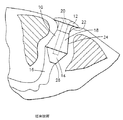
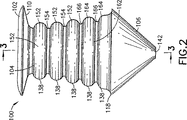
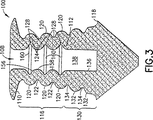
JP4106185B2 - 点口プラグ - Google Patents
点口プラグ Download PDFInfo
- Publication number
- JP4106185B2 JP4106185B2 JP2000580554A JP2000580554A JP4106185B2 JP 4106185 B2 JP4106185 B2 JP 4106185B2 JP 2000580554 A JP2000580554 A JP 2000580554A JP 2000580554 A JP2000580554 A JP 2000580554A JP 4106185 B2 JP4106185 B2 JP 4106185B2
- Authority
- JP
- Japan
- Prior art keywords
- shaft
- plug
- point
- wall
- bore
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
- 238000003780 insertion Methods 0.000 claims description 13
- 230000037431 insertion Effects 0.000 claims description 13
- 210000004083 nasolacrimal duct Anatomy 0.000 claims description 10
- 230000003068 static effect Effects 0.000 claims description 7
- 230000002093 peripheral effect Effects 0.000 claims description 5
- 210000003128 head Anatomy 0.000 description 33
- 238000007373 indentation Methods 0.000 description 8
- 239000012530 fluid Substances 0.000 description 6
- 238000000034 method Methods 0.000 description 4
- 210000001519 tissue Anatomy 0.000 description 4
- 230000000903 blocking effect Effects 0.000 description 3
- 239000000463 material Substances 0.000 description 3
- 206010041232 sneezing Diseases 0.000 description 3
- 206010015958 Eye pain Diseases 0.000 description 2
- 230000037237 body shape Effects 0.000 description 2
- 238000013461 design Methods 0.000 description 2
- 238000001746 injection moulding Methods 0.000 description 2
- 239000007788 liquid Substances 0.000 description 2
- 238000000465 moulding Methods 0.000 description 2
- 229910052710 silicon Inorganic materials 0.000 description 2
- 239000010703 silicon Substances 0.000 description 2
- 238000001721 transfer moulding Methods 0.000 description 2
- 230000002792 vascular Effects 0.000 description 2
- 208000003556 Dry Eye Syndromes Diseases 0.000 description 1
- 206010013774 Dry eye Diseases 0.000 description 1
- 206010019233 Headaches Diseases 0.000 description 1
- 206010020751 Hypersensitivity Diseases 0.000 description 1
- 206010041235 Snoring Diseases 0.000 description 1
- 206010070488 Upper-airway cough syndrome Diseases 0.000 description 1
- 230000007815 allergy Effects 0.000 description 1
- 210000003484 anatomy Anatomy 0.000 description 1
- 231100000869 headache Toxicity 0.000 description 1
- 238000011065 in-situ storage Methods 0.000 description 1
- 206010023332 keratitis Diseases 0.000 description 1
- 201000010666 keratoconjunctivitis Diseases 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 238000007789 sealing Methods 0.000 description 1
- 201000009890 sinusitis Diseases 0.000 description 1
- 239000000243 solution Substances 0.000 description 1
- 210000005239 tubule Anatomy 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M39/00—Tubes, tube connectors, tube couplings, valves, access sites or the like, specially adapted for medical use
- A61M39/02—Access sites
- A61M39/04—Access sites having pierceable self-sealing members
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F9/00—Methods or devices for treatment of the eyes; Devices for putting in contact-lenses; Devices to correct squinting; Apparatus to guide the blind; Protective devices for the eyes, carried on the body or in the hand
- A61F9/007—Methods or devices for eye surgery
- A61F9/00772—Apparatus for restoration of tear ducts
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/12—Surgical instruments, devices or methods for ligaturing or otherwise compressing tubular parts of the body, e.g. blood vessels or umbilical cord
- A61B17/12022—Occluding by internal devices, e.g. balloons or releasable wires
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/12—Surgical instruments, devices or methods for ligaturing or otherwise compressing tubular parts of the body, e.g. blood vessels or umbilical cord
- A61B17/12022—Occluding by internal devices, e.g. balloons or releasable wires
- A61B17/12099—Occluding by internal devices, e.g. balloons or releasable wires characterised by the location of the occluder
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods
- A61B17/12—Surgical instruments, devices or methods for ligaturing or otherwise compressing tubular parts of the body, e.g. blood vessels or umbilical cord
- A61B17/12022—Occluding by internal devices, e.g. balloons or releasable wires
- A61B17/12131—Occluding by internal devices, e.g. balloons or releasable wires characterised by the type of occluding device
- A61B17/12159—Solid plugs; being solid before insertion
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Surgery (AREA)
- Heart & Thoracic Surgery (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Biomedical Technology (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Engineering & Computer Science (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Vascular Medicine (AREA)
- Reproductive Health (AREA)
- Medical Informatics (AREA)
- Molecular Biology (AREA)
- Ophthalmology & Optometry (AREA)
- Plastic & Reconstructive Surgery (AREA)
- Pulmonology (AREA)
- Anesthesiology (AREA)
- Hematology (AREA)
- Prostheses (AREA)
- Orthopedics, Nursing, And Contraception (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US09/188,011 US6041785A (en) | 1997-03-27 | 1998-11-06 | Punctum plug |
| US09/188,011 | 1998-11-06 | ||
| PCT/US1999/026152 WO2000027321A1 (en) | 1998-11-06 | 1999-11-05 | Punctum plug |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2002529144A JP2002529144A (ja) | 2002-09-10 |
| JP2002529144A5 JP2002529144A5 (enExample) | 2006-12-14 |
| JP4106185B2 true JP4106185B2 (ja) | 2008-06-25 |
Family
ID=22691406
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2000580554A Expired - Lifetime JP4106185B2 (ja) | 1998-11-06 | 1999-11-05 | 点口プラグ |
Country Status (8)
| Country | Link |
|---|---|
| US (1) | US6041785A (enExample) |
| EP (1) | EP1126801B1 (enExample) |
| JP (1) | JP4106185B2 (enExample) |
| KR (1) | KR20010092435A (enExample) |
| AU (1) | AU752839B2 (enExample) |
| CA (1) | CA2349590A1 (enExample) |
| HK (1) | HK1040178A1 (enExample) |
| WO (1) | WO2000027321A1 (enExample) |
Families Citing this family (51)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6371122B1 (en) | 2000-06-20 | 2002-04-16 | Robert M. Mandelkorn | Gauge/dilator apparatus |
| ES2203269B1 (es) * | 2001-02-05 | 2005-06-16 | Juan Murube Del Castillo | Protesis para drenar la lagrima del oja de la fosa nasal. |
| US6605108B2 (en) * | 2001-04-13 | 2003-08-12 | Eagle Vision, Inc. | Monocanalicular stent |
| US7278430B2 (en) * | 2002-03-01 | 2007-10-09 | Arvik Enterprises, Llc | Blood vessel occlusion device |
| US7060083B2 (en) * | 2002-05-20 | 2006-06-13 | Boston Scientific Scimed, Inc. | Foldable vaso-occlusive member |
| US7017580B2 (en) * | 2003-05-22 | 2006-03-28 | Clarity Corporation | Punctum plug system including a punctum plug and passive insertion tool therefor |
| US7204253B2 (en) * | 2003-05-22 | 2007-04-17 | Clarity Corporation | Punctum plug |
| JP4104137B2 (ja) | 2003-05-30 | 2008-06-18 | 有限会社エム・エル・シー | 涙点プラグ |
| US6994684B2 (en) * | 2003-06-16 | 2006-02-07 | Alphamed Inc. | Punctum plugs having fluid collecting recesses and methods of punctal occlusion |
| US20050232972A1 (en) * | 2004-04-15 | 2005-10-20 | Steven Odrich | Drug delivery via punctal plug |
| US7156821B2 (en) * | 2004-04-23 | 2007-01-02 | Massachusetts Eye & Ear Infirmary | Shunt with enclosed pressure-relief valve |
| US7186233B2 (en) * | 2004-04-23 | 2007-03-06 | Massachusetts Eye & Ear Infirmary | Dry eye treatment |
| CA2572592C (en) | 2004-07-02 | 2015-09-08 | Eliot Lazar | Treatment medium delivery device and methods for delivery of such treatment mediums to the eye using such a delivery device |
| ES2380651T3 (es) * | 2005-09-01 | 2012-05-17 | Bristol-Myers Squibb Company | Biomarcadores y procedimientos para determinar la sensibilidad a moduladores de recpetor 2 del factor de crecimiento endotelial vascular |
| US7862532B2 (en) * | 2005-10-07 | 2011-01-04 | Delta Life Sciences, Inc. | Punctum plugs having insertion guides and strengthening beams |
| SG170806A1 (en) | 2006-03-31 | 2011-05-30 | Qlt Plug Delivery Inc | Nasolacrimal drainage system implants for drug therapy |
| US8652090B2 (en) * | 2006-05-18 | 2014-02-18 | Cannuflow, Inc. | Anti-extravasation surgical portal plug |
| US20080086101A1 (en) * | 2006-08-25 | 2008-04-10 | David Freilich | Ophthalmic insert |
| UY30883A1 (es) | 2007-01-31 | 2008-05-31 | Alcon Res | Tapones punctales y metodos de liberacion de agentes terapeuticos |
| MX2010002619A (es) | 2007-09-07 | 2010-06-01 | Qlt Plug Delivery Inc | Deteccion de implante lagrimal. |
| ES2732555T3 (es) | 2007-09-07 | 2019-11-25 | Mati Therapeutics Inc | Implantes lagrimales y métodos relacionados |
| CN102670352A (zh) * | 2007-09-07 | 2012-09-19 | Qlt股份有限公司 | 用于泪腺植入物的插入和抽出工具 |
| ES2533359T3 (es) * | 2007-09-07 | 2015-04-09 | Mati Therapeutics Inc. | Núcleos de fármaco para liberación sostenida de agentes terapéuticos |
| WO2009105178A2 (en) * | 2008-02-18 | 2009-08-27 | Qlt Plug Delivery, Inc. | Lacrimal implants and related methods |
| US20090227938A1 (en) * | 2008-03-05 | 2009-09-10 | Insitu Therapeutics, Inc. | Wound Closure Devices, Methods of Use, and Kits |
| CN104623741A (zh) | 2008-04-30 | 2015-05-20 | 马缇医疗股份有限公司 | 复合泪管植入物及相关方法 |
| EP2276492A2 (en) * | 2008-05-09 | 2011-01-26 | QLT Plug Delivery, Inc. | Sustained release delivery of active agents to treat glaucoma and ocular hypertension |
| US20100217309A1 (en) * | 2009-02-20 | 2010-08-26 | Boston Scientific Scimed, Inc. | Plug for arteriotomy closure and method of use |
| CA2752645C (en) * | 2009-02-23 | 2017-10-03 | Qlt Inc. | Lacrimal implants and related methods |
| TWI495459B (zh) * | 2009-03-31 | 2015-08-11 | Johnson & Johnson Vision Care | 淚管塞(二) |
| TW201043211A (en) * | 2009-03-31 | 2010-12-16 | Johnson & Johnson Vision Care Inc | Punctal plugs |
| US9421127B2 (en) | 2009-03-31 | 2016-08-23 | Johnson & Johnson Vision Care, Inc. | Punctal plugs |
| US8979821B2 (en) | 2009-04-14 | 2015-03-17 | Fezza Family Properties, Llc | Lacrimal filler |
| US9259352B2 (en) | 2010-03-29 | 2016-02-16 | Johnson & Johnson Vision Care, Inc. | Punctal plugs |
| US9259351B2 (en) | 2010-03-29 | 2016-02-16 | Johnson & Johnson Vision Care, Inc. | Punctal plugs |
| US9022967B2 (en) | 2010-10-08 | 2015-05-05 | Sinopsys Surgical, Inc. | Implant device, tool, and methods relating to treatment of paranasal sinuses |
| US20120157938A1 (en) * | 2010-12-16 | 2012-06-21 | Tokarski Jason M | Punctal plug with drug core retention features |
| US9974685B2 (en) | 2011-08-29 | 2018-05-22 | Mati Therapeutics | Drug delivery system and methods of treating open angle glaucoma and ocular hypertension |
| DK3290024T3 (da) | 2011-08-29 | 2019-06-03 | Mati Therapeutics Inc | Vedvarende indgivelse af aktive midler til behandling af glaukom og okulær hypertension |
| US9320526B1 (en) * | 2011-10-13 | 2016-04-26 | Enteroptyx | Punctum plug |
| WO2013154843A1 (en) * | 2012-04-11 | 2013-10-17 | Sinopsys Surgical, Inc. | Implantation tools, tool assemblies, kits and methods |
| KR102189608B1 (ko) | 2013-01-25 | 2020-12-11 | 사이놉시스 서지칼 인코포레이티드 | 부비강 접근 임플란트 디바이스들 및 관련 툴들, 방법들 및 키트들 |
| US10271949B2 (en) * | 2013-03-12 | 2019-04-30 | St. Jude Medical, Cardiology Division, Inc. | Paravalvular leak occlusion device for self-expanding heart valves |
| US9339274B2 (en) | 2013-03-12 | 2016-05-17 | St. Jude Medical, Cardiology Division, Inc. | Paravalvular leak occlusion device for self-expanding heart valves |
| KR101425636B1 (ko) * | 2013-07-05 | 2014-08-01 | 임한웅 | 누액통과부를 포함하는 비루관 튜브 |
| WO2015069433A1 (en) | 2013-10-16 | 2015-05-14 | Sinopsys Surgical, Inc. | Apparatuses, tools and kits relating to fluid manipulation treatments of paranasal sinuses |
| WO2016015002A1 (en) | 2014-07-24 | 2016-01-28 | Sinopsys Surgical, Inc. | Paranasal sinus access implant devices and related products and methods |
| US10172740B2 (en) | 2015-11-06 | 2019-01-08 | David E Freilich | Lacrimal stent |
| ES3012747T3 (en) | 2017-09-20 | 2025-04-10 | Sinopsys Surgical Inc | Paranasal sinus fluid access implantation tools and assemblies |
| US12213916B2 (en) | 2020-01-17 | 2025-02-04 | Quest Medical, Inc. | Nasolacrimal duct stent assembly and method |
| EP4297707A4 (en) | 2021-02-24 | 2025-03-19 | Ocular Therapeutix, Inc. | INTRACANALICULAR DEPOSIT INSERTION DEVICE |
Family Cites Families (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2487038A (en) * | 1944-03-25 | 1949-11-08 | Sonotone Corp | Ear insert for earphones |
| US3949750A (en) * | 1974-10-07 | 1976-04-13 | Freeman Jerre M | Punctum plug and method for treating keratoconjunctivitis sicca (dry eye) and other ophthalmic aliments using same |
| US4915684A (en) * | 1988-06-21 | 1990-04-10 | Mackeen Donald L | Method and apparatus for modulating the flow of lacrimal fluid through a punctum and associated canaliculus |
| FR2700265B1 (fr) * | 1993-01-08 | 1995-03-31 | France Chirurgie Instr | Bouchon méatique pour pathologie lacrymale. |
| US5723005A (en) * | 1995-06-07 | 1998-03-03 | Herrick Family Limited Partnership | Punctum plug having a collapsible flared section and method |
| US6016806A (en) * | 1997-03-27 | 2000-01-25 | Eaglevision, Inc | Punctum plug |
| US5830171A (en) | 1997-08-12 | 1998-11-03 | Odyssey Medical, Inc. | Punctal occluder |
-
1998
- 1998-11-06 US US09/188,011 patent/US6041785A/en not_active Expired - Lifetime
-
1999
- 1999-11-05 AU AU13437/00A patent/AU752839B2/en not_active Ceased
- 1999-11-05 CA CA002349590A patent/CA2349590A1/en not_active Abandoned
- 1999-11-05 HK HK02101363.5A patent/HK1040178A1/zh unknown
- 1999-11-05 WO PCT/US1999/026152 patent/WO2000027321A1/en not_active Ceased
- 1999-11-05 KR KR1020017005558A patent/KR20010092435A/ko not_active Ceased
- 1999-11-05 JP JP2000580554A patent/JP4106185B2/ja not_active Expired - Lifetime
- 1999-11-05 EP EP99956938A patent/EP1126801B1/en not_active Expired - Lifetime
Also Published As
| Publication number | Publication date |
|---|---|
| KR20010092435A (ko) | 2001-10-24 |
| EP1126801B1 (en) | 2012-05-23 |
| AU752839B2 (en) | 2002-10-03 |
| AU1343700A (en) | 2000-05-29 |
| CA2349590A1 (en) | 2000-05-18 |
| US6041785A (en) | 2000-03-28 |
| HK1040178A1 (zh) | 2002-05-31 |
| EP1126801A1 (en) | 2001-08-29 |
| WO2000027321A1 (en) | 2000-05-18 |
| EP1126801A4 (en) | 2003-02-05 |
| JP2002529144A (ja) | 2002-09-10 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP4106185B2 (ja) | 点口プラグ | |
| CA2284743C (en) | Punctum plug | |
| AU756757B2 (en) | Punctum plug | |
| US6629533B1 (en) | Punctum plug with at least one anchoring arm | |
| US6027470A (en) | Punctum plug and method for inserting the same into the punctual opening | |
| US6306114B1 (en) | Valved canalicular plug for lacrimal duct occlusion | |
| US6605054B2 (en) | Multiple bypass port phaco tip | |
| AU2007248163B2 (en) | Multi-purpose phacoemulsification needle | |
| US6033376A (en) | Wound shaper sleeve | |
| US7601136B2 (en) | Infusion sleeve | |
| AU2002359782B2 (en) | Prosthesis with foldable flange | |
| US6398754B1 (en) | Phaco tip with fluid bypass port | |
| JP2001502211A (ja) | 水晶体超音波吸引装置用の、スリーブで保護された手術用ニードル | |
| BRPI0406329B1 (pt) | Mecanismo de fecho rotacional para um trocarte | |
| BRPI0406033B1 (pt) | Luva de trocarte | |
| US20060253056A1 (en) | Multi-purpose phacoemulsification needle | |
| WO1999018900A1 (en) | Corneal endothelium protective device | |
| JPH07178130A (ja) | 涙病理学用の道口キャップ | |
| JP7610237B1 (ja) | 医療用挿入器 | |
| KR200411226Y1 (ko) | 흉관 삽입기 | |
| AU2013201760B2 (en) | Multi-purpose phacoemulsification needle |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20061019 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20061019 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20070517 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20070809 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20070907 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20080107 |
|
| A911 | Transfer to examiner for re-examination before appeal (zenchi) |
Free format text: JAPANESE INTERMEDIATE CODE: A911 Effective date: 20080228 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20080319 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20080331 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 4106185 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20110404 Year of fee payment: 3 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20130404 Year of fee payment: 5 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20140404 Year of fee payment: 6 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| S111 | Request for change of ownership or part of ownership |
Free format text: JAPANESE INTERMEDIATE CODE: R313113 |
|
| S531 | Written request for registration of change of domicile |
Free format text: JAPANESE INTERMEDIATE CODE: R313531 |
|
| R350 | Written notification of registration of transfer |
Free format text: JAPANESE INTERMEDIATE CODE: R350 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| EXPY | Cancellation because of completion of term |