JP4934678B2 - 新規デバイス - Google Patents
新規デバイス Download PDFInfo
- Publication number
- JP4934678B2 JP4934678B2 JP2008547168A JP2008547168A JP4934678B2 JP 4934678 B2 JP4934678 B2 JP 4934678B2 JP 2008547168 A JP2008547168 A JP 2008547168A JP 2008547168 A JP2008547168 A JP 2008547168A JP 4934678 B2 JP4934678 B2 JP 4934678B2
- Authority
- JP
- Japan
- Prior art keywords
- delivery device
- removal means
- mouthpiece insert
- mouthpiece
- insert
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
- 239000003814 drug Substances 0.000 claims description 18
- 238000000034 method Methods 0.000 claims description 14
- 238000004140 cleaning Methods 0.000 claims description 13
- 229940079593 drug Drugs 0.000 claims description 13
- 238000009825 accumulation Methods 0.000 claims description 6
- 229940112141 dry powder inhaler Drugs 0.000 claims description 3
- 239000000843 powder Substances 0.000 description 16
- -1 limiterol Chemical compound 0.000 description 5
- 108090000765 processed proteins & peptides Proteins 0.000 description 4
- 229920001184 polypeptide Polymers 0.000 description 3
- 102000004196 processed proteins & peptides Human genes 0.000 description 3
- 150000003839 salts Chemical class 0.000 description 3
- 239000000126 substance Substances 0.000 description 3
- 102100037611 Lysophospholipase Human genes 0.000 description 2
- 108010058864 Phospholipases A2 Proteins 0.000 description 2
- UCTWMZQNUQWSLP-UHFFFAOYSA-N adrenaline Chemical compound CNCC(O)C1=CC=C(O)C(O)=C1 UCTWMZQNUQWSLP-UHFFFAOYSA-N 0.000 description 2
- 239000005557 antagonist Substances 0.000 description 2
- 239000002220 antihypertensive agent Substances 0.000 description 2
- 239000003795 chemical substances by application Substances 0.000 description 2
- 150000002148 esters Chemical class 0.000 description 2
- 239000003172 expectorant agent Substances 0.000 description 2
- 239000003112 inhibitor Substances 0.000 description 2
- 229960005486 vaccine Drugs 0.000 description 2
- JWZZKOKVBUJMES-UHFFFAOYSA-N (+-)-Isoprenaline Chemical compound CC(C)NCC(O)C1=CC=C(O)C(O)=C1 JWZZKOKVBUJMES-UHFFFAOYSA-N 0.000 description 1
- XWTYSIMOBUGWOL-UHFFFAOYSA-N (+-)-Terbutaline Chemical compound CC(C)(C)NCC(O)C1=CC(O)=CC(O)=C1 XWTYSIMOBUGWOL-UHFFFAOYSA-N 0.000 description 1
- NUBLQEKABJXICM-FQEVSTJZSA-N (1r)-1-(4-amino-3,5-dichlorophenyl)-2-[6-(2-pyridin-2-ylethoxy)hexylamino]ethanol Chemical compound C1=C(Cl)C(N)=C(Cl)C=C1[C@@H](O)CNCCCCCCOCCC1=CC=CC=N1 NUBLQEKABJXICM-FQEVSTJZSA-N 0.000 description 1
- PDNHLCRMUIGNBV-UHFFFAOYSA-N 1-pyridin-2-ylethanamine Chemical compound CC(N)C1=CC=CC=N1 PDNHLCRMUIGNBV-UHFFFAOYSA-N 0.000 description 1
- LSLYOANBFKQKPT-DIFFPNOSSA-N 5-[(1r)-1-hydroxy-2-[[(2r)-1-(4-hydroxyphenyl)propan-2-yl]amino]ethyl]benzene-1,3-diol Chemical compound C([C@@H](C)NC[C@H](O)C=1C=C(O)C=C(O)C=1)C1=CC=C(O)C=C1 LSLYOANBFKQKPT-DIFFPNOSSA-N 0.000 description 1
- 108010001857 Cell Surface Receptors Proteins 0.000 description 1
- 102000009025 Endorphins Human genes 0.000 description 1
- 108010049140 Endorphins Proteins 0.000 description 1
- 102000004190 Enzymes Human genes 0.000 description 1
- 108090000790 Enzymes Proteins 0.000 description 1
- 229940123457 Free radical scavenger Drugs 0.000 description 1
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 1
- GIIZNNXWQWCKIB-UHFFFAOYSA-N Serevent Chemical compound C1=C(O)C(CO)=CC(C(O)CNCCCCCCOCCCCC=2C=CC=CC=2)=C1 GIIZNNXWQWCKIB-UHFFFAOYSA-N 0.000 description 1
- 239000004480 active ingredient Substances 0.000 description 1
- 239000000048 adrenergic agonist Substances 0.000 description 1
- 229940126157 adrenergic receptor agonist Drugs 0.000 description 1
- NDAUXUAQIAJITI-UHFFFAOYSA-N albuterol Chemical compound CC(C)(C)NCC(O)C1=CC=C(O)C(CO)=C1 NDAUXUAQIAJITI-UHFFFAOYSA-N 0.000 description 1
- 239000000730 antalgic agent Substances 0.000 description 1
- 239000003242 anti bacterial agent Substances 0.000 description 1
- 230000001078 anti-cholinergic effect Effects 0.000 description 1
- 239000000043 antiallergic agent Substances 0.000 description 1
- 229940088710 antibiotic agent Drugs 0.000 description 1
- 229940121375 antifungal agent Drugs 0.000 description 1
- 239000003429 antifungal agent Substances 0.000 description 1
- 239000000739 antihistaminic agent Substances 0.000 description 1
- 229940125715 antihistaminic agent Drugs 0.000 description 1
- 229940030600 antihypertensive agent Drugs 0.000 description 1
- 229940127088 antihypertensive drug Drugs 0.000 description 1
- 239000002246 antineoplastic agent Substances 0.000 description 1
- 239000003963 antioxidant agent Substances 0.000 description 1
- 239000003435 antirheumatic agent Substances 0.000 description 1
- 239000003443 antiviral agent Substances 0.000 description 1
- 206010003119 arrhythmia Diseases 0.000 description 1
- 230000006793 arrhythmia Effects 0.000 description 1
- 208000006673 asthma Diseases 0.000 description 1
- 102000016966 beta-2 Adrenergic Receptors Human genes 0.000 description 1
- 108010014499 beta-2 Adrenergic Receptors Proteins 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 230000023555 blood coagulation Effects 0.000 description 1
- 229940124630 bronchodilator Drugs 0.000 description 1
- 239000000168 bronchodilator agent Substances 0.000 description 1
- 229940097217 cardiac glycoside Drugs 0.000 description 1
- 239000002368 cardiac glycoside Substances 0.000 description 1
- 108010015046 cell aggregation factors Proteins 0.000 description 1
- 229960001117 clenbuterol Drugs 0.000 description 1
- STJMRWALKKWQGH-UHFFFAOYSA-N clenbuterol Chemical compound CC(C)(C)NCC(O)C1=CC(Cl)=C(N)C(Cl)=C1 STJMRWALKKWQGH-UHFFFAOYSA-N 0.000 description 1
- 229960000265 cromoglicic acid Drugs 0.000 description 1
- VLARUOGDXDTHEH-UHFFFAOYSA-L disodium cromoglycate Chemical compound [Na+].[Na+].O1C(C([O-])=O)=CC(=O)C2=C1C=CC=C2OCC(O)COC1=CC=CC2=C1C(=O)C=C(C([O-])=O)O2 VLARUOGDXDTHEH-UHFFFAOYSA-L 0.000 description 1
- 230000000694 effects Effects 0.000 description 1
- 230000003419 expectorant effect Effects 0.000 description 1
- 229940066493 expectorants Drugs 0.000 description 1
- 229960001022 fenoterol Drugs 0.000 description 1
- 229940043075 fluocinolone Drugs 0.000 description 1
- FEBLZLNTKCEFIT-VSXGLTOVSA-N fluocinolone acetonide Chemical compound C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@]1(F)[C@@H]2[C@@H]2C[C@H]3OC(C)(C)O[C@@]3(C(=O)CO)[C@@]2(C)C[C@@H]1O FEBLZLNTKCEFIT-VSXGLTOVSA-N 0.000 description 1
- 229960002848 formoterol Drugs 0.000 description 1
- BPZSYCZIITTYBL-UHFFFAOYSA-N formoterol Chemical compound C1=CC(OC)=CC=C1CC(C)NCC(O)C1=CC=C(O)C(NC=O)=C1 BPZSYCZIITTYBL-UHFFFAOYSA-N 0.000 description 1
- 239000003102 growth factor Substances 0.000 description 1
- 229940088597 hormone Drugs 0.000 description 1
- 239000005556 hormone Substances 0.000 description 1
- 239000002955 immunomodulating agent Substances 0.000 description 1
- 229960004078 indacaterol Drugs 0.000 description 1
- QZZUEBNBZAPZLX-QFIPXVFZSA-N indacaterol Chemical compound N1C(=O)C=CC2=C1C(O)=CC=C2[C@@H](O)CNC1CC(C=C(C(=C2)CC)CC)=C2C1 QZZUEBNBZAPZLX-QFIPXVFZSA-N 0.000 description 1
- 238000003780 insertion Methods 0.000 description 1
- 230000037431 insertion Effects 0.000 description 1
- 229960001361 ipratropium bromide Drugs 0.000 description 1
- KEWHKYJURDBRMN-ZEODDXGYSA-M ipratropium bromide hydrate Chemical compound O.[Br-].O([C@H]1C[C@H]2CC[C@@H](C1)[N@@+]2(C)C(C)C)C(=O)C(CO)C1=CC=CC=C1 KEWHKYJURDBRMN-ZEODDXGYSA-M 0.000 description 1
- 229960001317 isoprenaline Drugs 0.000 description 1
- 239000008101 lactose Substances 0.000 description 1
- 150000002617 leukotrienes Chemical class 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 102000006240 membrane receptors Human genes 0.000 description 1
- LMOINURANNBYCM-UHFFFAOYSA-N metaproterenol Chemical compound CC(C)NCC(O)C1=CC(O)=CC(O)=C1 LMOINURANNBYCM-UHFFFAOYSA-N 0.000 description 1
- 239000000203 mixture Substances 0.000 description 1
- 229960001664 mometasone Drugs 0.000 description 1
- QLIIKPVHVRXHRI-CXSFZGCWSA-N mometasone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(Cl)[C@@H]1[C@@H]1C[C@@H](C)[C@@](C(=O)CCl)(O)[C@@]1(C)C[C@@H]2O QLIIKPVHVRXHRI-CXSFZGCWSA-N 0.000 description 1
- 229940066491 mucolytics Drugs 0.000 description 1
- 229960002259 nedocromil sodium Drugs 0.000 description 1
- 229960002657 orciprenaline Drugs 0.000 description 1
- 239000000813 peptide hormone Substances 0.000 description 1
- 229960002288 procaterol Drugs 0.000 description 1
- FKNXQNWAXFXVNW-BLLLJJGKSA-N procaterol Chemical compound N1C(=O)C=CC2=C1C(O)=CC=C2[C@@H](O)[C@@H](NC(C)C)CC FKNXQNWAXFXVNW-BLLLJJGKSA-N 0.000 description 1
- 239000002599 prostaglandin synthase inhibitor Substances 0.000 description 1
- 102000004169 proteins and genes Human genes 0.000 description 1
- 108090000623 proteins and genes Proteins 0.000 description 1
- 239000002516 radical scavenger Substances 0.000 description 1
- 230000000241 respiratory effect Effects 0.000 description 1
- 229960002052 salbutamol Drugs 0.000 description 1
- 229960004017 salmeterol Drugs 0.000 description 1
- 239000000932 sedative agent Substances 0.000 description 1
- 229940125723 sedative agent Drugs 0.000 description 1
- 208000010110 spontaneous platelet aggregation Diseases 0.000 description 1
- 229930002534 steroid glycoside Natural products 0.000 description 1
- 150000008143 steroidal glycosides Chemical class 0.000 description 1
- 238000003786 synthesis reaction Methods 0.000 description 1
- 229960000195 terbutaline Drugs 0.000 description 1
- 239000003204 tranquilizing agent Substances 0.000 description 1
- 230000002936 tranquilizing effect Effects 0.000 description 1
- 229960002117 triamcinolone acetonide Drugs 0.000 description 1
- YNDXUCZADRHECN-JNQJZLCISA-N triamcinolone acetonide Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@H]3OC(C)(C)O[C@@]3(C(=O)CO)[C@@]1(C)C[C@@H]2O YNDXUCZADRHECN-JNQJZLCISA-N 0.000 description 1
- 239000011782 vitamin Substances 0.000 description 1
- 229940088594 vitamin Drugs 0.000 description 1
- 229930003231 vitamin Natural products 0.000 description 1
- 235000013343 vitamin Nutrition 0.000 description 1
- 150000003722 vitamin derivatives Chemical class 0.000 description 1
- 210000004885 white matter Anatomy 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M11/00—Sprayers or atomisers specially adapted for therapeutic purposes
- A61M11/001—Particle size control
- A61M11/002—Particle size control by flow deviation causing inertial separation of transported particles
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0001—Details of inhalators; Constructional features thereof
- A61M15/0021—Mouthpieces therefor
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M15/00—Inhalators
- A61M15/0065—Inhalators with dosage or measuring devices
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2202/00—Special media to be introduced, removed or treated
- A61M2202/06—Solids
- A61M2202/064—Powder
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2206/00—Characteristics of a physical parameter; associated device therefor
- A61M2206/10—Flow characteristics
- A61M2206/14—Static flow deviators in tubes disturbing laminar flow in tubes, e.g. archimedes screws
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2209/00—Ancillary equipment
- A61M2209/10—Equipment for cleaning
Landscapes
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Heart & Thoracic Surgery (AREA)
- Hematology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Anesthesiology (AREA)
- Biomedical Technology (AREA)
- Veterinary Medicine (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Pulmonology (AREA)
- Medical Preparation Storing Or Oral Administration Devices (AREA)
- Medicinal Preparation (AREA)
- Infusion, Injection, And Reservoir Apparatuses (AREA)
Description
さらに本発明は、送達デバイスをクリーニングする方法に関する。
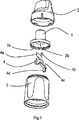
2:マウスピースインサートホルダ、
3:マウスピースインサート、3a,3b:マウスピースインサートの一部分、
4:除去手段、4a,4b:螺旋部の螺旋領域、4c:除去部、4d:突起部。
Claims (11)
- 粒状薬剤を投薬するための送達デバイスであって、
複数の面で形成された、使用時にユーザにより吸引される空気フローが流れる流路と、
本体部(1)とを備え、
流路は空気フローの入口および出口を含むチャンバを有し、
チャンバは、本体部(1)に対して相対的に回転可能であり、少なくとも部分的にマウスピースインサート(3)により構成され、
除去手段(4)がチャンバの内側表面に当接して配置され、
除去手段およびマウスピースインサートは、互いに対して相対的に回転可能であり、
除去手段(4)は、使用時、送達デバイスに吸引された空気に回転移動を与えるために、送達デバイスの入口および出口の間に配設された螺旋部を有し、
螺旋部は、マウスピースインサートの内部に当接するように配置され、除去手段およびマウスピースインサートが互いに対して回転するとき、マウスピースインサートの内側表面が螺旋部によりクリーニングされ、その結果、チャンバの内部への薬剤の蓄積が抑制されることを特徴とする送達デバイス。 - 請求項1に記載の送達デバイスであって、
螺旋部は複数のねじれ螺旋部分(4a,4b)を有することを特徴とする送達デバイス。 - 請求項2に記載の送達デバイスであって、
螺旋部は2つのねじれ螺旋部分(4a,4b)を有することを特徴とする送達デバイス。 - 請求項3に記載の送達デバイスであって、
ねじれ螺旋部分は弾性を有し、マウスピースインサート(3)の内部に付勢力を与え、付勢力は送達デバイスの長手方向軸に対して実質的に垂直方向に加えられることを特徴とする送達デバイス。 - 請求項1に記載の送達デバイスであって、
マウスピースインサート(3)は第1の部分(3a)および第2の部分(3b)を有し、
除去手段(4)は、マウスピースインサートの第1の部分(3a)の内側表面に当接して配置される除去部(4c)をさらに有し、
螺旋部は、マウスピースインサートの第2の部分(3b)の内側表面に当接して配置され、
除去手段およびマウスピースインサートが互いに対して回転するとき、第1の部分(3a)の内側表面が除去部(4c)によりクリーニングされ、第2の部分(3b)の内側表面が螺旋部によりクリーニングされることを特徴とする送達デバイス。 - 請求項1に記載の送達デバイスであって、
送達デバイスはマウスピースインサートホルダ(2)をさらに備え、
マウスピースインサート(3)およびマウスピースインサートホルダはそれぞれ、互いに対して係合する部分を有し、
除去手段(4)およびマウスピースインサート(3)は、インサートホルダをねじ回して固定または外す際に、互いに対して相対的に回転することを特徴とする送達デバイス。 - 請求項1に記載の送達デバイスであって、
除去手段(4)および本体部(1)はそれぞれ、本体部および除去手段(4)が互いに対して確実に固定されるように、互いに対して係合する部分を有することを特徴とする送達デバイス。 - 請求項7に記載の送達デバイスであって、
除去手段(4)は、本体部および除去手段(4)が互いに対して確実に固定されるように、本体部(1)の切り込みに係合するように構成された突起部(4d)を有することを特徴とする送達デバイス。 - 送達デバイスをクリーニングする方法であって、
この送達デバイスは、
複数の面で形成された、使用時にユーザにより吸引される空気フローが流れる流路と、
本体部(1)とを備え、
流路は空気フローの入口および出口を含むチャンバを有し、
チャンバは、本体部(1)に対して回転可能であり、少なくとも部分的にマウスピースインサート(3)により構成され、
除去手段がチャンバの内側表面に当接して配置され、
除去手段およびマウスピースインサートは、互いに対して相対的に回転可能であり、
除去手段(4)は、使用時、送達デバイスに吸引された空気に回転移動を与えるために、送達デバイスの入口および出口の間に配設された螺旋部を有し、
螺旋部は、マウスピースインサートの内部に当接するように配置され、
マウスピースインサートの内部が螺旋部によりクリーニングされ、その結果、チャンバの内部への薬剤の蓄積が抑制されるように、除去手段(4)およびマウスピースインサートを互いに対して回転させるステップを有することを特徴とする方法。 - 請求項9に記載の方法であって、
吸入する前において、除去手段(4)およびマウスピースインサートを互いに対して回転させるステップを有することを特徴とする方法。 - 乾燥粉末吸入器を有する請求項1〜9のいずれか1に記載の送達デバイス。
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| SE0502909-5 | 2005-12-23 | ||
| SE0502909 | 2005-12-23 | ||
| PCT/SE2006/001466 WO2007073302A1 (en) | 2005-12-23 | 2006-12-21 | New device |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2009521256A JP2009521256A (ja) | 2009-06-04 |
| JP2009521256A5 JP2009521256A5 (ja) | 2009-11-26 |
| JP4934678B2 true JP4934678B2 (ja) | 2012-05-16 |
Family
ID=38188933
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2008547168A Expired - Fee Related JP4934678B2 (ja) | 2005-12-23 | 2006-12-21 | 新規デバイス |
Country Status (5)
| Country | Link |
|---|---|
| US (1) | US8025051B2 (ja) |
| EP (1) | EP1965848A4 (ja) |
| JP (1) | JP4934678B2 (ja) |
| CN (1) | CN101346158B (ja) |
| WO (1) | WO2007073302A1 (ja) |
Families Citing this family (19)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP2534958A1 (en) | 2007-12-14 | 2012-12-19 | AeroDesigns, Inc | Delivering aerosolizable food products |
| US8539951B1 (en) | 2008-05-27 | 2013-09-24 | Trudell Medical International | Oscillating positive respiratory pressure device |
| US8327849B2 (en) | 2008-10-28 | 2012-12-11 | Trudell Medical International | Oscillating positive expiratory pressure device |
| US8485179B1 (en) | 2009-02-23 | 2013-07-16 | Trudell Medical International | Oscillating positive expiratory pressure device |
| US9149589B2 (en) | 2009-02-23 | 2015-10-06 | Trudell Medical International | Method and device for performing orientation dependent oscillating positive expiratory pressure therapy |
| AU2012265933B2 (en) | 2011-06-06 | 2016-03-17 | Trudell Medical International Inc. | Oscillating positive expiratory pressure device |
| US9517315B2 (en) | 2012-11-30 | 2016-12-13 | Trudell Medical International | Oscillating positive expiratory pressure device |
| US9492646B2 (en) * | 2013-03-06 | 2016-11-15 | Nordson Corporation | Applicator and method for dispensing a fluid and a particulate |
| WO2015003249A1 (en) | 2013-07-12 | 2015-01-15 | Trudell Medical International | Huff cough simulation device |
| US9849257B2 (en) | 2013-08-22 | 2017-12-26 | Trudell Medical International | Oscillating positive respiratory pressure device |
| US10363383B2 (en) | 2014-02-07 | 2019-07-30 | Trudell Medical International | Pressure indicator for an oscillating positive expiratory pressure device |
| US10004872B1 (en) | 2015-03-06 | 2018-06-26 | D R Burton Healthcare, Llc | Positive expiratory pressure device having an oscillating valve |
| EP4134118A3 (en) | 2015-07-30 | 2023-05-03 | Trudell Medical International | Combined respiratory muscle training and oscillating positive expiratory pressure device |
| USD780906S1 (en) | 2015-09-02 | 2017-03-07 | Trudell Medical International | Respiratory treatment device |
| USD778429S1 (en) | 2015-09-02 | 2017-02-07 | Trudell Medical International | Respiratory treatment device |
| EP3383465B1 (en) | 2015-12-04 | 2021-02-03 | Trudell Medical International | Huff cough simulation device |
| US10206426B2 (en) * | 2016-04-08 | 2019-02-19 | Funai Electric Co., Ltd. | Maintenance apparatus and method for vaporizing device |
| CA3240360A1 (en) | 2017-05-03 | 2018-11-08 | Trudell Medical International | Combined oscillating positive expiratory pressure therapy and huff cough simulation device |
| US10953278B2 (en) | 2018-02-02 | 2021-03-23 | Trudell Medical International | Oscillating positive expiratory pressure device |
Family Cites Families (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| IT1237118B (it) * | 1989-10-27 | 1993-05-18 | Miat Spa | Inalatore multidose per farmaci in polvere. |
| GB9015522D0 (en) | 1990-07-13 | 1990-08-29 | Braithwaite Philip W | Inhaler |
| GB9218937D0 (en) * | 1992-09-08 | 1992-10-21 | Norton Healthcare Ltd | Medicament dispensing device |
| JPH09264638A (ja) * | 1996-03-28 | 1997-10-07 | Mitsubishi Electric Corp | オリフィス制御弁 |
| TW469832U (en) * | 1997-03-14 | 2001-12-21 | Astra Ab | Inhalation device |
| SE9700937D0 (sv) * | 1997-03-14 | 1997-03-14 | Astra Ab | Powder inhaler I |
| SE9801114D0 (sv) * | 1998-03-30 | 1998-03-30 | Astra Ab | Inhalation device |
| US6116469A (en) * | 1998-07-06 | 2000-09-12 | Dart Industries Inc. | Condiment shaker |
| DE60013416T2 (de) * | 2000-06-13 | 2005-03-03 | Andi-Ventis Ltd. | Mundstück für einen Pulverinhalator |
| CA2562386C (en) * | 2004-04-21 | 2014-11-18 | Innovata Biomed Limited | Inhaler |
-
2006
- 2006-12-21 WO PCT/SE2006/001466 patent/WO2007073302A1/en active Application Filing
- 2006-12-21 CN CN2006800489242A patent/CN101346158B/zh not_active Expired - Fee Related
- 2006-12-21 US US12/097,969 patent/US8025051B2/en not_active Expired - Fee Related
- 2006-12-21 JP JP2008547168A patent/JP4934678B2/ja not_active Expired - Fee Related
- 2006-12-21 EP EP06835881A patent/EP1965848A4/en not_active Withdrawn
Also Published As
| Publication number | Publication date |
|---|---|
| US20080289653A1 (en) | 2008-11-27 |
| CN101346158A (zh) | 2009-01-14 |
| EP1965848A1 (en) | 2008-09-10 |
| CN101346158B (zh) | 2012-02-22 |
| US8025051B2 (en) | 2011-09-27 |
| WO2007073302A1 (en) | 2007-06-28 |
| JP2009521256A (ja) | 2009-06-04 |
| EP1965848A4 (en) | 2012-12-05 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP4934678B2 (ja) | 新規デバイス | |
| JP4138019B2 (ja) | 吸入器 | |
| JP5651233B2 (ja) | 簡易なカプセル・ベースの吸入器 | |
| FI112920B (fi) | Inhalaatiolaite | |
| JPH08507704A (ja) | 吸入装置 | |
| SE527190C2 (sv) | Inhalatoranordning samt kombinerade doser av en beta2-agonist, ett antikolinergiskt medel och ett antiinflammatorisk steroid | |
| US20100136121A1 (en) | Medicaments | |
| CA2528863A1 (en) | Combined doses | |
| WO2014012930A2 (en) | Metering element for an inhalation device and inhalation device comprising a metering element | |
| US20160051777A1 (en) | Metering element for an inhalation device and assembly for an inhalation device containing same |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20091006 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20091006 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20110929 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20111004 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20111226 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20120214 |
|
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20120220 |
|
| R150 | Certificate of patent or registration of utility model |
Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20150224 Year of fee payment: 3 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| LAPS | Cancellation because of no payment of annual fees |