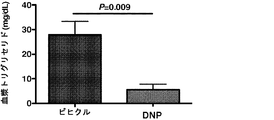
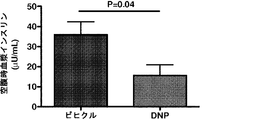
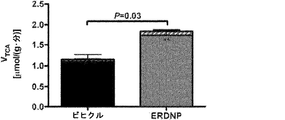
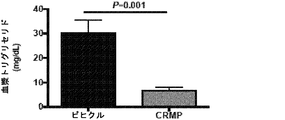
JP6706204B2 - 新規2,4−ジニトロフェノール製剤およびその使用法 - Google Patents
新規2,4−ジニトロフェノール製剤およびその使用法 Download PDFInfo
- Publication number
- JP6706204B2 JP6706204B2 JP2016537894A JP2016537894A JP6706204B2 JP 6706204 B2 JP6706204 B2 JP 6706204B2 JP 2016537894 A JP2016537894 A JP 2016537894A JP 2016537894 A JP2016537894 A JP 2016537894A JP 6706204 B2 JP6706204 B2 JP 6706204B2
- Authority
- JP
- Japan
- Prior art keywords
- subject
- dnp
- compound
- liver
- erdnp
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/045—Hydroxy compounds, e.g. alcohols; Salts thereof, e.g. alcoholates
- A61K31/05—Phenols
- A61K31/06—Phenols the aromatic ring being substituted by nitro groups
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K45/00—Medicinal preparations containing active ingredients not provided for in groups A61K31/00 - A61K41/00
- A61K45/06—Mixtures of active ingredients without chemical characterisation, e.g. antiphlogistics and cardiaca
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0053—Mouth and digestive tract, i.e. intraoral and peroral administration
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
- A61K9/50—Microcapsules having a gas, liquid or semi-solid filling; Solid microparticles or pellets surrounded by a distinct coating layer, e.g. coated microspheres, coated drug crystals
- A61K9/5005—Wall or coating material
- A61K9/501—Inorganic compounds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
- A61K9/50—Microcapsules having a gas, liquid or semi-solid filling; Solid microparticles or pellets surrounded by a distinct coating layer, e.g. coated microspheres, coated drug crystals
- A61K9/5005—Wall or coating material
- A61K9/5021—Organic macromolecular compounds
- A61K9/5026—Organic macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds, e.g. polyvinyl pyrrolidone, poly(meth)acrylates
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
- A61K9/50—Microcapsules having a gas, liquid or semi-solid filling; Solid microparticles or pellets surrounded by a distinct coating layer, e.g. coated microspheres, coated drug crystals
- A61K9/5005—Wall or coating material
- A61K9/5021—Organic macromolecular compounds
- A61K9/5036—Polysaccharides, e.g. gums, alginate; Cyclodextrin
- A61K9/5042—Cellulose; Cellulose derivatives, e.g. phthalate or acetate succinate esters of hydroxypropyl methylcellulose
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
- A61K9/50—Microcapsules having a gas, liquid or semi-solid filling; Solid microparticles or pellets surrounded by a distinct coating layer, e.g. coated microspheres, coated drug crystals
- A61K9/5005—Wall or coating material
- A61K9/5021—Organic macromolecular compounds
- A61K9/5036—Polysaccharides, e.g. gums, alginate; Cyclodextrin
- A61K9/5042—Cellulose; Cellulose derivatives, e.g. phthalate or acetate succinate esters of hydroxypropyl methylcellulose
- A61K9/5047—Cellulose ethers containing no ester groups, e.g. hydroxypropyl methylcellulose
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/16—Drugs for disorders of the alimentary tract or the digestive system for liver or gallbladder disorders, e.g. hepatoprotective agents, cholagogues, litholytics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/02—Drugs for disorders of the nervous system for peripheral neuropathies
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/04—Anorexiants; Antiobesity agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/06—Antihyperlipidemics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/08—Drugs for disorders of the metabolism for glucose homeostasis
- A61P3/10—Drugs for disorders of the metabolism for glucose homeostasis for hyperglycaemia, e.g. antidiabetics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P39/00—General protective or antinoxious agents
- A61P39/06—Free radical scavengers or antioxidants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P5/00—Drugs for disorders of the endocrine system
- A61P5/48—Drugs for disorders of the endocrine system of the pancreatic hormones
- A61P5/50—Drugs for disorders of the endocrine system of the pancreatic hormones for increasing or potentiating the activity of insulin
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Epidemiology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Organic Chemistry (AREA)
- Diabetes (AREA)
- Obesity (AREA)
- Hematology (AREA)
- Neurology (AREA)
- Biomedical Technology (AREA)
- Endocrinology (AREA)
- Neurosurgery (AREA)
- Inorganic Chemistry (AREA)
- Nutrition Science (AREA)
- Physiology (AREA)
- Gastroenterology & Hepatology (AREA)
- Child & Adolescent Psychology (AREA)
- Biochemistry (AREA)
- Toxicology (AREA)
- Rheumatology (AREA)
- Emergency Medicine (AREA)
- Pain & Pain Management (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
- Medicinal Preparation (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2019162622A JP6913137B2 (ja) | 2013-08-30 | 2019-09-06 | 新規2,4−ジニトロフェノール製剤およびその使用法 |
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201361872294P | 2013-08-30 | 2013-08-30 | |
| US61/872,294 | 2013-08-30 | ||
| US201361919003P | 2013-12-20 | 2013-12-20 | |
| US61/919,003 | 2013-12-20 | ||
| PCT/US2014/053406 WO2015031756A1 (en) | 2013-08-30 | 2014-08-29 | Novel 2,4-dinitrophenol formulations and methods using same |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2019162622A Division JP6913137B2 (ja) | 2013-08-30 | 2019-09-06 | 新規2,4−ジニトロフェノール製剤およびその使用法 |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2016530279A JP2016530279A (ja) | 2016-09-29 |
| JP2016530279A5 JP2016530279A5 (enExample) | 2017-09-07 |
| JP6706204B2 true JP6706204B2 (ja) | 2020-06-03 |
Family
ID=52587363
Family Applications (4)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2016537894A Active JP6706204B2 (ja) | 2013-08-30 | 2014-08-29 | 新規2,4−ジニトロフェノール製剤およびその使用法 |
| JP2019162622A Active JP6913137B2 (ja) | 2013-08-30 | 2019-09-06 | 新規2,4−ジニトロフェノール製剤およびその使用法 |
| JP2021113956A Active JP7359809B2 (ja) | 2013-08-30 | 2021-07-09 | 新規2,4-ジニトロフェノール製剤およびその使用法 |
| JP2023127746A Pending JP2023133603A (ja) | 2013-08-30 | 2023-08-04 | 新規2,4-ジニトロフェノール製剤およびその使用法 |
Family Applications After (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2019162622A Active JP6913137B2 (ja) | 2013-08-30 | 2019-09-06 | 新規2,4−ジニトロフェノール製剤およびその使用法 |
| JP2021113956A Active JP7359809B2 (ja) | 2013-08-30 | 2021-07-09 | 新規2,4-ジニトロフェノール製剤およびその使用法 |
| JP2023127746A Pending JP2023133603A (ja) | 2013-08-30 | 2023-08-04 | 新規2,4-ジニトロフェノール製剤およびその使用法 |
Country Status (5)
| Country | Link |
|---|---|
| US (4) | US10786466B2 (enExample) |
| EP (1) | EP3038611B1 (enExample) |
| JP (4) | JP6706204B2 (enExample) |
| CN (2) | CN110302385A (enExample) |
| WO (1) | WO2015031756A1 (enExample) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2021165309A (ja) * | 2013-08-30 | 2021-10-14 | イエール ユニバーシティ | 新規2,4−ジニトロフェノール製剤およびその使用法 |
Families Citing this family (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN116617195A (zh) | 2015-01-22 | 2023-08-22 | 米托充制药公司 | 脑源性神经营养因子的诱导表达 |
| DE102015016801A1 (de) * | 2015-12-23 | 2017-06-29 | Christian Windisch | Chemotherapeutisches Verfahren zur Behandlung maliger Geschwülste durch "Entkopplung" der oxidativen Phosphorilierung ("Krebsatmung") in aerob glycolysierenden Zellen (Krebszellen) |
| JP7021098B2 (ja) * | 2016-03-07 | 2022-02-16 | マイトコン ファーマシューティカルズ, インコーポレイテッド | 神経筋、神経変性、自己免疫、発達、外傷性脳損傷、震とう、ドライアイ疾患および/または代謝性疾患のdnpおよびdnpプロドラッグ処置 |
| GEP20217227B (en) | 2017-01-06 | 2021-02-25 | Pharmaceuticals Inc Rivus | Novel phenyl derivatives |
| WO2019217164A1 (en) * | 2018-05-09 | 2019-11-14 | Reyoung Corporation | Compositions and methods for treating cancer and other diseases |
| US20200397807A1 (en) | 2019-06-18 | 2020-12-24 | MitoPower, LLC | Nicotinyl riboside compounds and their uses |
| CN110237033A (zh) * | 2019-07-30 | 2019-09-17 | 成都医学院 | 一种2,4二硝基苯酚注射液及其制备方法和用途 |
| CN110302154B (zh) * | 2019-07-30 | 2021-09-10 | 成都医学院 | 一种2,4-二硝基苯酚脂肪乳及其制备方法和用途 |
| CN113045428B (zh) * | 2019-12-26 | 2022-04-22 | 中国农业大学 | 化合物及其在减脂中的用途 |
Family Cites Families (23)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CA774573A (en) | 1967-12-26 | Sehring Richard | Derivatives of 2-hydroxy-3,5-dinitrobenzyl alcohol | |
| US2183589A (en) | 1939-12-19 | Derivatives of compounds of the | ||
| US1695656A (en) | 1927-03-10 | 1928-12-18 | Firm Gehe & Co A G | Method of producing narcotica |
| US3081224A (en) | 1959-01-21 | 1963-03-12 | American Cyanamid Co | Methods of removing helminths employing halogenated nitrophenols and their derivatives |
| NL301998A (enExample) | 1962-12-27 | 1900-01-01 | ||
| DE3313905A1 (de) | 1983-04-16 | 1984-10-25 | Henkel KGaA, 4000 Düsseldorf | Neue 2.4-diaminophenolether als kuppler fuer oxidationshaarfaerbemittel |
| DE3515339A1 (de) | 1985-04-27 | 1986-10-30 | Cassella Ag, 6000 Frankfurt | Verfahren zur herstellung von 2,4-dinitrophenylethern |
| CN1011660B (zh) * | 1986-04-23 | 1991-02-20 | 吴佩璇 | 一种含三硝基苯、田螺的药品合成方法 |
| US5078908A (en) | 1989-10-02 | 1992-01-07 | Allergan, Inc. | Methods for generating chlorine dioxide and compositions for disinfecting |
| US5169645A (en) | 1989-10-31 | 1992-12-08 | Duquesne University Of The Holy Ghost | Directly compressible granules having improved flow properties |
| IN192400B (enExample) * | 1995-06-14 | 2004-04-10 | Council Scient Ind Res | |
| CA2180903A1 (en) | 1995-07-11 | 1997-01-12 | Kunio Sugisawa | Pesticidal composition to noxious organisms |
| EP1073693A1 (en) | 1998-04-24 | 2001-02-07 | Eastman Chemical Company | Coprecipitation of cellulose esters with functional additives and compositions thus obtainable |
| US7635722B1 (en) * | 1998-07-27 | 2009-12-22 | Saint Jude Pharmaceuticals, Inc. | Chemical induced intracellular hyperthermia |
| US6664297B1 (en) | 2000-10-18 | 2003-12-16 | Universidade Federal Do Rio De Janeiro | Methods for inhibition and dissolution of amyloidoses by administration of compositions comprising 2,4-dinitrophenol |
| EP1429734B1 (en) * | 2001-09-21 | 2007-12-26 | Egalet A/S | Controlled release solid dispersions of carvedilol |
| EP1545477A4 (en) | 2002-09-13 | 2006-11-22 | Cydex Inc | CAPSULES CONTAINING AQUEOUS FILLING COMPOSITIONS STABILIZED WITH DERIVED CYCLODEXTRIN |
| EP1575575B1 (en) | 2002-11-08 | 2010-05-19 | High Point Pharmaceuticals, LLC | Safe chemical uncouplers for the treatment of obesity |
| WO2007050673A2 (en) | 2005-10-25 | 2007-05-03 | University Of Florida | Cyclin dependent kinase inhibitors |
| EP2196453A1 (en) | 2008-12-10 | 2010-06-16 | Cellvir | Novel substituted aryl derivatives, their process of preparation and their therapeutical uses as anti-HIV agents |
| WO2011053825A2 (en) * | 2009-10-30 | 2011-05-05 | The United States Of America, As Represented By The Secretary, Department Of Health And Human Services | Compositions and methods for the treatment or prevention of mitochondrial diseases |
| JP2013209295A (ja) * | 2010-10-13 | 2013-10-10 | Takeda Chem Ind Ltd | ペプチド誘導体 |
| CN110302385A (zh) * | 2013-08-30 | 2019-10-08 | 耶鲁大学 | 新型2,4-二硝基苯酚制剂和使用其的方法 |
-
2014
- 2014-08-29 CN CN201910726120.2A patent/CN110302385A/zh active Pending
- 2014-08-29 WO PCT/US2014/053406 patent/WO2015031756A1/en not_active Ceased
- 2014-08-29 JP JP2016537894A patent/JP6706204B2/ja active Active
- 2014-08-29 EP EP14839060.2A patent/EP3038611B1/en active Active
- 2014-08-29 CN CN201480059363.0A patent/CN105682653B/zh active Active
- 2014-08-29 US US14/911,322 patent/US10786466B2/en active Active
-
2019
- 2019-09-06 JP JP2019162622A patent/JP6913137B2/ja active Active
-
2020
- 2020-07-22 US US16/936,316 patent/US11433033B2/en active Active
-
2021
- 2021-07-09 JP JP2021113956A patent/JP7359809B2/ja active Active
-
2022
- 2022-05-16 US US17/663,586 patent/US11883369B2/en active Active
-
2023
- 2023-08-04 JP JP2023127746A patent/JP2023133603A/ja active Pending
-
2024
- 2024-08-22 US US18/812,381 patent/US20250228793A1/en active Pending
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2021165309A (ja) * | 2013-08-30 | 2021-10-14 | イエール ユニバーシティ | 新規2,4−ジニトロフェノール製剤およびその使用法 |
| JP7359809B2 (ja) | 2013-08-30 | 2023-10-11 | イエール ユニバーシティ | 新規2,4-ジニトロフェノール製剤およびその使用法 |
Also Published As
| Publication number | Publication date |
|---|---|
| CN105682653A (zh) | 2016-06-15 |
| CN110302385A (zh) | 2019-10-08 |
| US20250228793A1 (en) | 2025-07-17 |
| US11883369B2 (en) | 2024-01-30 |
| JP2023133603A (ja) | 2023-09-22 |
| JP6913137B2 (ja) | 2021-08-04 |
| US20200352879A1 (en) | 2020-11-12 |
| EP3038611A1 (en) | 2016-07-06 |
| WO2015031756A1 (en) | 2015-03-05 |
| HK1225607A1 (en) | 2017-09-15 |
| JP2021165309A (ja) | 2021-10-14 |
| US10786466B2 (en) | 2020-09-29 |
| JP7359809B2 (ja) | 2023-10-11 |
| US20220339121A1 (en) | 2022-10-27 |
| JP2016530279A (ja) | 2016-09-29 |
| EP3038611B1 (en) | 2024-04-17 |
| US20160199310A1 (en) | 2016-07-14 |
| JP2020002166A (ja) | 2020-01-09 |
| US11433033B2 (en) | 2022-09-06 |
| CN105682653B (zh) | 2022-10-21 |
| EP3038611A4 (en) | 2017-03-29 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP7359809B2 (ja) | 新規2,4-ジニトロフェノール製剤およびその使用法 | |
| US12171731B2 (en) | Methods and compositions for the treatment of steatosis-associated disorders | |
| US10806735B2 (en) | Use of neutrophil elastase inhibitors in liver disease | |
| EP2773354B1 (en) | Oral immediate release formulations for substituted quinazolinones | |
| Ho et al. | Liposome-encapsulated curcumin attenuates HMGB1-mediated hepatic inflammation and fibrosis in a murine model of Wilson’s disease | |
| US11517540B2 (en) | Restoring physiology in iron-deficient organisms using small molecules | |
| JP2009506043A (ja) | Lynキナーゼの活性を調節し、関連する疾患を治療するための方法および製剤 | |
| KR20190034550A (ko) | 간성 뇌병증의 치료 방법 | |
| HK1225607B (en) | Sustained-release pharmaceutical composition comprising 2,4-dinitrophenol | |
| Cetin et al. | Paradigm shift in obesity treatment: an extensive review of current pipeline agents | |
| US20240100001A1 (en) | Denatonium salt for use in preventing, preventing progression and treating fatty liver diseases | |
| WO2025014754A1 (en) | Compositions containing, and combination therapies using bempedoic acid and a glp-1 receptor agonist | |
| Ouyang | The Involvement of Receptor Interacting Protein Kinase 1 (RIPK1) in the Pathogenesis of Acute Pancreatitis | |
| HK1201751B (en) | Oral immediate release formulations for substituted quinazolinones |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20160608 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20170725 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20170725 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20180411 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20180710 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20181206 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20190306 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20190509 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20190906 |
|
| A911 | Transfer to examiner for re-examination before appeal (zenchi) |
Free format text: JAPANESE INTERMEDIATE CODE: A911 Effective date: 20190917 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20191111 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20200210 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20200402 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20200420 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20200515 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 6706204 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |