219 brazing Methods 0.000 description 1
- 150000001720 carbohydrates Chemical class 0.000 description 1
- 229910021387 carbon allotrope Inorganic materials 0.000 description 1
- 238000005266 casting Methods 0.000 description 1
- 239000000919 ceramic Substances 0.000 description 1
- 239000000788 chromium alloy Substances 0.000 description 1
- BIJOYKCOMBZXAE-UHFFFAOYSA-N chromium iron nickel Chemical compound [Cr].[Fe].[Ni] BIJOYKCOMBZXAE-UHFFFAOYSA-N 0.000 description 1
- 239000011530 conductive current collector Substances 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 229910052802 copper Inorganic materials 0.000 description 1
- 239000010949 copper Substances 0.000 description 1
- 230000007423 decrease Effects 0.000 description 1
- 230000008021 deposition Effects 0.000 description 1
- 238000000151 deposition Methods 0.000 description 1
- 210000000613 ear canal Anatomy 0.000 description 1
- 230000005611 electricity Effects 0.000 description 1
- 239000008151 electrolyte solution Substances 0.000 description 1
- 229940021013 electrolyte solution Drugs 0.000 description 1
- 238000004146 energy storage Methods 0.000 description 1
- 150000002148 esters Chemical class 0.000 description 1
- 238000005530 etching Methods 0.000 description 1
- 229920005570 flexible polymer Polymers 0.000 description 1
- 239000011888 foil Substances 0.000 description 1
- 238000009472 formulation Methods 0.000 description 1
- 230000002209 hydrophobic effect Effects 0.000 description 1
- 229920013821 hydroxy alkyl cellulose Polymers 0.000 description 1
- 150000003949 imides Chemical class 0.000 description 1
- 229920000831 ionic polymer Polymers 0.000 description 1
- 238000005304 joining Methods 0.000 description 1
- 229920005684 linear copolymer Polymers 0.000 description 1
- 229920000092 linear low density polyethylene Polymers 0.000 description 1
- 239000011244 liquid electrolyte Substances 0.000 description 1
- 239000011159 matrix material Substances 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 239000002905 metal composite material Substances 0.000 description 1
- 239000000203 mixture Substances 0.000 description 1
- 239000002105 nanoparticle Substances 0.000 description 1
- 210000003928 nasal cavity Anatomy 0.000 description 1
- 229910052759 nickel Inorganic materials 0.000 description 1
- 229910000623 nickel–chromium alloy Inorganic materials 0.000 description 1
- 229910000510 noble metal Inorganic materials 0.000 description 1
- 230000000149 penetrating effect Effects 0.000 description 1
- 210000003800 pharynx Anatomy 0.000 description 1
- 238000007747 plating Methods 0.000 description 1
- 229920000412 polyarylene Polymers 0.000 description 1
- 229920000570 polyether Polymers 0.000 description 1
- 229920000573 polyethylene Polymers 0.000 description 1
- 229920002959 polymer blend Polymers 0.000 description 1
- 229920005597 polymer membrane Polymers 0.000 description 1
- 239000002952 polymeric resin Substances 0.000 description 1
- 238000006116 polymerization reaction Methods 0.000 description 1
- 229920001296 polysiloxane Polymers 0.000 description 1
- 239000004814 polyurethane Substances 0.000 description 1
- 229920002635 polyurethane Polymers 0.000 description 1
- 229920002451 polyvinyl alcohol Polymers 0.000 description 1
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 1
- 239000001267 polyvinylpyrrolidone Substances 0.000 description 1
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- RUOJZAUFBMNUDX-UHFFFAOYSA-N propylene carbonate Chemical compound CC1COC(=O)O1 RUOJZAUFBMNUDX-UHFFFAOYSA-N 0.000 description 1
- 238000011084 recovery Methods 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 229920005989 resin Polymers 0.000 description 1
- 239000011347 resin Substances 0.000 description 1
- 239000000523 sample Substances 0.000 description 1
- 239000010935 stainless steel Substances 0.000 description 1
- 229920003002 synthetic resin Polymers 0.000 description 1
- 239000004634 thermosetting polymer Substances 0.000 description 1
- 239000004416 thermosoftening plastic Substances 0.000 description 1
- 239000010936 titanium Substances 0.000 description 1
- 125000001889 triflyl group Chemical group FC(F)(F)S(*)(=O)=O 0.000 description 1
- PAPBSGBWRJIAAV-UHFFFAOYSA-N ε-Caprolactone Chemical compound O=C1CCCCCO1 PAPBSGBWRJIAAV-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/01—Introducing, guiding, advancing, emplacing or holding catheters
- A61M25/0105—Steering means as part of the catheter or advancing means; Markers for positioning
- A61M25/0133—Tip steering devices
- A61M25/0158—Tip steering devices with magnetic or electrical means, e.g. by using piezo materials, electroactive polymers, magnetic materials or by heating of shape memory materials
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B1/00—Instruments for performing medical examinations of the interior of cavities or tubes of the body by visual or photographical inspection, e.g. endoscopes; Illuminating arrangements therefor
- A61B1/00064—Constructional details of the endoscope body
- A61B1/0011—Manufacturing of endoscope parts
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B1/00—Instruments for performing medical examinations of the interior of cavities or tubes of the body by visual or photographical inspection, e.g. endoscopes; Illuminating arrangements therefor
- A61B1/005—Flexible endoscopes
- A61B1/0051—Flexible endoscopes with controlled bending of insertion part
- A61B1/0052—Constructional details of control elements, e.g. handles
- A61B1/0053—Constructional details of control elements, e.g. handles using distributed actuators, e.g. artificial muscles
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B1/00—Instruments for performing medical examinations of the interior of cavities or tubes of the body by visual or photographical inspection, e.g. endoscopes; Illuminating arrangements therefor
- A61B1/005—Flexible endoscopes
- A61B1/0051—Flexible endoscopes with controlled bending of insertion part
- A61B1/0057—Constructional details of force transmission elements, e.g. control wires
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L29/00—Materials for catheters, medical tubing, cannulae, or endoscopes or for coating catheters
- A61L29/02—Inorganic materials
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L29/00—Materials for catheters, medical tubing, cannulae, or endoscopes or for coating catheters
- A61L29/04—Macromolecular materials
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L29/00—Materials for catheters, medical tubing, cannulae, or endoscopes or for coating catheters
- A61L29/04—Macromolecular materials
- A61L29/041—Macromolecular materials obtained by reactions only involving carbon-to-carbon unsaturated bonds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L29/00—Materials for catheters, medical tubing, cannulae, or endoscopes or for coating catheters
- A61L29/08—Materials for coatings
- A61L29/085—Macromolecular materials
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L29/00—Materials for catheters, medical tubing, cannulae, or endoscopes or for coating catheters
- A61L29/14—Materials characterised by their function or physical properties, e.g. lubricating compositions
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/0009—Making of catheters or other medical or surgical tubes
- A61M25/0012—Making of catheters or other medical or surgical tubes with embedded structures, e.g. coils, braids, meshes, strands or radiopaque coils
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/0043—Catheters; Hollow probes characterised by structural features
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/0043—Catheters; Hollow probes characterised by structural features
- A61M25/0045—Catheters; Hollow probes characterised by structural features multi-layered, e.g. coated
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/0043—Catheters; Hollow probes characterised by structural features
- A61M25/005—Catheters; Hollow probes characterised by structural features with embedded materials for reinforcement, e.g. wires, coils, braids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/0043—Catheters; Hollow probes characterised by structural features
- A61M25/005—Catheters; Hollow probes characterised by structural features with embedded materials for reinforcement, e.g. wires, coils, braids
- A61M25/0052—Localized reinforcement, e.g. where only a specific part of the catheter is reinforced, for rapid exchange guidewire port
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/01—Introducing, guiding, advancing, emplacing or holding catheters
- A61M25/09—Guide wires
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/01—Introducing, guiding, advancing, emplacing or holding catheters
- A61M25/09—Guide wires
- A61M25/09041—Mechanisms for insertion of guide wires
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/0043—Catheters; Hollow probes characterised by structural features
- A61M2025/0058—Catheters; Hollow probes characterised by structural features having an electroactive polymer material, e.g. for steering purposes, for control of flexibility, for locking, for opening or closing
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/0043—Catheters; Hollow probes characterised by structural features
- A61M2025/0063—Catheters; Hollow probes characterised by structural features having means, e.g. stylets, mandrils, rods or wires to reinforce or adjust temporarily the stiffness, column strength or pushability of catheters which are already inserted into the human body
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/01—Introducing, guiding, advancing, emplacing or holding catheters
- A61M25/09—Guide wires
- A61M2025/09116—Design of handles or shafts or gripping surfaces thereof for manipulating guide wires
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/01—Introducing, guiding, advancing, emplacing or holding catheters
- A61M25/09—Guide wires
- A61M2025/09133—Guide wires having specific material compositions or coatings; Materials with specific mechanical behaviours, e.g. stiffness, strength to transmit torque
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/01—Introducing, guiding, advancing, emplacing or holding catheters
- A61M25/09—Guide wires
- A61M2025/09175—Guide wires having specific characteristics at the distal tip
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2205/00—General characteristics of the apparatus
- A61M2205/02—General characteristics of the apparatus characterised by a particular materials
- A61M2205/0238—General characteristics of the apparatus characterised by a particular materials the material being a coating or protective layer
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2205/00—General characteristics of the apparatus
- A61M2205/02—General characteristics of the apparatus characterised by a particular materials
- A61M2205/0272—Electro-active or magneto-active materials
- A61M2205/0283—Electro-active polymers [EAP]
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Veterinary Medicine (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Engineering & Computer Science (AREA)
- Biophysics (AREA)
- Biomedical Technology (AREA)
- Heart & Thoracic Surgery (AREA)
- Pulmonology (AREA)
- Anesthesiology (AREA)
- Hematology (AREA)
- Epidemiology (AREA)
- Surgery (AREA)
- Chemical & Material Sciences (AREA)
- Molecular Biology (AREA)
- Optics & Photonics (AREA)
- Pathology (AREA)
- Radiology & Medical Imaging (AREA)
- Physics & Mathematics (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Medical Informatics (AREA)
- Inorganic Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Manufacturing & Machinery (AREA)
- Media Introduction/Drainage Providing Device (AREA)
- Electrotherapy Devices (AREA)
- Surgical Instruments (AREA)
- Infusion, Injection, And Reservoir Apparatuses (AREA)
- Micromachines (AREA)
Description
Claims (23)
- 医療機器であって、
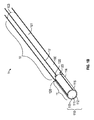
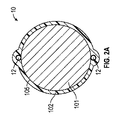
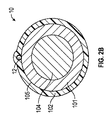
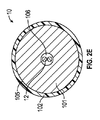
少なくとも1つのイオン電気活性ポリマーアクチュエータであって、少なくとも1つの表面を規定している少なくとも1つのポリマー電解質部材、及び前記少なくとも1つのポリマー電解質部材の前記表面の周囲に配置された複数の電極を備える少なくとも1つのイオン電気活性ポリマーアクチュエータと、
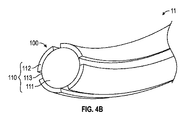
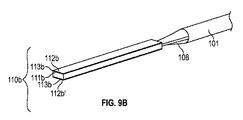
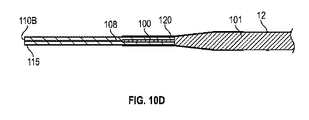
細長可撓性部であって、近位端及び、前記イオン電気活性ポリマーアクチュエータに近接して固定された遠位端を規定しており、コア及び前記コアを取り囲むスリーブをさらに備える、細長可撓性部と、
複数の導電性ワイヤであって、それぞれが近位端、及び前記複数の電極のうちの少なくとも1つに連結された遠位端を有する複数の導電性ワイヤとを備え、
前記少なくとも1つのポリマー電解質部材が、前記複数の導電性ワイヤのうちの少なくとも1つを通じて前記複数の電極のうちの少なくとも1つに供給される電位の印加に応答して非対称に変形し、
前記コアが、前記細長可撓性部の前記遠位端に近接するテーパ部を備え、
前記コアが、前記ポリマー電解質部材の内部に延びている、医療機器。 - 前記スリーブが、前記細長可撓性部の前記遠位端から延びて、前記イオン電気活性ポリマーアクチュエータの少なくとも一部を取り囲んでいる、請求項1に記載の医療機器。
- 前記導電性ワイヤが、前記スリーブと前記コアの間に固定され、前記イオン電気活性ポリマーアクチュエータの少なくとも一部分に固定されている、請求項2に記載の医療機器。
- 前記ポリマー電解質部材が、電解質と、フッ素ポリマー及び導電性ポリマーからなる群から選択されたポリマーとを含む、請求項1に記載の医療機器。
- 前記フッ素ポリマーが、ペルフルオロイオノマー、ポリフッ化ビニリデン(PVDF)、またはそれらのコポリマーである、請求項4に記載の医療機器。
- 前記導電性ポリマーが、ポリアニリン(PANI)、ポリピロール(Ppy)、ポリ(3,4−エチレンジオキシチオフェン)(PEDOT)、ポリ(p−フェニレンスルファイド)(PPS)、またはこれらの組み合わせを含む、請求項4に記載の医療機器。
- 前記電極のうちのそれぞれが、プラチナ、金、もしくは炭素系材料、またはこれらのうちの2つ以上の組み合わせを含む、請求項1に記載の医療機器。
- 前記炭素系材料が、炭化物由来炭素、カーボンナノチューブ、グラフェン、炭化物由来炭素とポリマー電解質部材との複合物、及びカーボンナノチューブとポリマー電解質部材との複合物のうちの少なくとも1つを含む、請求項7に記載の医療機器。
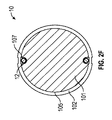
- 前記電極が、前記電極間の角度を等しくして、少なくとも1つのポリマー電解質部材の周囲に周方向に分散して配置されている、請求項1に記載の医療機器。
- 前記導電性ワイヤのそれぞれが、前記コアの外表面に沿って直線的に配置されている、請求項1に記載の医療機器。
- 前記細長可撓性部が、直線的に延び且つ前記コアの外表面に沿って周方向に互いに離間して配置された複数の溝をさらに備え、各溝が前記導電性ワイヤのうちの1つを受容している、請求項1に記載の医療機器。
- 前記複数の導電性ワイヤのそれぞれが、その上を覆っている絶縁被覆をさらに含む、請求項1に記載の医療機器。
- 前記ポリマー電解質部材の表面と前記電極のうちの少なくとも1つとの間に延びる導電性ブリッジをさらに備える、請求項1に記載の医療機器。
- 前記コアが、内部に前記導電性ワイヤが延びる内部ルーメンをさらに備える、請求項1に記載の医療機器。
- 前記導電性ワイヤが、さらに前記内部ルーメンを通って貫通している、請求項14に記載の医療機器。
- 前記複数の導電性ワイヤのうちのそれぞれは、螺旋状にまたは織り合わせるように、前記コアの周囲に巻き付けられている、請求項1に記載の医療機器。
- 前記コアが、さらなる導電性導管の役割を果たし且つ前記複数の電極のうちの少なくとも1つに連結される金属材料を備える、請求項1に記載の医療機器。
- 前記イオン電気活性ポリマーアクチュエータが、2自由度の動作を与えるように構成された、請求項1に記載の医療機器。
- 4つの電極が、前記ポリマー電解質部材の前記表面の周囲に、等しい角度で周方向に互いに分散して配置されている、請求項18に記載の医療機器。
- 前記イオン電気活性ポリマーアクチュエータが、1自由度の動作を与えるように構成された、請求項1に記載の医療機器。
- 前記ポリマー電解質部材が、棒状の形状であり、前記ポリマー電解質部材の前記表面の周囲に、2つの電極が、等しい角度で周方向に分散して配置されている、請求項20に記載の医療機器。
- 前記ポリマー電解質部材が長方形の形状であり、頂表面と対応する底表面とを規定しており、2つの電極が、前記ポリマー電解質部材の前記頂表面と前記底表面の周囲に対称に周方向に分散して配置されてサンドイッチ構造を形成している、請求項20に記載の医療機器。
- 前記コアが、前記ポリマー電解質部材の遠位端へと、内部に延びている、請求項22に記載の医療機器。
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201762539346P | 2017-07-31 | 2017-07-31 | |
| US201762539338P | 2017-07-31 | 2017-07-31 | |
| US62/539,338 | 2017-07-31 | ||
| US62/539,346 | 2017-07-31 | ||
| PCT/US2018/044059 WO2019027826A2 (en) | 2017-07-31 | 2018-07-27 | MEDICAL DEVICE ORIENTABLE AND METHOD FOR PREPARING THE SAME |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2020513863A JP2020513863A (ja) | 2020-05-21 |
| JP2020513863A6 JP2020513863A6 (ja) | 2020-09-24 |
| JP6831455B2 true JP6831455B2 (ja) | 2021-02-17 |
Family
ID=65234016
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2019520807A Active JP6831455B2 (ja) | 2017-07-31 | 2018-07-27 | 操向可能な医療機器及びその作製方法 |
| JP2019520901A Active JP6798020B2 (ja) | 2017-07-31 | 2018-07-27 | 編組構造の操向可能な医療機器及びその作製方法 |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2019520901A Active JP6798020B2 (ja) | 2017-07-31 | 2018-07-27 | 編組構造の操向可能な医療機器及びその作製方法 |
Country Status (11)
| Country | Link |
|---|---|
| US (3) | US11229775B2 (ja) |
| EP (3) | EP4424352A2 (ja) |
| JP (2) | JP6831455B2 (ja) |
| KR (2) | KR102086979B1 (ja) |
| CN (2) | CN109843368B (ja) |
| AU (2) | AU2018311843B2 (ja) |
| BR (2) | BR112019007355A8 (ja) |
| CA (2) | CA3039267C (ja) |
| MX (2) | MX2019004430A (ja) |
| TW (2) | TWI679036B (ja) |
| WO (2) | WO2019027826A2 (ja) |
Families Citing this family (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US11986150B2 (en) | 2009-12-15 | 2024-05-21 | Lumendi Ltd. | Method and apparatus for manipulating the side wall of a body lumen or body cavity so as to provide increased visualization of the same and/or increased access to the same, and/or for stabilizing instruments relative to the same |
| US11877722B2 (en) | 2009-12-15 | 2024-01-23 | Cornell University | Method and apparatus for manipulating the side wall of a body lumen or body cavity |
| WO2019133438A1 (en) | 2017-12-29 | 2019-07-04 | Xcath, Inc. | Steerable surgical robotic system |
| CN110789129A (zh) * | 2019-06-28 | 2020-02-14 | 东莞科威医疗器械有限公司 | 一种增强型医用插管及其制作方法 |
| CN110575603A (zh) * | 2019-10-14 | 2019-12-17 | 苏州法兰克曼医疗器械有限公司 | 一种具有可视功能的导丝输送装置 |
| WO2021123757A1 (en) * | 2019-12-16 | 2021-06-24 | Galvani Bioelectronics Limited | Stent-electrode intravascular neuromodulator and associated methods for activation of a nerve |
| CN111729106B (zh) * | 2020-06-30 | 2021-08-13 | 北京航空航天大学 | 一种柔性低温等离子体灭菌装置 |
| CN112409627A (zh) * | 2020-10-12 | 2021-02-26 | 浙江理工大学 | 一种新型混合纤维素ipmc材料的制备方法 |
| CN112406252B (zh) * | 2020-10-12 | 2023-01-17 | 浙江理工大学 | 基于c-cnc纤维素的高性能电驱动ipmc柔性驱动器的制备方法 |
| EP4232131A4 (en) * | 2020-10-23 | 2024-10-16 | Vicora Inc | ACTUATED THROMBECTOMY DEVICE |
| WO2022104262A1 (en) * | 2020-11-16 | 2022-05-19 | Lumendi Ltd. | Methods and apparatus for inverting a hollow sleeve and thereafter reverting an inverted hollow sleeve |
| TWI759076B (zh) * | 2021-01-15 | 2022-03-21 | 陳映臻 | 治療內腔室傷口之藥劑推進裝置及其藥劑推進管 |
| CN114225174A (zh) * | 2021-10-09 | 2022-03-25 | 邹最 | 导芯式防舌后坠低氧导管及应用 |
| CN115430020A (zh) * | 2022-11-08 | 2022-12-06 | 山东百多安医疗器械股份有限公司 | 一种带柔性电极的导尿管 |
| CN116617545B (zh) * | 2023-06-09 | 2024-03-22 | 株洲茂物医疗科技有限公司 | 导丝的制作方法和导丝 |
Family Cites Families (26)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4808157A (en) * | 1987-07-13 | 1989-02-28 | Neuro Delivery Technology, Inc. | Multi-lumen epidural-spinal needle |
| US5415633A (en) | 1993-07-28 | 1995-05-16 | Active Control Experts, Inc. | Remotely steered catheterization device |
| JPH0810336A (ja) * | 1994-06-30 | 1996-01-16 | Agency Of Ind Science & Technol | 医療用チューブ |
| US6616996B1 (en) * | 1994-10-28 | 2003-09-09 | Medsource Trenton, Inc. | Variable stiffness microtubing and method of manufacture |
| US5755704A (en) * | 1996-10-29 | 1998-05-26 | Medtronic, Inc. | Thinwall guide catheter |
| US7494474B2 (en) | 1997-06-04 | 2009-02-24 | Advanced Cardiovascular Systems, Inc. | Polymer coated guidewire |
| US5891114A (en) * | 1997-09-30 | 1999-04-06 | Target Therapeutics, Inc. | Soft-tip high performance braided catheter |
| US6117296A (en) | 1998-07-21 | 2000-09-12 | Thomson; Timothy | Electrically controlled contractile polymer composite |
| US6464684B1 (en) * | 1998-09-09 | 2002-10-15 | Scimed Life Systems, Inc. | Catheter having regions of differing braid densities and methods of manufacture therefor |
| US6679836B2 (en) * | 2002-06-21 | 2004-01-20 | Scimed Life Systems, Inc. | Universal programmable guide catheter |
| EP1957141A1 (en) * | 2005-11-17 | 2008-08-20 | MicroMuscle AB | Medical devices and methods for their fabrication and use |
| JP2007209554A (ja) * | 2006-02-09 | 2007-08-23 | Terumo Corp | カテーテル |
| US8414632B2 (en) | 2006-03-06 | 2013-04-09 | Boston Scientific Scimed, Inc. | Adjustable catheter tip |
| US20070249909A1 (en) * | 2006-04-25 | 2007-10-25 | Volk Angela K | Catheter configurations |
| US7766896B2 (en) * | 2006-04-25 | 2010-08-03 | Boston Scientific Scimed, Inc. | Variable stiffness catheter assembly |
| US7909844B2 (en) * | 2006-07-31 | 2011-03-22 | Boston Scientific Scimed, Inc. | Catheters having actuatable lumen assemblies |
| US9370640B2 (en) | 2007-09-12 | 2016-06-21 | Novasentis, Inc. | Steerable medical guide wire device |
| US8920369B2 (en) * | 2009-06-24 | 2014-12-30 | Shifamed Holdings, Llc | Steerable delivery sheaths |
| US9717553B2 (en) * | 2010-12-29 | 2017-08-01 | Biosence Webster (Israel) Ltd. | Braid with integrated signal conductors |
| EP2747634A4 (en) | 2011-08-22 | 2015-05-06 | Lake Region Mfg Inc D B A Lake Region Medical | MULTILADER GUIDING WIRE WITH LOW PROFILE |
| US9147825B2 (en) * | 2012-03-07 | 2015-09-29 | Board of Regents of the Nevada System of Higher Education on behalf of the University of Nevado, Reno | Methods of fabricating multi-degree of freedom shaped electroactive polymer actuators/sensors for catheters |
| CN103860258A (zh) * | 2012-12-12 | 2014-06-18 | 北京中孵友信医药科技股份有限公司 | 自适应式环向定位腔内导管 |
| US9364635B2 (en) | 2013-09-20 | 2016-06-14 | Covidien Lp | Computer controlled steerable tip guide catheter |
| EP3060289B1 (en) * | 2013-10-25 | 2018-06-27 | Intuitive Surgical Operations, Inc. | Flexible instrument with grooved steerable tube |
| FR3019993B1 (fr) * | 2014-04-16 | 2019-07-19 | Institut National Des Sciences Appliquees De Lyon | Fil de guidage a flexibilite variable controlee |
| CN112138264B (zh) * | 2016-02-05 | 2022-10-25 | 得克萨斯系统大学董事会 | 用于制备医疗装置的离子电活性聚合物致动器的方法 |
-
2018
- 2018-07-27 WO PCT/US2018/044059 patent/WO2019027826A2/en active Application Filing
- 2018-07-27 BR BR112019007355A patent/BR112019007355A8/pt active IP Right Grant
- 2018-07-27 TW TW107126073A patent/TWI679036B/zh active
- 2018-07-27 CA CA3039267A patent/CA3039267C/en active Active
- 2018-07-27 JP JP2019520807A patent/JP6831455B2/ja active Active
- 2018-07-27 AU AU2018311843A patent/AU2018311843B2/en active Active
- 2018-07-27 MX MX2019004430A patent/MX2019004430A/es unknown
- 2018-07-27 CA CA3039269A patent/CA3039269C/en active Active
- 2018-07-27 EP EP24181489.6A patent/EP4424352A2/en active Pending
- 2018-07-27 WO PCT/US2018/044057 patent/WO2019027825A1/en active Application Filing
- 2018-07-27 US US16/478,425 patent/US11229775B2/en active Active
- 2018-07-27 EP EP18841377.7A patent/EP3548130B1/en active Active
- 2018-07-27 US US16/478,435 patent/US10835716B2/en active Active
- 2018-07-27 MX MX2020001267A patent/MX2020001267A/es unknown
- 2018-07-27 KR KR1020197010800A patent/KR102086979B1/ko active IP Right Grant
- 2018-07-27 CN CN201880003922.4A patent/CN109843368B/zh active Active
- 2018-07-27 EP EP18842340.4A patent/EP3548131A4/en active Pending
- 2018-07-27 CN CN201880003921.XA patent/CN110461400B/zh active Active
- 2018-07-27 KR KR1020197010799A patent/KR102118837B1/ko active IP Right Grant
- 2018-07-27 AU AU2018311844D patent/AU2018311844B9/en active Active
- 2018-07-27 JP JP2019520901A patent/JP6798020B2/ja active Active
- 2018-07-27 TW TW107126074A patent/TWI683678B/zh active
- 2018-07-27 BR BR112019007377-9A patent/BR112019007377B1/pt active IP Right Grant
-
2021
- 2021-12-16 US US17/553,371 patent/US20220105315A1/en active Pending
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6831455B2 (ja) | 操向可能な医療機器及びその作製方法 | |
| JP2020513863A6 (ja) | 操向可能な医療機器及びその作製方法 | |
| JP7126597B2 (ja) | 操向自在な腔内医療機器 | |
| US10682177B2 (en) | Medical device having laminate-coated braid assembly | |
| WO2017149904A1 (ja) | 心腔内除細動カテーテル | |
| JP2019122862A (ja) | 心腔内除細動カテーテル |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20190705 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20190919 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20200207 |
|
| A871 | Explanation of circumstances concerning accelerated examination |
Free format text: JAPANESE INTERMEDIATE CODE: A871 Effective date: 20200207 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20200221 |
|
| A975 | Report on accelerated examination |
Free format text: JAPANESE INTERMEDIATE CODE: A971005 Effective date: 20200602 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20200707 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20210105 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20210128 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 6831455 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |