JP6236451B2 - セグメント化スキャフォールドの構造 - Google Patents
セグメント化スキャフォールドの構造 Download PDFInfo
- Publication number
- JP6236451B2 JP6236451B2 JP2015527447A JP2015527447A JP6236451B2 JP 6236451 B2 JP6236451 B2 JP 6236451B2 JP 2015527447 A JP2015527447 A JP 2015527447A JP 2015527447 A JP2015527447 A JP 2015527447A JP 6236451 B2 JP6236451 B2 JP 6236451B2
- Authority
- JP
- Japan
- Prior art keywords
- segment
- scaffold
- segments
- ring
- undulation
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
- 229910003460 diamond Inorganic materials 0.000 claims description 57
- 239000010432 diamond Substances 0.000 claims description 57
- 210000004204 blood vessel Anatomy 0.000 claims description 34
- 229920000642 polymer Polymers 0.000 claims description 25
- 229920001432 poly(L-lactide) Polymers 0.000 claims description 6
- 210000004027 cell Anatomy 0.000 description 26
- 238000007906 compression Methods 0.000 description 24
- 238000000034 method Methods 0.000 description 24
- 230000006835 compression Effects 0.000 description 23
- 230000007423 decrease Effects 0.000 description 18
- 230000008878 coupling Effects 0.000 description 17
- 238000010168 coupling process Methods 0.000 description 17
- 238000005859 coupling reaction Methods 0.000 description 17
- 238000005452 bending Methods 0.000 description 16
- 238000011282 treatment Methods 0.000 description 16
- 239000000463 material Substances 0.000 description 15
- 238000010586 diagram Methods 0.000 description 13
- 230000002792 vascular Effects 0.000 description 12
- 238000002788 crimping Methods 0.000 description 11
- 230000008859 change Effects 0.000 description 10
- 230000002093 peripheral effect Effects 0.000 description 10
- 238000013461 design Methods 0.000 description 9
- 230000008569 process Effects 0.000 description 9
- 229920002988 biodegradable polymer Polymers 0.000 description 8
- 239000004621 biodegradable polymer Substances 0.000 description 8
- 210000004351 coronary vessel Anatomy 0.000 description 8
- 208000037803 restenosis Diseases 0.000 description 8
- 230000033001 locomotion Effects 0.000 description 7
- 230000000541 pulsatile effect Effects 0.000 description 7
- 210000001367 artery Anatomy 0.000 description 6
- 210000001105 femoral artery Anatomy 0.000 description 6
- 238000003698 laser cutting Methods 0.000 description 6
- 238000002399 angioplasty Methods 0.000 description 5
- 230000006378 damage Effects 0.000 description 5
- 238000002513 implantation Methods 0.000 description 5
- 239000002184 metal Substances 0.000 description 5
- 229910052751 metal Inorganic materials 0.000 description 5
- 238000011084 recovery Methods 0.000 description 5
- 238000012360 testing method Methods 0.000 description 5
- 208000031481 Pathologic Constriction Diseases 0.000 description 4
- 239000003814 drug Substances 0.000 description 4
- 239000012634 fragment Substances 0.000 description 4
- 210000003090 iliac artery Anatomy 0.000 description 4
- 238000012986 modification Methods 0.000 description 4
- 230000004048 modification Effects 0.000 description 4
- 210000005259 peripheral blood Anatomy 0.000 description 4
- 239000011886 peripheral blood Substances 0.000 description 4
- 238000012545 processing Methods 0.000 description 4
- 230000001681 protective effect Effects 0.000 description 4
- 230000009467 reduction Effects 0.000 description 4
- 238000004904 shortening Methods 0.000 description 4
- 230000036262 stenosis Effects 0.000 description 4
- 208000037804 stenosis Diseases 0.000 description 4
- -1 Nitinol Chemical class 0.000 description 3
- 210000003484 anatomy Anatomy 0.000 description 3
- 230000008901 benefit Effects 0.000 description 3
- 210000004369 blood Anatomy 0.000 description 3
- 239000008280 blood Substances 0.000 description 3
- 230000017531 blood circulation Effects 0.000 description 3
- 238000005520 cutting process Methods 0.000 description 3
- 210000002216 heart Anatomy 0.000 description 3
- 230000003902 lesion Effects 0.000 description 3
- 239000003550 marker Substances 0.000 description 3
- 229920001610 polycaprolactone Polymers 0.000 description 3
- 230000000644 propagated effect Effects 0.000 description 3
- 238000007665 sagging Methods 0.000 description 3
- 230000011218 segmentation Effects 0.000 description 3
- JJTUDXZGHPGLLC-IMJSIDKUSA-N 4511-42-6 Chemical compound C[C@@H]1OC(=O)[C@H](C)OC1=O JJTUDXZGHPGLLC-IMJSIDKUSA-N 0.000 description 2
- 241001465754 Metazoa Species 0.000 description 2
- 208000007536 Thrombosis Diseases 0.000 description 2
- 208000035868 Vascular inflammations Diseases 0.000 description 2
- 239000013543 active substance Substances 0.000 description 2
- 230000002411 adverse Effects 0.000 description 2
- 230000006399 behavior Effects 0.000 description 2
- 230000015572 biosynthetic process Effects 0.000 description 2
- 238000001815 biotherapy Methods 0.000 description 2
- 230000036760 body temperature Effects 0.000 description 2
- HVYWMOMLDIMFJA-DPAQBDIFSA-N cholesterol Chemical compound C1C=C2C[C@@H](O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H]([C@H](C)CCCC(C)C)[C@@]1(C)CC2 HVYWMOMLDIMFJA-DPAQBDIFSA-N 0.000 description 2
- 239000011248 coating agent Substances 0.000 description 2
- 238000000576 coating method Methods 0.000 description 2
- 239000002131 composite material Substances 0.000 description 2
- 230000008602 contraction Effects 0.000 description 2
- 125000004122 cyclic group Chemical group 0.000 description 2
- 229940079593 drug Drugs 0.000 description 2
- 230000000694 effects Effects 0.000 description 2
- 210000003038 endothelium Anatomy 0.000 description 2
- 238000002474 experimental method Methods 0.000 description 2
- 230000035876 healing Effects 0.000 description 2
- 230000003993 interaction Effects 0.000 description 2
- 210000003734 kidney Anatomy 0.000 description 2
- 210000002751 lymph Anatomy 0.000 description 2
- 230000014759 maintenance of location Effects 0.000 description 2
- 238000004519 manufacturing process Methods 0.000 description 2
- 230000007246 mechanism Effects 0.000 description 2
- 150000002739 metals Chemical class 0.000 description 2
- 229910001000 nickel titanium Inorganic materials 0.000 description 2
- HLXZNVUGXRDIFK-UHFFFAOYSA-N nickel titanium Chemical compound [Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni] HLXZNVUGXRDIFK-UHFFFAOYSA-N 0.000 description 2
- 230000000737 periodic effect Effects 0.000 description 2
- 229920003023 plastic Polymers 0.000 description 2
- 239000004033 plastic Substances 0.000 description 2
- 229920002463 poly(p-dioxanone) polymer Polymers 0.000 description 2
- 229920002791 poly-4-hydroxybutyrate Polymers 0.000 description 2
- 239000004632 polycaprolactone Substances 0.000 description 2
- 239000000622 polydioxanone Substances 0.000 description 2
- 229920000166 polytrimethylene carbonate Polymers 0.000 description 2
- 238000007634 remodeling Methods 0.000 description 2
- 210000002254 renal artery Anatomy 0.000 description 2
- 229920006126 semicrystalline polymer Polymers 0.000 description 2
- 238000011272 standard treatment Methods 0.000 description 2
- 229940124597 therapeutic agent Drugs 0.000 description 2
- 210000001519 tissue Anatomy 0.000 description 2
- 210000005166 vasculature Anatomy 0.000 description 2
- RKDVKSZUMVYZHH-UHFFFAOYSA-N 1,4-dioxane-2,5-dione Chemical compound O=C1COC(=O)CO1 RKDVKSZUMVYZHH-UHFFFAOYSA-N 0.000 description 1
- 200000000007 Arterial disease Diseases 0.000 description 1
- 206010051113 Arterial restenosis Diseases 0.000 description 1
- 229910000684 Cobalt-chrome Inorganic materials 0.000 description 1
- 208000005189 Embolism Diseases 0.000 description 1
- 206010061218 Inflammation Diseases 0.000 description 1
- 239000004677 Nylon Substances 0.000 description 1
- 208000005764 Peripheral Arterial Disease Diseases 0.000 description 1
- 208000030831 Peripheral arterial occlusive disease Diseases 0.000 description 1
- 229920002614 Polyether block amide Polymers 0.000 description 1
- 229920000954 Polyglycolide Polymers 0.000 description 1
- 206010070995 Vascular compression Diseases 0.000 description 1
- 208000027418 Wounds and injury Diseases 0.000 description 1
- 230000001154 acute effect Effects 0.000 description 1
- 210000000709 aorta Anatomy 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- 210000000617 arm Anatomy 0.000 description 1
- 230000003143 atherosclerotic effect Effects 0.000 description 1
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 1
- 230000004323 axial length Effects 0.000 description 1
- 238000010009 beating Methods 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- 210000000013 bile duct Anatomy 0.000 description 1
- 239000012867 bioactive agent Substances 0.000 description 1
- 230000008512 biological response Effects 0.000 description 1
- 230000005540 biological transmission Effects 0.000 description 1
- 229920001400 block copolymer Polymers 0.000 description 1
- 210000000988 bone and bone Anatomy 0.000 description 1
- 210000004556 brain Anatomy 0.000 description 1
- 230000002308 calcification Effects 0.000 description 1
- 210000001715 carotid artery Anatomy 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 238000003486 chemical etching Methods 0.000 description 1
- 235000012000 cholesterol Nutrition 0.000 description 1
- 239000010952 cobalt-chrome Substances 0.000 description 1
- 238000007887 coronary angioplasty Methods 0.000 description 1
- 208000029078 coronary artery disease Diseases 0.000 description 1
- 238000005336 cracking Methods 0.000 description 1
- 230000007547 defect Effects 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 230000008021 deposition Effects 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 230000018109 developmental process Effects 0.000 description 1
- 201000010099 disease Diseases 0.000 description 1
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 1
- 238000010494 dissociation reaction Methods 0.000 description 1
- 230000005593 dissociations Effects 0.000 description 1
- 239000003937 drug carrier Substances 0.000 description 1
- 238000012377 drug delivery Methods 0.000 description 1
- 229910000701 elgiloys (Co-Cr-Ni Alloy) Inorganic materials 0.000 description 1
- 230000010102 embolization Effects 0.000 description 1
- 238000001125 extrusion Methods 0.000 description 1
- 238000007667 floating Methods 0.000 description 1
- 239000012530 fluid Substances 0.000 description 1
- 210000002683 foot Anatomy 0.000 description 1
- 230000009477 glass transition Effects 0.000 description 1
- 210000003709 heart valve Anatomy 0.000 description 1
- 210000001621 ilium bone Anatomy 0.000 description 1
- 239000007943 implant Substances 0.000 description 1
- 238000001727 in vivo Methods 0.000 description 1
- 230000004054 inflammatory process Effects 0.000 description 1
- 230000028709 inflammatory response Effects 0.000 description 1
- 238000001746 injection moulding Methods 0.000 description 1
- 208000014674 injury Diseases 0.000 description 1
- 230000007794 irritation Effects 0.000 description 1
- 229960000448 lactic acid Drugs 0.000 description 1
- JVTAAEKCZFNVCJ-UHFFFAOYSA-N lactic acid Chemical compound CC(O)C(O)=O JVTAAEKCZFNVCJ-UHFFFAOYSA-N 0.000 description 1
- 210000002414 leg Anatomy 0.000 description 1
- 238000003754 machining Methods 0.000 description 1
- 238000012423 maintenance Methods 0.000 description 1
- 230000013011 mating Effects 0.000 description 1
- 210000004165 myocardium Anatomy 0.000 description 1
- 229920001778 nylon Polymers 0.000 description 1
- 210000000056 organ Anatomy 0.000 description 1
- 229910052760 oxygen Inorganic materials 0.000 description 1
- 239000001301 oxygen Substances 0.000 description 1
- 239000006069 physical mixture Substances 0.000 description 1
- 229920001072 poly(l-lactide-co-caprolactone) Polymers 0.000 description 1
- 239000004631 polybutylene succinate Substances 0.000 description 1
- 229920002961 polybutylene succinate Polymers 0.000 description 1
- 229920002643 polyglutamic acid Polymers 0.000 description 1
- 210000003137 popliteal artery Anatomy 0.000 description 1
- 229920005604 random copolymer Polymers 0.000 description 1
- 230000035945 sensitivity Effects 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 239000010935 stainless steel Substances 0.000 description 1
- 229910001220 stainless steel Inorganic materials 0.000 description 1
- 210000002784 stomach Anatomy 0.000 description 1
- 210000003270 subclavian artery Anatomy 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 230000001225 therapeutic effect Effects 0.000 description 1
- 230000008467 tissue growth Effects 0.000 description 1
- 210000003708 urethra Anatomy 0.000 description 1
- 230000000283 vasomotion Effects 0.000 description 1
- 238000012800 visualization Methods 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/82—Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/86—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure
- A61F2/90—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure characterised by a net-like or mesh-like structure
- A61F2/91—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure characterised by a net-like or mesh-like structure made from perforated sheet material or tubes, e.g. perforated by laser cuts or etched holes
- A61F2/915—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure characterised by a net-like or mesh-like structure made from perforated sheet material or tubes, e.g. perforated by laser cuts or etched holes with bands having a meander structure, adjacent bands being connected to each other
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/82—Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/86—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/82—Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/86—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure
- A61F2/89—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure the wire-like elements comprising two or more adjacent rings flexibly connected by separate members
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/82—Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/86—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure
- A61F2/90—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure characterised by a net-like or mesh-like structure
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/95—Instruments specially adapted for placement or removal of stents or stent-grafts
- A61F2/958—Inflatable balloons for placing stents or stent-grafts
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B29—WORKING OF PLASTICS; WORKING OF SUBSTANCES IN A PLASTIC STATE IN GENERAL
- B29C—SHAPING OR JOINING OF PLASTICS; SHAPING OF MATERIAL IN A PLASTIC STATE, NOT OTHERWISE PROVIDED FOR; AFTER-TREATMENT OF THE SHAPED PRODUCTS, e.g. REPAIRING
- B29C35/00—Heating, cooling or curing, e.g. crosslinking or vulcanising; Apparatus therefor
- B29C35/02—Heating or curing, e.g. crosslinking or vulcanizing during moulding, e.g. in a mould
- B29C35/08—Heating or curing, e.g. crosslinking or vulcanizing during moulding, e.g. in a mould by wave energy or particle radiation
- B29C35/0805—Heating or curing, e.g. crosslinking or vulcanizing during moulding, e.g. in a mould by wave energy or particle radiation using electromagnetic radiation
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B29—WORKING OF PLASTICS; WORKING OF SUBSTANCES IN A PLASTIC STATE IN GENERAL
- B29C—SHAPING OR JOINING OF PLASTICS; SHAPING OF MATERIAL IN A PLASTIC STATE, NOT OTHERWISE PROVIDED FOR; AFTER-TREATMENT OF THE SHAPED PRODUCTS, e.g. REPAIRING
- B29C65/00—Joining or sealing of preformed parts, e.g. welding of plastics materials; Apparatus therefor
- B29C65/56—Joining or sealing of preformed parts, e.g. welding of plastics materials; Apparatus therefor using mechanical means or mechanical connections, e.g. form-fits
- B29C65/64—Joining a non-plastics element to a plastics element, e.g. by force
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B29—WORKING OF PLASTICS; WORKING OF SUBSTANCES IN A PLASTIC STATE IN GENERAL
- B29C—SHAPING OR JOINING OF PLASTICS; SHAPING OF MATERIAL IN A PLASTIC STATE, NOT OTHERWISE PROVIDED FOR; AFTER-TREATMENT OF THE SHAPED PRODUCTS, e.g. REPAIRING
- B29C66/00—General aspects of processes or apparatus for joining preformed parts
- B29C66/01—General aspects dealing with the joint area or with the area to be joined
- B29C66/05—Particular design of joint configurations
- B29C66/10—Particular design of joint configurations particular design of the joint cross-sections
- B29C66/12—Joint cross-sections combining only two joint-segments; Tongue and groove joints; Tenon and mortise joints; Stepped joint cross-sections
- B29C66/124—Tongue and groove joints
- B29C66/1248—Interpenetrating groove joints
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B29—WORKING OF PLASTICS; WORKING OF SUBSTANCES IN A PLASTIC STATE IN GENERAL
- B29C—SHAPING OR JOINING OF PLASTICS; SHAPING OF MATERIAL IN A PLASTIC STATE, NOT OTHERWISE PROVIDED FOR; AFTER-TREATMENT OF THE SHAPED PRODUCTS, e.g. REPAIRING
- B29C66/00—General aspects of processes or apparatus for joining preformed parts
- B29C66/70—General aspects of processes or apparatus for joining preformed parts characterised by the composition, physical properties or the structure of the material of the parts to be joined; Joining with non-plastics material
- B29C66/71—General aspects of processes or apparatus for joining preformed parts characterised by the composition, physical properties or the structure of the material of the parts to be joined; Joining with non-plastics material characterised by the composition of the plastics material of the parts to be joined
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/82—Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2002/826—Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents more than one stent being applied sequentially
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/82—Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/86—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure
- A61F2/90—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure characterised by a net-like or mesh-like structure
- A61F2/91—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure characterised by a net-like or mesh-like structure made from perforated sheet material or tubes, e.g. perforated by laser cuts or etched holes
- A61F2/915—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure characterised by a net-like or mesh-like structure made from perforated sheet material or tubes, e.g. perforated by laser cuts or etched holes with bands having a meander structure, adjacent bands being connected to each other
- A61F2002/9155—Adjacent bands being connected to each other
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/82—Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/86—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure
- A61F2/90—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure characterised by a net-like or mesh-like structure
- A61F2/91—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure characterised by a net-like or mesh-like structure made from perforated sheet material or tubes, e.g. perforated by laser cuts or etched holes
- A61F2/915—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure characterised by a net-like or mesh-like structure made from perforated sheet material or tubes, e.g. perforated by laser cuts or etched holes with bands having a meander structure, adjacent bands being connected to each other
- A61F2002/9155—Adjacent bands being connected to each other
- A61F2002/91591—Locking connectors, e.g. using male-female connections
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2210/00—Particular material properties of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2230/00—Geometry of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2230/0002—Two-dimensional shapes, e.g. cross-sections
- A61F2230/0004—Rounded shapes, e.g. with rounded corners
- A61F2230/0013—Horseshoe-shaped, e.g. crescent-shaped, C-shaped, U-shaped
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2230/00—Geometry of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2230/0002—Two-dimensional shapes, e.g. cross-sections
- A61F2230/0028—Shapes in the form of latin or greek characters
- A61F2230/0054—V-shaped
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2240/00—Manufacturing or designing of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2240/001—Designing or manufacturing processes
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B29—WORKING OF PLASTICS; WORKING OF SUBSTANCES IN A PLASTIC STATE IN GENERAL
- B29C—SHAPING OR JOINING OF PLASTICS; SHAPING OF MATERIAL IN A PLASTIC STATE, NOT OTHERWISE PROVIDED FOR; AFTER-TREATMENT OF THE SHAPED PRODUCTS, e.g. REPAIRING
- B29C35/00—Heating, cooling or curing, e.g. crosslinking or vulcanising; Apparatus therefor
- B29C35/02—Heating or curing, e.g. crosslinking or vulcanizing during moulding, e.g. in a mould
- B29C35/08—Heating or curing, e.g. crosslinking or vulcanizing during moulding, e.g. in a mould by wave energy or particle radiation
- B29C35/0805—Heating or curing, e.g. crosslinking or vulcanizing during moulding, e.g. in a mould by wave energy or particle radiation using electromagnetic radiation
- B29C2035/0838—Heating or curing, e.g. crosslinking or vulcanizing during moulding, e.g. in a mould by wave energy or particle radiation using electromagnetic radiation using laser
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B29—WORKING OF PLASTICS; WORKING OF SUBSTANCES IN A PLASTIC STATE IN GENERAL
- B29K—INDEXING SCHEME ASSOCIATED WITH SUBCLASSES B29B, B29C OR B29D, RELATING TO MOULDING MATERIALS OR TO MATERIALS FOR MOULDS, REINFORCEMENTS, FILLERS OR PREFORMED PARTS, e.g. INSERTS
- B29K2067/00—Use of polyesters or derivatives thereof, as moulding material
- B29K2067/04—Polyesters derived from hydroxycarboxylic acids
- B29K2067/046—PLA, i.e. polylactic acid or polylactide
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B29—WORKING OF PLASTICS; WORKING OF SUBSTANCES IN A PLASTIC STATE IN GENERAL
- B29K—INDEXING SCHEME ASSOCIATED WITH SUBCLASSES B29B, B29C OR B29D, RELATING TO MOULDING MATERIALS OR TO MATERIALS FOR MOULDS, REINFORCEMENTS, FILLERS OR PREFORMED PARTS, e.g. INSERTS
- B29K2995/00—Properties of moulding materials, reinforcements, fillers, preformed parts or moulds
- B29K2995/0037—Other properties
- B29K2995/0059—Degradable
- B29K2995/006—Bio-degradable, e.g. bioabsorbable, bioresorbable or bioerodible
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B29—WORKING OF PLASTICS; WORKING OF SUBSTANCES IN A PLASTIC STATE IN GENERAL
- B29L—INDEXING SCHEME ASSOCIATED WITH SUBCLASS B29C, RELATING TO PARTICULAR ARTICLES
- B29L2031/00—Other particular articles
- B29L2031/753—Medical equipment; Accessories therefor
- B29L2031/7532—Artificial members, protheses
- B29L2031/7534—Cardiovascular protheses
Landscapes
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Oral & Maxillofacial Surgery (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Veterinary Medicine (AREA)
- General Health & Medical Sciences (AREA)
- Cardiology (AREA)
- Transplantation (AREA)
- Heart & Thoracic Surgery (AREA)
- Vascular Medicine (AREA)
- Life Sciences & Earth Sciences (AREA)
- Physics & Mathematics (AREA)
- Mechanical Engineering (AREA)
- Optics & Photonics (AREA)
- Thermal Sciences (AREA)
- Electromagnetism (AREA)
- Toxicology (AREA)
- Prostheses (AREA)
- Media Introduction/Drainage Providing Device (AREA)
Description
個々の刊行物又は特許明細書を、あたかも特別にかつ個々に参照して組み込んでいるかのごとく、及び、上記個々の刊行物又は特許明細書が、すべての図を含みつつ、本明細書に完全に記載されているかのごとく、本明細書に記載するすべての刊行物及び特許明細書を参照して本明細書に組み込む。
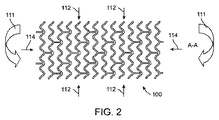
1. 第1の態様のセグメント化スキャフォールドは、
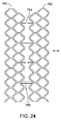
端部同士を突き合わせるように並べて配置された2つ以上の径方向に拡張可能で分離されたスキャフォールドのセグメントを備え、
各セグメントは、ストラットから構成された、2つ以上の起伏した円筒形リングを含み、
各セグメントの一端におけるリングは、前記セグメントの前記一端から長手方向外方に突出する山起伏を有すると共に、前記セグメントに向けて長手方向に延在する谷起伏を有し、
隣接したリングの前記山起伏及び前記谷起伏は互いにオーバーラップしている。
2. 第2の態様のセグメント化スキャフォールドは、上記第1の態様において、前記オーバーラップは、各隣接したリングに対して前記谷起伏間に延在する山起伏を備える。
3. 第3の態様のセグメント化スキャフォールドは、上記第1の態様において、前記セグメントは、血管内への送達のために縮小されクリンプされた状態にある。
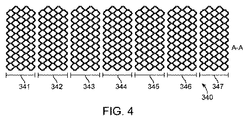
4. 第4の態様のセグメント化スキャフォールドは、上記第1の態様において、前記セグメントは、送達バルーンに被さる、縮小されクリンプされた状態にある。
5. 第5の態様のセグメント化スキャフォールドは、上記第1の態様において、各セグメントの前記起伏したリングは複数のダイヤモンド形セルを形成し、各端部における前記リングに沿って、交互のダイヤモンド形が、ダイヤモンド形セルの長手方向長さである長手方向長さを有する山起伏及び谷起伏を形成するように省かれている。
6. 第6の態様のセグメント化スキャフォールドは、上記第5の態様において、一端で省かれた前記ダイヤモンド形は、反対端で省かれたダイヤモンド形と長手方向に整列される。
7. 第7の態様のセグメント化スキャフォールドは、上記第5の態様において、一端で省かれた前記ダイヤモンド形は、反対端で省かれたダイヤモンド形と長手方向に整列されず、周方向でオフセットされる。
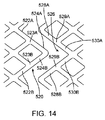
8. 第8の態様のセグメント化スキャフォールドは、上記第1の態様において、前記山起伏は、前記山起伏から周方向に延在する連結側壁面を含む前記山起伏の山に位置するヘッド部を有し、1つのリングの前記連結側壁面は、前記セグメントがクリンプされた状態にあるときに、隣接したリングの対応する連結側壁面と係合するように構成されている。
9. 第9の態様のスキャフォールドを送達する方法は、
送達バルーンにクリンプされたセグメント化スキャフォールドを提供する工程を備え、前記セグメント化スキャフォールドは、端部同士を突き合わせるように並べて配置された2つ以上の径方向に拡張可能な分離されたスキャフォールドセグメントを有し、
前記セグメントの各端部は、ストラットから構成された起伏した円筒形リングを有し、隣接したセグメントの起伏はオーバーラップし、
前記スキャフォールドセグメントを展開直径へ拡張する工程をさらに備え、前記隣接したセグメントの前記起伏は、前記展開直径でオーバーラップする。
10. 第10の態様のスキャフォールドを送達する方法は、上記第9の態様において、前記クリンプされたセグメント化スキャフォールドを血管内の治療部位に置く工程をさらに備える。
11. 第11の態様のスキャフォールドを送達する方法は、上記第10の態様において、スキャフォールドセグメントによって支持されない、血管壁の全周囲の前記治療部位における長手方向位置はない。
12. 第12の態様のセグメントは、
径方向に拡張可能なスキャフォールドのセグメントであって:
ストラットから構成された2つ以上の接続された起伏した円筒形リングを備え、
各セグメントの前記起伏したリングは、2対の対向する頂点を有するダイヤモンド形セルを複数形成し、一対が長手方向に整列され、一対が周方向に整列され、
少なくとも1つの端部リングに沿った交互のダイヤモンド形が省かれて、前記ダイヤモンド形セルの長手方向長さである長手方向長さを有する前記少なくとも1つの端部リングに沿って山起伏及び谷起伏を形成する。
13. 第13の態様のセグメントは、上記第12の態様において、前記交互のダイヤモンド形は、両方の端部リングに沿って省かれる。
14. 第14の態様のセグメントは、上記第13の態様において、1つの端部リングに沿う前記起伏の山は、対向する端部リングに沿う前記起伏の山と長手方向に整列される。
15. 第15の態様のセグメントは、上記第13の態様において、1つの端部リングにおける前記起伏の山は、対向する端部リングに沿う前記起伏の谷と長手方向に整列される。
16. 第16の態様のセグメントは、上記第13の態様において、一端において省かれたダイヤモンド形は、反対端で省かれたダイヤモンド形と長手方向に整列される。
17. 第17の態様のセグメントは、上記第13の態様において、反対端で省かれたダイヤモンド形が周方向にオフセットされるように、一端において省かれたダイヤモンド形は、反対端で省かれたダイヤモンド形と長手方向に整列されない。
18. 第18の態様のセグメントは、上記第13の態様において、前記少なくとも1つの端部リングの前記山起伏は、前記山起伏から周方向に延在する連結側壁面を含む前記山起伏の山に位置するヘッド部を有し、前記連結側壁面は、前記セグメントがクリンプされた状態にある場合に、隣接するリングの対応する連結側壁面と係合するように構成されている。
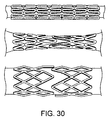
19. 第19の態様のスキャフォールドは、
クリンプされ縮小された構成の複数のスキャフォールドのセグメントと;
隣接したセグメント間に位置する切れ目を有する前記隣接したセグメント間の少なくとも1つの不連続連結要素とを備える。
20. 第20の態様のスキャフォールドは、上記第19の態様において、前記複数のセグメントは送達バルーンに被さるよう位置決めされる。
21. 第22の態様のスキャフォールドは、上記第19の態様において、前記スキャフォールドは、前記隣接したセグメント同士を接続する連結要素をまったく備えない。
22. 第22の態様のスキャフォールドは、上記第19の態様において、前記切れ目は、前記不連続連結要素の長さの10%未満である。
23. 第23の態様のスキャフォールドは、上記第19の態様において、異なる隣接したセグメント間の前記不連続連結要素は、周方向にオフセットされている。
24. 第24の態様のスキャフォールドは、上記第19の態様において、異なる隣接したセグメント間の前記不連続連結要素は、連続連結要素が前記スキャフォールドの第1の端部から前記スキャフォールドの第2の端部へ螺旋パターンで配置されるように、周方向にオフセットされる。
25. 第25の態様のスキャフォールドは、上記第19の態様において、さらに、隣接したセグメント同士を接続する少なくとも1つの連続連結要素を備える。
26. 第26の態様のスキャフォールドは、上記第25の態様において、前記連続連結要素は、前記スキャフォールドの展開時に前記連続連結要素の崩壊を容易にする弱い部分を備える。
27. 第27の態様のスキャフォールドを改変する方法は、
クリンプされ縮小された構成のスキャフォールドを提供する工程を備え、前記スキャフォールドは、長手方向スキャフォールドのセグメントと、隣接するスキャフォールドのセグメント同士を接続する連結要素とを有し;
さらに、少なくとも1セットの隣接したセグメント間の少なくとも1つの連結要素に切れ目を生成する工程を備える。
28. 第28の態様のスキャフォールドを改変する方法は、上記第27の態様において、前記切れ目は、前記少なくとも1つの連結要素をレーザー切断することによって生成される。
Claims (8)
- 端部同士を突き合わせるように並べて配置された、2つ以上の、径方向に拡張可能でかつ分離されたポリマースキャフォールドのセグメントを備え、前記ポリマーはポリ(L−ラクチド)を含み、
各セグメントは、ストラットから構成された、2つ以上の起伏した円筒形リングを含み、
第1のセグメントの一端におけるリングは、前記第1のセグメントの前記一端から長手方向外方に突出する山起伏を有すると共に、前記第1のセグメントに向けて長手方向に延在する谷起伏を有し、
前記第1のセグメントと端部同士を突き合わせるように並べて配置された第2のセグメントの一端におけるリングは、前記第1のセグメントの前記リングに隣接して配設された谷起伏を有すると共に、前記第2のセグメントの前記一端から長手方向外方に突出する山起伏を有し、
各リングの前記山起伏及び前記谷起伏は互いにオーバーラップしており、
各セグメントの前記起伏したリングは複数のダイヤモンド形セルを形成し、各端部における前記リングに沿って、交互のダイヤモンド形が、ダイヤモンド形セルの長手方向長さである長手方向長さを有する山起伏及び谷起伏を形成するように省かれ、
前記第1のセグメントの一端で省かれた前記ダイヤモンド形は、前記第1のセグメントの反対端で省かれたダイヤモンド形と長手方向に整列される、
セグメント化スキャフォールド。 - 前記オーバーラップは、一対の谷起伏間に延在する各山起伏を備える、
請求項1に記載のセグメント化スキャフォールド。 - 前記第1のセグメント及び前記第2のセグメントは、血管内への送達のために縮小されクリンプされた状態にある、
請求項1に記載のセグメント化スキャフォールド。 - 前記第1のセグメント及び前記第2のセグメントは、送達バルーンに被さる、縮小されクリンプされた状態にある、
請求項1に記載のセグメント化スキャフォールド。 - 前記山起伏は、前記山起伏から周方向に延在する連結側壁面を含む前記山起伏の山に位置するヘッド部を有し、前記第1のセグメントの前記リングの前記連結側壁面は、前記第1のセグメント及び前記第2のセグメントがクリンプされた状態にあるときに、前記第2のセグメントの前記リングの対応する連結側壁面と係合するように構成された、
請求項1に記載のセグメント化スキャフォールド。 - 前記交互のダイヤモンド形は、前記第1のセグメント及び前記第2のセグメントの両端で省かれた、
請求項1に記載のセグメント化スキャフォールド。 - 前記第1のセグメントの最小幅は、前記ダイヤモンド形セルの長手方向長さである、
請求項1に記載のセグメント化スキャフォールド。 - 前記第2のセグメントの谷起伏は複数の第1の部分と複数の第2の部分とを有し、前記第1の部分及び前記第2の部分はダイヤモンド形セルの幅に等しく、前記第2の部分は前記第1の部分よりも大きな角度で内側に曲がっている、
請求項4に記載のセグメント化スキャフォールド。
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US13/584,678 | 2012-08-13 | ||
| US13/584,678 US8834556B2 (en) | 2012-08-13 | 2012-08-13 | Segmented scaffold designs |
| PCT/US2013/033141 WO2014028062A1 (en) | 2012-08-13 | 2013-03-20 | Segmented scaffold designs |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2015529102A JP2015529102A (ja) | 2015-10-05 |
| JP2015529102A5 JP2015529102A5 (ja) | 2016-04-28 |
| JP6236451B2 true JP6236451B2 (ja) | 2017-11-22 |
Family
ID=48087725
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2015527447A Expired - Fee Related JP6236451B2 (ja) | 2012-08-13 | 2013-03-20 | セグメント化スキャフォールドの構造 |
Country Status (6)
| Country | Link |
|---|---|
| US (4) | US8834556B2 (ja) |
| EP (1) | EP2882380B1 (ja) |
| JP (1) | JP6236451B2 (ja) |
| CN (1) | CN104540477B (ja) |
| HK (1) | HK1209019A1 (ja) |
| WO (1) | WO2014028062A1 (ja) |
Cited By (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US10722631B2 (en) | 2018-02-01 | 2020-07-28 | Shifamed Holdings, Llc | Intravascular blood pumps and methods of use and manufacture |
| US11185677B2 (en) | 2017-06-07 | 2021-11-30 | Shifamed Holdings, Llc | Intravascular fluid movement devices, systems, and methods of use |
| US11511103B2 (en) | 2017-11-13 | 2022-11-29 | Shifamed Holdings, Llc | Intravascular fluid movement devices, systems, and methods of use |
| US11654275B2 (en) | 2019-07-22 | 2023-05-23 | Shifamed Holdings, Llc | Intravascular blood pumps with struts and methods of use and manufacture |
| US11724089B2 (en) | 2019-09-25 | 2023-08-15 | Shifamed Holdings, Llc | Intravascular blood pump systems and methods of use and control thereof |
| US11964145B2 (en) | 2019-07-12 | 2024-04-23 | Shifamed Holdings, Llc | Intravascular blood pumps and methods of manufacture and use |
| US12102815B2 (en) | 2019-09-25 | 2024-10-01 | Shifamed Holdings, Llc | Catheter blood pumps and collapsible pump housings |
Families Citing this family (29)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8814930B2 (en) * | 2007-01-19 | 2014-08-26 | Elixir Medical Corporation | Biodegradable endoprosthesis and methods for their fabrication |
| US8128677B2 (en) | 2007-12-12 | 2012-03-06 | Intact Vascular LLC | Device and method for tacking plaque to a blood vessel wall |
| US9254212B2 (en) * | 2012-04-06 | 2016-02-09 | Abbott Cardiovascular Systems Inc. | Segmented scaffolds and delivery thereof for peripheral applications |
| US8834556B2 (en) * | 2012-08-13 | 2014-09-16 | Abbott Cardiovascular Systems Inc. | Segmented scaffold designs |
| WO2014134568A2 (en) | 2013-03-01 | 2014-09-04 | The Regents Of The University Of California | Apparatus and methods for bidirectional hyperelastic stent covers |
| US9687239B2 (en) | 2014-04-15 | 2017-06-27 | Abbott Cardiovascular Systems Inc. | Intravascular devices supporting an arteriovenous fistula |
| US9259339B1 (en) | 2014-08-15 | 2016-02-16 | Elixir Medical Corporation | Biodegradable endoprostheses and methods of their fabrication |
| US9855156B2 (en) | 2014-08-15 | 2018-01-02 | Elixir Medical Corporation | Biodegradable endoprostheses and methods of their fabrication |
| US9480588B2 (en) | 2014-08-15 | 2016-11-01 | Elixir Medical Corporation | Biodegradable endoprostheses and methods of their fabrication |
| US9730819B2 (en) | 2014-08-15 | 2017-08-15 | Elixir Medical Corporation | Biodegradable endoprostheses and methods of their fabrication |
| US9433520B2 (en) | 2015-01-29 | 2016-09-06 | Intact Vascular, Inc. | Delivery device and method of delivery |
| US9375336B1 (en) | 2015-01-29 | 2016-06-28 | Intact Vascular, Inc. | Delivery device and method of delivery |
| EP3265037A4 (en) * | 2015-03-03 | 2018-10-31 | Efemoral Medical LLC | Multi-element bioresorbable intravascular stent |
| US10993824B2 (en) | 2016-01-01 | 2021-05-04 | Intact Vascular, Inc. | Delivery device and method of delivery |
| US11622872B2 (en) | 2016-05-16 | 2023-04-11 | Elixir Medical Corporation | Uncaging stent |
| EP3457985B1 (en) | 2016-05-16 | 2021-02-17 | Elixir Medical Corporation | Uncaging stent |
| CN105796217B (zh) * | 2016-05-17 | 2018-10-26 | 常州市第二人民医院 | 一种血管支架 |
| US11564816B2 (en) * | 2016-10-07 | 2023-01-31 | Efemoral Medical, Inc. | Radially rigid and longitudinally flexible multi-element intravascular stent |
| US10231856B2 (en) | 2016-10-27 | 2019-03-19 | Cook Medical Technologies Llc | Stent with segments capable of uncoupling during expansion |
| US11660218B2 (en) | 2017-07-26 | 2023-05-30 | Intact Vascular, Inc. | Delivery device and method of delivery |
| CN107569299B (zh) * | 2017-09-04 | 2022-02-22 | 上海脉全医疗器械有限公司 | 一种可吸收支架 |
| JP2021512730A (ja) * | 2018-02-23 | 2021-05-20 | エフェモラル メディカル インコーポレイテッド | 静脈閉塞性疾患の治療のための吸収性血管内デバイス |
| DE102018105925A1 (de) * | 2018-03-14 | 2019-09-19 | Malte Neuss | Doppelstent |
| JP2021526901A (ja) * | 2018-06-08 | 2021-10-11 | エフェモラル メディカル インコーポレイテッド | 拡張時に短縮して血管運動のための間隔を作成する吸収可能な血管内デバイス |
| CN114615956A (zh) * | 2019-10-11 | 2022-06-10 | 埃夫莫拉尔医疗有限公司 | 随时间降低血管的径向刚性的可吸收血管内装置 |
| CN110731835A (zh) * | 2019-10-25 | 2020-01-31 | 郑州美港高科生物科技有限公司 | 聚合物肾动脉支架 |
| CN116456940A (zh) * | 2020-11-23 | 2023-07-18 | 埃夫莫拉尔医疗有限公司 | 用于在弯曲期间保持动脉管腔的分段球囊可扩张支架系统 |
| CN114948362A (zh) * | 2021-02-16 | 2022-08-30 | 奥林巴斯株式会社 | 支架 |
| CN113065264B (zh) * | 2021-05-12 | 2021-12-31 | 四川大学 | 一种交叉支撑螺旋腘动脉支架及其制作方法 |
Family Cites Families (60)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4733665C2 (en) * | 1985-11-07 | 2002-01-29 | Expandable Grafts Partnership | Expandable intraluminal graft and method and apparatus for implanting an expandable intraluminal graft |
| US5064435A (en) * | 1990-06-28 | 1991-11-12 | Schneider (Usa) Inc. | Self-expanding prosthesis having stable axial length |
| US5316023A (en) * | 1992-01-08 | 1994-05-31 | Expandable Grafts Partnership | Method for bilateral intra-aortic bypass |
| EP0795304B1 (en) * | 1996-03-10 | 2004-05-19 | Terumo Kabushiki Kaisha | Implanting stent |
| WO1998020810A1 (en) * | 1996-11-12 | 1998-05-22 | Medtronic, Inc. | Flexible, radially expansible luminal prostheses |
| US8353948B2 (en) | 1997-01-24 | 2013-01-15 | Celonova Stent, Inc. | Fracture-resistant helical stent incorporating bistable cells and methods of use |
| US5827321A (en) * | 1997-02-07 | 1998-10-27 | Cornerstone Devices, Inc. | Non-Foreshortening intraluminal prosthesis |
| US7357942B2 (en) | 1997-09-26 | 2008-04-15 | Abbott Laboratories | Compositions, systems, and kits for administering zotarolimus and paclitaxel to blood vessel lumens |
| US6623521B2 (en) | 1998-02-17 | 2003-09-23 | Md3, Inc. | Expandable stent with sliding and locking radial elements |
| US6171334B1 (en) * | 1998-06-17 | 2001-01-09 | Advanced Cardiovascular Systems, Inc. | Expandable stent and method of use |
| US6261319B1 (en) * | 1998-07-08 | 2001-07-17 | Scimed Life Systems, Inc. | Stent |
| WO2000015151A1 (en) * | 1998-09-16 | 2000-03-23 | Isostent, Inc. | Linkage stent |
| US20060122691A1 (en) | 1998-12-03 | 2006-06-08 | Jacob Richter | Hybrid stent |
| US20070219642A1 (en) | 1998-12-03 | 2007-09-20 | Jacob Richter | Hybrid stent having a fiber or wire backbone |
| US20050033399A1 (en) | 1998-12-03 | 2005-02-10 | Jacob Richter | Hybrid stent |
| US6096072A (en) * | 1999-01-26 | 2000-08-01 | Uni-Cath Inc. | Self-exchange stent with effective supporting ability |
| US7018401B1 (en) | 1999-02-01 | 2006-03-28 | Board Of Regents, The University Of Texas System | Woven intravascular devices and methods for making the same and apparatus for delivery of the same |
| US6251134B1 (en) * | 1999-02-28 | 2001-06-26 | Inflow Dynamics Inc. | Stent with high longitudinal flexibility |
| US6258117B1 (en) | 1999-04-15 | 2001-07-10 | Mayo Foundation For Medical Education And Research | Multi-section stent |
| US6409753B1 (en) * | 1999-10-26 | 2002-06-25 | Scimed Life Systems, Inc. | Flexible stent |
| US20020055768A1 (en) | 1999-11-24 | 2002-05-09 | Kathy Hess | Method of manufacturing a thin-layered, endovascular, polymer-covered stent device |
| JP4754714B2 (ja) * | 2000-06-01 | 2011-08-24 | テルモ株式会社 | 管腔内留置物 |
| WO2002015824A2 (en) | 2000-08-25 | 2002-02-28 | Kensey Nash Corporation | Covered stents, systems for deploying covered stents |
| MXPA03008465A (es) * | 2001-03-20 | 2005-03-07 | Gmp Cardiac Care Inc | Endoprotesis de rail. |
| GB0121980D0 (en) | 2001-09-11 | 2001-10-31 | Cathnet Science Holding As | Expandable stent |
| US7294146B2 (en) | 2001-12-03 | 2007-11-13 | Xtent, Inc. | Apparatus and methods for delivery of variable length stents |
| US20040186551A1 (en) * | 2003-01-17 | 2004-09-23 | Xtent, Inc. | Multiple independent nested stent structures and methods for their preparation and deployment |
| US20050182477A1 (en) * | 2001-12-20 | 2005-08-18 | White Geoffrey H. | Intraluminal stent and graft |
| US6945995B2 (en) * | 2002-08-29 | 2005-09-20 | Boston Scientific Scimed, Inc. | Stent overlap point markers |
| AU2004224415B2 (en) * | 2003-03-19 | 2011-07-14 | Vactronix Scientific, Llc | Endoluminal stent having mid-interconnecting members |
| US7625401B2 (en) | 2003-05-06 | 2009-12-01 | Abbott Laboratories | Endoprosthesis having foot extensions |
| US7175654B2 (en) * | 2003-10-16 | 2007-02-13 | Cordis Corporation | Stent design having stent segments which uncouple upon deployment |
| US8157855B2 (en) | 2003-12-05 | 2012-04-17 | Boston Scientific Scimed, Inc. | Detachable segment stent |
| US20050182479A1 (en) | 2004-02-13 | 2005-08-18 | Craig Bonsignore | Connector members for stents |
| US7794490B2 (en) | 2004-06-22 | 2010-09-14 | Boston Scientific Scimed, Inc. | Implantable medical devices with antimicrobial and biodegradable matrices |
| US20050288766A1 (en) * | 2004-06-28 | 2005-12-29 | Xtent, Inc. | Devices and methods for controlling expandable prostheses during deployment |
| US8317859B2 (en) * | 2004-06-28 | 2012-11-27 | J.W. Medical Systems Ltd. | Devices and methods for controlling expandable prostheses during deployment |
| US7481835B1 (en) | 2004-10-29 | 2009-01-27 | Advanced Cardiovascular Systems, Inc. | Encapsulated covered stent |
| US7291166B2 (en) * | 2005-05-18 | 2007-11-06 | Advanced Cardiovascular Systems, Inc. | Polymeric stent patterns |
| EP1998716A4 (en) * | 2006-03-20 | 2010-01-20 | Xtent Inc | APPARATUS AND METHODS FOR ESTABLISHING RELATED PROSTHETIC SEGMENTS |
| US20090076584A1 (en) | 2007-09-19 | 2009-03-19 | Xtent, Inc. | Apparatus and methods for deployment of multiple custom-length prostheses |
| CN101411651B (zh) * | 2007-10-17 | 2011-03-09 | 微创医疗器械(上海)有限公司 | 一种嵌套式结构的人体管腔内支架 |
| CN201135513Y (zh) * | 2007-10-17 | 2008-10-22 | 微创医疗器械(上海)有限公司 | 一种嵌套式结构的人体管腔内支架 |
| WO2009069113A1 (en) * | 2007-11-28 | 2009-06-04 | The Provost, Fellows And Scholars Of The College Of The Holy And Undivided Trinity Of Queen Elizabeth Near Dublin | A luminal prosthesis |
| US8926688B2 (en) | 2008-01-11 | 2015-01-06 | W. L. Gore & Assoc. Inc. | Stent having adjacent elements connected by flexible webs |
| GB0803302D0 (en) * | 2008-02-22 | 2008-04-02 | Barts & London Nhs Trust | Blood vessel prosthesis and delivery apparatus |
| US20090248131A1 (en) | 2008-03-31 | 2009-10-01 | Medtronic Vascular, Inc. | Covered Stent and Method of Making Same |
| US20100042202A1 (en) | 2008-08-13 | 2010-02-18 | Kamal Ramzipoor | Composite stent having multi-axial flexibility |
| US20100174357A1 (en) * | 2009-01-07 | 2010-07-08 | Lemaitre Vascular, Inc. | Vascular Prosthesis of Varying Flexibility |
| US20100191323A1 (en) | 2009-01-23 | 2010-07-29 | Mitchell Wayne Cox | Biodegradable stent graft |
| US20100256728A1 (en) | 2009-04-07 | 2010-10-07 | Medtronic Vascular, Inc. | Semi-Permiable Biodegradable Stent Graft and Uses Thereof |
| US8425587B2 (en) | 2009-09-17 | 2013-04-23 | Abbott Cardiovascular Systems Inc. | Method of treatment with a bioabsorbable stent with time dependent structure and properties and regio-selective degradation |
| US8808353B2 (en) | 2010-01-30 | 2014-08-19 | Abbott Cardiovascular Systems Inc. | Crush recoverable polymer scaffolds having a low crossing profile |
| US8568471B2 (en) * | 2010-01-30 | 2013-10-29 | Abbott Cardiovascular Systems Inc. | Crush recoverable polymer scaffolds |
| US8844113B2 (en) * | 2010-04-30 | 2014-09-30 | Abbott Cardiovascular Systems, Inc. | Methods for crimping a polymeric stent scaffold onto a delivery balloon |
| US9402754B2 (en) * | 2010-05-18 | 2016-08-02 | Abbott Cardiovascular Systems, Inc. | Expandable endoprostheses, systems, and methods for treating a bifurcated lumen |
| US10406009B2 (en) | 2010-09-15 | 2019-09-10 | Abbott Cardiovascular Systems Inc. | Bioabsorbable superficial femoral stent patterns with designed to break links |
| US9839540B2 (en) * | 2011-01-14 | 2017-12-12 | W. L. Gore & Associates, Inc. | Stent |
| US9254212B2 (en) | 2012-04-06 | 2016-02-09 | Abbott Cardiovascular Systems Inc. | Segmented scaffolds and delivery thereof for peripheral applications |
| US8834556B2 (en) * | 2012-08-13 | 2014-09-16 | Abbott Cardiovascular Systems Inc. | Segmented scaffold designs |
-
2012
- 2012-08-13 US US13/584,678 patent/US8834556B2/en not_active Expired - Fee Related
-
2013
- 2013-03-20 CN CN201380042731.6A patent/CN104540477B/zh not_active Expired - Fee Related
- 2013-03-20 JP JP2015527447A patent/JP6236451B2/ja not_active Expired - Fee Related
- 2013-03-20 EP EP13715828.3A patent/EP2882380B1/en not_active Not-in-force
- 2013-03-20 WO PCT/US2013/033141 patent/WO2014028062A1/en active Application Filing
-
2014
- 2014-07-16 US US14/333,344 patent/US9585778B2/en active Active
- 2014-07-16 US US14/333,354 patent/US9585779B2/en active Active
-
2015
- 2015-10-12 HK HK15109942.3A patent/HK1209019A1/xx unknown
-
2017
- 2017-01-24 US US15/414,528 patent/US20170196718A1/en not_active Abandoned
Cited By (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US11185677B2 (en) | 2017-06-07 | 2021-11-30 | Shifamed Holdings, Llc | Intravascular fluid movement devices, systems, and methods of use |
| US11717670B2 (en) | 2017-06-07 | 2023-08-08 | Shifamed Holdings, LLP | Intravascular fluid movement devices, systems, and methods of use |
| US11511103B2 (en) | 2017-11-13 | 2022-11-29 | Shifamed Holdings, Llc | Intravascular fluid movement devices, systems, and methods of use |
| US10722631B2 (en) | 2018-02-01 | 2020-07-28 | Shifamed Holdings, Llc | Intravascular blood pumps and methods of use and manufacture |
| US11229784B2 (en) | 2018-02-01 | 2022-01-25 | Shifamed Holdings, Llc | Intravascular blood pumps and methods of use and manufacture |
| US12076545B2 (en) | 2018-02-01 | 2024-09-03 | Shifamed Holdings, Llc | Intravascular blood pumps and methods of use and manufacture |
| US11964145B2 (en) | 2019-07-12 | 2024-04-23 | Shifamed Holdings, Llc | Intravascular blood pumps and methods of manufacture and use |
| US11654275B2 (en) | 2019-07-22 | 2023-05-23 | Shifamed Holdings, Llc | Intravascular blood pumps with struts and methods of use and manufacture |
| US11724089B2 (en) | 2019-09-25 | 2023-08-15 | Shifamed Holdings, Llc | Intravascular blood pump systems and methods of use and control thereof |
| US12102815B2 (en) | 2019-09-25 | 2024-10-01 | Shifamed Holdings, Llc | Catheter blood pumps and collapsible pump housings |
Also Published As
| Publication number | Publication date |
|---|---|
| US9585778B2 (en) | 2017-03-07 |
| US8834556B2 (en) | 2014-09-16 |
| US20140330361A1 (en) | 2014-11-06 |
| US9585779B2 (en) | 2017-03-07 |
| EP2882380B1 (en) | 2017-08-02 |
| US20170196718A1 (en) | 2017-07-13 |
| US20140046431A1 (en) | 2014-02-13 |
| HK1209019A1 (en) | 2016-03-24 |
| CN104540477B (zh) | 2017-03-08 |
| WO2014028062A1 (en) | 2014-02-20 |
| JP2015529102A (ja) | 2015-10-05 |
| US20140330360A1 (en) | 2014-11-06 |
| CN104540477A (zh) | 2015-04-22 |
| EP2882380A1 (en) | 2015-06-17 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6236451B2 (ja) | セグメント化スキャフォールドの構造 | |
| JP6064030B2 (ja) | セグメント化スキャフォールドおよび抹消血管への適用のための送達 | |
| EP2616020B1 (en) | Stents with low strut thickness and variable strut geometry | |
| EP2394611B1 (en) | Hybrid stent | |
| EP3119354B1 (en) | Reduced granulation and inflammation stent design | |
| US7381217B2 (en) | Serpentine stent pattern | |
| US20120239136A1 (en) | Flexible intraluminal stent | |
| US8425586B2 (en) | Vascular prosthesis with stress relief slots | |
| US8348993B2 (en) | Flexible stent design | |
| US20070260304A1 (en) | Bifurcated stent with minimally circumferentially projected side branch |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20160307 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20160307 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20170117 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20170118 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20170412 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20171003 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20171030 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 6236451 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| LAPS | Cancellation because of no payment of annual fees |