JP5437331B2 - 心臓機能を改善するための医薬組成物及びその方法 - Google Patents
心臓機能を改善するための医薬組成物及びその方法 Download PDFInfo
- Publication number
- JP5437331B2 JP5437331B2 JP2011184310A JP2011184310A JP5437331B2 JP 5437331 B2 JP5437331 B2 JP 5437331B2 JP 2011184310 A JP2011184310 A JP 2011184310A JP 2011184310 A JP2011184310 A JP 2011184310A JP 5437331 B2 JP5437331 B2 JP 5437331B2
- Authority
- JP
- Japan
- Prior art keywords
- kmup
- pharmaceutical composition
- salts
- myocardial infarction
- group
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D473/00—Heterocyclic compounds containing purine ring systems
- C07D473/02—Heterocyclic compounds containing purine ring systems with oxygen, sulphur, or nitrogen atoms directly attached in positions 2 and 6
- C07D473/04—Heterocyclic compounds containing purine ring systems with oxygen, sulphur, or nitrogen atoms directly attached in positions 2 and 6 two oxygen atoms
- C07D473/06—Heterocyclic compounds containing purine ring systems with oxygen, sulphur, or nitrogen atoms directly attached in positions 2 and 6 two oxygen atoms with radicals containing only hydrogen and carbon atoms, attached in position 1 or 3
- C07D473/08—Heterocyclic compounds containing purine ring systems with oxygen, sulphur, or nitrogen atoms directly attached in positions 2 and 6 two oxygen atoms with radicals containing only hydrogen and carbon atoms, attached in position 1 or 3 with methyl radicals in positions 1 and 3, e.g. theophylline
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/505—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim
- A61K31/519—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim ortho- or peri-condensed with heterocyclic rings
- A61K31/52—Purines, e.g. adenine
- A61K31/522—Purines, e.g. adenine having oxo groups directly attached to the heterocyclic ring, e.g. hypoxanthine, guanine, acyclovir
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/10—Drugs for disorders of the cardiovascular system for treating ischaemic or atherosclerotic diseases, e.g. antianginal drugs, coronary vasodilators, drugs for myocardial infarction, retinopathy, cerebrovascula insufficiency, renal arteriosclerosis
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Epidemiology (AREA)
- Urology & Nephrology (AREA)
- Vascular Medicine (AREA)
- Cardiology (AREA)
- Heart & Thoracic Surgery (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Description
実験動物としての雄性ラット(250-300g)は、台湾台北の国立実験動物繁殖及び研究センター(National Laboratory Animal Breeding and Research Center)によって提供され、一定の温度と制御照明の下に収容されている。又、食べ物と水は、自由に利用可能である。
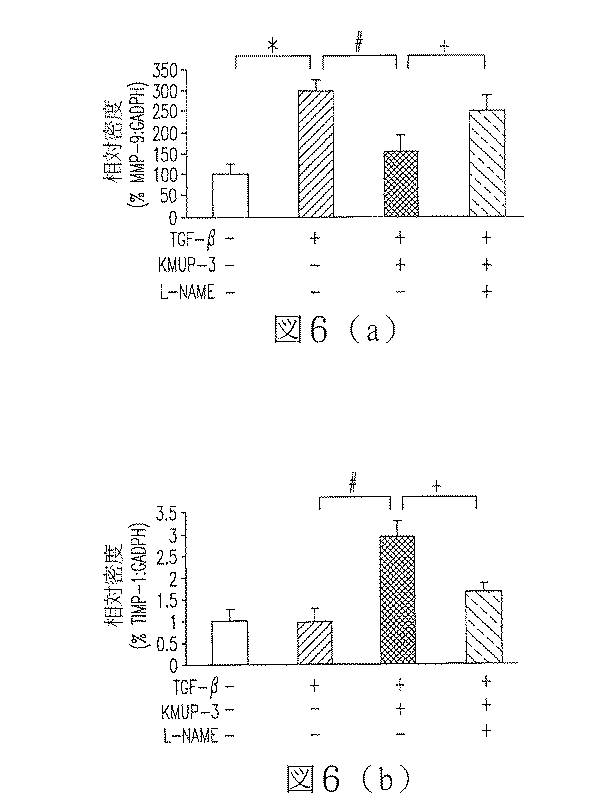
心臓は、50 mmol・L-1のトリスヒドロキシメチルアミノメタン(tris(hydroxymethyl)aminomethane、Tris)において、10秒2回超音波処理し、4℃、13,000 rpmで30分間遠心分離する。ウシ血清アルブミンを標準液として使用することにより、上澄部のタンパク質濃度を決定する。細胞抽出物は、100 mmol・L-1のTris、20%のグリセロール、4%の硫酸ドデシルナトリウム(SDS)、及び0.2%のブロモフェノールブルーを含むpH6.8のサンプルバッファー(sample buffer)により、4:1の比率で煮沸されている。電気泳動は、10%のポリアクリルアミドゲル電気泳動(Poly-Acrylamide Gel Electrophoresis)で実行し、硝酸セルロース膜(ミリポア社、ビルリカ、マサチューセッツ州、アメリカ)に転送される。前記膜は、20 mmol・L-1のTris及び137 mmol・L-1 のpH7.6のNaClからなるTris緩衝食塩水と、0.1%のTween-20(TTBS)と、5%の脱脂粉乳によって室温で反応してブロックされる。TTBSで洗浄した後、MMP-9、TIMP-1(ミリポア社、テメキュラ、カリフォルニア州、アメリカ)、またはeNOSの一次抗体(BD Transduction Laboratories、フランクリンレイクス、ニュージャージー州、アメリカ)において、4℃で一晩恒温放置する。前記膜は、マウスやウサギIgG(Santa Cruz Biotechnology、サンタクルーズ、カリフォルニア州、アメリカ)に対する西洋ワサビペルオキシダーゼ複合抗体と1時間恒温放置する前に、TTBSで洗浄する。その後、前記膜は、TTBSで更に洗浄し、強化された化学発光性(chemiluminescence)を発展して特定抗原を検出する。バンドの強度は濃度測定によって定量化される。
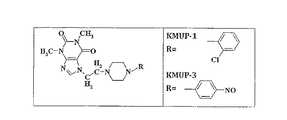
KMUP-3(8.3 g)は、エタノール(10 ml)と1N塩酸(60 ml)の混合物に溶解し、それらからなる溶液は50℃で20分間反応させ、室温下でエタノール(またはメタノール)をその中に加え、前記溶液を結晶化するように一晩恒温放置し、KMUP-3塩酸塩(6.4 g)を取得するように濾過する。
KMUP-3(8.3 g)は、がをエタノール(10 ml)及びクエン酸(4 g)の混合物に溶解し、それらからなる溶液は50℃で20分間反応させ、室温下でエタノール(またはメタノール)をその中に加え、前記溶液を結晶化するように一晩恒温放置し、KMUP-3-クエン酸塩(10.5 g)を取得するように濾過する。
KMUP-3(8.3 g)は、がをエタノール(10 ml)及びニコチン酸(2.4 g)の混合物に溶解し、それらからなる溶液は50℃で20分間反応させ、室温下でエタノール(またはメタノール)をその中に加え、前記溶液を結晶化するように一晩恒温放置し、KMUP-3-ニコチン酸塩(8.3 g)を取得するように濾過する。
錠剤を製造するために、次の成分をそれぞれ混合した後、重み付けをし、打錠機に充填する。
乳糖 適量
トウモロコシ澱粉 適量
1.Creemers EE, Davis JN, Parkhurst AM, Leenders P, Dowdy KB, Hapke E, et al. (2003). Deficiency of TIMP-1 exacerbates LV remodeling after MI in mice. Am J Physiol Heart Circ Physiol 284: H364-371.
2.Halapas A, Zacharoulis A, Theocharis S, Karavidas A, Korres D, Papadopoulos K, et al. (2008). Serum levels of the osteoprotegerin, receptor activator of nuclear factor kappa-B ligand, metalloproteinase-1 (MMP-1) and tissue inhibitors of MMP-1 levels are increased in men 6 months after acute MI. Clin Chem Lab Med 46: 510-516.
3.Harada M, Qin Y, Takano H, Minamino T, Zou Y, Toko H et al. (2005). G-CSF prevents cardiac remodeling after myocardial infarction by activating the Jak-Stat pathway in cardiomyocytes. Nat Med 11: 305-311.
4.Lee TM, Lin MS, Chang NC (2008). Effect of ATP-sensitive potassium channel agonists on ventricular remodeling in healed rat infarcts. J Am Coll Cardiol 51: 1309-1318.
5.Li GH, Shi Y, Chen Y, Sun M, Sader S, Maekawa Y, et al. (2009). Gelsolin regulates cardiac remodeling after myocardial infarction through DNase I-mediated apoptosis. Circ Res 104: 896-904.
6.Liang JC, Chen HR, Chiu CC, Liou SF, Chen IJ, Yeh JL (2006). Protective effect of labedipinedilol-A, a novel dihydropyridine-type calcium channel blocker, on myocardial apoptosis in ischemiareperfusion injury. Life Sci 79: 1248-1256.
7.Lin RJ, Wu BN, Lo YC, An LM, Dai ZK, Lin YT, et al. (2006). A xanthine-based epitheliumdependent airway relaxant KMUP-3 (7-[2-[4- (4-nitrobenzene)piperazinyl]ethyl]-1,3-dimethylxanthine) increases respiratory performance and protects against tumor necrosis factoralpha-induced tracheal contraction, involving nitric oxide release and expression of cGMP and protein kinase G. J Pharmacol Exp Ther 316: 709-717.
8.Lin TH, Chiu HC, Lee YT, Su HM, Juo SH, Voon WC, et al. (2007). The C-allele of tissue inhibitor of metalloproteinases 2 is associated with increased magnitude of QT dispersion prolongation in elderly Chinese - 4-year follow-up study. Clin Chim Acta 386: 87-93.
9.Lin X, Jo H, Ishii TM, Fujita M, Fu M, Tambara K, et al. (2009). Controlled release of matrix metalloproteinase-1 plasmid DNA prevents left ventricular remodeling in chronic myocardial infarction of rats. Circ J 73: 2315-2321.
10.Louhelainen M, Vahtola E, Kaheinen P, Leskinen H, Merasto S, Kyto V, et al. (2007). Effects of levosimendan on cardiac remodeling and cardiomyocyte apoptosis in hypertensive Dahl/Rapp rats. Br J Pharmacol 150: 851-861.
11.Vandegriff KD, Young MA, Lohman J, Bellelli A, Samaja M, Malavalli A, et al. (2008). CO-MP4, a polyethylene glycol-conjugated haemoglobin derivative and carbon monoxide carrier that reduces myocardial infarct size in rats. Br J Pharmacol 154: 1649-1661.
12.Wu BN, Lin RJ, Lin CY, Shen KP, Chiang LC, Chen IJ (2001). A xanthine-based KMUP-1 with cyclic GMP enhancing and K (+) channels opening activities in rat aortic smooth muscle. Br J Pharmacol 134: 265-274.
13.Wu BN, Chen IC, Lin RJ, Chiu CC, An LM, Chen IJ (2005). Aortic smooth muscle relaxants KMUP-3 and KMUP-4, two nitrophenylpiperazine derivatives of xanthine, display cGMP-enhancing activity: roles of endothelium, phosphodiesterase, and K+ channel. J Cardiovasc Pharmacol 46(5): 600-608.
14.Yeh JL, Hsu JH, Wu PJ, Liou SF, Liu CP, Chen IJ, et al. (2010). KMUP-1 attenuates isoprenalineinduced cardiac hypertrophy in rats through NO/cGMP/PKG and ERK1/2/calcineurin A pathways. Br J Pharmacol 159: 1151-1160.
15.Yoshimura S, Nishimura Y, Nishiuma T, Yamashita T, Kobayashi K, Yokoyama M (2006). Overexpression of nitric oxide synthase by the endothelium attenuates bleomycin-induced lung fibrosis and impairs MMP-9/TIMP-1 balance. Respirology 5: 546-556.
Claims (5)
- 心筋梗塞の治療及び心筋のリモデリングと心筋梗塞後の心機能不全の予防に使用し、心臓機能を改善するための医薬組成物であって、
薬学的に許容される担体と、
KMUP−3化合物及びその塩類の一つである活性成分の有効量を含み、
前記医薬組成物は、マトリックスメタロプロテアーゼ9(MMP−9)の蛋白発現量を減少させること特徴とする医薬組成物。 - 前記医薬組成物は、内皮型一酸化窒素合成酵素(eNOS)の蛋白発現量を増加させること特徴とする請求項1に記載の医薬組成物。
- 前記塩類は、KMUP−3−クエン酸塩およびKMUP−3−ニコチン酸塩からなる群から選ばれたこと特徴とする請求項1に記載の医薬組成物。
- 第一アルコール及び酸を含む溶液にKMUP化合物を溶解する工程と、
第二アルコールを前記溶液に加える工程と、
前記溶液からKMUP−3塩類を分離する工程と、
を含むこと特徴とする、請求項1に記載のKMUP−3塩類を製造するための方法。 - 前記KMUP−3塩類は、前記溶液中での結晶であり、
前記第一アルコールは、エタノールであり、
前記第二アルコールは、エタノール及びメタノールのいずれかであり、
前記酸は、塩酸、クエン酸、及びニコチン酸からなる群から一つを選び、
前記KMUP化合物は、第一温度で溶解し、前記第二アルコールは、前記第一温度より高い第二温度で加え、
前記第二温度は、室温である、こと特徴とする請求項4に記載のKMUP−3塩類を製造するための方法。
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| TW099135491 | 2010-10-18 | ||
| TW099135491A TWI462923B (zh) | 2010-10-18 | 2010-10-18 | Kmup-3之心肌梗塞疾患用途 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2012087115A JP2012087115A (ja) | 2012-05-10 |
| JP5437331B2 true JP5437331B2 (ja) | 2014-03-12 |
Family
ID=45934663
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2011184310A Expired - Fee Related JP5437331B2 (ja) | 2010-10-18 | 2011-08-26 | 心臓機能を改善するための医薬組成物及びその方法 |
Country Status (3)
| Country | Link |
|---|---|
| US (1) | US20120095013A1 (ja) |
| JP (1) | JP5437331B2 (ja) |
| TW (1) | TWI462923B (ja) |
Family Cites Families (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6451807B1 (en) * | 1999-04-30 | 2002-09-17 | Lilly Icos, Llc. | Methods of treating sexual dysfunction in an individual suffering from a retinal disease, class 1 congestive heart failure, or myocardial infarction using a PDE5 inhibitor |
| US20080064705A1 (en) * | 2006-09-12 | 2008-03-13 | Kaohsiung Medical University | Theophylline-based nitophenylpiperazine derivatives for enhancing aortic smooth muscle relaxation |
| US20080081816A1 (en) * | 2006-10-03 | 2008-04-03 | Kaohsiung Medical University | Anti-inflammation activity of newly synthesized xanthine derivatives kmup-1 and kmup-3 |
-
2010
- 2010-10-18 TW TW099135491A patent/TWI462923B/zh not_active IP Right Cessation
-
2011
- 2011-04-12 US US13/085,017 patent/US20120095013A1/en not_active Abandoned
- 2011-08-26 JP JP2011184310A patent/JP5437331B2/ja not_active Expired - Fee Related
Also Published As
| Publication number | Publication date |
|---|---|
| TWI462923B (zh) | 2014-12-01 |
| US20120095013A1 (en) | 2012-04-19 |
| JP2012087115A (ja) | 2012-05-10 |
| TW201217376A (en) | 2012-05-01 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR101762999B1 (ko) | 종양 치료제 | |
| US9938320B2 (en) | Peptides for promoting angiogenesis and use thereof | |
| JP7002444B2 (ja) | 医薬 | |
| WO2014190015A1 (en) | Cryopyrin inhibitors for preventing and treating inflammation | |
| CN108430475B (zh) | 用于治疗慢性咳嗽的奥维匹坦 | |
| US20020143030A1 (en) | Treatment of gastroparesis in certain patient groups | |
| ES2994095T3 (en) | Saracatinib for use in the treatment of idiopathic pulmonary fibrosis | |
| WO2021252630A1 (en) | Methods for treating or preventing chronic kidney disease | |
| JP4942297B2 (ja) | 肺高血圧症の処置を目的としたn−{5−[4−(4−メチルピペラジノメチル)−ベンゾイルアミド]−2−メチルフェニル}−4−(3−ピリジル)−2−ピリジン−アミンの使用 | |
| EP1293213A1 (en) | Preventives/remedies for postoperative stress | |
| CA2249330C (en) | Method of treating heart failure with endothelin antagonists | |
| JP5437331B2 (ja) | 心臓機能を改善するための医薬組成物及びその方法 | |
| US9249185B2 (en) | Peptides for promoting angiogenesis and an use thereof | |
| WO2021032643A1 (en) | Compounds suitable for the treatment and prophylaxis of muscle wasting and other conditions | |
| CN109806263A (zh) | 一种药物组合物及其制备方法和用途 | |
| CN116139115A (zh) | 厚朴酚和/或和厚朴酚芳环氨基取代类衍生物的抗低氧/缺氧损伤用途及药物组合物 | |
| US20250099455A1 (en) | Treatment for congestive heart failure | |
| US6562838B2 (en) | Treatment of cardiovascular disease with quinolinone enantiomers | |
| WO2013103148A1 (ja) | 側副血行路発達促進剤 | |
| JP5118648B2 (ja) | 疾患の処置および予防のための組成物および方法 | |
| US20250213550A1 (en) | Compounds and methods for treating inflammatory bowel disease | |
| KR20030087074A (ko) | 아릴에텐술폰아미드 유도체의 신규 용도 | |
| CN118526503A (zh) | 治疗或预防败血症或与败血症相关的病情的化合物 | |
| ITRM940625A1 (it) | Uso di esteri ftalidilidenici della carnitina e di alcanoil carnitine per il trattamento dello shock endotossico | |
| JP2002338467A (ja) | アデニル酸シクラーゼ5型阻害剤 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20130514 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20130814 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20130819 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20131114 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20131203 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20131211 |
|
| R150 | Certificate of patent or registration of utility model |
Free format text: JAPANESE INTERMEDIATE CODE: R150 Ref document number: 5437331 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| LAPS | Cancellation because of no payment of annual fees |