JP5721310B2 - 酸素除去 - Google Patents
酸素除去 Download PDFInfo
- Publication number
- JP5721310B2 JP5721310B2 JP2008558910A JP2008558910A JP5721310B2 JP 5721310 B2 JP5721310 B2 JP 5721310B2 JP 2008558910 A JP2008558910 A JP 2008558910A JP 2008558910 A JP2008558910 A JP 2008558910A JP 5721310 B2 JP5721310 B2 JP 5721310B2
- Authority
- JP
- Japan
- Prior art keywords
- hydrogen
- gas
- catalyst
- hydrocarbon
- free oxygen
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 title claims abstract description 69
- 239000001301 oxygen Substances 0.000 title claims abstract description 69
- 229910052760 oxygen Inorganic materials 0.000 title claims abstract description 69
- 239000007789 gas Substances 0.000 claims abstract description 118
- 239000003054 catalyst Substances 0.000 claims abstract description 81
- 229910052739 hydrogen Inorganic materials 0.000 claims abstract description 78
- 239000001257 hydrogen Substances 0.000 claims abstract description 78
- 229930195733 hydrocarbon Natural products 0.000 claims abstract description 70
- 150000002430 hydrocarbons Chemical class 0.000 claims abstract description 70
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 claims abstract description 68
- 239000000203 mixture Substances 0.000 claims abstract description 49
- 239000004215 Carbon black (E152) Substances 0.000 claims abstract description 46
- 238000006243 chemical reaction Methods 0.000 claims abstract description 38
- 238000000034 method Methods 0.000 claims abstract description 20
- 238000002156 mixing Methods 0.000 claims abstract description 11
- VNWKTOKETHGBQD-UHFFFAOYSA-N methane Chemical compound C VNWKTOKETHGBQD-UHFFFAOYSA-N 0.000 claims description 54
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 39
- 239000003345 natural gas Substances 0.000 claims description 24
- 238000002485 combustion reaction Methods 0.000 claims description 21
- 238000007254 oxidation reaction Methods 0.000 claims description 20
- 230000003647 oxidation Effects 0.000 claims description 19
- 238000000629 steam reforming Methods 0.000 claims description 13
- 238000002453 autothermal reforming Methods 0.000 claims description 12
- 150000002431 hydrogen Chemical class 0.000 claims description 11
- 229910000510 noble metal Inorganic materials 0.000 claims description 10
- 229910052697 platinum Inorganic materials 0.000 claims description 9
- 229910052717 sulfur Inorganic materials 0.000 claims description 9
- 238000011144 upstream manufacturing Methods 0.000 claims description 9
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical compound [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 claims description 8
- 239000011593 sulfur Substances 0.000 claims description 8
- 229910052759 nickel Inorganic materials 0.000 claims description 7
- 230000015572 biosynthetic process Effects 0.000 claims description 6
- 229910052763 palladium Inorganic materials 0.000 claims description 6
- 229910052703 rhodium Inorganic materials 0.000 claims description 6
- 238000001816 cooling Methods 0.000 claims description 5
- 230000002745 absorbent Effects 0.000 claims description 4
- 239000002250 absorbent Substances 0.000 claims description 4
- 229910052741 iridium Inorganic materials 0.000 claims description 4
- 229910052707 ruthenium Inorganic materials 0.000 claims description 4
- 239000002574 poison Substances 0.000 claims description 3
- 231100000614 poison Toxicity 0.000 claims description 3
- 229910052723 transition metal Inorganic materials 0.000 claims description 3
- 150000003624 transition metals Chemical class 0.000 claims description 3
- 238000003763 carbonization Methods 0.000 claims 1
- UGFAIRIUMAVXCW-UHFFFAOYSA-N Carbon monoxide Chemical compound [O+]#[C-] UGFAIRIUMAVXCW-UHFFFAOYSA-N 0.000 description 15
- 229910002091 carbon monoxide Inorganic materials 0.000 description 15
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 12
- 238000002407 reforming Methods 0.000 description 11
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 description 10
- MCMNRKCIXSYSNV-UHFFFAOYSA-N Zirconium dioxide Chemical compound O=[Zr]=O MCMNRKCIXSYSNV-UHFFFAOYSA-N 0.000 description 10
- PNEYBMLMFCGWSK-UHFFFAOYSA-N aluminium oxide Inorganic materials [O-2].[O-2].[O-2].[Al+3].[Al+3] PNEYBMLMFCGWSK-UHFFFAOYSA-N 0.000 description 10
- GWEVSGVZZGPLCZ-UHFFFAOYSA-N Titan oxide Chemical compound O=[Ti]=O GWEVSGVZZGPLCZ-UHFFFAOYSA-N 0.000 description 8
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 6
- XLOMVQKBTHCTTD-UHFFFAOYSA-N Zinc monoxide Chemical compound [Zn]=O XLOMVQKBTHCTTD-UHFFFAOYSA-N 0.000 description 6
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 5
- 150000001335 aliphatic alkanes Chemical class 0.000 description 5
- CPLXHLVBOLITMK-UHFFFAOYSA-N Magnesium oxide Chemical compound [Mg]=O CPLXHLVBOLITMK-UHFFFAOYSA-N 0.000 description 4
- 229910002092 carbon dioxide Inorganic materials 0.000 description 4
- 239000001569 carbon dioxide Substances 0.000 description 4
- 229910002090 carbon oxide Inorganic materials 0.000 description 4
- 230000003197 catalytic effect Effects 0.000 description 4
- 238000006356 dehydrogenation reaction Methods 0.000 description 4
- 229910052753 mercury Inorganic materials 0.000 description 4
- 229910052751 metal Inorganic materials 0.000 description 4
- 239000002184 metal Substances 0.000 description 4
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 3
- CETPSERCERDGAM-UHFFFAOYSA-N ceric oxide Chemical compound O=[Ce]=O CETPSERCERDGAM-UHFFFAOYSA-N 0.000 description 3
- 229910000422 cerium(IV) oxide Inorganic materials 0.000 description 3
- 229910052802 copper Inorganic materials 0.000 description 3
- 239000010949 copper Substances 0.000 description 3
- 238000006477 desulfuration reaction Methods 0.000 description 3
- 230000023556 desulfurization Effects 0.000 description 3
- 238000004519 manufacturing process Methods 0.000 description 3
- QSHDDOUJBYECFT-UHFFFAOYSA-N mercury Chemical compound [Hg] QSHDDOUJBYECFT-UHFFFAOYSA-N 0.000 description 3
- VLKZOEOYAKHREP-UHFFFAOYSA-N n-Hexane Chemical compound CCCCCC VLKZOEOYAKHREP-UHFFFAOYSA-N 0.000 description 3
- OFBQJSOFQDEBGM-UHFFFAOYSA-N n-pentane Natural products CCCCC OFBQJSOFQDEBGM-UHFFFAOYSA-N 0.000 description 3
- 229910052757 nitrogen Inorganic materials 0.000 description 3
- 239000000377 silicon dioxide Substances 0.000 description 3
- 239000011787 zinc oxide Substances 0.000 description 3
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 2
- ATUOYWHBWRKTHZ-UHFFFAOYSA-N Propane Chemical compound CCC ATUOYWHBWRKTHZ-UHFFFAOYSA-N 0.000 description 2
- 239000006096 absorbing agent Substances 0.000 description 2
- 229910052785 arsenic Inorganic materials 0.000 description 2
- RQNWIZPPADIBDY-UHFFFAOYSA-N arsenic atom Chemical compound [As] RQNWIZPPADIBDY-UHFFFAOYSA-N 0.000 description 2
- 239000000919 ceramic Substances 0.000 description 2
- 239000010779 crude oil Substances 0.000 description 2
- 239000006260 foam Substances 0.000 description 2
- 238000002347 injection Methods 0.000 description 2
- 239000007924 injection Substances 0.000 description 2
- 239000000395 magnesium oxide Substances 0.000 description 2
- 238000012423 maintenance Methods 0.000 description 2
- 229910052976 metal sulfide Inorganic materials 0.000 description 2
- 150000002739 metals Chemical class 0.000 description 2
- 239000008188 pellet Substances 0.000 description 2
- 238000000926 separation method Methods 0.000 description 2
- 229910018072 Al 2 O 3 Inorganic materials 0.000 description 1
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical group [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 1
- 229910052684 Cerium Inorganic materials 0.000 description 1
- RWSOTUBLDIXVET-UHFFFAOYSA-N Dihydrogen sulfide Chemical compound S RWSOTUBLDIXVET-UHFFFAOYSA-N 0.000 description 1
- OTMSDBZUPAUEDD-UHFFFAOYSA-N Ethane Chemical compound CC OTMSDBZUPAUEDD-UHFFFAOYSA-N 0.000 description 1
- GYHNNYVSQQEPJS-UHFFFAOYSA-N Gallium Chemical compound [Ga] GYHNNYVSQQEPJS-UHFFFAOYSA-N 0.000 description 1
- 241000264877 Hippospongia communis Species 0.000 description 1
- 229910020068 MgAl Inorganic materials 0.000 description 1
- 229910002837 PtCo Inorganic materials 0.000 description 1
- 229910002836 PtFe Inorganic materials 0.000 description 1
- 229910002847 PtSn Inorganic materials 0.000 description 1
- ATJFFYVFTNAWJD-UHFFFAOYSA-N Tin Chemical compound [Sn] ATJFFYVFTNAWJD-UHFFFAOYSA-N 0.000 description 1
- 239000003570 air Substances 0.000 description 1
- 239000000956 alloy Substances 0.000 description 1
- 229910045601 alloy Inorganic materials 0.000 description 1
- 239000001273 butane Substances 0.000 description 1
- 239000011651 chromium Substances 0.000 description 1
- UOUJSJZBMCDAEU-UHFFFAOYSA-N chromium(3+);oxygen(2-) Chemical class [O-2].[O-2].[O-2].[Cr+3].[Cr+3] UOUJSJZBMCDAEU-UHFFFAOYSA-N 0.000 description 1
- 239000003426 co-catalyst Substances 0.000 description 1
- 239000011248 coating agent Substances 0.000 description 1
- 238000000576 coating method Methods 0.000 description 1
- 239000000571 coke Substances 0.000 description 1
- 239000000567 combustion gas Substances 0.000 description 1
- 239000000112 cooling gas Substances 0.000 description 1
- TVZPLCNGKSPOJA-UHFFFAOYSA-N copper zinc Chemical compound [Cu].[Zn] TVZPLCNGKSPOJA-UHFFFAOYSA-N 0.000 description 1
- OMZSGWSJDCOLKM-UHFFFAOYSA-N copper(II) sulfide Chemical compound [S-2].[Cu+2] OMZSGWSJDCOLKM-UHFFFAOYSA-N 0.000 description 1
- PGTIPSRGRGGDQO-UHFFFAOYSA-N copper;oxozinc Chemical compound [Zn].[Cu]=O PGTIPSRGRGGDQO-UHFFFAOYSA-N 0.000 description 1
- 230000007797 corrosion Effects 0.000 description 1
- 238000005260 corrosion Methods 0.000 description 1
- 238000000354 decomposition reaction Methods 0.000 description 1
- 230000000694 effects Effects 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 238000000605 extraction Methods 0.000 description 1
- 239000012530 fluid Substances 0.000 description 1
- 239000000446 fuel Substances 0.000 description 1
- 229910052733 gallium Inorganic materials 0.000 description 1
- 239000008246 gaseous mixture Substances 0.000 description 1
- 231100001261 hazardous Toxicity 0.000 description 1
- 230000020169 heat generation Effects 0.000 description 1
- 238000010438 heat treatment Methods 0.000 description 1
- 229910000037 hydrogen sulfide Inorganic materials 0.000 description 1
- 238000009434 installation Methods 0.000 description 1
- 229910052742 iron Inorganic materials 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- IJDNQMDRQITEOD-UHFFFAOYSA-N n-butane Chemical compound CCCC IJDNQMDRQITEOD-UHFFFAOYSA-N 0.000 description 1
- 239000003921 oil Substances 0.000 description 1
- 125000001741 organic sulfur group Chemical group 0.000 description 1
- 230000001590 oxidative effect Effects 0.000 description 1
- 239000002245 particle Substances 0.000 description 1
- 230000000737 periodic effect Effects 0.000 description 1
- 231100000572 poisoning Toxicity 0.000 description 1
- 230000000607 poisoning effect Effects 0.000 description 1
- 229920001343 polytetrafluoroethylene Polymers 0.000 description 1
- 239000001294 propane Substances 0.000 description 1
- 238000010926 purge Methods 0.000 description 1
- 238000000746 purification Methods 0.000 description 1
- 238000004064 recycling Methods 0.000 description 1
- 238000007670 refining Methods 0.000 description 1
- 230000008929 regeneration Effects 0.000 description 1
- 238000011069 regeneration method Methods 0.000 description 1
- 239000010457 zeolite Substances 0.000 description 1
Images
Classifications
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J8/00—Chemical or physical processes in general, conducted in the presence of fluids and solid particles; Apparatus for such processes
- B01J8/02—Chemical or physical processes in general, conducted in the presence of fluids and solid particles; Apparatus for such processes with stationary particles, e.g. in fixed beds
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J8/00—Chemical or physical processes in general, conducted in the presence of fluids and solid particles; Apparatus for such processes
- B01J8/02—Chemical or physical processes in general, conducted in the presence of fluids and solid particles; Apparatus for such processes with stationary particles, e.g. in fixed beds
- B01J8/04—Chemical or physical processes in general, conducted in the presence of fluids and solid particles; Apparatus for such processes with stationary particles, e.g. in fixed beds the fluid passing successively through two or more beds
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J8/00—Chemical or physical processes in general, conducted in the presence of fluids and solid particles; Apparatus for such processes
- B01J8/02—Chemical or physical processes in general, conducted in the presence of fluids and solid particles; Apparatus for such processes with stationary particles, e.g. in fixed beds
- B01J8/04—Chemical or physical processes in general, conducted in the presence of fluids and solid particles; Apparatus for such processes with stationary particles, e.g. in fixed beds the fluid passing successively through two or more beds
- B01J8/0446—Chemical or physical processes in general, conducted in the presence of fluids and solid particles; Apparatus for such processes with stationary particles, e.g. in fixed beds the fluid passing successively through two or more beds the flow within the beds being predominantly vertical
- B01J8/0449—Chemical or physical processes in general, conducted in the presence of fluids and solid particles; Apparatus for such processes with stationary particles, e.g. in fixed beds the fluid passing successively through two or more beds the flow within the beds being predominantly vertical in two or more cylindrical beds
- B01J8/0457—Chemical or physical processes in general, conducted in the presence of fluids and solid particles; Apparatus for such processes with stationary particles, e.g. in fixed beds the fluid passing successively through two or more beds the flow within the beds being predominantly vertical in two or more cylindrical beds the beds being placed in separate reactors
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01B—NON-METALLIC ELEMENTS; COMPOUNDS THEREOF; METALLOIDS OR COMPOUNDS THEREOF NOT COVERED BY SUBCLASS C01C
- C01B3/00—Hydrogen; Gaseous mixtures containing hydrogen; Separation of hydrogen from mixtures containing it; Purification of hydrogen
- C01B3/02—Production of hydrogen or of gaseous mixtures containing a substantial proportion of hydrogen
- C01B3/32—Production of hydrogen or of gaseous mixtures containing a substantial proportion of hydrogen by reaction of gaseous or liquid organic compounds with gasifying agents, e.g. water, carbon dioxide, air
- C01B3/34—Production of hydrogen or of gaseous mixtures containing a substantial proportion of hydrogen by reaction of gaseous or liquid organic compounds with gasifying agents, e.g. water, carbon dioxide, air by reaction of hydrocarbons with gasifying agents
- C01B3/38—Production of hydrogen or of gaseous mixtures containing a substantial proportion of hydrogen by reaction of gaseous or liquid organic compounds with gasifying agents, e.g. water, carbon dioxide, air by reaction of hydrocarbons with gasifying agents using catalysts
- C01B3/382—Multi-step processes
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01B—NON-METALLIC ELEMENTS; COMPOUNDS THEREOF; METALLOIDS OR COMPOUNDS THEREOF NOT COVERED BY SUBCLASS C01C
- C01B3/00—Hydrogen; Gaseous mixtures containing hydrogen; Separation of hydrogen from mixtures containing it; Purification of hydrogen
- C01B3/02—Production of hydrogen or of gaseous mixtures containing a substantial proportion of hydrogen
- C01B3/32—Production of hydrogen or of gaseous mixtures containing a substantial proportion of hydrogen by reaction of gaseous or liquid organic compounds with gasifying agents, e.g. water, carbon dioxide, air
- C01B3/34—Production of hydrogen or of gaseous mixtures containing a substantial proportion of hydrogen by reaction of gaseous or liquid organic compounds with gasifying agents, e.g. water, carbon dioxide, air by reaction of hydrocarbons with gasifying agents
- C01B3/48—Production of hydrogen or of gaseous mixtures containing a substantial proportion of hydrogen by reaction of gaseous or liquid organic compounds with gasifying agents, e.g. water, carbon dioxide, air by reaction of hydrocarbons with gasifying agents followed by reaction of water vapour with carbon monoxide
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J2208/00—Processes carried out in the presence of solid particles; Reactors therefor
- B01J2208/00008—Controlling the process
- B01J2208/00017—Controlling the temperature
- B01J2208/00106—Controlling the temperature by indirect heat exchange
- B01J2208/00168—Controlling the temperature by indirect heat exchange with heat exchange elements outside the bed of solid particles
- B01J2208/00203—Coils
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J2208/00—Processes carried out in the presence of solid particles; Reactors therefor
- B01J2208/00008—Controlling the process
- B01J2208/00017—Controlling the temperature
- B01J2208/00504—Controlling the temperature by means of a burner
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J2219/00—Chemical, physical or physico-chemical processes in general; Their relevant apparatus
- B01J2219/00002—Chemical plants
- B01J2219/00004—Scale aspects
- B01J2219/00006—Large-scale industrial plants
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J2219/00—Chemical, physical or physico-chemical processes in general; Their relevant apparatus
- B01J2219/00002—Chemical plants
- B01J2219/00027—Process aspects
- B01J2219/00038—Processes in parallel
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J2219/00—Chemical, physical or physico-chemical processes in general; Their relevant apparatus
- B01J2219/00002—Chemical plants
- B01J2219/00027—Process aspects
- B01J2219/0004—Processes in series
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01B—NON-METALLIC ELEMENTS; COMPOUNDS THEREOF; METALLOIDS OR COMPOUNDS THEREOF NOT COVERED BY SUBCLASS C01C
- C01B2203/00—Integrated processes for the production of hydrogen or synthesis gas
- C01B2203/02—Processes for making hydrogen or synthesis gas
- C01B2203/0205—Processes for making hydrogen or synthesis gas containing a reforming step
- C01B2203/0227—Processes for making hydrogen or synthesis gas containing a reforming step containing a catalytic reforming step
- C01B2203/0244—Processes for making hydrogen or synthesis gas containing a reforming step containing a catalytic reforming step the reforming step being an autothermal reforming step, e.g. secondary reforming processes
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01B—NON-METALLIC ELEMENTS; COMPOUNDS THEREOF; METALLOIDS OR COMPOUNDS THEREOF NOT COVERED BY SUBCLASS C01C
- C01B2203/00—Integrated processes for the production of hydrogen or synthesis gas
- C01B2203/02—Processes for making hydrogen or synthesis gas
- C01B2203/025—Processes for making hydrogen or synthesis gas containing a partial oxidation step
- C01B2203/0261—Processes for making hydrogen or synthesis gas containing a partial oxidation step containing a catalytic partial oxidation step [CPO]
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01B—NON-METALLIC ELEMENTS; COMPOUNDS THEREOF; METALLOIDS OR COMPOUNDS THEREOF NOT COVERED BY SUBCLASS C01C
- C01B2203/00—Integrated processes for the production of hydrogen or synthesis gas
- C01B2203/02—Processes for making hydrogen or synthesis gas
- C01B2203/0283—Processes for making hydrogen or synthesis gas containing a CO-shift step, i.e. a water gas shift step
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01B—NON-METALLIC ELEMENTS; COMPOUNDS THEREOF; METALLOIDS OR COMPOUNDS THEREOF NOT COVERED BY SUBCLASS C01C
- C01B2203/00—Integrated processes for the production of hydrogen or synthesis gas
- C01B2203/12—Feeding the process for making hydrogen or synthesis gas
- C01B2203/1205—Composition of the feed
- C01B2203/1211—Organic compounds or organic mixtures used in the process for making hydrogen or synthesis gas
- C01B2203/1235—Hydrocarbons
- C01B2203/1241—Natural gas or methane
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01B—NON-METALLIC ELEMENTS; COMPOUNDS THEREOF; METALLOIDS OR COMPOUNDS THEREOF NOT COVERED BY SUBCLASS C01C
- C01B2203/00—Integrated processes for the production of hydrogen or synthesis gas
- C01B2203/12—Feeding the process for making hydrogen or synthesis gas
- C01B2203/1258—Pre-treatment of the feed
- C01B2203/1264—Catalytic pre-treatment of the feed
- C01B2203/127—Catalytic desulfurisation
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01B—NON-METALLIC ELEMENTS; COMPOUNDS THEREOF; METALLOIDS OR COMPOUNDS THEREOF NOT COVERED BY SUBCLASS C01C
- C01B2203/00—Integrated processes for the production of hydrogen or synthesis gas
- C01B2203/12—Feeding the process for making hydrogen or synthesis gas
- C01B2203/1276—Mixing of different feed components
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01B—NON-METALLIC ELEMENTS; COMPOUNDS THEREOF; METALLOIDS OR COMPOUNDS THEREOF NOT COVERED BY SUBCLASS C01C
- C01B2203/00—Integrated processes for the production of hydrogen or synthesis gas
- C01B2203/14—Details of the flowsheet
- C01B2203/142—At least two reforming, decomposition or partial oxidation steps in series
Landscapes
- Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Organic Chemistry (AREA)
- Inorganic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Combustion & Propulsion (AREA)
- General Health & Medical Sciences (AREA)
- Health & Medical Sciences (AREA)
- Hydrogen, Water And Hydrids (AREA)
- Catalysts (AREA)
- Industrial Gases (AREA)
- Gyroscopes (AREA)
- Solid-Sorbent Or Filter-Aiding Compositions (AREA)
Description
天然ガス、LPG又はLNGのようなガス状炭化水素は少量の遊離酸素、すなわちO2ガスを含有しうる。遊離酸素は、ガス状炭化水素をストリッパーガスとして使用することによって又は空気とブレンドすることによって意図せず導入されうる。例えば、天然ガスは、メンテナンス後のパージ不良、ストリッパーポンプへの空気漏れ、ガス乾燥機用ストリッパーガスとしての天然ガスの使用、ウォーターインジェクション用ストリッパーガスとしての天然ガスの使用の結果として、またダウンホールに注入された流体中の溶存空気に由来して遊離酸素を含有しうる。これらのプロセスから回収された天然ガス中の遊離酸素の量は70〜100ppm(体積)の範囲であろう。あるいは、遊離酸素は、発熱量を削減するためのいわゆる“エア・バランシング(air−balancing)”で空気とブレンドするプロセスによってLPG又はLNGに導入されうる。このようにしてLPG又はLNGに導入される遊離酸素の量は0.5体積%ほどであろう。
そこで本発明は、ガス状炭化水素ストリーム中の遊離酸素を削減する方法を提供する。該方法は、
(i)炭化水素から水素を含有するガス混合物を形成するステップと;
(ii)前記ガス混合物を遊離酸素を含有するガス状炭化水素ストリームと混合するステップと;そして
(iii)得られた炭化水素ガス混合物を、ガス状炭化水素中に存在する遊離酸素の少なくとも一部を水蒸気に変換する変換触媒上に通すステップと
を含む。
CH4 + 2O2 → CO2 + 2H2O
CH4 + H2O → CO + 3H2
CO + H2O → CO2 + H2
となる。
水性ガスシフト触媒は、貴金属ベース、鉄ベース又は銅ベースでありうる。例えば、25〜35%wtのCuO、30〜60%wtのZnO及び5〜40%のAl2O3を含有する粒状銅−亜鉛アルミナ低温シフト触媒が200〜250℃の範囲の温度で使用できる。あるいは、水性ガスシフト触媒はセリア又はチタニア上のPtであってもよい。
水素形成が、ATR、POx又はcPOxのいずれによるものであろうと、水性ガスシフト反応の有無にかかわらず、得られたガス混合物を冷却してから遊離酸素を含有する炭化水素と接触させるのが望ましいであろう。好ましくは、ガス混合物の温度は、遊離酸素を含有する炭化水素と合わせる場合、≦300℃、さらに好ましくは≦200℃、さらに好ましくは≦150℃である。ガス混合物の冷却は公知の熱交換器技術を用いて実施できる。例えばガス混合物は高及び中圧水蒸気生成における圧力下で水を用いて冷却できる。
1/2O2 + CO → CO2
変換触媒は、低温での水素及び/又は一酸化炭素の酸化に関して活性を示すことが示されている任意のものであってよく、好ましくは担持8族遷移金属触媒である。例えば、該触媒は、セリア、マグネシア、アルミナ、チタニア、ジルコニア又はシリカのような酸化物担体上の一つ又は複数のCo、Ni、Pt、Pd、Rh、Ir又はRuを含みうる。Auが存在してもよい。金属硫化物担体も使用できる。好ましくは、触媒はアルミナ上Au、PtSn、PtFe、PtCo、Pt、Pd、Co又はNi、例えばアルミナ上≦5%wt Ptである。変換触媒は、織物、不織布又は編物タイプのメッシュ、ペレット又は押出物のような粒子、発泡体、モノリス又は不活性担体上のコーティングの形態であってよい。遊離酸素の変換は、好ましくは≦300℃、さらに好ましくは≦200℃、最も好ましくは≦150℃で実施され、入口ガス温度は好ましくは<100℃、さらに好ましくは<50℃である。
所望であれば、水素含有ガスの一部を例えば適切な膜技術を用いて水素分離ステップにかけ、回収された水素を例えば水素化脱硫のために上流に送ることもできる。
本発明のプロセスに使用される装置は、特にサイドストリームの部分燃焼が影響を受ける場合、都合よくコンパクト化されうる。
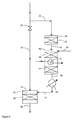
あるいは、水素形成手段は、炭化水素及び水蒸気入口手段、酸素含有ガス入口手段、生成物ガス出口手段を有し、そして入口及び出口手段の間に部分酸化触媒と水蒸気改質触媒が配置された自己熱改質装置を含みうる。
Claims (6)
- ガス状炭化水素ストリーム中の遊離酸素を削減する方法であって、
(i)遊離酸素を含有するガス状炭化水素ストリームの一部から水素含有ガス混合物を形成するステップ;
ここで、水素含有ガス混合物は、
炭化水素/水蒸気混合物の酸素含有ガスによる部分酸化の後、部分酸化されたガス混合物を担持Ni又は貴金属水蒸気改質触媒上に直接通すステップを含む自己熱改質によって形成されるか、または、
炭化水素を酸素含有ガスで部分酸化することによって形成され、
かつ、該形成された水素含有ガス混合物を水性ガスシフト触媒上で水性ガスシフト反応にかけ、該ガス混合物の水素含有量が増加される、
(ii)前記(i)で得られた水素含有ガス混合物を、遊離酸素を含有する炭化水素のガス状ストリームと混合して炭化水素ガス混合物を形成するステップ;及び
(iii)前記(ii)で得られた炭化水素ガス混合物を、前記炭化水素ガス混合物中に存在する遊離酸素の少なくとも一部を水蒸気に変換する、Co、Ni、Pt、Pd、Rh、Ir及びRuの一つ又は複数を含有する担持8族遷移金属変換触媒上に300℃以下で通すステップ、を含む、方法。 - 遊離酸素を含有する炭化水素が、ストリッパーガスとして使用された天然ガスを含む天然ガスである、請求項1に記載の方法。
- 遊離酸素の変換が≦300℃の温度で実施される、請求項1または請求項2に記載の方法。
- 硫黄吸収剤が(i)の水素形成ステップの上流に提供され、水素含有ガスの形成に使用される炭化水素から硫黄触媒毒が除去される、請求項1〜3のいずれか一項に記載の方法。
- ガス状炭化水素ストリームの遊離酸素含有量を削減するための装置であって、
遊離酸素を含有するガス状の炭化水素の入口手段と生成物ガスの出口手段とを有する変換容器、
前記容器内の前記入口手段及び出口手段の間に配置された、Co、Ni、Pt、Pd、Rh、Ir及びRuの一つ又は複数を含有する担持8族遷移金属変換触媒、及び
前記変換容器に接続された水素形成手段、を含み、
前記水素形成手段は、水素含有ガスを、ガス状の炭化水素が前記水素含有ガスと混合されて前記触媒上を300℃以下で通過するように前記容器に提供し、
ここで、
前記水素形成手段が、炭化水素入口手段、水蒸気入口手段、酸素含有ガス入口手段、生成物ガス出口手段を有し、そして前記入口手段及び出口手段の間に部分酸化手段と水蒸気改質触媒が配置された自己熱改質装置を含むか、または、
前記水素形成手段が、炭化水素及び酸素含有ガス入口手段、生成物ガス出口手段を有し、そして、前記入口手段及び出口手段の間に部分酸化触媒を有する部分燃焼容器を含み、
ここで、遊離酸素含有ガス状炭化水素のサイドストリーム部分が前記自己熱改質装置または前記部分燃焼容器に供給され、
水性ガスシフト触媒を有する水性ガスシフト容器が、自己熱改質装置又は部分燃焼容器と変換容器との間に、自己熱改質装置又は部分燃焼容器からのガス状生成物ストリームが遊離酸素含有ガス状炭化水素ストリームと混合される前に水素富化できるように接続される、
前記装置。 - 熱交換器手段が水素形成手段からの水素含有ガスを冷却するために提供される、請求項5に記載の装置。
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| GB0605232.8 | 2006-03-16 | ||
| GBGB0605232.8A GB0605232D0 (en) | 2006-03-16 | 2006-03-16 | Oxygen removal |
| PCT/GB2007/050100 WO2007105012A1 (en) | 2006-03-16 | 2007-03-05 | Oxygen removal |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2009530435A JP2009530435A (ja) | 2009-08-27 |
| JP2009530435A5 JP2009530435A5 (ja) | 2010-04-08 |
| JP5721310B2 true JP5721310B2 (ja) | 2015-05-20 |
Family
ID=36292814
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2008558910A Expired - Fee Related JP5721310B2 (ja) | 2006-03-16 | 2007-03-05 | 酸素除去 |
Country Status (12)
| Country | Link |
|---|---|
| US (1) | US9017642B2 (ja) |
| EP (1) | EP1993719B9 (ja) |
| JP (1) | JP5721310B2 (ja) |
| CN (1) | CN101394922B (ja) |
| AT (1) | ATE528070T1 (ja) |
| AU (1) | AU2007226323B2 (ja) |
| BR (1) | BRPI0708866A2 (ja) |
| CA (1) | CA2641183C (ja) |
| ES (1) | ES2373987T3 (ja) |
| GB (1) | GB0605232D0 (ja) |
| NO (1) | NO20083689L (ja) |
| WO (1) | WO2007105012A1 (ja) |
Families Citing this family (16)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP5274802B2 (ja) * | 2006-09-15 | 2013-08-28 | 株式会社日本触媒 | 酸素除去方法 |
| JP5033829B2 (ja) * | 2008-08-27 | 2012-09-26 | 株式会社神鋼環境ソリューション | 消化ガスの脱酸素方法及び装置 |
| EP2336083A1 (en) * | 2009-12-17 | 2011-06-22 | Topsøe Fuel Cell A/S | Gas generator and processes for the conversion of a fuel into an oxygen-depleted gas and/or hydrogen-enriched gas |
| GB201000097D0 (en) * | 2010-01-05 | 2010-12-29 | Johnson Matthey Plc | Apparatus and process for treating natural gas |
| JP5545719B2 (ja) * | 2010-01-28 | 2014-07-09 | 住友精化株式会社 | メタンを主成分とするガスの処理方法および処理装置 |
| CA2709722A1 (en) | 2010-07-15 | 2012-01-15 | Alakh Prasad | Integrated biogas cleaning a system to remove water, siloxanes, sulfur, oxygen, chlorides, and volatile organic compounds |
| JP5389753B2 (ja) * | 2010-07-27 | 2014-01-15 | 株式会社日立製作所 | 石炭ガス化ガスのco2分離回収装置 |
| JP2012142173A (ja) * | 2010-12-28 | 2012-07-26 | Jx Nippon Oil & Energy Corp | 燃料電池用脱硫システム、燃料電池用水素製造システム及び燃料電池システム、並びに、炭化水素系燃料の脱硫方法及び水素の製造方法 |
| CN102161924A (zh) * | 2011-01-25 | 2011-08-24 | 任国平 | 一种煤层气用生物质炭低温脱氧法 |
| JP2012211213A (ja) * | 2011-03-30 | 2012-11-01 | Tokyo Gas Co Ltd | バイオガスの精製方法 |
| JP2012207145A (ja) * | 2011-03-30 | 2012-10-25 | Sumitomo Seika Chem Co Ltd | メタンを主成分とするガスの処理方法 |
| CN103160352B (zh) * | 2013-03-11 | 2014-09-03 | 大连天元气体技术有限公司 | 一种含氧煤层气的脱氧方法 |
| US9238601B2 (en) * | 2013-10-15 | 2016-01-19 | Uop Llc | Method for providing oxygen free regeneration gas for natural gas dryers |
| DE102014223759A1 (de) * | 2014-11-20 | 2016-05-25 | Wacker Chemie Ag | Entfernung von Sauerstoff aus Kohlenwasserstoff-haltigen Gasgemischen |
| US11993514B2 (en) * | 2019-12-06 | 2024-05-28 | Palo Alto Research Center Incorporated | Liquid metal condensate catalyzed hydrocarbon pyrolysis |
| WO2023209423A1 (en) * | 2022-04-28 | 2023-11-02 | Kara Technologies Inc. | Method of deoxygenation of a hydrocarbon in the presence of methane-containing gas environment and catalyst structure |
Family Cites Families (27)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2384065A (en) | 1941-07-18 | 1945-09-04 | Air Reduction | Purification of gases |
| GB844971A (en) | 1957-11-26 | 1960-08-17 | Johnson Matthey Co Ltd | Improvements in and relating to the purification of gases or gaseous mixtures |
| NL270250A (ja) * | 1960-10-19 | |||
| US4181503A (en) * | 1978-10-30 | 1980-01-01 | United Technologies Corporation | Process for alternately steam reforming sulfur containing hydrocarbons that vary in oxygen content |
| NO152209C (no) | 1982-09-13 | 1985-08-28 | Norsk Hydro As | Fremgangsmaate ved pumping og avgassing av vann |
| GB2149391B (en) * | 1983-11-10 | 1987-10-07 | Westinghouse Electric Corp | Method for removing dissolved oxygen from aqueous media |
| US4579723A (en) | 1985-03-28 | 1986-04-01 | The Boc Group, Inc. | Methods for purifying inert gas streams |
| NO158283C (no) | 1986-02-13 | 1988-08-17 | Norsk Hydro As | Fremgangsmaate og anordning for behandling av blandinger vaeske/gass. |
| GB8610196D0 (en) | 1986-04-25 | 1986-05-29 | Ici Plc | Sulphur compounds removal |
| US4755498A (en) * | 1986-04-30 | 1988-07-05 | International Fuel Cells Corporation | Steam reforming catalyst |
| GB8623482D0 (en) | 1986-09-30 | 1986-11-05 | Johnson Matthey Plc | Catalytic generation of hydrogen |
| GB8805447D0 (en) * | 1988-03-08 | 1988-04-07 | British Petroleum Co Plc | Chemical process |
| EP0391839B1 (de) | 1989-04-05 | 1992-06-03 | GebràDer Sulzer Aktiengesellschaft | Verfahren zum Anfahren eines Prozesses zum Deoxidieren von Wasser, insbesondere von Meerwasser |
| GB9022060D0 (en) | 1990-10-10 | 1990-11-21 | Ici Plc | Mercury removal |
| US5204075A (en) | 1991-05-30 | 1993-04-20 | The Boc Group, Inc. | Process for the purification of the inert gases |
| US5446232A (en) | 1994-02-14 | 1995-08-29 | Occidental Chemical Corporation | Removing oxygen from hydrocarbon gases |
| FR2731693B1 (fr) | 1995-03-16 | 1997-05-23 | Air Liquide | Procede et installation de generation d'azote pour le traitement thermique |
| US20020007595A1 (en) | 1997-06-24 | 2002-01-24 | Uli Maier-Roeltgen | Method for reforming hydrocarbons autothermally |
| GB9806199D0 (en) | 1998-03-24 | 1998-05-20 | Johnson Matthey Plc | Catalytic generation of hydrogen |
| AU2188900A (en) * | 1998-12-15 | 2000-07-03 | Advanced Technology Materials, Inc. | Apparatus and method for point-of-use treatment of effluent gas streams |
| ATE302737T1 (de) | 1999-05-03 | 2005-09-15 | Nuvera Fuel Cells | Autothermen dampfreformierungsystem mit integrierten shift betten , reaktor für präferentielle oxidation ,hilfsreaktor und systemsteuerungen |
| US6280864B1 (en) * | 1999-06-18 | 2001-08-28 | Uop Llc | Control system for providing hydrogen for use with fuel cells |
| US6623720B2 (en) * | 2000-03-31 | 2003-09-23 | The Regents Of The University Of Michigan | Transition metal carbides, nitrides and borides, and their oxygen containing analogs useful as water gas shift catalysts |
| GB0223300D0 (en) * | 2002-10-08 | 2002-11-13 | Bp Chem Int Ltd | Process |
| NO20030647D0 (no) | 2003-02-10 | 2003-02-10 | Minox Technology As | Fremgangsmåte og anlegg for rensing av produsert vann |
| US20040159584A1 (en) | 2003-02-18 | 2004-08-19 | Ke Liu | Mini-CPO providing hydrogen for hydrogen desulfurization of hydrocarbon feeds |
| DE112004000293T5 (de) * | 2003-02-18 | 2006-10-19 | HydrogenSource LLC, South Windsor | Wasserstoffgenerator zur Wasserstoffentschwefelung für Kohlenwasserstoff-Rohrstoffe |
-
2006
- 2006-03-16 GB GBGB0605232.8A patent/GB0605232D0/en not_active Ceased
-
2007
- 2007-03-05 US US12/293,175 patent/US9017642B2/en not_active Expired - Fee Related
- 2007-03-05 BR BRPI0708866-3A patent/BRPI0708866A2/pt active Search and Examination
- 2007-03-05 AT AT07705384T patent/ATE528070T1/de not_active IP Right Cessation
- 2007-03-05 JP JP2008558910A patent/JP5721310B2/ja not_active Expired - Fee Related
- 2007-03-05 ES ES07705384T patent/ES2373987T3/es active Active
- 2007-03-05 WO PCT/GB2007/050100 patent/WO2007105012A1/en active Application Filing
- 2007-03-05 AU AU2007226323A patent/AU2007226323B2/en not_active Ceased
- 2007-03-05 EP EP07705384.1A patent/EP1993719B9/en not_active Not-in-force
- 2007-03-05 CA CA2641183A patent/CA2641183C/en not_active Expired - Fee Related
- 2007-03-05 CN CN200780007687.XA patent/CN101394922B/zh not_active Expired - Fee Related
-
2008
- 2008-08-27 NO NO20083689A patent/NO20083689L/no not_active Application Discontinuation
Also Published As
| Publication number | Publication date |
|---|---|
| BRPI0708866A2 (pt) | 2011-06-14 |
| US20090242462A1 (en) | 2009-10-01 |
| CA2641183A1 (en) | 2007-09-20 |
| WO2007105012A1 (en) | 2007-09-20 |
| ES2373987T3 (es) | 2012-02-10 |
| EP1993719B9 (en) | 2014-04-16 |
| US9017642B2 (en) | 2015-04-28 |
| ATE528070T1 (de) | 2011-10-15 |
| AU2007226323A1 (en) | 2007-09-20 |
| CN101394922B (zh) | 2012-06-27 |
| CN101394922A (zh) | 2009-03-25 |
| AU2007226323B2 (en) | 2011-03-17 |
| GB0605232D0 (en) | 2006-04-26 |
| EP1993719B1 (en) | 2011-10-12 |
| EP1993719A1 (en) | 2008-11-26 |
| NO20083689L (no) | 2008-10-08 |
| JP2009530435A (ja) | 2009-08-27 |
| CA2641183C (en) | 2013-04-30 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5721310B2 (ja) | 酸素除去 | |
| KR100908867B1 (ko) | 단일 챔버 콤팩트 연료 처리장치 | |
| CA2705640C (en) | Steam-hydrocarbon reforming with reduced carbon dioxide emissions | |
| EP1977993B1 (en) | Catalytic steam reforming with recycle | |
| JP2004515444A (ja) | 単一チャンバーのコンパクトな燃料処理装置 | |
| US9932229B2 (en) | Method and system for production of hydrogen rich gas mixtures | |
| AU2007333978A1 (en) | Hybrid combustor for fuel processing applications | |
| US7867411B2 (en) | Method for producing synthesis gas and apparatus for producing synthesis gas | |
| US8216324B2 (en) | Process for the production of hydrogen with a thermally-integrated desulfurization unit | |
| US20100028229A1 (en) | Oxygen removal | |
| JP5348938B2 (ja) | 一酸化炭素ガス発生装置および方法 | |
| EA046288B1 (ru) | Низкоуглеродное водородное топливо | |
| EA040289B1 (ru) | Система и способ для получения синтез-газа |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20100222 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20100222 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20120730 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20120903 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20121203 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20130902 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20131030 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20140617 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20140902 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20150313 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20150324 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 5721310 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| LAPS | Cancellation because of no payment of annual fees |