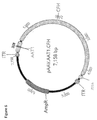
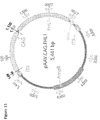
JP2021500922A - 加齢黄斑変性を処置するための組成物および方法 - Google Patents
加齢黄斑変性を処置するための組成物および方法 Download PDFInfo
- Publication number
- JP2021500922A JP2021500922A JP2020542710A JP2020542710A JP2021500922A JP 2021500922 A JP2021500922 A JP 2021500922A JP 2020542710 A JP2020542710 A JP 2020542710A JP 2020542710 A JP2020542710 A JP 2020542710A JP 2021500922 A JP2021500922 A JP 2021500922A
- Authority
- JP
- Japan
- Prior art keywords
- promoter
- cfh
- vector
- aav
- aav vector
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
- 238000000034 method Methods 0.000 title claims abstract description 110
- 239000000203 mixture Substances 0.000 title claims abstract description 78
- 208000002780 macular degeneration Diseases 0.000 title claims description 42
- 206010064930 age-related macular degeneration Diseases 0.000 title claims description 40
- 239000013598 vector Substances 0.000 claims abstract description 266
- 108090000623 proteins and genes Proteins 0.000 claims abstract description 188
- 241000702421 Dependoparvovirus Species 0.000 claims abstract description 15
- 210000004027 cell Anatomy 0.000 claims description 240
- 101000737574 Homo sapiens Complement factor H Proteins 0.000 claims description 182
- 102100035432 Complement factor H Human genes 0.000 claims description 180
- 239000012634 fragment Substances 0.000 claims description 141
- 125000003729 nucleotide group Chemical group 0.000 claims description 128
- 239000002773 nucleotide Substances 0.000 claims description 125
- 230000014509 gene expression Effects 0.000 claims description 109
- 102000004169 proteins and genes Human genes 0.000 claims description 83
- 239000013607 AAV vector Substances 0.000 claims description 65
- 102100040132 Complement factor H-related protein 1 Human genes 0.000 claims description 40
- 101000890732 Homo sapiens Complement factor H-related protein 1 Proteins 0.000 claims description 40
- 102000040430 polynucleotide Human genes 0.000 claims description 38
- 108091033319 polynucleotide Proteins 0.000 claims description 38
- 239000002157 polynucleotide Substances 0.000 claims description 38
- 230000035772 mutation Effects 0.000 claims description 34
- 230000000295 complement effect Effects 0.000 claims description 30
- 210000001525 retina Anatomy 0.000 claims description 24
- 125000003275 alpha amino acid group Chemical group 0.000 claims description 23
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims description 23
- 241000702423 Adeno-associated virus - 2 Species 0.000 claims description 22
- 229960000723 ampicillin Drugs 0.000 claims description 22
- AVKUERGKIZMTKX-NJBDSQKTSA-N ampicillin Chemical compound C1([C@@H](N)C(=O)N[C@H]2[C@H]3SC([C@@H](N3C2=O)C(O)=O)(C)C)=CC=CC=C1 AVKUERGKIZMTKX-NJBDSQKTSA-N 0.000 claims description 22
- 230000008488 polyadenylation Effects 0.000 claims description 22
- 230000000694 effects Effects 0.000 claims description 16
- 208000017442 Retinal disease Diseases 0.000 claims description 14
- 230000002207 retinal effect Effects 0.000 claims description 13
- 208000003098 Ganglion Cysts Diseases 0.000 claims description 11
- 208000005400 Synovial Cyst Diseases 0.000 claims description 11
- 230000003612 virological effect Effects 0.000 claims description 11
- 102100030386 Granzyme A Human genes 0.000 claims description 9
- 101001009599 Homo sapiens Granzyme A Proteins 0.000 claims description 9
- 239000003937 drug carrier Substances 0.000 claims description 9
- 210000003494 hepatocyte Anatomy 0.000 claims description 9
- 239000003550 marker Substances 0.000 claims description 8
- 230000003115 biocidal effect Effects 0.000 claims description 7
- 102100035431 Complement factor I Human genes 0.000 claims description 6
- 206010025421 Macule Diseases 0.000 claims description 6
- 108091005804 Peptidases Proteins 0.000 claims description 6
- 239000004365 Protease Substances 0.000 claims description 6
- 230000000670 limiting effect Effects 0.000 claims description 6
- 239000012528 membrane Substances 0.000 claims description 6
- 208000011580 syndromic disease Diseases 0.000 claims description 6
- 101150030967 CFH gene Proteins 0.000 claims description 5
- 101150091459 CFI gene Proteins 0.000 claims description 5
- 108090001126 Furin Proteins 0.000 claims description 5
- 230000024203 complement activation Effects 0.000 claims description 5
- 210000004379 membrane Anatomy 0.000 claims description 5
- 239000003242 anti bacterial agent Substances 0.000 claims description 4
- 230000015556 catabolic process Effects 0.000 claims description 4
- 238000006731 degradation reaction Methods 0.000 claims description 4
- 210000004185 liver Anatomy 0.000 claims description 4
- 210000004126 nerve fiber Anatomy 0.000 claims description 4
- 102220599936 Disks large-associated protein 5_G69E_mutation Human genes 0.000 claims description 3
- 102220612381 Mitogen-activated protein kinase kinase kinase 1_S58A_mutation Human genes 0.000 claims description 3
- 210000001775 bruch membrane Anatomy 0.000 claims description 3
- 210000004899 c-terminal region Anatomy 0.000 claims description 3
- 230000002949 hemolytic effect Effects 0.000 claims description 3
- 239000012466 permeate Substances 0.000 claims description 3
- 210000003583 retinal pigment epithelium Anatomy 0.000 claims description 3
- 102200027734 rs118092776 Human genes 0.000 claims description 3
- 102200031710 rs121913059 Human genes 0.000 claims description 3
- 102200031950 rs121913062 Human genes 0.000 claims description 3
- 102220130078 rs143237092 Human genes 0.000 claims description 3
- 102200147378 rs398123072 Human genes 0.000 claims description 3
- 102220047868 rs570523689 Human genes 0.000 claims description 3
- 102200037607 rs771019366 Human genes 0.000 claims description 3
- 210000002987 choroid plexus Anatomy 0.000 claims description 2
- 230000008359 toxicosis Effects 0.000 claims description 2
- 230000002485 urinary effect Effects 0.000 claims description 2
- 102100037486 Reverse transcriptase/ribonuclease H Human genes 0.000 claims 2
- 102100021244 Integral membrane protein GPR180 Human genes 0.000 claims 1
- 210000000981 epithelium Anatomy 0.000 claims 1
- 230000004154 complement system Effects 0.000 abstract description 31
- 208000030533 eye disease Diseases 0.000 abstract description 21
- 101710127220 Four and a half LIM domains protein 1 Proteins 0.000 abstract description 20
- 102100038651 Four and a half LIM domains protein 1 Human genes 0.000 abstract description 20
- 108700019146 Transgenes Proteins 0.000 description 76
- 108090000765 processed proteins & peptides Proteins 0.000 description 67
- 229940024606 amino acid Drugs 0.000 description 64
- 102000004196 processed proteins & peptides Human genes 0.000 description 64
- 210000001508 eye Anatomy 0.000 description 63
- 229920001184 polypeptide Polymers 0.000 description 61
- 150000001413 amino acids Chemical class 0.000 description 57
- 150000007523 nucleic acids Chemical class 0.000 description 53
- 230000006870 function Effects 0.000 description 51
- 238000011282 treatment Methods 0.000 description 41
- 239000013608 rAAV vector Substances 0.000 description 38
- 239000002245 particle Substances 0.000 description 35
- 102000039446 nucleic acids Human genes 0.000 description 34
- 108020004707 nucleic acids Proteins 0.000 description 34
- 238000004519 manufacturing process Methods 0.000 description 33
- 108091028043 Nucleic acid sequence Proteins 0.000 description 32
- -1 antibodies Proteins 0.000 description 32
- 241000700605 Viruses Species 0.000 description 27
- 239000007924 injection Substances 0.000 description 27
- 238000002347 injection Methods 0.000 description 27
- 210000000234 capsid Anatomy 0.000 description 26
- 230000001105 regulatory effect Effects 0.000 description 26
- 108091008695 photoreceptors Proteins 0.000 description 24
- 108020004414 DNA Proteins 0.000 description 22
- 102100035326 Complement factor H-related protein 2 Human genes 0.000 description 21
- 102100035321 Complement factor H-related protein 3 Human genes 0.000 description 21
- 102100035324 Complement factor H-related protein 4 Human genes 0.000 description 21
- 102100035325 Complement factor H-related protein 5 Human genes 0.000 description 21
- 101000878135 Homo sapiens Complement factor H-related protein 2 Proteins 0.000 description 21
- 101000878136 Homo sapiens Complement factor H-related protein 3 Proteins 0.000 description 21
- 101000878133 Homo sapiens Complement factor H-related protein 4 Proteins 0.000 description 21
- 101000878134 Homo sapiens Complement factor H-related protein 5 Proteins 0.000 description 21
- 108020004684 Internal Ribosome Entry Sites Proteins 0.000 description 20
- 239000000306 component Substances 0.000 description 20
- 238000004806 packaging method and process Methods 0.000 description 20
- 239000008194 pharmaceutical composition Substances 0.000 description 20
- 208000002267 Anti-neutrophil cytoplasmic antibody-associated vasculitis Diseases 0.000 description 19
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 17
- 201000010099 disease Diseases 0.000 description 17
- 239000013612 plasmid Substances 0.000 description 17
- 238000012360 testing method Methods 0.000 description 17
- 210000001519 tissue Anatomy 0.000 description 17
- 239000013643 reference control Substances 0.000 description 15
- 108090000565 Capsid Proteins Proteins 0.000 description 14
- 102100023321 Ceruloplasmin Human genes 0.000 description 14
- 238000012217 deletion Methods 0.000 description 14
- 230000037430 deletion Effects 0.000 description 14
- 239000000243 solution Substances 0.000 description 14
- 238000006467 substitution reaction Methods 0.000 description 14
- 108091032973 (ribonucleotides)n+m Proteins 0.000 description 13
- 101100118093 Drosophila melanogaster eEF1alpha2 gene Proteins 0.000 description 13
- 229920002451 polyvinyl alcohol Polymers 0.000 description 13
- 241000701161 unidentified adenovirus Species 0.000 description 13
- 238000001262 western blot Methods 0.000 description 13
- 239000000047 product Substances 0.000 description 12
- 235000020945 retinal Nutrition 0.000 description 12
- 239000011604 retinal Substances 0.000 description 12
- NCYCYZXNIZJOKI-UHFFFAOYSA-N vitamin A aldehyde Natural products O=CC=C(C)C=CC=C(C)C=CC1=C(C)CCCC1(C)C NCYCYZXNIZJOKI-UHFFFAOYSA-N 0.000 description 12
- 239000003814 drug Substances 0.000 description 11
- 239000003623 enhancer Substances 0.000 description 11
- 230000001225 therapeutic effect Effects 0.000 description 11
- 201000004569 Blindness Diseases 0.000 description 10
- 241000282412 Homo Species 0.000 description 10
- 108700028146 Genetic Enhancer Elements Proteins 0.000 description 9
- 241000125945 Protoparvovirus Species 0.000 description 9
- 102100028001 Retinaldehyde-binding protein 1 Human genes 0.000 description 9
- 238000001415 gene therapy Methods 0.000 description 9
- 239000002953 phosphate buffered saline Substances 0.000 description 9
- 230000002265 prevention Effects 0.000 description 9
- 230000000069 prophylactic effect Effects 0.000 description 9
- 125000006850 spacer group Chemical group 0.000 description 9
- 230000004393 visual impairment Effects 0.000 description 9
- 241001164825 Adeno-associated virus - 8 Species 0.000 description 8
- 241000701022 Cytomegalovirus Species 0.000 description 8
- ZHNUHDYFZUAESO-UHFFFAOYSA-N Formamide Chemical compound NC=O ZHNUHDYFZUAESO-UHFFFAOYSA-N 0.000 description 8
- 108010043121 Green Fluorescent Proteins Proteins 0.000 description 8
- 102000004144 Green Fluorescent Proteins Human genes 0.000 description 8
- 241001465754 Metazoa Species 0.000 description 8
- 108091034117 Oligonucleotide Proteins 0.000 description 8
- 230000002068 genetic effect Effects 0.000 description 8
- 239000005090 green fluorescent protein Substances 0.000 description 8
- 239000002609 medium Substances 0.000 description 8
- 230000004048 modification Effects 0.000 description 8
- 238000012986 modification Methods 0.000 description 8
- 210000000608 photoreceptor cell Anatomy 0.000 description 8
- 239000011780 sodium chloride Substances 0.000 description 8
- 241000894007 species Species 0.000 description 8
- 238000002560 therapeutic procedure Methods 0.000 description 8
- 241001655883 Adeno-associated virus - 1 Species 0.000 description 7
- 241000580270 Adeno-associated virus - 4 Species 0.000 description 7
- 241001634120 Adeno-associated virus - 5 Species 0.000 description 7
- 101000734572 Homo sapiens Phosphoenolpyruvate carboxykinase, cytosolic [GTP] Proteins 0.000 description 7
- 101001078886 Homo sapiens Retinaldehyde-binding protein 1 Proteins 0.000 description 7
- 241000699666 Mus <mouse, genus> Species 0.000 description 7
- 102100034796 Phosphoenolpyruvate carboxykinase, cytosolic [GTP] Human genes 0.000 description 7
- 238000007792 addition Methods 0.000 description 7
- 239000000872 buffer Substances 0.000 description 7
- 239000003795 chemical substances by application Substances 0.000 description 7
- 230000003292 diminished effect Effects 0.000 description 7
- 229940079593 drug Drugs 0.000 description 7
- 238000003780 insertion Methods 0.000 description 7
- 230000037431 insertion Effects 0.000 description 7
- 230000010076 replication Effects 0.000 description 7
- 235000000346 sugar Nutrition 0.000 description 7
- 239000013603 viral vector Substances 0.000 description 7
- 241000202702 Adeno-associated virus - 3 Species 0.000 description 6
- 241000972680 Adeno-associated virus - 6 Species 0.000 description 6
- 241001164823 Adeno-associated virus - 7 Species 0.000 description 6
- 241000649045 Adeno-associated virus 10 Species 0.000 description 6
- 108010069112 Complement System Proteins Proteins 0.000 description 6
- 102000000989 Complement System Proteins Human genes 0.000 description 6
- 102000053602 DNA Human genes 0.000 description 6
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 6
- 241000829100 Macaca mulatta polyomavirus 1 Species 0.000 description 6
- 241000283973 Oryctolagus cuniculus Species 0.000 description 6
- 239000000969 carrier Substances 0.000 description 6
- 208000015181 infectious disease Diseases 0.000 description 6
- 108020004999 messenger RNA Proteins 0.000 description 6
- 239000000126 substance Substances 0.000 description 6
- 238000003786 synthesis reaction Methods 0.000 description 6
- 238000001890 transfection Methods 0.000 description 6
- 238000005406 washing Methods 0.000 description 6
- 241000649046 Adeno-associated virus 11 Species 0.000 description 5
- 241000649047 Adeno-associated virus 12 Species 0.000 description 5
- 108700028369 Alleles Proteins 0.000 description 5
- 201000007737 Retinal degeneration Diseases 0.000 description 5
- 208000007014 Retinitis pigmentosa Diseases 0.000 description 5
- 230000015572 biosynthetic process Effects 0.000 description 5
- 230000003247 decreasing effect Effects 0.000 description 5
- 208000035475 disorder Diseases 0.000 description 5
- 238000002474 experimental method Methods 0.000 description 5
- 238000009396 hybridization Methods 0.000 description 5
- 238000003384 imaging method Methods 0.000 description 5
- 230000001939 inductive effect Effects 0.000 description 5
- 239000000463 material Substances 0.000 description 5
- 210000002569 neuron Anatomy 0.000 description 5
- 238000000746 purification Methods 0.000 description 5
- 239000001509 sodium citrate Substances 0.000 description 5
- NLJMYIDDQXHKNR-UHFFFAOYSA-K sodium citrate Chemical compound O.O.[Na+].[Na+].[Na+].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O NLJMYIDDQXHKNR-UHFFFAOYSA-K 0.000 description 5
- 208000024891 symptom Diseases 0.000 description 5
- 230000008685 targeting Effects 0.000 description 5
- 238000013519 translation Methods 0.000 description 5
- 230000004304 visual acuity Effects 0.000 description 5
- 102100027211 Albumin Human genes 0.000 description 4
- 241000283690 Bos taurus Species 0.000 description 4
- 101710163595 Chaperone protein DnaK Proteins 0.000 description 4
- 108020004705 Codon Proteins 0.000 description 4
- 102100031181 Glyceraldehyde-3-phosphate dehydrogenase Human genes 0.000 description 4
- 101710178376 Heat shock 70 kDa protein Proteins 0.000 description 4
- 101710152018 Heat shock cognate 70 kDa protein Proteins 0.000 description 4
- 101000693913 Homo sapiens Albumin Proteins 0.000 description 4
- 102100038300 Metabotropic glutamate receptor 6 Human genes 0.000 description 4
- 102000035195 Peptidases Human genes 0.000 description 4
- 102000010292 Peptide Elongation Factor 1 Human genes 0.000 description 4
- 108010077524 Peptide Elongation Factor 1 Proteins 0.000 description 4
- 206010038923 Retinopathy Diseases 0.000 description 4
- DBMJMQXJHONAFJ-UHFFFAOYSA-M Sodium laurylsulphate Chemical compound [Na+].CCCCCCCCCCCCOS([O-])(=O)=O DBMJMQXJHONAFJ-UHFFFAOYSA-M 0.000 description 4
- 230000002378 acidificating effect Effects 0.000 description 4
- 230000003444 anaesthetic effect Effects 0.000 description 4
- UCMIRNVEIXFBKS-UHFFFAOYSA-N beta-alanine Chemical compound NCCC(O)=O UCMIRNVEIXFBKS-UHFFFAOYSA-N 0.000 description 4
- 238000004113 cell culture Methods 0.000 description 4
- 230000004456 color vision Effects 0.000 description 4
- 150000001875 compounds Chemical class 0.000 description 4
- 230000006378 damage Effects 0.000 description 4
- 238000011161 development Methods 0.000 description 4
- 230000004438 eyesight Effects 0.000 description 4
- 108020004445 glyceraldehyde-3-phosphate dehydrogenase Proteins 0.000 description 4
- 210000002287 horizontal cell Anatomy 0.000 description 4
- 230000001976 improved effect Effects 0.000 description 4
- 239000012535 impurity Substances 0.000 description 4
- 239000007788 liquid Substances 0.000 description 4
- 108010038450 metabotropic glutamate receptor 6 Proteins 0.000 description 4
- 238000010369 molecular cloning Methods 0.000 description 4
- 210000004498 neuroglial cell Anatomy 0.000 description 4
- 229920001983 poloxamer Polymers 0.000 description 4
- 239000000523 sample Substances 0.000 description 4
- FSYKKLYZXJSNPZ-UHFFFAOYSA-N sarcosine Chemical compound C[NH2+]CC([O-])=O FSYKKLYZXJSNPZ-UHFFFAOYSA-N 0.000 description 4
- 210000002966 serum Anatomy 0.000 description 4
- 238000010561 standard procedure Methods 0.000 description 4
- 229940124597 therapeutic agent Drugs 0.000 description 4
- 238000013518 transcription Methods 0.000 description 4
- 230000035897 transcription Effects 0.000 description 4
- 241001529453 unidentified herpesvirus Species 0.000 description 4
- 210000002845 virion Anatomy 0.000 description 4
- 230000000007 visual effect Effects 0.000 description 4
- 102000040650 (ribonucleotides)n+m Human genes 0.000 description 3
- 102000053642 Catalytic RNA Human genes 0.000 description 3
- 108090000994 Catalytic RNA Proteins 0.000 description 3
- 108010005939 Ciliary Neurotrophic Factor Proteins 0.000 description 3
- 102100031614 Ciliary neurotrophic factor Human genes 0.000 description 3
- 108091026890 Coding region Proteins 0.000 description 3
- 102000004190 Enzymes Human genes 0.000 description 3
- 108090000790 Enzymes Proteins 0.000 description 3
- 102000004961 Furin Human genes 0.000 description 3
- 101000834253 Gallus gallus Actin, cytoplasmic 1 Proteins 0.000 description 3
- 208000010412 Glaucoma Diseases 0.000 description 3
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 3
- 101000729271 Homo sapiens Retinoid isomerohydrolase Proteins 0.000 description 3
- PMMYEEVYMWASQN-DMTCNVIQSA-N Hydroxyproline Chemical compound O[C@H]1CN[C@H](C(O)=O)C1 PMMYEEVYMWASQN-DMTCNVIQSA-N 0.000 description 3
- 108091092195 Intron Proteins 0.000 description 3
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical compound OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 description 3
- ROHFNLRQFUQHCH-YFKPBYRVSA-N L-leucine Chemical compound CC(C)C[C@H](N)C(O)=O ROHFNLRQFUQHCH-YFKPBYRVSA-N 0.000 description 3
- LRQKBLKVPFOOQJ-YFKPBYRVSA-N L-norleucine Chemical compound CCCC[C@H]([NH3+])C([O-])=O LRQKBLKVPFOOQJ-YFKPBYRVSA-N 0.000 description 3
- 241000699670 Mus sp. Species 0.000 description 3
- 208000006550 Mydriasis Diseases 0.000 description 3
- 102000010175 Opsin Human genes 0.000 description 3
- 108050001704 Opsin Proteins 0.000 description 3
- 102100031176 Retinoid isomerohydrolase Human genes 0.000 description 3
- 108010034546 Serratia marcescens nuclease Proteins 0.000 description 3
- JLCPHMBAVCMARE-UHFFFAOYSA-N [3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-hydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methyl [5-(6-aminopurin-9-yl)-2-(hydroxymethyl)oxolan-3-yl] hydrogen phosphate Polymers Cc1cn(C2CC(OP(O)(=O)OCC3OC(CC3OP(O)(=O)OCC3OC(CC3O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c3nc(N)[nH]c4=O)C(COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3CO)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cc(C)c(=O)[nH]c3=O)n3cc(C)c(=O)[nH]c3=O)n3ccc(N)nc3=O)n3cc(C)c(=O)[nH]c3=O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)O2)c(=O)[nH]c1=O JLCPHMBAVCMARE-UHFFFAOYSA-N 0.000 description 3
- 239000000370 acceptor Substances 0.000 description 3
- 210000000411 amacrine cell Anatomy 0.000 description 3
- 238000004458 analytical method Methods 0.000 description 3
- 230000002491 angiogenic effect Effects 0.000 description 3
- 230000000692 anti-sense effect Effects 0.000 description 3
- 238000003556 assay Methods 0.000 description 3
- 230000008901 benefit Effects 0.000 description 3
- 230000033228 biological regulation Effects 0.000 description 3
- 230000000903 blocking effect Effects 0.000 description 3
- 239000011575 calcium Substances 0.000 description 3
- 239000000356 contaminant Substances 0.000 description 3
- 238000004132 cross linking Methods 0.000 description 3
- 210000000695 crystalline len Anatomy 0.000 description 3
- 230000007850 degeneration Effects 0.000 description 3
- 238000010790 dilution Methods 0.000 description 3
- 239000012895 dilution Substances 0.000 description 3
- PMMYEEVYMWASQN-UHFFFAOYSA-N dl-hydroxyproline Natural products OC1C[NH2+]C(C([O-])=O)C1 PMMYEEVYMWASQN-UHFFFAOYSA-N 0.000 description 3
- 230000009977 dual effect Effects 0.000 description 3
- 238000001914 filtration Methods 0.000 description 3
- 238000009472 formulation Methods 0.000 description 3
- 239000000499 gel Substances 0.000 description 3
- 238000003306 harvesting Methods 0.000 description 3
- 229960002591 hydroxyproline Drugs 0.000 description 3
- 238000003364 immunohistochemistry Methods 0.000 description 3
- 230000006872 improvement Effects 0.000 description 3
- 238000001727 in vivo Methods 0.000 description 3
- 230000001965 increasing effect Effects 0.000 description 3
- 238000011068 loading method Methods 0.000 description 3
- 238000005259 measurement Methods 0.000 description 3
- 229910052751 metal Inorganic materials 0.000 description 3
- 239000002184 metal Substances 0.000 description 3
- 150000002739 metals Chemical class 0.000 description 3
- 238000002360 preparation method Methods 0.000 description 3
- 230000008569 process Effects 0.000 description 3
- 238000005215 recombination Methods 0.000 description 3
- 230000006798 recombination Effects 0.000 description 3
- 238000011160 research Methods 0.000 description 3
- 230000004258 retinal degeneration Effects 0.000 description 3
- 239000000790 retinal pigment Substances 0.000 description 3
- 210000000844 retinal pigment epithelial cell Anatomy 0.000 description 3
- 108091092562 ribozyme Proteins 0.000 description 3
- 150000003839 salts Chemical class 0.000 description 3
- 230000035945 sensitivity Effects 0.000 description 3
- 238000004659 sterilization and disinfection Methods 0.000 description 3
- 238000003756 stirring Methods 0.000 description 3
- 239000006228 supernatant Substances 0.000 description 3
- 230000008719 thickening Effects 0.000 description 3
- 238000010361 transduction Methods 0.000 description 3
- 230000026683 transduction Effects 0.000 description 3
- 238000003151 transfection method Methods 0.000 description 3
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 3
- GVJHHUAWPYXKBD-UHFFFAOYSA-N (±)-α-Tocopherol Chemical compound OC1=C(C)C(C)=C2OC(CCCC(C)CCCC(C)CCCC(C)C)(C)CCC2=C1C GVJHHUAWPYXKBD-UHFFFAOYSA-N 0.000 description 2
- CPKVUHPKYQGHMW-UHFFFAOYSA-N 1-ethenylpyrrolidin-2-one;molecular iodine Chemical compound II.C=CN1CCCC1=O CPKVUHPKYQGHMW-UHFFFAOYSA-N 0.000 description 2
- FUOOLUPWFVMBKG-UHFFFAOYSA-N 2-Aminoisobutyric acid Chemical compound CC(C)(N)C(O)=O FUOOLUPWFVMBKG-UHFFFAOYSA-N 0.000 description 2
- FWMNVWWHGCHHJJ-SKKKGAJSSA-N 4-amino-1-[(2r)-6-amino-2-[[(2r)-2-[[(2r)-2-[[(2r)-2-amino-3-phenylpropanoyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoyl]amino]hexanoyl]piperidine-4-carboxylic acid Chemical compound C([C@H](C(=O)N[C@H](CC(C)C)C(=O)N[C@H](CCCCN)C(=O)N1CCC(N)(CC1)C(O)=O)NC(=O)[C@H](N)CC=1C=CC=CC=1)C1=CC=CC=C1 FWMNVWWHGCHHJJ-SKKKGAJSSA-N 0.000 description 2
- CIWBSHSKHKDKBQ-JLAZNSOCSA-N Ascorbic acid Chemical compound OC[C@H](O)[C@H]1OC(=O)C(O)=C1O CIWBSHSKHKDKBQ-JLAZNSOCSA-N 0.000 description 2
- 102100026189 Beta-galactosidase Human genes 0.000 description 2
- 102100022002 CD59 glycoprotein Human genes 0.000 description 2
- 101150044789 Cap gene Proteins 0.000 description 2
- 241000283707 Capra Species 0.000 description 2
- 108010035563 Chloramphenicol O-acetyltransferase Proteins 0.000 description 2
- 102100031191 Cilia- and flagella-associated protein 91 Human genes 0.000 description 2
- 108091062157 Cis-regulatory element Proteins 0.000 description 2
- 108010078015 Complement C3b Proteins 0.000 description 2
- 102100025680 Complement decay-accelerating factor Human genes 0.000 description 2
- 108091035707 Consensus sequence Proteins 0.000 description 2
- SRBFZHDQGSBBOR-IOVATXLUSA-N D-xylopyranose Chemical compound O[C@@H]1COC(O)[C@H](O)[C@H]1O SRBFZHDQGSBBOR-IOVATXLUSA-N 0.000 description 2
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 2
- 108090000204 Dipeptidase 1 Proteins 0.000 description 2
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 2
- 238000002965 ELISA Methods 0.000 description 2
- 241000196324 Embryophyta Species 0.000 description 2
- 241000991587 Enterovirus C Species 0.000 description 2
- 241000283074 Equus asinus Species 0.000 description 2
- 101000823089 Equus caballus Alpha-1-antiproteinase 1 Proteins 0.000 description 2
- 208000036357 GUCY2D-related recessive retinopathy Diseases 0.000 description 2
- 102000018932 HSP70 Heat-Shock Proteins Human genes 0.000 description 2
- 108010027992 HSP70 Heat-Shock Proteins Proteins 0.000 description 2
- 101710154606 Hemagglutinin Proteins 0.000 description 2
- 241000238631 Hexapoda Species 0.000 description 2
- 101000897400 Homo sapiens CD59 glycoprotein Proteins 0.000 description 2
- 101000776592 Homo sapiens Cilia- and flagella-associated protein 91 Proteins 0.000 description 2
- 101000856022 Homo sapiens Complement decay-accelerating factor Proteins 0.000 description 2
- 101000961414 Homo sapiens Membrane cofactor protein Proteins 0.000 description 2
- 108091006905 Human Serum Albumin Proteins 0.000 description 2
- 102000008100 Human Serum Albumin Human genes 0.000 description 2
- 108700005091 Immunoglobulin Genes Proteins 0.000 description 2
- 201000003533 Leber congenital amaurosis Diseases 0.000 description 2
- ROHFNLRQFUQHCH-UHFFFAOYSA-N Leucine Natural products CC(C)CC(N)C(O)=O ROHFNLRQFUQHCH-UHFFFAOYSA-N 0.000 description 2
- 108060001084 Luciferase Proteins 0.000 description 2
- 241000124008 Mammalia Species 0.000 description 2
- 102100039373 Membrane cofactor protein Human genes 0.000 description 2
- 108010025020 Nerve Growth Factor Proteins 0.000 description 2
- 102000007072 Nerve Growth Factors Human genes 0.000 description 2
- 101710093908 Outer capsid protein VP4 Proteins 0.000 description 2
- 101710135467 Outer capsid protein sigma-1 Proteins 0.000 description 2
- 239000002202 Polyethylene glycol Substances 0.000 description 2
- 101710176177 Protein A56 Proteins 0.000 description 2
- 101800001295 Putative ATP-dependent helicase Proteins 0.000 description 2
- 101800001006 Putative helicase Proteins 0.000 description 2
- 206010038848 Retinal detachment Diseases 0.000 description 2
- 101710101931 Retinaldehyde-binding protein 1 Proteins 0.000 description 2
- 101710137010 Retinol-binding protein 3 Proteins 0.000 description 2
- 102100038247 Retinol-binding protein 3 Human genes 0.000 description 2
- 240000004808 Saccharomyces cerevisiae Species 0.000 description 2
- 102000012479 Serine Proteases Human genes 0.000 description 2
- 108010022999 Serine Proteases Proteins 0.000 description 2
- 108091027967 Small hairpin RNA Proteins 0.000 description 2
- 241001492404 Woodchuck hepatitis virus Species 0.000 description 2
- 208000027418 Wounds and injury Diseases 0.000 description 2
- DHKHKXVYLBGOIT-UHFFFAOYSA-N acetaldehyde Diethyl Acetal Natural products CCOC(C)OCC DHKHKXVYLBGOIT-UHFFFAOYSA-N 0.000 description 2
- 125000002777 acetyl group Chemical class [H]C([H])([H])C(*)=O 0.000 description 2
- 239000002253 acid Substances 0.000 description 2
- 230000004913 activation Effects 0.000 description 2
- 230000003044 adaptive effect Effects 0.000 description 2
- 125000000539 amino acid group Chemical group 0.000 description 2
- 230000003321 amplification Effects 0.000 description 2
- PYMYPHUHKUWMLA-UHFFFAOYSA-N arabinose Natural products OCC(O)C(O)C(O)C=O PYMYPHUHKUWMLA-UHFFFAOYSA-N 0.000 description 2
- 235000019568 aromas Nutrition 0.000 description 2
- SRBFZHDQGSBBOR-UHFFFAOYSA-N beta-D-Pyranose-Lyxose Natural products OC1COC(O)C(O)C1O SRBFZHDQGSBBOR-UHFFFAOYSA-N 0.000 description 2
- 108010005774 beta-Galactosidase Proteins 0.000 description 2
- 102000006635 beta-lactamase Human genes 0.000 description 2
- 230000004071 biological effect Effects 0.000 description 2
- 229910052799 carbon Inorganic materials 0.000 description 2
- 210000003161 choroid Anatomy 0.000 description 2
- 210000000349 chromosome Anatomy 0.000 description 2
- 238000004140 cleaning Methods 0.000 description 2
- 238000007796 conventional method Methods 0.000 description 2
- 238000009295 crossflow filtration Methods 0.000 description 2
- 230000001086 cytosolic effect Effects 0.000 description 2
- YSMODUONRAFBET-UHFFFAOYSA-N delta-DL-hydroxylysine Natural products NCC(O)CCC(N)C(O)=O YSMODUONRAFBET-UHFFFAOYSA-N 0.000 description 2
- 230000001419 dependent effect Effects 0.000 description 2
- 230000008021 deposition Effects 0.000 description 2
- 239000000645 desinfectant Substances 0.000 description 2
- 238000001514 detection method Methods 0.000 description 2
- 238000005538 encapsulation Methods 0.000 description 2
- YSMODUONRAFBET-UHNVWZDZSA-N erythro-5-hydroxy-L-lysine Chemical compound NC[C@H](O)CC[C@H](N)C(O)=O YSMODUONRAFBET-UHNVWZDZSA-N 0.000 description 2
- 210000000720 eyelash Anatomy 0.000 description 2
- 210000000744 eyelid Anatomy 0.000 description 2
- 238000000799 fluorescence microscopy Methods 0.000 description 2
- BTCSSZJGUNDROE-UHFFFAOYSA-N gamma-aminobutyric acid Chemical compound NCCCC(O)=O BTCSSZJGUNDROE-UHFFFAOYSA-N 0.000 description 2
- 230000009368 gene silencing by RNA Effects 0.000 description 2
- 239000008103 glucose Substances 0.000 description 2
- 102000045512 human CFH Human genes 0.000 description 2
- 230000002209 hydrophobic effect Effects 0.000 description 2
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 2
- 238000000338 in vitro Methods 0.000 description 2
- 238000010348 incorporation Methods 0.000 description 2
- 230000002458 infectious effect Effects 0.000 description 2
- 208000014674 injury Diseases 0.000 description 2
- 230000010354 integration Effects 0.000 description 2
- 238000001990 intravenous administration Methods 0.000 description 2
- 208000017169 kidney disease Diseases 0.000 description 2
- 238000002372 labelling Methods 0.000 description 2
- 239000006166 lysate Substances 0.000 description 2
- 210000004962 mammalian cell Anatomy 0.000 description 2
- 238000013507 mapping Methods 0.000 description 2
- 239000003900 neurotrophic factor Substances 0.000 description 2
- 230000007935 neutral effect Effects 0.000 description 2
- 238000003199 nucleic acid amplification method Methods 0.000 description 2
- 238000012014 optical coherence tomography Methods 0.000 description 2
- 230000005043 peripheral vision Effects 0.000 description 2
- 239000000546 pharmaceutical excipient Substances 0.000 description 2
- 239000000049 pigment Substances 0.000 description 2
- 229920001223 polyethylene glycol Polymers 0.000 description 2
- 102000054765 polymorphisms of proteins Human genes 0.000 description 2
- 230000001124 posttranscriptional effect Effects 0.000 description 2
- 238000012545 processing Methods 0.000 description 2
- 125000006239 protecting group Chemical group 0.000 description 2
- 238000003127 radioimmunoassay Methods 0.000 description 2
- 230000009467 reduction Effects 0.000 description 2
- 230000011514 reflex Effects 0.000 description 2
- 230000022532 regulation of transcription, DNA-dependent Effects 0.000 description 2
- 101150066583 rep gene Proteins 0.000 description 2
- 230000004264 retinal detachment Effects 0.000 description 2
- 230000028327 secretion Effects 0.000 description 2
- 230000001568 sexual effect Effects 0.000 description 2
- 238000001542 size-exclusion chromatography Methods 0.000 description 2
- 239000004055 small Interfering RNA Substances 0.000 description 2
- FQENQNTWSFEDLI-UHFFFAOYSA-J sodium diphosphate Chemical compound [Na+].[Na+].[Na+].[Na+].[O-]P([O-])(=O)OP([O-])([O-])=O FQENQNTWSFEDLI-UHFFFAOYSA-J 0.000 description 2
- 229940048086 sodium pyrophosphate Drugs 0.000 description 2
- 239000007787 solid Substances 0.000 description 2
- 239000003381 stabilizer Substances 0.000 description 2
- 150000008163 sugars Chemical class 0.000 description 2
- 230000001629 suppression Effects 0.000 description 2
- 230000009885 systemic effect Effects 0.000 description 2
- 235000019818 tetrasodium diphosphate Nutrition 0.000 description 2
- 239000001577 tetrasodium phosphonato phosphate Substances 0.000 description 2
- 230000000699 topical effect Effects 0.000 description 2
- KBPHJBAIARWVSC-XQIHNALSSA-N trans-lutein Natural products CC(=C/C=C/C=C(C)/C=C/C=C(C)/C=C/C1=C(C)CC(O)CC1(C)C)C=CC=C(/C)C=CC2C(=CC(O)CC2(C)C)C KBPHJBAIARWVSC-XQIHNALSSA-N 0.000 description 2
- 230000005026 transcription initiation Effects 0.000 description 2
- 241000701447 unidentified baculovirus Species 0.000 description 2
- 108700026220 vif Genes Proteins 0.000 description 2
- 235000013343 vitamin Nutrition 0.000 description 2
- 239000011782 vitamin Substances 0.000 description 2
- 229930003231 vitamin Natural products 0.000 description 2
- 229940088594 vitamin Drugs 0.000 description 2
- JKQXZKUSFCKOGQ-JLGXGRJMSA-N (3R,3'R)-beta,beta-carotene-3,3'-diol Chemical compound C([C@H](O)CC=1C)C(C)(C)C=1/C=C/C(/C)=C/C=C/C(/C)=C/C=C/C=C(C)C=CC=C(C)C=CC1=C(C)C[C@@H](O)CC1(C)C JKQXZKUSFCKOGQ-JLGXGRJMSA-N 0.000 description 1
- ORKBYCQJWQBPFG-WOMZHKBXSA-N (8r,9s,10r,13s,14s,17r)-13-ethyl-17-ethynyl-17-hydroxy-1,2,6,7,8,9,10,11,12,14,15,16-dodecahydrocyclopenta[a]phenanthren-3-one;(8r,9s,13s,14s,17r)-17-ethynyl-13-methyl-7,8,9,11,12,14,15,16-octahydro-6h-cyclopenta[a]phenanthrene-3,17-diol Chemical compound OC1=CC=C2[C@H]3CC[C@](C)([C@](CC4)(O)C#C)[C@@H]4[C@@H]3CCC2=C1.O=C1CC[C@@H]2[C@H]3CC[C@](CC)([C@](CC4)(O)C#C)[C@@H]4[C@@H]3CCC2=C1 ORKBYCQJWQBPFG-WOMZHKBXSA-N 0.000 description 1
- NCYCYZXNIZJOKI-IOUUIBBYSA-N 11-cis-retinal Chemical compound O=C/C=C(\C)/C=C\C=C(/C)\C=C\C1=C(C)CCCC1(C)C NCYCYZXNIZJOKI-IOUUIBBYSA-N 0.000 description 1
- BLCJBICVQSYOIF-UHFFFAOYSA-N 2,2-diaminobutanoic acid Chemical compound CCC(N)(N)C(O)=O BLCJBICVQSYOIF-UHFFFAOYSA-N 0.000 description 1
- JKMHFZQWWAIEOD-UHFFFAOYSA-N 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid Chemical compound OCC[NH+]1CCN(CCS([O-])(=O)=O)CC1 JKMHFZQWWAIEOD-UHFFFAOYSA-N 0.000 description 1
- OYIFNHCXNCRBQI-UHFFFAOYSA-N 2-aminoadipic acid Chemical compound OC(=O)C(N)CCCC(O)=O OYIFNHCXNCRBQI-UHFFFAOYSA-N 0.000 description 1
- RDFMDVXONNIGBC-UHFFFAOYSA-N 2-aminoheptanoic acid Chemical compound CCCCCC(N)C(O)=O RDFMDVXONNIGBC-UHFFFAOYSA-N 0.000 description 1
- BRMWTNUJHUMWMS-UHFFFAOYSA-N 3-Methylhistidine Natural products CN1C=NC(CC(N)C(O)=O)=C1 BRMWTNUJHUMWMS-UHFFFAOYSA-N 0.000 description 1
- PECYZEOJVXMISF-UHFFFAOYSA-N 3-aminoalanine Chemical compound [NH3+]CC(N)C([O-])=O PECYZEOJVXMISF-UHFFFAOYSA-N 0.000 description 1
- 229940117976 5-hydroxylysine Drugs 0.000 description 1
- SLXKOJJOQWFEFD-UHFFFAOYSA-N 6-aminohexanoic acid Chemical compound NCCCCCC(O)=O SLXKOJJOQWFEFD-UHFFFAOYSA-N 0.000 description 1
- ZCYVEMRRCGMTRW-UHFFFAOYSA-N 7553-56-2 Chemical compound [I] ZCYVEMRRCGMTRW-UHFFFAOYSA-N 0.000 description 1
- 101150016901 ALB1 gene Proteins 0.000 description 1
- 208000010370 Adenoviridae Infections Diseases 0.000 description 1
- 206010060931 Adenovirus infection Diseases 0.000 description 1
- 102000002260 Alkaline Phosphatase Human genes 0.000 description 1
- 108020004774 Alkaline Phosphatase Proteins 0.000 description 1
- 108020005544 Antisense RNA Proteins 0.000 description 1
- 229940088872 Apoptosis inhibitor Drugs 0.000 description 1
- 101100274294 Arabidopsis thaliana CHLD gene Proteins 0.000 description 1
- 101100365087 Arabidopsis thaliana SCRA gene Proteins 0.000 description 1
- 241000972773 Aulopiformes Species 0.000 description 1
- 208000037663 Best vitelliform macular dystrophy Diseases 0.000 description 1
- 102100022794 Bestrophin-1 Human genes 0.000 description 1
- 108010087504 Beta-Globulins Proteins 0.000 description 1
- 208000019838 Blood disease Diseases 0.000 description 1
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 1
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 1
- 241000282832 Camelidae Species 0.000 description 1
- 241000282472 Canis lupus familiaris Species 0.000 description 1
- KXDHJXZQYSOELW-UHFFFAOYSA-M Carbamate Chemical compound NC([O-])=O KXDHJXZQYSOELW-UHFFFAOYSA-M 0.000 description 1
- 108091006146 Channels Proteins 0.000 description 1
- 208000005590 Choroidal Neovascularization Diseases 0.000 description 1
- 206010060823 Choroidal neovascularisation Diseases 0.000 description 1
- 206010010356 Congenital anomaly Diseases 0.000 description 1
- 206010010541 Congenital melanosis Diseases 0.000 description 1
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 1
- ZZZCUOFIHGPKAK-UHFFFAOYSA-N D-erythro-ascorbic acid Natural products OCC1OC(=O)C(O)=C1O ZZZCUOFIHGPKAK-UHFFFAOYSA-N 0.000 description 1
- HMFHBZSHGGEWLO-SOOFDHNKSA-N D-ribofuranose Chemical compound OC[C@H]1OC(O)[C@H](O)[C@@H]1O HMFHBZSHGGEWLO-SOOFDHNKSA-N 0.000 description 1
- 230000004543 DNA replication Effects 0.000 description 1
- 230000006820 DNA synthesis Effects 0.000 description 1
- 102000016928 DNA-directed DNA polymerase Human genes 0.000 description 1
- 108010014303 DNA-directed DNA polymerase Proteins 0.000 description 1
- 108090000626 DNA-directed RNA polymerases Proteins 0.000 description 1
- 102000004163 DNA-directed RNA polymerases Human genes 0.000 description 1
- 108010053770 Deoxyribonucleases Proteins 0.000 description 1
- 102000016911 Deoxyribonucleases Human genes 0.000 description 1
- 206010012689 Diabetic retinopathy Diseases 0.000 description 1
- 101001031598 Dictyostelium discoideum Probable serine/threonine-protein kinase fhkC Proteins 0.000 description 1
- 239000006144 Dulbecco’s modified Eagle's medium Substances 0.000 description 1
- 102100037024 E3 ubiquitin-protein ligase XIAP Human genes 0.000 description 1
- 241000282326 Felis catus Species 0.000 description 1
- 229920001917 Ficoll Polymers 0.000 description 1
- 239000007995 HEPES buffer Substances 0.000 description 1
- 208000032087 Hereditary Leber Optic Atrophy Diseases 0.000 description 1
- 101000903449 Homo sapiens Bestrophin-1 Proteins 0.000 description 1
- 101001065295 Homo sapiens Fas-binding factor 1 Proteins 0.000 description 1
- 101001031607 Homo sapiens Four and a half LIM domains protein 1 Proteins 0.000 description 1
- 101000887490 Homo sapiens Guanine nucleotide-binding protein G(z) subunit alpha Proteins 0.000 description 1
- 101000878605 Homo sapiens Low affinity immunoglobulin epsilon Fc receptor Proteins 0.000 description 1
- 101000597428 Homo sapiens Nucleoredoxin-like protein 1 Proteins 0.000 description 1
- 101000594760 Homo sapiens Nucleoredoxin-like protein 2 Proteins 0.000 description 1
- 101000741544 Homo sapiens Properdin Proteins 0.000 description 1
- 101000829506 Homo sapiens Rhodopsin kinase GRK1 Proteins 0.000 description 1
- 241000700588 Human alphaherpesvirus 1 Species 0.000 description 1
- LCWXJXMHJVIJFK-UHFFFAOYSA-N Hydroxylysine Natural products NCC(O)CC(N)CC(O)=O LCWXJXMHJVIJFK-UHFFFAOYSA-N 0.000 description 1
- 206010020772 Hypertension Diseases 0.000 description 1
- 208000026350 Inborn Genetic disease Diseases 0.000 description 1
- 102100037644 Kelch-like protein 41 Human genes 0.000 description 1
- 108050003242 Kelch-like protein 41 Proteins 0.000 description 1
- SNDPXSYFESPGGJ-BYPYZUCNSA-N L-2-aminopentanoic acid Chemical compound CCC[C@H](N)C(O)=O SNDPXSYFESPGGJ-BYPYZUCNSA-N 0.000 description 1
- AHLPHDHHMVZTML-BYPYZUCNSA-N L-Ornithine Chemical compound NCCC[C@H](N)C(O)=O AHLPHDHHMVZTML-BYPYZUCNSA-N 0.000 description 1
- AGPKZVBTJJNPAG-UHNVWZDZSA-N L-allo-Isoleucine Chemical compound CC[C@@H](C)[C@H](N)C(O)=O AGPKZVBTJJNPAG-UHNVWZDZSA-N 0.000 description 1
- SNDPXSYFESPGGJ-UHFFFAOYSA-N L-norVal-OH Natural products CCCC(N)C(O)=O SNDPXSYFESPGGJ-UHFFFAOYSA-N 0.000 description 1
- 201000000639 Leber hereditary optic neuropathy Diseases 0.000 description 1
- 206010024380 Leukoderma Diseases 0.000 description 1
- NNJVILVZKWQKPM-UHFFFAOYSA-N Lidocaine Chemical compound CCN(CC)CC(=O)NC1=C(C)C=CC=C1C NNJVILVZKWQKPM-UHFFFAOYSA-N 0.000 description 1
- 102100038007 Low affinity immunoglobulin epsilon Fc receptor Human genes 0.000 description 1
- 239000005089 Luciferase Substances 0.000 description 1
- 208000015439 Lysosomal storage disease Diseases 0.000 description 1
- 241000282567 Macaca fascicularis Species 0.000 description 1
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 1
- 241000713869 Moloney murine leukemia virus Species 0.000 description 1
- 101100434646 Mus musculus Alb gene Proteins 0.000 description 1
- 101000597433 Mus musculus Nucleoredoxin-like protein 1 Proteins 0.000 description 1
- DTERQYGMUDWYAZ-ZETCQYMHSA-N N(6)-acetyl-L-lysine Chemical compound CC(=O)NCCCC[C@H]([NH3+])C([O-])=O DTERQYGMUDWYAZ-ZETCQYMHSA-N 0.000 description 1
- JDHILDINMRGULE-LURJTMIESA-N N(pros)-methyl-L-histidine Chemical compound CN1C=NC=C1C[C@H](N)C(O)=O JDHILDINMRGULE-LURJTMIESA-N 0.000 description 1
- JJIHLJJYMXLCOY-BYPYZUCNSA-N N-acetyl-L-serine Chemical compound CC(=O)N[C@@H](CO)C(O)=O JJIHLJJYMXLCOY-BYPYZUCNSA-N 0.000 description 1
- OLNLSTNFRUFTLM-BYPYZUCNSA-N N-ethyl-L-asparagine Chemical compound CCN[C@H](C(O)=O)CC(N)=O OLNLSTNFRUFTLM-BYPYZUCNSA-N 0.000 description 1
- YPIGGYHFMKJNKV-UHFFFAOYSA-N N-ethylglycine Chemical compound CC[NH2+]CC([O-])=O YPIGGYHFMKJNKV-UHFFFAOYSA-N 0.000 description 1
- 108010065338 N-ethylglycine Proteins 0.000 description 1
- PYUSHNKNPOHWEZ-YFKPBYRVSA-N N-formyl-L-methionine Chemical compound CSCC[C@@H](C(O)=O)NC=O PYUSHNKNPOHWEZ-YFKPBYRVSA-N 0.000 description 1
- AKCRVYNORCOYQT-YFKPBYRVSA-N N-methyl-L-valine Chemical compound CN[C@@H](C(C)C)C(O)=O AKCRVYNORCOYQT-YFKPBYRVSA-N 0.000 description 1
- 206010028980 Neoplasm Diseases 0.000 description 1
- 208000001140 Night Blindness Diseases 0.000 description 1
- 108091092724 Noncoding DNA Proteins 0.000 description 1
- 108020004485 Nonsense Codon Proteins 0.000 description 1
- 101710163270 Nuclease Proteins 0.000 description 1
- 102100035399 Nucleoredoxin-like protein 1 Human genes 0.000 description 1
- 102100036205 Nucleoredoxin-like protein 2 Human genes 0.000 description 1
- 108010038807 Oligopeptides Proteins 0.000 description 1
- 102000015636 Oligopeptides Human genes 0.000 description 1
- AHLPHDHHMVZTML-UHFFFAOYSA-N Orn-delta-NH2 Natural products NCCCC(N)C(O)=O AHLPHDHHMVZTML-UHFFFAOYSA-N 0.000 description 1
- UTJLXEIPEHZYQJ-UHFFFAOYSA-N Ornithine Natural products OC(=O)C(C)CCCN UTJLXEIPEHZYQJ-UHFFFAOYSA-N 0.000 description 1
- 229910019142 PO4 Inorganic materials 0.000 description 1
- 235000019483 Peanut oil Nutrition 0.000 description 1
- ABLZXFCXXLZCGV-UHFFFAOYSA-N Phosphorous acid Chemical group OP(O)=O ABLZXFCXXLZCGV-UHFFFAOYSA-N 0.000 description 1
- 102100029533 Photoreceptor-specific nuclear receptor Human genes 0.000 description 1
- 101710164507 Photoreceptor-specific nuclear receptor Proteins 0.000 description 1
- 102100035846 Pigment epithelium-derived factor Human genes 0.000 description 1
- 208000008601 Polycythemia Diseases 0.000 description 1
- 229920001213 Polysorbate 20 Polymers 0.000 description 1
- 229920000153 Povidone-iodine Polymers 0.000 description 1
- 241000288906 Primates Species 0.000 description 1
- 108010076504 Protein Sorting Signals Proteins 0.000 description 1
- 238000012228 RNA interference-mediated gene silencing Methods 0.000 description 1
- 108091030071 RNAI Proteins 0.000 description 1
- 238000011529 RT qPCR Methods 0.000 description 1
- 241000700159 Rattus Species 0.000 description 1
- 108010008281 Recombinant Fusion Proteins Proteins 0.000 description 1
- 102000007056 Recombinant Fusion Proteins Human genes 0.000 description 1
- 108091081062 Repeated sequence (DNA) Proteins 0.000 description 1
- 208000004453 Retinal Dysplasia Diseases 0.000 description 1
- 206010057430 Retinal injury Diseases 0.000 description 1
- 206010038910 Retinitis Diseases 0.000 description 1
- 102100040756 Rhodopsin Human genes 0.000 description 1
- 108090000820 Rhodopsin Proteins 0.000 description 1
- 102100023742 Rhodopsin kinase GRK1 Human genes 0.000 description 1
- 108091028664 Ribonucleotide Proteins 0.000 description 1
- PYMYPHUHKUWMLA-LMVFSUKVSA-N Ribose Natural products OC[C@@H](O)[C@@H](O)[C@@H](O)C=O PYMYPHUHKUWMLA-LMVFSUKVSA-N 0.000 description 1
- 102000005801 Rod Opsins Human genes 0.000 description 1
- 108010005063 Rod Opsins Proteins 0.000 description 1
- 241000283984 Rodentia Species 0.000 description 1
- 101150105073 SCR1 gene Proteins 0.000 description 1
- 101100134054 Saccharomyces cerevisiae (strain ATCC 204508 / S288c) NTG1 gene Proteins 0.000 description 1
- 108010077895 Sarcosine Proteins 0.000 description 1
- 206010040844 Skin exfoliation Diseases 0.000 description 1
- 108020004459 Small interfering RNA Proteins 0.000 description 1
- 241000282887 Suidae Species 0.000 description 1
- 101710191977 Survival factor 2 Proteins 0.000 description 1
- 108010022394 Threonine synthase Proteins 0.000 description 1
- 102000006601 Thymidine Kinase Human genes 0.000 description 1
- 108020004440 Thymidine kinase Proteins 0.000 description 1
- 102000040945 Transcription factor Human genes 0.000 description 1
- 108091023040 Transcription factor Proteins 0.000 description 1
- 102000006612 Transducin Human genes 0.000 description 1
- 108010087042 Transducin Proteins 0.000 description 1
- 108020004566 Transfer RNA Proteins 0.000 description 1
- 239000013504 Triton X-100 Substances 0.000 description 1
- 229920004890 Triton X-100 Polymers 0.000 description 1
- 108091034131 VA RNA Proteins 0.000 description 1
- 241000700618 Vaccinia virus Species 0.000 description 1
- 229930003268 Vitamin C Natural products 0.000 description 1
- 229930003427 Vitamin E Natural products 0.000 description 1
- 108700031544 X-Linked Inhibitor of Apoptosis Proteins 0.000 description 1
- JKQXZKUSFCKOGQ-LQFQNGICSA-N Z-zeaxanthin Natural products C([C@H](O)CC=1C)C(C)(C)C=1C=CC(C)=CC=CC(C)=CC=CC=C(C)C=CC=C(C)C=CC1=C(C)C[C@@H](O)CC1(C)C JKQXZKUSFCKOGQ-LQFQNGICSA-N 0.000 description 1
- QOPRSMDTRDMBNK-RNUUUQFGSA-N Zeaxanthin Natural products CC(=C/C=C/C=C(C)/C=C/C=C(C)/C=C/C1=C(C)CCC(O)C1(C)C)C=CC=C(/C)C=CC2=C(C)CC(O)CC2(C)C QOPRSMDTRDMBNK-RNUUUQFGSA-N 0.000 description 1
- HCHKCACWOHOZIP-UHFFFAOYSA-N Zinc Chemical compound [Zn] HCHKCACWOHOZIP-UHFFFAOYSA-N 0.000 description 1
- 230000005856 abnormality Effects 0.000 description 1
- 238000009825 accumulation Methods 0.000 description 1
- 230000021736 acetylation Effects 0.000 description 1
- 238000006640 acetylation reaction Methods 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 125000002015 acyclic group Chemical group 0.000 description 1
- 208000011589 adenoviridae infectious disease Diseases 0.000 description 1
- 238000001042 affinity chromatography Methods 0.000 description 1
- 108010081667 aflibercept Proteins 0.000 description 1
- 229960002833 aflibercept Drugs 0.000 description 1
- 238000013019 agitation Methods 0.000 description 1
- 235000015107 ale Nutrition 0.000 description 1
- 125000003342 alkenyl group Chemical group 0.000 description 1
- 125000000217 alkyl group Chemical group 0.000 description 1
- JKQXZKUSFCKOGQ-LOFNIBRQSA-N all-trans-Zeaxanthin Natural products CC(=C/C=C/C=C(C)/C=C/C=C(C)/C=C/C1=C(C)CC(O)CC1(C)C)C=CC=C(/C)C=CC2=C(C)CC(O)CC2(C)C JKQXZKUSFCKOGQ-LOFNIBRQSA-N 0.000 description 1
- HMFHBZSHGGEWLO-UHFFFAOYSA-N alpha-D-Furanose-Ribose Natural products OCC1OC(O)C(O)C1O HMFHBZSHGGEWLO-UHFFFAOYSA-N 0.000 description 1
- 230000004075 alteration Effects 0.000 description 1
- 229940059260 amidate Drugs 0.000 description 1
- 150000001412 amines Chemical class 0.000 description 1
- 229940124277 aminobutyric acid Drugs 0.000 description 1
- 229960002684 aminocaproic acid Drugs 0.000 description 1
- 208000007502 anemia Diseases 0.000 description 1
- 210000004102 animal cell Anatomy 0.000 description 1
- 238000010171 animal model Methods 0.000 description 1
- 238000005349 anion exchange Methods 0.000 description 1
- 238000005571 anion exchange chromatography Methods 0.000 description 1
- 230000002424 anti-apoptotic effect Effects 0.000 description 1
- 239000000427 antigen Substances 0.000 description 1
- 108091007433 antigens Proteins 0.000 description 1
- 102000036639 antigens Human genes 0.000 description 1
- 239000003963 antioxidant agent Substances 0.000 description 1
- 235000006708 antioxidants Nutrition 0.000 description 1
- 229910052586 apatite Inorganic materials 0.000 description 1
- 239000000158 apoptosis inhibitor Substances 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- PYMYPHUHKUWMLA-WDCZJNDASA-N arabinose Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)C=O PYMYPHUHKUWMLA-WDCZJNDASA-N 0.000 description 1
- 210000004507 artificial chromosome Anatomy 0.000 description 1
- 125000003118 aryl group Chemical group 0.000 description 1
- 230000003416 augmentation Effects 0.000 description 1
- 239000003855 balanced salt solution Substances 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- 229940000635 beta-alanine Drugs 0.000 description 1
- 229940064804 betadine Drugs 0.000 description 1
- 229960000397 bevacizumab Drugs 0.000 description 1
- 238000006664 bond formation reaction Methods 0.000 description 1
- 229940098773 bovine serum albumin Drugs 0.000 description 1
- 239000007975 buffered saline Substances 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- 239000001506 calcium phosphate Substances 0.000 description 1
- 229910000389 calcium phosphate Inorganic materials 0.000 description 1
- 235000011010 calcium phosphates Nutrition 0.000 description 1
- 201000011510 cancer Diseases 0.000 description 1
- 125000002837 carbocyclic group Chemical group 0.000 description 1
- 125000004432 carbon atom Chemical group C* 0.000 description 1
- UHBYWPGGCSDKFX-UHFFFAOYSA-N carboxyglutamic acid Chemical compound OC(=O)C(N)CC(C(O)=O)C(O)=O UHBYWPGGCSDKFX-UHFFFAOYSA-N 0.000 description 1
- 238000005277 cation exchange chromatography Methods 0.000 description 1
- 239000006143 cell culture medium Substances 0.000 description 1
- 230000030833 cell death Effects 0.000 description 1
- 230000003915 cell function Effects 0.000 description 1
- 230000006037 cell lysis Effects 0.000 description 1
- 238000002659 cell therapy Methods 0.000 description 1
- 230000007248 cellular mechanism Effects 0.000 description 1
- 229920002301 cellulose acetate Polymers 0.000 description 1
- 238000005119 centrifugation Methods 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 238000012512 characterization method Methods 0.000 description 1
- 239000002738 chelating agent Substances 0.000 description 1
- 238000006243 chemical reaction Methods 0.000 description 1
- 238000004587 chromatography analysis Methods 0.000 description 1
- 239000008395 clarifying agent Substances 0.000 description 1
- 238000010367 cloning Methods 0.000 description 1
- 230000004186 co-expression Effects 0.000 description 1
- 239000011248 coating agent Substances 0.000 description 1
- 238000000576 coating method Methods 0.000 description 1
- 238000011284 combination treatment Methods 0.000 description 1
- 238000004891 communication Methods 0.000 description 1
- 239000002299 complementary DNA Substances 0.000 description 1
- 239000003184 complementary RNA Substances 0.000 description 1
- 230000001276 controlling effect Effects 0.000 description 1
- 229910052802 copper Inorganic materials 0.000 description 1
- 239000010949 copper Substances 0.000 description 1
- 238000012937 correction Methods 0.000 description 1
- 239000012228 culture supernatant Substances 0.000 description 1
- 238000012258 culturing Methods 0.000 description 1
- 125000000392 cycloalkenyl group Chemical group 0.000 description 1
- 125000000753 cycloalkyl group Chemical group 0.000 description 1
- 230000009089 cytolysis Effects 0.000 description 1
- 230000004452 decreased vision Effects 0.000 description 1
- 230000006735 deficit Effects 0.000 description 1
- 239000007857 degradation product Substances 0.000 description 1
- 230000005016 dendritic process Effects 0.000 description 1
- 238000000326 densiometry Methods 0.000 description 1
- 239000005547 deoxyribonucleotide Substances 0.000 description 1
- 125000002637 deoxyribonucleotide group Chemical group 0.000 description 1
- 230000035618 desquamation Effects 0.000 description 1
- 239000003599 detergent Substances 0.000 description 1
- 230000006866 deterioration Effects 0.000 description 1
- 229960000633 dextran sulfate Drugs 0.000 description 1
- 239000008121 dextrose Substances 0.000 description 1
- 206010012601 diabetes mellitus Diseases 0.000 description 1
- 238000002405 diagnostic procedure Methods 0.000 description 1
- 235000005911 diet Nutrition 0.000 description 1
- 230000037213 diet Effects 0.000 description 1
- 230000029087 digestion Effects 0.000 description 1
- 102000004419 dihydrofolate reductase Human genes 0.000 description 1
- 239000003085 diluting agent Substances 0.000 description 1
- 239000000539 dimer Substances 0.000 description 1
- NAGJZTKCGNOGPW-UHFFFAOYSA-K dioxido-sulfanylidene-sulfido-$l^{5}-phosphane Chemical compound [O-]P([O-])([S-])=S NAGJZTKCGNOGPW-UHFFFAOYSA-K 0.000 description 1
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 1
- 238000009826 distribution Methods 0.000 description 1
- 238000004945 emulsification Methods 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 230000002708 enhancing effect Effects 0.000 description 1
- 238000006911 enzymatic reaction Methods 0.000 description 1
- NPUKDXXFDDZOKR-LLVKDONJSA-N etomidate Chemical compound CCOC(=O)C1=CN=CN1[C@H](C)C1=CC=CC=C1 NPUKDXXFDDZOKR-LLVKDONJSA-N 0.000 description 1
- 238000011156 evaluation Methods 0.000 description 1
- 231100000040 eye damage Toxicity 0.000 description 1
- 230000004424 eye movement Effects 0.000 description 1
- 201000008065 familial hemophagocytic lymphohistiocytosis 4 Diseases 0.000 description 1
- 239000000945 filler Substances 0.000 description 1
- 238000001943 fluorescence-activated cell sorting Methods 0.000 description 1
- 238000011990 functional testing Methods 0.000 description 1
- 108020001507 fusion proteins Proteins 0.000 description 1
- 102000037865 fusion proteins Human genes 0.000 description 1
- 229960003692 gamma aminobutyric acid Drugs 0.000 description 1
- WIGCFUFOHFEKBI-UHFFFAOYSA-N gamma-tocopherol Natural products CC(C)CCCC(C)CCCC(C)CCCC1CCC2C(C)C(O)C(C)C(C)C2O1 WIGCFUFOHFEKBI-UHFFFAOYSA-N 0.000 description 1
- 238000001476 gene delivery Methods 0.000 description 1
- 238000003197 gene knockdown Methods 0.000 description 1
- 208000016361 genetic disease Diseases 0.000 description 1
- 238000010353 genetic engineering Methods 0.000 description 1
- 230000002518 glial effect Effects 0.000 description 1
- 229960001031 glucose Drugs 0.000 description 1
- 230000013595 glycosylation Effects 0.000 description 1
- 238000006206 glycosylation reaction Methods 0.000 description 1
- 239000003102 growth factor Substances 0.000 description 1
- 239000001963 growth medium Substances 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 239000000185 hemagglutinin Substances 0.000 description 1
- 208000014951 hematologic disease Diseases 0.000 description 1
- 208000018706 hematopoietic system disease Diseases 0.000 description 1
- HNDVDQJCIGZPNO-UHFFFAOYSA-N histidine Natural products OC(=O)C(N)CC1=CN=CN1 HNDVDQJCIGZPNO-UHFFFAOYSA-N 0.000 description 1
- 239000012510 hollow fiber Substances 0.000 description 1
- 102000057125 human FHL1 Human genes 0.000 description 1
- 102000052301 human GNAZ Human genes 0.000 description 1
- 238000004191 hydrophobic interaction chromatography Methods 0.000 description 1
- QJHBJHUKURJDLG-UHFFFAOYSA-N hydroxy-L-lysine Natural products NCCCCC(NO)C(O)=O QJHBJHUKURJDLG-UHFFFAOYSA-N 0.000 description 1
- 208000000069 hyperpigmentation Diseases 0.000 description 1
- 230000003810 hyperpigmentation Effects 0.000 description 1
- 230000005847 immunogenicity Effects 0.000 description 1
- 238000010324 immunological assay Methods 0.000 description 1
- 230000002779 inactivation Effects 0.000 description 1
- 206010022000 influenza Diseases 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 208000017532 inherited retinal dystrophy Diseases 0.000 description 1
- 108091006086 inhibitor proteins Proteins 0.000 description 1
- 230000000977 initiatory effect Effects 0.000 description 1
- 229910052500 inorganic mineral Inorganic materials 0.000 description 1
- 230000002452 interceptive effect Effects 0.000 description 1
- 238000007918 intramuscular administration Methods 0.000 description 1
- 239000007928 intraperitoneal injection Substances 0.000 description 1
- 239000011630 iodine Substances 0.000 description 1
- 229910052740 iodine Inorganic materials 0.000 description 1
- 150000002500 ions Chemical class 0.000 description 1
- RGXCTRIQQODGIZ-UHFFFAOYSA-O isodesmosine Chemical compound OC(=O)C(N)CCCC[N+]1=CC(CCC(N)C(O)=O)=CC(CCC(N)C(O)=O)=C1CCCC(N)C(O)=O RGXCTRIQQODGIZ-UHFFFAOYSA-O 0.000 description 1
- 238000001738 isopycnic centrifugation Methods 0.000 description 1
- 239000000644 isotonic solution Substances 0.000 description 1
- 235000015110 jellies Nutrition 0.000 description 1
- 101150066555 lacZ gene Proteins 0.000 description 1
- 238000002430 laser surgery Methods 0.000 description 1
- 229960004194 lidocaine Drugs 0.000 description 1
- 230000029226 lipidation Effects 0.000 description 1
- 208000018769 loss of vision Diseases 0.000 description 1
- 231100000864 loss of vision Toxicity 0.000 description 1
- 238000004020 luminiscence type Methods 0.000 description 1
- 235000012680 lutein Nutrition 0.000 description 1
- 229960005375 lutein Drugs 0.000 description 1
- KBPHJBAIARWVSC-RGZFRNHPSA-N lutein Chemical compound C([C@H](O)CC=1C)C(C)(C)C=1\C=C\C(\C)=C\C=C\C(\C)=C\C=C\C=C(/C)\C=C\C=C(/C)\C=C\[C@H]1C(C)=C[C@H](O)CC1(C)C KBPHJBAIARWVSC-RGZFRNHPSA-N 0.000 description 1
- 239000001656 lutein Substances 0.000 description 1
- ORAKUVXRZWMARG-WZLJTJAWSA-N lutein Natural products CC(=C/C=C/C=C(C)/C=C/C=C(C)/C=C/C1=C(C)CCCC1(C)C)C=CC=C(/C)C=CC2C(=CC(O)CC2(C)C)C ORAKUVXRZWMARG-WZLJTJAWSA-N 0.000 description 1
- 210000003712 lysosome Anatomy 0.000 description 1
- 230000001868 lysosomic effect Effects 0.000 description 1
- 239000011777 magnesium Substances 0.000 description 1
- 229910052749 magnesium Inorganic materials 0.000 description 1
- 238000012423 maintenance Methods 0.000 description 1
- 230000014759 maintenance of location Effects 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- 239000012092 media component Substances 0.000 description 1
- 239000012533 medium component Substances 0.000 description 1
- YACKEPLHDIMKIO-UHFFFAOYSA-N methylphosphonic acid Chemical compound CP(O)(O)=O YACKEPLHDIMKIO-UHFFFAOYSA-N 0.000 description 1
- 108091070501 miRNA Proteins 0.000 description 1
- 239000002679 microRNA Substances 0.000 description 1
- 244000005700 microbiome Species 0.000 description 1
- 238000007814 microscopic assay Methods 0.000 description 1
- 238000013508 migration Methods 0.000 description 1
- 230000005012 migration Effects 0.000 description 1
- 239000011707 mineral Substances 0.000 description 1
- 239000002480 mineral oil Substances 0.000 description 1
- 235000010446 mineral oil Nutrition 0.000 description 1
- 210000003470 mitochondria Anatomy 0.000 description 1
- 238000001823 molecular biology technique Methods 0.000 description 1
- 238000010172 mouse model Methods 0.000 description 1
- 239000002637 mydriatic agent Substances 0.000 description 1
- 238000001728 nano-filtration Methods 0.000 description 1
- 239000013642 negative control Substances 0.000 description 1
- 230000007524 negative regulation of DNA replication Effects 0.000 description 1
- 210000005036 nerve Anatomy 0.000 description 1
- 230000004297 night vision Effects 0.000 description 1
- 230000000422 nocturnal effect Effects 0.000 description 1
- 230000037434 nonsense mutation Effects 0.000 description 1
- 210000004940 nucleus Anatomy 0.000 description 1
- 235000003170 nutritional factors Nutrition 0.000 description 1
- 201000005111 ocular hyperemia Diseases 0.000 description 1
- 239000003921 oil Substances 0.000 description 1
- 235000019198 oils Nutrition 0.000 description 1
- 238000002515 oligonucleotide synthesis Methods 0.000 description 1
- 235000020660 omega-3 fatty acid Nutrition 0.000 description 1
- 229940012843 omega-3 fatty acid Drugs 0.000 description 1
- 239000006014 omega-3 oil Substances 0.000 description 1
- 235000020665 omega-6 fatty acid Nutrition 0.000 description 1
- 229940033080 omega-6 fatty acid Drugs 0.000 description 1
- 210000001328 optic nerve Anatomy 0.000 description 1
- 210000000056 organ Anatomy 0.000 description 1
- 210000003463 organelle Anatomy 0.000 description 1
- 229960003104 ornithine Drugs 0.000 description 1
- NPKKRSHVJIQBKU-UHFFFAOYSA-N ornogenin Natural products CC(OC(=O)C=Cc1ccccc1)C2(O)CCC3(O)C4(O)CC=C5CC(O)CCC5(C)C4CC(OC(=O)C=Cc6ccccc6)C23C NPKKRSHVJIQBKU-UHFFFAOYSA-N 0.000 description 1
- 230000002018 overexpression Effects 0.000 description 1
- 230000001590 oxidative effect Effects 0.000 description 1
- 230000036961 partial effect Effects 0.000 description 1
- 239000000312 peanut oil Substances 0.000 description 1
- 239000008188 pellet Substances 0.000 description 1
- VSIIXMUUUJUKCM-UHFFFAOYSA-D pentacalcium;fluoride;triphosphate Chemical compound [F-].[Ca+2].[Ca+2].[Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O VSIIXMUUUJUKCM-UHFFFAOYSA-D 0.000 description 1
- YVBBRRALBYAZBM-UHFFFAOYSA-N perfluorooctane Chemical compound FC(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)F YVBBRRALBYAZBM-UHFFFAOYSA-N 0.000 description 1
- 210000002824 peroxisome Anatomy 0.000 description 1
- 239000003208 petroleum Substances 0.000 description 1
- 235000021317 phosphate Nutrition 0.000 description 1
- 125000002467 phosphate group Chemical group [H]OP(=O)(O[H])O[*] 0.000 description 1
- 150000003013 phosphoric acid derivatives Chemical class 0.000 description 1
- 230000026731 phosphorylation Effects 0.000 description 1
- 238000006366 phosphorylation reaction Methods 0.000 description 1
- BZQFBWGGLXLEPQ-REOHCLBHSA-N phosphoserine Chemical compound OC(=O)[C@@H](N)COP(O)(O)=O BZQFBWGGLXLEPQ-REOHCLBHSA-N 0.000 description 1
- 230000000649 photocoagulation Effects 0.000 description 1
- 238000002428 photodynamic therapy Methods 0.000 description 1
- 108090000102 pigment epithelium-derived factor Proteins 0.000 description 1
- 229920000729 poly(L-lysine) polymer Polymers 0.000 description 1
- 238000003752 polymerase chain reaction Methods 0.000 description 1
- 239000000256 polyoxyethylene sorbitan monolaurate Substances 0.000 description 1
- 235000010486 polyoxyethylene sorbitan monolaurate Nutrition 0.000 description 1
- 229920000136 polysorbate Polymers 0.000 description 1
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 1
- 239000001267 polyvinylpyrrolidone Substances 0.000 description 1
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 1
- 239000011148 porous material Substances 0.000 description 1
- 230000001323 posttranslational effect Effects 0.000 description 1
- 229960001621 povidone-iodine Drugs 0.000 description 1
- 239000000843 powder Substances 0.000 description 1
- 239000002243 precursor Substances 0.000 description 1
- 230000002028 premature Effects 0.000 description 1
- 239000003755 preservative agent Substances 0.000 description 1
- 230000002250 progressing effect Effects 0.000 description 1
- 230000000750 progressive effect Effects 0.000 description 1
- 230000001737 promoting effect Effects 0.000 description 1
- 230000000644 propagated effect Effects 0.000 description 1
- 230000001179 pupillary effect Effects 0.000 description 1
- 210000002763 pyramidal cell Anatomy 0.000 description 1
- 230000005855 radiation Effects 0.000 description 1
- 230000002285 radioactive effect Effects 0.000 description 1
- 229960003876 ranibizumab Drugs 0.000 description 1
- 238000003753 real-time PCR Methods 0.000 description 1
- 102000005962 receptors Human genes 0.000 description 1
- 108020003175 receptors Proteins 0.000 description 1
- 108010054624 red fluorescent protein Proteins 0.000 description 1
- 230000002829 reductive effect Effects 0.000 description 1
- 230000003252 repetitive effect Effects 0.000 description 1
- 230000003362 replicative effect Effects 0.000 description 1
- 210000003994 retinal ganglion cell Anatomy 0.000 description 1
- 208000004644 retinal vein occlusion Diseases 0.000 description 1
- 102000029752 retinol binding Human genes 0.000 description 1
- 108091000053 retinol binding Proteins 0.000 description 1
- 239000002336 ribonucleotide Substances 0.000 description 1
- 125000002652 ribonucleotide group Chemical group 0.000 description 1
- 108020004418 ribosomal RNA Proteins 0.000 description 1
- 235000019515 salmon Nutrition 0.000 description 1
- 239000012266 salt solution Substances 0.000 description 1
- 210000003786 sclera Anatomy 0.000 description 1
- 238000012216 screening Methods 0.000 description 1
- 238000005204 segregation Methods 0.000 description 1
- 108010079094 short-wavelength opsin Proteins 0.000 description 1
- 238000004904 shortening Methods 0.000 description 1
- 239000002924 silencing RNA Substances 0.000 description 1
- 108091069025 single-strand RNA Proteins 0.000 description 1
- 239000001488 sodium phosphate Substances 0.000 description 1
- 229910000162 sodium phosphate Inorganic materials 0.000 description 1
- 239000012064 sodium phosphate buffer Substances 0.000 description 1
- 239000002904 solvent Substances 0.000 description 1
- 238000000527 sonication Methods 0.000 description 1
- 239000003549 soybean oil Substances 0.000 description 1
- 235000012424 soybean oil Nutrition 0.000 description 1
- 230000007480 spreading Effects 0.000 description 1
- 238000003892 spreading Methods 0.000 description 1
- 239000008223 sterile water Substances 0.000 description 1
- 238000007920 subcutaneous administration Methods 0.000 description 1
- 239000000758 substrate Substances 0.000 description 1
- 239000013595 supernatant sample Substances 0.000 description 1
- 239000004094 surface-active agent Substances 0.000 description 1
- 238000001356 surgical procedure Methods 0.000 description 1
- 230000004083 survival effect Effects 0.000 description 1
- 239000000725 suspension Substances 0.000 description 1
- 238000010998 test method Methods 0.000 description 1
- MPLHNVLQVRSVEE-UHFFFAOYSA-N texas red Chemical compound [O-]S(=O)(=O)C1=CC(S(Cl)(=O)=O)=CC=C1C(C1=CC=2CCCN3CCCC(C=23)=C1O1)=C2C1=C(CCC1)C3=[N+]1CCCC3=C2 MPLHNVLQVRSVEE-UHFFFAOYSA-N 0.000 description 1
- 239000002562 thickening agent Substances 0.000 description 1
- RYYWUUFWQRZTIU-UHFFFAOYSA-K thiophosphate Chemical compound [O-]P([O-])([O-])=S RYYWUUFWQRZTIU-UHFFFAOYSA-K 0.000 description 1
- YSMODUONRAFBET-WHFBIAKZSA-N threo-5-hydroxy-L-lysine Chemical compound NC[C@@H](O)CC[C@H](N)C(O)=O YSMODUONRAFBET-WHFBIAKZSA-N 0.000 description 1
- 238000003325 tomography Methods 0.000 description 1
- 231100000167 toxic agent Toxicity 0.000 description 1
- 239000003440 toxic substance Substances 0.000 description 1
- 239000003053 toxin Substances 0.000 description 1
- 231100000765 toxin Toxicity 0.000 description 1
- 108700012359 toxins Proteins 0.000 description 1
- FGMPLJWBKKVCDB-UHFFFAOYSA-N trans-L-hydroxy-proline Natural products ON1CCCC1C(O)=O FGMPLJWBKKVCDB-UHFFFAOYSA-N 0.000 description 1
- 230000002103 transcriptional effect Effects 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
- 230000007704 transition Effects 0.000 description 1
- 230000000472 traumatic effect Effects 0.000 description 1
- QORWJWZARLRLPR-UHFFFAOYSA-H tricalcium bis(phosphate) Chemical compound [Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O QORWJWZARLRLPR-UHFFFAOYSA-H 0.000 description 1
- RYFMWSXOAZQYPI-UHFFFAOYSA-K trisodium phosphate Chemical compound [Na+].[Na+].[Na+].[O-]P([O-])([O-])=O RYFMWSXOAZQYPI-UHFFFAOYSA-K 0.000 description 1
- YTZALCGQUPRCGW-ZSFNYQMMSA-N verteporfin Chemical compound N1C(C=C2C(=C(CCC(O)=O)C(C=C3C(CCC(=O)OC)=C(C)C(N3)=C3)=N2)C)=C(C=C)C(C)=C1C=C1C2=CC=C(C(=O)OC)[C@@H](C(=O)OC)[C@@]2(C)C3=N1 YTZALCGQUPRCGW-ZSFNYQMMSA-N 0.000 description 1
- 229940061392 visudyne Drugs 0.000 description 1
- 235000019154 vitamin C Nutrition 0.000 description 1
- 239000011718 vitamin C Substances 0.000 description 1
- 235000019165 vitamin E Nutrition 0.000 description 1
- 229940046009 vitamin E Drugs 0.000 description 1
- 239000011709 vitamin E Substances 0.000 description 1
- 150000003722 vitamin derivatives Chemical class 0.000 description 1
- 201000007790 vitelliform macular dystrophy Diseases 0.000 description 1
- 208000020938 vitelliform macular dystrophy 2 Diseases 0.000 description 1
- 230000001755 vocal effect Effects 0.000 description 1
- 238000009736 wetting Methods 0.000 description 1
- 239000000080 wetting agent Substances 0.000 description 1
- FJHBOVDFOQMZRV-XQIHNALSSA-N xanthophyll Natural products CC(=C/C=C/C=C(C)/C=C/C=C(C)/C=C/C1=C(C)CC(O)CC1(C)C)C=CC=C(/C)C=CC2C=C(C)C(O)CC2(C)C FJHBOVDFOQMZRV-XQIHNALSSA-N 0.000 description 1
- 108091005957 yellow fluorescent proteins Proteins 0.000 description 1
- 235000010930 zeaxanthin Nutrition 0.000 description 1
- 229940043269 zeaxanthin Drugs 0.000 description 1
- 239000001775 zeaxanthin Substances 0.000 description 1
- 229910052725 zinc Inorganic materials 0.000 description 1
- 239000011701 zinc Substances 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/63—Introduction of foreign genetic material using vectors; Vectors; Use of hosts therefor; Regulation of expression
- C12N15/79—Vectors or expression systems specially adapted for eukaryotic hosts
- C12N15/85—Vectors or expression systems specially adapted for eukaryotic hosts for animal cells
- C12N15/86—Viral vectors
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K48/00—Medicinal preparations containing genetic material which is inserted into cells of the living body to treat genetic diseases; Gene therapy
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K48/00—Medicinal preparations containing genetic material which is inserted into cells of the living body to treat genetic diseases; Gene therapy
- A61K48/005—Medicinal preparations containing genetic material which is inserted into cells of the living body to treat genetic diseases; Gene therapy characterised by an aspect of the 'active' part of the composition delivered, i.e. the nucleic acid delivered
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K48/00—Medicinal preparations containing genetic material which is inserted into cells of the living body to treat genetic diseases; Gene therapy
- A61K48/005—Medicinal preparations containing genetic material which is inserted into cells of the living body to treat genetic diseases; Gene therapy characterised by an aspect of the 'active' part of the composition delivered, i.e. the nucleic acid delivered
- A61K48/0058—Nucleic acids adapted for tissue specific expression, e.g. having tissue specific promoters as part of a contruct
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P27/00—Drugs for disorders of the senses
- A61P27/02—Ophthalmic agents
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/46—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates
- C07K14/47—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates from mammals
- C07K14/4701—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates from mammals not used
- C07K14/472—Complement proteins, e.g. anaphylatoxin, C3a, C5a
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2750/00—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA ssDNA viruses
- C12N2750/00011—Details
- C12N2750/14011—Parvoviridae
- C12N2750/14111—Dependovirus, e.g. adenoassociated viruses
- C12N2750/14134—Use of virus or viral component as vaccine, e.g. live-attenuated or inactivated virus, VLP, viral protein
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2750/00—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA ssDNA viruses
- C12N2750/00011—Details
- C12N2750/14011—Parvoviridae
- C12N2750/14111—Dependovirus, e.g. adenoassociated viruses
- C12N2750/14141—Use of virus, viral particle or viral elements as a vector
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2750/00—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA ssDNA viruses
- C12N2750/00011—Details
- C12N2750/14011—Parvoviridae
- C12N2750/14111—Dependovirus, e.g. adenoassociated viruses
- C12N2750/14141—Use of virus, viral particle or viral elements as a vector
- C12N2750/14143—Use of virus, viral particle or viral elements as a vector viral genome or elements thereof as genetic vector
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2750/00—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA ssDNA viruses
- C12N2750/00011—Details
- C12N2750/14011—Parvoviridae
- C12N2750/14111—Dependovirus, e.g. adenoassociated viruses
- C12N2750/14141—Use of virus, viral particle or viral elements as a vector
- C12N2750/14145—Special targeting system for viral vectors
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2800/00—Nucleic acids vectors
- C12N2800/22—Vectors comprising a coding region that has been codon optimised for expression in a respective host
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2800/00—Nucleic acids vectors
- C12N2800/60—Vectors containing traps for, e.g. exons, promoters
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2830/00—Vector systems having a special element relevant for transcription
- C12N2830/50—Vector systems having a special element relevant for transcription regulating RNA stability, not being an intron, e.g. poly A signal
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2840/00—Vectors comprising a special translation-regulating system
- C12N2840/007—Vectors comprising a special translation-regulating system cell or tissue specific
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Genetics & Genomics (AREA)
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Biotechnology (AREA)
- General Health & Medical Sciences (AREA)
- Organic Chemistry (AREA)
- Molecular Biology (AREA)
- Zoology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Biomedical Technology (AREA)
- Medicinal Chemistry (AREA)
- Wood Science & Technology (AREA)
- General Engineering & Computer Science (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Veterinary Medicine (AREA)
- Pharmacology & Pharmacy (AREA)
- Biochemistry (AREA)
- Epidemiology (AREA)
- Biophysics (AREA)
- Virology (AREA)
- Plant Pathology (AREA)
- Physics & Mathematics (AREA)
- Microbiology (AREA)
- Gastroenterology & Hepatology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Toxicology (AREA)
- Ophthalmology & Optometry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201762574814P | 2017-10-20 | 2017-10-20 | |
| US62/574,814 | 2017-10-20 | ||
| PCT/US2018/056709 WO2019079718A1 (en) | 2017-10-20 | 2018-10-19 | COMPOSITIONS AND METHODS FOR TREATING AGE-RELATED MACULAR DEGENERATION |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2021500922A true JP2021500922A (ja) | 2021-01-14 |
| JP2021500922A5 JP2021500922A5 (enExample) | 2021-11-25 |
Family
ID=66174265
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2020542710A Withdrawn JP2021500922A (ja) | 2017-10-20 | 2018-10-19 | 加齢黄斑変性を処置するための組成物および方法 |
Country Status (7)
| Country | Link |
|---|---|
| US (1) | US20210188927A1 (enExample) |
| EP (1) | EP3697920A4 (enExample) |
| JP (1) | JP2021500922A (enExample) |
| CN (1) | CN111788311A (enExample) |
| AU (1) | AU2018351491A1 (enExample) |
| CA (1) | CA3079553A1 (enExample) |
| WO (1) | WO2019079718A1 (enExample) |
Families Citing this family (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP3824095A4 (en) * | 2018-07-20 | 2022-04-20 | University of Utah Research Foundation | GENE THERAPY FOR MACULATE GENERATION |
| US20210371480A1 (en) * | 2018-10-23 | 2021-12-02 | Gemini Therapeutics Inc. | Compositions and methods for treating age-related macular degeneration and other diseases |
| GB201821089D0 (en) * | 2018-12-21 | 2019-02-06 | Gyroscope Therapeutics Ltd | Codon-optimised complement factor I |
| KR20230019062A (ko) * | 2019-10-22 | 2023-02-07 | 어플라이드 제네틱스 테크놀로지스 코퍼레이션 | 연령-관련 황반 변성 및 다른 안구 질환 및 장애의 치료를 위한 아데노-연관 바이러스 (aav) 벡터 |
| US20220395557A1 (en) * | 2019-10-23 | 2022-12-15 | Gemini Therapeutics Sub, Inc. | Methods for treating patients having cfh mutations with recombinant cfh proteins |
| IL299048A (en) | 2020-06-14 | 2023-02-01 | Vertex Pharma | Variants of complement factor - 1 fusion structures and preparations containing them and their uses |
| AU2021362770A1 (en) * | 2020-10-16 | 2022-12-08 | Gyroscope Therapeutics Limited | Nucleic acid encoding an anti-VEGF entity and a negative complement regulator and uses thereof for the treatment of age-related macular degeneration |
| WO2022094340A1 (en) * | 2020-10-30 | 2022-05-05 | Gemini Therapeutics Sub, Inc. | Methods for treating inflammatory ocular diseases with complement factor h |
| CN115786308B (zh) * | 2022-11-24 | 2024-05-31 | 江苏海洋大学 | 一种提高右旋糖酐酶热稳定性的方法及突变体 |
Family Cites Families (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2005333845A (ja) * | 2004-05-25 | 2005-12-08 | Tokyoto Igaku Kenkyu Kiko | ミトコンドリア局在型DsRed2及びECFP発現ベクター |
| EP2357257B1 (en) * | 2005-02-14 | 2019-07-31 | University of Iowa Research Foundation | Methods and reagents for treatment and diagnosis of age-related macular degeneration |
| RU2727411C2 (ru) * | 2015-09-24 | 2020-07-21 | Дзе Трастиз Оф Дзе Юниверсити Оф Пенсильвания | Композиция и способ для лечения заболевания, опосредованного комплементом |
| GB201519086D0 (en) * | 2015-10-28 | 2015-12-09 | Syncona Partners Llp | Gene Therapy |
-
2018
- 2018-10-19 JP JP2020542710A patent/JP2021500922A/ja not_active Withdrawn
- 2018-10-19 WO PCT/US2018/056709 patent/WO2019079718A1/en not_active Ceased
- 2018-10-19 US US16/757,268 patent/US20210188927A1/en not_active Abandoned
- 2018-10-19 EP EP18869436.8A patent/EP3697920A4/en not_active Withdrawn
- 2018-10-19 CA CA3079553A patent/CA3079553A1/en not_active Abandoned
- 2018-10-19 CN CN201880078342.1A patent/CN111788311A/zh active Pending
- 2018-10-19 AU AU2018351491A patent/AU2018351491A1/en not_active Abandoned
Also Published As
| Publication number | Publication date |
|---|---|
| CA3079553A1 (en) | 2019-04-25 |
| WO2019079718A1 (en) | 2019-04-25 |
| EP3697920A1 (en) | 2020-08-26 |
| AU2018351491A1 (en) | 2020-05-07 |
| EP3697920A4 (en) | 2022-03-02 |
| CN111788311A (zh) | 2020-10-16 |
| US20210188927A1 (en) | 2021-06-24 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP2021500922A (ja) | 加齢黄斑変性を処置するための組成物および方法 | |
| US10383922B2 (en) | AAV-mediated gene therapy for RPGR X-linked retinal degeneration | |
| EP2844302B1 (en) | Viral vectors for the treatment of retinal dystrophy | |
| US20210371480A1 (en) | Compositions and methods for treating age-related macular degeneration and other diseases | |
| JP2020028308A (ja) | 変異体キャプシドを有するアデノ関連ウイルスビリオンおよびその使用方法 | |
| EP3795180A1 (en) | Gene therapy for ocular disorders | |
| CN113474461A (zh) | 治疗bietti晶体营养不良的组合物和方法 | |
| KR102799528B1 (ko) | Rdh12가 연루된 장애 및 질환의 치료를 위한 방법 및 조성물 | |
| JP7211960B2 (ja) | 眼疾患の遺伝子治療 | |
| US20210261625A1 (en) | Modified adeno-associated viral capsid proteins for ocular gene therapy and methods of use thereof | |
| US20220396807A1 (en) | Methods for treating patients having cfh mutations with cfh-encoding vectors | |
| US20220226507A1 (en) | Optimized gene therapy targeting retinal cells | |
| HK40036018A (en) | Compositions and methods for treating age-related macular degeneration | |
| CN113795279A (zh) | 靶向akt通路的神经保护性基因疗法 | |
| WO2024229049A1 (en) | Dual aav vectors for treating stargardt disease | |
| HK40049894A (en) | Gene therapy for ocular disorders | |
| HK40013460B (zh) | 用於治疗涉及rdh12的病症和疾病的方法和组合物 | |
| HK40013460A (en) | Methods and compositions for treatment of disorders and diseases involving rdh12 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20211018 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20211018 |
|
| A761 | Written withdrawal of application |
Free format text: JAPANESE INTERMEDIATE CODE: A761 Effective date: 20220826 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20220913 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A821 Effective date: 20220829 |