JP5568461B2 - 血液パラメータをモニタリングするための装置及び方法 - Google Patents
血液パラメータをモニタリングするための装置及び方法 Download PDFInfo
- Publication number
- JP5568461B2 JP5568461B2 JP2010500432A JP2010500432A JP5568461B2 JP 5568461 B2 JP5568461 B2 JP 5568461B2 JP 2010500432 A JP2010500432 A JP 2010500432A JP 2010500432 A JP2010500432 A JP 2010500432A JP 5568461 B2 JP5568461 B2 JP 5568461B2
- Authority
- JP
- Japan
- Prior art keywords
- blood
- body part
- light
- emitter
- reflectance
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
- 210000004369 blood Anatomy 0.000 title claims description 75
- 239000008280 blood Substances 0.000 title claims description 75
- 238000000034 method Methods 0.000 title claims description 58
- 238000012544 monitoring process Methods 0.000 title description 14
- 238000005259 measurement Methods 0.000 claims description 72
- 238000005534 hematocrit Methods 0.000 claims description 55
- 238000010521 absorption reaction Methods 0.000 claims description 43
- 238000002834 transmittance Methods 0.000 claims description 28
- 230000002829 reductive effect Effects 0.000 claims description 25
- 239000012503 blood component Substances 0.000 claims description 23
- 108010054147 Hemoglobins Proteins 0.000 claims description 18
- 102000001554 Hemoglobins Human genes 0.000 claims description 18
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical group [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 claims description 15
- 229910052760 oxygen Inorganic materials 0.000 claims description 15
- 239000001301 oxygen Substances 0.000 claims description 15
- INGWEZCOABYORO-UHFFFAOYSA-N 2-(furan-2-yl)-7-methyl-1h-1,8-naphthyridin-4-one Chemical compound N=1C2=NC(C)=CC=C2C(O)=CC=1C1=CC=CO1 INGWEZCOABYORO-UHFFFAOYSA-N 0.000 claims description 14
- 108010064719 Oxyhemoglobins Proteins 0.000 claims description 14
- 108010002255 deoxyhemoglobin Proteins 0.000 claims description 14
- 230000008859 change Effects 0.000 claims description 13
- 210000004204 blood vessel Anatomy 0.000 claims description 9
- 230000003595 spectral effect Effects 0.000 claims description 9
- 210000000624 ear auricle Anatomy 0.000 claims description 3
- 210000000883 ear external Anatomy 0.000 claims description 3
- 210000003371 toe Anatomy 0.000 claims description 3
- 210000003454 tympanic membrane Anatomy 0.000 claims description 3
- 210000001519 tissue Anatomy 0.000 description 49
- 230000009102 absorption Effects 0.000 description 39
- 230000003287 optical effect Effects 0.000 description 23
- 239000000306 component Substances 0.000 description 22
- 210000003743 erythrocyte Anatomy 0.000 description 21
- 238000005516 engineering process Methods 0.000 description 12
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 10
- 238000010586 diagram Methods 0.000 description 9
- 238000002310 reflectometry Methods 0.000 description 9
- 230000006870 function Effects 0.000 description 8
- 230000005540 biological transmission Effects 0.000 description 7
- 208000007502 anemia Diseases 0.000 description 4
- 230000017531 blood circulation Effects 0.000 description 4
- 238000000691 measurement method Methods 0.000 description 4
- 230000005855 radiation Effects 0.000 description 4
- 238000000862 absorption spectrum Methods 0.000 description 3
- 238000001514 detection method Methods 0.000 description 3
- 238000000502 dialysis Methods 0.000 description 3
- 230000031700 light absorption Effects 0.000 description 3
- 238000006213 oxygenation reaction Methods 0.000 description 3
- 238000012795 verification Methods 0.000 description 3
- 208000006545 Chronic Obstructive Pulmonary Disease Diseases 0.000 description 2
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 2
- 208000014767 Myeloproliferative disease Diseases 0.000 description 2
- 238000002835 absorbance Methods 0.000 description 2
- 230000009471 action Effects 0.000 description 2
- 238000004458 analytical method Methods 0.000 description 2
- 238000013459 approach Methods 0.000 description 2
- 230000003796 beauty Effects 0.000 description 2
- 230000009286 beneficial effect Effects 0.000 description 2
- 238000009534 blood test Methods 0.000 description 2
- 210000000988 bone and bone Anatomy 0.000 description 2
- 230000006378 damage Effects 0.000 description 2
- 230000018044 dehydration Effects 0.000 description 2
- 238000006297 dehydration reaction Methods 0.000 description 2
- 238000011026 diafiltration Methods 0.000 description 2
- 238000009792 diffusion process Methods 0.000 description 2
- 239000012530 fluid Substances 0.000 description 2
- OVBPIULPVIDEAO-LBPRGKRZSA-N folic acid Chemical compound C=1N=C2NC(N)=NC(=O)C2=NC=1CNC1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 OVBPIULPVIDEAO-LBPRGKRZSA-N 0.000 description 2
- 238000001631 haemodialysis Methods 0.000 description 2
- 230000000322 hemodialysis Effects 0.000 description 2
- 208000014674 injury Diseases 0.000 description 2
- 239000010410 layer Substances 0.000 description 2
- 238000005086 pumping Methods 0.000 description 2
- 238000001356 surgical procedure Methods 0.000 description 2
- 206010009944 Colon cancer Diseases 0.000 description 1
- 208000005156 Dehydration Diseases 0.000 description 1
- 208000032843 Hemorrhage Diseases 0.000 description 1
- 206010021143 Hypoxia Diseases 0.000 description 1
- 208000026350 Inborn Genetic disease Diseases 0.000 description 1
- 208000002720 Malnutrition Diseases 0.000 description 1
- OVBPIULPVIDEAO-UHFFFAOYSA-N N-Pteroyl-L-glutaminsaeure Natural products C=1N=C2NC(N)=NC(=O)C2=NC=1CNC1=CC=C(C(=O)NC(CCC(O)=O)C(O)=O)C=C1 OVBPIULPVIDEAO-UHFFFAOYSA-N 0.000 description 1
- 206010028980 Neoplasm Diseases 0.000 description 1
- 241001274197 Scatophagus argus Species 0.000 description 1
- 208000027418 Wounds and injury Diseases 0.000 description 1
- 230000001154 acute effect Effects 0.000 description 1
- 239000000853 adhesive Substances 0.000 description 1
- 230000001070 adhesive effect Effects 0.000 description 1
- 230000008901 benefit Effects 0.000 description 1
- 208000034158 bleeding Diseases 0.000 description 1
- 230000000740 bleeding effect Effects 0.000 description 1
- 210000000601 blood cell Anatomy 0.000 description 1
- 210000001124 body fluid Anatomy 0.000 description 1
- 239000010839 body fluid Substances 0.000 description 1
- 210000001185 bone marrow Anatomy 0.000 description 1
- 238000004364 calculation method Methods 0.000 description 1
- 201000011510 cancer Diseases 0.000 description 1
- 238000005119 centrifugation Methods 0.000 description 1
- FDJOLVPMNUYSCM-WZHZPDAFSA-L cobalt(3+);[(2r,3s,4r,5s)-5-(5,6-dimethylbenzimidazol-1-yl)-4-hydroxy-2-(hydroxymethyl)oxolan-3-yl] [(2r)-1-[3-[(1r,2r,3r,4z,7s,9z,12s,13s,14z,17s,18s,19r)-2,13,18-tris(2-amino-2-oxoethyl)-7,12,17-tris(3-amino-3-oxopropyl)-3,5,8,8,13,15,18,19-octamethyl-2 Chemical compound [Co+3].N#[C-].N([C@@H]([C@]1(C)[N-]\C([C@H]([C@@]1(CC(N)=O)C)CCC(N)=O)=C(\C)/C1=N/C([C@H]([C@@]1(CC(N)=O)C)CCC(N)=O)=C\C1=N\C([C@H](C1(C)C)CCC(N)=O)=C/1C)[C@@H]2CC(N)=O)=C\1[C@]2(C)CCC(=O)NC[C@@H](C)OP([O-])(=O)O[C@H]1[C@@H](O)[C@@H](N2C3=CC(C)=C(C)C=C3N=C2)O[C@@H]1CO FDJOLVPMNUYSCM-WZHZPDAFSA-L 0.000 description 1
- 208000029742 colonic neoplasm Diseases 0.000 description 1
- 239000002131 composite material Substances 0.000 description 1
- 238000011109 contamination Methods 0.000 description 1
- 230000008602 contraction Effects 0.000 description 1
- 238000007405 data analysis Methods 0.000 description 1
- 230000007812 deficiency Effects 0.000 description 1
- 238000003745 diagnosis Methods 0.000 description 1
- 201000010099 disease Diseases 0.000 description 1
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 1
- 230000000857 drug effect Effects 0.000 description 1
- 230000000694 effects Effects 0.000 description 1
- 238000013213 extrapolation Methods 0.000 description 1
- 210000001508 eye Anatomy 0.000 description 1
- 239000000835 fiber Substances 0.000 description 1
- 229960000304 folic acid Drugs 0.000 description 1
- 235000019152 folic acid Nutrition 0.000 description 1
- 239000011724 folic acid Substances 0.000 description 1
- 208000016361 genetic disease Diseases 0.000 description 1
- 230000007954 hypoxia Effects 0.000 description 1
- 229910052742 iron Inorganic materials 0.000 description 1
- 229960003284 iron Drugs 0.000 description 1
- 210000003734 kidney Anatomy 0.000 description 1
- 230000004807 localization Effects 0.000 description 1
- 210000004072 lung Anatomy 0.000 description 1
- 230000001071 malnutrition Effects 0.000 description 1
- 235000000824 malnutrition Nutrition 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 210000000214 mouth Anatomy 0.000 description 1
- 210000003205 muscle Anatomy 0.000 description 1
- 208000015380 nutritional deficiency disease Diseases 0.000 description 1
- 239000013307 optical fiber Substances 0.000 description 1
- 239000002245 particle Substances 0.000 description 1
- 230000007170 pathology Effects 0.000 description 1
- 230000035515 penetration Effects 0.000 description 1
- 230000001902 propagating effect Effects 0.000 description 1
- 239000011241 protective layer Substances 0.000 description 1
- 230000010349 pulsation Effects 0.000 description 1
- 238000002106 pulse oximetry Methods 0.000 description 1
- 230000000717 retained effect Effects 0.000 description 1
- 230000002441 reversible effect Effects 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 210000003491 skin Anatomy 0.000 description 1
- 210000004872 soft tissue Anatomy 0.000 description 1
- 238000001228 spectrum Methods 0.000 description 1
- 230000002123 temporal effect Effects 0.000 description 1
- 238000012360 testing method Methods 0.000 description 1
- 239000003053 toxin Substances 0.000 description 1
- 231100000765 toxin Toxicity 0.000 description 1
- 108700012359 toxins Proteins 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
- 230000008733 trauma Effects 0.000 description 1
- 238000011144 upstream manufacturing Methods 0.000 description 1
- 210000002700 urine Anatomy 0.000 description 1
- 239000002699 waste material Substances 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/145—Measuring characteristics of blood in vivo, e.g. gas concentration, pH value; Measuring characteristics of body fluids or tissues, e.g. interstitial fluid, cerebral tissue
- A61B5/1455—Measuring characteristics of blood in vivo, e.g. gas concentration, pH value; Measuring characteristics of body fluids or tissues, e.g. interstitial fluid, cerebral tissue using optical sensors, e.g. spectral photometrical oximeters
- A61B5/14551—Measuring characteristics of blood in vivo, e.g. gas concentration, pH value; Measuring characteristics of body fluids or tissues, e.g. interstitial fluid, cerebral tissue using optical sensors, e.g. spectral photometrical oximeters for measuring blood gases
- A61B5/14552—Details of sensors specially adapted therefor
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/145—Measuring characteristics of blood in vivo, e.g. gas concentration, pH value; Measuring characteristics of body fluids or tissues, e.g. interstitial fluid, cerebral tissue
- A61B5/14535—Measuring characteristics of blood in vivo, e.g. gas concentration, pH value; Measuring characteristics of body fluids or tissues, e.g. interstitial fluid, cerebral tissue for measuring haematocrit
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/68—Arrangements of detecting, measuring or recording means, e.g. sensors, in relation to patient
- A61B5/6801—Arrangements of detecting, measuring or recording means, e.g. sensors, in relation to patient specially adapted to be attached to or worn on the body surface
- A61B5/6813—Specially adapted to be attached to a specific body part
- A61B5/6825—Hand
- A61B5/6826—Finger
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/68—Arrangements of detecting, measuring or recording means, e.g. sensors, in relation to patient
- A61B5/6801—Arrangements of detecting, measuring or recording means, e.g. sensors, in relation to patient specially adapted to be attached to or worn on the body surface
- A61B5/683—Means for maintaining contact with the body
- A61B5/6838—Clamps or clips
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/72—Signal processing specially adapted for physiological signals or for diagnostic purposes
- A61B5/7235—Details of waveform analysis
- A61B5/7239—Details of waveform analysis using differentiation including higher order derivatives
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Physics & Mathematics (AREA)
- Engineering & Computer Science (AREA)
- General Health & Medical Sciences (AREA)
- Biomedical Technology (AREA)
- Heart & Thoracic Surgery (AREA)
- Medical Informatics (AREA)
- Molecular Biology (AREA)
- Surgery (AREA)
- Animal Behavior & Ethology (AREA)
- Biophysics (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Pathology (AREA)
- Optics & Photonics (AREA)
- Artificial Intelligence (AREA)
- Physiology (AREA)
- Psychiatry (AREA)
- Signal Processing (AREA)
- Spectroscopy & Molecular Physics (AREA)
- Computer Vision & Pattern Recognition (AREA)
- Measurement Of The Respiration, Hearing Ability, Form, And Blood Characteristics Of Living Organisms (AREA)
- Investigating Or Analysing Materials By Optical Means (AREA)
- Investigating Or Analysing Biological Materials (AREA)
Description
ここで、
μa,blood=組織サンプル内の全血液成分についての吸収係数;
μa,water=組織サンプル内の水分に関する吸収係数(血液内の水分は含まない);
μa,rest=水及び血液以外の組織サンプル(例えば皮膚、骨)内の全ての成分についての吸収係数;
Cb=全組織サンプル内の血液の容積百分率;
Cw=全組織サンプル(血液中の水分を含まない)内の水分の容積百分率;及び、
Crest=水分及び血液以外の全組織サンプル内の全ての成分の容積百分率。
μ’s,blood =組織サンプルサンプル内の全血液成分についての削減散乱係数;及び、
μ’s,rest=水分と血液以外の組織サンプル内の全ての成分についての削減散乱係数。
ここで、
Hct=ヘマトクリット値(血液中のRBCの容積百分率);
V=RBCの平均容量;
SAT=血液酸素減衰の比(全RBCに対する酸素化さらたRBCの量);
σox=酸素化されたRBCの吸収断面;
σdeox=非酸素化されたRBCの吸収断面;及び
μa,plasma =血液内の血漿についての吸収係数。単純化の目的のために、このパラメータは、RBC以外の血液内の全成分による吸収を含む。
ここで、
zb=組織から、フルエンス率がゼロにされる外挿境界までの距離;及び、
ρ=光が組織に入射する点からの半径方向距離。
ここで、
T=媒体の透過率;
Iout=媒体を通過する光の強度;
Iin=示される光の強度;
d =光が媒体を通過する距離;
F(μ,d)=μsとdの複合関数。
Claims (15)
- 被験者の血液に関する少なくとも1つのパラメータを測定するための方法であって、
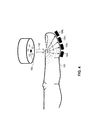
380−980nmのスペクトル範囲内において少なくとも1つの測定される波長で、少なくとも1つのエミッタで、少なくとも1つの血管を含む身体の部分に向かって、光を放射すること、
少なくとも1つの反射率検出器で、前記身体の部分によって反射された光を捕捉することにより、空間的に分解された光の反射率の測定を得ること、
前記測定から、少なくとも1つの前記測定される各波長で、前記身体の部分の血液成分の吸収係数(μa)及び削減散乱係数(μs’)のうちの少なくとも1つを引き出すこと、
前記吸収係数及び前記削減散乱係数のうちの少なくとも1つにおける時間導関数(∂μ s ’/∂t及び∂μ a /∂t)を引き出すこと、及び、
単独の波長での前記時間導関数の比率を用いて、少なくとも1つの前記測定される各波長について、前記パラメータを計算すること、
の工程を含む方法。 - 前記光を放射する行程は、オキシヘモグロビン及びデオキシヘモグロビンに関する等吸収波長で光を放射することを含む請求項1に記載の方法。
- 前記等吸収波長は、
約390nm;
約422nm;
約452nm;
約500nm;
約529nm;
約545nm;
約570nm;
約584nm;及び
約803nm;
からなるリストから選択される請求項2に記載の方法。 - 前記身体の部分を横切って前記エミッタの反対側に配置される少なくとも1つの透過率検出器で、前記身体の部分を通過する光を捕捉することにより、拡散反射率測定を得るという工程をさらに含む請求項1に記載の方法。
- 前記身体の部分は、
指;
つま先;
外側の耳;
鼓膜;
耳たぶ;
口;
目;
手のひら;及び
指の間の皮膜;
からなるリストから選択される請求項1の方法。 - 前記パラメータは、
酸素飽和レベル;
ヘモグロビン濃度;
血中濃度の時間変化;
平均RBCサイズ;
平均RBCサイズ分布;
からなるリストから選択される請求項1に記載の方法。 - 前記光を放射する工程は、少なくとも2つの測定される波長で光を放射することを含み、前記計算する工程は、少なくとも2つの前記測定される波長で、前記被験者の酸素飽和レベル及びヘマトクリット値を解くことを含む請求項1に記載の方法。
- 前記光を放射する工程は、少なくとも3つの測定される波長で、光を放射することを含み、そして、前記計算する工程は、少なくとも3つの前記測定される各波長で、前記被験者の酸素飽和レベル、血液濃度の時間変化、及びヘマトクリット値を解くことを含む請求項1に記載の方法。
- 被験者の血液に関する少なくとも1つのパラメータを測定するための装置であって、
380−980nmのスペクトル範囲内で、少なくとも1つの測定される波長で、少なくとも1つの血管を含む身体の部分に向かって、光を放射するための少なくとも1つのエミッタ;
前記身体の部分によって反射される光を捕捉することにより、空間的に分解された光の反射率の測定を得るための少なくとも1つの反射率検出器;
及び、前記測定から、少なくとも1つの前記測定される各波長で、前記身体の部分の血液成分の吸収係数(μa)及び削減散乱係数(μs’)のうちの少なくとも1つを引き出し、前記吸収係数及び前記削減散乱係数のうちの少なくとも1つにおける時間導関数(∂μ s ’/∂t及び∂μ a /∂t)を引き出し、そして、単独の波長での前記時間導関数の比率を使用して、少なくとも1つの前記測定される各波長について、前記パラメータを計算するためのプロセッサ、
を含む装置。 - 前記身体の部分を通過する光を捕捉することによって、拡散透過率測定を得るために、前記身体の部分を横切って前記エミッタの反対側に配置される少なくとも1つの透過率検出器をさらに含む請求項9に記載の装置。
- 前記身体の部分は、
指;
つま先;
外側の耳;
鼓膜;
耳たぶ;
口;
目;
手のひら;及び
指の間の皮膜;
からなるリストから選択される請求項9に記載の装置。 - 前記パラメータは、
酸素飽和レベル;
ヘモグロビン濃度;
血中濃度の時間変化;
平均RBCサイズ;
平均RBCサイズ分布;
からなるリストから選択される請求項9に記載の装置。 - 前記エミッタは、オキシヘモグロビン及びデオキシヘモグロビンに関して、等吸収波長で光を放射する請求項9に記載の装置。
- 前記等吸収波長は、
約390nm;
約422nm;
約452nm;
約500nm;
約529nm;
約545nm;
約570nm;
約584nm;及び
約803nm;
からなるリストから選択される請求項13に記載の装置。 - 前記エミッタ及び前記反射率検出器の作動を制御するために、前記エミッタ及び前記反射率検出器に接続されるコントローラ;
前記計算されるパラメータを表示するために、前記プロセッサに接続されるディスプレイ;
前記身体の部分に設けられ、前記身体の部分を保護し、前記放射された光及び前記反射された光を実質的に垂直な方向に導くための身体接触手段;及び、
前記装置を安定した方式で保持し、前記放射された光を正確にかつ効率的に前記身体の部分に導くための保持手段;
からなるグループから選択される少なくとも1つをさらに含む請求項9に記載の装置。
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US92011607P | 2007-03-27 | 2007-03-27 | |
| US60/920,116 | 2007-03-27 | ||
| PCT/IL2008/000413 WO2008117286A2 (en) | 2007-03-27 | 2008-03-26 | Device and method for monitoring blood parameters |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2010522603A JP2010522603A (ja) | 2010-07-08 |
| JP2010522603A5 JP2010522603A5 (ja) | 2011-05-19 |
| JP5568461B2 true JP5568461B2 (ja) | 2014-08-06 |
Family
ID=39789121
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2010500432A Expired - Fee Related JP5568461B2 (ja) | 2007-03-27 | 2008-03-26 | 血液パラメータをモニタリングするための装置及び方法 |
Country Status (4)
| Country | Link |
|---|---|
| US (1) | US8017407B2 (ja) |
| EP (1) | EP2129288B1 (ja) |
| JP (1) | JP5568461B2 (ja) |
| WO (1) | WO2008117286A2 (ja) |
Families Citing this family (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8781546B2 (en) * | 2008-04-11 | 2014-07-15 | Covidien Lp | System and method for differentiating between tissue-specific and systemic causes of changes in oxygen saturation in tissue and organs |
| WO2011068998A2 (en) * | 2009-12-02 | 2011-06-09 | Duke University | Systems and methods for determining hemoglobin concentration utilizing diffuse reflectance at isosbestic wavelengths |
| US9002655B2 (en) * | 2010-05-03 | 2015-04-07 | Gambro Lundia Ab | Medical apparatus for extracorporeal blood treatment and method for determining a blood parameter value in a medical apparatus thereof |
| US20130248695A1 (en) * | 2010-10-29 | 2013-09-26 | Duncan MacIntyre | Method and apparatus for analyte detection |
| WO2012135100A1 (en) * | 2011-03-30 | 2012-10-04 | Robert Steuer | Method and apparatus for non-invasive photometric blood constituent diagnosis |
| US10092226B2 (en) | 2011-12-23 | 2018-10-09 | General Electric Company | Method, arrangement, sensor, and computer program product for non-invasively measuring hemoglobin concentrations in blood |
| US20160113530A1 (en) * | 2014-10-23 | 2016-04-28 | Samsung Electronics Co., Ltd. | Method and apparatus for acquiring biological information, and wrist watch-type terminal using the same |
| NZ773812A (en) | 2015-03-16 | 2022-07-29 | Magic Leap Inc | Methods and systems for diagnosing and treating health ailments |
| JP2018534962A (ja) * | 2015-09-23 | 2018-11-29 | コーニンクレッカ フィリップス エヌ ヴェKoninklijke Philips N.V. | マルチカラーパルスオキシメータ |
| EP3417276B1 (en) * | 2016-02-16 | 2021-06-30 | Stichting Het Nederlands Kanker Instituut- Antoni van Leeuwenhoek Ziekenhuis | Method, apparatus and computer program for estimating a property of an optically diffuse medium |
| CN106940290A (zh) * | 2017-01-22 | 2017-07-11 | 广州地理研究所 | 基于高光谱数据的水库水体氨氮含量估算方法 |
| WO2020044337A1 (en) * | 2018-08-29 | 2020-03-05 | Tel Hashomer Medical Research Infrastructure And Services Ltd. | System and method for determining oxygenated-blood content of biological tissue |
| US11744930B2 (en) * | 2020-04-06 | 2023-09-05 | Fresenius Medical Care Holdings, Inc. | Intradialytic monitoring of hemodynamic status based on detection of oxygen signature phase shift |
| WO2022061262A1 (en) * | 2020-09-21 | 2022-03-24 | PAVmed Inc. | Systems and methods for non-invasive solute measurement |
Family Cites Families (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS5725217B2 (ja) * | 1974-10-14 | 1982-05-28 | ||
| US4142101B1 (en) | 1977-07-20 | 1991-02-19 | Low intensity x-ray and gamma-ray imaging device | |
| US6681128B2 (en) | 1990-10-06 | 2004-01-20 | Hema Metrics, Inc. | System for noninvasive hematocrit monitoring |
| JP2002501803A (ja) * | 1998-02-05 | 2002-01-22 | イン−ラインダイアグノスティックスコーポレイション | 非観血的血液成分モニタ方法および装置 |
| US6662031B1 (en) | 1998-05-18 | 2003-12-09 | Abbott Laboratoies | Method and device for the noninvasive determination of hemoglobin and hematocrit |
| JP2000155090A (ja) * | 1998-11-20 | 2000-06-06 | Fuji Photo Film Co Ltd | 血管の画像化装置 |
| DE19921928C2 (de) * | 1999-05-12 | 2002-11-14 | Bosch Gmbh Robert | Elektrisches Gerät |
| US6587704B1 (en) | 1999-06-16 | 2003-07-01 | Orsense Ltd. | Method for non-invasive optical measurements of blood parameters |
| US6213952B1 (en) | 1999-09-28 | 2001-04-10 | Orsense Ltd. | Optical device for non-invasive measurement of blood related signals utilizing a finger holder |
| US6505060B1 (en) | 2000-09-29 | 2003-01-07 | Datex-Ohmeda, Inc. | Method and apparatus for determining pulse oximetry differential values |
| US6434408B1 (en) * | 2000-09-29 | 2002-08-13 | Datex-Ohmeda, Inc. | Pulse oximetry method and system with improved motion correction |
| US6660995B1 (en) * | 2001-06-25 | 2003-12-09 | Regents Of The University Of California | Particle size analysis in a turbid media with a single-fiber, optical probe while using a visible spectrometer |
| WO2005025399A2 (en) * | 2003-09-12 | 2005-03-24 | Or-Nim Medical Ltd. | Noninvasive optical monitoring of region of interest |
| EP3505052A1 (en) | 2005-04-25 | 2019-07-03 | University of Massachusetts | Systems and methods for correcting optical reflectance measurements |
-
2008
- 2008-03-26 WO PCT/IL2008/000413 patent/WO2008117286A2/en active Search and Examination
- 2008-03-26 EP EP08720038.2A patent/EP2129288B1/en not_active Not-in-force
- 2008-03-26 US US12/593,246 patent/US8017407B2/en not_active Expired - Fee Related
- 2008-03-26 JP JP2010500432A patent/JP5568461B2/ja not_active Expired - Fee Related
Also Published As
| Publication number | Publication date |
|---|---|
| US20100076281A1 (en) | 2010-03-25 |
| WO2008117286A2 (en) | 2008-10-02 |
| US8017407B2 (en) | 2011-09-13 |
| WO2008117286A3 (en) | 2010-02-25 |
| JP2010522603A (ja) | 2010-07-08 |
| EP2129288A2 (en) | 2009-12-09 |
| EP2129288A4 (en) | 2013-11-06 |
| EP2129288B1 (en) | 2016-12-21 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5568461B2 (ja) | 血液パラメータをモニタリングするための装置及び方法 | |
| JP4176480B2 (ja) | 非侵襲性のヘマトクリット測定値の精度を改善するための方法および装置 | |
| US10912504B2 (en) | Near-infrared spectroscopy and diffuse correlation spectroscopy device and methods | |
| JP4465271B2 (ja) | 対象組織内の血液酸素飽和度を非侵襲的に決定する装置 | |
| US12076116B2 (en) | Sensor for tissue measurements | |
| JP3625475B2 (ja) | 非侵入的にヘマトクリット値をモニタするシステム | |
| US9591999B2 (en) | Determination of tissue oxygenation in vivo | |
| JP4903980B2 (ja) | パルスオキシメータ及びその操作方法 | |
| EP2503935B1 (en) | Apparatus for spectrophotometric blood oxygenation monitoring of organs in the body | |
| US20080004513A1 (en) | VCSEL Tissue Spectrometer | |
| Paul et al. | Design and development of non invasive glucose measurement system | |
| JP2007509718A (ja) | 体液および電解質障害をモニタリングするデバイスおよび方法 | |
| US10925525B2 (en) | Combined pulse oximetry and diffusing wave spectroscopy system and control method therefor | |
| US10342488B2 (en) | Probes and pressure modulation algorithms for reducing extratissue contamination in hemodynamic measurement | |
| CA2375635A1 (en) | Adaptive calibration pulsed oximetry method and device | |
| JPH11244267A (ja) | 血中成分濃度測定装置 | |
| US20150217056A1 (en) | Therapy systems and methods utilizing tissue oxygenation detection | |
| RU2524131C2 (ru) | Способ оптического детектирования и устройство для оптического детектирования состояния суставов | |
| Reuss et al. | The pulse in reflectance pulse oximetry: modeling and experimental studies | |
| McMurdy et al. | Photonics‐based In Vivo total hemoglobin monitoring and clinical relevance | |
| Patil et al. | Methods and devices to determine hemoglobin non invasively: A review | |
| Kraitl et al. | Optical non-invasive methods for characterization of the human health status | |
| Kraitl et al. | Analysis of time series for non-invasive characterization of blood components and circulation patterns | |
| Lu et al. | Portable near-infrared spectroscopy for detecting peripheral arterial occlusion | |
| IL201199A (en) | A method for controlling blood parameters |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20110325 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20110325 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20120921 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20121005 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20121226 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20130108 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20130204 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20130212 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20130226 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20130305 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20130403 |
|
| RD04 | Notification of resignation of power of attorney |
Free format text: JAPANESE INTERMEDIATE CODE: A7424 Effective date: 20130730 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20130906 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20140606 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20140623 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 5568461 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| LAPS | Cancellation because of no payment of annual fees |