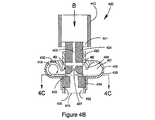
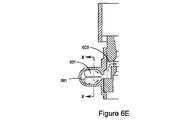
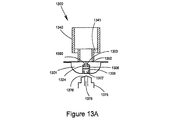
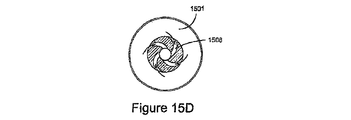
JP5528336B2 - デリバリー装置及び関連方法 - Google Patents
デリバリー装置及び関連方法 Download PDFInfo
- Publication number
- JP5528336B2 JP5528336B2 JP2010514883A JP2010514883A JP5528336B2 JP 5528336 B2 JP5528336 B2 JP 5528336B2 JP 2010514883 A JP2010514883 A JP 2010514883A JP 2010514883 A JP2010514883 A JP 2010514883A JP 5528336 B2 JP5528336 B2 JP 5528336B2
- Authority
- JP
- Japan
- Prior art keywords
- chamber
- fluid
- drug
- housing
- mouthpiece
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
- 238000000034 method Methods 0.000 title description 12
- 239000012530 fluid Substances 0.000 claims description 389
- 239000003814 drug Substances 0.000 claims description 317
- 229940079593 drug Drugs 0.000 claims description 291
- 238000004891 communication Methods 0.000 claims description 114
- 238000011144 upstream manufacturing Methods 0.000 claims description 9
- 239000006185 dispersion Substances 0.000 description 86
- 238000012377 drug delivery Methods 0.000 description 82
- 239000000463 material Substances 0.000 description 41
- 230000007246 mechanism Effects 0.000 description 26
- 238000005520 cutting process Methods 0.000 description 23
- 239000002245 particle Substances 0.000 description 23
- 210000000214 mouth Anatomy 0.000 description 15
- 239000004033 plastic Substances 0.000 description 12
- 230000013011 mating Effects 0.000 description 11
- 239000011888 foil Substances 0.000 description 10
- 238000001647 drug administration Methods 0.000 description 8
- 210000004072 lung Anatomy 0.000 description 8
- 239000000843 powder Substances 0.000 description 8
- 230000004048 modification Effects 0.000 description 7
- 238000012986 modification Methods 0.000 description 7
- 230000002829 reductive effect Effects 0.000 description 7
- 230000029058 respiratory gaseous exchange Effects 0.000 description 7
- 238000009513 drug distribution Methods 0.000 description 6
- 239000007788 liquid Substances 0.000 description 6
- 238000007789 sealing Methods 0.000 description 6
- 238000003860 storage Methods 0.000 description 6
- 238000011109 contamination Methods 0.000 description 5
- 230000000994 depressogenic effect Effects 0.000 description 5
- 230000000694 effects Effects 0.000 description 5
- 210000003811 finger Anatomy 0.000 description 5
- 238000005243 fluidization Methods 0.000 description 5
- 239000000203 mixture Substances 0.000 description 5
- 238000013519 translation Methods 0.000 description 5
- 230000009286 beneficial effect Effects 0.000 description 4
- 230000003134 recirculating effect Effects 0.000 description 4
- 230000001225 therapeutic effect Effects 0.000 description 4
- 241001465754 Metazoa Species 0.000 description 3
- 230000015556 catabolic process Effects 0.000 description 3
- 239000003795 chemical substances by application Substances 0.000 description 3
- 239000000356 contaminant Substances 0.000 description 3
- 238000006731 degradation reaction Methods 0.000 description 3
- 238000009826 distribution Methods 0.000 description 3
- 230000001788 irregular Effects 0.000 description 3
- 230000036961 partial effect Effects 0.000 description 3
- 230000037361 pathway Effects 0.000 description 3
- 238000009517 secondary packaging Methods 0.000 description 3
- 125000006850 spacer group Chemical group 0.000 description 3
- 230000000087 stabilizing effect Effects 0.000 description 3
- 239000010409 thin film Substances 0.000 description 3
- 230000032258 transport Effects 0.000 description 3
- 230000009471 action Effects 0.000 description 2
- 239000000853 adhesive Substances 0.000 description 2
- 230000001070 adhesive effect Effects 0.000 description 2
- 238000004873 anchoring Methods 0.000 description 2
- 230000004888 barrier function Effects 0.000 description 2
- 230000008901 benefit Effects 0.000 description 2
- 230000000903 blocking effect Effects 0.000 description 2
- 230000003247 decreasing effect Effects 0.000 description 2
- 230000008021 deposition Effects 0.000 description 2
- 230000000881 depressing effect Effects 0.000 description 2
- 229920001971 elastomer Polymers 0.000 description 2
- 235000013305 food Nutrition 0.000 description 2
- 230000006872 improvement Effects 0.000 description 2
- 238000002347 injection Methods 0.000 description 2
- 239000007924 injection Substances 0.000 description 2
- 238000003780 insertion Methods 0.000 description 2
- 230000037431 insertion Effects 0.000 description 2
- 230000000670 limiting effect Effects 0.000 description 2
- 238000004519 manufacturing process Methods 0.000 description 2
- 238000002156 mixing Methods 0.000 description 2
- 239000002105 nanoparticle Substances 0.000 description 2
- 238000002360 preparation method Methods 0.000 description 2
- 238000005096 rolling process Methods 0.000 description 2
- 239000003566 sealing material Substances 0.000 description 2
- 238000002560 therapeutic procedure Methods 0.000 description 2
- YCKRFDGAMUMZLT-UHFFFAOYSA-N Fluorine atom Chemical compound [F] YCKRFDGAMUMZLT-UHFFFAOYSA-N 0.000 description 1
- 241000282412 Homo Species 0.000 description 1
- 125000002066 L-histidyl group Chemical group [H]N1C([H])=NC(C([H])([H])[C@](C(=O)[*])([H])N([H])[H])=C1[H] 0.000 description 1
- 206010035664 Pneumonia Diseases 0.000 description 1
- 238000009825 accumulation Methods 0.000 description 1
- 230000001154 acute effect Effects 0.000 description 1
- 230000004075 alteration Effects 0.000 description 1
- 229940035674 anesthetics Drugs 0.000 description 1
- 230000000844 anti-bacterial effect Effects 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- 208000006673 asthma Diseases 0.000 description 1
- 239000008376 breath freshener Substances 0.000 description 1
- 206010006451 bronchitis Diseases 0.000 description 1
- 238000000576 coating method Methods 0.000 description 1
- 230000001010 compromised effect Effects 0.000 description 1
- 239000012141 concentrate Substances 0.000 description 1
- 235000009508 confectionery Nutrition 0.000 description 1
- 239000002872 contrast media Substances 0.000 description 1
- 230000000593 degrading effect Effects 0.000 description 1
- 239000000975 dye Substances 0.000 description 1
- 239000000806 elastomer Substances 0.000 description 1
- 230000007613 environmental effect Effects 0.000 description 1
- 150000002148 esters Chemical class 0.000 description 1
- 238000004299 exfoliation Methods 0.000 description 1
- 230000001815 facial effect Effects 0.000 description 1
- 229910052731 fluorine Inorganic materials 0.000 description 1
- 239000011737 fluorine Substances 0.000 description 1
- 239000003205 fragrance Substances 0.000 description 1
- 239000003193 general anesthetic agent Substances 0.000 description 1
- 238000001802 infusion Methods 0.000 description 1
- 230000003993 interaction Effects 0.000 description 1
- 238000002372 labelling Methods 0.000 description 1
- 230000004199 lung function Effects 0.000 description 1
- 230000014759 maintenance of location Effects 0.000 description 1
- 238000002483 medication Methods 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 230000002906 microbiologic effect Effects 0.000 description 1
- 239000011859 microparticle Substances 0.000 description 1
- 239000002991 molded plastic Substances 0.000 description 1
- 238000000465 moulding Methods 0.000 description 1
- 239000002324 mouth wash Substances 0.000 description 1
- 235000016709 nutrition Nutrition 0.000 description 1
- 230000000050 nutritive effect Effects 0.000 description 1
- 230000001151 other effect Effects 0.000 description 1
- 230000035515 penetration Effects 0.000 description 1
- 230000008447 perception Effects 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 239000003380 propellant Substances 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 210000002345 respiratory system Anatomy 0.000 description 1
- 230000000717 retained effect Effects 0.000 description 1
- 238000007493 shaping process Methods 0.000 description 1
- 239000007779 soft material Substances 0.000 description 1
- 230000006641 stabilisation Effects 0.000 description 1
- 238000011105 stabilization Methods 0.000 description 1
- 238000010254 subcutaneous injection Methods 0.000 description 1
- 239000007929 subcutaneous injection Substances 0.000 description 1
- 235000000346 sugar Nutrition 0.000 description 1
- 239000013589 supplement Substances 0.000 description 1
- 229940126585 therapeutic drug Drugs 0.000 description 1
- 210000003813 thumb Anatomy 0.000 description 1
- 239000011782 vitamin Substances 0.000 description 1
- 229940088594 vitamin Drugs 0.000 description 1
- 229930003231 vitamin Natural products 0.000 description 1
- 235000013343 vitamin Nutrition 0.000 description 1
- 239000002699 waste material Substances 0.000 description 1
- 238000003466 welding Methods 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0001—Details of inhalators; Constructional features thereof
- A61M15/0005—Details of inhalators; Constructional features thereof with means for agitating the medicament
- A61M15/0006—Details of inhalators; Constructional features thereof with means for agitating the medicament using rotating means
- A61M15/0008—Details of inhalators; Constructional features thereof with means for agitating the medicament using rotating means rotating by airflow
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0028—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M11/00—Sprayers or atomisers specially adapted for therapeutic purposes
- A61M11/001—Particle size control
- A61M11/002—Particle size control by flow deviation causing inertial separation of transported particles
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M11/00—Sprayers or atomisers specially adapted for therapeutic purposes
- A61M11/006—Sprayers or atomisers specially adapted for therapeutic purposes operated by applying mechanical pressure to the liquid to be sprayed or atomised
- A61M11/008—Sprayers or atomisers specially adapted for therapeutic purposes operated by applying mechanical pressure to the liquid to be sprayed or atomised by squeezing, e.g. using a flexible bottle or a bulb
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0001—Details of inhalators; Constructional features thereof
- A61M15/0013—Details of inhalators; Constructional features thereof with inhalation check valves
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0001—Details of inhalators; Constructional features thereof
- A61M15/002—Details of inhalators; Constructional features thereof with air flow regulating means
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0001—Details of inhalators; Constructional features thereof
- A61M15/0021—Mouthpieces therefor
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0028—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up
- A61M15/003—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using capsules, e.g. to be perforated or broken-up
- A61M15/0033—Details of the piercing or cutting means
- A61M15/0035—Piercing means
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0028—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up
- A61M15/003—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using capsules, e.g. to be perforated or broken-up
- A61M15/0033—Details of the piercing or cutting means
- A61M15/004—Details of the piercing or cutting means with fixed piercing or cutting means
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0028—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up
- A61M15/003—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using capsules, e.g. to be perforated or broken-up
- A61M15/0033—Details of the piercing or cutting means
- A61M15/0041—Details of the piercing or cutting means with movable piercing or cutting means
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0028—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up
- A61M15/003—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using capsules, e.g. to be perforated or broken-up
- A61M15/0043—Non-destructive separation of the package, e.g. peeling
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0028—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up
- A61M15/0045—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using multiple prepacked dosages on a same carrier, e.g. blisters
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0028—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up
- A61M15/0045—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using multiple prepacked dosages on a same carrier, e.g. blisters
- A61M15/0046—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using multiple prepacked dosages on a same carrier, e.g. blisters characterized by the type of carrier
- A61M15/0048—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using multiple prepacked dosages on a same carrier, e.g. blisters characterized by the type of carrier the dosages being arranged in a plane, e.g. on diskettes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0028—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up
- A61M15/0045—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using multiple prepacked dosages on a same carrier, e.g. blisters
- A61M15/0046—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using multiple prepacked dosages on a same carrier, e.g. blisters characterized by the type of carrier
- A61M15/005—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using multiple prepacked dosages on a same carrier, e.g. blisters characterized by the type of carrier the dosages being arranged on a cylindrical surface
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0028—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up
- A61M15/0061—Inhalators using prepacked dosages, one for each application, e.g. capsules to be perforated or broken-up using pre-packed dosages having an insert inside
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0065—Inhalators with dosage or measuring devices
- A61M15/0068—Indicating or counting the number of dispensed doses or of remaining doses
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0065—Inhalators with dosage or measuring devices
- A61M15/0068—Indicating or counting the number of dispensed doses or of remaining doses
- A61M15/007—Mechanical counters
- A61M15/0071—Mechanical counters having a display or indicator
- A61M15/0075—Mechanical counters having a display or indicator on a disc
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0065—Inhalators with dosage or measuring devices
- A61M15/0068—Indicating or counting the number of dispensed doses or of remaining doses
- A61M15/0081—Locking means
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0086—Inhalation chambers
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0086—Inhalation chambers
- A61M15/0088—Inhalation chambers with variable volume
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/009—Inhalators using medicine packages with incorporated spraying means, e.g. aerosol cans
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M16/00—Devices for influencing the respiratory system of patients by gas treatment, e.g. ventilators; Tracheal tubes
- A61M16/04—Tracheal tubes
- A61M16/0488—Mouthpieces; Means for guiding, securing or introducing the tubes
- A61M16/049—Mouthpieces
- A61M16/0495—Mouthpieces with tongue depressors
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M11/00—Sprayers or atomisers specially adapted for therapeutic purposes
- A61M11/02—Sprayers or atomisers specially adapted for therapeutic purposes operated by air or other gas pressure applied to the liquid or other product to be sprayed or atomised
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M11/00—Sprayers or atomisers specially adapted for therapeutic purposes
- A61M11/06—Sprayers or atomisers specially adapted for therapeutic purposes of the injector type
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2202/00—Special media to be introduced, removed or treated
- A61M2202/06—Solids
- A61M2202/064—Powder
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2205/00—General characteristics of the apparatus
- A61M2205/07—General characteristics of the apparatus having air pumping means
- A61M2205/071—General characteristics of the apparatus having air pumping means hand operated
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2205/00—General characteristics of the apparatus
- A61M2205/07—General characteristics of the apparatus having air pumping means
- A61M2205/071—General characteristics of the apparatus having air pumping means hand operated
- A61M2205/075—Bulb type
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2205/00—General characteristics of the apparatus
- A61M2205/60—General characteristics of the apparatus with identification means
- A61M2205/6045—General characteristics of the apparatus with identification means having complementary physical shapes for indexing or registration purposes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2205/00—General characteristics of the apparatus
- A61M2205/60—General characteristics of the apparatus with identification means
- A61M2205/6063—Optical identification systems
- A61M2205/6081—Colour codes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2205/00—General characteristics of the apparatus
- A61M2205/82—Internal energy supply devices
- A61M2205/8218—Gas operated
- A61M2205/8225—Gas operated using incorporated gas cartridges for the driving gas
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2206/00—Characteristics of a physical parameter; associated device therefor
- A61M2206/10—Flow characteristics
- A61M2206/16—Rotating swirling helical flow, e.g. by tangential inflows
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2209/00—Ancillary equipment
- A61M2209/06—Packaging for specific medical equipment
Landscapes
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Life Sciences & Earth Sciences (AREA)
- Anesthesiology (AREA)
- General Health & Medical Sciences (AREA)
- Biomedical Technology (AREA)
- Heart & Thoracic Surgery (AREA)
- Hematology (AREA)
- Veterinary Medicine (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Pulmonology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Biophysics (AREA)
- Otolaryngology (AREA)
- Emergency Medicine (AREA)
- Mechanical Engineering (AREA)
- Infusion, Injection, And Reservoir Apparatuses (AREA)
- Medicinal Preparation (AREA)
Applications Claiming Priority (7)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US94833107P | 2007-07-06 | 2007-07-06 | |
| US60/948,331 | 2007-07-06 | ||
| US97181207P | 2007-09-12 | 2007-09-12 | |
| US60/971,812 | 2007-09-12 | ||
| US5263208P | 2008-05-12 | 2008-05-12 | |
| US61/052,632 | 2008-05-12 | ||
| PCT/US2008/008303 WO2009009013A2 (en) | 2007-07-06 | 2008-07-06 | Inhalation devices for storing and delivering medicament |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2013021615A Division JP5651200B2 (ja) | 2007-07-06 | 2013-02-06 | デリバリー装置 |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2010532677A JP2010532677A (ja) | 2010-10-14 |
| JP2010532677A5 JP2010532677A5 (enExample) | 2011-08-04 |
| JP5528336B2 true JP5528336B2 (ja) | 2014-06-25 |
Family
ID=39790456
Family Applications (4)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2010514883A Expired - Fee Related JP5528336B2 (ja) | 2007-07-06 | 2008-07-06 | デリバリー装置及び関連方法 |
| JP2013021615A Active JP5651200B2 (ja) | 2007-07-06 | 2013-02-06 | デリバリー装置 |
| JP2014231220A Expired - Fee Related JP6497893B2 (ja) | 2007-07-06 | 2014-11-14 | デリバリー装置及び関連方法 |
| JP2017011539A Pending JP2017070849A (ja) | 2007-07-06 | 2017-01-25 | デリバリー装置及び関連方法 |
Family Applications After (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2013021615A Active JP5651200B2 (ja) | 2007-07-06 | 2013-02-06 | デリバリー装置 |
| JP2014231220A Expired - Fee Related JP6497893B2 (ja) | 2007-07-06 | 2014-11-14 | デリバリー装置及び関連方法 |
| JP2017011539A Pending JP2017070849A (ja) | 2007-07-06 | 2017-01-25 | デリバリー装置及び関連方法 |
Country Status (6)
| Country | Link |
|---|---|
| US (5) | US8291901B2 (enExample) |
| EP (3) | EP3453418A1 (enExample) |
| JP (4) | JP5528336B2 (enExample) |
| CN (1) | CN101795723B (enExample) |
| DK (1) | DK2898914T3 (enExample) |
| WO (1) | WO2009009013A2 (enExample) |
Families Citing this family (89)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9006175B2 (en) | 1999-06-29 | 2015-04-14 | Mannkind Corporation | Potentiation of glucose elimination |
| AU2003220125B2 (en) | 2002-03-20 | 2006-06-15 | Mannkind Corporation | Inhalation apparatus |
| PL1786784T3 (pl) | 2004-08-20 | 2011-04-29 | Mannkind Corp | Kataliza syntezy diketopiperazyn |
| EP2314298B1 (en) | 2004-08-23 | 2015-05-27 | MannKind Corporation | Microparticles comprising diketopiperazine salts for drug delivery |
| US8763605B2 (en) | 2005-07-20 | 2014-07-01 | Manta Devices, Llc | Inhalation device |
| AU2006290870B2 (en) | 2005-09-14 | 2013-02-28 | Mannkind Corporation | Method of drug formulation based on increasing the affinity of active agents for crystalline microparticle surfaces |
| DK1986679T3 (da) | 2006-02-22 | 2017-11-20 | Mannkind Corp | Fremgangsmåde til forbedring af mikropartiklers farmaceutiske egenskaber omfattende diketopiperazin og et aktivt indholdsstof |
| EP3453418A1 (en) | 2007-07-06 | 2019-03-13 | Manta Devices, LLC | Delivery device and related methods |
| US11224704B2 (en) | 2007-07-06 | 2022-01-18 | Manta Devices, Llc | Dose delivery device for inhalation |
| WO2009079078A1 (en) | 2007-12-14 | 2009-06-25 | Labogroup S.A.S. | Delivering aerosolizable food products |
| MX2010006657A (es) | 2007-12-20 | 2010-10-05 | Astrazeneca Ab | Dispositivo y metodo para desagregar polvo 854. |
| EP2082764A1 (en) * | 2008-01-24 | 2009-07-29 | Boehringer Ingelheim International GmbH | Inhaler |
| US8485180B2 (en) | 2008-06-13 | 2013-07-16 | Mannkind Corporation | Dry powder drug delivery system |
| CN103252007B (zh) | 2008-06-13 | 2016-06-22 | 曼金德公司 | 干粉吸入器和用于药物输送的系统 |
| JP5479465B2 (ja) | 2008-06-20 | 2014-04-23 | マンカインド コーポレイション | 吸入努力をリアルタイムにプロファイルする対話式機器および方法 |
| TWI614024B (zh) | 2008-08-11 | 2018-02-11 | 曼凱公司 | 超快起作用胰島素之用途 |
| WO2010039200A2 (en) | 2008-09-30 | 2010-04-08 | Oriel Therapeutics, Inc. | Dry powder inhalers with endless strips and cooperating piercers and related methods |
| US9050427B2 (en) * | 2008-09-30 | 2015-06-09 | Oriel Therapeutics, Inc. | Dry powder inhalers with multi-facet surface deagglomeration chambers and related devices and methods |
| AT507631B1 (de) | 2008-11-26 | 2012-05-15 | Haas Rouven Mag | Vorrichtung zum ansaugen von pulver- oder granulat-material und kapsel hierfür |
| US8314106B2 (en) | 2008-12-29 | 2012-11-20 | Mannkind Corporation | Substituted diketopiperazine analogs for use as drug delivery agents |
| DE102009006431B4 (de) * | 2009-01-23 | 2010-12-30 | Ing. Erich Pfeiffer Gmbh | Austragvorrichtung |
| EP2676695A3 (en) | 2009-03-11 | 2017-03-01 | MannKind Corporation | Apparatus, system and method for measuring resistance of an inhaler |
| KR101875969B1 (ko) | 2009-06-12 | 2018-07-06 | 맨카인드 코포레이션 | 한정된 비표면적을 갖는 디케토피페라진 마이크로입자 |
| JP2012531961A (ja) | 2009-07-01 | 2012-12-13 | アストラゼネカ・アクチエボラーグ | 粉末を気流に引き込むためのディスペンサーおよび方法 |
| WO2011056889A1 (en) | 2009-11-03 | 2011-05-12 | Mannkind Corporation | An apparatus and method for simulating inhalation efforts |
| WO2011116293A2 (en) * | 2010-03-19 | 2011-09-22 | Manta Devices, Llc | Delivery device and related methods |
| RU2531455C2 (ru) | 2010-06-21 | 2014-10-20 | Маннкайнд Корпорейшн | Системы и способы доставки сухих порошковых лекарств |
| WO2012010877A2 (en) * | 2010-07-21 | 2012-01-26 | Astrazeneca Ab | New device |
| WO2012054878A2 (en) * | 2010-10-21 | 2012-04-26 | Gliders, LLC | Delivery systems and method thereof |
| ES2492679T3 (es) * | 2011-02-02 | 2014-09-10 | Sulzer Mixpac Ag | Dispositivo de descarga para material fluido |
| CN105667994B (zh) | 2011-04-01 | 2018-04-06 | 曼金德公司 | 用于药物药盒的泡罩包装 |
| US11273099B2 (en) | 2011-05-16 | 2022-03-15 | The Technology Partnership, Plc. | Dose container |
| US9750901B2 (en) * | 2011-06-13 | 2017-09-05 | Shanghai Xiuxin Chenpon Pharmaceutical Technology Co., Ltd. | Dry powder drug-dosing device |
| WO2012174472A1 (en) | 2011-06-17 | 2012-12-20 | Mannkind Corporation | High capacity diketopiperazine microparticles |
| US11103659B2 (en) * | 2011-07-06 | 2021-08-31 | Manta Devices, Llc | Delivery device and related methods |
| FR2979058B1 (fr) * | 2011-08-19 | 2013-08-30 | Valois Sas | Dispositif d'inhalation de poudre. |
| WO2013036881A2 (en) * | 2011-09-07 | 2013-03-14 | Syphase, Llc | Dry powder inhalation device |
| WO2013063160A1 (en) | 2011-10-24 | 2013-05-02 | Mannkind Corporation | Methods and compositions for treating pain |
| US10463815B2 (en) * | 2012-02-21 | 2019-11-05 | Respira Therapeutics, Inc. | Inhaler to deliver substances for prophylaxis or prevention of disease or injury caused by the inhalation of biological or chemical agents |
| SG11201500218VA (en) | 2012-07-12 | 2015-03-30 | Mannkind Corp | Dry powder drug delivery systems and methods |
| US9248247B2 (en) * | 2012-07-26 | 2016-02-02 | Nathaniel Gerald Portney | Capsular medication delivery and inhalation device |
| EP2708219A1 (en) * | 2012-09-12 | 2014-03-19 | PARI Pharma GmbH | Opening element for opening an ampoule in an aerosol generation device and aerosol generation device comprising the opening element |
| EP2911690A1 (en) | 2012-10-26 | 2015-09-02 | MannKind Corporation | Inhalable influenza vaccine compositions and methods |
| WO2014089174A2 (en) * | 2012-12-06 | 2014-06-12 | Aerodesigns, Inc. | Aerosol dispenser with edible cartridge |
| WO2014102658A1 (en) * | 2012-12-26 | 2014-07-03 | Koninklijke Philips N.V. | Intuitive overlaid readiness indicator for defibrillators |
| US8920364B2 (en) | 2013-02-28 | 2014-12-30 | Medtronic Xomed, Inc. | Biomaterial delivery device |
| US8845578B2 (en) | 2013-02-28 | 2014-09-30 | Medtronic Xomed, Inc. | Biomaterial delivery device |
| EP2970149B1 (en) | 2013-03-15 | 2019-08-21 | MannKind Corporation | Microcrystalline diketopiperazine compositions and methods |
| US10835691B2 (en) | 2013-05-17 | 2020-11-17 | Koninklijke Philips N.V. | Substance delivery module |
| JP6499160B2 (ja) * | 2013-05-17 | 2019-04-10 | コーニンクレッカ フィリップス エヌ ヴェKoninklijke Philips N.V. | 物質送達モジュール用のカートリッジ |
| EP3021919B1 (en) * | 2013-07-16 | 2017-10-04 | Emphasys Importadora, Exportadora E Distribuidora De Embalagens Ltda. | Powder inhaler |
| CN105451716A (zh) | 2013-07-18 | 2016-03-30 | 曼金德公司 | 热稳定性干粉药物组合物和方法 |
| WO2015021064A1 (en) | 2013-08-05 | 2015-02-12 | Mannkind Corporation | Insufflation apparatus and methods |
| CN103417466B (zh) * | 2013-08-15 | 2015-01-07 | 江南大学 | 一种天然保湿护肤组合物及其在化妆品中的应用 |
| US10307464B2 (en) | 2014-03-28 | 2019-06-04 | Mannkind Corporation | Use of ultrarapid acting insulin |
| SE538399C2 (sv) * | 2014-04-03 | 2016-06-14 | Iconovo Ab | Torrpulverinhalator |
| WO2015168572A2 (en) * | 2014-05-02 | 2015-11-05 | Manta Devices, Llc | Delivery device and related methods |
| WO2016014153A1 (en) * | 2014-07-23 | 2016-01-28 | Microdose Therapeutx, Inc. | Dry powder nebulizer |
| DE102014215064A1 (de) | 2014-07-31 | 2016-02-04 | Pari GmbH Spezialisten für effektive Inhalation | Vernebler |
| US10561806B2 (en) | 2014-10-02 | 2020-02-18 | Mannkind Corporation | Mouthpiece cover for an inhaler |
| JP2018501935A (ja) * | 2015-01-08 | 2018-01-25 | コンベクシティ サイエンティフィック エルエルシーConvexity Scientific Llc | ネブライザー装置 |
| CN108144168A (zh) * | 2015-05-16 | 2018-06-12 | 苏州汉方医药有限公司 | 一种手动悬浮微颗粒发生器与化学药物制剂组成的治疗药盒 |
| ES2742148T3 (es) * | 2015-07-18 | 2020-02-13 | Hoefliger Harro Verpackung | Procedimiento y dispositivo para la separación y la transferencia de gránulos |
| JP6957454B2 (ja) * | 2015-11-06 | 2021-11-02 | ベーリンガー インゲルハイム インターナショナル ゲゼルシャフト ミット ベシュレンクテル ハフツング | ネブライザと容器とを有するシステム |
| DE102016001408A1 (de) * | 2016-02-09 | 2017-08-10 | Drägerwerk AG & Co. KGaA | HME-Vorrichtung zur Verwendung in einem Atemkreislauf eines Beatmungssystems |
| WO2017179071A1 (en) * | 2016-04-15 | 2017-10-19 | Atul Sardana | An integrated packing, reconstitution and delivery device for oral vaccines and drugs containing multiple components |
| CN115591066A (zh) | 2017-01-10 | 2023-01-13 | 波士顿科学国际有限公司(Us) | 用于输送粉末药剂的装置和方法 |
| CN108567448A (zh) * | 2017-03-10 | 2018-09-25 | 阿普洛维克斯公司 | 用于阴道流体收集的自取样设备 |
| CA3057683A1 (en) | 2017-03-28 | 2018-10-04 | Concentrx Pharmaceuticals, Inc. | Devices and methods for delivering dry powder medicaments |
| US11690964B2 (en) | 2017-05-31 | 2023-07-04 | Virginia Commonwealth University | Devices, systems, and methods for dry powder therapies |
| GB201713899D0 (en) * | 2017-08-30 | 2017-10-11 | Indosys Ltd | Multi-dose medicament delivery device |
| EP3884980B1 (en) * | 2018-11-19 | 2024-06-26 | Shin Nippon Biomedical Laboratories, Ltd. | Medicine dispensing device |
| RU2767698C1 (ru) * | 2018-12-06 | 2022-03-18 | Филип Моррис Продактс С.А. | Мундштук с внутренней и внешней трубчатыми секциями |
| KR102852206B1 (ko) * | 2019-01-14 | 2025-09-02 | 필립모리스 프로덕츠 에스.에이. | 건조 분말 흡입기 장치 |
| CN113329779B (zh) * | 2019-01-14 | 2023-08-08 | 菲利普莫里斯生产公司 | 干粉吸入器 |
| US10813386B1 (en) * | 2019-08-29 | 2020-10-27 | Puff Corporation | Transportable mouthpiece |
| JP7386651B2 (ja) | 2019-09-02 | 2023-11-27 | 株式会社キャタラー | 排ガス浄化用触媒 |
| CN110575446A (zh) * | 2019-09-09 | 2019-12-17 | 上海臣邦医药科技股份有限公司 | 一种复方吸入组合物及其制备方法 |
| WO2021089485A1 (en) * | 2019-11-05 | 2021-05-14 | Jt International Sa | Inhaler |
| CN121102700A (zh) * | 2019-12-03 | 2025-12-12 | 波士顿科学国际有限公司 | 药剂施用医疗装置 |
| RU2742406C1 (ru) * | 2020-09-29 | 2021-02-05 | Общество с ограниченной ответственностью "ВКМ групп" | Ингалятор |
| US11000067B1 (en) | 2020-10-05 | 2021-05-11 | Puff Corporation | Portable electronic vaporizing device |
| CN115120819B (zh) * | 2021-03-24 | 2024-02-09 | 心诚镁行动医电股份有限公司 | 雾化装置以及喷嘴模块 |
| US11576431B1 (en) | 2021-09-28 | 2023-02-14 | Puff Corporation | Portable water pipe assembly |
| WO2023186758A1 (en) * | 2022-03-28 | 2023-10-05 | Astrazeneca Ab | Dry powder formulation inhaler and capsule |
| FR3134520B1 (fr) * | 2022-04-14 | 2024-04-19 | Aptar France Sas | Inhalateur unidose de poudre |
| DE102022121402A1 (de) * | 2022-08-24 | 2024-02-29 | Nebu-Tec Med. Produkte Eike Kern Gmbh | Strömungsoptimiertes Mundstück mit Luftspalt für einen Inhalator |
| GB202218332D0 (en) | 2022-12-06 | 2023-01-18 | Cambridge Healthcare Innovations Ltd | Drug-delivery device and method |
| CN119656437B (zh) * | 2024-12-19 | 2025-10-10 | 江西侨明医疗器械有限公司 | 一种防气体倒流污染的医疗雾化器 |
Family Cites Families (134)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2603216A (en) * | 1952-07-15 | Powder inhaler | ||
| US1410556A (en) * | 1919-03-07 | 1922-03-28 | Frank C Dorment | Inhaler |
| US2307986A (en) | 1940-02-15 | 1943-01-12 | Bolte | Insufflator |
| US2436878A (en) * | 1945-03-02 | 1948-03-02 | Joseph B Biederman | Inhaler |
| US2590832A (en) | 1949-03-29 | 1952-03-25 | Frederick M Turnbull | Inhaler with closure |
| US2860638A (en) | 1956-02-21 | 1958-11-18 | Bartolomeo Frank | Smoking device |
| US2893392A (en) | 1958-01-08 | 1959-07-07 | American Cyanamid Co | Article of manufacture for intracutaneous injections |
| US2974787A (en) | 1960-05-10 | 1961-03-14 | American Cyanamid Co | Single use, prepackaged vaccinator |
| GB983870A (en) * | 1961-12-29 | 1965-02-17 | Longworth Scient Instr Company | Improvements in or relating to anaesthetising apparatus |
| FR1591549A (enExample) | 1967-11-17 | 1970-04-27 | ||
| US3888253A (en) | 1972-08-04 | 1975-06-10 | Beecham Group Ltd | Device for administration of medicines |
| GB1521000A (en) * | 1975-06-13 | 1978-08-09 | Syntex Puerto Rico Inc | Inhalation device |
| US4104027A (en) | 1977-11-21 | 1978-08-01 | Carroll Robert B | Process for the presumptive identification of narcotics and drugs of abuse |
| DE2960616D1 (en) | 1978-05-03 | 1981-11-12 | Fisons Plc | Inhalation device |
| IT1116047B (it) | 1979-04-27 | 1986-02-10 | Sigma Tau Ind Farmaceuti | Dispositivo per la rapida inalazione di farmaci in polvere da parte di persone sofferenti di asma |
| US4601896A (en) | 1984-03-21 | 1986-07-22 | Mark Nugent | Pharmaceutical capsule compositions and structures for gastric sensitive materials |
| AT396872B (de) | 1985-07-30 | 1993-12-27 | Glaxo Group Ltd | Gerät zur verabreichung von medikamenten in pulverform |
| AT384552B (de) * | 1985-08-01 | 1987-12-10 | Hurka Wilhelm | Inhalationsgeraet zur dosierung und verteilung von festkoerpern in die atemluft |
| DK479189D0 (da) * | 1989-01-06 | 1989-09-28 | Hans Gernot Schenk | Inhalator |
| FR2649323B1 (fr) | 1989-07-04 | 1995-06-30 | Valois | Dispositif de projection et de pulverisation d'une dose d'un produit divisable |
| DE4004904A1 (de) * | 1990-02-16 | 1990-09-13 | Gerhard Brendel | Trommel-applikator |
| US6536427B2 (en) * | 1990-03-02 | 2003-03-25 | Glaxo Group Limited | Inhalation device |
| US5167242A (en) | 1990-06-08 | 1992-12-01 | Kabi Pharmacia Aktiebolaq | Nicotine-impermeable container and method of fabricating the same |
| ATE209938T1 (de) * | 1990-09-26 | 2001-12-15 | Pharmachemie Bv | Wirbelkammer-pulverinhalator |
| FR2667509B1 (fr) | 1990-10-04 | 1995-08-25 | Valois | Inhalateur a poudre, dispositif de conditionnement de microdoses de poudre sous forme de bandes adaptees a etre utilisees dans un inhalateur a poudre, et procede de fabrication de ces bandes. |
| FR2676929B1 (fr) * | 1991-05-30 | 1994-02-11 | Aerosols Bouchage Ste Fse | Inhalateur de poudres. |
| CA2112674C (en) * | 1991-07-02 | 2005-10-04 | John S. Patton | Method and device for delivering aerosolized medicaments |
| US5337740A (en) * | 1991-08-01 | 1994-08-16 | New England Pharmaceuticals, Inc. | Inhalation devices |
| US5476093A (en) | 1992-02-14 | 1995-12-19 | Huhtamaki Oy | Device for more effective pulverization of a powdered inhalation medicament |
| US5239993A (en) * | 1992-08-26 | 1993-08-31 | Glaxo Inc. | Dosage inhalator providing optimized compound inhalation trajectory |
| GB2270293A (en) | 1992-09-05 | 1994-03-09 | Medix Ltd | Drug dispensing system |
| UA27961C2 (uk) * | 1992-12-18 | 2000-10-16 | Шерінг Корпорейшн | Інгалятор для порошкових ліків |
| US5533502A (en) * | 1993-05-28 | 1996-07-09 | Vortran Medical Technology, Inc. | Powder inhaler with aerosolization occurring within each individual powder receptacle |
| SE9302550D0 (sv) | 1993-07-30 | 1993-07-30 | Ernst Hoerlin | Powder inhaler |
| US5388572A (en) | 1993-10-26 | 1995-02-14 | Tenax Corporation (A Connecticut Corp.) | Dry powder medicament inhalator having an inhalation-activated piston to aerosolize dose and deliver same |
| DE4400083C2 (de) | 1994-01-04 | 1997-07-03 | Asta Medica Ag | Verpackung für kleine vordosierte Mengen eines fein verteilten Feststoffs |
| DE59409856D1 (de) | 1994-05-19 | 2001-10-11 | Pari Gmbh | Vorrichtung zur Trocknung und Pufferung von Aerosolen |
| US5483954A (en) * | 1994-06-10 | 1996-01-16 | Mecikalski; Mark B. | Inhaler and medicated package |
| CZ289029B6 (cs) | 1994-09-21 | 2001-10-17 | Inhale Therapeutic Systems | Způsob aerosolizace práąku, zejména práąkového léku a zařízení k provádění tohoto způsobu |
| JPH08103499A (ja) * | 1994-10-04 | 1996-04-23 | Unisia Jecs Corp | 吸入式投薬器 |
| DE4440734A1 (de) | 1994-11-15 | 1996-05-23 | Bayer Ag | Abscheidesystem für einen Pulverinhalator |
| US5622166A (en) | 1995-04-24 | 1997-04-22 | Dura Pharmaceuticals, Inc. | Dry powder inhaler delivery system |
| US5921237A (en) | 1995-04-24 | 1999-07-13 | Dura Pharmaceuticals, Inc. | Dry powder inhaler |
| JP3320261B2 (ja) * | 1995-06-01 | 2002-09-03 | 株式会社ユニシアジェックス | 吸入式投薬器 |
| US6209538B1 (en) | 1995-08-02 | 2001-04-03 | Robert A. Casper | Dry powder medicament inhalator having an inhalation-activated flow diverting means for triggering delivery of medicament |
| US5823183A (en) | 1995-08-02 | 1998-10-20 | Innovative Devices | Dry powder medicament inhalator having an inhalation-activated flow diverting means for triggering delivery of medicament |
| SE9502800D0 (sv) | 1995-08-10 | 1995-08-10 | Astra Ab | Disposable inhaler |
| JPH11513641A (ja) | 1995-10-20 | 1999-11-24 | ファルマシア・アンド・アップジョン・カンパニー | ブリスターパッケージ |
| US5673793A (en) | 1995-10-27 | 1997-10-07 | Seidler; David | Blister pack with built-in product ejection system |
| US5669378A (en) | 1995-12-21 | 1997-09-23 | Pera; Ivo | Inhaling device |
| US5694920A (en) * | 1996-01-25 | 1997-12-09 | Abrams; Andrew L. | Inhalation device |
| US6470884B2 (en) * | 1996-01-29 | 2002-10-29 | Aventis Pharma Limited | Capsule opening arrangement for use in a powder inhaler |
| FR2744991B1 (fr) | 1996-02-15 | 1998-03-20 | Oreal | Melangeur monocorps,pour le conditionnement separe et le melange d'au moins deux produits |
| HU220949B1 (en) | 1996-04-25 | 2002-06-29 | Astrazeneca Ab | Inhaler |
| GB9626263D0 (en) | 1996-12-18 | 1997-02-05 | Innovata Biomed Ltd | Powder inhaler |
| EE200000373A (et) * | 1997-12-22 | 2001-10-15 | Astrazeneca Ab | Inhalatsiooniseade |
| US6257233B1 (en) * | 1998-06-04 | 2001-07-10 | Inhale Therapeutic Systems | Dry powder dispersing apparatus and methods for their use |
| US6234169B1 (en) * | 1998-08-14 | 2001-05-22 | Arthur Slutsky | Inhaler |
| FI108518B (fi) | 1999-04-23 | 2002-02-15 | Orion Yhtymae Oyj | Jauheinhalaattori yhdistelmälääkkeelle |
| GB9909357D0 (en) * | 1999-04-24 | 1999-06-16 | Glaxo Group Ltd | Medicament carrier |
| US6606992B1 (en) * | 1999-06-30 | 2003-08-19 | Nektar Therapeutics | Systems and methods for aerosolizing pharmaceutical formulations |
| EP1072530A1 (en) | 1999-07-19 | 2001-01-31 | Société des Produits Nestlé S.A. | An assembly for two products to be mixed just prior to use |
| US7464706B2 (en) * | 1999-07-23 | 2008-12-16 | Mannkind Corporation | Unit dose cartridge and dry powder inhaler |
| US7305986B1 (en) | 1999-07-23 | 2007-12-11 | Mannkind Corporation | Unit dose capsules for use in a dry powder inhaler |
| EP1220698B1 (en) | 1999-10-12 | 2004-07-21 | SHL Medical AB | Inhaler |
| US6810872B1 (en) | 1999-12-10 | 2004-11-02 | Unisia Jecs Corporation | Inhalant medicator |
| US6679256B2 (en) | 1999-12-17 | 2004-01-20 | Nektar Therapeutics | Systems and methods for extracting powders from receptacles |
| US6443307B1 (en) | 2000-01-25 | 2002-09-03 | Michael D. Burridge | Medication dispenser with an internal ejector |
| US20030034271A1 (en) | 2000-01-25 | 2003-02-20 | Burridge Michael D. | Internal ejector punch for blister-pack type containers |
| US7069929B2 (en) | 2000-02-01 | 2006-07-04 | Quadrant Technologies Limited | Dry powder inhaler |
| US6427688B1 (en) * | 2000-02-01 | 2002-08-06 | Dura Pharmaceuticals, Icn. | Dry powder inhaler |
| DE10011120A1 (de) * | 2000-03-09 | 2001-09-13 | Pfeiffer Erich Gmbh & Co Kg | Spender für Medien |
| US6948494B1 (en) * | 2000-05-10 | 2005-09-27 | Innovative Devices, Llc. | Medicament container with same side airflow inlet and outlet and method of use |
| US6668827B2 (en) | 2000-05-16 | 2003-12-30 | Nektar Therapeutics | Systems devices and methods for opening receptacles having a powder to be fluidized |
| US20020020408A1 (en) | 2000-05-25 | 2002-02-21 | Invivotech, Inc. | Inhalation medicament delivery device |
| AR028747A1 (es) | 2000-06-23 | 2003-05-21 | Norton Health Care Ltd | Desaglomerador para inhalador de polvo seco accionado por la respiracion, un inhalador de polvo seco y un metodo de desaglomeracion de polvo seco. |
| AR028746A1 (es) * | 2000-06-23 | 2003-05-21 | Norton Health Care Ltd | Cartucho de dosis previamente medidas para inhalador de polvo seco accionado por la respiracion, el inhalador y un metodo de provision de dosis previamente medidas de polvo seco |
| JP3974748B2 (ja) | 2000-11-29 | 2007-09-12 | 株式会社日立製作所 | 粉体吸引具 |
| US6595210B2 (en) | 2000-11-27 | 2003-07-22 | Unisia Jecs Corporation | Inhalator for administering powder composition |
| US6595203B1 (en) * | 2000-11-28 | 2003-07-22 | Forrest M. Bird | Apparatus for administering intermittent percussive ventilation and unitary breathing head assembly for use therein |
| US6443152B1 (en) | 2001-01-12 | 2002-09-03 | Becton Dickinson And Company | Medicament respiratory delivery device |
| US6722364B2 (en) | 2001-01-12 | 2004-04-20 | Becton, Dickinson And Company | Medicament inhalation delivery devices and methods for using the same |
| JP2005506855A (ja) | 2001-05-10 | 2005-03-10 | ベクトゥラ デリバリー デバイシーズ リミテッド | 吸入器 |
| GB2375308A (en) * | 2001-05-10 | 2002-11-13 | Cambridge Consultants | Inhalers |
| SE0101825D0 (sv) | 2001-05-22 | 2001-05-22 | Astrazeneca Ab | An Inhalation device |
| GB0113881D0 (en) | 2001-06-07 | 2001-08-01 | Innovate Biomed Ltd | Foil cutting system |
| US7540284B2 (en) | 2001-06-20 | 2009-06-02 | Novartis Pharma Ag | Powder aerosolization apparatus and method |
| US6681768B2 (en) | 2001-06-22 | 2004-01-27 | Sofotec Gmbh & Co. Kg | Powder formulation disintegrating system and method for dry powder inhalers |
| GB0120018D0 (en) | 2001-08-16 | 2001-10-10 | Meridica Ltd | Pack containing medicament and dispensing device |
| CN100506312C (zh) * | 2002-02-28 | 2009-07-01 | 赛氏联合企业 | 手提式电池供电吸气器 |
| GB0205572D0 (en) | 2002-03-09 | 2002-04-24 | Chawla Brinda P S | Medicament delivery and packaging |
| AU2003220125B2 (en) * | 2002-03-20 | 2006-06-15 | Mannkind Corporation | Inhalation apparatus |
| US20030192532A1 (en) * | 2002-04-12 | 2003-10-16 | Hopkins Andrew David | Nebulizer |
| AU2003215347A1 (en) | 2002-04-19 | 2003-11-03 | 3M Innovative Properties Company | A spacer for inertial removal of the non-respirable fraction of medicinal aerosols |
| JP4074481B2 (ja) | 2002-06-11 | 2008-04-09 | 株式会社日立製作所 | 投薬補助装置 |
| GB0215904D0 (en) | 2002-07-09 | 2002-08-21 | Team Holdings Uk Ltd | Drug delivery system and method |
| US20060108877A1 (en) | 2002-09-24 | 2006-05-25 | Tegel Robert G | Extensible coil for biaxial driver |
| GB0227128D0 (en) | 2002-11-20 | 2002-12-24 | Glaxo Group Ltd | A capsule |
| BR0316924A (pt) | 2002-12-02 | 2005-10-18 | Univ Alberta | Dispositivo e método para desaglomeração de pó para inalação |
| EA007730B1 (ru) * | 2002-12-13 | 2006-12-29 | Оцука Фармасьютикал Ко., Лтд. | Ингаляционное устройство для транспульмонального введения |
| US6941947B2 (en) | 2002-12-18 | 2005-09-13 | Quadrant Technologies Limited | Unit dose dry powder inhaler |
| US20040206350A1 (en) | 2002-12-19 | 2004-10-21 | Nektar Therapeutics | Aerosolization apparatus with non-circular aerosolization chamber |
| US7669596B2 (en) | 2002-12-31 | 2010-03-02 | Novartis Pharma Ag | Aerosolization apparatus with rotating capsule |
| GB0303870D0 (en) | 2003-02-20 | 2003-03-26 | Norton Healthcare Ltd | Pre-metered dose magazine for breath-actuated dry powder inhaler |
| FR2853302B1 (fr) | 2003-04-02 | 2006-02-24 | Nestle Waters Man & Technology | Capsule a opercule percable, bouchon muni d'une telle capsule contenant une substance destinee a etre ajoutee au contenu du recipient ainsi bouche et recipient correspondant |
| GB0312007D0 (en) * | 2003-05-24 | 2003-07-02 | Innovata Biomed Ltd | Container |
| EP1488819A1 (en) | 2003-06-16 | 2004-12-22 | Rijksuniversiteit te Groningen | Dry powder inhaler and method for pulmonary inhalation of dry powder |
| GB0315509D0 (en) * | 2003-07-02 | 2003-08-06 | Meridica Ltd | Dispensing device |
| JP3887356B2 (ja) | 2003-07-08 | 2007-02-28 | エスアイアイ・ナノテクノロジー株式会社 | 薄片試料作製方法 |
| GB2406088B (en) * | 2003-09-10 | 2006-07-19 | Rexam Med Packaging Ltd | Packaging |
| GB2405798A (en) * | 2003-09-15 | 2005-03-16 | Vectura Ltd | Dry powder inhaler with primary and secondary piercing elements and a medicament pack for use with an inhalation device. |
| GB0322544D0 (en) | 2003-09-26 | 2003-10-29 | Innovata Biomed Ltd | Apparatus |
| GB2407042B (en) | 2003-10-17 | 2007-10-24 | Vectura Ltd | Inhaler |
| US7861712B2 (en) * | 2004-04-23 | 2011-01-04 | Manta Product Development | Sealed capsule including an integrated puncturing mechanism |
| GB0427028D0 (en) * | 2004-12-09 | 2005-01-12 | Cambridge Consultants | Dry powder inhalers |
| GB0427858D0 (en) | 2004-12-20 | 2005-01-19 | Glaxo Group Ltd | Manifold for use in medicament dispenser |
| GB0503738D0 (en) * | 2005-02-23 | 2005-03-30 | Optinose As | Powder delivery devices |
| JP4475530B2 (ja) * | 2005-03-31 | 2010-06-09 | 株式会社吉野工業所 | 吸引式の粉体投与器 |
| GB0507711D0 (en) * | 2005-04-15 | 2005-05-25 | Vectura Group Plc | Improved blister piercing |
| EP1901793A1 (en) * | 2005-07-13 | 2008-03-26 | Cipla Ltd. | Inhaler device |
| US8763605B2 (en) | 2005-07-20 | 2014-07-01 | Manta Devices, Llc | Inhalation device |
| KR20080032136A (ko) * | 2005-08-01 | 2008-04-14 | 아스트라제네카 아베 | 흡입기 밸브 |
| GB0520794D0 (en) | 2005-10-12 | 2005-11-23 | Innovata Biomed Ltd | Inhaler |
| AR058289A1 (es) * | 2005-12-12 | 2008-01-30 | Glaxo Group Ltd | Colector para ser usado en dispensador de medicamento |
| EP1844809A1 (de) | 2006-04-13 | 2007-10-17 | Boehringer Ingelheim Pharma GmbH & Co. KG | Medikamentenmagazin für einen Inhalator, sowie Mehrdosispulverinhalator |
| EP1844806A1 (de) * | 2006-04-13 | 2007-10-17 | Boehringer Ingelheim Pharma GmbH | Medikamenten-Ausgabevorrichtung, Medikamentenmagazin dafür, und Verfahren zur Entnahme eines Medikaments aus einer Medikamentenkammer |
| GR1005620B (el) * | 2006-05-09 | 2007-09-03 | Βελτιωση συσκευης εισπνοων ξηρης σκονης | |
| PT103481B (pt) | 2006-05-16 | 2008-08-01 | Hovione Farmaciencia S A | Inalador de uso simples e método de inalação |
| EP3453418A1 (en) | 2007-07-06 | 2019-03-13 | Manta Devices, LLC | Delivery device and related methods |
| HUE027246T2 (en) | 2008-01-24 | 2016-10-28 | Vectura Delivery Devices Ltd | inhaler |
| CN103252007B (zh) | 2008-06-13 | 2016-06-22 | 曼金德公司 | 干粉吸入器和用于药物输送的系统 |
| WO2010021589A1 (en) | 2008-08-20 | 2010-02-25 | Astrazeneca Ab | Inhaler |
| WO2011116293A2 (en) | 2010-03-19 | 2011-09-22 | Manta Devices, Llc | Delivery device and related methods |
| WO2013036881A2 (en) | 2011-09-07 | 2013-03-14 | Syphase, Llc | Dry powder inhalation device |
-
2008
- 2008-07-06 EP EP18178534.6A patent/EP3453418A1/en not_active Withdrawn
- 2008-07-06 DK DK15150445.3T patent/DK2898914T3/en active
- 2008-07-06 CN CN2008801060089A patent/CN101795723B/zh not_active Expired - Fee Related
- 2008-07-06 WO PCT/US2008/008303 patent/WO2009009013A2/en not_active Ceased
- 2008-07-06 JP JP2010514883A patent/JP5528336B2/ja not_active Expired - Fee Related
- 2008-07-06 EP EP08826238.1A patent/EP2170444B1/en not_active Not-in-force
- 2008-07-06 EP EP15150445.3A patent/EP2898914B1/en active Active
- 2008-07-07 US US12/168,445 patent/US8291901B2/en active Active
-
2012
- 2012-10-09 US US13/647,881 patent/US8607787B2/en active Active
-
2013
- 2013-02-06 JP JP2013021615A patent/JP5651200B2/ja active Active
- 2013-12-13 US US14/105,412 patent/US9713684B2/en not_active Expired - Fee Related
-
2014
- 2014-04-10 US US14/249,409 patent/US9919115B2/en active Active
- 2014-11-14 JP JP2014231220A patent/JP6497893B2/ja not_active Expired - Fee Related
-
2017
- 2017-01-25 JP JP2017011539A patent/JP2017070849A/ja active Pending
-
2018
- 2018-03-19 US US15/924,906 patent/US20180207374A1/en not_active Abandoned
Also Published As
| Publication number | Publication date |
|---|---|
| JP2013121528A (ja) | 2013-06-20 |
| EP2170444A2 (en) | 2010-04-07 |
| JP2010532677A (ja) | 2010-10-14 |
| US8291901B2 (en) | 2012-10-23 |
| EP2898914A2 (en) | 2015-07-29 |
| US9919115B2 (en) | 2018-03-20 |
| CN101795723A (zh) | 2010-08-04 |
| WO2009009013A2 (en) | 2009-01-15 |
| US8607787B2 (en) | 2013-12-17 |
| US20090013994A1 (en) | 2009-01-15 |
| JP2017070849A (ja) | 2017-04-13 |
| JP5651200B2 (ja) | 2015-01-07 |
| DK2898914T3 (en) | 2018-09-03 |
| JP2015037613A (ja) | 2015-02-26 |
| CN101795723B (zh) | 2013-06-19 |
| JP6497893B2 (ja) | 2019-04-10 |
| EP2898914A3 (en) | 2015-10-21 |
| US20180207374A1 (en) | 2018-07-26 |
| WO2009009013A8 (en) | 2009-11-05 |
| EP2170444B1 (en) | 2016-09-07 |
| US20140216454A1 (en) | 2014-08-07 |
| EP2898914B1 (en) | 2018-06-20 |
| US20140102451A1 (en) | 2014-04-17 |
| US9713684B2 (en) | 2017-07-25 |
| EP3453418A1 (en) | 2019-03-13 |
| US20130032144A1 (en) | 2013-02-07 |
| WO2009009013A3 (en) | 2009-04-23 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5528336B2 (ja) | デリバリー装置及び関連方法 | |
| US5161524A (en) | Dosage inhalator with air flow velocity regulating means | |
| KR100321814B1 (ko) | 건분말흡입기 | |
| US11224704B2 (en) | Dose delivery device for inhalation | |
| JP4395642B2 (ja) | 簡易吸入器 | |
| US5483954A (en) | Inhaler and medicated package | |
| US9283336B2 (en) | Delivery device and related methods | |
| EP2866867B1 (en) | Nasal dry powder delivery system for vaccines and other treatment agents | |
| CN106061531B (zh) | 粉末吸入器和粉末吸入组 | |
| US20020108611A1 (en) | Inhalation device and method | |
| US20170106154A1 (en) | Dry powder inhaler | |
| MXPA06002544A (es) | Cartucho de dosis unitaria e inhalador de polvo seco. | |
| WO2000044426A1 (en) | Inhalation type drug dispenser | |
| PL204303B1 (pl) | Urządzenie do emitowania proszku | |
| WO2008042951A2 (en) | Inhalation devices and related methods | |
| US9649454B2 (en) | Delivery device and related methods | |
| CN109821117B (zh) | 粉末释放装置及方法 | |
| CN111182934A (zh) | 多剂量药物输送装置 | |
| CN109821118B (zh) | 新型肺部药物输送系统 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20100810 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20110617 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20110617 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20121106 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20121108 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20130206 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20131001 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20140326 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20140415 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 5528336 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| LAPS | Cancellation because of no payment of annual fees |