JP6491188B2 - 皮膚移植片採取用の吸収性基材 - Google Patents
皮膚移植片採取用の吸収性基材 Download PDFInfo
- Publication number
- JP6491188B2 JP6491188B2 JP2016502366A JP2016502366A JP6491188B2 JP 6491188 B2 JP6491188 B2 JP 6491188B2 JP 2016502366 A JP2016502366 A JP 2016502366A JP 2016502366 A JP2016502366 A JP 2016502366A JP 6491188 B2 JP6491188 B2 JP 6491188B2
- Authority
- JP
- Japan
- Prior art keywords
- substrate
- layer
- base layer
- wicking
- absorbent
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
- 239000000758 substrate Substances 0.000 title claims description 130
- 239000002250 absorbent Substances 0.000 title claims description 109
- 230000002745 absorbent Effects 0.000 title claims description 109
- 239000012530 fluid Substances 0.000 claims description 93
- 239000000463 material Substances 0.000 claims description 84
- 238000007789 sealing Methods 0.000 claims description 48
- 239000000853 adhesive Substances 0.000 claims description 40
- 230000001070 adhesive effect Effects 0.000 claims description 40
- 238000004891 communication Methods 0.000 claims description 29
- 239000011148 porous material Substances 0.000 claims description 27
- 239000004814 polyurethane Substances 0.000 claims description 17
- 229920002635 polyurethane Polymers 0.000 claims description 16
- 239000000499 gel Substances 0.000 claims description 15
- 229920001296 polysiloxane Polymers 0.000 claims description 14
- 230000002093 peripheral effect Effects 0.000 claims description 11
- 229920001577 copolymer Polymers 0.000 claims description 9
- 239000006260 foam Substances 0.000 claims description 9
- 229920000642 polymer Polymers 0.000 claims description 8
- 229920000098 polyolefin Polymers 0.000 claims description 8
- 238000003306 harvesting Methods 0.000 claims description 7
- 230000008878 coupling Effects 0.000 claims description 6
- 238000010168 coupling process Methods 0.000 claims description 6
- 238000005859 coupling reaction Methods 0.000 claims description 6
- 239000007788 liquid Substances 0.000 claims description 5
- 239000003522 acrylic cement Substances 0.000 claims description 4
- 229920001410 Microfiber Polymers 0.000 claims description 3
- 239000000560 biocompatible material Substances 0.000 claims description 3
- 239000000416 hydrocolloid Substances 0.000 claims description 3
- 239000000017 hydrogel Substances 0.000 claims description 3
- 239000003658 microfiber Substances 0.000 claims description 3
- 239000002657 fibrous material Substances 0.000 claims 3
- 125000003011 styrenyl group Chemical class [H]\C(*)=C(/[H])C1=C([H])C([H])=C([H])C([H])=C1[H] 0.000 claims 1
- 239000010410 layer Substances 0.000 description 179
- 210000003491 skin Anatomy 0.000 description 95
- 210000001519 tissue Anatomy 0.000 description 18
- 238000002513 implantation Methods 0.000 description 16
- 238000000034 method Methods 0.000 description 14
- 238000000576 coating method Methods 0.000 description 8
- 239000011248 coating agent Substances 0.000 description 7
- 238000005520 cutting process Methods 0.000 description 7
- 230000002209 hydrophobic effect Effects 0.000 description 6
- 230000007246 mechanism Effects 0.000 description 6
- 210000004027 cell Anatomy 0.000 description 4
- 239000007943 implant Substances 0.000 description 4
- 208000014674 injury Diseases 0.000 description 4
- -1 polyethylene Polymers 0.000 description 4
- 239000002904 solvent Substances 0.000 description 4
- 230000008733 trauma Effects 0.000 description 4
- 229920002134 Carboxymethyl cellulose Polymers 0.000 description 3
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 3
- 239000004952 Polyamide Substances 0.000 description 3
- 239000004372 Polyvinyl alcohol Substances 0.000 description 3
- 230000000845 anti-microbial effect Effects 0.000 description 3
- 235000010948 carboxy methyl cellulose Nutrition 0.000 description 3
- 230000006870 function Effects 0.000 description 3
- 235000019645 odor Nutrition 0.000 description 3
- 229920002647 polyamide Polymers 0.000 description 3
- 229920000728 polyester Polymers 0.000 description 3
- 229920002451 polyvinyl alcohol Polymers 0.000 description 3
- 239000004800 polyvinyl chloride Substances 0.000 description 3
- 229910052708 sodium Inorganic materials 0.000 description 3
- 239000011734 sodium Substances 0.000 description 3
- 229920002554 vinyl polymer Polymers 0.000 description 3
- 238000003466 welding Methods 0.000 description 3
- WCDDVEOXEIYWFB-VXORFPGASA-N (2s,3s,4r,5r,6r)-3-[(2s,3r,5s,6r)-3-acetamido-5-hydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-4,5,6-trihydroxyoxane-2-carboxylic acid Chemical compound CC(=O)N[C@@H]1C[C@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](C(O)=O)O[C@@H](O)[C@H](O)[C@H]1O WCDDVEOXEIYWFB-VXORFPGASA-N 0.000 description 2
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 2
- 108010010803 Gelatin Proteins 0.000 description 2
- 239000004698 Polyethylene Substances 0.000 description 2
- 229920002125 Sokalan® Polymers 0.000 description 2
- 208000027418 Wounds and injury Diseases 0.000 description 2
- 238000010521 absorption reaction Methods 0.000 description 2
- 239000000783 alginic acid Substances 0.000 description 2
- 235000010443 alginic acid Nutrition 0.000 description 2
- 229920000615 alginic acid Polymers 0.000 description 2
- 229960001126 alginic acid Drugs 0.000 description 2
- 150000004781 alginic acids Chemical class 0.000 description 2
- 239000004599 antimicrobial Substances 0.000 description 2
- 230000004888 barrier function Effects 0.000 description 2
- 210000000270 basal cell Anatomy 0.000 description 2
- 230000015572 biosynthetic process Effects 0.000 description 2
- 229910052799 carbon Inorganic materials 0.000 description 2
- 239000001768 carboxy methyl cellulose Substances 0.000 description 2
- 239000008112 carboxymethyl-cellulose Substances 0.000 description 2
- 210000002615 epidermis Anatomy 0.000 description 2
- 239000000835 fiber Substances 0.000 description 2
- 229920000159 gelatin Polymers 0.000 description 2
- 239000008273 gelatin Substances 0.000 description 2
- 235000019322 gelatine Nutrition 0.000 description 2
- 235000011852 gelatine desserts Nutrition 0.000 description 2
- 230000035876 healing Effects 0.000 description 2
- 229940014041 hyaluronate Drugs 0.000 description 2
- 208000015181 infectious disease Diseases 0.000 description 2
- 239000002121 nanofiber Substances 0.000 description 2
- 229920000058 polyacrylate Polymers 0.000 description 2
- 239000004584 polyacrylic acid Substances 0.000 description 2
- 229920000570 polyether Polymers 0.000 description 2
- 229920000573 polyethylene Polymers 0.000 description 2
- 238000010094 polymer processing Methods 0.000 description 2
- 229920000915 polyvinyl chloride Polymers 0.000 description 2
- 239000000126 substance Substances 0.000 description 2
- 238000006467 substitution reaction Methods 0.000 description 2
- 238000001356 surgical procedure Methods 0.000 description 2
- 238000012546 transfer Methods 0.000 description 2
- 238000002054 transplantation Methods 0.000 description 2
- 125000000391 vinyl group Chemical group [H]C([*])=C([H])[H] 0.000 description 2
- 239000011800 void material Substances 0.000 description 2
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 2
- 229920001661 Chitosan Polymers 0.000 description 1
- VEXZGXHMUGYJMC-UHFFFAOYSA-M Chloride anion Chemical compound [Cl-] VEXZGXHMUGYJMC-UHFFFAOYSA-M 0.000 description 1
- 102000008186 Collagen Human genes 0.000 description 1
- 108010035532 Collagen Proteins 0.000 description 1
- 239000004803 Di-2ethylhexylphthalate Substances 0.000 description 1
- 229920002943 EPDM rubber Polymers 0.000 description 1
- VGGSQFUCUMXWEO-UHFFFAOYSA-N Ethene Chemical compound C=C VGGSQFUCUMXWEO-UHFFFAOYSA-N 0.000 description 1
- 239000005977 Ethylene Substances 0.000 description 1
- 229920000181 Ethylene propylene rubber Polymers 0.000 description 1
- 206010016807 Fluid retention Diseases 0.000 description 1
- 208000032843 Hemorrhage Diseases 0.000 description 1
- 244000043261 Hevea brasiliensis Species 0.000 description 1
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 1
- 241001124569 Lycaenidae Species 0.000 description 1
- 241001465754 Metazoa Species 0.000 description 1
- 229920000459 Nitrile rubber Polymers 0.000 description 1
- 239000005062 Polybutadiene Substances 0.000 description 1
- 229920002614 Polyether block amide Polymers 0.000 description 1
- 239000004721 Polyphenylene oxide Substances 0.000 description 1
- 239000004743 Polypropylene Substances 0.000 description 1
- 239000004793 Polystyrene Substances 0.000 description 1
- 239000004820 Pressure-sensitive adhesive Substances 0.000 description 1
- 206010058141 Skin graft rejection Diseases 0.000 description 1
- 239000002174 Styrene-butadiene Substances 0.000 description 1
- 206010060872 Transplant failure Diseases 0.000 description 1
- BZHJMEDXRYGGRV-UHFFFAOYSA-N Vinyl chloride Chemical compound ClC=C BZHJMEDXRYGGRV-UHFFFAOYSA-N 0.000 description 1
- 238000002679 ablation Methods 0.000 description 1
- DHKHKXVYLBGOIT-UHFFFAOYSA-N acetaldehyde Diethyl Acetal Natural products CCOC(C)OCC DHKHKXVYLBGOIT-UHFFFAOYSA-N 0.000 description 1
- 150000001241 acetals Chemical class 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- 150000007513 acids Chemical class 0.000 description 1
- NIXOWILDQLNWCW-UHFFFAOYSA-N acrylic acid group Chemical group C(C=C)(=O)O NIXOWILDQLNWCW-UHFFFAOYSA-N 0.000 description 1
- 230000003213 activating effect Effects 0.000 description 1
- 239000012790 adhesive layer Substances 0.000 description 1
- 230000002411 adverse Effects 0.000 description 1
- 239000003242 anti bacterial agent Substances 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- 239000012298 atmosphere Substances 0.000 description 1
- 230000001580 bacterial effect Effects 0.000 description 1
- 230000033228 biological regulation Effects 0.000 description 1
- BJQHLKABXJIVAM-UHFFFAOYSA-N bis(2-ethylhexyl) phthalate Chemical compound CCCCC(CC)COC(=O)C1=CC=CC=C1C(=O)OCC(CC)CCCC BJQHLKABXJIVAM-UHFFFAOYSA-N 0.000 description 1
- 208000034158 bleeding Diseases 0.000 description 1
- 230000000740 bleeding effect Effects 0.000 description 1
- MTAZNLWOLGHBHU-UHFFFAOYSA-N butadiene-styrene rubber Chemical compound C=CC=C.C=CC1=CC=CC=C1 MTAZNLWOLGHBHU-UHFFFAOYSA-N 0.000 description 1
- 229920005549 butyl rubber Polymers 0.000 description 1
- 239000003575 carbonaceous material Substances 0.000 description 1
- 238000005266 casting Methods 0.000 description 1
- 239000001913 cellulose Substances 0.000 description 1
- 229920002678 cellulose Polymers 0.000 description 1
- 239000003153 chemical reaction reagent Substances 0.000 description 1
- 229920001436 collagen Polymers 0.000 description 1
- 150000001875 compounds Chemical class 0.000 description 1
- 239000000356 contaminant Substances 0.000 description 1
- 238000012258 culturing Methods 0.000 description 1
- 230000006378 damage Effects 0.000 description 1
- 230000035614 depigmentation Effects 0.000 description 1
- 210000004207 dermis Anatomy 0.000 description 1
- 201000010099 disease Diseases 0.000 description 1
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 1
- 238000009826 distribution Methods 0.000 description 1
- 229920001971 elastomer Polymers 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 239000003256 environmental substance Substances 0.000 description 1
- 235000020774 essential nutrients Nutrition 0.000 description 1
- HQQADJVZYDDRJT-UHFFFAOYSA-N ethene;prop-1-ene Chemical group C=C.CC=C HQQADJVZYDDRJT-UHFFFAOYSA-N 0.000 description 1
- 238000001125 extrusion Methods 0.000 description 1
- 239000004744 fabric Substances 0.000 description 1
- 229920005570 flexible polymer Polymers 0.000 description 1
- 239000006261 foam material Substances 0.000 description 1
- 239000004088 foaming agent Substances 0.000 description 1
- 230000036074 healthy skin Effects 0.000 description 1
- 238000010438 heat treatment Methods 0.000 description 1
- 229910052739 hydrogen Inorganic materials 0.000 description 1
- 239000001257 hydrogen Substances 0.000 description 1
- 230000002706 hydrostatic effect Effects 0.000 description 1
- 238000002955 isolation Methods 0.000 description 1
- 238000003754 machining Methods 0.000 description 1
- 238000004519 manufacturing process Methods 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 239000000203 mixture Substances 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 238000000465 moulding Methods 0.000 description 1
- 239000002105 nanoparticle Substances 0.000 description 1
- 229920003052 natural elastomer Polymers 0.000 description 1
- 229920001194 natural rubber Polymers 0.000 description 1
- 210000000056 organ Anatomy 0.000 description 1
- 244000052769 pathogen Species 0.000 description 1
- 230000035699 permeability Effects 0.000 description 1
- 229920001084 poly(chloroprene) Polymers 0.000 description 1
- 229920002857 polybutadiene Polymers 0.000 description 1
- 229920001195 polyisoprene Polymers 0.000 description 1
- 229920006254 polymer film Polymers 0.000 description 1
- 229920001155 polypropylene Polymers 0.000 description 1
- 229920002223 polystyrene Polymers 0.000 description 1
- 229920006264 polyurethane film Polymers 0.000 description 1
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 1
- 239000001267 polyvinylpyrrolidone Substances 0.000 description 1
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 230000008439 repair process Effects 0.000 description 1
- 239000005060 rubber Substances 0.000 description 1
- 150000003839 salts Chemical class 0.000 description 1
- 230000035807 sensation Effects 0.000 description 1
- 235000019615 sensations Nutrition 0.000 description 1
- 239000013464 silicone adhesive Substances 0.000 description 1
- 229920002379 silicone rubber Polymers 0.000 description 1
- 229910052709 silver Inorganic materials 0.000 description 1
- 239000004332 silver Substances 0.000 description 1
- 230000037380 skin damage Effects 0.000 description 1
- 239000007779 soft material Substances 0.000 description 1
- 239000011115 styrene butadiene Substances 0.000 description 1
- 229920003048 styrene butadiene rubber Polymers 0.000 description 1
- 230000001225 therapeutic effect Effects 0.000 description 1
- 210000000689 upper leg Anatomy 0.000 description 1
- 239000002699 waste material Substances 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/10—Hair or skin implants
- A61F2/105—Skin implants, e.g. artificial skin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods, e.g. tourniquets
- A61B17/32—Surgical cutting instruments
- A61B17/322—Skin grafting apparatus
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F13/00—Bandages or dressings; Absorbent pads
- A61F13/00051—Accessories for dressings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F13/00—Bandages or dressings; Absorbent pads
- A61F13/02—Adhesive bandages or dressings
- A61F13/0203—Adhesive bandages or dressings with fluid retention members
- A61F13/0206—Adhesive bandages or dressings with fluid retention members with absorbent fibrous layers, e.g. woven or non-woven absorbent pads or island dressings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F13/00—Bandages or dressings; Absorbent pads
- A61F13/02—Adhesive bandages or dressings
- A61F13/0203—Adhesive bandages or dressings with fluid retention members
- A61F13/0213—Adhesive bandages or dressings with fluid retention members the fluid retention member being a layer of hydrocolloid, gel forming material
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F13/00—Bandages or dressings; Absorbent pads
- A61F13/02—Adhesive bandages or dressings
- A61F13/0203—Adhesive bandages or dressings with fluid retention members
- A61F13/0223—Adhesive bandages or dressings with fluid retention members characterized by parametric properties of the fluid retention layer, e.g. absorbency, wicking capacity, liquid distribution
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F13/00—Bandages or dressings; Absorbent pads
- A61F13/02—Adhesive bandages or dressings
- A61F13/0203—Adhesive bandages or dressings with fluid retention members
- A61F13/0226—Adhesive bandages or dressings with fluid retention members characterised by the support layer
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F13/00—Bandages or dressings; Absorbent pads
- A61F13/02—Adhesive bandages or dressings
- A61F13/0246—Adhesive bandages or dressings characterised by the skin-adhering layer
- A61F13/025—Adhesive bandages or dressings characterised by the skin-adhering layer having a special distribution arrangement of the adhesive
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F13/00—Bandages or dressings; Absorbent pads
- A61F13/02—Adhesive bandages or dressings
- A61F13/0259—Adhesive bandages or dressings characterised by the release liner covering the skin adhering layer
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/0095—Packages or dispensers for prostheses or other implants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods, e.g. tourniquets
- A61B17/32—Surgical cutting instruments
- A61B17/322—Skin grafting apparatus
- A61B2017/3225—Skin grafting apparatus with processing of harvested tissue
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Engineering & Computer Science (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Heart & Thoracic Surgery (AREA)
- Biomedical Technology (AREA)
- Vascular Medicine (AREA)
- Transplantation (AREA)
- Dermatology (AREA)
- Cardiology (AREA)
- Oral & Maxillofacial Surgery (AREA)
- Surgery (AREA)
- Chemical & Material Sciences (AREA)
- Dispersion Chemistry (AREA)
- Plastic & Reconstructive Surgery (AREA)
- Molecular Biology (AREA)
- Medical Informatics (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Prostheses (AREA)
- Materials For Medical Uses (AREA)
- Hematology (AREA)
- Materials Engineering (AREA)
- Epidemiology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Media Introduction/Drainage Providing Device (AREA)
- Laminated Bodies (AREA)
Description
本出願は、2013年7月31日出願の米国仮特許出願第61/860,822号(「Absorbent Substrates For Harvesting Skin Grafts」)、および2013年3月14日出願の米国仮特許出願第61/782,385号(「Absorbent Dressing With Hybrid Drape」)の優先権を主張し、その開示全体を、参照することにより本書に援用する。
Claims (18)
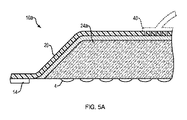
- 皮膚移植片を移植するための吸収性基材において、
複数の細孔を有するベース層であって、少なくとも1つの切除された皮膚移植片と接触しかつドナー部位から除去するために前記移植片を捕捉するように適合された少なくとも1つの接着剤部位を有するベース層と;
前記ベース層に周辺で接合されかつそれとの間にシールされた囲いを規定するシーリング部材と;
前記シールされた囲い内に配置された吸収性材料と、
前記囲いにおいて前記ベース層と前記吸収性材料との間に配置された少なくとも第1のウィッキング層と
を含み、
前記第1のウィッキング層が、前記第1のウィッキング層の表面に沿って横方向に流体を輸送するように適合された粒状構造を有し、第2のウィッキング層が、前記シーリング部材に隣接して配置され、前記第1及び第2のウィッキング層が接合されて前記吸収性材料を囲むシールを形成し、
前記ベース層の少なくとも一部分は前記吸収性材料と流体連通して、流体を捕捉することを特徴とする、吸収性基材。 - 請求項1に記載の基材において、前記ベース層が、シリコーンなどの生体適合性材料を含み、かつ皮膚移植片採取装置の室内に位置決めされるように構成されており、前記ベース層が、複数の皮膚移植片を同時に捕捉するように構成されていることを特徴とする、基材。
- 請求項2に記載の基材において、前記生体適合性材料が、シリコーン、シリコーンゲル、軟質シリコーン、親水コロイド、ヒドロゲル、ポリウレタン、ポリウレタンゲル、ポリオレフィン、ポリオレフィンゲル、水素化スチレン共重合体、水素化スチレン共重合体ゲル、ゲル発泡体およびこれらの組み合わせからなる群から選択されることを特徴とする、基材。
- 請求項1乃至3の何れか1項に記載の基材において、前記ベース層の前記少なくとも1つの接着剤部位の平均厚さが、500ミクロン(μm)〜1000ミクロン(μm)であり、および、硬度が、5ショアOO〜80ショアOOであるか、またはそれらの組み合わせであることを特徴とする、基材。
- 請求項1乃至4の何れか1項に記載の基材において、前記基材が、さらに、前記少なくとも1つの接着剤部位に関連付けられた接着剤を含み、および、前記少なくとも1つの接着剤部位は、複数の接着剤部位を含み、各部位は、皮膚移植片採取装置によって隆起された対応する皮膚移植片に係合するように配置および構成されていることを特徴とする、基材。
- 請求項5に記載の基材において、前記接着剤がアクリル接着剤を含むことを特徴とする、基材。
- 請求項1乃至6の何れか1項に記載の基材において、前記ベース層が、さらに、複数の細孔を含んで、レシピエント部位から前記吸収性材料へ流体を輸送するための通路をもたらし、および前記ベース層は、さらに、発泡ポリマー、流体透過性のポリマー性材料、または繊維性材料を含むことを特徴とする、基材。
- 請求項7に記載の基材において、前記ベース層は前記発泡ポリマー又は前記流体透過性のポリマー性材料ではなく繊維性材料を含み、前記繊維性材料が、平均直径が0.1マイクロメートル〜10マイクロメートルであるか、または平均直径が1ナノメートル〜100ナノメートルである複数のマイクロファイバーを含むことを特徴とする、基材。
- 請求項8に記載の基材において、前記ベース層が、さらに、少なくともいくつかの前記捕捉部位間に配置された細孔のネットワークを含み、および前記細孔が、全体的に円形であり、かつその平均的な横断面寸法が1ナノメートル〜1ミリメートルに及ぶことを特徴とする、基材。
- 請求項1に記載の基材において、さらに、前記囲いにおいて前記ベース層と前記吸収性材料との間に配置されかつ前記吸収性材料に流体を分配するように適合された少なくとも第1のウィッキング層を含むことを特徴とする、基材。
- 請求項10に記載の基材において、さらに、前記囲いにおいて前記吸収性材料と前記シーリング部材との間に配置された少なくとも第2のウィッキング層を含むことを特徴とする、基材。
- 請求項11に記載の基材において、前記第1のウィッキング層が、前記第1のウィッキング層の面に沿って流体を吸い上げるように適合された粒状構造を有し、および前記第2のウィッキング層が、前記第2のウィッキング層の面に沿って流体を吸い上げるように適合された粒状構造を有し;および、前記第1のウィッキング層の周辺部分が前記第2のウィッキング層の周辺部分に結合され、前記第1のウィッキング層と前記第2のウィッキング層との間の前記吸収性層を取り囲むウィッキング層の囲いを提供することを特徴とする、基材。
- 請求項12に記載の基材において、前記吸収性材料が複数の吸収性層を含み、および前記複数の吸収性層が、前記第1のウィッキング層と前記第2のウィッキング層との間に流体連通されて位置決めされていることを特徴とする、基材。
- 請求項13に記載の基材において、さらに、前記吸収性層間を流体連通させて配置された少なくとも1つの中間ウィッキング層を含むことを特徴とする、基材。
- 請求項1乃至14の何れか1項に記載の基材において、前記吸収性材料が、流体を吸収するように適合されている親水性材料を含み;および前記シーリング部材が液不透過性であり、かつポリウレタンを含むことを特徴とする、基材。
- 請求項2又は3に記載の基材において、さらに、前記基材から、溜まった流体を抜き取るために、減圧源に結合するための少なくとも1つのポートを含み;前記少なくとも1つのポートが、さらに、前記室内の前記吸収性材料または少なくとも1つのウィッキング層から外部流体レセプタクルまでの流体連通をもたらす弁または導管を含むことを特徴とする、基材。
- 請求項1乃至16の何れか1項に記載の基材において、さらに、皮膚移植片ハーベスター内にまたはレシピエント部位に位置決めする前に前記基材を取り扱うために、少なくとも1つの取り外し可能なバッキング、または皮膚移植片ハーベスター内に位置決めする前に前記基材を取り扱うために、前記ベース層に関連付けられた少なくとも第1の取り外し可能なバッキングと、レシピエント部位に位置決めする前に前記基材および関連の皮膚移植片を取り扱うために、第2の取り外し可能なバッキングとを含むことを特徴とする、基材。
- 請求項1乃至17の何れか1項に記載の基材において、前記ベース層および前記吸収性材料が、一体的構造として形成されていることを特徴とする、基材。
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201361782385P | 2013-03-14 | 2013-03-14 | |
| US61/782,385 | 2013-03-14 | ||
| US201361860822P | 2013-07-31 | 2013-07-31 | |
| US61/860,822 | 2013-07-31 | ||
| PCT/US2014/027205 WO2014152319A2 (en) | 2013-03-14 | 2014-03-14 | Absorbent substrates for harvesting skin grafts |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2018207340A Division JP2019048104A (ja) | 2013-03-14 | 2018-11-02 | 皮膚移植片採取用の吸収性基材 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2016512138A JP2016512138A (ja) | 2016-04-25 |
| JP6491188B2 true JP6491188B2 (ja) | 2019-03-27 |
Family
ID=51531295
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2016502366A Expired - Fee Related JP6491188B2 (ja) | 2013-03-14 | 2014-03-14 | 皮膚移植片採取用の吸収性基材 |
| JP2018207340A Pending JP2019048104A (ja) | 2013-03-14 | 2018-11-02 | 皮膚移植片採取用の吸収性基材 |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2018207340A Pending JP2019048104A (ja) | 2013-03-14 | 2018-11-02 | 皮膚移植片採取用の吸収性基材 |
Country Status (7)
| Country | Link |
|---|---|
| US (2) | US9962254B2 (ja) |
| EP (2) | EP3289988A1 (ja) |
| JP (2) | JP6491188B2 (ja) |
| CN (1) | CN105636532B (ja) |
| AU (1) | AU2014239952B2 (ja) |
| CA (1) | CA2905481C (ja) |
| WO (1) | WO2014152319A2 (ja) |
Families Citing this family (40)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| BRPI0919260B8 (pt) | 2008-09-24 | 2021-06-22 | Massachusetts Inst Technology | equipamentos para formar pluralidade de tecidos de enxerto, para expandir membrana, para formar pluralidade de microenxertos a partir de pelo menos parte de tecido de enxerto e para produzir bolhas de sucção e método para afetar região de pele |
| US8978234B2 (en) * | 2011-12-07 | 2015-03-17 | MoMelan Technologies, Inc. | Methods of manufacturing devices for generating skin grafts |
| US9610093B2 (en) | 2010-08-06 | 2017-04-04 | Kci Licensing, Inc. | Microblister skin grafting |
| US9597111B2 (en) | 2010-08-06 | 2017-03-21 | Kci Licensing, Inc. | Methods for applying a skin graft |
| WO2013066426A2 (en) | 2011-06-24 | 2013-05-10 | Kci Licensing, Inc. | Reduced-pressure dressings employing tissue-fixation elements |
| AU2014239952B2 (en) | 2013-03-14 | 2018-09-27 | Solventum Intellectual Properties Company | Absorbent substrates for harvesting skin grafts |
| US9180001B2 (en) * | 2013-08-16 | 2015-11-10 | C.R. Bard, Inc. | Methods of breast surgery |
| EP3243453B1 (en) | 2013-12-31 | 2019-10-09 | KCI Licensing, Inc. | Skin graft harvesting device |
| EP3089681B1 (en) | 2013-12-31 | 2021-09-08 | 3M Innovative Properties Company | Fluid-assisted skin graft harvesting |
| EP3804774A1 (en) * | 2015-04-09 | 2021-04-14 | 3M Innovative Properties Co. | System for harvesting skin grafts |
| GB2537841B (en) * | 2015-04-27 | 2020-12-09 | Medtrade Products Ltd | Wound dressing |
| CN107530531B (zh) | 2015-04-27 | 2021-07-13 | 史密夫及内修公开有限公司 | 减压装置 |
| US11006974B2 (en) | 2015-11-03 | 2021-05-18 | Kci Licensing, Inc. | Devices for creating an epidermal graft sheet |
| CA3009878A1 (en) * | 2015-12-30 | 2017-07-06 | Smith & Nephew Plc | Negative pressure wound therapy apparatus |
| SE541418C2 (en) | 2016-01-22 | 2019-09-24 | S2Medical Ab | Minimally invasive tissue harvesting device |
| JP6911043B2 (ja) | 2016-03-07 | 2021-07-28 | スミス アンド ネフュー ピーエルシーSmith & Nephew Public Limited Company | 陰圧源が創傷被覆材内に一体化された創傷治療装置及び方法 |
| DE102016106097B3 (de) * | 2016-04-04 | 2017-05-18 | Leibniz-Institut Für Polymerforschung Dresden E.V. | Gewebe- und Organtransportvorrichtung |
| CN109121396B (zh) | 2016-04-26 | 2022-04-05 | 史密夫及内修公开有限公司 | 与具有流体侵入抑制部件的一体化负压源一起使用的伤口敷料和方法 |
| CA3038206A1 (en) | 2016-05-03 | 2017-11-09 | Smith & Nephew Plc | Optimizing power transfer to negative pressure sources in negative pressure therapy systems |
| US11305047B2 (en) | 2016-05-03 | 2022-04-19 | Smith & Nephew Plc | Systems and methods for driving negative pressure sources in negative pressure therapy systems |
| US11096831B2 (en) | 2016-05-03 | 2021-08-24 | Smith & Nephew Plc | Negative pressure wound therapy device activation and control |
| CA3034789A1 (en) | 2016-08-25 | 2018-03-01 | Smith & Nephew Plc | Absorbent negative pressure wound therapy dressing |
| EP3519001A1 (en) | 2016-09-30 | 2019-08-07 | Smith & Nephew PLC | Negative pressure wound treatment apparatuses and methods with integrated electronics |
| WO2018162613A1 (en) | 2017-03-08 | 2018-09-13 | Smith & Nephew Plc | Negative pressure wound therapy device control in presence of fault condition |
| DE102017106310B4 (de) | 2017-03-23 | 2018-10-11 | Lavenir Bioscience Ag | Vorrichtung und Verfahren zur Erzeugung einer Mikrograftmatrix aus Vollhaut |
| AU2018265052B2 (en) | 2017-05-09 | 2023-08-31 | Smith & Nephew Plc | Redundant controls for negative pressure wound therapy systems |
| EP3634513B1 (en) * | 2017-05-12 | 2021-06-30 | KCI USA, Inc. | Adherent, bioresorbable transplant substrate |
| CN107496053A (zh) * | 2017-08-11 | 2017-12-22 | 京东方科技集团股份有限公司 | 电子皮肤、制作方法和驱动方法 |
| US10559742B1 (en) * | 2017-08-24 | 2020-02-11 | Mainstream Engineering Corporation | Mounting pad and method for deterring theft and securing air conditioning units against high winds |
| US11701265B2 (en) | 2017-09-13 | 2023-07-18 | Smith & Nephew Plc | Negative pressure wound treatment apparatuses and methods with integrated electronics |
| GB201718070D0 (en) | 2017-11-01 | 2017-12-13 | Smith & Nephew | Negative pressure wound treatment apparatuses and methods with integrated electronics |
| GB201718072D0 (en) | 2017-11-01 | 2017-12-13 | Smith & Nephew | Negative pressure wound treatment apparatuses and methods with integrated electronics |
| EP3703632B1 (en) | 2017-11-01 | 2024-04-03 | Smith & Nephew plc | Negative pressure wound treatment apparatuses and methods with integrated electronics |
| GB201718054D0 (en) | 2017-11-01 | 2017-12-13 | Smith & Nephew | Sterilization of integrated negative pressure wound treatment apparatuses and sterilization methods |
| WO2019108738A1 (en) * | 2017-11-29 | 2019-06-06 | Kci Licensing, Inc. | Nanomodified transfer drape for epidermal grafting |
| USD898925S1 (en) | 2018-09-13 | 2020-10-13 | Smith & Nephew Plc | Medical dressing |
| GB201903774D0 (en) | 2019-03-20 | 2019-05-01 | Smith & Nephew | Negative pressure wound treatment apparatuses and methods with integrated electronics |
| EP3721822A1 (en) * | 2019-04-11 | 2020-10-14 | ETH Zurich | Device for taking essentially two-dimensional plane material sections, tensioning device, system for tensioning |
| GB201907716D0 (en) | 2019-05-31 | 2019-07-17 | Smith & Nephew | Systems and methods for extending operational time of negative pressure wound treatment apparatuses |
| US20220015950A1 (en) * | 2020-07-15 | 2022-01-20 | Joseph Smith | Bandage with removable cap |
Family Cites Families (136)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2579039A (en) | 1943-05-17 | 1951-12-18 | Us Electrical Motors Inc | Lubricating system |
| US2379574A (en) | 1943-11-05 | 1945-07-03 | Claude R Wickard | Method of producing surgical bandages with improved elastic properties |
| US2721555A (en) | 1952-12-03 | 1955-10-25 | John A Jenney | Dermatome |
| US3054404A (en) | 1961-08-14 | 1962-09-18 | Cicero P Meek | Skin graft applicator |
| US3782387A (en) | 1972-02-29 | 1974-01-01 | R Falabella | Apparatus and methods for obtaining and making skin grafts |
| US4345374A (en) | 1974-01-14 | 1982-08-24 | The Gillette Company | Razor with means to adjust blade geometry |
| SU772544A1 (ru) | 1979-01-04 | 1980-10-23 | Тартусский Ордена Трудового Красного Знамени Государственный Университет | Дерматом |
| EP0099748B1 (en) * | 1982-07-21 | 1987-05-27 | Smith and Nephew Associated Companies p.l.c. | Adhesive wound dressing |
| DE3247387C2 (de) | 1982-12-22 | 1984-11-22 | Rolf Prof. Dr.med. 7400 Tübingen Hettich | Verfahren zur Herstellung eines Transplantats und Vorrichtung zur Durchführung des Verfahrens |
| US4605010A (en) | 1984-05-17 | 1986-08-12 | Western Clinical Engineering Ltd. | Pressurizing cuff |
| GB8428921D0 (en) | 1984-11-15 | 1984-12-27 | Gillette Co | Safety razor blades |
| US4600533A (en) * | 1984-12-24 | 1986-07-15 | Collagen Corporation | Collagen membranes for medical use |
| US4666447A (en) | 1985-01-30 | 1987-05-19 | Mentor Corporation | Skin expansion device and method of making the same |
| US5460939A (en) | 1986-04-18 | 1995-10-24 | Advanced Tissue Sciences, Inc. | Temporary living skin replacement |
| US5015584A (en) | 1987-10-14 | 1991-05-14 | Board Of Regents, The University Of Texas System | Epidermal graft system |
| US4917086A (en) | 1988-05-26 | 1990-04-17 | Snyder Laboratories, Inc. | Dermatome blade assembly |
| IE911869A1 (en) | 1990-06-01 | 1991-12-04 | Regeneron Pharma | A family of map2 protein kinases |
| SE9101022D0 (sv) | 1991-01-09 | 1991-04-08 | Paal Svedman | Medicinsk suganordning |
| US5163955A (en) | 1991-01-24 | 1992-11-17 | Autogenics | Rapid assembly, concentric mating stent, tissue heart valve with enhanced clamping and tissue alignment |
| US5329846A (en) | 1991-08-12 | 1994-07-19 | Bonutti Peter M | Tissue press and system |
| US6503277B2 (en) | 1991-08-12 | 2003-01-07 | Peter M. Bonutti | Method of transplanting human body tissue |
| US6436078B1 (en) | 1994-12-06 | 2002-08-20 | Pal Svedman | Transdermal perfusion of fluids |
| CN2125374U (zh) | 1992-06-12 | 1992-12-23 | 王诚 | 负压自动取皮机 |
| US6251100B1 (en) | 1993-09-24 | 2001-06-26 | Transmedica International, Inc. | Laser assisted topical anesthetic permeation |
| US5386633A (en) | 1993-12-27 | 1995-02-07 | Kanno; Yukio | Hamburger patty knife with blade attachment |
| US5476478A (en) | 1994-04-18 | 1995-12-19 | Providence Hospital | Preoperative skin stretching apparatus and method |
| US5489304A (en) | 1994-04-19 | 1996-02-06 | Brigham & Women's Hospital | Method of skin regeneration using a collagen-glycosaminoglycan matrix and cultured epithelial autograft |
| EP0750473A1 (en) | 1994-04-21 | 1997-01-02 | Medchem Products, Inc. | Skin stretching device |
| US5496339A (en) | 1994-05-17 | 1996-03-05 | Koepnick; Russell G. | Universal automated keratectomy apparatus and method |
| US5433221A (en) | 1994-10-05 | 1995-07-18 | Adair; Edwin L. | Windowed self-centering drape for surgical camera |
| US5571098A (en) | 1994-11-01 | 1996-11-05 | The General Hospital Corporation | Laser surgical devices |
| US5595570A (en) | 1994-12-06 | 1997-01-21 | S.C.M.D., Ltd. | Keratome with spring loaded adjustable plate, cutting length adjustment plate, method of cutting a corneal flap, and gauge-mounted bracket for adjusting plate on keratome head |
| US5520635A (en) | 1994-12-16 | 1996-05-28 | Gelbfish; Gary A. | Method and associated device for removing clot |
| US5686303A (en) | 1994-12-30 | 1997-11-11 | Korman; Joshua | Method of growing vertebrate skin in vitro |
| US6693077B1 (en) | 1995-02-14 | 2004-02-17 | Human Genome Sciences, Inc. | Keratinocyte growth factor-2 |
| GB9508606D0 (en) | 1995-04-27 | 1995-06-14 | Svedman Paul | Suction blister sampling |
| US5792173A (en) | 1995-07-10 | 1998-08-11 | Stuart D. Edwards | Wound closure hemostasis device |
| US5817115A (en) | 1995-12-04 | 1998-10-06 | Chiron Vision Corporation | Apparatus for resecting corneal tissue |
| US6080166A (en) | 1995-12-05 | 2000-06-27 | Mcewen; James Allen | Direct linear drive dermatome |
| IL116282A (en) | 1995-12-07 | 2000-10-31 | L R Surgical Instr Ltd | Adjustable mesher device and a system for using the same |
| US6071247A (en) | 1996-07-21 | 2000-06-06 | Kennedy; William R. | Skin blister biopsy apparatus and method |
| NL1004276C2 (nl) | 1996-10-15 | 1998-04-20 | Willem Marie Ysebaert | Werkwijzen voor het vervaardigen van huideilandjes, voor het verplaatsen van huid of huideilandjes, voor het spreiden van huideilandjes en het aanbrengen hiervan op een brandwond, alsmede een houder, snijraam, snijtafel, contradrager, klemorgaan, membraan, transportorgaan en spreidingsorgaan om te worden toegepast voor dergelijke werkwijzen. |
| US6626901B1 (en) | 1997-03-05 | 2003-09-30 | The Trustees Of Columbia University In The City Of New York | Electrothermal instrument for sealing and joining or cutting tissue |
| AU8224598A (en) | 1997-06-26 | 1999-01-19 | Smith & Nephew Plc | Cell culture products |
| US5858019A (en) * | 1997-10-15 | 1999-01-12 | Ashraf; Bahman | Graft site cutter |
| US6610075B1 (en) | 1997-10-24 | 2003-08-26 | Becton, Dickenson And Company | Keratome with suspended stabilized blade, improved suction ring with applanator and guided engagement with keratome cutter head, automated translation of the cutter head, and blade insertion tool |
| US5972476A (en) | 1997-11-21 | 1999-10-26 | Means Industries, Inc. | Laminated parts and method of making same |
| US5921980A (en) | 1997-12-03 | 1999-07-13 | University Of Kentucky Research Foundation | Laser skin graft harvesting apparatus and related method |
| US6071267A (en) | 1998-02-06 | 2000-06-06 | Kinetic Concepts, Inc. | Medical patient fluid management interface system and method |
| US6358260B1 (en) | 1998-04-20 | 2002-03-19 | Med-Logics, Inc. | Automatic corneal shaper with two separate drive mechanisms |
| US7540875B2 (en) | 1998-06-01 | 2009-06-02 | Avatar Design & Development, Inc. | Surgical cutting tool with automatically retractable blade assembly |
| US6402770B1 (en) | 1998-06-01 | 2002-06-11 | Avatar Design & Development, Inc. | Method and apparatus for placing and maintaining a percutaneous tube into a body cavity |
| US6599305B1 (en) | 1998-08-12 | 2003-07-29 | Vladimir Feingold | Intracorneal lens placement method and apparatus |
| US6083236A (en) | 1998-08-12 | 2000-07-04 | Feingold; Vladimir | Keratome method and apparatus |
| US6165189A (en) | 1999-02-10 | 2000-12-26 | Sis, Ltd. | Microkeratome for performing lasik surgery |
| US6585939B1 (en) | 1999-02-26 | 2003-07-01 | Orchid Biosciences, Inc. | Microstructures for use in biological assays and reactions |
| IL138710A0 (en) | 1999-10-15 | 2001-10-31 | Newman Martin H | Atomically sharp edge cutting blades and method for making same |
| WO2001097866A2 (en) | 2000-06-22 | 2001-12-27 | Visualization Technology, Inc. | Windowed medical drape |
| CA2415102C (en) | 2000-06-30 | 2010-09-14 | I.V. House, Inc. | Infusion site guard |
| DE10051215A1 (de) | 2000-10-16 | 2002-05-08 | Gebauer Gmbh | Klinge mit amorpher Schneidkante |
| US6629983B1 (en) | 2000-10-27 | 2003-10-07 | Edge Systems Corporation | Apparatus and method for skin/surface abrasion |
| US7078582B2 (en) | 2001-01-17 | 2006-07-18 | 3M Innovative Properties Company | Stretch removable adhesive articles and methods |
| US7666192B2 (en) | 2001-02-16 | 2010-02-23 | Kci Licensing, Inc. | Skin grafting devices and methods |
| US7700819B2 (en) * | 2001-02-16 | 2010-04-20 | Kci Licensing, Inc. | Biocompatible wound dressing |
| US7651507B2 (en) | 2003-03-03 | 2010-01-26 | Kci Licensing, Inc. | Tissue processing system |
| AU2002326781A1 (en) | 2001-08-29 | 2003-03-18 | Artemis Medical, Inc. | Undamaged tissue collection assembly and method |
| US7513902B2 (en) | 2001-10-01 | 2009-04-07 | The Cleveland Clinic Foundation | Skin lesion exciser and skin-closure device therefor |
| US7468242B2 (en) | 2001-11-05 | 2008-12-23 | Medgenics, Inc. | Dermal micro organs, methods and apparatuses for producing and using the same |
| WO2003040686A2 (en) | 2001-11-05 | 2003-05-15 | Medgenics, Inc. | Dosing and administration of therapeutic micro-organs in living subjects and devices and methods for same |
| US8466116B2 (en) | 2001-12-20 | 2013-06-18 | The Unites States Of America As Represented By The Secretary Of The Department Of Health And Human Services | Use of CpG oligodeoxynucleotides to induce epithelial cell growth |
| AU2003235675A1 (en) | 2002-01-15 | 2003-07-30 | I.V. House, Inc. | Site guard for intravenous sites and other sensitive areas |
| WO2003068120A1 (en) * | 2002-01-18 | 2003-08-21 | Levin John M | Force-transmission-control system and devices employing same |
| DE10209122B4 (de) | 2002-03-01 | 2006-04-13 | Fleischmann, Wilhelm, Dr.med. | Instrument zur Gewebedehnung der Haut |
| EP1549738B1 (en) | 2002-04-30 | 2010-04-21 | Stratatech Corporation | Keratinocytes expressing exogenous angiogenic growth factors |
| US20030212357A1 (en) | 2002-05-10 | 2003-11-13 | Pace Edgar Alan | Method and apparatus for treating wounds with oxygen and reduced pressure |
| US7666134B2 (en) | 2002-09-28 | 2010-02-23 | Kci Licensing, Inc. | System and method for transplantation of dermal tissue |
| US7964390B2 (en) | 2002-10-11 | 2011-06-21 | Case Western Reserve University | Sensor system |
| US7976519B2 (en) * | 2002-12-31 | 2011-07-12 | Kci Licensing, Inc. | Externally-applied patient interface system and method |
| WO2004071313A2 (en) | 2003-02-10 | 2004-08-26 | Applied Tissue Technologies, Llc | Apparatus for dermal tissue harvesting |
| CN2596950Y (zh) * | 2003-02-24 | 2004-01-07 | 孙文红 | 一种表皮取皮器 |
| US7625384B2 (en) | 2003-02-27 | 2009-12-01 | Wright Medical Technology, Inc. | Method and apparatus for processing dermal tissue |
| US7708746B2 (en) | 2003-02-27 | 2010-05-04 | Wright Medical Technology, Inc. | Method and apparatus for processing dermal tissue |
| US20040175690A1 (en) | 2003-03-03 | 2004-09-09 | Kci Licensing, Inc. | Tissue harvesting device and method |
| US7285576B2 (en) * | 2003-03-12 | 2007-10-23 | 3M Innovative Properties Co. | Absorbent polymer compositions, medical articles, and methods |
| US20040181950A1 (en) | 2003-03-17 | 2004-09-23 | Rodgers Murray Steven | Alignment of microkeratome blade to blade handle |
| US7137979B2 (en) | 2003-05-31 | 2006-11-21 | Tyrell, Inc. | Methods and devices for the treatment of skin lesions |
| US6997092B2 (en) | 2003-06-02 | 2006-02-14 | Chau Lih Rong Enterprise Co., Ltd. | Cutting apparatus |
| US10583220B2 (en) | 2003-08-11 | 2020-03-10 | DePuy Synthes Products, Inc. | Method and apparatus for resurfacing an articular surface |
| US20050101972A1 (en) | 2003-11-06 | 2005-05-12 | University Of Florida Research Foundation, Inc. | Devices and systems for separating and preparing skin |
| ATE464855T1 (de) | 2004-03-31 | 2010-05-15 | Cook Inc | Transplantatmaterial und gefässprothese mit extrazellulärer kollagenmatrix und dessen herstellungsverfahren |
| US7790945B1 (en) * | 2004-04-05 | 2010-09-07 | Kci Licensing, Inc. | Wound dressing with absorption and suction capabilities |
| US20050244967A1 (en) | 2004-05-03 | 2005-11-03 | Pearlman Andrew L | Closed automated system for tissue based therapy |
| US8932233B2 (en) | 2004-05-21 | 2015-01-13 | Devicor Medical Products, Inc. | MRI biopsy device |
| EP1765253A4 (en) | 2004-06-09 | 2009-08-19 | Flowmedic Ltd | PORTABLE COMPLETED DEVICE FOR IMPROVING THE CIRCULATION |
| TWI270362B (en) | 2004-12-28 | 2007-01-11 | Ind Tech Res Inst | Cell subcloning device |
| US20060287696A1 (en) | 2005-06-21 | 2006-12-21 | Wright David W | Heat and light therapy treatment device and method |
| WO2007034438A2 (en) | 2005-09-26 | 2007-03-29 | Koninklijke Philips Electronics N.V. | Substance sampling and/or substance delivery via skin |
| US8568333B2 (en) | 2006-05-01 | 2013-10-29 | Devicor Medical Products, Inc. | Grid and rotatable cube guide localization fixture for biopsy device |
| FR2904103B1 (fr) | 2006-07-18 | 2015-05-15 | Airbus France | Dispositif a ecoulement de chaleur |
| US8579852B2 (en) | 2006-09-14 | 2013-11-12 | Omeed Memar | Apparatus and methods for repairing tissue defects |
| CN101053528A (zh) | 2007-06-01 | 2007-10-17 | 张磊 | 一种能分离大面积表皮的吸头 |
| US7946585B2 (en) | 2007-09-26 | 2011-05-24 | T.E. Brangs, Inc. | Mechanical ball projection game devices |
| GB2453308B (en) | 2007-10-03 | 2012-07-25 | Acell Group Ltd | Composite products |
| JP4899019B2 (ja) * | 2007-10-17 | 2012-03-21 | アルケア株式会社 | 傷手当用品とその製造方法 |
| US8002779B2 (en) | 2007-12-13 | 2011-08-23 | Zimmer Surgical, Inc. | Dermatome blade assembly |
| CN201139613Y (zh) * | 2008-01-22 | 2008-10-29 | 耿文军 | 表皮移植器 |
| US20110251602A1 (en) | 2008-04-01 | 2011-10-13 | The General Hospital Corporation | Method and apparatus for tissue expansion |
| JP5882733B2 (ja) | 2008-04-01 | 2016-03-09 | ザ ジェネラル ホスピタル コーポレイション | 組織移植の方法と装置 |
| WO2010014716A1 (en) | 2008-07-31 | 2010-02-04 | Clevex, Inc. | Unitary shave biopsy blade |
| US20100043694A1 (en) | 2008-08-20 | 2010-02-25 | Patel Gordhanbhai N | Tamper evident indicating devices |
| BRPI0919260B8 (pt) | 2008-09-24 | 2021-06-22 | Massachusetts Inst Technology | equipamentos para formar pluralidade de tecidos de enxerto, para expandir membrana, para formar pluralidade de microenxertos a partir de pelo menos parte de tecido de enxerto e para produzir bolhas de sucção e método para afetar região de pele |
| US8568363B2 (en) | 2008-10-31 | 2013-10-29 | The Invention Science Fund I, Llc | Frozen compositions and methods for piercing a substrate |
| ATE546387T1 (de) | 2009-05-30 | 2012-03-15 | Bayer Innovation Gmbh | Produkt mit bioresorbierbaren trägermaterialien und verpackung |
| US9919072B2 (en) * | 2009-08-21 | 2018-03-20 | Novan, Inc. | Wound dressings, methods of using the same and methods of forming the same |
| US20110077664A1 (en) | 2009-09-28 | 2011-03-31 | Wright Medical Technology, Inc. | Device for processing dermal tissue |
| WO2011059441A1 (en) | 2009-11-13 | 2011-05-19 | Smith & Nephew, Inc. | Mesher |
| US8900181B2 (en) | 2009-12-18 | 2014-12-02 | Srgi Holdings, Llc | Skin treatment and drug delivery device |
| US9061095B2 (en) * | 2010-04-27 | 2015-06-23 | Smith & Nephew Plc | Wound dressing and method of use |
| US20120021186A1 (en) | 2010-06-07 | 2012-01-26 | Uwe Schneider | Seam structure and method for making a seam |
| US8562626B2 (en) | 2010-08-06 | 2013-10-22 | MoMelan Technologies, Inc. | Devices for harvesting a skin graft |
| US8926631B2 (en) * | 2010-08-06 | 2015-01-06 | MoMelan Technologies, Inc. | Methods for preparing a skin graft without culturing or use of biologics |
| US8617181B2 (en) | 2010-08-06 | 2013-12-31 | MoMelan Technologies, Inc. | Methods for preparing a skin graft |
| US8978234B2 (en) | 2011-12-07 | 2015-03-17 | MoMelan Technologies, Inc. | Methods of manufacturing devices for generating skin grafts |
| US9597111B2 (en) | 2010-08-06 | 2017-03-21 | Kci Licensing, Inc. | Methods for applying a skin graft |
| US9173674B2 (en) | 2010-08-06 | 2015-11-03 | MoMelan Technologies, Inc. | Devices for harvesting a skin graft |
| US9610093B2 (en) | 2010-08-06 | 2017-04-04 | Kci Licensing, Inc. | Microblister skin grafting |
| US8226663B2 (en) | 2010-09-22 | 2012-07-24 | Irina Remsburg | Microdermabrasion hand piece providing automatic limitation of skin hyperextension |
| US20120197267A1 (en) | 2011-01-27 | 2012-08-02 | MoMelan Technologies, Inc. | Devices for generating and transferring micrografts and methods of use thereof |
| US9107990B2 (en) * | 2011-02-14 | 2015-08-18 | Kci Licensing, Inc. | Reduced-pressure dressings, systems, and methods for use with linear wounds |
| US9468459B2 (en) * | 2011-04-20 | 2016-10-18 | Kci Licensing, Inc. | Skin graft devices and methods |
| US20130041385A1 (en) | 2011-08-10 | 2013-02-14 | Joseph Giovannoli | Apparatus for skin reduction |
| PT2780073T (pt) | 2011-11-15 | 2017-12-18 | Neurometrix Inc | Aparelho para alívio da dor que utiliza estimulação nervosa elétrica transcutânea |
| CN108272557A (zh) * | 2012-03-01 | 2018-07-13 | 爱乐康株式会社 | 伤口处理用品 |
| JP2015515906A (ja) | 2012-05-09 | 2015-06-04 | メドック リミテッド. | 改良された温度刺激プローブ及び方法 |
| AU2014239952B2 (en) | 2013-03-14 | 2018-09-27 | Solventum Intellectual Properties Company | Absorbent substrates for harvesting skin grafts |
| EP3089681B1 (en) | 2013-12-31 | 2021-09-08 | 3M Innovative Properties Company | Fluid-assisted skin graft harvesting |
-
2014
- 2014-03-14 AU AU2014239952A patent/AU2014239952B2/en active Active
- 2014-03-14 US US14/211,026 patent/US9962254B2/en active Active
- 2014-03-14 WO PCT/US2014/027205 patent/WO2014152319A2/en active Application Filing
- 2014-03-14 EP EP17186324.4A patent/EP3289988A1/en not_active Withdrawn
- 2014-03-14 CN CN201480027486.6A patent/CN105636532B/zh active Active
- 2014-03-14 JP JP2016502366A patent/JP6491188B2/ja not_active Expired - Fee Related
- 2014-03-14 EP EP14769345.1A patent/EP2967627B1/en active Active
- 2014-03-14 CA CA2905481A patent/CA2905481C/en active Active
-
2018
- 2018-04-20 US US15/958,811 patent/US20180235749A1/en not_active Abandoned
- 2018-11-02 JP JP2018207340A patent/JP2019048104A/ja active Pending
Also Published As
| Publication number | Publication date |
|---|---|
| CN105636532A (zh) | 2016-06-01 |
| AU2014239952B2 (en) | 2018-09-27 |
| AU2014239952A1 (en) | 2015-10-01 |
| US9962254B2 (en) | 2018-05-08 |
| EP2967627B1 (en) | 2017-08-30 |
| CA2905481C (en) | 2021-05-04 |
| EP2967627A4 (en) | 2016-04-13 |
| JP2019048104A (ja) | 2019-03-28 |
| US20140277454A1 (en) | 2014-09-18 |
| WO2014152319A3 (en) | 2014-12-18 |
| WO2014152319A2 (en) | 2014-09-25 |
| EP2967627A2 (en) | 2016-01-20 |
| US20180235749A1 (en) | 2018-08-23 |
| JP2016512138A (ja) | 2016-04-25 |
| EP3289988A1 (en) | 2018-03-07 |
| CN105636532B (zh) | 2019-06-21 |
| CA2905481A1 (en) | 2014-09-25 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6491188B2 (ja) | 皮膚移植片採取用の吸収性基材 | |
| JP7000419B2 (ja) | 加圧下で使用する医療システムおよびドレッシング | |
| US11864980B2 (en) | Medical system and dressing for use under compression | |
| US20230201042A1 (en) | Hybrid drape having a gel-coated perforated mesh | |
| US10912861B2 (en) | Soft-tack, porous substrates for harvesting skin grafts | |
| CN110944688A (zh) | 组织接触接口 | |
| WO2019125962A1 (en) | Wound dressing for the harvesting of superficial epidermal grafts | |
| US20240148560A1 (en) | Medical dressing full indicator | |
| US20240099897A1 (en) | Medical System And Dressing For Use Under Compression |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20160408 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20170216 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20171115 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20171121 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20180221 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20180703 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20181102 |
|
| A911 | Transfer to examiner for re-examination before appeal (zenchi) |
Free format text: JAPANESE INTERMEDIATE CODE: A911 Effective date: 20181214 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20190129 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20190228 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 6491188 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| S111 | Request for change of ownership or part of ownership |
Free format text: JAPANESE INTERMEDIATE CODE: R313113 |
|
| R360 | Written notification for declining of transfer of rights |
Free format text: JAPANESE INTERMEDIATE CODE: R360 |
|
| R360 | Written notification for declining of transfer of rights |
Free format text: JAPANESE INTERMEDIATE CODE: R360 |
|
| R371 | Transfer withdrawn |
Free format text: JAPANESE INTERMEDIATE CODE: R371 |
|
| S111 | Request for change of ownership or part of ownership |
Free format text: JAPANESE INTERMEDIATE CODE: R313113 |
|
| R350 | Written notification of registration of transfer |
Free format text: JAPANESE INTERMEDIATE CODE: R350 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| LAPS | Cancellation because of no payment of annual fees |