JP5114426B2 - 制御可能に血管を閉塞する方法及び装置 - Google Patents
制御可能に血管を閉塞する方法及び装置 Download PDFInfo
- Publication number
- JP5114426B2 JP5114426B2 JP2008547244A JP2008547244A JP5114426B2 JP 5114426 B2 JP5114426 B2 JP 5114426B2 JP 2008547244 A JP2008547244 A JP 2008547244A JP 2008547244 A JP2008547244 A JP 2008547244A JP 5114426 B2 JP5114426 B2 JP 5114426B2
- Authority
- JP
- Japan
- Prior art keywords
- balloon
- volume
- inflation
- expansion
- fluid
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
- 210000004204 blood vessel Anatomy 0.000 title claims description 157
- 238000000034 method Methods 0.000 title description 94
- 239000012530 fluid Substances 0.000 claims description 198
- 230000007246 mechanism Effects 0.000 claims description 97
- 239000000463 material Substances 0.000 claims description 91
- 230000008602 contraction Effects 0.000 description 158
- 230000008569 process Effects 0.000 description 50
- 238000011282 treatment Methods 0.000 description 50
- 238000013461 design Methods 0.000 description 32
- 239000003795 chemical substances by application Substances 0.000 description 28
- 239000002872 contrast media Substances 0.000 description 28
- 230000006870 function Effects 0.000 description 28
- 230000017531 blood circulation Effects 0.000 description 20
- 239000012298 atmosphere Substances 0.000 description 19
- 210000001519 tissue Anatomy 0.000 description 18
- 230000014759 maintenance of location Effects 0.000 description 17
- 238000001802 infusion Methods 0.000 description 16
- 239000007788 liquid Substances 0.000 description 16
- 230000008859 change Effects 0.000 description 15
- 230000010412 perfusion Effects 0.000 description 15
- 239000008280 blood Substances 0.000 description 14
- 210000004369 blood Anatomy 0.000 description 14
- 239000007924 injection Substances 0.000 description 13
- 238000002347 injection Methods 0.000 description 13
- 239000000243 solution Substances 0.000 description 11
- 210000004351 coronary vessel Anatomy 0.000 description 10
- 239000003814 drug Substances 0.000 description 10
- 230000004044 response Effects 0.000 description 10
- 230000007704 transition Effects 0.000 description 9
- 238000004891 communication Methods 0.000 description 8
- 229940079593 drug Drugs 0.000 description 8
- 238000002594 fluoroscopy Methods 0.000 description 8
- 206010053648 Vascular occlusion Diseases 0.000 description 7
- 238000005452 bending Methods 0.000 description 7
- 230000007423 decrease Effects 0.000 description 7
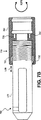
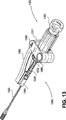
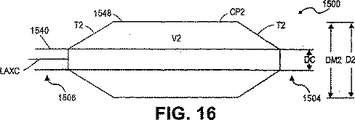
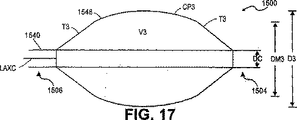
- 238000010586 diagram Methods 0.000 description 7
- 238000001125 extrusion Methods 0.000 description 7
- 238000003384 imaging method Methods 0.000 description 7
- 208000021331 vascular occlusion disease Diseases 0.000 description 7
- 210000005166 vasculature Anatomy 0.000 description 7
- 230000015572 biosynthetic process Effects 0.000 description 6
- 239000007789 gas Substances 0.000 description 6
- 229920000642 polymer Polymers 0.000 description 6
- 230000001105 regulatory effect Effects 0.000 description 6
- 238000003780 insertion Methods 0.000 description 5
- 230000037431 insertion Effects 0.000 description 5
- 239000000203 mixture Substances 0.000 description 5
- 238000000465 moulding Methods 0.000 description 5
- 238000013459 approach Methods 0.000 description 4
- 210000001367 artery Anatomy 0.000 description 4
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 4
- 230000001276 controlling effect Effects 0.000 description 4
- 239000012216 imaging agent Substances 0.000 description 4
- 238000004519 manufacturing process Methods 0.000 description 4
- 239000001301 oxygen Substances 0.000 description 4
- 229910052760 oxygen Inorganic materials 0.000 description 4
- 239000000126 substance Substances 0.000 description 4
- 230000001225 therapeutic effect Effects 0.000 description 4
- 229920002614 Polyether block amide Polymers 0.000 description 3
- 230000008901 benefit Effects 0.000 description 3
- 230000036772 blood pressure Effects 0.000 description 3
- 210000004027 cell Anatomy 0.000 description 3
- 239000003623 enhancer Substances 0.000 description 3
- 238000012986 modification Methods 0.000 description 3
- 230000004048 modification Effects 0.000 description 3
- 239000004033 plastic Substances 0.000 description 3
- 229920003023 plastic Polymers 0.000 description 3
- 238000002604 ultrasonography Methods 0.000 description 3
- 230000002792 vascular Effects 0.000 description 3
- 210000003462 vein Anatomy 0.000 description 3
- 241001631457 Cannula Species 0.000 description 2
- 239000004677 Nylon Substances 0.000 description 2
- 208000031481 Pathologic Constriction Diseases 0.000 description 2
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 2
- 239000002998 adhesive polymer Substances 0.000 description 2
- 230000000712 assembly Effects 0.000 description 2
- 238000000429 assembly Methods 0.000 description 2
- 230000009286 beneficial effect Effects 0.000 description 2
- 210000002798 bone marrow cell Anatomy 0.000 description 2
- 230000006835 compression Effects 0.000 description 2
- 238000007906 compression Methods 0.000 description 2
- 238000010276 construction Methods 0.000 description 2
- 230000003247 decreasing effect Effects 0.000 description 2
- 230000010339 dilation Effects 0.000 description 2
- 230000000694 effects Effects 0.000 description 2
- 238000010438 heat treatment Methods 0.000 description 2
- 210000005260 human cell Anatomy 0.000 description 2
- 239000003978 infusion fluid Substances 0.000 description 2
- 238000005304 joining Methods 0.000 description 2
- 238000002595 magnetic resonance imaging Methods 0.000 description 2
- 230000013011 mating Effects 0.000 description 2
- 238000005259 measurement Methods 0.000 description 2
- 238000002844 melting Methods 0.000 description 2
- 230000008018 melting Effects 0.000 description 2
- 229920001778 nylon Polymers 0.000 description 2
- 238000002428 photodynamic therapy Methods 0.000 description 2
- -1 polyethylene Polymers 0.000 description 2
- 238000012545 processing Methods 0.000 description 2
- 208000037803 restenosis Diseases 0.000 description 2
- 150000003839 salts Chemical class 0.000 description 2
- 238000004513 sizing Methods 0.000 description 2
- 239000011780 sodium chloride Substances 0.000 description 2
- 210000000130 stem cell Anatomy 0.000 description 2
- 208000037804 stenosis Diseases 0.000 description 2
- 230000036262 stenosis Effects 0.000 description 2
- 229940124597 therapeutic agent Drugs 0.000 description 2
- 238000002560 therapeutic procedure Methods 0.000 description 2
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 2
- 206010002091 Anaesthesia Diseases 0.000 description 1
- 206010003210 Arteriosclerosis Diseases 0.000 description 1
- 208000037260 Atherosclerotic Plaque Diseases 0.000 description 1
- 101100350187 Caenorhabditis elegans odd-2 gene Proteins 0.000 description 1
- VGGSQFUCUMXWEO-UHFFFAOYSA-N Ethene Chemical compound C=C VGGSQFUCUMXWEO-UHFFFAOYSA-N 0.000 description 1
- 241000282412 Homo Species 0.000 description 1
- 241001465754 Metazoa Species 0.000 description 1
- 108700001094 Plant Genes Proteins 0.000 description 1
- 239000004698 Polyethylene Substances 0.000 description 1
- 239000003570 air Substances 0.000 description 1
- 230000037005 anaesthesia Effects 0.000 description 1
- 210000003484 anatomy Anatomy 0.000 description 1
- 210000004102 animal cell Anatomy 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- 239000011230 binding agent Substances 0.000 description 1
- 238000009530 blood pressure measurement Methods 0.000 description 1
- 230000005587 bubbling Effects 0.000 description 1
- 238000005266 casting Methods 0.000 description 1
- 238000012790 confirmation Methods 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 230000008878 coupling Effects 0.000 description 1
- 238000010168 coupling process Methods 0.000 description 1
- 238000005859 coupling reaction Methods 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 230000000916 dilatatory effect Effects 0.000 description 1
- 238000002224 dissection Methods 0.000 description 1
- 238000005553 drilling Methods 0.000 description 1
- 230000009977 dual effect Effects 0.000 description 1
- 230000002526 effect on cardiovascular system Effects 0.000 description 1
- 230000005611 electricity Effects 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 239000000835 fiber Substances 0.000 description 1
- 239000006260 foam Substances 0.000 description 1
- 238000005242 forging Methods 0.000 description 1
- 230000023266 generation of precursor metabolites and energy Effects 0.000 description 1
- 239000011521 glass Substances 0.000 description 1
- 229920001903 high density polyethylene Polymers 0.000 description 1
- 239000004700 high-density polyethylene Substances 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 238000001746 injection moulding Methods 0.000 description 1
- 230000009191 jumping Effects 0.000 description 1
- 239000004816 latex Substances 0.000 description 1
- 229920000126 latex Polymers 0.000 description 1
- 229920001684 low density polyethylene Polymers 0.000 description 1
- 239000004702 low-density polyethylene Substances 0.000 description 1
- 238000003754 machining Methods 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 235000015097 nutrients Nutrition 0.000 description 1
- 239000013307 optical fiber Substances 0.000 description 1
- 210000000056 organ Anatomy 0.000 description 1
- 229920000573 polyethylene Polymers 0.000 description 1
- 229920001296 polysiloxane Polymers 0.000 description 1
- 229920002635 polyurethane Polymers 0.000 description 1
- 239000004814 polyurethane Substances 0.000 description 1
- 108090000623 proteins and genes Proteins 0.000 description 1
- 230000000717 retained effect Effects 0.000 description 1
- 238000004904 shortening Methods 0.000 description 1
- 230000002269 spontaneous effect Effects 0.000 description 1
- 238000001356 surgical procedure Methods 0.000 description 1
- 230000008961 swelling Effects 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
- 238000013519 translation Methods 0.000 description 1
- 230000004865 vascular response Effects 0.000 description 1
- 230000024883 vasodilation Effects 0.000 description 1
- 239000011800 void material Substances 0.000 description 1
- 238000005406 washing Methods 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/12—Surgical instruments, devices or methods for ligaturing or otherwise compressing tubular parts of the body, e.g. blood vessels or umbilical cord
- A61B17/12022—Occluding by internal devices, e.g. balloons or releasable wires
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/12—Surgical instruments, devices or methods for ligaturing or otherwise compressing tubular parts of the body, e.g. blood vessels or umbilical cord
- A61B17/12022—Occluding by internal devices, e.g. balloons or releasable wires
- A61B17/12027—Type of occlusion
- A61B17/1204—Type of occlusion temporary occlusion
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/10—Balloon catheters
- A61M25/1002—Balloon catheters characterised by balloon shape
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/10—Balloon catheters
- A61M25/1018—Balloon inflating or inflation-control devices
- A61M25/10181—Means for forcing inflation fluid into the balloon
- A61M25/10182—Injector syringes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/10—Balloon catheters
- A61M25/1027—Making of balloon catheters
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/10—Balloon catheters
- A61M2025/1043—Balloon catheters with special features or adapted for special applications
- A61M2025/1052—Balloon catheters with special features or adapted for special applications for temporarily occluding a vessel for isolating a sector
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/0021—Catheters; Hollow probes characterised by the form of the tubing
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M25/00—Catheters; Hollow probes
- A61M25/10—Balloon catheters
- A61M25/1018—Balloon inflating or inflation-control devices
- A61M25/10184—Means for controlling or monitoring inflation or deflation
- A61M25/10187—Indicators for the level of inflation or deflation
- A61M25/10188—Inflation or deflation data displays
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Heart & Thoracic Surgery (AREA)
- Animal Behavior & Ethology (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- General Health & Medical Sciences (AREA)
- Surgery (AREA)
- Child & Adolescent Psychology (AREA)
- Biophysics (AREA)
- Pulmonology (AREA)
- Anesthesiology (AREA)
- Hematology (AREA)
- Molecular Biology (AREA)
- Medical Informatics (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Vascular Medicine (AREA)
- Reproductive Health (AREA)
- Surgical Instruments (AREA)
- Media Introduction/Drainage Providing Device (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US11/313,477 | 2005-12-20 | ||
| US11/313,477 US7674240B2 (en) | 2005-12-20 | 2005-12-20 | Method and apparatus for controlled vessel occlusion |
| PCT/US2006/045205 WO2007078455A1 (en) | 2005-12-20 | 2006-11-21 | Method and apparatus for controlled vessel occlusion |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2009519809A JP2009519809A (ja) | 2009-05-21 |
| JP2009519809A5 JP2009519809A5 (enExample) | 2010-04-15 |
| JP5114426B2 true JP5114426B2 (ja) | 2013-01-09 |
Family
ID=37808054
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2008547244A Expired - Fee Related JP5114426B2 (ja) | 2005-12-20 | 2006-11-21 | 制御可能に血管を閉塞する方法及び装置 |
Country Status (4)
| Country | Link |
|---|---|
| US (5) | US7674240B2 (enExample) |
| EP (1) | EP1962936A1 (enExample) |
| JP (1) | JP5114426B2 (enExample) |
| WO (1) | WO2007078455A1 (enExample) |
Families Citing this family (57)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7674240B2 (en) | 2005-12-20 | 2010-03-09 | Abbott Cardiovascular Systems Inc. | Method and apparatus for controlled vessel occlusion |
| JP5184360B2 (ja) * | 2006-08-21 | 2013-04-17 | 株式会社カネカ | バルーンカテーテル |
| EP2086573B1 (en) | 2006-10-09 | 2020-11-25 | Neurofluidics, Inc. | Cerebrospinal fluid purification system |
| US10632237B2 (en) | 2006-10-09 | 2020-04-28 | Minnetronix, Inc. | Tangential flow filter system for the filtration of materials from biologic fluids |
| US10850235B2 (en) | 2006-10-09 | 2020-12-01 | Minnetronix, Inc. | Method for filtering cerebrospinal fluid (CSF) including monitoring CSF flow |
| EP2157909A2 (en) * | 2007-06-27 | 2010-03-03 | Flip Technologies Limited | A system, device and a method for dilating a stricture in a lumen and for determining the transverse cross-sectional area of a lumen or cavity |
| AU2007361589B9 (en) * | 2007-11-21 | 2014-05-08 | Invatec S.P.A. | Balloon for the treatment of stenosis and method for manufacturing the balloon |
| WO2009094522A1 (en) * | 2008-01-24 | 2009-07-30 | Cedars-Sinai Medical Center | Tulip-shaped balloon catheter and methods for pericardiocentesis and percutaneous pericardiotomy |
| US8372029B2 (en) * | 2008-03-12 | 2013-02-12 | Olympus Medical Systems Corp. | Fluid feeder, balloon catheter and fluid feeder supporting device |
| US20090234281A1 (en) * | 2008-03-12 | 2009-09-17 | Olympus Medical Systems Corp. | Fluid feeder and balloon catheter |
| WO2009125380A1 (en) * | 2008-04-09 | 2009-10-15 | Flip Technologies Limited | A system and a method for inflating an inflatable element with a liquid inflating medium and a balloon catheter inflated by the system and method |
| US8348111B2 (en) * | 2008-10-01 | 2013-01-08 | Jac Products, Inc. | System and method for vehicle article carrier having stowable cross bars |
| US11045300B2 (en) | 2008-12-19 | 2021-06-29 | Cvdevices, Llc | Systems, devices, and methods for organ retroperfusion along with regional mild hypothermia |
| US9968727B2 (en) * | 2008-12-19 | 2018-05-15 | Cvdevices, Llc | Systems, devices, and methods for organ retroperfusion along with regional mild hypothermia |
| US8702689B2 (en) | 2009-09-01 | 2014-04-22 | Boston Scientific Scimed, Inc. | Systems and methods for twisting an expansion element of a cryoablation system |
| US20110082450A1 (en) * | 2009-10-02 | 2011-04-07 | Cardiofocus, Inc. | Cardiac ablation system with inflatable member having multiple inflation settings |
| US8480650B2 (en) | 2010-04-30 | 2013-07-09 | Abbott Cardiovascular Systems Inc. | Method for increased uptake of beneficial agent and ejection fraction by postconditioning procedures |
| US9155869B2 (en) | 2010-04-30 | 2015-10-13 | Abbott Cardiovascular Systems Inc. | Catheter having inflation and deflation lumen useful for preventing or reducing reperfusion injury |
| WO2011136813A1 (en) * | 2010-04-30 | 2011-11-03 | Abbott Cardiovascular Systems Inc. | Catheter having inflation and deflation lumen useful for preventing or reducing reperfusion injury |
| US8708996B2 (en) | 2010-04-30 | 2014-04-29 | Abbott Cardiovascular Systems, Inc. | Methods and device for synergistic mitigation of reperfusion injury after an ischemic event |
| US9168361B2 (en) | 2010-04-30 | 2015-10-27 | Abbott Cardiovascular Systems Inc. | Balloon catheter exhibiting rapid inflation and deflation |
| US8540669B2 (en) | 2010-04-30 | 2013-09-24 | Abbott Cardiovascular Systems Inc. | Catheter system providing step reduction for postconditioning |
| US8992540B2 (en) * | 2010-07-22 | 2015-03-31 | Kyphon Sarl | Adjustable surgical instruments and methods of use and fabrication |
| WO2012061940A1 (en) | 2010-11-08 | 2012-05-18 | Colibri Technologies Inc. | Systems and methods for improved visualization during minimally invasive procedures |
| US9533124B2 (en) | 2011-04-14 | 2017-01-03 | Abbott Cardiovascular Systems Inc. | Reperfusion injury devices |
| USD699841S1 (en) * | 2011-07-29 | 2014-02-18 | Nordson Corporation | Female body of connector for fluid tubing |
| USD698440S1 (en) * | 2011-07-29 | 2014-01-28 | Nordson Corporation | Connector for fluid tubing |
| USD699840S1 (en) * | 2011-07-29 | 2014-02-18 | Nordson Corporation | Male body of connector for fluid tubing |
| KR101177279B1 (ko) * | 2012-01-10 | 2012-08-27 | 이영희 | 의료용 3-way 스톱콕 및 이에 연결되는 약액세트 연결구 |
| US9522257B2 (en) | 2012-03-30 | 2016-12-20 | Abbott Cardiovascular Systems Inc. | Integrated controlled volume inflator device, components, and methods of use |
| EP2879751B1 (en) * | 2012-08-03 | 2019-09-25 | Harold Carrison | Vessel flow control devices |
| GB201219491D0 (en) | 2012-10-30 | 2012-12-12 | Rainbow Medical Ltd | Surgical controller |
| US9358042B2 (en) | 2013-03-13 | 2016-06-07 | The Spectranetics Corporation | Expandable member for perforation occlusion |
| KR102227469B1 (ko) | 2013-03-13 | 2021-03-15 | 라보리 | 카테터 어셈블리 |
| CN103252012A (zh) * | 2013-05-13 | 2013-08-21 | 宁波圣宇瑞医疗器械有限公司 | 医用弯曲导管 |
| WO2015026774A1 (en) * | 2013-08-20 | 2015-02-26 | The Charlotte-Mecklenburg Hospital Authority D/B/A Carolinas Healthcare System | Device for placement of an intrauterine balloon |
| US9027877B1 (en) * | 2014-04-10 | 2015-05-12 | Google Inc. | Filling apparatus for high-altitude balloons |
| US20160206298A1 (en) * | 2015-01-21 | 2016-07-21 | Biolife, L.L.C. | Combination Hemostatic Tablet or Powder and Radial Arterial Compression Band with Syringe Assembly |
| US10315017B2 (en) * | 2015-01-27 | 2019-06-11 | Cook Medical Technologies Llc | Incremental inflation tool for extraction balloons |
| US11147540B2 (en) | 2015-07-01 | 2021-10-19 | Minnetronix, Inc. | Introducer sheath and puncture tool for the introduction and placement of a catheter in tissue |
| ES2906952T3 (es) | 2015-08-05 | 2022-04-21 | Minnetronix Inc | Sistema de filtro de flujo tangencial para la filtración de materiales a partir de fluidos biológicos |
| US10449336B2 (en) | 2015-08-11 | 2019-10-22 | The Spectranetics Corporation | Temporary occlusions balloon devices and methods for preventing blood flow through a vascular perforation |
| US10499892B2 (en) | 2015-08-11 | 2019-12-10 | The Spectranetics Corporation | Temporary occlusion balloon devices and methods for preventing blood flow through a vascular perforation |
| ES2944452T3 (es) | 2015-12-04 | 2023-06-21 | Minnetronix Inc | Sistemas de acondicionamiento de fluido cerebrospinal |
| US9963216B1 (en) | 2016-02-26 | 2018-05-08 | X Development Llc | Filling apparatus for high-altitude balloons |
| WO2018144095A2 (en) | 2016-11-14 | 2018-08-09 | Menorrx, LLC | System and method for delivering therapeutic agents to the uterine cavity |
| EP3773858B1 (en) * | 2018-04-13 | 2023-11-29 | Merit Medical Systems, Inc. | Multi-stage balloon catheter systems |
| CN109009413B (zh) * | 2018-08-13 | 2024-02-06 | 北京安和加利尔科技有限公司 | 一种具有注液结构的内镜用高频电刀 |
| CN109350204A (zh) * | 2018-12-12 | 2019-02-19 | 韩敏 | 一种妇产科止血囊 |
| WO2020197801A1 (en) * | 2019-03-27 | 2020-10-01 | Gynion, Llc | System and method for delivering therapeutic agents to the uterine cavity |
| US11813417B2 (en) | 2019-08-13 | 2023-11-14 | Medtronic Vascular, Inc. | Catheter modification device |
| AU2021206169A1 (en) * | 2020-01-07 | 2022-06-09 | CRM Medical Devices, Inc. | Catheter with a click connector |
| KR102372360B1 (ko) * | 2020-06-24 | 2022-03-08 | 권승열 | 액상물의 분사량 조절이 가능한 복합 카테터 |
| CN111803171B (zh) * | 2020-06-30 | 2021-08-24 | 重庆大学附属肿瘤医院 | 一种便于操作的血管外科手术用封堵装置 |
| CN112386298B (zh) * | 2020-11-19 | 2022-05-27 | 张慧 | 一种临床用止血抢救装置 |
| CN113133747B (zh) * | 2021-03-31 | 2024-01-02 | 中国人民解放军联勤保障部队第九二〇医院 | 医用集成式检测装置 |
| CN113520318B (zh) * | 2021-07-08 | 2022-03-08 | 哈尔滨医科大学 | 一种集成oct成像和pdt的导管设计 |
Family Cites Families (37)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4583974A (en) * | 1984-04-04 | 1986-04-22 | Kokernak Denis T | Syringe for balloon dilation catheters |
| US4739768B2 (en) * | 1986-06-02 | 1995-10-24 | Target Therapeutics Inc | Catheter for guide-wire tracking |
| US4990139A (en) | 1986-09-10 | 1991-02-05 | Jang G David | Tandem independently inflatable/deflatable multiple diameter balloon angioplasty catheter systems |
| US4744366A (en) | 1986-09-10 | 1988-05-17 | Jang G David | Concentric independently inflatable/deflatable multiple diameter balloon angioplasty catheter systems and method of use |
| US4795431A (en) * | 1987-01-14 | 1989-01-03 | Walling Peter T | Syringe and catheter apparatus |
| US5236659A (en) | 1988-10-04 | 1993-08-17 | Cordis Corporation | Tailoring expansion properties of balloons for medical devices |
| US5795325A (en) * | 1991-07-16 | 1998-08-18 | Heartport, Inc. | Methods and apparatus for anchoring an occluding member |
| US5348538A (en) | 1992-09-29 | 1994-09-20 | Scimed Life Systems, Inc. | Shrinking balloon catheter having nonlinear or hybrid compliance curve |
| US5389070A (en) | 1993-03-16 | 1995-02-14 | Wake Forest University | Syringe apparatus with a fluid reservoir for injection and aspiration of fluids |
| US5843116A (en) * | 1996-05-02 | 1998-12-01 | Cardiovascular Dynamics, Inc. | Focalized intraluminal balloons |
| WO1995023625A1 (en) | 1994-03-01 | 1995-09-08 | Boston Scientific Corporation | Asymmetric dilatation balloon |
| US5533985A (en) | 1994-04-20 | 1996-07-09 | Wang; James C. | Tubing |
| US5634910A (en) * | 1995-09-22 | 1997-06-03 | Ryder International Corporation | Syringe instrument |
| WO1997026027A1 (en) | 1996-01-16 | 1997-07-24 | Advanced Cardiovascular Systems, Inc. | Lubricous and readily bondable catheter shaft |
| US20030094736A1 (en) | 1996-05-03 | 2003-05-22 | Chuan Qin | Method of surface modifying a medical tubing |
| US6270477B1 (en) | 1996-05-20 | 2001-08-07 | Percusurge, Inc. | Catheter for emboli containment |
| WO1998036290A1 (en) | 1997-02-14 | 1998-08-20 | Hamamatsu Photonics K.K. | Radiation detection device and method of producing the same |
| US6258080B1 (en) | 1997-07-01 | 2001-07-10 | Target Therapeutics, Inc. | Kink-free spiral-wound catheter |
| US6056721A (en) | 1997-08-08 | 2000-05-02 | Sunscope International, Inc. | Balloon catheter and method |
| US6368316B1 (en) * | 1998-06-11 | 2002-04-09 | Target Therapeutics, Inc. | Catheter with composite stiffener |
| US6641573B1 (en) | 1999-03-25 | 2003-11-04 | Arteria Medical Science, Inc. | Device and method of guide wire balloon inflation and deflation to prevent cerebral embolization during carotid stenting |
| US6234996B1 (en) | 1999-06-23 | 2001-05-22 | Percusurge, Inc. | Integrated inflation/deflation device and method |
| US7037318B2 (en) * | 2000-12-18 | 2006-05-02 | Boston Scientific Scimed, Inc. | Catheter for controlled stent delivery |
| US6585718B2 (en) * | 2001-05-02 | 2003-07-01 | Cardiac Pacemakers, Inc. | Steerable catheter with shaft support system for resisting axial compressive loads |
| US7112357B2 (en) * | 2002-01-23 | 2006-09-26 | Boston Scientific Scimed, Inc. | Medical devices comprising a multilayer construction |
| US7169170B2 (en) * | 2002-02-22 | 2007-01-30 | Cordis Corporation | Self-expanding stent delivery system |
| US20050256508A1 (en) * | 2002-05-07 | 2005-11-17 | Cardiac Pacemakers, Inc. | Guide catheter system having relative markings |
| US20050256503A1 (en) * | 2002-05-07 | 2005-11-17 | Cardiac Pacemakers, Inc. | Tapered catheter delivery system |
| GB0216081D0 (en) * | 2002-07-11 | 2002-08-21 | Imp College Innovations Ltd | Methods of making biological materials and uses thereof |
| NO20024883D0 (no) * | 2002-10-09 | 2002-10-09 | Amersham Health As | Slange |
| US7628784B2 (en) | 2003-01-06 | 2009-12-08 | C. R. Bard, Inc. | Balloon catheter with improved resistance to non-deflation |
| US20050015048A1 (en) | 2003-03-12 | 2005-01-20 | Chiu Jessica G. | Infusion treatment agents, catheters, filter devices, and occlusion devices, and use thereof |
| US11000670B2 (en) * | 2003-04-28 | 2021-05-11 | Cook Medical Technologies Llc | Flexible sheath with varying durometer |
| JP5178198B2 (ja) * | 2004-10-11 | 2013-04-10 | アブビサー メディカル リミテッド ライアビリティ カンパニー | 腹腔内圧モニタリング用の機器および方法 |
| US7931619B2 (en) * | 2005-01-04 | 2011-04-26 | C. R. Bard, Inc. | Power injection catheters |
| US8221348B2 (en) * | 2005-07-07 | 2012-07-17 | St. Jude Medical, Cardiology Division, Inc. | Embolic protection device and methods of use |
| US7674240B2 (en) * | 2005-12-20 | 2010-03-09 | Abbott Cardiovascular Systems Inc. | Method and apparatus for controlled vessel occlusion |
-
2005
- 2005-12-20 US US11/313,477 patent/US7674240B2/en not_active Expired - Fee Related
-
2006
- 2006-11-21 EP EP06838275A patent/EP1962936A1/en not_active Withdrawn
- 2006-11-21 WO PCT/US2006/045205 patent/WO2007078455A1/en not_active Ceased
- 2006-11-21 JP JP2008547244A patent/JP5114426B2/ja not_active Expired - Fee Related
-
2010
- 2010-02-12 US US12/705,556 patent/US8419714B2/en not_active Expired - Fee Related
-
2013
- 2013-02-25 US US13/776,579 patent/US8936568B2/en not_active Expired - Fee Related
- 2013-02-25 US US13/776,568 patent/US9179919B2/en not_active Expired - Fee Related
-
2015
- 2015-11-09 US US14/936,211 patent/US20160058984A1/en not_active Abandoned
Also Published As
| Publication number | Publication date |
|---|---|
| JP2009519809A (ja) | 2009-05-21 |
| US20100191220A1 (en) | 2010-07-29 |
| US20130165903A1 (en) | 2013-06-27 |
| EP1962936A1 (en) | 2008-09-03 |
| US20130172923A1 (en) | 2013-07-04 |
| WO2007078455A1 (en) | 2007-07-12 |
| US20070142818A1 (en) | 2007-06-21 |
| US8936568B2 (en) | 2015-01-20 |
| US9179919B2 (en) | 2015-11-10 |
| US20160058984A1 (en) | 2016-03-03 |
| US8419714B2 (en) | 2013-04-16 |
| US7674240B2 (en) | 2010-03-09 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5114426B2 (ja) | 制御可能に血管を閉塞する方法及び装置 | |
| US6234996B1 (en) | Integrated inflation/deflation device and method | |
| US6086559A (en) | Method and device for pressure-controlled handling of a fluid, in particular for medical purposes | |
| US6179815B1 (en) | Low compliance inflation/deflation system | |
| US7104981B2 (en) | Apparatus and method for inserting an intra-aorta catheter through a delivery sheath | |
| EP1631197B1 (en) | Apparatus for sealing a vascular puncture | |
| US4919121A (en) | Inflation device for angioplasty catheter | |
| US7033344B2 (en) | Methods for reducing distal embolization | |
| US6802825B2 (en) | Access catheter apparatus for use in minimally invasive surgery and diagnostic procedures in the uterus and fallopian tubes | |
| CN102316924A (zh) | 球囊导管膨胀装置和方法 | |
| CN104918535A (zh) | 锚定工作通道 | |
| WO1998056440A1 (en) | Syringe and method for inflating low volume catheter balloons | |
| US9220532B2 (en) | Translumenal peritoneal access and catheter therefor | |
| WO2000029060A2 (en) | Low volume syringe and method for inflating surgical balloons | |
| EP0962232A2 (en) | Balloon catheter | |
| US9089663B2 (en) | Percutaneous access device | |
| US20250161641A1 (en) | Devices and Methods for Insertion and/or Pressurization of a Balloon Catheter for Balloon Dilation of the Eustachian Tube and Other Anatomical Passageways Accessible Through the Nostril of a Human | |
| CN111182933A (zh) | 注射装置和系统以及其使用方法 | |
| CN111135431B (zh) | 一种用于锚定导丝的球囊导管及连接器组件 | |
| CN114618071B (zh) | 微导管及使用方法 | |
| CN218944129U (zh) | 一种球囊扩张导管 | |
| US12329920B2 (en) | Balloon angioplasty inflation device and method of preparing a balloon for angioplasty |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20091124 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20100217 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20111130 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20111206 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20120306 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20121002 |
|
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20121015 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20151019 Year of fee payment: 3 |
|
| R150 | Certificate of patent or registration of utility model |
Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| LAPS | Cancellation because of no payment of annual fees |