JP4738794B2 - 人工修復器具 - Google Patents
人工修復器具 Download PDFInfo
- Publication number
- JP4738794B2 JP4738794B2 JP2004340863A JP2004340863A JP4738794B2 JP 4738794 B2 JP4738794 B2 JP 4738794B2 JP 2004340863 A JP2004340863 A JP 2004340863A JP 2004340863 A JP2004340863 A JP 2004340863A JP 4738794 B2 JP4738794 B2 JP 4738794B2
- Authority
- JP
- Japan
- Prior art keywords
- absorbent material
- repair device
- sheet
- absorbent
- artificial
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L31/00—Materials for other surgical articles, e.g. stents, stent-grafts, shunts, surgical drapes, guide wires, materials for adhesion prevention, occluding devices, surgical gloves, tissue fixation devices
- A61L31/14—Materials characterised by their function or physical properties, e.g. injectable or lubricating compositions, shape-memory materials, surface modified materials
- A61L31/148—Materials at least partially resorbable by the body
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L31/00—Materials for other surgical articles, e.g. stents, stent-grafts, shunts, surgical drapes, guide wires, materials for adhesion prevention, occluding devices, surgical gloves, tissue fixation devices
- A61L31/14—Materials characterised by their function or physical properties, e.g. injectable or lubricating compositions, shape-memory materials, surface modified materials
- A61L31/146—Porous materials, e.g. foams or sponges
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/0063—Implantable repair or support meshes, e.g. hernia meshes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2250/00—Special features of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2250/0014—Special features of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof having different values of a given property or geometrical feature, e.g. mechanical property or material property, at different locations within the same prosthesis
- A61F2250/003—Special features of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof having different values of a given property or geometrical feature, e.g. mechanical property or material property, at different locations within the same prosthesis differing in adsorbability or resorbability, i.e. in adsorption or resorption time
Landscapes
- Health & Medical Sciences (AREA)
- Heart & Thoracic Surgery (AREA)
- Surgery (AREA)
- Vascular Medicine (AREA)
- Epidemiology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Chemical & Material Sciences (AREA)
- Dispersion Chemistry (AREA)
- Prostheses (AREA)
- Materials For Medical Uses (AREA)
Description
第2の吸収性材料は、非吸収性材料又は包封状態の非吸収性材料を植え込み後或る期間にわたり内部又は腹部内臓又は組織及び器官を隔離するよう機能することができる。加うるに、第2の吸収性材料は、非吸収性材料と内部又は腹部内臓との術後癒着を防止する癒着バリヤとして機能することができる。第2の吸収性材料は、第1の吸収性材料又はコンポーネントの吸収速度よりも早い吸収速度を有するのがよい。1以上の第2の吸収性材料を修復器具に用いるのがよい。
公称直径が8インチ(20.32cm)、加熱能力が最高170℃の金属ローラを備えた積層システムを利用してヘルニア修復器具を作製した。金属ローラの回転速度は、毎分1〜10フィート(0.3048m〜3.048m)であった。ジュロメータが40、加圧力が最高直線1フィート(0.3048m)当たり150ポンド(68.04kg)のソフトフェースのポリウレタン加圧ローラを更に有していた。0.8milのポリ(1,4−ジオキサン−2−オン)(PDS)フィルムの一方の側部を第1の剥離紙(ニュージャージー州07605レオニア所在のテッコテ・コーポレイションから市販されている)で被覆し、PDSフィルムの他方の側部をプロレン(Prolene(登録商標))ポリプロピレンメッシュ(PSM)製品(ニュージャージー州ソマービル所在のエシコン・インコーポレイテッドから市販されている)の滑らかな側部と接触させた。第2の剥離紙をPSM製品のざらざらの側部上に配置してコンポーネントが積層システムのローラに粘着しないようにした。第1の剥離紙/PDS/PSM/第2の剥離紙から成る構造体を、157℃の温度に設定されると共に毎分2フィート(0.6096m)で回転する金属ローラを備えた積層システム内に配置し、他方、第1の剥離剤が加熱された金属ローラに接触した状態で圧力ローラをそのフェースを横切って変位する直線1インチ(2.54cm)当たり70ポンド(31.75kg)の荷重を加えるよう設定し、これにより、PDSをメッシュ中へ移動させた。この工程を3回繰り返した。
上記構造体を積層システムから取り出し、ジュロメータが40のソフトフェースのポリウレタンローラにより手押し圧力だけを用いて構造体の剥離紙側上で手動転動させた。その直後、剥離紙を取り外し、構造をポリウレタンローラで手押しによる圧力だけを用いてPDS側上で2回手動で転動させた。次に、サンプルを約1〜7時間、貯蔵ビン内に置き、次に移送し、ヘルニア修復器具を裁断して包装する時期に至るまで真空下で貯蔵した。
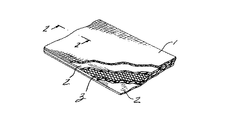
(1)第1の側部及び第2の側部を備えた非吸収性材料と、第1の側部及び第2の側部を備えた第1の吸収速度の第1の吸収性材料と、第1の側部及び第2の側部を備えた第1の吸収速度よりも早い吸収速度の第2の吸収性材料とから成り、非吸収性材料の第2の側部は、第1の吸収性材料の第1の側部の近くに位置し、第2の吸収性材料は、第1の吸収性材料の第2の側部の近くに位置していることを特徴とする人工修復器具。
(2)非吸収性材料は、ポリオレフィン、ポリエステル、フルオロポリマー、ポリアミド及びこれらの混合物から成る群から選択され、第1の吸収性材料は、ポリジオキサノン、有機ヒドロキシエステルのポリマー又はコポリマー、ポリグリコリド、ポリラクチド、ポリヒドロキシ酪酸、ポリカプロラクトン、ポリトリメチレンカーボネート及びポリビニルアルコールから成る群から選択され、第2の吸収性材料は、酸化再生セルロース、ゼラチンフィルム、有機ヒドロキシエステルのポリマー又はコポリマー、ポリグリコリド、ポリラクチド、ポリジオキサノン、ポリヒドロキシ酪酸、ポリカプロラクトン、ポリトリメチレンカーボネート及びポリビニルアルコールから成る群から選択されていることを特徴とする上記実施形態(1)記載の人工修復器具。
(3)非吸収性材料の第1の側部の近くに位置した第1の吸収性材料の第2のシートを更に有していることを特徴とする上記実施形態(1)記載の人工修復器具。
(4)第1の吸収性材料は、融点が非吸収性材料及び第2の吸収性材料の融点よりも低く、第1の吸収性材料は、非吸収性材料を第2の吸収性材料に接合していることを特徴とする上記実施形態(1)記載の人工修復器具。
(5)非吸収性材料は、ポリプロピレンメッシュであり、第1の吸収性材料は、ポリジオキサノンフィルムであり、第2の吸収性材料は、酸化再生セルロースファブリックであることを特徴とする上記実施形態(1)記載の人工修復器具。
(7)第1の吸収性コンポーネントは、ポリジオキサノンであり、人工修復器具は、少なくとも5分の保持時間を有することを特徴とする上記実施形態(6)記載の人工修復器具。
(8)非吸収性多孔質材料は、ポリジオキサノン、ポリラクチド、ポリグリコリド及びカプロラクトンのコポリマーから成る群から選択された第1の吸収性コンポーネントで包封されたポリエチレン、ポリプロピレン、ポリエステル、フルオロポリマー又はポリアミドメッシュであり、第2の吸収性材料は、酸化再生セルロースであることを特徴とする上記実施形態(6)記載の人工修復器具。
2 第1の吸収性コンポーネント
3 非吸収性多孔質材料
Claims (10)
- 人工修復器具であって、
第1の側部及び第2の側部を備えた非吸収性材料のシートと、
第1の側部及び第2の側部を備えた第1の吸収性材料の第1のシートと、
第1の側部及び第2の側部を備えた第2の吸収性材料のシートと、
前記第1の吸収性材料の第2のシートと、
を含み、
前記非吸収性材料のシートの第2の側部が、前記第1の吸収性材料の第1のシートの第1の側部に接合されており、前記第2の吸収性材料のシートは、前記第1の吸収性材料の第1のシートの第2の側部に接合されており、前記第1の吸収性材料の第2のシートは、前記非吸収性材料のシートの第1の側部に接合されており、
前記第1の吸収性材料の第1および第2のシートは、ポリジオキサノンフィルムであり、前記第2の吸収性材料のシートは、酸化再生セルロースファブリックであり、
前記第1の吸収性材料の第1のシートは、溶融時に、前記非吸収性材料のシートを前記第2の吸収性材料のシートに接合する手段としての役目を果たす、人工修復器具。 - 前記人工修復器具は、内部又は腹部内臓又は組織及び器官に向けられる第1の側部と、その反対側の第2の側部とを有しており、前記第1の吸収性材料の第2のシートが前記人工修復器具の第1の側部に位置付けられている、請求項1記載の人工修復器具。
- 前記非吸収性材料のシートは、ポリプロピレンメッシュである、請求項1または2記載の人工修復器具。
- 前記第1の吸収性材料は、融点が前記非吸収性材料及び前記第2の吸収性材料の融点よりも低く、前記第1の吸収性材料の第1のシートは、前記非吸収性材料のシートを前記第2の吸収性材料のシートに接合している、請求項3記載の人工修復器具。
- 人工修復器具であって、
(i)第1の吸収性材料で包封された非吸収性材料の多孔質シートと、
(ii)前記包封された非吸収性材料の多孔質シートに接合された第2の吸収性材料のシートと、
を含み、
前記第1の吸収性材料は、ポリジオキサノンであり、前記第2の吸収性材料は、酸化再生セルロースであり、前記第1の吸収性材料は、溶融時に、前記非吸収性材料の多孔質シートを前記第2の吸収性材料のシートに接合する手段としての役目を果たす、人工修復器具。 - 前記人工修復器具は、少なくとも5分の保持時間を示す、請求項5記載の人工修復器具。
- 前記非吸収性材料の多孔質シートは、ポリエチレン、ポリプロピレン、ポリエステル、フルオロポリマー、又はポリアミドのメッシュである、請求項5または6記載の人工修復器具。
- 前記人工修復器具は、内部又は腹部内臓又は組織及び器官に向けられる第1の側部と、その反対側の第2の側部とを有しており、前記第2の吸収性材料のシートが前記人工修復器具の第2の側部に位置付けられている、請求項5〜7のいずれか記載の人工修復器具。
- 人工修復器具であって、
(i)ポリ(1,4−ジオキサン−2−オン)で封入されたポリプロピレンメッシュと、
(ii)前記封入されたポリプロピレンメッシュに接合された酸化再生セルロースファブリックと、
を含み、
前記ポリ(1,4−ジオキサン−2−オン)は、溶融時に、前記ポリプロピレンメッシュを前記酸化再生セルロースファブリックに接合する手段としての役目を果たし、
当該人工修復器具は、少なくとも5分の保持時間を有している、人工修復器具。 - 前記人工修復器具は、内部又は腹部内臓又は組織及び器官に向けられる第1の側部と、その反対側の第2の側部とを有しており、前記酸化再生セルロースファブリックが前記人工修復器具の第2の側部に位置付けられている、請求項9記載の人工修復器具。
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US10/723,720 US20050113849A1 (en) | 2003-11-26 | 2003-11-26 | Prosthetic repair device |
| US723720 | 2003-11-26 |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2005152651A JP2005152651A (ja) | 2005-06-16 |
| JP2005152651A5 JP2005152651A5 (ja) | 2011-02-03 |
| JP4738794B2 true JP4738794B2 (ja) | 2011-08-03 |
Family
ID=34522994
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2004340863A Expired - Fee Related JP4738794B2 (ja) | 2003-11-26 | 2004-11-25 | 人工修復器具 |
Country Status (4)
| Country | Link |
|---|---|
| US (2) | US20050113849A1 (ja) |
| EP (1) | EP1541183B1 (ja) |
| JP (1) | JP4738794B2 (ja) |
| DE (1) | DE602004022503D1 (ja) |
Families Citing this family (109)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| AU2003249310A1 (en) * | 2002-07-17 | 2004-02-02 | Proxy Biomedical Limited | Soft tissue implants and methods for making same |
| US7758654B2 (en) * | 2004-05-20 | 2010-07-20 | Kensey Nash Corporation | Anti-adhesion device |
| US8263102B2 (en) | 2004-09-28 | 2012-09-11 | Atrium Medical Corporation | Drug delivery coating for use with a stent |
| US9801982B2 (en) | 2004-09-28 | 2017-10-31 | Atrium Medical Corporation | Implantable barrier device |
| US8367099B2 (en) | 2004-09-28 | 2013-02-05 | Atrium Medical Corporation | Perforated fatty acid films |
| US8312836B2 (en) * | 2004-09-28 | 2012-11-20 | Atrium Medical Corporation | Method and apparatus for application of a fresh coating on a medical device |
| WO2006036984A2 (en) | 2004-09-28 | 2006-04-06 | Atrium Medical Corporation | Stand-alone film and methods for making the same |
| US9012506B2 (en) | 2004-09-28 | 2015-04-21 | Atrium Medical Corporation | Cross-linked fatty acid-based biomaterials |
| US9000040B2 (en) | 2004-09-28 | 2015-04-07 | Atrium Medical Corporation | Cross-linked fatty acid-based biomaterials |
| US9566370B2 (en) * | 2004-12-23 | 2017-02-14 | Novus Scientific Ab | Mesh implant for use in reconstruction of soft tissue defects |
| US9717825B2 (en) | 2004-12-23 | 2017-08-01 | Novus Scientific Ab | Mesh implant for use in reconstruction of soft tissue defects |
| WO2006119142A2 (en) * | 2005-04-29 | 2006-11-09 | Kassab Ghassan S | Tissue engineering of blood vessels |
| EP1937183B1 (en) | 2005-09-12 | 2018-11-28 | Proxy Biomedical Limited | Soft tissue implants |
| US9427423B2 (en) | 2009-03-10 | 2016-08-30 | Atrium Medical Corporation | Fatty-acid based particles |
| US9278161B2 (en) | 2005-09-28 | 2016-03-08 | Atrium Medical Corporation | Tissue-separating fatty acid adhesion barrier |
| CA2626030A1 (en) | 2005-10-15 | 2007-04-26 | Atrium Medical Corporation | Hydrophobic cross-linked gels for bioabsorbable drug carrier coatings |
| EP2114298B1 (en) | 2006-02-08 | 2022-10-19 | Medtronic, Inc. | Temporarily stiffened mesh prostheses |
| US8315700B2 (en) | 2006-02-08 | 2012-11-20 | Tyrx, Inc. | Preventing biofilm formation on implantable medical devices |
| US8591531B2 (en) | 2006-02-08 | 2013-11-26 | Tyrx, Inc. | Mesh pouches for implantable medical devices |
| ATE499068T1 (de) * | 2006-06-22 | 2011-03-15 | Novus Scient Pte Ltd | Gewebeimplantat zur verwendung in der rekonstruktion von weichgewebedefekten |
| US8083755B2 (en) * | 2006-06-22 | 2011-12-27 | Novus Scientific Pte. Ltd. | Mesh implant for use in reconstruction of soft tissue defects |
| EP2083875B1 (en) | 2006-11-06 | 2013-03-27 | Atrium Medical Corporation | Coated surgical mesh |
| US9492596B2 (en) * | 2006-11-06 | 2016-11-15 | Atrium Medical Corporation | Barrier layer with underlying medical device and one or more reinforcing support structures |
| US9023114B2 (en) | 2006-11-06 | 2015-05-05 | Tyrx, Inc. | Resorbable pouches for implantable medical devices |
| US8016841B2 (en) * | 2007-06-11 | 2011-09-13 | Novus Scientific Pte. Ltd. | Mesh implant with an interlocking knitted structure |
| US20090036996A1 (en) * | 2007-08-03 | 2009-02-05 | Roeber Peter J | Knit PTFE Articles and Mesh |
| US20090187197A1 (en) * | 2007-08-03 | 2009-07-23 | Roeber Peter J | Knit PTFE Articles and Mesh |
| US9308068B2 (en) | 2007-12-03 | 2016-04-12 | Sofradim Production | Implant for parastomal hernia |
| US8206632B2 (en) * | 2007-12-18 | 2012-06-26 | Ethicon, Inc. | Methods of making composite prosthetic devices having improved bond strength |
| US8299316B2 (en) * | 2007-12-18 | 2012-10-30 | Ethicon, Inc. | Hemostatic device |
| US8629314B2 (en) * | 2007-12-18 | 2014-01-14 | Ethicon, Inc. | Surgical barriers having adhesion inhibiting properties |
| US20090163936A1 (en) * | 2007-12-21 | 2009-06-25 | Chunlin Yang | Coated Tissue Engineering Scaffold |
| US8808314B2 (en) | 2008-02-18 | 2014-08-19 | Covidien Lp | Device and method for deploying and attaching an implant to a biological tissue |
| US9833240B2 (en) | 2008-02-18 | 2017-12-05 | Covidien Lp | Lock bar spring and clip for implant deployment device |
| US9044235B2 (en) | 2008-02-18 | 2015-06-02 | Covidien Lp | Magnetic clip for implant deployment device |
| US9398944B2 (en) | 2008-02-18 | 2016-07-26 | Covidien Lp | Lock bar spring and clip for implant deployment device |
| WO2009104182A2 (en) | 2008-02-18 | 2009-08-27 | Polytouch Medical Ltd | A device and method for deploying and attaching a patch to a biological tissue |
| US9034002B2 (en) | 2008-02-18 | 2015-05-19 | Covidien Lp | Lock bar spring and clip for implant deployment device |
| US8317808B2 (en) | 2008-02-18 | 2012-11-27 | Covidien Lp | Device and method for rolling and inserting a prosthetic patch into a body cavity |
| US9393093B2 (en) | 2008-02-18 | 2016-07-19 | Covidien Lp | Clip for implant deployment device |
| US9393002B2 (en) | 2008-02-18 | 2016-07-19 | Covidien Lp | Clip for implant deployment device |
| US9301826B2 (en) | 2008-02-18 | 2016-04-05 | Covidien Lp | Lock bar spring and clip for implant deployment device |
| US8758373B2 (en) | 2008-02-18 | 2014-06-24 | Covidien Lp | Means and method for reversibly connecting a patch to a patch deployment device |
| US9242026B2 (en) | 2008-06-27 | 2016-01-26 | Sofradim Production | Biosynthetic implant for soft tissue repair |
| EP2792307B1 (en) | 2008-10-20 | 2017-10-04 | Covidien LP | A device for attaching a patch to a biological tissue |
| RU2011116449A (ru) * | 2008-11-20 | 2012-12-27 | Лайфселл Корпорейшн | Способ лечения и предотвращения парастомальных грыж |
| US20110038910A1 (en) | 2009-08-11 | 2011-02-17 | Atrium Medical Corporation | Anti-infective antimicrobial-containing biomaterials |
| WO2011021083A1 (en) | 2009-08-17 | 2011-02-24 | PolyTouch Medical, Inc. | Articulating patch deployment device and method of use |
| EP3508144B1 (en) | 2009-08-17 | 2021-04-07 | Covidien LP | Patch deployment device |
| FR2949688B1 (fr) | 2009-09-04 | 2012-08-24 | Sofradim Production | Tissu avec picots revetu d'une couche microporeuse bioresorbable |
| US8349354B2 (en) * | 2009-09-22 | 2013-01-08 | Ethicon, Inc. | Composite layered hemostasis device |
| AU2011218066B2 (en) * | 2010-02-19 | 2015-04-23 | Lifecell Corporation | Abdominal wall treatment devices |
| US8821585B2 (en) | 2010-06-14 | 2014-09-02 | Ethicon, Inc. | Composite anisotropic tissue reinforcing implants having alignment markers and methods of manufacturing same |
| US8517174B2 (en) * | 2010-06-22 | 2013-08-27 | Ethicon, Inc. | Dispensing packages for medical devices having two components that are mechanically interlocked and methods therefor |
| FR2962646B1 (fr) | 2010-07-16 | 2012-06-22 | Sofradim Production | Prothese avec element radio-opaque |
| WO2012009707A2 (en) | 2010-07-16 | 2012-01-19 | Atrium Medical Corporation | Composition and methods for altering the rate of hydrolysis of cured oil-based materials |
| WO2012064552A1 (en) | 2010-11-12 | 2012-05-18 | C.R. Bard, Inc. | Fabric prosthesis for repairing a tissue wall defect in proximity of a tube-like structure |
| EP2637713B1 (en) | 2010-11-12 | 2016-04-20 | Tyrx, Inc. | Anchorage devices comprising an active pharmaceutical ingredient |
| DE102011004239A1 (de) * | 2011-02-16 | 2012-08-16 | Gelita Ag | Verwendung eines medizinischen Implantats als Adhäsionsbarriere |
| FR2972626B1 (fr) | 2011-03-16 | 2014-04-11 | Sofradim Production | Prothese comprenant un tricot tridimensionnel et ajoure |
| US8579990B2 (en) | 2011-03-30 | 2013-11-12 | Ethicon, Inc. | Tissue repair devices of rapid therapeutic absorbency |
| FR2977789B1 (fr) | 2011-07-13 | 2013-07-19 | Sofradim Production | Prothese pour hernie ombilicale |
| FR2977790B1 (fr) | 2011-07-13 | 2013-07-19 | Sofradim Production | Prothese pour hernie ombilicale |
| CA2849052C (en) | 2011-09-30 | 2019-11-05 | Sofradim Production | Reversible stiffening of light weight mesh |
| US9005308B2 (en) | 2011-10-25 | 2015-04-14 | Covidien Lp | Implantable film/mesh composite for passage of tissue therebetween |
| EP3842078A1 (en) | 2011-12-20 | 2021-06-30 | LifeCell Corporation | Sheet tissue products |
| EP2793965B1 (en) | 2011-12-20 | 2019-02-20 | LifeCell Corporation | Flowable tissue products |
| FR2985271B1 (fr) | 2011-12-29 | 2014-01-24 | Sofradim Production | Tricot a picots |
| FR2985170B1 (fr) | 2011-12-29 | 2014-01-24 | Sofradim Production | Prothese pour hernie inguinale |
| ES2705823T3 (es) | 2012-01-24 | 2019-03-26 | Lifecell Corp | Matrices de tejidos alargadas |
| US10206769B2 (en) | 2012-03-30 | 2019-02-19 | Covidien Lp | Implantable devices including a film providing folding characteristics |
| US9999637B2 (en) | 2012-04-24 | 2018-06-19 | Lifecell Corporation | Functionalized tissue matrices |
| US9867880B2 (en) | 2012-06-13 | 2018-01-16 | Atrium Medical Corporation | Cured oil-hydrogel biomaterial compositions for controlled drug delivery |
| FR2992662B1 (fr) | 2012-06-28 | 2014-08-08 | Sofradim Production | Tricot avec picots |
| FR2992547B1 (fr) | 2012-06-29 | 2015-04-24 | Sofradim Production | Prothese pour hernie |
| FR2994185B1 (fr) | 2012-08-02 | 2015-07-31 | Sofradim Production | Procede de preparation d’une couche poreuse a base de chitosane |
| FR2995778B1 (fr) | 2012-09-25 | 2015-06-26 | Sofradim Production | Prothese de renfort de la paroi abdominale et procede de fabrication |
| FR2995788B1 (fr) | 2012-09-25 | 2014-09-26 | Sofradim Production | Patch hemostatique et procede de preparation |
| FR2995779B1 (fr) | 2012-09-25 | 2015-09-25 | Sofradim Production | Prothese comprenant un treillis et un moyen de consolidation |
| US10159555B2 (en) | 2012-09-28 | 2018-12-25 | Sofradim Production | Packaging for a hernia repair device |
| FR3006578B1 (fr) | 2013-06-07 | 2015-05-29 | Sofradim Production | Prothese a base d’un textile pour voie laparoscopique |
| FR3006581B1 (fr) | 2013-06-07 | 2016-07-22 | Sofradim Production | Prothese a base d’un textile pour voie laparoscopique |
| US10130457B2 (en) | 2014-03-05 | 2018-11-20 | Tela Bio, Inc. | Surgical attachment device |
| EP3000432B1 (en) | 2014-09-29 | 2022-05-04 | Sofradim Production | Textile-based prosthesis for treatment of inguinal hernia |
| EP3000433B1 (en) | 2014-09-29 | 2022-09-21 | Sofradim Production | Device for introducing a prosthesis for hernia treatment into an incision and flexible textile based prosthesis |
| EP3029189B1 (en) | 2014-12-05 | 2021-08-11 | Sofradim Production | Prosthetic porous knit, method of making same and hernia prosthesis |
| US20160175082A1 (en) * | 2014-12-23 | 2016-06-23 | Novus Scientific Ab | Resorbable medical mesh implant for repair or prevention of parastomal hernia |
| EP3059255B1 (en) | 2015-02-17 | 2020-05-13 | Sofradim Production | Method for preparing a chitosan-based matrix comprising a fiber reinforcement member |
| EP3085337B1 (en) | 2015-04-24 | 2022-09-14 | Sofradim Production | Prosthesis for supporting a breast structure |
| ES2676072T3 (es) | 2015-06-19 | 2018-07-16 | Sofradim Production | Prótesis sintética que comprende un tejido de punto y una película no porosa y método para formarla |
| EP3317448B1 (en) | 2015-06-30 | 2021-09-08 | Tela Bio, Inc. | Corner-lock stitch patterns |
| US10426587B2 (en) | 2015-07-21 | 2019-10-01 | Tela Bio, Inc. | Compliance control stitching in substrate materials |
| DE102015013989A1 (de) * | 2015-10-30 | 2017-05-04 | Johnson & Johnson Medical Gmbh | Chirurgisches Implantat |
| WO2017074671A1 (en) * | 2015-10-30 | 2017-05-04 | Ethicon Llc | Surgical implant and process of manufacturing thereof |
| DE102015013992A1 (de) | 2015-10-30 | 2017-05-04 | Johnson & Johnson Medical Gmbh | Chirgurgisches Implantat und Verfahren zu dessen Herstellung |
| EP3195830B1 (en) | 2016-01-25 | 2020-11-18 | Sofradim Production | Prosthesis for hernia repair |
| EP3448308B1 (en) | 2016-04-26 | 2024-08-14 | Tela Bio, Inc. | Hernia repair grafts having anti-adhesion barriers |
| US10933174B2 (en) | 2016-05-03 | 2021-03-02 | Medtronic, Inc. | Hemostatic devices and methods of use |
| US10980922B2 (en) * | 2016-05-03 | 2021-04-20 | Medtronic, Inc. | Hemostatic devices and methods of use |
| US10765782B2 (en) | 2016-05-03 | 2020-09-08 | Medtronic, Inc. | Hemostatic devices and methods of use |
| EP3312325B1 (en) | 2016-10-21 | 2021-09-22 | Sofradim Production | Method for forming a mesh having a barbed suture attached thereto and the mesh thus obtained |
| EP3398554A1 (en) | 2017-05-02 | 2018-11-07 | Sofradim Production | Prosthesis for inguinal hernia repair |
| EP3761963A4 (en) | 2018-03-09 | 2021-12-08 | Tela Bio, Inc. | SURGICAL REPAIR TRANSPLANT |
| EP3653171B1 (en) | 2018-11-16 | 2024-08-21 | Sofradim Production | Implants suitable for soft tissue repair |
| WO2020185688A1 (en) | 2019-03-08 | 2020-09-17 | Tela Bio, Inc. | Textured medical textiles |
| US12064330B2 (en) | 2020-04-28 | 2024-08-20 | Covidien Lp | Implantable prothesis for minimally invasive hernia repair |
| US20220023488A1 (en) | 2020-07-21 | 2022-01-27 | Ethicon, Inc. | Sealant Dressing with Removable Intermediate Separating Layer |
| US20220062492A1 (en) | 2020-08-31 | 2022-03-03 | Ethicon, Inc. | Sealant Dressing with Protected Reactive Components |
| CN114748208B (zh) * | 2022-04-15 | 2024-01-12 | 柔脉医疗(深圳)有限公司 | 一种可原位检测多种化学、生物成分的组织工程支架 |
Family Cites Families (37)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5007916A (en) * | 1985-08-22 | 1991-04-16 | Johnson & Johnson Medical, Inc. | Method and material for prevention of surgical adhesions |
| US5002551A (en) * | 1985-08-22 | 1991-03-26 | Johnson & Johnson Medical, Inc. | Method and material for prevention of surgical adhesions |
| US5092884A (en) * | 1988-03-24 | 1992-03-03 | American Cyanamid Company | Surgical composite structure having absorbable and nonabsorbable components |
| DE69121587T3 (de) * | 1990-12-06 | 2000-05-31 | W.L. Gore & Associates, Newark | Implantierbare bioresorbierbare artikel |
| US5254133A (en) * | 1991-04-24 | 1993-10-19 | Seid Arnold S | Surgical implantation device and related method of use |
| WO1993017635A1 (en) * | 1992-03-04 | 1993-09-16 | C.R. Bard, Inc. | Composite prosthesis and method for limiting the incidence of postoperative adhesions |
| US5766246A (en) * | 1992-05-20 | 1998-06-16 | C. R. Bard, Inc. | Implantable prosthesis and method and apparatus for loading and delivering an implantable prothesis |
| US5743917A (en) * | 1993-01-13 | 1998-04-28 | Saxon; Allen | Prosthesis for the repair of soft tissue defects |
| US5725577A (en) * | 1993-01-13 | 1998-03-10 | Saxon; Allen | Prosthesis for the repair of soft tissue defects |
| CA2114282A1 (en) * | 1993-01-28 | 1994-07-29 | Lothar Schilder | Multi-layered implant |
| US5626611A (en) * | 1994-02-10 | 1997-05-06 | United States Surgical Corporation | Composite bioabsorbable materials and surgical articles made therefrom |
| US6093200A (en) * | 1994-02-10 | 2000-07-25 | United States Surgical | Composite bioabsorbable materials and surgical articles made therefrom |
| ATE182443T1 (de) * | 1994-05-26 | 1999-08-15 | Johann Seiger | Sonnenschutzabdeckung |
| US5629077A (en) * | 1994-06-27 | 1997-05-13 | Advanced Cardiovascular Systems, Inc. | Biodegradable mesh and film stent |
| US5768246A (en) * | 1994-08-30 | 1998-06-16 | Samsung Electronics Co., Ltd. | Method and apparatus for recording and reproducing digital data using frequency domain conversion and detection |
| US20020131933A1 (en) * | 1996-01-16 | 2002-09-19 | Yves Delmotte | Biopolymer membrane and methods for its preparation |
| US5634944A (en) * | 1995-02-23 | 1997-06-03 | The Nemours Foundation | Body membrane prosthesis |
| GB9510624D0 (en) * | 1995-05-25 | 1995-07-19 | Ellis Dev Ltd | Textile surgical implants |
| DE19613730C2 (de) * | 1996-03-26 | 2002-08-14 | Ethicon Gmbh | Flächiges Implantat zum Verstärken oder Verschließen von Körpergewebe |
| US5791352A (en) * | 1996-06-19 | 1998-08-11 | Fusion Medical Technologies, Inc. | Methods and compositions for inhibiting tissue adhesion |
| JP3754528B2 (ja) * | 1997-03-31 | 2006-03-15 | ユニ・チャーム株式会社 | 体液処理用吸収性物品 |
| US6120539A (en) * | 1997-05-01 | 2000-09-19 | C. R. Bard Inc. | Prosthetic repair fabric |
| FR2766716B1 (fr) * | 1997-08-01 | 2000-02-18 | Cogent Sarl | Prothese composite pour la prevention des adherences post-chirurgicales et son procede d'obtention |
| FR2766717B1 (fr) * | 1997-08-01 | 2000-06-09 | Cogent Sarl | Prothese composite pour la prevention des adherences post-chirurgicales et son procede d'obtention |
| US6319264B1 (en) * | 1998-04-03 | 2001-11-20 | Bionx Implants Oy | Hernia mesh |
| US6270630B1 (en) * | 1998-12-03 | 2001-08-07 | Li Xing | Process and apparatus for producing hydrocarbons from residential trash or waste and/or organic waste materials |
| US20030040809A1 (en) * | 1999-03-20 | 2003-02-27 | Helmut Goldmann | Flat implant for use in surgery |
| DE19912648A1 (de) * | 1999-03-20 | 2000-09-21 | Aesculap Ag & Co Kg | Flächiges Implantat, Verfahren zu seiner Herstellung und Verwendung in der Chirurgie |
| US6258124B1 (en) * | 1999-05-10 | 2001-07-10 | C. R. Bard, Inc. | Prosthetic repair fabric |
| US6383201B1 (en) * | 1999-05-14 | 2002-05-07 | Tennison S. Dong | Surgical prosthesis for repairing a hernia |
| DE19954166A1 (de) * | 1999-11-10 | 2001-05-17 | Inst Textil & Faserforschung | Flächiges Implantat, Verfahren zu seiner Herstellung und Verwendung in der Chirurgie |
| ES2228648T3 (es) * | 1999-12-17 | 2005-04-16 | Genzyme Corporation | Protesis ortopedica. |
| WO2001075501A1 (fr) * | 2000-03-31 | 2001-10-11 | Nikon Corporation | Procede et dispositif de soutien d'un element optique, dispositif optique, appareil d'exposition, et procede de fabrication d'un dispositif |
| JP2004531292A (ja) * | 2001-01-02 | 2004-10-14 | アドヴァンスト セラミックス リサーチ インコーポレイテッド | 生物医学的に適用される組成物及び方法 |
| US6800082B2 (en) * | 2001-10-19 | 2004-10-05 | Ethicon, Inc. | Absorbable mesh device |
| DE10155842A1 (de) * | 2001-11-14 | 2003-05-28 | Ethicon Gmbh | Flächiges Implantat |
| JP3719659B2 (ja) * | 2001-12-26 | 2005-11-24 | 株式会社日立製作所 | 情報受信システム及び情報受信端末 |
-
2003
- 2003-11-26 US US10/723,720 patent/US20050113849A1/en not_active Abandoned
-
2004
- 2004-11-25 EP EP04257324A patent/EP1541183B1/en active Active
- 2004-11-25 JP JP2004340863A patent/JP4738794B2/ja not_active Expired - Fee Related
- 2004-11-25 DE DE602004022503T patent/DE602004022503D1/de active Active
-
2007
- 2007-11-27 US US11/945,568 patent/US20080071300A1/en not_active Abandoned
Also Published As
| Publication number | Publication date |
|---|---|
| US20080071300A1 (en) | 2008-03-20 |
| JP2005152651A (ja) | 2005-06-16 |
| EP1541183B1 (en) | 2009-08-12 |
| DE602004022503D1 (de) | 2009-09-24 |
| EP1541183A1 (en) | 2005-06-15 |
| US20050113849A1 (en) | 2005-05-26 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP4738794B2 (ja) | 人工修復器具 | |
| US11547543B2 (en) | Reinforcement device with dissolvable layer and its use | |
| US8562633B2 (en) | Tissue repair device with a bioabsorbable support member | |
| US8206632B2 (en) | Methods of making composite prosthetic devices having improved bond strength | |
| US9750594B2 (en) | Soft tissue implants and methods for making same | |
| US9788930B2 (en) | Soft tissue implants and methods for making same | |
| JP6608356B2 (ja) | 開口部を有する層を含む外科用インプラント | |
| KR102231871B1 (ko) | 외과용 임플란트 | |
| KR20070120513A (ko) | 외과용 임플란트 | |
| KR20160045802A (ko) | 외과용 임플란트 | |
| CN108348316A (zh) | 外科植入物及其制造方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20071120 |
|
| RD04 | Notification of resignation of power of attorney |
Free format text: JAPANESE INTERMEDIATE CODE: A7424 Effective date: 20071128 |
|
| RD04 | Notification of resignation of power of attorney |
Free format text: JAPANESE INTERMEDIATE CODE: A7424 Effective date: 20080912 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20100202 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20100430 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20100510 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20100517 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20100817 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20101203 |
|
| A524 | Written submission of copy of amendment under article 19 pct |
Free format text: JAPANESE INTERMEDIATE CODE: A524 Effective date: 20101203 |
|
| A911 | Transfer to examiner for re-examination before appeal (zenchi) |
Free format text: JAPANESE INTERMEDIATE CODE: A911 Effective date: 20110216 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20110329 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20110427 |
|
| R150 | Certificate of patent or registration of utility model |
Free format text: JAPANESE INTERMEDIATE CODE: R150 Ref document number: 4738794 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20140513 Year of fee payment: 3 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| LAPS | Cancellation because of no payment of annual fees |