JP5005532B2 - 10−プロパルギル−10−デアザアミノプテリンを用いるt細胞リンパ腫の治療 - Google Patents
10−プロパルギル−10−デアザアミノプテリンを用いるt細胞リンパ腫の治療 Download PDFInfo
- Publication number
- JP5005532B2 JP5005532B2 JP2007515512A JP2007515512A JP5005532B2 JP 5005532 B2 JP5005532 B2 JP 5005532B2 JP 2007515512 A JP2007515512 A JP 2007515512A JP 2007515512 A JP2007515512 A JP 2007515512A JP 5005532 B2 JP5005532 B2 JP 5005532B2
- Authority
- JP
- Japan
- Prior art keywords
- cell
- cell lymphoma
- lymphoma
- pharmaceutical composition
- deazaaminopterin
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
- 0 CC(C*#[N+])(c1ccc(*)cc1)C(C=C(C)C1*)=Nc2c1nc(C)nc2[*+] Chemical compound CC(C*#[N+])(c1ccc(*)cc1)C(C=C(C)C1*)=Nc2c1nc(C)nc2[*+] 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/4985—Pyrazines or piperazines ortho- or peri-condensed with heterocyclic ring systems
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/505—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim
- A61K31/519—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim ortho- or peri-condensed with heterocyclic rings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/505—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim
- A61K31/519—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim ortho- or peri-condensed with heterocyclic rings
- A61K31/525—Isoalloxazines, e.g. riboflavins, vitamin B2
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7042—Compounds having saccharide radicals and heterocyclic rings
- A61K31/7052—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides
- A61K31/706—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom
- A61K31/7064—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom containing condensed or non-condensed pyrimidines
- A61K31/7068—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom containing condensed or non-condensed pyrimidines having oxo groups directly attached to the pyrimidine ring, e.g. cytidine, cytidylic acid
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Medicinal Chemistry (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Pharmacology & Pharmacy (AREA)
- Chemical & Material Sciences (AREA)
- Epidemiology (AREA)
- Molecular Biology (AREA)
- Organic Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
Description
(a)胸腺からの原始的リンパ性前駆細胞に悪性腫瘍が生じる、リンパ芽球性リンパ腫;
(b)T細胞前リンパ球性白血病、T細胞顆粒リンパ球性白血病、活動性NK細胞白血病、皮膚T細胞性リンパ腫(菌状息肉腫セザリー症候群)、未分化大細胞リンパ腫、T細胞型、腸症型T細胞リンパ腫、HTLV−1に関連するものを含む成人T細胞白血病/リンパ腫、及び血管免疫芽球性T細胞リンパ腫、及び皮下脂肪織炎性T細胞リンパ腫を含む、成熟又は末梢T細胞新生物;並びに
(c)最初にリンパ節副皮質を冒し、真の濾胞状パターンに成長することのない、末梢T細胞リンパ腫。
診断: 末梢T細胞リンパ腫、ステージIV
人口統計: 48歳男性
前治療: CHOP×4サイクル(2002年7月〜2002年11月)−難治性
ICE×2サイクル(2002年12月)−難治性
キャンパス(2003年3月〜2003年6月)−複合反応
治療前病期診断:広範な疾患、皮膚疾患
試験対象の治療:PDX 135mg/m2×1投与
毒性: グレード3の口内炎;グレード3の好中球減少症;敗血症
反応: PETスキャンによる本質的に完全な寛解
コメント: この患者は最終的には、グラム陽性菌による皮膚外傷開口部からの菌血症と敗血症を発症した後に死亡した。
診断: リンパ芽球性リンパ腫、T細胞前駆体、ステージIV
人口統計: 65歳女性
前治療: 2002年5月よりL−20複合体併用化学治療が行われ、2年間にわたって投与された。2002年5月から2004年2月までMTXを受けた。2004年12月に再発した。
治療前病期診断:広範囲に広がる再発
試験対象の治療:PDX 30mg/m2×3週間、4週間毎。これまでに3サイクルを完了
毒性: なし
反応: PET及びCTスキャンによる完全な寛解
コメント: 広範囲の洞に起因する疾患を伴う、本質的にメトトキサレート抵抗性の疾患を患っている患者であるが、PDXを1投与後に解消し始めた。
診断: T細胞リンパ腫に関連するHTLV
人口統計: 38歳男性
前治療: EPOCH−注入投与併用化学治療 2003年10月から2004年2月
治療前病期診断:左腋窩疾患
試験対象の治療:PDX 毎週30mg/m2×3を4週間毎×2サイクル
毒性: なし
反応: 完全な寛解
コメント: 最初のサイクル完了までに臨床的に明白な疾患は完全に消滅、非常に良好に耐容され、毒性なし。
診断: 脂肪織炎性T細胞リンパ腫
人口統計: 25歳男性
前治療: Ontak(難治性)、2002年9月〜2002年11月;Targretin及びIFNα 2003年1月〜2003年10月(永続的な部分的寛解);CHOP 2004年4月〜2004年6月;ICE 2004年6月、CyPen 2004年7月〜2004年8月、Targretin/MTX 2004年9月から2005年2月。
試験対象の治療:PDX 毎週30mg/m2×4
反応: PETによる臨床的に完全な寛解
毒性: なし
コメント: 皮下傷治癒、多数のためカウント不可、大きな潰瘍性肉芽傷
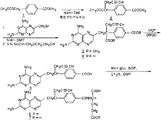
図4は、本発明による10−プロパルギル−10dAMの調製に有用な合成スキームを示す。18mlのシーブ乾燥THF中の油分散液における60%NaH(1.06g、26.5mmol)の混合物を0℃に冷却した。この低温混合物を、乾燥THF(7mL)中のホモテレフタル酸ジメチルエステル(5.0g、24mmol、図4における化合物1)の溶液で処理し、この混合物を0℃で1時間撹拌した。臭化プロパルギル(26.4mmol)を加え、混合物を0℃においてさらに1時間、次いで室温において16時間撹拌した。生じた混合物を2.4mLの50%酢酸で処理し、次いで240mLの水中に注いだ。この混合物を、エーテル(2×150mL)で抽出した。このエーテル抽出物を混合し、Na2SO4上で乾燥させて、橙黄色油状物になるまで濃縮した。シクロヘキサン−EtOAc(8:1)によって溶離させるシリカゲル(600mlの230〜400メッシュ)上でのクロマトグラフィにより、生成物α−プロパルギルホモテレフタル酸ジメチルエステル(化合物2)が白色固体(4.66)として得られ、これはTLC(シクロヘキサン−EtOAc、3:1)によって均質であるように見受けられた。しかしながら、この生成物の質量スペクトルデータは、これが、目的生成物2とジプロパルギル化化合物との混合物であることを示した。出発物質1は検出されなかった。HPLCは、モノプロパルギル化生成物のジプロパルギル化生成物に対する比率が約3:1であることを示している。化合物1とは異なり、ジプロパルギル化生成物は次の工程の反応において好ましくない副産物を生成する可能性がないため、この物質は化合物3への変換のために適切であった。合成を進行させるために用いられる生成物中に出発化合物1が存在しないことは、最終生成物を生じる変換中の10−dAMの逐次形成を避けるために非常に重要であるが、これは10−プロパルギル−1−dAMから10−dAMを完全に除去することが非常に困難であるからである。
Claims (18)
- T細胞リンパ腫の治療用医薬組成物の調製における、10−プロパルギル−10−デアザアミノプテリンの使用。
- 前記10−プロパルギル−10−デアザアミノプテリンが、10−デアザアミノプテリンを実質的に含んでいない請求項1に記載の使用。
- 前記医薬組成物が、1用量当たり10−プロパルギル−10−デアザアミノプテリンを30から275mg/m2の量で投与するように調製される請求項1又は2に記載の使用。
- 患者が診断されたT細胞リンパ腫が、
(a)胸腺からの原始的リンパ性前駆細胞に悪性腫瘍が生じる、リンパ芽球性リンパ腫、
(b)T細胞前リンパ球性白血病、T細胞顆粒リンパ球性白血病、活動性NK細胞白血病;菌状息肉腫又はセザリー症候群を含む皮膚T細胞性リンパ腫、未分化大細胞リンパ腫、T細胞型、腸症型T細胞リンパ腫、HTLV−1関連T細胞リンパ腫を含む成人T細胞白血病又はリンパ腫、及び血管免疫芽球性T細胞リンパ腫、及び皮下脂肪織炎性T細胞リンパ腫からなる群から選択される、成熟又は末梢T細胞新生物、並びに
(c)最初にリンパ節副皮質を冒し、真の濾胞状パターンに成長することのない、末梢T細胞リンパ腫
からなる群から選択される、請求項1から3の何れか1項に記載の使用。 - 前記T細胞リンパ腫が、前記リンパ芽球性リンパ腫である、請求項4に記載の使用。
- 前記T細胞リンパ腫が、前記末梢T細胞リンパ腫である、請求項4に記載の使用。
- 前記T細胞リンパ腫が、HTLV−1関連T細胞リンパ腫である、請求項4に記載の使用。
- 前記T細胞リンパ腫が、皮下脂肪織炎性T細胞リンパ腫である、請求項4に記載の使用。
- 前記T細胞リンパ腫が、セザリー症候群である、請求項4に記載の使用。
- 10−プロパルギル−10−デアザアミノプテリンを含む、T細胞リンパ腫を治療するための医薬組成物。
- 10−デアザアミノプテリンを実質的に含んでいない請求項10に記載の医薬組成物。
- 1用量当たり10−プロパルギル−10−デアザアミノプテリンを30から275mg/m2の量で投与するように調製される、請求項10又は11に記載の医薬組成物。
- 患者が診断されたT細胞リンパ腫が、
(a)胸腺からの原始的リンパ性前駆細胞に悪性腫瘍が生じる、リンパ芽球性リンパ腫、
(b)T細胞前リンパ球性白血病、T細胞顆粒リンパ球性白血病、活動性NK細胞白血病;菌状息肉腫又はセザリー症候群を含む皮膚T細胞性リンパ腫、未分化大細胞リンパ腫、T細胞型、腸症型T細胞リンパ腫、HTLV−1関連T細胞リンパ腫を含む成人T細胞白血病又はリンパ腫、及び血管免疫芽球性T細胞リンパ腫、及び皮下脂肪織炎性T細胞リンパ腫からなる群から選択される、成熟又は末梢T細胞新生物、並びに
(c)最初にリンパ節副皮質を冒し、真の濾胞状パターンに成長することのない、末梢T細胞リンパ腫
からなる群から選択される、請求項10から12の何れか1項に記載の医薬組成物。 - 前記T細胞リンパ腫が、リンパ芽球性リンパ腫である、請求項13に記載の医薬組成物。
- 前記T細胞リンパ腫が、前記末梢T細胞リンパ腫である、請求項13に記載の医薬組成物。
- 前記T細胞リンパ腫が、前記HTLV−1関連T細胞リンパ腫である、請求項13に記載の医薬組成物。
- 前記T細胞リンパ腫が、皮下脂肪織炎性T細胞リンパ腫である、請求項13に記載の医薬組成物。
- 前記T細胞リンパ腫が、セザリー症候群である、請求項13に記載の医薬組成物。
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US52159304P | 2004-05-30 | 2004-05-30 | |
| US60/521,593 | 2004-05-30 | ||
| PCT/US2005/019169 WO2005117891A1 (en) | 2004-05-30 | 2005-05-31 | Treatment of t-cell lymphoma using 10-propargyl-10-deazaaminopterin |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2008501038A JP2008501038A (ja) | 2008-01-17 |
| JP2008501038A5 JP2008501038A5 (ja) | 2008-06-26 |
| JP5005532B2 true JP5005532B2 (ja) | 2012-08-22 |
Family
ID=34971703
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2007515512A Expired - Lifetime JP5005532B2 (ja) | 2004-05-30 | 2005-05-31 | 10−プロパルギル−10−デアザアミノプテリンを用いるt細胞リンパ腫の治療 |
Country Status (23)
| Country | Link |
|---|---|
| US (4) | US7939530B2 (ja) |
| EP (1) | EP1750716B1 (ja) |
| JP (1) | JP5005532B2 (ja) |
| KR (1) | KR101189693B1 (ja) |
| CN (2) | CN102824346B (ja) |
| AT (1) | ATE405272T1 (ja) |
| AU (1) | AU2005249516B2 (ja) |
| BR (1) | BRPI0510895A (ja) |
| CA (1) | CA2565968C (ja) |
| DE (1) | DE602005009176D1 (ja) |
| DK (1) | DK1750716T3 (ja) |
| ES (1) | ES2313365T3 (ja) |
| HR (1) | HRP20080569T3 (ja) |
| ME (1) | ME01087B (ja) |
| MX (1) | MXPA06013559A (ja) |
| NO (1) | NO337276B1 (ja) |
| NZ (2) | NZ576849A (ja) |
| PL (1) | PL1750716T3 (ja) |
| PT (1) | PT1750716E (ja) |
| RS (1) | RS50622B (ja) |
| SI (1) | SI1750716T1 (ja) |
| WO (2) | WO2005117891A1 (ja) |
| ZA (1) | ZA200609266B (ja) |
Families Citing this family (16)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6835750B1 (en) | 2000-05-01 | 2004-12-28 | Accera, Inc. | Use of medium chain triglycerides for the treatment and prevention of alzheimer's disease and other diseases resulting from reduced neuronal metabolism II |
| US8263354B2 (en) * | 2004-05-30 | 2012-09-11 | Sloan-Kettering Institute For Cancer Research | Methods for assessing cancer for increased sensitivity to 10-propargyl-10-deazaaminopterin |
| DE602005009176D1 (de) * | 2004-05-30 | 2008-10-02 | Sloan Kettering Inst Cancer | Behandlung von t-zellen-lymphom mittels 10-propargyl-10-deazaaminopterin |
| US20080188479A1 (en) * | 2004-05-30 | 2008-08-07 | Sloan-Kettering Institute For Cancer Research | Methods to Treat Cancer with 10-propargyl-10-deazaaminopterin and Methods for Assessing Cancer for Increased Sensitivity to 10-propargyl-10-deazaaminopterin |
| ES2608286T3 (es) | 2007-07-31 | 2017-04-07 | Accera, Inc. | Uso de ensayos genómicos y compuestos cetogénicos para tratamiento de una función cognitiva reducida |
| US20100248249A1 (en) * | 2007-08-17 | 2010-09-30 | Allos Therapeutics, Inc. | Methods for Assessing Cancer for Increased Sensitivity to 10-Propargyl-10-Deazaaminopterin by Assessing Egfr Levels |
| US9901578B2 (en) | 2007-08-17 | 2018-02-27 | Allos Therapeutics, Inc. | Combination of 10-propargyl-10-deazaaminopterin and erlotinib for the treatment of non-small cell lung cancer |
| US20090048262A1 (en) * | 2007-08-17 | 2009-02-19 | Allos Therapeutics, Inc. | Combination of 10-propargyl-10-deazaaminopterin and erlotinib for the treatment of non-small cell lung cancer |
| CN105640931A (zh) | 2008-07-03 | 2016-06-08 | 艾克塞拉公司 | 用于治疗神经性障碍的乙酰乙酸的单甘油酯及其衍生物 |
| WO2010022277A2 (en) * | 2008-08-20 | 2010-02-25 | O'connor Owen A | Combination of 10-propargyl-10-deazaaminopterin and bortezomib for the treatment of cancers |
| CN106892923A (zh) | 2010-02-02 | 2017-06-27 | 艾洛斯治疗学有限公司 | 10‑炔丙基‑10‑去氮杂氨基蝶呤之光学纯非对映体以及使用所述非对映体的方法 |
| CA2800900A1 (en) * | 2010-06-02 | 2011-12-08 | Allos Therapeutics, Inc. | Methods for treating methotrexate-resistant disorders with 10-propargyl-10-deazaaminopterin |
| MX2013001640A (es) * | 2010-08-10 | 2013-07-22 | Allos Therapeutics Inc | Metodos para la extension de supervivencia libre de progresion usando 10-propargil-10-deazaaminopterina. |
| WO2013164856A1 (en) | 2012-05-04 | 2013-11-07 | Avra Laboratories Private Limited | A process for preparing intermediates of 10-propargyl-10-deazaaminopterin (pralatrexate) synthesis and the intermediates thereof |
| CN103274943B (zh) * | 2013-05-24 | 2015-01-21 | 苏州明锐医药科技有限公司 | 4-[1-(2-丙炔基)-3,4-二氧代正丁基]苯甲酸酯及其制备方法 |
| EP3310350A4 (en) | 2015-06-16 | 2019-01-09 | Spectrum Pharmaceuticals, Inc. | COMBINATION THERAPY WITH BELINOSTAT AND PRALATREXATE FOR THE TREATMENT OF LYMPHOMA |
Family Cites Families (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4393064A (en) | 1976-03-05 | 1983-07-12 | Sri International | Process and composition for treatment of leukemia and process for preparing the same |
| US4652533A (en) | 1983-04-28 | 1987-03-24 | Pandex Laboratories, Inc. | Method of solid phase immunoassay incorporating a luminescent label |
| US4843155A (en) | 1987-11-19 | 1989-06-27 | Piotr Chomczynski | Product and process for isolating RNA |
| EP0674178B1 (en) | 1990-11-20 | 2002-08-28 | Dade Behring Marburg GmbH | Cyclosporin immunoassay |
| US5354751A (en) * | 1992-03-03 | 1994-10-11 | Sri International | Heteroaroyl 10-deazaamino-pterine compounds and use for rheumatoid arthritis |
| US5981592A (en) * | 1995-03-13 | 1999-11-09 | Loma Linda University Medical Center | Method and composition for treating cystic fibrosis |
| US6323205B1 (en) * | 1996-07-17 | 2001-11-27 | Sloan-Kettering Institute For Cancer Research | Combinations of 10-propargyl-10-deazaaminopterin and taxols and methods of using same in the treatment of tumors |
| US6028071A (en) * | 1996-07-17 | 2000-02-22 | Sloan-Kettering Institute For Cancer Research | Purified compositions of 10-propargyl-10-deazaaminopterin and methods of using same in the treatment of tumors |
| GB0128510D0 (en) * | 2001-11-28 | 2002-01-23 | Novartis Ag | Organic compounds |
| DE602005009176D1 (de) * | 2004-05-30 | 2008-10-02 | Sloan Kettering Inst Cancer | Behandlung von t-zellen-lymphom mittels 10-propargyl-10-deazaaminopterin |
-
2005
- 2005-05-31 DE DE602005009176T patent/DE602005009176D1/de not_active Expired - Lifetime
- 2005-05-31 HR HR20080569T patent/HRP20080569T3/xx unknown
- 2005-05-31 US US11/568,254 patent/US7939530B2/en active Active
- 2005-05-31 RS RSP-2008/0470A patent/RS50622B/sr unknown
- 2005-05-31 NZ NZ576849A patent/NZ576849A/en not_active IP Right Cessation
- 2005-05-31 CN CN201210342831.8A patent/CN102824346B/zh not_active Expired - Lifetime
- 2005-05-31 BR BRPI0510895-0A patent/BRPI0510895A/pt not_active Application Discontinuation
- 2005-05-31 CA CA2565968A patent/CA2565968C/en not_active Expired - Lifetime
- 2005-05-31 SI SI200530392T patent/SI1750716T1/sl unknown
- 2005-05-31 US US11/141,868 patent/US7622470B2/en not_active Expired - Lifetime
- 2005-05-31 CN CNA2005800173160A patent/CN1960734A/zh active Pending
- 2005-05-31 JP JP2007515512A patent/JP5005532B2/ja not_active Expired - Lifetime
- 2005-05-31 WO PCT/US2005/019169 patent/WO2005117891A1/en not_active Ceased
- 2005-05-31 KR KR1020117012373A patent/KR101189693B1/ko not_active Expired - Lifetime
- 2005-05-31 DK DK05756183T patent/DK1750716T3/da active
- 2005-05-31 MX MXPA06013559A patent/MXPA06013559A/es active IP Right Grant
- 2005-05-31 AU AU2005249516A patent/AU2005249516B2/en not_active Expired
- 2005-05-31 WO PCT/US2005/019170 patent/WO2005117892A1/en not_active Ceased
- 2005-05-31 EP EP05756183A patent/EP1750716B1/en not_active Expired - Lifetime
- 2005-05-31 ES ES05756183T patent/ES2313365T3/es not_active Expired - Lifetime
- 2005-05-31 ME MEP-2008-310A patent/ME01087B/me unknown
- 2005-05-31 PL PL05756183T patent/PL1750716T3/pl unknown
- 2005-05-31 AT AT05756183T patent/ATE405272T1/de active
- 2005-05-31 ZA ZA200609266A patent/ZA200609266B/xx unknown
- 2005-05-31 NZ NZ551082A patent/NZ551082A/en not_active IP Right Cessation
- 2005-05-31 PT PT05756183T patent/PT1750716E/pt unknown
-
2006
- 2006-12-22 NO NO20065971A patent/NO337276B1/no unknown
-
2009
- 2009-10-21 US US12/603,117 patent/US8299078B2/en not_active Expired - Lifetime
-
2012
- 2012-10-29 US US13/663,234 patent/US20130053391A1/en not_active Abandoned
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US8299078B2 (en) | Treatment of T-cell lymphoma using 10-propargyl-10-deazaaminopterin | |
| EP2389375B1 (en) | Hydroxamic acid derivatives | |
| WO2021108672A1 (en) | Combination therapy involving diaryl macrocyclic compounds | |
| US6028071A (en) | Purified compositions of 10-propargyl-10-deazaaminopterin and methods of using same in the treatment of tumors | |
| KR101401220B1 (ko) | 방사선 치료 증강제 | |
| US6323205B1 (en) | Combinations of 10-propargyl-10-deazaaminopterin and taxols and methods of using same in the treatment of tumors | |
| JPWO2021093839A5 (ja) | ||
| CN113811302B (zh) | 激酶抑制剂的用途 | |
| JP2023509191A (ja) | 癌を治療するための組み合わせ療法 | |
| KR101189692B1 (ko) | 10-프로파르질-10-데아자아미노프테린을 사용하는 t-세포림프종의 치료 | |
| JP2767176B2 (ja) | 抗癌剤 | |
| HK1177901B (en) | Treatment of t-cell lymphoma using 10-propargyl-10-deazaaminopterin | |
| EP1891957A1 (en) | Purified Compositions of 10-Propargyl-10-Deazaaminopterin and Methods of Using Same in the Treament of Tumors | |
| HK1110238A (en) | Purified compositions of 10-propargyl-10-deazaaminopterin and methods of using same in the treament of tumors |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20080508 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20080508 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20110610 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20110912 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20110920 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20111011 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20111018 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20111109 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A821 Effective date: 20111109 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20111213 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20120221 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20120427 |
|
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20120523 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20150601 Year of fee payment: 3 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 5005532 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R153 | Grant of patent term extension |
Free format text: JAPANESE INTERMEDIATE CODE: R153 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |