JP5070373B2 - 組枠によって支持される充填構造を有する移植片システムおよびその使用方法 - Google Patents
組枠によって支持される充填構造を有する移植片システムおよびその使用方法 Download PDFInfo
- Publication number
- JP5070373B2 JP5070373B2 JP2008509201A JP2008509201A JP5070373B2 JP 5070373 B2 JP5070373 B2 JP 5070373B2 JP 2008509201 A JP2008509201 A JP 2008509201A JP 2008509201 A JP2008509201 A JP 2008509201A JP 5070373 B2 JP5070373 B2 JP 5070373B2
- Authority
- JP
- Japan
- Prior art keywords
- filling structure
- wall
- filling
- aneurysm
- lumen
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
- 238000000034 method Methods 0.000 title description 15
- 206010002329 Aneurysm Diseases 0.000 claims description 74
- 239000000463 material Substances 0.000 claims description 44
- 210000004204 blood vessel Anatomy 0.000 claims description 15
- 230000017531 blood circulation Effects 0.000 claims description 14
- 239000012530 fluid Substances 0.000 claims description 12
- 239000012528 membrane Substances 0.000 claims description 9
- 239000002184 metal Substances 0.000 claims description 9
- 239000000835 fiber Substances 0.000 claims description 8
- 230000004048 modification Effects 0.000 claims description 7
- 238000012986 modification Methods 0.000 claims description 7
- 239000006260 foam Substances 0.000 claims description 6
- 238000007788 roughening Methods 0.000 claims description 5
- 239000008280 blood Substances 0.000 claims description 4
- 210000004369 blood Anatomy 0.000 claims description 4
- 229920002635 polyurethane Polymers 0.000 claims description 4
- 239000004814 polyurethane Substances 0.000 claims description 4
- 238000000576 coating method Methods 0.000 claims description 3
- 239000003814 drug Substances 0.000 claims description 3
- 229940079593 drug Drugs 0.000 claims description 3
- 229920000126 latex Polymers 0.000 claims description 3
- 239000004816 latex Substances 0.000 claims description 3
- 239000011248 coating agent Substances 0.000 claims description 2
- 229920001296 polysiloxane Polymers 0.000 claims description 2
- 238000007789 sealing Methods 0.000 claims description 2
- 229910052710 silicon Inorganic materials 0.000 claims 1
- 239000010703 silicon Substances 0.000 claims 1
- 238000011144 upstream manufacturing Methods 0.000 claims 1
- 208000007474 aortic aneurysm Diseases 0.000 description 38
- 210000003090 iliac artery Anatomy 0.000 description 27
- 208000002223 abdominal aortic aneurysm Diseases 0.000 description 26
- 210000000709 aorta Anatomy 0.000 description 21
- 210000002254 renal artery Anatomy 0.000 description 15
- 239000000945 filler Substances 0.000 description 11
- 239000010410 layer Substances 0.000 description 9
- 229920000642 polymer Polymers 0.000 description 9
- 238000011282 treatment Methods 0.000 description 9
- 230000008439 repair process Effects 0.000 description 7
- 201000008982 Thoracic Aortic Aneurysm Diseases 0.000 description 6
- 210000002376 aorta thoracic Anatomy 0.000 description 6
- 230000007704 transition Effects 0.000 description 6
- 239000007943 implant Substances 0.000 description 5
- 239000000126 substance Substances 0.000 description 5
- 230000002792 vascular Effects 0.000 description 5
- 210000001367 artery Anatomy 0.000 description 4
- 208000003457 familial thoracic 1 aortic aneurysm Diseases 0.000 description 4
- 208000007536 Thrombosis Diseases 0.000 description 3
- 238000003384 imaging method Methods 0.000 description 3
- 230000002787 reinforcement Effects 0.000 description 3
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 2
- 239000013543 active substance Substances 0.000 description 2
- 239000000853 adhesive Substances 0.000 description 2
- 230000001070 adhesive effect Effects 0.000 description 2
- 230000001919 adrenal effect Effects 0.000 description 2
- 210000003484 anatomy Anatomy 0.000 description 2
- 230000000975 bioactive effect Effects 0.000 description 2
- 239000003795 chemical substances by application Substances 0.000 description 2
- -1 flocking Substances 0.000 description 2
- 210000004013 groin Anatomy 0.000 description 2
- 238000011065 in-situ storage Methods 0.000 description 2
- 238000004519 manufacturing process Methods 0.000 description 2
- 239000004005 microsphere Substances 0.000 description 2
- 238000002355 open surgical procedure Methods 0.000 description 2
- 229920000728 polyester Polymers 0.000 description 2
- 239000004810 polytetrafluoroethylene Substances 0.000 description 2
- 229920001343 polytetrafluoroethylene Polymers 0.000 description 2
- 230000001737 promoting effect Effects 0.000 description 2
- 239000002356 single layer Substances 0.000 description 2
- 239000011780 sodium chloride Substances 0.000 description 2
- 210000001519 tissue Anatomy 0.000 description 2
- 210000005166 vasculature Anatomy 0.000 description 2
- 102000008186 Collagen Human genes 0.000 description 1
- 108010035532 Collagen Proteins 0.000 description 1
- 229920004934 Dacron® Polymers 0.000 description 1
- 208000002251 Dissecting Aneurysm Diseases 0.000 description 1
- 239000004593 Epoxy Substances 0.000 description 1
- 208000008952 Iliac Aneurysm Diseases 0.000 description 1
- 206010061307 Neck deformity Diseases 0.000 description 1
- 239000002202 Polyethylene glycol Substances 0.000 description 1
- 229910000639 Spring steel Inorganic materials 0.000 description 1
- 239000004826 Synthetic adhesive Substances 0.000 description 1
- 210000001015 abdomen Anatomy 0.000 description 1
- WYTGDNHDOZPMIW-RCBQFDQVSA-N alstonine Natural products C1=CC2=C3C=CC=CC3=NC2=C2N1C[C@H]1[C@H](C)OC=C(C(=O)OC)[C@H]1C2 WYTGDNHDOZPMIW-RCBQFDQVSA-N 0.000 description 1
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 1
- 239000000227 bioadhesive Substances 0.000 description 1
- 229920000229 biodegradable polyester Polymers 0.000 description 1
- 239000004622 biodegradable polyester Substances 0.000 description 1
- 230000036760 body temperature Effects 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 230000002490 cerebral effect Effects 0.000 description 1
- 229920001436 collagen Polymers 0.000 description 1
- 238000004891 communication Methods 0.000 description 1
- 238000011960 computer-aided design Methods 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 238000013461 design Methods 0.000 description 1
- 230000010339 dilation Effects 0.000 description 1
- 239000013013 elastic material Substances 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 208000037834 fusiform aneurysm Diseases 0.000 description 1
- 239000007789 gas Substances 0.000 description 1
- 239000000499 gel Substances 0.000 description 1
- 230000005484 gravity Effects 0.000 description 1
- 239000007952 growth promoter Substances 0.000 description 1
- 230000002008 hemorrhagic effect Effects 0.000 description 1
- 210000001621 ilium bone Anatomy 0.000 description 1
- 238000002513 implantation Methods 0.000 description 1
- 238000007689 inspection Methods 0.000 description 1
- 230000001788 irregular Effects 0.000 description 1
- 210000003734 kidney Anatomy 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 239000003550 marker Substances 0.000 description 1
- 230000005012 migration Effects 0.000 description 1
- 238000013508 migration Methods 0.000 description 1
- 238000000465 moulding Methods 0.000 description 1
- 239000001301 oxygen Substances 0.000 description 1
- 229910052760 oxygen Inorganic materials 0.000 description 1
- 229920000052 poly(p-xylylene) Polymers 0.000 description 1
- 229920002401 polyacrylamide Polymers 0.000 description 1
- 229920001223 polyethylene glycol Polymers 0.000 description 1
- 229920000139 polyethylene terephthalate Polymers 0.000 description 1
- 239000005020 polyethylene terephthalate Substances 0.000 description 1
- 229920006254 polymer film Polymers 0.000 description 1
- 238000006116 polymerization reaction Methods 0.000 description 1
- 239000011148 porous material Substances 0.000 description 1
- 239000000843 powder Substances 0.000 description 1
- 230000001681 protective effect Effects 0.000 description 1
- 238000011084 recovery Methods 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 229910001285 shape-memory alloy Inorganic materials 0.000 description 1
- 239000002002 slurry Substances 0.000 description 1
- 239000010935 stainless steel Substances 0.000 description 1
- 229910001220 stainless steel Inorganic materials 0.000 description 1
- 238000001356 surgical procedure Methods 0.000 description 1
- 238000002560 therapeutic procedure Methods 0.000 description 1
- 230000001732 thrombotic effect Effects 0.000 description 1
- 230000008467 tissue growth Effects 0.000 description 1
- 238000007740 vapor deposition Methods 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/04—Hollow or tubular parts of organs, e.g. bladders, tracheae, bronchi or bile ducts
- A61F2/06—Blood vessels
- A61F2/07—Stent-grafts
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods, e.g. tourniquets
- A61B17/12—Surgical instruments, devices or methods, e.g. tourniquets for ligaturing or otherwise compressing tubular parts of the body, e.g. blood vessels, umbilical cord
- A61B17/12022—Occluding by internal devices, e.g. balloons or releasable wires
- A61B17/12099—Occluding by internal devices, e.g. balloons or releasable wires characterised by the location of the occluder
- A61B17/12109—Occluding by internal devices, e.g. balloons or releasable wires characterised by the location of the occluder in a blood vessel
- A61B17/12113—Occluding by internal devices, e.g. balloons or releasable wires characterised by the location of the occluder in a blood vessel within an aneurysm
- A61B17/12118—Occluding by internal devices, e.g. balloons or releasable wires characterised by the location of the occluder in a blood vessel within an aneurysm for positioning in conjunction with a stent
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods, e.g. tourniquets
- A61B17/12—Surgical instruments, devices or methods, e.g. tourniquets for ligaturing or otherwise compressing tubular parts of the body, e.g. blood vessels, umbilical cord
- A61B17/12022—Occluding by internal devices, e.g. balloons or releasable wires
- A61B17/12131—Occluding by internal devices, e.g. balloons or releasable wires characterised by the type of occluding device
- A61B17/12136—Balloons
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods, e.g. tourniquets
- A61B17/12—Surgical instruments, devices or methods, e.g. tourniquets for ligaturing or otherwise compressing tubular parts of the body, e.g. blood vessels, umbilical cord
- A61B17/12022—Occluding by internal devices, e.g. balloons or releasable wires
- A61B17/12131—Occluding by internal devices, e.g. balloons or releasable wires characterised by the type of occluding device
- A61B17/12181—Occluding by internal devices, e.g. balloons or releasable wires characterised by the type of occluding device formed by fluidized, gelatinous or cellular remodelable materials, e.g. embolic liquids, foams or extracellular matrices
- A61B17/12195—Occluding by internal devices, e.g. balloons or releasable wires characterised by the type of occluding device formed by fluidized, gelatinous or cellular remodelable materials, e.g. embolic liquids, foams or extracellular matrices comprising a curable material
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods, e.g. tourniquets
- A61B17/22—Implements for squeezing-off ulcers or the like on the inside of inner organs of the body; Implements for scraping-out cavities of body organs, e.g. bones; Calculus removers; Calculus smashing apparatus; Apparatus for removing obstructions in blood vessels, not otherwise provided for
- A61B2017/22051—Implements for squeezing-off ulcers or the like on the inside of inner organs of the body; Implements for scraping-out cavities of body organs, e.g. bones; Calculus removers; Calculus smashing apparatus; Apparatus for removing obstructions in blood vessels, not otherwise provided for with an inflatable part, e.g. balloon, for positioning, blocking, or immobilisation
- A61B2017/22065—Functions of balloons
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/82—Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/86—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure
- A61F2/90—Stents in a form characterised by the wire-like elements; Stents in the form characterised by a net-like or mesh-like structure characterised by a net-like or mesh-like structure
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/04—Hollow or tubular parts of organs, e.g. bladders, tracheae, bronchi or bile ducts
- A61F2/06—Blood vessels
- A61F2002/065—Y-shaped blood vessels
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/04—Hollow or tubular parts of organs, e.g. bladders, tracheae, bronchi or bile ducts
- A61F2/06—Blood vessels
- A61F2/07—Stent-grafts
- A61F2002/077—Stent-grafts having means to fill the space between stent-graft and aneurysm wall, e.g. a sleeve
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2230/00—Geometry of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2230/0002—Two-dimensional shapes, e.g. cross-sections
- A61F2230/0028—Shapes in the form of latin or greek characters
- A61F2230/0034—D-shaped
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Surgery (AREA)
- Animal Behavior & Ethology (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Heart & Thoracic Surgery (AREA)
- Vascular Medicine (AREA)
- Reproductive Health (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Medical Informatics (AREA)
- Molecular Biology (AREA)
- Transplantation (AREA)
- Oral & Maxillofacial Surgery (AREA)
- Cardiology (AREA)
- Pulmonology (AREA)
- Gastroenterology & Hepatology (AREA)
- Neurosurgery (AREA)
- Prostheses (AREA)
Description
Claims (23)
- 血管内の動脈瘤を治療するシステムであって、当該システムは、
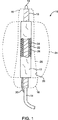
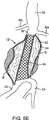
外壁と内壁を有する第1の二重壁充填構造と、
前記第1の二重壁充填構造の第1のほぼ管状の管腔の少なくとも一部内に拡張させることができる、前記第1の二重壁充填構造とは別の、第1の組枠と、
外壁と内壁を有する第2の二重壁充填構造と、
前記第2の二重壁充填構造の第2のほぼ管状の管腔の少なくとも一部内に拡張させることができる、前記第1の組枠と前記第1および第2の二重壁充填構造とは別の、少なくとも第2の組枠と、
を備え、
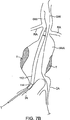
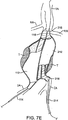
前記第1の二重壁充填構造が硬化可能流体充填媒体で充填されるように構成されることで、前記第1の二重壁充填構造の外壁は、前記動脈瘤にわたって位置決めされた場合に前記動脈瘤の内側面と適合し、かつ前記第1の二重壁充填構造の内壁は、前記第1のほぼ管状の管腔を流れる血液と直接接触するように、血流経路を提供する前記第1のほぼ管状の管腔を形成し、
前記第2の二重壁充填構造が、前記動脈瘤内において前記第1の二重壁充填構造に隣接し、かつ並行して配置され、また、硬化可能流体充填媒体で充填されるように構成されることで、前記第2の二重壁充填構造の外壁は、前記動脈瘤にわたって位置決めされた場合に前記動脈瘤の内側面と適合し、かつ前記第2の二重壁充填構造の内壁は、前記第2のほぼ管状の管腔を流れる血流と直接接触するように、血流経路を提供する前記第2のほぼ管状の管腔を形成し、
前記第2の二重壁充填構造は、前記第1の二重壁充填構造からは分離した別個の構造である
ことを特徴とするシステム。 - 前記組枠は、前記ほぼ管状の管腔のほぼ全長にわたって拡張することを特徴とする請求項1に記載のシステム。
- 前記組枠は、隣接する血管内に前記ほぼ管状の管腔の少なくとも一端部から外向きに拡張することを特徴とする請求項1乃至2のいずれかに記載のシステム。
- 前記組枠は、隣接する血管内に前記ほぼ管状の管腔の両端部から外向きに拡張することを特徴とする請求項3に記載のシステム。
- それぞれの前記管状の管腔内に配置する少なくとも2つの組枠を備えることを特徴とする請求項1乃至4のいずれかに記載のシステム。
- 前記組枠は連続して置かれるように構成されていることを特徴とする請求項5に記載のシステム。
- 前記組枠は、互いに重ね合わされ、および/または互いに取り付けられるようになっていることを特徴とする請求項5に記載のシステム。
- 前記組枠は弾性があり、制約された構成から展開された構成まで自己拡張するように構成されていることを特徴とする請求項1乃至7のいずれかに記載のシステム。
- 前記組枠は自己拡張メッシュであることを特徴とする請求項8に記載のシステム。
- 前記組枠は可鍛性で、小さい直径の構成から展開された構成まで拡張されるバルーン状に構成されていることを特徴とする請求項1乃至9のいずれかに記載のシステム。
- 前記組枠は金属フレームからなることを特徴とする請求項1乃至10のいずれかに記載のシステム。
- 前記金属フレームの少なくとも一部は膜で覆われていることを特徴とする請求項11に記載のシステム。
- 前記管状の管腔に位置決めされる前記組枠の一部は前記膜で覆われており、前記管状の管腔を越えて拡張する部分は覆われていないことを特徴とする請求項1乃至12のいずれかに記載のシステム。
- 前記充填構造の少なくとも外壁は、シリコン、ポリウレタン、ラテックス、またはこれらの組合せより構成される順応性材料で形成されていることを特徴とする請求項1に記載のシステム。
- 前記充填構造全体は、シリコン、ポリウレタン、ラテックス、またはこれらの組合せより構成される順応性材料で形成されていることを特徴とする請求項1乃至14のいずれかに記載のシステム。
- 前記充填構造の外側面の少なくとも一部は、密封または組織内部成長を促進するように改質されることを特徴とする請求項1乃至15のいずれかに記載のシステム。
- 前記表面改質は、粗面処理、泡層、繊維、フロック加工、スティップル、または薬物コーティングを含むことを特徴とする請求項16に記載のシステム。
- 前記充填構造の内側面の少なくとも一部は、前記構造の硬化を促進するように改質されることを特徴とする請求項1乃至17のいずれかに記載のシステム。
- 前記表面改質は、粗面処理、リング、スティップル、フロック加工、泡層、または繊維を含むことを特徴とする請求項18に記載のシステム。
- 前記二重壁充填構造を運ぶように前記第1の管状の管腔内に位置決めすることができる拡張可能な管状支持体を有する搬送カテーテルをさらに備えていることを特徴とする請求項1乃至19のいずれかに記載のシステム。
- 前記拡張可能な管状支持体は、管状支持体が、前記血管に前記充填構造の各端部を位置合わせし、これと適合させるように、前記二重壁充填構造から上流側と下流側に拡張することを特徴とする請求項20に記載のシステム。
- 前記管状支持体は、膨張可能な支持バルーンを備えていることを特徴とする請求項20に記載のシステム。
- 前記管状支持体は、1つまたは複数の固定直径まで拡張可能な機械的構造を備えていることを特徴とする請求項22に記載のシステム。
Applications Claiming Priority (7)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US67515805P | 2005-04-28 | 2005-04-28 | |
| US60/675,158 | 2005-04-28 | ||
| US11/187,471 | 2005-07-22 | ||
| US11/187,471 US7530988B2 (en) | 2004-07-22 | 2005-07-22 | Methods and systems for endovascular aneurysm treatment |
| US73660205P | 2005-11-14 | 2005-11-14 | |
| US60/736,602 | 2005-11-14 | ||
| PCT/US2006/016403 WO2006116725A2 (en) | 2005-04-28 | 2006-04-28 | Graft systems having filling structures supported by scaffolds and methods for their use |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2008539050A JP2008539050A (ja) | 2008-11-13 |
| JP2008539050A5 JP2008539050A5 (ja) | 2009-05-28 |
| JP5070373B2 true JP5070373B2 (ja) | 2012-11-14 |
Family
ID=37215576
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2008509201A Expired - Fee Related JP5070373B2 (ja) | 2005-04-28 | 2006-04-28 | 組枠によって支持される充填構造を有する移植片システムおよびその使用方法 |
Country Status (5)
| Country | Link |
|---|---|
| EP (1) | EP1874231B1 (ja) |
| JP (1) | JP5070373B2 (ja) |
| AU (1) | AU2006239228A1 (ja) |
| CA (1) | CA2599460A1 (ja) |
| WO (1) | WO2006116725A2 (ja) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2019526389A (ja) * | 2016-09-09 | 2019-09-19 | ダブリュ.エル.ゴア アンド アソシエイツ,インコーポレイティドW.L. Gore & Associates, Incorporated | 全体アーチ概念 |
Families Citing this family (25)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2004026183A2 (en) | 2002-09-20 | 2004-04-01 | Nellix, Inc. | Stent-graft with positioning anchor |
| US8048145B2 (en) | 2004-07-22 | 2011-11-01 | Endologix, Inc. | Graft systems having filling structures supported by scaffolds and methods for their use |
| WO2007008600A2 (en) | 2005-07-07 | 2007-01-18 | Nellix, Inc. | Systems and methods for endovascular aneurysm treatment |
| US20070150041A1 (en) | 2005-12-22 | 2007-06-28 | Nellix, Inc. | Methods and systems for aneurysm treatment using filling structures |
| JP5329542B2 (ja) * | 2007-08-23 | 2013-10-30 | ダイレクト フロウ メディカル、 インク. | インプレース形成支持部を有する経腔的に移植可能な心臓弁 |
| CN101902988A (zh) | 2008-04-25 | 2010-12-01 | 耐利克斯股份有限公司 | 支架移植物的输送系统 |
| US20090287145A1 (en) * | 2008-05-15 | 2009-11-19 | Altura Interventional, Inc. | Devices and methods for treatment of abdominal aortic aneurysms |
| US20100305686A1 (en) * | 2008-05-15 | 2010-12-02 | Cragg Andrew H | Low-profile modular abdominal aortic aneurysm graft |
| CN102076282A (zh) * | 2008-06-04 | 2011-05-25 | 耐利克斯股份有限公司 | 泊入装置和使用方法 |
| WO2009149294A1 (en) | 2008-06-04 | 2009-12-10 | Nellix, Inc. | Sealing apparatus and methods of use |
| CA2782385A1 (en) | 2009-12-01 | 2011-06-09 | Altura Medical, Inc. | Modular endograft devices and associated systems and methods |
| US20110276078A1 (en) | 2009-12-30 | 2011-11-10 | Nellix, Inc. | Filling structure for a graft system and methods of use |
| US9603708B2 (en) | 2010-05-19 | 2017-03-28 | Dfm, Llc | Low crossing profile delivery catheter for cardiovascular prosthetic implant |
| US8801768B2 (en) | 2011-01-21 | 2014-08-12 | Endologix, Inc. | Graft systems having semi-permeable filling structures and methods for their use |
| JP5976777B2 (ja) * | 2011-04-06 | 2016-08-24 | エンドーロジックス インコーポレイテッド | 血管内動脈瘤治療のための方法およびシステム |
| US9445897B2 (en) | 2012-05-01 | 2016-09-20 | Direct Flow Medical, Inc. | Prosthetic implant delivery device with introducer catheter |
| CA2881535A1 (en) | 2012-08-10 | 2014-02-13 | Altura Medical, Inc. | Stent delivery systems and associated methods |
| WO2014110231A2 (en) * | 2013-01-10 | 2014-07-17 | Trivascular, Inc. | Sac liner for aneurysm repair |
| WO2014159093A1 (en) * | 2013-03-14 | 2014-10-02 | Endologix, Inc. | Method for forming materials in situ within a medical device |
| WO2014144809A1 (en) | 2013-03-15 | 2014-09-18 | Altura Medical, Inc. | Endograft device delivery systems and associated methods |
| CN108403257B (zh) * | 2014-05-30 | 2021-03-16 | 安德乐吉克斯有限责任公司 | 使用膨胀式填充结构的模块化支架移植物系统和方法 |
| JP6672286B2 (ja) | 2014-10-23 | 2020-03-25 | トリバスキュラー・インコーポレイテッドTriVascular, INC. | アクセス導管を有するステントグラフト送達システム |
| EP3397199A4 (en) * | 2015-12-31 | 2019-08-07 | Endologix, Inc. | SYSTEMS AND METHODS WITH FENESTRY GRAFT AND FILLING STRUCTURE |
| EP3576673A4 (en) * | 2017-02-01 | 2021-01-06 | Endologix LLC | LONGITUDINALLY EXPANDABLE ENDOPROTHESIS SYSTEMS AND PROCESSES |
| EP3784169A4 (en) | 2018-04-23 | 2022-02-16 | Endologix LLC | MODULATION OF INFLAMMATORY RESPONSE FOLLOWING ENDOVASCULAR TREATMENT |
Family Cites Families (35)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1980000007A1 (en) | 1978-06-02 | 1980-01-10 | A Rockey | Medical sleeve |
| US4728328A (en) | 1984-10-19 | 1988-03-01 | Research Corporation | Cuffed tubular organic prostheses |
| AU678350B2 (en) | 1992-05-08 | 1997-05-29 | Schneider (Usa) Inc. | Esophageal stent and delivery tool |
| AU1091095A (en) | 1993-11-08 | 1995-05-29 | Harrison M. Lazarus | Intraluminal vascular graft and method |
| JP3182301B2 (ja) | 1994-11-07 | 2001-07-03 | キヤノン株式会社 | マイクロ構造体及びその形成法 |
| US5785679A (en) | 1995-07-19 | 1998-07-28 | Endotex Interventional Systems, Inc. | Methods and apparatus for treating aneurysms and arterio-venous fistulas |
| US5769882A (en) | 1995-09-08 | 1998-06-23 | Medtronic, Inc. | Methods and apparatus for conformably sealing prostheses within body lumens |
| US5824037A (en) | 1995-10-03 | 1998-10-20 | Medtronic, Inc. | Modular intraluminal prostheses construction and methods |
| US6193745B1 (en) | 1995-10-03 | 2001-02-27 | Medtronic, Inc. | Modular intraluminal prosteheses construction and methods |
| US5591195A (en) | 1995-10-30 | 1997-01-07 | Taheri; Syde | Apparatus and method for engrafting a blood vessel |
| US5665117A (en) | 1995-11-27 | 1997-09-09 | Rhodes; Valentine J. | Endovascular prosthesis with improved sealing means for aneurysmal arterial disease and method of use |
| US6576009B2 (en) | 1995-12-01 | 2003-06-10 | Medtronic Ave, Inc. | Bifurcated intraluminal prostheses construction and methods |
| US5824040A (en) | 1995-12-01 | 1998-10-20 | Medtronic, Inc. | Endoluminal prostheses and therapies for highly variable body lumens |
| ATE290832T1 (de) | 1996-01-05 | 2005-04-15 | Medtronic Inc | Expandierbare endoluminale prothesen |
| US6395019B2 (en) | 1998-02-09 | 2002-05-28 | Trivascular, Inc. | Endovascular graft |
| GB9904722D0 (en) | 1999-03-03 | 1999-04-21 | Murch Clifford R | A tubular intraluminal graft |
| US6409757B1 (en) | 1999-09-15 | 2002-06-25 | Eva Corporation | Method and apparatus for supporting a graft assembly |
| US6663667B2 (en) | 1999-12-29 | 2003-12-16 | Edwards Lifesciences Corporation | Towel graft means for enhancing tissue ingrowth in vascular grafts |
| PT1259192E (pt) * | 2000-03-03 | 2004-04-30 | Cook Inc | Dispositivo endovascular com uma endoprotese |
| US6773454B2 (en) | 2000-08-02 | 2004-08-10 | Michael H. Wholey | Tapered endovascular stent graft and method of treating abdominal aortic aneurysms and distal iliac aneurysms |
| GB0114918D0 (en) * | 2001-06-19 | 2001-08-08 | Vortex Innovation Ltd | Devices for repairing aneurysms |
| AUPR847201A0 (en) * | 2001-10-26 | 2001-11-15 | Cook Incorporated | Endoluminal graft |
| US20060292206A1 (en) * | 2001-11-26 | 2006-12-28 | Kim Steven W | Devices and methods for treatment of vascular aneurysms |
| US7147660B2 (en) * | 2001-12-20 | 2006-12-12 | Boston Scientific Santa Rosa Corp. | Advanced endovascular graft |
| US20030130725A1 (en) | 2002-01-08 | 2003-07-10 | Depalma Donald F. | Sealing prosthesis |
| US20030135269A1 (en) | 2002-01-16 | 2003-07-17 | Swanstrom Lee L. | Laparoscopic-assisted endovascular/endoluminal graft placement |
| US7131991B2 (en) | 2002-04-24 | 2006-11-07 | Medtronic Vascular, Inc. | Endoluminal prosthetic assembly and extension method |
| US20030204249A1 (en) | 2002-04-25 | 2003-10-30 | Michel Letort | Endovascular stent graft and fixation cuff |
| US6918926B2 (en) | 2002-04-25 | 2005-07-19 | Medtronic Vascular, Inc. | System for transrenal/intraostial fixation of endovascular prosthesis |
| US7175652B2 (en) | 2002-08-20 | 2007-02-13 | Cook Incorporated | Stent graft with improved proximal end |
| WO2004026183A2 (en) * | 2002-09-20 | 2004-04-01 | Nellix, Inc. | Stent-graft with positioning anchor |
| US20040098096A1 (en) * | 2002-10-22 | 2004-05-20 | The University Of Miami | Endograft device to inhibit endoleak and migration |
| EP1613242B1 (en) * | 2003-03-26 | 2013-02-20 | The Foundry, LLC | Devices for treatment of abdominal aortic aneurysms |
| US20050065592A1 (en) | 2003-09-23 | 2005-03-24 | Asher Holzer | System and method of aneurism monitoring and treatment |
| WO2006012567A2 (en) * | 2004-07-22 | 2006-02-02 | Nellix, Inc. | Methods and systems for endovascular aneurysm treatment |
-
2006
- 2006-04-28 JP JP2008509201A patent/JP5070373B2/ja not_active Expired - Fee Related
- 2006-04-28 AU AU2006239228A patent/AU2006239228A1/en not_active Abandoned
- 2006-04-28 CA CA002599460A patent/CA2599460A1/en not_active Abandoned
- 2006-04-28 WO PCT/US2006/016403 patent/WO2006116725A2/en active Application Filing
- 2006-04-28 EP EP06751879.5A patent/EP1874231B1/en not_active Not-in-force
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2019526389A (ja) * | 2016-09-09 | 2019-09-19 | ダブリュ.エル.ゴア アンド アソシエイツ,インコーポレイティドW.L. Gore & Associates, Incorporated | 全体アーチ概念 |
| US10646323B2 (en) | 2016-09-09 | 2020-05-12 | W. L. Gore & Associates, Inc. | Total arch concept |
Also Published As
| Publication number | Publication date |
|---|---|
| AU2006239228A1 (en) | 2006-11-02 |
| EP1874231B1 (en) | 2016-01-06 |
| JP2008539050A (ja) | 2008-11-13 |
| WO2006116725A3 (en) | 2007-09-27 |
| CA2599460A1 (en) | 2006-11-02 |
| EP1874231A4 (en) | 2013-05-15 |
| EP1874231A2 (en) | 2008-01-09 |
| WO2006116725A2 (en) | 2006-11-02 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US11957608B2 (en) | Graft systems having filling structures supported by scaffolds and methods for their use | |
| JP5070373B2 (ja) | 組枠によって支持される充填構造を有する移植片システムおよびその使用方法 | |
| US7530988B2 (en) | Methods and systems for endovascular aneurysm treatment | |
| US11596413B2 (en) | Methods and systems for aneurysm treatment using filling structures | |
| US20210093473A1 (en) | System and methods for endovascular aneurysm treatment | |
| JP6297023B2 (ja) | 耐キンク性ステントグラフト | |
| AU2009256084A1 (en) | Sealing apparatus and methods of use | |
| US20180021045A1 (en) | Dual inflatable arterial prosthesis | |
| US11723668B2 (en) | Systems and methods with anchor device for fixation of filling structures in blood vessels | |
| CN113164246A (zh) | 具有套囊和支干的支架移植物系统和方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20090410 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20090410 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20110621 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20110916 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20120321 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20120420 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20120510 |
|
| A711 | Notification of change in applicant |
Free format text: JAPANESE INTERMEDIATE CODE: A711 Effective date: 20120514 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20120514 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A821 Effective date: 20120514 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 5070373 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20150831 Year of fee payment: 3 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| LAPS | Cancellation because of no payment of annual fees |