JP6004498B2 - 耳用足場 - Google Patents
耳用足場 Download PDFInfo
- Publication number
- JP6004498B2 JP6004498B2 JP2014503204A JP2014503204A JP6004498B2 JP 6004498 B2 JP6004498 B2 JP 6004498B2 JP 2014503204 A JP2014503204 A JP 2014503204A JP 2014503204 A JP2014503204 A JP 2014503204A JP 6004498 B2 JP6004498 B2 JP 6004498B2
- Authority
- JP
- Japan
- Prior art keywords
- scaffold
- ear
- cartilage
- shape
- applicator
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
- 239000000463 material Substances 0.000 claims description 32
- 210000004728 ear cartilage Anatomy 0.000 claims description 5
- 230000001131 transforming effect Effects 0.000 claims 1
- 210000000845 cartilage Anatomy 0.000 description 59
- 238000000034 method Methods 0.000 description 35
- 239000012781 shape memory material Substances 0.000 description 15
- 238000007920 subcutaneous administration Methods 0.000 description 15
- 229920001169 thermoplastic Polymers 0.000 description 15
- 239000004416 thermosoftening plastic Substances 0.000 description 14
- 210000005069 ears Anatomy 0.000 description 13
- 238000003780 insertion Methods 0.000 description 11
- 230000037431 insertion Effects 0.000 description 11
- 230000008901 benefit Effects 0.000 description 10
- 230000008859 change Effects 0.000 description 7
- 229910001000 nickel titanium Inorganic materials 0.000 description 7
- 229920000642 polymer Polymers 0.000 description 7
- 238000001356 surgical procedure Methods 0.000 description 7
- JJTUDXZGHPGLLC-IMJSIDKUSA-N 4511-42-6 Chemical compound C[C@@H]1OC(=O)[C@H](C)OC1=O JJTUDXZGHPGLLC-IMJSIDKUSA-N 0.000 description 6
- 238000013461 design Methods 0.000 description 6
- HLXZNVUGXRDIFK-UHFFFAOYSA-N nickel titanium Chemical group [Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ti].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni].[Ni] HLXZNVUGXRDIFK-UHFFFAOYSA-N 0.000 description 6
- 238000007634 remodeling Methods 0.000 description 6
- 229920000954 Polyglycolide Polymers 0.000 description 5
- 238000012937 correction Methods 0.000 description 5
- 238000000465 moulding Methods 0.000 description 5
- 238000011282 treatment Methods 0.000 description 5
- AEMRFAOFKBGASW-UHFFFAOYSA-N Glycolic acid Polymers OCC(O)=O AEMRFAOFKBGASW-UHFFFAOYSA-N 0.000 description 4
- PXHVJJICTQNCMI-UHFFFAOYSA-N Nickel Chemical compound [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 description 4
- 229910045601 alloy Inorganic materials 0.000 description 4
- 239000000956 alloy Substances 0.000 description 4
- 238000010586 diagram Methods 0.000 description 4
- 238000010438 heat treatment Methods 0.000 description 4
- 239000007943 implant Substances 0.000 description 4
- 238000001727 in vivo Methods 0.000 description 4
- 238000002161 passivation Methods 0.000 description 4
- -1 polyethylene Polymers 0.000 description 4
- 210000001519 tissue Anatomy 0.000 description 4
- YFHICDDUDORKJB-UHFFFAOYSA-N trimethylene carbonate Chemical compound O=C1OCCCO1 YFHICDDUDORKJB-UHFFFAOYSA-N 0.000 description 4
- 240000006409 Acacia auriculiformis Species 0.000 description 3
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 description 3
- 238000005452 bending Methods 0.000 description 3
- 150000002009 diols Chemical class 0.000 description 3
- 230000000694 effects Effects 0.000 description 3
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 3
- 239000010931 gold Substances 0.000 description 3
- 229910052737 gold Inorganic materials 0.000 description 3
- 210000003128 head Anatomy 0.000 description 3
- 229910001092 metal group alloy Inorganic materials 0.000 description 3
- 229920003023 plastic Polymers 0.000 description 3
- 239000004033 plastic Substances 0.000 description 3
- 229920002463 poly(p-dioxanone) polymer Polymers 0.000 description 3
- 229920001610 polycaprolactone Polymers 0.000 description 3
- 230000008569 process Effects 0.000 description 3
- 239000012815 thermoplastic material Substances 0.000 description 3
- 239000010936 titanium Substances 0.000 description 3
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 3
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 description 2
- 208000002193 Pain Diseases 0.000 description 2
- 229910000831 Steel Inorganic materials 0.000 description 2
- 230000000740 bleeding effect Effects 0.000 description 2
- 239000002537 cosmetic Substances 0.000 description 2
- 230000006378 damage Effects 0.000 description 2
- 238000011065 in-situ storage Methods 0.000 description 2
- 230000007774 longterm Effects 0.000 description 2
- 229910052751 metal Inorganic materials 0.000 description 2
- 239000002184 metal Substances 0.000 description 2
- 239000000203 mixture Substances 0.000 description 2
- 238000012986 modification Methods 0.000 description 2
- 230000004048 modification Effects 0.000 description 2
- 229910052759 nickel Inorganic materials 0.000 description 2
- 230000036407 pain Effects 0.000 description 2
- 238000005498 polishing Methods 0.000 description 2
- 229920001281 polyalkylene Polymers 0.000 description 2
- 239000000622 polydioxanone Substances 0.000 description 2
- 239000010959 steel Substances 0.000 description 2
- 229910052719 titanium Inorganic materials 0.000 description 2
- RKDVKSZUMVYZHH-UHFFFAOYSA-N 1,4-dioxane-2,5-dione Chemical compound O=C1COC(=O)CO1 RKDVKSZUMVYZHH-UHFFFAOYSA-N 0.000 description 1
- MVLAJMHRDGFLMO-UHFFFAOYSA-N 1,5-dioxocane-2,4-dione Chemical compound O=C1CC(=O)OCCCO1 MVLAJMHRDGFLMO-UHFFFAOYSA-N 0.000 description 1
- VOXZDWNPVJITMN-ZBRFXRBCSA-N 17β-estradiol Chemical compound OC1=CC=C2[C@H]3CC[C@](C)([C@H](CC4)O)[C@@H]4[C@@H]3CCC2=C1 VOXZDWNPVJITMN-ZBRFXRBCSA-N 0.000 description 1
- LCSKNASZPVZHEG-UHFFFAOYSA-N 3,6-dimethyl-1,4-dioxane-2,5-dione;1,4-dioxane-2,5-dione Chemical compound O=C1COC(=O)CO1.CC1OC(=O)C(C)OC1=O LCSKNASZPVZHEG-UHFFFAOYSA-N 0.000 description 1
- 206010067484 Adverse reaction Diseases 0.000 description 1
- 208000032544 Cicatrix Diseases 0.000 description 1
- 201000004624 Dermatitis Diseases 0.000 description 1
- 206010049119 Emotional distress Diseases 0.000 description 1
- KMTRUDSVKNLOMY-UHFFFAOYSA-N Ethylene carbonate Chemical compound O=C1OCCO1 KMTRUDSVKNLOMY-UHFFFAOYSA-N 0.000 description 1
- 208000032843 Hemorrhage Diseases 0.000 description 1
- 208000002260 Keloid Diseases 0.000 description 1
- 208000034693 Laceration Diseases 0.000 description 1
- 241001465754 Metazoa Species 0.000 description 1
- 229910000990 Ni alloy Inorganic materials 0.000 description 1
- 239000004677 Nylon Substances 0.000 description 1
- MUBZPKHOEPUJKR-UHFFFAOYSA-N Oxalic acid Chemical compound OC(=O)C(O)=O MUBZPKHOEPUJKR-UHFFFAOYSA-N 0.000 description 1
- 206010033799 Paralysis Diseases 0.000 description 1
- 239000004698 Polyethylene Substances 0.000 description 1
- 229920000331 Polyhydroxybutyrate Polymers 0.000 description 1
- 239000004743 Polypropylene Substances 0.000 description 1
- 239000004793 Polystyrene Substances 0.000 description 1
- 206010040893 Skin necrosis Diseases 0.000 description 1
- 229920002334 Spandex Polymers 0.000 description 1
- 239000004809 Teflon Substances 0.000 description 1
- 229920006362 Teflon® Polymers 0.000 description 1
- 206010057040 Temperature intolerance Diseases 0.000 description 1
- 229910001069 Ti alloy Inorganic materials 0.000 description 1
- 230000002159 abnormal effect Effects 0.000 description 1
- 230000005856 abnormality Effects 0.000 description 1
- 230000002745 absorbent Effects 0.000 description 1
- 239000002250 absorbent Substances 0.000 description 1
- 238000010521 absorption reaction Methods 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- NIXOWILDQLNWCW-UHFFFAOYSA-N acrylic acid group Chemical group C(C=C)(=O)O NIXOWILDQLNWCW-UHFFFAOYSA-N 0.000 description 1
- 230000001154 acute effect Effects 0.000 description 1
- 239000000853 adhesive Substances 0.000 description 1
- 230000001070 adhesive effect Effects 0.000 description 1
- 230000002411 adverse Effects 0.000 description 1
- 230000006838 adverse reaction Effects 0.000 description 1
- 238000002048 anodisation reaction Methods 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 208000034158 bleeding Diseases 0.000 description 1
- 230000010478 bone regeneration Effects 0.000 description 1
- 229910052799 carbon Inorganic materials 0.000 description 1
- 229910002092 carbon dioxide Inorganic materials 0.000 description 1
- 239000001569 carbon dioxide Substances 0.000 description 1
- 229920002678 cellulose Polymers 0.000 description 1
- 239000001913 cellulose Substances 0.000 description 1
- 230000035601 cold sensitivity Effects 0.000 description 1
- 238000001816 cooling Methods 0.000 description 1
- 229920001577 copolymer Polymers 0.000 description 1
- 238000002316 cosmetic surgery Methods 0.000 description 1
- 230000034994 death Effects 0.000 description 1
- 238000011982 device technology Methods 0.000 description 1
- 229920001971 elastomer Polymers 0.000 description 1
- 239000000806 elastomer Substances 0.000 description 1
- 230000013020 embryo development Effects 0.000 description 1
- 230000007613 environmental effect Effects 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 230000006870 function Effects 0.000 description 1
- 238000002695 general anesthesia Methods 0.000 description 1
- 239000003365 glass fiber Substances 0.000 description 1
- 229920001519 homopolymer Polymers 0.000 description 1
- 230000007062 hydrolysis Effects 0.000 description 1
- 238000006460 hydrolysis reaction Methods 0.000 description 1
- 230000001969 hypertrophic effect Effects 0.000 description 1
- 238000002513 implantation Methods 0.000 description 1
- 208000015181 infectious disease Diseases 0.000 description 1
- 208000014674 injury Diseases 0.000 description 1
- 238000012966 insertion method Methods 0.000 description 1
- 230000001788 irregular Effects 0.000 description 1
- 210000001117 keloid Anatomy 0.000 description 1
- JJTUDXZGHPGLLC-UHFFFAOYSA-N lactide Chemical compound CC1OC(=O)C(C)OC1=O JJTUDXZGHPGLLC-UHFFFAOYSA-N 0.000 description 1
- 239000004816 latex Substances 0.000 description 1
- 229920000126 latex Polymers 0.000 description 1
- 230000014759 maintenance of location Effects 0.000 description 1
- 238000005272 metallurgy Methods 0.000 description 1
- VUZPPFZMUPKLLV-UHFFFAOYSA-N methane;hydrate Chemical compound C.O VUZPPFZMUPKLLV-UHFFFAOYSA-N 0.000 description 1
- 238000002324 minimally invasive surgery Methods 0.000 description 1
- 229920001778 nylon Polymers 0.000 description 1
- 230000035515 penetration Effects 0.000 description 1
- 230000000704 physical effect Effects 0.000 description 1
- 229920001308 poly(aminoacid) Polymers 0.000 description 1
- 239000005015 poly(hydroxybutyrate) Substances 0.000 description 1
- 239000004632 polycaprolactone Substances 0.000 description 1
- 229920000728 polyester Polymers 0.000 description 1
- 229920000573 polyethylene Polymers 0.000 description 1
- 229920000139 polyethylene terephthalate Polymers 0.000 description 1
- 239000005020 polyethylene terephthalate Substances 0.000 description 1
- 239000004633 polyglycolic acid Substances 0.000 description 1
- 229920001444 polymaleic acid Polymers 0.000 description 1
- 229920001155 polypropylene Polymers 0.000 description 1
- 229920001296 polysiloxane Polymers 0.000 description 1
- 229920002223 polystyrene Polymers 0.000 description 1
- 229920000166 polytrimethylene carbonate Polymers 0.000 description 1
- 229920002635 polyurethane Polymers 0.000 description 1
- 239000004814 polyurethane Substances 0.000 description 1
- 239000004800 polyvinyl chloride Substances 0.000 description 1
- 229920000915 polyvinyl chloride Polymers 0.000 description 1
- 230000002980 postoperative effect Effects 0.000 description 1
- 230000002035 prolonged effect Effects 0.000 description 1
- 238000011084 recovery Methods 0.000 description 1
- 238000005096 rolling process Methods 0.000 description 1
- 231100000241 scar Toxicity 0.000 description 1
- 230000037387 scars Effects 0.000 description 1
- 229910001285 shape-memory alloy Inorganic materials 0.000 description 1
- 229920000431 shape-memory polymer Polymers 0.000 description 1
- 229920002379 silicone rubber Polymers 0.000 description 1
- 239000004945 silicone rubber Substances 0.000 description 1
- 239000004759 spandex Substances 0.000 description 1
- 238000001228 spectrum Methods 0.000 description 1
- KDYFGRWQOYBRFD-UHFFFAOYSA-L succinate(2-) Chemical compound [O-]C(=O)CCC([O-])=O KDYFGRWQOYBRFD-UHFFFAOYSA-L 0.000 description 1
- 238000011477 surgical intervention Methods 0.000 description 1
- 229920002994 synthetic fiber Polymers 0.000 description 1
- 238000002560 therapeutic procedure Methods 0.000 description 1
- 230000003685 thermal hair damage Effects 0.000 description 1
- 229920002725 thermoplastic elastomer Polymers 0.000 description 1
- 238000012549 training Methods 0.000 description 1
- 230000009466 transformation Effects 0.000 description 1
- 230000007704 transition Effects 0.000 description 1
- 230000008733 trauma Effects 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/02—Inorganic materials
- A61L27/04—Metals or alloys
- A61L27/06—Titanium or titanium alloys
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F11/00—Methods or devices for treatment of the ears or hearing sense; Non-electric hearing aids; Methods or devices for enabling ear patients to achieve auditory perception through physiological senses other than hearing sense; Protective devices for the ears, carried on the body or in the hand
- A61F11/20—Ear surgery
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/18—Internal ear or nose parts, e.g. ear-drums
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/34—Trocars; Puncturing needles
- A61B17/3468—Trocars; Puncturing needles for implanting or removing devices, e.g. prostheses, implants, seeds, wires
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/0059—Cosmetic or alloplastic implants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/18—Internal ear or nose parts, e.g. ear-drums
- A61F2002/183—Ear parts
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2210/00—Particular material properties of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2210/0014—Particular material properties of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof using shape memory or superelastic materials, e.g. nitinol
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2230/00—Geometry of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2230/0002—Two-dimensional shapes, e.g. cross-sections
- A61F2230/0004—Rounded shapes, e.g. with rounded corners
- A61F2230/0013—Horseshoe-shaped, e.g. crescent-shaped, C-shaped, U-shaped
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Biomedical Technology (AREA)
- Engineering & Computer Science (AREA)
- Heart & Thoracic Surgery (AREA)
- Vascular Medicine (AREA)
- Otolaryngology (AREA)
- Transplantation (AREA)
- Oral & Maxillofacial Surgery (AREA)
- Cardiology (AREA)
- Pulmonology (AREA)
- Acoustics & Sound (AREA)
- Physics & Mathematics (AREA)
- Surgery (AREA)
- Biophysics (AREA)
- Psychology (AREA)
- Chemical & Material Sciences (AREA)
- Dermatology (AREA)
- Inorganic Chemistry (AREA)
- Medicinal Chemistry (AREA)
- Epidemiology (AREA)
- Prostheses (AREA)
- Professional, Industrial, Or Sporting Protective Garments (AREA)
- Materials For Medical Uses (AREA)
- Telescopes (AREA)
- Pens And Brushes (AREA)
Description
(a)足場を第1の構造に圧縮してカテーテルの内腔内に挿入するステップ、
(b)挿入に望ましい位置を外部から皮膚に印を付けるステップ、
(c)小さい切開部を皮膚に形成するステップ、
(d)足場を収容しているカテーテルを印を付けた経路に従って挿入する、または足場が係合部材を備える場合は、予めプログラムされた構造の足場を収容するために皮下空間を形成し、次いで足場を挿入するステップ、
(e)スライダを前方に移動させ、これにより足場を留置し、足場をその第2の予めプログラムされた構造にするステップ、
(f)アプリケータを除去するステップ。
1.Alexander K.S.,Stott D.J.,Sivakumar B.and Kang N.A morphometric study of the human ear.J Plast Reconstr Aesthet Surg 2010;
2.Janz B.A.,Cole P.,Hollier L.H.,Jr.and Stal S.Treatment of prominent and constricted ear anomalies.Plast Reconstr Surg 2009;124;27e−37e.
3.Bulstrode N.W.,Huang S.and Martin D.L.Otoplasty by percutaneous anterior scoring.Another twist to the story:a long−term study of 114 patients.Br J Plast Surg 2003;56;1459.
4.Firmin F.,Sanger C.and O’Toole G.Ear reconstruction following severe complications of otoplasty.J Plast Reconstr Aesthet Surg 2008;61 Suppl 1;S13−20.
5.Furnas D.W.Complications of surgery of the external ear.Clin Plast Surg 1990;17;305−18.
6.Jeffery S.L.Complications following correction of prominent ears:an audit review of 122 cases.Br J Plast Surg 1999;52;588−90.
7.Tan S.T.,Abramson D.L.,MacDonald D.M.and Mulliken J.B.Molding therapy for infants with deformational auricular anomalies.Ann Plast Surg 1997;38;263−8.
8.Ullmann Y.,Blazer S.,Ramon Y.,Blumenfeld I.and Peled I.J. Early nonsurgical correction of congenital auricular deformities.Plast Reconstr Surg 2002;109;907−13.
9.Sorribes M.M.and Tos M.Nonsurgical treatment of prominent ears with the Auri method.Arch Otolaryngol Head Neck Surg 2002;128;1369−76.
10.Iatrou I,Theologie−Lygidakis N,Tzerbos F,Alexandridis K.The use of biodegradable plates in oral and maxillofacial surgery in children.The XVIIIth Congress of the European Association for Cranio−Maxillofacial Surgery,Barcelona,Spain,September 12−15,2006.
11.Nieminen T,Rantala I,Hiidenheimo I,Keraenen J,Kainulainen H,Wuolijoki E,Kallela I.Degradative and mechanical properties of a novel resorbable plating system during a 3−year follow−up in vivo and in vitro.J Mater Sci:Mater Med 19:1155−1163,2008.
Claims (13)
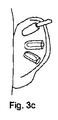
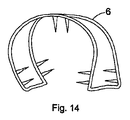
- 耳の軟骨部分(3)を再成形するための足場(6)において、前記足場が、係合部材を具え、かつ第1の構造から第2の予めプログラムされた構造に変態することができる形状記憶材料から形成されており、前記第2の予めプログラムされた構造にあるときに前記足場が馬蹄形に一致するように構成されており、前記馬蹄形が先細にされた接線方向延長部(9)を含むことを特徴とする足場。
- 請求項1に記載の足場(6)において、前記足場が耳の耳輪の形状に一致するように構成されていることを特徴とする足場。
- 請求項1または2に記載の足場(6)において、前記接線方向延長部(9)が1〜5mmの長さであることを特徴とする足場。
- 請求項3に記載の足場(6)において、前記接線方向延長部の長さが、少なくとも1mmの長さであることを特徴とする足場。
- 請求項4に記載の足場(6)において、前記接線方向延長部の長さが、2.3mmの長さであることを特徴とする足場。
- 請求項1乃至5のいずれか1項に記載の足場(6)において、前記足場が、少なくとも10mmの長さ、少なくとも1mmの幅、および最大2mmの厚みであることを特徴とする足場。
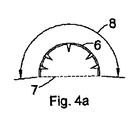
- 請求項1乃至6のいずれか1項に記載の足場(6)において、曲率半径(7)が少なくとも3mmであることを特徴とする足場。
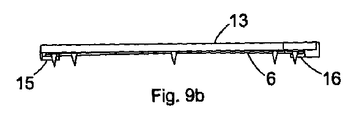
- 請求項1乃至7のいずれか1項に記載の足場(6)において、4つの係合部材が、前記足場の端部における各縁に位置していることを特徴とする足場。
- 請求項1乃至8のいずれか1項に記載の足場において、前記係合部材が歯であることを特徴とする足場。
- 請求項1乃至9のいずれか1項に記載の足場において、前記歯が円柱形であることを特徴とする足場。
- 請求項1乃至10のいずれか1項に記載の足場において、前記足場が電解研磨または不動態化されていることを特徴とする足場。
- 請求項1乃至11のいずれか1項に記載の足場において、前記足場が金めっきされていることを特徴とする足場。
- 請求項1乃至12のいずれか1項に記載の足場を耳の中に挿入するためのアプリケータであって、ハンドル、保持手段、および留置用の手段を具え、前記保持手段が、近位リップ(15)および遠位リップ(16)の形態であることを特徴とするアプリケータ。
Applications Claiming Priority (7)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| GBGB1105744.5A GB201105744D0 (en) | 2011-04-05 | 2011-04-05 | Ear scaffold |
| GBGB1105746.0A GB201105746D0 (en) | 2011-04-05 | 2011-04-05 | Mouldable ear scaffold |
| GBGB1105745.2A GB201105745D0 (en) | 2011-04-05 | 2011-04-05 | Wire ear scaffold |
| GBGB1105744.5 | 2011-04-05 | ||
| GBGB1105745.2 | 2011-04-05 | ||
| GBGB1105746.0 | 2011-04-05 | ||
| PCT/GB2012/000282 WO2012136950A1 (en) | 2011-04-05 | 2012-03-28 | Ear scaffold |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2014519349A JP2014519349A (ja) | 2014-08-14 |
| JP2014519349A5 JP2014519349A5 (ja) | 2015-05-21 |
| JP6004498B2 true JP6004498B2 (ja) | 2016-10-12 |
Family
ID=46027991
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2014503204A Expired - Fee Related JP6004498B2 (ja) | 2011-04-05 | 2012-03-28 | 耳用足場 |
Country Status (16)
| Country | Link |
|---|---|
| US (1) | US9554895B2 (ja) |
| EP (1) | EP2693983B1 (ja) |
| JP (1) | JP6004498B2 (ja) |
| KR (1) | KR101867120B1 (ja) |
| CN (2) | CN103561685A (ja) |
| AU (1) | AU2012238470C1 (ja) |
| BR (1) | BR112013025784B1 (ja) |
| CA (1) | CA2830079C (ja) |
| DK (1) | DK2693983T3 (ja) |
| ES (1) | ES2641646T3 (ja) |
| HU (1) | HUE034991T2 (ja) |
| MX (1) | MX349504B (ja) |
| PL (1) | PL2693983T3 (ja) |
| SG (1) | SG193632A1 (ja) |
| WO (1) | WO2012136950A1 (ja) |
| ZA (1) | ZA201308189B (ja) |
Families Citing this family (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2014107532A1 (en) * | 2013-01-02 | 2014-07-10 | The Children's Hospital Of Philadelphia | Method and apparatus for correcting auricular deformities |
| GB201405414D0 (en) * | 2014-03-26 | 2014-05-07 | Northwood Medical Innovations Ltd | Surgical introducer |
| GB2525040B (en) * | 2014-04-11 | 2020-04-01 | Northwood Medical Innovation Ltd | Ear tie |
| GB2578703A (en) * | 2014-04-11 | 2020-05-20 | Northwood Medical Innovation Ltd | Ear tie introducer |
| CA159015S (en) * | 2014-04-14 | 2015-05-06 | Northwood Medical Innovation Ltd | Ear scaffold for the correction of prominent ear deformity |
| US10485665B2 (en) | 2015-12-09 | 2019-11-26 | Reconstrata, Llc | Apparatus and method for constructing implantable cartilage structures |
| CN105997301A (zh) * | 2016-04-25 | 2016-10-12 | 哈尔滨工业大学 | 基于形状记忆聚合物的人工假体的智能可变形方法 |
| WO2018220242A1 (es) * | 2017-05-31 | 2018-12-06 | Tt Innovation Healtools, S.L. | Dispositivo de recarga de implantes de otoplastia |
| KR101895546B1 (ko) | 2018-07-09 | 2018-09-05 | 윤영묵 | 코 성형 인공보형물 |
| CN111183918A (zh) * | 2020-02-28 | 2020-05-22 | 成都睿畜电子科技有限公司 | 一种猪耳仿形骨架及无创式猪耳测温装置 |
| CN111700719B (zh) * | 2020-06-24 | 2024-11-15 | 王恒 | 一种双侧耳软骨取骨术后固定装置 |
| KR102567697B1 (ko) * | 2021-04-05 | 2023-08-16 | 연세대학교 산학협력단 | 귀 재건 시 사용되는 귀 후면 보형물 |
Family Cites Families (19)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3174851A (en) | 1961-12-01 | 1965-03-23 | William J Buehler | Nickel-base alloys |
| US5477864A (en) | 1989-12-21 | 1995-12-26 | Smith & Nephew Richards, Inc. | Cardiovascular guidewire of enhanced biocompatibility |
| US5433748A (en) | 1991-12-04 | 1995-07-18 | Porex Technologies Corp. | Auricular implant |
| JP2605559Y2 (ja) * | 1993-12-21 | 2000-07-24 | 株式会社パイオラックス | 管状器官の治療具 |
| GB2304579A (en) * | 1995-08-31 | 1997-03-26 | David Thomas Gault | Ear splint |
| JP3842844B2 (ja) * | 1996-07-02 | 2006-11-08 | 財団法人脳科学・ライフテクノロジー研究所 | 耳介の形態異常治療用矯正具 |
| JPH10201783A (ja) * | 1997-01-20 | 1998-08-04 | Masanori Kobayashi | 手指腱保護具 |
| US6375826B1 (en) * | 2000-02-14 | 2002-04-23 | Advanced Cardiovascular Systems, Inc. | Electro-polishing fixture and electrolyte solution for polishing stents and method |
| US6485503B2 (en) * | 2000-05-19 | 2002-11-26 | Coapt Systems, Inc. | Multi-point tissue tension distribution device, a brow and face lift variation, and a method of tissue approximation using the device |
| CA2441883A1 (en) * | 2000-09-01 | 2002-03-07 | Onux Medical, Inc. | Multi-fastener surgical apparatus and method |
| US7195640B2 (en) * | 2001-09-25 | 2007-03-27 | Cordis Corporation | Coated medical devices for the treatment of vulnerable plaque |
| US7182771B1 (en) * | 2001-12-20 | 2007-02-27 | Russell A. Houser | Vascular couplers, techniques, methods, and accessories |
| JP4945772B2 (ja) | 2002-04-09 | 2012-06-06 | ストラウマン ホールディング アーゲー | 生体親和性の改善された医療補綴器具 |
| GB0517499D0 (en) * | 2005-08-26 | 2005-10-05 | West Hertfordshire Hospitals N | Surgical scaffold |
| US8157837B2 (en) * | 2006-03-13 | 2012-04-17 | Pneumrx, Inc. | Minimally invasive lung volume reduction device and method |
| CN1861018B (zh) * | 2006-06-08 | 2010-12-15 | 上海索康医用材料有限公司 | 复合型耳支架 |
| US8454653B2 (en) * | 2008-02-20 | 2013-06-04 | Covidien Lp | Compound barb medical device and method |
| CN201213852Y (zh) * | 2008-04-25 | 2009-04-01 | 上海交通大学医学院附属第九人民医院 | 骨水泥颅耳角支撑支架 |
| CA2800386A1 (en) * | 2009-05-26 | 2010-12-02 | Oc2, Llc | Filamentous tissue implant |
-
2012
- 2012-03-28 BR BR112013025784-9A patent/BR112013025784B1/pt not_active IP Right Cessation
- 2012-03-28 JP JP2014503204A patent/JP6004498B2/ja not_active Expired - Fee Related
- 2012-03-28 HU HUE12719022A patent/HUE034991T2/en unknown
- 2012-03-28 CN CN201280016009.0A patent/CN103561685A/zh active Pending
- 2012-03-28 KR KR1020137027202A patent/KR101867120B1/ko active IP Right Grant
- 2012-03-28 PL PL12719022T patent/PL2693983T3/pl unknown
- 2012-03-28 MX MX2013011632A patent/MX349504B/es active IP Right Grant
- 2012-03-28 CA CA2830079A patent/CA2830079C/en active Active
- 2012-03-28 DK DK12719022.1T patent/DK2693983T3/en active
- 2012-03-28 SG SG2013072608A patent/SG193632A1/en unknown
- 2012-03-28 US US14/009,196 patent/US9554895B2/en active Active
- 2012-03-28 CN CN201710565225.5A patent/CN107260364B/zh not_active Expired - Fee Related
- 2012-03-28 EP EP12719022.1A patent/EP2693983B1/en active Active
- 2012-03-28 WO PCT/GB2012/000282 patent/WO2012136950A1/en active Application Filing
- 2012-03-28 ES ES12719022.1T patent/ES2641646T3/es active Active
- 2012-03-28 AU AU2012238470A patent/AU2012238470C1/en not_active Ceased
-
2013
- 2013-11-01 ZA ZA2013/08189A patent/ZA201308189B/en unknown
Also Published As
| Publication number | Publication date |
|---|---|
| CN107260364B (zh) | 2020-03-31 |
| HUE034991T2 (en) | 2018-05-02 |
| CA2830079C (en) | 2019-02-26 |
| ES2641646T3 (es) | 2017-11-10 |
| WO2012136950A1 (en) | 2012-10-11 |
| SG193632A1 (en) | 2013-10-30 |
| US20140228953A1 (en) | 2014-08-14 |
| CN107260364A (zh) | 2017-10-20 |
| BR112013025784B1 (pt) | 2021-03-16 |
| MX349504B (es) | 2017-07-31 |
| AU2012238470B2 (en) | 2016-03-03 |
| KR101867120B1 (ko) | 2018-06-12 |
| MX2013011632A (es) | 2014-07-28 |
| EP2693983A1 (en) | 2014-02-12 |
| US9554895B2 (en) | 2017-01-31 |
| WO2012136950A8 (en) | 2013-09-12 |
| EP2693983B1 (en) | 2017-06-28 |
| ZA201308189B (en) | 2016-07-27 |
| BR112013025784A8 (pt) | 2019-11-26 |
| AU2012238470C1 (en) | 2016-06-09 |
| KR20140059165A (ko) | 2014-05-15 |
| CN103561685A (zh) | 2014-02-05 |
| JP2014519349A (ja) | 2014-08-14 |
| PL2693983T3 (pl) | 2018-01-31 |
| CA2830079A1 (en) | 2012-10-11 |
| AU2012238470A1 (en) | 2013-09-19 |
| BR112013025784A2 (pt) | 2016-12-20 |
| DK2693983T3 (en) | 2017-10-09 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6004498B2 (ja) | 耳用足場 | |
| AU2012238470B9 (en) | Ear scaffold | |
| US7799075B2 (en) | Surgical scaffold | |
| WO2016033196A1 (en) | Nasal implants and systems and method of use | |
| MX2008002537A (en) | Surgical scaffold |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20150327 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20150327 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20160208 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20160223 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20160816 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20160901 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 6004498 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| S111 | Request for change of ownership or part of ownership |
Free format text: JAPANESE INTERMEDIATE CODE: R313113 |
|
| R350 | Written notification of registration of transfer |
Free format text: JAPANESE INTERMEDIATE CODE: R350 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| LAPS | Cancellation because of no payment of annual fees |