JP5778183B2 - セミフレキシブル弁形成リング - Google Patents
セミフレキシブル弁形成リング Download PDFInfo
- Publication number
- JP5778183B2 JP5778183B2 JP2012552041A JP2012552041A JP5778183B2 JP 5778183 B2 JP5778183 B2 JP 5778183B2 JP 2012552041 A JP2012552041 A JP 2012552041A JP 2012552041 A JP2012552041 A JP 2012552041A JP 5778183 B2 JP5778183 B2 JP 5778183B2
- Authority
- JP
- Japan
- Prior art keywords
- annuloplasty ring
- ring according
- annulus
- commissure
- tricuspid
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 210000000591 tricuspid valve Anatomy 0.000 claims description 24
- 239000000463 material Substances 0.000 claims description 17
- 230000002787 reinforcement Effects 0.000 claims description 16
- 239000003550 marker Substances 0.000 claims description 15
- 239000003351 stiffener Substances 0.000 claims description 9
- 229920001187 thermosetting polymer Polymers 0.000 claims description 8
- 239000012779 reinforcing material Substances 0.000 claims description 5
- 230000002452 interceptive effect Effects 0.000 claims description 3
- 230000003014 reinforcing effect Effects 0.000 claims 5
- 230000000452 restraining effect Effects 0.000 claims 1
- 239000003356 suture material Substances 0.000 claims 1
- 201000001943 Tricuspid Valve Insufficiency Diseases 0.000 description 18
- 206010044640 Tricuspid valve incompetence Diseases 0.000 description 18
- 210000004115 mitral valve Anatomy 0.000 description 8
- 238000001356 surgical procedure Methods 0.000 description 8
- 230000002861 ventricular Effects 0.000 description 8
- 230000008439 repair process Effects 0.000 description 7
- 238000002513 implantation Methods 0.000 description 5
- 238000000034 method Methods 0.000 description 5
- 210000005241 right ventricle Anatomy 0.000 description 5
- 239000008280 blood Substances 0.000 description 3
- 210000004369 blood Anatomy 0.000 description 3
- 230000001419 dependent effect Effects 0.000 description 3
- 239000000835 fiber Substances 0.000 description 3
- 210000003709 heart valve Anatomy 0.000 description 3
- 230000007246 mechanism Effects 0.000 description 3
- 229910052751 metal Inorganic materials 0.000 description 3
- 239000002184 metal Substances 0.000 description 3
- 208000012336 Mitral valvular disease Diseases 0.000 description 2
- 239000000956 alloy Substances 0.000 description 2
- 229910045601 alloy Inorganic materials 0.000 description 2
- 210000003484 anatomy Anatomy 0.000 description 2
- TZCXTZWJZNENPQ-UHFFFAOYSA-L barium sulfate Chemical compound [Ba+2].[O-]S([O-])(=O)=O TZCXTZWJZNENPQ-UHFFFAOYSA-L 0.000 description 2
- 239000000560 biocompatible material Substances 0.000 description 2
- 230000017531 blood circulation Effects 0.000 description 2
- 230000010339 dilation Effects 0.000 description 2
- 230000000694 effects Effects 0.000 description 2
- 230000004927 fusion Effects 0.000 description 2
- 238000010438 heat treatment Methods 0.000 description 2
- 210000005246 left atrium Anatomy 0.000 description 2
- 210000005240 left ventricle Anatomy 0.000 description 2
- 210000004072 lung Anatomy 0.000 description 2
- 238000002844 melting Methods 0.000 description 2
- 230000008018 melting Effects 0.000 description 2
- 210000003205 muscle Anatomy 0.000 description 2
- 229920000728 polyester Polymers 0.000 description 2
- 229920000642 polymer Polymers 0.000 description 2
- 210000001147 pulmonary artery Anatomy 0.000 description 2
- 210000003102 pulmonary valve Anatomy 0.000 description 2
- 210000005245 right atrium Anatomy 0.000 description 2
- 239000000523 sample Substances 0.000 description 2
- 229920002379 silicone rubber Polymers 0.000 description 2
- 210000000115 thoracic cavity Anatomy 0.000 description 2
- 210000001519 tissue Anatomy 0.000 description 2
- 238000002604 ultrasonography Methods 0.000 description 2
- 206010002091 Anaesthesia Diseases 0.000 description 1
- ANLKZVDMILBJDJ-UHFFFAOYSA-N CC(C=C(C)C)N=O Chemical compound CC(C=C(C)C)N=O ANLKZVDMILBJDJ-UHFFFAOYSA-N 0.000 description 1
- DJDZSUKPWHDHQQ-UHFFFAOYSA-O CCCC(CC(CCCO)C(CN)C(C)(CC)C(C1)C1=CC[NH3+])C(CC)CCCC[U] Chemical compound CCCC(CC(CCCO)C(CN)C(C)(CC)C(C1)C1=CC[NH3+])C(CC)CCCC[U] DJDZSUKPWHDHQQ-UHFFFAOYSA-O 0.000 description 1
- 206010027727 Mitral valve incompetence Diseases 0.000 description 1
- 239000004677 Nylon Substances 0.000 description 1
- 239000004721 Polyphenylene oxide Substances 0.000 description 1
- 239000004743 Polypropylene Substances 0.000 description 1
- 206010067171 Regurgitation Diseases 0.000 description 1
- 230000037005 anaesthesia Effects 0.000 description 1
- 230000003444 anaesthetic effect Effects 0.000 description 1
- 210000000709 aorta Anatomy 0.000 description 1
- 210000001765 aortic valve Anatomy 0.000 description 1
- 230000004872 arterial blood pressure Effects 0.000 description 1
- 230000001746 atrial effect Effects 0.000 description 1
- 210000001992 atrioventricular node Anatomy 0.000 description 1
- 230000000747 cardiac effect Effects 0.000 description 1
- 238000007675 cardiac surgery Methods 0.000 description 1
- 239000003638 chemical reducing agent Substances 0.000 description 1
- 230000006835 compression Effects 0.000 description 1
- 238000007906 compression Methods 0.000 description 1
- 230000007797 corrosion Effects 0.000 description 1
- 238000005260 corrosion Methods 0.000 description 1
- 230000023753 dehiscence Effects 0.000 description 1
- 201000010099 disease Diseases 0.000 description 1
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 1
- 239000003814 drug Substances 0.000 description 1
- 229940079593 drug Drugs 0.000 description 1
- 230000002526 effect on cardiovascular system Effects 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 210000003238 esophagus Anatomy 0.000 description 1
- 239000007943 implant Substances 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 238000009434 installation Methods 0.000 description 1
- 239000000203 mixture Substances 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 229920001778 nylon Polymers 0.000 description 1
- 230000008447 perception Effects 0.000 description 1
- 230000035479 physiological effects, processes and functions Effects 0.000 description 1
- 229920000570 polyether Polymers 0.000 description 1
- -1 polypropylene Polymers 0.000 description 1
- 229920001155 polypropylene Polymers 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 238000004393 prognosis Methods 0.000 description 1
- 238000011084 recovery Methods 0.000 description 1
- 238000010992 reflux Methods 0.000 description 1
- 239000004945 silicone rubber Substances 0.000 description 1
- 230000004083 survival effect Effects 0.000 description 1
- WFKWXMTUELFFGS-UHFFFAOYSA-N tungsten Chemical compound [W] WFKWXMTUELFFGS-UHFFFAOYSA-N 0.000 description 1
- 229910052721 tungsten Inorganic materials 0.000 description 1
- 239000010937 tungsten Substances 0.000 description 1
- 210000001631 vena cava inferior Anatomy 0.000 description 1
- 210000002620 vena cava superior Anatomy 0.000 description 1
- 230000000007 visual effect Effects 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/24—Heart valves ; Vascular valves, e.g. venous valves; Heart implants, e.g. passive devices for improving the function of the native valve or the heart muscle; Transmyocardial revascularisation [TMR] devices; Valves implantable in the body
- A61F2/2442—Annuloplasty rings or inserts for correcting the valve shape; Implants for improving the function of a native heart valve
- A61F2/2445—Annuloplasty rings in direct contact with the valve annulus
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/24—Heart valves ; Vascular valves, e.g. venous valves; Heart implants, e.g. passive devices for improving the function of the native valve or the heart muscle; Transmyocardial revascularisation [TMR] devices; Valves implantable in the body
- A61F2/2442—Annuloplasty rings or inserts for correcting the valve shape; Implants for improving the function of a native heart valve
- A61F2/2466—Delivery devices therefor
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2250/00—Special features of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2250/0014—Special features of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof having different values of a given property or geometrical feature, e.g. mechanical property or material property, at different locations within the same prosthesis
- A61F2250/0018—Special features of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof having different values of a given property or geometrical feature, e.g. mechanical property or material property, at different locations within the same prosthesis differing in elasticity, stiffness or compressibility
Landscapes
- Health & Medical Sciences (AREA)
- Cardiology (AREA)
- Oral & Maxillofacial Surgery (AREA)
- Transplantation (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Heart & Thoracic Surgery (AREA)
- Vascular Medicine (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Prostheses (AREA)
Description
Claims (14)
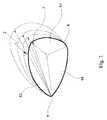
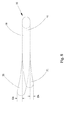
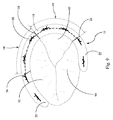
- 前方/後方交連(A/P交連)、後方/中隔交連(P/S交連)および中隔/前方交連(S/A交連)でそれぞれ連結された前方弁尖、後方弁尖および中隔弁尖を含む三尖弁の弁輪に取り付けるための弁形成リングであって、
中央部分ならびに第1および第2の端部分を含む細長い縫合可能材料チューブと、
前記チューブの中央部分に収容され、該中央部分の長さにわたって延びる弧状補強材であって、前記チューブに対する補強材の円周方向の移動を防ぐために、前記中央部分内に周方向の移動を拘束されている弧状補強材と、を備え、
前記チューブの第1と第2の端部分に補強材が無く、前記第1と第2の端は軸線方向に変形可能であり、
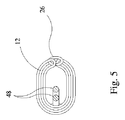
前記弧状補強材は、密巻コイルスプリングと該密巻コイルスプリングのバネキャビティ内に収容された補強ワイヤとを備え、
前記弧状補強材は、該補強ワイヤを前記弧状補強材の各端の端キャップによって密巻コイルスプリング内に長手方向に固定するように、構成されている、
ことを特徴とする弁形成リング。 - 弁形成リングが三尖弁の弁輪に作動的に取り付けられ、その中央部分が、S/P交連の周辺からA/P交連までの範囲に及ぶように形成されている、
請求項1に記載の弁形成リング。 - 弧状補強材が、後方半径を有する後方部分および前方半径を有する前方部分を含み、前方半径が後方半径よりも大きい、
請求項1に記載の弁形成リング。 - 後方半径および前方半径が、弁形成リングが三尖弁の弁輪に作動的に取り付けられ、後方半径が、拡張前の状態の少なくとも弁輪の後方部分に適合し、前方半径が、拡張前の状態の弁輪の前方部分の少なくとも一部に適合するように選択される、
請求項3に記載の弁形成リング。 - 第1と第2の端が、軸線方向に充分に変形可能であり、それにより、弁形成リングが作動的に三尖弁の弁輪に取り付けられた場合、第1と第2の端が弧状補強材により定まる平面より容易に逸脱して、正常な動作と実質的に干渉をせずに、三尖弁の解剖学的形態に適合することができる、
請求項1に記載の弁形成リング。 - 細長い縫合可能材料チューブが、弧状補強材で定まる平面内でC形状に熱硬化する編んだ熱硬化性材料を含む、
請求項1に記載の弁形成リング。 - Cの開口部が弁形成リングの公称寸法の約50%超の長さを有するギャップを規定する、
請求項6に記載の弁形成リング。 - ギャップが公称寸法の約60%の長さである、
請求項7に記載の弁形成リング。 - 弧状補強材が、弧状補強材の各端での縫合線により中央部分内に周方向移動を拘束されている、
請求項1に記載の弁形成リング。 - 各第1と第2の端部分にX線マーカをさらに含む、
請求項1に記載の弁形成リング。 - X線マーカが、軸線方向および半径方向に容易に変形可能である、
請求項10に記載の弁形成リング。 - 第1と第2の端部分が、軸線方向および半径方向に容易に変形可能である、
請求項1に記載の弁形成リング。 - 周方向に間隔をあけて設けた約11以下の取り付け縫合糸をさらに含む、
請求項1に記載の弁形成リング。 - 弧状補強材が弧状補強材の各端の縫合線により縫合可能材料に固定される、
請求項8に記載の弁形成リング。
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US30115810P | 2010-02-03 | 2010-02-03 | |
| US61/301,158 | 2010-02-03 | ||
| US30153210P | 2010-02-04 | 2010-02-04 | |
| US61/301,532 | 2010-02-04 | ||
| PCT/US2011/023386 WO2011097251A1 (en) | 2010-02-03 | 2011-02-01 | Semi-flexible annuloplasty ring |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2013518671A JP2013518671A (ja) | 2013-05-23 |
| JP2013518671A5 JP2013518671A5 (ja) | 2014-03-20 |
| JP5778183B2 true JP5778183B2 (ja) | 2015-09-16 |
Family
ID=44355742
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2012552041A Active JP5778183B2 (ja) | 2010-02-03 | 2011-02-01 | セミフレキシブル弁形成リング |
Country Status (5)
| Country | Link |
|---|---|
| US (2) | US10932907B2 (ja) |
| EP (1) | EP2531143B1 (ja) |
| JP (1) | JP5778183B2 (ja) |
| CN (1) | CN102985031B (ja) |
| WO (1) | WO2011097251A1 (ja) |
Families Citing this family (102)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2006097931A2 (en) | 2005-03-17 | 2006-09-21 | Valtech Cardio, Ltd. | Mitral valve treatment techniques |
| US8951285B2 (en) | 2005-07-05 | 2015-02-10 | Mitralign, Inc. | Tissue anchor, anchoring system and methods of using the same |
| CA2653358C (en) * | 2006-06-02 | 2012-03-13 | Medtronic, Inc. | Annuloplasty ring and method |
| US11259924B2 (en) | 2006-12-05 | 2022-03-01 | Valtech Cardio Ltd. | Implantation of repair devices in the heart |
| WO2008068756A2 (en) | 2006-12-05 | 2008-06-12 | Valtech Cardio, Ltd. | Segmented ring placement |
| WO2010004546A1 (en) | 2008-06-16 | 2010-01-14 | Valtech Cardio, Ltd. | Annuloplasty devices and methods of delivery therefor |
| US9974653B2 (en) | 2006-12-05 | 2018-05-22 | Valtech Cardio, Ltd. | Implantation of repair devices in the heart |
| US11660190B2 (en) | 2007-03-13 | 2023-05-30 | Edwards Lifesciences Corporation | Tissue anchors, systems and methods, and devices |
| EP2185107B1 (en) | 2007-09-07 | 2017-01-25 | Edwards Lifesciences Corporation | Active holder for annuloplasty ring delivery |
| US8382829B1 (en) | 2008-03-10 | 2013-02-26 | Mitralign, Inc. | Method to reduce mitral regurgitation by cinching the commissure of the mitral valve |
| US8926696B2 (en) | 2008-12-22 | 2015-01-06 | Valtech Cardio, Ltd. | Adjustable annuloplasty devices and adjustment mechanisms therefor |
| US8241351B2 (en) | 2008-12-22 | 2012-08-14 | Valtech Cardio, Ltd. | Adjustable partial annuloplasty ring and mechanism therefor |
| US9011530B2 (en) | 2008-12-22 | 2015-04-21 | Valtech Cardio, Ltd. | Partially-adjustable annuloplasty structure |
| US8715342B2 (en) | 2009-05-07 | 2014-05-06 | Valtech Cardio, Ltd. | Annuloplasty ring with intra-ring anchoring |
| US8911494B2 (en) | 2009-05-04 | 2014-12-16 | Valtech Cardio, Ltd. | Deployment techniques for annuloplasty ring |
| US10517719B2 (en) | 2008-12-22 | 2019-12-31 | Valtech Cardio, Ltd. | Implantation of repair devices in the heart |
| US8556965B2 (en) * | 2008-12-31 | 2013-10-15 | Medtronic, Inc. | Semi-rigid annuloplasty ring and band |
| US8353956B2 (en) | 2009-02-17 | 2013-01-15 | Valtech Cardio, Ltd. | Actively-engageable movement-restriction mechanism for use with an annuloplasty structure |
| US9968452B2 (en) | 2009-05-04 | 2018-05-15 | Valtech Cardio, Ltd. | Annuloplasty ring delivery cathethers |
| WO2013069019A2 (en) | 2011-11-08 | 2013-05-16 | Valtech Cardio, Ltd. | Controlled steering functionality for implant-delivery tool |
| US10098737B2 (en) | 2009-10-29 | 2018-10-16 | Valtech Cardio, Ltd. | Tissue anchor for annuloplasty device |
| US9180007B2 (en) | 2009-10-29 | 2015-11-10 | Valtech Cardio, Ltd. | Apparatus and method for guide-wire based advancement of an adjustable implant |
| US9011520B2 (en) | 2009-10-29 | 2015-04-21 | Valtech Cardio, Ltd. | Tissue anchor for annuloplasty device |
| EP2506777B1 (en) | 2009-12-02 | 2020-11-25 | Valtech Cardio, Ltd. | Combination of spool assembly coupled to a helical anchor and delivery tool for implantation thereof |
| US8870950B2 (en) | 2009-12-08 | 2014-10-28 | Mitral Tech Ltd. | Rotation-based anchoring of an implant |
| US8579964B2 (en) | 2010-05-05 | 2013-11-12 | Neovasc Inc. | Transcatheter mitral valve prosthesis |
| US11653910B2 (en) | 2010-07-21 | 2023-05-23 | Cardiovalve Ltd. | Helical anchor implantation |
| US8932350B2 (en) | 2010-11-30 | 2015-01-13 | Edwards Lifesciences Corporation | Reduced dehiscence annuloplasty ring |
| US9554897B2 (en) | 2011-04-28 | 2017-01-31 | Neovasc Tiara Inc. | Methods and apparatus for engaging a valve prosthesis with tissue |
| US9308087B2 (en) | 2011-04-28 | 2016-04-12 | Neovasc Tiara Inc. | Sequentially deployed transcatheter mitral valve prosthesis |
| US9402721B2 (en) | 2011-06-01 | 2016-08-02 | Valcare, Inc. | Percutaneous transcatheter repair of heart valves via trans-apical access |
| US10792152B2 (en) | 2011-06-23 | 2020-10-06 | Valtech Cardio, Ltd. | Closed band for percutaneous annuloplasty |
| US8858623B2 (en) * | 2011-11-04 | 2014-10-14 | Valtech Cardio, Ltd. | Implant having multiple rotational assemblies |
| WO2013088327A1 (en) | 2011-12-12 | 2013-06-20 | David Alon | Heart valve repair device |
| EP2819619B1 (en) | 2012-02-29 | 2019-01-16 | ValCare, Inc. | Percutaneous annuloplasty system with anterior-posterior adjustment |
| US9180008B2 (en) | 2012-02-29 | 2015-11-10 | Valcare, Inc. | Methods, devices, and systems for percutaneously anchoring annuloplasty rings |
| US9345573B2 (en) | 2012-05-30 | 2016-05-24 | Neovasc Tiara Inc. | Methods and apparatus for loading a prosthesis onto a delivery system |
| CA2885354A1 (en) | 2012-09-29 | 2014-04-03 | Mitralign, Inc. | Plication lock delivery system and method of use thereof |
| EP3730084A1 (en) | 2012-10-23 | 2020-10-28 | Valtech Cardio, Ltd. | Controlled steering functionality for implant-delivery tool |
| EP2911593B1 (en) | 2012-10-23 | 2020-03-25 | Valtech Cardio, Ltd. | Percutaneous tissue anchor techniques |
| US9730793B2 (en) | 2012-12-06 | 2017-08-15 | Valtech Cardio, Ltd. | Techniques for guide-wire based advancement of a tool |
| WO2014115149A2 (en) | 2013-01-24 | 2014-07-31 | Mitraltech Ltd. | Ventricularly-anchored prosthetic valves |
| US9724084B2 (en) | 2013-02-26 | 2017-08-08 | Mitralign, Inc. | Devices and methods for percutaneous tricuspid valve repair |
| US10449333B2 (en) | 2013-03-14 | 2019-10-22 | Valtech Cardio, Ltd. | Guidewire feeder |
| EP2967700B1 (en) | 2013-03-15 | 2020-11-25 | Valcare, Inc. | Systems for delivery of annuloplasty rings |
| WO2014152503A1 (en) | 2013-03-15 | 2014-09-25 | Mitralign, Inc. | Translation catheters, systems, and methods of use thereof |
| US9572665B2 (en) | 2013-04-04 | 2017-02-21 | Neovasc Tiara Inc. | Methods and apparatus for delivering a prosthetic valve to a beating heart |
| FR3004335A1 (fr) * | 2013-04-11 | 2014-10-17 | Cormove | Prothese d'annuloplastie partiellement deformable |
| WO2014189974A1 (en) * | 2013-05-20 | 2014-11-27 | Twelve, Inc. | Implantable heart valve devices, mitral valve repair devices and associated systems and methods |
| US10813751B2 (en) | 2013-05-22 | 2020-10-27 | Valcare, Inc. | Transcatheter prosthetic valve for mitral or tricuspid valve replacement |
| US20160120642A1 (en) | 2013-05-24 | 2016-05-05 | Valcare, Inc. | Heart and peripheral vascular valve replacement in conjunction with a support ring |
| EP3013253B1 (en) | 2013-06-28 | 2021-01-06 | ValCare, Inc. | Device for securing an article to a tissue |
| CN104010593B (zh) * | 2013-07-29 | 2016-05-25 | 金仕生物科技(常熟)有限公司 | 人工心脏瓣膜成形环 |
| US10070857B2 (en) | 2013-08-31 | 2018-09-11 | Mitralign, Inc. | Devices and methods for locating and implanting tissue anchors at mitral valve commissure |
| WO2015059699A2 (en) | 2013-10-23 | 2015-04-30 | Valtech Cardio, Ltd. | Anchor magazine |
| AU2014348469B2 (en) * | 2013-11-15 | 2019-08-15 | Guy's And St Thomas' Nhs Foundation Trust | Information markers for heart prostheses and methods of using same |
| US9610162B2 (en) | 2013-12-26 | 2017-04-04 | Valtech Cardio, Ltd. | Implantation of flexible implant |
| EP4066786A1 (en) | 2014-07-30 | 2022-10-05 | Cardiovalve Ltd. | Articulatable prosthetic valve |
| WO2016059639A1 (en) | 2014-10-14 | 2016-04-21 | Valtech Cardio Ltd. | Leaflet-restraining techniques |
| CN107205818B (zh) | 2015-02-05 | 2019-05-10 | 卡迪尔维尔福股份有限公司 | 带有轴向滑动框架的人工瓣膜 |
| US20160256269A1 (en) | 2015-03-05 | 2016-09-08 | Mitralign, Inc. | Devices for treating paravalvular leakage and methods use thereof |
| SG10202010021SA (en) | 2015-04-30 | 2020-11-27 | Valtech Cardio Ltd | Annuloplasty technologies |
| US10314707B2 (en) * | 2015-06-09 | 2019-06-11 | Edwards Lifesciences, Llc | Asymmetric mitral annuloplasty band |
| WO2017035002A1 (en) | 2015-08-21 | 2017-03-02 | Twelve Inc. | Implantable heart valve devices, mitral valve repair devices and associated systems and methods |
| EP4112006A1 (en) | 2015-12-15 | 2023-01-04 | Neovasc Tiara Inc. | Transseptal delivery system |
| US10751182B2 (en) | 2015-12-30 | 2020-08-25 | Edwards Lifesciences Corporation | System and method for reshaping right heart |
| EP3397207A4 (en) | 2015-12-30 | 2019-09-11 | Mitralign, Inc. | SYSTEM AND METHOD FOR REDUCING TRISCUPIDAL FLUTE REGULATION |
| DE202017007326U1 (de) | 2016-01-29 | 2020-10-20 | Neovasc Tiara Inc. | Klappenprothese zum Verhindern einer Abflussobstruktion |
| US10531866B2 (en) | 2016-02-16 | 2020-01-14 | Cardiovalve Ltd. | Techniques for providing a replacement valve and transseptal communication |
| GB2548891B (en) * | 2016-03-31 | 2018-07-04 | I Birdi Ltd | A prosthetic device for mitral valve repair |
| US10702274B2 (en) | 2016-05-26 | 2020-07-07 | Edwards Lifesciences Corporation | Method and system for closing left atrial appendage |
| GB201611910D0 (en) | 2016-07-08 | 2016-08-24 | Valtech Cardio Ltd | Adjustable annuloplasty device with alternating peaks and troughs |
| US20190231525A1 (en) | 2016-08-01 | 2019-08-01 | Mitraltech Ltd. | Minimally-invasive delivery systems |
| US10856975B2 (en) | 2016-08-10 | 2020-12-08 | Cardiovalve Ltd. | Prosthetic valve with concentric frames |
| CN107753153B (zh) | 2016-08-15 | 2022-05-31 | 沃卡尔有限公司 | 用于治疗心脏瓣膜关闭不全的装置和方法 |
| EP3541462A4 (en) | 2016-11-21 | 2020-06-17 | Neovasc Tiara Inc. | METHODS AND SYSTEMS FOR RAPID RETRACTION OF A TRANSCATHETER HEART VALVE DELIVERY SYSTEM |
| CN108618871A (zh) | 2017-03-17 | 2018-10-09 | 沃卡尔有限公司 | 具有多方向锚部的二尖瓣或三尖瓣修复系统 |
| CN106821548B (zh) * | 2017-04-01 | 2020-11-03 | 上海纽脉医疗科技有限公司 | 经导管人工二尖瓣成形环装置及系统 |
| US11045627B2 (en) | 2017-04-18 | 2021-06-29 | Edwards Lifesciences Corporation | Catheter system with linear actuation control mechanism |
| RU2738014C1 (ru) * | 2017-07-18 | 2020-12-07 | Кефалиос С.А.С. | Регулируемые чрескожные устройства для аннулопластики, доставляющие системы, способ чрескожного развертывания устройства для аннулопластики и способ, осуществляемый с помощью одного или более обрабатывающих устройств |
| US11793633B2 (en) | 2017-08-03 | 2023-10-24 | Cardiovalve Ltd. | Prosthetic heart valve |
| US12064347B2 (en) | 2017-08-03 | 2024-08-20 | Cardiovalve Ltd. | Prosthetic heart valve |
| US10856984B2 (en) | 2017-08-25 | 2020-12-08 | Neovasc Tiara Inc. | Sequentially deployed transcatheter mitral valve prosthesis |
| US10835221B2 (en) | 2017-11-02 | 2020-11-17 | Valtech Cardio, Ltd. | Implant-cinching devices and systems |
| US11135062B2 (en) | 2017-11-20 | 2021-10-05 | Valtech Cardio Ltd. | Cinching of dilated heart muscle |
| CN111655200B (zh) | 2018-01-24 | 2023-07-14 | 爱德华兹生命科学创新(以色列)有限公司 | 瓣环成形术结构的收缩 |
| WO2019145941A1 (en) | 2018-01-26 | 2019-08-01 | Valtech Cardio, Ltd. | Techniques for facilitating heart valve tethering and chord replacement |
| CN108478310B (zh) * | 2018-06-08 | 2024-05-03 | 倪一鸣 | 一种可调节长度和角度的三尖瓣成型环 |
| ES2974082T3 (es) | 2018-07-12 | 2024-06-25 | Edwards Lifesciences Innovation Israel Ltd | Sistemas de anuloplastia y herramientas de bloqueo para los mismos |
| CA3104687A1 (en) | 2018-07-30 | 2020-02-06 | Edwards Lifesciences Corporation | Minimally-invasive low strain annuloplasty ring |
| WO2020093172A1 (en) | 2018-11-08 | 2020-05-14 | Neovasc Tiara Inc. | Ventricular deployment of a transcatheter mitral valve prosthesis |
| CN113613593A (zh) | 2018-12-03 | 2021-11-05 | 沃卡尔有限公司 | 用于控制微创二尖瓣/三尖瓣修复系统的稳定和调节工具 |
| CN109662805B (zh) * | 2019-01-18 | 2024-02-13 | 西安增材制造国家研究院有限公司 | 一种三尖瓣成形环及其制造方法 |
| WO2020206012A1 (en) | 2019-04-01 | 2020-10-08 | Neovasc Tiara Inc. | Controllably deployable prosthetic valve |
| CN113924065A (zh) | 2019-04-10 | 2022-01-11 | 内奥瓦斯克迪亚拉公司 | 具有自然血流的假体瓣膜 |
| WO2020236931A1 (en) | 2019-05-20 | 2020-11-26 | Neovasc Tiara Inc. | Introducer with hemostasis mechanism |
| CA3143344A1 (en) | 2019-06-20 | 2020-12-24 | Neovasc Tiara Inc. | Low profile prosthetic mitral valve |
| EP3998995A4 (en) | 2019-07-15 | 2023-08-23 | ValCare, Inc. | TRANSCATHETER BIOPROSTHESIS ELEMENT AND SUPPORT STRUCTURE |
| WO2021084407A1 (en) | 2019-10-29 | 2021-05-06 | Valtech Cardio, Ltd. | Annuloplasty and tissue anchor technologies |
| WO2021236634A2 (en) | 2020-05-20 | 2021-11-25 | Cardiac Implants, Llc | Reducing the diameter of a cardiac valve annulus with independent control over each of the anchors that are launched into the annulus |
| WO2022015966A1 (en) * | 2020-07-15 | 2022-01-20 | The Board Of Trustees Of The Leland Stanford Junior University | Selectively flexible mitral annuloplasty devices for optimal annulus dynamics and biomechanics |
| US11723773B2 (en) * | 2021-06-15 | 2023-08-15 | “National Research Cardiac Surgery Center” Npjsc | Surgery for correcting tricuspid valve regurgitation |
Family Cites Families (17)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| FR2306671A1 (fr) * | 1975-04-11 | 1976-11-05 | Rhone Poulenc Ind | Implant valvulaire |
| WO1993015690A2 (en) * | 1992-01-27 | 1993-08-19 | Medtronic, Inc. | Annuloplasty and suture rings |
| DE69630235T2 (de) * | 1995-12-01 | 2004-08-05 | Medtronic, Inc., Minneapolis | Anuloplastik-Prothese |
| DE19910233A1 (de) * | 1999-03-09 | 2000-09-21 | Jostra Medizintechnik Ag | Anuloplastieprothese |
| US6187040B1 (en) * | 1999-05-03 | 2001-02-13 | John T. M. Wright | Mitral and tricuspid annuloplasty rings |
| US6955689B2 (en) * | 2001-03-15 | 2005-10-18 | Medtronic, Inc. | Annuloplasty band and method |
| US6786924B2 (en) * | 2001-03-15 | 2004-09-07 | Medtronic, Inc. | Annuloplasty band and method |
| US6908482B2 (en) * | 2001-08-28 | 2005-06-21 | Edwards Lifesciences Corporation | Three-dimensional annuloplasty ring and template |
| US20090088838A1 (en) * | 2006-05-12 | 2009-04-02 | Shaolian Samuel M | Adjustable annuloplasty ring and activation system |
| EP1854429A1 (en) * | 2006-05-12 | 2007-11-14 | Micardia Corporation | Intraoperative and post-operative adjustment of an annuloplasty ring |
| US7879087B2 (en) * | 2006-10-06 | 2011-02-01 | Edwards Lifesciences Corporation | Mitral and tricuspid annuloplasty rings |
| EP2124825B1 (en) * | 2007-01-26 | 2018-10-24 | Medtronic, Inc. | Annuloplasty device for tricuspid valve repair |
| US7993395B2 (en) * | 2008-01-25 | 2011-08-09 | Medtronic, Inc. | Set of annuloplasty devices with varying anterior-posterior ratios and related methods |
| US20090287303A1 (en) * | 2008-05-13 | 2009-11-19 | Edwards Lifesciences Corporation | Physiologically harmonized tricuspid annuloplasty ring |
| US8556965B2 (en) * | 2008-12-31 | 2013-10-15 | Medtronic, Inc. | Semi-rigid annuloplasty ring and band |
| US20110160849A1 (en) * | 2009-12-22 | 2011-06-30 | Edwards Lifesciences Corporation | Bimodal tricuspid annuloplasty ring |
| US8932350B2 (en) * | 2010-11-30 | 2015-01-13 | Edwards Lifesciences Corporation | Reduced dehiscence annuloplasty ring |
-
2011
- 2011-02-01 US US13/576,982 patent/US10932907B2/en active Active
- 2011-02-01 CN CN201180015528.0A patent/CN102985031B/zh active Active
- 2011-02-01 WO PCT/US2011/023386 patent/WO2011097251A1/en active Application Filing
- 2011-02-01 JP JP2012552041A patent/JP5778183B2/ja active Active
- 2011-02-01 EP EP11740273.5A patent/EP2531143B1/en active Active
-
2021
- 2021-02-05 US US17/169,068 patent/US20210161665A1/en active Pending
Also Published As
| Publication number | Publication date |
|---|---|
| JP2013518671A (ja) | 2013-05-23 |
| US20210161665A1 (en) | 2021-06-03 |
| EP2531143A4 (en) | 2015-12-02 |
| WO2011097251A1 (en) | 2011-08-11 |
| US20130204361A1 (en) | 2013-08-08 |
| US10932907B2 (en) | 2021-03-02 |
| CN102985031A (zh) | 2013-03-20 |
| CN102985031B (zh) | 2015-09-30 |
| EP2531143A1 (en) | 2012-12-12 |
| EP2531143B1 (en) | 2018-01-17 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5778183B2 (ja) | セミフレキシブル弁形成リング | |
| JP7055992B2 (ja) | 心臓弁修復装置およびその植込み方法 | |
| US10398547B2 (en) | Implant with anchoring device for heart valve disease | |
| US9414919B2 (en) | Semi-rigid annuloplasty ring and band | |
| US10004598B2 (en) | Implantable device for use in the human and/or animal body to replace an organ valve | |
| JP6441297B2 (ja) | ステント及びステントグラフト | |
| US20120078360A1 (en) | Prosthetic devices, systems and methods for replacing heart valves | |
| CN108348321B (zh) | 心脏瓣膜 | |
| CN115515533A (zh) | 具有低压缩的高柔性植入物导管 | |
| JP2023059960A (ja) | 非対称な僧帽弁形成バンド | |
| US20070203391A1 (en) | System for Treating Mitral Valve Regurgitation | |
| US20070078297A1 (en) | Device for Treating Mitral Valve Regurgitation | |
| JP2019524378A (ja) | 心臓弁ドッキング・コイルとシステム | |
| EP3269328A2 (en) | Cardiac valve implant | |
| JP2004528885A (ja) | 輪形成バンド及び方法 | |
| CN209377809U (zh) | 一种人工心脏瓣膜假体及其支架 | |
| EP4003233A1 (en) | Tethering system for a prosthetic heart valve | |
| JP2024508254A (ja) | 二重フレーム置換心臓弁 | |
| CN113952082A (zh) | 一种防返流多功能心脏瓣膜假体 | |
| US20240374380A1 (en) | Devices, Systems, and Methods for a Valve Replacement | |
| US20240374381A1 (en) | Devices, Systems, and Methods for a Valve Replacement | |
| CN114948345A (zh) | 心脏瓣膜定位装置、心脏瓣膜置换组件及植入方法 | |
| CN117136038A (zh) | 双框架置换心脏瓣膜 | |
| IL311654A (en) | Valve replacement devices, systems and methods |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20140130 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20140130 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20141017 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20141029 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20150129 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20150608 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20150708 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 5778183 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |