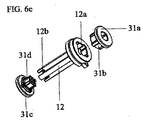
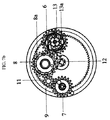
JP5885353B2 - 乾燥粉末吸入器のマウスピースボタン - Google Patents
乾燥粉末吸入器のマウスピースボタン Download PDFInfo
- Publication number
- JP5885353B2 JP5885353B2 JP2013504863A JP2013504863A JP5885353B2 JP 5885353 B2 JP5885353 B2 JP 5885353B2 JP 2013504863 A JP2013504863 A JP 2013504863A JP 2013504863 A JP2013504863 A JP 2013504863A JP 5885353 B2 JP5885353 B2 JP 5885353B2
- Authority
- JP
- Japan
- Prior art keywords
- inhaler
- cover
- mouthpiece
- mouthpiece cover
- wheel
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0028—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up
- A61M15/0045—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using multiple prepacked dosages on a same carrier, e.g. blisters
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0001—Details of inhalators; Constructional features thereof
- A61M15/0021—Mouthpieces therefor
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0001—Details of inhalators; Constructional features thereof
- A61M15/0021—Mouthpieces therefor
- A61M15/0025—Mouthpieces therefor with caps
- A61M15/0026—Hinged caps
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0028—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up
- A61M15/003—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using capsules, e.g. to be perforated or broken-up
- A61M15/0043—Non-destructive separation of the package, e.g. peeling
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0028—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up
- A61M15/0045—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using multiple prepacked dosages on a same carrier, e.g. blisters
- A61M15/0046—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using multiple prepacked dosages on a same carrier, e.g. blisters characterized by the type of carrier
- A61M15/0051—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using multiple prepacked dosages on a same carrier, e.g. blisters characterized by the type of carrier the dosages being arranged on a tape, e.g. strips
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0028—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up
- A61M15/0045—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using multiple prepacked dosages on a same carrier, e.g. blisters
- A61M15/0053—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using multiple prepacked dosages on a same carrier, e.g. blisters characterized by the type or way of disposal
- A61M15/0055—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using multiple prepacked dosages on a same carrier, e.g. blisters characterized by the type or way of disposal the used dosages being coiled
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0065—Inhalators with dosage or measuring devices
- A61M15/0068—Indicating or counting the number of dispensed doses or of remaining doses
- A61M15/007—Mechanical counters
- A61M15/0071—Mechanical counters having a display or indicator
- A61M15/0075—Mechanical counters having a display or indicator on a disc
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0065—Inhalators with dosage or measuring devices
- A61M15/0068—Indicating or counting the number of dispensed doses or of remaining doses
- A61M15/0081—Locking means
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2202/00—Special media to be introduced, removed or treated
- A61M2202/06—Solids
- A61M2202/062—Desiccants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2202/00—Special media to be introduced, removed or treated
- A61M2202/06—Solids
- A61M2202/064—Powder
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2205/00—General characteristics of the apparatus
- A61M2205/27—General characteristics of the apparatus preventing use
- A61M2205/276—General characteristics of the apparatus preventing use preventing unwanted use
Landscapes
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Life Sciences & Earth Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Veterinary Medicine (AREA)
- Biomedical Technology (AREA)
- Heart & Thoracic Surgery (AREA)
- Hematology (AREA)
- Pulmonology (AREA)
- Animal Behavior & Ethology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Public Health (AREA)
- Anesthesiology (AREA)
- Biophysics (AREA)
- Medical Preparation Storing Or Oral Administration Devices (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicinal Preparation (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
- Packages (AREA)
Applications Claiming Priority (7)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| TR2010/02877 | 2010-04-13 | ||
| TR2010/02877A TR201002877A2 (tr) | 2010-04-13 | 2010-04-13 | Blister ambalaj içeren inhalasyon cihazı |
| TR2010/03091 | 2010-04-20 | ||
| TR201003091 | 2010-04-20 | ||
| TR201003238 | 2010-04-26 | ||
| TR2010/03238 | 2010-04-26 | ||
| PCT/TR2011/000089 WO2011129789A1 (en) | 2010-04-13 | 2011-04-13 | Dry powder inhaler mouthpiece button |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2013526910A JP2013526910A (ja) | 2013-06-27 |
| JP2013526910A5 JP2013526910A5 (cg-RX-API-DMAC7.html) | 2014-05-29 |
| JP5885353B2 true JP5885353B2 (ja) | 2016-03-15 |
Family
ID=44211848
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2013504863A Expired - Fee Related JP5885353B2 (ja) | 2010-04-13 | 2011-04-13 | 乾燥粉末吸入器のマウスピースボタン |
| JP2013504866A Active JP5855642B2 (ja) | 2010-04-13 | 2011-04-13 | 乾燥粉末吸入器のマウスピースボタン |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2013504866A Active JP5855642B2 (ja) | 2010-04-13 | 2011-04-13 | 乾燥粉末吸入器のマウスピースボタン |
Country Status (15)
| Country | Link |
|---|---|
| EP (2) | EP2566547B1 (cg-RX-API-DMAC7.html) |
| JP (2) | JP5885353B2 (cg-RX-API-DMAC7.html) |
| CY (1) | CY1117758T1 (cg-RX-API-DMAC7.html) |
| DK (1) | DK2558148T3 (cg-RX-API-DMAC7.html) |
| EA (2) | EA024468B1 (cg-RX-API-DMAC7.html) |
| ES (1) | ES2576992T3 (cg-RX-API-DMAC7.html) |
| HR (1) | HRP20160515T1 (cg-RX-API-DMAC7.html) |
| HU (1) | HUE030056T2 (cg-RX-API-DMAC7.html) |
| IN (2) | IN2012DN04991A (cg-RX-API-DMAC7.html) |
| PL (1) | PL2558148T3 (cg-RX-API-DMAC7.html) |
| PT (1) | PT2558148T (cg-RX-API-DMAC7.html) |
| RS (1) | RS54842B1 (cg-RX-API-DMAC7.html) |
| SI (1) | SI2558148T1 (cg-RX-API-DMAC7.html) |
| SM (1) | SMT201600210B (cg-RX-API-DMAC7.html) |
| WO (2) | WO2011129793A1 (cg-RX-API-DMAC7.html) |
Families Citing this family (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9345848B2 (en) | 2009-10-20 | 2016-05-24 | Sima Patent Ve Lisanslama Hizmetleri Ltd. Sti. | Dry powder inhaler |
| USD733287S1 (en) | 2011-04-13 | 2015-06-30 | Mahmut Bilgic | Dry powder inhaler |
| USD744087S1 (en) | 2013-10-01 | 2015-11-24 | Mahmut Bilgic | Dry powder inhaler |
| CN109045427B (zh) * | 2016-10-03 | 2021-06-25 | 捷普科技(上海)有限公司 | 药剂分配器 |
| WO2020049289A1 (en) | 2018-09-03 | 2020-03-12 | Ttp Plc | Medicament delivery device |
| GB2580562B (en) * | 2018-09-03 | 2021-03-10 | Ttp Plc | Medicament delivery device |
| CN116672549A (zh) * | 2023-06-07 | 2023-09-01 | 艾特美(苏州)医药科技有限公司 | 准纳器 |
| WO2025185416A1 (zh) * | 2024-03-05 | 2025-09-12 | 传思生物公司 | 粉末吸入器、刺穿机构及粉末递送机构 |
| CN119158126B (zh) * | 2024-10-28 | 2025-04-01 | 浩英生物科技(苏州)有限公司 | 一种具有计量监测功能的吸入器 |
Family Cites Families (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB9930602D0 (en) * | 1999-12-24 | 2000-02-16 | Glaxo Group Ltd | Inhalation device |
| GB0026647D0 (en) * | 2000-10-31 | 2000-12-13 | Glaxo Group Ltd | Medicament dispenser |
| BRPI0507714B8 (pt) * | 2004-02-16 | 2021-07-27 | Glaxo Group Ltd | contador de dose para uso com um dispensador de medicamento, e, dispensador de medicamento |
| GB0509952D0 (en) * | 2005-05-16 | 2005-06-22 | Glaxo Group Ltd | Fault indicator |
| GB0515584D0 (en) * | 2005-07-28 | 2005-09-07 | Glaxo Group Ltd | Medicament dispenser |
| DE102006043637A1 (de) * | 2006-05-18 | 2007-11-22 | Boehringer Ingelheim Pharma Gmbh & Co. Kg | Zerstäuber |
| GB0704928D0 (en) * | 2007-03-14 | 2007-04-25 | Cambridge Consultants | Dry powder inhalers |
| GB0712803D0 (en) * | 2007-07-02 | 2007-08-08 | Glaxo Group Ltd | Medicament dispenser |
| TR200803523A1 (tr) * | 2008-05-16 | 2009-12-21 | Bi̇lgi̇ç Mahmut | Şerit ambalaj içeren düzenek |
-
2011
- 2011-04-13 DK DK11720618.5T patent/DK2558148T3/en active
- 2011-04-13 EA EA201201392A patent/EA024468B1/ru unknown
- 2011-04-13 RS RS20160424A patent/RS54842B1/sr unknown
- 2011-04-13 EP EP11720616.9A patent/EP2566547B1/en active Active
- 2011-04-13 WO PCT/TR2011/000093 patent/WO2011129793A1/en not_active Ceased
- 2011-04-13 ES ES11720618.5T patent/ES2576992T3/es active Active
- 2011-04-13 WO PCT/TR2011/000089 patent/WO2011129789A1/en not_active Ceased
- 2011-04-13 EA EA201201390A patent/EA026563B1/ru unknown
- 2011-04-13 SI SI201130862A patent/SI2558148T1/sl unknown
- 2011-04-13 JP JP2013504863A patent/JP5885353B2/ja not_active Expired - Fee Related
- 2011-04-13 HR HRP20160515TT patent/HRP20160515T1/hr unknown
- 2011-04-13 PL PL11720618.5T patent/PL2558148T3/pl unknown
- 2011-04-13 HU HUE11720618A patent/HUE030056T2/en unknown
- 2011-04-13 JP JP2013504866A patent/JP5855642B2/ja active Active
- 2011-04-13 PT PT117206185T patent/PT2558148T/pt unknown
- 2011-04-13 EP EP11720618.5A patent/EP2558148B1/en active Active
-
2012
- 2012-06-06 IN IN4991DEN2012 patent/IN2012DN04991A/en unknown
- 2012-06-06 IN IN4988DEN2012 patent/IN2012DN04988A/en unknown
-
2016
- 2016-06-24 CY CY20161100571T patent/CY1117758T1/el unknown
- 2016-07-05 SM SM201600210T patent/SMT201600210B/it unknown
Also Published As
| Publication number | Publication date |
|---|---|
| IN2012DN04988A (cg-RX-API-DMAC7.html) | 2015-10-02 |
| JP2013523380A (ja) | 2013-06-17 |
| HRP20160515T1 (hr) | 2016-07-01 |
| EP2566547B1 (en) | 2015-08-19 |
| WO2011129793A1 (en) | 2011-10-20 |
| IN2012DN04991A (cg-RX-API-DMAC7.html) | 2015-10-02 |
| EP2558148A1 (en) | 2013-02-20 |
| EA201201392A1 (ru) | 2013-05-30 |
| EA201201390A1 (ru) | 2013-05-30 |
| JP5855642B2 (ja) | 2016-02-09 |
| RS54842B1 (sr) | 2016-10-31 |
| EA024468B1 (ru) | 2016-09-30 |
| EP2566547A1 (en) | 2013-03-13 |
| EA026563B1 (ru) | 2017-04-28 |
| WO2011129789A1 (en) | 2011-10-20 |
| HUE030056T2 (en) | 2017-04-28 |
| EP2558148B1 (en) | 2016-04-13 |
| ES2576992T3 (es) | 2016-07-12 |
| PT2558148T (pt) | 2016-07-13 |
| JP2013526910A (ja) | 2013-06-27 |
| PL2558148T3 (pl) | 2016-09-30 |
| SI2558148T1 (sl) | 2016-10-28 |
| SMT201600210B (it) | 2016-08-31 |
| CY1117758T1 (el) | 2017-05-17 |
| DK2558148T3 (en) | 2016-07-25 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5885353B2 (ja) | 乾燥粉末吸入器のマウスピースボタン | |
| US10842952B2 (en) | Dry powder inhaler | |
| JP6290969B2 (ja) | 乾燥粉末吸入器のマウスピースボタン | |
| EP2566546B1 (en) | Dry powder inhaler mouthpiece button | |
| JP5860869B2 (ja) | 吸入デバイス | |
| JP5857034B2 (ja) | ブリスター包装体を有する吸入器 | |
| JP2013523378A (ja) | 乱流を生成する吸入器 | |
| WO2011129788A1 (en) | User-friendly dry powder inhaler | |
| JP2013523377A (ja) | 乾燥粉末吸入器のマウスピースボタン | |
| JP5873477B2 (ja) | 乾燥粉末形態の薬剤の送達に使用する吸入器 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A711 | Notification of change in applicant |
Free format text: JAPANESE INTERMEDIATE CODE: A711 Effective date: 20131009 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20140410 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20140410 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20150127 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20150130 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20150401 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20150707 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20160203 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20160208 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 5885353 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| LAPS | Cancellation because of no payment of annual fees |